BIBR 1532_321674-73-1_CoA_MedChemExpress
影响因子_Chem2012
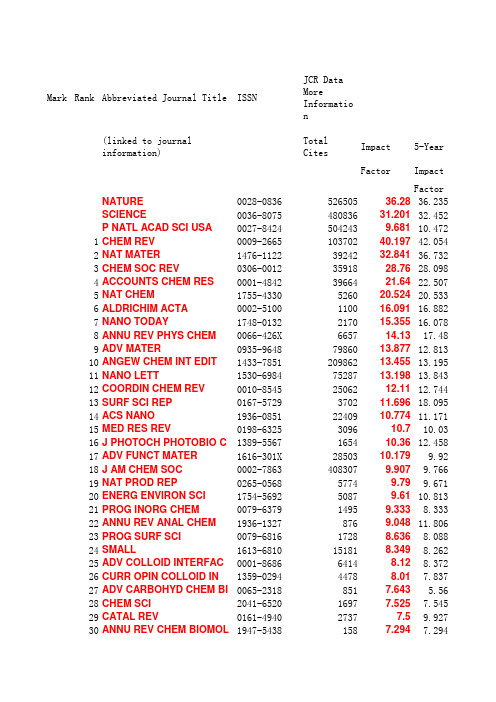
Mark Rank Abbreviated Journal Title ISSN JCR Data More Information (linked to journal information)Total Cites Impact5-YearFactor ImpactFactor NATURE0028-0836********.2836.235SCIENCE0036-807548083631.20132.452P NATL ACAD SCI USA0027-84245042439.68110.4721CHEM REV0009-266510370240.19742.0542NAT MATER1476-11223924232.84136.7323CHEM SOC REV0306-00123591828.7628.0984ACCOUNTS CHEM RES0001-48423966421.6422.5075NAT CHEM1755-4330526020.52420.5336ALDRICHIM ACTA0002-5100110016.09116.8827NANO TODAY1748-0132217015.35516.0788ANNU REV PHYS CHEM0066-426X665714.1317.489ADV MATER0935-96487986013.87712.81310ANGEW CHEM INT EDIT1433-785120986213.45513.19511NANO LETT1530-69847528713.19813.84312COORDIN CHEM REV0010-85452506212.1112.74413SURF SCI REP0167-5729370211.69618.09514ACS NANO1936-0851*******.77411.17115MED RES REV0198-6325309610.710.0316J PHOTOCH PHOTOBIO C1389-5567165410.3612.45817ADV FUNCT MATER1616-301X2850310.1799.9218J AM CHEM SOC0002-78634083079.9079.76619NAT PROD REP0265-056857749.799.67120ENERG ENVIRON SCI1754-569250879.6110.81321PROG INORG CHEM0079-637914959.3338.33322ANNU REV ANAL CHEM1936-13278769.04811.80623PROG SURF SCI0079-681617288.6368.08824SMALL1613-6810151818.3498.26225ADV COLLOID INTERFAC0001-868664148.128.37226CURR OPIN COLLOID IN1359-029444788.017.83727ADV CARBOHYD CHEM BI0065-23188517.643 5.5628CHEM SCI2041-652016977.5257.54529CATAL REV0161-494027377.59.92730ANNU REV CHEM BIOMOL1947-54381587.2947.29431CHEM MATER0897-4756699267.286 6.94932ADV ORGANOMET CHEM0065-30559517 6.63633NANO RES1998-01242017 6.977.46134CHEMSUSCHEM1864-56313040 6.8277.17135GREEN CHEM1463-926212023 6.32 6.76136TRAC-TREND ANAL CHEM0165-99366620 6.273 6.87337J PHYS CHEM LETT1948-71854695 6.213 6.217 38CHEM COMMUN1359-7345105562 6.169 6.082 39ADV SYNTH CATAL1615-415014629 6.048 5.904 40J CATAL0021-951733570 6.002 6.201 41J MATER CHEM0959-942846166 5.968 5.992 42INT REV PHYS CHEM0144-235X1533 5.967 6.977 43CHEM-EUR J0947-653954127 5.925 5.866 44NANOSCALE2040-33643187 5.914 5.914 45ORG LETT1523-706068838 5.862 5.558 46ANAL CHEM0003-270095262 5.856 5.983 47J CONTROL RELEASE0168-365926071 5.7327.112 48LAB CHIP1473-019713729 5.67 6.497 49APPL CATAL B-ENVIRON0926-337320525 5.625 6.052 50BIOSENS BIOELECTRON0956-566320029 5.602 5.637 51BIOMACROMOLECULES1525-779721280 5.479 5.646 52CARBON0008-622327798 5.378 6.008 53J MED CHEM0022-262356481 5.248 5.321 54J CHEM THEORY COMPUT1549-96188327 5.215 5.673 55PROG NUCL MAG RES SP0079-65651765 5.214 6.296 56CHEMCATCHEM1867-38801502 5.207 5.207 57FARADAY DISCUSS1364-549853725 4.687 58BIOCONJUGATE CHEM1043-180213657 4.93 5.224 59CURR MED CHEM0929-867311387 4.859 4.806 60J PHYS CHEM C1932-744760782 4.805 5.049 61CRYST GROWTH DES1528-748319082 4.72 4.877 62J CHEM INF MODEL1549-959611209 4.675 4.305 63INORG CHEM0020-166982190 4.601 4.553 64J COMPUT CHEM0192-865122334 4.583 4.795 65ANAL CHIM ACTA0003-267038343 4.555 4.144 66J CHROMATOGR A0021-967360179 4.531 4.362 67CHEM-ASIAN J1861-47284472 4.5 4.461 67ELECTROANAL CHEM0070-9778451 4.56 69J ORG CHEM0022-326398614 4.45 4.204 70SOFT MATTER1744-683X10988 4.39 4.998 71CHEM REC1527-89991256 4.377 4.161 72ANALYST0003-265414243 4.23 4.119 73PROG SOLID STATE CH0079-67861516 4.188 3.551 74LANGMUIR0743-7463103776 4.186 4.514 75CURR TOP MED CHEM1568-02664646 4.174 4.417 76PHARM RES-DORDR0724-874117160 4.093 4.668 77INT J HYDROGEN ENERG0360-319926939 4.054 4.402 78J AM SOC MASS SPECTR1044-03058794 4.002 3.746 79ORGANOMETALLICS0276-733339562 3.963 3.78680CHEMBIOCHEM1439-42279059 3.944 3.719 81APPL CATAL A-GEN0926-860X26533 3.903 3.961 82CRIT REV ANAL CHEM1040-8347849 3.902 3.956 83SENSOR ACTUAT B-CHEM0925-400527323 3.898 3.751 84MAR DRUGS1660-33971161 3.854 3.704 85CRYSTENGCOMM1466-80339036 3.842 4.023 86DALTON T1477-922637605 3.838 3.887 87TALANTA0039-914024981 3.794 3.747 88CHEM RES TOXICOL0893-228X10444 3.779 3.969 89ANAL BIOANAL CHEM1618-264218911 3.778 3.733 90J PHYS CHEM B1520-6106118812 3.696 4.061 90ORG BIOMOL CHEM1477-052015667 3.696 3.652 92ACS CHEM NEUROSCI1948-7193310 3.676 3.676 93ADV CATAL0360-05641395 3.667 5.04 94FOOD CHEM0308-814636842 3.655 4.268 95CARBOHYD POLYM0144-861715055 3.628 3.987 96PHYS CHEM CHEM PHYS1463-907631819 3.573 3.931 97EXPERT OPIN THER PAT1354-37761563 3.571 2.122 98ULTRASON SONOCHEM1350-41774594 3.567 3.83 99GOLD BULL0017-15571139 3.517 3.162 100STRUCT BOND0081-59931940 3.475 6.315 101FOOD HYDROCOLLOID0268-005X5607 3.473 3.46 102COLLOID SURFACE B0927-77658715 3.456 3.354 103CURR ORG SYNTH1570-1794665 3.434 3.842 104J CHEMINFORMATICS1758-2946179 3.419 3.419 105CHEMPHYSCHEM1439-423510354 3.412 3.553 106J COMB CHEM1520-47663046 3.408 2.893 107CATAL TODAY0920-586122303 3.407 3.584 108ACS MED CHEM LETT1948-5875511 3.355 3.355 109J INORG BIOCHEM0162-01349265 3.354 3.495 110EUR J MED CHEM0223-523411332 3.346 3.509 111EUR J ORG CHEM1434-193X18591 3.329 3.289 112ELECTROPHORESIS0173-083517540 3.303 2.917 113J BIOL INORG CHEM0949-82573803 3.289 3.488 114J NANOPART RES1388-07644770 3.287 3.574 115MICROPOR MESOPOR MAT1387-181113858 3.285 3.254 116CHEM CENT J1752-153X292 3.281 3.033 117J MASS SPECTROM1076-51745549 3.268 3.301 117PHYTOMEDICINE0944-71134731 3.268 3.3 119BIOANALYSIS1757-6180829 3.223 3.223 120J ANAL ATOM SPECTROM0267-94776969 3.22 2.966 121J PHYS CHEM REF DATA0047-26894693 3.172 3.804 122MOL DIVERS1381-19911114 3.153 3.236123CHEMMEDCHEM1860-71793364 3.151 3.445 124J NAT PROD0163-386418661 3.128 3.164 125DYES PIGMENTS0143-72086586 3.126 3.28 126MAR CHEM0304-42036836 3.074 3.551 127J COLLOID INTERF SCI0021-979742700 3.07 3.263 128CURR ORG CHEM1385-27283649 3.064 3.468 129J PHARM SCI-US0022-354916617 3.055 3.324 130EUR J INORG CHEM1434-194815553 3.049 2.904 131ADV INORG CHEM0898-******** 3.048 3.049 131MICROCHEM J0026-265X3125 3.048 2.878 133MICROCHIM ACTA0026-36724182 3.033 2.508 134TETRAHEDRON0040-402053591 3.025 3.06 135J ETHNOPHARMACOL0378-874121075 3.014 3.728 136ANAL BIOCHEM0003-269740002 2.996 3.247 137PLASMONICS1557-1955654 2.989 4.026 138CATAL COMMUN1566-73677950 2.986 3.299 139J PHARMACEUT BIOMED0731-708514454 2.967 2.979 140J MOL CATAL A-CHEM1381-116917457 2.947 3.336 141J PHYS CHEM A1089-563953462 2.946 2.941 142FUEL PROCESS TECHNOL0378-******** 2.945 3.131 143BIOORGAN MED CHEM0968-089622584 2.921 3.157 144J ELECTROANAL CHEM1572-665722586 2.905 2.731 145J CHROMATOGR B1570-023221252 2.888 3.057 146DRUG DES DEV THER1177-8881203 2.877147ELECTROANAL1040-039710069 2.872 2.856 148ANTI-CANCER AGENT ME1871-52061203 2.862149J SUPERCRIT FLUID0896-84465365 2.86 3.145 150J AGR FOOD CHEM0021-856171104 2.823 3.239 151MEDCHEMCOMM2040-2503223 2.8 2.8 152RAPID COMMUN MASS SP0951-419813453 2.79 2.782 153PURE APPL CHEM0033-454512669 2.789 2.987 154J MOL CATAL B-ENZYM1381-11774576 2.735 2.701 155J SEP SCI1615-93067275 2.733 2.725 156SYNLETT0936-521417866 2.71 2.567 157TETRAHEDRON LETT0040-403976620 2.683 2.588 158TETRAHEDRON-ASYMMETR0957-416613382 2.652 2.652 159SOLID STATE IONICS0167-273822111 2.646 3.097 160RUSS CHEM REV+0036-021X3090 2.644 2.941 161PHYTOCHEM ANALYSIS0958-03441883 2.633 2.32 162TOP CATAL1022-55284801 2.624 2.973 163SEP PURIF REV1542-2119175 2.615 3.629 164NEW J CHEM1144-05469359 2.605 2.775 165INT J MOL SCI1661-65962744 2.598 2.617166PHOTOCH PHOTOBIO SCI1474-905X3988 2.584 2.688 167ENVIRON CHEM1448-25171188 2.57 2.694 168BIOORG MED CHEM LETT0960-894X31475 2.554 2.539 169MINI-REV MED CHEM1389-55752649 2.528 2.772 170FUTURE MED CHEM1756-8919616 2.522 2.522 171BEILSTEIN J ORG CHEM1860-5397812 2.517 2.078 172PLANT FOOD HUM NUTR0921-96681448 2.505 2.889 173J ANAL APPL PYROL0165-23704356 2.487 2.687 174REACT FUNCT POLYM1381-51484207 2.479 2.727 175APPL CLAY SCI0169-13175168 2.474 3.06 176SYNTHESIS-STUTTGART0039-788118726 2.466 2.448 177J CHEM THERMODYN0021-96145451 2.422 2.173 178J PHOTOCH PHOTOBIO A1010-603012665 2.421 2.925 179MINI-REV ORG CHEM1570-193X460 2.406 2.283 180ORG PROCESS RES DEV1083-61603609 2.391 2.38 181MOL INFORM1868-1743171 2.39 2.407 182MOLECULES1420-30495118 2.386 2.411 183J ORGANOMET CHEM0022-328X22966 2.384 2.085 184CHIRALITY0899-******** 2.35 2.232 185AUST J CHEM0004-94255762 2.342 2.258 186CHEM PHYS LETT0009-261455511 2.337 2.215 187CARBOHYD RES0008-621514992 2.332 2.386 188COLLOID POLYM SCI0303-402X5510 2.331 2.077 189J ALLOY COMPD0925-838836296 2.289 2.104 190CHEM BIOL DRUG DES1747-02771504 2.282 2.477 191ADV QUANTUM CHEM0065-3276754 2.275 1.356 192CATAL LETT1011-372X9443 2.242 2.314 193COLLOID SURFACE A0927-775717780 2.236 2.359 194BIOPHYS CHEM0301-46224908 2.203 2.163 195J CHEM TECHNOL BIOT0268-25756388 2.168 2.27 196THEOR CHEM ACC1432-881X4587 2.162 2.909 197MATCH-COMMUN MATH CO0340-62531420 2.161 1.826 198J SOLID STATE CHEM0022-459617779 2.159 2.412 199PLANTA MED0032-094311107 2.153 2.338 200SUPRAMOL CHEM1061-02782323 2.145 2.021 201FLUID PHASE EQUILIBR0378-******** 2.139 2.225 202J FLUORESC1053-05092486 2.107 2.197 203APPL SURF SCI0169-433228315 2.103 2.032 204PHYTOTHER RES0951-418X7721 2.086 2.469 204SAR QSAR ENVIRON RES1062-936X715 2.086 1.874 206J FOOD COMPOS ANAL0889-15753582 2.079 3.257 207APPL ORGANOMET CHEM0268-26052774 2.061 1.849 208POLYHEDRON0277-538713245 2.057 2.118209J BIOMOL SCREEN1087-05712131 2.049 2.066 210J FLUORINE CHEM0022-11394628 2.033 1.95 211SOLVENT EXTR ION EXC0736-******** 2.024 1.81 212J ANAL TOXICOL0146-47602651 2.022 1.994 213ADSORPTION0929-560713282 2.215 214SURF SCI0039-602823707 1.994 1.661 215J ENVIRON MONITOR1464-03253800 1.991 2.245 216J IND ENG CHEM1226-086X1844 1.977 1.647 216PROG ORG COAT0300-94404252 1.977 2.167 218INORG CHEM COMMUN1387-70036187 1.972 1.975 219BIOMED CHROMATOGR0269-38792728 1.966 1.88 220J PHYS ORG CHEM0894-32302668 1.963 1.648Eigenfactor® MetricsMore Information Immediacy Articles Cited Eigenfactor®Article Influence®Index Half-life Score Score9.698419.4 1.6565820.3536.0758719.4 1.4128217.5081.87436147.8 1.6033 4.8927.1581967.90.2146413.3056.246134 4.70.2208917.8915.471314 3.20.13678.0693.4612670.10127.2865.308120 1.80.032847.94472 5.70.00328 5.2462.32437 2.80.0121 5.1443.267309.70.016877.4672.15578950.26226 4.0612.8982002 5.40.513933.372.082955 4.20.34576 5.0592.1791517.70.04173.2063.2588.70.009037.8051.631114120.12069 3.5842.12524 6.80.00678 2.7330.37516 6.60.00367 3.3871.51453340.112652.9391.86531767.50.816772.7922.61172 5.70.01594 2.9722.049548 1.90.01802 2.6940>10.00.00081 2.9341.0520 3.10.0052 3.930.51290.00413 3.9451.221430 3.20.072332.5070.633797.80.01312 2.3720.829707.40.00919 2.2640.3336>10.00.00074 1.6142.8483280.90.00453 2.4890.4297>10.00.00219 2.9011.41724 1.40.000932.9691.637673 6.40.15823 1.910.52>10.00.00074 1.8340.918122 2.30.01112 2.3841.1142012.40.01339 1.8641.09442 4.20.03184 1.4971.022138 5.60.01667 1.8071.405529 1.40.024172.072 1.5033408 5.50.24077 1.547 1.003398 4.10.04809 1.4850.842389.60.04417 1.5691.0882527 4.30.12631 1.571 1.75127.10.004032.549 1.29416973.90.1688 1.527 1.187653 1.40.01164 1.561 1.48516804.90.18767 1.411 0.93313287.70.17541 1.5470.915331 6.70.04644 1.6491.143538 3.60.05012 1.7050.858471 5.40.04732 1.4211.102704 4.20.05043 1.2040.752509 4.90.06046 1.3951.081641 6.30.06074 1.595 1.0497097.40.09638 1.3 1.145413 3.10.04017 1.716 1.55207.60.004652.345 1.049224 1.60.00601 1.352 1.3771389.20.01265 1.752 0.577300 5.50.03426 1.347 0.474403 5.20.03177 1.297 0.723166 2.90.28453 1.339 0.7237333.50.053380.912 0.595289 6.30.017020.7660.82814997.40.127630.9641.0763438.40.03476 1.516 0.597626 6.80.070850.9630.622110470.09860.9211.0453772.70.02083 1.243>10.00.00011 1.506 1.09912429.90.13784 1.05 0.8981353 2.50.05461 1.584 0.3182260.0032 1.1690.75869480.0249 1.0571.25>10.00.00172 1.053 0.711936 6.50.22306 1.179 0.706201 4.70.01384 1.168 0.7392618.50.02793 1.196 0.5911753 3.30.059170.7180.711228 5.80.02249 1.0541.013827 6.90.067680.830.819337 4.30.03418 1.204 0.5245527.10.045390.929 0.235177.20.001430.864 0.355904 5.50.057310.801 0.404171 1.90.004220.839 0.8441018 2.90.021720.749 0.831492 5.50.077660.883 0.447854 5.10.05450.81 0.68921970.02114 1.039 0.702967 4.10.062970.967 0.7151689 6.70.2465 1.159 0.8941061 3.90.051860.961 0.69669 1.40.00158 1.21405>10.00.00072 1.57 0.7321483 4.70.079720.839 0.64835 4.90.028380.744 0.91231440.11162 1.241 0.70896 2.90.005410.609 0.964197 4.70.009170.739 0.28257.30.002590.91611980.00341 1.67 0.825234 5.40.011580.756 0.716524 4.20.021180.7390.43848 4.40.002050.9771.4250 1.50.000460.805 0.71410 4.50.04139 1.1870 4.50.008370.668 0.45566870.04130.919 0.793179 1.30.00205 1.044 0.635222 6.80.015560.791 0.484672 3.50.026580.705 0.766789 4.80.049630.8180.424413 6.90.029650.6261.018111 5.60.01044 1.028 0.287669 3.80.015170.929 0.796368 5.20.03430.799 0.20986 3.20.001390.839 0.58143 6.50.013621 0.441195 5.40.009240.65 0.795171 1.60.001670.422 0.6142647.70.010820.673 0.21414>10.00.004512.295 0.734943.90.002830.6860.765226 3.30.014480.8530.4644018.60.025550.6821.052213 4.90.013750.659 0.5487>10.00.01419 1.433 0.6410467.80.070860.835 0.47215 5.80.008280.868 0.6764449.50.023950.793 0.605583 5.20.036290.663 0.310>10.00.00060.798 0.686185 5.30.005860.592 0.5226 4.60.008520.479 0.588119180.085760.724 0.344756 6.80.024870.586 0.475524>10.00.034330.912 0.276105 3.30.00338 1.185 0.46378 3.80.026420.746 0.602487 5.90.028250.647 0.496280 6.20.034720.765 0.5991610 6.30.124690.81 0.402326 5.90.013060.799 0.52978550.057240.716 0.526409>10.00.020090.672 0.3015487.20.038990.75 0.64937 1.90.000820.528362 5.80.018750.583 0.495103 3.30.005940.555200 4.80.011950.645 0.36816487.70.106370.722 0.591164 1.10.000580.7 0.56342660.032820.763 0.928139>10.00.014250.916 0.574183 5.70.0090.576 0.26453 3.80.026470.7 0.59358360.04060.66 0.51817349.50.098910.574 0.33328870.023130.591 0.29421100.028010.852 0.14854>10.00.004550.917 0.533757.10.00310.519 0.237152 5.20.014030.848 0.3339 4.70.000610.947 0.684358 6.80.01790.68 0.205611 2.60.01090.6360.62216 4.70.011730.744 0.56760 5.20.00460.935 0.5051516 5.10.073520.5710.402102 4.70.008450.7241.286112 1.70.002350.689 0.491962.10.002940.478 0.311617.50.00190.52 0.2641407.50.007770.652 0.623151 5.60.009150.618 0.30427650.011510.675 0.5575198.10.032360.622 0.531271 6.80.01050.542 0.3713267.50.020820.69 0.1503.90.001480.557 0.6211774.90.008970.574 0.37777 1.40.000560.519 0.325756 2.80.015320.517 0.658584>10.00.024370.447 0.4051265.80.006880.602 0.6922089.50.008880.572 0.451010>10.00.078030.694 0.401404>10.00.017360.539 0.51319590.007720.464 0.7552050 3.80.097330.507 0.376165 3.30.006880.645 0.66712>10.00.00110.509 0.4782497.40.017690.585 0.3366606.60.036250.587 0.5871098.60.00970.678 0.3982017.80.010420.532 0.974265 5.60.011680.86 0.575120 3.70.005470.581 0.3184918.50.029120.67 0.328274>10.00.010820.469 0.26411090.003750.484 0.4863257.70.015030.544 0.237257 5.10.005680.505 0.3831811 5.30.077130.549 0.29290 6.70.012010.475 0.2563950.001450.383 0.448163 5.60.008040.74 0.4131267.20.004260.402 0.4064617.20.019290.3870.46126 5.50.006040.602 0.2781807.30.008730.496 0.15645>10.00.001770.415 0.2139480.004840.536 0.29100 5.90.003030.562 0.511319>10.00.034280.619 0.318371 5.40.010620.649 0.2145 3.70.005160.354 0.275204 6.70.007280.481 0.454476 4.80.011950.353 0.422161 4.80.006940.423 0.466146 6.60.004980.398。
分子体积排阻高效液相色谱法测定低浓度重组人源化抗BCMA/CD3双特异性抗体药物的浓度

药物分析杂志Journal of Pharmaceutical Analysis药物分析杂志 线性关系考察 精密吸取混合对照品储备液、4、6、8、10 mL ,分别置10 mL 量瓶中,用70%甲醇水溶液定容至刻度,即得系列混合对照品溶液。
分别精密吸取上述系列混合对照品溶液10 μL ,按“2.1”项下色谱条件进样分析,测定峰面积。
以峰面积(Y )葛根素(puerarin ) 2. 连翘酯苷A (forsythiaside A ) 3. 黄芩苷(baicalin 牛蒡苷(arctiin )混合对照品(mixed reference substances ) B. 样品(sample ) C. 缺葛根阴性样品(negative sample of Puerariae Lobatae Radix ) D. 缺连翘阴性样negative sample of Forsythiae Fructus ) E. 缺黄芩阴性样品(negative sample of Scutellariae Radix ) F. 缺炒牛蒡子阴性样品(negative sample ofArctii Fructus ) 小儿解表颗粒HPLC 色谱图 HPLC chromatograms of Xiao ’er Jiebiao granules表2 4个成分的线性回归方程、相关系数(r )及线性范围Tab. 2 The regression equation ,correlation coefficients and linear ranges of 4 components成分(component )回归方程(regression equation )r线性范围(linear range )/(μg ·mL -1)葛根素(puerarin )Y =45.19X +7.5460.999 9 4.799~47.99连翘酯苷A forsythiaside A )Y =16.61X +1.3590.999 93.968~39.68黄芩苷(baicalin )Y =32.55X +13.430.999 916.76~167.6牛蒡苷(arctiin )Y =5.843X +0.992 80.999 97.082~70.82药物分析杂志药物分析杂志药物分析杂志药物分析杂志。
WHO_TRS_937__annex8_eng

© World Health OrganizationWHO Technical Report Series, No. 937, 2006Annex 8Proposal to waive in vivo bioequivalence requirements for WHO Model List of Essential Medicines immediate-release, solid oral dosage forms Introduction1. Background2. WHO revisions to the criteria for Biopharmaceutics Classifi cation Systemclassifi cation3. WHO extensions to the scope of application of the biowaiver4. WHO additional criteria for application of the biowaiver procedure5. Explanation of the tables6. Biowaiver testing procedure according to WHOIntroductionThis proposal is closely linked to the Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchange-ability (WHO Technical Report Series, No. 937, Annex 7). It aims to give national authorities suffi cient background information on the various orally administered active pharmaceutical ingredients (APIs) on the WHO Model List of Essential Medicines (EML), also taking into account local usage of the API, to enable them to make an informed decision as to whether generic formulations should be subjected to in vivo bioequivalence (B E) studies or whether a biowaiver can be granted. In light of scientifi c work and dis-cussion in the last decade, some of the criteria used to evaluate the API in terms of potential for a biowaiver have been revised to allow a broadened scope of application. The result is that many APIs on the EML can now be considered for the biowaiver procedure, subject to the usage and risks in the national setting.1. Background1.1Initiatives to allow biowaivers based on the BiopharmaceuticsClassifi cation SystemIn 1995 the American Department of Health and Human Services, US Food and Drug Administration (HHS-FDA) instigated the B iopharmaceutics391Classifi cation System (BCS), with the aim of granting so-called biowaiv-ers for scale-up and post-approval changes (SUPAC) (/cder/ guidance/cmc5.pdf). A biowaiver means that in vivo bioavailability and/or bioequivalence studies may be waived (i.e. not considered necessary for product approval). Instead of conducting expensive and time-consuming in vivo studies, a dissolution test could be adopted as the surrogate basis for the decision as to whether two pharmaceutical products are equivalent. At that time the biowaiver was only considered for SUPAC to pharmaceutical products.More recently, the application of the biowaiver concept has been extended to approval of certain orally administered generic products (/ cder/guidance/3618fnl.htm).Within the context of the documents cited above, only APIs with high solu-bility and high permeability and which are formulated in solid, immediate-release (IR) oral formulations can be approved on the basis of the biowaiver procedure. A major advantage of the biowaiver procedure is the simplifi ca-tion of the product approval process and the reduction of the time required, thus reducing the cost of bringing new products to market.1.2What is the Biopharmaceutics Classifi cation System?The Biopharmaceutics Classifi cation System (BCS) was proposed in 1995 by Amidon et al.1 It is a scientifi c framework which divides APIs into four groups, according to their solubility and permeability properties.1.3 Classifi cation of active pharmaceutical ingredients accordingto the Biopharmaceutics Classifi cation SystemAccording to the HHS-FDA defi nitions in the documents cited above, the four possible categories for an API according to the BCS are:•BCS class I: “high” solubility – “high” permeability•BCS class II: “low” solubility – “high” permeability•BCS class III: “high” solubility – “low” permeability•BCS class IV: “low” solubility – “low” permeability.Depending on the classifi cation, the oral availability of the API may be expected to range from being heavily dependent on the formulation and manufacturing method (e.g. Class II APIs: poorly soluble yet highly perme-able) to being mostly dependent on the API permeability properties (e.g.Class III APIs: highly soluble yet poorly permeable).1Amidon GL, Lennemas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classifi cation: the correlation of in vitro drug product dissolution and in vivo bioavailability. Phar-maceutics Research, 1995, 12:413–420.3921.4How is high or low solubility currently defi ned by the Departmentof Health and Human Services, US Food and Drug Administration?The aqueous solubility of a drug substance is considered as high according to the HHS-FDA BCS criteria when:• the ratio of the highest orally administered dose (in mg) to the solubility (mg/ml) is 250 ml or lower.—This criterion is met over the pH range 1–7.5 at 37 °C.According to HHS-FDA guidances, the determination of the equilibrium solubility should be carried out with the shake-fl ask method (other methods such as acid or base titration are permitted when their ability to predict the equilibrium solubility is justifi ed). The experiments should be carried out at a temperature of 37 ± 1°C. Further, a suffi cient number of pH conditions should be chosen to cover the pH range of 1–7.5 and each determination should be carried out at least in triplicate. The buffer solutions given in the United States Pharmacopeia (USP) are appropriate for the tests, but other buffers are also allowed for these experiments. The pH value of each buffer solution should be checked before and after each experiment. Degradation of the API due to pH or buffer composition should be reported together with other stability data.The reason for the 250-ml cut-off criterion for the dose:solubility ratio is that in pharmacokinetic bioequivalence studies, the API formulation is to be ingested with a large glass of water (8 ounces corresponds to about 250 ml). If the highest orally administered dose can be completely dissolved in this amount of water, independent of the physiological pH value (hence the determination over the pH range 1–7.5), solubility problems are not expected to hinder the uptake of the API in the small intestine.The other important parameter for the BCS is the intestinal permeability of the API.1.5How is high or low permeability currently defi ned by the Departmentof Health and Human Services, US Food and Drug Administration?According to HHS-FDA a drug is considered highly permeable, when 90 % or more of the orally administered dose is absorbed in the small intestine.Permeability can be assessed by pharmacokinetic studies (for example, mass balance studies), or intestinal permeability methods, e.g. intestinal perfusion in humans, animal models, Caco 2 cell lines or other suitable, validated cell lines. In vivo or in situ animal models or in vitro models (cell lines) are only considered appropriate by HHS-FDA for passively trans-ported drugs. It should be noted that all of these measurements assess the fraction absorbed (as opposed to the bioavailability, which can be reduced substantially by fi rst-pass metabolism).393HHS-FDA suggests use of two different methods for determining the per-meability classifi cation if results with one method are inconclusive.1.6Which pharmaceutical formulations can currently be consideredfor a biowaiver according to the Department of Health andHuman Services, US Food and Drug Administration?To be considered bioequivalent according to the HHS-FDA biowaiver pro-cedure, a pharmaceutical product:• should contain a Class I API;• should be rapidly dissolving, meaning it should release at least 85% of its content in 30 minutes in three different media (pH 1.2, pH 4.5 and pH6.8, composition see “Multisource document”)1 in a paddle (50 rpm) orbasket (100 rpm) apparatus at 37 °C and a volume of 900 ml;• should not contain excipients which could infl uence the absorption of the API;• should not contain an API with a narrow therapeutic index; and• should not be designed to be absorbed from the oral cavity.The reasoning for the above-mentioned dissolution restrictions is that whena highly soluble, highly permeable API dissolves rapidly, it behaves like asolution in the gastrointestinal tract. If this is the case, the pharmaceutical composition of the product is insignifi cant, provided that excipients which infl uence the uptake across the gut wall are excluded from the formulation.The API is not prone to precipitation after its dissolution due to its good solu-bility under all pH conditions likely to be found in the upper gastrointestinal tract. The high permeability ensures the complete uptake (> 90%) of the API during its passage through the small intestine. The rapid dissolution of the product guarantees that the API is available long enough for the uptake in the small intestine (the passage time in the small intestine is approximately four hours) and negates any slight differences between the formulations.Pharmaceutical products containing an API with a narrow therapeutic index should always be tested with in vivo methods, because the risk to the patient resulting from a possible incorrect bioequivalence decision using the bio-waiver procedure is considered too high with these kinds of APIs.As the BCS is only applicable to APIs which are absorbed from the small intestine; drugs absorbed from other sites (e.g. from the oral cavity) are not eligible for a biowaiver.It is clear that the HHS-FDA requirements for the classifi cation of APIs and eligibility criteria for the biowaiver are very strict. During the last decade,1Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability (WHO Technical Report Series, No. 937, Annex 7).394several publications and continuing scientifi c discussions have suggested that the original HHS-FDA criteria for application of the biowaiver pro-cedure could be relaxed without substantially increasing the risk to public health or to the individual patient. On the basis of these publications and dialogue, WHO has proposed revised BCS criteria and additional consid-erations for the eligibility of a pharmaceutical product for the biowaiver procedure in the “Multisource document”.12.WHO revisions to the criteria for BCS classifi cationWHO revisions to the BCS criteria are as follows:•WHO high-solubility defi nitionWhen an API shows a dose:solubility ratio of 250 ml or lower at 37 °C over a pH range of 1.2–6.8, it can be classifi ed as “highly soluble”. The decrease in pH from 7.5 in the FDA guidances to 6.8 refl ects the need to dissolve the drug before it reaches the mid-jejunum to ensure absorption from the gastrointestinal tract.• Furthermore, the dose that is to be used for the calculation is the highestdose indicated in the Model List of Essential Medicines (EML). In some countries, products may be available at doses exceeding the highest dose on the EML. In such cases, the classifi cation given in the tables at the end of this Annex may no longer be appropriate and the dose:solubil-ity ratio and the permeability will have to be reassessed at the product dose.•WHO permeability defi nitionWhen an API is absorbed to an extent of 85% or more, it is considered to be “highly permeable”. The permeability criterion was relaxed from 90% in the FDA guidance to 85% in the WHO “Multisource document”.Some examples of APIs now included in BCS Class I that were previ-ously considered to be in Class III are paracetamol, acetylsalicylic acid, allopurinol, lamivudine and promethazine.Application of these revised criteria has changed the classifi cation of some APIs in the list. Thus, the classifi cations in the tables attached to this docu-ment supersede those in previous publications. As new APIs appear on the EML, it will be necessary to classify them according to the revised BCS;so it is therefore anticipated that the tables will be revised regularly. In addition, some APIs have not yet been suffi ciently characterized to assign them a BCS classifi cation. As the tables evolve, it is anticipated that more concrete information will be generated for these APIs as well.1Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability (WHO Technical Report Series, No. 937, Annex 7).395the basket apparatus (applies to pharmaceutical products containingClass III APIs);—rapidly dissolving (release of > 85% of the labelled amount of drug in 30 minutes) in standard media at pH 1.2, 4.5 and 6.8, at a rota-tional speed of 75 rpm in the paddle apparatus or 100 rpm in the bas-ket apparatus (applies to pharmaceutical products containing Class IAPIs and/or Class II APIs which are weak acids and meet the 250 mldose:solubility requirement at pH 6.8).(4)Considerations relating to excipientsThe national authority should be aware that some excipients can infl uencemotility and/or permeability in the gastrointestinal tract. Therefore, the ex-cipients used in the multisource product formulation should be scrutinized.In this regard, the national authority can draw on the experience relat-ing to formulations which have been approved on the basis of humanbioequivalence studies in their own or in other jurisdictions.If the multisource product under consideration contains excipients thathave been used before in similar amounts in other formulations of thesame API, it can be reasonably concluded that these excipients will haveno unexpected consequences for the bioavailability of the product. If,however, the formulation contains different excipients, or amounts ofthe same excipients that are very different from usual, the national au-thority may choose to declare the biowaiver procedure inapplicable.A list of usual and acceptable excipients can be found at the following website: /cder/iig/iigfaqWEB.htm; formulations of some productscan be found on the web sites of some national drug regulatory authorities.5.Explanation of the tablesThe decision of a national authority to allow a biowaiver based on the BCS should take into consideration the solubility and permeability char-acteristics as well as the therapeutic use and therapeutic index of the API, its pharmacokinetic properties, the similarity of the dissolution profi les of the multisource and the comparator products in standard buffers with a pH of 1.2, pH 4.5 and pH 6.8 at 37 °C. Data related to the excipients compo-sition in the multisource product are also required. A systematic approach to the biowaiver decision has been established by the International Pharma-ceutical Federation (FIP) and published in the Journal of Pharmaceutical Sciences (/cgi-bin/jhome/68503813).The relevant documents can also be downloaded from the FIP web site at: http://www.fi/. These monographs provide detailed information which should be taken into account whenever available in the biowaiver consideration.3985.1Which active pharmaceutical ingredients are included in thetables?The substances listed in the 14th WHO Model List of Essential Medicines (EML) of March 2005 have been evaluated and classifi ed according to the revised criteria given above.5.2Where do the data come from?The solubility and permeability values were found in the publicly available literature, such as Martindale’s, the Merck Index and scientifi c journals.Please note that the doses used for the calculation of the dose:solubility ratio are those stated in the EML.The indications given in the tables are reproduced directly from the EML. If the EML specifi es the dosage form (e.g. sublingual tablet) this is indicated under “comments”.5.3“Worst case” approach to the Biopharmaceutics Classifi cationSystemThe drugs listed in the EML were classifi ed according to the criteria explained above. Where no clear classifi cation could be made, the “worst case” was as-sumed. For example if a substance is highly soluble, but absolute bioavailability data were not available, the test conditions for BCS Class III substances have been proposed. The same procedure was adopted for fi xed combinations, for example amoxicillin and clavulanic acid, the testing procedure was always fi xed according to the “worst” BCS classifi cation, in this example clavulanic acid (BCS Class III/1), because amoxicillin is a BCS Class I drug. This com-bination would therefore be tested according to BCS Class III requirements.The results of the revised classifi cation can be found in Tables 1–3.5.4Why are there three Tables?Table 1 lists all APIs on the EML that are administered orally, with the excep-tion of the APIs listed as complementary. Table 2 summarizes the APIs listed as complementary in the EML and Table 3 lists the APIs for which no classifi cation had previously been assigned, or that had been introduced with the 14th EML (March 2005), together with a more detailed explanation of their classifi cation.5.5 Risk assessmentTo minimize the risks of an incorrect biowaiver decision in terms of public health and risks to individual patients, the therapeutic indications of the API, known pharmacokinetic variations, food effects, etc. should be evalu-ated based on local clinical experience, taking into account the indications399for which the API is prescribed in that country as well as specifi c pharmaco-kinetic population variations (for example CYP polymorphisms). Known potential risks are listed under “potential risks” in the tables. The absence of an entry under “potential risks” should not, however, be misconstrued as meaning that there are no risks associated with the use of the medicine. 6.Biowaiver testing procedure according to WHODepending on the BCS classifi cation of the API, based on solubility and permeability characteristics listed in the accompanying tables, the testing procedure is defi ned in section 9.2.1 of the “Multisource document”1:6.1For pharmaceutical products containing BiopharmaceuticsClassifi cation System Class I (highly soluble, highlypermeable) APIsFor rapidly dissolving (as defi ned above) pharmaceutical products contain-ing BCS Class I APIs, more than 85% dissolution of the labelled amount is required within 30 minutes in standard media at pH 1.2, 4.5 and 6.8 using the paddle apparatus at 75 rpm or the basket apparatus at 100 rpm. The dis-solution profi les of the comparator and the multisource products should be compared by an f> 50 or an equivalent statistical criterion.2If within 15 minutes more than 85% of the API are released from the compar-ator and the multisource formulation under the above-mentioned conditions the products will be considered very rapidly dissolving. In this case the prod-ucts are deemed to be equivalent and a profi le comparison is not required.6.2For pharmaceutical products containing BiopharmaceuticsClassifi cation System Class III (highly soluble, lowpermeability) APIsA biowaiver can be considered only if both the multisource and the com-parator product are very rapidly dissolving. Eighty-fi ve per cent or more dissolution of the labelled amount of the API should be achieved within15 minutes in standard media at pH 1.2, 4.5 and 6.8 using the paddle ap-paratus at 75 rpm or the basket apparatus at 100 rpm.Generally, the risks of an inappropriate biowaiver decision should be more critically reviewed (e.g. site-specifi c absorption, induction/competition at the absorption site, excipient composition and therapeutic risks) for prod-ucts containing BCS Class III APIs than for BCS Class I drugs.1Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability (WHO Technical Report Series, No. 937, Annex 7).4006.3For pharmaceutical products containing APIs with highsolubility at pH 6.8 but not at pH 1.2 or 4.5 and with highpermeability (by defi nition, BCS Class II compoundswith weak acidic properties)These are eligible for a biowaiver provided that the multisource product:• is rapidly dissolving, i.e. 85% or more dissolution of the labelled amount of the API should be achieved within 30 minutes in standard media at pH 6.8 using the paddle apparatus at 75 rpm or the basket apparatus at 100 rpm; and• the multisource product exhibits similar dissolution profiles, as deter-mined with the f2 value or equivalent statistical evaluation, to those ofthe comparator product in buffers at all three pH values (pH 1.2, 4.5 and6.8).For multisource products containing BCS Class II APIs with dose:solubility ratios of 250 ml or less, at pH 6.8, the excipients should also be critically evaluated in terms of type and amounts of surfactants in the formulation.Further details of eligibility for the biowaiver and appropriate test proce-dures can be found in sections 5 and 9 of the “Multisource document”.11Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability (WHO Technical Report Series, No. 937, Annex 7).401405c h l o r p h e n a -m i n e h yd r o ge n m a l e a t e 4 m g h i g hB A 25-59%, fi r s t p a s s 3/19.2.1.2C Y P 2D 6 p o l y -m o r p h i s m a n t i a l l e r g i ce x t e n t of fi r s t -p a s s m e t a b o l i s m u n c e r t a i nc h l o r p r o m a z i n e h yd r o c h l o r i de 100 m gh i g hl o w39.2.1.2 p s y c h o t h e r a p e u -t i c m e d i c i n e c i p r o fl o x a c i nh y d r o c h l o r i d e 250 m g h i g hB A 70–82%, p o s s i b l e fi r s t p a s s , h i g h i nC a c o -2 c e l l s3/19.2.1.2 a n t i b a c t e r i a le x t e n t of fi r s t - p a s s m e t a b o l i s m u n c e r t a i nc l o f a z i m i n e100 m gi n s u f fi c i e n t l i t e r a t u r e l o w 4/3N o t e l i g i b l e f o r b i o w a i v e r a t p r e s e n t a n t i l e p r o s y m e d i c i n ec l o m i f e n e c i t r a t e50 m g h i g h i n s u f fi c i e n t l i t e r a t u r e 3/19.2.1.2o v u l a t i o n i n d u c e rc l o m i p r a m i n e h yd r o c h l o r i de 25 m g h i g h66% e x c r e t e d i n t h e u r i n e , t h e r e m a i n d e r b e i n g e l i m i -n a t e d i n t h e f a e c e s 3/19.2.1.2p s y c h o t h e r a p e u -t i c m e d i c i n el a c k o f a b s o l u t e b i o a v a i l a b i l i t y d a t ac l o x a c i l l i n (a s s od i u m s a l t )1000 m g h i g hl o w 39.2.1.2a n t i b a c t e r i a lc ode i n e p h o s p h a t e 30 m g h i g h l o w39.2.1.2r i s k o f a b u s eo p i o i d a n a l g e s i c ,d i a r r h oe a i n a d u l t sd a p s o n e100 m gl o w (w e a k b a s e ) h i g h 2N o t e l i g i b l e f o r b i o w a i v e rG 6P D d e fi -c i e n c ya n t i l e p r o s y m e d i c i n ed i a ze p a m 5 m g h i g hh i g h19.2.1.1p s y c h o t h e r a p e u -t i c m e d i c i n e s c o r e d t a b l e tB A , B i o a v a i l a b i l i t y ; G 6P D , g l u c o s e -6-p h o s p h a t e d e h y d r o g e n a s e .409g l y c e r y l t r i n i t r a t e 500 μgh i g hs u b l i n g u a l a p p l i c a t i o n ,p e r m e a b i l -i t y i n t h e o r a l c a v i t y m o r e i m p o r t a n t t h a n G I p e r m e a b i l i t y3/1N A hl o c a l a b s o r p t i o n a n t i a n g i n a l m e d i c i n e s u b l i n g u a l a p p l i c a t i o ng r i s e o f u l v i n 250 m gl o w (n e u t r a l ) h i g h2N o t e l i g i b l e f o r b i o w a i v e r a n t i f u n g a lh a l o p e r i d o l2 m gb o r d e r l i n e < 0.01 m g /m l 2l o w 4/3N o t e l i g i b l e f o r b i o w a i v e rp s y c h o t h e r a p e u -t i c m e d i c i n eh y d r a l a z i n e h y d r o c h l o r i d e50 m g h i g hl o w 39.2.1.2a n t i h y p e r t e n s i v e m e d i c i n eh y d r o c h l o r o -t h i a z i d e 25 m g h i g h l o w 39.2.1.2a n t i h y p e r t e n s i v e m e d i c i n e , d i u r e t i c a n d u s e d i n h e a r t f a i l u r es c o r e d t a b l e ti b u p r o f e n 400 m gl o w , w e a k a c i d (p K a 4.4,5.2)h i g h 29.2.1.3N S A I D , a n t i m i -g r a i n e m e d i c i n ei n d i n a v i r s u l f a t e 400 m g l o w l o w (?)4/2N o t e l i g i b l e f o r b i o w a i v e r C Y P 450 3A 4, f o o d e f f e c t (–)a n t i r e t r o v i r a lu n k n o w n w h e t h e r p o o r B A i s d u e t o p o o r s o l u b i l i t y o r p o o r s o l u b i l i t y a n d p o o r p e r m e a b i l i t yD :S , D o s e :s o l u b i l i t y r a t i o ; B A , b i o a v a i l a b i l i t y .426T a b l e 3C o m p o u n d s i n t r o d u c e d t o t h e W H O M o d e l L i s t o f E s s e n t i a l M e d i c i n e s s i n c e M a r c h 2005 f o r w h i c h n o c e r t a i n c l a s s i fi c a t i o n h a d b e e n p r e v i o u s l y r e p o r t e d (t h e s e c o m p o u n d s a l s o a p p e a r i n T a b l e 1 a n d T a b l e 2)M e d i c i n e aH i g h e s t o r a l s t r e n g t h a c c o r d i n g t o W H O E s s e n t i a l M e d i c i n e s L i s t a S o l u b i l i t y bP e r m e a b i l i t y c B C S c l a s s dD i s s o l u t i o n t e s t (f o r b i o w a i v e r )e P o t e n t i a l r i s k s fI n d i c a t i o n (s )a c c o r d i n g t o W H O E s s e n t i a l M e d i c i n e s L i s t (E M L )aC o m m e n t s a n d s p e c i a l d o s a g e f o r m i n d i c a t i o n s aa m l o d i p i n e 5 m gs l i g h t l y s o l u b l e (1),D :S 5 m lB A a b s60–65%,e x c r e t i o n o f d r u g m e t a b o -l i t e s i n u r i n e 90–95% (2)19.2.1.1a n t i h y p e r t e n s i v e m e d i c i n eB A a b s < 85% a s c r i b e d t o fi r s t -p a s s m e t a b o l i s ma m o d i a q u i n e(b a s e )200 m g45 m g /m l 2,D :S 4.4 m lB A > 75% (3)3/19.2.1.2C Y P 2C 8p o l y m o r p h i s m ,i n c r e a s e d r i s k f o r a g r a n u l o c y -t o s i s a n d h e p a -t o t o x i c i t y (4)a n t i m a l a r i a la m o x i c i l l i n + c l a v u l a n i c a c i d 500 m g + 125 m gf r e e l y s o l u b l e i n w a t e r (1),D :S 1.25 m la b s o r p t i o n > 73% (5)1 + 3/19.2.1.2a n t ib ac t e r i a lt e s t s b a s e d o n c l a v u l a n i c a c i d c l a s s i fi c a t i o na r t e s u n a t e 50 m gv e r ys l i g h t l y s o l u b l e (6),D :S 500 m l ;(w e a k a c i d ,p K a ~ 6.4)B A a b s 82% (1),B A a b s 88% (7),B A a b s 61% (8)4/2N o t e l i g i b l e f o r b i o w a i v e ra n t i m a l a r i a lp e r m e a b i l i t y d e p e n d s o n s e v e r i t y o f d i s e a s eD :S , D o s e : s o l u b i l i t y ; B A , B i o a v a i l a b i l i t y .427a z i t h r o m y c i n 500 m gp r a c t i c a l l y i n s o l u b l e i n w a t e r (1)< 0.01m g /m l , D :S 50 000 m lB A a b s 16% (9);B A 37%(10, 11); 4/2N o t e l i g i b l e f o r b i o w a i v e ra n t ib ac t e r i a l u n k n o w n w h e t h e r p o o r B A i sd ue t o p o o r s o l u b i l i t y o r p o o r s o l u b i l i t y a n d p o o r p e r m e a b i l i t yc a l c i u m f o l i n a t e 15 m gs p a r i n g l y s o l u b l e i n w a t e r (P h . E u r . 5.2); v e r y s o l u b l e (U S P 28); D :S 15 m l a n d 0.015 m l , r e s p e c -t i v e l yB A a b s 92% 25 m g (12, 13);B A a b s 73.4%(15 m g ) (14);f u l l y a b s o r b e d ;A UC a n d t 1/2s i m i l a r a f t e r i.v . & p .o (15)19.2.1.1 a n t i c y t o t o x i c m e d i c i n el e v o d o p a (l ) + c a r b i d o p a (c )(l ) 250 m g + (c ) 25 m g(l ) h i g h +(c ) s o l u b l e 1 i n 500 o f w a t e r , f r e e l y s o l u b l e i n 3 M H C l (1)(l ) h i g h +(c ) B A 58% (16); B A a b s88% (d o g s ) (17)(l ) 1 +(c ) 3/19.2.1.2n a r r o w t h e r a p e u t i c i n d e xa n t i p a r k i n s o n m e d i c i n et e s t s b a s e d o n c a r b i d o p a c l a s s i fi c a t i o nc e fi x i m e 400 m gs l i g h t l ys o l u b l e (2),D :S 400 m l22–54% (2)4N o t e l i g i b l e f o r b i o w a i v e ra n t ib ac t e r i a lD :S , D o s e : s o l u b i l i t y ; B A : B i o a v a i l a b i l i t y ; P h .E u r ., E u r o p e a n P h a r m a c o p o e i a ; U S P , U n i t e d S t a t e s P h a r m a c o p o e i a ; A U C , a r e a u n d e r t h e c u r v e ; i.v ., i n t r a v e n o u s .。
SCI_Chem 影响因子

Abbreviated JournalTitle(linked to journal information)Total CitesImpact Factor5-YearImpactFactor Immediacy Index综合1Nature 0028-0836********.59738.1599.2432Science0036-807550848931.02733.587 6.6913P Natl Acad Sci Usa 0027-84245349519.73710.583 1.893化学综合1Chem Rev 0009-266511259641.29845.79514.3352Nat Mater 1476-11224634835.74942.3768.4113Nat Nanotechnol 1748-33872192031.1736.011 5.8764Chem Soc Rev 0306-00124764624.89230.1817.9975Prog Mater Sci 0079-6425592123.19422.3337.2176Nat Chem 1755-4330865221.75723.02 5.5327Accounts Chem Res 0001-48424211220.83324.633 5.2958Nano Today 1748-0132294417.68918.1920.7849Annu Rev Mater Res 1531-7331525916.17914.4950.66710Surf Sci Rep 0167-5729411515.33322.2817.7511Adv Mater 0935-96489195214.82913.86 2.55712Mat Sci Eng R 0927-796X 485013.90218.9740.66713Angew Chem Int Edit 1433-785122989413.73413.56 2.95914Annu Rev Phys Chem 0066-426X 700213.36518.121 4.13815Nano Lett 1530-69848843113.02514.132 2.47116Acs Nano 1936-0851*******.06212.524 1.9417Energ Environ Sci 1754-56921284911.65312.462 3.08718Coordin Chem Rev 0010-85452560111.01612.257 2.29419J Am Chem Soc 0002-786343128610.67710.237 2.16420Nat Prod Rep 0265-0568660910.17810.072 2.38521Adv Energy Mater 1614-6832199510.04310.05 2.11822Adv Funct Mater 1616-301X 347589.76510.342 1.81523Med Res Rev 0198-632532979.5839.978 1.81124Npg Asia Mater 1884-40493019.0428.556 2.41425Annu Rev Anal Chem 1936-132711498.612.2830.69626Top Curr Chem 0340-102255078.456 6.205 4.27327Chem Sci 2041-652048458.3148.33 2.77128Chem Mater 0897-4756746518.2387.627 1.12329J Photoch Photobio C 1389-556720268.06911.9520.7530Small 1613-6810181377.8238.084 1.42931Prog Photovoltaics 1062-799545357.7127.023 3.22332J Control Release 0168-3659297557.6338.078 1.13633Annu Rev Chem Biomol 1947-54383317.5127.512 1.04534Int Mater Rev 0950-660822557.487.1491.188RankISSNJCR Data35Chemsuschem1864-563150567.4757.951 1.189 36Prog Solid State Ch0079-678614747.429 3.3380 37Nano Res1998-012430317.3927.8010.979 38Prog Surf Sci0079-681619047.1369.140.273 39Adv Carbohyd Chem Bi0065-23188337.133 5.8460.75 40Green Chem1463-926215554 6.828 6.992 1.269 41Adv Organomet Chem0065-3055841 6.758.9410 42Curr Opin Colloid In1359-02944670 6.6297.0360.884 43J Phys Chem Lett1948-71858575 6.585 6.651 1.301 44Top Organometal Chem1436-60021152 6.384 1.762 45Chem Commun1359-7345122728 6.378 6.226 1.53 46Catal Rev0161-49402529 6.37510.1750.889 47Trac-Trend Anal Chem0165-99367327 6.351 6.7610.92 48Nanoscale2040-33647835 6.233 6.262 1.167 49Adv Colloid Interfac0001-86867115 6.1698.01 1.32 50Org Lett1523-706073440 6.142 5.563 1.572 51Mater Today1369-70213769 6.0718.677 1.977 52Prog Nucl Mag Res Sp0079-65651993 6.022 6.065 1.389 53Crit Rev Solid State1040-8436744 5.9477.3681 54Carbon0008-622332742 5.868 6.35 1.197 55Chem-Eur J0947-653960788 5.831 5.623 1.241 56Appl Catal B-Environ0926-337323011 5.825 6.0310.965 57J Catal0021-951734516 5.787 6.249 1.025 58Wires Comput Mol Sci1759-0876570 5.738 5.738 3.518 59Lab Chip1473-019716485 5.697 6.136 1.256 60Anal Chem0003-270096794 5.695 5.7690.948 61J Med Chem0022-262359227 5.614 5.383 1.225 62Adv Synth Catal1615-415015502 5.535 5.323 1.109 63Curr Opin Solid St M1359-02862508 5.4387.3290.913 64Biosens Bioelectron0956-566322068 5.437 5.389 1.105 65J Chem Theory Comput1549-961811067 5.389 5.936 1.067 66Biomacromolecules1525-779724209 5.371 5.750.721 67Acs Catal2155-54351461 5.265 5.265 1.222 68Adv Catal0360-05641399 5.25 5.2860 69Chemcatchem1867-38802718 5.181 5.3070.941 70Mrs Bull0883-******** 5.024 5.590.569 71Acs Appl Mater Inter1944-82448635 5.008 5.040.683 72Electroanal Chem0070-977837757.50.25 73J Comb Chem1520-47662925 4.933 3.10274Int Rev Phys Chem0144-235X1524 4.92 5.595 2.231 75J Phys Chem C1932-744778595 4.814 5.1520.738 76Pharm Res-Dordr0724-874119035 4.742 5.0460.735 77Cryst Growth Des1528-748322310 4.689 4.8730.869 78Sol Energ Mat Sol C0927-024818447 4.63 5.205 1.215 79J Chromatogr A0021-967363419 4.612 4.5820.71480Inorg Chem0020-166985446 4.593 4.5510.956 81Bioconjugate Chem1043-180213900 4.58 4.7960.768 82Chem-Asian J1861-47286084 4.572 4.488 1.04683J Org Chem0022-326396723 4.564 4.135 1.101 84Anal Chim Acta0003-267039448 4.387 4.3440.684 85Chem Rec1527-89991375 4.377 4.814 1.533 86Int J Plasticity0749-64195276 4.356 4.70.862 87J Chem Inf Model1549-959611250 4.304 4.0670.795 88Langmuir0743-7463106920 4.187 4.4160.793 89Organometallics0276-733339735 4.145 3.653 1.004 90Ultraschall Med0172-46141247 4.116 2.723 1.286 91J Flow Chem2062-249X61 4.091 4.091 1.143 92Curr Med Chem0929-867312773 4.07 4.4710.627 93Struct Bond0081-59931899 4.068 4.24894Mar Drugs1660-33971871 3.978 3.9110.428 95Analyst0003-265416152 3.969 3.9040.785 96Acta Mater1359-645434860 3.941 4.3950.781 97Soft Matter1744-683X15943 3.909 4.35 1.013 98Crystengcomm1466-803312988 3.879 4.0690.863 99Acs Chem Neurosci1948-7193634 3.871 3.9570.729 100Nanotechnology0957-448434133 3.842 3.8380.697 101Org Electron1566-11995856 3.836 4.0210.63 102J Comput Chem0192-865124682 3.835 4.4010.847 103Phys Chem Chem Phys1463-907640969 3.829 3.976 1.052 104Faraday Discuss1359-66405758 3.821 4.148 2.369 105Dalton T1477-922638660 3.806 3.8890.947 106Catal Sci Technol2044-47531079 3.753 3.753 1.024 107Sci Technol Adv Mat1468-69962352 3.752 3.6440.235 108Chembiochem1439-42279616 3.74 3.670.78 109Curr Top Med Chem1568-02664850 3.702 3.8850.655 110Chem Res Toxicol0893-228X10785 3.667 4.0130.807 111Anal Bioanal Chem1618-264221971 3.659 3.7560.727 112Acs Comb Sci2156-8952379 3.636 3.6360.596 113Corros Sci0010-938X18210 3.615 4.0160.757 114J Phys Chem B1520-6106119722 3.607 3.7020.66 115J Am Soc Mass Spectr1044-03058760 3.592 3.5030.925 116J Cheminformatics1758-2946315 3.59 3.6710.314 117Org Biomol Chem1477-052018355 3.568 3.490.952 118Ultrasound Obst Gyn0960-76928490 3.557 3.7080.756 119Colloid Surface B0927-776510312 3.554 3.4170.707 120Int J Hydrogen Energ0360-319933119 3.548 4.0860.584 121Sensor Actuat B-Chem0925-400529909 3.535 3.6680.527 122Dyes Pigments0143-72087664 3.532 3.4330.897 123Expert Opin Ther Pat1354-37761742 3.525 2.5230.6 124Ultrason Sonochem1350-41775008 3.516 3.708 1.234125J Phys Chem Ref Data0047-26894996 3.5 3.7790.391 126Eur J Med Chem0223-523414818 3.499 3.8490.541 127Talanta0039-914026966 3.498 3.7330.531 128Food Hydrocolloid0268-005X6307 3.494 3.5250.953 129Drug Des Dev Ther1177-8881377 3.4860.256 130Carbohyd Polym0144-861718471 3.479 3.9420.665 131Microchim Acta0026-36724743 3.434 2.8830.5 132Appl Catal A-Gen0926-860X27726 3.41 3.910.506 133J Mech Phys Solids0022-509610460 3.406 3.7460.784 134Pure Appl Chem0033-454513333 3.386 3.1150.382 135Micropor Mesopor Mat1387-181115134 3.365 3.4140.869 136J Biol Inorg Chem0949-82574011 3.353 3.3550.713 137Chemphyschem1439-423511279 3.349 3.3880.768 138Eur J Org Chem1434-193X19346 3.344 3.1370.746 139Food Chem0308-814641375 3.334 4.0720.589 140Acs Med Chem Lett1948-58751087 3.311 3.3180.699 141Future Med Chem1756-89191081 3.31 3.2450.933 142J Nat Prod0163-386419898 3.285 3.2670.787 143Electrophoresis0173-083516985 3.261 2.8690.479 144Bioanalysis1757-61801318 3.253 3.0440.723 145J Mass Spectrom1076-51745573 3.214 3.2270.495 146J Inorg Biochem0162-013410014 3.197 3.430.672 147J Mol Catal A-Chem1381-116917999 3.187 3.3190.496 148J Colloid Interf Sci0021-979744929 3.172 3.390.747 149J Anal Atom Spectrom0267-94777362 3.155 2.9530.725 150Sep Purif Rev1542-2119222 3.154 3.5430.3 151J Pharm Sci-Us0022-354917986 3.13 3.3850.6 152Eur J Inorg Chem1434-194816310 3.12 2.9510.728 153Cement Concrete Res0008-884613854 3.112 3.7460.441 154Curr Org Chem1385-27283953 3.039 3.2220.253 155Isr J Chem0021-21481263 3.025 1.9460.478 156Mar Chem0304-420368723 3.3150.466 157Catal Today0920-586123325 2.98 3.4640.515 158Phytomedicine0944-71135244 2.972 3.2580.401 159New J Chem1144-054610014 2.966 2.920.698 160J Pharmaceut Biomed0731-708514648 2.947 2.8530.562 161Photoch Photobio Sci1474-905X4541 2.923 2.810.538 162Catal Commun1566-73679190 2.915 3.3280.535 163Mater Design0261-30699587 2.913 2.8050.703 164J Agr Food Chem0021-856176046 2.906 3.2880.417 165Bioorgan Med Chem0968-089624911 2.903 3.1510.617 166Crit Rev Anal Chem1040-8347979 2.892 3.690.591 167Microchem J0026-265X3428 2.879 2.85 1.048 168Mini-Rev Med Chem1389-55752893 2.865 2.9210.496 169Mol Divers1381-19911289 2.861 3.090.44170Chemmedchem1860-71793723 2.835 3.0750.788 171J Mol Catal B-Enzym1381-11774943 2.823 2.8050.542 172Scripta Mater1359-646219677 2.821 3.1450.534 173Adv Phys Org Chem0065-3160391 2.818 2.609174Electroanal1040-039710646 2.817 2.8620.462 175Fuel Process Technol0378-******** 2.816 3.4930.49 176Tetrahedron0040-402052981 2.803 2.8990.636 177Beilstein J Org Chem1860-53971405 2.801 2.4750.386 178J Phys Chem A1089-563955641 2.771 2.8560.658 179J Ethnopharmacol0378-874121278 2.755 3.3220.519 180Org Process Res Dev1083-61604311 2.739 2.6670.808 181J Supercrit Fluid0896-84466044 2.732 3.1380.472 182Medchemcomm2040-2503690 2.722 2.7220.603 183Adv Inorg Chem0898-******** 2.714 3.3780.25 184J Electroanal Chem1572-665721687 2.672 2.6760.545 185Int J Photoenergy1110-662X945 2.663 2.240.671 186Synlett0936-521417034 2.655 2.4520.604 187Environ Chem1448-25171390 2.652 2.701 1.075 188Opt Mater Express2159-3930593 2.616 2.6220.815 189Anti-Cancer Agent Me1871-52061386 2.610.239 190Top Catal1022-55284896 2.608 2.7080.238 191J Sep Sci1615-93068094 2.591 2.6380.296 192Anal Biochem0003-269739746 2.582 2.9690.558 193Rsc Adv2046-20691816 2.562 2.5670.695 194J Anal Appl Pyrol0165-23705102 2.56 3.0740.447 195Nanoscale Res Lett1931-75733998 2.524 2.7830.2980951-419813285 2.509 2.6110.49 196Rapid Commun Mass Sp196Sci Adv Mater1947-2935553 2.509 2.5610.301 198React Funct Polym1381-51484515 2.505 2.6530.282 199J Chem Technol Biot0268-25756796 2.504 2.4790.573 200Synthesis-Stuttgart0039-788117999 2.5 2.3840.615 201Microsc Microanal1431-92762043 2.495 3.0770.276 202J Chromatogr B1570-023220776 2.487 2.90.319 203Phytochem Analysis0958-03441895 2.48 2.280.292 204Adv Heterocycl Chem0065-2725888 2.478 2.6170.455 205Chem Biol Drug Des1747-02771940 2.469 2.4090.511 206Int J Mol Sci1422-00674706 2.464 2.7320.313 207Ultrasound Med Biol0301-56297839 2.455 2.8440.251 208Gold Bull0017-15571073 2.434 3.1220.04 209Molecules1420-30497552 2.428 2.6790.329 210Plasmonics1557-1955877 2.425 2.9880.525 211J Photoch Photobio A1010-603013061 2.416 2.6910.299 212Tetrahedron Lett0040-403973763 2.397 2.3760.561 213J Alloy Compd0925-838839264 2.39 2.1610.629 214Phys Status Solidi-R1862-62541437 2.388 2.4320.527215Fluid Phase Equilibr0378-******** 2.379 2.3380.544 216Solvent Extr Ion Exc0736-******** 2.375 2.6090.345 217Beilstein J Nanotech2190-4286309 2.374 2.3740.573 218Plant Food Hum Nutr0921-96681569 2.358 2.7620.25 219Acta Pharmacol Sin1671-40835577 2.354 2.5210.596 220Planta Med0032-094311009 2.348 2.4620.304 221Appl Clay Sci0169-13175590 2.342 2.7980.337 222Bioorg Med Chem Lett0960-894X33460 2.338 2.4270.584 222Macromol Mater Eng1438-74922983 2.338 2.3920.509 222Mol Inform1868-1743344 2.338 2.3460.347 225Russ Chem Rev+0036-021X3092 2.299 2.8130.204 226J Chem Thermodyn0021-96145905 2.297 2.2560.735 227Constr Build Mater0950-06187337 2.293 2.8180.391 228Chemometr Intell Lab0169-74394880 2.291 2.4320.253 229Biophys Chem0301-46224822 2.283 2.0940.649 230Arab J Chem1878-5352299 2.2660.343 231J Ginseng Res1226-8453349 2.2590.34 232Materials1996-19441176 2.247 2.3380.222 233Catal Lett1011-372X9373 2.244 2.2610.351 234Theor Chem Acc1432-881X5484 2.233 3.1510.812 235Fitoterapia0367-326X4706 2.231 2.1390.349 236Mater Lett0167-577X23419 2.224 2.3220.489 237Food Addit Contam A1944-00495142 2.22 2.4420.508 238J Biomol Screen1087-05712357 2.207 2.0890.719 239Platin Met Rev0032-1400735 2.194 2.4760.579 240J Nanopart Res1388-07645724 2.175 2.7210.222 241J Mater Sci0022-246131538 2.163 2.10.543 242Adv Quantum Chem0065-3276869 2.161 1.6940.308 242Colloid Polym Sci0303-402X5657 2.161 2.1140.433 244Chem Phys Lett0009-261455163 2.145 2.150.485 244J Ind Eng Chem1226-086X2264 2.145 1.9550.329 246Tetrahedron-Asymmetr0957-416611707 2.115 2.1430.384 247Appl Surf Sci0169-433231193 2.112 2.0990.33 248Synthetic Met0379-677913916 2.109 2.1020.334 249Colloid Surface A0927-775718414 2.108 2.3330.333 249Mat Sci Eng A-Struct0921-509340513 2.108 2.3490.307 251J Anal Toxicol0146-47602660 2.107 1.7580.429 252Solid State Nucl Mag0926-20401202 2.1 2.0570.711 253J Food Compos Anal0889-15753737 2.088 2.7430.19 254J Environ Monitor1464-03254378 2.085 2.1370.322 255Mater Chem Phys0254-058417174 2.072 2.3950.286 256J Pept Sci1075-26171942 2.071 1.8280.434 257Phytother Res0951-418X8059 2.068 2.4380.444 258Nano-Micro Lett2150-5551205 2.057 1.910.35 259Solid State Ionics0167-273820728 2.046 2.5640.26260Carbohyd Res0008-621514176 2.044 2.1780.357 261J Solid State Chem0022-459618166 2.04 2.2950.392 262Curr Org Synth1570-1794673 2.038 2.9140.76 263Ultrasonics0041-624X3651 2.028 2.0540.456 264Smart Mater Struct0964-17267120 2.024 2.3770.289 265Inorg Chem Commun1387-70036416 2.016 1.8810.434 266Appl Organomet Chem0268-26052866 2.011 1.9220.272 267Electrochem Solid St1099-00628883 2.01 2.0260.596 268J Chem Eng Data0021-956815169 2.004 2.1150.295 269Comb Chem High T Scr1386-207314532 1.9750.268 269J Organomet Chem0022-328X217932 1.9920.527 271Thermochim Acta0040-603111408 1.989 2.0460.327 272J Mol Model1610-29403157 1.984 2.3010.378 273J Therm Anal Calorim1388-61509934 1.982 1.7420.243 274Int J Fatigue0142-11235248 1.976 1.9740.352 275J Vib Control1077-54631649 1.966 1.7360.672 276Chem Phys0301-010412935 1.957 2.0590.592 277J Mater Process Tech0924-013618426 1.953 2.1760.332 277Sensors-Basel1424-82207082 1.953 2.3950.321 279Biomed Chromatogr0269-38792861 1.945 1.8150.385 280J Fluorine Chem0022-11394998 1.939 1.9490.465ArticlesCited Half-life Eigenfactor ®Metrics ScoreArticleInfluenc e® Score8699.6 1.5750820.8448329.7 1.3598717.71238018 1.55663 4.8921768.20.2266114.294141 5.20.2278819.481121 3.70.1543615.607390 3.50.178418.8542370.015018.643126 2.40.054018.927207 6.50.108177.90837 3.20.01489 5.62318>10.00.013647.55389.40.008828.821867 5.10.27819 4.24368.40.00872 6.542227 5.50.53637 3.497299.90.01888.1211078 4.40.37491 5.1891191 2.40.20333 4.012473 1.80.05534 3.461368.20.03818 3.07130997.70.83183 2.99465 5.90.01757 3.141169 1.40.00944 3.344569 4.20.12336 3.006377.20.00647 2.73229 1.90.00155 3.27323 3.90.00716 4.441557.70.01045 2.068458 1.40.02121 2.833576 6.70.15215 2.032167.90.00342 3.148457 3.60.07856 2.503112 4.60.01202 1.97501 6.90.05008 1.9062220.00197 2.73916>10.00.003282.792Eigenfactor®Metrics286 2.60.0208 2.0046>10.00.00142 1.13896 2.80.01517 2.301119.20.00397 3.974>10.00.00086 1.894 44940.03848 1.5233>10.00.00076 2.537437.60.01013 2.301 63220.04884 2.393 2150.003483173 4.80.28954 1.5629>10.00.00211 3.009 138 5.80.01695 1.794 1015 1.70.02966 1.597 508.20.01226 2.37 160850.18182 1.40244 5.40.01448 3.213188.30.00431 2.295 107.10.00156 2.34 674 6.40.06437 1.6 1916 4.10.1766 1.469 480 5.40.05059 1.419 284>10.00.04014 1.60356 1.20.00194 1.703 617 3.70.05475 1.603 14797.80.17236 1.533 8907.80.09182 1.35 404 4.40.04405 1.352 239.20.00403 2.705 448 4.10.05929 1.252 507 3.50.04948 1.865 480 5.30.05919 1.416 315 1.30.00585 1.6362>10.00.00057 1.543 255 2.10.01222 1.495 116 6.20.01779 2.376 953 2.30.03563 1.2844>10.00.00016 2.2160 5.10.006940.708137.90.00319 2.303 3283 3.40.34957 1.353 2798.70.02859 1.299 773 3.90.057280.943 493 5.30.04212 1.304 11447.10.090460.92115617.50.124780.98 259 5.70.03346 1.295 372 2.80.02555 1.219 1289>10.00.12482 1.03 7277.10.06585 1.03830 5.80.00343 1.39794 6.80.01439 1.685 303 6.60.019140.892 2119 6.70.21487 1.164 9987.10.059110.76263 3.40.002370.436140.000190.962 475 5.50.03101 1.1898.60.00225 1.225 194 2.50.00680.908 817 6.60.031350.985 68170.09849 1.714 1358 2.50.073 1.334 1194 2.60.032550.749 10720.00309 1.26 1021 4.40.12987 1.194 459 3.20.02416 1.192 2618.90.03446 1.396 1804 3.70.15067 1.296 1418.60.01298 1.609 1709 4.50.08330.842 327 1.30.00320.94181 4.90.00904 1.23 327 4.70.03579 1.258 17750.0131 1.044 2597.10.02119 1.078 877 4.30.068460.99799 1.40.001410.915 449 6.30.028910.786 16157.50.18356 1.081 22860.020170.96535 1.90.00176 1.224 1160 3.80.055440.914 201 6.30.017610.949 426 4.10.025740.772 2120 3.70.074070.708 1016 5.80.054290.757 321 5.50.013530.63690 3.30.006210.726 184 4.80.010070.73123>10.00.00228 1.513 610 3.80.032170.72 842 5.40.057730.829 213 5.40.01270.77439 2.40.001731002 4.80.033670.729 208 4.30.00930.537 5247.60.043570.93 116>10.00.02104 1.812 173>10.00.013330.936 589 5.30.032210.807 108 6.10.009190.931 487 4.70.03951 1.142 76450.047770.779 1666 5.20.091160.854 193 1.80.004620.921 120 2.20.004670.894 3338.70.027140.756 4327.10.027040.625 19520.003610.533 194 6.90.011710.909 2597.30.013360.716 266 6.80.026850.752 87080.070140.869 2187.70.01310.75610 5.60.00062 1.012 4189.20.026590.815 622 5.20.034970.666 1709.90.01712 1.222 178 5.80.009120.836 907.70.00320.65258>10.00.01221 1.371 4877.30.041850.896 187 5.60.009820.662 338 6.50.019280.711 443 6.30.026840.651 20850.01330.806 409 4.50.027060.735 774 3.20.034350.762 15287.90.107180.745 739 5.40.053960.715 227.60.001180.774 105 5.10.007480.647 129 4.90.00780.74475 3.50.002990.639212 3.70.013920.784 201 5.60.009290.599 500 6.30.06138 1.238 >10.00.00042 1.049 290 6.10.019640.625 251 5.60.017330.895 12498.40.075460.68 241 2.20.005560.628 1341 6.70.120620.829 651 6.90.026690.572 224 5.10.010210.672 290 5.30.013220.702 194 1.60.002660.7278>10.00.000620.792 314>10.00.019270.649 32830.001390.381 520 6.30.034550.62153 5.20.004350.904 200 1.30.002460.856 109 3.70.00563143 5.80.013670.825 439 4.20.025470.66 441>10.00.03210.853 17290.80.002020.494 1527.40.009010.741 624 2.40.016810.714 337 6.40.02850.71 173 1.90.002010.614 124 6.30.008010.593 2207.60.010810.602 4708.20.028730.596 221 5.20.00853 1.29 4957.50.036490.714 967.20.003150.52311>10.00.000450.543 184 3.50.006730.588 1064 2.80.017560.666 2437.60.014140.744 257.10.002380.868 105130.022770.574 10130.003550.834 2518.20.017620.63 16669.90.081330.508 1478 4.10.10840.548 14830.008560.8753427.80.015630.587 559.80.002470.6496 1.50.001250.668648.10.002270.561 178 6.20.010850.6 247>10.00.011750.526 202 5.20.013550.701 1484 5.40.069550.546 116 6.40.005740.60572 2.10.00140.58954>10.00.004010.856 351 6.60.011110.57 960 3.90.021660.681 1549.50.006150.597 5790.008180.646 6720.0008353 2.30.00061176 2.60.005320.633 1947.80.016490.569 223 5.60.014960.968 25280.006360.457 1626 5.50.049760.537 1977.10.008730.585 128 5.40.00580.57919>10.00.001080.734 650 3.70.017210.673 9419.10.050830.59113>10.00.00130.598 21090.007690.475 906>10.00.066080.687 350 3.90.005590.396 2247.80.01640.479 1789 5.50.074220.539 419>10.00.014550.491 579 6.80.034280.585 11717.30.084950.73 918.60.003920.471 458.60.00220.655 100 6.20.007370.666 335 5.10.012850.644 964 5.50.038290.59199 5.20.004810.478 302 6.60.012460.48640 2.10.000610.345 392>10.00.021350.719339>10.00.01560.489 5138.90.025150.615 50 4.50.001620.659 1368.50.005580.639 353 5.90.018730.713 495 4.80.011170.321 1147.60.003960.391 156 6.70.022520.706 508 6.50.027410.476 82 5.10.003380.472 410>10.00.020760.421 400>10.00.012770.531 463 4.70.008250.53 728 5.70.012880.253 2367.10.012090.669 180 3.80.004490.45 360>10.00.018270.679 2957.80.035210.653 950 3.40.026580.586 218 5.20.006840.446 2547.50.007820.487。
JCR2015影响因子(所有期刊从高到低排序)+中科院分区
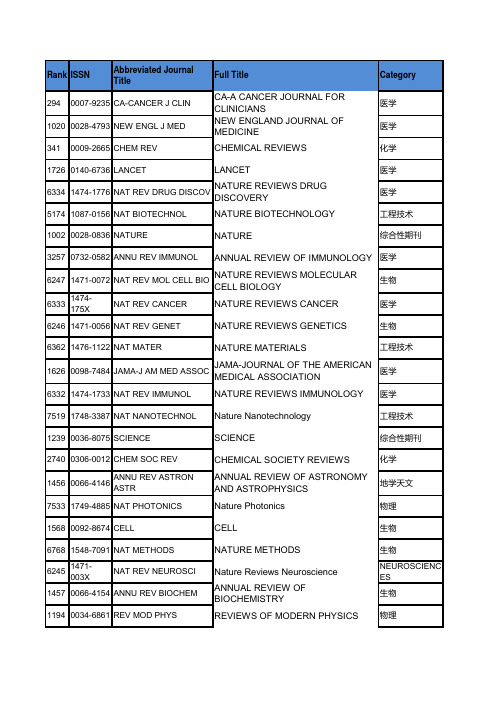
Rank ISSN Abbreviated JournalTitleFull Title Category2940007-9235CA-CANCER J CLIN CA-A CANCER JOURNAL FOR CLINICIAN医学10200028-4793NEW ENGL J MED NEW ENGLAND JOURNAL OF MEDICINE医学3410009-2665CHEM REV CHEMICAL REVIEWS化学17260140-6736LANCET LANCET医学63341474-1776NAT REV DRUG DISCOV NATURE REVIEWS DRUG DISCOVERY医学51741087-0156NAT BIOTECHNOL NATURE BIOTECHNOLOGY工程技术10020028-0836NATURE NATURE综合性期刊32570732-0582ANNU REV IMMUNOL ANNUAL REVIEW OF IMMUNOLOGY医学62471471-0072NAT REV MOL CELL BIO NATURE REVIEWS MOLECULAR CELL B生物63331474-175X NAT REV CANCER NATURE REVIEWS CANCER医学62461471-0056NAT REV GENET NATURE REVIEWS GENETICS生物63621476-1122NAT MATER NATURE MATERIALS工程技术16260098-7484JAMA-J AM MED ASSOC JAMA-JOURNAL OF THE AMERICAN ME医学63321474-1733NAT REV IMMUNOL NATURE REVIEWS IMMUNOLOGY医学75191748-3387NAT NANOTECHNOL Nature Nanotechnology工程技术12390036-8075SCIENCE SCIENCE综合性期刊27400306-0012CHEM SOC REV CHEMICAL SOCIETY REVIEWS化学14560066-4146ANNU REV ASTRON ASTR ANNUAL REVIEW OF ASTRONOMY AND地学天文75331749-4885NAT PHOTONICS Nature Photonics物理15680092-8674CELL CELL生物67681548-7091NAT METHODS NATURE METHODS生物62451471-003X NAT REV NEUROSCI Nature Reviews Neuroscience NEUROSCIENCES 14570066-4154ANNU REV BIOCHEM ANNUAL REVIEW OF BIOCHEMISTRY生物11940034-6861REV MOD PHYS REVIEWS OF MODERN PHYSICS物理48751061-4036NAT GENET NATURE GENETICS生物15060079-6425PROG MATER SCI PROGRESS IN MATERIALS SCIENCE工程技术51051078-8956NAT MED NATURE MEDICINE医学11140031-9333PHYSIOL REV PHYSIOLOGICAL REVIEWS医学15100079-6700PROG POLYM SCI PROGRESS IN POLYMER SCIENCE化学76261755-4330NAT CHEM Nature Chemistry化学62371470-2045LANCET ONCOL LANCET ONCOLOGY医学74001740-1526NAT REV MICROBIOL NATURE REVIEWS MICROBIOLOGY生物66151535-6108CANCER CELL CANCER CELL医学67091543-5008ANNU REV PLANT BIOL ANNUAL REVIEW OF PLANT BIOLOGY生物63191473-3099LANCET INFECT DIS LANCET INFECTIOUS DISEASES医学150001-4842ACCOUNTS CHEM RES ACCOUNTS OF CHEMICAL RESEARCH化学80331934-5909CELL STEM CELL Cell Stem Cell生物57941364-6613TRENDS COGN SCI TRENDS IN COGNITIVE SCIENCES医学63351474-4422LANCET NEUROL LANCET NEUROLOGY医学14660066-4308ANNU REV PSYCHOL ANNUAL REVIEW OF PSYCHOLOGY医学50621074-7613IMMUNITY IMMUNITY医学19000163-769X ENDOCR REV ENDOCRINE REVIEWS医学580001-8732ADV PHYS ADVANCES IN PHYSICS物理17250140-525X BEHAV BRAIN SCI BEHAVIORAL AND BRAIN SCIENCES医学76141754-5692ENERG ENVIRON SCI Energy & Environmental Science化学74791745-2473NAT PHYS Nature Physics物理29820370-1573PHYS REP PHYSICS REPORTS-REVIEW SECTION 物理65501529-2908NAT IMMUNOL NATURE IMMUNOLOGY医学61991465-7392NAT CELL BIOL NATURE CELL BIOLOGY生物84372159-8274CANCER DISCOV Cancer discovery ONCOLOGY 18180147-006X ANNU REV NEUROSCI ANNUAL REVIEW OF NEUROSCIENCE医学60511433-8351LIVING REV RELATIV Living Reviews in Relativity物理28850360-1285PROG ENERG COMBUST PROGRESS IN ENERGY AND COMBUST工程技术医学68241553-4006ANNU REV PATHOL-MECH Annual Review of Pathology-Mechanisms o14640066-4278ANNU REV PHYSIOL ANNUAL REVIEW OF PHYSIOLOGY医学32580732-183X J CLIN ONCOL JOURNAL OF CLINICAL ONCOLOGY医学29200362-1642ANNU REV PHARMACOL ANNUAL REVIEW OF PHARMACOLOGY 医学1520003-4819ANN INTERN MED ANNALS OF INTERNAL MEDICINE医学地学天文39930935-4956ASTRON ASTROPHYS REV ASTRONOMY AND ASTROPHYSICS REV71031614-4961LIVING REV SOL PHYS LIVING REVIEWS IN SOLAR PHYSICS ASTRONOMY & AS 67881550-4131CELL METAB Cell Metabolism生物39970935-9648ADV MATER ADVANCED MATERIALS工程技术76391756-1833BMJ-BRIT MED J BMJ-British Medical Journal MEDICINE, GENER 36650893-8512CLIN MICROBIOL REV CLINICAL MICROBIOLOGY REVIEWS医学1830003-9926ARCH INTERN MED ARCHIVES OF INTERNAL MEDICINE医学10940031-6997PHARMACOL REV PHARMACOLOGICAL REVIEWS医学680002-5100ALDRICHIM ACTA ALDRICHIMICA ACTA化学11890034-4885REP PROG PHYS REPORTS ON PROGRESS IN PHYSICS物理生物26600301-5556ADV ANAT EMBRYOL CEL ADVANCES IN ANATOMY EMBRYOLOGY化学14630066-426X ANNU REV PHYS CHEM ANNUAL REVIEW OF PHYSICAL CHEMIS5210016-5085GASTROENTEROLOGY GASTROENTEROLOGY医学51251081-0706ANNU REV CELL DEV BI ANNUAL REVIEW OF CELL AND DEVELO生物医学32960735-1097J AM COLL CARDIOL JOURNAL OF THE AMERICAN COLLEGE20440169-5347TRENDS ECOL EVOL TRENDS IN ECOLOGY & EVOLUTION生物71041614-6832ADV ENERGY MATER Advanced Energy Materials CHEMISTRY, PHYS 52791097-6256NAT NEUROSCI NATURE NEUROSCIENCE医学化学59481389-5567J PHOTOCH PHOTOBIO C JOURNAL OF PHOTOCHEMISTRY AND P81491946-6234SCI TRANSL MED Science Translational Medicine CELL BIOLOGY医学64501520-765X EUR HEART J SUPPL EUROPEAN HEART JOURNAL SUPPLEM14600066-4197ANNU REV GENET ANNUAL REVIEW OF GENETICS生物39160927-796X MAT SCI ENG R MATERIALS SCIENCE & ENGINEERING 工程技术80491936-122X ANNU REV BIOPHYS Annual Review of Biophysics生物76891759-4758NAT REV NEUROL Nature Reviews Neurology医学21640195-668X EUR HEART J EUROPEAN HEART JOURNAL医学37130896-6273NEURON NEURON医学20380169-409X ADV DRUG DELIVER REV ADVANCED DRUG DELIVERY REVIEWS医学75131748-0132NANO TODAY Nano Today工程技术86088755-1209REV GEOPHYS REVIEWS OF GEOPHYSICS地学81551947-5454ANNU REV CONDEN MA P Annual Review of Condensed Matter Physi PHYSICS, CONDEN 19620167-5729SURF SCI REP SURFACE SCIENCE REPORTS化学11550033-2909PSYCHOL BULL PSYCHOLOGICAL BULLETIN医学5530017-5749GUT GUT医学51931088-9051GENOME RES GENOME RESEARCH生物生物52311092-2172MICROBIOL MOL BIOL R MICROBIOLOGY AND MOLECULAR BIOL83072047-7538LIGHT-SCI APPL Light-Science & Applications OPTICS 76811758-678X NAT CLIM CHANGE Nature climate change ENVIRONMENTAL 57581359-4184MOL PSYCHIATR MOLECULAR PSYCHIATRY医学1820003-990X ARCH GEN PSYCHIAT ARCHIVES OF GENERAL PSYCHIATRY医学3590009-7322CIRCULATION CIRCULATION医学67721549-1676PLOS MED PLOS MEDICINE MEDICINE, GENER 49041063-5157SYST BIOL SYSTEMATIC BIOLOGY生物81041941-1405ANNU REV MAR SCI Annual Review of Marine Science地学73441723-8617WORLD PSYCHIATRY World Psychiatry医学64881523-9829ANNU REV BIOMED ENG ANNUAL REVIEW OF BIOMEDICAL ENG工程技术76901759-4774NAT REV CLIN ONCOL Nature Reviews Clinical Oncology医学58401369-7021MATER TODAY Materials Today工程技术52771097-2765MOL CELL MOLECULAR CELL生物26720302-2838EUR UROL EUROPEAN UROLOGY医学14580066-4170ANNU REV ENTOMOL ANNUAL REVIEW OF ENTOMOLOGY生物65641530-6984NANO LETT NANO LETTERS工程技术19380166-2236TRENDS NEUROSCI TRENDS IN NEUROSCIENCES医学67381545-9993NAT STRUCT MOL BIOL NATURE STRUCTURAL & MOLECULAR BBIOCHEMISTRY & 76941759-5029NAT REV ENDOCRINOL Nature Reviews Endocrinology医学19350166-0616STUD MYCOL STUDIES IN MYCOLOGY生物20150168-6445FEMS MICROBIOL REV FEMS MICROBIOLOGY REVIEWS生物医学7260021-9738J CLIN INVEST JOURNAL OF CLINICAL INVESTIGATION84752168-6106JAMA INTERN MED JAMA Internal Medicine MEDICINE, GENER医学50501073-449X AM J RESP CRIT CARE AMERICAN JOURNAL OF RESPIRATORY68091552-4450NAT CHEM BIOL Nature Chemical Biology生物57731360-1385TRENDS PLANT SCI TRENDS IN PLANT SCIENCE生物14610066-4219ANNU REV MED ANNUAL REVIEW OF MEDICINE医学80481936-0851ACS NANO ACS Nano工程技术医学67671548-5943ANNU REV CLIN PSYCHO ANNUAL REVIEW OF CLINICAL PSYCHO76951759-5045NAT REV GASTRO HEPAT Nature Reviews Gastroenterology & Hepat医学10000027-8874JNCI-J NATL CANCER I JNCI-Journal of the National Cancer Institu ONCOLOGY医学7570022-1007J EXP MED JOURNAL OF EXPERIMENTAL MEDICINE44551001-0602CELL RES CELL RESEARCH生物68181552-5260ALZHEIMERS DEMENT Alzheimers & Dementia医学79881931-3128CELL HOST MICROBE Cell Host & Microbe生物1050002-953X AM J PSYCHIAT AMERICAN JOURNAL OF PSYCHIATRY医学3970010-8545COORDIN CHEM REV COORDINATION CHEMISTRY REVIEWS化学14620066-4227ANNU REV MICROBIOL ANNUAL REVIEW OF MICROBIOLOGY生物770002-7863J AM CHEM SOC JOURNAL OF THE AMERICAN CHEMICA化学84802168-622X JAMA PSYCHIAT JAMA Psychiatry PSYCHIATRY 43000962-8924TRENDS CELL BIOL TRENDS IN CELL BIOLOGY生物19870167-7799TRENDS BIOTECHNOL TRENDS IN BIOTECHNOLOGY工程技术76871759-0876WIRES COMPUT MOL SCI W IREs Computational Molecular Science CHEMISTRY, MUL 65801531-7331ANNU REV MATER RES ANNUAL REVIEW OF MATERIALS RESE工程技术1150003-0007B AM METEOROL SOC BULLETIN OF THE AMERICAN METEOR地学71151616-301X ADV FUNCT MATER ADVANCED FUNCTIONAL MATERIALS工程技术68331554-8627AUTOPHAGY Autophagy生物75871752-0894NAT GEOSCI Nature Geoscience地学医学19310165-6147TRENDS PHARMACOL SCI TRENDS IN PHARMACOLOGICAL SCIEN15560091-6749J ALLERGY CLIN IMMUN JOURNAL OF ALLERGY AND CLINICAL I医学82642041-1723NAT COMMUN Nature communications Biological Science Disciplines 20200168-8278J HEPATOL JOURNAL OF HEPATOLOGY医学化学60491433-7851ANGEW CHEM INT EDIT ANGEWANDTE CHEMIE-INTERNATIONA19030163-8998ANNU REV NUCL PART S ANNUAL REVIEW OF NUCLEAR AND PA物理43630968-0004TRENDS BIOCHEM SCI TRENDS IN BIOCHEMICAL SCIENCES生物14690067-0049ASTROPHYS J SUPPL S ASTROPHYSICAL JOURNAL SUPPLEME地学天文物理14590066-4189ANNU REV FLUID MECH ANNUAL REVIEW OF FLUID MECHANICS24880270-9139HEPATOLOGY HEPATOLOGY医学3600009-7330CIRC RES CIRCULATION RESEARCH医学940002-9297AM J HUM GENET AMERICAN JOURNAL OF HUMAN GENE生物74601744-4292MOL SYST BIOL Molecular Systems Biology生物61981465-6906GENOME BIOL GENOME BIOLOGY BIOTECHNOLOGY 36240890-9369GENE DEV GENES & DEVELOPMENT生物370001-6322ACTA NEUROPATHOL ACTA NEUROPATHOLOGICA医学930002-9270AM J GASTROENTEROL AMERICAN JOURNAL OF GASTROENTE医学61551461-023X ECOL LETT ECOLOGY LETTERS环境科学67111543-592X ANNU REV ECOL EVOL S ANNUAL REVIEW OF ECOLOGY EVOLU生物2530006-4971BLOOD BLOOD医学84252157-1724KIDNEY INT SUPPL Kidney International Supplements UROLOGY & NEPHROLOGY 23760261-4189EMBO J EMBO JOURNAL生物35670887-6924LEUKEMIA LEUKEMIA医学62881471-4906TRENDS IMMUNOL TRENDS IN IMMUNOLOGY医学医学1580003-4967ANN RHEUM DIS ANNALS OF THE RHEUMATIC DISEASES85252211-2855NANO ENERGY Nano Energy CHEMISTRY, PHYS 2400006-3223BIOL PSYCHIAT BIOLOGICAL PSYCHIATRY医学16200098-2997MOL ASPECTS MED MOLECULAR ASPECTS OF MEDICINE医学57341355-4786HUM REPROD UPDATE HUMAN REPRODUCTION UPDATE医学16720105-2896IMMUNOL REV IMMUNOLOGICAL REVIEWS医学79641884-4049NPG ASIA MATER NPG Asia Materials MATERIALS SCIEN 81361943-8206ADV OPT PHOTONICS Advances in Optics and Photonics OPTICS24090265-0568NAT PROD REP NATURAL PRODUCT REPORTS化学85472214-109X LANCET GLOB HEALTH Lancet Global Health PUBLIC, ENVIRON 19010163-7827PROG LIPID RES PROGRESS IN LIPID RESEARCH生物28830360-0564ADV CATAL ADVANCES IN CATALYSIS化学26360301-0082PROG NEUROBIOL PROGRESS IN NEUROBIOLOGY医学29480364-5134ANN NEUROL ANNALS OF NEUROLOGY医学20260168-9525TRENDS GENET TRENDS IN GENETICS生物76911759-4790NAT REV RHEUMATOL Nature Reviews Rheumatology医学7130021-9525J CELL BIOLJOURNAL OF CELL BIOLOGY 生物18980163-7258PHARMACOL THERAPEUT P HARMACOLOGY & THERAPEUTICS 医学66051534-5807DEV CELL DEVELOPMENTAL CELL 生物9990027-8424P NATL ACAD SCI USA PROCEEDINGS OF THE NATIONAL ACA 综合性期刊76111754-2189NAT PROTOC Nature Protocols 生物61911464-7931BIOL REV BIOLOGICAL REVIEWS 生物85402213-2600LANCET RESP MED Lancet Respiratory Medicine RESPIRATORY SYSTEM 14650066-4286ANNU REV PHYTOPATHOL ANNUAL REVIEW OF PHYTOPATHOLOG 生物62141467-5463BRIEF BIOINFORM BRIEFINGS IN BIOINFORMATICS 生物33610742-3098J PINEAL RES JOURNAL OF PINEAL RESEARCH 医学42810960-9822CURR BIOL CURRENT BIOLOGY 生物84242157-1422CSH PERSPECT MED Cold Spring Harbor perspectives in medicin MEDICINE, RESEA 62891471-4914TRENDS MOL MED TRENDS IN MOLECULAR MEDICINE 医学47001043-2760TRENDS ENDOCRIN MET TRENDS IN ENDOCRINOLOGY AND MET 医学47361046-6673J AM SOC NEPHROL JOURNAL OF THE AMERICAN SOCIETY 医学67321545-7885PLOS BIOL PLoS Biology BIOCHEMISTRY &46501040-4651PLANT CELL PLANT CELL 生物47171044-579X SEMIN CANCER BIOL SEMINARS IN CANCER BIOLOGY 医学3220008-5472CANCER RES CANCER RESEARCH 医学83162050-084X ELIFE eLife BIOLOGY 84112155-5435ACS CATAL ACS catalysis CHEMISTRY, PHYSICAL 75601751-7362ISME J ISME Journal 环境科学82692041-6520CHEM SCI Chemical science CHEMISTRY, MULT 2610006-8950BRAIN BRAIN 医学11620033-3190PSYCHOTHER PSYCHOSOM PSYCHOTHERAPY AND PSYCHOSOMAT 医学43460966-842X TRENDS MICROBIOL TRENDS IN MICROBIOLOGY 生物85462213-8587LANCET DIABETES ENDO Lancet Diabetes & Endocrinology ENDOCRINOLOGY 76931759-5002NAT REV CARDIOL Nature Reviews Cardiology 医学26130300-5771INT J EPIDEMIOL INTERNATIONAL JOURNAL OF EPIDEMI 医学58301368-7646DRUG RESIST UPDATE DRUG RESISTANCE UPDATES 医学27240305-1048NUCLEIC ACIDS RES NUCLEIC ACIDS RESEARCH 生物33250737-4038MOL BIOL EVOL MOLECULAR BIOLOGY AND EVOLUTION 生物62311469-221X EMBO REP EMBO REPORTS 生物84392160-3308PHYS REV X Physical Review X PHYSICS, MULTIDISCIPLINAR 32930734-9750BIOTECHNOL ADV BIOTECHNOLOGY ADVANCES 工程技术65391527-8204ANNU REV GENOM HUM G A NNUAL REVIEW OF GENOMICS AND H 生物48421058-4838CLIN INFECT DIS CLINICAL INFECTIOUS DISEASES 医学80501936-1327ANNU REV ANAL CHEM Annual Review of Analytical Chemistry CHEMISTRY, ANAL 18530149-7634NEUROSCI BIOBEHAV R NEUROSCIENCE AND BIOBEHAVIORAL 医学49101063-6706IEEE T FUZZY SYST IEEE TRANSACTIONS ON FUZZY SYSTE 工程技术56991350-9462PROG RETIN EYE RES PROGRESS IN RETINAL AND EYE RESE 医学50971078-0432CLIN CANCER RES CLINICAL CANCER RESEARCH 医学81271943-0264CSH PERSPECT BIOL Cold Spring Harbor perspectives in biology CELL BIOLOGY 81541947-5438ANNU REV CHEM BIOMOL Annual review of chemical and biomolecula CHEMISTRY, APPL 47411047-3211CEREB CORTEX CEREBRAL CORTEX 医学76581757-4676EMBO MOL MED EMBO Molecular Medicine 医学15250084-6597ANNU REV EARTH PL SC ANNUAL REVIEW OF EARTH AND PLAN地学天文85372212-6864PHYS DARK UNIVERSE Physics of the Dark Universe ASTRONOMY & ASTROPHYSI 15260085-2538KIDNEY INT KIDNEY INTERNATIONAL医学17610142-9612BIOMATERIALS BIOMATERIALS工程技术76961759-5061NAT REV NEPHROL Nature Reviews Nephrology医学51771087-0792SLEEP MED REV SLEEP MEDICINE REVIEWS医学41100950-6608INT MATER REV INTERNATIONAL MATERIALS REVIEWS工程技术化学18720161-4940CATAL REV CATALYSIS REVIEWS-SCIENCE AND EN41800955-0674CURR OPIN CELL BIOL CURRENT OPINION IN CELL BIOLOGY生物41120950-9232ONCOGENE ONCOGENE医学31840586-7614SCHIZOPHRENIA BULL SCHIZOPHRENIA BULLETIN医学21910198-6325MED RES REV MEDICINAL RESEARCH REVIEWS医学18490149-5992DIABETES CARE DIABETES CARE医学37140896-8411J AUTOIMMUN JOURNAL OF AUTOIMMUNITY医学71011613-6810SMALL SMALL工程技术21950199-9885ANNU REV NUTR ANNUAL REVIEW OF NUTRITION医学85242211-1247CELL REP Cell reports CELL BIOLOGY37210897-4756CHEM MATER CHEMISTRY OF MATERIALS工程技术1570003-4932ANN SURG ANNALS OF SURGERY医学13180040-6376THORAX THORAX医学10150028-3878NEUROLOGY NEUROLOGY医学62101467-2960FISH FISH FISH AND FISHERIES农林科学56481342-937X GONDWANA RES GONDWANA RESEARCH地学56981350-9047CELL DEATH DIFFER CELL DEATH AND DIFFERENTIATION生物85532223-7755GEOCHEM PERSPECT Geochemical Perspectives GEOCHEMISTRY & 4220012-1797DIABETES DIABETES医学57201354-1013GLOBAL CHANGE BIOL GLOBAL CHANGE BIOLOGY环境科学77831838-7640THERANOSTICS THERANOSTICS MEDICINE, RESEA 61781463-9262GREEN CHEM GREEN CHEMISTRY化学78381863-8880LASER PHOTONICS REV Laser & Photonics Reviews物理62171467-7881OBES REV Obesity Reviews医学2760007-1250BRIT J PSYCHIAT BRITISH JOURNAL OF PSYCHIATRY医学74121741-7007BMC BIOL BMC BIOLOGY生物环境科学15570091-6765ENVIRON HEALTH PERSP ENVIRONMENTAL HEALTH PERSPECTIV11570033-295X PSYCHOL REV PSYCHOLOGICAL REVIEW医学69511568-9972AUTOIMMUN REV AUTOIMMUNITY REVIEWS医学3680009-9147CLIN CHEM CLINICAL CHEMISTRY医学医学3730009-9236CLIN PHARMACOL THER CLINICAL PHARMACOLOGY & THERAPE67011542-3565CLIN GASTROENTEROL H Clinical Gastroenterology and Hepatology医学4390012-8252EARTH-SCI REV EARTH-SCIENCE REVIEWS地学58361369-5266CURR OPIN PLANT BIOL CURRENT OPINION IN PLANT BIOLOGY生物医学27100304-419X BBA-REV CANCER BIOCHIMICA ET BIOPHYSICA ACTA-REV生物11730033-5835Q REV BIOPHYS QUARTERLY REVIEWS OF BIOPHYSICS化学560001-8686ADV COLLOID INTERFAC ADVANCES IN COLLOID AND INTERFAC1990004-3591ARTHRITIS RHEUM-US ARTHRITIS & RHEUMATISM-ARTHRITIS医学78321863-2297SEMIN IMMUNOPATHOL Seminars in Immunopathology医学46731040-9238CRIT REV BIOCHEM MOL CRITICAL REVIEWS IN BIOCHEMISTRY 生物25510277-7037MASS SPECTROM REV MASS SPECTROMETRY REVIEWS物理20130168-3659J CONTROL RELEASE JOURNAL OF CONTROLLED RELEASE医学10210028-646X NEW PHYTOL NEW PHYTOLOGIST生物78421864-5631CHEMSUSCHEM ChemSusChem化学37510903-1936EUR RESPIR J EUROPEAN RESPIRATORY JOURNAL医学27360305-7372CANCER TREAT REV CANCER TREATMENT REVIEWS医学48921062-7995PROG PHOTOVOLTAICS PROGRESS IN PHOTOVOLTAICS工程技术生物42350959-437X CURR OPIN GENET DEV CURRENT OPINION IN GENETICS & DEV68281553-7366PLOS PATHOG PLoS Pathogens生物68291553-7390PLOS GENET PLoS Genetics生物11050031-9007PHYS REV LETT PHYSICAL REVIEW LETTERS物理65491529-1839OPTOMETRY OPTOMETRY OPHTHALMOLOGY4270012-3692CHEST CHEST医学41370952-7915CURR OPIN IMMUNOL CURRENT OPINION IN IMMUNOLOGY医学69851571-0645PHYS LIFE REV Physics of Life Reviews生物81631948-7185J PHYS CHEM LETT The journal of physical chemistry letters CHEMISTRY, PHYS 83212050-7488J MATER CHEM A Journal of Materials Chemistry A CHEMISTRY, PHYS环境科学66791540-9295FRONT ECOL ENVIRON FRONTIERS IN ECOLOGY AND THE ENV环境科学38970926-3373APPL CATAL B-ENVIRON APPLIED CATALYSIS B-ENVIRONMENTA8310022-3417J PATHOL JOURNAL OF PATHOLOGY医学1840003-9942ARCH NEUROL-CHICAGO ARCHIVES OF NEUROLOGY医学64781523-0864ANTIOXID REDOX SIGN ANTIOXIDANTS & REDOX SIGNALING生物82562040-3364NANOSCALE Nanoscale CHEMISTRY, MUL80181933-0219MUCOSAL IMMUNOL Mucosal Immunology医学75341749-5016SOC COGN AFFECT NEUR Social Cognitive and Affective Neuroscienc医学42930962-4929ACTA NUMER ACTA NUMERICA MATHEMATICS80611936-8798JACC-CARDIOVASC INTE JACC. Cardiovascular interventions CARDIAC & CARDI 52851098-3600GENET MED GENETICS IN MEDICINE医学1190003-0090B AM MUS NAT HIST BULLETIN OF THE AMERICAN MUSEUM环境科学84892190-5991J CACHEXIA SARCOPENI Journal of Cachexia Sarcopenia and Muscl MEDICINE, GENER 84762168-6149JAMA NEUROL JAMA Neurology CLINICAL NEUROLOGY医学36210890-8567J AM ACAD CHILD PSY JOURNAL OF THE AMERICAN ACADEMY74131741-7015BMC MED BMC Medicine医学76241755-263X CONSERV LETT Conservation Letters BIODIVERSITY CONSERVATIO物理15070079-6565PROG NUCL MAG RES SP PROGRESS IN NUCLEAR MAGNETIC RE19850167-7659CANCER METAST REV CANCER AND METASTASIS REVIEWS医学医学7860022-202X J INVEST DERMATOL JOURNAL OF INVESTIGATIVE DERMATO28540342-4642INTENS CARE MED INTENSIVE CARE MEDICINE医学42370959-440X CURR OPIN STRUC BIOL CURRENT OPINION IN STRUCTURAL BI生物80601936-878X JACC-CARDIOVASC IMAG JACC. Cardiovascular imaging CARDIAC & CARD33330738-8551CRIT REV BIOTECHNOL CRITICAL REVIEWS IN BIOTECHNOLOG工程技术84792168-6203JAMA PEDIATR JAMA Pediatrics PEDIATRICS42210958-1669CURR OPIN BIOTECH CURRENT OPINION IN BIOTECHNOLOG工程技术74501743-8977PART FIBRE TOXICOL Particle and fibre toxicology TOXICOLOGY生物42960962-8436PHILOS T R SOC B PHILOSOPHICAL TRANSACTIONS OF THNEUROPSYCHOPHARMACOLOGY医学36560893-133X NEUROPSYCHOPHARMACO38530923-7534ANN ONCOL ANNALS OF ONCOLOGY医学15500091-3022FRONT NEUROENDOCRIN FRONTIERS IN NEUROENDOCRINOLOG医学17840144-235X INT REV PHYS CHEM INTERNATIONAL REVIEWS IN PHYSICA化学82261998-0124NANO RES Nano Research化学化学14450065-3055ADV ORGANOMET CHEM ADVANCES IN ORGANOMETALLIC CHEM4410012-9615ECOL MONOGR ECOLOGICAL MONOGRAPHS环境科学17300140-7791PLANT CELL ENVIRON PLANT CELL AND ENVIRONMENT生物7120021-9517J CATAL JOURNAL OF CATALYSIS工程技术11830033-8419RADIOLOGY RADIOLOGY医学11250032-0889PLANT PHYSIOL PLANT PHYSIOLOGY生物50551073-8584NEUROSCIENTIST NEUROSCIENTIST医学57701359-7345CHEM COMMUN CHEMICAL COMMUNICATIONS化学35080884-0431J BONE MINER RES JOURNAL OF BONE AND MINERAL RES医学生物58211367-5931CURR OPIN CHEM BIOL CURRENT OPINION IN CHEMICAL BIOLO8170022-3050J NEUROL NEUROSUR PS JOURNAL OF NEUROLOGY NEUROSUR医学COMPUTER SCIEN 68301553-877X IEEE COMMUN SURV TUT IEEE COMMUNICATIONS SURVEYS AND83942150-7511MBIO mBio MICROBIOLOGY 72461674-2788J MOL CELL BIOL Journal of molecular cell biology CELL BIOLOGY 890002-9165AM J CLIN NUTR AMERICAN JOURNAL OF CLINICAL NUT医学52751096-7176METAB ENG METABOLIC ENGINEERING工程技术14380065-2660ADV GENET ADVANCES IN GENETICS生物51221080-6040EMERG INFECT DIS EMERGING INFECTIOUS DISEASES医学81421944-8244ACS APPL MATER INTER ACS Applied Materials & Interfaces工程技术57651359-6446DRUG DISCOV TODAY DRUG DISCOVERY TODAY医学4230012-186X DIABETOLOGIA DIABETOLOGIA医学21520193-936X EPIDEMIOL REV EPIDEMIOLOGIC REVIEWS医学医学47911053-2498J HEART LUNG TRANSPL JOURNAL OF HEART AND LUNG TRANS50601074-5521CHEM BIOL CHEMISTRY & BIOLOGY生物42360959-4388CURR OPIN NEUROBIOL CURRENT OPINION IN NEUROBIOLOGY医学化学15120079-6786PROG SOLID STATE CH PROGRESS IN SOLID STATE CHEMISTR66181535-9476MOL CELL PROTEOMICS MOLECULAR & CELLULAR PROTEOMIC生物75431750-1326MOL NEURODEGENER Molecular Neurodegeneration医学82652041-210X METHODS ECOL EVOL Methods in ecology and evolution ECOLOGY环境科学62071466-822X GLOBAL ECOL BIOGEOGR GLOBAL ECOLOGY AND BIOGEOGRAPH59381388-9842EUR J HEART FAIL EUROPEAN JOURNAL OF HEART FAILU医学49671066-5099STEM CELLS STEM CELLS医学17070129-0657INT J NEURAL SYST International Journal of Neural Systems工程技术25530278-0046IEEE T IND ELECTRON IEEE TRANSACTIONS ON INDUSTRIAL E工程技术42900962-1083MOL ECOL MOLECULAR ECOLOGY生物21560194-911X HYPERTENSION HYPERTENSION医学19340165-9936TRAC-TREND ANAL CHEM TRAC-TRENDS IN ANALYTICAL CHEMIS化学18990163-7525ANNU REV PUBL HEALTH ANNUAL REVIEW OF PUBLIC HEALTH医学41020950-1991DEVELOPMENT DEVELOPMENT生物医学7220021-9630J CHILD PSYCHOL PSYC JOURNAL OF CHILD PSYCHOLOGY AND物理46661040-8436CRIT REV SOLID STATE CRITICAL REVIEWS IN SOLID STATE AN81451945-4589AGING-US Aging CELL BIOLOGY 59271387-6473NEW ASTRON REV NEW ASTRONOMY REVIEWS地学天文74431743-5390NANOTOXICOLOGY Nanotoxicology医学41970956-5663BIOSENS BIOELECTRON BIOSENSORS & BIOELECTRONICS工程技术11870034-4257REMOTE SENS ENVIRON REMOTE SENSING OF ENVIRONMENT环境科学43220964-6906HUM MOL GENET HUMAN MOLECULAR GENETICS生物64871523-7060ORG LETT ORGANIC LETTERS化学61691462-8902DIABETES OBES METAB DIABETES OBESITY & METABOLISM医学81681949-2553ONCOTARGET Oncotarget Oncogenes 47971053-8119NEUROIMAGE NEUROIMAGE医学24860270-6474J NEUROSCI JOURNAL OF NEUROSCIENCE医学63411474-9718AGING CELL AGING CELL生物72451674-2052MOL PLANT Molecular Plant生物8020022-2593J MED GENET JOURNAL OF MEDICAL GENETICS医学79091874-9399BBA-GENE REGUL MECH Biochimica et Biophysica Acta-Gene Regul生物15320090-3493CRIT CARE MED CRITICAL CARE MEDICINE医学81051941-1413ANNU REV FOOD SCI T Annual review of food science and technoloFOOD SCIENCE & 12760038-6308SPACE SCI REV SPACE SCIENCE REVIEWS地学天文81441945-0877SCI SIGNAL Science Signaling BIOCHEMISTRY & 26820303-4240REV PHYSIOL BIOCH P REVIEWS OF PHYSIOLOGY BIOCHEMIS医学84482162-4011ONCOIMMUNOLOGY OncoImmunology ONCOLOGY生物51631084-9521SEMIN CELL DEV BIOL SEMINARS IN CELL & DEVELOPMENTAL9450025-6196MAYO CLIN PROC MAYO CLINIC PROCEEDINGS医学工程技术57541359-0286CURR OPIN SOLID ST M CURRENT OPINION IN SOLID STATE & M79001873-9946J CROHNS COLITIS Journal of Crohns & Colitis医学64971525-0016MOL THER MOLECULAR THERAPY医学14460065-308X ADV PARASIT ADVANCES IN PARASITOLOGY医学68991560-2745FUNGAL DIVERS FUNGAL DIVERSITY生物51441083-4419IEEE T SYST MAN CY B IEEE TRANSACTIONS ON SYSTEMS MA工程技术81121941-7640CIRC-CARDIOVASC INTE Circulation: Cardiovascular Interventions CARDIAC & CARDI 30630378-7753J POWER SOURCES JOURNAL OF POWER SOURCES工程技术生物33830748-3007CLADISTICS CLADISTICS-THE INTERNATIONAL JOUR生物58771383-5742MUTAT RES-REV MUTAT MUTATION RESEARCH-REVIEWS IN MU7250021-972X J CLIN ENDOCR METAB JOURNAL OF CLINICAL ENDOCRINOLO医学62901471-4922TRENDS PARASITOL TRENDS IN PARASITOLOGY医学61641462-2912ENVIRON MICROBIOL ENVIRONMENTAL MICROBIOLOGY环境科学3240008-6223CARBON CARBON工程技术47121044-3983EPIDEMIOLOGY EPIDEMIOLOGY医学36600893-3952MODERN PATHOL MODERN PATHOLOGY医学18730161-5505J NUCL MED JOURNAL OF NUCLEAR MEDICINE医学68751558-3724POLYM REV Polymer Reviews工程技术67831549-9634NANOMED-NANOTECHNOL Nanomedicine-Nanotechnology Biology an工程技术18760161-6420OPHTHALMOLOGY OPHTHALMOLOGY医学29840370-2693PHYS LETT B PHYSICS LETTERS B物理63151473-0197LAB CHIP LAB ON A CHIP工程技术46281029-8479J HIGH ENERGY PHYS JOURNAL OF HIGH ENERGY PHYSICS PHYSICS, PARTICL10420029-6643NUTR REV NUTRITION REVIEWS医学79501879-6257CURR OPIN VIROL Current Opinion in Virology VIROLOGY 41750954-6820J INTERN MED JOURNAL OF INTERNAL MEDICINE医学76151754-6834BIOTECHNOL BIOFUELS Biotechnology for Biofuels工程技术医学62321469-493X COCHRANE DB SYST REV Cochrane Database of Systematic Reviews36740894-1491GLIA GLIA医学16730105-4538ALLERGY ALLERGY医学74331742-7061ACTA BIOMATER Acta Biomaterialia工程技术46641040-841X CRIT REV MICROBIOL CRITICAL REVIEWS IN MICROBIOLOGY生物76631757-7004WIRES RNA WIRES RNA CELL BIOLOGY工程技术35420885-8993IEEE T POWER ELECTR IEEE TRANSACTIONS ON POWER ELEC51121079-5642ARTERIOSCL THROM VAS ARTERIOSCLEROSIS THROMBOSIS AN医学7830022-1899J INFECT DIS JOURNAL OF INFECTIOUS DISEASES医学2090004-637X ASTROPHYS J ASTROPHYSICAL JOURNAL地学天文28390340-5761ARCH TOXICOL ARCHIVES OF TOXICOLOGY医学42700960-7412PLANT J PLANT JOURNAL生物49631065-9471HUM BRAIN MAPP HUMAN BRAIN MAPPING医学5280016-6731GENETICS GENETICS生物14410065-2776ADV IMMUNOL ADVANCES IN IMMUNOLOGY医学34470820-3946CAN MED ASSOC J CANADIAN MEDICAL ASSOCIATION JOU医学3260008-6363CARDIOVASC RES CARDIOVASCULAR RESEARCH医学11560033-2917PSYCHOL MED PSYCHOLOGICAL MEDICINE医学43190964-4563TOB CONTROL TOBACCO CONTROL医学57881364-0321RENEW SUST ENERG REV R ENEWABLE & SUSTAINABLE ENERGY工程技术医学25140272-6386AM J KIDNEY DIS AMERICAN JOURNAL OF KIDNEY DISEA58371369-5274CURR OPIN MICROBIOL CURRENT OPINION IN MICROBIOLOGY生物环境科学67121543-5938ANNU REV ENV RESOUR ANNUAL REVIEW OF ENVIRONMENT AN81081941-3289CIRC-HEART FAIL Circulation-Heart Failure医学35880889-1591BRAIN BEHAV IMMUN BRAIN BEHAVIOR AND IMMUNITY医学1350003-3022ANESTHESIOLOGY ANESTHESIOLOGY医学47501049-250X ADV ATOM MOL OPT PHY ADVANCES IN ATOMIC MOLECULAR AN物理医学53951180-4882J PSYCHIATR NEUROSCI JOURNAL OF PSYCHIATRY & NEUROSC47961053-5888IEEE SIGNAL PROC MAG IEEE SIGNAL PROCESSING MAGAZINE工程技术57551359-0294CURR OPIN COLLOID IN CURRENT OPINION IN COLLOID & INTE化学31020390-6078HAEMATOLOGICA HAEMATOLOGICA HEMATOLOGY地学天文63551475-7516J COSMOL ASTROPART P JOURNAL OF COSMOLOGY AND ASTRO生物60021420-682X CELL MOL LIFE SCI CMLS-Cellular and Molecular Life Sciences9170024-9297MACROMOLECULES MACROMOLECULES工程技术84962192-2640ADV HEALTHC MATER Advanced healthcare materials工程技术82952046-2441OPEN BIOL Open biology BIOCHEMISTRY &工程技术18870162-8828IEEE T PATTERN ANAL IEEE TRANSACTIONS ON PATTERN ANA54041198-743X CLIN MICROBIOL INFEC CLINICAL MICROBIOLOGY AND INFECT医学84402161-1653ACS MACRO LETT ACS Macro Letters化学62151467-7644PLANT BIOTECHNOL J PLANT BIOTECHNOLOGY JOURNAL生物62121467-3037CURR ISSUES MOL BIOL CURRENT ISSUES IN MOLECULAR BIOL生物65071525-7797BIOMACROMOLECULES BIOMACROMOLECULES化学36340891-5849FREE RADICAL BIO MED FREE RADICAL BIOLOGY AND MEDICIN医学40690947-6539CHEM-EUR J CHEMISTRY-A EUROPEAN JOURNAL化学50391072-4710ARCH PEDIAT ADOL MED ARCHIVES OF PEDIATRICS & ADOLESC医学医学24640269-2813ALIMENT PHARM THER ALIMENTARY PHARMACOLOGY & THER9590025-7974MEDICINE MEDICINE医学12840039-2499STROKE STROKE医学69011560-7917EUROSURVEILLANCE EUROSURVEILLANCE INFECTIOUS DISEASES 66541538-7933J THROMB HAEMOST JOURNAL OF THROMBOSIS AND HAEM医学84262157-6564STEM CELL TRANSL MED Stem cells translational medicine CELL & TISSUE ENGINEERING 15130079-6816PROG SURF SCI PROGRESS IN SURFACE SCIENCE工程技术84982192-8606NANOPHOTONICS-BERLIN N anophotonics NANOSCIENCE &66161535-7163MOL CANCER THER MOLECULAR CANCER THERAPEUTICS医学医学70591600-6135AM J TRANSPLANT AMERICAN JOURNAL OF TRANSPLANTA35210885-3185MOVEMENT DISORD MOVEMENT DISORDERS医学35010883-7694MRS BULL MRS BULLETIN工程技术26690301-9268PRECAMBRIAN RES PRECAMBRIAN RESEARCH地学71071615-4150ADV SYNTH CATAL ADVANCED SYNTHESIS & CATALYSIS化学42180957-9672CURR OPIN LIPIDOL CURRENT OPINION IN LIPIDOLOGY医学81131941-7705CIRC-CARDIOVASC QUAL Circulation: Cardiovascular Quality and Ou CARDIAC & CARDI1320003-2700ANAL CHEM ANALYTICAL CHEMISTRY化学26970304-3835CANCER LETT CANCER LETTERS医学84312158-3188TRANSL PSYCHIAT Translational Psychiatry PSYCHIATRY43740969-2126STRUCTURE STRUCTURE生物78331863-2653BRAIN STRUCT FUNCT Brain Structure & Function医学27410306-2619APPL ENERG APPLIED ENERGY工程技术450001-690X ACTA PSYCHIAT SCAND ACTA PSYCHIATRICA SCANDINAVICA医学17750143-5221CLIN SCI CLINICAL SCIENCE医学57371355-6037HEART HEART医学82852045-2322SCI REP-UK Scientific reports Natural Science Disciplines 47861052-9276REV MED VIROL REVIEWS IN MEDICAL VIROLOGY医学34040749-6419INT J PLASTICITY INTERNATIONAL JOURNAL OF PLASTIC工程技术24600268-960X BLOOD REV BLOOD REVIEWS医学64761522-8517NEURO-ONCOLOGY NEURO-ONCOLOGY医学18600160-4120ENVIRON INT ENVIRONMENT INTERNATIONAL环境科学24800269-9370AIDS AIDS医学2780007-1323BRIT J SURG BRITISH JOURNAL OF SURGERY医学13480043-1354WATER RES WATER RESEARCH环境科学57241354-3784EXPERT OPIN INV DRUG EXPERT OPINION ON INVESTIGATIONA医学7550022-0957J EXP BOT JOURNAL OF EXPERIMENTAL BOTANY生物7450022-0477J ECOL JOURNAL OF ECOLOGY环境科学77031759-9954POLYM CHEM-UK POLYMER CHEMISTRY POLYMER SCIENCE 82141994-0416CRYOSPHERE Cryosphere GEOGRAPHY, PHY 18630160-6689J CLIN PSYCHIAT JOURNAL OF CLINICAL PSYCHIATRY医学67821549-9618J CHEM THEORY COMPUT J ournal of Chemical Theory and Computat化学65401527-8999CHEM REC CHEMICAL RECORD化学61951465-542X BREAST CANCER RES BREAST CANCER RESEARCH ONCOLOGY。
血管外膜细胞钙化及其钙化机制研究

血管外膜细胞钙化及其钙化机制研究谭小青,张旭升,樊小容,黄战军摘要 目的:研究经体外诱导钙化建立大鼠血管外膜细胞钙化模型,检测钙化过程中成骨相关指标及凋亡㊁自噬相关蛋白的表达变化,旨在为心血管疾病模型提供更精确的细胞模型,并初步探讨其钙化机制㊂方法:原代提取大鼠胸主动脉外膜纤维细胞,取3~6代细胞使用诱导培养基(高糖DMEM +10%胎牛血清+10mmol/L β-甘油磷酸+0.05mmol/L 抗坏血酸+100mmol/L 地塞米松)诱导钙化,诱导时间为3d ㊁6d ㊁9d ㊁12d ㊁15d ,筛选出诱导细胞钙化的最佳时间㊂对细胞采用茜素红S 染色㊁细胞内钙含量测定和碱性磷酸酶(ALP )活性检测,鉴定是否成功构建钙化模型㊂采用实时定量聚合酶链式反应(PT -PCR )检测成骨相关因子骨形态生成蛋白2(BMP2)和核心结合因子α1(Runx2)的mRNA 含量,蛋白免疫印迹法(Western Blot )检测凋亡蛋白Bax ㊁Bcl -2和自噬相关蛋白微血管相关蛋白(LC3)㊁Beclin -1的表达水平,找出血管外膜细胞钙化的潜在机制㊂结果:当诱导钙化时间为15d 时,血管外膜细胞中主要钙化指标胞内钙含量及ALP 活性上调(P <0.05),茜素红S 染色显示钙化组有明显钙盐沉积㊂血管外膜细胞经钙化诱导后,BMP2和Runx2的mRNA 水平上调,Bax 蛋白水平上调,Bcl -2和Beclin -1蛋白水平下调,LC3-Ⅱ/LC3-Ⅰ比值上调(P <0.05)㊂结论:钙化诱导培养基培养血管外膜细胞15d 可成功构建钙化细胞模型,血管外膜细胞钙化可能与细胞向成骨样表型转化有关,血管外膜细胞钙化过程涉及细胞自噬及凋亡调控㊂关键词 血管外膜细胞;钙化;成骨样表型转化;自噬与凋亡;实验研究d o i :10.12102/j.i s s n .1672-1349.2023.18.010 Calcification of Vascular Adventitial Cells and Its MechanismTAN Xiaoqing,ZHANG Xusheng,FAN Xiaorong,HUANG Zhanjun Longgang District People 's Hospital of Shenzhen,Shenzhen 518172,Guangdong,China Corresponding Author ZHANG Xusheng,E -mail:*****************Abstract Objective:To investigate the mananism of calcification of rat vascular adventitial cells,establish the calcification model of rat vascular adventitial cells,and detect the expression changes of osteogenesis -related indicators,apoptosis,and autophagy -related proteins during the calcification process.It aimed to provide more accurate cell models for cardiovascular disease and initially explore the mechanism of calcification.Methods:Rat thoracic aortic adventitial fibroblasts were extracted from the primary generation,and the 3rd to 6th generation cells were used for induction medium(high glucose DMEM +10%fetal bovine serum +10mmol/L β-glycerophosphate +0.05mmol/L ascorbic acid +100mmol/L dexamethasone)to induce calcification,the induction time was 3,6,9,12,and 15d,and the optimal time for inducing cell calcification was selected.The cells were stained with alizarin red S,detected by intracellular calcium content and alkaline phosphatase(ALP)to identify whether the calcification model was successfully constructed.Real -time quantitative reverse transcription polymerase chain reaction(RT -PCR)was used to detect the mRNA levels of osteogenesis -related factors bone morphogenetic protein(BMP2)and runt -related transcription factor 2(Runx2);Western Blot was used to detect the apoptosis proteins Bax,Bcl -2,the autophagy -related proteins LC -3,and Beclin -1expression level;then the potential mechanism of vascular adventitial cell calcification would be revealed.Results:When calcification was induced for 15days,the intracellular calcium content in the adventitial cells of the main calcification indicators and ALP activity were up -regulated(P <0.05).Alizarin red S staining showed obvious calcium deposits in the calcification group.After calcification was induced in adventitial cells,the mRNA levels of BMP2and Runx2up -regulated,the protein levels of Bax up -regulated,the protein levels of Bcl -2and Beclin -1down -regulated,and the ratio of LC3-Ⅱ/LC3-Ⅰdown -regulated(P <0.05).Conclusion:Adventitial cells cultured in the calcification -inducing medium for 15days could successfully construct a calcified cell model.calcification of adventitial cells might be related to the transformation of cells to an osteoblast -like phenotype.The Calcification process of adventitial cells involved autophagy and apoptosis regulation.Keywords adventitial cells;calcification;osteogenic phenotype transformation;autophagy and apoptosis;experimental study血管钙化常见于动脉粥样硬化㊁血脂异常㊁高血压㊁糖尿病㊁慢性肾病及衰老等人群[1],血管钙化引起血管硬度增加㊁顺应性降低,导致心肌缺血㊁心力衰竭㊁血栓形成等,增加脑卒中㊁心脏病㊁动脉粥样硬化斑块破裂等的风险,被认为是影响心血管疾病的重要因素之一[2-4]㊂目前关于血管内膜㊁中膜和心脏瓣膜钙化的关注和研究相对较多㊂临床工作中发现,血管外膜也可发生钙化,然而调查发现,现阶段对血管外膜钙化的作者单位 深圳市龙岗区人民医院(广东深圳518172)通讯作者 张旭升,E -mail :*****************引用信息 谭小青,张旭升,樊小容,等.血管外膜细胞钙化及其钙化机制研究[J ].中西医结合心脑血管病杂志,2023,21(18):3347-3350.关注较少,因此,需要更多的研究来阐明血管钙化的致病机制㊂最初血管钙化被认为是被动和退行性病变,标志着血管老化,但是越来越多研究表明血管钙化是类似于胚胎骨形成的病理生物学过程[5-6]㊂Bostr öm 等[7-8]研究发现,钙化过程中大鼠血管中膜细胞由原有收缩表型转变成为成骨样细胞表型,原有的收缩标志物如平滑肌肌动蛋白α(α-SMA )等表达减少,并表达核心结合因子α1(Runx2)㊁骨形态生成蛋白2(BMP2)等多种成骨样标志物,从而介导骨基质在血管中沉积㊂细胞凋亡与自噬为2种细胞死亡的方式,与血管钙化息息相关,研究表明,血管中膜细胞在细胞凋亡过程中释放凋亡小体,促进细胞钙化,而细胞自噬通过多种机制调控细胞钙化[9-10]㊂本研究对大鼠血管外膜细胞进行体外诱导钙化,建立大鼠血管外膜细胞钙化模型,并检测钙化过程中成骨相关指标及凋亡㊁自噬相关蛋白的表达变化,旨在为心血管疾病模型提供更精确的细胞模型,并初步探讨其钙化机制㊂1材料与方法1.1试剂胎牛血清(FBS,Gibco),青霉素,链霉素(Gibco,美国),茜素红S溶液,β-甘油磷酸,抗坏血酸,地塞米松(Sigma,美国),抗GAPDH抗体(Bioworld),抗Bcl-2, Bax,Bcelin1和微血管相关蛋白(LC3)抗体(CST),碱性磷酸酶检测试剂盒㊁钙(Ca)检测试剂盒(南京建城生物工程研究所)㊂1.2大鼠血管外膜细胞分离与培养取10只4~6周龄雄性Wistar-Kyoto大鼠(体质量120~180g)胸主动脉分离血管外膜,采用组织黏附法培养㊂使用添加10%胎牛血清的高糖DMEM培养基(Gibco dmem)在37ħ㊁5%二氧化碳条件下培养细胞㊂当细胞增殖至80%~90%融合时,用0.25%胰酶消化传代㊂使用第3代至第6代的细胞进行后续实验㊂1.3体外钙化模型的建立钙化诱导培养基为含10%胎牛血清,10mmol/L β-甘油磷酸钠,0.05mmol/L抗坏血酸和100mmol/L 地塞米松的高糖DMEM培养液㊂将第3代至第6代细胞分为对照组和钙化组,待细胞长至50%融合时,使用钙化诱导培养基培养,每3d更换1次培养基,连续培养15d㊂1.4碱性磷酸酶(ALP)酶活测定细胞钙化诱导后,弃去培养基,1ˑ磷酸缓冲盐溶液(PBS)洗细胞3次,加入裂解液500μL(1%T ritonX-100),冰上裂解40min后,离心,取上清液㊂使用上清液根据试剂盒说明书检测ALP活性及总蛋白含量㊂1.5细胞内钙含量检测细胞钙化诱导后,弃去培养基,1ˑPBS洗细胞3次,每孔加入500μL0.6mol/L的盐酸4ħ脱钙过夜,取上清,根据钙测试试剂盒说明书检测钙含量㊂将脱钙后的细胞用4ħPBS洗3次,每孔加入500μL NaOH/0.1%SDS裂解细胞,取上清,用二喹啉甲酸法(BCA)测定细胞蛋白含量㊂1.6茜素红S染色细胞钙化诱导15d,弃去培养基,1ˑPBS洗细胞3次,加入0.5mL4%多聚甲醛室温固定15min,用双蒸水洗3次,加入1mL0.1%茜素红室温孵育15min,吸去染液,双蒸水洗3次,在倒置显微镜下观察㊂1.7实时定量聚合酶链式反应(RT-PCR)检测细胞钙化诱导后,弃去培养基,1ˑPBS洗细胞3次,使用TaKaRa MiniBEST Universal RNA Extraction Kit提取总RNA,使用PrimeScrip TM RT reagent Kit将所提取的RNA逆转录合成cDNA,以cDNA为模板,通过SYBR Green I嵌合荧光定量RT-PCR检测BMP-2㊁Runx2和GAPDH的表达量㊂引物序列见表1㊂表1引物序列基因方向序列Runx2正向5'-TGGCTTTGGTTTCAGGTTAGG-3'反向5'-TGGAGATGTTGCTCTGTTCG-3' BMP-2正向5'-TGAGGATTAGCAGGTCTTTGC-3'反向5'-TCTCGTTTGTGGAGTGGATG-3' GAPDH正向5'-GGCTGCCCAGAACATCAT-3'反向5'-CGGACACATTGGGGGTAG-3'1.8蛋白免疫印迹法(Western Blot)检测细胞钙化诱导15d,弃去培养基,1ˑPBS洗细胞3次,提取细胞总蛋白㊂使用12%SDS-PAGE胶电泳分离,并转移到聚偏二氟乙烯膜(PVDF)上,封闭后,加入一抗(Bax1ʒ1000,Bcl-21ʒ1000,Beclin11ʒ1000, LC31ʒ1000,GAPDH1:1000)稀释液,4ħ孵育过夜;加入二抗稀释液(1ʒ10000)室温孵育1h后,使用ECL发光试剂盒显影并计算灰度值㊂1.9统计学处理应用SPSS19.0软件进行统计处理,符合正态分布的定量资料以均数ʃ标准差(xʃs)表示,比较采用t检验,以P<0.05为差异有统计学意义㊂2结果2.1大鼠血管外膜细胞可在体外被诱导钙化为验证高磷是否能诱导大鼠血管外膜细胞钙化,使用钙化诱导培养基培养细胞,在不同时间点检测ALP活性和胞内钙含量㊂随着培养时间延长,ALP活性逐渐上升,在培养第12天达到峰值,与对照组比较差异有统计学意义(P<0.05);诱导第3天开始所测得的胞内钙含量与对照组比较升高(P<0.05),ALP 活性和钙含量升高具有时间依赖性㊂详见图1㊁图2㊂诱导15d所测得钙含量最高,因此,后续实验选择的诱导时间为15d㊂对钙化诱导15d的细胞进行茜素红S染色,结果显示,对照组细胞呈长梭形,而钙化组细胞变成菱形㊂茜素红S染色后,钙化组可观察到大量的橘红色钙结节(见图3),而对照组完全没有㊂这也证明大鼠血管外膜细胞可在体外被钙化培养基诱导钙化㊂图1钙化诱导培养基诱导外膜细胞后ALP含量(与0d时比较,*P<0.05)图2钙化诱导培养基诱导外膜细胞后胞内钙含量(与0d时比较,*P<0.05)图3培养15d时细胞经茜素S红染色切片图(ˑ100)2.2血管外膜细胞钙化与细胞向成骨样表型转化有关血管钙化的增加与成骨细胞特异性标志物如BMP2㊁和Runx2的增加有关[11]㊂RT-PCR结果显示,与对照组比较,钙化组的成骨细胞特异性标志物BMP2和Runx2mRNA表达量增加,与对照组比较差异有统计学意义(P<0.05)㊂详见图4㊂图4外膜细胞钙化过程中BMP2和Runx2mRNA表达量(与对照组比较,*P<0.05)2.3血管外膜细胞钙化过程涉及细胞自噬及凋亡调控通过Western Blot检测凋亡和自噬相关蛋白的表达量变化㊂与对照组比较,钙化组促凋亡蛋白Bax表达上调,抑凋亡蛋白Bcl-2表达下调(P<0.05)㊂详见图5㊂钙化组自噬相关蛋白Beclin1表达上调,LC3-Ⅱ/ LC3-Ⅰ比例上调(P<0.05),说明钙化诱导培养后细胞内凋亡水平上调㊁自噬水平升高㊂详见图6㊂图5诱导钙化后促凋亡蛋白及抑凋亡蛋白表达变化图6诱导钙化后凋亡及自噬蛋白Beclin1等表达变化3讨论血管钙化作为心血管疾病病人的并发症之一,其发病率与严重程度逐年增高及加重,是导致心血管疾病病人高死亡率的重要因素㊂血管钙化缺乏有效的治疗药物㊂因此,探究血管钙化发病机制,在分子水平寻找有效的诊断和防治靶点是急需开展的基础研究工作㊂本研究证明,使用10mmol/Lβ-甘油磷酸+0.05 mmol/L抗坏血酸+100mmol/L地塞米松培养外膜细胞即可诱导大鼠血管外膜细胞在体外发生钙化,这是通过茜素红S染色㊁ALP活性检测及胞内钙含量检测结果得以确定的㊂血管钙化过程中,血管中膜细胞向成骨样细胞表型转变并表达相关成骨标志物,从而引起骨基质的沉积,是血管钙化的重要特点及机制[5]㊂本实验所用的血管外膜细胞钙化条件与血管中膜细胞钙化条件一致,说明血管外膜细胞钙化的机制可能与中膜细胞钙化的机制部分一致㊂血管中膜细胞钙化过程中,细胞表达成骨相关的转录因子如Runx2等,进而促进下游表达骨相关蛋白如骨形态发生蛋白BMP2等的表达,从而促使细胞向成骨样细胞主动分化[12-13],本研究也观察到类似的机制㊂通过PT-PCR检测,发现钙化培养基培养大鼠血管外膜细胞15d后,BMP2和Runx2的mRNA表达水平升高㊂本研究通过对钙盐沉积与成骨样细胞表型转变2个维度的探讨,证明血管外膜细胞可在体外被诱导钙化,丰富了血管钙化的分型㊂血管钙化的发生机制复杂,涉及多种信号通路,如细胞自噬和凋亡㊁Wnt/β-catenin信号通路激活㊁内质网应激等均参与调控血管钙化的过程㊂自噬作为一种细胞应激的适应性反应,在维持血管结构与功能中十分关键㊂研究表明,血管钙化过程中自噬水平增高[14-15]㊂在体外实验中,高磷可提高大鼠血管中膜细胞的自噬水平,增加细胞内自噬体数量,从而抑制凋亡与钙化[16]㊂还有研究表明,自噬可通过抑制大鼠血管中膜细胞氧化应激,抑制血管内皮细胞的炎症反应,对三酰甘油等脂代谢进行调控,从而减轻血管钙化[17-18]㊂LC3和Beclin1是2种典型的自噬标志物,Western Blot实验结果表明,用钙化培养基诱导大鼠血管外膜细胞15d,LC3-Ⅱ/LC3-Ⅰ比率升高,Beclin1蛋白水平表达升高,说明细胞内自噬水平升高㊂多项研究表明,细胞凋亡参与促进血管钙化的发生,抑制细胞凋亡和抑制钙化[16-17]㊂在对大鼠的体内研究发现,成纤维细胞生长因子21通过内质网应激调控Caspase-12信号通路来减少血管内中膜细胞凋亡,从而抑制血管钙化[18]㊂另外,提高培养基中的Pi 或Ca2+浓度,可诱导细胞质膜形成并释放基质囊泡(如凋亡小体),从而导致细胞外基质钙化,这种基质钙化可能成为血管钙化的成核位点[19]㊂Bax和Bcl-2是2种典型的凋亡和抑制凋亡蛋白,本实验结果证明,利用钙化培养基对血管外膜细胞诱导钙化过程中,细胞内凋亡水平升高㊂同时细胞内自噬水平也升高,这可能是细胞自我调控以对抗钙化的结果㊂本研究证实血管外膜细胞可在体外被诱导钙化,且外膜钙化过程与骨组织钙化过程类似,为主动可调控的过程㊂血管钙化是一个复杂的过程,涉及细胞凋亡和自噬等调控通路,仍需进一步研究㊂参考文献:[1]梁英权,段亚君,韩际宏.血管钙化分子机制研究进展[J].中国动脉硬化杂志,2020,28(11):921-929.[2]NICOLL R,HENEIN M Y.The predictive value of arterial andvalvular calcification for mortality and cardiovascular events[J].Int J Cardiol Heart Vessel,2014,3:1-5.[3]JOHNSON R C,LEOPOLD J A,LOSCALZO J.Vascularcalcification:pathobiological mechanisms and clinical implications[J].Circulation Research,2006,99(10):1044-1059.[4]YAMADA S,GIACHELLI C M.Vascular calcification in CKD-MBD:roles for phosphate,FGF23,and Klotho[J].Bone,2017,100:87-93.[5]LIN M E,CHEN T M,WALLINGFORD M C,et al.Runx2deletion insmooth muscle cells inhibits vascular osteochondrogenesis andcalcification but not atherosclerotic lesion formation[J].Cardiovascular Research,2016,112(2):606-616.[6]DURHAM A L,SPEER M Y,SCATENA M,et al.Role of smoothmuscle cells in vascular calcification:implications in atherosclerosis andarterial stiffness[J].Cardiovascular Research,2018,114(4):590-600.[7]BOSTRÖM K I,RAJAMANNAN N M,TOWLER D A.The regulationof valvular and vascular sclerosis by osteogenic morphogens[J].Circulation Research,2011,109(5):564-577.[8]SPEER M Y,YANG H Y,BRABB T,et al.Smooth muscle cells giverise to osteochondrogenic precursors and chondrocytes incalcifying arteries[J].Circulation Research,2009,104(6):733-741.[9]PROUDFOOT D,SKEPPER J N,HEGYI L,et al.Apoptosisregulates human vascular calcification in vitro:evidence forinitiation of vascular calcification by apoptotic bodies[J].Circulation Research,2000,87(11):1055-1062.[10]AN S J,BOYD R,ZHU M,et al.NADPH oxidase mediatesangiotensin II-induced endothelin-1expression in vascularadventitial fibroblasts[J].Cardiovascular Research,2007,75(4):702-709.[11]ZEADIN M,BUTCHER M,WERSTUCK G,et al.Effect of leptin onvascular calcification in apolipoprotein E-deficient mice[J].Arterioscler Thromb Vasc Biol,2009,29(12):2069-2075. [12]LEOPOLD J A.Vascular calcification:mechanisms of vascularsmooth muscle cell calcification[J].Trends in CardiovascularMedicine,2015,25(4):267-274.[13]刘聿秀.高尿酸诱导血管钙化的机制研究[D].青岛:青岛大学,2015.[14]LIU Q,LUO Y,ZHAO Y,et al.Nano-hydroxyapatite acceleratesvascular calcification via lysosome impairment and autophagydysfunction in smooth muscle cells[J].Bioact Mater,2022,8:478-493.[15]LIANG J,HUANG J,HE W,et al.β-Hydroxybutyric Inhibits vascularcalcification via autophagy enhancement in models induced byhigh phosphate[J].Front Cardiovasc Med,2021,8:685748. [16]CICERI P,ELLI F,CAPPELLETTI L,et al.A new in vitro model todelay high phosphate-induced vascular calcification progression[J].Mol Cell Biochem,2015,410(1/2):197-206.[17]BYON C H,JAVED A,DAI Q,et al.Oxidative stress inducesvascular calcification through modulation of the osteogenictranscription factor Runx2by AKT signaling[J].The Journal ofBiological Chemistry,2008,283(22):15319-15327.[18]OUIMET M,FRANKLIN V,MAK E,et al.Autophagy regulatescholesterol efflux from macrophage foam cells via lysosomal acidlipase[J].Cell Metabolism,2011,13(6):655-667.[19]REYNOLDS J L,JOANNIDES A J,SKEPPER J N,et al.Humanvascular smooth muscle cells undergo vesicle-mediatedcalcification in response to changes in extracellular calcium andphosphate concentrations:a potential mechanism for acceleratedvascular calcification in ESRD[J].Journal of the AmericanSociety of Nephrology,2004,15(11):2857-2867.(收稿日期:2022-03-30)(本文编辑王雅洁)。
益生菌对阿尔茨海默病作用的研究进展

益生菌对阿尔茨海默病作用的研究进展发布时间:2021-12-14T06:08:15.523Z 来源:《中国结合医学杂志》2021年12期作者:宋鑫萍1,2,李盛钰2,金清1[导读] 阿尔茨海默病已成为威胁全球老年人生命健康的主要疾病之一,患者数量逐年攀升,其护理的经济成本高,给全球经济造成重大挑战。
近年来研究显示,益生菌在适量使用时作为有益于宿主健康的微生物,在防治阿尔茨海默病方面具有积极影响,其作用机制可能通过调节肠道菌群,影响神经免疫系统,调控神经活性物质以及代谢产物,通过肠-脑轴影响该病发生和发展。
宋鑫萍1,2,李盛钰2,金清11.延边大学农学院,吉林延吉 1330022.吉林省农业科学院农产品加工研究所,吉林长春 130033摘要:阿尔茨海默病已成为威胁全球老年人生命健康的主要疾病之一,患者数量逐年攀升,其护理的经济成本高,给全球经济造成重大挑战。
近年来研究显示,益生菌在适量使用时作为有益于宿主健康的微生物,在防治阿尔茨海默病方面具有积极影响,其作用机制可能通过调节肠道菌群,影响神经免疫系统,调控神经活性物质以及代谢产物,通过肠-脑轴影响该病发生和发展。
本文综述了近几年来国内外益生菌对阿尔茨海默病的作用进展,以及其预防和治疗阿尔茨海默病的潜在作用机制。
关键词:益生菌;阿尔茨海默病;肠道菌群;机制Recent Progress in Research on Probiotics Effect on Alzheimer’s DiseaseSONG Xinping1,2,LI Shengyu2,JI Qing1*(1.College of Agricultural, Yanbian University, Yanji 133002,China)(2.Institute of Agro-food Technology, Jilin Academy of Agricultural Sciences, Chanchun 130033, China)Abstract:Alzheimer’s disease has become one of the major diseases threatening the life and health of the global elderly. The number of patients is increasing year by year, and the economic cost of nursing is high, which poses a major challenge to the global economy. In recent years, studies have shown that probiotics, as microorganisms beneficial to the health of the host, have a positive impact on the prevention and treatment of Alzheimer’s disease. Its mechanism may be through regulating intestinal flora, affecting the nervous immune system, regulating the neuroactive substances and metabolites, and affecting the occurrence and development of the disease through thegut- brain axis. This paper reviews the progress of probiotics on Alzheimer’s disease at home and abroad in recent years, as well as its potential mechanism of prevention and treatment.Key words:probiotics; Alzheimer’s disease; gut microbiota; mechanism阿尔茨海默病(Alzheimer’s disease, AD),系中枢神经系统退行性疾病,属于老年期痴呆常见类型,临床特征主要包括:记忆力减退、认知功能障碍、行为改变、焦虑和抑郁等。
Expression, Purification and Crystallization of

Expression, Purification and Crystallization of the Mycobacterium Tuberculosis HSP16.3 Molecular Chaperone Background of Mycobacterium Tuberculosis HSP16.3HSP16.3, a 16.3 kDa protein from Mycobacterium Tuberculosis, was originally identified as a prominent antigen (Kingston et al., 1987). During the stationary phase, HSP16.3 is maximally expressed and becomes a main protein of the latent phase (Yuan et al., 1996). Previous studies showed that HSP16.3 can make the cell structure stable and prevent stationary Mycobacterium Tuberculosis from autolysing (Cunningham et al., 1998). In previous studies, HSP16.3 was found as one of theα-crystallin-related small heat shock proteins (sHSP) with molecular chaperone activity. Experiments in vitro revealed that HSP16.3 can suppress the thermal aggregation of citrate synthase at 39.5˚C, without consumption of A TP (Chang et al., 1996).Now the Mycobacterium Tuberculosis HSP16.3 gene was cloned to the plasmid pSTE-HSP16.3, and transformed to E.Coli. BL21(DE3) strain.Material and MethodExpressionThings to have ready before Starting.-Plate or glycerol culture-Sterile LB 25ml in a 50mL shaker flasker, 250ml in a 500mL shaker flasker, all together autoclaved, antibiotic added afterword.- antibiotic and sterile water- TipsPrepare the LB and autoclave:Fomula of the LB medium for 1 Liter:Bacto Tryptone (BT) 10 gBacto Y east Extract (BYE) 10 gNaCl 10gThe LB medium, dd H2O and the tips all together autoclaved at 121 ˚C for 20 minutes.Method:1 Innoculate 25 ml LB Medium ( containing 100 ug) and grow culture overnight(37˚C, 200rpm).2 Next morning inoculate 250 ml prewarmed LB Medium ( containing 100 ug) with the 25 ml overnight culture and grow at 37 ˚C, 200rpm, HSP16.3 was overexpressed in soluble form intracellularly without IPTG induction.3 Incubate the Culture for 10 hours before havesting the cell at 4000 g for 20 minutes.4 Resuspend the cell pellet in 30 ml Butter A and freeze the Sample in -80˚C refigerator.PurificationDE52 Ion-Exchange columnThings to have ready before Starting.-Butter A: 50 mM Imidazole pH 6.5 (1 liter)-Butter B: 50 mM Imidazole pH 6.5 , 300mM NaClall together Fitrate with 0.2 um membrane.- DE52 medium , column ,Gradient maker, UV-monitor and Fractioner- TipsMethod:1 Thaw the cell pellet and vortex .2 Add 0.4ml 100 mM PMSF and sonicate (400kw, 4s-6s 50 cycle* 5 )3 Centrifuge 15000 rpm, 30 minutes to pellet debris4 Transfer supernatant to a 50 ml conicale tube and discard the pellet.5 The supernatant dilute to 50 ml with Buffer A and then load to DE52 ion-exchange columns (20ml), which was pre-equibrated with 100ml Buffer A. And then wash the unbound proteins with 100 ml Buffer A.6 Elute the protein with a linear gradient : 200ml buffer A plus 200ml buffer B, 2ml/min, 6ml each fraction.7 Run 15% SDS-PAGE to determine the HSP16.3 peak.Desalting by dialysis1 Preparation of the dialysis tubeCut the tube in a suitable length (20-30 cm)Boil the tube in solution containing 10 mM NaHCO3 for a few minutes.Boil the tube in solution containing 10 mM EDTA for a few minutes.Rasin the tube with de-ion water2 Pool the HSP16.3 peak and dialysis the Sample against 1000ml Buffer A for more than 6hours.Q-Separose (HP) Ion-Exchange Column1 load the sample to Q-Separose (HP) Ion-Exchange column (20ml), which was pre-equibrated with 100ml Buffer A. And then wash the unbound proteins with 100 ml Buffer A.2 Elute the protein with a linear gradient : 200ml buffer A plus 200ml buffer B, 2ml/min, 6ml each fraction.3 Run 15% SDS-PAGE to determine the purity of the HSP16.3 peak.Gel filtration ColumnThe HSP peak was a final volumn 0.3ml and then run though a Superdex75 (HR, 10/30mm) gel filtration column in 150mM NaCl and 5mM Imdazole, pH6.5. Crystallization1 The purified HSP16.3 was solvent-exchanged to water and concentrated to 20mg/ml before crystallization trails (Bradford). All the crystallization trials were carried out using the hanging-drop vapor-diffusion method at 291K: drops consisted of2 microlitres of HSP16.3 protein solution plus 2 microlitres of the precipitant. The drops were equilibrated against 0.2 ml precipitant at room temperature. The crystallization conditions were investigated with a PEG4000 Kit.Result and discussionThe purity of the final HSP16.3 was over 95% by SDS-PAGE. The crystallization trials of HSP16.3 yielded Cubic crystals with a size of 0.8*0.8*0.6mm in a few days.20040060080010001200mAUBuffer Tris-HCL pH 8.5 Precipitant PEG 4000 MethodV apor Diffusion Temperature 293 K Size0.8*0.8*0.6mmReferencesChang Z., Primm, T.P., Jakana J., Lee H. I., Serysheva I., Chiu W., Gilber H. F., Quiocho F. A., (1996) J Biol Chem 271:7218-7223Cunningham A. F., Spreadbury C. L., (1998) J. Bacteriol. 184:801-808Kingston A. E., Salgame P. R., Mitchison N.A., Colston M. J. (1987) Infect. Immun 55,3149-3154Yuan Y., Crane D. D., Barry C. E. III (1996) J Bacteriol178: 4484-4492。
1,4,5-三磷酸肌醇受体与神经变性疾病

1,4,5-三磷酸肌醇受体与神经变性疾病赵吉利1,岳雅蓉1,张鑫1,杜文倩1,王云霞1综述,薛慧2,项文平2,孟天予2审校摘要:1,4,5-三磷酸肌醇受体(inositol 1,4,5-trisphosphate receptors,IP3Rs)是细胞内质网上的钙离子(cal‑cium ion,Ca2+)通道,通过调控Ca2+参与细胞生物学功能,是维持中枢神经系统正常功能的关键分子。
近年来,越来越多的研究发现,IP3Rs结构和功能异常与神经变性疾病如阿尔茨海默病、帕金森病、亨廷顿病、脊髓小脑共济失调等的发病机制密切相关,这些结构和功能异常如何影响IP3Rs功能,及相关钙信号,并且如何在这些疾病的发病和严重程度中发挥作用,仍尚不清楚。
IP3Rs如何在神经变性疾病中发挥作用将于本文中进行综述。
关键词:1,4,5-三磷酸肌醇受体;钙离子;神经变性疾病;认知障碍中图分类号:R741 文献标识码:AInositol 1,4,5-trisphosphate receptors and neurodegenerative diseases ZHAO Jili,YUE Yarong,ZHANG Xin,et al.(Central School of Clinical Medicine,Baotou Medical College,Inner Mongolia University of Science and Technology,Baotou 014040,China)Abstract:Inositol 1,4,5-trisphosphate receptor (IP3R),which is a calcium ion (Ca2+) channel in the endoplasmic re‑ticulum,participates in cellular biological functions through regulating the Ca2+ signal,and it is a key molecule in maintaining the normal function of the central nervous system. In recent years,more and more studies have found that the structural and functional abnormalities of IP3Rs are closely related to the pathogenesis of neurodegenerative diseases such as Alzheimer's disease,Parkinson disease,Huntington disease,and spinocerebellar ataxia. However,it remains unclear how these structural and functional abnormalities affect the function of IP3Rs and the related calcium signal as well as the pathogenesis and sever‑ity of neurodegenerative diseases. This paper reviews the role of IP3Rs in neurodegenerative diseases.Key words:Inositol 1,4,5-trisphosphate receptor;Calcium ion;Neurodegenerative disease;Cognitive disorder1,4,5-三磷酸肌醇受体(inositol 1,4,5-trisphos‑phate receptors,IP3Rs)是一种位于内质网上的配体门控的Ca2+通道,1998年Supattapone等人[1]首次在大鼠小脑中发现IP3Rs,其广泛表达于单细胞原生动物在内的动物细胞中,通过调节内质网中Ca2+的释放,产生钙信号。
德谷门冬双胰岛素注射液治疗2_型糖尿病临床效果及安全性探讨

DOI:10.16658/ki.1672-4062.2023.17.098德谷门冬双胰岛素注射液治疗2型糖尿病临床效果及安全性探讨林生,谢平,陈予福州市长乐区人民医院内分泌科,福建福州350200[摘要]目的研究德谷门冬双胰岛素注射液治疗2型糖尿病的临床效果及安全性。
方法选取于2022年7月—2023年4月福州市长乐区人民医院收治的2型糖尿病患者98例为研究对象,采用随机抓阄法分为两组,每组49例。
两组均联用常规降糖药物治疗,对照组采用甘精胰岛素注射液治疗,观察组采用德谷门冬双胰岛素注射液治疗。
对比两组临床治疗效果、临床症状好转时间和胰岛素用量情况、糖代谢指标、胰岛素功能指标、不良反应发生情况、心血管不良事件发生情况。
结果观察组总有效率高于对照组,差异有统计学意义(P<0.05)。
观察组尿酮体转阴时间、血糖达标时间、胰岛素用量均优于对照组,差异有统计学意义(P< 0.05)。
观察组空腹血糖、餐后2 h血糖、糖化血红蛋白均低于对照组,差异有统计学意义(P<0.05)。
观察组胰岛β细胞功能指数高于对照组,胰岛素抵抗指数、空腹胰岛素低于对照组,差异有统计学意义(P<0.05)。
两组恶心呕吐、倦怠乏力、低血糖总发生率比较,差异无统计学意义(P>0.05)。
两组心绞痛、心力衰竭总发生率比较,差异无统计学意义(P>0.05)。
结论德谷门冬双胰岛素注射液治疗2型糖尿病临床效果显著优于甘精胰岛素注射液,但是治疗安全性无显著变化。
[关键词] 2型糖尿病;德谷门冬双胰岛素注射液;不良反应;心血管不良事件[中图分类号] R59 [文献标识码] A [文章编号] 1672-4062(2023)09(a)-0098-04Discussion on the Clinical Effect and Safety of Insulin Degludec and Insu⁃lin Aspart Injection in the Treatment of Type 2 Diabetes MellitusLIN Sheng, XIE Ping, CHEN YuDepartment of Endocrinology, Changle District People's Hospital, Fuzhou, Fujian Province, 350200 China[Abstract] Objective To study the clinical effect and safety of insulin degludec and insulin aspart injection in the treatment of type 2 diabetes mellitus. Methods A total of 98 patients with type 2 diabetes admitted to Fuzhou Changle District People's Hospital from July 2022 to April 2023 were selected as the study objects and divided into two groups with 49 cases in each group by random lottery method. Both groups were treated with conventional hypoglycemic drugs, the control group was treated with insulin glargine injection, and the observation group was treated with Degu asparton double insulin injection. The clinical therapeutic effect, time of improvement of clinical symptoms, insulin dosage, glucose metabolism index, insulin function index, occurrence of adverse reactions and cardiovascular adverse events were compared between the two groups. Results The total effective rate of the observation group was higher than that of the control group, and the difference was statistically significant (P<0.05). The time of urine ketone body turning negative, blood glucose reaching standard and insulin dosage in observation group were better than those in control group, and the differences were statistically significant (P<0.05). Fasting plasma glucose, 2-hour postprandial blood glucose and glycated hemoglobin in the observation group were lower than those in the control group, and the differences were statistically significant (P<0.05). The function index of islet β cells in observation group was higher than that in control group, the insulin resistance index and fasting insulin was lower than that in control group, the dif⁃ference was statistically significant (P<0.05). There was no statistically significant difference in the total incidence of [作者简介]林生(1981-),男,本科,副主任医师,研究方向为糖尿病及其并发症的相关临床研究。
BIBR 1532_端粒酶新型的选择性抑制剂_321674-73-1_Apexbio

化学性质
产品名: Cas No.: 分子量: 分子式:
BIBR 1532 321674-73-1 331.36 C21H17NO3
产品名: BIBR 1532 修订日期: 6/30/2016
化学名: SMILES: 溶解性: 储存条件: 一般建议:
运输条件:
2-[[(E)-3-naphthalen-2-ylbut-2-enoyl]amino]benzoic acid
ApexBio Technology
和三氧化二砷抑制细胞增殖能力和端粒酶活性[3]。
参考文献: [1]. Damm, K.; Hemmann, U.; Garin-Chesa, P.; Hauel, N.; Kauffman, I.; Priepke, H.; Niestroj, C.; Daiber, C.; Enenkel, B.; Guilliard, B.; Lauritsch, I.; Muller, E.; Pascolo, E.; Sauter, G.; Pantic, M.; Martens, U. M.; Wenz, C.; Linger, J.; Kraut, N.; Rettig, W. J.;Schnapp, A. A highly selective telomerase inhibitor limiting human cancer cell proliferation. EMBO J. 2001, 20, 69586968. [2]. Bashash D1, Ghaffari SH, Mirzaee R, Alimoghaddam K, Ghavamzadeh A. Telomerase inhibition by non-nucleosidic compound BIBR1532 causes rapid cell death in pre-B acute lymphoblastic leukemia cells. Leuk Lymphoma. 2013 Mar;54[4]:561-8. doi: 10.3109/10428194.2012.704034. Epub 2012 Sep 28. [3]. Bashash D1, Ghaffari SH, Zaker F, Kazerani M, Hezave K, Hassani S, Rostami M, Alimoghaddam K, Ghavamzadeh A. Anticancer Agents Med Chem. 2013 Sep;13(7):1115-25. BIBR 1532 increases arsenic trioxide-mediated apoptosis in acute promyelocytic leukemia cells: therapeutic potential fo应用
小分子抑制剂、激动剂、拮抗剂--DNA损伤DNA修复信号通路

DNA损伤/DNA修复人类细胞中的DNA每天会受到数以万计的外源性损伤(化学污染、紫外线、电离辐射、烷基化/甲基化等引起)和内源性损伤(碱的氧化、烷基化、水解等引起)。
损伤后DNA会发生单链和双链断裂,未修复的DNA损伤会导致细胞衰老、凋亡和恶性肿瘤等等。
为了避免此类情况,激发了细胞的DNA损伤反应(DDR)。
DNA损伤反应(DDR)能够检测DNA的损伤并介导其修复,包含DNA损伤、细胞周期停滞、DNA复制调控、DNA损伤修复旁路等一系列通路的调控,以维持基因组的稳定性和细胞活力。
异常的DNA损伤反应与衰老、癌症和免疫疾病有关。
DNA损伤/DNA修复通路转导过程当DNA受到外源性或内源性损伤后,会发生单链和双链断裂,DNA损伤反应会被激活。
DNA双链断裂激活DNA-PK与ATM/ATR激酶。
DNA-PK诱导DNA修复,涉及错配、碱基切除、核苷酸切除修复等多种机制。
RPA、Rad51和fanconi贫血蛋白等也可直接用于DNA修复。
ATM/ATR激酶经过两个并行级联最终将CyclinB-cdc2复合体失活:1)ATM/ATR激酶激活Chk2激酶,Chk2激酶磷酸化并使失活Cdc25,同时也通过Chk1抑制Cdc25,从而阻止cdc2的激活,快速抑制细胞有丝分裂从G2期进入M期。
此外,ATR通过刺激Cdk1抑制激酶Wee1来抑制Cyclinb/Cdk1的激活,防止DNA损伤的细胞进入有丝分裂。
2)另一级联反应稍慢,Chk2激酶磷酸化p53,使其从MDM2和MDM4(MdmX)上分离,激活p53下游调节基因,从而抑制CyclinB-cdc2复合体活性或将其从细胞核中排出。
p300/PCAF对p53乙酰化可进一步增强其转录能力。
同时,p53也可诱导细胞凋亡。
DNA单链断裂后,激活ATR激酶,ATR通过Chk1进一步激活Cdc25A。
Cdc25A是一种CDK2激活所需的磷酸酶。
CDK2抑制周期素E/A,使有丝分裂无法从G1期进入S期。
