氧化铝颗粒的表面改性及其在C平面(0001)蓝宝石衬底上的化学机械抛光(CMP)性质
LED用蓝宝石衬底抛光技术进展_卓志国

磁流变液是一种新型的可控智能材料,是由磁敏微粒、表面 活性剂以及其他一ቤተ መጻሕፍቲ ባይዱ添加剂按一定比例分散在基载液中形成的 悬浮液。当抛光液被喷洒至转轮的凹槽,并随着凹槽的转动进入 磁场区时,磁敏微粒被磁化,成链状或纤维状排列,这导致整个流 体的粘度增大,从而表现出类固体性质对晶片进行塑性切削加 工,随后又恢复液体状态,如图 1 所示。由于磁流变抛光属于可控
抛光主要以化学和机械作用将晶片表面材料去除,以获得 光洁、无晶格缺陷的表面,在这些加工方法中有的以机械作用为 主,如机械抛光、液体喷射抛光;有的以化学作用为主,如化学抛 光、水合抛光;或化学机械相平衡起作用,如化学机械抛光。
2 以机械作用为主的抛光方法
传统的抛光以机械抛光为主,主要采用比晶片材料硬的物
1 引言
单晶蓝宝石具有优良的物理和化学性质,如耐磨损、抗化学 腐蚀、光透过率高等,广泛用于光学透镜,同时蓝宝石单晶因为与 半导体 GaN 的晶格系数失配率较小、机械强度高、价格便宜等成 为发光二极管的主要衬底材料。而 GaN 膜的无缺陷生长很大程 度上依赖于蓝宝石晶片的表面加工质量,但高硬度和高化学稳定 性导致其难以加工。为获得无损伤层的晶片表面,人们提出各种 加工工艺以期能够获得更高的加工速率、加工质量,降低加工成 本,其中有些已经应用于大规模生产加工,有的还处于实验室阶 段。而在蓝宝石晶片的超精密加工中以抛光最为常用。
1991年ibm首次将化学机械抛光技术成功应用到dram的生产中后cmp引人注目地得到用其他任何平面化加工不能得到的表面形貌变化化学机械抛光时旋转的工件以一定的压力压在旋转的抛光垫上由亚微米或纳米磨粒和化学溶液组成的抛光液在晶片与抛光垫之间流动抛光液在抛光垫的传输和旋转离心力的作用下均匀分布其上在晶片和抛光垫之间形成一层液体薄膜液体中的化学成分与晶片产生化学反应生成一层比原材料软的反应层然后通过磨粒的微机械摩擦将这些化学反应物从蓝宝石晶片表面去除随流动的液体带走即在化学成膜和机械去膜的交替过程中实现超精密表面加工
氧化铝纳米颗粒表面修饰及其应用研究

氧化铝纳米颗粒表面修饰及其应用研究氧化铝是一种广泛应用的功能性材料,常用于制备陶瓷材料、涂层、催化剂等。
随着纳米技术的发展,氧化铝纳米颗粒的应用越来越广泛。
在实际应用中,氧化铝纳米颗粒的表面性质对其性能有着很重要的影响。
因此,对氧化铝纳米颗粒表面进行修饰,成为了研究热点之一。
本文将介绍氧化铝纳米颗粒表面修饰及其应用研究的相关进展。
1. 氧化铝纳米颗粒表面修饰方法氧化铝纳米颗粒的表面修饰方法可以分为物理方法和化学方法两种。
1.1 物理方法物理方法是指通过物理手段改变氧化铝纳米颗粒表面结构的方法。
常见的物理方法包括离子束照射、等离子体处理、激光辐照等。
离子束照射是通过离子束轰击氧化铝纳米颗粒表面,使其发生化学反应,从而改变表面性质。
等离子体处理和激光辐照则是通过热、光等作用,改变氧化铝纳米颗粒表面结构。
1.2 化学方法化学方法是指通过化学反应改变氧化铝纳米颗粒表面结构的方法。
常见的化学方法包括溶胶-凝胶法、水热法、化学气相沉积法等。
溶胶-凝胶法是一种将溶胶浸泡在胶体中,通过溶胶与胶体分子相互作用形成氧化铝纳米颗粒的方法。
水热法是将胶体溶液加热至高温高压条件下形成氧化铝纳米颗粒的方法。
化学气相沉积法则是将氧化铝前体沉积在纳米颗粒表面的方法。
2. 氧化铝纳米颗粒表面修饰的应用研究进展氧化铝纳米颗粒表面修饰后,可以用于制备催化剂、涂层、传感器等材料。
以下是一些应用研究的进展。
2.1 催化剂改变氧化铝纳米颗粒表面的化学性质,可以调节其催化活性。
例如,对表面进行氢气还原可使氧化铝纳米颗粒表面亲水性增强,从而提高其选择性催化还原CO2的能力。
同时,还发现通过表面修饰后的氧化铝纳米颗粒可以用于制备光催化剂、催化剂载体等。
2.2 涂层将氧化铝纳米颗粒表面修饰后,可以用于制备涂层材料。
例如,将表面修饰后的氧化铝纳米颗粒与树脂或涂料复合,可以得到具有高硬度、高耐磨性、抗腐蚀性好等特性的涂层材料。
2.3 传感器表面修饰后的氧化铝纳米颗粒可用于制备气敏传感器、生物传感器等。
蓝宝石化学机械抛光专利技术分析
工艺与装备163蓝宝石化学机械抛光专利技术分析高玉江刘洋刘腾达李春雨(国家知识产权局专利局专利审查协作天津中心,天津300304)摘要:本文首先对蓝宝石化学机械抛光专利申请状况进行概述,然后重点从蓝宝石化学机械抛光装置结构 抛光垫、修整器、C M P停止、抛光液、蓝宝石应用及加工工艺等方面,分析蓝宝石C M P专利技术。
关键词:蓝宝石化学机械抛光C M P专利引言蓝宝石(S a卯h i r e)的主要成分为单晶氧化铝(a-A l2〇3),具有高硬度、高熔点、耐磨损、透光性好、电绝缘性优良和化学性能稳定的特点,被广泛应用于医学、工业、国防、航空航天及生活领域,特别适于作为L r o衬 底材料。
同时,由于蓝宝石优异的机械性能,它也逐渐被 用于i P h o n e、i W a t c h等显示面板上。
化学机械抛光(C h e m i c a l M e c h a n i c a l P o l i s h i n g,简 称C M P)技术是机械削磨和化学腐蚀的组合技术。
它借助超 微粒子的研磨作用和浆料的化学腐蚀作用,在被研磨的介质 表面形成光洁平坦的平面。
化学机械抛光是目前唯一可以实 现全局平坦化的抛光方法,现己成为半导体加工行业的主导 技术,同时也成为对蓝宝石表面进行平坦化的主要加工方法。
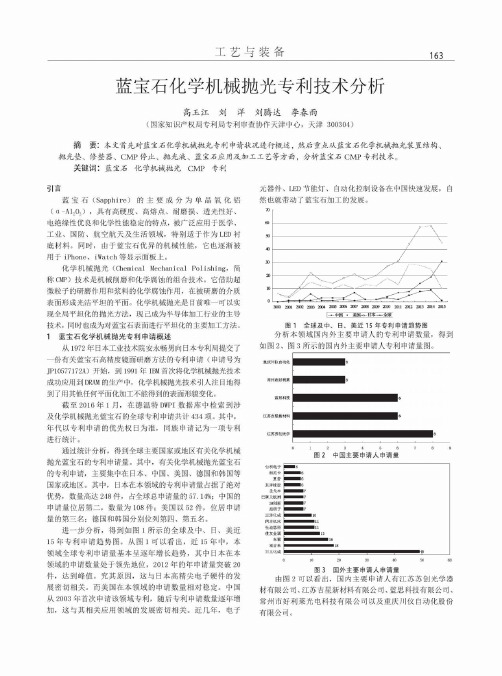
1蓝宝石化学机械抛光专利申请概述从1972年日本工业技术院安永畅男向日本专利局提交了 一份有关蓝宝石高精度镜面研磨方法的专利申请(申请号为 J P1*******A)开始,到1991年I B M首次将化学机械抛光技术 成功应用到D R A M的生产中,化学机械抛光技术引人注目地得 到了用其他任何平面化加工不能得到的表面形貌变化。
截至2016年1月,在德温特D W P I数据库中检索到涉 及化学机械抛光蓝宝石的全球专利申请共计434项。
其中,年代以专利申请的优先权日为准,同族申请记为一项专利 进行统计。
通过统计分析,得到全球主要国家或地区有关化学机械 抛光蓝宝石的专利申请量。
蓝宝石衬底CMP中氧化硅磨粒粒度分布对抛光液体系性能影响研究

蓝宝石衬底CMP中氧化硅磨粒粒度分布对抛光液体系性能影响研究王晓剑;李薇薇;钟荣锋;肖银波;许宁徽;孙运乾【期刊名称】《表面技术》【年(卷),期】2024(53)2【摘要】目的化学机械抛光(CMP)包含化学腐蚀和机械磨削两方面,抛光液p H、磨粒粒径和浓度等因素均会不同程度地影响其化学腐蚀和机械磨削能力,从而影响抛光效果。
方法采用30~150 nm连续粒径磨粒抛光液、120 nm均一粒径磨粒抛光液、50 nm和120 nm配制而成的混合粒径磨粒抛光液,分别对蓝宝石衬底晶圆进行循环CMP实验,研究CMP过程中抛光液体系的变化。
结果连续粒径磨粒抛光液中磨粒大规模团聚,满足高材料去除率的抛光时间仅有4 h,抛光后的晶圆表面粗糙度为0.665 nm;均一粒径磨粒抛光液中磨粒稳定,无团聚现象,抛光9 h内材料去除率较连续粒径磨粒抛光液高94.7%,能至少维持高材料去除率18 h,抛光后的晶圆表面粗糙度为0.204 nm;混合粒径磨粒抛光液初始状态下磨粒稳定性较高,抛光9 h内材料去除率较连续粒径磨粒抛光液高114.8%,之后磨粒出现小规模团聚现象,后9h材料去除率仅为均一粒径磨粒抛光液的59.6%,18 h内材料去除率仅为均一粒径磨粒抛光液的87.7%,但抛光后的晶圆表面粗糙度为0.151 nm。
结论一定时间内追求较高的材料去除率和较好的晶圆表面粗糙度选用混合粒径磨粒抛光液,但需要长时间CMP使用均一粒径磨粒抛光液更适合,因此,在工业生产中需要根据生产要求配合使用混合粒径磨粒抛光液和均一粒径磨粒抛光液。
【总页数】8页(P168-174)【作者】王晓剑;李薇薇;钟荣锋;肖银波;许宁徽;孙运乾【作者单位】河北工业大学电子信息工程学院;广东惠尔特纳米科技有限公司【正文语种】中文【中图分类】TG175;TG356.28【相关文献】1.蓝宝石衬底CMP中温度控制对速率的影响2.纳米碳化硅抛光液的制备及其对蓝宝石晶片抛光性能的研究3.混合磨粒CMP抛光液的制备及抛光效果研究4.单晶蓝宝石衬底磨粒抛光技术研究现状5.硅衬底超精密CMP中抛光液的研究因版权原因,仅展示原文概要,查看原文内容请购买。
氧化铝复合磨粒的抛光特性研究

Ab ta tI r e o e h n e te d s eso tb l y o l mia a rsv n r v n g lmeain i h mia — sr c :n od rt n a c h ip rin sa ii fau n a ie a d p e e ta go rto n c e c lme t b
雷 红 卢 海参 严 琼林 褚 风灵 丘海能
( . 上 海 大 学 纳 米 中心 1 上海 20 4 ;2 深圳 开 发磁 记 录股 份 有 限 公 司 04 4 . 广 东 深圳 5 8 3 ) 10 5
摘要:为提高氧化铝磨料分散稳定性 ,利用接枝聚合对氧化铝粒子进行 了表面改性 ,并研究 了改性后氧化铝粒子在
hn efr n e fh rprdau n o p st a rsv ndgt o at i C ig p r ma c s epe ae lmiac m oi baieo i a cmp c s o ot e i l d c( D)gassb t t w r n et a ls u sr e eeiv s g — a i
i dc tst a lmi ap rilswi atn d f ain h v etrd s e in sa i t h n t a n d f d. h o i— n ia e h tau n a ce t g f ig mo ii t a e b te ip r o tbl y t a h tu mo ii T e p l t hr c o s i e s
蓝宝石衬底研磨抛光方法

蓝宝石衬底研磨抛光方法The polishing and grinding of sapphire substrates is a crucial step in the production of high-quality sapphire wafers for use in various electronic and optoelectronic applications. 蓝宝石衬底的研磨抛光是生产高质量蓝宝石晶片的关键步骤,用于各种电子和光电应用。
This process involves the use of abrasive materials and precision equipment to achieve a smooth, flat surface that is free from defects and impurities. 这个过程涉及使用磨料和精密设备,以达到光滑、平整的表面,并且没有缺陷和杂质。
Sapphire substrates are known for their hardness and durability, which makes them challenging to polish and grind effectively. 蓝宝石衬底以其硬度和耐久性而闻名,这使得它们在研磨和抛光方面具有挑战性。
The process requires careful control of parameters such as pressure, speed, and the type of abrasive material used to achieve the desired surface finish. 这个过程需要仔细控制压力、速度和使用的磨料类型等参数,以达到期望的表面光洁度。
One of the key considerations in sapphire substrate polishing and grinding is the choice of abrasive materials and the techniques usedto apply them. 在蓝宝石衬底的研磨抛光中,一个关键考虑因素是选择磨料材料和应用它们的技术。
氧化铝纳米颗粒的制备及其表面改性研究

氧化铝纳米颗粒的制备及其表面改性研究随着生活水平的提高和科技的不断发展,纳米材料的研究与应用变得越来越重要。
氧化铝纳米颗粒作为一种重要的纳米材料,具有广泛的应用前景,如陶瓷材料、催化剂、纳米生物材料等领域。
本文将介绍氧化铝纳米颗粒的制备方法和表面改性研究,以期对该材料的研究和应用提供一定的参考价值。
一、氧化铝纳米颗粒的制备方法氧化铝纳米颗粒的制备方法主要包括物理法、化学法和生物法三种。
其中,物理法包括溅射法、电弧法、激光烧结法等;化学法包括溶胶-凝胶法、水热法、水热合成法等;生物法则包括微生物法、植物法、动物法等。
通过不同的制备方法,可以获得不同形态和大小的氧化铝纳米颗粒。
以化学法制备氧化铝纳米颗粒为例,下面介绍一种常见的方法——溶胶-凝胶法。
该方法的步骤包括:制备氧化铝溶胶;将溶胶转化为凝胶;将凝胶进行热处理,得到氧化铝纳米颗粒。
其中,制备氧化铝溶胶的方法主要是通过水热、超声等方法进行制备,一般使用氧化铝前驱体(如AlCl3、NaAlO2)和适量的碱性物质(如NaOH、NH3)反应得到。
得到的氧化铝溶胶可以通过离心等方法得到相应的白色沉淀。
将氧化铝溶胶转化为凝胶的方法主要是通过脱水水解实现。
一般来说,制得的氧化铝溶胶和稀硝酸或其他脱水剂混合。
混合物在室温下搅拌均匀,形成黑色凝胶。
最后是热处理步骤。
将制备好的氧化铝凝胶放入干燥器中,经过蒸发方法除去水分。
再将干燥后的凝胶放在热炉中,进行升温处理。
在高温下,凝胶会分解,形成氧化铝纳米颗粒。
这种方法所得到的氧化铝纳米颗粒具有较高的结晶度和分散性,是一种常用的制备方法。
二、氧化铝纳米颗粒的表面改性研究氧化铝纳米颗粒的表面改性研究旨在改善其特性和应用。
常用的表面改性方法包括包覆法、改性剂法和上转换法等。
包覆法是将氧化铝纳米颗粒表面包覆一层其他材料,如氧化钛、二氧化硅等材料,以获得更好的性能。
包覆后,氧化铝纳米颗粒的比表面积会减小,表面活性也会得到降低,但表面稳定性和化学反应性会有所提高。
超细氧化铝表面改性及其抛光特性

L ih n ' L i n Z a g Zea g ' Xio Ba q u Has e e Ho g h n fn a o i '
( . eerhC n r f a osi c n aot h o g ,hnh i nvr t,h nh i 0 44, h a 1R sac et n ・ e ea dN n— c nl y S aga U i sy S aga 2 04 C i ; eoN cn e o ei n
2 S h o fE vr n n a n h mia n i e r g。 h n h iUn v ri , h n h i 01 0 C i a . c o l n i me tl d C e c lE g n ei S a g a ie st S a g a 8 0, h n ; o o a n y 2
蓝宝石衬底的化学机械抛光工艺研究

蓝宝石衬底的化学机械抛光工艺研究
赵之雯
【期刊名称】《纳米科技》
【年(卷),期】2007(004)005
【摘要】系统地分析了蓝宝石抛光工艺过程的性能参数,通过大量实验总结出了其影响因素并提出了优化方案.结果表明,采用粒径为40nm、低分散度的SiO2溶胶磨料并配合以适当参数进行抛光,可以获得良好的表面状态和较高的去除速率,能够有效提高蓝宝石表面的性能及加工效率.
【总页数】4页(P45-48)
【作者】赵之雯
【作者单位】福建工程学院电子信息与电气工程系,福建,福州,350014
【正文语种】中文
【中图分类】TB34;TQ02
【相关文献】
1.蓝宝石衬底的化学机械抛光工艺研究 [J], 赵之雯
2.蓝宝石衬底的化学机械抛光工艺研究 [J], 赵之雯
3.氧化铝颗粒的表面改性及其在C平面(0001)蓝宝石衬底上的化学机械抛光(CMP)性质 [J], 汪为磊;刘卫丽;白林森;宋志棠;霍军朝
4.用于GaN生长的蓝宝石衬底片化学机械抛光工艺研究 [J], 赵之雯
5.单晶蓝宝石衬底晶片的化学机械抛光工艺研究 [J], 余青;刘德福;陈涛
因版权原因,仅展示原文概要,查看原文内容请购买。
蓝宝石衬底材料CMP抛光工艺研究

目前国内蓝宝石批量生产的技术还很不成熟, 在生产蓝宝石衬底片的时候产生裂痕和崩边现象比 例较高,占总数的5%-8%,在之后的研磨工序完 成以后,检查发现有50%的宝石片上有短而粗的 加工痕迹在抛光时无法抛掉,并且很多经过加工之 后的蓝宝石片由于表面划痕较重,有Z0%左右的 宝石片表面有粗深划痕[830-12],需要重新研磨抛 光,从而导致返工,而部分经过返工的蓝宝石片由 于研磨抛光过度,导致厚度过薄而报废,这样就大 大提高了蓝宝石衬底片加工的成本。目前国际七的
水平是,去除速率低于1斗n以;表面粗糙度达到 0.4m左右”1;平整度小于5¨m Eg]。
尽管CMP技术发展的速度很快,但它们需要 解决的理论及技术问题还很多。如人们对诸如抛光 参数(如压力、转速、温度等)对平面度的影响、 抛光垫.浆料-片子之问的相互作用、浆料化学性质 (如组成、pH值、颗粒度等)对各种参数的影响等 比较基本的基础机理了解甚少,因而定量确定最佳 CMP工艺,系统地研究CMP工艺过程参数,建立 完善的CMP理论模型,满足各种器件对CMP工艺 的不同要求,是研究CMP技术的重大课题;另外 片子表面残留浆料的清除也是CMP后清洗的主要 课题,研制合适的CMP工艺、设备及抛光液以使 去除速度高而稳定、片子的膜内均匀性和片内均匀 性都理想,而且产生的缺陷少,是CMP技术发展
Abstract:Chemical mechanical polishing(CMP)technique of sapphire substrate was introduced, and the foreground application of sapphire substrate,the problems existed in the CMP technique weIe summarized.and the factors that influence the CMP process were summarized.The capability parameters and the itffluence factors of the sapphire CMP technique were systematically analyzed. Feasible pmjcots of using abrasive of large particle size and low dispersity,appending the organic alkali and surfactant were suggested.It could advance the surface performance and machining efficiency of sapphire substance effectively.
氧化铝表面特性改性的方法[发明专利]
![氧化铝表面特性改性的方法[发明专利]](https://img.taocdn.com/s3/m/c7421cff55270722182ef735.png)
专利名称:氧化铝表面特性改性的方法
专利类型:发明专利
发明人:B·巴鲁瓦蒂,S·马哈帕特拉,A·萨卡,Y·索马斯森达申请号:CN201580005678.1
申请日:20150116
公开号:CN105980044A
公开日:
20160928
专利内容由知识产权出版社提供
摘要:本发明公开了一种对氧化铝的表面特性进行改性的方法,所述方法包括以下步骤:(i)形成水性混合物,该水性混合物包含一重量份的氧化铝,以及相当于0.001至0.1重量份铝含量的铝单体或聚合化合物;(ii)使所述混合物平衡至少五分钟;(iii)将混合物的pH提高到6至8;和(iv)干燥所述混合物,其中当所述铝化合物为单体时,所述方法包括在步骤(i)和(ii)之间将混合物的pH提高到3.0至5.0的额外步骤。
申请人:荷兰联合利华有限公司
地址:荷兰鹿特丹
国籍:NL
代理机构:中国专利代理(香港)有限公司
更多信息请下载全文后查看。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
氧化铝颗粒的表面改性及其在C平面(0001)蓝宝石衬底上的化学机械抛光(CMP)性质汪为磊;刘卫丽;白林森;宋志棠;霍军朝【摘要】为了提高氧化铝颗粒的CMP性能,本工作探索了一种合适的改性方法.同时,为了改善其化学机械性能,通过与其表面羟基的硅烷化化学反应和与Al和仲胺的络合两种作用,用N-(2-氨基乙基)-3-氨基丙基三甲氧基硅烷表面改性氧化铝颗粒.本工作给出了化学反应机理,即N-(2-氨基乙基)-3-氨基丙基三甲氧基硅烷接枝到氧化铝表面.通过傅里叶变换红外光谱(FTIR)和X射线光电子能谱(XPS)表征了改性氧化铝颗粒的组成和结构.结果表明:N-(2-氨基乙基)-3-氨基丙基三甲氧基硅烷已被成功地接枝到氧化铝颗粒的表面,导致改性比未改性的氧化铝颗粒具有更好的化学和机械性能.测试了未改性和改性的氧化铝颗粒在蓝宝石基底上的CMP性能.结果显示:改性氧化铝颗粒比未改性氧化铝颗粒有更高的材料去除速率和更好的表面质量.即,改性氧化铝颗粒在pH=10时比未改性氧化铝颗粒在pH=13.00时表现出更高的材料去除率,这将为减少设备腐蚀提供新思路.%To improve the Chemical Mechanical Polishing (CMP) performance of alumina particles in aqueous solu-tion, a suitable modification method was explored. Meanwhile, in order to improve their chemical mechanical per-formance, alumina particles were surface modified with N-(2-aminoethyl)-3-aminopropyltrimethoxysilane through si-lanization chemical reaction with their surface hydroxyl groups and complexation with Al and secondary amine. This work gives a detailed and thorough chemical reaction mechanism that N-(2-aminoethyl)-3-aminopropyltrimethoxysilane grafted onto the surface of alumina. The compositions and structures of themodified alumina particles were character-ized by Fourier transform infrared spectroscopy (FTIR), and X-ray photoelectron spectroscopy (XPS). The results supported that the N-(2-aminoethyl)-3-aminopropyltrimethoxysilane was perfectly grafted onto the surface of alumina particles, which led to the modified alumina particles with better surface chemical and mechanical properties than un-modified alumina particles. Then, CMP performance of the unmodified and modified alumina particles on the sapphire substrate was tested. The results showed that the modified alumina particles exhibited higher material removal rate (MRR) and better surface quality than unmodified alumina particle. The focus is that the modified alumina particles manifested higher MRR at pH 10.00 than the unmodified alumina particles at PH 13.00, which may open a way to reduce corrosion of equipment.【期刊名称】《无机材料学报》【年(卷),期】2017(032)010【总页数】6页(P1109-1114)【关键词】改性方法;N-(2-氨基乙基)-3-氨基丙基三甲氧基硅烷;化学机械抛光;CMP;氧化铝抛光液【作者】汪为磊;刘卫丽;白林森;宋志棠;霍军朝【作者单位】中国科学院上海微系统与信息技术研究所, 信息材料国家重点实验室, 纳米技术实验室, 上海 200050;中国科学院大学, 北京100049;上海新安纳电子科技有限公司, 上海 201506;中国科学院上海微系统与信息技术研究所, 信息材料国家重点实验室, 纳米技术实验室, 上海 200050;上海新安纳电子科技有限公司, 上海201506;上海新安纳电子科技有限公司, 上海 201506;中国科学院上海微系统与信息技术研究所, 信息材料国家重点实验室, 纳米技术实验室, 上海 200050;上海新安纳电子科技有限公司, 上海 201506;中国科学院上海微系统与信息技术研究所, 信息材料国家重点实验室, 纳米技术实验室, 上海 200050;上海新安纳电子科技有限公司, 上海 201506【正文语种】中文【中图分类】TQ174Smart phones, tablet computers and other mobile terminal have entered lato thousands of families. For example, smart phones, with the progressof society and the development of communications, are also developing rapidly. As an integral part of mobile phone, the phone screen is the large size, high definition and high scratch resistance. At present, compared to the glass material which is mainly applied in the mobile phone screen plate, sapphire material MOHS's hardness is up to nine, whose hardness and scratch resistance are three times as much as the IPHONE with corning gorilla glass. Its unique physical and optical properties make it a choice of mobile terminal window material optimization and upgrading. In addition, with the rapid development of light emitting diodes (LED), sapphire substrate material has been given more attention. Single crystal sapphire has vastly applied as a substrate in gallium nitride (GaN) based LED which has good heat stability and chemical and mechanical properties[1-3]. The surface quality of sapphire has a vast important role in the performance ofLED devices, so that the surface of sapphire is required to be smooth and have no defects. In order to reach this goal, chemical mechanical polishing (CMP) is the only accepted global planarization technology for sapphire. Alumina, as an abrasive, has been used in sapphire substrate chemical mechanical polishing (CMP) for many years[4-6]. The alumina powder used for polishing actually has a same material with sapphire, except that the difference between polycrystalline and single crystals. However, so far, α- Al2O3 slurry is not used on a large scale on the polishing of sapphire. In the process of dispersion, Al2O3 particles are easily agglomerated, and these aggregates hard and compact and it is difficult to effectively obtain dispersion in the polishing slurry. As a result, agglomeration of alumina particles in the polishing process can easily produce scratches. These shortcomings seriously hamper the alumina polishing slurry in the application of sapphire precision polishing. In this work, to improve the CMP performance of alumina particles in aqueous solution, a modi- fication method, as a suitable method, was explored.Alumina polishing slurry was divided into alkaline and acidic polishing liquid. Compared with the alkaline pol- ishing slurry, acidic polishing slurry on equipment corro- sion is more serious, so the markets are mainly composed of alkaline polishing slurry. Yet for alkaline alumina pol- ishing slurry, alkalinity for alkaline polishing slurry has played a very important role, so the pH is 12.00 (from market research) above to boost material removal rate, which will seriously damage the equipment. So the prepa- ration of a kind of low alkaline alumina polishing slurry has become a newtopic. Surface modification[7-11] became a preferred choice. Shen, et al[11] employed Υ-aminoprop- yltriethoxysilane (APS) to modify magnetite particles. Zhang, et al[12] used Υ-aminopropyltriethoxysilane to modify ceria particles and investigated its CMP perform- ance. Tang, et al[7] modified zinc oxide (ZnO) particles with polymethacrylic acid (PMAA) in aqueous solution system. The dispersion stability of ZnO particles was cru- cially improved due to the introduction of grafted polymer on the surface of particles. Lei, et al[8–10] modified alumina particles with silica or polymer obtaining a series of modi- fied alumina particles and studied their CMP performance. As a silane coupling agent, N-(2-aminoethyl)-3-amino- propyltrimeth-oxysilane for double amino functional si- lane can change the material surface properties. In the present study, in order to reduce the alkaline of alumina slurry without lowering chemical mechanical polishing performance, we used N-(2-aminoethyl)-3-aminopropyltr- imethoxysilane to modified alumina particles. We studied the reaction mechanism of the specific modification and established a model of rationalization modification reactions, and investigated its CMP performance on c-plane (0001) sapphire substrate.N-(2-aminoethyl)-3-aminopropyltrimethoxysilane and KOH was purchased from Sinopharm Chemical Reagent Co., Ltd.. Alumina particles powder with a Primary parti- cle diameter of about 40 nm was purchased from Nan- jing new materials Co., Ltd.. All the chemicals were AR.In the work, the modified alumina particles were pre- pared in two steps. First, the N-(2-aminoethyl)-3-amino- propyltrimethoxysilane, ethanol andwater were mixed in mass ratio of 5:18: 2 in a beaker, which were stirred for 30 min for hydrolysis at 50℃. Second, the above solution were mixed with the pure alumina particles in a mass ratio of 2:5 together, which were stirred unti l the liquid will evaporate completely at 80℃, using residual heat to com- pletely evaporate the liquid. Then after several times of grinding, washing, centrifugation, we finally obtained the modified alumina particles by grinding.The quality of the sapphire wafer before and after CMP were measured by the analytical balance (METTLER TOLEDO) to calculate the material removal rate accord- ing to Eq.(1):Here, is quality variation in sapphire wafer before and after polishing, T is the material removal time of sap-phire wafer. The content of alumina particles synthe- sized through an ALFOL method. The abrasive concen- tra-tion in the slurries was maintained 5wt%. The pH of the slurries were 10.00 and 13.00 adjusted by dilute KOH (2 mol/L). The polishing experiments were carried out using a CMP tester (BRUKER CP-4), with SUBA 600 stacked pad. To get repeatable results, the initial pad was cleaned for 30 min of breakout time. The polishing process parameters were set as follows: pad rotation speed, 100 r/min; wafer rota-tion speed, 90 r/min; down force, 6 psi; feed rate of the slurry, 125 mL/min; and polishing time, 30 min; each time mass of slurry, 500 g. Polishing rate in the work was an average of two polishing runs. All experi- ments were con-ducted at room temperature.FTIR spectra were obtained on a Thermo Nicolet Nexus 470 FTIRspectrometer.Changes in the surface chemistry of the unmodified alumina and the modified alumina were studied by using an X-ray photoelectron spectroscope system (Axis Ultra, Kratos, Britain).Fourier transform infrared spectroscopy was used to characterize structure of functional groups on the surface of the alumina. In Fig. 1, unmodified alumina surface had a very weak stretching vibration mode of hydroxyl groups at 3472 cm-1 and bending vibration mode of hydroxyl groups at 1338 cm-1. It showed that the presence of hydroxyl groups on the surface of the alumina before modification. By comparing the unmodified alumina, modified alumina had a series of absorption peak at 3371 cm-1 and 3294 cm-1, 2927 cm-1 which belong to primary amine, secondary amine, reveal that alumina particles were modified successfully . After the alumina was modified, there was a special peak at 1116 cm-1 which belongs to alumino silicates that is Al–O–Si[11]. This explained the occurrence of a silanization reaction with hydroxyl groups.Another method used for structure characterization was XPS. First of all, Fig. 2 showed that survey photoelectron spectra of alumina particles before and after modification. Both the unmodified alumina and modified alumina presents peaks of Al, C, and O, while added peak of element N and Si appears for modified alumina. In addition, Table 1 showed binding energy of abrasives containing before and after modified alumina particles. By comparison, after modification of the surface of the alumina Al and O bonding energy could be reduced, which indirectly indicated that N-(2-aminoethyl)-3-aminopropyltrimethoxysilane had been successfully modified alumina surface. Finally, Table 2 gave that the composition of elements on the surface of alumina particles before and after modification. By comparison with that only elements Al, O, and C existed on the surface of unmodified alumina, element Si and N were introduced on the surface of modified alumina. The introduction of Si and N elements, the increasing of the C elements, the reduction of Al and O elements only indicated N-(2-aminoethyl)-3-aminopropyltrimethoxysilane had been successfully modified alumina surface, but did not reveal N-(2-aminoethyl)-3-aminopropyl-trimethoxysilane modified alumina surface.To demonstrate the chemical reaction produced on the surface of alumina, modified alumina was analyzed by XPS. The spectra of the Al2p, O1s, were showed in Fig. 3 and their BE were displayed in Table 3 and Table 4. In Fig. 3, the black line (a) and the red line (b) showed the real total intensity measured by XPS test, and the red line (a) and the blue line (b) showed the total intensity after curve fitting by using the XPSPEAK software. The closer between the real result and the fitting result means more precise measurement results.The peak at the BE of 73.8 eV (Fig. 3(a)) corresponds to Al(2p) in (–Si(OCH3)2O–)xAlyand the peak at the BE of 531.10 eV (Fig. 3(b)) corresponds to O(1s) in (–Si (OCH3)2O–)xAly, which demonstrated that the first step reaction happened in the Fig. 4. The peak at the BE of 73.1 eV (Fig. 3(a)) corresponds to Al(2p) in AlN and the peak at the BE of 530.30 eV (Fig. 3(b)) corresponds to O(1s) in Al2O3 which showed that alumina atomshappened the effect of aluminum ions. It is well known that aluminum ions are complexed with amines, but aluminum atoms cannot. The secondary amine with lone pair electrons was more likely to react with aluminum ions than primary amines of small electron donor groups and tertiary amines with large steric hindrance. Why is there a complex reaction between the aluminum atom and the secondary ammonia? As the first step of the reaction in Fig. 4 occurred, the alumina binding energy on the surface of the alumina was weakened so that the change in the state of the aluminum atom.Although it could be seen in Fig. 3(a) that the area under the forestgreen line ((–Si(OCH3)2O–)xAly) was much larger than the fuchsia line (AlN), indicating that the first step was the primary role, the two-step reaction also played an important role and cannot be ignored. The above results illustrate that these two effects (Fig. 4) make N-(2-aminoethyl)- 3-aminopropyltrimethoxysilane solid alumina surface. Based on the above conclusions, Fig. 4 gave that the chemical reaction occurred in the process of modification.It is understood that abrasive loading has an important role on frictional interactions during CMP[13-14]. The friction force is wise to be seriously dependent on properties of the opposing surfaces, surface conditions, and the abrasive size, which all influence the contact area between the opposing surfaces[15]. A friction force is proportional to constant COF[16]. COF as a function of polishing time for sapphire substrates polished by unmodified alumina particles and modified alumina particles are showedin Fig. 5. In Fig. 5, it is observed that COF of sapphire CMP using modified alumina particles is higher than that of unmodified alumina particles.In order to understand the difference in polishing performance between the unmodified alumina particles and the modified alumina particles at pH 10.00 and 13.00, the MRR and RMS of sapphire substrate were shown in Table 5. It can be found that the MRR of alumina particles slurries at pH 13.00 is two times larger than that of alumina particles slurries at pH 10.00, which suggest that alkaline play a very important role. In addition, it cannot be only seen that the MRR of modified alumina particles is three times larger than that of pure alumina particles at pH 10.00, but also surface quality is better. Above all, it can be displayed that the MRR of modified alumina particles at pH 10.00 exceeded that of pure alumina particles at 13.00, which insinuated that low alkaline slurry can achieve polishing effect of high alkaline slurry, in other words, this can greatly weak the chemical corrosion effect of polishing equipment. In order to further investigate the difference in polishing performance between unmodified alumina particles and modified alumina particles, the topographical micrographs of polished sapphire substrate surfaces were tested by AFM. As shown in Fig. 6, the surface before polishing rough and Rq is about 0.900. After polishing with pure alumina particles, Rq decrease to about 0.500.However, after polishing with modified alumina particles, Rq decrease to about 0.300. In other words, the modified alumina abrasive possesses higher surface planarization than pure alumina abrasive.In this work, to improve the CMP performance of alumina particles in aqueous solution, a modification method, as a suitable method, was explored. Firstly, The alumina particles modified by N-(2-aminoethyl)- 3-aminopropyltrimethoxysilane have a better CMP performance than unmodified alumina. Secondly, FTIR only indirectly proves that N-(2-amino-ethyl)-3-aminopropyltrimethoxysilane have successful grafted on the surface of alumina particle, and XPS experiment directly proved this point. Innovatively, this paper gives a detailed chemical reaction mechanism that N-(2-aminoethyl)-3-aminopropyltrimethoxy-silane grafted onto the surface of alumina, which N-(2-aminoethyl)-3-aminopropyltrimethoxysilane through salanization reaction and complexation with Al and secondary amine firmly grafted onto the surface of alumina, in particular, complexation with Al and secondary amine is not negligiable. The consequence is that the modified alumina friction coefficient is bigger that unmodified alumina, resulting the modified alumina particles exhibit higher material removal rate and better surface quality than unmodified alumina particle. Most important of all, the focus is that the modified alumina particles manifested higher MRR at pH 10.00 than the unmodified alumina particles at pH 13.00, which will provide ideas to reduce corrosion of equipment.[1] SAITO T, HIRAYAMA T, YAMAMOTO T, et al. Lattice strain and dislocations in polished surfaces on sapphire. J. Am. Ceram. Soc., 2005, 88: 2277–2285.[2] NIU X H, LIU Y L, TAN B M, et al. Method of surface treatment onsapphire substrate. Transactions of Nonferrous Metals Society of China, 2006, 16: 732–734.[3] TAKEUCHI T, TAKEUCHI H, SOTA S, et al. Optical properties of strained AlGaN and GaInN on GaN. Jpn. J. Appl. Phys., 1997, 36: L177–L179.[4] LIMA R S, MARPLE B R. Thermal spray coatings engineered from nanostructured ceramic agglomerated powders for structural, thermal barrier and biomedical applications: a review. J. Therm. Spray Technol., 2007, 16: 40–63.[5] KIM K T, KOO H Y, LEE G G, et al. Synthesis of alumina nanoparticle-embedded-bismuth telluride matrix thermoelectric composite powders. Mater. Lett. , 2012, 82: 141–144.[6] ZOIS D, LEKATOU A, VARDAVOULIAS M, et al. Nanostructured alumina coatings manufactured by air plasma spraying: correlation of properties with the raw powder microstructure. J. Alloys Compd., 2010, 495: 611–616.[7] TANG E J, CHENG G X, MA X L, et al. Surface modification of zinc oxide nanoparticle by PMAA and its dispersion in aqueous system. Appl. Surf. Sci., 2006, 252: 5227–5232.[8] LEI H, LU H S, LUO J B, et al. Preparation of α-alumina- g-polyacrylamide composite abrasive and chemical mechanical polishing behavior. Thin Solid Films, 2008, 516: 3005–3008.[9] LEI H, ZHANG P Z. Preparation of alumina/silica core-shell abrasives and their CMP behavior. Appl. Surf. Sci., 2007, 253: 8754–8761.[10] ZHANG Z F, LEI H. Preparation of α-alumina/polymethacrylic acidcomposite abrasive and its CMP performance on glass substrate. Microelectron. Eng., 2008, 85: 714–720.[11] SHEN X C, FANG X Z, ZHOU Y H, et al. Synthesis and characterization of 3-aminopropyltriethoxysilane-modified superpar- amagnetic magnetite nanoparticles. Chem. Lett., 2004, 33: 1468–1469.[12] ZHANG Z F, YU L, LIU W L, et al. Surface modification of ceria nanoparticles and their chemical mechanical polishing behavior on glass substrate. Appl. Surf. Sci., 2010, 256: 3856-3861.[13] HOMMA Y. Dynamical mechanism of chemical mechanical polishing analyzed to correct Preston's empirical model. J. Electroanal. Chem., 2006, 153: G587–G590.[14] MATSUDA T, TAKAHASHI H, TSURUGAYA M, et al. Characteristics of abrasive-free micelle slurry for copper CMP. J. Electrochem. Soc., 2003, 150: G532–G536.[15] ABIADE J T, CHOI W, SINGH R K. Effect of pH on ceria–silica interactions during chemical mechanical. J. Mater. Res., 2005, 20: 1139–1145.[16] LIANG H, CRAVEN D R. Tribology in Chemical–Mechanical Planarization. Taylor & Francis, Boca Raton, Fla., 2005.。
