黑金属和有色金属Ferrous and Non-Ferrous Metals
有色金属诠释(non-ferrous metals)

有色金属诠释(non-ferrous metals)摘要: 有色金属non-ferrous metal,狭义的有色金属又称非铁金属,是铁、锰、铬以外的所有金属的统称。
广义的有色金属还包括有色合金。
有色合金是以一种有色金属为基体(通常大于50%),加入一种或几种其他元素而构成的合金 ...有色金属non-ferrous metal,狭义的有色金属又称非铁金属,是铁、锰、铬以外的所有金属的统称。
广义的有色金属还包括有色合金。
有色合金是以一种有色金属为基体(通常大于50%),加入一种或几种其他元素而构成的合金。
有色金属[Metallurgy] nonferrous metals 简介:定义:狭义的有色金属又称非铁金属,是铁、锰、铬以外的所有金属的统称。
广义的有色金属还包括有色合金,有色合金是以一种有色金属为基体(通常大于50%),加入一种或几种其他元素而构成的合金。
有色金属通常指除去铁(有时也除去锰和铬)和铁基合金以外的所有金属。
有色金属可分为四类:1、重金属:一般密度在4.5g/cm3以上,如铜、铅、锌等;2、轻金属:密度小(0.53~4.5g/cm3),化学性质活泼,如铝、镁等;3、贵金属:地壳中含量少,提取困难,价格较高,密度大,化学性质稳定,如金、银、铂等;4、稀有金属:如钨、钼、锗、锂、镧、铀等。
详细信息:有色金属是指铁、铬、锰三种金属以外所有的金属。
中国在1958年,将铁、铬、锰列入黑色金属;并将铁、铬、锰以外的64种金属列入有色金属。
这64种有色金属包括:铝有色金属分布、镁、钾、钠、钙、锶、钡、铜、铅、锌、锡、钴、镍、锑、汞、镉、铋、金、银、铂、钌、铑、钯、锇、铱、铍、锂、铷、铯、钛、锆、铪、钒、铌、钽、钨、钼、镓、铟、铊、锗、铼、镧、铈、镨、钕、钐、铕、钆、铽、镝、钬、铒、铥、镱、镥、钪、钇、硅、硼、硒、碲、砷、钍。
种类面积:有色金属表面积也是非常重要的,有色金属的比表面积检测数据只有采用BET方法检测出来的结果才是真实可靠的,国内目前有很多仪器只能做直接对比法的检测,现在国内也被淘汰了。
有色金属冶金

? 19世纪的后期,世界黄金生产飞速发展,在美国, 南非,前苏联等发现了许多大型的金矿。
? 历史上经历过俄罗斯黄金热,加利福尼亚热,澳 大利亚热,南非热,
2 国内
? 中国为世界上认识黄金和开发利用黄金 最早的国家之一,在 4000多年以前就有关 于黄金的文字记载, 18\世纪以前黄金的产 量名列前茅。
是当时世界的第五大产金国。
黄金的生产方式:
? 从矿石中生产:总产量的 2/3 ? 从阳极泥中生产:总产量的 1/3
黄金的主要产地: 山东, 河南
4 稀有金属(rare metals) :
是一种习惯称呼,是沿用至今的一个历史名词;
或在地壳中丰度小,天然资源少; 或虽丰度大,赋存分散,经济提取难; 或性质接近难分离成单一金属; 或开发较晚,过去使用的较少,而给人留下“稀有”
Ge、Se、Te 、Re
特点: 在地壳中的分散性:大部分不能形成
或极少能形成独立的矿物。一般是以同晶型杂 质的形态存在于其它矿物晶格中。因此,稀散 元素往往是从冶金、化工过程的废渣中顺便提 取。
稀土金属 rare earth metals:Sc、Y、镧系元素(57
3100
乌兹别克 澳大利亚 加拿大
3100
3000
1400
世界银的贮量50%分布在加 拿大、墨西哥美国、澳大利亚和 秘鲁五国。
表2 世界银的贮量分布
总计(万T) 墨西哥 加拿大 美国 澳大利亚秘鲁
280
37
37
31
29
25
二、 生产
? 黄金生产和贮量相对应, ? 我国在4-5名之间。
考试重点+单词
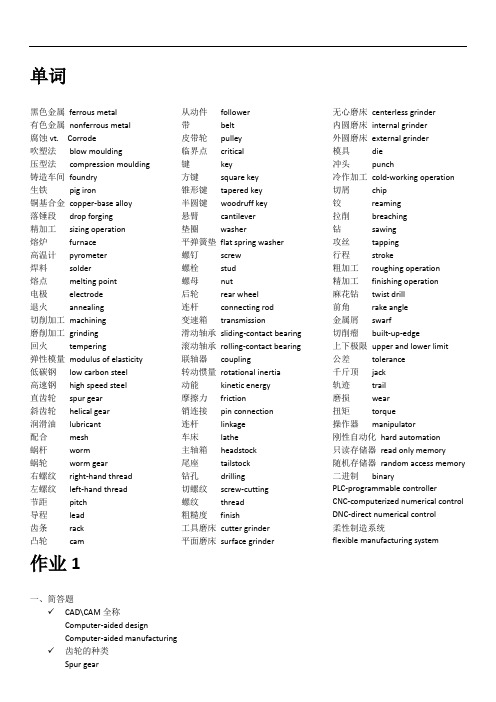
单词黑色金属ferrous metal有色金属nonferrous metal腐蚀vt. Corrode吹塑法blow moulding压型法compression moulding 铸造车间foundry生铁pig iron铜基合金copper-base alloy落锤段drop forging精加工sizing operation熔炉furnace高温计pyrometer焊料solder熔点melting point电极electrode退火annealing切削加工machining磨削加工grinding回火tempering弹性模量modulus of elasticity 低碳钢low carbon steel高速钢high speed steel直齿轮spur gear斜齿轮helical gear润滑油lubricant配合mesh蜗杆worm蜗轮worm gear右螺纹right-hand thread左螺纹left-hand thread节距pitch导程lead齿条rack凸轮cam 从动件follower带belt皮带轮pulley临界点critical键key方键square key锥形键tapered key半圆键woodruff key悬臂cantilever垫圈washer平弹簧垫flat spring washer螺钉screw螺栓stud螺母nut后轮rear wheel连杆connecting rod变速箱transmission滑动轴承sliding-contact bearing滚动轴承rolling-contact bearing联轴器coupling转动惯量rotational inertia动能kinetic energy摩擦力friction销连接pin connection连杆linkage车床lathe主轴箱headstock尾座tailstock钻孔drilling切螺纹screw-cutting螺纹thread粗糙度finish工具磨床cutter grinder平面磨床surface grinder无心磨床centerless grinder内圆磨床internal grinder外圆磨床external grinder模具die冲头punch冷作加工cold-working operation切屑chip铰reaming拉削breaching钻sawing攻丝tapping行程stroke粗加工roughing operation精加工finishing operation麻花钻twist drill前角rake angle金属屑swarf切削瘤built-up-edge上下极限upper and lower limit公差tolerance千斤顶jack轨迹trail磨损wear扭矩torque操作器manipulator刚性自动化hard automation只读存储器read only memory随机存储器random access memory二进制binaryPLC-programmable controllerCNC-computerized numerical controlDNC-direct numerical control柔性制造系统flexible manufacturing system作业1一、简答题✓CAD\CAM全称Computer-aided designComputer-aided manufacturing ✓齿轮的种类Spur gearHelical gear✓铸造工艺包括几步Make moldsPrepare and melt the metalPour the metal into the moldsClean the castings✓列举几种机械零件GearKeyShaftScrewCamBearingSpringWorm gear set✓皮带传动是否精确?为什么Inaccuracy, belt drives depend on friction on the pulleys and some slippage is inherent in their operation.✓刀具磨损的类型Flank wear 后刀面磨损Crater wear 前刀面磨损Notch wear V形凹口磨损Brittle fracture/edge chipping 已解决的磨损形式✓钢的力学性能Strength 强度Stiffness 刚度Ductility 延展Impact strength 冲击强度Hardness 硬度Toughness 韧性二、汉译英✓对于深沟球轴承,沟槽的深度大约是球直径的1/4In deep-groove ball bearings, the races沟槽are approximately one fourth as deep as the ball diameter.✓把金属件连接在一起的方法有很多,用哪一种方法要根据金属的种类和要连接的焊件的种类来定There are a number of methods of joining metal articles together, depending on the type of metal and the strength of the joint which is required.✓软焊是要用熔融状态下的第三种金属来使两件金属连接在一起的工艺Soldering is the process of joining two metals by a third metal to be applied in the molten state.✓力学性能是材料施加压力的反应特性Mechanical properties are the characteristic responses of a material to applied forces.✓用于冷作加工的机器叫压力机The machine used for most cold-working operations is known as a press.作业2一、简答题1、柔性系统的主要功能To create the cells from which the system is to be constructed.2、金属分为哪两类Simple metalsAlloys3、热处理的类型Annealing 退火Tempering 回火Quench 淬火Normalizing 正火4、热加工工艺有哪些Casting 铸造Forging 锻造Welding 焊接5、传动扭矩的键包括Square key 方键Tapered key 锥形键Woodruff key 半圆键6、车床的主要部分Headstock 主轴箱Tailstock 尾座Tool-post 刀架7、主轴箱内有哪些部件机构Driving 驱动Gear mechanism 齿轮装置Spindle 卡盘二、汉译英1、作为一种形状制造方法,切削是所有制造过程中最普遍使用的。
普通冶金学专题培训

根据冶炼措施旳不同,钢铁生产中产生旳 炉渣分为:高炉渣和炼钢炉渣;
按渣中化学成份含量旳不同又分为: 碱性渣和酸性渣。
41
2).煤气 钢铁生产中还能取得大量可燃气体,这是
钢铁联合企业内部旳主要气体燃料。
多种煤气旳性能、成份及用途入下表
18
这些都是主要旳工业材料.
但生产量最大,且用以主要部分旳是金属材料。
再把金属材料细分:
根据1981年联合国统计鉴定记载,全世界占金属材料 产量约95%旳是钢,即钢94.9%.
铝2.5%,铜1.3%,锌0.7%,铅0.5%,其他和计为0.1%。
所以能够说,当代仍是“钢旳时代”。 19
钢铁材料在当代社会中一直保持优势旳原因
高温高压过程,温度为200~300 ℃)
共同工序:浸出
分离
富集
提取
7
3、电冶金 (电热冶金、电
化学冶Байду номын сангаас)
特点:用电能提取和精炼金属
按电能 形式分
电热冶金—— 电能转变为热 能。
Cu、Zn Al
电化学冶金—— 利用电化学反应 (电解和电积)从 盐类水溶液或熔体 中析出金属。
8
金属分类
黑色金属和有色金属; 黑色金属有:铁、铬、锰,除此之外旳为有
1)资源丰富 铁元素占地壳旳5%,是居第4位旳丰富元素, 有高端旳大矿床,需求资源量可维持423年。
2)冶炼轻易 大生产旳方式早已确立,其性能价格比好。
3)在强度、硬度、韧性等方面,作为使用量 最大旳一般构造材料,具有优越旳特征(见下 图)。
20
21
钢
22
有色金属概述

第一节 第二节 第三节 第四节 第五节 第六节 铝及铝合金 铜及铜合金 钛及钛合金 轴承合金 镁及镁合金 粉末冶金材料
工业生产中,通常把钢铁材料称为黑色金属 (ferrous metal),而把其它的金属材料称为有
色金属(nonferrous metal)。
有色金属的产量和用量不如黑色金属多,但由
压气机叶片
Al-Cu-Mg-Fe-Ni系耐热锻铝合金:常用牌号有LD7
(2A70)、LD8(2A80)、LD9(2A90)等 。用于制造
150 ~225℃下工作的零件,如压气机叶片、超音 速飞机蒙皮等。
2、铸造铝合金
牌号表示:ZAl+主要合金元素符号和平均百分含量 注:若平均含量小于1%,一般不标数字。
过渡相θ'
平衡相θ
时效机理:过饱和固溶体中形成溶质原子偏聚区
和由之脱溶出微粒弥散分布——沉淀硬化。 时效强化条件:固溶体有一定溶解度,且随T↓, 溶解度↓↓。 过饱和程度越大,时效温度越高,时效进行得越 强烈。
自然时效:在室温下进 行的时效,以GP区强 化为主; 人工时效:在加热条件 下进行的时效,以过渡 沉淀相强化为主。
加热固溶
时效析出
固溶处理:指将合金加热
到固溶线以上,保温并 淬火后获得过饱和的单 相固溶体组织的处理。
淬火过饱和
时效:指将过饱和的固溶体加热到固溶线以下某温度
保温,以析出弥散强化相的热处理。
时效过程中的组织变化
以Al-Cu合金为例:
GPⅠ区 GPⅡ区(过
渡相θ″) 溶质原子(Cu)富集区, 可以是片状、针状或球状 溶质原子继续富集,且GP区 有序化→弹性畸变更严重 与CuAl2化学成分相当,与 母相共格关系开始破坏 θ '相长大到一定尺寸时与 母相完全脱离(非共格)
材料导论 (28)

Module 4 (Metallic Materials金属材料)Video 6 Nonferrous Metals有色金属Hello, everyone. Today, we will talk about nonferrous metals.译文:大家好!今天我们来谈谈有色金属。
Nonferrous metals are the metals that are not iron based. Compared with ferrous alloys, nonferrous metals are much lighter, so well-suited for use in the aircraft or canning industries. They also have higher conductivity, resistance to corrosion, and non-magnetic property, so some perfect for use in small electronics and as electrical wiring.译文:有色金属为非铁基的金属。
与黑色金属相比,有色金属更轻,因此很适合用于飞机和罐头行业。
有色金属具有高的导电性、耐腐蚀性能和非磁性,因此非常适合制造小型电子产品和电线。
The commonly used nonferrous metals include copper, aluminum, magnesium, titanium, the refractory metals, the superalloys, noble metals, and the white metals.译文:常用的有色金属包括:铜、铝、镁、钛、难熔金属、超级合金、贵金属,以及白色金属。
有缘学习更多驾卫星ygd3076Copper is a soft, malleable, and ductile metal, with very high thermal and electrical conductivity. Also, it is highly resistant to corrosion in the ambient atmosphere, seawater, and some industrial chemicals. The mechanical and corrosion-resistance properties of copper may be improved by alloying.译文:铜是一种较软的、可锻的韧性金属,具有很高的导热性和导电性。
黑色金属、钢和有色金属

一、黑色金属、钢和有色金属在介绍钢的分类之前先简单介绍一下黑色金属、钢与有色金属的基本概念。
1、黑色金属是指铁和铁的合金。
如钢、生铁、铁合金、铸铁等。
钢和生铁都是以铁为基础,以碳为主要添加元素的合金,统称为铁碳合金。
生铁是指把铁矿石放到高炉中冶炼而成的产品,主要用来炼钢和制造铸件。
把铸造生铁放在熔铁炉中熔炼,即得到铸铁(液状),把液状铸铁浇铸成铸件,这种铸铁叫铸铁件。
铁合金是由铁与硅、锰、铬、钛等元素组成的合金,铁合金是炼钢的原料之一,在炼钢时做钢的脱氧剂和合金元素添加剂用。
2、把炼钢用生铁放到炼钢炉内按一定工艺熔炼,即得到钢。
钢的产品有钢锭、连铸坯和直接铸成各种钢铸件等。
通常所讲的钢,一般是指轧制成各种钢材的钢。
钢属于黑色金属但钢不完全等于黑色金属。
3、有色金属又称非铁金属,指除黑色金属外的金属和合金,如铜、锡、铅、锌、铝以及黄铜、青铜、铝合金和轴承合金等。
另外在工业上还采用铬、镍、锰、钼、钴、钒、钨、钛等,这些金属主要用作合金附加物,以改善金属的性能,其中钨、钛、钼等多用以生产刀具用的硬质合金。
以上这些有色金属都称为工业用金属,此外还有贵重金属:铂、金、银等和稀有金属,包括放射性的铀、镭等。
二、钢的分类钢是含碳量在0.04%-2.3%之间的铁碳合金。
为了保证其韧性和塑性,含碳量一般不超过1.7%。
钢的主要元素除铁、碳外,还有硅、锰、硫、磷等。
钢的分类方法多种多样,其主要方法有如下七种:1、按品质分类(1) 普通钢(P≤0.045%,S≤0.050%)(2) 优质钢(P、S均≤0.035%)(3) 高级优质钢(P≤0.035%,S≤0.030%)2.、按化学成份分类(1) 碳素钢:a.低碳钢(C≤0.25%);b.中碳钢(C≤0.25~0.60%);c.高碳钢(C≤0.60%)。
(2) 合金钢:a.低合金钢(合金元素总含量≤5%);b.中合金钢(合金元素总含量>5~10%);c.高合金钢(合金元素总含量>10%)。
黑色金属和有色金属

黑色金属和有色金属浅析黑色金属和有色金属这名字,常常使人误会,以为黑色金属一定是黑的,其实不然。
黑色金属只有三种:铁、锰、铬。
而它们三个都不是黑色的!纯铁是银白色的;锰是银白色的;铬是灰白色的。
因为铁的表面常常生锈,盖着一层黑色的四氧化三铁与棕褐色的三氧化二铁的混合物,看去就是黑色的。
所以人们称之为“黑色金属”。
常说的“黑色冶金工业”,主要是指钢铁工业。
因为最常见的合金钢是锰钢与铬钢,这样,人们把锰与铬也算成是“黑色金属”了。
除了铁、锰、铬以外,其他的金属,都算是有色金属。
在有色金属中,还有各种各样的分类方法。
比如,按照比重来分,铝、镁、锂、钠、钾等的比重小于4.5,叫做“轻金属”,而铜、锌、镍、汞、锡、铅等的比重大于4.5,叫做“重金属”。
像金、银、铂、锇、铱等比较贵,叫做“贵金属”,镭、铀、钍、钋等具有放射性,叫做“放射性金属”,还有像铌、钽、锆、镥、金、镭、铪、铀等因为地壳中含量较少,或者比较分散,人们又称之为“稀有金属”。
黑色金属补充说明黑色金属材料乃工业上对铁、铬和锰的统称。
亦包括这三种金属的合金,尤其是合金钢及钢铁。
与黑色金属相对的是有色金属。
事实上纯净的铁及铬是银白色的,而锰是银灰色。
由于钢铁表面通常覆盖一层黑色的四氧化三铁,而锰及铬主要应用于冶炼黑色的合金钢。
所以才会被“错误分类”为黑色金属。
黑色金属的分类也有其意义,因为这三种金属都是冶炼钢铁的主要原料,而钢铁在国民经济中占有极其重要的地位,亦是衡量一国家国力的重要标志。
据统计,黑色金属的产量约占世界金属总产量的95%。
黑色金属用途铬性质银白色,硬度最高的金属,不容易腐蚀。
用途进行电镀时的必用金属产品生产方式性质用途铬钢在钢里掺入铬。
硬而耐腐蚀。
制造机械、枪炮筒、坦克、装甲车。
不锈钢炼钢时掺入占全体12%以上的铬,及掺入一定量的镍。
不生锈。
建筑材料、餐具锰性质灰白色,脆而硬。
产品生产方式性质用途锰钢炼钢时里掺入12%以上的锰。
坚硬、强韧、不容易磨损。
有色金属的地位及作用

有色金属的地位及作用————于文俊(09874125)有色金属行业是对有色金属(non-ferrousmetal)进行采选、冶炼、压延加工等一系列工作的生产部门的总称。
根据中国官方行业分类标准,有色金属行业分为有色金属采选和有色金属冶炼及压延加工业两个部门。
有色金属采选业是指对常用有色金属矿、贵金属矿,以及稀有稀土金属矿的开采、选矿活动;有色金属冶炼及压延加工业包含有色金属冶炼业、有色金属合金制造、有色金属压延加工三个部门。
有色金属冶炼业指通过熔炼、精炼、电解或其他方法从有色金属矿、废杂金属料等有色金属原料中提炼常用有色金属的生产活动;有色金属合金制作业指以有色金属为基体,加入一种或几种其他元素所构成的合金生产活动;有色金属压延加工业指对有色金属及合金的压延加工生产活动。
有色金属(non-ferrousmetal)指除铁、锰、铬及其合金以外的金属。
统计上,将有色金属行业产品分为有色纯金属、有色合金与有色材三个部分。
有色纯金属分为重金属、轻金属、贵金属、半金属和稀有金属五类。
有色合金按合金系统分:重有色金属合金、轻有色金属合金、贵金属合金、稀有金属合金等;按合金用途则可分:变形(压力加工用合金)、铸造合金、轴承合金、印刷合金、硬质合金、焊料、中间合金、金属粉未等。
有色材按化学成份分类:铜和铜合金材、铝和铝合金材、铅和铅合金材、镍和镍合金材、钛和钛合金材。
按形状分类时,可分为:板、条、带、箔、管、棒、线、型等品种。
有色金属行业是我国的基础工业,是我国较早与国际接轨的行业之一。
其发展受国内经济发展速度和国家行业性政策影响,同时,也受国际经济形势的影响。
目前,国内的有色金属市场已基本与国际接轨,国际市场上有色金属的价格波动对国内有色金属价格的波动有着直接的影响,国内有色金属期现货的价格已基本与国际期现货的有色金属价格同步波动。
有色金属企业属于资金密集型企业,规模效益显著。
企业规模大,就会在成本降低中获得好处。
2有色金属

金属和黑色金属金属分为两大类-黑色金属和有色金属黑色金属是含有铁的金属,有色金属是不含铁的金属。
然而,某些有色金属的杂质中可能也含有少量的铁。
钢和铸铁是常用的最普通的,钢主要是含有铁和碳以及不同数量的某些别的元素的合金,控制钢的含碳量和其他合金元素的含量,并对钢进行不同的热处理,可以使钢获得各种不同的物理性能。
普通的碳钢除含有铁和碳之外,通常还含有少量的硅,硫,磷和锰。
加进下列一种或几种元素可以制成各种合金钢,这些元素是:镍、铬、钼、钒、钨、锰、硅和少量别的合金元素。
碳是目前钢里所含的最为重要的合金元素,正是钢的含碳量对其所能获得的最大硬度起着主要的决定性作用,含碳量越高,抗拉强度就越高,并且钢热处理后所达到的硬度也越高。
低碳钢通常用来制造需要进行多次成形加工的低强度零件。
中碳钢用于锻件以及制造另一些需要强度比较高,并具有一定的韧性的部件。
,高碳钢用来制造弹簧、刀具和模具等高强度零件。
合金钢具有各种特殊的性质,这取决于所加进金属的种类及数量,对于从事矿冶的冶金工作者来说,含有除碳、磷、硫和硅之外别的微量元素的钢都称作合金钢。
每一种合金钢都各有其特点。
汽车就是用约100种不同的合金钢制成的。
一些常见的合金元素如下:锰锰用来同硫化合以降低硫的某些不良的影响,也可以同碳化合以增加硬度和韧性,锰具有增加淬硬层深度的作用,锰还能降低轧制和锻造温度时的脆性以改善锻造质量。
硅硅通常在钢中不超过 3.00%。
少量的硅能改善韧性。
硅主要是与其他合金元素化合以提高冲击强度。
硫通常认为,硫对钢的热加工是有害的,对经过处理达到很高的抗拉强度的钢的冲击性能也是有害的。
然而,硫对机加工是非常有用的东西,所以常在钢中再加硫.使含硫量达到0.30%以利用这种有用的性能.磷磷对钢会产生不良的影响。
因为它会使钢变脆.有某种迹象表明,含有少量的、低于 0. 05%的磷会提高抗拉强度。
镍镍很容易溶解在钢水里。
普通的镍钢里的含镍量为0.40-5.00%。
金属种类总结

黑色金属(Ferrous Metal)是工业上对铁(Fe),铬(Cr)和锰(Mn)的统称,也包括这三种金属的合金。
与之对应的是有色金属(Non-ferrous Metal),即非铁金属,除铁,猛,铬以外的所有金属的统称,其可分为:重金属:铜(Cu)铅(Pb)锌(Zn)等;轻金属:镁(Mg)铝(Al)等;贵金属:金(Au)银(Ag)等;稀有金属:镍(Ni)钴(Co)铌(Nb)钽(Ta)等;有色金属亦可分为基本金属和小金属。
基础金属一般定义为使用范围广,使用量较多的常用金属。
包括铁,猛,铜,铝,铅,锌等,一般每年消耗量为百万吨甚至上亿吨。
小金属相对单位较小,故称之为小金属。
公司对业务分类分为:Base Metal: 铜(包括铜精矿Copper Concentrate,铜阳极板Copper Anodes,粗铜Copper Blister),铅(铅精矿Lead Concentrate,氧化铅Lead Oxide),锌(锌精矿Zinc Concentrate),金(金精矿Gold Concentrate),银(银精矿Silver Concentrate)等Minor Metal: 镍(镍精矿Nickel Concentrate和水合氢氧化镍Intermediate Product of Cobalt Metallurgy),钶钽铁矿Coltan,钽铁矿Tantalite,钨Tungsten等Industrial Material: 矾土Bauxite,重晶石Barite,天青石Celestite等Ferroalloys: 铬铁矿Ferro Chrome,硅铁矿Ferro Silicon等Steel Raw Materials: 铬矿Chrome,锰金属Manganese Metal Lumps等。
金属分那几类?什么是重金属?与它对应的金属是什么

金属分那几类?什么是重金属?与它对应的金属是什么金属分那几类?什么是重金属?与它对应的金属是什么1、黑色金属和有色金属黑色金属包括铁、铬、锰;(外层常被黑色的氧化膜覆蓋,名称由此而来)有色金属是指铁、铬、锰三种金属以外的所有金属.2、按其密度分:轻金属:指密度小于4.5g/cm*3重金属:指密度大于4.5g/cm*33、按价格、在地壳中的储量分贵金属:指在地壳中含量少,开采和提取都比较困难,对氧和其它试剂稳定,价格比一般金属贵的有色金属.包括金、银、铂、钯、锇、铱、钌、铑.4、按在地壳中的分布情况稀有金属:稀有金属并不是说稀少,只是指在地壳中分布不广,开采冶炼较难,在工业应用较晚,故称为稀有金属.包括锂、铍、铷、铯、钛、锆、铪、钒、铌、钽、钨、钼、铼、镓、铟、锗、铊等.5、按导电效能分半金属:一般指矽、硒、碲、砷和硼五种元素.其物理化学性质介于金属和非金属之间.如砷是非金属,但它能传热和导电.为什么Cr是重金属Fe却不是重金属呢重金属这是些通俗的命名。
Fe,Co,Ni在炼钢中常用称黑色金属。
而Cr,Mo,W等却称为重金属。
铁是重金属吗什么叫重金属铁不是重金属重金属是汞、铬、镉、锰等什么是重金属重金属化学重金属1 重金属指比重大于5的金属,(一般指密度大于4.5克每立方厘米的金属)约有45种,如铜、铅、锌、铁、钴、镍、锰、镉、汞、钨、钼、金、银等。
尽管锰、铜、锌等重金属是生命活动所需要的微量元素,但是大部分重金属如汞、铅、镉等并非生命活动所必须,而且所有重金属超过一定浓度都对人体有毒。
如汞中毒的临床表现有,全身症状为头痛、头昏、乏力、发热。
口腔及消化道症状表现为齿龈红肿酸痛、糜烂出血、牙齿松动、龈槽溢脓,口腔有臭味,并有恶心、呕吐、食欲不振、腹痛、腹泻。
面板接触可出现红色斑丘疹,以四肢及头面部分布较多。
少数患者可有肾损害,个别严重者可有咳嗽、胸痛、呼吸困难、绀紫等急性间质性肺炎的表现。
重金属中毒会使体内的蛋白质凝固,这个你可以从高三的化学书看到,如果轻微中毒,就大量喝牛奶,牛奶中的蛋白质会和重金属反应,这样不会损伤到你自身的身体机能,喝了以后马上就医。
实用英语词汇系列:机械翻译词汇_Part2

glazing磷化预处理phosphating pretreatment薄膜磷化处理thin amorphous phosphating湿碰湿wet-to-wet钝化passivating结皮现象skinning起皱wrinkling遮蔽masking凉干flash off气幕air seal喷漆室booth干式喷漆室dry spray booth水过滤式喷漆室water wash spray booth下吸式喷漆室down draft spray booth侧抽风喷漆室spray booth of side exhaust油漆调制室paint mixing room打磨间scuff sand booth通过式烘干室passing through type baking oven 桥式烘干室bridge type baking oven辐射对流烘干室joint radiation convection oven 浸漆设备dip equipment浸漆槽dip tank循环槽recirculating tank自动喷漆机automatic sprayer热喷漆机flow coating equipment淋漆设备flow coating equipment手工浸漆设备hand dip equipment无空气喷涂设备airless spray equipment电泳涂漆设备electro apparatus电涂漆设备electrocoating installation静电喷漆设备electrostatic spray equipment 喷枪spray gun双头喷枪double head spray gun空气喷枪air spray gun吊具sling推杆悬链double-chain conveyer悬挂运输链overhead conveyer油水分离器oil-water separator油漆调制罐paint mixing tank除尘装置dust collector静电除尘装置electrostatic dust collector阴极罩cathode shiedl超滤装置ultrafiltration device喷砂机sand blasting machine红外线固化IR curing紫外线固化UV curing电子束固化electron beam curing辐射固化漆膜radiation cured coating (RCC) 光泽计glossmeter比重计gravimeter粘度计viscosimetr细度计fineness gauge厚度计thickness gauge遮盖力测定仪cryptometer电镀electroplating预镀(冲镀)striking防镀(止镀)resist无氰电镀non-cyanide plating化学电镀electroless plating气相电镀vapor plating挂镀rack用假阴极电镀dummying机械电镀mechanical plating浸镀immersion plating退镀stripping滚镀barrel plating电解沉积electrowinning混浊度turbidity侵蚀fretting侵渍作用maceration亲水的hydrophilic疏水的hydrophobic絮凝剂flocculant光亮镀层bright coating防腐镀层coating for protection against corrosion 冲洗flush打光lapping抛光burnishing滚光tumbling变色tarnishing电解electrolysis沉淀法decantation抗暗处理anti-tarnish treatment均镀力levelling power深镀能力covering power酸洗pickling磷化phosphating镀锌galvanizing漂白whiten出斑点spotting out电镀机plating machine自动电镀装置automatic plating machine直线式电镀机straight-line plating machine挂式电镀机plating machine held on racks钟形滚镀机oblique-barrel plating machine微型滚镀机portable plating barrel冷水槽cold rinse bank双联冷水槽double countercurrent rinse tank 电解去油槽electrolytic cleaner衬铅槽lead lined steel tank哈林电槽haring cell热水槽hot rise tank超声波去油槽ultrasonic cleaning tank浸酸槽acid dip tank温水槽warm rinse tank赫尔槽hull cell镀锌槽zinc-plating tank固定槽plating tank回收槽reclaim tank水平全浸式滚筒horizontally fully-immersed barrel 六角滚筒hexagonal plating barrel孔板流量计orifie meter浊度计nephelometer库仑计coulometer滴重计stalagmometer汽车制造设备manufactruing equipment of automobile 万能铣床universal milling machine内外圆研磨床inner-outer happing machine仿形车床copy lathe龙门刨床double housing planer牛头刨床shaper攻丝机threading machine双柱坐标镗床double pillar jig borer滚丝机thread roller滚齿机hobbing machine钻床drilling machine插床slotting machine珩齿机gear honing machine平衡-钻中心孔机床balancing centering machine曲轴车床crankshaft turning lathe双工位曲轴车床double-post carnkshafttruning blathe曲轴(主轴颈)车床crankshaft (main journal turning lathe) 凸轮轴(轴颈)车床camshaft(journal )turning lathe液压仿形车床dydraulic coping lathe凸轮轴仿形车床camsahft coping lathe多轴深孔钻床multi-spindle deep hole drilling machine曲轴轴颈铣削专用机床crankshaft journal milling machine数控曲轴铣床N/C crankshaft milling machine螺纹铣床thread milling mahcine球面蜗杆铣床cone-worm milling machine花键轴铣床spline shaft milling mahcine转鼓铣床drum milling machine旋风式曲轴铣床whirl-wind type crankshaft milling machine曲轴主轴颈磨床cranksahft journal grinding machine凸轮轴磨床camshaft grinding machine螺纹磨床thread grinding machine活塞销超精加工机piston pin superfinishing machine弹簧端面磨床grinding machine for spiral spring活塞椭圆磨床piston oval -grinding mahcine多砂轮平面磨床multiple abrasive wheels surface grinding machine 宽砂轮外圆磨床wide abrasive wheel cylindrical grinding machine 蜗杆磨床worm grinding machine靠模磨床copy grinding mahcine花键磨床spline shaft grinding machine齿轮磨床gear grinding machine活塞环内外圆无心磨床internal and cylindrical centerless grinder for pistone ring活塞环卧轴双端面磨床horizontal spindle double ended grinding machine for piston ring 活塞环立轴双端面磨床vertical -spindle double ended grinding mahcine for piston ring 活塞环倒角磨床piston ring chamfering grinding machine金刚镗床diamond fine boring machine坐标镗床jig-boring machine花键轴冷打机床spline shaft cold-striking machine气缸体侧拉床cylinder block side broaching machine气缸体专用拉床cylinder block broaching machine立式双溜板外拉床vertical double-apron external broaching machine卧式连续式拉床horizontal continuous broaching machine活塞加工多工位组合机床multiple station combined machine for piston machining转鼓式双面钻镗组合机床drum type double face drilling and boring combined machine气缸盖加工自动线automatic production line for cylinder head气缸体加工自动线automatic production line for cylinder block曲轴动平衡自动线automatic production line for cranshaft dynamic balance连杆加工自动线automatic production line for connecting rod汽车主减速器壳加工自动线automatic production line for automobile final drive housing曲轴超精磨机床crankshaft superfinishing machine凸轮轴超精磨机床camshaft polishing machine曲轴轴颈抛光机床crankshaft polishing machine凸轮轴抛光机床camshaft polishing machine直齿锥齿轮刨齿机straight bevel gear generator弧齿锥齿轮铣齿机curve-tooth bevel gear milling machine弧齿锥齿轮铣刀盘刃curve-tooth bevel gear milling工具磨床cutter grinding machine剃齿机gear shaving machine挤齿机gear burnishing machine珩齿机gear honing machine直齿锥齿轮拉齿机straight bevel gear broaching machine活塞环外圆超精加工机piston ring superfinishing machine珩磨机honing machine气门座研磨机valve seat lapping machine双盘研磨机two-lap lapping machine自动换刀数控机床automatic tool changing N/C machine自动更换主轴箱数控机床automatic headstock changing N/C machine工业用机器人industrial robot砂带磨床abrasive belt grinding machine校正活塞重量半自动机床semi-automatic machine tool for correcting weight of piston 活塞环弹性自动检验机automatic inspection machine for piston ring tension液压仿形砂轮修整器hydraulic copy wheel dresser液压伺服对刀装置hydraulic servo tool setting device液压跟踪中心架hydraulic traching center test镗刀磨损自动补偿装置automatic compensating device for boring cutter wear磨圆锥齿轮孔和端面用的卡盘chuck for grinding hole and end face of bevel gear电接触式检验夹具electical -contacted type detecting fixture连杆称重去重自动线automatic line for weighting connecting rod连杆综合检验专用夹具special fixure for comprehensive detection of connecting rod 曲轴综合检验专用夹具special fixture for comrehensive detection of crankshaft压铸机die casting machine高压造型机high pressure moulding machine摆动碾压机oscillating rolling machine齿轮滚轧机gearrolling mill缸体自动造型生产线automatic moulding productio line ofr cylinder block卧式锻造机horizontal forging machine电镦机electric upsetting machine径向锻造机radial forging machine辊锻机roll forging machine多工位自动冷镦机automatic multi-stage cold fromer汽车从动锥齿轮淬火压模die quenching of automotive driven bevel gear曲轴轴颈表面淬火机床crankshaft journal face hardening machine气体渗碳自动线automatic production line for gas carburization曲轴动平衡机crankshaft dynamic balancing machine齿轮淬火压床gear hardening press汽车用材料materials used in auto manufacturing金属metal黑金属ferrous metal有色金属non=ferrous metal重金属heavy metal贵金属precious metal铸铁cast iron灰铸铁grey cast iron白口铸铁white cast iron可锻铸铁malleable cast iron球墨铸铁nodular cast iron合金铸铁alloy cast iron耐磨铸铁wear resisting cast rion耐蚀铸铁corrosion resisting cast iron冷激铸铁chilled cast rion普通碳素结构钢carbon structureal steel优质碳素结构钢improved carbon structural steel 碳素工具钢carbon tool steel合金工具钢alloy tool steel高速工具钢high speed steel合金结构钢alloy structureal steel渗碳碳素钢carburizing carbon steel渗碳合金钢carburizing alloy steel氮化钢nitriding steel调质钢hardened and tempered steel热轧弹簧钢hot rolling spring steel冷拉弹簧钢cold drawing spring steel轴承钢bearing steel不锈钢stainless steel耐热钢heat resisting steel易削钢free cutting steel镇静钢(脱氧钢)killed steel半镇静钢half-killed steel沸腾钢(不脱氧钢)rimmed steel硼钢boron steel磁钢magnetic steel铸钢cast steel软钢mild steel多层钢multi-steel平炉钢siemens-martin steel顶吹氧转炉钢Luft Dushe steel冷拔低碳无缝钢管cold drawing low carbon seamless steel tube 镀锌钢板galvanized steel sheet镀铅钢板lead-covered steel sheet渗铝钢板aluminied steel sheet不锈(耐热)钢板stainless steel sheet镇静钢冷轧钢板cold rolling killed steel sheet沸腾钢冷钆钢板cold rolling rimmed steel sheet高强度底合金钢板low alloy steel sheet with high strength 冷轧薄钢板cold rolled thin steel sheet热轧薄钢板hot rolled thin steel sheet减震复合钢板shock-resistant complex steel sheet塑料夹层消声钢板multi-steel sheet with plastic plate铬酸锌复合钢板complex steel sheet with chromate zinc 深冲压用薄钢板deep drawing sheet紫铜copper青铜bronze工业用铜commercial bronze熟青铜wrought bronze锻造青铜cast bronze铅青铜lead bronze铝青铜aluminium bronze镍青铜nickel bronze硅青铜silicon bronze锆青铜zirconium bronze钛青铜titanium bronze铬青铜chrome bronze磷青铜phosphor bronze锰青铜manganese bronze硅锰青铜silico-manganese bronze铍青铜beryllium bronze镉青铜cadmium bronze黄铜brass熟黄铜wrought brass红铜red brass硅黄铜silicon brass锰黄铜manganese brass镍黄铜nickel brass锰铜manganin顿巴黄铜tombac康铜constantan铅合金lead alloy锌合金zinc alloy锌合金锭zinc alloy ingots轴承合金bearing alloy含油轴承合金self-lubricant bearing alloy高锡铝合金双金属带bimetal strip from tin-aluminium alloy 烧结合金sintered metal形变铝合金wrought aluminium alloy铸造铝合金aluminium casting alloy 碱土巴氏合金alkali-earth babbitt铅基巴氏合金lead base babbitt铝合金avional白合金white metal伍德合金wood's metal李彼威特合金lipowit's metal因科镍合金inconelY合金y alloy铝硅合金silumin共晶合金eutectic alloy减摩合金antifriction alloy铬镍合金chromel铝镍合金alumel蒙乃尔合金monel电子合金electron (dow metal)轻金属合金light metal alloy中间合金intermediate alloy耐蚀双金属anticorrosive bimetal铸造双金属cst bimetal高强度锻造铝合金high strength duralumin 超硬铝superduralumin钢化玻璃hardened glass有机玻璃plexiglass橡胶rubber天然橡胶natural rubber合成橡胶synthetic rubber(elastomer)再生橡胶regeneratd rubber苯乙烯聚丁橡胶styrene-butadiene rubber(SBR) 乙烯丙烯橡胶ethylene-propylene rubber (EPR) 硫化橡胶vulcanized rubber氯丁橡胶chloroprene rubber多孔橡胶porous rubber软橡胶soft rubber钠-丁二烯橡胶sodium butadiene rubber丁腈橡胶nitrile rubber腈基丁二烯橡胶nitrile butadiene rubber(NBR)海棉橡皮spongy rubber油漆(涂料)paint汽车漆automotive enamel汽车面漆automotive topcoat汽车用喷漆automotive alcquer封底漆seal coat皱纹漆crepon finish喷底漆电泳底漆electrophoretic primer环氧电泳底漆epoxy resin electrophoretic primer 头道底漆primer末道底漆finish primer硝基底漆nitrocellulose primer磷化底漆etch primer硝基面漆nitrocellulose enamel高光泽面漆high gloss finish锤纹面漆hammer finish二道漆second coat铝粉漆(银色漆)aluminum powder paint防蚀漆anticorrosive paint防污漆antifouling paint(copper paint)防锈漆antirust paint沥青漆bituminous paint环氧树脂漆epoxy paint底漆priming paint瓷漆enamel paint烘漆baking paint耐热漆heat-resistant paint绝缘漆insulating paint绝缘涂料红丹漆red lead paint发光漆luminous paint耐火漆nonflammable paint酚醛漆phenolic paint树脂漆resin paint乙烯基树脂漆vinyl resin paint水胶涂料water paint铅白漆white lead paint锌白漆white zinc paint清漆varnish罩光清漆finish varnish水性腻子water base filler油性腻子oil-base filler环氧腻子epoxy resin filler硝化腻子nitrocellulose filler氨基腻子amino-filler水性涂料water borne coating阳离子型电泳涂料cation electrophoretic coating 阴离子型电泳涂料anion electrophoretic coating 耐油涂料oil resistant coating耐酸涂料acid-prrof coating特种涂料special coating粉未涂料powder coating防霉涂料fungus proof paint防锈涂料rust-preventing coating 表面活性剂surfactant消泡剂antifoaming agent防声胶deadener脱胶剂glue stripper脱漆剂paint stripper树脂(树胶)resin合成树脂synthetic resin人造树胶artificial resin树胶脂gum resin树脂粘结剂resinoid bond动物胶gelatin丙阶(不溶)酚醛树脂resite乙阶(半溶)酚醛树脂resitol甲阶(可溶)酚醛树脂resol聚丙烯polypropylene(PP)聚甲醛polyformaldehyde(POM) 聚氯乙烯polyvinyl chloride(PVC) 聚苯乙烯polystyrene(PS)聚碳酸酯polycarbonate(PC)聚乙烯polyethylene(PE)聚酰胺polyamide(PA)聚砜polysulfone(PSF)ABS 共聚体acrylonitrile-butadiene- styrene polymer 聚氨酯polyurethane(PU)聚酯polyester酚醛树脂phenolric resin(PF)环氧树脂epoxy resin(EP)塑料plastics酚塑料phenola plastics氨基塑料amino plastics醋酸纤维塑料acetate fiber plastics聚氯乙烯塑料vinylene chloride plastics泡沫塑料foam plastics注射成型塑料injected-formative plastics玻璃纤维增强塑料glass-fiber reinforced plastics碳纤维增强塑料carbon-fiber reinforced plastics多孔塑料(泡沫塑料)expanded plastics热塑塑料thermoplastic plastics热固性塑料thermosetting plastics尼龙nylon纯料。
有色金属及合金

硬铝合金 硬铝合金为Al-Cu-Mg系合金,还含有少量的Mn。各种硬铝合金都 可以进行时效强化,属于可以热处理强化的铝合金,亦可进行变形强化。 其牌号用“LY”表示,“LY”是“铝硬”二字的汉语拼音字首。 硬铝主要分为三种:低合金硬铝,合金中Mg、Cu含量低;标准硬 铝,合金元素含量中等;高合金硬铝,合金元素含量较多。
超硬铝合金 超硬铝合金为Al-Mg-Zn-Cu系合金,并含有少量的Cr和Mn。牌号 有LC4、LC6等。Zn、Cu、Mg与Al可以形成固溶体和多种复杂的第二相。 其牌号用”LC“表示,”LC“是”铝超“二字的拼音字首。 所以经过固溶处理和人工时效后,可获得很高的强度和硬度。它是 强度最高的一种铝合金。但这种合金的抗蚀性较差,高温下软化快。可 以用包铝法提高抗蚀性。超硬铝合金多用来制造受力大的重要构件,如 飞机大梁、起落架等。
Al-Zn铸造铝合金
Al-Zn合金(ZL401、ZL402)价格便宜,铸造性能优良,经变质处 理和时效处理后强度较高,但抗蚀性差,热裂倾向大。常用于制造汽车、 拖拉机的发动机零件及形状复杂的仪器零件,也可用于制造日用品。 铸造铝合金的铸件,由于形状较复杂,组织粗糙,化合物粗大,并 有严重的偏析,因此它的热处理与变形铝合金相比,淬火温度应高一些, 加热保温时间要长一些,以使粗大析出物完全溶解并使固溶体成分均匀 化。淬火一般用水冷却,并多采用人工时效。
铸造铝合金 铸造铝合金按照主要合金元素的不同,可分为四类:Al-Si铸造铝合 金,如ZL101、ZL105等;Al-Cu铸造铝合金,如ZL201、ZL203等;AlMg铸造铝合金,如ZL301、ZL302等;Al-Zn铸造铝合金,如ZL401、 ZL402等。 Al-Si铸造铝合金 Al-Si铸造铝合金通常称为铝硅明,铝硅明包括简单铝硅明(Al-Si二 元合金)和复杂铝硅明(Al-Si-Mg-Cu等多元合金)。其牌号为ZL10~ 系列,含11~13%Si的简单铝硅明(ZL102)铸造后几乎全部是共晶组 织。 内燃机中的活塞,是在高速、高温、高压、变负荷下工作的,所以 要求制造活塞的材料必须比重小、高耐磨、高的耐蚀性、耐热性,还要 求活塞材料的线膨胀系数接近汽缸体的线膨胀系数。复杂铝硅明基本上 能满足这一要求,它是制造活塞的理想材料。
最新整理金属基本常识.doc

一、黑色金属、钢和有色金属在介绍钢的分类之前先简单介绍一下黑色金属、钢与有色金属的基本概念。
1、黑色金属是指铁和铁的合金。
如钢、生铁、铁合金、铸铁等。
钢和生铁都是以铁为基础,以碳为主要添加元素的合金,统称为铁碳合金。
生铁是指把铁矿石放到高炉中冶炼而成的产品,主要用来炼钢和制造铸件。
把铸造生铁放在熔铁炉中熔炼,即得到铸铁(液状),把液状铸铁浇铸成铸件,这种铸铁叫铸铁件。
铁合金是由铁与硅、锰、铬、钛等元素组成的合金,铁合金是炼钢的原料之一,在炼钢时做钢的脱氧剂和合金元素添加剂用。
2、把炼钢用生铁放到炼钢炉内按一定工艺熔炼,即得到钢。
钢的产品有钢锭、连铸坯和直接铸成各种钢铸件等。
通常所讲的钢,一般是指轧制成各种钢材的钢。
钢属于黑色金属但钢不完全等于黑色金属。
3、有色金属又称非铁金属,指除黑色金属外的金属和合金,如铜、锡、铅、锌、铝以及黄铜、青铜、铝合金和轴承合金等。
另外在工业上还采用铬、镍、锰、钼、钴、钒、钨、钛等,这些金属主要用作合金附加物,以改善金属的性能,其中钨、钛、钼等多用以生产刀具用的硬质合金。
以上这些有色金属都称为工业用金属,此外还有贵重金属:铂、金、银等和稀有金属,包括放射性的铀、镭等。
二、钢的分类钢是含碳量在0.04%-2.3%之间的铁碳合金。
为了保证其韧性和塑性,含碳量一般不超过1.7%。
钢的主要元素除铁、碳外,还有硅、锰、硫、磷等。
钢的分类方法多种多样,其主要方法有如下七种:1、按品质分类(1) 普通钢(P≤0.045%,S≤0.050%)(2) 优质钢(P、S均≤0.035%)(3) 高级优质钢(P≤0.035%,S≤0.030%)2.、按化学成份分类(1) 碳素钢:a.低碳钢(C≤0.25%);b.中碳钢(C≤0.25~0.60%);c.高碳钢(C≤0.60%)。
(2) 合金钢:a.低合金钢(合金元素总含量≤5%);b.中合金钢(合金元素总含量>5~10%);c.高合金钢(合金元素总含量>10%)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Heat treatment – tempering
19 of 24
© Boardworks Ltd 2005
Heat treatment – annealing
20 of 24
© Boardworks Ltd 2005
Working with metals – hand tools
21 of 24
13 of 24
© Boardworks Ltd 2005
A closer look at alloys
14 of 24
© Boardworks Ltd 2005
Random alloy generator
15 of 24
© Boardworks Ltd 2005
Metals and their properties
4 of 24
© Boardworks Ltd 2005
Primary processes – rolling
5 of 24
© Boardworks Ltd 2005
Primary processes – extrusion
6 of 24
© Boardworks Ltd 2005
Stock forms of metals
16 of 24
© Boardworks Ltd 2005
Changing the properties of metals
17 of 24
© Boardworks Ltd 2005
Heat treatment – haks Ltd 2005
Product Design Ferrous and Non-Ferrous Metals
These icons indicate that teacher’s notes or useful web addresses are available in the Notes Page. This icon indicates the slide contains activities created in Flash. These activities are not editable. For more detailed instructions, see the Getting Started presentation.
Because metals in their most basic form are natural resources, designers and manufacturers need to be careful and socially responsible about how much they use, and reuse or recycle metals where possible.
Metals are available in several raw forms. Each form is suitable for different manufacturing processes depending on the type of equipment used, the cost of the metal, the scale of production and the properties of the finished product. round rod flat strip round tube
22 of 24 © Boardworks Ltd 2005
Finishing techniques
Several surface finishing techniques can be used on metals. The most common ones are detailed below: Paint
square hexagonal
square tube angle channel sheet
© Boardworks Ltd 2005
octagonal
7 of 24
Categories of metals
Metals can be broken down into these main categories:
Ferrous metals
Non-ferrous metals
Pure metals do not contain any other metals or elements.
Pure metals
8 of 24
Alloys
Alloys
© Boardworks Ltd 2005
Ferrous metals
The elements of all metals are found naturally in the earth. However, they need to be extracted and processed before they can be used for manufacturing purposes.
11 of 24
© Boardworks Ltd 2005
A closer look at non-ferrous metals
12 of 24
© Boardworks Ltd 2005
Alloys
Sometimes ferrous and non-ferrous metals require different properties in order to function better in specific situations. Alloying metals involves mixing two or more metals and other elements to improve their properties. Alloying metals can:
1 of 24 © Boardworks Ltd 2005
Learning objectives
Learning objectives
2 of 24
To understand where metals come from and how they are prepared for use. To look at examples of ferrous metals, non-ferrous metals and alloys, and to know the properties of different metals. To understand how heat treatment can change the properties of metals. To be able to use hand tools to work with metals. To be familiar with the industrial processes used to manufacture metal goods.
Non-ferrous facts Aluminium is the most common non-ferrous metal, found in abundance in bauxite ore. Non-ferrous metals are not magnetic.
Non-ferrous metals: contain no iron are not attracted by a magnet.
9 of 24
© Boardworks Ltd 2005
A closer look at ferrous metals
10 of 24
© Boardworks Ltd 2005
Non-ferrous metals
Non-ferrous metals do not contain iron. These are pure metals used by designers, manufacturers and engineers in a wide variety of applications.
Ferrous metals are obtained from iron ore. You might recognize the letters ‘Fe’ from the periodic table, where they represent iron.
Ferrous facts Iron replaced bronze as the principal metal by 1000 BC. Early pots and pans made from iron poisoned the users! Early steels were made by adding carbon to iron as it was melted over a charcoal fire. Ferrous metals: contain iron will corrode unless protected are attracted by a magnet are strong, rigid and cheap.
lower the melting point alter thermal and electrical properties make a material harder for cutting purposes improve resistance to corrosion help metal to flow better into a cast.
© Boardworks Ltd 2005
Industrial processes
Milling machines are used to remove thin layers from a billet (block of material) which is clamped to the bed (base) of the machine. The material is fed past a cutting tool which has many sharp teeth and can remove material quickly. When manufacturers want to make cylindrical products, they use a centre lathe. Metals and plastics can be used on this machine. The work is held in a chuck and a cutting tool is moved towards the work while being held in a tool holder, mounted on the tool post.
