2015,cell reports,myosin影响细胞形态和骨架重组,促进迁移
SDF-1/_CXCR4_信号轴在MSCs_修复损伤组织中作用的研究进展

第43卷㊀第2期2024年㊀4月北京生物医学工程BeijingBiomedicalEngineeringVol 43㊀No 2April㊀2024㊃综㊀述㊃基金项目:重庆市自然科学基金(2009bb5040)资助作者单位:1㊀重庆市第六人民医院(重庆㊀400060)2㊀重庆市红十字会医院(江北区人民医院)(重庆㊀400020)通信作者:宋关君,副主任医师㊂E⁃mail:song9973@126 comSDF⁃1/CXCR4信号轴在MSCs修复损伤组织中作用的研究进展杨凌霄1㊀宋关君2摘㊀要㊀骨髓间充质干细胞(mesenchymalstemcells,MSCs)具有自我更新和多向分化潜能,在损伤组织修复中起着重要作用㊂基质细胞衍生因子-1(stromalcell⁃derivedfactor⁃1,SDF⁃1)/CXC趋化因子受体4(CXCchemokinereceptor4,CXCR4)信号轴是由SDF⁃1与其受体CXCR4相互作用构成的耦联分子对,能够进行细胞间信号转导㊁诱导细胞的定向迁移,参与细胞的多种生物学过程㊂研究证实,SDF⁃1/CXCR4信号轴在MSCs参与心肌缺血㊁肾脏病变㊁骨组织损伤等损伤组织修复过程中有重要的促趋化和增殖的作用㊂本文简要介绍了SDF⁃1和CXCR4的分子结构,重点阐述了SDF⁃1/CXCR4信号轴在MSCs参与相关损伤组织修复中的作用,归纳总结了该领域的研究进展,并展望了该领域未来的发展方向,为深入理解SDF⁃1/CXCR4信号轴及其在MSCs参与组织损伤修复过程中的作用提供理论基础,也为临床上更好地将MSCs应用于损伤组织修复提供参考㊂关键词㊀基质细胞衍生因子-1;CXC趋化因子受体4;间充质干细胞;组织损伤;组织修复DOI:10 3969/j.issn.1002-3208 2024 02 014.中图分类号㊀R318㊀㊀文献标志码㊀A㊀㊀文章编号㊀1002-3208(2024)02-0205-06本文著录格式㊀杨凌霄,宋关君.SDF⁃1/CXCR4信号轴在MSCs修复损伤组织中作用的研究进展[J].北京生物医学工程,2024,43(2):205-210.YANGLingxiao,SONGGuanjun.ResearchprogressontheroleofSDF⁃1/CXCR4signalaxisinMSCsrepairinginjuredtissues[J].BeijingBiomedicalEngineering,2024,43(2):205-210.ResearchprogressontheroleofSDF⁃1/CXCR4signalaxisinMSCsrepairinginjuredtissuesYANGLingxiao1,SONGGuanjun21㊀TheSixthPeople sHospitalofChongqing,Chongqing㊀400060;2㊀TheRedCrossHospitalofChongqing(JiangbeiDistrictPeople sHospitalofChongqing),Chongqing㊀400020Correspondingauthor:SONGGuanjun(E⁃mail:song9973@126 com)ʌAbstractɔ㊀Bonemarrow⁃derivedmesenchymalstemcells(MSCs)haveaself⁃renewalcapacityandmultilineagedifferentiationpotential,andplayanimportantroleintherepairofinjuredtissue.Stromalcell⁃derivedfactor⁃1(SDF⁃1)/CXCchemokinereceptor4(CXCR4)signalaxisisacoupledmolecularpairformedbytheinteractionbetweenSDF⁃1andCXCR4,whichcancarryoutsignaltransduction,inducecellmigration,andparticipateinavarietyofbiologicalprocessesofcells.StudieshaveconfirmedthatSDF⁃1/CXCR4signalaxisplaysapivotalroleinpromotingchemotaxisandproliferationinMSCs⁃mediatedtissuerepairofmyocardialischemia,kidneydisease,andbonetissueinjuryandsoon.ThisreviewpaperbrieflyintroducesthemolecularstructureofSDF⁃1andCXCR4,thendiscussestheroleofSDF⁃1/CXCR4signalaxisinMSCs⁃mediatedrepairofrelatedinjuredtissue.Finally,wesummarizetheresearchprogressandprospectthefuturedevelopmentdirectionsinthisfield.ThisreviewprovidesatheoreticalbasisforbetterunderstandingofSDF⁃1/CXCR4axisanditsroleinMSCs⁃mediatedtissuerepair,andbringsareferenceforbetterapplicationofMSCsintissuerepairinclinic.ʌKeywordsɔ㊀stromalcell⁃derivedfactor⁃1;CXCchemokinereceptor4;mesenchymalstemcell;tissueinjury;tissuerepair0㊀引言骨髓间充质干细胞(mesenchymalstemcells,MSCs)是一类多能成体干细胞,在特定环境条件下可分化为成骨细胞㊁软骨细胞㊁脂肪细胞等多种细胞㊂除具有易于分离获取㊁体外增殖能力强㊁不涉及伦理㊁低免疫原性等特点外,MSCs还具有趋化㊁迁移特性,在损伤组织的修复中起着重要作用[1]㊂基质细胞衍生因子-1(stromalcell⁃derivedfactor⁃1,SDF⁃1)主要由骨髓基质细胞和不成熟的成骨细胞分泌,是一种对免疫细胞有趋化作用且相对分子量较小的趋化因子蛋白㊂SDF⁃1又叫前B细胞生长刺激因子(pre⁃B⁃cellgrowthstimulatingfactor,PBSF),在分类上归为趋化因子CXC亚组,系统命名为CXCL12(CXCchemokineligand12),有SDF⁃1α和SDF⁃1β两个异构体,其N-末端是绑定和激活趋化受体的主要功能区,具有7个耦合到G蛋白上的跨膜结构域[2]㊂CXC趋化因子受体4(CXCchemokinereceptor4,CXCR4)属于一种G蛋白耦联受体,是目前人们了解最清楚的SDF⁃1主要受体,包括7个跨膜螺旋,由352个氨基酸组成㊂激活后的SDF⁃1/CXCR4信号能够诱导细胞的定向迁移或参与细胞的多种生物学过程,如血管生成㊁造血作用㊁免疫应答㊁炎症响应㊁癌症转移等[3]㊂越来越多的研究发现,SDF⁃1/CXCR4轴在组织损伤及修复中起着重要的作用㊂本文主要介绍SDF⁃1/CXCR4轴在MSCs参与损伤组织修复中作用的相关研究进展㊂1㊀在MSCs参与心肌梗死修复中的作用心肌梗死(myocardialinfarction,MI)导致的心脏功能失调是当今人类面临的重大健康问题之一,主要表现为长期的肌肉损伤㊁瘢痕形成㊁心脏功能衰退和冠状动脉瞬时堵塞㊂由SDF⁃1参与的基于MSCs的细胞疗法是治疗MI的潜在手段之一[4]㊂在对MI模型的研究中,Tang等[5]发现SDF⁃1α修饰后的MSCs能够提高成活率并且促进MSCs表达SDF⁃1㊁血管内皮生长因子(vascularendothelialgrowthfactor,VEGF),进而激活抗凋亡激酶ERK和AKT信号通路㊂SDF⁃1α修饰后的MSCs移植后具有心肌细胞的表型特征(如表达肌钙蛋白T)和内皮细胞的表型特征(如表达CD31)[6]㊂Zhang等[7]发现MSCs分泌的SDF⁃1能够有效地阻止由于组织部位的缺血导致的心肌细胞死亡,并能够使受损心肌处的胶原I(collagenI,ColI)㊁胶原III(collagenIII,ColIII)和基质金属蛋白酶2(metalloprotease2,MMP2)㊁基质金属蛋白酶9(metalloprotease9,MMP9)㊁转化生长因子β(transforminggrowthfactor⁃β,TGF⁃β)表达降低㊂Zhuang等[8]将SDF⁃1注入兔MI模型中,发现不但MSCs向受伤心肌处的迁移增加,而且受损处的新血管形成能力明显提高㊂采用SDF⁃1处理MSCs后再移植,都呈现不同程度的左心室壁厚度增加㊁梗死面积减少㊁毛细血管和小动脉数量增加㊁心室扩张减小等心脏功能改善的现象㊂有研究发现心肌中SDF⁃1的表达只在MI的早期阶段出现㊂将MSCs注射到缺血心肌处后的4d内能够起到改善心肌的效果,而在注射后的8d和16d观察这种积极的作用消失,与此同时心肌中SDF⁃1的表达也很低㊂最近的研究也证实,SDF⁃1/CXCR4介导的干细胞动员参与了电针对心肌梗死小鼠的心脏保护作用[9]㊂这些结果提示,SDF⁃1是募集MSCs的关键作用因子㊂同时,SDF⁃1在MI的早期阶段表达也提示,在应用MSCs进行MI治疗中,对患者进行MSCs治疗的最佳时间也是一个不容忽视的问题㊂总的来看,SDF⁃1/CXCR4信号轴能促进MSCs向MI部位定向迁移,迁移到损伤部位的MSCs能阻止心肌细胞凋亡,促进血管生成,对MI㊃602㊃北京生物医学工程㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀第43卷导致的心脏损伤组织表现出良好的修复作用,但由于SDF⁃1在MI中的表达呈现出时效性,因此,在临床上应用MSCs进行MI患者治疗中如何确定MSCs治疗的最佳时间以取得更好的疗效还需进一步探究㊂2㊀在MSCs参与肾脏疾病修复中的作用新近的研究发现,MSCs可能通过其旁分泌和自分泌的机制实现对肾脏疾病的修复,包括促有丝分裂㊁抗凋亡㊁抗炎㊁抗纤维化和促血管生成等作用实现,而在此过程中MSCs的分化效果却并不十分明显[10]㊂SDF⁃1能够增强低氧预处理(hypoxicpreconditioning,HP)后的MSCs对肾脏疾病的治疗作用,包括促进MSCs分泌SDF⁃1和其受体CXCR4㊁CXCR7[11]㊂其中,SDF⁃1/CXCR4提高MSCs的趋化性,而SDF⁃1/CXCR7增加迁移后MSCs的成活数量㊂通过建立肾脏疾病模型,Tögel等[12]发现SDF⁃1对高表达CXCR4受体的细胞起到重要的募集和归巢作用㊂SDF⁃1对肾脏缺血的这种响应是受低氧条件中调节细胞反应的主要转录因子HIF⁃1(hypoxia⁃induciblefactor⁃1)所调节㊂SDF⁃1还能够显著提高MSCs对其他细胞因子的旁分泌作用,比如:诱导血管内皮生长因子(vascularendothelialgrowthfactor,VEGF)㊁碱性成纤维细胞生长因子(basic⁃fibroblastgrowthfactor,b⁃FGF)㊁胰岛素样生长因子1(insulin⁃likegrowthfactor,IGF⁃1)㊁肝细胞生长因子(hepatocytegrowthfactor,HGF)等的分泌㊂另外,SDF⁃1也能诱导T细胞的排斥反应,从而呈现出在受损组织处的抗炎症反应㊂也有研究发现缺血肾脏处自身表达SDF⁃1也在一定程度上增加了MSCs向其部位的迁移㊁粘附功能,促进了MSCs对肾脏损伤的修复作用[13]㊂MSCs定向迁移到损伤部位后,主要以旁分泌和定向分化两种机制实现对损伤组织的修复作用[1]㊂在MSCs参与肾脏损伤组织修复研究中,发现SDF⁃1/CXCR4能提高MSCs的趋化性,促进其旁分泌作用,进而展现出促肾脏细胞增殖㊁促血管生成㊁抗凋亡㊁抗炎㊁抗纤维化等系列修复作用,但在该修复过程中MSCs的定向分化作用并不明显[10],其原因值得深入探讨㊂在该过程中若能同时发挥MSCs的旁分泌功能和定向分化两种作用,应该会收到更好的修复效果㊂3㊀在MSCs参与骨组织损伤修复中的作用在骨组织工程和骨组织损伤修复领域,提高MSCs向受损组织处的定向募集和归巢能力是一种有效的方法[14]㊂SDF⁃1能够刺激MSCs向异位植入位点的迁移㊂对骨形成蛋白2(bonemorphogeneticprotein2,BMP2)诱导的MSCs向成骨细胞分化的调节作用也是学者关注的关键问题之一[15]㊂Kitaori等[16]的研究发现,在骨修复的初期,骨移植处的SDF⁃1表达水平增高,进而SDF⁃1通过与其受体CXCR4之间的相互作用招募MSCs到达受伤位点,从而加速新骨形成㊂而在SDF⁃1诱导MSCs向骨细胞分化方面,有实验研究显示,阻断SDF⁃1/CXCR4信号显著降低BMP2诱导的MSCs成骨分化中前成骨细胞标志物碱性磷酸酶(alkalinephosphatase,ALP)的活性和成熟成骨细胞标志物骨钙蛋白(osteocalcin,OCN)的合成[17]㊂其次,在MSCs成骨分化过程中,破坏SDF⁃1信号会损害受伤位点处的骨结节矿化㊂阻断SDF⁃1信号也抑制BMP2诱导的MSCs成骨分化的两个关键因子Runx2(runt⁃relatedtranscriptionfactor⁃2)和Osterix(Osx)的早期表达[18]㊂进一步的研究发现,这种影响主要是通过SDF⁃1/CXCR4轴对细胞内的Smad和ERK的活性调节来实现的[19]㊂此外也有研究发现,在含BMP2的植入物中添加SDF⁃1,可以提高从骨髓中募集骨祖细胞的效率,增加BMP2诱导的异位骨的形成[20]㊂4㊀在MSCs参与脑损伤修复中的作用将MSCs移植到中枢神经系统紊乱的动物模型(如脑卒中)中,MSCs可以向中枢神经受损处募集㊁迁移,并且能够提高神经细胞特异性蛋白的表达,进而提高局部神经系统的功能[21]㊂Kortesidis等[22]深入探究了其分子机制,发现移植后的MSCs通过自分泌和旁分泌的方式上调SDF⁃1及其受体CXCR4的表达,促进自身的增殖和存活㊂Shichinohe等[23]首次直接通过体内CXCR4敲除的小鼠动物模型实验,发现脑卒中区域能够激活星形胶质细胞分泌SDF⁃1,SDF⁃1与MSCs上表达的CXCR4作用,诱导MSCs向卒中处的迁移㊂迁移后的MSCs又通过自身表达的SDF⁃1促进其本身在宿㊃702㊃第2期㊀㊀㊀㊀㊀㊀杨凌霄,等:SDF⁃1/CXCR4信号轴在MSCs修复损伤组织中作用的研究进展主大脑处的增殖和成活,通过调动体内的相关修复机制,最终参与神经系统功能的恢复㊂该研究结果揭示了SDF⁃1/CXCR4对移植后MSCs存活和增殖的作用机制㊂Wang等[24]的研究也发现,SDF⁃1α和其受体CXCR4在诱导干细胞向受伤组织处的迁移中发挥的积极作用,并且通过绿色荧光蛋白(greenfluorescenceprotein,GFP)标记的MSCs发现,在脑卒中损伤中,MSCs的迁移是沿着嗅神经-丘脑和海马-皮质路线这一轨迹进行的㊂在受伤脑组织中,SDF⁃1/CXCR4能够诱导MSCs的募集和迁移㊁粘附以及调节造血作用等[25]㊂同时,由于很多白细胞能够表达CXCR4受体,所以SDF⁃1也表现出了抗炎的潜在作用,即SDF⁃1能够调动脑卒中处的固有免疫反应[26]㊂Bakondi等[27]还发现大脑初级神经元中存在以SDF⁃1为基础的生存信号,以保护神经前体细胞免受缺氧造成脑部损伤引起的细胞凋亡,证明SDF⁃1具有抗凋亡的作用㊂近年来发现,SDF⁃1的另一受体CXCR7在这一过程中也发挥重要的作用[28],但对其分子机制尚缺乏深入认识㊂因此,CXCR4和CXCR7两种受体在该过程中的作用方式(独立或协同)以及贡献大小等问题都需要进一步明确㊂5㊀在MSCs参与肿瘤微环境重塑中的作用正常组织发生恶变可被视为一种特殊的组织损伤,炎性微环境是肿瘤组织的重要特征之一㊂肿瘤组织能募集MSCs参与肿瘤微环境的重塑,并对肿瘤细胞的生物学行为产生重要影响㊂肿瘤细胞与MSCs之间的交互对话及相互影响成为近年来肿瘤领域的研究热点,但是,目前人们对于MSCs如何参与肿瘤微环境的重塑以及MSCs如何影响肿瘤细胞的生物学行为还缺乏系统认识㊂有研究发现,迁移到肿瘤组织的MSCs对肿瘤细胞的增殖起抑制作用㊂Lu等[29]将小鼠骨髓来源MSCs与小鼠肝癌细胞系㊁淋巴瘤及大鼠胰岛瘤细胞系共培养,发现MSCs对鼠瘤的生长起抑制作用,并且抑制效果与MSCs的量成正比㊂Khakoo等[30]也发现MSCs对卡波西肉瘤的抑制是剂量相关的,提示MSCs对肿瘤细胞的抑制行为可能呈现出剂量依赖关系㊂皮下注射MSCs到黑色素瘤鼠体内发现肿瘤细胞凋亡明显增加,其生长也受到明显抑制[31]㊂多种细胞因子或趋化因子能促进MSCs向肿瘤组织迁移㊂研究发现,MSCs与肿瘤细胞(或其条件培养基)共培养时,MSCs能高表达SDF⁃1,诱导MSCs向肿瘤细胞迁移[32]㊂相关研究进一步探讨了后续信号的传递,发现SDF⁃1激活了信号通路JAK2/STAT3和MAPK,进而活化下游信号PAX(paxillin)和NF⁃kB,导致细胞骨架的重排和细胞迁移行为的变化[33]㊂SDF⁃1/CXCR4在诱导MSCs对急性髓性白血病(acutemyeloidlekemia,AML)的修复中也具有重要作用[34]㊂研究发现,AML患者的外周血中SDF⁃1的分泌量有所下降,对MSCs的迁移效率带来不利影响,但SDF⁃1的这种不足可以在外源加入MSCs之后得到明显改善[35]㊂在MSCs参与肿瘤微环境的重塑中,也有研究发现MSCs促进了多种类型肿瘤细胞的增殖㊁侵袭和转移[36-37],或者促进肿瘤血管形成[38],提示MSCs对肿瘤细胞的生物学行为呈现双向影响㊂SDF⁃1/CXCR4轴在肿瘤的侵袭转移中发挥了重要作用,对其有效干预可能成为肿瘤治疗的新靶点㊂但是,由于MSCs对肿瘤细胞的生物学行为呈现出双向影响效应,因此如果要应用MSCs进行肿瘤患者损伤组织的修复,应该特别警惕MSCs在肿瘤微环境重塑中的负面作用㊂将来的研究工作需进一步深入探究MSCs对肿瘤组织的作用并揭示其分子机制,这样不仅能更好地认识MSCs重塑肿瘤微环境后,肿瘤细胞生物学行为的变化特征,而且能为将MSCs发展成为安全有效的抗肿瘤和损伤组织修复工具提供理论指导㊂6㊀结语SDF⁃1及其受体CXCR4构成的SDF⁃1/CXCR4轴对细胞的多种生物学行为起着重要调控作用㊂近年来,越来越多的研究证实了SDF⁃1/CXCR4轴在MSCs对损伤组织进行修复过程中所扮演的重要角色㊂本文主要总结了MSCs在参与心肌梗死㊁肾脏疾病㊁骨组织损伤㊁脑损伤修复以及肿瘤微环境重塑中的主要生物学效应以及SDF⁃1/CXCR4信号轴在该过程中的关键信号介导作用(表1)㊂尽管人们在该领域的研究已取得了不少成果,但目前人们对于SDF⁃1/CXCR4轴参与MSCs介导的损伤组织修复的详细分子机制还缺乏系统㊁深入的认识㊂另一㊃802㊃北京生物医学工程㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀第43卷方面,近年的研究发现CXCR7是SDF⁃1的另一受体㊂对于SDF⁃1/CXCR7在MSCs参与的损伤组织修复中的作用以及CXCR4与CXCR7之间的关系,有许多工作尚需进一步深入探索㊂随着国内外学者对SDF⁃1/CXCR4和SDF⁃1/CXCR7影响MSCs增殖㊁迁移㊁分化等生物学行为研究的不断深入,SDF⁃1/CXCR4和SDF⁃1/CXCR7参与MSCs进行组织修复的分子机制及相关信号调控网络将被逐步阐明,这对更好地将MSCs应用于损伤组织修复和再生医学具有重要意义㊂表1㊀MSCs在不同损伤组织修复中的生物学效应Table1㊀ThebiologicaleffectsofMSCsintherepairofdifferentdamagedtissues损伤组织类型主要生物学效应参考文献心肌梗死SDF⁃1/CXCR4促进MSCs定向迁移;MSCs阻止心肌细胞凋亡,促进血管生成[5-8]肾脏组织损伤SDF⁃1/CXCR4提高MSCs趋化性,促进其旁分泌作用;MSCs促肾脏细胞增殖㊁抗凋亡㊁抗炎㊁抗纤维化和促血管生成[10-12]骨组织损伤SDF⁃1/CXCR4增强MSCs的募集和归巢;诱导MSCs的成骨分化,加速新骨形成[15-20]脑组织损伤MSCs上调SDF⁃1和CXCR4表达;诱导MSCs的迁移㊁粘附;调节脑卒中组织的免疫反应和造血作用[21-27]肿瘤微环境重塑SDF⁃1/CXCR4促进MSCs向肿瘤组织迁移;MSCs对肿瘤细胞增殖㊁侵袭和转移起抑制或促进作用,对肿瘤细胞生物学行为的影响呈现双向效应[29-38]参考文献[1]㊀FuX,LiuG,HalimA,etal.Mesenchymalstemcellmigrationandtissuerepair[J].Cells,2019,8(8):784.[2]㊀SadriF,RezaeiZ,FereidouniM.ThesignificanceoftheSDF⁃1/CXCR4signalingpathwayinthenormaldevelopment[J].MolecularBiologyReports,2022,49(4):3307-3320.[3]㊀LingL,HouJ,LiuD,etal.ImportantroleoftheSDF⁃1/CXCR4axisinthehomingofsystemicallytransplantedhumanamnion⁃derivedmesenchymalstemcells(hAD⁃MSCs)toovariesinratswithchemotherapy⁃inducedprematureovarianinsufficiency(POI)[J].StemCellResearch&Therapy,2022,13(1):79.[4]㊀FreitasC,WangX,GeY,etal.Comparisonoftroponinelevation,priormyocardialinfarction,andchestpaininacuteischemicheartfailure[J].CJCOpen,2020,2(3):135-144.[5]㊀TangJ,WangJ,GuoL,etal.Mesenchymalstemcellsmodifiedwithstromalcell⁃derivedfactor1αimprovecardiacremodelingviaparacrineactivationofhepatocytegrowthfactorinaratmodelofmyocardialinfarction[J].MoleculesandCells,2010,29(1):9-19.[6]㊀JiangQ,HuangK,LuF,etal.ModifyingstrategiesforSDF⁃1/CXCR4interactionduringmesenchymalstemcelltransplantation[J].GeneralThoracicandCardiovascularSurgery,2022,70(1):1-10.[7]㊀ZhangM,MalN,KiedrowskiM,etal.SDF⁃1expressionbymesenchymalstemcellsresultsintrophicsupportofcardiacmyocytesaftermyocardialinfarction[J].FASEBJournal,2007,21(12):3197-3207.[8]㊀ZhuangY,ChenX,XuM,etal.Chemokinestromalcell⁃derivedfactor1/CXCL12increaseshomingofmesenchymalstemcellstoinjuredmyocardiumandneovascularizationfollowingmyocardialinfarction[J].ChineseMedicalJournal,2009,122(2):183-187.[9]㊀ZhaoTT,LiuJJ,ZhuJ,etal.SDF⁃1/CXCR4⁃mediatedstemcellmobilizationinvolvedincardioprotectiveeffectsofelectroacupunctureonmousewithmyocardialinfarction[J].OxidativeMedicineandCellularLongevity,2022,2022:4455183.[10]㊀Sierra⁃ParragaJM,MerinoA,EijkenM,etal.Reparativeeffectofmesenchymalstromalcellsonendothelialcellsafterhypoxicandinflammatoryinjury[J].StemCellResearch&Therapy,2020,11(1):352.[11]㊀LiuH,LiuS,LiY,etal.TheroleofSDF⁃1⁃CXCR4/CXCR7axisinthetherapeuticeffectsofhypoxia⁃preconditionedmesenchymalstemcellsforrenalischemia/reperfusioninjury[J].PLoSOne,2012,7(4):e34608.[12]㊀TögelF,IsaacJ,HuZ,etal.RenalSDF⁃1signalsmobilizationandhomingofCXCR4⁃positivecellstothekidneyafterischemicinjury[J].KidneyInternational,2005,67(5):1772-1784.[13]㊀KameishiS,DunnCM,OkaM,etal.Rapidandeffectivepreparationofclonalbonemarrow⁃derivedmesenchymalstem/stromalcellsheetstoreducerenalfibrosis[J].ScientificReports,2023,13(1):4421.[14]㊀SunX,LiX,QiH,etal.MiR⁃21nanocapsulespromoteearlybonerepairofosteoporoticfracturesbystimulatingtheosteogenicdifferentiationofbonemarrowmesenchymalstemcells[J].JournalofOrthopaedicTranslation,2020,24:76-87.[15]㊀StokovicN,IvanjkoN,MaticicD,etal.Bonemorphogeneticproteins,carriers,andanimalmodelsinthedevelopmentofnovelboneregenerativetherapies[J].Materials(Basel,Switzerland),2021,14(13):3513.[16]㊀KitaoriT,ItoH,SchwarzEM,etal.Stromalcell⁃derivedfactor1/CXCR4signalingiscriticalfortherecruitmentofmesenchymalstemcellstothefracturesiteduringskeletalrepairinamousemodel[J].ArthritisandRheumatism,2009,60(3):813-823.[17]㊀LiJ,ChenH,ZhangD,etal.Theroleofstromalcell⁃derived㊃902㊃第2期㊀㊀㊀㊀㊀㊀杨凌霄,等:SDF⁃1/CXCR4信号轴在MSCs修复损伤组织中作用的研究进展factor1oncartilagedevelopmentanddisease[J].OsteoarthritisCartilage,2021,29(3):313-322.[18]㊀YangJ,LiY,LiuY,etal.RoleoftheSDF⁃1/CXCR4signalingpathwayincartilageandsubchondralboneintemporomandibularjointosteoarthritisinducedbyoverloadedfunctionalorthopedicsinrats[J].JournalofOrthopaedicSurgeryandResearch,2020,15(1):330.[19]㊀VerheijenN,SuttorpCM,vanRhedenREM,etal.CXCL12-CXCR4interplayfacilitatespalatalosteogenesisinmice[J].FrontiersinCellandDevelopmentalBiology,2020,8:771.[20]㊀LauerA,WolfP,MehlerD,etal.BiofabricationofSDF⁃1functionalized3D⁃printedcell⁃freescaffoldsforbonetissueregeneration[J].InternationalJournalofMolecularSciences,2020,21(6):2175.[21]㊀PirzadJahromiG,PShabanzadehA,MokhtariHashtjiniM,etal.Bonemarrow⁃derivedmesenchymalstemcellandsimvastatintreatmentleadstoimprovedfunctionalrecoveryandmodifiedc⁃Fosexpressionlevelsinthebrainfollowingischemicstroke[J].IranianJournalofBasicMedicalSciences,2018,21(10):1004-1012.[22]㊀KortesidisA,ZannettinoA,IsenmannS,etal.Stromal⁃derivedfactor⁃1promotesthegrowth,survival,anddevelopmentofhumanbonemarrowstromalstemcells[J].Blood,2005,105(10):3793-3801.[23]㊀ShichinoheH,KurodaS,YanoS,etal.RoleofSDF⁃1/CXCR4systeminsurvivalandmigrationofbonemarrowstromalcellsaftertransplantationintomicecerebralinfarct[J].BrainResearch,2007,1183:138-147.[24]㊀WangY,DengY,ZhouGQ.SDF⁃1alpha/CXCR4⁃mediatedmigrationofsystemicallytransplantedbonemarrowstromalcellstowardsischemicbrainlesioninaratmodel[J].BrainResearch,2008,1195:104-112.[25]㊀FelkerS,ShresthaA,BaileyJ,etal.DifferentialCXCR4expressiononhematopoieticprogenitorcellsversusstemcellsdirectshomingandengraftment[J].JCIInsight,2022,7(9):e151847.[26]㊀MichalettosG,RuscherK.Crosstalkbetweengabaergicneurotransmissionandinflammatorycascadesinthepost⁃ischemicbrain:relevanceforstrokerecovery[J].FrontiersinCellularNeuroscience,2022,16:807911.[27]㊀BakondiB,ShimadaIS,PetersonBM,etal.SDF⁃1ɑsecretedbyhumanCD133⁃derivedmultipotentstromalcellspromotesneuralprogenitorcellsurvivalthroughCXCR7[J].StemCellsandDevelopment,2011,20(6):1021-1029.[28]㊀DongBC,LiMX,WangXY,etal.EffectsofCXCR7⁃neutralizingantibodyonneurogenesisinthehippocampaldentategyrusandcognitivefunctioninthechronicphaseofcerebralischemia[J].NeuralRegenerationResearch,2020,15(6):1079-1085.[29]㊀LuYR,YuanY,WangXJ,etal.Thegrowthinhibitoryeffectofmesenchymalstemcellsontumorcellsinvitroandinvivo[J].CancerBiology&Therapy,2008,7(2):245-251.[30]㊀KhakooAY,PatiS,AndersonSA,etal.HumanmesenchymalstemcellsexertpotentantitumorigeniceffectsinamodelofKaposi ssarcoma[J].JournalofExperimentalMedicine,2006,203(5):1235-1247.[31]㊀RautiainenS,LaaksonenT,KoivuniemiR.Angiogeniceffectsandcrosstalkofadipose⁃derivedmesenchymalstem/stromalcellsandtheirextracellularvesicleswithendothelialcells[J].InternationalJournalofMolecularSciences,2021,22(19):10890.[32]㊀MaM,YeJY,DengR,etal.MesenchymalstromalcellsmayenhancemetastasisofneuroblastomaviaSDF⁃1/CXCR4andSDF⁃1/CXCR7signaling[J].CancerLetters,2011,312(1):1-10.[33]㊀GaoH,PriebeW,GlodJ,etal.Activationofsignaltransducersandactivatorsoftranscription3andfocaladhesionkinasebystromalcell⁃derivedfactor1isrequiredformigrationofhumanmesenchymalstemcellsinresponsetotumorcell⁃conditionedmedium[J].StemCells,2009,27(4):857-865.[34]㊀Cuesta⁃GomezN,GrahamGJ,CampbellJDM.Chemokinesandtheirreceptors:predictorsofthetherapeuticpotentialofmesenchymalstromalcells[J].JournalofTranslationalMedicine,2021,19(1):156.[35]㊀LadikouEE,ChevassutT,PepperCJ,etal.DissectingtheroleoftheCXCL12/CXCR4axisinacutemyeloidleukaemia[J].BritishJournalofHaematology,2020,189(5):815-825.[36]㊀HillBS,SarnellaA,D AvinoG,etal.Recruitmentofstromalcellsintotumourmicroenvironmentpromotethemetastaticspreadofbreastcancer[J].SeminarinCancerBiology,2020,60:202-213.[37]㊀CeccarigliaS,CargnoniA,SiliniAR,etal.Autophagy:apotentialkeycontributortothetherapeuticactionofmesenchymalstemcells[J].Autophagy,2020,16(1):28-37.[38]㊀SirithammajakS,ManochantrS,TantrawatpanC,etal.Humanmesenchymalstemcellsderivedfromtheplacentaandchorionsuppresstheproliferationwhileenhancingthemigrationofhumanbreastcancercells[J].StemCellsInternational,2022,2022:4020845.(2023-06-29收稿,2023-09-07修回)㊃012㊃北京生物医学工程㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀第43卷。
促骨修复的重组生长因子的研究开发进展

摘要 :骨修复是一个复杂过程 , 需要细胞与各种生长 因子 的协 同作用 。生长因子是一类通过与特异 的、 高亲 和 的细胞膜受体结合 , 调 节细胞生 长与其他 细胞 功能 等多效应 的小分子多肽类物质 。 重组生 长因子是利用基 因工程技 术生产 的生长 因子产 品。由于生长因子在骨修复过程中的重要作 用以及传 统骨 移植方法暴 露出的一些不 良反应 和 并发症 问题 , 用重组生长因子治疗骨缺损 、 骨不连 以及重组生长因子在各种骨科 手术 中的应用越来越受到研究人员 的重视 。本文对重组人血小板衍生生 长因子( r h P D G F ) 、 重组人骨形成蛋 白( r h B MP s ) 促 骨修 复的实验和临床研究 以
的能力 。
生长 因子能加速骨愈合并能提高骨修复 的质
量 。在 骨损伤 后 的血肿 期 时 , 血小 板 一 颗 粒开 始释
放T G F — B 、 P D G F 、 V E G F 等 因子 。炎症反应后 , 巨噬 细 胞和 其他 炎性 细胞 分泌 F G F 、 P D G F和 T G F — B 。 在
Vo l _3 6 NO .4 8.201 3
促 骨修 复 的 重 组 生 长 因子 的研 究开 发 进 展
杨 阳 , 一 ,李玛琳 2 , 1 A
( 1 . 昆明医科 大学 药学 院暨省天然药物药理重点实验室 ,云南昆 明 6 5 0 5 0 0 ;2 . 云南 中医学 院药学院 ,云南昆明 6 5 0 5 0 0 )
连 以及 重 组 生 长 因子 在各 种 骨 科 手 术 中 的应 用 越
t e i n , B MP s ) 、转化 生 长 因子一 B( t r a n s f o r mi n g g r o w t h
《细胞生物学》——细胞8章 细胞骨架1
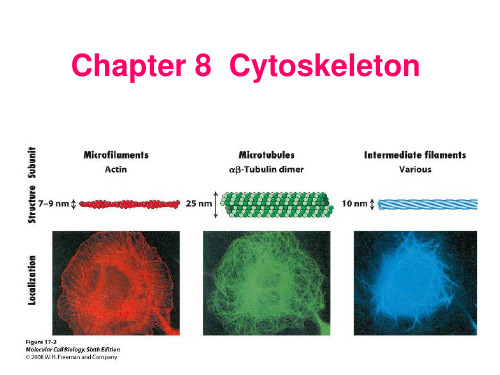
A 球状肌动蛋白 B 纤维状肌动蛋白
G蛋 C白 D Arp2/3
提交
3. 微丝的组装不包括下列哪个阶段() A. 成核反应 B. 纤维的延长 C. 踏车行为 D. 解离阶段
单选题 10分
3. 微丝的组装不包括下列哪个阶段()
A 成核反应 B 纤维的延长 C 踏车行为 D 解离阶段
即正极与负极之别
(2)肌动蛋白单体组装成微丝的过程
① 缓慢成核期:肌动蛋白单体与起始复合物结合→形成寡聚体(至少2-3
个单体)。
包括2种肌动蛋白相关蛋白(Arp2/3)和5种
其它蛋白。
② 快速延长期:肌动蛋白单体具有ATP酶活性,可利用水解ATP释放的能
量来快速组装单体。当微丝的组装速度快于肌动蛋白水解ATP的速度时,在
提交
本章主要内容
微丝与细胞运动 微管及其功能 中间丝
第一节 微丝与细胞运动
微丝(microfilament, MF) 肌动蛋白丝(actin filament) 纤维状肌动蛋白
(fibrous, F-actin)
由肌动蛋白单体组装而成的 直径为7 nm的纤维状结构 存在于所有真核细胞中 微丝的组装/去组装 微丝结合蛋白
(MF binding protein)
一、微丝的组成及其组装 二、微丝网络结构的调节与细胞运动
一、微丝的组成及其组装
(一)微丝的结构与成分 (二)微丝的组装及其动力学特性 (三)影响微丝组装的特异性药物
(一)微丝的结构与成分
• 微丝的主要结构成分:肌动蛋白(actin)。
• 肌动蛋白在细胞内有2种存在形式:①球状肌动蛋白(G-actin): 肌动蛋白单体;②纤维状肌动蛋白(F-actin):由多个单体组装 而成。
肌球蛋白磷酸化的研究进展

肌球蛋白磷酸化的研究进展摘要:肌球蛋白是肌原纤维粗丝的组成单位,由多条重链与多条轻链组成,被视为一种分子马达。
在肌肉收缩、趋化性胞质分裂、胞引作用、膜泡运输以及信号传导等生理过程中起重要作用。
目前肌球蛋白磷酸化是研究的一个热点,它对细胞的迁移、收缩、胞质分裂以及其他未知功能都有着至关重要的作用。
肌球蛋白磷酸化分为重链的嶙酸化与轻链的磷酸化。
根据国内外的最新相关研究报道,分别从肌球蛋白的结构与功能、磷酸化的作用机制、磷酸化的生物学功能以及最新研究成果等方面,对肌球蛋白的嶙酸化研究进展进行阐述。
关键词:肌球蛋白;β-抑制蛋白;肌球蛋白重链磷酸化;肌球蛋白轻链磷酸化中图分类号:Q71文献标识码:A 文章编号:1007-7847(2015)02-0154-06Progresses on Myosin PhosphorylationHAO Li-juan,KANG Zhi-qiong,MA Shang-shang,LU Peng,YAO Qin,CHEN Ke-ping*(Life Sciences Institute,Jiangsu University,Zhenjiang 212013,Jiangsu,China)Abstract :Myosin is the unit of myofibril raw silk,composed of multiple heavy chains and light chains,is regarded as a kind of molecular motors. It mainly works on muscle contraction,chemotaxis cytoplansmic* division,cell function,vesicular transport and signal transduction. Recently myosin phosphorylation is a hot topic,as it plays an important role in cell migration,contraction,cytokinesis and other unknown functions. Myosin phosphorylation is divided into heavy chain and light chain phosphorylation. According to the latest reports,it mainly elaborates the research progress on the phosphorylation of myosin on the structures and functions,the action mechanism of phosphorylation,the biological function of phosphorylation and the latest research results.Key words:myosin;β-Arrestin;phosphorylation of myosin heavy chain;phosphorylation of myosin light chain (LifeScienceResearch,2015,19(2):154-159)l肌球蛋白的结构与功能肌球蛋白主要存在于平滑肌中,它是肌原纤维粗丝的组成单位。
CD147研究现状

CD147研究现状摘要:在多种癌组织中,MMPs的表达主要由癌细胞相关的细胞外基质金属蛋白酶诱导子(extracellular matrix metalloproteinase inducer,Emmprin, CD147)介导的癌-基质相互作用来调节。
CD147 分子是一个高度糖基化的跨膜蛋白,属于IgSF类CAM,与细胞-细胞及细胞-ECM相互作用密切相关[22]。
CD147 分子的表达在原发性乳腺癌、卵巢癌等人类肿瘤中显著上调[23-28],能够刺激基质细胞MMPs的生产,但对MMPs的生理性抑制剂MMPs组织抑制子(tissue inhibitorof metalloproteinases,TIMPs)的表达没有影响,从而调节由MMPs激活介导的胶原平衡[29],促进ECM降解与重建,促进癌细胞的侵袭与转移。
CD147 分子是一种广泛存在于细胞表面的属于免疫球蛋白超家族的糖蛋白, 参与机体多种生理和病理的过程。
CD147是一种在细胞中高度表达的胞膜监视分子,能刺激肿瘤细胞周围的成纤维细胞及肿瘤细胞产生基质金属蛋白酶(MMPs)。
正常组织中 CD147 的出现和调节也伴随着 MMPs 表达的升高,这一现象提示CD147 介导的 MMPs 诱导作用是非肿瘤生理或病理状态下的一种常见调节机制。
影响 CD147 水平及其 MMPs 诱导活性——包括生长因子、激素、糖基化、膜脱落等。
研究发现,CD147 分子是一个多功能分子,在胚胎发育、神经系统功能、损伤修复、炎症发生、肿瘤进展等生理、病理状态下均发挥重要作用,尤其是在癌发生发展过程中,该分子参与了多个关键步骤,且其功能作用提示,该分子可能是参与癌—基质交互作用的一类重要分子。
CD147分子的研究概况CD147 分子是一种高度糖基化的、广泛表达于造血及非造血细胞系,分子量为 50-60kDa的跨膜糖蛋白,属免疫球蛋白超家族(IgSF)成员。
最早在 1989年,Altruda F等首次克隆出一个新的小鼠跨膜糖蛋白gp42 基因,与组织相容性抗原有同源区,属于免疫球蛋白超家族成员,具有潜在粘附分子的特性。
Rho-GTP酶

1 Rho GTP酶 到目前为止,Rho GTP酶超家族已发现约20个成员,根据结构和功能不同,大致分为5个亚家族,包括:(1)Rho亚家族,包括RhoA、RhoB和RhoC,在序列上具有高度同源性,并在多种细胞中高表达,主要参与张力纤维形成和粘着斑复合体(focal adhesion complexs,FACs)组装;(2)Rac亚家族:包括Rac1、Rac2、Rac3和RhoG,促进层状伪足和胞膜皱褶形成;(3)Cdc42亚家族,包括Cdc42、TC10、TCL、Wrch1和chp/Wrch2,其中Cdc42促进丝状伪足形成:(4)Rnd亚家族:包括Rnd1、Rnd3/RhoE和Rnd2,在细胞中组成性激活表达并具有不同的组织分布,可拮抗Rho信号通路;(5)Rho BTB亚家族,包括Rho BTB1和Rho BTB2,具体功能尚不清楚。在所有Rho GTP酶超家族成员中,Cdc42、Rac1和RhoA是目前研究最多的Rho GTP酶。Rho家族各成员在氨基酸序列上有50%~55%的同源性,在靠近催化位点处都有1个能和GTP结合的功能区,与催化GTP水解密切相关。Rho GTP酶同Ras超家族的其他成员一样,羧基端通常具有共同结构域,即由半胱氨酸残基,脂族残基和其他氨基酸残基组成的末端,是翻译后修饰的位点〔2〕。Rho GTP酶的翻译后修饰与其质膜定位有关,只有经翻译后修饰的Rho GTP酶才具有活性并能与细胞膜上适宜的脂质分子结合。在异戊烯基转移酶的作用下,半胱氨酸的巯基和异戊二烯基团间共价形成硫醚键,并在内切酶的作用下水解掉末端其余3个残基,最后异戊二烯基化的半胱氨酸残基在甲基转移酶的作用下发生甲基化,完成翻译后的修饰。Rho家族蛋白同Ras超家族的所有成员一样在活性型/GTP限制型和失活型/二磷酸鸟苷(guanosine diphosphate,GDP)限制型构象之间循环。调节这个循环过程的3类重要蛋白是:(1)鸟苷酸交换因子(guaninenucleotide exchanging factors,GEFs),催化GDP的释放和GTP的结合,活化Rho GTP酶。不同的Rho GEF在结构上都具有相同的功能域,包含1个DH(Dbl homology domain)区和1个PH(pleckstrin homologyv domain)区,前者与Rho GTP酶结合并催化其构象改变,后者通过和细胞膜上特定的脂质作用使GEF在膜上定位;(2)GTP酶活化蛋白(GTPase activating protein,GAP),作为负向调节因子加速Rho GTP酶的水解,使Rho GTP酶由活性状态变为无活性状态;(3)GDP解离抑制因子(GDP dissociation inhibitor,GDI),阻止GDP从Rho GTP酶上分离,抑制Rho GTP酶活性。Rho GTP酶是细胞内多条信号转导通路的关键分子,作为分子开关在胞内信号转导中发挥桥梁作用。Rho GTP酶可参与对正常细胞增殖、分化、凋亡的调节,并与肿瘤的发生和转移密切相关。实验研究发现,在多种肿瘤中可见Rho GTP酶表达异常,改变细胞内Rho GTP酶的表达水平可以直接影响肿瘤细胞侵袭和转移的过程。 2 Rho G足形成还依赖另一种关键调节子cofilin,cofilin可使肌动蛋白单体从肌动蛋白丝的顶端解离,诱导肌动蛋白丝从头部折断,产生新的末端。Rac和Cdc42可通过活化共同的底物p21激活激酶(p21activated kinase,PAK),分别激活LIM(3种同源异型结构域蛋白lin11、isl1和mec3)激酶1(LIM kinase1,LIMK1)和LIM激酶2(LIM kinase2,LIMK2),磷酸化cofilin使其失活而抑制肌动蛋白的解聚,稳定肌动蛋白细胞骨架。 22 细胞基质粘附 伪足形成启动细胞迁移的过程,但细胞持续的迁移需依赖细胞伪足与细胞外基质(extracellular matrix,ECM)的稳定粘附,提供细胞向前迁移的牵引支点。迁移的细胞头部与ECM的粘附和尾部与ECM的去粘附的不断交替使得细胞向前迁移,Rho GTP酶对这一过程发挥精确的调节。细胞表面的整合素受体与ECM中特异的配体结合,通过整合素聚集成簇而形成FACs,而整合素受体的胞内区与桩蛋白(paxillin),纽蛋白(vinculin)和踝蛋白(talin)等多种肌动蛋白结合蛋白相互作用形成分子桥,并与细胞骨架相连,提供细胞迁移的锚着位点。活化的Rac可诱导肌动蛋白的聚合和层状伪足的形成,同时也能诱导新的FACs的形成,而FACs的形成又能反过来活化Rac,这一正反馈的失控可增加肿瘤细胞的侵袭能力〔11〕。Jung〔12〕发现,活化的Rac1和Cdc42,可通过激活PAKl磷酸化下游的粘着斑激酶(focal adhesion kinase,FAK),活化的FAK作为分子支架招募胞浆中桩蛋白,纽蛋白和踝蛋白等至FACs,促进FACs的形成。p65激活激酶还可通过LIMK间接调节cofilin的活性,cofilin在活性型和失活型间的循环可调节肌动蛋白亚单位从肌动蛋白丝末端的解离和聚合,并对促进肌动蛋白纤维组装时踏车(treadmilling)现象的发生非常必要〔3〕。肿瘤细胞侵袭和转移与ECM的降解密切相关,Rho GTP酶可直接或间接调节下游效应子促进ECM的降解。对人乳腺癌细胞株MDAMB435的研究发现,Rac1和Cdc42可通过间接活化LIMKl上调丝氨酸蛋白酶尿激酶型纤溶酶原激活剂(urokinase type plasminogen activator,uPA)系统,增加uPA启动子活性,诱导uPA和uPA受体mRNA和蛋白表达及uPA的分泌,降解ECM胶原等成分,有助于细胞的侵袭转移〔13〕。 23 细胞骨架重组 侵袭和转移的肿瘤细胞的持续运动需依靠张力纤维收缩和肌动蛋白丝的延长提供动力,Rho GTP酶可通过调节细胞骨架的重组,为细胞迁移提供动力。张力纤维是真核细胞中一种稳定的、平行排列的微丝结构,由肌动蛋白、肌球蛋白、原肌球蛋白等组成,肌动肌球蛋白相对运动产生的收缩力是细胞迁移动力的主要来源。Rho及其下游的Rho相关卷曲螺旋形成蛋白激酶(Rho associated coiledcoil for
细胞骨架

(一) 微丝的成分及组装
1 微丝的成分
1)肌动蛋白: 分子近球形,具极性,头尾相接形成 螺旋状具极性的微丝。已分离6种,4种α (分别为横 纹肌、心肌、血管平滑肌和肠道平滑肌特有),β和γ 各 1种。 2)肌动蛋白结合蛋白
肌球蛋白 作用位点
2 微丝的组装
1)聚合过程: G-actin活化 ; G-actin聚集形成种子 G-actin在种子两端聚合而延长;聚合时正极较快
基体
中心粒和基粒 是同源的,可 相互转变,均 可自我复制。
AB
纤毛
C
左图显示藻类细胞鞭毛基 部的基体(荧光染色)
(四) 微管特异性药物 长春碱类和秋水仙素类药物是通过阻滞微管蛋白聚
合,使有丝分裂不能进行从而破坏肿瘤细胞增殖。 ◆秋水仙素(colchicine) 、鬼臼素和长春花 紫杉醇及紫杉特尔的作用则是促进微管蛋白聚合作 用和抑制微管解聚,它们主要作用于 β-微管蛋白的N碱:阻断微管蛋白组装成微管,可破坏纺 锤体结构。 末端31位氨基酸和 217-231氨基酸残基上,使具有可逆 变化的微管不能解聚,阻止有丝分裂,最后导致癌细 ◆紫杉酚(taxol):能促进微管的装配,并使 胞死亡。 已形成的微管稳定。 紫杉醇源于短叶紫杉的树皮,紫杉醇可明显减少 ◆为行使正常的微管功能,微管动力学不稳G1期 的细胞群体,而增加 G2期和M 期的细胞群。紫杉醇对 定性是其功能正常发挥的基础。 卵巢癌、乳腺癌及非小细胞肺癌等有突出的疗效,被 誉为近15年来最好的抗肿瘤新药。
纺锤体极
基体
高等植物功能性的MTOC——细胞核表面 高等植物细胞微管的成核能力仅在细胞核表面 得到证实.Mizuno[1993]发现,经过冻-融处理 的烟草细胞核或核颗粒具有微管成核作用,成核 的微管从细胞核表面或核颗粒呈放射状发出.说 明植物细胞核表面具有类似中心体的功能.
蜕膜化子宫内膜间质细胞细胞骨架重塑研究进展

蜕膜化子宫内膜间质细胞细胞骨架重塑研究进展黄媛【摘要】子宫内膜蜕膜化是成功妊娠的重要环节.蜕膜化可参与获得子宫内膜容受性.研究发现,早期胚胎着床失败与子宫内膜蜕膜化受损有关.蜕膜化涉及子宫内膜间质细胞细胞骨架重塑,包括中间纤维丝、微丝和微管等各种骨架成分的变化,以及相关骨架结合蛋白对骨架动态变化的影响.就蜕膜化过程中的细胞骨架成分具体变化、性激素对蜕膜化细胞骨架调节以及细胞骨架变化对蜕膜化的自身调节进行综述.【期刊名称】《国际生殖健康/计划生育杂志》【年(卷),期】2011(030)003【总页数】4页(P234-236,254)【关键词】蜕膜;细胞骨架;子宫内膜;性腺甾类激素【作者】黄媛【作者单位】200030,上海交通大学附属国际和平妇幼保健院【正文语种】中文子宫内膜接受胚胎是一短暂的生理过程,称为“子宫内膜容受期”,一般在月经周期的第20~24天。
子宫内膜容受性表型的获得不但要求子宫内膜上皮的质膜转化过程,同时需要子宫内膜间质蜕膜化转变。
近来有报道蜕膜化在胚胎着床阶段也有重大作用。
Salker等[1]通过对反复自然流产(RPL)的患者进行大样本的研究发现,导致RPL发生的原因之一是子宫内膜蜕膜化受损,导致母胎对话失败,引起反复流产。
同样,子宫内膜蜕膜化受损还可以影响胎盘形成,并造成各种妊娠并发症,如子痫前期和胎儿生长受限。
就蜕膜化进程中间质细胞细胞骨架变化及相关的分子调节机制做文献综述。
子宫内膜蜕膜化的基本特征子宫内膜蜕膜化是子宫内膜间质重构过程,包括巨细胞和自然杀伤细胞浸润、血管生成和细胞外基质重构,其中较受关注的是子宫内膜成纤维细胞转化为蜕膜化细胞。
显微镜下可见高度增生纺锤状的子宫内膜间质细胞(ESC)转变成多边上皮样或类圆形样上皮细胞[2]。
超微结构下蜕膜化细胞具备分泌特征,出现大量高尔基复合体、扩张的粗面内质网、致密的膜结合分泌颗粒等[3]。
蜕膜化细胞形状可增大,糖原和脂质含量上升。
胰岛素样生长因子结合蛋白-2在骨软骨疾病发生发展中的作用

胰岛素样生长因子结合蛋白-2在骨软骨疾病发生发展中的作用邵婉珍;王森;郭雄【摘要】胰岛素样生长因子结合蛋白-2(Insulin-like growth factor-binding proteins-2,IGFBP2)是胰岛素样生长因子结合蛋白(insulin-like growth factor binding proteins,IGFBPs)家族中的一个重要成员,具有调控和储存转运胰岛素样生长因子(Insulin-like growth factors,IGFs)的作用以及独立于IGFs的作用,在机体生长和发育中发挥重要的功能,在骨软骨疾病发生发展中其表达明显升高.本文对IGFBP2在正常软骨细胞、骨关节炎和大骨节病软骨细胞中的表达、过表达机制及其在疾病进展的作用等方面进行阐述,以期对骨软骨病的发病机制及其早期诊断提供新的思路.【期刊名称】《国外医学(医学地理分册)》【年(卷),期】2017(038)003【总页数】3页(P302-304)【关键词】IGFBP2;大骨节病;骨关节炎;机制【作者】邵婉珍;王森;郭雄【作者单位】西安交通大学医学部公共卫生学院地方病研究所,中国卫计委微量元素与地方病重点实验室,陕西西安 710061;西安交通大学医学部公共卫生学院地方病研究所,中国卫计委微量元素与地方病重点实验室,陕西西安 710061;西安交通大学医学部公共卫生学院地方病研究所,中国卫计委微量元素与地方病重点实验室,陕西西安 710061【正文语种】中文【中图分类】R599◇综述与介评◇人类胰岛素样生长因子-2(insulin-like growth factor-binding proteins-2,IGFBP2)基因位于2q 35上,是一段长度为32 kb 的单拷贝基因。
该基因含有 4个外显子,第1外显子主要编码氨基端序列,第3外显子及第4外显子主要编码羧基端序列,第2外显子编码中央区序列。
核盘菌通过类似整联蛋白SSITL...

核盘菌通过类似整联蛋白(SSITL)抑制寄主的抗病反应目 录摘 要 (I)ABSTRACT (IV)缩略词表 (VIII)1. 前言综述 (1)1.1 核盘菌的危害及其防治 (1)1.1.1 核盘菌的危害及其生物学特性 (1)1.1.2 作物菌核病的防治研究 (1)1.2 植物病原菌与寄主植物的互作 (5)1.2.1 植物天然的的物理及生理生化防卫屏障 (5)1.2.2 植物的先天免疫系统 (6)1.2.3 植物的后天免疫系统 (10)1.2.4 植物的非寄主抗性 (13)1.2.5 不同类型植物病原菌的侵染策略以及互作方式 (14)1.2.6 核盘菌的侵染策略 (16)1.3基因功能研究的策略 (19)1.3.1丝状真菌的遗传转化的研究进展 (19)1.3.2基因的超标达、敲除和沉默 (20)1.3.3 蛋白质的定位 (24)1.4 Integrin以及Integrin–like基因的研究进展 (26)1.4.1 整联蛋白的结构 (27)1.4.2 整联蛋白的信号传导 (29)1.4.3整联蛋白在微生物中的生物学功能 (30)1.5 本项研究的目的和意义 (32)2. 材料与方法 (33)2.1 菌株及植物材料 (33)2.2 基因的生物信息学分析 (33)2.3 核酸的实验操作 (34)2.3.1 DNA的提取 (34)2.3.2 质粒的提取 (34)2.3.3 总RNA的提取 (35)2.3.4 RT和Real–Time PCR (35)2.3.5 Northern blot (36)2.4 蛋白质的实验操作 (37)2.4.1 SSITL的原核表达 (37)2.4.2 抗体血清的制备、效价(ELISA)以及特异性(Western blot)的检测 (37)2.4.3 SSITL的免疫胶体金亚细胞定位 (39)2.4.4 核盘菌侵染洋葱表皮过程中SSITL的免疫荧光定位 (40)2.5 相关载体的构建 (40)2.6 ATMT介导的真菌和植物转化 (41)2.7 生物学特性的实验研究 (43)2.7.1 生长速度、致病力、菌丝顶端分支以及菌落形态的观察 (43)华中农业大学2012届博士研究生学位论文2.7.2 菌核的培养、大小及重量的测定和菌核萌发的研究 (43)2.7.3 核盘菌产草酸能力的测定 (44)2.7.4 核盘菌侵染拟南芥叶片过程的观察 (45)2.8 SSITL与植物诱导抗性的关系 (45)2.8.1 核盘菌侵染拟南芥过程中SSITL基因的表达情况 (45)2.8.2 核盘菌侵侵染拟南芥过程中拟南芥局部抗性的动态变化 (45)2.8.3 核盘菌侵侵染拟南芥过程中拟南芥系统抗性的动态变化 (46)2.8.4 SSITL在植物中表达对植物的抗病性的影响 (46)3. 结果与分析 (47)3.1 SSITL的生物信息学分析 (47)3.1.1 SSITL的序列分析 (47)3.1.2 SSITL蛋白的同源比对分析及高级结构预测 (49)3.2 SSITL对核盘菌生物学特性的影响 (53)3.2.1 SSITL基因在核盘菌不同生长时期的表达 (53)3.2.2 SSITL基因沉默对核盘菌生物学特性的影响 (53)3.3 SSITL抗体的制备以及免疫胶体金亚细胞定位 (61)3.3.1 SSITL的原核诱导表达 (61)3.3.2 抗血清效价以及特异性测定 (63)3.3.3 SSITL蛋白的亚细胞定位 (63)3.4 SSITL基因在核盘菌与植物互作过程中的作用 (67)3.4.1 核盘菌侵染拟南芥时,SSITL基因的表达情况 (67)3.4.2 核盘菌SSITL对拟南芥局部防卫反应的影响 (68)3.4.3 核盘菌SSITL对拟南芥系统防卫反应的影响 (70)3.4.4 SSITL在寄主植物中瞬时表达对植物抗病性的影响 (74)3.4.5 SSITL在寄主植物中组成型表达对植物抗病性的影响 (79)3.4.6 SSITL的表达对烟草的影响 (81)4. 讨论 (83)4.1 SSITL基因生物学功能的深入探讨 (83)4.1.1 SSITL基因的序列分析 (83)4.1.2 SSITL基因的功能分析 (85)4.2 SSITL参与抑制植物诱导抗性 (87)4.2.1 SSITL基因在核盘菌侵染过程中被诱导表达 (88)4.2.2 SSITL参与抑制植物的局部抗性 (88)4.2.3 SSITL参与抑制植物的系统抗性 (89)4.2.4 SSITL基因在植物中表达后,植物的抗性受到抑制 (90)4.3 研究SSITL的互作蛋白以及作用机理 (90)4.4 结论与展望 (92)5. 参考文献 (94)附录: (116)博士期间发表的论文 (121)致 谢 (122)核盘菌通过类似整联蛋白(SSITL)抑制寄主的抗病反应摘 要核盘菌(Sclerotinia sclerotiorum)属于子囊菌门,是一种世界性分布的典型的死体营养型病原真菌。
【细胞生物学】08 细胞骨架(思维导图)
肌球蛋白:依赖于微丝的分子马达
肌细胞的收缩运动
与运动相关
马达结构域
位于肌球蛋白的头部 包含一个肌动蛋白亚基的结合位点和一个具有ATP酶活性的ATP结合位点
负责将ATP水解释放的化学能转变成机械能 ATP的水解及磷酸基团的释放等会改变马达结构域和调控结构域的构象
三个功能结构域
调节结构域
位于马达结构域后部
γ-微管蛋白
存在于中心体等具有微管组织功能的细胞结构上 与α/β-微管蛋白二聚体中的α-微管蛋白结合,确定了微管的极性
Tn-T使原肌球蛋白移位到肌动蛋白双螺旋沟槽的深处,暴露出细肌丝肌动蛋白与 肌球蛋白头部的结合位点
解除了肌动蛋白与肌球蛋白结合的障碍
肌肉收缩的滑动模型
细肌丝与粗肌丝之间的相对滑动
肌球蛋白将ATP中储存的化学能转化成肌丝滑动的机械能,导致细肌丝和粗肌丝 之间发生相对方向的滑动
肌球蛋白的头部结构域与肌动蛋白丝之间的每一个机械运动周期消耗一分子ATP
肌动蛋白亚基所结合的ATP都被水解为ADP,这段微丝比较容易解聚
系统稳定
纤维正端组装的速度和负端解聚的速度相同
临界浓度Cc
此时体系中肌动蛋白单体的浓度
微丝的稳定性受多种结合蛋白的调控
微丝的解聚
负端更容易与帮助微丝解聚的蛋白质相互作用 两端解聚速度相近,正端稍快
踏车行为
Cc+ < 细胞质肌动蛋白单体浓度 < Cc微丝的正极端由于肌动蛋白亚基的不断添加而延长,而负极端由于解离而缩短
肌动蛋白单体(G-actin) 由单体装而成的纤维状肌动蛋白(F-actin)
比例约1:1
结构与成分
肌动蛋白(actin)
三维结构
外观呈蝶状
成骨细胞与破骨细胞的相互作用对骨重塑的调节

ste m cells f or treat m ent of therapy-resistant graft-versus -host disease1Trans p lantati on,2006;81(10):1390–139719 Bocelli-Tyndall C,B racci L,Spagnoli G et al1Bone mar2 r ow mesenchy mal str omal cells(BM-MSCs)fr om healthy donors and aut o-i m mune disease patients reduce the p r o2 liferati on of aut ol ogousand all ogeneic-sti m ulated ly mpho2 cytes in vitr o1Rheu mat ol ogy,2007;46(3):403–408 20 Gerdoni E,Gall o B,Casazza S et al1Mesenchy mal ste m cells effectively modulate pathogenic i m mune res ponse in experi m ental aut oi m mune encephal omyelitis1Ann Neur ol, 2007;61(3):219–22721 Augell o A,Tass o R,Negrini S M et al1Cell therapy using all ogeneic bone marr ow mesenchy mal ste m cells t o p reventtissue da mage in collagen-induced arthritis1A rthritis Rheu m,2007;56(4):1175–118622 English A,Jones E A,Corscadden D et al1A comparative assess ment of cartilage and j oint fat pad as a potential s ource of cells f or aut ol ogous therapy devel opment in knee osteoarthritis1Rheumat ol ogy,2007;46(11),1676–168323 Pisati F,Boss olasco P,MeregalliM et al1I nducti on of neu2 r otr ophin exp ressi on via hu man adult mesenchy mal ste m cells:i m p licati on f or cell therapy in neur odegenerative dis2 eases1Cell Trans p lant,2007;16(1):41–55(2009-04-14收稿)成骨细胞与破骨细胞的相互作用对骨重塑的调节汕头大学医学院第一附属医院(515041) 陈 斌综述 李学东 杜世新审校摘 要 骨骼是一个动态活性组织,它通过持续的重塑来维持其矿化平衡及自身的结构完整。
OSM诱导成骨分化及骨形成的研究进展

OSM诱导成骨分化及骨形成的研究进展抑瘤素M(OSM)是屬于IL-6家族的一类分泌型蛋白因子,在维持慢性炎症条件下机体内环境稳态中发挥重要作用。
它也是gp130膜蛋白家族的一员,在细胞增殖、分化、造血系统功能和炎症免疫等病理生理过程具有多功能调控作用。
过去很多研究报道了OSM在肿瘤、肝细胞再生、心血管疾病等疾病发生、发展中起着重要作用,对类风湿性关节炎、皮肤及肺部感染等炎症疾病也有重要的调控作用。
近年来,越来越多将研究重点聚焦于OSM对成骨分化过程中骨发生和骨成熟的调控。
1 OSM简介抑瘤素M(Oncostatin M,OSM)是IL-6家族中的一类蛋白因子,1986年Zarling等[1]首次从PMA活化U937细胞培养上清中分离纯化到一种因子,这种因子有明显抑制A375人黑素瘤细胞生长而命名为抑瘤素。
人OSM基因定位于22号染色体,基因组由3个外显子和2个内含子组成,OSM前体有252个氨基酸[2]。
OSM与白血病抑制因子(LIF)、粒细胞集落刺激因子(GM-CSF)、IL-6及睫状神经营养因子(CNTF)分子之间有一定的同源性和相似的二级结构[3]。
在机体细胞微环境内,OSM可通过集落刺激因子、脂多糖(LPS)、Toll样受体配体(TLR-ligands)、前列腺素E2(PGE2)刺激中性粒细胞、单核巨噬细胞和树突状细胞产生,从而参与、调节机体慢性感染性疾病及其他慢性疾病。
OSM 受体广泛分布于多种肿瘤细胞、内皮细胞和上皮细胞,在生理和病理过程中OSM 具有多种生物活性.细胞表面糖蛋白受体gp130是OSM受体的一个亚单位,是识别OSM的低亲和力受体,在组成OSM高亲和力受体中,除gp130亚单位外,还有白血病抑制因子受体(LIFR)的参与。
OSM与膜蛋白受体gp130结合后,在体内主要通过OSM受体(OSMR)和LIFR介导其生物学功能,其受体复合体gp130/OSMR、gp130/LIFR在多种细胞来源的组织中都有广泛表达。
星形胶质细胞主要细胞骨架研究的最新进展

星形胶质细胞(Astrocyte,Ast)是中枢神经系统的主要细胞成分之一,约占正常成人中枢神经系统细胞总数的 40%,其功能日益受到重视。
其细胞骨架主要由微丝(MF)、微管(MT)和中间纤维(IFs)3 种蛋白质组成,其细胞骨架的复杂变化在中枢神经系统的生理和病理变化中发挥重要作用,本文就生理病理条件下 Ast 细胞骨架的复杂变化(细胞骨架的生物学特性、信号转导途径、细胞骨架的构建与其在临床疾病中变化等)作一综述。
Ast 数量巨大,约占正常成人中枢神经系统细胞总数的 40%,具有十分重要的作用,在维持脑内平衡中起关键作用,通过合成和释放神经转导物质、调节突触的活性、支持及保护神经元的代谢来维持神经元的功能。
MF、MT 和 IFs 几乎参与细胞一切重要的生命活动,如细胞内各种细胞器的空间定位及其在细胞质的位置改变、细胞运动、细胞内外物质运输、细胞信号转导、细胞增殖分裂与分化等。
一、细胞骨架的生物学特性细胞骨架是由蛋白纤维交织成的立体网状结构。
如同一种内部框架,充满整个细胞质的空间,与外侧的细胞膜和内侧的核膜存在一定的结构联系。
真核细胞骨架主要由 MF、MT 和 IFs 组成,它们分别由不同的蛋白单体组装而成,这些细胞骨架在细胞的生命活动中具有重要的作用。
(一)MFMF 是三种骨架结构中最细的,其直径约 7 nm,主要由肌动蛋白组成,以游离球状肌动蛋白(G-actin)或聚合纤维状肌动蛋白(F-actin)形式存在,只有聚合态的 F-actin 才具有生物作用。
G-actin 聚合形成 F-actin,使丝状伪足伸长,F-actin 解聚引起丝状伪足回缩。
聚合及非聚合态的肌动蛋白能与多种结合蛋白相互作用,这些结合蛋白对肌动蛋白的聚合及 MF 的稳定、长度及分布具有调节作用。
MF 骨架是细胞骨架的一种,与维持细胞形态、细胞分裂、细胞运动、细胞内物质转运及信号转导等多种功能密切相关:一般认为细胞外基质一整合素一细胞骨架轴是细胞感受外界机械信号并将其转化为生化信号的主要途径。
细胞骨架的生物化学成分与功能

细胞骨架的生物化学成分与功能细胞骨架是维持细胞形态、机械支撑和细胞运动的关键结构,它由多种生物化学成分组成。
本文将详细介绍细胞骨架的主要成分及其功能,在探索细胞骨架的重要作用的同时,也将了解细胞骨架的组成对细胞整体功能的调控作用。
1. 微丝蛋白(Actin)微丝蛋白是细胞骨架的重要组成部分之一,具有极高的保守性和广泛存在性。
在细胞内,微丝蛋白通过与微丝相关蛋白相互作用形成细胞骨架结构。
微丝蛋白主要参与细胞形态的维持、细胞运动、细胞分裂等多种生物学过程。
2. 中间丝蛋白(Intermediate Filaments)中间丝蛋白是一类直径约为10纳米的纤维蛋白质,包括角蛋白、细胞核蛋白等。
中间丝蛋白主要定位于细胞核内和细胞质中,起到细胞结构支撑和机械强度保持的作用。
在细胞分裂和细胞运动过程中,中间丝蛋白也发挥着重要的作用。
3. 微管蛋白(Microtubules)微管蛋白是细胞骨架的另一个重要成分,由α-和β-微管蛋白活性亚单位组成,具有较高的极性。
微管蛋白在细胞的内分泌运输、细胞分裂、纤毛和鞭毛的形成等过程中发挥重要功能。
此外,微管蛋白还参与调节细胞极性和细胞骨架动态的重构。
4. 肌动蛋白(Myosin)肌动蛋白是微丝蛋白的结合伴侣,参与细胞的收缩运动以及负责细胞运动活动的调节。
肌动蛋白与微丝以及其他蛋白质相互作用,在细胞内形成肌动蛋白纤维结构,同时参与细胞骨架的动态调节和重塑。
细胞骨架的作用是多方面的,下面将重点介绍其主要功能:1. 细胞形态维持与细胞分裂细胞骨架通过与细胞膜和其他蛋白质相互作用,能够赋予细胞特定的外形和结构。
不仅如此,细胞骨架还参与细胞的极性维护、细胞外基质的组织和脏器的形成。
此外,在细胞分裂过程中,细胞骨架通过重新组织和调节,参与分裂骨架的形成和染色体的分离。
2. 细胞运动与细胞外基质的重塑细胞骨架通过参与细胞的伸缩、收缩、转动等运动过程,使细胞能够在体内外进行有针对性的定向移动。
细胞极性和细胞运动的分子机制

细胞极性和细胞运动的分子机制细胞是生命的基本单位,每个细胞都有其特有的形态和功能。
细胞的形态和功能不仅仅由其细胞器和分子组成,还受到细胞内分子之间的相互作用影响。
其中,细胞极性和细胞运动是两个十分重要的方面。
一、细胞极性的分子机制细胞极性是指细胞在空间上不对称的现象,包括极性细胞的两个极(头端和尾端)和细胞中的各个部分。
细胞极性的形成取决于细胞内的分子运输和定向,因此,其中涉及到的分子机制主要包括细胞内运输和细胞外信号传递。
1.细胞内运输细胞内运输主要通过细胞中的微管和微丝网络完成。
微管是由动力蛋白亚型kinesin和dynein完成,微丝则由动力蛋白myosin 完成。
这些蛋白分子在细胞内寻找特定的细胞器,将其从一个部分运输到另一个部分。
通过这种方式,细胞可以分子级别地控制细胞器的运输和定位,从而影响细胞极性的形成。
例如,在蛋白分泌的过程中,细胞利用TGN(trans-Golgi network)和ER(endoplasmic reticulum)之间的运输来控制细胞内蛋白的定向分泌。
2.细胞外信号传递除了细胞内运输,细胞外信号也会影响细胞极性的形成。
信号通过细胞膜上的受体识别和特定分子的结合来激活细胞内通路。
一个例子是Rho GTPase,这是细胞外信号调节细胞形态和行为的重要分子。
在Cdc42, Rac和Rho三个亚型中,Cdc42是一个特别重要的亚型,在细胞中引导杆状突起和cell-cell链接的形成,从而影响细胞极性的形成。
二、细胞运动的分子机制细胞运动是生命体系中的重要行为之一,它涉及到许多生命过程,如胚胎发育、组织修复等。
细胞运动主要有细胞集群的运动、个体细胞的运动和细胞内各种运动。
1.细胞集群的运动细胞集群的运动包括胚胎发育和组织修复等过程。
这些过程涉及到大量的细胞-细胞相互作用和细胞基质相互作用。
细胞-细胞相互作用的分子机制通常涉及到细胞黏附分子和细胞表面受体的相互作用。
此外,通过信号转导通路,这些细胞也能够影响它们周围的细胞,从而协调它们的运动。
多系统萎缩患者皮肤组织中泛素和α-突触核蛋白的表达

多系统萎缩患者皮肤组织中泛素和α-突触核蛋白的表达张瑞锋;殷竞争;周玉帅;滕军放【摘要】目的:检测多系统萎缩(MSA)患者皮肤组织中泛素(Ub)和αt-突触核蛋白表达的变化,探讨泛素蛋白酶体系统在MSA发病机制中的作用.方法:收集5例MSA患者及8例正常对照的皮肤标本,采用免疫组化法和Western blot方法分别检测Ub及α-突触核蛋白的细胞定位及表达情况.结果:与正常对照组相比,MSA患者皮肤表皮层中Ub[(0.021±0.006)vs(0.429±0.009)]及α-突触核蛋白[(0.035±0.015)vs(0.111±0.021)]的表达均上调(t=3.974和3.321,P<0.05).结论:泛素蛋白酶体系统可能参与了MSA的发病.【期刊名称】《郑州大学学报(医学版)》【年(卷),期】2015(050)003【总页数】4页(P358-361)【关键词】多系统萎缩;皮肤活检;泛素蛋白酶体系统;α-突触核蛋白【作者】张瑞锋;殷竞争;周玉帅;滕军放【作者单位】郑州大学第一附属医院神经内科郑州450052;河南省高等学校临床医学重点学科开放实验室郑州450052;郑州大学第一附属医院神经内科郑州450052;河南省高等学校临床医学重点学科开放实验室郑州450052;郑州大学第一附属医院神经内科郑州450052;河南省高等学校临床医学重点学科开放实验室郑州450052;郑州大学第一附属医院神经内科郑州450052;河南省高等学校临床医学重点学科开放实验室郑州450052【正文语种】中文【中图分类】R742.5#通信作者,男,1960年11月生,博士,教授,主任医师,研究方向:神经退行性疾病,E-mail:138****************多系统萎缩(multiple system atrophy,MSA)是一组成年期发病、呈散发性的神经系统变性疾病,临床较为少见,主要表现为不典型的帕金森综合征、小脑性共济失调、不同程度的自主神经功能障碍和锥体系统累及等症状[1-2]。
摘要:细胞骨架的结构与功能

细胞骨架的结构与功能摘要:细胞骨架一般是指真核细胞中的蛋白纤维网络结构,所组成的结构体系称为“细胞骨架系统”,是细胞的重要保守结构之一,主要包括微管,微丝和中间纤维;而广义的细胞骨架还包括核骨架、核纤层和细胞外基质,形成贯穿于细胞核、细胞质、细胞外的一体化网络结构(Alberts B et al.,2002)。
细胞骨架除了维持细胞的特定形状及细胞内部结构的有序性等基本功能外,还在细胞的物质运输、能量与信息传递、基因表达、细胞的运动、细胞的分裂分化及凋亡中起重要作用。
关键词:细胞骨架;微管;微丝;中间纤维;结构;功能20世纪60年代之前,电镜制样大多采用低温固定,而细胞骨架会在低温下解聚,所以科研工作者们一直没有注意到它。
直到1963年Slauterback首次用电镜在水螅刺细胞中第一次观察到微管以来,细胞骨架的重要作用被揭示,现在已知,细胞骨架的作用不仅在于维持细胞形态稳定,而且还参与了调节细胞的重要生命活动,如细胞的物质运输、能量与信息传递、基因表达、细胞的分裂分化以及凋亡等(Bershadsky ,A et al., 1988)。
细胞骨架不仅在维持细胞形态,承受外力、保持细胞内部结构的有序性方面起重要作用,而且还参与许多重要的生命活动,如:在植物细胞中细胞骨架指导细胞壁的合成;在细胞分裂中细胞骨架牵引染色体分离,在细胞物质运输中,各类小泡和细胞器可沿着细胞骨架定向转运;在肌肉细胞中,细胞骨架和它的结合蛋白组成动力系统;在白细胞的迁移、精子的游动、神经细胞轴突和树突的伸展等方面都与细胞骨架有关;维持细胞的形态,为各种细胞器的定位和实施功能提供基础,确保细胞中各种生命活动在时间和空间上有序进行;同时,细胞骨架对离子通道也有调节作用。
既然细胞骨架对生物体如此的必不可少,所以有必要从细胞骨架的基本成分微管、微丝和中间纤维的结构和功能来对细胞骨架的研究进展做一个概述。
微管的结构与功能微管是一种具有极性的细胞骨架,可在所有哺乳类动物细胞中存在,它是由13 条原纤维构成的中空管状结构,直径22—25纳米,除了红细胞外,所有微管均由约55kD结构相似的α及β微管蛋白组成,且每一条原纤维由微管蛋白二聚体线性排列而成(Claire E. Walczak et al.,2010) 。
胰岛素在氟诱导成骨作用中的地位

胰岛素在氟诱导成骨作用中的地位张秀云;王岩;赵志涛;杨晨;徐辉【期刊名称】《中国科技论文》【年(卷),期】2015(000)018【摘要】The gene expression of osteogenesis and insulin receptor for the rats exposed to fluoride was analyzed by realtime PCR. The insulin secretion by pancreas islet was observed by immunohistochemistry,and then the levels of insulin,blood glucose and serum fat were tested by means of biochemical methods.The effects of insulin receptor expression in osteoblasts and fluorine in combination with insulin on bone marrow mesenchymal stem cells into bone were studied by in vitro osteoblasts infected by fluo-rine.The results show that expression of Alkaline phosphatase (ALP),osteocalcin (OCN),Runt-related transcription factor 2 (Runx2)and Insulin receptor (InR)increase after low-dose fluoride administration for 3 months and high-dose fluoride also stim-ulate the expression of ALP,OCN and Runx2,which suggest the osteogenesis action of low and high dose of fluoride.The insu-lin level obviously enhances in rats treated with low-dose fluoride from 1 month to 3 months;however,it reduces after high-dose fluoride administration for 3 months,which is consistent with the expression of InR in rats after high-dose fluoride administration for 3 months.In vitro studies indicateρ(F-)= 2 mg/L and 8 mg/L stimulate the protein expression of insulin receptor by im-munofluorescence technique with anti-InR antibody.The 10 μg/mL insulin and co-treatment fluoride promote cell viability of bone marrow stromal cells,and single insulin administration significantly enhances cell viability.The fluoride ofρ(F-)= 1 mg/L and 4 mg/L markedly stimulates ALP activity.These data of fluorosis animal model show low-dose fluoride induced osteogenesis and insulin secretion and increased InR expression in rats,and which implies the role of insulin on the mechanism underlying the oste-ogenesis induced by fluoride.In vitro cell culture also indicates that insulin stimulates cell osteoblastic cell proliferation.All these results suggest the insulin is involved in the mechanism underlying low-dose of fluoride induced osteogenesis action.%采用实时 PCR 测试了投氟大鼠的骨组织成骨和胰岛素受体等基因表达情况,采用免疫组化技术观察了胰岛分泌胰岛素情况,以及采用生化方法检测血清中的胰岛素、血糖和血脂等变化,利用体外成骨细胞染氟考察了胰岛素受体在成骨细胞内表达及氟联合胰岛素对骨髓间充质干细胞的成骨作用影响。
神经元运动蛋白的结构与功能

神经元运动蛋白的结构与功能神经元是人体中最微小的细胞单元之一,其扮演着传递神经信号的重要角色。
而神经元的正常运作依赖于其内部各种蛋白物质的协同作用。
其中,神经元运动蛋白(Neuron Motor Protein)便是神经元中重要的一类蛋白,被广泛研究和应用于生物学领域中。
本文旨在探讨神经元运动蛋白的结构与功能,同时介绍基于其结构的科研应用和对人体生理健康的影响。
一、神经元运动蛋白的结构神经元运动蛋白是一类高度动态的蛋白质分子,其主要分为肌动蛋白(Myosin)、微管蛋白(Tubulin)和中间纤维蛋白(Intermediate Filament)三类。
这些蛋白质分子在结构和功能上的差异都很大,但它们都参与到神经元的运动和形态维持等方面。
其中,肌动蛋白、微管蛋白和中间纤维蛋白的结构分别如下:(一)肌动蛋白肌动蛋白是一种参与细胞肌肉运动的蛋白质,其通过肌肉收缩产生重要的生理功能。
在神经元中,肌动蛋白则主要发挥细胞运动和形态维持等重要作用。
肌动蛋白结构由两个重链和四个轻链构成,重链与重链之间通过肌动蛋白头、颈和尾部三个区域连接。
而轻链则分为两类,一类为肌钙蛋白,一类为肌钙蛋白酯化酶。
这些结构的组合和相互作用,才使得肌动蛋白发挥出其功能特性。
(二)微管蛋白微管蛋白是一种以α/β-图瑞德蛋白组装而成的管状蛋白质。
在神经元中,微管蛋白发挥细胞内物质运输的功能,参与神经信号传递。
微管蛋白结构由蛋白链组成,蛋白链环绕成管状结构,并进一步形成微管。
微管的稳定性和动态性,决定了微管蛋白的功能效果。
(三)中间纤维蛋白中间纤维蛋白是细胞内一种纤维状的蛋白质,可以维持细胞形态和机械强度,同时在细胞凋亡、神经元轴突损伤等细胞损伤情况下也扮演重要角色。
其结构由多个棒状鞭毛蛋白组成,这些棒状鞭毛蛋白之间通过交替排列的α-螺旋和非α-螺旋区域连接,从而形成具有强度和延伸性的中间纤维结构。
二、神经元运动蛋白的功能神经元运动蛋白在神经元的正常功能过程中发挥着重要的作用,常见的功能表现如以下几点:(一)细胞内物质运输神经元需要将细胞内的物质进行有效的分配和传递,这个过程主要由微管蛋白和肌动蛋白来完成。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Report Specific Myosins Control Actin Organization,Cell Morphology,and Migration in Prostate Cancer CellsGraphical AbstractHighlightsd Myo1b,Myo9b,Myo10,and Myo18a are highly expressed inmetastatic prostate cancerd Knockdown of individual myosins distinctly affects thecytoskeleton and cell migrationd Myosins act in concert to directly influence actin organizationand cell migrationd Misregulation of myosin expression may drive the metastaticphenotype AuthorsKatarzyna A.Makowska,Ruth E.Hughes, Kathryn J.White,Claire M.Wells, Michelle PeckhamCorrespondenceclaire.wells@(C.M.W.),m.peckham@(M.P.)In BriefMakowska et al.show that several myosin isoforms(Myo1b,Myo9b,Myo10, and Myo18a)are overexpressed in metastatic prostate cancer.Knockdown of each of the myosins resulted in distinct cell phenotypes,showing that they can contribute to metastasis through re-organization of the actin cytoskeleton in addition to motoractivity. Makowska et al.,2015,Cell Reports13,2118–2125December15,2015ª2015The Authors/10.1016/j.celrep.2015.11.012Cell ReportsReportSpecific Myosins Control Actin Organization,Cell Morphology,and Migrationin Prostate Cancer CellsKatarzyna A.Makowska,1Ruth E.Hughes,1Kathryn J.White,1Claire M.Wells,2,*and Michelle Peckham1,*1School of Molecular and Cellular Biology,Faculty of Biological Sciences,University of Leeds,Leeds LS29JT,UK2Division of Cancer Studies,King’s College London,London SE11UL,UK*Correspondence:claire.wells@(C.M.W.),m.peckham@(M.P.)/10.1016/j.celrep.2015.11.012This is an open access article under the CC BY-NC-ND license(/licenses/by-nc-nd/4.0/).SUMMARYWe investigated the myosin expression profile in prostate cancer cell lines and found that Myo1b, Myo9b,Myo10,and Myo18a were expressed at higher levels in cells with high metastatic potential. Moreover,Myo1b and Myo10were expressed at higher levels in metastatic ing an siRNA-based approach,we found that knockdown of each myosin resulted in distinct phenotypes.Myo10 knockdown ablatedfilopodia and decreased2D migration speed.Myo18a knockdown increased circumferential non-muscle myosin2A-associated actinfilament arrays in the lamella and reduced direc-tional persistence of2D migration.Myo9b knock-down increased stressfiber formation,decreased 2D migration speed,and increased directional persis-tence.Conversely,Myo1b knockdown increased numbers of stressfibers but did not affect2D migra-tion.In all cases,the cell spread area was increased and3D migration potential was decreased.There-fore,myosins not only act as molecular motors but also directly influence actin organization and cell morphology,which can contribute to the metastatic phenotype.INTRODUCTIONMyosins are a large and diverse family of molecular motors important for cell migration and motility.The human genome encodes39myosin genes,subdivided into12different classes (Berg et al.,2001;Peckham and Knight,2009).Class2is the largest(13genes).Ten of these are found exclusively in muscle. The remaining three encode the non-muscle(NM)myosin iso-forms2A,2B,and2C,which contribute to cell shape,adhesion, and cytokinesis(Mogilner and Keren,2009;Vicente-Manzanares et al.,2009).Myosin isoforms in the remaining classes contribute to a wide range of functions,including organelle trafficking, membrane tethering,Golgi organization,actin organization, and actin polymerization(Hartman and Spudich,2012).Individ-ual cell types only express a subset of myosin genes.Early studies have shown that 8–11different myosin isoforms are co-expressed in epithelial cell lines,leukocytes,liver cells,and myoblasts(Bement et al.,1994;Wells et al.,1997).Some myosin isoforms are expressed widely,whereas others(e.g.,Myo7a and Myo3)are restricted to a small tissue subset(Dose´and Burnside, 2000;Hasson et al.,1995).It has never been determined how variation in myosin expres-sion profile between closely related cell types contributes to a variation in cellular phenotype.Modulating myosin expression could help to drive a cell toward a more migratory phenotype and,therefore,metastasis in cancer.Here we determined the myosin isoform expression profile in a range of prostate cell lines and in silico and then investigated four of the overexpressed myosin isoforms to uncover how each contribute to the more highly metastatic phenotype of PC-3cells(Pulukuri et al.,2005).RESULTSMyo1b,Myo9b,Myo10,and Myo18a Are Overexpressed in More Highly Metastatic CellsWe analyzed myosin expression for all26of the non-muscle myosin genes in the three most widely used prostate cancer cell lines:PC-3,DU145,and LNCaP(Weber et al.,2004).PC-3 cells are considered to have a higher metastatic potential than LNCaP cells(Aalinkeel et al.,2004).Class2muscle myosin isoforms were excluded because they are not expressed in non-muscle cells.We also analyzed a matched pair of normal (1535NP)and cancerous(1535CT)cell lines derived from the prostate of the same patient(Bright et al.,1997).A core of12myosin genes were expressed in all cell lines tested,as demonstrated by RT-PCR(Table S1).However, DU145cells additionally expressed two myosin isoforms, Myo7a and Myo3,normally only expressed in the cochlea,retina, testis,lung,and kidney(Hasson et al.,1995)or in the retina and pancreas(Dose´and Burnside,2000)respectively,and, therefore,we did not use these cells in further experiments, although,for completeness,the qPCR analysis on these cells is included(Figure S1).Expression levels of MYO1B,MYO1D,MYO1E,MYO9B, MYO10,and MYO18A were significantly higher in PC-3than in LNCaP cells by qPCR(Figure1A).MYO1B and MYO10expression levels were also significantly higher in1535CT than in1535NP cells (Figure1B).An in silico analysis(Figure1C)showed that MYO1B,2118Cell Reports13,2118–2125,December15,2015ª2015The AuthorsMYO1D ,and MYO10levels were significantly higher in metastatic tumors than in benign tissue,suggesting that this trend is also found in vivo.MYO1E and MYO18A expression levels were also higher in 1535CT cells compared with 1535NP cells,although this difference was not significant,and the in silico analysis did not show any significant differences in expression (Figure 1C).However,the expression of MYO18A or MYO1E may be upregu-lated in some tumors.MYO6expression levels were significantly lower in PC-3cells compared with LNCaP (Figure 1A),lowerinFigure 1.Myosin Expression Profiles in Tumors and Prostate Cancer Cell Lines(A)Comparison of the expression levels for 12of the myosin isoforms expressed by LNCaP and PC-3cells,detected by qPCR.Data are presented as mean ±SD (n =3).(B)Comparison of the expression levels for six myosin isoforms expressed by a pair of matched (normal [1535NP]and cancerous [1535CT])pros-tate cancer cell lines,detected by qPCR.Data are presented as mean ±SD (n =3).(C)In silico analysis of the mRNA expression levels for eight myosin isoforms in 171(GEO:GSE6919)prostate tumor samples.Data are shown as the log2expression ratio.Clinical tumor classification and number of samples are indicated.*p <0.05,**p <0.01,***p <0.001.1535CT than in 1535NP cells (Figure 1B),and highest in localized medium-grade tu-mors (Figure 1C),as reported earlier (Dunn et al.,2006;Puri et al.,2010).MYO9B expression levels were increased in tumors compared with benign tissues (Figure 1C).Levels of MYH9,the only non-muscle myosin 2gene we found to be expressed in prostate cancer cells,did not change at the mRNA level (Figure 1A)between LNCaP and PC-3cells or between normal,tumor,or metastatic samples in the in silico analysis.Western blotting for Myo1b,NM2A,Myo6,Myo9b,Myo10,Myo18a,and NM2A in PC-3and LNCaP cells (Fig-ures 2A and 2B)showed similar trends in protein expression levels.In PC-3cells,high endogenous levels of Myo10were associated with a high num-ber of filopodia (Figures 2C and 2D),in which Myo10was localized to the tips (Fig-ure 2F),as expected from its known role in filopodium formation (Berg and Cheney,2002;Berg et al.,2000).In contrast,both fi-lopodium number and Myo10expression levels were low in LNCaP cells (Figure 2C),staining was diffuse,and Myo10was often absent from filopodial tips (Figures 2E and 2F).Upregulation of Myo10in breast can-cer cells has been linked to expression of mutant p53(Arjonen et al.,2014).However,LNCaP cells are p53wild-type,and PC-3cells are p53-null (Carroll et al.,1993),suggesting that,in this case,there is no link between Myo10overexpression and expression of mutant p53.In DU145cells,which do express mutant p53,Myo10expression is slightly higher,and numbers of filopodia are increased compared with LNCaP cells (Figures S1A and S1B),but both are lower compared with PC-3cells.Myo1b localized to organelles in both cell types (Figures 2D and 2E),as expected from its roles in trafficking of endosomes,Cell Reports 13,2118–2125,December 15,2015ª2015The Authors 2119multivesicular bodies,and lysosomes (Cordonnier et al.,2001;Raposo et al.,1999;Salas-Cortes et al.,2005).Higher Myo1b expression in PC-3cells was associated with an additional local-ization of Myo1b to actin-rich structures at the plasma mem-brane and filopodia (Figure 2D),consistent with an earlier study (Tang and Ostap,2001).Myo9b and Myo18a were both enriched in membrane ruffles/lamellipodia in PC-3cells (Figure 2D),consistent with Myo18a’s role in modifying actin organization in the lamellipodium (Hsu et al.,2010)and Myo9b’s role in cell polarity and recruitment of RhoGAP to the lamellipodium (Hanley et al.,2010).Staining for both was diffuse in LNCaP cells.The higher endogenous expression levels of Myo1b,Myo9b,and Myo10in more metastatic cell types/tissue suggested that they all contribute to the cellular phenotype of metastatic cells.We therefore used siRNA knockdown (KD)to determine the effects of reducing their expression levels in PC-3cells on cell morphology and cell migration.We also investigated Myo18a because the interaction of Myo18a regulates NM2A filaments (Billington et al.,2015)and,therefore,may also influence cell migration andphenotype.Figure 2.Expression and Localization of Myosin Isoforms in LNCaP and PC-3Cells(A and B)Example immunoblots (A)showing the variation in expression levels for five myosin iso-forms in PC-3and LNCaP cells.Molecular markers (in kilodalton)are shown on the right.Anti-Myo18a shows a single band with a molecular weight close to 230kDa,suggesting that only the a isoform is expressed (Mori et al.,2003).The results are quantified in (B).Data are presented as mean ±SD (n =3).Expression levels were compared with total p44/p42MAPK (ERK)for all isoforms except Myo9b,for which GAPDH was used.GAPDH gave similar results as ERK as a loading control.(C)Quantification of numbers of filopodia in LNCaP and PC-3cells (n =20).(D and E)Maximum intensity projection images for PC-3(D)and LNCaP (E)cells immunostained for F-actin (red)and either Myo1b,Myo9b,Myo10,or Myo18a (green).The arrows in (D)indicate Myo1b localization within filopodia,Myo9b in actin rich protrusions,Myo10at filopodial tips,and Myo18in actin-rich lamellae.The arrows in (E)indicate Myo1b localization to vesicles and lack of Myo9b in actin-rich protrusions.Merged images include nu-clear staining using DAPI (blue).Scale bar,30m m.(F)Many filopodia,and Myo10at their tips PC-3cells,contrasted with few filopodia with lack of Myo10localization in LNCaP cells.*p <0.05,**p <0.01,***p <0.001.Knockdown of Myo1b,Myo9b,Myo10,and Myo18a Results in Isoform-Specific Changes in Cell Morphology,Cell Migration,and Actin Bundle Organization in PC-3CellssiRNA-mediated KD for 72hr significantly reduced expression levels of each myosin isoform in PC-3cells (Figure 3A)andaltered their morphology (Figure 3B).The spread area of the cells increased up to 3-fold (Figure 3C).KD of Myo10,but not Myo1b,Myo9b,or Myo18a,also significantly reduced the numbers of fi-lopodia (Figure 3D).Although the increase in cell area in Myo10KD cells could be explained by the reduction in filopodia,as re-ported for COS-7and HeLa cells (Bohil et al.,2006),it does not explain the increased cell area for Myo1b,Myo9b,and Myo18a KD cells,where filopodia are still present.Myo9b and Myo10were most important for PC-3cell migra-tion in 2D.Knockdown of Myo9b and Myo10both significantly reduced cell speed 2-fold in a 2D random migration assay (Fig-ures 3E and 3F).Directional persistence was increased slightly for Myo9b KD cells but unaltered for Myo10.In contrast,knock-down of Myo1b and Myo18a did not affect speed in 2D random migration assays (Figures 3E and 3F).Knockdown of Myo18a significantly reduced directional persistence in 2D (Figures 3E and 3G),indicating that these cells are less able to polarize.How-ever,cell migration was inhibited for each myosin in a circular invasion assay (Figure 3H)that closely mimics 3D invasion (Yu and Machesky,2012).Staining for F-actin in circular migration2120Cell Reports 13,2118–2125,December 15,2015ª2015The Authorsassays (Figure 3I)showed an increase in actin stress fibers for cells at the border for each myosin knockdown compared with controls.We also observed distinct changes in the acto-myosin organization following KD of each myosin.Control PC-3cells (Figures 4A and 4B)contained few F-actin stress fibers,andFigure 3.Knockdown of Myosin 1b,9b,10,and 18a Affects Morphology and Cell Migra-tion of PC-3Cells(A)KD of Myo1b,Myo9b,Myo10,or Myo18a in PC-3cells analyzed by immunoblotting after 72hr confirms that myosin levels are reduced signifi-cantly.Loading control:total ERK or GAPDH.(B)Representative fields showing control and myosin-depleted PC-3cells stained for F-actin using fluorescent phalloidin,obtained by tiling using the 340objective on the Zeiss LSM 880.Images are shown in reverse contrast.Arrowheads show prominent stress fibers (Myo1b KD),pe-ripheral high-density actin staining (Myo9b KD),central stress fiber bundles (Myo10KD),and increased actin in lamellae (Myo18a KD).(C and D)Quantification of cell area (C)and number of filopodia (D)in control and myosin KD cells (n =20).NT,control cells treated with non-targeting siRNA.(E)Representative tracks for individual cells during an overnight time-lapse microscopy experiment for control (treated with non-targeting siRNA)and Myo1b,Myo9b,Myo10,and Myo18a-KD cells af-ter HGF stimulation.Each colored line represents the track taken by an individual cell in the field.(F and G)Quantification of speed (F)and direc-tional persistence (G)of cell migration (n =50cells).Data were plotted as box-and-whisker plots,with whiskers showing maximum and minimum values.(H)Circular invasion assay showing cells stained for F-actin and nuclei (DAPI).The dashed line marks the border of cell-free space that was created by stoppers before their removal.Scale bar,100m m and 90m m (Myo18a KD).(I)Magnified images of cells at the border of the cell-free space stained for F-actin.Scale bar,10m m.*p <0.05,**p <0.01,***p <0.001.NM2A staining was mostly localized to the lamellae.A marked increase in centripetal F-actin fibers running parallel to the plasma membrane in the lamella associated with NM2A filaments was characteristic of Myo18a KD (Figures 4A and 4B).The appearance of sparse,long stress fibers,associated with NM2A and extended along the length of the cells,was characteristic of Myo1b KD (Figures 4A–4C).A line profile analysis of the frequency of actin bun-dles in the lamellae of KD cells showed that the frequency of bundles was reduced significantly (2.1±0.1bun-dles/m m,mean ±SEM,n =9)compared with controls (2.6±0.2bundles/m m,mean ±SEM,n =9,p <0.5%)(Figures 4A and 4B),suggesting that the actin cytoskeleton is being re-organized.Myo9b KD cells contained a distinctive actin-rich area at the cell periphery from which NM2A was largely absent,in addition to an increase in stress fibers (Figures 4A and 4B).Cell Reports 13,2118–2125,December 15,2015ª2015The Authors 2121Myo10KD cells showed loss of filopodia and the appearance of distinctive actin bundles in the central region of the cell (Fig-ures 4A and 4B).Changes to the F-actin organization were associated with for-mation of focal adhesions at the edges of the cells,consistent with a more spread cell phenotype (Figure 4C).Knockdown of Myo1b,Myo10,or Myo18a did not change phosphorylation levels of myosin light chain (MLC)(Figure 4D),suggesting that NM2A is re-organized rather than activated as a result of their knockdown.Myo9b KD did increase MLC phosphorylation 2-fold in cells,and this is likely to contribute to the actomyosin re-organization observed (Figure 4D).Figure 4.Knockdown of Myosin 1b,9b,10,and 18a Affects Focal Adhesion and Actin and NM2A Organization in PC-3Cells(A and B)Control cells treated with non-targeting siRNA and myosin-depleted cells co-stained for F-actin (red)and NM2A (green)(A).An enlarged view of the region in the boxed area in (A)is shown in (B).Arrowheads indicate F-actin structures in KD cells.Scale bars,5m m (A)and 2m m (B).(C)Control NT cells and myosin-depleted cells co-stained for F-actin (red)and focal adhesions using paxillin (green).Arrowheads indicate focal adhesions at the ends of F-actin bundles in KD cells Scale bar,30m m.(D)Immunoblotting for phosphorylated myosin light chain (pMLC)and ERK or GAPDH (loading control)in control and KD cells.Quantification of the control and Myo9b KD blot shown here showed a 1.8-fold increase in pMLC in KD compared with NT cells.UT,untreated.DISCUSSIONThese data show that Myo10,Myo9b,and Myo1b are overexpressed in more highly metastatic cell lines and in metastatic tissue.High levels of Myo10in PC-3cells are linked to high numbers of filopodia,and high levels of Myo9b are linked to low levels of stress fibers.Both isoforms contribute to a more migratory phenotype,as shown by immunostaining,cell migra-tion,and KD experiments.Myo1b and Myo18a influence cell morphology and actin organization but have little effect on migration in 2D,whereas all four iso-forms inhibit cell migration in invasion assays.Therefore,changes in expression of several myosin isoforms may contribute to metastasis in prostate cancer.Our finding that Myo10-dependent filopodia are likely to be important in pros-tate cancer agrees with recent similar findings for breast cancer metastasis (Ar-jonen et al.,2014;Cao et al.,2014)and non-small lung cell cancer (Sun et al.,2015).Filopodia are important but notabsolutely required for cell migration because Myo10KD cells can migrate in 2D but with a reduced speed,and other cells lack-ing filopodia can migrate (Lundquist,2009).The increased cell area resulting from Myo10KD agrees with previous findings (Bohil et al.,2006).The central actin bundles in Myo10knock-down cells are reminiscent of actin bundles in filopodia.Fascin is also required for filopodial formation (Vignjevic et al.,2006),its overexpression results in multiple filopodia (Vignjevic et al.,2006),and fascin levels are also upregulated in prostate cancer (Darnel et al.,2009).Myo10KD may lead to actin bundling in the cell body by excess (non-phosphorylated)fascin.The role of two filopodial proteins,Myo10and fascin,in prostate (and other)2122Cell Reports 13,2118–2125,December 15,2015ª2015The Authorscancers suggest thatfilopodium formation is key for metastasis. Myo10has also been implicated in integrin-mediated adhesion, and any reduction in adhesion resulting from its KD could disrupt signaling to the actin cytoskeleton and,therefore,indirectly result in changes in actin organization.High levels of Myo9b expression in PC-3cells are likely to contribute to their lack of stressfibers and,therefore,to enhanced migration.The RhoGTPase-activating domain in Myo9b inhibits Rho,reducing the downstream activity of ROCK(RhoKinase), thereby increasing MLC phosphatase activity,reducing MLC phosphorylation(Reinhard et al.,1995;Wirth et al.,1996),and, therefore,reducing actin stressfiber formation.Knockdown of Myo9b is therefore expected to increase MLC phosphorylation and stressfiber formation,as we observed.In agreement with ourfindings,a previous report has shown that cell migration was reduced and MLC phosphorylation increased in macro-phages isolated from Myo9b knockout mice(Hanley et al., 2010).Myo9b has also been implicated in an increased risk of esophageal cancer(Menke et al.,2012).The high levels of Myo1b in PC-3cells and effects of knock-down on3D invasion,cell shape,and morphology suggest that it,too,has a role in prostate cancer.Myo1b has also been impli-cated in non-small-cell lung cancers(Ohmura et al.,2015). Myo1b(Myr1/MM1a;Gillespie et al.,2001)regulates actin as-sembly in vesicular transport(post-Golgi carriers[Almeida et al.,2011]and endocytic organelles[Cordonnier et al.,2001; Raposo et al.,1999]),and it maintains cortical tension at the plasma membrane,where it specifically associates with dy-namic,non-tropomyosin-containing actinfilaments(Coluccio and Geeves,1999;Tang and Ostap,2001).High endogenous levels of Myo1b in more highly metastatic cells might therefore increase cortical tension,allowing cells to move through stiff extracellular matrices in vivo,perhaps explaining why knock-down of Myo1b only affects migration in3D but not2D.Myo18a could contribute to metastasis in prostate cancer. The re-organization of actin and NM2A in Myo18a KD cells may arise from its interaction with non-muscle myosin2(NM2). NM2forms shortfilaments( 300nm long)containing 20mol-ecules(Billington et al.,2013).The assembly/disassembly of non-muscle myosin2filaments is dynamic(Shutova et al., 2014)and regulated by many different pathways(Vicente-Man-zanares et al.,2009).Myo18a and NM2A can form mixed bipolar filaments in vitro that are smaller that pure NM2Afilaments(Bill-ington et al.,2015).The re-organization of NM2A we observed after knocking down Myo18a,without a change to levels of light chain phosphorylation,supports the idea that an interaction be-tween Myo18a and NM2A modulates NM2Afilament formation and organization in PC-3cells.Therefore,Myo1b,Myo9b,Myo10,and Myo18a each contribute to the morphology and migration of more highly met-astatic PC-3cells,with each myosin having a specific effect on actin organization.Misregulation of their expression in cells with metastatic potential may allow them to work in concert to generate a torpedo-shaped cell with multiple protrusions that is better able to migrate through a3D matrix and,therefore, more able to metastasize.Many different drugs have now been developed that can inhibit specific myosin isoforms,including those in classes1,2,5,and6(Bond et al.,2013).Developing drugs to block specific myosin functions could be useful in pre-venting metastasis.Importantly,these results emphasize that myosin not only uses actin as tracks to walk along but that it is able to actively drive actin organization in cells. EXPERIMENTAL PROCEDURESCell CultureLNCaP,DU145,and PC-3cells(from the ATCC)were grown in RPMI1640me-dium with GlutaMAX(Gibco,Life Technologies)supplemented with10%heat-inactivated fetal bovine serum(FBS)and penicillin-streptomycin.1535NP and CT cells(Bright et al.,1997)were a gift from Suzanne Topalian(Johns Hopkins University School of Medicine).They were grown in keratinocyte medium (Gibco,Life Technologies)supplemented with10%heat-inactivated FBS, 1%L-glutamate,antibiotics,bovine pituitary extract,and epidermal growth factor.Antibodies and ReagentsThe antibodies used were as follows:Myo6(H-215,Santa Cruz Biotech-nology);Myo10(HPA024223,Sigma);total ERK(p44/42mitogen-activated protein kinase[MAPK],Cell Signaling Technology);Myo18a,a gift from Prof. Yu and Dr.Hsu(Chang Gung University,Taiwan;Hsu et al.,2010)or from Genscript;NM2A(PRB-440P,Covance);paxillin(SAB4502553,Sigma); Myo1b(HPA013607,Sigma);phospho-myosin light chain(Cell Signal); Myo9b(Proteintech);and glyceraldehyde3-phosphate dehydrogenase (GAPDH)(Abcam).HRP-conjugated secondary antibodies andfluorescent phalloidin were from Sigma,andfluorescent secondary antibodies were from Molecular Probes.TransfectionssiGENOME SMARTpool siRNA(GE Healthcare,Dharmacon)was used to silence myosins in PC-3cells.Cells were seeded at a density of20,000 cells/ml in growth media and allowed to adhere and grow overnight.Lipofect-amine RNAiMAX reagent(Invitrogen,Life Technologies)was used for transfec-tions.Maximum KD was achieved after72hr.PCRThe RNeasy mini kit(QIAGEN)was used to extract cellular RNA.cDNA was synthesized using avian myeloblastosis virus(AMV)reverse transcriptase. RT-PCR was used to detect which myosin isoforms were expressed(Table S1).Real-time PCR using SYBR Green was used to investigate the expression levels of expressed myosins(see Table S2for primer sequences).Data anal-ysis was performed using a Bio-Rad system and software.ImmunoblottingCells were lysed(30min,4 C)in lysis buffer(150mM NaCl,0.05M Tris[pH8], 1%Triton X-100,and1mM EDTA[pH8]with protease inhibitor cocktail (Thermo Scientific).Lysates were clarified by centrifugation,protein content was quantified by bicinchoninic acid(BCA)assay,and then samples were mixed with23Laemmli buffer for use in protein gels(4%–20%or7.5%)and blots.Chemiluminescence detection(Supersignal West Pico,Thermo Scien-tific)used multiple exposures to ensure signal linearity.If required,membranes were stripped using Restore western blot stripping buffer(Thermo Scientific) and re-probed.ImmunostainingCells were grown on glass coverslips,fixed with2%paraformaldehyde in PBS, and stained using standard procedures(Swailes et al.,2006).Cells were imaged using a DeltaVision deconvolution microscope or Zeiss880Airyscan.Migration AssaysFor2D assays,cells were plated onto a glass-bottomed96-well plates,trans-fected with non-targeting siRNA or with myosin KD siRNA(three replicates each),serum-starved48hr later for24hr,and then treated with hepatocyte growth factor(HGF)(25ng/ml)forfilming.A minimum of threefields from each replicate was selected for imaging,over14hr at5-min intervals usingCell Reports13,2118–2125,December15,2015ª2015The Authors2123differential interference contrast(DIC)optics,and a203lens(5123512total pixel size,232binning)on a DeltaVision system.Cell migration was analyzed using ImageJ software(MTrackJ plugin).To perform the3D-like circular inva-sion assay(Yu and Machesky,2012),cell-free space was created using cell stoppers(Ibidi).After removing the stopper,cells were covered with a thin layer of Matrigel(4mg/ml)and normal medium and allowed to grow and migrate for another24–48hr.Cells were thenfixed in2%paraformaldehyde(PFA)and stained.Data AnalysisImmunoblots and digitized images of immunostained cells were analyzed us-ing ImageJ.GraphPad Prism5.0was used to analyze data.Data are presented as mean±SD for at least three separate experiments(n R3).A two-way ANOVA was used to compare differences between groups,and statistical sig-nificance was accepted for p%0.05.SUPPLEMENTAL INFORMATIONSupplemental Information includes onefigure and two tables and can be found with this article online at /10.1016/j.celrep.2015.11.012.AUTHOR CONTRIBUTIONSConceptualization,C.M.W.and M.P.;Methodology,K.A.M.,C.M.W.,and M.P.;Investigation,K.A.M.,K.J.W.,R.H.,and M.P.;Writing–Original Draft, K.A.M.and M.P.;Writing–Review&Editing,K.A.M.,C.M.W.,and M.P.;Fund-ing Acquisition,C.M.W.and M.P.;Resources,C.M.W.and M.P.;Supervision, C.M.W.and M.P.ACKNOWLEDGMENTSThis work was supported by Yorkshire Cancer Research Pilot Grant MS/JF/ LPP044(to M.P.and K.J.W.);CRUK Studentship C37059/A11941(to K.M. and M.P.),an MRC studentship(to R.H.and M.P.),and Guys and St Thomas Charity funding(to C.M.W.).The Wellcome Trust(WT104918MA to M.P.) funded the Zeiss Airyscan confocal microscope.Received:June2,2015Revised:August27,2015Accepted:November2,2015Published:December3,2015REFERENCESAalinkeel,R.,Nair,M.P.,Sufrin,G.,Mahajan,S.D.,Chadha,K.C.,Chawda,R.P., and Schwartz,S.A.(2004).Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells.Cancer Res.64,5311–5321.Almeida,C.G.,Yamada,A.,Tenza,D.,Louvard,D.,Raposo,G.,and Coudrier, E.(2011).Myosin1b promotes the formation of post-Golgi carriers by regu-lating actin assembly and membrane remodelling at the trans-Golgi network. Nat.Cell Biol.13,779–789.Arjonen,A.,Kaukonen,R.,Mattila,E.,Rouhi,P.,Ho¨gna¨s,G.,Sihto,H.,Miller, B.W.,Morton,J.P.,Bucher,E.,Taimen,P.,et al.(2014).Mutant p53-associ-ated myosin-X upregulation promotes breast cancer invasion and metastasis. J.Clin.Invest.124,1069–1082.Bement,W.M.,Hasson,T.,Wirth,J.A.,Cheney,R.E.,and Mooseker,M.S. (1994).Identification and overlapping expression of multiple unconventional myosin genes in vertebrate cell A91,11767.Berg,J.S.,and Cheney,R.E.(2002).Myosin-X is an unconventional myosin that undergoes intrafilopodial motility.Nat.Cell Biol.4,246–250.Berg,J.S.,Derfler,B.H.,Pennisi,C.M.,Corey,D.P.,and Cheney,R.E.(2000). Myosin-X,a novel myosin with pleckstrin homology domains,associates with regions of dynamic actin.J.Cell Sci.113,3439–3451.Berg,J.S.,Powell,B.C.,and Cheney,R.E.(2001).A millennial myosin census. Mol.Biol.Cell12,780–794.Billington,N.,Wang,A.,Mao,J.,Adelstein,R.S.,and Sellers,J.R.(2013).Char-acterization of three full-length human nonmuscle myosin II paralogs.J.Biol. Chem.288,33398–33410.Billington,N.,Beach,J.R.,Heissler,S.M.,Remmert,K.,Guzik-Lendrum,S., Nagy,A.,Takagi,Y.,Shao,L.,Li,D.,Yang,Y.,et al.(2015).Myosin18A coas-sembles with nonmuscle myosin2to form mixed bipolarfilaments.Curr.Biol. 25,942–948.Bohil,A.B.,Robertson,B.W.,and Cheney,R.E.(2006).Myosin-X is a molecular motor that functions infilopodia A103, 12411–12416.Bond,L.M.,Tumbarello,D.A.,Kendrick-Jones,J.,and Buss,F.(2013).Small-molecule inhibitors of myosin proteins.Future Med.Chem.5,41–52.Bright,R.K.,Vocke,C.D.,Emmert-Buck,M.R.,Duray,P.H.,Solomon,D., Fetsch,P.,Rhim,J.S.,Linehan,W.M.,and Topalian,S.L.(1997).Generation and genetic characterization of immortal human prostate epithelial cell lines derived from primary cancer specimens.Cancer Res.57,995–1002.Cao,R.,Chen,J.,Zhang,X.,Zhai,Y.,Qing,X.,Xing,W.,Zhang,L.,Malik,Y.S., Yu,H.,and Zhu,X.(2014).Elevated expression of myosin X in tumours contrib-utes to breast cancer aggressiveness and metastasis.Br.J.Cancer111, 539–550.Carroll,A.G.,Voeller,H.J.,Sugars,L.,and Gelmann,E.P.(1993).p53onco-gene mutations in three human prostate cancer cell lines.Prostate23, 123–134.Coluccio,L.M.,and Geeves,M.A.(1999).Transient kinetic analysis of the 130-kDa myosin I(MYR-1gene product)from rat liver.A myosin I designed for maintenance of tension?J.Biol.Chem.274,21575–21580.Cordonnier,M.N.,Dauzonne,D.,Louvard,D.,and Coudrier,E.(2001).Actin filaments and myosin I alpha cooperate with microtubules for the movement of lysosomes.Mol.Biol.Cell12,4013–4029.Darnel,A.D.,Behmoaram,E.,Vollmer,R.T.,Corcos,J.,Bijian,K.,Sircar,K., Su,J.,Jiao,J.,Alaoui-Jamali,M.A.,and Bismar,T.A.(2009).Fascin regulates prostate cancer cell invasion and is associated with metastasis and biochem-ical failure in prostate cancer.Clin.Cancer Res.15,1376–1383.Dose´,A.C.,and Burnside,B.(2000).Cloning and chromosomal localization of a human class III myosin.Genomics67,333–342.Dunn,T.A.,Chen,S.,Faith,D.A.,Hicks,J.L.,Platz,E.A.,Chen,Y.,Ewing,C.M., Sauvageot,J.,Isaacs,W.B.,De Marzo,A.M.,and Luo,J.(2006).A novel role of myosin VI in human prostate cancer.Am.J.Pathol.169,1843–1854.Gillespie,P.G.,Albanesi,J.P.,Bahler,M.,Bement,W.M.,Berg,J.S.,Burgess, D.R.,Burnside,B.,Cheney,R.E.,Corey,D.P.,Coudrier,E.,et al.(2001). Myosin-I nomenclature.J.Cell Biol.155,703–704.Hanley,P.J.,Xu,Y.,Kronlage,M.,Grobe,K.,Scho¨n,P.,Song,J.,Sorokin,L., Schwab,A.,and Ba¨hler,M.(2010).Motorized RhoGAP myosin IXb(Myo9b) controls cell shape and A107,12145–12150.Hartman,M.A.,and Spudich,J.A.(2012).The myosin superfamily at a glance. J.Cell Sci.125,1627–1632.Hasson,T.,Heintzelman,M.B.,Santos-Sacchi,J.,Corey,D.P.,and Mooseker, M.S.(1995).Expression in cochlea and retina of myosin VIIa,the gene product defective in Usher syndrome A92,9815–9819.Hsu,R.M.,Tsai,M.H.,Hsieh,Y.J.,Lyu,P.C.,and Yu,J.S.(2010).Identification of MYO18A as a novel interacting partner of the PAK2/betaPIX/GIT1complex and its potential function in modulating epithelial cell migration.Mol.Biol.Cell 21,287–301.Lundquist,E.A.(2009).Thefiner points offilopodia.PLoS Biol.7,e1000142.Menke,V.,Van Zoest,K.P.,Moons,L.M.,Pot,R.G.,Siersema,P.D.,Kuipers, E.J.,and Kusters,J.G.(2012).Myo9B is associated with an increased risk of Barrett’s esophagus and esophageal adenocarcinoma.Scand.J.Gastroen-terol.47,1422–1428.Mogilner,A.,and Keren,K.(2009).The shape of motile cells.Curr.Biol.19, R762–R771.2124Cell Reports13,2118–2125,December15,2015ª2015The Authors。
