Guideline_Normothermia
MRSA_guideline
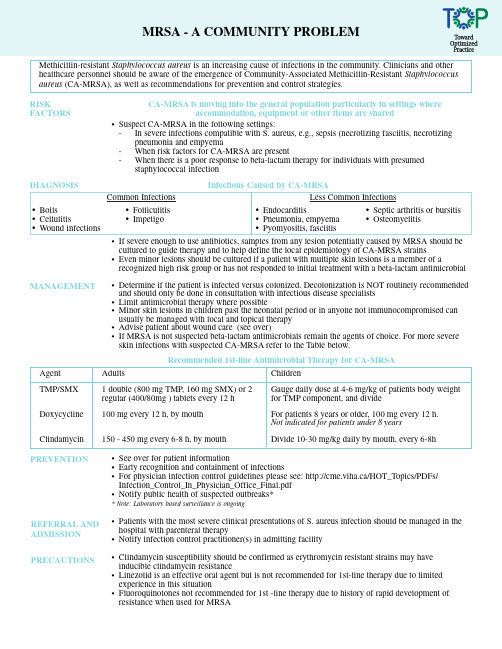
Methicillin-resistant Staphylococcus aureus is an increasing cause of infections in the community. Clinicians and other healthcare personnel should be aware of the emergence of Community-Associated Methicillin-Resistant Staphylococcus aureus (CA-MRSA), as well as recommendations for prevention and control strategies.RISK FACTORS CA-MRSA is moving into the general population particularly in settings where accommodation, equipment or other items are sharedDIAGNOSIS Infections Caused by CA-MRSA•Endocarditis•Pneumonia, empyema•Pyomyositis, fasciitis•If severe enough to use antibiotics, samples from any lesion potentially caused by MRSA should becultured to guide therapy and to help define the local epidemiology of CA-MRSA strains•Even minor lesions should be cultured if a patient with multiple skin lesions is a member of arecognized high risk group or has not responded to initial treatment with a beta-lactam antimicrobialMRSA - A COMMUNITY PROBLEMMANAGEMENT•Determine if the patient is infected versus colonized. Decolonization is NOT routinely recommended and should only be done in consultation with infectious disease specialists•Limit antimicrobial therapy where possible•Minor skin lesions in children past the neonatal period or in anyone not immunocompromised canusually be managed with local and topical therapy•Advise patient about wound care (see over)•If MRSA is not suspected beta-lactam antimicrobials remain the agents of choice. For more severeskin infections with suspected CA-MRSA refer to the Table below.Agent TMP/SMXDoxycyclineClindamycin Adults1 double (800 mg TMP, 160 mg SMX) or 2regular (400/80mg ) tablets every 12 h100 mg every 12 h, by mouth150 - 450 mg every 6-8 h, by mouthChildrenGauge daily dose at 4-6 mg/kg of patients body weightfor TMP component, and divideFor patients 8 years or older, 100 mg every 12 h.Not indicated for patients under 8 yearsDivide 10-30 mg/kg daily by mouth, every 6-8hRecommended 1st-line Antimicrobial Therapy for CA-MRSA•Boils •Cellulitis •Wound infections •Suspect CA-MRSA in the following settings:-In severe infections compatible with S. aureus, e.g., sepsis (necrotizing fasciitis, necrotizing pneumonia and empyema-When risk factors for CA-MRSA are present-When there is a poor response to beta-lactam therapy for individuals with presumed staphylococcal infection•See over for patient information•Early recognition and containment of infections•For physician infection control guidelines please see: http://cme.viha.ca/HOT_Topics/PDFs/ Infection_Control_In_Physician_Office_Final.pdf•Notify public health of suspected outbreaks** Note: Laboratory based surveillance is ongoing•Patients with the most severe clinical presentations of S. aureus infection should be managed in the hospital with parenteral therapy•Notify infection control practitioner(s) in admitting facility•Clindamycin susceptibility should be confirmed as erythromycin resistant strains may have inducible clindamycin resistance•Linezolid is an effective oral agent but is not recommended for 1st-line therapy due to limited experience in this situation•Fluoroquinolones not recommended for 1st -line therapy due to history of rapid development of resistance when used for MRSAPREVENTION REFERRAL AND ADMISSION PRECAUTIONS•Folliculitis•ImpetigoCommon Infections•Septic arthritis or bursitis•OsteomyelitisLess Common Infections•Clean hands regularly with soap and water or alcohol-based hand gel (if hands are not visibly soiled). Antibacterial soaps are NOT recommended •Always clean hands immediately after touching infected skin or any item that has come in direct contact with a draining wound •Keep wounds that are draining covered with clean, dry, bandages •Maintain good general hygiene with regular showering •Do not share items that may become contaminated with wound drainage, such as towels,clothing, bedding, bar soap, razors, and athletic equipment that touches the skin •Wash clothing that has come in contact with other peoples skin •If you are not able to keep your wound covered with a clean, dry bandage at all times, do not participate in activities where you have skin to skin contact with other persons (such as athletic activities) until your wound is healed•Clean equipment and other environmental surfaces with which multiple individuals have bare skin contact with an over the counter detergent/disinfectant that specifies Staphylococcus aureus on the product label and is suitable for the type of surface being cleaned KEY PREVENTION MESSAGES FOR PATIENTS WITH SKIN AND SOFT TISSUE INFECTIONShttp://www.cmaj.ca/cgi/reprint/175/2/145http://www.cmaj.ca/cgi/reprint/175/2/149http://www.cmaj.ca/cgi/reprint/175/2/161For more information:This information sheet for health workers and patients was developed in cooperation with Alberta Health and Wellness and Infectious Disease Specialists.September 2006.。
美国CDC-SSI预防指南2017

Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection,2017Sandra I.Berríos-Torres,MD;Craig A.Umscheid,MD,MSCE;Dale W.Bratzler,DO,MPH;Brian Leas,MA,MS;Erin C.Stone,MA;Rachel R.Kelz,MD,MSCE;Caroline E.Reinke,MD,MSHP;Sherry Morgan,RN,MLS,PhD;Joseph S.Solomkin,MD;John E.Mazuski,MD,PhD;E.Patchen Dellinger,MD;Kamal M.F.Itani,MD;Elie F.Berbari,MD;John Segreti,MD;Javad Parvizi,MD;Joan Blanchard,MSS,BSN,RN,CNOR,CIC;George Allen,PhD,CIC,CNOR;Jan A.J.W.Kluytmans,MD;Rodney Donlan,PhD;William P.Schecter,MD;for the Healthcare Infection Control Practices Advisory CommitteeInvited CommentarySupplemental contentAuthor Affiliations:Authoraffiliations are listed at the end of this article.Group Information:The Healthcare Infection Control Practices Advisory Committee members are listed at the end of this article.Corresponding Author:Erin C.Stone,MA,Division of Healthcare Quality Promotion,Centers for Disease Control and Prevention,1660Clifton Rd NE,Mail Stop A07Atlanta,GA 30329(ecstone@ ).Clinical Review &EducationJAMA Surgery |Special CommunicationE1S urgical site infections(SSIs)are infections of the incision or organ or space that occur after surgery.1Surgical patients ini-tially seen with more complex comorbidities2and the emer-gence of antimicrobial-resistant pathogens increase the cost and challenge of treating SSIs.3-5The prevention of SSI is increasingly im-portant as the number of surgical procedures performed in the United States continues to rise.6,7Public reporting of process,out-come,and other quality improvement measures is now required,8,9 and reimbursements10for treating SSIs are being reduced or de-nied.It has been estimated that approximately half of SSIs are preventable by application of evidence-based strategies.11MethodsThis guideline focuses on select areas for the prevention of SSI deemed important to undergo evidence assessment for the ad-vancement of the field.These areas of focus were informed by feed-back received from clinical experts and input from the Healthcare Infection Control Practices Advisory Committee(HICPAC),a fed-eral advisory committee to the Centers for Disease Control and Prevention(CDC).This guideline was a systematic review of the literature.No institutional review board approval or participant in-formed consent was necessary.This guideline’s recommendations were developed based on a targeted systematic review of the best available evidence on SSI prevention conducted in MEDLINE,EMBASE,CINAHL,and the Cochrane Library from1998through April2014.To provide explicit links between the evidence and recommendations,a modified Grad-ing of Recommendations,Assessment,Development,and Evalua-tion(GRADE)approach was used for evaluating the quality of evi-dence and determining the strength of recommendations.12-15The methods and structure of this guideline were adopted in2009by CDC and HICPAC.16,17The present guideline does not reevaluate sev-eral strong recommendations offered by CDC’s1999Guideline for Prevention of Surgical Site Infection18that are now considered to be accepted practice for the prevention of SSI.These recommen-dations are found in eAppendix1of the Supplement.A detailed description of the Guideline Questions,Scope and Purpose,and Methods,as well as the Evidence Summaries supporting the evi-dence-based recommendations,can also be found in eAppendix1 of the Supplement.The detailed literature search strategies,GRADE Tables,and Evidence Tables supporting each section can be found in eAppen-dix2of the Supplement.Results of the entire study selection pro-cess are shown in the Figure.Of5759titles and abstracts screened,896underwent full-text review by2independent reviewers.Full-text articles were excluded if:1)SSI was notFigure.Results of the Study Selection ProcessCDC indicates Centers for Disease Control and Prevention;GRADE,Grading of Recommendations,Assessment,Development,and Evaluation;RCTs,randomized controlled trials;SRs,expand;and SSI,surgical site infection.Clinical Review&Education Special Communication CDC Guideline for the Prevention of Surgical Site Infection,2017E2JAMA Surgery Published online May3,©2017American Medical Association.All rights reserved.reported as an outcome;2)all patients included had“dirty”surgi-cal procedures(except for Q2addressing the use of aqueous iodo-phor irrigation);3)the study only included oral or dental health procedures;4)the surgical procedures did not include primary closure of the incision in the operating room(eg,orthopedic pin sites,thoracotomies,or percutaneous endoscopic gastrostomy [PEG]procedures,or wounds healing by secondary intention);or 5)the study evaluated wound protectors used postincision. Evidence-based recommendations in this guideline were cross-checked with those from other guidelines identified in a system-atic search.CDC completed a draft of the guideline and shared it with the expert panel for in-depth review and then with HICPAC and mem-bers of the public at committee meetings(June2010to July2015). CDC posted notice in the Federal Register for the following2peri-ods of public comment:from January29to February28,2014,and from April8to May8,ments were aggregated and re-viewedwiththewritinggroupandatanotherHICPACmeeting.Based on the comments received,the literature search was updated,and new data were incorporated into a revised draft.Further input was provided by HICPAC during a public teleconference in May2015.Fi-nalHICPACinputwasprovidedviaavotebymajorityruleinJuly2015. After final HICPAC input,CDC updated the draft document and obtained final CDC clearance and coauthor approval.Recommendation CategoriesRecommendations were categorized using the following standard system that reflects the level of supporting evidence or regulations:•Category IA:A strong recommendation supported by high to mod-erate–quality evidence suggesting net clinical benefits or harms.•Category IB:A strong recommendation supported by low-quality evidence suggesting net clinical benefits or harms or an accepted practice(eg,aseptic technique)supported by low to very low–quality evidence.•Category IC:A strong recommendation required by state or fed-eral regulation.•Category II:A weak recommendation supported by any quality evidence suggesting a trade-off between clinical benefits and harms.•No recommendation/unresolved issue:An issue for which there is low to very low–quality evidence with uncertain trade-offs be-tween the benefits and harms or no published evidence on out-comes deemed critical to weighing the risks and benefits of a given intervention.RecommendationsCore SectionIn2006,approximately80million surgical procedures were per-formed in the United States at inpatient hospitals(46million)7and ambulatory hospital–affiliated or freestanding(32million)settings.6 Between2006and2009,SSIs complicated approximately1.9%of surgical procedures in the United States.19However,the number of SSIs is likely to be underestimated given that approximately50% of SSIs become evident after discharge.20Estimated mean attrib-utablecostsofSSIsrangefrom$10443in2005USdollarsto$25546 in2002US dollars per infection.3-5,11Costs can exceed$90000per infection when the SSI involves a prosthetic joint implant21,22or an antimicrobial-resistant organism.23The Core Section of this guide-line(eAppendix1of the Supplement)includes recommendations for the prevention of SSI that are generalizable across surgical proce-dures,with some exceptions as mentioned below.Parenteral Antimicrobial Prophylaxis1A.1.Administer preoperative antimicrobial agents only when indi-cated based on published clinical practice guidelines and timed such that a bactericidal concentration of the agents is established in the serum and tissues when the incision is made.(Category IB–strong recommendation;accepted practice.)1A.2.No further refinement of timing can be made for preoperative antimicrobial agents based on clinical outcomes.(No recommenda-tion/unresolved issue.)1B.Administer the appropriate parenteral prophylactic antimicro-bial agents before skin incision in all cesarean section procedures. (Category IA–strong recommendation;high-quality evidence.)1C.The literature search did not identify randomized controlled trials that evaluated the benefits and harms of weight-adjusted paren-teral antimicrobial prophylaxis dosing and its effect on the risk of SSI.Other organizations have made recommendations based on ob-servational and pharmacokinetic data,and a summary of these rec-ommendations can be found in the Other Guidelines section of the narrative summary for this question(eAppendix1of the Supple-ment).(No recommendation/unresolved issue.)1D.The search did not identify sufficient randomized controlled trial evidence to evaluate the benefits and harms of intraoperative re-dosing of parenteral prophylactic antimicrobial agents for the pre-vention of SSI.Other organizations have made recommendations based on observational and pharmacokinetic data,and a summary of these recommendations can be found in the Other Guidelines sec-tion of the narrative summary for this question(eAppendix1of the Supplement).(No recommendation/unresolved issue.)1E.In clean and clean-contaminated procedures,do not administer additional prophylactic antimicrobial agent doses after the surgical incision is closed in the operating room,even in the presence of a drain.(Category IA–strong recommendation;high-quality evidence.)Nonparenteral Antimicrobial Prophylaxis2A.1.Randomized controlled trial evidence suggested uncertain trade-offs between the benefits and harms regarding intraopera-tive antimicrobial irrigation(eg,intra-abdominal,deep,or subcuta-neous tissues)for the prevention of SSI.Other organizations have made recommendations based on the existing evidence,and a sum-mary of these recommendations can be found in the Other Guide-lines section of the narrative summary for this question(eAppen-dix1of the Supplement).(No recommendation/unresolved issue.)2A.2.The search did not identify randomized controlled trials that evaluated soaking prosthetic devices in antimicrobial solutions be-fore implantation for the prevention of SSI.(No recommendation/ unresolved issue.)CDC Guideline for the Prevention of Surgical Site Infection,2017Special Communication Clinical Review&Education JAMA Surgery Published online May3,2017E3©2017American Medical Association.All rights reserved.2B.1.Do not apply antimicrobial agents(ie,ointments,solutions,or powders)to the surgical incision for the prevention of SSI.(Cat-egory IB–strong recommendation;low-quality evidence.)2B.2.Application of autologous platelet-rich plasma is not neces-sary for the prevention of SSI.(Category II–weak recommendation; moderate-quality evidence suggesting a trade-off between clinical benefits and harms.)2C.Consider the use of triclosan-coated sutures for the prevention of SSI.(Category II–weak recommendation;moderate-quality evi-dence suggesting a trade-off between clinical benefits and harms.)2D.Randomizedcontrolledtrialevidencesuggesteduncertaintrade-offs between the benefits and harms regarding antimicrobial dress-ings applied to surgical incisions after primary closure in the oper-ating room for the prevention of SSI.(No recommendation/ unresolved issue.)Glycemic Control3A.1.Implement perioperative glycemic control and use blood glu-cose target levels less than200mg/dL in patients with and with-out diabetes.(Category IA–strong recommendation;high to mod-erate–quality evidence.)3A.2.The search did not identify randomized controlled trials that evaluated lower(<200mg/dL)or narrower blood glucose target lev-els than recommended in this guideline nor the optimal timing,du-ration,or delivery method of perioperative glycemic control for the prevention of SSI.Other organizations have made recommenda-tions based on observational evidence,and a summary of these rec-ommendations can be found in the Other Guidelines section of the narrative summary for this question(eAppendix1of the Supple-ment).(No recommendation/unresolved issue.)3B.The search did not identify randomized controlled trials that evaluated the optimal hemoglobin A1C target levels for the preven-tion of SSI in patients with and without diabetes.(No recommen-dation/unresolved issue.)Normothermia4.Maintain perioperative normothermia.(Category IA–strong rec-ommendation;high to moderate–quality evidence.)5.The search did not identify randomized controlled trials that evaluated strategies to achieve and maintain normothermia,the lower limit of normothermia,or the optimal timing and duration of normothermia for the prevention of SSI.Other organizations have made recommendations based on observational evidence,and a summary of these recommendations can be found in the Other Guidelines section of the narrative summary for this question (eAppendix1of the Supplement).(No recommendation/ unresolved issue.)Oxygenation6A.Randomizedcontrolledtrialevidencesuggesteduncertaintrade-offs between the benefits and harms regarding the administration of increased fraction of inspired oxygen(F IO2)via endotracheal in-tubation during only the intraoperative period in patients with nor-mal pulmonary function undergoing general anesthesia for the pre-vention of SSI.(No recommendation/unresolved issue.)6B.For patients with normal pulmonary function undergoing gen-eral anesthesia with endotracheal intubation,administer increased F IO2during surgery and after extubation in the immediate postop-erative period.To optimize tissue oxygen delivery,maintain peri-operative normothermia and adequate volume replacement.(Cat-egory IA–strong recommendation;moderate-quality evidence.)6C.Randomizedcontrolledtrialevidencesuggesteduncertaintrade-offs between the benefits and harms regarding the administrationof increased F IO2via face mask during the perioperative period in patients with normal pulmonary function undergoing general an-esthesia without endotracheal intubation or neuraxial anesthesia(ie, spinal,epidural,or local nerve blocks)for the prevention of SSI.(No recommendation/unresolved issue.)6D.Randomized controlled trial evidence suggested uncertain trade-offs between the benefits and harms regarding the admin-istration of increased F IO2via face mask or nasal cannula during only the postoperative period in patients with normal pulmonary function for the prevention of SSI.(No recommendation/ unresolved issue.)7.The search did not identify randomized controlled trials that evalu-ated the optimal target level,duration,and delivery method of F IO2 for the prevention of SSI.Other organizations have made recom-mendations based on observational studies,and a summary of these recommendations can be found in the Other Guidelines section of the narrative summary for this question(eAppendix1of the Supple-ment).(No recommendation/unresolved issue.)Antiseptic Prophylaxis8A.1.Advise patients to shower or bathe(full body)with soap(an-timicrobial or nonantimicrobial)or an antiseptic agent on at least the night before the operative day.(Category IB–strong recommenda-tion;accepted practice.)8A.2.Randomized controlled trial evidence suggested uncertain trade-offs between the benefits and harms regarding the optimal timing of the preoperative shower or bath,the total number of soap or antiseptic agent applications,or the use of chlorhexidine glu-conate washcloths for the prevention of SSI.(No recommendation/ unresolved issue.)8B.Perform intraoperative skin preparation with an alcohol-based antiseptic agent unless contraindicated.(Category IA–strong rec-ommendation;high-quality evidence.)8C.Application of a microbial sealant immediately after intraopera-tive skin preparation is not necessary for the prevention of SSI.(Cat-egory II–weak recommendation;low-quality evidence suggesting a trade-off between clinical benefits and harms.)8D.The use of plastic adhesive drapes with or without antimicro-bial properties is not necessary for the prevention of SSI.(Category II–weak recommendation;high to moderate–quality evidence sug-gesting a trade-off between clinical benefits and harms.)9A.Consider intraoperative irrigation of deep or subcutaneous tis-sues with aqueous iodophor solution for the prevention of SSI.In-traperitoneal lavage with aqueous iodophor solution in contami-nated or dirty abdominal procedures is not necessary.(Category II–weak recommendation;moderate-quality evidence suggesting a trade-off between clinical benefits and harms.)Clinical Review&Education Special Communication CDC Guideline for the Prevention of Surgical Site Infection,2017E4JAMA Surgery Published online May3,©2017American Medical Association.All rights reserved.9B.The search did not identify randomized controlled trials that evaluated soaking prosthetic devices in antiseptic solutions before implantation for the prevention of SSI.(No recommendation/ unresolved issue.)10.Randomized controlled trial evidence was insufficient to evalu-ate the trade-offs between the benefits and harms of repeat appli-cation of antiseptic agents to the patient’s skin immediately before closing the surgical incision for the prevention of SSI.(No recommendation/unresolved issue.)Prosthetic Joint Arthroplasty SectionPreventioneffortsshouldtargetallsurgicalproceduresbutespecially those in which the human and financial burden is greatest.In2011, primary total knee arthroplasty accounted for more than half of the 1.2million prosthetic joint arthroplasty procedures(primary and re-vision)performed in the United States,followed by total hip arthro-plastyandhiphemiarthroplasty.24Primaryshoulder,elbow,andankle arthroplasties are much less common.By2030,prosthetic joint ar-throplasties are projected to increase to3.8million procedures per year.25-27Infection is the most common indication for revision in total knee arthroplasty28and the third most common indication in total hip arthroplasty.28By2030,the infection risk for hip and knee arthroplasty is expected to increase from2.18%22to6.5% and6.8%,respectively.25In addition,owing to increasing risk and the number of individuals undergoing prosthetic joint arthro-plasty procedures,the total number of hip and knee prosthetic joint infections is projected to increase to221500cases per year by2030,at a cost of more than$1.62billion.22,25The Prosthetic Joint Arthroplasty section contains recommendations that are applicable to these procedures(eAppendix1of the Supplement).Blood Transfusion11A.Available evidence suggested uncertain trade-offs between the benefits and harms of blood transfusions on the risk of SSI in prosthetic joint arthroplasty.Other organizations have made rec-ommendations on this topic,and a reference to these recommen-dations can be found in the Other Guidelines section of the narra-tive summary for this question(eAppendix1of the Supplement). (No recommendation/unresolved issue.)11B.Do not withhold transfusion of necessary blood products from surgical patients as a means to prevent SSI.(Category IB–strong recommendation;accepted practice.)Systemic Immunosuppressive Therapy12and13.Available evidence suggested uncertain trade-offs between the benefits and harms of systemic corticosteroid or other immunosuppressive therapies on the risk of SSI in pros-thetic joint arthroplasty.Other organizations have made recom-mendations based on the existing evidence,and a summary of these recommendations can be found in the Other Guidelines section of the narrative summary for this question(eAppendix1of the Supplement).(No recommendation/unresolved issue.)14.For prosthetic joint arthroplasty patients receiving systemic corticosteroid or other immunosuppressive therapy,recommen-dation1E applies:in clean and clean-contaminated procedures, do not administer additional antimicrobial prophylaxis doses after the surgical incision is closed in the operating room,even in the presence of a drain.(Category IA–strong recommendation;high-quality evidence.)Intra-articular Corticosteroid Injection15and16.Available evidence suggested uncertain trade-offs between the benefits and harms of the use and timing of preop-erative intra-articular corticosteroid injection on the incidence of SSI in prosthetic joint arthroplasty.Other organizations have made recommendations based on observational studies,and a summary of these recommendations can be found in the Other Guidelines section of the narrative summary for this ques-tion(eAppendix1of the Supplement).(No recommendation/ unresolved issue.)Anticoagulation17.Available evidence suggested uncertain trade-offs between the benefits and harms of venous thromboembolism prophylaxis on the incidence of SSI in prosthetic joint arthroplasty.Other organizations have made recommendations based on the existing evidence,and these references can be found in the Other Guide-lines section of the narrative summary for this question(eAppen-dix1of the Supplement).(No recommendation/unresolved issue.)Orthopedic Surgical Space Suit18.Available evidence suggested uncertain trade-offs between the benefits and harms of orthopedic space suits or the health care personnel who should wear them for the prevention of SSI in prosthetic joint arthroplasty.(No recommendation/unresolved issue.)Postoperative Antimicrobial Prophylaxis DurationWith Drain Use19.In prosthetic joint arthroplasty,recommendation1E applies:in clean and clean-contaminated procedures,do not administer addi-tional antimicrobial prophylaxis doses after the surgical incision is closed in the operating room,even in the presence of a drain.(Cat-egory IA–strong recommendation;high-quality evidence.)Biofilm20A.Availableevidencesuggesteduncertaintrade-offsbetweenthe benefits and harms regarding cement modifications and the pre-vention of biofilm formation or SSI in prosthetic joint arthroplasty. (No recommendation/unresolved issue.)20B.The search did not identify studies evaluating prosthesis modi-fications for the prevention of biofilm formation or SSI in prosthetic joint arthroplasty.(No recommendation/unresolved issue.)20C.The search did not identify studies evaluating vaccines for the prevention of biofilm formation or SSI in prosthetic joint arthro-plasty.(No recommendation/unresolved issue.)CDC Guideline for the Prevention of Surgical Site Infection,2017Special Communication Clinical Review&Education JAMA Surgery Published online May3,2017E5©2017American Medical Association.All rights reserved.20D.The search did not identify studies evaluating biofilm control agents,such as biofilm dispersants,quorum sensing inhibitors,or novel antimicrobial agents,for the prevention of biofilm formation or SSI in prosthetic joint arthroplasty.(No recommendation/ unresolved issue.)ConclusionsSurgical site infections are persistent and preventable health care–associated infections.There is increasing demand for evidence-based interventions for the prevention of SSI.The last version of the CDC Guideline for Prevention of Surgical Site Infection18was pub-lished in1999.While the guideline was evidence informed,most rec-ommendations were based on expert opinion,in the era before evi-dence-based guideline methods.CDC updated that version of the guideline using GRADE as the evidence-based method that pro-videsthefoundationoftherecommendationsinthisguideline.These new and updated recommendations are not only useful for health care professionals but also can be used as a resource for profes-sional societies or organizations to develop more detailed imple-mentation guidance or to identify future research priorities.The pau-city of robust evidence across the entire guideline created challenges in formulating recommendations for the prevention of SSI.None-theless,the thoroughness and transparency achieved using a sys-tematic review and the GRADE approach to address clinical ques-tionsofinteresttostakeholdersarecriticaltothevalidityoftheclinical recommendations.The number of unresolved issues in this guideline reveals sub-stantial gaps that warrant future research.A select list of these un-resolved issues may be prioritized to formulate a research agenda toadvancethefield.Adequatelypowered,well-designedstudiesthat assess the effect of specific interventions on the incidence of SSI are needed to address these evidence gaps.Subsequent revisions to this guideline will be guided by new research and technological advance-ments for preventing SSIs.ARTICLE INFORMATIONAccepted for Publication:March1,2017. Published Online:May3,2017.doi:10.1001/jamasurg.2017.0904Correction:This article was corrected on June21, 2017,to fix corrupted numbering and update abstract and methods.Author Affiliations:Division of Healthcare Quality Promotion,Centers for Disease Control and Prevention,Atlanta,Georgia(Berríos-Torres,Stone, Donlan);Center for Evidence-Based Practice, University of Pennsylvania Health System, Philadelphia(Umscheid,Leas,Kelz,Morgan); College of Public Health,The University of Oklahoma Health Sciences Center,Oklahoma City (Bratzler);Carolinas Healthcare System,Charlotte, North Carolina(Reinke);Department of Surgery, University of Cincinnati College of Medicine, Cincinnati,Ohio(Solomkin);Section of Acute and Critical Care Surgery,Washington University School of Medicine in St Louis,Saint Louis,Missouri (Mazuski);American College of Surgeons Representative,University of Washington Medical Center,Seattle(Dellinger);Surgical Infection Society Representative,Veterans Affairs Boston Healthcare System,Boston University and Harvard Medical School,Boston,Massachusetts(Itani); Musculoskeletal Infection Society Representative, Mayo Clinic College of Medicine,Rochester, Minnesota(Berbari);American Academy of Orthopaedic Surgeons Representative,Rush University Medical Center,Chicago,Illinois (Segreti);American Academy of Orthopaedic Surgeons Representative,Rothman Institute, Philadelphia,Pennsylvania(Parvizi);Quality Department,Littleton Adventist Hospital,Denver, Colorado(Blanchard);Association of Perioperative Registered Nurses Representative,New York Methodist Hospital,Brooklyn(Allen);Laboratory for Microbiology and Infection Control,Amphia Hospital,Breda,the Netherlands(Kluytmans); Julius Center for Health Sciences and Primary Care, University Medical Center,Utrecht,the Netherlands (Kluytmans);Department of Surgery,San Francisco General Hospital,University of California,San Francisco(Schecter).Author Contributions:Dr Umscheid and Ms Stonehad full access to all of the data in the study andtake responsibility for the integrity of the data andthe accuracy of the data analysis.Study concept and design:Berríos-Torres,Umscheid,Bratzler,Leas,Stone,Kelz,Morgan,Mazuski,Dellinger,Itani,Berbari,Parvizi,Blanchard,Kluytmans.Acquisition,analysis,or interpretation of data:Berríos-Torres,Umscheid,Bratzler,Leas,Stone,Kelz,Reinke,Morgan,Solomkin,Mazuski,Dellinger,Segreti,Allen,Kluytmans,Donlan,Schecter.Drafting of the manuscript:Berríos-Torres,Umscheid,Leas,Stone,Kelz,Reinke,Morgan,Itani,Berbari,Segreti,Blanchard.Critical revision of the manuscript for importantintellectual content:Berríos-Torres,Umscheid,Bratzler,Leas,Stone,Kelz,Reinke,Solomkin,Mazuski,Dellinger,Itani,Berbari,Segreti,Parvizi,Allen,Kluytmans,Donlan,Schecter.Statistical analysis:Berríos-Torres,Umscheid,Leas.Administrative,technical,or material support:Berríos-Torres,Bratzler,Leas,Stone,Morgan,Itani,Parvizi.Study supervision:Berríos-Torres,Umscheid,Bratzler,Stone,Itani,Berbari,Parvizi.Conflict of Interest Disclosures:Drs Umscheid,Kelz,and Morgan and Mr Leas reported receivingfunding from the Centers for Disease Control andPrevention to support the guideline developmentprocess.Dr Bratzler reported being a consultant forthe Oklahoma Foundation for Medical Quality andfor Telligen(a nonprofit Medicaid external qualityreview organization)and reported that hisinstitution received payment for his lectures,including service on speakers’bureaus fromPremier and Janssen Pharmaceuticals.Dr Reinkereported receiving lecture fees from Covidien andreported being a paid consultant for Teleflex.DrSolomkin reported receiving grants for clinicalresearch from,receiving consulting fees regardingclinical trial data,serving on an advisory board for,or lecturing for honoraria from the following:Merck,Actavis,AstraZeneca,PPD,Tetraphase,Johnson&Johnson,and3M.Dr Mazuski reportedbeing a paid consultant for Bayer,CubistPharmaceuticals,Forest Laboratories,MedImmune,Merck/Merck Sharp and Dohme,and Pfizer;reported receiving lecture fees from ForestLaboratories,Merck/Merck Sharp and Dohme,andPfizer;and reported that his institution receivedfunding for his consultancy to AstraZeneca andgrants from AstraZeneca,Bayer,Merck/MSD,andTetraphase.Dr Dellinger reported receiving grantsfor clinical research from,serving on an advisoryboard for,or lecturing for honoraria from thefollowing:Merck,Baxter,Ortho-McNeil,Targanta,Schering-Plough,WebEx,Astellas,Care Fusion,Durata,Pfizer,Applied Medical,Rib-X,Affinium,and3M.Dr Itani reported that his institutionreceived grants from Merck,Cubist,Dr Reddy’s,Sanofi Pasteur,and Trius for research trials;reported clinical advisory board membership atForrest Pharmaceuticals;and reported payment fordevelopment of educational presentations for MedDirect and Avid Education.Dr Berbari reported thathis institution received a grant from Pfizer for aresearch trial for which he serves as the principalinvestigator.Dr Segreti reported receiving lecturefees from Pfizer,Merck,and Forest Laboratoriesand reported owning stocks in or having stockoptions from Pfizer.Dr Parvizi reported being a paidconsultant for Zimmer,Smith and Nephew,ConvaTec,TissueGene,CeramTech,and Medtronic;reported receiving royalties from Elsevier,WoltersKluwer,Slack Incorporated,Data Trace Publishing,and Jaypee Brothers Medical Publishers;andreported having stock options with Hip InnovationTechnologies,CD Diagnostics,and PRN.Dr Allenreported receiving lecture fees from Ethicon androyalties from Wolters Kluwer as an author forInfection Control:A Practical Guide for HealthcareFacilities.Dr Kluytmans reported being a paidconsultant for3M,Johnson&Johnson,and Pfizer.No other disclosures were reported.Funding/Support:The Centers for Disease Controland Prevention(CDC)supported the developmentof the guideline.The activities of Drs Umscheid,Kelz,and Morgan and Mr Leas were supportedthrough a short-term detail under contract at CDC(10IPA1004117,10IPA1004133,11IPA1106551,11IPA1106555,and11IPA1106565).Role of the Funder/Sponsor:Centers for DiseaseControl and Prevention conducted the full guidelinedevelopment process,directing the design andconduct of the systematic reviews;collection,Clinical Review&Education Special Communication CDC Guideline for the Prevention of Surgical Site Infection,2017E6JAMA Surgery Published online May3,©2017American Medical Association.All rights reserved.。
大面积脑梗死诊疗指南

.
• Large hemispheric infarction (LHI), also known as malignant middle cerebral infarction, is a devastating disease associated with significant disability and mortality.
原创力文档是网络服务平台方若您的权利被侵害侵权客服qq
Evidence-Based Guidelines for the Management of Large Hemispheric Infarction
The sixth affiliated hospital of KMU Wang hao 2015.05.21
• 对于NIHSS 评分较高以及内窥镜检查发现持续吞咽功能障碍者, 应该在ICU 主要1-3 周内和家属讨论放置PEG。(弱推荐,极低质 量证据)
.
Glucose Control
• Both hyperglycemia and hypoglycemia have been associated with increased morbidity and mortality in acute ischemic stroke.
• 1.疼痛、焦虑、躁动者推荐给予镇静和镇痛。(强推荐,极低质 量证据)
• 2.尽可能给予最低强度的镇静治疗,尽可能尽早停止镇静治疗, 同时应保持生理学稳定,防治患者的不适感。(强推荐,极低质 量证据)
.
Are daily wake-up trials recommended?
• Wake-up trials were initially reported to be beneficial regarding reduction of ventilation duration and outcome for some ICU populations.
以bundle防控SSI-(宋代馨)

Hair Removal 去除毛发 Patient Skin Preparation 术前皮肤准备 Surgical Hand Preparation 外科手消毒 Control of The Operating Room Environment 手术室环境控制
• • Air Quality 空气质量 Environmental Cleaning 环境清洁
Sterilization of Supplies And Instruments 供应室器械仪器灭菌 Appropriate Surgical Attire And Drapes 选择合适的手术衣及手术铺巾 Creation of Sterile Surface 手术区域创造无菌表面 Surgical Technique: 手术技术
Process Variability过程因素
Appropriate Antimicrobial Prophylaxis 适当给予预防性抗生素 Patient Management 患者管理包括体温管理,血糖控制及给氧
• • • Normothermia 保温 Glucose Control 血糖 Oxygenation 给氧
Incidence of Surgical Site Infections – North Asia 北亚洲国家SSI发生率
Published SSI research
Japan Nosocomial Infections Surveillance, 2013 report Kim E.S. et al., Infection Control and Hospital Epidemiology,2012,33(6): 572-580 Wang Y.J. et al., Chin J Evid-based Med, 2012,12(7):855-860 Lee M.K. et al., Journal of Hospital Infection, 2007,65:341-347 Taiwan CDC Nosocomial Infections Surveillance, 2012 report
最小二乘法的英文书籍

最小二乘法的英文书籍The Method of Least SquaresThe method of least squares is a statistical technique used to determine the line of best fit for a set of data points. This method is widely used in various fields of study, including engineering, physics, economics, and social sciences, to analyze and interpret data. The basic principle behind the method of least squares is to minimize the sum of the squared differences between the observed values and the predicted values. In other words, the method aims to find the line or curve that best represents the relationship between the independent and dependent variables in a dataset.The history of the method of least squares can be traced back to the early 19th century, when it was independently developed by several mathematicians and scientists. The most notable contributors to the development of this method include Carl Friedrich Gauss, Adrien-Marie Legendre, and Thomas Bayes. Gauss, in particular, is credited with the formalization and widespread use of the method, which he applied to various problems in astronomy and physics.The method of least squares is based on the assumption that theerrors or deviations between the observed values and the predicted values are normally distributed, with a mean of zero and a constant variance. This assumption is known as the Gauss-Markov assumption, and it is crucial for the validity of the method's statistical properties.The process of applying the method of least squares involves the following steps:1. Identify the independent and dependent variables: The first step in using the method of least squares is to identify the variables that are being studied. The independent variable (or variables) is the factor that is being manipulated or controlled, while the dependent variable is the outcome or response that is being measured.2. Collect the data: Once the variables have been identified, the next step is to collect the data. This typically involves measuring the values of the independent and dependent variables for a set of observations or data points.3. Fit the line of best fit: The method of least squares is used to determine the line or curve that best fits the data. This is done by minimizing the sum of the squared differences between the observed values and the predicted values. The resulting line or curve is known as the line of best fit or the regression line.4. Interpret the results: After the line of best fit has been determined, the next step is to interpret the results. This may involve calculating the slope and intercept of the line, as well as the goodness of fit, which measures how well the line of best fit represents the data.The method of least squares has several important properties that make it a powerful tool for data analysis. First, the method is unbiased, meaning that the predicted values are, on average, equal to the true values. Second, the method is efficient, in the sense that it produces the smallest possible variance of the predicted values. Finally, the method is consistent, which means that as the number of data points increases, the predicted values converge to the true values.Despite its many advantages, the method of least squares also has some limitations. For example, the method assumes that the errors are normally distributed and have constant variance, which may not always be the case in real-world data. Additionally, the method is sensitive to outliers, which can have a significant impact on the resulting line of best fit.In recent years, the method of least squares has been extended and refined to address some of these limitations. For example, robust regression techniques have been developed to deal with outliers, while Bayesian methods have been used to incorporate priorinformation into the analysis.Overall, the method of least squares is a powerful and widely used tool for data analysis. Its ability to identify the line or curve that best represents the relationship between variables makes it an essential tool in a wide range of scientific and mathematical disciplines.。
MyLab辅助功能最佳实践指南说明书

MyLab Accessibility Best Practices GuideStriving to ensure every learner succeeds MyLab course materials Alternative course materials Accessible eTextbooks Alternate format text AccessText Network Braille and large print MyLab accessibility features Turning on Accessibility Platform and navigation Assignments and assessments Question bank for homework and tests Custom Question Builder Microsoft PowerPoint decks Alternate text Videos Discipline-specific media In your MyLab course: New considerations Support and documentation VPATs and other accessibility status documents Table of Contents112222344456789910101010Introduction/OverviewStriving to ensure every learner succeedsMyLab course materialsEmpowering learners will always be central to Pearson’s mission and values. That includes designing instructional content for MyLab® to be fully accessible to students with disabilities while continually improving usability.By honoring the following best practices together, we can enable a wider array of students to thrive through their learning journey — and prepare everyone to prosper.Pearson’s investment in accessible product design and remediation is significant and ongoing as we strive to meet and exceed Web Content Accessibility Guidelines (WCAG 2.1 AA standards) for all our educational materials including those designed for MyLab courses.If for any reason any Pearson eTextbook doesn’t meet a student’s need, Pearson is prepared to provide other course material options. To save everyone time and effort, we’ve partnered with top providers and accessibility experts to expand our capabilities.Alternative course materialsAccessible eTextbooksPearson’s newest eTextbook platform, Pearson+, supports the majority of WCAG 2.1 AA standards and we are continuously updating to improve both accessibility and usability as well as compatibility with assistive technologies. Keep in mind that the most recent edition of each title will provide the most accessible user experience.Pearson+ eTextbooks can be accessed in two ways:1. Using a MyLab courseIf your course uses Pearson MyLab online learning platforms, students may already have access to an accessible eTextbook. To open, go to the MyLab course menu in your browser and select the eTextbook option.2. Logging in to Pearson+ directlyFor classes that do not use MyLab, students can log in to Pearson+ to access eTextbooks from a computer or mobile device (iOS and Android), even when offline.Please email us at ****************************** for title-specific details or if you encounter any issues.Alternate format textIf a Pearson+ eTextbook is not fully accessible for a student based on their needs, an alternate format text can be found within the Pearson VitalSource accessible digital textbooks library.You may purchase Pearson titles as eTextbooks from VitalSource and get instant access to eTextbooks through the VitalSource Bookshelf platform.Learn more about accessible digital eTextbooks from Pearson and get answers to common questions about them.AccessText NetworkPearson partners with the AccessText Network to ensure that students with print-based disabilities that would be better addressed by a non-digital textbook also have that option. Upon request and at no added cost, additional Pearson titles are available for qualified students who buy or rent our print or eTextbook versions through AccessText Network, a clearinghouse for files from all major higher education publishers.A disability services representative must make these title requests from the school who must register with the AccessText Network. The alternative text file will be delivered typically as an untagged PDF (meaning not fully accessible for digital consumption).This method is ideal for:• Schools intending to produce their own braille or large format documents• Students requiring a printed textbook versus digital book as a result of a disability Braille and large printPearson and Allyant, the leading provider of accessible textbook formats in North America, partnered to significantly reduce the turnaround time and cost of providing top-selling Pearson titles in braille and reflowed large print.T-Base can deliver top-selling braille or reflowed large-print textbooks within 10 business days at a substantial cost reduction to institutions. Purchasing accessible textbooks through this new, more efficient process enables educators to focus on helping students succeed in their studies. Explore the ever-expanding Allyant Catalog. Order textbooks in braille or reflowed large print by emailing Allyant at: **************** or calling: 1-800-563-0668.Platform and navigationAll MyLab website pages are screen reader and keyboard-accessible including student pages like Calendar, Homework and Tests, Results, Announcements, and Study Plan.The platform’s interface works with common screen magnifiers. Browser- or device-based contrast settings are respected.*Note: When the student is using keyboard-only navigation or assistive technology within MyLab, the Accessibility Mode is required to complete assignments. (Explore the command-line language students can use to type symbols when Accessibility Mode is turned on.)Assignments and assessments Question bank for homework and testsWhen giving students with disabilities any assignments, be sure their eTextbooks display a copyright year of 2014 or later, and keep in mind that all Pearson business products published in 2016 or later also have accessible eTextbooks.Contact ****************************** for questions about other eTextbooks. Accessible questions are noted with an icon to help instructors select accessible assessmentsand require alternative text.Pearson is actively and consistently increasing the number of accessible questions.Accessible questions and items are:• Screen reader and keyboard accessible• Compatible with common screen magnifiers• Contrast-optimized for vision-impaired access• Created with accessible media such as video with captions and/or video descriptions and images with alternative textCustom Question BuilderWhen editing existing questions or composing your own, remember to:• Add alternative text for images• Use only captioned media• Format fonts for optimal readability• Weigh the use of static vs algorithmic questions for ease of editing and to accommodate student needs• Want to learn more? Watch our MyLab Create Your Own Questions video.• Use questions from other texts and the new enhanced book search. Watch our MyLab Enhanced Book Search video.Microsoft PowerPoint decksMicrosoft PowerPoint decks copyrighted 2018 or later are accessible and include: • Slides that use a clear, simple language and layout• Slides that use accessible fonts of a minimum size• Defined reading order• Accessible color contrast ratios (4.5:1) for text and images of text and color combinations that can be read by students with color blindness• Alternative text descriptions for images• Accessible math, where applicable• Slide titles in the title placeholder for each slide that are unique and concise• Meaningful text or raw URLs to describe hyperlinks• Lists that are built as structured lists• Columns that are created with defined reading order• Tables that are created with PowerPoint’s table feature, are simple grids with predictable rows and columns, and are free of merged cells• Slides that are free of background images and flickering imagesAlternative Text• Alternative text (“Alt Text”) is a written description that conveys visual content of images to students who are blind or have low vision. Pearson eTextbooks with a copyright year of 2018 or later have alternative text for images.• Alternative text is read aloud by screen readers and other assistive technology devices.For complex images, a long description may also be provided via hyperlink to fullydescribe the image.VideosVideos:• Are navigable using screen readers and keyboards• Offer closed captioning in most Business, Reading & Writing, and Math & Statistics products. Note: MIS titles and products released prior to 2010 may vary.• Provide access to certain transcripts via the video player. (See the Further Problem Solving example.) Activate this feature in “Settings.” Some transcripts are available as supplemental materials within the course and do not require a request.• Will include audio descriptions in future releases of visual details on screen that are not apparent from the audio alone.In your MyLab course: New considerations Support and documentationVPATs and other accessibility status documents• Link an HTML eBook directly to its own tab.• Use screen-reader compatible questions. If using Skill Builder, select only the screen reader questions.• Extend testing time by 50% by using the individual student settings.• VPATs and Accessibility Conformance and Remediation Forms (ACC&R) are available for many products upon request and organized and described by feature and status. • To request this information, contact Pearson Disability Support .• Learn more about Pearson’s Accessibility online or contact your Pearson Sales Representative.Discipline-specific mediaMany MyLab products feature discipline-specific media (e.g.: animations, simulations, experiments, flashcards, etc.). For updated details on their accessibility, refer to discipline-specific accessibility status flyers.。
术中加温输血输液的重要临床意义

据文献报道,患者体内温度低于36
℃时就可以定义为低体温【1】。
体温降低是围手术期最常见的热紊乱 现象之一,50-80%的病人发生术后低 温。多数情况低温程, 度不重,体温通常 降低23C,即中心温度在3436C之 间。
一般认为该现象是麻醉药物抑制体温 调节中枢和病人暴露在相对寒冷的手术 室环境共同作用的结果。
成人静脉输入每1升环境温度下的液体或每输入1个单 位4℃血液,可降低平均体温0.25℃
围术期患者低体温的诱因
• 室温低 – 室温在20~21℃时,102例病人术中低温的发 生率约为79.4% – 室温在24~25℃时,137例病人术中低温的发 生率约为55.47%。
• 手术床温度低 • 皮肤消毒使热量蒸发
体温调节中枢
◆下丘脑前部--散热中枢
◆下丘脑后部--产热中枢
体温的生理调节
在下丘脑中,整合的温度信息与温度阈值进行比 较,从而触发相应的体温调节反应:
----当体温高于热反应阈值时产生出汗反应和主动血管扩 张; ----低于冷反应阈值时引起血管收缩和寒战。
正常情况下热反应阈值和冷反应阈值之间仅相 差约0.2℃,在该范围内,不触发任何体温调节 反应。
围术期患者低体温的诱因
• 麻醉气体温度低 • 半开放呼吸回路
– 机械通气时,给病人应用干燥、凉的气体通气,约有10%的代谢 热量经呼吸丢失。
• 静脉输液温度低 – 直接降低中心体温; – 成人静脉输注1L环境温度下的液体,或1单位4℃的血液,平均体 温约降低0.25 ℃左右。
• 灌洗液温度低 • 体腔开放蒸发
[1] Segers MJ. Hypothermia in the trauma patients. Der Unfallchirurg, 1998, 101(10): 741-749
Zoll Alsius Cooling Catheter 使用指南说明书

Therapeutic Hypothermia After Cardiac Arrest (Catheter Cooling) GuidelineProHealth CareGoals:The aim of this therapy is to suppress the chemical reactions that occur when vital signs have been absent and reperfusion has occurred after cardiac arrest. To initiate current evidence based research and best practice guidelines to the caregiver, which has been shown to improve patient neurologic outcomes post cardiac arrest, where resuscitation has been delayed or prolonged. Induction of mild hypothermia post cardiac arrest will slow bodily processes thereby decreasing the effects of hypoxia after the cardiac event.Zoll / Alsius Cooling Catheter:The cooling catheter should be placed in the femoral vein and functions as a closed loop interval cooling circuit, which cools the blood as it circulates past the catheter. The catheter is attached to the Zoll/Alsius thermal regulation system, which monitors catheter performance and measures temperature via a connection to the internal thermometer probe. The machine should ideally be plugged in 20 minutes prior to use and set to maximal cooling with a target temperature of 33°C for 24 hrs. ICY Cath for shorter patients, Quatro for taller patients.Equipment:1)CVP/PA or Esophageal, and bladder catheter temperature probe2)One cooling blanket devices3)Two liters of 4°C .09% Saline4)Cooling CatheterInclusion criteria:-Hypothermia should be initiated as soon as possible after the of return of spontaneous circulation (ROSC)-Must include the following:o Post cardiac arrest (does not need to be witnessed) : defined as absence of pulse requiring chest compressions, regardless of location of collapse, or initial rhythm, with subsequentROSC.o Comatose with Glasgow Motor Score (GMS) Less than 6§▪ 6 – Obeys commands§▪ 5 – Localizes painful stimuli§▪ 4 – Flexion/Withdrawal to painful stimuli§▪ 3 – Abnormal flexion to painful stimuli (decorticate response)§▪ 2 – Abnormal extension to painful stimuli (decerebrate response)§▪ 1 – Makes no movement to painful stimuliExclusion Criteria:-Patient is under18 years of age (for younger patients coordinate care plan with Children’s Hospital of Wisconsin).-Active DNR (Do Not Resuscitate) order-Presence of severe pre-arrest cognitive impairment (ie. Nonverbal and bed bound)-Time is greater than 12 hours from ROSC-Uncontrolled Bleeding-Evidence of Trauma – ie: Trauma as possible cause of cardiac arrest (because of active bleeding){note: hanging typically results in an anoxic arrest and therefore is not a contraindicationbased on mechanism alone}-Presence of other etiology for coma (ie.. Head Trauma, Hemorrhagic Stroke, Status Epilepticus, etc..) {note: narcotic overdose completely reversed with Narcan is not an exclusion criteria} -Pre-existing multi-organ dysfunction syndrome or severe sepsis-Comorbidities with minimal chance of meaningful survival independent of neurologic status-Cardiac instabilityo Refractory or recurrent life threatening dysrhythmiao Hypotension is not a contraindication unless caused by persistent dysrhythmia -Note: Respiratory arrest leading to cardiac arrest is not a contraindication to cooling.Relative Exclusion Criteria:-Environmental hypothermia exposure (warm patient to 32-34° C and continue protocol)-History of Bleeding disorder or current coagulopathy (Coumadin, Lovenox, Aspirin, etc.. are not contraindications) {note: target temp of 36° C does not affect coagulation – consider this option} -Controlled bleeding : target temp of 36° C does not affect coagulation – consider this option-Overdose : If ROSC, and sedative effect of overdose has been reversed, and patient remains altered with GMS less than 6, and there is concern for anoxic injury, patient is a candidate for cooling. Ifpatient remains comatose and there is a persistent pharmacologic reason for their mental status, theyare not a candidate for cooling.-Pregnancy: as per one case report, cooling can be performed safely on pregnant patients.For patients meeting criteria for 36° C target temperature, see appendix A below.Pre-induction phase:-Start 2 IVs each 20 ga or larger-Continuous ECG monitoring-Continuous S P O2 monitoring-Hyperoxia is harmful in ROSC. Titrate FiO2 to hold SpO2 between 92 – 96%-Insert Temp Probe Foley Catheter-Urine HCG on females of child bearing age-Order ABG,CBC, BMP, INR, LFT’s, Troponin, CPK with MB, Lactate, Mg, PO4-Order PCXR post intubation-Order Head CT to rule out intracranial bleed (Should be done at a convenient time before the “monitoring phase”. Should not delay cardiac cath unless there is a high suspicion of intracranialbleed)-Insert nasogastric or oral gastric tube-Document baseline vitals at a minimum of every 15 minutes-Assess and document baseline skin condition-Insert an arterial line-Insert two core temperature monitoring devices (CVP/PA cath or Esophageal temp probe) {connected to temp port #1 on Zoll machine} and (bladder or rectal probe) {connected to temp port#2 on Zoll machine} The second core temperature monitoring device should be placed as soon aspossible to allow complementary core temperature readings.-Place Cooling Catheter {Set target temp to 33°C} (catheter may be placed in cardiac cath lab)-Order KUB x-ray to confirm location of catheter tip (may be done with fluoroscopy as well)-Anticipate need for vasopressor support-Position cooling blanket under patient with sheet between blanket and patient if delay in placing cooling cath {preferred to place cooling blanket on bed prior to patient arrival}-Intubate patient with ETT-Sedate patient (consider Propofol 5-10 mcg/kg/min or Versed drip) and/or (Fentanyl 50-100mcg bolus and 50mcg/hr drip) {narcotics are important to control shivering and treat pain} -If monitoring sedation with Bispectral Index Monitoring (BIS), titrate to goal : 40-60-Paralyze patient with neuromuscular blocking agents as needed for shivering (consider drip)-Wrap hands and feet in towels to prevent frost bite and decrease shivering stimulusInduction Phase:-Hypothermia procedures should not delay interventional cardiology-Place cloth protected ice packs in groin and axilla – will be removed when temp is 34°C-Infuse 30ml/kg of 4°C saline rapid bolus via pressure bag (max of 2 liters) if {the patient is not already at target temperature, additional fluid is not contraindicated, a cold fluid bolus has notalready been administered}.-Do not administer cold saline bolus if obvious pulmonary edema is present-Place second cooling blanket on top of patient with sheet between blanket and sheet (if delay in placing cooling cath)-The cooling blankets should not make direct contact with the patient’s skin-Turn on the cooling blankets in manual mode and set temperature at 4°C (Gaymar flat blanket style) -Cooling blankets can be removed once cooling catheter has been placed.-Automated cooling devices (Zoll / Alsius) should be set with a target temp or (set point) of 33°C.-The patient’s temperature should be kept between 32°C and 34°C for 24 hrs from the time target temperature is reached-The patient should be cooled as fast as possible until target is reached-The temperature should not go below 32°C-Document core temperature every 15 min during cooling and rewarming.-Be aware of subclinical shivering (see appendix B below)-Consider continuous EEG monitoring for 48 hrs due to 10-15% incidence of seizures-Once catheter location confirmed, initiate max cool algorithm on Zoll / Alsius device (set point 33°C)Monitoring/Maintenance Phase:-Document core temperature every hour during maintenance.-Continue to assess patients skin every 2 hrs-Continuous ECG monitoring (bradycardic rhythms are common)-Keep Head of Bed elevated at 30°-Reposition patient q 2° and PRN-Do not perform invasive thoracic procedures or reposition the patient if temp is < 32°C-Monitor vital signs per ICU routine-Mean Arterial Pressure (MAP) goal (90-100 mmHg) or BPs 120.-Use vasopressor support as needed-Insulin drip as ordered for glucose management-Maintain CVP between 8-12 mmHg (euvolemia, unless other concerns, ie: CHF)-Monitor blood glucose every 4 hrs or as ordered per glucose management protocol-Be sure to avoid heated humidified oxygen on the ventilator-NG or OG to low intermittent suction-Avoid maintenance fluid containing dextrose-Constant assessment for shivering-Pepcid 20mg IVP q 12°-Tylenol 650mg NG or PR q6° x 24°-Avoid bed bath during administration of hypothermia-Often continuous neuromuscular blockade may be stopped during this phase and used only as needed for shivering. Be aware of subclinical shivering (see appendix B below)-Blood glucose should be maintained between 125-175 mg/dl-Once the temperature has reached the machine’s set point, record, and monitor the location of the cooling “power” indicator on the machine.Re-Warming Phase:-Consider increasing CVP to 15 mmHg just prior to warming, unless CHF as patient will vasodilate with warming-Automatically re-warm the patient after 24 hr hypothermic period complete with Zoll / Alsius cooling cath algorithm pre-programmed in machine. (target temp 37°C)o Hypothermic period starts once target temperature is reachedo The patient should be re-warmed at a rate no faster then 0.25°C per hour-Neuromuscular blocking agents may be needed during re-warming phase to prevent shivering but should no longer be needed once 36° C is reached.-Once warmed, patients may become hyperthermic.o Screen for sources of infection (ie. Blood cultures x 2, UA with culture, pCXR, etc.)o Tylenol 1000mg PO or PR q 6°PRN Temp > 37.5°C.o Consider using active cooling/warming to maintain normothermia for 48 hrs.-Anticipate increased CO2 production during rewarming and possible need for ventilator adjustment -Stop Potassium containing solutions unless hypokalemic (potassium will increase during rewarming)Special Considerations:-Rectal temperature monitoring is the least accurate and is not preferred.-Avoid IV solutions containing dextrose unless hypoglycemia has developed.-Watch for clinical symptoms of seizure in paralyzed patient (unexplained tachycardia)-Avoid hyperventilation-Perform routine neuro assessment q 4°once rewarming is complete-Obtain Neuropsychology consultation prior to dischargeReasons to Abort Cooling:•Significant hemorrhage•Severe and persistent arrhythmia causing hypotension•Decision to withdraw care or palliative care•Ethical reasons : ie. Previously unknown end-stage cancer or refractory shock with end-stage multiorgan failureDo not Abort Cooling if:•Cardiac arrest – perform ACLS as if patient was normothermico If regain ROSC, restart 24hr hypothermic monitoring phase at time of new ROSCDiagnostic Studies (Laboratory/Radiology – if not already done)•ABG, CBC, CMP, Mg, PO4, INR, PTT, Lactate q 6° x4•CPK, CPK-MB q8° x 3•Troponin q8° x3•Potassium q2° during re-warming phase until Temp ≥ 36°C•LABS: Include patient’s body temperature in order and on label if < 37°C•Blood sugar per protocol•PCXR – re: ETT placement, R/O aspiration•12 lead EKG q 12° x 2Neuroprognostication:•Standard prognostication techniques were developed prior to therapeutic hypothermia•Neurologic evaluation for ROSC patients based on:o Clinical neurological examination {including but not limited to: GCS, pupillary andcorneal reflexes, N20-peak on median nerve somatosensory evoked potentials (SSEP),and EEG}.•Recommend waiting 72hrs after rewarmed to 37°C before determining prognosis•Findings allowing for discontinuation of active intensive care:o Brain Death due to cerebral herniationo Severe myoclonus status in the first 24 hrs after admission and a bilateral absence of N20-peak on median nerve SSEPo Minimum of 72 hrs after warmed: persisting coma with GMS 1-2 and bilateral absence of N20-peak on median nerve SSEPo Minimum of 72 hrs after warmed: persisting coma with GMS 1-2 and a treatmentrefractory status epilepticus•Patients with GMS 1-2, 72 hrs after warmed with retained N20-peak on the SSEP or if SSEP not available:o Re-examination dailyo Consider withdrawal of intensive care if: No improvement in GMS and metabolic and pharmacological affection is ruled outAppendix A : Therapeutic Hypothermia with Target Temperature of 36° COn Nov 17th, 2013, Nielsen published an article in the NEJM that allows the option tocool patients to a target temp of 36°C instead of 32-34°C. There are many advantages tousing a target temp of 36°C. At this temperature, pharmacologic, electrolyte, metabolic,sedative, hemodynamic, coagulopathic, and shivering challenges are avoided. Apotential limitation to this study is that 70% of the patients had bystander CPR. Thiscould decrease the relative severity of the brain injury sustained by these patients, andtherefore, may not accurately reflect the patient population in every area. Data is stilllacking to demonstrate that 36°C is therapeutically equivalent to 33°C for patients withmoderate and severe brain injury. Since the severity of brain injury can not be accuratelypredicted prior initiating cooling, a target temp of 32-34°C is still recommended for mostpatients. If for clinical reasons, a provider feels that cooling a patient to a targettemperature of 33°C carries too much risk (perhaps due to cardiac instability or risk ofbleeding), there is good evidence to allow cooling to a target temperature of 36°C. Theremainder of the protocol is unchanged however management can be more consistent of atypical intensive care patient since rewarming, drug metabolism, electrolyte, andshivering problems are much less likely. It is possible that in the future, the targettemperature of 33°C will be abandoned for a target temp of 36°C on all patients. Appendix B : Subclinical ShiveringShivering can be either visible or invisible. The first indication of shivering maybe labored breathing, a fall in mixed venous O2 saturation, and heightenedmuscular tone. Visible shivering may be as subtle as involuntary facial and neckmuscle contractions or trembling on palpation of the thorax. Shivering can tripleoxygen consumption, causing hypoxemia, and organ ischemia. It will alsoincrease intracranial pressure. Therefore shivering is undesirable in critically illpatients and in post cardiac arrest patients. Shivering is most likely to occurbetween 34 and 36°C. Therefore, either cooling patients to 36°C or rapidlycooling patients beyond 34°C is ideal. Shivering is common with surface coolingtechniques but only occurs in 3.7% of patients cooled intravascularly. Make noteof cooling power indicator on cooling machine throughout maintenance phase. Ifthe cooling machine suddenly has to put more power into keeping the patientcool, they are likely shivering.Signs of subclinical shivering:•Increase heart rate without other cause•Patient’s rate of cooling is slow or has slowed•Cooling machine moves to colder mode vs previous baseline•Evidence of shivering on EEGConsider temporary neuromuscular blockadeAuthorscontactinfo:KayWalter,RN;*******************Dr.MarkSchultz;************.com(Updated 1-31-14)。
MANUSCRIPT SUBMISSION GUIDELINES.pdf

APPENDIX 3MANUSCRIPT SUBMISSION GUIDELINES1.MANUSCRIPT FILE FORMATThe Author must submit all manuscripts electronically via email or on CD-ROM and as part of 1 (one) file (e.g. all chapters of Book and any front matter must be a part of one document). The Publisher will only accept PC compatible files. Microsoft Word is the preferred software, but Microsoft Works (.wps) is also accepted. If the Author is using another program, save it as .txt.Prepare your manuscript as plain unformatted text with a simple tab at the beginning of each paragraph. You must not use “automatic formatting” or “styles” in your text.∙End Notes: If information is important enough to be said, try to work it into the text. If notes are necessary to your presentation, use End Notesonly. Do not use footnotes and do not use “footnote” or “end note” functions in word processing programs. Mark notes in text (superscript text orparenthesis with “Note 1” etc.) and type notes as regular text on a separate page at the end of your manuscript. Restrict use of headings to mainheadings and sub-headings only. Third-level headings are discouraged.∙DO NOT TYPE ANYTHING IN ALL CAPS (not even the title), and do not format text in any way: use your word processor’s defaults and DO NOTenter codes or styles for type size, font, paragraph definitions, paragraphstyles, indents, columns, and similar formatting. You MAY use italics(preferred) or bold for emphasis in your text.∙Quotations and Permissions to Quote: Direct quotations longer than three lines (approximately 50 words) will be set off as block quotations with indented margins. The Author must secure and submit to the Publisherpermission to quote “substantial amounts” of copyrighted material. ThePublisher shall not be responsible for any delays to the production processcaused by outstanding permissions.In general, permission from the copyright holder is required for quotationsexceeding 50 words and such protection extends to the author or theauthor’s heirs for quotation of any unpublished material. Unless the original author’s exact words are necessary to make a point, try to paraphrase theoriginal author’s words (with the proper citation) or use quoted phrases inthe body of the text. Paraphrase or direct quotation requires a page number in addition to the year for in-text citations. The Author must also secure and submit to Publisher permission to use previously published graphics.Graphics that are not the property of the Author must be acknowledged inthe graphic title.2.FRONT MATTERCertain books may contain a dedication, foreword, acknowledgments, preface, introduction, and prologue. All of these elements should be considered as part of the manuscript itself and be submitted as such. If the Author wishes to include one or more of these elements but cannot supply them when the rest of the manuscript is submitted, the Author acknowledges that additional layout costs may be incurred, unless layout is postponed until these elements are in place. If the Author decides to include one or more of these elements after the book has been formatted, there will be an extra charge of 400.00 SEK (four hundred Swedish kronor) per hour to repaginate and reformat the layout.3.IMAGE FILE FORMATImage files must be submitted separately and may not be embedded into THE Author’s manuscript. The Author must mark in the manuscript where the image should be inserted by using this protocol: [insert “image name” here].4.AUTHOR BIOGRAPHYRoughly one or two paragraphs of a page containing an Author biography is optional, but recommended, and is usually placed in the back matter. A brief Author bio can also be included on the back cover. Author photo is optional.5.NUMBERINGNotes, whether footnotes or endnotes, should be numbered consecutively, beginning with 1, throughout each article or chapter — not throughout an entire book unless the text has no internal divisions. If a work contains only a handful of footnotes, they may be referenced by asterisks rather than numbers.6.INDEXIf the Author requires an index, the Author must indicate this when the manuscript is submitted, before the layout commences, so that the Publisher can plan accordingly. The list of terms to be indexed should be supplied with the manuscript submission. The index will be scheduled after the final approval of the interior layout. Once scheduled, indexing can take anywhere from 5–10 (five to ten) business days, and the Author accepts the extra time this adds to the production process. There is an additional layout fee of 1,300.00 SEK (one thousand three hundred Swedish kronor) for any book that contains an index.。
Survey of clustering data mining techniques

A Survey of Clustering Data Mining TechniquesPavel BerkhinYahoo!,Inc.pberkhin@Summary.Clustering is the division of data into groups of similar objects.It dis-regards some details in exchange for data simplifirmally,clustering can be viewed as data modeling concisely summarizing the data,and,therefore,it re-lates to many disciplines from statistics to numerical analysis.Clustering plays an important role in a broad range of applications,from information retrieval to CRM. Such applications usually deal with large datasets and many attributes.Exploration of such data is a subject of data mining.This survey concentrates on clustering algorithms from a data mining perspective.1IntroductionThe goal of this survey is to provide a comprehensive review of different clus-tering techniques in data mining.Clustering is a division of data into groups of similar objects.Each group,called a cluster,consists of objects that are similar to one another and dissimilar to objects of other groups.When repre-senting data with fewer clusters necessarily loses certainfine details(akin to lossy data compression),but achieves simplification.It represents many data objects by few clusters,and hence,it models data by its clusters.Data mod-eling puts clustering in a historical perspective rooted in mathematics,sta-tistics,and numerical analysis.From a machine learning perspective clusters correspond to hidden patterns,the search for clusters is unsupervised learn-ing,and the resulting system represents a data concept.Therefore,clustering is unsupervised learning of a hidden data concept.Data mining applications add to a general picture three complications:(a)large databases,(b)many attributes,(c)attributes of different types.This imposes on a data analysis se-vere computational requirements.Data mining applications include scientific data exploration,information retrieval,text mining,spatial databases,Web analysis,CRM,marketing,medical diagnostics,computational biology,and many others.They present real challenges to classic clustering algorithms. These challenges led to the emergence of powerful broadly applicable data2Pavel Berkhinmining clustering methods developed on the foundation of classic techniques.They are subject of this survey.1.1NotationsTo fix the context and clarify terminology,consider a dataset X consisting of data points (i.e.,objects ,instances ,cases ,patterns ,tuples ,transactions )x i =(x i 1,···,x id ),i =1:N ,in attribute space A ,where each component x il ∈A l ,l =1:d ,is a numerical or nominal categorical attribute (i.e.,feature ,variable ,dimension ,component ,field ).For a discussion of attribute data types see [106].Such point-by-attribute data format conceptually corresponds to a N ×d matrix and is used by a majority of algorithms reviewed below.However,data of other formats,such as variable length sequences and heterogeneous data,are not uncommon.The simplest subset in an attribute space is a direct Cartesian product of sub-ranges C = C l ⊂A ,C l ⊂A l ,called a segment (i.e.,cube ,cell ,region ).A unit is an elementary segment whose sub-ranges consist of a single category value,or of a small numerical bin.Describing the numbers of data points per every unit represents an extreme case of clustering,a histogram .This is a very expensive representation,and not a very revealing er driven segmentation is another commonly used practice in data exploration that utilizes expert knowledge regarding the importance of certain sub-domains.Unlike segmentation,clustering is assumed to be automatic,and so it is a machine learning technique.The ultimate goal of clustering is to assign points to a finite system of k subsets (clusters).Usually (but not always)subsets do not intersect,and their union is equal to a full dataset with the possible exception of outliersX =C 1 ··· C k C outliers ,C i C j =0,i =j.1.2Clustering Bibliography at GlanceGeneral references regarding clustering include [110],[205],[116],[131],[63],[72],[165],[119],[75],[141],[107],[91].A very good introduction to contem-porary data mining clustering techniques can be found in the textbook [106].There is a close relationship between clustering and many other fields.Clustering has always been used in statistics [10]and science [158].The clas-sic introduction into pattern recognition framework is given in [64].Typical applications include speech and character recognition.Machine learning clus-tering algorithms were applied to image segmentation and computer vision[117].For statistical approaches to pattern recognition see [56]and [85].Clus-tering can be viewed as a density estimation problem.This is the subject of traditional multivariate statistical estimation [197].Clustering is also widelyA Survey of Clustering Data Mining Techniques3 used for data compression in image processing,which is also known as vec-tor quantization[89].Datafitting in numerical analysis provides still another venue in data modeling[53].This survey’s emphasis is on clustering in data mining.Such clustering is characterized by large datasets with many attributes of different types. Though we do not even try to review particular applications,many important ideas are related to the specificfields.Clustering in data mining was brought to life by intense developments in information retrieval and text mining[52], [206],[58],spatial database applications,for example,GIS or astronomical data,[223],[189],[68],sequence and heterogeneous data analysis[43],Web applications[48],[111],[81],DNA analysis in computational biology[23],and many others.They resulted in a large amount of application-specific devel-opments,but also in some general techniques.These techniques and classic clustering algorithms that relate to them are surveyed below.1.3Plan of Further PresentationClassification of clustering algorithms is neither straightforward,nor canoni-cal.In reality,different classes of algorithms overlap.Traditionally clustering techniques are broadly divided in hierarchical and partitioning.Hierarchical clustering is further subdivided into agglomerative and divisive.The basics of hierarchical clustering include Lance-Williams formula,idea of conceptual clustering,now classic algorithms SLINK,COBWEB,as well as newer algo-rithms CURE and CHAMELEON.We survey these algorithms in the section Hierarchical Clustering.While hierarchical algorithms gradually(dis)assemble points into clusters (as crystals grow),partitioning algorithms learn clusters directly.In doing so they try to discover clusters either by iteratively relocating points between subsets,or by identifying areas heavily populated with data.Algorithms of thefirst kind are called Partitioning Relocation Clustering. They are further classified into probabilistic clustering(EM framework,al-gorithms SNOB,AUTOCLASS,MCLUST),k-medoids methods(algorithms PAM,CLARA,CLARANS,and its extension),and k-means methods(differ-ent schemes,initialization,optimization,harmonic means,extensions).Such methods concentrate on how well pointsfit into their clusters and tend to build clusters of proper convex shapes.Partitioning algorithms of the second type are surveyed in the section Density-Based Partitioning.They attempt to discover dense connected com-ponents of data,which areflexible in terms of their shape.Density-based connectivity is used in the algorithms DBSCAN,OPTICS,DBCLASD,while the algorithm DENCLUE exploits space density functions.These algorithms are less sensitive to outliers and can discover clusters of irregular shape.They usually work with low-dimensional numerical data,known as spatial data. Spatial objects could include not only points,but also geometrically extended objects(algorithm GDBSCAN).4Pavel BerkhinSome algorithms work with data indirectly by constructing summaries of data over the attribute space subsets.They perform space segmentation and then aggregate appropriate segments.We discuss them in the section Grid-Based Methods.They frequently use hierarchical agglomeration as one phase of processing.Algorithms BANG,STING,WaveCluster,and FC are discussed in this section.Grid-based methods are fast and handle outliers well.Grid-based methodology is also used as an intermediate step in many other algorithms (for example,CLIQUE,MAFIA).Categorical data is intimately connected with transactional databases.The concept of a similarity alone is not sufficient for clustering such data.The idea of categorical data co-occurrence comes to the rescue.The algorithms ROCK,SNN,and CACTUS are surveyed in the section Co-Occurrence of Categorical Data.The situation gets even more aggravated with the growth of the number of items involved.To help with this problem the effort is shifted from data clustering to pre-clustering of items or categorical attribute values. Development based on hyper-graph partitioning and the algorithm STIRR exemplify this approach.Many other clustering techniques are developed,primarily in machine learning,that either have theoretical significance,are used traditionally out-side the data mining community,or do notfit in previously outlined categories. The boundary is blurred.In the section Other Developments we discuss the emerging direction of constraint-based clustering,the important researchfield of graph partitioning,and the relationship of clustering to supervised learning, gradient descent,artificial neural networks,and evolutionary methods.Data Mining primarily works with large databases.Clustering large datasets presents scalability problems reviewed in the section Scalability and VLDB Extensions.Here we talk about algorithms like DIGNET,about BIRCH and other data squashing techniques,and about Hoffding or Chernoffbounds.Another trait of real-life data is high dimensionality.Corresponding de-velopments are surveyed in the section Clustering High Dimensional Data. The trouble comes from a decrease in metric separation when the dimension grows.One approach to dimensionality reduction uses attributes transforma-tions(DFT,PCA,wavelets).Another way to address the problem is through subspace clustering(algorithms CLIQUE,MAFIA,ENCLUS,OPTIGRID, PROCLUS,ORCLUS).Still another approach clusters attributes in groups and uses their derived proxies to cluster objects.This double clustering is known as co-clustering.Issues common to different clustering methods are overviewed in the sec-tion General Algorithmic Issues.We talk about assessment of results,de-termination of appropriate number of clusters to build,data preprocessing, proximity measures,and handling of outliers.For reader’s convenience we provide a classification of clustering algorithms closely followed by this survey:•Hierarchical MethodsA Survey of Clustering Data Mining Techniques5Agglomerative AlgorithmsDivisive Algorithms•Partitioning Relocation MethodsProbabilistic ClusteringK-medoids MethodsK-means Methods•Density-Based Partitioning MethodsDensity-Based Connectivity ClusteringDensity Functions Clustering•Grid-Based Methods•Methods Based on Co-Occurrence of Categorical Data•Other Clustering TechniquesConstraint-Based ClusteringGraph PartitioningClustering Algorithms and Supervised LearningClustering Algorithms in Machine Learning•Scalable Clustering Algorithms•Algorithms For High Dimensional DataSubspace ClusteringCo-Clustering Techniques1.4Important IssuesThe properties of clustering algorithms we are primarily concerned with in data mining include:•Type of attributes algorithm can handle•Scalability to large datasets•Ability to work with high dimensional data•Ability tofind clusters of irregular shape•Handling outliers•Time complexity(we frequently simply use the term complexity)•Data order dependency•Labeling or assignment(hard or strict vs.soft or fuzzy)•Reliance on a priori knowledge and user defined parameters •Interpretability of resultsRealistically,with every algorithm we discuss only some of these properties. The list is in no way exhaustive.For example,as appropriate,we also discuss algorithms ability to work in pre-defined memory buffer,to restart,and to provide an intermediate solution.6Pavel Berkhin2Hierarchical ClusteringHierarchical clustering builds a cluster hierarchy or a tree of clusters,also known as a dendrogram.Every cluster node contains child clusters;sibling clusters partition the points covered by their common parent.Such an ap-proach allows exploring data on different levels of granularity.Hierarchical clustering methods are categorized into agglomerative(bottom-up)and divi-sive(top-down)[116],[131].An agglomerative clustering starts with one-point (singleton)clusters and recursively merges two or more of the most similar clusters.A divisive clustering starts with a single cluster containing all data points and recursively splits the most appropriate cluster.The process contin-ues until a stopping criterion(frequently,the requested number k of clusters) is achieved.Advantages of hierarchical clustering include:•Flexibility regarding the level of granularity•Ease of handling any form of similarity or distance•Applicability to any attribute typesDisadvantages of hierarchical clustering are related to:•Vagueness of termination criteria•Most hierarchical algorithms do not revisit(intermediate)clusters once constructed.The classic approaches to hierarchical clustering are presented in the sub-section Linkage Metrics.Hierarchical clustering based on linkage metrics re-sults in clusters of proper(convex)shapes.Active contemporary efforts to build cluster systems that incorporate our intuitive concept of clusters as con-nected components of arbitrary shape,including the algorithms CURE and CHAMELEON,are surveyed in the subsection Hierarchical Clusters of Arbi-trary Shapes.Divisive techniques based on binary taxonomies are presented in the subsection Binary Divisive Partitioning.The subsection Other Devel-opments contains information related to incremental learning,model-based clustering,and cluster refinement.In hierarchical clustering our regular point-by-attribute data representa-tion frequently is of secondary importance.Instead,hierarchical clustering frequently deals with the N×N matrix of distances(dissimilarities)or sim-ilarities between training points sometimes called a connectivity matrix.So-called linkage metrics are constructed from elements of this matrix.The re-quirement of keeping a connectivity matrix in memory is unrealistic.To relax this limitation different techniques are used to sparsify(introduce zeros into) the connectivity matrix.This can be done by omitting entries smaller than a certain threshold,by using only a certain subset of data representatives,or by keeping with each point only a certain number of its nearest neighbors(for nearest neighbor chains see[177]).Notice that the way we process the original (dis)similarity matrix and construct a linkage metric reflects our a priori ideas about the data model.A Survey of Clustering Data Mining Techniques7With the(sparsified)connectivity matrix we can associate the weighted connectivity graph G(X,E)whose vertices X are data points,and edges E and their weights are defined by the connectivity matrix.This establishes a connection between hierarchical clustering and graph partitioning.One of the most striking developments in hierarchical clustering is the algorithm BIRCH.It is discussed in the section Scalable VLDB Extensions.Hierarchical clustering initializes a cluster system as a set of singleton clusters(agglomerative case)or a single cluster of all points(divisive case) and proceeds iteratively merging or splitting the most appropriate cluster(s) until the stopping criterion is achieved.The appropriateness of a cluster(s) for merging or splitting depends on the(dis)similarity of cluster(s)elements. This reflects a general presumption that clusters consist of similar points.An important example of dissimilarity between two points is the distance between them.To merge or split subsets of points rather than individual points,the dis-tance between individual points has to be generalized to the distance between subsets.Such a derived proximity measure is called a linkage metric.The type of a linkage metric significantly affects hierarchical algorithms,because it re-flects a particular concept of closeness and connectivity.Major inter-cluster linkage metrics[171],[177]include single link,average link,and complete link. The underlying dissimilarity measure(usually,distance)is computed for every pair of nodes with one node in thefirst set and another node in the second set.A specific operation such as minimum(single link),average(average link),or maximum(complete link)is applied to pair-wise dissimilarity measures:d(C1,C2)=Op{d(x,y),x∈C1,y∈C2}Early examples include the algorithm SLINK[199],which implements single link(Op=min),Voorhees’method[215],which implements average link (Op=Avr),and the algorithm CLINK[55],which implements complete link (Op=max).It is related to the problem offinding the Euclidean minimal spanning tree[224]and has O(N2)complexity.The methods using inter-cluster distances defined in terms of pairs of nodes(one in each respective cluster)are called graph methods.They do not use any cluster representation other than a set of points.This name naturally relates to the connectivity graph G(X,E)introduced above,because every data partition corresponds to a graph partition.Such methods can be augmented by so-called geometric methods in which a cluster is represented by its central point.Under the assumption of numerical attributes,the center point is defined as a centroid or an average of two cluster centroids subject to agglomeration.It results in centroid,median,and minimum variance linkage metrics.All of the above linkage metrics can be derived from the Lance-Williams updating formula[145],d(C iC j,C k)=a(i)d(C i,C k)+a(j)d(C j,C k)+b·d(C i,C j)+c|d(C i,C k)−d(C j,C k)|.8Pavel BerkhinHere a,b,c are coefficients corresponding to a particular linkage.This formula expresses a linkage metric between a union of the two clusters and the third cluster in terms of underlying nodes.The Lance-Williams formula is crucial to making the dis(similarity)computations feasible.Surveys of linkage metrics can be found in [170][54].When distance is used as a base measure,linkage metrics capture inter-cluster proximity.However,a similarity-based view that results in intra-cluster connectivity considerations is also used,for example,in the original average link agglomeration (Group-Average Method)[116].Under reasonable assumptions,such as reducibility condition (graph meth-ods satisfy this condition),linkage metrics methods suffer from O N 2 time complexity [177].Despite the unfavorable time complexity,these algorithms are widely used.As an example,the algorithm AGNES (AGlomerative NESt-ing)[131]is used in S-Plus.When the connectivity N ×N matrix is sparsified,graph methods directly dealing with the connectivity graph G can be used.In particular,hierarchical divisive MST (Minimum Spanning Tree)algorithm is based on graph parti-tioning [116].2.1Hierarchical Clusters of Arbitrary ShapesFor spatial data,linkage metrics based on Euclidean distance naturally gener-ate clusters of convex shapes.Meanwhile,visual inspection of spatial images frequently discovers clusters with curvy appearance.Guha et al.[99]introduced the hierarchical agglomerative clustering algo-rithm CURE (Clustering Using REpresentatives).This algorithm has a num-ber of novel features of general importance.It takes special steps to handle outliers and to provide labeling in assignment stage.It also uses two techniques to achieve scalability:data sampling (section 8),and data partitioning.CURE creates p partitions,so that fine granularity clusters are constructed in parti-tions first.A major feature of CURE is that it represents a cluster by a fixed number,c ,of points scattered around it.The distance between two clusters used in the agglomerative process is the minimum of distances between two scattered representatives.Therefore,CURE takes a middle approach between the graph (all-points)methods and the geometric (one centroid)methods.Single and average link closeness are replaced by representatives’aggregate closeness.Selecting representatives scattered around a cluster makes it pos-sible to cover non-spherical shapes.As before,agglomeration continues until the requested number k of clusters is achieved.CURE employs one additional trick:originally selected scattered points are shrunk to the geometric centroid of the cluster by a user-specified factor α.Shrinkage suppresses the affect of outliers;outliers happen to be located further from the cluster centroid than the other scattered representatives.CURE is capable of finding clusters of different shapes and sizes,and it is insensitive to outliers.Because CURE uses sampling,estimation of its complexity is not straightforward.For low-dimensional data authors provide a complexity estimate of O (N 2sample )definedA Survey of Clustering Data Mining Techniques9 in terms of a sample size.More exact bounds depend on input parameters: shrink factorα,number of representative points c,number of partitions p,and a sample size.Figure1(a)illustrates agglomeration in CURE.Three clusters, each with three representatives,are shown before and after the merge and shrinkage.Two closest representatives are connected.While the algorithm CURE works with numerical attributes(particularly low dimensional spatial data),the algorithm ROCK developed by the same researchers[100]targets hierarchical agglomerative clustering for categorical attributes.It is reviewed in the section Co-Occurrence of Categorical Data.The hierarchical agglomerative algorithm CHAMELEON[127]uses the connectivity graph G corresponding to the K-nearest neighbor model spar-sification of the connectivity matrix:the edges of K most similar points to any given point are preserved,the rest are pruned.CHAMELEON has two stages.In thefirst stage small tight clusters are built to ignite the second stage.This involves a graph partitioning[129].In the second stage agglomer-ative process is performed.It utilizes measures of relative inter-connectivity RI(C i,C j)and relative closeness RC(C i,C j);both are locally normalized by internal interconnectivity and closeness of clusters C i and C j.In this sense the modeling is dynamic:it depends on data locally.Normalization involves certain non-obvious graph operations[129].CHAMELEON relies heavily on graph partitioning implemented in the library HMETIS(see the section6). Agglomerative process depends on user provided thresholds.A decision to merge is made based on the combinationRI(C i,C j)·RC(C i,C j)αof local measures.The algorithm does not depend on assumptions about the data model.It has been proven tofind clusters of different shapes,densities, and sizes in2D(two-dimensional)space.It has a complexity of O(Nm+ Nlog(N)+m2log(m),where m is the number of sub-clusters built during the first initialization phase.Figure1(b)(analogous to the one in[127])clarifies the difference with CURE.It presents a choice of four clusters(a)-(d)for a merge.While CURE would merge clusters(a)and(b),CHAMELEON makes intuitively better choice of merging(c)and(d).2.2Binary Divisive PartitioningIn linguistics,information retrieval,and document clustering applications bi-nary taxonomies are very useful.Linear algebra methods,based on singular value decomposition(SVD)are used for this purpose in collaborativefilter-ing and information retrieval[26].Application of SVD to hierarchical divisive clustering of document collections resulted in the PDDP(Principal Direction Divisive Partitioning)algorithm[31].In our notations,object x is a docu-ment,l th attribute corresponds to a word(index term),and a matrix X entry x il is a measure(e.g.TF-IDF)of l-term frequency in a document x.PDDP constructs SVD decomposition of the matrix10Pavel Berkhin(a)Algorithm CURE (b)Algorithm CHAMELEONFig.1.Agglomeration in Clusters of Arbitrary Shapes(X −e ¯x ),¯x =1Ni =1:N x i ,e =(1,...,1)T .This algorithm bisects data in Euclidean space by a hyperplane that passes through data centroid orthogonal to the eigenvector with the largest singular value.A k -way split is also possible if the k largest singular values are consid-ered.Bisecting is a good way to categorize documents and it yields a binary tree.When k -means (2-means)is used for bisecting,the dividing hyperplane is orthogonal to the line connecting the two centroids.The comparative study of SVD vs.k -means approaches [191]can be used for further references.Hier-archical divisive bisecting k -means was proven [206]to be preferable to PDDP for document clustering.While PDDP or 2-means are concerned with how to split a cluster,the problem of which cluster to split is also important.Simple strategies are:(1)split each node at a given level,(2)split the cluster with highest cardinality,and,(3)split the cluster with the largest intra-cluster variance.All three strategies have problems.For a more detailed analysis of this subject and better strategies,see [192].2.3Other DevelopmentsOne of early agglomerative clustering algorithms,Ward’s method [222],is based not on linkage metric,but on an objective function used in k -means.The merger decision is viewed in terms of its effect on the objective function.The popular hierarchical clustering algorithm for categorical data COB-WEB [77]has two very important qualities.First,it utilizes incremental learn-ing.Instead of following divisive or agglomerative approaches,it dynamically builds a dendrogram by processing one data point at a time.Second,COB-WEB is an example of conceptual or model-based learning.This means that each cluster is considered as a model that can be described intrinsically,rather than as a collection of points assigned to it.COBWEB’s dendrogram is calleda classification tree.Each tree node(cluster)C is associated with the condi-tional probabilities for categorical attribute-values pairs,P r(x l=νlp|C),l=1:d,p=1:|A l|.This easily can be recognized as a C-specific Na¨ıve Bayes classifier.During the classification tree construction,every new point is descended along the tree and the tree is potentially updated(by an insert/split/merge/create op-eration).Decisions are based on the category utility[49]CU{C1,...,C k}=1j=1:kCU(C j)CU(C j)=l,p(P r(x l=νlp|C j)2−(P r(x l=νlp)2.Category utility is similar to the GINI index.It rewards clusters C j for in-creases in predictability of the categorical attribute valuesνlp.Being incre-mental,COBWEB is fast with a complexity of O(tN),though it depends non-linearly on tree characteristics packed into a constant t.There is a similar incremental hierarchical algorithm for all numerical attributes called CLAS-SIT[88].CLASSIT associates normal distributions with cluster nodes.Both algorithms can result in highly unbalanced trees.Chiu et al.[47]proposed another conceptual or model-based approach to hierarchical clustering.This development contains several different use-ful features,such as the extension of scalability preprocessing to categori-cal attributes,outliers handling,and a two-step strategy for monitoring the number of clusters including BIC(defined below).A model associated with a cluster covers both numerical and categorical attributes and constitutes a blend of Gaussian and multinomial models.Denote corresponding multivari-ate parameters byθ.With every cluster C we associate a logarithm of its (classification)likelihoodl C=x i∈Clog(p(x i|θ))The algorithm uses maximum likelihood estimates for parameterθ.The dis-tance between two clusters is defined(instead of linkage metric)as a decrease in log-likelihoodd(C1,C2)=l C1+l C2−l C1∪C2caused by merging of the two clusters under consideration.The agglomerative process continues until the stopping criterion is satisfied.As such,determina-tion of the best k is automatic.This algorithm has the commercial implemen-tation(in SPSS Clementine).The complexity of the algorithm is linear in N for the summarization phase.Traditional hierarchical clustering does not change points membership in once assigned clusters due to its greedy approach:after a merge or a split is selected it is not refined.Though COBWEB does reconsider its decisions,its。
SAEJ诊断故障代码定义

SAE Technical Standards Board Rules provide that: “This report is published by SAE to advance the state of technical and engineering sciences. The use of this report is entirely voluntary, and its applicability and suitability for any particular use, including any patent infringement arising therefrom, is the sole responsibility of the user.”SAE reviews each technical report at least every five years at which time it may be reaffirmed, revised, or cancelled. SAE invites your written comments and suggestions. Copyright ©2002 Society of Automotive Engineers, Inc.All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of SAE.TO PLACE A DOCUMENT ORDER:Tel: 877-606-7323 (inside USA and Canada)Tel: 724-776-4970 (outside USA)Fax: 724-776-0790Email: custsvc@TABLE OF CONTENTS1Scope (4)1.1Purpose (4)1.2Differences from ISO Document (4)2References (4)2.1Applicable Publications (4)2.1.1SAE Publications (4)2.1.2ISO Documents (4)2.2Related Publications (4)2.2.1SAE Publications (4)3Terms and Definitions (4)4General Specifications (7)5Format Structure (7)5.1Description (7)5.2ISO/SAE Controlled Codes (Core DTCs) (8)5.3Manufacturer Controlled Codes (Non-Uniform DTCs) (9)5.4Body System Groupings (9)5.4.1B0XXX ISO/SAE controlled (9)5.4.2B1XXX Manufacturer Controlled (9)5.4.3B2XXX Manufacturer Controlled (9)5.4.4B3XXX Reserved by Document (9)5.5Chassis System Groupings (9)5.5.1C0XXX ISO/SAE Controlled (9)5.5.2C1XXX Manufacturer Controlled (9)5.5.3C2XXX Manufacturer Controlled (9)5.5.4C3XXX Reserved by Document (9)5.6Powertrain System Groupings - DTC Numbers and Descriptions are Given in Appendix B (9)5.6.1P0XXX ISO/SAE controlled (9)5.6.2P1XXX manufacturer control (9)5.6.3P2XXX ISO/SAE controlled (9)5.6.4P3XXX Manufacturer Controlled and ISO/SAE Reserved (9)5.7Network Groupings - DTC Numbers and Descriptions are Given in Appendix C (9)5.7.1U0XXX ISO/SAE Controlled (9)5.7.2U1XXX Manufacturer Controlled (9)5.7.3U2XXX Manufacturer Controlled (9)5.7.4U3XXX Reserved (9)6Diagnostic Trouble Code Descriptions (10)7Change Requests (11)Appendix A (Normative) Diagnostic Trouble Code Naming Guidelines (12)A.1Discussion (12)Appendix B (Normative) Powertrain System Diagnostic Trouble Code (14)B.1P00XX Fuel and Air Metering and Auxiliary Emission Controls (14)B.2P01XX Fuel and Air Metering (16)B.3P02XX Fuel and Air Metering (19)B.4P03XX Ignition System or Misfire (21)B.5P04XX Auxiliary Emission Controls (23)B.6P05XX Vehicle Speed, Idle Control, and Auxiliary Inputs (26)B.7P06XX Computer and Auxiliary Outputs (28)B.8P07XX Transmission (31)B.9P08XX Transmission (33)B.10P09XX Transmission (36)B.11P0AXX Hybrid Propulsion (38)B.12P0BXX Reserved by Document (39)B.13P0CXX Reserved by Document (39)B.14P0DXX Reserved by Document (39)B.15P0EXX Reserved by Document (39)B.16P0FXX Reserved by Document (39)B.17P10XX Manufacturer Controlled Fuel and Air Metering and Auxiliary Emission Controls (39)B.18P11XX Manufacturer Controlled Fuel and Air Metering (39)B.19P12XX Manufacturer Controlled Fuel and Air Metering (39)B.20P13XX Manufacturer Controlled Ignition System or Misfire (39)B.21P14XX Manufacturer Controlled Auxiliary Emission Controls (39)B.22P15XX Manufacturer Controlled Vehicle Speed, Idle Control, and Auxiliary Inputs (39)B.23P16XX Manufacturer Controlled Computer and Auxiliary Outputs (39)B.24P17XX Manufacturer Controlled Transmission (39)B.25P18XX Manufacturer Controlled Transmission (39)B.26P19XX Manufacturer Controlled Transmission (39)B.27P20XX Fuel and Air Metering and Auxiliary Emission Controls (40)B.28P21XX Fuel and Air Metering and Auxiliary Emission Controls (42)B.29P22XX Fuel and Air Metering and Auxiliary Emission Controls (45)B.30P23XX Ignition System or Misfire (47)B.31P24XX Auxiliary Emission Controls (48)B.32P25XX Auxiliary Inputs (50)B.33P26XX Computer and Auxiliary Outputs (52)B.34P27XX Transmission (54)B.35P28XX ISO/SAE Reserved (56)B.36P2AXX Fuel and Air Metering and Auxiliary Emission Controls (56)B.37P30XX Fuel and Air Metering and Auxiliary Emission Controls (56)B.38P31XX Fuel and Air Metering and Auxiliary Emission Controls (56)B.39P32XX Fuel and Air Metering and Auxiliary Emission Controls (56)B.40P33XX Ignition System or Misfire (56)B.41P34XX Cylinder Deactivation (56)B.42P35XX ISO/SAE Reserved (59)B.43P36XX ISO/SAE Reserved (59)B.44P37XX ISO/SAE Reserved (59)B.45P38XX ISO/SAE Reserved (59)B.46P39XX ISO/SAE Reserved (59)Appendix C(Normative) Network Communication Groupings (60)C.1U00XX Network Electrical (60)C.2U01XX Network Communication (62)C.3U02XX Network Communication (65)C.4U03XX Network Software (66)C.5U04XX Network Data (67)3.2Range/Performance—Circuit is in the normal operating range, but not correct for current operating conditions,it may be used to indicate stuck or skewed values indicating poor performance of a circuit, component, or system.3.3Low Input—Circuit voltage, frequency, or other characteristic measured at the control module input terminal orpin that is below the normal operating range.3.4High Input—Circuit voltage, frequency, or other characteristic measured at the control module input terminalor pin that is above the normal operating range.3.5Bank—Specific group of cylinders sharing a common control sensor, bank 1 always contains cylinder number1, bank 2 is the opposite bankNOTE—If there is only one bank, use bank #1 DTCs and the word bank may be omitted. With a single “bank”system using multiple sensors, use bank #1.3.6Sensor Location—Location of a sensor in relation the engine air flow, starting from the fresh air intakethrough to the vehicle tailpipe or fuel flow from the fuel tank to the engine in order numbering 1,2,3 and so on NOTE—See Figures 1 to 4.FIGURE 1—V6/V8/V12 CYLINDER ENGINE WITH 2 EXHAUST BANKS AND 4 CATALYSTS EXAMPLEFIGURE 2—V6/V8/V12 CYLINDER ENGINE WITH 2 EXHAUST BANKS AND 3 CATALYSTS EXAMPLEFIGURE 3—L4/L5/L6 CYLINDER ENGINE WITH 1 EXHAUST BANK AND 2 CATALYSTS EXAMPLEFIGURE 4—L4/L5/L6 CYLINDER ENGINE WITH 1 EXHAUST BANK AND 1 CATALYST EXAMPLEFIGURE 5—STRUCTURE OF DIAGNOSTIC TROUBLE CODESEXAMPLEThe data bus value $9234 would be displayed to technicians as the manufacturer controlled body code B1234, see the figure below.FIGURE 6—EXAMPLE OF TROUBLE CODE STRUCTURECodes have been specified to indicate a suspected trouble or problem area and are intended to be used as a directive to the proper service procedure. To minimize service confusion, fault codes should not be used to indicate the absence of problems or the status of parts of the system (e.g., powertrain system O.K., or MIL activated), but should be confined to indicate areas in need of service attention.Some ranges have been expanded beyond 100 numbers by using the hexadecimal base 16 number system. ISO/SAE Controlled Codes (Core DTCs)—ISO/SAE controlled diagnostic trouble codes are those codes where industry uniformity has been achieved. These codes were felt to be common enough across most manufacturers' applications that a common number and fault message could be assigned. All unspecified numbers in each grouping have been reserved for future growth. Although service procedures may differ widely amongst manufacturers, the fault being indicated is common enough to be assigned a particular fault code. Codes in this area are not to be used by manufacturers until they have been approved by ISO/SAE.5.3Manufacturer Controlled Codes (Non-Uniform DTCs)—Areas within each alpha designator have beenmade available for manufacturer-controlled DTCs. These are fault codes that will not generally be used by a majority of the manufacturers due to basic system differences, implementation differences, or diagnostic strategy differences. Each vehicle manufacturer or supplier who designs and specifies diagnostic algorithms, software, and diagnostic trouble codes are strongly encouraged to remain consistent across their product line when assigning codes in the manufacturer controlled area. For powertrain codes, the same groupings should be used as in the ISO /SAE controlled area, i.e., 100's and 200's for fuel and air metering, 300's for ignition system or misfire, etc.Code groupings for non-powertrain codes will be specified at a later date.While each manufacturer has the ability to define the controlled DTCs to meet their specific controller algorithms, all DTC words shall meet SAE J1930.5.4Body System Groupings5.4.1B0XXX ISO/SAE C O NTROLLED5.4.2B1XXX M ANUFACTURER C ONTROLLED5.4.3B2XXX M ANUFACTURER C ONTROLLED5.4.4B3XXX R ES ERVE D BY D O CUMENT5.5Chassis System Groupings5.5.1C0XXX ISO/SAE C ONTRO LLE D5.5.2C1XXX M ANUFACTURER C O NTROLLED5.5.3C2XXX M ANUFACTURER C O NTROLLED5.5.4C3XXX R ESERVED BY D O CUMENT5.6Powertrain System Groupings—DTC Numbers and Cescriptions are Given in Appendix B5.6.1P0XXX ISO/SAE C O NTROLLED5.6.2P1XXX M ANUFACTURER C ONTROL5.6.3P2XXX ISO/SAE C O NTROLLED5.6.4P3XXX M ANUFACTURER C ONTROLLED A ND ISO/SAE R ES ERV ED5.7Network Groupings—DTC Numbers and Descriptions are given in Appendix C5.7.1U0XXX ISO/SAE C ONTRO LLE D5.7.2U1XXX M ANUFACTURER C O NTROLLED5.7.3U2XXX M ANUFACTURER C O NTROLLED5.7.4U3XXX R ESERVED6.Diagnostic Trouble Code Descriptions—Each specified fault code has been assigned a description toindicate the circuit, component or system area that was determined to be at fault. The descriptions are organized such that different descriptions related to a particular sensor or system are grouped together. In cases where there are various fault descriptions for different types of faults, the group also has a “generic”description as the first code/message of the group. A manufacturer has a choice when implementing diagnostics, based on the specific strategy and complexity of the diagnostic.Where more specific fault descriptions for a circuit, component, or system exist, the manufacturer should choose the code most applicable to their diagnosable fault. The descriptions are intended to be somewhat general to allow manufacturers to use them as often as possible yet still not conflict with their specific repair procedures. The terms “low” and “high” when used in a description, especially those related to input signals, refer to the voltage, frequency, etc. at the pin of the controller. The specific level of “low” and “high” shall be specified by each manufacturer to best meet their needs.For example, in diagnosing a 5 V reference Throttle Position Sensor (TP Sensor), if the input signal at the Powertrain Control Module (PCM) is stuck at near 0 V, a manufacturer has the flexibility to select from either of two codes - P0120 (Throttle/Pedal Position Sensor/Switch A Circuit) or P0122 (Throttle/Pedal Position Sensor/ Switch A Circuit Low Input), depending on the manufacturer's diagnostic procedures. If the input signal at the PCM is stuck at near 5 V, a manufacturer has the flexibility to select from either of two codes - P0120 (Throttle/ Pedal Position Sensor/Switch A Circuit) or P0123 (Throttle/Pedal Position Sensor/Switch A Circuit High Input), depending on the manufacturer's diagnostic procedures. If the input signal at the PCM is stuck at 1.5 V at idle instead of the expected 1.0 V, the manufacturer has the flexibility to select from either of two codes - P0120 (Throttle/Pedal Position Sensor/Switch A Circuit) or P0121 (Throttle/Pedal Position Sensor/Switch A Circuit Range/Performance Problem), depending on the manufacturer's diagnostic procedures. The root cause of the higher than expected TP Sensor voltage may be either a faulty TP Sensor, corrosion in the TP Sensor connections or an improperly adjusted throttle plate. Identification of the root cause is done using the diagnostic procedures and is not implied by the DTC message, thus allowing the manufacturer the flexibility in assigning DTCs Change requests.7.Change Requests—Use this form to pass your request.Request Form for New SAE J2012 SAE Controlled DTCWhat is the purpose of the component, circuit, or system?Example: Exhaust Gas Recirculation.What is the purpose of the diagnostic?Example: detect low EGR flowRequested Group NumberRequested DTC NumberRequested DTC NomenclatureExample: EGR Low Flow DetectedRequested by:Phone/FaxEmailAddressDate:Please send completed form(s) to:SAE Headquarters755 West Big Beaver RoadSuite 1600Troy, MI 48084USAATTN: SAE J2012 PowertrainCommittee Chairman8.Notes8.1Marginal Indicia—The change bar (l) located in the left margin is for the convenience of the user in locatingareas where technical revisions have been made to the previous issue of the report. An (R) symbol to the left of the document title indicates a complete revision of the report.PREPARED BY THE SAE VEHICLE ELECTRICAL AND ELECTRONICS DIAGNOSTICSYSTEMS STANDARDS COMMITTEEAPPENDIX A(NORMATIVE)DIAGNOSTIC TROUBLE CODE NAMING GUIDELINESA.1Discussion—The following Table A1 is a guideline used to help in determining DTC descriptions. Appendix Bshows applications for recommended industry common trouble codes for the powertrain control system. These include systems that might be integrated into an electronic control module that would be used for controlling engine functions, such as fuel, spark, idle speed, and vehicle speed (cruise control) as well as those for transmission control. The fact that a code is recommended as a common industry code does not imply that it isa required code (legislated), an emission related code, nor that it indicates a fault that will cause themalfunction indicator to be illuminated.TABLE A1—DTC NAMING GUIDELINES FOR SIGNALS FROM COMPONENTSComponent/System SAE J19301)AcronymSAEJ19301)Modifier(if used) 1)Noun Name1Circuit1)Intermittent(if used) 1)State(if used) 1)Parameter(if used) 1)Location(if used) 1)Throttle Position TP Sensor Circuit Low Voltage Throttle Position TP Sensor Circuit PerformanceManifold AbsolutePressureMAP Sensor Circuit High VoltageEngine CoolantTemperatureECT Sensor Circuit Low Voltage Intake Air Temperature IAT Sensor Circuit High Voltage Vehicle Speed Sensor VSS included inacronymCircuit High VoltageVehicle Speed Sensor VSS included inacronymCircuit IntermittentHeated Oxygen Sensor HO2S included inAcronymHeaterCircuitHeated Oxygen Sensor HO2S included inAcronymHeater Circuit Low Voltage Bank (B1)Sensor 1 (S1)Idle Air Control IAC Valve Circuit Low VoltageMass Air Flow MAF Sensor Circuit High FrequencyMass Air Flow MAF Sensor Circuit PerformanceKnock Sensor KS included inacronymModule SensorCircuit Bank 1Knock Sensor KS included inacronymModule SensorCircuit PerformanceCrankshaft Position CKP Sensor CircuitEvaporative Emissions EVAP CanisterPurgeValve CircuitEngine Speed RPM Input CircuitAir Conditioning A/C ClutchStatusN/A Circuit Low VoltageHeated Oxygen Sensor HO2S Circuit TransitionTime Ratio Bank 1 (B1) Sensor (S1)Heated Oxygen Sensor HO2S Circuit Insufficient SwitchingBank 1 (B1)Sensor 1 (S1)Distributor Ignition DI Low ResolutionCircuitIntermittentDistributor IgnitionDIHigh Resolution CircuitNOTE 1) The Service Information uses Component/System from SAE J1930 or Acronym from SAE J1930, Modifier, Noun Name, Circuit, Intermittent, State, Parameter, and Location.TABLE A2—DTC NAMING GUIDELINES FOR SIGNALS TO COMPONENTSComponent/SystemSAE J19301)Acronym SAE J19301)Modifier (if used)1)Noun Name1)Control 1)Circuit 1)Intermittent (if used) 1)State (if used)1)Parameter (if used) 1)Location (if used) 1)Malfunction Indicator lamp MIL included in acronymControl Circuit Injector N/A Control Circuit Fan Control FC Relay 1Control Circuit Fan Control FC Relay 2Control Circuit Low Exhaust Gas RecirculationEGR Solenoid Control Circuit High Secondary Air Injection AIR Solenoid Control Circuit HighEvaporative Emissions EVAP Purge Solenoid Control Circuit Air Conditioning A/C ClutchRelay Control Circuit Idle Air Control IAC Valve Control Circuit Low Ignition Control IC N/A included in acronym Circuit Low Voltage Ignition ControlIC N/A included in acronym Circuit High VoltageTorque Converter ClutchTCCSolenoidControlCircuitStuck onNOTE 1) The Service Information uses Component/System from SAE J1930 or Acronym from SAE J1930, Modifier, Noun Name, Circuit, Intermittent, State, Parameter, and Location.TABLE A3—DTC NAMING GUIDELINES INVOLVING SEVERAL COMPONENTS OR SYSTEMSComponent/SystemSAE J19301)Acronym SAE J19301)Modifier 1)System 1)Intermittent 1)State 1)Parameter 1)Location 1)Exhaust Gas Recirculation EGR System Fuel TrimFT System LeanBank 1Secondary Air InjectionAIRSystemBank 1NOTE 1) The Service Information uses Component/System from SAE J1930 or Acronym from SAE J1930, Modifier, Noun Name, Circuit, Intermittent, State, Parameter, and Location.TABLE A1—DTC NAMING GUIDELINES FOR SIGNALS FROM COMPONENTSComponent/SystemSAE J19301)Acronym SAEJ19301)Modifier (if used) 1)Noun Name 1Circuit1)Intermittent (if used) 1)State (if used) 1)Parameter (if used) 1)Location (if used) 1)APPENDIX B(NORMATIVE)POWERTRAIN SYSTEM DIAGNOSTIC TROUBLE CODEB.1P00XX Fuel and Air Metering and Auxiliary Emission ControlsTABLE B1—P00XX FUEL AND AIR METERING AND AUXILIARY EMISSION CONTROLS DTC number DTC naming Location P0001 Fuel Volume Regulator Control Circuit/OpenP0002 Fuel Volume Regulator Control Circuit Range/PerformanceP0003 Fuel Volume Regulator Control Circuit LowP0004 Fuel Volume Regulator Control Circuit HighP0005 Fuel Shutoff Valve "A" Control Circuit/OpenP0006 Fuel Shutoff Valve "A" Control Circuit LowP0007 Fuel Shutoff Valve "A" Control Circuit HighP0008 Engine Position System Performance Bank 1 P0009 Engine Position System Performance Bank 2 P0010 a) "A" Camshaft Position Actuator Circuit Bank 1 P0011 a) "A" Camshaft Position - Timing Over-Advanced or System Performance Bank 1 P0012 a) "A" Camshaft Position - Timing Over-Retarded Bank 1 P0013 b) "B" Camshaft Position - Actuator Circuit Bank 1 P0014 b) "B" Camshaft Position - Timing Over-Advanced or System Performance Bank 1 P0015 b) "B" Camshaft Position - Timing Over-Retarded Bank 1 P0016 Crankshaft Position – Camshaft Position Correlation Bank 1 Sensor A P0017 Crankshaft Position – Camshaft Position Correlation Bank 1 Sensor B P0018 Crankshaft Position – Camshaft Position Correlation Bank 2 Sensor A P0019 Crankshaft Position – Camshaft Position Correlation Bank 2 Sensor B P0020 a) "A" Camshaft Position Actuator Circuit Bank 2 P0021 a) "A" Camshaft Position - Timing Over-Advanced or System Performance Bank 2 P0022 a) "A" Camshaft Position - Timing Over-Retarded Bank 2 P0023 b) "B" Camshaft Position - Actuator Circuit Bank 2 P0024 b) "B" Camshaft Position - Timing Over-Advanced or System Performance Bank 2 P0025 b) "B" Camshaft Position - Timing Over-Retarded Bank 2 P0026 Intake Valve Control Solenoid Circuit Range/Performance Bank 1 P0027 Exhaust Valve Control Solenoid Circuit Range/Performance Bank 1 P0028 Intake Valve Control Solenoid Circuit Range/Performance Bank 2 P0029 Exhaust Valve Control Solenoid Circuit Range/Performance Bank 2 P0030 HO2S Heater Control Circuit Bank 1 Sensor 1 P0031 HO2S Heater Control Circuit Low Bank 1 Sensor 1 P0032 HO2S Heater Control Circuit High Bank 1 Sensor 1 P0033 Turbo Charger Bypass Valve Control CircuitP0034 Turbo Charger Bypass Valve Control Circuit LowP0035 Turbo Charger Bypass Valve Control Circuit HighP0036 HO2S Heater Control Circuit Bank 1 Sensor 2TABLE B1—P00XX FUEL AND AIR METERING AND AUXILIARY EMISSION CONTROLS (CONTINUED) DTC number DTC naming Location P0037 HO2S Heater Control Circuit Low Bank 1 Sensor 2 P0038 HO2S Heater Control Circuit High Bank 1 Sensor 2 P0039 Turbo/Super Charger Bypass Valve Control Circuit Range/PerformanceP0040 O2 Sensor Signals Swapped Bank 1 Sensor 1/ Bank 2 Sensor 1P0041 O2 Sensor Signals Swapped Bank 1 Sensor 2/ Bank 2 Sensor 2P0042 HO2S Heater Control Circuit Bank 1 Sensor 3 P0043 HO2S Heater Control Circuit Low Bank 1 Sensor 3 P0044 HO2S Heater Control Circuit High Bank 1 Sensor 3 P0045 Turbo/Super Charger Boost Control Solenoid Circuit/OpenP0046 Turbo/Super Charger Boost Control Solenoid Circuit Range/PerformanceP0047 Turbo/Super Charger Boost Control Solenoid Circuit LowP0048 Turbo/Super Charger Boost Control Solenoid Circuit HighP0049 Turbo/Super Charger Turbine OverspeedP0050 HO2S Heater Control Circuit Bank 2 Sensor 1 P0051 HO2S Heater Control Circuit Low Bank 2 Sensor 1 P0052 HO2S Heater Control Circuit High Bank 2 Sensor 1 P0053 HO2S Heater Resistance Bank 1 Sensor 1 P0054 HO2S Heater Resistance Bank 1 Sensor 2 P0055 HO2S Heater Resistance Bank 1 Sensor 3 P0056 HO2S Heater Control Circuit Bank 2 Sensor 2 P0057 HO2S Heater Control Circuit Low Bank 2 Sensor 2 P0058 HO2S Heater Control Circuit High Bank 2 Sensor 2 P0059 HO2S Heater Resistance Bank 2 Sensor 1 P0060 HO2S Heater Resistance Bank 2 Sensor 2 P0061 HO2S Heater Resistance Bank 2 Sensor 3 P0062 HO2S Heater Control Circuit Bank 2 Sensor 3 P0063 HO2S Heater Control Circuit Low Bank 2 Sensor 3 P0064 HO2S Heater Control Circuit High Bank 2 Sensor 3 P0065 Air Assisted Injector Control Range/PerformanceP0066 Air Assisted Injector Control Circuit or Circuit LowP0067 Air Assisted Injector Control Circuit HighP0068 MAP/MAF – Throttle Position CorrelationP0069 Manifold Absolute Pressure – Barometric Pressure CorrelationP0070 Ambient Air Temperature Sensor CircuitP0071 Ambient Air Temperature Sensor Range/PerformanceP0072 Ambient Air Temperature Sensor Circuit LowP0073 Ambient Air Temperature Sensor Circuit HighP0074 Ambient Air Temperature Sensor Circuit IntermittentP0075 Intake Valve Control Solenoid Circuit Bank 1 P0076 Intake Valve Control Solenoid Circuit Low Bank 1 P0077 Intake Valve Control Solenoid Circuit High Bank 1 P0078 Exhaust Valve Control Solenoid Circuit Bank 1 P0079 Exhaust Valve Control Solenoid Circuit Low Bank 1TABLE B1—P00XX FUEL AND AIR METERING AND AUXILIARY EMISSION CONTROLS (CONTINUED) DTC number DTC naming Location P0080 Exhaust Valve Control Solenoid Circuit High Bank 1P0081 Intake Valve Control Solenoid Circuit Bank 2P0082 Intake Valve Control Solenoid Circuit Low Bank 2P0083 Intake Valve Control Solenoid Circuit High Bank 2P0084 Exhaust Valve Control Solenoid Circuit Bank 2P0085 Exhaust Valve Control Solenoid Circuit Low Bank 2P0086 Exhaust Valve Control Solenoid Circuit High Bank 2P0087 Fuel Rail/System Pressure - Too LowP0088 Fuel Rail/System Pressure - Too HighP0089 Fuel Pressure Regulator 1 PerformanceP0090 Fuel Pressure Regulator 1 Control CircuitP0091 Fuel Pressure Regulator 1 Control Circuit LowP0092 Fuel Pressure Regulator 1 Control Circuit HighP0093 Fuel System Leak Detected – Large LeakP0094 Fuel System Leak Detected – Small LeakP0095 Intake Air Temperature Sensor 2 CircuitP0096 Intake Air Temperature Sensor 2 Circuit Range/PerformanceP0097 Intake Air Temperature Sensor 2 Circuit LowP0098 Intake Air Temperature Sensor 2 Circuit HighP0099 Intake Air Temperature Sensor 2 Circuit Intermittent/Erratica)The "A" camshaft shall be either the "intake," "left," or "front" camshaft. Left/Right and Front/Rear are determined as if viewed from the driver'sseating position. Bank 1 contains cylinder number one, Bank 2 is the opposite bank.b)The "B" camshaft shall be either the "exhaust," "right," or "rear" camshaft. Left/Right and Front/Rear are determined as if viewed from thedriver's seating position. Bank 1 contains cylinder number one, Bank 2 is the opposite bank.B.2P01XX Fuel and Air MeteringTABLE B2—P01XX FUEL AND AIR METERINGDTC number DTC naming Location P0100 Mass or Volume Air Flow CircuitP0101 Mass or Volume Air Flow Circuit Range/PerformanceP0102 Mass or Volume Air Flow Circuit Low InputP0103 Mass or Volume Air Flow Circuit High InputP0104 Mass or Volume Air Flow Circuit IntermittentP0105 Manifold Absolute Pressure/Barometric Pressure CircuitP0106 Manifold Absolute Pressure/Barometric Pressure Circuit Range/PerformanceP0107 Manifold Absolute Pressure/Barometric Pressure Circuit Low InputP0108 Manifold Absolute Pressure/Barometric Pressure Circuit High InputP0109 Manifold Absolute Pressure/Barometric Pressure Circuit IntermittentP0110 Intake Air Temperature Sensor 1 CircuitP0111 Intake Air Temperature Sensor 1 Circuit Range/PerformanceP0112 Intake Air Temperature Sensor 1 Circuit LowP0113 Intake Air Temperature Sensor 1 Circuit HighP0114 Intake Air Temperature Sensor 1 Circuit IntermittentTABLE B2—P01XX FUEL AND AIR METERING (CONTINUED)DTC number DTC naming Location P0115 Engine Coolant Temperature CircuitP0116 Engine Coolant Temperature Circuit Range/PerformanceP0117 Engine Coolant Temperature Circuit LowP0118 Engine Coolant Temperature Circuit HighP0119 Engine Coolant Temperature Circuit IntermittentP0120 Throttle/Pedal Position Sensor/Switch "A" CircuitP0121 Throttle/Pedal Position Sensor/Switch "A" Circuit Range/PerformanceP0122 Throttle/Pedal Position Sensor/Switch "A" Circuit LowP0123 Throttle/Pedal Position Sensor/Switch "A" Circuit HighP0124 Throttle/Pedal Position Sensor/Switch "A" Circuit IntermittentP0125 Insufficient Coolant Temperature for Closed Loop Fuel ControlP0126 Insufficient Coolant Temperature for Stable OperationP0127 Intake Air Temperature Too HighP0128 Coolant Thermostat (Coolant Temperature Below Thermostat Regulating Temperature)P0129 Barometric Pressure Too LowP0130 O2 Sensor Circuit Bank 1 Sensor 1 P0131 O2 Sensor Circuit Low Voltage Bank 1 Sensor 1 P0132 O2 Sensor Circuit High Voltage Bank 1 Sensor 1 P0133 O2 Sensor Circuit Slow Response Bank 1 Sensor 1 P0134 O2 Sensor Circuit No Activity Detected Bank 1 Sensor 1 P0135 O2 Sensor Heater Circuit Bank 1 Sensor 1 P0136 O2 Sensor Circuit Bank 1 Sensor 2 P0137 O2 Sensor Circuit Low Voltage Bank 1 Sensor 2 P0138 O2 Sensor Circuit High Voltage Bank 1 Sensor 2 P0139 O2 Sensor Circuit Slow Response Bank 1 Sensor 2 P0140 O2 Sensor Circuit No Activity Detected Bank 1 Sensor 2 P0141 O2 Sensor Heater Circuit Bank 1 Sensor 2 P0142 O2 Sensor Circuit Bank 1 Sensor 3 P0143 O2 Sensor Circuit Low Voltage Bank 1 Sensor 3 P0144 O2 Sensor Circuit High Voltage Bank 1 Sensor 3 P0145 O2 Sensor Circuit Slow Response Bank 1 Sensor 3 P0146 O2 Sensor Circuit No Activity Detected Bank 1 Sensor 3 P0147 O2 Sensor Heater Circuit Bank 1 Sensor 3 P0148 Fuel Delivery ErrorP0149 Fuel Timing ErrorP0150 O2 Sensor Circuit Bank 2 Sensor 1 P0151 O2 Sensor Circuit Low Voltage Bank 2 Sensor 1 P0152 O2 Sensor Circuit High Voltage Bank 2 Sensor 1 P0153 O2 Sensor Circuit Slow Response Bank 2 Sensor 1 P0154 O2 Sensor Circuit No Activity Detected Bank 2 Sensor 1 P0155 O2 Sensor Heater Circuit Bank 2 Sensor 1 P0156 O2 Sensor Circuit Bank 2 Sensor 2 P0157 O2 Sensor Circuit Low Voltage Bank 2 Sensor 2。
EVS_EN_16454_2015_en_preview

© 2015 CEN All rights of exploitation in any form and by any means reserved worldwide for CEN national Members.
Ref. No. EN 16454:2015 E
EENVS1-6E4N5146:240541:520(1E5)
This document is a preview generated by EVS
EUROPEAN STANDARD NORME EUROPÉEN240.60
EN 16454
September 2015
Supersedes CEN/TS 16454:2013
Euroopa standardi EN 16454:2015 ingliskeelset consists of the English text of the European
teksti.
standard EN 16454:2015.
Standard on jõustunud sellekohase teate This standard has been endorsed with a
EUROPEAN COMMITTEE FOR STANDARDIZATION COMITÉ EUROPÉEN DE NORMALISATION EUROPÄISCHES KOMITEE FÜR NORMUNG
CEN-CENELEC Management Centre: Avenue Marnix 17, B-1000 Brussels
This document is a preview generated by EVS
Contents
Page
术中低体温发生原因及预防进展

术中低体温发生原因及预防进展发表时间:2018-07-05T14:10:12.793Z 来源:《医药前沿》2018年6月第18期作者:申文冬旷文娟[导读] 体温是人体重要的生理指标之一,常恒定在36.2~37.5℃,临床上将人体核心体温低于36℃时称为低体温[1],是临床手术的常见并发症。
(中山大学附属第三医院手术麻醉中心广东广州 510000)【摘要】综述了手术患者术中低体温的发生原因及预防进展,主要影响因素包括:麻醉药物影响,手术室环境,输血输液,手术因素及自身因素等;预防措施包括:监测体温,调节手术室温度,被动加温,主动加温,液体加温及预保温等。
【关键词】低体温;护理;预防【中图分类号】R614 【文献标识码】A 【文章编号】2095-1752(2018)18-0021-03体温是人体重要的生理指标之一,常恒定在36.2~37.5℃,临床上将人体核心体温低于36℃时称为低体温[1],是临床手术的常见并发症。
有文献报道,患者在手术期间核心体温会下降1~3℃[2],其发生率可高达50%~70%[3,4]。
术中低体温可使凝血功能发生异常、诱发心血管突发事件、抑制免疫功能、感染率增加[5,6]等;同时可引起病人疼痛和不适[7],导致麻醉苏醒延迟,延长出院时间[8]等,最终影响患者的手术安全和预后。
因此,术中体温的保护越来越受到医护人员的关注。
现将手术患者术中低体温的发生原因及预防进展综述如下。
1.术中低体温的原因1.1 麻醉药物影响全麻药物抑制温度调节中枢,使散热增加,同时产热减少,是大多数患者发生低体温的主要原因。
1.2 手术室环境正常层流洁净手术室的温度一般在22~24℃,这样可以为术者提供舒适的工作环境,但手术患者常常会感觉寒冷不适,手术室常规温度和室内空气快速对流这两个因素容易导致低体温。
有研究显示[9],当手术室温度低于21℃,时间超过3h,患者极易出现低体温。
1.3 输血输液术中输入大量未经加热的液体,特别是输入大量库存血时,体温会出现明显下降。
Gumbel分布函数与梯度的R包说明书

Package‘dgumbel’October13,2022Type PackageTitle The Gumbel Distribution Functions and GradientsVersion1.0.1Date2020-04-07Maintainer BerentÅnund Strømnes Lunde<*********************> Description Gumbel distribution functions(De Haan L.(2007)<doi:10.1007/0-387-34471-3>)implemented with the techniques of automatic differentiation(Griewank A.(2008)<isbn:978-0-89871-659-7>).With this tool,a user should be able to quickly model extremeevents for which the Gumbel distribution is the domain of attraction.The package makes available the density function,the distributionfunction the quantile function and a random generating function.Inaddition,it supports gradient functions.The package combines'Adept'(C++templated automatic differentiation)(Hogan R.(2017)<doi:10.5281/zenodo.1004730>)and'Eigen'(templated matrix-vectorlibrary)for fast computations of both objective functions and exactgradients.It relies on'RcppEigen'for easy access to'Eigen'andbindings to R.License GPL(>=2)URL https:///blunde1/dgumbelBugReports https:///blunde1/dgumbel/issuesEncoding UTF-8Imports Rcpp(>=1.0.2)LinkingTo Rcpp,RcppEigenRoxygenNote6.1.1NeedsCompilation yesAuthor BerentÅnund Strømnes Lunde[aut,cre,cph],Robin Hogan[ctb](Author of included Adept library),The University of Reading[cph](Copyright holder of included Adeptlibrary)Repository CRANDate/Publication2020-04-1621:00:03UTC1R topics documented:gumbel (2)Index4 gumbel The Gumbel Distribution and DerivativesDescriptionDensity function,distribution function,quantile function and random generation,and their gradient functions for the Gumbel distribution with location and scale parameters.Usagedgumbel(x,location=0,scale=1,log=FALSE,grad=FALSE)pgumbel(q,location=0,scale=1,lower.tail=TRUE,log.p=FALSE,grad=FALSE) qgumbel(p,location=0,scale=1,lower.tail=TRUE,grad=FALSE)rgumbel(n,location=0,scale=1)Argumentsx,q Vector of quantiles.p Vector of probabilities.n Number of observations.location,scaleLocation and scale parameters.log,log.p Logical;if TRUE,probabilities p are given as log(p).lower.tail Logical;if TRUE(default),probabilities are P[X<=x],otherwise,P[X>x] grad Logical;if TRUE,the gradient w.r.t.parameters location and scale is given instead of function value.DetailsThe Gumbel distribution function with parameters location=a and scale=b isG(z)=exp−exp−z−abfor all real z,where b>0.Gradients are exact numerical derivatives implemented using automatic differentiation.dgumbel builds on the Eigen linear algebra library,Adept for automatic differenti-ation and RcppEigen for bindings to R and loading Eigen.Valuedgumbel gives the density function,pgumbel gives the distribution function,qgumbel gives the quantile function,and rgumbel generates random deviates.If grad=TRUE is supplied,then the gradient is returned instead of the objective function.Examplesdgumbel(-1:2,-1,0.5)pgumbel(-1:2,-1,0.5)qgumbel(seq(0.9,0.6,-0.1),2,0.5)rgumbel(6,-1,0.5)p<-(1:9)/10pgumbel(qgumbel(p,-1,2),-1,2)##[1]0.10.20.30.40.50.60.70.80.9##Random number generationloc=.5scale=3.2n<-1000x<-rgumbel(n,loc,scale)##The densityhist(x,freq=FALSE)xs<-sort(x)fx<-dgumbel(xs,loc,scale)points(xs,fx,type="l",col=2,lwd=2)##The distributionedf<-sapply(xs,function(x){sum(xs<=x)/n})plot(xs,edf)Fx<-pgumbel(xs,loc,scale)points(xs,Fx,type="l",col=2,lwd=2)##The quantile functionq<-qgumbel(0.6,loc,scale)polygon(c(xs[xs<=q],q),c(Fx[xs<=q],0),col=3)##Negative log likelihood:Objective and gradientnll<-function(par,data)-sum(dgumbel(data,par[1],par[2],log=TRUE))dnll<-function(par,data)-rowSums(dgumbel(data,par[1],par[2],log=TRUE,grad=TRUE)) ##Parameter estimationpar_start<-c(3,1)opt<-nlminb(par_start,objective=nll,gradient=dnll,data=x,control=list(trace=5)) opt$convergenceopt$parIndex∗distributiongumbel,2dgumbel(gumbel),2gumbel,2pgumbel(gumbel),2qgumbel(gumbel),2rgumbel(gumbel),24。
Guide to Mitrefnch Skills Matrix说明书

Guide To Mitre nch Skills MatrixContents/contact-us /contact-us1 Mitre nch Skills Matrix 31.1 What is it? 31.2 What is it used for? 31.3 How does it work? 41 . Mitre nch Skills Matrix1.1 What is it?The Mitre nch Skills Matrix is an enhanced Resource Planning function available in the Mitre nch Time Management System.This quick and easy‐to‐use tool assists with the management, control and monitoring of skills levels within your organization.The Mitre nch Skills Matrix displays all current team members and the skills that have been assigned to them.1.2 What is it used for?The Mitre nch skills Matrix can:• Examine what your strengths and weaknesses are• Identify speci c training needs• Quickly identify available skills and possible future requirements• Organize adequate cover for vacation and sick leaveIn addition, when used in conjunction with the Mitre nch Future Work Planner and Group Rules within the Time Management System, the Mitr nch Skills Matrix allows managers to plan workforce availability based on sta skills.Skills can also be linked to the real‐time Onsite List, enabling managers/supervisors to clearly see who is onsite at a given time/contact-us/contact-us1.3 How does it work?Within the Mitre nch Time Management System, once you select Mitre nch Skills Matrix from the Group menu, you will see a list of all the members of your selected department, showing the skills that have been assigned to them (e.g. First Aid practitioners).You can still update any skills that an employee has acquired by ticking the relevant box and then clicking Apply. The total number of people in the department credited with each skill is shown at the bottom of the table./contact-us /contact-us With the Mitre nch Time Management System you can now link this to training records so that when you double click on a skill it will take you through to the associated training panel as well as adding a training record against that employee which you can edit.You can also customize the appearance of the skills matrix to show any value from the training record in the grid.Skills can be viewed at point in time e.g. who from the current rst aiders will still have his certi cate valid in January next year.To conduct an in‐depth assessment of your workforceskills you can use the arrow buttons to select the skillsfor which you wish to search in the relevant group.You can change the range of your search to the entiredatabase or a speci c group via the drop‐down list atthe bottom./contact-us/contact-usSAPPI Fine Paper Township of Brick Friendly’s Ice CreamLahey HealthBehavioral SciencesCountry - WideInsurance CompanyL&T InfotechWadhams Enterprises, Inc.Finger LakesMedical AssociatesChemprene MamaMia Produce LLCDavid E ZellerInsurance AgencyEnterasys NYU Hospitals Center A Selection of our customersChoice Logistics Heritage Automative Group/contact-us/contact-usAbout Mitre nchToday, Mitre nch products comprise an innovative collection of tools that can be con gured to suit an organization’s exact workforce management needs. Whether you have one particular goal to meet, or whether you are looking for a turnkey system designed to fully integrate all aspects of your organization’s workforce management, Mitre nch delivers.Mitre nch products includes:• Time & Attendance Systems• Employee Scheduling• Cloud Hosted Solutions• Absence Management• HR Management Software• Employee Self Service• Biometric Systems• Mobile Phone Clocking‐In• Performance Management• Auto ID Card Production• Fire Evacuation• Visitor ManagementContact details:Boston79A Chapel StreetNewton, MA 02458New York104 West 40th Street, Suite 400 New York, NY 10018Toll Free: (888) 238-8704Email: info@mitre Web Address: www.mitre Dallas9330 LBJ Freeway, Suite 900 Dallas, TX 75243La Jolla O ce888 Prospect Street, Suite 200 La Jolla, CA 92037。
Kaplan-Meier曲线的绘制步骤(八)

Kaplan-Meier曲线是生存分析中常用的一种图表,它可以用来描述患者在一定时间内生存的概率,是生存分析中不可或缺的工具。
本文将介绍Kaplan-Meier曲线的绘制步骤,帮助读者更好地理解和运用这一方法。
数据收集和整理绘制Kaplan-Meier曲线的第一步是收集患者的生存数据。
这些数据通常包括患者的入组时间、随访时间和事件发生情况(比如死亡或复发)。
在收集数据时,需要确保数据的准确性和完整性,避免出现漏报或错误的情况。
同时,还需要对收集到的数据进行整理和清洗,排除掉不完整或不合理的数据,保证数据的质量。
计算生存曲线在收集和整理好数据之后,就可以开始计算Kaplan-Meier曲线了。
首先要计算每个时间点的生存概率。
这可以通过Kaplan-Meier方法来实现,该方法考虑了患者在各个时间点的生存状态,根据事件发生的时间和事件发生的情况来计算生存概率。
通过逐步累积计算,可以得到各个时间点的生存概率。
绘制生存曲线一旦得到了各个时间点的生存概率,就可以开始绘制Kaplan-Meier曲线了。
通常情况下,这是通过统计软件来完成的。
在绘制曲线时,横轴通常表示随访时间,纵轴表示生存概率。
曲线的形状和走势可以直观地反映出患者的生存情况,同时也可以进行不同组别之间的比较分析。
评估曲线的可靠性绘制Kaplan-Meier曲线之后,需要对曲线的可靠性进行评估。
这可以通过计算曲线下的面积(即面积法)来实现,常用的方法包括log-rank检验和Cox比例风险模型。
通过这些方法,可以得到曲线的可靠性评估指标,从而判断曲线是否具有统计学意义。
解读曲线的意义最后,需要对绘制出的Kaplan-Meier曲线进行解读,从中得出有关患者生存情况的结论。
这包括对曲线的走势、不同组别之间的比较、曲线下的面积等进行分析,从而得出有关治疗效果、预后预测等方面的结论。
同时,也需要注意曲线所反映的生存情况和实际临床情况之间的关系,避免片面解读曲线所反映的结果。
Guidelines Briefs(指导手册)

Guidelines and template for producing IBE/IBRO briefs 10-10-17 Aims and ContentThe IBE/IBRO briefs are aimed at providing links between education and research from the sciences of mind and brain, and are aimed at a broad readership that includes educators, policy-makers and scientists. Additionally, when presenting research/perspectives please present, where possible, the practical implication of the concepts for education (e.g. policy-makers and/or classroom teachers). Your article should not exceed 3000 words (excluding references), although additional material can be provided in appendices.Cover and TemplateThe front of the report should begin with two pages (not numbered) (see Appendix 1 for Template). This should include a one-line affiliation including department or faculty, institution, country. It should also include a short biography of yourself (~200 words)Language and StylePlease frame your language/writing style to ensure it remains accessible and clear, no matter the reader’s e xperience in educational or scientific research, bearing in mind that many readers may not have English as their first language.Executive SummaryIn addition to your article please provide an executive summary (150-250 words), preferably in bullet point form, to be included at the beginning of the article.ReferencesPlease use the Nature reference style for ease of reading the main text (see Appendix 2) :Main text-Use no more than 2 levels of sub heading:•Main sub heading•Sections under the subheading please use italic.-Define an abbreviation the first time that it is used.-Use a Calibri 11 for main text.-Use the automatic page numbering function to number the pages in bottom right hand corner, from the first page of the main text (i.e. not including the first two pages in thetemplate in Appendix 1)-Do not use field functions or indent main text-Indent extended quotes and use double quote marks with reference provided in full.-Insert figures and tables as close as possible to where they are referred to in the main text.Include a legend for each table and text, referred to in the main text. Ensure no text in thediagram is smaller in font size than the main text.-Save your file in docx format (Word 2007 or higher) or doc format (older Word versions).-Manuscripts with mathematical content can also be submitted in LaTeX.-Abbreviations should be defined at first mention and used consistently thereafter.-Footnotes, rather than endnotes, can be used to give additional information – especially where this may not concern the non-specialist reader.-Footnotes to the text are labelled consecutively with Roman numerals (i, ii, iii etc) Permissions for diagrams and figuresAuthors wishing to include figures, tables, or text passages that have already been published elsewhere are required to obtain permission from the copyright owner(s) for both the print and online format and to include evidence that such permission has been granted when submitting their papers. Any material received without such evidence will be assumed to originate from the authors.Appendix 1 Nature Reference StyleReferences are each numbered, ordered sequentially as they appear in the text, tables, boxes, figure legends, online-only methods, Extended Data tables and Extended Data figure legends.When cited in the text, reference numbers are superscript, not in brackets unless they are likely to be confused with a superscript number.Do not use linked fields (produced by EndNote and similar programs). Please use the one-click button provided by EndNote to remove EndNote codes before saving your file.As a guideline, Articles allow up to 50 references and Letters allow up to 30 references. Only onepublication can be listed for each number.Only articles that have been published or accepted by a named publication, or that have been uploaded to a recognized preprint server (for example, arXiv, bioRxiv), should be in the reference list; papers in preparation should be mentioned in the text with a list of authors (or initials if any of the authors are co-authors of the present contribution).Published conference abstracts, numbered patents, preprints on recognized servers (preprints ofaccepted papers in the reference list should be submitted with the manuscript) and research datasets that have been assigned a digital object identifier may be included in reference lists, but text, grant details and acknowledgements may not. (An exception is the highlighted references which we ask authors of Reviews, Perspectives and Insights articles to provide.)All authors should be included in reference lists unless there are more than five, in which case only the first author should be given, followed by ‘et al.’.Please follow the style below in the published edition of Nature in preparing reference lists.∙Authors should be listed surname first, followed by a comma and initials of given names.∙Titles of all cited articles are required. Titles of articles cited in reference lists should be in upright, not italic text; the first word of the title is capitalized, the title written exactly as it appears in the work cited, ending with a full stop. Book titles are italic with all main words capitalized. Journal titles are italic and abbreviated according to common usage. Volume numbers are bold. The publisher and city ofpublication are required for books cited. (Refer to published papers in Nature for details.)∙Research datasets may be cited in the reference list if they have been assigned digital object identifiers (DOIs) and include authors, title, publisher (repository name), identifier (DOI expressed as a URL).Example:Hao, Z., AghaKouchak, A., Nakhjiri, N. & Farahmand, A. Global Integrated Drought Monitoring and Prediction System (GIDMaPS) data sets. figshare /10.6084/m9.figshare.853801 (2014). ∙Recognized preprints may be cited in the reference list. Example:Babichev, S. A., Ries, J. & Lvovsky, A. I. Quantum scissors: teleportation of single-mode optical states by means of a nonlocal single photon. Preprint at /quant-ph/0208066 (2002).∙References to web-only journals should give authors, article title and journal name as above, followed by URL in full - or DOI if known - and the year of publication in parentheses.∙References to websites should give authors if known, title of cited page, URL in full, and year of posting in parentheses.Appendix 1IBRO/IBE-UNESCO Science of Learning Briefings Title of BriefAuthor of BriefTitle Title of BriefSeries IBRO/IBE-UNESCO Science of Learning BriefingsAuthor of briefAuthor affiliationDate。
Shoulder Rumble Strip Guidelines说明书

Design Memorandum No. 02-02TO: Engineering Offices and Divisions Design Manual Reference: Districts Section III-06.04Consulting Engineers� RevisionFROM: Kenneth Birst, Design Engineer SupplementalDATE: May 7, 2002SUBJECT: SHOULDER RUMBLE STRIP GUIDELINESIntroductionThis Design Memorandum establishes guidelines for placement of shoulder rumble strips on rural state highways, including: Interstate highways, multilane divided and undivided highways, and two-lane highways. Rural is defined as roadway segments that have minimal residential or commercial development and little or no further development is anticipated in the near future.ImplementationThese guidelines shall be in effect for all projects with a scheduled bid opening date afterJune 14, 2002. District personnel should make every effort to implement these guidelines for projects which have been bid prior to June 14, 2002, and on which shoulder rumble strip construction has not yet begun.PurposeTo provide shoulder rumble strips to reduce run-off-the-road (ROR) crashes and prevent a drowsy or inattentive driver from traveling very far onto the shoulder and possibly striking a parked vehicle, a bicyclist, a pedestrian, or maintenance personnel. Additionally, the shoulder rumble strips may serve as a means to guide motorists and maintenance operators during inclement weather conditions when striping visibility is poor.GuidanceShoulder rumble strips will be installed on the following highways as defined in the NDDOT Highway Classification and Performance Guidelines:! All Rural Interstate highways.! Interregional highways with shoulder widths of 4 feet or greater.! State Corridor highways with shoulder widths of 4 feet or greater and the average daily traffic (ADT) is 2,000 or greater.Shoulder rumble strips may be considered on other highways at locations that have high ROR crash rates, provided there is adequate shoulder width to receive the rumble strips. The Planning and Programming Division - Traffic Operation Section will make recommendations regarding the crash analysis. The District Engineer will make recommendations regarding the structural adequacy of existing shoulders and slough to receive rumble strips.Shoulder rumble strips will be installed in conjunction with rural highway projects where paved shoulders are constructed, reconstructed, or overlaid as part of a highway construction contract and as a separate project for highways on which no reconstruction is scheduled in the near future. Shoulder rumble strips should not be installed where major surfacing work is scheduled or anticipated within the next three years.Shoulder rumble strips will not be installed on rural highways which have a paved shoulder width of less than 4.0 feet, except as provided for the left, or median shoulder on rural Interstate and divided highways.Shoulder rumble strips will not be installed within urban areas, where there is curb and gutter, where the highway posted speed is 45 mph or less, across bridge approaches and decks, or adjacent to guardrail if the clear path between the shoulder rumble strip and guardrail is less than 5 feet.Interstate Highways: The rumble strips will be installed by milling a continuous pattern on both the right and left shoulders as summarized in Table 1. Shoulder rumble strips may also be installed on the right shoulder of Interstate loop ramps. Existing formed-in shoulder rumble strips on the shoulders, initially installed at about a 48-foot spacing, will remain in-place rather than installing new, continuous milled-in rumble strips.Two-lane Highways and Multilane Highways: The rumble strips will be installed by milling an intermittent or continuous pattern on the shoulders as summarized in Table 1.Bicycle Travel Considerations: The AASHTO Guide for the Development of Bicycle Facilities 1999, indicates a paved shoulder width of 4 feet to accommodate bicycle travel. Further, the guide indicates shoulder rumble strips are not recommended where shoulders are used bybicyclists unless there is a minimum clear path of 1 foot from the rumble strip to the traveled way, 4 feet from the rumble strip to the outside edge of paved shoulder, or 5 feet to adjacent guardrail, curb, or other obstacle.The NDDOT guidelines accommodate bicycle travel on two-lane and multilane highways as follows:• For paved shoulders greater than, or equal to 6 feet, bicycle travel is accommodated between the rumble strip and outside edge of the shoulder. The shoulder rumble strip will have an intermittent design to provide bicyclists a chance to temporarily cross over in the travel way to avoid debris and object blocking the shoulder, and to make turningmovements.• For paved shoulders less than 6 feet, bicycle travel is accommodated along the outside edge of the edge of the travel lane. The shoulder rumble strip will have an a minimumoffset of 12 inches from the travel lane and a continuous design.• The width of the shoulder rumble strips have been narrowed to 12 inches.• The rumble strips will be discontinued if the clear path between the rumble strip and guardrail or other obstruction is less than 5 feet.• As shoulder rumble strips will require bicyclists to ride farther out from the vehicle induced wind-sweep shoulder edge, periodic sweeping as part of regular maintenanceactivities may be necessary to remove debris to safely accommodate bicycle travel.Maintenance Considerations: The shoulder rumble strips may be covered during patching and overlay activities. A reasonable attempt should be made to reinstall the rumble strips, thereby maintaining the consistency of shoulder rumble strips on the highway. If it is not possible or feasible to maintain the rumble strips due to construction methods or insufficient shoulder width, the rumble strips should be discussed in the project concept report and a decision item included for approval. Alternative installation methods may be considered, such as using the rolled-in type of rumble strip. The rolled-in method may be useful for patching and overlay completed by district personnel.The shoulder rumble strips may be covered during chip and sand sealing activities. The District Engineer may limit chips to the travel lanes only and sand the shoulders, or eliminate the chips or sand on the shoulders altogether.The shoulder rumble strips milled into new or existing bituminous pavements will be fog sealed to protect the milled rumble strips from oxidation and moisture. The fog seal will consist of an application of SS-1h or CSS-1h at a rate of 0.10 gal/sy across the full width of the milled rumble strip. The District Engineer may eliminate the requirement to fog seal the rumble strips.Table 1 - Types of Shoulder Rumble Strips Highway Type Shoulder Shoulder Rumble Strip TypesInterstate Right(Outside)Type 1 - 16" ContinuousContinuous milled rumble strips, 16" wide, located 2'from the edge of travel lane.Left(Inside/Median)Type 1 - 16" - ContinuousContinuous milled rumble strips, 16" wide, located 1'from the edge of travel lane. Install only when a 2' orgreater paved shoulder width exists.Multilane, Divided Right(Outside)Type 2 - 12" - Intermittent (40' milled strip/10' gap)Intermittent milled rumble strips, 12" wide, located 1'from the edge of travel lane.Left(Inside/Median)Type 2 - 12" - ContinuousContinuous milled rumble strips, 12" wide, located 1'from the edge of travel lane. Install only when a 2' orgreater paved shoulder width exists.Multilane, Undivided; Two-lane;Paved Shoulder $6' Both(Rt &Lt)Type 3 - 12" - Intermittent (40' milled strip/10' gap)Intermittent milled rumble strips, 12" wide, located 1'from the edge of travel lane.Two-lane; Paved Shoulder, $ 4' and < 6' Both(Rt &Lt)Type 4 - 12" - ContinuousContinuous milled rumble strips, 12" wide, located 1'from the edge of travel lane. Installed only whendocumented ROR crash problem and little or no bicycletraffic is expected.See attached Standard Drawing D-960-2, revised date March 21, 2002, for section and plan views of shoulder rumble strips on shoulders and guidelines for appropriate breaks in the shoulder rumble strips for exit and entrance ramps, turning lanes, intersections, paved or graveled approaches and private drives, field drives, and scenic and historical marker turnouts. QuestionsAny questions regarding the content or implementation of this memorandum should be referred toMarkS.Gaydos,DesignDivision,701-328-4417,******************.us.ApprovedFrancis G. Ziegler - Director, Infrastructure Support Services Date20/msg/~5804429.wpdattachmentc: FHWA。
围麻醉期促进正常体温管理流程

围麻醉期促进正常体温管理流程郭志红;王飞;李玉香;唐鲁【期刊名称】《山东医药》【年(卷),期】2013(053)020【总页数】2页(P97-98)【关键词】围麻醉期;体温;管理流程【作者】郭志红;王飞;李玉香;唐鲁【作者单位】济南军区总医院,济南250031;第二军医大学长海医院;第二军医大学长海医院;济南军区总医院,济南250031;第二军医大学长海医院【正文语种】中文【中图分类】R473.6围术期低体温是麻醉和外科手术期间常见的并发症,指在手术中非计划性的对机体有害的低体温[1,2]。
围术期低体温产生的不良影响包括从增加患者的不适到增加发病率和病死率[3~9]。
围术期低体温自20世纪80年代以来,受到了国内外的广泛关注并展开了循证实践研究。
2001年美国围麻醉护理协会(ASPAN)发布了《围术期低体温的防治指南》,8 a后遵循循证医学研究方法,ASPAN对该指南进行修订,并于2009年4月19日发布《围麻醉期促进正常体温的临床循证指南(第二版)》[10]。
尽管围手术期体温过低的现象引起越来越多的关注,但是临床实践中缺乏有力的执行依据,该指南旨在提供临床医务工作者以循证、实用、可行的方法,促进规范的围手术期低体温处理。
该指南同时适用于住院和门诊,以及包括麻醉镇静管理的其他领域[11]。
本文通过分析国内外围麻醉期体温管理的相关研究,参考ASPAN《围麻醉期促进正常体温的临床循证指南(第二版)》,对围麻醉期体温控制流程的研究与进展作一综述。
1 术前患者体温的评估与管理为减少围手术期体温过低相关并发症和费用,必须在整个手术过程中维持患者正常的体温。
术中或术后体温再次分配引起的体温过低是难以处理的,因为体表复温需要一定的时间才能达到身体中枢区域。
有效地预防围手术期体温过低的方法是预热[12]。
预热是指在麻醉诱导前对外周组织和体表进行加温[13]。
预热降低再分配性体温过低的机制有两个:首先是减少中枢到外周的体温梯度,其次是扩张血管。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
AST Guideline Statement for the Maintenance of Normothermiain the Perioperative PatientIntroductionThe maintenance of normothermia in the perioperative patient is essential during all phases of the surgical procedure. Measures to monitor and maintain body temperature should begin in the preoperative phase and continue into the postoperative phase of the surgical procedure. The monitoring of patient temperature is the responsibility of all surgical team members and not just the anesthesia provider.AST Guideline StatementMaintaining normothermia in the perioperative patient is a collaborative effort between the anesthesia provider, the surgeon, perioperative personnel, and perianesthesia personnel. Maintaining Perioperative NormothermiaPerioperative temperature management is imperative to positive surgical outcomes. The body maintains its temperature between 36°C and 38°C by balancing heat production and heat loss. The thermoregulatory mechanisms in the central nervous system (CNS) control this function. The body loses heat through radiation (from tissues), conduction (contact with cool surfaces), evaporation (respiration), and convection (exposure to the environment). Intraoperative hypothermia is one complication for patients receiving general anesthesia, especially in geriatric and pediatric populations. Under anesthesia, the average adult loses 0.5° to 1.5°C (0.9° to 2.7°F), and the greatest heat loss occurs during the first hour of anesthesia. The maintenance of normothermia during the intraoperative period prevents complications associated with hypothermia.Hyperthermia during the surgical procedure can be caused by dehydration, fever, pre-medication, excessive drapes, and a closed anesthesia breathing circuit. In some instances, the surgical procedure may be delayed to permit fluid administration and a reduction in patient temperature, including discontinuing the use of any warming devices. The patient’s body temperature should be continuously monitored throughout the surgical procedure in order to assess metabolic changes.Risk Factors for Hypothermia∙Large volume of irrigation∙Major blood or fluid loss∙Exposure of a large body cavity∙Patient’s age∙Patient’s physical status and preexisting conditions∙Cold surgical environment∙Length and type of surgical procedure∙Type of anesthesiaComplications Associated with Hypothermia∙Coagulopathy∙Altered metabolism, ie metabolic acidosis∙Wound infections∙Shivering∙Cardiovascular effects∙Surgical bleedingExample Protocol for Preventing Hypothermia in the Surgical Patient1.Limit the amount of skin exposed during all phases of the surgical procedure.Suggestions: Surgical team members coordinate efforts to keep patient covered andwarm during the preoperative and postoperative phases with the use of warm blankets or warming devices.2.Monitor patient’s temperature during all phases of the surgical procedure.Suggestions: This is primarily a role of the anesthesia provider, but the circulating CSTor surgical assistant can provide assistance in monitoring the patient’s temperature bychecking the temperature surface monitor that is placed on the patient’s forehead.e warmed irrigation and infusion fluids/solutions.Suggestions: The CST and surgical assistant should use irrigation fluids obtained fromthe blanket and solution warmer located in the substerile room.e of warmed anesthetic gasesSuggestions: The anesthesia provider is responsible for this function.5.Monitor operating room temperature and humidity closely.Suggestions: The CST and surgical assistant should follow established recommendations for temperature and humidity levels in the operating room, and periodically check andrecord these levels for each operating room.6.Utilize heat-maintenance devices (head coverings, leggings, forced-air warming systems,hypothermia/hyperthermia mattress, reflective blankets/head coverings, radiant heatsources).Suggestions: The CST and surgical assistant should know the proper procedures foroperating warming devices and the safety protocol associated with the use of any type of warming device as established by hospital policy and manufacturer’s recommendations.Competency StatementsCompetency Statement Measurable Criteria1. Surgical technologists and surgical assistants are qualified to identify potential complications, associated with hypothermia and hyperthermia in the perioperative environment, and appropriate interventions for treatment. 1. Educational standards as established by the Core Curriculum for Surgical Technology.12. The subject areas of normothermia, hyperthermia and hypothermia are included in the didactic studies as a student.3. The proper use of thermoregulatory methods and devices is included in the didactic studies as a student.4. Students demonstrate the proper application of thermoregulatory methods during clinical rotation, including the proper use and operation of thermoregulatory devices, and are evaluated by preceptors and instructors.5. CSTs and surgical assistants perform patient care duties by applying thermoregulatory methods and devices in the perioperative setting as practitioners.6. CSTs and surgical assistants identify potential patient complications associated with the use of thermoregulatory methods and devices in the perioperative setting as practitioners, including contributing to the decision-making process of proper interventions to treat hyperthermia and hypothermia.7. CSTs and surgical assistants complete continuing education to remain current in their knowledge of hyperthermia, hypothermia, and maintenance of normothermia for the surgical patient.DefinitionsCore Temperature: A temperature of the interior of the body, ranging from 36.8° to 37.7°C (98° to 100°F)Normothermia: A core temperature range of 36°C to 38°C (96.8°F to 100.4°F) Hypothermia: A core temperature less than 36°C (96.8°F)Hyperthermia: A core temperature greater than 38°C (100.4°F)Unplanned perioperative hypothermia: An unexpected core temperature decrease to less than 36°C (96.8° F) as a result of surgeryReferences1.Core Curriculum for Surgical Technology. 5th ed. Littleton, CO: Association ofSurgical Technologists; 2002.2.DeFazio-Quinn DM, Schick L, eds. PeriAnesthesia Nursing Core Curriculum:Preoperative, Phase I and Phase II PACU Nursing. St Louis, MO: WB Saunders;2004.3.Drain CB. Perianesthesia Nursing: A Critical Care Approach. 4th ed.St Loui,MO: WB Saunders; 2003.4.Meeker M, Rothrock J, eds. Alexander’s Care of the Patient in Surgery. 12th ed.5.St. Louis, MO: CV Mosby; 2003.6.Phillips N. Berry & Kohn’s Operating Room Technique. 10th ed. St Louis, MO:7.2004.8.Price P, Frey K, Junge TL, eds. Surgical Technology for the SurgicalTechnologist: A Positive Care Approach. 2nd ed. Clifton Park, NY: DelmarThomson Learning; 2004.9.Wagner VD. Impact of perioperative temperature management on patient safety.Surgical Services Management, 2003; 9 (4): 38-43.。
