化工原理课件
合集下载
化工原理-精馏课件

吸收
6.3 双组分连续精馏塔的计算
xD
xq
xF
e
d
xW
yq
a
e
d
a
f
c
yq
非理想溶液
理想溶液
xD
xq
xF
xW
c
吸收
6.3 双组分连续精馏塔的计算
NT,min 当操作线远离平衡线 NT减少,与对角线重合时达到 NT,min,一般由图解法求取。若体系为双组分理想溶液,则可通过解析法计算 (Fenske方程):
L
V
L’
V’
F
L
V
L’
V’
F
L
F
F
冷液进料
饱和液体进料
气液混合物进料
饱和蒸气进料
过热蒸气进料
吸收
6.3 双组分连续精馏塔的计算
不同 q值对应的 q线方程
进料状况
进料的焓 IF
q值
q线斜率 q/q-1
冷液体
IF<IL
q>1
+
饱和液体
IF=IL
q=1
无穷大
气液混合物
IL<IF<IV
0 < q <1
–
吸收
6.1 理想溶液的气液相平衡
6.1.2 相平衡——相平衡方程 纯液体的挥发度:该液体在一定温度下的饱和蒸气压。 溶液中各组分的挥发度:该组分在蒸气中的分压和与之相平衡的液相中的摩尔分率之比。 相对挥发度:是指溶液中两组分挥发度之比,常以易挥发组分的挥发度为分子。
吸收
6.1 理想溶液的气液相平衡
吸收
6.3 双组分连续精馏塔的计算
对加料板作物料衡算 V’-V=L’-L-F 令 则有: q 线方程,精馏段操作线和提馏段操作线的交点,但经过 点。
6.3 双组分连续精馏塔的计算
xD
xq
xF
e
d
xW
yq
a
e
d
a
f
c
yq
非理想溶液
理想溶液
xD
xq
xF
xW
c
吸收
6.3 双组分连续精馏塔的计算
NT,min 当操作线远离平衡线 NT减少,与对角线重合时达到 NT,min,一般由图解法求取。若体系为双组分理想溶液,则可通过解析法计算 (Fenske方程):
L
V
L’
V’
F
L
V
L’
V’
F
L
F
F
冷液进料
饱和液体进料
气液混合物进料
饱和蒸气进料
过热蒸气进料
吸收
6.3 双组分连续精馏塔的计算
不同 q值对应的 q线方程
进料状况
进料的焓 IF
q值
q线斜率 q/q-1
冷液体
IF<IL
q>1
+
饱和液体
IF=IL
q=1
无穷大
气液混合物
IL<IF<IV
0 < q <1
–
吸收
6.1 理想溶液的气液相平衡
6.1.2 相平衡——相平衡方程 纯液体的挥发度:该液体在一定温度下的饱和蒸气压。 溶液中各组分的挥发度:该组分在蒸气中的分压和与之相平衡的液相中的摩尔分率之比。 相对挥发度:是指溶液中两组分挥发度之比,常以易挥发组分的挥发度为分子。
吸收
6.1 理想溶液的气液相平衡
吸收
6.3 双组分连续精馏塔的计算
对加料板作物料衡算 V’-V=L’-L-F 令 则有: q 线方程,精馏段操作线和提馏段操作线的交点,但经过 点。
《化工原理吸收》课件

02 模拟方法可以预测不同操作条件下的吸收效果, 以及优化吸收设备的结构和操作参数。
03 常用的模拟方法包括物理模型模拟、数学模型模 拟和实验模拟等。
吸收过程的优化策略
01
吸收过程的优化策略是通过调整操作条件和设备参数
来提高吸收效果的方法。
02
优化策略通常包括选择合适的吸收剂、优化操作条件
、改进设备结构和操作参数等。
增加流速可以提高溶质的 传递速率,但同时会增加 设备的投资和能耗。
04
吸收设备与流程
吸收设备的类型与特点
填料塔
结构简单,易于制造, 适用于气体流量较小、 溶液组成较低的情况。
板式塔
传质效率高,处理能力 大,适用于气体流量较 大、溶液组成较高的情
况。
喷射器
结构简单,操作方便, 适用于气体流量较小、 溶液组成较低的情况。
THANK YOU
感谢各位观看
溶解度与相平衡的关系
物质在气液两相中的溶解度差异是吸收过程得以进行的驱动力。
亨利定律与相平衡
亨利定律:气体在液体中的溶解度与该气体在气液界 面上的分压成正比。
输标02入题
亨利定律的数学表达式:(Henry's Law):(c = kP)
01
03
亨利定律的应用:通过测量气体的溶解度和气液界面 上的分压,可以计算出亨利常数,进而了解物质在特
03
优化策略的目标是提高吸收效果、降低能耗和减少环
境污染等。
06
吸收的实际应用
工业废气的处理
工业废气处理
吸收法可用于处理工业生产过程中产生的废气,如硫氧化物 、氮氧化物等有害气体。通过吸收剂的吸收作用,将有害气 体转化为无害或低害物质,达到净化废气的目的。
03 常用的模拟方法包括物理模型模拟、数学模型模 拟和实验模拟等。
吸收过程的优化策略
01
吸收过程的优化策略是通过调整操作条件和设备参数
来提高吸收效果的方法。
02
优化策略通常包括选择合适的吸收剂、优化操作条件
、改进设备结构和操作参数等。
增加流速可以提高溶质的 传递速率,但同时会增加 设备的投资和能耗。
04
吸收设备与流程
吸收设备的类型与特点
填料塔
结构简单,易于制造, 适用于气体流量较小、 溶液组成较低的情况。
板式塔
传质效率高,处理能力 大,适用于气体流量较 大、溶液组成较高的情
况。
喷射器
结构简单,操作方便, 适用于气体流量较小、 溶液组成较低的情况。
THANK YOU
感谢各位观看
溶解度与相平衡的关系
物质在气液两相中的溶解度差异是吸收过程得以进行的驱动力。
亨利定律与相平衡
亨利定律:气体在液体中的溶解度与该气体在气液界 面上的分压成正比。
输标02入题
亨利定律的数学表达式:(Henry's Law):(c = kP)
01
03
亨利定律的应用:通过测量气体的溶解度和气液界面 上的分压,可以计算出亨利常数,进而了解物质在特
03
优化策略的目标是提高吸收效果、降低能耗和减少环
境污染等。
06
吸收的实际应用
工业废气的处理
工业废气处理
吸收法可用于处理工业生产过程中产生的废气,如硫氧化物 、氮氧化物等有害气体。通过吸收剂的吸收作用,将有害气 体转化为无害或低害物质,达到净化废气的目的。
《化工原理》课件

进行期末考试,综合评估学生在整个课程中的学 习成果。
学习资源
1 教材推荐
2 参考书目
除了《化工原理》教材外, 我们还推荐以下参考教材, 有助于更深入地理解化工 原理。
在课程中提供的参考书目 中,您可以找络资源
我们提供一些网络资源, 供学生进一步学习化工原 理和实际应用。
推荐使用《化工原理》教材, 该教材详细解释了化工原理 的基本概念和实际应用。
重要概念
1 反应原理
了解不同类型的化学反应和它们的原理,如 合成反应、分解反应和酸碱反应。
2 质量守恒与能量守恒
理解质量守恒定律和能量守恒定律,并学会 在化工过程中应用。
3 化学平衡
4 反应动力学
学习如何计算和控制化学反应中的平衡常数, 以及如何进行反应平衡的优化。
《化工原理》PPT课件
欢迎来到《化工原理》PPT课件!本课程将介绍化工基本原理和实际应用,帮 助您理解化工流程和反应动力学。
课程介绍
课程目标
掌握化工基本原理,理解反 应动力学,培养化工工艺设 计的能力。
课程概述
介绍化工原理相关的重要概 念和实际应用,涵盖质量守 恒、能量守恒和化学平衡等 方面。
教材介绍
掌握反应速率和化学动力学的概念,了解如 何改变反应速率和提高反应效率。
实际应用
化工工艺流程
了解化工工艺流程的基本原理,包括物料流动、反 应控制和产品分离等关键步骤。
催化剂的应用
探索催化剂在化工过程中的重要作用,了解如何选 择和使用催化剂以提高反应效率。
课程评估
课堂作业 期中考试 期末考试
通过完成课堂作业,巩固对课程知识的理解和应 用能力。 进行期中考试,评估学生对化工原理的掌握程度。
学习资源
1 教材推荐
2 参考书目
除了《化工原理》教材外, 我们还推荐以下参考教材, 有助于更深入地理解化工 原理。
在课程中提供的参考书目 中,您可以找络资源
我们提供一些网络资源, 供学生进一步学习化工原 理和实际应用。
推荐使用《化工原理》教材, 该教材详细解释了化工原理 的基本概念和实际应用。
重要概念
1 反应原理
了解不同类型的化学反应和它们的原理,如 合成反应、分解反应和酸碱反应。
2 质量守恒与能量守恒
理解质量守恒定律和能量守恒定律,并学会 在化工过程中应用。
3 化学平衡
4 反应动力学
学习如何计算和控制化学反应中的平衡常数, 以及如何进行反应平衡的优化。
《化工原理》PPT课件
欢迎来到《化工原理》PPT课件!本课程将介绍化工基本原理和实际应用,帮 助您理解化工流程和反应动力学。
课程介绍
课程目标
掌握化工基本原理,理解反 应动力学,培养化工工艺设 计的能力。
课程概述
介绍化工原理相关的重要概 念和实际应用,涵盖质量守 恒、能量守恒和化学平衡等 方面。
教材介绍
掌握反应速率和化学动力学的概念,了解如 何改变反应速率和提高反应效率。
实际应用
化工工艺流程
了解化工工艺流程的基本原理,包括物料流动、反 应控制和产品分离等关键步骤。
催化剂的应用
探索催化剂在化工过程中的重要作用,了解如何选 择和使用催化剂以提高反应效率。
课程评估
课堂作业 期中考试 期末考试
通过完成课堂作业,巩固对课程知识的理解和应 用能力。 进行期中考试,评估学生对化工原理的掌握程度。
化工原理完整教材课件 PPT

基本原理及其流动规律解决关问题。以
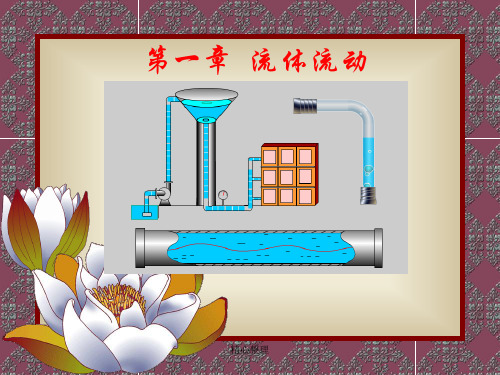
图1-1为煤气洗涤装置为例来说明: 流体动力学问题:流体(水和煤气)
在泵(或鼓风机)、流量计以及管道中 流动等;
流体静力学问题:压差计中流体、 水封箱中的水
图1-1 煤气洗涤装置
1.1 概述
确定流体输送管路的直径, 计算流动过程产生的阻力和 输送流体所需的动力。
根据阻力与流量等参数 选择输送设备的类型和型号, 以及测定流体的流量和压强 等。
流体流动将影响过程系 统中的传热、传质过程等, 是其他单元操作的主要基础。
图1-1 煤气洗涤装置
1.1.1 流体的分类和特性
气体和流体统称流体。流体有多种分类方法: (1)按状态分为气体、液体和超临界流体等; (2)按可压缩性分为不可压流体和可压缩流体; (3)按是否可忽略分子之间作用力分为理想流体与粘
化工原理完整教材课件
第一章 流体流动
Fluid Flow
--内容提要--
流体的基本概念 静力学方程及其应用 机械能衡算式及柏努 利方程 流体流动的现象 流动阻力的计算、管路计算
1. 本章学习目的
通过本章学习,重点掌握流体流动的基本原理、管 内流动的规律,并运用这些原理和规律去分析和解决流 体流动过程的有关问题,诸如:
气体的密度必须标明其状态。 纯气体的密度一般可从手册中查取或计算得到。当压
强不太高、温度不太低时,可按理想气体来换算:
(1-3)
式中
p ── 气体的绝对压强, Pa(或采用其它单位); M ── 气体的摩尔质量, kg/kmol;
性流体(或实际流体); (4)按流变特性可分为牛顿型和非牛倾型流体;
流体区别于固体的主要特征是具有流动性,其形状随容器形状 而变化;受外力作用时内部产生相对运动。流动时产生内摩擦从而 构成了流体力学原理研究的复杂内容之一
化工原理精馏PPT课件全

用饱和蒸气压表示的气液平衡关系
2)用相对挥发度表示 ☆挥发度定义
某组分在气相中的平衡分压与该组分在液相中
的摩尔分率之比
挥发度意义
vi
pi xi
某组分由液相挥发到气相中的趋势,是该组分 挥发性大小的标志
双组分理想溶液
vA
pA xA
pAo xA xA
pAo
vB
pB xB
pBo xB xB
pBo
☆相对挥发度定义
pA pyA
pB pyB p(1 yA )
p
o A
xA
pyA
yA
p
o A
xA
p
pBo xB pyB
yB
pBo xB p
yA
p
o A
x
A
p
xA
p pBo pAo pBo
yA
pAo p
p pBo pAo pBo
xA
p pBo pAo pBo
,
yA
pAo p
p pBo pAo pBo
解 (1)利用拉乌尔定律计算气液平衡数据
xA
p pBo pAo pBo
yA
p
o A
x
A
p
t/℃ x y
80.1 84 88 92 96 100 104 108 110.8 1.000 0.822 0.639 0.508 0.376 0.256 0.155 0.058 0.000 1.000 0.922 0.819 0.720 0.595 0.453 0.305 0.127 0.000
xF,y,x---原料液、气相、液相产品的组成,摩尔分数
y
1
F D
x
化工原理课件(天大版)

反应热与反应焓
反应方向与平衡常数
反应速率与活化能
反应熵与反应吉布斯能
05
化工动力学基础
反应速率方程
添加标题
添加标题
添加标题
添加标题
反应速率方程:描述反应速率与反应物浓度及其他因素关系的数学表达式
反应速率定义:单位时间内反应物浓度的减少量或生积成正比的比例系数
催化剂:使用催化剂可以降低反应活化能,提高反应速率
反应物浓度:反应物浓度增大,反应速率加快
06
分离过程原理及应用
分离过程分类与特点
分离过程的分类:根据不同的原理和操作方式,分离过程可以分为多种类型,如蒸馏、萃取、结晶、过滤等。
R
分离过程的特点:不同的分离过程具有不同的特点和应用范围,需要根据具体需求进行选择。
A
分离过程的原理:每种分离过程都有其特定的原理和操作方式,需要掌握其基本原理和操作方法。
C
分离过程的应用:分离过程在化工、医药、食品等领域有着广泛的应用,需要根据具体需求进行选择和应用。
I
单击此处输入你的智能图形项正文
文字是您思想的提炼
单击此处输入你的智能图形项正文
文字是您思想的提炼
单击此处输入你的智能图形项正文
07
化学反应器原理及应用
化学反应器分类与特点
塔式反应器的特点:适用于气液相反应,具有较大的接触面积和适宜的停留时间
固定床反应器的特点:催化剂固定在反应器内,适用于气固相或液固相反应
流化床反应器的特点:催化剂悬浮在反应器内,适用于气固相或液固相反应
反应器分类:釜式反应器、管式反应器、塔式反应器、固定床反应器、流化床反应器等
化学反应器的设备:介绍反应器的主要设备,如搅拌器、换热器、塔器等。
(完整版)化工原理课件(天大版)

以 F = 1000 kg/h 的流量送入蒸发器,在422K下蒸发 出部分水得到50%的浓KNO3溶液。然后送入冷却结晶器, 在311K下结晶,得到含水0.04 的KNO3结晶和含KNO3 0.375的饱和溶液。前者作为产品取出, 后者循环回到 蒸发器。过程为稳定操作,试计算KNO3结晶产品量P、 水分蒸发量W和循环的饱和溶液量R。
返回 30 03:06:50
4. 流体的特征
具有流动性; 无固定形状,随容器形状而变化; 受外力作用时内部产生相对运动。
不可压缩流体:流体的体积不随压力变化而变化, 如液体;
可压缩性流体:流体的体积随压力发生变化, 如气体。 返回 31
13.7
QL 13.7kW
热损失:
100% 6.54%
257.3 47.8
返回 23 03:06:50
例4 非稳定热量衡算举例
罐内盛有20t重油,初温
T1=20℃,用外循环加热法 水蒸气
进行加热,重油循环量
W=8t/h。循环重油经加热
冷 凝
器升温至恒定的100℃后又 水
W=8t/h T3=100℃
基本单位:7个,化工中常用有5 个,即长度(米),质量(千 克),时间(秒),温度(K), 物质的量(摩尔)
➢ 物理单位 基本单位:长度(厘米cm),质 制(CGS制) 量(克g),时间(秒s)
➢ 工程单 位制
基本单位:长度(米),重量或力 (千克力kgf),时间(秒)
我国法定单位制为国际单位制(即SI制) 返回 11
化工生产过程中,流体(液体、气体)的流动 是各种单元操作中普遍存在的现象。如:
传热 — 冷、热两流体间的热量传递; 传质 — 物料流间的质量传递。 流体流动的强度对热和质的传递影响很大。 强化设备的传热和传质过程需要首先研究流体的流动 条件和规律。 因此,流体流动成为各章都要研究的内容。流体 流动的基本原理和规律是“化工原理” 的重要基础。
返回 30 03:06:50
4. 流体的特征
具有流动性; 无固定形状,随容器形状而变化; 受外力作用时内部产生相对运动。
不可压缩流体:流体的体积不随压力变化而变化, 如液体;
可压缩性流体:流体的体积随压力发生变化, 如气体。 返回 31
13.7
QL 13.7kW
热损失:
100% 6.54%
257.3 47.8
返回 23 03:06:50
例4 非稳定热量衡算举例
罐内盛有20t重油,初温
T1=20℃,用外循环加热法 水蒸气
进行加热,重油循环量
W=8t/h。循环重油经加热
冷 凝
器升温至恒定的100℃后又 水
W=8t/h T3=100℃
基本单位:7个,化工中常用有5 个,即长度(米),质量(千 克),时间(秒),温度(K), 物质的量(摩尔)
➢ 物理单位 基本单位:长度(厘米cm),质 制(CGS制) 量(克g),时间(秒s)
➢ 工程单 位制
基本单位:长度(米),重量或力 (千克力kgf),时间(秒)
我国法定单位制为国际单位制(即SI制) 返回 11
化工生产过程中,流体(液体、气体)的流动 是各种单元操作中普遍存在的现象。如:
传热 — 冷、热两流体间的热量传递; 传质 — 物料流间的质量传递。 流体流动的强度对热和质的传递影响很大。 强化设备的传热和传质过程需要首先研究流体的流动 条件和规律。 因此,流体流动成为各章都要研究的内容。流体 流动的基本原理和规律是“化工原理” 的重要基础。
化工原理完整(天大版)PPT课件

化工原理
Principles of Chemical Engineering
使用教材: 姚玉英主编,化工原理,天津大学出版社,1999 参考教材: 陈敏恒主编,化工原理,化学工业出版社,2002 蒋维钧主编,化工原理,清华大学出版社,1993
可编辑课件
版权所有,未经授权禁止复制或建立镜像。谢谢!
返回 1 2021/4/25
0 绪论 1 流体流动
5 蒸馏 6 吸收
2 流体输送机械
3 非均相物系的分 离和固体流态化
4 传热
7 蒸馏和吸收塔设备 8 液-液萃取 9 干燥
可编辑课件
返回 2 2021/4/25
0 绪论
0.1 化工生产与单元操作 0.2 单位制与单位换算 0.3 物料衡算与能量衡算
可编辑课件
返回 3 2021/4/25
解:首先根据题意画出过程的物料流程图
可编辑课件
返回 16 2021/4/25
F=1000 20%
W, 0.0%
蒸发器 422K
S 50%
冷却结晶器 311K
R, 37.5%
P 1-0.04
解题思路:题求三个量,如何列物料衡算式。
首先考虑划定适宜的物衡范围以利于解题。
1.求KNO3结晶产品量P
按虚线框作为物料衡算范围,只涉及两个未知量。
0 绪论
0.1 化工原理课程的性质和基本内容 1. 化工生产过程
原料预处理
物理过程 单元操作
化学反应
化学反应过程 反应器
产物后处理
物理过程 单元操作
可编辑课件
返回 4 2021/4/25
可编辑课件
返回 5 2021/4/25
可编辑课件
返回 6 2021/4/25
Principles of Chemical Engineering
使用教材: 姚玉英主编,化工原理,天津大学出版社,1999 参考教材: 陈敏恒主编,化工原理,化学工业出版社,2002 蒋维钧主编,化工原理,清华大学出版社,1993
可编辑课件
版权所有,未经授权禁止复制或建立镜像。谢谢!
返回 1 2021/4/25
0 绪论 1 流体流动
5 蒸馏 6 吸收
2 流体输送机械
3 非均相物系的分 离和固体流态化
4 传热
7 蒸馏和吸收塔设备 8 液-液萃取 9 干燥
可编辑课件
返回 2 2021/4/25
0 绪论
0.1 化工生产与单元操作 0.2 单位制与单位换算 0.3 物料衡算与能量衡算
可编辑课件
返回 3 2021/4/25
解:首先根据题意画出过程的物料流程图
可编辑课件
返回 16 2021/4/25
F=1000 20%
W, 0.0%
蒸发器 422K
S 50%
冷却结晶器 311K
R, 37.5%
P 1-0.04
解题思路:题求三个量,如何列物料衡算式。
首先考虑划定适宜的物衡范围以利于解题。
1.求KNO3结晶产品量P
按虚线框作为物料衡算范围,只涉及两个未知量。
0 绪论
0.1 化工原理课程的性质和基本内容 1. 化工生产过程
原料预处理
物理过程 单元操作
化学反应
化学反应过程 反应器
产物后处理
物理过程 单元操作
可编辑课件
返回 4 2021/4/25
可编辑课件
返回 5 2021/4/25
可编辑课件
返回 6 2021/4/25
化工原理第一章流体流动课件

流体静力学基本方程
STEP 02
STEP 01
流体静力学基本方程是流 体静压强与其密度和重力 加速度的关系式。
STEP 03
该方程是流体静力学中的 基础方程,对于理解流体 静力学中的各种现象非常 重要。
该方程可以用来计算流体 的静压强、流体的密度和 重力加速度之间的关系。
静压力对流体的作用力
流体在静压力作用下会产生压缩或膨 胀,这与其弹性有关。
Part
04
流体流动的阻力
流动阻力的产生与分类
流动阻力
流体在管道中流动时,由于流体内部及 流体与管壁之间的摩擦而产生的阻力。
VS
阻力分类
直管阻力和局部阻力。直管阻力是流体在 管道中流动时,由于流体的粘性和管壁的 粗糙度引起的摩擦阻力;局部阻力则是流 体流经管路中的阀门、弯头等局部结构时 ,由于流体的方向和速度发生急剧变化而 引起的阻力。
流体微团的运动分析
流体微团的定义
流体微团是指流体中无限接近的、密合在一起的若干分子组成的微小团体。
流体微团的运动分析
通过对流体微团的运动分析,可以研究流体的宏观运动规律,如速度场、加速 度、角速度等。这些参数对于理解流体动力学的基本原理和工程应用非常重要 。
牛顿粘性定律及流体的分类
牛顿粘性定律的定义
绝对压力
以完全真空为零点测量的 压力,单位为帕斯卡(Pa )。
表压
以当地大气压为基准测量 的压力,单位也为帕斯卡 (Pa)。
真空度
与大气压相比的压力差值 ,单位为帕斯卡(Pa)。
流体静压强分布规律
流体静压强大小与流体的 密度、重力加速度和高度 有关。
在重力场中,流体静压强 随高度增加而减小。
在同一高度上,不同流体 的静压强不同。
化工原理-精选版课件.ppt
1、牛顿型流体与非牛顿型流体;
2、层流内层与边界层,边界层的分离。
化工原理
本章 内容
2019/12/17
1.1 流体静力学基本方程 1.2 流体流动的基本方程 1.3 流体流动现象 1.4 流体在管内的流动阻力 1.5 管路计算 1.6 流速和流量测量
化工原理
第一节 流体静力学基本方程
1 流体的密度
化工原理
3、液体密度的计算 通常液体可视为不可压缩流体,其密度仅随温度略有变化 (极高压强除外)。 (1)纯组分液体的密度其变化关系可从手册中查得。
(2)混合液体的密度
取1kg液体,令液体混合物中各组分的质量分率分别为:
xwA、xwB、、xwn ,
当m总 1kg时,xwi
其中xwi
mi
2019/12/17
化工原理
流体流动是最普遍的化工单元操作之一,研究流体流动问 题也是研究其它化工单元操作的重要基础。
掌握 内容
1、流体的密度和粘度的定义、单位、影响因 素及数据的求取;
2、压强的定义、表示法及单位换算; 3、流体静力学基本方程、连续性方程、柏努
利方程及应用; 4、流动型态及其判断,雷诺准数的物理意义
2019/12/17
化工原理
5、 与密度相关的几个物理量
(1)比容:单位质量的流体所具有的体积,用υ表示,单
位为m3/kg。
mi m总
假设混合后总体积不变:
2019/12/17
V总
xwA
A
xwB
B
xwn m总
n m
化工原理
1 xwA xwB xwn
m A B
n
——液体混合物密度计算式
2、层流内层与边界层,边界层的分离。
化工原理
本章 内容
2019/12/17
1.1 流体静力学基本方程 1.2 流体流动的基本方程 1.3 流体流动现象 1.4 流体在管内的流动阻力 1.5 管路计算 1.6 流速和流量测量
化工原理
第一节 流体静力学基本方程
1 流体的密度
化工原理
3、液体密度的计算 通常液体可视为不可压缩流体,其密度仅随温度略有变化 (极高压强除外)。 (1)纯组分液体的密度其变化关系可从手册中查得。
(2)混合液体的密度
取1kg液体,令液体混合物中各组分的质量分率分别为:
xwA、xwB、、xwn ,
当m总 1kg时,xwi
其中xwi
mi
2019/12/17
化工原理
流体流动是最普遍的化工单元操作之一,研究流体流动问 题也是研究其它化工单元操作的重要基础。
掌握 内容
1、流体的密度和粘度的定义、单位、影响因 素及数据的求取;
2、压强的定义、表示法及单位换算; 3、流体静力学基本方程、连续性方程、柏努
利方程及应用; 4、流动型态及其判断,雷诺准数的物理意义
2019/12/17
化工原理
5、 与密度相关的几个物理量
(1)比容:单位质量的流体所具有的体积,用υ表示,单
位为m3/kg。
mi m总
假设混合后总体积不变:
2019/12/17
V总
xwA
A
xwB
B
xwn m总
n m
化工原理
1 xwA xwB xwn
m A B
n
——液体混合物密度计算式
化工原理课件(天大版)

综合计算
涉及多个物理过程和化学反应的复杂传质过程的计算,需要对各个过程进行分别 处理,并综合考虑各过程之间的相互影响。
分子扩散传质及传质过程的计算
分子扩散
物质分子在运动过程中,从高浓度区 域向低浓度区域的定向迁移,产生物 质传递现象。
传质过程计算
根据分子扩散定律,通过求解浓度场 和扩散系数等参数,实现对传质过程 的模拟和预测。
01
流体的密度、压强、黏度等物理 性质的定义和测量方法。
02
流体静力学基本方程的推导和应 用,包括压力、重力和惯性力对 流体平衡状态的影响。
流体流动的基本方程及流量测量仪表
流体流动的基本方程,如质量守恒、 动量守恒和能量守恒方程。
流量测量仪表的工作原理和应用,如 节流式、涡轮式、电磁式和超声波式 流量计等。
化工原理课件(天大版)
汇报人:
2023-12-10
目录
• 化工原理绪论 • 流体流动 • 传热学 • 传质学 • 化工设备 • 化学反应工程 • 化工过程的控制与优化
01
化工原理绪论
化工原理的研究对象和内容
化工原理研究对象
以化学工程中各种单元操作(动 量传递、热量传递和质量传递) 为研究对象,研究其原理、方法 和过程。
05
化工设备
化工设备的基本类型及结构特点
分离设备
用于将混合物中的不同组分分 离出来的设备,如离心机、过 滤器等。
储罐和容器
用于储存和容纳液体的设备, 如储罐、水池等。
反应设备
用于化学反应的设备,如反应 釜、反应塔等。
换热设备
用于将热能从一个物质传递到 另一个物质的设备ห้องสมุดไป่ตู้如热交换 器、蒸发器等。
输送设备
涉及多个物理过程和化学反应的复杂传质过程的计算,需要对各个过程进行分别 处理,并综合考虑各过程之间的相互影响。
分子扩散传质及传质过程的计算
分子扩散
物质分子在运动过程中,从高浓度区 域向低浓度区域的定向迁移,产生物 质传递现象。
传质过程计算
根据分子扩散定律,通过求解浓度场 和扩散系数等参数,实现对传质过程 的模拟和预测。
01
流体的密度、压强、黏度等物理 性质的定义和测量方法。
02
流体静力学基本方程的推导和应 用,包括压力、重力和惯性力对 流体平衡状态的影响。
流体流动的基本方程及流量测量仪表
流体流动的基本方程,如质量守恒、 动量守恒和能量守恒方程。
流量测量仪表的工作原理和应用,如 节流式、涡轮式、电磁式和超声波式 流量计等。
化工原理课件(天大版)
汇报人:
2023-12-10
目录
• 化工原理绪论 • 流体流动 • 传热学 • 传质学 • 化工设备 • 化学反应工程 • 化工过程的控制与优化
01
化工原理绪论
化工原理的研究对象和内容
化工原理研究对象
以化学工程中各种单元操作(动 量传递、热量传递和质量传递) 为研究对象,研究其原理、方法 和过程。
05
化工设备
化工设备的基本类型及结构特点
分离设备
用于将混合物中的不同组分分 离出来的设备,如离心机、过 滤器等。
储罐和容器
用于储存和容纳液体的设备, 如储罐、水池等。
反应设备
用于化学反应的设备,如反应 釜、反应塔等。
换热设备
用于将热能从一个物质传递到 另一个物质的设备ห้องสมุดไป่ตู้如热交换 器、蒸发器等。
输送设备
化工原理 传热 完整ppt课件

─热导率或导热系数,W/(m·℃)或W/(m·K)。
精选
18
3、热导率
QAddxtAQdt
dx
(1) 为单位温度梯度下的热通量大小(物理意义)
物质的越大,导热性能越好
(2) 是分子微观运动的宏观表现
= f(结构,组成,密度,温度,压力)
(3) 各种物质的导热系数
金属固体 > 非金属固体 > 液体 > 气体
传热
精选
1
第一节 概述
一、传热过程在化工生产中的应用
加热或冷却 换热/能量回用 保温
强化传热过程 削弱传热过程
精选
2
能量回收:节能减排、资源回用! 同时,是化工厂提高经济效益的一个重要措施!
余热资源被认为是继煤、石油、天然气和水力之后的又一常规能源。
例如:钢铁行业烟气余热回收对比
余热没有回收
热交换器进行余热回收
流 体
间壁
流体与壁面之间的热量传递以对流方式为主,并伴有
流体分子热运动引起的热传导,通常把这一传热过程
称为对流传热。
精选
12
精选
13
6、传热速率方程式
传热过程的推动力是两流体的温度差,因沿传热 管长度不同位置的温度差不同,通常在传热计算 时使用平均温度差,以 t m 表示。经验指出,在稳 态传热过程中,传热速率Q与传热面积A和两流体 的温度差 t m 成正比。即得传热速率方程式为:
QKAtm1/tKmA总总 传热 热阻 推动力
式中 K ── 总传热系数,W/(m2·℃)或W/(m2·K); Q ── 传热速率,W或J/s;
A ── 总传热面积,m2;
tm ── 两流体的平均精选温差,℃或K。
14
精选
18
3、热导率
QAddxtAQdt
dx
(1) 为单位温度梯度下的热通量大小(物理意义)
物质的越大,导热性能越好
(2) 是分子微观运动的宏观表现
= f(结构,组成,密度,温度,压力)
(3) 各种物质的导热系数
金属固体 > 非金属固体 > 液体 > 气体
传热
精选
1
第一节 概述
一、传热过程在化工生产中的应用
加热或冷却 换热/能量回用 保温
强化传热过程 削弱传热过程
精选
2
能量回收:节能减排、资源回用! 同时,是化工厂提高经济效益的一个重要措施!
余热资源被认为是继煤、石油、天然气和水力之后的又一常规能源。
例如:钢铁行业烟气余热回收对比
余热没有回收
热交换器进行余热回收
流 体
间壁
流体与壁面之间的热量传递以对流方式为主,并伴有
流体分子热运动引起的热传导,通常把这一传热过程
称为对流传热。
精选
12
精选
13
6、传热速率方程式
传热过程的推动力是两流体的温度差,因沿传热 管长度不同位置的温度差不同,通常在传热计算 时使用平均温度差,以 t m 表示。经验指出,在稳 态传热过程中,传热速率Q与传热面积A和两流体 的温度差 t m 成正比。即得传热速率方程式为:
QKAtm1/tKmA总总 传热 热阻 推动力
式中 K ── 总传热系数,W/(m2·℃)或W/(m2·K); Q ── 传热速率,W或J/s;
A ── 总传热面积,m2;
tm ── 两流体的平均精选温差,℃或K。
14
化工原理完整(天大版)PPT课件

解:首先根据题意画出过程的物料流程图
.
返回 16 2020/5/23
F=1000 20%
W, 0.0%
蒸发器 422K
S 50%
冷却结晶器 311K
R, 37.5%
P 1-0.04
解题思路:题求三个量,如何列物料衡算式。
首先考虑划定适宜的物衡范围以利于解题。
1.求KNO3结晶产品量P
按虚线框作为物料衡算范围,只涉及两个未知量。
GI=GO+GA .
返回 17 2020/5/23
KNO3 组分的物料衡算: F20% = W 0% + P (100 - 4) % 1000 20% = 0 + P 96 % 则:P = 208.3 kg/h
2.水分蒸发量W (物衡范围同1.) 总物料衡算式: F = W + P 则:W = F-P = 1000-208.3 = 791.7 kg/h
.
返回 12 2020/5/23
0.3 物料衡算与能量衡算
☆ 稳定操作
以单位时间为基准, 如 : h , min , s 。 参数=f(x,y,z)
非稳定操作
以每批生产周期所用 的时间为基准。参数 =f(x,y,z,)
=0
=
uA恒定
.
uB 返回 13
2020/5/23
dy
dz
三维
微分衡算(非稳态)
.
返回 15 2020/5/23
例1(清华版,P6):稳态时的总物料衡算及组分物料衡算
生产KNO3的过程中,质量分率为0.2的KNO3水溶液, 以 F = 1000 kg/h 的流量送入蒸发器,在422K下蒸发 出部分水得到50%的浓KNO3溶液。然后送入冷却结晶器, 在311K下结晶,得到含水0.04 的KNO3结晶和含KNO3 0.375的饱和溶液。前者作为产品取出, 后者循环回到 蒸发器。过程为稳定操作,试计算KNO3结晶产品量P、 水分蒸发量W和循环的饱和溶液量R。
.
返回 16 2020/5/23
F=1000 20%
W, 0.0%
蒸发器 422K
S 50%
冷却结晶器 311K
R, 37.5%
P 1-0.04
解题思路:题求三个量,如何列物料衡算式。
首先考虑划定适宜的物衡范围以利于解题。
1.求KNO3结晶产品量P
按虚线框作为物料衡算范围,只涉及两个未知量。
GI=GO+GA .
返回 17 2020/5/23
KNO3 组分的物料衡算: F20% = W 0% + P (100 - 4) % 1000 20% = 0 + P 96 % 则:P = 208.3 kg/h
2.水分蒸发量W (物衡范围同1.) 总物料衡算式: F = W + P 则:W = F-P = 1000-208.3 = 791.7 kg/h
.
返回 12 2020/5/23
0.3 物料衡算与能量衡算
☆ 稳定操作
以单位时间为基准, 如 : h , min , s 。 参数=f(x,y,z)
非稳定操作
以每批生产周期所用 的时间为基准。参数 =f(x,y,z,)
=0
=
uA恒定
.
uB 返回 13
2020/5/23
dy
dz
三维
微分衡算(非稳态)
.
返回 15 2020/5/23
例1(清华版,P6):稳态时的总物料衡算及组分物料衡算
生产KNO3的过程中,质量分率为0.2的KNO3水溶液, 以 F = 1000 kg/h 的流量送入蒸发器,在422K下蒸发 出部分水得到50%的浓KNO3溶液。然后送入冷却结晶器, 在311K下结晶,得到含水0.04 的KNO3结晶和含KNO3 0.375的饱和溶液。前者作为产品取出, 后者循环回到 蒸发器。过程为稳定操作,试计算KNO3结晶产品量P、 水分蒸发量W和循环的饱和溶液量R。
化工原理课件

相对挥发度 v A vB
pA pB
xA xB
yA xA yB xB
显然对理想溶液,有:
p
0 A
p
0 B
y x 1( 1)x
6
液体混合物的蒸气压
1、对于二组分混合液,由于B组分的存在,使得A组 分在汽相中的蒸气分压比其在纯态下的饱和蒸气压 要小。
pA pA0xA ------拉 乌 尔 ( R a o u lt) 定 律
简 单 蒸 馏 平 衡 蒸 馏
精 馏
25
3、平衡级和精馏原理
1)平衡级定义 又称接触级
使不平衡的气液两相(气相 温度高、液相温度低)经过足够 长时间充分接触,离开时,气液 两相达到了平衡,这个过程称为 平衡级。
饱和液相
x0 t0 L
y V t
y y0
t
B
t-y
T0
t-x
B
接触级
A
t0 x
x x0
29
§6.4 二元连续精馏塔的计算
计算项目: 塔顶(或塔底)产量和浓度 塔内物流量 回流量 塔板数或填料层高度 进料位置 塔径
L
F, xF
L
D, xD
V
V
W, xW
30
6.4.1 全塔物料衡算
F DW FxF DxD WxW
F-原料流量,kmol/h
F, xF
D-塔顶产品(馏出液)流量,kmol/h
理想物系
p
A
pB
p
0 A
x
A
p
0 B
x
B
拉乌尔定律
理 想 物 系 的 t - x ( y ) 相 平 衡 关 系 :
对 理 想 物 系 , 汽 相 满 足 : P p A p B p0 AxpB 0(1x)
化工原理第五版完整版课件全

gdz dp udu 0
不可压缩性流体, Const.
zg 1 u2 p Const.
2
(1)
——伯努利方程式
34
(二)伯努利方程式的物理意义
各项意义:
zg ——单位质量流体所具有的位能,J/kg;
p
——单位质量流体所具有的静压能,J/kg ;
1 u2 ——单位质量流体所具有的动能,J/kg。
(2)下端面所受总压力 P2 p2 A 方向向上
(3)液柱的重力 G gA(z1 z2 ) 方向向下
14
p0 p1 G
z1 p2 z2
液柱处于静止时,上述三力的合力为零:
p2 A p1 A gA(z1 z2 ) 0
p2 p1 g(z1 z2 )
p1
z1 g
p2
z2g
压力形式 能量形式
——静力学基本方程 式
15
讨论:
(1)适用于重力场中静止、连续的同种不可压缩性 流体;
(2)物理意义:
zg ——单位质量流体所具有的位能,J/kg;
p
——单位质量流体所具有的静压能,J/kg。
在同一静止流体中,处在不同位置流体的位
能和静压能各不相同,但二者可以转换,其总和
保持不变 。
16
(3)在静止的、连续的同种流体内,处于同一水平 面上各点的压力处处相等。压力相等的面称为等压 面。 (4)压力具有传递性:液面上方压力变化时,液体 内部各点的压力也将发生相应的变化。
11
2、混合物的密度
混合气体 各组分在混合前后质量不变,则有
m 11 12 nn
1 ,2 n ——气体混合物中各组分的体积分数。
或
m
pM m RT
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
AB y yB A//x xB A(1yyA A )//x1 (A xA)
For a separation process in which α =1, the compositions of component A would be the same in both phases, separation is not possible when this occurs since the driving force for mass transfer is zero. When the value of α is above 1, a separation is possible. The value of α may change as concentration and total pressure change.
Diffusion in gases
• A simple theory for gases shows that D is proportional to the product of the average molecular velocity and the mean free path .
Boiling-Point Diagrams and x-y Plots
• the boiling-point diagram • x-y diagram
• maximum boiling azeotrope • minimum boiling azeotrope
7.2. Equilibrium-Stage Operations
• Raoult’s law applies to each component over entire concentration range, such mixtures are called ideal.
Relative Volatility of Vapor–Liquid Systems
• Definition
• The reciprocal of the overall resistance is an overall coefficient.
Chapter7.
7.1 Equilibrium Relations
Equilibrium data can be shown in tables, equations, or graphs.
7.1.1 gas-liquid equilibrium
• Henry's law
The equilibrium relation between partial pressure in the gas phase and xA The equilibrium relation between mole fraction in the gas phase and mole fraction in the liquid xA
• The Chapman-Enskog equation for binary diffusion
0 .0
D AB
0T 1 3 /2M 8A 5 M B 8/M A M B1 /2 PA 2 B D
Diffusion in liquids
• The diffusivities in liquids are generally 4 to 5 orders of magnitude smaller than in gases, but the fluxes for a given mole fraction gradient in liquid or gas may be nearly the same because of the much greater liquid densities.
D 1 u
3
• The mean free path for ideal gases varies inversely with pressure and increases with T1.0
• The mean molecular velocity depends on T0.5
D for ideal gases varies with T1.5 and varies inversely with pressure
化工原理课件
Equimolal diffusion
• For equimolal diffusion in gases, the net volumetric and molar flows are zero
NAJADzM cyAiyA
The concentration gradient for A is linear in the film, and the gradient for B has the same magnitude but the opposite sign
7.1.2 Vapor-Liquid Equilibrium Relations
• Raoult's Law
Equilibrium relation
• At low pressure the vapor of mixture approaches ideal behavior and fol• rectifying section • Stripping • Reboiler and vapor stream • Reflux • Total condenser and partial condenser • Vapor enriched in boiler • Liquid enriched in heavier boiler
Two-Film Theory
• In the two-film theory, equilibrium is assumed at the interface, and the resistances to mass transfer in the two phases are added to get an overall resistance, just as is done for heat transfer.
For a separation process in which α =1, the compositions of component A would be the same in both phases, separation is not possible when this occurs since the driving force for mass transfer is zero. When the value of α is above 1, a separation is possible. The value of α may change as concentration and total pressure change.
Diffusion in gases
• A simple theory for gases shows that D is proportional to the product of the average molecular velocity and the mean free path .
Boiling-Point Diagrams and x-y Plots
• the boiling-point diagram • x-y diagram
• maximum boiling azeotrope • minimum boiling azeotrope
7.2. Equilibrium-Stage Operations
• Raoult’s law applies to each component over entire concentration range, such mixtures are called ideal.
Relative Volatility of Vapor–Liquid Systems
• Definition
• The reciprocal of the overall resistance is an overall coefficient.
Chapter7.
7.1 Equilibrium Relations
Equilibrium data can be shown in tables, equations, or graphs.
7.1.1 gas-liquid equilibrium
• Henry's law
The equilibrium relation between partial pressure in the gas phase and xA The equilibrium relation between mole fraction in the gas phase and mole fraction in the liquid xA
• The Chapman-Enskog equation for binary diffusion
0 .0
D AB
0T 1 3 /2M 8A 5 M B 8/M A M B1 /2 PA 2 B D
Diffusion in liquids
• The diffusivities in liquids are generally 4 to 5 orders of magnitude smaller than in gases, but the fluxes for a given mole fraction gradient in liquid or gas may be nearly the same because of the much greater liquid densities.
D 1 u
3
• The mean free path for ideal gases varies inversely with pressure and increases with T1.0
• The mean molecular velocity depends on T0.5
D for ideal gases varies with T1.5 and varies inversely with pressure
化工原理课件
Equimolal diffusion
• For equimolal diffusion in gases, the net volumetric and molar flows are zero
NAJADzM cyAiyA
The concentration gradient for A is linear in the film, and the gradient for B has the same magnitude but the opposite sign
7.1.2 Vapor-Liquid Equilibrium Relations
• Raoult's Law
Equilibrium relation
• At low pressure the vapor of mixture approaches ideal behavior and fol• rectifying section • Stripping • Reboiler and vapor stream • Reflux • Total condenser and partial condenser • Vapor enriched in boiler • Liquid enriched in heavier boiler
Two-Film Theory
• In the two-film theory, equilibrium is assumed at the interface, and the resistances to mass transfer in the two phases are added to get an overall resistance, just as is done for heat transfer.
