phase change materials for latent heat thermal energy storage
相变微胶囊填充改性聚乙二醇

林业工程学报,2023,8(4):102-109JournalofForestryEngineeringDOI:10.13360/j.issn.2096-1359.202211007收稿日期:2022-11-07㊀㊀㊀㊀修回日期:2023-03-27基金项目:山东省重大科技创新工程(2019JZZY010305)㊂作者简介:林捷,男,研究方向为生物质复合材料㊂通信作者:孙丰文,男,研究员㊂E⁃mail:sunfw@njfu.edu.cn相变微胶囊填充改性聚乙二醇/酚醛泡沫的制备林捷,张茜,魏信义,孙丰文∗(南京林业大学材料科学与工程学院,南京210037)摘㊀要:相变微胶囊可以通过相变潜热的方式将能量储存在其内部,可作为储能材料用于保温㊁节能等领域㊂酚醛泡沫具有隔声㊁质轻㊁难燃㊁保温等优异性能,常用作城市建筑的墙体保温材料,但酚醛泡沫机械性能差㊁韧性低㊁粉化率高等缺点限制了其进一步发展㊂笔者以可发性酚醛树脂为原料,将其与聚乙二醇(PEG⁃400)和相变微胶囊(MPCM)进行共混发泡制备酚醛泡沫,研究不同质量分数的改性剂对酚醛泡沫物理力学性能㊁化学结构㊁微观结构㊁热学性能和隔声性能的影响㊂红外光谱测试结果表明,酚醛树脂中的羟基或羟甲基与聚乙二醇中的羟基发生了反应,将柔性长键引入到酚醛树脂的结构中㊂PEG⁃400降低了泡沫的粉化率,提高了泡沫的冲击韧性,MPCM的填充改性提高了泡沫的压缩强度㊂当添加质量分数为4%的PEG⁃400和10%的MPCM时,泡沫的冲击韧性㊁表观密度和压缩强度分别提高了56%,63.9%和95.9%,残炭率降低了11.6%,隔声量降低了1.2%㊂当PEG⁃400和MPCM的添加量分别控制在4%和10%时,泡沫的综合性能最佳㊂关键词:酚醛泡沫;相变材料;聚乙二醇;保温;增韧改性中图分类号:TQ328㊀㊀㊀㊀㊀文献标志码:A㊀㊀㊀㊀㊀文章编号:2096-1359(2023)04-0102-08Preparationandcharacterizationofmodifiedpolyethyleneglycol/phenolicfoamfilledwithphasechangemicrocapsulesLINJie,ZHANGQian,WEIXinyi,SUNFengwen∗(CollegeofMaterialsScienceandEngineering,NanjingForestryUniversity,Nanjing210037,China)Abstract:Utilizationofgreenenergy⁃savingbuildingmaterialsinthebuildingindustryisanimportantwaytoreducebuildingenergyconsumption.Becauseofthelowapparentdensityandthermalconductivity,thefoammaterialisoftenusedaswallinsulationmaterialforurbanbuildings.Traditionalinsulationfoammaterials,suchaspolyvinylchloride,polyurethane,andpolystyrene,havepoorflame⁃retardantperformanceandaredifficulttomeettherequirementsoffirepreventioninmodernbuildings.Onthecontrary,themostoutstandingadvantagesofphenolicfoamareflameresistance,lowsmokeproduction,andcreepresistanceathightemperatures,sothephenolicfoamhasattractedtheattentionofmanyresearchersandengineers.Butthephenolicfoamhasthedrawbacksofpoormechanicalproperties,lowtoughness,andhighpulverizationrate,whichlimititsfurtherapplications.Phasechangematerialsarethermallyfunctionalmaterials,inwhichenergyisstoredaslatentheat.Byaddingphasechangesubstancesintoexistingbuildingmaterials,energy⁃savingbuildinginsulationwallswithgoodheatstorageperformancecanbeprepared.However,mostphasechangematerialsusedintheconstructionindustryaresolid⁃liquidphasechange,whichareeasytoleakaftermelting.Therefore,microencapsulationtechnologyisagoodsolutiontoaddressthisissue.Inthisexperiment,thephenolicfoamwithgoodmechanicalproperties,energystorage,andsoundinsulationpropertieswaspreparedbyblendingphenolicresinwithpolyethyleneglycol(PEG⁃400)andmicrophasechangematerials(MPCM).Inthispaper,theeffectsofdifferentmassfractionsofmodifiersonthephysicalandmechanicalproperties,chemicalstruc⁃ture,microstructure,thermalproperties,andsoundinsulationpropertiesofthephenolicfoamwerestudied.TheFouriertransforminfraredspectralanalysisshowedthatthehydroxylorhydroxymethylgroupsofthephenolicresinreactedwiththehydroxylgroupsofpolyethyleneglycol,introducingflexiblelongbondsintothestructureofthephe⁃nolicresin.Thescanningelectronmicroscopeimagesshowedthatthephasechangemicrocapsuleswereevenlyfilledinthecellsofthephenolicfoam.TheadditionofPEG⁃400reducedthepulverizationrateoffoamandimprovedtheim⁃pacttoughnessoffoam,andtheMPCMmodificationenhancedthecompressionstrengthofthefoam.WhenthemassfrictionofPEG⁃400andMPCMwere4%and10%,respectively,theimpacttoughness,apparentdensity,andcom⁃㊀第4期林捷,等:相变微胶囊填充改性聚乙二醇/酚醛泡沫的制备pressivestrengthofthefoamincreasedby56%,63.9%,and95.9%,respectively.Thecarbonresidualdecreasedby11.6%,andthesoundinsulationcapacitydecreasedby1.2%.WhentheamountsofPEG⁃400andMPCMwere4%and10%,respectively,thefoamachievedtheoptimaloverallperformance.Keywords:phenolicfoam;phasechangematerials;polyethyleneglycol;insulation;tougheningmodification㊀㊀传统泡沫材料如聚氯乙烯㊁聚氨酯㊁聚苯乙烯等,具有优良的绝缘性能和机械性能,常用于建筑墙体的保温和货物的包装材料,但其阻燃性能较差,且燃烧时易产生刺激性的有毒气体[1],限制了其在建筑领域的进一步应用㊂酚醛泡沫作为新一代的泡沫保温材料,其成本低㊁质轻㊁低毒㊁阻燃㊁发烟量低㊁耐高温蠕变㊁耐腐蚀㊁化学性质稳定,受到众多研究者的广泛关注[2-3]㊂但是,酚醛树脂分子内部存在大量仅靠亚甲基键相连的类如苯环的刚性结构,缺乏柔性长链基团,由于亚甲基键键短,酚醛树脂分子空间位阻大,链节旋转自由度小[4],酚醛泡沫机械性能差㊁韧性低㊁粉化率高㊂针对酚醛泡沫的这些缺陷,众多学者对其进行增韧改性研究㊂目前,酚醛泡沫的改性方式可以分为两类:物理增韧改性和化学增韧改性[5]㊂物理增韧也称为外增韧,就是将可发性酚醛树脂与柔性的改性剂直接进行混合,两者之间不发生任何化学反应㊂目前常用的物理增韧剂主要有橡胶[6]㊁热塑性树脂[7]和纤维[8-9]㊂马玉峰等[10]先使用KH⁃550和碱液处理木纤维,再将其作为增韧改性剂制备酚醛泡沫,结果表明,木纤维经过KH⁃550和碱液处理后,其与酚醛树脂的界面相容性明显改善,木纤维的引入提高了泡沫的机械性能㊂化学增韧法是指在树脂合成反应中引入增韧剂,例如在羟甲基和酚羟基上接入烃基长链或者其他柔性基团㊂柔性链段的引入可以改变酚醛泡沫原有的机械性能㊂当外力作用在增韧改性后的酚醛泡沫上,材料可以通过自身形变来吸收一部分能量,避免材料出现应力集中,从而改善材料的韧性㊁降低粉化率[11]㊂宋飞[12]合成了1种含磷桐油基硅氧烷,并将其作为增韧剂制备酚醛泡沫,结果表明,柔性长链的引入提高了泡沫的压缩㊁弯曲强度,隔热性能,阻燃性以及抑烟性能㊂此外,将相变材料引入现有的建筑材料制成具有良好蓄热性能的节能建材也是当前的研究热点之一㊂相变材料是1种热功能性材料,可以相变潜热的方式将能量储存在材料内部㊂通过应用场景的转换和时间的推移,可以实现能量在不同时空位置上的传递与转换㊂与其他绝缘材料不同,相变材料在达到其熔化温度时会吸收大量热量,并随着固化释放其储存的潜热㊂相变储能具有相变潜热大㊁价格低廉㊁适用范围广㊁环境友好等优点[13]㊂但是,目前建筑行业所使用的相变材料大多数发生的是固⁃液相变,在熔融后相变材料容易泄露㊂对相变材料进行微胶囊化包覆,可有效解决其易泄露问题,并且微胶囊化可预防相变材料与外界其他物质发生反应,保护内部的相变材料并减少有害物质污染环境[14]㊂相变微胶囊可以直接添加在石膏板㊁混凝土中,也可以将其与聚氨酯㊁环氧树脂混合发泡制备保温泡沫材料[15-16]㊂因此,笔者通过聚乙二醇和相变微胶囊对酚醛泡沫进行复合改性,在改善泡沫韧性的同时赋予其一定的蓄热性能,以开发1种具有良好阻燃㊁隔声㊁蓄热性能的新型建筑材料㊂1㊀材料与方法1.1㊀材料和试剂正戊烷㊁吐温⁃80㊁磷酸㊁聚乙二醇400(polyethy⁃leneglycol⁃400,PEG⁃400),分析纯,均购于上海麦克林生化有限公司;对甲苯磺酸㊁盐酸,化学纯,均购于国药集团化学试剂有限公司;酚醛树脂,固体含量为83.76%,黏度为9220mPa㊃s,pH为6.7,含水率4.64%,由南京太尔化工提供;相变微胶囊(相变材料为正十八烷,囊壁材料为聚甲基丙烯酸甲酯),胶囊粒径5 10μm,购自安徽美科迪智能微胶囊科技有限公司㊂1.2㊀样品制备将40g可发性酚醛树脂㊁2g表面活性剂(吐温⁃80)㊁6g发泡剂(正戊烷)㊁6.4g固化剂(固化剂:自制,盐酸㊁对甲苯磺酸㊁磷酸㊁水质量比为1ʒ4ʒ3ʒ2)加入500mL烧杯中,使用高速机械搅拌机以400r/min的速率搅拌60s㊂将混合均匀后的原料倒入发泡模具,放入70ħ的烘箱中,固化30min㊂待样品冷却完毕后进行脱模取样,制备得到酚醛泡沫(phenolicresin,PF)㊂取40g树脂和一定质量分数(酚醛树脂质量的2%,4%和6%)的PEG⁃400加入烧杯中,使用高速机械搅拌机以800r/min的转速搅拌60s㊂然后,依次加入发泡剂,搅拌均匀后倒入发泡模具,放301林业工程学报第8卷入烘箱进行发泡,制备得到聚乙二醇改性酚醛泡沫(2%PEG⁃400/PF㊁4%PEG⁃400/PF和6%PEG⁃400/PF)㊂先将一定质量分数(酚醛树脂质量的5%,10%和15%)的相变微胶囊粉末(microphasechangematerials,MPCM)加入40g酚醛树脂中,在40 50ħ下超声分散20min㊂按上述配比,依次加入聚乙二醇和发泡剂,制备相变微胶囊填充改性聚乙二醇/酚醛泡沫(5%MPCM/PEG⁃400/PF㊁10%MPCM/PEG⁃400/PF和15%MPCM/PEG⁃400/PF)㊂将制备得到的泡沫切除外皮,置于通风橱中熟化48h,使泡沫中残余发泡剂挥发㊂1.3㊀材料测试与表征1.3.1㊀力学性能的测定表观密度按照国标GB/T6343 2009‘泡沫塑料及橡胶表观密度测定“进行测量;压缩强度按照GB/T8813 2008‘硬质泡沫塑料压缩性能的测定“,采用型号为CMT4303的微机控制电子万能材料试验机进行测定;冲击强度按GB/T1043.12008‘塑料简支梁冲击性能的测定“进行测试㊂每组制备9个样品进行性能测试,最终结果取9次测量后的平均值㊂1.3.2㊀粉化率的测定粉化率按照国标GB/T12812 2006‘硬质泡沫塑料易碎性的测定“进行测试㊂将试样制备成30mmˑ30mmˑ30mm的立方体㊂先将样品置于粒径48μm(300目)的砂纸上,再将质量为200g的砝码置于样品正上方,以恒定的力量在300目砂纸上摩擦250mm距离,反复摩擦30次㊂每组制备9个样品进行性能测试,最终结果取9次测量后的平均值㊂称量试验前后试样质量,利用摩擦后试样的质量损失率来表征粉化率㊂粉化率计算如下:mt=m1-m2m1ˑ100%(1)式中:mt为粉化率;m1为泡沫初始质量,g;m2为磨损后泡沫质量,g㊂1.3.3㊀傅里叶红外光谱测试将泡沫研磨成粉末,采用KBr压片法,使用VERTEX80V型红外光谱仪进行扫描分析,波数范围为400 4000cm-1㊂1.3.4㊀微观形貌观察采用Quanta200型环境扫描电子显微镜观察泡沫断面的微观结构,并根据扫描电镜图利用软件Nanomeasure1.2计算泡孔尺寸,绘制分布图㊂1.3.5㊀热重测试采用TG209F3型热重分析仪对试样进行热性能分析,测试条件为:氮气氛围,升温速率为10ħ/min,升温范围为30 800ħ,氮气流速为10mL/min,参比物为Al2O3坩埚㊂1.3.6㊀差示扫描量热仪测试采用DSC200F3型差示扫描量热仪对样品的焓值进行测试㊂氮气氛围,升温速率10ħ/min,0 80ħ循环3次㊂1.3.7㊀导热系数的测定采用HFM436型导热系数测定仪(德国耐驰公司)测定样品在25ħ时的导热系数,每组测试3次,取平均值㊂1.3.8㊀隔声性能测试按照GB/T18696.2 2002‘声学阻抗管中吸声系数和声阻抗的测量:第2部分:传递函数法“对酚醛泡沫的隔声性能进行测定,每组测试3次,取平均值㊂2㊀结果与分析2.1㊀聚乙二醇改性酚醛泡沫的粉化率和冲击韧性PEG⁃400是一种高分子聚合物,具有良好的水溶性,并且与高分子有机物有很好的相容性㊂聚乙二醇改性酚醛泡沫的粉化率和冲击韧性见图1㊂由图1可知,随着PEG⁃400的加入,酚醛泡沫的粉化率不断降低,冲击韧性呈现先增加后降低的趋势㊂图1结果表明,PEG⁃400提高了酚醛泡沫的冲击韧性,降低了酚醛泡沫的粉化率,可能是PEG⁃400与酚醛树脂分子发生化学反应,在酚醛树脂结构中接入了柔性长链,最终达到了增韧的效果㊂图1㊀聚乙二醇改性酚醛泡沫的粉化率和冲击韧性Fig.1㊀FragilityandimpacttoughnessofPEG⁃400modifiedphenolicfoams2.2㊀聚乙二醇改性酚醛泡沫的表观密度和压缩强度㊀㊀聚乙二醇改性酚醛泡沫的表观密度和压缩强401㊀第4期林捷,等:相变微胶囊填充改性聚乙二醇/酚醛泡沫的制备度见图2㊂由图2可知,泡沫的压缩强度随着聚乙二醇添加量的增加逐渐降低,而酚醛泡沫的表观密度随着聚乙二醇添加量的增加呈现先增加后降低的趋势㊂当体系中PEG⁃400的添加量较低时,PEG⁃400会填充部分泡沫空隙,导致密度变大;但添加量过多后会导致体系黏度降低,减小泡孔的图2㊀聚乙二醇改性酚醛泡沫的表观密度和压缩强度Fig.2㊀ApparentdensitiesandcompressionstrengthsofPEG⁃400modifiedphenolicfoams生长阻力,可能在泡沫形成过程中出现大孔,导致密度降低㊂结合PEG⁃400添加量对酚醛泡沫粉化率和冲击韧性的影响,当PEG⁃400添加量为4%时,酚醛泡沫的综合性能最优,满足后续实验要求㊂2.3㊀相变微胶囊填充改性酚醛泡沫的物理力学性能㊀㊀实验选用的MPCM具有良好的机械性能和蓄热性能㊂相变微胶囊填充改性酚醛泡沫的物理力学性能测定结果见图3㊂由图3可知,MPCM的加入对酚醛泡沫的粉化率影响不大,但会提高泡沫的冲击韧性,当MPCM添加量为10%时冲击韧性较PF提高了56.0%㊂随着MPCM添加量的增加,改性酚醛泡沫的表观密度和压缩强度显著提高,当MPCM添加量为10%时,表现密度和压缩强度分别提高了63.9%和95.9%㊂MPCM的加入,会导致体系黏度增大,影响泡孔生长,降低发泡倍率㊂同时由于MPCM的粒径较小,可以均匀地分散在泡孔中,起到了类似于填料的作用,提高了酚醛泡沫的压缩强度㊂图3㊀相变微胶囊填充改性酚醛泡沫的物理力学性能Fig.3㊀Physicalandmechanicalpropertiesofmodifiedphenolicfoamsfilledwithphasechangemicrocapsules2.4㊀酚醛泡沫的傅里叶红外光谱酚醛泡沫改性前后的红外光谱分析结果见图4㊂未改性酚醛泡沫红外光谱图显示,3434cm-1位置为羟基的伸缩振动峰,2924cm-1处为羟甲基键的伸缩振动峰,1617cm-1处为苯环中碳碳双键的伸缩振动峰㊂从图4可以看出:在1175 1275cm-1处出现C O C的特征吸收峰,说明聚乙二醇的羟基与酚醛树脂中的羟基发生了部分反应[17],成功在酚醛树脂的分子结构上引入了柔性长链,从而起到了增韧的效果㊂在MPCM/PEG⁃400/PF的红外图谱中,2915和2845cm-1处的高强度峰是正十八烷分子中甲基的对称伸缩振动峰和亚甲基的不对称伸缩振动峰,1468和1373cm-1处的吸收峰是亚甲基和甲基中C H的弯曲振动峰;2952和1734cm-1处的吸收峰分别为聚甲基丙烯酸甲酯的C H与C O的伸缩振动峰,而1199和1161cm-1处的双吸收峰属于C O的伸缩振动峰[18]㊂上述特征峰表明,相变微胶囊与酚醛树脂充分混合,形成了MPCM/PF复合材料,且MPCM的引入没有改变酚醛树脂基体的化学结构㊂图4㊀酚醛泡沫的傅里叶红外光谱Fig.4㊀FT⁃IRcurvesofphenolicfoams501林业工程学报第8卷2.5㊀酚醛泡沫的微观形貌分析酚醛泡沫的断面微观形貌见图5㊂图5a为PF的微观结构图,未改性泡沫的泡孔结构不均匀,开孔率较高,泡孔大多呈现椭圆形㊂相比之下,PEG⁃400改性后泡沫的泡孔结构更加均匀㊂但随着PEG⁃400含量的增加,泡沫的开孔率逐渐增大㊂泡孔孔径分布见图6,图6a显示纯PF的平均孔径为141.40μm,孔径大小分布范围为90 200μm㊂PEG⁃400的添加对酚醛泡沫的孔径影响不大,2%PEG⁃400/PF㊁4%PEG⁃400/PF和6%PEG⁃400/PF的平均孔径分别为136.23,160.59和142.56μm㊂综合来看,4%PEG⁃400改性后的酚醛泡沫具有最佳的泡孔结构㊂a)PF;b)2%PEG⁃400/PF;c)4%PEG⁃400/PF;d)6%PEG⁃400/PF;e)5%MPCM/PEG⁃400/PF;f,h)10%MPCM/PEG⁃400/PF;g,i)15%MPCM/PEG⁃400/PF㊂图5㊀酚醛泡沫的SEM图Fig.5㊀SEMimagesofphenolicfoamsa)PF;b)2%PEG⁃400/PF;c)4%PEG⁃400/PF;d)6%PEG⁃400/f)10%MPCM/PEG⁃400/PF;g)15%MPCM/PEG⁃400/PF㊂图6㊀酚醛泡沫的泡孔孔径分布Fig.6㊀Cellsizedistributionsofphenolicfoams㊀㊀图5e g为MPCM填充改性后的酚醛泡沫放大800倍的微观结构图㊂与纯酚醛泡沫相比,MPCM改性后泡沫的平均孔径减小㊂由图6e g可知,MPCM的添加会减小酚醛泡沫的孔径㊂5%MPCM/PEG⁃400/PF㊁10%MPCM/PEG⁃400/PF和15%MPCM/PEG⁃400/PF的平均孔径分别为85.68,99.18和110.20μm,与PF相比分别降低了39.4%,29.9%和22.1%㊂可能有以下两种原因:①分散均601㊀第4期林捷,等:相变微胶囊填充改性聚乙二醇/酚醛泡沫的制备匀的MPCM作为成核剂,可以降低临界成核自由能,从而增加了形成的气泡核的数量;②混合MPCM后泡沫的黏度增大,限制了细胞的生长㊂图5h㊁i为10%MPCM/PEG⁃400/PF和15%MPCM/PEG⁃400/PF放大2000倍的图片,可以很明显地看见MPCM较为均匀地分散在泡沫中㊂2.6㊀酚醛泡沫的热性能分析2.6.1㊀热稳定性改性泡沫的热重(thermogravimetry,TG)和微分热重(derivativethermogravimetry,DTG)曲线见图7㊂从图7a可看出,800ħ条件下酚醛泡沫的残炭率为57.8%㊂低于100ħ时,泡沫的质量损失是由于其内部的残余水分和发泡剂的挥发㊂在100200ħ,泡沫的降解主要是材料的进一步固化脱水和酚醛树脂的降解;300 500ħ时,泡沫开始分解,高分子链降解为单个苯环;500 800ħ时,苯环结构炭化程度进一步增大㊂图7㊀改性泡沫的TG和DTG曲线Fig.7㊀TGandDTGcurvesofthephenolicfoams㊀㊀PEG⁃400的分解温度与酚醛泡沫材料不同,所以PEG⁃400/PF的TG曲线较PF相比也有所差异㊂从图7b可以看出,PEG⁃400改性酚醛泡沫材料在200 300ħ时的分解速率高于酚醛泡沫,这主要是因为PEG⁃400在此段温度内发生了分解㊂随着PEG⁃400添加量的增加,泡沫的残炭率不断降低㊂800ħ时,2%,4%和6%PEG⁃400改性酚醛泡沫的残炭率分别为56.4%,55.3%和53.7%㊂相较于PF和PEG⁃400/PF,MPCM/PEG⁃400/PF在200 300ħ和400 500ħ两个阶段的分解速率远快于前两者㊂其中,200 300ħ的质量损失主要归因于囊芯相变材料正十八烷的分解;400 500ħ的失重是囊壁材料PMMA分解引起的㊂5%,10%和15%MPCM/PEG⁃400/PF在800ħ的残炭率分别为54.4%,51.1%和47.9%㊂由于PEG⁃400和MPCM的分解温度都低于酚醛泡沫,改性后试样的残炭率出现了一定程度的降低㊂当PEG⁃400的质量分数为4%㊁MPCM的质量分数不超过10%时,泡沫的残炭率均仍在50%以上,泡沫依然具有较好的热稳定性㊂2.6.2㊀热熔融结晶行为相变微胶囊酚醛泡沫的差示扫描量热(differ⁃entialscanningcalorimetry,DSC)曲线见图8㊂由图8可见,未添加相变微胶囊的酚醛泡沫在升温过程中无吸热峰㊂在添加相变微胶囊后,泡沫的DSC升温曲线出现了吸热峰,这是由于胶囊内部的相变材料发生固⁃液相变㊂添加相变微胶囊的酚醛泡沫在降温阶段出现了两个结晶峰,这是由于相变微胶囊内部的正十八烷在结晶时会发生异相成核和均相成核㊂吸热峰的出现表明了MPCM/PEG⁃400/PF具有了一定的蓄热保温㊁调节温度的功能㊂随着MPCM含量的增加,泡沫的熔融焓也逐渐增大㊂MPCM的添加量为5%,10%和15%时,样品的熔融焓分别为2.88,5.74和8.50J/g,结晶焓为2.36,4.97图8㊀酚醛泡沫DSC分析图Fig.8DSCanalysisofphenolicfoams2.6.3㊀导热性能酚醛泡沫的导热系数见图9㊂由图9可知,样品的导热系数随着PEG⁃400添加量的增加呈先上701林业工程学报第8卷升后下降的趋势㊂当PEG⁃400添加量较小时,泡沫的表观密度增加,导热系数随之变大;当PEG⁃400添加量进一步增加时,改性泡沫体的表观密度逐渐降低,导热系数随之变小㊂此时,泡沫的导热系数主要受到泡孔结构的影响㊂随着MPCM含量的增加,泡沫的导热系数也是呈先上升后下降的趋势㊂此时,样品的导热系数主要由表观密度和相变材料的吸热决定㊂当泡沫的表观密度增大,其导热系数随之增大;而当相变微胶囊添加量提高,泡沫的焓值增加,会导致泡沫的导热系数下降㊂当MPCM添加量为5%时,样品的导热系数最大,此时泡沫的表观密度对导热系数起到主导作用㊂随着MPCM含量的进一步增加,相变材料的吸热会导致泡沫导热系数逐渐降低㊂图9㊀酚醛泡沫的导热系数Fig.9㊀Thermalconductivitiesofphenolicfoams2.7㊀酚醛泡沫的隔声性能综合来看,当MPCM添加量为10%时,泡沫的综合性能最佳,所以此处选用PF㊁4%PEG⁃400和10%MPCM/PEG⁃400/PF3种泡沫进行隔声性能测试㊂酚醛泡沫隔声测试曲线见图10㊂由图10可见,随着频率的增加,酚醛泡沫的隔声量在750Hz处出现1个隔声低谷,此时入射波和泡沫中的弯曲波波长相同,泡沫的振动与入射声波之间形成共振,绝大部分的声波从泡沫中穿过㊂与之相反,4%PEG⁃400改性泡沫在频率700Hz处出现了1个波峰,这可能是PEG⁃400的引入改变了泡沫原有的结构,阻碍了泡沫的弯曲振动,降低了泡沫的振动幅度,进而削弱了共振现象,提高了隔声效果㊂在中高频段,泡沫的隔声量随着频率的增加不断提高㊂该频带下泡沫一般难以被激发振动,所以隔声性能逐渐增强㊂PF㊁4%PEG⁃400/PF和10%MPCM/PEG⁃400/PF平均隔声量分别为12.81,14.94和12.66dB㊂PEG⁃400的加入增强了泡沫的隔声性能,4%PEG⁃400/PF的平均隔声量相比PF提高了2.13dB㊂MPCM的引入会使泡沫密度增大㊁发泡倍率变小,从而影响泡沫的隔声效果㊂但10%MPCM/PEG⁃400/PF平均隔声量较PF变化不大,这可能是由于MPCM带来的负面效果与PEG⁃400增强的效果相抵消㊂图10㊀酚醛泡沫隔声测试曲线Fig.10㊀Soundinsulationcurvesofphenolicfoams3㊀结㊀论1)通过添加PEG⁃400和相变微胶囊与酚醛树脂进行共混发泡,制备出性能优异的改性泡沫㊂FT⁃IR的结果显示,PEG⁃400和酚醛树脂分子中的羟基或羟甲基发生了反应㊂SEM的结果显示,相变微胶囊均匀地填充在泡沫的泡孔中㊂2)经PEG⁃400改性后,泡沫的压缩强度和粉化率逐渐降低,冲击强度明显增大㊂PEG⁃400的加入在一定程度降低了泡沫的保温性和热稳定性,但提高了隔声性能㊂3)MPCM的加入一定程度上降低了泡沫的热稳定性㊁保温性能和隔声性能,但也让泡沫具有一定的蓄热保温㊁调节温度的功能㊂当添加质量分数为4%的PEG⁃400和10%的MPCM时,泡沫的综合性能最优㊂参考文献(References):[1]SONGF,LIZ,JIAPY,etal.Phosphorus⁃containingtungoil⁃basedsiloxanetoughenedphenolicfoamwithgoodmechanicalproperties,fireperformanceandlowthermalconductivity[J].Materials&Design,2020,192:108668.DOI:10.1016/j.matdes.2020.108668.[2]MOUGELC,GARNIERT,CASSAGNAUP,etal.Phenolicfoams:areviewofmechanicalproperties,fireresistanceandnewtrendsinphenolsubstitution[J].Polymer,2019,164:86-117.DOI:10.1016/j.polymer.2018.12.050.[3]崔亚平,朱时雪,方娟.天然纤维黄麻增强保温酚醛泡沫性能研究[J].塑料科技,2021,49(3):43-47.DOI:10.15925/j.cnki.issn1005-3360.2021.03.010.CUIYP,ZHUSX,FANGJ.Studyonpropertiesofnaturalfiberjutereinforcedinsulationphenolicfoam[J].PlasticsScienceandTechnology,2021,49(3):43-47.801㊀第4期林捷,等:相变微胶囊填充改性聚乙二醇/酚醛泡沫的制备[4]赵继永,王志鹏,程世婧,等.高性能聚合物泡沫材料的制备性能与应用研究进展[J].高分子材料科学与工程,2020,36(6):136-144.DOI:10.16865/j.cnki.1000-7555.2020.0115.ZHAOJY,WANGZP,CHENGSJ,etal.Preparation,pro⁃pertiesandapplicationsofhigh⁃performancepolymericfoammate⁃rials[J].PolymerMaterialsScience&Engineering,2020,36(6):136-144.[5]许国娟,贾晨辉,刘晶,等.酚醛树脂增韧改性研究进展及应用现状概述[J].复合材料科学与工程,2021(9):118-128.DOI:10.19936/j.cnki.2096-8000.20210928.018.XUGJ,JIACH,LIUJ,etal.Toughingmodificationdevelop⁃mentandtheapplicationstatusofphenolicresin[J].CompositesScienceandEngineering,2021(9):118-128.[6]刘书萌,吴东森,刘鹏波.硅橡胶增韧改性酚醛泡沫的性能研究[J].塑料科技,2014,42(11):57-60.DOI:10.15925/j.cnki.issn1005-3360.2014.11.029.LIUSM,WUDS,LIUPB.Studyonpropertiesofsiliconerub⁃bertoughenedphenolicfoam[J].PlasticsScienceandTechnology,2014,42(11):57-60.[7]陈永鑫.改性酚醛泡沫原位填充芳纶蜂窝复合材料的制备工艺研究[D].南京:南京航空航天大学,2014.CHENYX.Studyonthepreparationtechnologyofaramidcellularcorefilledwithphenolicfoamsinsitu[D].Nanjing:NanjingUniversityofAeronauticsandAstronautics,2014.[8]YANGYF,HEJM.Mechanicalcharacterizationofphenolicfoamsmodifiedbyshortglassfibersandpolyurethaneprepolymer[J].PolymerComposites,2015,36(9):1584-1589.DOI:10.1002/pc.23066.[9]谢梅竹,马磊,赵绘婷,等.植物纤维增强阻燃酚醛泡沫的制备及性能研究[J].塑料科技,2022,50(10):49-53.DOI:10.15925/j.cnki.issn1005-3360.2022.10.010.XIEMZ,MAL,ZHAOHT,etal.Preparationandpropertiesofplantfiberreinforcedflameretardantphenolicfoam[J].PlasticsScienceandTechnology,2022,50(10):49-53.[10]马玉峰,耿祥,王春鹏,等.桉木纤维预处理对酚醛泡沫复合材料性能的影响[J].林业工程学报,2018,3(1):97-102.DOI:10.13360/j.issn.2096-1359.2018.01.016.MAYF,GENGX,WANGCP,etal.Effectofpretreatmentofeucalyptusfiberonpropertiesofcompositephenolicfoams[J].JournalofForestryEngineering,2018,3(1):97-102.[11]刘海翔,杜官本,邓书端.酚醛泡沫材料改性的研究进展[J].化工新型材料,2022,50(11):42-48.DOI:10.19817/j.cnki.issn1006-3536.2022.11.009.LIUHX,DUGB,DENGSD.Researchprogressonmodifica⁃tionofphenolicfoammaterial[J].NewChemicalMaterials,2022,50(11):42-48.[12]宋飞.基于桐油的酚醛泡沫材料增韧和阻燃改性及其结构和性能研究[D].北京:中国林业科学研究院,2020.SONGF.Studyontougheningandflame⁃retardantmodificationofphenolicfoammaterialbasedontungoilanditsstructureandproperties[D].Beijing:ChineseAcademyofForestry,2020.[13]方桂花,张文涛,于孟欢.定形相变储能材料的研究进展[J].化工新型材料,2022,50(8):39-42.DOI:10.19817/j.cnki.issn1006-3536.2022.08.008.FANGGH,ZHANGWT,YUMH.Researchanddevelopmentofshape⁃stabilizedPCMforenergystorage[J].NewChemicalMaterials,2022,50(8):39-42.[14]ARSHADA,JABBALM,YANYY,etal.Themicro⁃/nano⁃PCMsforthermalenergystoragesystems:astateofartreview[J].InternationalJournalofEnergyResearch,2019,43:5572-5620.DOI:10.1002/er.4550.[15]YANGCG,FISCHERL,MARANDAS,etal.Rigidpolyure⁃thanefoamsincorporatedwithphasechangematerials:astate⁃of⁃the⁃artreviewandfutureresearchpathways[J].EnergyandBuildings,2015,87:25-36.DOI:10.1016/j.enbuild.2014.10.075.[16]AMARALC,PINTOSC,SILVAT,etal.Developmentofpoly⁃urethanefoamincorporatingphasechangematerialforthermalenergystorage[J].JournalofEnergyStorage,2020,28:101177.DOI:10.1016/j.est.2019.101177.[17]孙恒,杨鹏,刘蓓蓓.聚乙二醇对酚醛树脂泡沫增韧效果的研究[J].山东化工,2018,47(17):22-26.DOI:10.19319/j.cnki.issn.1008-021x.2018.17.009.SUNH,YANGP,LIUBB.Studyontougheningeffectofphe⁃nolicresinfoamsaddedpolyethyleneglycol[J].ShandongChemi⁃calIndustry,2018,47(17):22-26.[18]申晋琛,林明桂,马中义,等.十八烷@聚甲基丙烯酸甲酯微胶囊相变材料的制备及表征[J].燃料化学学报,2022,50(11):1511-1516.DOI:10.19906/j.cnki.jfct.2022056.SHENJC,LINMG,MAZY,etal.Preparationandcharacte⁃rizationofOct@PMMAmicrocapsulesphasechangematerials[J].JournalofFuelChemistryandTechnology,2022,50(11):1511-1516.(责任编辑㊀丁春香)901。
无机水合盐相变储热材料的过冷性研究_曾翠华

文章编号: 1008-8857(2005)01-0044-06
Vol. 21 No. 1 2005
无机水合盐相变储热材料的过冷性研究
曾翠华, 张仁元
(广东工业大学 材料与能源学院, 广州 510090)
摘 要: 无机水合盐相变储能材料通常存在过冷现象,影响了该类材料所蓄热量的排放性能, 如果过冷严重,储存的热量不能释放出来。就无机水合盐的过冷机理及非均匀形核的动力学 机理进行了探讨,加入成核剂是降低过冷度的有效措施。
式中:GS、GL 分别为固相和液相单位体积自由能。由 G = H − TS ,可得
∆Gv = (HS − HL) − T (SS − SL)
(1)
由于恒压下
∆HP = HS − HL = Lm
(2)
∆Sm = SL − SS = Lm
(3)
Tm
式中:Lm 为熔化热,表示固相转变为液相时体系向环境吸热,定义为正值;∆Sm 为固体的熔 化熵,它主要反映固体转变为液体时的组态熵的增加,可从熔化热与熔点的比值求得。
3 结论
(1) 依据非均匀成核机理,在无机水合盐相变储热材料中加入成核剂是降低无机水合盐 过冷度的有效也是较经济的措施。
48
能源研究与信息
2005 年 第 21 卷
(2) 成核剂对非均匀形核位置的促进作用取决于接触角θ。接触角θ 越小,成核剂对非均 匀形核的作用越大。
(3) 为了使θ 减小,应使 a -W 界面的比表明能σaW 尽可能降低,故要求成核剂与形核晶体 具有相近的结合键类型而且与晶核相接的彼此晶面具有相似的原子配置和小的点阵错配度 δ = | a − a1 | a ,其中 a 为晶核的相接晶面上的原子间距;a1 为成核剂相接面上的原子间距。 有些无机水合盐相变储热材料的成核剂与上述理论推断符合得较好[7~10]。但是,也有一些研究 结果表明,晶核与成核剂基底之间的点阵错配δ 并不象上述所强调的那样重要[6~9],因此在对 无机水合盐相变储热材料的成核剂的选取主要还是通过试验来确定有效的成核剂。
相变材料英文文献

P roc edia Materials Sc ienc e 4( 2014 )389 – 394Available online at ScienceDirect2211-8128 © 2014 Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license(/licenses/by-nc-nd/3.0/).Peer-review under responsibility of Scientific Committee of North Carolina State University doi: 10.1016/j.mspro.2014.07.579390J ianqing Chen et al. / P rocedia Materials Science 4( 2014 )389 – 394energy), thermal energy storage techniques have been paid great attention (Zalba et al. (2003) and Agyenim et al. (2010)). Latent heat storage using phase-change-materials (PCMs) is particularly attractive, since it provides benefits include reduction in temperature variability (thermal inertia) and high thermal energy storage density (Hoshi et al. (2005), Tian and Zhao (2013)).Various PCMs are generally divided into two main groups from their compositions (i.e. organic and inorganic PCMs) or two categories from their melting points (i.e. high-temperature PCMs above 200 °C and low-temperature one below 200 °C). The high-temperature PCMs can be used in solar power plants, while the low-temperature PCMs are mainly used in waste heat recovery systems and buildings. Organic substances exhibit desirable properties at low temperature applications, such as limited supercooling, no phase segregation and non-corrosion. I norganic PCMs have large latent heat and can be used in high temperature energy storage. But both of organic and inorganic PCMs present a low thermal conductivity (Zalba et al. (2003), Olives and Mauran (2001)).I n order to offset the heat storage/extraction rate during melting/solidification cycles, extensive investigations have been carried out to improve the thermal response of PCMs through adding various high thermal conductivity materials (Li et al. (2008)). The methods include dispersing high conductivity particles or fibers into PCMs, impregnating a porous metallic (or graphite) matrix with PCMs.Metal foam is a cellular structure consisting of a solid metal, containing a large volume fraction of gas-filled pores. The pores can be sealed (closed-cell foam) or form an interconnected network (open-cell foam). Due to the high surface-area to volume ratio and strong mixing capability, high porosity open-cell light-metallic foams have emerged as one of the most promising emerging materials for thermal energy storage (Cui et al. (2010), Hong and Herling (2006), and Bugaje (1997)).2.PCMs Embedded with Metal FoamI n many applications, thermal energy storage is required to receive, store and subsequently release heat. The major disadvantage of PCMs is their low thermal conductivities, which dramatically slows the phase change process and causes a wide temperature distribution within PCMs. Metal foams presents an order of magnitude higher thermal conductivity than PCMs. At the same time the random internal structure and high porosity of metal foam can enhance and accelerate the phase change process without significantly reducing PCMs’ heat storage capacity. The distribution of foam ligaments in PCMs makes the melting and solidification processes more uniform.There are many kinds of metal foams have been used in phase change materials, such as aluminum foam (Bauer and Wirtz (2000), Chintakrinda et al. (2011), Tong et al. (1995), Jiang et al. (2012)), copper foam (Chi et al. (2011), Sheng et al. (2013), Zhang and Yu (2007), and Cui (2012)) and Nickel foam (Shiina (2006), Weiqiang et al (2009), Xiao, (2013)).2.1.PCMs Embedded with Aluminum FoamBauer and Wirtz (2000) developed a plate like structure thermal energy storage composite consisting of a central core of foamed aluminum foam packed with PCM to store heat during peak power operation of variable power dissipating devices. Tong et al. (1995) inserted a matrix of continuously connected aluminum foam into phase change material (water) and investigated the solidification heat transfer of the water. Results show inserting metal-matrix into water provides a very effective way to enhance the solidification heat transfer.Jiang et al. (2012) prepared a shape-stabilized PCMs using bulk porous Al foams impregnated with organic PCMs (paraffin and stearic acid). The thermal-/dynamic-mechanical properties of the shape-stabilized PCMs were studied. The filling fraction of PCMs was approximately more than 80%, the latent value of the paraffin/Al foam and stearic acid/Al foam composite is 72.9kJ/kg and 66.7kJ/kg.2.2.PCMs Embedded with Copper FoamChi et al. (2011) made a new type of high efficiency energy storage devices consisted of cooper foam and water. The cold charging process of the new type energy storage devices was approved to be faster and more adequate due to the embedding of copper foam.Sheng et al. (2013) prepared a salt hydrate/metal foam composite phase change material by using barium391J ianqing Chen et al. / P rocedia Materials Science 4( 2014 )389 – 394hydroxide octahydrate (Ba(OH)2·8H2O) as latent heat storage PCM and copper foams as a supporting matrix. Thermal cycling and heat transfer performance was studied. Results show that high porosity copper foam not only enhance the heat transfer rate of Ba(OH)2·8H2O but also effectively reduce the supercooling of the PCM.Zhang and Yu (2007) investigated the thermal performance of solid-liquid phase change thermal storage device with 98% pure Heneicosane (C21H44) filled in copper foam through a vacuuming procedure. Experimental results show that the thermal conductivity and performance of the thermal storage device is obviously improved using copper foam as a heat transfer enhancement.Cui (2012) prepared a composite PCMs using paraffin as phase change materials and copper foam as filled materials. The results show that copper foam can not only lead to a more uniform temperature distribution within the thermal energy storage unit, but also extensively shorten the charging time.2.3.PCMs Embedded with Nickel FoamShiina (2006) studied the application of latent heat storage technology using a composite PCM (a copper or nickel foam saturated by PCM). The results indicate that composite PCM had increased effective thermal conductivity and could augment temperature change reduction of the heat transfer fluid.In order to improve the void distribution and thermal performance of phase change thermal storage devices, Xu et al (2009) designed and manufactured a thermal storage containers embedded with nickel foam cores. Embedding nickel foam into the PCMs enhanced both the void distribution and thermal performance of solid-liquid phase change process.Xiao, (2013) prepared paraffin/nickel foam and paraffin/copper foam composite phase change materials (PCMs) using a vacuum impregnation method. Results show that the thermal conductivity of the composite PCMs were drastically enhanced, e.g., the thermal conductivity of the paraffin/nickel foam composite was nearly three times larger than that of pure paraffin.3.Properties of PCMs Embedded with Metal Foam3.1.Effective Thermal ConductivityDue to the high thermal conductivity and porous structure, embedding metal foam into PCMs can enhance the heat transfer, thus improve the effective thermal conductivity of the composite PCMs.t is very difficult to predict the thermal conductivity of the PCMs embedded with metal foam for the complicated pore structure of the metal foam. Xu et al (2009) proposed a new phase distribution model of metal foam matrix PCMs. Simplified heat transfer model with void sub model was established and the effective thermal conductivity formula was derived by the equivalent thermal resistance method.Zhang et al (2010) investigated the thermal parameters (Effective thermal conductivity, thermal diffusivity and thermal capacity) of copper-foam/paraffin with four different porosities using transient plane source (TPS) method. The test results showed that the effective thermal conductivity is obviously improved by embedding the copper foam into paraffin and it reached 25 times compared to pure paraffin.3.2.ConvectionMetal foam with high thermal conductivity is generally considered to have high potential to enhance the heat transfer performance for PCMs. In an attempt to enhance the convective thermal transport, metal foam can be used for making advanced compact heat exchangers because of the high surface area to volume ratio as well as enhanced flow mixing due to the tortuosity of the pass ways. But in the study of Tian and Zhao (2011), the natural convection in liquid region of the PCMs is suppressed by the metal foam. Buoyancy-driven velocities are too weak to produce dominant convection due to high viscosity and low thermal expansion ratio of the PCM and the large flow resistance of metal foam.392J ianqing Chen et al. / P rocedia Materials Science 4( 2014 )389 – 3944.Research Methods of Heat Transfer in PCMsIn order to study the heat transfer and thermal storage capacity of the PCMs, extensive research methods have been developed, such as experimental study, theoretical analysis and numerical simulation.4.1.Experimental MethodLafdi et al (2007) built an experimental setup to measure the temperature profiles and capture the melting evolution of the PCM (low melting temperature paraffin wax) inside aluminum foam. Effects of porosity and pore size of the aluminum foam on the heat transfer performance were studied. For the higher porosity aluminum foam the steady-state temperature was reached faster as compared to the lower porosity foam. By using bigger pore size foam, the steady-state temperature was reached faster as compared to the foams with smaller pore size.Wu and Zhao (2011) investigated heat transfer enhancement performance of metal foam (copper foam) in high temperature thermal energy storage system using NaNO3 as phase change material. Effect of natural convection on the heat transfer rate was investigated under bottom and top heating conditions. The heat transfer rate can be enhanced by copper foam to 2.1 times compare to pure NaNO3. However, in the liquid PCM, the heat transfer rate was no longer better that of the pure NaNO3 because metal foam suppressed the natural convection severely. Under the top heating condition, the heat transfer rate was enhanced by 1.2 times.Shi et al (2010) studied phase change heat transfer in ice ball with porous metal foam experimentally. The results show that porous metal foams can enhance the phase change heat transfer in ice ball, lead to starting phase change earlier and shorten the whole phase change time.4.2.Theoretical Analysis MethodKrishnan et al (2004) employed a two-temperature model to account for the local thermal non-equilibrium. Separate energy equations for the solid and fluid respectively are written and closed using a steady-state interphase heat transfer coefficient between the two phases. A general momentum equation that includes the Brikman-forchheimer extension to Darcy flow is employed. Natural convection inside the fluid was analyzed using a two-temperature formulation. Results show that local thermal equilibrium is not ensured either during the transient or at steady state in the system.Peng et al (2009) investigated the phase change heat transfer in PCM (wax) embedded in high porosity aluminum foam. A two-temperature model was established according to the difference of the heat transfer between wax and Al foam. The temperature distributions and flow fields of the PMCs were simulated by apparent heat capacity method. The results showed that the heat transfer of the PCMs in Al foam was effectively improved compared to pure PCMs without Al foam. Chen et al (2010) studied the melting process of paraffin in high porosity aluminum foam using a similar two-temperature model. The simulation results indicate that aluminum foam makes the temperature distribution of the paraffin more even. There is big temperature difference between metal frame and PCM during phase change process. Local thermal non-equilibrium is obvious. Melting speed of the paraffin increases with the decreasing of metal foam porosity.4.3.Numerical Simulation MethodBased on local thermal non-equilibrium between the metal matrix and PCMs, Gao and Chen (2012) and Zhang et al (2013) developed a Lattice Boltzmann model to characterize the melting processes and heating conduction of PCMs in metal foams and the temperature filed of metal foams framework. An equation based on density distribution function was constructed to characterize the velocity field of melt fluid. An enthalpy-based method is employed to account the phase change problem. The melting front location as the function of time and the temperature distribution in metallic framework and the PCMs is simulated by Lattice-Boltzmann model.The effects of the porosity and pore size on the melting are also investigated and discussed. The results indicate that the effects of foam porosity play important roles in the overall heat transfer. For the lower porosity foams, the melting rate is comparatively greater than the higher porosity foams, due to greater heat conduction from metal foam with high heat conductivity. The foam pore size has a limited effect on the melting rate due to two counteracting effects between393 J ianqing Chen et al. / P rocedia Materials Science 4( 2014 )389 – 394conduction and convection heat transfer. Increasing of the pore density leads to increasing conduction heat transfer, decreasing convection heat transfer and decreasing heat storage capacity. Therefore, it is suggested to consider engineering requirements to determine porosity in the design of foam metal heat storage device.A phase filed model deals with free boundary problems without tracing their positions, and therefore provides potentials of being extended to consider more complicated mechanisms: multi-dimension and volume change. Han et al (2013) established a foam-PCM phase filed model to solve the phase change problem by introducing two phase files to deal with phase change and volume change. The coupled heat transfer between PCMs and metal foams is solved based on the non-equilibrium heat transfer theory. An effectiveness map distinguishing the conditions under which incorporating metal foam into the PCMs is sensitive, lowly sensitive or irrelevant is produced to guide the metal selection and structure design of metal foams when enhancing the heat transfer of PCMs.5.SummaryIn this paper the research progress of phase change materials (PCMs) embedded with metal foam is reviewed. The conduction, convection and phase change heat transfer process in the PCMs has been extensively investigated. Embedding metal foam into PCMs is an effective method to enhance the heat transfer in the PCMs. Due to the high thermal conductivity, surface area volume ratio, porosity and complicated three dimension network, metal foam in the composite PCMs increase the effective thermal conductivity of the composite PCMs, and thus improve the uniformity of the temperature distribution in the PCMs. Generally the metal foam embedded in the PCMs suppress the natural convection and reducing the convective heat transfer performance. Metal foam structure affects the heat transfer performance of the PCMs significantly. It is suggested to consider both the effects foam porosity and pore size on conduction and convection heat transfer as well as engineering requirements to determine porosity in the design of foam metal heat storage device.AcknowledgementsThis work is supported by the Supporting Program for Science and Technology of Changzhou (Industry, Grant. No. CE20120024) and Fundamental Research Funds for Central Universities of China (No. 2009B16114).ReferencesAgyenim, F., Hewitt, N., Eames, P., and Smyth, M., 2010, A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS), Renewable and Sustainable Energy Reviews, 14, 615-628.Akira, H., David, R.M., Antoine, B., Takeo, S.S., 2005, Screening of high melting point phase change materials (PCM) in solar thermal concentrating technology based on CLFR, Solar Energy Solar Energy, 79, 332-339.Bauer, C.A., and Wirtz, R.A., 2000, Thermal characteristics of a compact, passive thermal energy storage device, ASME Heat Transfer Div. Publ.HTD, 366, 283-289.Bugaje, M., 1997, Enhancing the thermal response of latent heat storage systems, Int. J. Energy Res., 21, 59-66.Chi, P., Xie, Y., Yu, J., and Yang, X., 2011, Experiment and analysis for cold charging process of new energy storage device, Journal of Beijing University of Aeronautics and Astronautics, 37, 1070-1075.Chintakrinda, K., Weinstein, R.D., and Fleischer, A.S., 2011, A direct comparison of three different material enhancement methods on the transient thermal response of paraffin phase change material exposed to high heat fluxes, International Journal of Thermal Sciences, 50, 39-47. Cui, H.T., 2012, Experimental investigation on the heat charging process by paraffin filled with high porosity copper foam, Appl. Therm. Eng., 39, 26-28.Gao, D., and Chen, Z., 2012, Influence of pore density on melting of phase change materials in metal foams-numerical simulation with Lattice Boltzmann method, Acta Energiae Solaris Sinica, 33, 1604-1609.Hai-ting, C., Feng-qing, L., Jin-da, Z., and Wei, L., 2010, Enhancement of high porosity metal foam to phase change energy storage, J. Hebei Univ. Sci. Technol., 31, 3-6.Han, X.X., Tian, Y., and Zhao, C.Y., 2013, An effectiveness study of enhanced heat transfer in phase change materials (PCMs), Int. J. Heat Mass Transfer, 60, 459-468.Hong, S., and Herling, D.R., 2006, Open-cell aluminum foams filled with phase change materials as compact heat sinks, Scr. Mater., 55, 887-890. Jinghua, J., Yingying, Z., Aibin, M., Donghui, Y., Fumin, L., Jianqing, C., Jun, S., Song, D., 2012, Preparation and performances of bulk porous Al foams impregnated with phase-change-materials for thermal storage, PNSC Progress in Natural Science: Materials International, 22, 440-444.Krishnan, S., Murthy, J.Y., and Garimella, S.V., 2004, A two-temperature model for the analysis of passive thermal control systems, Journal of Heat Transfer, 126, 28-37.394J ianqing Chen et al. / P rocedia Materials Science 4( 2014 )389 – 394 Lafdi, K., Mesalhy, O., and Shaikh, S., 2007, Experimental study on the influence of foam porosity and pore size on the melting of phase change materials, J. Appl. Phys., 102, 35-49.Li, K., Guo, N., and Wang, H., 2008, Progress on the Study of Improving the Phase Change Materials Conductivity, 1-6.Peng, D., Chen, Z., and Shi, M., 2009, Numerical simulations of phase change material thawing process in metallic foams, Journal of Engineering Thermophysics, 30, 1025-1028.Py, X., Olives, R., Mauran, S., 2001, Paraffin/porous-graphite-matrix composite as a high and constant power thermal storage material, International journal of heat and mass transfer., 44, 2727-2737.Sheng, Q., Xing, Y., and Wang, Z., 2013, Preparation and performance analysis of metal foam composite phase change material, Journal of Chemical Industry and Engineering, 64, 3565-3570.Shi, J., Chen, Z., and Shi, M., 2010, Experimental study on the effect of the porous metal foams in ice ball on the freezing heat transfer of fluid, Journal of Engineering Thermophysics, 31, 1395-1397.Shiina, Y., 2006, Reduction of temperature changes in heat transfer fluid by the use of latent heat storage technology, Transactions of the Atomic Energy Society of Japan, 5, 190-199.Tian, Y., and Zhao, C.Y., 2011, Natural convection investigations in porous, Phase Change Materials, 3, 769-772.Tian, Y., and Zhao, C.Y., 2013, A review of solar collectors and thermal energy storage in solar thermal applications, Appl. Energy, 104, 538-553.Tong, X., Khan, J.A., and Amin, M.R., 1995, Enhancement of solidification heat transfer by inserting metal-matrix into phase change material, 8.Weiqiang, X., Xiugan, Y., and Yuming, X., 2009, Effect of embedding nickel foam on solid-liquid phase change, Journal of Beijing University of Aeronautics and Astronautics, 35, 197-200.Wu, Z.G., and Zhao, C.Y., 2011, Experimental investigations of porous materials in high temperature thermal energy storage systems, Solar Energy, 85, 71-80.Xiao, X., Zhang, P., and Li, M., 2013, Preparation and thermal characterization of paraffin/metal foam composite phase change material, 112, 1357-1366.Xu, W., Yuan, X., and Li, Z., 2009, Study on effective thermal conductivity of metal foam matrix composite phase change materials, Journal of Functional Materials, 40, 1329-1332.Zalba, B., Marin, J. M., Cabeza, L. F., and Mehling, H., 2003, Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications, Appl. Therm. Eng., 23, 251-283.Zhang, T., and Yu, J., 2007, Experiment of solid-liquid phase change in copper foam, Journal of Beijing University of Aeronautics and Astronautics, 33, 1021-1024.Zhang, T., Yu, J., and Gao, H., 2010, Measurement of thermal parameters of Copper-foam/paraffin composite PCM using transient plane source (TPS) method, Acta Energiae Solaris Sinica, 31, 604-609.Zhang, Y., Gao, D., and Chen, Z., 2013, I nfluence of porosity on melting of phase change materials in metal foams with lattice Boltzmann method, Journal of Southeast University, 43, 94-98.Zhen-qian, C., Ming-wei, G., and Ming-heng, S., 2010, Numerical simulation on phase change heat transfer of paraffin in metal foams, Journal of Thermal Science and Technology, 9, 6-11.。
相变微胶囊的热导率测量郑兴华

中国工程热物理学会传热传质学学术会议论文编号:113473相变微胶囊的热导率测量郑兴华1,2,邱琳1,2,祝捷1,2,苏国萍1,2,唐大伟1 (⒈国科学院工程热物理研究所, 北京 100190; ⒉中国科学院研究生院, 北京 100039 )(Tel*************,Email:**********************)摘要:提出应用3ω谐波探测技术进行脲醛树脂-石蜡相变微胶囊的等效热导率测量方法。
测试了跨越相变温度区间的微胶囊等效热导率,分析了等效热导率随温度的变化关系。
在其相变温度区间内,热导率存在极大值,该极值点对应的温度与其相变温度峰值一致。
同样温度下,降温时的等效热导率略小于升温,这主要是由降温时相变材料的过冷引起。
关键词:谐波探测;等效热导率;相变微胶囊0前言利用成膜材料把固体或液体材料包覆形成微小粒子的技术,将相变材料进行胶囊化后形成的相变材料微胶囊(Micro encapsulated phase change material,简称MEPCM),囊心与外界环境隔开,可使相变材料免受外界湿度、氧气等因素的影响改善传统相变材料的稳定性,且可解决固-液相变材料相变后液相的流动、挥发、腐蚀及泄漏问题,降低相变材料的毒性。
另外由于微胶囊粒径均匀、直径较小且囊壁较薄,在有效提高相变材料的热传递及使用效率的同时也更易于分散到其他材料中制成性能更加优异的相变储能材料及各种新型功能材料。
目前MEPCM已经可以应用于智能调温纺织品、皮革工业、传热流体、建筑物、太阳能利用、余热回收及农业等领域中,相变储能微胶囊的研究正成为世界范围内的研究热点[1-7]。
目前,国内外关于MEPCM的研究主要集中在其制备方法、焓值、相变潜热及应用领域[8-14],而对于直接影响其传热能力及蓄热调温能力的等效热导率的测量研究较少。
这一方面是因为相变微胶囊目前的生产工艺尚不可量产,科研人员将主要的关注焦点集中在其制备方法上。
硬脂酸插层高岭石复合相变材料的制备和热性能研究

第49卷第12期人工晶体学报Vol.49No.12 2020年12月JOURNAL OF SYNTHETIC CRYSTALS December,2020硬脂酸/插层高岭石复合相变材料的制备和热性能研究张蒙1,赵炳新1,王娟1,程宏飞2(1.中国矿业大学(北京)地球科学与测绘工程学院,北京100083;2.长安大学地球科学与资源学院,西安710054)摘要:高岭石(K)是一种常见的黏土矿物,具有低成本、阻燃、多层结构等固有优点。
本文采用真空浸渍法将硬脂酸(SA)吸附到插层高岭石(IKL)和二甲基亚砜(DMSO)复合物的孔隙中来制备用于储热的复合相变材料。
通过X射线衍射(XRD)、红外光谱(FT-IR)、热重分析(TG)、差示扫描量热法(DSC)、扫描电子显微镜(SEM)和比表面积分析仪(BET)表征了复合材料的热性能、结构和主要组分。
由于插层复合物形成后高岭石层间距增大,对SA的吸附率达到32.3%,熔化和凝固潜热值分别为43.36J/g和43.16J/g,熔化和凝固温度分别为51.9t和51.7t。
此外,该复合相变材料具有较好的热稳定性。
由于SA/IKL复合相变材料具有高吸附量、高潜热、良好的热稳定和低成本等优点,因此,其在实际的应用中具有潜在的价值。
关键词:高岭石;硬脂酸;真空浸渍法;储热;潜热;相变材料中图分类号:TB34文献标识码:A文章编号:1000-985X(2020)12-236546Preparation and Thermal Properties of Stearic Acid/IntercalatedKaolinite Composite Phase Change MaterialZHANG Meng1,ZHAO Bingxin1,WANG Juan1,CHENG Hongfei2(1.School of Geoscience and Surveying Engineering,China U niversity of Mining and Technology,Beijing100083,China;2.School of Earth Science and Engineering,Chang'an U niversity,Xi'an710054,China)Abstract:Kaolinite(K)is a common clay mineral with inherent advantages such as low cost,flame retardant,and multilayer structure.In this paper,a vacuum impregnation method was used to adsorb stearic acid(SA)into dimethyl sulfoxide (DMSO)and intercalated kaolinite(IKL)pores to prepare a composite phase change material for heat storage.Thermal properties,structure and composition of the prepared composite were tested by X-ray diffraction(XRD),fourier transform infrared spectroscopy(FT-IR),thermal gravimetric(TG),differential scanning calorimety(DSC),scanning electron microscope(SEM),and Brunauer-Emmet-Tellerare(BET).The mass ratio of SA adsorbed into IKL without leakage is as high as32.3%,ascribed to the expansion of kaolinite interlayer.The latent heat of melting and freezing is43.36J/g and43.16J/g,and the melting and freezing temperatures is51.9t and51.7t.In addition,the composite phase changematerial has excellent thermal stability.Due to the high adsorption capacity,high latent heat,good thermal stability,as well as low cost,SA/IKL composite phase change material has potential application value in practical applications.Key words:kaolinite;stearic acid;vacuum impregnation method;heat storage;latent heat;phase change material0引言近年来,能源危机和环境污染已经成为十分严峻的问题,开发可再生能源和储能材料为解决这一问题提供了有效的方案[1]。
超低温-高温跨温区相变材料制备及物性调控综述

第 12 卷第 12 期2023 年 12 月Vol.12 No.12Dec. 2023储能科学与技术Energy Storage Science and Technology超低温-高温跨温区相变材料制备及物性调控综述折晓会1, 2,王星宇1,郭晓龙1,刘艺炫3,王家蕴1,韩鹏1, 2,任晓芬1, 2,赵学敏1, 2(1石家庄铁道大学机械工程学院,低温能量转换、存储与输运研究中心,河北石家庄050043;2河北省储能产业技术研究院,河北石家庄050000;3河北工程大学能源与环境工程学院,河北邯郸056038)摘 要:相变储能技术利用相变材料在相变过程中释放或吸收潜热的特性,将能量以潜热的形式储存或释放。
其具有高能量密度、长寿命、高功率的优势,在电动汽车、可再生能源储存、电网调峰、智能电网方面具有广泛应用前景,为能源转型和高效能源利用提供了一种可行的解决方案。
本文通过对相关文献的探讨,综述了不同温区相变材料的优缺点以及应用领域,包括超低温区(-190~-50 ℃)、低温区(-50~0 ℃)、普温区(0~100 ℃)和高温区(100~700 ℃)。
针对相变材料性能改善,阐述了导热系数提升、过冷度降低、相变温度调控、循环稳定性提高等方法。
此外,对于复合相变材料的制备方法,介绍了微胶囊化、浸渍法、溶胶-凝胶法和超声波法,并对后三者的不足进行阐述和说明。
最后,对于相变材料的未来应用进行了展望,为相变储能技术在能源储存领域的进一步研究提供了参考和指导。
关键词:相变材料;相变储能;物性调控;制备方法doi: 10.19799/ki.2095-4239.2023.0726中图分类号:TB 333 文献标志码:A 文章编号:2095-4239(2023)12-3818-18A review on the preparation of ultra-low-temperature,high-temperature, and cross-temperature zone phase change materials and the regulation of physical properties SHE Xiaohui1, 2, WANG Xingyu1, GUO Xiaolong1, LIU Yixuan3, WANG Jiayun1, Han Peng1, 2,REN Xiaofen1, 2, ZHAO Xuemin1, 2(1Low Temperature Energy Conversion, Storage and Transportation Research Center, School of Mechanical Engineering, Shijiazhuang Tiedao University, Shijiazhuang 050043, Hebei, China; 2Hebei Energy Storage Industry and Technology Research Institute, Shijiazhuang 050000, Hebei, China; 3School of Energy and Environmental Engineering, Hebei University of Engineering, Handan 056038, Hebei, China)Abstract:Phase change energy storage technology harnesses the unique properties of phase change materials to release or absorb latent heat during phase transitions, enabling energy storage in the form of latent heat. This technology holds promising applications in electric vehicles, renewable energy storage, grid peaking, and smart grids owing to its high energy density, extended lifespan, and high power. It presents a viable solution for energy收稿日期:2023-10-17;修改稿日期:2023-10-31。
pcm 相变 材料

PCM相变材料概述相变材料(Phase Change Materials,简称PCM)是一种具有特殊热性质的材料,能够在温度变化时发生相变,吸收或释放大量的潜热。
PCM广泛应用于热管理、节能和储能等领域。
本文将介绍PCM的原理、分类、应用以及未来发展方向。
原理PCM的相变过程是指物质在温度达到临界点时,由一种物态转变为另一种物态的过程。
常见的PCM包括固液相变和固气相变两种类型。
固液相变是指物质由固态转变为液态或由液态转变为固态的过程。
在这个过程中,物质吸收或释放大量的潜热,使得温度保持不变。
这种特性使得PCM能够在温度升高或降低时吸收或释放热量,起到调节温度的作用。
固气相变是指物质由固态直接转变为气态或由气态直接转变为固态的过程。
与固液相变类似,这个过程中也会伴随着潜热的吸收或释放。
分类根据不同的相变温度和应用需求,PCM可以分为有机相变材料和无机相变材料两种类型。
有机相变材料有机相变材料主要由碳、氢、氧等元素组成,具有较低的熔点和较高的热容量。
常见的有机相变材料包括蜡、聚合物等。
它们具有以下特点:•较低的熔点:有机相变材料通常具有较低的熔点,使得它们在常见温度范围内能够发生相变,实现热管理的目标。
•较高的热容量:由于其分子结构特殊,有机相变材料具有较高的热容量,在吸收或释放热量时能够提供更多的能量。
•可调节性:通过改变组成和结构,可以调节有机相变材料的熔点和热容量,以满足不同应用需求。
无机相变材料无机相变材料主要由金属、氧化物等元素组成,具有较高的熔点和较大的潜热。
常见的无机相变材料包括硅、铟锡合金等。
它们具有以下特点:•较高的熔点:无机相变材料通常具有较高的熔点,使得它们在高温环境下能够发挥稳定的性能。
•较大的潜热:由于其原子结构特殊,无机相变材料具有较大的潜热,在吸收或释放热量时能够提供更多的能量。
•耐久性:无机相变材料具有较好的耐久性和稳定性,能够在长期使用中保持良好的性能。
应用PCM广泛应用于热管理、节能和储能等领域。
相变潜热测定方法探讨

相变潜热测定方法探讨摘要:相变潜热是指物质在等温等压情况下,从一个相变化成另一个相所吸收或者放出的热量。
研究物质的性质以及开发储能材料时都需要对材料发生相变时的潜热进行测量。
本文简要介绍了相变潜热的几种测定方法。
关键词:潜热;相变;测量Discussion on determination methods of latent heat Abstract: Latent heat of a substance is the quantity of heat absorbed or released when the substance changes from one phase to another phase under isothermal isobaric conditions. Properties of substances and materials are required for phase change latent heat measured at the time of the development of energy storage materials. This paper briefly describes several measurement methods of latent heat.Key words: latent heat; phase change; measurement0引言相变传热过程在各种工业过程和材料开发领域有着重要的应用。
研究好相变的传热过程,测量出相变潜热的大小,对优化工业生产过程,开发优质储能材料有着极为重要的意义1756年英国化学家Joseph Black用32F冰与172F同等重量的水混合,得到平衡温度仍然为32F,而不是102F,这说明在冰溶解中,需要一些温度计所不能觉察的热量,进而发现各种物质在发生物态变化时都存在这种效应。
Joseph Black将这种不表现为温度升高的热称为潜热。
用于冷链的低温相变材料的研究进展

化工进展Chemical Industry and Engineering Progress2022年第41卷第1期用于冷链的低温相变材料的研究进展刘畅,陈艳军,张超灿(武汉理工大学材料科学与工程学院,湖北武汉430070)摘要:相变材料(PCM )具有较高的储能密度,有利于能源的储存和高效利用。
对于低温相变材料,其应用从相变温度为0℃至室温的空调和建筑等领域到零下的工业制冷和食品、药物等的运输储藏,非常广泛。
本文从水溶液相变材料体系和非水相变材料体系两方面对冷链用相变材料进行了系统介绍,并从过冷、长期稳定性和导热等角度综述了近年关于冷链用相变材料的研究。
指出对于水溶液相变材料体系存在的严重过冷及盐-水体系较强的金属腐蚀性,可通过使用合适的成核剂、改善相变材料对成核剂的浸润性、避免纳米粒子团聚及用不锈钢或聚合物材料封装等方法改善;对于非水相变材料体系,可通过引入高导热的纳米粒子和支撑材料,微胶囊化PCM 等方法来解决有机物热导率较低的问题。
关于纳米粒子的聚沉以及引入支撑材料和微胶囊化PCM 导致的大量潜热损失问题,指出改善纳米粒子和支撑材料与PCM 的亲和性是值得尝试的方向。
关键词:相变材料;水溶液相变材料体系;非水相变材料体系;热学性质中图分类号:TK01+8文献标志码:A文章编号:1000-6613(2022)01-0286-14Low temperature phase change materials for subzero applicationsLIU Chang ,CHEN Yanjun ,ZHANG Chaocan(School of Materials Science and Engineering,Wuhan University of Technology,Wuhan 430070,Hubei,China)Abstract:Phase change materials (PCM)show high energy storage density,which is conducive to the storage and efficient utilization of energy.For low temperature phase change materials,their applications range can be from air-conditioning and construction industrial to industrial refrigeration,transportation and storage of food and medicine.This paper provides a systematic introduction to phase change materials for subzero applications,and reviews recent research on phase change materials for subzero applications from the perspectives of supercooling,chronic stability and thermal conductivity.In view of the severe supercooling of the aqueous PCM systems and metal corrosion of salt solution,the relevant research in recent years show that these problems can be solved by using suitable nucleating agents and improving the compatibility between the PCM and the nucleating agent,avoiding nanoparticles agglomeration and encapsulating PCM with stainless steel or polymer materials.The problem of low thermal conductivity of non-aqueous PCM systems can be solved by introducing nanoparticles and supporting materials with high thermal conductivity or encapsulating PCM.Finally,as for the aggregation of nanoparticles and the latent heat loss caused by the introduction of support materials and microencapsulated PCM,it is worth trying to improve the compatibility of nanoparticles and support materials with PCM.综述与专论DOI :10.16085/j.issn.1000-6613.2021-0213收稿日期:2021-01-29;修改稿日期:2021-04-22。
相变材料作文模板英语

相变材料作文模板英语英文回答:Phase Change Materials: A Novel Approach to Thermal Energy Storage。
Phase change materials (PCMs) are substances that undergo a reversible change in physical state from solid to liquid or liquid to gas, with the absorption or release of heat. This unique property makes PCMs a promising candidate for thermal energy storage applications.PCMs offer several advantages over traditional thermal storage materials such as water or concrete:High latent heat capacity: PCMs can store asignificant amount of thermal energy per unit volume, making them more efficient for energy storage.Constant temperature storage: During the phase changeprocess, PCMs maintain a constant temperature, which can be beneficial for heating or cooling applications.Compact size: PCMs are typically denser than water, allowing for smaller and more compact storage systems.Environmental friendliness: PCMs are non-toxic and environmentally friendly.Common types of PCMs include organic compounds (e.g., paraffin waxes, fatty acids), inorganic compounds (e.g., salts, metals), and eutectic mixtures (combinations of two or more PCMs). The selection of a PCM depends on the specific application, considering factors such as desired operating temperature range, thermal conductivity, and chemical stability.PCM-based thermal storage systems have variouspotential applications, including:Building heating and cooling: PCMs can be integrated into walls, ceilings, and floors to provide passive heatingor cooling, reducing energy consumption.Industrial processes: PCMs can be used to store thermal energy from industrial processes, such as waste heat from furnaces or exhaust systems, for later use.Renewable energy backup: PCMs can store thermal energy from renewable sources, such as solar or geothermal, for use when the primary source is unavailable.Transportation: PCMs can be incorporated into vehicle seats or interiors to regulate cabin temperature, improving passenger comfort.The design and implementation of PCM-based thermal storage systems require careful considerations of several factors:Integration and packaging: PCMs need to be effectively integrated into the storage system and protected from degradation or leakage.Heat transfer enhancement: Increasing the thermal conductivity of PCMs can improve heat transfer rates and system efficiency.Thermal cycling: Repeated phase changes can lead to volume changes and potential degradation of PCMs, requiring proper design and maintenance strategies.Research and development efforts are ongoing to optimize PCMs and their applications. Ongoing work focuses on improving thermal conductivity, reducing costs, and developing new PCMs with tailored properties for specific applications.中文回答:相变材料,热能储存的新颖方法。
基于相变材料的动力电池热管理研究进展

第8卷 第6期 新 能 源 进 展Vol. 8 No. 62020年12月ADVANCES IN NEW AND RENEWABLE ENERGYDec. 2020* 收稿日期:2020-05-08 修订日期:2020-06-30基金项目:广东省重点领域研发计划项目(2019B090909001) † 通信作者:李恺翔,E-mail :*********************文章编号:2095-560X (2020)06-0493-09基于相变材料的动力电池热管理研究进展*吕少茵,曾维权,杨 洋,李恺翔†(广州汽车集团股份有限公司汽车工程研究院,广州 511434)摘 要:相变材料(PCM )由于具有相变潜热大、相变时体积变化小的优点,成为电池热管理研究的主要方向之一。
本文介绍了相变材料的蓄热原理,综述了主要相变材料石蜡以及针对其导热系数不高而进行的强化换热研究成果,介绍了相变材料耦合其他多种冷却方式在动力电池热管理上的应用,并展望了未来PCM 的研究方向。
关键词:相变材料;热管理;动力电池 中图分类号:TK02 文献标志码:A DOI :10.3969/j.issn.2095-560X.2020.06.007Research Progress on Power Battery Thermal Management SystemBased on Phase Change MaterialLÜ Shao-yin, ZENG Wei-quan, YANG Yang, LI Kai-xiang(GAC Automotive Research & Development Center, Guangzhou 511434, China)Abstract: Phase change material (PCM) has become one of the main research areas of battery thermal management because of its large latent heat and small volume change during its phase change. In this paper, the heat storage principle of PCM was introduced. The main phase change material paraffin and researches about enhancing the thermal conductivity of paraffin were reviewed. The applications of other cooling methods coupled with phase change materials in thermal management of power battery were summarized. Finally, the future application of the PCM in thermal management of battery was prospected.Key words: phase change material; thermal management; power battery0 引 言随着全球人口和经济的发展,能源短缺和环境污染问题引起了人类广泛关注。
水合无机盐及其复合相变储热材料的研究进展

CHEMICAL INDUSTRY AND ENGINEERING PROGRESS 2016年第35卷第6期·1820·化 工 进 展水合无机盐及其复合相变储热材料的研究进展苑坤杰,张正国,方晓明,高学农,方玉堂(华南理工大学传热强化与过程节能教育部重点实验室,广东 广州510640)摘要:水合无机盐具有适宜的相变温度、较大的相变潜热、原料廉价以及热导率较高(相对有机相变材料而言)等优势而有望成为中低温热利用领域理想的储热材料。
然而,水合盐具有过冷、相分离和液漏等缺陷,一直以来成为限制其应用的瓶颈缺陷。
水合无机盐作为相变储热材料的传统研究主要集中在成核剂和增稠剂的选择上,近几年一些研究者开始研究水合无机盐复合或封装工艺,极大地促进了水合盐性能提升方面的工作。
本文综述了常见的几种低温类水合无机盐过冷和相分离现象的解决方法以及基于这几种低温水合无机盐的复合相变储热材料的研究,复合相变材料较单一纯相变材料具有诸多优越的性能,预测采用多孔材料吸附封装技术或微胶囊封装技术来制备水合无机盐复合相变材料可能成为未来解决水合盐液漏问题的研究热点。
关键词:水合无机盐;储热;复合相变材料中图分类号:TK 02 文献标志码:A 文章编号:1000–6613(2016)06–1820–07 DOI :10.16085/j.issn.1000-6613.2016.06.023Research progress of inorganic hydrated salts and their phase change heatstorage compositesYUAN Kunjie ,ZHANG Zhengguo ,F ANG Xiaoming ,GAO Xuenong ,F ANG Yutang (Key Lab of Enhanced Heat Transfer and Energy Conservation ,Ministry of Education ,School of Chemistry andChemical Engineering ,South China University of Technology ,Guangzhou 510640,Guangdong ,China )Abstract :With the advantages of proper phase change temperature ,high latent heat ,cheap raw materials and high thermal conductivity (compared with organic phase change materials),the inorganic hydrated salts are considered as ideal heat storage materials in the field of middle and low temperature heat utilization. However ,the hydrated salts have also defects of supercooling ,phase separation and leakage ,which have significantly limited their applications. The traditional researches on hydrated salts focused on the choice of nucleating agents and thickening agents. Recently ,some researchers on the composite or encapsulation of inorganic hydrous salt showed up ,greatly promoting the work of improving the performance of hydrated salts. In this paper ,the solutions for supercooling and phase separation and the researches on phase change composites of several common inorganic hydrated salts are summarized. Compared with single phase change materials ,the phase change composites have many superior properties. Preparing inorganic hydrated salts phase change composites with porous materials package and micro encapsulation technology may become the research hotspots for solving the problem of inorganic hydrated salts leakage in the future.Key words :inorganic hydrated salt ;heat storage ;phase change composites第一作者:苑坤杰(1990—),女,博士研究生。
相变材料在调温沥青路面中的研究进展

#色建材 Green Building Materials相变材料在调温沥青路面中的研究进展Phase-Change Material Applied in Temperature Self- Adjusting Asphalt Road PavementP击美荣(上海市建筑科学研究院有限公司,上海201108)摘要:相变材料(PCMs)因具有很高的熔融潜热特性,可以储存大量的热能,将其加入路面用于调控路面温度成为近年来研究的热点。
对相 变材料及定形相变材料合成方法进行了概述,重点阐述了相变材料的掺入对沥青胶浆及沥青路面性能影响的研究进展。
同时,对该研究领域 现存的问题进行了总结。
最后对相变材料在沥青路面中的应用进行展望。
关键词:相变材料;熔融潜热;调温沥青路面;材料合成;沥青胶浆中图分类号:TU535 文献标识码:A文章编号:1674-814X随着我国公路事业的迅速发展,沥青路面以行车舒适、平稳和噪声低等水泥混凝土路面难以替代的优点被广泛运 用。
据统计,我国现有公路中沥青路面约占85%,已成为 我国铺装道路中最主要的路面形式。
随着全球气候变暖,我 国夏季炎热地区气温往往超过35 °C甚至在40 °C以上。
由于沥青是一种黑色吸热的温度敏感性材料,吸收阳光辐射后 表面温度会超过60 °C,导致沥青路面产生车辙、拥包、推 挤等热稳性病害,严重影响了沥青路面的路用性能。
为了缓解沥青路面的高温病害,研究人员从提高材料的 热稳定性着手,研究了沥青改性u_31、优化矿料配比141、添加 纤维材料等技术对沥青胶浆性能的影响。
其研究结果的运用 对沥青路面的高温病害有所缓解,但多年来沥青路面病害情况仍然严峻。
近年来,相变材料(Phase Change Material, PCMs)在道路温度控制领域的应用逐渐引起了人们的关注。
相变材料在相变过程中可以吸收或释放大量的热能,但 温度不变,是一种优良的储能材料。
Methods and apparatus for latent heat (phase chang
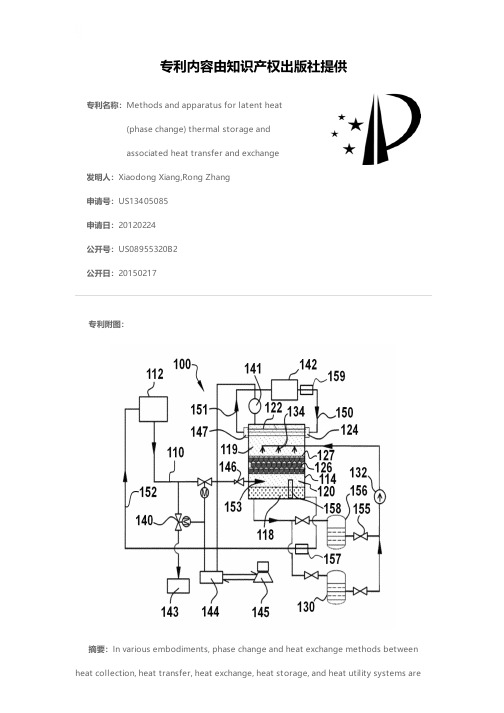
专利名称:Methods and apparatus for latent heat(phase change) thermal storage andassociated heat transfer and exchange发明人:Xiaodong Xiang,Rong Zhang申请号:US13405085申请日:20120224公开号:US08955320B2公开日:20150217专利内容由知识产权出版社提供专利附图:摘要:In various embodiments, phase change and heat exchange methods between heat collection, heat transfer, heat exchange, heat storage, and heat utility systems aredescribed. In certain embodiments, the heat transfer fluids/heat exchange fluids, heat storage media, and working media in the system are all phase change materials with transition temperatures close to each other and in decreasing order and perform their respective function through phase changes within a relatively narrow temperature range. Methods to control heat transfer rate, heat exchange and/or heat charging/discharging rate between heat collection, thermal energy storage and heat utility apparatus at will are provided. Methods of controlling such systems are also provided.申请人:Xiaodong Xiang,Rong Zhang地址:Danville CA US,Cupertino CA US国籍:US,US代理机构:Weaver Austin Villeneuve & Sampson LLP更多信息请下载全文后查看。
materialsforadvancedheatstorageinbuildings

P rocedia Engineering 57 ( 2013 )837 – 8431877-7058 © 2013 The Authors. Published by Elsevier Ltd.Selection and peer-review under responsibility of the Vilnius Gediminas Technical Universitydoi: 10.1016/j.proeng.2013.04.106Available online at Open access under CC BY -NC-ND license.838 M ilan Ostry and Pavel Charvat / P rocedia Engineering 57 ( 2013 ) 837 – 843 building technologies with high energy consumption. Therefore, the attention must be paid to the materials with low weight and higher heat storage ability that can increase thermal comfort by the passive radiant cooling or active heating. There are two common approaches to thermal energy storage – sensible heat storage and latent heat storage. Both approaches have certain advantages and disadvantages.2. Sensible heat storageIn this technique heat is stored by changing the temperature of the storage medium. The amount of stored heat depends on the heat capacity of the medium, the temperature change and the amount of storage medium. Sensible heat storage can utilize solid or liquid storage mediums. The amount of energy stored in the medium depends on the:• the weight of the building structures; • the specific heat capacity of the materials of the structures; • the difference between initial and final temperature during the storage process. The materials used for sensible heat storage do not undergo a phase change. The most popular liquid medium is water, which usually needs to be contained in some kind of container. Water is very often used as a heat carrier in thermal energy systems and it makes a lot of sense to use it as a heat storage medium in such systems.Air is the heat carrier that accounts for most of the heat transfer in the built environments and that makes other materials more suitable for building-integrated thermal storage. Stone, reinforced concrete or brick masonry are the common materials used in building construction that can be employed for thermal energy storage [2]. The main advantages of sensible heat storage systems are low costs and long working stability. The massive building structures contribute to the thermal stability of indoor environment during hot days. Indoor temperature swings are very much dampened by the heat storage effect of the building structures. However, sensible heat storage is the least efficient method of heat storage, since much less thermal energy is involved in raising the temperature of a material than in melting a crystalline compound or in breaking the chemical bonds. The amount of stored heat is given by following equation [3]:ff i i T T p p T T Q m c dT V c dT ==ρ∫∫, (1)where Q is stored heat (J)m mass of the storage medium (kg) c p average thermal capacity between T i a T f (J.kg -1.K -1)ρ density of the storage medium (kg.m -3)V volume of the storage medium (m 3)T i initial temperature (K)T f final temperature (K)Table 1. Characteristics of selected material applicable for sensible heat storage (at 20 °C)Material Densitykg.m -3Specific heat J.kg –1.K –1 Thermal capacity 106.J.m –3.K –1 watersteelreinforced concretegraniteplain concretesolid brickwoodgypsum board 998 7850 2500 2500 2100 1800 400 750 4182 440 1020 950 1020 900 2510 1060 4,17 3,45 2,55 2,38 2,14 1,62 1,00 0,803. Latent heat storageIn latent heat storage the heat is stored by means of a reversible change of state or a phase change of the storage medium. The solid-liquid transformations are most commonly utilized, though solid-solid transitions have been investigated as well.839M ilan Ostry and Pavel Charvat / P rocedia Engineering 57 ( 2013 ) 837 – 843 The latent heat storage systems also make use of some sensible heat capacity of the medium during the increase of temperature (outside of the melting range). The heat of fusion usually dominates, but considerable heat can be added by sensible storage.The storage capacity of latent heat storage systems is influenced by the:• weight of heat storage medium; • specific heat in solid and liquid phase; • difference between initial and melting temperature; • difference between melting and final temperature; • specific heat of fusion. The molecules of the material have much larger freedom of movement when the material is in the liquid state than when it is in the solid state and therefore the molecules have higher energy in the liquid state. The molecules of the material that is in the state of gas have a still higher degree of freedom, being almost entirely free of the intermolecular attraction, and correspondingly possess very high energies [4]. Solid-gas and liquid-gas phase changes are not generally used for thermal energy storage in building structures in spite of their highest latent heats because of the high volume changes.The latent heat storage media are called the Phase Change Materials (PCMs). The PCMs can be integrated in building structures to provide thermal storage capacity and thus to increase the thermal comfort of occupants in the buildings with light-weight envelope. The use of the phase change materials for latent heat storage has lagged behind that of sensible heat storage. This is partly because the latent heat storage systems represent a higher level of technology. Also, while concrete and masonry can serve a double purpose – structural strengths and thermal storage capacity – the PCM cannot be used as a material for load bearing structures and they need to be integrated with building structures usually in some form of surface finishing (plaster, wallboards, suspended ceiling tiles, etc.).Another very important issue with building integrated thermal storage is heat transfer between the storage material and the indoor environment.Many massive load bearing structures with high sensible heat storage capacity are “insulated” from the indoor environment by floor finishes, suspended ceilings, furnishing, etc. That significantly reduces convective and radiant heat transfer between the thermal storage material and the indoor environment. Indoor air that is the heat carrier for convective heat transfer in this case is a rather ineffective medium. The values of heat transfer coefficient at internal surfaces in a built environment almost never exceed 10 W.m –2.K –1. In order to increase the value of the heat transfer coefficient the indoor air velocities w ould need to be increased and that w ould have a negative impact on the thermal comfort of the occupants. The radiant heat transfer betw een an occupant and the storage mass requires direct visibility, therefore, the unobstructed internal surfaces are the best location for building integrated thermal storage.Because of relatively small changes in the volume of the material the amount of stored heat during the phase change from solid to liquid state is equal to the change in enthalpy [5]:.Q m h Δ=Δ (2) where Q is stored heat (J)m mass of medium (kg) h enthalpy (J.kg -1)Considering only solid-liquid transformation, heat added to the medium causes at first sensible heating of the solid followed by a solid – liquid phase change and sensible heating of the liquid. The amount of stored heat can be described by following equation [6]:()()f T Tm ps pl pl Ti Tm Q m c T dT l c T dT ⎡⎤⎢⎥=++⎢⎥⎣⎦∫∫. (3) where Q is stored heat (J)m mass of the medium (kg) T i initial temperature (K)T f final temperature (K)T m melting temperature (K)l pl latentheat (J.kg -1) c ps specific heat of solid (J.kg -1.K -1)c pl specific heat of liquid (J.kg -1.K -1)840M ilan Ostry and Pavel Charvat / P rocedia Engineering 57 ( 2013 )837 – 843841M ilan Ostry and Pavel Charvat / P rocedia Engineering 57 ( 2013 ) 837 – 843Fig. 2. Gypsum plaster with microencapsulated PCMs and system of capillary tubes (author)Fig. 2 show s the application of commercial organic microencapsulated PCMs Micronal DS 5008X (manufactured by BASF) in the full-scale experiments carried out at the Faculty of Civil Engineering. The microcapsules with paraffin were mixed with gypsum plaster. The gypsum plaster with PCM covers a system of capillary tubes for discharge of stored heat. The system can be used for active cooling as well.Table 2. Characteristics of microencapsulated PCMs (heating phase) Material Peak temperature (K) Stored heat(kJ.kg -1)Micronal DS 5008X Micronal DS 5029X 24.3 23.3 86.875.8The wallboard containing phase change material looks similar to a common gypsum wallboard and it can be used on the walls or suspended ceilings. The microencapsulated phase change materials can be combined with other building materials, e.g. gypsum plaster, bricks, mortar, reinforced or plain concrete and some insulation materials. Special attention has to be given to the choice of the capsule's material to avoid chemical reactions between the capsules and the building material [12]. The third kind of thermal energy storage technology is the use of novel shape-stabilized phase change materials. A low thermal conductivity is a disadvantage of this storage technology. The thermal conductivity of shape-stabilized phase change material can be improved by addition of graphite.Fig. 3. PCMs encapsulated in aluminium panel (author)842 M ilan Ostry and Pavel Charvat / P rocedia Engineering 57 ( 2013 ) 837 – 843Yet another technique used for integration of phase change materials in buildings is macro-capsulation. In this case the PCMs are contained in various types of containers. The flat-shaped containers are the best for building use but spherical containers can be employed in storage tanks or packed-bed thermal storages.Macro encapsulated phase change material can be installed in suspended ceiling and on the w alls. A phase change material can be encapsulated in:• flat aluminium or plastic panel; • aluminium or plastic containers; • pouches; • plastic tubes; • plastic balls.Table 3. Characteristics of selected commercial PCMs proposed for macroencapsulationMaterial Peak temperature (K) Stored heat(kJ.kg -1)DELTA®-COOL 24Parafol 18-97Rubitherm SP 25 A8Rubitherm RT 27 28.2 28.9 26.6 27.8 126.6 221.3 71.4 139.7The aluminum containers have higher thermal conductivity then the macrocapsules made of plastics but there is a risk of corrosion in case of inorganic phase change materials. The plastics are compatible w ith a lot of inorganic phase change materials.Fig. 4. Encapsulated PCMs for installation in suspended ceiling (author)5. ConclusionsThe energy conservation is the driving factor behind many innovations in the building sector. Unfortunately, a solution to one problem can bring about some new problems. The w idespread use of light-w eight building envelopes has helped to reduce the transmission heat loss of buildings but it has brought about the problems w ith overheating during the w arm season. A problem that did not typically occurred in buildings in the moderate climate. This new challenge can be dealt with in a traditional way by installation of air-conditioning systems or some new energy-efficient solutions can be found to solve it.The purpose provided thermal storage capacity for light-w eight buildings is one of the options in dealing w ith overheating. This solution is not suitable for all light-weight buildings and all operation modes but it has the potential to reduce or eliminate the need for mechanical cooling. Even a simple shift of cooling loads from daytime to night hours reduces the peak electricity demand. A number of latent heat storage options for integration with building structures already exist and some new options are being proposed and investigated.843 M ilan Ostry and Pavel Charvat / P rocedia Engineering 57 ( 2013 )837 – 843AcknowledgementsThis work was supported by the Czech Grant Agency under project No.P104/12/1838 ”Utilization of latent heat storagein phase change materials to reduce primary energy consumption in buildings”.References[1] Dinçer, I., Rosen, M. A., 2002. Thermal Energy Storage: Systems and Applications. John Wiley & Sons, Ltd,p. 579.[2] Škramlik, J.,Novotný, M.,Šuhajda, K., Modelling of diffusion in porous medium, in Proc. of the International Conference on Numerical Analysis andApplied Mathematics: Numerical Analysis and Applied Mathematics (ICNAAM 2011),2011, Halkidiky, Greece[3] Khalifa, A. J. N., Abbas, E. F., 2009. A comparative performance study of some thermal storage materials used for solar space heating, Energy andBuildings 41(4), pp. 407-415.[4] Garg, H. P., Mullick, S. C., Bhargava, A. K., 1985. Solar Thermal Energy Storage. D. ReidelPublishing Comnpany, p. 642.[5] Silva, T., Vicente, R., Soares, N., Ferreira, V., 2012. Experimental testing and numerical modelling of masonry wall solution with PCM incorporation:A passive construction solution, Energy and Buildings 49, pp. 235-245.[6] Regin, F. A., Solanki, S.C., Saini, J.S., 2008. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review, Renewableand Sustainable Energy Reviews 12(9), pp. 2438-2458.[7] Klimes, L., Charvat, P., Ostry, M., 2012. Challenges in computer modelling of phase change materials, Materiali in tehnologije 46(4), pp. 335-338.ISSN 1580-2949.[8] Zalba, B., Marín, J. M., Cabeza, L. F., Mehling, H., 2003 Review on thermal energy storage with phase change: materials, heat transfer analysis andapplications, Applied Thermal Engineering 23(3), pp. 251-283.[9] Tyagi, V. V., Buddhi, D., 2007. PCM thermal storage in buildings: A state of art, Renewable and Sustainable Energy Reviews 11(6), pp. 1146-1166.[10] Mehling, H., Cabeza, L. F., 2008. Heat and cold storage with PCM.An up to date introduction into basics and applications.Springer-Verlag BerlinHeidelberg. 308 p.[11] Zhao, C.Y., Zhang, G. H., 2011. Review on microencapsulated phase change materials (MEPCMs): Fabrication, characterization and applications,Renewable and Sustainable Energy Reviews 15(8), pp. 3813-3832.[12] Kuznik, F., David, D., Johannes, K., Roux, J.-J., 2011. A review on phase change materials integrated in building walls, Renewable and SustainableEnergy Reviews 15(1), pp. 379–391.[13] Tyagi, V. V., Kaushik, S. C., Tyagi, S. K., Akiyama, T., 2011. Development of phase change materials based microencapsulated technology forbuildings: A review, Renewable and Sustainable Energy Reviews 15(2), pp. 1373-1391.。
应用于低温集热蓄热的二十二烷-十二醇复合相变材料的制备和性能

第 12 卷第 12 期2023 年 12 月Vol.12 No.12Dec. 2023储能科学与技术Energy Storage Science and Technology应用于低温集热蓄热的二十二烷-十二醇复合相变材料的制备和性能陈红兵1,李春阳1,王聪聪1,李璊2,卢浩阳1,刘宇航1,张岩1(1北京建筑大学供热、供燃气、通风及空调工程重点实验室,北京100044;2北京市热力集团有限责任公司石景山分公司,北京100043)摘 要:为使相变材料更好覆盖太阳能PV/T系统中电池背板的工作温度范围,提高系统的储热能力。
本工作首先采用熔融混合法制备了不同质量分数的二十二烷-十二醇(DE-CP)二元相变材料,并对其潜热进行测试分析。
其中,二元相变材料的相变温度区间为20~50 ℃,基本覆盖PV/T系统中电池背板的工作温度;相比于其他二元相变材料,DE质量分数为60%的二元相变材料的潜热值最大,为243.8 kJ/kg;并且DE与CP质量配比为6∶4的二元相变材料的高温度相变峰面积大于低温度相变峰面积,两个相变峰的分布说明,DE质量分数为60%的二元相变材料更加适合在太阳能PV/T系统中应用。
然后在二元相变材料中添加EG(膨胀石墨)作为支撑材料,通过吸附增强其导热能力,最终得到复合相变材料。
并对其潜热、相容性、导热性进行测量和分析,结果表明DE-CP/EG复合相变材料的相变温度区间仍为20~50 ℃,三种原材料的相容性好,导热系数由原来二元相变材料的0.135 W/(m·K)提升至1.383 W/(m·K),解决了相变材料自身导热系数低的问题,为后续复合相变材料在太阳能PV/T系统中的应用提供借鉴参考。
关键词:复合相变材料;二十二烷;十二醇;膨胀石墨;热性能;储热doi: 10.19799/ki.2095-4239.2023.0482中图分类号:TK 519;TB 34 文献标志码:A 文章编号:2095-4239(2023)12-3663-07 Preparation and properties of binary composite phase-change materials based on solar low-temperature heat storageCHEN Hongbing1, LI Chunyang1, WANG Congcong1, LI Men2, LU Haoyang1,LIU Yuhang1, ZHANG Yan1(1Beijing Municipal Key Laboratory of HVAC, Beijing University of Civil Engineering and Architecture, Beijing 100044, China; 2Beijing Thermal Power Group Co., Ltd. Shijingshan Branch, Beijing 100043, China)Abstract:The working temperature range for the battery backplane and the heat storage capacity in solar PV/T systems using phase-change materials need to be improved. In this report, docosane-dodecanol (DE-CP) binary phase-change materials with different mass fractions were prepared using the melt-mixing method, and their latent heat was tested and analyzed. The phase-change temperature range for the binary phase-change material is 20–50 ℃, which essentially covers the working temperature for the battery backplane in the PV/Tsystem. Compared with other binary phase-change materials, the latent heat of the binary phase-change material with 60% DE mass fraction is the largest at 243.8 kJ/kg. Moreover, the收稿日期:2023-07-17;修改稿日期:2023-08-26。
相变材料对储热系统换热速率的影响

统最大理论蓄 / 释功率 Φ max ( W) ꎬ蓄热为正值ꎬ释
热为负值:
碳基微纳米材料因其具有高导热系数的固有特性
常被用 于 改 善 PCMs 的 导 热 性ห้องสมุดไป่ตู้能 且 效 果 明 显.
1293
Φ max = mc pHTF ( t in - t m ) .
storage systemꎬ and phase change temperatureꎬ thermal conductivity and energy storage density of
PCMs are the three most critical factors for selecting PCMs. These effects of three key factors of
r PCM =
相变潜热过程. 引入换热器的换热效能
(7)
由图 1a 可知ꎬ单元热阻 R it 与系统热阻 R t 为
并联的关系ꎬ因此可得系统总热阻:
分析ꎬ可忽略 PCMs 显热影响ꎬ只考虑 PCMs 系统
[18 - 19]
r PCM
1
ln
.
2πλ PCM L
r0
ꎬ它
Copyright©博看网. All Rights Reserved.
对储热系统换热速率的影响效果进行综合分析ꎬ
从而在工程应用上对这三大因素进行综合衡量选
取给予指引和帮助.
本文通过常用的管壳式结构的储热系统作为
研究对象ꎬ引入常用的热阻模型 [17] 简化进行分析
比较ꎬ说明导热系数、相变温度及储能密度对管壳
因此本文引入热阻模型方法对相变过程中的热阻
phase_Change_materials

USE OF RENEWABLE PHASE CHANGE MATERIALSTO REDUCE CARBON DIOXIDE EMISSIONSGalen J. Suppes, Michael J. Goff, and Shailesh Lopes,Department of Chemical Engineering, W2028 Engineering Bldg. East,Columbia, MO, 65211AbstractPhase change materials (PCM) can reduce heating, ventilation, and air conditioning (HVAC) costs by a number of different mechanisms, including: eliminating air conditioning costs by storing nighttime coolness for use during the day, eliminating heating costs by storing daytime warmness for use during the night, and by load-shifting of electricity through thermal storage. Well-designed strategies and utilization of phase change materials could easily reduce HVAC-related carbon dioxide emissions by more than 25% from current levels. It is anticipated that these reductions in emissions can be achieved while saving consumers money and creating new markets for agricultural commodities. Greenhouse gas reduction potential of PCSs is further enhanced by producing them from fats and oils. This paper summarizes experimental data on the synthesis of PCMs from beef tallow and quantifies the cost and greenhouse gas savings from their use.IntroductionFigure 1 summarizes carbon dioxide emissions in the U.S. by source i[1]. As evident by Figure 1, electrical power generation is the largest producer of carbon dioxide emissions, and furthermore, emissions in this sector are growing faster than any other sector. When electricity is combined with residential and commercial fossil fuel heating, the sum of 33.9%, 5.4%, and 3.4% total a massive 42.7% of the total greenhouse gas emissions. Due to magnitude of this contribution to greenhouse gas emissions, thermal energy storage can pave the way to reducing carbon dioxide emissions to 1990 levels or less for decades to come. In many if not most instances, reductions in greenhouse gas emissions can be achieved while saving the consumer money.Thermal energy storage can reduce greenhouse gas emissions through multiple mechanisms, including: 1) Peak load shifting of electricity, 2) Eliminating part of the heating or air conditioning loads, and 3) Enhance the performance of alternatives to fossil fuel combustion heating. The purpose of this paper is to provide an introduction into the topic of phase change materials and to describe how phase change materials can reduce greenhouse gas emissions through each of the three indicated mechanisms.Phase change material store coolness or warmness, typically through a latent heat of freezing that is about one hundred fold greater than sensible heat. I is the most commonly used phase change material and is used to keep food cool in a cooler or a drink cold in a cup.While a 0︒C storage in ice can be used to store coolness for a house, it would require a more expensive air conditioner and consume more energy to go to this low temperature as compared to typical evaporator operation at about 10︒C. Alternatively, a phase change material thatfreezes at 15︒C freezes at a high enough temperature to be frozen by a conventional air conditioner while freezing at a low enough temperature to provide a temperature driving force to keep the house cool. Particularly good and efficient strategies can be developed to enhance HVAC applications through the proper choice of phase change material with a freezing point typically between 15︒C and 35︒C.Figure 1. Summary of carbon dioxide emissions by source in the United States.While some inexpensive salts have been demonstrated for HVAC applications, these salts have two performance problems: 1) they are corrosive and 2) the hydrates typically used in the 15-35︒C temperature range are often not stable for prolonged storage and cycling in HVAC applications.Narrow freezing point range paraffin waxes distilled from petroleum are often the product of choice. These waxes typically sell for prices greater than $0.35 per pound. When compared to the price of fats and oils ranging in price from $0.10 to $0.30, derivatives of fats and oils can be prepared at lower costs than paraffin waxes. These new fat and oil products are made from renewable resources and have the potential to undercut the paraffin waxes on price. Fatty acid derivatives of fats and oils are able to meet a range of energy storage means in the 15-35︒C temperature range. Current research by the authors of this paper has a focus on low-cost and high conversions of natural fat and oil products for PCM applications.Efficiency for Energy StorageWhen evaluating chemicals for energy storage, thermal storage options have two important performance advantages over battery, water, or other storage options.The most important performance advantage is the fact that thermal storage and recovery can approach efficiencies of 100% while chemical energy or potential energy storage and retrieval will typically have overall efficiencies of less than 80%. When HVAC is the final application, thermal energy storage can approach 100% efficiency because it is governed by heat transfer that is governed by the first law of thermodynamics. In practical terms this means that in a well-insulated system no energy is lost because it is conserved. In real applications some air handling losses are incurred, but these can be minimized.Alternatively, battery and pump storage include the transfer of electrical energy to chemical energy (in the case of a battery) or shaft work then potential energy (in the case of pump storage). Fundamentally each of these energy conversions can approach 100% efficiency, but in practice chemical energy and potential energy conversion are each rarely better than 90% efficient and electric motor production of shaft work is rarely better than 95% efficient. Alternatively, conversion of electrical energy to heat and transfer of heat is routinely performed at first law efficiencies greater than 99%.Thermal energy storage is the most efficient way to store energy when the final application is in HVAC. Matching the PCM freezing points with applications is the most important design criteria to minimize or eliminate any inefficiencies in the energy storage process.Peak Load ShiftingPeak load shifting is the easiest application of phase change materials and is also the application capable of producing the greatest reductions in carbon dioxide emissions. The concept is quite simple. In the summer time, air conditioners and most industrial equipment tends to naturally operate during the middle of the day when the sun’s heat is the greatest and people are at work running equipment. Without the intervention of electrical power providers, peak demand for electricity would be many times greater than the baseline load at 2:00 in the morning. With intervention through cooperation with customers, peak demand is often reduced to being 50% to 100% greater of baseline load.To meet peak demand needs, electrical power companies need electrical power generation that can start up and shut down quickly. The preferred generation means are gas turbines typically operating at thermal efficiencies less than 30%. As compared to a modern natural gas combined cycle plant operating at 50% to 53% thermal efficiency, the peak demand unit produces about 40% more carbon dioxide per kilowatt hour of electricity produced.Also, the use of wind and nuclear energy is limited due to their inflexible nature in meeting peak demand needs. Wind is available on nature’s schedule and nuclear power is capital intensive and best operated a full load at all times. Energy storage can greatly enhance the ability of nuclear and wind power to meet all electrical needs including peak demand needs.When replacing peak demand gas turbines with wind or nuclear power, nearly 100% of the carbon dioxide emissions are eliminated.While wind and nuclear energy are unable to meet much of electrical power needs on their own, they can fully meet electrical power needs when combined with energy storage. The sky is the limit when it come to potential reductions in carbon dioxide emissions due to electrical power and HVAC when wind or nuclear energy is combined with energy storage.Eliminating HVAC LoadsPhase change materials can be used to eliminate HVAC electrical power or fossil fuel loads in some instances. One means of doing this is to store solar energy accumulated during the day and used during the night. Most buildings simply do not have enough built-in heat capacity to substantially moderate temperatures in a 24 hour cycle—PCM devices can be used as a active means to provide this energy storage while tapping into natures sources of heat or coolness available during only part of the 24 hour cycle.In the winter time, solar energy provides a source of warmth that can be tapped into with varying degrees of success. In the summer time, evaporative cooling that typically achieves much lower temperatures during the night provides a source of coolness. Various means are available to tap into these sources of heat and coolness.In addition to active storage of solar-originated energy during the winter, passive storage can be achieved by incorporating PCM into wall boards. This passive method simply increases the heat capacity of a building and should be performed on a surface (such as wallboards) that readily allow heat transfer between the room contents and the PCM.Enhancing the Performance of AlternativesThe previous section described how enhancing the performance of solar heating or evaporative coolers can be used to eliminate heating or cooling costs. In these examples, significant reductions in electrical costs can be achieved. Phase change materials can also be used in combination with vapor-compression cycles to increase the utility of vapor-compression cycles.SummaryPCM devices can be used to substantially reduce greenhouse gas emissions originating from electrical power generation and residential/commercial heating. While such PCM devices are typically used with HVAC applications, their ability to moderate peak demand impacts most electrical power generation. The ability of electrical power generation means such as wind and nuclear energy to meet consumer needs are greatly enhanced when used with energy storage such as PCM devices.AcknowledgementsThis work was supported by the USDA NRI Competitive Grants Program/USDA award number 2001-355504-11208. Experimental results were presented at the conference and were in the process of being reviewed for publication at the time of the conference.Referencesi[1]Emissions by Economic Sector, Excerpt from the Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990-2000, U.S. EPA, Office of Atmospheric Programs, September 2001, see /globalwarming/publications/emissions/index.html.用于CO_2吸收的离子液体的合成、表征及吸收性能--《精细化工》2007年04期本文献来源中国知网以N-甲基咪唑和3-溴丙胺氢溴酸盐为起始原料,合成了一种含氨基的离子液体(ionic liquid,IL)——1-(1-氨基丙基)-3-甲基咪唑溴盐(简写为[NH2p-mim]Br),总产率为90.1%,溴含量为0.980 mol Br/mol IL,胺含量为0.948 mol NH2/mol IL。
相变材料作文模板英语翻译

相变材料作文模板英语翻译Title: Essay Template on Phase Change Materials。
Introduction。
Phase change materials (PCMs) are a type of material that can store and release large amounts of energy when they undergo a change in their physical state, such as from solid to liquid or vice versa. They have gained significant attention in recent years due to their potential applications in various fields, including energy storage, thermal management, and temperature regulation. In this essay, we will explore the properties, applications, and future prospects of phase change materials.Properties of Phase Change Materials。
Phase change materials exhibit unique properties that make them suitable for a wide range of applications. One of the key characteristics of PCMs is their high energy storage capacity. When a PCM undergoes a phase change, it can absorb or release a large amount of energy in the form of latent heat. This property makes them ideal for use in thermal energy storage systems, where they can store excess heat or cold and release it when needed.Another important property of PCMs is their ability to maintain a constant temperature during the phase change process. This property, known as thermal inertia, allows PCMs to regulate temperature fluctuations in their surroundings, making them useful for applications such as building insulation and temperature-controlled packaging.Applications of Phase Change Materials。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Stearic acid/polymethylmethacrylate composite as form-stable phase change materials for latent heat thermal energy storageYi Wang a ,b ,c ,*,Tian Dong Xia a ,b ,Hui Xia Feng c ,Han Zhang caState Key Laboratory of Gansu Advanced Non-ferrous Metal Materials,Lanzhou University of Technology,Lanzhou 730050,China bKey Laboratory of Non-ferrous Metal Alloys,Ministry of Education,Lanzhou University of Technology,Lanzhou 730050,China cCollege of Petrochemical Technology,Lanzhou University of Technology,Lanzhou 730050,Chinaa r t i c l e i n f oArticle history:Received 15September 2010Accepted 20December 2010Available online 8January 2011Keywords:Form-stable composite PCM LHTESEncapsulationThermal properties Thermal reliability PMMAa b s t r a c tThe aim of this research is to prepare of a novel form-stable composite phase change material (PCM)for the latent heat thermal energy storage (LHTES)in buildings,passive solar space heating or functional fluid by entrapping of SA into PMMA cell through ultraviolet curing dispersion polymerization.The composite PCM was characterized using scanning electron microscope (SEM)and Fourier transformation infrared (FT-IR)analysis technique.The results show that the form-stable microencapsulated PCM with core/shell structure was formed and the maximum encapsulated proportion of SA in the composite was 51.8wt.%without melted PCM seepage from the composite.In the shape stabilized microencapsulated PCM,the polymer acts as supporting material to form the microcapsule cell preventing the leakage of PCM from the composite and the SA acts as a PCM encapsulated in the cell of PMMA resin.The oxygen atom of carbonyl group of skeleton is interacted with the hydrogen atom of hydroxyl group of SA.Thermal properties,thermal reliability and heat storage/release performance of the composite PCM were determined by differential scanning calorimetry (DSC),FT-IR and thermal cycling test analysis.The melting and freezing temperatures and the latent heats of the composite PCM were measured as 60.4 C,50.6 C and 92.1J/g,95.9J/g,respectively.The results of DSC,FT-IR and thermal cycling test are all show that the thermal reliability of the composite PCM has an imperceptible change.This conclusion indicates that the composite has a good thermal and chemical stability.Ó2010Elsevier Ltd.All rights reserved.1.IntroductionThe direct solar radiation is considered to be one of the most prospective sources of energy to avert the growing concern about greenhouse gas emissions and the climb in fuel prices.However,the large-scale utilization of this form of energy is possible only if the effective technology for its storage can be developed with acceptable capital and running costs.The storage of energy in suitable forms,which can conventionally be converted into the required form,is a present day challenge to the technologists.The scientists all over the world are in search of new technologies to develop solar energy storage devices,which are as important as developing new sources of energy.Energy storage not only reduces the mismatch between supply and demand,decreases the excess energy wasted,but also improves the performance and reliabilityof energy systems and plays an important role in conserving the energy.Among all ways of energy storage,latent heat thermal energy storage (LHTES)using phase change material (PCM)is particularly attractive technique because the PCM can absorb or release large latent heat during the melting or solidifying process when surrounding temperature increases and decreases [1,2].When compared to conventional sensible heat energy storage systems,the LHTES provides much higher energy storage density with a smaller temperature difference between storing and releasing heat.Therefore,these features make them more func-tional and widely used than the traditional PCM in heat pumps,spacecraft thermal control,functional fibers thermal insulation,heat transfer,solar energy utilization,building materials and shifting peak energy demand applications.Many kind of inorganic and organic compounds such as salt hydrates,paraf fins,fatty acids,fatty acid esters and their binary and ternary mixtures are de fined as PCM and they can be poten-tially used for LHTES in many fields due to their different phase change intervals.Unfortunately,prior to the large-scale practical application of this technology,it is necessary to resolve numerous problems.For their employment as latent heat storage materials,*Corresponding author.State Key Laboratory of Gansu Advanced Non-ferrous Metal Materials,Lanzhou University of Technology,Lanzhou,Gansu 730050,China.Tel.:þ8613919809076;fax:þ869312973648.E-mail addresses:haoyunwangyi1977@ ,wangyi@ (Y.Wang).Contents lists available at ScienceDirectRenewable Energyjournal ho me page:www.elsevier.co m/locate/renene0960-1481/$e see front matter Ó2010Elsevier Ltd.All rights reserved.doi:10.1016/j.renene.2010.12.022Renewable Energy 36(2011)1814e 1820these materials must exhibit certain desirable thermodynamic, kinetic and chemical properties.Moreover,economic consider-ations and easy availability of these materials has to be kept in mind.The major drawbacks such as leakage,increases not only the heat resistance but the cost of the LHTES system,and lower thermal conductivity,which limits the power that can be extracted from the energy storage system,have restricted their extensive application. Various methods have been developed for overcoming these problems.The most potential and actually useful method is preparing form-stable PCM.The form-stable PCM can be prepared by encapsulation of PCM into a polymeric structure,some articles defined as microencapsulated phase change material(MEPCM),by physical-mixing or chemical synthesis.Its main advantages of these materials is it can maintain the shape and prevent PCM leakage during the phase change process and it can be directly used in thermal energy storage system without extra encapsulation due to the PCM has been encapsulated in three-dimensional net structure formed by polymer materials[3].In addition,thermal conductivity of the form-stable PCM composites can be increased remarkably by the loading of carbonfibers,carbonfiber brushes,graphite and etc due to their higher thermal conductivity[4,5].There are many literatures have concentrated on form-stable PCM.A literature survey on form-stable PCM indicates that mela-mine-formaldehyde(MF)resin[6],urea e formaldehyde(UF)resin [7],polyurethane(PU)[8],high density polyethylene(HDPE) [9e11],styrene-butadiene-styrene(SBS)copolymer[12]and pol-ymethyl methacrylate(PMMA)[3,13]are usually selected as microcapsule shell materials for the PCM protection.PMMA is a thermoplastic,transparent and commercially available acrylic polymer.It has moderate properties,easy handling and processing, high impact strength,relatively good chemical resistance and low cost.From this point of view,PMMA is a versatile material and the promising polymer as shell material containing PCM for thermal energy storage applications.Alkan et al.[3]prepared stearic acid (SA),palmitic acid(PA),myristic acid(MA),and lauric acid(LA)/ PMMA composite as form-stable PCM by encapsulation of fatty acids into PMMA which acts as supporting material though solution casting method.Wang and Meng[13]synthesized a series of PMMA microcapsules containing CA-LA,CA-MA,CA-SA and LA-MA eutectics acting as the heat-absorbing materials using the method of self-polymerization.Alkan and their colleagues fabricated microencapsulated PCM by emulsion polymerization using doco-sane[14],n-octacosane[15]and n-heptadecane[16]as core material and PMMA resin as shell materials.Stearic acid(SA)is known as linear chain fatty acid and widely utilized as PCM.It is chemically stable,showing no phase segre-gation during phase change,odorless,nontoxic,easily available and large thermal storage capacity[17e19].Therefore,it may be used as energy storage material for passive solar space heating,functional fluid or decreasing indoor temperature swing in buildings.As can be seen from the literature survey given above,there are some studies on microencapsulation or form-stable PCM with PMMA.However,as far as the authors are aware,there is no investigation reported in literature on preparation and comprehensive characterization of PMMA blends as PCM by UV photoinitiated dispersion polymerization.Ultraviolet photo-polymerization is a high efficiency,wide enabling,energy saving, environmental friendly and environmental protection tech-nology.It has been widely used in chemical industry,machinery, electronics,light industry and communication[20,21].In this regard,the objective of this study is to prepare microcapsules made of PMMA as shell material and SA as core by UV photoinitiated dispersion polymerization.Fourier transfor-mation infrared spectroscope(FT-IR)and scanning electronic microscope(SEM)were used to determine the chemical characterization and microstructure of composites,respectively. Differential scanning calorimetry(DSC)and thermal perfor-mance test were employed to investigate the thermal properties and thermal stability of the prepared composite.In addition, thermal cycling test was conducted to determine the thermal reliability of the PCM.2.Experimental2.1.MaterialsStearic acid(SA,commercial grade,T e¼66.5 C,ΔH¼177.8J/g) was used as phase change material(core material)and supplied by Beijing Chemical Co.Ltd.Methyl methacrylate(MMA,AR) purchased from Tianjin Kermel Chemical Reagent Co.Ltd.was used as shell-forming monomers.MMA was washed by5%NaOH solu-tion(V MMA:V NaOH¼5:1)in a separatory funnel for several times to remove the polymerization inhibitor hydroquinone and then double distilled before use.The polyethylene glycol octyl phenyl-ether and poly(vinyl alcohol)(PVA)were obtained from Shanghai Chemical Reagent Co.Ltd.and used as surfactant and dispersing agent,respectively.Ethylene glycol dimethacrylate(EGDMA)was bought from Shanghai Chuanghe Chemical Reagent Company and used as crosslinking agent.Photosensitive solicitation reagent 2-hydroxyl-2-methyl-1-phenyl acetone(1173)which chemical formula are showing in Fig.1and deionized water was self-made by our laboratory.2.2.Preparation of the form-stable SA/PMMA composite PCMIn preparation of the SA/PMMA form-stable PCM,dispersion polymerization through UV photoinitiated method was conducted. Reactionflask with100mL deionized water,5g SA,0.01g PVA and 0.02g polyethylene glycol octyl phenylether was heated above melting point of SA under nitrogen atmosphere.Then the mixture was violent mixed for half an hour before polymerization.Then 4mL methyl methacrylate monomer and EGDMA photoinitiator were added and stirred vigorously at the same temperature for1h. The mixture contained the monomer was reacted at UV radiation for30min and the precipitate was separated form the solution by casting water.The particles was dried under vacuum at80 C for 24h.This process was recognized as leakage test and the composite which did not leak melted PCM from the microcapsules was defined as form-stable PCM.2.3.Characteristic of the form-stable SA/PMMA composite PCMPhase change properties,melting and freezing temperatures and latent heats,of the SA and SA/PMMA composite were deter-mined by differential scanning calorimeter(DSC,NETZSCH STA-449C model)at a heating rate of5 C per minute and temperature range from room temperature to100 C in a purified nitrogen atmosphere.The specimens for DSC analysis with the mass of 5e10mg were taken from the brittle failure surface of the form-stable PCM.The extrapolated onset temperature(T S),peakFig.1.Chemical structure of1173.Y.Wang et al./Renewable Energy36(2011)1814e18201815temperature(T P )and extrapolated end temperature (T E )of the SA and SA/PMMA microcapsules were obtained by the special soft-ware of the DSC.The phase change latent heats were determined by numerical integration of the area under the peaks.In order to prove the compatibility between the components of composite,the form-stable PCM was characterized by spectroscopy methods.The spectroscopic analysis after and before thermal cycling was per-formed on a KBr disk in the range of 4000e 400cm À1by using a Nicolet AVTAR 360FT-IR.The scanning electron microscope (SEM)images of form-stable composite were taken by JSM-6701model SEM.The form-stable PCM specimens were broken and the frac-tured surfaces were coated with gold before SEM imaging.2.4.Thermal performance and thermal stability analysisTo con firm the heat storage/release performance of PCM composite,thermal performance test was conducted using the experimental set-up in Fig.2.In this experimental set-up,the water was circulated throughout the surrounding of test cell for the melting process of the composite (5g)at a constant temperature of 80 C [17].After melting process,the sample were immediately subjected to solidi fication process in the refrigerator (cooling facility)at 30 C.The temperature variations of the PCM and reference substance during these periods were automatically recorded to a PC via data logger with a temperature measuring accuracy of Æ0.1 C at time intervals of 2s.The thermal cycling test was also used to determine thermal reliability of form-stable composite PCM in terms of the change in phase change temperatures and latent heats with respect to the thermal cycling number.A thermal cycling test consisted of exposing composite PCM to a melting and freezing process.The tests were performed consecutively up to 500thermal cycling using a thermal cycler.DSC and FT-IR analysis were also performed to determine the thermal and chemical stability of the composite PCM after thermal cycling.3.Results and discussion3.1.Characterization of the form-stable SA/PMMA composite PCM The morphologies of the microcapsules,particle size and shape especially,are crucial for achieving the desired properties.The solubility,stability,chemical reactivity,opacity,flow ability,and strength of the materials are affected by the size and characteristics of the microcapsules.The surface morphology of the SA/PMMA form-stable PCM was investigated by SEM analysis and the image was shown in Fig.3.As shown in Fig.3(a),the SA/PMMA micro-capsules have a single phase surface,regular spherical shape,relatively uniform sizes and smooth surface without edges or sharp dents.The surface of the microcapsules is adhered with small PMMA resin particles.The similar appearance was coincided indifferent microcapsules appears as quite homogenous and the diameters of the microcapsules vary within the narrow range,2e 3m m.As can be seen in the image of Fig.3(b),the dark colored area is pore which entraps the core material and the light colored region is microcapsule shell materials.This result veri fied strongly the core/shell structure of microcapsules was formed and the skeleton was stable against the leakage of SA from the blends.The formation of microcapsule,the PMMA shell fabricated on the surface of SA droplets through monomers dispersion polymeriza-tion,is implemented by ultraviolet curing dispersion polymeriza-tion.Schematic fabrication of the microcapsules has been described in Fig.4.FT-IR spectroscopy can be used to reveal speci fic interactions in PCM/polymer composite [22].Fig.5displays the FT-IR absorption spectra of the characteristic peaks form SA,PMMA and SA/PMMA composite.In the pure SA spectrum,there is an adsorption peak at the wave number of 3017cm À1and the 2848cm À1which usually overlaps with the absorption band of aliphatic C e H vibration caused by stretching vibration of O e H group.The peak at 1702cm À1is the characteristic absorption peak for the stretching vibration of carbonyl group.The peak at 1463cm À1is the e CH 2bending peaks,1389cm À1represents C e H and C e C bending and 729cm À1and 718cm À1corresponds to rocking vibration and bending,which are all characteristic for aliphatic chain of SA.In the spectrum of PMMA,the peak at 1731cm À1is the most intensive absorption band representing the carbonyl group.The peaks at 1076cm À1and 1198cm À1can be assigned to the C e O stretching of the ester paring the FT-IR spectra of SA and PMMA,it is clearly seen that the FT-IR spectrum of the microcapsules consti-tutes of both the peaks of SA and PMMA.The only difference is the peak positions in the SA/PMMA spectrum slightly deviates from the positions in their pure spectra.This changes are attributed to the interactions between oxygen atom of carbonyl group (C ]O)of PMMA and hydrogen atom of hydroxyl group (O e H)of SA,likely.Fig.2.Experimental set-up for studying the thermal behavior ofPCM.Fig.3.The SEM images of the composite.Y.Wang et al./Renewable Energy 36(2011)1814e 18201816The infrared spectrum has no signi ficant new peaks indicates there are no chemical reaction between fatty acid with PMMA.The FT-IR analysis conclusions assumed as another evidence of successful formation of bined with the result of SEM images,it can be see clearly that the SA and PMMA have good compatibility.The DSC results of the form-stable composite and pure SA samples are shown in Fig.6.Form the curves,the T S ,T P ,and T E values of SA can be determined as 54.2 C,59.9 C and 66.5 C for melting phase change and 54.9 C,50.9 C and 48.3 C for freezing phase change.And the result for the composite can be determined as 54.8 C,60.4 C and 66.1 C for melting and 53.8 C,50.6 C and 48.1 C for crystallization pared the phase change temperatures value of SA and composite,it can be seen that the change in melting point was 0.5 C and 0.3 C in freezing point,respectively.These results can be considered as an indicator of the interaction to be occurred between the functional groups of PMMA and the SA.In the curve of DSC,the latent heats of melting and freezing were found to be 177.8J/g and 185.6J/g for SA and 92.1J/g and 95.9J/g for form-stable composite PCM,respectively.The percentage of PCM in microcapsules is the key factor for form-table PCM.It determines directly enthalpy and thus energy storage ef ficiency of the PCM.The encapsulation ratio can becalculated with the equation of SA (wt.%)¼D H(SA/PMMA composite PCM)/D H(pure SA)based on enthalpy values as 51.8%in the experiments.Where D H (SA/PMMA composite PCM)and D H (pure SA)are measured enthalpies of form-stable SA/PMMA composite PCM and SA itself,respectively.These latent heat values were slightly lower than the calculated values based on the mass ratio of the SA in parison of LHTES properties of form-stable composite PCM prepared in this study with that of the different composite PCMs in literature [17],it can remarkably be noted that the form-stable composite PCM has an important LHTES potential.3.2.Thermal reliability of the composite PCMA high-Quality composite PCM must be thermally and chemi-cally stable over a large number of melting and freezing cycling.The superior composite should be shown no or less change in its thermal properties and chemical structure after long term utility period.Therefore,thermal cycling test was performed to determine the change in thermal properties of the composite PCM and their DSC curves and the results before and after 500cycling are given in Fig.7and Table 1,respectively.As seen form the Fig.7and Table 1,Fig.4.The formation process of SA/PMMAmicrocapsules.Fig.5.FT-IR spectra of PMMA,SA and SA/PMMA compositePCM.Fig.6.DSC thermogram of SA and SA/PMMA composite.Y.Wang et al./Renewable Energy 36(2011)1814e 18201817the thermal properties (melting and freezing temperature,latent heat)of SA/PMMA after 500cycling have irregular and reasonable changes compared with the SA/PMMA composite.In other words,the reasonable changes of temperature or enthalpy indicated the composite has good thermal reliability in terms of the changes in its melting and freezing temperatures.Fig.8shows the TG curves of before and after 500cycled SA/PMMA composite and pure SA.The variations of weight loss rate about SA/PMMA composite before and after 500cycling are nearly constant at intervals of 30e 90 C.It is also known that the weight loss rates of SA is larger than that of SA/PMMA composite which indicate the SA/PMMA has good thermal stability under used temperature.On the other hand,the chemical stability of composite after repeated thermal cycling was also investigated by FT-IR.Fig.9shows the FT-IR spectra of form-stable composite PCM before andafter 500thermal cycling.The shape and frequency value of all peaks are consistent in the samples before and after thermal cycling indicates that the chemical structure of the composite was not affected by repeated melting/freezing cycling.3.3.The heat storage and release performance of PCM composites The heat storage or release performance was veri fied by temperature curves for melting and freezing time of the form-stable SA/PMMA composite PCM which are shown in Fig.10(a)and (b),respectively.The melting and solidi fication time was estimated from the temperature curves by constructiontheirFig.7.DSC curves of form-stable SA/PMMA composite PCM before and after thermal cycling.Table 1Thermal properties of form-stable SA/PMMA composites before and after thermal cycling.SampleMelting FreezingT S ( C)T P ( C)T E ( C)Latent heat (J/g)T S ( C)T P ( C)T E ( C)Latent heat(J/g)SA/PMMA composite before thermal cycling54.860.466.192.153.850.648.195.9SA/PMMA composite after thermal cycling 55.159.365.590.953.150.947.797.8Changes 0.03À1.1À0.6À1.2À0.70.3À0.41.9Fig.8.TG curves of pure SA and SA/PMMA composite PCM before and after thermalcycling.Fig.9.FT-IR spectra of SA/PMMA composite PCM before and after 500cycling.Fig.10.Melting and solidi fication temperature curves of SA,PMMA and SA/PMMA composite.Y.Wang et al./Renewable Energy 36(2011)1814e 18201818outer tangent lines.The interval time between two points of intersection is de fined as melting and solidi fication time.As seen from Fig.10(a),the melting times of pure SA and the composite PCM were determined as 580s and 600s,respectively,showing SA and SA/PMMA have the approximate melting times.This indicated that the composite PCM have an almost equal heat absorbtion velocity supplied from a heat source.As show in Fig.10(b),the solidi fication time estimated as 940s for pure SA and 620s for the composite PCM.This result also suggested that the SA/PMMA has an excellent heat storage property.The total times of heating process were less than those of cooling process because of the heat transfer rate of heating process in the hot water were higher than that of cooling process in the standing cold air of the refrigerator.The melting and freezing peak posi-tion of samples were evidently less than those obtained from DSC.This because of DSC measurement is an express method and differs from classical thermo-physical methods.The thermal performances of a PCM in a thermal cycling with a slow heating/cooling rate are very different from those in a rapid heating/cooling process [23].The heat storage and release reliability of the composite PCM after 500cycling were also determined and the result are show in Fig.11.According to Fig.11,it can be obviously observed that the composite has heat storage and release process.Moreover,the heat storage and release process curves before and after 500cycling of the form-stable SA/PMMA composite PCM have less change.This result indicated that the composite can be implemented to transfer energy in different time and space.4.ConclusionA novel form-stable SA/PMMA composite PCM for LHTES applications was prepared by entrapping of SA into PMMA cell by ultraviolet curing dispersion polymerization.The structure,morphology,thermal properties,thermal reliability and heat storage/release performance were determined by SEM,FT-IR,DSC and melting/freezing cycling test respectively.The form-stable composite PCM can be formed by 51.8wt.%SA encapsulated in three-dimensional net structure of PMMA supporting material.The melting and freezing temperatures and the latent heats of form-stable SA/PMMA composite PCM were measured as 60.4 C,50.6 C and 92.1J/g,95.9J/g,respectively.The results of DSC,FT-IR and thermal cycling test are all showed that the form-stable composite PCM had good thermal reliability and chemical stability although it was subjected to 500melting/freezing cycling.So,the composite can be used as energy storage material for passive solar space heating or decreasing indoor temperature swing in buildings.AcknowledgmentsAuthor would like to acknowledge the financial support of National Natural Science Foundation of China (Grant No.51063003)and outstanding young teachers ’project by Lanzhou University of Technology (Grant No.2008012).References[1]Bayés-GarcíL,Ventol L,Cordobilla R,Benages R,Calvet T,Cuevas-Diarte MA.Phase change materials (PCM)microcapsules with different shell composi-tions:preparation,characterization and thermal stability.Sol Energy Mater Sol Cells 2010;94:1235e 40.[2]Sari A,Alkan C,Karaipekli A,Uzun O.Microencapsulated n-octacosane asphase change material for thermal energy storage.Solar Energy 2009;83:1757e 63.[3]Alkan C,Sari A.Fatty acid/poly(methyl methacrylate)(PMMA)blends as form-stable phase change materials for latent heat thermal energy storage.Solar Energy 2008;82:118e 24.[4]Sari A.Form-stable paraf fin/high density polyethylene composites as solid-liquid phase change materials for thermal energy storage:preparation and thermal properties.Energ Convers Manag 2004;45:2033e 42.[5]Sari A,Karaipekli A.Thermal conductivity and latent heat thermal energystorage characteristics of paraf fin/expanded graphite composite as phase change material.Appl Energ Eng 2007;27:1271e 7.[6]Su JF,Wang LX,Ren L.Preparation and characterization of double-MF shellmicroPCMs used in building materials.J Appl Polym Sci 2005;97:1755e 62.[7]Su JF,Wang LX,Ren L.Fabrication and thermal properties of microPCMs:used melamine e formaldehyde resin as shell material.J Appl Polym Sci 2006;101:1522e 8.[8]Kim EY,Kim HD.Preparation and properties of microencapsulated octadecanewith waterborne polyurethane.J Appl Polym Sci 2005;96:1596e 604.[9]Cai YB,Song L,He QL.Preparation,thermal and flammability propertiesof a novel form-stable phase change materials based on high density polyethylene/poly(ethylene-co-vinyl acetate)/organophilic montmoril-lonite nanocomposites/paraf fin compounds.Energ Convers Manag 2008;49:2055e 62.[10]Cai YB,Wei QF,Huang FL.Thermal stability,latent heat and flame retardantproperties of the thermal energy storage phase change materials based on paraf fin/high density polyethylene composites.Renewable Energy 2009;34:2117e 23.[11]Xiao M,Feng B,Gong KC.Preparation and performance of shape stabilizedphase change thermal storage materials with high thermal conductivity.Energ Convers Manag 2002;43:103e 8.[12]Zhang YP,Lin KP,Yang R.Preparation,thermal performance and applicationof shape-stabilized PCM in energy ef ficient buildings.Energy Build 2006;38:1262e 9.[13]Wang LJ,Meng D.Fatty acid eutectic/polymethyl methacrylate composite asform-stable phase change material for thermal energy storage.Appl Energy 2010;87:2660e 5.[14]Alkan C,Sari A,Karaipekli A.Preparation,characterization,and thermalproperties of microencapsulated phase change material for thermal energy storage.Sol Energy Mater Sol Cells 2009;93:143e 7.[15]Sari A,Alkan C,Karaipekli A.Microencapsulated n-octacosane as phasechange material for thermal energy storage.Solar Energy 2009;83:1757e63.Fig.11.Heat storage (release)performance of the form-stable composite.Y.Wang et al./Renewable Energy 36(2011)1814e 18201819。
