Sol-gel prepared beta-Ga2O3 thin films for ultraviolet photodetectors
TCO薄膜简介

TCO薄膜简介透明导电氧化物(transparentconductiveoxide简称TCO)薄膜主要包括In、Sb、Zn 和Cd的氧化物及其复合多元氧化物薄膜材料,具有禁带宽、可见光谱区光透射率高和电阻率低等共同光电特性,广泛地应用于太阳能电池、平面显示、特殊功能窗口涂层及其他光电器件领域。
透明导电薄膜以掺锡氧化铟(tindopedindiumoxide简称ITO)为代表,研究与应用较为广泛、成熟,在美日等国已产业化生产。
近年来ZnO薄膜的研究也不断深入,掺铝的ZnO薄膜(简称AZO)被认为是最有发展潜力的材料之一。
同时,人们还开发了Zn2SnO4、In4Sn3O12、MgIn2O4、CdIn2O4等多元透明氧化物薄膜材料。
TCO薄膜的制备工艺以磁控溅射法最为成熟,为进一步改善薄膜性质,各种高新技术不断被引入,制备工艺日趋多样化。
本文综述以ITO和AZO为代表的TCO薄膜的研究进展及应用前景。
一、TCO薄膜的发展TCO薄膜最早出现于20世纪初,1907年Badeker首次制成了CdO透明导电薄膜,引起了人们的较大兴趣。
但是,直到第二次世界大战,由于军事上的需要,TCO薄膜才得到广泛的重视和应用。
1950年前后出现了SnO2基和In2O3基薄膜。
ZnO基薄膜兴起于20世纪80年代。
相当长一段时间,这几种材料在TCO薄膜中占据了统治地位。
直到上世纪90年代中期,才有新的TCO薄膜出现,开发出了多元TCO薄膜、聚合物基体TCO薄膜、高迁移率TCO薄膜以及P型TCO薄膜。
而SnO2基和In2O3基材料也通过掺加新的元素而被制成了高质量TCO薄膜。
最近,据媒体报导,美国俄勒冈大学研究人员对TCO材料的研究取得重大突破,他们研制出一种便宜、可靠且对环境无害的透明导电薄膜材料。
该材料可用于制作透明晶体管,用来制造非常便宜的一次性电子产品、大型平面显示器和可折叠又方便携带的电器。
科学家称,这项研究成果将引导新产业和消费领域的发展。
【国家自然科学基金】_透明导电氧化物薄膜_基金支持热词逐年推荐_【万方软件创新助手】_20140803

2013年 序号 1 2 3 4 5 67 18 19 20
科研热词 透明导电氧化物 透过率 透明氧化物半导体 透明导电薄膜 综述 磁控溅射 石墨烯氧化物 石墨烯 溶胶-凝胶法 有机器件 显微组织 常压烧结法 射频磁控溅射 光电性质 光电器件 低温 低损伤 zno薄膜 sn掺杂 gzo
2011年
2012年 科研热词 推荐指数 序号 科研热词 推荐指数 透明导电氧化物 2 1 透明导电氧化物 2 透明导电薄膜 1 2 透明导电薄膜 1 透明导电氧化物(tco)衬底 1 3 透明导电氧化物薄膜 1 退火 1 4 设计原理 1 还原 1 5 缺陷 1 表面形貌 1 6 缓冲层 1 石墨氧化物 1 7 磁控溅射 1 直流磁控溅射 1 8 溶胶-凝胶法 1 电阻率 1 9 掺杂 1 氧分压 1 10 性能退化 1 微晶硅(μ c-si:h)电池 1 11 功函数 1 射频磁控溅射 1 12 力学性能 1 品质 1 13 光致发光 1 含氧官能团 1 14 sol-gel, transparent conductive 1 oxide, sb doped s 可见光透过率 1 15 sb掺杂sno2 1 单层 1 16 p型 1 制备方法 1 17 n型 1 光电性能 1 18 gzo(zno:ga)薄膜 1 光学带隙 1 修复 1 三结电池 1 zno背反射层(br) 1 x射线光电子能谱 1 pin型si基薄膜太阳电池 1 n掺杂β -ga2o3 1 iwo薄膜 1 gzo薄膜 1 graphene, graphene oxide, reparative 1 reduction, transparent flexible electrode
2008年 序号 1 2 3 4 5 6 7 8
科研热词 高透射率 高迁移率 退火处理 近红外 薄膜 磁控溅射 掺钨氧化铟 iwo
Series 6 电磁炉说明书

Series 6, Induction hob, 60 cm, Black, surface mount without frame PVQ651FC5EHEZ390090 Wok para radiantes e inducciónHEZ390210 Sartén antiadherente de 15 cm. de base. HEZ9ES100 Cafetera 4 tazasHEZ9FE280 Sartén de hierro Ø 18 / 28 cmHEZ9SE040 set de 4 piezasHEZ9SE060 set de 6 piezas Encimera de inducción con PerfectFry: logra resultados de fritura perfectos gracias al control automático de temperatura.• DirectSelect: Directo, simple selección de la zona de cocción deseada, potencia y funciones adicionales.• CombiZone: más flexibilidad al combinar dos zonas de cocción para cocinar con distribución uniforme del calor, para asadores y similares.• PerfectFry: Para un perfecto dorado de los alimentos gracias al control del sensor con 4 niveles de potencia con ajuste automático• Terminación biselada: diseño limpio con cristal biselado en la parte delantera y los laterales.• PowerBoost: hasta un 50% más de potencia para una cocción más rápida.Familia de Producto: ..........................Placa independie.vitrocerámica Tipo de construcción: .........................................................Integrable Entrada energética: ...............................................................Eléctrico Número de posiciones que se pueden usar al mismo tiempo: . (4)Medidas del nicho de encastre: ...............51 x 560-560 x 490-500 mm Anchura: .................................................................................592 mm Dimensiones aparato (alto, ancho, fondo): ...........51 x 592 x 522 mm Medidas del producto embalado: ........................126 x 753 x 608 mm Peso neto: ...............................................................................11.7 kg Peso bruto: ..............................................................................13.9 kg Indicador de calor residual: .................................................Separado Ubicación del panel de mandos: ...............Frontal de la vitrocerámica Material de la superficie básica: ....................................Vitrocerámica Color de superficie superior: .....................................................Negro Longitud del cable de alimentación eléctrica: ......................110.0 cm Sealed Burners: ..............................................................................No Zonas con booster: ....................................................................Todas Potencia del segundo elemento calentador: ............................3.6 kW Power of heating element (kW in boost): ..............................[3.7] kW Potencia: .................................................................................7400 W Tensión: ..............................................................................220-240 V Frecuencia: ..........................................................................50; 60 Hz Entrada energética: ...............................................................Eléctrico Tipo de clavija: ..................................................................sin enchufe Appliance Dimensions (h x w x d) (in): ..........................................x x Dimensions of the packed product: ....................4.96 x 23.93 x 29.64 Net weight: .........................................................................26.000 lbs Gross weight: .....................................................................31.000 lbs Número de posiciones que se pueden usar al mismo tiempo: . (4)Longitud del cable de alimentación eléctrica: ......................110.0 cm Medidas del nicho de encastre: ...............51 x 560-560 x 490-500 mm Dimensiones aparato (alto, ancho, fondo): ...........51 x 592 x 522 mm Medidas del producto embalado: ........................126 x 753 x 608 mm Peso neto: ...............................................................................11.7 kg Peso bruto: ..............................................................................13.9 kgSeries 6, Induction hob, 60 cm,Black, surface mount without frame PVQ651FC5EEncimera de inducción con PerfectFry: logra resultados de fritura perfectos gracias al control automático de temperatura.Prestaciones profesionalesDiseño:- Terminación biseladaRapidez:- Función Sprint en todas las zonasConfort:- 4 zonas de inducción- DirectSelect con 17 niveles de cocción- 2 Combi zones- 2-steps- Sensores touchControl- Programación de tiempo de cocción para cada zona y avisador acústico- Avisador acústico- sí- síPotencia y tamaño:- Regulación electrónica con 17 niveles de potenciaSeguridad:- Indicador de calor residual dual (H/h)- Main Switch- Detección de recipiente- Posibilidad de limitar la potencia total de la encimera- Desconexión de seguridad de la placa- Bloqueo de seguridad para niños automático o manual- Función Clean: bloqueo temporal del control- Display de consumo de energíaMedidas:- Dimensiones para instalación: (Al/An/F) 51 mm x 560 mm x 490 mm- Dimensiones del producto: (An/F) 592 mm x 522 mm- Min. espesor de mesa: 16 mm- Cable incluido- Potencia de conexión: 7.4 kwSeries 6, Induction hob, 60 cm, Black, surface mount without frame PVQ651FC5E。
水凝胶 骨修复Mineralization of Hydrogels for Bone Regeneration

See discussions, stats, and author profiles for this publication at: https:///publication/45826681 Mineralization of Hydrogels for Bone RegenerationARTICLE in TISSUE ENGINEERING PART B REVIEWS · DECEMBER 2010Impact Factor: 4.64 · DOI: 10.1089/ten.TEB.2010.0462 · Source: PubMedCITATIONS 64READS 1395 AUTHORS, INCLUDING:Timothy E L DouglasGhent University61 PUBLICATIONS 814 CITATIONSSEE PROFILEAvailable from: Timothy E L DouglasRetrieved on: 29 January 2016Mineralization of Hydrogels for Bone RegenerationKaterina Gkioni,M.Sc.,1Sander C.G.Leeuwenburgh,Ph.D.,1Timothy E.L.Douglas,Ph.D.,1Antonios G.Mikos,Ph.D.,2and John A.Jansen,D.D.S.,Ph.D.1Hydrogels are an important class of highly hydrated polymers that are widely investigated for potential use in soft tissue engineering.Generally,however,hydrogels lack the ability to mineralize,preventing the formation of chemical bonds with hard tissues such as bone.A recent trend in tissue engineering involves the development of hydrogels that possess the capacity to mineralize.The strategy that has attracted most interest has been the incorporation of inorganic phases such as calcium phosphate ceramics and bioglasses into hydrogel matrices.These inorganic particles act as nucleation sites that enable further mineralization,thus improving the me-chanical properties of the composite material.A second route to create nucleation sites for calcification of hydrogels involves the use of features from the physiological mineralization process.Examples of these bio-mimetic mineralization strategies include (1)soaking of hydrogels in solutions that are saturated with respect to calcium phosphate,(2)incorporation of enzymes that catalyze deposition of bone mineral,and (3)incorporation of synthetic analogues to matrix vesicles that are the initial sites of biomineralization.Functionalization of the polymeric hydrogel backbone with negatively charged groups is a third mechanism to promote mineralization in otherwise inert hydrogels.This review summarizes the main strategies that have been developed in the past decade to calcify hydrogel matrices and render these hydrogels suitable for applications in bone regeneration.IntroductionBone substitution materialsBone is a composite material comprised of a collage-nous fibrous matrix that is enriched with platelet-shaped nanocrystals of carbonated apatite (average dimensions:50nm long,25nm wide,and 3nm thick).This complex na-nostructure makes bone a unique tissue with exceptional mechanical and biological properties.1,2Despite several decades of research on synthetic bone substitutes,the use of autografts is still the gold standard in clinical practice.Autografting requires a surgery in which parts of healthy bone from the patient are harvested from,for instance,the iliac crest and subsequently transferred to the site of application.Alternative options include the use of bone harvested from another donor (allografts)or from an-imals (xenografts).3,4Even though these surgical treatments have resulted into good clinical outcome,they are accom-panied by strong drawbacks such as infections,pain,and morbidity at the donor site,high costs,and the necessity of additional surgery.5,6To eliminate these severe problems,there is a pressing need for novel synthetic materials that can substitute bone sufficiently.Several materials,such as metals 7,ceramics,and poly-mers 8,have been used for bone replacement.In the era ofregenerative medicine,the poor degradability of metallic and ceramic scaffolds has become the major disadvantage that inhibits complete regeneration of bone tissue.Polymers,on the other hand,are known for the ease by which degradation can be tailored by controlling the chemical composition of the monomer units during synthesis.Until recently,the majority of polymeric bone substitutes were premade con-structs that were implanted surgically via invasive surgery.Clinically,there is a growing need for materials that can be inserted using minimally invasive methods such as a simple injection.9Ideally,such a material should be of viscosity low enough to be injected and harden after injection,thereby enabling incorporation of drugs,cells,and growth factors in the viscous solution before administration.10Hydrogels are a specific,highly hydrated class of polymers that fulfill all of the abovementioned requirements.HydrogelsHydrogels are hydrophilic crosslinked polymers that are formed by the reaction of one or more monomers,by associ-ation of hydrogen bonds or van der Waals interactions be-tween the chains.11,12The crosslinking can be achieved either physically or chemically.While in chemical crosslinking co-valent bonds must be formed,physical crosslinking happens when physical interaction between the chains occurs.131Department of Biomaterials,Radboud University Nijmegen Medical Center,Nijmegen,The Netherlands.2Department of Bioengineering,Rice University,Houston,Texas.TISSUE ENGINEERING:Part B Volume 16,Number 6,2010ªMary Ann Liebert,Inc.DOI:10.1089/ten.teb.2010.0462577Hydrogels can be classified according to their origin(natural or synthetic),14method of preparation(homopolymer,co-polymer,multipolymer,and interpenetrating hydrogels),io-nic charges(neutral,anionic,cationic,and ampholytic hydrogels),and physical structure(amorphous,semicrystal-line,and hydrogen bonded structures).11When hydrogels are in contact with water,they swell and form an insoluble three-dimensional network.Other than injectability,hydrogels display many properties15that make them desirable candidates for tissue engineering applica-tions.One of the most important advantages is their aqueous environment,which protects cells and sensitive drugs that can be incorporated in the network for controlled delivery at the site of injury.The aqueous environment allows trans-portation of substances,such as nutrients and by-products from cell metabolism,in and out of the hydrogels.16Hy-drogels can also be derivatized with functional groups that mediate processes such as cell attachment and subsequent spreading.17Until recently,hydrogels have been mainly considered for soft tissue regeneration.In the last few years, however,the interest to test the feasibility of using the ben-eficial properties of hydrogels for hard tissue regeneration has increased.Still,for applications in hard tissue engineer-ing,hydrogels are associated with a number of disadvan-tages such as their poor mineralization upon implantation.18 Further,the inherent mechanical weakness of hydrogels is a limiting factor that restricts their use to non-load-bearing applications15,19,even though reinforcement can be achieved by the addition of other phases.6,18,20,21Finally,many hy-drogels are difficult to sterilize due to their high water con-tent and the polymer reactivity under UV light.22It is not in the scope of this study to review a list of all the hydrogels—natural or synthetic—used in thefield of tissue engineering,since there are many excellent reviews that thoroughly elaborate on this subject.19,23–28This article will focus on the strategies developed during the past decade to induce mineralization in inert,nonmineralizing hydrogels in vitro(immersion in simulated bodyfluids[SBF])or in vivo for use in bone regeneration.Three major strategies used for calcification of hydrogels will be reviewed,including(1)the addition of inorganic particles aiming at mineralization and improvement of the mechanical properties of hydrogels,(2) the creation of nucleation sites by biomimetic methods,such as soaking treatments and the use of enzymes and vesicles that play an important role in physiological biomineraliza-tion,and(3)the derivatization of the polymeric hydrogel backbone with anionic functional groups.In addition,some indirect methods of mineralization such as growth factors and cell incorporation or addition of demineralized bone matrix will be briefly discussed.Mineralization by Adding Inorganic PhasesThe capacity of a specific class of bone-substituting ma-terials to induce calcification is often referred to as bioac-tivity,which implies that these materials possess the capacity to promote nucleation and subsequent proliferation of cal-cium phosphate crystals.Generally,most polymeric materi-als do not possess this capacity,but the addition of a ceramic phase can still render the resulting composites bioactive by providing nucleation sites for the promotion of hydroxyap-atite(HA)precipitation.The concept of combining a hydro-gel with an inorganic phase is inspired by the composite nature of bone itself.One of the many advantages of adding an inorganic phase is that the dispersed mineral will provide nucleation sites for HA formation as well as cell adhesion sites that enable integration with surrounding bone tis-sue.29,30Further,degradation of the temporary hydrogel implant will allow for replacement by new bone formation, thus increasing mechanically stability.Degradation times and mechanical properties of organic–inorganic composite materials can be controlled to a large extent by the addition of inorganic phases.20,21,31Moreover, the handling characteristics of such composite materials can be greatly improved,since brittle ceramic particles can be delivered in moldable or even injectable formulations using the elasticity of the hydrogels.5Finally32,the addition of carbonated apatites in polymers can have a neutralizing ef-fect on the acidic pH caused by the degradation by-products, thus minimizing excessive inflammation around the im-plantation site.There are many bioactive inorganic materials that can be used to render hydrogels mineralizable.These ceramic ma-terials are able to create afirm bond with bone at the site of implantation by forming an intermediate layer of HA on their surface.33The most commonly used inorganic phases are calcium phosphates and bioglasses.Many calcium phosphate ce-ramics can be found in literature with the most representa-tive being b-tricalcium phosphate(b-TCP),amorphous calcium phosphate,and HA.This group of ceramics shows strong resemblance to the mineral phase of bone and it is found in many normal or pathological calcified sites in the human body.34Thorough reviews of all relevant calcium phosphates that are present in the human body can be found elsewhere.35–37Bioactive glasses are amorphous solids con-taining<60wt%SiO2that are bioactive due to their high reactivity in aqueous media.Modern preparation techniques such as the sol–gel process have yielded a wide range of mesoporous,highly bioactive,and bioresorbable materials for the production of bone implants.38It has been shown39,40 that the formation of HA on the surface of these materials is due to the formation of–OH groups when the glass contacts bodyfluids.41,42Composites based on natural hydrogelsAdvantages of natural hydrogels include their biocom-patibility,biodegradability,and commercial availability. Composites of natural hydrogels and bioactive phases have been shown to accelerate osteogenesis and sometimes pos-sess osteoconductive properties that were even superior to monolithic HA implants.43There are many natural poly-mers23used for tissue engineering most commonly collagen and its denatured derivative gelatin44,fibrin,as well as chitin and its deacetylated derivative chitosan.Collagen(mostly collagen type I)is the main polymer phase of bone,45and it is highly biocompatible,degrades enzymatically,and can be processed easily into different forms such as sponges,46fibers,47tubes,and sheets.48,49An example of a collagen hydrogel that was combined with an inorganic calcium phosphate phase was reported by Zou et al.49The collagenfibers were crosslinked by using glu-taraldehyde.Ceramic b-TCP particles were homogeneously578GKIONI ET AL.dispersed inside the collagen matrix,but also afirm bond between the ceramic particles and the hydrogel was formed. In addition,the scaffolds showed bone tissue regeneration after12weeks of implantation in animals.For more spe-cific information on the use of collagen as matrix phase, the reader is referred to a thorough review about collagen-HA composites for hard tissue engineering by Wahl and Czermuszka.50Fibrin glue51,52is a synthetic analogue of the blood coagu-lation process that creates afibrin clot upon mixing of the two componentsfibrinogen and thrombin and it can be used as tissue adhesive in many surgical applications due to its fa-vorable biological behavior.Le Nihouannen et al.53combined these beneficial properties offibrin glue in terms of clinical handling and biocompatibility with the bioactive characteris-tics of an additional ceramic phase to develop a composite material for bone regeneration.Micro-and macroporous biphasic calcium phosphate granules(HA and b-TCP in a weight ratio of60/40,respectively)were mixed with afibrin glue matrix inducing mineralization within thefibrin network. Tan et al.54prepared an injectable biomaterial consisting of calcium alginate and nano-HA.The injectability and the setting time of the material could be easily tuned by altering the absolute and relative concentrations of the components. Alginate has the unique capacity to gel in the presence of dissolved calcium ions,which is a very mild method to create crosslinks into an organic matrix.The particles of HA had a diameter of50m m and thefinal concentration of HA in the gel was kept at3%g/mL.CaSO4was used to crosslink the alginate gel.It was concluded that that thefinal com-posite material is a good candidate for bone repair and bone tissue engineering.Alginates for bone reconstruction re-inforced with HA55and octacalcium phosphate56have also been shown to be bioactive.Addition of SiO2,which is the main component of bio-glasses,inside polymeric matrices also aims to trigger the calcification of polymer matrix.Madhumathi et al.57pre-pared a scaffold by dispersing silica nanoparticles inside a chitin hydrogel.The scaffold showed HA formation only after7days of immersion in SBF.Similar particles were also introduced inside chitosan hydrogels58and significant min-eralization of the matrix was observed after immersion in SBF as well as implantation in rat calvaria for3weeks.Si-milarly,addition of sol-gel prepared SiO2-CaO-P2O5bioglass nanoparticles inside a chitosan-based hydrogel also induced bone-like apatite after immersion in SBF.59Composites based on synthetic hydrogelsEven though naturally derived hydrogels have desirable biological properties,they often exhibit degradation profiles that are too fast for hard tissue regeneration.60Moreover, chemical characteristics of natural hydrogels such as the molecular weight usually display a wide distribution due to their natural origin,which limits the reproducibility and functionality of the materials.On the contrary,synthetic hydrogels can be prepared with tailored and highly repro-ducible chemical characteristics,thereby allowing for careful degradation properties.61The combination of the different monomer units results in hydrogels with controlled charac-teristics in terms of degradation rate,swelling ratios,and mechanical properties.62Polymeric chains can befinely tuned based on the clinical requirements of the various applications in hard tissue engi-neering.As a result,a wide range of crosslinking techniques can be used to form the hydrogels such as photo-polymerization or radical polymerization in the presence of small crosslinking agents.10,17,61The most common synthetic hydrogels that are studied for bone tissue engineering pur-poses include hydrogels based either on polyethylene glycol (PEG)62,63,poly(2-hydroxyethyl methacrylate)(pHEMA),or poly(N-isopropylacrylamide).64,65A recent example of the use of PEG-based hydrogels as matrix for the addition of inorganic HA nanoparticles was described by Sarvestani et al.,66,67who exploited the calcium-binding capacity of a6-glutamic acid sequence(as found in the terminal sequences of osteonectin)to increase the inter-action strength between inorganic HA nanoparticles and (L-lactide-co-ethylene oxide-co-fumarate).The other end of the peptide was functionalized with an acrylate group that enabled the establishment of covalent bonds between the peptide and the organic polymer.In this way,the functio-nalized peptide acted as a linker between inorganic and organic composite components.Patel et al.68developed cyclic acetal hydrogels reinforced with nanoparticles of HA for craniofacial tissue engineering application.Incorporation of HA nanoparticles into cyclic acetal hydrogels resulted into enhanced differentiation of bone marrow stromal cells by promotion of endogenous osteogenic signal expression.Composites based on pHEMA with high mineral content of about37%–50%were prepared by Song et al.69The group used pHEMA polymer that was crosslinked in the presence of HA crystals using viscous ethylene glycol as solvent to facilitate the easy dispersion and prevent sedimentation of the HA particles.Even though the material had a mineral content similar to that of human bone,it possessed elasto-meric properties that allowed for press-fitting the composites into bone defects.After implantation in rats,the material supported osteoblastic differentiation and promoted bone mineralization.The combination of the excellent mechanical properties along with the beneficial biological response, confirm the promising concept of using pHEMA in combi-nation with HA crystals.Similarly,pHEMA has been reinforced with inorganic particles such as such as TiO2na-noparticles,70nanocarbonate-substituted apatite,71and SiO2 nanoparticles.72Biomimetic MineralizationHydrogels can also be mineralized by means of biomi-metic methods that take their inspiration from the biomi-neralization process by which native apatite nanocrystals are formed in vivo.Several features from this biomineralization process have been studied for their potential to be used in hydrogel mineralization,including(alternate)soaking treat-ments influids that are saturated with respect to apatite deposition,enzyme-directed mineralization,and the incor-poration of synthetic analogs of matrix vesicles as initial sites of biomineralization.Soaking in solutions containing Ca2þand PO43ÀDu et al.73used collagen matrices presoaked in PO43Àthat were subsequently immersed in Ca2þsolutions.ByMINERALIZATION OF HYDROGELS579controlling the parameters of their method,different crystal polymorphs could be created,whereas the materials were shown to be able to promote mineralization upon implan-tation in rats.Furuichi et al.74prepared a calcium phosphate-polyacrylic acid composite hydrogel by crosslinking a polyacrylic acid polymer in the presence of(NH4)HPO4so-lution and then immersing it in a calcium containing solu-tion.The diffusion of Ca2þinto the polyacrylic acid hydrogel that contained phosphate ions induced calcification of the hydrogel matrix resulting in a hierarchically organized composite architecture that resembled bone.By alternately incubating a cellulose hydrogel in calcium and phosphate solutions,Hutchens et al.75was able to prepare biomimetic composites.The mineral phase of these compos-ites was characterized as calcium-deficient HA.X-ray dif-fraction also revealed that the crystallites formed were elongated along the c-axis and had a length of*50nm,which is similar to the apatite crystals found in natural bone.The same mechanism was utilized to induce HA mineralization in a chitosan hydrogel by Madhumathi et al.,76who used chit-osan hydrogel membranes that were alternately soaked in solutions of CaCl2and Na2HPO4.HA deposits were homo-geneously dispersed throughout the matrix afterfive cycles. Similarly,Hong et al.77used a cellulose hydrogel that wasfirst treated with a CaCl2solution and then immersed in SBF. Uniform and dense biomimetic mineralization was observed after immersion for14days in the SBF solution.Using a urea-containing solution,Kim et al.managed to precipitate calcium phosphate crystals on top and inside a PEG-based hydrogel.78The PEG-fumarate polymer was crosslinked with ethylene glycol methacrylate phosphate, which acted as a source of phosphorous for the formation of apatitic crystalline platelets with a ratio of Ca/P equal to1.60. Vesicles loaded with Ca2þand PO43ÀAnother aspect of bone biomineralization that has been exploited to calcify hydrogel matrices relates to the vesicular nature of physiological calcification.Initial mineralization occurs in the so-called matrix vesicles,which are cellularly derived structures of40–200nm in diameter that are sepa-rated from other structures in the extracellular matrix by a limiting phospholipid membrane enclosing a central aque-ous core.After their formation in specific regions of the outer membrane of osteoblasts,these vesicles migrate toward the calcification front of growing bones.Here,the vesicles secrete apatitic crystals that subsequently calcify periodically ar-ranged,calcium-binding hole zones with specific amino acid composition in collagenfibers of the extracellular matrix.79,80 Liu et al.81created liquid vesicles that entered the hydrogel matrix using a current-mediated ion diffusion method that resulted in mineralization at the interior of a pHEMA hy-drogel.The dense hydrogel acted as binding site for the Ca ions and promoted mineralization of nanoapatite.The min-eral that was formed inside the entire volume of the hydrogel exhibited a structure very similar to the inorganic component of bone.A similar strategy to promote mineralization ac-cording to vesicular mineralization was developed by Ped-erson et al.82and Westhaus and Messersmith.83In the latter studies the vesicles were designed to melt at body temper-ature to release the Ca2þand PO43Àions necessary for mineralization of the surrounding hydrogel matrix.Enzymatic mineralizationAlkaline phosphatase(ALP)84is an enzyme that plays an important role in the remodeling of bone and more specifi-cally in the resorption of bone and the mineralization of carbonated apatite.The enzyme acts as a catalyst for the hydrolysis of the organic phosphoesters,thereby increasing the local concentration of inorganic phosphate groups that results into enzyme-directed deposition of carbonated apa-tites.85,86Moreover,ALP decreases the concentration of py-rophosphates that act as inhibitors of apatite crystal growth. Recently,several groups have tried to immobilize this en-zyme onto implant surfaces or into hydrogels to induce local mineralization of implant surfaces and scaffolds.87ALP has been immobilized88onto afibrin gel by activating the–COOH groups fromfibrin glue using1-ethyl-3-(di-methylaminopropyl)carbodiimide hydrochloride.Subse-quently,these scaffolds were incubated in ALP solutions resulting in covalent bonding between the enzyme and the fibrin ing a mouse calvarial defect model,it was demonstrated that thefibrin scaffold with the immobilized ALP enhanced new bone formation.Similarly89,ALP has been immobilized onto a pHEMA hydrogel using a copolymerization technique.The enzyme retained its activity after copolymerization,and after im-mersion in SBF containing organophosphates for17days, mineral deposition was observed.Spoerke et al.90report the synthesis of a novel gel com-posed of amphiphilic nanofibers functionally enriched with phosphorylated and acidic groups.The hydrogels were formed in the presence of cell culture media supplemented with calcium chloride and immersed in calcification media containing b-glycerolphosphate and ALP among others. After8days of immersion the mineralization was visibly apparent throughout the hydrogel.Chemical Modification of HydrogelsA different approach to induce mineralization in hydrogels involves the introduction of negatively charged functional groups onto the backbone or side chains of hydrogel poly-mers.This mechanism resembles the biomineralization pro-cess in bone tissue,where noncollagenous,calcium-binding proteins are essential as modulators of nucleation and growth of apatitic biomineral nanocrystals.Generally,these proteins are acidic and phosphorylated and accumulate in mineraliz-ing bone matrix91.Important mineral-inducing proteins such as osteonectin and bone sialoprotein(BSP)are enriched in anionic glutamate(Glu).92,93These acidic sequences are re-sponsible for the attraction of Ca2þand subsequent creation of a local supersaturation that is necessary for CaP precipitation, which make them quintessential for biomineralization of hard tissues.Similarly,alternating sequences of anionic carboxyl-ate,phosphate,or hydroxyl groups along the backbone of synthetic or natural polymers can endow the resulting hy-drogels in swollen state with apatite-nucleating properties. Therefore,the implementation of acidic sequences into hy-drogels opens up new perspectives for the development of hydrogels with mineral-attracting capacity.The following section will address the functionalization of hydrogels with negatively charged groups(PO43À,COOH,and OH)that are either present as isolated functional groups or as part of peptide sequences.580GKIONI ET AL.PO43À,-COOH,and-OH groupsAddition of negatively charged groups such as phosphate, carboxylate,and hydroxyl groups is commonly performed by copolymerization of the hydrogel-forming polymer with monomers containing one or more of these groups. Stancu et al.94developed copolymers of diethyl amino ethyl methacrylate and methacryloyloxyethyl phosphate (MOEP),as well as copolymers of MOEP with1-vinyl-2-pyrrolidinone and compared the calcification ability of both types of copolymer.Samples with different phosphate con-tent were prepared and immersed in SBF for15days.The results revealed that globular mineralization occurred on the surface of the MOEP-diethyl amino ethyl methacrylate hy-drogels.The absence of mineral deposition onto the MOEP-1-vinyl-2-pyrrolidinone copolymers was attributed to the fact that each calcium ion was double bonded by two phosphate groups from adjacent MOEP units formed during copolymerization.Nuttelman et al.95coupled ethylene glycol methacrylate phosphate groups to PEG-diacrylate hydrogels.The polymers were immersed in human mesenchymal stem cell culture media that were supplemented with b-glycerophosphate, which resulted in mineral formation on their surface.The precipitated mineral was found to resemble biological apa-tites not only in composition,but also in molecular structure. Wang et al.96also modified a PEG hydrogel by copolymeri-zation with a phosphoester.Upon immersion in osteogenic media for3weeks,extensive mineralization was observed throughout the three-dimensional network of the copolymer. The introduction of carboxymethyl groups on the pHEMA backbone was described by Filmon et al.97,98The prepared carboxylated scaffolds were immersed in SBF supplemented with antibiotics for15days.The results showed that min-eralization was induced only by the functionalized pHEMA-carboxymethyl hydrogel,whereas the unfunctionalized pHEMA hydrogels did not display any mineral formation. Crosslinked pHEMA has also been modified by exposing carboxylate groups on the surface of the hydrogel using urea to hydrolyze the2-hydroxyethyl esters of the polymer by Song et al.,99who also prepared libraries of pHEMA-based hydrogels100copolymerized with negatively charged monomers in a separate study.Both types of carboxylate-functionalized hydrogels were reported to induce mineralization after im-mersion in SBF.The introduction of hydroxyl-containing silanol(Si-OH) groups on thermosensitive poly(N-isopropylacrylamide)–PEG dimethacrylate copolymer is described by Ho et al.101 These silanol groups were introduced to the main polymer backbone by reacting with trimethacryloxypropyltrimetho-xysilane(MPS).It was reported102that MPS could be added at various concentrations,thereby improving the mechanical properties of thefinal hydrogel without altering its lower critical solution temperature.Similar to carboxylate and phosphate groups,Si-OH groups present in MPS provided sites that bound calcium and induced subsequent mineral deposition upon soaking in SBF.Peptide-mediated mineralization—acidic peptidesAcidic peptide sequences can be conjugated on a hydro-gel,but there are also formulations of hydrogels composed of polymerized polypeptides.The mineralization capacity of a hydrogel made from crosslinked polyglutamic acid was studied by Sugino et al.103The hydrogel samples studied were injectable and bioresorbable,and after crosslinking they were treated with different concentrations of CaCl2solutions for24h at body temperature.Upon soaking in SBF for7 days,HA was formed on the surface of the treated hydrogels irrespective of the CaCl2concentration of the solution used for the pretreatment.The HA nucleation potency of BSP-collagen hydrogels was tested and compared with agarose-BSP gels by Baht et al.104To assess the mineralization potency,the hydrogel scaffolds were perfused with buffers containing either Ca(NO3)2or Na2HPO4 with a steadyflow state.The results showed that collagen favors the BSP nucleation potency by nearly a factor of10 when compared to agarose gels.The synergistic interaction between collagen and BSP appeared to improve the mineral-ization capacity of these natural hydrogels.Chirila et al.105immobilized three different artificial protein sequences onto pHEMA hydrogels.These sequences(two of them can be found in nacrein and the third is present in dentin matrix acidic phosphoprotein)were tested in vitro and their ability to nucleate calcium phosphate was assessed in solu-tions.Disks prepared from the peptide conjugated polymers were immersed in Ca2þand PO43Àcontaining media for a total period of6weeks.The peptide sequences were shown to have no or an enhancing effect on calcium mineralization. Gungormus et al.106developed a peptide-based hydrogel that mediated the formation of HA.The27residue peptide MDG1self-assembles into a hydrogel by changing its form when alternating the ionic strength of the solution.By entrapping ALP in the hydrogel and immersing it in a b-glycerophosphate solution,mineralization of the hydrogel was achieved.Indirect MineralizationEven though it is not the scope of this review to address drug or cell delivery systems,it should be emphasized that hydrogels are often used to deliver osteoinductive growth factors such as bone morphogenetic proteins,demineralized bone matrix,107–114and/or cells,115–125and in many of these cases extensive mineralization is observed as a secondary consequence.According to this mechanism,growth factors trigger cell signaling pathways that stimulate stem cells in the direct vicinity of the hydrogel to differentiate into the osteogenic lineage and produce biomineral.In the case of cell delivery,cells that have been differentiated into the osteo-genic lineage are encapsulated directly into hydrogels before implantation that subsequently calcify the carrier hydrogel. Introduction of the Arginine–Glysine–Aspartate amino acid sequence(RGD or Arg-Gly-Asp)is commonly used to provide attachment and differentiation sites for cells inside the hydrogels resulting in indirect mineralization.126–128 For further information on mineralization induced by growth factors release and/or cell encapsulation,the reader is referred to reviews by Salinas and Anseth,123Hunt and Grover,129and Schmidt et al.130ConclusionsTraditionally,hydrogels have been considered for soft tissue regeneration only,but recently successful attempts have been made to render hydrogels suitable for hard tissueMINERALIZATION OF HYDROGELS581。
八面体结构ZnGa2O4微晶的制备及其光催化性能

八面体结构ZnGa2O4微晶的制备及其光催化性能刘亮亮;曹丽云;黄剑锋;张晓薇;吴建鹏;王开通【摘要】以乙酸锌和氧化镓为反应原料,以乙二胺四乙酸(EDTA)为配位剂,采用溶胶-凝胶法制备了八面体结构的ZnGa2O4微晶.通过TG-DSC,XRD、SEM等分析方法对ZnGa2O4微晶进行了测试和表征.研究了其物相组成、显微结构、形成机理及光催化性能.结果表明,在700℃、4~6 h时可以成功制备出八面体结构的ZnGa2O4单晶,其暴露的晶面族{111};八面体结构ZnGa2O4的合成是一个受ZnO的产生速率所控制的过程;光催化降解罗丹明B的实验表明,八面体结构ZnGa2O4微晶有着较好的光催化性能.%The octahedral ZnGa2O4 crystallines were synthesized using a EDTA complexation sol-gel process including the zinc acetate and gallium oxide, with the ethylenediamine tetraacetate for complexing agent. The crystalline phase, compositions, morphology, microstructure of the ZnGa2O4 are characterized by Thermogravimetry and Differential Scanning Caborimetry (TG-DSC), X-ray diffraction (XRD) and Scanning Electron Microscopy (SEM) measurements. Results indicate that the single crystalline of ZnGa2O4 with the octahedral structure can be obtained at calcining temperature of 700 ℃ from 4 h to 6 h; The preparation process of ZnGa2O4 with the octahedral structure is controlled by the generating rate of ZnO. The degradation of rhodamine B shows that octahedral ZnGa2O4 crystallites have good photocatalytic performance.【期刊名称】《无机化学学报》【年(卷),期】2012(028)010【总页数】6页(P2091-2096)【关键词】ZnGa2O4单晶;EDTA配位溶胶-凝胶法;光催化;降解速率常数【作者】刘亮亮;曹丽云;黄剑锋;张晓薇;吴建鹏;王开通【作者单位】陕西科技大学材料科学与工程学院,教育部轻化工助剂化学与技术重点实验室,西安710021;陕西科技大学材料科学与工程学院,教育部轻化工助剂化学与技术重点实验室,西安710021;陕西科技大学材料科学与工程学院,教育部轻化工助剂化学与技术重点实验室,西安710021;陕西科技大学材料科学与工程学院,教育部轻化工助剂化学与技术重点实验室,西安710021;陕西科技大学材料科学与工程学院,教育部轻化工助剂化学与技术重点实验室,西安710021;陕西科技大学材料科学与工程学院,教育部轻化工助剂化学与技术重点实验室,西安710021【正文语种】中文【中图分类】TN383+.2ZnGa2O4是具有立方晶系尖晶石晶体结构的复合氧化物,其应用前景比较广泛,可以应用于场发射显示器[1]、薄膜电致发光显示器[2]、和真空荧光显示器,同时由于有着优越的热学和化学稳定性,能承受较高的电流冲击,从而能代替硫化物在发光二极管、光电探测器、低电压发光材料中使用[3-5];光催化方面,Zhang[6]等采用水热法合成了ZnGa2O4介孔材料,并证明了其在降解苯系列污染物中优于TiO2及其掺杂物的光催化活性;Shi等[7]在室温下成功合成出的ZnGa2O4介孔光催化材料,能用于CO2的光还原,成功地将CO2转化为碳氢化合物燃料。
【国家自然科学基金】_光致发光性质_基金支持热词逐年推荐_【万方软件创新助手】_20140802

水溶性 氰化银基配合物 氮化镓 氧流量 氧化锌薄膜 材料 有机电致发光器件 有机电致发光 有效质量近似模型 晶体结构 无机非金属材料 施主受主对 施主-受主对 掺杂 扩散 快速热退火(rta) 微结构 微乳液 应力 带电激子 射频辅助 多孔硅锗 多元醇方法 壳/核结构 团簇离子注入 咔唑 同轴送氧 同质结 受主能级 发光薄膜 发光 双β -二酮配体 原位生长 半导体纳米材料 半导体材料 半导体光致发光 化学溶液工艺技术 化学溶液工艺 化学氧化 包络波函数 分子开关 光谱电化学 光致荧光 光致发光(pl) 光学增益 偏振光致发光 低维纳米结构 低温pl谱 二芳基冠醚 二维电子气 乙酰水杨酸 ⅳ-ⅵ半导体光学 ⅱ型量子阱 β -ga2o3 棒
107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160
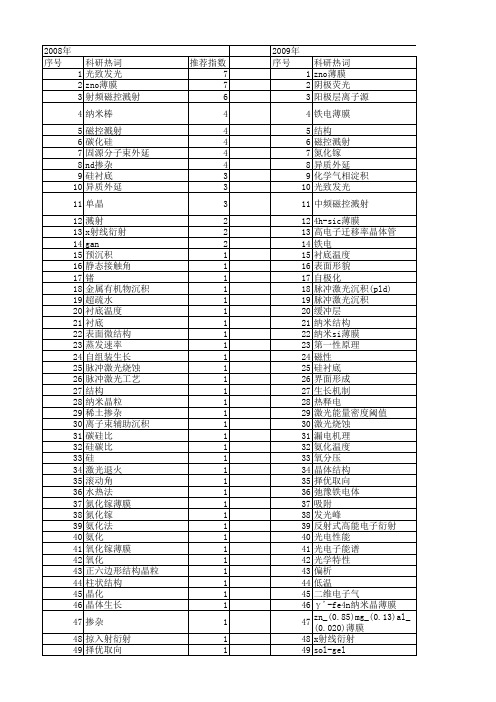
2009年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 聚合物发光二极管 聚(3,6-咔唑) 聚(2,5-二丁氧基)对苯乙炔 罗丹明b 缺陷发光 缓冲层 结构特性 纳米颗粒 纳米阵列 纳米硅 纳米棒 纳米复合薄膜 纳米zno/sno2复合材料 纳米zno 红移 紫外峰 粗糙度 稀土配合物 离子注入 碳化硅 碲化镉/硫化镉 碲化镉 碱金属元素 硫化铋 硅基纳米孔阵列结构 砷掺杂 电致发光 电沉积 电化学刻蚀 特性 燃烧法 激光染料 溶胶凝胶 溶液法 深能级瞬态谱 水热合成 氮化铟 氨气掺杂 氧化锡 氧化铝 氟化 柠檬酸 极化效应 择优取向 应力分析 席夫碱 局域态 射频磁控溅射 多量子阱 多孔阳极氧化铝模板 多壁碳纳米管 复合材料
硫酸氧化锆催化正庚烷异构化

摘要过渡金属氧化物作为固体酸多相催化剂在催化研究中占有重要的地位。
氧化锆由于具有酸性和碱性表面活性中心,作为催化剂和催化剂载体受到广泛关注。
硫酸氧化锆因其具有超强酸性,在正庚烷异构化反应上有着很好的催化活性。
然而硫酸氧化锆却存在着比表面不高,硫组易流失等问题,限制其在工业中的应用。
通过掺杂金属元素的方法可以提高氧化锆的稳定性,增强酸性和相关的反应性能。
本论文致力于用溶胶-凝胶法制备掺铝介孔硫酸氧化锆,并通过引入助剂等,力争获得在正庚烷异构化反应中具有高催化性能的硫酸氧化锆催化剂。
本论文主要开展了以下几个方面的工作。
1.通过溶胶-凝胶法制备掺铝介孔硫酸氧化锆催化剂,并考察不同铝含量对催化剂性能的影响。
2.在铝含量为5%的情况下考察不同焙烧温度对催化剂性能的影响,寻找最佳的焙烧温度。
3.在催化剂中掺入稀土元素,考察稀土元素的影响。
4.通过红外,XRD等表征技术研究催化剂的结构。
关键词:氧化锆;介孔;溶胶-凝胶;硫酸化;正庚烷异构化AbstractTransition-metal oxides play an important role in catalysis as solid acid catalyst.Among them,zirconia has been paid much attention and has been used as acidic catalyst and catalyst support because of the presence of acidic and basic surface active center.As a strong acidic catalyst .SO42-/ZrO2 exhibit unique catalytic performance on n-heptane isomerization.However,SO42-/ZrO2 has main disadvantages of loss of sulfur species and relative low surface area,which limits its industrial process.Doping other metal species can improve the stability of zirconia to enhance the performance of the acid and related reactions.In this thesis ,the research work was mainly focused on the preparation of Al-SO42-/ZrO2with intracystalline mesopore by sol-gel method and the exploration of catalysis with high performance over n-heptane isomerization through lead into assistant.The major work may be summarized as the follows:1.composing Al-SO42-/ZrO2 with intracystalline mesopore by sol-gel method and considering the effect of the proportion of Al on the performance of catalyst.2.considering the effect of different calcination temperature on the performance of catalyst when the content of aluminium was 5%.3.leading rare earth into catalyst and observe the change of the performance of catalyst.4.Characterization by XRD,IR Techniques.Key words:zirconia ;mesoporous ;sol-gel ;sulfated ;n-heptane isomerization目录第1章概述 (1)1.1 氧化锆的研究及应用进展 (1)1.2 正庚烷异构化反应简介 (4)第2章实验部分 (12)2.1 实验所用试剂及仪器 (12)2.2 实验方法 (12)2.3 表征方法 (14)第3章制备条件对催化剂结构及性能的影响 (15)3.1 催化剂的表征 (16)3.2正庚烷临氢异构化反应测试结果 (18)结论 (22)参考文献 (23)致谢 .............................................................................................. 错误!未定义书签。
尖晶石型化合物的制备及光催化性能

靳玲玲等:氧化钇透明陶瓷的研究进展· 527 ·第38卷第3期尖晶石型化合物的制备及光催化性能林仕伟,段文杰,李建保(海南大学,硅锆钛资源综合开发与利用海南省重点实验室,海口 570228)摘要:本文总结了近年来尖晶石型化合物光催化研究方面的工作,包括尖晶石型化合物的常用制备方法、光催化机理以及光催化制氢和降解有机物等性能;同时介绍提高尖晶石型光催化剂活性的主要途径,并展望未来尖晶石型化合物光催化领域的研究工作。
关键词:尖晶石;制备;光催化性能;可见光响应;综合评述中图分类号:TQ 426.6 文献标志码:A 文章编号:0454–5648(2010)03–0527–08PREPARATION AND PHOTOCATALYTIC PROPERTIES OF SPINEL COMPOUNDSLIN Shiwei,DUAN Wenjie,LI Jianbao(Hainan Provincial Key Laboratory of Research on Utilization of Si–Zr–Ti Resources, Hainan University, Haikou 570228, China) Abstract: This paper summarizes the research progresses on spinel compounds in recent years, including preparation, photocatalytic mechanisms, hydrogen production, and organic-compound decomposition. The main ways to improve spinel photocatalytic efficiency are introduced, and their photocatalytic prospects are predicted.Key words: spinels; preparation; photocatalytic properties; visible-light response; review光催化是近几十年来研究的热点之一。
【国家自然科学基金】_si(111)衬底_基金支持热词逐年推荐_【万方软件创新助手】_20140802
53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86
射频溅射参数 多铁性复合薄膜 图形衬底 取向 发光 反射式高能电子衍射 原子力显微镜 厚度 化合物半导体 光致发光谱 光致发光\ 催化荆 位错运动 一维gan纳米结构 β -ga2o3 棒 zno缓冲层 zno纳米棒 znmgo薄膜 zn0.85-xco0.15cuxo薄膜 x射线衍射分析 xps pld mg0.05zn0.95o薄膜 lpcvd lanio3(镍酸镧) gan 纳米棒 ga2o3/al膜 ga-n共掺杂 cu、ag、pt薄膜 cocu共掺杂 ba0.7sr0.3tio3薄膜 aln/si(111)衬底 4h-sic薄膜 3c-sic
推荐指数 7 7 6 4 4 4 4 4 3 3 3 2 2 2 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
2009年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52
科研热词 推荐指数 退火 3 磁控溅射 3 溅射 3 光学特性 3 zno 3 gan 3 薄膜 2 纳米线 2 择优生长 2 择优取向 2 分子束外延 2 光致发光 2 x射线衍射 2 si衬底 2 锰氧化物薄膜 1 铁电性 1 钴 1 钯催化 1 金属有机化学气相沉积 1 超高真空扫描隧道显微镜 1 超声波振动 1 衬底温度 1 表面增强效应 1 脉冲激光烧蚀 1 脉冲激光沉积法 1 结晶 1 纳米结构 1 纳米si晶粒 1 磁性薄膜 1 直流磁控反应溅射 1 电阻率 1 电阻开关 1 电脉冲诱发电阻转变 1 电滞回线 1 电沉积 1 电子结构 1 生长机制 1 环境气体 1 漏电流 1 溶胶-凝胶法 1 溶胶-凝胶 1 温度 1 水蒸气 1 氨化 1 氧空位 1 氧化锌柱 1 氧化锌 1 气相输运 1 气流 1 正磁电阻 1 晶须 1 晶格畸变 1
TCO简介

TCO薄膜的简介透明导电氧化物(transparentconductiveoxide简称TCO)薄膜主要包括In、Sb、Zn和Cd的氧化物及其复合多元氧化物薄膜材料,具有禁带宽、可见光谱区光透射率高和电阻率低等共同光电特性,广泛地应用于太阳能电池、平面显示、特殊功能窗口涂层及其他光电器件领域。
透明导电薄膜以掺锡氧化铟(tindopedindiumoxide简称ITO)为代表,研究与应用较为广泛、成熟,在美日等国已产业化生产。
近年来ZnO薄膜的研究也不断深入,掺铝的ZnO薄膜(简称AZO)被认为是最有发展潜力的材料之一。
同时,人们还开发了Zn2SnO4、In4Sn3O12、MgIn2O4、CdIn2O4等多元透明氧化物薄膜材料。
TCO薄膜的制备工艺以磁控溅射法最为成熟,为进一步改善薄膜性质,各种高新技术不断被引入,制备工艺日趋多样化。
本文综述以ITO和AZO为代表的TCO 薄膜的研究进展及应用前景。
一、TCO薄膜的发展TCO薄膜最早出现于20世纪初,1907年Badeker首次制成了CdO透明导电薄膜,引起了人们的较大兴趣。
但是,直到第二次世界大战,由于军事上的需要,TCO薄膜才得到广泛的重视和应用。
1950年前后出现了SnO2基和In2O3基薄膜。
ZnO基薄膜兴起于20世纪80年代。
相当长一段时间,这几种材料在TCO薄膜中占据了统治地位。
直到上世纪90年代中期,才有新的TCO薄膜出现,开发出了多元TCO薄膜、聚合物基体TCO薄膜、高迁移率TCO薄膜以及P型TCO薄膜。
而SnO2基和In2O3基材料也通过掺加新的元素而被制成了高质量TCO 薄膜。
最近,据媒体报导,美国俄勒冈大学研究人员对TCO材料的研究取得重大突破,他们研制出一种便宜、可靠且对环境无害的透明导电薄膜材料。
该材料可用于制作透明晶体管,用来制造非常便宜的一次性电子产品、大型平面显示器和可折叠又方便携带的电器。
科学家称,这项研究成果将引导新产业和消费领域的发展。
重组贻贝粘蛋白的表征及功效评价

生物技术进展 2023 年 第 13 卷 第 4 期 596 ~ 603Current Biotechnology ISSN 2095‑2341研究论文Articles重组贻贝粘蛋白的表征及功效评价李敏 , 魏文培 , 乔莎 , 郝东 , 周浩 , 赵硕文 , 张立峰 , 侯增淼 *西安德诺海思医疗科技有限公司,西安 710000摘要:为了推进重组贻贝粘蛋白在医疗、化妆品领域的应用,对大肠杆菌规模化发酵及纯化生产获得的重组贻贝粘蛋白进行了表征及功效评价。
经Edman 降解法、基质辅助激光解吸电离飞行时间质谱、PITC 法、非还原型SDS -聚丙烯酰胺凝胶电泳法、凝胶法、改良的Arnow 法对重组贻贝粘蛋白进行氨基酸N 端测序、相对分子量分析、氨基酸组成分析、蛋白纯度分析、内毒素含量测定、多巴含量测定;通过细胞迁移、斑马鱼尾鳍修复效果对重组贻贝粘蛋白进行功效评价。
结果显示,获得的重组贻贝粘蛋白与理论的一级结构一致,蛋白纯度达95%以上,内毒素<10 EU ·mg -1,多巴含量大于5%;重组贻贝粘蛋白浓度为60 μg ·mL -1时能够显著促进细胞增殖的活性(P <0.01);斑马鱼尾鳍面积样品组与模型对照组相比极显著增加(P <0.001)。
研究结果表明,重组贻贝粘蛋白具有显著的促细胞迁移和修复愈合的功效,具备作为生物医学材料的潜质。
关键词:贻贝粘蛋白;基因重组;生物材料;表征;功效评价DOI :10.19586/j.20952341.2023.0021 中图分类号:S985.3+1 文献标志码:ACharacterization and Efficacy Evaluation of Recombinant Mussel Adhesive ProteinLI Min , WEI Wenpei , QIAO Sha , HAO Dong , ZHOU Hao , ZHAO Shuowen , ZHANG Lifeng ,HOU Zengmiao *Xi'an DeNovo Hith Medical Technology Co., Ltd , Xi'an 710000, ChinaAbstract :In order to promote the application of recombinant mussel adhesive protein in the medical and cosmetics field , the recombi⁃nant mussel adhesive protein obtained from scale fermentation and purification of Escherichia coli was characterized and its efficacy was evaluated. Amino acid N -terminal sequencing , relative molecular weight analysis , amino acid composition analysis , protein purityanalysis , endotoxin content , dihydroxyphenylalanine (DOPA ) content of recombinant mussel adhesive protein were determined by the following methods : Edman degradation , matrix -assisted laser desorption ionization time -of -flight mass spectrometry (MALDI -TOF -MS ), phenyl -isothiocyanate (PITC ), nonreductive SDS -polyacrylamide gel electrophoresis (SDS -PAGE ), gel method , modified Ar⁃now. The efficacy of recombinant mussel adhesive protein was evaluated by cell migration and repairing effect of zebrafish tail fin. Re⁃sults showed that the obtained recombinant mussel adhesive protein was confirmed to be consistent with the theoretical primary structure , protein purity of more than 95%, endotoxin <10 EU ·mg -1, DOPA content above 5%. When the recombinant mussel adhesive protein concentration was 60 μg ·mL -1, the effect of promoting cell proliferation was the most obvious , and it had very significant activity (P <0.01). The caudal fin area of zebrafish in sample group was significantly increased compared with model control group (P <0.001). The results indicated that recombinant mussel adhesive protein can promote cell migration and repair healing and has the potential to be used as biomedical materials.Key words :mussel adhesive protein ; gene recombination ; biological materials ; representation ; efficacy evaluation贻贝粘蛋白(mussel adhesive protein , MAP )也称作贻贝足丝蛋白(mussel foot protein ,Mfps ),收稿日期:2023⁃02⁃24; 接受日期:2023⁃03⁃31联系方式:李敏 E -mail:*******************;*通信作者 侯增淼 E -mail:***********************.cn李敏,等:重组贻贝粘蛋白的表征及功效评价是海洋贝类——紫贻贝(Mytilus galloprovincalis)、厚壳贻贝(Mytilus coruscus)、翡翠贻贝(Perna viri⁃dis)等分泌的一种特殊的蛋白质,贻贝中含有多种贻贝粘蛋白,包括贻贝粘蛋白(Mfp 1~6)、前胶原蛋白(precollagens)和基质蛋白(matrix proteins)等[1]。
【国家自然科学基金】_硅基薄膜_基金支持热词逐年推荐_【万方软件创新助手】_20140731

科研热词 推荐指数 溶胶-凝胶法 5 非晶硅 2 集成薄膜 2 锆钛酸铅 2 磁控溅射法 2 漏电流 2 掺锰铁酸铋 2 发光学 2 光学薄膜 2 pzt 2 非晶ni-al阻挡层 1 静电驱动 1 铒硅酸盐 1 铁电膜 1 铁电性能 1 退火工艺 1 退火 1 软磁 1 薄膜光学 1 薄膜 1 脉冲激光晶化 1 绝缘介电层参数 1 结构设计 1 结晶 1 纳米结构铁电膜 1 纳米结构 1 紫外敏感 1 粒径 1 硅纳米晶 1 硅基薄膜电池 1 硅基微电感 1 真空蒸发法 1 电润湿效应 1 玻璃基lsco/pzt/lsco电容器 1 物性及微结构表征 1 激光打孔 1 溶胶凝胶法 1 浓硼扩散区 1 氧化铒 1 氧化硅 1 氢化非晶硅 1 氢化微晶硅 1 气敏特性 1 椭圆偏振光谱学 1 晶体硅 1 无机非金属材料 1 快速退火 1 微结构 1 微磁通门 1 微机械陀螺 1 微悬臂梁 1 异质结 1
科研热词 电致发光 光致发光 铁电薄膜 纳米硅 二氧化硅 靶负压 非晶掺氮碳化硅基薄膜(a-sicnx:h) 非晶a-sic:h薄膜 银岛膜 铜扩散 铒 量子阱 量子点 衰减时间 表面等离激元 薄膜厚度 薄膜制备 耐磨寿命 纳米硬度 稀土离子 硅基发光材料 硅 沉积速率 氮化硅 染料敏化太阳能电池 有机无机复合 择优取向 抛光 感光溶胶-凝胶 微图案 应变 单晶硅 半导体光电子 光阳极薄膜 光电集成 光学常数 光功率分配器 光刻印 介质扩散阻挡层 一维纳米氧化物 β -fesi2薄膜 zno y分支 sol-gel si基板 sinx:tb3+薄膜 pfcvad pb_(1-x)ge_xte薄膜 mos器件 mn2+ hfo2微结构 batio3
2011年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45
表面活性剂辅助Sol-gel法SrTiO3粉体的形状控制合成

水 中溶 解和 10℃加 热浓 缩后 加入溶 液 。氯化 锶: 0 酞 酸 丁酯: 酸 : 丙酮 : 活性 剂 单体 的 摩尔 配 柠檬 乙酰 表面
比为 11 :.: 。然 后用 甲醇 调节 至两 个溶 液 的金 :: 01 0 5 3
学 术 研 究
5
图 1示 出 应 用 两 种 表 面 活 性 剂 作 模 版 合 成
STO 粉 体 的 X D图谱 。两种粉 体 均为纯 的 。聚 乙 ri R 烯醇 作模 版合 成 的粉体 有较 强 的衍 射 峰表 明有 大 的 结 晶度 。 由 3 . 处 的最 强衍 射 峰 ( 1 ) 据谢 乐逊 24 。 10 根 公式 计算 的聚 乙二 醇和 聚 乙烯 醇合 成 的粉体 的粒 子
分 别 干燥 1 、2h 1 、 2 。缓慢 干 燥 的 目的是 5h 1 、2h 1 h
电性 。 究表 明合理 的化 学溶液 和快 速升温烧成等 研
可 以使 制 备 的 c 取 向 的柱 状 薄膜 具 有 大 的 ca比 一 / 值, 因而 具有 大的铁 电性 。此 外 , 状控 制 STO 粉 形 ri
子形 貌 。
本 研 究 所 用 的 原 料 均 为 分 析 纯 ,有 酞 酸 丁 酯
2 结果与讨论
(8 9%,TtH, H) 1, i ( 204 氯化 锶 , C C 3 ) 甲醇 为溶 剂 , 乙酰丙
酮为 稳定 剂 , 檬 酸为 螯合 剂 , 乙二 醇 ( E 一 0 , 柠 聚 P G 2 0 C O (HC 2) C 2H H2H一C 2H 02 HO )和 聚 乙 烯 醇 ( V 一 一 P A, [H C ( H 卜 ) C 2 H O ) 为表 面活性剂模 板 。
TCO透明导电薄膜简介

TCO透明导电薄膜简介前言透明导电氧化物transparentconductiveoxide简称TCO薄膜主要包括In、Sb、Zn和Cd的氧化物及其复合多元氧化物薄膜材料具有禁带宽、可见光谱区光透射率高和电阻率低等共同光电特性广泛地应用于太阳能电池、平面显示、特殊功能窗口涂层及其他光电器件领域。
透明导电薄膜以掺锡氧化铟tindopedindiumoxide简称ITO为代表研究与应用较为广泛、成熟在美日等国已产业化生产。
近年来ZnO薄膜的研究也不断深入掺铝的ZnO薄膜简称AZO被认为是最有发展潜力的材料之一。
同时人们还开发了Zn2SnO4、In4Sn3O12、MgIn2O4、CdIn2O4等多元透明氧化物薄膜材料。
TCO薄膜的制备工艺以磁控溅射法最为成熟为进一步改善薄膜性质各种高新技术不断被引入制备工艺日趋多样化。
本文综述以ITO和AZO为代表的TCO薄膜的研究进展及应用前景。
一、TCO薄膜的发展TCO薄膜最早出现于20世纪初1907年Badeker首次制成了CdO透明导电薄膜引起了人们的较大兴趣。
但是直到第二次世界大战由于军事上的需要TCO薄膜才得到广泛的重视和应用。
1950年前后出现了SnO2基和In2O3基薄膜。
ZnO基薄膜兴起于20世纪80年代。
相当长一段时间这几种材料在TCO薄膜中占据了统治地位。
直到上世纪90年代中期才有新的TCO薄膜出现开发出了多元TCO薄膜、聚合物基体TCO薄膜、高迁移率TCO 薄膜以及P型TCO薄膜。
而SnO2基和In2O3基材料也通过掺加新的元素而被制成了高质量TCO薄膜。
最近据媒体报导美国俄勒冈大学研究人员对TCO材料的研究取得重大突破他们研制出一种便宜、可靠且对环境无害的透明导电薄膜材料。
该材料可用于制作透明晶体管用来制造非常便宜的一次性电子产品、大型平面显示器和可折叠又方便携带的电器。
科学家称这项研究成果将引导新产业和消费领域的发展。
这种薄膜材料的成分是无定型重金属阳离子氧化物与导电物质碳相比具有很多优点相对于有机聚合体导电物质来说亦具有较高的灵活性和化学稳定性容易制造也更加坚硬。
氮化镓的合成制备及展望

氮化镓的合成制备及展望氮化镓的合成制备及展望摘要:氮化镓作为第三代半导体的代表,具有优越的电学性能,它在光电子器件如:蓝光、紫外、紫光等光发射二极管和激光二极管方面有着重要的应用.。
氮化镓的合成制备,对全球半导体产业的发展具有重要意义,目前已经成为世界的研究热点。
本文对氮化镓薄膜以及纳米氮化镓的合成制备方法进行了综述。
引言GaN 是一种优异的直接带隙半导体材料,室温下禁带宽度为3.4 eV,具有优良的光电性能、热稳定性及化学稳定性,是制作高亮度蓝绿发光二极管( LED) 、激光二极管( LD) 以及大功率、高温、高速和恶劣环境条件下工作的光电子器件的理想材料。
最近有报道发现GaN 基纳米材料具有吸收可见光使水解离产生氢的性能,这使得GaN 纳米材料的研究获得了很多的关注。
半导体纳米粒子由于小尺寸效应,往往会呈现不同于体材料的发光特性。
但要实现高效可靠的光发射,尤其是可在柔性衬底上制作器件并可供日常使用的光发射材料仍然是个巨大的挑战。
目前,合成GaN纳米粒子方法主要有氨热法、金属有机化合物化学气相沉积( MOCVD) 法、高温热解法、胶体化学法等。
一、氮化镓薄膜制备GaN 薄膜的合成技术,近年来在文献中有很多的报导。
由于GaN 的熔点很高,且饱和蒸汽压较高,在自然界中无法以单晶形式存在,而且用一般的体单晶生长方法来制备薄膜也相当困难,必须采用外延法进行制备。
MOCVD,MBE,HVPE 等是比较传统的GaN 薄膜制备方法。
1.金属有机物气相沉积法MOCVD(金属有机物气相沉积法)是在气相外延生长的基础上发展起来的一种新型气相外延生长技术。
在采用MOCVD 法制备GaN 单晶的传统工艺中,通常以三甲基镓作为镓源,氨气作为氮源,以蓝宝石(Al2O3)作为衬底,并用氢气和氮气的混合气体作为载气,将反应物载入反应腔内,加热到一定温度下使其发生反应,能够在衬底上生成GaN 的分子团,在衬底表面上吸附、成核、生长,最终形成一层GaN 单晶薄膜。
Amersham Biosciences ProteoGel IPG Strips 产品说明书

ProteoGel™ IPG StripsStorage Temperature –20 °CTECHNICAL BULLETINProduct DescriptionTwo-dimensional electrophoresis separates proteins inthe first dimension by their isoelectric point (pI) and by molecular weight in the second dimension. Isoelectric point separation is achieved by electrophoresis (focusing) of solubilized proteins in a gel containing an immobilized pH gradient (ProteoGel™ IPG Strips). Following the first dimension separation, the ProteoGel IPG Strip is equilibrated with ProteoGel™ IPG Equilibration Buffer (Product Code I 7281) to denaturethe proteins with a detergent (SDS) and urea. The ProteoGel IPG Strip is then placed in the well of aSDS-PAGE 2D gel and electrophoresed to separate the proteins by molecular weight.ProteoGel IPG Strips are produced using acrylamido buffers to create stable, immobilized pH gradients in a polyacrylamide matrix. The polyacrylamide matrix isdried onto a plastic backing to increase the shelf-life ofthe IPG strips and to allow the strip to be rehydratedwith the protein sample.ProteoGel IPG Strips are available with a wide rangepH gradient (pH 3-10) or 5 different narrow range pH gradients (3-5, 4-7, 5-8, 6-11, and 8-11) to optimize the separation of the proteins. The strips are available in three different lengths (7 cm, 11 cm, and 18 cm). The strips are conveniently labeled with a “+” on the acidic (anode) end of the strip for orientation in the focusing unit.Table 1.ProteoGel IPG Strip Product CodespH RangeLength 3-10 3-5 4-7 5-8 6-11 8-11 7 cm I 2531 I 3031 I 2906 I 3156 I 7406 I 3281 11 cm I 3406 I 3656 I 3531 I 3781 I 7531 I 3906 18 cm I 4031 I 4281 I 4156 I 4406 I 7656 I 4531 Products Required But Not ProvidedUltrapure Water (18 megohm or equivalent) Rehydration trayForceps (Product Code F 3767)Mineral oil (Product Code M 3516)IPG strip focusing apparatusProteoGel IPG Equilibration Buffer (ProductCode I 7281)SDS-PAGE apparatusGel Protein StainCoomassie Brilliant Blue G-250 stain(EZBlue™ Gel Staining Reagent, ProductCode G 1041) orSilver Stain(ProteoSilver™ Silver Stain Kit, Product CodePROT-SIL1) or (ProteoSilver™ Plus, ProductCode PROT-SIL2 for samples prepared forMALDI-MS analysis.)DuraSeal laboratory stretch film (Product CodeD 3172) or Parafilm (Product Code P 7793) Precautions and DisclaimerThese products are for laboratory use only, not for drug, household, or other uses. Consult the MSDS for information regarding hazards and safe handling practices.Storage/StabilityThe ProteoGel IPG strips are stable at −20 °C for at least 1 year in an unopened package.Procedure1. Prepare the protein sample in an appropriatesolubilization/extraction solution. Sigma offersthree different ProteoPrep™ kits (PROT-TOT,PROT-TWO, and PROT-MEM) for2D electrophoresis sample preparation. Each kitcontains solubilization/extraction solutions, atributylphosphine solution for reduction of thesample, and iodoacetamide for protein alkylation.2. Dilute an aliquot of the prepared sample to thedesired concentration. Pipette the appropriateamount of sample (see Table 2) along the edge ofthe rehydration tray.Table 2.ProteoGel IPG strip rehydration volumesIPG strip length Sample volume7 cm 125 µl11 cm 200 µl18 cm 320 µl3. Remove the protective plastic from the ProteoGelIPG strip gel surface and place the strip, gel sidedown, on the sample such that the entire gel is incontact with sample.Note: When the gel side is down, the writing on the strip will appear in the correct orientation. Wrap the rehydration tray with laboratory stretch film toprevent evaporation. Allow the strips to rehydrateat room temperature for 5 hours or until essentially all the sample has been absorbed into the gel.Lower temperatures may cause the urea toprecipitate. Less than 3 µl of the sample shouldremain in the tray after rehydration.Note: Water-saturated blotting paper may be added to an empty lane of the rehydration tray to reduceevaporation or overlay mineral oil (Product CodeM 3516) on the strips.4. Assemble the strip into the IPG strip focusingapparatus following the manufacturer's instructions.The acidic end (+) of the ProteoGel IPG stripshould be at the anode (red/+). Ensure that the gel on the IPG strip has made contact with theelectrode or a moist electrode wick. Overlaymineral oil on the strips to minimize evaporationduring the focusing.5. See Table 3 for the recommended electrophoresisprotocols for focusing of the ProteoGel IPG strips.The recommended temperature is 20 °C. Lowertemperatures may cause the urea to precipitate.Increasing the total volt hours may improve thefocusing. The maximum current allowed per strip is50 µA, otherwise damage to the strip fromoverheating may occur. Table 3.ProteoGel IPG Strip Focusing ConditionsStep VoltageTimeVoltHours 7 cm stripConditioningRampFocusing250 V250 – 6000 V6000 V1 hour2 hours60,00011 cm stripConditioningRampFocusing250 V250 – 6000 V6000 V1 hour2 hours80,00018 cm stripConditioningRampFocusing250 V250 – 6,000 V6,000 V1 hour2 hours100,0006. If necessary, the focused ProteoGel IPG strips maybe wrapped with laboratory stretch film and storedbelow –20 °C for up to 1 week, prior to running thesecond dimension gel.7. After focusing, equilibrate the focused IPG Stripwith ProteoGel IPG Equilibration Buffer for 20 to30 minutes at room temperature.8. Fill the well of a SDS-PAGE 2D gel with electrodebuffer and place the ProteoGel IPG Strip into thewell, with forceps, so that the side of the stripmakes complete contact with the top of thepolyacrylamide gel. Avoid air bubbles between thestrip and the top of the gel.Note: An agarose overlay is not necessary.9. Assemble the SDS-PAGE 2D gel into theelectrophoresis unit and electrophorese the gel untilthe blue dye front is within 1 cm of the bottom of thegel.10. Stain the SDS-PAGE gel using Coomassie BrilliantBlue (EZBlue Gel Staining Reagent, Product CodeG 1041) or silver stain (ProteoSilver Silver Stain Kit,Product Code PROT-SIL1) to visualize the proteins.Proteosilver Plus (Product Code PROT-SIL2) isrecommended for samples prepared for MALDI-MSanalysis.Related Products Product Code ProteoPrep KitsTotal Extraction SampleMembrane Protein Extraction Universal Extraction PROT-TOT PROT-MEM PROT-TWOCellular and Organelle MembraneSolubilizing ReagentC 0356 Chaotropic Membrane ExtractionReagent 2C 0606 Dithiothreitol (DTT)D 5545 Iodoacetamide (IAA) A 3221 Tributylphosphine (TBP) T 7567 ProteoGel IPG EquilibrationBufferI 7281 ProteoGel Tris-Tricine-SDSElectrode BufferT 2821 Hi/Lo Profile Rocker Z36,774-5 References1. Gorg, Angelika, Two-Dimensional Electrophoresisof Proteins Using Immobilized pH Gradients. ALaboratory Manual. Technical University of Munich (Munich, Germany: 1998).Coomassie Brilliant Blue is a registered trademark of Imperial Chemical Industries.Duraseal is a trademark of Diversified Biotech. Parafilm is a registered trademark of American National Can Company.Technology developed in partnership with Proteome Systems™.MDS/MAM 3/02Sigma brand products are sold through Sigma-Aldrich, Inc.Sigma-Aldrich, Inc. warrants that its products conform to the information contained in this and other Sigma-Aldrich publications. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see reverse side ofthe invoice or packing slip.。
Ceragel CPS72和CPS72D ORP电род,分析型和数字型,具有Memosens技术

Products Solutions Services TI00374C/07/EN/13.1371212355Technical InformationCeragel CPS72 and CPS72DORP electrodes, analog and digital with MemosenstechnologyFor process technology, hygienic and sterile applicationswith poison-resistant reference with ion trapApplication•Hygienic and sterile applications (sterilizable, autoclavable)–Fermenters–Biotechnology–Pharmaceutical industry–Food industry•Process technology and monitoring of processes with:–Rapidly changing ORP measured values–High proportion of electrode poisons such as H2SWith ATEX, FM and CSA approval for use in hazardous areasYour benefits•Certified biocompatibility, no cytotoxicity•Acrylamide-free bridging electrolyte•Integrated temperature sensor in the case of Memosens•Poison-resistant reference with ion trap, resulting in a very long service life•Bridging electrolyte free of silver ions•Suitable for CIP/SIP cleaning and autoclavable, depending on version up to 140 ˚C(284 °F)CPS72/CPS72D2Function and system designMeasuring principle ORP measurementThe oxidation-reduction potential is an indicator of the equilibrium between oxidizing and reducingsubstances in the medium. The oxidation-reduction potential is measured with a gold or platinumelectrode instead of the pH-sensitive glass membrane. As in the case of pH measurement, anintegrated Ag/AgCl reference system is used as the reference electrode.General characteristics•Short response timesThe ceramic junction allows sufficiently fast diffusion of the medium, thereby enabling shortresponse times.•Long service lifeUse of an ion trap as standard ensures that the reference is protected against poisoning, results in asignificantly longer service life and guarantees immunity to temperature and pressure fluctuations.The ion trap also effectively prevents the diffusion of silver ions into the bridging electrolyte.Communication and data processing CPS72D Measuring system data which digital sensors can save in the sensor include: •Manufacturer data–Serial number–Order code–Date of manufacture•Calibration data–Date of calibration–Calibrated offset ("mV" measuring mode)–% slope ("%" measuring mode)–Number of calibrations–Serial number of the transmitter used to perform the last calibration•Operating data–Temperature application range–ORP area of application–Date of initial commissioning–Operating hoursYou can display the data listed above using the Liquiline CM44x or Liquiline M CM42 transmitter.CPS72D dependability Maximum process safetyWith its inductive transmission of the measured value via a non-contact plug-in connection,Memosens guarantees maximum process safety and offers the following advantages:•All problems caused by moisture are eliminated:–The plug-in connection is free from corrosion–Moisture cannot corrupt the measured value–Plug-in system can even be connected under water•The transmitter is galvanically decoupled from the medium.This means there is no need to choose between "symmetrical high-impedance" or "unsymmetrical"solutions and impedance converters when it comes to pH/ORP measurement.•EMC safety is guaranteed by screening measures in the digital measured value transmission.•Can easily be used in hazardous areas thanks to intrinsically safe electronics.Memosens technology digitizes the measured values in the sensor and transmits them to thetransmitter via a non-contact connection in a way that is free from any potential interference. Theresult:•Automatic error message generation if the sensor fails or the connection between sensor andtransmitter is interrupted•Immediate error detection increases measuring point availabilityCPS72/CPS72D3Ease of useSensors with Memosens technology have integrated electronics that save calibration data and other information, such as total hours of operation and operating hours at very high temperatures etc. When the sensor is mounted, the sensor data are automatically sent to the transmitter and used to calculate the current ORP potential.Saving the calibration data makes it possible to calibrate and adjust the sensor irrespective of the measuring point. The result:•Convenient calibration in the measuring lab under optimum external conditions improves the quality of the calibration.•Measuring point availability is dramatically increased by the quick and easy replacement of precalibrated sensors.•Installing the transmitter in the measuring container with integrated measuring devices reduces the amount of fastening material and cabling work required.•The availability of the sensor data makes it possible to accurately determine the maintenance intervals of the measuring point and enables predictive maintenance.•The sensor history can be documented with external storage media and evaluation programs. The sensor's field of application can be determined based on its previous history.Communication with the transmitterAlways connect digital sensors with Memosens technology to a transmitter with Memosens technology. It is not possible to transfer data to a transmitter for analog sensors.Measuring systemA complete measuring system comprises:•ORP electrode CPS72 or CPS72D•Transmitter, e.g. Liquiline CM42 or Liquiline CM44x (for CPS72D with Memosens technology)•Special measuring cable. e.g. CPK9 or Memosens data cable CYK10 for CPS72D •Immersion, flow or retractable assembly, e.g. Cleanfit H CPA475Fig.1: Measuring system for ORP measurement1Process assembly Cleanfit H CPA4752ORP electrode CPS72 / CPS72D3Special measuring cable CPK9 (for electrodes with TOP68 plug-in head) / CYK10 for digital sensors 4Liquiline CM42 transmitterCPS72/CPS72D4InputMeasured variables ORP Measuring rangePay attention to the application conditions in the process.InstallationInstallation instructionsDo not install the electrodes upside down. The inclination angle must be at least 15° from the horizontal. A smaller installation angle is not permitted as it could cause an air bubble to form, preventing contact between the reference and the reference lead.Before screwing in, make sure the threaded connection of the assembly is clean and runs smoothly.‣Screw in the electrode finger-tight (3 Nm)! (Information valid only when installing inEndress+Hauser assemblies.)‣Also pay attention to the installation instructions provided in the Operating Instructions of theassembly used.Electrode installation; installation angle at least 15˚ from the horizontal A Permitted orientation BForbidden orientationORP-1500 to 1500 mVTemperature:-15 to 140 ˚C (5 to 284 ˚F)0 to 135 ˚C (32 to 275 ˚F) for sensors with Ex approval and analog sensorsCPS72/CPS72D5EnvironmentAmbient temperature rangeRisk of damage due to frost‣The sensor must not be used at temperatures below –15 ˚C (5 ˚F).Storage temperature 0 to 50 ˚C (32 to 120 ˚F)Degree of protectionProcessProcess temperature rangeProcess pressure (absolute)CAUTION!Sensor is exposed to pressure when used for longer periods under increased process pressure Risk of injury due to glass breakage‣Do not apply too much heat to sensors of this type if they are being used under reduced processpressure or under atmospheric pressure.‣Wear protective goggles and suitable gloves when handling this type of sensor.Pressure-temperature ratingsPressure-temperature ratingsApplicationHygienic and sterile applications, but also applications such as chromate reduction and chlorine metering in swimming poolsNOTICERisk of damage to electrode‣Never use the electrode outside of the listed specifications!IP 67:GSA plug-in head (with closed connector system)IP 68:ESA plug-in head (1m (3.3 ft) water column, 50˚C (120 ˚F), 168h)IP 68:Memosens plug-in head (10 m (33 ft) water column, 25 ˚C (77 ˚F), 45 days, 1 M KCl)-15 to 140 ˚C (5 to 284 ˚F)-15 to 135 ˚C (5 to 275 ˚F) for sensors with Ex approval and analog sensors 0.8 to 11 bar (12 to 160 psi)CPS72/CPS72D6Mechanical constructionDesign, dimensions CPS72 Design, dimensions CPS72DCPS72 with GSA plug-in head, dimensions in mm (inch)1GSA electrode plug-in head, Pg 13.52Viton O-ring with thrust collar3External reference lead with ion trap4Diaphragm5Ag wire6Platinum elementCPS72 with ESA plug-in head, dimensions in mm (inch)1ESA electrode plug-in head, Pg 13.52Viton O-ring with thrust collar3External reference lead with ion trap4Diaphragm5Ag wire6Platinum elementCPS72D with Memosens plug-in head, dimensions in mm (inch)1Memosens plug-in head2Viton O-ring with thrust collar3External reference lead with ion trap4Temperature sensor5Diaphragm6Ag wire7Platinum elementCPS72/CPS72D7Weight 0.1 kg (0.22 lbs)MaterialsProcess connection Pg 13.5Temperature sensorPlug-in headsReference systemAg/AgCl reference lead with gel, acrylamide-free bridging electrolyte, non-cytotoxic, AgCl-free, ion trapCertificates and approvalsEx approval CPS72 (ESA) and CPS72DATEX/NEPSI• II 1G Ex ia IIC T3/T4/T6 Ga FM/CSA•IS/NI CL. I. Div 1, Group A-D IECEx•Ex ia IIC T3/T4/T6 GaBiocompatibilityBiocompatibility certified in accordance with:•ISO 10993?5:1993•USP <87>, agar diffusion test and decoloration testTÜV certificate ESA and Memosens plug-in head Pressure resistance 16 bar (232 psi), process overpressure minimum three times the safety pressureElectromagneticcompatibility CPS72DInterference emission and interference immunity as per EN 61326: 2006Electrode shaft Glass to suit process Metal lead Ag/AgClJunctionCeramic, sterilizable and autoclavable ORP-sensitive element PlatinumGelBridging electrolyte acrylamide-free, no cytotoxicity In contact with medium polyacrylamide-freeCPS72D:NTC 30KCPS72:ESA:Threaded plug-in head Pg 13.5, TOP68, 16bar (232 psi), Ex GSA:Threaded plug-in head Pg 13.5, non-ExCPS72D:Memosens plug-in head for digital, non-contact data transmission, 16 bar (232 psi), Ex or non-ExCPS72/CPS72D8Ordering informationProduct page You can create a valid and complete order code on the Internet with the Configurator tool.Enter the following addresses in the browser to access the relevant product page:/cps72/cps72dProduct structure The navigation area is located on the right of the product page.1.Under "Device support" click "Configure your selected product".The Configurator opens in a separate window.2.Configure the device as per your requirements by selecting all the options.This results in a valid and complete order code.3.Export the order code as a PDF or Excel file. To do so, click the appropriate button at the top of thescreen.CPS72/CPS72D9AccessoriesThe most important accessories available at the time this document went to print are listed below. Contact your service department or sales center for accessories that are not listed here.AssembliesCleanfit W CPA450•Manual retractable assembly for pH/ORP electrodes for installation of 120 mm electrodes in tanks and pipes•Order according to product structure (-> online Configurator, /cpa450)•Technical Information TI183C/07/ENCleanfit P CPA471•Compact stainless steel retractable assembly for installation in tanks and pipes, for manual or pneumatically remote -controlled operation•Order according to product structure (-> online Configurator, /cpa471)•Technical Information TI217C/07/ENCleanfit P CPA472•Compact plastic retractable assembly for installation in tanks and pipes, for manual or pneumatically remote-controlled operation•Order according to product structure (-> online Configurator, /cpa472)•Technical Information TI223C/07/ENCleanfit P CPA472D•Robust retractable assembly for pH, ORP and other industrial sensors, for manual or pneumatically remote-controlled operation, heavy?duty version made from very durable materials•Order according to product structure (-> online Configurator, /cpa472d)•Technical Information TI403C/07/ENCleanfit P CPA473•Stainless steel process retractable assembly with ball valve shutoff for particularly reliable separation of the medium from the environment•Order according to product structure (-> online Configurator, /cpa473)•Technical Information TI344C/07/ENCleanfit P CPA474•Plastic process retractable assembly with ball valve shutoff for particularly reliable separation of the medium from the environment•Order according to product structure (-> online Configurator, /cpa474)•Technical Information TI345C/07/ENCleanfit H CPA475•Retractable assembly for pH/ORP measurement in tanks and pipes under sterile measuring conditions•Order according to product structure (-> online Configurator, /cpa475)•Technical Information TI240C/07/ENUnifit H CPA442•Process assembly for food, biotechnology and chemicals; for 120mm electrodes•Order according to product structure (-> online Configurator, /cpa442)•Technical Information TI306C/07/ENDipfit W CPA111•Immersion and installation assembly made of plastic for open and closed containers•Order according to product structure (-> online Configurator, /cpa111)•Technical Information TI112C/07/ENDipfit P CPA140•pH/ORP immersion assembly with flange connection for very demanding processes•Order according to product structure (-> online Configurator, /cpa140)•Technical Information TI178C/07/ENFlowfit P CPA240•pH/ORP flow assembly for very demanding processes•Order according to product structure (-> online Configurator, /cpa240)•Technical Information TI179C/07/ENCPS72/CPS72D10Flowfit W CPA250•Flow assembly for pH/ORP measurement•Order according to product structure (-> online Configurator, /cpa250)•Technical Information TI041C/07/ENEcofit CPA640•Set comprising adapter for 120mm pH sensors and sensor cable with TOP68 coupling •Order according to product structure (-> online Configurator, /cpa640)•Technical Information TI264C/07/ENBuffer solutions Technical ORP buffer solutions•+220 mV, pH 7, 100 ml (3.4 fl.oz.); Order No. CPY3-0•+468 mV, pH 0.1, 100 ml (3.4 fl.oz.); Order No. CPY3-1Measuring cable Measuring cable•For sensors with ESA plug-in head, for high-temperature and high-pressure applications, IP68•Order according to product structure•Technical Information TI00501C/07/ENSpecial measuring cable CPK1•For pH/ORP electrodes with GSA plug-in head•Order according to product structure•Technical Information TI00501C/07/ENMemosens data cable CYK10•For digital sensors with Memosens technology•Order according to product structure (-> online Configurator, /cyk10)•Technical Information TI00118C/07/ENVersions of the CYK10 that are suitable for use in hazardous areas are marked by an orange/redcoupling end element.CPS72/CPS72D11。
ThinPrep 扩散胶片协议 - 萨库拉 Tissue-Tek Prisma 和 Prisma P

0:00:10
==
Activada
6 ThinPrep Rinse Solution 7 Agua destilada o de alimentación del instrumento1
0:01:00
==
0:00:30
==
Activada Activada
8 ThinPrep Bluing Solution
0:00:30
==
Activada
9 Agua destilada o de alimentación del instrumento1
0:00:30
**
Activada
10 Alcohol reactivo o etílico: 50 %
0:00:30
**
Activada
11 Alcohol reactivo o etílico: 95 %
14
6 AGUA DESTILADA
9
11
12
54
55
14 (D1b)
15
17
19
21
23
25
27
29 (D2a)
ALCOHOL AL 95 %
16
THINPREP EA 15
THINPREP BLUING 8
16
ALCOHOL AL 95 %
17
18
ALCOHOL AL 100 %
18
20
AGUA DESTILADA
7
22
24
26
28
30 (D2b)
31
ALCOHOL AL 100 %
20
33
ALCOHOL AL 100 %
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Sol-gel prepared β-Ga2O3 thin films for ultraviolet photodetectorsYoshihiro Kokubun, Kasumi Miura, Fumie Endo, and Shinji NakagomiCitation: Appl. Phys. Lett. 90, 031912 (2007); doi: 10.1063/1.2432946View online: /10.1063/1.2432946View Table of Contents: /resource/1/APPLAB/v90/i3Published by the American Institute of Physics.Related ArticlesLarge enhanced perpendicular magnetic anisotropy in CoFeB/MgO system with the typical Ta buffer replaced by an Hf layerAIP Advances 2, 032151 (2012)Effect of adatom surface diffusivity on microstructure and intrinsic stress evolutions during Ag film growthJ. Appl. Phys. 112, 043503 (2012)Photoexpansion and nano-lenslet formation in amorphous As2S3 thin films by 800nm femtosecond laser irradiationJ. Appl. Phys. 112, 033105 (2012)Thickness effects on the magnetic and electrical transport properties of highly epitaxial LaBaCo2O5.5+δ thin films on MgO substratesAppl. Phys. Lett. 101, 021602 (2012)Temperature dependence of nanometer-size metallic phase texture and its correlation with bulk magnetic and transport properties and defects of a (La0.4Pr0.6)0.67Ca0.33MnO3 filmAppl. Phys. Lett. 101, 022404 (2012)Additional information on Appl. Phys. Lett.Journal Homepage: /Journal Information: /about/about_the_journalTop downloads: /features/most_downloadedInformation for Authors: /authorsSol-gel prepared-Ga2O3thinfilms for ultraviolet photodetectors Yoshihiro Kokubun,a͒Kasumi Miura,Fumie Endo,and Shinji NakagomiDepartment of Information Technology and Electronics,Faculty of Science and Engineering,Ishinomaki Senshu University,1Shinmito,Minamisakai,Ishinomaki,Miyagi986-8580,Japan͑Received4November2006;accepted15December2006;published online19January2007͒-Ga2O3thinfilms have been prepared on͑0001͒sapphire substrates by the sol-gel method.X-ray diffraction showed that-Ga2O3polycrystallinefilms were formed at heat-treatment temperatures above600°C.With increasing heat-treatment temperature above900°C,the lattice constants of the-Ga2O3films decreased,while the band gap increased.Planar geometry photoconductive detectors based on the sol-gel prepared-Ga2O3thinfilms have been fabricated.They showed the photoresponse only for the wavelengths shorter than270nm,which correspond to the solar-blind region.The peak wavelength in the spectral response depended on the heat-treatment temperature in the sol-gel process.©2007American Institute of Physics.͓DOI:10.1063/1.2432946͔Monoclinic structured gallium oxide͑-Ga2O3͒is a wide band gap͑ϳ4.9eV͒semiconductor1and is a chemi-cally and thermally stable material.Therefore,-Ga2O3has attracted interest as a material for gas sensors,2phosphors,3 transparent conductors,4and transparent electronic devices.5 Moreover,-Ga2O3is the promising candidate for the ultra-violet͑UV͒photodetector that is blind to wavelengths above 280nm,the so-called solar-blind photodetector.It is appli-cable to aflame detector,which is useful for applications in flame safeguard andfire control areas.Although solar-blind photodetectors based on AlGaN with high Al composition have been intensively investigated,6AlGaN photodetectors tend to show degraded characteristics with increasing Al composition.Recently,Feng et al.7have demonstrated that -Ga2O3nanowires are promising materials for realizing solar-blind photodetectors.However,UV photodetectors based on-Ga2O3thinfilms have not been investigated.At present,-Ga2O3films are prepared by various meth-ods such as sputtering,8chemical vapor deposition͑CVD͒,9 pulsed laser deposition,10spray pyrolysis,11and sol-gel method.12In recent years,the sol-gel method has been ex-tensively used to prepare various kinds of oxidefilms,such as TiO2,13LiNbO3,14and ZnO15,16films.The sol-gel method is more convenient and less expensive and has such general advantages as easier composition control and superior uni-formity.However,there have been few detailed reports on the preparation of-Ga2O3films by the sol-gel method ex-cept for the applications to gas sensors and phosphors,par-ticularly from the viewpoint of the influence of the prepara-tion conditions on thefilm properties.In this letter,we describe the sol-gel prepared-Ga2O3films as ultraviolet photodetectors,relating to the heat-treatment temperature in the sol-gel process.-Ga2O3films were prepared on͑0001͒sapphire sub-strates by the sol-gel method.The sapphire substrates were degreased in ultrasonically agitated organic solvents prior to film preparation.As a starting material,gallium isopropoxide was used.2-Methoxyethanol and monoethanolamine were used as the solvent and stabilizer,respectively.Gallium isopropoxide wasfirst dissolved in a mixture of 2-methoxyethanol and monoethanolamine at60°C.The mo-lar ratio of monoethanolamine to gallium isopropoxide was maintained at1.0and the concentration of gallium isopro-poxide was0.4mol/l.After the solution was stirred for1h, a homogeneous and transparent solution was obtained.The films were spin coated on the substrates at3000rpm.After spin coating,the substrates werefirst heated at90°C in air for10min to evaporate the solvent,and then at300°C for 20min to eliminate the organic component in thefilm.This procedure was repeated six times.Thefilms were then heat treated at400–1200°C in air for1h.The thickness of the films was around150–200nm.The structural properties of thefilms were studied by x-ray diffraction͑XRD͒in the−2mode using Cu K␣ra-diation.Optical properties were evaluated using ultraviolet-visible transmission spectroscopy.Planar geometry photo-conductive detectors were fabricated by forming two Au Ohmic contacts with1.5mm spacing on the-Ga2O3film surface by vacuum evaporation.Spectral photoresponse was measured using a monochromator with a xenon-arc lamp as the optical excitation source.Photocurrent was measured un-der a10V bias condition with an Advantest R8340A ultra-high resistance meter.The photocurrent was also normalized to allow for incident light intensity variation as a function of wavelength.Figure1shows the spectral responses of the photocon-ductive detectors fabricated by using-Ga2O3films prepareda͒Electronic mail:kokubun@isenshu-u.ac.jpFIG. 1.Spectral responses of the photoconductive detectors based on-Ga2O3films prepared at various temperatures.APPLIED PHYSICS LETTERS90,031912͑2007͒0003-6951/2007/90͑3͒/031912/3/$23.00©2007American Institute of Physics90,031912-1at various heat-treatment temperatures in the sol-gel process.The measurements were carried out by illuminating the top surface of the film as illustrated in the inset of Fig.1.The photocurrent increases as the wavelength is scanned from 200nm.In the films prepared at 600and 800°C,the pho-tocurrent reaches its peak value at 250nm and then falls sharply.For wavelengths longer than 280nm,no photocur-rent is observed.The peak wavelength of photocurrent shifts toward shorter wavelengths with increasing heat-treatment temperature above 900°C.The peak value of the photocur-rent slightly increased with increasing heat-treatment tem-perature up to 1000°C,but it abruptly decreased at 1200°C.The maximum responsivity was about 8ϫ10−5A/W for the film prepared at 1000°C.The decrease of photocurrent at wavelengths less than the peak wave-length can be ascribed to the strong absorption near the film surface,where the lifetime for the recombination of photo-generated carriers may be smaller than in the bulk.17All samples showed no photoresponse for wavelengths longer than 280nm.This result indicates that -Ga 2O 3films have potential for solar-blind photodetectors such as flame sen-sors.In order to investigate the cause for the peak wavelength shift in the spectral response due to the heat-treatment tem-perature,optical absorption,and XRD measurements were performed.The optical transmission spectra for the -Ga 2O 3films showed high transmittance above 80%in the wavelength region above 280nm independent of the heat-treatment temperature.Figure 2shows ͑␣h ͒2vs h plots,where ␣and h are the absorption coefficient and photon energy,respectively.The absorption coefficient increases rapidly at the photon energy range around 4.9–5.6eV de-pending on the heat-treatment temperature,and ͑␣h ͒2as a function of h fits the straight line quite well.The result indicates that the absorption in this energy region is due to the direct transition.The direct band gap was obtained from the intersection of the straight line and horizontal axis in Fig.2.The band gap of the films prepared at heat-treatment tem-peratures below 800°C is 4.95eV,which is approximately in agreement to the reported value for the -Ga 2O 3films prepared by the pulsed laser deposition.10However,the bandgap increases with increasing heat-treatment temperature above 900°C.The enlargement of the band gap is consistent with the peak shift in the spectral response of photocurrent.Figure 3shows XRD patterns of the films prepared at various heat-treatment temperatures.At 400°C,no peaks appeared except for sapphire-related diffraction peaks,indi-cating that the prepared film was amorphous.In the films prepared at heat-treatment temperatures above 600°C,dif-fraction peaks corresponding to the Ga 2O 3monoclinic phase were observed,indicating that -Ga 2O 3polycrystalline films were formed.The diffraction peaks indexed as ͑2¯01͒and higher order diffractions were dominant.The full width at half maximum ͑FWHM ͒values and the peak intensities of the diffractions for the -Ga 2O 3films decrease and increase with increasing heat-treatment temperature,respectively.The crystallite sizes estimated from the FWHM values of the XRD peaks using Scherrer formula increased from 15to 40nm with increasing heat-treatment temperature.Each diffraction peak position gradually shifts toward higher angles with increasing heat-treatment temperature above 900°C.The lattice constants of the -Ga 2O 3films were esti-mated by using the software CELLCALC ,18which is a program that calculates unit cell parameters from observed d value and hkl data by the method of least squares.Figure 4shows the heat-treatment temperature dependence of the lattice con-stants of the -Ga 2O 3films.At heat-treatment temperatures below 800°C,the a -,b -,and c -axis lattice constants of the sol-gel prepared -Ga 2O 3films are comparable to the stan-dard values for bulk -Ga 2O 3͑a =12.23Å,b =3.04Å,and c =5.80Å͒.19However,they decrease with increasing heat-treatment temperature above 900°C.The diffusion of Al from the alumina substrates during postannealing at higher temperatures above 1000°C has been observed in the -Ga 2O 3films prepared by sputtering 8and CVD.9Therefore,the observed decrease in the lattice constants at higher heat-treatment temperatures is supposed to be caused by the dif-fusion of Al from the sapphire substrates during the heat-treatment in the sol-gel process.Since the ionic radius of Al 3+͑0.54Å͒is smaller than that of Ga 3+͑0.62Å͒,the lat-tice constant would be reduced if Al substitutes for Ga site in the -Ga 2O 3lattice.If Al is incorporated into the -Ga 2O 3lattice as discussed above,the band gap would increasewithFIG.2.Square of absorption coefficient as a function of photon energy for -Ga 2O 3films prepared at varioustemperatures.FIG.3.X-ray diffraction patterns of -Ga 2O 3films prepared at various temperatures.the Al content because Al 2O 3has a wider band gap than Ga 2O 3.Therefore,the conclusion based on the lattice con-stant is consistent with the results obtained from the optical measurements.In conclusion,-Ga 2O 3films have been prepared on ͑0001͒sapphire substrates by the sol-gel method.-Ga 2O 3polycrystalline films were obtained at heat-treatment tem-peratures above 600°C.With increasing heat-treatment tem-perature above 900°C,the lattice constants decreased while the band gap increased,which are ascribed to the Al diffu-sion from the sapphire substrate during the heat-treatment ing the sol-gel prepared -Ga 2O 3thin film,asolar-blind UV photodetector possessing the sensitivity only for the wavelengths shorter than 270nm was demonstrated.The peak in the spectral response shifted toward shorter wavelengths with increasing heat-treatment temperature above 900°C.1H.H.Tippins,Phys.Rev.140,A316͑1965͒.2M.Fleischer,L.Höllbauer,and H.Meixner,Sens.Actuators B 18–19,119͑1994͒.3T.Minami,H.Yamada,Y .Kubota,and T.Miyata,Jpn.J.Appl.Phys.,Part 236,L1191͑1997͒.4N.Ueda,H.Hosono,R.Waseda,and H.Kawazoe,Appl.Phys.Lett.70,3561͑1997͒.5K.Matsuzaki,H.Yanagi,T.Kamiya,H.Hiramatsu,K.Nomura,M.Hirano,and H.Hosono,Appl.Phys.Lett.88,092106͑2006͒.6A.Hirano, C.Pernot,M.Iwaya,T.Detchprohm,H.Amano,and I.Akasaki,Phys.Status Solidi A 188,293͑2001͒.7P.Feng,J.Y .Zhang,Q.H.Li,and T.H.Wang,Appl.Phys.Lett.88,153107͑2006͒.8M.Fleischer,W.Hanrieder,and H.Meixner,Thin Solid Films 190,93͑1990͒.9G.A.Battiston,R.Gerbasi,M.Porchia,R.Bertoncello,and F.Caccavale,Thin Solid Films 279,115͑1996͒.10M.Orita,H.Ohta,M.Hirano,and H.Hosono,Appl.Phys.Lett.77,4166͑2000͒.11A.Oritiz,J.C.Alonso,E.Andrade,and C.Urbiola,J.Electrochem.Soc.148,F26͑2001͒.12Y .X.Li,A.Trinchi,W.Wlodarski,K.Galatsis,and K.Kalantar-zadeh,Sens.Actuators B 93,431͑2003͒.13Y .Takahashi and Y .Matsuoka,J.Mater.Sci.23,2259͑1988͒.14S.Hirano and K.Kato,J.Non-Cryst.Solids 100,538͑1988͒.15M.Ohyama,H.Kozuka,T.Yoko,and S.Sakka,J.Ceram.Soc.Jpn.104,296͑1996͒.16Y .Kokubun,H.Kimura,and S.Nakagomi,Jpn.J.Appl.Phys.,Part 242,L904͑2003͒.17R.H.Bube,Electronic Properties of Crystalline Solids ͑Academic,New York,1974͒,Chap.12,p.455.18H.Miura,J.Crystallogr.Soc.Jpn.45,145͑2003͓͒in Japanese ͔.19JCPDS-International Centre for Diffraction Data,Card No.43-1012.FIG.4.Heat-treatment temperature dependence of the lattice constants of ͑a ͒a ,͑b ͒b ,and ͑c ͒c axes for -Ga 2O 3films ͑*Ref.19͒.。
