T-type Ca2+
细胞生物学-细胞社会性

主要功能是对保护细胞、参与细胞识别。
细胞之间及细胞与基质之间的 相互作用首先发生在细胞表面
细胞连接的不同方式
细胞间相互作用以细胞识别为基础
识别的选择作用
两栖类胚性细 胞识别:
分别用蛋白 酶将外胚层和 中胚层水解成 单细胞,并分 别标记上不同 的颜色,然后 将这两种细胞 混合→
凝集素( 结合糖的蛋白质 )
糖蛋白的糖-肽连接
N 连接 ( N-Glycosidic or N-linked type ): 门冬酰氨残基的酰氨N与糖基相连
GlcNAc β1 --- Asn (NXS/T). NXS/T:代表蛋白质肽链中由Asn-氨基酸X -Ser/Thr 组成的三肽序列;氨基酸X
GalNAcα→Ser/Thr Galβ1↗3
Core 3:GlcNAcβ1 → 3 GalNACα→Ser/Thr
Core 4:GlcNAcβ1↘6 GalNAcα→Ser/Thr
GlcNAcα1↗3 在Core 2 及Core 4 的GlcNAc 糖基上还可以进一步发生糖基化,使糖链延长,形成长 的骨架(backbone),例如,生成多聚 N 乙酰氨基乳糖结构;其非还原末端的Gal基还可发 生唾液酸化或岩藻糖化。故O糖链可以具有和N糖链相似的末端结构。 Core 5:GalNAcα1 → 3 GalNAcα→Ser/Thr,存在于人类的腺癌及胚胎肠道细胞。 Core 6:GlcNAcβ1 → 6 GalNAcα→Ser/Thr,存在于人类胚胎肠道及卵巢囊泡粘蛋白 Core 7:GalNAcα1 → 6 GalNAcα→Ser/Thr,存在于牛颌下腺粘蛋白 Core 8:Gal α1 → 3 GalNAcα → Ser/Thr
人卫版药理学第8版21钙拮抗药

【分类】
1. 电压依赖性钾通道: 1)延迟整流钾通道(KV):Ik〔IKr IKs〕→AP 3相 2)瞬时外向钾通道(KA):IA 或Ito →AP1相 3)起搏电流(If) →心脏传导系统起搏电流之一
2. 钙依赖性钾通道(KCa): IK(Ca) →血管 SM 松弛 3. 内向整流钾通道(KIR) : IK1 →维持 4相 RP 4. ATP敏感钾通道(KATP) : IK(ATP) →调节机体C功
第21章 离子通道及 钙通道阻滞药
Chapter 21 Ion Channel and Calcium Channel Blocking Agents
【目的要求】
1.掌握钙通道阻滞药的药理作用与临床 应用; 2.熟悉作用于钠、钾通道的药物应用; 熟悉离子通道的特性和分类,离子通道的 生理功能,通道分子构造及门控机制; 3.了解离子通道概念及研究简史;了解 钙通道阻滞药的分类和作用机制。
4.抗充血性心衰: 尼可地尔〔↓心脏负荷,↑CO〕 5.平喘,镇咳: 克罗卡林, 吡那地尔
机制:对抗PGE、5-HT等引起的支气管痉挛、 阿片样镇咳效应
第3节 钙通道阻滞药
钙通道阻滞药(calcium channel blockers, CCBs) 是一类选择性阻滞钙通道,抑制胞外Ca2+ 内流,降低胞内Ca2+浓度的药物,又称钙拮抗药 (calcium antagonists)。
电压依赖性钙通道(VDCC):
〔2〕T-型(transient type): 激活电压低,失活速度快。 分布于全身细胞,参与心脏自律性调节、SM收
缩、N 放电、激素分泌等。 与多种疾病有关,如心律失常、心肌肥厚/纤
维化,高BP、癫痫、精神病等。
电压依赖性钙通道(VDCC):
基于Type-2 T-S的模糊脉冲控制

摘要 : 用 T p. 利 ye2模糊 系统在描 述 复杂非 线性 系统 诸 多不确 定性 问题 时 的有效 性 , Tp- 模 糊 在 ye1
脉 冲控 制 的基础 上 , 出 了区间 T p - T S模 糊 脉 冲控 制 方 法. 方 法利 用 T p 一 糊 隶属 函数 提 y e2 — 该 ye2模
Absr c :A o e mp lie c n r lsr tg a e n it r a p 一 S f zy mo e s p o o e o ta t n v li u sv o to tae y b s d o ne v lTy e 2 T— u z d lwa r p s d fr
sa ii n lss o h y tmsb n e rt d a p o c e fc mp rs n meh d a d l e rmarx i e u l— tb lt a ay i ft e s se y i tg a e p r a h so o a io t o n i a ti n q ai y n te .T e smu ai n o h nv  ̄e e d l m d l c n e sl ee mi e t mb r hp f n to s wi i s h i l t ft e i e d p n u u mo e a a i d tr n he me e s i u cin t o y h
I 江荨大 圜——■圈圈 .o / 一.一 34 3 1 N
J UR NA L O F J A NG SU UN 1 o l VERSI TY ( tr l ce c dt n Naua in eE i o ) S i
d i 0 3 6 /.sn 17 —7 7 . 0 2 0 .2 o:1 . 9 9 ji . 6 1 7 5 2 1 .4 0 3 s
第4章 药理学心血管药理

第二十一章离子通道理论及钙通道阻滞药离子通道(ion channels)是细胞膜中的跨膜蛋白质分子,对某些离子能选择通透,其功能是细胞生物电活动的基础。
离子通道按激活方式分两类:电压门控离子通道(voltage-gated channels)和化学门控离子通道(ligand gated channels)。
(一)钠通道(sodium channels):是选择性允许钠离子跨膜通过的离子通道。
均为电压门控性离子通道,主要功能是维持细胞膜的兴奋性及其传导。
根据对钠通道阻滞剂TTX和μ-CTX的敏感性不同分三类:1、神经类钠通道:对TTX敏感性高,而对μ-CTX敏感性低。
2、骨骼肌类钠通道:对TTX和μ-CTX敏感性均高。
3、心肌类钠通道:对TTX和μ-CTX敏感性均低。
钠通道的特征:1、电压依赖性。
2、激活和失活速度快。
3、有特异激活剂和阻滞剂:激活剂:树蛙毒素(batrachotoxin,BTX)、木藜芦毒素(grayanotoxin,GTX)。
阻滞剂:河豚毒素(tetrodotoxin,TTX)、蛤蚌毒素(saxitoxin,STX)。
(二)钾通道(potassium channels):是选择性允许钾离子跨膜通过的离子通道。
是目前发现的亚型最多、作用最复杂的一类离子通道。
广泛分布于骨骼肌、神经、心脏、血管、气管、胃肠道、血液及腺体等细胞。
钾通道在调节细胞膜电位和兴奋性以及平滑肌舒缩活动中起重要作用。
钾通道按其电生理特征不同分三类:1、电压依赖性钾通道(voltage-dependent K+ channels):其活性受膜电位变化的调控。
(1)延迟整流钾通道(delayed rectifier K+ channels):其电流为I k,与膜的复极化有关。
在心肌细胞存在两种主要的延迟整流钾通道:1)慢激活整流钾电流—I ks2)快激活整流钾电流—I kr二者为心肌细胞动作电位复极3期的主要离子流。
离子通道与心律失常

也参与心脏节律的调节和细胞间信号交流
电压门控钾离子通道
瞬时外向钾通道IA或Ito1 延迟整流钾通道 IK 内向整流钾通道 IK1
1
配体门控钾离子通道
乙酰胆碱激活的钾通道IK(Ach) ATP敏感性钾通道IK(ATP)
广泛存在 种类最多 作用最复杂
瞬时外向钾通道 (transient outward K+ channels)
电流为If 由K+和Na+共同携带 If为超极化激活的时间依赖性内向整流电流 是窦房结/房室结和希浦系统的起搏电流 之一 Adr激活If而Ach抑制If
乙酰胆碱激活的钾通道(acetylcholine-activated
K+ channels)
电流为IK(Ach) 具内向整流特性 存在于窦房结、心房肌、房室结、浦肯野纤维和心室肌 细胞分布广泛
2期复极化 L型钙电流(long-lasting Ca2+ current,L-type Ca2+ current, ICa-L) Ca2+内流 IK1 IK1通道内向整流特性 阻止了K+的进一步外流 随着动作电位复极化到接近静息电位时 内向整流现象解除 K+又可经IK1通道外流而加速最后的复极化过程 IK延迟整流钾电流(delayed rectifier K+ current, IK)
快反应心肌细胞膜 开放时选择性允许Na+内流
特征
电压依赖性 去极化达一定水平被激活 →开放产生内 向钠电流(Ina) →达最大效应→失活关闭 激活和失活速度快 前者1ms, 后者10ms内完成。
根据电压依赖性和对TTX的敏感性不同分为:
快(瞬时)钠通道:参与AP 0期去极化。 慢(持久)钠通道:参与AP 2期平台的形成。
IP3R1调控CaMKII和VDAC1在海洛因致心肌细胞节律异常中的作用

IP3R1调控CaMKII 和VDAC1在海洛因致心肌细胞节律异常中的作用*管雅玲1, 肖锦玲1, 苏丽萍2, 庄梦婕1, 刘丽2, 季敏2, 朱森森1,刘静宇1, 戴晨璐1, 蒲红伟3△(1新疆医科大学基础医学院,新疆 乌鲁木齐 830017;2新疆医科大学第一附属医院病理科,新疆 乌鲁木齐830054;3新疆医科大学第一附属医院学科建设科,新疆 乌鲁木齐 830054)[摘要] 目的:探讨1,4,5-三磷酸肌醇1型受体(inositol 1,4,5-trisphosphate receptor type 1, IP3R1)调控钙/钙调蛋白依赖性蛋白激酶II (calcium/calmodulin -dependent protein kinase II , CaMKII )和电压依赖性阴离子通道1(voltage -dependent anion channel 1, VDAC1)在海洛因(heroin , HE )致心肌细胞节律异常中的作用。
方法:联合蛋白组学和GEO (Gene Expression Omnibus )数据库分析心律失常芯片数据,寻找关键调控因子。
构建IP3R1基因敲减慢病毒并感染原代乳大鼠心肌细胞(neonatal rat cardiomyocytes , NRCMs ),实验分为对照(control )组、HE 组和HE+shIP3R1组。
结晶紫染色观察心肌细胞形态;ELISA 法检测乳酸脱氢酶(lactate dehydrogenase , LDH )和天冬氨酸转氨酶(aspartate aminotransferase , AST )水平;透射电镜观察线粒体形态学变化;Fluo -4/AM 探针法检测细胞内Ca 2+浓度;DCFH -DA 荧光探针检测细胞内活性氧(reactive oxygen species , ROS )含量;JC -1染色法检测线粒体膜电位(mitochondrial membrane potential , MMP )水平;ATP 检测试剂盒检测细胞内ATP 水平;免疫共沉淀(co -immunopre‑cipitation , Co -IP )分析IP3R1与CaMKIIδ和VDAC1蛋白之间的相互作用;Western blot 检测IP3R1、CaMKIIδ、p -CaM‑KIIδ(T287)和VDAC1的蛋白水平。
免疫学重点

第八章主要组织相容性复合体及其编码分子名词解释1.主要组织相容性复合体(major histocompatibility complex MHC):是存在于人和脊椎动物体内,编码主要组织相容性抗原的一组紧密连锁的基因群,其主要功能是以其产物提呈抗原肽进而激活了T淋巴细胞,启动适应性免疫应答。
2.人类白细胞抗原(human leukocyte antigen HLA):是人类的只要组织相容性抗原,因其首先在人白细胞表面发现而命名。
根据结构和功能分为HLAⅠ类分子和HLAⅡ类分子两种。
3.HLA I类分子:由重链(α链)和β2m组成的异二聚体,分布于所有有核细胞表面,是加工提呈内源性抗原的关键分子。
4.HLA ‖类分子:由α链和β链组成,主要表达于专职抗原提呈细胞表面,是加工提呈外源性抗原的关键分子。
5.共显性(co-dominance):即同源染色体对应座位上的两个等位基因都能得到表达,HLA 基因具有共显性特点,所以一个免疫细胞的表面通常可以检测到分别来自于父母双方的6对共12种HLAⅠ类和Ⅱ类分子。
6.MHC多态性(MHC polymorphism):是一个群体概念,指一个基因座上存在多个等位基因,即群体中不同个体在等位基因拥有状态上存在差别。
7.连锁不平衡(linkage disequilibrium):指分属于两个或两个以上基因座位的等位,同时出现在一条染色体上的概率高于随机出现的概率。
8.HLA单体型(HLA haplotype):指染色体上MHC不同座位等位基因的特定组合。
9.锚定残基(anchor residue):抗原肽往往带有两个或两个以上和HLA分子凹槽相结合的特定部位,称为锚定位,该位置上的氨基酸残基称为锚定残基。
10.MHC包容性(MHC flexibility):HLA分子对抗原肽的识别并非呈现严格的一对一关系,而是一种类型的HLA分子可以识别一群带有特定共用基序的肽段。
ion channel

二、离子通道的分类
钠离子通道 钙离子通道 钾离子通道 氯离子通道
钠离子通道的分类
钠离子通道广泛分布于可兴奋细胞中, 钠离子通道广泛分布于可兴奋细胞中 , 现已克隆出至 少9种类型的钠通道,其中多数对钠通道选择性拮抗剂 种类型的钠通道, 河豚毒素(TTX)较敏感, 河豚毒素(TTX)较敏感,如Ⅰ、Ⅱ、ⅡA、Ⅲ,Na-g Na和NaCh6等亚型均主要分布于中枢神经系统,SKM1和 NaCh6等亚型均主要分布于中枢神经系统, SKM2(分别为对TTX敏感型和不敏感型)主要分布于 分别为对TTX敏感型和不敏感型) 骨骼肌中, 骨骼肌中 , 而心肌细胞中存在的一种主要类型的钠通 道 ( hl 型 ) , 则对 TTX的敏感度较低 , ( 较其它钠通道 hl型 则对TTX 的敏感度较低, 亚型低约200倍 亚型低约200倍)
钠离子通道的功能
引起细胞去极化, 引起细胞去极化 , 兴奋的产生和兴奋的传导 心肌、神经细胞) (心肌、神经细胞)完成兴奋性细胞功能所必 如心肌收缩,神经冲动传导等。 需,如心肌收缩,神经冲动传导等。 麻醉,镇静。 麻醉,镇静。
钙离子通道的功能
平滑肌收缩 血压升高 心肌收缩力↑ 心肌收缩力↑ 内分泌增加 神经介质释放 细胞因子 炎症介质 释放
钠通道 ENaC 钠通道 ENaC
1. 电压门控钙通道
钙 通 道
(1)低血钾周期性瘫痪 (2)家族性偏瘫型偏头痛
L型钙通道CACNL1A1基因突变 脑P/Q型钙通道的α1A亚单位基因突变,
染色体1q21,Kca通道基因突变
(3)2—型发作性共济失调 (4)先天性Lambert—Eaton 脑P/Q型钙通道的α1A亚单位基因突变 突触前电压门控钙通道缺陷
细胞膜离子通道基本概念
Invitrogen Neon

Neon™ Transfection SystemFor transfecting mammalian cells, including primary and stem cells, with high transfection efficiency. Catalog Numbers MPK5000, MPK1025, MPK1096, MPK10025, MPK10096Doc. Part No. 25-1056 Pub. No. MAN0001632 Rev.B.0WARNING! For safety and biohazard guidelines, see the “Safety” appendix in the Neon™ Transfection System User Guide (Pub.No. MAN0001557). Read the Safety Data Sheets (SDSs) and follow the handling instructions. Wear appropriate protective eyewear, clothing, and gloves.Note: This Quick Reference is intended as a benchtop reference for experienced users of the Neon™ Transfection System User Guide (Pub. No. MAN0001557). For detailed instructions, supplemental procedures, and troubleshooting, see the Neon™ Transfection System User Guide (Pub. No. MAN0001557).General guidelines•Prepare high-quality plasmid DNA at a concentration of 1 to 5 μg/μL in deionized water or TE buffer, or high quality RNAi duplex at a concentration of 100–250 μM in nuclease-free water.•Use an appropriate GFP (green fluorescent protein) construct or siRNA control to determine transfection efficiency. See the Neon™Transfection System User Guide (Pub. No. MAN0001557) for details.•Use Resuspension Buffer R for established adherent and suspension cells, as well as primary adherent cells. Use Resuspension Buffer T with high voltage protocols of 1900 V or more. If arcing occurs with Resuspension Buffer R, consider switching toResuspension Buffer T.•Based on your initial results, you may need to optimize the electroporation parameters for your experiment using an 18-well or pre-programmed 24-well optimization protocol.•Discard the Neon™ Tips after 2 usages and Neon™ Tubes after 10 usages as a biological hazard. Change the tube and buffer when switching to a different plasmid DNA/siRNA or cell type.•The volume of plasmid DNA or siRNA added to the tranfection reaction should not exceed 10% of the total transfection volume.•Visit for a library of electroporation protocols for a variety of commonly used cell types.Prepare cellsFor the appropriate volume of medium to use based on cell density, or plating volumes for other plate formats, see “Amount of reagents” on page 2.1.Cultivate the required number of cells (70% to 90%confluent on the day of transfection) by seeding a flaskcontaining fresh growth medium 1 to 2 days prior toeletroporation.2.On the day of the experiment, pre-warm aliquots of culturemedium containing serum, PBS (without Ca2+and Mg2+), and Trypsin/EDTA solution to 37°C.3.Rinse the cells with PBS (without Ca2+and Mg2+), thentrypsinize the cells with the Trypsin/EDTA solution.4.Take an aliquot of trypsinized cell suspension, then countcells to determine the cell density.5.Harvest the cells in growth medium containing serum.6.Transfer cells to a 1.5-mL microcentrifuge tube or a 15-mLconical tube, then centrifuge the cells at 100 - 400 × g for 5 minutes at room temperature.7.Wash cells with PBS (without Ca2+and Mg2+) bycentrifugation at 100 - 400 × g for 5 minutes at roomtemperature.8.Aspirate the PBS, then resupsend the cell pellet inResuspension Buffer R (or Resuspension Buffer T forprograms ≥ 1900 V) at a final density of 1.0 × 107 cells/mL for adherent cells or 2.0 × 107 cells/ml for suspension cells.Gently pipette the cells to obtain a single cell suspension.IMPORTANT! Avoid storing the cell suspension for more than 15 to 30 minutes at room temperature. This will reduce cell viability and transfection efficiency.9.Prepare 24-well plates by filling the wells with 500 μL ofculture medium containing serum and supplements, butwithout antibiotics. Pre-incubate plates in a 37°C, 5% CO2 humidified incubator.Amount of reagentsFor each electroporation sample, the amount of plasmid DNA/siRNA, cell number, and volume of plating medium per well are listed in the following table. Use Resuspension Buffer T for cell types that require high voltage protocols of 1900 V or more. For all other cell types, use Resuspension Buffer R.[1]Use Resuspension Buffer T for primary suspension blood cells.Using the Neon ™Transfection SystemFor details on setting up the Neon ™device and Neon ™PipetteStation, see the Neon ™Transfection System User Guide (Pub. No.MAN0001557).1.Select the appropriate protocol for your cell type. Use one ofthe following options:•Input the electroporation parameters in the Input window if you already have the electroporation parameters for your cell type.•Tap Database , then select the cell-specificelectroporation parameters that you have added for various cell types.•Tap Optimization to perform the optimization protocol for your cell type.2.Fill the Neon ™Tube with 3 mL of Electrolytic Buffer (useBuffer E for the 10 μL Neon ™Tip and Buffer E2 for the 100μL Neon ™Tip).Note: Make sure that the electrode on the side of the tube is completely immersed in buffer. 3.Insert the Neon ™ Tube into the Neon ™Pipette Station untilyou hear a click sound (Figure 1).Figure 1 Schematic of Neon ™ Tube and Neon ™ Pipette Station.4.Transfer the appropriate amount of plasmid DNA/siRNA intoa sterile, 1.5 mL microcentrifuge tube.5.Add cells to the tube containing plasmid DNA/siRNA, thengently mix. See “Amount of reagents” on page 2 for cell number, DNA/siRNA amount, and plating volumes to use.6.To insert a Neon ™Tip into the Neon ™Pipette, press thepush-button on the pipette to the second stop to open the clamp.7.Insert the top-head of the Neon ™Pipette into the Neon ™Tipuntil the clamp fully picks up the mount stem of the piston (Figure 2).Figure 2 Schematic of Neon ™ Pipette and Neon ™Tip.8.Gently release the push-button, continuing to apply adownward pressure on the pipette, ensuring that the tip is sealed onto the pipette without any gaps.9.Press the push-button on the Neon ™Pipette to the first stopand immerse the Neon ™Tip into the cell-DNA/siRNA mixture.Slowly release the push-button on the pipette to aspirate thecell-DNA/siRNA mixture into the Neon ™Tip (Figure 3).Figure 3 Schematic of Neon ™Tip.Note: Avoid air bubbles during pipetting as air bubbles cause arcing during electroporation leading to lowered or failed transfection. If you notice air bubbles in the tip,discard the sample, then carefully aspirate the fresh sample into the tip again without any air bubbles.10.Insert the Neon ™Pipette with the sample vertically into theNeon ™ Tube placed in the Neon ™Pipette Station until youhear a click sound (Figure 4).Figure 4 Schematic of Neon ™ Tube and Neon ™ Pipette Station.Note: Ensure that the metal head of the Neon ™pipette projection is inserted into the groove of the pipette station.11.Ensure that you have selected the appropriateelectroporation protocol, then press Start on the touchscreen.12.The Neon ™device automatically checks for the properinsertion of the Neon ™ Tube and Neon ™Pipette before delivering the electric pulse.13.After delivering the electric pulse, Complete is displayed onthe touchscreen to indicate that electroporation is complete.14.Slowly remove the Neon ™Pipette from the Neon ™PipetteStation. Immediately transfer the samples from the Neon ™Tip by pressing the push-button on the pipette to the first stop into the prepared culture plate containing prewarmed medium with serum and supplements but without antibiotics.Note: Discard the Neon ™ Tip into an appropriate biologicalhazardous waste container. To discard the Neon ™Tip, press the push-button to the second stop into an appropriate biological hazardous waste container.15.Repeat step 6 to step 14 for the remaining samples.Note: Be sure to change the Neon ™Tips after using it twiceand Neon ™ Tubes after 10 usages. Use a new Neon ™Tip andNeon ™Tube for each new plasmid DNA sample.16.Gently rock the plate to ensure even distribution of the cells.Incubate the plate at 37℃ in a humidified CO 2 incubator.17.If you are not using the Neon ™device, turn the power switchon the rear to OFF .18.Assay samples to determine the transfection efficiency(e.g., fluorescence microscopy or functional assay) or geneknockdown (for siRNA).19.Based on your initial results, you may need to optimizedthe electroporation parameters for your cell type. For more information, see the Neon™ Transfection System User Guide (Pub. No. MAN0001557).Life Technologies Corporation | 5781 Van Allen Way | Carlsbad, California 92008 USAFor descriptions of symbols on product labels or product documents, go to /symbols-definition.The information in this guide is subject to change without notice.DISCLAIMER: TO THE EXTENT ALLOWED BY LAW, THERMO FISHER SCIENTIFIC INC. AND/OR ITS AFFILIATE(S) WILL NOT BE LIABLE FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE, OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR ARISING FROM THIS DOCUMENT, INCLUDING YOUR USE OF IT.Important Licensing Information: These products may be covered by one or more Limited Use Label Licenses. By use of these products, you accept the terms and conditions of all applicable Limited Use Label Licenses.©2021 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified./support | /askaquestion。
凝血项目临床应用

APTT N /
PT
N
/
T
T
FIB
FIB缺陷 原发纤溶
肝素
APTT
PT
T
T
FIB
DIC 严重肝脏病
混合血浆纠正实验、肝素纠正实验
PT/APTT延长
质控正常、复查一致
TT排除肝素污染
怀疑凝血因子缺乏或抗凝物 (因子抗体或狼疮抗凝物)
正常人与患者血浆1:1混合37℃孵育2h及 正常人与患者血浆分别37℃孵育2h后混合
下一节
➢ Ⅰ因子 — 纤维蛋白原含量(FIB)
FIB项目检测原理(Clauss法)
缺失 缺失
血浆
ⅩⅢ
Ⅻ Ⅺ Ⅹ Ⅸ Ⅷ Ⅶ Ⅴ Ⅳ Ⅲ Ⅱ Ⅰ
咪唑
37℃
Ⅱ Ⅰ
Ⅳ
FIB试剂
Ⅱ
过量凝血酶
Ⅳ
Ca2+
测量时间
秒值→含量 2-4g/l
不同FIB检测方法的比较
注:摘自《临床检验基础》(人卫第5版)
FIB常见的临床意义
INR范围 1.5~2.5 2.0~2.5 2.0~2.8 2.5~3.0 2.5~3.0
下一节 ➢ 活化部分凝血活酶时间(APTT)
APTT项目检测原理
血浆
ⅩⅢ
Ⅻ
Ⅺ
Ⅹ
Ⅸ
Ⅷ
Ⅶ
Ⅴ
缺失
Ⅳ
缺失
Ⅲ
Ⅱ
Ⅰ
37℃
Ⅺ
Ⅻ
激活剂
Ⅸ PL Ⅳ Ⅷ
Ⅹ PL Ⅳ Ⅴ
Ⅱ Ⅰ
Ⅳ
APTT 试剂
激活剂 PL
硅藻土 磷脂
Ⅳ
Ca2+
A:FIB存在异常,或者凝血酶受到了干扰;
伊士达高Tg、低CTE、多功能填充环氧树脂和酚醛固化层压板和预浸料IT-180ABS IT-180A

IT-180ABS/IT-180ATCHigh Tg, Low CTE, Multifunctional Filled Epoxy Resin and Phenolic-Cured Laminate & PrepregIT-180A is an advanced high Tg (175℃ by DSC) multifunctional filled epoxy with low CTE, high thermal reliability and CAF resistance. It’s design for high layer PCB and can pass 260℃ Lead free assembly and sequential lamination process.Key Features =============================== Advanced High Tg Resin TechnologyIndustrial standard material with high Tg (175℃ by DSC) multifunctional filled epoxy resin and excellent thermal reliability.Lead-Free Assembly CompatibleRoHS compliant and suitable for high thermal reliability needs, and Lead free assemblies with a maximum reflow temperature of 260℃. Friendly Processing and CAF ResistanceFriendly PCB process like high Tg FR4. Users can short the learning curve when using this material.CAF ResistanceLow thermal expansion coefficient (CTE) helps to excellent thermal reliability and CAF resistance providing long-term reliability for industrial boards and automobile application.Available in Variety of ConstructionsAvailable in a various of constructions, copper weights and glass styles, including standard(HTE), RTF and VLP copper foil. ApplicationsMultilayer and High Layer PCB AutomobileBackplanesServers and Networking TelecommunicationsData StorageHeavy Copper ApplicationIndustrial ApprovalUL 94 V-0IPC-4101C Spec / 99/ 101/ 126 RoHS CompliantGlobal AvailabilityArea Address Contact e-mail TELTaiwan 22,Kung Yen 1st Rd. Ping Chen Industry Zone. Ping Chen,Taoyuan, Taiwan, R.O.C.Sales: *************.twTechnician: *****************.tw886-3-4152345 #3168886-3-4152345 #5300East China Chun Hui Rd., Xishan Economic Development Zone,Wuxi City, Jiangsu Province, ChinaSales : ****************Technician: *********************86-510-8223-5888 #516886-510-8223-5888 #3000South China168, Dongfang Road, Nanfang Industrial Park, BeiceVillage, Humen Town, Dongguan City, Guangdong Province, China Sales: ***********.cnTechnician : ***************.cn86-769-88623268 #32086-769-88623268 #550Japan No.2, Huafang Rd, Yonghe Economic Zone, Economic andTechnological Development Zone, Guangzhou,Guangdong Province, ChinaSales: ****************.cnTechnician : *****************.tw86-20-6286-8088 #8027886-3-4152345 #5388USA Tapco Circuit Supply1225 Greenbriar Drive, Suite AAddison, IL 60101, USASales: *******************************Technician : ********************************1-614-937-52051-310-699-8028Europe ITEQ Europe,Via L. Pergher, 16 38121 Trento, Italy Sales: ********************Technician : *********************39-0461-82052639-0461-820526REV 06-12ITEQ Laminate/ Prepreg : IT-180ATC / IT-180ABS IPC-4101C Spec / 99 / 101 / 126LAMINATE( IT-180ATC)Thickness<0.50 mm[0.0197 in] Thickness≧0.50 mm[0.0197 in] Units T est MethodPropertyTypical Value Spec Typical Value SpecMetric(English)IPC-TM-650(or as noted)Peel Strength, minimumA. Low profile copper foil and very low profile copperfoil - all copper weights > 17µm [0.669 mil]B. Standard profile copper foil1.After Thermal Stress2.At 125°C [257 F]3.After Process Solutions 0.88 (5.0)1.23 (7.0)1.05 (6.0)1.05 (6.0)0.70 (4.00)0.80 (4.57)0.70 (4.00)0.55 (3.14)0.88 (5.0)1.40 (8.0)1.23 (7.0)1.23 (7.0)0.70 (4.00)1.05 (6.00)0.70 (4.00)0.80 (4.57)N/mm(lb/inch)2.4.82.4.8.22.4.8.3Volume Resistivity, minimumA. C-96/35/90B. After moisture resistanceC. At elevated temperature E-24/125 3.0x1010--5.0x1010106--103--3.0x10101.0x1010--104103MΩ-cm 2.5.17.1Surface Resistivity, minimumA. C-96/35/90B. After moisture resistanceC. At elevated temperature E-24/125 3.0x1010--4.0x1010104--103--3.0x10104.0x1010--104103MΩ 2.5.17.1Moisture Absorption, maximum -- -- 0.12 0.8 % 2.6.2.1 Dielectric Breakdown, minimum -- -- 60 40 kV 2.5.6 Permittivity (Dk, 50% resin content)(Laminate & Laminated Prepreg)A. 1MHzB. 1GHzC. 2GHzD. 5GHzE. 10GHz 4.44.44.24.14.05.44.44.44.34.14.15.4 --2.5.5.92.5.5.13Loss Tangent (Df, 50% resin content) (Laminate & Laminated Prepreg)A. 1MHzB. 1GHzC. 2GHzD. 5GHzE. 10GHz 0.0150.0150.0150.0160.0170.0350.0140.0150.0150.0160.0160.035 --2.5.5.92.5.5.13Flexural Strength, minimumA. Length directionB. Cross direction ----------------500-530(72,500-76,850)410-440(59,450-63,800)415(60,190)345(50,140)N/mm2(lb/in2)2.4.4Arc Resistance, minimum 125 60 125 60 s 2.5.1 Thermal Stress 10 s at 288°C [550.4F],minimumA. UnetchedB. Etched PassPassPass VisualPass VisualPassPassPass VisualPass VisualRating 2.4.13.1Electric Strength, minimum(Laminate & Laminated Prepreg)45 30 -- -- kV/mm 2.5.6.2 Flammability,(Laminate & Laminated Prepreg)V-0 V-0 V-0 V-0 Rating UL94 Glass Transition Temperature(DSC) 175 170 minimum 175 170 minimum ˚C 2.4.25Decomposition Temperature-- -- 345 340 minimum ˚C2.4.24.6 (5% wt loss)X/Y Axis CTE (40℃ to 125℃) -- -- 10-13 -- PPM/˚C 2.4.24 Z-Axis CTEA. Alpha 1B. Alpha 2C. 50 to 260 Degrees C ------------452102.760 maximum300 maximum3.0 maximumPPM/˚CPPM/˚C%2.4.24Thermal ResistanceA. T260B. T288 -------->60>3030 minimum15 minimumMinutesMinutes2.4.24.1CAF Resistance -- -- Pass AABUS Pass/Fail 2.6.25The above data and fabrication guide provide designers and PCB shop for their reference. We believe that these information are accurate, however, the data may vary depend on the test methods and specification used. The actual sales of the product should be according to specification in the agreement between ITEQ and its customer. ITEQ reserves the right to revise its data at any time without notice and maintain the best information available to users.REV 06-12。
中英对照外文文献(标题)对照

1. Effects of an N-Type Calcium Antagonist on Angiotensin II-Renin Feedback1. N型钙拮抗剂在肾素-血管紧张素II中的效果反馈2. Beneficial Effects of L- and N-type Calcium Channel Blocker on Glucose andLipid Metabolism and Renal Function in Patients with Hypertension and TypeII Diabetes Mellitus2. L型和N型钙通道拮抗剂对葡萄糖以及对高血压和II型糖尿病患者脂质代谢和肾功能的有益作用3. Expression of N-type calcium channels in human adrenocortical cells andtheir contribution to corticosteroid synthesis3.N型钙通道在人类肾上腺细胞中的表达以及对糖皮质激素合成的促进4. Blood pressure lowering effect and duration of action of calcium channelblocker cilnidipine by home blood pressure measurement4. 通过家庭自测血压检测钙通道拮抗剂西尼地平的降压效果以及药效持续时间5. Anti-leishmanial and anti-trypanosomal activities of 1, 4-dihydropyridines:In vitro evaluation and structure-activity relationship study5. 1,4二氢砒啶的抗利什曼虫和抗锥虫效果:体外评价以及构效关系研究6. The N-type and L-type calcium channel blocker cilnidipine suppresses renalinjury in Dahl rats fed a high-salt diet6. N型和L型钙通道拮抗剂西尼地平对高盐饲料喂养的Dahl大鼠肾损伤的抑制作用7. Antiproteinuric effect of cilnidipine in hypertensive japanese treated withrenin-angiotensin-system inhibitors-A multicenter, open, randomized trialusing 24-hour urine collection7. 西尼地平对使用肾素-血管紧张素系统进行治疗的日本患者的抗蛋白尿药效——多通道的、开放的、随机的试验8. A new-generation N/L-type calcium channel blocker leads to less activationof the renin-angiotensin system compared with conventional L type calciumchannel blocker8. 与传统的类型相比,新一代的N/L型钙通道拮抗剂肾素-血管紧张素系统的激活作用较弱9. Effect of cilnidipine on normal to marginally elevated urinealbumin-creatinine ratio in asymptomatic non-diabetic hypertensivepatients: An exponential decay curve analysis9. 西尼地平对尿白蛋白肌酐比例正常至略微偏高的无症状的非糖尿病高血压患者的效果:指数衰变曲线分析10. Comparison between the antiproteinuric effects of the calcium channelblockers benidipine and cilnidipine in combination with angiotensinreceptor blockers in hypertensive patients with chronic kidney disease10. 钙通道拮抗剂贝尼地平与西尼地平和血管紧张素结合的抗蛋白尿效果比较11. Cilnidipine additively inhibits the progression of renal impairment indiabetic rats when used in combination with an angiotensin II receptorblocker (ARB)11. 西尼地平在与血管紧张素II受体拮抗剂(ARB)同时使用时,可抑制糖尿病大鼠体内肾损害的发展12. T-type Ca channel blockade as a determinant of kidney protection12. T型钙通道接抗体为保护肾脏的决定因素13. Renal protection effect of cilnidipine for long term in chronic kidneydisease patients13. 西尼地平对慢性肾病患者肾脏保护作用的长效性14. The L/N-type calcium channel blocker, cilnidipine, reduces heart rate andalbuminuria in patients with type 214. L/N型钙通道拮抗剂,西尼地平,可降低2型糖尿病患者的心率15. Dual actions of cilnidipine in human internal thoracic artery: Inhibitionof calcium channels and enhancement of endothelial nitric oxide synthase15. 西尼地平对人类胸廓内动脉的双重作用:钙通道抑制和内皮型一氧化氮合成酶的提高16. Cilnidipine inhibited renal injury and reninangiotensin-aldosterone systemin deoxycorticosterone-salt hypertensive rats16. 西尼地平对去氧皮质酮醋酸盐高血压鼠的肾损伤和肾素-血管紧张素-醛固酮系统的抑制作用17. Reno-protective effect of cilnidipine in metabolic syndrome rats; possibleinvolvement of N-type calcium channel in podocyte17. 西尼地平对代谢综合症大鼠的肾脏保护作用;可能涉及足细胞中的N型钙通道18. Comparative effects of amlodipine and cilnidipine on sympathetic nervousmodulation in patients with hypertension18. 氨录地平与西尼地平对高血压患者的交感神经调节的对比效果19. Benidipine, a dihydropyridine L-type/T-type calcium channel blocker,affords additive benefits for prevention of cardiorenal injury inhypertensive rats19. 贝尼地平,一种二氢砒啶L型/T型钙通道拮抗剂,同时还可预防高血压鼠的心肾损伤20. A cross-over comparison of anti-albuminuric effects among 4 types calciumchannel blockers on chronic kidney disease20. 针对慢性肾病的四种钙通道接抗体的抗蛋白尿性交叉对比21. The effect of irbesartan on blood pressures and albuminuria in hypertensivetype 2 diabetic patients21. 厄贝沙坦对2型糖尿病合并高血压患者的降血压、控制蛋白尿的作用22. Cilnidipine suppresses podocyte injury and proteinuria in metabolicsyndrome rats: Possible involvement of N-type calcium channel in podocyte22. 西尼地平对新陈代谢症状大鼠的足细胞损伤和蛋白尿的抑制作用:可能涉及足细胞中的N型钙通道23. Bioequivalence of two cilnidipine formulations in healthy chinesevolunteers23. 在中国志愿者中俩种西尼地平配方的生物等效性24. Effect of cilnidipine vs losartan on cerebral blood flow in hypertensivepatients with a history of ischemic stroke: A randomized controlled trial24. 西尼地平与氯沙坦对有缺血性脑卒中史的高血压患者的脑血流的作用对比:随机对照试验25. Calcium antagonists: current and future applications based on new evidence.Calcium channel blockers and autonomic nervous system25. 钙拮抗剂:基于新论据而作出的现今的以及将来的应用。
Dispensing system for t-shirt type bags

专利名称:Dispensing system for t-shirt type bags 发明人:Richard M. Wile申请号:US08/431101申请日:19950428公开号:US05524763A公开日:19960611专利内容由知识产权出版社提供摘要:In the dispensing system disclosed herein, an aligned stack of T-shirt type bags are assembled within a disposable tubular cartridge with the loop handles and a significant portion of the bottoms of the bags extending beyond the ends of the tubular cartridge. The central portions of the bag mouths are releasably attached to an adjacent portion of the cartridge and a rack is provided for supporting the cartridge essentially horizontally with the handles and the bottom portions of the bags hanging freely therefrom. Accordingly, the top bag in the stack can be removed by grasping the exposed bottom portion and pulling the remainder through the tubular cartridge, tearing the mouth of the bag free from the attachment.申请人:BPI PACKAGING TECHNOLOGIES, INC.代理人:Henry D. Pahl, Jr.更多信息请下载全文后查看。
type t_teacher is record -回复

type t_teacher is record -回复“t_teacher”是一个记录类型,可以用来描述一个老师的信息。
这篇文章将回答关于“t_teacher”的问题,并提供有关如何创建和使用这个记录类型的详细步骤。
第一步:了解t_teacher记录类型的结构在开始创建一个“t_teacher”记录之前,了解它的结构是非常重要的。
一个“t_teacher”记录类型通常包含以下信息:姓名、性别、年龄、教育背景、教学经验等。
这些信息是用来描述一个老师的基本属性和背景的。
第二步:创建一个新的t_teacher记录在数据库或者存储记录的系统中创建一个新的“t_teacher”记录是非常简单的。
首先,确定记录的唯一标识,通常是一个独一无二的ID。
然后,按照记录类型的结构,填充相应的字段。
例如,输入老师的姓名、性别、年龄、教育背景和教学经验等信息。
第三步:添加额外的字段来描述教师的其他信息除了基本的信息,有时候还需要添加一些额外的字段来描述教师的其他信息,比如教授科目、执教学校、荣誉奖项等。
这些字段的添加可以根据具体需要进行,以使记录更加完整和准确。
第四步:更新和编辑t_teacher记录创建一个t_teacher记录并不意味着它是静态不变的。
在教师的信息发生变化时,可以随时更新和编辑记录。
这可能涉及到更改教师的任职学校、更新教育背景、增加教学经验等。
确保在每次更新记录时,所有的字段都是最新的和准确的。
第五步:使用t_teacher记录一旦有了完整且准确的t_teacher记录,它就可以在各种场景中使用了。
例如,在教育机构招聘老师时,可以使用这些记录作为参考。
在安排教师授课时,可以查看他们的特长和经验。
在评估教师的绩效时,可以根据记录中的数据进行评估。
总之,t_teacher记录提供了一个方便和系统化的方式来管理和利用关于教师的信息。
第六步:存档和备份t_teacher记录最后,为了确保数据的安全性和持久性,建议定期存档和备份t_teacher 记录。
netinetin.h
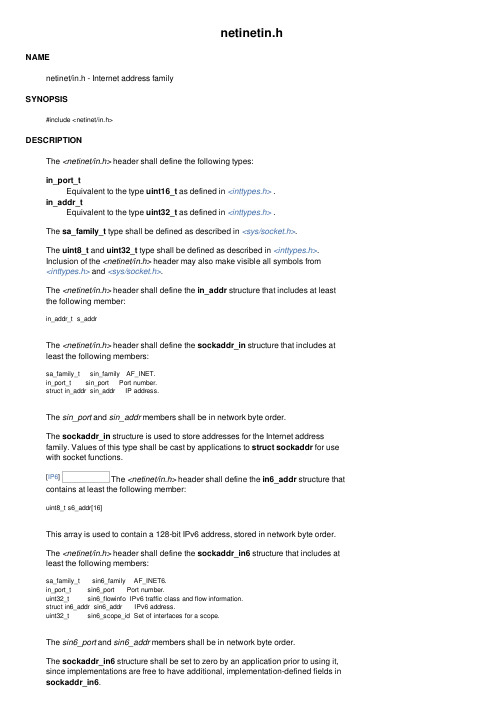
netinetin.hNAMEnetinet/in.h - Internet address familySYNOPSIS#include <netinet/in.h>DESCRIPTIONThe <netinet/in.h> header shall define the following types:in_port_tEquivalent to the type uint16_t as defined in <inttypes.h> .in_addr_tEquivalent to the type uint32_t as defined in <inttypes.h> .The sa_family_t type shall be defined as described in <sys/socket.h>.The uint8_t and uint32_t type shall be defined as described in <inttypes.h>.Inclusion of the <netinet/in.h> header may also make visible all symbols from<inttypes.h> and <sys/socket.h>.The <netinet/in.h> header shall define the in_addr structure that includes at least the following member:in_addr_t s_addrThe <netinet/in.h> header shall define the sockaddr_in structure that includes at least the following members:sa_family_t sin_family AF_INET.in_port_t sin_port Port number.struct in_addr sin_addr IP address.The sin_port and sin_addr members shall be in network byte order.The sockaddr_in structure is used to store addresses for the Internet addressfamily. Values of this type shall be cast by applications to struct sockaddr for use with socket functions.[IP6] The <netinet/in.h> header shall define the in6_addr structure that contains at least the following member:uint8_t s6_addr[16]This array is used to contain a 128-bit IPv6 address, stored in network byte order.The <netinet/in.h> header shall define the sockaddr_in6 structure that includes at least the following members:sa_family_t sin6_family AF_INET6.in_port_t sin6_port Port number.uint32_t sin6_flowinfo IPv6 traffic class and flow information.struct in6_addr sin6_addr IPv6 address.uint32_t sin6_scope_id Set of interfaces for a scope.The sin6_port and sin6_addr members shall be in network byte order.The sockaddr_in6 structure shall be set to zero by an application prior to using it, since implementations are free to have additional, implementation-defined fields in sockaddr_in6.The sin6_scope_id field is a 32-bit integer that identifies a set of interfaces as appropriate for the scope of the address carried in the sin6_addr field. For a link scope sin6_addr, the application shall ensure that sin6_scope_id is a link index. For a site scope sin6_addr, the application shall ensure that sin6_scope_id is a site index. The mapping of sin6_scope_id to an interface or set of interfaces is implementation-defined.The <netinet/in.h> header shall declare the following external variable:const struct in6_addr in6addr_anyThis variable is initialized by the system to contain the wildcard IPv6 address. The <netinet/in.h> header also defines the IN6ADDR_ANY_INIT macro. This macro must be constant at compile time and can be used to initialize a variable of type struct in6_addr to the IPv6 wildcard address.The <netinet/in.h> header shall declare the following external variable:const struct in6_addr in6addr_loopbackThis variable is initialized by the system to contain the loopback IPv6 address. The <netinet/in.h> header also defines the IN6ADDR_LOOPBACK_INIT macro. This macro must be constant at compile time and can be used to initialize a variable of type struct in6_addr to the IPv6 loopback address.The <netinet/in.h> header shall define the ipv6_mreq structure that includes at least the following members:struct in6_addr ipv6mr_multiaddr IPv6 multicast address.unsigned ipv6mr_interface Interface index.The <netinet/in.h> header shall define the following macros for use as values of the level argument of getsockopt() and setsockopt():IPPROTO_IPInternet protocol.IPPROTO_IPV6[IP6] Internet Protocol Version 6.IPPROTO_ICMPControl message protocol.IPPROTO_RAW[RS] Raw IP Packets Protocol.IPPROTO_TCPTransmission control protocol.IPPROTO_UDPUser datagram protocol.The <netinet/in.h> header shall define the following macros for use as destination addresses for connect(), sendmsg(), and sendto():INADDR_ANYIPv4 local host address.INADDR_BROADCASTIPv4 broadcast address.The <netinet/in.h> header shall define the following macro to help applications declare buffers of the proper size to store IPv4 addresses in string form:INET_ADDRSTRLEN16. Length of the string form for IP.The htonl(), htons(), ntohl(), and ntohs() functions shall be available as defined in<arpa/inet.h>. Inclusion of the <netinet/in.h> header may also make visible all symbols from <arpa/inet.h>.[IP6] The <netinet/in.h> header shall define the following macro to help applications declare buffers of the proper size to store IPv6 addresses in string form: INET6_ADDRSTRLEN46. Length of the string form for IPv6.The <netinet/in.h> header shall define the following macros, with distinct integer values, for use in the option_name argument in the getsockopt() or setsockopt() functions at protocol level IPPROTO_IPV6:IPV6_JOIN_GROUPJoin a multicast group.IPV6_LEAVE_GROUPQuit a multicast group.IPV6_MULTICAST_HOPSMulticast hop limit.IPV6_MULTICAST_IFInterface to use for outgoing multicast packets.IPV6_MULTICAST_LOOPMulticast packets are delivered back to the local application.IPV6_UNICAST_HOPSUnicast hop limit.IPV6_V6ONLYRestrict AF_INET6 socket to IPv6 communications only.The <netinet/in.h> header shall define the following macros that test for special IPv6 addresses. Each macro is of type int and takes a single argument of type const struct in6_addr *:IN6_IS_ADDR_UNSPECIFIEDUnspecified address.IN6_IS_ADDR_LOOPBACKLoopback address.IN6_IS_ADDR_MULTICASTMulticast address.IN6_IS_ADDR_LINKLOCALUnicast link-local address.IN6_IS_ADDR_SITELOCALUnicast site-local address.IN6_IS_ADDR_V4MAPPEDIPv4 mapped address.IN6_IS_ADDR_V4COMPATIPv4-compatible address.IN6_IS_ADDR_MC_NODELOCALMulticast node-local address.IN6_IS_ADDR_MC_LINKLOCALMulticast link-local address.IN6_IS_ADDR_MC_SITELOCALMulticast site-local address.IN6_IS_ADDR_MC_ORGLOCALMulticast organization-local address.IN6_IS_ADDR_MC_GLOBALMulticast global address.The following sections are informative.APPLICATION USAGENone.RATIONALENone.FUTURE DIRECTIONSNone.SEE ALSOHost and Network Byte Orders, <arpa/inet.h>, <inttypes.h>, <sys/socket.h>, theSystem Interfaces volume of IEEE Std 1003.1-2001, connect(), getsockopt(), htonl(), htons(), ntohl(), ntohs(), sendmsg(), sendto(), setsockopt()CHANGE HISTORYFirst released in Issue 6. Derived from the XNS, Issue 5.2 specification.The sin_zero member was removed from the sockaddr_in structure as per TheOpen Group Base Resolution bwg2001-004.IEEE Std 1003.1-2001/Cor 1-2002, item XBD/TC1/D6/12 is applied, adding const qualifiers to the in6addr_any and in6addr_loopback external variables.IEEE Std 1003.1-2001/Cor 2-2004, item XBD/TC2/D6/22 is applied, making it clear which structure members are in network byte order.。
T型钙通道和心血管及神经系统疾病

T型钙通道和心血管及神经系统疾病薛全福;王振纲【摘要】低电压T型钙通道广泛分布在各种类型细胞中,包括心血管和神经元细胞中,与高电压钙通道不同,在接近膜静息电位的低度去极化时即能被激活,因而有利于心脏起博和神经元细胞在生理状态下接近静息时,对兴奋和电反应的调节.但对T型钙通道在疾病中的作用所知有限.近年来因为克隆出3种T型钙通道α1亚单位的基因,(Cav3.1,Cav3.2和Cav 3.3),使深入研究实验动物及人体疾病时T型钙通道的性质、药理、体内分布、基因调节成为可能.并且为新药研发提供有力工具.转基因动物实验已证明T型钙通道不仅是治疗肾性高血压、心律失常也是治疗意识丧失型癫痫及神经性疼痛的重要药物靶点.此外,细胞内钙超载还与房颤、心衰、偏头痛、阿尔采末病、睡眠障碍等的发病有关,所以盼望有新的T型钙通道阻断剂出现.有报道Efonidipine能选择性地抑制低电压 T型钙通道,有望成为选择性阻断剂.此外,近年来调控T型钙通道活性的分子机制的研究取得新的达展,对新药的开发有所启迪.作者建议,对治疗心血管及神经系统疾病的药物靶点T型钙通道及其阻断剂的研究给予更多的关注.【期刊名称】《中国药理学通报》【年(卷),期】2010(026)009【总页数】4页(P1250-1253)【关键词】T型钙通道;调控;阻断剂;体内分布;心血管疾病;神经系统疾病【作者】薛全福;王振纲【作者单位】中国医学科学院基础医学研究所·北京协和医学院基础学院生理与病理生理学系,北京,100005;中国医学科学院基础医学研究所·北京协和医学院基础学院药理学系,北京,100005【正文语种】中文【中图分类】R329.25;R348.1;R54;R7411 T型钙通道胞内钙离子(Ca2+)来源于胞外,但因为细胞的脂质双层膜形成屏障,限制水和离子的进入,故胞内Ca2+含量极微,仅为胞外的2万分之一。
己知Ca2+主要通过嵌在膜上的特种蛋白质形成的孔道进入胞內,称之为Ca2+通道。
JS中常用的邮箱验证代码

<! DOCTYP E HTML PUBLIC "-//W3C//DTD HTML 4.0//EN""/TR/REC-html140/strict.dtd"><html><head><meta http-equiv="Conten t-Type" conten t="text/html; charse t=gb2312"><title>Sample Page!</title><script langua ge="JavaSc ript" type="text/javasc ript"><!--functi on EmailA ddres sTest(){//获取用户输入的邮箱地址相关信息var EmailS tring=docume nt.MyForm.MyEmai l.value;var strLen gth=EmailS tring.length;var index1=EmailS tring.indexO f("@");var index2=EmailS tring.indexO f(".",index1);var msg="验证邮箱地址实例:\n\n";msg+=" 邮箱地址: "+EmailS tring+"\n";msg+=" 验证信息: ";//返回相关验证信息if(index1==-1||index2==-1||index2<=index1+1||index1==0||index2==strLen gth-1) {msg+="邮箱地址不合法!\n\n"msg+="不能同时满足如下条件:\n";msg+=" 1、邮件地址中同时含有'@'和'.'字符;\n";msg+=" 2、'@'后必须有'.',且中间至少间隔一个字符;\n"msg+=" 3、'@'不为第一个字符,'.'不为最后一个字符。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
T-type Ca 2+channels contribute to IBMX/forskolin-and K +-induced Ca 2+transients in porcine olfactory receptor neuronsShree Hari Gautam a ,Ken-ichi Otsuguro b ,Shigeo Ito b ,Toshiyuki Saito c ,Yoshiaki Habara a ,*aLaboratory of Physiology,Department of Biomedical Sciences,Graduate School of Veterinary Medicine,Hokkaido University,Sapporo 060-0818,JapanbLaboratory of Pharmacology,Department of Biomedical Sciences,Graduate School of Veterinary Medicine,Hokkaido University,Sapporo 060-0818,JapancLaboratory of Neurobiology,National Institute of Agrobiological Sciences,Tsukuba 305-0901,JapanReceived 3August 2006;accepted 25September 2006Available online 30October 2006AbstractT-type Ca 2+channels are low-voltage-activated Ca 2+channels that control Ca 2+entry in excitable cells during small depolarization above resting ing Ca 2+imaging with a laser scanning confocal microscope we investigated the involvement of T-type Ca 2+channels in IBMX/forskolin-and sparingly elevated extracellular K +-induced Ca 2+transients in freshly isolated porcine olfactory receptor neurons (ORNs).In the presence of mibefradil (10m M)or Ni 2+(100m M),the selective T-type Ca 2+channel inhibitors,IBMX/forskolin-induced Ca 2+transients in the soma were either strongly (>60%)inhibited or abolished completely.However,the Ca 2+transients in the knob were only partially (<60%)inhibited.Ca 2+transients induced by 30mM K +were also partially ($60%)inhibited at both the knob and soma.Furthermore,ORNs responded to as little as a 2.5mM increase in the extracellular K +concentration (7.5mM K +),and such responses were completely inhibited by mibefradil or Ni 2+.These results reveal functional expression of T-type Ca 2+channels in porcine ORNs,and suggest a role for these channels in the spread Ca 2+transients from the knob to the soma during activation of the cAMP cascade following odorant binding to G-protein-coupled receptors on the cilia/knob of ORNs.#2006Elsevier Ireland Ltd and the Japan Neuroscience Society.All rights reserved.Keywords:Olfaction;Signal transduction;Ca 2+dynamics;cAMP;Mibefradil;Ni 2+;Pig1.IntroductionIn most mammalian species,odor signals play critical roles in several vital functions such as feeding,reproduction,and social organization.These behaviors require the fundamental operation of odor detection and discrimination.Olfactory receptor neurons (ORNs),the sensory neurons present in the nasal cavity,are primarily involved in coding sensory information from odorous stimuli.An understanding of the mechanism of signal transduction in ORNs is thus fundamental for an appreciation of how odor signals affect animal behavior.Based on the evidence available so far,cyclic AMP is known as the universal second messenger of olfactory signaltransduction in vertebrates (Schild and Restrepo,1998;Chen et al.,2000;Takeuchi and Kurahashi,2003;Delay and Restrepo,2004;Madrid et al.,2005).Other possible messengers such as InsP 3,NO,and CO are now believed to play modulatory roles for some odorants (Schild and Restrepo,1998;Gold,1999;Chen et al.,2000;Takeuchi and Kurahashi,2003).According to the cAMP-mediated signal transduction cascade,odorant binding to G-protein-coupled receptors stimulates an adenylyl cyclase (type III),and the resulting increase in cAMP directly activates cyclic nucleotide-gated (CNG)channels,leading to an influx of Ca 2+(Schild and Restrepo,1998;Ronnett and Moon,2002).The subsequent rise in the Ca 2+concentration activates Ca 2+-dependent Cl Àconductance,giving depolarizing receptor potential during excitatory responses (Kurahashi and Yau,1993;Lowe and Gold,1993;Reuter et al.,1998;Reisert et al.,2003)or Ca 2+-dependent K +conductance,giving hyperpolarizing receptor/locate/neuresNeuroscience Research 57(2007)129–139*Corresponding author.Tel.:+81117065199;fax:+81117065202.E-mail address:habara@vetmed.hokudai.ac.jp (Y .Habara).0168-0102/$–see front matter #2006Elsevier Ireland Ltd and the Japan Neuroscience Society.All rights reserved.doi:10.1016/j.neures.2006.09.016potential during inhibitory responses(Delgado et al.,2003; Delay and Restrepo,2004;Madrid et al.,2005).Notably,all of these major transduction events are localized to the cilia and knob,the apical portion of the dendrite exposed to the mucus layer in the nasal cavity(Menco et al.,1992;Menco,1997; Lowe and Gold,1991;Schild et al.,1994;Hallani et al.,1998; Kaur et al.,2001;Morales et al.,1997;Gautam et al.,2006). The signal generated at the cilia/knob has to propagate to the soma,the site of action potential generation located deep within the olfactory epithelium,for the transmission of the sensory information from odorous stimuli to the second-order neurons in the specific glomeruli in the olfactory bulb(Narusuye et al., 2003;Lowe,2003).However,the mechanisms underlying the propagation of chemoelectrical signals from the cilia/knob to the soma remain unclear.Experiments carried out in the last decade have also indicated that Ca2+is the sole‘third messenger’of olfactory signal transduction regardless of the possible involvement of multiple second messengers(Schild and Restrepo,1998). However,the role of Ca2+in ORNs is not limited to being the third messenger of signal transduction mediating receptor potential generation,it is,rather involved in the entire sensory process taking place from the cilia to the soma,including negative feedback actions on various stages of the odor transduction mechanism and initiation of the repolarization process(by activating Ca2+-dependent K+channels)after the generation of action potentials(Menini,1999;Matthews and Reisert,2003).Consistent with the complex actions of Ca2+,it has been suggested that Ca2+is tightly regulated in a compartmentalized manner within ORNs(Restrepo et al., 1993;Schild et al.,1994;Tareilus et al.,1995;Leinders-Zufall et al.,1997,1998;Gautam et al.,2006).However,the mechanism underlying the spread of Ca2+transients from the cilia/knob to the soma is not fully understood.So far,based on thefindings from a number of vertebrate species,it is known that Ca2+transients in the soma involve activation of high-voltage-activated(HVA)Ca2+channels(Schild and Restrepo, 1998).In salamander ORNs it has also been reported that store-operated Ca2+release in the soma may amplify the Ca2+ transients primarily evoked by the activation of HVA Ca2+ channels(Zufall et al.,2000).In a recent report,we have shown in rat ORNs that the spread of Ca2+transients from the cilia/knob to the soma can also be a function of the intensity of stimulation(Gautam et al.,2006).Based on the electro-physiological properties low-voltage-activated(LVA)(T-type)Ca2+channels have been reported in newt ORNs (Kawai et al.,1996;Kawai and Miyachi,2001).However,no information is yet available about their localization or on how the presence of T-type Ca2+channels may influence the spatiotemporal dynamics of Ca2+during activation of the signal transduction cascade.The relevance of T-type Ca2+ channels in the ORNs of mammalian species also remains to be investigated.In the present study,using a combination of IBMX(a phosphodiesterase inhibitor)and forskolin(an adenylyl cyclase activator)as the pharmacological activator of the cAMP cascade,and mibefradil and Ni2+as selective inhibitors of T-type Ca2+channels(Perez-Reyes,2003),we herein show how T-type Ca2+channels contribute to the spread of Ca2+ transients from the knob to the soma during activation of the cAMP cascade in isolated pig ORNs.Additionally,the data from the elevated extracellular K+-induced responses are shown to facilitate understanding of the role of T-type Ca2+ channels during activation of the cAMP cascade by IBMX/ forskolin.2.Materials and methods2.1.Isolation of porcine ORNsAll protocols for experiments on animals described in this work were approved by the Animal Research Committee of the Graduate School of Veterinary Medicine,Hokkaido University.Crossbred male piglets(Sus scrofa domestica,LW,4–6weeks after birth,8–12kg)purchased from Nishihara Farm(Rusutsu,Hokkaido,Japan)were housed with food and water ad libitum at room temperature(20–268C)for2–4days before use.Piglets were deeply anaesthetized with pentobarbital sodium(30mg/kg,i.v.,Dainippon Pharma-ceutical Co.Ltd.,Osaka,Japan)after sedation with a combination of mid-azolam(0.5mg/kg,i.m.,Dormicum,Astellas,Tokyo,Japan)and ketamine (10mg/kg,i.m.,Ketalar,Sankyo,Tokyo,Japan);and then sacrificed by exsanguination from the carotid arteries and jugular veins.All efforts were made to minimize animal suffering.The head was quickly separated from the torso,de-skinned and sawed sagittally into two halves to expose the nasal cavity.The nasal septum and turbinates were quickly removed and transferred to ice-cold divalent cation-free Ringer’s solution.Olfactory epithelium was dissected out of the underlying septal and turbinate bone/cartilage followed by mincing with dissecting scissors(1–2mm3)and then digested with 10U per ml papain in divalent cation-free Ringer’s solution for10min at room temperature.The tissue was then transferred to normal Ringer’s solution, washed several times and gently triturated with a disposable plastic Pasteur pipette to dissociate the cells.The cell suspension wasfiltered through a nylon mesh,centrifuged at60Âg for5min and resuspended in1–2ml of normal Ringer’s solution.The data presented in this study were obtained from a total of34isolations(one piglet per isolation).In general,responses from3to4 cells were recorded per isolation and each experiment employed cells from multiple isolations.2.2.Dye loadingTwelve microliters of1mM Fluo-4AM(Molecular Probes,Eugene,OR, USA)in DMSO was mixed with990m l of normal Ringer’s solution by sonication for40–50s.Then1ml of the cell suspension was mixed with the Fluo-4solution to give afinal concentration of6m M Fluo-4AM and loading proceeded for1h at room temperature.The cell suspension was then gently centrifuged,thoroughly washed and resuspended in2ml of normal Ringer’s solution.To begin the experiment,100m l of the suspension of Fluo-4-loaded cells was transferred to Cell-Tak(Becton Dickinson Labware,Bedford,MA,USA)-coated coverslips that were attached to the bottoms of recording chambers (volume100m l)with odourless dental wax.The chambers containing the cell suspension were left undisturbed for about10min and centrifuged at60Âg for 5min.This method was sufficient to ensurefirm attachment of the dendritic knob and soma of each isolated ORN onto the coverslip of the chamber.2.3.Calcium imagingWe recorded changes in the intracellular Ca2+concentration,[Ca2+]i,using a confocal laser scanning imaging system(Fluoview FV500,Olympus,Tokyo, Japan),which allowed recording of the stimulus-induced spatiotemporal dynamics of Ca2+transients in individual ORNs.The recording chamber was set on the stage of an inverted microscope with a perfusion system and the cells were continuously perfused with normal Ringer’s solution at aflow rateS.H.Gautam et al./Neuroscience Research57(2007)129–139 130of2ml per min for10–15min prior to and throughout the experiments.A forced air-cooled argon ion laser beam(488nm)with a total laser output of10mWand linear polarization was used,and the emittedfluorescence(>505nm)was guided through aÂ40water immersion objective to a pinhole diaphragm. Photodamage was minimized by operating the laser at its lowest power setting and by attenuating the laser intensity by interposing a neutral densityfilter(1% transmission)into the illumination path.Isolated individual cells were imaged atÂ4–5magnification with the electronic zoom setting of the imaging software. The resting levelfluorescence intensity was generally lower at the dendrite and knob than at the soma,possibly due to the differences in their optical thicknesses and[Ca2+]i(Restrepo et al.,1993;Leinders-Zufall et al.,1997). The restingfluorescence signal was adjusted to the minimum by changing the PMT sensitivity in such a way that thefluorescence at the knob was still visible. Images were acquired every2s by normal XYT scanning.All the scan settings were kept constant over the series of experiments.To reduce photobleaching, scanning was paused during washings between two successive stimulus appli-cations.All the cells used in this study were clearly identifiable as ORNs by their characteristic morphology,having an oval to round soma with a slender dendrite that ended in a small knob-like swelling.Cilia were too thin to be seen clearly unless magnified above the normal experimental level.Only isolated ORNs maintaining morphological integrity were used for the experi-ments.We discarded the cells in which the knob and soma lay in different focal planes,those lacking proper attachment onto the coverslips of the recording chamber,cells showing a relatively higher level of restingfluor-escence intensity and those with retracted dendrites.Bath solution and the stimulus solutions were superfused by gravity feed from the reservoirs. Stimulus solutions were pulse-applied by switching the bath solution;this allowed a complete change of bath solution within3–4s.For the experiments involving repetitive stimulations the interval between the successive stimula-tions was set at more than3min to ensure that the cells were not desensitized by the previous stimulation.2.4.ChemicalsNormal Ringer’s solution contained(in mM)138NaCl,5KCl,2CaCl2,1 MgCl2,10HEPES,10glucose and1Na-pyruvate supplemented with0.1% BSA(pH7.4).For elevated extracellular K+solutions,NaCl was replaced with an equimolar concentration of KCl.For divalent cation-free isolation solution, CaCl2and MgCl2were omitted and replaced with1mM EDTA(pH7.4). Forskolin,Na-pyruvate,3-isobutyl-1-methyl-xanthine(IBMX),and mibefradil were purchased from Sigma Chemical Co.(St.Louis,MO,USA).Other chemicals were obtained from Wako Pure Chemicals(Osaka,Japan)unless otherwise specified.2.5.Data analysisInitially the time courses of the changes influorescence intensities at the selected regions of ORNs were recorded in arbitrary units using Fluoview4.2 with Tiempo(Olympus,Tokyo,Japan).These data were then converted to relative changes influorescence intensity(%D F/F),where D F and F refer to the stimulus-induced change in thefluorescence and the resting-levelfluores-cence,respectively.The averagefluorescence intensity during20–30s prior to the stimulus application was taken as the resting-levelfluorescence.The magnitude of the response was assessed by taking the peak%D F/F values during stimulation.The average results are expressed as meansÆS.E.of independent experiments(n),where n refers to the number of ORNs.Statistical analysis was done using the paired and unpaired Student’s t-tests.Results were considered significant at P<0.05.3.Results3.1.Dissociated porcine ORNsThe population of dissociated pig ORNs consisted of cells with dendrites of various lengths(Fig.1),probably representing their stage of maturity and/or position of the soma within the olfactory epithelium.Since the cilia were hardly visible under the normal scanning mode set for Ca2+imaging,the brightness and contrast of these images were adjusted later using Adobe Photoshop to assure the visibility of the cilia.While the majority of the cells were relatively small in size(Fig.1A–C), some of them were unusually large(Fig.1D).We could not record responses from such large cells since none of them was viable at the time of imaging.As ORNs senesce and die at a regular rate,the large cells possibly represented old cells approaching degeneration,and hence their vulnerability to the experimental manipulations.3.2.IBMX/forskolin-and slightly elevated extracellularK+-induced Ca2+transientsFig.2A(a)shows a transmission image of an isolated pig ORN used for an experiment and Fig.2A(b–h)pseudocolour images of this cell at various time points during the course of the experiment as indicated in Fig.2B.Fig.2B shows the time courses of the changes in the intracellular Ca2+concentration in response to30s pulse stimulation with10m M IBMX plus 1m M forskolin(Fig.2B,left)and10mM K+(Fig.2B,right). The IBMX/forskolin-induced increase in thefluorescence intensity appearedfirst at the knob(Fig.2A(c)and B),and then extended to the dendrite followed by the soma(Fig.2B).The elevated extracellular K+-induced response,however,showed simultaneous onset of the responses at the knob,dendrite and soma(Fig.2B,right).3.3.Effects of T-type Ca2+channel inhibitors on IBMX/ forskolin-induced Ca2+transients3.3.1.Effect of Ni2+Micromolar concentrations of Ni2+(such as100m M)are known to selectively inhibit T-type Ca2+channels in various mammalian cell types(Perez-Reyes,2003).Kawai et al.(1996) also demonstrated the specificity of Ni2+on T-type Ca2+ channels in newt ORNs.We used100m M Ni2+to test the involvement of T-type Ca2+channels in the spatiotemporal dynamics of[Ca2+]i during IBMX/forskolin-induced activation of the cAMP cascade in pig ORNs(Fig.3).The presence of Ni2+in the bath solution1min prior to and during the test stimulations inhibited IBMX/forskolin-induced Ca2+transients in two different ways:(1)In5of12cells,the presence of Ni2+ almost completely abolished the response at the dendrite and soma,resulting in a knob-confined response with reduced magnitude(Type-I inhibition).(2)In the remaining7cells, however,the Ca2+transients were only partially inhibited throughout the knob,dendrite and soma(Type-II inhibition). Fig.3A(a)shows an example of Ni2+-induced Type-I inhibition, and Fig.3A(b)summarizes the results fromfive such experiments in which the extents of inhibition in the knob and soma were54Æ9%(P<0.05)and95Æ2%(P<0.001), respectively.The extent of inhibition at the dendrite was similar to that at the soma(data not shown).Likewise,Fig.3B(a)shows an example of Ni2+-induced Type-II inhibition,and Fig.3B(b)S.H.Gautam et al./Neuroscience Research57(2007)129–139131summarizes the results from seven such experiments where the extents of inhibition in the knob and soma were 48Æ9%(P <0.01)and 64Æ4%(P <0.001),respectively.Taking all 12cells together,Ni 2+inhibited the IBMX/forskolin-induced Ca 2+transients by 50Æ6%and 77Æ5%in the knob and soma,respectively.3.3.2.Effect of mibefradilMibefradil was basically developed as a specific blocker of T-type Ca 2+channels (Clozel et al.,1997),and now is a well-known selective nondihydropyridine blocker of native as well as recombinant T-type channels (Mishra and Hermsmeyer,1994;Cribbs et al.,1998;Martin et al.,2000;Michels et al.,2002;Perez-Reyes,2003).While T-type Ca 2+channels may differ considerably in their pharmacological properties depending on the tissue or cell type (Huguenard,1996),10m M mibefradil has been shown to completely inhibit LVA Ca 2+currents/transients in dissociated rat retinal bipolar cells (Pan et al.,2001).In the present study also,10m M mibefradil was sufficient to almost completely abolish 7.5or 10mM K +-induced Ca 2+transients (Section 3.4.1).Hence,we examined the effect of 10m M mibefradil on the spatiotemporal dynamics of [Ca 2+]i during IBMX/forskolin-induced activation of the cAMP cascade (Fig.4).As in the case of Ni 2+,the presence ofmibefradil in the bath solution 1min prior to and during the test stimulations caused Type-I inhibition in 7cells and Type-II inhibition in the other 6of the 13cells tested.Fig.4A(a)shows an example of mibefradil-induced Type-I inhibition,and Fig.4A(b)summarizes the results from seven such experi-ments where the extents of inhibition in the knob and soma were 49Æ8%(P <0.01)and 93Æ2%(P <0.001),respec-tively.In a similar way,Fig.4B(a)shows an example of mibefradil-induced Type-II inhibition,and Fig.4B(b)sum-marizes the results from six such experiments where the extents of inhibition in the knob and soma were 52Æ8%(P <0.01)and 66Æ7%(P <0.001),respectively.Taking all 13experiments together,mibefradil inhibited IBMX/forsko-lin-induced Ca 2+transients by 50Æ5%and 81Æ5%in the knob and soma,respectively.parison of the response susceptible to Type-I inhibition and that susceptible to Type-II inhibition by Ni 2+or mibefradilTo understand why the presence of Ni 2+or mibefradil during IBMX/forskolin-induced responses caused some ORNs to exhibit Type-I inhibition while others showed Type-II inhibition,we compared the averaged peak amplitude of the responses susceptible to Type-I inhibition with that susceptibleS.H.Gautam et al./Neuroscience Research 57(2007)129–139132Fig.1.Representative examples of cells from the population of isolated pig olfactory receptor neurons (ORNs).The majority of ORNs had a small soma with dendrites of various lengths (A–C).Scale bar =10m m.Some ORNs were unusually large (D).Contrast and brightness were adjusted using Adobe Photoshop to increase visibility of the cilia.to Type-II inhibition,combining the data from Ni2+and mibefradil(Fig.5).The amplitude of the response susceptible to Type-II inhibition(n=13)was significantly higher than that susceptible to Type-I inhibition(n=12)(P<0.01,knob; P<0.001,soma).It was,thus,revealed that cells showing relatively smaller responses were susceptible to Type-I inhibition whereas those showing larger ones were susceptible to Type-II inhibition.3.4.Ca2+transients induced by7.5mM K+3.4.1.Effect of mibefradilThe responsiveness of pig ORNs to a slightly elevated extracellular K+concentration(10mM K+)has already been shown in Fig.2.We found that even7.5mM K+was enough to induce significant Ca2+transients in pig ORNs.Considering the magnitude of depolarization likely to be induced by such a small increase in the extracellular K+concentration,we suspected that such responses should be mediated,almost exclusively,by T-type Ca2+channels.Hence,we studied the effects of different concentrations of mibefradil on7.5mM K+-induced responses to determine the optimum dose of mibefradil to inhibit T-type Ca2+channels in isolated pig ORNs. Mibefradil,perfused for1min prior to and during the test stimulations,reversibly reduced the Ca2+transients evoked by 7.5mM K+in a dose-dependent manner.An example of the effect of4,7and10m M mibefradil on7.5mM K+-induced Ca2+transients is shown in Fig.6A.The effect of4m M mibefradil was examined in six cells that were also used to observe the effect of7m M mibefradil as shown in Fig.6A(a). The effect of7m M mibefradil was also examined in11other cells.The effect of10m M mibefradil was recorded for16cells. The effects of the different concentrations of mibefradil are summarized in Fig.6B.The extent of inhibition increased from 4m M(40Æ11%,knob;42Æ7%,soma;P<0.01)to7m M (79Æ16%,knob;76Æ12%,soma;P<0.001)and at10m M the responses were almost completely abolished(96Æ2%, knob;94Æ2%,soma;P<0.001).S.H.Gautam et al./Neuroscience Research57(2007)129–139133Fig.2.Confocal Ca2+imaging of a Fluo-4-loaded pig ORN.(A)(a)Transmission image of an isolated ORN.The black-dotted markings over the knob,dendrite and soma correspond to the area analyzed for the time courses shown in(B).Scale bar=5m m.(b–h)Pseudocolourfluorescence images of the cell at various time points as depicted in(B).The IBMX/Fk-induced increase in thefluorescence intensity appearedfirst at the knob(c),followed by the dendrite and soma(d).(B)Time courses of Ca2+transients at the knob(black trace),dendrite(light grey trace)and soma(dark grey trace).Note that unlike the IBMX/forskolin-induced response,the10mM K+-induced response showed simultaneous onset of the responses at the knob,dendrite and soma.Stimulus was applied for30s.IBMX/Fk,10m M IBMX plus1m M forskolin.134S.H.Gautam et al./Neuroscience Research57(2007)129–139Fig.3.Effects of100m M Ni2+on IBMX/forskolin-induced Ca2+transients.(A)Type-I inhibition.(a)A representative example of Type-I inhibition;that is,complete abolishment of Ca2+transients at the soma(grey trace)with partial inhibition at the knob(black trace).(b)Average peak amplitudes of Ca2+transients from5ORNs showing Type-I inhibition.(B)Type-II inhibition.(a)A representative example of Type-II inhibition;that is,partial inhibition of Ca2+transients at both the soma(grey trace)and the knob(black trace).(b)Averaged peak amplitudes of Ca2+transients from7ORNs showing Type-II inhibition.Stimulus was applied for20s.*P<0.05, **P<0.01,***P<0.001paired Student’s t-test vs.control.Fig.4.Effects of10m M mibefradil on IBMX/forskolin-induced Ca2+transients.(A)Type-I inhibition.(a)A representative example of Type-I inhibition;that is, complete abolishment of Ca2+transients at the soma(grey trace)with partial inhibition at the knob(black trace).(b)Average peak amplitudes of Ca2+transients from 7ORNs showing Type-I inhibition.(B)Type-II inhibition.(a)A representative example of Type-II inhibition;that is,partial inhibition of Ca2+transients at both the soma(grey trace)and the knob(black trace).(b)Averaged peak amplitudes of Ca2+transients from6ORNs showing Type-II inhibition.Stimulus was applied for20s. **P<0.01,***P<0.001paired Student’s t-test vs.control.3.4.2.Effect of Ni 2+Like mibefradil,Ni 2+almost completely abolished the 7.5mM K +-induced Ca 2+responses in ORNs (Fig.7).The effect of Ni 2+was examined in 14cells as shown in Fig.7A.On average,Ni 2+reduced the 7.5mM K +-induced response by 93Æ3%and 94Æ2%(P <0.0001)in the knob and soma,respectively (Fig.7B).3.5.Effects of mibefradil and Ni 2+on 30mM K +-induced Ca 2+transientsTo assess the contribution of T-type Ca 2+channels to the Ca 2+transients evoked during moderate depolarization of ORNs we examined the effects of mibefradil and Ni 2+on 30mM K +-induced Ca 2+transients.Fig.8shows repre-sentative examples and the averaged results of the experi-ments performed.Mibefradil (10m M)reduced the peak amplitudes of 30mM K +-induced Ca 2+transients by 64Æ5%and 62Æ3%(n =9;P <0.001)in the knob and soma,respectively (Fig.8A).Likewise,Ni 2+inhibited the peak amplitudes of the response by 60Æ6%and 58Æ3%(n =8;P <0.01)in the knob and soma,respectively (Fig.8B).Cells were exposed to 30mM K +for 20s and the drugs were applied for 1min prior to and during the test stimulations.3.6.Effects of mibefradil and Ni 2+on 100mM K +-induced Ca 2+transientsTo demonstrate the specificities of 10m M mibefradil and 100m M Ni 2+for the low-voltage-activated T-type Ca 2+channels in the isolated pig ORNs,we next examined the effects of these drugs on Ca 2+transients induced by 100mM K +(Fig.9).Consistent with the well-known specificities ofmibefradil and Ni 2+for T-type Ca 2+channels,these drugs had no effect on 100mM K +-induced Ca 2+transients while the same drugs almost completely abolished the 10mM K +-induced Ca 2+transients in the same ORNs.Similar results were obtained with four cells tested for Ni 2+and 5cells tested for mibefradil.Cells were exposed to 10mM K +for 20s and to 100mM K +for 6s.The traces shown in Fig.9represent means of the responses at the knob and soma.The drugs were applied in the same way as in other experiments described above.S.H.Gautam et al./Neuroscience Research 57(2007)129–139135Fig.5.Cells showing relatively small responses were susceptible to Type-I inhibition by Ni 2+or mibefradil.The Averaged peak amplitudes of IBMX/forskolin-induced Ca 2+transients that were susceptible to Type-I inhibition (n =12;5,Ni 2+and 7,mibefradil)were compared with those susceptible to Type-II inhibition (n =13;7,Ni 2+and 6,mibefradil).**P <0.01,***P <0.001unpaired Student’s t-test.Fig.6.Effects of mibefradil on 7.5mM K +-induced Ca 2+transients.(A)Representative time courses corresponding to the knob (black trace)and soma (grey trace)in the presence and absence of 4or 7m M (a),or 10m M (b)mibefradil.(B)Averaged peak amplitudes of Ca 2+transients in the presence and absence of 4m M (n =6),7m M (n =17)or 10m M (n =16)mibefradil.Stimulus was applied for 20s.Cells were bathed in normal Ringer’s solution containing 5mM K +.**P <0.01,***P <0.001paired Student’s t -test vs.control.。
