2015版质量管理体系程序文件-组织知识控制程序(中英文版)
ISO9001:2015全套文件英文版(含质量手册及全套程序文件)

Ningbo XXX Material TechnologyCo.,LtdISO9001:2015 Quality ManualRevision [A/0] - [2018/3/1](c) [Copyright Year Of 2018] [Ningbo XXX Material Technology Co.,Ltd]; all rights reserved. This document may contain proprietary information and may only be released to third parties with approval of management. Document is uncontrolled unless otherwise marked; uncontrolled documents are not subject to update notification.Revision [A/0] - [2018/3/1]Page 1 of 19TABLE OF CONTENTS0.0 Revision History and Approval ...................................................................................................................... 3 1.0 Welcome to Ningbo XXX Material Technology Co.,Ltd ................................................................................ 4 2.0 XXX Material: Who We Are ........................................................................................................................... 4 2.1 Determining Our Strategic Direction ......................................................................................................... 4 2.2 Scope of the Management System ........................................................................................................... 4 2.2.1 Scope Statement ............................................................................................................................... 4 2.2.2 Facilities Within the Scope ................................................................................................................ 4 2.2.3 Permissible Exclusions ..................................................................................................................... 5 2.2.4 Scope of the ISO9001:2015 Quality Manual ..................................................................................... 5 3.0 Quality Policy................................................................................................................................................. 5 4.0 Management System Structure and Controls ............................................................................................... 5 4.1 Process Approach .................................................................................................................................... 5 4.1.1 Process Identification ........................................................................................................................ 5 4.1.2 Process Controls & Objectives .......................................................................................................... 6 4.1.3 Outsourced Processes ...................................................................................................................... 7 4.2 Documentation & Records ....................................................................................................................... 7 4.2.1 General .............................................................................................................................................. 7 4.2.2 Control of Documents ....................................................................................................................... 7 4.2.3 Control of Records ............................................................................................................................ 7 4.3 Change Management ................................................................................................................................ 8 4.4 Risks and Opportunities ............................................................................................................................ 8 5.0 Management & Leadership ........................................................................................................................... 8 5.1 Management Leadership and Commitment .............................................................................................. 8 5.2 Customer Focus ........................................................................................................................................ 9 5.3 Quality Policy ............................................................................................................................................. 9 5.4 Organizational Roles Responsibilities & Authorities ................................................................................. 9 5.5 Internal Communication ............................................................................................................................ 9 5.6 Management Review .............................................................................................................................. 10 6.0 Resources ................................................................................................................................................... 10 6.1 Provision of Resources ........................................................................................................................... 10 6.2 Human Resources .................................................................................................................................. 10 6.3 Infrastructure ........................................................................................................................................... 11 6.4 Work Environment ................................................................................................................................... 11 6.5 Organizational Knowledge ...................................................................................................................... 11 7.0 Operation ..................................................................................................................................................... 11 7.1 Operational Planning and Control ........................................................................................................... 12 7.2 Customer-Related Activities .................................................................................................................... 12 7.2.1 Capture of Customer Requirements ............................................................................................... 12 7.2.2 Review of Customer Requirements ................................................................................................ 12 7.2.3 Customer Communication ............................................................................................................... 12 7.3 Design and Development ........................................................................................................................ 13 7.4 Purchasing .............................................................................................................................................. 13 7.5 Provision of [Production of adhesive tape] .............................................................................................. 13 7.5.1 Control of Provision of [Production of adhesive tape] ..................................................................... 13 7.5.2 Identification and Traceability .......................................................................................................... 14 7.5.3 Property Belonging to Third Parties ................................................................................................ 14 7.5.4 Preservation .................................................................................................................................... 14 7.5.5 Post-Delivery Activities .................................................................................................................... 14 7.5.6 Process Change Control ................................................................................................................. 15 7.5.7 Measurement and Release of [Production of adhesive tape] ......................................................... 15 7.5.8 Control of Nonconforming Outputs .. (15)Revision [A/0] - [2018/3/1]Page 2 of 198.0 Improvement ............................................................................................................................................... 15 8.1 General .................................................................................................................................................... 15 8.2 Customer Satisfaction ............................................................................................................................. 15 8.3 Internal Audit ........................................................................................................................................... 16 8.4 Corrective and Preventive Action ............................................................................................................ 16 Appendix A: Overall Process Sequence & Interaction ........................................................................................ 17 Appendix B: ISO 9001:2015 Cross Reference . (18)Revision [A/0] - [2018/3/1] 0.0 Revision History and ApprovalRev. Nature of changes Approval DateA/0 Original release. XXX 2018/3/1Page 3 of 19Revision [A/0] - [2018/3/1] 1.0 Welcome to Ningbo XXX Material Technology Co.,LtdNingbo XXX Material Technology Co., Ltd. Was established in 2000, with the UAE businessmen to build the joint venture company; 2004 Ming Shan founded Nissin special adhesive tape and adhesive tape company; 2008 due to the strategic adjustment of 3 company merger and reorganization, the establishment of Ningbo letter mountains adhesive products Manufacturing Co., Ltd..PVC insulation tape is one of China’s national standard drafting unit, the field of adhesive products governing unit of China, Ningbo, adhesives and related products Industry Association, honorary president of the unit.We produce more than 1000 varieties of products of 11 categories. The annual throughput more than 100,000,000 square of the adhesive and 10,000 ton of the adhesive.Our company located in Ningbo which the city of the east China sea, beside 500 kilometers of shanghai, 20 kilometers from Ningbo list airport and 50 kilograms of the Ningbo seaport. The transport is very convenient (Easy to ship to everywhere on the world).2.0 XXX Material: Who We Are2.1 Determining Our Strategic DirectionXXX Material has reviewed and analyzed key aspects of itself and its stakeholders to determine the strategic direction of the company. This involves:∙Understanding our core products and services, and scope of management system (see 2.2 below).∙Identifying “interested parties” (stakeholders) who receive our [Production of adhesive tape], or who may be impacted by them, or those parties who may otherwise have a significant interest in our company. These parties are identified in the document [Requirements and expectations list of interested parties].∙Understanding internal and external issues that are of concern to XXX Material and its interested parties; also identified in the document [Requirements and expectations list of interested parties]. Many such issues are identified through an analysis of risks facing either XXX Material or the interested parties. Such issues are monitored and updated as appropriate, and discussed as part of management reviews.This information is then used by senior management to determine the company’s strategic direction. This is defined in records of management review, and periodically updated as conditions and situations change.2.2 Scope of the Management System2.2.1 Scope StatementBased on an analysis of the above issues of concern, interests of stakeholders, and in consideration of its products and services, XXX Material has determined the scope of the management system as follows:Production of adhesive tapePage 4 of 19Revision [A/0] - [2018/3/1]Page 5 of 192.2.2 Facilities Within the ScopeThe quality system applies to all processes, activities and employees within the company. The facility is located at: Factory Add :XXX Tel :XXX Fax :XXX XXX XXX2.2.3 Permissible ExclusionsThe following clauses of ISO 9001 were determined to be not applicable to XXX Material.∙ 8.3 Design and development of products and services 2.2.4Scope of the ISO9001:2015 Quality ManualThis manual is prepared for the purpose of defining the company’s interpretations of the ISO 9001:2015 international standard, as well as to demonstrate how the company complies with that standard.This manual does not follow the numbering structure of ISO 9001. Instead, Appendix B presents a cross reference between the sections of this manual and the clauses of ISO 9001:2015.This manual presents “Notes” which are used to define how XXX Material has tailored its management system to suit its purposes. These are intended to clarify implementation approaches and interpretations for concepts which are not otherwise clearly defined in ISO 9001:2015. Notes appear in italics, with gray background.Where subordinate or supporting documentation is reference in this manual, these are indicated by bold italics .∙ Quality PolicyThe Quality Policy of XXX Material is as follows:Quality first, customer satisfaction;Scientific management, continuous improvement. ∙ Management SystemStructure and Controls 4.1Process Approach4.1.1 Process IdentificationXXX Material has adopted a process approach for its management system. By identifying thetop-level processes within the company, and then managing each of these discretely, this reduces the potential for nonconforming [Production of adhesive tape] discovered during final processes or after delivery. Instead, nonconformities and risks are identified in real time, by actions taken within each of the top-level processes.Note: not all activities are considered “processes” – the term “process” in this context indicates the activity has been elevated to a higher level of control and management oversight.The controls indicated herein are applicable only to the top-level processes identified.。
ISO9001:2015全套程序文件英文版

ISO9001:2015全套程序文件英文版(本人辛苦原创)Code QM-COP-01Date2018.10.24Date2018.10.241.0 PurposeAll the documents required by the Company’s quality management system should be controlled to ensure the version applied by all the relevant departments is valid.2.0 ScopeIt is applicable to all the documents pertaining to the quality management system including external documents.3.0 Definition3.1 Controlled document: The document applied in and out of the Company is controlled in modifications, identities, versions, version numbers, formats, fonts, etc.3.2 DCC: Document Controlling Center3.3 External document: It refers to the document that has been handled by outside individuals like national/international standards, laws and regulations, documents provided by customers or suppliers, material certificates, amendment advice, etc.3.3.1 Administrative documents on quality management system or product, released from local government authorities and regulatory agencies such as the notices from Guangdong Food and Drug Administration.3.3.2 National laws and regulations such as Product Quality Law of the People’s Republic of China, Regulation on the Supervision and Administration of Medical Devices, 93/42/EEC, etc.3.3.3 International standards such as Medical devices—Quality management systems—Requirements for regulatory purposes.3.3.4 National standards such as Medical electrical equipment – Part 1: General requirements for safety.3.3.5 Regulations and standards provided by customers such as agreements and commitments signed with customers.3.3.6 Drawings provided by customers such as drawings, mold drawings provided by a certain customer.3.3.7 Other important external documents relating to the product, including official materials like customer’s notice.4.0 Duties4.1 General Manager: Responsible for approval of the Company’s quality manual.4.2 Management Representative: Responsible for the Company’s procedure files, quality plans and cross-department three-order files and approval of external documents.4.3 Principals of each department: Responsible for approval of three-order files and all kinds of tables as well as department-related external documents.4.4 Department: Responsible for compilation, number and review of the documents dominated by the department.Code QM-COP-01Date2018.10.24Date2018.10.244.5 Quality Management Department: Responsible for all the controlled documents of the Company to ensure the electronic document is the latest version, and responsible for the updating of the controlled document list of all the departments.5.0 Procedures5.1 Document classification: The management system documents includes four layers and external documents5.1.1The Quality Manual (including policies and goals) is a principle-based and master document guiding the implementation of the quality management system. As the first level document, it does not just explain the scope of application but also describe the interaction among all the procedures in the quality management system.5.1.2 The procedure document is the expansion and specification of the Quality Manual, providing the process, methods and controlling means for carrying out quality management. It belongs to the second level document.5.1.3 Supporting documents (operation/technical specifications, process/inspection standards, technical guidance and position description) specify the quality management goals, duties of the posts of all levels and specific operation methods. It belongs to the third level document.5.1.4 The table is applied to record the state and result of activities, belonging to the fourth level document.5.1.5 External document: It refers to the document directly obtained from outside and cited by the Company, including national/international standards, laws and regulations, documents provided by customers or suppliers, material certificates and amendment advice.5.1.6 The document is drawn up mainly in written or electronic form, and both shall be under control.5.2 Document compilation and approval5.2.1 The formats of the second and third level documents are the same as that of the document.5.2.2 The date of the document must be written in the form of “year month day”.5.2.3 Limits for examination and approving authority for documentsS/N Order Type ofdocumentPrepared byReviewedbyJointreviewed byApprovedbyRemark1 First ManagementManualQualityManagementDepartmentManagementRepresentativeSupervisorof eachdepartmentTopmanagement2 SecondProceduredocumentAlldepartmentDepartmentRelevantdepartmentManagementCodeQM-COP-01Date2018.10.24 Date2018.10.245.3 Document’s number and version/version number5.3.1 Number: The document compiler numbers the newly compiled documents according to the Basic Rules for Numbering the Controlled Documents and the document list of the department, and confirms the uniqueness of the numbers with the controlling center.5.3.2 Version/Version number: The version or version number of the controlled document is compiled insmanagermanagerRepresen tative3ThirdManagement documentAll department sDepartme nt managerRelevant department manager and Managemen tRepresentat iveManage ment Represen tativeJob Description of the personnel below the manager level is reviewed by the department manager and approved by the manager of HR Department.4ThirdProcess, inspection standarddocument and specification (including external document)All department sQuality Manageme nt Departme ntDepartm ent manager5Fourt hTablesAlldepartment sQualityManageme nt Departme ntDepartm entmanagerAdditional remarks: 1) The document can be compiled by the compilers or above the compiler level but must be approved by the personnel upper than the compiler.2) The relevant department refers to the departments having ties with others involved in this system. 3) When the approver of the above documents is absent, his agent or Management Representative can sign it up instead to make the document effective.Code QM-COP-01Date2018.10.24Date2018.10.24the form of 26 alphabets from A to Z. The initial version number is “A/0”, the next revised version is “A/1” and so on. Changing Arabic numbers is enough for minor revisions while changing alphabets, for instance, from “A” to “B”, is necessary in case of major revisions.5.4 Document distribution and storage5.4.1 The document compiler sends the copy of the approved document and its electronic version to the Quality Management Department where the document will be checked whether it has been approved by designated personnel. After that, the document will be registered, controlled with the controlled document list updated.5.4.2 The document controller determines the scope of distribution, makes copies of the electronic file ina required number according to the List of distributed controlled documents, add the watermarks of correspondent departments on these copies, save them to the folder for controlled documents of each department and notify the departments for making and using the documents by email.5.4.3 All the department are responsible for checking if the controlled document is correct or not.5.4.4 The authority for the controlled document folder of each department shall be set as follows:①Document controller is permitted to modify, delete the content or add new content to the document.②Each department can only read but cannot delete, modify or add the content of controlled documents.5.4.5 The document controller must copy the electronic document as a backup.5.4.6 Visual management of documentsAs for the documents which are frequently applied at production site, all the departments should take correspondent measures such as hanging them on the wall, beside the equipment or enveloping them with plastic so to make it easy for operators to use.5.5 Document reading5.5.1 In case of reading the documents, the relevant personnel can open the PDF file which are saved in the Company’s share disk.5.6 Document review, modification, recovery, invalidation and destruction5.6.1 Review①The documents of the quality management system should be reviewed once a year by the Quality Management Department and internal review team organized by the Management Representative along with the Company’s internal review and reviewed with the result put down in the internal review record.②In case of special circumstances, some documents should be reviewed by the relevant department.③The review must take into account the influence of both the internal factors like the Company’s organization and position changes and the external factors like laws, regulations, relevant standards and market demands upon the sufficiency and applicability of the documents with the Review Record filled in.5.6.2 Revision/alteration①The director and executor of each unit should check the effect after implementing the documents. If the documents are not applicable or in doubt in addition to the opinions on the content of the documents from other units, the documents can be revised or modified by the department which revised or compiledCode QM-COP-01Date2018.10.24Date2018.10.24them last time after the discussion among the relevant departments. Relevant approval process is the same as that in 5.2.2.②All the modifications or alterations must be underlined (“___”). In case of version change, the previous underline should be substituted by the latest one.③The revision record should be written on the first page of the documents, containing the content of the revision, identification of the affected documents, signature of the approver, date of approval and effective time.④The relevant departments shall be notified of review and confirmation of the alteration, and personnel training will be provided if necessary.⑤In the following circumstances that there is any alteration to the documents of the quality management system or the documents relating to the Company’s medical device products, the top management or Management Representative of the Company should be notified of deciding whether to inform the competent authority or notified bodies about it. If it is necessary, the notification should be implemented in accordance with the local laws and administrative regulations.a. Major alterations to the Quality Manual.b. Major alterations to the product’s functions, performance, safety, reliability and electromagnetic compatibility, caused by altering product standards.c. Major alterations to the product’s functions, performance, safety, reliability and electromagnetic compatibility, caused by changing key components of products.d. Stipulated by laws and regulations.5.6.3 Once the new version of controlled document is distributed, the old one becomes invalid automatically. The document controller should delete the copies of invalid controlled documents in the controlled document folder, upload the latest version and keep the original documents printed with an “invalid” stamp at the document controlling center till the expiry date (at least five years) before destruction.5.6.4 As for the invalid original documents, the document controlling center should destruct them uniformly after Document/Record Destruction Registration Form filled in by the center is approved by the Management Representative.5.7 The non-controlled document is identified as the “Reference”. If a Company’s customer or other personnel need it for their jobs, they must have the copies of the Company’s controlled documents and get its copies approved by the Management Representative and stamped with the ‘Reference’ seal by the Quality Management Department. The ‘Reference’ documents will not be withdrawn or changed to the latest version.5.8 Temporary documentIt is not yet official for some reasons but needed by each department. Such document should have a ‘Temporarily Controlled’ stamp as well as the time limit and distribution department on them. The temporary document cannot be valid for more than 3 months.Code QM-COP-01Date2018.10.24Date2018.10.245.9 Management of external documents5.9.1 Each department of the Company can collect external documents through the following channels.a. National, provincial, municipal governments and their relevant functional departments.b. All kinds of meetings, professional newspapers, magazines, publishers and suppliers.c. Internet, telephone and fax.5.9.2 The external document collected by each department should be selected timely and delivered to the relevant department to recognize its contents and decide whether make it a controlled document.a. The collected technical standards on our products should be delivered to the Technical Department to recognize its year, version and applicable articles.b. The laws, regulations and rules that are issued by the state on the quality and safety of the product should be delivered to the Quality Management Department to identify the required department and scope.c. Policy documents issued by the superior should be delivered to the administration department for recognition.d. The technical documents provided by suppliers or customers should be delivered to the Technical Department and Quality Management Department for recognition.f. The design input documents provided by customers should be delivered by the Market Department to the R&D Department for recognition. Saved in DHF format, they don’t have to be controlled by document controller.5.9.3 Numbering of external documentsAs for the external documents on technology and standards as well as other external documents, the Quality Management Department should number them in accordance with the Basic rules on numbering controlled documents.5.9.4 Distribution of external documentsa. After being recognized, the external documents should be kept on a file and put down on a list.b. The external documents should be distributed after the distribution scope is confirmed according to 5.4 of this procedure.5.9.5 Updating of external documentsAs for the external documents which need updating, the new version should be distributed with the invalid ones withdrawn immediately.5.9.6 Preservation and destruction of external documentsThe preservation and destruction should be implemented according to 5.6.3 of this procedure.5.10 The Quality Management Department should supervise and inspect irregularly the controlling process implemented by each department.6.0 Records and Tables6.1 Controlled Document Directory。
2015版ISO9001知识管理控制程序

知识管理控制程序1.目的确定并管理公司拥有的知识,有效避免知识的流失,达成公司知识传承、应用共享、创新增值的效果。
2.适用范围适用于与管理体系过程运行及提供合格产品和服务有关的知识的确定、获取、保持、共享、应用和创新等知识管理过程的控制。
3.权责3.1管理体系档案科(简称档案科):主导公司知识管理工作,包括1)知识管理的专兼职队伍建设;2)建立知识管理运行、考核和激励制度;3)知识管理规划编制;4)定期组织知识管理绩效评价并策划知识更新和改进措施;5)负责知识管理方法和工具(如标杆学习、导师制和教练制、头脑风暴法等)的选择、创建和在公司内的推广和应用。
6)归口管理公司获取的知识,建立受控知识清单。
3.2人力资源中心:1)构建公司培训体系,组织公司各类领域专业人员参与各类课程开发、知识与经验传授,将隐性知识转化成培训课件和教材;2)公司行政管理制度的制定、相关法律法规的获取、保持与管理;3)负责政府、权威机构等信息的收集与管理。
3.3企划科:负责媒体信息的收集与管理。
3.4信息科:公共网盘、ERP系统等知识管理基础设施的建设、应用和维护管理。
3.5产品技术中心:1)产品标准、技术与研发成果等知识的创造、获取、保持与管理;2)技术与研发方面新知识的推广与应用;3)公司知识产权的申报与维护。
3.6生产技术运营各部门:1)生产制造过程有关知识的获取、保持、改进与创新;2)生产制造过程中经验、教训的总结,形成知识,根据需要在公司内部进行交流和共享。
3)新技术、新工艺、新材料、新设备方面知识的获取、保持与应用。
3.7商务运营部门:1)销售渠道、客户服务、竞争对手信息等知识的获取、保持与管理;2)客户意见和满意度信息的收集、整理与分析;3)主导知识管理工作(见3.1)。
3.8品质保障中心:产品质量检验、质量改进方面知识的获取、保持与管理。
3.9实验工程中心:测试标准、测试方法方面知识的获取、保持与管理。
3.10体系管理中心:体系管理方面知识的获取、保持与管理。
ISO9001-2015中文版(完整)

ISO9001:2015标准目录1 范围2 规范性引用文件3 术语和定义4 组织的背景4.1 理解组织及其背景4.2 理解相关方的需求和期望4.3 质量管理体系范围的确定4.4 质量管理体系5 领导作用5.1 领导作用和承诺5.2 质量方针5.3 组织的作用、职责和权限6 策划6.1 风险和机遇的应对措施6.2 质量目标及其实施的策划6.3 变更的策划7 支持7.1 资源7.2 能力7.3 意识7.4 沟通7.5 形成文件的信息8 运行8.1 运行的策划和控制8.2 市场需求的确定和顾客沟通8.3 运行策划过程8.4 外部供应产品和服务的控制8.5 产品和服务开发8.6 产品生产和服务提供8.7 产品和服务放行8.8 不合格产品和服务9 绩效评价9.1 监视、测量、分析和评价9.2 内部审核9.3 管理评审10 持续改进10.1 不符合和纠正措施10.2 改进附录A 质量管理原则文献1 范围本标准为有下列需求的组织规定了质量管理体系要求:a)需要证实其具有稳定地提供满足顾客要求和适用法律法规要求的产品和服务的能力;b)通过体系的的有效应用,包括体系持续改进的过程,以及保证符合顾客和适用的法律法规要求,旨在增强顾客满意。
注1:在本标准一中,术语“产品”仅适用于:a) 预期提供给顾客或顾客所要求的商品和服务;b) 运行过程所产生的任何预期输出。
注2:法律法规要求可称作为法定要求。
2 规范性引用文件下列文件中的条款通过本标准的引用而构成本标准的条款。
凡是注日期的引用文件,只有引用的版本适用。
凡是不注日期的引用文件,其最新版本(包括任何修订)适用于本标准。
ISO9000:2015 质量管理体系基础和术语3 术语和定义本标准采用ISO9000:2015 中所确立的术语和定义。
4 组织的背景环境4.1 理解组织及其背景环境组织应确定外部和内部那些与组织的宗旨、战略方向有关、影响质量管理体系实现预期结果的能力的事务。
ISO9001:2015文件控制程序英文版
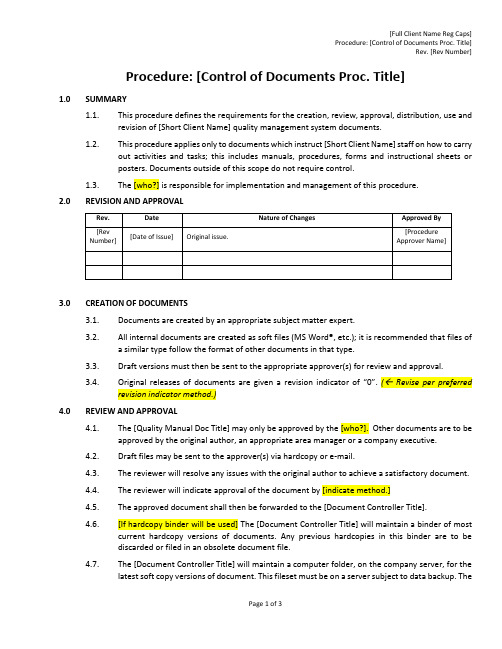
Procedure: [Control of Documents Proc. Title]1.0SUMMARY1.1.This procedure defines the requirements for the creation, review, approval, distribution, useand revision of [Short Client Name] quality management system documents.1.2.This procedure applies only to documents which instruct [Short Client Name] staff on how tocarry out activities and tasks; this includes manuals, procedures, forms and instructional sheetsor posters. Documents outside of this scope do not require control.1.3.The [who?] is responsible for implementation and management of this procedure.2.0REVISION AND APPROVAL3.0CREATION OF DOCUMENTS3.1.Documents are created by an appropriate subject matter expert.3.2.All internal documents are created as soft files (MS Word®, etc.); it is recommended that files ofa similar type follow the format of other documents in that type.3.3.Draft versions must then be sent to the appropriate approver(s) for review and approval.3.4.O riginal releases of documents are given a revision indicator of “0”. ( Revise per preferredrevision indicator method.)4.0REVIEW AND APPROVAL4.1.The [Quality Manual Doc Title] may only be approved by the [who?]. Other documents are to beapproved by the original author, an appropriate area manager or a company executive.4.2.Draft files may be sent to the approver(s) via hardcopy or e-mail.4.3.The reviewer will resolve any issues with the original author to achieve a satisfactory document.4.4.The reviewer will indicate approval of the document by [indicate method.]4.5.The approved document shall then be forwarded to the [Document Controller Title].4.6.[If hardcopy binder will be used] The [Document Controller Title] will maintain a binder of mostcurrent hardcopy versions of documents. Any previous hardcopies in this binder are to bediscarded or filed in an obsolete document file.4.7.The [Document Controller Title] will maintain a computer folder, on the company server, for thelatest soft copy versions of document. This fileset must be on a server subject to data backup.The [Document Controller Title] will place new or revised documents into that folder, settingeach file’s permission to READ ONLY, or converting the released vers ions to a non-editable fileformat.4.8.Any previous soft versions are then moved to a separate folder identified for obsoletedocuments which are kept for historical purposes.4.9.The directory of official released documents shall act as a “master list” of docume nts, indicatingthe current versions of all documents. No other master list is required.5.0DISTRIBUTION OF DOCUMENTS5.1.[If intranet is used] Controlled documents will be available via the intranet for all employees.Employees receive training on the file and folder locations for most current documents.5.2.[If hardcopy document distribution is used] The [Document Controller Title] will maintain a listof where controlled hardcopy documents are to be distributed. The [Document Controller Title]will be responsible for distributing updated copies of such controlled hardcopies to properlocations. Controlled hardcopies shall be stamped CONTROLLED in red ink on the first page, todistinguish them from uncontrolled documents or photocopies.5.3.Controlled hardcopies may not be altered or modified by users, and must remain legible andreadily identifiable. This includes hand mark-ups by unauthorized personnel. The only exceptionto this rule is for Forms (see below.)5.4.Controlled hardcopies may not be photocopied, unless for the purposes of sending to a recipientwho is authorized to receive uncontrolled versions of [Short Client Name] documents (i.e., avendor or customer). The only exception to this rule is for Forms (see below.)6.0RE-EVALUATION6.1.Documents must be reviewed by the original author or another subject matter expert or top6.2.The [Document Controller Title] will ensure re-evaluation is conducted and that documents areupdated if required. The [Document Controller Title] will maintain a record of document re-evaluations, to identify when documents are due for re-evaluation.6.3.If a document is determined to require updating, the changes shall be made and a new versionissued per the rules below.6.4.If a document is determined not to require updating, no action on the document is necessary.7.0REVISING DOCUMENTS7.1.Changes to documents go through the same steps as original issue, except that their revisionlevel is advanced upon approval.7.2.Only authorized personnel may change documents, although any employee can request achange to their Manager, or by filing a [CAR Form Name] [or other document change requestform.]Wherever possible, the document shall include a change history table within its text.[Other methods for identifying changes might be use of Track Changes feature in document,。
ISO9001:2015中文完整版

ISO/DIS 9001:2015质量管理体系—要求 Quality management system—Requirements目录 0 引言 (04)0.1 总则 (04)0.2 ISO标准的质量管理 (04)0.3 过程方法 (05)0.4 PDCA 循环 (06)0.5 基于风险的思维 (06)0.6 与其他管理体系标准的相容性 (06)1 范围 (08)2 规范性引用文件 (08)3 术语和定义 (08)4 组织的背景 (20)4.1 理解组织及其背景 (20)4.2 理解利益相关方的需求和期望 (20)4.3 确定质量管理体系的范围 (20)4.4 质量管理体系及其过程 (21)5 领导 (21)5.1 领导和承诺 (21)5.1.1 领导和质量管理体系承诺 (21)5.1.2 以顾客为关注焦点 (21)5.2 质量方针 (22)5.3 组织角色、职责和权限 (22)6 质量管理体系策划 (22)6.1 应对风险和机遇的措施 (22)6.2 质量目标及其实现策划 (23)6.3 变更策划 (23)7 支持 (23)7.1 资源 (23)7.1.1 总则 (23)7.1.2 人 (24)7.1.3 基础设施 (24)7.1.4 过程作业环境 (24)7.1.5 监视和测量资源 (24)7.1.6 组织的知识 (25)7.3 意识 (25)7.4 沟通..................................................... .................. .. (25)7.5 文件信息................................................... .................. (25)7.5.1 总则 (25)7.5.2 创建和更新............................................................... (26)7.5.3 文件信息控制....................................................... (26)8 运作........................................................................ . (26)8.1 运作策划和控制......................... .................. .. (26)8.2 产品和服务要求的确定 (27)8.2.1 顾客沟通............................................................... .. (27)8.2.2 与产品和服务有关的要求的确定................................... (27)8.2.3 与产品和服务有关的要求的评审 (27)8.3 产品和服务的设计与开发............................. .................. .. (27)8.3.1 总则 (27)8.3.2 设计和开发策划....................................... .. (28)8.3.3 设计和开发输入 (28)8.3.4 设计和开发控制 (28)8.3.5 设计和开发输出 (28)8.3.6 设计和开发变更 (29)8.4 外部供应产品和服务的控制 (29)8.4.1 总则 (29)8.4.2 外部供应的控制类型和程度 (29)8.4.3 外部供应商的信息 (29)8.5 产品和服务提供 (30)8.5.1 产品和服务提供的控制 (30)8.5.2 标识和可追溯性 (30)8.5.3 属于顾客或外部供应商的财产 (30)8.5.4 防护 (30)8.5.5 交付后活动 (30)8.5.6 变更控制 (31)8.6 产品和服务的放行 (31)8.7 不合格过程输出、产品和服务的控制 (31)9 绩效评价 (31)9.1 监视、测量、分析和评价 (31)9.1.2 顾客满意 (32)9.1.3 分析和评价 (32)9.2 内部审核 (32)9.3 管理评审 (33)10 改进 (33)10.1 总则 (33)10.2 不合格和纠正措施 (34)10.3 持续改进 (34)附录A(规范性附录)新结构、术语和概念的说明(省略) (35)附录B(规范性附录)质量管理原则(省略) (35)附录C(规范性附录)ISO10000 质量管理标准组合(略) (35)参考文献(省略) (35)0 引言 0.1 总则采用质量管理体系应该是组织的一项战略性决策。
ISO9001:2015完全翻译版

ISO9001:2015质量管理体系要求原文翻译完全版1 范围本标准为有下列需求的组织规定了质量管理体系要求:a)需要证实其具有稳定地提供满足顾客要求和适用法律法规要求的产品和服务的能力;b)通过体系的的有效应用,包括体系持续改进的过程,以及保证符合顾客和适用的法律法规要求,旨在增强顾客满意。
注 1:在本标准一中,术语“产品”仅适用于:预期提供给顾客或顾客所要求的商品和服务;注 2:法律法规要求可称作为法定要求。
2 规范性引用文件下列文件中的条款通过本标准的引用而构成本标准的条款。
凡是注日期的引用文件,只有引用的版本适用。
凡是不注日期的引用文件,其最新版本(包括任何修订)适用于本标准。
ISO9000:2015 质量管理体系-基础和术语3 术语和定义本标准采用ISO9000:2015 中所确立的术语和定义。
4 组织的背景环境4.1 理解组织及其背景环境组织应确定哪些与其宗旨和战略方向有关且影响质量管理体系实现其预期结果的能力的外部和内部情况。
组织应监视和评审这外部和内部情况的信息。
注 1:事宜可能是正面和负面的因素或要考虑的情况注 2:理解外部的环境,可以通过考虑源于国际、国家、地区的或本地的法律法规、技术、竞争、市场、文化、社会和经济环境的情况,促进对外部情境的了解。
注3:理解组织内部环境,可以通过考虑与价值、文化、知识和组织绩效有关的情况,促进对内部情境的了解。
4.2 理解相关方的需求和期望:出于对组织持续地提供满足顾客和适用法律法规要求的产品和服务的能力的影响或潜在影响,组织应确定:a)与质量管理体系有关的相关方;b)与质量管理体系有关的相关方的要求。
组织应监视和评审有关相关方及其有关要求的信息。
4.3 确定质量管理体系的范围组织应界定质量管理体系的边界和应用,以确定其范围。
在确定此范围时,组织应考虑:a)标准 4.1 条款中提到的内部和外部情况;b)标准 4.2 条款中涉及的有关相关方的要求;c)组织的产品和服务。
最新版质量管理体系程序文件(2015版)

程序文件汇编依据GB/T19001-2015idtISO9001:2015标准编制文件编号:HT-CX-2020版次状态:A/O发放编号:编制:行政部审核:批准:发布日期:2020年4月10日实施日期:2020年4月10日0.1目录1组织环境与相关方要求管理程序 (3)2风险和机遇的应对措施控制程序 (5)3人力资源控制程序 (8)4基础设施和过程运行环境控制程序 (11)5组织知识控制程序 (13)6沟通控制程序 (15)7文件控制程序 (17)8记录控制程序 (20)9运行策划和控制程序 (22)10产品和服务的要求控制程序 (24)11外部提供产品、服务和过程控制程序 (26)12销售和服务控制控制程序 (30)13组织更改管理控制程序 (32)14过程的监视和测量控制程序 (36)15产品的监视和测量控制程序 (38)16不合格品控制程序 (39)17顾客满意程度测量程序 (41)18分析和评价控制程序 (43)19内部审核程序 (46)20管理评审控制程序 (49)21改进控制程序 (52)1组织环境与相关方要求管理程序1目的为保证公司质量管理体系的策划能实现预期的结果,识别、监视并评审与公司的宗旨、战略方向相关的内外部环境;识别、监视并评审与公司质量管理体系有关的相关方期望或要求;根据内外部因素与相关方期望或要求,结合本公司已有的优势和劣势,识别出风险和机会;针对识别的风险和机会,策划应对措施。
2范围适用质量管理体系所有相关的部门与过程。
3职责3.1行政部:确定内外部环境因素和相关方期望或要求。
3.2总经理:批准风险和机会的应对措施。
3.3管理者代表:组织各部门进行内外部环境因素和相关方期望或要求的识别评价、并拟定应对措施,对结果进行审核整理。
3.4各部门:配合进行内外部环境因素和相关方期望或要求的识别评价、并拟定应对措施。
4程序4.1组织环境管理4.1.1在建立与持续改进质量管理体系时,公司将充分识别理解并考虑那些与公司的宗旨、战略方向相关,并影响公司实现质量管理体系预期结果能力的内部和外部环境。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
XXX有限公司质量管理体系程序文件
组织知识控制程序(中英文版)
Organizational knowledge control procedure
组织知识控制程序(中英文版)
Organizational knowledge control procedure
1、目的purpose
为了对知识实行统一、有效的控制和管理。
In order to unify knowledge, effective control and management.
2、范围scope
适用于公司内部知识的交流和共享的管理、外部知识管理、企业知识资产的管理。
Applicable to internal knowledge exchange and sharing management, external knowledge management, enterprise knowledge asset management.
3、职责Responsibility
3.1总经理:知识资料的限制级别的审批。
General Manager: limited level of approval of knowledge materials.
3.2企管部:负责组织知识资料的收集、整理、发布工作。
Enterprise Management Department: responsible for organizing the collection, collation and release of knowledge materials.
3.3其他部门:负责配合总务人事部提供相应的资料。
Other departments: responsible for supporting the General personnel Department to provide the corresponding information.
4、程序 procedure
4.1企管部: Department of Enterprise Management:
负责每半年组织各部门收集本部门的知识内容,并对收集过程提供协助。
Responsible for organizing the departments to collect the knowledge content of the department every six months, and provide assistance to the collection process
1)负责收集公司内部管理制度,公司信息公告。
Responsible for collecting the company's internal management system, company information bulletin.
2)负责外部国家法律法规,行业法规、标准等。
Responsible for external national laws and regulations, industry regulations, standards, etc.
3)负责公司知识产权、商誉等管理维护。
Responsible for the management and maintenance of intellectual property, goodwill, etc.
4)负责组织对各部门提供的知识信息资料进行整理,形成知识资产清单。
Responsible for organizing and organizing knowledge information materials provided by various departments to form a list of knowledge assets.
5)负责按限制级别在共享平台发布相关知识资料,发布的资料需为PDF扫描版式。
Responsible for publishing related knowledge on the shared platform according to the restriction level. The published information should be PDF scanning format.
6)负责组织审核组成员对相关更改资料进行重新整理。
Responsible for organizing audit team members to reorganize relevant changes.
7)负责公司知识资料的持续有效性管理。
每年12月对相关文件进行跟踪,确定均为最新版本。
Responsible for the continuous effectiveness management of the company's knowledge materials. Follow up the relevant documents on December each year and confirm that they are the latest version.
4.2管理者代表: Management representatives:
负责公司方针、目标、质量手册以及程序文件的收集。
Responsible for the collection of company policies, objectives, quality manuals and procedures.
4.3工程部:Engineering Department:。
