药明康德有机合成2013年3月晋升测试题I (1)
化学合成部专业知识培训测试题 (Part III)-071124.doc
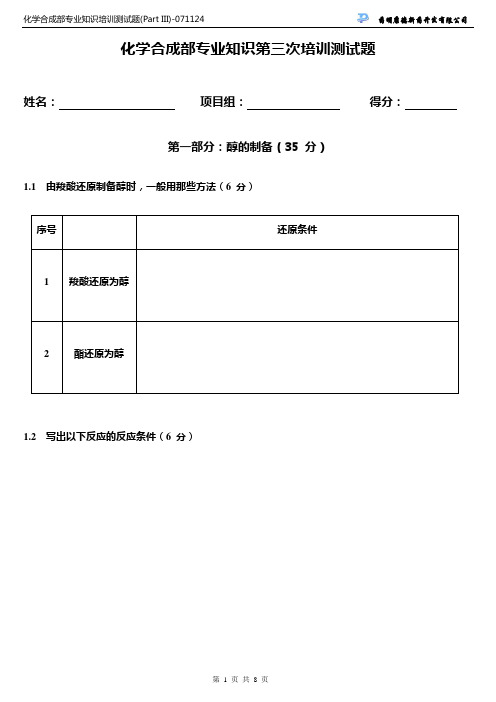
化学合成部专业知识第三次培训测试题姓名:项目组:得分:第一部分:醇的制备(35 分)1.1由羧酸还原制备醇时,一般用那些方法(6 分)序号还原条件1 羧酸还原为醇2 酯还原为醇1.2写出以下反应的反应条件(6 分)OHNOO OH OHHNO ONH2OHOOH1.3写出以下转换的条件(9 分)S Br SOH S CHO SOHS CHO SCOOEt HO1.4写出以下反应的产物。
(4 分):OX -LAH or HydrogenaionRS -NaN 31.5 解释一下硼氢化反应并写出通式(4 分)1.6 写出以下反应的产物(6 分)OsO PhMgBr (3 eq.)THF, rtN COOEt第二部分:芳(酰)胺化反应(20分)2.1 一般芳(酰)胺化反应常用的方法是那三种条件,用芳基硼酸做芳(酰)胺化反应有什么优点(6 分)?2.2 请填写完成以下转化的条件或反应产物(6 分)OH NH2NHAc Br 5 mol % Pd2(dba)3 20 mol % P(2-furyl)3Cs2CO3, toluene 100-110℃2.3 请设计以下合成路线(8 分)N HONHONONH N NHOO第三部分:腈的合成(15 分)3.1 填写合适的反应条件或产物 (12 分)N BocNH 2O N BocCNN ONNOOO CHOCNOHEtOOCCNEtOOCNNH ONN3.2 请设计以下合成路线(3 分)CNNCCOOEtBr第四部分:磺酰氯的合成(10 分)4.1 写出一般三种以上磺酰氯的合成方法(3 分)4.2 写出三种以上方法完成以下转换(3 分)NBrNSO2Cl 4.3 请设计以下合成路线(4 分)N OHN NSNHO OO第五部分:吲哚的合成(20 分)5.1 写出Fisher 吲哚合成的通式(反应原料,产物及条件,可不描述机理)(4 分)5.1 填写下列转换的条件与中间体(6 分)BnONO 2BnONHNH 2Br N H5.3 请设计以下合成路线(4 分)MeO ClClMeO ClClN H。
有机合成工考试题库完整

一、单项选择题1.在安全疏散中,厂房内主通道宽度不少于(D)A.0.5mB.0.8mC.1.0mD.1.2m2.NaOH滴定H3PO4以酚丈为指示剂,终点时生成(B)。
(已知H3PO4的各级离解常数:Ka1=6.9X10-3,Ka2=6.2X10-8,Ka3=4.8X10-13)ANaH2P04B.Na2HPO4C.Na3PO4D.NaH2PO4+Na2HPO414.烷姓①正庚烷、②正己烷、③2-甲基戊烷、④正癸烷的沸点由高到低的顺序是(D)A.①②③④B.③②①④C.④③②①D.④①②③15下列烯姓中哪个不是最基本的有机合成原料土烯”中的一个(B)A.乙烯B.丁烯C.丙烯D.1,3-丁二烯16目前有些学生喜欢使用涂改液,经实验证明,涂改液中含有许多挥发性有害物质,二氯甲烷就是其中一种。
下面关于二氯甲烷(CH2cl2)的几种说法:①它是由碳、氢、氯三种元素组成的化合物;②它是由氯气和甲烷组成的混合物;③它的分子中碳、氢、氯元素的原子个数比为1:2:2;④它是由多种原子构成的一种化合物。
说法正确的是(A)A.①③B②④C.②③D.①④17在一个绝热刚性容器中发生一化学反应,使系统的温度从T1升高到T2,压力从P1升高到P2,则(B)A.Q<0,W<0,AU<0B.Q=0,W=0,△U=OC.Q=0,W<0,△U<OD.Q<0,W=0,△U<O18磺化能力最强的是(A)A.三氧化硫B.氯磺酸C.硫酸D.二氧化硫19磺化剂中真正的磺化物质是(A)A.SO3B.SO2C.H2SO4D.H2SO320不属于硝化反应加料方法的是(D)A.并加法B.反加法C.正加法D.过量法21侧链上卤代反应的容器不能为(C)A.玻璃质B.搪瓷质C.铁质D.衬锲22氯化反应进料方式应为(B)A.逆流B.并流C.层流D.湍流A.酰氯〉酸酊>竣酸B.竣酸〉酰氯〉酸酊C.酸酊〉酰氯〉竣D.酰氯〉竣酸>酸酊24化学氧化法的优点是(D)A.反应条件温和B.反应易控制C.操作简便,工艺成熟D.以上都对25不同电解质对铁屑还原速率影响最大的(A23比较下列物质的反应活性(A)A.NH4ClB.FeCl226氨解反应属于(B)A.一级反应B二级反应27卤烷烷化能力最强的是(A.RIB.RBr28醛酮缩合中常用的酸催化剂A.硫酸B硝酸C.NaClD.NaOHC.多级反应D.零级反应A)C.RClDRF(A)C.磷酸D.亚硝酸29在工业生产中,芳伯胺的水解可看做是羟基氨解反应的逆过程,方法有(D)A.酸性水解法B碱性水解法C亚硫酸氢钠水解法D以上都对30下列能加速重氮盐分解的是(A)。
化学合成部专业知识培训测试题 (Part I)-070818.doc

化学合成部专业知识培训试题姓名:项目组:得分:第一部分:Beilstein 查询(30 分)1.1在检索的主界面上Allow一栏,前面五个选择后代表什么(5 分)?1.21.2 看以下的检索设定,回答问题(5 分):a) 以下的检索中小图虚线代表什么意思?b) 芳环的四个Cx代表什么?c) 检索主界面的Search field两栏,LAH, >90%各代表什么?1.3 按要求在虚框中填入检索信息(7 分)硝基苯表方法N吡啶氮氧化物表示方法NR 普通芳香环:R芳香杂环:R 任意官能团:R卤原子通式:R任意取代的杂原子:1.4 在一个分子中有一个仲羟基和一个伯羟基我们需要选定一个方法将硅的保护基放在仲羟基上而不是常规的伯羟基上,请写出检索的设定方法(5 分):1.5 一般缩酮比N-Boc 对酸更敏感,如何通过设定检索方法找到可以脱N-Boc 但同一分子内缩酮基团还稳定的文献(7 分):1.6 由于很多杂环的单双键可以移动,一般我们如何设定以防漏检?以如下的分子画出设定方法(3 分)。
ClNHN ONH 2ClNH N NH 2还原附加:1.7 在检索设定一览如何利用快捷键将单键改为双键 (2 分)第二部分:酰胺的合成(40 分)2.1 使用碳二亚胺类缩合剂一般需要加入酰化催化剂或活化剂,如4-N,N-二甲基吡啶(DMAP )、1-羟基苯并三氮唑(HOBt)等等,其主要原因是什么,写出DMAP 作为催化剂的过渡态(8 分).R N O R 2OHN CO RCxN Cx R 1+HNR 2R 12.2 以HATU 的缩合反应为例,说明其反应机理(8 分)。
NN NNOHOROR N H O R 1+NH 2R 1NNH 2R 12.3 请列举出至少十种以上的胺的酰化方法(10 分)2.4 胺酯交换一种最为通用的方法是什么,请写出通用反应式(3 分)?2.5 以下各类胺可以通过那些交换方法得到酰胺(5 分):序号胺的分类胺酯交换方法1 氨2 脂肪伯胺3 脂肪仲胺4 芳香伯胺5 芳香仲胺2.6 腈基水解一般条件都比较苛刻,写出一种较为温和的反应条件(3 分):OCl CN ClNH2 2.7 请写出Ritter 反应的原理和反应通式(3 分):第三部分:羟基的保护(20 分)3.1 以下四种羟基的硅保护基在使用时各有什么优缺点(8 分)?序号硅保护基结构式使用特点1 TMS2 TBS3 TIPS4 TBDPS3.2 硅醚保护为什么用咪唑作碱(2 分)3.3 羟基的硅醚脱保护一般使用什么试剂,使用过程中有那些注意事项(3 分)3.4 写出EE, THP, MOM, SEM-等保护基的结构,为什么我们在分子中有受性中心时,一般尽量避免用EE和THP作为保护基(5 分)3.5.当分子中同时有游离的仲胺和伯胺,我们一般选择用什么作为羟基的保护基,为什么?(2 分)第四部分:Mitsunobu 反应(10 分)4.1 请写出Mitsunobu 反应的机理,并说明什么样亲核试剂适合于Mitsunobu 反应(4 分)RNuR'OHNuHN N COOR, PPh 3ROOC4.2以下那些亲核试剂可以用于Mitsunobu 反应(对打勾,不对打叉)(4 分)NHOHNNH O ONHOOHO OHNHSHOHO SHNN HOO F PhN H O NN H N PhNH OBocN H Ns备注:4.3和4.2共5分,每错两个扣一分,错九个扣全分4.3以下底物中那些羟基可以用于Mitsunobu 反应 (对打勾,不对打叉)(1 分)OHOHOHOHOEtOH4.4 一般做Mitsunobu 的实验注意事项是什么(2 分)。
成都药明康德化学合成类部门入职考试

成都药明康德化学合成类部门入职考试1、在溶剂提取法中,更换新鲜溶剂可以创造新的(),从而使有效成分能够继续被提取出来。
()[单选题] *A极性差B压力差C浓度差(正确答案)D体积差2、在简单萃取法中,一般萃取几次即可()[单选题] *A3~4次(正确答案)B1~2次C4~5次D3~7次3、不属于木脂素类化合物的物理性质的是()[单选题] *A一般没有挥发性B有光学活性C易溶于有机溶剂D有色晶体(正确答案)4、七叶内酯的结构类型为()[单选题] *A简单香豆素(正确答案)B简单木脂素C呋喃香豆素D异香豆素5、即有一定亲水性,又能与水分层的是()[单选题] * A正丁醇B乙酸乙酯C二者均是(正确答案)D二者均非6、具有挥发性的生物碱是()[单选题] *A吗啡碱B麻黄碱(正确答案)C苦参碱D小檗碱7、萃取时易发生乳化现象的是()[单选题] *A简单萃取法(正确答案)B逆流连续萃取法C二者均是D二者均不是8、关于肿节风,说法正确的有(多选)()*A别名:接骨金粟兰、九节茶等(正确答案)B功能主治抗菌消炎凉血清热解毒(正确答案)C肿节风为白色针晶(正确答案)D不易溶于甲醇,乙醇9、萃取法是利用混合物中各成分在两相溶剂中的分配.系数不同而到达分离的方法,所谓两相溶剂是指()[单选题] *A两种相互接触而又不相溶的溶剂(正确答案)B两种不相互接触而又互相溶的溶剂C两种不相互接触而又不相容的溶剂D两种互相接触而又互相溶的溶剂10、很少含有挥发油的植物科为()[单选题] *A菊科B唇形科C茜草科(正确答案)D姜科11、纸色谱是分配色谱中的一种,它是以滤纸为(),以纸上所含的水分为固定相的分配色谱。
()[单选题] *A固定相B吸附剂C展开剂D支持剂(正确答案)12、E何首乌(正确答案)下列不含蒽醌类成分的中药是()*A丹参(正确答案)B决明子C芦荟D紫草(正确答案)13、巴豆的致泻成分是()[单选题] *A丁二酸B巴豆油酸(正确答案)C绿原酸D抗内毒素14、与明胶反应生成沉淀的成分是()[单选题] *A强心苷B皂苷C有机酸D鞣质(正确答案)15、苯丙素类的基本母核是具有一个或数个()单元的天然化合物()[单选题] *AC6-C3基团(正确答案)BC6-C6基团CC5-C3基团DC8-C8基团16、药材虎杖中的醌结构类型为()[单选题] *A苯醌类B萘醌类C蒽醌类(正确答案)D菲醌类17、E连续回流提取法(正确答案)从中药中水提取液中萃取偏于亲水性的成分的溶剂是()[单选题] *A正丁醇(正确答案)B乙醇C乙醚D三氯甲烷18、可沉淀具有羧基或邻二酚羟基成分的沉淀法是()[单选题] * A溶剂沉淀法B醋酸铅沉淀法(正确答案)C酸碱沉淀法D水提醇沉法19、有机溶剂加热提取中药成分应采用()[单选题] *A回流装置(正确答案)B蒸馏装置C萃取装置D分馏装置20、从香豆素类的结构看,香豆素是一种()[单选题] *A内酯(正确答案)B羧酸C酰胺D糖21、没有挥发性也不能升华的是()[单选题] *A香豆素苷类(正确答案)B游离蒽醌类C樟脑D游离香豆素豆素类22、酸碱沉淀法中的酸提碱沉法主要适用于()[单选题] *A黄酮类B香豆素类C醌类D生物碱类(正确答案)23、连续回流提取法与回流提取法比较,其优越性是()[单选题] * A节省时间且效率高B节省溶剂且效率高(正确答案)C受热时间短D提取量较大24、淀粉含量多的药材提取时不宜用()[单选题] * A浸渍法B渗漉法C煎煮法(正确答案)D回流提取法25、生物碱碱性的表示方法常用()[单选题] * ApKBBKBCpH(正确答案)DpKA26、极性最大的溶剂是()[单选题] *A酸乙酯(正确答案)B苯C乙醚D氯仿27、能溶于水的生物碱是()[单选题] *A莨菪碱B小檗碱(正确答案)C长春新碱D长春碱28、具有挥发性的生物碱是()[单选题] *A苦参碱B莨菪碱C麻黄碱(正确答案)D小檗碱29、组成缩合鞣质的基本单元是()[单选题] *A黄烷-3-醇(正确答案)B酚羟基C环戊烷D哌啶环30、萃取时,混合物中各成分越易分离是因为()[单选题] * A分配系数一样B分配系数相差越大(正确答案)C分配系数越小D以上都不是。
药明康德有机合成2015年3月晋升测试题I_Answer

WuXi AppTec Promotion Exam(for Assistant Director and above)March 13, 2015Note: 1. the answers for >85% of the questions can be found from company training website;2. 20% of the questions are from Science Seminars.1. Vorapaxar (brand name Zontivity ) is a protease-activated receptor (PAR-1) antagonist based on the natural product Himbacine . On May 5, 2014, Vorapaxar obtained FDA approval. It is a pharmaceutical treatment for acute coronary syndrome chest pain caused by coronary artery disease. Please design a reasonable synthetic route to prepare Himbacine from compounds A , B, C and other commercially available reagents. (Ref: J. Am. Chem. Soc. 1996, 9812) (18 points)Key points: Takai reaction; DA cyclization; epimerization 所属主任:___________姓名:___________pound A was identified as a potent S1P1 receptor agonist with good selectivity vs S1P3 receptor. Please provide a synthesis of this compound from compounds B, C and other readily available reagents. (Ref: J. Med. Chem. 2014, 57, 10424−10442) (18 points)3.Please review the below synthetic scheme for the core synthesis of Daphnilongeranin B and answer the related questions.(Ref: Org. Lett.2011, 1812) (20 points)Question 1: Please provide structure for intermediate A and mechanism from Compound 2to Compound 3, and explain the stereochemistry (6 points)Answer:Question 2: Please provide the conditions for Step 4 (Condition A) and Step 7 (Condition B) (6 points)Answer: Na2CO3/MeOH;Question 3: Please explain the stereoselectivity of Step 8 (3 points)Answer: “H” delivered from less hindered faceQuestion 4: Please provide a stepwise mechanism for Step 9 (5 points)Answer:4.Please review the synthetic scheme below for total synthesis of (+)-Clavilactone A and (-)-Clavilactone B and answer the related questions.(Ref: Org. Lett.2013, 15, 5582) (18 points)Question 1: Please draw the structures for intermediate A, reagent C and conditions for Step 2B (9 points)Si(OTf)2/Py, DCM, -78 o CAnswer:, (t-Bu)Question 2: In Step 3, please explain the reason for stereoselectivity. (4 point)Answer:Oxygen atom can’t attack from down side easily.Question 2: Please provide a stepwise mechanism of Ring Closing Metathesis (5 points)Answer:5. Please answer the following questions from recent seminars at Wuxi. (20 points)Scheme 5-1Question 1: In his design of total synthesis of Macfarlandin E and Aplyviolene, Professor Larry Overman claimed that a dialdehyde strategy will likely fail. Please have a look at his analysis and propose a mechanism for the formation of B and C from A. (4 points)Answer:Scheme 5-2Question 2: Based on the mechanism from Question 1, what’s the product from above transformation?(3 points)Answer:Scheme 5-3Question 3: What’s the product from above transformation? (3 points)Answer:Scheme 5-4Question 4: Please provide the mechanism of Step A and the product from step B. (7 points)Answer:product 3 points; mechanism 4 pointsScheme 5-5Question 5: What’s the product from above transformation? (3 points)Answer:6. English (6 points)Please translate following words to English1)过渡金属transition metal2)抑制剂inhibitor3)不对称合成asymmetric synthesis4)亲核试剂nucleophile5)脂肪族的aliphatic6 ) 螺环化合物spiro-cyclic compound。
药明康德Level 2题目-Reduction

还原反应考试试题一、是非题1, Raney 镍在碱性体系中的还原能力大于酸性体系下的还原能力()2, 催化氢化主要是顺式加成,反式产物为主()3,硼烷还原速度顺序为RCOOH>RCOOEt>RCONH2( )4, 叠氮化合物还原可保持相连碳的立体构型 ( )5, 活泼金属还原硝基化合物为自由基型反应机理,经过了亚硝基、羟胺、偶氮中间体 ( )6, 硼烷可以将醛基、酮羰基、羧酸、酯基还原成羟基()7, 催化氢化反应中,炔烃的反应活性高于烯烃,位阻小的不饱和键的活性高于位阻大的不饱和键,氢气的压力越高反应越快()8, 脱氢解反应中,卤代烃的反应活性为RI <RBr< RCl ( )9, 选用位阻较大的硼烷试剂可以提高硼烷加成反应的选择性()10,Birth反应中芳环上供电子取代基加速反应,吸电子取代基阻碍反应()11,底物中硫原子的存在会阻碍钯催化氢化反应()12,采用Clemmensen反应时,酮还原较好,羧酸、酯、酰胺不受影响()LiAlH4>LiBH4>NaBH4>KBH4>NaBH2S3>i-Bu3BHLi ()14,氢化二异丁基铝(DIBAlH)可将酯还原为醛,双键、卤素、硝基均受影响()15,林德拉催化剂(Lindar Catalyst)可以将二取代炔烃还原为反式烯烃()。
16,烯烃的硼氢化氧化制备醇得到马氏规则产物。
()17,Clemmensen反应可以选择性还原酮,羧酸、酯、酰胺不受影响。
()18,催化氢化反应: 氢压越高,反应越快()19,二酰亚胺(diimide,NH=NH)为选择性还原剂时,末端双键及顺式双键活性高;双键上取代基越多,位阻越大,收率及速率明显下降。
()20,芳烃的还原反应: 芳稠环活性>苯,ArOH>ArNH2>ArH>ArCOOH>ArMe()21,芳基酮类化合物含酸敏感官能团用Clemmensen还原,含碱敏感官能团用Wolff-Kishner-黄鸣龙反应。
有机合成中新药研发中原料药的关键制备考核试卷

B.缩合反应
C.环加成反应
D.烷基化反应
11.以下哪种技术通常用于原料药合成过程中的手性拆分?()
A.液-液萃取
B.色谱分离
C.晶体工程
D.离子交换
12.关于原料药的杂质分析,以下哪种方法不常用于杂质的鉴定?()
A. HPLC
B. NMR
C. IR
D. GC-MS
13.在有机合成中,以下哪个条件通常不用于催化加氢反应?()
A.使用可回收溶剂
B.优化工艺减少废弃物
C.严格控制排放标准
D.避免使用有害化学品
20.以下哪些是原料药合成中可能使用的安全措施?()
A.使用防护装备
B.紧急停车系统
C.防止静电火花
D.定期安全培训
(以下继续其他题型)
三、填空题(本题共10小题,每小题2分,共20分,请将正确答案填到题目空白处)
1.在有机合成中,用于保护羧基的常见保护基是______。
B.超临界二氧化碳
C.乙醇
D.苯
14.在原料药的生产中,哪些措施有助于提高产品的安全性?()
A.控制杂质
B.严格工艺参数
C.使用高质量原料
D.避免交叉污染
15.以下哪些技术可用于原料药的结晶过程优化?()
A.结晶动力学
B.晶体工程
C.过程分析技术
D.计算机模拟
16.原料药的固态表征中,以下哪些参数是重要的?()
4.分析在原料药的生产过程中,可能出现的质量问题和杂质来源,并提出相应的解决策略和预防措施,以确保最终产品的质量和安全性。
标准答案
一、单项选择题
1. A
2. B
3. C
4. A
5. D
有机合成工考试试题(题库版)

有机合成工考试试题(题库版)1、单选下列试剂中属于硝化试剂的是()A.浓硫酸B.氨基磺酸C.混酸D.三氧化硫正确答案:C2、单选20%发烟硝酸换算成硫酸的浓度为()。
A、104.5%B、10(江南博哥)6.8%C、108.4%D、110.2%正确答案:A3、判断题工业生产上所用的还原铁粉一般采用50~60目的铁粉正确答案:对4、单选滴定管、移液管、刻度吸管和锥形瓶是滴定分析中常用的四种玻璃仪器,在使用前不必用待装溶液润洗的是()。
A.滴定管B.移液管C.刻度吸管D.锥形瓶正确答案:D5、判断题缩聚反应一定要通过两种不同的单体才能发生。
正确答案:错6、判断题由于sp杂化轨道对称轴夹角是180°。
所以乙炔分子结构呈直线型。
正确答案:对7、单选用熔融碱进行碱熔时,磺酸盐中无机盐的含量要求控制在()A.5%以下B.10%以下C.15%以下D.20%正确答案:B8、判断题有毒气体气瓶的燃烧扑救,应站在下风口,并使用防毒用具。
正确答案:错9、判断题丁苯橡胶的单体是丁烯和苯乙烯。
正确答案:错10、判断题氮氧化合物和碳氢化合物在太阳光照射下,会产生二次污染——光化学烟雾。
正确答案:对11、单选关于我国技术性贸易壁垒体系的说法,不正确的是()A.建立了全国技术性贸易壁垒措施部级联席会议制度B.已经能够有效地应对国外的技术性贸易壁垒措施的挑战C.建立了技术性贸易措施国家通报、评议和咨询的国内协调机制D.建立了技术性贸易措施信息服务体系正确答案:B12、判断题氨解指的是氨与有机物发生复分解反应而生产仲胺的反应。
正确答案:错13、判断题SN2反应分为两步进行。
正确答案:错14、判断题油脂在碱性条件下的水解称为皂化反应。
正确答案:对15、单选下列酰化剂在进行酰化反应时,活性最强的是()。
A、羧酸B、酰氯C、酸酐D、酯正确答案:B16、判断题和烯烃相比,炔烃与卤素的加成是较容易的正确答案:错17、单选国家标准规定的实验室用水分为()级。
药明康德有机合成2012年9月晋升测试题I

(for Assistant Director and above)
September 7, 2012
Note: 1. the answers for >85% of the questions can be found from company training website; 2. ca. 25% of the questions are from Science Seminars.
答
题
时
请
勿
超
越
Question 6-4: Please provide a suitable condition in the last step to prepare compound 10
此
(2 points)
虚
线
, 谢
谢
!
7. AS shown below, oxidative removal of PMB group in 1 afforded an unknown byproduct with MW +331.36. and 1H NMR (DMSO) spectrum given. (8 points)
O
O
N O
Oห้องสมุดไป่ตู้OH 1
MW: 333.38
CAN, CH3CN/H2O
O
NH
O O OH 2
MW: 213.23
by-product 3
答 题 时 请 勿 超 越 此 虚 线 , 谢 谢 !
Question 7-1: Please propose the correct structure of the unknown byproduct according to the information supplied and explain the process. (6 points)
药明康德有机合成2013年9月晋升测试题I_Answer

Scheme 5-3: De Novo Arene Synthesis
Question 5: Please provide the structure for Product A. (3 points) Answer:
Question 6: Please provide a stepwise mechanism in the step 6 to form compound 5. (5 points) Answer: Mechanism of the Mukaiyama Aldol Addition
Scheme 5-1: Enantioselective Cyanohydrin Ether Synthesis
Question 1: Please provide a stepwise mechanism for the above transformation. (5 points) Answer:
O O O
O
O OEt
NH2
HN NH2. HOAc
O O O
O
N N
OH
POCl3
O O O
O
N N
Cl
H2N
O
N
O
O
N
O
HN
Erlotinib (TarcevaTM), Genentech
2. Aspidosperma alkaloids represent the largest family of monoterpenoid indole alkaloids. Compound A is an intermediate of (-)-Aspidospermidine. Please design a reasonable synthetic route to prepare Compound A from Compound B and other commercially available reagents. (Ref: Angew. Chem. Int. Ed. 2013, 4117) (20 points)
药物合成考试题及答案

第一章卤化反应试题
一.填空题。
(每空2分共20分)
1.和与烯烃加成属于(亲电 )(亲电or 亲核)加成反应,其过2Cl 2Br 渡态可能有两种形式:①(
桥型卤正离子
)②(
开放式碳正离子 )
2.
在醇的氯置换反应中,活性较大的叔醇,苄醇可直接用(浓HCl
)
或( HCl )气体。
而伯醇常用( LUCas )进行氯置换反应。
3.双键上有苯基取代时,同向加成产物(增多 ){增多,减少,不变},烯烃与卤素反应以( 对向加成
)机理为主。
4在卤化氢对烯烃的加成反应中,HI 、HBr 、HCl 活性顺序为(
HI>HBr>HCl )
烯烃RCH=CH2、CH2=CH2、CH2=CHCl 的活性顺序为(
RCH=CH2>CH2=CH2>CH2=CHCl )
答案:
B
C2H5ONa C2H5OH COOC2H5
Cl
O
以上四个方程式分别属于()A
反应中先加热后水解的终产物是()B.NH2RCHO C.RNH2。
药明康德有机题库

药明康德有机题库一、单项选择题1、有机化合物中,哪个官能团代表羧酸?A. -OHB. -COOHC. -CHOD. -COCl2、下列哪个化合物属于醇类?A. CH3CH2ClB. CH3CH2OHC. CH3COOHD. CH3CHO3、在有机化学中,哪个术语用于描述碳碳双键?A. 烯烃B. 炔烃C. 烷烃D. 芳香烃4、下列哪个反应属于酯化反应?A. 醇与酸的反应B. 醇与碱的反应C. 醇与卤代烃的反应D. 醇与酸的脱羧反应5、哪个化合物是乙酸乙酯?A. CH3COOC2H5B. CH3COClC. CH3CH2OHD. CH3COOH6、在有机反应中,SN1和SN2分别代表什么类型的反应?A. 取代和加成B. 消除和取代C. 亲核取代和单分子取代D. 双分子取代和自由基取代7、下列哪个化合物属于酮类?A. CH3COOHB. CH3COCH3C. CH3CH2OHD. CH3CHO8、有机化合物中的卤代烃可以通过什么反应转化为醇?A. 水解B. 酯化C. 还原D. 氧化9、下列哪个反应是烯烃与溴水的加成反应?A. 取代反应C. 消除反应D. 重排反应10、下列哪个化合物属于芳香烃?A. 苯B. 环己烷C. 丙酮D. 乙酸乙酯11、有机化合物中的醛基可以通过什么反应转化为羧酸?A. 氧化B. 还原C. 水解D. 酯化12、下列哪个化合物属于酯类?A. 乙酸乙酯B. 乙酸C. 乙醇D. 乙醛13、在有机反应中,哪个术语用于描述碳碳三键?A. 烯烃B. 炔烃C. 烷烃14、下列哪个反应是醇的氧化反应?A. 醇与酸的反应B. 醇与卤代烃的反应C. 醇的催化氧化D. 醇的酯化反应15、下列哪个化合物属于醚类?A. CH3OCH3B. CH3COOHC. CH3CH2OHD. CH3CHO16、有机化合物中的羧酸可以通过什么反应转化为酯?A. 酯化反应B. 水解反应C. 还原反应D. 氧化反应17、下列哪个反应是烯烃的聚合反应?A. 加成反应B. 取代反应C. 聚合反应D. 消除反应18、下列哪个化合物属于胺类?B. CH3COOHC. CH3CH2OHD. CH3CHO19、有机化合物中的硝基可以通过什么反应转化为氨基?A. 还原反应B. 氧化反应C. 水解反应D. 酯化反应20、下列哪个反应是羧酸与醇的酯化反应?A. 醇与酸的反应B. 醇与卤代烃的反应C. 醇的催化氧化D. 醇的酯化反应21、下列哪个化合物属于酰胺类?A. CH3COOHB. CH3COClC. CH3CONH2D. CH3CHO22、在有机反应中,哪个术语用于描述碳碳单键?A. 烯烃B. 炔烃D. 芳香烃23、下列哪个反应是醇的脱水反应?A. 醇与酸的反应B. 醇与卤代烃的反应C. 醇的催化脱水D. 醇的酯化反应24、下列哪个化合物属于腈类?A. CH3CNB. CH3COOHC. CH3CH2OHD. CH3CHO25、有机化合物中的醛基可以通过什么反应转化为醇?A. 氧化B. 还原C. 水解D. 酯化26、下列哪个反应是羧酸的脱羧反应?A. 羧酸与醇的反应B. 羧酸与碱的反应C. 羧酸与卤代烃的反应D. 羧酸与酸的反应27、下列哪个化合物属于磺酸类?A. CH3SO3HB. CH3COOHC. CH3CH2OHD. CH3CHO28、有机化合物中的羰基可以通过什么反应转化为羟基?A. 加成反应B. 还原反应C. 氧化反应D. 消除反应29、下列哪个反应是醇与卤代烃的取代反应?A. 醇与酸的反应B. 醇与卤代烃的反应C. 醇的催化氧化D. 醇的酯化反应30、下列哪个化合物属于异氰酸酯类?A. R-NCOB. R-COOR'C. R-CND. R-COOH。
有机合成测试题

有机合成本试卷可能用到的相对原子质量:H 1 C 12 O 16 Na 23第Ⅰ卷(选择题,共48分)一、单项选择题(本题包括8小题,每小题3分,共24分。
每小题只有一个....选项符合题意。
)1.到2008年,北京举办奥运盛会时,将有望用生物芯片这种对兴奋抽查率更高的检测方式,用"科技奥运"净化体育道德。
国际奥委会公布的违禁药物目前有138种,某种兴奋剂的结构如下,关于它的说法中正确的是()A.它的化学式是:C15H18O2NClB.1mol该物质最多能与2molNaOH反应C.从结构上看,它属于芳香烃D.它的分子中所有碳原子共平面2.一氧化碳、烯烃和氢气在催化剂作用下发生烯烃的醛化反应,又叫羰基的合成。
由乙烯可制丙醛:CH2=CH2+CO+H2−−催化剂CH3CH2CHO由丁烯进行醛化反应也可以得到醛,在它的同分−→异构体中,属于醛的有()A.2种B.3种C.4种D.5种3.只用水就能鉴别的一组物质是()A.苯、乙醇、四氯化碳B.乙醇、乙醛、苯磺酸C.乙醛、乙二醇、硝基苯D.苯酚、乙醇、甘油4.下列物质中,分子里的所有原子不可能在同一平面上的是()A.乙炔B.苯甲醛C.异戊二烯D.丙烯醛(CH2=CH—CHO)5.香草醛、本品广泛用于食品、饮料、烟草、酒类、医药、化工和各类化妆用品等,是一种性能稳定、香味纯正、留香持久的优良香料和食品添加剂。
其结构如图所示,下列说法不正确的是()A香草醛的分子式为C7H8O3B加FeCl3溶液,溶液不显紫色C能与NaOH发生中和反应D能发生银镜反应6.洗涤做过银镜反应的试管和制取酚醛树脂的试管,最好选用下列试剂中的()A.浓氨水和酒精B.盐酸溶液和氨水C.稀硝酸溶液和酒精D.烧碱溶液和盐酸7.下列配制银氨溶液的操作中,正确的是()A.在洁净的试管中加入1~2mL AgNO3溶液,再加入过量浓NH3水,振荡,混合均匀B.在洁净的试管中加入1~2mL 稀NH3水,再逐滴加入2% AgNO3溶液至过量C.在洁净的试管中加入1~2mL AgNO3溶液,再逐滴加入浓NH3水至过量D.在洁净的试管中加入2% AgNO3溶液1~2mL,逐滴加入2% 稀NH3水,边滴边振荡,至沉淀恰好溶解时为止8.使用哪组试剂,可鉴别在不同试剂瓶内的1—己烯、甲苯和丙醛。
药明康德有机题库

药明康德有机题库全文共四篇示例,供读者参考第一篇示例:药明康德是一家专注于生物医药研发服务领域的企业,致力于为全球客户提供高质量的研发解决方案。
在为客户提供定制化的服务的过程中,药明康德建立了一个庞大的有机题库,以帮助客户更好地理解有机化学的知识,并提供必要的帮助和支持。
有机化学是理解和掌握生物制药研发过程中必不可少的基础学科之一。
在药明康德的有机题库中,包含了大量涉及有机分子结构、反应机理、合成路线等方面的题目,涵盖了从基础到高级的各个层次。
这些题目既有选择题、填空题、判断题等传统的题型,也有复杂的综合题目,帮助客户全面地了解和掌握有机化学的相关知识。
除了传统的题目外,药明康德的有机题库还包含了大量的案例分析题目,这些题目结合了实际的生物医药研发案例,帮助客户将有机化学知识与实际应用相结合,更好地理解学科的应用场景。
通过这些案例分析题目的练习,客户可以更快地提升自己的解决问题的能力,并在实际工作中更加得心应手。
药明康德的有机题库还提供了丰富的学习资源和辅助工具,例如教学视频、实验模拟等,帮助客户更轻松地学习和掌握有机化学知识。
客户可以根据自己的学习需求和进度随时随地地进行学习,提高学习的效率和质量。
药明康德的有机题库还提供了在线答疑服务,客户可以随时向专业的老师提问,及时得到问题的解答和指导,加深对知识的理解。
药明康德的有机题库致力于为客户提供全方位的有机化学学习解决方案,帮助客户更好地掌握,理解和应用有机化学知识。
通过学习和练习,客户可以不断提升自己的专业技能和解决问题的能力,为未来的职业发展打下坚实的基础。
作为生物医药研发领域的专业服务提供商,药明康德将继续致力于提供更优质、更全面的有机化学学习资源,与客户共同成长,共同进步。
第二篇示例:药明康德有机题库是一个专门为有机化学领域的学习者设计的题库,旨在帮助学生更好地提高对有机化学知识的掌握和理解。
有机化学是一个相对复杂的学科,涉及到许多化学反应和结构的理解,因此需要大量的练习和题目来巩固知识。
药物合成试题及答案1

研发部合成人员转正试题(一)一、单项选择题(每题2分,共40分)1、BP是( A )的英文缩写A 英国药典B 欧洲药典C 美国药典D 日本药局2、中国新药注册分类中3.1类新药是指(B )A 改变给药途径且尚未在国内上市销售的制剂;B 已在国外上市销售但尚未在国内上市销售的原料药及制剂;C 尚未在国内外上市销售的新的复方制剂;D 改变已上市销售盐类药物的酸根、碱基或金属元素,但不改变其药理作用的原料药及制剂。
3、在离子交换色谱法中,为了改变药品的分离选择性,尤其是对分离可电离的有机化合物尤为有利时,此时可加入(B)A 水B 缓冲盐C 有机溶剂D 表面活性剂4、易发生水解反应的药物结构是(D )A 醚B 醛C 含有巯基的药物D 苷类药物5、在人工合成过程中掺入放射性14C的用途是( D )A 催化剂B 媒介质C 组成元素D 示踪原子6、高效液相色谱法的定量方法有( C )和外标法。
A 焰色法B 蛋白沉淀法C 内标法D 酸碱滴定法7、有关影响滤过因素的叙述,错误的是( C )A 滤过面积增大,滤速加快B 降低药液粘度,可加快滤速C常压滤过速度大于减压滤过速度D 增加滤器上下压力差,可加快滤速8、下列关于增加药物溶解度方法的叙述,正确的是( A )A 加助溶剂B 搅拌C 粉碎D 加热9、综合治理“白色污染”的各种措施中,最有前景的是A 填埋或向海中倾倒处理B 热分解或熔融再生处理C 积极寻找纸等纤维类制品替代塑料D 开发研制降解塑料10、关于药物代谢的叙述正确的是( D )A 药物通过吸收、分布主要以原药的形式排泄B 将具有活性的药物经过结构修饰变成无活性的化合物C 增加药物的解离度使其生物活性增强,有利于代谢D 在酶的作用下将药物转化成极性分子,再通过正常系统排泄至体外的过程11、一般而言,采用高效液相色谱法进行药物分析时,精密度的RSD值应小于( C )A 0.5%B 1.5%C 2%D 4%12、药典所指的“精密称定”,系指称取重量应准确至所取重量的(B)A 百分之一B 千分之一C 万分之一D 十万分之一E 百万分之一13、最新版药典USP( B )。
药物合成题库

07/08第二学期药物合成反应试题一、填空题(0.5分×40=20分)1、轨道的节越多,它的能量越 高 。
2、1S 轨道呈 球 对称,对应于氢原子的电子的 最低可能的轨道 。
3、写出以下原子的基态电子结构:Be: 1S22S2 C: 1S22S22Px12Py1 F: 1S22S2SPx22Py22Pz1 Mg: 1S22S22Px22Py22Pz23S2 4、据分子轨道理论,两个原子轨道重叠形成两个分子轨道,其中一个叫成键轨道 ,具有比原来的原子轨道更低的能量;一个叫反键轨道 ,具有较高的能量。
5、场效应和分子的几何构型 有关,而诱导效应只依赖于键 。
6、碳-碳键长随S 成分的增大而变短 ,键的强度随S 成分的增大而增大 。
7、判断诱导效应(-Is )强弱:-F,-I,-Br,-Cl 。
-F>-Cl>-Br>-I 8、判断诱导效应(+Is )强弱:-C(CH 3)3>-CH(CH 3)2>-CH 2CH 3>-CH3。
9、一般情况下重键的不饱和程度愈大,吸电性 愈强 ,即-Is 诱导效应愈大。
10、在共轭体系中,形成共轭体系的原子以及和这些原子直接结合的原子都在同一平面里 ;各个键上的电子云密度在一定程度上都趋于平均化 。
11、布朗斯特酸的定义是给出质子的物质 ,路易斯酸的定义是有空轨道的任何一种物质 。
12、卤素对双键亲电加成的活性顺序是:Cl 2>Br 2>I 2 。
13、卤素对双键亲电加成是按分步机理进行的, X +先对双键做亲电进攻,而后X _再加上去。
14、判断共轭类型,并用箭头标明电子云偏移方向:π-π共轭p -π 共轭p -π 共轭15、溴素或氯对烯烃的加成一般属于 亲电加成 机理。
16、在卤素对烯烃的加成反应中,当双键上有苯基取代时,同向加成的机会 增C(CH 3)3CH 2CH 3CH 3CH(CH 3)2C H CH 2CH 2H 2CH加 。
药明康德化学合成部2012年3月晋升测试题II_Answer

1.3 组成有机化合物的原子许多有同位素,所以在质谱中会出现不同质量的同位素峰。 有机物分子中同时含一个溴一个氯时,在质谱中会出现明显同位素峰的比例是 ( D )
A. 1: 2: 1; B. 9: 6: 1; C. 9: 3: 1; D. 3: 4: 1
第 1 页 共 12 页
2012 年 3 月药明康德化学合成部晋升测试(II)试题
2H 1H 2H
O
1H
2H 2H
第 7 页 共 12 页
2012 年 3 月药明康德化学合成部晋升测试(II)试题
四、机理 (10 分)
4.1 请写出 Mitsunobu 的反应通式及机理 (5 分)
4.2 请画出 Sonogashira coupling 的反应通式及机理(5 分)
带格式的: 行距: 单倍行距
反应完成后,将反应混合物冷却至-10 ~ 0 ℃,搅拌下,缓慢滴加1 mL 的水猝灭反应。完毕,再缓慢 滴加滴 1 mL 的1 0%NaOH 溶液。滤除产生的固体,用反应溶剂洗涤数次,滤液减压浓缩即得产品。 必要时,固体可用乙醇或甲醇浸泡洗涤,浓缩收更多的产品(此时多半含无机盐)。一般情况下,加完 后,沙状固体悬浮于反应溶剂中,非常容易过滤。有时在加水过程中,反应物较为粘稠,可适当加入 一些反应溶剂稀释。或用10水硫酸钠后处理
4-氯吡啶的活性也更高. D. Pd 直接插入 C-X 健的过程中, 反应的活性大小次序为: X=I>Br~OTf>Cl>F
1.12 按照药明康德公司安全监查部规定,无水溶剂允许存放期限为(D)
A:1 天 B:3 天
C:5 天 D:7 天
1.13 NaH、Na、LiAlH4 着火了,只能用哪种灭火器材?(A)
有机合成工(高级)理论试卷A和答案.doc

江阴市职业技能鉴定试题 有机合成工(高级)理论知识试卷A 注意事项 1、 本试卷依据《有机合成丄》行业标准命制,考试时间:60分钟。
2、 请在试卷标封处填写姓名、准考证号和所在单位的名称。
3、 请仔细阅读答题耍求,在规定位置填写答案。
一、 判断题(将判断结果填入括号中。
正确的填“V”,错误的填“X”。
) (X )1、由于KMnO4具有很强的氧化性,所以KMnO4法只能用于测定还原性物质。
(X )2、原子吸收法是根据基态原子和激发态原子对特征波长吸收而建立起來的分析方 法。
(X )3、为保证安全,在给焊炬点火时,最好先开氧气,点燃后再开乙炔。
(V )4、限制火灾爆炸事故蔓延的措施足分区隔离、配置消防器材和设S 安全阻火装S 。
(X )5、绝热过程都是等熵过程。
(V )6、以石墨为电极,电解氯化铜水溶液,阴极的产物是铜。
(X )7、单环芳烃类奋机化合物-•般情况下与很多试剂易发生加成反应,不易进行取代反 应。
(X )8、硝基属于供电子基团。
(X )9、丁苯橡胶的单体是丁烯和苯乙烯。
(7 )10、普通的衣物防皱整理剂含有甲醛,新买服装先用水溃洗以除掉残留的甲醛。
(X )11、冷热流体进行热交换吋,流体的流动速度越快,对流传热热阻越人。
(X )12、全回流吋理论塔板数最多。
(V )13、化工管路中通常在管路的相对低点安装冇排液阀。
(X )14、离心泵开车之前,必须打开进口阀和出口阀。
(V )15、层流内层影响俾热、传质,其厚度越大,传热、传质的町力越大。
(V )16、根据相平衡理论,低温高压有利于吸收,因此吸收压力在一定范围内越高越好。
(V )17,吸收操作屮,所选用的吸收剂的粘度要低。
(X )18、利用萃取操作可分离煤汕和水的混合物。
(V )19、蒸馏是以液体混合物中各组分挥发能力不M 为依裾,仙进行分离的一种操作。
(X )20、萃取和精馏的分离对象相同,而分离的原理不M)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Question 4: What effect is responsible for the high diastereoselectivity observed in the methylation of ketone 6? (3 points)
答 题 时 请 勿 超 越 此 虚 线 , 谢 谢 !
4. Please review the below synthetic scheme for the first total synthesis of (±)-oxerine and answer the
related questions. (Ref: Tetrahedron, 1994, 50, 13575.)
Question 3:
When the mixture of 4A & 4B react with P-tolSH, only 5A was obtained as major product.
Compound 4B was observed to be converted into 4A under this basic condition.
(14 points)
[Step a] ?
N
Br
N
1
O
b. NaBH4, MeOH c. PhCH2Br, NaH
OTHP d. Pd ()), TMSC CH
Br 2
OBn OTHP
N
( )-3
TMS
e. p-TsOH, MeOH f. TBAF, THF
OBn
g. PPh3, NBS, CH2Cl2
1. Crizotinib (PF-02341066) is a potent and selective c-MET/ALK dual inhibitor. It has gained FDA
姓
approval for ALK-positive cancers. Please design reasonable synthetic route to prepare racemic
谢
!
Question 4: Please propose the mechanism for step 9;
(5 points)
答
题
时
请
勿
6. Please review the below synthetic scheme and answer the related questions.
超
(Ref: J. Org. Chem., 2012, 77, 4017)
5. Please review the below synthetic scheme and answer the related questions.
(Ref: Org. Lett., 2012, 14, 4886)
(18 points)
OTES
O
H CO2Et
1
L-Selectride THF, - 80 oC
2)异吲哚啉
3)环加成
4)亲核试剂
5)硼氢化钠
6)脱水
7) 偶合常数
答
8)核磁共振题Fra bibliotek时请
勿
超
越
此
虚
线
, 谢
谢
!
2. Lorcaserin, a selective Serotonin 5-HT2c Receptor agonist, was approved by FDA for the treatment of Obesity in 2012. Dr. Graeme Semple (Vice President of Arena Pharmaceuticals) discussed in details about the discovery, SAR and synthesis of Lorcaserin during his visiting seminar at WuXi. Please answer below questions. (15 points)
Please provide a rationale on how this racemization occurred
(5 points)
答
题
时
请
勿
超
越
此
虚
线
, 谢
谢
Question 4: Please provide the structure of compound 7.
!
(3 points)
(12 points)
越
此
虚
线
, 谢
谢
!
Question 1: Please provide reaction conditions for Steps 2 and 3;
(4 points)
Question 2: Please explain the stereo-selectivity for Step 2;
Question 1: Design a reasonable synthesis route for Lorcaserin started from compound 1 (10 points)
答
题
时
请
勿
超
越
此
虚
Question 2: Describe the strategy & methods to obtain enantiomeric pure Lorcasrin in
Step 1
OTES H CO2Et 4
1. Swern oxidation 2. MePPh3Br, nBuLi
Step 4/5
NaHMDS, CS2 MeI, THF, - 78 oC
Step 2 2
1. DiBAL-H, DCM
H CO2Et 5
2. Swern oxidation 3. CH2=CHMgBr, DCM
线
manufacturing scales
(5 points)
,
谢
谢
!
3. During his WX visit, Professor Yujiro Hayashi discussed many new methodologies developed in his group and their applications in the natural product synthesis (WX Science Seminar series #9, Nov 02th 2012). Please study below scheme and answer the related questions. (18 points)
Scheme 3-1: High Efficient Synthesis of (-)-oseltamivir
答
题
时
请
Question 1: The author used catalyst 1 in the preparation of enantiomeric enriched compound 2.
OH h. SmI2, THF/HMPA, -78oC
N
N
4
OBn 5 CH2
i, O3
OBn
N O
6
j, MeMgBr
OBn
答
N
( )-Oxerine
题
HO CH3
时
( )-7
请
勿
超
Question 1: What’s the condition for Step a? (3 points)
越
此
虚
(4 points)
答
Question 3: Please draw mechanism for Step 5;
(4 points)
题
时
请
勿
超
越
此
虚
线
, 谢
谢
!
7. English (8 points) Please translate following words to english
1)重结晶
WuXi AppTec Promotion Exam
(for Assistant Director and above)
March 1, 2013
Note: 1. the answers for >85% of the questions can be found from company training website; 2. 30% of the questions are from Science Seminars.
Step 6/7/8
Bu3SnH, AIBN Toluene, refulx
Step 3 3
H OH
6
Grubbs II catalyst Step 9
H OH 7
Dess-martin oxidation Step 10
HO 8
Question 1: Please draw the products from steps 1 and 2;
(4 points)
Question 2: Please explain the stereochemistry of step 1;
(4 points)
答
题
时
请
勿
超
越
Question 3: Please propose the mechanism for step 3;
(5 points)
此
虚
线
, 谢
勿
Please provide an explanation on the origin of enantioselectivity
