Heck反应及金属催化偶联反应
偶联反应在有机合成中的应用研究

偶联反应在有机合成中的应用研究引言:有机合成是研究有机分子之间的化学反应,以构建新的有机化合物,这在药物合成和材料科学领域具有重要的应用价值。
偶联反应是一类常用的有机合成方法,通过连接两个或多个有机分子,形成新的键,构建目标分子的分子骨架。
本文将讨论几种重要的偶联反应及其在有机合成中的应用研究。
一、金属催化的偶联反应金属催化的偶联反应是现代有机合成中最具代表性和广泛应用的方法之一。
其中最著名的是钯催化的偶联反应,如Suzuki偶联、Heck偶联和Negishi偶联等。
这些反应通过利用钯催化剂促使芳环和烯烃之间的偶联反应,具有高效、高选择性和底物广泛适应性的优点。
这些反应在药物合成和材料科学中得到了广泛的应用。
例如,Suzuki偶联反应被用于合成药物、农药和功能材料,而Heck偶联反应则被广泛应用于合成天然产物和聚合物材料。
二、碳-碳键形成反应碳-碳键形成反应是有机合成中另一类重要的偶联反应,其原理是通过碳原子之间的偶联反应来构建目标分子的骨架。
这些反应通常通过碱金属或过渡金属催化剂来实现。
最常用的碳-碳键形成反应是格氏反应和克鲁普斯基反应。
格氏反应通过将硝酚和醛缩合得到苯酚类化合物,而克鲁普斯基反应则通过将已有的碳酸盐与酸酐醇缩合得到β-酮酸盐。
这些反应广泛应用于药物合成和天然产物的合成。
三、氧化还原偶联反应氧化还原偶联反应是一类利用氧化还原反应构建碳-碳键的方法。
这些反应通常通过金属催化剂或有机小分子催化剂来实现。
最常用的氧化还原偶联反应是巴甫洛夫反应和维特igler反应。
巴甫洛夫反应通过将两个醛或酮经过氢转移催化之后结合形成α-羰基酮,而维特igler反应则是通过将亚砜和羧酸酐反应生成α-烯酮。
这些反应在合成脂肪酸、天然产物和有机化学品中具有重要的应用价值。
结论:在有机合成中,偶联反应为构建新的有机化合物提供了强大的工具。
金属催化的偶联反应、碳-碳键形成反应和氧化还原偶联反应是其中最重要和最常用的方法。
金属催化反应在有机合成中的应用

金属催化反应在有机合成中的应用随着有机化学的发展,金属催化反应成为了有机合成中不可或缺的一部分。
金属催化反应可以提供高效、高选择性、经济的合成方法,已成为有机合成中不可或缺的一部分。
在本文中,我们将会探讨金属催化反应在有机合成中的应用。
一、Pd催化反应1. Suzuki偶联反应Suzuki偶联反应是一种重要的碳-碳键形成反应。
它利用了Pd 的具有活性的交叉耦合机制,通过芳芯片内的针对芳芯片和芳基和芳芯片和芳酯化合物反应来形成碳-碳键。
Suzuki偶联反应已被广泛地应用于糖化学、生命有机化学和天然产物合成领域等。
使用催化剂和底物的种类以及反应条件、配体等条件的优化是Suzuki偶联反应成功的关键。
2. Heck反应Heck反应是一种发现于1972年的碳-碳键形成反应。
它利用Pd为催化剂,在氧气存在下将芳基溴化物和烯烃偶联。
Heck反应可以合成许多化合物,包括药物、担体、香料等。
3. 点击化学点击化学是21世纪最激动人心的新领域之一。
它是由Sharpless教授等人发明的,利用Pd催化百里醇和炔烃的化学反应,构成环状化合物。
这种反应具有高效性、高选择性和优良的底物范围等特点,成为抗癌药物和治疗癌症的前沿技术。
二、Ru催化反应1. 环氧化环氧化是一种在有机合成中应用广泛的方法,也是一种重要的氧化反应。
Ru是一种高效的催化剂,可以实现对基因环戊烷环氧化反应。
2. 吡啶脱氢在化学学科中,吡啶脱氢是一种在有机合成中广泛应用的反应。
这种反应可以通过氧气替代常用的氢气,来具有绿色和环保的特点。
使用Ru催化剂和新型配体可以高效实现吡啶脱氢。
三、其他金属催化反应1. Cu催化偶联反应Cu催化偶联反应是一种具有极大应用潜力的反应,其使用成本低、操作温和。
Cu催化偶联反应可以实现碳-碳键、键和碳-氮键等多种键合成。
2. Fe催化环化Fe催化环化是一种非常受欢迎的反应方式,是一种新型环化方法,具有底物范围广、环化度高、反应数量大等优点。
Heck反应及金属催化偶联反应

Heck reaction
通常把在碱性条件下钯
催化的芳基或乙烯基卤代物 和活性烯烃之间的偶联反应 称为Heck反应。自从20世纪 60年代末Heck 和Morizoki独 立发现该反应以来,通过对催 化剂和反应条件的不断改进 使其的应用范围越来越广泛, 使该反应已经成为构成C-C 键的重要反应之一。另外, Heck反应具有很好的Trans 选择性
OTIPS
O
N H
1,2,2,6,6-pentamethylpiperidine, DMA, 100 oC
N
H
1
2
A mixture of Pd2(dba)3·CHCl3 (360 mg, 0.347 mol), (s)-BINAP (504 mg, 0.809 mol), and N,N-dimethylacetamide (DMA, 21 mL) was stirred at room temperature for 65 min. To the resulting orange solution was added a solution of compound 1 (1.82 g, 3.51 mol), 1,2,2,6,6-pentamethylpiperidine (3.2 mL, 18 mmol), and DMA (18 mL), and the reaction was heated at 100 ℃ for 90 min. The result dark solution was poured into half-saturated aqueous NaHCO3 (100 mL) and extracted with ether (3 × 150 mL). The combined organic extracts were washed with brine (100 mL), dried (MgSO4), and concentrated, and the residue was purified by sgc (9:1 → 1:1 hexane-EtOAc) to give oxindole enoxysilane compound 2 (1.29 g, 94%) as a 98:2
卤代芳烃的c-c键耦合反应

卤代芳烃的c-c键耦合反应
卤代芳烃的C-C键耦合反应是一种在有机合成中构建新的碳碳键的重要方法。
这类反应通常涉及到过渡金属催化,尤其是钯催化剂,在卤代芳烃或卤代烯烃与另一分子(如烯烃、炔烃、硼酸或有机锌试剂等)之间形成新的C-C键。
以下是几种常见的卤代芳烃C-C键耦合反应:
1. Heck反应:这是一种卤代芳烃或卤代烯烃与烯烃在碱和钯催化下生成取代烯烃的反应。
2. Suzuki偶联反应:在这个反应中,卤代芳烃与活化的硼酸在钯催化剂作用下发生偶联,生成联苯类化合物。
这个反应对水不敏感,可以容忍多种活性官能团存在,并且副产物无毒且易于除去,适合工业化生产。
3. Negishi偶联反应:这是卤代芳烃与有机锌试剂在钯催化剂作用下进行的偶联反应,适用于一些其他方法难以偶联的底物。
4. 亲电交叉偶联反应:这种反应可以在两个不同亲电试剂间发生,通常是在电化学条件下,通过阴极还原生成碳负离子,然后与另一亲电试剂发生偶联。
此外,在选择耦合反应时,需要考虑底物的活性、位阻效应以及电子效应。
一般来说,碘代和三氟甲磺酸盐的活性较高,溴代次之,氯代最低。
对于贫电子的卤代芳烃,可能需要过量使用以提高反应效果。
总的来说,这些耦合反应由于条件温和、效率高,已经成为有机合成中不可或缺的工具,广泛应用于药物合成、材料科学等领域。
有机化学四大偶联反应

有机化学四大偶联反应有机化学中的偶联反应是合成有机分子的重要方法之一,广泛应用于药物合成、材料科学等领域。
以下介绍有机化学的四大偶联反应。
第一种偶联反应是格氏偶联反应(Giemsa),它是20世纪初由法国化学家格氏首次提出的。
这种反应是通过有机金属化合物与芳香化合物进行反应,形成碳-碳键。
通常使用有机锡化合物和芳香卤化物作为底物,在碱性条件下,在加热的情况下进行反应。
这种反应是高度选择性的,并且能够合成具有天然产物活性的有机化合物。
第二种偶联反应是索尼赫德烯偶联反应(Suzuky-Miyaura),该反应是由日本化学家索尼赫德和宫浦在20世纪70年代提出的。
这种反应是通过有机金属化合物与芳香卤化物进行反应,形成碳-碳键。
通常使用有机锌化合物或有机硼化合物和芳香卤化物作为底物,在碱性条件下,在加热的情况下进行反应。
索尼赫德烯偶联反应是高度选择性的合成方法,可以合成具有天然产物活性的有机化合物。
第三种偶联反应是肾上腺素偶联反应(Heck),是由丹麦化学家肯格赫首次提出的。
这种反应是通过有机金属化合物与不饱和化合物(通常是烯烃)进行反应,形成碳-碳键。
通常使用有机铜化合物和不饱和化合物作为底物,在碱性条件下,在加热的情况下进行反应。
肾上腺素偶联反应具有高效、高选择性和底物适用范围广的特点,广泛应用于药物合成和天然产物的全合成。
第四种偶联反应是叠氮偶联反应(Azide-Alkyne),又称为"CuAAC"反应,由美国化学家哈斯利首次提出。
在这种反应中,叠氮化合物与炔烃发生反应,生成1,4-二取代三氮唑化合物。
这种反应是通过铜催化剂的存在实现的,即铜催化的炉二碳合成反应。
这种反应具有高效、高选择性和底物适用范围广的特点,并且它是药物合成中的重要方法。
以上是有机化学的四大偶联反应的介绍。
这些偶联反应不仅拓宽了有机合成的范围,还为合成具有特定结构和功能的有机化合物提供了重要的手段。
研究人员可以根据这些偶联反应的特点选择合适的反应体系,并结合自己的研究目标进行合成路线的设计。
有机合成中的金属催化反应

有机合成中的金属催化反应金属催化反应是有机合成领域中一种重要的合成策略。
通过金属催化反应,可以实现高效、高选择性的化学转化,为有机化学合成提供了广阔的发展空间。
本文将介绍金属催化反应的原理、应用以及一些成功的案例。
一、金属催化反应的原理金属催化反应主要是指在有机化合物的转化过程中,通过金属配合物作为催化剂来促进反应的进行。
金属催化反应的原理可以归结为以下几个关键步骤:1. 活化底物:金属催化剂能够与底物形成键合,从而活化底物,使其更容易进行反应。
这种活化可以发生在底物的氢、氧、氮等原子上,也可以通过有机分子的C-C和C-X键上发生。
2. 氧化还原:金属催化剂在反应过程中可以参与氧化还原反应,促进底物的氧化或还原。
金属催化剂作为氧化剂或还原剂可以转移电子,从而改变底物的电子状态,使其发生化学转化。
3. 配位或成键:金属催化剂与底物之间发生配位或成键反应,形成活性中间体。
这些中间体在反应过程中发挥重要作用,可以进一步催化底物的转化。
二、金属催化反应的应用金属催化反应在有机合成中具有广泛的应用。
能够实现的转化类型包括但不限于碳-碳键、碳-氮键、碳-氧键、碳-硫键以及氢转移反应等。
通过选择合适的金属催化剂以及反应条件,可以高效地合成各种有机化合物。
1. 碳-碳键形成:金属催化反应可以实现碳-碳键的形成,包括交叉偶联反应、烯烃和炔烃的环化反应、直接烷基化等。
这些反应对于药物和天然产物的合成具有重要意义。
2. 碳-氮键形成:金属催化反应在碳-氮键形成反应中也发挥着重要的作用,例如羟胺和羧酸的缩合反应、亲电取代反应以及氨基化反应等。
这些反应可以方便地合成含有氮元素的有机化合物。
3. 碳-氧键形成:金属催化反应可以实现碳-氧键的形成,例如醇和醚的合成、酯和酸的加成反应等。
这些反应对于合成酯、酮等化合物具有重要意义。
4. 碳-硫键形成:金属催化反应还可以实现碳-硫键的形成,包括硫醚的合成以及烯烃和硫醇的环化反应等。
有机合成中的金属催化反应研究进展

有机合成中的金属催化反应研究进展有机合成是一门极其重要的化学学科,它是用于制备基础化合物、添加剂、催化剂、药物等大量有机化合物的学科。
与传统的常规有机合成方法相比,金属催化反应作为一种高效且环保的有机化合物制备方法,近年来得到了广泛应用。
本文将从金属催化反应机理的解析、金属催化剂的研究进展、金属催化反应在有机合成中的应用等方面综合探讨有机合成中的金属催化反应研究进展。
一、金属催化反应机理的解析金属催化反应机理指的是在特定的催化剂作用下,无机物或有机物能够发生化学反应。
金属催化反应机理包括配位结构、配体效果和反应动力学等方面的研究。
1. 配位结构金属催化剂由金属原子和配体构成,其中金属原子是反应中心,配体扮演辅助和稳定反应中心的角色。
不同的配体对反应活性、反应选择性及催化剂稳定性均产生重要影响。
2. 配体效应配体是影响金属催化剂选择性和反应活性的重要因素。
不同配体在反应体系中具有不同的电子性质、空间位阻及反应中心的性质,具有很大的影响。
3. 反应动力学反应动力学研究主要包括催化剂与底物反应,以及反应过程中中间体的性质和作用等方面。
反应动力学研究对于反应条件的优化,催化剂的选择和设计等都具有重要意义。
二、金属催化剂的研究进展金属催化剂是金属有机化学研究中的重要组成部分。
目前,常用的催化剂主要包括钯、铂、铑、铜、钼、铁等金属。
1. 钯催化剂钯催化反应已成为有机合成领域的研究热点。
钯催化合成芳香化合物、脂肪族化合物、螺环化合物等已有了重要进展。
尤其是钯催化的交叉偶联反应、氨基甲酸酯基烷基化反应、催化升格等反应具有广泛的应用前景。
2. 铂催化剂铂催化反应广泛应用于有机合成和生物医药领域。
铂催化的烯烃同分异构化、有机合成中的糖苷化反应、DNA连接反应等已取得了重要进展。
3. 铑催化剂铑是一种高效的催化剂,在不对称催化剂的合成和应用中具有广泛的应用前景。
铑催化的孪晶化、精细化学品和生理活性物质的合成等领域取得了重要进展。
过渡金属催化反应的基础和应用

过渡金属催化反应的基础和应用过渡金属催化反应是现代有机化学领域的一个重要分支。
它以过渡金属作为催化剂,可以有效促进各种有机反应的进行,从而实现高效、高选择性和绿色化学合成。
一、过渡金属催化反应的基础过渡金属催化反应的基础在于过渡金属催化剂具有一定的电子调控和立体效应。
其特点在于过渡金属能够参与反应,并且能够在反应过程中发挥关键作用。
同时,过渡金属催化反应还需要考虑反应中反应物的选择性、可控性和立体匹配性等因素。
1. 过渡金属的电子调控作用在有机反应中,催化剂通常需要通过调控反应物的电子结构,将其转化为更容易与其他反应物作用的中间体。
而过渡金属催化反应的催化剂,则能够通过调控反应物的活化能和键能,实现对反应的选择性控制。
这种电子调控作用与过渡金属的电子排布有关,其中有些过渡金属具有不对称电子密度分布。
例如,palladium配合物具有单个电子分布不均的d8电子结构,这使得palladium成为许多有机反应的优良催化剂。
2. 过渡金属的立体效应在有些反应中,由于反应物之间的取向关系或者过渡态的立体构型等因素,反应的产物结构及其选择性会受到很大影响。
而过渡金属催化剂能够通过调控反应物的旋转和取向,实现反应产物的立体选择性控制。
此外,过渡金属催化剂在反应中会发挥配体效应,即通过改变配体结构来影响活化剂和底物的相互作用。
这种立体调控效应可以通过改变配体电荷、主、辅配体之间的取向关系等因素来实现。
二、过渡金属催化反应的应用过渡金属催化反应在有机合成中广泛应用,可用于构建多种化学键、环化反应、开环反应等。
以下具体介绍一些常见的过渡金属催化反应及其应用。
1. Suzuki反应Suzuki反应是一种通过palladium催化的偶联反应,常用于构造芳基-碳基键。
该反应的底物是芳基卤化物和芳基硼酸酯,产物为具有芳香性的偶联物。
2. Heck反应Heck反应也是一种通过palladium催化的偶联反应,常用于构造芳基-烯基键。
有机化学四大偶联反应

有机化学四大偶联反应有机化学是研究碳元素及其化合物的科学,是化学学科中的一个重要分支。
在有机化学中,有机合成反应是一项重要的研究内容。
有机化学四大偶联反应是有机合成中常用的四种反应类型,包括:Suzuki偶联反应、Stille偶联反应、Heck偶联反应和Sonogashira 偶联反应。
这些反应在有机合成中起到了重要的作用,为有机化学的发展做出了巨大的贡献。
我们来介绍Suzuki偶联反应。
Suzuki偶联反应是一种重要的芳香化合物合成方法,它是基于钯催化剂的反应。
该反应将有机硼酸酯和有机卤化物或磺酸酯作为底物,在适当的条件下,经过交叉偶联反应,生成目标产物。
Suzuki偶联反应在药物合成和材料科学中有着广泛的应用,可以高效地合成出具有重要生物活性和物理性质的化合物。
接下来是Stille偶联反应,它是一种重要的碳-碳键形成反应。
该反应是通过钯催化剂催化下的亲核取代反应来实现的,底物包括有机卤化物和有机锡化合物。
Stille偶联反应具有底物适用范围广、反应条件温和等优点,在天然产物的合成和药物研发中得到了广泛的应用。
第三种偶联反应是Heck偶联反应,它是一种重要的芳香化合物合成方法。
该反应是通过钯催化下的芳香取代反应实现的,底物包括有机卤化物和烯烃。
Heck偶联反应是一种高效、高选择性的反应,在药物研发和天然产物的合成中得到了广泛的应用。
最后是Sonogashira偶联反应,它是一种重要的炔烃合成方法。
该反应是通过钯催化下的炔烃与有机卤化物的偶联反应实现的。
Sonogashira偶联反应可以高效地合成炔烃化合物,对于合成具有炔烃结构的药物和功能材料具有重要意义。
在有机化学四大偶联反应中,每一种反应都有其独特的应用领域和优点。
这些反应的发展和应用为有机合成提供了新的思路和方法,为有机化学的发展做出了重要贡献。
总结起来,有机化学四大偶联反应包括Suzuki偶联反应、Stille偶联反应、Heck偶联反应和Sonogashira偶联反应。
现代有机合成方法与技术

现代有机合成方法与技术
现代有机合成方法与技术是指通过化学反应在实验室或工业生产
中合成有机化合物的方法和技术。
随着化学的发展和进步,现代
有机合成方法与技术得到了极大的发展和创新。
以下列举了一些
常见的现代有机合成方法与技术:
1. 金属催化反应:如铂、钯、铜等金属催化下的氢化、氧化、氨化、烯烃与烯烃的偶联等反应,具有高效、高选择性和底物范围
广的特点。
2. 环化反应:包括常见的合成环状化合物的方法,如氧化硅法、
磷化硅法、氰化法等,可以合成各种环状化合物。
3. 偶联反应:如格氏反应、Suzuki反应、Heck反应等,可以将碳-
碳键或碳-氮键等形成新的键连接两个或多个分子。
4. 碳氢键功能化反应:如分子间芳香基的碳氢键活化反应,通过
金属催化作用,将芳香化合物中的碳氢键功能化为官能团,实现
目标化合物的合成。
5. 能源节约合成:如超临界流体、微波辐射等条件下的有机合成,可以节省能源、缩短反应时间,并且还可以改善反应的选择性和
产率。
6. 生物催化法:利用酶或生物催化剂催化有机反应,具有温和反
应条件、高选择性和底物范围广等特点。
7. 无机合成法:利用无机化学合成有机化合物,如Grignard试剂、化学还原法等。
构建碳碳三键的方法(一)

构建碳碳三键的方法(一)构建碳碳三键介绍碳碳三键是有机化学中一种非常重要的化学键,具有很高的稳定性和特殊的化学性质。
在有机合成中,构建碳碳三键是一项非常重要的工作。
本文将介绍几种常见的构建碳碳三键的方法。
方法一:烯烃加成烯烃加成是最常见的构建碳碳三键的方法之一。
当碳原子上的双键与另一个分子反应时,可以形成新的碳碳三键。
常见的烯烃加成反应有马可夫尼科夫规则和安莫夫规则等。
方法二:金属催化的偶联反应金属催化的偶联反应是一种有效构建碳碳三键的方法。
在该反应中,金属催化剂可以促使两个分子发生偶联反应,生成新的碳碳三键。
典型的金属催化偶联反应有Suzuki偶联和Negishi偶联等。
方法三:Carbene插入Carbene插入是一种常用的构建碳碳三键的方法。
Carbene分子可以插入到碳碳双键中,形成新的碳碳三键,并释放出稳定的分子。
此方法可在有机合成中产生多样的碳碳三键。
方法四:自由基反应自由基反应是构建碳碳三键的又一常见方法。
在自由基反应中,自由基通过主链上的断裂、迁移或加成,可以生成新的碳碳三键。
典型的自由基反应包括链转移、自由基加成和氢氟化反应等。
方法五:炔基化合物的反应炔基化合物的反应是一种特殊的构建碳碳三键的方法。
通过炔烃与其他试剂反应,可以构建碳碳三键并形成新的有机化合物。
常见的炔基化合物反应有Sonogashira偶联和Glaser偶联等。
结论构建碳碳三键是有机合成中的重要步骤之一。
在本文中,我们介绍了烯烃加成、金属催化的偶联反应、Carbene插入、自由基反应以及炔基化合物的反应等几种常见的构建碳碳三键的方法。
这些方法在有机化学合成中发挥着重要作用,为合成有机化合物提供了有效的手段。
希望本文对你在有机化学研究中的学习和工作有所帮助。
方法六:分子内环化反应分子内环化反应是一种特殊的构建碳碳三键的方法,也被广泛应用于有机合成领域。
在该反应中,一个分子内部的官能团与分子其他部分反应,形成新的碳碳三键。
有机合成中的金属有机化学反应应用

有机合成中的金属有机化学反应应用金属有机化学反应是有机合成领域中一类重要的反应类型,通过引入金属作为反应媒介或催化剂,可以实现特定的化学转化,拓展了有机合成的范围和效率。
本文将介绍金属有机化学反应在有机合成中的应用,并探讨其在合成策略和新化合物合成中的重要作用。
一. 金属有机化学反应金属有机化学反应是指金属与有机物之间的化学反应,通常涉及金属与有机物中的碳-碳、碳-氧、碳-氮等化学键的形成或断裂。
金属有机化学反应可分为一般金属有机反应和金属有机催化反应两类。
1. 一般金属有机反应一般金属有机反应是指以金属作为反应媒介或试剂来改变有机物分子结构的反应。
典型的一般金属有机反应包括金属与有机卤化物的反应、金属与有机硼化合物的反应、金属与有机锡化合物的反应等。
这些反应通常能实现碳-碳键的形成或断裂,从而在有机合成中起到关键的作用。
2. 金属有机催化反应金属有机催化反应是指利用金属催化剂来促进有机物之间的反应。
金属催化剂可以通过提供反应表面、活化底物、调节反应的路径等方式,显著加速反应速率,提高反应的选择性和收率。
常见的金属有机催化反应包括金属催化的偶联反应、金属催化的环化反应、金属催化的氧化还原反应等。
二. 金属有机化学反应在有机合成中的应用金属有机化学反应在有机合成中具有广泛的应用,可以用于合成复杂的天然产物、药物和功能材料,以及合成新型的共轭体系和手性化合物等。
以下将介绍几个金属有机化学反应在有机合成中的典型应用。
1. 金属有机催化的偶联反应金属有机催化的偶联反应是有机合成中最常用的反应之一。
该反应通过金属催化剂引发的碳原子偶联,实现不同碳基团之间的键合,从而构建复杂的有机分子结构。
典型的金属有机催化的偶联反应有Suzuki偶联、Negishi偶联、Heck反应等,这些反应在药物合成、天然产物合成以及有机电子材料的合成中发挥了重要作用。
2. 金属有机化学反应的环化反应金属有机化学反应的环化反应是指通过金属催化剂促使有机分子中的碳原子与其他原子或官能团形成环状结构的反应。
有机合成中的金属有机催化反应

有机合成中的金属有机催化反应有机合成是化学领域中的一个重要分支,它涉及合成和制备有机化合物的方法和策略。
而金属有机催化反应是有机合成中一种十分有效的方法。
本文将探讨金属有机催化反应在有机合成中的应用与意义,以及一些常见的金属有机催化反应。
一、金属有机催化反应的基本原理与应用1.1 基本原理金属有机催化反应是指在反应中使用金属有机化合物作为催化剂,起到促进和加速反应的作用。
一般而言,金属有机催化反应可以分为两类:配位催化和氧化还原催化。
配位催化是指金属有机物与底物发生配位作用,形成一个配位体-金属络合物。
这个络合物可以提供亲电性的金属中心,从而使底物发生反应。
例如,Pd(0)催化剂在Suzuki反应中起到了重要作用。
Pd(0)与底物和配体(通常是磷配体)发生配位,形成一个稳定的Pd(II)配位体络合物。
这个络合物可以与溴化物交换反应,生成最终的产物。
氧化还原催化是指催化剂在反应过程中发生氧化还原反应,从而使底物发生反应。
常见的氧化还原催化反应包括羰基还原、烯烃和炔烃的氢化等。
例如,Wilkinson催化剂在烯烃的氢化反应中起到了重要作用。
Wilkinson催化剂是由RhCl(PPh3)3和NaBH4组成的络合物,它能够与烯烃发生氧化还原反应,将烯烃还原为烷烃。
1.2 应用意义金属有机催化反应在有机合成中具有重要的应用意义。
首先,金属有机催化反应可以提供高效、高选择性的反应方法。
相比于其他催化剂,金属有机催化剂通常具有高反应活性和催化活性。
这使得金属有机催化反应在有机合成中能够实现一些常规方法无法实现的反应。
其次,金属有机催化反应可以进行复杂的生成物构建。
有机合成中的一些复杂结构需要经过多步反应才能合成,而金属有机催化反应可以直接将简单的起始物转化为复杂的产物,从而大大简化了合成的过程。
最后,金属有机催化反应在绿色化学合成中具有潜在的应用。
传统合成方法通常需要高温、高压或有毒的底物和试剂,而金属有机催化反应通常在较温和的条件下进行。
交叉偶联反应的类型

交叉偶联反应的类型1.引言1.1 概述交叉偶联反应(Cross-coupling reaction)是一类重要的有机合成反应,它可以在两个或更多的有机分子之间建立键合,形成新的混合物。
在交叉偶联反应中,通常会使用过渡金属催化剂来引发反应,并使反应发生在选择性、高效的条件下。
交叉偶联反应源于20世纪70年代的发现,由于其广泛的应用领域和高度的化学选择性,成为了有机合成领域中的重要工具之一。
不仅可以构建碳-碳键,还可以构建碳-氮键、碳-氧键等重要的化学键。
它不仅可以用于药物合成、材料化学、天然产物合成等多个领域,还可以通过调整反应条件和催化剂选择,实现对底物的高度选择性修饰。
交叉偶联反应的类型繁多,常见的包括苯基-锌、叠氮-钯、硼基-钯、锡基-钯等反应类型。
每种类型的反应都有其独特的特点和应用领域,具体选择哪种类型的反应也需要根据具体的研究目的和底物结构来确定。
本文将详细介绍交叉偶联反应的各种类型,并重点阐述它们的反应机理、优缺点以及在有机合成中的应用。
通过对不同类型交叉偶联反应的比较和分析,有助于读者更好地理解和掌握这一重要的有机合成工具。
为了更好地组织内容,下文将根据各个类型的交叉偶联反应进行分类和详细介绍。
1.2 文章结构文章结构部分的内容可以按照以下方式编写:文章结构本文共分为引言、正文和结论三个部分。
引言部分概述了交叉偶联反应的背景和意义,介绍了文章的结构和目的;正文部分详细阐述了两种交叉偶联反应类型的要点;结论部分对全文进行总结,并对未来的研究方向进行展望。
正文部分按照交叉偶联反应类型分为两个小节,分别介绍了交叉偶联反应类型1和类型2。
每个小节中又分别列出了要点1和要点2,以便更好地说明交叉偶联反应的特点和应用。
通过以上结构的安排,本文能够完整而清晰地呈现交叉偶联反应的类型及其相关要点,使读者能够更好地理解和掌握这一研究领域。
目的部分的内容可以按照以下方式进行撰写:1.3 目的本文的目的是探讨交叉偶联反应的类型。
有机化学基础知识点整理偶联反应与交叉偶联反应

有机化学基础知识点整理偶联反应与交叉偶联反应有机化学基础知识点整理:偶联反应与交叉偶联反应有机化学是研究有机物结构与特性的科学,其中偶联反应和交叉偶联反应是有机合成中常用的重要手段,本文将对这两种反应进行基础知识点整理。
一、偶联反应偶联反应是指两个有机分子中的两个不同官能团在反应条件下发生连接形成新的键,从而生成一个新的有机分子。
常见的偶联反应有Heck反应、Suzuki反应、Stille反应等。
1. Heck反应Heck反应是通过钯催化下的芳香化合物与烯烃发生的偶联反应,生成具有烯烃结构的芳香化合物。
该反应需要碱性条件和适量的氧气存在。
反应机理包括反应前的氧化加成、钯催化下的反应、脱氧等步骤。
2. Suzuki反应Suzuki反应是通过钯催化下的芳香化合物与硼酸酯发生的偶联反应,生成具有芳香环和烷基或芳基基团的化合物。
该反应需有碱性条件和无氧环境。
反应机理包括反应前的亲核加成、钯催化下的反应、脱氧等步骤。
3. Stille反应Stille反应是通过钯催化下的芳香化合物与有机锡化合物发生的偶联反应,生成具有烷基或芳基基团的化合物。
该反应需有碱性条件、无氧环境和适量的溴化物存在。
反应机理包括反应前的亲核加成、钯催化下的反应、脱溴等步骤。
二、交叉偶联反应交叉偶联反应是指两个不同有机物之间的偶联反应,生成具有两个不同基团的化合物。
常见的交叉偶联反应有Negishi反应、Kumada反应、Suzuki-Miyaura反应等。
1. Negishi反应Negishi反应是通过钯催化下的有机锌化合物和卤代化物发生的交叉偶联反应,生成具有不同基团的化合物。
该反应需有碱性条件和适量的酸存在。
反应机理包括反应前的亲核加成、钯催化下的反应、脱卤等步骤。
2. Kumada反应Kumada反应是通过钯催化下的有机镁卤化物和卤代化物发生的交叉偶联反应,生成具有不同基团的化合物。
该反应需有碱性条件和无氧环境。
反应机理与Negishi反应类似。
HECK 金属钯 串联反应 金属催化

1 - 苄基- 5 -(2 - 溴- 乙基)- 十氢-苯并[cd]〕吲哚-2-酮的合成俞可(巢湖学院化学化工与生命科学学院,安徽巢湖238000)1 引言多步骤串联反应是通过将原先多步独立合成的反应组合成为一个合成的操作,金属钯催化的多步串联反应近年来得到了广泛的应用。
1.1 串联反应许多复杂分子的合成经常需要多步完成,涉及繁琐的分离和提纯。
从经济和环保角度看,有必要减少步骤, 最大化地避免中间体的分离与提纯,具有这种合成理念的反应就是通常所说的串联反应(tandem reactions), 串联反应不是在一个反应瓶内简单地接连进行二步独立反应,而是第一反应生成的活泼中间体接着进行第二步、第三步的反应[1]。
串联反应在有机合成中具有以下优点:串联反应的中间体不需分离,直接用于原位反应,从而简化了操作步骤、对于敏感的、不稳定的中间体,这一优点尤为突出、串联反应减少了溶剂、洗脱剂的用量和副产物的产生,有利于环保、串联反应经常可以得到独特的化学结构,大多具有很高的选择性,所以近年来在有机合成领域得到了广泛的应用。
[2]串联反应一般可分为串联加成反应、串联取代反应、串联环化反应和串联重排反应以及金属催化的串联反应五大类。
1.1.1加成反应在众多串联加成反应中,Baylis-Hillman反应(BH)最具代表性(Scheme 1)。
早期的Baylis-Hillman反应(BH),一直没有被广泛接受和应用,直到2005年,由Kristin E. Price等人根据物理数据提出的机理才被人广泛接受。
[3]NN +OOMeHN NOO HH ArO HArOArONN O O H ArO NN O O Ar O Me Me Me H NN ArOHOO Ar O MeScheme 11.1.2 取代反应串联取代反应我们以咪唑并[1,5-a]吡啶羧酸酯衍生物的合成为例:王建武等发现了咪唑甲醛与γ-2溴代巴豆酸酯在弱碱条件下首先进行亲核取代反应,然后生成γ-2碳负离子并转移到α-2位,再进行分子内亲核进攻成环、脱水的串联反应(Scheme 2),这一发现为咪唑并[1,5-a]吡啶羧酸酯衍生物的合成提供了一种新的方法。
偶联反应
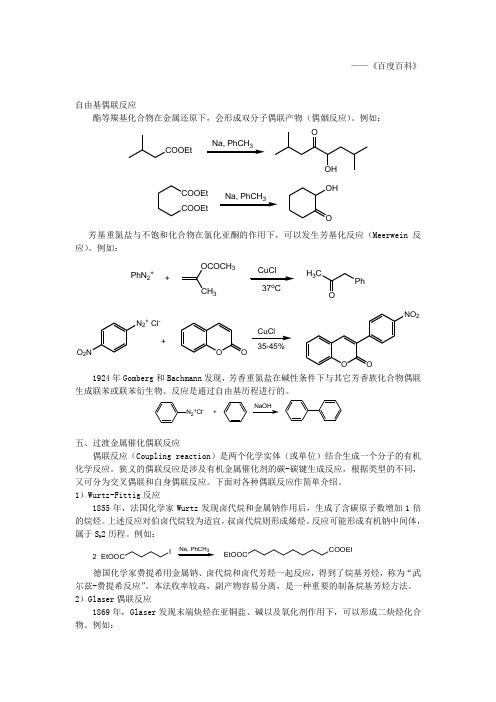
——《百度百科》自由基偶联反应酯等羰基化合物在金属还原下,会形成双分子偶联产物(偶姻反应)。
例如:COOEt3OOHCOOEtCOOEt OHO 3芳基重氮盐与不饱和化合物在氯化亚酮的作用下,可以发生芳基化反应(Meerwein 反应)。
例如:PhN 2++OCOCH 3CH 337oCH 3CPhON 2+ Cl-O 2NOOOONO 235-45%+CuCl1924年Gomberg 和Bachmann 发现,芳香重氮盐在碱性条件下与其它芳香族化合物偶联生成联苯或联苯衍生物。
反应是通过自由基历程进行的。
N 2+Cl -+五、过渡金属催化偶联反应偶联反应(Coupling reaction )是两个化学实体(或单位)结合生成一个分子的有机化学反应。
狭义的偶联反应是涉及有机金属催化剂的碳-碳键生成反应,根据类型的不同,又可分为交叉偶联和自身偶联反应。
下面对各种偶联反应作简单介绍。
1)Wurtz-Fittig 反应1855年,法国化学家Wurtz 发现卤代烷和金属钠作用后,生成了含碳原子数增加1倍的烷烃。
上述反应对伯卤代烷较为适宜,叔卤代烷则形成烯烃。
反应可能形成有机钠中间体,属于S N 2历程。
例如:EtOOCI2Na, PhCH 3COOEtEtOOC德国化学家费提希用金属钠、卤代烷和卤代芳烃一起反应,得到了烷基芳烃,称为“武尔兹-费提希反应”。
本法收率较高,副产物容易分离,是一种重要的制备烷基芳烃方法。
2)Glaser 偶联反应1869年,Glaser 发现末端炔烃在亚铜盐、碱以及氧化剂作用下,可以形成二炔烃化合物。
例如:4O 260%3)Ullmann 反应Ullmann 偶合反应是有机合成中构建碳—碳键最重要的方法之一。
Ullmann 偶合反应首次报道1901年, 它通常是利用铜作为催化剂, 催化卤代芳烃发生偶合反应生成联苯及其衍生物。
一般反应式为:2 ArXPd(0)或Pd(II)X= Cl 、Br 、I Ar-Ar目前该反应的底物范围、反应条件以及催化剂等都有了较大的改进。
偶联反应
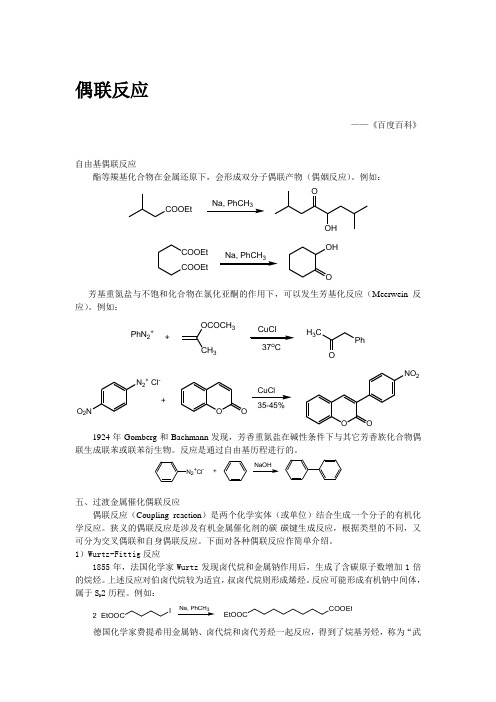
偶联反应——《百度百科》自由基偶联反应酯等羰基化合物在金属还原下,会形成双分子偶联产物(偶姻反应)。
例如:COOEt3OOHCOOEtCOOEt OHO 3芳基重氮盐与不饱和化合物在氯化亚酮的作用下,可以发生芳基化反应(Meerwein 反应)。
例如:PhN 2++OCOCH 3337o CH 3CPhON 2+ Cl-O 2NOOOONO 235-45%+CuCl1924年Gomberg 和Bachmann 发现,芳香重氮盐在碱性条件下与其它芳香族化合物偶联生成联苯或联苯衍生物。
反应是通过自由基历程进行的。
N 2+Cl -+五、过渡金属催化偶联反应偶联反应(Coupling reaction )是两个化学实体(或单位)结合生成一个分子的有机化学反应。
狭义的偶联反应是涉及有机金属催化剂的碳-碳键生成反应,根据类型的不同,又可分为交叉偶联和自身偶联反应。
下面对各种偶联反应作简单介绍。
1)Wurtz-Fittig 反应1855年,法国化学家Wurtz 发现卤代烷和金属钠作用后,生成了含碳原子数增加1倍的烷烃。
上述反应对伯卤代烷较为适宜,叔卤代烷则形成烯烃。
反应可能形成有机钠中间体,属于S N 2历程。
例如:EtOOCI23COOEtEtOOC德国化学家费提希用金属钠、卤代烷和卤代芳烃一起反应,得到了烷基芳烃,称为“武尔兹-费提希反应”。
本法收率较高,副产物容易分离,是一种重要的制备烷基芳烃方法。
2)Glaser 偶联反应1869年,Glaser 发现末端炔烃在亚铜盐、碱以及氧化剂作用下,可以形成二炔烃化合物。
例如:4260%3)Ullmann 反应Ullmann 偶合反应是有机合成中构建碳—碳键最重要的方法之一。
Ullmann 偶合反应首次报道1901年, 它通常是利用铜作为催化剂, 催化卤代芳烃发生偶合反应生成联苯及其衍生物。
一般反应式为:2 ArXPd(0)或Pd(II)X= Cl 、Br 、I Ar-Ar目前该反应的底物范围、反应条件以及催化剂等都有了较大的改进。
化学反应中的有机金属催化研究

化学反应中的有机金属催化研究自从20世纪以来,有机金属催化研究领域得到了广泛的关注和发展。
有机金属催化的研究属于有机化学和配位化学交叉领域,是一项颇具挑战的科学研究。
随着有机金属催化的理论研究逐渐深入,它的应用范围也不断拓展,成为有机化学发展的重要组成部分之一。
一、有机金属催化的起源1939年,人们发现钯盐可以催化石墨与乙烯反应。
钯盐催化乙烯与石墨的反应,开始了有机金属催化的研究历程。
此后,人们又发现许多其他的过渡金属也可以催化有机反应,从而开辟了有机金属催化反应的新路。
20世纪70年代以后,有机金属催化反应的研究逐步深入,完善了有机金属催化的理论基础,促进了这一领域的快速发展。
二、有机金属催化原理有机金属催化反应的基础是配合物现象。
金属催化剂主要是通过与反应物中的有机分子发生配位反应,形成有机金属化学中间体,然后通过反应中间体进行反应。
该过程不仅包括与反应物分子的配位发生消化,还包括已经与反应物分子配位的金属离子与分子间的化学反应和自由基反应产生的中间体的催化作用。
三、有机金属催化反应的分类有机金属催化反应的分类是根据有机金属催化剂的种类进行划分的。
它们包括:配合物催化(Pd、Rh、Ir)、半夹心催化(Co、Ni、Fe)、砷基催化(As)等。
其中,配合物催化剂是应用最广泛的一种,也是有机合成中最热门的反应类型。
四、有机金属催化在有机合成中的应用相较于传统的化学反应,有机金属催化反应的优点明显,例如反应温度低、效率高、选择性广、废产物少等。
因此,有机金属催化在化学合成中具有广泛的应用前景。
它广泛应用于合成烯烃、芳香族化合物、脂肪族化合物等多个领域。
以下是几个有名的有机金属催化反应:(1)Β-羰基化反应:由醛羰化成为酮,氧离子具有还原性,借助利用有机金属催化剂的氧化还原反应,将醛与酮相互转化的反应。
(2)Heck 反应:在有机金属催化剂的催化下,芳基卤化物与烯烃反应,发生反向的β-消除产生的为一种新的芳香族化合物。
染料合成催化剂

染料合成催化剂染料合成催化剂是在染料制备过程中使用的一种化学物质,它能够加速化学反应的速率,使原料更快地转化为目标染料分子。
催化剂通过降低反应的活化能来实现这一作用,而不被反应本身消耗。
在染料工业中,催化剂的应用对于提高合成效率、降低成本、减少环境污染和提升产品质量具有重要意义。
染料合成涉及到多种化学反应,包括偶联反应、还原反应、氧化反应、重氮化反应等。
在这些反应中,催化剂可以是无机物质,如金属盐、酸或碱;也可以是有机物质,如有机金属络合物、酶等。
以下是一些常见的染料合成催化剂及其应用:1. 金属催化剂:贵金属如钯(Pd)、铂(Pt)、铑(Rh)等的络合物常用于催化偶联反应,如Suzuki反应、Heck反应等,这些反应是构建复杂芳香结构的关键步骤。
非贵金属催化剂如铁(Fe)、铜(Cu)等也在染料合成中有所应用。
2. 酸催化剂:硫酸(H2SO4)、盐酸(HCl)、醋酸(CH3COOH)等无机酸或有机酸可以催化某些染料的合成反应,如重氮化反应中常用盐酸作为催化剂。
3. 碱催化剂:氢氧化钠(NaOH)、碳酸钠(Na2CO3)等碱性物质可用于催化某些缩合反应,如合成靛蓝类染料时就需要碱性催化剂。
4. 生物催化剂:酶作为一种高效的生物催化剂,可以在温和的条件下催化特定的化学反应。
例如,过氧化物酶可以用于合成某些类型的染料。
5. 有机金属催化剂:有机金属化合物如有机锡、有机锌等也可以作为染料合成的催化剂,特别是在形成某些特殊化学键时表现出独特的催化活性。
在选择催化剂时,需要考虑反应的类型、所需的选择性、成本、环境影响以及催化剂的回收和再利用等因素。
理想的催化剂应具有高活性、高选择性、稳定性好、易于分离和可重复使用的特点。
随着绿色化学的发展,环境友好型催化剂的研发和应用越来越受到重视。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
总的说来,Heck反应可以分为两大类:分子内反应 和分子间反应。第一篇该反应的报道是Heck 在1972年 发表的。
I +
Pd(OAc)2 (20 mol%) nBu3N (1 eq)
NMP, 100°C, 2h
Nolley, J.P.; Heck, R. F.; Tetrahedron 1972, 37, 2320
2.2 形成季碳中心的反应
从20世纪80年代早期研究以来得到了广泛的应用。 1989年,Shibasaki 和Overman 首先报道不对称Heck反 应。
CO2Me I
Pd(OAc)2 (3 mol%) (R)-BINAP (9 mol%) cyclohexene (6 mol%)
Ag2CO3 (2 eq) NMP, 60°C
X
Ha
CO2Et
Hb
Hc
PdLn
Hb
CO2Et
Hc
X PdLn
Ha Hb
a PdLnX HcCO2Et
b
Ha
PdLnX
Hb
HcCO2Et
A
Hb
PdLnX
Ha
HcCO2Et
B
Hb
CO2Et
Hc
E
Ha PdLnX
Hc Ph
Hb CO2Et
CO2Et Haa
Ph CO2Et
Heck 反应
汝杰
南京航空航天大学 仿生结构与材料防护研究所
过渡金属催化的交叉偶联反应
Transition Metal Catalyzed Cross Coupling
Organic Chemist
A
B
A
B
The formation of carbon–carbon bonds is a fundamental reaction in organic synthesis the efficiency of which has interested organic chemists for a long time ago. Aryl–aryl bond formation has been known for more than a century and was one of the first reactions involving a transition metal.1 Modern synthetic chemistry is also sustained by the use of transition-metal catalysts as powerful tools for carbon– carbon bond-forming processes.2 Among these, carbon–carbon coupling reactions through the activation of carbon–hydrogen bonds,3 as well as addition reactions,4 have experienced an increasing interest in the preparation of molecules, the access to which is not so straightforward using other methodologies. On the other hand, the transition-metal catalysed carbon–carbon bond formation developed in the 1970s represented a milestone in synthetic organic chemistry that allowed the cross coupling of substrates in ways that would have previously been thought impossible
Glaser (Hay) Coupling
Suzuki Coupling
[Pd] or [Ni]
+ R1 X 2R
B(OH)2
Base
1R
R2
Sonogashira Reaction
R1 X + R2
H Pd-Complex, CuI
Amine
R2
R1
Amination Reaction
Stille reaction
.Chem.1989,54,5846
像天然产物physostigmine的合成,成功 的运用和Heck反应构成手性的季碳中心。
MeO
I O
N Me
OTIPS Pd2(dba)3-CHCl3 (10 mol%) (S)-BINAP (23 mol%)
PMP, DMA, 100°C
MeO
OTIPS
O N Me
3 M HCl THF, rt
MeO
CHO O N Me
84% (95% ee)
MeNH3Cl, Et3N LiAlH4, THF, reflux
(88%)
MeO
NMe NH Me (-)-esermethole
1) BBr3, CH2Cl2, rt 2) Na, Et2O, MeNCO
O
OTBS
H OO
O 49%
O
(紫杉醇)
Danishefsky, S. J. J. Am. Chem. Soc. 1996, 118, 2843
分子内Heck反应化生成环外双键
MeO O
OMe I
OMe
Pd(PPh3)4 , NEt3(12eq)
NO NCO2Me
MeCN, 80℃, 10h
MeO O
1
OMe
OMe
O N
NCO2Me
2
A stirred solution of 1, triethylmine and catalytic tetrakispalladium(0) in 2.4 mL of acetonitrile was heated at 80 ℃ in a sealed tube under an argon atmosphere for 10 h. The reaction mixture turned dark orange after ca10 minand the catalyst plated out on the walls of the tube as a shiny layer of palladium metal upon completion of the reactionThe reaction mixture was cooled to room temperaturethe reaction was quenched with aqueous NaHCO3andthe mixture was extracted with EtOAc The organic extracts were washed with aqueous NaHSO3 water and brine and dried over MgSO4 Filtrationconcentrationand purification of the orange residue by flash column chromatography gave 66mg of19 as acolorless solidmp 193-194 ℃Rf = 0.29
MeCN, 80°C, 10h
OMe
OMe
MeO O
O N
NCO2Me
90%
Danishefsky, S. J. J. Am. Chem. Soc. 1993, 115, 6094
该反应还被Danishefsky应用到全合成Taxol上。
OTf
OTBS
O OH O
Pd(PPh3)4 (1.1 eq) K2CO3, MeCN, 85°C
(31%)
MeNHCO2
NMe NH Me
(-)-physostigmine
Matsuura, T.; Overman, L.E. J.Am.Chem.Soc. 1998, 120, 6500
2.2.1 分子内不对称Heck反应
MeO
I
OTIPS
O
10 % Pd2(dba)3, 23 % (s)-BINAP, CHCl3 MeO
Mori 和 Ban于1977年首次报道了分子内的Heck反应:
CO2Me Br
N Ac
Pd(OAc)2 (2 mol%) PPh3, DMF
TMEDA (2 equaiv) 125°C, 5h
CO2Me
N Ac 43%
Indole product formed as result of Pd-H isomerization of product clefin
Heck reaction
通常把在碱性条件下钯
催化的芳基或乙烯基卤代物 和活性烯烃之间的偶联反应 称为Heck反应。自从20世纪 60年代末Heck 和Morizoki独 立发现该反应以来,通过对催 化剂和反应条件的不断改进 使其的应用范围越来越广泛, 使该反应已经成为构成C-C 键的重要反应之一。另外, Heck反应具有很好的Trans 选择性
Mori, M.; Ban, K.; Tetrahedron 1977, 12, 1037
2. 分子内的Heck反应
2.1 生成烯基取代的反应
该类反应主要用于生成环外双键。环外双键是合 成上一大难题,该反应成功的应用具有重大意义。
目前已有合成的报道。
MeO O
OMe I
OMe
O N
NCO2Me
Pd(PPh3)4 (cat) NEt3 (12 eq)
