聚多巴胺-纳米金修饰玻碳电极检测芦丁
电化学法对珍菊降压片中芦丁的测定

电化学法对珍菊降压片中芦丁的测定
谢靓;付艳;金晶;李群芳
【期刊名称】《化学传感器》
【年(卷),期】2016(036)003
【摘要】目的:建立电化学法对珍菊降压片中芦丁的含量测定方法.方法:将碱法恒电位法活化的玻碳电极置于氯金酸溶液中在-0.2V条件下恒电位沉积一层纳米金,发现电极对芦丁有良好的电化学响应.结果:在最优条件下对芦丁标准溶液进行测定,当芦丁浓度在0.02~ 12 μmol/L与DPV峰电流呈良好的线性关系,检测限为
0.007.μmol/L(S/N=3).结论:该方法准确、可靠,可用于珍菊降压片中芦丁含量的测定.
【总页数】5页(P58-62)
【作者】谢靓;付艳;金晶;李群芳
【作者单位】湖北民族学院化学与环境工程学院,湖北恩施445000;湖北民族学院化学与环境工程学院,湖北恩施445000;湖北民族学院化学与环境工程学院,湖北恩施445000;湖北民族学院化学与环境工程学院,湖北恩施445000
【正文语种】中文
【相关文献】
1.电化学法对苦荞茶中芦丁含量的测定 [J], 胡海洋;陈红艳
2.HPLC同时测定珍菊降压片中氢氯噻嗪与芦丁的含量 [J], 代龙
3.珍菊降压片中芦丁的含量测定 [J], 姜慧祯;林娜
4.高效液相色谱法测定珍菊降压片中氢氯噻嗪和芦丁的含量 [J], 周汉升
5.珍菊降压片中芦丁和蒙花苷的含量测定方法研究 [J], 潘玉华;刘佳丽
因版权原因,仅展示原文概要,查看原文内容请购买。
聚多巴胺-纳米金修饰玻碳电极检测芦丁

聚多巴胺-纳米金修饰玻碳电极检测芦丁张英;张述林;任旺;蔡述兰;蒲勤;何华锋;凌淋;吴路宇;钟宵宇【期刊名称】《化学研究与应用》【年(卷),期】2013(000)009【摘要】采用一锅法制备聚多巴胺-纳米金修饰玻碳电极(PDA-AuNPs/GCE),用扫描电子显微镜(SEM)对修饰电极进行表面形貌分析,并研究芦丁在该修饰电极上的电化学行为。
实验表明,PDA-AuNPs/GCE对芦丁有较好的电催化氧化性能,芦丁的氧化峰电流与其浓度在1.0×10-6~1.0×10-4 mol· L-1范围内成线性关系,检测下限为2.3×10-7mol· L-1(S/N=3)。
该修饰电极可用于复方芦丁片中芦丁含量的检测,效果良好。
%The polydopamine-gold nanoparticle modified glassy carbon electrode ( PDA-AuNPs/GCE ) was fabricated by one-pot method.The surface morphology of modified electrode was characterized by scanning electron microscopy ( SEM).The electrochemi-cal behavior of rutin at the modified electrode was studied ,and the experimental results indicated that the proposed sensor exhibits good electro-catalytic activity towards the oxidation of rutin.The oxidative peak currents(ipa)increase linearly with the concentration of rutin from 1.0 ×10-6 to 1.0 ×10-4 mol· L-1 with a detection limit of 2.3 ×10-7 mol· L-1.The modified electrode can be applied to the analysis of rutin in complex rutin tablet with good results.【总页数】4页(P1299-1302)【作者】张英;张述林;任旺;蔡述兰;蒲勤;何华锋;凌淋;吴路宇;钟宵宇【作者单位】四川理工学院化学与制药工程学院,四川自贡 643000; 绿色催化四川省高校重点实验室,四川自贡 643000;四川理工学院材料与化学工程学院,四川自贡 643000;四川理工学院化学与制药工程学院,四川自贡 643000; 绿色催化四川省高校重点实验室,四川自贡 643000;四川理工学院化学与制药工程学院,四川自贡 643000;四川理工学院化学与制药工程学院,四川自贡 643000;四川理工学院化学与制药工程学院,四川自贡 643000;四川理工学院化学与制药工程学院,四川自贡 643000;四川理工学院化学与制药工程学院,四川自贡 643000;四川理工学院化学与制药工程学院,四川自贡 643000【正文语种】中文【中图分类】O657.1【相关文献】1.聚(三聚氰胺)与金纳米粒共修饰玻碳电极用于芦丁的电化学测定 [J], 冯利彬;齐崴;苏荣欣;何志敏2.吲哚乙酸在纳米金/碳纳米管/壳聚糖修饰玻碳电极上的电化学行为及其检测 [J], 张学钰;刘兴梅;刘伟禄;杨明;张志权3.纳米金/碳纳米管/聚硫堇修饰玻碳电极检测甲基对硫磷 [J], 李振;罗启枚;刘登友;王辉宪;周华;王玲4.二氧化铈纳米棒修饰玻碳电极的制备及其对芦丁的检测 [J], 汪美芳;张伟;方宾5.芦荟大黄素在石墨烯/聚多巴胺/金复合纳米材料修饰电极上的电化学行为及检测[J], 阳敬;兰慧;吴其国;蓝伦礼;庄晨曦;赵佳因版权原因,仅展示原文概要,查看原文内容请购买。
聚多巴胺微纳米球制备及金属纳米粒子修饰研究

聚多巴胺微纳米球制备及金属纳米粒子修饰研究一、本文概述本文旨在探讨聚多巴胺微纳米球的制备方法,以及如何通过金属纳米粒子修饰来进一步优化其性能。
聚多巴胺微纳米球作为一种具有优良生物相容性和多功能性的材料,在生物医学、药物递送、生物传感器等领域具有广泛的应用前景。
然而,其性能的提升和功能的拓展仍需要深入的研究。
因此,本文将对聚多巴胺微纳米球的制备过程进行详细阐述,同时探讨金属纳米粒子修饰对其性能的影响,以期为未来聚多巴胺微纳米球的应用提供理论和实践指导。
本文将介绍聚多巴胺微纳米球的基本性质和应用背景,为后续研究提供理论基础。
接着,将详细介绍聚多巴胺微纳米球的制备方法,包括原料选择、反应条件优化等方面,以便读者能够了解并重复实验。
在此基础上,本文将探讨金属纳米粒子修饰对聚多巴胺微纳米球性能的影响,包括金属纳米粒子的种类、尺寸、修饰方法等因素对聚多巴胺微纳米球性能的影响。
本文将总结研究成果,展望未来的研究方向和应用前景,为相关领域的研究者提供参考和借鉴。
二、材料与方法本研究所使用的主要材料包括多巴胺盐酸盐、聚乙烯吡咯烷酮(PVP)、乙醇、去离子水等。
多巴胺盐酸盐是制备聚多巴胺微纳米球的关键原料,其含有的儿茶酚基团和氨基基团可以在碱性环境下自聚合形成聚多巴胺。
PVP作为表面活性剂,有助于控制微纳米球的尺寸和形态。
乙醇和去离子水用于配制反应溶液。
将多巴胺盐酸盐溶解在去离子水中,加入适量的乙醇和PVP,搅拌均匀。
然后,将混合溶液在室温下搅拌一定时间,使多巴胺盐酸盐充分自聚合形成聚多巴胺微纳米球。
通过离心和洗涤得到纯净的聚多巴胺微纳米球。
为了对聚多巴胺微纳米球进行金属纳米粒子的修饰,我们首先选择了几种常见的金属盐,如银氨溶液、氯化金等。
将聚多巴胺微纳米球分散在含有金属盐的水溶液中,利用聚多巴胺中的儿茶酚基团对金属离子的强吸附能力,使金属离子在微纳米球表面还原成金属纳米粒子。
通过控制反应条件和金属盐的种类,可以实现对聚多巴胺微纳米球表面金属纳米粒子的种类、大小和分布的控制。
金纳米管阵列修饰玻碳电极用于示差脉冲伏安法测定多巴胺

金纳米管阵列修饰玻碳电极用于示差脉冲伏安法测定多巴胺徐国良;李羚;杨光明【期刊名称】《理化检验-化学分册》【年(卷),期】2012(048)012【摘要】以聚碳酸酯模板为工作电极,采用电沉积法从氯金酸和高氯酸溶液中制得金纳米管。
将沉积了金纳米管的模板固定在玻碳电极表面,用氯仿溶解7min将模板溶解。
制得了金纳米管阵列修饰电极,采用循环伏安法和微分示差脉冲伏安法研究了多巴胺在修饰电极上的电化学行为,结果表明:多巴胺在该电极上有一对氧化还原峰,提出了示差脉冲伏安法测定多巴胺的方法。
在电位+0.170V处,多巴胺的氧化峰电流与其浓度在4.95×10^-7~9.9×10^-2mol·L^-1范围内呈线性关系,方法的检出限(3σ)为1.06×10^-8mol·L^-1。
应用该修饰电极测定人尿样品中多巴胺含量,加标回收率在96.9%~101.4%之间,相对标准偏差(n=5)在3.1%~4.2%之间。
【总页数】5页(P1470-1473,1477)【作者】徐国良;李羚;杨光明【作者单位】保山学院资源环境学院,保山678000;保山学院资源环境学院,保山678000;保山学院资源环境学院,保山678000【正文语种】中文【中图分类】O657.1【相关文献】1.以聚(3-己基噻吩)-石墨烯-Nafion修饰的玻碳电极为工作电极示差脉冲伏安法测定细颗粒物PM2.5中铅的含量 [J], 金党琴;龚爱琴;丁邦东;周慧;田连生;韩磊;黄文江2.石墨烯/纳米金复合修饰玻碳电极差示脉冲伏安法测定盐酸吗啡 [J], 常艳兵;刘艳玲;杨晓丽;何琼3.氧化锌纳米簇-金纳米颗粒-壳聚糖复合膜修饰电极用于示差脉冲伏安法测定吗啡[J], 陶满兰;谢宗元;罗娟娟;陈雅倩;华梅;杨云慧4.单壁碳纳米管Nafion复合膜修饰玻碳电极用于示差脉冲伏安法测定多巴胺 [J], 欧陵斌;韩立路;柏杨5.纳米修饰玻碳电极阳极示差脉冲伏安法测定盐酸异丙嗪 [J], 刘娜;胡效亚;王赤贞胤;郭荣因版权原因,仅展示原文概要,查看原文内容请购买。
利用聚中性红/碳纳米管修饰玻碳电极测定多巴胺

De t e r mi n a t i o n o f d o p a mi n e b y P o l y ( n e u t r a l r e d ) / Ca r b o n N a n o t u b e s mo d i i f d e
e l e c t r o d e
ZHOU Gu — z h e n , LI U ra n g , S UN Yu a n - x i
( C o l l e g e o f C h e mi s t r y a n d C h e mi c a l E n g i n e e r i n g , H u n a n Un i v e r s i t y o f A r t s a n d S c i e n c e , C h a n g d e 4 1 5 0 0 0 , C h i n a )
b u f e r s o l u t i o n( P B S p H =6 . 0 ) , C y c l i c V o l t a mme t r y( C V ) nd a D i f f e r e n t i a l P u l s e V o l t a mme g r o ms ( DP V ) w e r e u s e d t o s ud t y t h e e l e c t r o c h e mi c a l b e h a v i o r o f t h e P o l y ( n e u t r a l r e d l l / c r a b o n n no a t u b e s i f l m mo d i i f e d e l e c t r o d e s ( f o r s h o r t P NR / C N T E ) . T h e r e d u c t i o n p e a k c u r r e n t w a s p r o p o r a t i o n a l t o he t c o n c e n r t a t i o n o f d o p a mi n e( D A) o v e r t h e r ng a e s
基于聚亚甲基蓝导电膜的芦丁电化学传感器
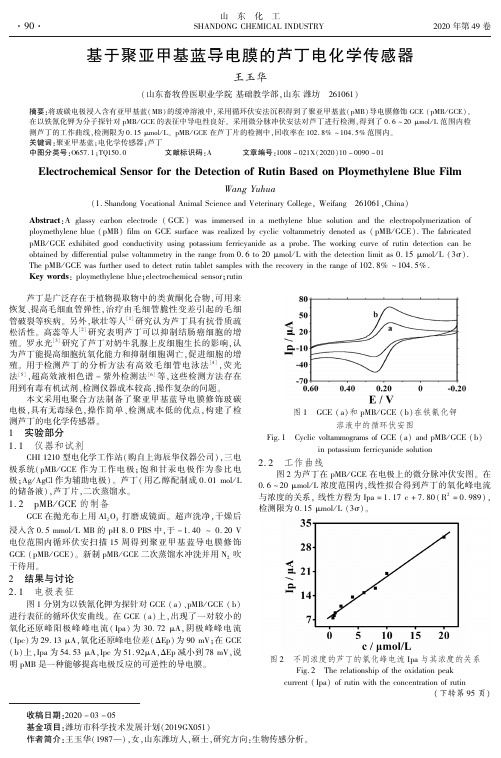
The pMB / GCE was further used to detect rutin tablet samples with the recovery in the range of 102. 8% ~ 104. 5% .
在以铁氰化钾为分子探针对 pMB / GCE 的表征中导电性良好ꎮ 采用微分脉冲伏安法对芦丁进行检测ꎬ得到了 0. 6 ~ 20 μmol / L 范围内检
测芦丁的工作曲线ꎬ检测限为 0. 15 μmol / Lꎮ pMB / GCE 在芦丁片的检测中ꎬ回收率在 102. 8% ~ 104. 5% 范围内ꎮ
本文采用电聚合方法制备了聚亚甲基蓝导电膜修饰玻碳
电极ꎬ具有无毒绿色ꎬ操作简单、检测成本低的优点ꎬ构建了检
测芦丁的电化学传感器ꎮ
1 实验部分
1. 1 仪器和试剂
CHI 1210 型电化学工作站( 购自上海辰华仪器公司) ꎬ三电
极系统( pMB / GCE 作为工作电极ꎻ 饱和甘汞电极作为参比电
极ꎻAg / AgCl 作为辅助电极) ꎮ 芦丁( 用乙醇配制成 0. 01 mol / L
干待用ꎮ
2 结果与讨论
2. 1 电极表征
图 1 分别为以铁氰化钾为探针对 GCE ( a) 、pMB / GCE ( b)
进行表征的循环伏安曲线ꎮ 在 GCE ( a) 上ꎬ出现了一对较小的
氧化还原峰阳极峰峰电流 ( Ipa) 为 30. 72 μAꎬ 阴 极 峰 峰 电 流
苝酐-纳米金修饰电极同时检测抗坏血酸、多巴胺和尿酸的研究

苝酐-纳米金修饰电极同时检测抗坏血酸、多巴胺和尿酸的研究归国风;宋鹏;孙小媛【摘要】多孔结构的3,4,9,10-苝四甲酸二酐(PTCDA)作为骨架,用抗坏血酸做还原剂制备纳米金(GNPs),制备了高催化活性的PTC-GNPs复合纳米材料.将该材料用于玻碳电极的修饰(GCE),制得PTC-GNPs复合材料修饰的电极(PTC-GNPs/GCE).该修饰电极能够同时对尿酸(UA)、多巴胺(DA)和抗坏血酸(AA)进行检测.分别使用循环伏安法(CV)和差分脉冲伏安法(DPV)对UA、DA和AA和在修饰电极上的电化学行为进行研究.实验结果表明,在pH=5.0的PBS缓冲体系中,该修饰电极对UA、DA和AA的线性响应范围分别为0.002~0.462 mol/L、0.002~0.352 mol/L和0.04~1.54 mol/L.该传感在临床医学检测领域具有一定的应用前景.【期刊名称】《化学传感器》【年(卷),期】2014(034)004【总页数】5页(P34-38)【关键词】苝四甲酸二酐;纳米金;多巴胺;尿酸;抗坏血酸;伏安法【作者】归国风;宋鹏;孙小媛【作者单位】贵州工程应用技术学院化学工程学院,贵州毕节551700;贵州工程应用技术学院化学工程学院,贵州毕节551700;贵州工程应用技术学院化学工程学院,贵州毕节551700【正文语种】中文生物活性小分子物质在哺乳动物及人类体内发挥着重要的作用。
不同的活性小分子物质在细胞信号转导、基因表达调控和生物学效应上形成复杂的调节网络,承担着调节机体稳态的重要使命。
目前,在电化学领域研究比较多的生物活性小分子物质主要有H2O2[1],多巴胺(DA)[2],抗坏血酸(AA)[3],尿酸(UA)[4]等。
其中DA是一种重要的神经递质[5],UA是人体最基础的代谢产物[6],AA是维持人体健康必需的维生素[7],三者作为生物活性小分子同时存在于体液当中,其含量对于人体的健康有着极大的影响。
金纳米修饰电极 电化学检测

金纳米修饰电极电化学检测金纳米修饰电极是一种常用的电化学检测方法,它能够提高电极的灵敏度和稳定性,广泛应用于生物传感器、环境监测和医学诊断等领域。
本文将从人类视角出发,描述金纳米修饰电极的原理、制备方法以及应用前景。
一、原理金纳米修饰电极利用纳米金颗粒的独特性质,增加了电极表面的活性区域,提高了电化学反应的速率和效率。
金纳米颗粒具有较大的比表面积和良好的导电性,可以提供更多的反应位点和电子传递通道,从而增强了电极的灵敏度。
此外,金纳米颗粒还具有优良的生物相容性和生物亲和性,可用于固定生物分子,实现生物传感器的构建。
二、制备方法金纳米修饰电极的制备方法多种多样,常见的方法包括溶液法、溶胶-凝胶法和电化学沉积法等。
其中,溶液法是最常用的方法之一。
首先,将金盐加入溶液中,通过还原剂将金离子还原成金纳米颗粒,然后将金纳米颗粒沉积在电极表面。
通过控制反应条件和处理参数,可以调节金纳米颗粒的尺寸和分布,从而优化电极的性能。
三、应用前景金纳米修饰电极具有广阔的应用前景。
在生物传感器领域,金纳米修饰电极可以用于检测生物分子,如蛋白质、核酸和细胞等,具有高灵敏度和高选择性。
在环境监测领域,金纳米修饰电极可以用于检测重金属离子、有机污染物和环境激素等,具有快速、准确和便捷的特点。
在医学诊断领域,金纳米修饰电极可以用于检测生物标志物,如血糖、胆固醇和肿瘤标志物等,有助于早期诊断和治疗。
金纳米修饰电极是一种重要的电化学检测方法,具有很大的应用潜力。
通过合理设计和制备,可以获得高性能的金纳米修饰电极,为生物传感器、环境监测和医学诊断等领域的研究提供有力支持。
相信在不久的将来,金纳米修饰电极将在多个领域展现出更加广阔的应用前景。
【35】金修饰在聚咪唑修饰的玻碳电极同时检测多巴胺尿素等

Analytica Chimica Acta 741 (2012) 15–20Contents lists available at SciVerse ScienceDirectAnalytica ChimicaActaj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /a caSimultaneous determination of ascorbic acid,dopamine,uric acid and tryptophan on gold nanoparticles/overoxidized-polyimidazole composite modified glassy carbon electrodeCun Wang,Ruo Yuan ∗,Yaqin Chai,Shihong Chen,Fangxin Hu,Meihe ZhangEducation Ministry Key Laboratory on Luminescence and Real-Time Analysis,College of Chemistry and Chemical Engineering,Southwest University,Chongqing 400715,Chinah i g h l i g h t sThe electropolymerization of imida-zole (Im)on GCE was first reported. The PIm film can be overoxidized to form the overoxidized polyimidazole (PImox).PImox allows dispersing of Au and generates additional electrocatalytic sites. The overlapping voltammetric response of AA,DA,UA and Trp is well-resolved.g r a p h i c a la b s t r a c tElectropolymerization of Im on the GCE,the PIm modified electrode was denoted as PIm/GCE.Subse-quently,the PIm/GCE was washed with doubly distilled water,and then transferred to 0.1M PBS (pH 4.0)for electrochemical oxidation at +1.8V for 250s.The obtained electrode was denoted as PImox/GCE (Fig.A).Then,the deposition of GNPs on PImox/GCE was carried out.The obtained electrode was described as GNPs/PImox/GCE (Fig.B).a r t i c l ei n f oArticle history:Received 13March 2012Received in revised form 18June 2012Accepted 19June 2012Available online 4 July 2012Keywords:ImidazoleGold nanoparticles Ascorbic acid Dopamine Uric acid Tryptophana b s t r a c tA novel electrode was developed through electrodepositing gold nanoparticles (GNPs)on overoxidized-polyimidazole (PImox)film modified glassy carbon electrode (GCE).The combination of GNPs and the PImox film endowed the GNPs/PImox/GCE with good biological compatibility,high selectivity and sensi-tivity and excellent electrochemical catalytic activities towards ascorbic acid (AA),dopamine (DA),uric acid (UA)and tryptophan (Trp).In the fourfold co-existence system,the peak separations between AA–DA,DA–UA and UA–Trp were large up to 186,165and 285mV,respectively.The calibration curves for AA,DA and UA were obtained in the range of 210.0–1010.0M,5.0–268.0M and 6.0–486.0M with detection limits (S/N =3)of 2.0M,0.08M and 0.5M,respectively.Two linear calibrations for Trp were obtained over ranges of 3.0–34.0M and 84.0–464.0M with detection limit (S/N =3)of 0.7M.In addition,the modified electrode was applied to detect AA,DA,UA and Trp in samples using standard addition method with satisfactory results.© 2012 Elsevier B.V. All rights reserved.1.IntroductionAscorbic acid (AA)is very popular for its antioxidant property,and present in the human diet as a vital vitamin.Moreover,it is∗Corresponding author at:College of Chemistry and Chemical Engineer-ing,Southwest University,Chongqing Key Laboratory of Analytical Chemistry,Chongqing,400715,PR China.Tel.:+862368252277;fax:+862368253172.E-mail address:yuanruo@ (R.Yuan).also used for the prevention and treatment of common cold,men-tal illness,infertility,cancer and AIDS [1].Dopamine (DA)is one of the most significant catecholamines.It plays a very important role in the functioning of the central nervous,cardiovascular,renal and hormonal systems as well as in drug addiction and Parkin-son’s disease [2].Uric acid (UA)is the primary end product of purine metabolism.Abnormal levels of UA are symptoms of several diseases,including gout,hyperuricemia and Lesch Nyan disease [3,4].Tryptophan (Trp)is an essential amino acid for human and0003-2670/$–see front matter © 2012 Elsevier B.V. All rights reserved./10.1016/j.aca.2012.06.04516 C.Wang et al./Analytica Chimica Acta741 (2012) 15–20herbivores[5].In addition,it has been implicated as a possible cause of schizophrenia in people who cannot properly metabo-lize it[6].It is known to all,AA,DA,UA and Trp usually coexist in biological samples.Therefore,a sensitive and selective method for their simultaneous determination is highly desirable for analyt-ical application and diagnostic research.A major problem for their simultaneous determination is that AA,DA,UA and Trp undergo the same potential at conventional electrodes with a pronounced foul-ing effect,resulting in poor reproducibility.Besides,many reported detection methods were complicated,expensive,and suffered from sensitivity as well as selectivity.To overcome these problems,many materials have been employed to modify electrodes,such as gold nanocluster/overoxidized-polypyrrole composite[7]and iron(III) doped zeolite[5].Recently,polymerfilm and metal nanoparticles have been arose great attentions due to their wide applications in thefields of chemical modified electrodes[8,9].As is well-known,metal nanoparticles especially gold nanoparticles(GNPs)can enhance the conductivity,facilitate the electron transfer and improve the detection limit of electrode[10].Among various types of conduc-tive polymers[11–14],polyimidazole(PIm)has many attractive features,for example thefilm is very stable and hard to be taken off from the surface unless the electrode is heavily polished.It has high selectivity and sensitivity due to the chemically stable homogeneousfilm with controlled thickness[12].Furthermore, the PImfilm can be further overoxidized at higher potentials to form the overoxidized-polyimidazole(PImox),resulting in an insulating membrane with lower background current.The overox-idizedfilm has large surface area because of the porous structure. Moreover,the porous structure of the PImoxfilm is in favor of dispersing metal nanoparticles into the polymer matrix and gener-ates additional electrocatalytic sites[7,15].Since the combination of the polymerfilms and metal nanoparticles not only signifi-cantly improve the electrocatalytic properties of substrates and the stability and reproducibility of the electrode but also decrease the overpotential and increase the reaction rate[14].For exam-ple,gold nanocluster/overoxidized-polypyrrole composite[7]and overoxidized poly(N-acetylaniline)[16]modified electrodes have been used for the determination of DA or serotonin.However, compared with the methods mentioned above,the modified electrode in our work exhibits its peculiar advantages,such as higher sensitivity,selectivity,reproducibility and relatively low cost.In this study,a novel electrode based on the PImoxfilm incorporated with GNPs was constructed for the simultaneous determination of AA,DA,UA and Trp.Due to the synergic effect between PImox and GNPs,the overlapping voltammetric response of AA,DA,UA and Trp is well-resolved from each other with lowered oxidation pared with the GNPs/GCE,PIm/GCE and PImox/GCE,the GNPs/PImox/GCE exhibited obviously enhanced responses towards AA,DA,UA and Trp.Furthermore,the practi-cal application was investigated using standard addition method and satisfactory results were obtained.2.Materials and methods2.1.ChemicalsChloroauric acid(HAuCl4)and imidazole(Im)were pur-chased from Sigma Chemical Co.(St.Louis,Mo,USA).Sodium dodecylsulfate(SDS),uric acid,tryptophan,ascorbic acid and dopamine were purchased from Chemical Reagent Co.(Chongqing, China).Phosphate buffer solutions(PBS)(0.1M)at various pH were prepared using0.1M Na2HPO4,0.1M KH2PO4,and 0.1M KCl.Double-distilled water was used throughout the experiments.2.2.ApparatusAll electrochemical experiments were performed with a CHI 660D electrochemical workstation(Shanghai Chenhua Instrument Co.,China).The conventional three-electrode system included a modified glassy carbon electrode(GCE)as working electrode,a platinum wire as auxiliary electrode and a saturated calomel electrode(SCE)as a reference electrode.The morphological char-acterization of thefilms was examined by scanning electron microscope(SEM,S-4800,Hitachi,Japan).All measurements were carried out at room temperature.2.3.Preparation of GNPs/PImox modified electrodeThe glassy carbon electrode(diameter4.0mm)was successively polished to a mirror using0.3and0.05m alumina slurry.After-ward,the electrode was washed thoroughly with ethanol and water and dried at room temperature.Electropolymerization of Im on the GCE was carried out by8 circles between–0.2and0.8V at0.1V s–1in0.1M SDS containing 0.1M Im[14].The SDS was used as assisted reagent and support-ing electrolyte for the electropolymerization of Im[14].Moreover, the SDS could form a complex with Im,resulting in porous struc-ture with a large surface area[14,17].Eight circles were chosen to deposit the Imfilm because the highest sensitivity towards the biomolecules was obtained under this case(Fig.1S).Subsequently, the PIm modified electrode(PIm/GCE)was washed with double distilled water,and then transferred to0.1M PBS(pH4.0)for elec-trochemical oxidation at+1.8V to250s,obtaining PImox modified electrode(PImox/GCE).The deposition of GNPs on PImox/GCE was carried out in a mixture of H2SO4(0.5M)and HAuCl4(1mM)at –0.2V for80s[9].The80s was the optimal deposition time accord-ing to our studies(Fig.2S).The obtained electrode was described as GNPs/PImox/GCE.For comparison,PIm/GCE,PImox/GCE and GNPs/GCE were pre-pared using the same procedure.3.Results and discussion3.1.SEM of the modified electrodeThe morphology of modifiedfilms was investigated by scanning electron microscopy(SEM).As shown in Fig.1A,PImoxfilm appears a comparatively smooth and homogeneous surface.However,after electrodepositing GNPs,flower-like nanostructures were observed in Fig.1B,indicating that GNPs were obtained.As seen from Fig.1B, the particle size of theflower-like GNPs was ranging from250to 450nm.Also,the GNPs look very pretty,and the keen-edged leaves make the surface of GNPs very rough and many leaves possess a large specific surface area.The results indicated that the PImox and GNPs/PImoxfilms were effectively immobilized on the electrode surface.3.2.The influence of pH on the oxidation of AA,DA,UA and Trp at the GNPs/PImox/GCEThe effect of pH value for the simultaneous determination of AA, DA,UA and Trp at GNPs/PImox/GCE was thoroughly investigated by differential pulse voltammetry(DPV).Fig.2showed the effect of pH value on the peak current and peak potential.From Fig.2A,the peak current of AA and Trp initially increased and reached a maximum value at pH4.0,then,another maximum value was observed at neu-tral pH with pH increasing.DA and UA give the highest response at pH4.0with pH increasing.The reason may be related to the change in electrostatic interaction between the four substances and GNPs/PImoxfilm.In Fig.2B,the linear regression equations for AA,C.Wang et al./Analytica Chimica Acta741 (2012) 15–2017Fig.1.SEM images of(A)PImox/GCE and(B)GNPs/PImox/GCE.DA,and Trp were obtained as:E DA(mV)=421.30–18.08pH,E UA (mV)=628.59–27.23pH and E Trp(mV)=789.21–17.37pH.All the peak potentials of AA,DA,UA and Trp shifted to more negative val-ues with the pH increasing.This is a consequence of a deprotonation step involved in all oxidation processes that is facilitated at higher pH values[18].AA(p K a=4.10),DA(p K a=8.87),UA(p K a=5.7)and Trp(p K a=5.89)exist as cationic form in pH4.0PBS(0.1M)[12]. Moreover,overoxidation in the Im ring leads to effective collection of the cationic species[7,19].It was expected that cationic forms of AA,DA,UA and Trp were electrostatically interacted with the poly-mer backbone at pH4.0[12].In addition,the maximum separation of peak potentials for AA–DA,DA–UA and UA–Trp is observed at pH 4.0.In order to obtain a high sensitivity and selectivity,pH4.0PBS (0.1M)was selected for further experiments.3.3.Cyclic voltammetric behaviors of the modified electrodeFig.3A showed the cyclic voltammograms(CVs)of different modified electrodes in0.1M PBS(pH4.0).As seen from Fig.3A, PImox/GCE(curve b)showed almost the same background current with the bare GCE(curve a),indicating anodic polarization at+1.8V maybe turn the PImfilm into an insulating PImoxfilm with the loss of electroactivity[20].However,after modified with GNPs,the background signal increased for GNPs/PImox/GCE(curve c),and this may be resulted from the larger effective surface area of GNPs. The inset Fig.3A showed the electropolymerization process.The Fig.2.Effect of pH on(A)the peak current and(B)the peak potential for the oxi-dation of150M AA,15M DA,80M UA and80M Trp in0.1M PBS(pH4.0). DPV conditions:scan rate,20mV s–1;amplitude,50mV;pulse width,50ms;pulse period,200ms.anodic peak potentials tended to be stable after8circles,suggesting a self-adjustment of the polymerfilm thickness at the GCE[21,22].The ability of GNPs/PImox/GCE to promote the voltammetric resolution of AA,DA,UA and Trp was investigated.Control exper-iments for the simultaneous determination of AA,DA,UA and Trp were carried out by CV at PIm/GCE,PImox/GCE,GNPs/GCE and GNPs/PImox/GCE,respectively.As can be seen from Fig.3B, PIm/GCE(curve a)and GNPs/GCE(curve c)only showed a broad and overlapped anodic peaks in the fourfold mixture.On the PImox/GCE (curve b)three peaks appeared at295,485and795mV for DA, UA and Trp,respectively,but simultaneous determination of AA, DA,UA and Trp could not be obtained due to the indistinguish-able and small response to AA.In contrast,the modification of the GCE surface with GNPs/PImoxfilm(curve d)resolved the merged voltammetric peak into four well-defined peaks at poten-tials around136,320,485and770mV with a remarkable increase in peak current for AA,DA,UA and Trp,respectively.The reasons for the oxidation of AA,DA,UA and Trp at a wide potential sep-aration are ascribed as follows:Firstly,the GNPs enhanced the catalytic activity of PImox/GCE by increasing the peak current due to the increasing electronic conductivity and effective surface area [21].Secondly,during the overoxidization process,higher density of groups such as C O,COO−,C OH and OH can be generated on the backbone of PImoxfilm.The existence of C O,COO−,C OH and OH on the PImoxfilm could provide a selective interface18 C.Wang et al./Analytica Chimica Acta 741 (2012) 15–20Fig.3.(A)CV responses of (a)bare GCE,(b)PImox/GCE and (c)GNPs/PImox/GCE in 0.1M PBS (pH 4.0).Inset of part (A)shows the CVs of polymerization of Im (0.1M)in 0.1M SDS solution on GCE in the potential range from –0.2to 0.8V.Scan rate,0.1V s –1;8circles.(B)CVs at (a)PIm/GCE,(b)PImox/GCE,(c)GNPs/GCE and (d)GNPs/PImox/GCE in 0.1M PBS (pH 4.0)containing 200M AA,20M DA,50M UA and 50M Trp.for the molecular interaction of AA,DA,UA and Trp via hydro-gen bonds with the proton-donating group such as NH and OH [15,16,21].Thirdly,AA,DA,UA and Trp exist as cationic form at pH 4.0.In addition,overoxidation in the Im ring leads to an effective rejection of the anionic species and preferential collection of the cationic species [19,23].It was expected that protonated forms of AA,DA,UA and Trp were electrostatically interacted with the poly-mer backbone.Thus,it effectively catalyzed the oxidation of four substances at low pH.Fourthly,the selective promising feature of PImox film especially the composite with GNPs imparted superior selectivity and sensitivity towards the AA,DA,UA and Trp [13].In a word,the synergic effect of PImox film and the GNPs endowed the GNPs/PImox/GCE with a lower detection limit,wider linear range,better electricity and higher selectivity and sensitivity than that of other electrodes in the literature (Table 1).3.4.Simultaneous determination of AA,DA,UA and TrpDPV was employed to detect AA,DA,UA and Trp because of its higher current sensitivity and better resolution than CV.In four-fold mixture,the electro-oxidation processes of AA,DA,UA and Trp in the mixture were investigated when the concentration of one species changed,whereas those of other three species are kept con-stant.Fig.4A depicted the DPV of AA containing 50M UA,15M DA and 50M Trp.The peak current of AA increased linearly with an increase in AA concentration from 210.0to 1010.0M,with theT a b l e 1C o m p a r i s o n o f t h e r e s p o n s e c h a r a c t e r i s t i c s o f d i f f e r e n t m o d i fie d e l e c t r o d e s .E l e c t r o d e m a t e r i a l sL i n e a r r a n g e (M )D e t e c t i o n l i m i t (M )P e a k p o t e n t i a l (V )R e f e r e n c eA AD AU AT r pA AD AU AT r pA AD AU AT r pF e 3+Y /Z C M E 0.6–100–0.3–7000.2–1500.21–0.080.06––––[5]M W C N T –F e N A Z –C H 7.77–8337.35–8330.23–83.30.074–34.51.111.050.0330.011––––[6]G N P /c h o l i n e –0.2–801.2–100––0.120.6––0.230.37–[9]2-a m i n o -1,3,4-t h i a d i a z o l e 30–3005–5010–100–2.010.330.19–0.200.330.49–[12]O v e r o x i d i z e d p o l y (N -a c e t y l a n i l i n e )–0.50–20.0–––0.0168–––0.12––[24]p o l y -c h r o m o t r o p e 2B –2.0–80.0–––0.30–––0.349––[25]G N P s /P I m o x210.0–1010.05.0–268.06.0–486.03.0–34.084.0–464.02.00.080.50.70.1360.3200.4850.770T h i s w o r kC.Wang et al./Analytica Chimica Acta741 (2012) 15–2019Fig.4.DPVs at the GNPs/PImox/GCE in0.1M PBS(pH4.0)(A)containing50M UA,15M DA,50M Trp and different concentrations of AA(from inner to outer):210, 310,460,610,710,810,960,1010M;(B)containing100M AA,50M UA,50M Trp and different concentrations of DA(from inner to outer):5,10,18,28,38,88,168, 268M;(C)containing100M AA,15M DA,50M Trp and different concentrations of UA(from inner to outer):6,11,36,86,166,266,366,486M and(D)containing 150M AA,20M UA,5M DA and different concentrations of Trp(from inner to outer):3,6,11,19,34,84,164,314,464M.Insets:plots of Ip vs.concentration for AA, DA,UA and Trp,respectively.linear function I p,AA(A)=23.02+0.01584C AA(M)(R=0.9975),and a detection limit of2.0M(S/N=3).Fig.4B illustrated the DPVof DA containing100M AA,50M UA and50M Trp.The linearrelationship between the peak current and the concentration of DAwas obtained in the concentration range of5.0to268.0M.Thelinear function was expressed as I p,DA(A)=17.26+0.07882C DA(M)(R=0.9827),and a detection limit of0.08M(S/N=3)wasobtained.Similarly,Fig.5C depicted the DPV response of UAinFig.5.DPVs of simultaneous determination of AA,DA,UA and Trp using GNPs/PImox modified GCE in0.1M PBS(pH4.0).Concentrations of the four compounds:AA(60, 120,180,280,380,480,680,920M),DA(5,10,15,20,25,30,40,60M),UA(5, 10,15,20,28,36,54,63M)and Trp(5,10,15,20,25,30,40,60M).the presence of100M AA,15M DA and50M Trp,which exhibited a linear relationship in the concentration range of6.0–486.0M,and its linear regression equation was defined asI p,UA(A)=10.59+0.03220C UA(M)(R=0.9898).The detection limit of0.5M(S/N=3)for UA was observed.Fig.5D illustrated the DPV of Trp containing50M AA,20M UA and5M DA.Two lin-ear calibration ranges of3.0–34.0M,with the linear function I p,Trp (A)=9.549+0.2414C Trp(M)(R=0.9362)and84.0–464.0M, and the linear function I p,Trp(A)=18.18+0.03716C Trp(M) (R=0.9632)were obtained for the Trp determination.The detec-tion limit was found to be0.7M(S/N=3).The relative standard deviations(RSD)for determining AA,DA,UA and Trp(n=10)were 7.09%,4.37%,5.67%and2.23%,respectively.The drift of the oxida-tion peaks of AA,DA,UA and Trp were observed for further addition of the respective analytes.In addition,DPVs were also performed at GNPs/PImox/GCE while changing the concentrations of AA,DA, UA and Trp simultaneously(Fig.5).3.5.Interferences,stability and reproducibilityIn order to evaluate the ability of anti-interference,several com-pounds were selected.It was found that no significant interference for the detection of AA(150M),DA(10M),UA(50M)and Trp(50M)was observed for these compounds:NaCl,KCl,KNO3, CaCl2,MgSO4,ZnCl2,methenamine,ethylenediaminetetraacetic acid disodium salt,glucose,l-cysteine,glutathione,folic acid and levodopa.Stability of the GNPs/PImox/GCE was also investigated. The modified electrode was stored at room temperature when not used.The response of the electrode lost approximately6.3%,20 C.Wang et al./Analytica Chimica Acta741 (2012) 15–20Table2Determination of AA,DA,UA and Trp in dopamine hydrochloride injection,vitamin C tablets,human urine and serum samples.Sample Detected a(M)Added(M)Found a(M)Recovery(%) Urine1Uric acid36.3±1.710.045.0±1.397.2Ascorbic acid40.039.8±1.199.5Dopamine0.50.5±0.02100.0tryptophan30.030.5±0.7101.7Urine2Uric acid26.8±1.120.046.4±0.999.1Ascorbic acid80.081.3±2.4101.6Dopamine 2.5 2.4±0.196.0tryptophan40.040.1±1.0100.3 Serum1Uric acid12.1±0.415.026.8±0.698.9Ascorbic acid100.0100.2±3.7100.2Dopamine 2.0 1.9±0.195.0tryptophan50.050.2±1.8100.4 Serum2Uric acid13.6±0.620.035.0±0.9104.2Ascorbic acid150.0148.2±4.598.8Dopamine 1.0 1.0±0.04100.0tryptophan40.039.8±1.099.5 Vitamin C tablets Ascorbic acid399.0±10.250.0487.7±19.2108.6Dopamine 1.0 1.0±0.08100.0Uric acid10.09.9±0.699.0tryptophan40.039.0±1.197.5 Dopamine injection Ascorbic acid200.0207.7±4.9103.9Dopamine93.2±3.5 1.592.5±1.297.7Uric acid50.051.8±2.2103.6tryptophan50.051.3±1.8102.6All samples were analyzed using standard addition method(n=3).a Mean value±standard deviation(n=3).3.2%,5.6%and5.4%for AA,DA,UA and Trp of its original response after13days,respectively.The relative standard deviation(RSD) (n=10)for all these species was less than7.09%.Thus,the pro-posed electrode showed a high stability and good reproducibility and anti-interference ability.3.6.Sample analysisDopamine hydrochloride injection,vitamin C tablets,human urine and serum samples were selected for analysis using the stan-dard addition method.All samples were diluted with0.1M PBS (pH4.0)before the measurements to prevent the matrix effect of authentic samples.Then,appropriate amount of each sample was added into5mL0.1M PBS(pH4.0),respectively.To ascer-tain the correctness of the results,the samples were spiked with certain amounts of standard AA,DA,UA and Trp and then the total values were detected using DPV.The results were listed in Table2.The recovery rates of the samples ranged between95.0% and108.6%,showing that the proposed method could be effectively used for the determination of AA,DA,UA and Trp in commercial samples.4.ConclusionsIn conclusion,a novel GNPs/PImox/GCE was constructed to determine AA,DA,UA and Trp in this work.The modified elec-trode not only showed high selectivity towards the oxidation of AA, DA,UA and Trp,but also resolved their overlapped oxidation peaks into four well-defined peaks,respectively.This was attributed to a decrease of the reduction potential ability of PImox,the very high electron transfer rate of GNPs that can remarkable increase in peak current towards the compounds,and the remarkable syner-gistic effects of PImox and GNPs.The simple fabrication procedure, low detection limit,high selectivity,good stability and sensitivity, suggest that this modified electrode is an attractive candidate for practical applications.AcknowledgmentsThe National Natural Science Foundation of China(21075100), the Ministry of Education of China(Project708073),the Nature Science Foundation of Chongqing City(CSTC-2009BA1003, 2011BA7003)and State Key Laboratory of Electroanalytical Chem-istry(SKIEAC2010009),China supported this work.Appendix A.Supplementary dataSupplementary data associated with this article can be found,in the online version,at /10.1016/j.aca.2012.06.045. References[1]N.F.Atta,M.F.El-Kady,G.Ahmed,Anal.Biochem.400(2010)78–88.[2]J.W.Mo,B.Ogorevc,Anal.Chem.73(2001)1196–1202.[3]P.Elena,Y.Kubota,D.A.Tryk,A.Fujishima,Anal.Chem.72(2000)1724–1727.[4]J.M.Zen,P.J.Chen,Anal.Chem.69(1997)5087–5093.[5]A.Babaei,M.Zendehdel,B.Khalilzadeh,A.Taheri,Colloids Surf.B66(2008)226–232.[6]M.Noroozifar,M.Khorasani-Motlagh,R.Akbari,M.B.Parizi,Biosens.Bioelec-tron.28(2011)56–63.[7]J.Li,X.Q.Lin,Sens.Actuators B124(2007)486–493.[8]A.L.Liu,S.B.Zhang,W.Chen,X.H.Lin,X.H.Xia,Biosens.Bioelectron.23(2008)1488–1495.[9]P.Wang,Y.X.Li,X.Huang,L.Wang,Talanta73(2007)431–437.[10]S.Thiagarajan,S.M.Chen,Talanta74(2007)212–222.[11]K.C.Lin,T.H.Tsai,S.M.Chen,Biosens.Bioelectron.26(2010)608–614.[12]P.Kalimuthu,S.A.John,Talanta80(2010)1686–1691.[13]X.H.Jiang,X.Q.Lin,Anal.Chim.Acta537(2005)145–151.[14]Y.X.Li,P.Wang,L.Wang,X.Q.Lin,Biosens.Bioelectron.22(2007)3120–3125.[15]S.Ulubay,Z.Dursun,Talanta80(2010)1461–1466.[16]X.M.Tu,Q.J.Xie,S.Y.Jiang,S.Z.Yao,Biosens.Bioelectron.22(2007)2819–2826.[17]D.R.Albano,F.Sevilla,Sens.Actuators B121(2007)129–134.[18]B.Habibia,M.H.Pournaghi-Azar,Electrochim.Acta55(2010)5492–5498.[19]Z.D.Chen,Y.Takei,B.A.Deore,T.Nagaoka,Analyst125(2000)2249–2254.[20]L.S.Van Dyke,C.R.Martin,Langmuir6(1990)1118–1123.[21]A.A.Ensafi,M.Taei,T.Khayamian,A.Arabzadeh,Sens.Actuators B147(2010)213–221.[22]H.Yao,Y.Sun,X.Lin,Y.Tang,L.Huang,Electrochim.Acta52(2007)6165–6171.[23]M.S¸ahin,Y.S¸ahin,A.Özcan,Sens.Actuators B133(2008)5–14.[24]L.Z.Zheng,S.G.Wu,X.Q.Lin,L.Nie,L.Rui,Analyst126(2001)736–738.[25]X.H.Lin,Q.Zhuang,J.H.Chen,S.B.Zhang,Y.J.Zheng,Sens.Actuators B125(2007)240–245.。
聚多巴胺-氧化石墨烯修饰玻碳电极测定痕量Cu(Ⅱ)

聚多巴胺-氧化石墨烯修饰玻碳电极测定痕量Cu(Ⅱ)杨澜;王纯太;朱国栋;刘建允【期刊名称】《分析科学学报》【年(卷),期】2018(34)3【摘要】采用吸附自聚法制备聚多巴胺-氧化石墨烯复合材料修饰玻碳电极(PDA-GO/GCE),并研究了该修饰电极的电化学性能。
结果表明,在0.1mol/L的HAc-NaAc缓冲溶液中,最优实验条件下,于-0.9V电位富集10min,溶出峰电流与Cu(Ⅱ)的浓度在0.1~1.0μg/L和1.0~30.0μg/L范围内呈良好线性关系,线性相关系数为0.991和0.995,检出限为0.02μg/L。
采用标准加入法对水样中Cu(Ⅱ)进行测定,回收率达91.3%~96.9%,测量结果和电感耦合等离子体质谱法一致。
PDA-GO/GCE 具有良好选择性、稳定性和重现性,且制备简单、灵敏度高,可用于自来水和湖水中痕量Cu(Ⅱ)的检测。
【总页数】4页(P393-396)【关键词】氧化石墨烯;聚多巴胺;Cu(Ⅱ);方波溶出伏安法【作者】杨澜;王纯太;朱国栋;刘建允【作者单位】东华大学环境科学与工程学院国家环境保护纺织工业污染防治工程技术中心【正文语种】中文【中图分类】O657.15【相关文献】1.以聚(3-己基噻吩)-石墨烯-Nafion修饰的玻碳电极为工作电极示差脉冲伏安法测定细颗粒物PM2.5中铅的含量 [J], 金党琴;龚爱琴;丁邦东;周慧;田连生;韩磊;黄文江2.聚茜素红-还原态氧化石墨烯修饰玻碳电极测定苯二酚 [J], 朱超云;宋伟;许红林;姚琪3.纳米Cu2O-还原石墨烯复合修饰玻碳电极用于多巴胺的检测 [J], 贺全国;李广利;刘军;刘晓鹏;梁静;邓培红4.聚对氨基苯磺酸/石墨烯修饰玻碳电极伏安法测定痕量汞 [J], 许春萱;熊小琴;吴莹莹;马文杰5.聚苏木精/氧化石墨烯修饰玻碳电极测定水样中痕量铅和镉 [J], 许春萱;熊小琴;金紫荷;杨晶晶因版权原因,仅展示原文概要,查看原文内容请购买。
芦丁在石墨烯修饰电极上的电化学行为及其灵敏检测

芦丁在石墨烯修饰电极上的电化学行为及其灵敏检测刘坤平;李惠茗;何钢;颜军;郭晓强;赵琦;苟小军【期刊名称】《安徽农业科学》【年(卷),期】2013(041)010【摘要】[目的]研究了芦丁在石墨烯修饰电极上的电化学行为及其测定方法.[方法]采用化学还原法,制备了氨基功能化的石墨烯,并用于构建灵敏的芦丁电化学传感器.采用循环伏安法和差分脉冲法,研究芦丁在该修饰电极上的电化学行为,并用差分脉冲法对芦丁进行检测.[结果]高比表面积和高导电性的石墨烯使芦丁在该传感器上表现出增强的电化学活性.电极反应动力学研究表明,芦丁在该修饰电极表面经历了一个受表面控制的准可逆过程.在最优试验条件下,芦丁的还原峰电流与其浓度在2×10-8~1×10-5 mol/L范围内呈良好的线性关系,检出限(S/N=3)为1.0×10-8mol/L.[结论]该修饰电极具有良好的选择性和稳定性,可实现实际样品中芦丁含量的灵敏检测.【总页数】5页(P4238-4241,4349)【作者】刘坤平;李惠茗;何钢;颜军;郭晓强;赵琦;苟小军【作者单位】成都大学中药化学实验室,四川成都610106;成都大学生物产业学院,四川成都610106;成都大学生物产业学院,四川成都610106;成都大学中药化学实验室,四川成都610106;成都大学生物产业学院,四川成都610106;成都大学中药化学实验室,四川成都610106;成都大学生物产业学院,四川成都610106;成都大学中药化学实验室,四川成都610106;成都大学生物产业学院,四川成都610106;成都大学中药化学实验室,四川成都610106;成都大学中药化学实验室,四川成都610106;成都大学生物产业学院,四川成都610106【正文语种】中文【中图分类】S131;Q652.9【相关文献】1.鞣酸功能化石墨烯修饰电极上芦丁的电化学行为及灵敏检测 [J], 李惠茗;张惠怡;赖祥文;梁立;刘坤平;苟小军2.芦丁在Nafion/纳米金@石墨烯修饰碳糊电极上的电化学行为及测定 [J], 匡云飞;邹建陵;李薇;杨颖群;许金生;冯泳兰;李玉明3.DNA修饰电极的研究Ⅷ.1,10-菲咯啉存在时钴离子在ssDNA修饰金电极上的电化学行为及痕量钴的检测 [J], 庞代文;陆琪;赵元弟;张敏4.芦荟大黄素在石墨烯/聚多巴胺/金复合纳米材料修饰电极上的电化学行为及检测[J], 阳敬;兰慧;吴其国;蓝伦礼;庄晨曦;赵佳5.核黄素在电化学还原石墨烯/Nafion修饰电极上的电化学行为及分析检测 [J], 马琦;李坤;张素芳;宋金萍;郭永;董川因版权原因,仅展示原文概要,查看原文内容请购买。
芦丁在纳米金修饰玻碳电极上的电化学行为及其测定

一、 实验目的
1.初步掌握电化学工作站的使用方法,掌握循环伏安法和差分脉冲伏安法 的基本原理和测量技术 2. 通过对体系的测量,了解如何根据峰电流、峰电势及峰电势差和扫描速 度之间的函数关系来判断电极反应过程的可逆性, 以及求算有关的热力学参数和 动力学参数。 3. 学习固体电极表面的处理方法 二、 实验原理
七、思考题: 1. 在三电极体系中,工作电极、辅助电极和参比电极各起什么作用? 2. 若实验中测得的条件电位值和值与文献值有差异,试说明为什么? 3. 通过扫速与峰电流的关系,可以说明什么问题?
pa/V
ipa/A
pc/V
ipc/A
ipa/ipc
5. 考察峰电流与浓度的关系 在 15 mL 分别含芦丁标准液 0.1、0.2、0.5 、1.0、2.0 µM 的电解液中。其他 实验条件同上,分别记录从 0.8 ~ 0 V 扫描的差示脉冲伏安图,并作标准曲线。
五、注意事项: 1. 为了使液相传质过程只受扩散控制,应在加入电解质和溶液处于静止下
在脉冲施加前 20ms,只有电容电流 iC; 在脉冲期后 20ms, 所测电流为电解电 流和电容电流的和,DPV 是两次电流相减得到 Δi,因此杂质的氧化还原电流导 致的背景电流也被大大的扣除了,因而具有更高的检测灵敏度和更低的检出限, 使其能够应用于浓度低至 10-8mol/L(约 1µg/L)的场合。 纳米材料从兴起到现在,研究发展历程大致可分为以下 3 个阶段 :第一阶段 (18 世纪中期到 20 世纪 90 年代初) ,在美国巴尔的摩召开的首届国际纳米科学技 术会议标志着正式把纳米技术作为材料学科的一个新的分支 ; 第二阶段 (1990 — 1994 年) ,第二届国际纳米材料学术会议提出了对纳米材料微结构的研究应着眼 于对不同类型材料的具体描述;第三阶段(1994 年至现在) ,纳米材料的特点在于按 人们的意愿设计、组装和创造新的体系,即以纳米颗粒、纳米线和纳米管为基本 单元在一维、二维和三维空间组装纳米结构的体系。研究表明,纳米材料具有大 量的界面,界面原子可达到 50% 以上,使得纳米材料具有常规材料不具备的独特 性质,产生了四大效应:尺寸效应、量子效应、表面效应和界面效应。 纳米金是指金粒子直径在 1 ~100nm 之间的金材料,是最稳定的贵金属纳米 粒子之一。它属于介观粒子,具有特殊的电子结构,在一些特定的晶面上存在着表 面电子态,其费米能级恰好位于体能带结构沿该晶向的禁带之中。因此,处于此表 面态的电子由于功函数的束缚而不能逸出外围;又由于体能态的限制而不能深入 内层,形成了只能平行于表面方向运动的二维电子云。这就是纳米金颗粒所具有 表面效应、量子效应和宏观量子隧道效应等的物理基础。纳米金的颜色随其直径 大小和周围化学环境的不同而呈现红色至紫色 , 并具有很强的二次电子发射能 力。
纳米金修饰分子印迹聚合膜电极检测双酚A

纳米金修饰分子印迹聚合膜电极检测双酚A姚丹妮;王鑫宏;路莹;秦伟超;于洪斌【期刊名称】《环境监测管理与技术》【年(卷),期】2018(030)006【摘要】以纳米金修饰玻碳电极为基础电极,以双酚A为模板分子,以邻氨基苯硫酚为聚合单体,采用循环伏安法电聚合制备分子印迹聚合膜,利用循环伏安和交流阻抗法研究电极的电化学特性.结果表明,双酚A在修饰电极表面的反应是一个受吸附控制的等电子、质子转移的不可逆反应.采用差分脉冲伏安法检测双酚A,线性范围为5.0×10-6 mol/L~4.0×10-4 mol/L,检出限为2.3×10-7 mol/L.将该电极用于自来水和牛奶样品的测定,结果均为未检出,加标回收率分别为93.5%和95.4%,3次测定结果的RSD分别为4.0%和5.7%.【总页数】4页(P44-46,68)【作者】姚丹妮;王鑫宏;路莹;秦伟超;于洪斌【作者单位】东北师范大学环境学院,吉林省城市污水处理与水质保障科技创新中心,吉林长春 130117;吉林农业大学资源与环境学院,吉林长春 130118;东北师范大学环境学院,吉林省城市污水处理与水质保障科技创新中心,吉林长春 130117;东北师范大学环境学院,吉林省城市污水处理与水质保障科技创新中心,吉林长春130117;东北师范大学环境学院,吉林省城市污水处理与水质保障科技创新中心,吉林长春 130117【正文语种】中文【中图分类】O657.15【相关文献】1.基于纳米金膜的双酚A分子印迹传感器研究 [J], 赵广超;石勇;肇启东;李新勇;李春艳;薛方红2.还原氧化石墨烯-纳米金修饰的分子印迹传感器选择性检测水与牛奶中盐酸洛美沙星 [J], 利健文;韦寿莲;姚夙;刘永3.核壳结构的双酚A磁性纳米分子印迹聚合物的合成及应用 [J], 朱丽丽;曹玉华;曹光群4.基于碳纳米管/分子印迹聚合物修饰电极对双酚A的测定研究 [J], 杜春瑶;陈莹莹;詹伟玲;祝程凤;李泽林;曾延波;李蕾5.基于纳米金增敏的双酚A二氧化钛凝胶分子印迹电化学传感器的制备及应用 [J], 李玲玲;杨绍明;丁绍卿;尚培玲;杨杰;曹嫱;查文玲因版权原因,仅展示原文概要,查看原文内容请购买。
聚精氨酸修饰玻碳电极上多巴胺的电化学特性及其检测[1]
![聚精氨酸修饰玻碳电极上多巴胺的电化学特性及其检测[1]](https://img.taocdn.com/s3/m/eed0c009cc1755270722083e.png)
的氧化峰分开 200 mV ,消除了二者之间的相互干 扰 。500倍 K+ 、C l- 、NO3- 及 10 倍酒石酸 、150 倍 柠檬酸 、10 倍 L - 苯丙氨酸 、葡萄糖 、蔗糖 、甘氨 酸 、酪氨酸和乳酸 :均不干扰 DA 的测定 。以上结 果表明 ,此修饰电极的抗干扰能力强 ,对 DA 有较 好的选择性 。 216 样品分析
极在空气中放置 20 天后 ,其 CV 曲线和对 DA 的 催化作用基本不变 。表明聚精氨电极具有高度的
化学 、机械和电化学稳定性即较长的寿命 ,这对实 际应用有重要意义 。
在 10~350mV / s扫速范围内 , 210 ×10 - 5 mo l/ L DA 的 ipa和 ipc均与扫速呈线性关系 ,线性回归方 程分别为 : ipa (μA ) = 013435v (mV / s) + 319536, r = 019989; i (μA ) = - 012581v (mV / s) + 013727, r = - 019990。多巴胺的氧化峰电流与扫描速度成 正比 (图 2 ) ,这表明 DA 在修饰电极上有吸附特 性 ,氧化还原受表面控制 。此外 ,峰电位之差随扫 速的增加而增大 ,说明 DA 在修饰电极上为准可逆 反应 。
第 201086卷年第6月6期
化学研究与应用 Chem ical Research and App lication
文章编号 : 1004 - 1656 (2006) 06 - 0659 - 04
聚精氨酸修饰玻碳电极上多 巴胺的电化学特性及其检测
Vol. 18, No. 6 Jun. , 2006
氧化还原反应 ,其氧化峰电流与 DA 的浓度在 3. 0 ×10 - 7 ~8. 0 ×10 - 4 mol/L 范围内呈良好的线性关
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
m e t h o d . T h e s u r f a c e m o r p h o l o g y o f m di o i f e d e l e c t r de o w a s c h a r a c t e r i z e d b y s c a n n i n g e l e c t r o n mi c r o s c o p y ( S E M) . T h e e l e c t r o c h e mi -
De t e r mi na t i o n o f r ut i n by po l y do p a mi ne - na no Au
mo d i ie f d g l a s s y c a r b o n e l e c t r o d e
ZHANG Yi n g , “
2. Ke y L a b o r a t o r y o f Gr e e n C a t a l y s i s o f S i c h u n a I n s t i t u t e s o f HJ i s h E d u c a i t o n, Z i g o n g 43 6 0 0 0, C h i n a ;
3 . C o H e g e o f Ma t e i r ls a a n d C h e m i c l a E n g i n e e i r n g , S i e h u n a U n i v e si r y t o f S c i e n C e nd a E n i g n e e i f n g , Z i g o n g 43 6 0 0 0, C h i n a )
摘要 : 采用一锅 法制备聚 多巴胺一 纳米金修饰 玻碳 电极 ( P D A — A u N P s / G C E) , 用扫描 电子显 微镜 ( S E M) 对修 饰 电极进 行表面形貌 分析 , 并研 究芦丁在该修 饰电极上 的电化 学行 为。实验表明 , P D A - A u N P s / G C E对芦 丁有较 好 的电催 化氧化性 能 , 芦 丁的氧化峰电流 与其浓 度在 1 . 0 x l 0 ~ ~1 . 0 x l 0 -  ̄ m o l ・ L - 范 围 内成 线性关 系 , 检测 下
,
Z H A N G S h u . 1 i n , R E N Wa n g , C A I S h u — l a n , P U Q i n ,
HE Hu a — f e n g , L I NG L i n , WU L u . y u , Z HONG Xi a o . y u
聚 多 巴胺 一 纳 米 金 修 饰 玻 碳 电极 检 测 芦 丁
张 英 , 张述 , 任 旺 2 , 蔡述兰 , 蒲 勤 , 何华锋 , 凌 淋 , 吴路宇 , 钟 宵宇
( 1 . 四川理工学院化学与制药工程学院, 四川 自贡 6 4 3 0 0 0 ; 2 . 绿色 催化 四川 省 高 校重 点实 验 室 , 四川 自贡 6 4 3 0 0 0 ) 3 . 四川理 工 学 院材料 与 化学 工程 学 院 , 四川 自贡 6 4 3 0 0 0 )
( 1 . C o l l e g e o f C h e m i c l a a n d P h a r ma c e u i t c l a E n g i n e e i f n g , S i c h u n a U n i v e r s i t y o f S c i e n c e a n d E n g i n e e r i n g , Z i g o n g 6 4 3 0 0 0 , C h i n a ;
Ab s t r a c t : T h e p o l y d o p a mi n e - g o l d n no a p a r t i e l e m o d i f i e d g l a s s y c a r b o n e l e c t r o d e( P D A — A u N P s / G C E) W s a f a b i r c a t e d b y o n e - p o t
第2 5 卷第 9期
2 0 1 3年 9月
化 学 研 究 与 应 用
Ch e mi c a l Re s e a r c h a n d Ap p l i c a t i o n
Vo 1 . 2 5. No . 9 S e p ., 2 0 1 3
文章 编 号 : 1 0 0 4 - 1 6 5 6 ( 2 0 1 3 ) 0 9 — 1 2 9 9 - 0 4
限为 2 . 3 x 1 0 一 o t o l ・ L ( S / N= 3 ) 。该修饰 电极可用 于复方芦丁片 中芦丁含量 的检测 , 效果 良好 。
关键 词 : 聚多巴胺 ; 纳米金 ; 修 饰电极 ; 芦 丁
中图分类号 : 0 6 5 7 . 1 文献标 志码 Nhomakorabea: A
