进口药品登记表
进口药材申请表

相关证件:1.《营业执照》编号:****许可证》编号:****
法定代表人:****职位:****
注册地址:****邮政编码:****
生产地址:****邮政编码:****
注册申请负责人:****签名:****职位:****
电话(含区号及分机号):****传真:****
21.机构(国外加工企业):****□本机构负责缴费
名称:****
组织机构代码:****
相关证件:《营业执照》编号:****
法定代表人:****职位:****
注册地址:****邮政编码:****
生产地址:****邮政编码:****
联系人:****电话:****
XXXXXX
申明
22.我们保证:①本申请遵守《中华人民共和国药品管理法》、《中华人民共和国药品管理法实施条例》和《进口药材管理办法》等法律、法规和规章的规定;②申请表内容及所提交资料、样品均真实、来源合法,未侵犯他人的权益,其中试验研究的方法和数据均为本药品所采用的方法和由本药品得到的试验数据;③一并提交的电子文件与打印文件内容完全一致。
8.出口地(国家):****
9.申请进口数量(公斤):****
10.包装材料:****
11.包装规格:****
12.合同号:****
13.检验标准:〇中国药典版
〇进口药材质量标准,标准来源
〇部颁药材标准,标准来源
〇省、自治区、直辖市药材标准,标准来源____________
〇自拟药材质量标准(仅限于无法定标准进口药材)
国家食品药品监督管理局
进口药材申请表
申请编号:XXXXXXXXX
申请事项
1.申请分类:〇首次进口药材:〇已有法定标准药材〇无法定标准药材
进口药品批件
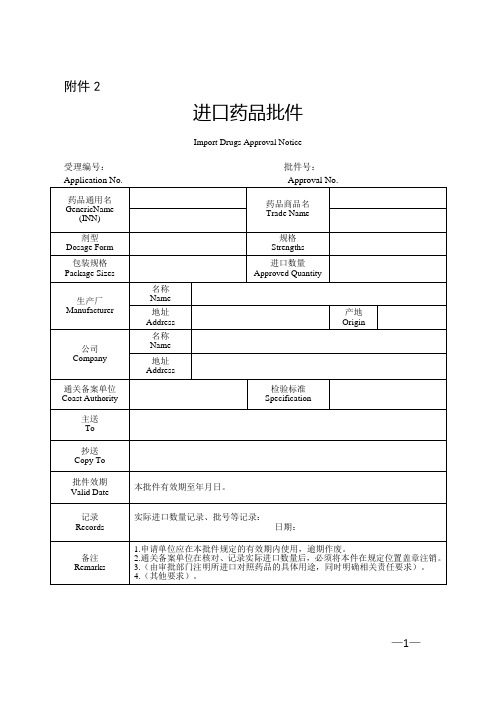
进口药品批件
Import Drugs Approval Notice
受理编号:批件号:
Application No.Approval No.
药品通用名GenericName (INN)
药品商品名
Trade Name
剂型
Dosage Form
规格
Strengths
包装规格
Package Sizes
进口数量
Approved Quantity
生产厂
Manufacturer名称NFra bibliotekme地址
Address
产地
Origin
公司
Company
名称
Name
地址
Address
通关备案单位Coast Authority
检验标准
Specification
主送
To
抄送
Copy To
批件效期
Valid Date
本批件有效期至年月日。
记录
Records
实际进口数量记录、批号等记录:
日期:
备注
Remarks
1.申请单位应在本批件规定的有效期内使用,逾期作废。
2.通关备案单位在核对、记录实际进口数量后,必须将本件在规定位置盖章注销。
3.(由审批部门注明所进口对照药品的具体用途,同时明确相关责任要求)。
4.(其他要求)。
进口申请注册表填表说明

进口申请注册表填表说明1.申请分类:按药品注册申请的分类填写,属新药的,选新药申请;属已有国家标准的药品,选已有国家标准药品的申请;属进口药品,选进口药品申请。
本项为必选项目。
2.申报时期:按照该申请申报时期选择,属临床前研究时期申报临床的,选临床试验的;属申报生产的,选择生产。
本项为必选项目。
3.注册分类:按照《药品注册治理方法》附件一、附件二、附件三的注册分类填写,未列入上述附件的注册事项,选“其他”,并应当简要填写注册事项,如新的药用辅料、体外生物诊断试剂(《关于体外诊断试剂实施分类治理的公告》国药监办[2002]324号)等。
如临床批件为试行方法类别的,做完临床试验申报生产时,直截了当按《药品注册治理方法》类别填写,不再填写原试行方法附件的类别。
注册分类中的“X类”为《药品注册治理方法》中的分类,“原X类”为《药品注册治理方法》(试行)中的分类为,“一~五类”指的是原《新药审批方法》或《新生物制品审批方法》中的类别,申请人应按照《关于实施《药品注册治理方法》(试行)有关事项的通知》(国药监注[2002]437号)中的有关要求填写。
本项为必选项目。
4.附加申请事项:在申请分类和注册分类选定后,如同时申请非处方药,则选非处方药;同时申请减免临床研究,则选减或免临床研究;属于国际多中心临床研究,则选国际多中心临床研究。
选择“其他”的,应当简要填写申请事项。
5.药品名称:应当使用正式颁布的国家药品标准或者国家药典委员会《中国药品通用名称》或其增补本收载的药品通用名称。
申报复方制剂或者中药制剂自拟药品名称的,应当预先进行药品名称查重工作。
本项为必选项目。
6.英文名/拉丁名:英文名填写INN英文名;中药制剂没有英文名的,能够免填;申报中药材的需提供拉丁名。
7.汉语拼音:均需填写,注意正确区分字、词。
8.化学名称:应当以文字正确表达药物活性物质的化学结构,不要采纳结构式。
9.其他名称:系指曾经作为药品名称使用,但现在已被国家规范的药品通用名称取代者。
Application form for Imported Drug Supplementary Registretion 进口药品补充申请表格

Application form for Imported Drug Supplementary Registretion 进口药品补充申请表格State Food and Drug Administration Drug Supplementary Registration Application–for Foreign ApplicantsEntry Number:Acceptance No:StatementWe guarantee:①T his application complies with laws and regulations such as Drug Administration Law of The People’s Republic of China, Implementing Regulation of the Drug Administration Law of The People’s Republic of China, and Drug Registration Regulation;②The content of application form, thesubmitted information and the samples are true and legal, without infringing any other’s rights. Any methods and data is results of research and the drug tests conducted on the drugs;②T he accompanied electronic version is in perfect accordance with the printed version.We will take all the legal consequences of any false statements.Other Statement Items in Particular That:Application Items1The Application for: Import registration2 Drug category:3 whether OTC or not :4 Status of the initial registration:5 Registration category:〇Supplemental applications to be approved by SFDA: □Application for Drug Approval Number of a new drug by the New Drug Certificate holder of the drug.□Use of the name of the Trade Name of drugs. □Additional indications or functions of TCM or natural drug, or theindication approved in China for chemical drugor biological products. □Change in the usage or dosage of the drugs, or the group of patient to use the drug, but without change in route of administration.□Change of strength of drugs □Change of the supplementive in the formula of the drugs, where there is a medial requirement for it. □A change in the drug manufacture technology and process affecting drug quality. □Amendment of drug registration standards.□Substitute or removal of the drug material listed in formula of National Drug Standards as toxic or endangered.□Change of the immediate packing material or container of the import drugs, domestic injection, ophthalmologic, spray, powder Aerosol, Inhaler and Spray. Use of new immediate packing material or container.□Application for combined packing of drug.□The transfer of new drug technology.□Addition or amendment of items in insert sheet of TCM or natural drug, such as pharmacology and toxicology, clinical trial and Pharmacokinetic.□A change in items within the import drug registration certificate, such as name of the drug, drug enterprise name, registered location, packing specification.□Change of the location where the import drug is manufactured.□Change of the location where the import drug is packed overseas.□Repacking of import drugs in China.□Change of the location where the raw material for import preparation is manufactured.〇Supplemental applications to be approved by PDA and be filed for record at SFDA, or directly be filed for record at SFDA: □Change of the name of a domestic drug manufacturer.□Internal change of the manufacture workshop of a domestic drug manufacturer.□Change of immediate packing material or container (except for the item 10 as above)□Change of valid period of domestic drugs□Change of manufacture location of import drugs□Change of appearance of the drug withoutchange of drug standards.□Amendment of insert sheet of the drugs according to national drug standards or required by SFDA.□Supplementing and perfecting of the drug safety part of the insert sheet.□Modification of design of packing and label of the drugs according to the regulation.□Change of the agent for import drug registration.□Others〇Supplemental applications to be filed for record at PDA: □Amendment of insert sheet of the domestic drugs according to national drug standards or required by SFDA.□Supplementing and perfecting of the domestic drug safety part of the insert sheet.□Modification of design of packing and label of the domestic drugs according to the regulation.□Change of the packing specification of domestic drugs.□Change of manufacture location of domestic drugs□Change of appearance of the domestic drug without change of drug standards.□othersDrugs Information6 Generic Name:7 Generic Name Source:8 English / Latin name:9 Chinese Phonetic Alphabet:10 Chemical Name:10 Trade Names:11 Product category:12 strength:13 Other accepted or submitted preparation and Strength at the same time:14 Packaging: immediate packing material:Packaging size:15 Date of Expiration: 36 months16 Prescriptions (Including Prescription Volume):API/materials in TCM(TraditionalChinese medicine):Accessories:17 Materials /Accessories SourceSerial NO.Materials/AccessoriesNameApproval No/RegistrationNo/Accepted NoManufacturerImplementationStandardsVariationor notVariationapproved statusand approvedinstitution1218 Chinese Medicinal Materials Standard:Serial Numbe r Materials/Accessories NameWhetherlegal ornotStandardreferenceImplementationStandardsVariation ornotVariationapprovedstatus andapprovedinstitution1219 Indications or Attending Functions: Indications category:Supplementary contents:20 Supplementary contents:21 Rational to propose this supplementary:22 Initial approved registration contents and relevant information:Initial acceptance No:Clinical Trial Approval No:Initial IDL No:Drug specification No.:Relevant Conditions23 Patents:□Have Chinese patent: □chemical compound patent; □formulation patent; □process patent; □other patent;Patent No.: __________________Patentee: __________________Patent licensing/Publication date __________________□Have foreign patentPatent No.: __________________Patentee: __________________Patent licensing/Publication date __________________Patent Ownership Statement: __________________We state that: the application does not cause patent infringement.24 Variety Protection of Chinese Medicine: Variety Protection of Chinese Medicine expirydate:25 Monitoring Time With Same Variety of New Drugs:Expiry date: __________________26 Times for Applications:〇First time application 〇multi-times application the times application □Withdrew before, date__________________ reason: ______□not approved, date__________________ reason: ______The Applicant and Commissioned Research Institutions27. Institutions 1 (Foreign Pharmaceutical Companies):Chinese Name:English Name:Legal Representative: Position: Registered Address:Country or Region:Head of An Application for Registration: Positions:Tel: Fax:E-mail:Legal Representative (Signatures): (Department Official Seal)Month Day, Year28. Institutions 2 (Imported Drugs Production Plant):Chinese Name:English Name:Legal Representative: Position: Registered Address:Country or Region:Head of An Application for Registration: Positions:Tel: Fax:E-mail:Legal Representative (Signatures): (Department Official Seal)Month Day, Year29 Institutions 3 (Imported Drugs Foreign Packaging Factory):Chinese Name:English Name:Legal Representative: Position: Registered Address:Country or Region:Head of An Application for Registration: Positions:Tel: Fax:E-mail:Legal Representative (Signatures): (Department Official Seal)Month Day, Year30 Institutions 4 (Imported Drugs sub- Packaging Factory):Chinese Name:English Name:Organization code:Pharmaceutical production license No.: Legal Representative: Position: Registered Address: zip code: Postal address: zip code: Head of An Application for Registration: Positions:Tel: Fax:E-mail:Mobile phone:Legal Representative (Signatures): (Department Official Seal)Month Day, Year31 Institutions 5 (Registration Agency of Imported Drugs):This agency is responsible for paymentChinese Name:English Name:Organization Code:Legal Representative: Position: Registered Address:Zip Code:Contact Address:Zip Code:Head of an Application for Registration:Position:Contact: Position: Phone : Fax :E-mail: phone:Legal Representative (Signatures):(Department Official Seal)Month Day, Year32 Commissioned Research Institutions:No program forResearch Name oftheinstitutionResponsiblepersonTel.uthoritiesAfter reviewed, the table is in line with the formwith the request.Authorities: Reviewer (Signatures) Date:。
山西省特殊药品进口申请表
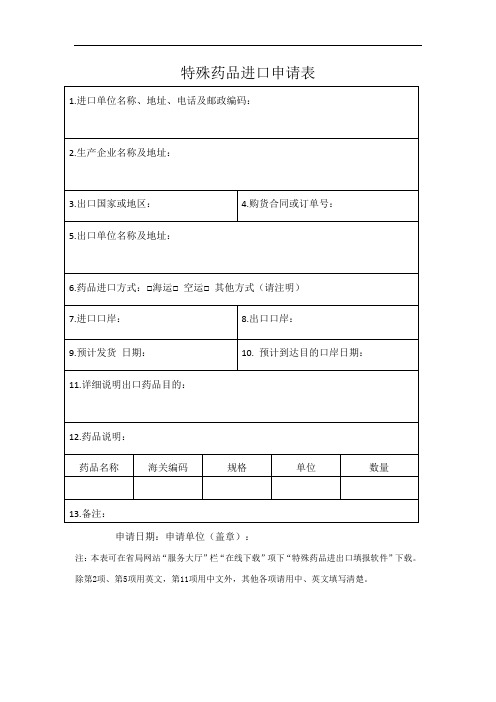
药品名称
海关编码
规格
单位
数量
13.备注:
申请日期:申请单位(盖章):
注:本表可在省局网站“服务大厅”栏“在线下载”项下“特殊药品进出口填报软件”下载。除第2项、第5项用英文,第11项用中文外,其他各项请用中、英文填写清楚。
特殊药品进口申请表
1.进口单位名称、地址、电话及邮政编码:
2.生产企业名称及地址:
3.出口国家或地区:
4.购货合同或订单号:
5.出口□其他方式(请注明)
7.进口口岸:
8.出口口岸:
9.预计发货 日期:
10.预计到达目的口岸日期:
11.详细说明出口药品目的:
放射性药品及其原料进口审批表

放射性药品及其原料进口审批表
申请文号:受理编号:批准文号:
1.本表一式3份,商务部、进口单位、用户单位各1份,有效期为自然年末。
2.本表格式与内容不得擅自更改,清单容量不够的,审批表分多页打印,每页加盖进口单位和用户单位公章。
3.申请单位应持获得批准的“放射性药品及其原料进口审批表”在商务部办理进口许可证,海关凭商务部签发的进口许可证办理验放手续。
4.进口活动完成20日内,进口单位和用户单位应分别向所在地省级环境保护部门备案,所有进口备案必须在次年1月15日前完成。
中华人民共和国环境保护部监制。
进口药品抽样记录单

进口药品抽样记录单
记录单编号:抽样日期:年月日
药品名称:商品名:
注册证号:检验通知号:
1. 存货地现场情况记录
1.1存货地点:
1.2抽样地点:
1.3储存条件等:
2. 货物包装情况记录
2.1外包装是否完整□;是否封固□(铅封□;塑料插封□;胶纸封□;其他封:)
2.2外包装为:铁桶□;纤维纸桶□;铝听□;硬纸板箱□;木箱□;牛皮纸袋□;蛇皮袋□;其他;
2.3内包装为:玻瓶□;纸盒□;塑料袋□;其他;
3. 药品包装标签与注册证核对情况记录:
3.1 □品名、规格、包装规格、有效期、生产厂商、注册证号等与注册证所载内容
一致;
3.2 □批号、数量和件数与报验时一致;
3.3 □不一致内容:(详细列出)
4 抽样情况记录,包括所抽桶(箱、听、袋)号、批号、数量:
5 抽样结论:
抽样单位:药检所经手人:
报检单位:经手人:
(请注意背面“注意事项”) 国家食品药品监督管理局制〖LM〗
注意事项
1.此记录单一式四份,由口岸药品检验所填写。
一份交负责通关备案的口岸药品监督管理局,一份交报验单位,一份留档。
对需进入海关监管区抽样的品种,抽样完成后,尚应将一份交负责海关。
2.表中注“□”处,应当根据现场查验的实际,是该情况则用“ ”标出,不是则用“×”标出。
3.现场查验完毕,口岸药品检验所应当在“抽样结论”一栏明确标出“符合规定,已予抽样”或“不符合规定,不予抽样”的字样,以便于口岸药品监督管理局据此妥善处理通关备案事项。
4.此单填写完毕,口岸药品检验所和报验单位对其内容核实无误后,双方经手人签字后生效。
