la1007846_Hierarchical Carbon Foams with Independently Tunable Mesopore and Macropore Size Distribut
格力公司环保产品中有害物质控制管理规定
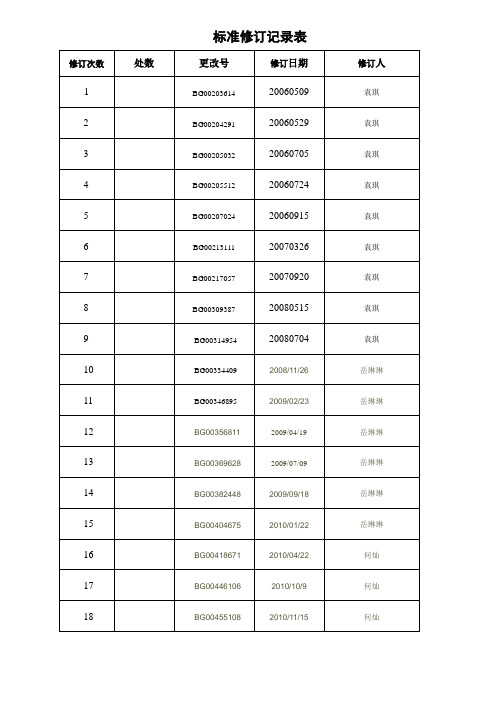
标准修订记录表QJ环保产品中有害物质控制管理规定珠海格力电器股份有限公司发布目次前言 (II)引言 (III)1 范围 (1)2 规范性引用文件 (1)3 术语和定义 (1)4 Ⅰ级有害物质具体管理标准 (5)5 Ⅱ级有害物质具体管理标准 (23)6 有害物质环保标识管理及对供应商的要求 (25)附录A(资料性附录)RoHS同质材料计算方法及部分环境管理物质的详细信息 (26)附录B(资料性附录) 各主要国家和地区就有害物质使用实施的法律法规 (35)附录C(资料性附录) 免于注册的物质清单 (39)附录D(资料性附录)注册需提交的信息 (40)附录E(资料性附录) 我司重点物料关注表 (42)附录F(规范性附录) REACH限制物质清单及使用要求 (43)附录G(资料性附录)第1批十五种高关注物质的检测流程 (59)参考文献 (62)前言格力电器股份有限公司技术标准是公司标准化委员会发布的标准,是公司内部使用的技术法规性文件。
本标准规定了格力电器产品需要满足的有害物质控制标准,检验分析方法等,旨在使原材料供应商和公司内部生产采用一致的控制标准,以符合欧盟及其他有关国家和地区或客户对电子电器产品中有毒有害物质的控制要求。
本标准与上次下发的版本相比的主要变化如下:--根据欧盟RoHS豁免条款修订决议修改本标准中关于RoHS各项豁免的表述(见4.1中表4,表6,表8,表9)本标准的附录A、附录B、附录C、附录D、附录F为资料性附录,附录E为规范性附录。
本标准由珠海格力电器股份有限公司提出。
本标准由珠海格力电器股份有限公司标准化委员会归口.本标准由珠海格力电器股份有限公司制冷技术研究院起草。
本标准主要起草人:王茜、袁琪、曾伟强、蔡小洪、颜小琳、龙新文、郑文力、古文育、唐君、高海涛、刘思红、张华、岳琳琳。
本标准于2005年12月首次发布,2006年5月第一次修订,2006年5月第二次修订,2006年7月第三次修订,2006年7月第四次修订,2006年9月第五次修订,2007年3月第六次修订,2007年9月第七次修订,2008年5月第八次修订,2008年7月第九次修订,2008年11月第十次修订,2009年2月第十一次修订,2009年4月第十二次修订,2009年7月第十三次修订,2009年9月第十四次修订,2010年1月第十五次修订,2010年4月第十六次修订,2010年10月第十七次修订,本次第十八次修订。
油漆名称中英文对照

油漆名称中英文对照Epoxy Zinc Rich Primer 环氧富锌漆MIO Epoxy Primer 环氧云铁漆Two‐pack Chlorinated Rubber Paint 2层氯化橡胶漆Acrylic Topcoat 丙烯酸面漆Incrganic Zinc Silicate Primer 无机硅酸锌底漆Modifed Polyester High‐temperature Paint 改性聚酯耐高温漆Heat resistant aluminium silicon paint 耐热铝硅漆Zinc silicate primer 硅酸锌底漆Epoxy Primer 环氧漆Polyurethane Paint 脂肪族聚氨酯面漆Silicone Aluminum 硅铝漆Penguard midcoat 环氧中间漆Epoxy mio barrio paint ,penguard Midcoat MIO 环氧云铁中间漆Hardtop AS Polyuerthane top 聚氨酯面漆Organic silicon high temperature tesistant paint 有机硅耐高温面漆Epoxy Primer(Zinc Free) 环氧底漆(无锌)Epoxy Mastic 环氧树脂乳香(RAL7035) Hempel 45880Epoxy Phenolic 环氧酚醛Hempel 8561Zinc Silicate Primer 硅酸锌Hempel 15700/1570GInorganic Silicate Topcoat 无机硅酸盐(珍珠灰)Amercoat 04Y31858A Epoxy Mastic 环氧树脂乳香Hempel 45880Zinc Rich Epoxy Primer 环氧富锌底漆Hempel 17360 Polyurethane Acrylic 聚氨酯丙烯酸(RAL7035) Hempel 55210 Polyamide cured epoxy primer 环氧聚酰胺底漆Polyamide cured epoxy(high build) 环氧聚酰胺底漆(高固性)Two‐pack high build aliphatic Polyurethan 2层高固性脂肪族聚氨酯漆(RAL9010) Inorgaic Zinc Silicate 无机硅酸锌(Gavosil 15700)Aluminium pigments of Silicone铝颜料硅树脂(Silicone Aluminium 56914) Polyamide cured epoxy primer环氧聚酰胺底漆Polyamide cured epoxy primer(high build)环氧聚酰胺底漆(高固性)Two‐pack high build aliphatic polyurethane 2层高固性脂肪族聚氨酯漆(RAL9010) Epoxy Zinc Rich Primer 环氧富锌漆MIO Epoxy Primer 环氧云铁漆Acrylic Topcoat 丙烯酸面漆(RAL9006)Epoxy Zinc Rich Primer 环氧富锌漆MIO Epoxy Primer 环氧云铁漆Two‐pack Chlorinated Rubber Paint 2层氯化橡胶漆(RAL9006)Inorgaic Zinc Silicate 无机硅酸锌Silicone Aluminium 硅胶铝Incrganic Zinc Silicate Primer 无机硅酸锌底漆Modifed Polyester High‐temperature Paint 改性聚酯耐高温漆Inorganic Zinc Silicate 无机硅酸锌Modifed Silicone Acrylics 改良有机硅丙烯漆zinc silicate primer 硅酸锌底漆Heat resistant aluminium silicon paint 耐热铝硅漆(RAL N7.0)Epoxy Zinc Rich Primer环氧富锌漆MIO Epoxy Primer 环氧云铁漆Futura AS,Acrylic Polyurethane top Coat 丙烯酸聚氨酯面漆(RAL7035)Zinc silicate primer 硅酸锌底漆Epoxy Primer 环氧漆Polyurethane Paint 脂肪族聚氨酯面漆 (RAL9010)Silicone aluminum 铝粉硅树脂Organic Primer 有机锌MIO Epoxy Primer 环氧漆Polyurethane Paint聚氨酯面漆(RAL 6024)Zinc silicate primer硅酸锌底漆Heat resistant aluminium silicon paint耐热铝硅漆(RAL N7.0)Epoxy phenolic resin paint环氧酚醛树脂漆Epoxy phenolic paint环氧酚醛漆Epoxy asphalt thick blade Primer环氧沥青厚桨底漆Epoxy asphalt thick blade Finish Coat(RAL 9005)环氧沥青厚桨底漆(RAL 9005) Epoxy Zinc Rich Primer 环氧富锌底漆Epoxy Polyamide paint环氧聚酰胺漆Two Pack Poly‐urethane Paint双组份聚亚安酯(RAL 1014)Poly‐urethane Paint聚亚安酯(RAL 1014)TwoPack Silicon acrylic Paint双组份硅丙烯酸(RAL 9006)Silicone heat-resistant aluminum paint 有机硅耐热铝漆High build epoxy intermediate paint 环氧厚浆中间漆。
好威皮具护理产品目录英文翻译

目录一步翻新色浆168A(油性).. 错误!未定义书签。
One-step refurbished mill base168 A (oil)一步翻新色浆168B(水性) .. 错误!未定义书签。
One-step refurbished mill base168 B(water)绒面磨砂翻新剂168C.............. 错误!未定义书签。
Suede nubuck refurbished agent 168C绒面皮改色剂............................. 错误!未定义书签。
Suede leather recolor agent绒面磨砂增艳剂......................... 错误!未定义书签。
Suede nubuck brightener agent红光去除剂................................. 错误!未定义书签。
Red dye remover环保超细无酪色膏(水性).......... 错误!未定义书签。
Environmental protection ultrafine no cream color paste (water)超艳荧光无酪色膏(水性).......... 错误!未定义书签。
Super bright fluorescent no cream color paste (water)金属颜料..................................... 错误!未定义书签。
Metallic pigment优丽高浓缩染料水..................... 错误!未定义书签。
Optimal high concentrated dye water皮革超能净面液......................... 错误!未定义书签。
Leather chaoneng net surface fluid强力去膜剂................................. 错误!未定义书签。
袄128小雯材料汇总

袄128小雯材料汇总袄128小雯是一种新型纤维素袄料。
具有优异的性能和广泛的适用性。
下面对袄128小雯的材料进行了详细介绍和分析,希望能够为读者提供有价值的信息。
一、原材料1、竹浆醋酸纤维竹浆醋酸纤维是袄128小雯的主要原材料之一,它是通过将竹浆用纯碱和醋酸进行酯化反应得到的。
竹浆醋酸纤维具有优异的抗菌、防臭、透气、吸湿、快干等特性,可以为袄128小雯的品质提供保障。
2、涤纶丝涤纶丝是一种合成纤维,它是袄128小雯的另一个原材料。
涤纶具有优异的强度、耐磨性、耐腐蚀性和易于染色等特点,可以为袄128小雯的外观和品质提供保证。
3、其他原材料除了竹浆醋酸纤维和涤纶丝,袄128小雯的生产还需要其他一些原材料,例如染料、酵素、纤维增性剂等。
这些原材料的选择和使用对袄128小雯的性能和质量都有着重要的影响。
二、生产工艺1、原料制备首先需要将涤纶丝和竹浆醋酸纤维进行拼混,以确保袄128小雯的透气性和吸湿性。
然后需要将拼混后的原料与染料、酵素、纤维增性剂等进行混合,以达到想要的颜色、手感、抗皱性等要求。
2、纺织加工在经过原料制备之后,就需要进行纺织加工了。
首先将原料分成纤维束,然后通过纺丝机将其纺织成纤维线。
接着,将纤维线通过织机进行织造,直到袄128小雯的成品形态出现。
3、后处理在经过纺织加工后,袄128小雯还需要进行后处理才能软化、定型和防皱。
后处理的过程包括洗涤、烘干、卷边、整烫等等。
这些后处理过程是非常重要的,可以保证袄128小雯的品质和性能符合标准要求。
三、应用领域1、服装袄128小雯的透气性和吸湿性非常好,适合用于制作夏季服装、内衣等。
而且它的手感柔软,质量轻,穿着舒适,是极受欢迎的面料之一。
2、家居用品袄128小雯的耐磨性强,易于清洁,具有抗菌防臭、透气吸湿等特性,适合用于制作各种家居用品,如窗帘、坐垫、桌布等等。
3、医疗卫生袄128小雯具有优异的抗菌、防臭、透气、吸湿等特性,适合用于制作医疗卫生用品,如口罩、披肩、手术衣等等。
科聚亚聚氨酯

HMDI耐黄变系统
品名 LW 520 LW 570 NCO % 4.75 7.5 硬度 90A 75D 固化剂种类 二胺类 二胺类 适用期(分钟) 4~6 1.5 特性 耐水解性佳 高定伸强度、耐水解性佳
6
LF-TDI聚醚低游离系统
品名 LF 800A LF 900A LF 930A LF 950A LF 600D LF 650D LF 700D LF 750D NCO % 2.9 3.8 5.05 6.05 7.25 7.7 8.25 8.9 硬度 80A 90A 93A 95A 60D 65D 70D 75D 固化剂种类 MOCA MOCA MOCA MOCA MOCA MOCA MOCA MOCA 适用期(分钟) 14 9 8.5 7.3 5.25 4.25 3.3 2.8 特性 易加工 动态性能佳、高回弹 动态性能佳、耐屈曲 高温动态性能佳 高温动态性能佳 高温动态性能佳 高温动态性能佳 高温动态性能佳
不断改善是科聚亚的使命。 致力于向客户提供更好的业务方案; 帮助员工学习与成长; 帮助投资者实现创新思考,稳固的发展规划以及满意结果。
1
科聚亚
聚氨酯业务
科聚亚聚氨酯在全球聚氨酯热浇注模塑预聚体、特殊水性聚氨酯分散体以及聚酯多元醇应用上处于领 先地位。我们的业务特性在于了解关注客户所需,提供适合的规格产品和应用开发以及完善的技术服 务与支持。 我们与客户的关系透过广泛全面的产品线和技术能力得以紧密联系,使得我们在业界以创造客户价值 的解决方案者著称。将您复杂而最具挑战的方案交给科聚亚,让我们和您一起开展双赢的方案。 科聚亚在全球提供超过300种聚氨酯预聚体和交联剂,包含Adiprene®, Vibrathane® 和Vibracure®等 高性能产品。 我们产品范围包含各种耐撕裂、耐磨浇注弹性体。它们在硬度、承载能力和耐刮、耐切上的杰出表现 在全球范围内赢得了客户的赞同。 科聚亚产品的主要应用领域包含: 工业和印刷滚轮; 矿山,原油和天然气田开采设备; 机械设备零件; 工业轮; 电器零件和输送设备; 各式运动器械和耗材。 除了传统的MDI、TDI预聚体,科聚亚也提供各种 TDI、 MDI、 PPDI和HDI种类低游离异氰酸酯预聚 体。这些低游离预聚体除了提供更好的物性,操作便利外,在确保环境安全和健康卫生方面也有杰出 的表现。我们的产品系列包括聚酯、聚醚、聚己内酰酯和聚碳酸酯类。 科聚亚提供Fomrez®聚酯多元醇应用于多种软质泡沫、热塑性弹性体、涂层、黏合剂和其它弹性体应 用。Witcobond®聚氨酯分散体具有优异的涂布性和接着性,广泛应用于木材、塑料、玻纤、化纤、皮 革、橡胶和其它多种材料。 商品名: 浇注型聚氨酯: Adiprene®和Vibrathane® 水性聚氨酯: Witcobond® 聚酯多元醇: Fomrez® 高硬度塑料系统: Royalcast®
华奇(中国)化工有限公司

华奇(中国)化工有限公司
佚名
【期刊名称】《橡胶科技》
【年(卷),期】2015(000)001
【摘要】<正>责任严谨创新全球大型橡胶及轮胎行业用酚醛树脂及间苯二酚树脂厂商主要产品"对-特辛基苯酚甲醛增粘树脂"SL-1800系列主要牌号有:SL-1801,SL-1802,SL-1801LFP(低游离酚增粘树脂),SL-1805。
SL-1800系列产品是非热反应性的热塑性树脂,具有优异的增粘性能,可用于任何需要高粘度粘接的复合橡胶制品,尤其是合成橡胶制品。
"对-叔丁基酚醛增粘树脂"SL-1400系列主要牌号有:SL-1401,SL-1402,SL-1403,SL-1405,SL-1407,SL-1408,SL-T421。
【总页数】1页(P2)
【正文语种】中文
【中图分类】F416.72
【相关文献】
1.华奇(中国)化工有限公司 [J], ;
2.华奇(中国)化工有限公司 [J], ;
3.华奇(张家港)化工有限公司 [J], ;
4.华奇(中国)化工有限公司 [J], ;
5.智能、开放、融合,铸就研华新格局——访研华(中国)有限公司工业自动化事业群中国区总经理蔡奇男先生 [J],
因版权原因,仅展示原文概要,查看原文内容请购买。
标准红外光谱图谱

Go to: home • ir • proton nmr • carbon nmr• mass specTable of Contents - IRI. HydrocarbonsII. Halogenated HydrocarbonsIII. Nitrogen Containing CompoundsIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O And P(=O)-O) VI. Sulfur Containing CompoundsVII. Oxygen Containing Compounds (Except -C(=O)-)VIII. Compounds Containing Carbon To Oxygen Double BondsI. HydrocarbonsA. Saturated Hydrocarbons1. Normal Alkanes2. Branched Alkanes3. Cyclic AlkanesB. Unsaturated Hydrocarbons1. Acyclic Alkenes2. Cyclic Alkenes3. AlkynesC. Aromatic Hydrocarbons1. Monocyclic (Benzenes)2. PolycyclicII. Halogenated HydrocarbonsA. Fluorinated Hydrocarbons1. Aliphatic2. AromaticB. Chlorinated Hydrocarbons1. Aliphatic2. Olefinic3. AromaticC. Brominated Hydrocarbons1. Aliphatic2. Olefinic3. AromaticD. Iodinated Hydrocarbons1. Aliphatic and Olefinic2. AromaticIII. Nitrogen Containing CompoundsA. Amines1. Primarya. Aliphatic and Olefinicb. Aromatic2. Secondarya. Aliphatic and Olefinicb. Aromatic3. Tertiarya. Aliphatic and Olefinicb. AromaticB. PyridinesC. QuinolinesD. Miscellaneous Nitrogen HeteroaromaticsE. HydrazinesF. Amine SaltsG. Oximes (-CH=N-OH)H. Hydrazones (-CH=N-NH2)I. Azines (-CH=N-N=CH-)J. Amidines (-N=CH-N)K. Hydroxamic AcidsL. Azo Compounds (-N=N-)M. Triazenes (-N=N-NH-)N. Isocyanates (-N=C=O)O. Carbodiimides (-N=C=N-)P. Isothiocyanates (-N=C=S)Q. Nitriles (-C≡N)1. Aliphatic2. Olefinic3. AromaticR. Cyanamides (=N-C≡N)S. Thiocyanates (-S-C≡N)T. Nitroso Compounds (-N=O)U. N-Nitroso Compounds (=N-N=O)V. Nitrites (-O-N=O)W. Nitro Compounds (-NO2)1. Aliphatic2. AromaticX. N-Nitro-Compounds (=N-NO2)IV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsA. Sulfides (R-S-R)1. Aliphatic2. Heterocyclic3. AromaticB. Disulfides (R-S-S-R)C. Thiols1. Aliphatic2. AromaticD. Sulfoxides (R-S(=O)-R)E. Sulfones (R-SO2-R)F. Sulfonyl Halides (R-SO2-X)G. Sulfonic Acids (R-SO2-OH)1. Sulfonic Acid Salts (R-SO2-O-M)2. Sulfonic Acid Esters (R-SO2-O-R)3. Sulfuric Acid Esters (R-O-S(=O)-O-R)H. Thioamides (R-C(=S)-NH2)I. Thioureas (R-NH-C(=S)-NH2)J. Sulfonamides (R-SO2-NH2)K. Sulfamides (R-NH-SO2-NH-R)VII. Oxygen Containing Compounds (Except -C(=O)-)A. Ethers1. Aliphatic Ethers (R-O-R)2. Acetals (R-CH-(-O-R)2)3. Alicyclic Ethers4. Aromatic Ethers5. Furans6. Silicon Ethers (R3-Si-O-R)7. Phosphorus Ethers ((R-O)3-P)8. Peroxides (R-O-O-R)B. Alcohols (R-OH)1. Primarya. Aliphatic and Alicyclicb. Olefinicc. Aromaticd. Heterocyclic2. Secondarya. Aliphatic and Alicyclicb. Olefinicc. Aromatic3. Tertiarya. Aliphaticb. Olefinicc. Aromatic4. Diols5. Carbohydrates6. PhenolsVIII. Compounds Containing Carbon To Oxygen Double BondsA. Ketones (R-C(=O)-R)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. α-Diketones and β-DiketonesB. Aldehydes (R-C(=O)-H)C. Acid Halides (R-C(=O)-X)D. Anhydrides (R-C(=O)-O-C(=O)-R)E. Amides1. Primary (R-C(=O)-NH2)2. Secondary (R-C(=O)-NH-R)3. Tertiary (R-C(=O)-N-R2)F. Imides (R-C(=O)-NH-C(=O)-R)G. Hydrazides (R-C(=O)-NH-NH2)H. Ureas (R-NH-C(=O)-NH2)I. Hydantoins, Uracils, BarbituratesJ. Carboxylic Acids (R-C(=O)-OH)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. Amino Acids5. Salts of Carboxylic AcidsK. Esters1. Aliphatic Esters of Aliphatic Acids2. Olefinic Esters of Aliphatic Acids3. Aliphatic Esters of Olefinic Acids4. Aromatic Esters of Aliphatic Acids5. Esters of Aromatic Acids6. Cyclic Esters (Lactones)7. Chloroformates8. Esters of Thio-Acids9. Carbamates10. Esters of Phosphorus AcidsPublished by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specTable of Contents - Proton NMRI. HydrocarbonsII. Halogenated HydrocarbonsIII. Nitrogen Containing CompoundsIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsVII. Oxygen Containing Compounds (Except -C(=O)-)VIII. Compounds Containing Carbon To Oxygen Double BondsI. HydrocarbonsA. Saturated Hydrocarbons1. Normal Alkanes2. Branched Alkanes3. Cyclic AlkanesB. Unsaturated Hydrocarbons1. Acyclic Alkenes2. Cyclic Alkenes3. AlkynesC. Aromatic Hydrocarbons1. Monocyclic (Benzenes)2. PolycyclicII. Halogenated HydrocarbonsA. Fluorinated Hydrocarbons1. Aliphatic2. AromaticB. Chlorinated Hydrocarbons1. Aliphatic2. AromaticC. Brominated Hydrocarbons1. Aliphatic2. AromaticD. Iodinated Hydrocarbons1. Aliphatic2. AromaticIII. Nitrogen Containing CompoundsA. Amines1. Primarya. Aliphaticb. Aromatic2. Secondarya. Aliphaticb. Aromatic3. Tertiarya. Aliphaticb. AromaticB. PyridinesC. Quaternary Ammonium SaltsD. HydrazinesE. Amine SaltsF. Ylidene Compounds (-CH=N-)G. Oximes (-CH=N-OH)H. Hydrazones (-CH=N-NH2)I. Azines (-CH=N-N=CH-)J. Amidines (-N=CH-N)K. Hydroxamic AcidsL. Azo Compounds (-N=N-)M. Isocyanates (-N=C=O)N. Carbodiimides (-N=C=N-)O. Isothiocyanates (-N=C=S)P. Nitriles (-C≡N)1. Aliphatic2. Olefinic3. AromaticQ. Cyanamides (=N-C≡N)R. Isocyanides (-N≡C )S. Thiocyanates (-S-C≡N)T. Nitroso Compounds (-N=O)U. N-Nitroso Compounds (=N-N=O)V. Nitrates (-O-NO2)W. Nitrites (-O-N=O)X. Nitro Compounds (-NO2)1. Aliphatic2. AromaticY. N-Nitro-Compounds (=N-NO2)IV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsA. Sulfides (R-S-R)1. Aliphatic2. AromaticB. Disulfides (R-S-S-R)C. Thiols1. Aliphatic2. AromaticD. Sulfoxides (R-S(=O)-R)E. Sulfones (R-SO2-R)F. Sulfonyl Halides (R-SO2-X)G. Sulfonic Acids (R-SO2-OH)1. Sulfonic Acid Salts (R-SO2-O-M)2. Sulfonic Acid Esters (R-SO2-O-R)3. Sulfuric Acid Esters (R-O-S(=O)-O-R)4. Sulfuric Acid Salts (R-O-SO2-O-M)H. Thioamides (R-C(=S)-NH2)I. Thioureas (R-NH-C(=S)-NH2)J. Sulfonamides (R-SO2-NH2)VII. Oxygen Containing Compounds (Except -C(=O)-)A. Ethers1. Aliphatic Ethers (R-O-R)2. Alicyclic Ethers3. Aromatic Ethers4. Furans5. Silicon Ethers (R3-Si-O-R)6. Phosphorus Ethers ((R-O)3-P)B. Alcohols (R-OH)1. Primarya. Aliphaticb. Olefinicc. Aromatic2. Secondarya. Aliphaticb. Aromatic3. Tertiarya. Aliphaticb. Aromatic4. Diols and Polyols5. Carbohydrates6. PhenolsVIII. Compounds Containing Carbon To Oxygen Double BondsA. Ketones (R-C(=O)-R)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. a-Diketones and b-DiketonesB. Aldehydes (R-C(=O)-H)C. Acid Halides (R-C(=O)-X)D. Anhydrides (R-C(=O)-O-C(=O)-R)E. Amides1. Primary (R-C(=O)-NH2)2. Secondary (R-C(=O)-NH-R)3. Tertiary (R-C(=O)-N-R2)F. Imides (R-C(=O)-NH-C(=O)-R)G. Hydrazides (R-C(=O)-NH-NH2)H. Ureas (R-NH-C(=O)-NH2)I. Hydantoins, Uracils, BarbituratesJ. Carboxylic Acids (R-C(=O)-OH)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. Amino Acids5. Salts of Carboxylic AcidsK. Esters1. Aliphatic Esters of Aliphatic Acids2. Olefinic Esters of Aliphatic Acids3. Aromatic Esters of Aliphatic Acids4. Cyclic Esters (Lactones)5. Chloroformates6. Carbamates7. Esters of Phosphorus AcidsPublished by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specTable of Contents - Carbon NMRI. HydrocarbonsII. Halogenated HydrocarbonsIII. Nitrogen Containing CompoundsIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O And P(=O)-O) VI. Sulfur Containing CompoundsVII. Oxygen Containing Compounds (Except -C(=O)-)VIII. Compounds Containing Carbon To Oxygen Double BondsI. HydrocarbonsA. Saturated Hydrocarbons1. Normal Alkanes2. Branched Alkanes3. Cyclic AlkanesB. Unsaturated Hydrocarbons1. Acyclic Alkenes2. AlkynesC. Aromatic Hydrocarbons1. Monocyclic (Benzenes) and PolycyclicII. Halogenated HydrocarbonsA. Fluorinated Hydrocarbons1. Aliphatic2. AromaticB. Chlorinated Hydrocarbons1. Aliphatic2. AromaticC. Brominated Hydrocarbons1. Aliphatic2. AromaticD. Iodinated Hydrocarbons1. Aliphatic2. AromaticIII. Nitrogen Containing CompoundsA. Amines1. Primarya. Aliphaticb. Aromatic2. Secondarya. Aliphaticb. Aromatic3. Tertiarya. Aliphaticb. AromaticB. PyridinesC. Amine SaltsD. Oximes (-CH=N-OH)E. Quaternary Ammonium SaltsF. Nitriles (-C≡N)1. Aliphatic2. Olefinic3. AromaticG. Thiocyanates (-S-C≡N)H. Nitro Compounds (-NO2)1. Aliphatic2. AromaticIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsA. Sulfides (R-S-R)1. Aliphatic2. AromaticB. Disulfides (R-S-S-R)C. Thiols1. Aliphatic2. AromaticD. Sulfones (R-SO2-R)VII. Oxygen Containing Compounds (Except -C(=O)-)A. Ethers1. Aliphatic Ethers (R-O-R)2. Alicyclic Ethers3. Aromatic EthersB. Alcohols (R-OH)1. Primarya. Aliphatic and Alicyclicb. Aromatic2. Secondarya. Aliphatic and Alicyclic3. Tertiarya. Aliphatic4. PhenolsVIII. Compounds Containing Carbon To Oxygen Double BondsA. Ketones (R-C(=O)-R)1. Aliphatic and Alicyclic2. AromaticB. Aldehydes (R-C(=O)-H)C. Acid Halides (R-C(=O)-X)D. Anhydrides (R-C(=O)-O-C(=O)-R)E. Amides1. Primary (R-C(=O)-NH2)2. Secondary (R-C(=O)-NH-R)3. Tertiary (R-C(=O)-N-R2)F. Carboxylic Acids (R-C(=O)-OH)1. Aliphatic and Alicyclic2. AromaticG. Esters1. Aliphatic Esters of Aliphatic Acids2. Olefinic Esters of Aliphatic Acids3. Aromatic Esters of Aliphatic AcidsPublished by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specTable of Contents - MSComing SoonI. HydrocarbonsII. Halogenated HydrocarbonsIII. Nitrogen Containing CompoundsIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O And P(=O)-O) VI. Sulfur Containing CompoundsVII. Oxygen Containing Compounds (Except -C(=O)-)VIII. Compounds Containing Carbon To Oxygen Double BondsI. HydrocarbonsA. Saturated Hydrocarbons1. Normal Alkanes2. Branched Alkanes3. Cyclic AlkanesB. Unsaturated Hydrocarbons1. Acyclic Alkenes2. Cyclic Alkenes3. AlkynesC. Aromatic Hydrocarbons1. Monocyclic (Benzenes)2. PolycyclicII. Halogenated HydrocarbonsA. Fluorinated Hydrocarbons1. Aliphatic2. AromaticB. Chlorinated Hydrocarbons1. Aliphatic2. Olefinic3. AromaticC. Brominated Hydrocarbons1. Aliphatic2. Olefinic3. AromaticD. Iodinated Hydrocarbons1. Aliphatic and Olefinic2. AromaticIII. Nitrogen Containing CompoundsA. Amines1. Primarya. Aliphatic and Olefinicb. Aromatic2. Secondarya. Aliphatic and Olefinicb. Aromatic3. Tertiarya. Aliphatic and Olefinicb. AromaticB. PyridinesC. QuinolinesD. Miscellaneous Nitrogen HeteroaromaticsE. HydrazinesF. Amine SaltsG. Oximes (-CH=N-OH)H. Hydrazones (-CH=N-NH2)I. Azines (-CH=N-N=CH-)J. Amidines (-N=CH-N)K. Hydroxamic AcidsL. Azo Compounds (-N=N-)M. Triazenes (-N=N-NH-)N. Isocyanates (-N=C=O)O. Carbodiimides (-N=C=N-)P. Isothiocyanates (-N=C=S)Q. Nitriles (-C≡N)1. Aliphatic2. Olefinic3. AromaticR. Cyanamides (=N-C≡N)S. Thiocyanates (-S-C≡N)T. Nitroso Compounds (-N=O)U. N-Nitroso Compounds (=N-N=O)V. Nitrites (-O-N=O)W. Nitro Compounds (-NO2)1. Aliphatic2. AromaticX. N-Nitro-Compounds (=N-NO2)IV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsA. Sulfides (R-S-R)1. Aliphatic2. Heterocyclic3. AromaticB. Disulfides (R-S-S-R)C. Thiols1. Aliphatic2. AromaticD. Sulfoxides (R-S(=O)-R)E. Sulfones (R-SO2-R)F. Sulfonyl Halides (R-SO2-X)G. Sulfonic Acids (R-SO2-OH)1. Sulfonic Acid Salts (R-SO2-O-M)2. Sulfonic Acid Esters (R-SO2-O-R)3. Sulfuric Acid Esters (R-O-S(=O)-O-R)H. Thioamides (R-C(=S)-NH2)I. Thioureas (R-NH-C(=S)-NH2)J. Sulfonamides (R-SO2-NH2)K. Sulfamides (R-NH-SO2-NH-R)VII. Oxygen Containing Compounds (Except -C(=O)-)A. Ethers1. Aliphatic Ethers (R-O-R)2. Acetals (R-CH-(-O-R)2)3. Alicyclic Ethers4. Aromatic Ethers5. Furans6. Silicon Ethers (R3-Si-O-R)7. Phosphorus Ethers ((R-O)3-P)8. Peroxides (R-O-O-R)B. Alcohols (R-OH)1. Primarya. Aliphatic and Alicyclicb. Olefinicc. Aromaticd. Heterocyclic2. Secondarya. Aliphatic and Alicyclicb. Olefinicc. Aromatic3. Tertiarya. Aliphaticb. Olefinicc. Aromatic4. Diols5. Carbohydrates6. PhenolsVIII. Compounds Containing Carbon To Oxygen Double BondsA. Ketones (R-C(=O)-R)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. α-Diketones and β-DiketonesB. Aldehydes (R-C(=O)-H)C. Acid Halides (R-C(=O)-X)D. Anhydrides (R-C(=O)-O-C(=O)-R)E. Amides1. Primary (R-C(=O)-NH2)2. Secondary (R-C(=O)-NH-R)3. Tertiary (R-C(=O)-N-R2)F. Imides (R-C(=O)-NH-C(=O)-R)G. Hydrazides (R-C(=O)-NH-NH2)H. Ureas (R-NH-C(=O)-NH2)I. Hydantoins, Uracils, BarbituratesJ. Carboxylic Acids (R-C(=O)-OH)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. Amino Acids5. Salts of Carboxylic AcidsK. Esters1. Aliphatic Esters of Aliphatic Acids2. Olefinic Esters of Aliphatic Acids3. Aliphatic Esters of Olefinic Acids4. Aromatic Esters of Aliphatic Acids5. Esters of Aromatic Acids6. Cyclic Esters (Lactones)7. Chloroformates8. Esters of Thio-Acids9. Carbamates10. Esters of Phosphorus AcidsPublished by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specSaturated HydrocarbonsNormal Alkanes1. C-H stretching vibration:CH3 asymmetric stretching, 2972-2952 cm-1CH3 symmetric stretching, 2882-2862 cm-1CH2 asymmetric stretching, 2936-2916 cm-1CH2 symmetric stretching, 2863-2843 cm-12. C-H bending vibration:CH3 asymmetric bending, 1470-1430 cm-1CH2 asymmetric bending, 1485-1445 cm-1(overlaps band due to CH3 asymmetricbending)3. C-H bending vibration:CH3 symmetric bending, 1380-1365 cm-1(when CH3 is attached to a C atom)4. C-H wagging vibration:CH2 out-of-plane deformations wagging, 1307-1303 cm-1 (weak) 5. CH2 rocking vibration:(CH2)2 in-plane deformations rocking, 750-740 cm-1(CH2)3 in-plane deformations rocking, 740-730 cm-1(CH2)4 in-plane deformations rocking, 730-725 cm-1(CH2) ≥ 6 in-plane deformations rocking, 722 cm-1Splitting of the absorption band occurs in most cases (730 and 720 cm-1) when the long carbon-chain alkane is in the crystalline state (orthorombic or monoclinic form).Coming Soon!Click on a vibrational mode link in the table to the leftor the spectrum above to visualize the vibrational mode here.Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Saturated HydrocarbonsBranched Alkanes1. C-H stretching vibration:CH3 asymmetric stretching, 2972-2952 cm-1CH3 symmetric stretching, 2882-2862 cm-1CH2 asymmetric stretching, 2936-2916 cm-1CH2 symmetric stretching, 2863-2843 cm-12. C-H bending vibration:CH3 asymmetric bending, 1470-1430 cm-1CH2 asymmetric bending, 1485-1445 cm-1(overlaps band due to CH3 symmetric bending)3. C-H bending vibration:-C-C(CH3)-C-C- symmetric bending, 1380-1365 cm-1(when CH3 is attached to a C atom)-C-C(CH3)-C(CH3)-C-C- symmetric bending, 1380-1365 cm-1(when CH3 is attached to a C atom)(CH3)2CH- symmetric bending, 1385-1380 cm-1and 1365 cm-1(two bands of about equal intensity)-C-C(CH3)2-C- symmetric bending,1385-1380 cm-1and 1365 cm-1 (two bands of about equal intensity).(CH3)3C- symmetric bending, 1395-1385 cm-1and 1365 cm-1(two bands of unequal intensity with the 1365 cm-1 band as the much stronger component of the doublet).4. Skeletal vibration:-C-C(CH3)-C-C-,1159-1151cm-1-C-C(CH3)-C(CH3)-C-C-,1130-1116 cm-1(CH3)CH-,1175-1165 cm-1 and 1170-1140 cm-1-C-C(CH3)2-C-,1192-1185 cm-1(CH3)3C-, 1255-1245 cm-1 and 1250-1200 cm-15. C-H rocking vibration:(CH2)2 in-plane deformations rocking, 750-740 cm-1(CH2)3 in-plane deformations rocking, 740-730 cm-1(CH2)4 in-plane deformations rocking, 730-725 cm-1(CH2) ≥ 6 in-plane deformations rocking, 722 cm-1Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Saturated Hydrocarbons Cyclic AlkanesCyclopropanes1. C-H stretching vibration:ring CH 2 asymmetric stretching, 3100-3072 cm -1 ring CH 2 symmetric stretching, 3030-2995 cm -12. Ring deformation vibration:ring deformation, 1050-1000 cm -13. C-H deformation vibration: CH 2 wagging, 860-790 cm -1Cyclobutanes1. C-H stretching vibration:ring CH 2 asymmetric stretching, 3000-2974 cm -1 ring CH 2 symmetric stretching, 2925-2875 cm -12. C-H deformation vibration:ring CH 2 asymmetric bending, ca 1444 cm -13. Ring deformation vibration:ring deformation, 1000-960 cm -1 888-838 cm -14. C-H deformation vibration:ring CH 2 rocking, 950-900 cm -1Cyclopentanes1. C-H stretching vibration:ring CH 2 asymmetric stretching, 2960-2952 cm -1 ring CH 2 symmetric stretching, 2866-2853 cm -1 2. C-H deformation vibration:ring CH 2 asymmetric bending, ca 1455 cm -1 3. Ring deformation vibration:ring deformation, 1000-960 cm -1 4. C-H deformation vibration:ring CH 2rocking, 930-890 cm -1Cyclohexanes1. C-H stretching vibration:ring CH 2 asymmetric stretching, ca 2927 cm -1ring CH 2 symmetric stretching, ca 2854 cm -1 2. C-H deformation vibration:ring CH 2 asymmetric bending, ca 1462 cm -1 3. C-H deformation vibration:ring CH 2 wagging, ca 1260 cm -1 4. Ring deformation vibration:ring deformation, 1055-1000 cm -1 1000- 952 cm -1 5. C-H deformation vibration:ring CH 2 rocking, 890-860 cm -16. The spectra of cyclic alkanes of five or more ring carbons show ring CH 2 stretching frequencies which overlap those of CH 3 and CH 2 groups of their alkyl substituents. These frequencies also overlap thoseof the CH 3 and CH 2 stretching frequencies of acylic alkanes. When samples of unknown composition are examined for the presence of such ring structures, the absorption bands of their spectra at the C-H stretching region should havethe best possible resolution.Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Numerous references cite the spectral region of 2800-2600 cm-1 for obtainingconfirmatory evidence of the presence of saturated simple ring structures. Absorptionat this region consists of a weak band or bands whose pattern and band locations arehelpful in confirming or indicating the presence of these rings. Although such absorptionfeatures have a limited diagnostic value, it is most reliable when the absorption occursin the spectra of simple saturated aliphatic hydrocarbons.Cycloalkanes (8, 9, and 10 C atoms)1 C-H stretching vibration:ring CH2 asymmetric stretching, ca 2930 cm-1ring CH2 symmetric stretching, ca 2850 cm-12. C-H deformation vibration:ring CH2 asymmetric bending, 2 or 3 absorption bands,1487-1443 cm-1Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specUnsaturated HydrocarbonsAcyclic AlkenesMonosubstituted Alkenes (vinyl)1. C=C stretching vibration:C=C stretching, 1648-1638 cm-12. C-H deformation vibration:trans CH wagging, 995-985 cm-1CH2 wagging, 910-905 cm-13. C-H stretching vibration:CH2 asymmetric stretching, 3092-3077 cm-1CH2 symmetric stretching and CH stretching, 3025-3012 cm-1 4. C-H deformation vibration:CH2 asymmetric bending, 1420-1412 cm-15. C-H deformation vibration overtone:overtone of CH2 wagging, 1840-1805 cm-1Asymmetric Disubstituted Alkenes (vinylidine)1. C=C stretching vibration:C=C stretching, 1661-1639 cm-12. C-H deformation vibration:CH2 wagging, 895-885 cm-13. C-H stretching vibration:CH2 stretching asymmetric, 3100-3077 cm-14. C-H deformation vibration overtone:overtone of CH2 wagging, 1792- 1775 cm-1Symmetric Disubstituted Alkenes (cis)1. C=C stretching vibration:C=C stretching, 1662- 1631 cm-12. C-H deformation vibration:cis CH wagging, 730- 650 cm-13. C-H stretching vibration:CH stretching, 3050-3000 cm-1Symmetric Disubstituted Alkenes (trans)1. C=C stretching vibration:C=C stretching, ca 1673 cm-1, very weak or absent2. C-H deformation vibration:trans CH wagging, 980-965 cm-13. C-H stretching vibration:CH stretching, 3050-3000 cm-1Trisubstituted Alkenes1. C=C stretching vibration:C=C stretching, 1692-1667 cm-12. C—H deformation vibration:C-H wagging, 840-790 cm-13. C-H stretching vibration:C-H stretching, 3050-2990 cm-1Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Tetrasubstituted Alkenes1. C=C stretching vibration:C=C stretching, 1680-1665 cm-1, very weak or absentNOTES: The C=C stretching vibration of molecules which maintain acenter of symmetry absorbs very weakly, if at all, in the infrared region and,usually, is difficult to detect. This is true of the trans isomers and thetetrasubstitutedC=C linkages.When two or more olefinic groups occur in the hydrocarbon molecule, the infraredabsorption spectrum shows the additive and combined absorption of theunsaturatedgroups. However, if the unsaturated groups are subject to conjugation, the C=Cstretchingfrequency, usually, is lowered and a splitting of the C=C stretching frequencyband occurs.Conjugation also intensifies the C=C stretching frequency of trans unsaturatedgroups.Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specUnsaturated Hydrocarbons Cyclic AlkenesEndocyclic C=CEndocyclic C=C corresponds to cis symmetrically disubstituted C=C of acyclic alkenes.1. C=C stretching, vibration:C=C stretching, near 1650 cm -1(except cyclobutene, 1560 cm -1 and cyclopentene, 1611 cm -1)2. C-H deformation vibration: CH wagging, 730- 650 cm -13. C-H stretching vibration:CH stretching, 3075- 3010 cm -1(usually two bands, asymmetric stretching and symmetric stretching for 4, 6, 7, and 8 membered rings)1- substituted endocyclic C=C1- substituted endocyclic C=C corresponds to trisubstituted acyclic alkenes.1. C=C stretching vibration:C=C stretching, near 1650 cm -1 (frequency raised)2. C-H deformation vibration: CH wagging, 840-790 cm -13. C-H stretching vibration:CH stretching, near 3000 cm -11.2- disubstituted endocyclic C=C1. C=C stretching vibration:C=C stretching, 1690-1670 cm -1 (4, 5, and 6 membered rings)Exocyclic C=CH 2Exocyclic C=CH 2 corresponds to the asymmetrically disubstituted C=C of acyclic alkenes (vinylidine).1. C=C stretching,1678-1650 cm -1 (4, 5, and 6 membered rings)2. C-H deformation vibration:=CH 2 wagging, 895-885 cm -13. C-H stretching vibration:=CH 2 stretching, near 3050 cm -1NOTES: The C=C stretching frequency of both the endocyclic HC=CH and the exocyclic C=CH 2 is sensitive to ring strain. As the ring size decreases from 6 to 4 members, the C=C stretching frequency of the endocyclic HC=CH is lowered. However, for the C=C stretching frequency of exocyclic C=CH 2, a gradual increase in the C=C stretching frequency occurs as the ring gets smaller. Substitution of methyl groups for the hydrogens of the endocyclic HC=CH and the exocyclic C=CH 2 cause an increase in the C=C stretching frequency.When two or more C=C groups occur in the hydrocarbon molecule, the infrared absorption spectrum shows the additive and combined absorption effects of the unsaturated groups. If such groups are subject to conjugation, the C=C stretching frequency is lowered and asplitting of the C=C stretching frequency band occurs.Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Unsaturated Hydrocarbons AlkynesMonosubstituted Alkynes (RC ≡CH)1. C ≡C stretching vibration:C ≡C stretching, 2140-2100 cm -12. C-H stretching vibration:≡CH bending, ca 3300 cm -13. C-H deformation vibration: ≡CH bending, 642-615 cm -14. C-H deformation vibration overtone:overtone of ≡CH deformation, 1260-1245 cm -1Disubstituted Alkynes (RC ≡CR')1. C ≡C stretching vibration:C ≡C stretching, 2260-2190 cm -1 (unconjugated)NOTES: Although the intensity of the absorption band caused bythe C ≡C stretching vibration is variable, it is strongest when the alkyne group is monosubstituted. When this group is disubstituted in open chain compounds, the intensity of the C ≡C stretching vibration band diminishesas its position in the molecule tends to establish a pseudo center of symmetry. In some instances this band is too weak to be detected and, thus, its absence in the spectrum does not, necessarily, establish proof of the absence of this linkage.Occasionally, the spectra of disubstituted alkynes show two or more bands at the C ≡C stretching region.Conjugation with olefinic double bonds or aromatic rings tend to slightly increase the intensity of the C ≡C stretching vibration band and shift it toa lower frequency.Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Published by Bio-Rad Laboratories, Inc., Informatics Division . © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.。
汉高产品表
铝材用,不含铬,环保产品,须与 4830 合用 25K G 铝材用,不含铬,环保产品,须与 4831 合用 25KG 铝材用,为无铬处理后提高耐蚀性能 铝材用,为无铬处理后提高耐蚀性能 压铸铝材用,不含铬,环保产品 卷材用,不含铬,环保产品 25KG 25KG 25KG 25KG
碱性液体清洗剂 PARCO CLEANER 338 L 卷材用清洗 铝合金碱性脱脂剂 P3-ALMECO 18 铝材用,适用于浸泡处理
30KG 25KG 30KG 35KG 25KG 25KG 25KG
铜拉丝专用
180KG 20KG 25KG 30KG 25KG
铝碱蚀亚光添加剂
30KG 25KG 20KG
专用于有机颜料染色后高温封闭
25KG 25KG
25KG 25KG 25K G 25KG 25KG 25KG 25KG 25KG 35KG 35KG 25KG 35KG 35KG 25KG 25KG 25KG 25KG
压铸铝材用,环保产品
25KG
锌钙系磷化 锰系磷化前表调 锰系厚膜磷化 锌系低温磷化配槽剂 锌系低温磷化添加剂 喷淋法低温三元磷化配槽剂 喷淋法低温三元磷化添加剂 三元磷化配槽剂 三元磷化添加剂 浸渍法低温三元磷化配槽剂 浸渍法低温三元磷化添加剂 喷淋法三元磷化配槽剂 喷淋法三元磷化添加剂 常温磷化 无磷环保磷化 可同时磷化铁铝锌等 磷化同时可除油 磷化同时可除油 与 NT1 配套 磷化配套 磷化配套
汉高产品表
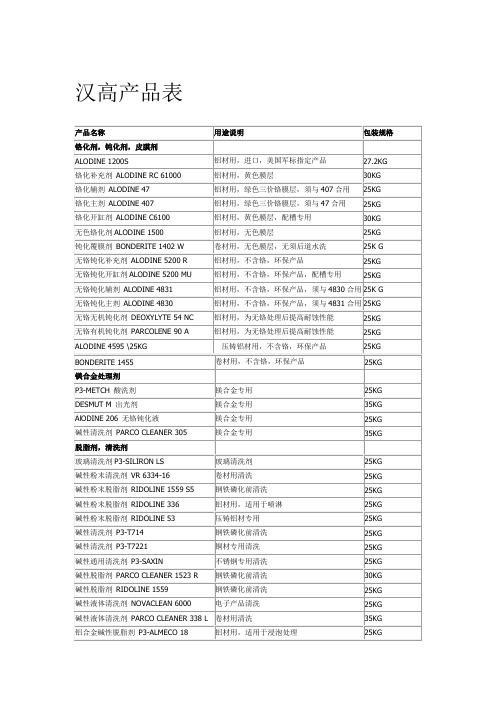
产品名称 铬化剂,钝化剂,皮膜剂 ALODINE 1200S 铬化补充剂 ALODINE RC 61000 铬化辅剂 ALODINE 47 铬化主剂 ALODINE 407 铬化开缸剂 ALODINE C6100 无色铬化剂 ALODINE 1500 钝化覆膜剂 BONDERITE 1402 W 无铬钝化补充剂 ALODINE 5200 R 无铬钝化开缸剂 ALODINE 5200 MU 无铬钝化辅剂 ALODINE 4831 无铬钝化主剂 ALODINE 4830 无铬无机钝化剂 DEOXYLYTE 54 NC 无铬有机钝化剂 PARCOLENE 90 A ALODINE 4595 \25KG BONDERITE 1455 镁合金处理剂 P3-METCH 酸洗剂 DESMUT M 出光剂 AlODINE 206 无铬钝化液 碱性清洗剂 PARCO CLEANER 305 脱脂剂,清洗剂 玻璃清洗剂 P3-SILIRON LS 碱性粉末清洗剂 VR 6334-16 碱性粉末脱脂剂 RIDOLINE 1559 S5 碱性粉末脱脂剂 RIDOLINE 336 碱性粉末脱脂剂 RIDOLINE 53 碱性清洗剂 P3-T714 碱性清洗剂 P3-T7221 碱性通用清洗剂 P3-SAXIN 碱性脱脂剂 PARCO CLEANER 1523 R 碱性脱脂剂 RIDOLINE 1559 碱性液体清洗剂 NOVACLEAN 6000 玻璃清洗剂 卷材用清洗 钢铁磷化前清洗 铝材用,适用于喷淋 压铸铝材专用 钢铁磷化前清洗 铜材专用清洗 不锈钢专用清洗 钢铁磷化前清洗 钢铁磷化前清洗 电子产品清洗 25KG 25KG 25KG 25KG 25KG 25KG 25KG 25KG 30KG 25KG 25KG 35KG 25KG 镁合金专用 镁合金专用 镁合金专用 镁合金专用 25KG 35KG 25KG 35KG 铝材用,进口,美国军标指定产品 铝材用,黄色膜层 铝材用,绿色三价铬膜层,须与 407 合用 铝材用,绿色三价铬膜层,须与 47 合用 铝材用,黄色膜层,配槽专用 铝材用,无色膜层 卷材用,无色膜层,无须后道水洗 铝材用,不含铬,环保产品 铝材用,不含铬,环保产品,配槽专用 27.2KG 30KG 25KG 25KG 30KG 25KG 25K G 25KG 25KG 用途说明 包装规格
纸业专业英语词汇翻译

纸业专业英语词汇翻译abrasive paper 砂纸abrasive base paper 砂纸原纸absorbing paper 吸水纸;吸收性纸account book paper 帐簿纸accounting machine paper 计算机用纸acid-free paper 无酸纸acid-proof paper 耐酸纸acid resistant paper 耐酸纸acoustic paper 隔音纸actinometer paper 溴化银印相纸active paper 吸湿纸adding machine paper 计算机用纸address label paper 地址标签纸adhesive paper 胶粘纸advertising paper 广告纸,招贴纸agate paper 仿大理石纸agate marble paper 仿玛瑙大理五石纹纸age resistant paper 耐老化纸air paper 航空信纸air-dried paper 风干纸air-filtration paper 空气滤纸air-knife coated paper 气刀涂布纸air-mail paper 航空信纸air-proof paper 不透气纸air-tight paper 气密纸alabaster paper 名片纸alabaster paper 薄纸albumenized paper 蛋白胶纸,蛋白纸albumin paper 蛋白胶纸aligning paper 地图纸alkali-proof paper 耐碱纸all rag paper 全棉纤维纸all-wood paper 全木浆纸allogator imitation paper 仿鳄皮纸alpha printing paper 西班牙草浆印刷纸alpha-writing paper 西班牙草浆书写纸aluminum paper 铝纸aluminum-castling paper 铝衬纸aluminum-coated paper 铝涂布纸aluminum-dusted paper 铝粉纸aluminum-foil backing paper 铝箔衬纸aluminum diaphragm paper 石棉隔膜纸aluminum (electrical)insulating paper 石棉(电)绝缘纸aluminum wall paper 石棉壁纸aseptic paper 消毒纸,防腐纸ash less paper 无灰纸ash filter paper 无灰滤纸aluminum laminated paper 铝箔夹层纸amber laid paper 琥柏条纹纸ammunition paper 弹药筒纸amplitude response recording paper 频率感应记录纸analytical filter paper 分析化学用滤纸angle paper 斜面纸angle-cut yarer 斜截面纸angular paper 斜角纸animal tub-sized paper 动物胶表面施胶纸anti-acid paper 耐酸纸,抗酸纸anti-acid manila paper 耐酸马尼拉纸anti-corrosion paper 防蚀纸anti-falsification paper 仿伪造纸anti-fungicide paper 防霉纸anti-fusion paper 服装剪裁用纸anti-tarnish paper 防锈纸anti-rust base paper 防锈原纸anti-tarnish paper 防锈纸antique paper 低光泽纸,仿古纸antique book paper 仿古书籍纸antique-bristol paper 仿古整饰光泽厚纸antique-cover paper 仿古整饰封面纸antique-eggshell paper 粗糙表面仿白纸粗糙表面仿白纸antique-glazed paper 低光泽纸antique-printing paper 仿古印刷纸,低光泽印刷纸anti-rust paper 防锈纸antiseptic paper 杀菌纸antitarnish paper 保光泽纸apricot paper 粉红色水果包装纸aquare(le) paper 水彩图画纸,水彩画纸archival paper 档案纸armature paper 绝缘纸aromatic paper 香料纸arsenical paper 含砷纸art paper 美术纸,铜版纸,涂料纸art-cover paper 美术装饰纸art-drawing paper 水彩例纸art-poster paper 美术广告纸;美工宣传纸articulating paper 牙科用纸artificial leather paper 人造革纸artificial parchment paper 仿羊皮纸asbestos paper 石棉纸asbestos base paper 钡地原纸,照相原纸asphalt paper 防潮纸,沥青纸,柏油纸asphalt base paper 沥青原纸asphalt coated paper 沥青涂布纸asphalt laminated paper 沥青层合纸asphalt saturated paper 防潮纸,沥青纸asphalt moisture proof paper 防潮纸,沥青纸asphalt sheathing paper 涂布防潮纸,沥青涂布纸asphalting paper 防潮纸,沥青纸asthma paper 防喘纸atlas paper 地图纸,绘图纸,印图纸autochion printing paper 彩色印刷纸auto copy paper 压感复写纸autograph paper 纪念册纸autographic register paper 自动(划线)记录纸autographic transfer paper 复印纸automobile-bag paper 汽车袋用纸automobile tire roll paper 轮胎包装纸autotype paper 复制纸,影印纸avenue paper 食品包装纸azure laid(writing) paper 蓝条纹书写纸A printing A级印刷纸(含漂白磨木浆)absolute humidity 绝对湿度absolute temperature 绝对温度absorbency of paper 纸张吸收性能absorbent 吸收性的,吸收剂absorbent paper 吸水纸,易吸墨的印刷纸addition agent 添加剂additional agent 添加剂autotype pigment paper 碳素纸autogtaphic printing paper 转写纸autographic stencil paper 誊写版蜡纸articulating paper 双面复写纸artificial parchment 仿硫酸纸artist's illustration board 绘图用厚纸ash content 灰分纸张Ashcroft tester 纸张耐破裂度测试仪ashless filter paper 无灰滤纸art board 涂料板纸art vellum 仿羊皮纸antitarnish paper 防锈纸antiquarian 纸张尺寸antifalsification paper 防伪造纸animal parchment 羊皮纸anopisthographic block book 单面印刷的木版纸A flute A级瓦楞纸波形数abaca(Musa texilis) 马尼拉麻,蕉麻abele(Populus alba) 银白杨abienol 松香醇abies 冷杉属abietate 松香酸酯abieteae(Abietoideae) 冷杉(亚科),松亚科abietene 松香烯abietic acid 松香酸abrader 研磨机abrading machine 研磨机abrasion 磨损,磨蚀abrasion resistance 抗磨性能abrasion test 耐磨试验abrasion t3ester 耐磨试验机abrasive 磨损的,磨蚀的abrasive acrion 磨蚀作用abrasive fiber 砂纸原纸用纤维abrasive grain 磨(料)粒(度)abrasive machine 研磨机abrasive resistance 抗磨性能abrasive tester 耐磨试验机abrasiveness 磨蚀absolute alcohol 无水酒精absolute alcohol dry 绝干absolute alcohol humidity 绝对湿度absolute alcohol temperature 绝对温度absolute unit 绝对单位absorb 吸收absorbability 吸收性能absorbed water 吸收水absorbency 吸收能力;吸收本领absorbent 吸收剂absorbent felt 吸水毛毯absorber 吸收器,吸收剂,减震器absorbing capacity 吸收能力absorbing capacity column 吸收塔absorbing capacity pad 防震垫absorbing capacity power 吸收能力absorbing capacity quality 吸收性能;吸收能力absorbing capacity tower 吸收塔absorption 吸收作用absorption ability 吸收能力absorption band 吸收光带absorption coefficient 吸收系数absorption measurement 吸收测定absorption rate 吸收率absorption spwctrum 吸收光谱absorption tester 吸收试验仪absorption tower 吸收塔absorptive capacity 吸收能力absorptivity 吸收能力,吸收率acacia (Acacia) 金合欢(属)acacia false (Robinia psendon cacia L.) 刺槐;洋槐acacia gum 金合欢胶accelerant 催速剂,促进剂,加速剂accelerated ageing 加速老化accelerated cement 速凝水泥accelerated oxidation 加速氧化accelerated weathering 人工加速风干accelerating agent 催速剂,促进剂accelerator 加速器,促进剂acceptability 合格率acceptable fiber 合格纤维accepted chips 合格木片accepted chips product 合格产品accepted chips stock 合格浆料acceptance sampling system 合格率抽样系统acceptance sampling system test 合格率检查accepts 合格品;良浆access time 存取时间,选取时间,信息发送时间accessibility 可及度accessory 附件;零件a.c. commutator motor 整流式交流电动机accident prevention 技术保安措施accidental error 偶(然误)差accordion fold 手风琴式折纸法accumulation 累积,蓄积,储积,堆积accumulator 储存槽,回收槽;蓄电池;污热水槽accumulator acid (亚硫酸盐制浆)回收酸accumulator relief 储存槽排气,回收槽排气accumulator tank 储存槽,回收槽,污热水槽accuracy 准确(度,性);精密(度,性)acer 枫树,槭树<BR>acetal 乙缩醛,乙醛缩二乙醇acetaldehyde 乙醛acetate 醋酸盐;醋酸酯;醋酸根(或基)acetate fiber 醋酸纤维acetate film 醋酸盐胶片acetate rayon 醋酸人造丝acetic acid 醋酸acetic acid anhydride 醋(酸水)解acetone 丙酮acetonitrile 乙腈aceto-veratrone 乙酰藜芦酮acetyl 乙酰(基)acetyl cellulose 醋酸纤维素acetyl vanilloyl 乙酰基香草酰acetylate 乙酰化,乙酰化产物acetylation 乙酰化(作用)achromatic 消色的,消色差的acid accumulator (酸液)回收锅(或槽)acid accumulator alizarin dye 茜素染料,1,2-二羟基蒽醌染料acid accumulator alum 酸性明矾acid accumulator bath 酸溶;脱酸槽acid accumulator bleaching 酸性漂白acid accumulator chloride 酸性氯化物acid accumulator circulation 酸液循环acid accumulator composition 酸液组成acid accumulator dye(stuff) 酸性染料acid accumulator extract 酸抽提acid accumulator fastness 耐酸度acid accumulator filter 滤酸器acid accumulator fortitying system 酸液强化系统acid accumulator free 无酸的,脱酸的,不含酸的acid accumulator group 酸根(或基)acid accumulator halide 酸性卤化物acid accumulator insoluble lignin 酸不溶木素acid accumulator lignin 酸木素acid accumulator line 酸液管道acid accumulator liquor 酸液acid accumulator maker 制酸工acid accumulator making 制酸acid accumulator number 酸值acid accumulator penetration 酸液渗透,酸液浸透acid accumulator plant 制酸车间acid accumulator preheater 酸液预热器acid accumulator press (羊皮纸机用)压酸辊acid accumulator proof enamel 防酸搪瓷acid accumulator prmp 酸泵acid accumulator recovery plant 酸液回收车间acid accumulator reduction 酸性还原acid accumulator resistance 耐酸性能,耐酸强度acid accumulator resistant 耐酸合金acid accumulator tesistant brick 耐酸砖acid accumulator tesisting bronze 耐酸铜acid accumulator resisting felt 耐酸毛毯acid accumulator resisting mortar 耐酸灰泥acid accumulator resisting paint 耐酸油漆acid accumulator resisting steel 耐酸铜acid accumulator resisting tile 耐酸砖acid accumulator rosin size 酸性松香胶acid accumulator settling basin 酸液澄清槽acid accumulator salt 酸式盐acid accumulator size 酸性施胶剂acid accumulator soluble 酸溶(性)acid accumulator soluble lignin 酸溶性木素acid accumulator souring 酸处理acid accumulator stable size 酸稳性施胶剂acid accumulator storage 贮酸槽acid accumulator strength 酸液浓度acid accumulator sulfite 酸性亚硫酸盐acid accumulator tank 酸槽acid accumulator tower 制酸塔,酸塔acid accumulator trap 分酸器acid accumulator treatment 酸处理acid accumulator treatment tower 酸处理塔acid accumulator tub 酸槽acid accumulator wash 酸洗acid accumulator water 酸水acidic 酸的,酸性的acidification 酸化(作用)acidifier 酸化器;酸化剂acidified water 酸化水acidify 酸化acidity 酸度acidity control 酸度控制acidolysis 酸解acidometer 酸度计,PH计,酸(液)比重计acidproof 耐酸的acidproof brick 耐酸砖acidproof cement 耐酸水泥acidproof lining 耐酸衬里acifulate 酸化acoustic insulation 隔音acoustic insulation properties 声学性质Aeroart 高密度聚乙烯合成纸(美国Aeroline产品,商业名称)across grain 横纹理acrylamide 丙烯酰胺acrylate 丙烯酸盐acrylic acid 丙烯酸acrylic acid bound coating 丙烯酸盐涂布acrylic acid compound 丙烯酯化合物acrylic acid emulsion 丙烯乳液acrylic acid fiber 丙烯纤维acrylonitrile 丙烯腈acrylonitrile butadiene rubber latex 丙烯腈,丁二烯其聚体乳胶activared carbon 活性碳activared carbon clay 活性白上activared carbon silica 活性硅activared carbon sludge 活性污泥activared carbon sulfur 活性硫酸activation 活性(作用)active alkali 活性碱active alkali alkali to wood ration 碱比,活性碱对木材量的比率active alkali carbon 活性碳active alkali chemical to wood ratio 碱比,用碱量active alkali chlorine 有效氯active alkali lime 有效石灰active alkali sulfur 有效硫酸active alkali surface 活性表面activity 活性度actual volume 有效容积,实际容积actual volume weight 实际重量,有效重量actuating signal 驱动信号,作用信号actuation time 动作时间actuator 驱动器;执行机构;激励器acylate 酰化;酰化产物acylation 酰化(作用)adapter 接合器;连接器;接头addition compound 加成化合物addition compound polymer 加成聚合物addition compound product 加成产物addition compound reaction 加成反应additional acid tank 辅助贮酸槽,辅助酸液槽additional dryer 附加烘缸additive 添加剂additive reaction 加成反应adherence 粘附adherent 粘附的adhesion 粘附(现象);粘附力adhesion of wet web 湿纸胎粘附现象adhesion strength 粘附强度adhesion tester 粘附力测定仪adhesive 粘附剂,粘合剂,胶粘剂,粘着剂;胶粘的adhesive capacity 粘附能力;胶粘度adhesive dissolving tank 溶胶桶adhesive felt 衬底用纸板adhesive force 胶粘力,粘着力,粘附力adhesive glassine tank 粘胶槽adhesive (glassine)tape 胶(带)纸adhesive migration 胶粘剂的迁移adiabatic condition 绝热状态,绝热情况adiabatic condition eficiency 绝热效率adiabatic cindition expansion 绝热膨胀adiabatic cindition throtling 绝热调节adipo-cellulose 含脂纤维素adjective color 间接染料adjust 调节,调整;修正adjustable bow curved roll (可调节)弧形辊,(可调)弓形辊adjustable bow curved roll orifice 可调锐板adjustable bow curved roll speed motor 调速电动机adjusting color 调色adjusting color device 调节装置adjusting color screw 调节螺旋adjusting color controls 调节控制器admission valve 进气阀;进浆阀admixture 掺和剂;掺和,混合adsorb 吸附adsorbability 吸附性(能)adsorbate (被)吸附物;吸附质adsorbed water 吸附水adsorbent 吸附剂,吸附的adsorption 吸附(作用)adsorptive capncity 吸附能力adsorptivity 吸附性(能)adulf wood 成年材advanced water treatment AWT 污水处理法,(污水)三级处理<BR>aerate 曝气,充气aerated lagoon 曝气塘aeration 曝气,充气aeration basin 曝气塘aeration tank 充气槽,充气罐aerator 曝气机aerobic 需氧的aerobic bacteria 好氧细菌,需氧细菌aerobic treatment 充氧处理aerogel 气凝胶aeromix wet scrubber 文丘里型洗涤塔affinity 亲力,亲合势;亲合能,亲合性afforestation 造体after dryer 后部烘缸组after dryer sizing 后施胶,表面施胶afrer dryer treatment 后处理agalite 滑石agar 琼脂agave 龙舌兰aged wood 老化材ag(e)ing 老化agglomerate 附聚(作用);烧结(作用)agglutinant 烧结剂;凝集剂agglomeration 聚集体agitating valve 搅拌浆agitation 搅拌(作用)agitator 搅拌器aging quality 耐久性;老化性能aging quality resistance 抗老化性能aging quality test 老化试验Ahlfors screen Ahlfors 木片筛aid 促进剂,辅助剂air bells (水印辊构成的)气泡(纸病)air bells blade 气刀air bells blast duster 风选机aidr bells blast system 鼓风系统;风选系统air bells bleed press 抽气压榨,吸风压榨air bells blower 鼓风机air bells blowing roll 热风辊air bells borne drying 气垫干燥,气托干燥air bells brake 空气制动器,风闸air bells brush 气刷air bells bubble (空)气泡air bells cap(drying) 热风罩(干燥)air bells chamber 通风室air bells channel 通风道air bells chip distributor 风送木片分布器air bells compressor 压缩空气机,空压机air bells condenser 空气冷凝器air bells conditioner 空气调节装置,空调设施air bells conditioning equipment 空气调节装置,空调设施air bells content 空气含量air bells controlled 气控air bells centrolled dilution valve 气控稀释阀air bells cooled 空气冷却air bells cooling 空气冷却air bells curtain 气帘,气幕air bells cushion 气挚air bells damper 风挡air bells deekle 气控定边器air bells doctor 气刀,空气刮刀air bells drainer 空气助滤压榨air bells dried 风干air bells dried wood 风干材air bells dry 风干air bells dry basis 风干基础air bells dry weight 风干重量air bells dryer 空气干燥器,热风干燥器air bells drying 风干的;热风干燥air bells drying machine 用热风干燥的造纸机air bells ejector 空气喷射器air bells entrainment 空气含量air bells escape valve 排气阀,放空阀air bells exhauster 排风机air bells filter 空气过滤器air bells float dryer 气垫干燥装置,气托干燥装置air bells float(drying) 气垫(干燥),气托(干燥)air bells float table 气托堆纸台air bells flow 气流air bells foil 热风气翼air bells foil dryer 气翼箱式热风干燥装置air bells heater 空气加热器air bells intake 进风口,空气入口air bells jet 空气喷嘴air bells knife 气刀air bells knife coating 气刀涂布air bells knife mark 气刀痕(纸病)air bells laid 空气沉降air bells -lay drying 热风干燥air bells line 空气管道air bells loaded headbox 气垫网前箱,气垫式压力流浆箱air bells loaded tension device 气动张力装置air bells nozzle 喷气嘴air bells operated automatic control 气动调湿控制器air bells operated thermostat 气动恒温器air bells outlet flue 排气管air bells permeability 透气性,透气度air bells permeability tester 透气度测定仪air bells piping 风管air bells pollution 大气污染air bells preheater 空气预热器air bells proof 不透气的;密封的air bells press 空气压力air bells quality 空气质量air bells regulator 空气调节器air bells removing roll 排气辊,(伏辊上方)小压辊air bells reservoir 贮气箱;气库air bells resisTANCE 空气阻力air bells roll 压纸辊air bells scrubber 空气洗涤器,净气器air bells seal 气封air bells separator 吹(气分)离器air bells space 空域,大气层;空隙air bells spring 气垫air bells stripping 空气脱吸,空气抽提air bells sword (卸纸垛装置的)气刀air bells tight 不透气的air bells trap 空气阱air bells valve (空)气阀air bells velocity pressure 气流速度压力air bells vent 排气口air bells wash 浮气器Aladdin former (纸板机用)Aladdin夹网成形器(日本三菱制作所)albumen 蛋白(胶)albumin 白蛋白albuminous substances 白蛋白物质alburnum 边材alcohol 乙醇,酒精alcohol acid 醇酸alcohol benzene extractive 笨醇抽提物alcohol extractive 乙醇抽提物alcohol lignin 乙醇木素alcoholic extract 乙醇抽提物alcoholic extract fermentation 乙醇发酵alcoholic extract hydroxyl group 醇羟基alcoholysis 醇解alcoholytic splitting 醇分裂aldehyde 醛aldehyde resin 聚醛树腊alder(Alnus) 桤木(属)aldo-醛aldonic acid 糖醛酸,醛糖首酸aldose 醛糖alfa (Stipa tenacissima) 非洲蒲草alga(e) 藻类algicide 灭藻剂alginate 藻朊酸盐;藻朊酸纤维alginic acid 藻朊酸algorithm 算法alignment 顺序;划线;对准;调直aliphatic 脂(肪)族的aliphatic acid 脂族酸aliphatic compound 脂族化合物alizarine dyestuff 茜素染料alkali 碱alkali cellulose 碱纤维素alkali charge 用碱量alkali consumption 碱耗,耗碱量alkali extract 碱抽提物alkali extraction 碱抽提alkali extractive (substance) 碱抽提物alkali fastness 抗碱牢度,抗碱性alkali filler 碱性填料alkali free 无碱的,不含碱的alkali fusion 碱熔alkali hydrolysis 碱性水解alkali lignin 碱木素alkali loquor 碱液alkali proof 抗碱的alkali ratio 碱比alkali reeovery 碱回收alkali reslstance 抗碱性(能)alkali resisting 抗碱的alkali resisting cellulose 抗碱纤维素alkali sensitive 对碱活泼的alkali solubility 碱溶性alkali soluble 碱溶性的alkali stable 对碱稳定的alkali staining resistance 抗碱染性(能)alkali treatment 碱处理alkaline bleach liquor 碱性漂液alkaline bleach liquor cleavage 碱性分裂,碱性裂解alkaline bleach liquor degradation 碱性降解alkaline bleach liquor extraction 碱抽提alkaline bleach liquor extraction tower 碱抽提塔alkaline bleach liquor filler 碱性填料alkaline bleach liquor purification 碱处理;碱净化alkaline bleach liquor reducing agent 碱性还原剂alkaline bleach liquor sizing 碱性施胶alkaline bleach liquor solubility 碱溶性alkaline bleach liquor soluble 可溶于碱的alkaline bleach liquor solutioln 碱性溶液alkaline bleach liquor steeping test (溶解浆)碱浸试验alkaline bleach liquor treatment 碱处理alkaline bleach liquor wash 碱洗(涤)alkalinity 碱度,碱性alkaloid 生物碱alkyl 烷基alkyl hydrosulfide 烷基硫醇alkyl ketene 烷基烯酮alkyl ketene dimer 烷基烯酮二聚体alkyl sulfhydrate 烷基硫醇,烷基硫氢alkyl sulfide 硫醚,烷基硫alkylaryl sulfonate 烷基芳基磺酸盐alkylation 烷(基)化all purpose computer 通用电子计算机allowable current 容许电流allowable current deviation 容许误差allowable current error 容许误差allowable current load 容许负荷alloy 合金alloy steel 合金钢alpha cellulose a 纤维素alpha cellulose a gage a 射线仪alpha cellulose a protein a 蛋白质alpha cellulose a pinene a 蒎烯alum 明矾alum cake (明)矾块alum earth 铝矾土alum liquor 明矾液,矾土液alum speck (明)矾斑(点)纸病alum spot (明)矾斑(点)纸病alumina 氧化铝alumina baryta white 铝钡门alumina oxide 氧化铝aluminate 铝酸盐alumine 钡土,氧化铝aluminum foil 铝箱aluminum foil resinate 树脂酸铝aluminum foil stearate 硬酯酸铝aluminum foil sulfate 硫酸铝alumite 耐酸铝alunite 明矾石ambient conditions 环境条件,外界条件ambient comditions temperature 环境温度American arbor-vitae (Thujaoccidentalis L.) 香柏,美国侧柏,金钟柏American arbor-vitae (Thujaoccidentalis L.) aspen (Populus tremuloides Michx.) 颤杨,美国白杨amide 酰胺amine 胺amino acid 氨基酸amino acid ethyl cellulose 氨基乙基纤维素amion acid group 氨基amion acid polumer 氨基聚合物amion acid propyl cellulose 氨基丙基纤维素amion acid sugar 氨基糖ammeter 安培计,电流表ammonia 氮ammoniabase liquor 铵基蒸煮液ammonia liquor (粗)氨水ammonia test 氨试验ammonia water 氨水ammoniacal copper solution 铜氨(溶)液ammonium base 铵基ammonium base base liquor 铵基蒸煮液ammonium base bisulfate 硫酸氢铵ammonium base bisulfite 亚硫酸铵ammonium base compounds 铵基化合物ammonium base salts 铵盐ammonium base stearate 硬脂酸铵ammonium base sulfate 硫酸铵ammonium base sulfite 亚硫酸铵amorphous 无定形的amorphous cellulose 无定形纤维素,非结品纤维素amorphous region 无定形区,非结品区amorphous resin 无定形松香amphiphatic 偶极性amphoteric 两性的amphoteric reaction 两性反应amphoteric surface reactive agent 两性表面活性剂amplifier 放大器amplitude(of shake) 振幅amur corktree (Phellodendron amurense Rup.) 黄檗amylaceous 淀粉的amyulase 淀粉酶amylopectin 支链淀粉amylose 支链淀粉anaerobic 厌氧的anaerobic treatment 厌氧处理analog 类似,模似analog computer 模拟计算机analog speed/draw system 车速和牵引力模拟控制系统analog sensor 模拟传感器analog signal 模拟信号analogy 模拟,类似analysis of variance 方差分析analytical balance 分析天平anatase 锐钛矿anchot bolt 地脚螺丝Anderson moisture expeller Anderson 螺旋挤水机Anderson moisture expeller Anderson barker Andersson 刀式剥皮机anenometer 风速机angiosper 被子植物angiospermous wood 被子树木angle cutting machine 斜切机angle cutting machine steel 角钢angle cutting machine valve 角阀anhydride (酸)酐anhydroglucose 葡萄糖酐anhydrous 无水的anhydrous alcohol 无水酒精anhydro-xylan unit 无水多缩木糖aniline 苯胺aniline color 苯胺染料aniline dye 苯胺染料aniline printing 苯胺染色animal glue 动物胶animal glue size 动物胶anion 阴离子anion exchange 阴离子交换anion exchange resin 阴离了交换树脂anion exchanger 阴离子交换剂anionic 阴离子的anionic compound 阴离子化合物anionic starch 阴离子淀粉annealing temperature 退火温度annual growth 一年生annual growth layer 年轮(层)annual growth plant 一年生植物annual growth ring 年轮annual growth zone 年轮区annular 环形的;轮壮的;有环纹的annular vessel 环纹导管annuli 环壮体anode 阳极anode protection 阳极保护anode ray 阳极射线anthraquinone 蒽醌anti-acid 耐酸的anti-acidblocking agent 防粘附剂;防阻塞剂antichlor 脱氯剂anticorrosion 耐腐蚀anticorrosion paint 耐蚀漆anti-crawl agent 防滑动剂anti-crawl agentdefiection 抗挠anti-crawl agentdeflection press roll 中固(抗挠)压榨辊anti-crawl agentdefiection roll 抗挠辊anti-crawl agentdetonator 抗爆剂anti-crawl agentflocculant 防絮凝剂anti-crawl agentflocculation 防絮凝作用anti-crawl agentfoam 消泡anti-crawl agentfoam oil 消泡油anti-crawl agentfoaming agent 消泡剂anti-crawl agentfoggant 防翳的;防翳剂anti-crawl agentfogging compound 防腐剂;防污剂anti-crawl agentfriction bearing 抗磨轴承anti-crawl agentfroth oil 消沫油anti-crawl agentknock agent 抗爆剂anti-crawl agentoxidant 抗氧剂;防老化剂anti-crawl agentpollution sequence(漂白车间)污染防治流程antique bristol 仿光泽纸antique bristol finish 仿古整饰antique bristol laid bond 仿证券纸antique bristol woven 仿光泽布纹纸anti-rust 防锈anti-rustrusting paint 防锈漆anti-rustskid 防滑动anti-rustskid coating 防滑涂布anti-rustskid treatment 防滑动处理antiseptic 防腐antiseptic agent 防腐剂antiseptics 防腐剂anti-static agent 抗静电剂anti-static agenttarnish agent 防锈剂aperture 孔;筛孔;网孔apex 顶端apical zone 顶生区Apmew(centrifugal)screen (阿牟)离心式圆筛,A型圆筛apparatus 仪器;装置apparent density 表观密度apparent density specific gravity 表观比重apparent density specific volume 表观体积apparent density viscosity 表观粘度apparent density weight 表观重量appearance 外观appendage 附属部分;附件appendix 附录application valve 控制阀applicator 上涂装置;施胶装置applicator roll 涂料辊;施胶辊applying felt 专用毛毯approach flow(of stock) 浆料上网approach flow(of stock) folw system (纸机上)流浆系统aoproach flow(of stock) (onto wire) 放料上网apron board 下唇板,裙板apron board (cloth) 唇布,裙布apron board conveyor 带式干燥机aquapel(size)聚烷基烯酮胶料(商业名称);乙烯酮二聚物胶料aquapulper 水力碎浆机aqueous 含水的,液态的;水成的aqueous emulsion 水乳液aqueous lignin 水木素aqueous phase 液相aqueous solution 水溶液araban 聚阿拉伯糖,多阿拉伯糖arabic gum 阿拉伯胶arabinose 阿拉伯糖,阿戊糖Arathene 高密度聚乙稀合成纸(商业名称,比利时UCB产品)arbor-vitae(Thuja occidentalissL.) 香柏,美国侧柏,金钟柏are foil 弧形案板,弧形脱水板arch dryer 拱状热风干燥室,拱式烘房arching 搭桥Arcu formq Arcu 夹网成形装置area of bars 打浆面积areal(dried)wejight 定量argilla 泥土,铝氧土arithmetic and logic unit 算数与逻辑装置arithmetic mean 算术平均arithmetic mean mean temperature 算术平均平均温度arithmetic mean unit 运算器armature winding 电枢绕线aromatic 芳香族的,芳烃的aromatic acid 芳酸aromatic alcohol 芳醇aromatic compound 芳香族化合物aromatic group 芳烃基arrester 制动片,制动机构arresting device 制动机构Arrhenius equation Arrhenius 方程式,阿雷尼厄斯方程式arrow root starch 木薯淀粉art cover 装饰面板art cover (regetable)parchment 美术(植物)羊皮纸artificial aging 人工老化artificial aging cotton 人造棉artificial aging dyestuff 合成染料artificial aging fiber 人造纤维artificial aging grindstone 人造磨石artificial aging leather 人造革artificial aging parchment 仿羊皮纸artificial aging pulpstone 人造磨石artificial aging regeneration 人工再生artificial aging resin 合成树脂artificial aging silk 人造成丝artificial aging stone 人造磨石artificial aging stone roll 人造石辊asbestine 滑石棉asbestos 石棉asbestos felt 石棉毛毯asbestos fiber 石棉纤维asbestos packing 石棉垫asbestos roll 石棉辊asbestos roofing felt 屋顶石棉毡asbestos rope 石棉绳asbestos sheet 石棉板asbestos wall 石棉壁板(纸)asbestos washer 石棉垫圈asbestos waterproof(ing)felt 防水石棉毡ascending chromatography 上行色谱(分离)法ash(Fraxinus) 灰分;炉灰ash content 灰分含量ash content dissolving tank 黑灰溶解槽ash content free 无灰的ash content hopper (锅炉)灰斗ash content tester 灰分试验器Ashcroft tester Ashcroft 耐破度试验仪Ashcroft tester Ashcroft thickness gage Ashcroft 厚度计ashless 含灰分较少的;无灰的aspect ratio 纵横比(值)aspen(Populus) 杨属aspen(Populus tremula L.) 欧洲山杨asphalt 沥青asphalt coating 沥青涂布asphalt emulsion 沥青乳胶asphalt felt 沥青油毛毡asphalt laminator 沥青层压机asphalt roofing 油毡线asphalt saturated felt 沥青纸,油毡纸asphalt size 沥青胶料asphaltum 沥青aspirated pit (pair) 闭塞纹孔(对)aspitation 抽气aspirator 抽气机Asplund defibrator Asplund 单动纤维分离机Asplund defibrator Asplund digester Asplund 卧式连续蒸煮器assay 鉴定;分析assay procedure 分析程序assembly 机组;成套设备;联动装置;基团,组assimulation 同化(作用)assistant superintendent 车间副主任Astrom barker Astrom 链式剥皮机Astrom barker Astrom barking machine Astrom 链式剥皮机asynchronous motor 异步电机asymmetry 不对称(现象)at maximum temperature 保温atmospheric conditions 大气状态atmospheric conditions humidity 大气湿度atmospheric conditions pressure 大气压力atomic bond 原子键atomization 雾化atomized suspension technique AST法,(亚硫酸盐废液)雾化回收法atomizer 喷雾器;雾化器attachment 附件attapulgate 无水硅酸铝矿石attenuant 稀释剂;衰减器attenuation 衰减作用attrition mill 磨碎机;磨浆机auger method (for sampling pulp) (纸浆取样)钴取法Austrian pine(Pinus nigra Ahr.) 南欧黑松autoclave 高压釜,高压锅auto cut-out 自动断路(器)automatic control 自动控制automatic control electric feed 电控自动装料automatic control feed 自动进料,自动喂料automatic control felt guide 毛毯自动校正器automatic control felt stretcher 毛毯自动张紧器automatic control flashing apparatus 自动闪蒸设备automatic control fraction collector 自动分选机automatic control guide(roll) 自动导辊automatic control knife grinde 自动磨刀机automatic control line 自动线automatic control logging 自动记录automatic control operation 自动操作automatic control pick-up 自动递纸装置,自动引纸装置automatic control plant 自动化工厂;自动化车间automatic control pressure controller 压力自动控制器automatic control pressure vent 自动排气阀automatic control production 自动化生产automatic control proportioning and metering device 自动配浆箱automatic control regulating box 自动调节箱automatic control tegulating device 自动调节装置automatic control regulator 自动调节器automatic control set-up box machine 自动制盒机automatic control sheet counting device 自动数纸装置automatic control sheetfeeder 自动续纸器automatic control sheet handling machine 自动码纸机automatic control sorter 自动选纸机automatic control stoker 自动加煤器automatic control stuff box 自动调节箱automatic control temperature controller 温度自动控制器automatic control tip time service 自动定时转换automatic control valve 自动阀automatic control wire guide (roll) 自动校网器,自动校网辊automatically feed 自动进料的,自动喂料的automation 自动化automobile storage bag 汽车轮胎包装用纸袋auto-oxidation 自动氧化auto-panel 自动控制批示板autc-slice 真空刮刀auto tire wrap 汽车轮胎包装用纸autumn wood 晚材,秋材auxiljaries 辅助装置auxiliary air 补给空气,二次风auxiliary air caustization 辅助苛化auxiliary air causticizer 辅助苛化器auxiliary air equipment 辅助装置auxiliary air screen 辅助筛auxiliary air separator 辅助分离器auxiliary air sizing agent 辅助施胶剂auxiliary air strainer 辅助滤带;辅助筛浆机available alkali 有效碱available alkali capacity 有效容量available alkali chlorine 有效氯available alkali crosssection 有效截面average fiber length 纤维平均长度average fiber lengthincrement 平均增量average fiber length moisture(of pulp bales) 成捆浆板平均水分含量average fiber length pressure 平均压力average fiber length temperature 平均温度average fiber length velocity 平均速度avometer 安伏欧计,万能(电)表,三用电表axial 轴向axial bond 主键;轴键axial flow pump 轴流泵axis 轴Aylesford refiner (实验室用)Aylesford盘磨机(英国制)azo compounds 偶氮化合物azo compounds dye(stuff) 偶氮染料absorbing board 吸收纸板accordion board 手风琴纸板acoustic(al) board 隔音纸板advertisement board 广告招贴用纸板air-dried board 风干纸板album board 相册纸板alkaline-proof soap box board 肥皂包装用抗碱纸板ammunition board 弹筒纸板antique board 仿古纸板anti-tarnish board 防锈纸板art board 铜版纸板artist board 绘画纸板artist's illustration board 绘画纸板asbestos board 石棉纸板asbestos asbestos board 石棉洋灰板asphalt board 沥青纸板,防潮纸板automobile board 汽车用纸板auto(mobiole) panel board 汽车仪表盘纸板abrasive coater 砂纸涂布机air brush coater 气刷涂布机air blade coater 气刀涂布机air doctor coater 气刀涂布机air knife coater 气刀涂布机arch bed brush coater 刷式拱形涂布机asphalt coater 沥青涂布机acid cooking 酸法蒸煮alkaline cooking 碱法蒸煮actic acid 乳酸actic acid method (纸板施胶度)abrasive paper 砂纸abrasive base paper 砂纸原纸absorbent paper 吸水纸absorbing paper 吸水纸;吸收纸account book paper 帐簿纸accunting machine paper 计算机用纸acid free paper 无酸纸acid proof paper 耐酸纸acid resistant paper 耐酸纸acoustic paper 隔音纸actinometer paper 溴化银印相纸active paper 吸湿纸adding machine paper 计算机纸address label paper 地址标签纸adhesive paper 胶粘纸advertising paper 广告纸,招贴纸agate paper 仿大理石纸agate marble paper 仿大理石纸age resistant paper 耐老化纸air paper 航空信纸air dried paper 风干纸。
CleanGredients 产品指南:更安全的选择成分说明书

Second generation bio-based water soluble polymer which is more sustainably sourced and readily biodegradable.
• Anti-redeposition, anti-encrustation and anti-film forming • Best-in-class readily biodegradable water soluble polymer • Dispersant and anti-scalent with improved calcium, iron and
• Available as aqueous solution or powder
Unique, high-purity hydrotroping acrylic/styrene copolymer.
• Hydrotroping of surfactants into heavy duty liquids • Particulate and oily soil dispersion/suspension • Protective corrosion inhibition • Hypochlorite stable • Calcium binding
• Hard surface cleaning • Vehicle cleaning • Water based alkaline cleaners • Microemulsions • All Purpose Cleaners (APC)
• All Purpose Cleaners (APC) • Hydrotroping • Acidic and alkaline cleaners
化学品英文名称

Filling material A AC 45 UBI / FR 310 5000 Acetic acid (5% at 20°C) Acetic acid (5% at 50°C) Acetic acid (at 40°C) Acetic acid (at 50°C) Acetic acid anhydride Acetoacetate, ethylAcetoacetate, methylAcetone Acetulan Acetyl chloride Acricid 40 EC Acricid liquid Acryl amide Acrylat 38092, solvent base Acrylic acid Acrylic Polymere RDZ 1263 Acrylic Polymere RDZ 2738 Acrylic Polymere RDZ 2771 Acrylic Polymere RDZ 958 Acrylic Polymere RZ 20810 Acrylic Polymere RZ 21376 Acrylic Polymere RZ 421 Activated carbon Activoll EFL Addipast 350 WD black Additol XK 391 Additol XK 406 Adhesion Promoter AMS 70 After Shave Afugan Aircraft fuel Aircraft fuel, Jet A1 Akypo RO 90 VG Akypo RO 90 VG Aldehyde C12 Aldehyde C8 Aldurol VUP 21 Aldurol VUP 51 Alkydal R 35 W Allylic heptylate
芳纶纤维介绍

芳纶纤维全称为"聚对苯二甲酰对苯二胺",英文为Aramid fiber(杜邦公司的商品名为Kevlar),是一种新型高科技合成纤维,具有超高强度、高模量和耐高温、耐酸耐碱、重量轻等优良性能,其强度是钢丝的5~6倍,模量为钢丝或玻璃纤维的2~3倍,韧性是钢丝的2倍,而重量仅为钢丝的1/5左右,在560度的温度下,不分解,不融化。
它具有良好的绝缘性和抗老化性能,具有很长的生命周期。
芳纶的发现,被认为是材料界一个非常重要的历史进程。
芳纶纤维是重要的国防军工材料,为了适应现代战争的需要,目前,美、英等发达国家的防弹衣均为芳纶材质,芳纶防弹衣、头盔的轻量化,有效提高了军队的快速反应能力和杀伤力。
在海湾战争中,美、法飞机大量使用了芳纶复合材料。
除了军事上的应用外,现已作为一种高技术含量的纤维材料被广泛应用于航天航空、机电、建筑、汽车、体育用品等国民经济的各个方面。
在航空、航天方面,芳纶由于质量轻而强度高,节省了大量的动力燃料,据国外资料显示,在宇宙飞船的发射过程中,每减轻1公斤的重量,意味着降低100万美元的成本。
除此之外,科技的迅猛发展正在为芳纶开辟着更多新的民用空间。
据报道,目前,芳纶产品用于防弹衣、头盔等约占7~8%,航空航天材料、体育用材料大约占40%;轮胎骨架材料、传送带材料等方面大约占20%左右,还有高强绳索等方面大约占13%。
芳纶主要分为两种,对位芳酰胺纤维(PPTA)和间位芳酰胺纤维(PMIA),自20世纪60年代由美国杜邦(DuPont)公司成功地开发出芳纶纤维并率先产业化后,在30多年的时间里,芳纶纤维走过了由军用战略物资向民用物资过渡的历程,价格也降低了将近一半。
现在国外芳纶无论是研发水平还是规模化生产都日趋成熟。
在芳纶纤维生产领域,对位芳酰胺纤维发展最快,产能主要集中在日本和美国、欧洲。
如美国杜邦的Kevlar纤维,荷兰阿克苏诺贝尔(Akzo Nobel)公司(已与帝人合并)的Twaron纤维,日本帝人公司的Technora纤维及俄罗斯的Terlon纤维等。
01 多异氰酸酯

制法:
TDI 工业化生产主要由甲苯经硝化生成二硝基甲苯,然后经催化 氢化生成二氨基甲苯,最后与光气反应制得。
三种 TDI 异构体产品的工业化光气法生产工艺流程如下:
3
硝化反应式如下: 二硝基甲苯(DNT)加氢还原成甲苯二胺(TDA)反应如下: 以 2,4-TDA 为例,光气化反应式为:
特性及用途: 甲苯二异氰酸酯是聚氨酯合成最重要的二异氰酸酯产品,广泛用 于软质聚氨酯泡沫塑料、涂料、弹性体、胶粘剂、密封胶及其它聚氨 酯产品。 TDI 是一种芳香族二异氰酸酯,反应性较脂肪族异氰酸酯高,TDI 本身及其聚氨酯产品长期暴露在自然光下会发生黄变。TDI 所含的 NCO 基团活性很大,易于水、醇、胺等起反应,在一定条件下可自 聚,这些反应可被碱性物质和许多种金属化合物催化,若操作不当可 引起急遽放热升温而产生质量或安全事故,故需小心对待。 TDI 工业品中以 TDI-80 用途最广,用量最多;TDI-100 结构规整, 可用于合成特殊的预聚体,主要用于聚氨酯弹性体;TDI-65 主要用 于聚酯型聚氨酯泡沫塑料等。 毒性及防护: 大鼠经口LD50=4130 mg/kg,大鼠吸入LC50=14 mL/m3/4h,吸入 LCLo: 600 mL/m3/6h;小鼠经口LD50=1950 mg/kg,吸入LC50=10 mL/m3/4h;兔经皮LD50>10 mL/kg。本品急性吸入毒性较高,经口毒 性较低。主要有明显刺激作用。对眼、呼吸道粘膜和皮肤有刺激作用, 并引起支气管哮喘。人的嗅觉阈为 0.35~0.92mg/m3。浓度达 3~ 3.6mg/m3时,对粘膜有刺激;27.8mg/m3时眼和呼吸道严重刺激。 车间空气卫生标准:中国MAC 0.2mg/m3;美国ACGIH TLV-TWA 0.036mg/m3、短期暴露极限浓度STEL 0.14mg/m3;英国对所有异氰酸 酯规定 8h的TWA按NCO计为 0.02 mg/m3,10min短期暴露极限浓度
冲锋衣面料科技详解

1、AIRPOLAR 100系列高功能性纤维轻便,保暖,不吸收水分和快干保证体表适宜的温度,适用于登山等运动,是优良的冬季户外面料2、Coolmax纤维针织面料具有四沟槽的Coolmax纤维,能将人体活动时所产生的汗水迅速排至服装表层蒸发,保持肌肤清爽,令活动倍感舒适。
它有着良好的导湿性,与棉纤维交织的针织面料具有良好的导湿效果,广泛的用来缝制T恤衫、运动装等。
特点透气功能英威达的COOLMAX®面料的优异功能来源于其本身的结构。
有了这种专门设计的纤维,设计师们才能制造出柔软、透气、吸湿排汗的优质面料。
易于护理用COOLMAX®面料制作的服装适宜机洗和烘干,但是,洗涤时不可使用织物柔软剂或含氯漂白剂。
其中某些服装可能因为款式(并非面料)而需要进行特殊护理。
请仔细阅读服装上的洗水标。
节约能源COOLMAX®面料快干,有助您减少使用烘干机。
只要将湿衣服挂起来,不久即可晾干。
其速度之快,足以令旅行者感到满意!全天候舒适天气寒冷时,特别是在进行有氧运动时,英威达的COOLMAX®面料提供理想的第一层里衬。
该面料可以带走皮肤表面的湿冷汗液,将其排向面料外层进而蒸发。
您在保持身体干燥的同时亦保持温暖感觉。
3、Conduit :优点:防雨、耐磨、防风缺点:透气差这是MountainHardware公司自己研制的一款防雨防风透气的材料,主要用于旗下的各款户外服装。
此材料被称为活的干燥剂,大自然中风雨雪的克星。
4、杜邦CORDURA面料,具有轻、速干、柔软、耐久性强的功能性面料,长时间使用也不易变色。
Cordura??面料的特殊结构赋予它优异的耐磨性,耐撕裂性,无与伦比的强度,良好的手感以有质轻、柔软、色泽稳定用易于护理等特点。
现今,Cordura??广泛用于箱包、鞋类等多种产品。
所有的Cordura??面料的织造,染色及后整理过程都是根据杜邦公司的生检测标准进行的。
Cordura??丝的纤维细度范围为从30旦到2000旦,这就意味着你熟悉和依赖的耐用布料将被应用到一个全新系列的产品中。
食品添加剂中英文对照表

Calcium phosphates (i)Monocalcium phosphate (ii)Dicalcium phophate (iii)Tricalcium phosphate
59 E 222 Sodium hydrogen sulphite 60 E 223 Sodium metabisulphite 61 E 224 Potassium metabisulphite 62 E 226 Calcium sulphite 63 E 227 Calcium hydrogen sulphite 64 E 228 Potassium hydrogen sulphite 65 E 230 Biphenyl, diphenyl 66 E 231 Orthophenyl phenol 67 E 232 Sodium orthophenyl phenol 68 E 233 Thiabendazole 69 E 234 Nisin 70 E 235 Natamycin or pimaricin 71 E 239 Hexamethylene tetramine 72 E 242 Dimethyl dicarbonate 73 E 249 Potassium nitrite 74 E 250 Sodium nitrite 75 E 251 Sodium nitrate 76 E 252 Potassium nitrate 77 E 260 Acetic acid 78 E 261 Potassium acetate or potassium diacetate
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
/Langmuir©2010American Chemical SocietyHierarchical Carbon Foams with Independently Tunable Mesopore andMacropore Size DistributionsAdam F.Gross*and Andrew P.NowakHRL Laboratories,LLC,3011Malibu Canyon Road,Malibu,California90265Received February23,2010.Revised Manuscript Received April28,2010Hierarchical carbon foams with independently tunable mesopore and macropore size distributions were formed in a high internal phase emulsion(HIPE)template.The HIPE consists of an internal oil phase that controls the macropore dimensions and an aqueous resorcinol-formaldehyde precursor solution external phase that directs the mesopore size distribution.Once the emulsion is formed,the precursor solution is cured,fluid elements are extracted from the monolith via solvent exchange, and then the sample is pyrolyzed to create a hierarchical open-cell foam consisting of macropores with mesoporous carbon xerogel walls.Both mesopore and macropore size distributions may be independently tuned by changing the synthesis parameters.These samples have a peak in the mesopore size distribution that may be tuned to between5and8nm and macropore average diameters that may be tuned to between0.7and2.1μm.Furthermore,the0.7and2.1μm average diameter macropores have0.18and0.53μm diameter macropore windows between adjacent pores,respectively.Pore volumes up to 5.26cm3/g and electrical conductivities as high as0.34S/cm are observed after1200°C carbonization of the framework.These foams may have potential applications as3-D current collectors in batteries and as fuel cell catalyst supports.IntroductionMany porous materials may be formed from emulsion-templated solutions.1,2Emulsions are an advantageous tool for structural control because of their ease of fabrication,wide range of possible pore sizes,and scalability.Furthermore,porous silica,metal oxides, carbons,and metals have been templated with emulsions and these materials demonstrate the flexibility of this technique to form solid materials.1We are particularly interested in forming porous mate-rials in high internal phase emulsions(HIPEs)that have greater than74%internal phase volumes,which is the free space occupied by packed uniform spheres.3,4At higher than74%internal phase volume,the droplets become abutted and the degree of interconnec-tion between resulting pores increases.By forming solid materials in the continuous phase of a HIPE,an open cell foam monolith consisting of polymers,silica,or carbons may be produced.1-3,5-7 Furthermore,when the solid material formed in the external phase of the HIPE is mesoporous silica,a hierarchical macroporous and mesoporous foam with increased surface area and pore volume is obtained.6,7Our goal is to produce hierarchical macroporous carbons with mesoporous carbons walls made from inexpensive and easily removable fluidic precursors templated in HIPEs where both the mesopore and macropore dimensions may be indepen-dently tailored by altering the synthetic composition.Porous carbon materials with hierarchical macropores and mesopores have potential applications as3-D current collectors,electrodes,and catalyst supports.1,2,8-10The carbon framework provides electrical conductivity,and the macropore dimensions and interconnectivity provide control of mass transport and pore volume.1Furthermore,the mesopore structure influences the sur-face area,which is important for catalyst supports,and enables additional ion-transport channels in batteries.Finally,the carbon structure is chemically inert and will survive a variety of reaction conditions.There are many templating approaches that produce hierarchical porous carbons with different degrees of pore structure tunability.A porous carbon may be formed by carbonizing a HIPE in which the continuous phase consists of a polymerized styrene monomer.5This approach produces porous carbons with nan-ometer length scale pores in the walls that are induced as a result of densification during carbonization;however,no peak in the mesopore size distribution was shown because this approach does not actively template mesopores during synthesis.Additionally, hierarchical foams may be formed from agglomerated colloids of a carbonaceous precursor.11,12The packed colloids form macropores whereas surfactant templating in the colloids,or their densification during carbonization,produces mesopores.Pluronic surfactants may also be mixed with water and phenolic resins to form a macroporous and mesoporous hierarchical carbon structured by phase separation during hydrothermal treatment.13In another approach used by many groups,a resin may be polymerized around solid micrometer-sized polymer or silica colloids and then carbonized to form a hierarchical carbon.1,2,8-10,14-18Macropores*Corresponding author.E-mail:afgross@.(1)Zhang,H;Cooper,A.I.Soft Matter2005,1,107.(2)Yuan,Z.;Su,B.J.Mater.Chem.2006,16,663.(3)Cameron,N.R.Polymer2005,46,1439.(4)Barbetta,A.;Cameron,N.R.;Cooper,mun.2000,221.(5)Wang,D.;Smith,N.L.;Budd,P.M.Polym.Int.2005,54,297.(6)Hu,Y.;Nareen,M.;Humphries,A.;Christian,P.J.Sol-Gel Sci.Technol. 2010,53,300.(7)Carn,F.;Colin,A.;Achard,M.F.;Deleuze,H.;Sellier,E.;Birot,M.;Bakov, R.J.Mater.Chem.2004,14,1370.(8)Yu,J.-S.;Kang,S;Yoon,S.B.;Chai,G.J.Am.Chem.Soc.2002,124,9382.(9)Chai,G.S.;Yoon,S.B.;Yu,J.S.;Choi,J.H.;Sung,Y.E.J.Phys.Chem.B 2004,108,7074.(10)Lee,K.T.;Lytle,J.C.;Ergang,N.S;Oh,S.M.;Stein,A.Adv.Funct. Mater.2005,15,547.(11)Carriazo,D.;Pico,F.;Gutierrez,M.C.;Rubio,F.;Rojo,J.M.;del Monte,F.J.Mater.Chem.2010,20,773.(12)Zou,C.;Wu,D.;Li,M.;Zeng,Q.;Xu,F.;Huang,Z.;Fu,R.J.Mater. Chem.2010,20,731.(13)Huang,Y.;Cai,H.;Feng,D.;Gu,D.;Deng,Y.;Tu,B.;Wang,H.;Webley, P.A.;Zhao,mun.2008,2641.(14)Zhao,J.;Cheng,F.;Yi,C.;Liang,J.;Tao,Z.;Chen,J.J.Mater.Chem. 2009,19,4108.(15)Wang,Z.;Kiesel,E.R.;Stein,A.J.Mater.Chem.2008,18,2194.(16)Lukens,W.W.;Stucky,G.D.Chem.Mater.2002,14,1665.(17)(a)Baumann,T. F.;Satcher,J.H.Chem.Mater.2003,15,3745.(b)Baumann,T.F.;Satcher,J.H.J.Non-Cryst.Solids2004,350,120.(18)Woo,S.W.;Dokko,K.;Sasajima,K.;Takei,T.;Kanamura,K.Chem. Commun.2006,4099.Gross and Nowak Articleform after removal of the colloids,and their dimensions are adjusted by changing the initial colloid diameter.Further-more,very uniform macropores were created by first forming a colloidal crystal(instead of using agglomerated colloids)and then using this ordered solid as a template for the carbona-ceous material.2,8-10,14,17-19An additional level of order in hierarchical carbons may be induced by arranging smaller colloids around larger colloids in a templating colloidal crystal.2,18,19Mesoporosity is induced without a template in many of these colloid-templated carbons as a result of carbo-nization and densification of the resin.In a few reports,the mesopore dimensions were more precisely controlled when larger colloids were surrounded by a mesoporous carbon instead of a resin.16,17One drawback with solid colloidal particle templating is that it requires the initial synthesis of particles and creating porosity in these materials may necessi-tate subsequent etching with hydrofluoric acid if silica-based materials are used.Finally,a macroporous polymer foam may be infiltrated with mesoporous silica in order to form a hierarchical carbon.20The macropore size is controlled by the initial polymer foam pore dimensions,and the mesopore dimensions are controlled by the synthesis parameters of the mesoporous silica.Mesoporous carbon is formed through the vapor deposition of a precursor on the silica,carbonization of the precursor,and the removal of the silica template with HF. Our goal is to fabricate hierarchical porous carbon monoliths with independently tunable mesopore and macropore dimensions without templating from solid materials.By using an oil-in-water soft matter template,we are able to form an open-cell hierarchical carbon foam using inexpensive and easily removable templating materials.We template our materials with a high internal phase emulsion formed by the high-speed mixing of a silicone oil internal phase and an aqueous continuous phase.The aqueous phase contains a resorcinol-formaldehyde precursor solution that enables the tuning of mesoporosity with synthetic variables.21,22 Additionally,the macropore dimensions are tuned with oil viscosity.23A carbon foam is fabricated by blending the oil and aqueous phases,thermally curing the precursor solution,remov-ing the oil phase via solvent extraction,exchanging water for organic solvent,drying the monolith,and finally carbonizing the sample.This process results in a hierarchical macroporous foam made from mesoporous carbon xerogel.Our material differs from carbonized polymer HIPEs because the meosporosity is con-trolled by the xerogel synthetic variables rather than being a byproduct of polymer densification during pyrolysis.We believe that our material is the first hierarchical carbon monolith formed from all fluidic precursors with tunable pore size mesoporous xerogel walls.In this study,the composition of the aqueous and oil phases of the HIPE are varied to demonstrate independent tuning of the mesopore and macropore pore size distributions followed by the characterization of the resulting foams.Finally, we measure the electrical conductivity of the carbon foam mono-liths to determine if these materials could be candidates for fuel cell and battery applications.Experimental SectionAll chemicals were purchased from Aldrich and used as received.Resorcinol-formaldehylde precursor solutions were synthesized by mixing a2:1:0.002molar ratio of formaldehyde (37wt%solution in water)to resorcinol(99%)to sodium carbonate(99%)in deionized water.22The resorcinol-formalde-hyde precursor solutions contained30or40wt%organic content (formaldehydeþresorcinol mass).The resorcinol-formaldehyde precursor solutions were stirred until they dissolved and allowed to rest for1h before use in carbon foam fabrication.Carbon foams were fabricated by dissolving0.360g of sodium dodecylbenzenesulfonate surfactant(technical grade)in12.5g of a resorcinol-formaldehyde precursor solution that was then com-bined with37.5g of silicone oil(100and1000cP viscosity,Fluka brand DC200).The mixture was transferred to a Waring laboratory blender and blended for10min in all experiments.A white viscoelastic emulsion was obtained and transferred in equal fractions to three60mL Nalgene polypropylene jars.The jars were sealed and aged for72h at85°C,which resulted in bright orange resorcinol-formaldehyde foam monoliths formed in the shape of the Nalgene containers.The foams were then soaked in chloroform to extract the silicone oil.The chloroform bath was poured off and refilled with fresh solvent twice,and there was at least a4h soak time during each of the first two soaking cycles and 8h during the third cycle.The foams then were then removed from chloroform and placed in an acetone bath to exchange solvent for water in the monolith.The acetone bath was poured off and refilled two times with at least2h between each soaking cycle. Finally,the foam monoliths were removed from the acetone bath, allowed to dry in air,and heated in a tube furnace under flowing nitrogen from room temperature to800°C at2.6°C/min and maintained at800°C for6h to pyrolyze the resorcinol-formalde-hyde gel.In one case,a foam was pyrolyzed under flowing nitrogen and heated from room temperature to1200°C at2°C/ min and maintained at1200°C for6h in order to measure the effect of different pyrolysis conditions.There are no significant chemical hazards encountered during the preparation of the carbon foams described in this study.Samples carbonized at800°C are denoted throughout this article on the basis of the organic loading of the xerogel and the viscosity of the silicone oil.For example,a40%/1000sample was made by blending a40wt%organic resorcinol-formaldehyde precursor solution with1000cP silicone oil.The water/oil volume ratios of the40%/100,40%/1000,and30%/1000foams were 23.5:76.5,23.5:76.5,and23.9:76.1,respectively.The ratio is slightly higher for the30%/1000emulsions as compared to that of the40%/100and40%/1000emulsions because of the lower density of the30wt%resorcinol-formaldehyde precursor solu-tion.If the sample was carbonized at a temperature other than 800°C,then the carbonization temperature is listed after the orga-nic loading of the xerogel and the viscosity of the silicone oil. The sample pore size,surface area,and pore volume were characterized with N2adsorption and Hg intrusion at Micro-meritics Analytical Services(Norcross,GA).N2data were ana-lyzed using the Brunner-Emmett-Teller(BET)and Barrett-Joyner-Halenda(BJH)methods.24Hg intrusion experiments were sensitive to pores between3nm and360μm in diameter. SEM images were acquired on a Hitachi S-4800.Electrical conductivity was measured with an HP34401A multimeter using a four-wire probe.Current and voltage probes were placed on opposite faces of a carbon foam monolith,and the resistance was measured.This resistance was converted to conductivity by inverting the resistance value and dividing by the thickness of the monolith.Results and DiscussionCarbon foams were synthesized as described in the Experi-mental Section from a HIPE template consisting of an external(19)Chai,G.S.;Shin,I.S.;Yu,J.S.Adv.Mater.2004,16,2057.(20) Alvarez,S.;Fuertes,A.B.Mater.Lett.2007,61,2378.(21)Al-Muhtaseb,S.A.;Ritter,J.A.Adv.Mater.2003,15,101.(22)Li,W.;Lu,A.;Weidenthaler,C.;Sch€u th,F.Chem.Mater.2004,16,5676.(23)Mason,T.G.;Wilking,J.N.;Meleson,K.;Chang,C.B.;Graves,S.M. J.Phys.:Condens.Matter2006,18,R635–R666.(24)Lowell,S.;Shields,J.E.;Thomas,M.A.;Thommes,M.Characterization of Porous Solids and Powders:Surface Area,Pore Size and Density;Springer: Dordrecht,The Netherlands,2006.Article Gross and Nowak phase resorcinol-formaldehyde precursor solution and an internaloil phase.Carbon xerogel is formed in the external phase,and themesoporosity is controlled by the resorcinol-formaldehyde pre-cursor solution composition.The water fraction of the resorcinol-formaldehyde precursor solution acts as a porogen and templatesthe mesopores.Additionally,the organic fraction and amountof catalyst in the resorcinol-formaldehyde precursor solutioncontrol the micropore structure and shrinkage during dryingand pyrolysis.(Smaller amounts of catalyst relative to resorcinolcreate microporosity in the carbon particles that make up thexerogel and better resist shrinkage during drying.21)Althoughthe cured resorcinol-formaldehyde gel from the continuousphase of the HIPE provides structural rigidity in the car-bon foam,the oil phase creates regions where the resorcinol-formaldehyde precursor solution is excluded to produce macro-pores.3Additionally,the oil droplets are abutted,which resultsin windows forming between the macropores and creates an open-cell foam.We refer to these openings between macropores as“macropore windows”to differentiate them from the largermacropores.A conceptual diagram of the HIPE is shown inFigure S1.The HIPE that templates the carbon foam is formed using highshear mixing,and both the macropore and macropore windowdimensions are controlled by the oil viscosity.Higher oil viscosityresults in increased viscous resistance against oil droplet deforma-tion and separation into larger droplets.Thus a lower interfacialsurface area is created between oil and water for a given amountof mechanical energy deposited into the system(with the samemixing speed used in all experiments).25This reduced surface arearesults in larger droplets,larger macropores,and larger macro-pore windows.We varied both the oil viscosity and resorcinol-formaldehyde precursor solution composition to demonstratecontrol of both mesopore and macropore textural properties.Sample Fabrication.The monolithic structures of an as-synthesized resorcinol-formaldehyde HIPE-templated foam andof a subsequently800°C pyrolyzed carbon foam are shown inFigure S2.The foams take the shape of the cylindrical jars inwhich the emulsions are cured and maintain this shape with someshrinkage during carbonization.SEM images of the carbon foamsare shown in Figures1(top)and2and demonstrate that the foamretained the HIPE structure through carbonization.The macro-pores reproduce the morphology of the templating silicone oilphase with macropore windows resulting from abutted droplets.Similar openings between macropores are also observed incolloidally templated carbons because adjacent templates aretouching.2,8-14,17,18The higher-magnification image of themacropore wall in Figure1(bottom)clearly shows the hierarchi-cal structure of the mesoporous carbon xerogel forming the wallsof the macropores.SEM images in Figures2and S3of the30%/1000and40%/1000materials show larger macropores than observed in the40%/100sample and demonstrate that the macropore size may be tuned with the silicone oil viscosity. Higher-resolution images of the xerogel structure,such as in Figure1(bottom),show no discernible differences for all three samples.Additionally,multiple SEM images for each sample were used to calculate the mean macropore diameter by averaging the diameters of at least60macropores.When100cP silicone oil is used in the40%/100foam,the macropores have a mean diameter of0.7μm,whereas both the30%/1000and40%/1000samples have a mean macropore diameter of2.1μm.This represents a 3-fold increase in the macropore mean diameter from a10-fold increase in oil viscosity.To determine if the macropore pore diameters between samples were distinct,the statistical signifi-cance between the means of macropore distributions was mea-sured by applying Welch’s t test for unequal variances for all sample pairs.Differences in the means for each macropore distribution with differing oil viscosity(40%/100vs30%/1000 and40%/100vs40%/1000)were both found to be statistically different(two-tailed p<0.0001)whereas macropore populations between identical oil viscosity samples(30%/1000vs40%/1000) were not(two-tailed p=0.97).Porosimetry.The carbon foams fabricated in this study were characterized with Hg intrusion and N2adsorption to understand the pore structures.Hg intrusion is most sensitive to pores with diameters>50nm whereas the small size and gaseous delivery of N2atoms make their adsorption best suited to characterizing mesopores and micropores.24Pore size distributions for all800°C Figure1.SEM images of a40%/100carbon foam showing the HIPE-templated structure of the carbon foam.Scale bars are shown in the images.Macropores and macropore windows formed from abutted silicone oil droplets are observed in the top image. This image is indistinguishable from a HIPE-templated polymer, demonstrating that the original emulsion structure is retained.3 The mesoporous xerogel structure of the walls is observed under higher magnification(bottom).Figure2.SEM images of40%/1000(top)and30%/1000(bottom) carbon foams.Scale bars are shown in the images.The structure of a high internal phase emulsion is observed in both samples.(25)Meleson,K.;Graves,S.;Mason,T.G.Soft Mater.2004,2,109.Gross and Nowak Articlepyrolyzed sample were derived from Hg intrusion data(Figure S4)and are shown in Figure3.The peak positions for the30%/ 1000and40%/1000samples are both at0.53μm,and the peak for the40%/100sample is at0.18μm.Additionally,the pore size distributions for30%/1000and40%/1000samples overlap almost perfectly and are insensitive to resorcinol-formaldehyde precursor solution composition.We note that these pore dimen-sions are much smaller than those observed in SEM,which is due to Hg intrusion measuring the smallest openings between pores.24 These openings are the macropore windows seen in Figures1,2, and S3,and the macropore window dimensions observed in SEM images are in good agreement with the Hg intrusion data.In addition,the macropore window diameters have the same∼3-fold increase in diameter from a10-fold increase in oil viscosity observed for macropores in SEM.Because macropore and macropore window dimensions were tuned with silicone oil viscosity but not with the resorcinol-formaldehyde precursor solution,we conclude that the silicone oil viscosity alone mainly controls macropore and macropore window structure.All textural parameters from Hg intrusion are presented in Table1.All carbon foams have large pore volumes and porosities; however,the40%/100sample is the most distinct from the other samples.The40%/100sample is templated with a volume of silicone oil that is equal to that used for other samples;however,it is formed from lower-viscosity oil that is sheared into smaller droplets with an increased surface area to volume ratio during HIPE formation.The greater surface area of the smaller oil droplets should produce a carbon foam with greater surface area, which agrees with the data in Table1.Additionally,the smaller droplets result in the aqueous phase being distributed over a larger interfacial area,which yields thinner xerogel walls.The larger Hg intrusion pore volume of the40%/100sample may result from abutted droplets more easily producing macropore windows between thinner walls and creating more open space as compared to the thicker walls in the40%/1000and30%/1000samples.N2adsorption BJH pore size distributions calculated from N2 adsorption isotherms(Figure S5)are shown in Figure S6for all 800°C pyrolyzed samples.The40%/100and40%/1000samples have very similar pore size distribution peak positions,and larger pores are found in the30%/1000sample as shown by the1.33-fold larger pore size distribution peak position.This demonstrates that the composition of the aqueous phase,and not the templating oil, mainly controls mesopore pore size distribution peak positions. We note that the oil viscosity has some effect on mesopore structure as shown by comparing the100and1000cP oil-templated samples in Figure S6.The40%/100sample has some mesopore volume above20nm,but the pore volumes of the40%/ 1000and30%/1000samples are negligible in this region.This may be explained by the thinner macropore walls in the40%/100 sample having less internal volume as compared to that in the 40%/1000and30%/1000samples.There will be more xerogel material exposed to the oil-water interface in the40%/100 sample,and with fewer surrounding carbon particles on the interface,some surface pores may have diameters greater than 20nm.The calculated N2adsorption textural parameters of all samples are presented in Table2.All foams are microporous as well as mesoporous.The presence of micropores is confirmed by the t-plot micropore volume derived from the N2adsorption data(Table2).This micropore volume is formed during pyrolysis in the carbon particles that make up the mesopore walls.26Thus,these carbon foams have two levels of hierarchy:(1)the microporous carbon particles that make up the mesoporous xerogel and(2)the mesoporous xerogel composing the walls of the macropores. The data in Tables1and2demonstrates that the resorcinol-formaldehyde precursor solution controls the mesopore diameter but has little effect on the macropore and macropore window structure.Although the silicone oil viscosity controls the macro-pore volume and size,it minimally affects the peak position of the mesopore pore size distribution.Thus,the mesopore and macro-pore/macropore window dimensions in these hierarchical carbon foams may be independently adjusted.Furthermore,the macro-pore and macropore window average diameters should be tunable between0.7and2.1μm and0.18and0.53μm,respectively,by incorporating oil into the HIPE with the appropriate viscosity between100and1000cP(with200,350,and500cP currently available).Larger or smaller macropores and macropore win-dows than those made in this study are likely accessible using silicone oils with viscosity greater than1000cP or less than100cP. The mesopore size should also be tunable over a wider range of values than those shown in this study by using alternative aerogel or xerogel materials as discussed later in this article.We note that the mesopore pore size distribution peak posi-tions,pore volumes,and surface areas are significantly smaller than those observed in neat xerogels made with the same recipe. (These materials have also been referred to as aerogels in the literature,and they have sample compositions and processing conditions that are similar to those of our materials.22,27)The mesopore textural parameters may have been altered by the curing temperature profile or the presence of surfactant.The carbon foams in this study were cured for3days at85°C in contrast to the1day at room temperature,1day at50°C,and1 day at90°C previously used for a neat xerogel in ref22.When a neat40wt%organic xerogel control solution was cured for3 days at85°C and solvent exchanged with chloroform and acetone in an identical manner to that for the carbon foams,we obtained the same pore volume of1.39cm3/g and a similar surface area of 641m2/g as found in a traditionally cured sample.28However,the peak position in the pore size distribution increased from25 to51nm.Thus,the different curing temperature profile does alterFigure3.Hg intrusion pore size distributions of800°C pyrolyzedcarbon foams.Samples are indicated by the legend on the graph.Hg intrusion is sensitive to the smallest opening between pores,andthis data is indicative of the macropore windows.The40%/1000and30%/1000carbon foams were templated with higher-viscositysilicone oil than was the40%/100sample,and this results in largermacropores and macropore windows.This data demonstrates thatthe macropore window pore size is insensitive to the composition ofthe resorcinol-formaldehyde precursor solution and is tunable withsilicone oil viscosity.(26)Gavalda,S.;Gubbins,K.E.;Hanzawa,Y.;Kaneko,K.;Thomson,K.T.Langmuir2002,18,2141.(27)Wu,D.;Fu,R.;Dresselhaus,M.S.;Dresselhaus,G.Carbon2006,44,675.(28)Gross,A.F.;Vajo,J.J.;Van Atta,S.L.;Olson,G.L.J.Phys.Chem.C2008,112,5651.Article Gross and Nowakthe pore structure and probably contributes to differences in morphology,but it cannot explain the decrease in the pore volume,surface area,or pore size distribution peak position found in Table2.Another possible reason for the differences in mesopore morphology is that our samples contain2.8wt% sodium dodecylbenzenesulfonate surfactant in the resorcinol-formaldehyde precursor solution,which is not present in neat carbon xerogel syntheses.Equal and lower surfactant levels are known to densify aerogels/xerogels and result in lower pore volumes,pore diameters,and surface areas than observed in unmodified materials.27,29Thus,the large amount of surfactant in the HIPEs is likely the primary cause of the morphological changes that we observe in the xerogel framework.In the HIPEs, a significant fraction of surfactant is isolated at the oil-water interface and is not available to alter xerogel structure.Because of uncertainty about the distribution of surfactant between the oil/ water interface and the aqueous phase,we chose not to run a control experiment using only surfactant and our resorcinol-formaldehyde precursor solution because the interpretation of results would be not be meaningful.In addition to the previously discussed samples,we attempted to form a30%/100material for this study and obtained a resorcinol-formaldehyde foam that partially collapsed after sol-vent exchange and ambient drying.We measured a Hg intrusion pore volume of only1.30cm3/g in the30%/100carbonized foam, and the macropore walls buckled during drying as shown in the SEM image in Figure S7.Because lower organic loading xerogels contain less material to resist shrinkage and because the macro-pore walls are thinner in samples made with100cP silicon oil,it is understandable that the30%/100sample collapsed.This indi-cates that our method of forming carbon foams is limited by the strength of the xerogel walls.We believe our method could overcome these limitations and produce macroporous carbon foams with a larger range of densities and average mesopore diameters by incorporating supercritically dried carbon aerogel formulations or by reducing the amount of surfactant during HIPE fabrication.27,30Supercritically dried carbon aerogel for-mulations may allow the formation of lower-density carbon foams because of their lower organic content and gentler drying procedure.Additionally,reducing the surfactant concentration may allow larger mesopores.According to ref27,less surfactant in our xerogel formulation should increase the peak position of the mesopore size distribution up to values as large as25nm.This approach will work only if the surfactant preferentially migrates to the oil-water interface and sufficient surfactant is present to enable HIPE formation.A40%/100sample was pyrolyzed at1200°C where partial graphitization and sintering of the framework occur to determine the maximum possible electrical conductivity of our materials.21 After1200°C pyrolysis,the sample monoliths remained intact and SEM images in Figure S8shows that the macropore, macropore window,and xerogel structure were all maintained. Elevated firing temperatures are known to degrade the surface area and pore volume of xerogels and we characterized the porosity of this foam to quantify any reduced porosity.31-33 The macropore textural characteristics are shown in Table1, and a comparison of the macropore pore size distributions from 40%/100foams pyrolyzed at800and1200°C is shown in Figure S9.The40%/1001200°C sample shows a reduced macropore window diameter,which is expected because of the sintering of the carbon framework,and a slightly decreased foam density,which has been observed in another report on high-temperature-fired porous carbons.34However,the sample showed an unexpected large increase in macropore volume and surface area.We hy-pothesize that closed macropores originally formed around non-abutted oil droplets and may have opened as a result of the1200°C treatment of the carbon framework.The mesopore textural data for the40%/1001200°C sample is shown in Table2,and a comparison of the mesopore pore size distributions from40%/100foams pyrolyzed at800and1200°C is shown in Figure S10.The increase in the peak position of the mesopore size distribution and the reduced micropore volume fraction are both expected from the sintering of carbon particles that template the mesopores.21,31-33However,the vastly increased surface area and pore volume do not agree with known trends for surfactant-free carbon aerogels and xerogels.31,33The40%/100 1200°C sample is different from materials in previous reports on sintering effects because it contains surfactant in the synthesis solution,which results in denser networks of carbon particles forming the mesoporous framework.27,29The more closely packed carbon particles may form inaccessible pores,and the modifica-tions in the carbon framework that occur at1200°C may enableTable1.Calculated Textural Parameters of Carbon Foams from Hg Intrusion DatasampleHg intrusionsurface area(m2/g)Hg intrusionpore volume(cm3/g)foam density(g/cm3)skeletaldensity(g/cm3)porosity(%)Hg intrusion pore sizedistribution peak position(μm)40%/100155 4.370.197 1.41860.18 40%/1000102 3.270.263 1.88860.53 30%/1000118 3.220.262 1.68840.53 40%/1001200°C254 5.260.166 1.34870.15 Table2.Calculated Textural Parameters of Carbon Foams from N2Adsorption DatasampleN2adsorptionsurface area(m2/g)N2adsorption single-pointadsorption total porevolume(cm3/g)N2adsorption t-plotmicropore volume(cm3/g)N2adsorptionV micropores/V totalN2adsorption BJH poresize distribution peakposition(nm)40%/1001870.1930.0330.17 5.3 40%/10001740.1440.0350.24 5.7 30%/10002470.2490.0420.177.6 40%/1001200°C5930.5770.0680.127.6(29)Worsley,M.A.;Satcher,J.H.;Baumann,T.F.J.Non-Cryst.Solids2010, 356,172.(30)Liu,N.;Zhang,S.;Fu,R.;Dresselhaus,M.S.;Dresselhaus,G.J.Appl. Polym.Sci.2007,104,2849.(31)Zanto,E.J.;Al-Muhtaseb,S.A.;Ritter,J.A.Ind.Eng.Chem.Res.2002,41, 3151.(32)Gross,J.;Alviso,C.T.;Pekala,R.W.Mater.Res.Soc.Symp.Proc.1996,431, 123(also available at /bridge/product.biblio.jsp?osti_id=231318).(33)Lin,C.;Ritter,J.A.Carbon2000,38,849.(34)Wiener,M.;Reichenauer,G.;Hemberger,F.;Ebert,H.-P.Int.J.Thermo-phys.2006,27,1826.。
