冬斯说明书
冬斯说明书

技术参数垒块类型403 405/407 410/412 415最大供给压强200毫巴(20千帕)360毫巴(36千帕)IP等级IP54外界温度外界温度-15℃至+70℃电磁阀DIN EN 161,A类,2组电压/频率~(AC)50-60Hz220-230V-15%至+10%~(AC)50-60Hz230V-15%至+10%功率/需求24V A~(AC)230V20℃28VA~(AC)230V20℃50VA~(AC)230V20℃50V A~(AC)230V20℃除尘装置过滤器网眼规格为0.8mm,不许移动气体吸发装置即可更换过滤器组件部署图右失(刃)垒块由以下部分组成1-法兰1-过滤器2-气体压强测试点1-气体压强开关3-法兰固定螺丝1-压强稳定器4-阻尼器调整装置2-电磁阀5-制动器调整装置-可快速打开安全阀6-输出装置螺母-缓慢打开调整阀7-用于螺母固定螺钉(未密封)图1. 8-低气压开关MB DLE 405-407-410-412-415 B01 S20 MB DLE 403 B01 S20非必需情况下需移动六角栓,请根据第3页指示操作。
气体吸收装置组装气体吸收装置必需在燃烧器左侧安装,如安装需在右侧进行时,有必需将另一侧压强测试点移动。
(2)对于型号MB DLE 403 B01,有必需移动气压开关位置。
(8)如气体吸收器直径和燃烧器直径不一致,就需要在二者之间安装转换器气体供给管道和气体吸收器之间连接,经过使用气体吸收器法兰和将其固定起来螺母实现(3)最好使用十字头螺钉在任何情况下,安装阀时候线圈不能处于朝地一面,当完成安装后,必需检验是否存在漏气并确保气体吸收器正常工作。
压强稳定器调整(4)使用适宜螺丝起子来固定压强调整器:顺时针方向位拧紧,逆时针方向为松开。
当压强阀安装达成理想效果后,盖上盖子并将电线末端用铅封好,使穿过孔间线圈尽可能短。
阀调整缓慢点火输出(阀初始开启相位)调整:再打开外壳(5)并根据+/—方向旋转螺钉以后进行翻转外壳(5)能够作为调整工具。
冬斯电磁阀参数说明

冬斯电磁阀参数说明全文共四篇示例,供读者参考第一篇示例:冬斯电磁阀参数说明冬斯电磁阀是一种常用的控制元件,在工业自动化领域广泛应用。
它通过电磁力来控制阀门的开启和关闭,从而实现流体的控制。
在选择使用冬斯电磁阀时,了解其参数是非常重要的。
下面将详细介绍冬斯电磁阀的参数说明。
1. 电压范围:冬斯电磁阀一般适用于直流电压或交流电压,通常电压范围在12V至240V之间。
在选择电磁阀时,需根据实际情况选取合适的电压范围。
2. 通径大小:通径大小是指电磁阀进出口的口径大小,通常以英寸或毫米为单位。
通径大小决定了电磁阀适用的流体流量大小,通常通径越大,流量也就越大。
3. 工作压力:工作压力是指电磁阀能够承受的最大压力,通常以MPa或bar为单位。
在选择电磁阀时,需根据工作介质的压力选择适合的工作压力范围。
5. 寿命参数:寿命参数是指电磁阀的使用寿命,通常以次数或时间为单位。
一般来说,电磁阀的寿命与其制造质量和使用环境有关,因此在选择电磁阀时,需考虑其寿命参数。
6. 控制方式:控制方式是指电磁阀的开启和关闭方式,常见的控制方式有直接式、间接式和脉冲式等。
在选择电磁阀时,需根据控制方式来确定适合的电磁阀。
以上是关于冬斯电磁阀的参数说明,希望对大家选择和使用电磁阀有所帮助。
冬斯电磁阀是一种常用的控制元件,具有广泛的应用领域,我们可以根据实际需要选择不同参数的电磁阀,以满足不同的控制需求。
通过了解和掌握电磁阀的参数,可以更加准确地选择合适的电磁阀,从而确保系统的稳定运行。
希望本文能够为大家提供一些参考,谢谢阅读!第二篇示例:冬斯电磁阀参数说明冬斯电磁阀是一种常见的控制装置,广泛应用于工业、农业、生活等各个领域。
它通过控制电磁铁的电流来控制阀门的开启和关闭,从而实现流体的控制。
在选择和应用冬斯电磁阀时,了解其参数是至关重要的。
下面我们将详细介绍冬斯电磁阀的各项参数。
1. 阀体材质:冬斯电磁阀的阀体通常采用优质的不锈钢材质制成,具有耐腐蚀、耐高温、抗压能力强的特点,适用于各种恶劣环境下的使用。
Viltepso (Viltolarsen) 使用说明说明书

UnitedHealthcare ® Community PlanViltepso ® (Viltolarsen)Policy Number : CS2023D0095KEffective Date : September 1, 2023 Instructions for Use Table of Contents Page Application ..................................................................................... 1 Coverage Rationale ....................................................................... 1 Applicable Codes .......................................................................... 2 Background ................................................................................... 2 Clinical Evidence ........................................................................... 3 U.S. Food and Drug Administration ............................................. 4 References ..................................................................................... 4 Policy History/Revision Information ............................................. 5 Instructions for Use ....................................................................... 5 This Medical Benefit Drug Policy does not apply to the states listed below; refer to the state-specific policy/guideline, if noted: State Policy/Guideline FloridaRefer to the state’s Medicaid clinical policy KansasNone LouisianaRefer to the state’s Medicaid clinical policy New JerseyViltepso ® (Viltolarsen) (for New Jersey Only) North CarolinaNone OhioViltepso ® (Viltolarsen) (for Ohio Only) PennsylvaniaViltepso ® (Viltolarsen) (for Pennsylvania Only) TexasRefer to drug-specific criteria found within the Texas Medicaid Provider Procedures ManualViltepso (viltolarsen) may be covered for the treatment of Duchenne muscular dystrophy (DMD) in patients who meet all of the following criteria :• For initial therapy , all of the following:o Diagnosis of Duchenne muscular dystrophy by, or in consultation with, a neurologist with expertise in the diagnosis of DMD; and o Submission of medical records (e.g., chart notes, laboratory values) confirming the mutation of the DMD gene is amenable to exon 53 skipping; ando One of the following: ▪ Submission of medical records (e.g., chart notes, laboratory values) confirming that the patient has a 6-MinuteWalk Test (6MWT) ≥ 300 meters while walking independently (e.g., without side-by-side assist, cane, walker,wheelchair, etc.) prior to beginning Viltepso therapy; or▪ Both of the following:Commercial Policy • Viltepso ® (Viltolarsen)Submission of medical records (e.g., chart notes) confirming that the patient is ambulatory without needing anassistive device (e.g., without side-by-side assist, cane, walker, wheelchair, etc.); and o One of the following:▪Patient has achieved a score of greater than 17 on the North Star Ambulatory Assessment (NSAA); or▪Patient has achieved a time to rise from the floor (Gower’s test) of less than 7 seconds;ando Viltepso is prescribed by, or in consultation with, a neurologist with expertise in the treatment of DMD; ando Viltepso dosing for DMD is in accordance with the United States Food and Drug Administration approved labeling; and o Viltepso is not used concomitantly with other exon skipping therapies for DMD; ando Initial authorization will be for no more than 6 monthsFor continuation of therapy, all of the following:o Patient has previously received Viltepso; ando Viltepso is prescribed by, or in consultation with, a neurologist with expertise in the treatment of DMD; ando Submission of medical records (e.g., chart notes) confirming that the patient is ambulatory without needing an assistive device (e.g., without side-by-side assist, cane, walker, wheelchair, etc.); ando Viltepso dosing for DMD is in accordance with the U.S. Food and Drug Administration (FDA) approved labeling; and o Viltepso is not used concomitantly with other exon skipping therapies for DMD; ando Reauthorization will be for no more than 12 monthsViltepso will not be covered for other forms of muscular dystrophy.The following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. Listing of a code in this policy does not imply that the service described by the code is a covered or non-covered health service. Benefit coverage for health services is determined by federal, state, or contractual requirements and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Guidelines may apply.HCPCS Code DescriptionJ1427 Injection, viltolarsen, 10 mgDiagnosis Code DescriptionG71.01 Duchenne or Becker muscular dystrophyDuchenne muscular dystrophy (DMD) is an X-linked disease that affects 1 in 3,600-6,000 live male births. DMD occurs as a result of mutations (mainly deletions) in the dystrophin gene. These mutations lead to an absence or a defect of the protein, dystrophin, resulting in progressive muscle degeneration, leading to loss of ambulation and additional respiratory, orthopedic, and cardiac complications. If left untreated, mean age of death is approximately 19 years of age.2-4Viltolarsen is an antisense oligonucleotide of the phosphorodiamidate morpholino oligomer (PMO) subclass. PMOs are synthetic molecules in which the five-membered ribofuranosyl rings found in natural DNA and RNA are replaced by a six-membered morpholino ring. Each morpholino ring is linked through an uncharged phosphorodiamidate moiety rather than the negatively charged phosphate linkage that is present in natural DNA and RNA. Each phosphorodiamidate morpholino subunit contains one of the heterocyclic bases found in DNA (adenine, cytosine, guanine, or thymine).1Viltolarsen is designed to bind to exon 53 of dystrophin pre-mRNA, resulting in exclusion of this exon during mRNA processing in patients with genetic mutations that are amenable to exon 53 skipping. Approximately 8% of DMD patients have out of frame deletion mutations amenable to exon 53 skipping. Exon skipping is intended to allow for production of an internally truncated dystrophin protein.1Eteplirsen (Exondys 51) was the first PMO approved by the U.S. Food and Drug Administration for treatment of DMD patients with confirmed genetic mutations amenable to exon 51 skipping. Approximately 13% of DMD patients have out of frame deletion mutations amenable to exon 51 skipping. This indication was approved under accelerated approval based on an increase in dystrophin in skeletal muscle observed in some patients treated with eteplirsen. A clinical benefit of eteplirsen has not been established. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.5Golodirsen (Vyondys 53) was the second PMO approved by the US Food and Drug Administration for treatment of DMD patients with confirmed genetic mutations amenable to exon 53 skipping, the same population as viltolarsen. Approximately8-10% of DMD patients have out of frame deletion mutations amenable to exon 53 skipping. This indication was also approved under accelerated approval based on an increase in dystrophin in skeletal muscle.6Casimersen (Amondys 45) is an antisense oligonucleotide that is designed to bind to exon 45 of dystrophin pre-messenger RNA, resulting in exon 45 skipping during messenger RNA processing in patients with amenable deletion mutations of the DMD gene. The FDA granted accelerated approval of casimersen for the treatment of patients with DMD who have a confirmed mutation of DMD that is amenable to exon 45 skipping, which is thought to cause approximately 8 percent of DMD cases.14Viltolarsen is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 53 skipping.1A phase II study evaluated two doses of viltolarsen in 16 ambulatory boys aged 4 to 9 years with a DMD diagnosis and DMD gene amenable to exon 53 skipping over 24 weeks. Ambulatory boys on a stable corticosteroid regimen for at least 3 months who could complete time to stand from supine, time to run/walk 10 m, and time to climb four stairs assessments were included. The study was a multicenter, two period dose-finding clinical trial. The first study period, which corresponded to the first 4 weeks of treatment following enrollment, was double-blinded and placebo-controlled. Participants in both dose cohorts were randomized 3:1 to receive viltolarsen or placebo. The second study period began at week 5 for each participant. During this period, all participants received viltolarsen according to their cohort dose for a 20-week open-label treatment. Primary study outcomes included safety, tolerability, and pharmacokinetics of low-dose (40 mg/kg per week) and high-dose (80 mg/kg per week) viltolarsen in ambulant boys with DMD. Efficacy was assessed based on change from baseline in dystrophin protein level (measured as % of the dystrophin level in healthy subjects, i.e., % of normal) at week 25. Muscle dystrophin production was assessed as protein production by Western blot for the primary study efficacy outcome and as dystrophin mRNA splicing on RT-PCR, dystrophin protein production by MS, and dystrophin localization by IF staining for secondary study efficacy outcomes. Additional secondary efficacy outcomes were gross motor skill assessments of timed function tests, including time to stand from supine, time to run/walk 10 m, time to climb four stairs, North Star Ambulatory Assessment, and 6-minute walk test as well as quantitative muscle testing. These outcomes were compared with a matched natural history control group from the Cooperative International Neuromuscular Research Group (CINRG) Duchenne Natural History Study (DNHS).In patients who received viltolarsen 80 mg/kg once weekly, mean dystrophin levels increased from 0.6% of normal at baseline to 5.9% of normal by week 25, with a mean change in dystrophin of 5.3% of normal levels (p = 0.01) as assessed by validated Western blot; the median change from baseline was 3.8%. All patients demonstrated an increase in dystrophin levels over their baseline values. As assessed by mass spectrometry, mean dystrophin levels increased from 0.6% of normal at baseline to 4.2% of normal by week 25, with a mean change in dystrophin of 3.7% of normal levels; the median change from baseline was 1.9%.Comparison of viltolarsen-treated participants with 65 age-matched and treatment matched natural history controls from CINRG DNHS suggested evidence of clinical benefit of viltolarsen treatment. Viltolarsen-treated participants showed improvement or stabilization of function over the 25-week period, whereas the CINRG DNHS external comparator group exhibited a decline in all timed function tests, except for time to climb four stairs. Velocity in the time to run/walk 10 m test significantly improved in viltolarsen-treated participants at weeks 13 and 25 compared with a decline in controls from CINRG DNHS (change at 25 weeks compared with baseline: viltolarsen, 0.23 m/s; control, −0.04m/s). The 6-minute walk test showed significant improvement at week 25 in viltolarsen treated participants, whereas results from CINRG DNHS controls declined over the same period (change at 25 weeks compared with baseline: viltolarsen, 28.9 m; control, −65.3 m). Significant improvements in time to stand from supine were observed (change at 25 weeks compared with baseline: viltolarsen, −0.19 s; control, 0.66 s). Velocity in the time toimprovement or stabilization, but the differences between viltolarsen treatment and external comparator controls were not significant. Measures of muscle strength by isometric testing showed no differences between viltolarsen-treated participants and the CINRG DNHS external comparator control group.7RACER53 is an ongoing 48-week, phase 3 double-blind, placebo controlled, randomized clinical trial that will evaluate the efficacy of viltolarsen in ambulatory DMD patients with out-of-frame deletion mutations amenable to skipping exon 53. The study will enroll 74 boys from 4 to 8 years of age with genotypically confirmed DMD on a stable dose of corticosteroids who can walk independently without assistive devices with a time to stand of less than 10 seconds. The primary endpoint is the change from baseline to week 48 in the time to stand. Secondary outcomes include the change in time to run/walk 10 meters, change in 6MWT, change in the NSAA, change in time to climb four steps, and change in muscle force contraction measured by dyanometry.8The SKIP-NMD trial of golodirsen is a U.S.-based, blinded, placebo-controlled, dose-escalation two-part Phase I/II RCT of male patients aged six to 15 years with a DMD diagnosis and DMD gene amenable to exon 53 skipping. Patients age 6 to 15 years with stable cardiac and pulmonary function, and on a stable dose of corticosteroids for at least six months were included. Additional inclusion criteria included a baseline six-minute walk test (6MWT) of greater than 250 m, a North Star Ambulatory Assessment (NSAA) score of greater than 17 or a rise time of less than 7 seconds. In part one, 12 patients were randomized to receive once-weekly intravenous infusions at escalating doses of 4, 10, 20, 30 mg/kg of golodirsen or matching placebo for 12 weeks. Part two consists of an open-label period of all patients from part one and 13 newly recruited patients who are receiving once-weekly infusions of 30 mg/kg of golodirsen for up to 168 weeks.Part one of the SKIP-NMD trial assessed safety and tolerability. In part two, the primary endpoints are change from baseline in 6MWT at 144 weeks and change in dystrophin protein levels at 48 weeks. Secondary endpoints include drug pharmacokinetics, change from baseline in FVC percent predicted, and change from baseline in dystrophin intensity at 144 weeks.At the time of pre-planned interim analysis, data from baseline and week 48 muscle biopsies, exon 53 skipping, and dystrophin localization were available for 25 patients on golodirsen. The study is ongoing, and results for the primary efficacy endpoint of 6MWT at week 144 are not yet available. Mean baseline of dystrophin in the trial was reported to be 0.095% of normal. At 48 weeks, the mean level of dystrophin had increased to 1.019% of normal resulting in an absolute increase of 0.918% of normal (p < 0.001). A clinically meaningful change in level of dystrophin has not yet been established in humans. As such, the clinical significance of these results is not clear. Among individual patients, dystrophin levels at week 48 ranged from 0.09% to4.30%.9-11ESSENCE is an ongoing 96-week, Phase 3, double-blind, placebo controlled, randomized clinical trial that will evaluate the efficacy of golodirsen and casimersen in DMD patients with out-of-frame deletion mutations amenable to skipping exon 53 and exon 45, respectively. The study will enroll 222 boys from 7 to 13 years of age with genotypically confirmed DMD and 6MWT ≥ 300 m and ≤ 450 m. The primary endpoint is the change from baseline to week 96 in 6MWT.12Viltolarsen or golodirsen have not been studied in DMD that is not amenable to exon 53 skipping, nor in other forms of muscular dystrophy (e.g., Becker muscular dystrophy).1,6This section is to be used for informational purposes only. FDA approval alone is not a basis for coverage.Viltepso is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 53 skipping. This indication is approved under accelerated approval based on an increase in dystrophin in skeletal muscle observed in some patients treated with Viltepso. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.1.Viltepso [package insert]. Paramus, NJ; NS Pharma, Inc, March 2021.2.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne musculardystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol; 2010 Jan; 9(1):77 93.3.Bushby K, Finkel R, Birnkrant DJ, et al. (2010) Diagnosis and management of Duchenne muscular dystrophy, part 2:implementation of multidisciplinary care. Lancet Neurol; 2010 Jan; 9(2):177-189.4.Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis,and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol.2018;17(3):251-267. doi: 10.1016/S1474-4422(18)30024.5.Exondys 51 [package insert]. Cambridge, MA: Sarepta Therapeutics, Inc, January 2022.6.Vyondys 53 [package insert]. Cambridge, MA: Sarepta Therapeutics, Inc, February 2021.7.Clemens PR, Rao VK, Connolly AM, et al. Safety, tolerability, and efficacy of viltolarsen in boys with Duchenne musculardystrophy amenable to exon 53 skipping: a phase 2 randomized clinical trial. JAMA Neurol. 2020; 26;77(8):1-10. doi:10.1001/jamaneurol.2020.2025.8.Study to Assess the Efficacy and Safety of Viltolarsen in Ambulant Boys With DMD (RACER53)https:///ct2/show/NCT04060199?term=viltolarsen&draw=2&rank=1. Accessed June 5, 2023.9.Frank DE, Mercuri E, Servais, L, et al. Golodirsen induces exon skipping leading to sarcolemmal dystrophin expression inpatients with genetic mutations amenable to exon 53 skipping. Poster presented at: Annual Clinical Genetics Meeting of the American College of Medical Genetics and Genomics; April 2-6, 2019; Seattle, WA.10.Muntoni F, Frank DE, Morgan J, et al. Golodirsen induces exon skipping leading to sarcolemmal dystrophin expression inpatients with genetic mutations amenable to exon 53 skipping [abstract]. Neuromuscul Disord. 2018;28:S5. Abstract D01.11.Muntoni F, Frank DE, Sardone V, et al. SRP-4053 induces exon skipping leading to sarcolemmal dystrophin expression inDuchenne muscular dystrophy patients amenable to exon 53 skipping. Poster presented at: 22nd International Annual Congress of the World Muscle Society; October 3-7, 2017; Saint Malo, France.12.Study of SRP-4045 and SRP-4053 in DMD Patients (ESSENCE)https:///ct2/show/NCT02500381?term=golodirsen&cond=Duchenne+Muscular+Dystrophy&rank=3.Accessed June 5, 2023.13.Institute for Clinical and Economic Review (ICER). Deflazacort, eteplirsen, and golodirsen for Duchenne musculardystrophy: Effectiveness and value: Evidence Report. https:///wp-content/uploads/2020/10/Corrected_ICER_DMD-Final-Report_042222.pdf. April 22, 2022. Accessed June 5, 2023. 14.Amondys 45 [package insert]. Cambridge, MA; Sarepta Therapeutics, Inc.: March 2023.Date Summary of Changes09/01/2023 ApplicationLouisianaReplaced reference link to the state-specific policy version with instruction to refer to the state’sMedicaid clinical policySupporting InformationArchived previous policy version CS2023D0095JThis Medical Benefit Drug Policy provides assistance in interpreting UnitedHealthcare standard benefit plans. When deciding coverage, the federal, state, or contractual requirements for benefit plan coverage must be referenced as the terms of the federal, state, or contractual requirements for benefit plan coverage may differ from the standard benefit plan. In the event of a conflict, the federal, state, or contractual requirements for benefit plan coverage govern. Before using this policy, please check the federal, state, or contractual requirements for benefit plan coverage. UnitedHealthcare reserves the right to modify its Policies and Guidelines as necessary. This Medical Benefit Drug Policy is provided for informational purposes. It does not constitute medical advice.UnitedHealthcare may also use tools developed by third parties, such as the InterQual® criteria, to assist us in administering health benefits. The UnitedHealthcare Medical Benefit Drug Policies are intended to be used in connection with the independent professional medical judgment of a qualified health care provider and do not constitute the practice of medicine or medical advice.。
Tissue-Tek Cryo 3 Flex 冻剂切片仪产品说明书

Feather is a registered trademark of Feather Safety Razor Co. and is used with permission.
7
A long tradition of excellence
Known for best-in-class automation and reliability Sakura Finetek remains a privately-held company in business for over 160 years. Sakura Finetek has achieved its success and solidified its reputation by providing timely, ingenious solutions to the real challenges laboratories face on a day-to-day basis.
• Intermittent* sectioning: 1 to 99 sections or total thickness of 1 to 99 microns
* Optional features available on Disinfection (D) and Disinfection/Motorized (DM) models
1 to 99 microns in 1 microns steps
1 to 100 microns in 1 microns steps
IEC 61010-1:2010 Ed:3, IEC 61010-2-010 Ed:3, IEC 61010-2-101 Ed:2, UL 61010-1 Ed:3, CAN/CSA C22.2 61010-1:2012 Ed:3 EMC: CENELEC IEC/EN 61326-1, EMC: CANELEC IEC/EN 61326-2-6
冬斯说明书

技术参数垒块类型 403 405/407410/412415最大供应压强200毫巴(20千帕)360毫巴(36千帕) IP 等级 IP54外界温度 外界温度—15C 至+ 70 C 电磁阀DIN EN 161 , A 类,2 组电压/频率〜(AC )50-60Hz220-230V —15腕 + 10% 〜(AC ) 50-60HZ230V —15%至 + 10%功率/需求24VA 〜(AC )230V 20 C28VA 〜(AC ) 230V 20 C50VA 〜(AC ) 230V 20 C50VA 〜(AC ) 230V 20 C除尘装置过滤器网眼规格为 0.8mm ,不许移动气体吸发装置即可更换过滤器组件垒块由以下部分构成 1-过滤器1-气体压强开关 1- 压强稳定器 2- 电磁阀-可迅速打开的安全阀 -缓慢打开的调整阀 图1.MB DLE 405-407-410-412-415 B01 S20布置图右失(刃) 1- 法兰2- 气体压强测试点 3- 法兰固定螺丝 4- 阻尼器调节装置 5- 制动器调节装置 6- 输出装置螺母7- 用于螺母的固定螺钉(未密封的)8- 低气压开关nIT ®r V 吒毕―4248632CCn◎OGQ 0J —«B• 叮6A 8LU非必要情况下需移动六角栓,请按照第3页的指示操作。
气体吸收装置的组装气体吸收装置必须在燃烧器的左侧安装,如安装需在右侧进行时,有必要将另一侧的压强测试点移动。
(2)对于型号MB DLE 403 B01,有必要移动气压开关的位置。
(8)如气体吸收器直径与燃烧器直径不一致,就需要在两者之间安装转换器气体供应管道与气体吸收器之间的的连接,通过使用气体吸收器的法兰与将其固定起来的螺母实现(3)最好使用十字头的螺钉在任何情况下,安装阀的时候线圈不能处于朝地的一面,当完成安装后,必须检查是否存在漏气并确保气体吸收器正常工作。
压强稳定器的调整(4)使用合适的螺丝起子来固定压强调整器:顺时针方向位拧紧,逆时针方向为松开。
冬斯MB -ZR420 说明书

Meßanschluß G 1/8 nach Ventil 1, beidseitig möglich
Test point connection G 1/8 downstream of valve 1, possible on both sides
Gasflußrichtung Gas flow direction
Ausgangsflansch Output flange
Meßanschluß G 1/8 nach Ventil 2 Test point connection G 1/8 downstream of valve 2
Hydraulikbremse oder Einstellteller Hydraulic brake or setting plate
Druckabgriffe / Pressure taps
0
5
1
4 23
Einbaumaße / Dimensions / [mm]
32 4
02 pe
3
4
pa
pBr
1
2
5
pe
5
3
4
pa
pBr
1,2,3,4,5 1,2,3,4,5 1,2,3,4,5
Verschlußschraube G 1/8 G 1/8 screwed sealing plug
pmax. = 360 mbar
°C
+70
0 -15
Umgebungstemperatur Ambient temperature
-15 °C … +70 °C
冬斯GW-A5压力监测器中文说明书

26
DIN EN 175 301-803
3…6
13,5
42
12,5
1 2 3 4
1 2 3
2 pe 4 1
3 pa
4 2 pe 3 pa
3
2
1
1,3, 2 4
P L
Mp N
P L
6
7 1 2 � � 43 5
6
1
2
7
3 pe
4 pa
5 pBr 6
1 2 3 4 5 6 7
5 6
5 34
2
3
4 1
2
4…6
1
4 4 4 4
2
3 4
4
1 2 3 4
2 1
3
2 1
V1
1 2
V2
3
1,2,4 G 1/8 DIN ISO 228 3
M4
2 1 3 2 1
1 4
4 4 2 3 4 4
2
3 4
MBC-300/700/1200
1
V1
1
V2
2 3
1
1 2 3 4
� � �
� �
2
3
4
�
� � �
GW...A5 GW...A5/1
5.02
适合燃气类1, 2, 3的燃气以及其他中 性气态介质。 许可证 根据欧盟气体装置准则颁发的欧盟样 机检查证:
242 576
GW…A5 CE-0085 AO 3220 根据欧盟高压装置准则颁发的欧盟样 机检查证:
02.10
GW…A5 CE0036 按照EN 1854为“S”级。 其它重要的气体使用国家的许可证。
221 630
Thermo King SLXe 寒冷箱说明书

Fácil de seleccionarFunción de bloqueo de alta velocidadFácil de utilizarFuncionamiento eléctrico SmartPower™ (OPCIONAL)Para obtener más informacióno conocer las sesiones de tutoría,póngase en contacto con suresponsable de servicio deThermo King.La función de bloqueo de alta velocidadse utiliza, generalmente, en zonasacústicamente sensibles para reducir elruido que emite el motor diesel. Estafunción no tiene efecto alguno cuandola unidad se encuentra en modo defuncionamiento eléctrico.1. Pulse la tecla Bloqueo de alta velocidad.• Se encenderá el indicador luminosoámbar para indicar que la unidad seencuentra en modo de bloqueo dealta velocidad.2. Si vuelve a pulsar la tecla Bloqueode alta velocidad, se desactivaráesta función.1. Conecte el suministro de corriente delvoltaje apropiado al receptáculo dealimentación de la unidad.2. Pulse la tecla de ENCENDIDO paraencender la unidad.• La alarma sonora de precalentamientosonará durante 20 segundos antesde que el motor eléctrico se pongaen marcha.Visualización de los códigos de alarma1. Pulse simultáneamente y mantenga pulsadas la tecla de ENCENDIDO y la teclaREV ANT VIA.TK 61004-8-PC-ES (Vers. 0, 01/13) ©Thermo King Corporationconmutación automática de eléctrico a dieselSi la función conmutación automática de eléctrico a diesel activada estáconfigurada en SÍ, la unidad conmutará automáticamente de modo eléctrico a mododiesel cuando se retire o falle la fuente de alimentación eléctrica.conmutación automática de diesel a eléctricoSi la función conmutación automática de diesel a eléctrico activada estáconfigurada en SÍ, la unidad conmutará automáticamente de modo diesel amodo eléctrico cuando se detecte o se conecte la fuente de alimentación eléctrica.muestra en pantalla. Una vez borradastodas las alarmas, la pantalla mostraráúnicamente ceros para indicar que noexiste ningún código de alarma.• Antes de borrar cualquier alarma, deben visualizarse todas ellas.• Si una alarma no se borra, puede que todavía exista. Si la alarma no se corrige,no se borrará o puede que se vuelva a generar inmediatamente.• No es posible borrar algunas de las alarmas con la HMI.consulte el manual del operador para obtener más información en relacióncon los códigos de alarma.1. Pulse la tecla de ENCENDIDO para encender la unidad.• Aparecerán guiones tanto en la parte superior como en la parte inferior de la pantalla mientras se enciende.• A continuación, se muestranbrevemente los contadores horarios relativos al tiempo de funcionamiento.• Aparecerá la pantalla estándar mostrando la temperatura del compartimento y la del punto de consigna.• El motor diesel se precalentará y arrancará según sea necesario cuando la unidad se encuentre1. Pulse la tecla CYCLE-SENTRY/Continuo para pasar del modo CYCLE-SENTRY al modo continuo.• El indicador luminoso de color ámbar indica que la unidad está funcionando en modo CYCLE-SENTRY.• Si no hay ningún indicadorluminoso encendido, la unidad está funcionando en modo continuo.1. Pulse la tecla DESCARCH para iniciar un descarche manual.• Un indicador luminoso ámbar junto a la tecla DESCARCH indica que la unidad se encuentra en descarche.NOTA: el ciclo de descarche finaliza automáticamente cuando el serpentín delevaporador alcanza una temperatura predeterminada o cuando haya transcurrido el tiempo establecido por el temporizador de descarche. También se puede finalizar un ciclo de descarche apagando y volviendo a encender la unidad.prueba de reVisiÓn antes del ViaJe completaNOTA: la revisión antes del viaje completa debe realizarse con la unidad apagada.encendida. En las unidades equipadas con SmartPower, el motor eléctrico se pondrá en marcha si la unidad está conectada a una fuente de alimentación eléctrica.2. Pulse la tecla de APAGADO para apagar la unidad.• La unidad se apagará inmediatamente y la pantalla quedará en blanco.1. Pulse las teclas de dirección hacia ARRIBA o hacia ABAJO hasta que se muestre el punto de consigna deseado.2. Pulse la tecla ENTRAR para confirmar el nuevo punto de consigna.1. Encienda la unidad, borre todos los códigos de alarma y apague la unidad.2. Vuelva a encender la unidad y espere a que se muestren los contadores horarios del tiempo de funcionamiento de la unidad. Cuando se muestren los contadores horarios, pulse y mantenga pulsada la tecla REV ANT VIA durante 5 segundos.• Un indicador luminoso parpadeante indica que se ha iniciado la revisión antes del viaje.• Un indicador luminoso de color ámbar iluminado permanentemente indica que la revisión antes del viaje está en curso.• La prueba de revisión antes del viaje completa tarda, generalmente, entre 20 y 30 minutos.• El indicador luminoso ámbar se apagará cuando se complete la prueba de revisión antes del viaje o si se produce una alarma de apagado.prueba de reVisiÓn antes del ViaJe con el motor en FuncionamientoNOTA: la prueba de revisión antes del viaje con el motor en funcionamiento debe realizarse con la unidad en funcionamiento.1. Encienda la unidad, borre todos los códigos de alarma y permita que la unidad se ponga en marcha.2. Con la unidad en funcionamiento, pulse y mantenga pulsada la tecla REV ANT VIA durante 5 segundos.• Un indicador luminoso parpadeante indica que se ha iniciado la revisión antes del viaje.• Un indicador luminoso de color ámbar iluminado permanentemente indica que la revisión antes del viaje está en curso.• La prueba de revisión antes del viaje completa tarda, generalmente, entre 20 y 25 minutos.• El indicador luminoso ámbar se apagará cuando se complete la prueba de revisión antes del viaje o si se produce una alarma de apagado.Para detener una prueba de revisión antes del viaje en cualquier momento, pulse la tecla de APAGADO para apagar la unidad. Esta acción generará el código de alarma 28: Interrupción de la revisión antes del viaje.resultados de la prueba de reVisiÓn antes del ViaJe Superación de la prueba de revisión antes del viaje• Si la unidad supera la prueba de revisión antes del viaje, se apagará el indicador luminoso ámbar correspondiente a esta cuando finalice la prueba y la unidad seguirá funcionando según sea necesario.Fallo de la prueba de revisión antes del viaje con alarmas de corrección• Si la unidad no supera la prueba de revisión antes del viaje y se generan alarmas de corrección, aparecerá el icono de alarma cuando se produzca la condición de alarma. Seguirá realizándose la prueba de revisión antes del viaje a menos que se genere una alarma de apagado.consulte el manual del operador para obtener más información en relación con los códigos de alarma.。
DUNGS冬斯MB - ZR (DLE) B01电磁阀中文说明书
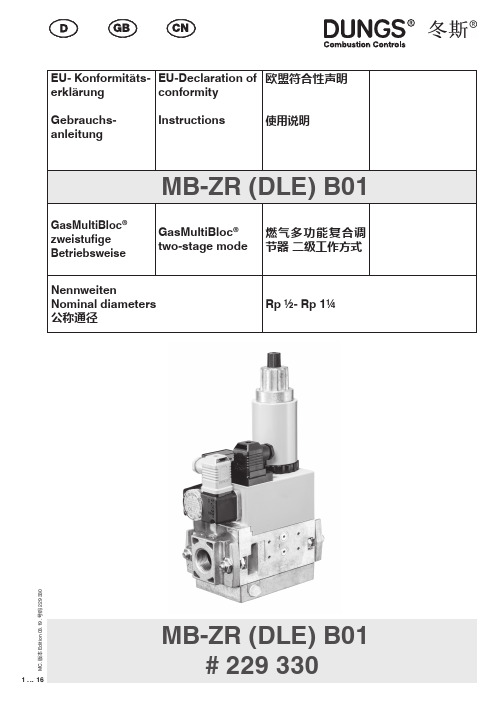
MB-ZR (DLE) B01# 229 3302 (16)M C . 版本 E d i t i o n 03.19 .号码 229 33Dr.-Ing. Karl-Günther Dalsaß,Geschäftsführer / Chief Operating Officer 总经理Urbach, 2018-04-213 (16)M C . 版本 E d i t i o n 03.19 .号码 229 3304 (16)M C . 版本 E d i t i o n 03.19 .号码 229 330GasMultiBloc ® zweistu-fi ge Betriebsweise T yp MB-ZR (DLE) B01NennweitenRp 1/2 - Rp 1 1/4GasMultiBloc ® two-stage operation T yp MB-ZR (DLE) B01Nominal diametres Rp 1/2 - Rp 1 1/4操作和安装说明燃气多功能复合调节器 二级工作方式MB - ZR (DLE) B01 型公称内径Rp 1/2 - Rp 1 1/4Max. BetriebsdruckMax. operating pressure 最大 工作压力p max.V1+V2 Klasse A, Gruppe 2V1+V2 Class A, Group 2V1+V2 A级2类nach / acc. / 根据EN 161SchutzartDegree of protection 保护程度IP 54 nach / acc. / 根据IEC 529 ( DIN 40 050)Familie 1 + 2 + 3Family 1 + 2 + 3系列 1 + 2 + 3Ausgangsdruckbereich Outlet pressure range 输出压力范围S 20 / S 22: 4 - 20 mbar (0,4 - 2 kPa)S 50 / S 52: 4 - 50 mbar (0,4 - 5 kPa)Druckwächter/ Pressure Switch/压力监控器T yp/T ype/型号GW…A5, GW…A2, NB…A2, ÜB…A2nach / acc. / 根据EN 1854FeinsiebFine-mesh sieve A级2类nach / acc. / 根据U n ~(AC) 220 V-15 % …- 230 V+10 %Einschaltdauer/Switch-on duration/ 开关时间 100 %fl e!在液化气设备中使用时,mb-ZR不得在低于0°C下运行。
Tissue-Tek Cryo3 Flex Microtome Cryostat 说明书

Imagine... smooth frozen sections with optimal disinfection Tissue-Tek® Cryo3® Flex Microtome/CryostatEFFORTLESSLY OBTAIN FROZEN SECTIONS FASTER WITH THE NEW CRYO3® FLEXThe Tissue-Tek® Cryo3® Flex is the 3rd generation of the trustedCryo3platform for fast sectioning of frozen tissue specimens. This cryostat is equipped with new, innovative features designed to get complete sections faster and to preserve valuable tissue.All required configurationsThe Tissue-Tek Cryo3Flex is available in three configurations, to meet every laboratories needs and budget demands. The range starts with the Basic, the cryostat with manual microtome. The second model is equipped with automated disinfection and the third model offers a combination of automated sectioning and a motorised microtome. No matter which model you choose, you will benefit from time-tested methods, operator simplicity, minimal maintenance and of course renowned Sakura Tissue-Tek reliability.Faster sectioningOf the new features, the most important is the fine angle adjustment of the 3D Precision Chuck on all 3 axes. It uniquely ensures accurate alignment of the block face to the blade, reduces users’ trimming time and preserves patients’ specimens. Furthermore, the sturdy chuck retains its position when locked and unlocked, eliminating any time and effort spent realigning the cutting angle.Innovative ozone disinfectionProtect yourself from pathogens with an unprecedented disinfection. TheTissue-Tek Cryo3Flex D and DM-models are equipped with two ozone generating lamps, which significantly shorten the short disinfection cycle to 45 minutes only. Ozone engulfs the entire chamber, notonly light exposed areas. Precise adjustment of face angle.Precise and reliable performanceThe maintenance-free microtome is located outside the chamber. This provides space for 12 Cryobar® specimen positions, including 4 Cryo+® positions that enable rapid cooling up to -50°C.Menu-driven, user- friendly controls are available via the colour touch screen. It provides a clear view on settings like chamber temperature, specimen and trim thickness and speed.Adjustments can be made in an improved and more intuitive manner. Equipped with the new anti-roll rake and anti-roll plate, specimen transfer to the slide is further simplified, increasing ease of use, especially for the less experienced.Dynamic debris removal systemThis vacuum system offers practical and easy removal of excess debris and O.C.T.™ Compound shavings. Unwanted sections can be swept into the vacuum head during sectioning, keeping the chamber clean. The attachment hose vacuums away debris inside the chamber. Eventually debris is collected in a bacterial/viral filter located under the multipurposeutility tray.By offering a total solution for cryotomy, SakuraFinetek makes your laboratories ready to deliverstandardised, superior quality results.Sakura Finetek is the innovative company inhistopathology, continuously looking forpossibilities to improve laboratories andsucceeds to meet present and future standardsin the histopathology laboratories around theworld.New Anti-Roll Rake.Sakura Finetek Cryo programEnhance the cryotomy process with the new Cryo 3 Flex combined with accessories and consumables, providing a total solution to quickly create a diagnosable slide of the highest quality.With our complete line of accessories you are able to fine tune the Tissue-Tek Cryo 3 Flex to perfectly suit your needs.PRODUCT RANGEBR-EU Cryo3 Flex 2018 v1Item code 620362046205Item code 621162105807 - 5809581958206213 + 6214Related productsTissue-Tek ® O.C.T. Compound; 12/cs Low Profile Cryo Sectioning Blades AutoWrite ® Adhesive SlidesRound Cryomold ® Standard ø 23 mm Round Cryomold ® Biopsy ø 15 mm Cryomold ® Standard; 25 x 20x 5 mm Cryomold ® Biopsy; 15 mm Cryomold ® Biopsy; 15 mm Manual Slide Staining setVisionTek ® Digital MicroscopeDescriptionCryo 3® Flex Cryostat, Basic Cryo 3® Flex Cryostat, Disinfection Cryo 3® Flex Cryostat, D. MotorizedKey accessoiries Anti-Roll Rake Assembly Anti-Roll Plate AssemblySpecimen Holders, Small, Medium, Large Cryobar ® Insert, 4-CavityCryobar ® Insert, 4-Post Utility TraysPlease visit our website sakura.euSakura Finetek Europe B.V., Flemingweg 10a, 2408 AV Alphen aan den Rijn P.O. Box 362, 2400 AJ Alphen aan den Rijn, The NetherlandsConsumables and related productsTissue-Tek ® O.C.T.™ Compound cryo embedding medium provides the most convenient specimen matrix for cryostat sectioning at -5°C and below.Accu-Edge ® Low-Profile Cryo section blades are recommended for optimal section results. These are made of carbon steel, resistant to high humidity circumstances.Besides this, you can choose our slides andcryo-molds to help you obtain consistent sections in the most reliable way.Item code 4583481095974728473045574565456644519002。
Ultomiris (ravulizumab-cwvz) 产品说明书

Ultomiris® (ravulizumab-cwvz)(Intravenous)Document Number: IC-0427 Last Review Date: 07/01/2021Date of Origin: 02/04/2019Dates Reviewed: 02/2019, 10/2019, 12/2019, 11/2020, 07/2021I.Length of AuthorizationCoverage will be provided for twelve months and may be renewed.II.Dosing LimitsA.Quantity Limit (max daily dose) [NDC Unit]:−Ultomiris 10 mg/mL****** – 30 mL SDV: 10 vials on day zero followed by 13 vials starting on day 14 and every 8 weeks thereafter−Ultomiris 100 mg/mL – 3 mL SDV: 10 vials on day zero followed by 13 vials starting on day 14 and every 8 weeks thereafter−Ultomiris 100 mg/mL – 11 mL SDV: 3 vials on day zero followed by 3 vials starting on day 14 and every 8 weeks thereafterB.Max Units (per dose and over time) [HCPCS Unit]:Indication Loading Dose Units Maintenance Dose UnitsPNH/aHUS 300 units on Day 0 360 units on Day 14 and every 8 weeks thereafter III.Initial Approval Criteria 1Coverage is provided in the following conditions:•Patient is at least 1 month of age; ANDANDAND•Patients must be administered a meningococcal vaccine at least two weeks prior to initiation of therapy (If urgent Ultomiris therapy is indicated in an unvaccinated patient,administer meningococcal vaccine(s) as soon as possible and provide patients with twoweeks of antibacterial drug prophylaxis.); ANDANDANDUniversal Criteria 1•Prescriber is enrolled in the Ultomiris Risk Evaluation and Mitigation Strategy (REMS) ANDprogram; AND• Confirmation that the patient will revaccinated with the meningococcal vaccine accordingto current medical guidelines for vaccine use; AND AND AND • Will not be used in combination with other complement-inhibitor therapy (i.e., eculizumab); AND AND Paroxysmal Paroxysmal Nocturnal Hemoglobinuria Nocturnal Hemoglobinuria Nocturnal Hemoglobinuria (PNH)(PNH)(PNH) † Ф 1,4,8• Used as Soliris switch therapy (please refer to Section IV for the pertinent renewal criteria); OR• Diagnosis must be accompanied by detection of PNH clones of at least 5% by flow cytometrydiagnostic testing; AND ANDAND o Demonstrate the presence of at least 2 different glycosylphosphatidylinositol (GPI)protein deficiencies (e.g., CD55, CD59, etc.) within at least 2 different cell lines (granulocytes, monocytes, erythrocytes); AND AND AND o Patient has one of the following indications for therapy:▪ Presence of a thrombotic event▪ Presence of organ damage secondary to chronic hemolysis▪ Patient is pregnant and potential benefit outweighs potential fetal risk ▪ Patient is transfusion dependent▪ Patient has high LDH activity (defined as ≥1.5 x ULN) with clinicalsymptoms; AND ANDAND o Documented baseline values for one or more of the following (necessary for renewal):serum lactate dehydrogenase (LDH), hemoglobin level, and packed RBC transfusion requirementAtypical Hemolytic Uremic Syndrome Atypical Hemolytic Uremic Syndrome (aHUS (aHUS) †) †) † 1,5,7• Used as Soliris switch therapy (please refer to Section IV for the pertinent renewalcriteria); OR OR • Patient shows signs of thrombotic microangiopathy (TMA) (e.g., changes in mental status,seizures, angina, dyspnea, thrombosis, increasing blood pressure, decreased platelet count, increased serum creatinine, increased LDH, etc.); AND ANDAND o Thrombotic Thrombocytopenic Purpura (TTP) has been ruled out by evaluatingADAMTS-13 level (ADAMTS-13 activity level > 10%); AND AND AND o Shiga toxin E. coli related hemolytic uremic syndrome (STEC-HUS) has been ruled out; AND AND AND o Other causes have been ruled out such as coexisting diseases or conditions (e.g.,bone marrow transplantation, solid organ transplantation, malignancy, autoimmune disorder, drug-induced, etc.) or known genetic defect in cobalamin C metabolism; ANDANDo Documented baseline values for one or more of the following (necessary for renewal): serum lactate dehydrogenase (LDH), serum creatinine/eGFR, platelet count, anddialysis requirement†FDA Approved Indication(s); ‡‡ Compendia Recommended Indication(s); Ф Orphan Drug IV.Renewal Criteria 1Coverage may be renewed based upon the following criteria:•Used as switch therapy, for a diagnosis of PNH or aHUS, if a patient has shown a beneficial disease response and absence of unacceptable toxicity while on Soliris; OROR •Patient continues to meet the universal and other indication-specific relevant criteria identified in section III; ANDANDANDo Absence of unacceptable toxicity from the drug. Examples of unacceptable toxicity include: serious meningococcal infections (septicemia and/or meningitis), infusion-related reactions, other serious infections (i.e. Streptococcus pneumoniae,Haemophilus influenzae, Neisseria gonorrhoeae), thrombotic microangiopathy(TMA) complications, etc.; ANDANDo Disease response indicated by one or more of the following::▪PNH1,4,8Decrease in serum LDH from pretreatment baselineStabilization/improvement in hemoglobin level from pretreatmentbaselineDecrease in packed RBC transfusion requirement from pretreatmentbaseline▪aHUS1,5,7Decrease in serum LDH from pretreatment baselineStabilization/improvement in serum creatinine/eGFR from pretreatmentbaselineIncrease in platelet count from pretreatment baselinePatient no longer requires dialysis treatmentsV.Dosage/Administration 1Paroxysmal nocturnal hemoglobinuria (PNH) & Atypical Hemolytic ComplementComplement--Inhibitor TherapyInhibitor Therapy Naïve*Naïve*Naïve*Administer the doses based on the patient’s body weight. Starting 2 weeks after the loading dose, begin maintenance doses once every 4 weeks or every 8 weeks (depending on body weight)Uremic Syndrome (aHUS)≥5 kg - <10 kg 600300Every 4 weeks ≥10 kg - <20 kg 600600Every 4 weeks ≥20 kg - <30 9002,100Every 8 weeks ≥30 kg - <40 kg 1,2002,700Every 8 weeks ≥40 kg - <60 kg 2,4003,000Every 8 weeks ≥60 kg - <100 kg 2,7003,300Every 8 weeks ≥100 kg 3,0003,600Every 8 weeks *Note: for Soliris switch therapy please refer to the package insert for appropriate switch dosing.VI.Billing Code/Availability InformationHCPCS Code:•J1303 − Injection, ravulizumab-cwvz, 10 mg; 1 billable unit = 10 mgNDC:•Ultomiris 300 mg/3 mL single-use vials for injection: 25682-0025-xx•Ultomiris 300 mg/30 mL single-use vials for injection: 25682-0022-xx******•Ultomiris 1100 mg/11 mL single-use vials for injection: 25682-0028-xx****Note: This NDC has been discontinued as of 06/11/2021.VII.References1.Ultomiris [package insert]. Boston, MA; Alexion Pharmaceuticals, Inc; June 2021. AccessedJune 2021.2.Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria andrelated disorders by flow cytometry. Borowitz MJ, Craig FE, DiGiuseppe JA, IllingworthAJ, Rosse W, Sutherland DR, Wittwer CT, Richards SJ. Cytometry B Clin Cytom. 2010Jul;78(4):211-30. doi: 10.1002/cyto.b.20525.3.Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnalhemoglobinuria. Blood. 2005 Dec 1. 106(12):3699-709.4.Sahin F, Akay OM, Ayer M, et al. Pesg PNH diagnosis, follow-up and treatment guidelines.Am J Blood Res. 2016;6(2): 19-27.5.Loirat C, Fakhouri F, Ariceta G, et al. An international consensus approach to themanagement of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016Jan;31(1):15-39.6.Taylor CM, Machin S, Wigmore SJ, et al. Clinical practice guidelines for the managementof atypical haemolytic uraemic syndrome in the United Kingdom. Br J Haematol. 2010Jan;148(1):37-47.7.Cheong HI, Kyung Jo S, Yoon SS, et al. Clinical Practice Guidelines for the Management ofAtypical Hemolytic Uremic Syndrome in Korea. J Korean Med Sci. 2016 Oct;31(10):1516-1528.8.Brodsky RA, Peffault de Latour R, Rottinghaus ST, et al. Characterization of breakthroughhemolysis events observed in the phase 3 randomized studies of ravulizumab versuseculizumab in adults with paroxysmal nocturnal hemoglobinuria. Haematologica. 2020 Jan16. pii: haematol.2019.236877. doi: 10.3324/haematol.2019.236877. [Epub ahead of print] Appendix 1 – Covered Diagnosis CodesD59.3 Hemolytic-uremic syndromeD59.5 Paroxysmal nocturnal hemoglobinuria [Marchiafava-Micheli]Appendix 2 – Centers for Medicare and Medicaid Services (CMS)Medicare coverage for outpatient (Part B) drugs is outlined in the Medicare Benefit Policy Manual (Pub. 100-2), Chapter 15, §50 Drugs and Biologicals. In addition, National Coverage Determination (NCD), Local Coverage Articles (LCAs) and Local Coverage Determinations (LCDs) may exist and compliance with these policies is required where applicable. They can be found at: /medicare-coverage-database/search/advanced-search.aspx. Additional indications may be covered at the discretion of the health plan.Medicare Part B Covered Diagnosis Codes (applicable to existing NCD/LCA/LCD): N/AJurisdiction Applicable State/US Territory ContractorE (1) CA, HI, NV, AS, GU, CNMI Noridian Healthcare Solutions, LLCF (2 & 3) AK, WA, OR, ID, ND, SD, MT, WY, UT, AZ Noridian Healthcare Solutions, LLC5 KS, NE, IA, MO Wisconsin Physicians Service Insurance Corp (WPS)6 MN, WI, IL National Government Services, Inc. (NGS)H (4 & 7) LA, AR, MS, TX, OK, CO, NM Novitas Solutions, Inc.8 MI, IN Wisconsin Physicians Service Insurance Corp (WPS) N (9) FL, PR, VI First Coast Service Options, Inc.J (10) TN, GA, AL Palmetto GBA, LLCM (11) NC, SC, WV, VA (excluding below) Palmetto GBA, LLCNovitas Solutions, Inc.L (12) DE, MD, PA, NJ, DC (includes Arlington &Fairfax counties and the city of Alexandria in VA)K (13 & 14) NY, CT, MA, RI, VT, ME, NH National Government Services, Inc. (NGS)15 KY, OH CGS Administrators, LLC。
第四元素干潜水服保暖层指南说明书

10°C to 16°CArctic 5°C to 13°CArctic +Xerotherm3°C to 11°CArctic +Xerotherm +J2-2°C to 7°CArctic +Xerotherm +X-Core +J20°C to 12°CHalo 3D-2°C to 6°CHalo 3D +Xerotherm +J2-1°C to 8°CHalo 3D +XerothermA BEGINNER’S GUIDE TO DRYSUIT LAYERINGChoose an undersuit. The guide on the left helps to illustrate theways fourth element products can be combined for use in differenttemperatures. In general, the Arctic undersuit is the best startingpoint for neoprene suits and the Arctic Expedition or the Halo 3Dare the best choices under a trilaminate suit.Add a wicking baselaye r. Use the J2 for maximum moisturemanagement or the Xerotherm to make your undergarmentsuitable for cooler temperatures or longer dive durations.Look after the core. In extreme temperatures, add an additionalbaselayer or other insulation to the body core. We developed theX-Core vest and leggings as a passive heating garment. It should beworn either next to the skin or over the baselayer.Insulate key exposure areas. The body loses heat from differentareas at different rates and when diving this is even moresignificant. Your head is particularly exposed during a dive and awell fitting hood is essential. Looking after the extremities also has ahuge impact on your enjoyment – cold hands with no dexterity canspoil a dive. The Arctic Expedition and Halo 3D both have additionalinsulation on the inside of the forearms to maximise the comfort ofhands, as well as panels on the thighs and chest where exposure isgreatest in the horizontal trim position.Choosing the best combination of thermal layers is a matter of assessing your needs, but the most significant factor, apart from the temperature of the water itself, is your drysuit. Neoprene drysuits tend to offer more thermal protection than trilaminate.As a general rule, under a neoprene suit you will need lighter undergarments than you would with a trilam, but the principle is still the same.This is intended as a guide only. Individuals experience cold temperatures differently.。
Viltepso (viltolarsen) 产品说明书

Viltepso® (viltolarsen)(Intravenous)Document Number: IC-0562 Last Review Date: 08/01/2022Date of Origin: 09/01/2020Dates Reviewed: 09/2020, 01/2021, 04/2021, 08/2021, 08/2022I.Length of AuthorizationCoverage will be for 6 months and may be renewed.II.Dosing LimitsA.Quantity Limit (max daily dose) [NDC Unit]:•Viltepso 250 mg/5 mL single-dose vial: 36 vials per 7 daysB.Max Units (per dose and over time) [HCPCS Unit]:•920 billable units (9200 mg) every 7 daysIII.Initial Approval CriteriaCoverage is provided in the following conditions:Universal Criteria 1,5•Patient is not on concomitant therapy with other DMD-directed antisense oligonucleotides(e.g., eteplirsen, golodirsen, casimersen, etc.); AND•Patient does not have symptomatic cardiomyopathy; AND•Serum cystatin C, urine dipstick, and urine protein-to-creatinine ratio (UPCR) are measured prior to starting therapy and periodically during treatment; AND Duchenne Muscular Dystrophy (DMD) † Ф1-8•Patient has a confirmed mutation of the DMD gene that is amenable to exon 53 skipping;AND•Patient has been on a stable dose of corticosteroids, unless contraindicated or intolerance, for at least 3 months; AND•Patient retains meaningful voluntary motor function (e.g., patient is able to speak, manipulate objects using upper extremities, ambulate, etc.); AND•Patient is receiving physical and/or occupational therapy; AND•Baseline documentation of one or more of the following:o Dystrophin levelo Timed function tests (e.g., 6-minute walk test [6MWT], time to stand [TTSTAND], time to run/walk 10 meters [TTRW], time to climb 4 stairs [TTCLIMB], etc.)o Upper limb function (ULM) testo North Star Ambulatory Assessment (NSAA) scoreo Forced Vital Capacity (FVC) percent predicted† FDA-labeled indication(s); ‡ Compendia recommended indication(s); Ф Orphan DrugIV.Renewal Criteria 1,5,6Coverage may be renewed based upon the following criteria:•Patient continues to meet the universal and other indication-specific relevant criteria such as concomitant therapy requirements (not including prerequisite therapy), etc. identified in section III; AND•Absence of unacceptable toxicity from the drug. Examples of unacceptable toxicity include: kidney toxicity (e.g., fatal glomerulonephritis, persistent increase in serum cystatin C,proteinuria, etc.), etc.; AND•Patient has responded to therapy compared to pretreatment baseline in one or more of the following (not all-inclusive):o Increase in dystrophin levelo Improvement in quality of lifeo Stability, improvement, or slowed rate of decline in one or more of the following: ▪Timed function tests (e.g., 6-minute walk test [6MWT], time to stand [TTSTAND], time to run/walk 10 meters [TTRW], time to climb 4 stairs [TTCLIMB], etc.) ▪Upper limb function (ULM) test▪North Star Ambulatory Assessment (NSAA) score▪Forced Vital Capacity (FVC) percent predictedV.Dosage/Administration 1Duchenne MuscularAdminister 80 mg/kg intravenously once weekly.DystrophyVI.Billing Code/Availability InformationHCPCS Code:•J1427 – Injection, viltolarsen, 10 mg; 1 billable unit = 10 mgNDC:•Viltepso 250 mg/5 mL single-dose vial: 73292-0011-xxVII.References1.Viltepso [package insert]. Paramus, NJ; NS Pharma, Inc.; March 2021. Accessed June 2022.2.Topaloglu H, Gloss D, Moxley RT 3rd, et al. Practice guideline update summary:Corticosteroid treatment of Duchenne muscular dystrophy: Report of the GuidelineDevelopment Subcommittee of the American Academy of Neurology. Neurology. 2016 Jul12;87(2):238.3.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchennemuscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management.Lancet Neurol; 2010 Jan; 9(1):77‑93.4.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchennemuscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol; 2010Jan; 9(2):177-189.5.Clemens PR, Rao VK, Connolly AM, et al; CINRG DNHS Investigators. Safety, Tolerability,and Efficacy of Viltolarsen in Boys With Duchenne Muscular Dystrophy Amenable to Exon53 Skipping: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2020 May 26. doi:10.1001/jamaneurol.2020.1264. [Avail at:https:///pmc/articles/PMC7251505/]6.Bushby K, Connor E. Clinical outcome measures for trials in Duchenne musculardystrophy: report from International Working Group meetings. Clin Investig (Lond).2011;1(9):1217-12357.Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchennemuscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, andgastrointestinal and nutritional management. Lancet Neurol 2018; 17:251.8.Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchennemuscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedicmanagement. Lancet Neurol 2018; 17:347.Appendix 1 – Covered Diagnosis CodesG71.01 Duchenne or Becker muscular dystrophyAppendix 2 – Centers for Medicare and Medicaid Services (CMS)Medicare coverage for outpatient (Part B) drugs is outlined in the Medicare Benefit Policy Manual (Pub. 100-2), Chapter 15, §50 Drugs and Biologicals. In addition, National CoverageDetermination (NCD), Local Coverage Articles (LCAs) and Local Coverage Determinations (LCDs) may exist and compliance with these policies is required where applicable. They can be found at: https:///medicare-coverage-database/search.aspx. Additional indications may be covered at the discretion of the health plan.Medicare Part B Covered Diagnosis Codes (applicable to existing NCD/LCA/LCD): N/AJurisdiction Applicable State/US Territory ContractorE (1) CA, HI, NV, AS, GU, CNMI Noridian Healthcare Solutions, LLCF (2 & 3) AK, WA, OR, ID, ND, SD, MT, WY, UT, AZ Noridian Healthcare Solutions, LLC5 KS, NE, IA, MO Wisconsin Physicians Service Insurance Corp (WPS)6 MN, WI, IL National Government Services, Inc. (NGS)H (4 & 7) LA, AR, MS, TX, OK, CO, NM Novitas Solutions, Inc.8 MI, IN Wisconsin Physicians Service Insurance Corp (WPS) N (9) FL, PR, VI First Coast Service Options, Inc.J (10) TN, GA, AL Palmetto GBA, LLCM (11) NC, SC, WV, VA (excluding below) Palmetto GBA, LLCNovitas Solutions, Inc.L (12) DE, MD, PA, NJ, DC (includes Arlington &Fairfax counties and the city of Alexandria in VA)K (13 & 14) NY, CT, MA, RI, VT, ME, NH National Government Services, Inc. (NGS)15 KY, OH CGS Administrators, LLC。
丹福斯(Danfoss)Vane类单泵服务与零件手册说明书

I-3167-SRevised 10-01-89Vane Type Single Pump35VQ**A-86* - 20(L)36VQ**A-86 - 20(L)Service and Parts ManualDanfoss ®Vane PumpsAX432876936851en-000101– 3 –Model CodeViton SealsSeriesVane TypeSAE Rated CapacityPort ConnectionsMounting & Shaft SealsAssemblyShaftsPort PositionsDesignRotation(Omit if not required.)(Rating @ 1200 RPM - 100 psi)21 - 21 USgpm25 - 25 USgpm30 - 30 USgpm35 - 35 USgpm38 - 38 USgpmF - Foot (Single Shaft Seal Assy.)S - Flange (Double Shaft Seal Assy.)Omitted-Flange (Single Shaft SealAssy.)1 - Square Key4 - Splined11 - Splined86 - Square KeySee Chart BelowL - Left Hand (CCW Rotation)Omitted - Right Hand RotationFor satisfactory service life of these components, use full ow ltration to provide uid which meets ISO cleanliness code 16/13or cleaner. Selections from pressure, return, and in-line lter series are recommended.A - SAE 4-Bolt FlangePrinted in U.S.A.Danfoss Power Solutions is a global manufacturer and supplier of high-quality hydraulic and electric components. We specialize in providing state-of-the-art technology and solutions that excel in the harsh operating conditions of the mobile off-highway market as well as the marine sector. Building on our extensive applications expertise, we work closely with you to ensure exceptional performance for a broad range of applications. We help you and other customers around the world speed up system development, reduce costs and bring vehicles and vessels to market faster.Danfoss Power Solutions – your strongest partner in mobile hydraulics and mobile electrification.Go to for further product information.We offer you expert worldwide support for ensuring the best possible solutions foroutstanding performance. And with an extensive network of Global Service Partners, we also provide you with comprehensive global service for all of our components.Local address:DanfossPower Solutions GmbH & Co. OHG Krokamp 35D-24539 Neumünster, Germany Phone: +49 4321 871 0DanfossPower Solutions ApS Nordborgvej 81DK-6430 Nordborg, Denmark Phone: +45 7488 2222DanfossPower Solutions (US) Company 2800 East 13th Street Ames, IA 50010, USA Phone: +1 515 239 6000DanfossPower Solutions Trading (Shanghai) Co., Ltd.Building #22, No. 1000 Jin Hai Rd Jin Qiao, Pudong New District Shanghai, China 201206Phone: +86 21 2080 6201Danfoss can accept no responsibility for possible errors in catalogues, brochures and other printed material. Danfoss reserves the right to alter its products without notice. This also applies to products already on order provided that such alterations can be made without subsequent changes being necessary in specifications already agreed.All trademarks in this material are property of the respective companies. Danfoss and the Danfoss logotype are trademarks of Danfoss A/S. All rights reserved.Products we offer:•Cartridge valves •DCV directional control valves•Electric converters •Electric machines •Electric motors •Gear motors •Gear pumps •Hydraulic integrated circuits (HICs)•Hydrostatic motors •Hydrostatic pumps •Orbital motors •PLUS+1® controllers •PLUS+1® displays •PLUS+1® joysticks and pedals•PLUS+1® operator interfaces•PLUS+1® sensors •PLUS+1® software •PLUS+1® software services,support and training •Position controls and sensors•PVG proportional valves •Steering components and systems •TelematicsHydro-GearDaikin-Sauer-Danfoss。
2022年版Honda Odyssey车型说明书

VISUAL INDEXQuickly locate items in the vehicle’s interior.Steering Wheel and Nearby Controls1Lights/turn signals/LaneWatch button*2 SEL/RESET knob*Brightness control3Wipers/washers 4Cruise control buttons 5Horn6Bluetooth HandsFreeLink/voice recognition buttons 7 Audio controlsMENU button*Display button* 8 Instrument panel9 Information display10 Hood release handle 11 Fuel fill door handle12ENGINE START/STOP button* 13 ECON button*14Vehicle Stability Assist (VSA) OFF buttonLane Departure Warning (LDW) button* Tire Pressure Monitoring System (TPMS) button* Forward Collision Warning (FCW) button*Heated windshield button*VISUAL INDEX821713394VISUAL INDEXDashboard and Other Controls1 Touchscreen*2 Hazard warning button3 Climate control systemSeat heater switches* 4 USB/HDMI* port Auxiliary input jack* Accessory power socket5Power window switches Door lock switchesDoor mirror controls 6 Electric parking brake Brake hold switch7 Shift lever*Manual transmission**if equipped1546327INSTRUMENT PANELLearn about the indicators, gauges, and displays related to driving the vehicle.Indicators briefly appear with each engine start and then go out. Red and amber indicators are most critical. Blue and green indicators are used for general information.Malfunction IndicatorsThese are the most critical indicators. If they come on and stay lit while driving or at any other time, there may be a problem. See your dealer if necessary.Brake system• Brake fluid is low.• The brake system has a problem.Press the brake pedal lightly to check pedal pressure. If normal, check the brake fluid level when you stop. If abnormal, take immediate action. If necessary, downshift the transmission to slow the vehicle using engine braking. Have your vehicle repaired immediately.Low oil pressureEngine oil pressure is low. Stop in a safe place. Open the hood. Check the oil level, and add oil if necessary. If the indicator does not turn off, have your vehicle repaired immediately.Charging systemThe battery is not charging. Turn off all electrical items, but do not turn off the vehicle to prevent further battery discharge. Have your vehicle repaired immediately.Supplemental Restraint System (SRS)There is a problem with one of the airbag systems or seat belt tensioners.Smart Entry system*There is a problem with the smart entry system.CanadaU.S.INSTRUMENT PANELINSTRUMENT PANEL Malfunction indicator lamp (check engine light)• The emissions control system may have a problem, or the fuel cap is loose or missing.• (Blinks) A misfire in the engine’s cylinders is detected. Stop in a safe place, and wait for the engine to cool down.Anti-lock Brake System (ABS)There is a problem with the anti-lock brake system. Your vehicle still has normal braking ability, but no anti-lock function.Tire Pressure Monitoring System (TPMS) (U.S. models only)There is a problem with the tire pressure monitoring system, or the vehicle is fitted with the compact spare tire.Forward Collision Warning (FCW)* (Canadian models only)There is a problem with the FCW system.Lane Departure Warning (LDW)* (Canadian models only)There is a problem with the LDW system.Vehicle Stability Assist (VSA)There is a problem with the VSA system or hill start assist system.Brake systemThere is a problem with the automatic brake hold system.Electric parking brake systemThere is a problem with the electric parking brake system. Avoid using the parking brake. Have your vehicle inspected immediately.Electric Power Steering (EPS)There is a problem with the EPS system. Stop in a safe place, and restart the vehicle.Starter system*There is a problem with the starter system.All Wheel Drive (AWD)*There is a problem with the AWD system. If the indicator blinks, the system is overheated and inactive. Stop in a safe place, and idle until the indicator goes off.High temperatureThe engine coolant temperature is high. Drive slowly to prevent overheating. If the indicator remains on, immediately stop the vehicle in a safe place. Low temperatureThe engine coolant temperature is low. If the indicator remains on for more than 10 minutes (2–8 minutes is normal), there may be a problem withINSTRUMENT PANELCondition IndicatorsThese indicators may require you to perform an action.Seat belt reminderMake sure seat belts are fastened for you and all passengers. The indicator blinks and beeps sound continuously if you or your front passenger has not fastened your seat belts when you begin driving. If the indicator remains on after seat belts are fastened, see your dealer.Parking brakeRelease the parking brake before driving. You will hear a beep if you drive with it not fully released.Door/tailgate openA door or the tailgate is open.Low fuelRefuel as soon as possible. If the indicator blinks, there is a problem with the fuel gauge. See your dealer.Low tire pressureStop in a safe place, check tire pressures, and inflate tire(s) if necessary. If the indicator remains on after tire inflation, you need to calibrate thesystem. The indicator also appears if your vehicle is fitted with the compact spare tire. Have your regular tire repaired or replaced as soon as possible.U.S.CanadaLane Departure Warning (LDW)* (Canadian models only)(Blinks) Take appropriate action to keep the vehicle within the lane.INSTRUMENT PANELWasher fluid level (Canadian models only)Washer fluid is low. Refill the reservoir.Brake depress•During automatic brake hold operation, the automatic brake hold button is pressed without pressing the brake pedal. Make sure you press the brake pedal.•(Blinks) Automatic brake hold is canceled during operation. Immediately press the brake pedal.Forward Collision Warning (FCW)* (Canadian models only)(Blinks) The system detects a likely collision with a vehicle in front of you, and a beep sounds. Take appropriate action to prevent a collision.Maintenance MinderScheduled maintenance for your vehicle is due.ImmobilizerYour key or remote transmitter cannot be recognized by the vehicle. If the indicator blinks, you may not be able to start the engine. Turn the vehicle off, and then on again. If it continues to blink, there may be a problem with the system. See your dealer.CVT modelsMT modelsOn/Off IndicatorsThese indicators remind you when an item is on or off.VSA off VSA on (blinks)CRUISE MAIN on CRUISE CONTROL on Turn signals/hazards on Fog lights* on High beams onExterior lights on ECON mode* on Brake hold system on Brake hold activated FCW* off LDW* offAlarm system setINSTRUMENT PANELInformation DisplayConsists of several displays that provide you with useful information.Press the select/reset knob to change information in the center of the display.Odometer Trip meter A Engine oil lifeRangeTrip meter AAverage fuel economy ATrip meter AAverage fuel economy BTrip meter B The meter ring illumination in the instrument panel can be changed to a color of your choice. When the vehicle is in the ACCESSORY or OFF mode and the meter ring is illuminated, press the select/reset knob to cycle through various color options.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
技术参数
垒块类型403 405/407 410/412 415
最大供应压强200毫巴(20千
帕)
360毫巴(36千帕)
IP等级IP54
外界温度外界温度-15℃至+70℃电磁阀DIN EN 161,A类,2组
电压/频率~(AC)50-60Hz
220-230V
-15%至+10%
~(AC)50-60Hz
230V
-15%至+10%
功率/需求
24VA
~(AC)
230V
20℃
28VA
~(AC)
230V
20℃
50VA
~(AC)
230V
20℃
50VA
~(AC)
230V
20℃
除尘装置过滤器网眼规格为0.8mm,不许移动气体吸发装置即可更换过滤器
组件布置图右失(刃)
垒块由以下部分构成 1-法兰
1-过滤器 2-气体压强测试点
1-气体压强开关 3-法兰固定螺丝
1-压强稳定器 4-阻尼器调节装置
2-电磁阀 5-制动器调节装置
-可迅速打开的安全阀 6-输出装置螺母
-缓慢打开的调整阀 7-用于螺母的固定螺钉(未密封的)
图1. 8-低气压开关
MB DLE 405-407-410-412-415 B01 S20 MB DLE 403 B01 S20
气体吸收装置的组装
气体吸收装置必须在燃烧器的左侧安装,如安装需在右侧进行时,有必要将另一侧的压强测试点移动。
(2)
对于型号MB DLE 403 B01,有必要移动气压开关的位置。
(8)
如气体吸收器直径与燃烧器直径不一致,就需要在两者之间安装转换器
气体供应管道与气体吸收器之间的的连接,通过使用气体吸收器的法兰与将其固定起来的螺母实现(3)
最好使用十字头的螺钉
在任何情况下,安装阀的时候线圈不能处于朝地的一面,当完成安装后,必须检查是否存在漏气并确保气体吸收器正常工作。
压强稳定器的调整(4)
使用合适的螺丝起子来固定压强调整器:顺时针方向位拧紧,逆时针方向为松开。
当压强阀的安装达到理想效果后,盖上盖子并将电线末端用铅封好,使穿过孔间的线圈尽可能的短。
阀的调整
缓慢点火输出(阀的初始开启相位)的调整:再打开外壳(5)并按照+/—方向旋转螺钉之后进行
翻转外壳(5)可以作为调整的工具。
点火阶段中,通过操作液压制动器来控制,输出逐渐增加,在未封死螺钉(7)被
松开的情况下,通过转换螺母来调整即时输出。
输出优化
通过如下手段,第一步操作阀的开启,第二步调整压强稳定器,是可以对垒块气体吸收器的工作进行优化以获得理想的输出。
如未到到预期效果,重复以上操作步骤。
低气压开关的调整
在执行初期阶段所有有关燃烧器压强开关的调整工作后,通过调节刻度尺转盘来对气体压强开关(8)进行调整,使燃烧器处于正常输出的工作状态下。
慢慢关闭阀门直到压强(盖亚强在气体压强开关检具上测量出来)降低5-6mbar 慢慢旋转气体压强开关的把手,直到该气体压强开关以及燃烧器停止工作,完全打开阀门。
过滤器的维护
以下操作必须由技术熟练人员进行
至少每年对过滤器进行一次检查
在不移动吸收器的情况下即可对过滤器进行更换,如果过滤器经常更换,最好紧固螺丝也经常更换。
维护步骤如下:
>关闭阀门及切断气流
>拧开螺钉并打开外壳
>取出旧过滤器并更换新的
>重新盖上外壳,拧紧螺钉,但不可过紧
>进行测试操作并检查是否漏气
线路结构(见图2)
气体吸收器出厂设置线路连接图见图2
注意事项
在启动任何工作状态前需确保电力供应被切断
对于带接线板的燃烧器,必须按照以下指示来对
吸收器连接六角栓对进移动。
>拧开栓的螺钉并打开外壳
>拧开接线柱螺钉并移开连接线路
>认真按照相关操作手册的指导,将连接线路和燃烧器接线板相连接。
注意
对于RS,RLS,RS/M系列的燃烧器:必须按照燃烧器操作指示手册中的线路示意图来移动吸收器连接六角栓
气体吸收装置压强损耗
气体吸收装置压强损耗△p请见以下示意图
a=气体
n=天然气(G20)
p=丙烷(G30)
c=城市气体(G14 0)只能用于(气体用途指令90/396/EEC)所规定之外的用途,通过对压强稳定器的调整,示意图中的重要性有所变化。
Welcome To Download !!!
欢迎您的下载,资料仅供参考!。
