拉帕替尼杂质结构式
舒尼替尼杂质汇总

专业<杂质对照品>解决方案,代理中检所/EP/BP/USP/LGC/TRC/DR/TLC/MC/SIGMA/BACHEM/STD等品牌。 1814+064+3157
项目报批 纯度高于98%
舒尼替尼杂质3 Sunitinib Impurity 3 356068-86-5
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
舒尼替尼杂质4 Sunitinib Impurity 4
N/A
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
舒尼替尼杂质5 Sunitinib Impurity 5
N/A
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
扬信医药代理各品种杂质对照品:舒更葡糖钠杂质,达托霉素杂质,依维莫司杂质,他克莫司杂质,阿奇霉素杂质,克拉维酸钾杂质, 红霉素杂质,克拉霉素杂质,林可霉素杂质,罗红霉素杂质,克林霉素杂质,恩曲他滨杂质,艾地那非杂质,瑞卢戈利杂质,艾氟康唑
舒尼替尼杂质列表集
中文名称
英文名称Biblioteka CAS舒尼替尼杂质1 Sunitinib Impurity 1 452105-33-8
规格
10mg 25mg 50mg 100mg 更大规格请咨询
用途
项目报批 纯度高于98%
结构式
舒尼替尼杂质2 Sunitinib Impurity 2 356068-97-8
10mg 25mg 50mg 100mg 更大规格请咨询
拉帕替尼说明书

HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to useTYKERB safely and effectively. See full prescribing information for TYKERB.TYKERB (lapatinib) tabletsInitial U.S. Approval: 2007WARNING: HEPATOTOXICITYSee full prescribing information for complete boxed warning.Hepatotoxicity has been observed in clinical trials and postmarketing experience. The hepatotoxicity may be severe and deaths have been reported. Causality of the deaths is uncertain. [See Warnings andPrecautions (5.2).]---------------------------RECENT MAJOR CHANGES--------------------Indications and Usage. (1) Month Year Dosage and Administration. (2) Month Year Contraindications. (4) Month Year----------------------------INDICATIONS AND USAGE---------------------TYKERB, a kinase inhibitor, is indicated in combination with: (1) •capecitabine, for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress HER2 and who have received prior therapyincluding an anthracycline, a taxane, and trastuzumab.•letrozole for the treatment of postmenopausal women with hormone receptor positive metastatic breast cancer that overexpresses the HER2 receptor for whom hormonal therapy is indicated.TYKERB in combination with an aromatase inhibitor has not been compared to a trastuzumab-containing chemotherapy regimen for the treatment of metastatic breast cancer.-----------------------DOSAGE AND ADMINISTRATION ----------------The recommended dosage of TYKERB for advanced or metastatic breastcancer is 1,250 mg (5 tablets) given orally once daily on Days 1-21 continuously in combination with capecitabine 2,000 mg/m2/day (administered orally in 2 doses approximately 12 hours apart) on Days 1-14 ina repeating 21 day cycle. (2.1)The recommended dose of TYKERB for hormone receptor positive, HER2 positive metastatic breast cancer is 1500 mg (6 tablets) given orally once daily continuously in combination with letrozole. When TYKERB is coadministered with letrozole, the recommended dose of letrozole is 2.5 mg once daily. (2.1)•TYKERB should be taken at least one hour before or one hour after a meal. However, capecitabine should be taken with food or within 30 minutes after food. (2.1)•TYKERB should be taken once daily. Do not divide daily doses of TYKERB.(2.1, 12.3)•Modify dose for cardiac and other toxicities, severe hepatic impairment, and CYP3A4 drug interactions. (2.2)FULL PRESCRIBING INFORMATION CONTENTS*:FULL PRESCRIBING INFORMATIONWARNING: HEPATOTOXICITY1 INDICATIONS AND USAGE2 DOSAGE AND ADMINISTRAT ONI2.1 Recommended Dosing2.2 Dose Modification Guidelines3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS5.1 Decreased Left Ventricular Ejection Fraction5.2 Hepatotoxicity5.3 Patients with Severe Hepatic Impairment5.4 Diarrhea5.5 Interstitial Lung Disease/Pneumonitis5.6 QT Prolongation5.7 Use in Pregnancy6 AD ERSE REACTIONSV6.1 Clinical Trials Experience6.2 Postmarketing Experience7 DRUG INTERACTIONS7.1 Effects of Lapatinib on Drug Metabolizing Enzymesand Drug Transport Systems7.2 Drugs that Inhibit or Induce Cytochrome P450 3A4Enzymes7.3 Drugs that Inhibit Drug Transport Systems ---------------------DOSAGE FORMS AND STRENGTHS --------------250 mg tablets (3)-------------------------------CONTRAINDICATIONS------------------------Known severe hypersensitivity (e.g., anaphylaxis) to this product or any of its components. (4)-----------------------WARNINGS AND PRECAUTIONS ----------------•Decreases in left ventricular ejection fraction have been reported. Confirm normal LVEF before starting TYKERB and continue evaluations during treatment. (5.1)•Lapatinib has been associated with hepatotoxicity. Monitor liver function tests before initiation of treatment, every 4 to 6 weeks during treatment, and as clinically indicated. Discontinue and do not restart TYKERB if patients experience severe changes in liver function tests. (5.2)•Dose reduction in patients with severe hepatic impairment should be considered.(2.2, 5.3, 8.7)•Diarrhea, including severe diarrhea, has been reported during treatment. Manage with anti-diarrheal agents, and replace fluids and electrolytes if severe.(5.4)•Lapatinib has been associated with interstitial lung disease and pneumonitis. Discontinue TYKERB if patients experience severe pulmonary symptoms. (5.5) •Lapatinib may prolong the QT interval in some patients. Consider ECG and electrolyte monitoring. (5.6, 12.6)•Fetal harm can occur when administered to a pregnant woman. Women should be advised not to become pregnant when taking TYKERB. (5.7)------------------------------ADVERSE REACTIONS -----------------------The most common (>20%) adverse reactions during treatment with TYKERB plus capecitabine were diarrhea, palmar-plantar erythrodysesthesia, nausea, rash, vomiting, and fatigue. The most common (≥20%) adverse reactionsduring treatment with TYKERB plus letrozole were diarrhea, rash, nausea,and fatigue. (6.1)To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or/medwatch.-------------------------------DRUG INTERACTIONS------------------------•TYKERB is likely to increase exposure to concomitantly administered drugs which are metabolized by CYP3A4 or CYP2C8. (7.1)•Avoid strong CYP3A4 inhibitors. If unavoidable, consider dose reduction of TYKERB in patients coadministered a strong CYP3A4 inhibitor. (2.2, 7.2) •Avoid strong CYP3A4 inducers. If unavoidable, consider gradual dose increase of TYKERB in patients coadministered a strong CYP3A4 inducer. (2.2, 7.2) See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.Revised: Month Year8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.3 Nursing Mothers8.4 Pediatric Use8.5 Geriatric Use8.6 Renal Impairment8.7 Hepatic Impairment10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.3 Pharmacokinetics12.4 QT Prolongation13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility14 CLINICAL STUDIES14.1 HER2 Positive Metastatic Breast Cancer14.2 Hormone Receptor Positive, HER2 Positive MetastaticBreast Cancer16 HOW SUPPLIED/STORAGE AND HANDLING17 PATIENT COUNSELING INFORMATION17.1 Information for Patients17.2 FDA-Approved Patient Labeling*Sections or subsections omitted from the full prescribing information are not listed.______________________________________________________________________12 3 4 56 7 8 91011121314151617181920212223242526272829303132333435 FULL PRESCRIBING INFORMATIONWARNING: HEPATOTOXICITYHepatotoxicity has been observed in clinical trials and postmarketing experience. The hepatotoxicity may be severe and deaths have been reported. Causality of the deaths is uncertain. [See Warnings and Precautions (5.2).]1 INDICATIONS AND USAGETYKERB® is indicated in combination with:• capecitabine for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress HER2 and who have received prior therapy including an anthracycline, a taxane, and trastuzumab.• letrozole for the treatment of postmenopausal women with hormone receptor positive metastatic breast cancer that overexpresses the HER2 receptor for whom hormonal therapy is indicated.TYKERB in combination with an aromatase inhibitor has not been compared to a trastuzumab-containing chemotherapy regimen for the treatment of metastatic breast cancer.2 DOSAGE AND ADMINISTRATION2.1 RecommendedDosingHER2 Positive Metastatic Breast Cancer: The recommended dose of TYKERB is 1,250 mg given orally once daily on Days 1-21 continuously in combination with capecitabine 2,000 mg/m2/day (administered orally in 2 doses approximately 12 hours apart) on Days 1-14 in a repeating 21 day cycle. TYKERB should be taken at least one hour before or one hour after a meal. The dose of TYKERB should be once daily (5 tablets administered all at once); dividing the daily dose is not recommended [see Clinical Pharmacology (12.3)]. Capecitabine should be taken with food or within 30 minutes after food. If a day’s dose is missed, the patient should not double the dose the next day. Treatment should be continued until disease progression or unacceptable toxicity occurs.Hormone Receptor Positive, HER2 Positive Metastatic Breast Cancer: The recommended dose of TYKERB is 1,500 mg given orally once daily continuously in combination with letrozole. When coadministered with TYKERB, the recommended dose of letrozole is 2.5 mg once daily. TYKERB should be taken at least one hour before or one hour after a meal. The dose of TYKERB should be once daily (6 tablets administered all at once); dividing the daily dose is not recommended [see Clinical Pharmacology (12.3)].2.2 DoseModificationGuidelinesCardiac Events: TYKERB should be discontinued in patients with a decreased left ventricular ejection fraction (LVEF) that is Grade 2 or greater by National Cancer Institute36 Common Terminology Criteria for Adverse Events (NCI CTCAE) and in patients with an LVEF37 that drops below the institution’s lower limit of normal [see Warnings and Precautions (5.1) and38 Adverse Reactions (6.1)]. TYKERB in combination with capecitabine may be restarted at a39 reduced dose (1,000 mg/day) and in combination with letrozole may be restarted at a reduced40 dose of 1,250 mg/day after a minimum of 2 weeks if the LVEF recovers to normal and the41 patient is asymptomatic.42 Hepatic Impairment: Patients with severe hepatic impairment (Child-Pugh Class C)43 should have their dose of TYKERB reduced. A dose reduction from 1,250 mg/day to44 750 mg/day (HER2 positive metastatic breast cancer indication) or from 1,500 mg/day to45 1,000 mg/day (hormone receptor positive, HER2 positive breast cancer indication) in patients46 with severe hepatic impairment is predicted to adjust the area under the curve (AUC) to the47 normal range and should be considered. However, there are no clinical data with this dose48 adjustment in patients with severe hepatic impairment.49 Concomitant Strong CYP3A4 Inhibitors: The concomitant use of strong CYP3A450 inhibitors should be avoided (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir,51 indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole). Grapefruit52 may also increase plasma concentrations of lapatinib and should be avoided. If patients must be53 coadministered a strong CYP3A4 inhibitor, based on pharmacokinetic studies, a dose reduction54 to 500 mg/day of lapatinib is predicted to adjust the lapatinib AUC to the range observed without55 inhibitors and should be considered. However, there are no clinical data with this dose56 adjustment in patients receiving strong CYP3A4 inhibitors. If the strong inhibitor is57 discontinued, a washout period of approximately 1 week should be allowed before the lapatinib58 dose is adjusted upward to the indicated dose. [See Drug Interactions (7.2).]59 Concomitant Strong CYP3A4 Inducers: The concomitant use of strong CYP3A460 inducers should be avoided (e.g., dexamethasone, phenytoin, carbamazepine, rifampin, rifabutin,61 rifapentin, phenobarbital, St. John’s Wort). If patients must be coadministered a strong CYP3A462 inducer, based on pharmacokinetic studies, the dose of lapatinib should be titrated gradually63 from 1,250 mg/day up to 4,500 mg/day (HER2 positive metastatic breast cancer indication) or64 from 1,500 mg/day up to 5,500 mg/day (hormone receptor positive, HER2 positive breast cancer65 indication) based on tolerability. This dose of lapatinib is predicted to adjust the lapatinib AUC66 to the range observed without inducers and should be considered. However, there are no clinical67 data with this dose adjustment in patients receiving strong CYP3A4 inducers. If the strong68 inducer is discontinued the lapatinib dose should be reduced to the indicated dose. [See Drug69 Interactions (7.2).]70 Other Toxicities: Discontinuation or interruption of dosing with TYKERB may be71 considered when patients develop ≥Grade 2 NCI CTCAE toxicity and can be restarted at72 1,250 mg/day when the toxicity improves to Grade 1 or less. If the toxicity recurs, then73 TYKERB in combination with capecitabine should be restarted at a lower dose (1,000 mg/day)74 and in combination with letrozole should be restarted at a lower dose of 1,250 mg/day.75 See manufacturer’s prescribing information for the coadministered product dosage76 adjustment guidelines in the event of toxicity and other relevant safety information or77 contraindications.78 3 DOSAGE FORMS AND STRENGTHS79 250 mg tablets — oval, biconvex, orange, film-coated with GS XJG debossed on one80 side.81 4 CONTRAINDICATIONS82 TYKERB is contraindicated in patients with known severe hypersensitivity (e.g.,83 anaphylaxis) to this product or any of its components.84 5 WARNINGS AND PRECAUTIONS85 5.1 Decreased Left Ventricular Ejection Fraction86 TYKERB has been reported to decrease LVEF [see Adverse Reactions (6.1)]. In clinical87 trials, the majority (>57%) of LVEF decreases occurred within the first 12 weeks of treatment;88 however, data on long-term exposure are limited. Caution should be taken if TYKERB is to be89 administered to patients with conditions that could impair left ventricular function. LVEF should90 be evaluated in all patients prior to initiation of treatment with TYKERB to ensure that the91 patient has a baseline LVEF that is within the institution’s normal limits. LVEF should continue92 to be evaluated during treatment with TYKERB to ensure that LVEF does not decline below the93 institution’s normal limits [see Dosage and Administration (2.2)].94 5.2 Hepatotoxicity95 Hepatotoxicity (ALT or AST >3 times the upper limit of normal and total bilirubin96 >2 times the upper limit of normal) has been observed in clinical trials (<1% of patients) and97 postmarketing experience. The hepatotoxicity may be severe and deaths have been reported.98 Causality of the deaths is uncertain. The hepatotoxicity may occur days to several months after99 initiation of treatment. Liver function tests (transaminases, bilirubin, and alkaline phosphatase) 100 should be monitored before initiation of treatment, every 4 to 6 weeks during treatment, and as 101 clinically indicated. If changes in liver function are severe, therapy with TYKERB should be 102 discontinued and patients should not be retreated with TYKERB [see Adverse Reactions (6.1)]. 103 5.3 Patients with Severe Hepatic Impairment104 If TYKERB is to be administered to patients with severe pre-existing hepatic impairment, 105 dose reduction should be considered [see Dosage and Administration (2.2) and Use in Specific 106 Populations (8.7)]. In patients who develop severe hepatotoxicity while on therapy, TYKERB 107 should be discontinued and patients should not be retreated with TYKERB [see Warnings and 108 Precautions (5.2)].109 5.4 Diarrhea110 Diarrhea, including severe diarrhea, has been reported during treatment with TYKERB 111 [see Adverse Reactions (6.1)]. Proactive management of diarrhea with anti-diarrheal agents is 112 important. Severe cases of diarrhea may require administration of oral or intravenous electrolytes 113 and fluids, and interruption or discontinuation of therapy with TYKERB.Disease/PneumonitisLung114 5.5 Interstitial115 Lapatinib has been associated with interstitial lung disease and pneumonitis in116 monotherapy or in combination with other chemotherapies [see Adverse Reactions (6.1)].117 Patients should be monitored for pulmonary symptoms indicative of interstitial lung disease or 118 pneumonitis. TYKERB should be discontinued in patients who experience pulmonary symptoms 119 indicative of interstitial lung disease/pneumonitis which are ≥Grade 3 (NCI CTCAE).Prolongation120 5.6 QT121 QT prolongation was observed in an uncontrolled, open-label dose escalation study of 122 lapatinib in advanced cancer patients [see Clinical Pharmacology (12.4)]. Lapatinib should be 123 administered with caution to patients who have or may develop prolongation of QTc. These124 conditions include patients with hypokalemia or hypomagnesemia, with congenital long QT125 syndrome, patients taking anti-arrhythmic medicines or other medicinal products that lead to QT 126 prolongation, and cumulative high-dose anthracycline therapy. Hypokalemia or127 hypomagnesemia should be corrected prior to lapatinib administration.128 5.7 Use in Pregnancy129 TYKERB can cause fetal harm when administered to a pregnant woman. Based on130 findings in animals, TYKERB is expected to result in adverse reproductive effects. Lapatinib 131 administered to rats during organogenesis and through lactation led to death of offspring within 132 the first 4 days after birth [see Use in Specific Populations (8.1)].133 There are no adequate and well-controlled studies with TYKERB in pregnant women. 134 Women should be advised not to become pregnant when taking TYKERB. If this drug is used 135 during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be 136 apprised of the potential hazard to the fetus.REACTIONS137 6 ADVERSE138 6.1 Clinical Trials Experience139 Because clinical trials are conducted under widely varying conditions, adverse reaction 140 rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical 141 trials of another drug and may not reflect the rates observed in practice.142 HER2 Positive Metastatic Breast Cancer: The safety of TYKERB has been evaluated 143 in more than 12,000 patients in clinical trials. The efficacy and safety of TYKERB in144 combination with capecitabine in breast cancer was evaluated in 198 patients in a randomized, 145 Phase 3 trial. [See Clinical Studies (14.1).] Adverse reactions which occurred in at least 10% of 146 patients in either treatment arm and were higher in the combination arm are shown in Table 1. 147 The most common adverse reactions (>20%) during therapy with TYKERB plus148 capecitabine were gastrointestinal (diarrhea, nausea, and vomiting), dermatologic (palmar149 plantar erythrodysesthesia and rash), and fatigue. Diarrhea was the most common adverse150 reaction resulting in discontinuation of study medication.151 The most common Grade 3 and 4 adverse reactions (NCI CTCAE v3) were diarrhea and 152 palmar-plantar erythrodysesthesia. Selected laboratory abnormalities are shown in Table 2.153 154Table 1. Adverse Reactions Occurring in ≥10% of Patients TYKERB 1,250 mg/day +Capecitabine 2,000 mg/m 2/day(N = 198) Capecitabine2,500 mg/m 2/day (N = 191) All Grades a Grade 3 Grade 4 All Grades aGrade 3 Grade4Reactions % % % % % %Gastrointestinal disordersDiarrhea 65 13 1 40 10 0 Nausea 44 2 0 43 2 0 Vomiting 26 2 0 21 2 0 Stomatitis 14 0 0 11 <1 0 Dyspepsia 11 <1 0 3 0 0Skin and subcutaneous tissuedisorders Palmar-plantar erythrodysesthesia 53 12 0 51 14 0 Rash b 28 2 0 14 1 0 Dry skin 10 0 0 6 0 0General disorders andadministrative site conditions Mucosal inflammation 15 0 0 12 2 0Musculoskeletal and connectivetissue disorders Pain in extremity 12 1 0 7 <1 0 Back pain 11 1 0 6 <1 0Respiratory, thoracic, andmediastinal disordersDyspnea 12 3 0 8 2 0Psychiatric disordersInsomnia 10 <1 0 6 0 0 155 aNational Cancer Institute Common Terminology Criteria for Adverse Events, version 3. 156 bGrade 3 dermatitis acneiform was reported in <1% of patients in TYKERB plus capecitabine 157 group. 158159 Table 2. Selected Laboratory AbnormalitiesTYKERB 1,250 mg/day +Capecitabine 2,000 mg/m2/day Capecitabine 2,500 mg/m2/dayAll Grades a Grade 3 Grade 4 All Grades a Grade 3 Grade 4 Parameters % % % % % % HematologicHemoglobin 56 <1 0 53 1 0 Platelets 18 <1 0 17 <1 <1Neutrophils 22 3 <1 31 2 1HepaticBilirubin 45 4 0 30 3 0 TotalAST 49 2 <1 43 2 0ALT 37 2 0 33 1 0 160 a National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.161162 Hormone Receptor Positive, Metastatic Breast Cancer: In a randomized clinical163 trial of patients (N = 1,286) with hormone receptor positive, metastatic breast cancer, who had164 not received chemotherapy for their metastatic disease, patients received letrozole with or165 without TYKERB. In this trial, the safety profile of TYKERB was consistent with previously166 reported results from trials of TYKERB in the advanced or metastatic breast cancer population.167 Adverse reactions which occurred in at least 10% of patients in either treatment arm and were168 higher in the combination arm are shown in Table 3. Selected laboratory abnormalities are169 shown in Table 4.170171Table 3. Adverse Reactions Occurring in ≥10% of PatientsTYKERB 1,500 mg/day + Letrozole 2.5 mg/day (N = 654) Letrozole 2.5 mg/day(N = 624) All Grades a Grade 3 Grade 4 All Grades aGrade 3 Grade4Reactions % % % % % %Gastrointestinal disorders Diarrhea 64 9 <1 20 <1 0 Nausea 31 <1 0 21 <1 0 Vomiting 17 1 <1 11 <1 <1 Anorexia 11 <1 0 9 <1 0Skin and subcutaneous tissuedisorders Rash b 44 1 0 13 0 0 Dry skin 13 <1 0 4 0 0 Alopecia 13 <1 0 7 0 0 Pruritus 12 <1 0 9 <1 0 Nail Disorder 11 <1 0 <1 0 0General disorders andadministrative site conditions Fatigue 20 2 0 17 <1 0 Asthenia 12 <1 0 11 <1 0Nervous system disorders Headache 14 <1 0 13 <1 0Respiratory, thoracic, andmediastinal disorders Epistaxis 11 <1 0 2 <1 0 172 173 174 175 abNational Cancer Institute Common Terminology Criteria for Adverse Events, version 3. In addition to the rash reported under "Skin and subcutaneous tissue disorders", 3 additional subjects in each treatment arm had rash under "Infections and infestations"; none were Grade 3 or 4. 176177 Table 4. Selected Laboratory AbnormalitiesTYKERB 1,500 mg/day +Letrozole 2.5 mg/day Letrozole 2.5 mg/dayAll Grades a Grade 3 Grade 4 All Grades a Grade 3 Grade 4% % % % % % HepaticParametersAST 53 6 0 36 2 <1 ALT 46 5 <1 35 1 0Total Bilirubin 22 <1 <1 11 1 <1178 a National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.179180 Decreases in Left Ventricular Ejection Fraction: Due to potential cardiac toxicity181 with HER2 (ErbB2) inhibitors, LVEF was monitored in clinical trials at approximately 8-week182 intervals. LVEF decreases were defined as signs or symptoms of deterioration in left ventricular183 cardiac function that are ≥Grade 3 (NCI CTCAE), or a ≥20% decrease in left ventricular cardiac184 ejection fraction relative to baseline which is below the institution's lower limit of normal.185 Among 198 patients who received TYKERB/capecitabine combination treatment, 3 experienced186 Grade 2 and one had Grade 3 LVEF adverse reactions (NCI CTCAE v3). [See Warnings and187 Precautions (5.1).] Among 654 patients who received TYKERB/letrozole combination188 treatment, 26 patients experienced Grade 1 or 2 and 6 patients had Grade 3 or 4 LVEF adverse189 reactions.190 Hepatotoxicity: TYKERB has been associated with hepatotoxicity [see Boxed Warning191 and Warnings and Precautions (5.2)].192 Interstitial Lung Disease/Pneumonitis: TYKERB has been associated with interstitial193 lung disease and pneumonitis in monotherapy or in combination with other chemotherapies [see194 Warnings and Precautions (5.5)].Experience195 6.2 Postmarketing196 The following adverse reactions have been identified during post-approval use of197 TYKERB. Because these reactions are reported voluntarily from a population of uncertain size,198 it is not always possible to reliably estimate their frequency or establish a causal relationship to199 drug exposure.200 Immune System Disorders: Hypersensitivity reactions including anaphylaxis [see201 Contraindications (4)].202 Skin and Subcutaneous Tissue Disorders: Nail disorders including paronychia.INTERACTIONS203 7 DRUGofLapatinib on Drug Metabolizing Enzymes and Drug Transport204 7.1 Effects205 Systems206 Lapatinib inhibits CYP3A4 and CYP2C8 in vitro at clinically relevant concentrations.207 Caution should be exercised and dose reduction of the concomitant substrate drug should be208 considered when dosing lapatinib concurrently with medications with narrow therapeutic209 windows that are substrates of CYP3A4 or CYP2C8. Lapatinib did not significantly inhibit the210 following enzymes in human liver microsomes: CYP1A2, CYP2C9, CYP2C19, and CYP2D6 or 211 UGT enzymes in vitro, however, the clinical significance is unknown.212 Lapatinib inhibits human P-glycoprotein. If TYKERB is administered with drugs that are 213 substrates of P-gp, increased concentrations of the substrate drug are likely, and caution should 214 be exercised.215 Paclitaxel: In cancer patients receiving TYKERB and the CYP2C8 substrate paclitaxel, 216 24-hour systemic exposure (AUC) of paclitaxel was increased 23%. This increase in paclitaxel 217 exposure may have been underestimated from the in vivo evaluation due to study design218 limitations.219 7.2 Drugs that Inhibit or Induce Cytochrome P450 3A4 Enzymes220 Lapatinib undergoes extensive metabolism by CYP3A4, and concomitant administration 221 of strong inhibitors or inducers of CYP3A4 alter lapatinib concentrations significantly (see222 Ketoconazole and Carbamazepine sections, below). Dose adjustment of lapatinib should be223 considered for patients who must receive concomitant strong inhibitors or concomitant strong 224 inducers of CYP3A4 enzymes [see Dosage and Administration (2.2)].225 Ketoconazole: In healthy subjects receiving ketoconazole, a CYP3A4 inhibitor, at226 200 mg twice daily for 7 days, systemic exposure (AUC) to lapatinib was increased to227 approximately 3.6-fold of control and half-life increased to 1.7-fold of control.228 Carbamazepine: In healthy subjects receiving the CYP3A4 inducer, carbamazepine, at 229 100 mg twice daily for 3 days and 200 mg twice daily for 17 days, systemic exposure (AUC) to 230 lapatinib was decreased approximately 72%.231 7.3 Drugs that Inhibit Drug Transport Systems232 Lapatinib is a substrate of the efflux transporter P-glycoprotein (P-gp, ABCB1). If233 TYKERB is administered with drugs that inhibit P-gp, increased concentrations of lapatinib are 234 likely, and caution should be exercised.235 8 USE IN SPECIFIC POPULATIONS236 8.1 Pregnancy237 Pregnancy Category D [see Warnings and Precautions (5.7)].238 Based on findings in animals, TYKERB can cause fetal harm when administered to a 239 pregnant woman. Lapatinib administered to rats during organogenesis and through lactation led 240 to death of offspring within the first 4 days after birth. When administered to pregnant animals 241 during the period of organogenesis, lapatinib caused fetal anomalies (rats) or abortions (rabbits) 242 at maternally toxic doses. There are no adequate and well-controlled studies with TYKERB in 243 pregnant women. Women should be advised not to become pregnant when taking TYKERB. If 244 this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the 245 patient should be apprised of the potential hazard to the fetus.246 In a study where pregnant rats were dosed with lapatinib during organogenesis and247 through lactation, at a dose of 120 mg/kg/day (approximately 6.4 times the human clinical248 exposure based on AUC following 1,250 mg dose of lapatinib plus capecitabine), 91% of the。
一例拉帕替尼致皮疹病例分析

一例拉帕替尼致皮疹病例分析一、案例背景知识简介拉帕替尼(lapatinib)是一种新型靶向抗肿瘤药物,2007年3月13日获得FDA批准在美国上市。
该药是一种口服片剂,主要作用于表皮生长因子受体1和2,属于双靶点的受体酪氨酸激酶抑制剂,能通过干扰肿瘤细胞增殖和生长所需的信号转导途径发挥抗肿瘤活性。
目前拉帕替尼在乳腺癌中的作用已明确,尤其适用于表皮生长因子受体2(HER2)过度表达且经蒽环类、紫杉类药物和曲妥珠单抗治疗后复发的晚期或转移性乳腺癌。
常见不良反应为胃肠道反应,包括恶心、腹泻、口腔炎和消化不良等,皮肤干燥、皮疹,其他有背痛、呼吸困难及失眠等。
本案例对拉帕替尼致皮疹的病例进行分析,旨在为临床安全用药提供参考。
二、病例内容简介患者,女,38岁,汉族。
因“左侧乳腺癌多发转移1年余,左乳癌改良根治术后5个月余”收治入院。
已行22周期化疗,此次入院行三线治疗,即第23周期化疗,化疗方案为曲妥珠单抗、拉帕替尼联合重酒石酸长春瑞滨注射液。
患者无吸烟史、饮酒史。
既往史、家族史、药物食物过敏史无特殊。
入院查体:体温36.6℃,脉搏94次/分,呼吸18次/分,血压152/72mmHg,身高161cm,体重58kg,体表面积1.57m2,KPS评分90分。
入院诊断:乳腺癌多发转移,左乳腺癌改良根治术后。
三、主要治疗经过及典型事件该患者上次在院治疗期间无明显不良反应的发生。
出院后即口服拉帕替尼(1250mg/d,1次服),大约10天后出现从下肢开始蔓延至上肢及头面部的皮疹,表现为瘙痒、红肿。
该患者坚持服用拉帕替尼直至此次入院。
在与患者充分沟通征得患者同意后调整拉帕替尼的药物剂量为1000mg/d,1次服用,患者的皮疹较前减轻。
四、讨论拉帕替尼致皮疹目前尚无统一的诊断标准,应用拉帕替尼后出现皮疹的患者,排除其他可能的原因,且降低拉帕替尼用量后皮疹明显好转,可考虑患者皮疹与拉帕替尼相关。
(一)患者皮疹的发生可能与口服拉帕替尼有关拉帕替尼是一种新型的分子靶向药物,常见的副作用为肠胃消化道系统反应,即恶心、呕吐、腹泻等症状,其他不良反应还有皮疹,表现为红肿、瘙痒、疼痛等。
乌帕替尼结构式范文

乌帕替尼结构式范文乌帕替尼(Upadacitinib)是一种新型的Janus激酶抑制剂,可以用于治疗多种疾病,包括类风湿性关节炎(RA)、银屑病关节炎(PsA)和溃疡性结肠炎(UC)等。
乌帕替尼的化学名称为(3R,4R)-3-ethyl-4-(((1r,2s)-2-fluoro-1-methylpropyl)amino)-1-(4-phenoxyphenyl)butan-1-ol,其结构式如下:HH\C/H4HN(+)-C(-)---C\CH3N\H-PhPh乌帕替尼的化学结构使其能够与Janus激酶相互作用,从而起到抑制其活性的作用。
Janus激酶是一种接头蛋白激酶,可以介导多种细胞信号途径,从而调控细胞的增殖、分化和炎症反应等生物学过程。
通过抑制Janus激酶的活性,乌帕替尼可以减少炎症反应,从而缓解类风湿性关节炎等疾病的症状。
乌帕替尼的药理特性使其成为一种有希望的治疗类风湿性关节炎、银屑病关节炎和溃疡性结肠炎的药物。
临床试验显示,乌帕替尼可以显著减轻关节疼痛和肿胀,改善关节功能,并且具有较好的耐受性和安全性。
乌帕替尼的用法和剂量在不同的疾病中略有差异。
一般情况下,治疗类风湿性关节炎的剂量为每天15毫克,治疗银屑病关节炎和溃疡性结肠炎的剂量为每天30毫克。
乌帕替尼可口服,建议在饭后或者与食物一起服用。
虽然乌帕替尼在临床试验中显示出良好的疗效和安全性,但在使用时仍然需要谨慎。
乌帕替尼可能会引起一些不良反应,包括感染、肝功能异常、血液病变、高血压等。
因此,在使用乌帕替尼之前,应该进行全面的评估并监测患者的相关指标。
总的来说,乌帕替尼是一种有潜力的Janus激酶抑制剂,可以用于治疗类风湿性关节炎、银屑病关节炎和溃疡性结肠炎等疾病。
它的化学结构使其具有良好的药理特性,并且在临床试验中显示出良好的疗效和安全性。
然而,在使用乌帕替尼时仍然需要注意其可能的不良反应和禁忌症,以确保患者的安全和疗效。
化工产品中对甲苯磺酸检测方法进展

化工产品中对甲苯磺酸检测方法进展对甲苯磺酸(PTSA),白色,粉末或针状结晶,易溶于水、醚和醇,是一种重要的化工中间体,在农药、医药等领域有着广泛且重要的应用。
就现代化工工业生产而言,对甲苯磺酸既可以作为催化剂,推动各类酯化、脱水、缩醛反应的正向进行,还可以应用于有机合成反应,作为对甲苯磺酰胺、甲苯磺酰氯以及对甲酚等产品的基本原料。
对甲苯磺酸属于腐蚀性物品,酸性较强,且易于潮解,可借助大气与水体间的循环污染环境,如环境中对甲苯磺酸的浓度超标,就容易对相关操作人员的身体造成损伤。
本文即围绕对甲苯磺酸检测,就几种主要的对甲苯磺酸检测技术和方法,进行了分析和探讨,具体内容如下。
1 目前主要的对甲苯磺酸检测技术分析对甲苯磺酸检测是对甲苯磺酸应用的关键环节,从原始的产物异构体含量检测,到混酸含量检测,再到当前的复杂基质痕量检测,对甲苯磺酸检测技术始终在发展和完善。
目前,涉及对甲苯磺酸含量测定的技术方法主要有紫外分光光度法、气相色谱法、离子色谱法等几种,具体技术方法如下。
1.1 紫外分光光度法分析紫外分光光度法可同时完成对甲基苯磺酸和强力霉素废水中磺基水杨酸含量的检测,并通过KH2PO3-NaHPO3缓冲溶液体系的引入,有效排除了废水中甲醇、硫酸钠等基质的检测干扰,提高了检测精度。
对甲基苯磺酸与磺基水杨酸间的定量可借助相应的计算分離完成。
相关学者以工业废水稀释液作为实验材料,应用紫外分光光度法进行对甲苯磺酸检测,发现对甲苯磺酸浓度与其紫外光谱相关二阶导数值间具有良好线性关系。
另外一批学者使用紫外分光光度法针对对甲基磺酸中母体的实际含量进行了测定,并联合NaOH滴定法对杂质游离酸含量的实际数值进行了确定。
1.2 离子色谱法分析部分学者通过实验和研究创建了离子色谱法,实现了丙烯酸丁酯生产废水中对甲基磺酸和丙烯酸的有效检测。
根据实际废水样品的检测分析结果,常规无极阴离子不会对对甲基苯磺酸和丙烯酸的检测造成干扰(硫酸根离子除外)。
高效液相色谱法测定二甲苯磺酸拉帕替尼片有关物质2

LIU Jun-hua,LIANG Xing-hui,WANG Bin-rong( Zhuhai United Laboratories Co. ,Ltd. ,Zhongshan 528467,China)
t / min
0 5 10 25 35 35. 01 45
Mobile phase A / %
70 65 65 20 20 70 70
Mobile phase B / %
30 35 35 80 80 30 30
中国药学杂志 2013 年 4 月第 48 卷第 8 期
图 1 拉帕替尼杂质 A、 of lapatinib impurity A,LAPA-1 and LAPA-2
Tel / Fax: ( 0760) 86655310
E-mail: ljhua123@ 126. com 中国药学杂志 2013 年 4 月第 48 卷第 8 期
格: 250 mg,批号: R520553,葛兰素史克公司) 。
2 方法与结果 2. 1 色谱条件
色谱柱( 1) Waters Xterra MS C18 ( 4. 6 mm × 250 mm,5 μm) ,色谱柱( 2) 大连依利特 Hypersil C18 ( 4. 6 mm × 250 mm,5 μm) 。除耐用性实验采用两根色谱 柱外,其他实验均采用色谱柱 ( 1 ) ; 以 0. 01 mol · L - 1 磷酸二氢钠溶液( 用磷酸调节 pH 值至 3. 2) 为 流动相 A,以 乙 腈 为 流 动 相 B,流 速 为 1. 0 mL · min - 1 ,线性梯度洗脱程序见表 1,检测波长为 265 nm。柱温为 40 ℃ ,进样体积为 10 μL。 2. 2 溶液的制备
普纳替尼杂质

943320-61-4
普纳替尼杂质
18087-73-5
普纳替尼杂质
694499-26-8
普纳替尼杂质
doimidazo[1,2b]pyridazine
普纳替尼杂质
2-Methyl-5nitrobenzotrifluor ide
89976-12-5
普纳替尼杂质
3-Iodo-4methylbenzoic acid
82998-57-0
普纳替尼杂质
3-ethynyl-4methylbenzoic acid
1001203-03-7
普纳替尼杂质
3-Bromo[1,2,4]triazolo[4, 3-a]pyridine
4922-68-3
更多杂质信息欢迎咨询(13410717707),深圳卓越专业提供
普纳替尼杂质
中文名称 英文名称 CAS号 结构式
普纳替尼
Ponatinib
943319-70-8
普纳替尼中间体
Ponatinib intermediates 3Bromoimidazo[1,2b]pyridazine 4-(4Methylpiperazin-1ylmethyl)-3trifluoromethylani line 1-Methyl-4-[[4nitro-2(trifluoromethyl)p henyl]methyl]piperazine
普纳替尼杂质中文名称英文名称结构式ponatinib普纳替尼中间体ponatinibintermediates943320614普纳替尼杂质18087735普纳替尼杂质694499268普纳替尼杂质694499246普纳替尼杂质普纳替尼杂质89976125普纳替尼杂质82998570普纳替尼杂质1001203037cas号9433197083bromoimidazo12bpyridazine44methylpiperazin1ylmethyl3trifuoromethylaniline1methyl44nitro2trifuoromethylphenylmethylpiperazine3iodoimidazo12bpyridazine2methyl5nitrobenzotrifuoride3iodo4methylbenzoicacid3ethynyl4methylbenzoicacid普纳替尼杂质49226833bromo124triazolo43apyridine更多杂质信息欢迎咨询13410717707深圳卓越专业提供
乌帕替尼质量标准

乌帕替尼(Upadacitinib,又称RINVOQ)是一种Janus激酶(JAK)抑制剂,主要用于治疗风湿性关节炎、银屑病性关节炎、特应性皮炎等疾病。
在质量标准方面,乌帕替尼的主要指标包括以下几点:
1. 纯度:乌帕替尼的纯度要求较高,通常需达到99%以上。
纯度越高,表明药物的有效成分含量越高,疗效和安全性更有保障。
2. 生物活性:乌帕替尼作为JAK抑制剂,其生物活性表现为对JAK家族成员的选择性抑制,从而抑制特定与疾病相关的信号通路。
生物活性强的新冠病毒意味着药物能在体内有效抑制病理性信号传导,从而发挥治疗作用。
3. 溶解性:乌帕替尼的溶解性对其疗效和患者用药便利性有很大影响。
一般来说,药物溶解性越好,患者用药时口感更好,药物吸收更快,疗效更佳。
4. 制剂工艺:乌帕替尼的制剂工艺对其疗效和安全性也有重要影响。
例如,缓释片剂能够使药物在体内持续释放,延长作用时间,降低药物浓度波动,从而提高患者依从性和疗效。
5. 杂质控制:乌帕替尼生产过程中,需要严格控制杂质含量。
杂质过多可能影响药物的纯度和生物活性,甚至引发不良反应。
因此,国家药品监督管理局对乌帕替尼杂质含量有明确的规定。
6. 药品质量检测:在乌帕替尼的生产和销售过程中,需要进行严格的质量检测,确保药品质量达到标准。
这包括药品的有效性、安全性、含量测定、体外溶出度等检测项目。
Lazertinib(拉泽替尼)合成检索总结报告
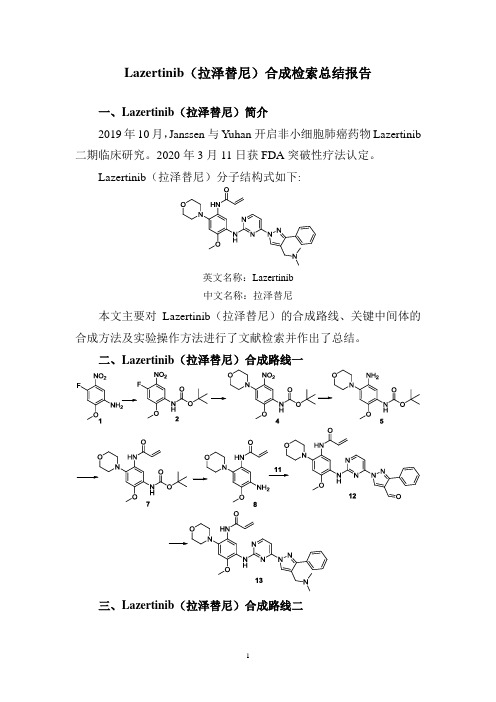
Lazertinib(拉泽替尼)合成检索总结报告
一、Lazertinib(拉泽替尼)简介
2019年10月,Janssen 与Yuhan 开启非小细胞肺癌药物Lazertinib 二期临床研究。
2020年3月11日获FDA突破性疗法认定。
Lazertinib(拉泽替尼)分子结构式如下:
英文名称:Lazertinib
中文名称:拉泽替尼
本文主要对Lazertinib(拉泽替尼)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Lazertinib(拉泽替尼)合成路线一
三、Lazertinib(拉泽替尼)合成路线二
四、Lazertinib(拉泽替尼)合成路线一检索总结报告(一) Lazertinib(拉泽替尼)中间体2的合成(路线一)
(二) Lazertinib(拉泽替尼)中间体4的合成(路线一)。
舒尼替尼杂质列表
杂质,奥拉帕利杂质,阿加曲班杂质,法莫替丁杂质,他达拉非杂质,厄洛替尼
杂质,替格瑞洛杂质等;并提供 COA、NMR、HPLC、MS、定量核磁等结构确证
Hale Waihona Puke 图谱。177 二 813 八 655
代理中检所/EP/BP/USP/LGC/TRC/TLC/MC/SIGMA/等品牌。
2199-62- 10mg-25mg-50mg
5
≥99
22
Impurity 22
4
-100mg
舒尼替尼杂质
Sunitinib
2199-61- 10mg-25mg-50mg
6
≥99
23
Impurity 23
3
-100mg
广州隽沐生物主营各种杂质对照品:紫杉醇杂质,莫西沙星杂质,达托霉素杂质,
阿奇霉素杂质,帕拉米韦杂质,克拉霉素磷酸酯杂质,西格列汀杂质,林可霉素
舒尼替尼杂质列表(部分)
序号 产品名称 英文名称 CAS 号
规格
纯度
结构式
Sunitinib 452105-3 10mg-25mg-50mg
1 舒尼替尼杂质 1
≥99
Impurity 1
3-8
-100mg
Sunitinib 356068-9 10mg-25mg-50mg
2 舒尼替尼杂质 2
≥99
Impurity 2
7-8
-100mg
Sunitinib 356068-8 10mg-25mg-50mg
3 舒尼替尼杂质 3
≥99
Impurity 3
6-5
-100mg
Sunitinib 356068-9 10mg-25mg-50mg
拉帕替尼说明书

说明:网上资料,包括两个版本的说明书,仅供参考。
【药品名称】通用名称:甲苯磺酸拉帕替尼片商品名称:甲苯磺酸拉帕替尼片(泰立沙)英文名称:Lapatinib ditosylate拼音全码:JiaBenHuangSuanLaPaTiNiPian(TaiLiSha)【主要成份】甲苯磺酸拉帕替尼,N-(3-氯-4-((3-氟苯基)甲氧基)苯基)-6-(5-(((2-(甲磺酰基)乙基)氨基)甲基)-2-呋喃基)-4-喹唑啉胺二对甲苯磺酸盐。
【成份】分子式:C29H26ClFN4O4S.2(C7H8O3S)分子量:943.48【性状】本品为黄色薄膜衣片,一侧平面,另一侧刻有凹陷GS XJG刻痕。
【适应症/功能主治】本品用于联合卡培他滨治疗ErbB-2过度表达的,既往接受过包括蒽环类、紫杉醇、曲妥珠单抗(赫赛汀)治疗的晚期或转移性乳腺癌。
【规格型号】250mg*10s【用法用量】推荐剂量为1250mg,每日1次,第1~21天服用,与卡培他宾2000mg/d,第1~14天分2次服联用。
拉帕替尼,应每日服用1次,不推荐分次服用。
饭前1h或饭后2h后服用。
如漏服1剂,第2天不需剂量加倍。
妊娠级别D,孕妇禁用。
是否通过乳汁分泌尚不清楚,哺乳期妇女应停止授乳。
老年人用药与年轻患者未发现有明显差异。
未对肾脏严重损害及透析患者做过临床试验,中重度肝损害的患者应酌减剂量。
【不良反应】临床试验中观察到的大于10%的不良反应主要为胃肠道反应,包括恶心、腹泻、口腔炎和消化不良等,皮肤干燥、皮疹,其他有背痛、呼吸困难及失眠等[4]。
与卡培他宾合用,不良反应有恶心、腹泻及呕吐,掌跖肌触觉不良等。
个别患者可出现左心室射血分数下降,间质性肺炎。
其最常见之副作用为肠胃消化道系统方面的副作用,即是恶心、呕吐、腹泻等症状,其他还有皮肤方面的红肿、搔痒、疼痛,以及疲倦等。
另外还有极少见但是严重的副作用,包括心脏方面以及肺部方面。
当病患出现二级(New York Heart Association,NYHA class 2)以上的心脏左心室搏出分率(Left Ventricle Ejection Fraction,LVEF)下降时,必须停止使用,以避免产生心脏衰竭。
尼达尼布杂质、 阿西替尼杂质(0222更新部分)

22/02/22杂质上新 | 尼达尼布杂质、阿西替尼杂质等2类目新增3种药物杂质对照品2022年02月22日,深圳恒丰万达医药官网中又有2类药物杂质新增杂质对照品了,这2类药物杂质分别是尼达尼布杂质、阿西替尼杂质。
此次总共新增了3种药物杂质对照品,下面我们将为您介绍这些产品的详细信息,包括货号、中文名、英文名、同义词、CAS号、分子式、分子量等,以下是具体的产品信息。
1、尼达尼布杂质51(T:185766-96176)货号:N004051,中文名:尼达尼布杂质51,英文名:Nintedanib Impurity 51,同义词:(Z)-methyl 3-(methoxy(phenyl)methylene)-2-oxoindoline-6-carboxylate,CAS号:,分子式:C18H15NO4,分子量:309.32。
2、尼达尼布杂质52(Q:2851922763)货号:N004052,中文名:尼达尼布杂质52,英文名:Nintedanib Impurity 52,同义词:(Z)-methyl 1-acetyl-3-(((4-(N-methyl-2-(4-methylpiperazin-1-yl)acetamido)phenyl)amino)(phenyl)methylene)-2-oxoindoline-6-carboxylate,CAS号:,分子式:C33H35N5O5,分子量:581.26。
3、阿西替尼杂质15货号:A020015,中文名:阿西替尼杂质15,英文名:Axitinib Impurity 15,同义词:(Z)-N-methyl-2-((3-(2-(pyridin-2-yl)vinyl)-1H-indazol-6-yl)thio)benzamide,CAS号:,分子式:C22H18N4OS,分子量:386.47。
以上就是恒丰万达今日新增的药物杂质对照品了,另外,本平台还有法匹拉韦杂质15、托伐普坦杂质3、布南色林杂质C、瑞舒伐他汀杂质59、克立硼罗杂质20、伊曲茶碱杂质5等上千种黄金现货杂质正在热销中。
拉帕替尼的新药用盐[发明专利]
![拉帕替尼的新药用盐[发明专利]](https://img.taocdn.com/s3/m/14391ff2ff00bed5b8f31dcf.png)
专利名称:拉帕替尼的新药用盐专利类型:发明专利
发明人:不公告发明人
申请号:CN201210469363.0申请日:20121119
公开号:CN102964339A
公开日:
20130313
专利内容由知识产权出版社提供
摘要:拉帕替尼的新药用盐,如图所示x=1、2、3n=0、0.5、1、1.5、2、2.5、3R=草酸、马来酸、柠檬酸、氢溴酸、盐酸、苯磺酸、对甲苯磺酸、硫酸、甲磺酸、乙酸、丙酸、丙二酸、葡萄糖酸、琥珀酸、富马酸、乳酸、酒石酸、苹果酸、丙酮酸、羟基丁酸、己二酸、水杨酸、邻苯二甲酸、扁桃酸。
申请人:北京阜康仁生物制药科技有限公司
地址:100070 北京市丰台区科技园2号楼2层B216
国籍:CN
更多信息请下载全文后查看。
乳腺癌的靶向药物

乳腺癌的靶向药物曲妥珠单抗是一种重组的DNA衍生单克隆抗体,可选择性地作用于HER2的细胞外部。
拉帕替尼则是一种口服的抗HER1和HER2的小分子酪氨酸激酶抑制剂。
帕妥珠单抗:也是重组的单克隆抗体,可与HER2受体胞外结构II区进行结合,抑制二聚体形成,抑制受体介导的信号转导通路。
Neratinib是一种不可逆的抑制EGFR、HER2、HER4的口服酪氨酸激酶抑制剂,对HER2阳性乳腺癌有一定的抗癌作用。
阿法替尼是一种对HER1、 2和4有不可逆抑制作用的口服小分子药物。
在曲妥单抗抵抗的转移性乳腺癌患者中进行的一个II期试验显示,部分缓解率为4/35,不良反应包括腹泻和皮疹。
值得一提的是,乳腺癌虽然可以使用靶向药物治疗,但在一定的时候后,也许是6个月也许是一年后,患者会产生耐药性。
所以靶向治疗也常常作为一种辅助治疗方式存在,同作为辅助治疗的还有中医调理。
如扶正祛邪的去壁灵芝孢子粉,不仅达到祛邪降低放化疗副作用、防止肿瘤增生和转移,还能扶正扶植正气、增强患者抵抗力免疫力、提高患者生活质量水平。
1.激素异常引起的乳腺癌某些乳腺癌是因雌激素或孕酮异常产生的。
活组织检查可以判断肿瘤的雌激素受体及孕酮受体是否呈阳性。
三分之二的乳腺癌是激素异常引起的,但有药物可以抑制激素异常。
2.HER2阳性乳腺癌约20%乳腺癌患者癌细胞中的HER2受体都超量,这种癌症叫做HER2阳性乳腺癌,它比其他乳腺癌的传播更快。
对这类乳腺癌有特殊的治疗方法,因此应该尽早确诊是否是这种乳腺癌。
1.乳房自查女性曾被建议每月进行一次乳房自查,但研究表明这对发现癌症的帮助很小。
现在认为比起自查,女性更应对自己乳房的变化了若指掌。
如要做自查,也应提前咨询医生相关事项。
2.乳腺活组织检查确定肿块是否是癌的唯一有效方式是活组织检查:提取部分或全部肿块的组织进一步实验室检测来确认是否是癌症以及何种类型。
乳腺癌种类不同,因此治疗方式应对症下药。
3.乳腺癌和乳腺X光片乳腺癌越早发现越易治疗。
