FDA临床试验常见词汇中译文对照.doc
(完整)临床试验常用中英文词汇

(完整)临床试验常用中英文词汇编辑整理:尊敬的读者朋友们:这里是精品文档编辑中心,本文档内容是由我和我的同事精心编辑整理后发布的,发布之前我们对文中内容进行仔细校对,但是难免会有疏漏的地方,但是任然希望((完整)临床试验常用中英文词汇)的内容能够给您的工作和学习带来便利。
同时也真诚的希望收到您的建议和反馈,这将是我们进步的源泉,前进的动力。
本文可编辑可修改,如果觉得对您有帮助请收藏以便随时查阅,最后祝您生活愉快业绩进步,以下为(完整)临床试验常用中英文词汇的全部内容。
SFDA Glossary: GCP,GLP,TRIAL Accuracy 准确度Active control, AC 阳性对照,活性对照Adverse drug reaction, ADR 药物不良反应Adverse event, AE 不良事件Adverse medical events 不良医学事件Adverse reaction 药物不良反应Alb 白蛋白ALD(Approximate Lethal Dose)近似致死剂量ALP 碱性磷酸酶Alpha spending function 消耗函数ALT 丙氨酸氨基转换酶Analysis sets 统计分析的数据集Approval 批准Assistant investigator 助理研究者AST 天门冬酸氨基转换酶ATR 衰减全反射法AUCss 稳态血药浓度-时间曲线下面积Audit 稽查Audit or inspection 稽查/视察Audit report 稽查报告Auditor 稽查员Bias 偏性,偏倚Bioequivalence 生物等效应Blank control 空白对照Blind codes 编制盲底Blind review 盲态审核Blind review 盲态检查Blinding method 盲法Blinding/ masking 盲法,设盲Block 分段Block 层Block size 每段的长度BUN 尿素氮Carryover effect 延滞效应Case history 病历Case report form 病例报告表Case report form/ case record form, CRF 病例报告表,病例记录表Categorical variable 分类变量Cav 平均浓度CD 圆二色谱CL 清除率Clinical equivalence 临床等效应Clinical study 临床研究Clinical study report 临床试验的总结报告Clinical trial 临床试验Clinical trial application, CTA 临床试验申请Clinical trial exemption, CTX 临床试验免责Clinical trial protocol, CTP 临床试验方案Clinical trial/ study report 临床试验报告Cmax 峰浓度Co-investigator 合作研究者Comparison 对照Compliance 依从性Composite variable 复合变量Computer—assisted trial design, CATD 计算机辅助试验设计Confidence interval 可信区间Confidence level 置信水平Consistency test 一致性检验Contract research organization, CRO 合同研究组织Contract/ agreement 协议/合同Control group 对照组Coordinating committee 协调委员会Crea 肌酐CRF(case report form)病例报告表Crossover design 交叉设计Cross-over study 交叉研究Css 稳浓度Cure 痊愈Data management 数据管理Database 建立数据库Descriptive statistical analysis 描述性统计分析DF 波动系统Dichotomies 二分类Diviation 偏差Documentation 记录/文件Dose—reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模拟Double dummy technique 双盲双模拟技术Double—blinding 双盲Drop out 脱落DSC 差示扫描热量计Effectiveness 疗效Electronic data capture, EDC 电子数据采集系统Electronic data processing, EDP 电子数据处理系统Emergency envelope 应急信件End point 终点Endpoint criteria/ measurement 终点指标Equivalence 等效性Essential documentation 必须文件Ethics committee 伦理委员会Excellent 显效Exclusion criteria 排除标准Factorial design 析因设计Failure 无效,失败Final point 终点Fixed—dose procedure 固定剂量法Forced titration 强制滴定Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Global assessment variable 全局评价变量GLU 血糖Good clinical practice, GCP 药物临床试验质量管理规范Good manufacture practice, GMP 药品生产质量管理规范Good non-clinical laboratory practice, GLP 药物非临床研究质量管理规范Group sequential design 成组序贯设计Health economic evaluation, HEV 健康经济学评价Hypothesis test 假设检验Hypothesis testing 假设检验International Conference of Harmonization, ICH 人用药品注册技术要求国际技术协调会,国际协调会议Improvement 好转Inclusion criteria 入选标准Independent ethics committee, IEC 独立伦理委员会Information consent form, ICF 知情同意书Information gathering 信息收集Informed consent, IC 知情同意Initial meeting 启动会议Inspection 视察/检查Institution inspection 机构检查Institution review board, IBR 机构审查委员会Intention to treat 意向治疗(——临床领域)Intention-to –treat, ITT 意向性分析(-统计学)Interactive voice response system, IVRS 互动式语音应答系统Interim analysis 期中分析Investigator 研究者Investigator's brochure, IB 研究者手册IR 红外吸收光谱Ka 吸收速率常Last observation carry forward, LOCF 最接近一次观察的结转LC-MS 液相色谱-质谱联用LD50 板数致死剂量Logic check 逻辑检查LOQ (Limit of Quantitation)定量限LOCF, Last observation carry forward 最近一次观察的结转Lost of follow up 失访Marketing approval/ authorization 上市许可证Matched pair 匹配配对Missing value 缺失值Mixed effect model 混合效应模式Monitor 监查员Monitoring 监查Monitoring report 监查报告MRT 平均滞留时间MS 质谱MS-MS 质谱-质谱联用MTD(Maximum Tolerated Dose)最大耐受剂量Multicenter trial 多中心试验Multi—center trial 多中心试验New chemical entity, NCE 新化学实体New drug application, NDA 新药申请NMR 核磁共振谱Non-clinical study 非临床研究Non-inferiority 非劣效性Non-parametric statistics 非参数统计方法Obedience 依从性ODR 旋光光谱Open-blinding 非盲Open-label 非盲Optional titration 随意滴定Original medical record 原始医疗记录Outcome 结果Outcome assessment 结果指标评价Outcome measurement 结果指标Outlier 离群值Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient history 病历Per protocol, PP 符合方案集Placebo 安慰剂Placebo control 安慰剂对照Polytomies 多分类Power 检验效能Precision 精密度Preclinical study 临床前研究Primary endpoint 主要终点Primary variable 主要变量Principal investigator 主要研究者Principle investigator, PI 主要研究者Product license, PL 产品许可证Protocol 试验方案Protocol 试验方案Protocol amendment 方案补正Quality assurance unit, QAU 质量保证部门Quality assurance, QA 质量保证Quality control, QC 质量控制Query list, query form 应用疑问表Randomization 随机化Randomization 随机Range check 范围检查Rating scale 量表Regulatory authorities, RA 监督管理部门Replication 可重复RSD 日内和日间相对标准差Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample size 样本含量Sample size 样本量,样本大小Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Serious adverse event, SAE 严重不良事件Serious adverse reaction, SAR 严重不良反应Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single blinding 单盲Single-blinding 单盲Site audit 试验机构稽查SOP 试验室的标准操作规程Source data verification, SDV 原始数据核准Source data, SD 原始数据Source document, SD 原始文件Specificity 特异性Sponsor 申办者Sponsor—investigator 申办研究者Standard curve 标准曲线Standard operating procedure, SOP 标准操作规程Statistic 统计量Statistical analysis plan 统计分析计划Statistical analysis plan 统计参数计划书Statistical analysis plan, SAP 统计分析计划Statistical model 统计模型Statistical tables 统计分析表Stratified 分层Study audit 研究稽查Subgroup 亚组Sub-investigator 助理研究者Subject 受试者Subject diary 受试者日记Subject enrollment 受试者入选Subject enrollment log 受试者入选表Subject identification code, SIC 受试者识别代码Subject recruitment 受试者招募Subject screening log 受试者筛选表Superiority 检验Survival analysis 生存分析SXRD 单晶X-射线衍射System audit 系统稽查T1/2 消除半衰期Target variable 目标变量T-BIL 总胆红素T-CHO 总胆固醇TG 热重分析TLC、HPLC 制备色谱Tmax 峰时间TP 总蛋白Transformation 变量变换Treatment group 试验组Trial error 试验误差Trial master file 试验总档案Trial objective 试验目的Trial site 试验场所Triple blinding 三盲Two one—side test 双单侧检验Unblinding 揭盲Unblinding 破盲Unexpected adverse event, UAE 预料外不良事件UV-VIS 紫外-可见吸收光谱Variability 变异Variable 变量Visual analogy scale 直观类比打分法Visual check 人工检查Vulnerable subject 弱势受试者Wash—out 清洗期Washout period 洗脱期Well-being 福利,健康。
FDA常用英文词汇翻译

FDA常用英文词汇翻译FDA(FOOD AND DRUG ADMINISTRA TION):(美国)食品药品管理局IND(INVESTIGA TIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICATION):新药申请ANDA(ABBREVIATED NEW DRUG APPLICATION):简化新药申请EP诉(EXPORT APPLICATION):出口药申请(申请出口不被批准在美国销售的药品) TREATMENT IND:研究中的新药用于治疗ABBREVIATED(NEW)DRUG:简化申请的新药DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其内容)HOLDER:DMF持有者CFR(CODE OF FEDERAL REGULATION):(美国)联邦法规PANEL:专家小组BA TCH PRODUCTION:批量生产;分批生产BA TCH PRODUCTION RECORDS:生产批号记录POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督INFORMED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)PRE<I>script</I>ION DRUG:处方药OTC DRUG(OVER—THE—COUNTER DRUG):非处方药U.S.PUBLIC HEALTH SERVICE:美国卫生福利部NIH(NATIONAL INSTITUTE OF HEALTH):(美国)全国卫生研究所CLINICAL TRIAL:临床试验ANIMAL TRIAL:动物试验ACCELERATED APPROV AL:加速批准STANDARD DRUG:标准药物INVESTIGATOR:研究人员;调研人员PREPARING AND SUBMITTING:起草和申报SUBMISSION:申报;递交BENIFIT(S):受益RISK(S):受害DRUG PRODUCT:药物产品DRUG SUBSTANCE:原料药ESTABLISHED NAME:确定的名称GENERIC NAME:非专利名称PROPRIETARY NAME:专有名称;INN(INTERNATIONAL NONPROPRIETARY NAME):国际非专有名称NARRA TIVE SUMMARY记叙体概要ADVERSE EFFECT:副作用ADVERSE REACTION:不良反应PROTOCOL:方案ARCHIV AL COPY:存档用副本REVIEW COPY:审查用副本OFFICIAL COMPENDIUM:法定药典(主要指USP、NF).USP(THE UNITED STATES PHARMACOPEIA):美国药典(现已和NF合并一起出版) NF(NATIONAL FORMULARY):(美国)国家药品集OFFICIAL=PHARMACOPEIAL= COMPENDIAL:药典的;法定的;官方的AGENCY:审理部门(指FDA) 医学全在线www.med126.c omSPONSOR:主办者(指负责并着手临床研究者)IDENTITY:真伪;鉴别;特性STRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)LABELED AMOUNT:标示量REGULATORY SPECIFICATION:质量管理规格标准(NDA提供)REGULATORY METHODOLOGY:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)REGULATORY METHODS V ALIDATION:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品PRE<I>script</I>ION DRUG:处方药OTC DRUG(OVER—THE—COUNTER DRUG):非处方药U.S.PUBLIC HEALTH SERVICE:美国卫生福利部NIH(NATIONAL INSTITUTE OF HEALTH):(美国)全国卫生研究所CLINICAL TRIAL:临床试验ANIMAL TRIAL:动物试验ACCELERATED APPROV AL:加速批准STANDARD DRUG:标准药物INVESTIGATOR:研究人员;调研人员PREPARING AND SUBMITTING:起草和申报SUBMISSION:申报;递交BENIFIT(S):受益医.学全,在.线,提供w w w.m e d126.c omRISK(S):受害DRUG PRODUCT:药物产品DRUG SUBSTANCE:原料药ESTABLISHED NAME:确定的名称GENERIC NAME:非专利名称PROPRIETARY NAME:专有名称;INN(INTERNATIONAL NONPROPRIETARY NAME):国际非专有名称NARRA TIVE SUMMARY记叙体概要ADVERSE EFFECT:副作用ADVERSE REACTION:不良反应PROTOCOL:方案ARCHIV AL COPY:存档用副本REVIEW COPY:审查用副本OFFICIAL COMPENDIUM:法定药典(主要指USP、NF).USP(THE UNITED STATES PHARMACOPEIA):美国药典(现已和NF合并一起出版) NF(NATIONAL FORMULARY):(美国)国家药品集OFFICIAL=PHARMACOPEIAL= COMPENDIAL:药典的;法定的;官方的AGENCY:审理部门(指FDA)SPONSOR:主办者(指负责并着手临床研究者)IDENTITY:真伪;鉴别;特性STRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)LABELED AMOUNT:标示量REGULATORY SPECIFICATION:质量管理规格标准(NDA提供)REGULATORY METHODOLOGY:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)REGULATORY METHODS V ALIDATION:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品。
临床试验英语词汇

专业术语、缩略语中英对照表缩略语英文全称中文全称ABE Average Bioequivalence 平均生物等效性AC Active control 阳性对照ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者ALB Albumin 白蛋白ALD Approximate Lethal Dose 近似致死剂量ALP Alkaline phosphatase 碱性磷酸酶ALT Alanine aminotransferase 丙氨酸转氨酶ANDA Abbreviated New Drug Application 简化新药申请ANOVA Analysis of variance 方差分析AST Aspartate aminotransferase 天冬氨酸转氨酶ATR Attenuated total reflection 衰减全反射法BA Bioavailability 生物利用度BE Bioequivalence 生物等效性BMI Body Mass Index 体质指数BUN Blood urea nitrogen 血尿素氮CATD Computer-assisted trial design 计算机辅助试验设计CDER Center of Drug Evaluation and Research 药品评价和研究中心CFR Code of Federal Regulation 美国联邦法规CI Co-Investigator 合作研究者CI Confidence Interval 可信区间COI Coordinating Investigator 协调研究者CRC Clinical Research Coordinator 临床研究协调者CRF Case Report/Record Form 病历报告表/病例记录表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告CTX Clinical Trial Exemption 临床试验免责CHMP Committee for Medicinal 人用药委会Products for Human UseDSC Differential scanning 差示扫描热量计DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统EWP Europe Working Party 欧洲工作组FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规GCP Good Laboratory Practice 药物非临床试验质量管理规GLU Glucose 葡萄糖GMP Good Manufacturing Practice 药品生产质量管理规HEV Health economic evaluation 健康经济学评价IB Investigator’s Brochure 研究者手册IBE IndividualBioequivalence 个体生物等效性IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会ITT Intention-to –treat 意向性分析IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统LD50 Medial lethal dose 半数致死剂量LLOQ Lower Limit of quantitation 定量下限LOCF Last observation carry forward 最接近一次观察的结转LOQ Limit of Quantitation 检测限MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部MRT Mean residence time 平均滞留时间MTD Maximum Tolerated Dose 最大耐受剂量ND Not detectable 无法定量NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)NMR Nuclear Magnetic Resonance 核磁共振PD Pharmacodynamics 药效动力学PI Principal Investigator 主要研究者PK Pharmacokinetics 药物动力学PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PP Per protocol 符合方案集PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QAU Quality Assurance Unit 质量保证部门QC Quality Control 质量控制QWP Quality Working Party 质量工作组RA Regulatory Authorities 监督管理部门REV Revision 修订SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SFDA State Food and Drug Administration 国家食品药品监督管理局SI Sponsor-Investigator 申办研究者SI Sub-investigator 助理研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂T-BIL Total Bilirubin 总胆红素T-CHO Total Cholesterol 总胆固醇TG Thromboglobulin 血小板球蛋白Tmax Time of maximum concentration 达峰时间TP Total proteinum 总蛋白UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO- WHO International Conference WHO 国际药品管理当局会议ICDR A of Drug Regulatory AuthoritiesAberrant result 异常结果Absorption phase 吸收相Absorption 吸收Accuracy 准确度Accurate 精密度Administer 给药Amendment修正案Approval 批准Assess 估计Audit Report 稽查报告Audit 稽查Auditor 稽查员Analytical run/batch:分析批Benefit 获益Bias 偏性,偏倚Bioequivalence 生物等效Biosimilar /Follow-on biologics 生物仿制药Blank Control 空白对照Blind codes 编制盲底Blind review 盲态检查/盲态审核Blinding method 盲法Blinding/masking 盲法/设盲Block size 每段的长度Block 层/分段BCS 生物药剂学分类系统Carryover effect 延滞效应Case history 病历Clinical equivalence 临床等效性Clinical study 临床研究Clinical Trial Report 临床试验报告Comparison 对照Compensation 补偿,赔偿金Compliance 依从性Concomitant 伴随的Conduct 行为Confidence level 置信水平Consistency test 一致性检验Contract/ agreement 协议/合同Control group 对照组Coordinating Committee 协调委员会Crossover design 交叉设计Cross-over Study 交叉研究Cure 痊愈Data management 数据管理Descriptive statistical analysis 描述性统计分析Dichotomies 二分类Dispense 分布Diviation 偏差Documentation 记录/文件Dosage forms 剂型Dose dumping 剂量倾卸(药物迅速释放入血而达到危险浓度)Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模Drop out 脱落Effectiveness 疗效Elimination phase 消除相Emergency envelope 应急信件Enantiomers 对映体End point 终点Endpoint criteria/ measurement 终点指标Enterohepatic recycling 肠肝循环Essential Documentation 必需文件Ethical 伦理的Ethics committee 伦理委员会Evaluate 评估Exclusion Criteria 排除标准Excretion 排泄Expedite 促进Extrapolated 外推的Essentially similar product:基本相似药物Factorial design 析因设计Failure 无效,失败Finacing 财务,资金Final point 终点First pass metabolism 首过代Fixed-dose procedure 固定剂量法Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Gene mutation 基因突变Genotoxicity tests 生殖毒性试验Global assessment variable 全局评价变量Group sequential design 成组序贯设计Hypothesis test 假设检验Highly permeable:高渗透性Highly soluble:高溶解度Highly variable drug:高变异性药物Highly:Variable Drug 高变异性药物HVDP:高变异药物制剂Identification 鉴别,Improvement 好转In vitro 体外In vivo 体Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Instruction 指令,说明Integrity 完整,正直Intercurrent 中间发生的,间发的Inter-individual variability 个体间变异性Interim analysis 期中分析Investigational Product 试验药物Investigator 研究者Involve 引起,包括IR 红外吸收光谱Innovator Product:原创药Ka 吸收速率常LC-MS 液相色谱-质谱联用logarithmic transformation 对数转换Logic check 逻辑检查Lost of follow up 失访Mask 面具,掩饰Matched pair 匹配配对Metabolism 代Missing value 缺失值Mixed effect model 混合效应模式Modified release products 改良释放剂型Monitor 监查员Monitoring Plan 监察计划Monitoring Report 监察报告MS-MS 质谱-质谱联用Multi-center Trial 多中心试验Negative 阴性,否定的Non-clinical Study 非临床研究Non-inferiority 非劣效性Non-Linear Pharmacokinetics 非线性药代动力学Non-parametric statistics 非参数统计方法NTID:窄治疗指数制剂Obedience 依从性Open-blinding 非盲Open-label 非盲Original Medical Record 原始医疗记录Outcome Assessment 结果评价Outcome measurement 结果指标Outlier 离群值OIP 经口服吸收药物Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient History 病历Per protocol,PP 符合方案集Permeability 渗透性Pharmacodynamic characteristics 药效学特征Pharmacokinetic characteristics 药代学特征Placebo Control 安慰剂对照Placebo 安慰剂Polytomies 多分类Post-dosing postures 给药后坐姿Potential 潜在的Power 检验效能Precision 精密度Preclinical Study 临床前研究Precursor 母体前体Premature 过早的,早发Primary endpoint 主要终点Primary variable 主要变量Prodrug 药物前体Protocol amendment 方案补正Protocol Amendments 修正案Protocol 试验方案Quality Control Sample:质控样品Rapidly dissolving:快速溶出Racemates 外消旋物Randomization 随机/随机化Range check 围检Rating scale 量表Recruit 招募,新会员Replication 可重复Retrieval 取回,补修Revise 修正Risk 风险Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample Size 样本量、样本大小Sampling schedules 采血计划Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single Blinding 单盲Site audit 试验机构稽查Solubility 溶解度Specificity 特异性Specify 叙述,说明Sponsor-investigator 申办研究者Standard curve 标准曲线Statistical model 统计模型Statistical tables 统计分析表Steady state 稳态Storage 储存Stratified 分层Study Audit 研究稽查Study Site 研究中心Subgroup 亚组Sub-investigator 助理研究者Subject Enrollment Log 受试者入选表Subject Enrollment 受试者入选Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Subject Screening Log 受试者筛选表Subject 受试者Submit 交付,委托Superiority 检验Supplemental 增补的Supra-bioavailability 超生物利用度(试验药的生物利用度大于对照药)Survival analysis 生存分析System Audit 系统稽查SmPC:药品说明书Standard Sample:标准样品Target variable 目标变量Treatment group 试验组Trial error 试验误差Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Trial site 试验场所Triple Blinding 三盲Two one-side test 双单侧检验Therapeutic equivalence:治疗等效性Un-blinding 破盲/揭盲Verify 查证、核实Visual analogy scale 直观类比打分法Vulnerable subject 弱势受试者Wash-out Period 洗脱期Well-being 福利,健康Withdraw 撤回,取消药代动力学参数Ae(0-t):给药到t时尿中排泄的累计原形药。
临床研究常用术语英文对照

临床研究常用术语英文对照Glossary of Common Terms常用术语表Clinical Research临床研究Clinical research is medical research that involves people to test new treatments and therapies.临床研究是涉及人们测试新疗法和疗法的医学研究。
Clinical Trial临床试验A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.前瞻性地将一项或多项人类受试者分配给一项或多项干预措施(可能包括安慰剂或其他对照)的研究,以评估这些干预措施对健康相关的生物医学或行为结局的影响。
Healthy Volunteer健康志愿者(又称:健康受试者)A Healthy volunteer is a person with no known significant healthproblems who participates in clinical research to test a new drug, device, or intervention.健康志愿者是指没有已知重大健康问题的人,他们参与临床研究以测试新药,新设备或新干预措施。
Inclusion/Exclusion Criteria纳入/排除标准Inclusion/Exclusion Criteria are factors that allow someone to participate in a clinical trial are inclusion criteria. Those that exclude or not allow participation are exclusion criteria.纳入/排除标准是允许某人参加临床试验的因素,是纳入标准。
临床试验英语词汇

专业术语、缩略语中英对照表缩略语英文全称中文全称ABE Average Bioequivalence 平均生物等效性AC Active control 阳性对照ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者ALB Albumin 白蛋白ALD Approximate Lethal Dose 近似致死剂量ALP Alkaline phosphatase 碱性磷酸酶ALT Alanine aminotransferase 丙氨酸转氨酶ANDA Abbreviated New Drug Application 简化新药申请ANOV A Analysis of variance 方差分析AST Aspartate aminotransferase 天冬氨酸转氨酶ATR Attenuated total reflection 衰减全反射法BA Bioavailability 生物利用度BE Bioequivalence 生物等效性BMI Body Mass Index 体质指数BUN Blood urea nitrogen 血尿素氮CATD Computer-assisted trial design 计算机辅助试验设计CDER Center of Drug Evaluation and Research 药品评价和研究中心CFR Code of Federal Regulation 美国联邦法规CI Co-Investigator 合作研究者CI Confidence Interval 可信区间COI Coordinating Investigator 协调研究者CRC Clinical Research Coordinator 临床研究协调者CRF Case Report/Record Form 病历报告表/病例记录表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告CTX Clinical Trial Exemption 临床试验免责CHMP Committee for Medicinal 人用药委会Products for Human UseDSC Differential scanning 差示扫描热量计DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统EWP Europe Working Party 欧洲工作组FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GCP Good Laboratory Practice 药物非临床试验质量管理规范GLU Glucose 葡萄糖GMP Good Manufacturing Practice 药品生产质量管理规范HEV Health economic evaluation 健康经济学评价IB Investigator’s Brochure研究者手册IBE IndividualBioequivalence 个体生物等效性IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会ITT Intention-to –treat 意向性分析IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统LD50 Medial lethal dose 半数致死剂量LLOQ Lower Limit of quantitation 定量下限LOCF Last observation carry forward 最接近一次观察的结转LOQ Limit of Quantitation 检测限MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部MRT Mean residence time 平均滞留时间MTD Maximum Tolerated Dose 最大耐受剂量ND Not detectable 无法定量NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)NMR Nuclear Magnetic Resonance 核磁共振PD Pharmacodynamics 药效动力学PI Principal Investigator 主要研究者PK Pharmacokinetics 药物动力学PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PP Per protocol 符合方案集PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QAU Quality Assurance Unit 质量保证部门QC Quality Control 质量控制QWP Quality Working Party 质量工作组RA Regulatory Authorities 监督管理部门REV Revision 修订SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SFDA State Food and Drug Administration 国家食品药品监督管理局SI Sponsor-Investigator 申办研究者SI Sub-investigator 助理研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂T-BIL Total Bilirubin 总胆红素T-CHO Total Cholesterol 总胆固醇TG Thromboglobulin 血小板球蛋白Tmax Time of maximum concentration 达峰时间TP Total proteinum 总蛋白UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO- WHO International Conference WHO 国际药品管理当局会议ICDR A of Drug Regulatory AuthoritiesAberrant result 异常结果Absorption phase 吸收相Absorption 吸收Accuracy 准确度Accurate 精密度Administer 给药Amendment修正案Approval 批准Assess 估计Audit Report 稽查报告Audit 稽查Auditor 稽查员Analytical run/batch:分析批Benefit 获益Bias 偏性,偏倚Bioequivalence 生物等效Biosimilar /Follow-on biologics 生物仿制药Blank Control 空白对照Blind codes 编制盲底Blind review 盲态检查/盲态审核Blinding method 盲法Blinding/masking 盲法/设盲Block size 每段的长度Block 层/分段BCS 生物药剂学分类系统Carryover effect 延滞效应Case history 病历Clinical equivalence 临床等效性Clinical study 临床研究Clinical Trial Report 临床试验报告Comparison 对照Compensation 补偿,赔偿金Compliance 依从性Concomitant 伴随的Conduct 行为Confidence level 置信水平Consistency test 一致性检验Contract/ agreement 协议/合同Control group 对照组Coordinating Committee 协调委员会Crossover design 交叉设计Cross-over Study 交叉研究Cure 痊愈Data management 数据管理Descriptive statistical analysis 描述性统计分析Dichotomies 二分类Dispense 分布Diviation 偏差Documentation 记录/文件Dosage forms 剂型Dose dumping 剂量倾卸(药物迅速释放入血而达到危险浓度)Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模Drop out 脱落Effectiveness 疗效Elimination phase 消除相Emergency envelope 应急信件Enantiomers 对映体End point 终点Endpoint criteria/ measurement 终点指标Enterohepatic recycling 肠肝循环Essential Documentation 必需文件Ethical 伦理的Ethics committee 伦理委员会Evaluate 评估Exclusion Criteria 排除标准Excretion 排泄Expedite 促进Extrapolated 外推的Essentially similar product:基本相似药物Factorial design 析因设计Failure 无效,失败Finacing 财务,资金Final point 终点First pass metabolism 首过代谢Fixed-dose procedure 固定剂量法Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Gene mutation 基因突变Genotoxicity tests 生殖毒性试验Global assessment variable 全局评价变量Group sequential design 成组序贯设计Hypothesis test 假设检验Highly permeable:高渗透性Highly soluble:高溶解度Highly variable drug:高变异性药物Highly:Variable Drug 高变异性药物HVDP:高变异药物制剂Identification 鉴别,身份证Improvement 好转In vitro 体外In vivo 体内Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Instruction 指令,说明Integrity 完整,正直Intercurrent 中间发生的,间发的Inter-individual variability 个体间变异性Interim analysis 期中分析Investigational Product 试验药物Investigator 研究者Involve 引起,包括IR 红外吸收光谱Innovator Product:原创药Ka 吸收速率常LC-MS 液相色谱-质谱联用logarithmic transformation 对数转换Logic check 逻辑检查Lost of follow up 失访Mask 面具,掩饰Matched pair 匹配配对Metabolism 代谢Missing value 缺失值Mixed effect model 混合效应模式Modified release products 改良释放剂型Monitor 监查员Monitoring Plan 监察计划Monitoring Report 监察报告MS-MS 质谱-质谱联用Multi-center Trial 多中心试验Negative 阴性,否定的Non-clinical Study 非临床研究Non-inferiority 非劣效性Non-Linear Pharmacokinetics 非线性药代动力学Non-parametric statistics 非参数统计方法NTID:窄治疗指数制剂Obedience 依从性Open-blinding 非盲Open-label 非盲Original Medical Record 原始医疗记录Outcome Assessment 结果评价Outcome measurement 结果指标Outlier 离群值OIP 经口服吸收药物Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient History 病历Per protocol,PP 符合方案集Permeability 渗透性Pharmacodynamic characteristics 药效学特征Pharmacokinetic characteristics 药代学特征Placebo Control 安慰剂对照Placebo 安慰剂Polytomies 多分类Post-dosing postures 给药后坐姿Potential 潜在的Power 检验效能Precision 精密度Preclinical Study 临床前研究Precursor 母体前体Premature 过早的,早发Primary endpoint 主要终点Primary variable 主要变量Prodrug 药物前体Protocol amendment 方案补正Protocol Amendments 修正案Protocol 试验方案Quality Control Sample:质控样品Rapidly dissolving:快速溶出Racemates 外消旋物Randomization 随机/随机化Range check 范围检Rating scale 量表Recruit 招募,新会员Replication 可重复Retrieval 取回,补修Revise 修正Risk 风险Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample Size 样本量、样本大小Sampling schedules 采血计划Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single Blinding 单盲Site audit 试验机构稽查Solubility 溶解度Specificity 特异性Specify 叙述,说明Sponsor-investigator 申办研究者Standard curve 标准曲线Statistical model 统计模型Statistical tables 统计分析表Steady state 稳态Storage 储存Stratified 分层Study Audit 研究稽查Study Site 研究中心Subgroup 亚组Sub-investigator 助理研究者Subject Enrollment Log 受试者入选表Subject Enrollment 受试者入选Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Subject Screening Log 受试者筛选表Subject 受试者Submit 交付,委托Superiority 检验Supplemental 增补的Supra-bioavailability 超生物利用度(试验药的生物利用度大于对照药)Survival analysis 生存分析System Audit 系统稽查SmPC:药品说明书Standard Sample:标准样品Target variable 目标变量Treatment group 试验组Trial error 试验误差Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Trial site 试验场所Triple Blinding 三盲Two one-side test 双单侧检验Therapeutic equivalence:治疗等效性Un-blinding 破盲/揭盲Verify 查证、核实Visual analogy scale 直观类比打分法Vulnerable subject 弱势受试者Wash-out Period 洗脱期Well-being 福利,健康Withdraw 撤回,取消药代动力学参数Ae(0-t):给药到t时尿中排泄的累计原形药。
FDA常用词中英对照

FDA常用词中英对照FDA(food and drug adminisration):(美国)食品药品监督管理局NDA(new drug application):新药申请ANDA(abbreviated new drug application):简化新药申请EP(export application):出口药申请(申请出口不被批准在美国销售的药品)treatment IND:研究中的新药用于治疗abbreviated(new)drug:简化申请的新药DMF(drug master file):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、 NDA、ANDA时才能参考其内容)holder:DMF持有者CFR(code of federal regulation):(美国)联邦法规PANEL:专家小组batch production:批量生产;分批生产batch production records:生产批号记录post or pre-market surveillance:销售前或销售后监督informed consent:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)prescription drug:处方药OTC drug(over—the—counter drug):非处方药U.S. public health service:美国卫生福利部NIH(national institute of health):(美国)全国卫生研究所animal trail:动物试验accelerated approval:加速批准standard drug:标准药物investigator :研究人员;调研人员preparing and submitting:起草和申报submission:申报;递交benefit(s):受益risk(s):受害drug product:药物产品drug substance:原料药established name:确定的名称generic name:非专利名称proprietary name:专有名称;INN(international nonproprietary name):国际非专有名称narrative summary: 记叙体概要adverse effect:副作用adverse reaction:不良反应protocol:方案archival copy:存档用副本review copy:审查用副本official compendium:法定药典(主要指USP、 NF).USP(the united state pharmacopeia):美国药典(现已和NF合并一起出版)NF(national formulary):(美国)国家药品集official=pharmacopeial = compendial:药典的;法定的;官方的agency:审理部门(指FDA)sponsor:主办者(指负责并着手临床研究者)identity:真伪;鉴别;特性strength:规格;规格含量(每一剂量单位所含有效成分的量)labeled amount:标示量regulatory specification:质量管理规格标准(NDA提供)regulatory methodology:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)regulatory methods validation:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use)人用药物注册技术要求国际协调会议ICH:Quality-质量Q1A(R2): Stability Testing of New Drug Substances and Products (Second Revision) 新原料药和制剂的稳定性试验(第二版)Q1B: Photostability Testing of New Drug Substances and Products新原料药和制剂的光稳定性试验Q1C: Stability Testing for New Dosage Forms新制剂的稳定性试验Q1D: Bracketing and Matrixing Designs for Stability Testing of Drug Substances and Drug Products原料药和制剂稳定性试验的交叉和矩阵设计Q1E: Evaluation of Stability Data对稳定性数据的评估处理Q1F: Stability Data Package for Registration Applications in Climatic Zones III and IV在气候带III和IV,药物注册申请所提供的稳定性数据Q2A: Text on Validation of Analytical Procedures分析程序的验证Q2B: Validation of Analytical Procedures: Methodology分析程序的验证:方法学Q3A(R): Impurities in New Drug Substances (Revised Guideline)新原料药中的杂质(修订版)Q3B(R): Impurities in New Drug Products (Revised Guideline)新制剂中的杂质(修订版)Q3C: Impurities: Guideline for Residual Solvents杂质:残留溶剂指南Q3C(M): Impurities: Guideline for Residual Solvents (Maintenance)杂质:残留溶剂指南(修改内容)Q4: Pharmacopoeias药典Q4A: Pharmacopoeial Harmonisation 药典的协调Q4B: Regulatory Acceptance of Pharmacopoeial Interchangeability药典互替在法规上的可接受性Q5A: Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin来源于人或者动物细胞系的生物技术产品的病毒安全性评估Q5B: Quality of Biotechnological Products: Analysis of the Expression Construct in Cells Used for Production of r-DNA Derived Protein Products生物技术产品的质量:源于重组DNA的蛋白质产品的生产中所用的细胞中的表达构建分析Q5C: Quality of Biotechnological Products: Stability Testing of Biotechnological/Biological Products生物技术产品的质量:生物技术/生物产品的稳定性试验Q5D: Derivation and Characterisation of Cell Substrates Used for Production of Biotechnological/Biological Products用于生产生物技术/生物产品的细胞底物的起源和特征描述Q5E: Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process基于不同生产工艺的生物技术产品/生物产品的可比较性Q6: Specifications for New Drug Substances and Products新原料药和制剂的质量规格Q6A: Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances质量规格:新原料药和新制剂的检验程序和可接收标准:化学物质Q6B: Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products质量规格:生物技术/生物产品的检验程序和可接收标准Q7: Good Manufacturing Practices for Pharmaceutical Ingredients活性药物成份的GMPQ7A: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients活性药物成份的GMP指南Q8: Pharmaceutical Development药物研发Q9: Quality Risk Management质量风险管理ICH:Safety-安全S1A: Guideline on the Need for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究需要的指南S1B: Testing for Carcinogenicity of Pharmaceuticals药物致癌性的检验S1C: Dose Selection for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究之剂量选择S1C(R): Addendum: Addition of a Limit Dose and Related Notes附录:极限剂量和有关注释的的补充S2A: Guidance on Specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals受法规管辖的药物基因毒性检验的特定方面的指南S2B: Genotoxicity: A Standard Battery for Genotoxicity Testing for Pharmaceuticals 基因毒性:药物基因毒性检验的标准S3A: Note for Guidance on Toxicokinetics: The Assessment of Systemic Exposure in Toxicity Studies毒物代谢动力学指南的注释:毒性研究中的全身性暴露量的评估S3B: Pharmacokinetics: Guidance for Repeated Dose Tissue Distribution Studies 药物代谢动力学:重复剂量的组织分布研究指南S4: Single Dose Toxicity Tests单剂量毒性检验S4A: Duration of Chronic Toxicity Testing in Animals (Rodent and Non-Rodent Toxicity Testing)动物体内慢性毒性持续时间的检验(啮齿动物和非啮齿动物毒性检验)S5A: Detection of Toxicity to Reproduction for Medicinal Products药物对生殖发育的毒性的检验S5B(M): Maintenance of the ICH Guideline on Toxicity to Male Fertility: An Addendum to the Guideline on Detection of Toxicity to Reproduction for Medicinal Products 对男性生殖能力的毒性的指南的变动:药物对生殖发育的毒性的检验指南增加了一个附录S6: Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals生物技术生产的药物的临床前安全评价S7A: Safety Pharmacology Studies for Human Pharmaceuticals人用药的安全药理学研究S7B: The Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization(QT Interval Prolongation) By Human Pharmaceuticals药物延迟心室复极化(QT间期)潜在作用的非临床评价S8: Immunotoxicology Studies for Human Pharmaceuticals人用药免疫毒理学研究M3(M): Maintenance of the ICH Guideline on Non-Clinical Safety Studies for the Conduct of Human Clinical Trials for Pharmaceuticals药物的对人临床试验的非临床安全研究指南的变动E-Efficacy(有效)E1: The Extent of Population Exposure to Assess Clinical Safety for Drugs Intended for Long-Term Treatment of Non-Life-Threatening Conditions对用于无生命危险情况下长期治疗的药物进行临床安全评估的族群暴露量范围E2A: Clinical Safety Data Management: Definitions and Standards for Expedited Reporting临床安全数据管理:速报制度的定义和标准E2B(R): Revision of the E2B(M) ICH Guideline on Clinical Safety Data Management Data Elements for Transmission of Individual Case Safety Reports个案安全报告送交的临床安全数据管理的数据要素指南(E2B(M))的修订版E2B (M): Maintenance of the Clinical Safety Data Management including: Data Elements for Transmission of Individual Case Safety Reports临床安全数据管理的变动包括:个案安全报告送交的数据要素E2B(M): Maintenance of the Clinical Safety Data Management including Questions and Answers临床安全数据管理的变动,包括问答E2C: Clinical Safety Data Management: Periodic Safety Update Reports for Marketed Drugs临床安全数据管理:已上市药品的周期性安全数据更新报告Addendum to E2C: Periodic Safety Update Reports for Marketed DrugsE2C的附录:已上市药品的周期性安全数据更新报告E2D: Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting批准后的安全数据管理:速报制度的定义和标准E2E: Pharmacovigilance Planning药物警戒计划E3: Structure and Content of Clinical Study Reports临床研究报告的结构和内容E4: Dose-Response Information to Support Drug Registration支持药品注册的剂量-效应资料E5: Ethnic Factors in the Acceptability of Foreign Clinical Data引入海外临床数据时要考虑的人种因素E6: Good Clinical Practice: Consolidated GuidelineGCP:良好的临床规范:统一的指南E7: Studies in Support of Special Populations: Geriatrics对特定族群的支持的研究:老人病学E8: General Considerations for Clinical Trials对临床试验的总的考虑E9: Statistical Principles for Clinical Trials临床试验的统计原则E10: Choice of Control Group and Related Issues in Clinical Trials临床试验中控制组和有关课题的选择E11: Clinical Investigation of Medicinal Products in the Pediatric Population 小儿科药物的临床调查E12A: Principles for Clinical Evaluation of New Antihypertensive Drugs新抗高血压药物的临床评价原则E14: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs非抗心率失常药物的QT/QTc 间期和致心率失常潜在作用的临床评价Multidisciplinary Guidelines 多学科兼容的指南M1: Medical Terminology医学术语M2: Electronic Standards for Transmission of Regulatory Information (ESTRI)药政信息传递之电子标准M3: Timing of Pre-clinical Studies in Relation to Clinical Trials (See Safety Topics) 有关临床试验的临床前研究的时间安排M4: The Common Technical Document (See CTD section for complete Status of the guidelines)通用技术文件(见有关CTD章节)M5: Data Elements and Standards for Drug Dictionaries药物词典的数据要素和标准临床试验常用的英文缩略语TTP: time-to-progression 疾病进展时间SAE: severity Adverse Event 严重不良事件AE: Adverse Event 不良事件SOP: Standard Operating Procedure 标准操作规程CRF: Case Report form 病例报告表DLT:剂量限制毒性MTD:最大耐受剂量KPS: Karnofsky Performance Status行为状态评分CR: complete response完全缓解PR: partial response部分缓解SD:病情稳定PD: progressive disease病情进展CTC:常用药物毒性标准IEC: independent ethics committee 独立伦理委员会IRB : institutional review board 伦理委员会CRA:临床研究助理CRO: Contract Research Organization 合同研究组织DFS: Disease Free Survival 无病生存期OS:(Overall Survival)总生存时间IC: Informed consent 知情同意ADR: Adverse Drug Reaction 不良反应GAP:Good Agricultural Practice 中药材种植管理规范GCP:Good Clinical Practice 药物临床试验质量管理规范GLP:Good Laboratory Practice 药品实验室管理规范GMP:Good Manufacturing Practice 药品生产质量管理规范GSP:Good Supply Practice 药品经营质量管理规范GUP:Good Use Practice 药品使用质量管理规范PI :Principal investigator 主要研究者CI: Co-inveatigator 合作研究者SI :Sub-investigator 助理研究者COI :Coordinating investigtor 协调研究者DGMP:医疗器械生产质量管理规范ICF: Informed consent form 知情同意书RCT : randomized controlled trial, 随机对照试验NRCCT: non-randomized concurrent controlled trial, 非随机同期对照试验EBM: evidence-based medicine 循证医学RCD: randomized cross-over design 随机交叉对照试验HCT: historical control trial, 历史对照研究RECIST: Response Evaluation Criteria In Solid Tumors. 实体瘤疗效反应的评价标准QC: Quality Control质量控制UADR: Unexpected Adverse Drug Reaction,非预期药物不良反应。
临床试验中所有涉及到得英文翻译

险分别是:养老保险:单位每个月为你缴纳21%,你自己缴纳8%;医疗保险:单位每个月为你缴纳9%,你自己缴纳2%外加10块钱的大病统筹(大病统筹主要管住院这块);失业保险:单位每个月为你缴纳2%,你自己缴纳1%;工伤保险:单位每个月为你缴纳0.5%,你自己一分钱也不要缴;生育保险:单位每个月为你缴纳0.8%,你自己一分钱也不要缴;住房公积金:单位每个月为你缴纳8%,你自己缴纳8%以上,这么算下来,单位每个月为你缴纳的社保比例应该是21%+9%+2%+0.5%+0.8%+8%=41.3%你自己每个月为你缴纳的社保比例应该是8%+2%+10块+1%+8%=19%+10块statement of agreement 协议声明Participant Information 参加者信息Informed Consent Form 知情同意书Sponsor 申办者Study Site 研究地址approve 批准moderate to severe psoriasis 中度至重度银屑病local operation entity 当地运营实体Research and Development, Inc 研发公司Xian-Janssen Pharmaceutical Ltd 西安杨森制药有限公司the electrical activity of the heartover time心脏随时间推移的电活动be required to fast from food andliquid禁用食物和液体qualitative analysis 定性分析quantitative analysis 定量分析instrumental analysis 仪器分析法flow injection analysis;FIA 流动注射分析法determinate error 可定误差coefficient of variation 变异系数confidence level 置信水平level of significance 显著性水平pooled standard debiation 合并标准偏差(组合标准差)rejection quotient ;Q 舍弃商volumetric analysis 容量分析法titrametric analysis 滴定分析法stoichiometric point 化学计量点equivalent point 等当点charge balance 电荷平衡charge balance equation 电荷平衡式mass balance 质量平衡material balance 物料平衡mass balance equation 质量平衡式acid-base indicator 酸碱指示剂acid-base titrations 酸碱滴定法autoprotolysis reaction 质子自递反应constant 常数proton balance equation 质子条件式colour change interval 变色范围protonic solvent 质子溶剂aprotic solvent 无质子溶剂differentiating effect 均化效应differentiating solvent 区分性溶剂amphototeric solvent 两性溶剂dissociation 离解crystal violet 结晶紫α-naphthalphenol benzyl alcohol 萘酚苯甲醇quinadinered 奎哪啶红thymol blue 百里酚蓝azo violet 偶氮紫bromophenol blue 溴酚蓝compleximetry 配位滴定法ischemic preconditioning 缺血预适应simple ischemia-reperfusion injury单纯缺血与再灌注损伤组groupLeukocyte 白血球Floating gel 漂浮凝胶acid concentration of the medium 介质酸浓度Alginic acid 海藻酸Aluminium hydroxide 氢氧化铝antacid activity 抗酸活性Antacid agent 抗酸剂Anti-reflux agent 抗返流剂benzyl alcohol 苯甲醇blinding agent 粘合剂Bulking agent 填充剂Calcium carbonate 碳酸钙carbonate 碳酸盐combinations 复方Comparative active ingredient活性成分组成的比较compositionsDetermination of pH gradient in vitro 体外PH梯度测定Dextrates 葡萄糖结合剂drug product 制剂drug substance 原料药duration 持续时间Excipients 辅料Filling agent 填充剂Flavour 香精/香料Formation of a floating gel in vitro 漂浮凝胶在体外的形成function of the acid concentration of介质酸浓度函数the mediumGaviscon? tablets Gaviscon?片Glucose monohydrate 一水葡萄糖Granulating agent 制粒溶剂Heavy magnesium carbonate 重质碳酸镁In-house monograph 内部专论Lemon cream flavour 柠檬奶油香精/香料Lubricant 润滑剂magnesium carbonate 碳酸镁magnesium carbonate gel 碳酸镁凝胶magnesium chloride 氯化镁Magnesium stearate 硬脂酸镁Magnesium trisilicate 三硅酸镁Mean “raft” pH平均“筏”PhPeak “intra-gastric” pH胃内峰PHpeppermint flavour 薄荷香料/香精pH gradient pH 梯度pharmaceutical development 制药开发Povidone 聚维酮Quantitative composition 定量组成raft 筏Raft booster 筏推动剂Rennie? Dual Action tablets 罗内?双效片Rennie? Duo, chewable tablets 罗内?Duo咀嚼片Rennie? Duo, oral suspension 罗内?Duo口服混悬剂RENNIE? LIQUID 罗内?液体剂Saccharin sodium 糖精钠Sodium alginate海藻酸钠 Sodium bicarbonate碳酸氢钠 sodium chloride氯化钠 Sodium hydrogen carbonate碳酸氢钠 sodium propyl p-hydroxybenzoate对羟基苯甲酸丙酯钠 stearate硬脂酸盐 Sucrose蔗糖 Sweetener甜味剂 symptomatic treatment症状性治疗 Talc滑石粉 Xanthan gum黄原胶 6-1 :David Grimes 教授演讲部分 long acting contraception长效避孕法 The role of long acting contraception in family planning长效避孕法在计划生育中发挥的作用 long-acting reversible contraception 长效可逆性避孕法 forgettable contraception遗忘式避孕法 overt act专门措施 Coital frequency性交频率 Progestin injection黄体酮注射 Depot medroxyprogesterone acetate 长效醋酸甲羟孕酮(DMPA )Intrauterine Contraception 宫内避孕法 Levonorgestrel Releasing System 左炔诺孕酮释放系统 Levonorgestrel intrauterine system (LNG-IUS)左炔诺孕酮宫内节育系统(LNG-IUS ) Single-rod progestin implant单棒黄体酮植入物 fibroids, hemoglobinopathy纤维瘤,血红蛋白病 surrogate end points替代终点 risk of ectopic pregnancies异位妊娠风险 tubal infertility输卵管性不孕 Gross Removal Rates总取出率 Levonorgestrel IUS左炔诺孕酮IUS Barrier methods屏障法 Venous thromboembolism静脉血栓形成 hysterectomy子宫切除术 endometriosis子宫内膜异位症 perimenopausal symptoms围绝经期症状 Hemoglobin and ferritin血红蛋白和铁蛋白 parous women经产妇 nulliparous women未产妇 menorrhagia / dysmenorrhea 月经过多/痛经uterine involution 子宫复旧Perforation rate 穿孔率Expulsion frequency 排出率estrogen and progesterone receptors 雌激素和孕酮受体expulsion / salpingitis 排出/输卵管炎curettage 刮除术mifepristone 米非司酮Lactation 哺乳期/泌乳partum 分娩spotting and bleeding 出血和点状出血Full breast-feeding 完全母乳喂养antiphospholipid syndrome 抗磷脂综合征anticoagulation 抗凝药estradiol 雌二醇6-2 :Dr. Ritva Hurskainen 演讲部分endometrial resection / ablation 子宫内膜切除/消融术Submucous fibroids 黏膜下纤维化Endometrial polyps 子宫内膜息肉Ovarian tumours or cysts 卵巢肿瘤或囊肿uterine malformation 子宫畸形acne 痤疮Levonorgestrel-ReleasingIntrauterine System左炔诺孕酮宫内缓释系统Health-Related Quality of Life 健康相关生存质量Bladder-emptying 膀胱排空Urge incontinence 尿失禁Stress incontinence 压力性失禁Tranexamic acid 氨甲环酸Norethisterone 炔诺酮Myomectomy or uterine artery embolisation 子宫肌瘤剔除术或子宫动脉栓塞术endometrial ablation 子宫内膜消融术6-2 :Yu Qi 教授演讲部分Heavy menstrual bleeding (HMB) 月经过多 (HMB) injected progestogens 孕激素注射剂Health Economics 卫生经济学Oophorectomy with hysterectomy 卵巢切除术联合子宫切除术Dilatation and curettage 扩刮术Impedance-controlled bipolar 阻抗控制双极射频消融术radiofrequency ablationFluid-filled thermal balloon endometrial ablation (TBEA) 充液热球囊子宫内膜消融术Microwave endometrial ablation(MEA)微波子宫内膜消融术Free fluid thermal endometrial ablation 自由流体热子宫内膜消融术Care Pathway for HMB (1) HMB的诊治路径Intermenstrual bleeding 月经间期出血Anovulatory DUB 无排卵型DUB Ovulatory DUB 排卵型DUB gynecologic complaints 妇科主诉Anti-fibrinolysis drugs 抗纤溶药Endometrial Atrophy Therapy 子宫内膜萎缩疗法Inhibitor of prostaglandin synthesis 前列腺素合成抑制剂Flufenamic Acid 氟芬那酸6-2:Session 4 (Day 2) 部分的词汇menstrual disorders 月经紊乱Cervical glands 宫颈腺体Abortifacient 堕胎者Cervical smears 宫颈涂片Pelvic infection 盆腔感染Valvular heart disease 心脏瓣膜疾病Amenorrhea 闭经Menache 初潮Menstruation 行经Menopause 绝经cyclical norethisterone 环炔诺酮Non steroidal antiinflammatory drugs 非甾体类抗炎药Strong premenstrual symptoms 重度经前症状 (PMS) Contraceptive patch or ring 避孕贴或避孕环Progestin oral pills 孕激素口服片剂drospirenon 屈螺酮Migraine 偏头痛Migraine without aura 无预兆的偏头痛Ovarian cysts 卵巢囊肿flange 凸缘Paracervical blockade 宫颈旁阻滞麻醉Ibuprofen 布洛芬anteverted uterus 前倾子宫retroverted uterus 后倾子宫misoprostol 米索前列醇bleeding pattern 出血模式endometrial hyperplasia 子宫内膜增生tamoxifen 他莫西芬Clin Conf 1 - Contraception &ProfGrimes1 词汇Anovulation 停止排卵Premature ovarian failure 卵巢早衰Hyperprolactinaemia 高泌乳素血症Hypothyroidism 甲减Transvaginal ultrasound 经阴道超声Polycystic ovarian syndrome (PCOS) 多囊卵巢综合征(PCOS) Combined oral contraception 联合口服药避孕说明书Packaging Insert药品名称Article Name通用名称Generic Name汉语拼音Name In Bopomofo成分Ingredients作用类别/主治功能Function and indication 规格Strengths注意事项Precautions药物相互作用Drug Interaction有效期Expire date执行标准Executive Standard国家药品标准National drug standard批准文号Approval Document No 国药准字Guo yao zhun zi修订日期Revision Date生产地址Address of Facility如果有问题Please contact the manufacturer in case of any problem止痒Relieving Itching消炎Diminishing Inflammation非处方non-prescription (OTC) 英文中文Fly Sheet 扉页intra-individually controlled 个体自身对照dose-eomparative 剂量比较open-label 公开标签Clinical trial phase 临床试验阶段diagnostic confidence 诊断置信度qualitative evaluation 定性评价quantitative evaluation 定量评价global evaluation 总体评价physical examination 体格检查Synopsis 纲要confidence intervals. 置信区间Duration of treatment 治疗期/治疗持续时间mode of admin. 给药方式Reference therapy 参照疗法Criteria for evaluation 评价标准Efficacy 有效性signal intensity ratio 信号强度比overall visualization 总体显影c1inieal differenee 临床差异Trial Manager 试验主管Trial Director 试验总监Co-investigator 助理研究者Formulation 制剂Type of formulation 剂型Specific radioactivity 比放射性drug substance 原料药Vehicle composition 赋形剂成分Generic name 通用名Study design and plan 研究设计和计划description of rationale 原理说明Overview and justification 概述和论证Study configuration: 研究结构Level of blinding: 设盲水平Investigational product 试验性药物Interim analyses 中期分析steering committees 指导委员会Protocol amendments 方案修正Sampie size 样本量Molecular weight 分子量Structural formula 结构式Molecular formula: 分子式osmolality 克分子渗透压浓度viscosity 粘度Qualitative evaluation 定量评价localisation of lesion ? 病灶定位visualization of lesion ? 病灶显影characterization of lesion ? 病灶特征记述Equivocal 模糊No contrast 无差异Referral diagnosis 转诊诊断Pre-conifastt MRI diagnosis 增强前MRI诊断Drug relationship 药物相关性Intensity 严重程度Flow chart of trial activities 试验流程图imaging 影像学检查Baseline period 基线期Drop-outs 脱落Deviations from the trial protocol 与试验方案的背离Target variabies 靶变量Disposition of sUbjects 受试者安排Ethnic group 种族Medication history 治疗史Medical and surgical history 病史和手术史abnormal findings 异常发现pulse rate 脉率systolic blood pressure 收缩压diastolic blood pressure 舒张压general appearanee 一般状态primary tumor 原发肿瘤metastases 转移灶multiple sclerosis 多发性硬化症angiography 血管造影myelography 脊髓造影Data sets analyzed 数据组分析Diagnostic confidence: 诊断置信度Optimal injection 最佳注射Overall visualization 总体显影度signal intensity ratio 信号强度比contrast to noise ratio 对比噪声比Total drug exposure 总的药物暴露test article 供试品Text tables 正文表格Box plot 箱线图Scatter diagrams 散点图contrast agents造影剂 efficacy evaluation有效性评价 plain scans平扫 worsened变差 extent of exposure暴露程度 Total drug exposure总的药物暴露 Display and analysis of adverse events 不良事件的陈述和分析 weakness of extension伸展无力 involuntary tremor不自主震颤 tolerance indicators容许指示剂 Title Page标题页 Good Clinical Practice (GCP).药物临床试验质量管理规范(GCP ) Analysis set分析集 Intent - to - treat population意向治疗人群 Preferred population首选人群 Reader 1读片者1 blinded reading盲态读片 False positive lesions假阳性病变 Sensitivity and specificity in liver segment involvement肝段受累的敏感性和特异性 liver lobes肝叶 pooled segments混合段 pre-contrast MRI造影前MRI combined pre-and post MRI联合造影前/后MRI Lesion classification病变分类 lesion type病变类型 Assessment of enhancement增强的评估 (dynamic imaging and hepatocytephase)动态影像和肝细胞相 Signal-to-noise ratio信噪比 Contrast-to-noise ratio对比噪声比 Independent Ethics Committee (IEC) 独立伦理委员会(IEC )Institutional Review Board (IRB)机构审查委员会(IRB ) Ethical conduct伦理学实施 study administrative structure研究行政结构 Comparators对比方法 Prior and concomitant therapy既往和目前的合用药物 Diffuse liver disease弥漫性肝病 Focal liver lesions 肝脏局灶性病变 Trackable/untrackable focal liver 可追踪性/不可追踪性肝lesions脏局灶性病变 Liver maps肝脏图谱 Lesion detection病变检出 Lesion characterization病变鉴定 Morphology形态学 Biliary system imaging增强的评估 Artifacts伪像 pre-contrast T2-weighted sequences 造影前T2加权序列 Intraoperative ultrasound (IOUS)术中超声(IOUS ) Required pulse sequences规定的脉冲序列 Adjustments of image size and contrast 图像大小和对比度的调整 Biliary system imaging胆道系统成像 presence of thrombus有血栓 Adjustments for covariates对协变量的调整 Examination of subgroups亚组检查 Drug-drug and drug-diseaseinteractions药物-药物相互作用和药物-疾病相互作用 specified diffuse liver disease特定弥漫性肝病 matched lesions匹配病变 Number of correctly and incorrectly classified lesions正确和错误分类病变的数量 Mass Effect占位效应 Enhancement patterns 增强模式 New Atrial Extrasystoles Postbaseline 基线后新出现房性期外收缩New Ventricular ExtrasystolesPostbaseline基线后新出现室性期外收缩 PRODUCT MONOGRAPH药品专论 Intravenous contrast enhancement agent for magnetic resonance imaging (MRI)静脉注射的磁共振成像(MRI )造影剂 Submission Control No:提交文件控制号 Elimination清除 Hepatic Insufficiency肝功能不全 DOSAGE FORMS, COMPOSITION ANDPACKAGING剂型、成分和包装 Proper name专有名称 Physical form外观 Solubility溶解性 pH in water水溶液的pH Osmolality 渗透压Density密度 Bi-phasic enhanced spiral CT双相增强螺旋CT Animal Pharmacology动物药理学 Human Pharmacology人体药理学 Insufficiency功能不全 Repeated-Dose Toxicity多次给药毒性 Genotoxic Potential遗传毒性可能性 Tumorigenicity and Carcinogenicity 致肿瘤性和致癌性 Reproductive Toxicology生殖毒理学 Local Tolerance and SensitizingPotential局部耐受性和致敏可能性 Formulation number制剂编号 Substance code number原料药代码编号 specifications规格 release date发布日期 This edition supersedes替代版本 Property of Bayer Schering Pharma 所有权归Bayer ScheringPharma 所有Physical, chemical and pharmaceutical properties and formulation 物理、化学以及药理学特性和剂型Description of Drug Substance 药品说明Product interaction 产品相互作用Special Populations 特殊人群Mean (SD) serum concentrations 平均(SD )血清浓度fecal excretion 粪便排泄量compartment model dependent (CMD ) 间室模型依赖 renal clearance 肾清除率total clearance 总清除率beats per minute(bpm) 每分钟心跳次数end stage renal failure (ESRF ) 终末阶段肾衰focal nodular hyperplasia(FNH) 局灶结节性增生field of view(FOV) 视野gradient echo(GRE) 梯度回波Hoechst Adverse Events Reaction Thesaurus System(HARTS) Hoechst 不良事件反应词典系统Gd-EOB-DTPA 钆-EOB-DTPA ,钆塞酸high pressure liquidchromatography(HPLC)高压液相色谱 Inductively Coupled Plasma Atomic Emission Spectroscopy(ICPAES )电感耦合等离子体原子发射光谱法 Specific Rotation 比旋光度Partition Coefficient 分配系数Time Profile 时间特征Elimination Profile 消除曲线Biotransformation 生物转化volume of distribution at steady state 稳态下分布体积repetition time 保留时间time of echo 回声时间terminal half-life 终末半衰期initial half-life 初始半衰期no observable effect level 不可观察的反应水平microsoft disk operating system 微软磁盘操作系统mean residence time 平均停留时间magnetic resonance imaging 磁共振成像magnetic resonance 核磁共振minimum lethal dose 最低致死剂量Medical Dictionary for Regulatory国际医学用语词典Activitiesmean corpuscular volume 平均血细胞体积mean corpuscular hemoglobin 红细胞平均血红蛋白含量Intraoperative ultrasound 术中超声医学英语中的缩写词aa——各et——及、和Rp.——取、请取sig./S.——用法、指示St./Stat.——立即、急速Cit.——急速s.o.s.——需要时p.r.n——必要时a.c.——饭前p.c.——饭后a.m.——上午p.m.——下午q.n.——每晚h.s.——睡前q.h.——每小时q.d.——每日1次B.i.d.——每日2次T.i.d.——每日3次Q.i.d.——每日4次q.4h.——每4小时1次p.o.——口服ad us.int.——内服ad us.ext.——外用H.——皮下注射im./M.——肌肉注射iv./V.——静脉注射iv gtt.——静脉滴注Inhal.——吸入O.D.——右眼O.L.——左眼O.S.——单眼O.U.——双眼No./N.——数目、个s.s——一半ug.——微克mg.——毫克g.——克kg.——千克(公斤)ml.——毫升L.——升q.s——适量Ad.——加至Aq.——水Aq.dest.——蒸馏水Ft.——配成Dil——稀释M.D.S.——混合后给予Co./Comp.——复方的Mist——合剂Pulv.——散剂Amp.——安瓿剂Emul.——乳剂Syr.——糖浆剂Tr.——酊剂Neb.——喷雾剂Garg.——含漱剂rtt./gutt.——滴、滴眼剂collyr.——洗眼剂Ocul.——眼膏Liq.——溶液剂Sol.——溶液Lot.——洗剂Linim.——擦剂Crem.——乳膏剂(冷霜)Ung.——软膏剂Past.——糊剂Ol.——油剂Enem.——灌肠剂Supp.——栓剂Tab.——片剂Pil.——丸剂Caps.——胶囊剂Inj.——注射剂。
临床试验常用中英文词汇

SFDA Glossary:GCP,GLP,TRIAL Accuracy 准确度Active control,AC 阳性对照,活性对照Adverse drug reaction,ADR 药物不良反响Adverse event,AE 不良事件Adverse medical events 不良医学事件Adverse reaction 药物不良反响Alb 白蛋白ALD〔Approximate Lethal Dose〕近似致死剂量ALP 碱性磷酸酶Alpha spending function 消耗函数ALT 丙氨酸氨基转换酶Analysis sets 统计分析的数据集Approval 批准Assistant investigator 助理研究者AST 天门冬酸氨基转换酶ATR 衰减全反射法AUCss 稳态血药浓度-时间曲线下面积Audit 稽查Audit or inspection 稽查/视察Audit report 稽查报告Auditor 稽查员Bias 偏性,偏倚Bioequivalence 生物等效应Blank control 空白对照Blind codes 编制盲底Blind review 盲态审核Blind review 盲态检查Blinding method 盲法Blinding/ masking 盲法,设盲Block 分段Block 层Block size 每段的长度BUN 尿素氮Carryover effect 延滞效应Case history 病历Case report form 病例报告表Case report form/ case record form,CRF 病例报告表,病例记录表Categorical variable 分类变量Cav 平均浓度CD 圆二色谱CL 去除率Clinical equivalence 临床等效应Clinical study 临床研究Clinical study report 临床试验的总结报告Clinical trial 临床试验Clinical trial application,CTA 临床试验申请Clinical trial exemption,CTX 临床试验免责Clinical trial protocol,CTP 临床试验方案Clinical trial/ study report 临床试验报告Cmax 峰浓度Co-investigator 合作研究者Comparison 对照Compliance 依从性Composite variable 复合变量Computer-assisted trial design,CATD 计算机辅助试验设计Confidence interval 可信区间Confidence level 置信水平Consistency test 一致性检验Contract research organization,CRO 合同研究组织Contract/ agreement 协议/合同Control group 对照组Coordinating committee 协调委员会Crea 肌酐CRF〔case report form〕病例报告表Crossover design 交叉设计Cross-over study 交叉研究Css 稳浓度Cure 痊愈Data management 数据管理Database 建立数据库Descriptive statistical analysis 描述性统计分析DF 波动系统Dichotomies 二分类Diviation 偏差Documentation 记录/文件Dose-reaction relation 剂量-反响关系Double blinding 双盲Double dummy 双模拟Double dummy technique 双盲双模拟技术Double-blinding 双盲Drop out 脱落DSC 差示扫描热量计Effectiveness 疗效Electronic data capture,EDC 电子数据采集系统Electronic data processing,EDP 电子数据处理系统Emergency envelope 应急信件End point 终点Endpoint criteria/ measurement 终点指标Equivalence 等效性Essential documentation 必须文件Ethics committee 伦理委员会Excellent 显效Exclusion criteria 排除标准Factorial design 析因设计Failure 无效,失败Final point 终点Fixed-dose procedure 固定剂量法Forced titration 强制滴定Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Global assessment variable 全局评价变量GLU 血糖Good clinical practice,GCP 药物临床试验质量管理标准Good manufacture practice,GMP 药品生产质量管理标准Good non-clinical laboratory practice,GLP 药物非临床研究质量管理标准Group sequential design 成组序贯设计Health economic evaluation,HEV 健康经济学评价Hypothesis test 假设检验Hypothesis testing 假设检验International Conference of Harmonization,ICH 人用药品注册技术要求国际技术协调会,国际协调会议Improvement 好转Inclusion criteria 入选标准Independent ethics committee,IEC 独立伦理委员会Information consent form,ICF 知情同意书Information gathering 信息收集Informed consent,IC 知情同意Initial meeting 启动会议Inspection 视察/检查Institution inspection 机构检查Institution review board,IBR 机构审查委员会Intention to treat 意向治疗〔——临床领域〕Intention-to –treat,ITT 意向性分析〔-统计学〕Interactive voice response system,IVRS 互动式语音应答系统Interim analysis 期中分析Investigator 研究者Investigator's brochure,IB 研究者手册IR 红外吸收光谱Ka 吸收速率常Last observation carry forward,LOCF 最接近一次观察的结转LC-MS 液相色谱-质谱联用LD50 板数致死剂量Logic check 逻辑检查LOQ 〔Limit of Quantitation〕定量限LOCF,Last observation carry forward 最近一次观察的结转Lost of follow up 失访Marketing approval/ authorization 上市许可证Matched pair 匹配配对Missing value 缺失值Mixed effect model 混合效应模式Monitor 监查员Monitoring 监查Monitoring report 监查报告MRT 平均滞留时间MS 质谱MS-MS 质谱-质谱联用MTD〔Maximum Tolerated Dose〕最大耐受剂量Multicenter trial 多中心试验Multi-center trial 多中心试验New chemical entity,NCE 新化学实体New drug application,NDA 新药申请NMR 核磁共振谱Non-clinical study 非临床研究Non-inferiority 非劣效性Non-parametric statistics 非参数统计方法Obedience 依从性ODR 旋光光谱Open-blinding 非盲Open-label 非盲Optional titration 随意滴定Original medical record 原始医疗记录Outcome 结果Outcome assessment 结果指标评价Outcome measurement 结果指标Outlier 离群值Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient history 病历Per protocol,PP 符合方案集Placebo 抚慰剂Placebo control 抚慰剂对照Polytomies 多分类Power 检验效能Precision 精密度Preclinical study 临床前研究Primary endpoint 主要终点Primary variable 主要变量Principal investigator 主要研究者Principle investigator,PI 主要研究者Product license,PL 产品许可证Protocol 试验方案Protocol 试验方案Protocol amendment 方案补正Quality assurance unit,QAU 质量保证部门Quality assurance,QA 质量保证Quality control,QC 质量控制Query list,query form 应用疑问表Randomization 随机化Randomization 随机Range check 范围检查Rating scale 量表Regulatory authorities,RA 监督管理部门Replication 可重复RSD 日内和日间相对标准差Run in 准备期Safety evaluation 平安性评价Safety set 平安性评价的数据集Sample size 样本含量Sample size 样本量,样本大小Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Serious adverse event,SAE 严重不良事件Serious adverse reaction,SAR 严重不良反响Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single blinding 单盲Single-blinding 单盲Site audit 试验机构稽查SOP 试验室的标准操作规程Source data verification,SDV 原始数据核准Source data,SD 原始数据Source document,SD 原始文件Specificity 特异性Sponsor 申办者Sponsor-investigator 申办研究者Standard curve 标准曲线Standard operating procedure,SOP 标准操作规程Statistic 统计量Statistical analysis plan 统计分析方案Statistical analysis plan 统计参数方案书Statistical analysis plan,SAP 统计分析方案Statistical model 统计模型Statistical tables 统计分析表Stratified 分层Study audit 研究稽查Subgroup 亚组Sub-investigator 助理研究者Subject 受试者Subject diary 受试者日记Subject enrollment 受试者入选Subject enrollment log 受试者入选表Subject identification code,SIC 受试者识别代码Subject recruitment 受试者招募Subject screening log 受试者筛选表Superiority 检验Survival analysis 生存分析SXRD 单晶X-射线衍射System audit 系统稽查T1/2 消除半衰期Target variable 目标变量T-BIL 总胆红素T-CHO 总胆固醇TG 热重分析TLC、HPLC 制备色谱Tmax 峰时间TP 总蛋白Transformation 变量变换Treatment group 试验组Trial error 试验误差Trial master file 试验总档案Trial objective 试验目的Trial site 试验场所Triple blinding 三盲Two one-side test 双单侧检验Unblinding 揭盲Unblinding 破盲Unexpected adverse event,UAE 预料外不良事件UV-VIS 紫外-可见吸收光谱Variability 变异Variable 变量Visual analogy scale 直观类比打分法Visual check 人工检查Vulnerable subject 弱势受试者Wash-out 清洗期Washout period 洗脱期Well-being 福利,健康。
临床试验英语词汇
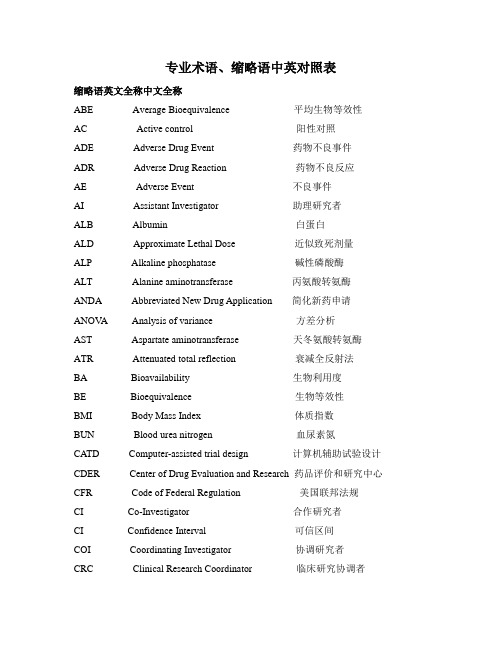
专业术语、缩略语中英对照表缩略语英文全称中文全称ABE Average Bioequivalence 平均生物等效性AC Active control 阳性对照ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者ALB Albumin 白蛋白ALD Approximate Lethal Dose 近似致死剂量ALP Alkaline phosphatase 碱性磷酸酶ALT Alanine aminotransferase 丙氨酸转氨酶ANDA Abbreviated New Drug Application 简化新药申请ANOV A Analysis of variance 方差分析AST Aspartate aminotransferase 天冬氨酸转氨酶ATR Attenuated total reflection 衰减全反射法BA Bioavailability 生物利用度BE Bioequivalence 生物等效性BMI Body Mass Index 体质指数BUN Blood urea nitrogen 血尿素氮CATD Computer-assisted trial design 计算机辅助试验设计CDER Center of Drug Evaluation and Research 药品评价和研究中心CFR Code of Federal Regulation 美国联邦法规CI Co-Investigator 合作研究者CI Confidence Interval 可信区间COI Coordinating Investigator 协调研究者CRC Clinical Research Coordinator 临床研究协调者CRF Case Report/Record Form 病历报告表/病例记录表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告CTX Clinical Trial Exemption 临床试验免责CHMP Committee for Medicinal 人用药委会Products for Human UseDSC Differential scanning 差示扫描热量计DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统EWP Europe Working Party 欧洲工作组FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GCP Good Laboratory Practice 药物非临床试验质量管理规范GLU Glucose 葡萄糖GMP Good Manufacturing Practice 药品生产质量管理规范HEV Health economic evaluation 健康经济学评价IB Investigator’s Brochure研究者手册IBE IndividualBioequivalence 个体生物等效性IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会ITT Intention-to –treat 意向性分析IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统LD50 Medial lethal dose 半数致死剂量LLOQ Lower Limit of quantitation 定量下限LOCF Last observation carry forward 最接近一次观察的结转LOQ Limit of Quantitation 检测限MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部MRT Mean residence time 平均滞留时间MTD Maximum Tolerated Dose 最大耐受剂量ND Not detectable 无法定量NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)NMR Nuclear Magnetic Resonance 核磁共振PD Pharmacodynamics 药效动力学PI Principal Investigator 主要研究者PK Pharmacokinetics 药物动力学PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PP Per protocol 符合方案集PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QAU Quality Assurance Unit 质量保证部门QC Quality Control 质量控制QWP Quality Working Party 质量工作组RA Regulatory Authorities 监督管理部门REV Revision 修订SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SFDA State Food and Drug Administration 国家食品药品监督管理局SI Sponsor-Investigator 申办研究者SI Sub-investigator 助理研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂T-BIL Total Bilirubin 总胆红素T-CHO Total Cholesterol 总胆固醇TG Thromboglobulin 血小板球蛋白Tmax Time of maximum concentration 达峰时间TP Total proteinum 总蛋白UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO- WHO International Conference WHO 国际药品管理当局会议ICDR A of Drug Regulatory AuthoritiesAberrant result 异常结果Absorption phase 吸收相Absorption 吸收Accuracy 准确度Accurate 精密度Administer 给药Amendment修正案Approval 批准Assess 估计Audit Report 稽查报告Audit 稽查Auditor 稽查员Analytical run/batch:分析批Benefit 获益Bias 偏性,偏倚Bioequivalence 生物等效Biosimilar /Follow-on biologics 生物仿制药Blank Control 空白对照Blind codes 编制盲底Blind review 盲态检查/盲态审核Blinding method 盲法Blinding/masking 盲法/设盲Block size 每段的长度Block 层/分段BCS 生物药剂学分类系统Carryover effect 延滞效应Case history 病历Clinical equivalence 临床等效性Clinical study 临床研究Clinical Trial Report 临床试验报告Comparison 对照Compensation 补偿,赔偿金Compliance 依从性Concomitant 伴随的Conduct 行为Confidence level 置信水平Consistency test 一致性检验Contract/ agreement 协议/合同Control group 对照组Coordinating Committee 协调委员会Crossover design 交叉设计Cross-over Study 交叉研究Cure 痊愈Data management 数据管理Descriptive statistical analysis 描述性统计分析Dichotomies 二分类Dispense 分布Diviation 偏差Documentation 记录/文件Dosage forms 剂型Dose dumping 剂量倾卸(药物迅速释放入血而达到危险浓度)Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模Drop out 脱落Effectiveness 疗效Elimination phase 消除相Emergency envelope 应急信件Enantiomers 对映体End point 终点Endpoint criteria/ measurement 终点指标Enterohepatic recycling 肠肝循环Essential Documentation 必需文件Ethical 伦理的Ethics committee 伦理委员会Evaluate 评估Exclusion Criteria 排除标准Excretion 排泄Expedite 促进Extrapolated 外推的Essentially similar product:基本相似药物Factorial design 析因设计Failure 无效,失败Finacing 财务,资金Final point 终点First pass metabolism 首过代谢Fixed-dose procedure 固定剂量法Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Gene mutation 基因突变Genotoxicity tests 生殖毒性试验Global assessment variable 全局评价变量Group sequential design 成组序贯设计Hypothesis test 假设检验Highly permeable:高渗透性Highly soluble:高溶解度Highly variable drug:高变异性药物Highly:Variable Drug 高变异性药物HVDP:高变异药物制剂Identification 鉴别,身份证Improvement 好转In vitro 体外In vivo 体内Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Instruction 指令,说明Integrity 完整,正直Intercurrent 中间发生的,间发的Inter-individual variability 个体间变异性Interim analysis 期中分析Investigational Product 试验药物Investigator 研究者Involve 引起,包括IR 红外吸收光谱Innovator Product:原创药Ka 吸收速率常LC-MS 液相色谱-质谱联用logarithmic transformation 对数转换Logic check 逻辑检查Lost of follow up 失访Mask 面具,掩饰Matched pair 匹配配对Metabolism 代谢Missing value 缺失值Mixed effect model 混合效应模式Modified release products 改良释放剂型Monitor 监查员Monitoring Plan 监察计划Monitoring Report 监察报告MS-MS 质谱-质谱联用Multi-center Trial 多中心试验Negative 阴性,否定的Non-clinical Study 非临床研究Non-inferiority 非劣效性Non-Linear Pharmacokinetics 非线性药代动力学Non-parametric statistics 非参数统计方法NTID:窄治疗指数制剂Obedience 依从性Open-blinding 非盲Open-label 非盲Original Medical Record 原始医疗记录Outcome Assessment 结果评价Outcome measurement 结果指标Outlier 离群值OIP 经口服吸收药物Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient History 病历Per protocol,PP 符合方案集Permeability 渗透性Pharmacodynamic characteristics 药效学特征Pharmacokinetic characteristics 药代学特征Placebo Control 安慰剂对照Placebo 安慰剂Polytomies 多分类Post-dosing postures 给药后坐姿Potential 潜在的Power 检验效能Precision 精密度Preclinical Study 临床前研究Precursor 母体前体Premature 过早的,早发Primary endpoint 主要终点Primary variable 主要变量Prodrug 药物前体Protocol amendment 方案补正Protocol Amendments 修正案Protocol 试验方案Quality Control Sample:质控样品Rapidly dissolving:快速溶出Racemates 外消旋物Randomization 随机/随机化Range check 范围检Rating scale 量表Recruit 招募,新会员Replication 可重复Retrieval 取回,补修Revise 修正Risk 风险Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample Size 样本量、样本大小Sampling schedules 采血计划Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single Blinding 单盲Site audit 试验机构稽查Solubility 溶解度Specificity 特异性Specify 叙述,说明Sponsor-investigator 申办研究者Standard curve 标准曲线Statistical model 统计模型Statistical tables 统计分析表Steady state 稳态Storage 储存Stratified 分层Study Audit 研究稽查Study Site 研究中心Subgroup 亚组Sub-investigator 助理研究者Subject Enrollment Log 受试者入选表Subject Enrollment 受试者入选Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Subject Screening Log 受试者筛选表Subject 受试者Submit 交付,委托Superiority 检验Supplemental 增补的Supra-bioavailability 超生物利用度(试验药的生物利用度大于对照药)Survival analysis 生存分析System Audit 系统稽查SmPC:药品说明书Standard Sample:标准样品Target variable 目标变量Treatment group 试验组Trial error 试验误差Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Trial site 试验场所Triple Blinding 三盲Two one-side test 双单侧检验Therapeutic equivalence:治疗等效性Un-blinding 破盲/揭盲Verify 查证、核实Visual analogy scale 直观类比打分法Vulnerable subject 弱势受试者Wash-out Period 洗脱期Well-being 福利,健康Withdraw 撤回,取消药代动力学参数Ae(0-t):给药到t时尿中排泄的累计原形药。
FDA常用词汇中英文,请收好!

FDA常用词汇中英文,请收好!AAccelerated: 加速条件 Accuracy: 准确性AIP(Application Integrity Policy): 申请完全政策制裁ANDA(Abbreviation New Drug Application):仿制药或仿制新药申请API(Active Pharmaceutical Ingredient):原料药或活性药。
原简称BPC(Bulk Pharmaceutical Chemical),现常用API。
在药典和一些论文中也常用Drug Substance 或Substance 来代表原料药。
Appearance: 外观Assay:含量Assessment: 药厂自我评估(厂家组织进行的对药厂本身设施有关文件的模拟FDA检查)Audit:审查(预审查,多用于美方原料药用户,在FDA和PAI之前到药厂进行现场预检查)Auditor:审核员Audit trail: 审计踪迹BBasket:篮子式Batch production records: 批生产纪录Batch records:批号(量)纪录(即batch production and control records 批量生产和检验纪录)BPC:(Bulk Pharmaceutical chemical)原料药Bracketing stability design:稳定性试验的括号分组设计Blend uniformity: 均匀度CCalibration: 校正或校准(对设备,仪器和衡器等的准确度进行校正)Certification of Areas for GMP compliance: (检验企业实施现行药品生产管路规范部门的标准操作规程)CFR 21 Part 11(Code of federal Registry Part11):联邦法规法典标题21第11部分CGMP(Current Good Manufacturing Practice):现行药品生产质量管理规范Change control form:也简称CCF 变更控制表Change control:变更控制Cleaning validation:清洁验证CMC(Chemistry and manufacture control)化学和生产的控制Compliance:符合性Compatibility:共存性或兼容性Content uniformity test: 产品含量均匀性测定Container closure system: 容器封闭系统 COA(Certification of analysis ):分析合格证书,检验报告或检验报告单DDelayed release:延期放行Design qualification: 设计确认Dissolution test:溶出度测试 Deviation records:偏差纪录DMF(drug master file):药物主文件或原料药档案Drug product:成品药 Drug substance:原料药EEIR(Establishment inspection report):确定检查报告Electronic signature:电子签名Equipment qualification:对设备,设施,仪器等性能的鉴定Excipients:赋形剂或辅料Excit meeting:现场检查结束会 Extended release: 缓慢释放EMEA(The European Medicines Evaluation Agency):欧洲医药评审委员会FFinished pharmaceuticals (drug product, finishes product, finished dosage form):制剂药(成品药)其定义位已原料药为起始物料,加一定的赋形剂,制成具有一定剂型可直接用于治疗的药剂。
临床试验中所有涉及到得英文翻译(14页)

临床试验中所有涉及到的英文翻译(第1页)1. 临床试验(Clinical Trial)2. 研究参与者(Research Participant)3. 研究方案(Protocol)4. 伦理审查委员会(Institutional Review Board, IRB)5. 知情同意书(Informed Consent Form)6. 试验药物(Investigational Product)7. 对照组(Control Group)8. 实验组(Experimental Group)9. 随机化(Randomization)10. 双盲试验(Doubleblind Trial)11. 病例报告表(Case Report Form, CRF)12. 不良事件(Adverse Event)13. 严重不良事件(Serious Adverse Event)14. 数据监控委员会(Data Monitoring Committee, DMC)15. 统计分析计划(Statistical Analysis Plan, SAP)16. 研究终点(Study Endpoint)17. 临床终点(Clinical Endpoint)18. 效力(Efficacy)19. 安全性(Safety)20. 药物代谢动力学(Pharmacokinetics)21. 药物效应动力学(Pharmacodynamics)22. 生物利用度(Bioavailability)23. 生物等效性(Bioequivalence)24. 药物相互作用(Drug Interaction)临床试验中所有涉及到的英文翻译(第2页)25. 研究目标(Study Objective)26. 研究假设(Research Hypothesis)27. 入组标准(Inclusion Criteria)28. 排除标准(Exclusion Criteria)29. 受试者筛选(Subject Screening)30. 基线评估(Baseline Assessment)31. 随访(Followup Visit)32. 疗程(Treatment Regimen)33. 药物剂量(Drug Dosage)34. 给药途径(Route of Administration)35. 药物耐受性(Drug Tolerance)36. 药物依赖性(Drug Dependence)37. 药物副作用(Side Effect)38. 药物毒性(Toxicity)39. 最大耐受剂量(Maximum Tolerated Dose)40. 疗效评估(Efficacy Evaluation)41. 安全性评估(Safety Assessment)42. 生命体征(Vital Signs)43. 实验室检查(Laboratory Tests)44. 影像学检查(Imaging Studies)45. 病历记录(Medical Records)46. 病历报告(Medical Report)47. 病历审查(Medical Review)48. 病历编码(Medical Coding)49. 病历数据库(Medical Database)临床试验中所有涉及到的英文翻译(第3页)50. 研究协调员(Study Coordinator)51. 主要研究者(Principal Investigator)52. 研究团队(Research Team)53. 监查员(Monitor)54. 申办者(Sponsor)55. 研究资助(Research Funding)56. 研究预算(Research Budget)57. 研究合同(Research Contract)58. 知识产权(Intellectual Property)59. 专利保护(Patent Protection)60. 研究注册(Study Registration)61. 临床试验注册(Clinical Trials Registration)62. 公开透明(Transparency)63. 研究结果发表(Publication of Results)64. 数据共享(Data Sharing)65. 隐私保护(Privacy Protection)66. 受试者隐私(Subject Privacy)67. 保密协议(Confidentiality Agreement)68. 信息安全(Information Security)69. 数据保护(Data Protection)70. 研究合规(Research Compliance)71. 法律法规(Regulatory Requirements)72. 质量保证(Quality Assurance)73. 质量控制(Quality Control)74. 标准操作程序(Standard Operating Procedures, SOPs)。
临床试验常用中英文词汇

SFDA Glossary: GCP,GLP,TRIALAccuracy 准确度Active control, AC 阳性对照,活性对照Adverse drug reaction, ADR 药物不良反应Adverse event, AE 不良事件Adverse medical events 不良医学事件Adverse reaction 药物不良反应Alb 白蛋白ALD(Approximate Lethal Dose)近似致死剂量ALP 碱性磷酸酶Alpha spending function 消耗函数ALT 丙氨酸氨基转换酶Analysis sets 统计分析的数据集Approval 批准Assistant investigator 助理研究者AST 天门冬酸氨基转换酶ATR 衰减全反射法AUCss 稳态血药浓度-时间曲线下面积Audit 稽查Audit or inspection 稽查/视察Audit report 稽查报告Auditor 稽查员Bias 偏性,偏倚Bioequivalence 生物等效应Blank control 空白对照Blind codes 编制盲底Blind review 盲态审核Blind review 盲态检查Blinding method 盲法Blinding/ masking 盲法,设盲Block 分段Block 层Block size 每段的长度BUN 尿素氮Carryover effect 延滞效应Case history 病历Case report form 病例报告表Case report form/ case record form, CRF 病例报告表,病例记录表Categorical variable 分类变量Cav 平均浓度CD 圆二色谱CL 清除率Clinical equivalence 临床等效应Clinical study 临床研究Clinical study report 临床试验的总结报告Clinical trial 临床试验Clinical trial application, CTA 临床试验申请Clinical trial exemption, CTX 临床试验免责Clinical trial protocol, CTP 临床试验方案Clinical trial/ study report 临床试验报告Cmax 峰浓度Co-investigator 合作研究者Comparison 对照Compliance 依从性Composite variable 复合变量Computer-assisted trial design, CATD 计算机辅助试验设计Confidence interval 可信区间Confidence level 置信水平Consistency test 一致性检验Contract research organization, CRO 合同研究组织Contract/ agreement 协议/合同Control group 对照组Coordinating committee 协调委员会Crea 肌酐CRF(case report form)病例报告表Crossover design 交叉设计Cross-over study 交叉研究Css 稳浓度Cure 痊愈Data management 数据管理Database 建立数据库Descriptive statistical analysis 描述性统计分析DF 波动系统Dichotomies 二分类Diviation 偏差Documentation 记录/文件Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模拟Double dummy technique 双盲双模拟技术Double-blinding 双盲Drop out 脱落DSC 差示扫描热量计Effectiveness 疗效Electronic data capture, EDC 电子数据采集系统Electronic data processing, EDP 电子数据处理系统Emergency envelope 应急信件End point 终点Endpoint criteria/ measurement 终点指标Equivalence 等效性Essential documentation 必须文件Ethics committee 伦理委员会Excellent 显效Exclusion criteria 排除标准Factorial design 析因设计Failure 无效,失败Final point 终点Fixed-dose procedure 固定剂量法Forced titration 强制滴定Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Global assessment variable 全局评价变量GLU 血糖Good clinical practice, GCP 药物临床试验质量管理规范Good manufacture practice, GMP 药品生产质量管理规范Good non-clinical laboratory practice, GLP 药物非临床研究质量管理规范Group sequential design 成组序贯设计Health economic evaluation, HEV 健康经济学评价Hypothesis test 假设检验Hypothesis testing 假设检验International Conference of Harmonization, ICH 人用药品注册技术要求国际技术协调会,国际协调会议Improvement 好转Inclusion criteria 入选标准Independent ethics committee, IEC 独立伦理委员会Information consent form, ICF 知情同意书Information gathering 信息收集Informed consent, IC 知情同意Initial meeting 启动会议Inspection 视察/检查Institution inspection 机构检查Institution review board, IBR 机构审查委员会Intention to treat 意向治疗(——临床领域)Intention-to –treat, ITT 意向性分析(-统计学)Interactive voice response system, IVRS 互动式语音应答系统Interim analysis 期中分析Investigator 研究者Investigator's brochure, IB 研究者手册IR 红外吸收光谱Ka 吸收速率常Last observation carry forward, LOCF 最接近一次观察的结转LC-MS 液相色谱-质谱联用LD50 板数致死剂量Logic check 逻辑检查LOQ (Limit of Quantitation)定量限LOCF, Last observation carry forward 最近一次观察的结转Lost of follow up 失访Marketing approval/ authorization 上市许可证Matched pair 匹配配对Missing value 缺失值Mixed effect model 混合效应模式Monitor 监查员Monitoring 监查Monitoring report 监查报告MRT 平均滞留时间MS 质谱MS-MS 质谱-质谱联用MTD(Maximum Tolerated Dose)最大耐受剂量Multicenter trial 多中心试验Multi-center trial 多中心试验New chemical entity, NCE 新化学实体New drug application, NDA 新药申请NMR 核磁共振谱Non-clinical study 非临床研究Non-inferiority 非劣效性Non-parametric statistics 非参数统计方法Obedience 依从性ODR 旋光光谱Open-blinding 非盲Open-label 非盲Optional titration 随意滴定Original medical record 原始医疗记录Outcome 结果Outcome assessment 结果指标评价Outcome measurement 结果指标Outlier 离群值Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient history 病历Per protocol, PP 符合方案集Placebo 安慰剂Placebo control 安慰剂对照Polytomies 多分类Power 检验效能Precision 精密度Preclinical study 临床前研究Primary endpoint 主要终点Primary variable 主要变量Principal investigator 主要研究者Principle investigator, PI 主要研究者Product license, PL 产品许可证Protocol 试验方案Protocol 试验方案Protocol amendment 方案补正Quality assurance unit, QAU 质量保证部门Quality assurance, QA 质量保证Quality control, QC 质量控制Query list, query form 应用疑问表Randomization 随机化Randomization 随机Range check 范围检查Rating scale 量表Regulatory authorities, RA 监督管理部门Replication 可重复RSD 日内和日间相对标准差Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample size 样本含量Sample size 样本量,样本大小Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Serious adverse event, SAE 严重不良事件Serious adverse reaction, SAR 严重不良反应Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single blinding 单盲Single-blinding 单盲Site audit 试验机构稽查SOP 试验室的标准操作规程Source data verification, SDV 原始数据核准Source data, SD 原始数据Source document, SD 原始文件Specificity 特异性Sponsor 申办者Sponsor-investigator 申办研究者Standard curve 标准曲线Standard operating procedure, SOP 标准操作规程Statistic 统计量Statistical analysis plan 统计分析计划Statistical analysis plan 统计参数计划书Statistical analysis plan, SAP 统计分析计划Statistical model 统计模型Statistical tables 统计分析表Stratified 分层Study audit 研究稽查Subgroup 亚组Sub-investigator 助理研究者Subject 受试者Subject diary 受试者日记Subject enrollment 受试者入选Subject enrollment log 受试者入选表Subject identification code, SIC 受试者识别代码Subject recruitment 受试者招募Subject screening log 受试者筛选表Superiority 检验Survival analysis 生存分析SXRD 单晶X-射线衍射System audit 系统稽查T1/2 消除半衰期Target variable 目标变量T-BIL 总胆红素T-CHO 总胆固醇TG 热重分析TLC、HPLC 制备色谱Tmax 峰时间TP 总蛋白Transformation 变量变换Treatment group 试验组Trial error 试验误差Trial master file 试验总档案Trial objective 试验目的Trial site 试验场所Triple blinding 三盲Two one-side test 双单侧检验Unblinding 揭盲Unblinding 破盲Unexpected adverse event, UAE 预料外不良事件UV-VIS 紫外-可见吸收光谱Variability 变异Variable 变量Visual analogy scale 直观类比打分法Visual check 人工检查Vulnerable subject 弱势受试者Wash-out 清洗期Washout period 洗脱期Well-being 福利,健康。
FDA临床试验常见词汇中译文对照

FDA临床试验常见词汇中译文对照Aaction letter 决定通知active comparator 活性药物对照组active control = AC 阳性对照,活性对照active ingredient 有效成分Active Substance Master File (ASMF) 欧洲药物主文件acute myocardial infarction 急性心肌梗死acute tibial fractures 急性胫骨骨折adalimumab (Humira) 阿达木单抗adaptive design 自适应设计adaptive randomization 自适应随机ADE = adverse drug event 药物不良事件Adenoviral Vectors 腺病毒载体adequate and well-controlled studies 充分严格的对照研究ADHD = Attention-deficit hyperactivity disorder注意力缺陷多动障碍; 注意力不足过动症; 多动症adhesion barrier product 防黏著产品adjuvant 助剂; 佐剂auxiliary;adjuvant therapy 佐药疗法,辅助疗法ADL = activities of daily living 日常生活活动能力ADME = absorption, distribution, metabolism, and excretion(药物)吸收、分配、代谢和排除ADR = adverse drug reaction 药物不良反应adrenal cortex 肾上腺皮质adrenal cortical hormone 肾上腺皮质激素adrenal gland 肾上腺adrenaline 肾上腺素adulterated devices 掺假器械adverse drug reaction = ADR药物不良反应adverse effect 副作用adverse event = AE 不良事件adverse medical events 不良医学事件adverse reaction (adverse event) 药物不良反应advisory 提醒advocacy and support groups 倡导和支持团体AE = adverse event 不良事件AERS = Adverse Event Reporting System 不良事件报告系统BBIMO Bioresearch Monitoring Program 生物研究监测bioavailability (F) 生物利用度biochemical drugs 生化药品biocides 生物杀灭剂; 杀生物剂biocompatibility 生物相容性biodegradable 生物分解bio-engineered, transgenic food 转基因食物bioequivalence; bioequivalent 生物等效应biofilm 细菌薄膜, 生物膜biologic 生物制品biological response modifiers BRM 生物应答调节剂biological therapeutic agents 生物治疗药剂biomarker 生物标志物biometrics 生物统计; 生物识别技术bion stimulator 生物体刺激器bionic knee 仿生膝关节biopharma: biopharmaceutical products 生物药物产品bipolar 双相燥郁症birth defect 出生缺陷, 新生儿缺陷, 先天缺陷BLA = biologic license application 生物制品许可申请blank control 空白对照blend uniformity analysis 混合均匀度分析blind 盲法blind codes 编制盲底blind review 盲态审核blinding method 盲法blinding/ masking 盲法,设盲blister packaging 泡罩包装; 水泡眼block 分段;层block size 每段的长度blocked randomization 区组随机Ccase history 病历case record form = CRF病例报告表/病例记录表case report form 病例报告表cash curve 现金曲线cash trap 现金陷阱; 现金套牢categorical variable 分类变量CLIA Clinical Laboratory Improvement Amendments临床实验室改进修订案clinical (human) data 临床数据clinical endpoint临床终点clinical equivalence 临床等效应clinical hold 临床试验暂停通知clinical investigator 临床研究者Clinical Pharmacists 临床药师Clinical Research Coordinator = CRC临床研究协调者clinical study 临床研究Clinical Study Application = CSA临床研究申请clinical study report 临床试验的总结报告clinical trial 临床试验clinical trial application = CTA 临床试验申请clinical trial exemption = CTX 临床试验免责clinical trial protocol = CTP 临床试验方案Clinical Trial Report = CTR临床试验报告clinically significant results 有临床意义cohort 队列cohort studies 队列研究co-investigator = CI合作研究者comparison 对照Compassionate Use 体恤使用competitive labeling 优越标签Complementary And Alternative Therapy 补充性和非传统治疗Complete response 完全有效compliance 遵守;对遵守法规情况的监管composite variable 复合变量Compression Test 压缩试验computer-assisted trial design= CATD计算机辅助试验设计Con Meds = concomitant medications 联合用药confidence interval 可信区间confidence level 置信水平Confidentiality Regarding Trial Participants 为试验参与者保密control对照control group 对照组controlled clinical trials 临床对照实验Controlled Trials 对照试验Critical Path 关键路径CRM = continual reassessment method 连续重新评估方法crossover design 交叉设计cross-over study 交叉研究crossover therapy 交叉治疗CRF = case report form 病例报告表dosage form 剂型dosage regimen 给药方案dose-ranging study 剂量范围研究dose-reaction relation 剂量-反应关系dose-related adverse reactions 剂量相关的不良反应double blinding 双盲double dummy 双模拟double dummy 双模拟double dummy technique 双盲双模拟技术double-blind study 双盲研究Double-Masked Study 双盲研究DRGs = Diagnosis Related Group System 疾病诊断相关分组drop out 脱落drop test 落震试验;跌落试验drug eluting coronary stents 药物洗脱支架drug product 药物产品drug substance 原料药drug-drug interaction56 药物-药物相互作用drug-food interaction 药物-食物的相互作用EEPS = Electronic Entry Processing System 电子录入处理系统effectiveness 疗效efficacy 有效性测定efficacy (Of a drug or treatment) 药效;药品疗效EEMEA = European Medical Evaluation Agency; European Agency for the Evaluation of Medicinal Products; European Medicines Agency 药物评价机构; 欧洲医药品管理局emergency envelope 应急信件Empiric Bayesian Multiple Gamma-Poisson Shrinker经验性贝氏法(伽玛泊松分布缩检法)empirical 经验性endpoint 终点endpoint criteria 终点指标factorial design 析因设计factorial trial 析因试验failure 无效,失败Fair Packaging and Labeling Act (1966) 公平包装和标签法False Claims Act 防制不实请求法false therapeutic claims 错误的疗效声明full analysis set 全分析集full factorial design 全因子试验法Iinclusion criteria 入选标准inclusion/exclusion criteria 入选/排除标准incremental exposure 食品中递增摄入量incubation period/latency period 潜伏期IND = Investigational New Drug 临床研究新药INDA = investigational new drug application NDA前申报阶段indemnity insurance 赔偿保险Independent Data Monitoring = IDM独立数据监察Independent Data Monitoring Committee = IDMC独立数据监察委员会independent ethics committee = IEC 独立伦理委员会indications 适应症investigational new drug = IND 临床研究新药investigational product 试验药物investigator 调研人员investigator's brochure = IB 研究者手册Mmasked 设盲mean absorption time = MAT(药物在体内的)平均吸收时间mean disintegration time = MDIT(药物在体内的)平均崩解时间Mean Dissolution Time = MDT (药物在体内的)平均释放时间Mean Residence Time = MRT(药物在体内的)平均滞留时间medical governance 医药治理Medicare 老年医疗保险制度;联邦老年医保medication guides (for patients) 用药指南Medicines Control Agency = MCA英国药品监督局Misbranding 错误标签; 冒牌Miscoding 编码错误missing value 缺失值mixed effect model 混合效应模式MLD = minimal lethal dose 最小致死剂量MoA = Mechanism of Action 作用机制;作用机理monitor 监查员monitoring plan监查计划monitoring report 监查报告MR = moderate response 好转MRA = Agreement on Mutual Recognition 相互承认协定MTD = maximal tolerance dose 最大耐受剂量multicenter trial 多中心试验multi-drug resistance 多药物抗药性multiple arm trials 多治疗组的试验mutual recognition procedure (EU) 相互承认程序OOS = Overall survival 总生存率Pparallel group design 平行组设计parameter estimation 参数估计parametric release 参数放行parametric statistics 参数统计方法patient file 病人档案patient global; pt global 病人总体评价patient history 病历per protocol ( PP) analysis 符合方案分析PFS = progression-free survival 无疾病进展存活率PGE = patient global evaluation 病人总体评价PHA = preliminary hazards analysis 预先危险分析pharmaceutical equivalence 药剂等效性pharmaceutics药剂学pharmacodynamics=PD 药物效应动力学; 简称药效学pharmacoepidemiology 药物流行病学pharmacokinetics = PK 药代动力学; 简称药动学pharmacology 药理学Pharmacovigilance105 药物警戒pharmacy 配药学PharMetrics claims database 索赔数据库PhRMA = Pharmaceutical Research and Manufacturers of America美国药物研究与生产商协会PIC=Pharmaceutical Inspection Convention 药品检查协定PIC/S Pharmaceutical Inspection Cooperation Scheme 药物检查合作计划pipeline assets 开发中产品PK = pharmacokinetics 药物代谢动力学; 药动学,药代动力学placebo 安慰剂placebo control 安慰剂对照placebo controlled study 安慰剂对照研究placebo effect 安慰剂效应PMA = premarket approval 上市前许可; 销售前批准PMCs = post marketing commitments 承诺药品上市后的继续研究PMDRA = Post Marketing Drug Risk Assessment 上市后药品风险评估(办公室) PMHx = Past Medical History 既往病史PMN = Premarket Notification 销售前通知PMS = Premenstrual syndrome 经前综合症POC (Proof-of-concept) Clinical Trials 概念证明POC = point-of-care testing 床旁分析polytomies 多分类pooled analysis = PA 荟萃分析postmarket surveillance 上市后监督post-marketing surveillance; postmarket safety surveillance 销售(上市)后监督power 把握度; 检验效能Pp = Process Performance 工序绩效Ppk = Process Performance Index 工序绩效指数precautions 慎用;注意事项precision 精密度preclinical (animal) data 临床前(动物实验)数据preclinical study 临床前研究predicate device = legally marketed device that is not subject to Premarket Approval (PMA)和已合法在市场上销售的且不需要做PMA“销售前批准”的Pre-market Approval (Application) = PMA上市前许可(申请)premarket notification 上市前通知pre-marketing surveillance 销售(上市)前监督preparing and submitting 起草和申报prescription drug 处方药preservation 保藏prevalence 患病率prevention trials预防试验primary (coronary) event 原位病变primary endpoint 主要终点primary mode of action = PMOA 首要作用模式primary variable 主要变量principal investigator = PI主要研究者Principles of Qualification 确认(验证)原则process controls 工艺控制process validation 工艺验证product codes 产品的号码product differentiation 产品差异化,产品特色化product license = PL 产品许可证product life cycle (PLC) 产品生命周期prognosis 预后progression-free survival = PFS 无进展生存progressive Disease PD 病情进展proof of principle study 原理循证研究propensity score 倾向性评分protocol 试验方案; 方案protocol amendment 方案补正prototype design 原型设计protozoa 原生动物门proven acceptable Range = PAR 确定可接受范围PTC = Product Technical Complaints 药品技术投诉Qqualification system for licensed pharmacist 执业药师资格准入制度qualified health claims 有保留的健康宣称Qualified Person = QP 受权人quality assurance = QA质量保证quality assurance unit = QAU质量保证部门quality control = QC 质量控制quality management systems 质量管理体系quality of life trials or supportive care trials 生存质量试验quality risk management = QRM 质量风险管理quantitative risk assessment 量化风险评估Rrandomization 随机化randomized trial 随机化试验randomized, double blinded clinical trial 随机双盲对照研究range check 范围检查rating scale 量表RCT = randomized clinical trials 随机临床试验RCT = randomized controlled trial 随机对照试验RDE: remote data entry 远距数据输入ready-to-eat foods 即食食品reagents 试剂recall 召回; 强制回收RECIST = Response Evaluation Criteria in Solid Tumors 实体瘤的疗效评价标准reconditioning 整改; 货物重整理;货物重包装recycled plastics 可循环利用塑料制品reference product 参比制剂reference samples 标准样品regulatory methodology 质量管理方法regulatory methods validation 管理用分析方法的验证(FDA对NDA提供的方法进行验证)regulatory specification 质量管理规格标准(NDA提供)rejection 排异remote monitoring system 远程监测系统; 远程监控REMS = Risk Evaluation and Mitigation Strategies 风险评估和减缓战略risk 受害risk assessment (risk analysis + risk evaluation) 风险评估,论证risk classification 风险分类;Risk Communications Advisory Committee 风险交流咨询委员会risk evaluation (part of risk assessment) 风险评价risk/ benefit analysis 风险-获益分析risk-benefit ratio 效益/风险比route of administration 给药途径royalties 专利使用费RPN = Risk Priority Number 风险优先指数RR = Response rate 缓解率RSD = (intra-day and inter-day) relative standard deviations (日内和日间) 相对标准差Ssafety advisory 安全建议safety evaluation 安全性评价safety evaluators 安全性评估人员safety set 安全性评价的数据集screening trials 筛选性试验SD = standard deviation 标准(偏)差SE = substantial equivalence 实质上的等同Seal Strength Test 密封强度试验sequence 试验次序SFDA 129= State Food And Drug Administration 国家食品药品监督管理局SG & A= Sales, General and Administration 销售、管理和一般费用shaft 传动轴SHEA = Society for Healthcare Epidemiology of America 美国医院流行病学学会sheaths 护套shelf life 保存期限; 保质期SIC codes = Standard Industrial Classification codes 标准产业分类代码side effects 副作用significance level 显著性水平Significant Risk (SR) 显著的危险性simple randomization 简单随机simulation model 仿真模型single blinding单盲single-blind study 单盲研究single-masked study 单盲研究site assessment = SA现场评估site audit 试验机构稽查SMDA = Safe Medical Devices Act of 1990 1990年安全医疗器械法SMF = Site Master File 生产场所主文件sNDA = supplemental NDA 疗效补充新药上市申请sponsor-investigator = SI 申办研究者spontaneous reports; voluntary reports 药品不良反应自愿报告SPS = Agreement on the Application Of Sanitary and Phytosanitary Measures卫生与植物卫生措施实施协议;简称SPS协议SSI = surgical site infection 手术部位感染SSOPs = Sanitation Standard Operating Procedures 卫生标准操作程序standard curve 标准曲线standard deviation 标准偏差standard drug 标准药物standard operating procedure = SOP 标准操作规程standard treatment 标准治疗Standards Of Care131 医护标准State Food and Drug Administration = SFDA国家食品药品监督管理局statistic 统计量statistical analysis plan = SAP 统计分析计划statistical model 统计模型statistical significance 统计学意义statistical tables 统计分析表Statisticians in the Pharmaceutical Industry = PSI制药业统计学家协会steady-state Area Under the Curve = AUCss稳态药时曲线下面积/稳态血药浓度-时间曲线下面积stratified 分层study audit 研究稽查study endpoint 研究终点Study Personnel List = SPL研究人员名单study site研究中心study type 研究类型subchronic toxicity studies 亚慢性毒性研究subgroup 亚组sub-investigator 助理研究者subject 受试者subject diary = SD 受试者日记subject enrollment 受试者入选subject enrollment log = SEL受试者入选表Subject Identification Code List = SIC受试者识别代码表subject recruitment 受试者招募subject screening log = SSL受试者筛选表submission 申报;递交subspecialties, internal medicine 亚专科,内科substantial equivalence to legally marketed (predicate) device 和已合法在市场上销售的且不需要做PMA“销售前批准”的相似产品有实质上的等同Ttrain-the-trainer program 培训者培训计划treatment group 试验组treatment IND 治疗性试验性新药申请treatment trials 治疗性试验trial error 试验误差trial initial meeting 试验启动会议trial master file 试验总档案trial objective 试验目的trial site 试验场所TRICARE 军队医疗系统triple blinding 三盲two one-side test 双单侧检验UAE = unexpected adverse event 预料外不良事件unblinding 破盲;揭盲under reporting bias 少报偏差unexplained syncope 不明原因晕厥unresectable 不能手术切除variability 变异variable 变量WHO International Collaborating Center for Drug Monitoring(世界卫生组织)国际药物监测合作中心WHO International Conference of Drug Regulatory Authorities= WHO-ICDRAWHO国际药品管理当局会议WHO Programme for International Drug Monitoring = PIDMWHO 国际药物监测合作计划。
临床试验相关词汇中英对照模板

临床试验词汇中英文对照Accuracy 准确度Active control,AC 阳性对照,活性对照Adverse drug reaction,ADR 药物不良反应Adverse event,AE 不良事件Adverse medical events 不良医学事件Adverse reaction 药物不良反应Alb 白蛋白ALD(Approximate Lethal Dose)近似致死剂量ALP 碱性磷酸酶Alpha spending function 消耗函数ALT 丙氨酸氨基转换酶Analysis sets 统计分析的数据集Approval 批准Assistant investigator 助理研究者AST 天门冬酸氨基转换酶ATR 衰减全反射法AUCss 稳态血药浓度-时间曲线下面积Audit 稽查Audit or inspection 稽查/视察Audit report 稽查报告Auditor 稽查员Bias 偏性,偏倚Bioequivalence 生物等效应Blank control 空白对照Blind codes 编制盲底Blind review 盲态审核Blind review 盲态检查Blinding method 盲法Blinding/ masking 盲法,设盲Block 分段Block 层Block size 每段的长度BUN 尿素氮Carryover effect 延滞效应Case history 病历Case report form 病例报告表Case report form/ case record form,CRF 病例报告表,病例记录表Categorical variable 分类变量Cav 平均浓度CD 圆二色谱CL(clearance)清除率Clinical equivalence 临床等效性Clinical study 临床研究Clinical study report 临床试验的总结报告Clinical trial 临床试验Clinical trial application,CTA 临床试验申请Clinical trial exemption,CTX 临床试验免责Clinical trial protocol,CTP 临床试验方案Clinical trial/ study report 临床试验报告Cmax 峰浓度Co-investigator 合作研究者Comparison 对照Compliance 依从性Composite variable 复合变量Computer-assisted trial design,CATD 计算机辅助试验设计Confidence interval 可信区间Confidence level 置信水平Consistency test 一致性检验Contract research organization,CRO 合同研究组织Contract/ agreement 协议/合同Control group 对照组Coordinating committee 协调委员会Crea 肌酐CRF(case report form)病例报告表Crossover design 交叉设计Cross-over study 交叉研究Css 稳态浓度Cure 痊愈Data management 数据管理Database 建立数据库Descriptive statistical analysis 描述性统计分析DF 波动系统Dichotomies 二分类Diviation 偏差Documentation 记录/文件Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模拟Double dummy technique 双盲双模拟技术Double-blinding 双盲Drop out 脱落DSC 差示扫描热量计Effectiveness 疗效Electronic data capture,EDC 电子数据采集系统Electronic data processing,EDP 电子数据处理系统Emergency envelope 应急信件End point 终点Endpoint criteria/ measurement 终点指标Equivalence 等效性Essential documentation 必须文件Ethics committee 伦理委员会Excellent 显效Exclusion criteria 排除标准Factorial design 析因设计Failure 无效,失败Final point 终点Fixed-dose procedure 固定剂量法Forced titration 强制滴定Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Global assessment variable 全局评价变量GLU 血糖Good clinical practice,GCP 药物临床试验质量管理规范Good manufacture practice,GMP 药品生产质量管理规范Good non-clinical laboratory practice,GLP 药物非临床研究质量管理规范Group sequential design 成组序贯设计Health economic evaluation,HEV 健康经济学评价Hypothesis test 假设检验Hypothesis testing 假设检验International Conference of Harmonization,ICH 人用药品注册技术要求国际技术协调会,国际协调会议Improvement 好转Inclusion criteria 入选标准Independent ethics committee,IEC 独立伦理委员会Information consent form,ICF 知情同意书Information gathering 信息收集Informed consent,IC 知情同意Initial meeting 启动会议Inspection 视察/检查Institution inspection 机构检查Institution review board,IBR 机构审查委员会Intention to treat 意向治疗(——临床领域)Intention-to –treat,ITT 意向性分析(-统计学)Interactive voice response system,IVRS 互动式语音应答系统Interim analysis 期中分析Investigator 研究者Investigator's brochure,IB 研究者手册IR 红外吸收光谱Ka 吸收速率常Last observation carry forward,LOCF 最接近一次观察的结转LC-MS 液相色谱-质谱联用LD50 板数致死剂量Logic check 逻辑检查LOQ (Limit of Quantitation)定量限LOCF,Last observation carry forward 最近一次观察的结转Lost of follow up 失访Marketing approval/ authorization 上市许可证Matched pair 匹配配对Missing value 缺失值Mixed effect model 混合效应模式Monitor 监查员Monitoring 监查Monitoring report 监查报告MRT 平均滞留时间MS 质谱MS-MS 质谱-质谱联用MTD(Maximum Tolerated Dose)最大耐受剂量Multicenter trial 多中心试验Multi-center trial 多中心试验New chemical entity,NCE 新化学实体New drug application,NDA 新药申请NMR 核磁共振谱Non-clinical study 非临床研究Non-inferiority 非劣效性Non-parametric statistics 非参数统计方法Obedience 依从性ODR 旋光光谱Open-blinding 非盲Open-label 非盲Optional titration 随意滴定Original medical record 原始医疗记录Outcome 结果Outcome assessment 结果指标评价Outcome measurement 结果指标Outlier 离群值Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient history 病历Per protocol,PP 符合方案集Placebo 安慰剂Placebo control 安慰剂对照Polytomies 多分类Power 检验效能Precision 精密度Preclinical study 临床前研究Primary endpoint 主要终点Primary variable 主要变量Principal investigator 主要研究者Principle investigator,PI 主要研究者Product license,PL 产品许可证Protocol 试验方案Protocol 试验方案Protocol amendment 方案补正Quality assurance unit,QAU 质量保证部门Quality assurance,QA 质量保证Quality control,QC 质量控制Query list,query form 应用疑问表Randomization 随机化Randomization 随机Range check 范围检查Rating scale 量表Regulatory authorities,RA 监督管理部门Replication 可重复RSD 日内和日间相对标准差Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample size 样本含量Sample size 样本量,样本大小Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Serious adverse event,SAE 严重不良事件Serious adverse reaction,SAR 严重不良反应Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single blinding 单盲Single-blinding 单盲Site audit 试验机构稽查SOP 试验室的标准操作规程Source data verification,SDV 原始数据核准Source data,SD 原始数据Source document,SD 原始文件Specificity 特异性Sponsor 申办者Sponsor-investigator 申办研究者Standard curve 标准曲线Standard operating procedure,SOP 标准操作规程Statistic 统计量Statistical analysis plan 统计分析计划Statistical analysis plan 统计参数计划书Statistical analysis plan,SAP 统计分析计划Statistical model 统计模型Statistical tables 统计分析表Stratified 分层Study audit 研究稽查Subgroup 亚组Sub-investigator 助理研究者Subject 受试者Subject diary 受试者日记Subject enrollment 受试者入选Subject enrollment log 受试者入选表Subject identification code,SIC 受试者识别代码Subject recruitment 受试者招募Subject screening log 受试者筛选表Superiority 检验Survival analysis 生存分析SXRD 单晶X-射线衍射System audit 系统稽查T1/2 消除半衰期Target variable 目标变量T-BIL 总胆红素T-CHO 总胆固醇TG 热重分析TLC、HPLC 制备色谱Tmax 峰时间TP 总蛋白Transformation 变量变换Treatment group 试验组Trial error 试验误差Trial master file 试验总档案Trial objective 试验目的Trial site 试验场所Triple blinding 三盲Two one-side test 双单侧检验Unblinding 揭盲Unblinding 破盲Unexpected adverse event,UAE 预料外不良事件UV-VIS 紫外-可见吸收光谱Variability 变异Variable 变量Visual analogy scale 直观类比打分法Visual check 人工检查Vulnerable subject 弱势受试者Wash-out 清洗期Washout period 洗脱期Well-being 福利,健康临床采血相关词汇Intravenous injection(IV) 静脉注射Intravenous drip/Intravenously guttae(iv gtt)静脉滴注intramscular injection(IM) 肌内注射intradermal injections 皮内注射subcutaneous injections(SC)皮下注射disposable sterile injector 一次性无菌注射针injection set注射器disposable venous infusion needle一次性静脉输液针disposable infusion set 一次性使用输液器blood transfusion set输血器infusion bag液袋urine drainage bag集尿袋blood bag血袋medical catheter医用导管stainless steel needle不锈钢医用针管blood taking needle/ blood collection needle采血针Needle Holder 持针器Blood samples血样样本sample drawing/collection 采血/抽血needles 针vacutainer needles真空采血针tubes导管vacutainer tubes 真空管vacutainer holder 真空持针器tourniquet 止血带disinfection swabs 消毒海绵micropore tapedental rollsadhesive dressing 胶布敷料rubber gloves 橡皮手套pillow 枕头stopper 塞子(采血管的塞子)vacuum tubes真空管non-vacuum tubes 非真空管needle disposal box 穿刺针处理盒(利器处理盒)transfer and storage tubes 转移和存储管pipette 吸管吸液管移液管disposable pipettes一次性使用吸管centrifuge离心机swinging bucket rotor 浮桶式转头、吊桶式转头timer 计时器racks for tubes 试管架identification codes识别码mark/label the tubes标记导管freeze冷冻refrigerator 冰箱freezer 冰箱冷冻机Serum Separation Tube 血清分离试管Serum血清Plasma血浆whole blood 全血be inverted 倒垂的倒置的clot 凝块凝结Coagulate 凝固凝结clotting time 凝血时间minimally 最低限度地最低程度地maximally 最高限度地最高程度地Separation of serum or plasma 分离血清或血浆Hemolysis 溶血hemolyzed samples 溶血样本assays 含量测定analysis 分析(名词)analyze 分析(动词)analyzing 分析(动名词)non-frozen samples 非冷冻样本chemistry laboratories化学实验室Analytical procedures 分析程序Qualification 资质资格Personnel in the laboratory 实验室人员Field personnel 现场工作人员Internal quality control 内部质量控制External quality control 外部质量控制Hepatitis B 乙肝Hepatitis C 丙肝Positive 阳性Negative 阴性Measurement 测量pulse rate 脉搏Blood pressure 血压systolic blood pressure 收缩压diastolic blood pressure 舒张压hypertensives 高血压hypotension 低血压antihypertensive drug 抗高血压药mercury sphygmomanometer 水银式血压计mercury column 水银柱serum total cholesterol 血清总胆固醇lipid lowering drug 降脂药Obesity 肥胖症Blood Glucose 血糖BMI (body mass index) 体重指数Overweight 超重sample size 样本大小statistically 统计上地,统计地statistical precision 统计精度sub-groups亚群Recruitment招募Ethical 伦理的道德的Legal 法律的合法的Data in electronic format 电子版数据back-up data 备查资料常用临床医学英文术语cough 咳嗽asthma 哮喘pneumonia 肺炎heart disease 心脏病arrhythmia 心律不齐indigestion 消化不良gastritis 胃炎appendicitis 盲肠炎hepatitis 肝炎dermatitis皮炎freckle/ephelis 痣,雀斑acne 粉刺flu 流感diarrhoea 痢疾quarantine 检疫vaccinate 打疫苗endemic 水土不服relapse 复发症casualty急症stupor 昏迷sprain 扭伤scalding 烫伤graze 擦伤scratch 搔挠trauma 外伤bruise 淤伤fracture骨折dislocation 脱臼tinnitus耳鸣trachoma沙眼colour blindness 色盲nearsightedness/myopia近视astigmatism 散光gingivitis 牙龈炎cavity 龋齿fever 发烧discomfort/disorder 不适malnutrition 营养不良incubation 潜伏期asthenia 虚弱poisoning 中毒fatigue 疲劳heat stroke 中暑itching 发痒ache/pain 痛tetanus 破伤风night sweat 盗汗chill 打冷颤pale 脸色发白shuddering 发抖inflammation 炎症acute 急症chronic 慢性病congenital 先天性病nausea恶心vomit 呕吐diseases 疾病acute diseases 急性病advanced diseases 病沉重期,晚期疾病chronic diseases慢性病communicable diseases 传染病complicating diseases 并发病congenital diseases 先天性疾病acquired diseases 后天性疾病contagious diseases接触性传染病endemic diseases 地方病epidemic diseases 流行病functional diseases 机能病、官能病infectious diseases 传染病inherited diseases 遗传病malignant diseases恶性病nutritional diseases 营养病occupation diseases 职业病organic diseases 器质性病paroxysmal diseases 阵发性病periodical diseases 周期病primary(principal)diseases原发(主导)病secondary diseases 继发病sexual(venereal, social)diseases 性病terminal diseases 绝症wasting diseases 消耗性疾病chief complaint 主诉clinical manifestation 临床表现delivery history 分娩史etiology病因学family history 家族史history, medical history 病史precipitating(induced)诱因marital status婚姻状况menstrual history 月经史menarche 初潮menopause闭经past history既往史pathogenesis 发病机制personal history 个人史symptoms 症状cardinal symptom 主要症状classical symptom 典型症状concomitant symptom 伴发症状constitutional(systemic)symptom 全身症状indirect symptom 间接症状induced symptom 诱发症状local symptom 局部症状mental symptom精神症状symptom-complex (syndrome)symptom 综合症,症候群signs体征antecedent 前驱征assident (accessory)副征commemorative 后遗症sign of death 死征diagnostic诊断征sign of disease 病征subjective 自觉征,主观征vein sign静脉征vital sign 生命体征body length (height of the body)身高body weight 体重barrel chest 桶状胸cachexia 恶病质compulsive position 被动体位critical facies 病危面容emaciation 消瘦enophthalmos 眼球下陷entropion 睑内翻exophthalmos 眼球突出flushed face 面色潮红gain (loss)in weight 增加(减轻)体重lock-jaw 牙关紧闭lordosis 脊柱前凸nasal ala flap 鼻翼扇动nystagmus 眼震obesity 肥胖pallor 苍白scolisis 脊柱侧凸agitation 焦急不安debility, weakness 虚弱diaphoresis 出汗,大量出汗dizziness, vertigo 眩晕lassitude, fatigue 无力,倦怠malaise 不适night sweat 盗汗numbness 麻木rigor, chill 寒冷,发冷perspiration, sweating 出汗pruritus, itching 痒,somasthenia 躯体无力tingling 麻刺感abscess 脓肿acidosis 酸中毒adhesion 粘连alkalosis 碱中毒allergy 过敏coagulation defect 凝血不良congestion 充血dehydration 脱水distention 膨胀edema 水肿embolism 栓塞,栓塞形成fluid and electrolyte imbalance 水电解质紊乱gangrene 坏疽hematoma 血肿hemorrhage, bleeding 出血infarction 梗塞,梗死infection 传染inflammation 炎症ketoacidosis 酮酸中毒metastasis 转移perforation 穿孔necrosis 坏死shock 休克response 反应,应答reaction 反应,感应thrombosis 血栓形成ulceration 溃疡fever, pyrexia 发烧,发热continuous fever 稽留热intermittent fever 间歇热low-grade fever 低热remittent fever 驰张热relapsing fever 回归热pain 痛burning pain 灼痛chest(flank,…)pain 胸(胁腹…)痛cramp-like pain 痉挛性痛dull, diffused pain 弥漫性钝痛pleuritic pain 胸膜炎性痛radiating pain (pain radiating to…)放射性痛(放射到…疼痛)angina 绞痛cardiac angina 心绞痛backache 背痛colic 绞痛,急腹痛earache 耳痛headache 头痛neuralgia 神经痛migraine 偏头痛rebound tenderness 反跳痛somatalgia 躯体痛sore throat 咽喉痛stomachache 胃痛toothache 牙痛bloody sputum 带血的痰cough 咳嗽dry cough 干咳expectoration 咳痰expectoration of blood 咳血hemoptysis 咳血anoxia 缺氧apnea 呼吸暂停,窒息asthma 气喘,哮喘Cheyne Stokes respiration 切-斯氏呼吸,潮式呼吸dyspnea 呼吸困难hyperpnea hyperventilation 过度呼吸,换气过度hypopnea 呼吸不全,呼吸浅表hypoxia 低氧,缺氧orthopnea 端坐呼吸respiratory arrest 呼吸停止suffocation 窒息tachypnea 呼吸急促fetid breath 口臭fruity breath 呼吸有水果味arrhythmia 心律失常,心律不齐atelectasis 肺不张,肺膨胀不全cardiac arrest 心搏骤停cardiac hypertrophy 心脏肥大cyanosis 发绀,青紫distension of jugular vein 颈静脉怒张extrasystole 期外收缩gallop rhythm 奔马律hemopleura 血胸hepatojugular reflux 肝颈静脉回流hypovolemia (循环)血容量减少palpitation 心悸tachycardia 心动过速pneumothorax 气胸thrill 震颤absent breath sounds 呼吸音消失dull sound 浊音hyperresonant 鼓音rale 啰音rhonchus, rhonchi 鼾音,干啰音wheeze 哮鸣音anorexia, loss of appetite 食欲不振,厌食dysphagia 吞咽困难eructation 嗳气belching 嗳气flatulence 气胀flatus 肠胃气,屁gaseous distention 胃胀气hematemesis 呕血hiccough, hiccup 打呃,呃逆nausea 恶心pyrosis 胃灼热regurgitation 反胃,回流thirsty 口渴vomiting 呕吐anal fissure, crack in the anal canal 肛裂ascites 腹水board-like rigidity of the abdomen 板状腹decreased tactile fremitus 触觉性震颤减弱exophageal varices 食管静脉曲张fistula 瘘,瘘管hemorrhoid 痔hernia 疝hepatomegaly 肝肿大intussusception 肠套叠jaundice 黄疸muscle guarding, defence of the abdominal wall 腹壁肌卫peristalsis 蠕动loss of peristalsis 蠕动消失mass peristalsis 总蠕动retrograde (reversed)peristalsis 逆蠕动prolapse 脱垂prolapse of anus 脱肛rectal prolapse 直肠脱垂,脱肛volvulus 肠扭转calculus 结石,石biliary calculus 胆结石vesical calculus 膀胱结石constipation 便秘defecation 排便diarrhea 腹泻incontinence of feces 大便失禁hematochezia 便血fecal impaction 大便嵌塞occult blood 潜血painful straining with defecation 排便痛性牵动clay colored stools 陶土色便dark, granular/coffee ground emesis 咖啡样呕吐物fecal vomiting, stercoraceous vomiting 呕粪,吐粪foul fatty stools, steatorrhea 恶臭脂肪便,脂肪痢scanty and hard stools 便少而硬tarry (black)stools 柏油样便anuria 无尿burning sensation no urination 排尿时的灼烧感dysurea 排尿困难,尿痛enuresis, bed wetting 遗尿frequency of urination 尿频micturation 排尿uresis, urination, voiding 排尿nocturia 夜尿oliguria 少尿polyuria 多尿tenesmus 里急后重vesical tenesmus 排尿时里急后重uremia coma 尿毒症昏迷urgency of urination 尿急urinary incontinence 尿失禁aciduria 酸尿chyluria 乳糜尿cylindruia 管型尿glycosuria 糖尿hematuria 血尿ketonuria 酮尿pneumatinuria 气尿proteinuria 蛋白尿pyuria 脓尿amenorrhea 经闭,无月经dysmenorrhea 痛经menorrhagia 月经过多lochia 恶露menorrhea 行经,月经过多menstruation 月经uterine contraction 子宫收缩blotch 斑点bruise 挫伤,青肿acne 痤疮,粉刺desquamation 脱皮,脱屑ecchymosis 瘀斑loss of skin turgor 失去皮肤充盈nevus 痣papule 丘疹petechia 瘀点,瘀斑pigmentation 色素沉着pustule 脓疱purpura 紫癫red nodule 红结节roseola 玫瑰疹scar 伤疤senile plaque 老人斑spider anaioma 蛛形痣subcutaneous nodule 皮下结节urticaria 荨麻疹vesicle 小水疱vitiligo 白斑blindness 失明blurred vision, visual disturbance 视力模糊impaired vision 视力下降lacrimation 流泪papilledema 视神经乳头水肿photophobia 畏光,羞明retinal detachment 视网膜脱离deafness 聋hearing loss 听力丧失tinnitus 耳鸣epistaxis, nasal bleeding 鼻出血impaired smelling 嗅觉障碍nasal discharge 鼻涕nasal obstruction 鼻塞sneeze 喷嚏snore 打鼾aphonia, loss of voice 失音症hoarseness 嘶哑gum bleeding 齿龈出血herpes labialis 唇疱疹,感冒疮Koplik's spots 科普利克斑lead line of the gum 龈铅线salivation, drooling 流口水straw-berry tongue 草莓舌tremulous tongue 舌震颤atrophy 萎缩contracture 挛缩deformity 畸形,变形dislocation 脱位fracture 骨折closed (simple)fracture 无创骨折,单纯性骨折comminuted fracture 粉碎性骨折compound fracture 哆开(开放性)骨折knock-knee 膝外翻opisthotonos 角弓反张prosthesis 假体spasm 痉挛tetany (肌)强直,手足抽搦wrist drop 腕下垂aphasia 失语ataxia 共济失调coma 昏迷consciousness 知觉,意识convulsion 抽搐,惊厥delirium 谵妄delusion 妄想faint 昏厥hallucination 幻觉hemiplegia 偏瘫increased intracranial pressure 颅内压增高insanity 精神错乱loss of orientation 定向丧失mania 躁狂memory defects, amnesia 记忆缺损,遗忘症paraplegia 截瘫,下身麻痹projectile vomiting 喷射性呕吐somnolence, (lethargy)昏睡,嗜睡stupor 木僵,昏呆tetraplegia 四肢瘫痪unconsciousness 失去知觉yawning 打哈欠crisis 危象cerebral (febrile, hematic, hemolytic,hypertensive, thyrotoxic, ……)crisis脑(热、血性、溶血、高血压、甲状腺中毒…)危象failure 衰竭,故障centra(circulatory, cardiac, myocardiac, peripheral, congestive, renal, respiratory)failure中枢(循环、心力、心肌、周围循环、充血性、肾、呼吸…)衰竭diagnosis诊断auscultation 听诊inspection 视诊palpation触诊percussion 叩诊laboratory examination 实验室检查physical examination 体格检查rectal (vaginal)touch 直肠(阴道)指诊impression 印象tentative diagnosis 暂定诊断differential diagnosis 鉴别诊断final diagnosis 最后诊断prognosis 预后prescription 处方incubation (latent)period 潜伏期prodromal stage 前驱期incipient stage 初期quiescent stage 静止期alleviation 减轻,缓和remission 缓解attack 发作convalescence (recovery)stage 恢复期rehabilitation 康复relapse 复发sudden death 猝死moribund 濒死的course of the disease 病程course of the treatment 疗程indication 适应症,指征complication 并发症contraindication 禁忌症side-effect 副作用sequel (sequela), after effect 后遗症therapies 治疗方法acupuncture therapy 针刺疗法block therapy 封闭疗法chemical therpy (chemo-therapy)化学疗法combined therapy 综合疗法conservative therapy 保守疗法constitutional therapy 全身疗法dietetic therapy 饮食疗法operative treatment 手术疗法palliative treatment, alleviative treatment 姑息疗法physical therapy 物理疗法psychotherapy 精神疗法radical treatment 根治radio-therapy 放射性疗法supporting treatment 支持疗法symptomatic treatment对症疗法cardiac massage 心脏按摩cardiac pacing 心脏起博electrotherapy 电疗法electroshock treatment 电休克疗法hemodialysis 血液透析hyperbaric therapy 高压氧疗法insulin-shock treatment 胰岛素休克疗法light therapy 光疗法Urine Analyzer 尿液分析仪blood sugar(glucose )analyzer血糖分析仪test strip 测试条reagent 试剂Semi-automatic Biochemical Analyzer半自动生化分析仪Automatic Blood Cell Analyzer全自动血细胞分析仪Urine sediments analyzer尿沉渣Bio-safety Cabinet 生物安全柜Incubator培养箱High Frequency Electrotome 高频电刀shadowless lamp无影灯High speed refrigerated centrifuge高速冷冻离心机hot air sterilizer热空气消毒箱microbiological incubator微生物培养箱Halogen light 卤素灯needle destroyer针头销毁器automatic packer自动纸塑包装机scalp vein set头皮针uniprocessor version单机版network version网络版macromolecule-solvent 高分子溶解的macromolecule cold accumulation 高分子蓄冷cold treatment冷疗法ice pack冰袋eyeshade 眼罩Medical injection pump医用灌注泵lithotrite 碎石机extracorporeal shock wave lithotrite体外冲击碎石机Ballistic intracroporeal lithotrite 气压冲击体内碎石机Laparoscope 腹腔镜Urology 泌尿外科kidney stones 肾结石Multi-parameter monitor, 多参数监护仪maternal monitor/fetal monitor母亲/胎儿监护仪ICU monitor 重症监护仪anesthetic equipment 麻醉机respirator呼吸机electronic colposcope 电子阴道镜smog absorber烟雾吸收器digital film room 数字胶片室Permanent Magnet Open Magnetic Resonance system 永磁开放式磁共振系统Ultrasonic Color Doppler Diagnostic system彩色超声多普勒诊断系统Mobile CT system 移动CT系统X-ray Mammary Machine 乳腺X线机Mammography乳腺high precision Stereotaxic 高精度脑立体定向仪portable Type-B ultrasonic 便携式B超Sterilization and Disinfection Equipment消毒灭菌设备Radiotherapeutic equipment.放射疗法设备pharmaceutical equipments.制药设备horizontal pressurized steam sterilizer普通卧式压力蒸汽灭菌器medical electronic linear accelerator医用电子直线加速器high frequency X-rays diagnostic machine高频X射线诊断机simulated positioner模拟定位机high frequency mobile X-rays machine高频移动X射线机医疗卫生人员职衔职称主任医师(讲课):Professor of Medicine主任医师(医疗):Professor of Treatment儿科主任医师:Professor of Paediatrics主治医师:Doctor-in-charge外科主治医师:Surgeon-in-charge内科主治医师:Physician-in-charge眼科主治医师:Oculist-in-charge妇科主治医师:Gynaecologist-in-charge牙科主治医师:Dentist-in-charge医师:Doctor医士:Assistant Doctor主任药师:Professor of Pharmacy主管药师:Pharmacist-in-charge药师:Pharmacist药士:Assistant Pharmacist主任护师:Professor of Nursing主管护师:Nurse-in-charge护师:Nurse Practitioner护士:Nurse主任技师:Senior Technologist主管技师:Technologist-in-charge技师:Technologist技士:Technician常用抗菌药物青霉素类青霉素(G) Penicillin(G) Benzylpenicillin, 苄青霉素, 盘尼西林青霉素V Penicillin V Phenoxymethylpenicillin, Blinvan, Ospen, 苯氧甲基青霉素苄星青霉素Benzathine Penicillin 长效西林, 长效青霉素, 比西林, LPG, 苄星青氨苄西林Ampicillin 安比西林, 氨苄青霉素, 安必仙, 安必林, 安比林阿莫西林Amoxicillin 特力士, 弗来莫星, 羟氨苄青霉素, 益萨林, 阿莫仙, 安福喜, 本原莫星巴氨西林Bacampicillin 美洛平, 氨卡西林, 氨苄青霉素甲戊酯阿洛西林Azlocillin 阿乐欣, 咪氨苄西林, 氧咪苄青霉素, Azlin美洛西林Mezlocillin 天林, Baypen, Mezlin, Baycipen替卡西林Ticarcillin 羧噻吩青霉素, 的卡西林, Nonapen, Ticarpen酞氨西林Talampicillin 氨苄青霉素酞酯, 酞氨苄青霉素, 酞氨苄西林, TAPC, Talpen夫苄西林Furbenicillin 呋脲苄青霉素, 呋苄青霉素, 呋氨西林, 呋喃酰脲苄青霉素氟氯西林Flucloxacillin 氟氯苯唑青霉素, 氟沙星, 福氯平, Floxapen羧苄西林Carbenicillin 羧苄青霉素, 卡比西林, 羧苄青阿扑西林Aspoxicillim 天冬羟氨青霉素, Doyle, ASPC匹氨西林Pivampicillin 氨苄西林酯, 匹凡西林, 匹呋西林, 吡呋氨卡西林双氯西林Dicloxacillin 双氯青, Dynapen, Consaphyl, Stampen, Diflor甲氧西林Meticillin 新青霉素Ⅰ, 美替西林, Azapen, Penysol苯唑西林钠Oxacillin Sodium 新青霉素Ⅱ, 苯唑青霉素钠, 苯甲异噁唑青霉素奈夫西林Nafcillin 新青霉素Ⅲ, 乙氧萘青霉素匹美西林Pivmecillinam 氮卓咪青霉素双酯, Celfuron, Melysin仑氨苄西林Lenampicillin Varacillin, Takacillin美西林Mecillinam Selexidleo, Selexid, Coactin, Amdinocillin, 氮卓脒青霉素哌拉西林钠Piperacillin Sodium 氧哌嗪青霉素, 哔哌西林, 哌氨苄青霉素Avocin, Orocin, Pipril氯唑西林钠Cloxacillin Sodium 邻氯青霉素钠, 氯唑青, Orbenin阿帕西林钠Apalcillin Sodium 萘啶青霉素钠, APPC, Lumota, Elumota, Palcin磺苄西林钠Sulbenicillin Sodium 磺苄青霉素钠, 卡他西林, 格达西林Sulfocillin, Lilacillin, Kedacillin青霉素V钾Phenoxymethylpenicillin Potassiume 6–苯氧乙酰胺基青霉烷酸钾, Cillaphen Distaquaine VK, Compocillin VK, Dowpen VK Cilicaine VK, Apopen, Biopen海他西林钾Hetacillin Potassium 缩酮氨苄青霉素钾, Etacillin, Veisapen卡茚西林钠Carindacillin Sodium Carbenicillin Indanyl Sodium, Geopen, Geocillin 治平霉素替莫西林二钠Temocillin Disodium Temopen头孢菌素类第一代头孢噻吩钠Cefalotin Sodium 先锋Ⅰ号, 头孢金素, 噻孢霉素, CET头孢噻啶Cefaloridine 先锋Ⅱ号, 头孢利素, CER, Kafspor头孢来星Cefaloglycine 先锋Ⅲ号, 头孢甘酸, Kafocin, Kefglycin头孢氨苄Cefalexin 先锋Ⅳ号, 苯甘孢霉素, 头孢力新, CEX头孢唑啉钠Cefazolin Sodium 先锋Ⅴ号, 塞福宁, 西华乐林, 先锋啉, 凯复唑Cefalin, CEX, Cefamedin, Kefzol, Cramaxin头孢拉定Cefradine 先锋Ⅵ号, 头孢雷定, 头孢瑞丁, 头孢环已烯,泛捷复, Velosef, CED, 塞福定头孢乙腈Cefacetrile 先锋Ⅶ号, 头孢腈甲, 头孢赛曲头孢匹林Cefapirin 先锋Ⅷ号, 头孢吡硫, Ceta-Dri头孢硫脒Cefathiamidine 先锋18, 吡脒头孢头孢克洛Cefaclor 赐福乐素, 新达乐, 头孢氯氨苄, Ceclor, 希刻劳,头孢克罗, 可福乐, 氯头孢菌素, CCL头孢羟氨苄Cefadroxil Cefadril, Kefroxil, 羟氨苄头孢菌素, 欧意, CDX,Duracef, Bidocef, Cefamx头孢沙定Cefroxadine 头孢环烯氨, ORASPON, CGP-9000, CXD头孢罗齐Cefprozil 头孢丙烯, Cefzil头孢替唑Ceftezole 特子社复, Tezacef, Alomen第二代头孢呋辛钠Cefuroxime Sodium 头孢呋肟, 赐福乐信, 舒贝洛, 西力欣, 特力欣, 明可欣, CXM, Zinacef, Monacef, Ketocef, Furex Kesiut, Supero头孢替安Cefotiam 噻乙胺唑头孢菌素, 头孢噻乙胺唑, 泛司博林, 泛司颇灵, Pansparin, Halospor, CTM头孢呋辛酯Cefuroxime Axetil 头孢呋肟酯, 新菌灵, 西力达, 头孢呋新乙酰乙酯Zinnat头孢西丁Cefoxitin 头孢氧唑, 甲氧头霉噻吩, 噻吩甲氧头孢菌素, 美福仙, 甲氧头霉噻吩, CXT, CFX头孢尼西二钠Cefonicid Disodium 铭乐希, Monocid, Cefonicid, Cefodie, Cefol头孢孟多Cefamandole 头孢羟唑, 羟苄四唑头孢菌素, Mandolkef, CMT, Cefadole, Mandol头孢美唑钠Cefmetazole Sodium 先锋美他醇, 氰唑甲氧头孢菌素, 头孢氰四唑,头孢美他唑, CEFMETAZON, CMZ, Cemetol头孢布宗Cefbuperazone 头孢拉宗, Keiperazon, Tomiporan, Cefobutazine, CBPZ 头孢雷特Ceforanide 头孢氨甲苯唑, 头孢苄胺四唑, 氨苄唑头孢菌素Precef第三代头孢地尼Cefdinir Cefzon头孢布坦Ceftibuten 头孢布烯, Cedax头孢卡品Cefcapene Pivoxil 头孢卡喷新戊酰氧甲酯, Flomox头孢噻肟钠Cefotaxime Sodium 头孢氨噻肟, 泰可欣, 塞福隆, 菌必灭, 治菌必妥, 凯福隆, 凯复龙, 头孢噻肟, 喜福得, Claforan, CTX头孢曲松钠Ceftriaxone Sodium 头孢三嗪, 头孢泰克松, 罗氏芬, 罗噻嗪, 菌必治, 菌得治, 头孢三嗪噻肟, 泛生舒复, 西华瑞隆, Rocephin, Ro-139904, CTRX 头孢哌酮钠Cefoperazone Sodium 头孢氧哌唑, 先锋必, 头孢必, 达诺欣, 塞福必,Cefobid, T-1551, CPZ头孢他啶Ceftazidime 头孢塔齐定, 头孢羧甲噻肟, 复达欣, 凯复定, Glazidine, Fortaz, Eposerin, Modocin, Fotrum, Tazidime, CTZ, Spectrum, Eposerin, Modocin头孢唑肟Ceftizoxime 头孢去甲噻肟, 益保世灵, 安保速灵, Epocelin CZX, CEFTIZOX头孢克肟Cefixime 头孢烯噻羟肟, 世伏素, 世福素, FK027, Suprax, CFIX , CEFSPAN头孢甲肟Cefmenoxime 头孢氨噻肟唑, 倍司特克, BESTCALL, CMX头孢地嗪Cefodizime 头孢双唑, 莫敌, 莫敌威, Modivid, Timcef头孢匹胺Cefpiramide 先福吡兰, 甲吡唑头孢菌素, 头孢吡胺, CPM,Cefpiran, Sepatren头孢特仑酯Cefteram Pivoxil 头孢特仑新戊酰氧甲酯, 托米仑, 头孢他美酯,富山龙, Tomiron头孢磺吡苄钠Cefsulodin Sodium 头孢磺吡酮, 头孢磺啶钠, 磺吡苄头孢菌素钠,达克舒林, Cefsulodine, Takesulin, CFS, SCE129拉他头孢Latamoxef 羟羧氧酰胺菌素, 拉氧头孢, 头孢拉坦, 噻吗灵Moxalactam, Shiomarin, Moxam, LMOX头孢米诺Cefminox 氨羧甲氧头孢菌素, 美土灵, Meicelin头孢咪唑Cefpimizole Benilan, SPIE, Ajicef头孢替坦二钠Cefotetan Disodium 双硫唑甲氧头孢霉素, Yamatetan, CTT, Cefotan第四代头孢唑喃钠Cefuzonam Sodium Cosmosin, CZON氟氧头孢钠Flomoxef Sodium 氟莫克西, 6315-S头孢匹罗Cefpirome 头孢吡隆, 氨噻肟吡戊头孢其它Β-内酰胺类亚胺培南Imipenem 伊米配能, 亚胺硫霉素, NFT氯曲南Aztreonam 菌克单, 君刻单, 噻肟单酰胺菌素, AZACTAM,Primbactam, Primbactin, SQ-26776氟罗培南Faropenem 福劳派南, Farom苄西林/氯唑西林Ampicillin/Cloxacillin 复方安比西林, 爱罗苏, 氨氯西林, 白罗仙, 淋必清, Pinocine亚胺培南/西司他丁Imipenem/Cilastatin 亚胺硫霉素/西拉司丁, 泰能, TIENAM, PRIMAXIN阿莫西林/氟氯西林Amoxicillin/Flucloxacillin 氟羟西林, 新灭菌, Biflocin, Infectrin三羟阿莫西林/单羟双氯西林Amoxycillin Trihydrate/Dicloxacillin康彼身, 克菌, CompliscanΒ-内酰胺类抗生类+Β-内酰胺酶抑制剂氨苄西林/舒巴坦Ampicillin/Sulbactam 强力安必仙, 舒他西林, 优力新, 新凯兰欣, 舒氨欣, Unasyn, Sultamicillin头孢哌酮/舒巴坦Cefoperazone/Sulbactam 舒普深, 舒哌酮, Sulperazone头孢噻肟/舒巴坦Cefotaxime/Sulbactam 新治菌哌拉西林/他佐巴坦Piperacillin/Tazobactam Tazocin, YP-14阿莫西林/克拉维酸Amoxillin/Clavulanic Acid 安灭菌, 安美汀, 奥格门汀, 安美汀, 阿莫克拉Augmentin, BRL25000替卡西林/克拉维酸Ticarcillin/Clavulanic Acid 泰门汀, 特泯菌, 特美汀, Timentin舒巴坦Sulbactam 舒巴克坦, 陪他美, 青霉烷砜氨基糖甙类链霉素Streptomycin RIMACTANE, RIFAMPINN, ovostrep庆大霉素Gentamycin 庆大霉素C, 瑞贝克, 正泰霉素庆大霉素链Gentamicin Chains 塞透派勒链, Septopal妥布霉素Tobramycin 立可信软膏,点必舒眼药水(妥布+地塞米松)泰星,妥布拉霉素,托普霉素,NEBCIN,Factorb, Brulamycin, Tobra, Nebramycin, Gernebcin, Obracine小诺霉素Micronomicin 小诺米星, 沙加霉素, 相模霉素, Sagamicin, Kw-1062, MCR核糖霉素Ribostamycin 威他霉素, 维生霉素, Vistamycin, Ibistacin大观霉素Spectinomycin 奇霉素, 壮观霉素, 淋必治, 奈毒素, Spectram, 克淋, Trobicin, Kirin阿米卡星Amikacin 丁胺卡那霉素, Amikin, Likacin西索米星Sisomicin 西梭霉素, 西索霉素, 紫苏霉素, Sisomin, Sipeptin奈替米星Netilmicin 乙基西梭霉素, 奈特, 奈替霉素, 乙基西素米星, 立克菌星, 乙基紫苏毒素, Ethylsisomicin, Vectacin, Zetamicn, Certomycin, NETROMYCIN异帕米星Isepamicin 依克沙霉素, HAPA-B, Exacin阿司米星Astromicin 阿司霉素, 福提霉素, 武夷霉素, 强壮霉素FORTIMICIN, Kw-1070, ASTM, Fortimicin地贝卡星Dibekacin 双去氧卡那霉素B, Dideoxykanamycin B, DKB, 达苄霉素, Icacine依替米星Etimicin 悉能磷霉素类磷霉素Fosfomycin 福赐美仙, Phosphonomycin, Fosfocina, FOM磷霉素氨基丁三醇Fosfomycin Tromethamine Monurol喹诺酮类第二代吡哌酸Pipemidic Acid PPA第三代诺氟沙星Nofloxacin 氟哌酸, 淋克星, Fulgram, Noroxin, AM-715 MK-0366, Brazan, Baccidal环丙沙星Ciprofloxacin 悉复欣, 悉普欣, 悉普宁, 丽珠环丙, 特美力,环丙氟哌酸, 健宝灵, CIPRO, Bay-0-9867, Ciprobay, Ciproxin培氟沙星Pefloxacin 培福新, 甲氟哌酸, 倍泰, Peflacine洛美沙星Lomefloxacin 洛威, 罗氟酸, 欣美罗, 多龙, Bareon芦氟沙星Rufloxacin 如氟沙星左旋氧氟沙星Levofloxacin 可乐必妥, 左氟沙星, Cravit依诺沙星Enoxacin 氟啶酸, 福禄马, 复克, FLUMARK, GYRAMID,Flumark氟罗沙星Fleroxacin 复诺定, 多氟哌酸, 喹诺敌, 麦加乐定, 沃尔得FLX, Megelone, Quinodis, Ro-236240, AM-833氧氟沙星Ofloxacin 氟嗪酸, 秦利必妥, 泰利特, 奥复星, 康泰必妥, 竹安新, TARIVID, 赞诺欣, Zanoxin, Oflocin, 泰利得, 正康培氟沙星Pefloxacin 甲氟哌酸, 培氟哌酸, 培福新, 倍宁, Pefalcine。
临床试验相关词汇中英对照

临床试验词汇中英文对照Accuracy 准确度Active control,AC 阳性对照,活性对照Adverse drug reaction,ADR 药物不良反应Adverse event,AE 不良事件Adverse medical events 不良医学事件Adverse reaction 药物不良反应Alb 白蛋白ALD(Approximate Lethal Dose)近似致死剂量ALP 碱性磷酸酶Alpha spending function 消耗函数ALT 丙氨酸氨基转换酶Analysis sets 统计分析的数据集Approval 批准Assistant investigator 助理研究者AST 天门冬酸氨基转换酶ATR 衰减全反射法AUCss 稳态血药浓度-时间曲线下面积Audit 稽查Audit or inspection 稽查/视察Audit report 稽查报告Auditor 稽查员Bias 偏性,偏倚Bioequivalence 生物等效应Blank control 空白对照Blind codes 编制盲底Blind review 盲态审核Blind review 盲态检查Blinding method 盲法Blinding/ masking 盲法,设盲Block 分段Block 层Block size 每段的长度BUN 尿素氮Carryover effect 延滞效应Case history 病历Case report form 病例报告表Case report form/ case record form,CRF 病例报告表,病例记录表Categorical variable 分类变量Cav 平均浓度CD 圆二色谱CL(clearance)清除率Clinical equivalence 临床等效性Clinical study 临床研究Clinical study report 临床试验的总结报告Clinical trial 临床试验Clinical trial application,CTA 临床试验申请Clinical trial exemption,CTX 临床试验免责Clinical trial protocol,CTP 临床试验方案Clinical trial/ study report 临床试验报告Cmax 峰浓度Co-investigator 合作研究者Comparison 对照Compliance 依从性Composite variable 复合变量Computer-assisted trial design,CATD 计算机辅助试验设计Confidence interval 可信区间Confidence level 置信水平Consistency test 一致性检验Contract research organization,CRO 合同研究组织Contract/ agreement 协议/合同Control group 对照组Coordinating committee 协调委员会Crea 肌酐CRF(case report form)病例报告表Crossover design 交叉设计Cross-over study 交叉研究Css 稳态浓度Cure 痊愈Data management 数据管理Database 建立数据库Descriptive statistical analysis 描述性统计分析DF 波动系统Dichotomies 二分类Diviation 偏差Documentation 记录/文件Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模拟Double dummy technique 双盲双模拟技术Double-blinding 双盲Drop out 脱落DSC 差示扫描热量计Effectiveness 疗效Electronic data capture,EDC 电子数据采集系统Electronic data processing,EDP 电子数据处理系统Emergency envelope 应急信件End point 终点Endpoint criteria/ measurement 终点指标Equivalence 等效性Essential documentation 必须文件Ethics committee 伦理委员会Excellent 显效Exclusion criteria 排除标准Factorial design 析因设计Failure 无效,失败Final point 终点Fixed-dose procedure 固定剂量法Forced titration 强制滴定Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Global assessment variable 全局评价变量GLU 血糖Good clinical practice,GCP 药物临床试验质量管理规范Good manufacture practice,GMP 药品生产质量管理规范Good non-clinical laboratory practice,GLP 药物非临床研究质量管理规范Group sequential design 成组序贯设计Health economic evaluation,HEV 健康经济学评价Hypothesis test 假设检验Hypothesis testing 假设检验International Conference of Harmonization,ICH 人用药品注册技术要求国际技术协调会,国际协调会议Improvement 好转Inclusion criteria 入选标准Independent ethics committee,IEC 独立伦理委员会Information consent form,ICF 知情同意书Information gathering 信息收集Informed consent,IC 知情同意Initial meeting 启动会议Inspection 视察/检查Institution inspection 机构检查Institution review board,IBR 机构审查委员会Intention to treat 意向治疗(——临床领域)Intention-to –treat,ITT 意向性分析(-统计学)Interactive voice response system,IVRS 互动式语音应答系统Interim analysis 期中分析Investigator 研究者Investigator's brochure,IB 研究者手册IR 红外吸收光谱Ka 吸收速率常Last observation carry forward,LOCF 最接近一次观察的结转LC-MS 液相色谱-质谱联用LD50 板数致死剂量Logic check 逻辑检查LOQ (Limit of Quantitation)定量限LOCF,Last observation carry forward 最近一次观察的结转Lost of follow up 失访Marketing approval/ authorization 上市许可证Matched pair 匹配配对Missing value 缺失值Mixed effect model 混合效应模式Monitor 监查员Monitoring 监查Monitoring report 监查报告MRT 平均滞留时间MS 质谱MS-MS 质谱-质谱联用MTD(Maximum Tolerated Dose)最大耐受剂量Multicenter trial 多中心试验Multi-center trial 多中心试验New chemical entity,NCE 新化学实体New drug application,NDA 新药申请NMR 核磁共振谱Non-clinical study 非临床研究Non-inferiority 非劣效性Non-parametric statistics 非参数统计方法Obedience 依从性ODR 旋光光谱Open-blinding 非盲Open-label 非盲Optional titration 随意滴定Original medical record 原始医疗记录Outcome 结果Outcome assessment 结果指标评价Outcome measurement 结果指标Outlier 离群值Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient history 病历Per protocol,PP 符合方案集Placebo 安慰剂Placebo control 安慰剂对照Polytomies 多分类Power 检验效能Precision 精密度Preclinical study 临床前研究Primary endpoint 主要终点Primary variable 主要变量Principal investigator 主要研究者Principle investigator,PI 主要研究者Product license,PL 产品许可证Protocol 试验方案Protocol 试验方案Protocol amendment 方案补正Quality assurance unit,QAU 质量保证部门Quality assurance,QA 质量保证Quality control,QC 质量控制Query list,query form 应用疑问表Randomization 随机化Randomization 随机Range check 范围检查Rating scale 量表Regulatory authorities,RA 监督管理部门Replication 可重复RSD 日内和日间相对标准差Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample size 样本含量Sample size 样本量,样本大小Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Serious adverse event,SAE 严重不良事件Serious adverse reaction,SAR 严重不良反应Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single blinding 单盲Single-blinding 单盲Site audit 试验机构稽查SOP 试验室的标准操作规程Source data verification,SDV 原始数据核准Source data,SD 原始数据Source document,SD 原始文件Specificity 特异性Sponsor 申办者Sponsor-investigator 申办研究者Standard curve 标准曲线Standard operating procedure,SOP 标准操作规程Statistic 统计量Statistical analysis plan 统计分析计划Statistical analysis plan 统计参数计划书Statistical analysis plan,SAP 统计分析计划Statistical model 统计模型Statistical tables 统计分析表Stratified 分层Study audit 研究稽查Subgroup 亚组Sub-investigator 助理研究者Subject 受试者Subject diary 受试者日记Subject enrollment 受试者入选Subject enrollment log 受试者入选表Subject identification code,SIC 受试者识别代码Subject recruitment 受试者招募Subject screening log 受试者筛选表Superiority 检验Survival analysis 生存分析SXRD 单晶X-射线衍射System audit 系统稽查T1/2 消除半衰期Target variable 目标变量T-BIL 总胆红素T-CHO 总胆固醇TG 热重分析TLC、HPLC 制备色谱Tmax 峰时间TP 总蛋白Transformation 变量变换Treatment group 试验组Trial error 试验误差Trial master file 试验总档案Trial objective 试验目的Trial site 试验场所Triple blinding 三盲Two one-side test 双单侧检验Unblinding 揭盲Unblinding 破盲Unexpected adverse event,UAE 预料外不良事件UV-VIS 紫外-可见吸收光谱Variability 变异Variable 变量Visual analogy scale 直观类比打分法Visual check 人工检查Vulnerable subject 弱势受试者Wash-out 清洗期Washout period 洗脱期Well-being 福利,健康临床采血相关词汇Intravenous injection(IV) 静脉注射Intravenous drip/Intravenously guttae(iv gtt)静脉滴注intramscular injection(IM) 肌内注射intradermal injections 皮内注射subcutaneous injections(SC)皮下注射disposable sterile injector 一次性无菌注射针injection set注射器disposable venous infusion needle一次性静脉输液针disposable infusion set 一次性使用输液器blood transfusion set输血器infusion bag液袋urine drainage bag集尿袋blood bag血袋medical catheter医用导管stainless steel needle不锈钢医用针管blood taking needle/ blood collection needle采血针Needle Holder 持针器Blood samples血样样本sample drawing/collection 采血/抽血needles 针vacutainer needles真空采血针tubes导管vacutainer tubes 真空管vacutainer holder 真空持针器tourniquet 止血带disinfection swabs 消毒海绵micropore tapedental rollsadhesive dressing 胶布敷料rubber gloves 橡皮手套pillow 枕头stopper 塞子(采血管的塞子)vacuum tubes真空管non-vacuum tubes 非真空管needle disposal box 穿刺针处理盒(利器处理盒)transfer and storage tubes 转移和存储管pipette 吸管吸液管移液管disposable pipettes一次性使用吸管centrifuge离心机swinging bucket rotor 浮桶式转头、吊桶式转头timer 计时器racks for tubes 试管架identification codes识别码mark/label the tubes标记导管freeze冷冻refrigerator 冰箱freezer 冰箱冷冻机Serum Separation Tube 血清分离试管Serum血清Plasma血浆whole blood 全血be inverted 倒垂的倒置的clot 凝块凝结Coagulate 凝固凝结clotting time 凝血时间minimally 最低限度地最低程度地maximally 最高限度地最高程度地Separation of serum or plasma 分离血清或血浆Hemolysis 溶血hemolyzed samples 溶血样本assays 含量测定analysis 分析(名词)analyze 分析(动词)analyzing 分析(动名词)non-frozen samples 非冷冻样本chemistry laboratories化学实验室Analytical procedures 分析程序Qualification 资质资格Personnel in the laboratory 实验室人员Field personnel 现场工作人员Internal quality control 内部质量控制External quality control 外部质量控制Hepatitis B 乙肝Hepatitis C 丙肝Positive 阳性Negative 阴性Measurement 测量pulse rate 脉搏Blood pressure 血压systolic blood pressure 收缩压diastolic blood pressure 舒张压hypertensives 高血压hypotension 低血压antihypertensive drug 抗高血压药mercury sphygmomanometer 水银式血压计mercury column 水银柱serum total cholesterol 血清总胆固醇lipid lowering drug 降脂药Obesity 肥胖症Blood Glucose 血糖BMI (body mass index) 体重指数Overweight 超重sample size 样本大小statistically 统计上地,统计地statistical precision 统计精度sub-groups亚群Recruitment招募Ethical 伦理的道德的Legal 法律的合法的Data in electronic format 电子版数据back-up data 备查资料常用临床医学英文术语cough 咳嗽asthma 哮喘pneumonia 肺炎heart disease 心脏病arrhythmia 心律不齐indigestion 消化不良gastritis 胃炎appendicitis 盲肠炎hepatitis 肝炎dermatitis皮炎freckle/ephelis 痣,雀斑acne 粉刺flu 流感diarrhoea 痢疾quarantine 检疫vaccinate 打疫苗endemic 水土不服relapse 复发症casualty急症stupor 昏迷sprain 扭伤scalding 烫伤graze 擦伤scratch 搔挠trauma 外伤bruise 淤伤fracture骨折dislocation 脱臼tinnitus耳鸣trachoma沙眼colour blindness 色盲nearsightedness/myopia近视astigmatism 散光gingivitis 牙龈炎cavity 龋齿fever 发烧discomfort/disorder 不适malnutrition 营养不良incubation 潜伏期asthenia 虚弱poisoning 中毒fatigue 疲劳heat stroke 中暑itching 发痒ache/pain 痛tetanus 破伤风night sweat 盗汗chill 打冷颤pale 脸色发白shuddering 发抖inflammation 炎症acute 急症chronic 慢性病congenital 先天性病nausea恶心vomit 呕吐diseases 疾病acute diseases 急性病advanced diseases 病沉重期,晚期疾病chronic diseases慢性病communicable diseases 传染病complicating diseases 并发病congenital diseases 先天性疾病acquired diseases 后天性疾病contagious diseases接触性传染病endemic diseases 地方病epidemic diseases 流行病functional diseases 机能病、官能病infectious diseases 传染病inherited diseases 遗传病malignant diseases恶性病nutritional diseases 营养病occupation diseases 职业病organic diseases 器质性病paroxysmal diseases 阵发性病periodical diseases 周期病primary(principal)diseases原发(主导)病secondary diseases 继发病sexual(venereal, social)diseases 性病terminal diseases 绝症wasting diseases 消耗性疾病chief complaint 主诉clinical manifestation 临床表现delivery history 分娩史etiology病因学family history 家族史history, medical history 病史precipitating(induced)诱因marital status婚姻状况menstrual history 月经史menarche 初潮menopause闭经past history既往史pathogenesis 发病机制personal history 个人史symptoms 症状cardinal symptom 主要症状classical symptom 典型症状concomitant symptom 伴发症状constitutional(systemic)symptom 全身症状indirect symptom 间接症状induced symptom 诱发症状local symptom 局部症状mental symptom精神症状symptom-complex (syndrome)symptom 综合症,症候群signs体征antecedent 前驱征assident (accessory)副征commemorative 后遗症sign of death 死征diagnostic诊断征sign of disease 病征subjective 自觉征,主观征vein sign静脉征vital sign 生命体征body length (height of the body)身高body weight 体重barrel chest 桶状胸cachexia 恶病质compulsive position 被动体位critical facies 病危面容emaciation 消瘦enophthalmos 眼球下陷entropion 睑内翻exophthalmos 眼球突出flushed face 面色潮红gain (loss)in weight 增加(减轻)体重lock-jaw 牙关紧闭lordosis 脊柱前凸nasal ala flap 鼻翼扇动nystagmus 眼震obesity 肥胖pallor 苍白scolisis 脊柱侧凸agitation 焦急不安debility, weakness 虚弱diaphoresis 出汗,大量出汗dizziness, vertigo 眩晕lassitude, fatigue 无力,倦怠malaise 不适night sweat 盗汗numbness 麻木rigor, chill 寒冷,发冷perspiration, sweating 出汗pruritus, itching 痒,somasthenia 躯体无力tingling 麻刺感abscess 脓肿acidosis 酸中毒adhesion 粘连alkalosis 碱中毒allergy 过敏coagulation defect 凝血不良congestion 充血dehydration 脱水distention 膨胀edema 水肿embolism 栓塞,栓塞形成fluid and electrolyte imbalance 水电解质紊乱gangrene 坏疽hematoma 血肿hemorrhage, bleeding 出血infarction 梗塞,梗死infection 传染inflammation 炎症ketoacidosis 酮酸中毒metastasis 转移perforation 穿孔necrosis 坏死shock 休克response 反应,应答reaction 反应,感应thrombosis 血栓形成ulceration 溃疡fever, pyrexia 发烧,发热continuous fever 稽留热intermittent fever 间歇热low-grade fever 低热remittent fever 驰张热relapsing fever 回归热pain 痛burning pain 灼痛chest(flank,…)pain 胸(胁腹…)痛cramp-like pain 痉挛性痛dull, diffused pain 弥漫性钝痛pleuritic pain 胸膜炎性痛radiating pain (pain radiating to…)放射性痛(放射到…疼痛)angina 绞痛cardiac angina 心绞痛backache 背痛colic 绞痛,急腹痛earache 耳痛headache 头痛neuralgia 神经痛migraine 偏头痛rebound tenderness 反跳痛somatalgia 躯体痛sore throat 咽喉痛stomachache 胃痛toothache 牙痛bloody sputum 带血的痰cough 咳嗽dry cough 干咳expectoration 咳痰expectoration of blood 咳血hemoptysis 咳血anoxia 缺氧apnea 呼吸暂停,窒息asthma 气喘,哮喘Cheyne Stokes respiration 切-斯氏呼吸,潮式呼吸dyspnea 呼吸困难hyperpnea hyperventilation 过度呼吸,换气过度hypopnea 呼吸不全,呼吸浅表hypoxia 低氧,缺氧orthopnea 端坐呼吸respiratory arrest 呼吸停止suffocation 窒息tachypnea 呼吸急促fetid breath 口臭fruity breath 呼吸有水果味arrhythmia 心律失常,心律不齐atelectasis 肺不张,肺膨胀不全cardiac arrest 心搏骤停cardiac hypertrophy 心脏肥大cyanosis 发绀,青紫distension of jugular vein 颈静脉怒张extrasystole 期外收缩gallop rhythm 奔马律hemopleura 血胸hepatojugular reflux 肝颈静脉回流hypovolemia (循环)血容量减少palpitation 心悸tachycardia 心动过速pneumothorax 气胸thrill 震颤absent breath sounds 呼吸音消失dull sound 浊音hyperresonant 鼓音rale 啰音rhonchus, rhonchi 鼾音,干啰音wheeze 哮鸣音anorexia, loss of appetite 食欲不振,厌食dysphagia 吞咽困难eructation 嗳气belching 嗳气flatulence 气胀flatus 肠胃气,屁gaseous distention 胃胀气hematemesis 呕血hiccough, hiccup 打呃,呃逆nausea 恶心pyrosis 胃灼热regurgitation 反胃,回流thirsty 口渴vomiting 呕吐anal fissure, crack in the anal canal 肛裂ascites 腹水board-like rigidity of the abdomen 板状腹decreased tactile fremitus 触觉性震颤减弱exophageal varices 食管静脉曲张fistula 瘘,瘘管hemorrhoid 痔hernia 疝hepatomegaly 肝肿大intussusception 肠套叠jaundice 黄疸muscle guarding, defence of the abdominal wall 腹壁肌卫peristalsis 蠕动loss of peristalsis 蠕动消失mass peristalsis 总蠕动retrograde (reversed)peristalsis 逆蠕动prolapse 脱垂prolapse of anus 脱肛rectal prolapse 直肠脱垂,脱肛volvulus 肠扭转calculus 结石,石biliary calculus 胆结石vesical calculus 膀胱结石constipation 便秘defecation 排便diarrhea 腹泻incontinence of feces 大便失禁hematochezia 便血fecal impaction 大便嵌塞occult blood 潜血painful straining with defecation 排便痛性牵动clay colored stools 陶土色便dark, granular/coffee ground emesis 咖啡样呕吐物fecal vomiting, stercoraceous vomiting 呕粪,吐粪foul fatty stools, steatorrhea 恶臭脂肪便,脂肪痢scanty and hard stools 便少而硬tarry (black)stools 柏油样便anuria 无尿burning sensation no urination 排尿时的灼烧感dysurea 排尿困难,尿痛enuresis, bed wetting 遗尿frequency of urination 尿频micturation 排尿uresis, urination, voiding 排尿nocturia 夜尿oliguria 少尿polyuria 多尿tenesmus 里急后重vesical tenesmus 排尿时里急后重uremia coma 尿毒症昏迷urgency of urination 尿急urinary incontinence 尿失禁aciduria 酸尿chyluria 乳糜尿cylindruia 管型尿glycosuria 糖尿hematuria 血尿ketonuria 酮尿pneumatinuria 气尿proteinuria 蛋白尿pyuria 脓尿amenorrhea 经闭,无月经dysmenorrhea 痛经menorrhagia 月经过多lochia 恶露menorrhea 行经,月经过多menstruation 月经uterine contraction 子宫收缩blotch 斑点bruise 挫伤,青肿acne 痤疮,粉刺desquamation 脱皮,脱屑ecchymosis 瘀斑loss of skin turgor 失去皮肤充盈nevus 痣papule 丘疹petechia 瘀点,瘀斑pigmentation 色素沉着pustule 脓疱purpura 紫癫red nodule 红结节roseola 玫瑰疹scar 伤疤senile plaque 老人斑spider anaioma 蛛形痣subcutaneous nodule 皮下结节urticaria 荨麻疹vesicle 小水疱vitiligo 白斑blindness 失明blurred vision, visual disturbance 视力模糊impaired vision 视力下降lacrimation 流泪papilledema 视神经乳头水肿photophobia 畏光,羞明retinal detachment 视网膜脱离deafness 聋hearing loss 听力丧失tinnitus 耳鸣epistaxis, nasal bleeding 鼻出血impaired smelling 嗅觉障碍nasal discharge 鼻涕nasal obstruction 鼻塞sneeze 喷嚏snore 打鼾aphonia, loss of voice 失音症hoarseness 嘶哑gum bleeding 齿龈出血herpes labialis 唇疱疹,感冒疮Koplik's spots 科普利克斑lead line of the gum 龈铅线salivation, drooling 流口水straw-berry tongue 草莓舌tremulous tongue 舌震颤atrophy 萎缩contracture 挛缩deformity 畸形,变形dislocation 脱位fracture 骨折closed (simple)fracture 无创骨折,单纯性骨折comminuted fracture 粉碎性骨折compound fracture 哆开(开放性)骨折knock-knee 膝外翻opisthotonos 角弓反张prosthesis 假体spasm 痉挛tetany (肌)强直,手足抽搦wrist drop 腕下垂aphasia 失语ataxia 共济失调coma 昏迷consciousness 知觉,意识convulsion 抽搐,惊厥delirium 谵妄delusion 妄想faint 昏厥hallucination 幻觉hemiplegia 偏瘫increased intracranial pressure 颅内压增高insanity 精神错乱loss of orientation 定向丧失mania 躁狂memory defects, amnesia 记忆缺损,遗忘症paraplegia 截瘫,下身麻痹projectile vomiting 喷射性呕吐somnolence, (lethargy)昏睡,嗜睡stupor 木僵,昏呆tetraplegia 四肢瘫痪unconsciousness 失去知觉yawning 打哈欠crisis 危象cerebral (febrile, hematic, hemolytic,hypertensiv e, thyrotoxic, ……)crisis脑(热、血性、溶血、高血压、甲状腺中毒…)危象failure 衰竭,故障centra(circulatory, cardiac, myocardiac, peripheral, congestive, renal, respiratory)failure中枢(循环、心力、心肌、周围循环、充血性、肾、呼吸…)衰竭diagnosis诊断auscultation 听诊inspection 视诊palpation触诊percussion 叩诊laboratory examination 实验室检查physical examination 体格检查rectal (vaginal)touch 直肠(阴道)指诊impression 印象tentative diagnosis 暂定诊断differential diagnosis 鉴别诊断final diagnosis 最后诊断prognosis 预后prescription 处方incubation (latent)period 潜伏期prodromal stage 前驱期incipient stage 初期quiescent stage 静止期alleviation 减轻,缓和remission 缓解attack 发作convalescence (recovery)stage 恢复期rehabilitation 康复relapse 复发sudden death 猝死moribund 濒死的course of the disease 病程course of the treatment 疗程indication 适应症,指征complication 并发症contraindication 禁忌症side-effect 副作用sequel (sequela), after effect 后遗症therapies 治疗方法acupuncture therapy 针刺疗法block therapy 封闭疗法chemical therpy (chemo-therapy)化学疗法combined therapy 综合疗法conservative therapy 保守疗法constitutional therapy 全身疗法dietetic therapy 饮食疗法operative treatment 手术疗法palliative treatment, alleviative treatment 姑息疗法physical therapy 物理疗法psychotherapy 精神疗法radical treatment 根治radio-therapy 放射性疗法supporting treatment 支持疗法symptomatic treatment对症疗法cardiac massage 心脏按摩cardiac pacing 心脏起博electrotherapy 电疗法electroshock treatment 电休克疗法hemodialysis 血液透析hyperbaric therapy 高压氧疗法insulin-shock treatment 胰岛素休克疗法light therapy 光疗法Urine Analyzer 尿液分析仪blood sugar(glucose )analyzer血糖分析仪test strip 测试条reagent 试剂Semi-automatic Biochemical Analyzer半自动生化分析仪Automatic Blood Cell Analyzer全自动血细胞分析仪Urine sediments analyzer尿沉渣Bio-safety Cabinet 生物安全柜Incubator培养箱High Frequency Electrotome 高频电刀shadowless lamp无影灯High speed refrigerated centrifuge高速冷冻离心机hot air sterilizer热空气消毒箱microbiological incubator微生物培养箱Halogen light 卤素灯needle destroyer针头销毁器automatic packer自动纸塑包装机scalp vein set头皮针uniprocessor version单机版network version网络版macromolecule-solvent 高分子溶解的macromolecule cold accumulation 高分子蓄冷cold treatment冷疗法ice pack冰袋eyeshade 眼罩Medical injection pump医用灌注泵lithotrite 碎石机extracorporeal shock wave lithotrite体外冲击碎石机Ballistic intracroporeal lithotrite 气压冲击体内碎石机Laparoscope 腹腔镜Urology 泌尿外科kidney stones 肾结石Multi-parameter monitor, 多参数监护仪maternal monitor/fetal monitor母亲/胎儿监护仪ICU monitor 重症监护仪anesthetic equipment 麻醉机respirator呼吸机electronic colposcope 电子阴道镜smog absorber烟雾吸收器digital film room 数字胶片室Permanent Magnet Open Magnetic Resonance system 永磁开放式磁共振系统Ultrasonic Color Doppler Diagnostic system彩色超声多普勒诊断系统Mobile CT system 移动CT系统X-ray Mammary Machine 乳腺X线机Mammography乳腺high precision Stereotaxic 高精度脑立体定向仪portable Type-B ultrasonic 便携式B超Sterilization and Disinfection Equipment消毒灭菌设备Radiotherapeutic equipment.放射疗法设备pharmaceutical equipments.制药设备horizontal pressurized steam sterilizer普通卧式压力蒸汽灭菌器medical electronic linear accelerator医用电子直线加速器high frequency X-rays diagnostic machine高频X射线诊断机simulated positioner模拟定位机high frequency mobile X-rays machine高频移动X射线机医疗卫生人员职衔职称主任医师(讲课):Professor of Medicine主任医师(医疗):Professor of Treatment儿科主任医师:Professor of Paediatrics主治医师:Doctor-in-charge外科主治医师:Surgeon-in-charge内科主治医师:Physician-in-charge眼科主治医师:Oculist-in-charge妇科主治医师:Gynaecologist-in-charge牙科主治医师:Dentist-in-charge医师:Doctor医士:Assistant Doctor主任药师:Professor of Pharmacy主管药师:Pharmacist-in-charge药师:Pharmacist药士:Assistant Pharmacist主任护师:Professor of Nursing主管护师:Nurse-in-charge护师:Nurse Practitioner护士:Nurse主任技师:Senior Technologist主管技师:Technologist-in-charge技师:Technologist技士:Technician常用抗菌药物青霉素类青霉素(G) Penicillin(G) Benzylpenicillin, 苄青霉素, 盘尼西林青霉素V Penicillin V Phenoxymethylpenicillin, Blinvan, Ospen, 苯氧甲基青霉素苄星青霉素Benzathine Penicillin 长效西林, 长效青霉素, 比西林, LPG, 苄星青氨苄西林Ampicillin 安比西林, 氨苄青霉素, 安必仙, 安必林, 安比林阿莫西林Amoxicillin 特力士, 弗来莫星, 羟氨苄青霉素, 益萨林, 阿莫仙, 安福喜, 本原莫星巴氨西林Bacampicillin 美洛平, 氨卡西林, 氨苄青霉素甲戊酯阿洛西林Azlocillin 阿乐欣, 咪氨苄西林, 氧咪苄青霉素, Azlin美洛西林Mezlocillin 天林, Baypen, Mezlin, Baycipen替卡西林Ticarcillin 羧噻吩青霉素, 的卡西林, Nonapen, Ticarpen酞氨西林Talampicillin 氨苄青霉素酞酯, 酞氨苄青霉素, 酞氨苄西林, TAPC, Talpen夫苄西林Furbenicillin 呋脲苄青霉素, 呋苄青霉素, 呋氨西林, 呋喃酰脲苄青霉素氟氯西林Flucloxacillin 氟氯苯唑青霉素, 氟沙星, 福氯平, Floxapen羧苄西林Carbenicillin 羧苄青霉素, 卡比西林, 羧苄青阿扑西林Aspoxicillim 天冬羟氨青霉素, Doyle, ASPC匹氨西林Pivampicillin 氨苄西林酯, 匹凡西林, 匹呋西林, 吡呋氨卡西林双氯西林Dicloxacillin 双氯青, Dynapen, Consaphyl, Stampen, Diflor甲氧西林Meticillin 新青霉素Ⅰ, 美替西林, Azapen, Penysol苯唑西林钠Oxacillin Sodium 新青霉素Ⅱ, 苯唑青霉素钠, 苯甲异噁唑青霉素奈夫西林Nafcillin 新青霉素Ⅲ, 乙氧萘青霉素匹美西林Pivmecillinam 氮卓咪青霉素双酯, Celfuron, Melysin仑氨苄西林Lenampicillin Varacillin, Takacillin美西林Mecillinam Selexidleo, Selexid, Coactin, Amdinocillin, 氮卓脒青霉素哌拉西林钠Piperacillin Sodium 氧哌嗪青霉素, 哔哌西林, 哌氨苄青霉素Avocin, Orocin, Pipril氯唑西林钠Cloxacillin Sodium 邻氯青霉素钠, 氯唑青, Orbenin阿帕西林钠Apalcillin Sodium 萘啶青霉素钠, APPC, Lumota, Elumota, Palcin磺苄西林钠Sulbenicillin Sodium 磺苄青霉素钠, 卡他西林, 格达西林Sulfocillin, Lilacillin, Kedacillin青霉素V钾Phenoxymethylpenicillin Potassiume 6–苯氧乙酰胺基青霉烷酸钾, Cillaphen Distaquaine VK, Compocillin VK, Dowpen VK Cilicaine VK, Apopen, Biopen海他西林钾Hetacillin Potassium 缩酮氨苄青霉素钾, Etacillin, Veisapen卡茚西林钠Carindacillin Sodium Carbenicillin Indanyl Sodium, Geopen, Geocillin 治平霉素替莫西林二钠Temocillin Disodium Temopen头孢菌素类第一代头孢噻吩钠Cefalotin Sodium 先锋Ⅰ号, 头孢金素, 噻孢霉素, CET头孢噻啶Cefaloridine 先锋Ⅱ号, 头孢利素, CER, Kafspor头孢来星Cefaloglycine 先锋Ⅲ号, 头孢甘酸, Kafocin, Kefglycin头孢氨苄Cefalexin 先锋Ⅳ号, 苯甘孢霉素, 头孢力新, CEX头孢唑啉钠Cefazolin Sodium 先锋Ⅴ号, 塞福宁, 西华乐林, 先锋啉, 凯复唑Cefalin, CEX, Cefamedin, Kefzol, Cramaxin头孢拉定Cefradine 先锋Ⅵ号, 头孢雷定, 头孢瑞丁, 头孢环已烯,泛捷复, Velosef, CED, 塞福定头孢乙腈Cefacetrile 先锋Ⅶ号, 头孢腈甲, 头孢赛曲头孢匹林Cefapirin 先锋Ⅷ号, 头孢吡硫, Ceta-Dri头孢硫脒Cefathiamidine 先锋18, 吡脒头孢头孢克洛Cefaclor 赐福乐素, 新达乐, 头孢氯氨苄, Ceclor, 希刻劳,头孢克罗, 可福乐, 氯头孢菌素, CCL头孢羟氨苄Cefadroxil Cefadril, Kefroxil, 羟氨苄头孢菌素, 欧意, CDX,Duracef, Bidocef, Cefamx头孢沙定Cefroxadine 头孢环烯氨, ORASPON, CGP-9000, CXD头孢罗齐Cefprozil 头孢丙烯, Cefzil头孢替唑Ceftezole 特子社复, Tezacef, Alomen第二代头孢呋辛钠Cefuroxime Sodium 头孢呋肟, 赐福乐信, 舒贝洛, 西力欣, 特力欣, 明可欣, CXM, Zinacef, Monacef, Ketocef, Furex Kesiut, Supero头孢替安Cefotiam 噻乙胺唑头孢菌素, 头孢噻乙胺唑, 泛司博林, 泛司颇灵, Pansparin, Halospor, CTM头孢呋辛酯Cefuroxime Axetil 头孢呋肟酯, 新菌灵, 西力达, 头孢呋新乙酰乙酯Zinnat头孢西丁Cefoxitin 头孢氧唑, 甲氧头霉噻吩, 噻吩甲氧头孢菌素, 美福仙, 甲氧头霉噻吩, CXT, CFX头孢尼西二钠Cefonicid Disodium 铭乐希, Monocid, Cefonicid, Cefodie, Cefol头孢孟多Cefamandole 头孢羟唑, 羟苄四唑头孢菌素, Mandolkef, CMT, Cefadole, Mandol头孢美唑钠Cefmetazole Sodium 先锋美他醇, 氰唑甲氧头孢菌素, 头孢氰四唑,头孢美他唑, CEFMETAZON, CMZ, Cemetol头孢布宗Cefbuperazone 头孢拉宗, Keiperazon, Tomiporan, Cefobutazine, CBPZ 头孢雷特Ceforanide 头孢氨甲苯唑, 头孢苄胺四唑, 氨苄唑头孢菌素Precef第三代头孢地尼Cefdinir Cefzon头孢布坦Ceftibuten 头孢布烯, Cedax头孢卡品Cefcapene Pivoxil 头孢卡喷新戊酰氧甲酯, Flomox头孢噻肟钠Cefotaxime Sodium 头孢氨噻肟, 泰可欣, 塞福隆, 菌必灭, 治菌必妥, 凯福隆, 凯复龙, 头孢噻肟, 喜福得, Claforan, CTX头孢曲松钠Ceftriaxone Sodium 头孢三嗪, 头孢泰克松, 罗氏芬, 罗噻嗪, 菌必治, 菌得治, 头孢三嗪噻肟, 泛生舒复, 西华瑞隆, Rocephin, Ro-139904, CTRX 头孢哌酮钠Cefoperazone Sodium 头孢氧哌唑, 先锋必, 头孢必, 达诺欣, 塞福必,Cefobid, T-1551, CPZ头孢他啶Ceftazidime 头孢塔齐定, 头孢羧甲噻肟, 复达欣, 凯复定, Glazidine, Fortaz, Eposerin, Modocin, Fotrum, Tazidime, CTZ, Spectrum, Eposerin, Modocin头孢唑肟Ceftizoxime 头孢去甲噻肟, 益保世灵, 安保速灵, Epocelin CZX, CEFTIZOX头孢克肟Cefixime 头孢烯噻羟肟, 世伏素, 世福素, FK027, Suprax, CFIX , CEFSPAN头孢甲肟Cefmenoxime 头孢氨噻肟唑, 倍司特克, BESTCALL, CMX头孢地嗪Cefodizime 头孢双唑, 莫敌, 莫敌威, Modivid, Timcef头孢匹胺Cefpiramide 先福吡兰, 甲吡唑头孢菌素, 头孢吡胺, CPM,Cefpiran, Sepatren头孢特仑酯Cefteram Pivoxil 头孢特仑新戊酰氧甲酯, 托米仑, 头孢他美酯,富山龙, Tomiron头孢磺吡苄钠Cefsulodin Sodium 头孢磺吡酮, 头孢磺啶钠, 磺吡苄头孢菌素钠,达克舒林, Cefsulodine, Takesulin, CFS, SCE129拉他头孢Latamoxef 羟羧氧酰胺菌素, 拉氧头孢, 头孢拉坦, 噻吗灵Moxalactam, Shiomarin, Moxam, LMOX头孢米诺Cefminox 氨羧甲氧头孢菌素, 美土灵, Meicelin头孢咪唑Cefpimizole Benilan, SPIE, Ajicef头孢替坦二钠Cefotetan Disodium 双硫唑甲氧头孢霉素, Yamatetan, CTT, Cefotan第四代头孢唑喃钠Cefuzonam Sodium Cosmosin, CZON氟氧头孢钠Flomoxef Sodium 氟莫克西, 6315-S头孢匹罗Cefpirome 头孢吡隆, 氨噻肟吡戊头孢其它Β-内酰胺类亚胺培南Imipenem 伊米配能, 亚胺硫霉素, NFT氯曲南Aztreonam 菌克单, 君刻单, 噻肟单酰胺菌素, AZACTAM,Primbactam, Primbactin, SQ-26776氟罗培南Faropenem 福劳派南, Farom苄西林/氯唑西林Ampicillin/Cloxacillin 复方安比西林, 爱罗苏, 氨氯西林, 白罗仙, 淋必清, Pinocine亚胺培南/西司他丁Imipenem/Cilastatin 亚胺硫霉素/西拉司丁, 泰能, TIENAM, PRIMAXIN阿莫西林/氟氯西林Amoxicillin/Flucloxacillin 氟羟西林, 新灭菌, Biflocin, Infectrin三羟阿莫西林/单羟双氯西林Amoxycillin Trihydrate/Dicloxacillin康彼身, 克菌, CompliscanΒ-内酰胺类抗生类+Β-内酰胺酶抑制剂氨苄西林/舒巴坦Ampicillin/Sulbactam 强力安必仙, 舒他西林, 优力新, 新凯兰欣, 舒氨欣, Unasyn, Sultamicillin头孢哌酮/舒巴坦Cefoperazone/Sulbactam 舒普深, 舒哌酮, Sulperazone头孢噻肟/舒巴坦Cefotaxime/Sulbactam 新治菌哌拉西林/他佐巴坦Piperacillin/Tazobactam Tazocin, YP-14阿莫西林/克拉维酸Amoxillin/Clavulanic Acid 安灭菌, 安美汀, 奥格门汀, 安美汀, 阿莫克拉Augmentin, BRL25000替卡西林/克拉维酸Ticarcillin/Clavulanic Acid 泰门汀, 特泯菌, 特美汀, Timentin舒巴坦Sulbactam 舒巴克坦, 陪他美, 青霉烷砜氨基糖甙类链霉素Streptomycin RIMACTANE, RIFAMPINN, ovostrep庆大霉素Gentamycin 庆大霉素C, 瑞贝克, 正泰霉素庆大霉素链Gentamicin Chains 塞透派勒链, Septopal妥布霉素Tobramycin 立可信软膏,点必舒眼药水(妥布+地塞米松)泰星,妥布拉霉素,托普霉素,NEBCIN,Factorb, Brulamycin, Tobra, Nebramycin, Gernebcin, Obracine小诺霉素Micronomicin 小诺米星, 沙加霉素, 相模霉素, Sagamicin, Kw-1062, MCR核糖霉素Ribostamycin 威他霉素, 维生霉素, Vistamycin, Ibistacin大观霉素Spectinomycin 奇霉素, 壮观霉素, 淋必治, 奈毒素, Spectram, 克淋, Trobicin, Kirin阿米卡星Amikacin 丁胺卡那霉素, Amikin, Likacin西索米星Sisomicin 西梭霉素, 西索霉素, 紫苏霉素, Sisomin, Sipeptin奈替米星Netilmicin 乙基西梭霉素, 奈特, 奈替霉素, 乙基西素米星, 立克菌星, 乙基紫苏毒素, Ethylsisomicin, Vectacin, Zetamicn, Certomycin, NETROMYCIN异帕米星Isepamicin 依克沙霉素, HAPA-B, Exacin阿司米星Astromicin 阿司霉素, 福提霉素, 武夷霉素, 强壮霉素FORTIMICIN, Kw-1070, ASTM, Fortimicin地贝卡星Dibekacin 双去氧卡那霉素B, Dideoxykanamycin B, DKB, 达苄霉素, Icacine依替米星Etimicin 悉能磷霉素类磷霉素Fosfomycin 福赐美仙, Phosphonomycin, Fosfocina, FOM磷霉素氨基丁三醇Fosfomycin Tromethamine Monurol喹诺酮类第二代吡哌酸Pipemidic Acid PPA第三代诺氟沙星Nofloxacin 氟哌酸, 淋克星, Fulgram, Noroxin, AM-715 MK-0366, Brazan, Baccidal环丙沙星Ciprofloxacin 悉复欣, 悉普欣, 悉普宁, 丽珠环丙, 特美力,环丙氟哌酸, 健宝灵, CIPRO, Bay-0-9867, Ciprobay, Ciproxin培氟沙星Pefloxacin 培福新, 甲氟哌酸, 倍泰, Peflacine洛美沙星Lomefloxacin 洛威, 罗氟酸, 欣美罗, 多龙, Bareon芦氟沙星Rufloxacin 如氟沙星左旋氧氟沙星Levofloxacin 可乐必妥, 左氟沙星, Cravit依诺沙星Enoxacin 氟啶酸, 福禄马, 复克, FLUMARK, GYRAMID,Flumark氟罗沙星Fleroxacin 复诺定, 多氟哌酸, 喹诺敌, 麦加乐定, 沃尔得FLX, Megelone, Quinodis, Ro-236240, AM-833氧氟沙星Ofloxacin 氟嗪酸, 秦利必妥, 泰利特, 奥复星, 康泰必妥, 竹安新, TARIVID, 赞诺欣, Zanoxin, Oflocin, 泰利得, 正康培氟沙星Pefloxacin 甲氟哌酸, 培氟哌酸, 培福新, 倍宁, Pefalcine。
临床试验常用中英文词汇

SFDA Glossary: GCP,GLP,TRIALAccuracy 准确度Active control, AC 阳性对照,活性对照Adverse drug reaction, ADR 药物不良反应Adverse event, AE 不良事件Adverse medical events 不良医学事件Adverse reaction 药物不良反应Alb 白蛋白ALD(Approximate Lethal Dose)近似致死剂量ALP 碱性磷酸酶Alpha spending function 消耗函数ALT 丙氨酸氨基转换酶Analysis sets 统计分析的数据集Approval 批准Assistant investigator 助理研究者AST 天门冬酸氨基转换酶ATR 衰减全反射法AUCss 稳态血药浓度-时间曲线下面积Audit 稽查Audit or inspection 稽查/视察Audit report 稽查报告Auditor 稽查员Bias 偏性,偏倚Bioequivalence 生物等效应Blank control 空白对照Blind codes 编制盲底Blind review 盲态审核Blind review 盲态检查Blinding method 盲法Blinding/ masking 盲法,设盲Block 分段Block 层Block size 每段的长度BUN 尿素氮Carryover effect 延滞效应Case history 病历Case report form 病例报告表Case report form/ case record form, CRF 病例报告表,病例记录表Categorical variable 分类变量Cav 平均浓度CD 圆二色谱CL 清除率Clinical equivalence 临床等效应Clinical study 临床研究Clinical study report 临床试验的总结报告Clinical trial 临床试验Clinical trial application, CTA 临床试验申请Clinical trial exemption, CTX 临床试验免责Clinical trial protocol, CTP 临床试验方案Clinical trial/ study report 临床试验报告Cmax 峰浓度Co-investigator 合作研究者Comparison 对照Compliance 依从性Composite variable 复合变量Computer-assisted trial design, CATD 计算机辅助试验设计Confidence interval 可信区间Confidence level 置信水平Consistency test 一致性检验Contract research organization, CRO 合同研究组织Contract/ agreement 协议/合同Control group 对照组Coordinating committee 协调委员会Crea 肌酐CRF(case report form)病例报告表Crossover design 交叉设计Cross-over study 交叉研究Css 稳浓度Cure 痊愈Data management 数据管理Database 建立数据库Descriptive statistical analysis 描述性统计分析DF 波动系统Dichotomies 二分类Diviation 偏差Documentation 记录/文件Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模拟Double dummy technique 双盲双模拟技术Double-blinding 双盲Drop out 脱落DSC 差示扫描热量计Effectiveness 疗效Electronic data capture, EDC 电子数据采集系统Electronic data processing, EDP 电子数据处理系统Emergency envelope 应急信件End point 终点Endpoint criteria/ measurement 终点指标Equivalence 等效性Essential documentation 必须文件Ethics committee 伦理委员会Excellent 显效Exclusion criteria 排除标准Factorial design 析因设计Failure 无效,失败Final point 终点Fixed-dose procedure 固定剂量法Forced titration 强制滴定Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Global assessment variable 全局评价变量GLU 血糖Good clinical practice, GCP 药物临床试验质量管理规范Good manufacture practice, GMP 药品生产质量管理规范Good non-clinical laboratory practice, GLP 药物非临床研究质量管理规范Group sequential design 成组序贯设计Health economic evaluation, HEV 健康经济学评价Hypothesis test 假设检验Hypothesis testing 假设检验International Conference of Harmonization, ICH 人用药品注册技术要求国际技术协调会,国际协调会议Improvement 好转Inclusion criteria 入选标准Independent ethics committee, IEC 独立伦理委员会Information consent form, ICF 知情同意书Information gathering 信息收集Informed consent, IC 知情同意Initial meeting 启动会议Inspection 视察/检查Institution inspection 机构检查Institution review board, IBR 机构审查委员会Intention to treat 意向治疗(——临床领域)Intention-to –treat, ITT 意向性分析(-统计学)Interactive voice response system, IVRS 互动式语音应答系统Interim analysis 期中分析Investigator 研究者Investigator's brochure, IB 研究者手册IR 红外吸收光谱Ka 吸收速率常Last observation carry forward, LOCF 最接近一次观察的结转LC-MS 液相色谱-质谱联用LD50 板数致死剂量Logic check 逻辑检查LOQ (Limit of Quantitation)定量限LOCF, Last observation carry forward 最近一次观察的结转Lost of follow up 失访Marketing approval/ authorization 上市许可证Matched pair 匹配配对Missing value 缺失值Mixed effect model 混合效应模式Monitor 监查员Monitoring 监查Monitoring report 监查报告MRT 平均滞留时间MS 质谱MS-MS 质谱-质谱联用MTD(Maximum Tolerated Dose)最大耐受剂量Multicenter trial 多中心试验Multi-center trial 多中心试验New chemical entity, NCE 新化学实体New drug application, NDA 新药申请NMR 核磁共振谱Non-clinical study 非临床研究Non-inferiority 非劣效性Non-parametric statistics 非参数统计方法Obedience 依从性ODR 旋光光谱Open-blinding 非盲Open-label 非盲Optional titration 随意滴定Original medical record 原始医疗记录Outcome 结果Outcome assessment 结果指标评价Outcome measurement 结果指标Outlier 离群值Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient history 病历Per protocol, PP 符合方案集Placebo 安慰剂Placebo control 安慰剂对照Polytomies 多分类Power 检验效能Precision 精密度Preclinical study 临床前研究Primary endpoint 主要终点Primary variable 主要变量Principal investigator 主要研究者Principle investigator, PI 主要研究者Product license, PL 产品许可证Protocol 试验方案Protocol 试验方案Protocol amendment 方案补正Quality assurance unit, QAU 质量保证部门Quality assurance, QA 质量保证Quality control, QC 质量控制Query list, query form 应用疑问表Randomization 随机化Randomization 随机Range check 范围检查Rating scale 量表Regulatory authorities, RA 监督管理部门Replication 可重复RSD 日内和日间相对标准差Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample size 样本含量Sample size 样本量,样本大小Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Serious adverse event, SAE 严重不良事件Serious adverse reaction, SAR 严重不良反应Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single blinding 单盲Single-blinding 单盲Site audit 试验机构稽查SOP 试验室的标准操作规程Source data verification, SDV 原始数据核准Source data, SD 原始数据Source document, SD 原始文件Specificity 特异性Sponsor 申办者Sponsor-investigator 申办研究者Standard curve 标准曲线Standard operating procedure, SOP 标准操作规程Statistic 统计量Statistical analysis plan 统计分析计划Statistical analysis plan 统计参数计划书Statistical analysis plan, SAP 统计分析计划Statistical model 统计模型Statistical tables 统计分析表Stratified 分层Study audit 研究稽查Subgroup 亚组Sub-investigator 助理研究者Subject 受试者Subject diary 受试者日记Subject enrollment 受试者入选Subject enrollment log 受试者入选表Subject identification code, SIC 受试者识别代码Subject recruitment 受试者招募Subject screening log 受试者筛选表Superiority 检验Survival analysis 生存分析SXRD 单晶X-射线衍射System audit 系统稽查T1/2 消除半衰期Target variable 目标变量T-BIL 总胆红素T-CHO 总胆固醇TG 热重分析TLC、HPLC 制备色谱Tmax 峰时间TP 总蛋白Transformation 变量变换Treatment group 试验组Trial error 试验误差Trial master file 试验总档案Trial objective 试验目的Trial site 试验场所Triple blinding 三盲Two one-side test 双单侧检验Unblinding 揭盲Unblinding 破盲Unexpected adverse event, UAE 预料外不良事件UV-VIS 紫外-可见吸收光谱Variability 变异Variable 变量Visual analogy scale 直观类比打分法Visual check 人工检查Vulnerable subject 弱势受试者Wash-out 清洗期Washout period 洗脱期Well-being 福利,健康欢迎您的下载,资料仅供参考!致力为企业和个人提供合同协议,策划案计划书,学习资料等等打造全网一站式需求。
常用医药词汇翻译

FDA(FOOD AND DRUG ADMINISTRATION):(美国)食品药品管理局IND(INVESTIGA TIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICATION):新药申请ANDA(ABBREVIA TED NEW DRUG APPLICATION):简化新药申请EP诉(EXPORT APPLICA TION):出口药申请(申请出口不被批准在美国销售的药品)TREATMENT IND:研究中的新药用于治疗ABBREVIATED(NEW)DRUG:简化申请的新药DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其内容)HOLDER:DMF持有者CFR(CODE OF FEDERAL REGULATION):(美国)联邦法规PANEL:专家小组BATCH PRODUCTION:批量生产;分批生产BATCH PRODUCTION RECORDS:生产批号记录POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督INFORMED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)FDA(FOOD AND DRUG ADMINISTRATION):(美国)食品药品管理局IND(INVESTIGA TIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICATION):新药申请ANDA(ABBREVIA TED NEW DRUG APPLICATION):简化新药申请EP诉(EXPORT APPLICA TION):出口药申请(申请出口不被批准在美国销售的药品)TREATMENT IND:研究中的新药用于治疗ABBREVIATED(NEW)DRUG:简化申请的新药DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
临床试验相关词汇中英对照(精.选)

临床试验词汇中英文对照Accuracy 准确度Active control,AC 阳性对照,活性对照Adverse drug reaction,ADR 药物不良反应Adverse event,AE 不良事件Adverse medical events 不良医学事件Adverse reaction 药物不良反应Alb 白蛋白ALD(Approximate Lethal Dose)近似致死剂量ALP 碱性磷酸酶Alpha spending function 消耗函数ALT 丙氨酸氨基转换酶Analysis sets 统计分析的数据集Approval 批准Assistant investigator 助理研究者AST 天门冬酸氨基转换酶ATR 衰减全反射法AUCss 稳态血药浓度-时间曲线下面积Audit 稽查Audit or inspection 稽查/视察Audit report 稽查报告Auditor 稽查员Bias 偏性,偏倚Bioequivalence 生物等效应Blank control 空白对照Blind codes 编制盲底Blind review 盲态审核Blind review 盲态检查Blinding method 盲法Blinding/ masking 盲法,设盲Block 分段Block 层Block size 每段的长度BUN 尿素氮Carryover effect 延滞效应Case history 病历Case report form 病例报告表Case report form/ case record form,CRF 病例报告表,病例记录表Categorical variable 分类变量Cav 平均浓度CD 圆二色谱CL(clearance)清除率Clinical equivalence 临床等效性Clinical study 临床研究Clinical study report 临床试验的总结报告Clinical trial 临床试验Clinical trial application,CTA 临床试验申请Clinical trial exemption,CTX 临床试验免责Clinical trial protocol,CTP 临床试验方案Clinical trial/ study report 临床试验报告Cmax 峰浓度Co-investigator 合作研究者Comparison 对照Compliance 依从性Composite variable 复合变量Computer-assisted trial design,CATD 计算机辅助试验设计Confidence interval 可信区间Confidence level 置信水平Consistency test 一致性检验Contract research organization,CRO 合同研究组织Contract/ agreement 协议/合同Control group 对照组Coordinating committee 协调委员会Crea 肌酐CRF(case report form)病例报告表Crossover design 交叉设计Cross-over study 交叉研究Css 稳态浓度Cure 痊愈Data management 数据管理Database 建立数据库Descriptive statistical analysis 描述性统计分析DF 波动系统Dichotomies 二分类Diviation 偏差Documentation 记录/文件Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模拟Double dummy technique 双盲双模拟技术Double-blinding 双盲Drop out 脱落DSC 差示扫描热量计Effectiveness 疗效Electronic data capture,EDC 电子数据采集系统Electronic data processing,EDP 电子数据处理系统Emergency envelope 应急信件End point 终点Endpoint criteria/ measurement 终点指标Equivalence 等效性Essential documentation 必须文件Ethics committee 伦理委员会Excellent 显效Exclusion criteria 排除标准Factorial design 析因设计Failure 无效,失败Final point 终点Fixed-dose procedure 固定剂量法Forced titration 强制滴定Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Global assessment variable 全局评价变量GLU 血糖Good clinical practice,GCP 药物临床试验质量管理规范Good manufacture practice,GMP 药品生产质量管理规范Good non-clinical laboratory practice,GLP 药物非临床研究质量管理规范Group sequential design 成组序贯设计Health economic evaluation,HEV 健康经济学评价Hypothesis test 假设检验Hypothesis testing 假设检验International Conference of Harmonization,ICH 人用药品注册技术要求国际技术协调会,国际协调会议Improvement 好转Inclusion criteria 入选标准Independent ethics committee,IEC 独立伦理委员会Information consent form,ICF 知情同意书Information gathering 信息收集Informed consent,IC 知情同意Initial meeting 启动会议Inspection 视察/检查Institution inspection 机构检查Institution review board,IBR 机构审查委员会Intention to treat 意向治疗(——临床领域)Intention-to –treat,ITT 意向性分析(-统计学)Interactive voice response system,IVRS 互动式语音应答系统Interim analysis 期中分析Investigator 研究者Investigator's brochure,IB 研究者手册IR 红外吸收光谱Ka 吸收速率常Last observation carry forward,LOCF 最接近一次观察的结转LC-MS 液相色谱-质谱联用LD50 板数致死剂量Logic check 逻辑检查LOQ (Limit of Quantitation)定量限LOCF,Last observation carry forward 最近一次观察的结转Lost of follow up 失访Marketing approval/ authorization 上市许可证Matched pair 匹配配对Missing value 缺失值Mixed effect model 混合效应模式Monitor 监查员Monitoring 监查Monitoring report 监查报告MRT 平均滞留时间MS 质谱MS-MS 质谱-质谱联用MTD(Maximum Tolerated Dose)最大耐受剂量Multicenter trial 多中心试验Multi-center trial 多中心试验New chemical entity,NCE 新化学实体New drug application,NDA 新药申请NMR 核磁共振谱Non-clinical study 非临床研究Non-inferiority 非劣效性Non-parametric statistics 非参数统计方法Obedience 依从性ODR 旋光光谱Open-blinding 非盲Open-label 非盲Optional titration 随意滴定Original medical record 原始医疗记录Outcome 结果Outcome assessment 结果指标评价Outcome measurement 结果指标Outlier 离群值Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient history 病历Per protocol,PP 符合方案集Placebo 安慰剂Placebo control 安慰剂对照Polytomies 多分类Power 检验效能Precision 精密度Preclinical study 临床前研究Primary endpoint 主要终点Primary variable 主要变量Principal investigator 主要研究者Principle investigator,PI 主要研究者Product license,PL 产品许可证Protocol 试验方案Protocol 试验方案Protocol amendment 方案补正Quality assurance unit,QAU 质量保证部门Quality assurance,QA 质量保证Quality control,QC 质量控制Query list,query form 应用疑问表Randomization 随机化Randomization 随机Range check 范围检查Rating scale 量表Regulatory authorities,RA 监督管理部门Replication 可重复RSD 日内和日间相对标准差Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample size 样本含量Sample size 样本量,样本大小Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Serious adverse event,SAE 严重不良事件Serious adverse reaction,SAR 严重不良反应Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single blinding 单盲Single-blinding 单盲Site audit 试验机构稽查SOP 试验室的标准操作规程Source data verification,SDV 原始数据核准Source data,SD 原始数据Source document,SD 原始文件Specificity 特异性Sponsor 申办者Sponsor-investigator 申办研究者Standard curve 标准曲线Standard operating procedure,SOP 标准操作规程Statistic 统计量Statistical analysis plan 统计分析计划Statistical analysis plan 统计参数计划书Statistical analysis plan,SAP 统计分析计划Statistical model 统计模型Statistical tables 统计分析表Stratified 分层Study audit 研究稽查Subgroup 亚组Sub-investigator 助理研究者Subject 受试者Subject diary 受试者日记Subject enrollment 受试者入选Subject enrollment log 受试者入选表Subject identification code,SIC 受试者识别代码Subject recruitment 受试者招募Subject screening log 受试者筛选表Superiority 检验Survival analysis 生存分析SXRD 单晶X-射线衍射System audit 系统稽查T1/2 消除半衰期Target variable 目标变量T-BIL 总胆红素T-CHO 总胆固醇TG 热重分析TLC、HPLC 制备色谱Tmax 峰时间TP 总蛋白Transformation 变量变换Treatment group 试验组Trial error 试验误差Trial master file 试验总档案Trial objective 试验目的Trial site 试验场所Triple blinding 三盲Two one-side test 双单侧检验Unblinding 揭盲Unblinding 破盲Unexpected adverse event,UAE 预料外不良事件UV-VIS 紫外-可见吸收光谱Variability 变异Variable 变量Visual analogy scale 直观类比打分法Visual check 人工检查Vulnerable subject 弱势受试者Wash-out 清洗期Washout period 洗脱期Well-being 福利,健康临床采血相关词汇Intravenous injection(IV) 静脉注射Intravenous drip/Intravenously guttae(iv gtt)静脉滴注intramscular injection(IM) 肌内注射intradermal injections 皮内注射subcutaneous injections(SC)皮下注射disposable sterile injector 一次性无菌注射针injection set注射器disposable venous infusion needle一次性静脉输液针disposable infusion set 一次性使用输液器blood transfusion set输血器infusion bag液袋urine drainage bag集尿袋blood bag血袋medical catheter医用导管stainless steel needle不锈钢医用针管blood taking needle/ blood collection needle采血针Needle Holder 持针器Blood samples血样样本sample drawing/collection 采血/抽血needles 针vacutainer needles真空采血针tubes导管vacutainer tubes 真空管vacutainer holder 真空持针器tourniquet 止血带disinfection swabs 消毒海绵micropore tapedental rollsadhesive dressing 胶布敷料rubber gloves 橡皮手套pillow 枕头stopper 塞子(采血管的塞子)vacuum tubes真空管non-vacuum tubes 非真空管needle disposal box 穿刺针处理盒(利器处理盒)transfer and storage tubes 转移和存储管pipette 吸管吸液管移液管disposable pipettes一次性使用吸管centrifuge离心机swinging bucket rotor 浮桶式转头、吊桶式转头timer 计时器racks for tubes 试管架identification codes识别码mark/label the tubes标记导管freeze冷冻refrigerator 冰箱freezer 冰箱冷冻机Serum Separation Tube 血清分离试管Serum血清Plasma血浆whole blood 全血be inverted 倒垂的倒置的clot 凝块凝结Coagulate 凝固凝结clotting time 凝血时间minimally 最低限度地最低程度地maximally 最高限度地最高程度地Separation of serum or plasma 分离血清或血浆Hemolysis 溶血hemolyzed samples 溶血样本assays 含量测定analysis 分析(名词)analyze 分析(动词)analyzing 分析(动名词)non-frozen samples 非冷冻样本chemistry laboratories化学实验室Analytical procedures 分析程序Qualification 资质资格Personnel in the laboratory 实验室人员Field personnel 现场工作人员Internal quality control 内部质量控制External quality control 外部质量控制Hepatitis B 乙肝Hepatitis C 丙肝Positive 阳性Negative 阴性Measurement 测量pulse rate 脉搏Blood pressure 血压systolic blood pressure 收缩压diastolic blood pressure 舒张压hypertensives 高血压hypotension 低血压antihypertensive drug 抗高血压药mercury sphygmomanometer 水银式血压计mercury column 水银柱serum total cholesterol 血清总胆固醇lipid lowering drug 降脂药Obesity 肥胖症Blood Glucose 血糖BMI (body mass index) 体重指数Overweight 超重sample size 样本大小statistically 统计上地,统计地statistical precision 统计精度sub-groups亚群Recruitment招募Ethical 伦理的道德的Legal 法律的合法的Data in electronic format 电子版数据back-up data 备查资料常用临床医学英文术语cough 咳嗽asthma 哮喘pneumonia 肺炎heart disease 心脏病arrhythmia 心律不齐indigestion 消化不良gastritis 胃炎appendicitis 盲肠炎hepatitis 肝炎dermatitis皮炎freckle/ephelis 痣,雀斑acne 粉刺flu 流感diarrhoea 痢疾quarantine 检疫vaccinate 打疫苗endemic 水土不服relapse 复发症casualty急症stupor 昏迷sprain 扭伤scalding 烫伤graze 擦伤scratch 搔挠trauma 外伤bruise 淤伤fracture骨折dislocation 脱臼tinnitus耳鸣trachoma沙眼colour blindness 色盲nearsightedness/myopia近视astigmatism 散光gingivitis 牙龈炎cavity 龋齿fever 发烧discomfort/disorder 不适malnutrition 营养不良incubation 潜伏期asthenia 虚弱poisoning 中毒fatigue 疲劳heat stroke 中暑itching 发痒ache/pain 痛tetanus 破伤风night sweat 盗汗chill 打冷颤pale 脸色发白shuddering 发抖inflammation 炎症acute 急症chronic 慢性病congenital 先天性病nausea恶心vomit 呕吐diseases 疾病acute diseases 急性病advanced diseases 病沉重期,晚期疾病chronic diseases慢性病communicable diseases 传染病complicating diseases 并发病congenital diseases 先天性疾病acquired diseases 后天性疾病contagious diseases接触性传染病endemic diseases 地方病epidemic diseases 流行病functional diseases 机能病、官能病infectious diseases 传染病inherited diseases 遗传病malignant diseases恶性病nutritional diseases 营养病occupation diseases 职业病organic diseases 器质性病paroxysmal diseases 阵发性病periodical diseases 周期病primary(principal)diseases原发(主导)病secondary diseases 继发病sexual(venereal, social)diseases 性病terminal diseases 绝症wasting diseases 消耗性疾病chief complaint 主诉clinical manifestation 临床表现delivery history 分娩史etiology病因学family history 家族史history, medical history 病史precipitating(induced)诱因marital status婚姻状况menstrual history 月经史menarche 初潮menopause闭经past history既往史pathogenesis 发病机制personal history 个人史symptoms 症状cardinal symptom 主要症状classical symptom 典型症状concomitant symptom 伴发症状constitutional(systemic)symptom 全身症状indirect symptom 间接症状induced symptom 诱发症状local symptom 局部症状mental symptom精神症状symptom-complex (syndrome)symptom 综合症,症候群signs体征antecedent 前驱征assident (accessory)副征commemorative 后遗症sign of death 死征diagnostic诊断征sign of disease 病征subjective 自觉征,主观征vein sign静脉征vital sign 生命体征body length (height of the body)身高body weight 体重barrel chest 桶状胸cachexia 恶病质compulsive position 被动体位critical facies 病危面容emaciation 消瘦enophthalmos 眼球下陷entropion 睑内翻exophthalmos 眼球突出flushed face 面色潮红gain (loss)in weight 增加(减轻)体重lock-jaw 牙关紧闭lordosis 脊柱前凸nasal ala flap 鼻翼扇动nystagmus 眼震obesity 肥胖pallor 苍白scolisis 脊柱侧凸agitation 焦急不安debility, weakness 虚弱diaphoresis 出汗,大量出汗dizziness, vertigo 眩晕lassitude, fatigue 无力,倦怠malaise 不适night sweat 盗汗numbness 麻木rigor, chill 寒冷,发冷perspiration, sweating 出汗pruritus, itching 痒,somasthenia 躯体无力tingling 麻刺感abscess 脓肿acidosis 酸中毒adhesion 粘连alkalosis 碱中毒allergy 过敏coagulation defect 凝血不良congestion 充血dehydration 脱水distention 膨胀edema 水肿embolism 栓塞,栓塞形成fluid and electrolyte imbalance 水电解质紊乱gangrene 坏疽hematoma 血肿hemorrhage, bleeding 出血infarction 梗塞,梗死infection 传染inflammation 炎症ketoacidosis 酮酸中毒metastasis 转移perforation 穿孔necrosis 坏死shock 休克response 反应,应答reaction 反应,感应thrombosis 血栓形成ulceration 溃疡fever, pyrexia 发烧,发热continuous fever 稽留热intermittent fever 间歇热low-grade fever 低热remittent fever 驰张热relapsing fever 回归热pain 痛burning pain 灼痛chest(flank,…)pain 胸(胁腹…)痛cramp-like pain 痉挛性痛dull, diffused pain 弥漫性钝痛pleuritic pain 胸膜炎性痛radiating pain (pain radiating to…)放射性痛(放射到…疼痛)angina 绞痛cardiac angina 心绞痛backache 背痛colic 绞痛,急腹痛earache 耳痛headache 头痛neuralgia 神经痛migraine 偏头痛rebound tenderness 反跳痛somatalgia 躯体痛sore throat 咽喉痛stomachache 胃痛toothache 牙痛bloody sputum 带血的痰cough 咳嗽dry cough 干咳expectoration 咳痰expectoration of blood 咳血hemoptysis 咳血anoxia 缺氧apnea 呼吸暂停,窒息asthma 气喘,哮喘Cheyne Stokes respiration 切-斯氏呼吸,潮式呼吸dyspnea 呼吸困难hyperpnea hyperventilation 过度呼吸,换气过度hypopnea 呼吸不全,呼吸浅表hypoxia 低氧,缺氧orthopnea 端坐呼吸respiratory arrest 呼吸停止suffocation 窒息tachypnea 呼吸急促fetid breath 口臭fruity breath 呼吸有水果味arrhythmia 心律失常,心律不齐atelectasis 肺不张,肺膨胀不全cardiac arrest 心搏骤停cardiac hypertrophy 心脏肥大cyanosis 发绀,青紫distension of jugular vein 颈静脉怒张extrasystole 期外收缩gallop rhythm 奔马律hemopleura 血胸hepatojugular reflux 肝颈静脉回流hypovolemia (循环)血容量减少palpitation 心悸tachycardia 心动过速pneumothorax 气胸thrill 震颤absent breath sounds 呼吸音消失dull sound 浊音hyperresonant 鼓音rale 啰音rhonchus, rhonchi 鼾音,干啰音wheeze 哮鸣音anorexia, loss of appetite 食欲不振,厌食dysphagia 吞咽困难eructation 嗳气belching 嗳气flatulence 气胀flatus 肠胃气,屁gaseous distention 胃胀气hematemesis 呕血hiccough, hiccup 打呃,呃逆nausea 恶心pyrosis 胃灼热regurgitation 反胃,回流thirsty 口渴vomiting 呕吐anal fissure, crack in the anal canal 肛裂ascites 腹水board-like rigidity of the abdomen 板状腹decreased tactile fremitus 触觉性震颤减弱exophageal varices 食管静脉曲张fistula 瘘,瘘管hemorrhoid 痔hernia 疝hepatomegaly 肝肿大intussusception 肠套叠jaundice 黄疸muscle guarding, defence of the abdominal wall 腹壁肌卫peristalsis 蠕动loss of peristalsis 蠕动消失mass peristalsis 总蠕动retrograde (reversed)peristalsis 逆蠕动prolapse 脱垂prolapse of anus 脱肛rectal prolapse 直肠脱垂,脱肛volvulus 肠扭转calculus 结石,石biliary calculus 胆结石vesical calculus 膀胱结石constipation 便秘defecation 排便diarrhea 腹泻incontinence of feces 大便失禁hematochezia 便血fecal impaction 大便嵌塞occult blood 潜血painful straining with defecation 排便痛性牵动clay colored stools 陶土色便dark, granular/coffee ground emesis 咖啡样呕吐物fecal vomiting, stercoraceous vomiting 呕粪,吐粪foul fatty stools, steatorrhea 恶臭脂肪便,脂肪痢scanty and hard stools 便少而硬tarry (black)stools 柏油样便anuria 无尿burning sensation no urination 排尿时的灼烧感dysurea 排尿困难,尿痛enuresis, bed wetting 遗尿frequency of urination 尿频micturation 排尿uresis, urination, voiding 排尿nocturia 夜尿oliguria 少尿polyuria 多尿tenesmus 里急后重vesical tenesmus 排尿时里急后重uremia coma 尿毒症昏迷urgency of urination 尿急urinary incontinence 尿失禁aciduria 酸尿chyluria 乳糜尿cylindruia 管型尿glycosuria 糖尿hematuria 血尿ketonuria 酮尿pneumatinuria 气尿proteinuria 蛋白尿pyuria 脓尿amenorrhea 经闭,无月经dysmenorrhea 痛经menorrhagia 月经过多lochia 恶露menorrhea 行经,月经过多menstruation 月经uterine contraction 子宫收缩blotch 斑点bruise 挫伤,青肿acne 痤疮,粉刺desquamation 脱皮,脱屑ecchymosis 瘀斑loss of skin turgor 失去皮肤充盈nevus 痣papule 丘疹petechia 瘀点,瘀斑pigmentation 色素沉着pustule 脓疱purpura 紫癫red nodule 红结节roseola 玫瑰疹scar 伤疤senile plaque 老人斑spider anaioma 蛛形痣subcutaneous nodule 皮下结节urticaria 荨麻疹vesicle 小水疱vitiligo 白斑blindness 失明blurred vision, visual disturbance 视力模糊impaired vision 视力下降lacrimation 流泪papilledema 视神经乳头水肿photophobia 畏光,羞明retinal detachment 视网膜脱离deafness 聋hearing loss 听力丧失tinnitus 耳鸣epistaxis, nasal bleeding 鼻出血impaired smelling 嗅觉障碍nasal discharge 鼻涕nasal obstruction 鼻塞sneeze 喷嚏snore 打鼾aphonia, loss of voice 失音症hoarseness 嘶哑gum bleeding 齿龈出血herpes labialis 唇疱疹,感冒疮Koplik's spots 科普利克斑lead line of the gum 龈铅线salivation, drooling 流口水straw-berry tongue 草莓舌tremulous tongue 舌震颤atrophy 萎缩contracture 挛缩deformity 畸形,变形dislocation 脱位fracture 骨折closed (simple)fracture 无创骨折,单纯性骨折comminuted fracture 粉碎性骨折compound fracture 哆开(开放性)骨折knock-knee 膝外翻opisthotonos 角弓反张prosthesis 假体spasm 痉挛tetany (肌)强直,手足抽搦wrist drop 腕下垂aphasia 失语ataxia 共济失调coma 昏迷consciousness 知觉,意识convulsion 抽搐,惊厥delirium 谵妄delusion 妄想faint 昏厥hallucination 幻觉hemiplegia 偏瘫increased intracranial pressure 颅内压增高insanity 精神错乱loss of orientation 定向丧失mania 躁狂memory defects, amnesia 记忆缺损,遗忘症paraplegia 截瘫,下身麻痹projectile vomiting 喷射性呕吐somnolence, (lethargy)昏睡,嗜睡stupor 木僵,昏呆tetraplegia 四肢瘫痪unconsciousness 失去知觉yawning 打哈欠crisis 危象cerebral (febrile, hematic, hemolytic,hypertensive, thyrotoxic, ……)crisis脑(热、血性、溶血、高血压、甲状腺中毒…)危象failure 衰竭,故障centra(circulatory, cardiac, myocardiac, peripheral, congestive, renal, respiratory)failure中枢(循环、心力、心肌、周围循环、充血性、肾、呼吸…)衰竭diagnosis诊断auscultation 听诊inspection 视诊palpation触诊percussion 叩诊laboratory examination 实验室检查physical examination 体格检查rectal (vaginal)touch 直肠(阴道)指诊impression 印象tentative diagnosis 暂定诊断differential diagnosis 鉴别诊断final diagnosis 最后诊断prognosis 预后prescription 处方incubation (latent)period 潜伏期prodromal stage 前驱期incipient stage 初期quiescent stage 静止期alleviation 减轻,缓和remission 缓解attack 发作convalescence (recovery)stage 恢复期rehabilitation 康复relapse 复发sudden death 猝死moribund 濒死的course of the disease 病程course of the treatment 疗程indication 适应症,指征complication 并发症contraindication 禁忌症side-effect 副作用sequel (sequela), after effect 后遗症therapies 治疗方法acupuncture therapy 针刺疗法block therapy 封闭疗法chemical therpy (chemo-therapy)化学疗法combined therapy 综合疗法conservative therapy 保守疗法constitutional therapy 全身疗法dietetic therapy 饮食疗法operative treatment 手术疗法palliative treatment, alleviative treatment 姑息疗法physical therapy 物理疗法psychotherapy 精神疗法radical treatment 根治radio-therapy 放射性疗法supporting treatment 支持疗法symptomatic treatment对症疗法cardiac massage 心脏按摩cardiac pacing 心脏起博electrotherapy 电疗法electroshock treatment 电休克疗法hemodialysis 血液透析hyperbaric therapy 高压氧疗法insulin-shock treatment 胰岛素休克疗法light therapy 光疗法Urine Analyzer 尿液分析仪blood sugar(glucose )analyzer血糖分析仪test strip 测试条reagent 试剂Semi-automatic Biochemical Analyzer半自动生化分析仪Automatic Blood Cell Analyzer全自动血细胞分析仪Urine sediments analyzer尿沉渣Bio-safety Cabinet 生物安全柜Incubator培养箱High Frequency Electrotome 高频电刀shadowless lamp无影灯High speed refrigerated centrifuge高速冷冻离心机hot air sterilizer热空气消毒箱microbiological incubator微生物培养箱Halogen light 卤素灯needle destroyer针头销毁器automatic packer自动纸塑包装机scalp vein set头皮针uniprocessor version单机版network version网络版macromolecule-solvent 高分子溶解的macromolecule cold accumulation 高分子蓄冷cold treatment冷疗法ice pack冰袋eyeshade 眼罩Medical injection pump医用灌注泵lithotrite 碎石机extracorporeal shock wave lithotrite体外冲击碎石机Ballistic intracroporeal lithotrite 气压冲击体内碎石机Laparoscope 腹腔镜Urology 泌尿外科kidney stones 肾结石Multi-parameter monitor, 多参数监护仪maternal monitor/fetal monitor母亲/胎儿监护仪ICU monitor 重症监护仪anesthetic equipment 麻醉机respirator呼吸机electronic colposcope 电子阴道镜smog absorber烟雾吸收器digital film room 数字胶片室Permanent Magnet Open Magnetic Resonance system 永磁开放式磁共振系统Ultrasonic Color Doppler Diagnostic system彩色超声多普勒诊断系统Mobile CT system 移动CT系统X-ray Mammary Machine 乳腺X线机Mammography乳腺high precision Stereotaxic 高精度脑立体定向仪portable Type-B ultrasonic 便携式B超Sterilization and Disinfection Equipment消毒灭菌设备Radiotherapeutic equipment.放射疗法设备pharmaceutical equipments.制药设备horizontal pressurized steam sterilizer普通卧式压力蒸汽灭菌器medical electronic linear accelerator医用电子直线加速器high frequency X-rays diagnostic machine高频X射线诊断机simulated positioner模拟定位机high frequency mobile X-rays machine高频移动X射线机医疗卫生人员职衔职称主任医师(讲课):Professor of Medicine主任医师(医疗):Professor of Treatment儿科主任医师:Professor of Paediatrics主治医师:Doctor-in-charge外科主治医师:Surgeon-in-charge内科主治医师:Physician-in-charge眼科主治医师:Oculist-in-charge妇科主治医师:Gynaecologist-in-charge牙科主治医师:Dentist-in-charge医师:Doctor医士:Assistant Doctor主任药师:Professor of Pharmacy主管药师:Pharmacist-in-charge药师:Pharmacist药士:Assistant Pharmacist主任护师:Professor of Nursing主管护师:Nurse-in-charge护师:Nurse Practitioner护士:Nurse主任技师:Senior Technologist主管技师:Technologist-in-charge技师:Technologist技士:Technician常用抗菌药物青霉素类青霉素(G) Penicillin(G) Benzylpenicillin, 苄青霉素, 盘尼西林青霉素V Penicillin V Phenoxymethylpenicillin, Blinvan, Ospen, 苯氧甲基青霉素苄星青霉素Benzathine Penicillin 长效西林, 长效青霉素, 比西林, LPG, 苄星青氨苄西林Ampicillin 安比西林, 氨苄青霉素, 安必仙, 安必林, 安比林阿莫西林Amoxicillin 特力士, 弗来莫星, 羟氨苄青霉素, 益萨林, 阿莫仙, 安福喜, 本原莫星巴氨西林Bacampicillin 美洛平, 氨卡西林, 氨苄青霉素甲戊酯阿洛西林Azlocillin 阿乐欣, 咪氨苄西林, 氧咪苄青霉素, Azlin美洛西林Mezlocillin 天林, Baypen, Mezlin, Baycipen替卡西林Ticarcillin 羧噻吩青霉素, 的卡西林, Nonapen, Ticarpen酞氨西林Talampicillin 氨苄青霉素酞酯, 酞氨苄青霉素, 酞氨苄西林, TAPC, Talpen夫苄西林Furbenicillin 呋脲苄青霉素, 呋苄青霉素, 呋氨西林, 呋喃酰脲苄青霉素氟氯西林Flucloxacillin 氟氯苯唑青霉素, 氟沙星, 福氯平, Floxapen羧苄西林Carbenicillin 羧苄青霉素, 卡比西林, 羧苄青阿扑西林Aspoxicillim 天冬羟氨青霉素, Doyle, ASPC匹氨西林Pivampicillin 氨苄西林酯, 匹凡西林, 匹呋西林, 吡呋氨卡西林双氯西林Dicloxacillin 双氯青, Dynapen, Consaphyl, Stampen, Diflor甲氧西林Meticillin 新青霉素Ⅰ, 美替西林, Azapen, Penysol苯唑西林钠Oxacillin Sodium 新青霉素Ⅱ, 苯唑青霉素钠, 苯甲异噁唑青霉素奈夫西林Nafcillin 新青霉素Ⅲ, 乙氧萘青霉素匹美西林Pivmecillinam 氮卓咪青霉素双酯, Celfuron, Melysin仑氨苄西林Lenampicillin Varacillin, Takacillin美西林Mecillinam Selexidleo, Selexid, Coactin, Amdinocillin, 氮卓脒青霉素哌拉西林钠Piperacillin Sodium 氧哌嗪青霉素, 哔哌西林, 哌氨苄青霉素Avocin, Orocin, Pipril氯唑西林钠Cloxacillin Sodium 邻氯青霉素钠, 氯唑青, Orbenin阿帕西林钠Apalcillin Sodium 萘啶青霉素钠, APPC, Lumota, Elumota, Palcin磺苄西林钠Sulbenicillin Sodium 磺苄青霉素钠, 卡他西林, 格达西林Sulfocillin, Lilacillin, Kedacillin青霉素V钾Phenoxymethylpenicillin Potassiume 6–苯氧乙酰胺基青霉烷酸钾, Cillaphen Distaquaine VK, Compocillin VK, Dowpen VK Cilicaine VK, Apopen, Biopen海他西林钾Hetacillin Potassium 缩酮氨苄青霉素钾, Etacillin, Veisapen卡茚西林钠Carindacillin Sodium Carbenicillin Indanyl Sodium, Geopen, Geocillin 治平霉素替莫西林二钠Temocillin Disodium Temopen头孢菌素类第一代头孢噻吩钠Cefalotin Sodium 先锋Ⅰ号, 头孢金素, 噻孢霉素, CET头孢噻啶Cefaloridine 先锋Ⅱ号, 头孢利素, CER, Kafspor头孢来星Cefaloglycine 先锋Ⅲ号, 头孢甘酸, Kafocin, Kefglycin头孢氨苄Cefalexin 先锋Ⅳ号, 苯甘孢霉素, 头孢力新, CEX头孢唑啉钠Cefazolin Sodium 先锋Ⅴ号, 塞福宁, 西华乐林, 先锋啉, 凯复唑Cefalin, CEX, Cefamedin, Kefzol, Cramaxin头孢拉定Cefradine 先锋Ⅵ号, 头孢雷定, 头孢瑞丁, 头孢环已烯,泛捷复, Velosef, CED, 塞福定头孢乙腈Cefacetrile 先锋Ⅶ号, 头孢腈甲, 头孢赛曲头孢匹林Cefapirin 先锋Ⅷ号, 头孢吡硫, Ceta-Dri头孢硫脒Cefathiamidine 先锋18, 吡脒头孢头孢克洛Cefaclor 赐福乐素, 新达乐, 头孢氯氨苄, Ceclor, 希刻劳,头孢克罗, 可福乐, 氯头孢菌素, CCL头孢羟氨苄Cefadroxil Cefadril, Kefroxil, 羟氨苄头孢菌素, 欧意, CDX,Duracef, Bidocef, Cefamx头孢沙定Cefroxadine 头孢环烯氨, ORASPON, CGP-9000, CXD头孢罗齐Cefprozil 头孢丙烯, Cefzil头孢替唑Ceftezole 特子社复, Tezacef, Alomen第二代头孢呋辛钠Cefuroxime Sodium 头孢呋肟, 赐福乐信, 舒贝洛, 西力欣, 特力欣, 明可欣, CXM, Zinacef, Monacef, Ketocef, Furex Kesiut, Supero头孢替安Cefotiam 噻乙胺唑头孢菌素, 头孢噻乙胺唑, 泛司博林, 泛司颇灵, Pansparin, Halospor, CTM头孢呋辛酯Cefuroxime Axetil 头孢呋肟酯, 新菌灵, 西力达, 头孢呋新乙酰乙酯Zinnat头孢西丁Cefoxitin 头孢氧唑, 甲氧头霉噻吩, 噻吩甲氧头孢菌素, 美福仙, 甲氧头霉噻吩, CXT, CFX头孢尼西二钠Cefonicid Disodium 铭乐希, Monocid, Cefonicid, Cefodie, Cefol头孢孟多Cefamandole 头孢羟唑, 羟苄四唑头孢菌素, Mandolkef, CMT, Cefadole, Mandol头孢美唑钠Cefmetazole Sodium 先锋美他醇, 氰唑甲氧头孢菌素, 头孢氰四唑,头孢美他唑, CEFMETAZON, CMZ, Cemetol头孢布宗Cefbuperazone 头孢拉宗, Keiperazon, Tomiporan, Cefobutazine, CBPZ 头孢雷特Ceforanide 头孢氨甲苯唑, 头孢苄胺四唑, 氨苄唑头孢菌素Precef第三代头孢地尼Cefdinir Cefzon头孢布坦Ceftibuten 头孢布烯, Cedax头孢卡品Cefcapene Pivoxil 头孢卡喷新戊酰氧甲酯, Flomox头孢噻肟钠Cefotaxime Sodium 头孢氨噻肟, 泰可欣, 塞福隆, 菌必灭, 治菌必妥, 凯福隆, 凯复龙, 头孢噻肟, 喜福得, Claforan, CTX头孢曲松钠Ceftriaxone Sodium 头孢三嗪, 头孢泰克松, 罗氏芬, 罗噻嗪, 菌必治, 菌得治, 头孢三嗪噻肟, 泛生舒复, 西华瑞隆, Rocephin, Ro-139904, CTRX 头孢哌酮钠Cefoperazone Sodium 头孢氧哌唑, 先锋必, 头孢必, 达诺欣, 塞福必,Cefobid, T-1551, CPZ头孢他啶Ceftazidime 头孢塔齐定, 头孢羧甲噻肟, 复达欣, 凯复定, Glazidine, Fortaz, Eposerin, Modocin, Fotrum, Tazidime, CTZ, Spectrum, Eposerin, Modocin头孢唑肟Ceftizoxime 头孢去甲噻肟, 益保世灵, 安保速灵, Epocelin CZX, CEFTIZOX头孢克肟Cefixime 头孢烯噻羟肟, 世伏素, 世福素, FK027, Suprax, CFIX , CEFSPAN头孢甲肟Cefmenoxime 头孢氨噻肟唑, 倍司特克, BESTCALL, CMX头孢地嗪Cefodizime 头孢双唑, 莫敌, 莫敌威, Modivid, Timcef头孢匹胺Cefpiramide 先福吡兰, 甲吡唑头孢菌素, 头孢吡胺, CPM,Cefpiran, Sepatren头孢特仑酯Cefteram Pivoxil 头孢特仑新戊酰氧甲酯, 托米仑, 头孢他美酯,富山龙, Tomiron头孢磺吡苄钠Cefsulodin Sodium 头孢磺吡酮, 头孢磺啶钠, 磺吡苄头孢菌素钠,达克舒林, Cefsulodine, Takesulin, CFS, SCE129拉他头孢Latamoxef 羟羧氧酰胺菌素, 拉氧头孢, 头孢拉坦, 噻吗灵Moxalactam, Shiomarin, Moxam, LMOX头孢米诺Cefminox 氨羧甲氧头孢菌素, 美土灵, Meicelin头孢咪唑Cefpimizole Benilan, SPIE, Ajicef头孢替坦二钠Cefotetan Disodium 双硫唑甲氧头孢霉素, Yamatetan, CTT, Cefotan第四代头孢唑喃钠Cefuzonam Sodium Cosmosin, CZON氟氧头孢钠Flomoxef Sodium 氟莫克西, 6315-S头孢匹罗Cefpirome 头孢吡隆, 氨噻肟吡戊头孢其它Β-内酰胺类亚胺培南Imipenem 伊米配能, 亚胺硫霉素, NFT氯曲南Aztreonam 菌克单, 君刻单, 噻肟单酰胺菌素, AZACTAM,Primbactam, Primbactin, SQ-26776氟罗培南Faropenem 福劳派南, Farom苄西林/氯唑西林Ampicillin/Cloxacillin 复方安比西林, 爱罗苏, 氨氯西林, 白罗仙, 淋必清, Pinocine亚胺培南/西司他丁Imipenem/Cilastatin 亚胺硫霉素/西拉司丁, 泰能, TIENAM, PRIMAXIN阿莫西林/氟氯西林Amoxicillin/Flucloxacillin 氟羟西林, 新灭菌, Biflocin, Infectrin三羟阿莫西林/单羟双氯西林Amoxycillin Trihydrate/Dicloxacillin康彼身, 克菌, CompliscanΒ-内酰胺类抗生类+Β-内酰胺酶抑制剂氨苄西林/舒巴坦Ampicillin/Sulbactam 强力安必仙, 舒他西林, 优力新, 新凯兰欣, 舒氨欣, Unasyn, Sultamicillin头孢哌酮/舒巴坦Cefoperazone/Sulbactam 舒普深, 舒哌酮, Sulperazone头孢噻肟/舒巴坦Cefotaxime/Sulbactam 新治菌哌拉西林/他佐巴坦Piperacillin/Tazobactam Tazocin, YP-14阿莫西林/克拉维酸Amoxillin/Clavulanic Acid 安灭菌, 安美汀, 奥格门汀, 安美汀, 阿莫克拉Augmentin, BRL25000替卡西林/克拉维酸Ticarcillin/Clavulanic Acid 泰门汀, 特泯菌, 特美汀, Timentin舒巴坦Sulbactam 舒巴克坦, 陪他美, 青霉烷砜氨基糖甙类链霉素Streptomycin RIMACTANE, RIFAMPINN, ovostrep庆大霉素Gentamycin 庆大霉素C, 瑞贝克, 正泰霉素庆大霉素链Gentamicin Chains 塞透派勒链, Septopal妥布霉素Tobramycin 立可信软膏,点必舒眼药水(妥布+地塞米松)泰星,妥布拉霉素,托普霉素,NEBCIN,Factorb, Brulamycin, Tobra, Nebramycin, Gernebcin, Obracine小诺霉素Micronomicin 小诺米星, 沙加霉素, 相模霉素, Sagamicin, Kw-1062, MCR核糖霉素Ribostamycin 威他霉素, 维生霉素, Vistamycin, Ibistacin大观霉素Spectinomycin 奇霉素, 壮观霉素, 淋必治, 奈毒素, Spectram, 克淋, Trobicin, Kirin阿米卡星Amikacin 丁胺卡那霉素, Amikin, Likacin西索米星Sisomicin 西梭霉素, 西索霉素, 紫苏霉素, Sisomin, Sipeptin奈替米星Netilmicin 乙基西梭霉素, 奈特, 奈替霉素, 乙基西素米星, 立克菌星, 乙基紫苏毒素, Ethylsisomicin, Vectacin, Zetamicn, Certomycin, NETROMYCIN异帕米星Isepamicin 依克沙霉素, HAPA-B, Exacin阿司米星Astromicin 阿司霉素, 福提霉素, 武夷霉素, 强壮霉素FORTIMICIN, Kw-1070, ASTM, Fortimicin地贝卡星Dibekacin 双去氧卡那霉素B, Dideoxykanamycin B, DKB, 达苄霉素, Icacine依替米星Etimicin 悉能磷霉素类磷霉素Fosfomycin 福赐美仙, Phosphonomycin, Fosfocina, FOM磷霉素氨基丁三醇Fosfomycin Tromethamine Monurol喹诺酮类第二代吡哌酸Pipemidic Acid PPA第三代诺氟沙星Nofloxacin 氟哌酸, 淋克星, Fulgram, Noroxin, AM-715 MK-0366, Brazan, Baccidal环丙沙星Ciprofloxacin 悉复欣, 悉普欣, 悉普宁, 丽珠环丙, 特美力,环丙氟哌酸, 健宝灵, CIPRO, Bay-0-9867, Ciprobay, Ciproxin培氟沙星Pefloxacin 培福新, 甲氟哌酸, 倍泰, Peflacine洛美沙星Lomefloxacin 洛威, 罗氟酸, 欣美罗, 多龙, Bareon芦氟沙星Rufloxacin 如氟沙星左旋氧氟沙星Levofloxacin 可乐必妥, 左氟沙星, Cravit依诺沙星Enoxacin 氟啶酸, 福禄马, 复克, FLUMARK, GYRAMID,Flumark氟罗沙星Fleroxacin 复诺定, 多氟哌酸, 喹诺敌, 麦加乐定, 沃尔得FLX, Megelone, Quinodis, Ro-236240, AM-833氧氟沙星Ofloxacin 氟嗪酸, 秦利必妥, 泰利特, 奥复星, 康泰必妥, 竹安新, TARIVID, 赞诺欣, Zanoxin, Oflocin, 泰利得, 正康培氟沙星Pefloxacin 甲氟哌酸, 培氟哌酸, 培福新, 倍宁, Pefalcine。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
FDA 临床试验常见词汇中译文对照Aaction letter 决定通知active comparator 活性药物对照组active control = AC 阳性对照,活性对照active ingredient 有效成分Active Substance Master File (ASMF) 欧洲药物主文件acute myocardial infarction 急性心肌梗死acute tibial fractures 急性胫骨骨折adalimumab (Humira) 阿达木单抗adaptive design 自适应设计adaptive randomization 自适应随机ADE = adverse drug event 药物不良事件Adenoviral Vectors 腺病毒载体adequate and well-controlled studies 充分严格的对照研究ADHD = Attention-deficit hyperactivity disorder注意力缺陷多动障碍; 注意力不足过动症; 多动症adhesion barrier product 防黏著产品adjuvant 助剂 ; 佐剂 auxiliary;adjuvant therapy 佐药疗法,辅助疗法ADL = activities of daily living 日常生活活动能力ADME = absorption, distribution, metabolism, and excretion(药物 )吸收、分配、代谢和排除ADR = adverse drug reaction 药物不良反应adrenal cortex 肾上腺皮质adrenal cortical hormone 肾上腺皮质激素adrenal gland 肾上腺adrenaline 肾上腺素adulterated devices 掺假器械adverse drug reaction = ADR 药物不良反应adverse effect 副作用adverse event = AE 不良事件adverse medical events 不良医学事件adverse reaction (adverse event) 药物不良反应advisory 提醒advocacy and support groups 倡导和支持团体AE = adverse event 不良事件AERS = Adverse Event Reporting System 不良事件报告系统BBIMO Bioresearch Monitoring Program 生物研究监测bioavailability (F) 生物利用度biochemical drugs 生化药品biocides 生物杀灭剂 ; 杀生物剂biocompatibility 生物相容性biodegradable 生物分解bio-engineered, transgenic food 转基因食物bioequivalence; bioequivalent 生物等效应biofilm 细菌薄膜 , 生物膜biologic 生物制品biological response modifiers BRM 生物应答调节剂biological therapeutic agents 生物治疗药剂biomarker 生物标志物biometrics 生物统计 ; 生物识别技术bion stimulator 生物体刺激器bionic knee 仿生膝关节biopharma: biopharmaceutical products 生物药物产品bipolar 双相燥郁症birth defect 出生缺陷 , 新生儿缺陷 , 先天缺陷BLA = biologic license application 生物制品许可申请blank control 空白对照blend uniformity analysis 混合均匀度分析blind 盲法blind codes 编制盲底blind review 盲态审核blinding method 盲法blinding/ masking 盲法,设盲blister packaging 泡罩包装 ; 水泡眼block 分段 ;层block size 每段的长度blocked randomization 区组随机Ccase history 病历case record form = CRF 病例报告表 / 病例记录表case report form 病例报告表cash curve 现金曲线cash trap 现金陷阱 ; 现金套牢categorical variable 分类变量CLIA Clinical Laboratory Improvement Amendments临床实验室改进修订案clinical (human) data 临床数据clinical endpoint 临床终点clinical equivalence 临床等效应clinical hold 临床试验暂停通知clinical investigator 临床研究者Clinical Pharmacists 临床药师Clinical Research Coordinator = CRC 临床研究协调者clinical study 临床研究Clinical Study Application = CSA 临床研究申请clinical study reportclinical trialclinical trial application = CTAclinical trial exemption = CTXclinical trial protocol = CTPClinical Trial Report = CTRclinically significant resultscohortcohort studiesco-investigator = CIcomparisonCompassionate Usecompetitive labelingComplementary And Alternative Therapy Complete responsecompliancecomposite variableCompression Testcomputer-assisted trial design= CATD Con Meds = concomitant medications confidence intervalconfidence levelConfidentiality Regarding Trial Participants controlcontrol groupcontrolled clinical trialsControlled TrialsCritical PathCRM = continual reassessmentmethod crossover designcross-over studycrossover therapyCRF = case report formdosage formdosage regimendose-ranging studydose-reaction relationdose-related adverse reactionsdouble blindingdouble dummydouble dummydouble dummy techniquedouble-blind studyDouble-Masked Study 临床试验的总结报告临床试验临床试验申请临床试验免责临床试验方案临床试验报告有临床意义队列队列研究合作研究者对照体恤使用优越标签补充性和非传统治疗完全有效遵守;对遵守法规情况的监管复合变量压缩试验计算机辅助试验设计联合用药可信区间置信水平为试验参与者保密对照对照组临床对照实验对照试验关键路径连续重新评估方法交叉设计交叉研究交叉治疗病例报告表剂型给药方案剂量范围研究剂量-反应关系剂量相关的不良反应双盲双模拟双模拟双盲双模拟技术双盲研究双盲研究DRGs = Diagnosis Related Group System drop outdrop testdrug eluting coronary stentsdrug productdrug substancedrug-drug interaction56drug-food interaction 疾病诊断相关分组脱落落震试验 ;跌落试验药物洗脱支架药物产品原料药药物 -药物相互作用药物 -食物的相互作用effectivenessefficacyefficacy (Of a drug or treatment) 疗效有效性测定药效;药品疗效EEMEA = European Medical Evaluation Agency; European Agency for the Evaluation of Medicinal Products; European Medicines Agency 药物评价机构emergency envelope 应急信件 Empiric Bayesian MultipleGamma-Poisson Shrinker; 欧洲医药品管理局empiricalendpointendpoint criteriafactorial designfactorial trialfailureFair Packaging and Labeling Act (1966) False Claims Actfalse therapeutic claimsfull analysis setfull factorial design 经验性贝氏法(伽玛泊松分布缩检法)经验性终点终点指标析因设计析因试验无效,失败公平包装和标签法防制不实请求法错误的疗效声明全分析集全因子试验法Iinclusion criteria入选标准inclusion/exclusion criteria入选/排除标准incremental exposure食品中递增摄入量incubation period/latency period潜伏期IND = Investigational New Drug临床研究新药INDA = investigational new drug application NDA前申报阶段indemnity insurance赔偿保险Independent Data Monitoring = IDM独立数据监察Independent Data Monitoring Committee = IDMC独立数据监察委员会independent ethics committee = IEC indicationsinvestigational new drug= IND investigational product 独立伦理委员会适应症临床研究新药试验药物investigatorinvestigator's brochure = IB 调研人员研究者手册Mmaskedmean absorption time = MAT mean disintegration time = MDIT 设盲(药物在体内的(药物在体内的)平均吸收时间)平均崩解时间Mean Dissolution Time = MDT Mean Residence Time = MRT (药物在体内的(药物在体内的)平均释放时间)平均滞留时间medical governanceMedicaremedication guides (for patients) Medicines Control Agency = MCA MisbrandingMiscodingmissing valuemixed effect modelMLD = minimal lethal doseMoA = Mechanism of Actionmonitormonitoring planmonitoring reportMR = moderate responseMRA = Agreement on Mutual Recognition MTD = maximal tolerance dose multicenter trialmulti-drug resistancemultiple arm trialsmutual recognition procedure (EU) 医药治理老年医疗保险制度;联邦老年医保用药指南英国药品监督局错误标签 ; 冒牌编码错误缺失值混合效应模式最小致死剂量作用机制 ;作用机理监查员监查计划监查报告好转相互承认协定最大耐受剂量多中心试验多药物抗药性多治疗组的试验相互承认程序OOS = Overall survival 总生存率Pparallel group designparameter estimationparametric releaseparametric statisticspatient filepatient global; pt globalpatient historyper protocol ( PP) analysisPFS = progression-free survival PGE = patient global evaluation PHA = preliminary hazards analysis pharmaceutical equivalence pharmaceutics 平行组设计参数估计参数放行参数统计方法病人档案病人总体评价病历符合方案分析无疾病进展存活率病人总体评价预先危险分析药剂等效性药剂学pharmacodynamics=PD 药物效应动力学 ; 简称药效学pharmacoepidemiology 药物流行病学pharmacokinetics = PK 药代动力学 ; 简称药动学pharmacology 药理学Pharmacovigilance105 药物警戒pharmacy 配药学PharMetrics claims database 索赔数据库PhRMA = Pharmaceutical Research and Manufacturers of America美国药物研究与生产商协会PIC=Pharmaceutical Inspection Convention 药品检查协定PIC/S Pharmaceutical Inspection Cooperation Scheme 药物检查合作计划pipeline assets 开发中产品PK = pharmacokinetics 药物代谢动力学 ; 药动学,药代动力学placebo 安慰剂placebo control 安慰剂对照placebo controlled study 安慰剂对照研究placebo effect 安慰剂效应PMA = premarket approval 上市前许可 ; 销售前批准PMCs = post marketing commitments 承诺药品上市后的继续研究PMDRA = Post Marketing Drug Risk Assessment 上市后药品风险评估 (办公室 ) PMHx = Past Medical History 既往病史PMN = Premarket Notification 销售前通知PMS = Premenstrual syndrome 经前综合症POC (Proof-of-concept) Clinical Trials 概念证明POC = point-of-care testing 床旁分析polytomies 多分类pooled analysis = PA 荟萃分析postmarket surveillance 上市后监督post-marketing surveillance; postmarket safety surveillance 销售(上市)后监督power 把握度 ; 检验效能Pp = Process Performance 工序绩效Ppk = Process Performance Index 工序绩效指数precautions 慎用;注意事项precision 精密度preclinical (animal) data 临床前 (动物实验 )数据preclinical study 临床前研究predicate device = legally marketed device that is not subject to Premarket Approval (PMA)和已合法在市场上销售的且不需要做PMA“销售前批准”的Pre-market Approval (Application) = PMA 上市前许可(申请)premarket notification 上市前通知pre-marketing surveillance 销售(上市)前监督preparing and submitting 起草和申报prescription drug 处方药preservation 保藏prevalenceprevention trialsprimary (coronary) eventprimary endpointprimary mode of action = PMOA primary variableprincipal investigator = PIPrinciples of Qualificationprocess controlsprocess validationproduct codesproduct differentiationproduct license =PLproduct life cycle (PLC)prognosisprogression-free survival = PFS progressive Disease PDproof of principle studypropensity scoreprotocolprotocol amendmentprototype designprotozoaproven acceptable Range = PAR PTC = Product Technical ComplaintsQqualification system for licensed pharmacist qualified health claims Qualified Person = QPquality assurance = QAquality assurance unit = QAUquality control = QC quality management systemsquality of life trials or supportive care trials quality risk management = QRM quantitative risk assessmentRrandomizationrandomized trialrandomized, double blinded clinical trial range checkrating scaleRCT = randomized clinical trialsRCT = randomized controlled trial 患病率预防试验原位病变主要终点首要作用模式主要变量主要研究者确认(验证)原则工艺控制工艺验证产品的号码产品差异化,产品特色化产品许可证产品生命周期预后无进展生存病情进展原理循证研究倾向性评分试验方案 ; 方案方案补正原型设计原生动物门确定可接受范围药品技术投诉执业药师资格准入制度有保留的健康宣称受权人质量保证质量保证部门质量控制质量管理体系生存质量试验质量风险管理量化风险评估随机化随机化试验随机双盲对照研究范围检查量表随机临床试验随机对照试验RDE: remote data entry 远距数据输入ready-to-eat foods 即食食品reagents 试剂recall 召回 ; 强制回收RECIST = Response Evaluation Criteria in Solid Tumors 实体瘤的疗效评价标准reconditioning 整改 ; 货物重整理;货物重包装recycled plastics 可循环利用塑料制品reference product 参比制剂reference samples 标准样品regulatory methodology 质量管理方法regulatory methods validation 管理用分析方法的验证( FDA 对 NDA 提供的方法进行验证)regulatory specification 质量管理规格标准( NDA 提供)rejection 排异remote monitoring system 远程监测系统 ; 远程监控REMS = Risk Evaluation and Mitigation Strategies 风险评估和减缓战略risk 受害risk assessment (risk analysis + risk evaluation) 风险评估,论证risk classification 风险分类 ;Risk Communications Advisory Committee 风险交流咨询委员会risk evaluation (part of risk assessment) 风险评价risk/ benefit analysis 风险 -获益分析risk-benefit ratio 效益 / 风险比route of administration 给药途径royalties 专利使用费RPN = Risk Priority Number 风险优先指数RR = Response rate 缓解率RSD = (intra-day and inter-day) relative standard deviations (日内和日间 ) 相对标准差Ssafety advisory 安全建议safety evaluation 安全性评价safety evaluators 安全性评估人员safety set 安全性评价的数据集screening trials 筛选性试验SD = standard deviation 标准 (偏 )差SE = substantial equivalence 实质上的等同Seal Strength Test 密封强度试验sequence 试验次序SFDA 129= State Food And Drug Administration 国家食品药品监督管理局SG & A= Sales, General and Administration 销售、管理和一般费用shaft 传动轴SHEA = Society for Healthcare Epidemiology of America 美国医院流行病学学会sheaths 护套shelf life 保存期限 ; 保质期SIC codes = Standard Industrial Classification codes 标准产业分类代码side effects 副作用significance level 显著性水平Significant Risk (SR) 显著的危险性simple randomization 简单随机simulation model 仿真模型single blinding 单盲single-blind study 单盲研究single-masked study 单盲研究site assessment = SA 现场评估site audit 试验机构稽查SMDA = Safe Medical Devices Act of 1990 1990 年安全医疗器械法SMF = Site Master File 生产场所主文件sNDA = supplemental NDA 疗效补充新药上市申请sponsor-investigator = SI 申办研究者spontaneous reports; voluntary reports 药品不良反应自愿报告SPS = Agreement on the Application Of Sanitary and Phytosanitary Measures卫生与植物卫生措施实施协议;简称 SPS协议SSI = surgical site infection 手术部位感染SSOPs = Sanitation Standard Operating Procedures 卫生标准操作程序standard curve 标准曲线standard deviation 标准偏差standard drug 标准药物standard operating procedure = SOP 标准操作规程standard treatment 标准治疗Standards Of Care131 医护标准State Food and Drug Administration = SFDA 国家食品药品监督管理局statistic 统计量statistical analysis plan = SAP 统计分析计划statistical model 统计模型statistical significance 统计学意义statistical tables 统计分析表Statisticians in the Pharmaceutical Industry = PSI 制药业统计学家协会steady-state Area Under the Curve = AUCss稳态药时曲线下面积/ 稳态血药浓度-时间曲线下面积stratified 分层study audit 研究稽查study endpoint 研究终点Study Personnel List = SPL 研究人员名单study site 研究中心study type 研究类型subchronic toxicity studies 亚慢性毒性研究subgroup 亚组sub-investigator 助理研究者subject 受试者subject diary = SD 受试者日记subject enrollment 受试者入选subject enrollment log = SEL 受试者入选表Subject Identification Code List = SIC 受试者识别代码表subject recruitment 受试者招募subject screening log = SSL 受试者筛选表submission 申报;递交subspecialties, internal medicine 亚专科 ,内科substantial equivalence to legally marketed (predicate) device 和已合法在市场上销售的且不需要做 PMA“销售前批准”的相似产品有实质上的等同Ttrain-the-trainer program 培训者培训计划treatment group 试验组treatment IND 治疗性试验性新药申请treatment trials 治疗性试验trial error 试验误差trial initial meeting 试验启动会议trial master file 试验总档案trial objective 试验目的trial site 试验场所TRICARE 军队医疗系统triple blinding 三盲two one-side test 双单侧检验UAE = unexpected adverse event 预料外不良事件unblinding 破盲 ;揭盲under reporting bias 少报偏差unexplained syncope 不明原因晕厥unresectable 不能手术切除variability 变异variable 变量WHO International Collaborating Center for Drug Monitoring(世界卫生组织 )国际药物监测合作中心WHO International Conference of Drug Regulatory Authorities = WHO-ICDRAWHO 国际药品管理当局会议WHO Programme for International Drug Monitoring = PIDMWHO 国际药物监测合作计划。
