Protriptyline_hydrochloride_DataSheet_MedChemExpress
Tapentadol_hydrochloride_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Aug.-29-2017Print Date:Aug.-29-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Tapentadol (hydrochloride)Catalog No. :HY-70042ACAS No. :175591-09-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C14H24ClNOMolecular Weight:257.80CAS No. :175591-09-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
LUNA SENSATION 藻类杀菌剂数据安全表说明书

LUNA SENSATION®1/11Version 4.0 / USA Revision Date: 06/30/2020102000012886 Print Date: 06/30/2020SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKINGProduct identifierTrade nameLUNA SENSATION®Product code (UVP)84469882SDS Number102000012886EPA Registration No. 264-1090Relevant identified uses of the substance or mixture and uses advised againstUseFungicideRestrictions on useSee product label for restrictions.Information on supplierSupplier Bayer CropScience LP 800 North Lindbergh Blvd. St. Louis, MO 63167 USAResponsible DepartmentEmail: ************************Emergency telephone no.Emergency Telephone Number (24hr/ 7 days)1-800-334-7577Product Information Telephone Number 1-866-99BAYER (1-866-992-2937)SECTION 2: HAZARDS IDENTIFICATIONClassification in accordance with regulation HCS 29CFR §1910.1200 Acute toxicity(Oral): Category 4Reproductive toxicity: Effects on or via lactationLabelling in accordance with regulation HCS 29CFR §1910.1200Signal word : WarningHazard statementsHarmful if swallowed.May cause harm to breast-fed children.LUNA SENSATION®2/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Precautionary statementsWash thoroughly after handling.Do not eat, drink or smoke when using this product.Obtain special instructions before use.Do not breathe mist.Avoid contact during pregnancy/ while nursing.IF SWALLOWED: Call a POISON CENTER/doctor/physician if you feel unwell.Rinse mouth.IF exposed or concerned: Get medical advice/ attention.Dispose of contents/container in accordance with local regulation.Hazards Not Otherwise Classified (HNOC)No physical hazards not otherwise classified.No health hazards not otherwise classified.SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTSHazardous Component Name CAS-No.Concentration % by weight Fluopyram 658066-35-4 21.4 Trifloxystrobin 141517-21-7 21.4 SECTION 4: FIRST AID MEASURESDescription of first aid measuresGeneral advice When possible, have the product container or label with you whencalling a poison control center or doctor or going for treatment.Inhalation Move to fresh air. If person is not breathing, call 911 or an ambulance,then give artificial respiration, preferably mouth-to-mouth if possible.Call a physician or poison control center immediately.Skin contact Take off contaminated clothing and shoes immediately.Wash offimmediately with plenty of water for at least 15 minutes.Call aphysician or poison control center immediately.Eye contact Hold eye open and rinse slowly and gently with water for 15-20minutes.Remove contact lenses, if present, after the first 5 minutes,then continue rinsing eye.Call a physician or poison control centerimmediately.Ingestion Call a physician or poison control center immediately.Rinse out mouthand give water in small sips to drink.DO NOT induce vomiting unlessdirected to do so by a physician or poison control center.Never giveanything by mouth to an unconscious person.Do not leave victimunattended.Most important symptoms and effects, both acute and delayedSymptoms To date no symptoms are known.Indication of any immediate medical attention and special treatment neededLUNA SENSATION®3/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Treatment Appropriate supportive and symptomatic treatment as indicated by thepatient's condition is recommended.SECTION 5: FIREFIGHTING MEASURESExtinguishing mediaSuitable Water spray, Carbon dioxide (CO2), Alcohol-resistant foam, Sand Unsuitable High volume water jetSpecial hazards arising from the substance or mixture In the event of fire the following may be released:, Hydrogen chloride (HCl), Hydrogen cyanide (hydrocyanic acid), Hydrogen fluoride, Carbon monoxide (CO), Carbon dioxide (CO2), Nitrogen oxides (NOx)Advice for firefightersSpecial protective equipment for firefighters Firefighters should wear NIOSH approved self-contained breathing apparatus and full protective clothing.Further information Keep out of smoke. Fight fire from upwind position. Cool closedcontainers exposed to fire with water spray. Do not allow run-off fromfire fighting to enter drains or water courses.Flash point> 100 °CAuto-ignition temperature 380 °C / 716 °FLower explosion limit No data availableUpper explosion limit No data availableExplosivity Not explosive92/69/EEC, A.14 / OECD 113SECTION 6: ACCIDENTAL RELEASE MEASURESPersonal precautions, protective equipment and emergency proceduresPrecautions Keep unauthorized people away. Isolate hazard area. Avoid contactwith spilled product or contaminated surfaces.Methods and materials for containment and cleaning upMethods for cleaning up Soak up with inert absorbent material (e.g. sand, silica gel, acidbinder, universal binder, sawdust). Clean contaminated floors andobjects thoroughly, observing environmental regulations. Collect andtransfer the product into a properly labelled and tightly closedcontainer.Additional advice Use personal protective equipment. If the product is accidentallyspilled, do not allow to enter soil, waterways or waste water canal.LUNA SENSATION®4/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Reference to other sections Information regarding safe handling, see section 7.Information regarding personal protective equipment, see section 8.Information regarding waste disposal, see section 13.SECTION 7: HANDLING AND STORAGEPrecautions for safe handlingAdvice on safe handling Use only in area provided with appropriate exhaust ventilation. Handleand open container in a manner as to prevent spillage.Hygiene measures Wash hands thoroughly with soap and water after handling and beforeeating, drinking, chewing gum, using tobacco, using the toilet orapplying cosmetics.Remove Personal Protective Equipment (PPE) immediately afterhandling this product. Before removing gloves clean them with soap andwater. Remove soiled clothing immediately and clean thoroughly beforeusing again. Wash thoroughly with soap and water after handling. Conditions for safe storage, including any incompatibilitiesRequirements for storage areas and containers Store in a cool, dry place and in such a manner as to prevent cross contamination with other crop protection products, fertilizers, food, and feed. Store in original container and out of the reach of children, preferably in a locked storage area. Protect from freezing. Keep away from direct sunlight.SECTION 8: EXPOSURE CONTROLS/PERSONAL PROTECTIONControl parameters*OES BCS: Internal Bayer AG, Crop Science Division "Occupational Exposure Standard"Exposure controlsPersonal protective equipmentIn normal use and handling conditions please refer to the label and/or leaflet. In all other cases the following recommendations would apply.Respiratory protection When respirators are required, select NIOSH approved equipmentbased on actual or potential airborne concentrations and inaccordance with the appropriate regulatory standards and/or industryrecommendations.Hand protection Chemical resistant nitrile rubber glovesEye protection Safety glasses with side-shieldsLUNA SENSATION®5/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Skin and body protection Wear long-sleeved shirt and long pants and shoes plus socks. General protective measures Follow manufacturer's instructions for cleaning/maintaining PPE. Ifno such instructions for washables, use detergent and warm/tepidwater.Keep and wash PPE separately from other laundry.SECTION 9. PHYSICAL AND CHEMICAL PROPERTIESAppearance white to beigePhysical State suspensionOdor characteristicOdour Threshold No data availablepH 5.0 - 8.0 (100 %) (23 °C)Viscosity, kinematic No data availableVapor Pressure No data availableVapor Density (Air = 1)No data availableDensity ca. 1.17 g/cm³ (20 °C)Evaporation rate No data availableBoiling Point No data availableMelting / Freezing Point No data availableWater solubility suspensiveMinimum Ignition Energy Not applicableDecompositionStable under normal conditions.temperatureSelf-accelaratingNo data availabledecomposition temperature(SADT)Partition coefficient: n-Not applicableoctanol/waterViscosity240 - 350 mPa.s (20 °C) Velocity gradient 20 /sFlammability No data availableOxidizing properties No oxidizing propertiesFlash point> 100 °CAuto-ignition temperature 380 °C / 716 °FLower explosion limit No data availableUpper explosion limit No data availableLUNA SENSATION®6/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Explosivity Not explosive92/69/EEC, A.14 / OECD 113Particle size No data availableOther information Further safety related physical-chemical data are not known.SECTION 10: STABILITY AND REACTIVITYReactivityThermal decomposition Stable under normal conditions.Chemical stability Stable under recommended storage conditions.Possibility of hazardous reactions No hazardous reactions when stored and handled according to prescribed instructions.Conditions to avoid Extremes of temperature and direct sunlight.Incompatible materials No incompatible materials known.Hazardous decompositionproductsNo decomposition products expected under normal conditions of use. SECTION 11: TOXICOLOGICAL INFORMATIONExposure routes Skin Absorption, Ingestion, Inhalation, Eye contactImmediate EffectsSkin Harmful if absorbed through skin.Ingestion Harmful if swallowed.Inhalation Harmful if inhaled.Information on toxicological effectsAcute oral toxicity LD50 (female Rat) 2,000 mg/kgAcute inhalation toxicity LC50 (Rat) > 1.7 mg/lExposure time: 4 hDetermined in the form of liquid aerosol.Highest attainable concentration.No deathsAcute dermal toxicity LD50 (Rat) > 2,000 mg/kgSkin corrosion/irritation No skin irritation (Rabbit)Serious eye damage/eyeirritationNo eye irritation (Rabbit)LUNA SENSATION®7/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020Respiratory or skin sensitisation Skin: Non-sensitizing. (Mouse)OECD Test Guideline 429, local lymph node assay (LLNA)Assessment STOT Specific target organ toxicity – single exposureFluopyram: Based on available data, the classification criteria are not met.Trifloxystrobin: Based on available data, the classification criteria are not met.Assessment STOT Specific target organ toxicity – repeated exposureFluopyram did not cause specific target organ toxicity in experimental animal studies.Trifloxystrobin did not cause specific target organ toxicity in experimental animal studies.Assessment mutagenicityFluopyram was not mutagenic or genotoxic in a battery of in vitro and in vivo tests.Trifloxystrobin was not mutagenic or genotoxic in a battery of in vitro and in vivo tests.Assessment carcinogenicityFluopyram caused at high dose levels an increased incidence of tumours in rats in the followingorgan(s): Liver.Fluopyram caused at high dose levels an increased incidence of tumours in mice in the followingorgan(s): Thyroid.The tumours seen with Fluopyram were caused through a non-genotoxic mechanism, which is not relevant at low doses. The mechanism that triggers these tumours is not relevant to humans. Trifloxystrobin was not carcinogenic in lifetime feeding studies in rats and mice.ACGIHNone.NTPNone.IARCNone.OSHANone.Assessment toxicity to reproductionFluopyram caused reproduction toxicity in a two-generation study in rats only at dose levels also toxic to the parent animals. The reproduction toxicity seen with Fluopyram is related to parental toxicity. Trifloxystrobin caused reduced body weight development in offspring during lactation only at doses also producing systemic toxicity in adult rats.Assessment developmental toxicityFluopyram caused developmental toxicity only at dose levels toxic to the dams. The developmental effects seen with Fluopyram are related to maternal toxicity.Trifloxystrobin caused developmental toxicity only at dose levels toxic to the dams. The developmental effects seen with Trifloxystrobin are related to maternal toxicity.Aspiration hazardBased on available data, the classification criteria are not met.LUNA SENSATION®8/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Further informationOnly acute toxicity studies have been performed on the formulated product.The non-acute information pertains to the active ingredient(s).SECTION 12: ECOLOGICAL INFORMATIONToxicity to fish LC50 (Oncorhynchus mykiss (rainbow trout)) 0.091 mg/lExposure time: 96 hToxicity to aquatic invertebratesEC50 (Daphnia magna (Water flea)) 0.086 mg/lExposure time: 48 hLC50 (Mysidopsis bahia (mysid shrimp)) 0.00862 mg/l Exposure time: 96 hThe value mentioned relates to the active ingredient trifloxystrobin.Toxicity to aquatic plants IC50 (Raphidocelis subcapitata (freshwater green alga)) 0.292 mg/lGrowth rate; Exposure time: 72 hEC10 (Desmodesmus subspicatus (green algae)) 0.0025 mg/lGrowth rate; Exposure time: 72 hThe value mentioned relates to the active ingredient trifloxystrobin. Biodegradability Fluopyram:Not rapidly biodegradableTrifloxystrobin:Not rapidly biodegradableKoc Fluopyram: Koc: 279Trifloxystrobin: Koc: 2377Bioaccumulation Fluopyram: Bioconcentration factor (BCF) 18Does not bioaccumulate.Trifloxystrobin: Bioconcentration factor (BCF) 431Does not bioaccumulate.Mobility in soil Fluopyram: Moderately mobile in soilsTrifloxystrobin: Slightly mobile in soilsAdditional ecologicalinformationNo other effects to be mentioned.Environmental precautions Do not apply directly to water, to areas where surface water is presentor to intertidal areas below the mean high water mark.Drift and runoff from treated areas may be hazardous to aquaticorganisms in adjacent sites.Do not apply when weather conditions favor runoff or drift.Do not allow product to enter streams, sewers or other waterways.Do not contaminate surface or ground water by cleaning equipment ordisposal of wastes, including equipment wash water.Apply this product as specified on the label.LUNA SENSATION®9/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 SECTION 13: DISPOSAL CONSIDERATIONSWaste treatment methodsProduct Dispose in accordance with all local, state/provincial and federalregulations.Pesticide, spray mixture or rinse water that cannot be used according tolabel instructions may be disposed of on site or at an approved wastedisposal facility.Follow advice on product label and/or leaflet.Contaminated packaging Do not re-use empty containers.Triple rinse containers.Completely empty container into application equipment, then dispose ofempty container in a sanitary landfill, by incineration or by otherprocedures approved by state/provincial and local authorities.If burned, stay out of smoke.Follow advice on product label and/or leaflet.RCRA Information Characterization and proper disposal of this material as a special orhazardous waste is dependent upon Federal, State and local laws andare the user's responsibility. RCRA classification may apply.SECTION 14: TRANSPORT INFORMATION49CFR Not dangerous goods / not hazardous materialIMDGUN number 3082Class 9Packaging group IIIMarine pollutant YESProper shipping name ENVIRONMENTALLY HAZARDOUS SUBSTANCE, LIQUID,N.O.S.(TRIFLOXYSTROBIN SOLUTION)IATAUN number 3082Class 9Packaging group IIIEnvironm. Hazardous Mark YESProper shipping name ENVIRONMENTALLY HAZARDOUS SUBSTANCE, LIQUID,N.O.S.(TRIFLOXYSTROBIN SOLUTION )This transportation information is not intended to convey all specific regulatory information relating to this product. It does not address regulatory variations due to package size or special transportation requirements.LUNA SENSATION®10/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Freight Classification: INSECTICIDES OR FUNGICIDES, N.O.I., OTHER THANPOISONSECTION 15: REGULATORY INFORMATIONEPA Registration No.264-1090US Federal RegulationsTSCA listWater 7732-18-51,2-Propanediol 57-55-6Polyethylene-polypropylene copolymer 9003-11-6US. Toxic Substances Control Act (TSCA) Section 12(b) Export Notification (40 CFR 707, Subpt D) No export notification needs to be made.SARA Title III - Section 302 - Notification and InformationNot applicable.SARA Title III - Section 313 - Toxic Chemical Release ReportingNone.US States Regulatory ReportingCA Prop65This product does not contain any substances known to the State of California to cause cancer.This product does not contain any substances known to the State of California to causereproductive harm.US State Right-To-Know Ingredients1,2-Propanediol 57-55-6 MN, RINone.EPA/FIFRA Information:This chemical is a pesticide product registered by the Environmental Protection Agency and is subject to certain labeling requirements under federal pesticide law. These requirements differ from the classification criteria and hazard information required for safety data sheets, and for workplace labels of non-pesticide chemicals. Following is the hazard information required on the pesticide label:Signal word:Caution!Hazard statements:Harmful if swallowed, inhaled or absorbed through the skin.Avoid contact with skin, eyes and clothing.Avoid inhalation of vapour or mist.SAFETY DATA SHEETLUNA SENSATION®11/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 SECTION 16: OTHER INFORMATIONAbbreviations and acronyms49CFR Code of Federal Regulations, Title 49ACGIH US. ACGIH Threshold Limit ValuesATE Acute toxicity estimateCAS-Nr. Chemical Abstracts Service numberCERCLA Comprehensive Environmental Response, Compensation, and Liability Act EINECS European inventory of existing commercial substancesELINCS European list of notified chemical substancesIARC International Agency for Research on CancerIATA International Air Transport AssociationIMDG International Maritime Dangerous GoodsN.O.S. Not otherwise specifiedNTP US. National Toxicology Program (NTP) Report on CarcinogensOECD Organization for Economic Co-operation and DevelopmentTDG Transportation of Dangerous GoodsTWA Time weighted averageUN United NationsWHO World health organisationNFPA 704 (National Fire Protection Association):Health - 2 Flammability - 1 Instability - 0 Others - noneHMIS (Hazardous Materials Identification System, based on the Third Edition Ratings Guide) Health - 2 Flammability - 1 Physical Hazard - 0 PPE -0 = minimal hazard, 1 = slight hazard, 2 = moderate hazard, 3 = severe hazard, 4 = extreme hazard Reason for Revision: The following sections have been revised: Section 2: Hazards Identification. Section 3: Composition / Information on Ingredients. Section 11: Toxicological Information. Section 12. Ecological information. Reviewed and updated for general editorial purposes.Revision Date: 06/30/2020This information is provided in good faith but without express or implied warranty. The customer assumes all responsibility for safety and use not in accordance with label instructions. The product names are registered trademarks of Bayer.。
循能泰说明书(己酮可可碱外文说明书)
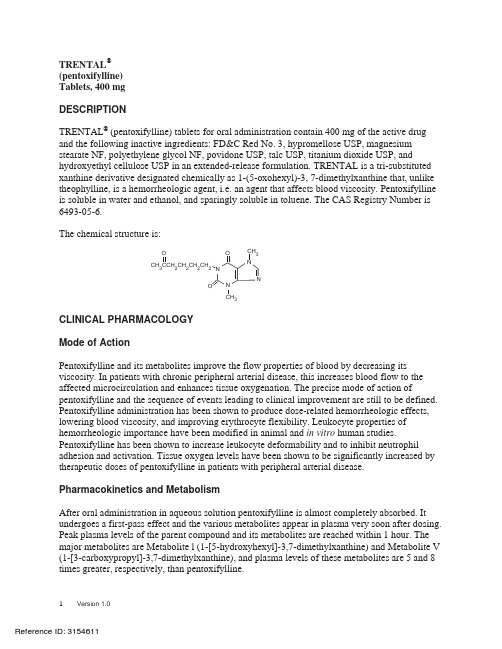
OCH CCH CH CH CH 32223 TRENTAL ®(pentoxifylline)Tablets, 400 mg DESCRIPTIONTRENTAL ® (pentoxifylline) tablets for oral administration contain 400 mg of the active drugand the following inactive ingredients: FD&C Red No. 3, hypromellose USP, magnesium stearate NF, polyethylene glycol NF, povidone USP, talc USP, titanium dioxide USP, andhydroxyethyl cellulose USP in an extended-release formulation. TRENTAL is a tri-substitutedxanthine derivative designated chemically as 1-(5-oxohexyl)-3, 7-dimethylxanthine that, unliketheophylline, is a hemorrheologic agent, i.e. an agent that affects blood viscosity. Pentoxifyllineis soluble in water and ethanol, and sparingly soluble in toluene. The CAS Registry Number is6493-05-6.The chemical structure is:CLINICAL PHARMACOLOGYMode of ActionPentoxifylline and its metabolites improve the flow properties of blood by decreasing itsviscosity. In patients with chronic peripheral arterial disease, this increases blood flow to theaffected microcirculation and enhances tissue oxygenation. The precise mode of action ofpentoxifylline and the sequence of events leading to clinical improvement are still to be defined.Pentoxifylline administration has been shown to produce dose-related hemorrheologic effects,lowering blood viscosity, and improving erythrocyte flexibility. Leukocyte properties of hemorrheologic importance have been modified in animal and in vitro human studies.Pentoxifylline has been shown to increase leukocyte deformability and to inhibit neutrophiladhesion and activation. Tissue oxygen levels have been shown to be significantly increased bytherapeutic doses of pentoxifylline in patients with peripheral arterial disease.Pharmacokinetics and MetabolismAfter oral administration in aqueous solution pentoxifylline is almost completely absorbed. Itundergoes a first-pass effect and the various metabolites appear in plasma very soon after dosing.Peak plasma levels of the parent compound and its metabolites are reached within 1 hour. Themajor metabolites are Metabolite l (1-[5-hydroxyhexyl]-3,7-dimethylxanthine) and Metabolite V(1-[3-carboxypropyl]-3,7-dimethylxanthine), and plasma levels of these metabolites are 5 and 8times greater, respectively, than pentoxifylline.Following oral administration of aqueous solutions containing 100 to 400 mg of pentoxifylline, the pharmacokinetics of the parent compound and Metabolite l are dose-related and not proportional (non-linear), with half-life and area under the blood-level time curve (AUC) increasing with dose. The elimination kinetics of Metabolite V are not dose-dependent. The apparent plasma half-life of pentoxifylline varies from 0.4 to 0.8 hours and the apparent plasma half-lives of its metabolites vary from 1 to 1.6 hours. There is no evidence of accumulation or enzyme induction (Cytochrome P450) following multiple oral doses.Excretion is almost totally urinary; the main biotransformation product is Metabolite V. Essentially no parent drug is found in the urine. Despite large variations in plasma levels of parent compound and its metabolites, the urinary recovery of Metabolite V is consistent and shows dose proportionality. Less than 4% of the administered dose is recovered in feces. Food intake shortly before dosing delays absorption of an immediate-release dosage form but does not affect total absorption. The pharmacokinetics and metabolism of TRENTAL have not been studied in patients with renal and/or hepatic dysfunction. The pentoxifylline AUC was increased and elimination rate decreased in an older population (60-68 years, n=6) compared to younger individuals (22-30 years, n=6) (see PRECAUTIONS, Geriatric Use).After administration of the 400 mg extended-release TRENTAL tablet, plasma levels of the parent compound and its metabolites reach their maximum within 2 to 4 hours and remain constant over an extended period of time. Coadministration of TRENTAL tablets with meals resulted in an increase in mean C max and AUC by about 28% and 13% for pentoxifylline, respectively. C max for Metabolite 1 also increased by about 20%. The extended release of pentoxifylline from the tablet eliminates peaks and troughs in plasma levels for improved gastrointestinal tolerance.INDICATIONS AND USAGETRENTAL is indicated for the treatment of patients with intermittent claudication on the basis of chronic occlusive arterial disease of the limbs. TRENTAL can improve function and symptoms but is not intended to replace more definitive therapy, such as surgical bypass, or removal of arterial obstructions when treating peripheral vascular disease.CONTRAINDICATIONSTRENTAL should not be used in patients with recent cerebral and/or retinal hemorrhage or in patients who have previously exhibited intolerance to this product or methylxanthines such as caffeine, theophylline, and theobromine.PRECAUTIONSGeneralAt the first sign of anaphylactic/anaphylactoid reaction, TRENTAL must be discontinued.Patients with chronic occlusive arterial disease of the limbs frequently show other manifestations of arteriosclerotic disease. TRENTAL has been used safely for treatment of peripheral arterial disease in patients with concurrent coronary artery and cerebrovascular diseases, but there have been occasional reports of angina, hypotension, and arrhythmia. Controlled trials do not show that TRENTAL causes such adverse effects more often than placebo, but, as it is a methylxanthine derivative, it is possible some individuals will experience such responses. Patients on Warfarin should have more frequent monitoring of prothrombin times, while patients with other risk factors complicated by hemorrhage (e.g., recent surgery, peptic ulceration, cerebral and/or retinal bleeding) should have periodic examinations for bleeding including, hematocrit and/or hemoglobin.Drug InteractionsAlthough a causal relationship has not been established, there have been reports of bleedingand/or prolonged prothrombin time in patients treated with TRENTAL with and without anticoagulants or platelet aggregation inhibitors. Patients on Warfarin should have more frequent monitoring of prothrombin times, while patients with other risk factors complicated by hemorrhage (e.g., recent surgery, peptic ulceration) should have periodic examinations for bleeding including hematocrit and/or hemoglobin.Concomitant administration of TRENTAL and theophylline-containing drugs leads to increased theophylline levels and theophylline toxicity in some individuals. Such patients should be closely monitored for signs of toxicity and have their theophylline dosage adjusted as necessary.TRENTAL has been used concurrently with beta blockers, digitalis, diuretics, antidiabetic agents, and antiarrhythmics, without observed problems. Small decreases in blood pressure have been observed in some patients treated with TRENTAL plus nifedipine or captopril; periodic systemic blood pressure monitoring is recommended for patients receiving concomitant antihypertensive therapy. If indicated, dosage of the antihypertensive agents should be reduced.Postmarketing cases of increased anticoagulant activity have been reported in patients concomitantly treated with pentoxifylline and vitamin K antagonists. Monitoring of anticoagulant activity in these patients is recommended when pentoxifylline is introduced or the dose is changed.Carcinogenesis, Mutagenesis and Impairment of FertilityLong-term studies of the carcinogenic potential of pentoxifylline were conducted in mice and rats by dietary administration of the drug at doses up to 450 mg/kg (approximately 19 times the maximum recommended human daily dose (MRHD) in both species when based on body weight; 1.5 times the MRHD in the mouse and 3.3 times the MRHD in the rat when based on body surface area). In mice, the drug was administered for 18 months, whereas in rats, the drug was administered for 18 months followed by an additional 6 months without drug exposure. In the rat study, there was a statistically significant increase in benign mammary fibroadenomas in females of the 450 mg/kg group. The relevance of this finding to human use is uncertain. Pentoxifylline was devoid of mutagenic activity in various strains of Salmonella (Ames test) andin cultured mammalian cells (unscheduled DNA synthesis test) when tested in the presence and absence of metabolic activation. It was also negative in the in vivo mouse micronucleus test. PregnancyCategory C. Teratogenicity studies have been performed in rats and rabbits using oral doses up to 576 and 264 mg/kg, respectively. On a weight basis, these doses are 24 and 11 times the maximum recommended human daily dose (MRHD); on a body-surface-area basis, they are 4.2 and 3.5 times the MRHD. No evidence of fetal malformation was observed. Increased resorption was seen in rats of the 576 mg/kg group. There are no adequate and well controlled studies in pregnant women. TRENTAL (pentoxifylline) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.Nursing MothersPentoxifylline and its metabolites are excreted in human milk. Because of the potential for tumorigenicity shown for pentoxifylline in rats, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.Pediatric UseSafety and effectiveness in pediatric patients have not been established.Geriatric UseClinical studies of TRENTAL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.The active metabolite is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.ADVERSE REACTIONSClinical trials were conducted using either extended-release TRENTAL tablets for up to 60 weeks or immediate-release TRENTAL capsules for up to 24 weeks. Dosage ranges in the tablet studies were 400 mg bid to tid and in the capsule studies, 200-400 mg tid. The table summarizes the incidence (in percent) of adverse reactions considered drug related, as well as the numbers of patients who received extended-release TRENTAL tablets, immediate-release TRENTAL capsules, or the corresponding placebos. The incidence of adverse reactions was higher in thecapsule studies (where dose related increases were seen in digestive and nervous system side effects) than in the tablet studies. Studies with the capsule include domestic experience, whereas studies with the extended-release tablets were conducted outside the U.S.The table indicates that in the tablet studies few patients discontinued because of adverse effects.INCIDENCE (%) OF SIDE EFFECTSExtended-Release Tablets Immediate-Release CapsulesCommercially Available Used only for Controlled ClinicalTrialsTRENTAL Placebo TRENTAL Placebo(Numbers of Patients at Risk) (321) (128) (177) (138)Discontinued for Side Effect 3.1 0 9.6 7.2CARDIOVASCULAR SYSTEMAngina/Chest Pain 0.3 - 1.1 2.2Arrhythmia/Palpitation -- 1.7 0.7Flushing -- 2.30.7 DIGESTIVE SYSTEMAbdominal Discomfort -- 4.0 1.4Belching/Flatus/Bloating 0.6 -9.0 3.62.9 Diarrhea --3.49.62.94.7Dyspepsia 2.88.728.80.8Nausea 2.20.7 Vomiting 1.2- 4.5NERVOUS SYSTEMAgitation/Nervousness -- 1.7 0.74.311.93.1Dizziness 1.95.8 Drowsiness -- 1.1Headache 1.2 1.6 6.2 5.82.2 Insomnia -- 2.3-0.8Tremor 0.3Blurred Vision -- 2.3 1.4TRENTAL has been marketed in Europe and elsewhere since 1972. In addition to the abovesymptoms, the following have been reported spontaneously since marketing or occurred in otherclinical trials with an incidence of less than 1%; the causal relationship was uncertain:Cardiovascular - dyspnea, edema, hypotension.Digestive - anorexia, cholecystitis, constipation, dry mouth/thirst.Nervous - anxiety, confusion, depression, seizures, aseptic meningitis.Respiratory - epistaxis, flu-like symptoms, laryngitis, nasal congestion.Skin and Appendages - brittle fingernails, pruritus, rash, urticaria, angioedema.Special Senses - blurred vision, conjunctivitis, earache, scotoma.Miscellaneous - bad taste, excessive salivation, leukopenia, malaise, sore throat/swollenneck glands, weight change.A few rare events have been reported spontaneously worldwide since marketing in 1972.Although they occurred under circumstances in which a causal relationship with pentoxifyllinecould not be established, they are listed to serve as information for physicians. Cardiovascular —angina, arrhythmia, tachycardia. Digestive — hepatitis, jaundice, cholestasis, increased liverenzymes; and Hemic and Lymphatic — decreased serum fibrinogen, pancytopenia, aplastic anemia, leukemia, purpura, thrombocytopenia. Immune system disorders — anaphylactic reaction, anaphylactoid reaction, anaphylactic shock.OVERDOSAGEOverdosage with TRENTAL has been reported in pediatric patients and adults. Symptoms appear to be dose related. A report from a poison control center on 44 patients taking overdoses of enteric-coated pentoxifylline tablets noted that symptoms usually occurred 4-5 hours after ingestion and lasted about 12 hours. The highest amount ingested was 80 mg/kg; flushing, hypotension, convulsions, somnolence, loss of consciousness, fever, and agitation occurred. All patients recovered. In addition to symptomatic treatment and gastric lavage, special attention must be given to supporting respiration, maintaining systemic blood pressure, and controlling convulsions. Activated charcoal has been used to absorb pentoxifylline in patients who have overdosed.DOSAGE AND ADMINISTRATIONThe usual dosage of TRENTAL in extended-release tablet form is one tablet (400 mg) three times a day with meals.While the effect of TRENTAL may be seen within 2 to 4 weeks, it is recommended that treatment be continued for at least 8 weeks. Efficacy has been demonstrated in double-blind clinical studies of 6 months duration.Digestive and central nervous system side effects are dose related. If patients develop these effects it is recommended that the dosage be lowered to one tablet twice a day (800 mg/day). If side effects persist at this lower dosage, the administration of TRENTAL should be discontinued.HOW SUPPLIEDTRENTAL (pentoxifylline) is available for oral administration as 400-mg pink film-coated oblong tablets imprinted Trental; supplied in bottles of 100 (NDC 0039-0078-10).Store between 59 and 86° F (15 and 30° C).Dispense in well-closed, light-resistant containers.Rx onlyRev. July 2010sanofi-aventis U.S. LLCBridgewater, NJ 08807©2010 sanofi-aventis U.S. LLC。
hydrochloride

hydrochloride Hydrochloride: An Essential Chemical CompoundIntroductionHydrochloride is a vital chemical compound that is widely used across various industries, including pharmaceuticals, water treatment, and chemical synthesis. In this document, we will explore the properties, uses, and production of hydrochloride, as well as its impact on human health and the environment.Properties of HydrochlorideHydrochloride, commonly known as HCl, is a colorless gas with a strong, pungent odor. It is highly soluble in water, forming hydrochloric acid, which is a strong acid with a pH less than 1. In its solid form, hydrochloride appears as a white crystalline powder.Uses of Hydrochloride1. Pharmaceuticals: Hydrochloride is extensively used in the pharmaceutical industry for the formulation of various medications. It is commonly employed as a salt form to enhance drug stability and solubility. Many drugs, such as antihistamines, decongestants, and analgesics, are synthesized as hydrochloride salts to improve their bioavailability and increase their efficacy.2. Water Treatment: Hydrochloride is an important chemical for water treatment processes. It is commonly used to adjust the pH levels of water, neutralize alkaline substances, and control the growth of bacteria and algae. Additionally, it is used in the disinfection of drinking water and swimming pools.3. Chemical Synthesis: Hydrochloride is a key reagent in various chemical synthesis reactions. It is used for the synthesis of dyes, pigments, detergents, and organic compounds, among other products. Hydrochloride is a versatile compound that can be employed in both small-scale laboratory reactions and large-scale industrial processes.Production of HydrochlorideHydrochloride can be produced through several methods, including the reaction of hydrochloric acid with various substances.1. Direct Synthesis: The most common method of producing hydrochloride involves the direct reaction of hydrogen gas (H2) with chlorine gas (Cl2). The reaction takes place in the presence of ultraviolet light, and the resulting gas mixture is then dissolved in water to obtain hydrochloric acid. This concentrated hydrochloric acid can be further concentrated or used directly in various applications.2. Indirect Synthesis: Another method of producing hydrochloride is the reaction of hydrochloric acid with a suitable metal carbonate or metal hydroxide. For example, the reaction between hydrochloric acid and calcium carbonate (CaCO3) results in the release of carbon dioxide gas (CO2) and the formation of calcium chloride (CaCl2). This calcium chloride can then be further processed to obtain hydrochloride.Health and Environmental ImpactHydrochloride is a corrosive substance that can cause severe burns or irritation to the skin, eyes, and respiratory system.Consequently, it is important to handle hydrochloride with caution and use appropriate safety measures.Hydrochloride is highly water-soluble, and its release into the environment can have detrimental effects on aquatic life and ecosystems. Discharges of hydrochloride-containing wastewater should be treated properly before being released into water bodies to minimize its impact.ConclusionHydrochloride is a widely used chemical compound with numerous applications across various industries. Its significance in pharmaceuticals, water treatment, and chemical synthesis cannot be understated. However, proper handling and disposal methods should be followed to ensure the safety of workers and prevent harm to the environment. Overall, hydrochloride plays a crucial role in our daily lives and will continue to be an essential chemical compound for the foreseeable future.。
tetracycline hydrochloride结构式名

tetracycline hydrochloride结构式名Tetracycline Hydrochloride: A Breakthrough Antibiotic that Revolutionized MedicineIntroduction:Tetracycline hydrochloride is a potent antibiotic that has played a significant role in the treatment of various infectious diseases since its discovery in the mid-20th century. In this article, we will explore the structural features of tetracycline hydrochloride and delve into its mechanism of action, clinical uses, and potential side effects. Join us on this journey as we uncover the impact this compound has had on the field of medicine.Structural Features and Composition:Tetracycline hydrochloride, also known by its chemical formula C22H24N2O8·HCl, is a semisynthetic derivative of a natural antibiotic produced by Streptomyces bacteria. The compound consists of four fused rings, referred to as rings A, B, C, and D, which are responsible for its antibacterial activity. The presence of various functional groups, such as dimethylamine, hydroxyl, and carbonyl moieties, confers different properties to tetracycline hydrochloride.Mechanism of Action:Tetracycline hydrochloride exerts its antimicrobial effect by inhibiting bacterial protein synthesis. It does so by binding reversibly to the 30S ribosomal subunit in susceptible bacterial cells, preventing the attachment of aminoacyl-tRNA to the messenger RNA-ribosome complex. Additionally, it interferes with the proofreading mechanism of the ribosome, leading to the incorporation of incorrect amino acids into the growing protein chain. This disruption of protein synthesis effectively inhibits bacterial growth and replication.Clinical Uses:Tetracycline hydrochloride has been widely employed in the treatment of various infectious diseases, including respiratory tract infections, urinary tract infections, sexually transmitted infections, and skin and soft tissue infections. Its broad-spectrum activity against both gram-positive and gram-negative bacteria has made it a valuable tool in combating bacterial infections, particularly in settings where other antibiotics are ineffective or contraindicated.Emerging Applications:Beyond its traditional uses, tetracycline hydrochloride has shown promise in a range of non-infectious conditions. Research suggests its potential in treating variousinflammatory disorders, such as rheumatoid arthritis and ocular inflammatory diseases. Additionally, studies have explored its potential role in the treatment of certain cancers, as it possesses anti-tumor properties. However, further research is necessary to fully understand and harness its therapeutic potential in these novel areas.Side Effects and Precautions:While tetracycline hydrochloride has proven to be an invaluable treatment option, it is not without its side effects. Common adverse reactions include gastrointestinal disturbances, such as nausea, vomiting, and diarrhea. Photosensitivity reactions can also occur, necessitating caution when exposed to sunlight or ultraviolet radiation. Long-term use of tetracycline hydrochloride can result in discoloration of teeth and bones, making it unsuitable for use in pregnant women and children under the age of eight.Conclusion:Tetracycline hydrochloride has emerged as a game-changer in the field of antibiotics, revolutionizing the treatment of infectious diseases. Its unique structural features, mechanism of action, and broad-spectrum activity have made it a cornerstone in the fight against bacterial infections.Moreover, ongoing research suggests potential applications in inflammatory disorders and cancer treatment. While side effects must be considered, the benefits of tetracycline hydrochloride far outweigh the risks when used judiciously. Continued exploration of this remarkable compound promises to uncover new therapeutic avenues and improve the health outcomes of countless patients worldwide.。
碧云天生物技术DEPC水说明书

碧云天生物技术/Beyotime Biotechnology 订货热线:400-1683301或800-8283301 订货e-mail :******************技术咨询:*****************网址:碧云天网站 微信公众号DEPC 水(DNase 、RNase free)产品编号 产品名称包装 R0021DEPC 水(DNase 、RNase free)100ml产品简介:碧云天生产的DEPC 水,即DEPC-treated Water ,是用DEPC(diethypyrocarbonate ,焦碳酸二乙酯)处理过并经高温高压消毒的Milli-Q 纯水。
经检测不含RNase 、DNase 和proteinase 。
DEPC 水可以用于RNA 沉淀的溶解,含有RNA 的各种反应体系如反转录、siRNA 的退火等,以及其它各种要求无RNase 、DNase 和proteinase 的反应体系。
DEPC 水不同于DEPC 。
DEPC 水是使用DEPC 处理过的水,基本上不含DEPC ,99.99%以上是水。
如需购买用于去除RNA 酶的DEPC ,可以选购碧云天的DEPC(ST036)。
包装清单:产品编号 产品名称包装 R0021 DEPC 水(DNase 、RNase free)100ml —说明书1份保存条件:室温保存,一年有效。
注意事项:如果每次的使用量很小,可以适当分装后再使用。
手上通常有RNase ,必须戴一次性手套操作,以防RNase 污染。
本产品仅限于专业人员的科学研究用,不得用于临床诊断或治疗,不得用于食品或药品,不得存放于普通住宅内。
为了您的安全和健康,请穿实验服并戴一次性手套操作。
相关产品:产品编号 产品名称包装 R0021 DEPC 水(DNase 、RNase free) 100ml R0022 DEPC 水(DNase 、RNase free)500ml ST036DEPC10g使用本产品的文献:1. Xu F, Yang T, Chen Y. Quantification of microRNA by DNA–Peptide Probe and Liquid Chromatography–Tandem MassSpectrometry-Based Quasi-Targeted Proteomics. Anal Chem. 2016 Jan 5;88(1):754-63. 2. Zheng Y, Liang W, Yuan Y, Xiong C, Xie S, Wang H, Chai Y, Yuan R. Wavelength-resolved simultaneous photoelectrochemical bifunctional sensor on single interface: A newly invitro approach for multiplexed DNA monitoring in cancer cells. Biosens Bioelectron. 2016 Jul 15;81:423-30. 3. Zheng X, Pang X, Yang P, Wan X, Wei Y, Guo Q, Zhang Q, Jiang X. A hybrid siRNA delivery complex for enhanced brain penetration and precise amyloid plaque targeting inAlzheimer's disease mice. Acta Biomater. 2017 Feb;49:388-401. 4. Liu L, Xu Q, Hao S, Chen Y. A Quasi-direct LC-MS/MS-based Targeted Proteomics Approach for miRNA Quantification via a Covalently Immobilized DNA-peptide Probe. SCI Rep-UK. 2017 Jul 18;7(1):5669. 5. Xu F, Zhou W, Cao J, Xu Q, Jiang D, Chen Y. A Combination of DNA-peptide Probes and Liquid Chromatography-Tandem MassSpectrometry (LC-MS/MS):A Quasi-Targeted Proteomics Approach for Multiplexed MicroRNA Quantification. Theranostics. 2017 Jul 8;7(11):2849-2862. 6. Zheng X, Pang X, Yang P, Wan X, Wei Y, Guo Q, Zhang Q, Jiang X. A hybrid siRNA delivery complex for enhanced brainpenetration and precise amyloid plaquetargeting in Alzheimer's disease mice. Acta Biomater. 2017 Feb;49:388-401. 7. Guo S, Meng XW, Yang XS, Liu XF, Ou-Yang CH, Liu C. Curcumin administration suppresses collagen synthesis inthe hearts ofrats with experimentaldiabetes. Acta Pharmacol Sin. 2018 Feb;39(2):195-204. 8. Han B,Zhang Y,Zhang Y,Bai Y,Chen X,Huang R,Wu F,Leng S,Chao J,Zhang JH,Hu G,Yao H. Novel insight into circular RNAHECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: implications for cerebral ischemic stroke. Autophagy. 2018;14(7):1164-1184.9.Xu L,Yu QW,Fang SQ,Zheng YK,Qi JC. MiR-650 inhibits the progression of glioma by targeting FAM83F. Eur Rev MedPharmaco. 2018 Dec;22(23):8391-8398.10.Yu C,Zhang X,Sun X,Long C,Sun F,Liu J,Li X,Lee RJ,Liu N,Li Y,Teng L. Ketoprofen and MicroRNA-124 Co-loaded poly (lactic-co-glycolic acid) microspheres inhibit progression of Adjuvant-induced arthritis in rats. Int J Pharmacol. 2018 Dec 1;552(1-2):148-153.11.Guo S,Meng XW,Yang XS,Liu XF,Ou-Yang CH,Liu C. Curcumin administration suppresses collagen synthesis in the hearts of ratswith experimental diabetes. Acta Pharmacol Sin. 2018 Feb;39(2):195-204.12.Xie Y,Hu JZ,Shi ZY. MiR-181d promotes steroid-induced osteonecrosis of the femoral head by targeting SMAD3 to inhibitosteogenic differentiation of hBMSCs. Eur Rev Med Pharmaco. 2018 Jul;22(13):4053-4062.13.Kang Q,Zou H,Zhou L,Liu LX,Cai JB,Xie N,Li WH,Zhang C,Shi WH,Wang LM,Zhang WH,Zhu H,Wang SF,Zhang XW. Role ofthe overexpression of TRAF4 in predicting the prognosis of intrahepatic cholangiocarcinoma. Int J Oncol. 2018 Jul;53(1):286-296.14.Wang P,Chen Y,Wang L,Wu Y,Wang L,Wu Y,Gong Z. The intervention mechanism of folic acid for benzo(a)pyrene toxic effects invitro and in vivo. Eur J Cancer Prev . 2018 Jul 16.15.Cai Y,Dong ZY,Wang JY. MiR-520b inhibited metastasis and proliferation of non-small cell lung cancer by targeting CHAF1A. EurRev Med Pharmaco. 2018 Nov;22(22):7742-7749.16.Pan SC,Cui HH,Qiu CG. HOTAIR promotes myocardial fibrosis through regulating URI1 expression via Wnt pathway. Eur RevMed Pharmaco. 2018 Oct;22(20):6983-6990.17.Zhang YQ,Chen Y,Ding YM,Yu TH. Protective effect of cyclosporine on inflammatory injury of renal tubular epithelial cells. EurRev Med Pharmaco. 2018 Oct;22(19):6551-6559.Version 2020.02.242 / 2 R0021 DEPC水(DNase、RNase free) 400-1683301/800-8283301 碧云天/Beyotime。
VTP-27999_Hydrochloride_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:VTP–27999 Hcl is an alkyl amine Renin inhibitor; VTP–27999 is useful for Hypertension and End–Organ Diseases.IC50 value:Target: ReninPROTOCOL (Extracted from published papers and Only for reference)Kinase assay [3]Renin was measured with the renin III IRMA (detection limit 1 pg/mL). This assay, which makes use of a monoclonal antibody (4G1)directed against renin's active site, also recognizes intact, open prorenin. This implies that intact prorenin can be measured with this assay after incubating it with acid or after exposing it, for 48 hours at 4°C, to 10 μM aliskiren because both procedures induce the conversion of all prorenin molecules into the open conformation. Additionally, we converted prorenin to renin by cleaving off the prosegment with immobilized trypsin (72 hours at 4°C). In plasma, this approach yields identical total renin (renin+prorenin) levels as aliskiren exposure and thus, subtracting the renin levels measured before trypsin treatment or aliskiren exposure from those after these procedures indirectly provides an indication of the prorenin levels. Intact, closed prorenin was measured with an ELISA(detection limit 10 pg/mL) that recognizes residues 32 to 39 of the prosegment. This prorenin assay was performed according to the instructions of the manufacturer, making use of the above–mentioned human recombinant prorenin to construct the standard curve.In a select set of samples, intact, open prorenin was measured on the basis of its prosegment, replacing the 125I–labeled activesite–directed monoclonal antibody of the Cisbio kit by a prosegment–directed 125I–labeled monoclonal antibody (F258–37–B1) in the IRMA (F258 IRMA; detection limit 10 pg/mL). F258–37–B1 is directed against the C–terminal part (p20–p43) of the propeptide and does not react (<0.1%) with renin. F258–37–B1 also does not react (<0.1%) with intact, closed prorenin. However, it does react with prorenin after the above treatment of prorenin with aliskiren. Thus, the aliskiren–induced nonproteolytic conformational change,causing the propeptide to move to the surface of the molecule, allows the recognition of prorenin by both the active site–directed antibody of the Cisbio kit and the prosegment–directed antibody of the prorenin IRMA. Finally, because VTP–27999 seemed to affect the outcome of the Cisbio IRMA, renin measurements in the presence of VTP–27999 were also performed with an alternative renin IRMA. This IRMA makes use of the active site–directed monoclonal antibody R1–20–5,10and has a detection limit of 0.9 pg/mL.References:[1]. Lanqi Jia et al. Discovery of VTP–27999, an Alkyl Amine Renin Inhibitor with Potential for Clinical Utility ACS Med. Chem. Lett., 2011, 2 (10), pp 747–751[2]. New renin inhibitor VTP–27999 alters renin immunoreactivity and does not unfold prorenin. Hypertension. 2013 May;61(5):1075–1082.[3]. Ishchenko A, et al. Structure–based design technology contour and its application to the design of Renin inhibitors.J Chem Inf Model. 2012 Aug 27;52(8):2089–97. Epub 2012 Jul 25.Product Name:VTP–27999 (Hydrochloride)Cat. No.:HY-76652CAS No.:1264191-73-2Molecular Formula:C 26H 42Cl 2N 4O 5Molecular Weight:561.54Target:Renin Pathway:Metabolic Enzyme/Protease Solubility:H 2O: ≥ 100 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
盐酸西维美林半水合物的制备

王信见,等:盐酸西维美林半水合物的制备/2019年第10期收稿日期:2019⁃07⁃11作者简介:王信见(1976-),男,四川彭州人,高级工程师,研究方向:药物合成,E⁃mail :4207678@ ;通讯作者:但国蓉,讲师,E⁃mail :liroy@ 。
doi :10.3969/j.issn.1672-5425.2019.10.016王信见,朱小锋,喻威,等.盐酸西维美林半水合物的制备[J ].化学与生物工程,2019,36(10):10⁃10.WANG X J ,ZHU X F ,YU W ,et al.Preparation of cevimeline hydrochloride hemihydrate [J ].Chemistry &Bioengineering ,2019,36(10):10⁃10.盐酸西维美林半水合物的制备王信见1,朱小锋1,喻 威1,但国蓉2(1.重庆惠源医药有限公司,重庆400039;2.陆军军医大学军事预防医学系,重庆400038)摘 要:在叔丁醇钠催化下,将3⁃喹咛环酮盐酸盐(Ⅱ)与三甲基碘化亚砜反应生成三亚甲基喹咛环氧化物(Ⅲ),再通入硫化氢开环加成得到3⁃羟基⁃3⁃巯基亚甲基喹咛(Ⅳ),化合物Ⅳ与乙醛缩合后生成西维美林碱基(Ⅴ),化合物Ⅴ再与消旋樟脑磺酸成盐并经过多次精制得到高纯度西维美林樟脑磺酸盐(Ⅵ),化合物Ⅵ碱化后与氯化氢成盐得到目标产物盐酸西维美林半水合物(Ⅰ),总收率20.5%。
该合成方法反应条件温和、操作简单、周期短、收率高,利于工业化生产。
关键词:西维美林半水合物;高收率;制备中图分类号:TQ460.31 文献标识码:A 文章编号:1672⁃5425(2019)10⁃0001⁃03Preparation of Cevimeline Hydrochloride HemihydrateWANG Xinjian 1,ZHU Xiaofeng 1,YU Wei 1,DAN Guorong 2(1.Chongqing Huiyuan Pharmaceutical Co.,Ltd ,Chongqing 400039,China ;2.College of Preventive Medicine ,Army Medical University ,Chongqing 400038,China )Abstract :Compound(Ⅲ)is obtained through the reaction between 3⁃quinuclidinone hydrochloride(Ⅱ)and trime⁃thylsulfoxonium iodide in the precence of sodium tert⁃butoxide,then compound Ⅳis obtained through ring⁃opening and addition of compound(Ⅲ)with hydrogen sulfide,and compound Ⅴis obtained through condensation of compound Ⅳwith acetaldehyde.Moreover,high⁃purity compound Ⅵis obtained from compound Ⅴvia reaction with racemic cam⁃phorsulfonic acid,recrystallization for resolution.Furthermore,Cevimeline hydrochloride hemihydrate (Ⅰ)is obtained from compound Ⅵvia alkalization,salt⁃forming reaction with hydrogen chloride with the total yield of 20.5%.This syn⁃thetic method has advantages such as moderate conditions,simple operation,saving time,and high yield,which is favora⁃ble for industrial production.Keywords :Cevimeline hydrochloride hemihydrate;high yield;preparation 盐酸西维美林半水合物,英文名Cevimeline hydro⁃chloride hemihydrate,化学名(+/-)⁃顺式⁃2⁃甲基螺(1,3⁃氧硫杂环戊烷⁃5,3'⁃喹咛环)盐酸半水合物,由Snow Brand 制药公司研制,2000年3月在美国首次上市(商品名Evoxac),2001年在日本批准上市,随后相继在台湾、香港等地上市。
哈迪兰产品公司安全数据表说明书

HAVILAND PRODUCTS COMPANYSAFETY DATA SHEETSection 1: IdentificationProduct Name: Havaclean Hand Sanitizer-E Product Code:H006616Haviland Products Company 421 Ann Street NWGrand Rapids, MI 49504 (616) 361-6691Emergency Phone:CHEMTREC: Canada and USA - (800) 424-9300 CHEMTREC:In Mexico - 01-800-681-9531Product Use: Hand SanitizerNot recommended for: NASection 2: Hazard(s) IdentificationGHS Ratings:Flammable liquid2Skin corrosive3Eye corrosive2BOrgan toxin single exposure3Organ toxin repeated exposure 1Flash point < 23°C and initial boiling point > 35°C (95°F)Reversible adverse effects in dermal tissue, Draize score: >=1.5 <2.3Mild eye irritant: Subcategory 2B, Reversible in 7 daysTransient target organ effects- Narcotic effects- Respiratorytract irritationSignificant toxicity in humans- Reliable, good quality humancase studies or epidemiological studies Presumedsignificant toxicity in humans- Animal studies with significantand/or severe toxic effects relevant to humans at generallylow exposure (guidanc e)GHS HazardsH225Highly flammable liquid and vapour H316Causes mild skin irritationH320Causes eye irritationH335May cause respiratory irritationH336May cause drowsiness ordizzinessH372Causes damage to organsthrough prolonged or repeatedexposure GHS PrecautionsP210Keep away from heat/sparks/openflames/hot surfaces – No smokingP233Keep container tightly closedP240Ground/bond container and receivingequipmentP241Use explosion-proofelectrical/ventilating/light/equipmentP242Use only non-sparking toolsP243Take precautionary measures againststatic dischargeP260Do not breathedust/fume/gas/mist/vapors/sprayP261Avoid breathingdust/fume/gas/mist/vapors/sprayP264Wash face, hands, and any exposedskin thoroughly after handlingP270Do not eat, drink or smoke when usingthis productP271Use only outdoors or in a well-ventilatedareaPowered by Plymouth Technolog yP280Wear protective gloves/protective clothing/eye protection/face protection P312Call a POISON CENTER ordoctor/physician if you feel unwellP314Get Medical advice/attention if you feel unwellP303+P361+P353If on skin (or hair): Remove / Take off immediately all contaminated clothing. Rinse skin with water / shower.P304+P340If inhales: Remove victim to fresh air and keep at rest in a position comfortable for breathing.P305+P351+P338If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.P332+P313If skin irritation occurs: Get medical advice / attentionP337+P313If eye irritation persists get medical advice / attentionP370+P378In case of fire: Use suitable media for extinctionP405Store locked upP403+P233Store in a well-ventilated place. Keep container tightly closed.P403+P235Store in a well ventilated place. Keep coolP501Dispose of contents/container in accordance withlocal/regional/national/international regulationsDangerSection 3: Composition/Information on IngredientsChemical Name / CAS No.OSHA Exposure Limits ACGIH Exposure Limits Other Exposure LimitsEthyl alcohol 64-17-570% - 80%Vapor Pressure: 42.979 mmHg1000 ppm TWA; 1900 mg/m3 TWA1000 ppm STELNIOSH: 1000 ppmTWA; 1900 mg/m3 TWAGlycerin 56-81-51% - 5%Vapor Pressure: .002 mmHg15 mg/m3 TWA (mist, total particulate); 5 mg/m3 TWA (mist, respirable fraction)Hydrogen peroxide 7722-84-10.1% - 1.0%1 ppm TWA; 1.4 mg/m3 TWA1 ppm TWANIOSH: 1 ppm TWA; 1.4 mg/m3 TWASection 4: First-aid MeasuresInhalationRescuers should put on appropriate protective gear. Remove from area of exposure. If not breathing,give artificial respiration. If breathing is difficult, give oxygen. Keep victim warm. Get immediate medical attention. Toprevent aspiration, keep head below knees.Eye ContactImmediately flush eyes with water. Flush eyes with water for a minimum of 15 minutes, occasionallylifting and lowering upper lids. Get medical attention promptly.Skin ContactRemove contaminated clothing. Wash skin with soap and water. Get medical attention. Wash clothingseparately and clean shoes before reuse.IngestionIf swallowed, do NOT induce vomiting. Give victim a glass of water. Call a physician or poison control centerimmediately. Never give anything by mouth to an unconscious person.Section 5: Fire-fighting MeasuresExtinguishing MediaWater spray. Alcohol-resistant foam. BC powder. Carbon dioxide.Do not use: Solid water jet ineffective as extinguishing medium.Specific Hazards Arising from the ChemicalDIRECT FIRE HAZARD. Highly flammable. Gas/vapour flammable with air within explosion limits.INDIRECT FIRE HAZARD. May be ignited by sparks. Gas/vapour spreads at floor level:ignition hazard. Reactions involving a fire hazard: see "Reactivity Hazard".Explosion hazard : DIRECT EXPLOSION HAZARD. Gas/vapour explosive with air within explosion limits.INDIRECT EXPLOSION HAZARD. may be ignited by sparks. Reactions with explosion hazards:see "Reactivity Hazard".Special Protective Equipment and Precautions for FirefightersSpecial Information: As in any fire, wear self-contained breathing apparatus pressure-demand (MSHA / NIOSHapproved or equivalent) and full protective gear.Section 6: Accidental Release MeasuresSpill and Leak ProceduresRemove ignition sources. Use special care to avoid static electric charges. No naked lights. Nosmoking.Keep upwind. Mark the danger area. Consider evacuation. Seal off low-lying areas.Close doors and windows of adjacent premises. Stop engines and no smoking. No naked flamesor sparks.Spark- and explosion-proof appliances and lighting equipment. Keep containersclosed. Wash contaminated clothes.Clean up methods:Take up liquid spill into a non combustible material e .g.: sand, earth, vermiculite or powderedlimestone. Scoop absorbed substance into closing containers. See "Material-handling"forsuitable container materials. Carefully collect the spill/leftovers. Damaged/cooled tanks must beemptied. Do not use compressed air for pumping over spills. Clean contaminated surfaces withan excess of water. Take collected spill to manufacturer/competent authority . Wash clothing andequipment after handling.Section 7: Handling and StoragePrinted: 4/6/2020 at 2:51:41PMHandling ProceduresProper grounding procedures to avoid static electricity should be followed. Ground/bond container and receiving equipment. Use explosion-proofelectrical/ventilating/lighting/equipment.Use with adequate ventilation. Avoid breathing dusts, mists, and vapors. Do not get in eyes, on skin, or on clothing. Wear eye protection and protective clothing . Wash thoroughly after handling.Storage RequirementsKeep container tightly closed. Keep only in the original container in a cool, well ventilated place away from : incompatible materials. Keep in fireproof place.Incompatible products : Strong bases. Strong acids.Incompatible materials : Sources of ignition. Direct sunlight. Heat sources.Heat and ignition sources : KEEP SUBSTANCE AWAY FROM: heat sources. ignition sources.Prohibitions on mixed storage : KEEP SUBSTANCE AWAY FROM: oxidizing agents. (strong) acids. water/moisture.Storage area : Keep out of direct sunlight. Store in a dry area. Ventilation at floor level. Fireproof storeroom.Provide for an automatic sprinkler system. Provide for a tub to collect spills. Provide the tank with earthing. Meet the legal requirements.Special rules on packaging : SPECIAL REQUIREMENTS: closing. dry. clean. correctly labelled. meet the legal requirements.Secure fragile packagings in solid containers.Packaging materials : SUITABLE MATERIAL: stainless steel. aluminium. iron. copper. nickel. synthetic material. glass.Section 8: Exposure Control/Personal ProtectionOther Exposure LimitsACGIH Exposure Limits OSHA Exposure Limits Chemical Name / CAS No.1000 ppm TWA; 1900 mg/m3 TWA1000 ppm STELNIOSH: 1000 ppm TWA; 1900 mg/m3 TWAEthyl alcohol 64-17-515 mg/m3 TWA (mist, total particulate); 5 mg/m3 TWA (mist, respirable fraction)Glycerin 56-81-51 ppm TWA; 1.4 mg/m3 TWA1 ppm TWANIOSH: 1 ppm TWA; 1.4 mg/m3 TWAHydrogen peroxide 7722-84-1ENGINEERING CONTROLS: Provide ventilation sufficient to maintain exposure below the recommended limits.RESPIRATORY PROTECTION: A respiratory protection program that meets OSHA 1910.134 and ANSI Z88.2 requirements must be followed whenever workplace conditions warrant the use of a respirator.SKIN PROTECTION: Wear impervious protective gloves. Wear protective gear as needed - apron, suit, boots. EYE PROTECTION: Wear safety glasses with side shields (or goggles) and a face shield.OTHER PROTECTIVE EQUIPMENT : Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower.HYGENIC PRACTICES: Do not eat, drink, or smoke in areas where this material is used. Avoid breathing vapors. Remove contaminated clothing and wash before reuse. Wash thoroughly after handling. Wash hands before eating.Section 9: Physical and Chemical PropertiesClear Colorless Liquid Appearance:AlcoholOdor:Not Available Vapor Pressure:Not AvailableOdor threshold:1.6Vapor Density: 6.5 - 8.5pH:Not AvailableDensity:Not AvailableMelting point:Not Available Freezing point:Complete Solubility:70° CBoiling range:25° C Flash point:8.3Evaporation rate (Ether=1):Not Available Flammability:Not AvailableExplosive Limits:0.837Specific Gravity Not AvailableAutoignition temperature:Not AvailableDecomposition temperature:Not Available Viscosity:Not AvailableGrams VOC less water:Section 10: Stability and Reactivity Chemical Stability:STABLEIncompatible MaterialsStrong acids. Strong bases.Conditions to AvoidDirect sunlight. Extremely high or low temperatures. Open flame.Hazardous Decomposition ProductsFume. Carbon monoxide. Carbon dioxide. May release flammable gases.Hazardous PolymerizationHazardous polymerization will not occur.Section 11: Toxicology InformationMixture ToxicityInhalation Toxicity LC50: 150mg/L Component Toxicity7722-84-1Hydrogen peroxideOral LD50: 801 mg/kg (Rat) Dermal LD50: 4,060 mg/kg (Rat) Inhalation LC50: 2 g/m3 (Rat)Routes of Entry:Inhalation Ingestion Skin contact Eye contactTarget OrgansBlood Eyes KidneysLiver Central Nervous System Reproductive System SkinRespiratory System Effects of OverexposureCarcinogen Rating CAS Number Description% Weight Hydrogen peroxide:7722-84-1Hydrogen peroxide 0.1% - 1.0%Section 12: Ecological InformationComponent Ecotoxicity Ethyl alcohol96 Hr LC50 Oncorhynchus mykiss: 12.0 - 16.0 mL/L [static]; 96 Hr LC50Pimephales promelas: >100 mg/L [static]; 96 Hr LC50 Pimephales promelas: 13400 - 15100 mg/L [flow-through]48 Hr LC50 Daphnia magna: 9268 - 14221 mg/L; 48 Hr EC50 Daphnia magna: 2 mg/L [Static]Glycerin96 Hr LC50 Oncorhynchus mykiss: 51 - 57 mL/L [static]Hydrogen peroxide96 Hr LC50 Pimephales promelas: 16.4 mg/L; 96 Hr LC50 Lepomis macrochirus:18 - 56 mg/L [static]; 96 Hr LC50 Oncorhynchus mykiss: 10.0 - 32.0 mg/L [static]48 Hr EC50 Daphnia magna: 18 - 32 mg/L [Static]Section 13: Disposal ConsiderationsDispose of in accordance with local, state and federal regulations.Section 14: Transportation InformationUN Code: 1993Proper Shipping Name: Flammable liquid, N.O.S. (Ethanol)Hazard Class: 3Packing Group: IIISection 15: Regulatory InformationOSHA Process Safety Management Highly Hazardous Chemicals7722-84-1 Hydrogen peroxideTSCA 8(b) Inventory7722-84-1 Hydrogen peroxide56-81-5 Glycerin64-17-5 Ethyl alcoholRegulationCountryAll Components ListedSection 16: Other InformationDate Prepared: 4/6/2020DisclaimerThe information herein is believed to be correct, but does not claim to be all inclusive and should beused only as a guide. Neither the above named supplier nor any of its affiliates or subsidiaries assumesany liability whatsoever for the accuracy or completeness of the information contained herein. Finaldetermination of suitability of any material is the sole responsibility of the user. All chemical reagentsmust be handled with the recognition that their chemical, physiological, toxicological, and hazardousproperties have not been fully investigated or determined. All chemical reagents should be handledonly by individuals who are familiar with their potential hazards and who have been fully trained in propersafety, laboratory, and chemical handling procedures. Although certain hazards are described herein,we can not guarantee that these are the only hazards which exist. Our SDS are based only on dataavailable at the time of shipping and are subject to change without notice as new information is obtained.Avoid long storage periods since the product is subject to degradation with age and may become moredangerous or hazardous. It is the responsibility of the user to request updated SDS for products that arestored for extended periods. Disposal of unused product must be undertaken by qualified personnelwho are knowledgeable in all applicable regulations and follow all pertinent safety precautions includingthe use of appropriate protective equipment (e.g. protective goggles, protective clothing, breathingequipment, face mask, fume hood). For proper handling and disposal, always comply with federal, stateand local regulations.。
碧云天生物技术 Propidium Iodide 产品说明书

碧云天生物技术/Beyotime Biotechnology订货热线:400-168-3301或800-8283301订货e-mail:******************技术咨询:*****************网址:碧云天网站微信公众号Propidium Iodide/碘化丙啶产品编号产品名称包装ST511 Propidium Iodide/碘化丙啶5mg产品简介:Propidium Iodide简称PI,中文名为碘化丙啶。
分子式为C27H34I2N4,分子量为668.40,纯度>95%。
进口分装,常用于细胞凋亡(apoptosis)或细胞坏死(necrosis)的检测,常用于流式细胞仪分析。
包装清单:产品编号产品名称包装ST511 Propidium Iodide/碘化丙啶5mg—说明书1份保存条件:4ºC避光保存。
注意事项:本产品对人体有刺激性,操作时请小心,并注意适当防护以避免直接接触人体或吸入体内。
本产品仅限于专业人员的科学研究用,不得用于临床诊断或治疗,不得用于食品或药品,不得存放于普通住宅内。
为了您的安全和健康,请穿实验服并戴一次性手套操作。
使用本产品的文献:1.Wu G, Liu ZS, Qian Q, Jiang CQ. Effects of Berberine on the Growth ofHepatocellular Carcinoma Cell lines. Medical Journal of Wuhan University. 2008 Jan;29(1).2.Liu Y, Sheng Z, Liu H, Wen D, He Q, Wang S, Shao W, Jiang RJ, An S,Sun Y, Bendena WG, Wang J, Gilbert LI, Wilson TG, Song Q, Li S.Juvenile hormone counteracts the bHLH-PAS transcription factors MET and GCE to prevent caspase-dependent programmed cell death in Drosophila. Development. 2009 Jun;136(12):2015-25.3.Li DL, Liu JJ, Liu BH, Hu H, Sun L, Miao Y, Xu HF, Yu XJ, Ma X, RenJ, Zang WJ. Acetylcholine inhibits hypoxia-induced tumor necrosis factor-α production via regulation of MAPKsphosphorylation in cardiomyocytes. J Cell Physiol. 2011 Apr;226(4):1052-9.4.Cao X, Deng W, Wei Y, Su W, Yang Y, Wei Y, Yu J, Xu X. Encapsulationof plasmid DNA in calcium phosphate nanoparticles: stem cell uptake and genetransfer efficiency. Int J Nanomedicine. 2011;6:3335-49.5.Meng LY, Liu HR, Shen Y, Yu YQ, Tao X. Cochinchina momordica seedextract induces G2/M arrest and apoptosis in human breast cancerMDA-MB-231 cells by modulating the PI3K/Akt pathway. Asian Pac J Cancer Prev. 2011;12(12):3483-8.6.Zhao Q, Xue Y, Wang JF, Li H, Long TT, Li Z, Wang YM, Dong P, XueCH. In vitro and in vivo anti-tumour activities of echinoside A and ds-echinoside A from Pearsonothuriagraeffei. J Sci Food Agric. 2012 Mar 15;92(4):965-74.7.Tu Z, Ma Y, Tian J, Li H, Akers W, Achilefu S, Gu Y. Estrogen receptor βpotentiates the antiproliferative effect of raloxifene and affects the cellmigration and invasion in HCT-116 colon cancer cells. J Cancer Res Clin Oncol. 2012 Jul;138(7):1091-103.8.Zhou Z, Wan Y, Zhang Y, Wang Z, Jia R, Fan Y, Nie H, Ying S, Huang P,Wang F. Follicular development and expression of nuclear respiratory factor-1 and peroxisome proliferator-activated receptor γ coactivator-1 alpha in ovaries of fetal and neonatal doelings. J Anim Sci.2012 Nov;90(11):3752-61.9.Jun Fang, Meihu Ma, Yongguo Jin, Ning Qiu, Chan Wang, Guodong Renand Xin Huang. Assessment of Salmonella enteritidis Viability in Egg White during Early Incubation Stages by Fluorescent Staining Method.Asian Journal of Animal and Veterinary Advances.7: 556-67. 10.Zhen-Jun S, Yuan-Yuan Z, Ying-Ying F, Shao-Ju J, Jiao Y, Xiao-Wei Z,Jian C, Yao X, Li-Ming Z.β,β-Dimethylacrylshikonin exerts antitumor activity via Notch-1 signaling pathway in vitro and invivo. Biochem Pharmacol. 2012 Aug 15;84(4):507-12.11.Tu Z, Li H, Ma Y, Tang B, Tian J, Akers W, Achilefu S, Gu Y. Theenhanced antiproliferative response to combined treatment of trichostatinA with raloxifene in MCF-7 breast cancer cells and its relevance toestrogen rec eptor β expression.Mol Cell Biochem. 2012 Jul;366(1-2):111-22.12.Feng C, Xu Z, Li Z, Zhang D, Liu Q, Lu L. Down-regulation of Wnt10aby RNA interference inhibits proliferation and promotes apoptosis in mouse embryonic palatal mesenchymal cells through Wnt/β-catenin signaling pathway. J Physiol Biochem. 2013 Dec;69(4):855-63.13.Hou Y, Chu M, Du FF, Lei JY, Chen Y, Zhu RY, Gong XH, Ma X, Jin J.Recombinant disintegrin domain of ADAM15 inhibits the proliferation and migration of Bel-7402 cells. Biochem Biophys Res Commun. 2013 Jun 14;435(4):640-5.14.Li Q, Zhou X, Shi Y, Li J, Zheng L, Cui L, Zhang J, Wang L, Han Z, HanY, Fan D. In vivo tracking and comparison of the therapeutic effects of MSCs and HSCs for liver injury. PLoS One. 2013 Apr 30;8(4):e62363. 15.Zhou R, Huang W, Yao Y, Wang Y, Li Z, Shao B, Zhong J, Tang M,Liang S, Zhao X, Tong A, Yang J.CA II, a potential biomarker by proteomic analysis, exerts significant inhibitory effect on the growth of colorectal cancer cells. Int J Oncol. 2013 Aug;43(2):611-21.16.Wang Y, Jiang XL, Peng SW, Guo XY, Shang GG, Chen JC, Wu Q, ChenGQ. Induced apoptosis of osteoblasts proliferating on polyhydroxyalk anoates. Biomaterials. 2013 May;34(15):3737-46.17.Yang F, Huang W, Li Y, Liu S, Jin M, Wang Y, Jia L, Gao Z. Anti-tumoreffects in mice induced by survivin-targeted siRNA delivered through polysaccharide nanoparticles. Biomaterials. 2013 Jul;34(22):5689-99..18.Zhou S, Wu H, Zeng C, Xiong X, Tang S, Tang Z, Sun X. ApolipoproteinE protects astrocytes from hypoxia and glutamate-induced apoptosis.FEBS Lett. 2013 Jan 16;587(2):254-8.19.Nie C, Yang D, Liu N, Dong D, Xu J, Zhang J. Thyrotropin-releasinghormone and its analogs accelerate wound healing. J Surg Res. 2014 Jun 15;189(2):359-65.Version 2016.12.08。
GM 缩写术语解释(英文)
Acronym DefinitionP ParkP PendingP PhosphorusP&A Parts & AccessoriesP&P Policy & ProceduresP&W Policy & WarrantyP.K. Setup Checking the fit-up of detail parts by assembling a complete body-in-white with screws or rivets.P/E Price / Earnings (ratio to gage stock performance)P-CR Piezo-Actuated Common RailP/MI CI Production / Manufacturing Integration Continuous ImprovementP/N Part Number.P/PD Product/Process DevelopmentP/PDP Product/Process Development ProcessP/PDS Product/Process Definition StageP/T Powertrain.P/U Pick Up.P/UBX Pick Up Box.P4P Pay For PerformancePa pascal: A unit of pressure corresponding to a force of 1 newton acting uniformly upon an area of 1 square meter. Hence 1 Pa = 1N/M2PA Park Avenue.PA Passenger Automobiles.PA Product Assurance.PA Production Achievement.PA Production Approval.PA Program AuthorizationPA Project Authorization.PA Purchase Authorization.PA Purchasing Agent.PAA Production Action Authorization (An official document used to authorize a temporary substitution, try out new parts or materials, rework existing parts, or use up excess orobsolete-stock that will be good for a limited number of pieces or a specific time period.) PAA Programmable Analog ArrayPABX Private Automatic Branch ExchangePAC Perceptual Audio Coder (Lucent encode / decode method for broadcast transmission / reception)PAC Performance Assessment Committee.PACE Program Assessment & Control EnvironmentPACS Program Assurance Control System.PACX Private Automatic Communication eXchangePAD Packet Assembly – DisassemblyPAD Personnel Administration & DevelopmentPAD Product Assembly DrawingPAD Product Assembly Document. Provides assembly plants with a written set of instructions and graphic illustrations of production operations to be performed on a given GM platform. Thisincludes the production parts required, their specific usage by model, style and optionconditions. A graphic Illustration depicting the operations and those procedures, and stepsand instructions required to assemble, install or adjust parts. also PDM.PAD Public Announcement Date.PAFC Phosphoric Acid Fuel CellPAG Programmable Automatic GagePAI Product Assurance InitiativePAIC Part Assessment & Information ControlPAIR Pulse Air Injection Reactor.PAL Pedagogic (for teaching) Algorithmic LanguagePAL Phase Alternation LinePAL Programmable Array LogicPAM Pulse Amplitude ModulationPAN Project Authorization Notice. A document issued by a vehicle platform to advise the design / release engineers of approved changes to a vehicle program.PAP Performance Achievement PlanPAPIR Powertrain Architecture Process Integration Review.PAPIR Product and Process Integration ReviewPAR Pilot Action Request.PAR Product Allegation Resolution. Part of CARS.PAR Police Accident ReportPar Tech Parts Technical groups (for inquiries on engineering or parts specifications.)PARC Production Area Record CenterPARD Periodic And Random DeviationPARIS Planning & Release Information System.Parms Parameters (an abbreviation)PARR Product Allegation Resolution Report.PARTS Part Readiness Tracking System.PAS Parking Assistance System.PAS Performance Algorithm ShiftingPAS Peripheral Acceleration Sensor (Bosch)PASCOM Product Announcement Strategy Committee.PASDS Power & Signal Distribution SystemPASER Particle Stream Erosion.Pass Passenger (an abbreviation)PASS PDIS Augmented Specification System (Canada)PASS Personalized Automotive Security System.PASS Proposal Assessment Study System.PATAC Pan Asian Technical Automotive CenterPATH Program on Advanced Technology for the HighwayPATP Patents & Technical PublicityPATS Parts Approval Tracking SystemPAV Pyrotechnically Actuated VentingPb Lead (Plumbium)PB Power Brakes.PBCR Preliminary BOM Change Request (Report)PBGA Plastic Ball Grid ArrayPBL Pickup Box, LongPBOM Preliminary Bill of MaterialsPBPCI Passive Backplane PCIPBS Personal Benefit SummaryPBS Pickup Box, ShortPBV Purpose Built VehiclesPBX Private Branch's eXchangePC Personal Computer.PC Plant ConcernsPC PolycarbonatePC Pressure Control (solenoid valve).PC Printed CircuitPC Product Characteristic.PC Product CostPC Programmable Controls or Controller.PC Problem Communication. An electronic network for documenting and reporting an unacceptable product build condition. When an approved PC is entered at any location, it iselectronically transmitted to the specific area and individual responsible for providing thenecessary corrective action. When a solution has been developed and approved, it iscommunicated to the reporting location via the PC network.PC&L Production, Control and Logistics. The engineering group responsible for ensuring smooth flow of needed material (physical and information). (refer to EPC&L).PC&S Production Control & Scheduling.PC/CODE Part Classification Code.PC/TCP Personal Computer / Transmission Control ProtocolPCA Product(ion) Compliance Audit.PCB Polychlorinated Biphenyl (waste oil residue)PCB Printed Circuit Board.PCC Paper Color Code.PCC Pontiac Centerpoint Campus (formerly TPC, Truck Product Center)PCC Powertrain Controls Center.PCC-c Pontiac Centerpoint Campus - Central (building)PCC-e Pontiac Centerpoint Campus – East (building)PCC-w Pontiac Centerpoint Campus – West (building)PCD Product Content DocumentPCDR Performance & Cost Detail ReportPCE Preliminary Cost Estimate.PCG Product Content Group.PCI Peripheral Component Interconnect (or Interface). PC bus that normally runs at 33 MHz on a 32-bit bus, but the specification allows for 64-bit and 66MHz operation that provides up to532MB per second.PCI Process Control InstructionsPCICN Piece Cost & Investment Change NoticePCIF Printed Circuit Interconnection FederationPCIT Piece Cost and Investment TrackingPCL Printer Control LanguagePCLL Product Compliance Legal Liaison.PCM Part Change Management (Saturn Service Parts)PCM Power Control ModulePCM Powertrain Control Module.PCM Program Content Monitor.PCM Program Control Module.PCM Propulsion Control Module.PCM Pulse Code ModulationPCMCIA Personal Computer Memory Card International (or Industry) Association.PCME Powertrain Control Module, EnginePCMIA People Can't Memorize Computer Industry AcronymsPCMT Powertrain Control Module, TransmissionPCN Personal Communications NetworkPCN Product (or Program) Change Notice.PCO Program Content & Objectives.PCP Performance Characterization ParametersPCP Process Control Plan.PCPA process control plan auditPCP Production Control PlanPCP Product Certification ProcessPCP Product Change Proposal.PCP Product Compliance Program (of GM), a.k.a. GMPCP.PCR Plan Change RequestPCR Process Change Request.PCR Product Change RequestPCR Product Change Request. Document used to change or modify the Corporate Product Plan between annual product plan approvals.PCS Personal Communication SystemPCS Pressure Control Solenoid.PCS Problem Communication SystemPCS Process Capability StudyPCS Product Content Sheet (defines vehicle content which generates an MCS.)PCSD President’s Council On Sustainable DevelopmentPCSE Powertrain Controls Service Engineering.PCU Power Control Unit.PCV Passenger Car VehiclePCV Positive Crankcase Ventilation.PCV Pressure Control Valve.Pd PalladiumPD Passenger Distribution.PD Problem Documentation.PD Production DesignPDA Personal Digital Assistant. Generally, small, hand-held, battery-operated, microprocessor-based devices that perform operations such as: Store telephone numbers, addresses, andreminders, Send and receive email and faxes (wirelessly), Receive pages (just like analphanumeric pager) .PDA Port De-ActivationPDA Preliminary Design ApproachPDB Platform Database.PDC Parts Distribution Center (Warehouse for GM Parts distribution)PDC Portfolio Development Center. Part of the NAO Design and Engineering Centers charged with providing advanced vehicle engineering for product portfolio planning and vehiclearchitecture definition. Develops integrated vehicle proposals, rationalized with the portfolioto improve and accelerate the vehicle launch process.PDCA Plan, Do, Check, Act; the basic philosophy behind the Engineering Management Process as developed by Deming over the first few years of his career; PLAN defines the systemthrough a set of objectives, and a strategy. The DO portion is the actual design of the partsto execute the vehicle and the tools and processes required to build the parts and cars. TheCHECK element of the cycle is the VALIDATION of the paper car and process, the productand the process. The ACT relates to continuous improvement where improvements arebased on the results of needs identified in the CHECK.PDCR Product Design Compliance ReportsPDCS Pulsat Dealer Communication System.PDD Parametric Die DesignPDD Product Definition DocumentPDD Product Description Department.PDES Product Data Exchange System (or Specification).PDES Product Data Exchange using STEP. Industrial consortium formed to expedite development of standards to be used in the exchange of product data (See IPO).PDF Portable Document FormatPDF Problem Definition Form.PDF Problem Description Form.PDG Product Description Group.PDI Pre Delivery InspectionPDI Preliminary Data IndicatorPDIF Pressure DIFferential.PDIP Plastic Dual In-Line PinPDIS Product Description Information System.PDIS Product Description Information Standard. A corporate standard used to transmitengineering specification data to other departments and divisions within General Motors. PDIT Product Design Improvement Team.PDIT Product Development & Improvement TeamPDIT Product Development Information Team.PDIT Product Development Integration Team.PDIT Program Development Integration Team.PDL Park & Directional LampPDL Process Description LanguagePDL Program Description LanguagePDL Program Development Library (A collection of GRIP and UFUNC programs from many of the organizations within GM.)PDM Passenger Door ModulePDM Product Data Management (GM's customized version of IMAN supporting a common process for managing product data.)PDM Product Data ManagerPDM Product Description Manual.PDM Pulse Duration Modulation.PDMS Product Data Management SystemPDN Product Discrepancy Notice.PDP Personal Development Plan.PDP Plant Data ProcessorPDP Powertrain Development Process. The common process within GM Powertrain for work directed toward bringing a quality product to market. The current PDP process used withinPowertrain is called 4 PDP. Being replaced by GPDP.PDP Process Development ProcessPDP Product Development ProcessPDQ Portage Double Quick, Inc. (An electronic machine used to pick points from the clay model.) PDQ Pretty Darn QuickPDR Parts Determination and ReleasePDR Physician's Desk ReferencePDR Preliminary Design ReviewPDRE Process Design Responsible EngineerPDS Passenger Door SwitchPDS Pre-Delivery Survey.PDS Product Description System. The corporate engineering specification system for program content. (See GPDS)PDS Proximity Detection SystemPDS Purchase and Delivery Satisfaction. A survey provided to owners of a new GM product for evaluating their purchase experience.PDT Product Delivery Team (as defined by ATV). The team role is to deliver a product and production system with high quality, within budget and on time. Responsibilities of the PDTare to have a clear understanding and agreement on functional requirements from SSTS,MTS & MMTS. Assure requirements of SSTS, MTS & MMTS are covered in a CTS.Manage initial release of and subsequent changes to all components. Manage financialanalysis (piece price & investment) and changes to meet or exceed business casecommitments. Source all components with qualified suppliers. Meet program timingmilestones. Ensure validation of components to CTS. Understand and implement TotalQuality Plan. Execute detailed manufacturing process. Respond to needs of build teams tosupport concept, prototype, pilot and production builds. Benchmark competitors.Documentation and change control of CTS’s.PDT Product Design TeamPDT Product Development Team. Function: generate designs which achieve targets.Responsibilities: Achieve targets allocated from PMT, negotiate proposed targetchanges/requirements with PMT, report status of targets to PMT on a regular basis, developan activity schedule by SIM Council and report issues, provide cross-functional evaluation ofalternatives to meet requirements and imperatives, assist in early source definition andprovide required sourcing packages, track the status of imperatives and performanceobjectives, track and control design changes, promote use of common systems/processes,assure Quality/Cost/Warranty/HPV goals for Non-GMT and GMX programs are achieved,Assure plant build concerns are resolved, assure KCD’s/KPC’s DFM/DFA are executedappropriately, assure generation of Subsystem and Component Technical Specs, assurefunctional representation at meetings as appropriate, publish meeting minutes and maintainPDT closed-loop action item/concerns log.PDT Program Development Team.PDU Protocol Data UnitPDW Process Development WallPE Packard Electric. See PED.PE PolyethylenePE Power EnrichmentPE Power EquivalentPE Price to Earnings. (normally written as P/E).PE Process EngineerPE Product Engineer(ing)PE Professional EngineerPEA Pontiac East AssemblyPEA Production Engineering Activity.PEAM Portable Electronic Airflow MachinePEB Power Electronics Bay.PEC Portfolio Engineering Center.PEC Pontiac Engineering CenterPECL Positive Emitter Coupled LogicPECT Program Execution Core TeamPED Packard Electric Division. Part of ACG.PED Process Engineering DocumentPED Product Engineering DepartmentPED Production Engineering Department.PED Production Engineering Documents.PED Program Engineering Director. This position is/was known as TVIE. PET member.PEDD Production Engineering Die DesignPEFC Polymer Electrolyte Fuel CellPEM Production Engineering Manual.PEM Program Engineering Manager. This position is/was known as TVIE. PET member. PEMFC Proton Exchange Membrane Fuel CellsPEP Product Evaluation Program (vehicle).PEPE Product Engineering Program ExecutionPER PersonnelPER Personnel Evaluation ReportPER Planning Experimental Records.PER Procedure Execution RequestPerf Performance (an abbreviation)PERL Practical Extraction and Report Language. A computer language used for scanning text and creating reports.PERT Planning Evaluation & Review Technique (ala PERT chart)PERT Program Evaluation and Review Technique. A computer code to detail project planning and monitoring.PEST Propulsion & Electrical Systems Team.PET Powertrain/Engine/TransmissionPET Product Education & TrainingPET Product Evaluation Team.PET Product, Education and Training Committee. The PET Committee consists of the five Marketing Divisions (includes the Dealer Business Center) (voting members), Allied Divisions(i.e. GM of Canada, Delphi Automotive Systems, Delco Electronics, GM Powertrain, ACDelco-SPO), TCO, STD, and other GM groups with an interest in knowing about orinfluencing technician training (i.e. STG Product Engineering, STG International Service).The committee is chaired by the STD Manager. The PET Committee provides a singlesource of service training plans for General Motors. All groups with a service trainingmessage to convey to Dealers should notify the Committee. In this way, economies of scaleare realized and redundant efforts minimized.PET Program Evaluation Team.PET Program Execution Team. Responsibilities are to: develop “Contract”, act as a single point of entry for assessing all program content and change requests, balance new product andprocess feature content to meet “Contract” (cost, quality, and productivity), resolve programcontent issues, keep program imperatives on track, prepare gate reviews, provide programreadiness coordination, achieve “Contract”, monitor customer satisfaction, use commonprocesses. Deliverables are: the “Contract”, documentation for action items and contentdecisions, recommendations to VLT-problem resolution recommendation and mechanics.Typical attendees include: Program Planning Manager/Asst. VLE (Chair), Program Manager(Agenda/Logistics), Finance, Vehicle Integration Engineer, Manufacturing IntegrationEngineer, Purchasing/PC&L, Quality, Manufacturing, Assistant Brand Managers - Product,International Product Development Manager, Design Center (As Required). Typical meetingcontent involves: vehicle and process integration review issues, monitor program progressthrough rigorous tracking, performance to “Contract”, content changes.PET Program Executive Team.PETC Product, Education & Training CommitteePETCM Powertrain Engine Transmission Control ModulePEU Power Electronic UnitPF Polyurethane FoamPF Power FactorPF Program Function [keys]PFA Pedestrian Federation of AmericaPFC Programmable Function Control.PFD Process Flow Diagram.PFEP Plan For Every PartPFI Port Fuel Injection.PFM Product Focus ManagerPFMA Process Failure Mode Analysis.PFMEA Process Failure Mode & Effects Analysis.PFMS Performance Feedback Measurement System.PFP Pay For PerformancePFSE Product Focus Support EngineerPFSSA Plant Floor Systems Service Agreement (between GM and EDS regarding plant floor Intranet systems services)PFT Product Focus Team.PG Proving Ground.PGA Pin Grid ArrayPGA Professional Graphics AdapterPGA Programmable Gate ArrayPGM Program ManagementPGR Policy Group Review.PGS Prime Graphics SystemPhn Phone (an abbreviation)PHT Parallel Hybrid Truck; one type of an Advanced Technology Vehicle.PHUD Parallel Hybrid Ultra-low-noise DetectorPI Preliminary Information.PI Priority Indicator.PIA Parts in Assembly.PIA Performance Integration AreasPIA Peripheral Interface Adapter.PIA Purchasing in Assembly.PIAD Plastics in Automotive Division (Specification)PIC Peripheral Integrated Circuit (Bosch)PIC Powertrain Information Converter.PIC Product Information CenterPICD Product Interface Control DocumentPICOS Purchased Input Concept Optimization With Supplier. Term used to describe process improvement at supplier plant.PICOS Purchasing Input Cost Optimization for SuppliersPICS Partners in Customer SatisfactionPID Primary Identifier (C2C)PID Purchase Identifier (ALB/UNIT)PID Parameter Identifier (or Identification).PIG Product Information Group.PIM Performance Integration Manager.PIM Power Inverter ModulePIM Product(ion) Information Management. A term applied to the topic of managing product data in a computer environment.PIM Program Implementation Managers.PIM Propulsion Inverter Module.PIMREP Project Incident Monitoring and Resolution Process. GMNA’s common issue solving tool to support the Issue Solving Process, to promote a common review process, and to ease data. PIMS Product Information Management System.PIN Personal Identification Number.PING Packet Inter Net Gopher (Part of the standard TCP/IP suite of protocols that allows you to check your connectivity with other devices, or to check whether your own TCP/IP stack isworking properly. Normally, you type in something like "ping 206.119.148.38," and you eitherget a response from that IP address or not.)PIO Powertrain Computer Input/Output.PIP Performance Improvement PlanPIPS Product in Process SimulatorPIT Performance Integration Team. Functions to provide leadership to the PMTs and PDTs and integrate and achieve total vehicle performance goals.PIT Process Improvement Team.PIW Product/Process Integration Wall.Pixel Picture ElementPJJ Toyota J-car (vehicle name).PKT Packet Data (C2C)PL/1Programming Language / 1 (IBM)PLA Project Labor Agreement.PLA Product Life Assessment.PLA Programmable Logic ArrayPLB Process Leadership BoardPLC Programmable Logic ControllerPLCC Plastic Leaded Chip Carrier (External connections consist of "J" leads around all four sides of the surface mount package.)PLCP Pre-Launch Control PlanPLD Part Locating DimensionPLD Programmable Logic DevicePLE Product Line Executive (replaced by Program Manager)PLEB Preloaded Enclosed Bushing.PLL Phase Locked Loop.PLM Plant Liaison ManagerPLP Principal Locating Points (for assembly fixture).PLP Production Launch Process.PLS Process Level Suffix.PLS Product Level SuffixPLT Planning Leadership TeamPLT Program Leadership Team.PLYRAT Residual Alignment Torque (tire measurement).PLZT Lead (Plumbium) Lanthanum Zirconte TitantePm PromethiumPM Particulate Matter. Pollution substance from diesel engine/fuelPM Performance MeasurementPM Phase MarginPM Phase ModulationPM Photo MultiplierPM Post MeridiemPM Preventative MaintenancePM Program ManagementPMC Process Monitoring and CompliancePMC Purchasing Material Control.PMCS Production & Manufacturing Control SystemPMFS Performance Measurement and Feedback System. Seven measures of NAO’s health; i.e.people development, product initiation, operations, marketing, sales and service, employeesatisfaction, retail customer satisfaction and shareholder satisfaction.PMI Process Modeling & Integration. The GM PMI Tool provides computer generation on the Top Flow Down chart. PMI Turbo consists of a set of Macros for Microsoft Word Version 6.0 thatautomate the creation of PMI Models. PMI97 Turbo consists of a set of Macros forMicrosoft Word Version 7.0.PMI Product / Manufacturing Implementation.PML Product Manufacturing LocationPMM Power Mode MasterPMM Process Management Methodology.PMM Program Manufacturing Manager.PMM Power Mode Master.PMO Program Management Office.PMOS Positive channel Metal Oxide SemiconductorPMP Performance Management Program (or Process or Plan)PMP Process Monitoring Point.PMP Program Management Plan.PMP Project Management Process. The common process for setting objectives, will be the same for all executives worldwide and all classified employees in the GMNA region (excludingMexico).PMQH toyota J-car (vehicle name).PMR Product (or Program) Manufacturability Requirements.PMs Performance MeasuresPMS Powertrain Management SystemPMS Payment Modification SystemPMS Production Material Scheduling.PMT Program Management TeamPMT Prototype Management Team.PMT Product Management Team. (in some departments, replaced by PFT)PMV Pressure Modulator Valve.PN Part NumberPNA Part Name.PNA Parts 'N Accessories (New or manufactured automotive Parts and Accessories marketed by GM Parts. Also P&A)PNC Part Number Control.PNC Pontiac North Campus (consists of GM Powertrain Headquarters and Pontiac Metal Center) PNG Pinion & Ring Gear.PNGV Partnership for a New Generation of VehiclesPNNL Pacific Northwest National LaboratoryPNP Park/Neutral Position.PNP Positive – Negative – Positive (type of transistor)PNSI Part Number Stocking Incentive (This incentive is based on part numbers stocked. Qualified dealers receive their PNSI rebate on a monthly basis.PO Paint Operations.PO Purchase Order. A document issued in response to an approved purchase requisition.POA Part of AssemblyPOA Purchase Order Alteration.POC Price Of Conformance.POC Proof Of Concept.POD Proof of Design.PODS Passive Occupant Detection System (supports the production order management and scheduling functions for all GM North American produced passenger car and light dutytrucks. This system simplifies the counting of forecasted orders for tool and productionplanning. The output of this system is in model code volumes.)POF Physics of FailurePOGEN Purchase Order Generation System-DelcoPOM Pulse-width-modulated Output ModulePOMS Product Order Management & Scheduling (System found within TSO that is used to determine whether a vehicle is buildable, based on available engineering specifications.) POMS Production Order Management System.PONC Price Of Non-Conformance.POP Pay on Production. A system where suppliers are paid for production material based on the customer's usage records, rather than physical receipt of the material.POP Personal Operating Principles.POP Point of Purchase.POP Post Office Protocol (The way e-mail software such as Eudora gets mail from a mail server.) POP Promote Our Products.POP Point of Presence. POP is a service provider's location for connecting to users. Generally, POP refer to the location where people can dial into the provider's host computer. Mostproviders have several POPs to allow low-cost access via telephone lines. .POPA Part of Production Assembly. Used on component parts that will not be serviced individually, but will be serviced using a production assembly.POR Power-On Reset.Pos Position (an abbreviation)POS Physics of SuccessPOS Point of SalesPOSA Part of Service Assembly. Used on component parts that will not be serviced individually, but will be serviced using a service assembly.POST Point of Sale Terminal.POSU Part of Service Unit, Kit. Used on parts (usually components) that will not be serviced individually, but will be serviced using a service unit or kit.POTS Plain Old Telephone SystemPOV Privately Owned VehiclePOX Partial OxidationPP Percentage PointPP PolypropylenePP Ported Purge.PP Program PlansPP&E Property, Plant, and Equipment. Capital investment or capital expenditures.PP&T Production Planning & Tooling.PPA Property Pass Administrator.PPAA Pre-Production Action AuthorizationPPAH Parallel Power Assist HybridPPAP Production Part Approval Process (formerly GP3). PPAP is a general procedure for “Supplier Submission of Material for Production Approval” included in “Targets for Excellence”. The process used to determine if all customer requirements are met by a supplier and ifthe process has the potential to produce product meeting these requirements on aproduction basis.PPB Program Plan Book.PPC Prestressed Piezoelectric Composites (Devices with ability to provide inordinately large mechanical output displacements, as high as 40 to 50 times the thickness of the deviceitself.)PPC Product Policy Committee.PPC Product Program Content. (Formally Plan Book) .PPC Production Possibilities CurvePPC Public Policy CommitteePPCO Project Plan Change Order.PPD Passenger Presence DetectionPPD Pilot Process Document.PPD Portfolio Planning DirectorPPD Price & Production Deviation.PPD Program Planning Director. The Program Planning Director / Manager acts as the “Chief of Staff” and is responsible for managing the activities of the Vehicle Line Team. The PPD /PPM is supported by a staff consisting of: Vehicle Manager(s), Assistant VehicleManager(s), Assistant Vehicle Timing Manager(s), Vehicle Team Analyst(s).PPDP Performance Planning and Development Process (Personnel Department blue sheet). PPDP Personal Performance Development PlanPPDR Product Planning & Design ReviewPPEC Product and Process Engineering CenterPPEC Product Problem Evaluation Committee.PPEI Platform/Powertrain Electrical Interface.PPG Pittsburgh Plate Glass.PPG Product Policy Group. Corporate group containing the chairman of the board and other chief executives. This group reviews and provides concept approval and final approval for everyGM program.。
英特尔商品说明书:RoHS限制有害物质数据表,2005年11月版

Material Declaration DatasheetRestriction on Hazardous Substances (RoHS) ComplianceManufacturer:IntelCorporation Date: 11 November 2005Equipment type: desktop board Product weight: 610.6 gramsModel designation: D915GUX Lead-free product: Lead-free Second Level Interconnects (SLI) onlyRoHS Definitions:Quantity limit of 0.1% by mass (1000 ppm) for; Lead (Pb); Mercury; Hexavalent Chromium; Polybrominated Biphenyls (PBB); Polybrominated Diphenyl Ethers (PBDE). Quantity limit of 0.010% by mass (100 ppm) for Cadmium.Intel understands RoHS compliance requires Lead and other materials banned in RoHS Directive are either (1) below all applicable substance thresholds as proposed by the EU, or (2) an approved or pending exemption applies. Note, RoHS implementation details are subject to change.RoHS Declaration:This product is RoHS directive compliant but does contain Lead, a RoHS restricted substance per the definitions above. This product uses the following applicable RoHS technology exemptions:- Lead in glass of electronic components- Lead in electronic ceramic parts (e.g. piezoelectronic devices)- Lead as an alloying element in aluminum containing up to 0.4% lead by weight- Lead in high melting temperature type solders (i.e. lead based alloys containing 85 % by weight or more lead)- Lead in solders to complete a viable electrical connection between semiconductor die and carrier within integrated circuit Flip Chip packagesThis product has been verified to be in conformance with EU directive 2002/95/EC as currently understood. To the best ofour knowledge the information contained in this declaration is true and correct.Level A Materials and Substances:Materials from Annex A of the EIA/EICTA/JGPSSI Material Composition Declaration Guide and listed below are not contained in this product in quantities above the threshold level for these materials, nor intentionally added to this product.AsbestosAzo colorantsCadmium/Cadmium compounds Hexavalent Chromium Hexavalent Chromium compounds Mercury/Mercury compoundsOzone Depleting SubstancesPolybrominated Biphenyls (PBBs)Polybrominated Diphenylethers (PBDEs)Polychlorinated Biphenyls (PCBs)Polychlorinated NaphthalenesRadioactive substancesShortchain Chlorinated ParaffinsTributyl Tin (TBT) and Triphenyl Tin (TPT)Tributyl Tin Oxide (TBTO)This product may contain Lead or Lead compounds in discreet component parts above the homogenous material threshold level of 1000 ppm per the RoHS exemptions above. In the aggregate, the Lead concentration for this product is 589 ppm. Level B Materials and Substances:This product does contain materials listed in Annex B of the EIA/EICTA/JGPSSI Material Composition Declaration Guide above the threshold level of 1000 ppm as listed below.Material / Substance Antimony/Antimony compounds Brominated flame retardants Nickel/Nickel compounds Description of Useflame retardantflame retardantplatingLocation in Productboard substrateboard substratecomponent platingMaterial Concentration3,410ppm33,200ppm4,520ppmCOMMENTS1.The data reported for Level A and B materials and substances are based on analytical testing of a representative sample product.Individual test results may vary due to differences in production and/or sensitivities of analytical testing methods. Data shown reflect analytical testing intended to validate Intel's RoHS compliance systems. Intel’s certification of RoHS compliance at the homogenous material level is based on Supplier Declarations of Conformance.2.This declaration is based on the product specified, with all product skus of this product eligible to be covered by this declaration.3.Material concentration data in parts per million (ppm) is representative of all product skus within the product family.4.Material mass can be estimated by multiplying concentration (in ppm) by product weight.5.The remainder of this product consists of non-reportable metals (i.e., copper, iron, tin), epoxy resin and other non-metal materials. INTEL ACCEPTS NO DUTY TO UPDATE THIS MDDS OR TO NOTIFY USERS OF THIS MDDS OF UPDATES OR CHANGES TO THIS MDDS. INTEL SHALL NOT BE LIABLE FOR ANY DAMAGES, DIRECT OR INDIRECT, CONSEQUENTIAL OR OTHERWISE, SUFFERED BY USERS OR THIRD PARTIES AS A RESULT OF THE USERS RELIANCE ON INFORMATION IN THIS MDDS THAT HAS BEEN UPDATED OR CHANGED.Page 1 of 2关于符合中国《电子信息产品污染控制管理办法》的声明Management Methods on Control of Pollution fromElectronic Information Products(China RoHS declaration)产品中有毒有害物质的名称及含量有毒有害物质或元素 部件名称(Parts) 铅(Pb)汞(Hg)镉(Cd)六价铬(Cr6+)多溴联苯(PBB)多溴二苯醚(PBDE)主板组件Motherboard Assembly× ○○○○○○:表示该有毒有害物质在该部件所有均质材料中的含量均在SJ/T 11363-2006标准规定的限量要求以下。
有机化学英文缩写

Ac acetyl 乙酰基acac acetylacetonate 乙酰基丙酮化物AIBN 2,2'-azobisisoblltyronitrile 偶氮二异丁腈Ar aryl 芳基的BBN borabicyclo[3.3.1]nonane 硼双环[3.3.1]壬烷BCME dis(chloromethyl)ether 双氯甲醚BHT butylated hydroxytoluene (2,6-di-t-butyl -p-cresol)别名抗氧化剂264 2,6-二叔丁基-4-甲基苯BINAL-H 2,2'-dihydroxy-1,1'-binaphthyl-lithium aluminum hydride 手性烷氧基联萘酚氢化铝锂BINAP 2,2' - bis(diphenylphosphino)-1,1' -binaphthyl双二苯基磷酰联萘BINOL 1,l'-bi-2,2'-naphthol 1,1'-联-2,2'-萘酚bipy 2,2' –bipyridyl 2,2'-联吡啶BMS borane-dimethyl sulfìde 硼烷吡啶Bn benzyl 苯甲基Boc t-butoxycarbonyl叔丁氧羰基BOM benzyloxymethyl苄氧甲基bp boiling point 沸点Bs brosyl (4-bromobenzenesulfonyl) 4-溴苯磺酰基BSA N, O-bis( trimethylsilyl )acetamide N,O-双三甲硅基乙酰胺Bu n-butyl 正丁基Bz benzoyl 苯甲酰CAN cerium(lV) ammonium nitrate 硝酸铈(Ⅳ)铵Cbz benzyloxycarbonyl 苄氧羰基CDI N,N-carbonyldiimidazole N,N'-羰基二咪唑CHIRAPHOS 2,3-bis(diphenylphosphino)butane 2,3-双(二苯基膦)丁烷Chx =Cy 环己基cod cyclooctadiene 环辛二烯cot cyclooctatetraene环辛四烯Cp cyclopentadienyl 环戊二烯基CRA complex reducing agent 复合还原试剂CSA 10-camphorsulfonic acid 10-樟脑磺酸CSI chlorosulfonyl isocyanate 氯磺酰异氰酸酯Cy cyclohexyl 环己基d density 密度DABCO 1,4-diazabicyclo[2.2.2]octane 1,4-重氮二环[2.2.2]辛烷DAST N,N'-diethylaminosulfur trifluoride二乙胺基三氟化硫dba dibenzylideneacetone二亚苄叉丙酮DBAD di-t-butyl azodicarboxylate偶氮二甲酸二叔丁酯DBN 1,5-diazabicyclo[4.3.0]non-5-ene 1,5-二氮杂二环[4,3,0]壬烯-5DBU 1 ,8-diazabicyclo[5.4.0]undec-7-ene 1,8-二氮杂二环-双环(5,4,0)-7-十一烯DCC N,N-dicyclohexylcarbodiimide N,N'二环己基碳二亚胺DCME dichloromethyl methyl ether二氯甲基甲醚DDO dimethyldioxirane双十二烷基二硫代乙二酰胺(又称钯试剂)DDQ 2,3-dichloro-5,6-dicyano-1,4-benzoquinone 2,3-二氯-5,6-二氰-1,4-苯醌de diastereomeric excess 非对映体过量DEAD diethyl azodicarboxylate偶氮二甲酸二乙酯DET diethyl tartrate酒石酸二乙酯DIBAL diisobutylaluminum hydride二异丁基氢化铝DIEA =DIPEA 二异丙基乙胺DIOP 2,3-O-isopropylidene-2,3-dihydroxy-1,4- bis-(diphenylphosphino)butane异丙烯-2,3-二羟-1,4-双二丙基膦丁烷DIPEA diisopropylethylamine二异丙基乙基胺diphos =dppe 1,2-双(二苯基磷酰)乙烷DIPT diisopropyl tartrate 二异丙基酒石酸盐DMA dimethylacetamid 二甲基乙酰胺DMAD dimethyl acetylenedicarboxylate 丁炔二酸二甲酯,别名:催泪瓦斯DMAP 4-(dimethylamino)pyridine 4-二甲基氨基吡啶DME 1,2-dimethoxyethane乙二醇二甲醚(二甲氧基乙烷)DMF dimethylformamide 二甲基甲酰胺dmg dimethylglyoximato 丁二酮肟(与Ni2+形成鲜红色螯合物)DMPU N,N' -dimethylpropyleneurea N,N-二甲基丙烯基脲DMS dimethyl sulfide 二甲基硫DMSO dimethyl sulfoxide 二甲基亚砜DMTSF dimethyl(methylthio)sulfonium tetrafluoroborate 二甲基(甲硫代)锍四氟硼酸盐dppb l ,4-bis(diphenylphosphino)butane 1,4-双(二苯基膦)丁烷dppe 1,2-bis(diphenylphosphino)ethane 1,2-双(二苯基磷)乙烷dppf l ,l'-bis(diphenylphosphino)ferrocene l , l'-双(二苯基磷)二茂铁dppp 1,3-bis(diphenylphosphino)propane 1,2-双(二苯基磷)丙烷DTBP di-t-butyl peroxide二叔丁基过氧化物EDA ethyl diazoacetate 重氮乙酸乙酯EDC l-ethyl-3-(3-dimethylaminopropyl)-carbodiimide 1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐EDCI = EDCee enantiomeric excess对映体过量EE l-ethoxyethyl 乙氧基乙基Et ethyl 乙基ETSA ethyltrimethylsilylacetate (三甲基硅基)醋酸乙酯EWG electron withdrawing group 吸电基团Fc ferrocenyl 二茂铁基Fmoc 9-fluorenylmethoxycarbonyl 9-芴甲氧羰酰基fp ftash point 闪点Hex n-hexyl 正己基HMDS hexamethyldisilazane六甲基二硅胺烷HMPA hexamethylphosphoric triamide六甲基膦酸三酰胺HOBt 1-hydroxybenzotriazole 1-羟基苯并三唑HOBT =HOBtHOSu N-hydroxysuccinimide N-羟基琥珀酰亚胺Im imidazole (imidazolyl) 咪唑Ipc isopinocampheyl 异松蒎基IR infrared 红外KHDMS potassium hexamethyldisilazide 六甲基二硅胺钾LAH lithium aluminum hydride 氢化铝锂LD50 dose that is lethal to 50% of test subjects 致死量为受试者的50%LDA lithium diisopropylamide 二异丙基氨基锂LDMAN lithium1-(dimethylamino)naphthalenide ? 1-(二甲氨基)萘锂LHMDS(LiHMDS)lithium hexamethyldisilazide 六甲基叠氮乙硅锂, 六甲基二硅氨基锂LICA lithiuim isopropylcyclohexylamide 异丙基环己氨基锂LiTMP(LTMP) lithium2,2,6,6-tetramethylpiperidide 2,2,6,6-四甲基哌啶锂哌啶(氮杂环己烷)LTA lead tetraacetate 四乙酸铅lut 2,6-lutidine 二甲基吡啶MCPBA(m-CPBA) m-chloroperbenzoic acid 间氯过苯酸MA maleic anhydride 顺丁烯二酸酐MAD methyl aluminum bis(2,6-di-t-butyl-4-methylphenoxide) ?MAT methyl aluminum bis(2,4,6-tri-t-butylphenoxide) ?Me methyl 甲基MEK methyl ethyl ketone 甲基乙基酮MEM 2-methoxyethoxymethyl (2-甲氧基乙氧基)甲基-MIC methyl isocyanate 甲基异氰酸酯MMPP magnesium monoperoxyphthalate 单过氧邻苯二甲酸镁MOM methoxymethyl 甲氧甲基MoOPH oxodiperoxomolybdenum(pyridine)-(hexamethylphosphoric triamide)mp melting point 熔点MPM methoxy(phenylthio)methyl 甲氧基(苯硫基)甲基,Ms methanesulfonyl (mesyl) 甲基磺酰基(保护羟基用)MS mass spectrometry 质谱MS Molecular sieves 分子筛MTEE (MTBE) methyl t-butyl ether 甲基叔丁基醚MTM methylthiomethyl 二甲硫醚MVK methyl vinyl ketone 甲基乙烯基酮n refractive index 折射率NaHDMS sodium hexamethyldisilazide 六甲基二硅胺钠Naph(Np) naphthyl 萘基NBA N-bromoacetamide N-溴乙酰胺NBD norbornadiene(bicyclo[2.2.1]hepta-2,5-diene) 二环庚二烯(别名:降冰片二烯)NBS N-bromosuccinimide N-溴代丁二酰亚胺(别名:N-溴代琥珀酰亚胺)NCS N-chlorosuccinimide N-氯代丁二酰亚胺. (别名:N-氯代琥珀酰亚胺)NIS N-iodosuccinimide N-碘代丁二酰亚胺(别名:N-碘代琥珀酰亚胺)NMO N-methylmorpholine N-oxide N-甲基氧化吗啉NMP N-methyl-2-pyrrolidone N-甲基-2-吡咯烷酮NMR nuclear magnetic resonance 核磁共振NORPHOS 5,6-bis(diphenylphosphino)-2-norbornene ?5,6-双(二苯基磷)-2-降冰片烯PCC pyridinium chlorochromate 吡啶氯铬酸盐PDC pyridinium dichromate 二氯吡啶酯Pent n-pentyl 正戊基Ph phenyl 苯基Phen 1,10-phenanthroline 1,10-菲罗啉Phth phthaloyl 邻苯二甲酰基Piv pivaloyl 新戊酰基PMB p-methoxybenzyl 对甲氧苄基;对甲氧苯甲基PMDTAPPA polyphosphoric acid 多聚磷酸PPE Polyphenylene Ether 聚苯醚PPTS pyridinium p-toluenesulfonate吡啶对甲苯磺酸Pr propyl丙基PTC phase-transfer catalysis (phase-transfer catalyst)相转移催化(相转移催化剂)PTSA(or TsOH) p-toluenesulfonic acid对甲苯磺酸Py (pyr) pyridine (or pyridyl)吡啶(或吡啶)PAMPrt room temperature 室温salen 双水杨酰胺乙基钴SAMP (S)-1-amino-2-(methoxymethyl)pyrrolidine(s)-1 -氨基- 2-(甲氧甲基)吡咯烷SET single electron transfer单电子转移Sia siamyl (s-isoamyl or 1,2-dimethylpropyl)TASF tris(diethylamino)sulfonium difluorotrimethylsilicateTBAB tetra-n-butylammonium bromide四丁基溴化铵TBAF tetra-n-butylammonium fluoride四丁基氟化TBADTBAI tetra-n-butylammonium iodide四丁基碘化TBAPTBDMS(TBS) t-butyldimethylsilyl二甲基硅烷TBDPS(BPS) t-butyldiphenylsilylTBHP t-butyl hydroperoxide叔丁基氢TBS t-butyldimethylsilyl二甲基硅烷TCNE tetracyanoethylene四氰基乙烯TCNQ 7,7,8,8-tetracyano-para-quinodimethaneTEA triethylamine三乙胺TEAB tetratehylammonium bromideTEBAC triethylbenzylammonium chloride三乙基氯化铵TEMPO 2,2,6,6-tetramethylpipedinyloxyTES triethylsilyl三乙基硅烷Tf trifluoromethanesulfonyl三氟甲基TFA trifluoroacetic acid三氟乙酸TFAA trifluoroacetic anhydride三氟乙酸酐THF tetrahydrofuran四氢呋喃THP 2-tetrahydropyranyl2 -吡喃ThxTIPS triisopropylsilylTMAO (TMANO) trimethylamine N-oxide三甲胺氮氧化物TMEDA N,N,N',N-tetramethyl- -hexaacetic acidTMG 1,1,3,3-tetramethylguanidineTMS tetramethylsilane四甲基Tol p-tolyl对甲苯TPAP tetra-n-propylammonium perruthenateTBHPTPP thiamine pyrophosphate5,10,15,20 -四苯基卟啉Tr triphenylmethyl (trityl)三苯(三苯甲基)Ts p-toluenesulfonyl (tosyl)对甲苯磺酰(磺酰)TTN thallium(III)-trinitrate硝酸铊(Ⅲ)UHP urea-hydrogen peroxide complex尿素过氧化氢复合Z benzyloxycarbonyl苄氧羰基。
高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼

·药物研发·高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼赵会明 张振洋 樊华军[英格尔检测技术服务(上海)有限公司 上海 201100]摘要建立了泮托拉唑钠原料药中的基因毒性杂质水合肼的高效液相色谱-串联质谱(LC-MSMS)检测方法。
采用反相色谱,以水-乙腈(含0.1%甲酸)为流动相,梯度洗脱,流速0.5 mL/min,以ESI正离子多反应监测(MRM)模式进行质谱检测。
结果显示,水合肼的检测限和定量限可达到0.23、0.47 ng/mL,其在0.47~9.37 ng/mL浓度范围内线性关系良好(r=0.999 9),准确度试验中低、中、高浓度回收率均在81.6%~90.9%之间。
在3批次泮托拉唑钠原料药中均未检出水合肼。
关键词高效液相色谱-串联质谱法基因毒性杂质泮托拉唑钠水合肼痕量检测中图分类号:R917; O657 文献标志码:A 文章编号:1006-1533(2022)11-0072-04引用本文 赵会明, 张振洋, 樊华军. 高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼[J]. 上海医药, 2022, 43(11): 72-75.Determination of hydrazine hydrate in pantoprazole sodium by high performance liquid chromatography-tandem mass spectrometryZHAO Huiming, ZHANG Zhenyang, FAN Huajun[ICAS Testing Technology Service (Shanghai) CO., LTD., Shanghai 201100, China]ABSTRACT To establish a high-performance liquid chromatography-tandem mass spectrometry (LC-MSMS) method for the determination of hydrazine hydrate in active pharmaceutical ingredient (API) pantoprazole sodium. HPLC was carried out by reverse chromatography using water-acetonitrile containing 0.1% formic acid as flow phase and gradient elution at a flow rate of 0.5 mL/min. Mass spectrometry was performed with multi-reaction monitoring (MRM) in positive ESI mode. The detection and quantitative limits of hydrazine hydrate reached 0.23, 0.47 ng/mL and hydrazine hydrate showed good linear relationship in the range of 0.47-9.37 ng/mL (r=0.999 9). The recoveries of samples at low, medium and high-level concentrations reached81.6% to 90.9% in the accuracy experiment. No hydrazine hydrate was detected in 3 batches of pantoprazole sodium.KEY WORDS HPLC-tandem mass spectrometry; genotoxic impurities; pantoprazole sodium; hydrazine hydrate; trace determination上消化道出血是近年的临床疾病中常见且多发的一种疾病,其临床表现为呕血、黑便等,如得不到及时有效治疗,可能引发失血性休克。
Tapentadol_hydrochloride_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Tapentadol (hydrochloride) is a potent agonist of the μ–opioid receptor and as a norepinephrine reuptake inhibitor (NRI).In Vitro: Tapentadol (0.5 μM) has a more pronounced effect than tramadol on the decrease of metabolic activity, and cell biomass.Toxicity caused by tramadol and tapentadol is not predominantly caused by oxidative damage. Tapentadol (200 μM) induces some signs of apoptosis, and significant increases in intracellular glucose and lactate. Both tramadol and tapentadol are shown to promote a decrease in ATP levels, suggesting alterations in energetic metabolism [1].In Vivo: Tapentadol (5.6, 10, 31.6, and 56.2 mg/kg) shows superior antinociceptive effect versus ketorolac in the mouse writhing test. The maximum effect of tapentadol is 99.29%, whereas than ketorolac of 80.14%[2].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Caspase–3 activity is determined as described. 50 μL of each lysate is mixed with 200 μL assay buffer (100 nM HEPES (pH 7.5), 20% (v/v) glycerol, 5 mM DTT, 0.5 mM EDTA) and 5 μL of the caspase–3 peptide substrate Ac–DEVD–pNA (final concentration 80 μM), in 96 well–plates, followed by incubation at 37°C for 24 h. Caspase–3 activity is determined at 405 nm, by quantifying the reaction product, using a microplate reader (Biotek Synergy 2).Cell Assay:[1]MTT assay measures dehydrogenase activity, an indicator of metabolically active mitochondria, and thus of cell viability.After 48 h of exposure to tramadol and tapentadol at 37°C and 5% CO 2, cells are incubated for 4 additional hours with 200 μL ofDMEM containing 0.5 mg/mL 3–(4,5–dimethylthiazol–2–yl)–2,5–diphenyltetrazolium (MTT). After incubation, the cell culture medium is removed, and formazan crystals are then solubilized by adding 100 μL of solubilization solution [(89% (v/v) isopropanol, 10% (v/v)Triton X–100, 1% (v/v) of HCl 0.37% (w/v)]. Upon homogenization, absorbance values are read at 550 nm in a plate reader andretrieved using Gene5 software. Results are presented as the percentage of cell viability versusconcentration. All drugs are tested in 5independent experiments, with each concentration tested in 6 replicates within each experiment.Animal Administration: Tapentadol is formulated in saline.[2]Different groups are used to characterize the dose–response curve of each individual drug. Tapentadol (5.6, 10, 31.6, and 56.2 mg/kg) or ketorolac (5.6, 10, 31.6, and 56.2 mg/kg) areadministered 15 min before acetic acid. Controls are administered saline. Once the dose–response curve of each drug isobtained, an experimental ED 50 value is determined for each and the tapentadol–ketorolac mixture is assessed in three different proportions (1:1, 3:1, and 1:3).References:[1]. Faria J, et al. Comparative study of the neurotoxicological effects of tramadol and tapentadol in SH–SY5Y cells. Toxicology. 2016 Jun 1;359–360:1–10.[2]. Zapata–Morales JR, et al. Isobolographic Analysis of the Interaction Between Tapentadol and Ketorolac in a Mouse Model of Visceral Pain. Drug Dev Res.Product Name:Tapentadol (hydrochloride)Cat. No.:HY-70042A CAS No.:175591-09-0Molecular Formula:C 14H 24ClNO Molecular Weight:257.80Target:Opioid Receptor; Opioid Receptor Pathway:GPCR/G Protein; Neuronal Signaling Solubility:H 2O: ≥ 2.6 mg/mL2016 Jun;77(4):187–91.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
罗地亚产品目录

HLB:8/10.5/11.4/12.1/13.3/13.7
浊点:48-50/64-66/69-72/73-75/57-59/75-77
FentacareF08
无溶剂C9-C11烷基乙氧基化物
RhodafacRP-710
有机磷酸酯阴离子表面活性剂
微浊液体
245KG/桶
低泡,良好清洗、润湿、乳化性能;高电流忍受能力,可用于电解除油、化学除油除蜡和PCB除油整孔,也可以和低泡非离子复配,用于喷淋。
RhodocalTS-60/MX
磷酸酯类阴离子表面活性剂
粘稠液体
200KG/桶
含氨基的阴离子表面活性剂,低泡,高电流负荷稳定,常与油酸类的表活复配用于电解除油粉
产品名称
化学成分
外观
产品特点及应用
MirapolWT
阳离子聚合物
淡黄色液体
200KG/桶
无氰碱性镀锌的走位剂、整平剂,能明显缩短电镀时间,改善镀层的均匀性和深镀能力,节省锌的用量,同时提高镀层的耐腐蚀能力。
PH:7.5-8.5;添加量:1.5g/L;消耗量:40-70g/KAh
沉降剂:主用于磨削液中吸附金属屑沉降
黄色液体
200KG/桶
用于水基清洗配方表面活性剂混合物,低泡、耐酸碱,对于清除油脂类的有机油污非常有效,经常可以取代溶剂,降低成本,适用于高压和低压清洗设备。
Antarox L61/62/64
EO/PO嵌段共聚物
透明液体
204.12KG/桶
泡沫控制能力优异,适用范围广。可替代陶氏TergitolL、巴斯夫Pluronic PE、亨斯曼Surfonic POA-L系列。
成都普瑞法科技开发有限公司 水飞蓟宾(Silybin) 说明书

成都普瑞法科技开发有限公司Chengdu Biopurify Phytochemicals Ltd.Material Safety Data Sheet Version 2.7Revision Date 11/01/20171. Chemical Product and company IdentificationProduct Name:水飞蓟宾English Name: Silybin(Mixture A&B)Catalogue No:22888-70-6Supplier: Chengdu Biopurify Phytochemicals Ltd.Address: No.11 Building,No.388 Rongtaidadao CNSTP,Wenjiang Zone,Chengdu Sichuan 611130 ChinaTel:+86-28-82633987 Fax:+86-28-82633165Website: Email: **********************2、Composition, Information on IngredientsAlias: Silybin(Mixture A&B)CAS Number:22888-70-6Mol. Formula: C25H22O10Mol. Weight :482.441Identified uses: Laboratory chemicals, for R&DStorage: Room temperature for transportation, 2~8℃ for long term storage, protected from strong light, keep package airproofed when not in use.3、Hazards identificationClassification of the substance or mixtureNot a hazardous substance or mixture according to Regulation (EC) No 1272/2008This substance is not classified as dangerous according to Directive 67/548/EEC.Label elementsNot a hazardous substance or mixture according to EC-directives 67/548/EEC or 1999/45/EC. Other hazards -The chemical, Physical and toxicological properties of this product have not been thoroughlyinvestigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.4、First-aid measuresIf inhaledIf breathed in, move person into fresh air. If not breathing, give artificial respiration.Consult a doctor.In case of skin contactWash off with soap and plenty of water. Consult a doctor.In case of eye contactFlush eyes with water as a precaution. Consult a doctor.If swallowedNever give anything by mouth to an unconscious person. Rinse mouth with water. Consult a doctor.Indication of any immediate medical attention and special treatment neededNo data availableShow this safety data sheet to the doctor in attendance.Immediate medical attention is required.5、Fire-fighting measuresConditions of flammabilityNot flammable or combustible.Suitable extinguishing mediaUse water spray, alcohol-resistant foam, dry chemical or carbon dioxide.Special protective equipment for firefightersWear self contained breathing apparatus for fire fighting if necessary.Hazardous combustion productsHazardous decomposition products formed under fire conditions. - Carbon oxides6、Accidental release measuresPersonal precautionsUse personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.Environmental precautionsDo not let product enter drains.Methods and materials for containment and cleaning upPick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed containers for disposal.7、Handing and storageHandling:Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation. Keep away from sources of ignition. Avoid prolonged or repeated exposure.Storage:Store in a well closed container. Protected from air and light, put into refrigerate or freeze forlong term storage.Specific end usesUse in a laboratory fume hood where possible. Refer to employer is COSHH risk assessment.8、Exposure controls, personal protectionContains no substances with occupational exposure limit values.Personal protective equipmentRespiratory protectionWhere risk assessment shows air-purifying respirators are appropriate use a full-face particle respirator type N100 (US) or type P3 (EN 143) respirator cartridges as a backup to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air respirator. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU).Hand protectionHandle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. Eye protectionFace shield and safety glasses Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).Skin and body protectionComplete suit protecting against chemicals, The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Hygiene measuresHandle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.9、Physical and chemical propertiesPhysical Description : Off-white powderProperty ValuespH No information availableMelting point/freezing point164-174ºCBoiling point793ºC at 760mmHgFlash point274.5ºCDensity 1.527g/cm3Evaporation rate No information availableUpper flammability limits No information availableLower flammability limit No information availableVapor pressure 1.63E-26mmHg at 25°CVapor density No information availableSpecific gravity No information availableWater solubility No information availableSolubility in other solvents No information availablePartition coefficient No information availableAutoignition temperature No information availableKinematic viscosity No information availableDecomposition temperature No information availableExplosive properties No information availableOxidizing properties No information available10、Stability and reactivityChemical stabilityStable under recommended storage conditions.Possibility of hazardous reactionsno data availableConditions to avoidno data availableMaterials to avoidStrong oxidizing agentsHazardous decomposition productsHazardous decomposition products formed under fire conditions. - Carbon oxidesOther decomposition products - no data available11、Toxicological informationAcute toxicityOral LD50: no data availableInhalation LC50: no data availableDermal LD50: no data availableOther information on acute toxicity: no data availableno data availableSerious eye damage/eye irritationno data availableRespiratory or skin sensitisationno data availableGerm cell mutagenicityno data availableCarcinogenicityIARC: No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at levels greater than or equal to 0.1% is identified as a carcinogen or potential carcinogen by ACGIH.NTP: No component of this product present at levels greater than or equal to 0.1% is identified as a known or anticipated carcinogen by NTP.OSHA: No component of this product present at levels greater than or equal to 0.1% is identified as a carcinogen or potential carcinogen by OSHA.Reproductive toxicityno data availableTeratogenicityno data availableSpecific target organ toxicity - single exposure (Globally Harmonized System)no data availableSpecific target organ toxicity - repeated exposure (Globally Harmonized System)no data availableAspiration hazardno data availablePotential health effectsInhalation May be harmful if inhaled. Causes respiratory tract irritation.Ingestion May be harmful if swallowed.Skin May be harmful if absorbed through skin. Causes skin irritation.Eyes May be caused eye irritation.Synergistic effectsno data availableAdditional InformationRTECS: Not available12、Ecological informationToxicityno data availablePersistence and degradabilityno data availableBioaccumulative potentialno data availableMobility in soilno data availablePBT and vPvB assessmentno data availableOther adverse effectsno data available13、Disposal considerationsProductOffer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed professional waste disposal service to dispose of this material.Contaminated packagingDispose of as unused product.14、Transport informationClassified according to the criteria of the UN Model Regulations as reflected in the IMDG Code, ADR, RID and IATA.UN numberDoes not meet the criteria for classification as hazardous for transport.UN proper shipping nameADR/RID: Not dangerous goodsIMDG: Not dangerous goodsIATA: Not dangerous goodsTransport hazard class(es)Does not meet the criteria for classification as hazardous for transport.Packaging groupDoes not meet the criteria for classification as hazardous for transport.Environmental hazardsThis product is not classified as environmentally hazardous according to the UN Model Regulations, nor a marine pollutant according to the IMDG Code.Special precautions for userno data available15、Regulatory informationThis safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.Safety, health and environmental regulations/legislation specific for the substance or mixtureno data availableChemical Safety AssessmentFor this product a chemical safety assessment was not carried out16、Other informationDISCLAIMERFor R&D use only. Not for drug, household or other uses.WARRANTYThe above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Biopurify and its Affiliates shall not be held liable for any damage resulting from handling or from contact with the above product. See and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.。
脯氨酸脱氢酶(ProDH)试剂盒说明书

脯氨酸脱氢酶(ProDH)试剂盒说明书脯氨酸脱氢酶(Proline dehydrogenase,ProDH)试剂盒说明书微量法100管/96样注意:正式测定前务必取23个预期差异较大的样本做预测定测定意义:ProDH是存在于线粒体内的催化脯氨酸降解的关键酶。
脯氨酸是分布Z广泛的一种渗透物质,在胁迫条件下很多植物可以通过增加合成、减少降解而在体内累积大量脯氨酸,降低ProDH活性对于调节渗透平衡、防止渗透胁迫对植物造成伤害、清除自由基、保护细胞结构具有重要意义。
测定原理:利用异硫氰酸甲酯检测ProDH催化的脱氢反应,600nm处吸光值的吸光值的变化反映酶活性的高低。
需自备的仪器和用品:可见分光光度计/酶标仪、台式离心机、可调式移液器、微量石英比色皿/96孔板、研钵、冰和蒸馏水。
试剂的组成和配制:提取液:100mL×1瓶,4℃保存;试剂一:液体2 mL×1支,4℃保存;试剂二:液体25mL×1瓶,4℃保存;试剂三:粉剂×1瓶,4℃保存;临用前加入4mL蒸馏水充分溶解待用,用不完的试剂4℃保存;试剂四:粉剂×1瓶,4℃保存;临用前加入4mL蒸馏水充分溶解待用,用不完的试剂4℃保存;试剂五:粉剂×4支,4℃保存;粗酶液提取:按照组织质量(g):提取液体积(mL)为1:5~10的比例(建议称取约0.1g样本,加入1mL提取液),进行冰浴匀浆,1500g 4℃离心15min,取上清液于一支新的EP管中,加入一滴试剂一(用10μL的枪头加入),涡旋混匀,冰浴放置30min后,16000g 4℃离心20min,取上清置冰上待测。
测定步骤:1、分光光度计或酶标仪预热30min以上,调节波长至600nm,蒸馏水调零。
2、样本测定(1)混合液的配制:首先将试剂三和试剂四配成溶液(见试剂的组成和配制),临用前根据用量按照试剂二(V):试剂三(V):试剂四(V)=2.4(mL):0.3(mL):0.3(mL)的比例充分混匀。
特佩洛酯溶液Trigonox BPIC-CP75产品数据说明说明书

Product Data SheetTrigonox BPIC-CP75tert-Butylperoxy isopropyl carbonate, 75% solution in odorless mineralspritsTrigonox® BPIC-CP75 can be used for the polymerization and copolymerization of styrene in the temperature range of 95-125°C. During polymerization the temperature is increased in steps.CAS number2372-21-6EINECS/ELINCS No.219-143-7TSCA statuslisted on inventoryMolecular weight176.2Active oxygen contentperoxide9.08%SpecificationsActive oxygen 6.72-6.90 %Appearance Clear liquidAssay74.0-76.0 %Color≤ 10 Pt-Co / APHAHydroperoxides as TBHP≤ 0.10 %Inorganic + organic hydrolysable chloride≤ 150 mg/kgViscosity, 20°C 2.3 mPa.sCharacteristicsDensity, 20 °C0.900 g/cm³Viscosity, 20 °C 2.3 mPa.sApplicationsTrigonox® BPIC-CP75 can be used for the market segments: polymer production, thermoset composites and acrylics production with their different applications/functions. For more information please check our website and/or contact us.Half-life dataThe reactivity of an organic peroxide is usually given by its half-life (t½) at various temperatures. For Trigonox® BPIC-CP75 in chlorobenzene half-life at other temperatures can be calculated by using the equations and constants mentioned below:0.1 hr at 137°C (279°F)1 hr at 117°C (243°C)10 hr at 98°C (208°F)Formula 1kd = A·e-Ea/RTFormula 2t½ = (ln2)/kdEa150.15 kJ/moleA 2.49E+16 s-1R8.3142 J/mole·KT(273.15+°C) KThermal stabilityOrganic peroxides are thermally unstable substances, which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition of a substance in the original packaging may occur is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT70°C (158°F)Method The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides a loss of quality can be detected over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts Max.25°C (77°F)Ts Min.-20°C (-4°F) to prevent crystallizationNote When stored according to these recommended storage conditions, Trigonox®BPIC-CP75 will remain within the Nouryon specifications for a period of at least 3months after delivery.Packaging and transportThe standard packaging is 10 kg of peroxide solution in 13 l HDPE drums. Both packaging and transport meet the international regulations. For the availability of other packed quantities consult your Nouryon representative. Trigonox®BPIC-CP75 is classified as Organic peroxide type C; liquid, Division 5. 2; UN 3103.Safety and handlingKeep containers tightly closed. Store and handle Trigonox® BPIC-CP75 in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalis and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for further information on the safe storage, use and handling of Trigonox® BPIC-CP75. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsCarbon dioxide, Methane, Acetone, tert-Butanol, IsopropanolAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Trigonox® is a registered trademark of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2022-11-2© 2022Polymer production Trigonox BPIC-CP75。
