TMT 蛋白标记试剂盒使用说明90064
tmt蛋白质组学验证方法

tmt蛋白质组学验证方法
TMT(Tandem Mass Tagging)蛋白质组学验证方法是一种用于
定量蛋白质的方法,它结合了质谱技术和化学标记技术。
TMT方法
可以同时比较多个样本中蛋白质的表达水平,是一种高通量、高灵
敏度的蛋白质组学方法。
TMT蛋白质组学验证方法的步骤通常包括样品制备、TMT标记、
蛋白质分离、质谱分析和数据解析等几个关键步骤。
首先,样品制
备阶段需要将不同条件下的蛋白质提取出来,并进行适当的前处理,如还原、碱解等。
接下来,TMT标记是该方法的关键步骤之一,它
涉及使用带有不同质荷比的化学标记剂对样品进行标记,以实现多
样品同时检测的目的。
标记后的样品通常会进行蛋白质分离,常用
的方法包括凝胶电泳、液相色谱等。
在质谱分析阶段,标记的样品
会被送入质谱仪进行分析,得到蛋白质的质谱数据。
最后,通过数
据解析,可以得到不同样品中蛋白质的相对丰度,进而进行蛋白质
表达差异的分析和验证。
TMT蛋白质组学验证方法具有很多优点,比如可以同时比较多
个样品,具有高通量和高灵敏度。
然而,该方法也存在一些局限性,比如标记效率可能存在差异,需要考虑数据分析的复杂性等。
总的来说,TMT蛋白质组学验证方法是一种强大的蛋白质组学技术,可以用于研究蛋白质在不同生理或病理条件下的表达差异,对于理解生物学过程和疾病发生发展具有重要意义。
TMT蛋白组学

TMT蛋白组学
TMT是一种化学标记技术,主要结合质谱应用于定量蛋白质组学的研究。
百泰派克生物科技提供基于质谱的TMT定量蛋白组学分析服务。
TMT蛋白组学
TMT蛋白组学,主要是指利用TMT技术对蛋白质组进行定量分析。
TMT(Tandem Mass Tags)技术是美国Thermo Fisher公司研发的一种化学标记技术,用于基于质谱(MS)的生物大分子(例如蛋白质、多肽和核酸)的定量和鉴定。
TMT属于被称为等压质量标签的试剂家族,它们提供了基于凝胶或抗体的定量方法的替代方法。
除了有助于蛋白质组定量分析外,TMT标签还可在RPLC-MS分析中提高某些高亲水性分析物(如磷酸肽)的检测灵敏度。
TMT蛋白组定量分析原理
TMT采用多个稳定同位素标签,特异性标记多肽的氨基基团后进行串联质谱分析,能够同时比较多达10种不同样本中蛋白质的相对含量。
TMT所有标签的化学结构均相同,由报告基团、平衡基团以及肽反应基团组成,但每个标签都包含在不同位置取代的同位素,因此报告基团和平衡集团在每个标签中具有不同的分子质量。
基团合并则具有相同的总分子量和结构,因此在色谱或电泳分离过程中,以及在单一MS模式下,用不同标签标记的分子是无法区分的。
在MS / MS模式下对标记分子进行片段化后,可从片段化离子获得序列信息,同时从标签的片段化可获取定量数据,从而实现蛋白组定量分析。
TMT蛋白组学。
tmt蛋白实验步骤

tmt蛋白实验步骤
tmt(Tandem Mass Tag)蛋白实验步骤包括以下阶段:
1.样品准备:从细胞、组织或生物流体中提取蛋白质。
蛋白质浓度测定:使用BCA、Bradford或其他蛋白定量方法测定蛋白质浓度。
2.蛋白质消化:酶解,常用胰蛋白酶进行酶解,将蛋白质分解成肽段。
3.TMT标记:肽段标记,使用TMT试剂标记肽段。
不同的样本用不同的TMT标签标记。
4.标记肽段混合:将不同样本中标记的肽段混合在一起,以便同时分析。
5.液相色谱分离:使用高效液相色谱(HPLC)或超高效液相色谱(UPLC)对混合的肽段进行分离。
6.质谱分析:使用质谱仪进行肽段的质谱分析。
TMT标签允许在MS/MS分析中通过检测标记离子来定量比较不同样本中的肽段。
7.数据处理与分析:使用专门的软件(如Proteome Discoverer、MaxQuant等)处理质谱数据,鉴定肽段和蛋白质,以及进行定量分析。
生物信息学分析:分析蛋白质的表达差异,进行生物学功能注释,通路和网络分析等。
8.验证实验:使用Western blot、ELISA等技术对关键发现进行验证。
以上步骤完成后,便完成了TMT蛋白实验。
请注意,根据具体实验需求和目标,某些步骤可能有所调整或需要进一步的优化。
建议咨
询专业人士了解具体的操作过程。
TMT标记定量蛋白质组学

广州辉骏生物科技有限公司TMT标记定量蛋白质组学一、技术概述TMT™(Tandem Mass Tag™)技术是由美国Thermo Scientific公司研发的一种多肽体外标记技术。
该技术采用10种同位素的标签,标记多肽的氨基基团,经过LC-MS/MS分析,可同时比较10组不同样品中蛋白质的相对含量。
TMT技术是常用的差异蛋白质组学技术,在疾病标记物筛选、药物作用靶点、动植物抗病/抗胁迫机制、动植物发育分化机理等领域都有广泛应用。
二、技术原理TMT试剂由三部分组成:质量报告基团、质量标准化基团和氨基反应基团。
质量报告基团有10种不同的分子量,质量标准化基团也10有种不同的分子量,与不同的报告基团搭配,能保证被标记的不同来源的同一肽段在一级质谱中具有相同的质荷比;氨基反应基团能与肽段N端及赖氨酸侧链氨基发生共价连接使肽段连上标记。
一级质谱中,任何一种TMT试剂标记的不同样品中的同一肽段表现出相同的质荷比;二级质谱中,可切割键(箭头所指)断裂释放出TMT报告离子,在质谱低质量区产生了10个TMT报告离子峰,其强度反应了该肽段在不同样品中的相对表达量信息,另外二级质谱中的肽段碎片离子峰质荷比反应了该肽段的序列信息;这些质谱原始数据经过数据库检索,可得到蛋白质的定性和相对定量信息。
三、技术优势1.灵敏度高:低丰度蛋白也能检测出;2. 适用范围广:几乎可对任何物种的各类蛋白质进行分离鉴定;3. 高通量:能同时对10组样本中包含的蛋白进行鉴定及表达差异分析;4. 高效:液相色谱与串联质谱连用,自动化操作,分析速度快,分离效果好。
四、技术流程蛋白样本制备——胰酶酶解——TMT标记——肽段混合——LC-MS/MS检测——数据库检索——数据分析广州辉骏生物科技有限公司。
Protocol _TMT labeling 6-plex
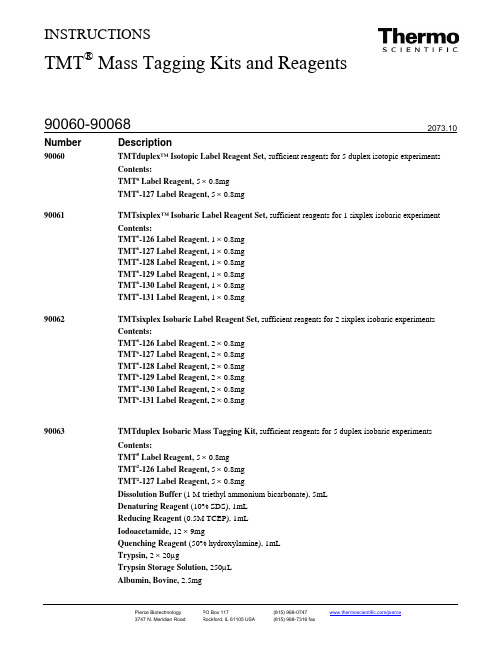
INSTRUCTIONSTMT® Mass Tagging Kits and Reagents90060 TMTduplex™ Isotopic Label Reagent Set, sufficient reagents for 5 duplex isotopic experiments Contents:TMT0 Label Reagent, 5 × 0.8mgTMT6-127 Label Reagent, 5 × 0.8mg90061 TMTsixplex™Isobaric Label Reagent Set, sufficient reagents for 1 sixplex isobaric experiment Contents:TMT6-126 Label Reagent, 1 × 0.8mgTMT6-127 Label Reagent, 1 × 0.8mgTMT6-128 Label Reagent, 1 × 0.8mgTMT6-129 Label Reagent, 1 × 0.8mgTMT6-130 Label Reagent, 1 × 0.8mgTMT6-131 Label Reagent, 1 × 0.8mg90062 TMTsixplex Isobaric Label Reagent Set, sufficient reagents for 2 sixplex isobaric experiments Contents:TMT6-126 Label Reagent, 2 × 0.8mgTMT6-127 Label Reagent, 2 × 0.8mgTMT6-128 Label Reagent, 2 × 0.8mgTMT6-129 Label Reagent, 2 × 0.8mgTMT6-130 Label Reagent, 2 × 0.8mgTMT6-131 Label Reagent, 2 × 0.8mg90063TMTduplex Isobaric Mass Tagging Kit, sufficient reagents for 5 duplex isobaric experiments Contents:TMT0 Label Reagent, 5 × 0.8mgTMT2-126 Label Reagent, 5 × 0.8mgTMT2-127 Label Reagent, 5 × 0.8mgDissolution Buffer (1 M triethyl ammonium bicarbonate), 5mLDenaturing Reagent (10% SDS), 1mLReducing Reagent (0.5M TCEP), 1mLIodoacetamide, 12 × 9mgQuenching Reagent (50% hydroxylamine), 1mLTrypsin, 2 × 20µgTrypsin Storage Solution, 250µLAlbumin, Bovine, 2.5mg90064TMTsixplex™ Isobaric Mass Tagging Kit, sufficient reagents for 5 sixplex isobaric experiments Contents:TMT0 Label Reagent, 5 × 0.8mgTMT6-126 Label Reagent, 5 × 0.8mgTMT6-127 Label Reagent, 5 × 0.8mgTMT6-128 Label Reagent, 5 × 0.8mgTMT6-129 Label Reagent, 5 × 0.8mgTMT6-130 Label Reagent, 5 × 0.8mgTMT6-131 Label Reagent, 5 × 0.8mgDissolution Buffer (1M triethyl ammonium bicarbonate), 5mLDenaturing Reagent (10% SDS), 1mLReducing Reagent (0.5 M TCEP), 1mLIodoacetamide, 12 × 9mgQuenching Reagent (50% hydroxylamine), 1mLTrypsin, 5 × 20µgTrypsin Storage Solution, 250µLAlbumin, Bovine, 2.5mg90065TMTduplex Isobaric Label Reagent Set, sufficient reagents for 5 duplex isobaric experiments Contents:TMT2-126 Label Reagent, 5 × 0.8mgTMT2-127 Label Reagent, 5 × 0.8mg90066TMTsixplex Label Reagent Set, sufficient reagents for 5 sixplex isobaric experimentsContents:TMT6-126 Label Reagent, 5 × 0.8mgTMT6-127 Label Reagent, 5 × 0.8mgTMT6-128 Label Reagent, 5 × 0.8mgTMT6-129 Label Reagent, 5 × 0.8mgTMT6-130 Label Reagent, 5 × 0.8mgTMT6-131 Label Reagent, 5 × 0.8mg90067TMTzero™Label Reagent, 5 × 0.8mg, sufficient reagents for 5 samples90068TMTsixplex Label Reagent Set, sufficient reagents for 12 sixplex isobaric experimentsContents:TMT6-126 Label Reagent, 2 × 5mgTMT6-127 Label Reagent, 2 × 5mgTMT6-128 Label Reagent, 2 × 5mgTMT6-129 Label Reagent, 2 × 5mgTMT6-130 Label Reagent, 2 × 5mgTMT6-131 Label Reagent, 2 × 5mgStorage: Upon receipt store at -20°C. Reagents are shipped with dry ice.Note: These products are for research use only − do not use for diagnostic procedures.ContentsIntroduction ................................................................................................................................................................................. 3 Procedure Summary ..................................................................................................................................................................... 4 Important Product Information .................................................................................................................................................... 4 Additional Materials Required ..................................................................................................................................................... 4 Material Preparation .................................................................................................................................................................... 5 Preparing and Labeling Peptides with the TMT Isobaric Mass Tags .......................................................................................... 5 Preparing and Labeling Intact Proteins with the TMT Isobaric Mass Tags ................................................................................. 6 Troubleshooting ........................................................................................................................................................................... 7 Additional Information ................................................................................................................................................................ 7 Related Thermo Scientific Products ............................................................................................................................................ 9 General References . (9)IntroductionThe Thermo Scientific TMT Isobaric and Isotopic Mass Tagging Kits and Reagents enable quantitative labeling of proteins extracted from cells and tissues. Each isobaric tagging reagent within a set has the same nominal parent (precursor) mass and is composed of an amine-reactive NHS-ester group, a spacer arm and an MS/MS reporter (Figure 1). The reagents label peptides prepared from cell-based or tissue samples, either two samples for the duplex kit or six samples for the sixplex kit. For each sample, a unique reporter mass results in the MS/MS spectrum (i.e., 126-127Da for TMT 2 and 126-131Da for TMT 6 Isobaric Label Reagents). These reporter ions are in the low mass region of the MS/MS spectrum and are used to report relative protein expression levels during peptide fragmentation.The TMT Reagents share an identical structure, allowing TMTzero and TMTsixplex Reagents to be used also as isotopic “light” and “heavy” duplex tags. These tags are used to quantitate protein expression changes in cell-based or tissue samples that may not be amenable to metabolic isotopic labeling strategies (e.g., SILAC). These isotopic pairs can also be used in targeted quantitation strategies, including selective reaction monitoring (SRM, see the Additional Information Section). Peptides and proteins labeled with all TMT Reagents can be enriched with the anti-TMT Antibody Resin and TMTEnrichment Kit (see the Additional Information and Related Thermo Scientific Products Sections).Figure 1. Chemical structure of the TMT Label Reagents. Panel A: Functional regions of the reagent structure. Panel B: Structures, isotope positions, MS/MS fragmentation sites and collision-induced reporter ions for each reagent.ABProcedure SummaryProtein extracts are isolated from cells grown in culture or from tissue samples. After removing amine-based buffers and thiol reagents, samples are reduced, alkylated and digested overnight. Samples are labeled with the TMT Reagents and then mixed at the duplex or the sixplex level. Strong-cation exchange (SCX) fractionation simplifies complex samples before LC-MS/MS analysis. Data analysis software is used to analyze the reporter ions in the low mass region (Figure 2).Peptides are typically labeled with TMT Reagents because it allows quantitation of every peptide, but intact proteins can also be labeled. There are several advantages to labeling intact proteins. For example, combining labeled samples earlier in the sample process will reduce sample variability. Also, mixed samples enable single processing for fractionation and digestion.Fractionation methods include ion exchange chromatography, 1D-PAGE and phosphoprotein enrichment.Figure 2. Schematic for using the Thermo Scientific TMTsixplex Isobaric Mass Tagging Reagents.Important Product Information• The TMT Reagents are moisture-sensitive. To avoid moisture condensation onto the product, vial must be equilibrated to room temperature before opening.• The TMT Reagents are amine-reactive and modify lysine residues and the peptide N-termini. All amine-containing buffers and additives must be removed before digestion and labeling.• All samples must be digested, labeled and then mixed equally before desalting, fractionation and LC-MS/MS. For optimal results, use 25-100µg of peptide for each labeling reaction.• To avoid contamination of MS samples, always wear gloves when handling samples and gels. Use ultrapure MS-grade reagents. Perform sample preparation in a cleaned work area cleaned with 70% methanol (Fisher Product No. A454-1). • The TMTzero Label Reagent can be used to optimize methods before multiplexed analysis of samples with the TMTduplex or TMTsixplex Reagent Set.Additional Materials Required• Anhydrous acetonitrile (Thermo Scientific Acetonitrile HPLC grade, Product No. 51101), 100 proof ethanol or, for protein labeling, anhydrous dimethyl sulfoxide (DMSO), Sequanal grade (Product No. 20688) • Glass syringe (100µL)• HPLC grade water (Fisher, Product No. W6-4) • Chilled (-20°C) acetone• Protease inhibitors (Thermo Scientific Halt Protease Inhibitor Single-Use Cocktail, EDTA-free, Product No. 78425) • Phosphatase inhibitors (Thermo Scientific Halt Phosphatase Inhibitor Cocktail, Product No. 78420)• Cell lysis reagent such as Thermo Scientific M-PER Mammalian Protein Extraction Reagent (Product No. 78501), RIPA Lysis and Extraction Buffer (Product No. 89901) or 8M Urea (Product No. 29700)• Protein assay such as Thermo Scientific Coomassie Plus (Bradford) Protein Assay (Product No. 23236), Pierce 660nm Protein Assay (Product No. 22600) or Pierce BCA Protein Assay Kit (Product No. 22235) • 75-300µm capillary C 18 reversed-phase column• Ion trap or time-of-flight (TOF) mass spectrometer with online or offline liquid chromatography (LC) system •Data analysis software such as Thermo Scientific Proteome Discoverer or Mascot Software (Matrix Science, Ltd.)•Optional: Thermo Scientific Zeba Spin Desalting Columns (Product No. 89882) or Slide-A-Lyzer Dialysis Cassettes,3.5K MWCO, 0.5mL (Product No. 66333)Material PreparationNote: The 50% hydroxylamine and 10% SDS stock solutions provided with the kit may precipitate during storage. Warm both solutions to room temperature and vortex before use.Albumin, Bovine (BSA) Reconstitute BSA (2.5mg) with 2.5mL of ultrapure water. Divide solution into 100µL aliquots and lyophilize to dryness.Add 50µL of the Dissolution Buffer (1M TEAB) to 450µL of ultrapure water.100mM TEAB (triethylammonium bicarbonate)2% SDS Add 50µL of the Denaturing Reagent (10% SDS) to 200µL of ultrapure water.200mM TCEP Add 70µL of the Reducing Reagent (0.5M TCEP) to 70µL of ultrapure water. Then add 35µL of the Dissolution Buffer (1M TEAB).5% Hydroxylamine Dilute the Quenching Reagent (50% hydroxylamine) 1:10 with 200mM TEAB.Preparing and Labeling Peptides with the TMT Isobaric Mass TagsA.Preparing Whole Cell Protein Extracts1.Culture cells to harvest at least 100µg of protein per condition. For best results, culture a minimum of 5 × 106 cells.2.Lyse cells in either RIPA buffer, M-PER® Reagent or 8M urea. Add protease and phosphatase inhibitors to the lysisreagent. Use 4mL of lysis reagent for each milliliter of cells.3.Perform a protein assay to determine the protein concentration. Use samples at ≥ 2mg/mL. Less concentrated samplescan be used; however, it might be necessary to use larger volumes of reducing/alkylating reagents.4.Place 100µg per condition (two for the TMTduplex or six for the TMTsixplex Label Reagents) in a polypropylenemicrocentrifuge tube.5.Add 45µL of 100mM TEAB to the sample and adjust to a final volume of 100µL with ultrapure water. For labelingreactions > 100µL use a large-volume centrifuge tube such as a 15mL or 50mL polypropylene conical tube.Optional: To solubilize complex protein mixtures, add 5µL of 2% SDS before adjusting to final volume.6.Add 5µL of the 200mM TCEP and incubate sample at 55°C for 1 hour.7.Immediately before use, dissolve one tube of iodoacetamide (9mg) with 132µL of 100mM TEAB to make375mM iodoacetamide. Protect solution from light.8.Add 5µL of the 375mM iodoacetamide (with TEAB) to the sample and incubate for 30 minutes protected from light.9.Add six volumes (~1mL) of pre-chilled (-20°C) acetone. Allow the precipitation to proceed overnight.10.Centrifuge the samples at 8000 ×g for 10 minutes at 4°C. Carefully invert the tubes to decant the acetone withoutdisturbing the white pellet. Allow the pellet to dry for 10 minutes.B.Protein DigestionNote: Use 25-100µg of purified or lyophilized protein per sample. If the protein is in solution, it must be free of amine-containing buffers. Use the BSA (100µg) as a control sample for method optimization.1.Suspend 100µg acetone-precipitated (or lyophilized) protein pellets with 100µL of 100mM TEAB.Note: An acetone-precipitated pellet might not completely dissolve; however, after proteolysis at 37°C, all the protein (peptides) will be solubilized.2.Immediately before use, add 20µL of the Trypsin Storage Solution to the bottom of the trypsin glass vial and incubate for5 minutes. Store any remaining reagent in single-use volumes at -80°C (e.g., 2.5µg of trypsin per 100µg of protein).3.Add 2.5µL of trypsin (i.e., 2.5µg) per 100µg of protein. Digest the sample overnight at 37°C.C. Peptide Labeling1.Immediately before use, equilibrate the TMT Label Reagents to room temperature. For the 0.8mg vials, add 41µL ofanhydrous acetonitrile or ethanol to each tube. For the 5mg vials, add 256µL of solvent to each tube. Allow the reagent to dissolve for 5 minutes with occasional vortexing. Briefly centrifuge the tube to gather the solution.Note: Reagents dissolved in anhydrous acetonitrile or ethanol are stable for one week when stored at -20°C and warmed to room temperature before opening.2.Carefully add 41µL of the TMT Label Reagent to each 25-100µg sample. Alternatively, transfer the reduced andaklyated protein to the TMT Reagent vial.Note: A 100µL glass syringe or positive displacement pipette may be necessary to accurately measure and dispense TMT Reagents in volatile acetonitrile solvent.3.Incubate the reaction for 1 hour at room temperature.4.Add 8µL of 5% hydroxylamine to the sample and incubate for 15 minutes to quench the reaction.bine samples at equal amounts and store at -80°C.Preparing and Labeling Intact Proteins with the TMT Isobaric Mass TagsNote: Protein labeling results in the modification of lysine residues and non-acetylated protein N-termini. Because trypsin does not recognize modified lysines, trypsin digestion cleaves only on the C-terminal side of arginine residues. The result is fewer, larger peptides and a less complex digest. Labeled proteins can be digested with other enzymes, including chymotrypsin and Glu-C.1.Solubilize and quantify protein samples as described above in Section A: Preparing Whole Cell Protein Extracts,steps 1-8.Note: If primary amine containing lysis buffers were used to prepare whole cell protein extracts, samples must be exchanged using dialysis or desalting into a suitable non-primary amine, containing buffer (e.g., TEAB, PBS, HEPES or bicine) at pH 7 to 9.2.Immediately before use, equilibrate the TMT Label Reagents to room temperature. For the 0.8mg vials, add 24µL ofanhydrous DMSO to each tube. For the 5mg vials, add 150µL DMSO to each tube. Allow the reagent to dissolve for5 minutes with occasional vortexing. Briefly centrifuge the tube to gather the solution.Note: Reagents dissolved in DMSO are stable for one week when stored at -20°C and warmed to room temperature before opening.3.Carefully add 24µL of the TMT Label Reagent to each 100µg sample. Alternatively, transfer the reduced and alkylatedprotein to TMT Reagent vial.4.Incubate the reaction for 1 hour at room temperature.5.Add 8µL of 5% hydroxylamine to the sample and incubate for 15 minutes to quench the reaction.bine samples at equal amounts. Store samples at -80°C or fractionate to remove excess tag (e.g., acetoneprecipitation, desalting or SDS-PAGE) before enzymatic digestion.TroubleshootingProblem Possible Cause SolutionPoor labeling An amine-based buffer was used Use a non-amine-based bufferIncorrect buffer pH Make sure the buffer pH is ~8.0Protein precipitation Lack of detergent present Add detergent, such as 0.05% SDS to the preparationpH decreased Make sure the pH is > 7.5Organic solvent too high For protein labeling, dissolve TMT Reagents in DMSOto minimize protein precipitationAdditional InformationA.Sample Clean-up, Enrichment and FractionationListed below are some options for peptide cleanup before MS analysis.•If SDS and DMSO were avoided during the preparation, acetonitrile may be removed by vacuum centrifugation and samples analyzed directly by LC-MS/MS. Collect MS data above 350Da to avoid signal from unincorporated tag.•SDS, solvent and unincorporated tags can be removed using TopTip™ Strong Cation-Exchange Tips (PolyLC, Product No. TT1000SEA-2003), according to the manufacturer’s instructions.•Salt and unincorporated tags can be removed using Thermo Scientific Pierce C18 Spin Columns (Product No. 89870).•Proteins labeled with TMT Reagents can be detected with the anti-TMT Antibody (Product No. 90075). Proteins and peptides labeled with TMT Reagents can be enriched with the Immobilized anti-TMT Antibody Resin and the TMT Enrichment Kit (Product No 90076 and 90077).•For best results, use an HPLC system to perform strong cation exchange fractionation to remove SDS and to fractionate complex proteomic extracts. Perform the separation with a strong-cation exchange column (PolyLC, Inc., Table 1). See also /Proteomics.v.2.htm and Trinidad, J.C. (2008).Table 1. Strong-cation exchange column information.PolyLC Part # Column Particle Pore Load Range Flow Range102SE0503 2.1 × 100 5µm 300Å 0.1-1.0mg 0.14-0.2mL/min104SE0503 4.6 × 100 5µm 300Å 0.4-4.0mg 0.7-1.0mL/minB.Data Acquisition Methods for Peptide QuantitationQuantitation of peptides labeled with Tandem Mass Tag® Reagents requires a mass spectrometer capable of MS/MS fragmentation, such as an ion trap, quadrupole time of flight, time of flight-time of flight (TOF-TOF) or triple quadrupole instrument. The choice of MS/MS fragmentation method(s) depends on the instrument capabilities such as collisionally induced dissociation (CID), pulsed-Q dissociation (PQD), higher energy collisional dissociation (HCD), or electron transfer dissociation (ETD), and the desire either to optimize one fragmentation method for both peptide identification and quantitation, or to use two methods that are each optimized for peptide identification or quantitation. For example, TMT Reagent reporter ions are not visible in ion traps following traditional CID fragmentation. Instead, quantify and identify peptides on an ion trap with PQD fragmentation or alternate PQD and CID methods optimized for identification and quantitation, respectively (Table 2). The TMT tags behave similarly to iTRAQ® Reagents (Life Technologies Corp.), although optimal chromatography and fragmentation energy settings are slightly different.Table 2. Instruments and MS/MS fragmentation options for peptide identification and quantitation with Thermo Scientific TMT Reagents.Instrument Fragmentation Method ReferenceThermo Scientific Orbitrap Velos, LTQ-Orbitrap XL, or MALDI-Orbitrap XL HCD, HCD/CID Schirle, et al. (2012), Bomgarden, etal. (2011), Lee, et al (2011), Xiong, etal. (2011), Strupat, et al. (2008)Thermo Scientific Orbitrap Velos, MS3, PTR-HCD Ting, et al. (2011), Wenger, et al.(2011)Thermo Scientific Velos Pro ion trap Trap HCD Biringer, et al. (2011)Thermo Scientific LTQ-Orbitrap Discovery or LTQ ion trap PQD, PQD/CID Bantscheff, et al. (2008), Schwartz,et al. (2008),Thermo Scientific LTQ-OrbitrapXL-ETDor LTQ-ETDETD Viner, et al. (2009) Q-TOF CID Van Ulsen, et al. (2009)TOF-TOF CID Dayon, et al. (2008)Triple Quadrupole CID, SRM Stella, et al (2011), Byers, et al.(2009)C.Data Analysis and QuantitationThe masses for peptide modification by the TMT zero, duplex, and sixplex reagents are present in the UNIMOD database () and are listed below. Several software packages directly support the modifications by TMT Reagents and the relative quantitation of reporter ions released from labeled peptides, including Thermo Scientific Proteome Discoverer (all versions) 1.1, Thermo Scientific Bioworks 3.1.1, Matrix Science Mascot 2.1 and above, and Proteome Software Scaffold Q+. For data acquired using a combination of fragmentation methods (i.e. HCD/CID or PQD/CID), Proteome Discoverer 1.1 or custom software might be necessary to merge spectra for identification and quantitation.D.Mass ModificationAll TMT Reagents share an identical chemical structure. Therefore, labeled samples behave identically during LC-MS or MALDI-MS analysis and can be quantified at either the MS/MS or MS level. For MS/MS quantitation, duplex or sixplex samples may be quantified with TMTduplex or TMTsixplex Reagent Sets. This strategy allows higher plexing and the ability to quantify specific, singly charged reporter ions without increasing sample complexity. For duplex MS quantitation, samples or internal standards labeled with TMTzero may be combined with samples labeled with a TMTsixplex Reagent, resulting in a modification of 224Da or 229Da for every labeled lysine residue, respectively. Paired peaks with a 5Da mass shift per labeled N-terminus and lysine residue are then quantified similarly to SILAC samples. This approach also may be used to quantitate specific parent and transition ions using selective reaction monitoring (SRM) strategies.Table 3. Modification masses of the Thermo Scientific TMT Label Reagents.Label ReagentModificationMass(monoisotopic)ModificationMass(average)CIDMonoisotopicReporter Mass*CID AverageReporterMass*ETDMonoisotopicReporter Mass**ETD AverageReporterMass**TMT0-126 224.152478 224.2994 126.127725 126.2193 114.127725 114.2086 TMT 2-126 225.155833 225.2921 126.127725 126.2193 114.127725 114.2086 TMT2-127 225.155833 225.2921 127.131079 127.2120 114.127725 114.2086 TMT6-126 229.162932 229.2634 126.127725 126.2193 114.127725 114.2086 TMT6-127 229.162932 229.2634 127.124760 127.2127 115.124760 115.2020TMT6-128 229.162932 229.2634 128.134433 128.2046 116.134433 116.1939 TMT6-129 229.162932 229.2634 129.131468 129.1981 117.131468 117.1874 TMT6-130 229.162932 229.2634 130.141141 130.1900 118.141141 118.1417TMT6-131 229.162932 229.2634 131.138176 131.1834 119.138176 119.1727 * CID, HCD, and PQD are collisional fragmentation methods that generate reporter ions from 126 to 131Da.**ETD is a non-ergodic fragmentation method that generates six unique reporter ions from 114 to 119Da.rmation Available from our Website•Tech Tip Protocol #49: Acetone precipitation of proteins•Tech Tip Protocol #19: Remove detergent from protein samplesRelated Thermo Scientific Products90076 Immobilized Anti-TMT Antibody Resin90075 Anti-TMT Antibody, 0.1mL88320 Pierce Peptide Retention Time Calibration Mixture, 50uL88321 Pierce Peptide Retention Time Calibration Mixture, 200uL89983 SILAC Protein Quantitation Kit – DMEM89982 SILAC Protein Quantitation Kit – RPMI 164088439 SILAC Protein Quantitation Kit - DMEM:F1287784 Pierce C18 Tips, 100µl bed, 96 tips89870 Pierce C18 Spin Columns, 25 columns28904 Trifluoroacetic Acid, Sequanal Grade23227 Pierce BCA Protein Assay23208 Pre-Diluted Protein Assay Standards88300 Fe-NTA Phosphopeptide Enrichment Kit88301 Pierce TiO2 Phosphopeptide Enrichment and Clean-up Kit90003 Pierce Phosphoprotein Isolation Kit88513 Pierce Concentrator, PES, 10K MWCO, 0.5mL89893 Zeba Spin Desalting Columns, 10mL, 5 columnsGeneral ReferencesBantscheff, M., et al. (2008). Robust and sensitive iTRAQ quantification on an LTQ Orbitrap Mass Spectrometer. Mol Cell Proteomics 7:1702-13. Biringer, R.G., et al. (2011). Quantitation of TMT-Labeled Peptides Using Higher-Energy Collisional Dissociation on the Velos Pro Ion Trap Mass Spectrometer. Application note # 520. Bomgarden, R.D., et al. (2011). Kinase Inhibitor Profiling of TrkA- and TrkB-Expressing Neuroblastoma Cell Lines Using Desthiobiotin Nucleotide Probes. Application note # 515. Byers, H.L. (2009). Candidate verification of iron-regulated Neisseria meningitidis proteins using isotopic versions of tandem mass tags (TMT) and single reaction monitoring, J Prot73(2):231-9.Dayon, L., et al. (2008). Relative quantification of proteins in human cerebrospinal fluids by MS/MS using 6-plex isobaric tags. Anal Chem80(8):2921-31. Dillon, R, et al. (2011). Discovery of a Novel B-Raf Fusion Protein Related to c-Met Drug Resistance. J Proteome Res10(11):5084-94.Lee, M.V., et al. (2011). A dynamic model of proteome changes reveals new roles for transcript alteration in yeast. Mol Syst Biol7: 514.Ross, P.L., et al. (2004). Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics 3(12):1154-69.Schirle, M., et al. (2012). Kinase inhibitor profiling using chemoproteomics. Methods Mol Biol795:161-77.Schwartz, J. et al. (2008). Relative quantitation of protein digests using tandem mass tags and pulsed-Q dissociation (PQD). Application note # 452.Stella, R., et al. (2011). Relative Quantification of Membrane Proteins in Wild-type and PrP-knockout Cerebellar Granule Neurons. J Proteome Res doi:10.1021/pr200759m. Strupat K., et al. (2008). Accurate MS and MS n Analysis with the Thermo Scientific MALDI LTQ Orbitrap. Application note # 30150.Ting, L., et at. (2011). MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nature Methods8: 937–940.Van Ulsen, P., et al. (2009). Identification of proteins of Neisseria meningitidis induced under iron-limiting conditions using the isobaric tandem mass tag (TMT) labeling approach. Proteomics9(7):1771-81.Viner, R.I., et al. (2009). Quantification of post-translationally modified peptides of bovine α-crystallin using tandem mass tags and electron transfer dissociation. J Proteomics72(5):874-85.Wenger, C.D., et al. (2011). Gas-phase purification enables accurate, multiplexed proteome quantification with isobaric tagging. Nat Methods8(11):933-5. Xiong, L., et al. (2011). Mass spectrometric studies on epigenetic interaction networks in cell differentiation. J Biol Chem 286(15):13657-68.Zhang, T., et al. (2010). Improving quantitation of TMT-labeled peptides using stepped higher-energy collisional dissociation. Application note # 483 Tandem Mass Tag and TMT are trademarks of Proteome Sciences plcTopTip is a trademark of PolyLC, Inc.iTRAQ is a trademark of Life Technologies Corp.This product (“Product”) is warranted to operate or perform substantially in conformance with published Product specifications in effect at the time of sale, as set forth in the Product documentation, specifications and/or accompanying package inserts (“Documentation”) and to be free from defects in material and workmanship. Unless otherwise expressly authorized in writing, Products are supplied for research use only. No claim of suitability for use in applications regulated by FDA is made. The warranty provided herein is valid only when used by properly trained individuals. Unless otherwise stated in the Documentation, this warranty is limited to one year from date of shipment when the Product is subjected to normal, proper and intended usage. This warranty does not extend to anyone other than the original purchaser of the Product (“Buyer”).No other warranties, express or implied, are granted, including without limitation, implied warranties of merchantability, fitness for any particular purpose, or non infringement. Buyer’s exclusive remedy for non-conforming Products during the warranty period is limited to replacement of or refund for the non-conforming Product(s).There is no obligation to replace Products as the result of (i) accident, disaster or event of force majeure, (ii) misuse, fault or negligence of or by Buyer, (iii) use of the Products in a manner for which they were not designed, or (iv) improper storage and handling of the Products.Current product instructions are available at /pierce. For a faxed copy, call 800-874-3723 or contact your local distributor.© 2012 Thermo Fisher Scientific Inc. All rights reserved. Unless otherwise indicated, all trademarks are property of Thermo Fisher Scientific Inc. and its subsidiaries. Printed in the USA.。
人免疫球蛋白酶联免疫分析试剂盒使用方法

人免疫球蛋白酶联免疫分析试剂盒使用方法本试剂盒仅供研究使用。
检测范围:96T2.5μg/ml -80μg/ml使用目的:本试剂盒用于测定人血清、血浆及相关液体样本中免疫球蛋白含量。
实验原理本试剂盒应用双抗体夹心法测定标本中人免疫球蛋白水平。
用纯化的抗-IgG抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入免疫球蛋白,再与HRP标记的羊抗人抗体结合,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB显色。
TMB在HRP酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的免疫球蛋白呈正相关。
用酶标仪在450nm波长下测定吸光度(OD值),通过标准曲线计算样品中人免疫球蛋白浓度。
标本要求1.标本采集后尽早进行提取,提取按相关文献进行,提取后应尽快进行实验。
若不能马上进行试验,可将标本放于-20℃保存,但应避免反复冻融2.不能检测含NaN3的样品,因NaN3抑制辣根过氧化物酶的(HRP)活性。
操作步骤1.标准品的稀释:本试剂盒提供原倍标准品一支,用户可按照下列图表在小试管中进行稀2.加样:分别设空白孔(空白对照孔不加样品及酶标试剂,其余各步操作相同)、标准孔、待测样品孔。
在酶标包被板上标准品准确加样50μl,待测样品孔中先加样品稀释液40μl,然后再加待测样品10μl(样品最终稀释度为5倍)。
加样将样品加于酶标板孔底部,尽量不触及孔壁,轻轻晃动混匀。
3.温育:用封板膜封板后置37℃温育30分钟。
4.配液:将20倍浓缩洗涤液用蒸馏水20倍稀释后备用5.洗涤:小心揭掉封板膜,弃去液体,甩干,每孔加满洗涤液,静置30秒后弃去,如此重复5次,拍干。
6.加酶:每孔加入酶标试剂50μl,空白孔除外。
7.温育:操作同3。
8.洗涤:操作同5。
9.显色:每孔先加入显色剂A50μl,再加入显色剂B50μl,轻轻震荡混匀,37℃避光显色15分钟.10.终止:每孔加终止液50μl,终止反应(此时蓝色立转黄色)。
TMT标记

百泰派克生物科技
TMT标记
串联质量标签TMT(Tandem Mass Tag)是美国Thermo Fisher公司研发的一种体外标记蛋白定量技术。
其基本原理是将水解后得到蛋白质多肽氨基酸N端和赖氨酸残基利用TMT试剂标签进行标记,然后进行质谱分析,通过串联质谱获得的离子峰强度和离子峰质荷比进行蛋白质的定性和定量分析。
TMT实验单次最高可同时对16种不同的生物样品进行检测,通量相比其他体外标记技术有很大的提高;此外,TMT报告基团间最小分子质量差达6/1000Da,在一定程度上降低了实验误差,提高了检测的分辨率。
百泰派克生物科技采用Thermo公司最新推出的Obitrap Fusion Lumos质谱仪结合Nano-LC,提供基于TMT标记定量蛋白质组学分析服务技术包裹,您只需要将您的实验目的告诉我们并将您的蛋白寄给我们,我们会负责项目后续所有事宜,包括蛋白酶切、肽段标价、肽段分离、质谱分析、质谱原始数据分析、生物信息学分析。
脑自然肽N端前体蛋白测定试剂盒(化学发光法)产品技术要求新产业

2.性能指标
2.1外观和性状
试剂盒各组分应齐全、完整、液体无渗漏;包装标签应清晰、准确、牢固;试剂盒内组分(磁性微球溶液除外)应为澄清的液体,无沉淀、无悬浮物、无絮状物。
2.2装量及允差
试剂盒的装量应不少于额定装量(见表1)。
表 1
2.3重复性
分别用高、中、低 3 个浓度的样本,各重复检测10 次,其变异系数(CV)应不大于8%。
2.4批间精密度
用3个批号试剂盒分别检测高、中、低3个浓度的样本,3个批号试剂盒之间的变异系数(CV)应不大于15%。
2.5准确度
相对偏差应在±10% 范围内。
2.6空白限
试剂盒的空白限应小于 2.0 pg/mL。
2.7检出限
试剂盒的检出限应≤5.0 pg/mL
2.8线性
在(5.0~35000.0)pg/mL 浓度范围内,线性相关系数(r)绝对值应大于0.9900。
tmt标记定量蛋白质组学原理

tmt标记定量蛋白质组学原理好嘞,今天我们聊聊TMT标记定量蛋白质组学,听起来挺复杂的吧?别急,咱们慢慢来,保证你听完后脑袋里还是清晰明了的。
咱得搞清楚什么是“蛋白质组学”。
简单说,就是研究细胞里的所有蛋白质,像是在探险,寻找那些隐藏在细胞角落里的“宝藏”。
想象一下,你在一个大宝藏里翻找,结果发现了各种金光闪闪的宝物,这就是蛋白质组学带来的惊喜。
然后,咱们得提到TMT。
TMT可不是个新潮的饮料,而是“标记定量”的一种方法。
想象一下,咱们把不同的蛋白质用不同颜色的标签标记上,就像给每个水果贴上了标签一样。
这样一来,我们就能轻松区分这些蛋白质,知道它们在细胞里到底有多重要。
就像在超市里,苹果、香蕉、橙子都有不同的标签,你一看就知道哪个是什么,省得你像个傻瓜一样在那儿找。
TMT的过程是怎样的呢?咱得从样本里提取蛋白质,听起来简单,但其实就像在寻找失散多年的老朋友。
有时候这还挺麻烦,特别是当样本复杂得像个麻辣烫,里面的成分五花八门。
然后,把提取出来的蛋白质切成小片,别担心,不是拿刀子乱切,而是用一种叫做酶的东西,把它们“剪”得整整齐齐,方便后面的工作。
咱们就要用到TMT的标记了。
每个小片蛋白质都被赋予了不同的标签,听起来像是给它们穿上了个性化的衣服。
这一标记就像是给每个蛋白质打了个二维码,等下再分析的时候,就能一眼看出哪个蛋白质是哪个。
真是妙不可言,对吧?一旦标记完成,咱们就把这些蛋白质混合在一起,像做沙拉一样,把各种各样的材料拌在一起。
通过一种叫做质谱的技术来分析这些标记。
质谱就像是一位优秀的侦探,能够把每个标签的信息都“捞”出来,帮我们识别和定量这些蛋白质。
最牛的地方在于,TMT技术可以一次性分析多个样本。
想想看,以前我们得一个一个样本分析,简直是慢得像乌龟。
但现在,通过TMT,咱们可以在一次实验中处理好几个样本,真是省时又省力,简直就像是参加了一个科学界的快闪活动,人人都来凑热闹。
说到这里,可能有人会问,为什么TMT这么重要呢?这可不得不提到它在生物医学研究中的应用。
tmt定量蛋白质组学的操作流程

tmt定量蛋白质组学的操作流程下载温馨提示:该文档是我店铺精心编制而成,希望大家下载以后,能够帮助大家解决实际的问题。
文档下载后可定制随意修改,请根据实际需要进行相应的调整和使用,谢谢!并且,本店铺为大家提供各种各样类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,如想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by theeditor. I hope that after you download them,they can help yousolve practical problems. The document can be customized andmodified after downloading,please adjust and use it according toactual needs, thank you!In addition, our shop provides you with various types ofpractical materials,such as educational essays, diaryappreciation,sentence excerpts,ancient poems,classic articles,topic composition,work summary,word parsing,copy excerpts,other materials and so on,want to know different data formats andwriting methods,please pay attention!TMT 定量蛋白质组学的操作流程一、实验准备阶段在开展 TMT 定量蛋白质组学实验之前,要做好充分的准备工作。
tmt标记定量蛋白质组学技术

tmt标记定量蛋白质组学技术好嘞,今天我们来聊聊一种特别酷的技术,叫做TMT标记定量蛋白质组学。
听起来好像很高大上对吧?别担心,我会把它说得轻松点。
蛋白质在我们身体里可是扮演了非常重要的角色,就像是建筑工地上的工人,负责建造和维护我们的细胞结构,调节生理过程,简直是个个都是“功臣”。
而要研究这些蛋白质,了解它们的功能、数量和变化,就需要用到一些高科技手段。
这时候,TMT就派上用场了。
TMT,全名是“天冬氨酸标记技术”,其实就是给蛋白质贴上小标签。
想象一下,你在一场盛大的派对上,所有人都穿着相同的衣服,那可就乱了套了。
可是如果每个人都有一个独特的标签,哎,那就清楚多了。
TMT的原理就是这样,给不同的样本加上不同的化学标签,然后再进行分析。
这样,无论你有多少个样本,都能一一分开,清清楚楚。
说到分析,哎呀,真是个“坑”。
在实验室里,科学家们可不是随便玩玩的。
搞这个的可得有一套高大上的设备,像是质谱仪,听起来就很厉害对吧?这些设备能够快速测量蛋白质的质量和数量,简直像是在高速公路上飞驰。
科学家们就像赛车手一样,精确地驾驶着这些设备,争分夺秒。
用TMT标记后,蛋白质的分析效率大大提高,真是“事半功倍”。
哎,说到这里,你可能会问,这样有什么用呢?其实TMT的应用可是相当广泛的。
在医学研究里,科学家们可以通过分析不同患者的蛋白质表达,找出疾病的潜在标志物。
这就像是给病症找到了“身份证”,为后续的治疗打下基础。
再比如,在农业研究中,TMT也能帮助科学家们分析作物的抗病性,培育出更优秀的品种。
这简直是农业界的“黑科技”呀。
TMT标记定量蛋白质组学也不是没有挑战。
想象一下,科学家们得处理海量的数据,有时候就像是在大海捞针。
数据分析的过程常常让人感到头疼,搞不好还得加班加点,简直是个“苦差事”。
不过,没关系,咱们的科学家们总能迎难而上,把问题一个个攻克,像是现代的“英雄”。
咱们还得提提TMT的优势,毕竟这技术可真不是盖的。
BioTeke 蛋白定量试剂使用说明书

蛋白定量试剂使用说明书【产品名称】产品通用名称:蛋白定量试剂产品商用名称:Bradford法蛋白定量试剂盒【包装规格】PP1101(250次)/PP1102(1000次)/PP1103(2500次)【检验原理】Bradford法蛋白浓度定量试剂盒是在世界上常用的蛋白浓度检测方法之一Bradford法基础上(考马斯亮蓝结合显色法)改进而成,当bradford染色液(考马斯亮蓝)和蛋白在酸性条件下结合时,最大吸光值波长立刻由465nm转移至595nm,同时颜色由褐色转为蓝色,通过测定吸光值大小并对照标准蛋白的吸光值,推算出蛋白浓度。
【产品特点】1.灵敏度高,检测浓度下限达到25μg/mL,最小检测蛋白量达到0.5μg,待测样品体积为1-20μL。
2.检测速度极快,10-20个样品只需不足10分钟即可完成。
3.在50-1000μg/mL浓度范围内有较好的线性关系。
【主要组成成分】试剂盒由蛋白标准(5mg/mL BSA),Bradford染色液,使用说明书和合格证组成。
主要组分及储存条件见表1。
表1试剂盒组成、储存试剂盒组成PP1101PP1102PP1103保存蛋白标准(5mg/mL0.25mL1mL 1.5mL×2-20℃BSA)Bradford染色液50mL200mL500mL4℃【储存条件及有效期】1、蛋白标准(5mg/mL BSA)在-20℃条件下保存;Bradford染色液在4℃条件下保存。
2、本产品有效期为一年,请在有效期内使用。
3、为了避免试剂长时间暴露于空气中发生挥发、氧化、pH值变化,各溶液使用后应及时盖紧盖子。
【使用方法】1.取10μL蛋白标准品(5mg/mL BSA)稀释至100μL,使终浓度为0.5mg/mL。
蛋白样品在什么溶液中,标准品也宜用什么溶液稀释。
但是为了简便起见,也可以用0.9%NaCl或PBS稀释标准品。
2.将稀释后标准品(0.5mg/mL BSA)按0,1,2,4,8,12,16,20μL分别加到酶标板中,加标准品稀释液将所有标准品补足到20μL。
thermo orbitrap tmt质谱方法详解

thermo orbitrap tmt质谱方法详解目录1. 引言1.1 背景和意义1.2 结构概述1.3 目的2. 正文2.1 Thermo Orbitrap技术介绍2.2 TMT质谱方法原理2.3 TMT质谱方法在蛋白质组学研究中的应用3. Thermo Orbitrap TMT质谱方法步骤详解3.1 样品处理与标记化学反应步骤3.2 质谱仪参数设置与数据采集步骤3.3 数据分析与结果解读步骤4. TMT质谱方法的优势和局限性探讨4.1 优势介绍4.2 局限性讨论5. 结论与展望5.1 结果总结5.2 研究前景展望及未来发展方向1. 引言1.1 背景和意义在蛋白质组学研究中,质谱技术是关键的分析手段之一。
随着科学技术的不断发展,各种高级质谱技术相继被引入,并取得了显著的进展。
其中,Thermo Orbitrap TMT(Tandem Mass Tagging)质谱方法作为一种先进的定量蛋白质组学方法,在生物医药领域得到了广泛应用。
1.2 结构概述Thermo Orbitrap TMT质谱方法结合了Thermo的精密仪器Orbitrap以及蛋白质组学中常用的TMT标记技术,具有高灵敏度、高精确性和高通量等特点。
该方法通过将不同样品中的蛋白质进行化学标记,并利用精密仪器进行定量分析,从而实现对多个样品中蛋白质的同时检测和定量。
1.3 目的本文旨在对Thermo Orbitrap TMT质谱方法进行详细介绍和解析,包括其原理、应用以及步骤详解。
通过对该方法的全面讲解,旨在帮助读者深入了解Thermo Orbitrap TMT质谱方法的工作原理和操作步骤,并掌握其在蛋白质组学研究中的应用价值。
(注:以上为文章“1. 引言”部分内容,使用了Markdown语言进行格式化)2. 正文2.1 Thermo Orbitrap技术介绍Thermo Orbitrap技术是一种高分辨质谱技术,由美国Thermo Fisher Scientific公司开发。
人的肌钙蛋白T(TNT)酶联免疫吸附测定试剂盒 说明书

Uscn Life Science Inc.Wuhan网址:电话:+862784259552传真:+862784259551E-mail:***************人的肌钙蛋白T(TnT)酶联免疫吸附测定试剂盒使用酶联免疫吸附测定试剂盒使用说明说明说明书书产品编号:E1820Hu规格:96T本试剂盒仅供体外研究使用,不用于临床诊断!预期应用本酶联免疫吸附测定试剂盒运用双抗体夹心ELISA 法定量测定人血清、血浆或其它相关生物液体中TnT 含量。
本试剂盒试剂盒未提供但需自备的设备及试剂未提供但需自备的设备及试剂1、450±10nm 滤光片的酶标仪(建议仪器使用前提前预热)2、单道和多道微量加液器及吸头3、稀释样品的EP 管4、蒸馏水或去离子水5、吸水纸6、盛放洗液的容器试剂盒的储存及有效期所有试剂均按试剂瓶标签上所示保存。
请注意,收到试剂盒后请尽快将标准品、检测溶液A 、检测溶液B 以及96孔板保存于-20。
开封后的酶标板要密封加干燥剂后保存于-20,避免潮湿。
有效期为6个月。
实验原理将TnT 抗体包被于96孔微孔板中,制成固相载体,向微孔中依次加入标准品和标本,其中的TnT 与连接于固相载体上的抗体结合,洗板之后加入生物素化的TnT 抗体,将未结合的生物素化抗体洗净后,加入HRP 标记的亲和素,再次彻底洗涤后加入底物(TMB)显色。
TMB在过氧化物酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的TnT呈正相关。
用酶标仪在450nm波长下测定吸光度(值),计算样品浓度。
标本的采集与与保存标本的采集1、血清:将收集于血清分离管的全血标本在室温放置30分钟或4过夜,然后1000g离心20分钟,取上清即可检测,或将上清置于-20或-80保存,但应避免反复冻融。
2、血浆:用EDTA或肝素作为抗凝剂采集标本,并将标本在采集后的30分钟内于2-81000g离心15分钟,取上清即可检测,或将上清置于-20或-80保存,但应避免反复冻融。
甲胎蛋白诊断试剂盒说明书

血清甲胎蛋白检测操作规程一.检验原理根据酶联免疫双抗体夹心法的作用原理,利用抗原、抗体反应的特异性与酶的高效催化性研制而成,其原理是将特异性抗体吸附到固相载体上。
加入待测的样品,与吸附于固相载体上的特异性抗体反应后,洗去未反应的样品,加入酶标记的特异性抗体与抗原反应,生成抗体-抗原-酶标抗体复合物,再加入底物和显色剂,生成可显色的产物,进行检测。
二.试剂盒组成96人份/盒:1.AFP标准品:5IU/ml、10IU/ml、20IU/ml、50IU/ml、100IU/ml、200IU/ml 各一瓶2.包被条96孔: AFP单抗;3.酶结合物(1号液)1瓶:酶标记单抗;4.洗液(2号液)1瓶:乳化剂OP;5.底物(3号液) 1瓶:过氧化脲;6.显色剂(4号液)1瓶:TMB;7.终止液1瓶:硫酸;8.说明书:1份不同批号的试剂不能混用。
三.储存条件及有效期本试剂盒在2-8℃保存,有效期为一年;打开包装后在4℃保存一个月。
四.适用仪器酶标仪、恒温培养箱、100µl移液器、1000µl移液器。
五.样本要求1.样本应为新鲜的血清。
2.溶血样本不可使用。
3.样本未能在24小时内使用时应-20℃保存。
4.样本使用前应恢复至室温。
5.禁止反复冻溶。
六.操作方法使用前试剂盒应恢复至室温,将冻干标准品各加入1ml蒸馏水,待完全溶解后与待测样品同时进行操作。
1.加样:将包被孔编号,每孔加标准品或待测样品各100μl。
2.孵育:37℃孵育,20分钟。
3.洗涤:在各孔中加入洗液2滴,轻轻摇动几下,弃之。
然后用蒸馏水或自来水加满各孔,甩掉,反复5次(洗涤要彻底,防止假阳性,但不可洗涤过猛过急而导致假阴性),最后在吸水纸上拍干。
4.加酶:每孔加酶结合物2滴(100μl)。
5.孵育:37℃孵育,15分钟。
6.洗涤:重复步骤“3”。
7.显色:加入底物1滴,随即加入显色剂1滴,轻轻摇动几下,室温反应2~5分钟。
8.终止:每孔加2滴(100μl)终止液。
TMT蛋白组学的具体步骤及方法

TMT蛋白组学的具体步骤及方法TMT(Tandem Mass Tag)是Thermo Fisher Scientific开发的一种多肽体外标记技术。
通过对不同样品中的肽段采用不同通道的TMT试剂进行标记,然后进行LC-MS/MS分析,实现样本蛋白组之间的相对定量。
技术流程TMT技术流程图技术原理TMT试剂盒每个通道的报告基团和平衡基团分子量总和是一致的,当肽段用TMT试剂标记后,来源于不同样本的同一序列的肽段的分子结构和分子量仍保持一致,它们在一级质谱中呈现为同一个信号,一起进入二级质谱分析阶段。
二级质谱中TMT试剂的平衡基团丢失,报告基团从肽段上碎裂下来,肽段自身序列也被碎裂。
肽段自身的碎片可用来定性分析肽段的序列组成。
报告基团的信号强度比值则能代表来源于不同样本的该肽段的含量比值。
TMT技术优势TMT试剂盒每个通道的报告基团和平衡基团分子量总和是一致的,当肽段用TMT试剂标记后,来源于不同样本的同一序列的肽段的分子结构和分子量仍保持一致,它们在一级质谱中呈现为同一个信号,一起进入二级质谱分析阶段。
因为标记后的肽段可以混合到一起,就可以将混合物一起进行色谱分级,然后再进行质谱分析,这样能有效增加鉴定数目。
粒成生物TMT技术流程:粒成生物TMT优势a.软硬件先进(仪器为Q Exactive,Q Exactive PLUS和Q Exactive HF-X,软件为PD2.2/Mascot);b.质控严格(预实验中每个样本都需要进行蛋白质水平和预质谱水平的质检,不合格的样本会给予合理建议。
正式实验中对标记效率和仪器状态也进行严格管控,发现不合格则重新开展实验,确保实验数据有效);c.专业的团队(团队成员经验丰富,能处理几乎所有类型的样本分析);d.强大的生信分析团队(除GO,KEGG等常规的分析外,还能进行IPA,趋势聚类,蛋白质相互作用以及其他个性化分析)。
粒成生物TMT成功案例:。
tmt蛋白组学试剂保存

tmt蛋白组学试剂保存TMT蛋白组学试剂保存TMT蛋白组学试剂是一种用于质谱分析的试剂,广泛应用于蛋白质组学研究领域。
在使用TMT蛋白组学试剂时,正确的保存方法可以确保试剂的稳定性和准确性,从而获得可靠的实验结果。
本文将介绍TMT蛋白组学试剂的保存方法及其重要性。
TMT蛋白组学试剂的保存温度是非常关键的。
一般来说,TMT蛋白组学试剂应保存在-20℃的冰箱中,避免长时间暴露于室温或高温环境中。
在保存过程中,要注意试剂瓶盖的密封性,确保试剂不受外界空气的影响。
TMT蛋白组学试剂应避免阳光直射。
阳光中的紫外线和可见光可以降解试剂的结构和性能,因此应将试剂存放在避光的环境中。
在实验室中,可以选择使用棕色或黑色的玻璃瓶来保存试剂,以阻挡光线的进入。
TMT蛋白组学试剂的保存时间也需要注意。
一般来说,TMT蛋白组学试剂的有效期一般为2年,但具体的有效期还是要根据试剂的具体规格和生产厂家的要求来确定。
在使用过程中,要及时查看试剂的有效期,避免使用过期试剂进行实验。
对于已经开封的TMT蛋白组学试剂,应尽量避免多次冻融。
多次冻融会导致试剂的活性和稳定性下降,影响实验结果的准确性。
因此,在使用过程中,要根据实验需要提前准备好所需的试剂量,避免频繁开封和冻融。
TMT蛋白组学试剂的保存还需要注意与其他试剂的隔离。
某些试剂可能对TMT试剂产生不利影响,例如酸性或碱性溶液、氧化剂等。
因此,在保存TMT试剂时,应与这些试剂分开存放,避免相互干扰。
TMT蛋白组学试剂在使用前应先进行充分的离心。
试剂瓶中的液体可能会出现沉淀或悬浮物,离心可以使试剂恢复均匀。
在取用试剂时,应使用洁净的移液器,避免污染试剂。
正确的保存TMT蛋白组学试剂对于获得可靠的实验结果非常重要。
通过遵循适当的保存方法,可以保证试剂的稳定性和准确性,提高实验的可重复性和可比性。
因此,在使用TMT蛋白组学试剂时,研究人员应严格按照上述的保存要求进行操作,以确保实验的成功和可靠性。
tmt蛋白组学试剂保存

tmt蛋白组学试剂保存TMT蛋白组学试剂保存随着科学技术的不断发展,蛋白质组学研究在生命科学领域扮演着越来越重要的角色。
蛋白质组学试剂的选择与保存对于研究结果的准确性和可靠性至关重要。
本文将重点介绍一种常用的蛋白质组学试剂——TMT蛋白组学试剂的保存方法。
TMT(Tandem Mass Tag)蛋白组学试剂是一种用于蛋白质组学研究的化学试剂。
它通过标记蛋白质样品中的不同肽段,实现对不同样品之间的定量比较。
TMT蛋白组学试剂的保存对于后续实验的成功进行至关重要。
TMT蛋白组学试剂应该保存在干燥、阴凉的地方,避免阳光直射。
高温和湿度会导致试剂的降解和失效,因此应该避免暴露在高温环境下。
同时,也需要避免试剂与空气中的水分接触,因为水分会导致试剂的水解和氧化。
TMT蛋白组学试剂应该保存在密封的容器中。
使用试剂前,应该先将试剂从冰箱中取出,并迅速封好。
保持试剂密封可以避免试剂受到空气中的污染物的影响,确保试剂的稳定性和活性。
TMT蛋白组学试剂的保存温度也是需要注意的。
一般来说,TMT蛋白组学试剂应该保存在-20℃的冰箱中。
在长时间保存时,可以考虑将试剂保存在更低的温度下,如-80℃。
冷冻保存可以有效延长试剂的保质期,并避免试剂的降解和失活。
在使用TMT蛋白组学试剂前,还需要注意试剂的有效期。
试剂的有效期是指在规定条件下,试剂可以保持稳定性和活性的时间。
过期的试剂可能会导致实验结果的偏差和不可靠性,因此在使用前务必检查试剂的有效期,并及时更新试剂。
除了以上保存方法外,还有一些其他值得注意的事项。
首先,使用TMT蛋白组学试剂前应该先阅读相关的说明书和操作手册,确保正确使用试剂。
其次,使用试剂时应该注意避免与其他化学物质的接触,以免产生不必要的化学反应。
最后,在使用试剂后应该及时清理试剂容器和实验台面,保持实验环境的整洁和安全。
TMT蛋白组学试剂的保存对于蛋白质组学研究的准确性和可靠性至关重要。
正确的保存方法包括干燥、阴凉的环境、密封的容器、适宜的保存温度和注意试剂的有效期。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
INSTRUCTIONSTMT Mass Tagging Kits and90060 TMTduplex Isotopic Label Reagent Set, sufficient reagents for 5 duplex isotopic experiments Contents:TMT0 Label Reagent, 5 × 0.8mgTMT6-127 Label Reagent, 5 × 0.8mg90061 TMTsixplex Isobaric Label Reagent Set, sufficient reagents for 1 sixplex isobaric experiment Contents:TMT6-126 Label Reagent, 1 × 0.8mgTMT6-127 Label Reagent, 1 × 0.8mgTMT6-128 Label Reagent, 1 × 0.8mgTMT6-129 Label Reagent, 1 × 0.8mgTMT6-130 Label Reagent, 1 × 0.8mgTMT6-131 Label Reagent, 1 × 0.8mg90062 TMTsixplex Isobaric Label Reagent Set, sufficient reagents for 2 sixplex isobaric experiments Contents:TMT6-126 Label Reagent, 2 × 0.8mgTMT6-127 Label Reagent, 2 × 0.8mgTMT6-128 Label Reagent, 2 × 0.8mgTMT6-129 Label Reagent, 2 × 0.8mgTMT6-130 Label Reagent, 2 × 0.8mgTMT6-131 Label Reagent, 2 × 0.8mg90063TMTduplex Isobaric Mass Tagging Kit, sufficient reagents for 5 duplex isobaric experiments Contents:TMT0 Label Reagent, 5 × 0.8mgTMT2-126 Label Reagent, 5 × 0.8mgTMT2-127 Label Reagent, 5 × 0.8mgDissolution Buffer (1 M triethyl ammonium bicarbonate), 5mLDenaturing Reagent (10% SDS), 1mLReducing Reagent (0.5M TCEP), 1mLIodoacetamide, 12 × 9mgQuenching Reagent (50% hydroxylamine), 1mLPierce™Trypsin Protease, MS Grade, 2 × 20µgTrypsin Storage Solution, 250µLAlbumin, Bovine, 2.5mg90064TMTsixplex Isobaric Mass Tagging Kit, sufficient reagents for 5 sixplex isobaric experiments Contents:TMT0 Label Reagent, 5 × 0.8mgTMT6-126 Label Reagent, 5 × 0.8mgTMT6-127 Label Reagent, 5 × 0.8mgTMT6-128 Label Reagent, 5 × 0.8mgTMT6-129 Label Reagent, 5 × 0.8mgTMT6-130 Label Reagent, 5 × 0.8mgTMT6-131 Label Reagent, 5 × 0.8mgDissolution Buffer (1M triethyl ammonium bicarbonate), 5mLDenaturing Reagent (10% SDS), 1mLReducing Reagent (0.5 M TCEP), 1mLIodoacetamide, 12 × 9mgQuenching Reagent (50% hydroxylamine), 1mLPierce Trypsin Protease, MS Grade, 5 × 20µgTrypsin Storage Solution, 250µLAlbumin, Bovine, 2.5mg90065TMTduplex Isobaric Label Reagent Set, sufficient reagents for 5 duplex isobaric experiments Contents:TMT2-126 Label Reagent, 5 × 0.8mgTMT2-127 Label Reagent, 5 × 0.8mg90066TMTsixplex Label Reagent Set, sufficient reagents for 5 sixplex isobaric experimentsContents:TMT6-126 Label Reagent, 5 × 0.8mgTMT6-127 Label Reagent, 5 × 0.8mgTMT6-128 Label Reagent, 5 × 0.8mgTMT6-129 Label Reagent, 5 × 0.8mgTMT6-130 Label Reagent, 5 × 0.8mgTMT6-131 Label Reagent, 5 × 0.8mg90067TMTzero Label Reagent, 5 × 0.8mg, sufficient reagents for 5 samples90068TMTsixplex Label Reagent Set, sufficient reagents for 12 sixplex isobaric experimentsContents:TMT6-126 Label Reagent, 2 × 5mgTMT6-127 Label Reagent, 2 × 5mgTMT6-128 Label Reagent, 2 × 5mgTMT6-129 Label Reagent, 2 × 5mgTMT6-130 Label Reagent, 2 × 5mgTMT6-131 Label Reagent, 2 × 5mgStorage: Upon receipt store at -20°C. Reagents are shipped with dry ice.Note: These products are for research use only − do not use for diagnostic procedures.ContentsIntroduction (3)Procedure Summary (4)Important Product Information (4)Additional Materials Required (4)Material Preparation (5)Preparing and Labeling Peptides with the TMT Isobaric Mass Tags (5)Troubleshooting (6)Additional Information (6)A.Data Acquisition Methods (6)B.Data Analysis and Quantitation (7)rmation Available from our Website (8)Related Thermo Scientific Products (8)General References (8)IntroductionThe Thermo Scientific™ TMT™ Isobaric Mass Tagging Kits and Reagents enable multiplex relative quantitation by mass spectrometry (MS). Each mass-tagging reagent within a set has the same nominal mass (i.e., isobaric) and chemical structure composed of an amine-reactive NHS-ester group, a spacer arm and an MS/MS reporter (Figure 1). The reagent sets can be used to label two or six peptide samples prepared from cells or tissues. For each sample, a unique reporter in the low mass region of the MS/MS spectrum (i.e., 126-127Da for TMT2 and 126-131Da for TMT6 Isobaric Label Reagents) is used to measure relative protein expression levels during peptide fragmentation.The TMTduplex™ Isotopic Label Reagent Set contains TMTzero™ and one of the TMTsixplex™ Reagents (TMT6-127) to be used as “light” and “heavy” tags for MS-level peptide quantitation similar to duplex isotopic metabolic labeling (e.g., SILAC) or isotopic dimethylation labeling. These isotopic pairs can also be used in targeted quantitation strategies, including selective reaction monitoring (SRM, see the Additional Information Section). Advantages of the TMTduplex and TMTsixplex Isobaric Label Reagents include increased sample multiplexing for relative quantitation, increased sample throughput and fewer missing quantitative channels among samples.Figure 1.Chemical structure of the TMTLabel Reagents. A. Functional regions of thereagent structure, including MS/MSfragmentation sites by higher energy collisiondissociation (HCD) and electron transferdissociation (ETD). B. TMTduplex Reagentstructures and isotope positions (*); only HCDdifferentiates between these two reporters.C. TMTsixplex Reagent structures and isotopepositions (*).Procedure SummaryProtein extracts isolated from cells or tissues are reduced, alkylated and digested overnight. Samples are labeled with the TMT Reagents and then mixed before sample fractionation and clean-up. Labeled samples are analyzed by high resolutionOrbitrap LC-MS/MS before data analysis to identify peptides and quantify reporter ion relative abundance (Figure 2).Figure 2. Schematic for using the Thermo Scientific TMTsixplex Isobaric Mass Tagging Reagents.Important Product Information• The TMT Reagents are moisture-sensitive. To avoid moisture condensation onto the product, vial must be equilibrated to room temperature before opening.•Anhydrous acetonitrile is the recommended solvent to dissolve reagents. Stock solutions are stable for one week when stored at -20°C. For long term storage of unused reagent, remove all solvent by drying and store with desiccant at -20°C. Anhydrous ethanol can be used as an alternative solvent to dissolve reagents but is not recommended for stock solution storage.• The TMT Reagents are amine-reactive and modify lysine residues and the peptide N-termini. All amine-containing buffers and additives must be removed before digestion and labeling.• All samples must be digested, labeled and then mixed equally before desalting, fractionation and LC-MS/MS. For optimal results, use 25-100µg of peptide for each labeling reaction.• To avoid contamination of MS samples, always wear gloves when handling samples and gels. Use ultrapure MS-grade reagents. Perform sample preparation in a cleaned work area.• The TMTzero Label Reagent can be used to optimize methods before multiplexed analysis of samples with the TMTduplex or TMTsixplex Reagent Set.Additional Materials Required• Microcentrifuge tubes• Anhydrous acetonitrile (Thermo Scientific™ Acetonitrile HPLC grade, Product No. 51101) • Water, LC-MS Grade (Product No. 51140) • Chilled (-20°C) acetone• Protein assay (e.g., Thermo Scientific™ BCA Protein Assay Kit, Product No. 22235) • 75-300µm capillary C 18 reversed-phase column• High-resolution Orbitrap Mass Spectrometer, ion trap or time-of-flight (TOF) mass spectrometer with online or offline liquid chromatography (LC) system• Data analysis software such as Thermo Scientific™ Proteome Discoverer™ or Mascot™ Software (Matrix Science, Ltd.)• Optional: C18 spin tips or columns (e.g., Thermo Scientific™ Pierce™ C18 Spin Columns, Product No. 89870 or Pierce™ C18 Tips, Product No. 87784)Material PreparationNote: The 50% hydroxylamine and 10% SDS stock solutions provided with the kit may precipitate during storage. Warm both solutions to room temperature and vortex before use. The amounts listed below are sufficient for preparing and labeling 6 samples.Add 500µL of the Dissolution Buffer (1M TEAB) to 4.5mL of ultrapure water.100mM TEAB (triethylammonium bicarbonate)Lysis Buffer Add 200µL of the Denaturing Reagent (10% SDS) to 1.8mL of 100mM TEAB.200mM TCEP Add 70µL of the Reducing Reagent (0.5M TCEP) to 70µL of ultrapure water. Then add 35µL of the Dissolution Buffer (1M TEAB).5% Hydroxylamine Add 50µL of the Quenching Reagent (50% hydroxylamine) to 450µL of 100mM TEAB. Preparing and Labeling Peptides with the TMT Isobaric Mass TagsNote: BSA can be used as a control sample for method optimization. Dissolve BSA to 1mg/mL using 100mM TEAB. Use 25-100µg of protein per labeling reaction. The Thermo Scientific™ Pierce™ Mass Spec Sample Prep Kit for Cultured Cells can also be used to prepare peptide digests for TMT reagent labeling.A.Preparing Whole Cell Protein Extracts1.Culture cells to harvest at least 100µg of protein per condition. For best results, culture a minimum of 2 × 106 cells.Note: Rinse cells 2-3 times with 1X PBS to remove cell culture media. Pellet cells using low-speed centrifugation(i.e., < 1000 × g) to prevent premature cell lysis.2.Lyse the cells by adding five cell-pellet volumes of Lysis Buffer (i.e., 100μL of Lysis Buffer for a 20μL cell pellet).Note: Lysis buffers such as 8M urea (Product No. 29700) in 50mM TEAB or HEPES buffer, pH 8 may be used as alternative denaturing cell lysis buffers. For urea-based lysis buffer, protein samples must be diluted to < 1M urea before digestion, and the final C18 desalting step (C.6) is not optional. Addition of protease and/or phosphatase inhibitors during lysis is optional and may interfere with MS analysis.Note: Depending on the Lysis Buffer used it may be necessary to reduce sample viscosity by shearing DNA using a microtip sonicator or addition of a nuclease (e.g., Thermo Scientific™ Pierce™ Universal Nuclease for Cell Lysis, Product No. 88700)3.Centrifuge lysate at 16,000 × g for 10 minutes at 4°C.4.Carefully separate the supernatant and transfer into a new tube.5.Determine the protein concentration of the supernatant using established methods such as the BCA Protein Assay Kit(Product No. 23227).Note: Use samples at ≥ 2mg/mL. Less concentrated samples may be used; however, it might be necessary to use larger volumes of reducing/alkylating reagents.6.Transfer 100µg per condition (two for the TMTduplex or six for the TMTsixplex Label Reagents) into a newmicrocentrifuge tube and adjust to a final volume of 100µL with 100mM TEAB.7.Add 5µL of the 200mM TCEP and incubate sample at 55°C for 1 hour.8.Immediately before use, dissolve one tube of iodoacetamide (9mg) with 132µL of 100mM TEAB to make375mM iodoacetamide. Protect solution from light.9.Add 5µL of the 375mM iodoacetamide to the sample and incubate for 30 minutes protected from light at roomtemperature.10.Add six volumes (~600µL) of pre-chilled (-20°C) acetone and freeze at -20°C. Allow the precipitation to proceed for atleast 4 hours up to overnight.Note: Methanol/chloroform is the recommended solvent for precipitation of proteins derived from tissue extracts.11.Centrifuge the samples at 8000 ×g for 10 minutes at 4°C. Carefully invert the tubes to decant the acetone withoutdisturbing the white pellet. Allow the pellet to dry for 2-3 minutes.B.Protein Digestion1.Resuspend 100µg of acetone-precipitated (or lyophilized) protein pellets with 100µL of 50mM TEAB.Note: An acetone-precipitated pellet might not completely dissolve; however, after proteolysis at 37°C, all the protein (peptides) will be solubilized.2.Immediately before use, add 20µL of the Trypsin Storage Solution to the bottom of the trypsin glass vial and incubate for5 minutes. Store any remaining reagent in single-use volumes at -80°C (e.g., 2.5µg of trypsin per 100µg of protein).3.Add 2.5µL of trypsin (i.e., 2.5µg) per 100µg of protein. Digest the sample overnight at 37°C.C. Peptide Labeling1.Immediately before use, equilibrate the TMT Label Reagents to room temperature. For the 0.8mg vials, add 41µL ofanhydrous acetonitrile to each tube. For the 5mg vials, add 256µL of solvent to each tube. Allow the reagent to dissolve for 5 minutes with occasional vortexing. Briefly centrifuge the tube to gather the solution.Note: Reagents dissolved in anhydrous acetonitrile are stable for one week when stored at -20°C. Anhydrous ethanol can be used as an alternative solvent to dissolve reagents but is not recommended for stock solution storage.2.Optional: Measure protein digest concentration using Thermo Scientific™ Pierce™ Quantitative Fluorescent PeptideAssay (Product No. 23290) or Thermo Scientific™ Pierce™ Quantitative Colorimetric Peptide Assay (Product No.23275).3.Carefully add 41µL of the TMT Label Reagent to each 100µL sample (25-100µg protein digest). Alternatively, transferthe reduced and alkylated protein digest to the TMT Reagent vial.4.Note: Labeling more than 100µg of protein digest per reaction requires additional TMT Label Reagent.5.Incubate the reaction for 1 hour at room temperature.6.Add 8µL of 5% hydroxylamine to the sample and incubate for 15 minutes to quench the reaction.bine samples at equal amounts in new microcentrifuge tube and store at -80°C.Note: TMT-labeled peptide concentration can be measured using Thermo Scientific™ Pierce™ QuantitativeColorimetric Peptide Assay. The Thermo Scientific™ Pierce™ Quantitative Fluorescent Peptide Assay cannot be used to measure TMT-labeled peptide concentrations.8.Optional: Clean-up samples with C18 spin tips (Product No. 87784) or columns (Product No. 89870)before LC-MSanalysis. Peptide clean up is recommended before LC-MS analysis but is not required. Fractionation of labeled peptides using Thermo Scientific™ Pierce™ High pH Reversed-Phase Peptide Fractionation Kit (Product No. 84868) isrecommended before LC-MS analysis to increase the number of peptide identifications.TroubleshootingProblem Possible Cause SolutionPoor labeling An amine-based buffer was used Use a non-amine-based bufferIncorrect buffer pH Make sure the buffer pH is ~8.0Too much sample was used Label 25-100µg per sampleProtein precipitation Lack of detergent present Add detergent, such as 0.05% SDS to the preparationpH decreased Make sure the pH is > 7.5Additional InformationA.Data Acquisition MethodsQuantitation of peptides labeled with Thermo Scientific™ Tandem Mass Tag™ Reagents requires a mass spectrometer capable of MS/MS fragmentation, such as an ion trap, quadrupole time of flight, time of flight-time of flight (TOF-TOF) or triple quadrupole instrument. Higher energy collision dissociation (HCD) is recommended for TMT reporter ion fragmentation. Optimal HCD fragmentation energy is instrument-dependent and can be optimized using TMTzero Reagents.Electron transfer dissociation (ETD) may be used as an alternative fragmentation method for peptide identification and quantitation. The choice of MS/MS fragmentation method(s) depends on the instrument capabilities such as collisionally induced dissociation (CID), pulsed-Q dissociation (PQD), higher energy collisional dissociation (HCD), or electron transfer dissociation (ETD). TMT Reagent reporter ions are not visible in ion traps following traditional CID fragmentation.Table 1. Instruments and MS/MS fragmentation options for peptide identification and quantitation withThermo Scientific TMT Reagents.Instrument Fragmentation Method Reference(s)Thermo Scientific Orbitrap™ Fusion™ Tribrid™ Mass Spectrometer HCD/SPS-MS3 McAllister, G.C., et al. (2014), Viner,et al. (2013)Thermo Scientific Orbitrap Elite™ Mass Spectrometer HCD/MS3 McAllister, G.C., et al. (2012), Viner,et al. (2012)Thermo Scientific Q Exactive™ MassSpectrometerHCD/MS2 Wühr, et al. (2012)Thermo Scientific Orbitrap Velos Pro™, LTQ-Orbitrap™ XL, or MALDI-Orbitrap™ XL Mass Spectrometer HCD/MS2 Ting, et al. (2011), Wenger, et al(2011), Schirle, et al. (2012), Lee, etal (2011), Xiong, et al. (2011),Strupat, et al. (2008)Thermo Scientific™ Velos Pro™ ion trap Trap HCD/MS2 Biringer, et al. (2011)Thermo Scientific Orbitrap Elite ETD, Velos Pro ETD, LTQ-OrbitrapXL ETD HCD/MS2 orETD/MS2Viner, et al. (2009)Q-TOF CID Van Ulsen, et al. (2009)TOF-TOF CID Dayon, et al. (2008)Triple Quadrupole CID/SRM Stella, et al (2011), Byers, et al.(2009)B.Data Analysis and QuantitationThe masses for peptide modification by the TMT zero, duplex, and sixplex reagents are present in the UNIMOD database () and are listed below. Several software packages directly support the modifications by TMT Reagents and the relative quantitation of reporter ions released from labeled peptides, including Thermo Scientific™ Proteome Discoverer™ 1.1 and above, Matrix Science Mascot™ 2.1 and above, and Proteome Software Scaffold™ Q+. For data acquired using a combination of fragmentation methods (i.e., HCD/MS3 or HCD/ETD), Proteome Discoverer may be necessary to merge spectra for identification and quantitation.Table 2. Modification masses of the Thermo Scientific TMT Label Reagents.Label ReagentReagentReporter IonModificationMass(monoisotopic)ModificationMass(average)HCDMonoisotopicReporter Mass*ETDMonoisotopicReporter Mass**TMT0-126 126 224.152478 224.2994 126.127726 114.127725TMT2-126 126 225.155833 225.2921 126.127726 114.127725TMT2-127 127C 225.155833 225.2921 127.131081 114.127725TMT6-126 126 229.162932 229.2634 126.127726 114.127725TMT6-127 127N 229.162932 229.2634 127.124761 115.124760TMT6-128 128C 229.162932 229.2634 128.134436 116.134433TMT6-129 129N 229.162932 229.2634 129.131471 117.131468TMT6-130 130C 229.162932 229.2634 130.141145 118.141141TMT6-131 131 229.162932 229.2634 131.138180 119.138176 * HCD is a collisional fragmentation method that generates six unique reporter ions from 126 to 131Da.**ETD is a non-ergodic fragmentation method that generates six unique reporter ions from 114 to 119Da.rmation Available from our Website•Tech Tip Protocol #49: Acetone precipitation of proteins•Tech Tip Protocol #19: Remove detergent from protein samplesRelated Thermo Scientific Products90110 TMT10plex™ Isobaric Label Reagent Set, 10 × 0.8mg90113 TMT10plex Isobaric Mass Tag Labeling Kit90406 TMT10plex Isobaric Label Reagent Set, 10 × 5mg90114 1M Triethylammonium bicarbonate (TEAB), 50mL90115 50% Hydroxylamine, 5mL90100 iodoTMTzero™ Label Reagent, 5 × 0.2mg90101 iodoTMTsixplex™ Label Reagent Set, 1 × 0.2mg90103 iodoTMTsixplex Isobaric Mass Tag Labeling Kit90076 Immobilized Anti-TMT Antibody Resin90075 Anti-TMT Antibody, 0.1mL90104 TMT Elution Buffer, 20mL84840 Pierce™ Mass Spec Sample Prep Kit for Cultured Cells23227 BCA Protein Assay Kit23275 Pierce Quantitative Colorimetric Peptide Assay23290 Pierce Quantitative Fluorescent Peptide Assay90057 Pierce Trypsin Protease, MS Grade90051 Lys-C Protease, MS Grade88300 Fe-NTA Phosphopeptide Enrichment Kit88301 Pierce TiO2 Phosphopeptide Enrichment and Clean-up Kit84868 Pierce High pH Reversed-Phase Peptide Fractionation Kit88321 Pierce Peptide Retention Time Calibration Mixture, 200µL87784 Pierce C18 Tips, 100µL bed, 96 tips89870 Pierce C18 Spin Columns, 25 columns28904 Trifluoroacetic Acid, Sequanal GradeGeneral ReferencesAltelaar A.F., et al. (2012). Benchmarking stable isotope labeling based quantitative proteomics. J Proteomics Oct 22. pii: S1874-3919(12)00704-X.doi: 10.1016/j.jprot.2012.10.009.Bantscheff, M., et al. (2008). Robust and sensitive iTRAQ quantification on an LTQ Orbitrap Mass Spectrometer. Mol Cell Proteomics7:1702-13.Biringer, R.G., et al. (2011). Quantitation of TMT-Labeled Peptides Using Higher-Energy Collisional Dissociation on the Velos Pro Ion Trap Mass Spectrometer. Application note # 520. Byers, H.L. (2009). Candidate verification of iron-regulated Neisseria meningitidis proteins using isotopic versions of tandem mass tags (TMT) and single reaction monitoring, J Prot73(2):231-9.Dayon, L., et al. (2008). Relative quantification of proteins in human cerebrospinal fluids by MS/MS using 6-plex isobaric tags. Anal Chem80(8):2921-31. Dillon, R, et al. (2011). Discovery of a Novel B-Raf Fusion Protein Related to c-Met Drug Resistance. J Proteome Res10(11):5084-94.Erikson, B.K., et al. (2015). Evaluating multiplexed quantitative phosphopeptide analysis on a hybrid quadrupole mass filter/linear ion trap/orbitrap mass spectrometer. Anal Chem87(2):1241-9.Keshishian, H., et al. (2015). Multiplexed, quantitative workflow for sensitive biomarker discovery in plasma yields novel candidates for early myocardial injury. Mol Cell Proteomics. 2015 Feb 27. pii: mcp.M114.046813Lee, M.V., et al. (2011). A dynamic model of proteome changes reveals new roles for transcript alteration in yeast. Mol Syst Biol 7:514.McAllister, G.C., et al. (2014). MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal Chem86(14):7150-8.McAllister, G.C., et al. (2012). Increasing the multiplexing capacity of TMTs using reporter ion isotopologues with isobaric masses. Anal Chem 84(17):7469-78.Murphy, J.P., et al. (2014). Combining amine metabolomics and quantitative proteomics of cancer cells using derivatization with isobaric tags. Proteomics 86(7):3585-93.Paulo, J.A., et al. (2014). A comprehensive proteomic and phosphoproteomic analysis of yeast deletion mutants of 14-3-3 orthologs and associated effects of rapamycin. Nature(2-3):474-86.Ross, P.L., et al. (2004). Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics 3(12):1154-69.Savitski, M.M., et al. (2014). Tracking cancer drugs in living cells by thermal profiling of the proteome. Science346(6205):1255784Schirle, M., et al. (2012). Kinase inhibitor profiling using chemoproteomics. Methods Mol Biol795:161-77.Schwartz, J. et al. (2008). Relative quantitation of protein digests using tandem mass tags and pulsed-Q dissociation (PQD). Application note # 452.Stella, R., et al. (2011). Relative Quantification of Membrane Proteins in Wild-type and PrP-knockout Cerebellar Granule Neurons. J Proteome Res doi: 10.1021/pr200759m. Strupat K., et al. (2008). Accurate MS and MSn Analysis with the Thermo Scientific MALDI LTQ Orbitrap. Application note # 30150.Ting, L., et al. (2011). MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nature Methods8: 937–940.Van Ulsen, P., et al. (2009). Identification of proteins of Neisseria meningitidis induced under iron-limiting conditions using the isobaric tandem mass tag (TMT) labeling approach. Proteomics9(7):1771-81.Viner, R.I., et al. (2013). Increasing the multiplexing of protein quantitation from 6- to 10-Plex with reporter ion isotopologues.PN_ASMS_W617_RViner_R1.Viner, R.I., et al. (2012). Relative quantitation of TMT-labeled proteomes – Focus on sensitivity and precision. Application note #566.Viner, R.I., et al. (2009). Quantification of post-translationally modified peptides of bovine α-crystallin using tandem mass tags and electron transfer dissociation. J Proteomics72(5):874-85.Wenger, C.D., et al. (2011). Gas-phase purification enables accurate, multiplexed proteome quantification with isobaric tagging. Nat Methods 8(11):933-5. Xiong, L., et al. (2011). Mass spectrometric studies on epigenetic interaction networks in cell differentiation. J Biol Chem 286(15):13657-68.Zhang, T., et al. (2010). Improving quantitation of TMT-labeled peptides using stepped higher-energy collisional dissociation. Application note # 483 Products are warranted to operate or perform substantially in conformance with published Product specifications in effect at the time of sale, as set forth in the Product documentation, specifications and/or accompanying package inserts (“Documentation”). No claim of suitability for use in applications regulated by FDA is made. The warranty provided herein is valid only when used by properly trained individuals. Unless otherwise stated in the Documentation, this warranty is limited to one year from date of shipment when the Product is subjected to normal, proper and intended usage. This warranty does not extend to anyone other than Buyer. Any model or sample furnished to Buyer is merely illustrative of the general type and quality of goods and does not represent that any Product will conform to such model or sample.NO OTHER WARRANTIES, EXPRESS OR IMPLIED, ARE GRANTED, INCLUDING WITHOUT LIMITATION, IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR ANY PARTICULAR PURPOSE, OR NON INFRINGEMENT. BUYER’S EXCLUSIVE REMEDY FOR NON-CONFORMING PRODUCTS DURING THE WARRANTY PERIOD IS LIMITED TO REPAIR, REPLACEMENT OF OR REFUND FOR THE NON-CONFORMING PRODUCT(S) AT SELLER’S SOLE OPTION. THERE IS NO OBLIGATION TO REPAIR, REPLACE OR REFUND FOR PRODUCTS AS THE RESULT OF (I) ACCIDENT, DISASTER OR EVENT OF FORCE MAJEURE, (II) MISUSE, FAULT OR NEGLIGENCE OF OR BY BUYER, (III) USE OF THE PRODUCTS IN A MANNER FOR WHICH THEY WERE NOT DESIGNED, OR (IV) IMPROPER STORAGE AND HANDLING OF THE PRODUCTS.Unless otherwise expressly stated on the Product or in the documentation accompanying the Product, the Product is intended for research only and is not to be used for any other purpose, including without limitation, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses, or any type of consumption by or application to humans or animals.Current product instructions are available at . For a faxed copy, call 800-874-3723 or contact your local distributor.© 2016 Thermo Fisher Scientific Inc. All rights reserved. Tandem Mass Tag and TMT are trademarks of Proteome Sciences plc. iTRAQ is a trademark of AB Sciex Pte. Ltd. Mascot is a trademark of Matrix Science. Scaffold is a trademark of Proteome Software. Unless otherwise indicated, all other trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries. Printed in the USA.。
