天津大学无机化学第五版习题答案解析
天津大学无机化学第五版习题答案

第1章 化学反应中的质量关系和能量关系 习题参考答案1.解:1.00吨氨气可制取2.47吨硝酸。
2.解:氯气质量为2.9×103g 。
3.解:一瓶氧气可用天数33111-1222()(13.210-1.0110)kPa 32L9.6d 101.325kPa 400L d n p p V n p V -⨯⨯⨯===⨯⨯4.解:pV MpVT nR mR== = 318 K 44.9=℃ 5.解:根据道尔顿分压定律ii n p p n=p (N 2) = 7.6⨯104 Pap (O 2) = 2.0⨯104 Pa p (Ar) =1⨯103 Pa6.解:(1)2(CO )n = 0.114mol; 2(CO )p = 42.87 10 Pa ⨯(2)222(N )(O )(CO )p p p p =--43.7910Pa =⨯ (3)4224(O )(CO ) 2.6710Pa0.2869.3310Pan p n p ⨯===⨯ 7.解:(1)p (H 2) =95.43 kPa (2)m (H 2) =pVMRT= 0.194 g 8.解:(1)ξ = 5.0 mol(2)ξ = 2.5 mol结论: 反应进度(ξ)的值与选用反应式中的哪个物质的量的变化来进行计算无关,但与反应式的写法有关。
9.解:∆U = Q p - p ∆V = 0.771 kJ 10.解: (1)V 1 = 38.3⨯10-3m 3= 38.3L(2) T 2 =nRpV 2= 320 K (3)-W = - (-p ∆V ) = -502 J (4) ∆U = Q + W = -758 J (5) ∆H = Q p = -1260 J11.解:NH 3(g) +45O 2(g) 298.15K−−−−→标准态NO(g) + 23H 2O(g) m r H ∆= - 226.2 kJ ·mol -1 12.解:m r H ∆= Q p = -89.5 kJ m r U ∆= m r H ∆- ∆nRT= -96.9 kJ13.解:(1)C (s) + O 2 (g) → CO 2 (g)m r H ∆ = m f H ∆(CO 2, g) = -393.509 kJ ·mol -121CO 2(g) + 21C(s) → CO(g)m r H ∆ = 86.229 kJ ·mol -1CO(g) +31Fe 2O 3(s) → 32Fe(s) + CO 2(g)m r H ∆ = -8.3 kJ ·mol -1各反应 m r H ∆之和m r H ∆= -315.6 kJ ·mol -1。
天津大学无机化学教研室《无机化学》复习全书(分子的结构与性质)

第6章分子的结构与性质6.1 复习笔记一、键参数1.键能(1)定义键能是指气体分子每断裂单位物质的量的某键(6.022×1023个化学键)时的焓变。
(2)特性①键能可作为衡量化学键牢固程度的键参数,键能越大,键越牢固;②对双原子分子,键能在数值上等于键解离能(D);③多原子分子中若某键不止一个,则该键键能为同种键逐级解离能的平均值;④可通过光谱实验测定键解离能以确定键能,还可利用生成焓计算键能。
2.键长(L b)(1)定义键长是指分子内成键两原子核间的平衡距离。
一些双原子分子的键长如表6-1所示:表6-1 一些双原子分子的键长(2)特性①一个键的性质主要取决于成键原子的本性;②两个确定的原子之间,如果形成不同的化学键,其键长越短,键能就越大,键就越牢固。
③键长可以用分子光谱或X射线衍射方法测得。
3.键角(1)定义键角是指在分子中两个相邻化学键之间的夹角。
(2)特性①键角可以用分子光谱或X射线衍射法测得;②可以通过分子内全部化学键的键长和键角数据来确定这个分子的几何构型。
二、价键理论1.共价键(1)共价键的形成共价键是指原子间由于成键电子的原子轨道重叠而形成的化学键。
(2)价键理论要点①两原子接近时,自旋方向相反的未成对的价电子可以配对,形成共价键;②成键电子的原子轨道如能重叠越多,则所形成的共价键就越牢固(最大重叠原理)。
(3)共价键的特征①共价键具有饱和性;②共价键具有方向性。
(4)原子轨道的重叠①两个原子轨道以对称性相同的部分相重叠(正重叠)图6-1所示为原子轨道几种正重叠的示意图。
(a)s-s (b)p x-s (c)p y-p y(d)d xy-p y图6-1 原子轨道几种正重叠示意图②两个原子轨道以对称性不同部分相重叠(负重叠)图6-2所示为原子轨道几种负重叠的示意图。
(a)p x-p y(b)p x-s (c)p y-p y(d)p x-d xy图6-2 原子轨道几种负重叠示意图(5)共价键的类型①按是否有极性来分类:②按原子轨道重叠部分的对称性来分类:a.键若原子轨道的重叠部分,对键轴(两原子的核间连线)具有圆柱形对称性,所形成的键称为键。
天津大学智慧树知到“药学”《无机化学》网课测试题答案4

天津大学智慧树知到“药学”《无机化学》网课测试题答案(图片大小可自由调整)第1卷一.综合考核(共15题)1.偶极矩可衡量分子极性大小,极化率可衡量分子变形性大小。
()A.正确B.错误2.下列反应式达到平衡时,,保持温度、压力不变,加入稀有气体He,使总体积增加一倍,则()。
A.平衡向左移动B.平衡向右移动C.平衡不发生移动D.条件不充足,不能判断3.下列分子或离子中,呈反磁性的是()。
A.B₂B.O₂C.N₂D.N₂⁻4.下列哪个物质可形成分子晶体?()A.H₂SB.KClC.SiD.Pt5.欲使Mg(OH)₂溶解,可加入()。
A.NaClB.NH₄ClC.NH₃·H₂OD.NaOH 6.判断下列各过程中,那个的△U最大?()A.体系放出了60KJ热,并对环境做了40KJ功B.体系吸收了60KJ热,环境对体系做了40KJ功C.体系吸收了40KJ热,并对环境做了60KJ功D.体系放出了40KJ热,环境对体系做了60KJ功7.关于pz原子轨道角度分布图与电子云角度分布图,下列叙述中错误的是()。
A.前者有正、负,后者全为正(习惯上不标出)B.前者为"双球形",后者为"双纺锤"形C.前者"胖些",后者"瘦些"D.前者值小,后者值大8.在一定标准下,CO₂(g)为下列哪个反应的值?()A.C(金刚石)+O₂(g)→CO₂(g)B.CO(g)+1/2O₂(g)→CO₂(g)C.C(石墨)+O₂(g)→CO₂(g)9.某元素最后填充的是2个n=3,l=0的电子,则该元素的原子序数为()。
A.12B.20C.19D.3010.下列各组原子轨道中不能叠加成键的是()。
A.px - pyB.px - pxC.s - pxD.s - pz11.下列叙述中正确的是()。
A.含有多种离子的溶液中,能形成溶度积小的沉淀者一定先沉淀B.凡溶度积大的沉淀一定会转化成溶度积小的沉淀C.某离子沉淀完全是指其完全变成了沉淀D.当溶液中难溶电解质的离子积小于其溶度积时,该难溶电解质就会溶解12.下列配离子中,具有平面正方构型的是()。
天大物化第五版下册答案

第十章界面现象10.1请回答下列问题:(1)常见的亚稳定状态有哪些?为什么会产生亚稳定状态?如何防止亚稳定状态的产生?解:常见的亚稳定状态有:过饱和蒸汽、过热或过冷液体和过饱和溶液等。
产生亚稳定状态的原因是新相种子难生成。
如在蒸气冷凝、液体凝固和沸腾以及溶液结晶等过程中,由于要从无到有生产新相,故而最初生成的新相,故而最初生成的新相的颗粒是极其微小的,其表面积和吉布斯函数都很大,因此在系统中产生新相极其困难,进而会产生过饱和蒸气、过热或过冷液体和过饱和溶液等这些亚稳定状态,为防止亚稳定态的产生,可预先在系统中加入少量将要产生的新相种子。
(2)在一个封闭的钟罩内,有大小不等的两个球形液滴,问长时间恒温放置后,会出现什么现象?解:若钟罩内还有该液体的蒸气存在,则长时间恒温放置后,出现大液滴越来越大,小液滴越来越小,并不在变化为止。
其原因在于一定温度下,液滴的半径不同,其对应的饱和蒸汽压不同,液滴越小,其对应的饱和蒸汽压越大。
当钟罩内液体的蒸汽压达到大液滴的饱和蒸汽压时。
该蒸汽压对小液滴尚未达到饱和,小液滴会继续蒸发,则蒸气就会在大液滴上凝结,因此出现了上述现象。
(3)物理吸附和化学吸附最本质的区别是什么?解:物理吸附与化学吸附最本质的区别是固体与气体之间的吸附作用力不同。
物理吸附是固体表面上的分子与气体分子之间的作用力为范德华力,化学吸附是固体表面上的分子与气体分子之间的作用力为化学键力。
(4)在一定温度、压力下,为什么物理吸附都是放热过程?解:在一定温度、压力下,物理吸附过程是一个自发过程,由热力学原理可知,此过程系统的厶GvO。
同时气体分子吸附在固体表面,有三维运动表为二维运动,系统的混乱度减小,故此r2,小汞滴的数4 33 r1N- 3 1 1310 91018个A2G dAA14 Nr22A2A1 N4 r22 2r1180.4865 101 10 9 1 10 3 2(1) P s 132 58.91 10-一-60.1 1063=1.178 10 kPa(2) p s 232 58.91 10一0.1 10-6=1.178 103kPa即:P s 3 2 58.91 10-30.1 10-6=2.356 103kPa过程的△ SvO。
(完整版)天津大学无机化学考试试卷(下册)及答案

天津大学无机化学考试试卷(下册)答案一、是非题(判断下列叙述是否正确,正确的在括号中画√,错误的画X)(每小题1分,共10分)1、( X)在周期表中,处于对角线位置的元素性质相似,这称为对角线规则。
2、( X)SnS溶于Na2S2溶液中,生成硫代亚锡酸钠。
3、( X )磁矩大的配合物,其稳定性强。
4、( X)氧族元素氢化物的沸点高低次序为H2O>H2S>H2Se>H3Te。
5、( √)已知[HgCl4]2-的K=1.0⨯10-16,当溶液中c(Cl-)=0.10mol·L-1时,c(Hg2+)/c([HgCl4]2-)的比值为1.0⨯10-12。
6、( √)如果某氢化物的水溶液为碱性,则此氢化物必为离子型氢化物。
7、( X)硼是缺电子原子,在乙硼烷中含有配位键。
8、( √)在浓碱溶液中MnO4-可以被OH-还原为MnO42-。
9、( √)配合物Na3[Ag(S2O3)2]应命名为二硫代硫酸根合银(Ⅰ)酸钠。
10、( X)Pb(OAc)2是一种常见的铅盐,是强电解质。
二、选择题(在下列各题中,选择出符合题意的答案,将其代号填入括号内)(每小题1分,共20分)1、在下列各种酸中氧化性最强的是............... ( B)。
(A)HClO3;(B)HClO;(C)HClO4;(D)HCl。
2、下列浓酸中,可以用来和KI(s)反应制取较纯HI(g)的是............... ( C)。
(A)浓HCl;(B)浓H2SO4;(C)浓H3PO4;(D)浓HNO3。
3、用于说明Pb(Ⅳ)具有强氧化性的是............... ( D)。
(A)熵效应;(B)螯合效应;(C)屏蔽效应;(D)惰性电子对效应。
4、美国的阿波罗飞船上的天线是用钛镍合金制成的,这是因为钛镍合金. ............... ( C)。
(A)机械强度大;(B)熔点高;(C)具有记忆性能;(D)耐腐蚀。
天津大学无机化学教研室《无机化学》(第4版)(上册)-章节题库-第1~2章【圣才出品】

第1章化学反应中的质量关系和能量关系一、选择题1.初始压强均为100kPa的2dm3N2和1dm3 O2充入抽空的1dm3容器中,如果温度保持不变,N2的分压是()。
A.100 kPaB.200 kPaC.300 kPaD.400 kPa【答案】B【解析】当n、T一定时,按波义耳定律的分压为2.在相同的温度和压强下,在两个体积相同的容器中分别充满N2和He,则两容器中物理量相等的是()。
A.分子数B.密度C.电子数D.原子数【答案】A【解析】根据理想气体状态方程pV=nRT,相同的温度、压强和体积的两种气体,物质的量相同。
3.下列实际气体中,性质最接近理想气体的是()。
A.H2B.HeC.N2D.O2【答案】B【解析】理想气体是指分子本身不占有体积、分子间没有作用力的气体。
在题中所给出的实际气体中,单原子分子He的体积和分子间作用力均最小,其性质最接近理想气体。
4.实际气体与理想气体性质接近的条件是()。
A高温高压B.低温高压C.高温低压D.低温低压【答案】C【解析】在高温低压条件下,实际气体分子之间的距离较远,分子之间的作用力很小,可忽略;同时,分子本身的体积与气体的体积相比小得多,可忽略。
5.扩散速率约为甲烷3倍的气体是()。
A.H2B.HeC.N2D.CO2【答案】A【解析】根据气体的扩散定律,气体的扩散速率与其相对分子质量的平方根成反比:6.下列各组气体中,在相同温度下两种气体扩散速率最接近整数倍的是()。
A.H2和HeB.He和N2C.He和O2D.H2和O2【答案】D【解析】气体的扩散速率与相对分子质量的平方根成反比:7.将5dm3300 K、300kPa的O2与8dm3 400K、200kPa的N2以及3.5dm3 350K、600kPa的He压入10dm3的容器中,维持体系温度300K,则下面判断中正确的是()。
A.O2的压强降低,N2和He的压强增加B.N2的压强增加,O2和He的压强降低C.N2的压强不变,总压比混合前的总压低D.O2、N2和He的压强均降低【答案】D【解析】根据理想气体状态方程,当n一定时,有混合气体中各气体的分压为8.气体的溶解度与气相中气体的分压成正比,可用c A=kp A表示。
天津大学无机化学考试试卷(上册) 答案

天津大学无机化学考试试卷(上册)答案天津大学无机化学考试试卷答案一、填表题 1. 原子序数33 23 2. 反应2CO (g) + O2 (g) 固定条件T、P P、V 3. 物质中心原子杂化类型分子空间构型 4. 物质SiC NH3 晶体类型原子晶体氢键型分子晶体晶格结点上粒子Si原子、C原子NH3 分子粒子间作用力共价键分子间力、氢键熔点相对高低高低HgCl2 sp 直线型SiCl4 sp3 正四面体型BBr3 sp2 正三角形PH3 不等性sp3 三角锥型改变条件加催化剂降低温度价层电子构型4s24p3 3d34s2 区p d 周期四四族ⅤA ⅤB 2CO2 (g) (?rHm k正增加减小k 逆增加减小υ正增加减小Kθ 不变增加平衡移动方向不移动向右移动 5. 在?L-1NH3?H2O中加入下列物质,写出NH3?H2O的解离度α和pH值变化趋势加入物质α pH值二、填空题 1. 随着溶液的pH值增加,下列电对Cr2O72-/Cr3+、Cl2/Cl-、MnO4-/MnO42-的E值将分别减小、不变、不变。
2. MgO晶体比金属Mg 的延展性差;石墨晶体比金刚石晶体的导电性好;SiO2晶体比SiF4晶体的硬度大;I2晶体比NaI晶体在水中的溶解度小。
3. 健康人血液的pH值为~。
患某种疾病的人的血液pH可暂时降到,此时血液中c(H+)为正常状态的28~35 倍。
4. 已知B2轨道的能级顺序为σ1sσ*1sσ2sσ*2sπ2pyπ2pzσ2pxπ*2pyπ*2p zσ*2px,则B2的分子轨道分布式为NH4Cl (s) 减小减小NaOH (s) 减小增大H2O 增大增大(σ1s)2(σ*1s)2(σ2s)2(σ*2s)2(π2py)1(π2pz) 1,成键数目及名称两个单电子π键,价键结构式为强的是PbO2 ,还原性最强的是Sn2+ 。
??H?6. 表示?rHm=fm(AgBr, s)的反应式为Ag(s) + 1/2 Br2(l) → AgBr(s) 。
(完整版)无机化学(天津大学版)

第一章化学反应中的质量关系和能量关系[学习指导]1.“物质的量”(n)用于计量指定的微观基本单元或其特定组合的物理量,其单位名称为摩[尔],单位符号为mol。
2.摩尔质量(M) M = m/n3.摩尔体积(V m)V m = V/n4.物质的量浓度(c B)c B = n B/V5.理想气体状态方程pV = nRT6.理想气体分压定律p= Σp B ;p B = (n B/n)p7.化学计量式和化学计量数O = ΣνB B ;νBB8.反应进度(ξ)表示化学反应进行程度的物理量,符号为ξ,单位为mol。
随着反应的进行,任一化学反应各反应物及产物的改变量:Δn B = νBξ9.状态函数状态函数的改变量只与体系的始、终态有关,而与状态变化的途径无关。
10.热和功体系和环境之间因温差而传递的热量称为热。
除热以外,其它各种形式被传递的能量称为功。
11.热力学能(U)体系内部所含的总能量。
12.能量守恒定律孤立体系中能量是不会自生自灭的,它可以变换形式,但总值不变。
13.热力学第一定律封闭体系热力学能的变化:ΔU = Q + WQ > 0, W > 0, ΔU > 0;Q < 0, W< 0, ΔU < 0。
14.恒压反应热(Q p)和反应焓变(Δr H m)H(焓) ≡ U + pV, Q p= Δr H m15.赫斯定律Q p= ∑Q B, Δr H m= ∑Δr H m(B)B B16.标准状况:p = 101.325kPa, T = 273.15 K标准(状)态:pθ= 100kPa下气体:纯气体物质液体、固体:最稳定的纯液体、纯固体物质。
溶液中的溶质:摩尔浓度为1mol·L-1标准态下17.标准摩尔生成焓()最稳定的单质─────—→ 单位物质的量的某物质=18.标准摩尔反应焓变()一般反应cC + dD = yY + zZ=[y(Y) + z(Z)] - [c(C)+ d(D)]=Σνi(生成物) + Σνi(反应物)第二章化学反应的方向、速率和限度[学习指导]1.反应速率:单位体积内反应进行程度随时间的变化率,即:2.活化分子:具有等于或超过E c能量(分子发生有效碰撞所必须具备的最低能量)的分子。
天津大学智慧树知到“药学”《无机化学》网课测试题答案卷5

天津大学智慧树知到“药学”《无机化学》网课测试题答案(图片大小可自由调整)第1卷一.综合考核(共10题)1.下列电对中,若H⁺浓度增大,哪种电对的电极电势增大?()A.Cl₂/Cl⁻B.MnO⁴⁻/Mn²⁺C.Fe(OH)₃/Fe(OH)₂D.I₂/I⁻2.关于pz原子轨道角度分布图与电子云角度分布图,下列叙述中错误的是()。
A.前者有正、负,后者全为正(习惯上不标出)B.前者为"双球形",后者为"双纺锤"形C.前者"胖些",后者"瘦些"D.前者值小,后者值大3.分子间力的本质是()。
A.化学键B.电性作用C.磁性作用D.原子轨道重叠4.某副族元素的A原子,电子最后填入3d,最高氧化数为+4,该元素的原子序数为()。
A.22B.32C.40D.295.在一定标准下,CO₂(g)为下列哪个反应的值?()A.C(金刚石)+O₂(g)→CO₂(g)B.CO(g)+1/2O₂(g)→CO₂(g)C.C(石墨)+O₂(g)→CO₂(g) 6.某反应的速率方程式是,当A的浓度减少50%时,v降低至原来的1/4,当B的浓度增大至2倍时,v增大1.41倍,则x=2,y=0.7。
()A.正确B.错误7.下列分子或离子中,呈反磁性的是()。
A.B₂B.O₂C.N₂D.N₂⁻8.下列各组量子数中,不合理的一组是()。
A.n=2,l=1,m=0B.n=2,l=2,m=-1C.n=3,l=1,m=1D.n=3,l=2,m=09.下列各说法正确的是()。
A.质量作用定律适用于任何化学反应B.反应速率常数取决于反应温度,与反应物的浓度无关C.反应活化能越大,反应速率也越大D.要加热才能进行的反应一定是吸热反应10.下列反应式达到平衡时,,保持温度、压力不变,加入稀有气体He,使总体积增加一倍,则()。
A.平衡向左移动B.平衡向右移动C.平衡不发生移动D.条件不充足,不能判断第1卷参考答案一.综合考核1.参考答案:B2.参考答案:D3.参考答案:B4.参考答案:A5.参考答案:C6.参考答案:B7.参考答案:C8.参考答案:B9.参考答案:B10.参考答案:A。
天津大学第五版物理化学下册习题解答

天津大学第五版物理化学下册习题解答天津大学第五版物理化学下册习题解答第六章相平衡6-1 指出下列平衡系统中的组分数C ,相数P 及自由度数F : (1)I 2(s )与其蒸气成平衡;(2)CaCO 3(s )与其分解产物CaO (s )和CO 2(g )成平衡;(3)NH 4HS(s)放入一抽空的容器中,并与其分解产物NH 3(g)和H 2S(g)成平衡;(4)取任意量的NH 3(g)和H 2S(g)与NH 4HS(s)成平衡;(5) I 2作为溶质在两不相互溶液体H 2O 和CCl 4中达到分配平衡(凝聚系统)。
解:(1)S-R-R '=1-0-0=1;P=2;F=C-P+2=1 (2)S-R-R '=3-1-0=2;P=3;F=C-P+2=1 (3)S-R-R '=3-1-1=1;P=2;F=C-P+2=1 (4)S-R-R '=3-1-0=2;P=2;F=C-P+2=2 (5) S-R-R '=3-0-0=3;P=2;F=C-P+1=2 6-2 常见的)(32s CO Na 水合物有)(10)(7),(232232232s O H CO Na s O H CO Na s O H CO Na 和(1)101.325kPa 下,与32CO Na 水溶液及冰平衡共存的水合物最多有几种?(2)20℃时,与水蒸气平衡共存的水合物最多可能有几种?解系统的物种数S=5,即H 2O 、)(32s CO Na 、)(10)(7),(232232232s O H CO Na s O H CO Na s O H CO Na 和。
独立的化学反应式有三个:)()()(232232s O H CO Na l O H s CO Na ?=+)(7)(6)(2322232s O H CO Na l O H s O H CO Na ?=+? )(10)(3)(72322232s O H CO Na l O H s O H CO Na ?=+?则R=3没有浓度限制条件 0'=R所以,组分数 C=S-R-'R =5-3-0=2在指定的温度或压力的条件下,其自由度数F=C-P+1=3-P 平衡条件下F=0时相数最多,因此上述系统最多只能有3相共存。
天津大学第五版有机化学标准答案

第一章 习题(一) 用简单的文字解释下列术语:(1)有机化合物:碳氢化合物及其衍生物。
(2) 键能:形成共价键时体系所放出的能量。
(3) 极性键:成键原子的电负性相差为~时所形成的共价键。
(4) 官能团:决定有机化合物的主要性质的原子或原子团。
(5) 实验式:能够反映有机化合物元素组成的相对比例的化学式。
(6) 构造式:能够反映有机化合物中原子或原子团相互连接顺序的化学式。
(7)均裂:共价键断裂时,两个成键电子均匀地分配给两个成键原子或原子团,形成两个自由基。
(8) 异裂:共价键断裂时,两个成键电子完成被某一个成键原子或原子团占有,形成正、负离子。
(9) sp 2杂化:由1 个s 轨道和2个p 轨道进行线性组合,形成的3个能量介于s 轨道和p 轨道之间的、能量完全相同的新的原子轨道。
sp 2杂化轨道的形状也不同于s 轨道或p 轨道,而是“一头大,一头小”的形状,这种形状更有利于形成σ键。
(10) 诱导效应:由于成键原子的电负性不同而引起的电子云的转移。
诱导效应只能通过σ键传递,并且随着碳链增长,诱导效应迅速减弱。
(11) 氢键:由氢原子在两个电负性很强的原子之间形成“桥梁”而导致的类似化学键的分子间或分子内作用力。
氢键具有饱和性和方向性,但作用力比化学键小得多,一般为20~30kJ/mol 。
(12) Lewis 酸:能够接受的电子的分子或离子。
(二) 下列化合物的化学键如果都为共价键,而且外层价电子都达到稳定的电子层结构,同时原子之间可以共用一对以上的电子,试写出化合物可能的Lewis 结构式。
(1)C H 3N H 2 (2) CH 3O C H 3 (3) CH 3C OH O(4) C H 3C H =C H 2 (5) C H 3C C H (6) CH 2O 解:分别以“○”表示氢原子核外电子,以“●”表示碳原子核外电子,以“★”表示氧原子核外电子,以“△”表示氮原子核外电子,题给各化合物的Lewis 结构式如下:(1)HH H H。
天津大学无机化学思考题

第一章思考题1.一气柜如下图所示:A假设隔板(A)两侧N2和CO2的T, P相同。
试问:(1)隔板两边气体的质量是否相等? 浓度是否相等?物质的量不等而浓度相等(2)抽掉隔板(假设不影响气体的体积和气柜的密闭性)后,气柜内的T和P 会改变?N2、CO2物质的量和浓度是否会改变?T和P 会不变,N2、CO2物质的量不变而浓度会改变2.标准状况与标准态有何不同? 标准状况指气体在27.315K和101325Pa下的理想气体,标准态是在标准压力下(100kPa)的纯气体、纯液体或纯固体3.化学反应方程式的系数与化学计量数有何不同?对某一化学反应方程式来说,化学反应方程式的系数和化学计量数的绝对值相同,但化学反应方程式的系数为正值,而反应物的化学计量数为负值,生成物的化学计量数为正值4.热力学能、热量、温度三者概念是否相同? 试说明之。
5.试用实例说明热和功都不是状态函数。
6.判断下列各说法是否正确:(1)热的物体比冷的物体含有更多的热量。
×(2)甲物体的温度比乙物体高,表明甲物体的热力学能比乙物体大。
×(3)物体的温度越高,则所含热量越多。
×(4)热是一种传递中的能量。
√(5)同一体系:(a)同一状态可能有多个热力学能值。
×(b)不同状态可能有相同的热力学能值。
√7.判断下列各过程中,那个ΔU最大:(1)体系放出了60kJ热,并对环境做了40kJ功。
(2)体系吸收了60kJ热,环境对体系做了40kJ功。
√(3)体系吸收了40kJ热,并对环境做了60kJ功。
(4)体系放出了40kJ热,环境对体系做了60kJ功。
根据ΔU=Q+W, (1) ΔU=-60+(-40)=-100KJ (2)ΔU=+60+40=+100KJ ,(3) ΔU=+40+(-60)=-20KJ (4)ΔU=-40+60=+20KJ因此通过计算可以看出,(2)过程的ΔU最大.8.下列各说法是否正确:(1)体系的焓等于恒压反应热。
天津大学无机化学第五版习题答案解析

第1章 化学反应中的质量关系和能量关系 习题参考答案1.解:1.00吨氨气可制取2.47吨硝酸。
2.解:氯气质量为2.9×103g 。
3.解:一瓶氧气可用天数 4.解:pV MpVT nR mR== = 318 K 44.9=℃ 5.解:根据道尔顿分压定律p (N 2) = 7.6⨯104 Pa p (O 2) = 2.0⨯104 Pa p (Ar) =1⨯103 Pa6.解:(1)2(CO )n = 0.114mol; 2(CO )p = 42.87 10 Pa ⨯(2)222(N )(O )(CO )p p p p =--43.7910Pa =⨯ (3)4224(O )(CO ) 2.6710Pa0.2869.3310Pan p n p ⨯===⨯7.解:(1)p (H 2) =95.43 kPa (2)m (H 2) =pVMRT= 0.194 g 8.解:(1)ξ = 5.0 mol(2)ξ = 2.5 mol结论: 反应进度(ξ)的值与选用反应式中的哪个物质的量的变化来进行计算无关,但与反应式的写法有关。
9.解:∆U = Q p - p ∆V = 0.771 kJ 10.解: (1)V 1 = 38.3⨯10-3m 3= 38.3L(2) T 2 =nRpV 2= 320 K (3)-W = - (-p ∆V ) = -502 J (4) ∆U = Q + W = -758 J (5) ∆H = Q p = -1260 J11.解:NH 3(g) +45O 2(g) 298.15K−−−−→标准态NO(g) + 23H 2O(g) m r H ∆= - 226.2 kJ ·mol -1 12.解:m r H ∆= Q p = -89.5 kJ m r U ∆= m r H ∆- ∆nRT= -96.9 kJ13.解:(1)C (s) + O 2 (g) → CO 2 (g)m r H ∆ =m f H ∆(CO 2, g) = -393.509 kJ ·mol -121CO 2(g) + 21C(s) → CO(g)m r H ∆ = 86.229 kJ ·mol -1CO(g) +31Fe 2O 3(s) → 32Fe(s) + CO 2(g)m r H ∆ = -8.3 kJ ·mol -1各反应 m r H ∆之和m r H ∆= -315.6 kJ ·mol -1。
天津大学无机化学第五版习题答案解析

8.解:(1)Kai(HClO)= 2.9×10-8;(2)Kspθ(AgI) = 8.51×10-17
(2) (298.15 K) = 5.76, (298.15 K) = 5.8105
7.解:(1) (l) = 2 (NO, g) = 173.1 kJ·mol1
= =30.32,故 = 4.81031
(2) (2) = 2 (N2O, g) =208.4 kJ·mol1
= =36.50,故 = 3.21037
反应后:c(HOAc) = 0.18 mol·L-1,c(OAc-) = 0.64 mol·L-1
设产生的H+变为x’mol·L-1,则
HOAc H++ OAc-
c平/(mol·L-1) 0.18-x’ x’ 0.64+x’
x’ = 5.1×10-6,pH =5.30
Δ(pH) = 5.30-5.72 = -0.42
7. 解:(1)设NH4Cl水解产生的H+为xmol·L-1,则
NH4++ H2O NH3·H2O + H+
c平/(mol·L-1) 0.010-xx x
x= 2.4×10-6,pH = 5.62
(2)设NaCN水解生成的H+为x’mol·L-1,则
CN-+ H2O HCN + OH-
c平/(mol·L-1) 0.10-x’x’x’
起始分压/105Pa 1.01 2.02 1.01 0.34
J= 0.168, = 1>0.168 =J,故反应正向进行。
12.解:(1)NH4HS(s)NH3(g) + H2S(g)
(完整版)天津大学无机化学考试试卷(上册)答案.docx
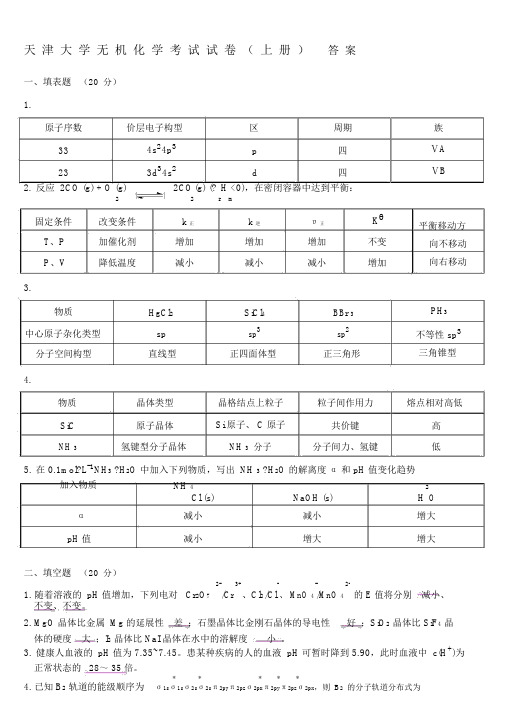
天津大学无机化学考试试卷(上册)答案一、填表题(20分)1.原子序数价层电子构型区周期334s24p3p四233d34s2d四2.反应 2CO (g) + O (g)2CO(g) (?H <0),在密闭容器中达到平衡:22r m固定条件改变条件k 正k 逆υ正KθT、P加催化剂增加增加增加不变P、V降低温度减小减小减小增加3.物质HgCl2SiCl4BBr 3中心原子杂化类型sp sp3sp2分子空间构型直线型正四面体型正三角形4.族ⅤAⅤB平衡移动方向不移动向右移动PH3不等性 sp3三角锥型物质晶体类型晶格结点上粒子粒子间作用力熔点相对高低SiC原子晶体Si 原子、 C 原子共价键高NH 3氢键型分子晶体NH 3分子分子间力、氢键低5.在 0.1mol?L-1NH3 ?H2O 中加入下列物质,写出 NH 3 ?H2O 的解离度α和 pH 值变化趋势加入物质NH 4NaOH (s)2 OCl (s)H α减小减小增大pH 值减小增大增大二、填空题(20分)2-/Cr 3+、Cl2-、 MnO 4-2-的 E 值将分别减小、1. 随着溶液的 pH 值增加,下列电对 Cr2O7/Cl/MnO 4不变、不变。
2. MgO 晶体比金属 Mg 的延展性差;石墨晶体比金刚石晶体的导电性好;SiO2晶体比 SiF4晶体的硬度大;I2晶体比 NaI 晶体在水中的溶解度小。
3.健康人血液的 pH 值为 7.35~7.45。
患某种疾病的人的血液 pH 可暂时降到 5.90,此时血液中 c(H+)为正常状态的28~ 35 倍。
*****4. 已知 B2轨道的能级顺序为σ1sσ1sσ2sσ2sπ2pyπ2pzσ2pxπ2pyπ2pzσ2px,则B2的分子轨道分布式为2* 2 2 * 2 1 1。
(σ1s ) (σ1s ) (σ2s ) (σ2s ) (π2py ) (π2pz ) ,成 数目及名称 两个 子 π ,价 构式θ θ - /Mn 2+ θ 4+ 2+5. 根据 E (PbO 2/PbSO 4) >E (MnO 4 ) >E (Sn /Sn ),可以判断在 成 的六种物 中,氧化性最的是 PbO 2 , 原性最 的是Sn 2+ 。
《无机化学》答案天津大学无机化学教研室编写高等教育出版社出版第8单元
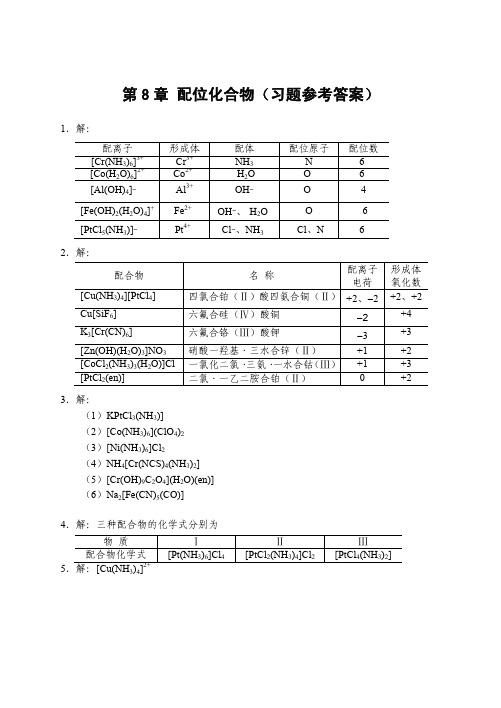
[Ag(CN)2] − y
+ I− y
\ (AgI) = (1.26×1021) × (8.52×10−17) = 1.07×105 K \ = K f\ ( [Ag (CN) 2 ] − ) · K sp
y = 0.49
可见 KCN 可溶解较多的 AgI。
10.解:设 1.0 L 1.0 mol·L−1 氨水可溶解 x mol AgBr,并设溶解达平衡时 c([Ag(NH3)2]+) = x mol·L−1(严格讲应略小于 x mol·L−1)c(Br− ) = x mol·L−1 AgBr(s) + 2NH3·H2O 平衡浓度/(mol·L ) 6.0 − 2 x
配体 NH3 -H2O OH― OH―、-H2OCl―、NH3-
配位原子 N OOO -Cl、N
配位数 664 -6 6-
四氯合铂(Ⅱ)酸四氨合铜(Ⅱ) +2、―2 六氟合硅(Ⅳ)酸铜 六氟合铬(Ⅲ)酸钾 硝酸一羟基·三水合锌(Ⅱ) 一氯化二氯· 三氨· 一水合钴 (Ⅲ) 二氯·一乙二胺合铂(Ⅱ)
[CoF6]3-
8. 解:混合后未反应前: c(Cu2+) = 0.050 mol·L−1 c(NH3) = 3.0 mol·L−1 Cu2+ + 4NH3·H2O
达平衡时: 平衡浓度/(mol·L−1)
2+ 3
[Cu(NH3)4]2+ + 4H2O 0.050 − x
K f\ =
{c ([Cu (NH ) ] )} {c (Cu )} { c (NH ) }
−1
[Ag(NH3)2]+ + Br− + 2H2O x x
\ K \ = K f\ ( [Ag (NH 3 ) 2 ]+ ) · K sp (AgBr) = 5.99×10−6
天津大学高等教育出版社第五版《物理化学》课后习题答案第四章

4.1有溶剂A与溶质B形成一定组成的溶液。
此溶液中B的浓度为c B,质量摩尔浓度为b B,此溶液的密度为。
以M A,M B分别代表溶剂和溶质的摩尔质量,若溶液的组成用B的摩尔分数x B表示时,试导出x B与c B,x B与b B之间的关系。
解:根据各组成表示的定义4.2D-果糖溶于水(A)中形成的某溶液,质量分数,此溶液在20℃时的密度。
求:此溶液中D-果糖的(1)摩尔分数;(2)浓度;(3)质量摩尔浓度。
解:质量分数的定义为4.3在25℃,1 kg水(A)中溶有醋酸(B),当醋酸的质量摩尔浓度b B介于和之间时,溶液的总体积求:(1) 把水(A )和醋酸(B )的偏摩尔体积分别表示成b B 的函数关系。
(2)时水和醋酸的偏摩尔体积。
解:根据定义当时4.460℃时甲醇的饱和蒸气压是84.4 kPa ,乙醇的饱和蒸气压是47.0 kPa 。
二者可形成理想液态混合物。
若混合物的组成为二者的质量分数各50 %,求60℃时此混合物的平衡蒸气组成,以摩尔分数表示。
解:甲醇的摩尔分数为58980049465004232500423250....x B =+=4.580℃时纯苯的蒸气压为100 kPa ,纯甲苯的蒸气压为38.7 kPa 。
两液体可形成理想液态混合物。
若有苯-甲苯的气-液平衡混合物,80℃时气相中苯的摩尔分数,求液相的组成。
解:4.6在18℃,气体压力101.352 kPa下,1 dm3的水中能溶解O2 0.045 g,能溶解N2 0.02 g。
现将 1 dm3被202.65 kPa空气所饱和了的水溶液加热至沸腾,赶出所溶解的O2和N2,并干燥之,求此干燥气体在101.325 kPa,18℃下的体积及其组成。
设空气为理想气体混合物。
其组成体积分数为:,解:显然问题的关键是求出O2和N2的亨利常数。
4.7 20℃下HCl 溶于苯中达平衡,气相中HCl 的分压为101.325 kPa 时,溶液中HCl 的摩尔分数为0.0425。
天大物化五版上册习题答案

第一章 气体pVT 性质1-1解:对于理想气体,pV=nRT111 )/(11-=⋅=⋅=⎪⎭⎫⎝⎛∂∂=⎪⎭⎫ ⎝⎛∂∂=T TVV p nR V T p nRT V T V V p p V α 1211 )/(11-=⋅=⋅=⎪⎪⎭⎫ ⎝⎛∂∂-=⎪⎪⎭⎫ ⎝⎛∂∂-=p p V V pnRT V p p nRT V p V V T T T κ 1-2解:设氯乙烯为理想气体,气柜内氯乙烯的物质的量为mol RT pV n 623.1461815.300314.8300106.1213=⨯⨯⨯==每小时90kg 的流量折合p 摩尔数为 133153.144145.621090109032-⋅=⨯=⨯=h mol M v Cl H C n/v=(14618.623÷1441.153)=10.144小时 1-3解:33714.015.273314.81016101325444--⋅=⨯⨯⨯=⋅=⋅=m kg M RT p M V n CH CH CHρ 1-4解:先求容器的容积33)(0000.10010000.100000.250000.1252cm cm V l O H ==-=ρn=m/M=pV/RTmol g pV RTm M ⋅=⨯-⨯⨯==-31.301013330)0000.250163.25(15.298314.841-5解:方法一:在题目所给出的条件下,气体的量不变。
并且设玻璃泡的体积不随温度而变化,则始态为 )/(2,2,1i i i i RT V p n n n =+=终态(f )时 ⎪⎪⎭⎫⎝⎛+=⎪⎪⎭⎫ ⎝⎛+=+=f f ff f f f f f f T T T T R Vp T V T V R p n n n ,2,1,1,2,2,1,2,1 kPaT T T T T p T T T T VR n p f f f f i i ff ff f 00.117)15.27315.373(15.27315.27315.373325.1012 2,2,1,2,1,2,1,2,1=+⨯⨯⨯=⎪⎪⎭⎫ ⎝⎛+=⎪⎪⎭⎫ ⎝⎛+=1-6解:将数据处理如下:P/kPa101.325 67.550 50.663 33.775 25.331 (ρ/p)/(g·dm -3·kPa )0.022770.022600.022500.022420.02237作(ρ/p)对p 图当p→0时,(ρ/p)=0.02225,则氯甲烷的相对分子质量为()10529.5015.273314.802225.0/-→⋅=⨯⨯==mol g RT p M p ρ1-7 解:设A 为乙烷,B 为丁烷。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
(3)
7.解:(1)p(H2) =95.43 kPa
(2)m(H2) = = 0.194 g
8.解:(1)=5.0 mol
(2)=2.5 mol
结论: 反应进度()的值与选用反应式中的哪个物质的量的变化来进行计算无关,但与反应式的写法有关。
9.解: U=Qpp V= 0.771 kJ
10.解:(1)V1= 38.3 10-3m3= 38.3L
(298.15 K) = 12.39 J·mol1·K1
(1573.15K)≈ (298.15 K)1573.15 (298.15 K)
= 70759 J ·mol1
(1573.15 K) =2.349, (1573.15 K) = 4.48103
10.解:H2(g) + I2(g) 2HI(g)
平衡分压/kPa 2905.74χ 2905.74χ 2χ
(2)T2= = 320 K
(3)W=(pV)=502 J
(4)U=Q+W= -758 J
(5)H=Qp= -1260 J
11.解:NH3(g) + O2(g) NO(g) + H2O(g) =226.2 kJ·mol1
12.解: = Qp=89.5 kJ
= nRT
=96.9 kJ
13.解:(1)C (s) + O2(g) → CO2(g)
= (CO2, g) =393.509 kJ·mol1
CO2(g) + C(s) → CO(g)
= 86.229 kJ·mol1
CO(g) + Fe2O3(s) → Fe(s) + CO2(g)
=8.3 kJ·mol1
各反应 之和 =315.6 kJ·mol1。
(2)总反应方程式为
C(s) + O2(g) + Fe2O3(s)→ CO2(g) + Fe(s)
起始分压/105Pa 1.01 2.02 1.01 0.34
J= 0.168, = 1>0.168 =J,故反应正向进行。
12.解:(1)NH4HS(s)NH3(g) + H2) = 5.76, (298.15 K) = 5.8105
7.解:(1) (l) = 2 (NO, g) = 173.1 kJ·mol1
= =30.32,故 = 4.81031
(2) (2) = 2 (N2O, g) =208.4 kJ·mol1
= =36.50,故 = 3.21037
第1章 化学反应中的质量关系和能量关系 习题参考答案
1.解:1.00吨氨气可制取2.47吨硝酸。
2.解:氯气质量为2.9×103g。
3.解:一瓶氧气可用天数
4.解:
= 318 K ℃
5.解:根据道尔顿分压定律
p(N2) = 7.6104Pa
p(O2) = 2.0104Pa
p(Ar) =1103Pa
6.解:(1) 0.114mol;
(2)Q =4141 kJ·mol1
16.解:(1) =151.1 kJ·mol1(2) =905.47 kJ·mol1(3) =71.7 kJ·mol1
17.解: =2 (AgCl, s)+ (H2O, l) (Ag2O, s)2 (HCl, g)
(AgCl, s) =127.3 kJ·mol1
18.解:CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
(3) (3) = 2 (NH3, g) =32.90 kJ·mol1
= 5.76, 故 = 5.8105
由以上计算看出:选择合成氨固氮反应最好。
8.解: = (CO2, g) (CO, g) (NO, g)
=343.94 kJ·mol1< 0,所以该反应从理论上讲是可行的。
9.解: (298.15 K) = (NO, g) = 90.25 kJ·mol1
= (CO2, g) + 2 (H2O, l) (CH4, g)
=890.36 kJ·mo1
Qp=3.69104kJ
第2章 化学反应的方向、速率和限度 习题参考答案
1.解: =3347.6 kJ·mol1; =216.64 J·mol1·K1; =3283.0 kJ·mol1< 0
该反应在298.15K及标准态下可自发向右进行。
=315.5 kJ·mol1
由上看出:(1)与(2)计算结果基本相等。所以可得出如下结论:反应的热效应只与反应的始、终态有关,而与反应的途径无关。
14.解: (3)= (2)×3- (1)×2=1266.47 kJ·mol1
15.解:(1)Qp= == 4 (Al2O3, s) -3 (Fe3O4, s) =3347.6 kJ·mol1
(298.15 K) =243.03 kJ·mol1
(298.15 K) = 40.92, 故 (298.15 K) = 8.31040
(373.15 K) = 34.02,故 (373.15 K) = 1.01034
6.解:(1) =2 (NH3, g) =32.90 kJ·mol1<0
该反应在298.15 K、标准态下能自发进行。
= 55.3
χ= 2290.12
p(HI) = 2χkPa = 4580.24 kPa
n= = 3.15 mol
11.解:p(CO) = 1.01105Pa,p(H2O) = 2.02105Pa
p(CO2) = 1.01105Pa,p(H2) = 0.34105Pa
CO(g) + H2O(g)CO2(g) + H2(g)
(2)由以上计算可知:
(298.15 K) =70.81 kJ·mol1; (298.15 K) =43.2 J·mol1·K1
= T· ≤0
T≥ = 1639 K
4.解:(1) = =
=
(2) = =
=
(3) = =
=
(4) = =
=
5.解:设 、 基本上不随温度变化。
= T·
(298.15 K) =233.60 kJ·mol1
2.解: = 113.4 kJ·mol1> 0
该反应在常温(298.15 K)、标准态下不能自发进行。
(2) = 146.0 kJ·mol1; = 110.45 J·mol1·K1; = 68.7 kJ·mol1> 0
该反应在700 K、标准态下不能自发进行。
3.解: =70.81 kJ·mol1; =43.2 J·mol1·K1; =43.9 kJ·mol1
