酵母手册
酵母双杂交操作手册 by shenao

酵母双杂交操作手册 by shenao Y2H所需材料:PJ69-4A PJ69-4α or AH109 Y187pGBKT7 DNA-BD Vector (bait)pGADT7 AD Vector (prey)pGBKT7-53 Control VectorpGBKT7-Lam Control VectorpGADT7-T Control VectorpCL1 Control Vector3' DNA-BD & AD Sequencing PrimersHerring testes carrier DNAYeast extract,Dextrose(glucose); SD base; DO Supplement; Peptone,with DMF) TE buffer DMSO PEG/LiAc (10X) TE/LiAc buffer (10X) X-a-gal(Yeast Phenotypes–––Ade, His, Leu, Requires adenine (Ade), histidine (His), leucine (Leu), or tryptophan (Trp) in the–or Trp medium to grow; is auxotrophic for at least one of these specific nutrients.Expresses the ADE2 reporter gene; i.e., does not require Ade in the medium to +Ade grow.+His Expresses the HIS3 reporter gene; i.e., does not require His in the medium to grow.+LacZ Expresses the lacZ reporter gene; i.e., is positive for b-galactosidase activity.+Mel1 Expresses the MEL1 reporter gene; i.e., is positive for a-galactosidase activity. MiscellaneousAde2p Protein encoded by the yeast ADE2 gene.3-AT 3-amino-1,2,4-triazole; a competitive inhibitor of the His3 protein.CHX CycloheximideDropout (supplement or solution); a mixture of specific amino acids and nucleosidesDO used to supplement SD base to make SD medium; DO solutions are missing one ormore of the nutrients required by untransformed yeast to grow on SD medium.His3p Protein encoded by the yeast HIS3gene.Minimal Synthetic Dropout medium; comprised of a nitrogen base, a carbon source SD medium (glucose or galactose), and a DO supplement.YPH Yeast Protocols HandbookSD/–Ade SD/–Met SD/–His SD/–Ura YPDA YPD/CHX SD/–Leu SD/–Trp StrainPJ69-4A – + – + + –––––– + + ––– PJ69-4α1SD/–Ade SD/–Met SD/–His SD/–Ura YPDA YPD/CHX SD/–Leu SD/–Trp StrainAH109 – + – + + –––––– + + ––– Y187Yeast selection Bacterial selection Fusion EpitopeCloning vectorspGBKT7 DNA/bait c-Myc TRP1 kanamycinpGADT7 AD/library HA LEU2 ampicillin Control vectorspCL1 GAL4 LEU2 ampicillinpGADT7-T AD/T-antigen HA LEU2 ampicillin pGBKT7-53 DNA-BD/p53 c-Myc TRP1 kanamycin pGBKT7-Lam DNA-BD/lamin C c-Myc TRP1 kanamycin .缩写:Ade腺嘌呤(adenine) His组氨酸(histidine) Leu亮氨酸(leucine) Trp色氨酸(tryptophan)培养基成分及配制方法:YPD & SD Base from CLONTECH already contains a carbonsource(Dextrose(glucose))YPD: 20g/L Peptone, 10g/L Yeast extract, 2% Dextrose(20g/L), 20 g/L Agar (for plates only) 加入上述药品,加水至1L,调节PH值到6.5。
毕赤酵母实验操作手册

毕赤酵母表达实验手册大肠杆菌表达系统最突出的优点是工艺简单、产量高、生产成本低。
然而,许多蛋白质在翻译后,需经过翻译后的修饰加工,如磷酸化、糖基化、酰胺化及蛋白酶水解等过程才能转化成活性形式。
大肠杆菌缺少上述加工机制,不适合用于表达结构复杂的蛋白质。
另外,蛋白质的活性还依赖于形成正确的二硫键并折叠成高级结构,在大肠杆菌中表达的蛋白质往往不能进行正确的折叠,是以包含体状态存在。
包含体的形成虽然简化了产物的纯化,但不利于产物的活性,为了得到有活性的蛋白,就需要进行变性溶解及复性等操作,这一过程比较繁琐,同时增加了成本。
与大肠杆菌相比,酵母是低等真核生物,具有细胞生长快,易于培养,遗传操作简单等原核生物的特点,又具有真核生物时表达的蛋白质进行正确加工,修饰,合理的空间折叠等功能,非常有利于真核基因的表达,能有效克服大肠杆菌系统缺乏蛋白翻泽后加工、修饰的不足。
因此酵母表达系统受到越来越多的重视和利用。
大肠杆菌是用得最多、研究最成熟的基因工程表达系统,当前已商业化的基因工程产品大多是通过大肠杆菌表达的,其主要优点是成本低、产量高、易于操作。
但大肠杆菌是原核生物,不具有真核生物的基因表达调控机制和蛋白质的加工修饰能力,其产物往住形成没有活性的包涵体,需要经过变性、复性等处理,才能应用。
近年来,以酵母作为工程菌表达外源蛋白日益引起重视,主更是因为酵母是单细胞真核生物,不但具有大肠杆菌易操作、繁殖快、易于工业化生产的特点,还具有真核生物表达系统基因表达调控和蛋白修饰功能,避免了产物活性低,包涵体变性、复性等等间题[1]。
与大肠杆菌相比,酵母是单细胞真核生物,具有比较完备的基因表达调控机制和对表达产物的加工修饰能力,人们对酿酒酵母(Saccharomyces.Cerevisiae)分子遗传学方面的认识最早,酿酒酵母也最先作为外源基因表达的酵母宿主.1981年酿酒酵母表达了第一个外源基因一干扰素基因,随后又有一系列外源基因在该系统得到表达。
酵母表达载体pPICZ手册

pPICZ A, B, and CPichia expression vectors for selection onZeocin™ and purification of recombinant proteins Catalog no. V190-20Rev. Date: 7 July 2010Manual part no. 25-0148MAN00000034User ManualiiTable of ContentsKit Contents and Storage (iv)Accessory Products (v)Introduction (1)Overview (1)Methods (3)Cloning into pPICZ A, B, and C (3)Pichia Transformation (9)Expression in Pichia (13)Purification (15)Appendix (17)Recipes (17)Zeocin™ (19)Map and Features of pPICZ A, B, and C (21)Lithium Chloride Transformation Method (23)Construction of In Vitro Multimers (24)Technical Support (32)Purchaser Notification (33)References (34)iiiKit Contents and StorageContents The following components are included with Catalog no. V190–20. Note that thepPICZ expression vectors are supplied in suspension.Component QuantityCompositionpPICZ A Expression Vector 20 μg 40 μl of 0.5 μg/μl vector in10 mM Tris–HCl, 1 mM EDTA,pH 8.0pPICZ B Expression Vector 20 μg 40 μl of 0.5 μg/μl vector in10 mM Tris–HCl, 1 mM EDTA,pH 8.0pPICZ C Expression Vector 20 μg 40 μl of 0.5 μg/μl vector in10 mM Tris–HCl, 1 mM EDTA,pH 8.0GS115/pPICZ/lacZ Positive1 stab --Control strainShipping/Storage The components included with Catalog no. V190–20 are shipped on wet ice.Upon receipt, store as directed below.For long-term storage of your positive control stab strain, we recommendpreparing a glycerol stock immediately upon receipt and storing at –80°C.Component ShippingStorage pPICZ A Expression Vector Wet ice Store at –20°CpPICZ B Expression Vector Wet ice Store at –20°CpPICZ C Expression Vector Wet ice Store at –20°CGS115/pPICZ/lacZ positive control strain Wet ice Store at 4°CivAccessory ProductsAdditional ProductsThe products listed in this section are intended for use with the pPICZ vectors.For more information, visit our web site at or contactTechnical Support (page 32).Product QuantityCatalogno. X-33 Pichia strain 1 stab C180-00GS115 Pichia strain 1 stab C181-00KM71H Pichia strain 1 stab C182-00SMD1168H Pichia strain 1 stab C184-00pPICZα A, B, and C 20 μg each V195-20pPIC6α A,B, and C 20 μg each V215-20pPIC6 A, B, and C 20 μg each V210-20pPIC6 Starter Kit 1 kit K210-01Original Pichia Expression Kit 1 kit K1710-01EasySelect™Pichia Expression Kit 1 kit K1740-01Pichia EasyComp™ Transformation Kit 1 kit K1730-01Pichia Protocols 1 book G100-01PureLink™ Gel Extraction Kit 50 preps250 prepsK2100–12K2100–25S.N.A.P ™ Gel Purification Kit 25 preps K1999–25PureLink™ Quick Plasmid Miniprep Kit 50 preps250 prepsK2100–10K2100–11PureLink™ HiPure Plasmid Midiprep Kit 25 preps50 prepsK2100–04K2100–13One Shot® TOP10 (chemically competent E. coli) 10 reactions20 reactionsC4040–10C4040–03One Shot® TOP10 Electrocompetent E. Coli 10 reactions20 reactionsC4040-50C4040-52TOP10 Electrocomp™ Kits 20 reactions C664–55Positope™ Control Protein 5 μg R900-50CIAP (Calf Intestinal Alkaline Phosphatase) 1,000 units 18009–019T4 DNA Ligase 100 units500 units15224–01715224–025Zeocin™ 1g5 gR250-01R250-05β-Gal Assay Kit 1 kit K1455-01β-Gal Staining Kit 1 kit K1465-01E-Gel® Agarose Gels E-Gel® Agarose Gels are bufferless, pre-cast agarose gels designed for fast, convenient electrophoresis of DNA samples. E-Gel® agarose gels are available in different agarose percentage and well format for your convenience.For more details on these products, visit our web site at or contact Technical Support (page 32).Continued on next pagevAccessory Products, ContinuedZeocin™Zeocin™ may be obtained from Invitrogen (see above). For your convenience, the drug is prepared in autoclaved, deionized water and available in 1.25 ml aliquotsat a concentration of 100 mg/ml. The stability of Zeocin™ is guaranteed for sixmonths if stored at –20°C.Detection of Fusion Protein A number of antibodies are available from Invitrogen to detect expression ofyour fusion protein from the pPICZ vector. Horseradish peroxidase (HRP)-conjugated antibodies allow one-step detection in Western blots usingcolorimetric or chemiluminescent detection methods. The amount of antibodysupplied is sufficient for 25 Western Blots.Antibody Epitope Catalogno.Anti-myc R950–25 Anti-myc-HRPDetects the 10 amino acid epitopederived from c-myc (Evans et al., 1985):EQKLISEEDLR951–25Anti-His(C-term) R930–25Anti-His(C-term)-HRPDetects the C-terminal polyhistidine(6xHis) tag (requires the free carboxylgroup for detection) (Lindner et al., 1997):HHHHHH-COOHR931–25Purification of Fusion Protein The polyhistidine (6xHis) tag allows purification of the recombinant fusionprotein using metal-chelating resins such as ProBond™. Ordering information forProBond™ resin is provided below.Product QuantityCatalogno. ProBond™ Purification System 6 purifications K850–01ProBond ™ Purification System with Anti-myc-HRP Antibody1 Kit K852–01ProBond ™ Purification System with Anti-His(C-term)-HRP Antibody1 Kit K853–01ProBond™ Nickel-Chelating Resin 50 ml150 mlR801–01R801–15Purification Columns 50 each R640–50viIntroductionOverviewIntroduction pPICZ A, B, and C are 3.3 kb expression vectors used to express recombinantproteins in Pichia pastoris. Recombinant proteins are expressed as fusions to aC-terminal peptide containing the c-myc epitope and a polyhistidine (6xHis) tag.The vector allows high-level, methanol inducible expression of the gene ofinterest in Pichia, and can be used in any Pichia strain including X33, GS115,SMD1168H, and KM71H. pPICZ contains the following elements:•5′ fragment containing the AOX1 promoter for tightly regulated, methanol-induced expression of the gene of interest (Ellis et al., 1985; Koutz et al., 1989;Tschopp et al., 1987a)•Zeocin™ resistance gene for selection in both E. coli and Pichia (Baron et al.,1992; Drocourt et al., 1990)•C-terminal peptide containing the c-myc epitope and a polyhistidine (6xHis)tag for detection and purification of a recombinant fusion protein (if desired)•Three reading frames to facilitate in-frame cloning with the C-terminalpeptideReference Sources The pPICZ A, B, and C expression vectors may be used with the Original Pichia Expression Kit, and are included in the EasySelect™Pichia Expression Kit (see page v for ordering information). Additional general information about recombinant protein expression in Pichia pastoris is provided in the manuals for the Original Pichia Expression Kit and the EasySelect™Pichia Expression Kit. For more information about the Original Pichia Expression Kit, the EasySelect™Pichia Expression Kit, or their manuals, visit our web site at or contact Technical Support (page 32).More detailed information and protocols dealing with Pichia pastoris may also be found in the following general reference:Higgins, D. R., and Cregg, J. M. (1998) Pichia Protocols. In Methods in Molecular Biology, Vol. 103. (J. M. Walker, ed. Humana Press, Totowa, NJ) (see page v for ordering information).Recommended Pichia Host Strain We recommend using the X-33 Pichia strain as the host for expression of recombinant proteins from pPICZ. Other Pichia strains including GS115, KM71H, and SMD1168H are suitable. The X-33 Pichia strain and other strains are available from Invitrogen (see page v for ordering information). The X-33 Pichia strain has the following genotype and phenotype:Genotype: Wild-typePhenotype: Mut+1Overview, ContinuedExperimental Overview The following table describes the basic steps needed to clone and express your gene of interest in pPICZ.Step Action1 Propagate pPICZ A, B, and C by transformation into a rec A, end A1E. coli strain such as TOP10, DH5 , or JM109.2 Develop a cloning strategy and ligate your gene into one of the pPICZvectors in frame with the C-terminal tag.3 TransformintoE. coli and select transformants on Low Salt LB platescontaining 25 μg/ml Zeocin™.4 Analyze 10–20 transformants by restriction mapping or sequencing toconfirm in-frame fusion of your gene with the C-terminal tag.5 Purify and linearize the recombinant plasmid for transformation intoPichia pastoris.6 TransformyourPichia strain and plate onto YPDS plates containing the appropriate concentration of Zeocin™.7 Select for Zeocin™-resistant transformants.8 Optimize expression of your gene.9 Purify your fusion protein on metal-chelating resin (i.e. ProBond™).Continued on next page2MethodsCloning into pPICZ A, B, and CIntroduction The pPICZ vector is supplied with the multiple cloning site in three readingframes (A, B, and C) to facilitate cloning your gene of interest in frame with theC-terminal peptide containing the c-myc epitope and a polyhistidine (6xHis) tag.Use the diagrams provided on pages 5–7 to help you design a strategy to cloneyour gene of interest in frame with the C-terminal peptide. Generalconsiderations for cloning and transformation are discussed in this section.General Molecular Biology Techniques For assistance with E. coli transformations, restriction enzyme analysis, DNA biochemistry, and plasmid preparation, refer to Molecular Cloning: A Laboratory Manual (Sambrook et al., 1989) or Current Protocols in Molecular Biology (Ausubel et al., 1994).E. coli Strain Many E. coli strains are suitable for the propagation of the pPICZ vectorsincluding TOP10, JM109, and DH5 . We recommend that you propagate thepPICZ vectors in E. coli strains that are recombination deficient (rec A) andendonuclease A deficient (end A).For your convenience, TOP10 E. coli are available as chemically competent orelectrocompetent cells from Invitrogen (page v).Transformation Method You may use any method of choice for transformation. Chemical transformation is the most convenient for many researchers. Electroporation is the most efficient and the method of choice for large plasmids.Maintenance of Plasmids The pPICZ vectors contain the Zeocin™ resistance (Sh ble) gene to allow selection of the plasmid using Zeocin™. To propagate and maintain the pPICZ plasmids, we recommend using the following procedure:e 10 ng of your vector to transform a rec A, end A E. coli strain like TOP10,DH5 , JM109, or equivalent (see above).2.Select transformants on Low Salt LB plates containing 25 μg/ml Zeocin™ (seepage 17 for a recipe).3.Prepare a glycerol stock from each transformant containing plasmid forlong-term storage (see page 8).Continued on next page3Cloning into pPICZ A, B, and C, ContinuedGeneral Considerations The following are some general points to consider when using pPICZ to express your gene of interest in Pichia:•The codon usage in Pichia is believed to be similar to Saccharomyces cerevisiae.•Many Saccharomyces genes have proven to be functional in Pichia.•The premature termination of transcripts because of "AT rich regions" has been observed in Pichia and other eukaryotic systems (Henikoff & Cohen, 1984; Irniger et al., 1991; Scorer et al., 1993; Zaret & Sherman, 1984). If you have problems expressing your gene, check for premature termination by northern analysis and check your sequence for AT rich regions. It may be necessary to change the sequence in order to express your gene (Scorer et al., 1993).•The native 5´ end of the AOX1 mRNA is noted in the diagram for each multiple cloning site. This information is needed to calculate the size of the expressed mRNA of the gene of interest if you need to analyze mRNA for any reason.Cloning Considerations For proper initiation of translation, your insert should contain an initiation ATG codon as part of a yeast consensus sequence (Romanos et al., 1992). An example of a yeast consensus sequence is provided below. The ATG initiation codon is shown underlined.(G/A)NNATG GTo express your gene as a recombinant fusion protein, you must clone your gene in frame with the C-terminal peptide containing the c-myc epitope and the polyhistidine tag. The vector is supplied in three reading frames to facilitate cloning. Refer to the diagrams on pages 5–7 to develop a cloning strategy.If you wish to express your protein without the C-terminal peptide, be sure to include a stop codon.Construction of Multimeric Plasmids pPICZ A, B, and C contain unique Bgl II and Bam H I sites to allow construction of plasmids containing multiple copies of your gene. For information on how to construct multimers, refer to pages 24–31.Continued on next page4Multiple CloningSite of pPICZ A Below is the multiple cloning site for pPICZ A. Restriction sites are labeled to indicate the cleavage site. The boxed nucleotides indicate the variable region.The multiple cloning site has been confirmed by sequencing and functionaltesting.You can download the complete sequence of pPICZ A from our web site at or by contacting Technical Support (see page 32).For a map and a description of the features of pPICZ, refer to the Appendix(pages 21–22). AAT AGC GCC GTC GAC CAT CAT CAT CAT CAT CAT TGTTCCTCAG TTCAAGTTGG GCACTTACGA GAAGACCGGT CTTGCTAGAT TCTAATCAAG AGGATGTCAG AATGCCATTT GCCTGAGAGA TGCAGGCTTC ATTTTTGATA CTTTTTTATTTGTAACCTAT ATAGTATAGG ATTTTTTTTG TCATTTTGTT 1218Asn Ser Ala Val Asp His His His His His His ***3´ AOX1 priming site TGA GTTTTAGCCT TAGACATGAC AACCTTTTTT TTTATCATCA TTATTAGCTT ACTTTCATAA TTGCGACTGG TTCCAATTGA CAAGCTTTTG ATTTTAACGA CTTTTAACGA CAACTTGAGA AGATCAAAAA ACAACTAATT ATTCGAAACG AGGAATTCAC GTGGCCCAGC CGGCCGTCTC GGATCGGTAC CTCGAGCCGC GGCGGCCGCC AGCTT GGGCCC GAA CAA AAA CTC ATC TCA GAA GAG GAT CTG 811Glu Gln Lys Leu Ile Ser Glu Glu Asp Leu 5´ AOX1 priming sitemyc epitope3´polyadenylation sitePolyhistidine tag5´ end of AOX1 mRNA Sfu I Eco R I Pml I Sfi I Bsm B I Asp 718 I Kpn I Xho ISac II Not I Apa I 104210981158871931991Continued on next pageMultiple CloningSite of pPICZ B Below is the multiple cloning site for pPICZ B. Restriction sites are labeled to indicate the cleavage site. The boxed nucleotides indicate the variable region.The multiple cloning site has been confirmed by sequencing and functionaltesting.You can download the complete sequence of pPICZ B from our web site at or by contacting Technical Support (see page 32).For a map and a description of the features of pPICZ, refer to the Appendix(pages 21–22). AAT AGC GCC GTC GAC CAT CAT CAT CAT CAT CAT TGA GTTTGTAGCC TTAGACATGA CTGTTCCTCA GTTCAAGTTG GGCACTTACG AGAAGACCGG TCTTGCTAGA TTCTAATCAA GAGGATGTCA GAATGCCATT TGCCTGAGAG ATGCAGGCTT CATTTTTGAT ACTTTTTTAT TTGTAACCTA TATAGTATAG GATTTTTTTT GTCATTTTGT TTC 1216Asn Ser Ala Val Asp His His His His His His ***3´ AOX1 priming site TGA GTTTGTAGCC TTAGACATGA AACCTTTTTT TTTATCATCA TTATTAGCTT ACTTTCATAA TTGCGACTGG TTCCAATTGA CAAGCTTTTG ATTTTAACGA CTTTTAACGA CAACTTGAGA AGATCAAAAA ACAACTAATTATTCGAAACG AGGAATTCAC GTGGCCCAGC CGGCCGTCTC GGATCGGTAC CTCGAGCCGC GGCGGCCGCC AGCTT TCTA GAA CAA AAA CTC ATC TCA GAA GAG GAT CTG 811Glu Gln Lys Leu Ile Ser Glu Glu Asp Leu 5´ AOX1 priming sitemyc epitope3´ polyadenylation site Polyhistidine tag5´ end of AOX1 mRNA Sfu I Eco R I Pml I Sfi I Bsm B I Asp 718 I Kpn I Xho ISac II Not I Xba I 104010961156871931991Continued on next pageMultiple CloningSite of pPICZ C Below is the multiple cloning site for pPICZ C. Restriction sites are labeled to indicate the cleavage site. The boxed nucleotides indicate the variable region.The multiple cloning site has been confirmed by sequencing and functionaltesting.You can download the complete sequence of pPICZ C from our web site at or by contacting Technical Support (see page 32).For a map and a description of the features of pPICZ, refer to the Appendix(pages 21–22). AAT AGC GCC GTC GAC CAT CAT CAT CAT CAT CAT TGA GTTTGTAGCC TTAGACATGA CTGTTCCTCA GTTCAAGTTG GGCACTTACG AGAAGACCGG TCTTGCTAGA TTCTAATCAA GAGGATGTCA GAATGCCATT TGCCTGAGAG ATGCAGGCTT CATTTTTGAT ACTTTTTTAT TTGTAACCTA TATAGTATAG GATTTTTTTT GTCATTTTGT TTC 1217Asn Ser Ala Val Asp His His His His His His ***3´ AOX1 priming siteTGA GTTTGTAGCC TTAGACATGA AACCTTTTTT TTTATCATCA TTATTAGCTT ACTTTCATAA TTGCGACTGG TTCCAATTGA CAAGCTTTTG ATTTTAACGA CTTTTAACGA CAACTTGAGA AGATCAAAAA ACAACTAATT ATTCGAAACG AGGAATTCAC GTGGCCCAGC CGGCCGTCTC GGATCGGTAC CTCGAGCCGC GGCGGCCGCC AGCTT ACGTA GAA CAA AAA CTC ATC TCA GAA GAG GAT CTG 811Glu Gln Lys Leu Ile Ser Glu Glu Asp Leu 5´ AOX1 priming sitemyc epitope3´ polyadenylation site Polyhistidine tag5´ end of AOX1 mRNA Sfu I Eco R I Pml I Sfi I Bsm B I Asp 718 I Kpn I Xho I Sac II Not I Sna B I 104110971157871931991Continued on next pageE. coli Transformation Transform your ligation mixtures into a competent rec A, end A E. coli strain(e.g. TOP10, DH5, JM109) and select on Low Salt LB agar plates containing25 μg/ml Zeocin™ (see below). Note that there is no blue/white screening for the presence of insert with pPICZ A, B, or C. Once you have obtained Zeocin™-resistant colonies, pick 10 transformants and screen for the presence and orientation of your insert.Important To facilitate selection of Zeocin™-resistant E. coli, the salt concentration of the medium must remain low (<90 mM) and the pH must be 7.5. Prepare Low Salt LB broth and plates using the recipe in the Appendix, page 17.Failure to lower the salt content of your LB medium will result in non-selection due to inhibition of the drug.C We recommend that you sequence your construct to confirm that your gene is in the correct orientation for expression and cloned in frame with the C-terminal peptide (if desired). Refer to the diagrams on pages 5–7 for the sequences and location of the priming sites.Preparing a Glycerol Stock Once you have identified the correct clone, be sure to purify the colony and make a glycerol stock for long-term storage. It is also a good idea to keep a DNA stock of your plasmid at –20°C.1.Streak the original colony out on an Low Salt LB plate containing 25 μg/mlZeocin™. Incubate the plate at 37°C overnight.2.Isolate a single colony and inoculate into 1–2 ml of Low Salt LB containing25 μg/ml Zeocin™.3.Grow the culture to mid-log phase (OD600 = 0.5–0.7).4.Mix 0.85 ml of culture with 0.15 ml of sterile glycerol and transfer to acryovial.5.Store at –80°C.Plasmid Preparation Once you have cloned and sequenced your insert, generate enough plasmid DNA to transform Pichia (5–10 μg of each plasmid per transformation). We recommend isolating plasmid DNA using the PureLink™ Quick Plasmid Miniprep Kit or the PureLink™ HiPure Plasmid Midiprep Kit (page v), or CsCl gradient centrifugation.Once you have purified plasmid DNA, proceed to Pichia Transformation, next page.Pichia TransformationIntroduction You should now have your gene cloned into one of the pPICZ vectors. Yourconstruct should be correctly fused to the C-terminal peptide (if desired). Thissection provides general guidelines to prepare plasmid DNA, transform yourPichia strain, and select for Zeocin™-resistant clones.Zeocin™ Selection We generally use 100 μg/ml Zeocin™ to select for transformants when using the X-33 Pichia strain. If you are transforming your pPICZ construct into anotherPichia strain, note that selection conditions may vary. We recommendperforming a dose response curve to determine the appropriate concentration ofZeocin™ to use for selection of transformants in your strain.Method of Transformation We do not recommend spheroplasting for transformation of Pichia with plasmids containing the Zeocin™ resistance marker. Spheroplasting involves removal of the cell wall to allow DNA to enter the cell. Cells must first regenerate the cell wall before they are able to express the Zeocin™ resistance gene. For this reason, plating spheroplasts directly onto selective medium containing Zeocin™ does not yield any transformants.We recommend electroporation for transformation of Pichia with pPICZ A, B, or C. Electroporation yields 103 to 104 transformants per μg of linearized DNA and does not destroy the cell wall of Pichia. If you do not have access to an electroporation device, use the LiCl protocol on page 23 or the Pichia EasyComp™Transformation Kit available from Invitrogen (see below).PichiaEasyComp™Transformation Kit If you wish to perform chemical transformation of your Pichia strain with pPICZ A, B, or C, the Pichia EasyComp™ Transformation Kit is available from Invitrogen (see page v for ordering information). The Pichia EasyComp™ Transformation Kit provides reagents to prepare 6 preparations of competent cells. Each preparation will yield enough competent cells for 20 transformations. Competent cells may be used immediately or frozen and stored for future use. For more information, visit our web site at or contact Technical Support (page 32).Important Since pPICZ does not contain the HIS4 gene, integration can only occur at the AOX1 locus. Vector linearized within the 5´ AOX1 region will integrate by gene insertion into the host 5´ AOX1 region. Therefore, the Pichia host that you use will determine whether the recombinant strain is able to metabolize methanol (Mut+) or not (Mut S). To generate a Mut+ recombinant strain, you must use a Pichia host that contains the native AOX1 gene (e.g. X-33, GS115, SMD1168H). If you wish to generate a Mut S recombinant strain, then use a Pichia host that has a disrupted AOX1 gene (i.e. KM71H).Continued on next pageHis4 Host Strains Host strains containing the his4 allele (e.g. GS115) and transformed with thepPICZ vectors require histidine when grown in minimal media. Add histidine toa final concentration of 0.004% to ensure growth of your transformants.The pPICZ vectors do not contain a yeast origin of replication. Transformantscan only be isolated if recombination occurs between the plasmid and the Pichiagenome.Materials Needed You will need the following items:Note: Inclusion of sorbitol in YPD plates stabilizes electroporated cells as they appear tobe somewhat osmotically sensitive.•5–10 μg pure pPICZ containing your insert•YPD Medium•50 ml conical polypropylene tubes• 1 liter cold (4°C) sterile water (place on ice the day of the experiment)•25 ml cold (4°C) sterile 1 M sorbitol (place on ice the day of the experiment)•30°C incubator•Electroporation device and 0.2 cm cuvettes•YPDS plates containing the appropriate concentration of Zeocin™ (seepage 18 for recipe)Linearizing YourpPICZ ConstructTo promote integration, we recommend that you linearize your pPICZ constructwithin the 5′ AOX1 region. The table below lists unique sites that may be used tolinearize pPICZ prior to transformation. Other restriction sites are possible.Note that for the enzymes listed below, the cleavage site is the same for versionsA, B, and C of pPICZ. Be sure that your insert does not contain the restriction siteyou wish to use to linearize your vector.Enzyme Restriction Site (bp) SupplierSac I 209 ManyPme I 414 New England BiolabsBst X I 707 ManyRestriction Digest 1.Digest ~5–10 μg of plasmid DNA with one of the enzymes listed above.2.Check a small aliquot of your digest by agarose gel electrophoresis forcomplete linearization.3.If the vector is completely linearized, heat inactivate or add EDTA to stopthe reaction, phenol/chloroform extract once, and ethanol precipitate using1/10 volume 3 M sodium acetate and 2.5 volumes of 100% ethanol.4.Centrifuge the solution to pellet the DNA, wash the pellet with 80% ethanol,air-dry, and resuspend in 10 μl sterile, deionized water. Use immediately orstore at –20°C.Continued on next pagePreparation of Pichia for Electroporation Follow the procedure below to prepare your Pichia pastoris strain for electroporation.1. Grow 5 ml of your Pichia pastoris strain in YPD in a 50 ml conical tube at30°C overnight.2. Inoculate 500 ml of fresh medium in a 2 liter flask with 0.1–0.5 ml of theovernight culture. Grow overnight again to an OD600 = 1.3–1.5.3. Centrifuge the cells at 1500 × g for 5 minutes at 4°C. Resuspend the pelletwith 500 ml of ice-cold (0–4°C), sterile water.4. Centrifuge the cells as in Step 3, then resuspend the pellet with 250 ml ofice-cold (0–4°C), sterile water.5. Centrifuge the cells as in Step 3, then resuspend the pellet in 20 ml of ice-cold (0–4°C) 1 M sorbitol.6. Centrifuge the cells as in Step 3, then resuspend the pellet in 1 ml of ice-cold(0–4°C) 1 M sorbitol for a final volume of approximately 1.5 ml. Keep the cells on ice and use that day. Do not store cells.Transformation by Electroporation 1.Mix 80 μl of the cells from Step 6 (above) with 5–10 μg of linearized pPICZDNA (in 5–10 μl sterile water) and transfer them to an ice-cold (0–4°C)0.2 cm electroporation cuvette.2.Incubate the cuvette with the cells on ice for 5 minutes.3.Pulse the cells according to the parameters for yeast (Saccharomycescerevisiae) as suggested by the manufacturer of the specific electroporation device being used.4.Immediately add 1 ml of ice-cold 1 M sorbitol to the cuvette. Transfer thecuvette contents to a sterile 15 ml tube.5.Let the tube incubate at 30°C without shaking for 1 to 2 hours.6.Spread 50-200 μl each on separate, labeled YPDS plates containing theappropriate concentration of Zeocin™.7.Incubate plates for 2–3 days at 30°C until colonies form.8.Pick 10–20 colonies and purify (streak for single colonies) on fresh YPD orYPDS plates containing the appropriate concentration of Zeocin™.Continued on next pageGenerally, several hundred Zeocin™-resistant colonies are generated using theprotocol on the previous page. If more colonies are needed, the protocol may bemodified as described below. Note that you will need ~20, 150 mm plates withYPDS agar containing the appropriate concentration of Zeocin™.1. Set up two transformations per construct and follow Steps 1 through 5 ofthe Transformation by Electroporation protocol, page 11.2. After 1 hour in 1 M sorbitol at 30°C (Step 5, previous page), add 1 ml YPDmedium to each tube.3. Shake (~200 rpm) the cultures at 30°C.4. After 1 hour, take one of the tubes and plate out all of the cells by spreading200 μl on 150 mm plates containing the appropriate concentration ofZeocin™.5. Optional: Continue incubating the other culture for three more hours (for atotal of four hours) and then plate out all of the cells by spreading 200 μl on150 mm plates containing the appropriate concentration of Zeocin™.6. Incubate plates for 2–4 days at 30°C until colonies form.Mut Phenotype If you used a Pichia strain containing a native AOX1 gene (e.g. X-33, GS115,SMD1168H) as the host for your pPICZ construct, your Zeocin™-resistanttransformants will be Mut+. If you used a strain containing a deletion in theAOX1 gene (e.g. KM71H), your transformants will be Mut S.If you wish to verify the Mut phenotype of your Zeocin™-resistant transformants,you may refer to the general guidelines provided in the EasySelect™PichiaExpression Kit manual or the Original Pichia Expression Kit manual or topublished reference sources (Higgins & Cregg, 1998).You are now ready to test your transformants for expression of your gene ofinterest. See Expression in Pichia, next page.。
毕赤酵母发酵手册
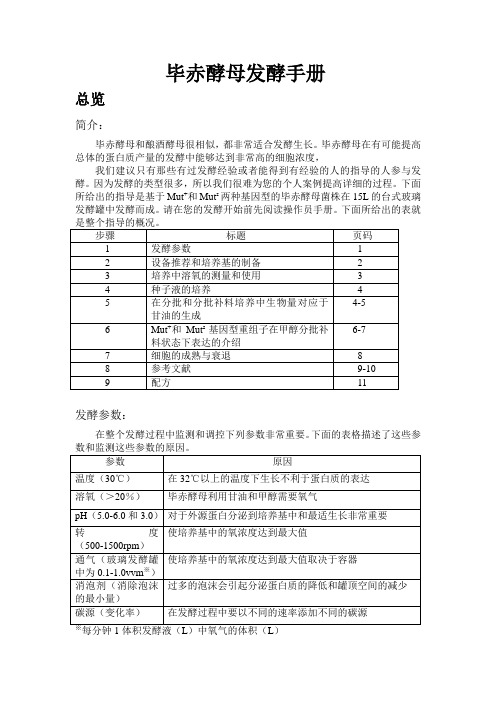
毕赤酵母发酵手册总览简介:毕赤酵母和酿酒酵母很相似,都非常适合发酵生长。
毕赤酵母在有可能提高总体的蛋白质产量的发酵中能够达到非常高的细胞浓度,我们建议只有那些有过发酵经验或者能得到有经验的人的指导的人参与发酵。
因为发酵的类型很多,所以我们很难为您的个人案例提高详细的过程。
下面所给出的指导是基于Mut+和Mut s两种基因型的毕赤酵母菌株在15L的台式玻璃发酵罐中发酵而成。
请在您的发酵开始前先阅读操作员手册。
下面所给出的表就发酵参数:在整个发酵过程中监测和调控下列参数非常重要。
下面的表格描述了这些参设备推荐:下面是所推荐设备的清单:·发酵罐的夹套需要在发酵过程中给酵母菌降温,尤其是在甲醇流加过程中。
你需要一个固定的来源来提供冷却水(5-10℃)。
这可能意味着你需要一个冷冻装置来保持水的冷却。
·一个泡沫探针就像消泡剂一样不可或缺。
·一个氧气的来源——空气(不锈钢的发酵罐需要1-2vvm)或者纯氧(玻璃发酵罐需要0.1-0.3vvm)。
·添加甘油和甲醇的补料泵。
·pH的自动控制。
培养基的准备:你需要准确配置下列溶液:·发酵所需的基本盐类(第11页)·PTM1补充盐类(第11页)·75ml的50%的甘油每升初始发酵液,12ml的PTM1补充盐每升甘油。
·740ml的100%的甲醇每升初始发酵液,12ml的PTM1补充盐每升甲醇。
毕赤酵母生长的测定:在不同的时间点通过测OD600的吸光值和湿细胞的重量来检测毕赤酵母的生长。
培养的代谢速率通过通过观察溶氧浓度对应于有效碳源来测定。
溶氧的测定:简介:溶解氧的浓度时指氧气在培养基中的相关比例,溶氧100%是指培养基中氧达到饱和。
毕赤酵母的生长需要消耗氧气,减少溶解氧的满度。
毕赤酵母在生长时会消耗氧气,减少溶氧的程度。
然而,因为代谢甲醇的最初阶段需要氧气,所以将溶氧浓度维持在一个适当的水平(>20%)来确保毕赤酵母在甲醇上的生长就至关重要。
毕赤酵母手册

毕赤酵母表达实验手册作者:Jnuxz 来源:丁香园时间:2007-9-5大肠杆菌表达系统最突出的优点是工艺简单、产量高、周期短、生产成本低。
然而,许多蛋白质在翻译后,需经过翻译后的修饰加工,如磷酸化、糖基化、酰胺化及蛋白酶水解等过程才能转化成活性形式。
大肠杆菌缺少上述加工机制,不适合用于表达结构复杂的蛋白质。
另外,蛋白质的活性还依赖于形成正确的二硫键并折叠成高级结构,在大肠杆菌中表达的蛋白质往往不能进行正确的折叠,是以包含体状态存在。
包含体的形成虽然简化了产物的纯化,但不利于产物的活性,为了得到有活性的蛋白,就需要进行变性溶解及复性等操作,这一过程比较繁琐,同时增加了成本。
大肠杆菌是用得最多、研究最成熟的基因工程表达系统,当前已商业化的基因工程产品大多是通过大肠杆菌表达的,其主要优点是成本低、产量高、易于操作。
但大肠杆菌是原核生物,不具有真核生物的基因表达调控机制和蛋白质的加工修饰能力,其产物往住形成没有活性的包涵体,需要经过变性、复性等处理,才能应用。
近年来,以酵母作为工程菌表达外源蛋白日益引起重视,原因是与大肠杆菌相比,酵母是低等真核生物,除了具有细胞生长快,易于培养,遗传操作简单等原核生物的特点外,又具有真核生物时表达的蛋白质进行正确加工,修饰,合理的空间折叠等功能,非常有利于真核基因的表达,能有效克服大肠杆菌系统缺乏蛋白翻译后加工、修饰的不足。
因此酵母表达系统受到越来越多的重视和利用。
[1]。
同时与大肠杆菌相比,作为单细胞真核生物的酵母菌具有比较完备的基因表达调控机制和对表达产物的加工修饰能力。
酿酒酵母(Saccharomyces.Cerevisiae)在分子遗传学方面被人们的认识最早,也是最先作为外源基因表达的酵母宿主。
1981年酿酒酵母表达了第一个外源基因----干扰素基因[2],随后又有一系列外源基因在该系统得到表达[3、4、5、6]。
干扰素和胰岛素虽然已经利用酿酒酵母大量生产并被广泛应用,当利用酿酒酵母制备时,实验室的结果很令人鼓舞,但由实验室扩展到工业规模时,其产量迅速下降。
酵母双杂实验操作手册和注意事项

酵母双杂(Yeast two-hybrid)实验操作手册和注意事项一. 酵母双杂的原理1989年,Song和Field建立了第一个基于酵母的细胞内检测蛋白间相互作用的遗传系统。
很多真核生物的位点特异转录激活因子通常具有两个可分割开的结构域,即DNA特异结合域(DNA-binding domain,BD)与转录激活域(Transcriptional activation domain ,AD)。
这两个结构域各具功能,互不影响。
但一个完整的激活特定基因表达的激活因子必须同时含有这两个结构域,否则无法完成激活功能。
不同来源激活因子的BD区与AD结合后则特异地激活被BD结合的基因表达。
基于这个原理,可将两个待测蛋白分别与这两个结构域建成融合蛋白,并共表达于同一个酵母细胞内。
如果两个待测蛋白间能发生相互作用,就会通过待测蛋白的桥梁作用使AD与BD形成一个完整的转录激活因子并激活相应的报告基因表达。
通过对报告基因表型的测定可以很容易地知道待测蛋白分子间是否发生了相互作用。
酵母双杂交系统由三个部分组成:(1)与BD融合的蛋白表达载体,被表达的蛋白称诱饵蛋白(bait)。
(2)与AD融合的蛋白表达载体,被其表达的蛋白称靶蛋白(prey)。
(3)带有一个或多个报告基因的宿主菌株。
常用的报告基因有HIS3,URA3,LacZ和ADE2等。
而菌株则具有相应的缺陷型。
双杂交质粒上分别带有不同的抗性基因和营养标记基因。
这些有利于实验后期杂交质粒的鉴定与分离。
根据目前通用的系统中BD来源的不同主要分为GAL4系统和LexA系统。
后者因其BD来源于原核生物,在真核生物内缺少同源性,因此可以减少假阳性的出现。
二.所用的载体及相关信息1. pGBKT7载体的图谱和相关信息The pGBKT7 vector expresses proteins fused to amino acids 1–147 of the GAL4 DNA binding domain (DNA-BD). In yeast, fusion proteins are expressed at high levels from the constitutive ADH1promoter (PADH1); transcription is terminated by the T7 and ADH1 transcription termination signals(TT7 & ADH1). pGBKT7 also contains the T7 promoter, a c-Myc epitope tag, and a MCS. pGBKT7replicates autonomously in both E. coli and S. cerevisiae from the pUC and 2 m ori, respectively. Thevector carries the Kan r for selection in E. coli and the TRP1 nutritional marker for selection in yeast.Yeast strains containing pGBKT7 exhibit a higher transformation efficiency than strains carrying other DNA-BD domain vectors (1).b. pGADT7载体的图谱和相关信息pGADT7-T encodes a fusion of the SV40 large T-antigen (a.a. 86–708) and the GAL4 AD (a.a. 768–881). The SV40 large T DNA (GenBank LocusSV4CG) was derived from a plasmid referenced in Li & Fields (1993) and was cloned into pGADT7 using the EcoR I and Xho I sites. pGADT7-T has not been sequenced.三.实验主要流程A.需要准备的药品和设备1.两种酵母菌种(AH109,Y187)2.酵母培养所需的药品: Yeast nitrogen base without amino acidsAgar (for plates only)sterile 10×Dropout Solution单缺-T,-L(clontech公司)二缺-T/-L (clontech公司)四缺-T/-L/-Ade/-His(clontech公司)3.酵母转化所需的药品: 10×TE buffer10×LiAc40%PEGcarrier DNA4.酵母显色所需要的药品: x- -GAL5.其他仪器设备: 30℃恒温培养箱30℃摇床.水浴锅分光光度计B.DNA-BD和DN-AD fusion protein 载体的分别构建。
毕赤酵母发酵工艺手册

毕赤酵母发酵工艺手册1. 引言欢迎使用毕赤酵母发酵工艺手册。
本手册旨在介绍毕赤酵母发酵的基本原理、工艺步骤以及相关注意事项。
通过遵循本手册,您可以更好地理解和掌握毕赤酵母的发酵过程,从而在生产中取得更好的效果。
2. 毕赤酵母发酵基本原理- 毕赤酵母是一种常见的酵母菌,其发酵能力强,适用于多种发酵产品的生产。
- 发酵是指通过酵母菌对底物中的糖类进行代谢,产生酒精和二氧化碳的过程。
- 毕赤酵母在发酵过程中需要适宜的温度、pH值和营养物质等条件。
3. 毕赤酵母发酵工艺步骤1. 发酵前准备:- 准备好所需的发酵基质,包括糖类、氮源和维生素等。
- 对基质进行消毒处理,确保无害菌的存在。
2. 接种毕赤酵母:- 选择合适的毕赤酵母培养液进行接种,注意接种量的控制。
- 将毕赤酵母培养液均匀加入发酵基质中。
3. 发酵条件控制:- 控制发酵温度在合适的范围内,一般为25-30摄氏度。
- 监测发酵基质的pH值,保持在适宜的范围内。
- 提供足够的氧气供给,促进酵母的生长和代谢。
4. 发酵过程监测:- 定期对发酵过程中的温度、pH值和酵母数量等进行监测和记录。
- 根据监测结果及时调整发酵条件,确保发酵过程稳定进行。
5. 发酵结束:- 当发酵基质中的糖类被完全代谢,产物达到预期时,发酵过程结束。
- 将发酵产物经过处理和提取,得到最终的产品。
4. 注意事项- 在发酵过程中,应注意卫生和消毒,以防止杂菌的污染。
- 严格控制发酵条件,避免过高或过低的温度、pH值对发酵效果产生不利影响。
- 根据不同的发酵产品,可能需要调整发酵步骤和条件,建议根据具体要求进行调整。
- 在使用本工艺手册时,请参考其他文献和专业意见,确保准确性和可靠性。
以上是关于毕赤酵母发酵工艺手册的简要介绍。
希望本手册能对您在毕赤酵母的发酵工艺中提供帮助和指导。
如有任何问题,请随时与我们联系。
谢谢!。
毕赤酵母表达操作手册(PDF精译版)

版权声明:本站几乎所有资源均搜集于网络,仅供学习参考,不得进行任何商业用途,否则产生的一切后 果将由使用者本人承担! 本站仅仅提供一个观摩学习与交流的平台, 将不保证所提供资源的完 整性,也不对任何资源负法律责任。
所有资源请在下载后 24 小时内删除。
如果您觉得满意, 请购买正版,以便更好支持您所喜欢的软件或书籍!☆☆☆☆☆生物秀[]☆☆☆☆☆中国生物科学论坛[/bbs/]☆☆☆☆☆生物秀下载频道[/Soft/]生物秀——倾力打造最大最专业的生物资源下载平台!■■■ 选择生物秀,我秀我精彩!!■■■欢迎到生物秀论坛(中国生物科学论坛)的相关资源、软件版块参与讨论,共享您的资源,获 取更多资源或帮助。
毕赤酵母多拷贝表达载体试剂盒用于在含多拷贝基因的毕赤酵母菌中表达并分离重组蛋白综述:基本特征:作为真核生物,毕赤酵母具有高等真核表达系统的许多优点:如蛋白加工、折叠、翻译后修饰等。
不仅如此,操作时与E.coli及酿酒酵母同样简单。
它比杆状病毒或哺乳动物组织培养等其它真核表达系统更快捷、简单、廉价,且表达水平更高。
同为酵母,毕赤酵母具有与酿酒酵母相似的分子及遗传操作优点,且它的外源蛋白表达水平是后者的十倍以至百倍。
这些使得毕赤酵母成为非常有用的蛋白表达系统。
与酿酒酵母相似技术:许多技术可以通用:互补转化基因置换基因破坏另外,在酿酒酵母中应用的术语也可用于毕赤酵母。
例如:HIS4基因都编码组氨酸脱氢酶;两者中基因产物有交叉互补;酿酒酵母中的一些野生型基因与毕赤酵母中的突变基因相互补,如HIS4、LEU2、ARG4、TR11、URA3等基因在毕赤酵母中都有各自相互补的突变基因。
毕赤酵母是甲醇营养型酵母:毕赤酵母是甲醇营养型酵母,可利用甲醇作为其唯一碳源。
甲醇代谢的第一步是:醇氧化酶利用氧分子将甲醇氧化为甲醛,还有过氧化氢。
为避免过氧化氢的毒性,甲醛代谢主要在一个特殊的细胞器-过氧化物酶体-里进行,使得有毒的副产物远离细胞其余组分。
酵母发酵手册

酵母发酵手册酵母发酵手册第一章:酵母的基本知识1.1 酵母的定义和分类\n酵母是一种单细胞真菌,常见于自然环境中的土壤、水体和植物表面。
根据其形态和生理特征,酵母可以分为两类:野生酵母和工业酵母。
野生酵母多样性较高,适应性强,但其发酵效果不稳定。
工业酵母则经过筛选和培养,具有较高的发酵效率和稳定性。
1.2 酵母的作用\n在食品加工中,酵母主要用于面包、啤酒、葡萄酒等食品的发酵过程。
它们通过分解碳水化合物产生二氧化碳和乙醇,使面团膨胀、提升口感,并赋予食品特殊的香味。
第章:使用酵母进行面包发酵2.1 面包发酵基础知识\n面包发酵是指将面粉、水和其他配料与适量的酵母混合后,在一定温度下进行发酵。
发酵过程中,酵母会分解面团中的糖分,产生二氧化碳气泡,使面团膨胀。
2.2 面包发酵步骤\n(1)准备面团:将面粉、水、盐和糖混合搅拌均匀,再加入适量的酵母。
\n(2)揉面:将面团放在台面上,用手揉搓至面团光滑有弹性。
\n(3)发酵:将揉好的面团放入一个大碗中,用湿布盖好,放置在温暖的地方进行发酵。
一般来说,温度应保持在25-30摄氏度之间。
\n(4)二次揉搓:发酵结束后,将面团取出,在台面上再次揉搓几分钟。
\n(5)成型:将揉好的面团按照所需形状进行成型。
\n(6)最后发酵:将成型好的面包放在烤盘上,在温暖的地方进行最后一次发酵。
\n(7)烘焙:预热烤箱至适当温度后,将最后发酵好的面包放入烤箱中烘焙。
第三章:酵母发酵的注意事项3.1 温度控制\n酵母的发酵效果与温度密切相关。
过高或过低的温度都会影响发酵效果。
一般来说,25-30摄氏度是最适宜的温度范围。
3.2 面团湿度\n面团湿度对发酵效果也有重要影响。
面团过于干燥会导致发酵不充分,面包口感较硬;而面团过于湿润则会导致面包松软无劲道。
3.3 面团揉搓时间\n揉搓时间也是影响发酵效果的重要因素。
揉搓时间过短会导致面筋结构不完善,影响面包体积和口感;揉搓时间过长则会使面筋过度伸展,导致面包口感较硬。
酵母发酵手册

酵母发酵手册(原创版)目录1.酵母发酵手册概述2.酵母发酵的基本原理3.酵母发酵的种类与应用4.酵母发酵的步骤与技巧5.酵母发酵的常见问题与解决方法6.酵母发酵的未来发展趋势正文一、酵母发酵手册概述酵母发酵手册是一本介绍酵母发酵技术及其应用的专着,旨在帮助读者深入了解酵母发酵的基本原理、操作方法以及在食品、饮料等产业中的广泛应用。
通过学习酵母发酵手册,读者可以掌握酵母发酵技术,从而在实际生产和生活中应用,提高产品的质量和口感。
二、酵母发酵的基本原理酵母发酵是一种生物技术,其基本原理是利用酵母菌对糖分进行代谢,产生酒精和二氧化碳的过程。
在发酵过程中,酵母菌通过分解糖分,产生能量,并释放出酒精和二氧化碳。
这个过程可以在无氧的环境下进行,因此被称为酵母发酵。
三、酵母发酵的种类与应用1.酒精发酵:酒精发酵是酵母发酵的一种,广泛应用于酿酒、制面包等方面。
通过酒精发酵,可以生产出各种酒类,如啤酒、葡萄酒、白酒等。
2.二氧化碳发酵:二氧化碳发酵主要应用于制作发酵食品,如馒头、面包、酸奶等。
在二氧化碳发酵过程中,酵母菌分解糖分,产生二氧化碳,使面团发酵,形成松软的口感。
四、酵母发酵的步骤与技巧1.酵母发酵的步骤:准备材料、激活酵母、混合面团、发酵、成型、再次发酵、烘焙(或蒸煮)等。
2.酵母发酵的技巧:掌握适宜的温度、湿度和时间;选择优质的酵母菌种;合理搭配面粉、糖、水等原料;注意卫生,防止污染等。
五、酵母发酵的常见问题与解决方法1.发酵不足:可能是酵母菌数量不足、温度过低或时间不够等原因导致。
解决方法是增加酵母菌数量、提高温度或延长发酵时间。
2.发酵过度:可能是酵母菌数量过多、温度过高或时间过长等原因导致。
解决方法是减少酵母菌数量、降低温度或缩短发酵时间。
3.面团发酸:可能是面团中的糖分过多,导致酵母菌过度发酵产生酸味。
解决方法是减少糖分,调整面团的配方。
六、酵母发酵的未来发展趋势随着科技的进步和人们生活品质的提高,酵母发酵技术在未来将不断创新和发展,更加注重绿色、环保、健康的理念。
酵母转化手册(译自Yeastmaker

质粒 DNA(浓度、纯度高) 变性的**Yeastmaker 宿主 DNA (10 µg/µl)
Small‐Scale (1.5 ml tube) 100 ng 5 µl
Library‐Scale (15 ml tube) 5–15 µg* 20µl
(* For example, use 5 µg of bait + 10 µg of prey for yeast two‐hybrid library cotransformation. )
50 µl 500 µl 30 min
600 µl 2.5 ml 45 min
6. 加入 DMSO,轻柔混匀
20 µl
160 µl
7. 在 42℃水浴锅中温浴
(注意:期间 Small‐Scale 每隔 5 min,Library‐Scale 每隔 10 min,轻轻倒混几次)
15 min
20 min
本手册仅供学习交流,不做其他用途,如需 word 版本请发送站内信。
B. 方法:转化酵母感受态细胞 1. 材料 Yeastmaker Yeast Transformation System 2 酵母感受态细胞(Section 6.A) PEG/LiAc (Section 4) 0.9% (w/v) NaCl DMSO 2. 将下列组分加入到已经预冷的无菌离心管中,混合均匀。
酵母单杂交手册(translatedbymony)

酵母单杂交手册(translatedbymony)酵母单杂交手册目录1简介2试剂盒包含的内容3缩写4 Y1H Gold 酵母菌株的相关信息5附加材料&酵母培养基A cDNA扩增、文库构建和筛选所需的附加材料B 相应的培养基要求6诱饵质粒及诱饵酵母菌株的构建A方法:合成、克隆p目的基因-AbAi质粒B方法:构建诱饵-受体酵母菌株7利用AbA r的表达检测诱饵菌株A方法:找到抑制诱饵菌株生长的最低AbA浓度8 cDNA文库的构建A方法:利用SMART技术合成cDNA第一链B方法:利用Long Distance PCR (LD-PCR)扩增cDNA第一链C方法:利用CHROMA SPIN+TE-400 Columns技术纯化合成的双链DNA9单杂交的文库的构建和筛选A方法:单杂交cDNA的文库的构建和筛选10结果分析11阳性克隆检验和猎物(prey)质粒分离A方法:通过划板确认受体表型B方法:酵母菌落PCR以消除Duplicate Clones(假阳性克隆?多拷贝?)C分离和纯化文库中可靠的具有受体活性的质粒D方法:区分阳性克隆和假阳性克隆E分析阳性克隆的序列12问题排除指南13参考文献附录A:质粒信息附录B:酵母生长培养基的配置及所需营养物附录C:SMART技术原理图1 利用Matchmaker Gold One-Hybrid System筛选蛋白-DNA的相互作用图2 将相应的片段整合到Y1HGold菌株,构建诱饵受体菌株图3 利用SMART技术和酵母的特点构建和筛选你的文库图4 通过菌落PCR确认pBait-AbAi构建成功图5 利用SMART技术合成cDNA第一链并因此合成相应的粘性末端能够整合到pGADT7-Rec 图6 利用SMART技术进行扩增图7 利用CHROMA SPIN+TE-400技术并收集产物图8 说明受体基因表达阳性克隆和假阳性克隆之间的活性差异图9 通过共转化选择性培养基验证相互作用图10 pAbAi载体和做对照的p53-AbAi载体图谱图11 pGADT7-Rec载体图谱图12 pGADT7载体图谱表格表1 Y1HGold酵母菌株基因型表2 利用各种SD培养基验证Y1HGold酵母菌株的表型表3 AbA r本底表达的预期结果表4 RNA总量与最佳循环数直接的关系表5 Matchmaker Gold One-Hybrid问题排除指南表6 Set 1酵母培养基和Set 1 Plus酵母培养基的组分表7 Matchmaker Gold Protocols所包含的每一个内容A介绍Matchmaker Gold Yeast One-Hybrid Library Screening System为建立酵母单杂交cDNA文库的建立提供了一个简单高效的方法。
酵母表达手册

酵母表达手册
酵母表达系统是一种常用于生产重组蛋白质的方法,其利用酵母细胞作为宿主来表达外源基因。
以下是酵母表达系统的基本步骤:
1. 基因克隆和转化:将目的基因克隆到酵母表达载体中,常用的载体有质粒和整合型载体。
转化方法包括电转化和化学转化。
2. 重组蛋白表达:将转化后的酵母细胞接种到发酵罐中进行培养,在适宜的温度、pH和营养条件下,目的基因在酵母细胞中表达出重组蛋白。
3. 蛋白质纯化:通过一系列的纯化技术,如离心、过滤、沉淀、亲和层析等,将重组蛋白从酵母细胞中分离出来并进行纯化。
4. 蛋白质后处理:根据需要,对纯化的重组蛋白进行进一步的后处理,如去盐、脱色、除菌等。
5. 蛋白质检测:通过SDS-PAGE、Western blot等方法检测重组蛋白的表达水平和纯度。
6. 蛋白质功能研究:对纯化的重组蛋白进行生物活性检测和功能研究,如酶活测定、免疫分析等。
在实际应用中,需要根据不同的需求选择不同的酵母表达系统,如酿酒酵母表达系统、毕赤酵母表达系统等。
同时,还需要对重组蛋白进行质量分析和稳定性研究,以确保其用于后续的实验或生产中具有可靠性和有效性。
酵母发酵手册

酵母发酵手册
摘要:
1.酵母发酵简介
2.酵母发酵的原理
3.酵母发酵的应用
4.酵母发酵的优缺点
5.酵母发酵的操作流程
6.酵母发酵的注意事项
7.酵母发酵的发展趋势
正文:
酵母发酵是一种古老的生物技术,通过利用酵母菌对糖分进行发酵,产生酒精和二氧化碳。
这种技术在食品、饮料、化工、医药等领域有着广泛的应用。
酵母发酵的原理是利用酵母菌在无氧条件下的代谢作用,将糖分分解成酒精和二氧化碳。
这一过程需要适当的温度、湿度、pH 值等条件,以保证酵母菌的正常生长和代谢。
酵母发酵的应用十分广泛。
在食品工业中,酵母发酵被用于制作面包、馒头、酒类等食品。
在饮料工业中,酵母发酵被用于制作啤酒、葡萄酒、果酒等。
在化工和医药领域,酵母发酵被用于生产乙醇、柠檬酸、抗生素等产品。
酵母发酵的优缺点需要综合考虑。
优点包括生产周期短、原料利用率高、产品收率高、污染少等。
缺点主要是技术要求高、设备投资大、生产过程中能
耗较高。
酵母发酵的操作流程包括酵母菌的培养、发酵液的制备、发酵过程的控制、产品的分离和提纯等。
其中,酵母菌的培养和发酵液的制备是关键步骤,需要严格控制温度、湿度、pH 值等条件。
酵母发酵的注意事项包括:保持无菌操作,防止细菌和其他微生物的污染;定期检查发酵过程,及时调整条件,保证酵母菌的正常生长和代谢;合理使用原料和能源,降低生产成本。
随着科技的进步,酵母发酵技术也在不断发展。
毕赤酵母表达手册

Pichia Expression KitVersion M01110225-0043Pichia Expression KitA Manual of Methods for Expression of Recombinant Proteins in Pichia pastorisCatalog no. K1710-01tech_service@iiINDIVIDUAL PICHIA EXPRESSION KIT LICENSE AGREEMENTThe Pichia Expression Kit is based on the yeast Pichia pastoris. Pichia pastoris was developed into an expression system by scientists at Salk Institute Biotechnology/Industry Associates (SIBIA) for high-level expression of recombinant proteins. All patents for Pichia pastoris and licenses for its use as an expression system are owned by Research Corporation Technologies, Inc. Tucson, Arizona. Invitrogen has an exclusive license to sell the Pichia Expression Kit to scientists for research purposes only, under the terms described below. Use of Pichia pastoris by commercial corporations requires the user to obtain a commercial license as detailed below. Before using the Pichia Expression Kit, please read the following license a greement. If you do not agree to be bound by its terms, contact Invitrogen within 10 days for authorization to return the unused Pichia Expression Kit and to receive a full credit. If you do agree to the terms of this Agreement, please complete the User Registration Card and return it to Invitrogen before using the kit.INDIVIDUAL PICHIA EXPRESSION KIT LICENSE AGREEMENTInvitrogen Corporation (INVITROGEN) grants you a non-exclusive license to use the enclosed Pichia Expression Kit (EXPRESSION KIT) for academic research or for evaluation purposes only. The EXPRESSION KIT is being transferred to you in furtherance of, and reliance on, such license. You may not use the EXPRESSION KIT, or the materials contained therein, for any commercial purpose without a license for such purpose from RESEARCH CORPORATION TECHNOLOGIES, INC., Tucson, Arizona. Commercial purposes include the use in or sale of expressed proteins as a commercial product, or use to facilitate or advance research or development of a commercial product. Commercial entities may conduct their evaluation for one year at which time this license automatically terminates. Commercial entities will be contacted by Research Corporation Technologies during the evaluation period regarding the purchase of a commercial license.Access to the EXPRESSION KIT must be limited solely to those officers, employees and students of your institution who need access thereto in order to perform the above-described research or evaluation. You must inform each of such officer, employee and student of the provisions of this Agreement and require them to agree, in writing, to be bound by the provisions of this Agreement. You may not distribute the EXPRESSION KIT to others, even those within your own institution. You may transfer modified, altered or original material from the EXPRESSION KIT to a third party following notification of INVITROGEN such that the recipient can be licensed. You may not assign, sub-license, rent lease or otherwise transfer this License or any of the rights or obligation hereunder, except as expressly permitted.This License is effective until terminated. You may terminate it at any time by destroying all Pichia expression products in your control. It will also terminate automatically if you fail to comply with the terms and conditions of the Agreement. You shall, upon termination of the License, destroy all Pichia Expression Kits in your control, and so notify INVITROGEN in writing.This License Shall be governed in its interpretation and enforcement by the laws of the State of California.Product User Registration CardPlease complete and return the enclosed Product User Registration Card for each Pichia Expression Kit that you purchase. This will serve as a record of your purchase and registration and will allow Invitrogen to provide you with technical support and manual updates. It will also allow Invitrogen to update you on future developments of and improvements to the Pichia Expression Kit. The agreement outlined above becomes effective upon our receipt of your User Registration Card or 10 days following the sale of the Pichia Expression Kit to you. Use of the kit at any time results in immediate obligation to the terms and conditions stated in this Agreement.Technical ServicesInvitrogen provides Technical Services to all of our registered Pichia Expression Kit users. Please contact us if you need assistance with the Pichia Expression Kit.United States Headquarters:Japanese Headquarters European Headquarters:Invitrogen Corporation1600 Faraday AvenueCarlsbad, CA 92008 USATel: 1 760 603 7200Tel (Toll Free): 1 800 955 6288 Fax: 1 760 602 6500E-mail:tech_service@ Invitrogen Japan K.K.Nihonbashi Hama-Cho Park Bldg. 4F2-35-4, Hama-Cho, NihonbashiTel: 81 3 3663 7972Fax: 81 3 3663 8242E-mail: jpinfo@Invitrogen Ltd3 Fountain DriveInchinnan Business ParkPaisley PA4 9RF, UKTel (Free Phone Orders): 0800 269 210Tel (General Enquiries): 0800 5345 5345Fax: +44 (0) 141 814 6287E-mail: eurotech@iiiivTable of ContentsMaterials (vii)Purchaser Notification (x)Product Qualification (xii)Introduction (1)Overview (1)Experimental Outline (3)Recombination and Integration in Pichia (7)Methods (11)Pichia Strains (11)E. coli Strains (13)Selecting a Pichia Expression Vector (14)pHIL-D2 (16)pPIC3.5 (17)pHIL-S1 (18)pPIC9 (19)Signal Sequence Processing (20)Cloning into the Pichia Expression Vectors (21)Transformation into E. coli (26)Preparation of Transforming DNA (27)Growth of Pichia for Spheroplasting (30)Preparation of Spheroplasts (32)Transformation of Pichia (34)Screening for Mut+ and Mut S Transformants (36)PCR Analysis of Pichia Integrants (40)Expression of Recombinant Pichia Strains (42)Analysis by SDS-Polyacrylamide Gel Electrophoresis (45)Optimization of Pichia Protein Expression (47)Scale-up of Expression (49)Protein Purification and Glycosylation (51)Recipes (53)E. coli Media Recipes (53)Pichia Media Recipes (54)Appendix (59)Electroporation of Pichia (59)PEG 1000 Transformation Method for Pichia (60)Lithium Chloride Transformation Method (61)Total DNA Isolation from Pichia (62)Detection of Multiple Integration Events (63)Procedure for Total RNA Isolation from Pichia (64)β-Galactosidase Assay (65)Technical Service (67)References (69)vviMaterialsKit Contents Box 1: Spheroplast Module. Store at room temperature.Reagent Amount ComponentsSOS media 20 ml 1 M Sorbitol0.3X YPD10 mM CaCl2Sterile Water 2 x 125 ml Autoclaved, deionized waterSE 2 x 125 ml 1 M Sorbitol25 mM EDTA, pH 8.0SCE 2 x 125 ml 1 M Sorbitol10 mM Sodium citrate buffer, pH 5.81 mM EDTA1 M Sorbitol2 x 125 ml --CaS 2 x 60 ml 1 M Sorbitol10 mM Tris-HCl, pH 7.5;10 mM CaCl240% PEG 25 ml 40% (w/v) PEG 3350 (Reagent grade) in waterCaT 25 ml 20 mM Tris-HCl, pH 7.520 mM CaCl2Stab Vials: Pichia and E. coli stabs. Store at +4°C.Phenotype(Pichia only)GenotypeStrain Amountstab his4Mut+GS115 1stab arg4 his4 aox1::ARG4 Mut S, Arg+KM71 1GS115 Albumin 1 stab HIS4Mut SGS115 β-Gal 1 stab HIS4Mut+stab F´ {pro AB, lac I q, lac Z∆M15, Tn10 (Tet R)} mcr A,TOP10F´ 1∆(mrr-hsd RMS-mcr BC), φ80lac Z∆M15, ∆lac X74,deo R, rec A1, ara D139, ∆(ara-leu)7697, gal U,gal K, rps L (Str R), end A1, nup G λ-.Box 2: Spheroplast Module. Store at -20°C.ComponentsReagent AmountZymolyase 10 x 20 µl 3 mg/ml Zymolyase in water(100,000 units/g lytic activity)1 M DTT 10 x 1 ml 1 M dithiothreitol in watercontinued on next pageviiKit Contents,continuedVector Box. Store at -20°C.Reagent DescriptionpHIL-D210 µg, lyophilized in TE, pH 8.0Vector for intracellular expression in PichiapPIC3.510 µg, lyophilized in TE, pH 8.0Vector for intracellular expression in PichiapHIL-S110 µg, lyophilized in TE, pH 8.0 Vector for secreted expression in Pichia. Uses the PHO1 signal sequencepPIC910 µg, lyophilized in TE, pH 8.0 Vector for secreted expression in Pichia. Uses the α-factor signal sequencePrimer Box. Store at -20°C.5´ AOX1 sequencing primer2 µg (312 pmoles), lyophilized5´-GACTGGTTCCAATTGACAAGC-3´3´ AOX1 sequencing primer2 µg (314 pmoles), lyophilized5´-GCAAATGGCATTCTGACATCC-3´α-Factor sequencing primer2 µg (315 pmoles), lyophilized5´-TACTATTGCCAGCATTGCTGC-3´Media The following prepackaged media is included for your convenience. Instructions for use are provided on the package.Media Amount Yield YP Base Medium 2 pouches 2 liters of YP mediumYP Base Agar Medium 2 pouches 2 liters of YP mediumYeast Nitrogen Base 1 pouch 500 ml of 10X YNBFor transformation of Pichia by spheroplasting, the Pichia Spheroplast Module isavailable separately from Invitrogen (see below for ordering information).Product Reactions or Amount Catalog no.Pichia Spheroplast Module 10 spheroplast preparations(50 transformations)K1720-01continued on next pageviiiRequired Equip-ment and Supplies (not provided) • 30°C rotary shaking incubator• Water baths capable of 37°C, 45°C, and 100°C• Centrifuge suitable for 50 ml conical tubes (floor or table-top)• Baffled culture flasks with metal covers (50 ml, 250 ml, 500 ml, 1000 ml, and 3 L)• 50 ml sterile, conical tubes• 6 ml and 15 ml sterile snap-top tubes (Falcon 2059 or similar)• UVSpectrophotometer• Mini agarose gel apparatus and buffers• Polyacrylamide Gel Electrophoresis apparatus and buffers• Media for transformation, growth, screening, and expression (see Recipes, pages 53-58) • 5% SDS solution (10 ml per transformation)• Sterile cheesecloth or gauze• Breaking Buffer (see Recipes, page 58)• Acid-washed glass beads (available from Sigma)• Replica-plating equipment (optional)• BeadBreaker™ (optional)ixPurchaser NotificationIntroduction The Pichia Expression Kit is based on the yeast Pichia pastoris. Pichia pastoris wasdeveloped into an expression system by scientists at Salk Institute Biotechnology/ IndustryAssociates (SIBIA) and Phillips Petroleum for high-level expression of recombinantproteins. All patents for Pichia pastoris and licenses for its use as an expression system areowned by Research Corporation Technologies (RCT), Inc., Tucson, Arizona. Forinformation on commercial licenses, please see page x.The Nature of the Invitrogen License Invitrogen has an exclusive license to sell the Pichia Expression Kit to scientists for research purposes only, under the terms described below. Use of Pichia pastoris by commercial entities for any commercial purpose requires the user to obtain a commercial license as detailed below. Before using the Pichia Expression Kit, please read the following license agreement. If you do not agree to be bound by its terms, contact Invitrogen within 10 days for authorization to return the unused Pichia Expression Kit and to receive a full credit. If you do agree to the terms of this license agreement, please complete the User Registration Card and return it to Invitrogen before using the kit.Pichia pastoris Patents Pichia pastoris is covered by one or more of the following U.S. patents and corresponding foreign patents owned and licensed by Research Corporation Technologies:4,683,293 4,808,537 4,812,405 4,818,700 4,837,148 4,855,231 4,857,467 4,879,231 4,882,279 4,885,242 4,895,800 4,929,555 5,002,876 5,004,688 5,032,516 5,122,465 5,135,868 5,166,329Individual Pichia Expression Kit License Agreement Invitrogen Corporation ("Invitrogen") grants you a non-exclusive license to use the enclosed Pichia Expression Kit ("Expression Kit") for academic research or for evaluation purposes only. The Expression Kit is being transferred to you in furtherance of, and reliance on, such license. You may not use the Expression Kit, or the materials contained therein, for any commercial purpose without a license for such purpose from Research Corporation Technologies, Inc., Tucson, Arizona.Definition of Commercial Purpose Commercial purposes include:(a) any use of Expression Products in a Commercial Product(b) any use of Expression Products in the manufacture of a Commercial Product(c) any sale of Expression Products(d) any use of Expression Products or the Expression Kit to facilitate or advanceresearch or development of a Commercial Product(e) any use of Expression Products or the Expression Kit to facilitate or advance anyresearch or development program the results of which will be applied to thedevelopment of Commercial Products"Expression Products" means products expressed with the Expression Kit, or with the use of any vectors or host strains in the Expression Kit. "Commercial Product" means any product intended for sale or commercial use.Commercial entities may conduct their evaluation for one year at which time this license automatically terminates. Research Corporation Technologies will contact commercial entities during the evaluation period regarding their desire for a commercial license.continued on next pagexPurchaser Notification, continuedIndividual Responsibilities Access to the Expression Kit must be limited solely to those officers, employees and students of your institution who need access to perform the above-described research or evaluation. You must inform each such officer, employee and student of the provisions of this license agreement and require them to agree, in writing, to be bound by the provisions of this license agreement. You may not distribute neither the Expression Kit nor the vectors or host strains contained in it to others, even to those within your own institution. You may only transfer modified, altered, or original material from the Expression Kit to a third party following written notification of, and written approval from, Invitrogen so that the recipient can be licensed. You may not assign, sub-license, rent, lease or otherwise transfer this license agreement or any of the rights or obligation thereunder, except as expressly permitted by Invitrogen and RCT.Termination of License This license agreement is effective until terminated. You may terminate it at any time by destroying all Pichia expression products in your control. It will also terminate auto-matically if you fail to comply with the terms and conditions of the license agreement. You shall, upon termination of the license agreement, destroy all Pichia Expression Kits in your control, and so notify Invitrogen in writing.This License shall be governed in its interpretation and enforcement by the laws of the State of California.Contact for Commercial Licensing Bennett Cohen, Ph.D.Research Corporation Technologies 101 North Wilmot Road, Suite 600 Tucson, Arizona 85711-3335 Phone: (520) 748-4400Fax: (520)748-0025User Registration Card Please complete and return the enclosed User Registration Card for each PichiaExpression Kit that you purchase. This will serve as a record of your purchase and regis-tration and will allow Invitrogen to provide you with technical support and manualupdates. It will also allow Invitrogen to update you on future developments and improve-ments to the Pichia Expression Kit. The agreement outlined above becomes effectiveupon our receipt of your User Registration Card or 10 days following the sale of thePichia Expression Kit to you. Use of the kit at any time results in immediate obligation tothe terms and conditions stated in this license agreement.xiProduct QualificationIntroduction This section describes the criteria used to qualify the components in the PichiaExpression Kit.Vectors All expression vectors are qualified by restriction enzyme digestion. Restriction digests must demonstrate the correct banding pattern when electrophoresed on an agarose gel.Spheroplast Reagents The spheroplast reagents are qualified by spheroplast preparation of GS115 following the protocol provided in the Pichia Expression Kit manual. At least 70% of the Pichia pastoris cells must form spheroplasts in 30 minutes or less.Pichia Strains The Pichia strains are by demonstrating viability of the culture. Single colonies should arise within 48 hours after streaking on YPD medium from the stabPrimers Sequencing primers are lot tested by automated DNA sequencing experiments.Buffers andSolutionsAll buffers and solutions are extensively tested for sterility.Media All Pichia growth and expression media are qualified by growing the GS115 Pichiastrain.xiiIntroductionOverviewReview Articles The information presented here is designed to give you a concise overview of the Pichia pastoris expression system. It is by no means exhaustive. For further information, pleaseread the articles cited in the text along with recent review articles (Buckholz and Gleeson,1991; Cregg et al., 1993; Sreekrishna et al., 1988; Wegner, 1990). A general review offoreign gene expression in yeast is also available (Romanos et al., 1992).General Characteristics of Pichia pastoris As a eukaryote, Pichia pastoris has many of the advantages of higher eukaryotic expression systems such as protein processing, protein folding, and posttranslational modification, while being as easy to manipulate as E. coli or Saccharomyces cerevisiae. It is faster, easier, and less expensive to use than other eukaryotic expression systems such as baculovirus or mammalian tissue culture, and generally gives higher expression levels. As a yeast, it shares the advantages of molecular and genetic manipulations with Saccharomyces, and has the added advantage of 10- to 100-fold higher heterologous protein expression levels. These features make Pichia very useful as a protein expression system.Similarity to Saccharomyces Many of the techniques developed for Saccharomyces may be applied to Pichia including: • transformation by complementation• genedisruption• genereplacementIn addition, the genetic nomenclature used for Saccharomyces has been applied to Pichia. For example, the HIS4 gene in both Saccharomyces and Pichia encodes histidinol dehydrogenase. There is also cross-complementation between gene products in both Saccharomyces and Pichia. Several wild-type genes from Saccharomyces complement comparable mutant genes in Pichia. Genes such as HIS4, LEU2, ARG4, TRP1, and URA3 all complement their respective mutant genes in Pichia.Pichia pastoris as a Methylotrophic Yeast Pichia pastoris is a methylotrophic yeast, capable of metabolizing methanol as its sole carbon source. The first step in the metabolism of methanol is the oxidation of methanol to formaldehyde using molecular oxygen by the enzyme alcohol oxidase. This reaction generates both formaldehyde and hydrogen peroxide. To avoid hydrogen peroxide toxicity, methanol metabolism takes place within a specialized cell organelle called the peroxisome, which sequesters toxic by-products from the rest of the cell. Alcohol oxidase has a poor affinity for O2, and Pichia pastoris compensates by generating large amounts of the enzyme. The promoter regulating the production of alcohol oxidase drives heterologous protein expression in Pichia.Two Alcohol Oxidase Proteins The AOX1 and AOX2 genes code for alcohol oxidase in Pichia pastoris. The AOX1 gene product accounts for the majority of alcohol oxidase activity in the cell. Expression of the AOX1 gene is tightly regulated and induced by methanol to high levels, typically > 30% ofthe total soluble protein in cells grown with methanol as the carbon source. The AOX1 gene has been isolated and the AOX1 promoter is used to drive expression of the gene of interest (Ellis et al., 1985; Koutz et al., 1989; Tschopp et al., 1987a). While AOX2 is about 97% homologous to AOX1, growth on methanol is much slower than with AOX1. This slowgrowth allows isolation of Mut S strains (aox1) (Cregg et al., 1989; Koutz et al., 1989).continued on next page1Overview, continuedExpression Expression of the AOX1 gene is controlled at the level of transcription. In methanol-grown cells approximately 5% of the polyA+ RNA is from the AOX1 gene. The regulation of theAOX1 gene is a two step process: a repression/derepression mechanism plus an inductionmechanism (e.g. GAL1 gene in Saccharomyces (Johnston, 1987)). Briefly, growth onglucose represses transcription, even in the presence of the inducer methanol. For thisreason, growth on glycerol is recommended for optimal induction with methanol. Pleasenote that growth on glycerol (derepression) is not sufficient to generate even minute levelsof expression from the AOX1 gene. The inducer, methanol, is necessary for detectablelevels of AOX1 expression (Ellis et al., 1985; Koutz et al., 1989; Tschopp et al., 1987a).Phenotype of aox1 mutants Loss of the AOX1 gene, and thus a loss of most of the cell's alcohol oxidase activity, results in a strain that is phenotypically Mut S (Methanol utilization slow). This has in the past been referred to as Mut. The Mut S designation has been chosen to accurately describe the phenotype of these mutants. This results in a reduction in the cells' ability to metabolize methanol. The cells, therefore, exhibit poor growth on methanol medium. Mut+ (Methanol utilization plus) refers to the wild type ability of strains to metabolize methanol as the sole carbon source. These two phenotypes are used when evaluating Pichia transformants for integration of your gene (Experimental Outline, page 3).Intracellular and Secretory Protein Expression Heterologous expression in Pichia can be either intracellular or secreted. Secretion requires the presence of a signal sequence on the expressed protein to target it to the secretory pathway. While several different secretion signal sequences have been used successfully, including the native secretion signal present on some heterologous proteins, success has been variable. The secretion signal sequence from the Saccharomyces cerevisiaeα factor prepro peptide has been used most successfully (Cregg et al., 1993; Scorer et al., 1993).The major advantage of expressing heterologous proteins as secreted proteins is that Pichia pastoris secretes very low levels of native proteins. That, combined with the very low amount of protein in the minimal Pichia growth medium, means that the secreted heterologous protein comprises the vast majority of the total protein in the medium and serves as the first step in purification of the protein (Barr et al., 1992). Note: If there are recognized glycosylation sites (Asn-X-Ser/Thr) in your protein's primary sequence, glycosylation may occur at these sites.Posttranslational Modifications In comparison to Saccharomyces cerevisiae, Pichia may have an advantage in the glyco-sylation of secreted proteins because it may not hyperglycosylate. Both Saccharomyces cerevisiae and Pichia pastoris have a majority of N-linked glycosylation of the high-mannose type; however, the length of the oligosaccharide chains added posttranslationally to proteins in Pichia (average 8-14 mannose residues per side chain) is much shorter than those in S. cerevisiae (50-150 mannose residues) (Grinna and Tschopp, 1989; Tschopp et al., 1987b). Very little O-linked glycosylation has been observed in Pichia.In addition, Saccharomyces cerevisiae core oligosaccharides have terminal α1,3 glycan linkages whereas Pichia pastoris does not. It is believed that the α1,3 glycan linkages in glycosylated proteins produced from Saccharomyces cerevisiae are primarily responsible for the hyper-antigenic nature of these proteins making them particularly unsuitable for therapeutic use. Although not proven, this is predicted to be less of a problem for glycoproteins generated in Pichia pastoris, because it may resemble the glycoprotein structure of higher eukaryotes (Cregg et al., 1993).2Experimental OutlineSelection of Vector and Cloning To utilize the strong, highly inducible P AOX1 promoter for expression of your protein, four expression vectors are included in this kit. pHIL-D2 and pPIC3.5 are used for intracellular expression while pHIL-S1 and pPIC9 are used for secreted expression (see pages 14-19 for more information). Before cloning your insert, you must...• decide whether you want intracellular or secreted expression.• analyze your insert for the following restriction sites: Sac I, Stu I, Sal I, Not I, and Bgl II. These sites are recommended for linearizing your construct prior to Pichiatransformation. If your insert has all of these sites, see pages 28-29 for alternate sites.Transformation and IntegrationTwo different phenotypic classes of His+ recombinant strains can be generated: Mut+ and Mut S. Mut S refers to the "Methanol utilization slow" phenotype caused by the loss of alcohol oxidase activity encoded by the AOX1 gene. A strain with a Mut S phenotype has a mutant aox1 locus, but is wild type for AOX2. This results in a slow growth phenotype on methanol medium. Transformation of strain GS115 can yield both classes of transformants, His+ Mut+ and His+Mut S, while KM71 yields only His+ Mut S since the strain itself is Mut S. Both Mut+ and Mut S recombinants are useful to have as one phenotype may favor better expression of your protein than the other. Due to clonal variation, you should test 6-10 recombinants per phenotype. There is no way to predict beforehand which construct or isolate will better express your protein. We strongly recommend that you analyze Pichia recombinants by PCR to confirm integration of your construct (see page 40).Once you have successfully cloned your gene, you will then linearize your plasmid to stimulate recombination when the plasmid is transformed into Pichia. The table below describes the types of recombinants you will get by selective digestion of your plasmid. RestrictionEnzymeIntegration Event GS115 Phenotype KM71 PhenotypeSal I or Stu I Insertion at his4His+ Mut+ His+ Mut SSac I Insertion at 5´AOX1 regionHis+ Mut+ His+ Mut SNot I or Bgl II Replacement atAOX1 locusHis+ Mut SHis+ Mut+His+ Mut S (notrecommended, see page 11)Expression and Scale-up After confirming your Pichia recombinants by PCR, you will test expression of both His+Mut+ and His+ Mut S recombinants. This will involve growing a small culture of each recombinant, inducing with methanol, and taking time points. If looking for intracellular expression, analyze the cell pellet from each time point by SDS polyacrylamide gel electrophoresis (SDS-PAGE). If looking for secreted expression, analyze both the cellpellet and supernatant from each time point. We recommend that you analyze your SDS-PAGE gels by both Coomassie staining and Western blot, if you have an antibody to your protein. We also suggest checking for protein activity by assay, if one is available. Not all proteins express to the level of grams per liter, so it is advisable to check by Western blotor activity assay, and not just by Coomassie staining of SDS-PAGE gels for production of your protein.Choose the Pichia recombinant strain that best expresses your protein and optimizeinduction based on the suggestions on pages 47-48. Once expression is optimized, scale-up your expression protocol to produce more protein.continued on next page3。
毕赤酵母菌种培养手册

毕赤酵母菌种培养手册1. 引言本手册旨在提供毕赤酵母菌种培养的详细步骤和注意事项。
毕赤酵母(Saccharomyces cerevisiae)被广泛应用于食品工业、酿酒业和生物学研究等领域。
通过正确的菌种培养技术,可以确保毕赤酵母的活力和纯度,从而保证实验和应用的可靠性和准确性。
2. 材料和方法2.1 培养基选择适合的培养基是培养毕赤酵母的关键。
常用的培养基包括YPD培养基、SD培养基和SC培养基等。
根据具体实验需求选择合适的培养基配方,并按照相应操作说明制备。
2.2 菌种的制备和传代1. 从冰冻保存的毕赤酵母菌种中取出适量菌种转移到无菌培养基中。
2. 在适当的温度(通常为30°C)下培养菌种至对数生长期。
3. 取适量无菌培养基转移菌种,传代培养。
2.3 菌种培养1. 取适量菌种转移到含有适量无菌培养基的培养瓶中。
2. 控制培养瓶中的菌液浓度,通常为OD600=0.5。
3. 在适当的温度(通常为30°C)下培养菌种至对数生长期或其他实验所需生长期。
2.4 菌种保存菌种的保存有助于长期维持活力和纯度。
常用的保存方法包括冷冻保存和制备冻干菌种等。
3. 结果和讨论通过本手册提供的方法,可以成功培养并维持毕赤酵母菌种的活力和纯度。
在培养过程中,应注意操作的无菌性和培养条件的合适性。
此外,根据具体实验需求,可适当调整菌液的浓度和培养温度等参数。
4. 总结本手册详细介绍了毕赤酵母菌种培养的步骤和注意事项。
正确的菌种培养技术对于保证实验和应用的可靠性和准确性至关重要。
通过遵循本手册的指南和方法,可以有效地培养毕赤酵母菌种,并取得可靠的实验结果。
请注意,本手册仅提供参考,并且在使用过程中应遵守相关的实验室安全操作和法律法规要求。
毕赤酵母发酵手册

Version B Pichia Fermentation Process GuidelinesOverviewIntroduction Pichia pastoris, like Saccharomyces cerevisiae, is particularly well-suited forfermentative growth. Pichia has the ability to reach very high cell densities duringfermentation which may improve overall protein yields.We recommend that only those with fermentation experience or those who have accessto people with experience attempt fermentation. Since there are a wide variety offermenters available, it is difficult to provide exact procedures for your particular case.The guidelines given below are based on fermentations of both Mut+ and Mut S Pichiastrains in a 15 liter table-top glass fermenter. Please read the operator's manual for yourparticular fermenter before beginning. The table below provides an overview of thematerial covered in these guidelines.Step Topic Page1 Fermentationparameters 12 Equipment needed and preparation of medium 23 Measurement and use of dissolved oxygen (DO) in the culture 34 Growth of the inoculum 45 Generation of biomass on glycerol in batch and fed-batch phases 4-56 Induction of expression of Mut+ and Mut S recombinants in themethanol fed-batch phase6-77 Harvesting and lysis of cells 88 References 9-109 Recipes 11Fermentation Parameters It is important to monitor and control the following parameters throughout thefermentation process. The following table describes the parameters and the reasons for monitoring them.Parameter Reason Temperature (30.0°C) Growth above 32°C is detrimental to protein expression Dissolved oxygen (>20%) Pichia needs oxygen to metabolize glycerol andmethanolpH (5.0-6.0 and 3.0) Important when secreting protein into the medium andfor optimal growthAgitation (500 to 1500 rpm) Maximizes oxygen concentration in the mediumAeration (0.1 to 1.0 vvm*for glass fermenters)Maximizes oxygen concentration in the medium whichdepends on the vesselAntifoam (the minimumneeded to eliminate foam)Excess foam may cause denaturation of your secretedprotein and it also reduces headspaceCarbon source (variablerate)Must be able to add different carbon sources at differentrates during the course of fermentationcontinued on next pageOverview, continuedRecommended Equipment Below is a checklist for equipment recommendations.• A jacketed vessel is needed for cooling the yeast during fermentation, especially during methanol induction. You will need a constant source of cold water (5-10°C). This requirement may mean that you need a refrigeration unit to keep the water cold. • A foam probe is highly recommended as antifoam is required.• A source of O2--either air (stainless steel fermenters at 1-2 vvm) or pure O2(0.1-0.3 vvm for glass fermenters).• Calibrated peristaltic pumps to feed the glycerol and methanol.• Automatic control of pH.Medium Preparation You will need to prepare the appropriate amount of following solutions:• Fermentation Basal Salts (page 11)• PTM1Trace Salts (page 11)• ~75 ml per liter initial fermentation volume of 50% glycerol containing 12 ml PTM1 Trace Salts per liter of glycerol.• ~740 ml per liter initial fermentation volume of 100% methanol containing 12 mlPTM1Trace Salts per liter of methanol.Monitoring the Growth of Pichia pastoris Cell growth is monitored at various time points by using the absorbance at 600 nm (OD600) and the wet cell weight. The metabolic rate of the culture is monitored by observing changes in the concentration of dissolved oxygen in response to carbon availability (see next page).Dissolved Oxygen (DO) MeasurementIntroduction The dissolved oxygen concentration is the relative percent of oxygen in the mediumwhere 100% is O2-saturated medium. Pichia will consume oxygen as it grows, reducing the dissolved oxygen content. However, because oxygen is required for the first step ofmethanol catabolism, it is important to maintain the dissolved oxygen (DO) concentra-tion at a certain level (>20%) to ensure growth of Pichia on methanol. Accuratemeasurement and observation of the dissolved oxygen concentration of a culture willgive you important information about the state and health of the culture. Therefore, it isimportant to accurately calibrate your equipment. Please refer to your operator's manual.Maintaining the Dissolved Oxygen Concentration (DO) 1. Maintaining the dissolved oxygen above 20% may be difficult depending on theoxygen transfer rates (OTR) of the fermenter, especially in small-scale glassvessels. In a glass vessel, oxygen is needed to keep the DO above 20%, usually~0.1-0.3 vvm (liters of O2per liter of fermentation culture per minute). Oxygen consumption varies and depends on the amount of methanol added and the protein being expressed.2. Oxygen can be used at 0.1 to 0.3 vvm to achieve adequate levels. This can beaccomplished by mixing with the air feed and can be done in any glass fermenter.For stainless steel vessels, pressure can be used to increase the OTR. Be sure toread the operator's manual for your particular fermenter.3. If a fermenter cannot supply the necessary levels of oxygen, then the methanol feedshould be scaled back accordingly. Note that decreasing the amount of methanol may reduce the level of protein expression.4. To reach maximum expression levels, the fermentation time can be increased todeliver similar levels of methanol at the lower feed rate. For many recombinantproteins, a direct correlation between amount of methanol consumed and theamount of protein produced has been observed.Use of DO Measurements During growth, the culture consumes oxygen, keeping the DO concentration low. Note that oxygen is consumed whether the culture is grown on glycerol or methanol. The DO concentration can be manipulated to evaluate the metabolic rate of the culture and whether the carbon source is limiting. The metabolic rate indicates how healthy the culture is. Determining whether the carbon source is limiting is important if you wish to fully induce the AOX1 promoter. For example, changes in the DO concentrations (DO spikes) allow you to determine whether all the glycerol is consumed from the culture before adding methanol. Secondly, it ensures that your methanol feed does not exceed the rate of consumption. Excess methanol (> 1-2% v/v) may be toxic.Manipulation of DO If carbon is limiting, shutting off the carbon source should cause the culture to decrease its metabolic rate, and the DO to rise (spike). Terminate the carbon feed and time how long it takes for the DO to rise 10%, after which the carbon feed is turned back on. If the lag time is short (< 1 minute), the carbon source is limiting.Fermenter Preparation and Glycerol Batch PhaseInoculum Seed Flask Preparation Remember not to put too much medium in the baffled flasks. Volume should be 10-30% of the total flask volume.1. Baffled flasks containing a total of 5-10% of the initial fermentation volume ofMGY or BMGY are inoculated with a colony from a MD or MGY plate or from a frozen glycerol stock.2. Flasks are grown at 30°C, 250-300 rpm, 16-24 hours until OD600= 2-6. Toaccurately measure OD600> 1.0, dilute a sample of your culture 10-fold before reading.Glycerol Batch Phase 1. Sterilize the fermenter with the Fermentation Basal Salts medium containing 4%glycerol (see page 11).2. After sterilization and cooling, set temperature to 30°C, agitation and aeration tooperating conditions (usually maximum rpm and 0.1-1.0 vvm air), and adjust the pH of the Fermentation Basal Salts medium to 5.0 with 28% ammonium hydroxide(undiluted ammonium hydroxide). Add aseptically 4.35 ml PTM1trace salts/liter of Fermentation Basal Salts medium.3. Inoculate fermenter with approximately 5-10% initial fermentation volume from theculture generated in the inoculum shake flasks. Note that the DO will be close to 100% before the culture starts to grow. As the culture grows, it will consumeoxygen, causing the DO to decrease. Be sure to keep the DO above 20% by adding oxygen as needed.4. Grow the batch culture until the glycerol is completely consumed (18 to 24 hours).This is indicated by an increase in the DO to 100%. Note that the length of timeneeded to consume all the glycerol will vary with the density of the initial inoculum.5. Sampling is performed at the end of each fermentation stage and at least twice daily.We take 10 ml samples for each time point, then take 1 ml aliquots from this 10 mlsample. Samples are analyzed for cell growth (OD600and wet cell weight), pH, microscopic purity, and protein concentrations or activity. Freeze the cell pellets and supernatants at -80°C for later analysis. Proceed to Glycerol Fed-Batch Phase,page 5.Yield A cellular yield of 90 to 150 g/liter wet cells is expected for this stage. Recombinant protein will not yet be produced due to the absence of methanol.Introduction Once all the glycerol is consumed from the batch growth phase, a glycerol feed isinitiated to increase the cell biomass under limiting conditions. When you are ready toinduce with methanol, you can use DO spikes to make sure the glycerol is limited.Glycerol Fed-Batch Phase 1. Initiate a 50% w/v glycerol feed containing 12 ml PTM1trace salts per liter of glycerol feed. Set the feed rate to 18.15 ml/hr /liter initial fermentation volume.2. Glycerol feeding is carried out for about four hours or longer (see below). A cellularyield of 180 to 220 g/liter wet cells should be achieved at the end of this stage while no appreciable recombinant protein is produced.Note The level of expressed protein depends on the cell mass generated during the glycerolfed-batch phase. The length of this feed can be varied to optimize protein yield. A rangeof 50 to 300 g/liter wet cells is recommended for study. A maximum level of 4%glycerol is recommended in the batch phase due to toxicity problems with higher levelsof glycerol.Important If dissolved oxygen falls below 20%, the glycerol or methanol feed should bestopped and nothing should be done to increase oxygen rates until the dissolvedoxygen spikes. At this point, adjustments can be made to agitation, aeration, pressure oroxygen feeding.Proteases In the literature, it has been reported that if the pH of the fermentation medium islowered to 3.0, neutral proteases are inhibited. If you think neutral proteases aredecreasing your protein yield, change the pH control set point to 3.0 during the glycerolfed-batch phase (above) or at the beginning of the methanol induction (next page) andallow the metabolic activity of the culture to slowly lower the pH to 3.0 over 4 to 5 hours(Brierley, et al., 1994; Siegel, et al., 1990).Alternatively, if your protein is sensitive to low pH, it has been reported that inclusion ofcasamino acids also decreases protease activity (Clare, et al., 1991).Introduction All of the glycerol needs to be consumed before starting the methanol feed to fullyinduce the AOX1 promoter on methanol. However, it has been reported that a "mixedfeed" of glycerol and methanol has been successful to express recombinant proteins(Brierley, et al., 1990; Sreekrishna, et al., 1989). It is important to introduce methanolslowly to adapt the culture to growth on methanol. If methanol is added too fast, it willkill the cells. Once the culture is adapted to methanol, it is very important to use DOspikes to analyze the state of the culture and to take time points over the course ofmethanol induction to optimize protein expression. Growth on methanol also generates alot of heat, so temperature control at this stage is very important.Mut+ Methanol Fed-Batch Phase 1. Terminate glycerol feed and initiate induction by starting a 100% methanol feedcontaining 12 ml PTM1trace salts per liter of methanol. Set the feed rate to 3.6 ml/hr per liter initial fermentation volume.2. During the first 2-3 hours, methanol will accumulate in the fermenter and thedissolved oxygen values will be erratic while the culture adapts to methanol.Eventually the DO reading will stabilize and remain constant.3. If the DO cannot be maintained above 20%, stop the methanol feed, wait for theDO to spike and continue on with the current methanol feed rate. Increaseagitation, aeration, pressure or oxygen feeding to maintain the DO above 20%. 4. When the culture is fully adapted to methanol utilization (2-4 hours), and is limitedon methanol, it will have a steady DO reading and a fast DO spike time (generally under 1 minute). Maintain the lower methanol feed rate under limited conditions for at least 1 hour after adaptation before doubling the feed. The feed rate is then doubled to ~7.3 ml/hr/liter initial fermentation volume.5 After 2 hours at the 7.3 ml/hr/liter feed rate, increase the methanol feed rate to~10.9 ml/hr per liter initial fermentation volume. This feed rate is maintainedthroughout the remainder of the fermentation.6. The entire methanol fed-batch phase lasts approximately 70 hours with a total ofapproximately 740 ml methanol fed per liter of initial volume. However, this may vary for different proteins.Note: The supernatant may appear greenish. This is normal.Yield The cell density can increase during the methanol fed-batch phase to a final level of 350 to 450 g/liter wet cells. Remember that because most of the fermentation is carried out ina fed-batch mode, the final fermentation volume will be approximately double the initialfermentation volume.Fermentation of Mut S Pichia Strains Since Mut S cultures metabolize methanol poorly, their oxygen consumption is very low. Therefore, you cannot use DO spikes to evaluate the culture. In standard fermentations of a Mut S strain, the methanol feed rate is adjusted to maintain an excess of methanol in the medium which does not exceed 0.3% (may be determined by gas chromatography). While analysis by gas chromatography will insure that nontoxic levels of methanol are maintained, we have used the empirical guidelines below to express protein in Mut S strains. A gas chromatograph is useful for analyzing and optimizing growth of Mut S recombinants.continued on next pageMethanol Fed-Batch Phase, continuedMut S Methanol Fed- Batch Phase The first two phases of the glycerol batch and fed-batch fermentations of the Mut S strains are conducted as described for the Mut+ strain fermentations. The methanol induction phases of the Mut+ and Mut S differ in terms of the manner and amount in which the methanol feed is added to the cultures.1. The methanol feed containing 12 ml PTM1trace salts per liter of methanol is initiated at 1 ml/hr/liter initial fermentation volume for the first two hours. It is then increased in 10% increments every 30 minutes to a rate of 3 ml/hr which ismaintained for the duration of the fermentation.2.. The vessel is then harvested after ~100 hours on methanol. This time may be variedto optimize protein expression.Harvesting and Lysis of CellsIntroduction The methods and equipment listed below are by no means complete. The amount of cells or the volume of supernatant will determine what sort of equipment you need.Harvesting Cells and Supernatant For small fermentations (1-10 liters), the culture can be collected into centrifuge bottles (500-1000 ml) and centrifuged to separate the cells from the supernatant.For large fermentations, large membrane filtration units (Millipore) or a Sharples centrifuge can be used to separate cells from the supernatant. The optimal method will depend on whether you need the cells or the supernatant as the source of your protein and what you have available.Supernatants can be loaded directly onto certain purification columns or concentrated using ultrafiltration.Cell Lysis We recommend cell disruption using glass beads as described in Current Protocols inMolecular Biology, page 13.13.4. (Ausubel, et al., 1990) or Guide to ProteinPurification (Deutscher, 1990). This method may be tedious for large amounts of cells.For larger amounts, we have found that a microfluidizer works very well. Frenchpressing the cells does not seem to work as well as the glass beads or the microfluidizer.ReferencesIntroduction Most of the references below refer to papers where fermentation of Pichia wasperformed. Note that some of these are patent papers. You can obtain copies of patentsusing any of the following methods.• Patent Depository Libraries. U. S. patents and international patents granted underthe Patent Cooperation Treaty (PCT) are available on microfilm. These can be copiedand mailed or faxed depending on length. There is a fee for this service. The referencelibrarian at your local library can tell you the location of the nearest Patent DepositoryLibrary.• Interlibrary Loan. If you are not near a Patent Depository Library, you may request acopy of the patent through interlibrary loan. There will be a fee for this service.• U. S. Patent Office. Requests may be made directly to the Patent Office, Arlington,VA. Please call 703-557-4636 for more information on cost and delivery.• Private Library Services. There are private companies who will retrieve and sendyou patents for a fee. Two are listed below:Library Connection: 804-758-3311Rapid Patent Services: 800-336-5010Citations Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A.,Struhl, K., eds (1990) Current Protocols in Molecular Biology. GreenePublishing Associates and Wiley-Interscience, New York.Brierley, R. A., Siegel, R. S., Bussineau, C. M. Craig, W. S., Holtz, G. C., Davis, G. R.,Buckholz, R. G., Thill, G. P., Wondrack, L. M., Digan, M. E., Harpold, M. M.,Lair, S. V., Ellis, S. B., and William, M. E. (1989) Mixed Feed RecombinantYeast Fermentation. International Patent (PCT) Application. Publication No.WO 90/03431.Brierley, R. A., Bussineau, C., Kosson, R., Melton, A., and Siegel, R. S. (1990)Fermentation Development of Recombinant Pichia pastoris Expressing theHeterologous Gene: Bovine Lysozyme. Ann. New York Acad. Sci.589: 350-362.Brierley, R. A., Davis, G. R. and Holtz, G. C. (1994) Production of Insulin-Like GrowthFactor-1 in Methylotrophic Yeast Cells. United States Patent5,324,639.Clare, J. J., Romanos, M. A., Rayment, F. B., Rowedder, J. E., Smith, M. A., Payne, M.M., Sreekrishna, K. and Henwood, C. A. (1991) Production of EpidermalGrowth Factor in Yeast: High-level Secretion Using Pichia pastoris StrainsContaining Multiple Gene Copies. Gene105: 205-212.Cregg, J. M., Tschopp, J. F., Stillman, C., Siegel, R., Akong, M., Craig, W. S.,Buckholz, R. G., Madden, K. R., Kellaris, P. A., Davis, G. R., Smiley, B. L.,Cruze, J., Torregrossa, R., Veliçelebi, G. and Thill, G. P. (1987) High-levelExpression and Efficient Assembly of Hepatitis B Surface Antigen in theMethylotrophic Yeast Pichia pastoris. Bio/Technology5: 479-485.Cregg, J. M., Vedvick, T. S. and Raschke, W. C. (1993) Recent Advances in theExpression of Foreign Genes in Pichia pastoris. Bio/Technology11: 905-910.Deutscher, M. P. (1990) Guide to Protein Purification. In: Methods in Enzymology (J.N. Abelson and M. I. Simon, eds.) Academic Press, San Diego, CA.continued on next pageReferences, continuedCitations, continuedDigan, M. E., Lair, S. V., Brierley, R. A., Siegel, R. S., Williams, M. E., Ellis, S. B., Kellaris, P. A., Provow, S. A., Craig, W. S., Veliçelebi, G., Harpold, M. M. andThill, G. P. (1989) Continuous Production of a Novel Lysozyme via Secretionfrom the Yeast Pichia pastoris. Bio/Technology7: 160-164.Hagenson, M. J., Holden, K. A., Parker, K. A., Wood, P. J., Cruze, J. A., Fuke, M., Hopkins, T. R. and Stroman, D. W. (1989) Expression of Streptokinase inPichia pastoris Yeast. Enzyme Microbiol. Technol.11: 650-656.Laroche, Y., Storme, V., Meutter, J. D., Messens, J. and Lauwereys, M. (1994) High-Level Secretion and Very Efficient Isotopic Labeling of Tick AnticoagulantPeptide (TAP) Expressed in the Methylotrophic Yeast, Pichia pastoris.Bio/Technology12: 1119-1124.Romanos, M. A., Clare, J. J., Beesley, K. M., Rayment, F. B., Ballantine, S. P., Makoff,A. J., Dougan, G., Fairweather, N. F. and Charles, I. G. (1991) RecombinantBordetella pertussis Pertactin p69 from the Yeast Pichia pastoris High LevelProduction and Immunological Properties. Vaccine9: 901-906.Siegel, R. S. and Brierley, R. A. (1989) Methylotrophic Yeast Pichia pastoris Produced in High-cell-density Fermentations With High Cell Yields as Vehicle forRecombinant Protein Production. Biotechnol. Bioeng.34: 403-404.Siegel, R. S., Buckholz, R. G., Thill, G. P., and Wondrack, L. M. (1990) Production of Epidermal Growth Factor in Methylotrophic Yeast Cells. International Patent(PCT) Application. Publication No. WO 90/10697.Sreekrishna, K., Nelles, L., Potenz, R., Cruse, J., Mazzaferro, P., Fish, W., Fuke, M., Holden, K., Phelps, D., Wood, P. and Parker, K. (1989) High LevelExpression, Purification, and Characterization of Recombinant Human TumorNecrosis Factor Synthesized in the Methylotrophic Yeast Pichia pastoris.Biochemistry28(9): 4117-4125.©2002 Invitrogen Corporation. All rights reservedRecipesFermentation Basal Salts Medium For 1 liter, mix together the following ingredients:Phosphoric acid, 85% (26.7 ml)Calcium sulfate 0.93 gPotassium sulfate 18.2 gMagnesium sulfate-7H2O 14.9gPotassium hydroxide 4.13 gGlycerol 40.0g Water to 1 literAdd to fermenter with water to the appropriate volume and sterilize.PTM1 Trace Salts Mix together the following ingredients:Cupric sulfate-5H2O 6.0gSodium iodide 0.08 gManganese sulfate-H2O 3.0gSodium molybdate-2H2O 0.2gBoric Acid 0.02 g Cobalt chloride 0.5 g Zinc chloride 20.0 gFerrous sulfate-7H2O 65.0gBiotin 0.2gSulfuric Acid 5.0 mlWater to a final volume of 1 literFilter sterilize and store at room temperature.Note: There may be a cloudy precipitate upon mixing of these ingredients. Filter-sterilize as above and use.11。
毕赤酵母手册

Pichia expression vectors for selection on Zeocin™ and purification of secreted, recombinant proteins
Cat. no. V195-20 Rev. Date: 7 July 2010 Manual part no. 25-0150
MAN0000035
User Manual
ii
Table of Contents
Important Information................................................................................................................................ v Accessory Products ................................................................................................................................... vii Introduction ................................................................................................................................................. 1 Overview .......................................................................................................................................................1 Methods........................................................................................................................................................ 2 Cloning into pPICZ A, B, and C...............................................................................................................2 Multiple Cloning Site of pPICZ A ...........................................................................................................5 Multiple Cloning Site of pPICZ B ............................................................................................................6 Multiple Cloning Site of pPICZ C............................................................................................................7 Pichia Transformation ..................................................................................................................................9 Expression in Pichia ....................................................................................................................................13 Purification ...........................................................................................................................15 Appendix .................................................................................................................................................... 17 Recipes .........................................................................................................................................................17 Zeocin™ ........................................................................................................................................................19 pPICZ Vector ............................................................................................................................................21 Lithium Chloride Transformation Method.............................................................................................23 Construction of In Vitro Multimers..........................................................................................................25 Technical Support.......................................................................................................................................33 Purchaser Notification ...............................................................................................................................34 References....................................................................................................................................................35
酵母发酵手册

酵母发酵手册摘要:一、酵母发酵概述1.酵母的定义与种类2.酵母发酵的应用领域二、酵母发酵原理1.酵母的生理特性2.酵母发酵的基本过程三、酵母发酵条件1.温度2.湿度3.氧气供应4.营养源四、酵母发酵的控制1.菌种选择与培养2.发酵过程监控3.产物分离与纯化五、酵母发酵的应用实例1.食品工业2.生物制药3.环保领域4.其他领域六、酵母发酵的发展趋势与展望正文:酵母发酵手册酵母是一种单细胞真核微生物,广泛应用于食品、生物制药、环保等领域。
酵母发酵在这些领域发挥着重要作用,为人类提供了丰富的产品与服务。
一、酵母发酵概述酵母是一种具有生理活性的微生物,能够将糖转化为酒精和二氧化碳。
在食品工业中,酵母发酵被用于制作面包、馒头、酒类等食品。
此外,酵母发酵还在生物制药领域发挥着重要作用,例如生产胰岛素、抗生素等药物。
二、酵母发酵原理酵母发酵的基本过程包括糖酵解、酒精发酵和二氧化碳发酵。
在糖酵解阶段,酵母将糖转化为丙酮酸,同时产生能量。
在酒精发酵阶段,丙酮酸被转化为酒精和二氧化碳。
最后,在二氧化碳发酵阶段,酒精和二氧化碳被转化为能量和更多的丙酮酸。
三、酵母发酵条件酵母发酵需要适宜的温度、湿度和氧气供应。
不同种类的酵母对温度的需求不同,一般而言,酵母的最适生长温度为25-30℃。
湿度方面,酵母发酵过程中需要保持相对湿度在70-90%。
此外,酵母发酵过程中氧气供应对发酵效果具有重要影响,因此需要根据实际需求进行调控。
四、酵母发酵的控制为了获得理想的发酵效果,需要对酵母发酵过程进行严格控制。
首先,要选择合适的菌种并进行培养。
其次,要实时监控发酵过程的各项参数,如温度、湿度、氧气供应等。
最后,要进行产物分离与纯化,确保产物的质量和收率。
五、酵母发酵的应用实例酵母发酵在食品工业中的应用包括面包、馒头、酒类等食品的生产。
在生物制药领域,酵母发酵被用于生产胰岛素、抗生素等药物。
此外,酵母发酵还在环保领域发挥重要作用,如废水处理、生物降解塑料等。
鲜酵母使用手册

鲜酵母使用手册1、鲜酵母是个什么东西?酵母是一种单细胞的真菌酵母微生物,有一定的生命周期.鲜酵母是酵母工业化生产的一个产品,干酵母是将鲜酵母干燥加工后的另一个产品。
目前生产的鲜酵母一般是块状,鲜酵母是由万亿个活的酵母细胞堆积而成,鲜酵母细胞内水份含量正常,细胞与细胞之间有少量水。
鲜酵母中干物质含量在34。
%左右(主要是活的酵母细胞),水份含量在66%左右.酵母的发面机理是酵母代谢面粉,产生利于人体吸收的营养和风味物质,并产生CO2气体,使面团膨胀、松软可口。
2、鲜酵母有哪三大优点?鲜酵母是一种没有经过干燥、造粒工艺的酵母。
与干酵母相比:A、鲜酵母具有活细胞多,发酵速度快、B、鲜酵母酶系健全,发酵风味好.C、生产成本低,使用成本低。
3、鲜酵母分几类?用法用量?鲜酵母分为高糖型和低糖型。
1)低糖型鲜酵母适用于每百斤面粉中加糖量5斤以下或不加糖的面制品,安琪低糖鲜酵母用于不加糖面制品更有优势,发面快,口感好。
如:馒头、包子、花卷、含糖量较少的饼。
2)高糖型鲜酵母适用于每百斤面粉中加糖量5斤以上的面制品.安琪高糖鲜酵母用于含糖量高的产品,发面稳定、后劲足。
如:各种面包、甜馒头、高档发酵型点心、饼等。
用法1)面制品制作时,将面粉及各种辅料放入和面机后,将鲜酵母直接搓碎均匀撒在面粉上,充分拌匀后加水搅拌至面筋形成。
2)也可将鲜酵母搓碎加入部分水中溶解,在和面操作中加水阶段时加入,水温根据气温定,气温低水温高,水温最高不能超过40℃。
用量:正确选用高、低糖酵母,一般面包用面粉量的2-3%,做馒头用面粉量的0。
5-1%。
用量随气温调整,温度高,用量少。
随鲜酵母存放时间延长,相应要加大用量4、安琪鲜酵母相对同类产品的优点?安琪公司有优良的菌种和原料,安琪公司采用先进的过程控制系统,一流的生产线,集成全自动化,先进的生产工艺,工艺控制严格,卫生条件好,严格控制了杂菌数和死细胞,加上多级产品质量检控及规范的物流体系,使得生产出来的安琪鲜酵母:活力强劲,发酵速度特别快.发酵风味足。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
6 9 25 52 52 61 62 63 64
Table II. Table III. Table IV. Table V. Table VI. Table VII. Table IX.
Table VIII. Matchmaker One-Hybrid System Cloning, Reporter & Control Plasmids
Yeast Protocols Handbook
FOR RESEARCH USE ONLY
Visit Visit sit our website for more details! click here…
PT3024-1 (PR742227) Published 11 February 2008
Yeast Protocols Handbook
List of Figures
Figure 1. Figure 2. Figure 3.
Sequence of GAL4 DNA-BD recognition sites in the GAL1, GAL2, MEL1 UASs and the UASG 17-mer Urea/SDS protein extraction method TCA protein extraction method
VIII. Analysis of Yeast Plasmid Inserts by PCR A. General Information B. Tips for Successful PCR of Yeast Plasmid Templates IX. Additional Useful Protocols A. Yeast Colony Hybridization B. Generating Yeast Plasmid Segregants C. Yeast Mating
Table of Contents
I. Introduction II. Introduction to Yeast Promoters III. Culturing and Handling Yeast IV. V. VI. VII. Preparation of Yeast Protein Extracts A. General Information B. Preparation of Yeast Cultures for Protein Extraction C. Preparation of Protein Extracts: Urea/SDS Method D. Preparation of Protein Extracts: TCA Method E. Troubleshooting Yeast Transformation Procedures A. General Information B. Reagents and Materials Required C. Tips for a Successful Transformation D. Integrating Plasmids into the Yeast Genome E. Small-scale LiAc Yeast Transformation Procedure F . Troubleshooting Yeast Transformation α- and β-Galactosidase Assays A. General Information B. In vivo Plate Assay Using X-gal in the Medium C. Colony-lift Filter Assay D. Liquid Culture Assay Using ONPG as Substrate E. Liquid Culture Assay Using CPRG as Substrate F . Liquid Culture Assay Using a Chemiluminescent Substrate G. α-Gal Quantitative Assay Working with Yeast Plasmids A. General Information B. Plasmid Isolation From Yeast C. Transforming E. coli with Yeast Plasmids 4 5 10 12 12 12 13 15 17 18 18 19 20 20 20 22 23 23 25 25 26 27 28 32 34 34 34 36 39 39 39 42 42 43 44 46 49 50 52 53 53 56 57 61 64
