一张图窥视有机官能团间的相互转化
高三有机化学中有机物间相互转化关系图

高三有机化学中有机物间相互转化关系图高三有机化学中有机物间相互转化关系图Document number:WTWYT-WYWY-BTGTT-YTTYU-2018GT一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等)⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等)⑸ 天然橡胶(聚异戊二烯) 2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐)⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 3 6FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2 ⑷ Zn 、Mg 等单质如⑸ -1价的I (氢碘酸及碘化物)变色⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;变色Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应)△下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴不饱和烃(烯烃、炔烃、二烯烃等)⑵不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等)⑶石油产品(裂化气、裂解气、裂化汽油等)⑷醇类物质(乙醇等)⑸含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等)⑹天然橡胶(聚异戊二烯)⑺苯的同系物2、无机物:⑴氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物)⑵ + 2价的Fe(亚铁盐及氢氧化亚铁)⑶-2价的S(硫化氢及硫化物)⑷ + 4价的S(二氧化硫、亚硫酸及亚硫酸盐)⑸双氧水(H2O2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A)官能团:无;通式:C n H2n+2;代表物:CH4B) 结构特点:键角为109°28′,空间正四面体分子。
高三有机化学中有机物间相互转化关系图

一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 36FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2⑷ Zn 、Mg 等单质 如 ⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO 3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应)△2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的Fe (亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 结构特点:键角为109°28′,空间正四面体分子。
高三有机化学中有机物间相互转化关系图word版本

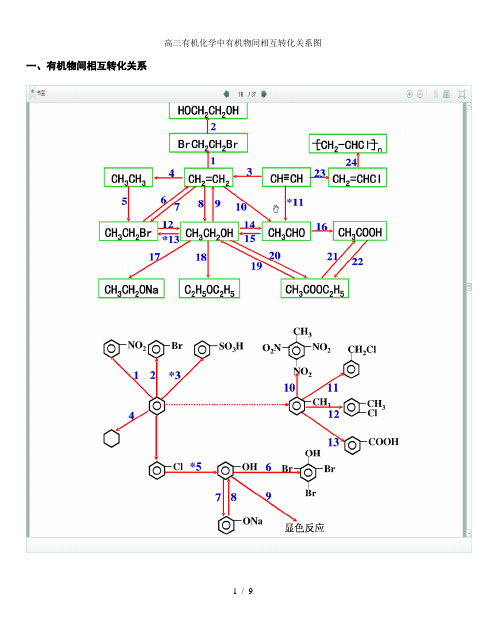
高三有机化学中有机物间相互转化尖系图、有机物间相互转化尖系HOCH地0HBrCH.CHM-ECHrCHC 比241CH3CH2ONa WC£H5GH3COOC2H5、能与漠水发生化学反应而使漠水褪色或变色的物质1有机物:(1)不饱和坯(烯泾、決屋、二烯怪等)⑵不饱和疑的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等)⑶石油产品(裂化气、裂解气、裂化汽油等)⑷含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等)⑸天然橡胶(聚异戊二烯)2、无机物:(1)—2价的S (硫化氢及硫化物)(2) + 4价的S (二氧化硫、亚硫酸及亚硫酸盐)(3) + 2 价的Fe6FeS0 4- 3Br2= 2F® (S04)3 +2FeB I6FeCl2 + 3Br2 = 4FeCla + 2FeBr3 J 父巳2Fel2 + 3Br2 = 2FeBr3 + 2b+,Mg + Br 2 === MgBr 2 (其中亦有Mg 与H+、Mg 与HBrO 的反应)(4) Zn、Mg等单质女口(5) -1价的I (氢碘酸及碘化物)变色(6)NaOH 等强碱、Na2CO3和AgNO3等盐Br2+H2O = HBr + HBrO2HBr + Na2CO3 = 2NaBr + CO21 + H2OHBrO + Na2CO3 = NaBrO + NaHCOa、能萃取漠而使漠水褪色的物质上层变无色的(P>1):卤代坯(CCI4、氯仿、漠苯等)、CS2等;下层变无色的(P V1 ):直镭汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷怪、液态饱和姪(如己烷等)等四、能使酸性高镒酸钾溶液褪色的物质1、有机物:(1)不饱和坯(烯怪、快姪、二烯坯等)(2)不饱和泾的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等)(3)石油产品(裂化气、裂解气、裂化汽油等)(4)醇类物质(乙醇等)(5)含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等)(6)天然橡胶(聚异戊二烯)⑺苯的同系物2、无机物:(1)氢卤酸及卤化物(氢漠酸、氢碘酸、浓盐酸、漠化物、碘化物)(2)+ 2价的Fe (亚铁盐及氢氧化亚铁)(3)-2价的S (硫化氢及硫化物)(4)+4价的S (二氧化硫、亚硫酸及亚硫酸盐)⑸双氧水(H2O2 )五、常见的各类有机物的官能团,结构特点及主要化学性质(1)烷姪A ) 官能团:无;通式:CnH2n +2 ;代表物:CH4B ) 结构特点:键角为109。
有机物的转化衍生关系

四、各类有机物之间的衍变和转化关系
掌握各类有机物间的相互联系,使有机化学知识形成体系,并根据此衍变关系合成和推断有机物 各类有机物之间的相互转化,实质是官能团之间的互换和变化,一般有以下几种 ★ 相互取代关系,如卤代烃与醇,实质是卤原子和羟基之间的相互取代 ★ 氧化还原关系:醇与醛、羧酸之间的相互转化 ★ 消去加成关系:如乙醇和乙烯
★ 结合重组关系;如醇和羧酸之间的酯化以及酯的水解
1、各类链烃及其衍生物间的关系
2、具体物质
C H 3CH
C H 2CH
2C H
COOH
O O
O O
CH 3C 2H 5OC 2H 5
CH 3COOH CH 3COOC 2H 5C H 3CH 2Br C H 2CH 2Br Br
C H 2CH O O
C OCH 2CH 2O C []n
CH 3CH 2OH CH 3CH 4
CHO
3、写方程式
(1)有机物转化网络图一
(2)有机物转化网络图二。
高三有机化学中有机物间相互转化关系图

一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 36FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2⑷ Zn 、Mg 等单质 如 ⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO 3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应)△2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的Fe (亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 结构特点:键角为109°28′,空间正四面体分子。
有机官能团转化规律

NO2
1
官能团转化与引入关系图
1. CH3—CH2 OH ( 消去反应
浓硫酸 170℃
) CH2=CH2
(加成反应)
CH2—CH2 Cl Cl
(水解或取代)
CH2—CH2 OH OH
(氧化反应 )
酯化
H—C—C—OH O O
(氧化 )
缩聚
HO—C—C—OH O O
生物发酵
葡萄糖 2. CH3—CH2 Cl
光合作用
[CH2—CH2—O]n CO2+H2O ) CH2=CH2 (加成反应 )
(加聚反应)
CH2—O—C=O
CH2—CH2 O ) CH2=CH Cl
OO HOCH2CH2-O-C-C-OH [OCH2CH2OCOCO]n
CH2—O—C=O
(消去反应
CH2—CH2 Cl Cl
(消去反应
(加成反应 )
CH2—CH—Cl Cl Cl
(加成反应 ) (水解或取代) CH2—CH—Br Br Cl 3. CH2=CH2 5. CH≡CH
3 分子成环 硝化取代 —NO2 加成 HCN
(水解或取代) CH2—CH OH O
加成 HCN
氧化
CH2—C—OH OH O CH2 = CH COOH
4. CH3—CH2 CN
CH3—COOH
CH2=CH—Cl
[CH2—CHCl]n
油 脂
动 植 物
蛋 白 质
CH3
H2 Ni 浓 H2SO4 HNO3 Cl —OH OH 浓 H2SO4 HNO3 CH3 浓 H2SO4 HNO3 O2N— —NO2 NO2 O2N— —NO2 —NO2 Fe 稀盐酸 —NH2
高三有机化学中有机物间相互转化关系图
一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的64 + 32 = 22(4)3 + 2362 + 32 = 43 + 2322 + 32 = 23 + 2I 2⑷ 、等单质 如⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ 等强碱、23和3等盐 2 + H 2O = +2 + 23 = 2 + 2↑+ H 2O + 23 = + 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(4、氯仿、溴苯等)、2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 + 2 2 (其中亦有与、与的反应) △2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的(亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:22;代表物:4B) 结构特点:键角为109°28′,空间正四面体分子。
有机物衍生关系及官能团的引入和消除

1、官能团的引入和转换: 、官能团的引入和转换:
(7)酯基的引入方法:①酯化反应的发生;②酯交换反 酯基的引入方法: 酯化反应的发生; 酯基的引入方法 应的发生。 应的发生。 (8)硝基的引入方法:硝化反应的发生。 硝基的引入方法: 硝基的引入方法 硝化反应的发生。 (9)氨基的引入方法:①-NO2的还原;②-CN的加氢 氨基的引入方法: 的还原; 氨基的引入方法 的加氢 还原; 蛋白质的水解。 还原;③肽、蛋白质的水解。 (10) 氰基的引入方法:①烯烃与HCN的加成;②炔烃 氰基的引入方法: 烯烃与 的加成; 的加成 的加成。 与HCN的加成。 的加成
有机合成题的常规解题方法
顺合成法: 顺合成法: 逆合成法: 逆合成法: 综合比较法: 综合比较法:
例题
以对二甲苯和乙醇为主要原料合成聚酯纤维
O HO C O C O CH2CH2O H n
例题
已知①溴单质通常与 起加成反应, 已知①溴单质通常与>C=C<起加成反应,但在高温下 起加成反应 溴易取代与>C=C<直接相连的碳原子上的氢原子②与 直接相连的碳原子上的氢原子② 溴易取代与 直接相连的碳原子上的氢原子 苯环直接相连的侧链碳原子上的氢原子在光照下也易被 溴取代
CH2 CH2 CH3 CH CH CH2 OH OH OH
例题
有机合成上通常通过下述两步反应在有机物分子碳 链上增加一个碳原子。 链上增加一个碳原子。
应用上述反应原理,试以乙炔,甲醇、HCN等物质 应用上述反应原理,试以乙炔,甲醇、 等物质 为主要原料, 为主要原料,通过六步反应合成
例题
已知: 已知:
CH 3 CH 2 CH 2 Br 消去→ CH 3 CH = CH 2 加成 → CH 3 C H CH 3 − HBr + HBr | Br
有机化学官能团相互转化

5-fH 3O +Hg 2+ / CH 3CN (aq)C=C-OR C=C-SROCH 3OH 3O+SCH 3OHg 2+3OH 3O Hg 2+3H 3O +OO O SSS SSRSR O O OR OR 5-gHg 2+ / H 3O +H 3O + / solv (aq)H 3O + / solv (aq)Hg 2+ / H 3O +OR OROH OHH 3O + / solv (aq)a very common protecting group, deprotect back to ketoneHC OEt OEt OEtRMgX / H 3O +HC OEt OEt OEtRMgXRCHON H+2Cr 2O 7-2N HCl.CrO 3Ag 2O:1. a mild oxidizing agent2. must be freshly prepared: NaOH into AgNO 3 (aq)3. may involve surface change, react with CO 2, lightSwern oxidation: (DMSO, oxalyl chloride, Et 3N)drawback: react at low T Collins reagent: (CrO 3 - 2 Py)1. drawback: use 6 equivalent, a messy reaction 2. must be very dry, fire easily; purify by CaH 23. an old oxidizing material, isolated by Collin.i. PCCii. PDCix. K 2R C OHO aldehyde1st alcohol2nd alcohol1st alcoholR C OHOR C ROR C HO 5-h i. PCC ii. PDCJOC, 1985, 50, 1332.N OCH 3OHPDC (pyridinium dichormate)(H 2Cr 2O 7 + 2 Py)PCC (pyridinium chlorochromate) (Py-HCl-CrO 3)most widely used use 1 - 1.2 eq.Pfitzner-Moffatt oxidationOO BrDMSOO OOH360 %Synth. Commun., 1986, 16, 1343.JOC, 1977, 42, 1991.Synthesis, 1981, 165.O I OOAcAcOpH 6: weak acid buffer, avoid interfere with ketal groupMcMurray reactioni. Corey approach: subtituted-quinone // H 3O +ii. Watt approacha. PhCHO // MCPBA // H 3O +b. ArPhO // MCPBA // H 3O +c. NBS // KOH // H 3O +PhOPh PhOPhNH 2Ph PhNH 2NC O H // H 3O +O O5-i.15-i.2i. Et 3N // H 3O +Nef reactionii. TiCl 3 / pH 1 or 6iii. SiO 2 / NaOH // H 3O +JACS, 1977, 99, 3861.iv. LDA / MoO 5-Py -v. NaOH // CH 3O OH 3O +vi. KMnO 4 / KOHChem. Rev. 1955, 55, 137.5-k IOOOH O(3 eq.)JACS, 2001, 123, 3183.CH 3CHO2. DDQ / TFA.Synthesis, 1979, 537.JCS, 1932, 1875.Ph-F / DMSO 3.1. SeO 2a select oxidantindrect: change to RC-OH followed by oxidation direct:1. DMAPO / DBU / CH 3CN i. DMSO / AgBF 4RBr DMSO / AgBF 4- Me 2SBull Soc. Jpn., 1981, 54, 2221.THL, 1974, 917.2. NaIO 4 / DMFO Br84 %oNaIO 4 / DMF a new method 3. DMSO reagents:ii. DMSO / ZnSRCHBrMeRC(O)MeDMSO BrOH OOH JACS, 2003, 68, 2480.ROAgBFTHL, 1975, 4467.C C R CHOHRR C C HC C R R'R C C HR C C ArR C C HC C R PhR C C Hsteric base, prevent Nu attack n -BuLi: not MeLi, or t -BuLi, fire easily RX: R-Br, R-TOS, RCHO, RC(O)Rn -BuLi / R'CHO // Ac 2O // KO t BuClOMeN Liiii.ii. (Ph 3P)2PdCl 2, CuI, Et 2NH / PhIi. n-BuLi / RX6-b6-a b c d e g 6-aC CC CC Csulfonic acid: PhSO 3H; sulfinic acid: PhSO 2H; sulfenic acid: PhSOHiv. CuI, NaI, Na 2CO 3, RC C CH 2ClR C C HCl CH 2CC R'RCCCH 2CCR'Synthesis, 2000, 691.RCH 2-SO 2Ph RC CHh f iRCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXin fact: convert to C=C firstlyii. protect - deprotecti. move to terminal 6-cNH 2NHKuse: KAPAuse: Co (CO)8 // Fe(NO 3)3, EtOHJACS, 1975, 97, 891.6-duse: i. Br 2 / CCl 4 // KO t Bu6-euse: Pb(OAc)4, LiCl // KO t Bu // Br 2/CCl 4 // KO t Fe(NO 3)3: weak oxidizing agentii. Br 2 // KOHJA CS, 1941, 63, 1180.PhPhPhPh6-fi. NaBH 4, H 3O +, Br 2, KOtBuii. NH 2OH, NaNO 2 / H 2SO 4 // Ac 2O / DMAPiii. LDA, ClPO(OEt)2ON NODMAP:4-N,N-D i m ethyl a minop y ridinemixture ofAc 2O / DMAP:N NC CH 3O6-guse: TsNHNH 2 / EtOH, heatTHL, 1967, 3943.OHO3(l)O(MVK)CH 3CH CH 2Robinson Annulation German invention, as acylating agentLDA: Li N(iPr)2, ignored a long time, re-introduced by Michigan State U. became famous, appeared every weekHORLiNH 2 / NH 3 (l)RXuse: LiNH 2 / NH 3 (l) / R-XO Cl6-h6-i.JA CS, 1958, 80, 4599.JA CS, 1955, 77, 3293.Me PhHOSO 2CF 3Me C C Phvia:Me CPhJOC, 1978, 43, 364.ArAr'H Br Ar C C Ar'NaOEtvia:Ar Ar'Br i. NaOEt (when X = Br)ii. BuLi (when X = -OSO 2CF 3)?heatRCH=CH 2:PBu RCH 2CH 2-O-PBu 3RCH 2CH 2-OHPh-Se-PBu 3Ph-Se-CNmechanism:MCPBA OAc CO 2MeOAcMeO MeO 2CNO 2SeNOAcCO 2MeOAc MeOMeO 2Cminorapplied for reactions: without rearrangement;no regiosiomerCC (CO 2H)2 / benzeneOH PhPhOO Cl ClClClOCl Cl NC NCO iii. Pd-C; or Ni; Pt, Rhii. chloronaili. DDQ use base: DBNi. CH 3I / Ag 2ii. HCHO / HCO 222use: heatuse: heatb 7-i. p-TSOH.H 2O or CSA ii. weak acid: HOAc; HCO 2H; H 2C 2O 4use:C C HIC C H NH 2C C H OC(S)SMe C C H OAc C C H OMs C C H OH a7-i h gCCX C C C CC CHC O C Cf e dc b a 7--C(O)-CH 3CH-CH CH-CX C-OHjCX-CYNaI / Zn (Cu)i. Zn / acetonei. CSCl 2/C COMs OMs C C BrBrCCOH OHc7-CCOH Iii. CSCl 2 / P(OMe)3P NNPhPOCl 3 / py // Snvia:C C IIapplication: i. protect alkene: via Br 2 // ZnCCCCC 36 o C CCCC=C 31 o C CCCC C Cl Cl148 o CS OR ORC C BrOAcZn / HOAcOAcO AcOAcOBrOAcZn OOAc OAcOAcJOC, 1978, 43, 364.HOAc, 1998, 2113.ii. In / MeOH ii. purify compoundd7-e7-i. WCl 6 / RLi ii. LiPPh 2 / CH 3I product retention product inversionNa R C HC HCH 2CH 2CH 2OH OClRiii. Na(special structure):7-d.7-d.S R 1R 2R 1R 2(EtO)3Puse: (EtO)3PSynthesis , 1977, 1134.via : betaine, oxaphosphetane (NMR)Onot good for Ph 3P=CH 2function as base:expensivedifficult to prepareOEtCNPPh 3CNPPh 3H OPPh 3O CO 2Me+notPh 3P CH EtH C OCO 2Me notPh 3P CH CO 2MeEtH O++++stable ylid gives trans (E)unstable ylid gives cis (Z)water soluble, removed by extraction(comparison: O=PPh 3 highly soluble in organic solvent)use:LiPh SON MeCH 2// Al (Hg)Me 3SiCHR -Li +Ph 3SiCH 2-Li + === Ph 3SiCH 2Br + n-BuLi (exchange)Me 3SiC -H-MgBr === Me 3SiCH 2Cl + Mg (metal reduction)Ph 3SiC -HCH 2Ph === Ph 3SiCH=CH 2 + PhLi (addition to vinylsilane)Me 3SiC -HCO 2Et === Me 3SiCH 2CO 2Et + Li (metalation)Me 3SiCH=PPh 3 === Me 3SiCH 2PPh 3+ X - + KHRO = MeO-, EtO-use: (RO)2PO-CHR'use: Ph 3P-CHR'vi. Sulfoximide (Johnson C.)iii. Silyl Wittig Reaction (Peterson Reaction)ii. Phosphonate Wittig Reaction (Horner-Emmons Modification)i. Wittig Reaction 7-f7-f.Synthesis, 1984, 384.THL, 1981, 2751.JOC, 1968, 33, 780.iv. CH 2(ZnI)2Chem. Lett, 1995, 259.Synlett, 1988, 12, 1369.2CH 2(ZnI)2v. CH 2CHBr 2, Sm, SnI 2 / CrCl 3, THFRO Rvii. Grignard reagent:1. TMSCH MgCluse: TMSCH 2MgClTHL, 1973, 3497.THL, 1988, 4339. 2. NaOAc, AcOHmethylenationOC RR'3H advantages over the Wittig:1. by-products are more easily removed,2. reaction suffers less from steric effects.via:(olefination reaction)1953 discover7-f.2not for Wittig, ylid unstableJOC, 1978, 43, 3253.JACS, 1974, 96, 4706.Chem. Lett, 1973, 1041.TiCl 3-LiAlH 4 / THF TiCl 3 / Mg TiCl 4 / Zn TiCl 4 / K ii. McMurry Couplingi. use: N 2H 4 / H 2S / Pb(OAc)4BASF, 1973, 2147.via:Zn-CuP(OEt)1. H 2S2. Pb(OAc)431. H 2S2. Pb(OAc)4OON SN N N OSN NSON ON NNSN NON NO OSO ON NOO OO OTiCl 3N 2H 4g7-form trans alkene:form cis alkene:i. Li / NH 3; or other IA metals ii. Li / EtNH 2iii. LiAlH 4 / THFi. H 2 / Ni 2B (P-2 catalyst)ii. H 2 / Pd-CaCO 3 (Lindlar catalyst)iii. H 2 / Pd-BaSO 4iv. B 2H 6 / HOAc (Diborane)v. N 2H 2vi. HCHO / Pd-C / Et 3Nnot use H 2 / Pt: might convert to alkaneh7-all form trans alkene:i. R 2BH / Br-CN (hydroboration)C CHR HHii. DIBAL / n-BuLi / CH 3I (hydroalumination)iii. Cp 2ZrClH / RX (hydrozirconation)application: protecting groupvia dihalidevia halohydrinvia epoxidevia diene-olefin additionC=C C CX XC CH XC=CC COC=CC=CC=C C=CC=CC CC Cnot for double bond might moveMnO2 / Ph3P CH3 Br- / MTBDNNNCH3MTBD via diradicalJA CS, 1998, 100, 877.Ph Ph7-i7-j8-a 8-a.28-a.38-a.41. HI 3. TsCl / C 62. PI 3JCS, 1905, 87, 1592.CH OH CH I PI 38-a.12. F 3S-NEt 21.(DAST)SN SF O OOO Chem. Rev., 1996, 96, 1737.2FCH 3SO O OH ONCHCl 2CH 3 1. CF 3CHFCF 2NEt 22. HOAc / i PrOH$ 65 / 500 g $ 80 / 50 gPBr 3PI 3$ 35 / 1000 g PBr 3$ 500 / 125 gJOC, 1993, 58, 3800.8-C-XC-OH C(O)Z d c b a C-NH 2C=O 2. PPh 3 / I 2e C-H8-d8-d.RC O OH1. AgNO 3/KOH 2R Br Ber. 1942, 75, 296.8-d.2ClOClRhCl(PPh 3)38-b NaNO 2 / HCl / HBF 4 /Chem Rev., 1956, 56, 219.8-c CF 2Br 2 / ZnFF JCS.PT I, 1993, 335.8-e8-e.1I86 %I 2 / HNO 3JACS, 1917, 39, 437.8-e.3I / HNO PhCH 2C(O)CH 3PhCHC(O)CH 3FN SFO OF +Chem. Rev., 1996, 96, 1737.F-TEDA-BF 4, 1994, 149.F 2-N 2 / CFCl 3-CHCl 3JOC, 1988, 53, 2803.90 %1. regioselective fluorination at the more substituted positions2. electrophilic in natureF -N 33ONXR 1R 3OOR 2HMg(ClO 4)2R 1R 3O OR 2XNBX / Mg(ClO 4)2JOC, 2002, 67, 7429.8-e.2X = Cl, Br, IX = Cl, Br, INBXNBX:i.ii.iii.iv.RRFR = CH 3CO, COCF 3, CCl 3, NO 2 HF / electrolysis1.4-1.6 Valready industrilized NF 3O / TBAH / CH 3CNv.TBHA: TetrabutylammoniumhydroxideTHL, 2003, 44, 2799.9-a9-C-CH 3C-X a (CH 3)3AlMe 3Al98 %Organomet. Chem. Rev., 1996, 4, 47.CH 2Cl 2bridgehead methylationB 2H 6 / H 2NOSO 3HB 2H 6 / H 2NO CH 3CN / H 3O +B 2H 6 / NH 2Cl C-C-NHCOCH 3C=CC-C-NH 24-freductive amination!Leuckart reactionmost generalvia: hydrazone4. PhNHNH 2 // Al (Hg)2. Me 3SiN 3 // LiAlH 43. NH 3 (excess) // RaNi / H 21. RNH 2 // NaBH 3CN5. NH 4+HCO 2-4-e6. RNH 2 / n -Bu 2SnClH / HMPASynthesis, 2000, 789.5. P 4S 10 // RaNi4. Et 3O + BF 4- // NaBH 43. B 2H 62. NaBH 3(OCOR)1. LiAlH 46. Lawesson's reagent // RaNi4-h4-g4-g.a 4-g.b R C NH 2R C NH 2R'formform AlH 3 / THF BrC NBr NH 2JOC, 2000, 65, 8152.AlH 3TH, 1989, 30, 5137.JOC, 1987, 52, 3901.R'Li // NaBH 4R'MgX // NaBH 4R'MgX // Li/NH 3(l)R'2CuLi // NaBH 4TH, 1989, 30, 5139.JOC, 1993, 58, 4313.R C NR C NH 2R'4-iNH 2ONHOCH 3O PhI(OAc)23JOC, 1993, 58, 2478.RCO NH 2RCO NIPh OAcRN C OR NH COOCH 3CH 3OHPhI, OAcRPhI(OAc)4-i.2C NH 2RCH 2PhI(OAc)2 // KOH / CH 3OHC(OR)2C(SR)2h C-NH 2C-NO 2C N C C 5-ag f d c b a 5-C=C-OR C=C-SR C-OH C=N-OH C=N-H C=S C=O C=Ov. via: epoxysilaneRCO CRRCO CH 2R42SOCl H 3O +23OO2-CrO 4OONaBH 3H 3O +3ZnTsNHNH 2MeLiTMSCl MCPBA LAH324CH 2CORRCH 2CORRMsClKOtBuHgCl 3SSCH 2CORRPhCHOi. via: α-CO 2Hii. via: α-haloketoneiii. via: aldol processiv. via: thioenol etherRCO CH 2Rdrawback: require simple structure, use many powerful agents: MeLi, LAH, MCPBAe i j C-Br k C-Hii. MCPBAi. hydrolysis5-b5-c C=N-OHC=N-Hi. RaNi ii. TiCl 3iii. KMnO 4 / Al 2O 3H 3O +5-dHg 2+ / H 2O JOC, 1972, 37, 2138.JOC, 1970, 35, 858.HgSO 4 / H 2O / H 2O5-c.15-c.2THL, 2001, 42, 4775.1. DIBAL / H 3O +5-eStenphen reductionmostly for.Syn, 1925, 3, 1874.2. HCl./ SnCl 2 / Et 2O 5-e.1R -CH 2-CN5-e.25-e.3-CH 2-C OHR -CH -C OH R'R -CH -C O R"R'R'X / n -BuLiCH 3I R''MgBr H 3O +3.H 3O +B 2H 6 / H 2NOSO 3HB 2H 6 / H 2NO CH 3CN / H 3O +B 2H 6 / NH 2Cl C-C-NHCOCH 3C=CC-C-NH 24-freductive amination!Leuckart reactionmost generalvia: hydrazone4. PhNHNH 2 // Al (Hg)2. Me 3SiN 3 // LiAlH 43. NH 3 (excess) // RaNi / H 21. RNH 2 // NaBH 3CN5. NH 4+HCO 2-4-e6. RNH 2 / n -Bu 2SnClH / HMPASynthesis, 2000, 789.5. P 4S 10 // RaNi4. Et 3O + BF 4- // NaBH 43. B 2H 62. NaBH 3(OCOR)1. LiAlH 46. Lawesson's reagent // RaNi4-h4-g4-g.a 4-g.b R C NH 2R C NH 2R'formform AlH 3 / THF BrC NBr NH 2JOC, 2000, 65, 8152.AlH 3TH, 1989, 30, 5137.JOC, 1987, 52, 3901.R'Li // NaBH 4R'MgX // NaBH 4R'MgX // Li/NH 3(l)R'2CuLi // NaBH 4TH, 1989, 30, 5139.JOC, 1993, 58, 4313.R C NR C NH 2R'4-iNH 2ONHOCH 3O PhI(OAc)23JOC, 1993, 58, 2478.RCO NH 2RCO NIPh OAcRN C OR NH COOCH 3CH 3OHPhI, OAcRPhI(OAc)4-i.2C NH 2RCH 2PhI(OAc)2 // KOH / CH 3OHC(OR)2C(SR)2h C-NH 2C-NO 2C N C C 5-ag f d c b a 5-C=C-OR C=C-SR C-OH C=N-OH C=N-H C=S C=O C=Ov. via: epoxysilaneRCO CRRCO CH 2R42SOCl H 3O +23OO2-CrO 4OONaBH 3H 3O +3ZnTsNHNH 2MeLiTMSCl MCPBA LAH324CH 2CORRCH 2CORRMsClKOtBuHgCl 3SSCH 2CORRPhCHOi. via: α-CO 2Hii. via: α-haloketoneiii. via: aldol processiv. via: thioenol etherRCO CH 2Rdrawback: require simple structure, use many powerful agents: MeLi, LAH, MCPBAe i j C-Br k C-Hii. MCPBAi. hydrolysis5-b5-c C=N-OHC=N-Hi. RaNi ii. TiCl 3iii. KMnO 4 / Al 2O 3H 3O +5-dHg 2+ / H 2O JOC, 1972, 37, 2138.JOC, 1970, 35, 858.HgSO 4 / H 2O / H 2O5-c.15-c.2THL, 2001, 42, 4775.1. DIBAL / H 3O +5-eStenphen reductionmostly for.Syn, 1925, 3, 1874.2. HCl./ SnCl 2 / Et 2O 5-e.1R -CH 2-CN5-e.25-e.3-CH 2-C OHR -CH -C OH R'R -CH -C O R"R'R'X / n -BuLiCH 3I R''MgBr H 3O +3.H 3O +5-fH 3O +Hg 2+ / CH3CN (aq)C=C-OR C=C-SROCH 3OH 3O+SCH 3OHg 2+3OH 3O Hg 2+3H 3O+OO O SSS SSRSR O O OR OR 5-gHg 2+ / H 3O +H 3O + / solv (aq)H 3O + / solv (aq)Hg 2+ / H 3O +OR OROH OHH 3O + / solv (aq)a very common protecting group, deprotect back to ketoneHC OEt OEt OEtRMgX / H 3O +HC OEt OEt OEtRMgXRCHON H+2Cr 2O 7-2N HCl.CrO 3Ag 2O:1. a mild oxidizing agent2. must be freshly prepared: NaOH into AgNO 3 (aq)3. may involve surface change, react with CO 2, lightSwern oxidation: (DMSO, oxalyl chloride, Et 3N)drawback: react at low T Collins reagent: (CrO 3 - 2 Py)1. drawback: use 6 equivalent, a messy reaction 2. must be very dry, fire easily; purify by CaH 23. an old oxidizing material, isolated by Collin.i. PCCii. PDCix. K 2R C OHO aldehyde1st alcohol2nd alcohol1st alcoholR C OHOR C ROR C HO 5-h i. PCC ii. PDCJOC, 1985, 50, 1332.N OCH 3OHPDC (pyridinium dichormate)(H 2Cr 2O 7 + 2 Py)PCC (pyridinium chlorochromate) (Py-HCl-CrO 3)most widely used use 1 - 1.2 eq.Pfitzner-Moffatt oxidationOO BrDMSOO OOH360 %Synth. Commun., 1986, 16, 1343.JOC, 1977, 42, 1991.Synthesis, 1981, 165.O I OOAcAcOpH 6: weak acid buffer, avoid interfere with ketal groupMcMurray reactioni. Corey approach: subtituted-quinone // H 3O +ii. Watt approacha. PhCHO // MCPBA // H 3O +b. ArPhO // MCPBA // H 3O +c. NBS // KOH // H 3O +PhOPh PhOPhNH 2Ph PhNH 2NC O H // H 3O +O O5-i.15-i.2i. Et 3N // H 3O +Nef reactionii. TiCl 3 / pH 1 or 6iii. SiO 2 / NaOH // H 3O +JACS, 1977, 99, 3861.iv. LDA / MoO 5-Py -v. NaOH // CH 3O OH 3O +vi. KMnO 4 / KOHChem. Rev. 1955, 55, 137.5-k IOOOH O(3 eq.)JACS, 2001, 123, 3183.CH 3CHO2. DDQ / TFA.Synthesis, 1979, 537.JCS, 1932, 1875.Ph-F / DMSO 3.1. SeO 2a select oxidantindrect: change to RC-OH followed by oxidation direct:1. DMAPO / DBU / CH 3CN i. DMSO / AgBF 4RBr DMSO / AgBF 4- Me 2SBull Soc. Jpn., 1981, 54, 2221.THL, 1974, 917.2. NaIO 4 / DMFO Br84 %oNaIO 4 / DMF a new method 3. DMSO reagents:ii. DMSO / ZnSRCHBrMeRC(O)MeDMSO BrOH OOH JACS, 2003, 68, 2480.ROAgBFTHL, 1975, 4467.C C R CHOHRR C C HC C R R'R C C HR C C ArR C C HC C R PhR C C Hsteric base, prevent Nu attack n -BuLi: not MeLi, or t -BuLi, fire easily RX: R-Br, R-TOS, RCHO, RC(O)Rn -BuLi / R'CHO // Ac 2O // KO t BuClOMeN Liiii.ii. (Ph 3P)2PdCl 2, CuI, Et 2NH / PhIi. n-BuLi / RX6-b6-a b c d e g 6-aC CC CC Csulfonic acid: PhSO 3H; sulfinic acid: PhSO 2H; sulfenic acid: PhSOHiv. CuI, NaI, Na 2CO 3, RC C CH 2ClR C C HCl CH 2CC R'RCCCH 2CCR'Synthesis, 2000, 691.RCH 2-SO 2Ph RC CHh f iRCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXin fact: convert to C=C firstlyii. protect - deprotecti. move to terminal 6-cNH 2NHKuse: KAPAuse: Co (CO)8 // Fe(NO 3)3, EtOHJACS, 1975, 97, 891.6-duse: i. Br 2 / CCl 4 // KO t Bu6-euse: Pb(OAc)4, LiCl // KO t Bu // Br 2/CCl 4 // KO t Fe(NO 3)3: weak oxidizing agentii. Br 2 // KOHJA CS, 1941, 63, 1180.PhPhPhPh6-fi. NaBH 4, H 3O +, Br 2, KOtBuii. NH 2OH, NaNO 2 / H 2SO 4 // Ac 2O / DMAPiii. LDA, ClPO(OEt)2ON NODMAP:4-N,N-D i m ethyl a minop y ridinemixture ofAc 2O / DMAP:N NC CH 3O6-guse: TsNHNH 2 / EtOH, heatTHL, 1967, 3943.OHO3(l)O(MVK)CH 3CH CH 2Robinson Annulation German invention, as acylating agentLDA: Li N(iPr)2, ignored a long time, re-introduced by Michigan State U. became famous, appeared every weekHORLiNH 2 / NH 3 (l)use: LiNH 2 / NH 3 (l) / R-XO Cl6-h6-i.JA CS, 1958, 80, 4599.JA CS, 1955, 77, 3293.Me PhHOSO 2CF 3Me C C Phvia:MeCPhJOC, 1978, 43, 364.ArAr'HBr Ar C C Ar'NaOEtvia:Ar Ar'Bri. NaOEt (when X = Br)ii. BuLi (when X = -OSO 2CF 3)?heatRCH=CH 2:PBu RCH 2CH 2-O-PBu 3RCH 2CH 2-OHPh-Se-PBu 3Ph-Se-CNmechanism:MCPBA OAc CO 2MeOAcMeO MeO 2CNO 2SeNOAcCO 2MeOAc MeOMeO 2Cminorapplied for reactions: without rearrangement;no regiosiomerCC (CO 2H)2 / benzeneOH PhPhOO Cl ClClClOCl Cl NC NCO iii. Pd-C; or Ni; Pt, Rhii. chloronaili. DDQ use base: DBNi. CH 3I / Ag 2ii. HCHO / HCO 222use: heatuse: heatb 7-i. p-TSOH.H 2O or CSA ii. weak acid: HOAc; HCO 2H; H 2C 2O 4use:C C HIC C H NH 2C C H OC(S)SMe C C H OAc C C H OMs C C H OH a7-i h gCCX C C C CC CHC O C Cf e dc b a 7--C(O)-CH 3CH-CH CH-CX C-OHjCX-CYNaI / Zn (Cu)i. Zn / acetonei. CSCl 2/C COMs OMs C C BrBrCCOH OHc7-CCOH Iii. CSCl 2 / P(OMe)3P NNPhPOCl 3 / py // Snvia:C C IIapplication: i. protect alkene: via Br 2 // ZnCCCCC 36 o C CCCC=C 31 o C CCCC C Cl Cl148 o CS OR ORC C BrOAcZn / HOAcOAcO AcOAcOBrOAcZn OOAc OAcOAcJOC, 1978, 43, 364.HOAc, 1998, 2113.ii. In / MeOH ii. purify compoundd7-e7-i. WCl 6 / RLi ii. LiPPh 2 / CH 3I product retention product inversionNa R C HC HCH 2CH 2CH 2OH OClRiii. Na(special structure):7-d.7-d.S R 1R 2R 1R 2(EtO)3Puse: (EtO)3PSynthesis , 1977, 1134.via : betaine, oxaphosphetane (NMR)Onot good for Ph 3P=CH 2function as base:expensivedifficult to prepareOEtCNPPh 3CNPPh 3H OPPh 3O CO 2Me+notPh 3P CH EtH C OCO 2Me notPh 3P CH CO 2MeEtH O++++stable ylid gives trans (E)unstable ylid gives cis (Z)water soluble, removed by extraction(comparison: O=PPh 3 highly soluble in organic solvent)use:LiPh SON MeCH 2// Al (Hg)Me 3SiCHR -Li +Ph 3SiCH 2-Li + === Ph 3SiCH 2Br + n-BuLi (exchange)Me 3SiC -H-MgBr === Me 3SiCH 2Cl + Mg (metal reduction)Ph 3SiC -HCH 2Ph === Ph 3SiCH=CH 2 + PhLi (addition to vinylsilane)Me 3SiC -HCO 2Et === Me 3SiCH 2CO 2Et + Li (metalation)Me 3SiCH=PPh 3 === Me 3SiCH 2PPh 3+ X - + KHRO = MeO-, EtO-use: (RO)2PO-CHR'use: Ph 3P-CHR'vi. Sulfoximide (Johnson C.)iii. Silyl Wittig Reaction (Peterson Reaction)ii. Phosphonate Wittig Reaction (Horner-Emmons Modification)i. Wittig Reaction 7-f7-f.Synthesis, 1984, 384.THL, 1981, 2751.JOC, 1968, 33, 780.iv. CH 2(ZnI)2Chem. Lett, 1995, 259.Synlett, 1988, 12, 1369.2CH 2(ZnI)2v. CH 2CHBr 2, Sm, SnI 2 / CrCl 3, THFRO Rvii. Grignard reagent:1. TMSCH MgCluse: TMSCH 2MgClTHL, 1973, 3497.THL, 1988, 4339. 2. NaOAc, AcOHmethylenationOC RR'3H advantages over the Wittig:1. by-products are more easily removed,2. reaction suffers less from steric effects.via:(olefination reaction)1953 discover7-f.2not for Wittig, ylid unstableJOC, 1978, 43, 3253.JACS, 1974, 96, 4706.Chem. Lett, 1973, 1041.TiCl 3-LiAlH 4 / THF TiCl 3 / Mg TiCl 4 / Zn TiCl 4 / K ii. McMurry Couplingi. use: N 2H 4 / H 2S / Pb(OAc)4BASF, 1973, 2147.via:Zn-CuP(OEt)1. H 2S2. Pb(OAc)431. H 2S2. Pb(OAc)4OON SN N N OSN NSON ON NNSN NON NO OSO ON NOO OO OTiCl 3N 2H 4g7-form trans alkene:form cis alkene:i. Li / NH 3; or other IA metals ii. Li / EtNH 2iii. LiAlH 4 / THFi. H 2 / Ni 2B (P-2 catalyst)ii. H 2 / Pd-CaCO 3 (Lindlar catalyst)iii. H 2 / Pd-BaSO 4iv. B 2H 6 / HOAc (Diborane)v. N 2H 2vi. HCHO / Pd-C / Et 3Nnot use H 2 / Pt: might convert to alkaneh7-all form trans alkene:i. R 2BH / Br-CN (hydroboration)C CHR HHii. DIBAL / n-BuLi / CH 3I (hydroalumination)iii. Cp 2ZrClH / RX (hydrozirconation)application: protecting groupvia dihalidevia halohydrinvia epoxidevia diene-olefin additionC=C C CX XC CH XC=CC COC=CC=CC=C C=CC=CC CC Cnot for double bond might moveMnO2 / Ph3P CH3 Br- / MTBDNNNCH3MTBD via diradicalJA CS, 1998, 100, 877.Ph Ph7-i7-j8-a 8-a.28-a.38-a.41. HI 3. TsCl / C 62. PI 3JCS, 1905, 87, 1592.CH OH CH I PI 38-a.12. F 3S-NEt 21.(DAST)SN SF O OOO Chem. Rev., 1996, 96, 1737.2FCH 3SO O OH ONCHCl 2CH 3 1. CF 3CHFCF 2NEt 22. HOAc / i PrOH$ 65 / 500 g $ 80 / 50 gPBr 3PI 3$ 35 / 1000 g PBr 3$ 500 / 125 gJOC, 1993, 58, 3800.8-C-XC-OH C(O)Z d c b a C-NH 2C=O 2. PPh 3 / I 2e C-H8-d8-d.RC O OH1. AgNO 3/KOH 2R Br Ber. 1942, 75, 296.8-d.2ClOClRhCl(PPh 3)38-b NaNO 2 / HCl / HBF 4 /Chem Rev., 1956, 56, 219.8-c CF 2Br 2 / ZnFF JCS.PT I, 1993, 335.8-e8-e.1I86 %I 2 / HNO 3JACS, 1917, 39, 437.8-e.3I / HNO PhCH 2C(O)CH 3PhCHC(O)CH 3FN SFO OF +Chem. Rev., 1996, 96, 1737.F-TEDA-BF 4, 1994, 149.F 2-N 2 / CFCl 3-CHCl 3JOC, 1988, 53, 2803.90 %adamantane1. regioselective fluorination at the more substituted positions2. electrophilic in natureF -N 33OO N XR 1R 3OOR 2H Mg(ClO 4)2R 1R 3O OR 2X NBX / Mg(ClO 4)2JOC, 2002, 67, 7429.8-e.2X = Cl, Br, IX = Cl, Br, INBXNBX:i.ii.iii.iv.RRFR = CH 3CO, COCF 3, CCl 3, NO 2 HF / electrolysis1.4-1.6 Valready industrilized NF 3O / TBAH / CH 3CNv.TBHA: TetrabutylammoniumhydroxideTHL, 2003, 44, 2799.9-a9-C-CH 3C-X a (CH 3)3AlMe 3Al98 %Organomet. Chem. Rev., 1996, 4, 47.CH 2Cl 2bridgehead methylation。
官能团互相转化

i 5-7-g f e d c b a e d c b a i h g f ed c ba h g f e d cb a h g f e dc b a 6-4-3-1-2-i h g f ed c b a C=C -C(O)-CH 3CH-CH CH-CX Functional Group InterconversionC=CC C C=CC C RCH 2-SO 2Ph RC CH CC C C C C CC C C=O C-C(O)Z C=C C=O C-OH C-X C-N C-H C-N C=O C---OH C-OC(O)R C-X C-OCH 2OR C-NH 2C-OR C-H C-OH C=C C-H C(O)OR C-(OR)2C-OH C-ORC-CHO C-CO 2H C-CN C=C C=O C-S C-X C-OH C-H C=C j C(O)XhC Nj kCC 8-C-Xi C-OHC-OH C(O)Z dc b a ed c b a f C-NH 2C-Hj CX-CYC CXC=O h g f iC CH RCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXjC O C-NH 2C-CN9-C-CH 3C-Xa e C=O1-atriflmesytosyS O O O RCH 2CF 3S OO O RCH 2CH 3CH 3CH 3CH 3OH (2). for 3' alcohol:LiAlH 4(1). for 1', 2' alcohol:1-i h g f e dc b a C-CHO C-CO 2HC-CN C=C C=O C-SC-NH 2C-X C-OH C-H CH 3CH3CH 3H n -Bu 3SnH C S O PhClRCH 2-HCH 3SOO O RCH 2CH 3S OOCl RCH 2OHjC(O)XPh 2SiHCl / InCl 3PhPhPhPhJOC, 2001, 66, 7741.ii. Ph 2SiHCl / InCl 3i. p -TsCl // LiAlH 4i. ClC(S)OPh // n -Bu 3SnH Cl 22via:a unique L accelerateInCl 3indium tri ii. Et 3SiH / Lewis acidJ. Org. Chem. 2000, 65, 6179JOC, 2000, 65, 6179.2rt, 3 hr1-bBu 3SnH: (l), easy to rem Ph 3SnH: (s), hard to rem Me 3SnH: too volatile, tounstable in acid, form H 2 gasNaBH 3CN: stable at pH 5-6hygroscopic, dried self, suggest: buy sma(Grignard reageH 2OMg / Et O JOC, 1969, 34, 3923.HBrNa / NH 3; Li / NH 3; Na / EtOH Zn; Fe; Sn; Mg(3). metal reduction(2). hydride reduction(1). free radical reductionJACS, 1972, 94, 8905.n -Bu 3SnH HBrNaBH 4 / InCl 3 / CH 3CNradical reagentn -Bu 3SnH / AlBN JA CS, 2002, 124, 906.i iii NaBH 3CNi LiAlH 4i ii ii NaBH 4ii THL, 1969, 3095.JOC, 1976, 41, 3064.iv LiBHEt 3 (super hydride)JCS Perkin Trans I, 1973,654.(3). L iAlH 4 / CuCl 2NaBH 4 / NiCl 2NaBHEt 3 / FeCl 2 (or CoCl 2, VCl 3)(2). Li / NH 3(1). Raney Ni BuLi1-d1-cRCH 2-HRCH 2NH 2radical mechanismChemistry:R-SH R-S-R R 2SO R 2SOR-SS-Rremove: Hg +; Ni(1).(2).Ar-H2H PO Ar-NH 2RCH 2NH 2RCH 2NMe 3R=CH 2R-CH 3(4).X-RCH 2NMe 3OH -p-TsClp-TsCl2(3).Ar-NH 2Ar-Hbasicneutral acidic1-e(2). thioketal:(3). Wolff-Kishner reduction:(5). Tosylhydrazone reduction (Shapiro reaction):(modified Wolff-Kishner reduction):)(6). enol derivatives: SHSH / BF 3, CH 2Cl 2 // RaNiN 2H 4, OH -, heatTsNHNH 2Tf 2O /N// H 2 / PtO 2preparation: HgCl 2 into Znsimilar: Sn / HCl(4). Pd-C / HCO 2NH 4: mild, efficient(7). Et 3SiH / CF 3COOHPhONO 21-fb.p. ~ 230 Chighly toxic, cancer suspected agent?= HMPT: h exa m ethyl p hosphoric t riamide (Me2N)3P=O(3). organic electrochemistryβ(1). particular structure:1-g(1). K / Al2O3K / HMPA(2). Na / NH31-h(2). normal structure: SOCl2JOC, 1980, 45, 3227HMPA: h exa m ethyl p hosphor a mide (Me2N)3P=Oyes for white mouse, uncertain for humanmodified to: N NO1-i(1). RhCl(PPh 3)3 (Wilkinson's cat)(2). Rh(DPPD)2+ Cl -DPPD = Ph 2P-CH 2CH 2-PPh 21-jHSiEt 3 / B(C 6F 5)3activator / hydride sourceRCH 2 OR OO RR OROR RCH 2 OCH 2CH2OH(3). AlCl 3 / LiAlH 4(2). HCl / NaBH 3(CN)(1). h / HSiCl 32-bN NH/ TBDMS-ClTBDPS-ClEt 3N / TMS-Clacid: H 2SO 4H 3PO 4BF 3-Et 2ORC-OCH 2CH=CH2RC-OCPh 3 = RC -OTr RC-O t BuRC-OCH 3RC-OSiR 3RC-OCH 2Ph = RC-OBZl = RC-OBni. Willianson s ii. stability of s iii. abbrev.: TBSilyl group:i. S N ii. abbreviation iii. advantage: Willianson st -Butyl group:i. abbreviati ii. deprotectiPhCH 2-ClPhCH 2-Br: reactivity goodPhCH 2-I: reactivity better than PhCH 2Br, generated in situ, PhCH 2Br + NaIPhCH 2-X: Benzyl- group:i. Williamson ii. not a goodMe group:CH 3-X: CH 3I; CH 3OSO 2R; (CH 3)3O + BF 4-, (CH 3)2SO 4base: NaH, n -BuLi, Ag 2O(4). t -Bu: acid cat /(3). allyl: base /Br (6). silyl: Et 3N / R 3SiCl(5). trityl: py // Ph 3C-Br(2). PhCH 2-: base / PhCH 2-X e d cb a 2-RC=C RC-H RC(O)ORRC-(OR)2RC-OH RC-OR (1). Me: base / CH 3-X2-a (7). acetal / ketal: (see 3e)fRC-CNgenerate H 2, or butane gasJOC, 1988, 53, 2985.trimethyloxonium tetrafluoroborateJCS, 1930, 2166.(8). ArF / CsFROHradical mechanism: SiCl 3t-BuORaNi with C=S2-c2-d (1). hv / HSiCl 3(2). HCl / tBu-OO-t Bu(4). BF 3 / NaBH 42-e2-e.vi. H 2O 2, t -BuOH, MnSO 4 // NaHCO 3, pH 8HO 2new, cheap,, simple, green chemistryOOHOOBr 2Br 2 / ROH2-f ROH / HClEtCNEt C OEt OEtOEtHClJA CS, 1942, 64, 1825.C-OH C-H C-OR C-NH 2C-X 3-a b c d3-a(1). [PhI(OAc)-O]2-Mn(TPP)(2). organic electrochemistry(3). X 2 / hv // OH -3-a.13-a.23-a.3(1) Me 3SiCl // MPCBA//H 3O +(2). O 2, LDA, (EtO)3PJA CS, 1975, 97, 6909.i h g f e C=O C---OH C-OC(O)RC-OCH 2OR C=Cj C O(1). Me: application: deprotecting (2). PhCH 2-(5). trityl:(6). silyl: (3). allyl: (4). t-Bu: RC-OCH 2Ph = RC-OBZl = RC-OBnRC-OSiR 3RC-OCH 3RC-OtBuRC-OCPh 3 = RC-OTr RC-OCH 2CH=CH2Ni. TMSIii. BF 3-Et 2O // R-SH (or HS-CH 2CH 2-SH)iii. BBr 3 / CH 2Cl 2, 0-10 Cvi.i. H 2 / Pd-C ii.CN CN Cl ClO, OH -O CH 2OCH 3RhCl(PPh 3)3, H 3O +Oi. TFA (CF 3CO 2H)ii. HBr / HOAc iii. TMS-Ii. HOAc: weak acid: good leaving groupii. H 2need stronger acidi. F - : HF, Py-H + F -; n +--SiMe 3-SiBuMe 2-SiBuPh 2if HOBr: OK for TMDMSJOC, 1987, 52, 4973.JOC, 1973, 38, 3224.iv. AlCl 3 / RSH THL, 2001, 42, 9207.v./ heatCl -N H3-b triphenylmethylorganic base: TMG3-c(1). OH -(2). KO 2 / DMSO 3-d not practically useful: R-OH cheaper than R-XJOC, 1975,40, 1678.(2). Na 2[Fe(CN)5(NO)] / K 2CO 3 / H 2O3-e(1). Symmetry:ketal: use H 3O +acetal: use H 3O +(2). unsymetry:RO-MOM RO-MEM RO-MTM RO-THPi. H 3O +p -TsOH / MeOHi. H 3O +; ii. ZnBr 2 / CH 2Cl 2HgCl 2 / CH 3CN (aq.)actually, acetal exchange (3). Ag 2O / H 2OTHL, 1975, 3183.JOC, 1986, 51, 3913RO 2C (CH 2)3CHRNH 2RO 2C (CH 2)3CHROHNa 2[Fe(CN)5(NO)]2323-f(1). base: KHCO 3 (or K 2CO 3, NH 3) / MeOH; NaOH (1 %, or 0.5 N)(3). RED: (2). acid: H 3O +PPh 3 / DEAD / RCO 2H // OH -3-gMitsunobu inversionSynthesis, 1981, 1.JOC, 1987, 52, 4235.common esters:formate = HCO 2R ------------------------ KHCO 3 (ortrifluoroacetate = CF 3CO 2R ------------ KHCO 3 (oracetate = CH 3CO 2R = ROAc --------- KHCO 3 (or Kbenzoate = PhCO 2R = ROBz -------- NaOH (1 %)pivalate = t Bu-CO 2R = ROPv ------ NaOH (0.5 N*HOi LiAlH 4ii. NaAlH 2(OCH 2CH 2OCH 3)CH 3O 2CCO 2CH 3HOOHNaAlH 2(OCH 2CH 2OCH 3)2C 6H 6, r.t.hydride:electron:Na / NH 3AGIEE, 2002, 41, 3028.JACS, 1972, 94, 7159.LAH ------------ almost all: ald, ketone, acie, ester, acyl X, anhydride NaBH 4 --------------- not for acid, ester (but LiBH 4 work for ester)B 2H 6 --------------- not for ester, acyl X, anhydride; from top:LiAlH 4; NaBH 4; Na / NH 3Al (O i Pr)3 / i PrOH ----------- Meerwein-Pondorf-Verley rxn IrCl 4 / i PrOH / P(OMe)3 ------ Henbest rxn LiBH(sec Bu)3 ------------------ H. C. Brownfrom bottom:(2). stereoselective:(1). regioselective:3-h (3). HCHO reagent:Me CHO MeOHHCHOKOHJACS, 1935, 511, 903.CH 3CHOC(CH 2OH)42Org.Syn, 1925, 4, 53.HCHO / KOHHCHO / Ca(OH)2Synthesis, 1994, 1007.PhNO 2OPhBH / SMe OBH 3 / THF solvent: THF, SMe 23-iR 3B, HOCH 2CH 2OH // H 2O 2 // NaOHJOC, 1986, 51, 4925.CORB RR 3BCRRCRR R 3CB OOH 2O 2R 3B O O OHOB R 3Cpractice3-k OOHOHOHOHOOHOH OHOHOHJOC, 1967, 32, 3452.H 2Hg ((3). B 2H 6, H 2O 2 / OH -, H 2O(2). Hg(OAc)2, H 2O // NaBH 4(1). H 3O +3-j3-j.13-j.2hydration:(1). KMnO 4 / NaOH (2). OsO 4(3). H 2O 2/HCO 2H (4). Na / EtOHcistran cis +trancis3Me 2NNN CH 3HClh νN CH 3HH +NCSN CH 3Cl NHCH21. LAH R 3C NH 2R C NR 2R C NHR R 3C OHR 2C OH RC OH R C NH 2tertiarysecondary primary Compare nomenclature class:not a very useful reactionC-N C-H C-N C-X C-OH C=OC=C 4-a b c d e f g 4-aSO 2NH 2Ph I OAcOAcS OON I Ph Fe (TPP)ClS OONH 2TPPN N2. NaN 3N C O1. SO 2Cl 2C O 2h i C N C (O)X C -C(O)XNH 22RC NO 2RC NH 2iii4-b CF 3CO 3H // Fe / HOAc1. many reducing agents4-b.14-b.21.2.3.4.Fe 3(CO)12 / CH 3OH JOC, 1972, 37, 930.NaBH 4 / Pd-C Na 2S Sn / HCl Vogel's 12.57Vogel's 12.58Vogel's 12.595.H 2 / Pt (S)-Csulfided platium not affect: aromatic rings, ketones, halides, nitrileJACS, 1933, 55, 4579.2HCHO NMe 2CO 2EtNH 2CO 2EtRC NCC NR C CRC NH 2iC N R NN+-C NRR'ii1. HCHO / HCO 2H 1. RBCl 2 / base1. HC(OEt)3 // NaBH 4;2. R 2CO // NaBH 3CN NH 2N CH 3CH 3HCHO N 3NHBCl 2NH 2COOHN COOHHCH 3NaBH 4b.3 2. HCHO // H 2 / Pd-CN 3NO 2MeO 2CNaBH 422rt NH 2NO 2MeO 2Cmild conditionhigh yieldnot affect:: NO2. NaBH 4 / CoCl 2-6H 2Onot good, usually2. Delepine3. NaN 3/ RED4-d4-c 5. Unpolung4. NaN 3 / RED3. Delepine2. Gabriel:1. NH 3N OO K N 2H 42Oi. LAH, NaBH 4ii. H 2 / catiii Zn / HCl; Al (Hg)i. Mg // NH 2Clii. Mg // PhSCH 2-N 3commercial available, tetramer of Me 3N24. CBr 4, PPh 3, NaN 3, DMF // PPh 3 / THFJOC, 2000, 65, 7110.urm heB 2H 6 / H 2NOSO 3HB 2H 6 / H 2NO CH 3CN / H 3O +B 2H 6 / NH 2Cl C-C-NHCOCH 3C=CC-C-NH 24-freductive amination!Leuckart reactionmost generalvia: hydrazone4. PhNHNH 2 // Al (Hg)2. Me 3SiN 3 // LiAlH 43. NH 3 (excess) // RaNi / H 21. RNH 2 // NaBH 3CN5. NH 4+HCO 2-4-e6. RNH 2 / n -Bu 2SnClH / HMPASynthesis, 2000, 789.5. P 4S 10// RaNi4. Et 3O + BF 4- // NaBH 43. B 2H 62. NaBH 3(OCOR)1. LiAlH 46. Lawesson's reagent // RaNi4-h4-g4-g.a 4-g.b R C NH 2R C NH 2R'formform AlH 3 / THF BrC NBr NH 2JOC, 2000AlH 3TH, 1989, 30, 5137.JOC, 1987, 52, 3901.R'Li // NaBH 4R'MgX // NaBH 4R'MgX // Li/NH 3(l)R'2CuLi // NaBH 4TH, 1989, 30, 5139.JOC, 1993, 58, 4313.R C N4-iNH 2ONHOCH 3O PhI(OAc)23JOC, 199RCO NH 2RCO NIPh OAcPhI, OARCPhI(OAc)4-i.2C NH 2RCH 2PhI(OAc)2 // KOH / CH 3OHC(OR)2C(SR)2hC-NH2C-NO2C NC C5-agfdcba5-C=C-ORC=C-SRC-OHC=N-OHC=N-HC=SC=OC=Ov. via: epoxysilaneR COCRR COCH2R42SOCl H3O+23OO2-HBr CrO4OOKOHNaBHCeCl3H3O+3ZnZnTsNHNH2MeLi TMSCl MCPBA LAH324CH2CORRCH2CORR3S SCH2CORRPhCHOi. via:α-CO2Hii. via: α-haloketoneiii. via: aldol processiv. via: thioenol etherR COCH2Rdrawback: require simple structure, use many powerful agents: MeLi, LAH, MCPBAeij C-Brk C-Hii. MCPBAi. hydrolysis5-b5-c C=N-OHC=N-Hi. RaNi ii. TiCl 3iii. KMnO 4 / Al 2O 3H 3O +5-dHg 2+ / H 2O JOC,JOC,HgSO 4 / H 2O / H 2O5-c.15-c.2THL, 2001, 42, 4775.1. DIBAL / H 3O +5-eStenphen reductionmostly for2. HCl./ SnCl 2 / Et 2O 5-e.1R -CH 2-C N5-e.25-e.3-CH 2-C OHR -CH -C OH R -CH -C O R"R'R'X / n -BuLiCH 3I R''MgBr H 3O +3.H 3O +5-fH 3O +Hg 2+ / CH3CN (aq)C=C-OR C=C-SROCH 3OH 3O+SCH 3OHg 2+3OH 3O Hg 2+3H 3O+O OO SSS SSR SR OO OR OR 5-gHg 2+ / H 3O +H 3O + / solv (aq)H 3O + / solv (aq)Hg 2+ / H 3O +OR OROH OHH 3O + / solv (aq)a very common protecting group, deprotect back to ketoneHC OEt OEt OEtRMgX / H 3O +HC OEt OEt OEtRMgXRCHON H+2Cr 2O 7-2N HCl.CrO 3Ag 2O:1. a mild oxidizing agent2. must be freshly prepared: NaOH into AgNO 3 (aq)3. may involve surface change, react with CO 2, lightSwern drawb Collins reagent: (CrO 3 - 2 Py)1. drawback: use 6 equivalent, a messy reaction 2. must be very dry, fire easily; purify by CaH 23. an old oxidizing material, isolated by Collin.i. PCCii. PDCix. K 2R C OHO aldehyde1st alcohol2nd alcohol1st alcoholR C OHOR C ROR C HO 5-h i. PCC ii. PDCN OCH 3OHPDC (pyridinium dichormate)(H 2Cr 2O 7 + 2 Py)PCC (pyridinium chlorochromate) (Py-HCl-CrO 3)most widely used use 1 - 1.2 eq.PfitzOO BrDMSOO OOH360 %Synth. Commun., 1986, 16, 1343.JOC, 1977, 42, 1991.O I OOAcAcOpH 6: weak acid buffer, aMcMurray reactioni. Corey approach: subtituted-quinone // H 3O +ii. Watt approacha. PhCHO // MCPBA // H 3O +b. ArPhO // MCPBA // H 3O +c. NBS // KOH // H 3O +5-iPhOPh PhOPhNH 2Ph PhNH 2NC O H // H 3O +OO5-i.15-i.2i. Et 3N // H 3O +Nef reactionii. TiCl 3 / pH 1 or 6iii. SiO 2 / NaOH // H 3O +JACS, 1977, 99, 3861.iv. LDA / MoO 5-Py -v. NaOH // CH 3O OH 3O +vi. KMnO 4 / KOHChem. Rev. 1955, 55, 137.5-j5-k IOOOH O(3 eq.)JACS, 2001, 123, 3183.CH 3CHO2. DDQ / TFA.Synthesis, 1979, 537.JCS, 1932, 1875.Ph-F / DMSO 3.1. SeO2a select oxidantindrect: change to RC-OH followed by oxidation direct:1. DMAPO / DBU / CH 3CN i. DMSO / AgBF 4RBrBull Soc. Jpn., 1981, 54, 2221.THL, 1974, 917.2. NaIO 4 / DMFO Br84 %oNaIO 4 / DMF a new method 3. DMSO reagents:ii. DMSO / ZnSRCHBrMeRC(O)MeDMSO BrOH OOH JACS, 2003, 68, 2480.AgBFTHL, 1975, 4467.C C R CHOHRR C C HC C R R'R C C HR C C ArR C C HC C RPhR C C Hsteric base, prevent Nu attackn -BuLi: not MeLi, or t -BuLi, fire easily RX: R-Br, R-TOS, RCHO, RC(O)Rn -BuLi / R'CHO // Ac 2O // KO t BuClOMeN Liiii.ii. (Ph 3P)2PdCl 2, CuI, Et 2NH / PhIi. n-BuLi / RX6-b6-a b c d e g 6-aC CC CC Csulfonic aciiv. CuI, NaI, Na 2CO 3, RC C CH 2ClR C C HCl CH 2CC R'RCCCH 2CRCH 2-SO 2Ph RC CHh f iRCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXin fact: convert to C=C firstlyii. protect - deprotecti. move to terminal 6-cNH 2NHKuse: KAPAuse: Co (CO)8 // Fe(NO 3)3, EtOHJACS, 1975, 97, 891.6-duse: i. Br 2 / CCl 4 // KO t Bu6-euse: Pb(OAc)4, LiCl // KO t Bu // Br 2/CCl 4 // KO t Fe(NO 3)3: weak oxidizing agentii. Br 2 // KOHJA CS, 1941, 63, 1180.PhPhPhPh6-fi. NaBH 4, H 3O +, Br 2, KOtBuii. NH 2OH, NaNO 2 / H 2SO 4 // Ac 2O / DMAPiii. LDA, ClPO(OEt)2OO6-guse: TsNHNH 2 / EtOH, heatTHL, 1967, 3943.OO(MVK)CH 3CH CH 2Robinson Annulation LDA: L bHORLiNH 2 / NH 3 (l)RXuse: LiNH 2 / NH 3 (l) / R-XO Cl6-h6-i.JA CS, 1958, 80, 4599.JA CS, 1955, 77, 3293.Me PhHOSO 2CF 3Me C C Phvia:Me CPhJOC, 1978, 43,ArAr'H Br Ar C C Ar'NaOEti. NaOEt (when X = Br)ii. BuLi (when X = -OSO 2CF 3)MCPBA OAc CO 2MeOAcMeO MeO 2CNO 2SeNOAcCO 2MeOAc MeOMeO 2C(CO 2H)2 / benzeneOH PhOO Cl ClClClOCl Cl NC NCO iii. Pd-C; or Ni; Pt, Rhii. chloronaili. DDQ use base: DBNi. CH 3I / Ag 2ii. HCHO / HCO 222use: heatuse: heatb 7-i. p-TSOH.H 2O or CSA ii. weak acid: HOAc; HCO 2H; H 2C 2O 4use:C C HIC C H NH 2C C H OC(S)SMe C C H OAc C C H OMs C C H OH a7-i h gCCX C C C CC CHC O C Cf e dc b a 7--C(O)-CH 3CH-CH CH-CX C-OHjCX-CYNaI / Zn (Cu)i. Zn / acetonei. CSCl 2/C COMs OMs C C BrBrCCOH OHc7-CCOH Iii. CSCl 2 / P(OMe)3P NNPhPOCl 3 / py // Snvia:C C IIapplication: i. protect alkene: via Br 2 // ZnCCCCC 36 o C CCCC=C 31 o C CCCC C Cl Cl148 o CC C BrOAcZn / HOAc, 1998, 2113.ii. In / MeOH ii. purify compoundd7-e7-i. WCl 6 / RLi ii. LiPPh 2 / CH 3I product retention product inversionNa R C HC HCH 2CH 2CH 2OH OClRiii. Na(special structure):7-d.7-d.S R 1R 2R 1R 2(EtO)3Puse: (EtO)3PSynthesis , 1977, 1134.via : betaine, oxaphosphetane (NMR)CNCO 2MeEtwater soluble, removed by extraction(comparison: O=PPh 3 highly soluble in organic solvent)use:LiPh SON MeCH 2// Al (Hg)Me 3SiCHR -Li +Ph 3SiCH 2-Li + === Ph 3SiCH 2Br + n-BuLi (exchange)Me 3SiC -H-MgBr === Me 3SiCH 2Ph 3SiC -HCH 2Ph === Ph 3SiCH=CH 2Me 3SiC -HCO 2Et === Me 3SiCH 2CO 2Et + Li (metalation)Me 3SiCH=PPh 3 === Me 3SiCH 2PPh 3+ X - + KHRO = MeO-, EtO-use: (RO)2PO-CHR'use: Ph 3P-CHR'vi. Sulfoximide (Johnson C.)iii. Silyl Wittig Reaction (Peterson Reaction)ii. Phosphonate Wittig Reaction (Horner-Emmons Modification)i. Wittig Reaction 7-f7-f.Synthesis, 1984, 384.THL, 1981, 2751.JOC, 1968, 33, 780.iv. CH 2(ZnI)2Chem. Lett, 1995, 259.Synlett, 1988, 12, 1369.2CH 2(ZnI)2v. CH 2CHBr 2, Sm, SnI 2 / CrCl 3, THFRO Rvii. Grignard reagent:1. TMSCH MgCluse: TMSCH 2MgClTHL, 1973, 3497.THL, 1988, 4339. 2. NaOAc, AcOHRR'H via:(olefination reaction)1953 discover7-f.2not for Wittig, ylid unstableJOC, 1978, 43, 3253.JACS, 1974, 96, 4706.Chem. Lett, 1973, 1041.TiCl 3-LiAlH 4 / THF TiCl 3 / Mg TiCl 4 / Zn TiCl 4 / K ii. McMurry Couplingi. use: N 2H 4 / H 2S / Pb(OAc)4BASF, 1973, 2147.31. H 2S2. Pb(OAc)4N NO OSO ON NOO OO ON 2H 4g7-form trans alkene:form cis alkene:i. Li / NH 3; or other IA metals ii. Li / EtNH 2iii. LiAlH 4 / THFi. H 2 / Ni 2B (P-2 catalyst)ii. H 2 / Pd-CaCO 3 (Lindlar catalyst)iii. H 2 / Pd-BaSO 4iv. B 2H 6 / HOAc (Diborane)v. N 2H 2vi. HCHO / Pd-C / Et 3Nnot use H 2 / Pt: might convert to alkaneh7-all form trans alkene:i. R 2BH / Br-CN (hydroboration)C CHR HHii. DIBAL / n-BuLi / CH 3I (hydroalumination)iii. Cp 2ZrClH / RX (hydrozirconation)application: protecting groupvia dihalidevia halohydrin via epoxidevia diene-olefin addition C=CC C X XC C H X C=C CC O C=C C=CC=CC=C C=C C CC C not fordouble bond might moveMnO 2 / Ph 3P CH 3 Br - / MTBDvia diradicalJA CS, 1998, 100, 877.PhPh7-i7-j8-a 8-a.28-a.38-a.41. HI 3. TsCl / C 62. PI 3JCS, 1905, 87, 1592.CH OH CH I PI 38-a.12. F 3S-NEt 21.(DAST)SN SF O OOO Chem. Rev., 1996, 96, 1737.CSO O OH ONCHCl 2CH 3 1. CF 3CHFCF 2NEt 2$ 500 / 125 gJOC, 1993, 58, 3800.8-C-XC-OH C(O)Z d c b a C-NH 2C=O 2. PPh 3 / I 2e C-H8-d8-d.RC O OH1. AgNO 3/KOH 2R Br Ber. 1942,8-d.2ClOClRhCl(PPh 3)38-b NaNO 2 / HCl / HBF 4 /Chem Rev., 1956, 56, 219.8-c CF 2Br 2 / ZnFF JCS.PT I, 1993, 335.8-e8-e.1I86 %I 2 / HNO 3JACS, 1917, 39, 437.8-e.3I / HNO PhCH 2C(O)CH 3PhCHC(O)CH 3FN SF OOF +Chem. Rev., 1996, 96, 1737.F-TEDA-BF 4, 1994, 149.F 2-N 2 / CFCl 3-CHCl 3JOC, 1988, 53, 2803.90 %adamantane1. regioselective2. electrophilic inF -N CFCl 3-CHCl 3OO N XR 1R 3OOR 2H Mg(ClO 4)2R 1R 3O OR 2X NBX / Mg(ClO 4)2JOC, 2002, 67, 7429.8-e.2X = Cl, Br, IX = Cl, Br, INBXNBX:i.ii.iii.iv.RRFR = CH 3CO, COCF 3, CCl 3, NO 2 HF / electrolysis1.4-1.6 V already industrilizedNF 3O / TBAH / CH 3CNv.TBHA: TetrabutylamTHL, 2003, 44, 279-a 9-C-CH 3C-X a (CH 3)3AlMe 3Al98 %Organomet. Chem.CH 2Cl 2bridgehead meth。
