奥美拉唑碳酸氢钠FDA说明书
奥美拉唑注射液

奥美拉唑【中文名称】:奥美拉唑【简写拼音】:AMLZ【所属类别】:抗酸及抗溃疡药【别名】安胃哌唑;奥美拉唑;奥咪拉唑;甲氧磺唑;沃必唑;渥米哌唑;亚枫咪唑,洛赛克【外文名】Omeprazole,Losec【性状】为白色至类白色结晶性粉末,熔点156℃。
【成份】主要成份为奥美拉唑。
化学名称:5-甲氧基-2-{[(4-甲氧基-3,5-二甲基-2-吡啶基)-甲基-亚砜}-1H-苯并咪唑。
分子式:C17H19N3O3S分子式量:345.42【药理作用】是近年来研究开发的作用机制不同于H2受体拮抗作用的全新抗消化性溃疡药。
它特异性地作用于胃粘膜壁细胞,降低壁细胞中的氢钾ATP酶的活性,从而抑制基础胃酸和刺激引起的胃酸分泌。
由于氢钾ATP酶又称做"质子泵",故本类药物又称为"质子泵抑制剂"。
【适应症】主要适用于十二指肠溃疡和卓-艾综合征,也可用于胃溃疡和反流性食管炎。
【作用与用途】本品作用于胃腺壁细胞,为H+-K+-ATP酶抑制剂,选择性对胃酸分泌有明显抑制作用,起效迅速,适用于胃及十二指肠溃疡,返流性食管炎和胃泌素瘤。
【用法】成人口服:治疗胃及十二指肠溃疡及反流性食管炎:每日早晨吞服20mg。
本品治疗十二指肠溃疡疗程通常为2-4周,治疗胃溃疡及反流性食管炎疗程为4 -8周。
【副作用】可有头痛、腹泻、便秘、腹痛、恶心或呕吐等,个别患者有转氨酶和胆红素增高、皮疹、眩晕、嗜睡、失眠等反应。
【禁忌证】①对本品过敏者禁用。
②孕妇及哺乳期妇女慎用。
③当怀疑胃溃疡时,应首先排除癌症的可能性,以免延误诊断。
④严重肝肾功能不全者慎用。
【注意事项】 1.不良反应及发生率与雷尼替丁相似,主要有恶心,上腹痛等。
皮疹也有发生,一般是轻微和短暂的,大多不影响治疗。
2.本品具有酶抑制作用,一些经肝脏细胞色素P450系统代谢的药物,如双香豆素、安定、苯妥英钠等,其药物半衰期可因合用本品而延长。
3.使用本品前,必须排除恶性肿瘤的可能性。
奥美拉唑钠注射液说明书

奥美拉唑钠注射液说明书奥美拉唑钠注射液作为当口服疗法不适用时下列病症的替代疗法:十二指肠溃疡、胃溃疡、反流性食管炎及Zollinger-Ellison综合征,那么奥美拉唑钠注射液说明书是什么样的呢?下面是店铺为你整理的奥美拉唑钠注射液说明书的相关内容,希望对你有用!奥美拉唑钠注射液说明书【药品类型】消化系统【中文名】奥美拉唑钠注射液【产品英文名】 Omeprazole Sodium for Injection【功能主治】主要用于:①消化性溃疡出血、吻合口溃疡出血。
②应激状态时并发的急性胃黏膜损害,和非甾体类抗炎药引起的急性胃黏膜损伤;③亦常用于预防重症疾病(如脑出血、严重创伤等)胃手术后预防再出血等;④全身麻醉或大手术后以及衰弱昏迷患者防止胃酸反流合并吸入性肺炎。
【药品性状】本品为白色疏松块状物或粉末,专用溶剂为无色的透明液体。
【药理作用】本品为胃壁细胞质子泵抑制剂,能特异性地抑制壁细胞顶端膜构成的分泌性微管和胞浆内的管状泡上的H+、K+-ATP酶,从而有效地抑制胃酸的分泌。
由于H+、K+-ATP酶是壁细胞泌酸的最后一个过程,故本品抑酸能力强大。
【药物相互作用】(1)本品可延长地西泮、苯妥英钠及其他经肝氧代谢药物的药效,如本品与苯妥英钠合用,则需小心监测病情,且苯妥英钠应酌情减量。
(2)与经细胞色素P450酶系统代谢的药物(如华法林)可能有相互作用。
不良反应】偶可见有一过性的轻度恶心、腹泻、腹痛、感觉异常、头晕或头痛等,但不影响治疗。
【产品规格】 40mg(按奥美拉唑计)【药品成分】奥美拉唑钠【孕妇用药】尽管动物实验未发现本品对妊娠期和哺乳期有不良作用,或对胎儿有毒性或致畸作用,但建议妊娠期和哺乳期妇女尽可能不用。
【儿童用药】儿童使用本品静滴的经验有限。
【老年患者用药】老年患者无需调整剂量。
【用法用量】静脉注射。
一次40mg,每日1~2次。
临用前将10ml专用溶剂注入冻干粉小瓶内,禁止用其它溶剂溶解。
奥美拉唑镁肠溶片说明书

13、使用质子泵抑制剂治疗可能会导致胃肠道感染风险轻微升高,如沙门氏 菌和弯曲杆菌感染。
14、对于长期服用本品的患者,特别是使用1年以上者,应定期进行监测。
15、长期反复出现消化不良和烧心症状的患者应定期就诊。
16、患者如果出现以下情况,应咨询医生:
· 既往患有胃溃疡或胃肠道手术史 · 因消化不良或烧心连续治疗4周以上 · 患有黄疸或重度肝病 · 年龄在55岁以上且出现新的或最近有症状变化 【孕妇及哺乳期妇女用药】 尚未在孕妇中开展充分且良好对照的研究。现有流行病学数据未能证明在 妊娠早期使用奥美拉唑时,重大先天性畸形或其他不良妊娠结局的风险增加。由 于在大鼠研究中观察到高剂量艾司奥美拉唑镁对发育中的骨骼具有影响,因此 只有对胎儿的潜在获益大于潜在风险时才应在妊娠期间使用本品。 奥美拉唑可被分泌入乳汁,哺乳期妇女慎用。 【儿童用药】 目前国内尚无儿童使用本品的经验。 【老年用药】 老年患者无需调整剂量。
6、与氯吡格雷的相互作用
应避免本品与氯吡格雷联合使用。氯吡格雷是一种前体药物,其活性代谢产
物抑制血小板聚集。与奥美拉唑等药物联合用药时,后者抑制CYP2C19活性,可
影响氯吡格雷代谢为活性代谢产物。联合使用氯吡格雷和80mg奥美拉唑可降低
氯吡格雷的药理活性,即使两者相隔12小时给药。当使用本品时,应考虑使用其
2、与其它质子泵抑制剂一样,奥美拉唑不应与奈非那韦合用。
【注意事项】 1、胃恶性肿瘤 当怀疑或者确诊胃溃疡,出现报警)时,应先排除恶性肿瘤,因为治疗可能会掩盖症状进而导 致延误诊断。
2、萎缩性胃炎
长期接受奥美拉唑治疗的患者,胃体病理活检时偶见萎缩性胃炎。
以C17H19N3O3S计 (1)10 毫克;(2)20 毫克 【用法用量】 必须整片吞服,至少用半杯液体送服。药片不可咀嚼或压碎,可将其分散于 水或微酸液体中(如:果汁),分散液必须在30分钟内服用。
奥美拉唑肠溶片说明书

奥美拉唑肠溶片说明书奥美拉唑肠溶片是一种常用的药物,主要用于治疗胃酸过多引起的胃病,如胃溃疡、消化性溃疡等。
下面,我们将通过详细阐述奥美拉唑肠溶片的药理特点、适应症、用法用量、不良反应等方面来进行介绍。
奥美拉唑肠溶片的药理特点主要表现在下面几个方面。
首先,它是一种质子泵抑制剂,能够有效地抑制胃酸分泌,从而降低胃酸浓度,减少胃酸对胃黏膜的刺激,有助于胃病的治疗。
其次,奥美拉唑肠溶片具有较长的半衰期,长时间服用可以达到稳态浓度,能够持续发挥药效。
另外,奥美拉唑肠溶片还具有较好的组织渗透性,能够穿过胃黏膜进入胃壁,发挥作用。
奥美拉唑肠溶片的适应症主要包括胃溃疡、消化性溃疡、胃食管反流性疾病、急性胃黏膜炎症等。
如果患者出现胃痛、胃灼烧、胃酸倒流等症状,可以考虑使用奥美拉唑肠溶片进行治疗。
奥美拉唑肠溶片的用法用量一般是口服,每次一片,每天一次。
如果患者严重的胃酸过多症状,医生可能会根据具体病情调整用药剂量和频次。
此外,奥美拉唑肠溶片应该在饭前30分钟到1小时内服用,以保持药物的疗效。
奥美拉唑肠溶片一般来说是比较安全的药物,但也不可避免地会出现一些不良反应。
常见的不良反应包括头痛、乏力、恶心、腹泻等轻度反应,一般可以自行缓解而无需特殊处理。
少数患者可能会出现过敏反应,如皮疹、荨麻疹、呼吸困难等,如果出现这些症状,应立即停药并就医处理。
在使用奥美拉唑肠溶片时,还需要注意以下几点。
首先,孕妇、哺乳期妇女、儿童和老年患者应在医生的指导下使用。
其次,奥美拉唑肠溶片与某些药物会发生相互作用,如抗凝药物、抗癫痫药物等,应注意避免同时使用,或在医生的指导下进行联合用药。
最后,长期使用奥美拉唑肠溶片的患者应定期进行胃镜检查,以评估治疗效果和监测胃病的变化。
综上所述,奥美拉唑肠溶片是一种有效治疗胃酸过多引起的胃病的药物。
它通过抑制胃酸分泌,减少胃酸对胃黏膜的刺激,起到治疗作用。
在使用奥美拉唑肠溶片时,患者应按照医生的建议用药,并注意可能出现的不良反应和药物相互作用。
注射用奥美拉唑使用说明

注射用奥美拉唑使用说明
奥美拉唑是一种口服溶液,主要用于治疗消化性溃疡疾病和胃食管反流病。
它属于质子泵抑制剂,能有效减少胃酸分泌,从而减轻胃部炎症和溃疡。
奥美拉唑也可以用于治疗幽门螺杆菌感染,该菌是导致消化性溃疡和胃炎的主要原因之一、奥美拉唑通过抑制幽门螺杆菌的酸产能力,帮助创造一个不利于细菌生长的环境,加速感染的治愈。
1.适应症:注射用奥美拉唑适用于治疗消化性溃疡、胃食管反流病以及幽门螺杆菌感染。
2.剂量和用法:注射用奥美拉唑通常以静脉滴注的方式给药。
剂量的选择和治疗时间的长短应根据患者的病情和临床需要来确定,并且应由医生根据患者的病情进行个体化调整。
3.用药频次:通常每天给药一次,根据医生的指示进行持续治疗。
4.注意事项:
-奥美拉唑注射剂只能由医疗专业人员按照规定的方法给药。
-如果患者对奥美拉唑过敏或有其他葡萄糖酶缺乏症等禁忌症,应立即停止使用并就医。
-在使用注射用奥美拉唑期间,应密切监测患者的病情和生命体征,并及时进行必要的调整。
-奥美拉唑可能与其他药物相互作用,使用时应告知医生正在使用的其他药物,并遵从医生的指导。
-注射用奥美拉唑不建议用于儿童和孕妇,如果需要使用,应在医生的指导下进行。
除了以上使用说明外,注射用奥美拉唑的不良反应也需要引起注意。
常见的不良反应包括头痛、恶心、腹泻、胃肠道不适等。
如果出现严重不良反应,如过敏反应、皮疹、呼吸困难等,应立即就医。
总之,注射用奥美拉唑是一种治疗消化性溃疡、胃食管反流病和幽门螺杆菌感染的有效药物。
在使用时应根据医生的指导,合理用药,并密切注意患者的病情和不良反应。
复方奥美拉唑干混悬剂项目介绍

复方奥美拉唑干混悬剂项目介绍一项目简介【项目名称】复方奥美拉唑干混悬剂【主要成份】奥美拉唑碳酸氢钠【适应证】十二指肠溃疡:本品适用于短期治疗活跃型十二指肠溃疡。
大多数患者会在4周内痊愈,少数患者需要追加4周的治疗方可痊愈。
胃溃疡:本品适用于短期治疗(4-8周)活跃型良性胃溃疡。
胃食管反流症(GERD):本品适用于治疗有烧心等症状的GERD患者。
糜烂性食管炎:本品适用于以内窥镜检查诊断为糜烂性食管炎的短期治疗(4-8周);本品用于超过8周治疗GERD的疗效尚未确定。
罕有患者经过8周的治疗后没有反应,建议放弃追加治疗。
如果是复发性糜烂性食管炎或GERD患者伴有烧心等症状,可以考虑追加4-8周的奥美拉唑治疗。
糜烂性食管炎的维持治疗:本品适用于糜烂性食管炎的维持治疗。
对照研究表明不要用药超过12个月。
减少危重患者的上消化道出血的风险:本品干混悬剂40mg/1680mg可以用来减少危重患者的上消化道出血的风险。
【规格】奥美拉唑/碳酸氢钠20mg/1.68g、奥美拉唑/碳酸氢钠40mg/1.68g 【项目分类】复方奥美拉唑干混悬剂由美国Santarus公司研制,商品名:ZEGERID®。
FDA于2004年6月15日批准其在美国上市,按照《药品注册管理办法》规定,本品属于已在国外上市销售但尚未在国内上市销售的三类新药。
二项目立题目的依据消化道溃疡主要指发生在胃和十二指肠的慢性溃疡,即胃溃疡和十二指肠溃疡,因溃疡的发生和形成与胃酸-胃蛋白酶的消化作用有关而得名。
消化性溃疡分布于全世界,是人们日常生活中常见的一种多发病。
根据资料统计,发病率约占人口的10%左右,中国消化系统发病率也呈逐年递增的趋势。
消化性溃疡药物治疗成为目前研究开发的重点和热点之一,主要有质子泵抑制剂、H受体拮抗剂、胃粘膜保护剂、抗酸剂等。
虽然治疗消化性溃疡的药物进展很2快,但是最古老的抗酸药在溃疡病的治疗上仍有相应的地位,抗酸剂主要是一些无机弱碱,常用药物有碳酸氢钠、氢氧化镁、三硅酸镁等,口服后能直接中和胃酸,受体拮抗剂的可减轻或解除胃酸对溃疡面的刺激和腐蚀作用;70年代西咪替丁等H2出现,使消化性溃疡并发症的发生率明显下降;80年代H+-K+-ATP酶(质子泵)抑制剂的问世,使消化性溃疡的治疗取得突破性进展,成为目前最强的新型抑酸药物。
奥美拉唑肠溶胶囊说明书范本2020

奥美拉唑肠溶胶囊说明书请仔细阅读说明书并按说明使用或在药师指导下购买和使用[药品名称]通用名称:奥美拉唑肠溶胶囊商品名称:英文名称:汉语拼音:[成份][性状]][作用类别]本品为抗酸类非处方药药品。
[适应症] 用于胃酸过多引起的烧心和反酸症状的短期缓解。
[规格] 20毫克[用法用量] 口服。
成人,一次1粒,一日1次(每24小时),用温开水送服。
本品必须整粒吞服,不可咀嚼或压碎,更不可将本品压碎于食物中服用。
[不良反应]全球临床试验中3096例患者(其中2631例来自双盲或开放的国际多中心研究)暴露于奥美拉唑,发生率≥2%的不良反应包括头痛(6.9%)、腹痛(5.2%)、恶心(4.0%)、腹泻(3.7%)、呕吐(3.2%)和胃肠胀气(2.7%)。
发生率≥1%的不良反应包括反酸(1.9%)、上呼吸道感染(1.9%)、便秘(1.5%)、头晕(1.5%)、皮疹(1.5%)、乏力(1.3%)、背痛(1.1%)和咳嗽(1.1%)。
在本品获准上市后使用过程中,已经发现如下不良反应。
由于这些不良反应由数量不明的人群自发报告,因此难以估算其实际发生率或确定其与药物暴露之间的因果关系。
按人体器官系统分类列出如下:全身性疾病:超敏反应包括速发过敏反应、速发过敏反应性休克、血管性水肿、支气管痉挛、间质性肾炎、荨麻疹,发热,疼痛,疲乏,不适;心血管系统:胸痛、心绞痛、心动过速、心动过缓、心悸、血压升高、外周水肿;内分泌系统:男性乳房发育;胃肠道系统:胰腺炎(某些可致命)、厌食、肠易激、粪便变色、食管念珠菌病、舌黏膜萎缩、口炎、口干、腹胀、显微镜下结肠炎。
奥美拉唑治疗期间,极罕见观察到患者出现胃底腺息肉。
这些息肉为良性,在停止治疗后可逆转。
患有卓-艾综合征的患者在接受奥美拉唑长期治疗时报告发生胃十二指肠类癌,该发现被认为与基础疾病有关。
肝胆系统:肝衰竭(某些可致命)、肝坏死(某些可致命)、肝性脑病、肝细胞疾病、胆汁淤积、混合型肝炎、黄疸、肝功能指标升高(ALT、AST、GGT、碱性磷酸酶和胆红素);感染:艰难梭状芽胞杆菌性腹泻;代谢疾病及营养不良:低血糖、低镁血症、低钙血症、低钾血症、低钠血症、体重增加;肌肉骨骼系统:肌无力、肌痛、肌痉挛、关节疼痛、腿部疼痛、骨折;神经系统/精神性疾病:抑郁、激动、攻击性、幻觉、意识模糊、失眠、紧张不安、淡漠、嗜睡、焦虑、梦异常、震颤、感觉异常、眩晕、味觉障碍;呼吸系统:鼻衄、咽痛;皮肤和皮下组织:中毒性表皮坏死松解症(某些可致命)、史蒂文斯-约翰逊综合征、多形性红斑、光敏性、荨麻疹、皮疹、皮炎、瘙痒、瘀点、紫癜、脱发、皮肤干燥、多汗;耳部和迷路系统:耳鸣;眼部疾病:视神经萎缩、前部缺血性视神经病变、视神经炎、干眼综合征、眼刺激、视物模糊、复视;泌尿生殖系统:间质性肾炎、血尿、蛋白尿、血肌酐升高、镜下脓尿、尿路感染、糖尿、尿频、睾丸疼痛;血液和淋巴系统:粒细胞缺乏症(某些可致命)、溶血性贫血、全血细胞减少症、中性粒细胞减少症、贫血、血小板减少症、白细胞减少症、白细胞增多症。
奥美拉唑胶囊说明书

核准日期:修改日期:奥美拉唑肠溶胶囊说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:奥美拉唑肠溶胶囊英文名称:Omeprazole Enteric-coated Capsules汉语拼音:Aomeilazuo Changrong Jiaonang【成份】本品主要成份为奥美拉唑。
化学名称:5-甲氧基-2-{[(4-甲氧基-3,5-二甲基-2-吡啶基)-甲基]-亚磺酰基}-1H-苯并咪唑。
化学结构式:分子式:C17H19N3O3S分子量:345.42【性状】本品内容物为白色或类白色肠溶小丸或颗粒。
【适应症】适用于胃溃疡、十二指肠溃疡、应激性溃疡、反流性食管炎和卓-艾综合征(胃泌素瘤)。
【规格】20mg【用法用量】口服,不可咀嚼。
1.消化性溃疡:一次20mg(1粒),一日1~2次。
每日晨起吞服或早晚各一次,胃溃疡疗程通常为4~8周,十二指肠溃疡疗程通常为2~4周。
2.反流性食管炎:一次20~60 mg(1~3粒),一日1~2次。
晨起吞服或早晚各一次,疗程通常为4~8周。
3.卓-艾综合征:一次60mg(3粒),一日1次,以后每日总剂量可根据病情调整为20~120mg(1~6粒),若一日总剂量需超过80mg(4粒)时,应分为两次服用。
【不良反应】本品耐受性良好,常见不良反应是腹泻、头痛、恶心、腹痛、胃肠胀气及便秘,偶见血清氨基转移酶(ALT,AST)增高、皮疹、眩晕、嗜睡、失眠等,这些不良反应通常是轻微的,可自动消失,与剂量无关。
长期治疗未见严重的不良反应,但在有些病例中可发生胃黏膜细胞增生和萎缩性胃炎。
【禁忌】对本品过敏者、严重肾功能不全者及婴幼儿禁用。
【注意事项】1.治疗胃溃疡时,应首先排除溃疡型胃癌的可能,因用本品治疗可减轻其症状,从而延误治疗。
2.肝肾功能不全者慎用。
3.本品为肠溶胶囊,服用时注意不要嚼碎,以免药物在胃内过早释放而影响疗效。
【孕妇及哺乳期妇女用药】虽然动物实验表明,本品无胎儿毒性或致畸作用,但对孕妇一般不用,对哺乳期妇女也应慎用。
碳酸氢钠片说明书

碳酸氢钠片说明书12020年4月19日碳酸氢钠片药品名称:通用名称:碳酸氢钠片英文名称:sodium bicarbonate tablets成份:碳酸氢钠适应症:用于缓解胃酸过多引起的胃痛、胃灼热感(烧心)、反酸。
规格:0.5g*100片用法用量:口服。
一次1~2片,每日3次。
不良反应:中和胃酸时所产生的二氧化碳可能引起嗳气、继发性胃酸分泌增加。
注意事项: 1. 本品连续使用不得超过7天,症状未缓解或消失请咨询医师或药师。
2. 6岁以下小儿不推荐使用。
3. 阑尾炎或有类似症状而未确诊者及消化道出血原因不明者不宜使22020年4月19日用。
4. 儿童用量请咨询医师或药师。
5. 如服用过量或出现严重不良反应,应立即就医。
6. 对本品过敏者禁用,过敏体质者慎用。
7. 本品性状发生改变时禁止使用。
8. 请将本品放在儿童不能接触的地方。
9. 儿童必须在成人监护下使用。
10. 如正在使用其它药品,使用本品前请咨询医师或药师。
药物相互作用:1.本品可加速酸性药物的排泄(如阿司匹林)。
2.本品可降低胃蛋白酶、维生素e的疗效。
3.如与其它药物同时使用可能会发生药物相互作用,详情请咨询医师或药师。
药理作用:本品为抗酸剂,口服后可迅速中和胃酸,解除胃酸过多或烧心症状,但作用较弱,持续时间较短。
贮藏:密封,干燥处保存保存。
有效期:36个月。
32020年4月19日批准文号:国药准字h41024197篇二:碳酸氢钠片说明书碳酸氢钠片说明书[药品名称]通用名:碳酸氢钠片曾用名:商品名:英文名:汉语拼音:[成份][性状][作用类别]本品为抗酸类非处方药药品。
[药理作用]本品为抗酸剂,口服后可迅速中和胃酸,解除胃酸过多或烧心症状,但作用较弱,持续时间较短。
[适应症]用于胃酸过多症。
[用法用量]口服,每次0.3-1克,每日3次。
[禁忌症][注意事项]本品连续使用不得超过7天,症状未缓解或消失请咨询医师或药师。
儿童用量请咨询医师或药师。
奥美拉唑肠溶片说明书

奥美拉唑肠溶片说明书
奥美拉唑肠溶片说明书
一、药品名称
奥美拉唑肠溶片
二、成分
每片含奥美拉唑20mg
三、适应症
适用于胃和十二指肠溃疡等病症的治疗。
四、用法用量
口服。
早餐前服用1片,每日1次,连续使用4周。
五、不良反应
可能会出现以下不良反应:
- 头晕
- 呕吐
- 胃部不适
- 腹泻等
如有以上不良反应,应立即停止使用并就医。
六、禁忌症
以下情况禁用此药品:
- 对奥美拉唑过敏的患者
- 妊娠期及哺乳期妇女
- 12岁以下儿童
七、注意事项
1. 使用本品应遵嘱医生的指导。
2. 本品可能会与其他药物发生相互作用,请告知医生正在使用的药物。
3. 长期使用本品可能会导致腹泻或其他消化道反应。
4. 服用本品期间应避免酗酒。
5. 如出现严重不适,请立即就医。
八、存储方法
请将药品存放在阴凉干燥的地方,避免阳光直射。
九、注意事项
请放置在儿童无法触及的地方。
十、生产厂家
XX药业有限公司
十一、联系方式
如有疑问,请拨打客服热线:XXX-XXXXXXX
以上为奥美拉唑肠溶片说明书内容,仅供参考,开封前请仔细阅读说明书,遵医嘱使用。
奥美拉唑碳酸氢钠胶囊优势
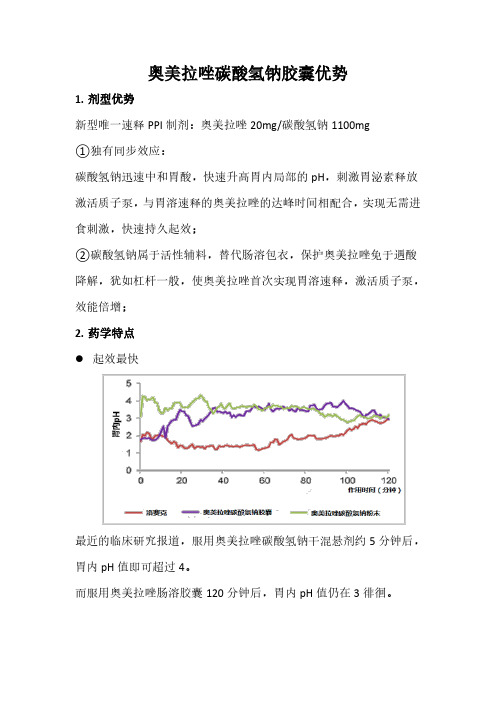
奥美拉唑碳酸氢钠胶囊优势1.剂型优势新型唯一速释PPI制剂:奥美拉唑20mg/碳酸氢钠1100mg①独有同步效应:碳酸氢钠迅速中和胃酸,快速升高胃内局部的pH,刺激胃泌素释放激活质子泵,与胃溶速释的奥美拉唑的达峰时间相配合,实现无需进食刺激,快速持久起效;②碳酸氢钠属于活性辅料,替代肠溶包衣,保护奥美拉唑免于遇酸降解,犹如杠杆一般,使奥美拉唑首次实现胃溶速释,激活质子泵,效能倍增;2.药学特点⚫起效最快最近的临床研究报道,服用奥美拉唑碳酸氢钠干混悬剂约5分钟后,胃内pH值即可超过4。
而服用奥美拉唑肠溶胶囊120分钟后,胃内pH值仍在3徘徊。
奥美拉唑碳酸氢钠胶囊这一剂型的改变,使得本品变革为速释剂型,是具有全新药代动力学的新药。
在美国药典的名称是Omeprazole Capsules;而奥美拉唑肠溶胶囊,在美国药典名称Omeprazole Delayed-Release Capsules,即”迟释胶囊”。
⚫强效持久抑酸——24小时期间胃内pH>4时间长达18.6h⚫强效持久抑酸—控制夜间酸突破,较泮托拉唑显著更优⚫强效持久抑酸—控制夜间酸突破,较埃索美拉唑显著更优除奥美拉唑碳酸氢钠和右旋兰索拉唑,所有的PPI均在饭前30-60分钟给药,以确保最大疗效。
奥美拉唑是一种速释PPI,和其他PPI相比,在睡前给药时,奥美拉唑碳酸氢钠被认为对睡后前4小时的夜间胃内pH控制更有效。
通常来说,传统PPI睡前服用则不那么有效。
这一规律对奥美拉唑碳酸氢钠不适用,奥美拉唑碳酸氢钠在睡前服用时可有效控制夜间pH。
⚫服用方便—可按需服用,可临睡前使用早间及临睡前按需给药,均可显著改善胃食管反流病(GERD)症状,没有酸反跳及夜间酸突破的风险。
3.临床优势⚫临床路径用药①.已列入中国临床用药路径2015版:胃十二指肠溃疡及胃食管反流病②.已列入美国临床用药路径2013版:胃食管反流病③.是FDA唯一批准用于降低危重患者上消化道出血风险的质子泵抑制剂⚫阿司匹林的最佳伴侣阿司匹林长期使用易引起胃溃疡及上消化道出血,而奥美拉唑碳酸氢钠是唯一获得FDA批准用于上消化道出血的PPI,起效最快,强效持久抑酸,两者联用无药物相互作用,可安心使用。
奥美拉唑肠溶胶囊说明书范本2020

胃肠道系统:胰腺炎(某些可致命)、厌食、肠易激、粪便变色、食管念珠菌病、舌黏膜萎缩、口炎、口干、腹胀、显微镜下结肠炎。奥美拉唑治疗期间,极罕见观察到患者出现胃底腺息肉。
这些息肉为良性,在停止治疗后可逆转。
8.使用质子泵抑制剂治疗可能会导致胃肠道感染风险轻微升高,如沙门氏菌和弯曲杆菌感染。
9.如果患者长期服用质子泵抑制剂,在用药过程中,要注意可能出现的骨折风险(尤其是老年患者);定期监测血镁水平,防止低镁血症的出现。
10.由于质子泵抑制剂与氯吡格雷存在相互作用,建议正在使用氯吡格雷类的患者在治疗前,与医生就用药安全性问题进行交流,以确保用药安全。
11.患者如果出现以下情况,应咨询医生:既往患有胃溃疡或胃肠道手术史、年龄在55岁以上且出现新的或最近有症状变化。
12.对本品过敏者禁用,过敏体质者慎用。
13.请将本品放在儿童不能接触的地方。
14.儿童使用本品应在医师指导下进行。
15.儿童必须在成人监护下使用。
16.本品性状发生改变时禁止使用。
17.如正在使用其他药品,使用本品前请咨询医师或药师。
[禁忌]
1.已知对奥美拉唑、其他苯并咪唑类或本品中任何其他成份过敏者禁用。超敏反应可能包括速发过敏反应、过敏性休克、血管性水肿、支气管痉挛、间质性肾炎和荨麻疹。
2.与其它质子泵抑制剂一样,奥美拉唑不应与阿扎那韦、奈非那韦合用。
3.对本品过敏者、严重肾功能不全者及婴幼儿禁用。
[注意事项]
1.使用不得超过7天,如症状未缓解,请咨询医师或药师。
耳部和迷路系统:耳鸣;
眼部疾病:视神经萎缩、前部缺血性视神经病变、视神经炎、干眼综合征、眼刺激、视物模糊、复视;
奥美拉唑肠溶胶囊(迪诺洛克)的说明书

奥美拉唑肠溶胶囊(迪诺洛克)的说明书个人健康问题永远都是大家比较关心的话题,谁都希望自己拥有一个健康的身体,然而健康的首要条件就是要求人们拥有一个健康的肠胃,这样才能更好的面对各种困境。
吃多了容易得疾病,不吃饭更容易患上疾病,因此及时服药进行治疗是很有必要的。
今天,我们为您介绍一种名叫奥美拉唑肠溶胶囊(迪诺洛克)的肠胃药物,该药物对于肠胃疾病具有很好的疗效。
【药品名称】通用名称:奥美拉唑肠溶胶囊商品名称:奥美拉唑肠溶胶囊(迪诺洛克)拼音全码:AoMeiLaZuoChangRongJiaoNang(DiRuiLuoKe)【主要成份】本品主要成份为奥美拉唑化学名:5-甲氧基-2-[[(4-甲氧基-3,5-二甲基-2-吡啶基)-甲基]-亚磺酰基]-1H-苯并咪唑分子式:C17H19N3O3S分子量:345.42【性状】本品内容物为白色或类白色肠溶小丸或颗粒。
【适应症/功能主治】适用于胃溃疡、十二指肠溃疡、应激性溃疡、反流性食管炎和卓-艾综合征(胃泌素瘤)。
【规格型号】20mg*14s【用法用量】口服,不可咀嚼。
1.消化性溃疡:一次20mg(1粒),一日1~2次。
每日晨起吞服或早晚各一次,胃溃疡疗程通常为4~8周,十二指肠溃疡疗程通常为2~4周。
2.反流性食管炎:一次20~60mg(1~3粒),一日1~2次。
晨起吞服或早晚各一次,疗程通常为4~8周。
3.卓-艾综合征:一次60mg(3粒),一日1次,以后每日总剂量可根据病情调整为20~120mg(1~6粒),若一日总剂量需超过80mg(4粒)时,应分为两次服用。
【不良反应】本药耐受性较好,不良反应可能包括:1.消化系统:可有口干、轻度恶心、呕吐、腹胀、便秘、腹泻、腹痛等;丙氨酸氨基转移酶(ALT)、天门冬氨酸氨基转移酶(AST)和胆红素可有升高,一般是轻微和短暂的,大多不影响治疗。
另有国外资料报道在长期使用奥美拉唑治疗的患者的胃体活检标本中可观察到胃粘膜细胞增生或萎缩性胃炎的表现。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Zegerid (omeprazole)Powder for Oral SuspensionDESCRIPTIONThe active ingredient in Zegerid (omeprazole) powder for oral suspension, is a substituted benzimidazole, 5-methoxy-2-[[(4-methoxy-3, 5-dimethyl-2-pyridinyl) methyl]sulfinyl]-1H-benzimidazole, a racemic mixture of two enantiomers that inhibits gastric acid secretion. Its empirical formula is C17H19N3O3S, with a molecular weight of 345.42. The structural formula is:Omeprazole is a white to off-white crystalline powder which melts with decomposition at about 155°C. It is a weak base, freely soluble in ethanol and methanol, and slightly soluble in acetone and isopropanol and very slightly soluble in water. The stability of omeprazole is a function of pH; it is rapidly degraded in acid media, but has acceptable stability under alkaline conditions.Zegerid Powder for Oral Suspension is supplied in unit dose packets as an immediate release formulation to be constituted with water for oral administration. Each packet contains 20 mg of omeprazole and the following excipients: sodium bicarbonate, sucrose, sucralose, xanthan gum, xylitol, and flavorings.CLINICAL PHARMACOLOGYOmeprazole is acid labile and thus rapidly degraded by gastric acid. Zegerid Powder for Oral Suspension is an immediate-release formulation that contains sodium bicarbonate to protect omeprazole from acid degradation. Pharmacokinetics:Absorptionobserved when doubling the dose to 40 mg. The bioavailability of omeprazole from Zegerid Powder for Oral Suspension increases upon repeated administration of Zegerid.Pharmacokinetic Parameters of Zegerid Following Oral 20 mg Once-Daily Dosing for 1 and 7 DaysValues represent arithmetic means.Values represent arithmetic means.When Zegerid is administered 1 hour after a meal, Cmax and AUC are reduced by 63% and 24%, respectively, relative to administration prior to a meal.DistributionOmeprazole is bound to plasma proteins. Protein binding is approximately 95%.MetabolismAbsolute bioavailability (compared to intravenous administration) is about 30-40% at doses of 20-40 mg, due in large part to pre-systemic metabolism.ExcretionIn healthy subjects, the mean plasma half-life is 1 hour (range 0.4 to 3.2 hours), and the total body clearance is 500-600 mL/min.Following single dose oral administration of omeprazole, little if any unchanged drug is excreted in urine. The majority of the dose (about 77%) is eliminated in urine as at least six metabolites. Two metabolites have been identified as hydroxyomeprazole and the corresponding carboxylic acid. The remainder of the dose was recoverable in feces. This implies a significant biliary excretion of the metabolites of omeprazole. Three metabolites have been identified in plasma — the sulfide and sulfone derivatives of omeprazole, and hydroxyomeprazole. These metabolites have very little or no antisecretory activity.Special Populations GeriatricThe elimination rate of omeprazole was somewhat decreased in the elderly, and bioavailability was increased. Omeprazole was 76% bioavailable when a single 40 mg oral dose of omeprazole (buffered solution) wasadministered to healthy elderly subjects, versus 58% in young subjects given the same dose. Nearly 70% of the dose was recovered in urine as metabolites of omeprazole and no unchanged drug was detected. The plasma clearance of omeprazole was 250 mL/min (about half that of young subjects) and its plasma half-life averaged one hour, similar to that of young healthy subjects.PediatricThe pharmacokinetics of Zegerid have not been studied in patients < 18 years of age.Parameter Day 1AUC(0-inf) (ng*hr/mL) 825 Coefficient of variation 72% Cmax (ng/mL) 672 Coefficient of variation 44% Tmax (min) 29.8 T½ (hr) 0.86Parameter Day 7AUC(0-inf) (ng*hr/mL) 1446Coefficient of variation 61% Cmax (ng/mL) 902Coefficient of variation 40% Tmax (min) 28.3 T½ (hr) 1.08GenderThere are no known differences in the absorption or excretion of omeprazole between males and females. Hepatic insufficiencyIn patients with chronic hepatic disease, the bioavailability of omeprazole increased to approximately 100% compared to an l.V. dose, reflecting decreased first-pass effect, and the mean plasma half-life of the drug increased to nearly 3 hours compared to the mean half-life of 1 hour in healthy subjects. Plasma clearance averaged 70 mL/min, compared to a value of 500-600 mL/min in normal subjects.Renal insufficiencyIn patients with chronic renal impairment, whose creatinine clearance ranged between 10 and 62 mL/min/1.73 m2, the disposition of omeprazole was very similar to that in healthy volunteers, although there was a slight increase in bioavailability. Because urinary excretion is a primary route of excretion of omeprazole metabolites, their elimination slowed in proportion to the decreased creatinine clearance.AsiansIn pharmacokinetic studies of single 20 mg omeprazole doses, an increase in AUC of approximately four fold was noted in Asian subjects compared to Caucasians.Dose adjustment, particularly where maintenance of healing of erosive esophagitis is indicated, for the hepatically impaired and Asian subjects should be considered.Drug-Drug InteractionsWhen omeprazole 40 mg once daily was given in combination with clarithromycin 500 mg every 8 hours to healthy adult male subjects, the steady-state plasma concentrations of omeprazole were increased by the concomitant administration of clarithromycin (Cmax, AUC 0-24 and T1/2 increased 30%, 89%, and 34%, respectively).PharmacodynamicsMechanism of ActionOmeprazole belongs to a class of antisecretory compounds, the substituted benzimidazoles, that do not exhibit anticholinergic or H2 histamine antagonistic properties, but that suppress gastric acid secretion by specific inhibition of the H+/K+ ATPase enzyme system at the secretory surface of the gastric parietal cell. Because this enzyme system is regarded as the acid (proton) pump within the gastric mucosa, omeprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production. This effect is dose-related and leads to inhibition of both basal and stimulated acid secretion irrespective of the stimulus. Animal studies indicate that after rapid disappearance from plasma, omeprazole can be found within the gastric mucosa for a day or more.Antisecretory ActivityResults from a study of the antisecretory effect of repeated once-daily dosing of 20 mg of Zegerid in healthy subjects (n = 28) is shown below.Effect of Zegerid 20 mg on Intragastric pH on Day 7(19)Parameter82%/(24%)*% Decrease from Baseline for Integrated IntragastricAcidity (mmol*hr/L)% Time Gastric pH > 4 (hours) 51% (12.2 h) /(43%)*Median pH 4.2/(37%)*Values represent medians. All parameters were measured over a 24-hour period.* Coefficient of variationThe antisecretory effect thus lasts far longer than would be expected from the very short plasma half-life(1 hour) apparently due to irreversible binding to the parietal H+/K+ ATPase enzyme. Repeated single daily oral doses of Zegerid20 mg have produced nearly 100% inhibition of 24-hour integrated intragastric acidity in some subjects.Enterochromaffin-like (ECL) Cell EffectsIn 24 month carcinogenicity studies in rats, a dose-related significant increase in gastric carcinoid tumors and ECL cell hyperplasia was observed in both male and female animals (see PRECAUTIONS, Carcinogenesis, Mutagenesis, Impairment of Fertility). Carcinoid tumors have also been observed in rats subjected to fundectomy or long-term treatment with other proton pump inhibitors or high doses of H2-receptor antagonists. Human gastric biopsy specimens have been obtained from more than 3000 patients treated with omeprazole in long-term clinical trials. The incidence of ECL cell hyperplasia in these studies increased with time; however, no case of ECL cell carcinoids, dysplasia, or neoplasia has been found in these patients. (See also CLINICAL PHARMACOLOGY, Pathological Hypersecretory Conditions.)However, these studies are of insufficient duration and size to rule out the possible influence of long-term administration of omeprazole on the development of any premalignant or malignant conditions.Serum Gastrin EffectsIn studies involving more than 200 patients, serum gastrin levels increased during the first 1 to 2 weeks of once-daily administration of therapeutic doses of omeprazole in parallel with inhibition of acid secretion. No further increase in serum gastrin occurred with continued treatment. In comparison with histamine H2-receptor antagonists, the median increases produced by 20 mg doses of omeprazole were higher (1.3 to 3.6 fold vs. 1.1 to 1.8 fold increase). Gastrin values returned to pretreatment levels, usually within 1 to 2 weeks after discontinuation of therapy.Other EffectsSystemic effects of omeprazole in the CNS, cardiovascular and respiratory systems have not been found to date. Omeprazole, given in oral doses of 30 or 40 mg for 2 to 4 weeks, had no effect on thyroid function, carbohydrate metabolism, or circulating levels of parathyroid hormone, cortisol, estradiol, testosterone, prolactin, cholecystokinin or secretin.No effect on gastric emptying of the solid and liquid components of a test meal was demonstrated after a single dose of omeprazole 90 mg. In healthy subjects, a single l.V. dose of omeprazole (0.35 mg/kg) had no effect on intrinsic factor secretion. No systematic dose-dependent effect has been observed on basal or stimulated pepsin output in humans. However, when intragastric pH is maintained at 4.0 or above, basal pepsin output is low, and pepsin activity is decreased.As do other agents that elevate intragastric pH, omeprazole administered for 14 days in healthy subjects produced a significant increase in the intragastric concentrations of viable bacteria. The pattern of the bacterial species was unchanged from that commonly found in saliva. All changes resolved within three days of stopping treatment.The course of Barrett’s esophagus in 106 patients was evaluated in a U.S. double-blind controlled study of omeprazole 40 mg b.i.d. for 12 months followed by 20 mg b.i.d. for 12 months or ranitidine 300 mg b.i.d. for 24 months. No clinically significant impact on Barrett’s mucosa by antisecretory therapy was observed. Although neosquamous epithelium developed during antisecretory therapy, complete elimination of Barrett’s mucosa was not achieved. No significant difference was observed between treatment groups in development of dysplasia in Barrett’s mucosa and no patient developed esophageal carcinoma during treatment. No significant differences between treatment groups were observed in development of ECL cell hyperplasia, corpus atrophic gastritis, corpus intestinal metaplasia, or colon polyps exceeding 3 mm in diameter (see also CLINICAL PHARMACOLOGY, Enterochromaffin-like (ECL) Cell Effects).Clinical StudiesDuodenal Ulcer DiseaseActive Duodenal Ulcer - In a multicenter, double-blind, placebo controlled study of 147 patients with endoscopically documented duodenal ulcer, the percentage of patients healed (per protocol) at 2 and 4 weeks was significantly higher with omeprazole 20 mg once a day than with placebo (p ≤ 0.01).Treatment of Active Duodenal Ulcer% of Patients HealedOmeprazole 20 mg a.m. (n = 99) Placebo a.m. (n = 48)Week 2 *41 13Week 4 *75 27*(p ≤ 0.01)Complete daytime and nighttime pain relief occurred significantly faster (p ≤ 0.01) in patients treated with omeprazole 20 mg than in patients treated with placebo. At the end of the study, significantly more patients who had received omeprazole had complete relief of daytime pain (p ≤ 0.05) and nighttime pain (p ≤ 0.01).In a multicenter, double-blind study of 293 patients with endoscopically documented duodenal ulcer, the percentage of patients healed (per protocol) at 4 weeks was significantly higher with omeprazole 20 mg once a day than with ranitidine 150 mg b.i.d. (p < 0.01).Treatment of Active Duodenal Ulcer% of Patients HealedOmeprazole 20 mg a.m. (n = 145)Ranitidine 150 mg b.i.d. (n = 148)Week 2 42 34Week 4 *82 63*(p < 0.01)Healing occurred significantly faster in patients treated with omeprazole than in those treated with ranitidine 150 mg b.i.d. (p < 0.01).In a foreign multinational randomized, double-blind study of 105 patients with endoscopically documented duodenal ulcer, 20 mg and 40 mg of omeprazole were compared to 150 mg b.i.d. of ranitidine at 2, 4 and 8 weeks. At 2 and 4 weeks both doses of omeprazole were statistically superior (per protocol) to ranitidine, but 40 mg was not superior to 20 mg of omeprazole, and at 8 weeks there was no significant difference between any of the active drugs.Treatment of Active Duodenal Ulcer% of Patients HealedOmeprazole Ranitidine20 mg (n = 34)40 mg(n = 36)150 mg b.i.d.(n = 35)Week 2 *83 *83 53Week 4 *97 *100 82Week 8 100 100 94*(p ≤ 0.01)Gastroesophageal Reflux Disease (GERD)Symptomatic GERDA placebo controlled study was conducted in Scandinavia to compare the efficacy of omeprazole 20 mg or 10 mg once daily for up to 4 weeks in the treatment of heartburn and other symptoms in GERD patients without erosive esophagitis. Results are shown below.% Successful Symptomatic Outcome aOmeprazole 20 mg a.m. Omeprazole10 mg a.m.Placeboa.m.All patients 46*,†(n = 205)31†(n = 199)13(n = 105)Patients with confirmed GERD56*,†(n = 11536†(n = 10914(n = 59)a Defined as complete resolution of heartburn*(p < 0.005) versus 10 mg†(p < 0.005) versus placeboErosive EsophagitisIn a US multicenter double-blind placebo controlled study of 20 mg or 40 mg of omeprazole in patients with symptoms of GERD and endoscopically diagnosed erosive esophagitis of grade 2 or above, the percentage healing rates (per protocol) were as follows:Week 20 mg Omeprazole(n = 83)40 mg Omeprazole(n = 87)Placebo(n = 43)4 39*45* 78 74*75* 14*(p < 0.01) Omeprazole versus placebo.In this study, the 40 mg dose was not superior to the 20 mg dose of omeprazole in the percentage healing rate. Other controlled clinical trials have also shown that omeprazole is effective in severe GERD. In comparisons with histamine H2-receptor antagonists in patients with erosive esophagitis, grade 2 or above, omeprazole in a dose of 20 mg was significantly more effective than the active controls. Complete daytime and nighttime heartburn relief occurred significantly faster (p < 0.01) in patients treated with omeprazole than in those taking placebo or histamine H2-receptor antagonists.In this and five other controlled GERD studies, significantly more patients taking 20 mg omeprazole (84%) reported complete relief of GERD symptoms than patients receiving placebo (12%).Long Term Maintenance Treatment of Erosive EsophagitisIn a U.S. double-blind, randomized, multicenter, placebo controlled study, two dose regimens of omeprazole were studied in patients with endoscopically confirmed healed esophagitis. Results to determine maintenance of healing of erosive esophagitis are shown below.Life Table AnalysisOmeprazole 20 mg q.d. (n = 138) Omeprazole20 mg 3 daysper week(n = 137)Placebo(n = 131)Percent inendoscopicremission at 6months *70 34 11*(p < 0.01) Omeprazole 20 mg q.d. versus Omeprazole 20 mg 3 consecutive days per week or placebo.In an international multicenter double-blind study, omeprazole 20 mg daily and 10 mg daily were compared to ranitidine 150 mg twice daily in patients with endoscopically confirmed healed esophagitis. The table below provides the results of this study for maintenance of healing of erosive esophagitis.Life Table AnalysisOmeprazole 20 mg q.d. (n = 131) Omeprazole10 mg q.d.(n = 133)Ranitidine150 mg b.i.d.(n = 128)Percent inendoscopicremission at 12months *77 ‡58 46*(p = 0.01) Omeprazole 20 mg q.d. versus Omeprazole 10 mg q.d. or Ranitidine.‡(p = 0.03) Omeprazole 10 mg q.d. versus Ranitidine.In patients who initially had grades 3 or 4 erosive esophagitis, for maintenance after healing 20 mg daily of omeprazole was effective, while 10 mg did not demonstrate effectiveness.INDICATIONS AND USAGEDuodenal UlcerZegerid is indicated for short-term treatment of active duodenal ulcer. Most patients heal within four weeks. Some patients may require an additional four weeks of therapy.Treatment of Gastroesophageal Reflux Disease (GERD)Symptomatic GERDZegerid is indicated for the treatment of heartburn and other symptoms associated with GERD.Erosive EsophagitisZegerid is indicated for the short-term treatment (4-8 weeks) of erosive esophagitis which has been diagnosed by endoscopy.(See CLINICAL PHARMACOLOGY, Clinical Studies.)The efficacy of Zegerid used for longer than 8 weeks in these patients has not been established. In the rare instance of a patient not responding to 8 weeks of treatment, it may be helpful to give up to an additional 4 weeks of treatment. If there is recurrence of erosive esophagitis or GERD symptoms (e.g. heartburn), additional 4-8 week courses of omeprazole may be considered.Maintenance of Healing of Erosive EsophagitisZegerid Powder for Oral Suspension is indicated to maintain healing of erosive esophagitis.Controlled studies do not extend beyond 12 months.CONTRAINDICATIONSZegerid is contraindicated in patients with known hypersensitivity to any components of the formulation. PRECAUTIONSGeneralSymptomatic response to therapy with omeprazole does not preclude the presence of gastric malignancy.Atrophic gastritis has been noted occasionally in gastric corpus biopsies from patients treated long-term with omeprazole.Zegerid contains 460 mg sodium per dose in the form of sodium bicarbonate. This should be taken into consideration for patients on a sodium-restricted diet.Zegerid contains 1680 mg (20 mEq) of sodium bicarbonate. Sodium bicarbonate is contraindicated in patients with metabolic alkalosis and hypocalcemia. Sodium bicarbonate should be used with caution in patients with Bartter’s syndrome, hypokalemia, and respiratory alkalosis. Long-term administration of bicarbonate with calcium or milk can cause milk-alkali syndrome.Information for PatientsZegerid is supplied as a powder for oral suspension. It should be taken on an empty stomach at least 1 hour prior to a meal.Zegerid is available as 20 mg single-dose packets. Directions for use: Empty packet contents into a small cup containing 2 tablespoons of water. DO NOT USE OTHER LIQUIDS OR FOODS. Stir well and drink immediately. Refill cup with water and drink.Drug InteractionsOtherOmeprazole can prolong the elimination of diazepam, warfarin and phenytoin, drugs that are metabolized by oxidation in the liver. There have been reports of increased INR and prothrombin time in patients receiving proton pump inhibitors, including omeprazole, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Patients treated with proton pump inhibitors and warfarin may need to be monitored for increases in INR and prothrombin time. Although in healthy subjects no interaction with theophylline or propranolol was found, there have been clinical reports of interaction with other drugs metabolized via the cytochrome P-450 system (e.g., cyclosporine, disulfiram, benzodiazepines). Patients should be monitored to determine if it is necessary to adjust the dosage of these drugs when taken concomitantly with Zegerid.Because of its profound and long-lasting inhibition of gastric acid secretion, it is theoretically possible that omeprazole may interfere with absorption of drugs where gastric pH is an important determinant of their bioavailability (e.g., ketoconazole, ampicillin esters, and iron salts). In the clinical trials, antacids were used concomitantly with the administration of omeprazole.Co-administration of omeprazole and clarithromycin have resulted in increases of plasma levels of omeprazole, clarithromycin, and 14-hydroxy-clarithromycin (see also CLINICAL PHARMACOLOGY, Pharmacokinetics). Carcinogenesis, Mutagenesis, Impairment of FertilityIn two 24-month carcinogenicity studies in rats, omeprazole at daily doses of 1.7, 3.4, 13.8, 44.0 and 140.8mg/kg/day (about 0.7 to 57 times the human dose of 20 mg per day, based on body surface area) produced gastric ECL cell carcinoids in a dose-related manner in both male and female rats; the incidence of this effect was markedly higher in female rats, which had higher blood levels of omeprazole. Gastric carcinoids seldom occur in the untreated rat. In addition, ECL cell hyperplasia was present in all treated groups of both sexes. In one of these studies, female rats were treated with 13.8 mg omeprazole/kg/day (about 5.7 times the human dose of 20 mg per day, based on body surface area) for one year, then followed for an additional year without the drug. No carcinoids were seen in these rats. An increased incidence of treatment-related ECL cell hyperplasia was observed at the end of one year (94% treated vs 10% controls). By the second year the difference between treated and control rats was much smaller (46% vs 26%) but still showed more hyperplasia in the treated group. Gastric adenocarcinoma was seen in one rat (2%). No similar tumor was seen in male or female rats treated for two years. For this strain of rat no similar tumor has been noted historically, but a finding involving only one tumor is difficult to interpret. In a 52-week toxicity study in Sprague-Dawley rats, brain astrocytomas were found in a small number of males that received omeprazole at dose levels of 0.4, 2, and 16 mg/kg/day (about 0.2 to 6.5 times the human dose of 20 mg/day, based on body surface area). Noastrocytomas were observed in female rats in this study. In a 2-year carcinogenicity study in Sprague-Dawley rats, no astrocytomas were found in males and females at the high dose of 140.8 mg/kg/day (about 57 times the human dose of 20 mg per day, based on body surface area). A 78-week mouse carcinogenicity study of omeprazole did not show increased tumor occurrence, but the study was not conclusive. A 26-week p53 (+/-) transgenic mouse carcinogenicity study was not positive.Omeprazole was positive for clastogenic effects in an in vitro human lymphocyte chromosomal aberration assay, in one of two in vivo mouse micronucleus tests, and in an in vivo bone marrow cell chromosomal aberration assay. Omeprazole was negative in the in vitro Ames Salmonella typhimurium assay, an in vitro mouse lymphoma cell forward mutation assay and an in vivo rat liver DNA damage assay.Omeprazole at oral doses up to 138.0 mg/kg/day (about 56 times the human dose of 20 mg per day, based on body surface area) was found to have no effect on fertility and reproductive performance.PregnancyPregnancy Category CThere are no adequate and well-controlled studies on the use of omeprazole in pregnant women. The vast majority of reported experience with omeprazole during human pregnancy is first trimester exposure and the duration of use is rarely specified, e.g., intermittent vs. chronic. An expert review of published data on experiences with omeprazole use during pregnancy by TERIS – the Teratogen Information System – concluded that therapeutic doses during pregnancy are unlikely to pose a substantial teratogenic risk (the quantity and quality of data were assessed as fair).Three epidemiological studies compared the frequency of congenital abnormalities among infants born to women who used omeprazole during pregnancy to the frequency of abnormalities among infants of women exposed to H2-receptor antagonists or other controls. A population-based prospective cohort epidemiological study from the Swedish Medical Birth Registry, covering approximately 99% of pregnancies, reported on 955 infants (824 exposed during the first trimester with 39 of these exposed beyond first trimester, and 131 exposed after the first trimester) whose mothers used omeprazole during pregnancy. In utero exposure to omeprazole was not associated with increased risk of any malformation (odds ratio 0.82, 95% CI 0.50-1.34), low birth weight or low Apgar score. The number of infants born with ventricular septal defects and the number of stillborn infants was slightly higher in the omeprazole exposed infants than the expected number in the normal population. The author concluded that both effects may be random.A retrospective cohort study reported on 689 pregnant women exposed to either H2-blockers or omeprazole in the first trimester (134 exposed to omeprazole). The overall malformation rate was 4.4% (95% CI 3.6-5.3) and the malformation rate for first trimester exposure to omeprazole was 3.6% (95% CI 1.5-8.1). The relative risk of malformations associated with first trimester exposure to omeprazole compared with nonexposed women was 0.9 (95% CI 0.3-2.2). The study could effectively rule out a relative risk greater than 2.5 for all malformations. Rates of preterm delivery or growth retardation did not differ between the groups.A controlled prospective observational study followed 113 women exposed to omeprazole during pregnancy (89% first trimester exposures). The reported rates of major congenital malformations was 4% for the omeprazole group, 2% for controls exposed to nonteratogens, and 2.8% in disease-paired controls (background incidence of major malformations 1-5%). Rates of spontaneous and elective abortions, preterm deliveries gestational age at delivery, and mean birth weight did not differ between the groups. The sample size in this study has 80% power to detect a 5-fold increase in the rate of major malformation.Several studies have reported no apparent adverse short term effects on the infant when single dose oral or intravenous omeprazole was administered to over 200 pregnant women as premedication for cesarean section under general anesthesia.Teratology studies conducted in pregnant rats at omeprazole doses up to 138 mg/kg/day (about 56 times the human dose of 20 mg/day, based on body surface area) and in pregnant rabbits at doses up to 69 mg/kg/day (about 56 times the human dose of 20 mg per day, based on body surface area) did not disclose any evidence for a teratogenic potential of omeprazole.In rabbits, omeprazole in a dose range of 6.9 to 69.1 mg/kg/day (about 5.6 to 56 times the human dose of 20 mg per day, based on body surface area) produced dose-related increases in embryo-lethality, fetal resorptions and pregnancy disruptions. In rats, dose-related embryo/fetal toxicity and postnatal developmental toxicity wereobserved in offspring resulting from parents treated with omeprazole at 13.8 to 138.0 mg/kg/day (about 5.6 to 56 times the human dose of 20 mg per day, based on body surface area).Chronic use of sodium bicarbonate may lead to systemic alkalosis and increased sodium intake can produce edema and weight increase. There are no adequate and well-controlled studies in pregnant women. Because animal studies and studies in humans cannot rule out the possibility of harm, omeprazole should be used during pregnancy only if the potential benefit to pregnant women justifies the potential risk to the fetus.Nursing MothersOmeprazole concentrations have been measured in breast milk of a woman following oral administration of 20 mg. The peak concentration of omeprazole in breast milk was less than 7% of the peak serum concentration. The concentration will correspond to 0.004 mg of omeprazole in 200 mL of milk. In rats, omeprazoleadministration during late gestation and lactation at doses of 13.8 to 138 mg/kg/day (about 5.6 to 56 times the human dose of 20 mg per day, based on body surface area) resulted in decreased weight gain in pups. Because omeprazole is excreted in human milk, because of the potential for serious adverse reactions in nursing infants from omeprazole, and because of the potential for tumorigenicity shown for omeprazole in rat carcinogenicity studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. In addition, sodium bicarbonate should be used with caution in nursing mothers.Pediatric UseThere are no adequate and well-controlled studies in pediatric patients with Zegerid.Geriatric UseOmeprazole was administered to over 2000 elderly individuals (≥ 65 years of age) in clinical trials in the US and Europe. There were no differences in safety and effectiveness between the elderly and younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.Pharmacokinetic studies with omeprazole have shown the elimination rate was somewhat decreased in the elderly and bioavailability was increased. The plasma clearance of omeprazole was 250 mL/min (about half that of young subjects). The plasma half-life averaged one hour, about the same as that in nonelderly, healthy subjects taking Zegerid. However, no dosage adjustment is necessary in the elderly. (See CLINICAL PHARMACOLOGY.)ADVERSE REACTIONSOmeprazole was generally well tolerated during domestic and international clinical trials in 3096 patients.In the US clinical trial population of 465 patients, the following adverse experiences were reported to occur in 1% or more of patients on therapy with omeprazole. Numbers in parentheses indicate percentages of the adverse experiences considered by investigators as possibly, probably or definitely related to the drug.Omeprazole (n = 465)Placebo (n = 64)Ranitidine (n = 195) Headache 6.9 (2.4) 6.3 7.7 (2.6) Diarrhea 3.0 (1.9) 3.1 (1.6) 2.1 (0.5) Abdominal Pain2.4 (0.4)3.12.1Nausea 2.2 (0.9) 3.1 4.1 (0.5) URI 1.91.62.6 Dizziness 1.5 (0.6) 0.0 2.6 (1.0) Vomiting1.5 (0.4) 4.71.5 (0.5)。
