Halogen Free and Flame Retardant Elastomeric Cable Compounds with Submicron Sized Fillers
聚烯烃无卤阻燃研究进展

第31卷第#期2019年7月常州大学学报(自然科学版)Journal of Changzhou University(Natural Science Edition)Vol.31No.#Jul.2019doi:10.3969/j.issn.2095-0411.2019.04.001聚烯烃无卤阻燃研究进展欧红香,叶青,蒋军成,薛洪来,徐家成,刘!(常州大学环境与安全工程学院,江苏常州213164)摘要:聚烯烃以其独特的性能得到广泛的使用,其易燃性带来的火灾风险使其阻燃研究备受关注&从聚烯烃的使用情况、受热分解机理以及阻燃原理进行论述,从无卤阻燃的角度介绍了磷系阻燃剂、氮系阻燃剂、金属氢氧化物阻燃剂、膨胀型阻燃剂和纳米阻燃剂,阐述了不同阻燃剂的性能特征、阻燃机理及在聚烯烃阻燃中的研究应用进展,并对无卤阻燃聚烯烃今后的发展提出了展望&关键词:聚烯烃;阻燃剂;阻燃机理;复合材料中图分类号:X937;TQ3222文献标志码:A文章编号:2095-0411(2019)04-0001-08 Research Progress on Halogen-Free Flame Retardant of Polyolefins OU Hongxiang,YE Qing,JIANG Juncheng,XUE Honglai,XU Jiacheng,LIU Ben (School of Environmental U Safety Engineering,Changzhou University,Changzhou213164,China)Abstract:Polyolefins have been widely used for their unique properties.Because of the fire risks caused by their flammability,the flame retardant research of polyolefins has attracted much attention.From theperspectiveofhalogen-freeflameretardant#thispaperintroducesvariousflameretardants#such asphosphorusflameretardants#nitrogenflameretardants#metalhydroxideflameretardants#intu-mescentflameretardantsandnanoflameretardants.Hheperformancecharacteristics#flameretardant mechanism#and research and application progress of di f erent flame retardants in polyolefinshave beensummarized.Hhefuturedevelopmentofhalogen-freeflameretardantpolyolefinsisalsoprospec-ted.Key words:polyolefins;flame retardant;flame retardant mechanism;composite materials随着高分子材料的发展,越来越多的天然高分子和合成聚合物被广泛应用,而它们本身固有的可燃性使其易被外部热源或火源点燃,并在燃烧过程中释放出大量的热、烟甚至有毒气体,世界各地每年都有许多由高分子材料引起的火灾事故,所造成的人员伤亡和财产损失已引起社会广泛关收稿日期:2019-06-11o基金项目:国家自然科学基金资助项目(21878026);江苏省研究生实践创新计划项目(KYCX18_2626);常州市科技支撑计划项目(CE20189003)&作者简介:欧红香(1976—),女,湖南宜章人,博士,教授&通信联系人:蒋军成(1967—),E-mail:jiangjc@ 引用本文:欧红香,叶青,蒋军成,等.聚烯烃无卤阻燃研究进展常州大学学报(自然科学版)2019,31(4):18.-2-常州大学学报(自然科学版)2019年注+12&聚烯烃作为一种由烯烃小分子聚合而成的高分子碳氢化合物[3#]#其中使用最广泛的包括聚乙烯(Polyethylene,PE)和聚丙烯(Polypropylene,PP)+5]。
广东风华 端华片式电阻器分公司产品安全数据说明书
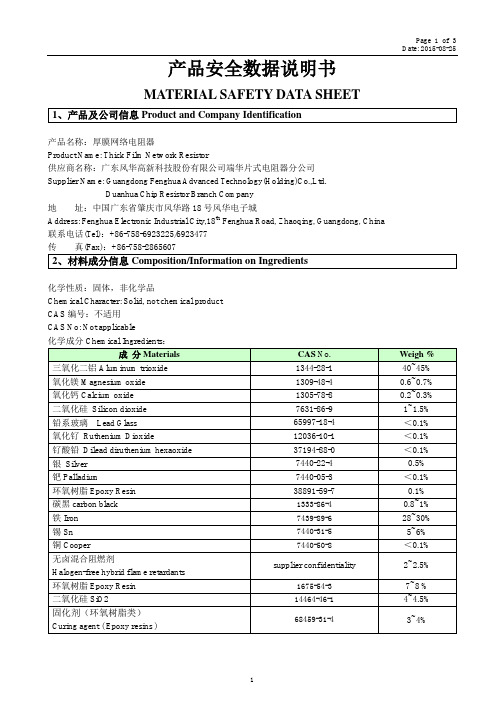
产品安全数据说明书MATERIAL SAFETY DATA SHEET1、产品及公司信息Product and Company Identification产品名称:厚膜网络电阻器Product Name: Thick Film Network Resistor供应商名称:广东风华高新科技股份有限公司端华片式电阻器分公司Supplier Name: Guangdong Fenghua Advanced Technology(Holding)Co.,Ltd.Duanhua Chip Resistor Branch Company地址:中国广东省肇庆市风华路18号风华电子城Address: Fenghua Electronic Industrial City,18th Fenghua Road, Zhaoqing, Guangdong, China联系电话(Tel):+86-758-6923225/6923477传真(Fax):+86-758-28656072、材料成分信息Composition/Information on Ingredients化学性质:固体,非化学品Chemical Character: Solid, not chemical productCAS编号:不适用CAS No: Not applicable化学成分Chemical Ingredients:成分Materials CAS No.Weigh % 三氧化二铝Aluminum trioxide 1344-28-1 40~45% 氧化镁Magnesium oxide 1309-48-4 0.6~0.7% 氧化钙Calcium oxide 1305-78-8 0.2~0.3% 二氧化硅Silicon dioxide 7631-86-9 1~1.5% 铅系玻璃Lead Glass 65997-18-4 <0.1% 氧化钌Ruthenium Dioxide 12036-10-1 <0.1% 钌酸铅Dilead diruthenium hexaoxide 37194-88-0 <0.1% 银Silver 7440-22-4 0.5% 钯Palladium 7440-05-3 <0.1% 环氧树脂Epoxy Resin 38891-59-7 0.1% 碳黑carbon black1333-86-4 0.8~1% 铁Iron7439-89-6 28~30% 锡Sn7440-31-55~6% 铜Cooper7440-50-8 <0.1% 无卤混合阻燃剂supplier confidentiality2~2.5% Halogen-free hybrid flame retardants环氧树脂Epoxy Resin1675-54-3 7~8 % 二氧化硅SiO214464-46-1 4~4.5% 固化剂(环氧树脂类)68459-31-4 3~4% Curing agent ( Epoxy resins )3、危害性信息Hazards Identification有害物质分类规定Hazards Material Classification:不适用Not Applicable理化特性Physical and Chemical Character:固体,非化学品Solid, not chemical product人体危害性Human Health Hazards:无危害Not Applicable环境危害性Environmental Hazards:无相关资料No relevant information found.4、急救方法First Aid Measures此类产品常态下极其稳定,无刺激性,一般不需急救。
Halogen-Free Flame~Retardant Rigid Polyurethane Foams;

Halogen-Free Flame-Retardant Rigid Polyurethane Foams: Effect of Alumina Trihydrate and Triphenylphosphate on the Properties of Polyurethane FoamsM.Thirumal,1Nikhil K.Singha,1Dipak Khastgir,1B.S.Manjunath,2Y.P.Naik21Rubber Technology Centre,Indian Institute of Technology,Kharagpur721302,India2Bhaba Atomic Research Centre,Trombay,Mumbai400085,IndiaReceived25March2009;accepted20October2009DOI10.1002/app.31626Published online14January2010in Wiley InterScience().ABSTRACT:Rigid polyurethane foam(PUF)filled with mixture of alumina trihydrate(ATH)and triphenyl phos-phate(TPP)as fire retardant additive was prepared with water as a blowing agent.In this study,the ATH content was varied from10to100parts per hundred polyol by weight(php),and TPP was used at a higher loading of ATH(75and100php)in a ratio of1:5to enhance the processing during PUF preparation.The effects of ATH on properties such as density,compressive strength,morpho-logical,thermal conductivity,thermal stability,flame-re-tardant(FR)behavior,and smoke characteristics were studied.The density and compressive strength of the ATH-filled PUF decreased initially and then increased with further increase in ATH content.There was no signif-icant change in the thermal stability with increasing ATH loading.We determined the FR properties of these foam samples by measuring the limiting oxygen index(LOI), smoke density,rate of burning,and char yield.The addi-tion of ATH with TPP to PUF significantly decreased the flame-spread rate and increased LOI.The addition of TPP resulted in easy processing and also improved FR charac-teristics of the foam.V C2010Wiley Periodicals,Inc.J Appl Polym Sci116:2260–2268,2010Key words:fillers;flame retardance;polyurethanes;thermo-gravimetric analysis(TGA);alumina trihydrate(ATH)INTRODUCTIONRigid polyurethane foams(PUFs)are widely used as thermal insulators and mechanical shock absorbers in transport overpacks and in air conditioning.They are also used as structural materials because of their light weight,greater strength to weight ratio,and energy-absorbing capabilities.1PUF,like other organic polymeric materials,tends to be flammable. Thus,the flammability of PUF has long been a factor limiting its use.To improve the flame-retardance properties,different flame retardants(FRs)are added to PUF.However,some of the FR additives used in PUF adversely affect its physical properties and pollute the environment by the evolution of undesirable gases on burning.In recent years, because of the stringent safety standards,both pub-lic and environmental,set by statutory authorities across the world,it has become imperative to de-velop better FR materials with improved FR effi-ciency that are economical and,at the same time, halogen free.2In general,alumina trihydrate(ATH)is unique in having a high proportion($34%)of water and is used as an FR additive and smoke-suppressant filler. Such inorganic fillers are assuming increasing im-portance in the industry because of their desirable combination of low cost,low smoke,and relatively high fire-retardant efficiency.ATH decomposes at about220 C to form Al2O3and water:Al2O3Á3H2OÀ!D Al2O3þ3H2OThe effectiveness of ATH as an FR additive depends primarily on its endothermic decomposi-tion,which withdraws heat from the substrate and, hence,retards the rate of flame propagation.Water vapor also reduces oxygen supply as it expands and envelops the interface boundary of foam and the environment.The expanding water vapor also cools the surface effectively because it takes away the ma-jority of heat supplied to the foam because of its high heat-carrying capacity at high temperatures.In contrast to the antimony oxide/halogenated fire-re-tardant system,ATH can provide equivalent fire retardancy at a lower cost and with significantly reduced emission of gases of low toxicity and corro-sivity on exposure to a flame environment.3One of the major drawbacks of adding these fillers is that the mechanical properties become inferiorCorrespondence to:N.K.Singha(nks@rtc.iitkgp.ernet.in). Contract grant sponsor:Board of Research in Nuclear Sciences(BRNS),Mumbai,India.Journal of Applied Polymer Science,Vol.116,2260–2268(2010) V C2010Wiley Periodicals,Inc.compared to the bare foam samples.This is possibly due to insufficient interactions between the polymer and the filler,which result in their inferior proper-ties.Bonding interactions between the foam and the FR additives may be improved by various techni-ques.The surface of the filler can be treated with various species that act as compatibilizers or sur-face-active agents.In general,ATH is surface-treated with chemicals,such as carboxylic acids,silanes, zirconates,and titanates,to improve its dispersion and distribution within the polymer ually, the content of ATH in the formulation is very high(>50%).ATH is used as an FR material in preparing FR rubber products(e.g.,cables,conveyor belts)and in plastic materials.4–13There have been reports of the use of ATH in polyurethane elastomers14,15and flexible16–19and rigid PUFs20–23as a low-cost FR and smoke-suppressant additive.In this investigation,we report the use of ATH as an FR nonreactive additive in the preparation of rigid PUF and the effects of ATH on the mechanical properties,thermal conductivity,thermal stability, FR,and smoke-density properties.At higher load-ings of ATH,the processing and preparation of PUFs were very difficult because of the resultant high viscosity.Therefore,at higher loadings of ATH, triphenyl phosphate(TPP)was used as a viscosity-suppressant and to improve the flame-resistant properties.EXPERIMENTALMaterialsPolymeric methane diphenyl diisocyanate(PMDI; NCO¼30.8%and functionality¼ 2.7)and poly (ether polyol)(OH content¼440mg of KOH/g,av-erage functionality¼ 3.0)were obtained from Huntsman International Pvt.,Ltd.(Mumbai,India). Distilled water was generated in our laboratory and was used as a chemical blowing agent.N,N,N0,N0,N0-Pen-tamethyldiethylenetriamine(PMDETA),obtained from Aldrich(St.Louis,MO),was used as a catalyst.Poly-ether dimethyl siloxane(TEGOSTAB B8460)supplied by Goldschmidt(Essen,Germany)was used as a surfac-tant.ATH,with a density of2.42g/cm3and an average particle size of200l m,and TPP,supplied by Phoenix Yule,Ltd.(Kolkata,India),were used as FR additives. All of the chemicals were used as received. Preparation of the foamATH-and TPP-filled PUF samples were prepared by a one-shot and free-rise method.The chemical com-positions of different filled foams are shown in Table I.Except for PMDI,all of the raw materials were well mixed in a plastic beaker,and then,FR was added,and the resulting mixture was thoroughly mixed with the help of a high-speed mechanical stirrer (3000rpm).Finally,PMDI was added to the mixture for a short duration with vigorous stirring for10s. The final resulting mixture was immediately poured into an open mold(30Â25Â15cm3)to produce free-rise foams.After preparation,the foam sample was kept in an oven at70 C for24h to complete the polymerization reaction.Different test samples were cut into specific foam shapes after curing.The sam-ples were rubbed with fine emery paper to get the proper dimensions.Different properties of the foams were analyzed with ASTM standard test methods. The amount of PMDI required for the reaction with polyether polyol and distilled water was calculated from their equivalent weight.For the completion of the reaction,excess PMDI(NCO/OH¼ 1.1)was used.Similarly,all foam samples were prepared by adjustment of the ATH content relative to polyol. Measurement of different propertiesMechanical propertiesThe apparent density of the PUF samples was meas-ured as per ASTM D1622-03;the average value of three samples is reported.The mechanical properties of the PUF samples were measured under ambient conditions with an Instron universal testing machine Hounsfield testing equipment(model H10KS).The compressive stress at10%strain in the parallel-to-foam rise direction was performed according to ASTM D1621-00.The size of the specimen was55Â55Â30mm3(LengthÂWidthÂThickness),the rate of crosshead movement was fixed at2.5mm/ min for each sample and the load cell used was 10kN.The strengths of five specimens per sample were measured,and the average of these values is reported.Scanning electron microscopy(SEM)analysisThe morphology of the PUF samples was studied with a scanning electron microscope(JEOL,JSM 5800,Tokyo,Japan).The samples were gold-coatedTABLE IChemical Composition of ATH/TPP-Filled Water-BlownRigid PUFMaterial php Polyether polyol100.0 PMDETA0.5 Tegostab B8460 2.0 Distilled water0.3 ATH10–50,75,100 TPP10,15,20 PMDI122.0RIGID POLYURETHANE FOAMS2261Journal of Applied Polymer Science DOI10.1002/appbefore scanning to provide an electrically conductive surface.An accelerating voltage of20kV was used while we recorded the scanning electron micrograms. Thermal conductivity testThe thermal conductivity of the PUFs was tested within a week of preparation of the PUFs with a guarded hot plate thermal conductivity meter as per ASTM C177-97.The size of the specimen was100Â100Â25mm3(LengthÂWidthÂThickness). Thermogravimetry(TG)studyThe decomposition temperature and char residue of the foams were analyzed on a TG analyzer Q50(TAInstruments,New Castle,DE)under a nitrogen envi-ronment at a heating rate of20 C/min over the tem-perature range30–800 C.Limiting oxygen index(LOI)testThe flammability test was performed with an LOI test instrument(Stanton Redcroft FTA unit,East Grinstead,UK)as per ASTM D2863-97.The speci-mens for the LOI measurement were120Â12Â12 mm3(LengthÂWidthÂThickness),five specimens per sample were measured,and their average values are reported.Test for flame propagationThe rate of flame spread was measured as per Fed-eral Motor Vehicle Safety Standard302.24A PUF specimen with dimensions of150Â10Â10mm3 (LengthÂWidthÂThickness)was exposed hori-zontally at its one end to a small flame for15s.The distance and time of burning or the time to burn between two specific marks were measured.The burn rate was expressed as the rate of flame spread according to the following formula:B¼60(L/T), where B,L,and T are the burn rate(mm/min), length of the flame travels(mm),and time(s)for the flame to travel L mm,respectively.Three specimens per sample were measured,and their average values are reported.Smoke-density testThe smoke density was measured with a smoke-density chamber(made by S.C.Dey and Co.,Kol-kata,India)as per ASTM D2843-04.The smoke gen-erated(flaming mode)in the process of burning the sample was measured by the change in light inten-sity.The size of the PUF specimen was100Â100Â12mm3(LengthÂWidthÂThickness).The maxi-mum smoke density was measured as the highest point of the light absorption versus time curve.This smoke-density rating represented the total amount of smoke present in the chamber for the4-min time and was measured with the following equation:Smoke-density rating¼A=TÂ100where A and T are the area under the light absorp-tion versus time curve and the total area of the curve,respectively.Determination of the char yields(CYs)We measured the CYs of the foams by heating the PUF in a muffle furnace at550 C for30min.The CY was calculated with the following equation:CY¼W b/W oÂ100,where W b and W o are the weights of the sample after and before burning.RESULTS AND DISCUSSIONDensityFoam density is a very important parameter that affects the mechanical properties of PUFs.25In gen-eral,the foam density is dependent on the degree of foaming,which in turn,depends in part on the type and amount of blowing agent.In this study,the amount of chemical blowing agent(distilled water) was kept constant.Table II shows the density of PUFs filled with ATH at different concentrations.It indicates that the density decreased with the addi-tion of small quantities of ATH-filled PUF and then increased with further increase in ATH loading.The density decreased at an initial loading of ATH.This was due to the increase in the cell size,as shown in the SEM figures(discussed later).However,beyond 20parts per hundred polyol by weight(php)of ATH loading,the density linearly increased with increasing ATH loading.This was due to a decreaseTABLE IIEffect of ATH/TPP on the Density and CompressiveStrength of PUFATHloading(php)TPPloading(php)Density(kg/m3)Compressivestrengthat10%strain(kg/cm2)Reducedcompressivestrength[MPa/(g/cm3)] 001038.17.910—88 5.5 6.320—81 5.0 6.230—13110.58.040—14011.38.150—15313.08.5501095 4.1 4.3751516514.48.7 1002020718.89.12262THIRUMAL ET AL. Journal of Applied Polymer Science DOI10.1002/appin the cell size and to the higher density of ATH (2420kg/m3)than that of neat PUF.The density of PUF filled with ATH(50php)and TPP(10php) was much lower than that of the PUF filled with ATH(50php)alone.This was because of the dilut-ing effect of TPP.However,with increasing ATH content,the density increased further(Table II).This was because the volume of PUF decreased after expansion as the amount of ATH increased,22which led to a greater amount of solid material(poly-urethane and ATH)instead of gaseous phase. Mechanical propertiesThe mechanical properties of PUF are important pa-rameters that determine its applications,such as in load bearing and as packaging materials.To study the effect of ATH loading on the compressive prop-erties of PUFs,the effect of foam density and the compressive strength of different foams were nor-malized by division by their respective densities.Ta-ble II shows the effects on the reduced compressive strength and compressive strength at10%strain of the PUFs filled with increasing loading of ATH and TPP.The table indicates that the reduced compres-sive strength and compressive strength at10%strain of PUFs filled with ATH initially decreased and then increased with further increases in the ATH loading. The initial decrease in properties was due to an increase in the average cell size of the PUFs,which also resulted in a decrease in the density.A higher loading of ATH caused a positive effect on the me-chanical properties of the PUFs.This was due to an increase in the cell wall thickness and also an increase in the density.It is known that the degree of foaming of PUFs depends on the viscosity and surface tension of the particular formulation.26 Higher loadings of ATH resulted in an increase in the viscosity(2Pa s for20php ATH from1.1Pa s for polyol),and this led to a decrease in the blowing or expansion of the PUFs.The mechanical properties of PUF filled with ATH(50php)and10php TPP decreased drastically compared to those of the PUF filled with only50php ATH.This decrease in the mechanical properties was due to the plasticizing effect of TPP with ATH,which was consistent with the change in density,as shown in Table II.In gen-eral,the metallic hydroxide of mineral fillers,such as ATH and magnesium hydroxide,act as nonrein-forcing fillers,because of its poor wetting or adhe-sion with the polymer matrix,and also,with inclu-sion of higher amounts,leads to agglomeration because the filler–filler interaction becomes more pronounced.Pinto et al.14observed poor mechanical properties in a polyurethane elastomer filled with ATH.In this case,the mechanical properties of PUF decreased at the initial loading of ATH,but they increased at higher loadings of ATH.This was due to an increase in the cell wall thickness.The interfa-cial contact between the polyurethane matrix and ATH modified at its surface improved the polymer–filler interaction and filler dispersion.This resulted in improved mechanical properties in the rigid PUF. Anorga et al.16also reported improved physical properties in flexible PUFs with the addition of ATH. MorphologyIn general,the physical properties of foam not only depend on the rigidity of the polymer matrix but also on the cellular structure of the foam.The mor-phology of a rigid PUF sample was studied with SEM.Figure1(a–d)shows the morphology of PUFs filled with ATH and TPP at different loadings.The shapes of the cells in the neat PUF and in the ATH-filled PUF were approximately spherical.As shown in Figure1(b),the average cell size of the PUF became bigger with the incorporation of lower amounts of ATH compared to the neat PUF[Fig. 1(a)].This was because ATH did not locate in the cell struts but between the cell walls.This caused an in-homogeneous cellular structure,which was responsi-ble for the lower compressive strength.26However, at higher loading of ATH(40php),the average cell size of PUF decreased because of less blowing[Fig. 1(c)].This may be due to the addition of a higher amount of ATH,which resulted in an increase in the viscosity(e.g.,2Pa s for20php ATH-filled polyol from1.1Pa s for polyol without ATH)of the foam formulation.The increased viscosity of the mixture led to a lower blowing tendency.Also,the morphol-ogy of the PUF was not very homogeneous because of the nonhomogeneous dispersion of ATH.The effi-ciency of foaming of PUF depends on the viscosity and surface tension of a particular formulation.27 Simioni et al.22also observed a decrease in average cell size with the addition of ATH(100php)in PUF. They found that the amount of polymer was drawn into the cell struts by the filler granules and also con-firmed the absence of interaction between the poly-mer and the filler.In this case,the addition of TPP to the PUF filled with a higher loading of ATH decreased the viscosity.For example,the viscosity of polyol filled with20php ATH was2Pa s;when TPP (4php)was added to this system,the viscosity dropped to1.6Pa s,which was due to the plasticiz-ing effect of TPP.This decrease in viscosity led to a good blowing efficiency,and thus,it increased the cell size[Fig.1(d)].Thermal conductivityThe thermal conductivity of PUF depends on the av-erage cell size,foam density,cell orientation,ratio ofRIGID POLYURETHANE FOAMS2263Journal of Applied Polymer Science DOI10.1002/appclosed-to open-cell content,and thermal conductiv-ity of filling materials.28Figure 2shows the effect of ATH and TPP on the thermal conductivity of the PUFs.The table indicates that the thermal conductiv-ity of PUF increased with increasing ATH loading.This was due to the high viscosity of the PUF for-mulation,which increased with increasing ATH loading and led to a nonhomogeneous dispersion of ATH.Therefore,the cellular structure of PUF was not very fine,and the bigger the average cell size was,the more the thermal conductivity increased.In addition,because of the greater volume of solid con-tent (polyurethane and ATH)in the ATH-filled PUF,there was a greater contribution to the thermal con-ductivity of PUF.Simioni et al.22also observed an increase in thermal conductivity with increasing ATH loading with PUFs.At the higher loading of ATH (along with TPP),PUF showed a decrease in the thermal conductivity;this was due to a decrease in the average cell size and an increase in the den-sity.It is well known that the cell size of a PUF depends on the viscosity and surface tension of the mixture.In this study,an increase in viscosity at higher loadings of ATH led to a reduction in the cell size.Thermal analysisFigure 3shows the TG/differential thermogravime-try (DTG)thermograms of ATH and TPP FR addi-tives under a nitrogen atmosphere.The figure reveals that the weight loss of ATH took place in three different temperature ranges,at 273,353,andabout 516 C,and their corresponding weight losses were about 1.2,20.5,and 32.2%,respectively.These weight losses were due to the removal of chemically bound water present in the ATH as shown:Al 2O 3Á3H 2OÀ!270À350C À2H 2OAl 2O 3ÁH 2O À!515CÀH 2OAl 2O 3This result was in good agreement with the resultsreported by Simioni and Modesti.23The onset tem-perature (temperature at 5%weight loss)of ATH was 303 C,which was higher than that of TPP (274 C).This indicated that the thermal stabilityofFigure 1Microphotographs of the ATH/TPP-filled PUF samples:(a)neat,(b)20php ATH,(c)40php ATH,(d)75php ATH þ15phpTPP.TPP was lower than that of ATH.The degradation pattern of TPP indicated that the TPP degraded completely to volatiles by 364 C and left no char res-idue.However,in the case of ATH,the weight loss was slow at the same temperature.The maximum degradation temperature (T max )of TPP was 356 C and was observed in a single step.The amount of residue (CY)of ATH was greater (67%)than that of TPP,which was almost zero at 550 C.Figure 4demonstrates the TG/DTG curves of PUF filled with and without ATH and ATH/TPP.In the neat and filled foam samples,the thermal degrada-tion took place in the range 250–420 C.The DTG curves of the PUFs filled with ATH and ATH/TPP showed a shoulder peak,which was probably due to the elimination of surface-active compounds used in ATH to improve its dispersion in the polymer matrix.With addition of TPP into the ATH,the weight loss of the samples was greater.T max for theneat and filled PUFs occurred at about 350 C,but CY was greater in case of filled PUFs compared to neat PUF.However,CY of the PUFs decreased with the addition of TPP into the ATH-filled PUF,as expected from the thermogravimetric analysis (TGA)curve of TPP (Fig.3,which shows no CY).This was probably due to the gas-phase mechanism of phos-phate additives.Different other phosphates,for example,ammonium polyphosphate (APP),have shown higher CYs because of the condensed-phase mechanism.29Table III shows the T max and CY values at 700 C of the PUFs filled with ATH and TPP under a nitro-gen atmosphere.There was no significant change in T max of PUF with ATH.Simioni and Modesti 23also found that ATH did not modify the TGA curves of their PUFs.In general,the degradation temperature of a polymer should increase with ATH loading.This is due to the endothermic decomposition of ATH,which decreases the temperature in the sur-roundings of the materials.Moreover,the water dilution and the formation of an aluminum oxide protective layer decrease the combustible gases and also act as barrier for transport of oxygen and fuel into polymer.Nachtigall et al.30observed an increase in the degradation temperature of modified PP on loading with ATH.In our case,there was no signifi-cant change in T max of the PUFs with or without the addition of ATH.This was probably due to the reac-tions between the water molecules released from ATH and the polyurethane degradation products (e.g.,isocyanate,carbodiimide),which were exother-mic in nature.The CY of ATH filled PUF increased with increasing ATH loading.However,the combi-nation of ATH with TPP decreased CY slightly,which might have been due to the gas-phase mecha-nism of TPP.In general,the addition of phosphate (APP)additives leads to the condensed-phase mech-anism of fire retardation.29Thus,it decreases the thermal degradation temperature of the polymer,which results in a greater quantity of CY.However,some phosphorus compounds may also be active in the gas phase by a radical trapping mechanism.In this case,TPP acted as gas phasemechanism,TABLE IIIEffect of ATH/TPP on T max of PUFSample ATH loading (php)TPP loading (php)T max in N 2( C)CY in TGA at 700 C under N 2(%)100361.710.5210—361.417.3330—362.318.9450—360.220.855010364.219.3610020361.417.3RIGID POLYURETHANE FOAMS 2265Journal of Applied Polymer Science DOI 10.1002/appthereby decreasing CY of the PUF filled with ATH/ TPP compared to the same with ATH alone.FR behaviorWe analyzed the FR behavior of PUFs filled with ATH and TPP at different loadings by determining the LOI,rate of flame spread,smoke density,and CY measurements.Table IV shows the effect of ATH on the LOI of PUF.It clearly shows that the LOI value slightly increased from22to25%with the addition of ATH in PUF.This lesser beneficial effect of ATH on the flame retardation of PUF occurred, because the initial water elimination process of ATH was hampered,as discussed in Thermal Analysis section.It may also have been due to the fact that lower amounts of ATH in the PUFs protected the dehydrating effect of ATH.An endothermic effect is only effective in PUFs having a higher amount of ATH.23The fact that the ATH did contain bound water is very important for its flame retardation in polymers.The slight increase in the LOI was due to the endothermic decomposition of ATH and water elimination from the third stage and also the forma-tion of aluminum oxide char on the surface of the polymer,which acted as an insulative protective layer.Table IV indicates that the LOI of PUF sample filled with50php ATH and10php TPP was higher than the PUF filled with same amount of ATH only. This was due to the volatilization of TPP and the formation of phosphorus acid at higher tempera-tures.The addition of APP improved the flame retardance of the polymers via the condensed-phase mechanism.In this case,APP first decomposed to produce polyphosphoric acid,which accelerated the formation of char via ester formation on reaction with hydroxyl precursor.29In this case,TPP first decomposed to form phosphorus acids(as shown in the following equation),which reacted with the A OH-containing moiety formed on the depolycon-densation of PUF at higher temperatures:31ðPhOÞ3P¼¼OÀ!D PhOHþH3PO4þH3PO3For a combination of additive systems,the numer-ical values of LOI may be shifted from those of the theoretically calculated ones.The upward shift is called synergism,and the downward shift is known as antagonism.The theoretical LOI values of the flame-retarded PUFs filled with ATH/TPP were cal-culated from knowledge of their experimental values under identical conditions with the individual addi-tives and without additive.For instance,the LOI val-ues of a polymer with binary combinations(LOI ab) can be calculated from the following equation:LOI ab¼LOI aþLOI bÀLOI cwhere LOI a,LOI b,and LOI c are the LOI values for samples containing‘‘a’’additive,‘‘b’’additive,and without additives,respectively.32According to the previous relationship,the experimental value of LOI of the ATH/TPP filled PUFs was greater(27.2%) than the theoretical value of LOI(26.2%).Hence,the PUFs filled with these combinations of additives showed synergistic behavior.The mechanism for this behavior may have been due to the combination of gas-phase(volatilization of TPP)and condensed-phase mechanisms of TPP and ATH.Simioni and Modesti23also found beneficial behaviors of fire retardants and easy processing of higher loaded ATH and dimethyl methyl phosphonate(DMMP) fire-retardant additives in PUF.Table IV shows the effect of ATH in the presence of TPP on the rate of flame spread of PUF at room temperature.The rate of flame spread or the rate ofTABLE IVEffect of ATH/TPP on LOI,Smoke Density,Rate of Flame Spread,and CYof the PUFSampleATHloading(php)TPPloading(php)LOI(%)Maximumsmokedensity(%)Smokedensityrating(%)Flamespread rate(mm/min)CY in the mufflefurnace at550 Cfor30min(%)1Neat—22.063622000.05210—22.2——182 3.2320—22.55451150 6.0430—23.0——11312.2540—23.7——10313.4650—25.045309417.37—1023.2——158 1.38501027.254368812.49751528.06461SE a18.2101002029.5——NB b26.0a Self-extinguished after15s.b Not burning(did not catch fire).2266THIRUMAL ET AL. Journal of Applied Polymer Science DOI10.1002/app。
电缆耐火性能术语

IEC 60332-3-22 (the test for bunched wires and cables)
IEC 60332-3-22(针对成束导线和电缆的测试) 60332- 22(针对成束导线和电缆的测试)
当着火后逃跑或灭火时,低烟雾散发非常重要
In practice, one should see the exit in order to survive.
实际上,一个人只有看到出口后才能逃离并生存下来
Smoke includes toxic gases for human beings.
烟雾含有对人体有害的有毒气体
阻燃电缆指的是电缆设计出的性能
Even if using the same material, one cable structure might fulfill the standard, another not.
即使使用同样的材料, 即使使用同样的材料,有的电缆因其结构设计而能够达到标 准要求,而其他的电缆却不能. 准要求,而其他的电缆却不能.
AEG工厂,7公斤聚氯乙烯燃烧造成了价值5000万德国马克设备的损失 AEG工厂,7公斤聚氯乙烯燃烧造成了价值5000万德国马克设备的损失
Hamburg-Altona Railway Station, fire April 1980, DM 230 000 fire damage finally assessed at DM 5,5 million as result of corrosive fumes from plastics.
The test has three categories A, B and C
HALOGEN-FREE, FLAME RETARDANT COMPOSITIONS

摘要:The present disclosure provides a flexible, halogen-free, flame retardant composition. The composition includes from about 25 wt % to about 95 wt % of a thermoplastic polyurethane (TPU); from about 5 wt % to about 50 wt % of an olefin block copolymer (OBC); and from about 30 wt % to about 70 wt % of a flame retardant. The flame retardant is selected from resorcinol bis(diphenyl 5 phosphate) (RDP), bis diphenyl phosphate (BDP), bisphenol-A bis(diphenyl phosphate) (BPADP), aluminum trihydrate (ATH), a nitrogen/phosphorus-based halogen-free flame retardant, epoxidized novolac resin, and combinations thereof. The composition requires no compatibilizer for the TPU and OBC. The composition finds application in wire and cable structures.
FLAME-RETARDANT THERMOPLASTIC ELASTOMER COMPOSITIO

专利名称:FLAME-RETARDANT THERMOPLASTICELASTOMER COMPOSITION WITHRESISTANCE TO SCRATCH-WHITENING 发明人:CHEN JING GIVEN,CHEN, JING GIVEN,GU FANGMING TONY,GU, FANGMINGTONY,CAO YURONG,CAO, YURONG,GUODAVID H,GUO, DAVID H.,MIAO XIAOXIONGSHAWN,MIAO, XIAOXIONG SHAWN申请号:CN2010/071336申请日:20100326公开号:WO2011/116525A1公开日:20110929专利内容由知识产权出版社提供摘要:Halogen-free, flame-retardant thermoplastic polyester elastomer (TPE-E) compositions that include at least one thermoplastic polyester elastomer, at least one low-melting, phosphorus-based flame retardant having a melting temperature no higher than 150℃, and a blend of solid intumescent flame retardants comprising a phosphorus-based, organic salt flame retardant and a nitrogen-based organic flame retardant are provided. The presence of the low-melting, phosphorus-based flame retardant renders the compositions more resistant to scratch-whitening.申请人:DOW GLOBAL TECHNOLOGIES LLC,CHEN, Jing Given,GU, Fangming Tony,CAO, Yurong,GUO, David H.,MIAO, Xiaoxiong Shawn地址:2040 Dow Center Midland, Michigan 48674 US,68-301 3536 Yindu Road, Minhang District Shanghai 201108 CN,Bldg. 4-2104 780 Siping Road, Hongkou District Shanghai200086 CN,14-502 1300 San Lin Road, Pudong District Shanghai 200123 CN,24-0303 666 Jin Xiu Road, Pudong District Shanghai 200135 CN,401, 35, Quarter 3 3118 Yindu Road, Minhang District Shanghai 201108 CN国籍:US,CN,CN,CN,CN,CN代理人:LIU, SHEN & ASSOCIATES更多信息请下载全文后查看。
Halogen-free flame retarder composition and flame

专利名称:Halogen-free flame retarder composition and flame retardant polyamide composition 发明人:Henrica Norberta Alberta MariaSteenbakkers-Menting,JohannesTijssen,Daniel Joseph Maria Tummers申请号:US11999124申请日:20071204公开号:US20080090946A1公开日:20080417专利内容由知识产权出版社提供摘要:The invention relates to a halogen-free, flame retarder composition for use in a thermoplastic composition, in particular a glassfibre-reinforced polyamide composition, which flame retarder composition contains at least 10-90 mass % phosphinate compound according to formula (I) and/or formula (II) and/or polymers thereof and 90-10 mass % polyphosphate salt of a 1,3,5-triazine compound according to formula (III) and 0-30 mass % olefin copolymer. When used as a flame retarder in glassfibre-reinforced compositions the halogen-free flame retardant composition results in a combination of a V-0 rating according to the UL 94 test of Underwriters Laboratories and excellent mechanical properties. The invention therefore also relates to the use of this flame retarder composition as a flame retarder in a polyamide composition, and a flame retardant polyamide composition that contains the flame retarder composition. The invention also relates to a moulded article containing the flame retardant polyamide composition, and the use thereof in the field of electrical and electronic applications.申请人:Henrica Norberta Alberta Maria Steenbakkers-Menting,JohannesTijssen,Daniel Joseph Maria Tummers 地址:Susteren NL,Beek NL,Geleen NL 国籍:NL,NL,NL更多信息请下载全文后查看。
HALOGEN-FREE FLAME-RETARDANT INSULATED WIRE

专利名称:HALOGEN-FREE FLAME-RETARDANTINSULATED WIRE发明人:FUJITA, Taro,藤田 太郎,HAYAMI, Hiroshi,早味 宏,NISHIKAWA, Shinya,西川 信也,OCHI,Yuji,越智 祐司,HORI, Kenji,堀 賢治申请号:JP2014/068776申请日:20140715公开号:WO2015/029621A1公开日:20150305专利内容由知识产权出版社提供专利附图:摘要:Provided is a halogen-free flame-retardant insulated wire which has high hotwater resistance, while achieving a good balance among insulation resistance, flame retardancy, wear resistance and thermal deformation resistance at high levels. A halogen-free flame-retardant insulated wire which comprises a conductor and a halogen-free insulating layer that covers the conductor. The insulating layer has: an outer layer that is formed of a crosslinked body of a resin composition which contains 100 parts by mass in total of resin components, wherein 25-30 parts by mass of a polyphenylene ether resin and 10-30 parts by mass of a styrene elastomer are finely dispersed in 40-65 parts by mass of a high-density polyethylene having a melt flow rate of 0.60 or less, and 5-50% by mass of a metal phosphinate, 6-25% by mass of a phosphoric acid ester and 1-10% by mass of a polyfunctional monomer relative to the resin components; and an inner layer that is formed of a crosslinked polyethylene. The thickness of the inner layer is 10-85% of the total thickness of the inner layer and the outer layer.申请人:SUMITOMO ELECTRIC INDUSTRIES,LTD.,住友電気工業株式会社地址:5-33, Kitahama 4-chome, Chuo-ku, Osaka-shi, Osaka 5410041 JP,〒5410041 大阪府大阪市中央区北浜四丁目5番33号 Osaka JP国籍:JP,JP代理人:NAKATA, Motomi et al.,中田 元己更多信息请下载全文后查看。
Heat stable halogen-free flame retardant copolyest

专利名称:Heat stable halogen-free flame retardant copolyester thermoplastic elastomercompositions发明人:Eleni Karayianni,David J. Wrigley申请号:US13211532申请日:20110817公开号:US08415415B2公开日:20130409专利内容由知识产权出版社提供摘要:Halogen-free flame retardant compositions exhibiting flame retardance and retention of mechanical properties, especially elongation at break, upon long-term high temperature exposure and comprising a) at least one copolyester thermoplastic elastomer, and b) from at or about 1 to at or about 30 weight percent, based on the total weight of the composition, of at least one flame retardant comprising a phosphinate, diphosphinate and/or polymers of these; c) from at or about 0.25 to at or about 15 weight percent, based on the total weight of the flame retardant composition, of one or more polyhydroxy polymers having a number average molecular weight of at least 2000 and selected from the group consisting of ethylene vinyl alcohol copolymers andpoly(vinyl alcohol)s.申请人:Eleni Karayianni,David J. Wrigley地址:Geneva CH,Athenaz CH国籍:CH,CH更多信息请下载全文后查看。
Halogen free flame retardant remixing system for g

专利名称:Halogen free flame retardant remixingsystem for glass fiber reinforced nylon andits application in halogen free flameretardant glass fiber reinforced nylonmaterials发明人:リー ジンヂョン,レイ ファ申请号:JP2018545271申请日:20180205公开号:JP2020504188A公开日:20200206专利内容由知识产权出版社提供摘要:The present invention discloses a flame retardant remixing system which does not contain halogen for glass fiber reinforced nylon, and calculates the weight percentage of the material, wherein the composition of the raw material is calculated from a weight percentage of 40 to 90% of aluminum phosphide digedium Contains 1 to 10% zinc.The present invention discloses a flame retardant remixing system without halogen for nylon fiber reinforced nylonFlame retardant remixing system without halogen is high flame retardantWithout transitionIt is characterized by the fact that it does not corrode the equipment.Overcoming the defect of phosphorus and nitrogen remixing systems based on aluminum phosphide diethyl used in existing glass fiber reinforced nylon materialsWell adapted to glass fiber reinforced nylon material systemsOverall performance is excellentHalogen free non flammable glass fiber reinforced nylon material can be obtained.申请人:ジィァンスー リースーデェァ ニュー マテリアル カンパニー リミテッド地址:中国 ジィァンスー タイヂョウ ジィァンイェン ディストリクト シェンガオ タウン グァンヂュゥァンホウバオ国籍:CN代理人:特許業務法人YKI国際特許事務所更多信息请下载全文后查看。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Halogen Free and Flame Retardant Elastomeric Cable Compoundswith Submicron Sized FillersDr. Annika Luks, Dr. Reiner SauerweinNabaltec AG, Division Functional FillersSchwandorf, Germany+49 9431 53522 · aluks@nabaltec.deAbstractIt is expected that during the next years the demand for low smoke fire retardant cable compounds will increase remarkably partly due to new regulations like e.g. the construction products directive (CPD) in Europe but also because of new technologies. For example the still rising focus on renewable energy lead to an increase in offshore windmill construction. For these vulnerable buildings special regulations have to be met. For example jacketing of cables on offshore wind mills or oil platforms are often made of HFFR elastomeric compounds to offer maximum mechanical performance for installation and service life and - very important for these applications - high flame resistance and low smoke performance in case of a fire according to IEC Standards 60331, 60332-3, 60754 and 61034.Keywords:ATH; aluminium trihydrate; HFFR; cable compound; EVM; EVA; submicron; Carbo Sea; drilling mud.1. IntroductionThe majority of HFFR cable compounds use fine precipitated aluminium trihydroxide (ATH) as flame retardant filler. These products have a typical medium particle size (D50 values) of 0.7-2 µm and a specific surface area (BET) from 2-12 m²/g.The properties of a compound have to be adjusted to the special needs for a certain application. One possibility to improve the fire resistance of a compound is to increase the filler load. Typical loadings for HFFR cable compounds are around 55 to 65 wt.-%.But the increase of the ATH content very often deteriorates the mechanical properties of the compound like tensile strength (TS) and elongation at break (E@B). The density of the compound is usually raised with increasing filler load. Moreover the viscosity of a compound increases drastically if the filler load exceeds a certain level [1].The negative impact on the mechanical properties of a compound can be reduced if a finer ATH is used. For elastomeric EVM HFFR cable compounds fine precipitated ATH with a specific surface area (BET) of 6-12 m²/g is typically used. Fine ATH types result in better flame retardancy of a compound. But on the other hand these very fine filler types reduce its processability [2, 3].To balance the compound properties there can also be used polymers like Ethylen-Vinyl Acetate (EVA or EVM) copolymers with a higher VA content. The effect of the VA content on the properties of a compound is one point of interest in this study. Another possibility to adjust the properties of a compound is to combine the advantages of two types of ATH. For this purpose Nabaltec developed two new submicron sized metal hydrate fillers named APYRAL®200 SM and ACTILOX® 400 SM. The main novelty is the fineness of the powder particles. The average diameter (D50) of these products is below 500 nm. With their moderately high specific surface area from 13 to 40 m²/g these special powders offer unique properties. The negative impact of a high BET on the melt volume rate (MVR) can be overcome if a compound is prepared using two types of ATH, a standard type which is optimised in regard to low viscosity in a resin e.g. APYRAL®40CD and a submicron sized APYRAL® 200 SM or ACTILOX® 400 SM respectively [2].The new kind of submicron sized ATH was presented at the 55th IWCS/Focus Conference in November 2006. This previous paper focused on the production of these special minerals and their performance in thermoplastic HFFR compounds [4].In the meantime investigations have been broadened to elastomeric HFFR cable compounds.The results of this work shall be presented here.2. Compound investigationCompounding was performed in a lab mixer and specimens were cut out of compression cured plaques. The curing process was done at 170°C over 25 min.Tensile strength (TS) and elongation at break (E@B) have been determined according to DIN 53504.The hardness of the compounds was measured according to Shore A, ISO 868.The Mooney viscosity of the uncured compounds was measured according to ISO 289.The fire performance of the materials was evaluated by Limiting Oxygen Index (LOI) according to ISO 4589 and Cone Calorimetry according to ISO 5660-1.2.1 MaterialsSeveral types of Ethylen-Vinyl Acetate copolymers (EVA or EVM) are widely used for the production of thermoplastic and elastomeric HFFR cable compounds.For this study three types of resin were used. Levapren® 600 HV (60 wt.-% VA, EVM 60), Levapren® 700 HV (70 wt.-% VA, EVM 70) and Levapren® 800 HV (80 wt.-% VA, EVM 80). These resins are commercial products of LANXESS Deutschland GmbH.The ATH qualities for these tests are APYRAL® 40CD (ATH 40), APYRAL®60CD (ATH 60), APYRAL®120E (ATH 120), three standard sized aluminium hydroxide fillers with specific surface area of 3.5, 6 and 11 m²/g. The submicron sized APYRAL® 200 SM (ATH 200) - an aluminium hydroxide with a specific surface area of 13 m²/g - was used as such or in combination with ATH 40. All ATH types are commercially available products of Nabaltec AG. Compounds consist of 100 phr of resin, 10 phr of zinc borate, 160 phr of filler and 22.5 phr of additives like stabilizers or softener.2.2 Mechanical dataThis study focuses on two aspects for the best choice of a compound.The influence of the ATH filler was investigated based on a fixed polymer basis.Additionally the effect of the VA content of the polymer on the compound properties was analyzed by running a series of filler compositions based on three different EVM polymer types.This shows a range of possibilities to combine the right polymer with the best filler to adjust certain properties of an elastomeric HFFR cable compound.In table 1 the combinations of resins and fillers used in this investigation are given. The compound numbers are used in the course of this article.Table 1: Combinations of polymers and fillers.PolymerFillerEVM 60 EVM70EVM800 ATH 1 6 11160 phr ATH 60 2 7 12160 phr ATH 120 3 8 13160 phr ATH 200 4 9 14100 phr ATH 40 + 60 phrATH 2005 10 15The Mooney viscosity of the uncured compounds as well as tensile strength (TS), elongation at break (E@B), LOI and Shore hardness of the cured compounds were determined. The data are given in the tables 2, 3 and 4.Table 2: Compound properties, resin EVM 60.Compound TS(MPa) E@B(%)LOI(%O2)ShoreA23°CML 1+4(100°C)1 747019.83514 2 10.223630.07632 3 13.218432.88036 4 14.519936.48448 5 11.820230.77629Table 3: Compound properties, resin EVM 70.Compound TS(MPa) E@B(%)LOI(%O2)ShoreA23°CML 1+4(100°C)6 4.5 449 19.9 31 137 8.9 264 32.6 75 328 11.621436.57736 9 12.622040.48145 10 1024834.57531 Table 4: Compound properties, resin EVM 80.Compound TS(MPa) E@B(%)LOI(%O2)ShoreA23°CML 1+4(100°C)11 6.252820.8321112 7.229835.57927 13 1022141.28231 14 11.419946.18743 15 7.925937.68027It is obvious and no surprise that tensile strength increases with increasing fineness of the filler. The combination of ATH 40 witha BET of 3.5 m²/g with the submicron sized ATH 200 leads tovalues which are in between the data analyzed for the standardfillers ATH 60 and ATH 120.Elongation at break shows in general the reverse tendency, whichwas expected as well. But surprisingly with the use of submicronsized material alone the best balance of mechanical data in EVM60 and EVM 70 is achieved. Tensile strength is increased, as expected, but the elongation at break is also improved comparedto ATH 120.The only exception from this behaviour was obtained when theresin EVM 80 was used. The reason for this is being investigated.Further investigation will also focus on a more detailed study ofthe influence of slight changes in the composition of the filler mixon the mechanical data of EVM compounds. Especially in resinswith a high VA content the balance of the mechanical data shouldbe further improved.The LOI values improve with increasing BET of the filler. Also ahigher VA content of the resin has a positive influence on the LOI value. Specimens which contain ATH burned with spark formation. This sparkling phenomenon was not observed whenthe compound contained no ATH.ATH 200 allows not only the production of a compound with thebest balance of mechanical data in this test but also with the bestflame resistance according to LOI.The Shore hardness of the compounds is increasing if the BET surface area of the filler is increased. The combination of standardand submicron sized material offers the possibility to reduce thisvalue gradually. With the given combination of ATH types theShore hardness is as low as with the coarsest grade in these investigations ATH 60 or even a bit lower.The Mooney viscosity is also increasing with increasing BET ofthe ATH in all resins. The difference between the standard gradeATH 120 and the submicron sized ATH 200 is around 10 points.The combination of standard ATH 40 and the submicron filler reduces the viscosity to a level which is as low as with the standard filler ATH 60 or even a bit lower. This is an indicationthat the processability is improved and this complies well withearlier results for thermoplastic EVA compounds [2, 4].Another possibility to influence the viscosity of the uncured compound is to use a resin which has a slightly lower or higher viscosity. With increasing VA content the viscosity of the polymer is reduced as well.2.3 Flame resistance, Cone Calorimeter testsSlight differences between the compounds can be seen in the Cone Calorimeter test. The tests have been performed with a fixed heat release rate of 50 kW/m². A frame and grid were used to keep the specimens in a constant distance to the cone shaped heater during the whole test.In tables 5 to 7 important values from the Cone Calorimeter tests are given. The peak of the heat release rate (PHRR) determines the time and the intensity of the maximum of the heat release rate during a test run. It should occur as late as possible and be as low as possible. The total heat release (THR) indicates the amount of energy which is released by a polymer compound over the whole test time. This value obviously is reduced by a flame retardant filler. The time to ignition (tti) should be as long as possible. MARHE is the maximum value of the ARHE curve which is calculated from the heat release rate (HRR). The smoke production rate (SPR) indicates how much smoke is released during the test and should be reduced as much as possible. But the SPR values are only comparable for a certain array of compounds. They do not comply with the ASTM E 662 smoke chamber test.Table 5: Data from Cone Calorimeter tests,resin EVM 60.Compound PHRR (kW/m²) THR(MJ/m²) tti(s) MARHE (kW/m²) SPR(m²/m²)1 654 62.8 39 278.6 9102 119.1 35.9 74 63.9 336.13 102.6 44.1 89 66 312.24 117.5 41.1 91 74.7 427.8 5 108.5 38.2 87 62.8 327.7Table 6: Data from Cone Calorimeter tests,resin EVM 70.Compound PHRR (kW/m²) THR(MJ/m²) tti(s) MARHE (kW/m²) SPR(m²/m²)6 686.7 80.4 35 307.5 1086 7 113.8 50.7 81 71.6 363 8 104.1 40.3 81 67.1 4049 127.5 57 93 78.1 43110 101.8 47.4 81 66 277The heat release rates and the smoke development show smalldifferences depending on the filler type and the polymer resin. The time to ignition can be increased when finer ATH is used as flame retardant. The use of ATH 200 increases the tti between two and 19 seconds compared to ATH 120. This improved tti indicates that a material which is made of a HFFR EVM compound containing ATH 200 can resist to a heat source longer without catching fire itself.Table 7: Data from Cone Calorimeter tests,resin EVM 80. Compound PHRR (kW/m²) THR(MJ/m²) tti(s) MARHE (kW/m²) SPR(m²/m²)11 622.6 73.8 34 279.3 1211 12 107.3 39.5 62 67.8 256.5 13 96.3 35.8 74 61.9 226.8 14 107.9 36.8 95 66.5 298.5 15 101.1 34 61 63.1 229.5 On the other hand the smoke production is increased when the submicron sized filler is used. This value can be reduced again by combining ATH 200 with the standard sized filler ATH 40 to achieve values comparable to those of standard ATH types. The smoke reducing effect of the combination of standard ATH with very small fillers is being analyzed further.The smoke production values have to be determined according to ASTM E 662 using the smoke chamber to give a final evaluation of this phenomenon.2.4 Influence of ageingAnother property which has to be taken into consideration for the application in underwater cable compounds is the retention ofmechanical properties of elastomeric compounds after immersion in oil or drilling muds as demanded in international standards suchas IEEE 1580 or NEK 606.The following table shows which ageing conditions were used inthis study to analyse this capability of the compounds. Table 8: Ageing conditions for this study. Test Ageing media Temperature (°C) Duration ofthe test (h) F no ageingA Hot air 135 168 BOil IRM 902 100 168 COil IRM 90370168D CalciumBromide 100 168 E Carbo Sea100168Tensile strength, elongation at break and Shore hardness at 23°C were determined after immersion tests and compared with the original compound.As examples Figure 1 and Figure 2 show elongation at break (E@B) and tensile strength (TS) after several ageing procedures for compounds containing the resin EVM 60.Figure 1: E@B for compounds 1 to 5 after immersion.Figure 2: TS for compounds 1 to 5 after immersion. The E@B suffers from the ageing procedures. The best stability of this value is achieved by the use of the standard sized filler ATH 60 or the mix of standard and submicron sized ATH.The highest TS values can be achieved if ATH 200 is used as flame retardant filler. But values of around 10 MPa can be achieved with all ATH qualities in this study.When EVM 70 and 80 are used, the retention of the mechanical data shows a comparable trend. The higher the VA content in the resin the higher is the E@B before and after the ageing processes. But on the other hand side the TS is reduced with increasing VA content.3. ConclusionThe investigation of elastomeric HFFR compounds based on EVM which were filled with different types of ATH - one of these a submicron sized ATH - was described.Based on these data it can be suggested that when highest mechanical values and flame retardancy have to be achieved submicron sized ATH should be used as this offers the best balance of tensile strength and elongation at break and the highest LOI values for all resins in this study. Shore hardness and Mooney viscosity can be reduced by substituting a certain amount of submicron ATH by a standard grade or by the use of another resin. Overall, the new type of flame retardant offers the possibility to adjust the compound properties over a wide range to special demands. Either it can be used alone or in combination with a standard sized ATH. This offers the user a new degree of freedom in compound development without using too many different types of ATH.The retention of mechanical data depends a lot on the medium in which the ageing is done. For aggressive oils a specific surface area which is not too high seems to be advantageous. In other media like CaBr2 and Carbo Sea the submicron sized ATH helps to keep the mechanical data at a higher level than the other fillers in this study. The level at which these values can be stabilised depends a lot on the resin in the compound. An increasing VA content makes it easier to reach higher values of elongation but the tensile strength is reduced. The compound has to be designed in accordance to the final application.Submicron sized fillers broaden the possibilities of the user to adjust a compound to his demand.More data have to be generated to get a deeper insight into the possibilities which are opened by the use of novel submicron sized aluminium hydroxide products. Another submicron sized material, ACTILOX® 400 SM a boehmite with an even higher specific surface area will be analyzed as well and the ratio of the standard and the submicron filler will be further optimised.4. AcknowledgmentsThe authors thank H. Gokorsch and C. Schmal (Nabaltec AG) for their contribution to this study.Especially the authors wish to thank M. La Rosa and F. Taschner from LANXESS Deutschland GmbH for their support in the development of this study the compounding work and analysis of values presented here.5. References[1]Wypych G., Handbook of Fillers 2nd edition, ChemTecPublishing (1999).[2]Brochure “Metal Hydrates for Cables”, Nabaltec AG (2007).[3]Rothon R., “Particulate-Filled Polymer Composites”,Longman Group Ltd. (1995).[4]Sauerwein R., “Application of Submicron Metal HydrateFillers in Flame Retardant Cables”, Proceedings of the 55thIWCS/Focus (2006).[5]La Rosa M., LANXESS Deutschland GmbH, Internal Studyregarding EVM and ATH6. AuthorsDr. Annika Luks is Regional Manager Sales and Technical Services Division Functional Fillers of Nabaltec AG. She graduated in inorganic chemistry at the Carl von Ossietzky University of Oldenburg.Dr. Reiner Sauerwein is General Manager Business Unit Additives of Division Functional Fillers of Nabaltec AG. He graduated in polymer chemistry at the Technical University of Darmstadt.For technical enquiries, please contact the authors atNabaltec AGAlustrasse 50-52D-92421 SchwandorfGermanyvia tel: +49 9431 53522 orvia e-mail: aluks@nabaltec.de.。
