Manidipine_dihydrochloride_LCMS_11968_MedChemExpress
米诺地尔分子量

米诺地尔分子量1. 什么是米诺地尔分子量?米诺地尔(Minoxidil)是一种用于治疗男性脱发和高血压的药物。
它是一种外用药,通过促进血液循环,刺激毛囊生长,从而增加头发的生长和密度。
米诺地尔分子量是指米诺地尔分子的相对分子质量,是衡量米诺地尔分子大小的指标。
2. 米诺地尔分子的组成米诺地尔的化学式为C9H15N5O,其分子结构如下所示:米诺地尔分子由9个碳原子、15个氢原子、5个氮原子和1个氧原子组成。
这些原子通过共价键相连,形成了一个稳定的分子结构。
3. 米诺地尔分子量的计算米诺地尔分子量的计算可以通过将每个原子的相对原子质量相加得到。
根据化学元素周期表,碳的相对原子质量为12.01,氢的相对原子质量为1.01,氮的相对原子质量为14.01,氧的相对原子质量为16.00。
因此,可以计算出米诺地尔的相对分子质量如下:碳的质量:9 * 12.01 = 108.09氢的质量:15 * 1.01 = 15.15氮的质量:5 * 14.01 = 70.05氧的质量:1 * 16.00 = 16.00米诺地尔的相对分子质量 = 108.09 + 15.15 + 70.05 + 16.00 = 209.29因此,米诺地尔的相对分子质量约为209.29。
4. 米诺地尔分子量的意义米诺地尔分子量的大小与其在化学反应和药物研发中的一些性质有关。
较大的分子量通常意味着较大的分子体积和较高的化学反应活性。
米诺地尔分子量较大,可能会对其在体内的吸收和代谢产生影响。
此外,米诺地尔分子量的大小也与其药物释放速率有关。
较大的分子量通常意味着较低的药物释放速率,从而延长药物在体内的作用时间。
5. 其他与米诺地尔分子量相关的研究除了米诺地尔分子量的计算和意义,科学家们还对米诺地尔分子的其他性质进行了深入研究。
例如,研究人员通过分子模拟方法,研究了米诺地尔分子与毛囊细胞的相互作用。
他们发现,米诺地尔分子可以与毛囊细胞表面的特定受体结合,从而刺激毛囊生长。
国际化妆品原料标准中文名称、INCI名、CAS号查询表(2010年版)

序号
1681 5894 10462 12033 1493 1494 1682 1679 1685 8598 10441 5892 5893 5896 5897 5895 8599 6505 6506 8241 8602 6523 66 71 660 14686 8209 8210 1683 3847 8600 3852 3851 3854 3855 3856 3857 3848 4272 8206 14846 4080 3860 3858 3859
中文名称
1,2-丁二醇 1,2-己二醇 1,2-戊二醇 1,3-丙二醇 1,3-双-(2,4-二氨基苯氧基)丙烷 1,3-双-(2,4-二氨基苯氧基)丙烷 HCl 1,4-丁二醇 1,4-丁二醇/琥珀酸/己二酸/HDI 共聚物 1,4-丁二醇二(甲基丙烯酸)酯 1,5-萘二酚 1,5-戊二醇 1,6-己二胺 1,6-己二醇 1,6-己二醇二水杨酸酯 1,6-己二醇二硬脂酸酯 1,6-己二醇蜂蜡酸酯 1,7-萘二酚 10-羟基癸酸 10-羟基癸烯酸 1-甲基乙内酰脲-2-酰亚胺 1-萘酚 1-羟乙基-4,5-二氨基吡唑硫酸盐 1-乙酰萘 1-乙酰氧基-2-甲基萘 2-(2-氨基乙氧基)乙醇 2,2'-硫代双(4-氯苯酚) 2,2'-亚甲基双 4-氨基苯酚 2,2'-亚甲基双-4-氨基苯酚 HCl 2,3-丁二醇
国际化妆品原料标准中文名称目录(2010年版)
序号 INCI名称 中文名称
酸 (C10-18 脂酸甘油三酯类聚甘油-3酯类) 磷酸酯类 (动物)肝水解产物 (动物)睾丸水解产物 (动物)脊髓索带提取物 (动物)脊髓索带脂质 (动物)脊髓脂质提取物 (动物)脐带提取物 (动物)气管水解产物 (动物)乳房提取物 (动物)神经提取物 (动物)胎盘蛋白 (动物)胎盘酶 (动物)胎盘脂质 (动物)心脏水解产物 (动物)心脏提取物 (动物)胸腺水解产物 (动物)胸腺提取物 (镁/钾/硅)(氟化物/氢氧化物/氧化 物) (牛)肝提取物 (牛)睾丸提取物 (牛)骨髓类脂质 (牛)骨髓提取物 (牛)肌肉提取物 (牛)卵巢提取物 (牛)脾提取物 (牛)网膜类脂质 (牛/猪)脑提取物 (日用)香精 (三油酰氧甲基)甲氨基乙醇硫酸酯 (神经)鞘磷脂 (神经)鞘脂类 (四丁氧基)丙基三硅氧烷 (天冬氨酸/谷氨酸)金 (辛/癸)酸异辛酯 (椰油/葵花籽油)酰胺丙基甜菜碱 (月桂/肉豆蔻)基二醇羟丙基醚 1-(3,4-二甲氧基苯基)-4,4-二甲基1,3-戊二酮 1,10-癸二醇 1,2,4-苯三酚三乙酸酯 1,2,4-三羟基苯 1,2,6-己三醇 1
伊布替尼 平面结构 溶解度

伊布替尼平面结构溶解度
【原创实用版】
目录
1.伊布替尼的简介
2.伊布替尼的平面结构
3.伊布替尼的溶解度
4.伊布替尼的应用领域
正文
伊布替尼是一种用于治疗慢性髓系白血病和费城染色体阳性急性淋巴细胞性白血病的药物,其化学名为“4-[(4-甲基 -1-哌嗪) 甲
基]-N-[4-[(3-甲基 -2-氧代 -5-咪唑啉 -4-基) 甲基]-2-甲基苯胺]-3-甲基 -2-氧代 -5-咪唑啉 -4-羧酸”。
它的平面结构由一个哌嗪环、一个咪唑啉环和一个苯胺环组成,这三个环通过共价键连接在一起,构成了伊布替尼的基本骨架。
伊布替尼的溶解度是指其在水中的溶解程度。
一般来说,伊布替尼在水中的溶解度较低,但在某些有机溶剂中的溶解度较高。
这一特性决定了伊布替尼在制备和应用过程中需要特别注意溶解度的问题,以保证药物的有效成分能够被充分吸收和利用。
伊布替尼的应用领域非常广泛,除了上述提到的治疗慢性髓系白血病和费城染色体阳性急性淋巴细胞性白血病外,还用于治疗多种实体瘤,如肺癌、胃癌等。
第1页共1页。
西咪替丁的化学结构式

西咪替丁的化学结构式1. 西咪替丁的概述西咪替丁(Simvastatin)是一种用于降低胆固醇和脂蛋白水平的药物,属于他汀类药物。
它通过抑制胆固醇合成的关键酶HMG-CoA还原酶,从而减少胆固醇在体内的合成。
西咪替丁是一种处方药,常用于治疗高胆固醇和高脂蛋白血症,预防心血管疾病的发生。
2. 西咪替丁的化学结构式西咪替丁的化学名为(1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-羟基-6-氧代-3,5-二甲基-4-甲硫基-4-氧代-5-氮-6-甲基-1,2,3,4-四氢-2-吡啶基]乙基}-3,7-二甲基-1,2,3,7,8,8a-六氢-1-萘酮。
西咪替丁的化学式为C25H38O5S,分子量为418.57 g/mol。
西咪替丁的结构式如下所示:3. 西咪替丁的合成途径西咪替丁的合成途径相对复杂,主要包括以下几个步骤:3.1 邻氨基苯甲酸的合成首先,通过邻氨基苯甲酸的合成作为起始原料。
邻氨基苯甲酸是通过对硝基苯甲酸的氢化还原得到的。
3.2 吡咯的合成邻氨基苯甲酸与乙酰乙酸乙酯反应生成吡咯化合物。
该反应需要碱催化。
3.3 吡咯的环化吡咯化合物通过烷基化反应得到环化产物。
该反应需要环化试剂和酸催化。
3.4 吡咯的氧化环化产物经氧化反应生成相应的醛。
该反应需要氧化剂。
3.5 醛的还原醛经还原反应生成相应的醇。
该反应需要还原试剂。
3.6 醇的酯化醇经酯化反应生成相应的酯。
该反应需要酯化试剂和酸催化。
3.7 酯的水解酯经水解反应生成相应的酸。
该反应需要水解试剂。
最终,通过以上合成步骤,得到西咪替丁。
4. 西咪替丁的药理作用西咪替丁通过抑制HMG-CoA还原酶的活性,阻断胆固醇的合成途径,从而降低体内胆固醇水平。
此外,西咪替丁还可以增加低密度脂蛋白受体的表达,促进低密度脂蛋白的清除,进一步降低胆固醇水平。
西咪替丁的主要药理作用包括:4.1 降低胆固醇水平西咪替丁通过抑制胆固醇的合成,可以显著降低总胆固醇、低密度脂蛋白胆固醇和甘油三酯的水平。
阿司咪唑

合成方法
合成方法
化合物(I)和碘甲烷在乙醇中回流8h,环合得到化合物(Ⅱ)。再水解脱去酯基,得到化合物(Ⅲ)。用对甲氧 基苯乙基溴进行N-烷基化,得化合物(Ⅳ)。再用对氟苄基溴烷基化,得阿司咪唑。
1. 1-[(4-氟苯基)甲基]-苯并咪唑-2-(3H)-酮的制备
在反应瓶中加入2-羟基苯并咪唑5.0g(37.3mmol)和NaH 1.6g(53mmol)(NaH含量大约为80%,浸入矿物油中) 的DMF 100ml的悬浮液.加毕.在60ºC.(最好有N2保护)搅拌反应1h.再加入4-氟苄基氯(FBC)5.4g(37mmol),加热 ( 6 0 ºC ) 搅 拌 反 应 5 . 5 h . 冷 却 至 室 温 后 加 入 冰 水 7 0 0 m l , 用 二 氯 甲 烷 ( 5 0 0 m l × 2 ) 提 取 . 有 机 层 用 食 盐 水 洗 . 无 水 N a 2 S O 4 干燥.过滤.滤液减压浓缩.剩余物用石油醚析晶.得1-[(4-氟苯基)甲基]-苯并咪唑-2-(3H)-酮固体8.0g,为无色 结 晶 m p 1 7 8 ~ 1 7 9 ºC , 收 率 8 8 % .
治疗措施
阿司咪唑中毒的治疗要点为: 1.大量摄入者予洗胃,后灌服活性炭和导泻。 2.对心肌抑制和Q-T间期延长者予5%碳酸氢钠250ml静注可能有效。 3.对症、支持治疗。
专家点评
专家点评
阿司咪阿司咪唑自1983年上市以来,在许多国家得到了广泛应用。国外研究显示阿司咪唑治疗荨麻疹的总有 效率为74%。国内的一项多中心双盲安慰剂对照试验表明阿司咪唑对急性荨麻疹的总有效率为82.9%,对慢性荨麻 疹的总有效率为86.0%,均显著高于安慰剂,主要不良反应为嗜睡、倦怠、口干等,连续用药3个月的患者中,半 数有食欲及体重增加。阿司咪唑的心脏毒性虽然发生率较低,但由于后果严重,已限制了它的应用。阿司咪唑为 强效和长效的H1受体拮抗剂,无中枢镇静和抗毒蕈碱样作用。代谢产物去甲阿司咪唑仍有抗胆胺作用。长期服用 可增进食欲和增加体重,服用过量可引起心脏Q-T间期延长和室性心律失常。适用于各种原因引起过敏性疾病。
高效液相色谱法测定注射用美罗培南的有关物质

第35卷第3期 长治医学院学报2021 年 6 月JOURNAL OF CHANGZHI MEDICAI COLLEGE167Vol. 35 No. 3Jun. 2021高效液相色谱法测定注射用美罗培南的有关物质李金格禹玉洪**作者单位山西医科大学药学院药剂教研室(030001)* 通信作者(E-mail :3024546064@ qq. com)摘要目的:探讨优化注射用美罗培南杂质A 、B 的测定方法。
方法:运用高效液相色谱法(HPLC) 进行检测,色谱柱以十八烷基硅烷键合硅胶为填充剂;流动相A :20. 0 mmol-L'1磷酸二氢钠-甲醇(89 :11, V/V),流动相B :甲醇,流速1.0 mL-min 1,检测波长220 nm,柱温30 P 。
结果:主成分峰与杂质峰可实现基线分离,杂质A 检测限和定量限分别为1. 62,5.15 ng,杂质B 检测限和定量限分别为0. 85,2. 51 ng ;1.2~24.0 ixg-mL -1的杂质A 具有良好的线性关系(r=0. 999 9) ,0.7-14.0 ixg-mL 1的杂质B 具有良好的线性 关系(r=0. 999 9);杂质A 平均加样回收率为101.2%(RSD= 1.38%,“ = 9),杂质B 平均加样回收率为100.2%(RSD=1.29%,n = 9)o 经破坏性试验,美罗培南可能的降解杂质A 、B 均不干扰美罗培南主峰的测定。
结论:检测限及定量限、精密度、稳定性、耐用性试验结果均符合HPLC 有关物质测定的方法学验证要求。
本HPLC 法专属性良好,可用于美罗培南的主要杂质A 、B 的定量控制。
关键词美罗培南;有关物质;高效液相色谱法中图分类号R97&1文献标识码 A 文章编号1006(2021)03-167-05Determination of Related Substances of Meropenem for Injection by High Performance Liquid ChromatographyLI Jinge , YU YuhongDepartment of Pharmacy , School of Pharmacy , Shanxi Medical UniversityAbstract Objective : To explore and optimize the determination method of impulity A andB of meropenem for injection. Meth ods :Using the high performance liquid chromatography ( HPLC ) to detection , Octadecylsilane-bonded silica gel was used as the fi ler ; The mobile phase A : 20. 0 mmol * L -1 sodium dihydrogen phosphate-methanol ( 89 : 11, V/V) . The mobile phase B : methanol ,the flow rate was 1. 0 mL *m in _1 and the detection wavelength was set at 220 nm. The column temperature was set at 30 % . Re sults :The principal component peak and impurity peak could achieve baseline separation. The detection limit and quantitative limit of impurity A were 1. 62 ng and 5. 15 ng respectively , and the detection limit and quantitative lim 让 of impurity B were 0. 85 ng and 2. 51 ng respectively. There was A good linear relationship between impurity A (r = 0. 999 9) and impurity B ( r= 0. 999 9 ) in therange of 1. 2-24. 0 |xg *m L _1 and 0. 7 ~ 14. 0 jig * mL -1. The average recovery of impurity A was 101. 2% ( RSD = 1. 38% , n = 9),and that of impurity B was 100. 2% ( RSD = 1. 29% , n= 9). After stressing test, both of impurities A and B of meropenem didn * tinterfere w 让h the determination of meropenem main peak. Conclusion : The test results of detection lim 让 and quant N ative lim 让,pre cision ,stabil 让y and durability all meet the methodological verification requirements of HPLC related substance determination. The HPLC method has good specificity and can be used for the quant N ative control of major impurities A and B.Key words meropenem ; related substances ; HPLC注射用美罗培南(Meropenem, C ”H25弘0申) 是由日本住友制药公司与英国ICI 制药公司共同 开发的第二代碳青霉烯抗生素,通过干扰细菌细胞壁的合成发挥杀菌作用,具有广谱耐酶的特 点[1_4]o 在美罗培南原料中常检测出杂质A 及杂质B,杂质A(C 17H 27N 3O 6S)为美罗培南四元内酰 胺环结构发生水解反应而形成,系美罗培南的降 解产物;杂质B(C 34H 50N 6O 10S 2)为美罗培南与杂质A 发生聚合反应而形成,系美罗培南的二聚体X 。
2-(甲氨基)-乙磺酸-n-椰油酰基衍生物钠盐

2-(甲氨基)-乙磺酸-n-椰油酰基衍生物钠盐下载提示:该文档是本店铺精心编制而成的,希望大家下载后,能够帮助大家解决实际问题。
文档下载后可定制修改,请根据实际需要进行调整和使用,谢谢!本店铺为大家提供各种类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by this editor. I hope that after you download it, it can help you solve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you! In addition, this shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts, other materials and so on, want to know different data formats and writing methods, please pay attention!2(甲氨基)乙磺酸n椰油酰基衍生物钠盐是一种具有抗菌、抗病毒和抗炎等多种生物活性的化合物。
抗抑郁药---盐酸维拉佐酮

2014.10.20
前言 • MDD治疗的主要药物种类
• 第一代经典抗抑郁药:主要包括单胺氧化酶抑制剂(maoi)和三环类 抗抑郁药(tca)。
• 第二代新型抗抑郁药:以选择性五羟色胺(5-ht)再摄取抑剂为主。 • 盐酸维拉佐酮(vilazodone hydrochloride)是首个吲哚烷基胺类新 型抗抑郁药。
2014.10.20
药物背景 • 安全性
• 盐酸维拉佐酮在8个临床试验共计2 177例MDD患者的临床研究中显 示具有良好的耐受性和安全性。在与安慰剂,对照的Ⅲ临床研究中, 因不良反应导致盐酸维拉佐酮治疗组中止治疗的患者占7.1%,安慰 剂对照组为3.2%,治疗组导致停药的常见不良反应主耍为恶心(1. 3%)和腹泻(1.2%) 。 • 由于抗抑郁药可增加儿童、青少年和l 8~24岁年轻人服药初期自杀想 法和自杀行为的风险,因此以上患者要慎用。 • 维拉唑酮在治疗终止时,尤其突然终止时,会出现戒断症状(烦躁不 安、易怒、眩晕、感觉障碍、意识模糊等症状)。
2014.10.20
报告框架
• 1.药物背景
• 2.专利介绍 • 3.合成路线
Viibryd(盐酸维拉佐酮)
2014.10.20
药物背景
• • • • • • • • • • • • • • • • 通用名称:维拉佐酮(Vilazodone) 商品名:Viibryd 原研公司:德国Merck KGaA 基本专利: DE19934333254 优先权:1993 年9 月30 日 相关中国专利: CN94116585 类别:抑郁症治疗药 化合物类型:新分子实体 5-[4-[4-( 5-cyano-1H-indol-3-yl) butyl]-1(New molecular entity) piperazinyl]-2-benzofurancarboxamide 分子式:C26H27N5O2 ·HCl hydrochloride 相对分子质量:477.99 CAS 号:163521-08-2 适应症:重度抑郁症(MDD) 化学名: 5-[4-[4-( 5-氰基-1H-吲哚-3-基) 丁基]-1-哌嗪基]-2-苯并呋喃草酰胺盐酸 盐; 获批单位:Trovis 制药有限责任公司 批准日期:2011 年1 月21 日
优泌乐25说明书

PV 5551 AMPHUMALOG® Mix75/25TM75% INSULIN LISPRO PROTAMINE SUSPENSION AND25% INSULIN LISPRO INJECTION(rDNA ORIGIN)100 UNITS PER ML (U-100)DESCRIPTIONHumalog® Mix75/25™ [75% insulin lispro protamine suspension and 25% insulin lispro injection, (rDNA origin)] is a mixture of insulin lispro solution, a rapid-acting blood glucose-lowering agent and insulin lispro protamine suspension, an intermediate-acting blood glucose-lowering agent. Chemically, insulin lispro is Lys(B28), Pro(B29) human insulin analog, created when the amino acids at positions 28 and 29 on the insulin B-chain are reversed. Insulin lispro is synthesized in a special non-pathogenic laboratory strain of Escherichia coli bacteria that has been genetically altered to produce insulin lispro. Insulin lispro protamine suspension (NPL component) is a suspension of crystals produced from combining insulin lispro and protamine sulfate under appropriate conditions for crystal formation.Insulin lispro has the following primary structure:Insulin lispro has the empirical formula C257H383N65O77S6 and a molecular weight of 5808, both identical to that of human insulin.Humalog Mix75/25 vials and Pens contain a sterile suspension of insulin lispro protamine suspension mixed with soluble insulin lispro for use as an injection.Each milliliter of Humalog Mix75/25 injection contains insulin lispro 100 units, 0.28 mg protamine sulfate, 16 mg glycerin, 3.78 mg dibasic sodium phosphate, 1.76 mg Metacresol, zinc oxide content adjusted to provide 0.025 mg zinc ion, 0.715 mg phenol, and Water for Injection. Humalog Mix75/25 has a pH of 7.0 to 7.8. Hydrochloric acid 10% and/or sodium hydroxide 10% may have been added to adjust pH.CLINICAL PHARMACOLOGYAntidiabetic ActivityThe primary activity of insulin, including Humalog Mix75/25, is the regulation of glucose metabolism. In addition, all insulins have several anabolic and anti-catabolic actions on many tissues in the body. In muscle and other tissues (except the brain), insulin causes rapid transport of glucose and amino acids intracellularly, promotes anabolism, and inhibits protein catabolism. In the liver, insulin promotes the uptake and storage of glucose in the form of glycogen, inhibits gluconeogenesis, and promotes the conversion of excess glucose into fat.Insulin lispro, the rapid-acting component of Humalog Mix75/25, has been shown to be equipotent to Regular human insulin on a molar basis. One unit of Humalog® has the sameglucose-lowering effect as one unit of Regular human insulin, but its effect is more rapid and of shorter duration. Humalog Mix75/25 has a similar glucose-lowering effect as compared with Humulin® 70/30 on a unit for unit basis.PharmacokineticsAbsorption — Studies in nondiabetic subjects and patients with type 1 (insulin-dependent) diabetes demonstrated that Humalog, the rapid-acting component of Humalog Mix75/25, is absorbed faster than Regular human insulin (U-100). In nondiabetic subjects given subcutaneous doses of Humalog ranging from 0.1 to 0.4 U/kg, peak serum concentrations were observed 30 to 90 minutes after dosing. When nondiabetic subjects received equivalent doses of Regular human insulin, peak insulin concentrations occurred between 50 to 120 minutes after dosing. Similar results were seen in patients with type 1 diabetes.Figure 1: Serum Immunoreactive Insulin (IRI) Concentrations, After Subcutaneous Injection of Humalog Mix75/25 or Humulin 70/30 in Healthy Nondiabetic Subjects. Humalog Mix75/25 has two phases of absorption. The early phase represents insulin lispro and its distinct characteristics of rapid onset. The late phase represents the prolonged action of insulin lispro protamine suspension. In 30 healthy nondiabetic subjects given subcutaneous doses(0.3 U/kg) of Humalog Mix75/25, peak serum concentrations were observed 30 to 240 minutes (median, 60 minutes) after dosing (see Figure 1). Identical results were found in patients with type 1 diabetes. The rapid absorption characteristics of Humalog are maintained with Humalog Mix75/25 (see Figure 1).Figure 1 represents serum insulin concentration versus time curves of Humalog Mix75/25 and Humulin 70/30. Humalog Mix75/25 has a more rapid absorption than Humulin 70/30, which has been confirmed in patients with type 1 diabetes.Distribution — Radiolabeled distribution studies of Humalog Mix75/25 have not been conducted. However, the volume of distribution following injection of Humalog is identical to that of Regular human insulin, with a range of 0.26 to 0.36 L/kg.Metabolism — Human metabolism studies of Humalog Mix75/25 have not been conducted. Studies in animals indicate that the metabolism of Humalog, the rapid-acting component of Humalog Mix75/25, is identical to that of Regular human insulin.Elimination — Humalog Mix75/25 has two absorption phases, a rapid and a prolonged phase, representative of the insulin lispro and insulin lispro protamine suspension components of the mixture. As with other intermediate-acting insulins, a meaningful terminal phase half-life cannot be calculated after administration of Humalog Mix75/25 because of the prolonged insulin lispro protamine suspension absorption.PharmacodynamicsStudies in nondiabetic subjects and patients with diabetes demonstrated that Humalog has a more rapid onset of glucose-lowering activity, an earlier peak for glucose-lowering, and a shorter duration of glucose-lowering activity than Regular human insulin. The early onset of activity of Humalog Mix75/25 is directly related to the rapid absorption of Humalog. The time course of action of insulin and insulin analogs, such as Humalog (and hence Humalog Mix75/25), may vary considerably in different individuals or within the same individual. The parameters of Humalog Mix75/25 activity (time of onset, peak time, and duration) as presented in Figures 2 and 3 should be considered only as general guidelines. The rate of insulin absorption and consequently the onset of activity is known to be affected by the site of injection, exercise, and other variables (see General under PRECAUTIONS).In a glucose clamp study performed in 30 nondiabetic subjects, the onset of action and glucose-lowering activity of Humalog, Humalog® Mix50/50™, Humalog Mix75/25, and insulin lispro protamine suspension (NPL component) were compared (see Figure 2). Graphs of mean glucose infusion rate versus time showed a distinct insulin activity profile for each formulation. The rapid onset of glucose-lowering activity characteristic of Humalog was maintained in Humalog Mix75/25.In separate glucose clamp studies performed in nondiabetic subjects, pharmacodynamics of Humalog Mix75/25 and Humulin 70/30 were assessed and are presented in Figure 3. Humalog Mix75/25 has a duration of activity similar to that of Humulin 70/30.Figure 2: Insulin Activity After Injection of Humalog, Humalog Mix50/50, Humalog Mix75/25, or Insulin Lispro Protamine Suspension (NPL Component) in 30 NondiabeticSubjects.Figure 3: Insulin Activity After Injection of Humalog Mix75/25 and Humulin 70/30 inNondiabetic Subjects.Figures 2 and 3 represent insulin activity profiles as measured by glucose clamp studies in healthy nondiabetic subjects.Figure 2 shows the time activity profiles of Humalog, Humalog Mix50/50, HumalogMix75/25, and insulin lispro protamine suspension (NPL component).Figure 3 is a comparison of the time activity profiles of Humalog Mix75/25 (see Figure 3a) and of Humulin 70/30 (see Figure 3b) from two different studies.Special PopulationsAge and Gender — Information on the effect of age on the pharmacokinetics of HumalogMix75/25 is unavailable. Pharmacokinetic and pharmacodynamic comparisons between men and women administered Humalog Mix75/25 showed no gender differences. In large Humalog clinical trials, sub-group analysis based on age and gender demonstrated that differences between Humalog and Regular human insulin in postprandial glucose parameters are maintained across sub-groups.Smoking — The effect of smoking on the pharmacokinetics and pharmacodynamics of Humalog Mix75/25 has not been studied.Pregnancy — The effect of pregnancy on the pharmacokinetics and pharmacodynamics of Humalog Mix75/25 has not been studied.Obesity — The effect of obesity and/or subcutaneous fat thickness on the pharmacokinetics and pharmacodynamics of Humalog Mix75/25 has not been studied. In large clinical trials, which included patients with Body Mass Index up to and including 35 kg/m2, no consistent differences were observed between Humalog and Humulin® R with respect to postprandial glucose parameters.Renal Impairment — The effect of renal impairment on the pharmacokinetics and pharmacodynamics of Humalog Mix75/25 has not been studied. In a study of 25 patients with type 2 diabetes and a wide range of renal function, the pharmacokinetic differences between Humalog and Regular human insulin were generally maintained. However, the sensitivity of thepatients to insulin did change, with an increased response to insulin as the renal function declined. Careful glucose monitoring and dose reductions of insulin, including HumalogMix75/25, may be necessary in patients with renal dysfunction.Hepatic Impairment — Some studies with human insulin have shown increased circulating levels of insulin in patients with hepatic failure. The effect of hepatic impairment on the pharmacokinetics and pharmacodynamics of Humalog Mix75/25 has not been studied. However, in a study of 22 patients with type 2 diabetes, impaired hepatic function did not affect the subcutaneous absorption or general disposition of Humalog when compared with patients with no history of hepatic dysfunction. In that study, Humalog maintained its more rapid absorption and elimination when compared with Regular human insulin. Careful glucose monitoring and dose adjustments of insulin, including Humalog Mix75/25, may be necessary in patients with hepatic dysfunction.INDICATIONS AND USAGEHumalog Mix75/25, a mixture of 75% insulin lispro protamine suspension and 25% insulin lispro injection, (rDNA origin), is indicated in the treatment of patients with diabetes mellitus for the control of hyperglycemia. Humalog Mix75/25 has a more rapid onset of glucose-lowering activity compared with Humulin 70/30 while having a similar duration of action. This profile is achieved by combining the rapid onset of Humalog with the intermediate action of insulin lispro protamine suspension.CONTRAINDICATIONSHumalog Mix75/25 is contraindicated during episodes of hypoglycemia and in patients sensitive to insulin lispro or any of the excipients contained in the formulation.WARNINGSHumalog differs from Regular human insulin by its rapid onset of action as well as a shorter duration of activity. Therefore, the dose of Humalog Mix75/25 should be given within 15 minutes before a meal.Hypoglycemia is the most common adverse effect associated with the use of insulins, including Humalog Mix75/25. As with all insulins, the timing of hypoglycemia may differ among various insulin formulations. Glucose monitoring is recommended for all patients with diabetes.Any change of insulin should be made cautiously and only under medical supervision. Changes in insulin strength, manufacturer, type (e.g., Regular, NPH, analog), species, or method of manufacture may result in the need for a change in dosage.PRECAUTIONSGeneralHypoglycemia and hypokalemia are among the potential clinical adverse effects associated with the use of all insulins. Because of differences in the action of Humalog Mix75/25 and other insulins, care should be taken in patients in whom such potential side effects might be clinically relevant (e.g., patients who are fasting, have autonomic neuropathy, or are using potassium-lowering drugs or patients taking drugs sensitive to serum potassium level). Lipodystrophy and hypersensitivity are among other potential clinical adverse effects associated with the use of all insulins.As with all insulin preparations, the time course of Humalog Mix75/25 action may vary in different individuals or at different times in the same individual and is dependent on site of injection, blood supply, temperature, and physical activity.Adjustment of dosage of any insulin may be necessary if patients change their physical activity or their usual meal plan. Insulin requirements may be altered during illness, emotional disturbances, or other stress.Hypoglycemia — As with all insulin preparations, hypoglycemic reactions may be associated with the administration of Humalog Mix75/25. Rapid changes in serum glucose concentrations may induce symptoms of hypoglycemia in persons with diabetes, regardless of the glucose value. Early warning symptoms of hypoglycemia may be different or less pronounced under certain conditions, such as long duration of diabetes, diabetic nerve disease, use of medications such as beta-blockers, or intensified diabetes control.Renal Impairment — As with other insulins, the requirements for Humalog Mix75/25 may be reduced in patients with renal impairment.Hepatic Impairment — Although impaired hepatic function does not affect the absorption or disposition of Humalog, careful glucose monitoring and dose adjustments of insulin, including Humalog Mix75/25, may be necessary.Allergy — Local Allergy — As with any insulin therapy, patients may experience redness, swelling, or itching at the site of injection. These minor reactions usually resolve in a few days to a few weeks. In some instances, these reactions may be related to factors other than insulin, such as irritants in the skin cleansing agent or poor injection technique.Systemic Allergy — Less common, but potentially more serious, is generalized allergy to insulin, which may cause rash (including pruritus) over the whole body, shortness of breath, wheezing, reduction in blood pressure, rapid pulse, or sweating. Severe cases of generalized allergy, including anaphylactic reaction, may be life threatening. Localized reactions and generalized myalgias have been reported with the use of cresol as an injectable excipient. Antibody Production — In clinical trials, antibodies that cross-react with human insulin and insulin lispro were observed in both human insulin mixtures and insulin lispro mixtures treatment groups.Information for PatientsPatients should be informed of the potential risks and advantages of Humalog Mix75/25 and alternative therapies. Patients should not mix Humalog Mix75/25 with any other insulin. They should also be informed about the importance of proper insulin storage, injection technique, timing of dosage, adherence to meal planning, regular physical activity, regular blood glucose monitoring, periodic hemoglobin A1c testing, recognition and management of hypo- and hyperglycemia, and periodic assessment for diabetes complications.Patients should be advised to inform their physician if they are pregnant or intend to become pregnant.Refer patients to the Patient Information leaflet for information on normal appearance, timing of dosing (within 15 minutes before a meal), storing, and common adverse effects.For Patients Using Insulin Pen Delivery Devices: Before starting therapy, patients should read the Patient Information leaflet that accompanies the drug product and the User Manual that accompanies the delivery device and re-read them each time the prescription is renewed. Patients should be instructed on how to properly use the delivery device, prime the Pen to a stream of insulin, and properly dispose of needles. Patients should be advised not to share their Pens with others.Laboratory TestsAs with all insulins, the therapeutic response to Humalog Mix75/25 should be monitored by periodic blood glucose tests. Periodic measurement of hemoglobin A1c is recommended for the monitoring of long-term glycemic control.Drug InteractionsInsulin requirements may be increased by medications with hyperglycemic activity such as corticosteroids, isoniazid, certain lipid-lowering drugs (e.g., niacin), estrogens, oral contraceptives, phenothiazines, and thyroid replacement therapy.Insulin requirements may be decreased in the presence of drugs that increase insulin sensitivity or have hypoglycemic activity, such as oral antidiabetic agents, salicylates, sulfa antibiotics, certain antidepressants (monoamine oxidase inhibitors), angiotensin-converting-enzyme inhibitors, angiotensin II receptor blocking agents, beta-adrenergic blockers, inhibitors of pancreatic function (e.g., octreotide), and alcohol. Beta-adrenergic blockers may mask the symptoms of hypoglycemia in some patients.Carcinogenesis, Mutagenesis, Impairment of FertilityLong-term studies in animals have not been performed to evaluate the carcinogenic potential of Humalog, Humalog Mix75/25, or Humalog Mix50/50. Insulin lispro was not mutagenic in a battery of in vitro and in vivo genetic toxicity assays (bacterial mutation tests, unscheduled DNA synthesis, mouse lymphoma assay, chromosomal aberration tests, and a micronucleus test). There is no evidence from animal studies of impairment of fertility induced by insulin lispro. PregnancyTeratogenic Effects — Pregnancy Category B — Reproduction studies with insulin lispro have been performed in pregnant rats and rabbits at parenteral doses up to 4 and 0.3 times, respectively, the average human dose (40 units/day) based on body surface area. The results have revealed no evidence of impaired fertility or harm to the fetus due to insulin lispro. There are, however, no adequate and well-controlled studies with Humalog, Humalog Mix75/25, or Humalog Mix50/50 in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Nursing MothersIt is unknown whether insulin lispro is excreted in significant amounts in human milk. Many drugs, including human insulin, are excreted in human milk. For this reason, caution should be exercised when Humalog Mix75/25 is administered to a nursing woman. Patients with diabetes who are lactating may require adjustments in Humalog Mix75/25 dose, meal plan, or both. Pediatric UseSafety and effectiveness of Humalog Mix75/25 in patients less than 18 years of age have not been established.Geriatric UseClinical studies of Humalog Mix75/25 did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients. In general, dose selection for an elderly patient should take into consideration the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in this population.ADVERSE REACTIONSClinical studies comparing Humalog Mix75/25 with human insulin mixtures did not demonstrate a difference in frequency of adverse events between the two treatments.Adverse events commonly associated with human insulin therapy include the following:Body as a Whole — allergic reactions (see PRECAUTIONS).Skin and Appendages — injection site reaction, lipodystrophy, pruritus, rash.Other — hypoglycemia (see WARNINGS and PRECAUTIONS).OVERDOSAGEHypoglycemia may occur as a result of an excess of insulin relative to food intake, energy expenditure, or both. Mild episodes of hypoglycemia usually can be treated with oral glucose. Adjustments in drug dosage, meal patterns, or exercise, may be needed. More severe episodes with coma, seizure, or neurologic impairment may be treated with intramuscular/subcutaneousglucagon or concentrated intravenous glucose. Sustained carbohydrate intake and observation may be necessary because hypoglycemia may recur after apparent clinical recovery. DOSAGE AND ADMINISTRATIONTable 1*: Summary of Pharmacodynamic Properties of Insulin Products (Pooled Cross-Study Comparison)Insulin Products Dose, U/kg Time of Peak Activity, Hours After Dosing Percent of Total Activity Occurring in the First 4 Hours Humalog 0.3 2.4 (0.8 - 4.3) 70% (49 - 89%) Humulin R 0.32 (0.26 - 0.37) 4.4 (4.0 - 5.5) 54% (38 - 65%) Humalog Mix75/25 0.3 2.6 (1.0 - 6.5) 35% (21 - 56%) Humulin 70/30 0.3 4.4 (1.5 - 16) 32% (14 - 60%) Humalog Mix50/50 0.3 2.3 (0.8 - 4.8) 45% (27 - 69%) Humulin 50/50 0.3 3.3 (2.0 - 5.5) 44% (21 - 60%) NPH 0.32 (0.27 - 0.40) 5.5 (3.5 - 9.5) 14% (3.0 - 48%) NPL component 0.3 5.8 (1.3 - 18.3) 22% (6.3 - 40%) * The information supplied in Table 1 indicates when peak insulin activity can be expected and the percent of the total insulin activity occurring during the first 4 hours. The information was derived from 3 separate glucose clamp studies in nondiabetic subjects. Values represent means, with ranges provided in parentheses.Humalog Mix75/25 is intended only for subcutaneous administration. Humalog Mix75/25 should not be administered intravenously. Dosage regimens of Humalog Mix75/25 will vary among patients and should be determined by the healthcare provider familiar with the patient’s metabolic needs, eating habits, and other lifestyle variables. Humalog has been shown to be equipotent to Regular human insulin on a molar basis. One unit of Humalog has the same glucose-lowering effect as one unit of Regular human insulin, but its effect is more rapid and of shorter duration. Humalog Mix75/25 has a similar glucose-lowering effect as compared with Humulin 70/30 on a unit for unit basis. The quicker glucose-lowering effect of Humalog is related to the more rapid absorption rate of insulin lispro from subcutaneous tissue.Humalog Mix75/25 starts lowering blood glucose more quickly than Regular human insulin, allowing for convenient dosing immediately before a meal (within 15 minutes). In contrast, mixtures containing Regular human insulin should be given 30 to 60 minutes before a meal. The rate of insulin absorption and consequently the onset of activity are known to be affected by the site of injection, exercise, and other variables. As with all insulin preparations, the time course of action of Humalog Mix75/25 may vary considerably in different individuals or within the same individual. Patients must be educated to use proper injection techniques.Humalog Mix75/25 should be inspected visually before use. Humalog Mix75/25 should be used only if it appears uniformly cloudy after mixing. Humalog Mix75/25 should not be used after its expiration date.HOW SUPPLIEDHumalog Mix75/25 [75% insulin lispro protamine suspension and 25% insulin lispro injection, (rDNA origin)] is available in the following package sizes: each presentation containing 100 units insulin lispro per mL (U-100).10 mL vials NDC 0002-7511-01 (VL-7511)5 x 3 mL prefilled insulin delivery devices (Pen) NDC 0002-8794-59 (HP-8794)5 x 3 mL prefilled insulin delivery devices (KwikPen™) NDC 0002-8797-59 (HP-8797)Storage — Humalog Mix75/25 should be stored in a refrigerator [2° to 8°C (36° to 46°F)], but not in the freezer. Do not use Humalog Mix75/25 if it has been frozen. Unrefrigerated [below 30°C (86°F)] vials must be used within 28 days or be discarded, even if they still contain Humalog Mix75/25. Unrefrigerated [below 30°C (86°F)] Pens, and KwikPens must be used within 10 days or be discarded, even if they still contain Humalog Mix75/25. Protect from direct heat and light. See table below:Not In-Use (Unopened) Room Temperature [Below 30°C (86°F)] Not In-Use (Unopened)RefrigeratedIn-Use (Opened) RoomTemperature [Below30°C (86°F)]10 mL Vial 28 days Until expiration date 28 days,refrigerated/roomtemperature.3 mL Pen and KwikPen (prefilled) 10 days Until expiration date 10 days. Do notrefrigerate.Literature revised March 16, 2009KwikPens manufactured byEli Lilly and Company, Indianapolis, IN 46285, USAPens manufactured byEli Lilly and Company, Indianapolis, IN 46285, USA orLilly France, F-67640 Fegersheim, FranceVials manufactured byEli Lilly and Company, Indianapolis, IN 46285, USA orLilly France, F-67640 Fegersheim, Francefor Eli Lilly and Company, Indianapolis, IN 46285, USACopyright © 2007, 2009, Eli Lilly and Company. All rights reserved.PV 5551 AMP PRINTED IN USA。
乙基己基三嗪酮 结构式 分子量 透皮

乙基己基三嗪酮的结构式是C18H26N2O,分子量为298.42 g/mol。
透皮是指物质穿过皮肤的过程,它可以通过皮肤表面透过皮肤下层进入血液循环系统。
乙基己基三嗪酮作为一种透皮增透剂,在化妆品和药物中起着重要作用。
1. 引言乙基己基三嗪酮是一种常用的化学成分,它在化妆品和药物中被广泛使用。
乙基己基三嗪酮的分子结构如下所示(图1):(图1: 乙基己基三嗪酮结构式)2. 乙基己基三嗪酮的分子量及作用乙基己基三嗪酮的分子量为298.42 g/mol,这意味着它的分子在摩尔质量方面比例为298.42克。
这一特性使得乙基己基三嗪酮在化妆品和药物中的使用非常方便,可以精确地控制用量,从而达到理想的效果。
乙基己基三嗪酮还具有良好的透皮性,能够快速透过皮肤进入到血液循环系统。
这种特性使其成为一种理想的透皮增透剂,在许多药物和化妆品中得到广泛应用。
3. 乙基己基三嗪酮在化妆品中的应用在化妆品中,乙基己基三嗪酮通常被添加到护肤品和化妆品中,以提高其透皮性,增加功效成分的渗透效果。
乙基己基三嗪酮可以帮助有效成分更好地渗透到皮肤深层,从而达到更好的护肤和美容效果。
4. 乙基己基三嗪酮在药物中的应用在药物中,乙基己基三嗪酮常常被用作透皮贴剂的增透剂,帮助药物有效成分透过皮肤层进入体内,从而实现缓慢释放和持续治疗的效果。
这种用法不仅能够提高药物的吸收效率,还可以降低药物对胃肠道的刺激,减轻药物的不良反应。
5. 个人观点和理解乙基己基三嗪酮作为一种透皮增透剂,在化妆品和药物领域发挥着重要作用。
它的分子结构和透皮性使得其在这两个领域具有广泛的应用前景。
然而,对于乙基己基三嗪酮的安全性和潜在的副作用问题,还需要更多的研究和探讨,以确保其在产品中的安全性和可靠性。
6. 总结与回顾本文通过介绍乙基己基三嗪酮的分子结构、分子量、透皮作用以及在化妆品和药物中的应用,深入探讨了这一化学成分的重要性和作用机制。
通过这些内容的讨论,读者不仅可以了解乙基己基三嗪酮的基本特性,还可以深入了解其在实际应用中的作用和潜在问题。
21cfr176.170 Tests for Paper and Paperborad

21 CFR Ch. I (4–1–05 Edition)§176.170films or grease-resistant papers con-form with appropriate food additive regulations.(c) The labeling of the food additive shall contain adequate directions for its use to insure compliance with the requirements of paragraphs (a) and (b) of this section.§176.170Components of paper and pa-perboard in contact with aqueous and fatty foods. Substances identified in this section may be safely used as components of the uncoated or coated food-contact surface of paper and paperboard in-tended for use in producing, manufac-turing, packaging, processing, pre-paring, treating, packing, transporting, or holding aqueous and fatty foods, subject to the provisions of this sec-tion. Components of paper and paper-board in contact with dry food of the type identified under Type VIII of table 1 in paragraph (c) of this section are subject to the provisions of §176.180. (a) Substances identified in para-graph (a) (1) through (5) of this section may be used as components of the food- contact surface of paper and paper-board. Paper and paperboard products shall be exempted from compliance with the extractives limitations pre-scribed in paragraph (c) of this section:Provided, That the components of the food-contact surface consist entirely of one or more of the substances identi-fied in this paragraph: And provided fur-ther, That if the paper or paperboard when extracted under the conditions prescribed in paragraph (c) of this sec-tion exceeds the limitations on extrac-tives contained in paragraph (c) of this section, information shall be available from manufacturing records from which it is possible to determine that only substances identified in this para-graph (a) are present in the food-con-tact surface of such paper or paper-board.(1) Substances generally recognized as safe in food.(2) Substances generally recognized as safe for their intended use in paper and paperboard products used in food packaging.(3) Substances used in accordance with a prior sanction or approval.(4) Substances that by regulation in parts 170 through 189 of this chapter may be safely used without extractives limitations as components of the uncoated or coated food-contact sur-face of paper and paperboard in contact with aqueous or fatty food, subject to the provisions of such regulation.(5) Substances identified in this para-graph, as follows:List of SubstancesLimitationsAcetyl peroxide ............................................................................For use only as polymerization catalyst.Acrylamide-methacrylic acid-maleic anhydride copolymers con-taining not more than 0.2 percent of residual acrylamide monomer and having an average nitrogen content of 14.9 percent such that a 1 percent by weight aqueous solution has a minimum viscosity of 600 centipoises at 75 °F, as de-termined by LVG-series Brookfield viscometer (or equivalent) using a No. 2 spindle at 30 r.p.m.For use only as a retention aid employed prior to the sheet- forming operation in the manufacture of paper and paper-board in such an amount that the finished paper and paper-board will contain the additive at a level not in excess of 0.05 percent by weight of dry fibers in the finished paper andpaperboard. Acrylamide-b -methacrylyloxyethyltrimethylammonium methyl sulfate copolymer resins containing not more than 10 molar percent of b -methacrylyloxyethyltrimethylammonium methyl sulfate and containing less than 0.2% of residual acrylamide monomer.For use only as a retention aid and flocculant employed prior to the sheet-forming operation in the manufacture of paper and paperboard. Acrylic acid, sodium salt copolymer with polyethyleneglycol allyl ether (CAS Reg. No. 86830–15–1).For use only in paper mill boilers. Acrylic acid copolymer with 2-acrylamido-2-methylpropane-sul-fonic acid (CAS Reg. No. 40623–75–4) and/or its ammo-nium/alkali metal mixed salts. The copolymer is produced by poly-merization of acrylic acid and 2-acrylamido-2- methylpropane-sulfonic acid in a weight ratio of 60/40, such that a 28 percent by weight aqueous solution of the polymer has a viscosity of 75–150 centipoises at 25 °C as deter-mined by LV-series Brookfield viscometer (or equivalent) using a No. 2 spindle at 60 r.p.m.For use only as a scale inhibitor prior to the sheet-forming op-eration in the manufacture of paper and paperboard andused at a level not to exceed 1.0 kilogram (2.2 pounds) of copolymer per 907 kilograms (1 ton) of dry paper and paper-board fibers.Food and Drug Administration, HHS§176.170List of SubstancesLimitationsAcrylonitrile polymer, reaction product with ethylenediamine sulfate having a nitrogen content of 22.5–25.0 percent (Kjel-dahl dry basis) and containing no more than 0.075 percent monomer as ethylenediamine. The finished resin in a 24 per-cent by weight aqueous solution has a viscosity of 1,000– 2,000 centipoises at 25 °C as determined by LVT-series Brookfield viscometer using a No. 4 spindle at 50 r.p.m. (or by other equivalent method).For use only as a size promoter and retention aid at a level not to exceed 0.5 percent by weight of the dry paper and paper-board. Acrylonitrile polymer with styrene, reaction product with ethyl-enediamine acetate, having a nitrogen content of 7.4–8.3 percent (Kjeldahl dry basis) and containing no more than 0.25 percent monomer as ethylenediamine. 1. For use only as a sizing material applied after the sheet- forming operation in the manufacture of paper and paper-board in such amount that the paper and paperboard will contain the additive at a level not in excess of 0.25 percentby weight of the dry paper and paperboard.2. For use only as a sizing material applied prior to the sheet- forming operation in the manufacture of paper and paper-board in such amount that the paper and paperboard will contain the additive at a level not in excess of 1.0 percent by weight of the dry paper and paperboard.1-Alkenyl olefins, containing not less than 72 percent of C 30 and higher olefins.For use only under the following conditions: 1. In coatings for paper and paperboard with food of Types I,II, IV-B, and VII-B described in table 1 of paragraph (c) of this section under conditions of use E, F, and G described in table 2 of paragraph (c) of this section.2. In coatings for paper and paperboard with food of Type VIII described in table I of paragraph (c) of this section under conditions of use A through H described in table 2 of para-graph (c) of this section.(2-Alkenyl) succinic anhydrides mixture, in which the alkenyl groups are derived from olefins which contain not less than 95 percent of C 15-C 21groups.For use only as a sizing agent employed prior to the sheet- forming operation in the manufacture of paper and paper-board and limited to use at a level not to exceed 1 percentby weight of the finished dry paper and paperboard fibers.Alkyl(C 12-C 20)methacrylatemethacrylic acid copolymers (CAS Reg. No. 27401–06–5).For use only as stabilizers employed prior to the sheet-forming operation in the manufacture of paper and paperboard. tert-Alkyl(C 8-C 16)mercaptans .......................................................For use only as polymerization-control agent. Aluminum acetate.2-Amino-2-methyl-1-propanol (CAS Reg. No. 124–68–5)..........For use as a dispersant for pigment suspension at a level notto exceed 0.25 percent by weight of pigment. The suspen-sion is used as a component of coatings for paper and pa-perboard under conditions of use described in paragraph (c) of this section, table 2, conditions of use E through G.Ammonium bis(N-ethyl-2-perfluoroalkylsulfonamido ethyl) phosphates, containing not more than 15% ammonium mono (N-ethyl-2-perfluoroalkylsulfonamido ethyl) phosphates, where the alkyl group is more than 95% C 8and the salts have a fluorine content of 50.2% to 52.8% as determined on a solids basis.For use only as an oil and water repellant at a level not to ex-ceed 0.17 pound (0.09 pound of fluorine) per 1,000 square feet of treated paper or paperboard of a sheet basis weight of 100 pounds or less per 3,000 square feet of paper or pa-perboard, and at a level not to exceed 0.5 pound (0.26 pound of fluorine) per 1,000 square feet of treated paper orpaperboard having a sheet basis weight greater than 100 lb. per 3,000 square feet as determined by analysis for total flu-orine in the treated paper or paperboard without correction for any fluorine that might be present in the untreated paper or paperboard, when such paper or paperboard is used as follows:1. In contact, under conditions of use C, D, E , F, G, or H de-scribed in table 2 of paragraph (c) of this section, with non-alcoholic food.2. In contact with bakery products of Type VII, VIII, and IX de-scribed in table I of paragraph (c) of this section under good manufacturing practices of commercial and institutional bak-ing.Ammonium persulfate.Ammonium thiosulfate.Ammonium zirconium carbonate (CAS Reg. No. 32535–84–5) and its tartaric acid adduct.For use only as an insolubilizer for binders used in coatings for paper and paperboard, and limited to use at a level not toexceed 2.5 percent by weight of coating solids.Ammonium zirconium citrate (CAS Reg. No. 149564–62–5), ammonium zirconium lactate-citrate (CAS Reg. No. 149564– 64–7), ammonium zirconium lactate (CAS Reg. No. 149564– 63–6).For use as insolubilizers with protein binders in coatings for paper and paperboard, at a level not to exceed 1.4 percent by weight of coating solids.21 CFR Ch. I (4–1–05 Edition) §176.170List of Substances LimitationsAnionic polyurethane, produced by reacting the preliminary adduct formed from the reaction of glyceryl monostearate and 2,4-toluenediisocyanate with not more than 10 mole per-cent N-methyldiethanolamine and not less than 90 mole per-cent dimethylolpropionic acid. The final product is a 15 to 20 percent by weight aqueous solution, having a Brookfield vis-cosity of 25 to 100 centipoises at 24 °C (75 °F).For use only as a surface sizing agent at a level not to exceed 0.1 percent by weight of dry paper and paperboard.9,10–Anthraquinone (Chemical Abstracts Service Registry No. 84–65–1) which has a purity of not less than 98 percent.For use only as a pulping aid in the alkaline pulping of lignocellulosic material at levels not to exceed 0.1 percent by weight of the raw lignocellulosic material.Aromatic petroleum hydrocarbon resin, hydrogenated (CAS Reg. No. 88526–47–0), produced by the catalytic polym-erization of aromatic substituted olefins from low boiling dis-tillates of cracked petroleum stocks with a boiling point no greater than 220 °C (428 °F), and the subsequent catalytic reduction of the resulting aromatic petroleum hydrocarbon resin. The resin meets the following specifications: softening point 85 °C (185 °F) minimum, as determined by ASTM Method E 28–67 (Reapproved 1982), ‘‘Standard Test Meth-od for Softening Point by Ring-and-Ball Apparatus,’’ and ani-line point 70 °C (158 °F) minimum, as determined by ASTM Method D 611–82, ‘‘Standard Test Methods for Aniline Point and Mixed Aniline Point of Petroleum Products and Hydro-carbon Solvents,’’ which are incorporated by reference in ac-cordance with 5 U.S.C. 552(a) and 1 CFR part 51. Copies may be obtained from the American Society for Testing and Materials, 1916 Race St., Philadelphia, PA 19103, or may be examined at the National Archives and Records Administra-tion (NARA). For information on the availability of this mate-rial at NARA, call 202–741–6030, or go to: http:// /federal l register/code l of l federal l regulations/ibr l locations.html..For use only as modifiers in wax polymer blend coatings for paper and paperboard at a level not to exceed 50 weight- percent of the coating solids under conditions of use E, F, and G identified in table 2 of paragraph (c) of this section.Azo-bisisobutyronitrile..................................................................For use only as polymerization catalyst.1,2-Benzisothiazolin-3-one (CAS Registry No. 2634–33–5).......For use only as a preservative in paper coating compositionsand limited to use at a level not to exceed 0.01 mg/in2(0.0016 mg/cm2) of the finished paper and paperboard. Benzoyl peroxide.........................................................................Do.N,N-Bis(2-hydroxyethyl)alkyl (C12-C18)amide..............................For use only as an adjuvant to control pulp absorbency andpitch content in the manufacture of paper and paperboardprior to the sheet forming operation.Bis(methoxymethyl)tetrakis-[(octadecyloxy)-methyl]melamine resins having a 5.8–6.5 percent nitrogen content (CAS Reg. No. 68412–27–1).For use only under the following conditions:1. As a water repellant employed prior to the sheet-forming op-eration in the manufacture of paper and paperboard in such amount that the finished paper and paperboard will contain the additive at a level not in excess of 1.6 percent by weight of the finished dry paper and paperboard fibers.2. The finished paper and paperboard will be used in contact with nonalcoholic foods only.3. As a water repellant employed after the sheet-forming oper-ation in the manufacture of paper and paperboard in such amount that the finished paper and paperboard will contain the additive at a level not to exceed 1.6 percent by weight of the finished dry paper and paperboard fibers. The finished paper and paperboard will be used only in contact with food of Types I, II, IV-B, VI, VII-B, and VIII described in table 1 of paragraph (c) of this section.2-Bromo-2-nitro-1,3-propanediol (CAS Reg. No. 52–51–7)........For use only as an antimicrobial/preservative in fillers, pigmentslurries, starch sizing solutions, and latex coatings at levelsnot to exceed 0.01 percent by weight of those components.Butanedioic acid, sulfo-1,4-di-(C9-C11alkyl) ester, ammonium salt (also known as butanedioic acid, sulfo-1,4-diisodecyl ester, ammonium salt [CAS Reg. No. 144093–88–9])..For use as a surface active agent in package coating inks at levels not to exceed 3 percent by weight of the coating ink.tert-Butyl hydroperoxide..............................................................For use only as polymerization catalyst.tert-Butyl peroxide.......................................................................Do.Calcium isostearate.....................................................................For use only with n-decyl alcohol as a stabilizing material foraqueous calcium stearate dispersions intended for use ascomponents of coatings for paper and paperboard. Carrageenan and salts of carrageenan as described in§§172.620 and 172.626 of this chapter.Castor oil, hydrogenated.Castor oil, sulfated, ammonium, potassium, or sodium salt.Cellulose, regenerated. Chloracetamide............................................................................For use only as polymerization-control agent.Cobaltous acetate........................................................................For use only as polymerization catalyst.Food and Drug Administration, HHS §176.170 List of Substances LimitationsCumene hydroperoxide...............................................................Do. Cyanoguanidine...........................................................................For use only:1. As a modifier for amino resins.2. As a fluidizing agent in starch and protein coatings for paperand paperboard.n-Decyl alcohol............................................................................For use only with calcium isostearate as a stabilizing materialfor aqueous calcium stearate dispersions intended for use ascomponents of coatings for paper and paperboard. Dialdehyde guar gum..................................................................For use only as a wet-strength agent employed prior to thesheet-forming operation in the manufacture of paper and pa-perboard and used at a level not to exceed 1% by weight ofthe finished dry paper and paperboard fibers.Dialdehyde locust bean gum.......................................................Do.Dialkyl(C16-C18)carbamoyl chloride (CAS Reg. No. 41319–54– 4) manufactured by the reaction of secondary amines de-rived from fatty acids of animal or vegetable sources with phosgene.For use as a sizing agent at a level not to exceed 0.2 percent by weight of the dry fiber.Diallyldimethyl ammonium chloride polymer with acrylamide and potassium acrylate, produced by copolymerizing either (1) diallyldimethyl ammonium chloride and acrylamide in a weight ratio of 50/50, with 4.4 percent of the acrylamide sub-sequently hydrolyzed to potassium acrylate or (2) polym-erized diallyldimethyl ammonium chloride, acrylamide and potassium acrylate (as acrylic acid) in a weight ratio of 50/ 47.8/2.2, respectively, so that the finished resin in a 1 per-cent by weight aqueous solution (active polymer) has a vis-cosity of more than 22 centipoises at 22 °C (72 °F) as deter-mined by LVF series, Brookfield Viscometer using No. 1 spindle at 60 RPM (or by other equivalent method) (CAS Reg. No. 25136–75–8).For use only as a retention and/or drainage aid employed prior to the sheet-forming operations in the manufacture of paper and paperboard and limited to use at a level not to exceed 0.05 percent by weight of the finished paper and paper-board.Diallyldimethylammonium chloride with acrylamide (CAS Reg. No. 26590–05–6). The copolymer is produced by copolym-erizing diallyldimethylammonium chloride with acrylamide in a weight ratio of 50–50 so that the finished resin in a 1 per-cent by weight aqueous solution (active polymer) has a vis-cosity of more than 22 centipoises at 22 °C (71.6 °F), as de-termined by LVF-series Brookfield viscometer using a No. 1 spindle at 60 r.p.m. (or by other equivalent method).For use only as a drainage and/or retention aid employed prior to the sheet-forming operation in the manufacture of paper and paperboard and limited to use at a level not to exceed 0.05 percent by weight of the finished paper and paper-board.Diallyldiethylammonium chloride polymer with acrylamide, and diallyldimethylammonium chloride, produced by copolym-erizing acrylamide, diallyldiethylammonium chloride, and diallyldimethylammonium chloride, respectively, in the fol-lowing weight ratios and having viscosities determined at 22 °C, by LVF-series Brookfield viscometer using a No. 1 spin-dle at 60 r.p.m. (or by other equivalent method), as follows:.1. Weight ratio: 50–2.5–47.5. The finished resin in a 1 per-cent by weight aqueous solution has a minimum vis-cosity of 22 centipoises.For use only as a retention aid employed prior to the sheet- forming operation in the manufacture of paper and paper-board and limited to use at a level not to exceed 0.05 per-cent by weight of the finished paper and paperboard.2. Weight ratio: 25–2.5–72.5. The finished resin in a 0.20 percent by weight aqueous solution has a minimum vis-cosity of 20 centipoises.For use only as a drainage and/or retention aid employed prior to the sheet-forming operation in the manufacture of paper and paperboard and limited to use at a level not to exceed 0.075 percent by weight of the finished paper and paper-board.3. Weight ratio: 80–2.5–17.5. The finished resin in a 0.30 percent by weight aqueous solution has a minimum vis-cosity of 50 centipoises.For use only as a drainage and/or retention aid employed prior to the sheet-forming operation in the manufacture of paper and paperboard and limited to use at a level not to exceed 0.075 percent by weight of the finished paper and paper-board.21 CFR Ch. I (4–1–05 Edition)§176.170List of SubstancesLimitationsDiallyldiethylammonium chloride polymer with acrylamide, po-tassium acrylate, and diallyldimethylammonium chloride. The polymer is produced by copolymerizing either: (1) acryl-amide, diallyldiethylammonium chloride, and diallyldimethylammonium chloride in a weight ratio of 50– 2.5–47.5, respectively, with 4.4 percent of the acrylamide subsequently hydrolyzed to potassium acrylate, or (2) acryl-amide, potassium acrylate (as acrylic acid),diallyldiethylammonium chloride, and diallyldimethylammonium chloride in a weight ratio of 47.8– 2.2–2.5–47.5, so that the finished resin in a 1 percent by weight aqueous solution has a minimum viscosity of 22 cen-tipoises at 22 °C, as determined by LVF-series Brookfield viscometer using a No. 1 spindle at 60 r.p.m. (or by other equivalent method).For use only as a retention aid employed prior to the sheet- forming operation in the manufacture of paper and paper-board and limited to use at a level not to exceed 0.05 per-cent by weight of the finished paper and paperboard. Diallyldimethylammonium chloride polymer with acrylamide, re-action product with glyoxal, produced by copolymerizing not less than 90 weight percent of acrylamide and not more than 10 weight percent of diallyldimethylammonium chloride, which is then cross-linked with not more than 30 weight per-cent of glyoxal, such that a 10 percent aqueous solution has a minimum viscosity of 25 centipoises at 25 °C as deter-mined by Brookfield viscometer Model RVF, using a No. 1 spindle at 100 r.p.m.For use only as a dry and wet strength agent employed prior to the sheet-forming operation in the manufacture of paper and paperboard in such an amount that the finished paper and paperboard will contain the additive at a level not in ex-cess of 2 percent by weight of the dry fibers in the finishedpaper and paperboard. 2,2-Dibromo-3-nitrilopropionamide (CAS Reg. No.10222–01–2). For use as a preservative at a level not to exceed 100 partsper million in coating formulations and in component slurries and emulsions, used in the production of paper and paper-board and coatings for paper and paperboard.2,5-Di-tert-butyl hydroquinone .....................................................For use only as an antioxidant for fatty based coating adju-vants provided it is used at a level not to exceed 0.005% by weight of coating solids.Diethanolamine ............................................................................For use only:1. As an adjuvant to control pulp absorbency and pitch content in the manufacture of paper and paperboard prior to the sheet-forming operation.2.In paper mill boilers.Diethanolamine salts of mono- and bis (1H ,1H ,2H ,2H -perfluo-roalkyl) phosphates where the alkyl group is even-numbered in the range C 8-C 18and the salts have a fluorine content of 52.4% to 54.4% as determined on a solids basis.For use only as an oil and water repellant at a level not to ex-ceed 0.17 pound (0.09 pound of fluorine) per 1,000 square feet of treated paper or paperboard, as determined by anal-ysis for total fluorine in the treated paper or paperboard with-out correction for any fluorine which might be present in theuntreated paper or paperboard, when such paper or paper-board is used in contact with nonalcoholic foods under the conditions of use described in paragraph (c) of this section, table 2, conditions of use (B) through (H).Diethyl(2-hydroxyethyl) methylammonium methyl sulfate, acry-late, polymer with acrylamide, chemical abstract service reg-istry No. [26796–75–8] having 90–95 mole pct. acrylamide, a nitrogen content of not more than 19.7 pct. (Kjeldahl, dry basis), and a residual acrylamide monomer content of not more than 0.1 pct. The finished polymer in a 1 pct. by weight aqueous solution has a minimum viscosity of 900 centipoises at 25 °C as determined by LVT-series Brookfield viscometer using a No. 2 spindle at 12 r.p.m. (or by equivalent method).For use only as a retention aid and drainage aid employed prior to the sheet-forming operation in the manufacture ofpaper and paperboard at a level not to exceed 0.15 pct. by weight of finished dry paper and paperboard fibers. Diethylenetriamine .......................................................................For use only as a modifier for amino resins.N,N-Diisopropanolamide of tallow fatty acids .............................For use only as an adjuvant to control pulp absorbency andpitch content in the manufacture of paper and paperboard prior to the sheet-forming operation.Dimethylamine-epichlorohydrin copolymer in which not more than 5 mole-percent of dimethylamine may be replaced by an equimolar amount of ethylenediamine and in which the ratio of total amine to epichlorohydrin does not exceed 1:1. The nitrogen content of the copolymer shall be 9.4 to 10.8 weight percent on a dry basis and a 10 percent by weight aqueous solution of the final product has a minimum vis-cosity of 5.0 centipoises at 25 °C, as determined by LVT-se-ries Brookfield viscometer using a No. 1 spindle at 60 r.p.m. (or by other equivalent method).For use only:1. As a retention aid employed before the sheet-forming oper-ation in the manufacture of paper and paperboard and lim-ited to use at a level not to exceed 1 percent by weight of the finished paper and paperboard.2. At the size press at a level not to exceed 0.017 percent by weight of the finished paper and paperboard.N-[(Dimethylamino)methyl]-acrylamide polymer with acrylamide and styrene having a nitrogen content of not more than 16.9 percent and a residual acrylamide monomer content of not more than 0.2 percent on a dry basis.For use only as a dry-strength agent employed prior to the sheet-forming operation in the manufacture of paper and pa-perboard and used at a level not to exceed 1 percent by weight of finished dry paper or paperboard fibers. N,N ′-Dioleoylethylenediamine.Food and Drug Administration, HHS §176.170 List of Substances Limitations Diphenylamine.............................................................................For use only as an antioxidant for fatty based coating adju-vants provided it is used at a level not to exceed 0.005% byweight of coating solids.Dipropylene glycol.Disodium salt of 1,4-dihydro-9,10-dihydroxyanthracene (CAS Reg. No. 73347–80–5).For use only as a catalyst in the alkaline pulping of lignocellulosic materials at levels not to exceed 0.1 percent by weight of the raw lignocellulosic materials.N,N′-Distearoylethylenediamine.n-Dodecylguanidine acetate........................................................For use only as an antimicrobial agent in paper and paper-board under the following conditions:1. For contact only with nonalcoholic food having a pH above 5and provided it is used at a level not to exceed 0.4 percentby weight of the paper and paperboard.2. For use in the outer ply of multiwall paper bags for contactwith dry food of Type VIII described in table I of paragraph(c) of this section and provided it is used at a level of 0.8percent by weight of the paper.n-Dodecylguanidine hydrochloride..............................................For use only as an antimicrobial agent in paper and paper-board under the following conditions:1. For contact only with nonalcoholic food having a pH above 5and provided it is used at a level not to exceed 0.4 percentby weight of the paper and paperboard.2. For use in the outer ply of multiwall paper bags for contactwith dry food of Type VIII described in table I of paragraph(c) of this section and provided it is used at a level of 0.8percent by weight of the paper.Fatty acids derived from animal and vegetable fats and oilsand salts of such acids, single or mixed, as follows:Aluminum.Ammonium.Calcium.Magnesium.Potassium.Sodium.Zinc.Ferric chloride.Ferrous ammonium sulfate.Fish oil, hydrogenated.Fish oil, hydrogenated, potassium salt.Furcelleran and salts of furcelleran as described in §§172.655and 172.660 of this chapter.Glutaraldehyde (CAS Reg. No. 111–30–8).................................For use only as an antimicrobial agent in pigment and fillerslurries used in the manufacture of paper and paperboard atlevels not to exceed 300 parts per million by weight of theslurry solids.Glyceryl lactostearate.Glyceryl mono-1,2-hydroxystearate.Glyceryl monoricinoleate.Guar gum modified by treatment with b-diethylamino- ethyl chloride hydrochloride.For use only as a retention aid and/or drainage aid employed prior to the sheet-forming operation in the manufacture of paper and paperboard.Guar gum modified by treatment with not more than 25 weight percent of 2,3-epoxypropyltri-methylammonium chloride such that the finished product has a maximum chlorine content of4.5 percent, a maximum nitrogen content of 3.0 percent, anda minimum viscosity in 1-percent-by-weight aqueous solution of 1,000 centipoises at 77 °F, as determined by RV-series Brookfield viscometer (or equivalent) using a No. 3 spindle at 20 r.p.m.For use only as a retention aid and/or internal size employed prior to the sheet-forming operation in the manufacture of paper and paperboard, and limited to use at a level: (1) Not to exceed 0.15 percent by weight of the finished dry paper and paperboard fibers intended for use in contact with all types of foods, except (2) not to exceed 0.30 pct. by weight of the finished dried paper and paperboard fibers for use with nonalcoholic and nonfatty food of types identified under Types I, II, IV-B, VI-B, VII-B, and VIII of table I in par. (c) of this section.N,N,N′,N′,N″,N″-Hexakis (methoxymethyl)-1,3,5-triazine-2,4,6- triamine polymer with stearyl alcohol, a-octadecenyl-omega- hydroxypoly(oxy-1,2-ethanediyl), and alkyl (C20+) alcohols (CAS Reg. No. 130328–24–4).For use only as a water-repellent applied to the surface of paper and paperboard at levels not to exceed 1 percent by weight of the finished dry paperboard fibers. The finished paper and paperboard will be used in contact with aqueous foods under conditions of use B through G as described in table 2 of paragraph (c) of this section.Hexamethylenetetramine.............................................................For use only as polymerization cross-linking agent for protein,including casein.Hydroquinone and the monomethyl or monoethyl ethers of hy-droquinone.For use only as an inhibitor for monomers.。
吩噻嗪类衍生物

1. Introduction Phenothiazines are important psychotropic compounds, but they also have further biological activities.1–3 For example, phenothiazines have recently been considered as potential drugs in the management of CreutzfeldtJacob disease.4 Metabolism of phenothiazine-based drugs often results in the formation of 7-hydroxylated derivatives or 5-sulfoxides.5–7 Because oxidation of asymmetrically substituted phenothiazines at the S(5) position introduces a new stereogenic center, these 5-sulfoxides are chiral. Although chiral 5-sulfoxide metabolites of the phenothiazine drug thioridazine in human plasma were separated by HPLC,8 to date optically active phenothiazine 5-oxides have not been obtained on preparative scale. Hence, stereoselective methods for the synthesis of optically active phenothiazine-5-oxides would extend the possibilities for investigation of the S -oxide metabolites of phenothiazine-based drugs.
去乙酰毛花苷注射液说明模板之欧阳育创编

去乙酰毛花苷注射液【药品名称】通用名:去乙酰毛花苷注射液英文名:Deslanoside Injection汉语拼音:QuyixianMaohuaganZhsheye本品主要成分及其化学名称:为3-[(O-β-D-药吡喃糖基-(1→4)-0-2,6-二脱氧-β-D-核-己吡喃糖基-(1→4)-0-2,6-二脱氧-β-D-核-己吡喃糖基-(1→4)-0-2,6-二脱氧-β-D-核-己吡喃糖基)氧化]-12,14-二羟基-心甾-20(22)-烯内酯。
其结构式为:分子式:C47H74O19分子量:943.09辅料:乙醇,甘油,注射用水。
【适应症】(1)主要用于心力衰竭。
由于其作用较快,适用于急性心功能不全或慢性心功能不全急性加重的患者。
(2)亦可用于控制伴快速心室率的心房颤动、心房扑动患者的心室率。
(3)终止室上性心动过速起效慢,已少用。
【规格】2ml:0.4mg【用法和用量】静脉注射成人常用量:用5%葡萄糖注射液稀释后缓慢注射,首剂0.4-0.6㎎,以后每2-4小时可再给0.2-0.4㎎,总量1-1.6㎎。
小儿常用量:按下列剂量分2-3次间隔3-4小时给予。
早产儿和足月新生儿或肾功能减退、心肌炎患儿,肌内或静脉注射按体重0.022㎎/㎏,2周-3岁,按体重0.025㎎/㎏。
本品静脉注射获满意疗效后,可改用地高辛常用维持量以保持疗效。
【不良反应】(1)常见的不良反应包括:新出现的心律失常、胃纳不佳或恶心、呕吐(刺激延髓中枢)、下腹痛、异常的无力、软弱。
(2)少见的反应包括:视力模糊或“黄视”(中毒症状)、腹泻、中枢神经系统反应如精神抑郁或错乱。
(3)罕见的反应包括:嗜睡、头痛及皮疹、寻麻疹(过敏反应)。
(4)在洋地黄的中毒表现中,心律失常最重要,最常见者为室性早搏,约占心脏反应的33%。
其次为房室传导阻滞,阵发性或加速性交界性心动过速,阵发性房性心动过速伴房室传导阻滞,室性心动过速、窦性停搏、心室颤动等。
奎尼丁化学结构__概述说明以及解释

奎尼丁化学结构概述说明以及解释1. 引言1.1 概述:本文探讨的主题是奎尼丁化学结构,旨在对其进行总结、说明和解释。
奎尼丁是一种重要的药物分子,具有广泛的应用领域,在心血管疾病治疗以及抗心律失常药物中起着重要作用。
通过深入了解奎尼丁的化学结构、特性和应用,我们可以更好地理解它在医药领域中的作用机制。
1.2 文章结构:本文将按照以下顺序进行论述:引言、奎尼丁化学结构、奎尼丁的化学特性与反应性、奎尼丁在医药领域中的应用以及结论部分。
首先,我们会给出奎尼丁定义和背景信息,并详细介绍其分子式和分子量。
接下来,我们将对奎尼丁的化学结构进行解析,并提供示意图以加深理解。
然后,我们将探讨奎尼丁的物理性质和化学性质,并列举一些典型反应示例以展示其反应性。
最后,我们将着重介绍奎尼丁在心血管疾病治疗和抗心律失常药物中的应用,并简要介绍其他医疗领域中奎尼丁的应用案例。
最后,我们将对全文进行总结和结论。
1.3 目的:本文的目的是通过描述和解释奎尼丁化学结构,帮助读者更好地理解奎尼丁这一重要分子的特性和在医药领域中的广泛应用。
同时,我们将为读者提供相关背景信息和具体示意图,以促使他们对奎尼丁有更深入的认识。
通过阐明奎尼丁在心血管疾病治疗和抗心律失常药物中的应用案例,我们可以增强人们对该化合物在医学中所起作用的认知。
最后,我们希望通过本文能够加深读者对于奎尼丁化学结构以及其重要性的理解,并为进一步研究和发展提供参考依据。
2. 奎尼丁化学结构2.1 奎尼丁的定义和背景信息奎尼丁是一种常用的药物,被广泛应用于心血管疾病治疗和抗心律失常药物中。
奎尼丁属于二萜类化合物,其具有多个环和官能团,这些特点使其在医药领域中具有重要作用。
2.2 奎尼丁的分子式和分子量奎尼丁的分子式为C20H24N2O2,相应的分子量为324.42 g/mol。
从分子式可以看出,奎尼丁由20个碳原子、24个氢原子、2个氮原子和2个氧原子组成。
2.3 奎尼丁的结构解析和示意图奎尼丁的化学结构包含多个环系统。
西布曲明分子成份

西布曲明分子成份摘要:一、西布曲明的基本信息二、西布曲明的分子成份三、西布曲明的药理作用四、西布曲明的临床应用五、西布曲明的副作用与禁忌六、总结正文:西布曲明(Sibutramine)是一种中枢神经系统食欲抑制剂,主要用于治疗肥胖症。
它于1997 年首次在德国上市,后来在全球范围内广泛使用。
2010 年,由于发现可能增加心血管风险,欧洲药品管理局(EMA)建议暂停使用该药物。
在我国,西布曲明于2000 年被批准上市,但在2010 年也被叫停。
西布曲明的分子成份主要包括两个部分:苯甲酸衍生物和氨甲酰基部分。
其化学名称为N,N-二甲基-α-苯基-4-(2-氨甲酰基-2,3-二氢-1H-苯并[d] 咪唑-5-基)苯甲酸。
分子式为C18H19N3O2,分子量为309.36。
西布曲明的药理作用主要是通过抑制5-羟色胺(5-HT)和去甲肾上腺素(NE)再摄取,从而增加这两种神经递质在突触间隙的浓度,达到抑制食欲的目的。
此外,西布曲明还可以通过作用于脑内的μ-阿片受体,产生一定的镇痛效果。
西布曲明主要用于治疗肥胖症,尤其是合并有心血管疾病风险的患者。
在临床试验中,西布曲明可有效减轻患者的体重,并有助于改善与肥胖相关的并发症,如高血压、高血脂等。
然而,西布曲明也存在一定的副作用,包括头痛、恶心、失眠、口干等。
此外,西布曲明具有潜在的心血管风险,可能导致心脏病发作、中风等严重后果。
因此,患有心脏病、中风、高血压等疾病的人群以及孕妇、哺乳期妇女应避免使用。
总之,西布曲明作为一种食欲抑制剂,曾在全球范围内广泛应用于肥胖症的治疗。
然而,由于潜在的心血管风险,该药物的使用受到了限制。
去乙酰毛花苷注射液说明模板之欧阳术创编

去乙酰毛花苷注射液【药品名称】通用名:去乙酰毛花苷注射液英文名:Deslanoside Injection汉语拼音:QuyixianMaohuaganZhsheye本品主要成分及其化学名称:为3-[(O-β-D-药吡喃糖基-(1→4)-0-2,6-二脱氧-β-D-核-己吡喃糖基-(1→4)-0-2,6-二脱氧-β-D-核-己吡喃糖基-(1→4)-0-2,6-二脱氧-β-D-核-己吡喃糖基)氧化]-12,14-二羟基-心甾-20(22)-烯内酯。
其结构式为:分子式:C47H74O19分子量:943.09辅料:乙醇,甘油,注射用水。
【适应症】(1)主要用于心力衰竭。
由于其作用较快,适用于急性心功能不全或慢性心功能不全急性加重的患者。
(2)亦可用于控制伴快速心室率的心房颤动、心房扑动患者的心室率。
(3)终止室上性心动过速起效慢,已少用。
【规格】2ml:0.4mg【用法和用量】静脉注射成人常用量:用5%葡萄糖注射液稀释后缓慢注射,首剂0.4-0.6㎎,以后每2-4小时可再给0.2-0.4㎎,总量1-1.6㎎。
小儿常用量:按下列剂量分2-3次间隔3-4小时给予。
早产儿和足月新生儿或肾功能减退、心肌炎患儿,肌内或静脉注射按体重0.022㎎/㎏,2周-3岁,按体重0.025㎎/㎏。
本品静脉注射获满意疗效后,可改用地高辛常用维持量以保持疗效。
【不良反应】(1)常见的不良反应包括:新出现的心律失常、胃纳不佳或恶心、呕吐(刺激延髓中枢)、下腹痛、异常的无力、软弱。
(2)少见的反应包括:视力模糊或“黄视”(中毒症状)、腹泻、中枢神经系统反应如精神抑郁或错乱。
(3)罕见的反应包括:嗜睡、头痛及皮疹、寻麻疹(过敏反应)。
(4)在洋地黄的中毒表现中,心律失常最重要,最常见者为室性早搏,约占心脏反应的33%。
其次为房室传导阻滞,阵发性或加速性交界性心动过速,阵发性房性心动过速伴房室传导阻滞,室性心动过速、窦性停搏、心室颤动等。
盐酸尼非卡兰的合成
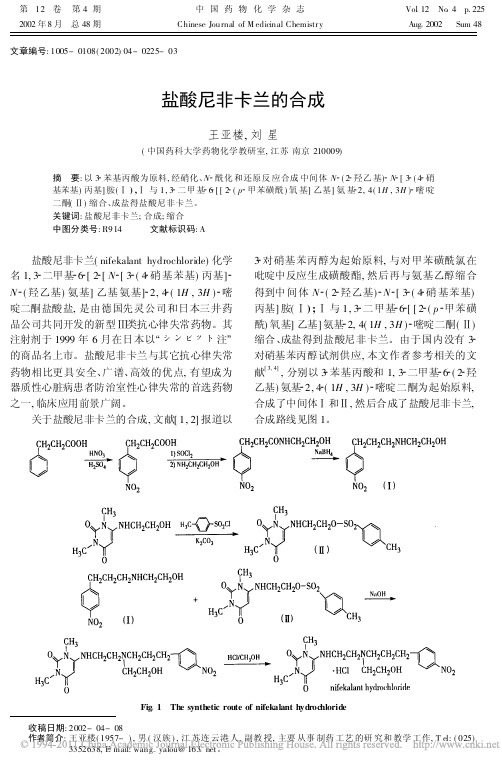
啶二酮盐酸盐, 是由德国先灵公司和日本三井药
品公司共同开发的新型 类抗心律失常药物。其
注射剂于 1999 年 6 月在日本以
! ∀注
的商品名上市。盐酸尼非卡兰与其它抗心律失常
药物相比更具安全、广谱、高效的优点, 有望成为
器质性心脏病患者防治室性心律失常的首选药物
之一, 临床应用前景广阔。
关于盐酸尼非卡兰的合成, 文献[ 1, 2] 报道以
盐酸尼非卡兰的合成
王亚楼, 刘 星
( 中国药科大学药物化学教研室, 江苏 南京 210009)
摘 要: 以 3 苯基丙酸为原料, 经硝化、N 酰化 和还原反 应合成 中间体 N ( 2 羟乙 基) N [ 3 ( 4 硝
基苯基) 丙基] 胺( ) , 与 1, 3 二甲 基 6 [ [ 2 ( p 甲苯磺酰 ) 氧 基] 乙基] 氨 基 2, 4( 1H , 3H ) 嘧 啶
参考文献:
[ 1] K atakami T , Yo koyama T , M iyamoto M , et al. Syn
thesis and pharmacolog ical studies of பைடு நூலகம் substituted 6
[ ( 2 aminoethyl) amino] 1, 3 dimethyl 2, 4( 1H , 3H )
在 50 mL 的反应瓶中, 加入 8 5 g( 0 04 mol) 1, 3 二甲基 6 ( 2 羟乙基) 氨基 2, 4 ( 1H , 3H ) 嘧 啶二酮及 35 mL 吡啶。将内温降至- 5 , 分批 加入 15 8 g ( 0 08 mol) 对甲苯磺酰氯, 使内温不 超过 5 , 加 完后 搅拌反 应 2 h, 将 反 应液 倒入 250 mL含 12 2 g K2 CO3 的冰水中, 放置, 析出结
