VDA 277 汽车内非金属材料的有机物散发测定
汽车行业汽车领域常见第三方计量检测项目【范本模板】

汽车行业汽车领域常见第三方计量检测项目广电计量杜亚俊汽车行业综述 (1)材料测试 (1)金属 (2)禁用物质测试 (3)ELV测试 (3)石棉测试 (4)气体腐蚀试验 (5)VOC测试 (5)机械冲击试验 (8)非金属 (8)禁用物质测试 (8)机械冲击试验 (9)材料试验 (9)气体腐蚀试验 (9)零部件 (10)汽车外饰件测试 (10)禁用物质测试 (11)气体腐蚀/臭氧腐蚀 (11)盐雾腐蚀 (12)光老化试验 (12)高低温/高低温湿热循环/温度冲击/快速温变 (12)沙尘试验 (13)淋雨试验 (13)振动 (14)综合环境可靠性试验 (14)机械冲击 (15)机械耐久/疲劳/寿命 (15)涂层/镀层特性测试 (15)内饰件 (16)VOC测试 (17)雾化测试 (19)气味测试 (20)禁用物质测试 (20)汽车内饰材料的燃烧特性 (21)温湿度环境试验 (21)光老化试验 (22)振动试验 (22)综合环境可靠性试验 (22)材料试验 (23)电子电器 (23)挥发性有机化合物测试 (24)禁用物质测试 (26)生物环境试验 (26)盐雾试验 (27)气体腐蚀试验 (27)综合环境可靠性试验 (27)沙尘试验 (28)淋雨试验 (28)振动试验 (28)高加速测试 (29)汽车电子部件 (29)新能源高压部件 (30)整车 (31)整车电磁兼容测试系统 (32)270立方米步入式高低温湿热试验室 (32)整车红外加热系统 (33)TG—1617P型控制气动综合测试仪 (33)整车室内道路模拟试验系统 (33)其它整车性能测试 (33)浮渡试验池 (34)制造 (34)仪器设备的计量校准 (35)环境空气与废气检测 (41)计量检测培训咨询 (45)产品认证 (45)可再利用率和可回收利用率计算服务 (46)AMASS车用材料基础数据库合作检测机构 (46)汽车行业综述在汽车计量检测技术服务领域具有10余年行业经验,我们秉承着科学与公正、准确与可靠、快捷与周到的服务理念,以丰富的专业知识和行业经验为客户提供技术解决方案。
车内非金属材料挥发性有机物及醛酮类物质测试方法和分级指标

车内⾮⾦属材料挥发性有机物及醛酮类物质测试⽅法和分级指标《车内⾮⾦属材料挥发性有机物及醛酮类物质测试⽅法及分级指标》编制说明⼀、⼯作简况(⼀)、⽴项的背景和意义车内挥发性有害物质(VOCs)具有散发周期长、毒性⼤、易受环境影响等特点,处理不当会严重威胁驾乘⼈员⾝体健康。
随着⼤众环保意识逐步提⾼,该问题引起了政府及⾏业各界的⼴泛关注。
⽬前,我国已出台《HJ/T 400-2007车内挥发性有机物和醛酮类物质采样测定⽅法》和《GB/T 27630-2011乘⽤车内空⽓质量评价指南》,明确规定了车内VOCs检测⽅法及限值标准。
在⾏业的推动下,整车VOCs限值要求标准正修订为强制性国家标准,修订后的强标预计将于2017年正式发布。
强标的发布将⼤⼤增强其对整车企业的约束⼒,同时也为政府部门进⼀步强化监管提供依据。
零部件VOCs标准⽅⾯,2015年国标委下达了编制《车内⾮⾦属部件挥发性有机物和醛酮类物质检测⽅法》的通知,⽬前该标准已形成初稿,预计于2017年底发布实施。
车内VOCs散发源于材料,管控的根源也在于材料。
然⽽我国还没有统⼀的材料VOCs采样测试⽅法及评价指标,车内⾮⾦属材料VOCs性能良莠不齐,⾏业发展缓慢。
⼀⽅⾯,国内整车企业材料VOCs 检测⽅法不统⼀,分散了企业研发精⼒及资⾦,抬⾼了⾏业成本;另⼀⽅⾯,由于缺乏材料VOCs散发性能评价⽅法,部分材料以次充好,严重扰乱了市场竞争环境。
制定车内⾮⾦属材料VOCs检测⽅法及分级指标,将填补国内汽车⾮⾦属材料VOCs检测标准空⽩,对于完善车⽤材料标准体系,规范⾏业⽆序发展现状具有重要推动作⽤。
(⼆)、国内外研究现状与发展趋势⽬前,材料级别VOCs检测⽅法基本成熟,国际上ISO、VDA、JASO 对车内⾮⾦属材料VOCs检测都有相关规定,依据主要检测仪器不同,可以分为袋⼦法、舱式法、顶空法。
袋⼦法以⽇系及国内⾃主品牌应⽤为主,舱式法以德系品牌应⽤为主,顶空法在材料企业中应⽤较多。
汽车内饰产品中苯系列挥发物含量的测试方法探讨
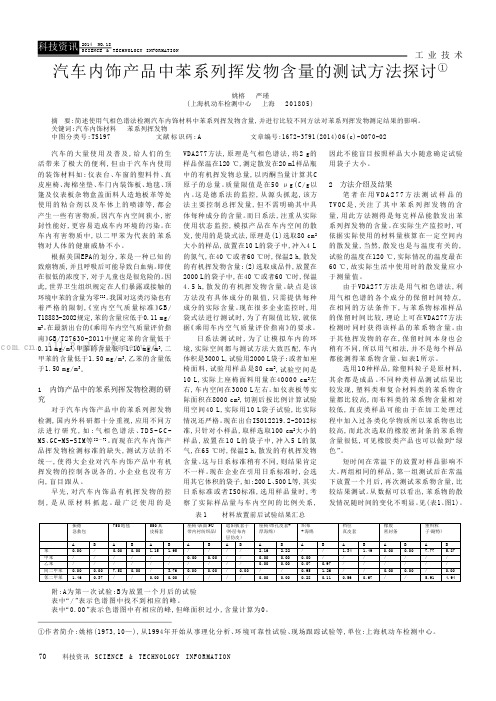
工 业 技 术70科技资讯 SC I EN C E & TE C HN O LO G Y I NF O R MA T IO N汽车的大量使用及普及,给人们的生活带来了极大的便利,但由于汽车内使用的装饰材料如:仪表台、车窗的塑料件、真皮座椅、海棉坐垫、车门内装饰板、地毯、顶篷及仪表板杂物盒盖面料人造地板革等处使用的粘合剂以及车体上的喷漆等,都会产生一些有害物质,因汽车内空间狭小,密封性能好,更容易造成车内环境的污染。
在车内有害物质中,以二甲苯为代表的苯系物对人体的健康威胁不小。
根据美国EPA的划分,苯是一种已知的致癌物质,并且呼吸后可能导致白血病。
即使在很低的浓度下,对于儿童也是很危险的。
因此,世界卫生组织规定在人们暴露或接触的环境中苯的含量为零[1]。
我国对这类污染也有着严格的限制,《室内空气质量标准》G B /T18883-2002规定,苯的含量应低于0.11mg/m 3。
在最新出台的《乘用车内空气质量评价指南》GB/T27630-2011中规定苯的含量低于0.11mg/m 3,甲苯的含量低于1.10mg/m 3,二甲苯的含量低于1.50mg/m 3,乙苯的含量低于1.50mg/m 3。
1 内饰产品中的苯系列挥发物检测的研究对于汽车内饰产品中的苯系列挥发物检测,国内外科研都十分重视,应用不同方法进行研究,如:气相色谱法、T D S -G C -MS、GC-MS-SIM等[2~7]。
而现在汽车内饰产品挥发物检测标准的缺失,测试方法的不统一,使得大企业对汽车内饰产品中有机挥发物的控制各说各的,小企业也没有方向,盲目跟从。
早先,对汽车内饰品有机挥发物的控制,是从原材料抓起。
最广泛使用的是VDA277方法,原理是气相色谱法,将2g的样品保温在120℃,测定散发在20ml样品瓶中的有机挥发物总量,以丙酮当量计算其C 原子的总量。
质量限值是在50μg(C/g以内。
这是德系法的监控,从源头抓起,该方法主要控制总挥发量,但不需明确其中具体每种成分的含量。
汽车内饰件测试方法详解modified

We Assure Your ExcellenceWe Assure Your ExcellenceWe Assure Your ExcellenceTVOC ØTotal Volatile Organic Compound :总挥发性有机化合物ØGB/T 18883-2002 中定义:利用Tenax GC 或Tenax TA 采样,非极性色谱柱(极性指数小于10)进行分析,保留时间在正己烷和正十六烷之间的挥发性有机化合物。
ØVDA277 中是指用HS-GC-FID 测试仪器,按照一定的测试条件,峰面积满足一定要求的化合物的总和,用丙酮含量计算,并折算成碳含量,故也称为总碳Ø福特VOC 总成测试方法中对TVOC 没有明确定义We Assure Your ExcellenceWe Assure Your Excellence汽车内饰件中非金属材料。
样品包装:聚乙烯袋包装后外面用铝箔包覆取样:天然材料(如棉、木头、皮革、羊毛等)剪碎后用CaCl2干燥24h再称样。
测试部件由不同材料组成,应该将不同材料分开测试。
金属上的涂层,胶粘剂等应该从金属上取下后取样2.3 测试流程We Assure Your ExcellenceWe Assure Your Excellence 加热温度:120℃加热时间:5小时进样器温度:200℃程序升温:起始50 ℃,恒温5分钟,以每分钟12度的速率升温到200 ℃,在这个温度恒温3分钟检测器温度:250℃分流比:大约1:20载气:He 载气流速:大约22-27cm/s2.4仪器参数We Assure Your Excellence2.5仪器工作原理-顶空(HS)原理:样品被封装在密闭的蒸汽瓶(顶空瓶)中,在预置温度下恒温加热;当固/液相和气相之间达到平衡状态时,顶空瓶中充满了易挥发物质;一定量的蒸汽被运送到GC 的柱子进行分析参数:温度:加热:120 ℃取样针:150 ℃传输线:180 ℃压力:载气压力:13psi取样压力:21psi时间:保温:5小时(标准品1小时)进样:0.05分钟加压:2分钟We Assure Your Excellence2.6仪器工作原理-气相色谱仪(GC-FID)We Assure Your Excellence •各组分在流动相和固定相中的分配系数不同•组分与固定相作用强弱不同2.6 仪器工作原理-(GC-FID)We Assure Your Excellence理论:在火焰头和收集极(电极)上加一电压。
车内非金属材料挥发性有机物和醛酮物质挥发量限值

JLYY-JT -08车内非金属材料挥发性有机物和醛酮物质挥发量限值(试行)编制:校对:审核:审定:标准:批准:浙江吉利汽车研究院有限公司二〇〇八年四月前 言车内非金属材料挥发性有害物质是造成车内空气污染的最主要原因之一。
为了防治车内空气污染,改善车内环境质量,实现对汽车非金属件环保质量的统一控制,确保汽车能够满足国内外汽车环保法规要求,提高汽车品质,为消费者营造一个安全环保的乘车环境,特制定本标准。
本标准第4章为强制性条款,其余为推荐性条款。
本标准由浙江吉利汽车研究院有限公司提出。
本标准由浙江吉利汽车研究院有限公司试验部负责起草。
本标准起草人:毛招凤。
本标准于2008年4月15日发布并实施。
1 范围本标准规定了车内非金属材料挥发性有机物和醛酮物质的术语和定义、限值要求、试验方法、检验规则、样件准备等内容。
本标准适用于汽车内饰、行李箱非金属材料及与汽车内室导入流动空气接触的零件。
2 规范性引用文件下列文件中的条款通过本标准的引用而成为本标准的条款。
凡是注日期的引用文件,其随后所有的修改单(不包括勘误的内容)或修订版均不适用于本标准,然而,鼓励根据本标准达成协议的各方研究是否可使用这些文件的最新版本。
凡是不注日期的引用文件,其最新版本适用于本标准。
GB/T 2918-1998 塑料试样状态调节和试验的标准环境JLYY-JT -08 车内非金属材料挥发性有机物和醛酮物质采样测定方法3 术语和定义下列术语和定义适用于本标准3.1车内非金属材料车内非金属材料是指由单一材料或复合材料制成的汽车用内饰部件非金属部分,如地毯、顶棚、隔音隔热垫、座椅面料、座椅泡棉、皮革、车内密封条、空调部件、后隔板、行李舱饰毯等,包括撞车时吸收碰撞能量的填料、缓冲装置、塑料类、纤维纺织类、皮革类、橡胶类等材料。
3.2挥发性有机组分挥发性有机组分是指利用TENAX等吸附剂采集,并用极性指数小于10的气相色谱柱分离,保留时间在正己烷到正十六烷之间的具有挥发性的化合物的总称。
VDA 277 汽车内非金属材料的有机物散发测定

Determination of Organic Emission of Non-metallic materials from vehicles Interior VDA 277Contents1. General2. Test preparations3. Testing sets and conditions4. Attempt realization5. Calibration6. Evaluation1. General1.1 PurposesAccording to this test method, the organic emission from non-metallic materials is determined with direct or indirect influence for the passenger's cell of automobiles stand. It measure the emission potential of a material, the sum of all release values of the emitted substances with gaschromatograph and detection with a Flame ionization detector. The test operated by means of steam space analysis (Head Space technology) at temperature of 120ºC.1.2 RequirementsThe accompanying sample, the respective requirements as well as special method is of the suitable specification to take drawing or the like.If materials are composed of different components, a separate check is required for every single material.2. Test preparationsTransport and storage of the tests has to occur in an aluminum coated Polyethylenbeutel. The sampling has immediately after incoming goods or in a condition, which corresponds to this, to take place. The times of the incoming goods and the sampling are to be labelled. There is no conditioning of the test as a rule. Except of it are physical materials (cotton, wood, leather, wool). These materials are dried before the weighted sample in the chopped up state for 24 hours with calcium chloride (CaCl2).The test is to be inferred at the agreed place about the whole test cross section from the component. The samples are chopped up in pieces with a weight more than 10 mg and less than 25 mg, without the test warms itself up. If necessary, another test preparations can be also undertaken and such deviation from standard should be mentioned in the final result.The test amount to be rocked to sleep is directed after the size of the Head Space of little glass whose least contents 5 ml must amount. Per 10 ml little glass volumes are to be rocked to sleep 1.000 g +0.001 g (i.e. maximum content error 0.1%) test material. Metal are to be removed before weighting. When metal parts are liable to organic substances, for example, varnish, pastes, should be separated mechanically at first and then to rock to sleep.The weighted test particles become in a Head Space little glasses (least 3 little glasses per test), then air-tighted with the use of a septums with the Teflon coating which points to the interior of the little glass.3. Testing sets and conditions3.1 testing setsGaschromatograph for capillary column with Head Space Tester, flame ionization detector (FID) and calculator/ Integrator.WCOT-capillary dividing column with a separating phase from 100% Polyethylene glycol (so-called Wax type, e.g., DBWax, Carbowax...)0.25 mm I.D., 0.25 µm film thickness, 30-m lengthAnalytic balance, in the order of 0.1 mgMicroliter syringe, 5 µl, sample in vitreous body3.2 measuring conditionsStove temperature program GC: 3 minutes isotherm at 50ºCHeating at 200ºC with a rate of 12 K / min4 minutes isotherm at 200ºCInjector temperature: 200ºCDetector temperature: 250ºCSplit ratio: approx. 1: 20Carrier gas: heliumMiddle carrier gas speed: approx. 22-27 cm /s.Remark: the substance 2,6-Di-tert-butyl-4-methylphenol (BHT) must show a retention time smaller than 16 minutes.4. Attempt realizationThe little glasses are tempered to the enrichment of the substances in the air standing about the test directly before the measurement 5 hours + 5 minutes with 120 + 1 ºC in the Head Space Tester and are analyzed directly afterwards. At least 3 tests are to be analyzed in each case.The control value is determined by average signal value of at least 3 measurements with empty test little glasses.The dosage must run off in all analyses of the tests, the control value and the calibration solution identically and reproduceable.The separating column must be brought to the bake once per week for 15 minutes at maximum temperature.5. CalibrationFor the quantitative determination of the total carbon emission as well as the amount of special single substances, calibration curve are compared with the method of the external standard.For the total carbon emission, acetone serves as a calibration substance, for the single substances and the respective materials.After installation of a new column and after changes in the device a basic calibration with 7 calibration concentration is to be carried out. In addition, a control calibration with at least 3 concentrations has to be carried out at least every 4 weeks.For basic calibration, 7 calibration solutions of acetone with concentration of 0.1/0.5/1/5/10/50/100grams of acetone in per liter of n-Butanol is made. For the control calibration solution at least three concentrations 0.5/5/50 grams of acetone per liter are required. It is to be guaranteed that in the used n-Butanol show no Peaks at the same time as acetone.For the single substances, calibration solution are to be produced in the same concentration like for the acetone-control calibration and in each case, a solvent is to be used which shows no peaks with the retention time of the relevant single substance and boiling point lies under 120ºC.All substances used for the calibration procedure should be checked at least each year to show quality.With the calibration measurement with one 5 μl - syringe in each case 2 μl + 0.02 μl (i.e. maximum injection error 1%) per 10 ml little glass volume in an empty, unlocked Head Space little glasses squirted. Specific attention should be paid to the fact that no air bubbles are in the cylinder when drawing the syringe. The little glass is closed directly after as under 2 described.The calibrated sample is kept at a moderate temperature for 1 hour with 120ºC in the Head space Tester and then analyzed in accordance with the general test specification, whereby the temperature program of the gaschromatograph can be broken off after elution of the solvent. At least 3 measurements are to be carried out for each calibration solution. With the concentrations of the calibration solutions (in g/l), determined for the respective calibration substance, a straight line can be drawn, whose upward gradient represents the calibration factor k (k(G) for the least squares to take place. The coefficient of correlation K must be thereby larger than 0,995.)6. EvaluationFrom the data of the gaschromatogram the total peak area as well as the surfaces of the peaks belonging to the single substance indicated in the design and/or TL must have to be extracted. For the computation of the total peak area only peaks are consulted,-their height greater than 10% of the triple value of the baseline isAnd-their area greater than 10% of the area of the Acetone peaks in the calibration solution with the concentration 0.5 g/l isThe detection limit of the analysis procedure must deliver a peak area and a peak height which is in each case smaller than 10% of the respective value which will receive for acetone in analysis of a calibration solution concentration of 0.5 g/l acetone.The desired emission values result from the results of measurement as follow:Total carbon emission E G:Ascertained from the total peak area which has arisen in the analysis of the tests, and the calibration factor k (G) from the acetone calibrationE G= Total peak area – Peak area of control x 2 x 0.6204K (G)The unit " µg carbon per g of sample ".The factor two is originated from the relation of " µg of sample " and arises by the fact that 1 g of sample, 2 µl calibration solution are given in 10 ml little glass.The factor 0.6204 shows the weight proportion of carbon in acetone.Single material – Emission Ei:Ascertained from the Peak area which has arisen in the analysis of the tests for the single material in request i, and the calibration factor k (i) from the single material calibrationEi = Peak area of the single material i x 2K (i)The unit " µg substance i per g of sample ".The factor two is originated from the relation of " g of sample " and arises by the fact that for 1 g of sample, 2 µl calibration solution are given in 10 ml little glass.For the results of 3 measurements of a part, not the average value, but all 3 measured values must fulfill the requirement. This is necessary to guarantee that at all places of the construction meet the requirements.。
汽车内饰非金属材料TVOC含量检测结果影响因素分析

汽车内饰非金属材料TVOC含量检测结果影响因素分析摘要:随着人们生活水平的提高和消费观念的改变,汽车已经成为了现代人生活中必不可少的交通工具。
而在汽车工业发展过程中,为了满足消费者对于舒适性、安全性等方面的需求,各种先进技术被应用到汽车制造当中。
其中,车内空气质量问题越来越受到关注。
目前,国内外学者针对汽车内饰件挥发性有机物含量进行了大量的研究工作。
然而,由于不同材质之间存在差异以及试验条件不一致等原因,导致所得出的结论不尽相同。
因此,本研究将重点探究汽车内饰非金属材料中苯乙烯、丙烯腈、甲醛等有害物质的含量检测方法及其影响因素。
关键词:汽车内饰;非金属材料;TVOC含量;检测结果;影响因素前言:作为汽车零部件中重要组成部分之一的塑料及其复合材料在车内使用过程中产生的挥发性有机物严重危害人体健康。
因此,对于汽车内饰件中的塑料及其复合材料进行VOC含量的检测具有非常重要的意义。
目前国内外关于汽车内饰件中非金属材料VOC含量的测试方法主要有袋式法、气相色谱-质谱联用法等。
但是由于不同材质之间存在差异以及实验操作过程中各种因素的干扰,导致测试出来的数据会出现一定程度上的误差,这就需要进一步探究其原因并提出相应的解决方案以保证测试结果准确可靠性。
一、汽车内饰非金属材料TVOC含量检测研究意义随着人们生活水平的提高,对于车内空气质量也越来越重视。
而作为车内重要组成部分之一的汽车内饰件,其挥发性有机物(TVOC)含量是评价车内空气品质的一个重要指标。
因此,准确测定汽车内饰件中的TVOC含量具有非常重要的现实意义和应用价值。
本文旨在通过对不同材质、不同部位的汽车内饰件进行测试分析,探究各种因素对TVOC含量检测结果的影响程度,从而得出一种较为精确可靠且操作简便的检测方法,以期更好地服务于广大消费者[1]。
二、汽车内饰非金属材料TVOC含量检测方法(一) TVOC简介挥发性有机物(volatile organic compounds)简称为TVOC,是指在常温下易挥发的有机化合物质。
非金属材料挥发性有害物质检测方法

非金属材料挥发性有害物质检测方法1 范围本标准规定了车内非金属材料挥发性有机物和醛酮物质的术语和定义、测试原理、试验设备、测量目标化合物、样件采集、气体捕集、分析方法、质量控制、结果报告。
选用固相吸附剂进行吸附后,用气质联用(GC-MS)和高效液相色谱(HPLC)测试。
本标准适用于汽车内饰、行李箱非金属材料及与汽车内室导入流动空气接触的零件。
2 规范性引用文件下列文件中的条款通过本标准的引用而成为本标准的条款。
凡是注日期的引用文件,其随后所有的修改单(不包括勘误的内容)或修订版均不适用于本标准,然而,鼓励根据本标准达成协议的各方研究是否可使用这些文件的最新版本。
凡是不注日期的引用文件,其最新版本适用于本标准。
车内挥发性有机物和醛酮类物质采样测定方法3 术语和定义下列术语和定义适用于本标准3.1挥发性有机组分挥发性有机组分是指利用tenax等吸附剂采集,并用极性指数小于10的气相色谱柱分离,保留时间在正己烷到正十六烷之间的具有挥发性的化合物的总称。
3.2醛酮组分醛酮组分是指利用采用高效液相色谱能测出的甲醛、乙醛、丙酮、丙烯醛、丙醛、烯醛、丁酮、丁醛、甲基丙烯醛、苯甲醛、戊醛、甲基苯甲醛、环己酮、己醛等化合物的总称。
4测试原理4.1 挥发性有机组分测试原理将试验材料置于密闭的氮气中在规定的温度下加热2h后,利用TENAX管采集一定体积的空气样品,样品中的挥发性有机组分被捕集在采样管中。
用干燥的惰性气体吹扫采样管后经二级脱附进入毛细管气相色谱质谱联用仪,从而对挥发性有机组分进行定性与定量分析。
4.2 醛酮组分测试原理将试验材料置于密闭的氮气中在规定的温度下加热2h后,利用DNPH管采集一定体积的空气样品,样品中的醛酮组分被捕集在采样管中。
用60%乙腈/40%水溶液洗脱,采用高效液相色谱对醛酮组分进行定性定量分析。
5试验设备5.1 测量用样袋样袋尺寸: 10L本测量应使用有2 处以上的阀门、进行过以下前处理的密封袋(以下简称样袋)。
汽车内饰件非金属材料(vda277)

05
非金属材料在汽车内饰件中的发展趋势
新型非金属材料的研发与应用
80%
高性能复合材料
如碳纤维复合材料,具有高强度 、轻量化和耐腐蚀等优点,在汽 车内饰件中逐渐得到应用。
100%
工程塑料
如聚碳酸酯、尼龙等,具有优良 的机械性能、耐热性和绝缘性, 广泛应用于汽车内部零件。
80%
生物可降解材料
如玉米塑料、竹纤维等,在环保 意识日益增强的背景下,这类材 料在汽车内饰件中的应用前景广 阔。
由两种或多种材料组成,具有 各组成材料的优点,如强度高 、质量轻等。
非金属材料在汽车内饰件中的应用实例
方向盘
通常采用塑料或橡胶制成,提供良好的握感和操 作稳定性。
座椅
使用海绵、皮革和织物等非金属材料制成,提供 舒适性和支撑力。
车门饰板
由塑料或复合材料制成,具有美观和防震作用。
仪表板
由多种非金属材料组成,包括塑料、橡胶和纤维 增强复合材料等。
02
VDA277标准概述
VDA277标准的定义与目的
定义
VDA277标准是德国汽车工业联合会(VDA)发布的一项针对汽车 内饰件非金属材料的质量标准。
目的
该标准旨在确保汽车内饰件非金属材料的质量、安全性和环保性 能,以保障驾乘人员的健康和安全,同时推动汽车工业的可持续 发展。
VDA277标准的关键要求和测试方法
04
VDA277标准对非金属材料的要求
VDA277标准对非金属材料的物理性能要求
抗冲击性
非金属材料应具备足够的抗冲击能力, 以承受汽车在行驶过程中产生的振动 和冲击。
耐热性
材料需能在汽车内部的高温环境下保 持稳定性,不易变形或产生有害气体。
汽车VDA278标准(中文版)

VDA278热脱附分析非金属汽车内饰材料中的有机挥发物目的:分析程序用于非金属材料(用于在机动车辆的内饰件,例如:纺织品,地毯,胶粘剂,密封剂,泡沫材料,皮革,塑料件,金属箔片漆或不同的材料的组合)的排放量的测定。
用本标准进行从有机质物质中释放出的物质的定性和定量分析。
此外,可以测定两个半定量的值,一是估计的挥发性有机化合物(VOC值),而是可能的散发的可冷凝物质的部分(雾值)。
此外,它也可以得到单一物质的排放量。
在分析样品排放时,将样品进行热提取、分离,进行气相色谱分析。
本测试方法是只有在本文中描述的条件下才有效。
然而,用该方法得到的结果,在其他一定的条件下是不适用的:●为了进行有关健康评估所排放的物质。
●在任何形式作为估计浓度基础评估那些的整车内饰,在驾驶或驾驶类似的条件。
关键词:释放非金属材料,热脱附(TDS),VOC值,雾度值内容列表1. 定义1.1热脱附分析1.2VDA278中VOC值1.3 VDA278中雾度值2.存储3.分析系统,仪器参数3.1设备系统的最低要求3.2热脱附系统适用性的检查3.3适用性检查实例4.进行分析4.1清洗吸附管4.2系统审核4.3校准4.4样品分析序列4.5色谱评价5验证5.1实际样品的测量值,实验室间测试5.2甲苯在Tenax中的检出限5.2甲苯在Tenax中的偏差和回收率6可能出现的错误及故障排除6.1样品准备6.2表面不均匀的复杂样品6.3冷却系统时的问题(KAS 3,Gerstel公司)6.4热脱附问题(turbo-matrix ATD,PE公司)6.5水分含量高的样品6.6物质的错误识别6.7高挥发值超过检测线性范围7附录7.1特定材料的重量7.2热脱附分析产生的漆膜7.3热脱附分析示意图7.4控制混合色谱示例7.5Excel报告介绍,小区配置7.6 Excel报告介绍,样品Excel报告1.定义1.1热脱附分析在热脱附分析(TDS)时,少量的样品在玻璃管中加热。
汽车-5种汽车内饰VOC含量分析采样方法

5种汽车内饰VOC含量分析采样方法作者:HSF中心2013-06-26 15:06 为了保证整车车内的空气质量符合GB/T27630要求,相关企业需要对汽车内饰零部件及其材料的VOC含量进行检测和控制,这项检测工作主要包括采样和样品分析两个步骤。
本文将针对采样方法进行特别介绍。
汽车内饰零部件及材料VOC含量分析的采样方法一般有5种,包括:采样袋法、检测舱法、项空法、热解析法和甲醛挥发法。
1.采样袋法采样袋法多为日系车企使用。
本方法进行VOC采样使用的采样袋由厚度为0.05 mm的聚氟乙烯(PVF)制成,根据采样袋容积分为大袋子和小袋子两种方法,分别用于测量总成零部件和材料的VOC 含量。
采样袋法主要被日系主机厂(包括丰田、日产和铃木等)及其合资企业采用。
该方法有如下的优点和缺点:1.1 优点•检测对象可以是零部件、总成,也可以是材料。
•可以同时采集用于检测苯烃类有机组分和检测醛酮组分的气体样品。
•本采样方法关于零部件、总成的VOC含量分析的采样与HJ/T400-2007《车内挥发性有机物和醛酮类物质采样测定方法》中车内空气VOC含量分析的采样方法相似,这样有利于建立整车车内空气质量技术要求与零部件/总成的VOC含量技术要求的对应关系。
1.2 缺点•由于采样袋使用次数有限,且需要消耗一次性的DNPH吸附管,因此与其他几种采样方法相比,采样袋法的测试成本较高,仅次于检测舱法。
•与检测舱法相比,采样袋法无法实时监控零部件、总成的VOC挥发情况。
总体而言,采样袋法是一种较为简便的测量零部件、总成VOC含量的采样方法,适合汽车企业和零部件供应商对零部件、总成的VOC含量进行检测和控制。
2.检测舱法检测舱法的试验空间是一个体积为(1±0.05)m3的密闭空间,内部装有调节空气均匀度的装置和样品支架。
为了调节空气交换率,试验箱体上安装有进气管和排气管。
使用试验气体(丙烷)和氮气对火焰离子探测仪进行校准,压缩空气需经过湿度调节装置以规定的湿度通入检测舱。
汽车车内有机挥发物(VOC)的检测

摘 要:本文介绍 了汽车 V C的危害 以及 国内外对汽 车 V C的一 些研究,同时对 汽车整车、零部件及材料的 V C检测方法进行简单介绍 , O O O 并分析了汽 车内饰件产 生 V C的主要来源。 O
Ab ta t T s rt c e n r d c s h h z r s f u o o l V C n s m r s a c o a t m bi e O a ho a d sr c : hi a i l i t o u e t e a a d o a t m bi e O a d o e e e r h n u o o l V C t me n
车 内的 主要 V C污 染 有乙 醛 、 二 苯基 甲烷 二异 氰 酸酯 O
部件 中都含有 一定量 的挥 发性有机 化合物 ,当气温达 到一定高
度 时 ,这些挥 发性物质就 会释放 出来 ,有些会 形成雾 翳并 凝结
在前 挡风玻璃 上 ,从而影 响驾驶 员的视线 ;有些则产 生令 人不
舒服 的气昧 ,甚至 引起头疼 、干咳和过 敏等不 适反应 ,从 而对 乘 员的 身体造成 伤害 。由于汽车 的某 些特点 ,使汽 车中的 V C O
其 中包括丙烯 醛、丙烯 腈、苯、 甲乙酮、苯 乙烯、四氯 乙烷、
三氧乙烷 、甲苯 、二 甲苯 、甲苯基二异氰酸酯等等 。
VC O 对人体 健康 有巨大 影响 。当居 室中的 V C 到一定 浓 O达 度 时,短 时间 内人 们会感 到头痛 、恶 心、呕吐 、乏 力等 ,严重
沸 点 沸点 <0 5 ℃ 名称 高挥发性有机化合物
Ke ywor ds: a t mo i e V C; d t r i a o uo bl; O e e m n ti n; m t ri a e al
汽车内装饰材料挥发性有机物GC-MS测定和分析

汽车内装饰材料挥发性有机物GC—MS测定和分析贺小凤*1,刘艳霖1,王桂霞2,钟文君2(1. 深圳信息职业技术学院,广东深圳518029;2。
深圳市高迪科技有限公司,广东深圳518057)摘要:采用恒温测试箱法采集汽车内部装饰材料释放的挥发性有机物,利用Nutech预浓缩仪对样品进行三级VOC富集,然后用GC—MS法(气相色谱-质谱法)对汽车内部装饰材料释放的挥发性有机物苯、甲苯、二甲苯、乙苯、苯乙烯进行了测定,检出限低于2.0 μg/m3.实验选择皮革表皮、橡胶密封条、灰色无纺布、海棉和仪表盘五种汽车内部装饰材料,进行挥发性有机物含量的测定,并对结果进行了分析和讨论。
结果表明五种汽车内部装饰材料均不同程度地释放挥发性有机物,其中热塑性材料仪表盘释放出的TVOC含量最高,橡胶密封条的TVOC释放量也较高,皮革表皮和灰色无纺布释放的甲苯较多,而消音材料海绵主要释放出二甲苯.汽车内部装饰材料释放挥发性有机物的情况复杂,因此,探索和建立有效的汽车内部装饰材料有害物质检测方法,对于选择低毒环保的汽车内部装饰材料非常有意义。
关键词:汽车内装饰材料;挥发性有机物;GC-MS法(气相色谱-质谱法)随着汽车进入家庭,汽车已逐步由生产资料变为人们重要的生活消费品,汽车与人民群众的联系越来越密切。
目前,国内已经出现了多起消费者由于汽车内部装饰材料和装饰用胶挥发的有毒有害气体超标严重、损害消费者身体健康而投诉汽车生产厂家的案例,汽车内部装饰材料的环保问题应该引起政府有关部门、质检机构和汽车及装饰材料生产厂家的高度重视。
探究汽车内部装饰材料的污染源,主要是车辆在生产时,内饰件要使用大量的“粘合剂"、“橡胶"、“针织制品”,这些都是产生车内环境污染的罪魁祸首,例如可以引起白血病的“苯”就来自于粘合用胶、人造革、漆面和皮革等,甲醛则主要来源于座椅套、车门衬板等针织品。
研究汽车内部不同装饰材料挥发性有机物的释放,对汽车内部装饰材料进行环保质量控制,有利于从源头上保证汽车内空气质量。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Determination of Organic Emission of Non-metallic materials from vehicles Interior VDA 277Contents1. General2. Test preparations3. Testing sets and conditions4. Attempt realization5. Calibration6. Evaluation1. General1.1 PurposesAccording to this test method, the organic emission from non-metallic materials is determined with direct or indirect influence for the passenger's cell of automobiles stand. It measure the emission potential of a material, the sum of all release values of the emitted substances with gaschromatograph and detection with a Flame ionization detector. The test operated by means of steam space analysis (Head Space technology) at temperature of 120ºC.1.2 RequirementsThe accompanying sample, the respective requirements as well as special method is of the suitable specification to take drawing or the like.If materials are composed of different components, a separate check is required for every single material.2. Test preparationsTransport and storage of the tests has to occur in an aluminum coated Polyethylenbeutel. The sampling has immediately after incoming goods or in a condition, which corresponds to this, to take place. The times of the incoming goods and the sampling are to be labelled. There is no conditioning of the test as a rule. Except of it are physical materials (cotton, wood, leather, wool). These materials are dried before the weighted sample in the chopped up state for 24 hours with calcium chloride (CaCl2).The test is to be inferred at the agreed place about the whole test cross section from the component. The samples are chopped up in pieces with a weight more than 10 mg and less than 25 mg, without the test warms itself up. If necessary, another test preparations can be also undertaken and such deviation from standard should be mentioned in the final result.The test amount to be rocked to sleep is directed after the size of the Head Space of little glass whose least contents 5 ml must amount. Per 10 ml little glass volumes are to be rocked to sleep 1.000 g +0.001 g (i.e. maximum content error 0.1%) test material. Metal are to be removed before weighting. When metal parts are liable to organic substances, for example, varnish, pastes, should be separated mechanically at first and then to rock to sleep.The weighted test particles become in a Head Space little glasses (least 3 little glasses per test), then air-tighted with the use of a septums with the Teflon coating which points to the interior of the little glass.3. Testing sets and conditions3.1 testing setsGaschromatograph for capillary column with Head Space Tester, flame ionization detector (FID) and calculator/ Integrator.WCOT-capillary dividing column with a separating phase from 100% Polyethylene glycol (so-called Wax type, e.g., DBWax, Carbowax...)0.25 mm I.D., 0.25 µm film thickness, 30-m lengthAnalytic balance, in the order of 0.1 mgMicroliter syringe, 5 µl, sample in vitreous body3.2 measuring conditionsStove temperature program GC: 3 minutes isotherm at 50ºCHeating at 200ºC with a rate of 12 K / min4 minutes isotherm at 200ºCInjector temperature: 200ºCDetector temperature: 250ºCSplit ratio: approx. 1: 20Carrier gas: heliumMiddle carrier gas speed: approx. 22-27 cm /s.Remark: the substance 2,6-Di-tert-butyl-4-methylphenol (BHT) must show a retention time smaller than 16 minutes.4. Attempt realizationThe little glasses are tempered to the enrichment of the substances in the air standing about the test directly before the measurement 5 hours + 5 minutes with 120 + 1 ºC in the Head Space Tester and are analyzed directly afterwards. At least 3 tests are to be analyzed in each case.The control value is determined by average signal value of at least 3 measurements with empty test little glasses.The dosage must run off in all analyses of the tests, the control value and the calibration solution identically and reproduceable.The separating column must be brought to the bake once per week for 15 minutes at maximum temperature.5. CalibrationFor the quantitative determination of the total carbon emission as well as the amount of special single substances, calibration curve are compared with the method of the external standard.For the total carbon emission, acetone serves as a calibration substance, for the single substances and the respective materials.After installation of a new column and after changes in the device a basic calibration with 7 calibration concentration is to be carried out. In addition, a control calibration with at least 3 concentrations has to be carried out at least every 4 weeks.For basic calibration, 7 calibration solutions of acetone with concentration of 0.1/0.5/1/5/10/50/100grams of acetone in per liter of n-Butanol is made. For the control calibration solution at least three concentrations 0.5/5/50 grams of acetone per liter are required. It is to be guaranteed that in the used n-Butanol show no Peaks at the same time as acetone.For the single substances, calibration solution are to be produced in the same concentration like for the acetone-control calibration and in each case, a solvent is to be used which shows no peaks with the retention time of the relevant single substance and boiling point lies under 120ºC.All substances used for the calibration procedure should be checked at least each year to show quality.With the calibration measurement with one 5 μl - syringe in each case 2 μl + 0.02 μl (i.e. maximum injection error 1%) per 10 ml little glass volume in an empty, unlocked Head Space little glasses squirted. Specific attention should be paid to the fact that no air bubbles are in the cylinder when drawing the syringe. The little glass is closed directly after as under 2 described.The calibrated sample is kept at a moderate temperature for 1 hour with 120ºC in the Head space Tester and then analyzed in accordance with the general test specification, whereby the temperature program of the gaschromatograph can be broken off after elution of the solvent. At least 3 measurements are to be carried out for each calibration solution. With the concentrations of the calibration solutions (in g/l), determined for the respective calibration substance, a straight line can be drawn, whose upward gradient represents the calibration factor k (k(G) for the least squares to take place. The coefficient of correlation K must be thereby larger than 0,995.)6. EvaluationFrom the data of the gaschromatogram the total peak area as well as the surfaces of the peaks belonging to the single substance indicated in the design and/or TL must have to be extracted. For the computation of the total peak area only peaks are consulted,-their height greater than 10% of the triple value of the baseline isAnd-their area greater than 10% of the area of the Acetone peaks in the calibration solution with the concentration 0.5 g/l isThe detection limit of the analysis procedure must deliver a peak area and a peak height which is in each case smaller than 10% of the respective value which will receive for acetone in analysis of a calibration solution concentration of 0.5 g/l acetone.The desired emission values result from the results of measurement as follow:Total carbon emission E G:Ascertained from the total peak area which has arisen in the analysis of the tests, and the calibration factor k (G) from the acetone calibrationE G= Total peak area – Peak area of control x 2 x 0.6204K (G)The unit " µg carbon per g of sample ".The factor two is originated from the relation of " µg of sample " and arises by the fact that 1 g of sample, 2 µl calibration solution are given in 10 ml little glass.The factor 0.6204 shows the weight proportion of carbon in acetone.Single material – Emission Ei:Ascertained from the Peak area which has arisen in the analysis of the tests for the single material in request i, and the calibration factor k (i) from the single material calibrationEi = Peak area of the single material i x 2K (i)The unit " µg substance i per g of sample ".The factor two is originated from the relation of " g of sample " and arises by the fact that for 1 g of sample, 2 µl calibration solution are given in 10 ml little glass.For the results of 3 measurements of a part, not the average value, but all 3 measured values must fulfill the requirement. This is necessary to guarantee that at all places of the construction meet the requirements.。
