Olaparib (AZD2281, Ku-0059436)_PARP1PARP2抑制剂_763113-22-0_Apexbio
厄贝沙坦说明书(英文)

Product InformationAVAPRO® (irbesartan)NAME OF THE MEDICINEAustralian Approved NameIrbesartan.Irbesartan is 2-butyl-3-[(2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl]-1,3-diazaspiro [4,4] non-1-en-4-one.Chemical StructureThe chemical structure of irbesartan isCAS number: 138402-11-6DESCRIPTIONIrbesartan is a white, to practically white, powder that is less than 0.1mg/mL soluble in water and slightly soluble in alcohol and methylene chloride.AVAPRO® (irbesartan) is a nonpeptide angiotensin II receptor (AT1 subtype) antagonist. It is available as 75, 150 or 300 mg film coated tablets for oral administration. The white, biconvex , oval-shaped tablets are marked with a heart on one side and on the other side 2871 (75mg) or 2872 (150mg) or 2873 (300mg). The inactive ingredients are: carnauba wax, croscarmellose sodium, hypromellose, lactose, macrogol 3000, magnesium stearate, microcrystalline cellulose, silicon dioxide, and titanium dioxide.PHARMACOLOGYPharmacodynamicsIrbesartan is a specific antagonist of angiotensin II receptors (AT1 subtype). Angiotensin II is an important component of the renin-angiotensin system and is involved in the pathophysiology of hypertension and in sodium homeostasis. Irbesartan does not require metabolic activation for its activity.Irbesartan blocks the potent vasoconstrictor and aldosterone-secreting effects of angiotensin II by selective antagonism of the angiotensin II (AT1 subtype) receptors localized on vascular smooth muscle cells and in the adrenal cortex. It has no agonist activity at the AT1 receptor and a much greater affinity (more than 8500-fold) for the AT1 receptor than for the AT2 receptor (a receptor that has not been shown to be associated with cardiovascular homeostasis).Irbesartan does not inhibit enzymes involved in the renin-angiotensin system (i.e., renin, angiotensin converting enzyme [ACE]) or affect other hormone receptors or ion channels involved in the cardiovascular regulation of blood pressure and sodium homeostasis.In healthy subjects, single oral irbesartan doses of up to 300mg produced dose-dependent inhibition of the pressor effect of angiotensin II infusions. Inhibition was complete (≥90%) at the time of peak irbesartan concentrations and sustained for 24 hours (60% and 40% at300mg and 150mg, respectively).In hypertensive patients, angiotensin II receptor inhibition following chronic administration of irbesartan causes a 1.5-2 fold rise in angiotensin II plasma concentration and a 2-3 fold increase in plasma renin levels. Aldosterone plasma concentrations generally decline following irbesartan administration, however serum potassium levels are not significantly affected at recommended doses.In hypertensive patients, chronic oral doses of irbesartan (up to 300mg) had no effect on glomerular filtration rate, renal plasma/blood flow or filtration fraction. In multiple dose studies in hypertensive patients, there were no notable effects on fasting triglycerides, total cholesterol or HDL-cholesterol or fasting glucose concentrations. There was no effect on serum uric acid during chronic oral administration. Following repeated doses of irbesartan, there was no uricosuric effect.PharmacokineticsIrbesartan is an orally active agent and does not require biotransformation for its activity. AbsorptionFollowing oral administration, irbesartan is rapidly and well absorbed. In studies employing an injection and an oral solution containing radio-labelled irbesartan the average absolute oral bioavailability of irbesartan was 60- 80%. Median peak plasma concentrations generally occurred 1.5 -2 hours after oral administration of irbesartan capsules and tablets. Food did not affect the bioavailability.DistributionIrbesartan is 90% protein-bound in the plasma, and has negligible binding to cellular components of blood. The volume of distribution (V ss) is 53-93 Litres.MetabolismFollowing oral or intravenous administration of 14C irbesartan more than 80% of the circulating plasma radioactivity was attributable to unchanged irbesartan. Irbesartan is metabolised by the liver via glucuronide conjugation and oxidation. The major circulating metabolite is irbesartan glucuronide (∼6%). Irbesartan undergoes oxidation primarily by thecytochrome P450 isoenzyme CYP2C9; isoenzyme CYP3A4 has negligible effect. It is not metabolised by, nor does it substantially induce or inhibit most isoenzymes commonly associated with drug metabolism (i.e., CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2D6, or CYP2E1). Irbesartan does not induce nor inhibit isoenzyme CYP3A4.ExcretionIrbesartan and its metabolites are excreted by both biliary and renal routes. About 20% of the administered radioactivity after an oral or intravenous dose of 14C irbesartan was recovered in urine with the remainder in the faeces. Less than 2% of the dose was excreted in urine as unchanged irbesartan.The terminal elimination half-life (t½) of irbesartan averaged 11 - 15 hours over a range ofirbesartan was 157 -176 mL/min, of which 3.0-3.5 mL/min was renal clearance. Irbesartan exhibits linear pharmacokinetics over the therapeutic dose range. Steady-state plasma concentrations are attained within 3 days after initiation of a once-daily dosing regimen. Limited accumulation (<20%) is observed in plasma upon repeated once-daily dosing.Special PopulationsIn male and female hypertensive subjects, higher (11-44%) plasma concentrations of irbesartan were observed in females than in males, although, following multiple dosing, males and females did not show differences in either accumulation or elimination half-life. No gender-specific differences in clinical effect have been observed.In elderly (male and female) normotensive subjects (65-80 years) with clinically normal renal and hepatic function, the plasma AUC and peak plasma concentrations (C max) of irbesartan are approximately 20%-50% greater than those observed in younger subjects (18-40 years). Regardless of age, the elimination half-life is comparable. No significant age-related differences in clinical effect have been observed.In black and white normotensive subjects, the plasma AUC and t½ of irbesartan are approximately 20-25% greater in blacks than in whites; the peak plasma concentrations (C max) of irbesartan are essentially equivalent.In patients with renal impairment (regardless of degree) and in haemodialysis patients, the pharmacokinetics of irbesartan are not significantly altered. Irbesartan is not removed by hemodialysis.In patients with hepatic insufficiency due to mild to moderate cirrhosis, the pharmacokinetics of irbesartan are not significantly altered.CLINICAL TRIALSThe antihypertensive effects of irbesartan were examined in seven (7) major placebo-controlled 8-12 week trials in patients with baseline diastolic blood pressures of 95-110 mmHg.The seven (7) studies of irbesartan monotherapy included a total of 1915 patients randomised to irbesartan (1-900mg) and 611 patients randomised to placebo. Once daily doses of 150 to900mg provided statistically and clinically significant decreases in systolic and diastolic blood pressure with a plateau in effect at doses above 300mg.Systolic/diastolic mean decreases in blood pressure at trough (24 hours post-dosing), compared to placebo, following 6 to 12 weeks of treatment were in the range of 7.5-9.9/4.6-6.2 mmHg with a 150mg dose, and 7.9-12.6/5.2-7.9 mmHg with a 300mg dose.Once-daily dosing with 150mg gave trough and mean 24 hour responses corresponding to those observed in patients receiving twice-daily dosing at the same total daily dose. Peak (3-6 hour) effects were uniformly, but moderately, larger than trough effects, with the trough-to-peak ratio for systolic and diastolic response generally between 60-70%.Two of the seven placebo-controlled trials identified above and two additional studies examined the antihypertensive effects of irbesartan and hydrochlorothiazide in combination. Addition of a low dose of hydrochlorothiazide (12.5mg) to irbesartan (75 to 300mg) once daily resulted in further diastolic blood pressure reductions at trough (24 hours post-dosing) of 2.3-4.8 mmHg and an overall systolic/diastolic placebo-subtracted reduction of up to13.6/11.5 mmHg at a dose of 300mg irbesartan and 12.5mg hydrochlorothiazide. Once daily dosing with 150mg irbesartan and 12.5 mg hydrochlorothiazide gave systolic/diastolic mean placebo-adjusted blood pressure reductions at trough (24 hours post-dosing) of 12.9/6.9 mmHg.In patients not adequately controlled (SeDBP≥90 mmHg) on irbesartan (up to 300mg) alone, the addition of 12.5mg hydrochlorothiazide gave an added reduction of up to 6.1 mmHg in trough (24 hours) diastolic blood pressure.In patients not adequately controlled (SeDBP 93-120 mmHg) on 25mg hydrochlorothiazide alone, the addition of irbesartan (75-150mg) gave an added systolic/diastolic mean reduction of 11.1/7.2 mmHg.Analysis of age, gender and race subgroups of patients showed that men and women, and patients over and under 65 years of age, had generally similar responses.The effect of irbesartan is apparent after the first dose, substantially present within 1-2 weeks, and reaches a maximal effect within 4-6 weeks. In long-term studies the effect of irbesartan appeared to be maintained for more than one year. After withdrawal of irbesartan, blood pressure gradually returned towards baseline; no rebound was observed. There was essentially no change in average heart rate in irbesartan-treated patients in controlled trials. Hypertension and type II diabetic renal diseaseThe Irbesartan Diabetic Nephropathy Trial (IDNT) was a multicentre, randomised, controlled, double-blind, morbidity and mortality trial comparing AVAPRO, amlodipine and placebo. In 1715 hypertensive patients with type II diabetes (proteinuria ≥ 900mg/day and serum creatinine 110-265 µmol/L in men and 90-265 µmol/L in women) the long-term effects (mean 2.6 years) of AVAPRO on the progression of renal disease and all-cause mortality were examined. In addition, a secondary end-point, the effect of AVAPRO on the risk of fatal or non-fatal cardiovascular events was assessed. Patients were randomised to receive AVAPRO 75mg, amlodipine 2.5mg, or matching placebo once-daily. Patients were thentitrated to a maintenance dose of 300mg AVAPRO, 10mg amlodipine, or placebo as tolerated. Additional antihypertensive agents (excluding ACE inhibitors, angiotensin II receptor antagonists and calcium channel blockers) were added as needed to help achieve blood pressure goal (≤ 135/85 or 10 mmHg reduction in systolic blood pressure if higher than 160 mmHg) for patients in all groups. AVAPRO demonstrated a 20% relative risk reduction in the composite primary endpoint (first occurrence of any of the following: doubling of serum creatinine, end-stage renal disease or all-cause mortality) compared to placebo (95% CI (3%, 34%), p =0.023) and a 23% relative risk reduction compared to amlodipine (95% CI (7%, 37%), p = 0.006). When the individual components of the primary endpoint were analysed, no effect in all cause mortality was observed, while a positive trend in the reduction in ESRD and a significant reduction in doubling of serum creatinine were observed. Similar blood pressure was achieved in the AVAPRO and amlodipine groups. There was no significant difference in the assessment of fatal or non-fatal cardiovascular events (cardiovascular death, non-fatal myocardial infarction, hospitalization for heart failure, permanent neurologic deficit attributed to stroke, or above-the-ankle amputation) among the three treatment groups.The study of the Effects of Irbesartan on MicroAlbuminuria in Hypertensive Patients with Type 2 Diabetes Mellitus (IRMA 2) was a multicentre, randomised, placebo-controlled, double-blind morbidity study, conducted in 590 hypertensive patients with type II diabetes, microalbuminuria (20 - 200mcg/min; 30 - 300mg/day) and normal renal function (serum creatinine ≤ 130 µmol/L in males and ≤ 100 µmol/L in females). The study examined as a primary endpoint the long-term effects (2 years) of AVAPRO on the progression to (overt) proteinuria (urinary albumin excretion rate [AER] > 200µg/min [> approximately300mg/day] and an increase in AER of at least 30% from baseline). In addition, after one and two years of treatment, the effect of AVAPRO on the change in overnight AER and the change in 24-hour creatinine clearance was assessed. Patients were randomised to receive AVAPRO 150mg, AVAPRO 300mg, or matching placebo once daily. Additional antihypertensive agents (excluding ACE inhibitors, angiotensin II receptor antagonists and dihydropyridine calcium blockers) were added as needed to help achieve blood pressure goal (≤ 135/85 mmHg) for patients in all groups. AVAPRO 300mg demonstrated a 70% relative risk reduction in the development of clinical (overt) proteinuria compared to placebo (95% CI (39%, 86%), p = 0.0004). AVAPRO 150mg demonstrated a 39% relative risk reduction in the development of proteinuria compared to placebo (95% CI (-8%, 66%), p = 0.085). In the intent to treat analysis, when the primary endpoint is adjusted for urinary albumin excretion rate and mean arterial pressure, AVAPRO 300mg demonstrated a 68% relative risk reduction, (95% CI (35%, 85%), p=0.002). The slowing of progression to clinical (overt) proteinuria was evident as early as three months and continued over the 2 year period. The decline in 24-hour creatinine clearance did not differ significantly among the 3 groups. Regression to normoalbuminuria (< 20µg/min; <30mg/day) was more frequent in the AVAPRO 300mg group (34%) than in the placebo group (21%).The adverse experiences reported in these two studies are summarised under ADVERSE REACTIONS- Hypertension and Type II Diabetic Renal Disease and PRECAUTIONS - Effect on Laboratory Tests.INDICATIONSAVAPRO is indicated for the treatment of hypertension.AVAPRO is indicated for delaying the progression of renal disease in hypertensive type II diabetics with persistent micro-albuminuria (≥ 30mg per 24 hours) or urinary protein in excess of 900mg per 24 hours.CONTRAINDICATIONSAVAPRO is contraindicated in patients who are hypersensitive to irbesartan or to any other component of the AVAPRO formulation.Pregnancy (See PRECAUTIONS-Use in Pregnancy)PRECAUTIONSHypotension - Volume-Depleted PatientsIrbesartan has been rarely associated with hypotension in hypertensive patients without other co-morbid conditions. Symptomatic hypotension, as with ACE inhibitors, may be expected to occur in sodium/volume-depleted patients such as those treated vigorously with diuretics and/or salt restriction, or on haemodialysis. Volume and/or sodium- depletion should be corrected before initiating therapy with irbesartan or a lower starting dose (e.g. 75 mg) should be considered. Patients undergoing haemodialysis should receive a starting dose of 75 mg and the dose should be adjusted according to B.P. response.Renal FunctionAs a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. In patients whose renal function depends on the activity of the renin-angiotensin-aldosterone system (e.g., hypertensive patients with renal artery stenosis in one or both kidneys, or patients with severe congestive heart failure), treatment with drugs that affect this system has been associated with oliguria and/or progressive azotemia and (rarely) with acute renal failure and/or death. The possibility of a similar effect occurring with the use of an angiotensin II receptor antagonist, including irbesartan cannot be excluded.In hypertensive type II diabetic patients with proteinuria (≥ 900mg/day), a population which has a high risk of renal artery stenosis, no patient treated with AVAPRO in IDNT had an early acute rise in serum creatinine attributable to renal artery disease. (See CLINICAL TRIALS)Experience is limited with irbesartan in patients with moderate to severe renal impairment; careful monitoring of renal function and potassium in such patients is advised. HyperkalaemiaWhile hyperkalaemia in uncomplicated patients with hypertension has not been reported with irbesartan, hyperkalaemia may occur during treatment with other drugs that affect the renin-angiotensin-aldosterone system, especially in the presence of renal impairment and/or heart failure. Adequate monitoring of serum potassium in patients at risk is recommended.Cardiac DisordersThe safety of irbesartan in the presence of heart failure has not been fully defined. Sudden death has occurred in some studies of patients with heart failure, and although such deaths may have reflected the natural history of the underlying heart failure, caution is recommended when treating such patients with irbesartan.At this time, experience is limited with irbesartan in the treatment of patients with ventricular dysfunction or cardiac arrhythmias; caution is advised.Effects on FertilityFertility and reproductive performance were not affected in studies of male and female rats at oral doses of up to 650 mg/kg/day (approximately 3 (male) and 8 (female) fold higher exposure, based on AUC, than that of humans at the maximum recommended clinical dose of 300mg/day).Use in Pregnancy Category DDrugs that act directly on the renin-angiotensin system can cause foetal and neonatal morbidity and death when administered to pregnant women. Several dozen cases have been reported in the world literature in patients who were taking angiotensin-converting enzyme inhibitors. When pregnancy is detected, AVAPRO should be discontinued as soon as possible.The use of drugs that act directly on the renin-angiotensin system during the second and third trimesters of pregnancy have been associated with foetal and neonatal injury, including hypotension, neonatal skull hypoplasia, anuria, reversible or irreversible renal failure, and death. Oligohydramnios has also been reported, presumably resulting from decreased foetal renal function; oligohydramnios in this setting has been associated with foetal limb contractures, craniofacial deformation and hypoplastic lung development. Prematurity, intrauterine growth retardation and patent ductus arteriosus have also been reported, although it is not clear whether these occurrences were due to exposure to the drug.These adverse effects do not appear to have resulted from intrauterine drug exposure that has been limited to the first trimester. Mothers whose embryos and foetuses are exposed to an angiotensin II receptor antagonist only during the first trimester should be so informed. Nonetheless, when patients become pregnant, physicians should have the patient discontinue the use of AVAPRO as soon as possible.Infants with histories of in utero exposure to an angiotensin II receptor antagonist should be closely observed for hypotension, oliguria and hyperkalemia.When pregnant rats were treated with irbesartan from day 0 to day 20 of gestation, at doses of 50mg/kg/day and higher, transient effects (increased renal pelvic cavitation, hypoureter or subcutaneous oedema) were noted in full term rat foetuses but not in the young animals necropsied after 6 weeks of age. In pregnant rabbits, at doses of 30mg/kg/day, maternal mortality, abortion and early foetal resorptions were noted. No teratogenic effects were observed in the rat or rabbit.Use in LactationIrbesartan is excreted in the milk of lactating rats. It is not known whether irbesartan or its metabolites are excreted in human milk. A decision should be made whether to discontinue breast feeding or to discontinue the drug, taking into account the importance of irbesartan to the therapy of the mother and the potential risk to the infant.Paediatric UseSafety and effectiveness in paediatric patients have not been established.Use in the ElderlyAmong patients who received irbesartan in clinical studies, no overall differences in efficacy or safety were observed between older patients (65 years or older) and younger patients. GenotoxicityIrbesartan was not genotoxic in a series of assays for mutagenic and clastogenic effects. CarcinogenicityThe carcinogenic potential of irbesartan was assessed in two 104 week studies in mice and rats. No carcinogenic potential was observed in either species at doses of up to 500mg/kg/day (males rats) and 1000 mg/kg/day (mice and female rats) The AUC based exposure levels were 3 - 6 fold higher in mice, 3 fold higher in male rats and 25 fold higher in female rats than that of humans at the maximum recommended clinical dose of 300 mg/day.Effect on Laboratory TestsNo clinically significant changes in laboratory test parameters occurred in controlled clinical studies of hypertension. No special monitoring of laboratory parameters is necessary for patients with uncomplicated essential hypertension. Monitoring of potassium levels and renal function is recommended for patients with heart failure and those with moderate to severe renal impairment (see PRECAUTIONS).In two clinical studies of patients with hypertension and type II diabetic renal disease (IDNT and IRMA2) the following was reportedHyperkalaemia: In IDNT the percent of subjects with hyperkalaemia (>6 mmol/L) was 18.6% in the AVAPRO group compared to 6.0% in the placebo group. In IRMA 2 the percent of subjects with hyperkalaemia (>6 mmol/L) was 1.0% in the AVAPRO groups and none in the placebo group. In IDNT discontinuations due to hyperkalaemia in the AVAPRO group were 2.1% vs 0.36% in the placebo group. In IRMA 2 discontinuations due to hyperkalaemia in the AVAPRO groups were 0.5% vs none in the placebo group.INTERACTIONS WITH OTHER MEDICINESBased on in vitro data, no interactions would be expected to occur with drugs whose metabolism is dependent upon cytochrome P450 isoenzymes CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2D6, CYP2E1 or CYP3A4. Irbesartan is primarily metabolised by CYP2C9, however, during clinical interaction studies, no significant pharmacodynamic interactions were observed when irbesartan was co-administered with warfarin (a drug metabolised by CYP2C9).Irbesartan does not affect the pharmacokinetics of digoxin. The pharmacokinetics of irbesartan is not affected by coadministration with nifedipine or hydrochlorothiazide.Potassium-sparing diuretics, potassium supplements, potassium containing salt substitutes. Based on experience with the use of other drugs that affect the renin-angiotensin system, concomitant use of potassium-sparing diuretics, potassium supplements, or salt substitutes containing potassium may lead to increases in serum potassium.LithiumReversible increases in lithium concentrations have been very rarely reported with irbesartan. Therefore if coadministration of AVAPRO and lithium proves necessary, careful monitoring of serum lithium levels is necessary.Combination use of ACE inhibitors or angiotensin receptor antagonists, anti-inflammatory drugs and thiazide diureticsConcomitant use of a renin-angiotensin system inhibiting drug (ACE-inhibitor or angiotensin receptor antagonist), and an anti-inflammatory drug (NSAID, including COX-2 inhibitor) alone or with a thiazide diuretic may increase the risk of renal impairment, including possible acute renal failure. These effects are usually reversible. This includes use in fixed-combination products containing more than one class of drug. The combination of these agents should be administered with caution, especially in the elderly, volume-depleted, and in patients with pre-existing renal impairment. Renal function (serum creatinine) should be monitored after initiation of concomitant therapy, and periodically thereafter. The antihypertensive effect of angiotensin II receptor antagonists, including irbesartan, may be attenuated by NSAIDs including selective COX-2 inhibitors.Effects on Ability to Drive or Use MachinesThe effect of irbesartan on ability to drive and use machines has not been studied. When driving vehicles or operating machines , it should be taken into account that occasionally dizziness or weariness may occur during treatment of hypertension.ADVERSE EFFECTSHypertensionIrbesartan has been evaluated for safety in approximately 5000 subjects in clinical studies, including 1300 hypertensive patients treated for over 6 months and over 400 patients treated for one year or more. Adverse events in patients receiving irbesartan were generally mild and transient with no relationship to dose. The incidence of adverse events was not related to age, gender, or race.In placebo-controlled clinical studies, including 1965 irbesartan-treated patients (usual duration of treatment 1 to 3 months), discontinuations due to any clinical or laboratory adverse event were 3.3 percent for irbesartan-treated patients and 4.5 percent for placebo-treated patients (p=0.029).Clinical adverse events, occurring in at least 1% of patients treated with irbesartan in placebo controlled trials are shown in the table below. The incidence of the same adverse events in the placebo control group is also shown.Clinical Adverse Events* in Placebo-Controlled Hypertension TrialsIncidencePercentage (%) of Patients*BODY SYSTEM/EVENT Irbesartann=1965Placebon=641GeneralFatigueInfluenza Chest pain 4.32.31.83.72.01.7CardiovascularOedemaTachycardia 1.51.22.30.9GastrointestinalDiarrhoeaNausea/VomitingDyspepsia/Heartburn Abdominal Pain 3.12.11.71.42.22.81.12.0Nervous SystemDizzinessHeadache aAnxiety/Nervousness 4.912.31.15.016.70.9DermatologicalRash 1.3 2.0 Musculoskeletal/ConnectiveMusc/Skel PainMusc/Skel Trauma a 6.61.96.60.5Renal/GenitourinaryUTI 1.1 1.4 RespiratoryUpper Resp. Infection Sinus AbnormalityCoughPharyngitisRhinitis 8.53.42.82.21.96.25.02.72.52.8a Statistically significant difference between irbesartan and placebo treatment groups.Adverse reactions that occurred in 2 or more hypertensive patients in clinical trials involving 3396 patients have been classified using standard terminology and in the following listing are categorised by body system and listed in order of decreasing frequency according to the following definitions: common adverse reactions are those occurring on one or more occasions in at least 1/100 but less than 1/10 patients; uncommon adverse reactions are those occurring in at least 1/1000 but less than 1/100 patients; rare adverse reactions are those occurring in less than 1/1000 patients.Cardiovascular : Uncommon: subjective rhythm disturbance, flushing, ECG abnormality, cardiac murmur, cardiac rhythm disturbance, orthostatic hypotension, atrial rhythm disturbance, bradycardia, hypotension; Rare:syncope, conduction disorder, myocardial infarction.Dermatologic : Uncommon: pruritus, facial erythema; Rare: dermatitis, acne,scalp-hair abnormality.Endocrine/Metabolic/Electrolyte Imbalance : Uncommon: sexual dysfunction, libido change; Rare: breast disorder, gout, hot flashes.Gastrointestinal :Uncommon: constipation, flatulence, dry mouth, abdomen distention; Rare: abnormal stool, decreased appetite, increased appetite, oral lesion, dysphagia, oesophagitis.General : Uncommon: weakness, hyperhidrosis, malaise, weight gain; Rare: cold sensation, warmth sensation, pain.Hematopoietic : Rare: anaemia.Immunology/Sensitivity Disorder : Uncommon: upper extremity oedema ; Rare: head/neck oedema.Musculoskeletal/Connective Tissue : Uncommon: muscle cramp, swelling extremity; Rare: arthritis, muscle ache, myalgia, extremity weakness, stiffness lower extremity.Nervous System : Uncommon: orthostatic dizziness, numbness, sleep disturbance, depression, emotion labile/disturbance, somnolence, vertigo, paresthesia; Rare: stress related disorder, tremor, coordination disturbance, disturbing dreams.Renal/Genitourinary : Uncommon: urination abnormality.Respiratory : Uncommon: epistaxis, dyspnea.Special Senses : Uncommon: vision disturbance, hearing abnormality; Rare: eye disturbance -other, eyelid abnormality, visual field abnormality, medication bad taste, taste disturbance. Hypertension and Type II Diabetic Renal DiseaseIn clinical studies (see CLINICAL TRIALS, Hypertension and type II diabetic renal disease), the adverse experiences were similar to those in clinical trials of hypertensive patients with the exception of orthostatic symptoms (dizziness, orthostatic dizziness and orthostatic hypotension) observed in IDNT (proteinuria ≥ 900mg/day, and serum creatinine from 90 - 265 µmol/L). In IDNT orthostatic symptoms occurred more frequently in the AVAPRO。
双三苯基膦二氯化钯 碳谱

双三苯基膦二氯化钯碳谱
碳谱是用来确定化合物的结构的一种分析方法,通过观察化合物中各个碳原子的化学位移来推断它们的化学环境和结构。
双三苯基膦二氯化钯(PdCl2(PCy3)2)是一种含有钯金属的化合物,其中配位了两个三苯基膦配体。
在这个化合物中,钯金属与配体通过配位键相连。
碳谱图通常以化学位移(δ)为横坐标,以峰的强度(相对强度或积分值)为纵坐标。
根据化学位移和峰的形状可以推测出化合物的结构和环境。
由于我无法提供图片,下面是双三苯基膦二氯化钯碳谱的一些可能的碳原子化学位移范围和特征:
- 膦基碳原子的化学位移通常在20-50 ppm左右,具体位置取决于配体和金属配位环境。
- 氯化钯(Pd-Cl)的化学位移通常在100-200 ppm之间。
- 如果还有其他有机基团和杂原子,它们的化学位移将根据它们的环境和相邻基团的影响而有所变化。
需要注意的是,每个化合物的碳谱都是独特的,具体的化学位移取决于化合物的结构和环境。
因此,要准确确定双三苯基膦二氯化钯的碳谱,需要通过实验测定并与已知化合物的碳谱进行比对。
Piezo1在呼吸系统疾病中的研究进展

Piezo1在呼吸系统疾病中的研究进展
蒋诗音;蒋永亮
【期刊名称】《临床肺科杂志》
【年(卷),期】2024(29)2
【摘要】Piezo是一种存在于细胞膜以及细胞质中介导阳离子通透性的通道蛋白,在细胞发育以及稳态中起到十分关键的作用。
在自然界广泛表达,在进化中高度保守,与其他的阳离子通道不具有同源性,在非兴奋细胞中介导阳离子的跨膜流动。
编码这种蛋白的是Fam38A以及Fam38B基因,分别命名为Piezo1以及Piezo2,其中Piezo1主要在非感觉组织中表达,如肺、膀胱、乳腺、前列腺等,Piezo2主要在背根神经节等感觉组织中表达。
Piezo1广泛参与机体各种生理以及病理过程的调控,并且在多种肺疾病如先天性肺发育不良、肺炎、肺纤维化、ARDS以及呼吸机相关性肺病、哮喘、肺动脉高压、肺癌等疾病中表达异常,因此,本综述主要对Piezo1与呼吸系统相关疾病进行回顾和总结。
【总页数】5页(P280-284)
【作者】蒋诗音;蒋永亮
【作者单位】湖南师范大学附属第一医院(湖南省人民医院)呼吸与危重症医学科【正文语种】中文
【中图分类】R73
【相关文献】
1.T淋巴细胞亚群在呼吸系统疾病发生发展中作用的研究进展
2.微生态制剂在呼吸系统疾病的研究进展及其在新冠肺炎中的应用价值
3.肾素血管紧张素系统在呼吸系统疾病发生和发展中的研究进展
4.吸气肌训练在呼吸系统疾病康复中的研究进展
因版权原因,仅展示原文概要,查看原文内容请购买。
lord 公司的水溶液性胶合剂 CHEMLOK 8560D 安全数据表 说明书

USA SAFETY DATA SHEET1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATIONProduct name:CHEMLOK 8560DProduct Use/Class: Aqueous AdhesiveLORD Corporation111 LORD DriveCary, NC 27511-7923 USATelephone: 814 868-3180Non-Transportation Emergency: 814 763-2345Chemtrec 24 Hr Transportation Emergency No.800 424-9300 (Outside Continental U.S. 703 527-3887)EFFECTIVE DATE: 06/30/20212. HAZARDS IDENTIFICATIONGHS CLASSIFICATION:Serious eye damage/eye irritation Category 2ASkin sensitization Category 1ACarcinogenicity Category 2Specific target organ systemic toxicity (single exposure) Category 2 blood systemSpecific target organ systemic toxicity (single exposure) Category 1 Respiratory system, Systemic toxicity Specific target organ systemic toxicity (repeated exposure) Category 2 blood systemSpecific target organ systemic toxicity (repeated exposure) Category 1 LungsHazardous to the aquatic environment - acute hazard Category 2Hazardous to the aquatic environment - chronic hazard Category 2GHS LABEL ELEMENTS:Symbol(s)Signal WordD ANGERHazard StatementsCauses serious eye irritation.May cause an allergic skin reaction.Suspected of causing cancer.May cause damage to organs.(blood system)Causes damage to organs.(Respiratory system, Systemic toxicity)May cause damage to organs through prolonged or repeated exposure.(blood system)Causes damage to organs through prolonged or repeated exposure.(Lungs)Toxic to aquatic life.Toxic to aquatic life with long lasting effects.Precautionary StatementsPreventionObtain special instructions before use.Do not handle until all safety precautions have been read and understood.Wear protective gloves/eye protection/face protection.Use personal protective equipment as required.Do not breathe dust/fume/gas/mist/vapors/spray.Wash thoroughly after handling.300000005458Do not eat, drink or smoke when using this product.Contaminated work clothing should not be allowed out of the workplace.Avoid release to the environment.ResponseGet medical advice/attention if you feel unwell.IF exposed: Call a POISON CENTER or doctor/physician.Specific treatment (see supplemental first aid instructions on this label).IF ON SKIN: Wash with plenty of soap and water.If skin irritation or rash occurs: Get medical advice/attention.IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.Continue rinsing.If eye irritation persists: Get medical advice/attention.Wash contaminated clothing before reuse.Collect spillage.StorageStore locked up.Disposal:Dispose of contents/container in accordance with waste/disposal laws and regulations of your country or particular locality.Other Hazards:This product contains component(s) which have the following warnings; however based on the GHS classification criteria of your country or locale, the product mixture may be outside the respective category(s).Acute: In elevated-temperature applications, product may release vapors that may produce cyanosis in the absence of sufficient ventilation or adequate respiratory protection. May be harmful if swallowed. Ingestion is not an expected route of entry in industrial or commercial uses. This product contains a residual amount of a chemical substance that may cause an allergic skin and/or respiratory reaction.Causes mild skin irritation.Chronic: Prolonged or repeated contact may result in dermatitis. The nitrogen substituted aromatic in this product gave positive results for mutagenicity in an Ames Assay study while two other mutagenicity studies proved negative.IARC has identified the proprietary curative in this product as an "animal suspected" carcinogen, Group 3, which downgrades a previous NCI report of it as an "animal positive" carcinogen. IARC has designated carbon black as Group 2B - inadequate evidence for carcinogenicity in humans, but sufficient evidence in experimental animals. In 2006 IARC reaffirmed its 1995 finding that there is "inadequate evidence" from human health studies to assesswhether carbon black causes cancer in humans. Further, epidemiological evidence from well-conductedinvestigations has shown no causative link between carbon black exposure and the risk of malignant or non-malignant respiratory disease in humans.withheld.The above Nonylphenol ethoxylate compound is listed by ECHA as an SVHC.FIRST AID - EYE CONTACT: Flush eyes immediately with large amount of water for at least 15 minutes holding eyelids open while flushing. Get prompt medical attention.FIRST AID - SKIN CONTACT: Flush contaminated skin with large amounts of water while removing contaminated clothing. Wash affected skin areas with soap and water. Get medical attention if symptoms occur.FIRST AID - INHALATION: Move person to fresh air. Restore and support continued breathing. If breathing is difficult, give oxygen. Get immediate medical attention.FIRST AID - INGESTION: If swallowed, do not induce vomiting. Call a physician or poison control center immediately for further instructions. Never give anything by mouth if victim is rapidly losing consciousness, unconscious or convulsing.SUITABLE EXTINGUISHING MEDIA: Carbon Dioxide, Dry Chemical, Foam, Water FogUNSUITABLE EXTINGUISHING MEDIA: Not determined for this product.SPECIFIC HAZARDS POSSIBLY ARISING FROM THE CHEMICAL: Keep containers tightly closed. Closed containers may rupture when exposed to extreme heat. Use water spray to keep fire exposed containers cool. WARNING: Due to the combustible nature of the dried film of this product and the potential for smoldering or fire, the accumulation and buildup of the dried film on spray booth walls and floors, spindles, fixtures and other surfaces should be avoided, and any buildup should be removed. Keep the dried film accumulations away from sparks, friction, impact, high heat (>235 F/>112 C) or other sources of ignition. These conditions could cause the dried film to ignite very readily and quickly, and the resulting smoldering or fire may be difficult to extinguish. During removal of accumulation/buildup of this product, take precautions to avoid heat, friction and impact during the cleaning process. Use paint stripper, brass brush, or plastic scraper for cleaning. In the event of smoldering or a fire involving the dried product, Cold Fire®** fire suppressing agent is preferred as the extinguishing medium. If Cold Fire® is not available, use water spray as the extinguishing medium. Take efforts to ensure that these agents reach the base of the smoldering or fire. Parker-LORD Corporation will not be responsible for personal injuries, property damage or any other damages arising from the accumulation (buildup, cleaning/removal or any related smoldering or fire) resulting from the use of this product. Refer to the Chemlok® Safe Handling Guide for additional information. **NOTE: Parker-LORD Corporation has determined Cold Fire® fire suppressing agent to be effective in extinguishing fires involving dried Chemlok® adhesives. Parker-LORD does not recommend any particular equipment or system for use in delivering or applying Cold Fire® products. Customer is responsible for determining that Cold Fire® products and any delivery equipment or system is appropriate and effective for customer's specific needs. During a fire, irritating and/or toxic gases and particulate may be generated by thermal decomposition or combustion.SPECIAL PROTECTIVE EQUIPMENT AND PRECAUTIONS FOR FIRE-FIGHTERS: Wear full firefighting protective clothing, including self-contained breathing apparatus (SCBA). If water is used, fog nozzles are preferable.PERSONAL PRECAUTIONS, PROTECTIVE EQUIPMENT AND EMERGENCY PROCEDURES: Avoid breathing vapors. Avoid contact. Use appropriate respiratory protection for large spills or spills in confined area.ENVIRONMENTAL PRECAUTIONS: Do not contaminate bodies of water, waterways, or ditches, with chemical or used container.METHODS AND MATERIALS FOR CONTAINMENT AND CLEANUP: Notify appropriate authorities if necessary. Contain and remove with inert absorbent material. Avoid contact. Keep non-essential personnel away from spill area. Before attempting cleanup, refer to hazard caution information in other sections of the SDS form.HANDLING: Keep closure tight and container upright to prevent leakage. Avoid skin and eye contact. Wash thoroughly after handling. Do not handle until all safety precautions have been read and understood. Empty containers should not be re-used. Use with adequate ventilation.STORAGE: Store only in well-ventilated areas. Keep from freezing. Keep container closed when not in use.INCOMPATIBILITY: Strong acids, bases, and strong oxidizers.Engineering controls: Sufficient ventilation in pattern and volume should be provided in order to maintain air contaminant levels below recommended exposure limits.PERSONAL PROTECTION MEASURES/EQUIPMENT:RESPIRATORY PROTECTION: Use a NIOSH approved chemical/mechanical filter respirator designed to remove a combination of particulates and organic vapor if occupational limits are exceeded. For emergencysituations, confined space use, or other conditions where exposure limits may be greatly exceeded, use an approved air-supplied respirator. For respirator use observe OSHA regulations (29CFR 1910.134) or use in accordance with applicable laws and regulations of your country or particular locality.SKIN PROTECTION: Use neoprene, nitrile, or rubber gloves to prevent skin contact.EYE PROTECTION: Use safety eyewear including safety glasses with side shields and chemical goggles where splashing may occur.OTHER PROTECTIVE EQUIPMENT: Remove and wash contaminated clothing before reuse.HYGIENIC PRACTICES: Wash hands before eating, smoking, or using toilet facility. Do not smoke in any chemical handling or storage area. Food or beverages should not be consumed anywhere this product is handled or stored. Wash thoroughly after handling.Typical values, not to be used for specification purposes.ODOR: Odorless VAPOR PRESSURE: N.D.APPEARANCE: Green/Black VAPOR DENSITY: Heavier than Air PHYSICAL STATE: Liquid LOWER EXPLOSIVE LIMIT: Not ApplicableUPPER EXPLOSIVE LIMIT: Not ApplicableFLASH POINT:≥ 201 °F, 93 °CSetaflash Closed CupBOILING RANGE: 100 °C EVAPORATION RATE: Slower than n-butyl-acetate AUTOIGNITION TEMPERATURE:N.D.DENSITY: 1.18 g/cm3 (9.83 lb/gal) DECOMPOSITION TEMPERATURE:N.D. VISCOSITY, DYNAMIC: N.D.ODOR THRESHOLD: N.D.VISCOSITY, KINEMATIC: N.D.SOLUBILITY IN H2O: Soluble VOLATILE BY WEIGHT: 52.14 %pH: 6.0VOLATILE BY VOLUME: 61.54 %FREEZE POINT: N.D. VOC CALCULATED: 0.02 lb/gal, 3 g/l COEFFICIENT OF WATER/OILN.D.DISTRIBUTION:LEGEND: N.A. - Not Applicable, N.E. - Not Established, N.D. - Not DeterminedHAZARDOUS POLYMERIZATION: Hazardous polymerization will not occur under normal conditions.STABILITY: Product is stable under normal storage conditions.CONDITIONS TO AVOID: High temperatures.; For dried product issues, refer to Section 5 of the (M)SDS. INCOMPATIBILITY: Strong acids, bases, and strong oxidizers.HAZARDOUS DECOMPOSITION PRODUCTS: Decomposition due to high temperatures or a fire causes the formation of irritating and/or toxic gases, organic vapors or fumes., May contain CO, CO2, oxides of nitrogen, oxides of sulfur, halogenated by-products, Metal oxidesEXPOSURE PATH: Refer to section 2 of this SDS.SYMPTOMS:Refer to section 2 of this SDS.TOXICITY MEASURES:Germ cell mutagenicity: No classification proposedCarcinogenicity: Category 2 - Suspected of causing cancer.Components contributing to classification: Curative.Reproductive toxicity: No classification proposedPERSISTENCE AND DEGRADABILITY:Not determined for this product.BIOACCUMULATIVE: Not determined for this product.MOBILITY IN SOIL: Not determined for this product.OTHER ADVERSE EFFECTS: Not determined for this product.DISPOSAL METHOD: Disposal should be done in accordance with Federal (40CFR Part 261), state and local environmental control regulations. If waste is determined to be hazardous, use licensed hazardous waste transporter and disposal facility. Waste streams, including the dried adhesive residue, resulting from the use of this product should be tested for RCRA characteristics, including ignitability, to determine any applicable waste classifications.US DOT RoadProper Shipping Name: Environmentally hazardous substances, liquid, n.o.s.Hazard Class: 9SECONDARY HAZARD: NoneUN/NA Number: 3082Packing Group: IIIEmergency Response Guide Number: 171For US DOT non-bulk road shipments this material may be classified as NOT REGULATED. For the mostaccurate shipping information, refer to your transportation/compliance department regarding changes inpackage size, mode of shipment or other regulatory descriptors.IATA CargoPROPER SHIPPING NAME: Environmentally hazardous substance, liquid, n.o.s.Hazard Class: 9HAZARD CLASS: NoneUN NUMBER: 3082PACKING GROUP: IIIEMS: 9LIMDGPROPER SHIPPING NAME: Environmentally hazardous substance, liquid, n.o.s.Hazard Class: 9HAZARD CLASS: NoneUN NUMBER: 3082PACKING GROUP: IIIEMS: F-AThe listed transportation classification applies to non-bulk shipments. It does not address regulatory variations due to changes in package size, mode of shipment or other regulatory descriptors. For the most accurate shipping information, refer to your transportation/compliance department.U.S. FEDERAL REGULATIONS: AS FOLLOWS:SARA SECTION 313This product contains the following substances subject to the reporting requirements of Section 313 of Title III of the Superfund Amendment and Reauthorization Act of 1986 and 40 CFR part 372.:Chemical Name CAS Number Weight % Less ThanZinc compound PROPRIETARY10.0%TOXIC SUBSTANCES CONTROL ACT:INVENTORY STATUSThe chemical substances in this product are on the active TSCA Section 8 Inventory or exempt.EXPORT NOTIFICATIONThis product contains the following chemical substances subject to the reporting requirements of TSCA 12(B) if exported from the United States:NoneUnder HazCom 2012 it is optional to continue using the HMIS rating system. It is important to ensure employees have been trained to recognize the different numeric ratings associated with the HazCom 2012 and HMIS schemes.HMIS RATINGS - HEALTH: 2* FLAMMABILITY: 1 PHYSICAL HAZARD: 0* - Indicates a chronic hazard; see Section 2Revision: Company LogoEffective Date: 06/30/2021The information contained herein is, to the best of our knowledge and belief, accurate. However, since the conditions of handling and use are beyond our control, we make no guarantee of results, and assume no liability for damages incurred by use of this material. It is the responsibility of the user to comply with all applicable federal, state and local laws and regulations.。
罗布林产品ARIA732-5102030操作手册说明书

NOTICEMARQUE: ROBLINREFERENCE:ARIA 732 - 5102030 CODIC:4297660Instructions Manual Manuel d’Instructions BedienungsanleitungINDEXRECOMMENDATIONS AND SUGGESTIONS (3)CHARACTERISTICS (6)INSTALLATION (7)USE (9)MAINTENANCE (10)SOMMAIRECONSEILS ET SUGGESTIONS (12)CARACTERISTIQUES (15)INSTALLATION (16)UTILISATION (18)ENTRETIEN (19)INHALTSVERZEICHNISEMPFEHLUNGEN UND HINWEISE (21)CHARAKTERISTIKEN (24)MONTAGE (25)BEDIENUNG (27)WARTUNG (28)CONSEILS ET SUGGESTIONSLes instructions pour l’utilisation se réfèrent aux différents modèles de cet appareil. Par conséquent, certaines descriptions de caractéristiquesparticulières pourraient ne pas appartenir spécifiquement à cet appareil. INSTALLATION • En aucun cas le fabricant ne peut être tenu pour responsable d’éventuels dommages dus à une installation ou à une utilisation impropre.• La distance de sécurité minimum entre le plan decuisson et la hotte aspirante est de 650 mm (certainsmodèles peuvent être installés à une hauteur inférieure ;voir le paragraphe concernant les dimensions de travailet l’installation).• Assurez-vous que la tension de votre secteur correspondà celle indiquée sur la plaque des données appliquée àl’intérieur de la hotte.• Pour les appareils de Classe I, s’assurer que l’installation électrique de votre intérieur dispose d’une mise à la terre adéquate.Relier l’aspirateur au conduit de cheminée avec un tube d’un diamètre minimum de 120 mm. Le parcours des fumées doit être le plus court possible.• Ne pas relier la hotte aspirante aux conduits de cheminée qui acheminent les fumées de combustion (par exemple de chaudières, de cheminées, etc.). • Si vous utilisez l’aspirateur en combinaison avec desappareils non électriques (par ex. appareils à gaz), vous devez garantir un degré d’aération suffisant dans la pièce,afin d’empêcher le retour du flux des gaz de sortie. Lacuisine doit présenter une ouverture communiquantdirectement vers l’extérieur pour garantir l’amenée d’airpropre. Si vous utilisez la hotte de cuisine en combinaison avec des appareils non alimentés à l’électricité, la pression négative dans la pièce ne doit pas dépasser 0,04 mbar afin d’éviter que la hotte ne réaspire les fumées dans la pièce.• Si le cordon d’alimentation est endommagé, veuillez le faire remplacer par le fabricant ou par un service après-vente agréé pour éviter tout risque d’accident.• Si les instructions d’installation du plan de cuisson à gaz spécifient unedistance supérieure à celle indiquée ci-dessus, veuillez impérativement entenir compte. Toutes les normes concernant l’évacuation de l’air doivent êtrerespectées.• Utiliser exclusivement des vis et des petites pièces du type adapté pour lahotte.Attention : toute installation des vis et des dispositifs de fixation nonconforme aux présentes instructions peut entraîner des risques dedécharges électriques.• Brancher la hotte à l’alimentation de secteur avec un interrupteur bipolaireayant une ouverture des contacts d’au moins 3 mm. UTILISATION• Cette hotte aspirante a été conçue exclusivement pour un usagedomestique, dans le but d’éliminer les odeurs de cuisine.• Ne jamais utiliser la hotte pour des objectifs différents de ceux pour lesquelselle a été conçue.• Ne jamais laisser un feu vif allumé sous la hotte lorsque celle-ci est enfonction.• Régler l’intensité du feu de manière à l’orienter exclusivement vers le fondde la casserole, en vous assurant qu’il ne déborde pas sur les côtés.• Contrôler constamment les friteuses durant leur Array utilisation : l’huile surchauffée risque de s’incendier.• Ne pas flamber des mets sous la hotte : sous risquede provoquer un incendie.• Cet appareil n’est pas destiné à être utilisé par desenfants d’un âge inférieur à 8 ans, ni par des personnes dont les capacitésphysiques, sensorielles ou mentales sont diminuées ou qui ont uneexpérience et des connaissances insuffisantes, à moins que ces enfants ouces personnes ne soient attentivement surveillés et instruits sur la manièred’utiliser cet appareil en sécurité et sur les dangers que cela comporte.Assurez-vous que les enfants ne jouent pas avec cet appareil. Le nettoyageet l’entretien de la part de l’utilisateur ne doivent pas être effectués par desenfants, à moins que ce ne soit sous la surveillance d’une personneresponsable.• ATTENTION : les parties accessibles peuvent devenir très chaudes durant l’utilisation des appareils de cuisson.ENTRETIEN•Avant d’effectuer toute opération de nettoyage et d’entretien, éteindre oudébrancher l’appareil du secteur.• Nettoyer et/ou remplacer les filtres après le délai indiqué (dangerd’incendie).• Nettoyer les filtres à graisse tous les 2 mois de fonctionnement ou plus souvent en cas d’utilisation particulièrement intense. Ces filtres peuvent être lavés au lave-vaisselle.• Le filtre à charbon actif ne peut être ni lavé ni régénéré et il doit être remplacé environ tous les 4 mois de fonctionnement ou plus souvent en cas d’utilisation particulièrement intense.• Effectuer le nettoyage selon les instructions, sous risque d'incendie.• Nettoyer la hotte avec un chiffon humide et un détergent liquide neutre.Le symbole marqué sur le produit ou sur son emballage indique que ce produit ne peut pas être éliminé comme déchet ménager normal. Lorsque ce produit doit être éliminé, veuillez le remettre à un centre de collecte prévu pour le recyclage du matériel électrique et électronique. En vous assurant que cet appareil est éliminé correctement, vous participez à prévenir desconséquences potentiellement négatives pour l'environnement et pour la santé, qui risqueraient de se présenter en cas d’élimination inappropriée. Pour toute information supplémentaire sur le recyclage de ce produit, contactez votre municipalité, votre déchetterie locale ou le magasin où vous avez acheté ce produit.CARACTERISTIQUESEncombrementComposantsRéf. Q.té Composants de Produit1 1 Corps Hotte équipé de: Commandes, Lumière, GroupeVentilateur, Filtres8 1 Grille en Direction Sortie Air9 1 Flasque de Réduction ø 150-120 mm101 Buse avec clapet ø 150 mmRéf. Q.té Composants pour l ’installation 12e 2 Vis 2,9 x 9,5Q.téDocumentation1Manueld’instructions109 1INSTALLATIONMontage du corps de hotteAVANT DE MONTER LA HOTTE DANS L’ARMOIRE MURALE SUIVRE LA MARCHE CI-DESSOUS :• Ouvrir le panneau en le tirant.• Retirer les filtres à graisse.• Débrancher le câblage des commandes en intervenant surles connecteurs.• Retirer le cadre en desserrant les 4 vis (2 à droite et2 à gauche).• La hotte peut être installée directement sur le plan inférieur desarmoires murales (650 mm min. par rapport au plan decuisson).• Faire une entaille sur le plan inférieur de l’armoire murale, dela manière indiquée.• Insérer la Hotte jusqu’à accrocher les Supports latéraux parencliquetage.• Bloquer définitivement en serrant les Vis Vf depuis le bas de laHotte.• Revisser le cadre avec les 4 vis précédemment retirées,rebrancher le câblage des commandes, remonter le filtre àgraisse et fermer le panneau.BranchementsSORTIE AIR VERSION ASPIRANTEl’installateur.Raccord tube ø 150•Insérer la bride avec soupape 10 ø 150 sur la sortiedu corps de hotte.•Fixer le tube avec des colliers serre-tube appropriés.Le matériel nécessaire n’est pas fourni.Raccord tube ø 120•Pour la liaison avec le tube ø120 mm, insérer la busede réduction 9 sur la bride ø 150 10 précédemmentinstallée.•Fixer le tube avec des colliers serre-tube appropriés.Le matériel nécessaire n’est pas fourni.•Dans les deux cas, retirer les filtres anti-odeur àcharbon actif éventuels.SORTIE AIR VERSION FILTRANTE•Percer un trou de ø 125 mm. sur l’éventuelle Tablettequi se trouve au-dessus de la Hotte.•Insérer le flasque de réduction 9 sur la sortie du corpsde la hotte.•Connecter la Flasque au trou de sortie sur la Tablettequi se trouve au-dessus de la Hotte, au moyen d’untuyau rigide ou flexible de ø120 mm.•Fixer le tube par des colliers appropriés. Le matériaunécessaire n’est pas fourni.•Fixer la Grille orientée 8 sur la sortie de l’air recycléà l’aide de 2 Vis 12e (2,9 x 9,5) fournies avecl’appareil.•S’assurer de la présence des filtres anti-odeur aucharbon actif.BRANCHEMENT ELECTRIQUE•Brancher la hotte sur le secteur en interposant un interrupteur bipolaire avec ouverture des contacts d’au moins 3 mm.UTILISATIONTableau des commandesT1T2T3T4LTOUCHE FONCTIONST1 MoteurCoupe le moteur. T2 VitesseDémarre le moteur à la première vitesse. Touche allumée fixe. T3 VitesseDémarre le moteur à la deuxième vitesse. Touche allumée fixe. T4 Vitesse Appuyée brièvement, elle démarre le moteur à la troisième vitesse. Toucheallumée fixe.Appuyée pendant 2 secondes. Touche clignotante.Elle démarre la quatrième vitesse avec une temporisation de 6 minutes,après lesquelles le moteur retourne à la vitesse précédemment program-mée. Fonction indiquée pour faire face aux pointes d’émission de fuméesde cuissonL Lumière Branche et débranche l’éclairage. Touche allumée fixe.ENTRETIENFiltres anti-graisseNETTOYAGE FILTRES ANTI-GRAISSE METALLIQUES AUTOPOR-TEURS• Lavables au lave-vaisselle, ils doivent être lavés environ tous les 2 mois d’emploi ou plus fréquemment en cas d’emploi par-ticulièrement intense.• Tirer sur les panneaux confort pour les ouvrir.• Retirer les filtres, un à un, en les poussant vers la partie posté-rieure du groupe tout en tirant vers le bas.• Laver les filtres en évitant de les plier, et les faire sécher avant de les remonter. (Tout changement de couleur sur la surface du filtre, susceptible de se produire avec le temps, ne nuit en rien à l’efficacité de ce dernier.)• Remonter les filtres en faisant attention de tenir la poignée vers la partie externe visible.• Refermer les panneaux confort.Filtres anti-odeur au charbon actif (version filtrante)Le filtre anti-odeur au charbon actif n’est pas lavable et ne peut pas être régénéré : il faut le remplacer tous les 4 mois de service environ, ou plus souvent en cas d’usage particulièrement intense.REMPLACEMENT• Tirer sur les panneaux confort pour les ouvrir.• Retirer les filtres à graisse.• Enlever les filtres anti-odeur au charbon actif saturés, comme indiqué (A).• Monter les nouveaux filtres, comme indiqué (B).• Remonter les filtres à graisse.• Refermer les panneaux confort.Éclairage• Pour le remplacement, contacter le Service après-vente (« Pour l’achat, s’adresser au service après-vente »).991.0439.148_01 - 1 212。
奥拉帕尼(Olaparib)合成检索总结报告
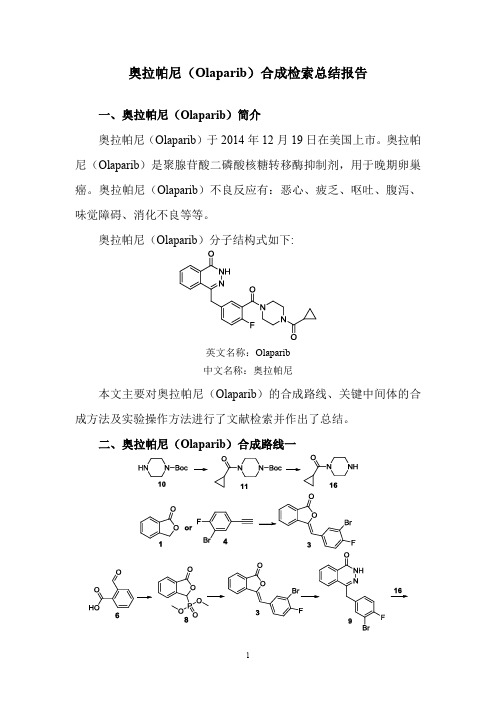
奥拉帕尼(Olaparib)合成检索总结报告
一、奥拉帕尼(Olaparib)简介
奥拉帕尼(Olaparib)于2014年12月19日在美国上市。
奥拉帕尼(Olaparib)是聚腺苷酸二磷酸核糖转移酶抑制剂,用于晚期卵巢癌。
奥拉帕尼(Olaparib)不良反应有:恶心、疲乏、呕吐、腹泻、味觉障碍、消化不良等等。
奥拉帕尼(Olaparib)分子结构式如下:
英文名称:Olaparib
中文名称:奥拉帕尼
本文主要对奥拉帕尼(Olaparib)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、奥拉帕尼(Olaparib)合成路线一
三、奥拉帕尼(Olaparib)合成路线二
四、奥拉帕尼(Olaparib)合成路线一检索总结报告
(一) 奥拉帕尼(Olaparib)中间体3的合成方法一(路线一)
(二) 奥拉帕尼(Olaparib)中间体3的合成方法二(路线一)
(三) 奥拉帕尼(Olaparib)中间体3的合成方法三(路线一)①奥拉帕尼(Olaparib)中间体8的合成。
舍曲林杂质汇总

611-71-2
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
扬信医药代理各品种杂质对照品:舒更葡糖钠杂质,达托霉素杂质,依维莫司杂质,他克莫司杂质,阿奇霉素杂质,克拉维酸钾杂质, 红霉素杂质,克拉霉素杂质,林可霉素杂质,罗红霉素杂质,克林霉素杂质,恩曲他滨杂质,艾地那非杂质,瑞卢戈利杂质,艾氟康唑
(Sertraline EP
52758-05-1
Impurity B)
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
舍曲林杂质3
Sertraline Impurity 3
ቤተ መጻሕፍቲ ባይዱ
(Sertraline EP
79559-98-1
Impurity C)
10mg 25mg 50mg 100mg 更大规格请咨询
舍曲林杂质列表集
中文名称
英文名称
CAS
舍曲林杂质1
Sertraline Impurity 1
(Sertraline EP
79617-99-5
Impurity A)
规格
10mg 25mg 50mg 100mg 更大规格请咨询
用途
项目报批 纯度高于98%
结构式
舍曲林杂质2
Sertraline Impurity 2
项目报批 纯度高于98%
Sertraline Impurity 4
舍曲林杂质4
(Sertraline EP
N/A
Impurity D)
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
舍曲林杂质5
Sertraline Impurity 5 (Sertraline EP Impurity E)
靶点PARP的抑制剂Olaparib

介绍:Olaparib (AZD2281, Ku-0059436)是一种选择性的PARP1/2抑制剂,IC50为5 nM/1 nM,比对Tankyrase-1的作用强300倍。
Phase 1/2。
靶点:PARP2,PARP1
体外研究:Olaparib是PARP抑制剂,也作用于BRCA1或BRCA2突变。
Olaparib对端锚(聚合)酶-1作用效果不大,IC50值大于。
Olaparib浓度为30-100 nM 作用于SW620细胞,使PARP-1失活。
与BRCA1和BRCA2充足细胞系(Hs578T,MDA-MB-231,T47D)相比,BRCA1缺陷细胞系(MDA-MB-463和HCC1937)对Olaparib过分敏感Olaparib抑制PARP,阻断碱基切除修复,导致KB2P细胞对Olaparib强烈敏感。
结果导致在DNA复制时由单链断裂转变为双链断裂,由此激活BRCA2依赖的重组途径。
体内研究:Olaparib有效作用于Brca1-/-;p53-/- 乳腺肿瘤 (每天按50 mg/kg 剂量腹腔注射),但是对 HR缺陷的Ecad-/-;p53-/-乳腺肿瘤没有效果。
Olaparib作用于携带肿瘤的鼠没有剂量限制毒性。
Olaparib已经用于治疗BRCA突变的肿瘤, 比如卵巢癌,胸腺癌,前列腺癌。
此外,Olaparib抑制ATM缺陷的肿瘤细胞具有选择性,说明Olaparib可作为治疗ATM突变的淋巴肿瘤的潜在试剂。
奥拉帕利片Olaparib-详细说明书与重点

奥拉帕利片Olaparib英文名:Olaparib Tablets,汉语拼音:Ao La Pa Li Pian。
【成份】本品活性成份为奥拉帕利,化学名称:4-(3-{[4-(环丙基羰基)哌嗪-1-基]羰基}4-氟苯基)甲基]酞嗪-1(2H)-酮。
化学结构式:分子式:C24H23FN4O3分子量:434.46。
【性状】本品为薄膜衣片150 mg:绿色至绿/灰色、椭圆形、双凸片,一面刻有“OP150”,另一面空白。
100 mg:黄色至深黄色、椭圆形、双凸片,一面刻有“OP100”,另一面空白。
【适应症】本品适用于铂敏感的复发性上皮性卵巢癌、输卵管癌或原发性腹膜癌成人患者在含铂化疗达到完全缓解或部分缓解后的维持治疗。
用于BRCA突变晚期卵巢癌的一线维持治疗。
携带BRCA突变的晚期上皮性卵巢癌、输卵管癌或原发性腹膜癌的维持治疗。
【规格】(1)150 mg ; (2)100 mg【用法用量】本品应在有抗肿瘤药物使用经验的医生的指导下使用。
推荐剂量:本品有150mg和100mg规格。
推荐剂量为300mg(2片150mg片剂),每日2次,相当于每日总剂量为600mg。
100mg片剂用于剂量减少时使用。
患者应在含铂化疗结束后的8周内开始本品治疗,持续治疗直至疾病进展或发生不可接受的毒性反应。
给药方法:口服给药。
本品应整片吞服,不应咀嚼、压碎、溶解或掰断药片。
本品在进餐或空腹时均可服用。
漏服:如果患者漏服一剂药物,应按计划时间正常服用下一剂量。
剂量调整-针对不良事件:为处理不良事件,比如恶心、呕吐、腹泻、贫血等,可考虑中断治疗或减量。
如果需要减量,推荐剂量减至250mg(1片150mg片剂,1片100mg片剂),每日服用2次(相当于每日总剂量为500mg)。
如果需要进一步减量,则推荐剂量减至200mg(2片100mg片剂),每日服用2次(相当于每日总剂量为400mg)。
合并使用细胞色素P450(CYP)3A抑制剂:在使用本品时,不推荐合并使用强效或中效CYP3A抑制剂,应考虑其他替代药物。
阿司咪唑

合成方法
合成方法
化合物(I)和碘甲烷在乙醇中回流8h,环合得到化合物(Ⅱ)。再水解脱去酯基,得到化合物(Ⅲ)。用对甲氧 基苯乙基溴进行N-烷基化,得化合物(Ⅳ)。再用对氟苄基溴烷基化,得阿司咪唑。
1. 1-[(4-氟苯基)甲基]-苯并咪唑-2-(3H)-酮的制备
在反应瓶中加入2-羟基苯并咪唑5.0g(37.3mmol)和NaH 1.6g(53mmol)(NaH含量大约为80%,浸入矿物油中) 的DMF 100ml的悬浮液.加毕.在60ºC.(最好有N2保护)搅拌反应1h.再加入4-氟苄基氯(FBC)5.4g(37mmol),加热 ( 6 0 ºC ) 搅 拌 反 应 5 . 5 h . 冷 却 至 室 温 后 加 入 冰 水 7 0 0 m l , 用 二 氯 甲 烷 ( 5 0 0 m l × 2 ) 提 取 . 有 机 层 用 食 盐 水 洗 . 无 水 N a 2 S O 4 干燥.过滤.滤液减压浓缩.剩余物用石油醚析晶.得1-[(4-氟苯基)甲基]-苯并咪唑-2-(3H)-酮固体8.0g,为无色 结 晶 m p 1 7 8 ~ 1 7 9 ºC , 收 率 8 8 % .
治疗措施
阿司咪唑中毒的治疗要点为: 1.大量摄入者予洗胃,后灌服活性炭和导泻。 2.对心肌抑制和Q-T间期延长者予5%碳酸氢钠250ml静注可能有效。 3.对症、支持治疗。
专家点评
专家点评
阿司咪阿司咪唑自1983年上市以来,在许多国家得到了广泛应用。国外研究显示阿司咪唑治疗荨麻疹的总有 效率为74%。国内的一项多中心双盲安慰剂对照试验表明阿司咪唑对急性荨麻疹的总有效率为82.9%,对慢性荨麻 疹的总有效率为86.0%,均显著高于安慰剂,主要不良反应为嗜睡、倦怠、口干等,连续用药3个月的患者中,半 数有食欲及体重增加。阿司咪唑的心脏毒性虽然发生率较低,但由于后果严重,已限制了它的应用。阿司咪唑为 强效和长效的H1受体拮抗剂,无中枢镇静和抗毒蕈碱样作用。代谢产物去甲阿司咪唑仍有抗胆胺作用。长期服用 可增进食欲和增加体重,服用过量可引起心脏Q-T间期延长和室性心律失常。适用于各种原因引起过敏性疾病。
小分子抑制剂、激动剂、拮抗剂--自噬信号通路

自噬自噬是一个吞噬自身细胞质蛋白或细胞器并使其包被进入囊泡,并与溶酶体融合形成自噬溶酶体,降解其所包裹的内容物的过程。
在哺乳动物细胞中,自噬主要有三种类型:微自噬、巨自噬和分子伴侣介导的自噬(CMA)。
微自噬通过溶酶体的膜直接包裹待降解物质等,并在溶酶体内降解。
巨自噬则由双层膜囊泡包裹待降解物质然后运送到溶酶体中降解。
而在分子伴侣介导的自噬(CMA)过程中,分子伴侣识别待降解蛋白,去折叠后并转移目的蛋白至溶酶体内。
自噬通常发生在营养缺乏的情况下,也与发育、分化、神经退行性疾病、应激、感染和癌症等生理和病理过程有关。
自噬机制的受损与肿瘤、神经退行性疾病、代谢相关疾病、免疫性疾病等发病过程密切相关。
自噬信号通路转导过程巨自噬:(1)起始:UKL和Beclin 1蛋白复合物起始自噬泡的形成。
(2)延伸:Atg5-Atg12-Atg16L复合物形成并与自噬泡融合;微管相关蛋白轻链3(LC3)由可溶解形式(LC3-I)转变为脂溶形式(LC3-II),与自噬泡结合形成自噬体。
(3)融合与降解:自噬体捕获需降解或清除的蛋白质、细胞器等物质;自噬体与溶酶体融合形成自噬溶酶体,释放内容物降解,完成自噬。
线粒体自噬:属于选择性自噬,主要是降解细胞中损伤和不需要的线粒体。
线粒体处于健康状态时,PINK 蛋白通过PARL的促进持续降解,而当线粒体功能障碍时,PINK蛋白会处于稳定状态,从细胞质中招募E3泛素连接酶Parkin,来诱导线粒体自噬。
Parkin会诱导线粒体膜蛋白的聚泛素化,由此导致通过LC3-作用区域(LIR)与LC3结合的自噬受体蛋白的SQSTM1/p62,NBR1和Ambra1聚集。
另外,在特定细胞中,BNIP3 和BNIP3L/NIX(也存在LIR)可通过非泛素反应机制聚集自噬的相关因子,从而促进自噬体的形成。
自噬信号通路图按靶点分类:*Autophagy*LRRK2*Mitophagy。
PARP抑制剂举例

PARP Poly(ADP-ribose) PolymerasePARP 抑制剂BMN 673结构式,子量: 380.35BMN 673是新型PARP 抑制剂,IC50为0.58 nM ,也有效抑制PARP-2,但不抑制PARG ,对PTEN 突变型高度敏感。
Phase 1。
Olaparib (AZD2281, Ku-0059436)结构式 分子量: 434.46Olaparib (AZD2281, KU0059436)是选择性的PARP1/2抑制剂,IC50为5 nM/1 nM,作用于 Tankyrase-1效果低300倍。
Phase 1/2。
Veliparib (ABT-888)结构式,子量: 244.29 ABT-888 (Veliparib, NSC 737664) 是有效的PARP1和PARP2抑制剂,Ki 分别为5.2 nM 和 2.9 nM ,抑制SIRT2活性。
Phase 1/2。
Rucaparib (AG-014699,PF-01367338)结构式分子量: 421.36Rucaparib (AG-014699, PF-01367338)是PARP 抑制剂,Ki 为1.4 nM 。
Phase 1/2。
Iniparib (BSI-201)结构式,子量: 292.03 BSI-201 (Iniparib, SAR240550) 是PARP1抑制剂,有效作用于三阴性乳腺癌(TNBC)。
Phase 3。
AG-14361结构式,子量: 320.39AG14361 是有效的PARP1抑制剂,Ki 为<5 nM ,比Benzamides 有效至少1000倍。
PJ34结构式,分子量: 295.34PJ-34是PARP 抑制剂,EC50为20 nM ,同等效果作用于PARP1/2。
PJ34 HCl 是PJ34的盐酸盐形式, 是PARP 抑制剂,EC50为20 nM ,同等有效作用于PARP1/2。
DDP锡铂、乙铂定、顺氯氨铂、氯氨铂、顺-双氯双氨络铂

【别名】锡铂、乙铂定、顺氯氨铂、氯氨铂、顺-双氯双氨络铂【英文名】Cisplatin,Cis-Diaminodichloroplatin,Platinol,Platistil,Neoplatin,【结构式】【作用特点】本品为无机金属络合物,将氯解离后与癌细胞的DNA交叉联结,从而破坏DNA功能。
可能与DNA形成链内或链间交联,亦可能与DNA及蛋白质形成交联,并抑制细胞有丝分裂,属细胞周期非特异性药物,除抗癌外,尚能抑制淋巴细胞转化,有免疫抑制作用。
【功能主治】本品抗瘤谱较广,可用于睾丸癌、头颈部肿瘤、鼻咽癌、恶性淋巴瘤、非小细胞肺癌、软组织肉瘤、卵巢癌、食管癌、膀胱癌、宫颈癌、前列腺癌、恶性胸水、消化道癌腹膜播散等,多用联合化疗,如睾丸癌用DDP十VLB十BLM方案,非小细胞肺癌用D DP十VCR十MMC方案。
【用法用量】静注或静滴:每次2Omg/m2,溶于温生理盐水或3%氯化钠注射液中,振摇使溶解,每日1次,连用5日为1段,隔1~2周重复第2段作为一个疗程,可用药4~6个疗程;或每次5 0~1OOmg/m2,每3~4周一个疗程。
动脉注射:每日20~30mg,溶于生理盐水2Oml中由插管推注,5日为一个疗程,间隔2周可重复用药。
腹腔内注入:用于消化道癌腹膜播散,每次100~160mg,通过腹腔内穿刺,注入腹水中,如静脉加注硫代硫酸钠5g则为“双路化疗”,可减少铂的全身毒性。
【不良反应】胃肠道反应多见,几乎全部均可致恶心呕吐。
应在给药前30分钟用5-HT3受体阻滞剂如恩丹西酮或格尼西酮。
大剂量时可致肾功能损伤和影响听力,亦可致神经毒性。
【注意事项】不可使用含有铝部件的注射器; 对既往有肾病史或中耳炎史者慎用。
在治疗中,出现下列症状之一者停用:1.周围白细胞低于3.5×109/L或血小板低于80×109/L。
2.用药后持续性严重呕吐。
3.早期肾脏毒性的表现,如血清肌酐大于2mg/dl 或尿素氮大于20mg/dl;或尿镜检在高倍视野中有白细胞10个、红细胞5个或管型5个。
Pyxis Lab ST-588 PTSA Fluorescent Polymer Dual Inl

ST-588PTSA/Fluorescent Polymer DualInline SensorUser ManualOctober12,2020Rev.2.00Pyxis Lab,Inc.1729Majestic Dr.Suite5Lafayette,CO80026USA©2017Pyxis Lab,Inc.Pyxis Lab Proprietary and ConfidentialTable of Contents1Introduction21.1Main Features (2)2Specifications3 3Unpacking Instrument43.1Standard Accessories (4)3.2Optional Accessories (5)4Installation64.1ST-588Piping (6)4.2ST-588SS Piping (6)4.3Wiring (7)4.4Connecting via Bluetooth (8)4.5Connecting via USB (8)5Setup and Calibration with uPyxis®Mobile App95.1Download uPyxis®Mobile App (9)5.2Connecting to uPyxis®Mobile App (9)5.3Calibration Screen and Reading (10)5.4Diagnosis Screen (11)5.5Device Info Screen (12)6Setup and Calibration with uPyxis®Desktop App126.1Install uPyxis®Desktop App (12)6.2Connecting to uPyxis®Desktop App (13)6.3Information Screen (13)6.4Calibration Screen (14)6.5Diagnosis Screen (14)7Outputs157.14–20mA Output Setup (15)7.2Communication using Modbus RTU (15)8Sensor Maintenance and Precaution158.1Methods to Cleaning the ST-588 (16)8.2Storage (16)9Troubleshooting17 10Contact Us18Warranty InformationConfidentialityThe information contained in this manual may be confidential and proprietary and is the property of Pyxis Lab,rmation disclosed herein shall not be used to manufacture,construct,or otherwise reproduce the goods rmation disclosed herein shall not be disclosed to others or made public in any manner without the express written consent of Pyxis Lab,Inc.Standard Limited WarrantyPyxis Lab warrants its products for defects in materials and workmanship.Pyxis Lab will,at its option,repair or replace instrument components that prove to be defective with new or remanufactured components (i.e.,equivalent to new).The warranty set forth is exclusive and no other warranty,whether written or oral, is expressed or implied.Warranty TermThe Pyxis warranty term is thirteen(13)months ex-works.In no event shall the standard limited warranty coverage extend beyond thirteen(13)months from original shipment date.Warranty ServiceDamaged or dysfunctional instruments may be returned to Pyxis for repair or replacement.In some in-stances,replacement instruments may be available for short duration loan or lease.Pyxis warrants that any labor services provided shall conform to the reasonable standards of technical com-petency and performance effective at the time of delivery.All service interventions are to be reviewed and authorized as correct and complete at the completion of the service by a customer representative,or des-ignate.Pyxis warrants these services for30days after the authorization and will correct any qualifying deficiency in labor provided that the labor service deficiency is exactly related to the originating event.No other remedy,other than the provision of labor services,may be applicable.Repair components(parts and materials),but not consumables,provided during a repair,or purchased individually,are warranted for90days ex-works for materials and workmanship.In no event will the in-corporation of a warranted repair component into an instrument extend the whole instrument’s warranty beyond its original term.Warranty ShippingA Repair Authorization(RA)Number must be obtained from Pyxis Technical Support before any product can be returned to the factory.Pyxis will pay freight charges to ship replacement or repaired products to the customer.The customer shall pay freight charges for returning products to Pyxis.Any product returned to the factory without an RA number will be returned to the customer.To receive an RMA you can generate a request on our website at https:///request-tech-support/.Pyxis Technical SupportContact Pyxis Technical Support at+1(866)203-8397,*********************,or by filling out a request for support at https:///request-tech-support/.1IntroductionThe Pyxis ST-588inline fluorometer probe simultaneously measures the concentration of PTSA and Fluores-cent Polymer in water.It can be simply inserted to the compression fitting port of a custom-made tee.The standard ST-001installation tee provided with each ST-588sensor,has two¾inch female NPT ports and can be placed to an existing¾inch sample water line.Pyxis Lab also offers2”and3”Tee formats for larger flow installations.The4–20mA current output of the ST-588probe can be connected to any controller that accepts an isolated or non-isolated4–20mA input.The ST-588probe is a smart device.In addition to mea-suring PTSA and Fluorescent Polymer,the ST-588probe has extra photo-electric components that monitor the color and turbidity of the sample water.This extra feature allows automatic color and turbidity com-pensation to eliminate interference commonly associated with real-world waters.The Pyxis ST-588probe has a short fluidic channel and can be easily cleaned.The fluidic and optical ar-rangement of the ST-588probe is designed to overcome shortcomings associated with other fluorometers that have a distal sensor surface or a long,narrow fluidic cell.Traditional inline fluorometers are susceptible to color and turbidity interference and fouling and are difficult to properly clean.1.1Main FeaturesThe ST-588measures PTSA and Fluorescent Polymer in a water sample and includes the following features:•Easy calibration with using uPyxis®Mobile or Desktop App.•Automatic compensation for turbidity up to150NTU and color created by up to10ppm iron or equivalent to10ppm humic acid.•Diagnostic information(probe fouling,color or turbidity over range,failure modes)are available in uPyxis®App or via Modbus RTU.•Easy to remove from the system for cleaning and calibration without the need for any tools.2SpecificationsTable1.ST-588Specifications*With Pyxis’s continuous improvement policy,these specifications are subject to change without notice.†The fluorescent polymer concentration scale is based on the polymer containing0.25mole%fluorescent monomer.Typical polymer specifications are attached below but may vary by producer.‡See Figure4for ST-588SS dimensions.3Unpacking InstrumentRemove the instrument and accessories from the shipping container and inspect each item for any damage that may have occurred during shipping.Verify that all accessory items are included.If any item is missing or damaged,please contact Pyxis Lab Customer Service at*********************.3.1Standard Accessories•Tee Assembly3/4”NPT(1x Tee,O-ring,and Nut)P/N:ST-001*NOTE*ST-001is not included for ST-588SS•8-Pin Female Adapter/Flying Leads Cable(1.5ft)•User Manual available online at https:///support/3.2Optional AccessoriesFigure1.4Installation4.1ST-588PipingThe provided ST-001Tee Assembly can be connected to a pipe system through the3/4”female ports,either socket or NPT threaded.To properly install the ST-588probe into the ST-001Tee Assembly,follow the steps below:1.Insert the provided O-ring into the O-ring groove on the tee.2.Insert the ST-588probe into the tee.3.Tighten the tee nut onto the tee to form a water-tight,compression seal.Figure2.Dimension of the ST-588and the ST-001Tee Assembly(mm)4.2ST-588SS PipingThe ST-588SS probe has3/4”female NPT threaded ports on the probe itself and therefore does not require a custom tee assembly.It is recommended that two3/4”NPT to1/4”tubing adapters are used to connect the probe to the sampling system.Sample water entering the probe must be cooled down to below104°F (40°C).The probe can be held by a1.75-inch pipe clamp or mounted to a panel with four1/4-28bolts.See Figure4for ST-588SS dimensions.Figure3.Dimension of the ST-588SS(inch)4.3WiringIf the power ground terminal and the negative4–20mA terminal in the controller are internally connected (non-isolated4–20mA input),it is unnecessary to connect the4–20mA negative wire(gray)to the4–20mA negative terminal in the controller.If a separate DC power supply other than that from the controller is used,make sure that the output from the power supply is rated for22–26VDC@85mA.*NOTE*The negative24V power terminal(power ground)and the negative4–20mA ter-minal on the ST-588probe are internally connected.Follow the wiring table below to connect the ST-588probe to a controller:Table2.*Internally connected to the power ground4.4Connecting via BluetoothA Bluetooth adapter(P/N:MA-WB)can be used to connect a ST-588probe to a smart phone with the uPyxis®Mobile App or a computer with the uPyxis®Desktop App.Figure4.Bluetooth connection to ST-588probe4.5Connecting via USBA USB-RS485adapter(P/N:MA-485)can be used to connect a ST-588probe to a computer with the uPyxis®Desktop App.*NOTE*Using non-Pyxis USB-RS485adapters may result in permanent damage of the ST-588probe communication hardware.B connection to ST-588probe5Setup and Calibration with uPyxis®Mobile App5.1Download uPyxis®Mobile AppDownload uPyxis®Mobile App from Apple App Store or Google Play.Figure6.5.2Connecting to uPyxis®Mobile AppTurn on Bluetooth on your mobile phone(Do not pair the phone Bluetooth to the ST-588probe).Open uPyxis®Mobile App.Once the app is open the app will start to search for the sensor.Once the uPyxis®Mobile App connects to the sensor,press the ST-588probe.Figure7.5.3Calibration Screen and ReadingWhen connected,the uPyxis®Mobile App will default to the Calibration screen.From the Calibration screen,you can perform calibrations by pressing on Zero Calibration,Slope Calibration,and4–20mA Span for either Fluorescent Polymer or PTSA,independently.Follow the screen instructions for each calibration step.Figure8.5.4Diagnosis ScreenFrom the Diagnosis screen,you can check the diagnosis condition.This feature may be used for technical support when communicating with*********************.To preform a probe cleaniness check,first select the Diagnosis Condition which defines the fluid type that the ST-588probe in currently measuring,then press Cleanliness Check.If the probe is clean,a Clean mes-sage will be shown.If the probe is severely fouled,a Dirty message will be shown.In this case,follow the procedure in the Methods to Cleaning the ST-588section of this manual.Figure9.5.5Device Info ScreenFrom the Device Info screen.You can name the Device or Product as well as set the Modbus address.Figure10.6Setup and Calibration with uPyxis®Desktop App6.1Install uPyxis®Desktop AppDownload the latest version of uPyxis®Desktop software package from:https:///upyxis/this setup package will download and install the Framework4.5(if not previously installed on the PC),the USB driver for the USB-Bluetooth adapter(MA-NEB),the USB-RS485adapter(MA-485),and the main uPyxis®Desktop application.Double click the uPyxis.Setup.exe file to install.Figure11.Click Install to start the installation process.Follow the screen instructions to complete the USB driver and uPyxis®installation.6.2Connecting to uPyxis®Desktop AppWhen the uPyxis®Desktop App opens,click on Device,then click either Connect via USB-Bluetooth or Connect via USB-RS485depending on the connection type.Figure12.6.3Information ScreenOnce connected to the device,a picture of the device will appear on the top left corner of the window and the uPyxis®Desktop App will default to the Information screen.On the Information screen you can set the information description for Device Name,Product Name,and Modbus Address,then click Apply Settings to save.Figure13.6.4Calibration ScreenTo calibrate the device,click on Calibration.On the Calibration screen there are six calibration options:•Fluorescent Polymer:Zero Calibration,Slope Calibration,and4-20mA Span•PTSA:Zero Calibration,Slope Calibration,and4-20mA SpanThe screen also displays the reading of the device.The reading refresh rate is every4seconds.Figure14.6.5Diagnosis ScreenAfter the device has been calibrated and installation has been completed,to check diagnosis,click on Di-agnosis.When in the Diagnosis screen you can view the Diagnosis Condition of the device.This feature may be used for technical support when communicating with*********************.To preform a probe Cleaniness Check,first select the Diagnosis Condition which defines the fluid type that the ST-588probe inCheck.If the probe is clean,a Clean message will be shown.message will be shown.In this case,follow the procedure in theof this manual.Figure15.7Outputs7.14–20mA Output SetupThe4–20mA output of the ST-588sensor is scaled as:•Fluorescent Polymer:–4mA=0ppm–20mA=20ppm•PTSA:–4mA=0ppb–20mA=200ppb7.2Communication using Modbus RTUThe ST-588probe is configured as a Modbus slave device.In addition to the ppm Fluorescent Polymer and ppb PTSA values,many operational parameters,including warning and error messages,are available via a Modbus RTU connection.Contact Pyxis Lab Customer Service(*********************)for more informa-tion.8Sensor Maintenance and PrecautionThe ST-588probe is designed to provide reliable and continuous Fluorescent Polymer and PTSA readings even when installed in moderately contaminated industrial cooling waters.Although the optics are com-pensated for the effects of moderate fouling,heavy fouling will prevent the light from reaching the sensor, resulting in low readings and the potential for product overfeed if the ST-588probe is used as part of an au-tomated control system.When used to control product dosing,it is suggested that the automation system be configured to provide backup to limit potential product overfeed,for example by limiting pump size or duration,or by alarming if the pumping rate exceeds a desired maximum limit.The ST-588probe is designed to be easily removed,inspected,and cleaned if required.It is suggested that the ST-588probe be checked for fouling and cleaned/calibrated on a monthly basis.Heavily contam-inated waters may require more frequent cleanings.Cleaner water sources with less contamination may not require cleaning for several months.The need to clean the ST-588probe can be determined by the Cleanliness Check using either the uPyxis®Mobile App(see the Mobile Diagnosis Screen section)or the uPyxis®Desktop App(see the Desktop Diagnosis Screen section).8.1Methods to Cleaning the ST-588Any equipment in contact with industrial cooling systems is subject to many potential foulants and con-taminants.Our inline probe cleaning solutions below have been shown to remove most common foulants and contaminants.A small,soft bristle brush,Q-Tips cotton swab,or soft cloth may be used to safely clean the probe housing and the quartz optical sensor channel.These components and more come with a Pyxis Lab Inline Probe Cleaning Solution Kit(P/N:SER-01)which can be purchased at our online Estore/Catalog https:///product/probe-cleaning-kit/Figure16.Inline Probe Cleaning Solution KitTo clean the ST-588probe,soak the lower half of the probe in100mL inline probe cleaning solution for 10minutes.Rinse the ST-588probe with distilled water and then check for the flashing blue light inside the ST-588probe quartz tube.If the surface is not entirely clean,continue to soak the ST-588probe for an e the small,soft bristle brush and Q-Tips cotton swabs as necessary to remove any remaining contaminants in the ST-588probe quartz tube.8.2StorageAvoid long term storage at temperature over100°F.In an outdoor installation,properly shield the ST-588 probe from direct sunlight and precipitation.9TroubleshootingIf the ST-588probe output signal is not stable and fluctuates significantly,make an additional ground con-nection––connect the clear(shield,earth ground)wire to a conductor that contacts the sample water electrically such as a metal pipe adjacent to the ST-588tee.Carry out routine calibration verification against a qualified Fluorescent Polymer and PTSA combined stan-dard.After properly cleaning the ST-588sensor,carry out the zero point calibration with distilled water and slope calibration using the qualified Fluorescent Polymer and PTSA combined standard.10Contact UsPyxis Lab,Inc1729Majestic Dr.Suite5Lafayette,CO80026USAPhone:+1(866)203-8397Email:*********************。
OlaparibPARP抑制剂-碧云天
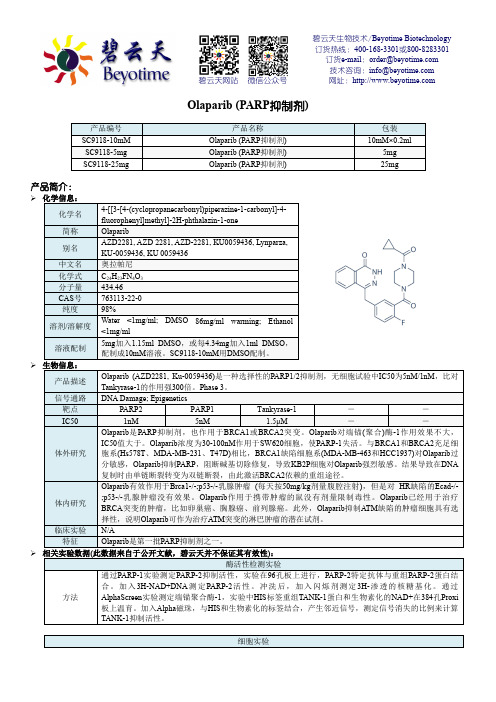
动物实验
动物模型 携带Brca1-/-;p53-/-乳腺癌的K14cre;Brca1F/F;p53F/F鼠
配制
50mg/ml储存在含10% 2-羟丙基-β-环糊精/PBS的DMSO中
剂量
50mg/kg
给药方式 按10μl/g剂量腹腔注射
参考文献:
1.Menear KA, et al. J Med Chem, 2008, 51(20), 6581-6591.
包装清单:
产品编号
SC9118-10mM SC9118-5mg SC9118-25mg
-
产品名称 Olaparib (PARP抑制剂) Olaparib (PARP抑制剂) Olaparib (PARP抑制剂)
说明书
包装 10mM×0.2ml
5mg 25mg 1份
保存条件:
-20ºC保存,至少一年有效。5mg和25mg包装也可以室温保存,至少6个月有效。如果溶于非DMSO溶剂,建议分装后-80ºC 保存,预计6个月有效。
Hale Waihona Puke 体外研究 胞系(Hs578T、MDA-MB-231、T47D)相比,BRCA1缺陷细胞系(MDA-MB-463和HCC1937)对Olaparib过
分敏感,Olaparib抑制PARP,阻断碱基切除修复,导致KB2P细胞对Olaparib强烈敏感。结果导致在DNA
复制时由单链断裂转变为双链断裂,由此激活BRCA2依赖的重组途径。
简称
Olaparib
别名
AZD2281, AZD 2281, AZD-2281, KU0059436, Lynparza, KU-0059436, KU 0059436
中文名 奥拉帕尼
化学式 分子量
小分子抑制剂、激动剂、拮抗剂--DNA损伤DNA修复信号通路

DNA损伤/DNA修复人类细胞中的DNA每天会受到数以万计的外源性损伤(化学污染、紫外线、电离辐射、烷基化/甲基化等引起)和内源性损伤(碱的氧化、烷基化、水解等引起)。
损伤后DNA会发生单链和双链断裂,未修复的DNA损伤会导致细胞衰老、凋亡和恶性肿瘤等等。
为了避免此类情况,激发了细胞的DNA损伤反应(DDR)。
DNA损伤反应(DDR)能够检测DNA的损伤并介导其修复,包含DNA损伤、细胞周期停滞、DNA复制调控、DNA损伤修复旁路等一系列通路的调控,以维持基因组的稳定性和细胞活力。
异常的DNA损伤反应与衰老、癌症和免疫疾病有关。
DNA损伤/DNA修复通路转导过程当DNA受到外源性或内源性损伤后,会发生单链和双链断裂,DNA损伤反应会被激活。
DNA双链断裂激活DNA-PK与ATM/ATR激酶。
DNA-PK诱导DNA修复,涉及错配、碱基切除、核苷酸切除修复等多种机制。
RPA、Rad51和fanconi贫血蛋白等也可直接用于DNA修复。
ATM/ATR激酶经过两个并行级联最终将CyclinB-cdc2复合体失活:1)ATM/ATR激酶激活Chk2激酶,Chk2激酶磷酸化并使失活Cdc25,同时也通过Chk1抑制Cdc25,从而阻止cdc2的激活,快速抑制细胞有丝分裂从G2期进入M期。
此外,ATR通过刺激Cdk1抑制激酶Wee1来抑制Cyclinb/Cdk1的激活,防止DNA损伤的细胞进入有丝分裂。
2)另一级联反应稍慢,Chk2激酶磷酸化p53,使其从MDM2和MDM4(MdmX)上分离,激活p53下游调节基因,从而抑制CyclinB-cdc2复合体活性或将其从细胞核中排出。
p300/PCAF对p53乙酰化可进一步增强其转录能力。
同时,p53也可诱导细胞凋亡。
DNA单链断裂后,激活ATR激酶,ATR通过Chk1进一步激活Cdc25A。
Cdc25A是一种CDK2激活所需的磷酸酶。
CDK2抑制周期素E/A,使有丝分裂无法从G1期进入S期。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
化学性质
产品名: Olaparib (AZD2281, Ku-0059436) 修订日期: 6/30/2016
产品名:
Cas No.: 分子量: 分子式: 别名: 化学名:
SMILES:
溶解性: 储存条件: 一般建议:
运输条件:
Olaparib (AZD2281, Ku-0059436)
763113-22-0
敏感性。在以背轴窗室模型(dorsal window chamber (DWC) model)建立的 Calu-6 异种移植 肺癌中,olaparib 可以增加血管灌流。
参考文献: Joana M. Senra, Brian A. Telfer, Kim E. Cherry, Cian M. McCrudden, David G. Hirst, Mark J. O’Connor, Stephen R. Wedge, and Ian J. Stratford. Inhibition of poly(ADP-ribose) polymerase-1 by olaparib (AZD2281) increases the radiosensitivity of a lung tumor xenograft. Mol Cancer Ther. 2011; 10(10): 1949-1958 Bastiaan Evers, Rinske Drost, Eva Schut, Michiel Bruin, Eline vab der Burg, Patrick W. B. Derksen, Henne Holstege, Xiaoling Liu, Ellen van Drunen, H. Berna Beverloo, Graeme C. M. Smith, Niall M. B. Martin, Alan Lau, Mark J. O’Connor, and Jos Jonkers. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and Cisplatin. Clin Cancer Res 2008; 14:3916-3925
实验操作
细胞实验: 细胞系 溶解方法
反应时间 应用
正常 LCL 细胞、ATM 缺失的 LCL 细胞
在 DMSO 中的溶解度>10 mM。为了获得更高的浓度,可以将离 心管在 37℃加热 10 分钟和/或在超声波浴中震荡一段时间。原液 可以在-20℃以下储存几个月。
细胞对 olaparib 的敏感性是由共济失调毛细血管扩张突变(ATM) 活性的缺失所介导的。免疫印迹分析表明,在 ATM 野生型,而 非 ATM 缺失的 LCLs 中,olaparib 以剂量依赖的方式诱导 ATM 依 赖的靶点 ATM S1981 和 SMC1 S966 的磷酸化。
特别声明
产品仅用于研究, 不针对患者销售,望谅解。 每个产品具体的储存和使用信息显示在产品说明书中。ApexBio 产品在推荐的条件下是稳定 的。产品会根据不同的推荐温度进行运输。许多产品短期运输是稳定的,运输温度不同于长 期储存的温度。我们确保我们的产品是在保持试剂质量的条件下运输的。收到产品后,按照 产品说明书上的要求进行储存。
434.46
C24H23FN4O3
AZD 2281,AZD-2281
4-[[3-[4-(cyclopropanecarbonyl)piperazine-1-carbonyl]-4-fluorophen yl]methyl]-2H-phthalazin-1-one
C1CC1C(=O)N2CCN(CC2)C(=O)C3=C(C=CC(=C3)CC4=NNC(=O)C5=CC= CC=C54)F
>21.7mg/mL in DMSO
Store at -20°C
For obtaining a higher solubility , please warm the tube at 37°C and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months.
引用文献
1. Raymond D, Harbison, Ph.D, et al. "The Influence of Oxygen Tension and Glycolytic and Citric Acid Cycle Substrates in Acroleininduced Cellular Injury in the Differentiated H9c2 Cardiac Cell Model."University of South Florida. 2016 Nov. 2. Cruz, Gladys Mae Saquilabon, et al. "Femtosecond near-infrared laser microirradiation reveals a crucial role for PARP signaling on factor assemblies at DNA damage sites." Nucleic acids research (2015): gkv976. PMID:26424850
产品描述:
Olaparib(AZD2281, Ku-0059436)是一种新型的选择性 PARP-1/2 抑制剂。Olaparib 强烈地抑 制 BRCA2 缺陷的小鼠肿瘤细胞的生长,已经成功用于治疗含有 BRCA 突变的肿瘤。在异种移 植非小细胞肺癌(NSCLC)细胞的裸鼠模型中,olaparib 可以增加非小细胞肺癌细胞的放射
请测试所有化合物在室内的溶解度,实际溶解度和理论值可能略 有不同。这是由实验系统的误差引起的,属于正常现象。
参考文献: [1] Weston V J, Oldreive C E, Skowronska A, et al. The PARP inhibitor olaparib induces significant killing of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood, 2010, 116(22): 4578-4587.
Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request
生物活性
靶点 :
Chromatin/Epigenetics
信号通路:
PARP
ApexBio Technology
动物实验: 动物模型 剂量 应用
注意事项
Granta-519 异种移植 NOD/SCID 小鼠
50 mg/kg/d,14 天;腹腔注射
通过 FACS 对人 CD45 染色的百分比分析表明,与对照组相比, olaparib 显著减少骨髓中 Granta-519 细胞的百分比,并减少小鼠 脾脏中肿瘤细胞负荷。
