BMS30314(943962-47-8)-HPLC-Biochempartner
不同亚型CHO_宿主细胞对抗体表达的影响

生物技术进展 2023 年 第 13 卷 第 5 期 698 ~ 703Current Biotechnology ISSN 2095‑2341进展评述Reviews不同亚型CHO 宿主细胞对抗体表达的影响曹辉 , 董静 , 贾宇 , 江一帆*华北制药集团新药研究开发有限责任公司,抗体药物河北省工程研究中心,抗体药物研究国家重点实验室,石家庄 050015摘要:CHO 细胞作为宿主细胞广泛应用于生物药工业化生产中。
其中,CHO -K1、CHO -DG44和CHO -S 是最常见的3种亚型。
虽然这些亚型是从共同的原始CHO 细胞分离出来的,但在不同的实验室或生物医药公司、研究人员、培养基或培养方式下连续传代、驯化和保存,使得CHO 细胞积累了大量变异,导致宿主细胞应用于抗体药生产时会在细胞生长状态、抗体表达量及以糖型为代表的质量属性方面表现出较大差异。
综述了CHO 细胞不同亚型的染色体差异、生长状态、表达差异以及糖型差异,以期为抗体药物研发中宿主细胞的选择提供参考。
关键词:CHO 细胞;抗体;表达量;糖型DOI :10.19586/j.2095‑2341.2023.0064中图分类号:Q28, R392-33 文献标志码:AEffects of Different Sources of CHO Host Cells on Antibody ExpressionCAO Hui , DONG Jing , JIA Yu , JIANG Yifan *State Key Laboratory of Antibody Drug Development , Hebei Engineering Research Center of Antibody Medicine , New Drug Research and Development Co. Ltd , North China Pharmaceutical Corporation , Shijiazhuang 050015, ChinaAbstract :CHO cells comprise a variety of lineages including CHO -K1, CHO -DG44 and CHO -S , which have been widely used in the industrial production of biological drugs. All CHO cell lines share a common ancestor , however , during the process of cell passage cultivation , cell domesticated , and preservation by different laboratories or companies , substantial genetic heterogeneity among them has been produced , that showed great differences in cell growth state , antibody titer , glycosylation and other product quality attributes. This article reviewed the difference in chromosome , growing status and expression , and glycoform in different sources ofCHO host cells , which was expected to be helpful in host cell selection during antibody drug research and development process.Key words :CHO cells ; monoclonal antibody ; antibody titer ; glycosylation生物药物在国际医药市场中占据主导地位,截至2023年,全球范围内已有100多个抗体药物被批准上市,近1 200个抗体药物处于不同临床试验阶段[1]。
一种快速分离检测产脂肽类生物表面活性剂枯草芽孢杆菌的方法

一种快速分离检测产脂肽类生物表面活性剂枯草芽孢杆菌的方法王大威;张健;姜伟;张凤久【摘要】脂肽(Lipopeptide)是由枯草芽孢杆菌(Bacillus subtilis)等微生物产生的一类具有较强表面活性的生物表面活性剂.枯革杆菌磷酸泛酰巯基转移酶基因(afp)是枯草芽孢杆菌中参与脂肽代谢的功能性基因.采用sfp基因PCR对从环境中得到的一组产生表面活性剂的微生物进行筛选,结合Tricine-SDS-PAGE电泳对PCR结果呈阳性的菌蛛的代谢粗初提物进行检测,初步鉴定得到两株枯草芽孢杆菌.进一步利用16S rDNA序列的系统发育学分析确定这两种菌株为枯草芽孢杆菌,并利用TLC、HPLC鉴定其产物为脂肽类表面活性剂,从而建立了一套快速分离检测产生脂肽类生物表面活性剂的枯草芽孢杆菌方法.【期刊名称】《生物技术通报》【年(卷),期】2011(000)009【总页数】5页(P142-146)【关键词】脂肽;枯草芽孢杆菌;sfp基因;Tricine-SDS-PAGE;电泳【作者】王大威;张健;姜伟;张凤久【作者单位】中海油研究总院,北京100027;海洋石油高效开发国家重点实验室,北京100027;中海油研究总院,北京100027;海洋石油高效开发国家重点实验室,北京100027;中国海洋石油总公司,北京100027;中国海洋石油有限公司,北京100027【正文语种】中文脂肽(Lipopeptide)又名脂酰肽(Acylpeptide),是由亲水的肽键和亲油的脂肪烃链两部分组成的小肽,由于其特殊的化学组成和两亲型分子结构,脂肽类生物表面活性剂显示了十分优良的特性,在医药、食品、化妆品、环境治理和微生物采油等领域都有广泛的应用[1] 。
大多数脂肽来源于微生物,而其中以来源于细菌的脂肽居多。
目前发现的脂肽类生物表面活性剂有10余种,主要包括Surfactin、Lichenysin、Iturin和Fengysin等[2] 。
常用多肽缩合试剂
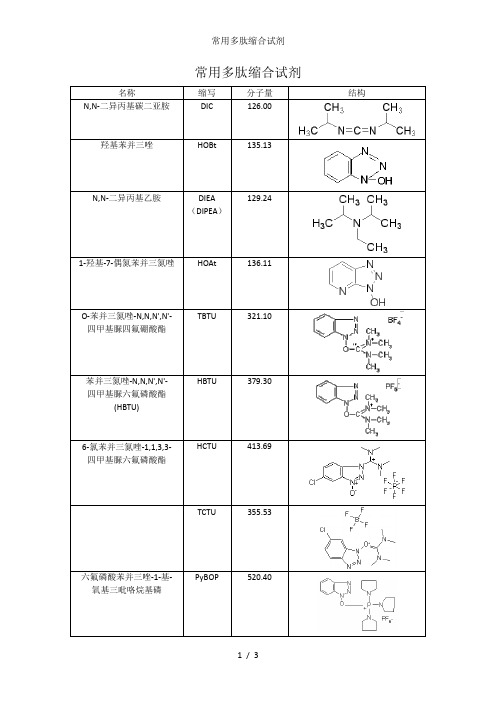
名称缩写分子量结构N,N-二异丙基碳二亚胺DIC 126.00羟基苯并三唑HOBt 135.13129.24N,N-二异丙基乙胺DIEA(DIPEA)1-羟基-7-偶氮苯并三氮唑HOAt 136.11TBTU 321.10O-苯并三氮唑-N,N,N',N'-四甲基脲四氟硼酸酯HBTU 379.30苯并三氮唑-N,N,N',N'-四甲基脲六氟磷酸酯(HBTU)HCTU 413.696-氯苯并三氮唑-1,1,3,3-四甲基脲六氟磷酸酯TCTU 355.53PyBOP 520.40六氟磷酸苯并三唑-1-基-氧基三吡咯烷基磷名称缩写分子量结构PyAOP 521.38(3H-1,2,3-三唑并[4,5-b]吡啶-3-氧基)三-1-吡咯烷基鏻六氟磷酸盐DCC 206.33N,N'-二环己基碳二亚胺4-二甲氨基吡啶DMAP 122.17DBU 152.241,8-二氮杂双环[5.4.0]十一碳-7-烯1,1’-羰基二咪唑CDI 162.15HATU 380.232-(7-偶氮苯并三氮唑)-N,N,N',N'-四甲基脲六氟磷酸酯HOOBt 163.103-羟基-1,2,3-苯并三嗪-4(3H)-酮Cl-HOBt 169.576-氯-1-羟基苯并三氮唑EDC.HCl 191.71-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐TATU 322.1O-(7-氮杂苯并三氮唑)-N,N,N',N'-四甲基脲四氟硼酸盐名称缩写分子量结构O-(1,2-二氢-2-氧-吡啶基)--1,1,3,3-四甲基脲四氟硼酸盐TPTU 297.10O-(N-琥珀酰亚胺基)-N NN'N'-四甲基四氟硼酸脲TSTU 301.10三吡咯烷基溴化鏻六氟磷酸盐PyBrOP 466.20N,N,N',N'-四甲基氯甲脒六氟磷酸盐TCFH 280.583 -二乙氧基磷酰基-1,2,3-苯唑4(3H)-酮DEPBT 299.23O-[(乙氧基羰基)氰基甲胺]-N,N,N',N'-四甲基硫尿四氟硼酸TOTU 328.1苯并三氮唑-1-基氧基三(二甲基氨基)磷鎓六氟磷酸盐BOP(卡特缩合剂)442.50N,N,N',N'-四甲基-O-(3,4-二氢-4-氧代-1,2,3-苯并三嗪-3-基)脲四氟硼酸盐TDBTU 349.09。
《中国药典》(2020年版)复方磺胺恶唑片中的甲氧苄啶含量测

《中国药典》(2020年版)复方磺胺噁唑片中的甲氧苄啶含量测甲氧苄啶(Trimethoprim, TMP),又称为甲氧苄氨嘧啶、甲氧苄嘧啶,是一种抗菌增效药,与磺胺类药物联合使用时,能使磺胺类药物抗菌谱扩大、抗菌活性大大增强。
由于其独特的作用,甲氧苄啶在养殖业病害防治中被广泛应用。
迪信泰检测平台采用高效液相色谱(HPLC)和液相色谱-三重四极杆质谱(LC-MS/MS)法,可高效、精准的检测甲氧苄啶的含量变化。
此外,我们还提供其他抗生素检测服务,以满足您的不同需求。
HPLC和LC-MS测定甲氧苄啶样本要求:1. 请确保样本量大于0.2g或者0.2mL。
周期:2~3周项目结束后迪信泰检测平台将会提供详细中英文双语技术报告,报告包括:1. 实验步骤(中英文)2. 相关质谱参数(中英文)3. 质谱图片4. 原始数据5. 甲氧苄啶含量信息应用范围:本方法采用高效液相色谱法测定复方磺胺甲恶唑片中磺胺甲恶唑和甲氧苄啶的含量。
本方法适用于复方磺胺甲恶唑片。
方法原理:供试品加甲醇稀释,最终用流动相定量稀释后,进入高效液相色谱仪进行色谱分离,用紫外吸收检测器,于波长240nm处检测磺胺甲恶唑和甲氧苄啶的吸收值,计算出其含量。
试剂: 1. 0.1mol/L盐酸2. 乙腈3. 三乙胺4. 氢氧化钠试液5. 冰醋酸仪器设备: 1. 仪器1.1 高效液相色谱仪1.2 色谱柱十八烷基硅烷键合硅胶为填充剂,理论塔板数按磺胺甲恶唑峰计算不低于4000。
磺胺甲恶唑和甲氧苄啶的分离度应符合要求。
1.3 紫外吸收检测器2. 色谱条件2.1 流动相:水乙腈三乙胺=799 200 1(用氢氧化钠试液或冰醋酸调节pH 值至5.9。
)2.2 检测波长:240nm2.3 柱温:室温试样制备:1. 称取供试品取本品10片,精密称定,研细,精密称取适量(约相当于磺胺甲恶唑44mg)置100mL量瓶中。
2. 对照品溶液的制备精密称取磺胺甲恶唑对照品和甲氧苄啶对照品适量,用0.1mol/L盐酸溶液溶解并定量稀释制成每1mL中约含有磺胺甲恶唑0.44mg的和甲氧苄啶89µg的溶液。
甲氧甲酰基亚甲基三苯基膦检测方法

甲氧甲酰基亚甲基三苯基膦检测方法简介甲氧甲酰基亚甲基三苯基膦是一种广泛应用于药物合成、有机合成和材料科学等领域的有机试剂。
它的化学结构为Ph3PCH2COOCH3,其中Ph表示苯基。
虽然甲氧甲酰基亚甲基三苯基膦在这些领域中的应用非常广泛,但它也具有一定的毒性和危险性。
因此,检测甲氧甲酰基亚甲基三苯基膦的含量和纯度是至关重要的。
本文将介绍一种基于高效液相色谱-质谱联用仪(HPLC-MS)的甲氧甲酰基亚甲基三苯基膦检测方法。
实验方法仪器和试剂- 高效液相色谱仪(Agilent 1200)- 三重四极质谱仪(API 2000)- 甲氧甲酰基亚甲基三苯基膦- 乙酸乙酯- 丙酮- 甲醇- 水- 链霉素色谱条件- 色谱柱:Zobrax Eclipse XDB-C18(4.6 mm×150 mm,5 μm)- 流动相A:甲醇- 流动相B:水/乙酸乙酯(9:1,v/v)- 色谱梯度:0-2 min,10% A;2-10 min,50% A;10-12 min,80% A;12-14 min,80% A;14-14.1 min,10% A;14.1-17 min,10% A- 流速:1 ml/min- 检测:UV检测器(210 nm)和质谱检测器(质荷比m/z 257.2/233.2)制备样品将甲氧甲酰基亚甲基三苯基膦加入适量的乙酸乙酯中,摇匀。
然后加入等体积的丙酮和链霉素,再次摇匀。
用干燥剂处理该混合物,安排在蒸发室中蒸发至干燥。
最后加入1 ml的甲醇重溶混合物,摇匀,取10 μl注射器注射(相当于100 μg/ml的标准溶液)。
结果与讨论样品在上述色谱条件下得到良好的分离,质量检测的检测限为0.5 μg/ml。
甲氧甲酰基亚甲基三苯基膦的保留时间为9.98 min,质荷比为m/z 257.2/233.2。
该方法针对其它扩散寻常法的优点包括:灵敏度高、选择性好、操作简单,并且适用于大样品量和高通量分析的需求。
胰胨-亚硫酸盐-环丝氨酸培养基配套试剂说明书

专注微生物监测控制为食药安全保驾护航
广东环凯微生物科技有限公司
网址: 地址:广州市黄埔区科学城神舟路788号
邮编:510663传真:************-8619销售热线:************转8602(分机)技术热线:************转8877、8876
(分机)产品说明书Product Manual
【产品名称】
通用名称:胰胨-亚硫酸盐-环丝氨酸培养基配套试剂
英文名称:Additives for Tryptose Sulfite Cycloserine Agar 【产品编号与包装规格】
产品编号
产品类型包装规格SR0290冻干试剂10支/盒
【产品用途】
每支添加于100mL (028020)中配成胰胨-亚硫酸盐-环丝氨酸培养基(TSC
)。
1、西林瓶的打开方法:本西林瓶在铝盖上有箭头的标志,开启西林瓶前,用75%酒精棉消毒西林瓶表面,在无菌条件下,按铝盖上的箭头方向打开铝盖,撕开铝盖,打开西林瓶胶塞。
2、试剂的使用方法:每支加入1ml 无菌生理盐水,使试剂完全溶解,再添加于100ml(028020)中配成完整的胰胨-亚硫酸盐-环丝氨酸培养基(TSC)。
【储存条件与保质期】
2-8℃保存,有效期两年。
【废物处理】
检测之后带菌物品置于121℃下高压灭菌30分钟后处理。
【执行标准】
Q/HK 0709微生物检测配套试剂
【说明版本】
2022年11月02日。
注射用泮托拉唑钠中依地酸二钠含量分析方法的建立

注射用泮托拉唑钠中依地酸二钠含量分析方法的建立雷小平杨明亮姚吉勰陈青连(杭州澳亚生物技术股份有限公司杭州 310018)摘要目的:建立高效液相色谱法测定注射用泮托拉唑钠中依地酸二钠的含量。
方法:采用十八烷基硅烷键合硅胶为填充剂的C18色谱柱(4.6 mm×250 mm,5 m m),以磷酸盐缓冲液-乙腈(90∶10)、乙腈进行梯度洗脱,流速为1.0 mL/min,检测波长为254 nm,柱温为35 ℃,进样体积为20 m L。
结果:依地酸二钠的检测不受其他成分干扰,不同浓度依地酸二钠的回收率均在98.0%~102.0%之间,回收率RSD为0.39%,浓度在27.52~64.20 m g/mL范围内线性良好(r=1.000 0)。
结论:本方法简便、迅速、专属性及重现性好,可用于注射用泮托拉唑钠中依地酸二钠含量的测定。
关键词依地酸二钠注射用泮托拉唑钠含量高效液相色谱法中图分类号:O657.72; R975.2 文献标志码:A 文章编号:1006-1533(2022)05-0077-04引用本文雷小平, 杨明亮, 姚吉勰, 等. 注射用泮托拉唑钠中依地酸二钠含量分析方法的建立[J]. 上海医药, 2022, 43(5): 77-80.Establishment of method for disodium edetate in pantoprazole sodium for injectionLEI Xiaoping, YANG Mingliang, YAO Jixie, CHEN Qinglian(Hangzhou Ausia Biological Tech. Co., Ltd., Hangzhou 310018, China)ABSTRACT Objective: To establish a high performance liquid chromatography (HPLC) method for the determination of disodium edetate in pantoprazole sodium for injection. Methods: HPLC was performed using C18 column (4.6 mm×250 mm, 5 μm) with gradient elution containing phosphate buffer-acetonitrile (90:10) (A) and acetonitrile (B) at a flow rate of 1.0 mL/min, detection wavelength 254 nm, column temperature 35 ℃ and sample volume 20 m L. Results: The determination of disodium edetate was not interfered by other components in the injection. The recovery rates of different concentrations of disodium edetate were between 98.0%-102.0% with RSD 0.39%. Standard curve of disodium edetate showed good linearity over the range of27.52-64.20 m g/mL with r=1.000 0. Conclusion: This method is simple, rapid, specific and reproduceable and can be used for thedetermination of disodium edetate in pantoprazole sodium for injection.KEy WORDS disodium edetate; pantoprazole sodium for injection; assay; HPLC依地酸和依地酸盐在药物制剂、化妆品和食品中被用作螯合剂,它们与碱土金属和重金属离子形成稳定的水溶性络合物(螯合剂)。
1,3-二(异氰酸根合甲基)环己烷在电池中的作用

1,3-二(异氰酸根合甲基)环己烷在电池中的作用1,3-二(异氰酸根合甲基)环己烷,也称为TDI,是一种有机化合物,化学式为C9H6N2O2。
它是一种重要的工业原料,广泛用于聚氨酯聚合物的生产中。
除了在聚氨酯行业中的应用外,1,3-二(异氰酸根合甲基)环己烷还具有电池相关领域的一些作用。
电池是一种将化学能转化为电能的装置,常见的电池种类包括干电池、铅酸电池、锂离子电池等。
不同种类的电池使用的电化学反应和材料有所不同,因此1,3-二(异氰酸根合甲基)环己烷在不同电池中的作用也有所差异。
首先,1,3-二(异氰酸根合甲基)环己烷在干电池中有一定的应用。
干电池是一种常见的电源设备,由电池罐、电解液、电极等组成。
在干电池的负极,通常使用的是锌或锌合金来提供电子供电,同时需要一个离子将电子输送到电解液中。
1,3-二(异氰酸根合甲基)环己烷可以作为一种配位剂,能够和锌离子形成络合物,稳定离子浓度并提高电解液导电性。
其次,1,3-二(异氰酸根合甲基)环己烷还可以用于铅酸电池的制造。
铅酸电池是一种常见的蓄电池,采用铅负极和铅过氧化物正极,中间通过硫酸电解液进行反应。
电池的放电过程中,铅负极会腐蚀产生电子,电子通过外部电路输出。
而正极的一种重要成分就是1,3-二(异氰酸根合甲基)环己烷。
1,3-二(异氰酸根合甲基)环己烷在铅酸电池的正极中起到稳定活性物质的作用,能够增加电极的寿命和循环性能;同时,它还能提供充电过程中所需的氧气,并减少电极的极化现象。
此外,1,3-二(异氰酸根合甲基)环己烷还可以在锂离子电池中被应用。
锂离子电池是一种高能量密度和轻量级的电池,广泛应用于电动汽车、手机、笔记本电脑等领域。
锂离子电池是通过锂离子在正负极之间的迁移来实现电能储存和释放的。
1,3-二(异氰酸根合甲基)环己烷在锂离子电池的正极材料中扮演了至关重要的角色。
它可以与锂离子发生化学反应,形成锂盐络合物,从而提高电池的电导率和循环性能。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
生物能源工程专业英语

bio-oil生物油aging 211–212 老化acetic acid309 ,,,,醋酸acetogens 316–317,,,,产乙酸菌adiabatic reactors 150,,,,绝热反应器air-blown gasifiers 95–97 ,空气吹制气化炉air-to-fuel ratio (A/F) 87气料比alcoholysis 262,,醇解alkali and alkaline earth metals (AAEMs) 28 碱和碱土金属(AAEMs)anhydro-oligosaccharides 30,,无水低聚糖ash effects灰分反应demineralization 180去矿化作用environmental conditions 180 环境条件inorganic phosphorus 183无机磷liquid yield and liquid’s composition 179液体产率和液体成分moisture content 181,,含湿量non-metal S 182非金属organic yield 181有机产量phase separation 184相分离sulfur and phosphorus180硫和磷total acid number (TAN) 185总酸值Becke-three parameter-Lee-Yang-Parr(B3LYP) functional 30bio-based chemicals 11生物基化学品bioenergy 87生物能源biofuels 10–11生物燃料biogas/landfill 53沼气/垃圾填埋场biomass combustion生物质燃烧baseload power generation 50基本载荷发电combustion properties燃烧性能composition of 59–62....的组成部分density and particle size 65密度和粒径heating value 63–64热值moisture content62水分含量,含湿量combustion stoichiometry燃烧化学计算air/fuel ratio 66气料比equilibrium 68平衡flame temperature 66–67火焰温度rates of reaction 68–71反应速率simplified global reaction 65–66combustors, types ofalternative combustion and powergeneration concepts 57–58燃烧器,供选择性的燃烧种类和发电概念co-firing 56–57共开火power and heat generation, large-scalesystems for 54–56发电和发热,大规模系统small-scale systems 53–54小规模系统electricity generation 51发电firewood gathering 50木柴收集fuel types可燃物类型gaseous fuel 52–53气态燃料solids 51–52固体fundamentals of 59基本原理magnetohydrodynamic energyconversion51磁流体能量转换plant photosynthesis and respiration 50植物光合和呼吸作用pollutant emissions and environmentalimpacts污染物排放和环境影响dioxin-like compounds 74–76二恶英类化合物greenhouse gas 温室气体emissions 77 排放heavy metals 76重金属incomplete combustion 74不完全燃烧oxides of nitrogen and sulfur 72–74氮和硫的氧化物(氮氧化物?)particulate matter 74颗粒物radioactive species 76–77放射性物质renewable and zero-carbon resources 51可再生和零碳资源biomass energy and carbon capture andsequestration (BECCS) 340生物能源与碳捕获和储存Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power, Second Edition. Edited by Robert C. Brown.© 2019 John Wiley & Sons Ltd. Published 2019 by John Wiley & Sons Ltd.biomass integrated gasification combined cycles (BIGCCs) 56生物质综合气化联合循环biomass particles 10生物质颗粒bio-methanol 11 生物甲醇aqueous phase 218水相biomass feedstock 221–222生物质进料器catalyst 221催化剂catalyst deactivation 223催化剂失活chemistry 221化学of lignocellulosic biomass 223 ....木质纤维生物质lower pyrolysis temperature and/or improve selectivity 218低温热解温度和提高选择性oil phase 218油相process configuration 222工艺配置process parameters 222–223工艺参数in situ and ex situ configuration 220 原位和非原位构型catalytic hydrotreating催化加氢处理condensation and polymerization reactions 214缩聚反应deep hydrotreating 217–220深度加氢处理hydrogen consumption 215 氢耗量nitrogen physisorption analysis 216 氮物理吸附分析Ru metal catalyst 215铷金属催化剂stabilization reactions 216平衡反应sulfur poisoning 216 硫中毒characteristics and quality特性和质量biomass composition 210生物质组成bio-oil viscosity 209生物油黏度boiler combustion applications 208 锅炉燃烧应用hemicellulose degradation 208 半纤维素降解inorganic content and composition209–210无机含量和组成water content of biomass 210 生物质含水量chemicals 232–235 化学物质composition of operating temperature effects 210工作温度效应的组成210。
LORD Corporation 产品说明书 - CHEMLOK 8560S-1

USA SAFETY DATA SHEET1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATIONProduct name: CHEMLOK 8560S-1Product Use/Class: Aqueous AdhesiveLORD Corporation111 LORD DriveCary, NC 27511-7923 USATelephone: 814 868-3180Non-Transportation Emergency: 814 763-2345 Chemtrec 24 Hr Transportation Emergency No.800 424-9300 (Outside Continental U.S. 703 527-3887)EFFECTIVE DATE: 12/20/20212. HAZARDS IDENTIFICATIONGHS CLASSIFICATION:Serious eye damage/eye irritation Category 2B Skin sensitization Category 1A Carcinogenicity Category 2Specific target organ systemic toxicity (single exposure) Category 2 blood system Specific target organ systemic toxicity (repeated exposure) Category 2 blood system Hazardous to the aquatic environment - acute hazard Category 2 Hazardous to the aquatic environment - chronic hazard Category 2GHS LABEL ELEMENTS:Symbol(s)Signal WordW ARNINGHazard StatementsCauses eye irritation.May cause an allergic skin reaction. Suspected of causing cancer.May cause damage to organs.(blood system)May cause damage to organs through prolonged or repeated exposure.(blood system) Toxic to aquatic life.Toxic to aquatic life with long lasting effects.Precautionary Statements PreventionObtain special instructions before use.Do not handle until all safety precautions have been read and understood. Wear protective gloves.Use personal protective equipment as required. Do not breathe dust/fume/gas/mist/vapors/spray. Wash thoroughly after handling.Do not eat, drink or smoke when using this product.Contaminated work clothing should not be allowed out of the workplace. Avoid release to the environment.Product: CHEMLOK 8560S-1, Effective Date: 12/20/2021ResponseGet medical advice/attention if you feel unwell.Call a POISON CENTER or doctor/physician if exposed or you feel unwell.Specific treatment (see supplemental first aid instructions on this label).IF ON SKIN: Wash with plenty of soap and water.If skin irritation or rash occurs: Get medical advice/attention.IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.Continue rinsing.If eye irritation persists: Get medical advice/attention.Wash contaminated clothing before reuse.Collect spillage.StorageStore locked up.Disposal:Dispose of contents/container in accordance with waste/disposal laws and regulations of your country or particular locality.Other Hazards:This product contains component(s) which have the following warnings; however based on the GHS classification criteria of your country or locale, the product mixture may be outside the respective category(s).Acute: May cause mild skin irritation. In elevated-temperature applications, product may release vapors that may produce cyanosis in the absence of sufficient ventilation or adequate respiratory protection. 4,4'-Diphenylmethane bis-maleimide is harmful by inhalation. Avoid breathing sanding dust from this product. May be harmful ifswallowed. Ingestion is not an expected route of entry in industrial or commercial uses.Chronic: Prolonged or repeated contact may result in dermatitis. The nitrogen substituted aromatic in this product gave positive results for mutagenicity in an Ames Assay study while two other mutagenicity studies proved negative.IARC has designated carbon black as Group 2B - inadequate evidence for carcinogenicity in humans, but sufficient evidence in experimental animals. In 2006 IARC reaffirmed its 1995 finding that there is "inadequate evidence"from human health studies to assess whether carbon black causes cancer in humans. Further, epidemiologicalevidence from well-conducted investigations has shown no causative link between carbon black exposure and the risk of malignant or non-malignant respiratory disease in humans. IARC has identified the proprietary curative in this product as an "animal suspected" carcinogen, Group 3, which downgrades a previous NCI report of it as an "animal positive" carcinogen.3. COMPOSITION/INFORMATION ON INGREDIENTSChemical Name CAS Number RangeNitrogen substituted aromatic PROPRIETARY15 - 20%Zinc compound PROPRIETARY 5 - 10%4,4'-Diphenylmethane bis-maleimide13676-54-5 1 - 5%Carbon black1333-86-4 1 - 5%Nonylphenol ethoxylate compound PROPRIETARY0.9 - 1%Curative PROPRIETARY0.1 - 0.9%Any "PROPRIETARY" component(s) in the above table is considered trade secret, thus the specific chemical and its exact concentration is being withheld.4. FIRST AID MEASURESFIRST AID - EYE CONTACT: Flush eyes immediately with large amount of water for at least 15 minutes holding eyelids open while flushing. Get prompt medical attention.FIRST AID - SKIN CONTACT: Flush contaminated skin with large amounts of water while removing contaminated clothing. Wash affected skin areas with soap and water. Get medical attention if symptoms occur.FIRST AID - INHALATION: Move person to fresh air. Restore and support continued breathing. If breathing is difficult, give oxygen. Get immediate medical attention.Product: CHEMLOK 8560S-1, Effective Date: 12/20/2021FIRST AID - INGESTION: If swallowed, do not induce vomiting. Call a physician or poison control center immediately for further instructions. Never give anything by mouth if victim is rapidly losing consciousness, unconscious or convulsing.5. FIRE-FIGHTING MEASURESSUITABLE EXTINGUISHING MEDIA: Carbon Dioxide, Dry Chemical, Foam, Water FogUNSUITABLE EXTINGUISHING MEDIA: Not determined for this product.SPECIFIC HAZARDS POSSIBLY ARISING FROM THE CHEMICAL: Keep containers tightly closed. Closed containers may rupture when exposed to extreme heat. Use water spray to keep fire exposed containers cool. WARNING: Due to the combustible nature of the dried film of this product and the potential for smoldering or fire, the accumulation and buildup of the dried film on spray booth walls and floors, spindles, fixtures and other surfaces should be avoided, and any buildup should be removed. Keep the dried film accumulations away from sparks, friction, impact, high heat (>235 F/>112 C) or other sources of ignition. These conditions could cause the dried film to ignite very readily and quickly, and the resulting smoldering or fire may be difficult to extinguish. During removal of accumulation/buildup of this product, take precautions to avoid heat, friction and impact during the cleaning process. Use paint stripper, brass brush, or plastic scraper for cleaning. In the event of smoldering or a fire involving the dried product, Cold Fire®** fire suppressing agent is preferred as the extinguishing medium. If Cold Fire® is not available, use water spray as the extinguishing medium. Take efforts to ensure that these agents reach the base of the smoldering or fire. Parker-LORD Corporation will not be responsible for personal injuries, property damage or any other damages arising from the accumulation (buildup, cleaning/removal or any related smoldering or fire) resulting from the use of this product. Refer to the Chemlok® Safe Handling Guide for additional information. **NOTE: Parker-LORD Corporation has determined Cold Fire® fire suppressing agent to be effective in extinguishing fires involving dried Chemlok® adhesives. Parker-LORD does not recommend any particular equipment or system for use in delivering or applying Cold Fire® products. Customer is responsible for determining that Cold Fire® products and any delivery equipment or system is appropriate and effective for customer's specific needs. During a fire, irritating and/or toxic gases and particulate may be generated by thermal decomposition or combustion. SPECIAL PROTECTIVE EQUIPMENT AND PRECAUTIONS FOR FIRE-FIGHTERS: Wear full firefighting protective clothing, including self-contained breathing apparatus (SCBA). If water is used, fog nozzles are preferable.6. ACCIDENTAL RELEASE MEASURESPERSONAL PRECAUTIONS, PROTECTIVE EQUIPMENT AND EMERGENCY PROCEDURES: Avoid breathing vapors. Avoid contact. Use appropriate respiratory protection for large spills or spills in confined area. See Section 5 for cautionary information on the dried residue of this product.ENVIRONMENTAL PRECAUTIONS: Do not contaminate bodies of water, waterways, or ditches, with chemical or used container.METHODS AND MATERIALS FOR CONTAINMENT AND CLEANUP: Notify appropriate authorities if necessary. Contain and remove with inert absorbent material. Avoid contact. Keep non-essential personnel away from spill area. Before attempting cleanup, refer to hazard caution information in other sections of this Safety Data Sheet.7. HANDLING AND STORAGEHANDLING: Keep closure tight and container upright to prevent leakage. Avoid skin and eye contact. Wash thoroughly after handling. Do not handle until all safety precautions have been read and understood. Empty containers should not be re-used. Use with adequate ventilation. See Section 5 for cautionary information on handling of the dried residue of this product. Avoid breathing sanding dust from this product.STORAGE: Store only in well-ventilated areas. Keep from freezing. Keep container closed when not in use. INCOMPATIBILITY: Strong acids, bases, and strong oxidizers.8. EXPOSURE CONTROLS/PERSONAL PROTECTIONCOMPONENT EXPOSURE LIMIT Chemical Name ACGIH TLV-TWA ACGIH TLV-STELOSHA PEL-TWAOSHA PEL-CEILINGSkinProduct: CHEMLOK 8560S-1, Effective Date: 12/20/2021Nitrogen substituted aromatic N.E.N.E.N.E. N.E. N.A. Zinc compound2 mg/m3 10 mg/m3 5 mg/m3 N.E. N.A. 4,4'-Diphenylmethane bis-maleimide N.E. N.E. N.E. N.E. N.A. Carbon black3 mg/m3 N.E. 3.5 mg/m3 N.E. N.A. Nonylphenol ethoxylate compound N.E. N.E. N.E. N.E. N.A. CurativeN.E.N.E.N.E.N.E.N.A.N.A. - Not Applicable, N.E. - Not Established, S - Skin DesignationEngineering controls: Sufficient ventilation in pattern and volume should be provided in order to maintain air contaminant levels below recommended exposure limits.PERSONAL PROTECTION MEASURES/EQUIPMENT:RESPIRATORY PROTECTION: Use a NIOSH approved chemical/mechanical filter respirator designed to remove a combination of particulates and organic vapor if occupational limits are exceeded. For emergencysituations, confined space use, or other conditions where exposure limits may be greatly exceeded, use an approved air-supplied respirator. For respirator use observe OSHA regulations (29CFR 1910.134) or use in accordance with applicable laws and regulations of your country or particular locality.SKIN PROTECTION: Use neoprene, nitrile, or rubber gloves to prevent skin contact.EYE PROTECTION: Use safety eyewear including safety glasses with side shields and chemical goggles where splashing may occur.OTHER PROTECTIVE EQUIPMENT: Remove and wash contaminated clothing before reuse.HYGIENIC PRACTICES: Wash hands before eating, smoking, or using toilet facility. Do not smoke in any chemical handling or storage area. Food or beverages should not be consumed anywhere this product is handled or stored. Wash thoroughly after handling.9. PHYSICAL AND CHEMICAL PROPERTIESTypical values, not to be used for specification purposes.ODOR:Odorless VAPOR PRESSURE:N.D.APPEARANCE: Green/BlackVAPOR DENSITY:Heavier than Air PHYSICAL STATE:Thixotropic liquid LOWER EXPLOSIVE LIMIT: Not Applicable FLASH POINT: ≥ 201 °F, 93 °C Setaflash Closed Cup UPPER EXPLOSIVE LIMIT:Not Applicable BOILING RANGE:100 °CEVAPORATION RATE: Slower than n-butyl-acetateAUTOIGNITION TEMPERATURE: N.D. DENSITY:1.2 g/cm3 (10.00 lb/gal) DECOMPOSITION TEMPERATURE:N.D. VISCOSITY, DYNAMIC: ≥50 mPa.s @ 25 °C ODOR THRESHOLD: N.D.VISCOSITY, KINEMATIC: ≥42 mm2/s @ 25 °C SOLUBILITY IN H2O:Water Dispersible VOLATILE BY WEIGHT: 56.93 % pH:6.0 VOLATILE BY VOLUME:66.72 % FREEZE POINT:N.D. VOC CALCULATED: 0.02 lb/gal , 3 g/l COEFFICIENT OF WATER/OILDISTRIBUTION:N.D.LEGEND: N.A. - Not Applicable, N.E. - Not Established, N.D. - Not Determined10. STABILITY AND REACTIVITYHAZARDOUS POLYMERIZATION: Hazardous polymerization will not occur under normal conditions.STABILITY: Product is stable under normal storage conditions.Product: CHEMLOK 8560S-1, Effective Date: 12/20/2021CONDITIONS TO AVOID: High temperatures.; For dried product issues, refer to Section 5 of the (M)SDS.; DO NOT ALLOW THIS MATERIAL TO DRY OUT. As a solid, p-benzoquinone dioxime is flammable, and it may explode if exposed to shock, friction or heat.INCOMPATIBILITY: Strong acids, bases, and strong oxidizers.HAZARDOUS DECOMPOSITION PRODUCTS: Decomposition due to high temperatures or a fire causes the formation of irritating and/or toxic gases, organic vapors or fumes., May contain CO, CO2, oxides of nitrogen, oxides of sulfur, halogenated by-products, Carbon dioxide, carbon monoxide, chlorine, hydrogen chloride, Phosgene, Metal oxides11. TOXICOLOGICAL INFORMATIONEXPOSURE PATH: Refer to section 2 of this SDS.SYMPTOMS: Refer to section 2 of this SDS.TOXICITY MEASURES:Chemical NameLD50/LC50 Nitrogen substituted aromatic Oral LD50: rat 1,100 mg/kgZinc compoundOral LD50: Rat > 5,000 mg/kg Dermal LD50: Rat > 2,000 mg/kgGHS LC50 (vapour): Acute toxicity point estimate 55 mg/l Inhalation LC50: Rat > 5,700 mg/m3 /4 h 4,4'-Diphenylmethane bis-maleimide Oral LD50: Rat > 5 g/kgDermal LD50: rat > 5,400 mg/kgGHS LC50 (dust and mist): rat 0.52 mg/l Inhalation ATE: 11 mg/l Carbon blackOral LD50: Rat > 15,400 mg/kg Dermal LD50: Rabbit > 3 g/kgGHS LC50 (vapour): Acute toxicity point estimate 55 mg/l : Nonylphenol ethoxylate compound N.D.CurativeOral LD50: Rat 464 mg/kgGHS LC50 (vapour): Acute toxicity point estimate 55 mg/l Inhalation LC50: Rat > 5 mg/l /4 hGerm cell mutagenicity: No classification proposedCarcinogenicity: Category 2 - Suspected of causing cancer. Components contributing to classification: Curative.Reproductive toxicity: No classification proposed12. ECOLOGICAL INFORMATIONECOTOXICITY:Chemical NameEcotoxicityNitrogen substituted aromatic N.D. Zinc compoundN.D.4,4'-Diphenylmethane bis-maleimide Fish: Oncorhynchus mykiss (rainbow trout) > 0.145 mg/l96 hCarbon blackN.D. Nonylphenol ethoxylate compound N.D.CurativeFish: Danio rerio (zebra fish) 24 mg/l96 h Static Invertebrates: Daphnia magna (Water flea) 3.5 mg/l48 h StaticPERSISTENCE AND DEGRADABILITY: Not determined for this product.BIOACCUMULATIVE: Not determined for this product.MOBILITY IN SOIL: Not determined for this product.OTHER ADVERSE EFFECTS: Not determined for this product.13. DISPOSAL CONSIDERATIONSDISPOSAL METHOD: Disposal should be done in accordance with Federal (40CFR Part 261), state and local environmental control regulations. If waste is determined to be hazardous, use licensed hazardous waste transporter and disposal facility. Waste streams, including the dried adhesive residue, resulting from the use of this product should be tested for RCRA characteristics, including ignitability, to determine any applicable waste classifications.14. TRANSPORT INFORMATIONUS DOT RoadProper Shipping Name: Environmentally hazardous substances, liquid, n.o.s.Hazard Class: 9SECONDARY HAZARD: NoneUN/NA Number: 3082Packing Group: IIIEmergency Response Guide Number: 171For US DOT non-bulk road shipments this material may be classified as NOT REGULATED. For the mostaccurate shipping information, refer to your transportation/compliance department regarding changes inpackage size, mode of shipment or other regulatory descriptors.IATA CargoPROPER SHIPPING NAME: Environmentally hazardous substance, liquid, n.o.s.Hazard Class: 9HAZARD CLASS: NoneUN NUMBER: 3082PACKING GROUP: IIIEMS: 9LIMDGPROPER SHIPPING NAME: Environmentally hazardous substance, liquid, n.o.s.Hazard Class: 9HAZARD CLASS: NoneUN NUMBER: 3082PACKING GROUP: IIIEMS: F-AThe listed transportation classification applies to non-bulk shipments. It does not address regulatory variations due to changes in package size, mode of shipment or other regulatory descriptors. For the most accurate shipping information, refer to your transportation/compliance department.15. REGULATORY INFORMATIONU.S. FEDERAL REGULATIONS: AS FOLLOWS:SARA SECTION 313This product contains the following substances subject to the reporting requirements of Section 313 of Title III of the Superfund Amendment and Reauthorization Act of 1986 and 40 CFR part 372.:Chemical Name CAS Number Weight % Less ThanZinc compound PROPRIETARY10.0%TOXIC SUBSTANCES CONTROL ACT:INVENTORY STATUSThe chemical substances in this product are on the active TSCA Section 8 Inventory or exempt.EXPORT NOTIFICATIONThis product contains the following chemical substances subject to the reporting requirements of TSCA 12(B) if exported from the United States:None16. OTHER INFORMATIONUnder HazCom 2012 it is optional to continue using the HMIS rating system. It is important to ensure employees have been trained to recognize the different numeric ratings associated with the HazCom 2012 and HMIS schemes.HMIS RATINGS - HEALTH: 2* FLAMMABILITY: 1 PHYSICAL HAZARD: 0* - Indicates a chronic hazard; see Section 2Revision: New GHS SDS FormatEffective Date: 12/20/2021DISCLAIMERThe information contained herein is, to the best of our knowledge and belief, accurate. However, since the conditions of handling and use are beyond our control, we make no guarantee of results, and assume no liability for damages incurred by use of this material. It is the responsibility of the user to comply with all applicable federal, state and local laws and regulations.。
HPLC法测定愈创木酚磺酸钾原料药中有关物质

HPLC 法测定愈创木酚磺酸钾原料药中有关物质摘要:目的HPLC法测定愈创木酚磺酸钾原料药中有关物质。
方法以十八烷基硅烷键合硅胶为填充剂(4.6 mm×250 mm,5 µm),以冰醋酸水溶液(每1000ml水中加入5ml冰醋酸,用三乙胺调节pH至3.5)为流动相A,以甲醇为流动相B,梯度洗脱(0~5 min,95%A;5~25 min,95% A→75% A;25~40 min,75% A→60% A;40~40.1 min,60% A→95% A),检测波长为279nm;柱温为30℃;流速为1.20ml/min;进样量20 µL。
结果:各杂质平均回收率在90%~110%之间,定量限浓度小于忽略限度(0.05%)。
结论该方法灵敏度高、准确度好,可用于愈创木酚磺酸钾原料药中有关物质的测定。
关键词: HPLC 愈创木酚磺酸钾原料药有关物质愈创木酚磺酸钾为刺激性祛痰药,通过刺激支气管黏膜腺体分泌,使痰液变稀容易咳出,常作为复方祛痰镇咳类口服液的主要成分之一[1]。
美国药典(USP)42版[2]收载的愈创木酚磺酸钾原料药为羟基甲氧基苯磺酸钾半水化合物(C7H7KO5S H2O),未对磺酸钾的取代位置作出规定,JP17版药典[3]中规定愈创木酚磺酸钾为4-羟基-3-甲氧基苯磺酸钾单体。
KP X药典中收载的也是4-羟基-3-甲氧基苯磺酸钾单体[4]。
本研究将美国药典收载的另一构型(3-羟基-4-甲氧基苯磺酸钾)作为已知杂质研究,同时还考察了愈创木酚(起始物料)、邻苯二酚(愈创木酚的起始物料)。
本研究采用普通C18柱,高有机相比例洗脱,延长色谱柱寿命,且方法准确,灵敏度较高。
1仪器与试药仪器Agilent 1260 InfinityII高效液相色谱仪(安捷伦科技有限公司);Sartorius型电子天平(赛多利斯有限公司),PHS-3C型pH计(上海越平科技仪器有限公司)。
试药甲醇(色谱纯;TEDIA);冰醋酸(分析纯;西陇科学股份有限公司);三乙胺(分析纯;国药集团化学试剂有限公司);水为纯化水。
BMS-303141_943962-47-8_DataSheet_MedChemExpress

Product Name:BMS-303141CAS No.:943962-47-8Cat No :HY 16107Product Data SheetCat. No.:HY-16107MWt:424.30Formula:C19H15Cl2NO4S Purity :>98%S l bilit Soluble to 10mM in DMSO andtoSolubility:Mechanisms:Biological Activity:Pathways:Others; Target:ATP citrate lyase Soluble to 10 mM in DMSO and to 50 mM in ethanolBMS-303141 is a potent ATP-citrate lyase (ACL) inhibitor with IC50 value of 0.13 uM (humanrecombinant ACL).IC50 value: 0.13 uM [1]Target: ATP citrate lyase in vitro: In HepG2 cells, BMS-303141 showed inhibition of total lipid syntheses with an IC50 of 8 μM.A cell based Alamar Blue cytotoxicity assay was used in parallel to differentiate the effect on the inhibition of lipid synthesis versus potential cytotoxicity. Under identical incubation conditions, BMS-303141 showed no cytotoxicity up to 50 μM, indicating the observed inhibition of lipid synthesis was t lt f d i d d t t i it [1]References:[1]. Li JJ, et al. 2-hydroxy-N-arylbenzenesulfonamides as ATP-citrate lyase inhibitors. Bioorg Med Ch L tt 2007J 117(11)320811 not a result of compound-induced cytotoxicity [1].in vivo: In mice, BMS-303141 showed an oral bioavailability of 55% but a relatively short half-life of2.1 h.20 We therefore decided to dose BMS-303141 admixed in the food to assure greater duration of exposure in subsequent chronic efficacy studies.There were a...Chem Lett. 2007 Jun 1;17(11):3208-11.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
拓扑替康结构式 -回复

拓扑替康结构式-回复拓扑替康(Tofacitinib)是一种属于JAK抑制剂的药物,用于治疗关节炎、银屑病和溃疡性结肠炎等自身免疫性疾病。
该药物的结构式如下所示:[拓扑替康结构式]拓扑替康属于一种小分子化合物,其化学名称为“3-{(3R,4R)-4-[(4-{(1E)-2-(5-氯-2-氧代苯基)乙-1-烯基}-1-苯基环己基)氨基]-3-甲基环己基}丙酸甲酯”。
它的化学式为C16H20N6O,分子量为312.37g/mol。
拓扑替康为无色结晶状固体,可溶于有机溶剂或水。
作用机制:拓扑替康通过抑制Janus激酶(JAK)的活性来发挥其治疗作用。
JAK是一类细胞内酪氨酸激酶,对细胞信号转导和调控起着重要作用。
通过抑制JAK的活性,拓扑替康可以干扰多个细胞因子途径的信号传递,从而调控免疫系统,减轻相关疾病的症状。
药物应用:拓扑替康已被美国食品药品监督管理局(FDA)批准用于治疗成人类风湿性关节炎、银屑病性关节炎、中度至重度溃疡性结肠炎以及活动性类风湿性关节炎。
它通常在其他治疗方法无效或无法耐受的情况下使用。
治疗效果:在临床试验中,拓扑替康已被证明能有效减少关节炎相关的关节疼痛、关节肿胀和关节运动受限等症状。
在银屑病和溃疡性结肠炎的治疗中,使用拓扑替康也能减少病情的恶化和复发。
注意事项:1. 在使用拓扑替康之前,患者应告知医生有关其过敏史、其他药物的使用情况以及存在的其他疾病,以便医生进行全面评估。
2. 拓扑替康可能会增加感染的风险。
在使用期间,患者应密切注意任何感染的症状,并及时向医生报告。
3. 服用拓扑替康可能导致一些不良反应,如头痛、腹泻、恶心、呕吐等。
如有不适,应立即告知医生。
总结:拓扑替康是一种抗关节炎和免疫性疾病药物,通过抑制JAK的活性,调控免疫系统,从而减轻相关疾病的症状。
尽管其可以有效改善患者的症状,但患者在使用该药物时需要密切关注可能产生的不良反应,并向医生报告任何异样症状。
通过科学的用药指导和临床监测,拓扑替康可以为患者提供更好的治疗效果。
锂离子电池电解液添加剂物性数据

锂离子电池电解液添加剂物性数据化学名称环己基苯(CHB) 亚硫酸亚乙酯(ES、DTO)硫酸亚乙酯(DTD) 亚硫酸丙烯酯(PS)碳酸亚乙烯酯(VC)别名苯基环己烷,苯基环乙烷亚硫酸乙二醇酯、乙二醇亚硫酸酯、亚硫酸乙烯酯硫酸乙烯酯、硫酸乙二醇酯、乙二醇硫酸酯、亚乙基硫酸酯Trimethylene Sulfite1,3,2-Dioxathiane 2-oxide1,3-Dioxo-2-one英文名称Cyclohexyl benzeneEthylene sulfite Ethylene Sulfate Propylenesulfite Vinylene carbonate CAS号827-52-1 3741-38-6 1072-53-5 4176-55-0 872-36-6分子式C12H16C2H4O3SC2H4O4SC3H6O3S C3H2O3分子结构分子量160.26 108.12124 122.186.05熔点/沸点/闪点7~8℃/239~240℃/98.0?/172~174℃/79℃97~99℃/?/? ?/76/?19~22℃/165℃/73℃密度(g/mL at 25℃)0.95 1.426 1.3225 1.355g/mL 粘度(40℃)折光率 1.5230±0.0050 1.445~1.447 1.420~1.422 外观无色油状液体无色液体白色结晶或白色结晶性粉末无色液体无色透明液体或白色固体特性易溶于醇、丙酮、苯、四氯化碳、二甲苯、不溶于水和甘油DTO的含量≥98%,氯乙醇含量≤1000ppm水溶性11.5 G/100 ML用途用于锂二次电池电解液的添加剂,具有防过充性能。
应用于锂电池高温溶剂。
作锂离子电池电解质的有机溶剂,又可作为锂离子电池电解液的添加剂,锂离子电池电解质添加了DTO 后将呈现出优异的儲存稳定性,可以提高电解液的低温性能,同时可以防止PC 分子嵌入石墨电极。
Incucyte

Product Information Presentation, Storage and StabilityThe Incucyte® Fabfluor-pH Antibody Labeling Reagents for antibody internalization are supplied as lyophilized solids in sufficient quantity to label 50 μg of test antibody, when used at the suggested molar ratio (1:3 of test antibody to labeling Fab). The lyophilized solid can be stored at 2-8° C for one year. Once re-hydrated, any unused reagent should be aliquoted and stored at -80° C for up to one year. Avoid repeated freeze-thaw cycles.Incucyte® Fabfluor-pH Antibody Labeling ReagentsFor Antibody Internalization AssaysAntibody Labeling Reagent Rehydrated: -80° C *Excitation and Emission maxima were determined at a pH of 4.5.Fabfluor_quick_guideBackgroundIncucyte ® Fabfluor-pH Antibody Labeling Reagents are designed for quick, easy labeling of Fc-containing test antibodies with a Fab fragment-conjugated pH-sensitive fluorophore. The pH-sensitive dye based system exploits the acidic environment of the lysosomes to quantify in-ternalization of the labeled antibody. As Fabfluor labeled antibodies reside in the neutral extracellular solution (pH 7.4), they interact with cell surface specific antigens and are internalized. Once in the lysosomes, they enter an acidic environment (pH 4.5–5.5) and a substantial in-crease in fluorescence is observed. In the absence of ex-pression of the specific antigen, no internalization occurs and the fluorescence intensity of the labeled antibodies remains low. With the Incucyte ® integrated analysis soft-ware, background fluorescence is minimized. These reagents have been validated for use with a number of different antibodies in a range of cell types. The Incucyte ® Live-Cell Analysis System enables real-time, kinetic eval -uation of antibody internalization.Recommended UseWe recommend that the Incucyte ® Fabfluor-pH Antibody Labeling Reagents are prepared at a stock concentration of 0.5 mg/mL by the addition of 100 μL of sterile water and triturated (centrifuge if solution not clear). The reagent may then be diluted directly into the labeling mixture with test antibody. Do NOT sonicate the solution.Additional InformationThe Fab antibody was purified from antisera by a combination of papain digestion and immunoaffinity chromatography using antigens coupled to agarose beads. Fc fragments and whole IgG molecules have been removed.Human Red (Cat. No. 4722) or Human Orange (Cat. No. 4812)—Based on immunoelectrophoresis and/ or ELISA, the antibody reacts with the Fc portion of human IgG heavy chain but not the Fab portion of human IgG. No antibody was detected against human IgM, IgA or against non-immunoglobulin serum proteins. The anti-body may cross-react with other immunoglobulins from other species.Mouse IgG1 (Cat. No. 4723), IgG2a (Cat. No. 4750) or IgG2b (Cat. No. 4751)—Based on antigen-binding assay and/or ELISA, the antibody reacts with the Fc portion of mouse IgG, IgG2a or IgG2b, respectively, but not the Fab portion of mouse immunoglobulins. No antibody was detected against mouse IgM or against non–immunoglobulin serum proteins. The antibody may cross-react with other mouse IgG subclasses or with immunoglobulins from other species.Rat (Cat. No. 4737)—Based on immunoelectrophoresis and/or ELISA, the antibody reacts with the Fc portion of rat IgG heavy chain but not the Fab portion of rat IgG. No antibody was detected against rat IgM, IgA or against non-immunoglobulin serum proteins. The antibody may cross-react with other immunoglobulins from other species.A.B.C.D.R e d O b j e c t A r e a (x 105 μm 2 p e r w e l l )Time (hours)A U C x 106 (0–12 h )log [α–CD71] (g/mL)Example DataFigure 1: Concentration-dependent increase in antibody internalization of Incucyte ® Fabfluor labeled-α-CD71 in HT1080 cells. α-CD71 and mouse IgG1 isotype control were labeled with Incucyte ® Mouse IgG1 Fabfluor-pH Red Antibody Labeling Reagent. HT1080 cells were treated with either Fabfluor-α-CD71 or Fabfluor-IgG1 (4 μg/mL); HD phase and red fluorescence images were captured every 30 minutes over 12 hours using a 10X magnification. (A) Images of cells treated with Fabfluor-α-CD71 display red fluorescence in the cytoplasm (images shown at 6 h). (B) Cells treated with labeled isotype control display no cellular fluorescence. (C) Time-course of Fabfluor-α-CD71 internalization with increasing concentrations of Fabfluor-α-CD71 (progressively darker symbols). Internalization has been quantified as the red object area for each time-point. (D) Concentration response curve to Fabfluor-α-CD71. Area under the curve (AUC) values have been determined from the time-course shown in panel C (0-12 hours) and are presented as the mean ± SEM, n=3 wells.CD71-FabfluorIgG-FabfluorProtocols and ProceduresMaterialsIncucyte® Fabfluor-pH Antibody Labeling ReagentTest antibody of interest containing human, mouse, or rat IgG Fc region (at known concentration)Target cells of interestTarget cell growth mediaSterile distilled water96-well flat bottom microplate (e.g. Corning Cat. No. 3595) for imaging96-well round black round bottom ULA plate (e.g. Corning Cat. No. 45913799) or amber microtube (e.g. Cole Parmer Cat. No. MCT-150-X, autoclaved) for conjugation step0.01% Poly-L-Ornithine (PLO) solution (e.g. Sigma Cat. No. P4957), optional for non-adherent cells Recommended control antibodiesIt is strongly recommended that a positive and negative control is run alongside test antibodies and cell lines. For example, CD71, which is a mouse anti-human antibody, is recommended as a positive control for the mouse Fab.Anti-CD71, clone MEM-189, IgG1 e.g. Sigma Cat. No. SAB4700520-100UGAnti-CD71, clone CYG4, IgG2a e.g. BioLegend Cat. No. 334102Isotype controls, depending on isotype being studied—Mouse IgG1, e.g. BioLegend Cat. No. 400124, Mouse IgG2a e.g. BioLegend Cat. No. 401501Preparation of Incucyte® Antibody Internalization Assay 1. Seed target cells of interest1.1 Harvest cells of interest and determine cell concentra-tion (e.g. trypan blue + hemocytometer).1.2 Prepare cell seeding stock in target cell growth mediawith a cell density to achieve 40–50% confluence be-fore the addition of labeled antibodies. The suggested starting range is 5,000–30,000 cells/well, although the seeding density will need to be optimized for each cell type.Note: For non-adherent cell types, a well coating may be required to maintain even cell distribution in the well. For a 96-well flat bottom plate, we recommend coating with 50 μL of either 0.01% Poly-L-Or-nithine (PLO) solution or 5 μg/mL fibronectin diluted in 0.1% BSA.Coat plates for 1 hour at ambient temperature, remove solution from wells and then allow the plates to dry for 30-60 minutes prior to cell addition.1.3 Using a multi-channel pipette, seed cells (50 µL perwell) into a 96-well flat bottom microplate. Lightly tapplate side to ensure even liquid distribution in well. Toensure uniform distribution of cells in each well, allowthe covered plate sit on a level surface undisturbed at room temperature in the tissue culture hood for 30minutes. After cells are settled, place the plate insidethe Incucyte® Live-Cell Analysis System to monitor cell confluence.Note: Depending on cell type, plates can be used in assay once cells have adhered to plastic and achieved normal cell morphology e.g.2-3 hours for HT1080 or 1-2 hours for non-adherent cell types. Some cell types may require overnight incubation.2. Label Test Antibody2.1 Rehydrate the Incucyte® Fabfluor-pH Antibody Label-ing Reagent with 100 µL sterile water to result in a final concentration of 0.5 mg/mL. Triturate to mix (centrifuge if solution is not clear).Note: The reagent is light sensitive and should be protected fromlight. Rehydrated reagent can be aliquoted into amber or foilwrapped tubes and stored at -80° C for up to 1 year (avoid freezing and thawing).2.2 Mix test antibody with rehydrated Incucyte® Fabfluor–pH Antibody Labeling Reagent and target cell growth media in a black round bottom microplate or ambertube to protect from light (50 µL/well).a. Add test antibody and Incucyte® Fabfluor–pH Anti-body Labeling Reagent at 2X the final concentration.We suggest optimizing the assay by starting with afinal concentration of 4 µg/mL of test antibody or theFabfluor-pH Antibody Labeling Reagent (i.e. 2Xworking concentration = 8 µg/mL).Note: A 1:3 molar ratio of test antibody to Incucyte® Fabfluor-pHAntibody Labeling Reagent is recommended. The labeling re-agent is a third of the size of a standard antibody (50 and 150KDa, respectively). Therefore, labeling equal quantities will pro-duce a 1:3 molar ratio of test antibody to labeling Fab.b. Make sufficient volume of 2X labeling solution for50 µL/well for each sample. Triturate to mix.c. Incubate at 37° C for 15 minutes protected from light.Note: If performing a range of concentrations of test antibody,e.g. concentration response-curve, it is recommended to createthe dilution series post the conjugation step to ensure consistentmolar ratio. We strongly recommend the use of both a negativeand positive control antibody in the same plate.3. Add labeled antibody to cells3.1 Remove cell plate from incubator.3.2 Using a multi-channel pipette, add 50 µL of 2X labeledantibody and control solutions to designated wells.Remove any bubbles and immediately place plate in the Incucyte® Live-Cell Analysis System and start scanning.Note: To reduce the risk of condensation formation on the lid priorto first image acquisition, maintain all reagents at 37° C prior toplate addition.4. Acquire images and analyze4.1 In the Incucyte® Software, schedule to image every15-30 minutes, depending on the speed of the specific antibody internalization.a Scan on schedule, standard. If the Incucyte® Cell-by-Cell Analysis Software Module (Cat. No. 9600-0031)is available, adherent cell-by-cell or non-adherentcell-by-cell scan types can be selected.b Channel selection: select “phase” and “red” or“phase” and "orange” (depending on reagent used).c Objective: 10X or 20X depending on cell types used,generally 10X is recommended for adherent cells,and 20X for non-adherent or smaller cells.NOTE: The optional Incucyte® Cell-by-Cell Analysis SoftwareModule enables the classification of cells into sub-populationsbased on properties including fluorescence intensity, size andshape. For further details on this analysis module and its appli-cation, please see: /cell-by-cell.4.2 To generate the metrics, user must create an AnalysisDefinition suited to the cell type, assay conditions andmagnification selected.4.3 Select images from a well containing a positiveinternalization signal and an isotype control well(negative signal) at a time point where internalizationis visible.4.4 In the Analysis Definition:Basic Analyzer:a. Set up the mask for the phase confluence measurewith fluorescence channel turned off.b. Once the phase mask is determined, turn the fluores-cence channel on: Exclude background fluorescencefrom the mask using the background subtractionfeature. The feature “Top-Hat” will subtract localbackground from brightly fluorescent objects withina given radius; this is a useful tool for analyzing ob-jects which change in fluorescence intensity overtime.i The radius chosen should reflect the size of thefluorescent object but contain enough backgroundto reliably estimate background fluorescence inthe image; 20-30 μm is often a useful startingpoint.ii The threshold chosen will ensure that objectsbelow a fluorescence threshold will not bemasked.iii Choose a threshold in which red or orange objectsare masked in the positive response image but lownumbers in the isotype control, negative responsewell. For a very sensitive measurement, for example,if interested in early responses, we suggest athreshold of 0.2.NOTE: The Adaptive feature can be used for analysis but maynot be as sensitive and may miss early responses. If interestedin rate of response, Top-Hat may be preferable.Cell-by-Cell (if available):a. Create a Cell-by-Cell mask following the softwaremanual.b. There is no need to separate phase and fluorescencemasks. The default setting of Top-Hat No Mask forthe fluorescence channel will enable backgroundsubtraction without generation of a mask. Ensurethat the Top-Hat radius is set to a value higher thanthe radius of the larger clusters to avoid excess back-ground subtraction.c. The threshold of fluorescence can be determined inCell-by-Cell Classification.Specifications subject to change without notice.© 2020. All rights reserved. Incucyte, Essen BioScience, and all names of Essen BioScience prod -ucts are registered trademarks and the property of Essen BioScience unless otherwise specified. Essen BioScience is a Sartorius Company. Publication No.: 8000-0728-A00Version 1 | 2020 | 04Sales and Service ContactsFor further contacts, visit Essen BioScience, A Sartorius Company /incucyte Sartorius Lab Instruments GmbH & Co. KGOtto-Brenner-Strasse 20 37079 Goettingen, Germany Phone +49 551 308 0North AmericaEssen BioScience Inc. 300 West Morgan Road Ann Arbor, Michigan, 48108USATelephone +1 734 769 1600E-Mail:***************************EuropeEssen BioScience Ltd.Units 2 & 3 The Quadrant Newark CloseRoyston Hertfordshire SG8 5HLUnited KingdomTelephone +44 (0) 1763 227400E-Mail:***************************APACEssen BioScience K.K.4th floor Daiwa Shinagawa North Bldg.1-8-11 Kita-Shinagawa Shinagawa-ku, Tokyo 140-0001 JapanTelephone: +81 3 6478 5202E-Mail:*************************5. Analysis GuidelinesAs the labeled antibody is internalized into the acidic environment of the lysosome, the area of fluorescence intensity inside the cells increases.This can be reported in two ways:Ways to Report Basic AnalyzerCell-by-Cell Analysis* To correct for cell proliferation, it is advisable to normalize the fluorescence area to the total cell area using User Defined Metrics.For Research Use Only. Not For Therapeutic or Diagnostic Use.LicensesFor non-commercial research use only. Not for therapeutic or in vivo applications. Other license needs contact Essen BioS cience.Fabfluor-pH Red Antibody Labeling Reagent: This product or portions thereof is manufactured under license from Carnegie Mellon University and U.S. patent numbers 7615646 and 8044203 and related patents. This product is licensed for sale only for research. It is not licensed for any other use. There is no implied license hereunder for any commercial use.Fabfluor-pH Orange Antibody Labeling Reagent: This product or portions thereof is manufactured under a license from Tokyo University and is covered by issued patents EP2098529B1, JP5636080B2, US8258171, and US9784732 and related patent applications. This product and related products are trademarks of Goryo Chemical. Any application of above mentioned technology for commercial purpose requires a separate li -cense from: Goryo Chemical, EAREE Bldg., SF Kita 8 Nishi 18-35-100, Chuo-Ku, Sapporo, 060-0008 Japan.SupportA complete suite of cell health applications is available to fit your experimental needs. Find more information at /incucyte Foradditionalproductortechnicalinformation,************************************************************/incucyte。
3-氧代环丁烷-1,1-二羧酸,二异丙酯 hplc

3-氧代环丁烷-1,1-二羧酸,二异丙酯hplc3-氧代环丁烷-1,1-二羧酸,二异丙酯(3-Oxocyclobutanecarboxylic Acid Diisopropyl Ester)是一种有机化合物。
HPLC(高效液相色谱,High-Performance Liquid Chromatography)是一种分离和分析化学物质的技术。
结合起来,3-氧代环丁烷-1,1-二羧酸,二异丙酯HPLC 可以指涉使用HPLC技术对3-氧代环丁烷-1,1-二羧酸,二异丙酯进行分析和检测。
HPLC是一种广泛应用的分离技术,它基于化学物质在液相中的相互作用和分配行为进行分离。
该技术涉及一组主要组件:流动相(溶剂)、色谱柱、样品进样系统、检测器和数据处理设备。
流动相:流动相是溶解化合物样品并在色谱柱中传输化合物的溶剂系统。
它通常是由溶剂混合物组成的,可根据目标分析的性质和要求选择不同的溶剂系统。
色谱柱:色谱柱是分离化合物的关键部件,根据分析目标的不同选择合适的柱子。
在HPLC中常用的柱子类型包括反相柱、离子交换柱、大小分子筛柱等。
反相柱是最常用的类型,其中常用的填充剂包括碳链和亲水基团。
样品进样系统:样品进样系统将要分析的化合物溶液引入色谱柱。
它可以是自动进样器或手动进样器,确保准确而重复的样品进样。
检测器:检测器用于检测样品在流动相中的组分。
常见的HPLC检测器包括紫外-可见检测器(UV-Vis)、荧光检测器、折光率检测器等。
选用适当的检测器可以根据化合物的属性和要求获得准确的定量和定性数据。
数据处理设备:HPLC仪器通常与计算机或数据处理设备连接,用于记录和分析检测器产生的信号。
数据处理设备可以对色谱图进行处理、数据整合和分析,并生成定量和定性的结果。
通过调节流动相的组成、柱温、流速和检测器参数等条件,对3-氧代环丁烷-1,1-二羧酸,二异丙酯的HPLC分析可以实现其分离和定量检测,同时可以通过与参考标准品比对来确定目标化合物的浓度。
UPLC-MS

UPLC-MS/MS法同时快速测定保健食品中10种降压类非法添加化学药王晓峰,许琨琨,卢文斌,吴芳海,蔡振世*,林晓明(泉州市食品药品检验所,福建泉州 362000)摘 要:目的:建立同时测定保健食品中10种降压类非法添加化学药的UPLC-MS/MS快速检测方法。
方法:液相采用ACQUITY UPLC®BEH C18(2.1 mm×100 mm,1.7 μm)柱,以超纯水(含0.1%甲酸)-乙腈(0.1%甲酸)为流动相,进行梯度洗脱,进样量2 μL,流速0.3 mL·min-1,柱温30 ℃。
质谱采用ESI,正、负离子多反应监测模式,进行定性分析和定量分析。
结果:盐酸普萘洛尔、酒石酸美托洛尔、艾司洛尔、比索洛尔、坎地沙坦、厄贝沙坦、替米沙坦、氯沙坦、缬沙坦和吲达帕胺的分离度良好,线性范围内相关性均较好,R2均大于0.996 3;平均回收率为93%~118%;RSD为1.4%~4.9%(n=6);方法检出限为0.10~5.00 μg·kg-1;方法定量限为0.30~15.00 μg·kg-1;单次分析仅需12 min。
结论:该方法操作便捷、检测时间短、灵敏度高、结果准确,适用于保健食品中10种降压类非法添加化学药的快速筛查及定量检测。
关键词:UPLC-MS/MS;保健食品;降压类药物Simultaneous and Rapid Determination of 10 illegally Added Antihypertensive Chemicals in Health Food by UPLC-MS/MS WANG Xiaofeng, XU Kunkun, LU Wenbin, WU Fanghai, CAI Zhenshi*, LIN Xiaoming(Quanzhou Institute for Food And Drug Control, Quanzhou 362000, China) Abstract: Objective: To establish a UPLC-MS/MS rapid detection method for simultaneously determining 10 illegally added chemical antihypertensive drugs in health foods. Method: ACQUITY UPLC®BEH C18( 2.1 mm×100 mm, 1.7 μm) column was used in the liquid phase with ultrapure water (containing 0.1% formic acid)-acetonitrile (0.1% formic acid) as the mobile phase for gradient elution, the injection volume was 2 μL, flow rate was 0.3 mL·m in-1, and column temperature was 30 ℃. The mass spectrometry was performed by ESI, positive/negative ion multiple reaction monitoring mode for qualitative and quantitative analysis. Result: The separation degree of propranolol, metoprolol, amlodipine, bisoprolol, candesartan, irbesartan, telmisartan, losartan, valsartan, and indapamide was good, and the linear range correlations were good, all R2 values were greater than 0.996 3. The average recovery rate was 93%~118%. RSD was 1.4%~4.9% (n=6). The LOD was between 0.10~5.00 μg·kg-1, and the LOQ was between 0.30~15.00 μg·kg-1. A single analysis only requires 12 min. Conclusion: The method is simple to operate, has short detection time, high sensitivity, and accurate results, and it is suitable for the rapid screening and quantitative detection of 10 illegally added chemical antihypertensive drugs in health foods.Keywords: UPLC-MS/MS; health food; antihypertensive drugs随着近年来我国经济的迅速发展,人们的生活质量得到了很大提高,同时老年人口不断增加,导致出现越来越多的高血压患者,市场上也出现了越来越多的辅助降压类保健食品。
1-氰基环丁基羧酸乙酯液相检测方法

1-氰基环丁基羧酸乙酯液相检测方法
氰基环丁基羧酸乙酯(也称为苯烷腈)是一种有机化合物,可通过液相检测方法进行分析和测量。
以下是一种可能的液相检测方法:
1. 毛细管气相色谱(Capillary Gas Chromatography):使用毛细管气相色谱仪对氰基环丁基羧酸乙酯进行分析。
样品首先被注入气相色谱仪的进样口,并通过毛细管柱进行分离。
然后,样品中的化合物会被分离并通过检测器进行检测和定量。
毛细管气相色谱可以提供对样品中氰基环丁基羧酸乙酯含量的精确测量。
2. 高效液相色谱(High Performance Liquid Chromatography,HPLC):使用高效液相色谱仪对氰基环丁基羧酸乙酯进行分析。
样品首先通过进样器被引入到色谱柱中,然后通过流动相的梯度洗脱,不同成分分离并检测。
HPLC方法可以提供对氰基环丁基羧酸乙酯样品中含量的准确测量。
3. 质谱联用技术(Mass Spectrometry Coupled Techniques):将质谱技术与气相色谱或液相色谱技术相结合,可以更加准确地鉴定和定量氰基环丁基羧酸乙酯。
质谱联用技术可以提供化合物的分子结构和分子量等信息,以及对样品中各种成分的定量分析。
以上液相检测方法仅提供了一些可能的选择,具体的方法选择应根据实际需求和实验条件进行决定。
同时,在进行液相检测
时,必须注意遵循安全操作,并根据相关法规和操作规程进行操作。
