3 GST CO_PCA
bw-gasalertmicro5-series-product-说明书

VOCs, CO 2, LEL, H 2S, CO, O 2, SO 2, PH 3, NH 3, NO 2, HCN, Cl 2, ClO 2, O3Protect yourselfCompact and lightweight, GasAlertMicro 5 Series instruments areavailable in diffusion or pumped instruments. The portable gas detectors simultaneously monitors and displays up to five potential atmospheric hazards. The GasAlertMicro 5 PID model also identifies PID detectable VOCs, while the GasAlertMicro 5 IR uses an NDIR sensor to monitorCO 2 levels. Adaptable to a variety of applications, GasAlertMicro 5 Series instruments have an extensive selection of user-settable field options. Use the passcode function to prevent unauthorized modifications of the instrument’s settings. Compatible with BW’s MicroDock II automatic test and calibration system, GasAlertMicro 5 Series instruments are unparalleled in their versatility, performance and overall value.Standard Package Contents• D etector complete with specified sensor(s), stainless steel alligator clip and concussion-proof housing• Rechargeable battery pack or alkaline pack with three AA batteries • Cradle charger and wall outlet charging adaptor (with rechargeable battery option)• Sample probe (with motorized pump option)• Screwdriver• Calibration/test adaptor and hose • Manual• Multi-language CD manual General SpecificationsSize 5.7 x 2.9 x 1.5 in. / 14.5 x 7.4 x 3.8 cm Weight 13.1 oz. / 370 gOperating temperature -4 to +122°F / -20 to +50°C14 to +104°F / -10 to +40°C (PID)Typical battery life 20 hours (15 hours PID/IR)**Based on a 5-gas instrument in diffusion mode at 68ºF / 20ºCCertifications and approvalsClass I, Div. 1, Gr. A, B, C, D American Bureau of Shipping (Toxic and PID models)ATEX: X g II 1 G Ga Ex ia IIC T4*X g II 2 G (IR model only)Ex d ia IIC T4*IECEx: Ga Ex ia IIC T4* Ex d ia IIC T4* (IR model only)*Temperature codes may vary as a function of the batteries installed. Please see owner’s manual for a complete listing of compatible batteries and codes.WarrantyFull two year warranty including sensors (one year Cl 2, NH3, O 3, ClO 2 and PID sensor)5612-15GasAlertMicro 5.....................................................................................50GasAlertMicro 5 PID ..............................................................................52GasAlertMicro 5 IR ................................................................................54GasAlertMicro 5 Series Accessories, Spares & Replacements ............56GasAlertMicro 5 Series Service Parts (60)GasAlertMicro 5 DetectorOrder NumberGasAlertMicro 5 Region CodesNote: Listed above are commonly ordered instrument configurations. To create a custom GasAlertMicro 5 detector, use the configurator table on the next page or contact BW Technologies for a copy of the electronic order number configurator.GasAlertMicro 5Pump versionGasAlertMicro 5Diffusion versionOrder Number ConfiguratorOrder number:For example, the order number for a GasAlertMicro 5 configured for O 2, %LEL, Cl 2, H 2S and CO and equipped with a rechargeable battery pack and cradle charger, motorized sampling pump, yellow housing and North American powerconnectivity would be M5-XWCY-R-P-D-Y-N-00.*Note: Some gases (e.g. SO 2 and NH 3) cannot be combined in the same GasAlertMicro 5 configuration. Please checkwith Customer Service for specific availability.†Note: HCN can only be ordered in the Toxic 1 location when the Duo-Tox (CO/H 2S) sensor is ordered in theToxic 2 location. In all other configurations, HCN must be ordered in the Toxic 2 location.USA 1.888.749.8878Canada 1.800.663.4164Latin & South America +55.11.3309.1030Europe +41 (0) 44.943.4300Germany +49 (0) 2137.17.6522France +33 (0) 442.98.17.70Middle East +971.4.4505852China +86.10.6786.7305S.E. Asia +65.6580.3468Australia +1.300.729.450Other Countries +1.403.248.9226 ********************Note: Listed above are commonly ordered instrument configurations. To create a custom GasAlertMicro 5 PID detector, use the configurator table on the next page or contact BW Technologies for a copy of the electronic order number configurator.GasAlertMicro 5 PIDPump versionGasAlertMicro 5 PIDDiffusion versionOrder Number ConfiguratorUSA 1.888.749.8878Canada 1.800.663.4164Latin & South America +55.11.3309.1030Europe +41 (0) 44.943.4300Germany +49 (0) 2137.17.6522France +33 (0) 442.98.17.70Middle East +971.4.4505852China +86.10.6786.7305S.E. Asia +65.6580.3468Australia +1.300.729.450Other Countries +1.403.248.9226 ********************Order number:For example, the order number for a GasAlertMicro 5 PID configured for O 2, %LEL, VOCs, H 2S and CO and equipped with a rechargeable battery pack and cradle charger, motorized sampling pump, yellow housing and North Americanpower connectivity would be M5PID-XWQY-R-P-D-Y-N-00.*Note: Some gases cannot be combined in the same GasAlertMicro 5 configuration. Please check with CustomerService for specific availability.GasAlertMicro 5 IR DetectorOrder NumberGasAlertMicro 5 IR Region CodesNote: Listed above are commonly ordered instrument configurations. To create a custom GasAlertMicro 5 IR detector, use the configurator table on the next page or contact BW Technologies for a copy of the electronic order number configurator.Gas LegendGasAlertMicro 5 IRPump versionGasAlertMicro 5 IRDiffusion versionOrder number:For example, the order number for a GasAlertMicro 5 IR configured for O 2, %LEL, CO 2, H 2S and CO and equipped with a rechargeable battery pack and cradle charger, motorized sampling pump, yellow housing and North American powerconnectivity would be M5IR-XWBY-R-P-D-Y-N-00.*Note: Some gases cannot be combined in the same GasAlertMicro 5 configuration. Please check with CustomerService for specific availability.Order Number ConfiguratorUSA 1.888.749.8878Canada 1.800.663.4164Latin & South America +55.11.3309.1030Europe +41 (0) 44.943.4300Germany +49 (0) 2137.17.6522France +33 (0) 442.98.17.70Middle East +971.4.4505852China +86.10.6786.7305S.E. Asia +65.6580.3468Australia +1.300.729.450Other Countries +1.403.248.9226 ********************Deluxe Confined Space KitM5-CK-DLConcussion-Proof BootGA-BM5-2For GasAlertMicro 5 Series pumped unitsCarrying HolsterGA-HM5Fits securely on beltConfined Space KitsOrder NumberCarrying & Protective Accessories Order Number Concussion-Proof BootGA-BM5-1For GasAlertMicro 5 Seriesdiffusion unitsSampling & Testing Equipment Order Number Note: For complete list of Sampling & Testing Equipment, see the Sampling Equipment section.ReplacementTest Cap and HoseM5-TC-1Pump ModuleM5-PUMPReplacementDiffusion CoverM5-DC-1Manual Aspirator PumpGA-AS02For remote sampling; complete withprobe, hose and aspirator pumpAuxiliary Pump Filter withHose ConnectorM5-QCONN-K1Auxiliary Pump FilterM5-AF-K2 / M5-AF-K2-100Kit of 5 or 100, for legacyGeneration 1 pump modules onlyAuxiliary Pump FilterM5-AF-K3 / M5-AF-K3-100Kit of 5 or 100, for Generation 2pump modulesPower Accessories Order Number*Note: Battery packs and chargers should be used with the new version GasAlertMicro 5 Series gas detectors (red circuit boards).MicroDock IIOrder NumberCradle ChargerM5-C01Cradle charger for rechargeable battery packs(can charge battery pack with or without detector attached)Vehicle Adaptor 12 V dcGA-V-CHRG4Vehicle adaptor cable for usewith cradle charger (M5-C01)Cradle Charger Kit with BatteryM5-C01-BAT08 / M5-C01-BAT08BComplete with charger and rechargeable battery packAlkaline Battery PackM5-BAT0501/M5-BAT0501BM5-BAT0502/M5-BAT0502BRechargeable Battery PackM5-BAT08/M5-BAT08B5062-26-NCL-EN59PID Cleaning KitM5PID-CLN-K1For PID sensorsReplacement Electrode StackM5PID-ES-1For PID sensorsReplacement 10.6 eV PID LampRL-PID10.6For PID sensorsReplacement Sensor Screens Order NumberReplacement SensorsOrder Number Note: All sensors come with a 2 year warranty unless marked with an asterisk (*), denoting 1 year warranty.†Advisory: Detectors are equipped on a standard basis with SR-W04 combustible sensors. This sensor includes a heavy duty silicone filter that make it ideal for use in environments that have known sources of silicone or vapors containing silicon. The SR-W04 silicone filter equipped sensor should not be usedwhen monitoring diesel, kerosene, jet fuel or other heavy hydrocarbon vapors with flashpoint temperatures above 38°C (100°F).Replacement IR SensorSR-B04Replacement PID SensorSR-Q0760 5062-26-NCL-ENNote: Please refer to the instrument’s documentation (shipped with the product or available at ) for complete instructions on common service procedures. Improper servicing or maintenance may affect warranty eligibility. Honeywell assumes no liability for damages resulting from improper servicing or maintenance.5062-26-NCL-EN61Note: Please refer to the instrument’s documentation (shipped with the product or available at ) for complete instructions on common serviceprocedures. Improper servicing or maintenance may affect warranty eligibility. Honeywell assumes no liability for damages resulting from improper servicing or maintenance.LegendService PartsOrder Number How to Replace a Sensor1. With detector OFF , use No. 1 Phillips screwdriver to remove2 screws (G) from back enclosure (F) on either side of the belt clip 2. Lift diffusion cover (A) or pump module straight up3. Remove sensor (O-R) by pulling straight up from PCB (E)4. Insert new sensor (O-R) into PCB (E)5. Replace diffusion cover (A) or pump module6. Replace 2 screws (G) in back enclosure (F) andhand-tighten until firmHow to Replace the Sensor Screen1. With detector OFF , use No. 1 Phillips screwdriver to remove2 screws (G) from back enclosure (F) on either side of the belt clip 2. Lift diffusion cover (A) or pump module straight up3. Remove sensor screen (B) by pulling it straight up fromdiffusion cover (A) or pump module4. Insert sensor screen (B) into diffusion cover (A) orpump module5. Replace diffusion cover (A) or pump module6. Replace 2 screws (G) in back enclosure (F) andhand-tighten until firm。
橄榄油 油橄榄果渣油 脂肪酸 成分 含量 气相色谱 归一法

MM_FS_CNJ_0326橄榄油油橄榄果渣油脂肪酸成分含量气相色谱归一法本文非本人原创,转载自橄榄油在线MM_FS_CNJ_0326橄榄油、油橄榄果渣油检验脂肪酸成分含量测定法1.适用范围本方法适用于商品橄榄油、油橄榄果渣油脂肪酸成分含量检验。
2.原理概要将橄榄油中的脂肪酸转化为脂肪酸甲酯,应用气相色谱仪将各甲酯分离,对照各脂肪酸甲酯标准样品保留时间定性,用归一法定量,以各甲酯的百分含量来表示各脂肪酸成分含量。
3.脂肪酸甲酯化(在通风橱内进行操作)3.1.三氟化硼-甲醇脂肪酸甲酯化法3.1.1.适用范围本方法适用于任何酸度的橄榄油、油橄榄果渣油。
应优先选用此法。
3.1.2.主要试剂和仪器3.1.2.1.主要试剂0.5mol/L氢氧化钠甲醇溶液(先用少许水将氢氧化钠溶解后再放入甲醇);市售三氟化硼乙醚溶液;甲醇;三氟化硼乙醚-甲醇溶液:将三氟化硼乙醚溶液与甲醇按1∶3(V BF3/3V CH3OH)配制;饱和食盐水溶液;石油醚(30~60℃沸程)。
3.1.2.2.仪器容量瓶:25mL;滴管:20mm;量筒:5mL;漏斗:直径30mm;试剂瓶:5~10mL;水浴锅。
3.1.3.过程简述3.1.3.1.用滴管取混匀油试样8~9滴(120~150mg)置于25mL容量瓶中,加入4mL 0.5mol/L氢氧化钠甲醇溶液,摇匀,置沸水浴上沸腾5min,使油试样呈透明状态。
3.1.3.2.待其冷却后加入5mL三氟化硼-甲醇溶液,再置水浴上沸腾2min,使甲酯化完全。
3.1.3.3.加入足够量的饱和食盐水溶液(到细颈底部),上层有黄色液体,用1~1.5mL 石油醚将其萃取到醚层(振摇、静止)。
再用滴管吸取上层甲酯石油醚溶液置于试剂瓶内,密封低温保存,待进入气相色谱仪进行脂肪酸成分含量测定。
3.2.氢氧化钾-甲醇脂肪酸甲酯化法3.2.1.适用范围本方法适用于酸度小于3%的橄榄油、油橄榄果渣油。
3.2.2.主要试剂和仪器3.2.2.1.试剂2mol/L氢氧化钾甲醇溶液:11.2g氢氧化钾溶于100mL甲醇中;正已烷。
PCA分析和分峰方法

界 面
SixPack
PCA
PCA软件的使用
新 版 集 成 在
软 件 中 的
X
和 射 数线 据吸 分收 析谱 讲学 习实 班验
北 京 同 步 辐 射 装 置
Beijing Synchrotron Radiation Facility
分 析 组 件
Athena
PCA
PCA软件的使用
北 京 同 步 辐 射 装 置
自吸收效应的产生
PCA软件的使用
北 京 同 步 辐 射 装 置
X
Beijing Synchrotron Radiation Facility
数据预处理——自吸收校正
需要注意自吸收效应的情况: 样品中所测元素的含量太高 样品太厚 在荧光模式下
和 射 数线 据吸 分收 析谱 讲学 习实 班验
PCA软件的使用
������ =
变换谱
������
组元数组
∙
������
������
∙
������
标样谱
组元数组的转置矩阵
和 射 数线 据吸 分收 析谱 讲学 习实 班验
目标变换—寻找合适的标样
PCA适用体系
北 京 同 步 辐 射 装 置
X
Beijing Synchrotron Radiation Facility
PCA软件的具体操作
和 射 数线 据吸 分收 析谱 讲学 习实 班验
PCA软件的使用
北 京 同 步 辐 射 装 置
X
Beijing Synchrotron Radiation Facility
软件主要操作步骤
1. 数据 2. 选择PCA范围 3. 选择模式 4. 进行主元拆分 5.重建数据和目标变换 6.作图选项 7.保存选项 6 5
球囊菌侵染对意大利蜜蜂幼虫肠道免疫与解毒相关基因表达及蛋白质、脂质和糖类含量的影响

NB-Designer软件操作手册(中文NB5和7适用).

Cat.No.NB 系列V106-CN5-01PNSPO!可编程终端NB-Designer用户手册1前言承蒙您惠购可编程终端NB 系列,谨致谢意。
NB 系列是指在FA 生产现场等地所产生的各种信息的可编程终端(PT)。
请在充分理解可编程终端的功能和性能等的基础上正确使用。
●读者对象本手册以下述人员为对象而编写。
具备电气知识(电气工程师或具备同等知识),且 y 负责引进FA 设备的人员; y 设计FA 系统的人员;y 安装、连接FA 设备的人员; y 管理FA 生产现场的人员。
●使用须知y 本手册除了对NB 系列的连接和设定进行说明之外,还介绍了其它必要的信息。
使用前请仔细阅读本手册,充分理解说明内容。
阅读后请妥善保管本手册,以便随时取阅。
●关于“使用时的承诺事项”1. 保修内容①保修期本公司产品的保修期为自购买之日或交付至指定场所之日起1年。
②保修范围在上述保修期内因本公司的责任而发生产品故障时,本公司将在产品购买地点免费予以更换或维修。
但当故障原因符合下列情况之一时,则不属于保修范围。
a)未按照产品目录或使用说明书等资料中说明的条件、环境、操作方法使用时; b)非本公司产品自身的原因时; c)未经本公司授权而改造或维修时; d)未按照本公司产品应有的方法使用时;e)以本公司产品出厂时的科技水平无法对故障进行预测时; f)因自然灾害等其它非本公司责任的不可抗力而导致故障时。
此外,以上的保修是指对本公司产品单件的保修,因本公司产品故障而造成的损失不属于保修对象。
2. 责任限制①因本公司产品而引起的特别损失、间接损失或消极损失,本公司概不负责。
②对于本公司的可编程产品,因非本公司人员编写的程序或由此而产生的后果,本公司概不负责。
23. 适用条件①将本公司产品与其它产品组合使用时,请确认适用的标准、法规或限制。
此外,请用户自行确认本公司的产品是否与您所使用的系统、机械和装置相兼容。
否则,本公司对自身产品的兼容性概不负责。
国际葡萄与葡萄酒组织(OIV)的全套酿酒法规以及检验标准_2008-Vol1

COMPENDIUM OF INTERNATIONAL MÉTHODS OF WINE AND MUST ANALYSIS
EDITION 2008
VOLUME 1
INCLUDED : Resolutions adopted in Budapest (Hungary) 5th A.G. – 15 June 2007
MA-E-INT-00-TABMAT 2008
1
COMPENDIUM OF INTERNATIONAL METHODS OF ANALYSIS-OIV Table of contents
Table of contents........................................................MA-E-INT-00-TABMAT VOLUME 1 Foreword ..................................................................MA-E-INT-01-AVPROP Layout and wording of OIV method of analysis ...............MA-E-INT-04-REDMET ANNEX A – METHODS OF ANALYSIS OF WINES AND MUSTS SECTION 1 – DEFINITIONS AND GENERAL PRINCIPLES General remarks ........................................................MA-E-AS1-02-REMGEN Classification of analytical methods (oeno 9/2000) .........MA-E-AS1-03-CLASMA Matrix effect for metals content analysis (oeno 5/2000) ............................................................MA-E-AS1-04-EFFMAT SECTION 2 – PHYSICAL ANALYSIS Density and Specific Gravity at 20oC (A 1).....................MA-E-AS2-01-MASVOL Evaluation by refractometry of the sugar concentration in grape musts, concentrated grape musts and rectified concentrated grape musts................MA-E-AS2-02-SUCREF Total dry matter (A 3)..................................................MA-E-AS2-03-EXTSEC Ash (A 6)...................................................................MA-E-AS2-04-CENDRE Alkalinity of Ash (A 7)..................................................MA-E-AS2-05-ALCCEN Oxidation-reduction potential (oeno 3/2000)...................MA-E-AS2-06-POTOXY Wine turbidity (oeno 4/2000)........................................MA-E-AS2-08-TURBID Method for isotopic ratio 18O/16O (Oeno 2/96)...............MA-E-AS2-09-MOUO18 Folin-Ciocalteu Index ..................................................MA-E-AS2-10-INDFOL Chromatic Characteristics (Oeno 1/2006) ......................MA-E-AS2-11-CARCHR SECTION 3 – CHIMICAL ANALYSIS SECTION 3.1 – ORGANIC COMPOUNDS SECTION 3.1.1 – SUGARS Reducing sugars (A 4) ................................................MA-E-AS311-01-SUCRED Glucose and fructose (enzymatic method) .....................MA-E-AS311-02-GLUFRU Dosage of sugars by HPLC (Oeno 23/2003) ..................MA-E-AS311-03-SUCRES Stabilisation of musts to detect Addition of sucrose (A 5) .............................................MA-E-AS311-04-STAMOU Detecting enrichment of musts, concentrat ed grape musts, rectified concentrated grape musts and wine by ²H-RMN.........................................MA-E-AS311-05-ENRRMN Polyols derived from sugars (Oeno 9/2006) ...................MA-F-AS311-06-POLYOL Glucose and fructose (pHmetry ) (Oeno 10/2006)............MA-F-AS311-07-GLCFR2 Glucose, fructose and saccharose (pHmetry ) (Oeno 11/2006)..........................................................MA-F-AS311-08-SACCHA
谷胱甘肽 S-转移酶(GST)活性检测试剂盒说明书__ 紫外分光光度法UPLC-MS-4496
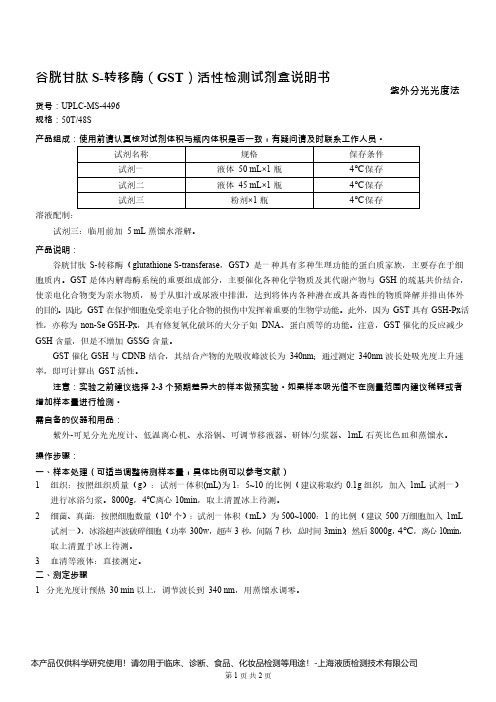
谷胱甘肽S-转移酶(GST)活性检测试剂盒说明书紫外分光光度法货号:UPLC-MS-4496规格:50T/48S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系工作人员。
试剂名称规格保存条件试剂一液体50mL×1瓶4℃保存试剂二液体45mL×1瓶4℃保存试剂三粉剂×1瓶4℃保存溶液配制:试剂三:临用前加5mL蒸馏水溶解。
产品说明:谷胱甘肽S-转移酶(glutathione S-transferase,GST)是一种具有多种生理功能的蛋白质家族,主要存在于细胞质内。
GST是体内解毒酶系统的重要组成部分,主要催化各种化学物质及其代谢产物与GSH的巯基共价结合,使亲电化合物变为亲水物质,易于从胆汁或尿液中排泄,达到将体内各种潜在或具备毒性的物质降解并排出体外的目的。
因此,GST在保护细胞免受亲电子化合物的损伤中发挥着重要的生物学功能。
此外,因为GST具有GSH-Px活性,亦称为non-Se GSH-Px,具有修复氧化破坏的大分子如DNA、蛋白质等的功能。
注意,GST催化的反应减少GSH含量,但是不增加GSSG含量。
GST催化GSH与CDNB结合,其结合产物的光吸收峰波长为340nm;通过测定340nm波长处吸光度上升速率,即可计算出GST活性。
注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:紫外-可见分光光度计、低温离心机、水浴锅、可调节移液器、研钵/匀浆器、1mL石英比色皿和蒸馏水。
操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)1.组织:按照组织质量(g):试剂一体积(mL)为1:5~10的比例(建议称取约0.1g组织,加入1mL试剂一)进行冰浴匀浆。
8000g,4℃离心10min,取上清置冰上待测。
2.细菌、真菌:按照细胞数量(104个):试剂一体积(mL)为500~1000:1的比例(建议500万细胞加入1mL试剂一),冰浴超声波破碎细胞(功率300w,超声3秒,间隔7秒,总时间3min);然后8000g,4℃,离心10min,取上清置于冰上待测。
欧洲药典5.5_GAOQS

COMMENTS CONCERNING SOME REVISED/ CORRECTED TEXTS PUBLISHED IN SUPPLEMENT 5.5Here follows information concerning certain technical modifications to some revised/corrected texts adopted by the European Pharmacopoeia Commission at the June 2005 session. This information completes the modifications indicated by lines in the margin in the supplement. Therefore, the information below is not necessarily exhaustive.ANALYTICAL METHODS2.4.29. Composition of fatty acids in oils rich in omega-3-acidsIn the test for system suitability, as in the case of thetest for oligomers in the omega-3-acid ethyl esters 90 monograph, the fi rst 3 requirements are consideredsuffi cient; the 4th requirement is not routinely performed by the producers who proposed the test, and is deleted. 2.6.15. Prekallikrein activatorThis general chapter has been revised based on the outcome of the international collaborative study BSP049 organised by the EDQM, to mention that a microtitre plate-based method, which is nowadays the most frequently used, may also be used instead of methods using autoanalysers, which were more appropriate whena large number of samples had to be analysed.2.6.21. Nucleic acid amplifi cation techniquesThis general chapter has been revised to makeit applicable to new applications such as the testfor mycoplasmas (see revised chapter 2.6.7) anda quantitative test system used to control anti-Dplasma for B19 virus (see monograph Human anti-D immunoglobulin (0557)).2.6.22. Activated coagulation factorsThis general chapter has been revised together with general chapters 2.7.11 and 2.7.22 to:— change protamine sulphate R to being an example of a suitable substance to neutralise the heparin;— replace the reference to cephalin R and platelet substitute R, which were obsolete, by a phospholipid preparation to act as a platelet substitute.2.7.4. Assay of human coagulation factor VIIIThe assay of human coagulation factor VIII using a chromogenic substrate was fi rst included in the European Pharmacopoeia in 1993, replacing the two-stage assay,in line with the recommendation of the Scientifi cand Standardisation Committee of the International Society on Thrombosis and Haemostasis (SSC ISTH). Commercial kits are used for the assay and the description of the method is generic to allow the use of all currently available kits with acceptable performance. The revision brings no essential major changes to the method. Work has been carried out recently to defi ne critical aspects of the method, particularly with respect to B domain-deleted factor VIII. Problems encountered with the latter product are best resolved by the useof a B domain-deleted factor VIII reference standardfor routine assay. The experimental work carried out recently indicates that it is best for these products to halt factor Xa generation when the factor Xa concentration has reached approximately 50 per cent of the maximum (plateau) level. Furthermore, since it has been shown that, from a statistical point of view, the potency found with independent or serial dilutions is not signifi cantly different, independent dilutions are no longer required. 2.7.11. Assay of human coagulation factor IXThis general chapter has been revised to:— mention factor IX-defi cient plasma as a predilution medium, since this is used routinely;— allow the use of commercial APTT reagents and omit the reference to cephalin-based reagents, which are now obsolete.2.7.21. Assay of human von Willebrand factorThis general chapter has been revised to improve and supplement the description of ristocetin cofactor activity, and in particular to introduce general statements on quantitative assays. Furthermore, since it has been shown that, from a statistical point of view, the potency found with independent or serial dilutions is not signifi cantly different, independent dilutions are no longer required. 2.7.22. Assay of human coagulation factor XIThe general chapter has been revised to:— mention factor XI-defi cient plasma as a predilution medium since this is used routinely;— allow the use of commercial APTT reagents and omit the reference to cephalin-based reagents, which are now obsolete.5.10. Control of impurities in substances for pharmaceutical useThe section on Interpretation of the test for related substances in the monographs on active substances has been modifi ed to replace the examples by a decision tree, which better illustrates the interpretation of monographs.^_^ ---VACCINES FOR HUMAN USEDOSAGE FORMSGENERAL MONOGRAPHSSubstances for pharmaceutical use (2034)In the section dealing with related substances, the possibility of exemptions to the general provisions has been introduced, since it is now seen to be appropriate to make exceptions in some specifi c monographs.The section on residual solvents has been modifi ed tostate explicitly that the content of residual solvents is taken into account for calculation of specifi c optical rotation and specifi c absorbance.Capsules (0016)In order to take account of the new harmonised chapteron Disintegration, adaptations have been introduced to the relevant sections.Ear preparations (0652)The test for Deliverable mass or volume has been the cause of some misunderstanding amongst users: it was not a quality control test, and aimed only to ensure that the fi lling was such that the labelled dose could be withdrawn; furthermore, it has been considered to be vague and impractical. It has therefore been replaced by an additional sentence under Production.Liquid preparations for cutaneous application (0927)Liquid preparations for oral use (0672)The test for Deliverable mass or volume has been the cause of some misunderstanding amongst users: it was not a quality control test, and aimed only to ensure that the fi lling was such that the labelled dose could be withdrawn; furthermore, it has been considered to be vague and impractical. It has therefore been replaced by an additional sentence under Production.Rectal preparations (1145)The test for deliverable mass or volume has been the cause of some misunderstanding amongst users: it was not a quality control test, and aimed only to ensure that the fi lling was such that the labelled dose could be withdrawn; furthermore, it has been considered to be vague and impractical. It has therefore been replaced by an additional sentence under Production.Also, in order to take account of the new harmonised chapters on Dissolution and Disintegration, adaptations have been introduced to the relevant sections.Semi-solid preparations for cutaneous application (0132)The test for deliverable mass or volume has been the cause of some misunderstanding amongst users: it was not a quality control test, and aimed only to ensure that the fi lling was such that the labelled dose could be withdrawn; furthermore, it has been considered to be vague and impractical. It has therefore been replaced by an additional sentence under Production.Tablets (0478)This monograph was published in Pharmeuropa 15.2 and 16.2 for enquiry related to uniformity of subdivided tablets and to oral lyophilisates. Due to the comments received, it was necessary to carry out a new enquiry as regards oral lyophilisates (Pharmeuropa 17.4). As regards subdivision of tablets, uniformity of mass is now tested on 30 parts; no 2nd test is required in case of failure. This revision is the result of the enquiry and the study performed by the OMCLs on the basis of Pharmeuropa 16.2. This text also takes into account the new harmonised chapters on Disintegration and Dissolution.Vaginal preparations (1164)The test for deliverable mass or volume has been the cause of some misunderstanding amongst users: it was not a quality control test, and aimed only to ensure that the fi lling was such that the labelled dose could be withdrawn; furthermore, it has been considered to be vague and impractical. It has therefore been replaced by an additional sentence under Production.Also, in order to take account of the new harmonised chapters on Dissolution and Disintegration, adaptations have been introduced to the relevant sections.Pneumococcal polysaccharide conjugate vaccine (adsorbed) (2150)The monograph has been revised to clarify that the test for sterility that is carried out on intermediates (the pneumococcal polysaccharides, the carrier protein and the monovalent bulk conjugate) uses 10 ml for each medium or the equivalent of 100 doses, whichever is less. Furthermore, it harmonises this monograph with the monograph on Meningococcal group C conjugate vaccine (2112).^_^---MONOGRAPHSAluminium hydroxide, hydrated, for adsorption (1664) Based on batch data, the limit for iron has been increased from 10 ppm to 15 ppm.Beclometasone dipropionate, anhydrous (0654) Beclometasone dipropionate monohydrate (1709) Following the establishment of beclometasone dipropionate for system suitability CRS and beclometasone dipropionate for peak identifi cation CRS, the identifi cation of impurities D and M has beenmodifi ed. In addition, relative retentions of the other detectable impurities have been deleted according to usual practice.Benzyl alcohol (0256)In the test for residue on evaporation, the temperatureof 100 °C indicated for evaporation on a water-bath was too low in view of the boiling point of benzyl alcohol (205 °C), therefore the evaporation method has been changed.In the assay, it is now specifi ed that the mixture is heated on a water-bath.Digoxin (0079)This monograph has been revised to replace the TLCtest for related substances by LC and to replace the assay with an LC test using the same system. For this product of natural origin having a complex impurity profi le, it has not proved possible to apply the general policy for impurities shown in the monograph on Substances for Pharmaceutical Use (2034). It has been necessary to give an acceptance criterion equivalent to 0.2 per cent for “any other impurities”. An acceptance criterion for the sum of impurities (specifi ed and unspecifi ed) has been defi ned and in order to provide a further degree of control, an acceptance criterion for the subtotal of unspecifi ed impurities has been added.Dipivefrine hydrochloride (1719)The following changes have been made:— an increase in the amount of concentrated ammonia solution used in the mobile phase to improve the symmetry of the peaks;— the introduction of a higher upper limit for the symmetry factor in the assay, i.e. 2.0 for the principal peak in the chromatogram obtained with referencesolution (c).Evening primrose oil, refi ned (2104)The unsaponifi able matter is largely composed of natural vitamins. It is therefore desirable that the oil is smoothly refi ned so that the unsaponifi able matter remains rather high. Based on batch data, the limit for unsaponifi able matter has been increased from 2.0 per cent to 2.5 per cent. Gemfi brozil (1694)Following a study in the EDQM laboratory, method C has been replaced by method F in the test for heavy metals. Furthermore, impurity D has been corrected.Glutathione (1670)Following the establishment of the CRS for glutathione, several batches have been tested and it appeared that the limits for impurity D and for the total of impurities were too strict. These 2 limits have therefore been increased. Human coagulation factor XI (1644)This monograph has been revised to harmonise thetotal protein test with other monographs. Reference to general chapter 2.5.33 is not appropriate since 7 different methods are described and it has not been shown that these 7 methods are equivalent. Since the determination of nitrogen by sulphuric acid digestion (Kjeldahl method) is the only method that can be performed without using a reference preparation, this is the method of choice. Indeed, this is a big advantage since results obtainedcan be compared directly. Other methods can also be used provided that they have been validated against the determination of nitrogen by the sulphuric acid digestion method described in the monograph.Ketorolac trometamol (1755)Following a study in the EDQM laboratory, method C has been replaced by method F in the test for heavy metals. Lauroyl macrogolglycerides (1231)Linoleoyl macrogolglycerides (1232)By analogy with the revision made to the monograph Stearoyl macrogolglycerides (1268) in 2004, the conditions used for the test for free glycerol have been modifi ed.Macrogol 20 glycerol monostearate (2044)In the test for hydroxyl value the method has been changed: method A yields better results, within the limits of 65 to 85.Methadone hydrochloride (0408)The silver nitrate titration in the assay has been replaced by the acid-base titration, which is more specifi c as it determines the active moiety; in addition, the titrant is low-cost, odourless, and easy to handle. This titration has been shown to be equivalent to the silver nitrate titration and to the previous perchloric acid titration.Oleoyl macrogolglycerides (1249)By analogy with the revision made to the monograph stearoyl macrogolglycerides (1268) in 2004, the conditions used for the test for free glycerol have been modifi ed.^_^ ---Pancuronium bromide (0681)2 new impurities have been added to the transparency list. Pancuronium bromide and its impurities cannot be analysed by LC because of insuffi cient sensitivity(little or no chromophores). For this reason, the current TLC method has been optimised to allow detection of impurities at 0.1 per cent.Sucrose (0204)As the amount of lead in the test solution is in practice extremely low, the system suitability criterion in the test for lead has been changed.Sumatriptan succinate (1573)As it is diffi cult to obtain samples of the individualimpurities to prepare replacement CRS batches, a method has been developed based on the injection of mixtures of sumatriptan with impurities obtained by evaporation, to identify the specifi ed impurities in the test for impurity A and impurity H and in the test for related substances.NEW : PHARMEUROPA SCIENTIFIC NOTESFor prices and ordering information please consult the catalogue on our internet site • Few Bicyclic Acetals at Reducing End of Low-Molecular-Weight Heparins: Might they Restrict Specifi cation of Pharmacopoeia?• The Control of Impurities in Chlortalidone Using a Reversed-Phase Stationary Phase • Factor VIII Test in Reference Preparations: Compensation for Different Dilutions • The Control of Impurities in Amitriptyline Hydrochloride Using a Reversed-Phase Hybrid Stationary Phase• A Precise Colour Determination Method for Tablets - an Application of Instrumental Colour Measurement in the Pharmaceutical Development• Development of an in vivo Test Procedure for the Ease of Breaking of Scored Tablets• Chromogenic Assay of Human Coagulation Factor VIII: Statistical Comparison of 2 Working Dilution Procedures• Impurity Profile of Amino Acids?• Batch Variability of Bacitracin: HPLC versus MEKC • Quality Criteria of Homoeopathic Mother Tinctures: Considerations Regarding Suitable Tests for Homoeopathic Monographs • Instructions for Authors Available now (English only)As from Pharmeuropa 17.4, Scientifi c Notes are principally published in a new publication called Pharmeuropa Scientifi c Notes.Articles published in Pharmeuropa Scientifi c Notes are indexed in the PubMed database of the National Library of Medecine, available on the internet site ().The fi rst edition of Pharmeuropa Scientifi c Notes (Pharmeuropa SN 2005-1) became available in August 2005.This issue is included in the subscription to Pharmeuropa, and is not available separately.SCIENTIFIC NOTES 2005-1________________________________________________________________________________^_^---。
气相色谱法测定1,1,3-三甲基-3-苯基茚满

气相色谱法测定1,1,3-三甲基-3-苯基茚满
王建刚;王文涛;李健秀;张云涛;郭慧
【期刊名称】《精细石油化工》
【年(卷),期】2003(000)003
【摘要】确立了1,1,3-三甲基-3-苯基茚满的气相色谱分析法.将试样用甲醇溶解,经5%SE-30/Chromosorb W不锈钢色谱柱(2 m × 3 mm)分离,在柱温为210 ℃,载气(H2)流量为20 mL/min时,在10 min内可完成样品分离,平均回收率为101.2%,相对标准偏差为0.15%.为1,1,3-三甲基-3-苯基茚满的质量控制提供了一个简便,准确,快速的工业分析方法.
【总页数】2页(P49-50)
【作者】王建刚;王文涛;李健秀;张云涛;郭慧
【作者单位】吉林化工学院分析测试中心,吉林,132022;吉林化工学院分析测试中心,吉林,132022;吉林化工学院分析测试中心,吉林,132022;哈尔滨制药总厂研究所,哈尔滨,150000;吉林化工学院分析测试中心,吉林,132022
【正文语种】中文
【中图分类】TQ2
【相关文献】
1.1,1,3-三甲基-3-苯基茚满的合成方法的研究 [J], 李海花;闫顺生
2.1,1,3-三甲基-3-苯基茚满的催化合成 [J], 颜莉;韩媛媛;路嫔;蔡清海
3.1,1,3-三甲基-3-苯基茚满的合成及表征 [J], 王文涛;文福姬;李健秀;王琨
4.5(6)-氨基-1-(4-氨基苯基)-1,3,3-三甲基茚满合成及工艺优化 [J], 凌钦才; 李晓雷; 谢国庆; 龚彦; 李明; 朱琦; 葛觉非; 钟佳琪; 翟金国
5.高效液相色谱法测定1,1,3-三甲基-3-苯基茚满 [J], 王建刚;李健秀;王文涛;孙红因版权原因,仅展示原文概要,查看原文内容请购买。
GSTpull-down和CoIP有何区别?实验结果该如何解读?

GSTpull-down和CoIP有何区别?实验结果该如何解读?前两天有小伙伴在后台咨询GST pull-down实验的相关问题,很遗憾前期并未整理相关的内容。
通过查阅资料,小P发现其实GST pull-down实验和CoIP实验原理是非常相似的!今天就简单介绍一下两者的异同,同时结合文献案例给大家分享一下CoIP和GST pull-down实验结果的分析方法。
01GST pull-down实验原理GST,全称glutathione-S-transferase,即谷胱甘肽-S-转移酶蛋白,能够和谷胱甘肽(Glutathione,GSH)稳定结合。
我们把GSH 与琼脂糖交联形成GSH琼脂糖珠,就能够将带有GST标签的诱饵蛋白固定,当体系中存在与诱饵蛋白有直接相互作用的靶标蛋白时,它就能够与微珠固相复合物结合而被“拉”下来。
02CoIP实验原理CoIP,Co-Immunoprecipitation,即免疫共沉淀实验,它通过一种偶联有Protein A/G的琼脂糖微珠或磁珠(Protein A/G能够特异性结合免疫球蛋白),将诱饵蛋白的抗体固定,随后再通过该抗体固定诱饵蛋白,当与诱饵蛋白有相互作用的靶标蛋白与此固相复合物混合时就可被吸附而“沉淀”下来。
从两者的实验原理我们不难发现,GST pull-down和CoIP实验都是通过一种多组分的固相复合物将诱饵蛋白固定,随后富集靶标蛋白,从而确定靶标蛋白和诱饵蛋白互相作用的事实,整体思路是很类似的;不过由于固定诱饵蛋白的方法不同,二者在适用范围上,以及实验难度上均有差异。
GST pull-down一般用于体外实验,因为诱饵蛋白是需要加上GST标签的重组蛋白,所以是非生理状态下的验证,蛋白质的结构和形式可能与天然状态下存在一定差异,一般用于体外验证两个已知蛋白的直接相互作用。
在实验难度上,重组诱饵蛋白一般采用原核表达体系,有高效的商业化载体(真核表表达体系也可以做,但需要找到合适的载体);并且固相复合物组分更少,有商业化的GSH琼脂糖珠,所以整个实验相对而言难度较低。
GST融合蛋白活性测定条件的优化

GST融合蛋白活性测定条件的优化梁斌;谢琦【期刊名称】《承德医学院学报》【年(卷),期】2016(033)005【总页数】3页(P413-415)【关键词】谷胱甘肽硫转移酶;分光光度法;活性测定【作者】梁斌;谢琦【作者单位】桂林医学院生物技术学院,广西桂林541004;桂林医学院生物技术学院,广西桂林541004【正文语种】中文【中图分类】Q78谷胱甘肽硫转移酶(GST,EC2.5.1.18)是一种广泛分布于动植物和微生物体内,具有多种生理功能的同工酶家族。
在医学研究中,由于它是生物体内重要的解毒酶系,其在体内活性的高低与代谢环境致癌物和化疗药物能力之间的关系受到人们的关注[1]。
在基因工程研究中,GST的基因已被克隆和表达,GST可被作为一种融合表达蛋白来提高重组蛋白的可溶性表达,与GST融合的重组蛋白很容易利用GST标签,通过亲和层析进行纯化,所以各种带有GST标签的重组蛋白被大量表达。
在各种与GST相关的研究中,都涉及到GST酶活性的测定。
目前,建立的GST酶活性测定方法有分光光度法、荧光底物法、滴定法、重叠氮法、氰化物法、对硝基酚法等。
张丹参等[2]比较了上述方法后认为,以1-氯-2,4-二硝基苯(CDNB)和还原型谷胱甘肽(GSH)为底物的分光光度法具有操作简便,灵敏度高,稳定性好的优势。
我学院在参照Habdous等[3-4]建立的以CDNB和GSH为底物的分光光度法的基础上,针对基因工程研究中大量使用的带有GST标签的融合蛋白的酶活测定条件进行了优化。
1.1 材料大肠杆菌BL21(DE3)[PGEX-4T-1],携带重组胸腺肽Tα1-TP5基因,由本实验室构建[5]。
CDNB(1-氯-2,4-二硝基苯),购自SIGMA公司。
GSH(还原型谷胱甘肽),购自上海生工。
大肠肝菌培养基:酵母粉2.4%,蛋白胨1.2%,甘油0.4%,磷酸二氢钾17mmol/L,磷酸氢二钾72mmol/L,硫酸镁1mmol/L,高压灭菌时磷酸盐和硫酸镁与其它组分分开,待溶液冷却至60℃以下后混合。
陆地棉GST基因家族全基因组分析

陆地棉GST基因家族全基因组分析许磊;陈文;司国阳;黄艺园;林毅;蔡永萍;高俊山【期刊名称】《遗传》【年(卷),期】2017(039)008【摘要】Glutathione-S-transferase (GST) is a ubiquitous multi-functional protein superfamily that plays important roles in plant primary and secondary metabolism,stress and intercellular signaltransduction.Concomitantly,it also functions as a ligand in the metabolism of plant hormones and substance transport.In order to understand the GST gene family in upland cotton (Gossypium hirsutum L.),herein we analyzed the species,evolutionary relationship,physical location,genestructure,conserved motifs and expression patterns.We identified 70 GST genes in the whole genome of upland cotton,and divided them intoU,F,T,Z,EF1Bγ and TCHQD groups by phylogenetic tree and gene structure analyses.The gene mapping analysis indicated that the GST genes were on every chromosome except chromosome AD/At2,AD/At4,AD/At5,AD/Dt5 and AD/Dt10.Moreover,the GST gene cluster appeared on four chromosomes (AD/At9,AD/Dt7,AD/Dt12 and AD/Dt13).qRT-PCR assays showed that eight genes (GhGSTF2-9) were expressed in theroot,stem,leave and fiber of different developmental stages while GhGSTF1 might be a bining qRT-PCR and bioinformatic analysis,we speculated that GhGSTF8 might be involved in the transport andaccumulation of proanthocyanidins/anthocyanins;GhGSTF4,6 and 9 might play roles in regulating the growth and stress response of upland cotton;the function of GhGSTF2,3,5 and 7 remains to be further investigated.Our work provides a theoretical basis for further studies on the molecular evolution and function of the GST gene family in upland cotton.%谷胱甘肽转移酶(glutathione-S-transferase,GST)是一种普遍存在的具有多功能的超家族蛋白,在植物初次生代谢、逆境胁迫、胞间信号传递等方面具有重要作用;同时,作为配体其在植物激素代谢以及物质转运方面也发挥作用.为了解析陆地棉(Gossypium hirsutumL.)GST基因家族的信息,本研究对该基因家族成员的种类、进化关系、物理定位、基因结构和保守基序以及表达模式进行了分析.结果显示,在陆地棉全基因组中共含有70个GST基因,进化树和基因结构分析将该家族分为U族、F族、T族、Z族、EF1Bγ族和TCHQD族.基因定位分析发现,除了AD/At2、AD/At4、AD/At5、AD/Dt5、AD/Dt 10号染色体上没有GST基因外,其他染色体上都有GST基因,并且在AD/At9、AD/Dt7、AD/Dt 12、AD/Dt 13这4条染色体上出现基因簇.对F族(Phi类)9个GST基因进行荧光定量分析,结果表明,除GhGSTF1可能为假基因外,GhGSTF2~9等8个基因在陆地棉根、茎、叶以及各个发育时期的纤维中均有表达;结合生物信息学分析,推测GhGSTF8可能参与原花青素/花青素的转运和积累;GhGSTF4,GhGSTF6和GhGSTF9可能在调节陆地棉的生长和胁迫反应中起作用,而GhGSTF2、GhGSTF3、GhGSTF5和GhGSTF7的功能还有待进一步研究.本研究为陆地棉GST基因家族的分子进化及功能研究提供了理论依据.【总页数】16页(P737-752)【作者】许磊;陈文;司国阳;黄艺园;林毅;蔡永萍;高俊山【作者单位】安徽农业大学生命科学学院,合肥230036;安徽农业大学生命科学学院,合肥230036;安徽农业大学生命科学学院,合肥230036;安徽农业大学生命科学学院,合肥230036;安徽农业大学生命科学学院,合肥230036;安徽农业大学生命科学学院,合肥230036;安徽农业大学生命科学学院,合肥230036【正文语种】中文【相关文献】1.陆地棉Dof基因家族的全基因组鉴定及分析 [J], 琚龙贞;赵汀;方磊;胡艳;张天真2.陆地棉NF-YA基因家族的全基因组鉴定与功能分析 [J], 潘奥;刘志;王静静;孙福来;张景霞;高阳;杜召海;焦梦佳;张军;王芙蓉3.陆地棉REM基因家族全基因组鉴定及表达分析 [J], 石荣康;张冬梅;孙正文;刘正文;解美霞;张艳;马峙英;王省芬4.陆地棉Nudix基因家族的全基因组鉴定及表达分析 [J], 窦玲玲;王文博;肖光辉;孙亚如;赵琴;田瑞洁;康洋洋;朱怡然;杨蕾蕾;王彩虹;冯宇5.陆地棉SPL基因家族的全基因组鉴定及表达分析 [J], 钟子达;钟晓真;沈超因版权原因,仅展示原文概要,查看原文内容请购买。
GST-pi与p53蛋白表达在肠癌和增生性病变中的关系

GST-pi与p53蛋白表达在肠癌和增生性病变中的关系王翠莲;李宗铉【期刊名称】《医药世界》【年(卷),期】2006(000)009【摘要】目的:探讨胎盘型谷胱甘肽转移酶(GST-pi)与p53蛋白在肠癌和增生性病变中表达及其关系.方法应用S-P免疫组化法检测36例肠腺癌,16例肠腺瘤伴非典型增生,20例肠腺瘤,10例肠息肉组织中GST-pi与p53蛋白表达的关系.结果GST-pi与p53蛋白在肠腺癌中阳性表达率分别为88.8%和86.1%,与其他组相比有显著差异(P<0.01),腺瘤伴非典型增生组的表达率为81.25%和68.75%.结论在肠道增生性病变中既有GST-pi与p53蛋白的表达,并随着细胞增生程度的增高、非典型增生到肠癌其表达率亦明显增高,说明肠道病变中细胞的异型性与GST-pi、p53蛋白表达呈正相关,可作为检测肠癌和肠道的癌前期病变的一个标志物.【总页数】2页(P52-53)【作者】王翠莲;李宗铉【作者单位】中国医科大学病理教研室,110001;长治医学院病理教研室,046000【正文语种】中文【中图分类】R73【相关文献】1.肠道肿瘤和增生性病变中GST-π与p53蛋白表达的关系 [J], 张海燕;方军;高宝辉;谢小志;谢丽微2.p53 cDNA突变和突变型p53蛋白表达与大肠癌预后的关系 [J],3.大肠癌中PCNA、c-erbB2、bc1-2和P53蛋白表达与临床病理关系 [J], 康旭;冼沛中;曹军;徐飞鹏4.散发性结直肠癌中p53蛋白表达与微卫星不稳定性的关系 [J], 张艳虹;高玉彤;来茂德5.结直肠癌患者血清p53抗体与肿瘤p53蛋白表达关系的研究 [J], 张宏;丛进春;陈春生;乔雷;刘恩卿因版权原因,仅展示原文概要,查看原文内容请购买。
十六烷基三甲基溴化铵水溶液热致相变的红外光谱研究

十六烷基三甲基溴化铵水溶液热致相变的红外光谱研究
王玮;李来明
【期刊名称】《分析化学》
【年(卷),期】1992(20)7
【摘要】在280~320K的温度范围内考察了30%十六烷基三甲基溴化铵水溶液的红外光谱随温度的变化。
结果表明该体系的凝聚胶-液晶相转变温度为300K。
在300K以下的凝聚胶相,分子的极性头部基团处于高度“固定”的状态,分子的碳氢链以有序的相互平行方式排列,极性头与碳氢链之间有一定的倾斜角。
在300K
以上的液晶相,极性头内部CH_3-(N^+)基团以及整个极性头与碳氢链之间发生了旋转,碳氢链变为以六方亚晶胞填充形式存在,旦扭曲式构象异构体数量显著增多,极性头与碳氢链之间已不存在倾斜角,分子的亲水极性头和疏水碳氢链部分都处于“融化”状态。
【总页数】5页(P769-773)
【作者】王玮;李来明
【作者单位】不详;不详
【正文语种】中文
【中图分类】O647.2
【相关文献】
1.结晶十六烷基三甲基溴化铵烷基链构象的红外光谱研究 [J], 王玮;李来明
2.十六烷基三甲基溴化铵在葫芦[6]脲水溶液中的胶束化行为及分子间包络行为 [J],
杨震宇;黄香丽;杨淑玲;曹迁永;张宁
3.乙醇-水溶液中十六烷基三甲基溴化铵的自组装特性 [J], 李(韦华);张铭;张金利;韩永才
4.振动光谱法研究水溶液中十六烷基三甲基溴化铵的分子构象 [J], 王玮;李来明
5.阴阳离子表面活性剂复配体系在水溶液中的表面吸附——三氧乙基化十二醇醚磺酸钠与十六烷基三甲基溴化铵体系 [J], 王仲妮;石明理
因版权原因,仅展示原文概要,查看原文内容请购买。
Empower 3 中的新增功能

1 只处理样品组 ........................................................................................................................ 1-1 概述 ...................................................................................................................................... 1-2 “只处理样品组”权限 .......................................................................................................... 1-3 创建 “只处理样品组” ......................................................................................................... 1-3 只处理样品组编辑器注意事项 ........................................................................................ 1-4 修改 “只处理样品组”的信息 ....................................................................................... 1-5 采集基于只处理样品组的新样品组 ....................................................................................... 1-5 报告只处理样品组 ................................................................................................................ 1-6 只处理样品组注意事项 ......................................................................................................... 保存只处理样品组 .......................................................................................................... 复制和删除 “只处理样品组” ........................................................................................ 自定义字段 ..................................................................................................................... 审计追踪和修订历史注释 ............................................................................................... 审计追踪记录 ................................................................................................................. 1-6 1-6 1-6 1-6 1-7 1-7
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Business Consulting Service
Profit Center Accounting v.s. Profitability Analysis
3
Confidential
2003 IBM Corporation
Business Consulting Service
Aims of Profit Center Accounting
Currency translation
Key figures
12
Confidential
2003 IBM Corporation
Business Consulting Service
Basic Settings: Contents
Profit Center Accounting Concepts Master Data Profit Center Assignments
COPA
COOM
Transaction Currency
Company Code Currency
Controlling Area Currency
ቤተ መጻሕፍቲ ባይዱ
Account Based Profitability Analysis
COPA
Operating Costing Based Concern Currency
Reporting
Multi-dimensional Reporting
Multi-dimensional Reporting
List-oriented Reporting
Organizational Aspects
Operating Concern Operating Concern Company Code Currency Profit related key figures
Return on Capital
Which asset value is attributed to a profit center?
Cost Management
Which responsibility areas exceeded their plan last months?
7
Confidential
8
Confidential
2003 IBM Corporation
Business Consulting Service
Profit Center Balance Sheet Items and Key Figures
Result
Balance sheet items
- Assets - Material stocks - Work in process - Payables/receivables - Down Payments
CO-PA account-based Market Profitability Cost-of-Sales Accounting
EC- PCA profit centers Enterprise Controlling COS & Period Accounting
Aims of Profitability Accounting Methods
10,000 -------------Contribution margin 1 300,000 Overhead 100,000 -------------Contribution margin 2 200,000 Research & develop. 10,000 Marketing 50,000 Sales and administration 40,000 -------------Contribution margin 3 100,000
The goal of EC-PCA is to measure the profitability of areas of responsibility within the organization. Company
goods/services
Billing Document SD
PrCtr 1
Operating Concern Transactional Company Code CO Area Currency Profit related key figures
Controlling Area Transactional, Company Code PCA Reporting Currency Profit related key figures Financial Performance Ind.
Company Code
R
Currency
10
Confidential
2003 IBM Corporation
Business Consulting Service
Comparison Summary I
Comparison
CO-PA costing-based Market Profitability Cost-of-Sales Accounting
1
2003 IBM Corporation
Business consulting service
陽慶電子新一代ERP/SAP 陽慶電子新一代ERP/SAP導入專案 ERP/SAP導入專案
Profit Center Accounting
Confidential
2
2003 IBM Corporation
2003 IBM Corporation
Business Consulting Service
Reporting
Profitability Analysis
Value Fields Revenues Sales deductions Net revenues Cost Variances 1,000,000 100,000 -------------900,000 590,000
Company
ECPCA
PrCtr 4
M
PrCtr 2
Company
M
PrCtr 3
PrCtr 5
4
Confidential
2003 IBM Corporation
Business Consulting Service
Profit Center v.s. Profitability Analysis Objects to be Evaluated
COPA ECPCA
Profitability segment Characteristic Characteristic values Value fields or nodes
Profit center Profit center hierarchies Profit center Accounts
Business Consulting Service
Typical Question in Profit Center Accounting
Contribution of an organizational unit
What is the operating profit for a profit center
13
Confidential
2003 IBM Corporation
Business Consulting Service
Profit Center Standard Hierarchy
Europe
Stock Products
External Services
Custom Products
Admin & Int . Service
Contribution of Individual Market Segments
Which are the largest and fastest growing customers?
Margin goals of Individual Sales Entities
Did the sales force reach their contribution margin goal? What was the success of the most recent sales promotion for a product line?
Success of Marketing Activities
Revenue and Cost Structure
What is the impact of a pricing strategy for a group of customers?
6
Confidential
2003 IBM Corporation
11
Confidential
2003 IBM Corporation
Business Consulting Service
Comparison Summary II
Comparison
CO-PA costing-based
CO-PA account-based
EC-PCA profit centers
PrCtr PrCtr
1
PrCtr
4
Product group
PrCtr 2
Region Distrib . channel
PrCtr
3
PrCtr
5
5
Confidential
2003 IBM Corporation
Business Consulting Service
Typical Question in Profitability Analysis
Profit Center Accounting
