饲料添加剂自由销售证明申请表
饲料添加剂及添加剂预混合饲料产品批准文号申请书

编号
饲料添加剂及添加剂预混合饲料
产品批准文号申请书
企业名称:(公章)注册地址:
生产地址:
申号名称:
产品名称:
法人代表:(签章)邮政编码:
电话:
联系人:
申报时间:
XX省农业厅制
填写说明
一、封面编号由农业厅行政审批管理处统一编写。
二、必须打印或铅印,不能随意涂改。
三、产品名称应科学、规范,并与生产许可证申请书中所用
名称一致,并按“一品一号”原则填报。
四、“申号名称”指饲料添加剂或添加剂预混合饲料。
五、本表一式三份。
六、申请书附件:
1.生产许可证复印件;
2产品配方、产品质量标准和检测方法;
3.产品产品标签样式和使用说明;
4.涵盖产品主成分指标的产品自检报告;
5.申请饲料添加剂产品批准文号的,还应当提供省级饲料管理部门指定的饲料检验机构出具的产品指标检测方法验证结论,但产品有国家和行业标准的除外。
6.提供有相应项目检验资质的检测机构出具的产品复核检验报告,产品复核检测应当涵盖产品质量标准规定的产品主成分指标和卫生指标。
七、本申请书自2018年6月1日启用。
饲料经营许可证申请表

《饲料经营许可证》申请表申请单位:申请日期:年月日受理日期:年月日开县畜牧兽医局制编号注:本表一式两份,县畜牧兽医局一份,自己留一份下面是余秋雨经典励志语录,欢迎阅读。
不需要的朋友可以编辑删除!!关于年龄1.一个横贯终生的品德基本上都是在青年时代形成的,可惜在那个至关重要的时代,青年人受到的正面的鼓动永远是为成功而搏斗,而一般所谓的成功总是带有排他性、自私性的印记。
结果,脸颊上还没有皱纹的他们,却在品德上挖下了一个个看不见的黑洞。
2.我不赞成太多地歌颂青年,而坚持认为那是一个充满陷阱的年代。
陷阱一生都会遇到,但青年时代的陷阱最多、最大、最险。
3.历史上也有一些深刻的哲人,以歌颂青年来弘扬社会的生命力。
但这里显然横亘着一种二律背反:越是坚固的对象越需要鼓动青年去对付,但他们恰恰因为年轻,无法与真正的坚持相斡旋。
4.青年时代的正常状态是什么,我想一切还是从真诚的谦虚开始。
青年人应该懂得,在我们出生之前,这个世界已经精精彩彩、复复杂杂地存在过无数年,我们什么也不懂,能够站正脚下的一角建设一点什么,已是万幸。
5.中年是对青年的延伸,又是对青年的告别。
这种告别不仅仅是一系列观念的变异,而是一个终于自立的成熟者对于能够随心所欲处置各种问题的自信。
6.中年人的当家体验是最后一次精神断奶。
你突然感觉到终于摆脱了父母、兄长、老师的某种依赖,而这种依赖在青年时代总是依稀犹在的;对于领导和组织,似乎更贴近了,却又显示出自己的独立存在,你成了社会结构网络中不可缺少的一个点;因此你在热闹中品尝了有生以来真正的孤立无援,空前的脆弱和空前的强大集于一身。
7.中年人一旦有了当家体验,就会明白教科书式的人生教条十分可笑。
当家管着这么一个大摊子,每个角落每时每刻都在涌现着新问题,除了敏锐而又细致地体察实际情况,实事求是地解开每一个症结,简直没有高谈阔论、把玩概念的余地。
这时人生变得很空灵,除了隐隐然几条人生大原则,再也记不得更多的条令。
饲料添加剂生产许可申请书

附件2 饲料添加剂生产许可申请书企业承诺书一、申报材料真实性承诺(一)本企业对《饲料和饲料添加剂管理条例》、《饲料和饲料添加剂生产许可管理办法》及其相关要求已经充分理解。
(二)本企业提供的纸质和电子申报材料均真实、完整、一致。
申报材料中如有虚假不实信息,自愿承担一切后果及法律责任。
二、遵纪守法承诺本企业严格遵守《饲料和饲料添加剂管理条例》及其配套规章和规范性文件的规定,严格遵守国家关于计量、环保、安全生产、劳动保护、消防安全、危险化学品生产使用、实验室管理等相关管理规定。
如有违纪违法行为,自愿承担一切后果及法律责任。
法定代表人(负责人)签名(企业公章)年月日生产许可证编号:饲料添加剂生产许可申请书企业名称:(公章)联系人:联系方式:申请事项:设立□续展□增加或更换生产线□增加产品品种□迁址□申报日期:年月日中华人民共和国农业部制二〇一二年表1 企业基本情况表2 产品基本情况表3 生产设备明细表(生产线)表4 检验仪器明细表表5 主要管理技术人员及特有工种人员登记表注1:“证书”指与企业签订了全日制用工劳动合同的管理人员、技术人员的职称证书、最高学历证书以及特有工种人员的职业资格证书。
特有工种人员已经参加鉴定且成绩合格,但尚未取得农业部职业技能鉴定机构颁发的职业资格证书的,在“获证书时间、种类及编号”一栏填写考试成绩。
注2:企业的检验化验员还应当在“获证书时间、种类及编号”一栏中填写身份证号码。
附件3 混合型饲料添加剂生产许可申请书企业承诺书一、申报材料真实性承诺(一)本企业对《饲料和饲料添加剂管理条例》、《饲料和饲料添加剂生产许可管理办法》和《混合型饲料添加剂生产企业许可条件》及其相关要求已经充分理解。
(二)本企业提供的纸质和电子申报材料均真实、完整、一致。
申报材料中如有虚假不实信息,自愿承担一切后果及法律责任。
二、遵纪守法承诺本企业严格遵守《饲料和饲料添加剂管理条例》及其配套规章和规范性文件的规定,严格遵守国家关于计量、环保、安全生产、劳动保护、消防安全、危险化学品使用、实验室管理等相关管理规定。
饲料添加剂生产许可申报材料一览表
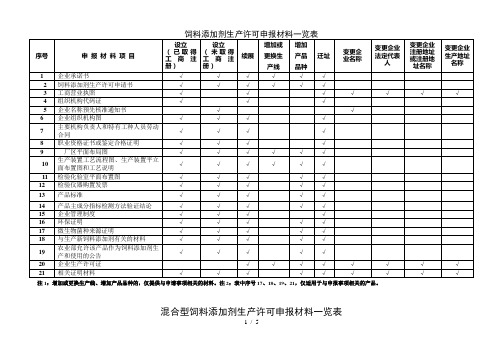
注1:增加或更换生产线、增加产品品种的,仅提供与申请事项相关的材料。
注2:表中序号17、18、19、21,仅适用于与申报事项相关的产品。
混合型饲料添加剂生产许可申报材料一览表
1 / 5
添加剂预混合饲料生产许可申报材料一览表
2 / 5
注2:表中序号12,仅适用于配料、混合工段采用计算机自动化控制系统的企业。
注3:表中序号13,不适用于液态添加剂预混合饲料生产企业。
浓缩饲料、配合饲料、精料补充料生产许可申报材料一览表
3 / 5
单一饲料生产许可申报材料一览表
4 / 5
注2:表中序号16、17、18、19,仅适用于与申请事项相关的产品。
5 / 5。
进口饲料、饲料添加剂申报指南

进口饲料、饲料添加剂申报指南Application Guidelines for Registration of Imported Feeds and FeedAdditivesNational Feed Industry OfficeApril, 2004Address: National Feed Industry Office, Ministry of AgricultureNo.11, Nongzhanguan Nanli, Beijing, P.R.of China. 100026Tel: 8610-64192831Fax: 8610-64192869IntroductionFeeds and Feed Additives Administration Regulation and Administrative Measures for the Registration of Imported Feeds and Feed Additives specify that foreign enterprises shall apply to the Ministry of Agriculture of the People's Republic of China (MOA) to obtain product registration license for their feed and feed additive products if they are to be place on the market for the first time within the territory of the People's Republic of China. For those feeds and feed additives with no registration license, they are not allowed to be sold or used in the territory of China. In the practice of application, we found that some producers and distributors are unfamiliar with China’s feed regulations, hence resulting in materials submitted in the applications being inadequate or presentations unclear. This Application Guidelines was then compiled to hopefully promote smooth registration procedures for imported feeds and feed additives.CONTENTS1 Scope and Coverage for Registration2 Common Terminology for Feed Industry3 Procedures of Application for licenses of imported feeds and feed additives3.1 Asking for Application Form and Category of Required Materials3.2 Application3.3 Appraisal of the Application Materials3.4 Product Inspection3.5 License Issuing3.6 Inquiries of registration License4 Application Form and Required Materials4.1 Format Requirement for Application Materials4.2 Filling in Application Form4.3 Contents of Required Materials4.4 Additional Information for Pet Food and Aquaculture Feed4.5 Related Regulations and Interpretation for License Application5 Sample Requirements6 Application for Re-registration6.1 Asking for Re-registration Form6.2 Required Materials for Re-registration6.3 Related Regulations for Re-registrationAppendix:1. Application Form for Registration of Imported Feeds and Feed Additives2. A Sample of the Application Form3. Flowchart of Appraisal of imported Feeds and Feed Additives4. A Sample of Chinese Label5. Product Classification6. Application Materials for Appraisal of Imported Products7. Re-registration Form for Imported Feeds and Feed AdditivesApplication Guidelines forRegistration of Imported Feeds and Feed Additives1 Scope and Coverage for RegistrationFeeds and feed additives to be imported to the P.R. of China.2 Common Terminology for Feed Industry (See GB10647- 89)2.1 Feedstuff (Single Feed)Feedstuff originated from only one kind of animal, plant, microbial or mineral source.2.1.1 Energy FeedFeedstuff with less than 18% crude fiber and less than 20% crude protein on dry matter basis.2.1.2 Protein FeedFeedstuff with less than 18% crude fiber and 20% or more crude protein on dry matter basis. 2.2 Complete FeedCompound feed which can meet the nutrition requirements from raised animals (except of water).2.3 Concentrate FeedMixture of protein ingredients, trace minerals, vitamins and non-nutritional feed additives according to a certain formula.2.4 Concentrate SupplementCompound feed mixed at a certain formula with many kinds of feed ingredients to supplement nutrition for herbivorous animals fed on roughage (dry, fresh or silage).2.5 Additive PremixPremix of more than two kinds of feed additives with carriers or diluents at a certain formula. 2.6 Compound PremixPremix of two or more kinds of feed additives in the category of trace minerals, vitamins, amino acids and non-nutritional feed additives with carriers or diluents at a certain formula.2.7 Feed AdditiveMinor or trace ingredients added during feed processing, production or utilization. They were categorized into nutritive and non-nutritive feed additives.2.7.1 Nutritive AdditiveTrace or minor substance to supplement nutrition in feed2.7.2 Non-nutritive AdditiveMinor or trace substance added in the feed to ensure or improve feed quality, promote animal production, ensure animal health and improve feed utilization efficiency.a. Anti-oxidant:Additive added to prevent or delay oxidation or damage of some active ingredients in the feed.b. Preservative:Additive added to prevent or inhibit fermentation or decaying of feed.c. Mould inhibitor:Additives added to prevent mold growth in the feed.d. Flavor enhancement:Additives added in the feed to improve feed palatability and animal appetite.e. Color pigment:Additives added to improve color of animal products or feed.f. Binder:Additive added in the feed to improve molding capacity of mash feed or anti-shape damaging capacity of pellet feed.2.7.3 See Appendix 5 for examples of different products.3 Procedures of Application for Licenses of Imported Feeds and Feed Additives (See Appendix 3 for Flow Chart)3.1Asking for Application Form and Category of Required Materials3.1.1 These documents can be acquired from National Feed Industry Office (NFIO).3.1.2 Downloaded from (Application Documents at left bottom section on home page).3.2 ApplicationAn Applicant shall submit original copy of application materials to NFIO. Application materials include Application Form for Registration of Imported Feeds and Feed Additives(see Appendix 1) and Materials Required for Submission.3.3 Appraisal of the Application MaterialsNFIO shall complete the appraisal of the application materials within 15 working days and inform the applicant of the appraisal result.a. If application materials do not meet requirements, NFIO shall inform the applicant via fax to make supplements or corrections. Application shall not be accepted unless required materials are supplemented or corrected in line with related regulations.Documents that fail to meet requirements will not be accepted.b. If application materials meet requirements, NFIO shall inform the applicant in a Note of Acceptance to send a copy of application documents, samples, Note of Acceptance and test fees (charged by test items) to MOA Feed Quality Inspection Center (FQIC) specified in the Note for product quality inspection.3.4 Product InspectionFQIC shall submit test results to NFIO.a. If a product is qualified, FQIC shall prepare and submit a test report to NFIO.b. If the product is not qualified, FQIC shall inform the applicant to submit samples for a second test. And if the second test still fail, this product shall not be registered in the P. R. of China.FQIC shall not release test report to the applicant and the applicant shall not request the report or its copies.3.5 License IssuingQualified Feeds and Feed additives shall be submitted to MOA for Appraisal. Once approved by MOA, a license will be issued.3.5.1 NFIO will inform the applicant about the date for releasing the license or failure of any product to be registered.3.5.2 The applicant shall pay registration fee of RMB 8300 yuan (remittance, cash or check is acceptable except for USD cash) to Department of Finance of MOA upon obtaining the license. 3.6 Inquiries of registration LicenseThe approval of registration will be announced on MOA Bulletin and also on the web site: . An applicant can inquire about commodity name, manufacturer and registration number of any approved product.4 Application Form and Required Materials Required4.1 Format requirement for application materialsa. One set of original application materials and a copy of it shall be submitted for each product.b. The application materials shall be in both Chinese and English, with Chinese version coming first.c. The English version shall be printed on formal stationery paper heading with manufacturer’s name and with signature or seals.d. The Chinese translation of the application materials shall be the same with the English version, and unrelated information about the products shall not be submitted.e. If materials submitted by the manufacturer are not in English, they shall be translated intoEnglish and Chinese.f. Application materials in Chinese shall be printed with A4 paper, in #4 or small#4 fonts.There’s no specific limitations for the English version.g. Application Form shall be on the first page of the whole set of application materials, whichshall be bounded in durable document folders.4.2 Filling in Application Form (Please see Appendix 2 for the Sample)4.2.1 Commodity nameImported feed and feed additives marketed in the P. R. of China shall have a Chinese name, which shall be defined according to GB/T 10647 Standard. A product shall be named after its real nature, instead of a simple translation of the English pronunciation.4.2.2 Common nameIt means a name that can reflect the nature of a feed and feed additive.4.2.3 Product classification (See Appendix 5)Refer to Common Terminology In Feed Industry (See Section 2).4.2.4 AppearanceIt means color, shape (mash, pellet, crystal and block) and physical properties (e.g. odor, solidity or liquidity)4.2.5 Major and minor ingredients and their contentsSpecification for key nutrition should be filled in the blank under “key ingredient and content”.Other items such as specification of hygiene and heavy metal shall be filled in under “other ingredient and content”.4.2.6 Manufacturer’s name and addressDetailed name and address of the manufacturer shall be filled in as exactly the same as indicated in the free sale certificate, except thata. An applicant is a group company:Name of the producer shall be given, though the group company, to which the producer is affiliated, shall be registered as the applicant. If the group company produces a same product in different countries, this product shall be registered by each of the producers in these countries before being exported to China. If the group company has several plants in different regions in one country, then this product shall also be registered by each of these plants, i.e., one license for one plant or one product. If there’s no specific name for a plant, it can be specified as “xxx plant” under “xxx group company”.b. An applicant places an order with another manufacturer for processing. The actual manufacturer should be filled in “manufacturer column” (co-manufacturer), and the consigner shall be filled in “applicant column”. Co-manufacturing contract or verification letter to the consigner by the co-manufacturer (printed on letterhead paper of the co-manufacturer, and signed by responsible person or properly stamped) is required to verify that the product is co-manufactured with the requirement from the consigner. Co-manufacturer is responsible for the production and the consigner for the sales of the product.c. An applicant is registering for fish meal. When the manufacturer applies, themanufacturer’s name and address shall be given under the columns of “manufacturer” and “applicant”. In this circumstance, distributor has nothing to do with the application.When the first distributor applies, the name and address of the first distributor shall be filled in the column of “applicant”. Under such circumstances, names and addresses of all theproducers shall be given in the one of “manufacturer”. Extra pages can be used if the space is not enough. (Distributors shall submit a list of producers with whom it has relations in fish meal trade, including producers’ name s and addresses). An original copy of application materials is needed only from one of the producers (see 4.3.1-4.3.9).4.2.7 Name and address of applicanta. For most products, manufacturers are the applicants.b. When a group company is an applicant, the plant manufacturing the product shall submit a letter (original and in English) to explain its relationship with the group company.c. When the first distributor is the applicant (only for fish meal), the plant shall submit a letter to explain that the distributor has been authorized to market the fish meal in the P. R. of China.4.2.8 AgencyName, telephone and fax numbers, as well address of the agency shall be provided.4.2.9 Signature and dateDocuments shall be signed by a person authorized by the applicant.4.3 Contents of required materials4.3.1 Certificate of Free Sale (CFS) approved by country or region of origin and materials for registration in other countriesCFS is a certificate that proves production and sales are permitted in a country of origin. CFS is issued by an official department that is in charge of manufacturing of feed or the product itself to certify that the producer is legal in manufacturing the product. CFS also certifies that the product can be safely used as feed or feed additive, and free to sell and export without limitations in and from the country of origin. Name and detailed address of the producer shall be provided in the certificate.If a country of origin is not English speaking, CFS, health certificate or official stamp, which is not in English, shall be notarized by the Chinese embassies in the country. Other application materials are not required for notarization.Chambers of commerce, associations or official notarization departments have no authorities to issue CFS for any products other than fish meal from Peru and Chile.It is operational to provide a copy of an import license once obtained in other countries.4.3.2 Origin, composition and manufacturing method4.3.2.1 OriginInformation about the origin shall be provided, either of animal, plant or basic chemicals for the synthetic process.4.3.2.2 Ingredient compositionComposition or effective ingredients shall be explained, including chemical structure, if there’s any, and detailed name of raw material. For plants and microbes, their names shall be detailed down to genus and strains.4.3.2.3 Manufacturing methodIt includes processing flowchart and literal explanation.Processing flowchart should be logical in describing technical conditions and methods for the key steps. Explanation shall focus on the control indicators for major steps and the whole process of quality control.For microbial products, name, origin and culture media ingredients of the strain shall be additionally provided.4.3.3 Quality Standard, Testing Method, Quality Testing Report and Health Certificate4.3.3.1 Quality StandardA producer shall apply with national standard, industrial standard or standard within the company.The quality standard covers appearance, sense perception, physi-chemical and hygiene indexes.Appearance and sense perception: color, smell and physical appearance.Physi-chemical index: Specification for key nutrition and processing quality of the product shall be provided with ceiling and bottom levels.Hygiene index: limitation for safe usage of toxins, harmful substances or pathogenic microbes of natural, secondary and exogenous sources. These include heavy metals (Pb, As, Hg, Cd), fluorine, nitrite (for fish meal only, calculated as NaNO2) and pathogenic microbes (salmonella, total bacteria count, molds and aflatoxin B1)4.3.3.2 Testing MethodsTesting methods shall be provided for key nutrition and hygienic conditions. Standard code is needed if the testing methods follow the international practice. For example, with national, AOAC and ISO standards, only standard code is needed for such nutrition as crude protein, crude fat, moisture, crude fiber and crude ash. Otherwise, some more information shall be provided like detailed steps of testing, reagents and concentrations, instruments and calculation methods.4.3.3.3 Quality testing reporta.For animal origin protein feed (such as fish meal, meat and bone meal, whey, etc.), a genuine and legal testing report shall be issued by department concerned in the country of origin.b. For other feed and feed additives, a testing reports issued by the producer is acceptable. It is required that the sample used for testing shall be in the same batch with the one to be registered. Moreover, all quality indicators shall be included in the testing report.4.3.3.4 Health certificatea. For fish meal, it shall be certified that no animal origin protein or fat other than fish meal is contained in the product.b. For meat and bone meal (MBM), it is to certify that what origin it is from (such as MBM originated from cattle, pig, or chicken) and that all raw materials are from healthy animals from non-epidemic areas.c. For whey, it is to certify that the fresh milk is supplied by healthy animals and no animal origin protein or fat other than dairy products is contained in the product.d. For fat type product, it is to certify that no dioxin exists in the product.e. Health certificate is a must for a product originated from epidemic areas and suspected of animal origin.f. In other cases, no health certificate is required.4.3.4 Label and trademark4.3.4.1 A label in original and Chinese is required.a. Original label includes the picture and literal explanation on an imported package. A Photo of the label is also acceptable.b. For feed and feed additives to be marketed within the territory of China, a label in Chinese shall be required according to Feed Label Standard (GB 10648-1999). (See appendix 4)4.3.4.2 TrademarkA trademark is necessary, only if the product has been registered in China.4.3.5 Application scope, method and dosageIt means coverage of animals, dosage and notices for using different kinds of feed products. 4.3.6 Package, storage conditions and shelf lifePackaging materials and net weight for each package shall be specified.Storage requirements cover location, conditions and ways of storage.Shelf life means the valid period since the date of production.4.3.7 Providing reports on safety evaluation and stability testing when necessaryThey are only necessary for the feed additives marked with “*” in No. 318 MOA Announcement and those products not listed in this Announcement.4.3.8 Report on feed efficiency and status of extensiona. Report on feed efficiency (i.e. trial feeding reports) and status of extension in other countries or regions are required for imported feed additives to be registered.b. It is not necessary for animal origin feeds permitted by China, such as fish meal, meat and bone meal, whey, fish oil, pet food and aquaculture feed.4.3.9 Letter of authorizationAn applicant shall submit a letter from the producer authorizing the applicant to register the product. No such document is required if the producer register by itself.4.4 Additional information about pet food, aquaculture feed and feed for ornamental fishes4.4.1 The application materials shall be separated respectively for cat food and dog food, and also for dry food and canned food. It means one set of application materials shall be prepared for each categories of dry cat food, canned cat food, dry dog food, and canned dog food. It also is the same case with the registration licenses.4.4.2 For ornamental fish (cat and dog) feed, application materials shall be submitted based on different breeds and ages. For instance, the materials shall be listed in the order of seedling carp, parr carp and matured carp; and in the case of golden fish, it shall be in the order of seedling golden fish, parr golden fish and matured golden fish.4.4.3 For the items mentioned in Section4.3.1-4.3.9. , the materials provided shall be different for the compound feeds of different varieties, with different tastes or for animals at different ages. Of course, some of the items are exempt from that, such as the free sale certificate, manufacturing method and testing methods.4.4.4 For the compound feeds with the same ingredients and of the same quality, but with different pellet size, it is required to describe in the manuals the reasonable sizes for animals at different ages.4.4.5 For the compound feeds of the same quality, but with different colors and tastes, it is required to specify the names of pigments and flavors added in the products.4.4.6 An sample for testing shall be of the same type with the one submitted for registration.A certain amount of testing fee will be collected based on different series of products. Additional 200 USD will be charged for one more taste or shape of the same series.4.5Related regulations and interpretations for license application4.5.1 There are 191 kinds of feed and feed additives that have been approved for use by China. Please see No. 318 MOA Announcement for details.4.5.2 There’s no requirements for a producer of any approved animal origin feed to submit the materials mentioned in Section 4.3.7 and 4.3.8. For instance, protein feeds like fish meal, meat and bone meal, compound feeds like aquaculture feed and pet food, and energy feeds like fish oil and animal fat.4.5.3 For feeds and feed additives that have not been approved or registered by their countries or origins of origin, trial feeding and safety evaluation must be conducted when applied for registration in China. The applicants will cover all the expenditures for the trials and evaluation. (Please see Article 8 of Administrative Measures for the Registration of Imported Feeds and Feed Additives)4.5.4 For feeds and feed additives that have been approved by exporting countries, while not approved for use in China, trial feeding and, if necessary, safety evaluation shall be conductedwhen they are imported by China. Both plans and implementer of the trial or evaluation shall be examined and approved by MOA. The applicants will cover all the expenditures for the trials and evaluation. (Please see Article 9 of Administrative Measures for the Registration of Imported Feeds and Feed Additives).4.5.5 For an imported feed or feed additive with complete application materials and qualified testing results, a license will be issued once upon the approval by the MOA. A product subjective to Article 8 and 9 will receive a license from the MOA after the results of trial feeding and safety evaluation are submitted for consideration of the China National Feed Appraisal Committee. (Please see Article 11 of Administrative Measures for the Registration of Imported Feeds and Feed Additives).4.5.6 For a feed or feed additive that has been approved for production and use in its country of origin, but not in China, its applicant is required to submit application materials according to the requirements from the Category of Application Materials for Appraisal of Imported Products(See Appendix 6). The National Feed Appraisal Committee will organize experts concerned to review these materials.5 Sample RequirementsThe registration of each product requires three samples originated from different batches of raw materials. Each sample shall weigh no less than 200 grams in solidity or 200 ml in liquidity. A testing report for the sample shall be enclosed in the application materials.6 Re-registration6.1 Asking for application form for Re-registration (See Appendix 7)6.1.1 The form is available from NFIO.6.1.2 It can also be downloaded from (See Application Materials at the bottom to the left of the home page)6.2 Requirements for the application materialsTwo copies of the previous license;Two copies of application forms for re-registration (printed in both Chinese and English);Two copies of quality standard specification and manuals;One original copy of authorization letter;and re-registration fee of RMB 4150 yuan for each product.6.2.1 Letter of authorizationA letter shall be submitted by a producer to authorize its distributor, partner or representative to re-register its product on behalf of the producer.No letter of authorization is required in case that a producer or its branch or representative office in China conducts the procedures of re-registration.6.3 Regulations for re-registration6.3.1 The license of registration for imported feeds and feed additives is valid for five years. If a product continues to be marketed within the territory of China after its license expires, re-registration shall be applied within 6 months before the expiry date of the previous license. (See Article 14 of Administrative Measures for the Registration of Imported Feeds and Feed Additives)6.3.2 For a feed or feed additive that are not re-registered on time or not qualified in the sample test for once, its samples shall be submitted for reexamination. A producer whose license has been suspended, however, has no chance for re-registration. (See Article 16 of Administrative Measures for the Registration of Imported Feeds and Feed Additives).6.3.3 Re-registration is not available for any feed or feed additive that has been suspended for production or use in its country or region of origin, or that has failed to pass through the sample test for twice successively in China. (See Article 17 of Administrative Measures for the Registration of Imported Feeds and Feed Additives).6.3.4 A feed or feed additive that has been changed in its producer’s address, quality standard, formula or application scope, shall be re-registered in time. (See Article 18 of Administrative Measures for the Registration of Imported Feeds and Feed Additives).*Applicant: Companies or individuals that apply for registration in MOA. They can be branch company, representative office, distributor, partner or authorized individuals of the producer or applying companies.Appendix 1进口饲料和饲料添加剂申请表Application Form for Registration of Imported Feeds and Feed AdditivesAppendix 2: A Sample of Application Form进口饲料和饲料添加剂申请表Application Form for Registration of Imported Feeds and Feed AdditivesAppendix 3进口饲料和饲料添加剂审批流程图Flowchart of Appraisal of Imported Feeds and Feed AdditivesAppendix 4 A sample of Chinese Labels进口饲料级乳清粉Imported Feed-Grade Whey许可证号:外饲准字200( )号 License Number: Foreign Registration 200 ( )1、本产品符合饲料卫生标准This product is in compliance with feed hygienic standard2、产品成分分析保证值 Product guaranteed analysis specification3Ingredients composition:This product is spray dried with pure whey4、产品标准编号:本产品符合加拿大XXXX 标准。
新饲料、新饲料添加剂申报指南

新饲料、新饲料添加剂申报指南全国饲料工作办公室鼓励研究、创制新饲料、新饲料添加剂是我国饲料工业坚持发展的基本产业政策之一。
为便于申报单位(生产者或研制者)更好地了解新饲料、新饲料添加剂的申报程序,提高申报材料的科学性和完整性,特制定本指南。
1范围本指南阐述了新饲料、新饲料添加剂的申报程序、申报材料要求及相关说明。
本指南适用于新饲料、新饲料添加剂申报。
2 名词解释2.1 新饲料、新饲料添加剂:是指我国研制、尚未批准使用的单-饲料和饲料添加剂。
2.2 创新型新饲料、新饲料添加剂:是指在我国境内研究、创制的,国外尚无报导的单一饲料及饲料添加剂。
2.3 国外批准生产、销售的产品:是指在其它国家己批准生产、销售,国内研制、尚未批准使用的单一饲料及饲料添加剂(受专利保护的产品除外)。
2.4 移植型新饲料、新饲料添加剂:是指已在我国境内其它行业使用,首次应用于饲料的单一饲料及饲料添加剂。
3 申报程序3.1 申报新产品的单位(生产者或研制者),到全国饲料工作办公室(以下简称全国饲料办)或全国饲料评审委员会办公室(以下简称评审办)索取申请表和申报材料项目表,也可从网上下载()。
3.2 填写申请表,并按要求准备申报材料。
申报材料一律使用A4规格纸,小4号字打印,装订成册。
申请表格式见附件1,申报材料项目表见附件2。
3.3 申报单位(生产者或研制者)到所在省(市)级饲料管理部门备案,由省(市)级饲料管理部门在申请表上盖章。
3.4 申报单位(生产者或研制者)将申请表和全套申报材料一式三份报送全国饲料办,由全国饲料办盖章接收,并将全套申报材料转交评审办进行形式审查。
3.5 形式审查后,申报材料完整的,评审办做出受理决定并通知申请单位(生产者或研制者)。
申报材料不完整的,通知申请单位(生产者或研制者)限期补齐,未按规定补齐材料的,不予受理。
申请单位(生产者或研制者)接到受理通知后,按规定缴纳新产品登记费8300元。
3.6 被受理产品,评审办组织专家预审并将预审意见反馈申请单位(生产者或研制者)。
饲料和饲料添加剂产品可办理“自由销售证明”

提供了有力支撑。目前,生猪存栏和
仔猪供给量已连续5个月恢复性增 长,预示7月份以后可出栏的商品
生猪养殖用地、抵押贷款试点和环 评等政策措施,尽快将补助资金兑 现到场到户。三是进一步加强指导
监测的情况看,6月份生猪生产恢复
该负责人表示,6月份以来猪肉 肥猪将会逐步增加,猪肉市场供应 服务。指导生猪规模养殖场改善基
有关负责人。
猪存栏连续5个月增长。三是补栏 效控制后的消费增长,上涨压力可 头。一是进一步抓好责任落实。近期
该负责人表示,今年以来,生猪 增养势头好。新冠肺炎疫情稳定控 能会更大。另外,南方部分地区强降 农业农村部将联合发展改革委等部
生产持续加快恢复,新建猪场多,补 制后,种猪和仔猪调运量逐月增加。 雨对生猪生产及产品调运产生了一 门开展生猪稳产保供工作和相关政
贡一任網强:一羌更一 % :.03)1785888123 E-mail:95746341 l@
来首次实现同比增长,是一个重要
涨,供需两方面都在增加,但消费增 加更快。从供应方面看,3月份以来, 生猪出栏量连续4个月环比增长,
其中6月份出栏量比5月增长 6.5%,比2月增长36.7%。今年前5 个月进口猪肉172.4万吨,同比增长 146.2%。从消费方面看,随着新冠肺
炎疫情得到有效控制,餐饮业恢复 经营,工厂复工,学校复课,猪肉消
计比去年增加100万吨以上,禽肉 产量增加120万吨以上。综合分析, 三季度之后猪肉供应紧张的局面将 逐步缓解,下半年猪肉市场供应总 体是有保障的。
该负责人表示,猪肉是重要的 民生产品,农业农村部按照党中央、 国务院决策部署,把恢复生猪生产 作为今年农业农村工作的重大任
快形成产能。通过龙头企业带动、财 政资金扶持、实用技术培训等多途 径支持中小养殖场户加快补栏增 养。四是毫不松懈抓好非洲猪瘟防. 控。继续严防严控.,落实落细常态化 防控措施,继续开展重点区域和场 点全覆盖入场采样检测,强化出栏 检疫、•屠宰监管和病死猪无害化处 理等措施,阻断疫情传播渠道。
饲料生产许可申请表

温馨提示本文档包含:1、企业承诺书2、单一饲料生产许可申请书3、浓缩饲料、配合饲料、精料补充料生产许可申请书请根据实际申请的行政许可类别选择性使用。
企业承诺书一、申报材料真实性承诺(一)本企业对《饲料和饲料添加剂管理条例》及其配套规章和规范性文件的要求已经充分理解。
(二)本企业提供的纸质和电子申报材料均真实、完整、一致。
申报材料中如有虚假不实信息,自愿承担一切后果及法律责任。
二、遵纪守法承诺本企业严格遵守《饲料和饲料添加剂管理条例》及其配套规章和规范性文件的规定,严格遵守国家关于计量、环保、安全生产、劳动保护、消防安全、危险化学品使用、实验室管理等相关管理规定。
如有违纪违法行为,自愿承担一切后果及法律责任。
法定代表人(负责人)签名(企业公章)年月日生产许可证编号:单一饲料生产许可申请书企业名称:(公章)联系人:联系方式:申请事项:设立□续展□增加或更换生产线□增加产品品种□迁址□申报日期:年月日中华人民共和国农业部制二〇一二年表1 企业基本情况表2 产品基本情况表3 生产设备明细表(生产线)表4 检验仪器明细表表5 主要管理技术人员及特有工种人员登记表注1:“证书”指与企业签订了全日制用工劳动合同的管理人员、技术人员的职称证书、最高学历证书以及特有工种人员的职业资格证书。
特有工种人员已经参加鉴定且成绩合格,但尚未取得农业部职业技能鉴定机构颁发的职业资格证书的,在“获证书时间、种类及编号”一栏填写考试成绩。
注2:企业的检验化验员还应当在“获证书时间、种类及编号”一栏中填写身份证号码。
生产许可证编号:浓缩饲料、配合饲料、精料补充料生产许可申请书产品类别:浓缩饲料□配合饲料□精料补充料□企业名称:(公章)联系人:联系方式:申请事项:设立□续展□增加或更换生产线□增加产品类别□增加产品系列□迁址□申报日期:年月日中华人民共和国农业部制二〇一二年表1 企业基本情况表2 产品基本情况表3 生产设备明细表表4 检验仪器明细表表5 主要管理技术人员及特有工种人员登记表特有工种人员已经参加鉴定且成绩合格,但尚未取得农业部职业技能鉴定机构颁发的职业资格证书的,在“获证书时间、种类及编号”一栏填写考试成绩。
饲料添加剂生产许可申请书(样板)

生产许可证编号:
饲料添加剂生产许可申请书
企业名称:XX市XXXXXXXXXXX有限公司(公章)
联系人:XXX
联系方式:0XX-XXXXXXX,1XXXXXXXXXX
申请事项:设立☑续展□增加或更换生产线□
增加产品品种□迁址□
申报日期:2020 年X 月XX 日
中华人民共和国农业部制
二〇一二年
表1 企业基本情况
表2 产品基本情况
表3 生产设备明细表(生产线)
表4 检验仪器明细表
表5 主要管理技术人员及特有工种人员登记表
注1:“证书”指与企业签订了全日制用工劳动合同的管理人员、技术人员的职称证书、最高学历证书。
注2:企业的检验化验员还应当在“获证书时间、种类及编号”一栏中填写身份证号码。
(注:农业部令2017年第8号删去提供“职业资格证书或鉴定合格证明”的要求。
)。
饲料及饲料添加剂申请书-推荐下载

饲料 商品名
通用名
主要 成分
使用范围
(动物名称及 生育阶段)
产 作用
品 说 用量 明
保质期
贮存条件
产品情况
(饲料及饲料添加剂)
英文
4
对全部高中资料试卷电气设备,在安装过程中以及安装结束后进行高中资料试卷调整试验;通电检查所有设备高中资料电试力卷保相护互装作置用调与试相技互术通关,1系电过,力管根保线据护敷生高设产中技工资术艺料0不高试仅中卷可资配以料置解试技决卷术吊要是顶求指层,机配对组置电在不气进规设行范备继高进电中行保资空护料载高试与中卷带资问负料题荷试2下卷2,高总而中体且资配可料置保试时障卷,各调需类控要管试在路验最习;大题对限到设度位备内。进来在行确管调保路整机敷使组设其高过在中程正资1常料中工试,况卷要下安加与全强过,看度并22工且22作尽22下可22都能22可地护以缩1关正小于常故管工障路作高高;中中对资资于料料继试试电卷卷保破连护坏接进范管行围口整,处核或理对者高定对中值某资,些料审异试核常卷与高弯校中扁对资度图料固纸试定,卷盒编工位写况置复进.杂行保设自护备动层与处防装理腐置,跨高尤接中其地资要线料避弯试免曲卷错半调误径试高标方中高案资等,料,编试要5写、卷求重电保技要气护术设设装交备备置底4高调、动。中试电作管资高气,线料中课并敷3试资件且、设卷料中拒管技试试调绝路术验卷试动敷中方技作设包案术,技含以来术线及避槽系免、统不管启必架动要等方高多案中项;资方对料式整试,套卷为启突解动然决过停高程机中中。语高因文中此电资,气料电课试力件卷高中电中管气资壁设料薄备试、进卷接行保口调护不试装严工置等作调问并试题且技,进术合行,理过要利关求用运电管行力线高保敷中护设资装技料置术试做。卷到线技准缆术确敷指灵设导活原。。则对对:于于在调差分试动线过保盒程护处中装,高置当中高不资中同料资电试料压卷试回技卷路术调交问试叉题技时,术,作是应为指采调发用试电金人机属员一隔,变板需压进要器行在组隔事在开前发处掌生理握内;图部同纸故一资障线料时槽、,内设需,备要强制进电造行回厂外路家部须出电同具源时高高切中中断资资习料料题试试电卷卷源试切,验除线报从缆告而敷与采设相用完关高毕技中,术资要资料进料试行,卷检并主查且要和了保检解护测现装处场置理设。备高中资料试卷布置情况与有关高中资料试卷电气系统接线等情况,然后根据规范与规程规定,制定设备调试高中资料试卷方案。
饲料添加剂越南注册资料201403

饲料添加剂在越南注册所需资料
1、自由销售证(需驻中国的越南领事馆认证)Certificate of Free sales
证书要有编号,厂家具体地址,签章,签发人的职务,签发日期
2、GMP 或ISO 证书(公证件)或第三方检测报告
GMP / ISO
3、产品成分证明(盖公司鲜章)Composition and guaranted Analysis
显示所有成分,每个成分的含量
4、产品功能说明(盖公司鲜章)Product Efficacy
5、产品分析单(盖公司鲜章)Certificate of Analysis
显示所有成分,每个成分的含量, 颜色,味道,颗粒/ 粉末/ 液体,检测方法
6、产品标签(盖公司鲜章)Label
除了基本的内容,还要加上:功用,用量,主要成分,含量,外观(颜色,颗粒/ 粉末/ 液体,味道),厂家名称,厂家地址,联系电话,MADE IN CHINA 7、产品信息(盖公司鲜章)Product Information
除了基本信息还要显示所有成分,每个成分的含量。
绿色通道饲料销售申请书模板

尊敬的审批部门:您好!我代表XXX饲料公司,向您提交一份关于绿色通道饲料销售申请书的申请。
在此,我们诚挚地希望贵部门能够审批通过我们的申请,以便我们更好地为广大养殖户提供优质、绿色的饲料产品。
首先,请允许我简要介绍一下我们的公司。
XXX饲料公司成立于上世纪90年代,是一家专注于研发、生产、销售绿色饲料的企业。
公司秉承“绿色、环保、高效”的理念,始终致力于为我国养殖业提供优质、健康的饲料产品。
经过多年的努力,我们的产品已经覆盖全国各地,赢得了广大养殖户的信任和支持。
随着我国养殖业的快速发展,绿色、环保的饲料产品越来越受到市场的欢迎。
为满足市场需求,我们公司积极引进先进的生产设备和技术,加强与科研院所的合作,不断优化产品配方,提高饲料的营养价值和环保性能。
同时,我们还在全国范围内建立了完善的销售网络,为养殖户提供专业、贴心的服务。
在此背景下,我们公司希望通过绿色通道饲料销售申请书,申请贵部门对我们公司的绿色饲料产品给予支持和认可。
具体申请内容如下:1. 对我们公司的绿色饲料产品进行审批,核发绿色通道饲料销售证书,以便我们能够在市场上更好地推广和销售绿色饲料。
2. 为我们公司的绿色饲料产品提供优惠政策,如减免税收、补贴等,以降低养殖户的使用成本,提高绿色饲料的市场竞争力。
3. 加大对我们公司的宣传力度,通过各种渠道宣传我们的绿色饲料产品,提高养殖户对我们公司的认知度和信任度。
4. 为我们公司提供技术支持,如在饲料配方、生产工艺等方面的指导和建议,以帮助我们公司不断提高产品质量和环保性能。
我们坚信,通过贵部门的支持和认可,我们的绿色饲料产品将更好地服务于我国养殖业,为养殖户创造更大的经济效益,同时为保护生态环境作出贡献。
在此,我们再次感谢贵部门对我们公司的关注和支持,期待您的审批通过。
敬请审批部门予以审批。
此致敬礼!申请人:XXX职务:XXX单位:XXX饲料公司联系电话:XXX申请日期:XXXX年XX月XX日。
四川省饲料添加剂产品批准文号申请表
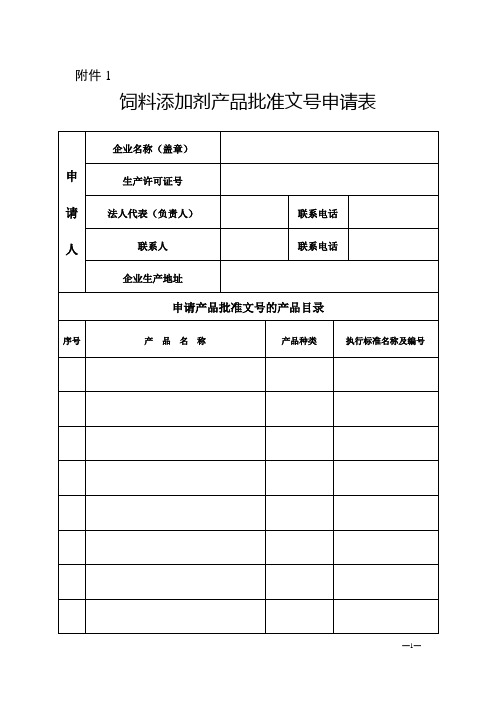
附件1饲料添加剂产品批准文号申请表—1—一、附件材料清单二、材料要求1.附件材料均用A4纸打印(或复印)。
2.产品执行标准须是合法有效的产品标准。
3.产品标签样稿须符合《饲料和饲料添加剂管理条例》第21条和GB10648的规定。
4.自检报告的项目指标应涵盖产品主成分指标,报告格式应符合检验报告的通用格式。
每个产品均需提交自检报告。
5.产品执行企业标准的,需附具主成分指标检测方法验证结论,此结论应由省级饲料管理部门指定的检测机构出具。
—2—附件2添加剂预混合饲料批准文号申请表一、附件材料清单1.生产许可证复印件2.产品配方3.产品执行标准复印件4.产品标签样稿5.自检报告二、材料要求1.附件材料均用A4纸打印(或复印)。
2.产品配方需加盖公司公章。
3.产品执行标准须是合法有效的产品标准。
4.产品标签样稿须符合《饲料和饲料添加剂管理条例》第21条和GB10648的规定。
每个产品均需提交标签样稿。
5.自检报告的项目指标应涵盖产品主成分指标,报告格式应符合检验报告的通用格式。
每个产品均需提交自检报告。
6.产品类别为复合预混合饲料或微量元素预混合饲料或维生素预混合饲料。
7.适用动物按猪、鸡、鸭、鹅、牛、羊、兔、鱼、虾、蟹等类进行确定。
8.添加比例为该预混料产品在动物日粮或配合饲料中的添加量。
—4—附件3饲料、饲料添加剂委托加工备案申请书产品类别:委托企业名称:联系电话:联系人:受托企业名称:联系电话:联系人:申请日期:四川省畜牧食品局印制—5——6——7—附件4贯彻饲料条例诚信规范经营新修订的《饲料和饲料添加剂管理条例》(以下简称条例),于2011年10月26日国务院第177次常务会议通过,国务院令第609号发布,自2012年5月1日起施行。
现将《条例》中对饲料经营者的管理规定和相应处罚告之如下:一、经营饲料须具备3个条件《条例》第二十二条规定:饲料、饲料添加剂经营者应当符合下列条件:1.有与经营饲料、饲料添加剂相适应的经营场所和仓储设施;2.有具备饲料、饲料添加剂使用、贮存等知识的技术人员;3.有必要的产品质量管理和安全管理制度。
南通特洛菲饲料科技有限公司

南通特洛菲饲料科技有限公司饲料订购/定制申请单(填写前请先阅读申请单后面的填写说明)(一)订购单棒状粉状或接近粉状液体颗粒状片状不辐照(喂养期间是普通环境)辐照(喂养期间是SPF环境)辐照(喂养期间是无菌环境)(二)委托收货人(如果收货人不是本人,请详细填写)(三)支付订购款的项目负责人(如果采取货到付款方式支付,并且订购人不是项目负责人,请详细填写)(四)填写说明和注意事项1.所有内容都必需如实填写。
【开发票要求的信息】中三项必须正确,其中,单位地址是指单位的地址,而不是订购人或收货人的地址,如果对三项中的任何一项不确定,请咨询贵单位财务部,只要有一项出错,将面临不能报销的尴尬。
2.满足以下订购方式时,不需要填写身份证号码:●预付款支付后生产;●不需要签订订购合同●不需要南通特洛菲饲料科技有限公司对饲料事项承担任何责任与义务。
其他情况均需提供真实的、与姓名相一致的身份证号码,用于合同准备。
3.有关饲料的事项,在填写前应当与南通特洛菲饲料科技有限公司技术部(QQ:1036 76 1036)充分交流后填写,如果有不清楚或不确定之处,应当咨询清楚。
4.填写前应当与经费提供人(项目负责人)充分沟通,在得到同意订购并且确认所定饲料符合研究设计要求后填写。
5.避免操作失误须知:如果生产的饲料与你的要求不一致,这将导致双方都不知道的后果。
为了杜绝这种情况发生,南通特洛菲饲料科技有限公司采取了严格措施,即按照申请单进行“一单一生产”的操作模式,完全按照订购单进行,这就要求你的所有要求或说明都应当在申请单中注明,除了订购单中的信息,其他任何方式(如QQ,电子邮件,手机短消息,微信等等)所提供的信息将有可能被忽视或得不到采纳。
因此,填写订购单后请做以下两方面的工作:(1) 认真逐一核查。
(2) 认真回顾科研设计对饲料的要求确定是否有其他需要补充说明的事项。
确保所有要求都体现在订购单中。
订购人在订购单中填写差错导致不良后果将承担全部责任。
江西省核发饲料添加剂批准文号申请表

江西省
饲料添加剂、添加剂预
混合饲料产品批准文号申请审批表
产品名称:
申报单位:(章)
生产地址:
联系电话:
联系人:
填表日期:年月日
江西省农业厅制
填表注意事项
一、本表由江西省农业厅统一印制。
二、本表由申报单位用钢笔填写或打印,字迹工整,内容准确,不能随意涂改。
本表一式三份,审批单位、质量复核检验单位及申报单位各留存一份。
三、本表只限于申报品种的一个含量规格,不同含量规格要另行填表。
四、申请新饲料添加剂、新添加剂预混合饲料产品批准文号,应提供农业部核发的新饲料添加剂、新添加剂预混合饲料证书。
五、填表的产品名称应科学、规范。
配方必须如实填写、不得省略。
六、本表各栏如不够填写时可加附页。
