8-Bacterial Infection and Immunity
本科生练习题_(医学生文献检索操作题)

本科生练习题一、网络资源检索1.利用百度检索出文献类型是PDF,文献内容是“冠心病”的文献,输入的是什么,检索的结果数量是多少?2. 利用Google查找网页标题中含“创新型大学”一词的页面,输入的是什么,检索结果数量是多少?3. 如何在google上查找造血干细胞的幻灯片?4. 利用google学术搜索查找我校李群伟教授在《泰山医学院学报》上发表的文章,并被引次数最高的文献。
此外,你能得到李教授的院系和邮件联系方式吗?5. 通过Google学术搜索检索论文“抗甲突汤治疗甲亢突眼症的临床疗效观察”被引用情况。
6.在google 或百度中检索关于网页制作的Powerpoint教学课件,给出检索策略和检索命令。
(提示:Powerpoint文件格式为ppt)7. 查找人类首次登月成功的时间、国家、宇航员姓名和宇宙飞船的名称。
8.提供SPECT中英文名称9. 《中华放射学杂志》被引频次最高的论文作者10. 以Google()为例,检索《中国循证医学杂志》上发表的篇名中包含“循证医学”(词组)的文章被引用情况。
要求提供检索词、检索步骤,检索结果数量,抄下被引频次最高一篇论文的题录和引用该文献且被引频次最高的他引文献题录。
11.查找2000年在《中国心理卫生杂志》上发表的心理健康(词组,出现在篇名)文献的被引情况,应使用何种搜索引擎,该如何检索?写出检索词和操作步骤,抄下被引频次最高的文献题录。
12. 你知道王老吉的历史是怎样的?创始人又是谁?你能找到他的图片吗?13.使用high wire press检索2005-至今西咪替丁治疗胃溃疡的文章,写出检索步骤和结果数。
二、中国生物医学文献数据库(CBMdisc)CBMdisc的检索步骤进入数据库系统:双击桌面快捷方式cbmWin2004,在出现的窗口上点击左键,进入CBM数据库,点击窗口右下方的开始键,进入数据库检索系统。
实习题一:检索肝癌的MRI诊断评价1. 进行主题词检索(1)进行MRI的主题词检索。
牙周病免疫英文版

Defense Mechanisms of Pefiodontal Tissues
• (四)saliva
The active ingredient in saliva, flow and the flow rate of saliva is closely related to protecting function. 1. Effective saliva flow and flow rate to provide the necessary lubrication to help deliver food, remove bacteria and epithelial shedding, add fresh antibacterial ingredient. 2. Salivary proteins form saliva biofilm, conducive to bacterial attachment and setting. 3. The antimicrobial composition of saliva : Lysozyme :cause that bacteria decomposition and death。 Peroxidase:Generating nascent oxygen, kill bacteria substance. Lactoferrin: Competition for nutrients iron with bacteria, inhibit bacterial growth.
Defense Mechanisms of Pefiodontal Tissues
• (一) epithelial barrier
1. Junctional epithelium update rate is about 5 days. Surface senescent cells shed into the gingival sulcus, while take away the bacteria attached to the junctional epithelium. 2. Junctional epithelium cell produce antibacterial substances: defensins, interleukin8 , interleukin 1, tumor necrosis factor a, they induce and activate special defensive cells such as polymorphonuclear leukocyte.
细菌的感染和免疫

▪ 〔nonpathogenic bacterium, nonpathogen〕
第二页,共四十二页。
正常菌群〔normal flora)
▪ 正常人的体表和同外界相通的口腔、鼻咽 腔、肠道、泌尿生殖道等腔道中都寄居着 不同种类和数量的微生物。当人体免疫功 能正常时,这些微生物对宿主无害,有些 对人还有利,是为正常微生物群,通称正 常菌群
皮肽
葡萄球菌、绿脓杆菌、白念珠菌 丙酸杆菌、类白喉杆菌、 非致病性分枝杆菌
尿道
白色葡萄球菌、类白喉杆菌、 非致病性分枝杆菌
肠道
大肠杆菌、产气杆菌、变形杆菌 绿脓杆菌、葡萄球菌、厌氧性细 菌 真菌、乳杆菌,双歧杆菌等
第五页,共四十二页。
健康壮年
粪便涂片
健康青年 粪便涂片
肠道的正常菌群
第六页,共四十二页。
致病条件
寄居部位的改变
免疫功能低下
菌群失调〔dysbacteriosis〕
第十一页,共四十二页。
医院获得性感染〔hospital acquired infection〕
病人在住院期间发生的感染,通称医院内 感染〔nosocomial infection〕。 ①交叉感染,由医院内病人或医务人员直 接或间接传播引起的感染; ②内源性感染,或称自身感染,由病人自 己体内正常菌群引起的感染; ③医源性感染,在治疗、诊断或预防过程 中,因所用器械等消毒不严而造成的感染。
第十五页,共四十二页。
第十六页,共四十二页。
毒素 〔toxin〕
外毒素〔exotoxin〕多数革兰阳性菌和少数革 兰阴性菌在生长繁殖过程中释放到菌体外的蛋 白质
内毒素〔endotoxin〕革兰阴性菌细胞壁的脂 多糖,菌体死亡崩解时游离出来
消化性溃疡穿孔诊断与治疗的最新进展

溃疡穿孔,能够降低病死率,减少并发症,存在着较 认为,对腹部x线检查阴性而又高度怀疑消化性溃
大争议。目前多数学者赞成手术治疗。
疡穿孑L者,可采取经胃管注入空气150~300 mL后再
1 消化性溃疡穿孔的早期诊断
行腹部X线检查的方法协助诊断[4】。曾连山等[51报
典型消化性溃疡穿孑L的诊断一般并不困难。急 道,行腹腔穿刺79例,阳性率为60.8%。术前有气
一些学者认为单纯穿孔修补术操作简单危险性较小对病情重不能耐受溃疡切除术或病史较短穿孔小瘢痕少青壮年患者修补术后积极内科治疗可治愈对穿孑l周围组织较硬或水肿严重者不宜缝合可将大网膜填入穿孔处周围用细丝线缝合固定于胃壁或十二指肠壁引
·10r7·
mucosal immunity despite in vivo MAdCAM一1 blockade of lympho- cyte homing[J].Ann Surg,2003,237(5):677-685.
复发而接受外科治疗的患者明显减少。但是,溃疡 (26.5%)。有相当一部分患者没有溃疡病史,x线
穿孔的发生率并未明显降低。溃疡穿孔通常发病急 检查也不见膈下游离气体,还有些老年人机体反应
骤,病情严重,是目前临床上常见的急腹症之一,处 差,对疼痛刺激不敏感,加上腹壁肌肉萎缩或脂肪堆
理不当甚至可危及生命旧J。对于采用何种方法治疗 积,使腹膜刺激征不典型,则易误诊或漏诊。有报道
收稿日期:2008-08·11修回日期:2008-10-08
消化性溃疡穿孔诊断与治疗的最新进展
ห้องสมุดไป่ตู้
中图分类号:11573.1
董迎(综述),崔华雷(审校)
(天津市儿童医院外科,天津300074)
文献标识码:A
J. Biol. Chem.-2007-Chen-28929-38

A Cellular Micro-RNA,let-7i,Regulates Toll-like Receptor4 Expression and Contributes to Cholangiocyte Immune Responses against Cryptosporidium parvum Infection* Received for publication,March27,2007,and in revised form,July26,2007Published,JBC Papers in Press,July27,2007,DOI10.1074/jbc.M702633200 Xian-Ming Chen1,2,Patrick L.Splinter1,Steven P.O’Hara1,and Nicholas Russo3From the Miles and Shirley Fiterman Center for Digestive Diseases,Division of Gastroenterology and Hepatology,Mayo Clinic College of Medicine,Rochester,Minnesota55905Toll-like receptors(TLRs)are important pathogen recogni-tion molecules and are key to epithelial immune responses to microbial infection.However,the molecular mechanisms that regulate TLR expression in epithelia are obscure.Micro-RNAs play important roles in a wide range of biological events through post-transcriptional suppression of target mRNAs.Here we report that human biliary epithelial cells(cholangiocytes) express let-7family members,micro-RNAs with complementa-rity to TLR4mRNA.We found that let-7regulates TLR4expres-sion via post-transcriptional suppression in cultured human cholangiocytes.Infection of cultured human cholangiocytes with Cryptosporidium parvum,a parasite that causes intestinal and biliary disease,results in decreased expression of primary let-7i and mature let-7in a MyD88/NF-B-dependent manner. The decreased let-7expression is associated with C.parvum-induced up-regulation of TLR4in infected cells.Moreover, experimentally induced suppression or forced expression of let-7i causes reciprocal alterations in C.parvum-induced TLR4 protein expression,and consequently,infection dynamics of C. parvum in vitro.These results indicate that let-7i regulates TLR4expression in cholangiocytes and contributes to epithelial immune responses against C.parvum infection.Furthermore, the data raise the possibility that micro-RNA-mediated post-transcriptional pathways may be critical to host-cell regulatory responses to microbial infection in general.Toll-like receptors(TLRs)4are an evolutionarily conserved family of cell surface pattern recognition molecules that play a key role in host immunity through detection of pathogens(1,2). Most TLRs,upon recognition of discrete pathogen-associated molecular patterns,activate a set of adaptor proteins(e.g.mye-loid differentiation protein88(MyD88))leading to the nuclear translocation of transcription factors,such as NF-B and AP-1, and thus transcriptionally regulate host-cell responses to pathogens,including parasites(3–5).TLRs may also recognize endogenous ligands induced during the inflammatory response (1–4).Evidence is accumulating that the signaling pathways associated with TLRs not only mediate host innate immunity but are also important to adaptive immune responses to micro-bial infection(6).Epithelial cells express TLRs and activation of TLRs triggers an array of epithelial defense responses,including production and release of cytokines/chemokines and anti-mi-crobial peptides(1–8).Expression of TLRs by epithelia is tightly regulated,reflecting the specific microenvironment and function of each epithelial cell type.This cell specificity is crit-ically important to assure that an epithelium will recognize invading pathogens but not elicit an inappropriate immune response to endogenous ligands or commensal microorgan-isms(4).Human bile is thought to be sterile under physiological con-ditions(9).Nevertheless,the biliary tract is connected and open to the intestinal tract and therefore,is potentially exposed to microorganisms from the gut.For example,duodenal microor-ganisms are believed to be a major source of bacterial infection in several biliary diseases(9,10).Indeed,Cryptosporidium par-vum,a coccidian parasite of the phylum Apicomplexa,prefer-entially infects the small intestine yet can infect biliary epithe-lial cells(i.e.cholangiocytes)causing biliary tract disease.We and others previously reported that human cholangiocytes express all10known TLRs and produce a variety of inflamma-tory cytokines/chemokines and antimicrobial peptides in response to microbial infection,suggesting a key but poorly understood role for cholangiocytes in epithelial defense(9–14). We also previously demonstrated that TLR2and TLR4signals mediate cholangiocyte responses including production of human-defensin2against C.parvum via TLR-associated acti-vation of NF-B(11).Dominant negative TLR/MyD88express-ing cholangiocytes have diminished defenses against C.parvum infection in vitro(11).Thus,TLRs and the mechanisms involved in their regulation are key elements for cholangiocyte defense against C.parvum infection.Micro-RNAs(miRNAs)are a newly identified class of endogenous small regulatory RNAs(15–17).In the cyto-*This work was supported by National Institutes of Health Grants AI071321 (to X.-M.C.),DK57993and DK24031(to N.F.L.)and the Mayo Foundation.The costs of publication of this article were defrayed in part by the pay-ment of page charges.This article must therefore be hereby marked “advertisement”in accordance with18U.S.C.Section1734solely to indi-cate this fact.1These authors contributed equally to this work.2To whom correspondence may be addressed:Dept.of Medical Microbiol-ogy and Immunology,Creighton University School of Medicine,2500Cal-ifornia Plaza,Omaha,NE68178.Tel.:402-280-3750;Fax:402-280-1875;E-mail:xianmingchen@.3To whom correspondence may be addressed:Mayo Clinic College of Medi-cine,200First St.,SW,Rochester,MN55905.Tel.:507-284-1006;Fax:507-284-0762;E-mail:larusso.nicholas@.4The abbreviations used are:TLRs,Toll-like receptors;miRNAs,micro-RNAs;MyD88,myeloid differentiation protein88;FITC,fluorescein isothiocya-nate;LPS,lipopolysaccharide;UTR,untranslated region;RT,reverse tran-scriptase;DN,dominant negative;EST,expressed sequence tag;ANOVA, analysis of variance.THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL.282,NO.39,pp.28929–28938,September28,2007©2007by The American Society for Biochemistry and Molecular Biology,Inc.Printed in the U.S.A.at ZHEJIANG UNIVERSITY, on January 10, Downloaded fromplasm,they associate with messenger RNAs(mRNAs)based on complementarity between the miRNAs and the target mRNAs.This binding causes either mRNA degradation or translational suppression resulting in gene suppression at a post-transcriptional level(15–17).Micro-RNAs exhibit tis-sue-specific or developmental stage-specific expression, indicating that their cellular expression is tightly regulated(15–17).Nevertheless,the molecular mechanisms underlying cellular regulation of miRNA expression are unclear.Recent studies have indicated that transcription factors,such as c-Myc and C/EBP␣,appear to be involved in the expression of miRNAs, suggesting a role for transcription factors in regulation of miRNA expression(18,19).It has become clear that miRNAs play essential roles in several biological processes,including development,differentiation,and apoptotic cell death(15–27). Also,an antiviral role for miRNAs has been described in plants (15).Direct evidence of the importance of miRNAs in verte-brates to control viral invasion has also recently emerged from studies using human retroviruses(20);a host-cell miRNA,miR-32,has been identified that can effectively suppress primate foamy virus type1replication(20).Conversely,a primate foamy virus type1-derived protein,Tas,alters host-cell miRNA expression(20).Thus,host-pathogen interactions can influ-ence host-cell miRNA-mediated post-transcriptional suppres-sion,a process potentially involved in the regulation of epithe-lial defenses in response to microbial infection.up-regulation of TLR4in infected cells,and consequently,the infection dynamics of C. parvum in vitro.Thus,a novel let-7-mediated regulatory path-way for TLR4expression has been identified in cholangiocytes, a process that is involved in epithelial responses against micro-bial infection.EXPERIMENTAL PROCEDURESC.parvum and Cholangiocyte Cell Lines—C.parvum oocysts of the Iowa strain were purchased from a commercial source (Bunch Grass Farm,Deary,ID).Before infecting cells,oocysts were excysted to release infective sporozoites as previously described(11,28).Freshly excysted sporozoites were tested for lipopolysaccharide(LPS)activity using the Limulus Amebocyte Lysate test kit(Bio-Whittaker,Walkersville,MD)as reported by others(29)and only Limulus Amebocyte Lysate-negative sporozoites were used in the study.H69cells are SV40-trans-formed normal human cholangiocytes originally derived from normal liver harvested for transplant.These cholangiocytes continue to express biliary epithelial cell markers,including cytokeratin19,␥-glutamyl transpeptidase and ion transporters consistent with biliary function and have been extensively char-acterized(11,28).In Vitro Infection Model—An in vitro model of human biliary cryptosporidiosis using H69cells was employed as previously described(11,28).Before infecting cells,oocysts were excysted to release infective sporozoites(11,28).Infection was done in a culture medium consisting of Dulbecco’s modified Eagle’s medium/F-12,100units/ml penicillin,and100g/ml strepto-mycin and freshly excysted sporozoites(1ϫ106sporozoites/ per slide well or culture plate).Inactivated organisms(treated at 65°C for30min)were used for sham infection controls.For some experiments,H69cells were exposed to LPS(100ng/ml, Invivogen)for12h.For the inhibitory experiments,SN50, one specific inhibitor of NF-B,was added in the medium at the same time as C.parvum.A concentration of50g/ml, which showed no cytotoxic effects on H69cells or on C. parvum sporozoites,was selected for the study.All the experiments were performed in triplicate. Immunofluorescent Microscopy—After exposure to C.par-vum as described above,cells were fixed with2%paraform-aldehyde and permeabilized with0.2%(v/v)Triton X-100in phosphate-buffered saline.For double-immunofluorescent labeling of TLR4with C.parvum,fixed cells were incubated with a monoclonal antibody TLR4(Imgenex)mixed with a polyclonal antibody against C.parvum(a gift from Dr.Guan Zhu,Texas A&M University,College Station,TX)followed by anti-mouse and anti-rabbit secondary antibodies(Molecular Probes)as we previously reported(11,28).In some experi-ments,4Ј,6-diamidino-2-phenylindole(5M)was used to stain cell beled cells were assessed by confocal laser scan-ning microscopy.Western Blot—A previously reported semi-quantitative Western blot approach was used to assess TLR4expression in cells(11).Briefly,total cell lysates were obtained from the cells after exposure to C.parvum and blotted for TLR4and actin. Antibodies to TLR4(Imgenex)and actin(Sigma)were used. TLR4levels were expressed as their ratio to actin(11). Microarray Analysis of Endogenous miRNA Expression—H69 cells were grown to confluence and total RNAs were isolated using the TRIzol kit(Invitrogen).Micro-RNA expression pro-file in H69cells was performed with a recently developed semi-quantitative microarray approach with the GenoExplorer TM micro-RNA Biochips by Genosensor(Tempe,AZ).5S rRNA was detected as the control and data were analyzed with the software provided(Genosensor).let-7i Precursor and Antisense Oligonucleotide—To manipu-late cellular function of let-7i in H69cells,we utilized an anti-sense approach to inhibit let-7i function and precursor trans-fection to increase let-7i expression.For experiments,H69cells were grown to subconfluence and then incubated in culture medium containing let-7i antisense2-methoxy oligonucleotide (Ambion,20ng/ml)for12h.For let-7i precursor transfection, H69cells were grown to60–70%confluence and transfected with the let-7i precursor(Ambion,20ng/ml)using the NeoFx transfection agent(Ambion).Those cells were usually used for experiments12h after transfection.Northern Blot—Total RNA from cultured cells was isolated using the conventional method of acid phenol:chloroform extraction using TRI Reagent(Sigma)and subsequent alcohol precipitation.The total RNA was then used as the starting material for the enrichment of miRNAs.Micro-RNAs were enriched using the mirVana miRNA Isolation kit(Ambion).let-7Regulates Cholangiocyte Immunityat ZHEJIANG UNIVERSITY, on January 10, Downloaded fromFor Northern blot detection,1g of miRNAs was separated on a15%polyacrylamide gel in1ϫTBE.Following electro-phoresis,miRNAs were transferred to nylon membrane using a semi-dry transfer and then UV cross-linked to the membrane using120mJ for30s.The probes for the detection of miRNAs and the5S rRNA were synthesized using an in vitro transcrip-tion approach with[␣-32P]UTP.The templates for the in vitro transcription of let-7i were:let-7i(5Ј-TGAGGTAGTAGTTT-GTGCTGTCCTGTCTC-3Ј)and5S rRNA(5Ј-GTTAGTACT-TGGATGGGAGACCGCCCTGTCTC-3Ј).The membranes were incubated with2.5ϫ106cpm per blot overnight at42°C. Subsequently,stringent washes were performed and the mem-branes were exposed to autoradiography film for24–48h.In Situ Hybridization—Cells were grown on4-well chamber slides and fixed with4%formaldehyde,5%acetic acid for15 min after a short washing with phosphate-buffered saline.After treatment with pepsin(0.1%in10m M HCl)for1min,cells were dehydrated through70,90,and100%ethanol.Treated cells were then hybridized with the fluorescent probes(10M)in probe dilution(EXIQON,Vedbaek,Denmark)for30min at 37°C followed by confocal microscopy(LSM510,Carl Zeiss). FITC-tagged antisense probe specific to let-7i was obtained from Ambion.Fluorescent intensity in the cytoplasm of cells was measured and calculated with an analysis system of the LSM510provided by Carl Zeiss,Inc.Luciferase Reporter Constructs and Luciferase Assay—Complementary59-mer DNA oligonucleotides containing the putative let-7target site within the3Ј-UTR of human TLR4mRNA were synthesized with flanking SpeI and HindIII restriction enzyme digestion sites(sense,5Ј-GATACTAGTA-TCGGGCCCAAGAAAGTCATTTCAACTCTTACCTCAT-CAAGTAAGCTTACA-3Ј;antisense,5Ј-TGTAAGCTTACT-TGATGAGGTAAGAGTTGAAATGACTTTCTTGGGCCC-GATACTAGTATC).The sense and antisense strands of the oligonucleotides were annealed by adding2g of each oligo-nucleotide to46l of annealing solution(100m M potassium acetate,30m M HEPES-KOH,pH7.4,and2m M magnesium acetate)and incubated at90°C for5min and then at37°C for 1h.The annealed oligonucleotides were digested with SpeI and HindIII and ligated into the multiple cloning site of the pMIR-REPORT Luciferase vector(Ambion,Inc.).In this vector,the post-transcriptional regulation of luciferase was potentially regulated by miRNA interactions with the TLR43Ј-UTR. Another pMIR-REPORT Luciferase construct containing TLR4mRNA3Ј-UTR with a mutant(ACCTCAT to ACCGAAT)at the putative seed region for let-7binding was also generated as a control.We then transfected cultured cholangiocytes with each reporter construct,as well as let-7i antisense oligonucleotide or precursor,followed by assessment of luciferase activity24h after transfection.Luciferase activity was then measured and normalized to the expression of the control TK Renilla construct as previously reported(11). Quantitative RT-PCR—A quantitative RT-PCR approach (LightCycler)to measure C.parvum infection was established by modification of a previous report(11).Briefly,total RNA was harvested from the cells after exposure to C.parvum and reversed transcribed to cDNA and amplified using Amplitaq Gold PCR master mixture(Roche).Primers specific for C.par-vum18S ribosomal RNA(forward,5Ј-TAGAGATTGGAGGT-TGTTCCT-3Јand reverse,5Ј-CTCCACCAACTAAGAACG-GCC-3Ј)were used to amplify the cDNA specific to the parasite.Primers specific for human plus C.parvum18S(11) were used to determine total18S cDNA.Data were expressed as copies of C.parvum18S versus total18S.Similarly,primers specific for human TLR4(forward:5Ј-TACTCACACCA-GAGTTGCTTTCA-3Јand reverse:5Ј-AGTTGACACT-GAGAGAGGTCCAG-3Ј)and let-7i primary transcript(Pri-let-7i)(forward,5Ј-CCTAGAAGGAATTGAGGGGAGT-3Јand reverse,5Ј-TGGCATTTAACTGCTGAAAGAA-3Ј)were used to amplify the cDNA specific to TLR4and Pri-let-7i.Dataof quantitative RT-PCR analysis were expressed as copies of targets versus18S.RESULTSHuman Cholangiocytes Express let-7Family Members, miRNAs That Mediate TLR4Expression—H69cells,SV40-transformed human cholangiocytes derived from normal liver harvested for transplantation(10),were used to test the miRNA expression profile in human cholangiocytes.Employing a recently developed semiquantitative microarray approach that detects385human miRNAs provided and performed by Genosensor,we detected a distinct expression profile of miRNAs in our cells(Table1).Using in silico computational target prediction analysis,we identified that of those miRNAs expressed in H69cells,at least three of the let-7family,let-7b,let-7i,and let-7g,have complementarity to TLR4mRNA withinthe3Ј-UTR(Fig.1A).let-7b and-7g are contained within known expressed sequence tags(EST),let-7i is also potentially within an intron of an EST(DA092355).However,we were unable to amplify this EST from H69cells(forward,5Ј-GGT-TABLE1Micro-RNAs detected in H69cells by microarray analysisExpression of miRNAs in H69cells as assessed by microarray analysis.H69cellswere grown to confluence and total RNAs isolated for miRNA microarray anal-ysis with the GenoExplorer TM micro-RNA Biochips by Genosensor.Data were expressed as the fluorescent intensity for each miRNA,representing the mean values from three independent analyses.Expression of5S rRNA was used as the control.miRNAs Fluorescentintensity miRNAsFluorescentintensity miR-001730Ϯ120miR-199a152Ϯ2.7miR-009140Ϯ9.1miR-214864Ϯ16miR-15a218Ϯ8.7miR-216163Ϯ2.0miR-15b188Ϯ7.0miR-296169Ϯ4.5miR-24230Ϯ2.1miR-299171Ϯ5.5miR-27b232Ϯ1.7miR-302a186Ϯ10miR-29a178Ϯ3.6miR-302d164Ϯ5.6miR-29b146Ϯ3.6miR-324-3p150Ϯ1.7miR-93141Ϯ3.6miR-337155Ϯ3.6miR-100211Ϯ2.3miR-338251Ϯ9.6miR-103151Ϯ1.5miR-339182Ϯ10miR-122a158Ϯ3.2miR-340172Ϯ8.0miR-124a710Ϯ230miR-342164Ϯ7.0miR-125a193Ϯ4.6miR-368162Ϯ11miR-125b3490Ϯ120miR-370175Ϯ1.2miR-128a330Ϯ40miR-371168Ϯ7.4miR-130a165Ϯ9.8miR-372298Ϯ19miR-130b202Ϯ7.5miR-373322Ϯ18miR-133a1880Ϯ120miRNA373*205Ϯ21miR-149181Ϯ11miR-374720Ϯ80miR-154161Ϯ4.0let-7b388Ϯ8.5miR-181b174Ϯ2.1let-7i161Ϯ11miR-194153Ϯ2.5let-7g172Ϯ4.0miR-197174Ϯ4.6rRNA-5s210Ϯ30let-7Regulates Cholangiocyte Immunityat ZHEJIANG UNIVERSITY, on January 10, Downloaded fromCACGTGGTGAGGAGTAGC-3Јand reverse,5Ј-CATTCTT-GTCATATTGAAAATACGC-3Ј),whereas the EST was amplified from human cerebellum (data not shown).Addition-ally,let-7i has promoter elements immediately upstream of the precursor transcript (30),suggesting that let-7i expression is potentially regulated independent of EST DA092355.Wetherefore selected let-7i to test a potential role of miRNA-me-diated post-transcriptional suppression in regulated TLR4expression.To further confirm the expression of let-7in H69cells,we used an antisense probe complementary to let-7i (Mayo DNA core facility)for Northern blot ing this technique,we cannot eliminate the possibility that other let-7family members are detected.However,the pattern of expres-sion of the let-7family can be discerned.let-7miRNAs were detected in our cells by Northern blot analysis,whereas a scrambled control probe showed no signal (Fig.1B ).To further assess the expression and intracellular distri-bution of let-7miRNAs in H69cells,we used a FITC-tagged anti-sense oligonucleotide comple-mentary to let-7i (Ambion)for in situ hybridization analysis followed by confocal microscopy.The anti-sense probe complementary to let-7i was visualized predominantly in the cytoplasm with smaller amounts in the nuclei of cells (Fig.1C ).Further-more,cells transfected with a let-7i precursor (Ambion)showed a signif-icantly increased let-7signal as assessed by both Northern blot analy-sis (Fig.1B )and in situ hybridization (Fig.1,D and F ).Cells transfected with a let-7i antisense 2-methoxy oli-gonucleotide (Ambion)showed a sig-nificant decrease of let-7signal by in situ hybridization (Fig.1,E and F )and a mild decrease by Northern blot (Fig.1B ).Having established the approaches to manipulate intracellular let-7lev-els in cholangiocytes,we next tested whether alteration of let-7cellular levels affects TLR4protein contentin H69cells.We transfected cells with the let-7i precursor or the let-7i antisense 2-methoxy oligonu-cleotide for 12h and then measured TLR4protein expression in cells by quantitative Western blotting.We found a dose-dependent increase of TLR4protein content in cultured cholangiocytes after treatment with the let-7i antisense 2-methoxy oli-gonucleotide (Fig.2A ).In contrast,overexpression of let-7i with the precursor decreased TLR4proteincontent in a dose-dependent manner (Fig.2A ).Because trans-fection of let-7i precursor and antisense 2-methoxy oligonu-cleotide is limited to a portion of the cell population,we tested whether alteration of TLR4protein content occurs only in directly transfected cells.To accomplish this,the let-7i precur-sor or antisense 2-methoxy oligonucleotide was tagged with FITC using the mir Vana miRNA Probe Construction kit (Ambion)to label transfected cells.Expression of TLR4in cul-tured cells was visualized by immunofluorescent microscopy.As shown in Fig.2,B and C ,an increase of TLR4protein (Fig.2C ,in red )was detected only in cells directly transfected with let-7i antisense 2-methoxy oligonucleotide (Fig.2B ,in green )compared with non-transfected cells.Quantitative analysis showed a significant increase of TLR4-specific fluorescence in cells transfected with let-7i antisense 2-methoxy oligonucleo-FIGURE plementarity of let-7family miRNAs expressed in cholangiocytes to the 3-UTR of TLR4mRNA and manipulation of let-7i expression in cultured human cholangiocytes.A ,complementarity of let-7family miRNAs detected in H69cells to the 3Ј-UTR of ing in silico computational target prediction analysis,we identified that the expressed let-7family members,let-7b ,let-7i ,and let-7g,have complementarity to the 3Ј-UTR of TLR4mRNA.B–F ,manipulation of let-7i expression in H69cells with specificlet-7i precursor or antisense oligonucleotide as assessed by Northern blot analysis (B )or by in situ hybridization (C–F ).let-7miRNAs were detected in H69cells by Northern blot analysis using a probe complementary to let-7i (B ).The control probe showed no signal.Whereas cells treated with a let-7i precursor (Ambion)showed anincreased signal indicating an increase of let-7,cells treated with a let-7i antisense 2-methoxy oligonucleotide(Ambion)showed a mild decrease of let-7signal (B ).5S rRNA was probed to confirm equal loading of mRNA.A FITC-tagged antisense oligonucleotide complementary to let-7i was used to visualize let-7miRNAs.The anti-sense probe was visualized predominantly in the cytoplasm with a limited detection in the nucleus (C ).Fur-thermore,cells treated with the precursor showed an increased fluorescence (D ),whereas cells treated with the antisense oligonucleotide showed a significant decrease of fluorescent signal (E ).F ,quantitative analysis of fluorescent intensity of the FITC-tagged let-7i antisense oligonucleotide.A total of about 200cells were ran-domly selected for each group and each data bar represents mean ϮS.D.from three independent experi-ments.*,p Ͻ0.05,ANOVA versus basal non-transfected control cells (Basal ).Bars ,5m.let-7Regulates Cholangiocyte Immunityat ZHEJIANG UNIVERSITY, on January 10, 2012 Downloaded fromtide and a significant decrease of TLR4specific fluorescence in cells treated with the let-7i precursor compared with non-transfected control cells,respectively (Fig.2D ).Taken together,these data reveal that human cholangiocytes express multiple endogenous miRNAs,including members of the let-7family,which have complementarity to TLR4mRNA,and that modu-lation of at least one of these,let-7i ,can mediate TLR4protein expression in cholangiocytes in vitro .let-7i Mediates TLR4Expression via Translational Sup-pression —Micro-RNAs mediate post-transcriptional suppres-sion via either mRNA cleavage or translational suppression.Totest whether let-7i can induce cleavage of TLR4mRNA,we measured the mRNA level of TLR4in cultured cholangiocytes transfected with either let-7i antisense 2-methoxy oligonucleo-tide or let-7i precursor by quantitative RT-PCR.No significant difference of TLR4mRNA was found in cells transfected with either let-7i antisense 2-methoxy oligonucleotide or let-7i pre-cursor (Fig.3A ).To directly address whether let-7i binds to the 3Ј-UTR of TLR4mRNA resulting in a translational suppres-sion,we generated a pMIR-REPORT luciferase construct con-taining the TLR4mRNA 3Ј-UTR with the putative let-7binding site (Fig.3B ).In addition,another pMIR-REPORT luciferase construct containing the TLR4mRNA 3Ј-UTR with a mutation at the putative let-7binding site (ACCTCAT to ACCGAAT)was generated as a control (Fig.3B ).We then transfected cul-tured cholangiocytes with each reporter construct,as wellasFIGURE 2.let-7i mediates TLR4expression in cultured cholangiocytes.A ,effects of let-7i on TLR4protein expression by Western analysis.H69cells were transfected with a let-7i precursor or let-7i antisense 2-methoxy oligo-nucleotide for 12h followed by Western blot for TLR4.A dose-dependent increase of TLR4protein content was detected after treatment with let-7i antisense oligonucleotide.In contrast,overexpression of let-7i with the let-7i precursor decreased TLR4protein content in a dose-dependent manner.B and C ,TLR4expression in cells transfected with a let-7i antisense oligonu-cleotide as assessed by immunofluorescence.H69cells were transfected with a FITC-conjugated antisense oligonucleotide complementary to let-7i for 12h followed by immunofluorescent staining for TLR4.An increased expression of TLR4protein (in red ,C )was detected in directly transfected cells (arrows ,in green ,B )compared with non-transfected cells.D ,effects of let-7i on TLR4protein expression by quantitative fluorescent analysis.H69cells were trans-fected with either let-7i precursor or let-7i antisense oligonucleotide for 12h followed by quantitative analysis of immunofluorescent signals for TLR4.A total of about 200cells were randomly selected for each group and each data bar represents mean ϮS.D.from three independent experiments.*,p Ͻ0.05,ANOVA versus with non-transfected control cells (Basal ).Bars ,5m.FIGURE 3.let-7i mediates TLR4protein expression via post-transcrip-tional suppression.A ,effects of let-7i on TLR4mRNA content.H69cells were transfected with either let-7i precursor or let-7i antisense oligonucleotide for 12h followed by quantitative RT-PCR for TLR4mRNA.Data were normalized to the 18S rRNA level and expressed as copies of TLR4mRNA/106copies 18S rRNA.B ,targeting of let-7i to the 3Ј-UTR of TLR4mRNA.A reporter construct with the potential binding site for let-7in the 3Ј-UTR of TLR4was generated.H69cells were transiently co-transfected for 24h with the reporter construct and either let-7i antisense oligonucleotide or let-7i precursor.Luciferase activ-ities were measured and normalized to the control TK Renilla luciferase level.Bars represent the mean ϮS.D.from three independent experiments.*,p Ͻ0.05,ANOVA versus cells transfected only with the reporter construct (3Ј-UTR Ctrl );#,p Ͻ0.05,ANOVA versus cells transfected with the reporter construct plus let-7i precursor (3ЈUTR ϩlet-7i precursor ).let-7Regulates Cholangiocyte Immunityat ZHEJIANG UNIVERSITY, on January 10, 2012 Downloaded fromlet-7i antisense 2-methoxy oligonucleotide or precursor,fol-lowed by assessment of luciferase activity 24h after transfec-tion.As shown in Fig.3B ,a significant decrease of luciferase activity was detected in cells trans-fected with the TLR43Ј-UTR con-struct under basal conditions (i.e.no antisense or precursor treatment).No change of luciferase was observed in cells transfected with the mutant TLR43Ј-UTR con-struct.Importantly,let-7i precursor significantly decreased luciferase reporter translation and in contrast,let-7i antisense 2-methoxy oligonu-cleotide markedly increased lucifer-ase reporter translation.A mutation in the binding sequence eliminated the let-7i precursor-induced de-crease of reporter translation.Taken together,the above data suggest that the seed region for let-7binding within the TLR43Ј-UTR is critical for TLR4trans-lational regulation in H69cells.Furthermore,manipulation of cel-lular levels of let-7i results in alter-ations of TLR4protein expression by suppressing translation via interactions with the 3Ј-UTR of TLR4mRNA rather than mRNA cleavage.LPS Stimulation and C.parvum Infection Decrease let-7i Expression in Cholangiocytes via a MyD88/NF-B Signaling-dependent Mech-anism —Having demonstrated that let-7i mediates TLR4translation in cholangiocytes,we then tested whether this regulation is of physio-logical or pathophysiological signif-icance.Expression of TLR4protein in epithelial cells is finely regulated and alterations of TLR4expression have been reported in intestinal andairway epithelial cells following microbial infection (1,31).There-fore,we measured let-7i expression in cholangiocytes upon LPS stimu-lation and following infection by C.parvum ,a parasite that infects both intestinal and biliary epithelium.We previously demonstrated that C.parvum infection activates TLR sig-nals in infected cholangiocytes in culture,including activation of the adaptor protein,MyD88,and nuclear translocation of NF-B in directly infected cells (28).Here,aprobe complementary to let-7i detected significantly less signal by Northern blot analysis following LPS stimulation (Fig.4A )or following C.parvum infection (Fig.4E ).However,given theFIGURE 4.LPS stimulation and C.parvum infection decrease let-7i expression in cholangiocytes in a NF-B dependent manner.A–H ,expression of let-7i in cholangiocytes after treatment with LPS (A–D )or infection by C.parvum (E–H ).H69cells,as well as cells stably transfected with a MyD88functionally deficient dominant negative mutant construct (MyD88-DN )or an empty control vector,were exposed to LPS (100ng/ml)for 4h or C.parvum for 12h followed by Northern blot (A and E ),quantitative RT-PCR (B and F )or in situ hybridization (C and G )analysis for let-7i .For Northern blot analysis,5S rRNA was blotted to confirm that an equal amount of total RNA was used.let-7signals,detected with the let-7i antisense probe,from three inde-pendent experiments were measured using a densitometric analysis and expressed as the ratio to 5S rRNA (A and E ).Quantitative RT-PCR analysis was performed with specific primers to let-7i primary transcript and expressed as copies/18S rRNA (B and F ).For in situ hybridization analysis,an FITC-tagged antisense probe complementary to let-7i (Ambion)was used to detect let-7family miRNAs.Cells were also stained with 4Ј,6-diamidino-2-phenylindole to label the nuclei in blue .Representative confocal images are shown in C and G .C.parvum was stained red using a specific antibody (arrowheads in G ).D and H are quantitative analyses of let-7expression detected with the fluorescently tagged antisense oligonucleotide complementary to let-7i in thecytoplasm of cultured cells by in situ hybridization after treatment with LPS (D )or infection by C.parvum (H ),respectively.A total of about 200cells were randomly selected for each group and each data bar represents mean ϮS.D.from three independent experiments.*,p Ͻ0.05,ANOVA versus no-LPS treated control cells (Ctrl ,in A ,B ,and D )or sham infected cells (Sham Inf.Ctrl ,in E ,F ,and H ).Bars ,5m.let-7Regulates Cholangiocyte Immunityat ZHEJIANG UNIVERSITY, on January 10, 2012 Downloaded from。
恶性胆道梗阻经皮胆道引流或支架置入术后感染的病原学特征

./0恶性胆道梗阻经皮胆道引流或支架置入术后感染的病原学特征李思茵,李 智,焦冰珂,洪祈源,姜小庆,邹建伟,倪才方苏州大学附属第一医院介入科,江苏苏州215006摘要:目的 探讨恶性胆道梗阻(MBO)患者经皮胆道引流或支架置入术后感染的病原学特征。
方法 收集2016年1月—2020年12月在苏州大学附属第一医院介入科接受介入治疗后存在或怀疑胆道感染、送检胆汁培养和/或无同期血培养的MBO患者的临床资料。
从病原学培养的阳性率、菌群分布、血培养与胆汁培养的一致性、主要致病菌的耐药率方面进行分析。
结果 共纳入患者219例,胆汁病原学培养阳性105例(47.95%),其中革兰氏阴性菌、革兰氏阳性菌、真菌的构成比分别为64.89%、28.24%、6.87%。
同期送检血培养者69例,阳性33例(47.82%)。
血培养和胆汁培养同为阳性的患者25例,一致性分析结果显示,完全一致占36%(9/25),部分一致占20%(5/25),完全不一致占44%(11/25)。
常见致病的革兰氏阴性菌为大肠埃希菌、肺炎克雷伯、阴沟肠杆菌,对其耐药率(<15%)较低的抗生素有头孢哌酮/舒巴坦、阿米卡星、亚胺培南。
常见致病的革兰氏阳性菌为屎肠球菌、粪肠球菌,对其耐药率较低的抗生素有万古霉素、利奈唑胺、替考拉宁。
结论 MBO患者经皮胆道引流或支架置入术后感染的常见致病菌为大肠埃希菌、肺炎克雷伯、肠球菌、阴沟肠杆菌。
血培养与胆汁培养的一致性较低,均应积极送检。
关键词:恶性胆管梗阻;细菌感染;抗药性,细菌基金项目:苏州大学“大学生创新创业训练计划”(202110285135Y)EtiologicalcharacteristicsofinfectionafterpercutaneousbiliarydrainageorstentimplantationformalignantbiliaryobstructionLISiyin,LIZhi,JIAOBingke,HONGQiyuan,JIANGXiaoqing,ZOUJianwei,NICaifang.(DepartmentofInterventionalRadiology,TheFirstAffiliatedHospitalofSoochowUniversity,Suzhou,Jiangsu215006,China)Correspondingauthor:LIZhi,lizhisoochow@suda.edu.cn(ORCID:0000-0001-7613-4193)Abstract:Objective Toinvestigatetheetiologicalcharacteristicsofinfectionafterpercutaneousbiliarydrainageorstentimplantationinpatientswithmalignantbiliaryobstruction(MBO).Methods ClinicaldatawerecollectedfromMBOpatientswhounderwentinterventionaltherapyinDepartmentofInterventionalRadiology,TheFirstAffiliatedHospitalofSoochowUniversity,fromJanuary2016toDecember2020andhadorweresuspectedofbiliarytractinfection,withsamplessubmittedforbilecultureand/orsimultaneousbloodculture.Analysiswasperformedfortheaspectsofpositiverateofculture,floradistribution,consistencybetweenbloodcultureandbileculture,anddrugresist ancerateofmajorpathogenicbacteria.Results Atotalof219patientswereenrolled,amongwhom105(47.95%)werepositiveforbileculture,andthecompositionratiosofGram-negativebacteria,Gram-positivebacteria,andfungiwere64.89%,28.24%,and6.87%,respectively.Atotalof69patientshadsamplessubmittedforbloodcultureduringthesameperiodoftime,amongwhom33(47.82%)hadpositiveresults.Positiveresultsofbothbilecultureandbloodculturewereobservedin25patients,andconsistencyanalysisshowedthatthepatientswithcompleteconsistency,partialconsistency,andcompleteinconsistencyaccountedfor36%(9/25),20%(5/25),and44%(11/25),respectively.CommonGram-negativebacteriawereEscherichiacoli,Klebsiellapneumoniae,andEnterobactercloacae,witharelativelylowlevelofdrugresistancetoantibioticsincludingcefoperazone/sulbactam,amikacin,andimipenem.CommonGram-positivebacteriawereEnterococcusfaeciumandEnterococcusfaecalis,witharelativelylowlevel(<15%)ofdrugresistancetoantibioticsincludingvancomycin,linezolid,andteicoplanin.Conclusion Commonpathogensofinfectionafterpercutaneousbiliarydrainageorstentimplanta tioninMBOpatientsincludeEscherichiacoli,Klebsiellapneumoniae,Enterococcus,andEnterobactercloacae.Thereisarelativelylowlevelofconsistencybetweenbloodcultureandbileculture,andthussamplesshouldbesubmittedforbothtests.Keywords:MalignantBiliaryObstruction;BacterialInfections;DrugResistance,BacterialResearchfunding:UndergraduateTrainingProgramforInnovationandEntrepreneurship,SoochowUniversity(202110285135Y)DOI:10.3969/j.issn.1001-5256.2022.06.024收稿日期:2021-09-29;录用日期:2021-11-22通信作者:李智,lizhisoochow@suda.edu.cn 经皮胆道引流或支架植入术是治疗恶性胆道梗阻(malignantbiliaryobstruction,MBO)的重要方法[1],与结石所致的良性胆道梗阻不同,MBO通常不合并显性感染。
非等位基因

非等位基因概述非等位基因是指同一基因座上的不同等位基因。
等位基因是指在某个给定的基因座上,可以存在多种不同的变体。
每个个体继承了一对等位基因,一对等位基因可能会导致不同的表型表达。
非等位基因的存在使得遗传学研究更加复杂,因为不同的等位基因会对个体的表型产生不同的影响。
背景在生物学中,基因座是指染色体上一个特定的位置,该位置上的基因决定了某个特征的表达方式。
每个基因座上可以有多种不同的等位基因。
等位基因是指在某个特定基因座上的不同基因变体。
每个个体都会继承一对等位基因,通过这对等位基因的不同组合,决定了个体的表型。
然而,并非所有基因座上的等位基因都具有相同的表现型。
非等位基因的影响非等位基因的存在导致不同等位基因会对个体表型产生不同的影响。
有些非等位基因会表现出显性效应,也就是说,当个体继承了一个突变的等位基因时,即使同时继承了一个正常的等位基因,但显性效应会使得突变的等位基因的表型表达得到体现。
相反,有些非等位基因会表现出隐性效应,当个体继承了两个突变的等位基因时,才会表现出突变的表型。
除了显性和隐性效应之外,非等位基因还可能发生两种其他类型的表型效应。
一种是共显效应,当个体继承了两个不同的突变等位基因时,在表型表达上会表现出一种新的特征,这个特征并不是单个突变等位基因所能导致的。
另一种是部分显性效应,当个体继承了两个不同的突变等位基因时,表型表达将介于两个单独突变等位基因的表型之间。
重组和非等位基因重组是指两个不同的染色体交换部分基因序列的过程。
在重组的过程中,非等位基因可能会发生改变,导致新的等位基因组合形成。
这一过程使得非等位基因的表型效应更加复杂,因为新的等位基因可能将不同基因座的效应组合起来。
非等位基因的重要性非等位基因对生物的适应性和多样性起着重要作用。
通过对等位基因的各种组合的研究,人们可以更好地理解基因与表型之间的关系,并揭示遗传变异对物种适应环境的重要性。
总结非等位基因是指同一基因座上的不同等位基因。
益生菌肠道微生物的基因组学英文论文及翻译

The genomics of probiotic intestinal microorganismsSeppo Salminen1 , Jussi Nurmi2 and Miguel Gueimonde1(1) Functional Foods Forum, University of Turku, FIN-20014 Turku, Finland(2) Department of Biotechnology, University of Turku, FIN-20014 Turku, FinlandSeppo SalminenEmail: *********************Published online: 29 June 2005AbstractAn intestinal population of beneficial commensal microorganisms helps maintain human health, and some of these bacteria have been found to significantly reduce the risk of gut-associated disease and to alleviate disease symptoms. The genomic characterization of probiotic bacteria and other commensal intestinal bacteria that is now under way will help to deepen our understanding of their beneficial effects.While the sequencing of the human genome [1, 2] has increased ourunderstanding of the role of genetic factors in health and disease, each human being harbors many more genes than those in their own genome. These belong to our commensal and symbiotic intestinal microorganisms - our intestinal 'microbiome' - which play an important role in maintaining human health and well-being. A more appropriate image of ourselves would be drawn if the genomes of our intestinal microbiota were taken into account. The microbiome may contain more than 100 times the number of genes in the human genome [3] and provides many functions that humans have thus not needed to develop themselves. The indigenous intestinal microbiota provides a barrier against pathogenic bacteria and other harmful food components [4–6]. It has also been shown to have a direct impact on the morphology of the gut [7], and many intestinal diseases can be linked to disturbances in the intestinal microbial population [8].The indigenous microbiota of an infant's gastrointestinal tract is originally created through contact with the diverse microbiota of the parents and the immediate environment. During breast feeding, initial microbial colonization is enhanced by galacto-oligosaccharides in breast milk and contact with the skin microbiota of the mother. This early colonization process directs the microbial succession until weaning and forms the basis for a healthy microbiota. The viable microbes in the adultintestine outnumber the cells in the human body tenfold, and the composition of this microbial population throughout life is unique to each human being. During adulthood and aging the composition and diversity of the microbiota can vary as a result of disease and the genetic background of the individual.Current research into the intestinal microbiome is focused on obtaining genomic data from important intestinal commensals and from probiotics, microorganisms that appear to actively promote health. This genomic information indicates that gut commensals not only derive food and other growth factors from the intestinal contents but also influence their human hosts by providing maturational signals for the developing infant and child, as well as providing signals that can lead to an alteration in the barrier mechanisms of the gut. It has been reported that colonization by particular bacteria has a major role in rapidly providing humans with energy from their food [9]. For example, the intestinal commensal Bacteroides thetaiotaomicron has been shown to have a major role in this process, and whole-genome transcriptional profiling of the bacterium has shown that specific diets can be associated with selective upregulation of bacterial genes that facilitate delivery of products of carbohydrate breakdown to the host's energy metabolism [10, 11]. Key microbial groups in the intestinal microbiota are highly flexible in adapting to changes in diet, and thus detailed prediction of their actions and effects may be difficult. Although genomic studies have revealed important details about the impact of the intestinal microbiota on specific processes [3, 11–14], the effects of species composition and microbial diversity and their potential compensatory functions are still not understood.Probiotics and healthA probiotic has been defined by a working group of the International Life Sciences Institute Europe (ILSI Europe) as "a viable microbial food supplement which beneficially influences the health of the host" [15]. Probiotics are usually members of the healthy gut microbiota and their addition can assist in returning a disturbed microbiota to its normal beneficial composition. The ILSI definition implies that safety and efficacy must be scientifically demonstrated for each new probiotic strain and product. Criteria for selecting probiotics that are specific for a desired target have been developed, but general criteria that must be satisfied include the ability to adhere to intestinal mucosa and tolerance of acid and bile. Such criteria have proved useful but cumbersome in current selection processes, as there are several adherence mechanisms and they influence gene upregulation differently in the host. Therefore, two different adhesion studies need to be conducted on each strain and theirpredictive value for specific functions is not always good or optimal. Demonstration of the effects of probiotics on health includes research on mechanisms and clinical intervention studies with human subjects belonging to target groups.The revelation of the human genome sequence has increased our understanding of the genetic deviations that lead to or predispose to gastrointestinal disease as well as to diseases associated with the gut, such as food allergies. In 1995, the first genome of a free-living organism, the bacterium Haemophilus influenzae, was sequenced [16]. Since then, over 200 bacterial genome sequences, mainly of pathogenic microorganisms, have been completed. The first genome of a mammalian lactic-acid bacterium, that of Lactococcus lactis, a microorganism of great industrial interest, was completed in 2001 [17]. More recently, the genomes of numerous other lactic-acid bacteria [18], bifidobacteria [12] and other intestinal microorganisms [13, 19, 20] have been sequenced, and others are under way [21]. Table 1lists the probiotic bacteria that have been sequenced. These great breakthroughs have demonstrated that evolution has adapted both microbes and humans to their current state of cohabitation, or even symbiosis, which is beneficial to both parties and facilitates a healthy and relatively stable but adaptable gut environment.Table 1Lessons from genomesLactic-acid bacteria and bifidobacteria can act as biomarkers of gut health by giving early warning of aberrations that represent a risk of specific gut diseases. Only a few members of the genera Lactobacillus and Bifidobacterium, two genera that provide many probiotics, have been completely sequenced. The key issue for the microbiota, for probiotics, and for their human hosts is the flexibility of the microorganisms in coping with a changeable local environment and microenvironments.This flexibility is emphasized in the completed genomes of intestinal and probiotic microorganisms. The complete genome sequence of the probiotic Lactobacillus acidophilus NCFM has recently been published by Altermann et al. [22]. The genome is relatively small and the bacterium appears to be unable to synthesize several amino acids, vitamins and cofactors. Italso encodes a number of permeases, glycolases and peptidases for rapid uptake and utilization of sugars and amino acids from the human intestine, especially the upper gastrointestinal tract. The authors also report a number of cell-surface proteins, such as mucus- and fibronectin-binding proteins, that enable this strain to adhere to the intestinal epithelium and to exchange signals with the intestinal immune system. Flexibility is guaranteed by a number of regulatory systems, including several transcriptional regulators, six PurR-type repressors and ninetwo-component systems, and by a variety of sugar transporters. The genome of another probiotic, Lactobacillus johnsonii [23], also lacks some genes involved in the synthesis of amino acids, purine nucleotides and numerous cofactors, but contains numerous peptidases, amino-acid permeases and other transporters, indicating a strong dependence on the host.The presence of bile-salt hydrolases and transporters in these bacteria indicates an adaptation to the upper gastrointestinal tract [23], enabling the bacteria to survive the acidic and bile-rich environments of the stomach and small intestine. In this regard, bile-salt hydrolases have been found in most of the sequenced genomes of bifidobacteria and lactic-acid bacteria [24], and these enzymes can have a significant impact on bacterial survival. Another lactic-acid bacterium, Lactobacillus plantarum WCFS1, also contains a large number of genes related to carbohydrate transport and utilization, and has genes for the production of exopolysaccharides and antimicrobial agents [18], indicating a good adaptation to a variety of environments, including the human small intestine [14]. In general, flexibility and adaptability are reflected by a large number of regulatory and transport functions.Microorganisms that inhabit the human colon, such as B. thetaiotaomicron and Bifidobacterium longum [12], have a great number of genes devoted to oligosaccharide transport and metabolism, indicating adaptation to life in the large intestine and differentiating them from, for example, L. johnsonii [23]. Genomic research has also provided initial information on the relationship between components of the diet and intestinal microorganisms. The genome of B. longum [12] suggests the ability to scan for nutrient availability in the lower gastrointestinal tract in human infants. This strain is adapted to utilizing the oligosaccharides in human milk along with intestinal mucins that are available in the colon of breast-fed infants. On the other hand, the genome of L. acidophilus has a gene cluster related to the metabolism of fructo-oligosaccharides, carbohydrates that are commonly used as prebiotics, or substrates to肠道微生物益生菌的基因组学塞波萨米宁,尤西鲁米和米格尔哥尔摩得(1)功能性食品论坛,图尔库大学,FIN-20014芬兰图尔库(2)土尔库大学生物技术系,FIN-20014芬兰图尔库塞波萨米宁电子邮件:seppo.salminen utu.fi线上发表于2005年6月29日摘要肠道有益的共生微生物有助于维护人体健康,一些这些细菌被发现显着降低肠道疾病的风险和减轻疾病的症状。
Infection and Immunity
Auti-Bacterium Immunity
一、Pathogenesis of Bacterium (一)Invasiveness endotoxin (二)Toxin exotoxin 二、Anti-Bacterium Immunity (一)HI (Ab): 1. Anti-toxin 2. Anti-Bacterium (二)CT CTL TDTH→CK TCRT
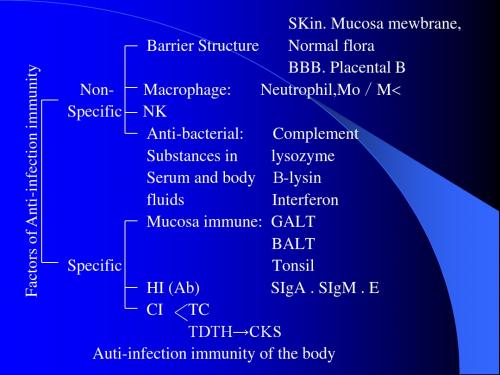
SKin. Mucosa mewbrane, Barrier Structure Normal flora BBB. Placental B NonMacrophage: Neutrophil,Mo/M Specific NK Anti-bacterial: Complement Substances in lysozyme Serum and body -lysin fluids Interferon Mucosa immune: GALT BALT Specific Tonsil HI (Ab) SIgA . SIgM . E CI TC TDTH→CKS Auti-infection immunity of the body
Anti-Virus Immunity
一、Pathogenesis of V (一)Target Cell damaged (二)Immunologic damage 二、Anti-V Immunity (一)HI 1. Neutralize Ab 2. ↑Phagocytosis 3. Lysis of infected cells (二)CI: 1. NK IL-2 2. CTL 3. TDTH→CK (IFN-r)
Examples of Infection Immunity
一、Meningococcus and Immunity 二、Influenza and Immunity 三、Polioviruses and Immunity
Immunization免疫,免疫系统,全英版

GENETICALLY ENGINEERED VACCINES
Hepatitis
B
American Academy of Family PhysiciansMorbidity and (AAFP) Mortality Weekly Report (MMWR).
Vaccine development and testing.
VACCINATION SCHEDULE
1. 2. 3. 4. 5. 6. 7.
The followings are the common infectious diseases against which world health organization(WHO) recommends routine immunization. Tuberculosis Diphtheria Pertusis Tetanus Polio Measles Hepatitis B.
HERD IMMUNITY
Herd immunity exists if the number of people in a community who have active immunity against an infection exceeds a critical level. If these level is achieved then even nonvaccinated individuals are protected from getting the disease. In this way transmission falls or stops without universal immunity.
EXPANDED PROGRAMME ON IMMUNIZATION (EPI)
潜伏性结核感染进展为活动性结核病的机制研究

• 276 •结核与肺部疾病杂志2020年〗2月第1卷第3期J Tubeir Limg DLs,December 2020. Vol. 1, No. 3综述潜伏性结核感染进展为活动性结核病的机制研究马慧敏张丽帆刘晓清【摘要】世界卫生组织将潜伏性结核感染(latent tuberculosis infection.LTB D定义为人体受到结核分枝杆菌抗原刺激后出现持续免疫反应,而没有表现出活动性结核病临床证据的…种状态。
全球约1/4的人感染了结核分枝杆菌.其中约5%〜10%的感染者最终会进展为活动性结核病。
因此.研究L T B I进展为活动性结核病的机制对结核病防控意义重大。
作者从结核分枝杆菌感染进程、I/TB丨进展为活动性结核病的免疫学改变,以及诱发LTBI 再活动的危险因素等方面对这一热点问题进行综述。
【关键词】潜伏性结核感染;活动性结核病;研究;综述Research progress on the mechanism of latent tuIxTculosis infection progressing to active tu!x*rcul(>sis M A H u i-m n i.Z H A N G L IU X ia o-q in g. Dix'isioti of Infectious Diseases, Peking Union Medical College Hospital *Chinese Academy of Medical Sciences, Peking Utiioti Medical College, Beijitig 100730, ChinaCorresponding a u th o r:L IU X iao-qi)ig, E m a il:liuxq@【Abstract】Latent tuberculosis infection (LTBI) is defined as a state of persistent immune response to stimulation by Mycobacterium tuberculosis antigens with no evidence of clinically manifest active tuberculosivS. Up to one third of the world^s population is estimated to be infected with Myrobacteriuni tuberculosis,and 5% —10% of those infected people will develop active tuberculosis disease sometime during their lives. Therefore, it is of great significance for tuberculosis prevention and control to study the mechanism of LTBI progressing to active tuberculosis. We reviewed this hot issue from the aspects of Myrobacterium tuberculosis infection process, immunological changes during LTBI progressing to active tuberculosis and the risk factors that induce LTBI reactivation, etc.【Key words】Latent tuberculosis infection; Active tuberculosis;Research;Review据世界卫生组织估计.2019年全球新发结核病患者近1000万例,死亡患者约141万例;估算我国新发结核病患者83. 3万例.占结核病患者总数的8. 4%.仅次于印度(26%)和印度尼西亚(8. 5%),居世界第三位。
欧洲药典7.5版

INDEX
To aid users the index includes a reference to the supplement in which the latest version of a text can be found. For example : Amikacin sulfate...............................................7.5-4579 means the monograph Amikacin sulfate can be found on page 4579 of Supplement 7.5. Note that where no reference to a supplement is made, the text can be found in the principal volume.
English index ........................................................................ 4707
Latin index ................................................................................. 4739
EUROPEAN PHARMACOPபைடு நூலகம்EIA 7.5
Index
Numerics 1. General notices ................................................................... 7.5-4453 2.1.1. Droppers...................
双语螺旋体专业知识讲座
抵抗力(resistance)-极弱(very weak)
• 对温度和干燥尤其敏感。
Ⅰ期(早期)梅毒:感染性极强,破坏性小
Primary syphilis: very highly infectious but little destructive. In about 3 weeks after infection, hard chancre develops at the site of infection ,usually at external genitalia.This primary lesion always heals spontaneously .
2.显微镜检验(microscopic examination) -------暗视野显微镜或荧光显微镜下找螺旋体
(spirochaete are demonstrated by dark field or immunofluorescence microscops)
3.血清学诊疗(serological tests)
2 vaccine------there is no vaccine against syphilis)疫苗:尚无 3 penicillin, as the treatment of choice, is given after
the accurate diagnosis of syphilis)梅毒确诊后,宜用青霉素等药 物及早予以彻底治疗
与 致病性(pathogenecity) 免疫性(immunity)
血小板在抵御细菌感染和增强免疫中的地位

DOI:10.12138力.issn.1671—9638.20217194・综述・血小板在抵御细菌感染和增强免疫中的地位易观群综述,谢双锋,马丽萍审校(中山大学孙逸仙纪念医院血液内科,广东广州510120)[摘要]血小板具有止血和维持血管完整的基本功能,但近年研究证明其在细菌感染中担任重要的免疫防御角色。
当机体出现细菌感染时,血小板第一个迁徙到感染前线,识别、捆绑、内吞病原菌或释放多种杀微生物蛋白直接杀灭细菌;同时增强中性粒细胞、单核细胞、补体系统的固有免疫功能,促T细胞、B细胞启动适应性免疫功能,协助共同清除机体细菌。
[关键词]血小板;细菌;感染;免疫[中图分类号]R515R446.11The role of platelet in resisting bacterial infection and enhancing immunity YI Guan-qun,XIE Shuang-f e ng,MA Li-ping(Department o f Hematology,Sun Yat-Sen Memorial Hospital,Sun Yat-Sen University,Guangzhou510120,China)[Abstract]Platelet has the basic function of hemostasis and maintaining vascular integrity,recent studies have provedthatitplaysanimportantroleinimmunedefenseagainstbacterialinfection Whenbacterialinfectionoccurs,platelet firstly migrate to the infection frontline to identify,bind,endocytose pathogens or release a variety of plate-letmicrobicidalprotein,whichcandirectlyki l bacteria,strengthentheinnateimmunefunctionofneutrophil,mon-ocyteand complementsystem,promote T lymphocyte and Blymphocytetoinitiateadaptiveimmuneresponses againstbacteria[Keywords]platelet;bacteria;infection;immunity很多无脊柱动物和早期脊柱动物有一种血细胞同时具有止血和免疫防御功能。
infection and immunity
1
This chapter focuses on
Definitions normal flora, opportunistic pathogen, dysbacteriosis, nosocomial infection, bacteremia, septicemia, pyemia , toxemia, carrier
Questions Under what conditions do opportunistic pathogens cause diseases? What factors are associated with pathogenicity of pathogens? What do the virulence factors of pathogens include? What is the difference between endotoxins and exotoxins?
Virulence factors
invasiveness toxin
Adherence factor Capsule and slime layer Invasive enzyme exotoxin
endotoxin
25
Pathogenicity
The amount of entry
species of bacteria Host defense state
28
Sources of infectious diseases
Nosocomial infections
Definitions: infections acquired in a hospital. Also called hospital-acquired infections.
博士生课程固有免疫模式识别ppt课件
Innate Immunity
机体在种系发生和进化 过程中逐渐形成的一种 天然免疫防御功能,构 成机体抵御病原生物入 侵的第一道防线.
monocyte macrophage
bacteria
The most ancient defense
Physical & chemical barriers and cellular line
•病原相关分子模式(Pathogen-associated molecular patterns, PAMP) :是病原微 生物(尤其是原核生物)表面存在一些人体所没有的,但可为 许多相关微生物所共享、结构恒定、进化保守的分子结构。
PAMP的特征
1.通常为病原微生物所特有,乃天然免疫系统区分“自己”与“非己(微生
stimulating
Liver: synthesis of APR proteins
Increase in the level of C-reactive protein & Mannose-binding lectin/MBL & Serum amyloid protein/SAP & fibrinogen)
模式识别受体( Pattern Recognition Receptors, PRRs)
固有免疫细胞表面、内体、溶酶体、细胞质中、可识
别一种或多种PAMPs或DAMPs的识别分子。
可溶性:体液和血液
甘露聚糖凝集素(MBL) C反应蛋白(CRP)
血清淀粉样蛋白 (SAP) 脂多糖结合蛋白(LBP)
PRR
C反应蛋白等(CRP)
补体
补体系统
细胞因子和黏附分子 细胞因子和免疫相关细胞表面分子
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Kห้องสมุดไป่ตู้iyang Liu Hebei North University
Some Basic Conceptions
致病性:是指细菌侵入机体生长繁殖、破坏组织、 引起病理变化的过程。 致病菌:能使宿主致病的细菌。 毒力:细菌的致病能力通常称为细菌的毒力。 LD50: The number of pathogens required to cause lethal disease in half of the exposed hosts is called an LD50. ID50: The number of pathogens required to cause disease (or, at least, infection) in half of the exposed hosts is called the ID50
Specific Humoral Cellular
T cells; other effectors cells
Cellular
macrophages, neutrophils
antibodies
Balance between Infection and Immunity
infection immunity
Exogenous infection : Patient, carrier, diseased animal or animal carrier. Endogenous condition : Most are normal flora, cause infection under abnormal condition.
毒性作用
外毒素
分子结构:A-B模式
A:活性亚单位 B:结合亚单位
可提纯制疫苗
白喉毒素 霍乱肠毒素 破伤风痉挛毒素 肉毒毒素
Phospholipid
• Immunity of host
Components of the Immune System
Nonspecific Humoral
complement, interferon, TNF etc.
Adhesion
BACTERIUM adhesin
receptor
Epithelium
E. coli with fimbriae
2. 侵袭性物质
3. Toxins
Property 来源 存在部位 化学成分 稳定性 抗原性 Exotoxin G+菌及部分G-菌 由活菌分泌或菌体溶解后释放 蛋白质 不稳定,60℃~80℃ 30min被破坏 强,刺激机体产生抗毒素;可经 甲醛液处理脱毒制成类毒素 强,对组织器官有选择性毒害作 用,引起特殊的临床表现。分为 三大类:神经毒素、细胞毒素、 肠毒素 G-菌 细胞壁组分,细菌裂解后释放 脂多糖 稳定,160 ℃ 2~4h 才被破坏 弱,刺激机体产生的中和抗体作用 弱;不能经甲醛液脱毒制成类毒素 较弱,各种细菌内毒素的毒性作用 大致相同,引起发热、白细胞增多、 休克、DIC等 Endotoxin
Some Basic Conceptions
构成毒力的物质基础主要为侵袭力和毒素
侵袭力:是指致病菌能突破机体的免疫防 御机制,进入体内生长繁殖和扩散的能力。
毒素:按其来源、性质和作用等不同,可 分为外毒素(exotoxin)和内毒素 (endotoxin)两种。
细菌的致病机制
1. 粘附因子
带菌状态:
致病菌在显性感染或隐性感染后未立即消失, 在体内继续存留一定时间,与机体免疫力处 于相对平衡状态,称为带菌状态,该宿主称 为带菌者。
Questions
内外毒素的主要区别? 什么是细菌的致病性? 什么是细菌的毒力,细菌的毒力包括什么? 什么是细菌的侵袭力,包括什么? 细菌感染的类型有什么? 显性感染的种类 及各类型的定义。 什么是带菌状态?
感染的类型
隐性感染 显性感染 带菌状态
According to infectious sites
1.
2.
3.
4.
5.
局部感染 全身感染 Toxemia : is the presence of exotoxins in the blood. Endotoxemia : is the presence of endotoxins in the blood. Bacteremia : is an invasion of the bloodstream by bacteria. Septicemia : illness that occurs when poisonous substances (toxins) produced by certain bacteria enter the bloodstream. Pyemia : is caused by pyogenic microorganisms in the blood.
Transmission
• 经粘膜感染 • 创伤感染 • 多途径感染
Routes of infection
Respiratory Gastroenteric Genitourinary tract Closely contact Insect biting Blood transfusion Mucous membranes
Detrimental:
Discomfort (inflammation) Damage to self (autoimmunity)
吞噬过程 接触 吞入 杀灭
Macrophage Attacking E.coli (SEM x8,800)
细菌感染的途径和类型
Source of infection
Thank you for your time and attention!
Disease =
Bolus of infection x virulence immunity
Significance of the Immune System
Beneficial:
Protection from Invaders Elimination of Altered Self
