Effects of pretreatment of substrates on the preparation of large scale ZnO nanotube arrays
CFS_预处理对不同秸秆原料酶解和理化结构的影响

山西农业科学 2023,51(12):1426-1434Journal of Shanxi Agricultural SciencesCFS 预处理对不同秸秆原料酶解和理化结构的影响田鑫,王雨萌,徐师苗,汪强杰,胡轲,张海波,程红艳(山西农业大学 资源环境学院,山西 太谷 030801)摘要:高铁酸钾复合液(CFS )是制备高铁酸钾的剩余滤液,其含有大量碱(OH -)和氧化剂(ClO -和Fe 6+),具有破坏木质纤维素顽固结构、提升酶解效率的潜力。
为实现秸秆的资源化利用与高铁酸钾制备废液的再利用,以山西储量丰富的玉米秸秆(CS )、高粱秸秆(SS )和谷子秸秆(MS )为原料,采用CFS 进行预处理,对比3种秸秆的酶解糖化率,分析秸秆的理化结构变化。
结果表明,CFS 预处理中碱和氧化剂共同参与了3种秸秆的降解,促进了酶解糖化率;在最佳预处理时间24 h 下,CS 、SS 和MS 的还原糖产量分别较对照提高252.77%、236.39%、216.66%,其中CS 的酶解效率最高;组分分析表明,CFS 处理能有效去除3种秸秆中木质素成分,增加纤维素相对含量,进而有利于纤维素酶的可及性;结构分析显示,CFS 处理后,3种秸秆的理化结构发生了不同程度变化,粗糙度增加,官能团发生断裂,纤维结晶度升高,热稳定性变差。
在3种秸秆中,CS 结构变化最明显,更有利于被生物转化。
综上,CFS 预处理可改变作物秸秆的理化结构,破坏其致密结构,促进后续酶解效率,是一种理想的预处理技术。
关键词:高铁酸钾复合液(CFS );预处理;作物秸秆;还原糖产量;理化结构中图分类号:S141.4 文献标识码:A 文章编号:1002‒2481(2023)12‒1426‒09Effects of CFS Pretreatment on Enzymatic Hydrolysis and PhysicochemicalStructure of Different Straw MaterialsTIAN Xin ,WANG Yumeng ,XU Shimiao ,WANG Qiangjie ,HU Ke ,ZHANG Haibo ,CHENG Hongyan(College of Resources and Environment ,Shanxi Agricultural University ,Taigu 030801,China )Abstract : Composite ferrate solution(CFS) is the residual filtrate for preparing potassium ferrate. It contains a lot of alkali (OH -) and oxidant(ClO - and Fe 6+), which has the potential to destroy the recalcitrant structure of lignocellulose and improve the efficiency of enzymatic hydrolysis. In order to realize the utilization of straw resources and reuse of preparation waste liquid of potassium ferrate, in this paper, corn straw(CS), sorghum straw(SS), and millet straw(MS), which are abundant in Shanxi province, were pretreated with CFS, the enzymolysis and saccharification rates of the three kinds of straw were compared, and the change of physicochemical structure of the straw was analyzed. The results showed that the alkali and oxidant in the pretreatment of CFS were involved in the degradation of three kinds of straw, which promoted the enzymatic hydrolysis rate and saccharification rate. Under the optimal pretreatment time of 24 h, the reducing sugar yield of CS, SS, and MS was increased by 252.77%, 236.39%, and 216.66% compared with that of the control, respectively, and the enzymatic hydrolysis efficiency of CS was the highest. Component analysis showed that CFS treatment could effectively remove lignin in three kinds of straw and increase the relative content of cellulose, which was beneficial to the accessibility of cellulase. Structural analysis showed that after CFS treatment, the physicochemical structure of the three kinds of straw changed in different degrees, roughness increased, functional group fractured, fiber crystallinity increased, and thermal stability decreased. Among the three kinds of straw, CS had the most obvious structural change and was more conducive to biotransformation. In conclusion, CFS pretreatment could change the physicochemical structure of crop straws, destroy the dense structure and promote the efficiency of subsequent enzymatic hydrolysis, so it was an ideal pretreatment technology.Key words :composite ferrate solution(CFS); pretreatment; crop straw; reducing sugar yield; physicochemical structuredoidoi:10.3969/j.issn.1002-2481.2023.12.11收稿日期:2023-01-04基金项目:山西省高等学校科技创新项目(2020L0137);山西农业大学科技创新基金项目 (2018YJ39);山西省优秀博士来晋工作奖励基金(SXYBKY201803);国家自然科学基金(52100149);山西省水利科学技术研究与推广项目(2022GM034)作者简介:田 鑫(1997-),女,山西汾阳人,在读硕士,研究方向:农业环境保护与废弃物资源化利用。
等离子体处理对紫云英种子萌发和生理特性的影响

第 32 卷第 10 期Vol.32,No.10129-1402023 年 10 月草业学报ACTA PRATACULTURAE SINICA李想,张梦,刘春增,等. 等离子体处理对紫云英种子萌发和生理特性的影响. 草业学报, 2023, 32(10): 129−140.LI Xiang,ZHANG Meng,LIU Chun-zeng,et al. Effects of dialectric barrier discharge plasma treatment on seed germination and physiological characteristics of Astragalus sinicus. Acta Prataculturae Sinica, 2023, 32(10): 129−140.等离子体处理对紫云英种子萌发和生理特性的影响李想1,张梦2,刘春增2,朱益飞3,叶晓馨1*(1.安徽大学资源与环境工程学院,安徽合肥 230601;2.河南省农业科学院植物营养与资源环境研究所,河南郑州 450002;3.空军工程大学航空工程学院,陕西西安 710038)摘要:为打破紫云英种子硬实,提高种子活力和发芽一致性,本研究以赣紫75-3-51和信紫1号为供试材料,采用室内发芽试验探讨了不同剂量等离子体处理(7、8、9 kV电压分别处理1、3、5、10 min)对紫云英种子活力、幼苗生长、抗氧化酶活性以及渗透调节物质含量的影响,以期为紫云英种子播前处理技术提供参考。
结果表明:等离子体处理对2种紫云英种子的发芽率和发芽势没有显著影响,但提高了紫云英种子活力。
不同品种紫云英对等离子体处理的响应存在差异。
等离子体处理对赣紫75-3-51生长存在低促高抑的现象,幼苗鲜重以及胚芽长度均随处理时间增加呈单峰曲线变化。
信紫1号幼苗鲜重在高剂量(9 kV处理10 min)等离子体处理条件下显著降低,较对照降低了20.5%。
pretreatment翻译

pretreatment翻译pretreatment的翻译是:预处理。
用法:pretreatment是一个名词,用于描述对某物进行预先处理的过程。
在科学、工业和医学领域,pretreatment常用于指代在实验、生产或治疗之前对样品、原料或病情进行预先处理的步骤。
预处理的目的可以是去除杂质、减少干扰、改善反应效果、提高效率等。
例句:1. The pretreatment of the soil sample involves removing debris and contaminants.土壤样品的预处理包括清除杂物和污染物。
2. For this experiment, the pretreatment of the testtubes includes sterilization and rinsing.在这个实验中,试管的预处理包括消毒和冲洗。
3. The pretreatment of the wastewater before treatmentwith a chemical agent is essential for optimal results.在用化学剂处理废水之前的预处理对于获得最佳结果至关重要。
4. Pretreatment of the raw material is necessary to ensure the quality of the final product.对原料进行预处理是为了确保最终产品的质量。
5. The pretreatment of the patient's condition involves conducting diagnostic tests and assessing medical history.对患者病情的预处理包括进行诊断检查和评估病史。
6. The pretreatment of the fabric involves washing and drying to remove any impurities.对面料的预处理包括清洗和烘干以去除任何杂质。
丁基苯酞研发的实践体验--冯亦璞

140 120
***
Vehicle
* *** ** *** *
dl-25
Nim 0.3
*** *** *** *** *** *** * *** *** ***
100
*
**
80
Drug
*
*
ischemia
60 40 20 0
norm 5
抑制谷氨酸释放
丁基苯酞抗脑缺血损伤的多种 作用机制(二)
增加内皮细胞 NO,eNOS,PGI2, (与增加rCBF有关) 降低[Ca2+]i (抑制细胞内钙库释放)
抑制炎症,抑制粘连分子
保护神经细胞(高K+,低糖低氧,NMDA,H2O2,Aβ)
脑保护作用是通过多种作用机制,以干预脑损伤的多个病理环节。
1.改善脑血流的药物
溶栓药:
rt-PA(重组组织型纤溶酶原激活剂) FDA批准,时间窗3h内,颅内出血6.4% 溶栓效果优于尿激酶 尿激酶,6h内, “九五”攻关随机双盲研究 初步结果(+) 原尿激酶,链激酶(-)
降纤药:
降纤酶、巴曲酶
可改善急性患者近期神经功能及日常生活能力 (需注意出血倾向)
及制剂高技术产业化示范工程”项目资助(计高技
[2002]2269号)。 4. 国家“十五”863重大科技专项“创新药物与中药现 代化”项目的支持(2002AA2Z3101, 2002,11~)
丁苯酞开发的背景和依据
脑卒中流行病学
死亡率和致残率很高 经济负担和社会负担 脑卒中的防治已成为医药卫生 工作中的重要课题
**
30
60
90
外文翻译--- 利用厌氧消化从微波加热的污泥中获取沼气

附录一Biogas recovery from microwave heated sludge byanaerobic digestionBiogas generated from sewage sludge, livestock waste, and food waste by anaerobic digestion is a valuable renewable energyresource. However, conventional anaerobic digestion is not an efficient process. A long hydraulic retention time and low biogasrecovery rate hinder the applications of those resources. An effective pretreatment method to destroy sludge microbial cellshas been one of the major concerns regarding improvement of the biogas production. This article focuses on the effects of microwave heating on sludge anaerobic digestion. Volatile suspended solid (VSS) and chemical organic demand solubilization of heated sludge were investigated. Microwave heating was found to be a rapid and efficient process for releasing organic substrates from sludge. The increase of organic dissolution ratio was not obvious when holding time was over 5 min with microwave heating. The effect of the VSS solubilization was primarily dependent on heating temperature. The highest value of VSS dissolving ratio, 36.4%, was obtained at 170°C for 30 min. The COD dissolving ratio was about 25% at 170°C. Total organic carbon of treated sludge liquor was 1.98 and 2.73 g/L at 150°C and 170°C for 5 min, respectively. A biochemical methane potential (BMP) test of excess sludge and a mixture of primary and excess sludge demonstrated an increase in biogas production. The total biogas from microwave treated mixture sludge increased by 12.9% to 20.2% over control after 30 days of digestion. Biogas production was 11.1% to 25.9% higher for excess sludge than for untreated sludge. The VS removal ratios of mixture sludge and excess sludge were 12% and 11% higher, respectively, compared to the untreated sludge.biogas recovery, microwave heating, sludge, anaerobic digestion 1 IntroductionWastewater treatment plants produce large amounts of primary and excess sludge that contains organic bacterial microbes and inorganic mineral components. State EPA reports have indicated that there are approximately 11 million tons of dewatered sludge cakes (about 80% moisture content) generated annually in China. In recent years, treatment and disposal of sludge have become a serious problem in many cities.Anaerobic digestion is a common process for sludge treatment. Compared with other processes, its advantages are lower energy requirement, better stabilized product, and generation of usable gas. However, the biological gel structure properties of sludge result in difficulties in anaerobic digestion. Pavlostathisetal.andVavilin et al.found that the bacterial cell wall restrained the biodegradability of sludge. An effective pretreatment method to destroy microbial cells has therefore been one of the major concerns in the sludge pretreatment process. Wang et al. Baier et al. Lin et al.and Tanaka et al.separately carried out sludge pretreatment research to improve biogasproduction and included ultrasonic, mechanical,alkaline, and thermal-chemical treatments for degradation of microbes. Heat treatment was a harsh process that disrupted bacterial cell wall, and released and hydrolyzed high molecular weight materials. Brook found that the hydrolysis of organics was a dominant characteristic that distinguished heat treatment from other methods. Industrial application has proven the effectiveness of heat treatment; for example, Kepp et al. stated that when sludge was heated with a Cambi process at 170°C, the volatile solids (VS) removal ratio of the treated sludge increasedfrom about 40% to approximately 60%. Using the advantages of the improved settling performance of heated sludge,Wang et bined heat treatment with an anaerobic sequenced batch reactor to increase the VS removal ratio to 60% with a lower hydraulic retention time (10 days).However, conventional heat treatment is time-consuming .For the purpose of heating sludge, microwave irradiation might serve as an alternative and much more rapid method .In recent years, the use of microwave as a novel technique to treat sludge has attracted much interest.A uniform microwave field generates energy through the realignment of dipoles with oscillating electric fields to generate heat both internally and at the surface of the treated material. Sludge is a multiphase medium containing water,mineral and organic substances, proteins, and cells of microorganisms.Due to its high water content, sewage sludge can absorb significant amounts of microwave energy.Zlotorzynski analyzed the application of microwave irradiation to analytical and environmental chemistry.Eskicioglu et ed sludge heated by microwave to 96°C in a batch anaerobic digestion test and found a 17% biogas increase over untreated sludge. Compared to conventional heat treatment, microwave treatment resulted in more soluble proteins and volatile fatty acids but a lower sugar content of the sludge. Park etal.reported that microwave treated sludge could produce 79% higher methane production than untreated sludge. Wojciechowska used microwave to condition sludge and found that after 180 s of microwave heating, the specific resistance to filtration (SRF) of mixed sludge (primary and secondary sludge)and anaerobic digested sludge decreased by 73% and 84%,respectively. Liao et al.reported that organic hydrolysis,induced by combing microwave with hydrogen peroxide and acid, could be used to recover sludge nutrients.It is evident that the effectiveness of microwave treatment has been recognized by many researchers. However,the exact nature of the sterilization effect, as well as whether this is due solely to thermal effects or to non- thermal effects, has continued to be a matter of controversy. In most conventional heat treatments, sludge is heated at a mild temperature using an open vessel. The higher temperature and pressure that are generated by microwave treatment of sludge in terms of overall biodegradability were investigated in the present paper.2 Materials and methods2.1 Sludge samplingSludge was sampled from three local municipal wastewater treatment plants (the Gaobeidian, Qinghe, and Beixiaohe wastewater treatment plants) in Beijing. These three wastewater works primarily treat municipal sewage. Table 1 presents the characteristics of the sludge. The mixturesludge (MS) was mixed by combining primary and excess sludge sampled from the gravity thickening tank in the Gaobeidian and Beixiaohe plants. Excess sludge (ES) collected from Qinghe plant was thickened in laboratory to a suspended solid (SS) content of 2.8%. After sampling, sludge wasscreened through a 3.2 mm×3.2 mm mesh sieve to remove large particles. The screened sludge was then stored in a refrigerator at 4°C until further testing.MS from Gaobeidian plant was used for the investigation of organics solubilization of sludge with microwave heating.Microwave treated MS from Beixiaohe plant and ES from Qinghe plant was used for evaluation of biodegradation by abiochemical methane potential (BMP) test. Table 1 shows the SS, VS, total COD, and pH.2.2 Microwave heating procedureA commercial domestic microwave oven (2450 MHz, 1000W, MSD6, Shanghai Sineo Co., Ltd) and PTFE vessels were used for microwave irradiation. This frequency of microwave energy has been widely used in scientific research.Sludge microwave heating was performed as batch tests using 30 mL of sludge in a 70 mL PTFE vessel. All test samples were subject to microwave heating at temperatures of 80, 120, 150 and 170°C. The microwave heating holding times were 1, 5, 10, 20 and 30 min. Sludge temperature and pressure were measured and controlled by the microwave oven.2.3 Biochemical methane potential (BMP) testA biochemical methane potential test was used to evaluate biogas recovery from sludge after microwave pretreatment.A 60 mL sample of microwave-heated sludge, seeded with 150 mL of anaerobic digestion sludge, was fed into a 250mL serum bottle. The seed sludge was collected from an anaerobic digestion tank at the Gaobeidian plant. In this plant, gravity thickened sludge was digested at 35°C with 30 days of HRT. A separate 60 mL sample of untreated sludge was used as a control sample. Each test was performed with parallel samples. The BMP tests were performed in a water bath at 35°C. The cumulative gas production was measured using a water displacement method. The serum bottles were shaken every 12 h to allow for sufficient blending. The methane content in the biogas was measured by a gas chromatograph equipped with a thermal conductivity detector.2.4 Analysis methodsThe total COD (TCOD) was determined by the potassium dichromate/ferrous ammoniumsulfate method. Sludge particles were kept uniformly suspended by a magnetic stirrerwhile sampling. The supernatants were separated from sludge by centrifuging (LG10-2.4A) at 2775 g for 10 min and were used for soluble COD (SCOD) determination. The total solid (TS) and SS were measured by drying sludge slurry at 105°C for 24 h; VS and VSS were tested by burning the dried sludge at 600°C for 2 h. For SS and subsequent VSS analysis, sludge was centrifuged prior to heating,to remove soluble solids as described in SCOD determination.TOC of sludge liquid was measured by Shimadzu’s TOC-5000.3 Results and discussion3.1 Temperature increases by microwave heatingCompared with conventional sludge heating, microwave heating is much more rapid. When materials are heated by high frequency electromagnetic waves, the heating effect arises from the interaction of the electric field component of the wave with charged particles in the material. Power absorbed by materials becomes higher as the penetration depth decreases. As a result of the complicated composition of sludge, the absorption of microwave energy will be influenced by organics (such as proteins, lipids, and carbohydrates)and solid concentration, as well as by the heatingLoad. Hong et al.reported that water absorbed microwave energy was in an exponential relationship with the heating load, and that the absorption efficiency could reach 80%.Figure 1 presents the heating and cooling curves in sludge microwave treatment at 120, 150 and 170°C for 5 min. Under microwave irradiation, sludge temperature increased rapidly, and the heating ratios were similar for the different temperatures. The microwave irradiation times to 120, 150 and 170°C were 4, 7 and 7.5 min, respectively. When the sludge was heated to pre-set temperature, sludge was kept at a stable temperature for 5 min. This time was called heating time. When the heating finished, the reactor filled with sludge was transferred from microwave oven into a cool water bath. The decline parts of the curves in Figure 1 representthe cooling of sludge.3.2 Organic sludge dissolving trendsThe conventional heat treatment performed by Wang et al. demonstrated that inorganic components dissolved at a lower dissolution ratio, and that the main part of the solid dissolution was due to VSS hydrolysis. Brooks presented a summary of the solid matter in the sludge and followed their pathways of dissolution and hydrolysis. First of all, the floc of microorganism was found to disperse anddisintegrate. The intracellular material was released, dissolved,and hydrolyzed as follows: lipids were hydrolyzed to palmitic acid, stearic acid, and oleic acid; proteins were degraded to a series of saturated and unsaturated acids,ammonia, and some carbon dioxide, while carbohydrates were broken down to polysaccharides of smaller molecular weight and, possibly, even to simple sugars. Therefore,volatile suspended solid (VSS) were generally taken as a principal parameter of organic hydrolysis.VSS dissolution depicted the tendency of sludge to become an inorganic product. Figure 2 presents changes in sludge VSS dissolution under different conditions. Holding times from 1 to 30 min were used at the temperatures of 80,120, 150 and 170°C. The VSS dissolution ratios substantially increased with rising temperature and prolonged holding time. However, the increases in dissolution were not obvious when the holding time was beyond 5 min. The effect onthe VSS dissolution was mainly dependent on the temperature. The highest value of VSS dissolution ratio,36.4%, was obtained for a treatment at 170°C for 30 min.The COD dissolution was the portion of TCOD in the sludge solid that was hydrolyzed into the liquor during the microwave irradiation. COD dissolution showed organicmatter dissolution. Microwave irradiation caused significant increases in COD concentrations. This corresponded to cell damage as a mechanism of microwave thermal treatment.The highest COD dissolution was 25.8% at 170°C for 10min (seen in Figure 3).The tendency toward COD dissolution, as affected by microwave heating temperature and time, was consistent with the VSS dissolution. Accordingly, SCOD concentration of treated sludge also showed a similar trend with temperature and holding time. As shown in Figure 4, at 170°C,the SCOD of sludge was about 10 g/L. As also shown in Figure 5, the mean value of TOC concentration increased with the microwave irradiation temperature and time, and reached the highest value, 3.4 g/L, with a treatment of 170°C for 30 min. The microwave thermal pretreatment caused a substantial dissolution and hydrolysis of organics.This suggests that microwave irradiation is capable of additionally decomposing complex chemical compounds and hydrolyzing them into simple compounds that can then be easily decomposed by bioprocesses. This effect can be used to enhance the sludge digestion process, as shown in the present results.3.3 Biogas recovery from microwave treated sludgePino-Jelcic et al. compared microwave treatment with conventional heat treatment at 60–65°C,and found that the sludge VS removal ratio of microwave-treated sludge by anaerobic digestion was 53.9%, while the ratio was 51.3%for conventional thermal treated sludge with anaerobic digestion.Microwave treatment was helpful in disrupting the cell membranes of sludge bacteria, destroying more E. Coli and releasing more intracellular materials. Heo et al. used a BMP test to evaluate the anaerobic digestibility of alkaline-treated sludge. A hydrolysis test showed that the VSS dissolution did not increase significantly with the prolongation of holding time beyond 5 min and that VSS dissolution was low at 80°C.In the present study, microwave heated sludge used for the BMP test was heated to temperatures of 120, 150 and 170°C for 5 and 10 min. Compared to ES, primary sludge and amixture of primary and ES could be readily digested.In order to analyze the microwave effect on different types of sludge, both MS from Beixiaohe and ES from Qinghe were tested. Cumulative biogas production of MS is shown in Figure 6. After microwave treatment, total biogas production increased by 12.9% to 20.2% over the control after 30 days of digestion. Figure 7 presents the cumulative total biogas production of ES. This production was 11.1% to 25.9% higher than untreated sludge. The highest biogas production was obtained from the sludge treated by microwave at 170°C for 10 min. Microwave heating as a pretreatment method for MS and ES therefore appeared to be effective in obtaining higher biogas production.Both batches used for BMP gas production showed a fast rate for the first 10 days, then the gas production ratio decreased and stabilized. As seen in Figures 6 and 7, the amount of biogas generated for MS from Beixiaohe plant was higher than that from ES. This was most likely due to differences in organic load, as MS contains more organic content than ES. However, microwave pretreatment improved the sludge anaerobic digestibility for both MS and ES. The microwave treatment temperature was more sensitive for MS than for ES.VS removal ratio in anaerobic digestion was another parameter that affected sludge biodegradability. Figures 8 and 9 present the VS removal ratios of the microwave treated MS from Beixiaohe plant and ES from Qinghe plant, respectively.The VS removal ratio of MS microwave treated at 170°C for 5 min was 12% higher than that for the untreated sludge. For ES, the VS removal ratio increased by 11%compared to untreated sludge.4 ConclusionsMicrowave heating using a domestic microwave oven with a frequency of 2450 MHz wasable to accomplish a rapid temperature increase in sludge. Therefore, as an alternative method, microwave treatment should also prove effective on an industrial scale. VSS dissolution approached values comparable to those by conventional heat treatment. The COD dissolution and the changes of TOC also indicated the same degree of organic component hydrolysis. At 170°C,the VSS dissolution ratio of treated sludge reached 36.4% and COD dissolution ratio was about 25%. Under this typical hydrolysis parameter, microwave irradiation could shorten holding time to 5 min, compared to conventional processes that require more than 30 min. This provided the possibility of shortening system sludge retention time,therefore saving energy and construction costs for industrial applications.Compared with microwave conditioning, higher temperature with a pressure vessel could also bring notable effects with relatively mild temperatures. Microwave irradiation was shown to be effective at improving sludge biodegradability for both MS and ES, allowing a greater recovery of biogas. The BMP test showed a significant improvement in biogas production and in the VS removal ratio. The results of this study indicate that higher biogas production is possible at temperatures no higher than 170°C.利用厌氧消化从微波加热的污泥中获取沼气通过厌氧消化的污水污泥,禽畜废物,食品废物产生沼气是一种宝贵的可再生能源资源。
荧光定量PCR法测定重组人干扰素α2b原液中宿主DNA的残留

荧光定量PCR法测定重组人干扰素α2b原液中宿主DNA的残留苏;周朝东;黄哲;尉然【摘要】目的:建立重组人干扰素α2 b原液中宿主DNA残留量测定的方法并进行验证,用于对该产品的质量控制。
方法通过wako DNA提取试剂盒提取干扰素原液中的宿主残留DNA,再利用SYBRGreen染料法对样品和标准DNA进行定量PCR测定,根据标准曲线对样品中的DNA残留量分析。
对建立的方法进行引物特异性以及结果准确性和精密性的验证,同时对企业提供的3批干扰素原液中的残留 DNA 测定。
结果该方法检测假单胞菌基因组 DNA 的最低准确定量浓度可达12 fg/μL, DNA 含量在12 fg/μL~120 ng/μL范围内线性良好,标准曲线的相关系数r=0.998; wako DNA提取试剂盒提取不同量的加标样品回收率均在50%~200%范围内;该方法检测3批干扰素原液的DNA残留量均低于标准限度,符合《中国药典》三部2010年版和2015年版中关于假单胞菌产重组人干扰素α2 b原液中宿主DNA残留量的要求。
结论 wako DNA提取试剂盒能解决干扰素原液中样品前处理的技术难点,与定量PCR法结合能够简便、快速、准确地对干扰素原液中残留的DNA定量测定。
%Objective To develop and verify a method for determination of residual host cell DNA in recombinant human interferon α2b substances, which is used for the quality control of the product.Methods The residual host cell DNA was extracted by wako DNA extractor kit and determined by SYBRGreen based q-PCR using standard DNA as control.The residual host cell DNA was analyzed according to the standard curve.The developed method was verified by primer specifity, results accuracy and precision and used for determinationof 3 batches of interferon substances. Results The minimum quantitative limit of residual host cell DNA by the developed method was 12 fg/μL, while the linear range was 12 fg/μL-120 ng/μL, with a correlation coefficient (r) of 0.998.The designed primers were specific to the DNA templates.The recovery rates of spiked samples with different DNA quantity were between 50%-200%.The residual host cell DNA determined by this method were not more than the limit, which were complied withthe requirements for residual host cell DNA in Chinese Pharmacopeia( volume III,2010 edition and 2015 edition) .Conclusion The wako DNA extractor kit could successfully solved the technical difficulties of sample pretreatment during residual DNA assay.The q-PCR method was simple, rapid and accurate for quantitation of residual host cell DNA in interferon substances.【期刊名称】《中国生化药物杂志》【年(卷),期】2016(036)004【总页数】3页(P193-195)【关键词】wako DNA提取试剂盒;荧光定量PCR;假单胞菌;干扰素;DNA残留;质量控制【作者】苏;周朝东;黄哲;尉然【作者单位】天津市药品检验所生化室,天津300070;天津市药品检验所生化室,天津 300070;天津市药品检验所生化室,天津 300070;天津华立达生物工程有限公司,天津 300457【正文语种】中文【中图分类】R917重组人干扰素α2b具有广谱抗病毒、抑制细胞增殖、提高机体免疫功能等作用[1-3]。
普萘洛尔对映体在经不同诱导剂诱导的人肝细胞中的代谢特征

本实验研究的手性药物是普萘洛尔(Propranolol, PPL)对映体。
普萘洛尔为非选择性β-肾上腺素受体阻滞剂,口服后药物达峰时间为1~3小时,t1/2为2~5小时。
主要在肝脏代谢,首过效应60%~70%,生物利用度仅为30%。
临床上使用的普萘洛尔是左旋异构体S(-)- PPL和右旋异构体R(+)- PPL等量混合的消旋品。
目前已证实S(- )型对映体的β受体阻断作用要比R (+)型约强100 倍[6]。
普萘洛尔在CYP450酶系中代谢, 各亚族均有不同程度的催化作用,文献显示以CYP 2D6和CYP 1A2 作用较强。
以往的研究主要是集中在两个酶对普萘洛尔的代谢,而对其它酶参与其代谢的研究则甚少涉及。
本课题选择CYP 1A1和CYP 3A4进行考察。
其主要的原因是CYP 1A1虽在肝脏中的含量很低,但此酶极容易被诱导、活化,使肝脏含量迅速增加。
许多外源性化合物经CYP 1A1代谢后可产生有毒的代谢产物,能诱发肿瘤的产生与发展;CYP 3A4是CYP 450酶系中最重要的亚型, 约占成人肝微粒体CYP450总量的30 %~40 %。
因此,有必要对这两种具代表性的肝药酶在普萘洛尔对映体的代谢过程中的参与情况进行研究和探讨。
肝细胞是在人类中检测CYP 450酶活性诱导或抑制最合适的实验研究平台,是最接近临床的研究系统。
普萘洛尔甚少体外药动学资料,特别是其对映体的药代动力学参数资料。
因此,本实验设计以体外培养人肝细胞作为代谢反应系统。
对普萘洛尔对映体在经诱导的肝细胞中代谢过程进行了研究,其目的是明确肝药酶CYP 1A1和CYP 3A4是否参与普萘洛尔对映体的代谢,并探究其酶动力学和药代动力学的特征,同时为分子生物学基础研究和临床合理用药提供参考和实验依据。
本实验包括四方面的内容。
一、肝细胞培养以及肝药酶CYP 1A1、CYP 3A4活性的测定目的采用人肝细胞作为体外代谢系统,测定经不同浓度的诱导剂诱导后酶代谢底物的情况,以此来描述酶活性的变化,并确定诱导剂的最佳诱导浓度。
固态发酵设备原理、设备与应用

固态发酵设备原理、设备与应用1.固态发酵设备是利用固体底物进行微生物发酵的装置。
Solid-state fermentation equipment is a device that uses solid substrates for microbial fermentation.2.固态发酵设备的原理是利用微生物在无水或低水条件下对有机底物进行降解和产物转化。
The principle of solid-state fermentation equipment is to use microorganisms to degrade and transform organic substrates under low or no water conditions.3.固态发酵设备通常包括发酵槽、通风系统、控制系统等组成部分。
Solid-state fermentation equipment typically includes fermentation tanks, ventilation systems, control systems, and other components.4.固态发酵设备可以用于生产食品添加剂、饲料、生物质能源等产品。
Solid-state fermentation equipment can be used to produce food additives, feed, biomass energy, and other products.5.固态发酵设备可以应用于传统发酵食品加工中,如豆制品、酿造、酸奶等。
Solid-state fermentation equipment can be applied in traditional fermented food processing, such as tofu, brewing, yogurt, etc.6.固态发酵设备可以利用废弃物和农副产品等资源进行资源化利用和产品开发。
染整相关术语中英文对照

染整相关术语中英文对照助剂类softening and antistatic agents柔软及抗静电剂water and oil-repellent agents防水防油整理剂insect-resist agents防虫剂thickeners增稠剂crosslinking agents交联剂emulsifiers乳化剂染料类direct dyestuffs直接染料reactive dyeings活性染料disperse dyestuffs分散染料设备类quickwash shrinkage tester快速洗水缩水试验机washing fastness tester水洗牢度机martindate aorasion tester耐磨损测试机standard light both标准光源箱universal strength tester万能强力测试机light fastness tester日晒牢度仪non-standard equipments非标设备ironing machine烫平机series three-legged centrifuger三足式系列离心机displacement heat exchanger容积式换热器reactor反应锅hank drier绞纱烘燥机bulk-fibre drier散毛烘燥机liquid-flow hank dyeing machine液流式绞纱染色机normal temperature oscillating dyeing machine常温振荡试样机sparging(jet)dyeing machine喷射式染色机high-temperature/high-pressure dyeing machine高温高压染色机program-controlled dyeing machine程控染色机high temperature overflow dyeing machine高温溢流染色机high temperature and high pressure yarn dyeing machine高温高压染纱机atmospheric overflow dyeing machine常温溢流染色机high temperature sample dyeing machine高温样品染色机stainless steal drying tumbler不锈钢烘筒烘燥机rope impregnating mangle绳状浸染机整理类resin finishing树脂starch finishing上浆现象类slightly reddish cast轻泛红现象electrolyte电解后整理摩擦轧光整理:friction finish轧光整理:calender finish起绒整理:fleece finish阻燃整理:flame retardant finish柔软整理:mellow finish落针,停经片:drop wireSCY:(single covered yarn)单包覆纱以一条硬纱缠绕于弹性纱外层。
LORD IMB TM 液体胶合质硅胶胶胶基础应用指南说明书

LORD® IMB TM Liquid Silicone Rubber Primers Application GuideLORD® In-Mold Bonding (IMB™) liquid silicone rubber (LSR) primers are non-tacky polymer-based coatings that when applied to a substrate provide a structural bond to a rigidor elastomeric polymer, which is formed under heat and pressure. LORD IMB 3000 series primers are designedfor bonding platinum-cured silicone rubber to a variety of thermoplastic and metal substrates during the injection molding process.Although a premium primer is the basis of a quality bond, it’s only the beginning; proper application is essential for maximum results. Whether you’re dipping or spraying, you’ll learn how to maximize efficiency and optimize results. This guide also shows how to troubleshoot commonbond problems. We hope this resource will become an indispensable part of your operation and a convenient,one-source solution to many of your bonding questions. Substrate Surface Preparation:One of the most important factors influencing adhesionin the bonding process is surface preparation. To ensure optimum bond performance and long-term environmental resistance, substrates must be free of organic and inorganic contaminants. Organic materials include grease, dirt and oils which can be removed by solvent or alkaline cleaning. Common inorganic contaminants are rust, scale and oxide layers. These can be cleaned by either mechanical or chemical processes, or a combination of both. Types of Surface Preparation:There are several ways to prepare substrates for primer application; however, the methods can be broadly divided into mechanical and chemical. Regardless of which method you choose, the essentials of good surface preparations include:• Removal of all surface contaminants and decomposition products.• Prevention of recontamination.• Careful handling through all processing steps. Mechanical preparation involves physically removing surface contamination and increasing surface area and substrate profile. This method includes:• Blasting – Abrasive particles (sand, grit or metal oxides) are projected against the surface with a stream of air. Blasting is especially effective for removing inorganic contamination and other corrosion compounds found on metal. Thecharacter or quality of the treatment is affected by duration of the blast; shape and size of the blasting media; particle velocity; and the hardness, porosity and other substrate properties.Chemical processes, on the other hand, utilize organic and inorganic chemicals to dissolve, suspend or eliminate soils and surface contaminants. Preparation methods include: • Alkaline cleaning• Acid passivationSelecting a Preparation Method: To determine which preparation method best suits your needs, consider:• Economy – In large volumes, chemical treatments are generally less expensive than mechanical methods.• Versatility – Mechanical preparation methods may be applicable to numerous metals, while chemical treatments may be metal-specific.• Adaptability to Existing Equipment – Existing facilities may favor either mechanical or chemical processing.• Adhesion Requirements – Adhesion requirements vary from product to product, and bond quality is affected by the particular application. Therefore, surface preparation will vary accordingly.• Environmental Resistance – Chemical conversion often provides enhanced environmental resistance compared to mechanical methods.• Government Regulations – Waste disposal regulations may prohibit the use of chemical treatments in certain areas.Maintaining Surface Conditions: Maintaining optimum surface cleanliness is essential until primer application is complete. To accomplish this:• Apply the primer immediately after the surface is prepared. • Avoid exposure to dust, moisture, chemical fumes, mold release agents and other possible contaminants.• Keep solvents and cleaning solutions free from contamination, and replace when necessary.• Ensure grits and abrasives remain clean and free of contaminants.• Check the purity of rinse water and “drying” air frequently, ensuring minimal contamination.The water break test can be used to check for oil and grease removal. If a surface can support an unbroken filmof deionized water for 60 seconds or more, it is considered essentially free from grease or oil.Surface Preparation for Various Substrates:Although the general principles are the same for preparing all substrates, some materials require special attention. Outlined below are guidelines for surface preparation of specific substrates.Stainless Steel (Mechanical Preparation)Preparing stainless steel with mechanical methods includes: 1. Blasting with sand or aluminum oxide. Steel grit shouldnot be used because it leaves ferrous deposits that can cause galvanic corrosion.2. One-hour layover maximum between blasting andprimer application.Stainless Steel (Chemical Preparation)Chemical treatment for the passivation of stainless steel involves the following:• Alkaline Wash1. Hot water rinse (70°C)2. Wash in sodium tripolyphosphate solution3. Hot water rinse (70°C)4. Hot air dry• Acid PassivationWashing step that uses mild acid solution such as citric or oxalic acidImmersion times, solution concentrations and operating temperatures may be adjusted to suit conditions and alloys. PlasticsLSR can be bonded to many rigid plastics. To prepare plastic surfaces:1. Solvent wipe. Hydrophobic solvents such as n-heptaneand Isopar TM can remove waxes and mold release.Alcohol such as ethanol or isopropanol can removepolar contaminants.2. Surface oxidation by plasma, flame, or corona treatment. Preparing the Primer:Temperature – Temperature affects the viscosity of LORD IMB primers. Recommended storage temperature is 21- 27°C (70-80°F) in original, unopened container. Cold storage is not recommended.Dilution – Regardless of dilution amounts, it is important in all cases that the appropriate diluent be added to the primer while stirring. Mixing guidelines are listed in the respective technical data sheets for each LORD IMB product. Applying the Primer:LORD IMB primers may be applied by brush or spray methods. General recommendation for dry film thickness is 2.0 to 5.0 micron (0.05 to 0.2 mil).Hand Brushing – LORD IMB solvent-based primers are suitable for hand brushing straight from the container. When using this method, wear the proper personal protective equipment, and work in a clean environment. Also make sure there are no dirty or greasy objects within reach.Spray Application – Spray application of primers is particularly applicable when coating one side or certain areas of a part. When spraying, however, it is important that the primer reach the substrate wet. If drying occurs before reaching the metal, adhesion will be poor.Hand-held guns may be used for small runs, while conveyorized or automated units are effective for large production operations. And for small, intricate parts, an air brush may be used. Regardless of size, properly adjusted equipment ensures delivery of uniform films – without sags and tears.During hand-spray operations, parts are often assembledon racks that incorporate masks wherever needed. If the application requires overall coating, parts can be rotated in front of the spray gun.Precision Spra y / Jetting – This technique follows the same principles as typical spray techniques outlined above but utilizes very precise application equipment to apply primer to small areas with minimal overspray. LORD IMB primers are compatible with this process.Drying Processes:All LORD IMB LSR primers can be dried at room temperature (21°C/ 70°F) in 30 minutes or less. During the drying process, no reaction is taking place – only solvent is evaporating. Thus, methods to speed up solvent evaporation are effective, such as increasing air flow or using hot air. Heating to 65°C (149°F) in a convection oven for 5 minutes is usually sufficient. Avoid drying temperatures of greater than 65°C (149°F). Avoid IR-based heating because this creates a high level of heat directly at the primer surface, potentially destroying the bonding ability.Handling Coated Parts:Both clean and coated parts should be kept free of contamination. Because fingerprints can adversely affect adhesion, gloves are highly recommended. Thin, white, cotton gloves are satisfactory, as they show soil easily, are economical enough to be discarded when necessary, and are thin and porous enough to be comfortable.Coated Parts Layover Stability: Mold as soon as possible, but store all coated parts properly to ensure maximum layover. Typically, this entails sealing primer-treated substrates in a clean plastic containerand storing the package in a cardboard box. These precautions ensure parts are protected from airborne contaminates. Refer to the applicable technical data sheet for recommended layover durations.Molding Considerations:One of the most important steps in the manufacturing process is molding. During this phase, the primer-coated substrate and elastomer are placed in the mold cavity, and under proper conditions of time, temperature and pressure the bonded assembly is formed.Controlling each step in the molding process is critical to bond success. Major variations in any step will cause bond failures. Minor alterations, though not detrimental individually, can collectively result in poor or marginal adhesion and above-average scrap rates.Considerations include:• Primer Dry Film Thickness (DFT) – One of the most important factors in environmental performance. Low and high DFT films can result in poor performance. Refer to the applicable technical data sheet for recommended DFT.• Molding Pressure – Optimum adhesion requires adequate pressure and intimate contact of elastomer and primer during vulcanization and cure. Molds that are either too tight or are too loose will hinder bond quality.• Temperature – Dramatic temperature variations from cavity to cavity may cause bond failure, lack of cure, or overcure conditions. Mold temperature should be checked periodically, particularly within the individual cavities. Tempilsticks®, or selective melting-point wax pencils, are excellent for spot-checking mold cavities. Thermocouples can also be used, but they must be calibrated regularly. • Mold Design – When designing the mold, provisions should be made to facilitate substrate loading as well as removal of the cured part.Post Treatment:Following part bonding, post-bake may be required to achieve maximum bonding performance. A typical post-bake condition for silicone parts is 150°C to 200°C (302°F to 392°F) for 2 to 4 hours. Troubleshooting:ASTM International provides a set of detailed symptom descriptions for bond failures. These descriptions allow complete and accurate problem assessment as well as quick solutions. (In this document, the terms “elastomer” and “primer” should be interpreted as “rubber” and “cement”, respectively.)Three basic ASTM designations are:• RC – failure at the rubber-cement interface.• CM – failure at the cover cement-metal interface; or at the primer-metal interface.• R – failure in the rubber.Rubber-Cement (RC) FailuresSeparation between rubber and cement is usually characterized by a hard, glossy surface on the metal with little or no visible rubber.The following list includes common causes of RC failures, as well as potential solutions:• Substrate not hot enough upon LSR injection.– Preheat the substrate inside of the mold by using aninjection delay or preheat the substrate in a batch oven to minimize cycle time.– Increase mold temperature.• Silicone rubber contains low concentration of functional groups.– Increase amount of B component in two-part primers.– If using single-component primer (LORD IMB 3050, forexample), mix with side B catalyst (LORD IMB 3040B, for example). Details are provided in the respective technical data sheets.• Color concentrate contains incompatible components.– Reduce or change color concentrate.• Primer is sweeping, or being removed by flowing silicone.– Reduce filling speed.– Change gate location.• Silicone is not compatible with primer.– Change to a different grade of silicone.Cement-Metal and Primer-Metal (CM) FailuresA clean separation between the primer and metal or other substrate indicates that no adhesion has occurred.The following list includes common causes of CM failure as well as potential solutions:• Substrate is not clean.– Clean substrate; often, oil, dirt, dust or othercontaminants inhibit bonding.• Primer is not sufficiently dried.– Reduce dry film thickness (apply thinner).– Increase drying time and/or temperature.• Substrate is not compatible with primer.– Mechanically roughen the substrate with abrasive.– Chemically activate the substrate with plasma, flame, or corona treatment.– Change to a different substrate.Parker LORDEngineered Materials Group 111 LORD DriveCary, NC 27511-7923USAphone +1 877 ASK LORD (275 5673)Values stated in this document represent typical values as not all tests are run on each lot of material produced. For formalized product specifications for specific product end uses, contact the Customer Support Center.Information provided herein is based upon tests believed to be reliable. In as much as Parker LORD has no control over the manner in which others may use this information, it does not guarantee the results to be obtained. In addition, Parker LORD does not guarantee the performance of the product or the results obtained from the use of the product or this information where the product has been repackaged by any third party, including but not limited to any product end-user. Nor does the company make any express or implied warranty of merchantability or fitness for a particular purpose concerning the effects or results of such use.WARNING — USER RESPONSIBILITY . FAILURE OR IMPROPER SELECTION OR IMPROPER USE OF THE PRODUCTS DESCRIBED HEREIN OR RELATED ITEMS CAN CAUSE DEATH, PERSONAL INJURY AND PROPERTY DAMAGE.This document and other information from Parker-Hannifin Corporation, its subsidiaries and authorized distributors provide product or system options for further investigation by users having technical expertise.The user, through its own analysis and testing, is solely responsible for making the final selection of the system and components and assuring that all performance, endurance, maintenance, safety and warning requirements of the application are met. The user must analyze all aspects of the application, follow applicable industry standards, and follow the information concerning the product in the current product catalog and in any other materials provided from Parker or its subsidiaries or authorized distributors.To the extent that Parker or its subsidiaries or authorized distributors provide component or system options based upon data or specifications provided by the user, the user is responsible for determining that such data and specifications are suitable and sufficient for all applications and reasonably foreseeable uses of the components or systems.©2020 Parker Hannifin - All Rights ReservedInformation and specifications subject to change without notice and without liability therefor. Trademarks used herein are the property of their respective owners.OD AG1024 06/20 Rev.1Rubber (R) FailuresRubber failures are separated into the following categories: SR (Spotty Rubber) – Often caused by pre-bond surface contaminants, this failure appears like splattered rubber on the substrate surface.TR (Thin Rubber) – Thin rubber failures are marked by even, but very light rubber residue on the substrate surface. These imperfections usually occur with butyl or rubber stocks that are highly oil-extended. When oils migrate to the RC interface, they create a bond layer that is part primer, part oil and part rubber. This weak layer easily fails when the part is stressed.HR (Heavy Rubber) – A thick or heavy layer of rubber remaining on the substrate surface indicates an excellent bond. The stock fails because it is stressed beyond its cohesive strength. This is the ideal failure mode.SB (Stock Break) – With stock breaks, the elastomer appears as if it was folded back on itself, then broken off. The break is jagged and at a sharp angle to the substrate surface. Although there are three primary bond failures, keep in mind that rubber-cement, cement-metal/substrate and rubber failures are often found in combination.Things to Avoid:• Certain chemicals are incompatible with LORD IMB primers and can cause failure. These include amines, sulfur, latex, or chemical compounds containing nitrogen, phosphorous or tin.• Certain silicone components commonly used in other primers. Make sure to avoid cross-contamination with silane/silicone-based primers.• Silicone-based mold release is not compatible with LORD IMB primers. Use PTFE-based release agents, such as McLube TM 1711L.Safe Handling:Proper handling of LORD IMB primers is essential for safe and effective application. We recommend these procedures be followed when using any LORD IMB LSR product: • Read labels, SDS and technical data sheets before use.• Ventilate application and storage areas. • Wear proper personal protective equipment.• Clean application and processing equipment regularly. • Dispose of waste according to federal, state and local regulations.Parker LORD Applications Laboratory:As an extension of our product development efforts, Parker LORD has injection molding machines in Erie, PA. Bysimulating customers’ applications, we can provide detailed technical support and more thoroughly evaluate optimum application characteristics of new products.。
染整相关术语中英文对照

染整相关术语中英文对照助剂类softening and antistatic agents柔软及抗静电剂water and oil-repellent agents防水防油整理剂insect-resist agents防虫剂thickeners增稠剂crosslinking agents交联剂emulsifiers乳化剂染料类direct dyestuffs直接染料reactive dyeings活性染料disperse dyestuffs分散染料设备类quickwash shrinkage tester快速洗水缩水试验机washing fastness tester水洗牢度机martindate aorasion tester耐磨损测试机standard light both标准光源箱universal strength tester万能强力测试机light fastness tester日晒牢度仪non-standard equipments非标设备ironing machine烫平机series three-legged centrifuger三足式系列离心机displacement heat exchanger容积式换热器reactor反应锅hank drier绞纱烘燥机bulk-fibre drier散毛烘燥机liquid-flow hank dyeing machine液流式绞纱染色机normal temperature oscillating dyeing machine常温振荡试样机sparging(jet)dyeing machine喷射式染色机high-temperature/high-pressure dyeing machine高温高压染色机program-controlled dyeing machine程控染色机high temperature overflow dyeing machine高温溢流染色机high temperature and high pressure yarn dyeing machine高温高压染纱机atmospheric overflow dyeing machine常温溢流染色机high temperature sample dyeing machine高温样品染色机stainless steal drying tumbler不锈钢烘筒烘燥机rope impregnating mangle绳状浸染机整理类resin finishing树脂starch finishing上浆现象类slightly reddish cast轻泛红现象electrolyte电解后整理摩擦轧光整理:friction finish轧光整理:calender finish起绒整理:fleece finish阻燃整理:flame retardant finish柔软整理:mellow finish落针,停经片:drop wireSCY:(single covered yarn)单包覆纱以一条硬纱缠绕于弹性纱外层。
有关酶解工艺的英文文献

Enzymatic Hydrolysis ProcessIntroductionEnzymatic hydrolysis is a process that involves the breakdown of complex organic compounds using enzymes. This process plays a crucial role in various industries, including biofuels, food, pharmaceuticals, and agriculture. Enzymes act as catalysts, accelerating the rate of chemical reactions and enabling the transformation of substrates into desired products. In this article, we will explore the different aspects of enzymatic hydrolysis, including its significance, applications, factors affecting the process, and future prospects.Importance of Enzymatic HydrolysisEnzymatic hydrolysis is a highly significant process due to its wide range of applications. Some key reasons for the importance of this process are:1.Biofuel Production: Enzymes are extensively utilized in theproduction of biofuels, such as ethanol and biodiesel. Enzymatichydrolysis of lignocellulosic biomass, such as agriculturalresidues and energy crops, breaks down complex carbohydrates intosimple sugars, which can then be fermented to produce biofuels.2.Food Industry: Enzymatic hydrolysis is employed in the foodindustry to enhance the nutritional properties of various foodproducts. For example, proteases are widely used to hydrolyzeproteins into amino acids, improving their digestibility.3.Pharmaceuticals: Enzymatic hydrolysis plays a critical role inthe pharmaceutical industry, particularly in drug formulation.Enzymes are used to break down active pharmaceutical ingredientsinto biologically active compounds, facilitating their absorptionand effectiveness.4.Waste Treatment: Enzymatic hydrolysis is utilized in thetreatment of various types of waste, including sewage andagricultural waste. Enzymes aid in the breakdown of organic matter,reducing the environmental impact and facilitating the productionof valuable by-products.Enzymatic Hydrolysis ProcessThe enzymatic hydrolysis process involves a series of steps, including substrate preparation, enzyme selection, enzymatic reaction, and product recovery.1.Substrate Preparation: The substrate used in enzymatic hydrolysisneeds to be properly prepared to ensure efficient enzyme-substrate interaction. This often involves pretreatment methods such as size reduction, chemical or physical treatment, and removal ofinhibitors.2.Enzyme Selection: The choice of enzyme is crucial as itdetermines the specificity and efficiency of the hydrolysisprocess. Different enzymes are suitable for different substrates,and factors such as pH, temperature, and enzyme concentration need to be considered.3.Enzymatic Reaction: The hydrolysis reaction typically occurs in acontrolled environment, with optimum pH and temperature conditions.The enzyme is added to the substrate, and the reaction is allowedto proceed for a specific period. During this time, the enzymebreaks down the substrate into smaller molecules.4.Product Recovery: After the enzymatic hydrolysis, the desiredproducts need to be separated from the reaction mixture. This mayinvolve techniques such as filtration, centrifugation, orchromatography, depending on the nature of the products andimpurities present.Factors Affecting Enzymatic HydrolysisSeveral factors can influence the efficiency of enzymatic hydrolysis. These factors need to be carefully considered and optimized to achieve the desired results. Some key factors include:1.pH: Enzymatic reactions are highly sensitive to pH. Differentenzymes have different pH optima, and maintaining the appropriatepH level ensures optimal enzyme activity.2.Temperature: Temperature significantly affects enzyme activity.Each enzyme has an optimum temperature range within which itfunctions best. Deviating from this range can either decrease the reaction rate or denature the enzyme.3.Enzyme Concentration: The concentration of enzymes in thereaction mixture influences the rate and efficiency of hydrolysis.Higher enzyme concentrations usually result in faster reactionrates, up to a certain limit.4.Substrate Concentration: The concentration of the substrate alsoaffects the hydrolysis process. Extremely high or low substrateconcentrations can inhibit enzyme activity and reduce overallefficiency.5.Inhibitors and Activators: Inhibitors can significantly affectenzymatic hydrolysis by interfering with the enzyme-substrateinteraction. Activators, on the other hand, can enhance enzymeactivity. Identifying and managing these factors is crucial foroptimum results.Future ProspectsEnzymatic hydrolysis continues to be an area of active research and development. Scientists are continually exploring new enzymes, optimizing reaction conditions, and developing innovative techniques to improve the efficiency and cost-effectiveness of the process. In addition, advancements in genetic engineering and enzyme immobilization techniques hold promising potential for further enhancing enzymatic hydrolysis processes.ConclusionEnzymatic hydrolysis is a vital process with significant applications in various industries. Understanding the factors influencing the process and optimizing reaction conditions are crucial for achieving efficient and cost-effective hydrolysis. Ongoing research efforts and technological advancements continue to drive progress in this field, opening doors to new possibilities and innovations in the future.。
t-剥离拉伸承载强度 英语

t-剥离拉伸承载强度英语T-Peel Tensile Strength: A Comprehensive Overview.Definition.T-peel tensile strength (TPT) is a measure of the force required to separate two bonded materials when the force is applied perpendicular to the bond line and parallel to the interface. It is a critical parameter in assessing the adhesion strength and durability of various materials, including adhesives, coatings, and composites.Measurement Methods.TPT is typically measured using a T-peel test, which involves bonding two materials together and then separating them using a tensile testing machine. The force required to initiate and propagate the separation is recorded as the TPT. Various testing standards, such as ASTM D1876 and ISO 8510-2, provide specific guidelines for conducting T-peeltests.Factors Influencing T-Peel Strength.Multiple factors can influence the TPT of a bonded joint, including:Material Properties: The chemical composition, surface roughness, and crystallinity of the bonded materials significantly impact their adhesion strength.Bonding Chemistry: The type of adhesive used, its chemical reactivity with the substrates, and the curing conditions play a crucial role in determining the TPT.Substrate Preparation: The cleanliness, surface treatment, and pretreatment of the substrates before bonding can affect the adhesion and TPT.Bond Thickness: The thickness of the adhesive layer can influence the stress distribution and affect the TPT.Testing Conditions: Factors such as temperature, humidity, and loading rate during the T-peel test can influence the measured TPT values.Sample Geometry: The shape and size of the specimens used for T-peel testing can affect the stress concentration and TPT results.Applications.TPT measurements have widespread applications in various industries, including:Adhesive Development: Assessing the adhesive performance of new formulations and comparing different adhesives for specific applications.Coating Evaluation: Evaluating the adhesion strength of coatings on substrates and their resistance to peeling and delamination.Composite Bonding: Determining the bond strengthbetween composite materials and adhesives or matrices.Packaging Industry: Ensuring the integrity of packaging materials and preventing product damage during handling and transportation.Medical Devices: Assessing the adhesion of medical adhesives and coatings in surgical and wound care applications.Importance.TPT is a crucial parameter for evaluating the performance and reliability of bonded systems. Strong TPT ensures:Structural Integrity: A high TPT indicates a strong bond between the materials, preventing premature failure or separation.Environmental Stability: Adhesives with high TPT exhibit better resistance to moisture, temperaturefluctuations, and other environmental factors that can weaken bonds.Product Durability: TPT measurements help predict the longevity of bonded products by assessing their resistance to peeling and delamination.Quality Control: T-peel testing is an essentialquality control tool to ensure that bonded products meet industry standards and customer specifications.Conclusion.T-peel tensile strength is a valuable metric for evaluating the adhesion strength and durability of bonded materials. By understanding the factors that influence TPT and the various testing methods available, engineers and researchers can optimize bonding processes, select appropriate adhesives, and ensure the reliability of bonded systems. TPT measurements play a significant role in advancing material science, manufacturing, and product design across numerous industries.。
北大考研-工学院研究生导师简介-王习东

爱考机构-北大考研-工学院研究生导师简介-王习东王习东目前任职:教授、博士生导师北京大学工学院能源与资源工程系、系主任北京大学资源高效与循环利用研究中心主任北京市“固体废弃物资源化技术与管理”重点实验室主任电话:86-10-82529083电子邮箱:教育经历:北京科技大学学士、硕士瑞典皇家工学院博士研究领域:(1)资源高效与循环利用(2)能源与环境材料背景资料:多年来,主要从事资源利用与环境材料的教学、研究工作。
先后主讲了本科生、硕士生、博士生课程等16门。
在资源综合利用物理化学与材料制备物理化学等领域做出了一定成绩。
承担或完成了包括国家杰出青年科学基金课题、国家“863”课题、国家“973”课题,国家攻关课题以及国家自然科学基金重点与面上课题在内的国家与省部级课题10余项,通过鉴定6项;申报国家发明专利30多项;获得国家与省部级科学技术奖励6项。
在国内外重要学术期刊发表学术论文100余篇,其中被“SCI”收录60余篇;出版学术专著2部。
2003年晋升教授,同年批准为博士生导师;2004年获得国家杰出青年科学基金;2005年获国务院颁发的政府特殊津贴,2006年入选“新世纪百千万人才工程”国家级人选。
获得荣誉:1996年,安徽省科技进步二等奖(排名第三)1997年,国家科技进步三等奖,(排名第三)2002年,北京市科技进步二等奖(排名第二)2002年,中国冶金科学技术二等奖(排名第二)2005年,北京市自然科学二等奖(排名第一)2006年,教育部提名国家自然科学二等奖(排名第一)发表论文(部分)[1]StudiesonthePEG-AssistedHydrothermalSynthesisandGrowthMechanismofZnOMicrorodandM esoporousMicrosphereArraysontheSubstrate,CRYSTALGROWTH&DESIGN2010,10(4):1500-15 07[2]EffectsofpretreatmentofsubstratesonthepreparationoflargescaleZnOnanotubearrays,RAREMETALS2010,29(1):21-25[3]ControllableSynthesisofHigh-puritybeta-SiAlONPowder,JOURNALOFINORGANICMATERI ALS2009,24(6):1163-1167[4]PreparationandCharacterizationofTiO2NanorodArraysviaHydrothermalApproach,RAREMETA LMATERIALSANDENGINEERING,2009,38:1060-1063[5]Thermodynamicstudyandsynthesesof-SiAlONceramics,ScienceinChinaSeriesE,2009,52(11):3122-3127[6]Copperextractionfromcopperorebyelectro-reductioninmoltenCaCl2-NaCl,ELECTROCHIMICA ACTA,2009,vol.54(18):4397-4402[7]ActivityofVO1.5inCaO-SiO2-MgO-Al2O3SlagsatLowVanadiumContentsandLowOxygenPress ures,STEELRESEARCHINTERNATIONAL,2009,Vol.80(4):251-255[8]ASimpleTwo-ParameterCorrelationModelforAqueousElectrolyteSolutionsacrossaWideRangeof Temperatures,JOURNALOFCHEMICALANDENGINEERINGDATA,2009,vol.54(2):179-186 [9]ThermodynamicActivityofChromiumOxideinCaO-SiO2-MgO-Al2O3-CrOxMelts,STEELRES EARCHINTERNATIONAL,2009,vol.80(3):202-208[10]HydrothermalsynthesisofSnO2nanoflowerarraysandtheiropticalproperties,SCRIPTAMATERI ALIA,2009vol.61(3):234-236[11]TheEffectoftheTextureandtheDensityofZnOSeedLayerontheOrientationofZnONanorodArrays, JOURNALOFNANOSCIENCEANDNANOTECHNOLOGY,2009,vol.9(10):5920-5926[12]HydrothermalPreparationandCharacterizationofNanocrystallinePorousTinDioxideThinFilms,J OURNALOFNANOSCIENCEANDNANOTECHNOLOGY,2009,vol.9(10):5770-5775[13]HydrothermalsynthesisandcharacterizationofTiO2nanorodarraysonglasssubstrates,MATERIA LSRESEARCHBULLETIN,2009,vol.44(6):1232-1237[14]PreparationandpropertiesofananoTiO2/Fe3O4compositesuperparamagneticphotocatalyst,RAR EMETALS,2009,Vol.28(5):423-427[15]EstimationofFreezingPointDepression,BoilingPointElevation,andVaporizationEnthalpiesofEle ctrolyteSolutions,INDUSTRIAL&ENGINEERINGCHEMISTRYRESEARCH,2009,vol.48(4):22 29-2235[16]Template-freehydrothermalsynthesisofsingle-crystallineSnO2nanocauliflowersandtheiroptical properties,RAREMETALS,2009,Vol.28(5):449-254[17]ThermalExpansionofMagnesiumAluminumOxynitride,HIGHTEMPERATUREMATERIALS ANDPROCESSES,2008,vol.27(2):97-101[18]EffectsofPVPonthepreparationandgrowthmechanismofmonodispersedNinanoparticles,RARE METALS,2008,vol.27(6):642-647[19]ThePreparationandCharacterizationofβ-SiAlONNanostructureWhiskers,JofNanomaterials,vol.2008,ArticleID282187[20]ExtensionoftheThree-Particle-InteractionModelforElectrolyteSolutions,MaterialsandManufact uringProcesses,23:737–742,2008[21]CorrelationandPredictionofThermodynamicPropertiesofSomeComplexAqueousElectrolytesby theModifiedThree-Characteristic-ParameterCorrelationModel,J.Chem.Eng.Data,2008,53,950–958[22]CorrelationandPredictionofThermodynamicPropertiesofNonaqueousElectrolytesbytheModifie dTCPCModel,J.Chem.Eng.Data2008,53,149–159[23]Effectsofpreparingconditionsontheelectrodepositionofwell-alignedZnOnanorodarrays,Electroc himicaActa,2008,53(14):4633-4641[24]ThermodynamicevaluationandhydrothermalpreparationofKxNa-xNbO3,RareMetals,2008,27(4) :371-377[25]Anewthree-particle-interactionmodeltopredictthethermodynamicpropertiesofdifferentelectroly tes,JournalofChemicalThermodynamics,v39,n4,April,2007,p602-612[26]Density-controlledhydrothermalgrowthofwell-alignedZnOnanorodarrays,Nanotechnology,v18, n3,Jan24,2007,p035605[27]Correlationandpredictionofactivityandosmoticcoefficientsofaqueouselectrolytesat298.15Kbyth emodifiedTCPCmodel;JournalofChemicalandEngineeringData,v52,n2,2007,p538-547[28]SynthesisandcharacterizationofMgAlON-BNcomposites,InternationalJournalofMaterialsResea rch,v98,n1,January,2007,p64-71[29]SynthesisandmicrostructureofLa-dopedCeriananoparticles,J.NanoscienceandNanotechnology, V.7No.8,2007,p2883-2888[30]Phaserelationshipofcomplexmulti-componentsystemchromatecleanerproduction,ProgressinNat uralScience,V17,No.72007,p838-844[31]SynthesisandthermodynamicanalysisofNan0-La2O3,ProgressinNaturalScience,V17,No.72007, p838-844[32]Compleximpedancestudyonnano-CeO2coatingTiO2,MATERIALS&DESIGN,2006,27(6):489-493[33]Optimizationofprocessparameterspreparinghollowfibrousnickelplaquebyweb-basedANN-GAs ystem,ACTAMETALLURGICASINICA,2005,41(12):1293-1297[34]Synthesis,evaluationandcharacterizationofaluminaceramicswithelongatedgrains,CERAMICSI NTERNATIONAL,2005,31(7):953-958[35]PropertiesandstructureofAlON-VNcompositessynthesizedbyhot-pressingtechnique,RAREME TALMATERIALSANDENGINEERING,2005,JUN.34:451-454[36]PreparationandferroelectricpropertiesofPZTfibers,CeramicsInternational,2005(31):281-286[37]Kineticstudiesofoxidationofγ-AlON-TiNcompositesJournalofAlloysandCompounds,2005,387(1-2):74-81[38]StudyoftheAlON-VNcompositeceramics,KeyEngineeringMaterials,Vols280-283,2005,1139-1 142[39]ManufactureandpropertiesofAlON-TiNparticulatecomposites,KeyEngineeringMaterials,Vols2 80-283,2005,1133-1138[40]ThermodynamicstudyofK2CrO4-K2AlO2-KOH-H2OandNa2CrO4-Na2AlO2-NaOH-H2Osys tems,J.ofUniv.Sci.Tech.Beijing,2004,(6):500-504.[41]Synthesis,MicrostructuresandPropertiesOfAluminumOxynitride,MaterialsScienceandEngineer ingA,2003,245-250[42]Influenceofdifferentseedsontransformationofaluminumhydroxidesandmorphologyofaluminagr ainsbyhot-pressing,MATERIALS&DESIGN,2003,24(3):209-214[43]SynthesisofTiN/AlONCompositeCeramics,J.Mineral,MetallurgyandMaterials,2003,10(1),49-5 3[44]Modelstoestimateviscositiesofternarymetallicmeltsandtheircomparisons,ScienceinChina,2003, (3):280-289[45]OxygenSwnsitivitynano-CeO2coatingTiO2materials,SensorsandActuatorsB,2003,92(1-2):167 -170[46]SilicaPhotonicCrystalswithQuasi-fullBandGapintheVisibleRegionPreparedinEthanol,Progressi nNaturalScience,2003,(9):717-720[47]Hightoughnessaluminaceramicswithelongatedgrainsdevelopedfromseeds,ScienceinChinaSerie sE2003,46(5):527-536[48]Kineticstudiesoftheoxidationof-aluminumoxynitride,MetallurgicalandMaterialsTransactionsB, V33B,April,2002:201~207[49]Estimationofviscosityofternary-metallicmelts,MetallurgicalandMaterialsTransactionsA,V33A, No.5,2002:201~207[50]SynthesisandcharacterisationofMgAlON,Z.Metallkde(InternationalJournalofMaterialsResearc handAdvancedTechniques),V93,No.6,2002,540-544[51]KineticstudyofoxidationofMgAlONandacomparisonoftheoxidationbehaviorofAlON,MgAlON, O’SiAlON-ZrO2andBN-ZCMceramics,Z.Metallkd(InternationalJournalofMaterialsResearchandAdv ancedTechniques),V93,No.6,2002,545-553[52]Slagcorrosionofgammaaluminumoxynitride,SteelResearch,V73,No.3,2002,91~96[53]PreparationofnanostructuredCeO2CoatedTiO2,MaterialsScienceandTechnology,V18,No.3,200 2,345~348[54]Investigationofconvertorsludgepelletsforsteelmaking,J.ofUniversityofSci.andTech.Beijing,No. 3,2002,266~269[55]Experimentalstudyandoptimizationofflamegunningparametersforsteelmakingfurnaces,Naihuo Cailiao,2002,36(6):318-321。
三元低共熔溶剂快速解离毛竹及提高酶水解得率研究

林业工程学报,2023,8(5):86-92JournalofForestryEngineeringDOI:10.13360/j.issn.2096-1359.202303023收稿日期:2023-03-23㊀㊀㊀㊀修回日期:2023-05-29基金项目:江苏省生物质能源与材料重点实验室基本科研业务费项目(JSBEM-S-202203)㊂作者简介:陈婷珺,女,研究方向为木质纤维原料的高值利用㊂通信作者:房桂干,男,研究员㊂E⁃mail:fangguigan@icifp.cn三元低共熔溶剂快速解离毛竹及提高酶水解得率研究陈婷珺1,周雪莲1,2,詹云妮1,刘旭泽1,黄晨1,邓拥军1,2,房桂干1,2∗(1.中国林业科学研究院林产化学工业研究所,江苏省生物质能源与材料重点实验室,南京210042;2.江苏省林业资源高效加工利用协同创新中心,南京210037)摘㊀要:以速生毛竹为原料,采用AlCl3辅助的多元醇基低共熔溶剂(氯化胆碱/1,4⁃丁二醇/AlCl3)进行预处理,实现毛竹组分温和㊁快速分离,并借助扫描电子显微镜(SEM)㊁X射线衍射(XRD)等手段考察低共熔溶剂(DES)预处理对纤维素酶水解的促进机制㊂研究表明:氯化胆碱/1,4⁃丁二醇/AlCl3体系可在10 30min大量脱除木质素和木聚糖,同时保留绝大部分纤维素;预处理物料纤维素酶水解得率均超过89%,溶解木质素可通过简单方式回收获得高纯度木质素,回收率超过90%㊂SEM结果显示,预处理后纤维表面出现明显的断裂和团聚,表明该体系对毛竹纤维的润涨断裂效果较强;XRD分析表明,经过该DES体系预处理60min后,纤维素结晶度由原料的59.59%增加至63.28%,而聚合度则由原料的773显著下降至341㊂在DES预处理过程中,木质素和半纤维素的大量脱除以及纤维的碎片化显著增加了酶与纤维素的接触,大幅提高了纤维素的糖化得率,且分离的木质素纯度高达96.34%㊂因此,氯化胆碱/1,4⁃丁二醇/AlCl3体系是一种快速㊁高效的预处理体系,可在提高预处理物料纤维素酶解得率的同时分离得到高得率㊁高纯度的木质素,进一步实现高值化利用㊂关键词:毛竹;低共熔溶剂(DES);快速解离;酶水解;高纯度木质素中图分类号:O636.2㊀㊀㊀㊀㊀文献标志码:A㊀㊀㊀㊀㊀文章编号:2096-1359(2023)05-0086-07RapidfractionationofmosobambooforenhancingenzymaticsaccharificationyieldusingternarydeepeutecticsolventCHENTingjun1,ZHOUXuelian1,2,ZHANYunni1,LIUXuze1,HUANGChen1,DENGYongjun1,2,FANGGuigan1,2∗(1.KeyLab.ofBiomassEnergyandMaterial,InstituteofChemicalIndustryofForestProducts,ChineseAcademyofForestry,Nanjing210042,China;2.JiangsuCo⁃InnovationCenterofEfficientProcessingandUtilizationofForestResources,NanjingForestryUniversity,Nanjing210037,China)Abstract:Deepeutecticsolvents(DES)composedofhydrogenbonddonorandhydrogenbondacceptorhavebeenwidelyusedforlignocellulosepretreatmentbecauseofitseasypreparation,biodegradability,environmentfriendliness,lowcostandnonflammability.Inthisstudy,rapidandgentlefractionationofmosobamboowasachievedusingAlCl3⁃assistedpolyoldeepeutecticsolvent(cholinechloride/1,4⁃butaneol/AlCl3)aspretreatmentmedium.ThemechanismofDESpretreatmentoncellulosehydrolysiswasinvestigatedbyscanningelectronmicroscopy(SEM)andX⁃raydiffractometry(XRD).Theresultsshowedthatthesystemcanachieveeffectivefractionationofbamboowithin30min,resultingin>61%ligninremoval,>84%xylanremovaland>90%glucanretention.Specifically,after10minpretreatment,theDESsystemcouldresultin61.34%ligninremovaland84.15%xylanremoval,indica⁃tingthattheshort⁃timefractionationcouldleadtosatisfyingfractionationperformance.Inaddition,theneartheoreticalglucancouldberecovered,indicatingtheresistanceofglucantotheDES.Remarkably,theglucanenzymatichydroly⁃sisyieldwasallbeyond89%,andtheligninrecoveryyieldcouldreachover90%withnearlynocarbohydrates.Thesignificantenhancementoftheenzymatichydrolysisyieldwasmainlyascribedtothesignificantligninremovalwhichgreatlyreducedbamboorecalcitrance,andthefitindexbetweentheligninremovalandenzymatichydrolysisyieldreachedashighas96.2%.TheSEMresultsshowedthatthefibersurfaceofthepretreatedsubstrateswasobviouslyfracturedandagglomerated,indicatingtheeffectiveswellinganddegradationeffectbytheDESsystem.Theshortenedandfracturedfibersofthepretreatedbamboofeedstockcouldefficientlyfacilitatethesubsequentenzymatichydrolysisbyincreasingthecontactareabetweencellulaseandcellulose.TheX⁃raydiffractionresultshowedthattheCrIofthe㊀第5期陈婷珺,等:三元低共熔溶剂快速解离毛竹及提高酶水解得率研究celluloseincreasedfrom59.59%to63.28%,whilethecelluloseDPdecreasedfrom773to341.TheincreasedCrIwasmainlyinducedbytheremovaloftheamorphousxylanandlignin,whiletheDPdecreasewasresultedfromthecellu⁃losefractureduringthefractionationprocess.ItshouldbenotedthattheenhancedCrIanddecreasedcelluloseDPwerealsothemainpromoterfortheenzymatichydrolysisprocess.Notably,theproposedDESsystemcouldobtainanex⁃cellentligninrecoveryyieldwhichisanessentialprerequisitefortheligninvalorization.Inaddition,theisolatedligninfeatureda96.34%purity,suggestingthattheDESsystemcouldobtainavaluableligninwithhighyieldandhighpuri⁃ty.Therefore,cholinechloride/1,4⁃butaneol/AlCl3isanefficientpretreatmentsystemwhichcouldyieldhighcellu⁃losesaccharificationandobtainligninwithhighyieldandhighpurityforitsfurthervalorization.Keywords:mosobamboo;deepeutecticsolvent;rapidfractionation;enzymatichydrolysis;highpuritylignin㊀㊀近年来,世界能源需求随飞速发展的经济状况而逐渐增长,化石能源危机和环境污染的双重考验应运而生,人们探寻清洁㊁可再生的新型能源势在必行㊂木质纤维原料具有热值高㊁储量大㊁成本低等优点,可用于制备各种可再生能源㊂木质纤维原料主要包括纤维素㊁半纤维素和木质素,其中半纤维素和木质素彼此共价交联,并通过氢键和范德瓦耳斯力与纤维素相互作用,阻碍了纤维素的糖化过程[1]㊂因此,采用预处理手段破除木质纤维天然屏障是实现其分组分转化的必要步骤[2]㊂目前研究中常用到的预处理方法主要有3种,包括物理法㊁化学法㊁生物法等[3]㊂其中,化学法是最常用的预处理方法,能够在短时间内实现三大素的有效分离,大幅提高纤维素酶解得率,但传统化学法(稀酸法㊁稀碱法等)存在反应温度高㊁污染严重等问题[4]㊂因此,寻求绿色㊁快速㊁可循环的绿色溶剂体系是当前生物质炼制的关键㊂低共熔溶剂(DES)是近年来兴起的新型溶剂体系[5]㊂相较于传统溶剂,具有低成本㊁热稳定性好㊁可循环利用等优点[6]㊂DES可有效断裂生物质内部的连接键,这依赖于其与木质纤维组分形成的强烈分子间氢键作用,从而实现组分的高效分离,大幅提高物料中纤维素的可消化率[7]㊂在DES中,氢键受体通常为氯化胆碱,氢键供体则主要包括有机酸(乳酸㊁甲酸等)㊁木质素衍生化合物(对羟基苯甲酸㊁愈创木酚等)和多元醇(乙二醇㊁甘油等)等[8]㊂其中,有机酸基低共熔体系可有效实现木质纤维原料的组分分离,但大多有机酸体系需较长的反应时间[5]㊂Shen等[9]采用氯化胆碱/乳酸体系对桉木进行预处理,在不同温度梯度下的处理时间均长达6h;Chen等[10]研究了氯化胆碱/对香豆酸体系对中草药残渣的预处理,结果表明实现84.62%的葡聚糖酶水解得率需在160ħ下处理5h㊂较长的预处理时间增加了成本,难以满足其工业化应用㊂因此,寻求一种快速㊁高效解离木质纤维原料的低共熔溶剂体系将有效解决上述问题㊂当以多元醇为氢键供体时,多元醇较低的黏度可显著增加DES体系的预处理效果,由此分离的木质素结构更加完整㊂但由于多元醇较弱的供氢能力,导致其形成的DES对木质纤维原料的解离效果较差[11],通常需要加入酸性催化剂㊂Liu等[12]研究了草酸辅助的氯化胆碱/乙二醇体系预处理桦木,可实现95.9%的葡聚糖转化,同时保留了木质素中大多数的芳基醚键,但该体系下草酸易同多元醇发生酯化反应,从而使得DES之间的氢键作用力减弱,减弱了回收后DES的预处理效果,不利于该溶剂的可持续发展㊂采用氯化铝等路易斯酸可以有效解决DES体系中副反应的发生,同时有效提高多元醇基DES体系的供氢能力,提高预处理效果并缩短预处理时间㊂通过向氯化胆碱/1,4⁃丁二醇体系中添加微量的AlCl3,快速解构木质纤维原料,并借助扫描电子显微镜(SEM)㊁X射线衍射(XRD)等分析手段系统探究三元低共熔体系促进组分解离和纤维素酶水解糖化的机制,以期实现温和条件下生物质的快速㊁高效预处理㊂1㊀材料与方法1.1㊀试验材料毛竹(主要化学组成为41.00%葡聚糖㊁12.78%木聚糖㊁30.99%木质素和1.12%乙醇抽出物),取自浙江仙鹤纸业有限公司,毛竹竹片于自来水中浸泡12h后,采用双螺杆挤压机进行挤压破碎,得到的物料经自然风干后保存于密封袋中备用㊂CTec2诺维信纤维素酶(250FPU/g)和X2753诺维信木聚糖酶(3490U/g)购自Sigma⁃Aldrich公司;AlCl3㊁氯化胆碱㊁1,4⁃丁二醇等购自麦克林生化科技(上海)有限公司㊂1.2㊀DES预处理将氯化胆碱㊁1,4⁃丁二醇和AlCl3按物质的量比25ʒ50ʒ0.1 25ʒ50ʒ3.0称量至250mL三口烧瓶中,在90ħ的油浴中加热直至形成透明澄清液体,78林业工程学报第8卷而后将DES体系转移至干燥器中备用㊂预处理实验在130ħ的温度条件下进行,在三口烧瓶中将10g的毛竹和100gDES充分混合,分别反应5,10,20,30和60min㊂反应结束后,向从油浴锅中取出的三口烧瓶中加入300mL丙酮⁃水溶液(体积比为1ʒ1)终止反应,通过磁力搅拌器对反应后的溶液进行2h搅拌,而后采用布氏漏斗进行固液分离㊂所得固体物料置于4ħ冰箱中保存,为去除预处理液中的丙酮,将其通过旋转蒸发(50ħ)后,加入1000mL去离子水,沉淀出预处理液中的木质素,木质素经离心㊁洗涤㊁冷冻干燥后称质量保存于干燥器中㊂1.3㊀酶水解试验称取相当于0.5g的预处理物料至150mL酶解瓶中,加入1mL乙酸⁃乙酸钠缓冲液(1mol/L),调整溶液pH4.8;加入40μL质量浓度为10g/L的四环素溶液,抑制微生物繁殖;加入0.1mL吐温80,降低残余木质素对酶的无效吸附;纤维素酶和半纤维素酶添加量为25FPU/g葡聚糖和150U/g木聚糖;最后加入去离子水,使反应体系总体积为20mL,并将酶解瓶置于温度50ħ㊁150r/min的摇床中振荡培养72h㊂将反应结束后的样品进行离心(10000r/min,5min),上清液经稀释后进行糖含量测定[7],获得其中的葡萄糖和木糖浓度㊂预处理物料葡聚糖㊁木聚糖的酶水解得率计算公式如下:yc=m1∕m2ˑ0.90ˑ100%(1)yx=m3∕m4ˑ0.88ˑ100%(2)式中:yc为葡聚糖酶水解得率,%;m1为水解液中葡萄糖质量,g;m2为初始底物中葡聚糖质量,g;yx为木聚糖酶水解得率,%;m3为水解液中木糖质量,g;m4为初始底物中木聚糖质量,g㊂1.4㊀各组分分析方法1.4.1㊀毛竹化学组分分析毛竹化学组分含量包括葡聚糖㊁木聚糖和木质素(酸溶木质素和酸不溶木质素)含量,均采用美国可再生能源实验室标准方法测定[13]㊂1.4.2㊀单糖含量的测定对样品中葡萄糖和木糖等单糖浓度进行测定,通过Agilent1260Ⅱ高效液相色谱仪(HPLC,美国安捷伦)完成㊂1.4.3㊀XRD分析实验对样品的结晶度进行测定,通过AdvancedD8X射线衍射仪(德国布鲁克)完成㊂样品的结晶度ICr计算公式如下[4]:ICr=I002-Iam()/I002ˑ100%(3)式中:I002为002晶格最大衍射峰强度;Iam为无定形区衍射散射峰强度㊂1.4.4㊀纤维素聚合度分析采用黏度法对样品的纤维素聚合度(DPv)进行测定㊂从毛竹原料和预处理物料中分离提取α⁃纤维素,然后根据ISO5351‘纸浆测定铜⁃乙二胺(CED)溶液中的极限黏度值“标准测定样品的特性黏度[14]㊂具体操作如下:将100mg质量恒定的α⁃纤维素加入锥形瓶中,加入10mL去离子水;随后通过磁力搅拌直至纤维均匀分散,向悬浮液中加入10mL双乙二胺氢氧化铜溶液溶解α⁃纤维素㊂上述溶液的黏度由乌氏黏度计测出,每样重复测量3次,取平均值以代表最终结果,DPv值的计算公式如下:DPv=0.75ˑη[]0.005æèçöø÷10.905(4)式中,[η]由黏度测量过程中的时间参数计算得出,具体参照文献[14]㊂2㊀结果与分析图1㊀不同AlCl3加入量对预处理效果的影响Fig.1㊀EffectsofdifferentamountofAlCl3onpretreatmentefficiency2.1㊀预处理对毛竹化学组成及回收率的影响AlCl3用量对预处理效果的影响结果如图1所示㊂由图1可知,随着氯化胆碱㊁1,4⁃丁二醇㊁氯化铝的摩尔比由25ʒ50ʒ0.2增加到25ʒ50ʒ1.0,木质素脱除率由40.26%增加到68.32%,半纤维素脱除率由60.23%增加到90.57%,而葡聚糖回收率相对稳定(96.6% 97.89%),表明随着AlCl3用量的增加,预处理效果显著增强㊂当物质的量比进一步增加到25ʒ50ʒ3.0时,木质素脱除率和半纤维素脱除率增加不明显,仅由68.32%和90.57%分别增加到88㊀第5期陈婷珺,等:三元低共熔溶剂快速解离毛竹及提高酶水解得率研究和氯化铝物质的量比由25ʒ50ʒ0.2增加至25ʒ50ʒ3.0时,预处理效果逐渐增强,当三者物质的量比为25ʒ50ʒ1.0时,半纤维素和木质素脱除率达到稳定值,再增加氯化铝用量对预处理效果的提升不明显,因此选择氯化胆碱㊁丁二醇和氯化铝物质的量比为25ʒ50ʒ1.0进行后续时间优化㊂以1,4⁃丁二醇为氢键供体㊁氯化胆碱为氢键受体,并添加微量AlCl3制备DES,设置5个不同的预处理时间㊂随着反应时间的延长,预处理物料得率㊁化学组分含量及其回收率/脱除率如表1所示㊂由表1可知,当低共熔溶剂预处理时间为5min时,仅65.50%的物料被回收,说明在短时间内,该DES即可实现木质纤维原料高效解聚㊂随着预处理时间从5min延长至30min,物料得率逐渐降低至61.54%,57.85%和50.39%㊂其中,木质素和木聚糖质量分数分别由22.69%和4.81%降低至12.19%和2.06%,而葡聚糖质量分数由61.58%增加至73.67%㊂当预处理时间延长至60min,物料得率提高至52.42%,此时葡聚糖含量略有下降,而木质素含量由12.19%(30min)增加至13.45%(60min),结果表明,过度延长预处理时间不利于木质纤维原料组分分离㊂此外,经过5min预处理即可实现52.03%的木质素脱除率,同时木聚糖脱除率达75.33%,而葡聚糖回收率高达98.39%㊂随着预处理时间由5min增加至30min,木质素脱除率逐渐增加至80.18%,木聚糖脱除率持续提高至91.87%,而葡聚糖回收率保持稳定㊂当预处理时间增加至60min时,葡聚糖回收率降低为84.71%,木聚糖脱除率增加至96.50%,而木质素脱除率降低至77.25%,表明持续延长预处理时间有助于木聚糖的降解,并对葡聚糖的降解作用增强㊂与此同时,延长预处理时间可能导致部分降解了的碳水化合物经过复杂的脱水和缩合反应形成假木质素[15]㊂因此,持续延长预处理时间将导致葡聚糖的降解损失以及假木质素的形成,不利于后续纤维素的糖化发酵以及木质素的高值化利用㊂表1㊀低共熔溶剂预处理时间对毛竹化学组分含量的影响Table1㊀EffectsofdifferentpretreatingtimeduringDESpretreatmentonchemicalcompositionsofmosobamboo不同预处理时间/min物料得率/%葡聚糖质量分数/%木聚糖质量分数/%木质素质量分数/%葡聚糖回收率/%木聚糖脱除率/%木质素脱除率/%565.50±0.1261.58±0.184.81±0.4422.69±0.1898.39±0.8975.33±0.5852.03±0.431061.54±0.1765.09±0.233.29±0.2619.46±0.2197.70±0.5684.15±0.6561.34±0.372057.85±0.2168.46±0.192.08±0.1915.88±0.3696.60±0.4390.57±0.3268.32±0.573050.39±0.2073.67±0.282.06±0.1812.19±0.1590.54±0.3691.87±0.4780.18±0.616052.42±0.3166.26±0.360.85±0.2013.45±0.2684.71±0.5996.50±0.1977.25±0.392.2㊀预处理对酶水解效率的影响酶水解是判断DES预处理效果的重要指标[16]㊂竹酶水解得率,以及木质素脱除率㊁木聚糖脱除率与葡聚糖酶水解得率的关系,结果如图2所示㊂㊀㊀a)预处理时间对酶水解效率的影响㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀b)木质素脱除率与酶水解得率的关系图2㊀预处理时间对酶水解得率影响以及木质素脱除率与酶水解得率的关系Fig.2㊀Effectsofdifferentpretreatmenttimeonhydrolysisyieldofglucanandxylan,andfittinglinearcorrelationsbetweenligninremovalandglucanhydrolysisyieldduringDESpretreatment㊀㊀由图2a可知,毛竹原料葡聚糖和木聚糖酶水解得率仅12.6%和1.7%㊂经过5min预处理后,葡聚糖酶水解得率由12.6%提高至79.9%,木聚糖酶水解得率由1.7%提高至96.3%,结果表明,该体系在极短时间内即可实现预处理物料葡聚糖和木聚糖的高效酶水解㊂随着预处理时间延长至10和98林业工程学报第8卷20min,葡聚糖酶水解得率继续提高到89.6%和96.2%,并且在预处理30min后达到100%;而木聚糖酶水解得率在预处理时间为10min后即已达100%㊂以上结果说明氯化胆碱/1,4⁃丁二醇/AlCl3体系在极短预处理时间内即可实现毛竹中葡聚糖和木聚糖的高效糖化,其葡聚糖酶解得率远高于传统的氯化胆碱/乳酸[17]㊁氯化胆碱/对香豆酸等有机酸体系[10]㊂植物细胞壁中,半纤维素和木质素通过物理或化学方式相连,并紧密包裹纤维素,形成了木质纤维原料致密的结构[18]㊂经过DES预处理,木质素被大量脱除,从而有效促进了毛竹酶水解性能㊂众所周知,木质素会增加对酶的无效吸附而抑制酶水解进程,本研究进一步探究了木质素脱除率和酶水解得率的关系,结果如图2b所示㊂从图2b可以看出,木质素的脱除和葡聚糖酶水解得率存在较强线性相关性,决定系数R2可达0.9262㊂当木质素脱除率由52.03%增加到80.18%,酶水解得率由79.9%增加至100%,表明木质素的去除可极大提高葡聚糖酶水解得率[4,18]㊂2.3㊀预处理前后物料表征分析2.3.1㊀物料表面微观形貌分析采用SEM观察毛竹原料以及不同预处理时间下毛竹纤维的微观结构,结果如图3所示㊂由图3可知,未经处理的毛竹原料纤维间聚集较少,其单根纤维较长且表面光滑㊂经过DES预处理后,物料的形貌发生显著变化,其纤维明显变短,单根纤维表面出现裂痕(白色箭头)和碎片沉积(红色箭头)现象㊂这是因为DES预处理过程中,纤维发生润涨,木质素与半纤维素的脱除使得纤维原始结构被破坏,同时溶出的木质素碎片部分沉积在纤维表面㊂随着时间的增加,预处理对纤维的切断作用增强,纤维变得更短,且出现团聚现象,这是由于随着预处理时间的延长,木质素和木聚糖脱除率增加,纤维素大量暴露,并通过氢键作用而聚集[4]㊂预处理后纤维变短以及裂痕增多,增大了酶解过程中纤维素与酶的接触面积,从而有效促进了葡聚糖㊁木聚糖的酶水解得率㊂a f)分别为0,5,10,20,30和60min预处理时间下的毛竹纤维扫描电镜图㊂图3㊀不同预处理时间下毛竹纤维的扫描电镜图Fig.3㊀ScanningelectronmicroscopyofmosobamboounderdifferentpretreatingtimesduringDESpretreatment2.3.2㊀纤维素结构分析为深入解析预处理前后物料纤维结构的变化,采用XRD测定了物料的纤维素结晶度指数(CrI),并进一步通过黏度法测定了纤维素聚合度,得出纤维素聚合度与葡聚糖酶水解得率的关系,结果见图4㊂由图4a可以看出,毛竹原料中纤维素结晶度为59.59%,随着预处理时间的延长,纤维素结晶度逐渐增加至59.63%(5min),61.49%(10min),61.95%(20min)和63.28%(30min),这是由于大量无定型的木质素与木聚糖的脱除,导致纤维素结晶度增加㊂当继续延长预处理时间至60min,纤维素结晶度略微下降至62.54%,该现象可能归因于两个方面:一是糖类降解产物在长时间的预处理条件下会通过脱水㊁缩合等复杂反应转化为假木质素,并沉积在纤维表面[15];二是随着预处理时间的延长,非结晶区纤维素部分发生水解,结晶区纤维素润涨加剧,最终导致纤维素结晶度降低㊂毛竹原料的纤维素聚合度为773;经过5min预处理后,聚合度显著下降至523;随着预处理时间延长,聚合度进一步下降至341(60min)㊂上述结果表明,尽管预处理过程葡聚糖回收率稳定,其聚合度显著降低,表明其中的糖苷键大量断裂从而有效增加了酶水解得率,但纤维素聚合度的过度下降会导致纤维素大量降解,因此,为了避免纤维素的回收率大幅09㊀第5期陈婷珺,等:三元低共熔溶剂快速解离毛竹及提高酶水解得率研究下降,控制预处理时间在30min内是较优选择㊂纤维素聚合度与葡聚糖的酶水解得率之间呈强相关的线性关系(图4b),其决定系数R2高达0.9331[17]㊂与上述研究结果相互验证,纤维素聚合度的降低同样有效促进了底物纤维素的酶水解转化㊂图4㊀预处理时间对纤维素结晶度和聚合度的影响以及酶水解得率与纤维素聚合度的关系Fig.4㊀EffectsofdifferentpretreatingtimeonthepolymerizationdegreeandcrystallinityofcelluloseduringDESpretreatmentandtherelationshipbetweentheyieldofglucanasehydrolysisandthedegreeofpolymerizationofcelluloseinDESdifferentpretreatmenttimes2.4㊀DES再生木质素分析对分离木质素的得率和纯度进行测定,结果如表2所示㊂由表2可知,经过不同时间的预处理,木质素回收率均可达95%以上,几乎实现木质素的全部回收,远高于报道的常见预处理体系如乙醇预处理体系[19]和离子液体预处理体系等[20]㊂众所周知,木质素的碎片化是导致其回收困难㊁得率低的重要原因㊂本研究中木质素的回收率高主要得益于本体系中丁二醇对木质素的保护作用,该机理在之前的研究中已报道[4],在反应过程中丁二醇同木质素侧链α位置的羟基发生醚化反应,该反应可有效抑制木质素中β⁃O⁃4芳基醚键的断裂,从而有效抑制木质素醚键的断裂和后续缩聚反应㊂此外,由于木质素侧链接上了丁二醇基团,这也导致木质素重量的增加而成为本研究中木质素得率高的原因之一㊂此外,回收木质素仅含有微量碳水化合物(0.04% 0.14%),酸不溶木质素占总量的84.56% 89.36%,酸溶木质素占木质素总量的5.69% 7.56%,木质素纯度可达90.88% 96.34%,表明该DES体系可回收获得高纯度木质素㊂综上所述,本研究所得的木质素同传统碱木质素和硫酸盐木质素相比,具备易回收㊁得率高㊁纯度高的优势,有利于其后续结构解析和高值化利用㊂表2㊀不同预处理时间下的木质素回收率及木质素糖含量分析Table2㊀Analysisofligninrecoveryandligninsugarcontentunderdifferentpretreatmenttimesindeepeutecticsystem不同预处理时间/min木质素回收率/%葡聚糖质量分数/%木聚糖质量分数/%阿拉伯糖质量分数/%酸不溶木质素质量分数/%酸溶木质素质量分数/%木质素纯度/%595.5400.14084.566.3290.881095.840.050.09085.655.6991.342096.7100.04086.327.5693.883095.35000.0387.327.2394.556096.2900089.366.9896.343㊀结㊀论以速生毛竹为原料,采用AlCl3辅助的多元醇基低共熔溶剂(氯化胆碱/1,4⁃丁二醇/AlCl3)进行预处理,实现了毛竹组分温和㊁快速分离,并借助扫描电子显微镜㊁X射线衍射等手段考察低共熔溶剂(DES)预处理对纤维素酶水解的促进机制,主要结论如下:1)氯化胆碱/1,4⁃丁二醇/AlCl3体系预处理在10 30min内实现毛竹中木质素和木聚糖有效脱除,同时保留绝大多数纤维素㊂2)DES预处理可在短时间内大幅提高物料的葡聚糖和木聚糖的酶水解得率㊂经过10 30min预处理后,毛竹预处理物料的葡聚糖酶水解得率即可达到89%以上,而在30min预处理后葡聚糖与木聚糖的酶水解得率均达到100%㊂19林业工程学报第8卷3)采用该DES体系,可回收超过95%的木质素,且回收的木质素几乎不含碳水化合物,纯度可达96.34%㊂研究结果可为建立高得率㊁高纯度木质素回收的DES体系提供方法参考㊂参考文献(References):[1]余燕燕,李以琳,楼雨寒,等.低共熔溶剂解离木纤维时木质素缩合对纤维素酶解的影响[J].林业工程学报,2021,6(6):101-108.DOI:10.13360/j.issn.2096-1359.202104022.YUYY,LIYL,LOUYH,etal.Effectoflignincondensationoncelluloseenzymatichydrolysisduringdeepeutecticsolventfractionationoflignocellulose[J].JournalofForestryEngineering,2021,6(6):101-108.[2]张玉静,陈皓钢,杨章旗,等.预处理对马尾松防腐材表面粗糙度和润湿性的影响[J].林业工程学报,2023,37(1):53-58.DOI:10.13360/j.issn.2096-1359.202201006.ZHANGYJ,CHENHG,YANGZQ,etal.Effectofpretreat⁃mentonsurfaceroughnessandwettabilityofpreservativetreatedMassonpine[J].JournalofForestryEngineering,2023,37(1):53-58.[3]MODENBACHAA,NOKESSE.Theuseofhigh⁃solidsloadingsinbiomasspretreatment:areview[J].BiotechnologyandBioen⁃gineering,2012,109(6):1430-1442.DOI:10.1002/bit.24464.[4]CHENGJ,HUANGC,ZHANY,etal.Effectivebiomassfrac⁃tionationandligninstabilizationusingadiolDESsystem[J].ChemicalEngineeringJournal,2022,443:136395.DOI:10.1016/j.cej.2022.136395.[5]益莎,杨波,杨光,等.竹产品加工剩余物有效成分的生物活性及应用研究进展[J].生物加工过程,2022,20(3):244-250.DOI:10.3969/j.issn.1672-3678.2022.03.002.YIS,YANGB,YANGG,etal.Progressonbioactivityandap⁃plicationofeffectivecomponentsfromprocessingresiduesofbam⁃booproducts[J].ChineseJournalofBioprocessEngineering,2022,20(3):244-250.[6]AMESHOKTT,CHENGPC,CHANGKL,etal.Microwave⁃assisteddeepeutecticsolvents/dimethylsulfoxidesystemforeffi⁃cientvalorizationofsugarbagassewasteintoplatformchemicals:abiorefineryapproachforcircularbioeconomy[J].BioresourceTechnology,2022,363:127969.DOI:10.1016/j.biortech.2022.127969.[7]XIEJ,XUJ,ZHANGZ,etal.Newternarydeepeutecticsol⁃ventswithcycleperformanceforefficientpretreatedradiatapineformingtolignincontainingcellulosenanofibrils[J].ChemicalEngineeringJournal,2023,451:138591.DOI:10.1016/j.cej.2022.138591.[8]HONGS,SHENXJ,XUEZM,etal.Structure⁃functionrela⁃tionshipsofdeepeutecticsolventsforligninextractionandchemi⁃caltransformation[J].GreenChemistry,2020,22(21):7219-7232.DOI:10.1039/D0GC02439B.[9]SHENXJ,WENJL,MEIQQ,etal.Facilefractionationoflignocellulosesbybiomass⁃deriveddeepeutecticsolvent(DES)pretreatmentforcelluloseenzymatichydrolysisandligninvaloriza⁃tion[J].GreenChemistry,2019,21(2):275-283.DOI:10.1039/C8GC03064B.[10]CHENL,YUQ,WANGQ,etal.Anoveldeepeutecticsolventfromlignin⁃derivedacidsforimprovingtheenzymaticdigestibilityofherbalresiduesfromcellulose[J].Cellulose,2019,26(3):1947-1959.DOI:10.1007/s10570-018-2190-8.[11]WANGZK,HONGS,WENJL,etal.Lewisacid⁃facilitateddeepeutecticsolvent(DES)pretreatmentforproducinghigh⁃purityandantioxidativelignin[J].ACSSustainableChemistryandEngineering,2020,8(2):1050-1057.DOI:10.1021/acs⁃suschemeng.9b05846.[12]LIUYZ,DEAKN,WANGZW,etal.Tunableandfunctionaldeepeutecticsolventsforlignocellulosevalorization[J].NatureCommunications,2021,12(1):5424.DOI:10.1038/s41467-021-25117-1.[13]SLUITERA,HAMESB,RUIZR,etal.Determinationofstruc⁃turalcarbohydratesandlignininBiomass⁃NREL/TP⁃510⁃42618[R].Colorado:LaboratoryAnalyticalProcedure(LAP),2012,17.http://www.nrel.gov/docs/gen/fy13/42618.pdf.[14]HED,WANGYX,YOOCG,etal.Thefractionationofwoodybiomassundermildconditionsusingbifunctionalphenol⁃4⁃sulfonicacidasacatalystandligninsolvent[J].GreenChemistry,2020,22(16):5414-5422.DOI:10.1039/D0GC01722A.[15]赵天畅,王杰,侯海旭,等.三元低共熔溶剂高效预处理提高杨木酶水解效率[J].林业工程学报,2022,7(3):86-92.DOI:10.13360/j.issn.2096-1359.202107037.ZHAOTC,WANGJ,HOUHX,etal.Theefficientpretreat⁃mentwithternary⁃deepeutecticsolventforfacilitatingtheenzy⁃matichydrolysisofpoplar[J].JournalofForestryEngineering,2022,7(3):86-92.[16]HUANGC,ZHANYN,CHENGJY,etal.Facilitatingenzy⁃matichydrolysiswithanovelguaiacol⁃baseddeepeutecticsolventpretreatment[J].BioresourceTechnology,2021,326:124696.DOI:10.1016/j.biortech.2021.124696.[17]LINWQ,XINGS,JINYC,etal.Insightintounderstandingtheperformanceofdeepeutecticsolventpretreatmentonimprovingenzymaticdigestibilityofbambooresidues[J].Biore⁃sourceTechnology,2020,306:123163.DOI:10.1016/j.biortech.2020.123163.[18]HUANGC,ZHANYN,WANGJ,etal.Valorizationofbamboobiomassusingcombinatorialpretreatments[J].GreenChemistry,2022,24(9):3736-3749.DOI:10.1039/D2GC00301E.[19]LANCEFIELDCS,PANOVICI,DEUSSPJ,etal.Pre⁃treat⁃mentoflignocellulosicfeedstocksusingbiorenewablealcohols:to⁃wardscompletebiomassvalorisation[J].GreenChemistry,2017,19(1):202-214.DOI:10.1039/C6GC02739C.[20]WEIHL,BUJ,ZHOUSS,etal.Afacileionicliquidandptoluenesulfonicacidpretreatmentofherbresidues:enzymatichydrolysisandligninvalorization[J].ChemicalEngineeringJour⁃nal,2021,419(3):129616.DOI:10.1016/j.cej.2021.129616.(责任编辑㊀李琦)29。
高效液相色谱法测定食品中乙酰磺胺酸钾含量
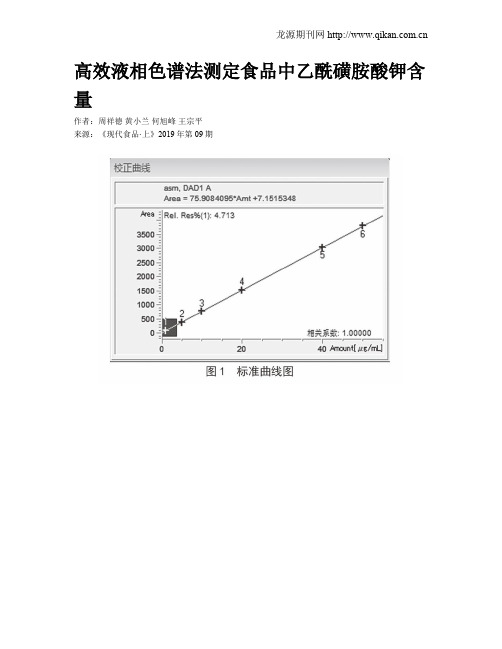
高效液相色谱法测定食品中乙酰磺胺酸钾含量作者:周祥德黄小兰何旭峰王宗平来源:《现代食品·上》2019年第09期摘要:建立食品中人工合成甜味剂乙酰磺胺酸钾(安赛蜜)的高效液相色谱(HPLC)检测方法。
样品经水提取,高脂肪样品经正己烷脱脂、高蛋白样品经蛋白沉淀剂沉淀蛋白,采用CAPCELL PAK C18(5 μm,4.6×250 mm)分离,以甲醇、0.02 mol·L-1乙酸铵水溶液作为流动相进行洗脱。
在1~50 μg·mL-1浓度范围内线性良好,相关系数0.999 9,乙酰磺胺酸钾在0.025、0.25、1.0 g·kg-1 3个添加水平的回收率范围为83.5%~101.0%,相对标准偏差为3.80%(n=7)。
当称样量为2 g时,该方法检出限为1 mg·kg-1。
该方法针对不同基质的樣品采用不同的前处理方法且操作简单,可以快速测定食品中的乙酰磺胺酸钾。
关键词:乙酰磺胺酸钾;高效液相色谱;不同基质食品;测定Abstract:A HPLC method for the determination of acesulfame potassium (Acesulfame) in food was established. The sample was extracted with water. The high fat sample was degreased in n-hexane, and the protein was precipitated by protein; precipitation agent. The target was separated by CAPCELL PAK C18 (5 μm, 4.6×250 mm). The mobile phase was methanol and 0.02 mol·L-1 ammonium acetate aqueous solution. The linearity was good in the concentration range of1~50 μg·mL-1, the correlation coefficient was 0.999 9, and the recovery rate of the three levels of addition of acesulfame potassium at 0.025, 0.25 g·kg-1 and 1.0 g·kg-1 was 83.5%~101.0%. The standard deviation is 3.80% (n=7). When the sample weight is 2 g, the detection limit of the method is 1 mg·kg-1. The method adopts different pretreatment methods for samples of different substrates and is simple to operate, andcanquickly determine potassium acesulfame in food.Key words:Acesulfame potassium; HPLC; Different substrate foods; Determination中图分类号:TS2乙酰磺胺酸钾,又名安赛蜜,是一种人工合成甜味剂[1],甜度高、价格低廉,添加到食品中可以降低产品成本,在食品领域使用广泛,但是经常食用含量超标的食品会对人体的肝脏和神经系统造成危害,如果短时间内大量食用,会引起血小板减少导致急性大出血,所以必须监控其添加量。
211237987_戊酸雌二醇预处理联合宫腔镜手术治疗中重度宫腔粘连的效果

[16] WU Z,YANG J,LIU J,et al.The relationship betweenmagnesium and osteoarthritis of knee:A MOOSE guided systematic review and meta-analysis[J/OL].Medicine (Baltimore),2019,98(45):e17774.https:///31702629/.[17]李晶晶,马卫兰,邓立琴,等.全膝关节置换术老年患者术后认知功能障碍的危险因素[J].中华麻醉学杂志,2019,39(2):158-161.[18]黎阳,刘金凤,李春莲,等.全膝关节置换术老年患者围术期镇痛管理的优化方案[J].中华麻醉学杂志,2019,39(12):1456-1460.[19]熊冰朗,林天烨,杨鹏,等.同期或分期双侧全髋关节置换临床疗效及围手术期安全性综合比较Meta 分析[J].海南医学院学报,2022,28(17):1327-1334,1342.[20]赵中溢,李勇阵,陈峰,等.同期双侧全膝关节置换和单髁置换治疗创伤性关节炎的比较[J].中国组织工程研究,2021,25(6):854-859.(收稿日期:2023-01-15) (本文编辑:田婧)*基金项目:惠州市科技计划项目(20210403)①广东省惠州市第一妇幼保健院 广东 惠州 516007通信作者:欧阳彦兰戊酸雌二醇预处理联合宫腔镜手术治疗中重度宫腔粘连的效果*欧阳彦兰①【摘要】 目的:探讨戊酸雌二醇预处理联合宫腔镜手术在治疗中重度宫腔粘连的临床应用。
方法:前瞻性选取惠州市第一妇幼保健院2021年3月-2022年1月收治的56例中重度宫腔粘连患者,按照随机数字表法分为常规组和联合组,每组28例。
两组均接受宫腔镜手术治疗,常规组在术后予以戊酸雌二醇治疗,联合组在常规组基础上予以戊酸雌二醇预处理治疗,观察两组患者子宫形态恢复情况、子宫内膜血流、细胞因子水平、月经恢复正常时间及宫腔再粘连发生率。
彩涂机组生产工艺流程

彩涂机组生产工艺流程英文回答:Coating Line Production Process.The production process of a coating line involves several key steps to transform raw materials into coated products. These steps include:1. Preparation of Substrates:The metal substrates (steel, aluminum) are cleaned thoroughly to remove any impurities or contaminants that could affect the adhesion of the coatings.2. Pretreatment:The pretreated substrates undergo a series of chemical and mechanical processes to improve their surface properties and enhance the bonding between the substrateand the coatings.3. Coating Application:Multiple layers of coatings are applied to the pretreated substrates using various methods such as roller coating, spray coating, or coil coating. These coatings provide the desired properties, such as corrosion resistance, aesthetics, and durability.4. Curing:The applied coatings are subjected to high temperatures in curing ovens to cross-link the polymer molecules and achieve optimum coating performance.5. Cooling:After curing, the coated products are cooled to room temperature in a controlled environment to prevent thermal stresses.6. Finishing:Additional processes, such as leveling, slitting, and packaging, are performed to prepare the finished coated products for shipment and use.中文回答:涂装生产工艺流程。
后续处理对SrTiO_(3)基晶界层电容器绝缘电阻的影响

第43卷第3期2021年5月湖北大学学报(自然科学版)Journal of Hubei University(Natural Science)Vol.43㊀No.3㊀㊀May 2021㊀收稿日期:20201201基金项目:国家自然科学基金(11674086)及贵州省经济和信息化委员会技术创新项目(2017021)资助作者简介:张木森(1995),男,硕士生;杨昌平,通信作者,教授,E-mail:cpyang@文章编号:10002375(2021)03028906后续处理对SrTiO 3基晶界层电容器绝缘电阻的影响张木森1,石大为2,徐玲芳1,王瑞龙1,肖海波1,梁世恒1,杨昌平1,3(1.湖北大学物理与电子科学学院,湖北武汉430062;2.湖北理工学院数理学院,湖北黄石435003;3.太原科技大学材料科学与工程学院,山西太原030024)摘要:采用二步法制备SrTiO 3晶界层电容器,并对其进行后续热㊁电和液氮处理,研究处理前后电容器电学性能的变化.实验结果表明,在50V 直流电压和200ħ条件下对SrTiO 3晶界层电容器进行后续快速退火和液氮处理后,其介电常数和介电损耗在基本保持不变的情况下,其绝缘电阻值可得到大幅提升,从最初30GΩ上升至200GΩ.通过处理,最后可获得平均介电常数为30000,损耗为0.003,绝缘电阻(50V 测量)为200GΩ的高性能SrTiO 3晶界层电容器.关键词:SrTiO 3晶界层电容器;介电性能;后续热电处理;绝缘电阻中图分类号:TQ174.75㊀㊀文献标志码:A㊀㊀DOI :10.3969/j.issn.1000-2375.2021.03.010著录信息:张木森,石大为,徐玲芳,等.后续处理对SrTiO 3基晶界层电容器绝缘电阻的影响[J].湖北大学学报(自然科学版),2021,43(3):289-294.Zhang M S,Shi D W,Xu L F,et al.Effect of subsequent thermal and electrical treatment on resistance of SrTiO 3grain boundary layer capacitors[J].Journal of Hubei University(Natural Science),2021,43(3):289-294.Effect of subsequent thermal and electrical treatment on resistance of SrTiO 3grain boundary layer capacitorsZHANG Musen 1,SHI Dawei 2,XU Lingfang 1,WANG Ruilong 1,XIAO Haibo 1,LIANG Shiheng 1,YANG Changping 1,3(1.Faculty of Physics and Electronic Technology,Hubei University,Wuhan 430062,China;2.College of Mathematical and Physical Sciences,Hubei Polytechnic University,Huangshi 435003,China;3.Faculty of Materials Science and Engineering,Taiyuan University of Science and Technology,Taiyuan 030024,China)Abstract :SrTiO 3grain boundary layer capacitors were fabricated by the two-step method and the dielectric properties were studied.The results show that the insulating resistance of SrTiO 3grain boundary layer capacitor can be dramatically enhanced from the initial 30GΩup to 200GΩby the subsequent treatment of rapid annealing at 200ħunder 50V with quenching in liquid nitrogen while the dielectric constant and dielectric loss remain basically unchanged.Herein,a SrTiO 3grain boundary layer ceramic capacitor with an average value of 30000for dielectric constant,dielectric loss 0.003,and insulating resistance 200GΩat 50V was obtained.Key words :SrTiO 3grain boundary layer capacitor;dielectric property;subsequent thermoelectrictreatment;insulating resistance290㊀湖北大学学报(自然科学版)第43卷0㊀引言单片层电容器由于体积小㊁介电常数高㊁温度稳定性好和应用频率宽等优点在电子对抗㊁雷达㊁导航㊁制导和卫星通讯等领域具有广泛应用[1-4].晶界层电容器是单片层电容器中的一种,主体成份一般为BaTiO3或SrTiO3,经过两次烧结制得[5-7].SrTiO3由于具有更大绝缘电阻㊁更高的耐压值以及更好的温度稳定性成为生产晶界层电容器的主要材料,占有市场主要份额[8].目前市场上单层片式半导体陶瓷材料的生产厂家,国际上主要有DLI㊁PRESIDIO COM-PENONT㊁TECDIA等厂家,国内生产单位主要有广州可纳瑞电子科技有限公司㊁广州金陶电子有限公司和电子科技大学.对于相同尺寸的SrTiO3晶界层电容器,国内产品在电容㊁损耗㊁电容温度变化系数和使用频率等诸多性能参数与国际产品相差不大,但绝缘电阻值和耐压值与国外先进产品相差较大,一般为国外产品20%~50%.因此提高国内SrTiO3(STO)晶界层电容器的绝缘电阻和耐压值,对提高国内产品质量,缩小与国外同类产品差距具有重要意义. STO晶界层电容器由表面㊁晶粒和晶界等部分构成[9].一般地,为保证电容器具有优良的介电性能和大的绝缘电阻值,金属电极与STO陶瓷表面应接触紧密,以保证两者接触为欧姆接触;STO晶粒应半导化,具有良好导电性和较小电阻值.STO电容器的介电性能和绝缘电阻等主要取决于晶界层性质,晶界层应尽可能的薄,以产生大的电容和介电常数;且绝缘电阻值应尽可能的大,以减小电容器的损耗和增大耐压值.由此可知晶界层的结构和性质决定了STO电容器的性能和使用.STO晶界层电容器绝缘电阻值取决于晶界电阻,该电阻包含两部分,一是由晶界层中玻璃化物质产生的欧姆电阻,二是通过在晶界层掺杂受主离子产生空间电荷区,由空间电荷区产生的势垒电阻[10].过去,人们利用氧化剂和玻璃化物质在高温条件下通过热扩散进入STO晶界以增大样品玻璃化物质的欧姆电阻和势垒电阻,但由于晶界层中的氧化剂和玻璃化物质处于原子排列无序的非晶状态,晶界层中的空位和缺陷较多,对STO的介电性能,绝缘电阻和损耗等均产生不利影响[11-12].因此,单纯依靠调整氧化剂和玻璃化物质成分和比例很难解决STO晶界层电容器综合性能的参数问题.本文中我们采用二步法制备SrTiO3晶界层电容器,并对其进行后续热电及液氮处理.实验结果表明,在50V直流电压和200ħ条件下对SrTiO3晶界层电容器进行后续快速退火处理,其绝缘电阻值可得到大幅提升.如果将样品置于液氮中,其介电常数和介电损耗在基本保持不变的情况下,绝缘电阻值将进一步得到提升,从原30GΩ大幅上升至200GΩ,表明后续热电及液氮处理方法是提高SrTiO3晶界层电容器绝缘电阻的有效方法.1㊀实验部分1.1㊀样品制备㊀本实验采用流延法及二步固相烧结法制备STO晶界层电容器.首先以SrCO3(纯度> 99%)㊁TiO2(纯度>99%)为主料加入无水乙醇,然后置于行星式球磨机球磨24h,取出用干燥箱进行干燥.然后在退火炉以4ħ/min升温至1150ħ,保温2h自然降温至室温得到SrTiO3前驱体.之后利用流延工艺将前驱体粉料在流延机上流延成片,得到STO基片(尺寸为40mm(长)ˑ40mm(宽)ˑ0.25 mm(厚)),将得到的STO基片以3ħ/min升温至600ħ后保温10h进行充分排胶.然后在充满N2和H2的还原性氛围中进行第一次烧结,即半导化烧结,烧结温度为1250~1480ħ,保温时间为2h.将得到的半导化STO陶瓷基片用旋涂法双面旋涂氧化剂,氧化剂为Pb3O4,Bi2O3,CuO,B2O3和溶剂(丁基卡必醇和N1载体按一定比例配料球磨得到).将涂覆好的基片在1000~1100ħ保温2.5h进行第二次烧结,即绝缘化烧结.之后样品从1000~1100ħ经0.5h降温至900ħ,再自然降温至室温.采用丝网印刷技术将得到的STO基片两面均匀的涂上银浆,在500~600ħ保温烧银制成样品电极.最后将样品切割为1mm(长)ˑ1mm(宽)ˑ0.25mm(厚)小型方片电容器待测.1.2㊀样品的热、电处理㊀将制好的SrTiO3电容器样品置于快速退火炉中(RTP-100004,合肥科晶材料技术有限公司),用钨丝将电容器电极面夹紧并施加50V的直流电压,电压由KEITHLEY2400提供.加压后,样品从室温以1ħ/s速度经200s后快速升至200ħ,保温100s后,关掉加压和升温电源,样品第3期张木森,等:后续处理对SrTiO3基晶界层电容器绝缘电阻的影响291㊀随炉温通过自然冷却降至室温.液氮处理过程为:对电容器样品施加50V的直流电压,以1ħ/s速度经200s快速从室温升至200ħ,保温100s后,关掉加压和升温电源后,将样品迅速从快速退火炉中取出置于液氮中急速冷却,1~3min后取出,在空气中静置24h,样品恢复至室温后待测.1.3㊀样品测量㊀样品形貌㊁晶界显微结构观察及EDS测量采用高分辨透射电子显微镜(FEI TECNAI G20200kV,美国FEI公司).SrTiO3电容器介电常数㊁介电损耗用介电谱仪(IM3590,日本HIOKI株式会社)进行测量,测量频率和电压分别为200kHz和1.0V.电容器电阻用绝缘电阻测试仪(TH2681,常州同惠电子股份有限公司)进行测量,测试电压为50V.2㊀结果与讨论SrTiO3晶界层电容器的绝缘电阻是其电学性能的一个重要参数,绝缘电阻值越大,电容器耐压值越大,介电损耗越小,电容器性能越稳定[13-14].因晶粒处于半导化状态,电阻值较小,STO电容器电阻主要由晶界层决定.该电阻由两部分组成,一部分由晶界处玻璃化物质产生的欧姆型电阻贡献,另一方部分由晶界层中受主离子与N型导电的STO晶粒之间的空间电荷层产生的势垒电阻贡献[15-17].STO陶瓷半导化后涂覆受主氧化剂并在氧化性气氛进行第二次烧结的过程实际上也是空气中的氧沿晶界向晶粒内部扩散的过程.氧向晶粒内部扩散时产生了扩散层,使晶界处的离子㊁电荷分布㊁载流子浓度等发生变化.由于晶界处大量受主态的存在,受主态离子和界面附近导电电子及带负电离子形成耗尽层,建立起Schottky势垒.势垒层宽度越宽,晶界势垒越高,电阻值越大.势垒层宽度与晶界中受主态离子浓度N D 有关,可以用下式表示[18]:X d=2εФ/qN D(1)式中X d为势垒层宽度,ε为晶界层玻璃化物质相对介电常数,Ф为势垒电势,q为单个受主离子电量, N D为有效受主掺杂浓度.从(1)式可以看出晶界受主杂质浓度越高则势垒层(耗尽层)越宽,Schottky势垒越大,电容器的绝缘电阻值越大.但同时由于样品晶界层变宽,样品的介电常数随之急剧减小,因此晶界层电容器的绝缘电阻与介电常数两者之间存在竞争,并相互制约.如何在不降低电容器介电常数的情况下提高电阻值是目前研究STO晶界层电容器的一个重要内容.我们通过实验发现后续热㊁电及液氮处理的方法在不显著改变电容器的介电常数和介电损耗的条件下,可极大提高电容器的绝缘电阻值.图1为不同放大倍数的STO显微结构TEM图.从图1(a)可以看到STO电容器为多晶陶瓷,晶界交汇处存在大量玻璃态物质.图1(b)~(c)可以看到,随TEM放大倍数进一步增加,STO晶界层中的玻璃化物质以纳米颗粒形式分散在晶界中,晶界层厚度在10nm量级.图1㊀不同放大倍数的STO晶粒及晶界TEM显微形貌图2为图1晶界层处的EDS图谱.从图2中可以看出,除Sr㊁Ti㊁O等STO基体组成元素外,还有Cu㊁Bi㊁Pb等涂敷剂(氧化剂)物质通过二次烧结经扩散作用也出现在晶界中.其中Bi对氧具有较强的吸附作用,从而有利于STO晶界的绝缘化,Pb具有较大的原子量和介电常数值,有利于提高晶界层玻璃化物质的介电常数,Cu离子价态(+1,+2)比Ti离子(+4)小,从而充当受主,带正电,与受氧空位N型半导化带负电的STO晶粒组成空间电荷层,为晶界层提供肖特基势垒和势垒电阻.这可以从图3的实验结果得到进一步说明.图3为经二步法烧结制得的STO晶界层电容器样品在50V测量电压下,绝缘电阻值随测量时间的变化.样品为小方片,尺寸为1mm(长)ˑ1mm(宽)ˑ0.25mm(厚),电容和损耗值分别为950pF292㊀湖北大学学报(自然科学版)第43卷㊀㊀㊀㊀㊀图2㊀STO 晶界处EDS 测量图3㊀加载电压50V 下电阻值随时间的变化(200kHz,1V 测量)和0.3%(200kHz,1V 测量).从图3可以看出STO 电容器绝缘电阻并非常数,随着负载时间的增加,电阻值逐渐变大.经70000s(约20h),从最初的30GΩ大幅增加到600GΩ左右后不再继续增大,表明该晶界层电容器电阻为势垒电阻,主要由晶界处空间电荷层的肖特基势垒贡献.该实验结果也说明在电场作用下,晶界处受主离子通过俘获载流子使得空间电荷层宽度增加从而提高界面处的势垒电势和绝缘电阻[19].当界面处受主离子俘获载流子与载流子逃逸受主陷阱速率相等时,绝缘电阻值达到最大并随时间不再继续增加.该实验结果同时也表示,STO 晶界层电容器的绝缘电阻值在50V 条件下,潜在值为600GΩ,远高于初始测量的30GΩ,表明电容器绝缘电阻具有很大的提升空间,电学处理是增加电阻值的有效方法.图4是图3经电学处理,样品静置24h 后,STO 样品的绝缘电阻值随测量时间的变化.电学处理过程为:将STO 电容器加载50V,经70000s 后将电压卸掉.由图4可以看出,STO 晶界层电容器电阻值在卸掉处理电压(50V)后,电阻值有所下降,从600GΩ降低至300GΩ.图5是样品在图4的基础上继续静置24h 后对其电阻进行的测量,可以看出随着静置时间的加长,电阻从之前的300GΩ继续减小至40GΩ左右.以上结果表明,STO 电容器通过简单的电学处理,绝缘电阻可显著增大,但其高绝缘电阻态无法维持,卸掉处理电压,静置48h 后,其绝缘电阻值重新恢复到原来小电阻状态,表明单纯的电学处理不能从根本上提高STO 电容器的绝缘电阻值.图4㊀经电处理后,室温㊁零电压下静置24h 后,负载50V 条件下STO 电容器电阻值随时间的变化图5㊀样品室温㊁零电压下再继续静置24h 后,负载50V 条件下STO 电容器电阻值随时间的变化㊀㊀为了将加载电压后STO 电容器的超高阻值(600GΩ)保留下来,我们在加载电压对电容器进行电处理的同时,也对电容器进行热处理.图6为STO 样品经电㊁热双重处理后,经不同时间(24h 和48h)静置,其绝缘电阻值随测量时间的变化.电㊁热双重处理过程如下:STO 电容器样品在加载50V 的直流电压后,在快速退火炉中以1ħ/s 速度经200s 迅速从室温升至200ħ,保温100s 后,关掉电压和升温电源,然后将样品迅速从快速退火炉中取出置于液氮中急速冷却1~3min 后取出在空气中静置.从图6可第3期张木森,等:后续处理对SrTiO 3基晶界层电容器绝缘电阻的影响293㊀以看出,尽管静置时间加长,但是经热㊁电以及液氮处理后的STO 电容器绝缘电阻值基本没有减小,经48h 静置后仍能维持在200GΩ的高阻态.表明电热及液氮处理不仅可显著提高STO 电容器绝缘电阻值,并且其高阻态能够保持和稳定住.考虑到陶瓷样品组分和电学性能存在一定的分散性,我们对多个STO 样品进行类似的处理和测量,结果示于图7.黑色方形点代表未经热㊁电和液氮处理电容器样品的绝缘电阻值;黑色圆点为同一批样品经热㊁电及液氮处理后,室温㊁零电压静置48h,然后在50V 条件测量的其绝缘电阻值;N 为样品序号.可以看出,所有测试样品经处理后绝缘电阻值均有大幅增加,最小增加量为100GΩ,最大增加为200GΩ.图6㊀热电处理后的STO 电容器经不同静置时间其绝缘电阻值随测量时间的变化图7㊀同批样品不同序号STO 电容器处理前后的电阻值㊀㊀图8和图9分别为图7样品处理前后的介电常数和介电损耗值(200kHz,1.0V 测量).可以看出经热电和液氮处理后,样品的介电常数均有所下降,最小下降量为216,最大下降量为6400,而介电损耗值在样品处理前后则无明显变化,变化范围基本保持在0.2%~0.4%.表明后续热电和液氮处理方法是在不改变样品介电性能条件下提高SrTiO 3晶界层电容器绝缘电阻的有效方法.图8㊀不同STO 电容器处理前后的介电常数图9㊀不同STO 电容器处理前后的介电损耗3 结论本文中采用二步法制备SrTiO 3晶界层电容器样品,对样品进行后续热电和液氮处理,研究该后续处理方法对样品介电性能及绝缘电阻值的影响.实验结果表明,经后续热电和液氮处理后,SrTiO 3晶界层电容器样品介电常数和损耗介电值基本保持不变,但绝缘电阻值得到大幅提升,从原30GΩ大幅上升至200GΩ.说明后续热电和液氮处理方法是提高SrTiO 3晶界层电容器绝缘电阻的有效方法.通过这样处理,我们可以获得平均介电常数为30000,损耗为0.003,绝缘电阻值为200GΩ的STO 电容器.294㊀湖北大学学报(自然科学版)第43卷4 参考文献[1]杨俊锋,冯毅龙,赵海飞,等.晶界层介电陶瓷及其单层电容器[J].材料研究与应用,2008,2(3):207-210.[2]Bao Lee P,Chin W L,Joon C J,et al.A review of synthesis and morphology of SrTiO3for energy and other applications [J].International Journal of Energy Research,2019,43(10):5151-5174.[3]Meyer B,Padilla J,Vanderbilt D.Theory of PbTiO3,BaTiO3,and SrTiO3surfaces[J].Faraday Discussions,1999,114: 395-405.[4]陈功田.高性能微波介质陶瓷的研制及其在小型GPS天线中的应用[D].长沙:湖南大学,2012.[5]Liu J,Liu Q L,Nie Z P,et al.Dielectric relaxations in fine-grained SrTiO3ceramics with Cu and Nb co-doping[J]. Ceramics International,2019,45(8):10334-10341.[6]汪春昌,李天宇.无铅非线性介电储能陶瓷材料研究进展[J].安徽大学学报(自然科学版),2019,43(4):1-11.[7]Paunovic V,Mitic V V,Djordjevic M,et al.Niobium doping effect on BaTiO3structure and dielectric properties[J]. Ceramics International,2020,46(6):8154-8164.[8]张士成,陈炳辰,韩跃新.钛酸锶系电子陶瓷的性能与应用研究进展[J].矿冶,2001,10(1):63-67.[9]孟凡明,孙兆奇.SrTiO3压敏材料研究进展[J].硅酸盐通报,2006,25(5):99-102.[10]钟吉品,王鸿,殷之文.晶界层电容器晶界势垒的研究[J].无机材料学报,1988,3(4):323-328.[11]赵双群,高斌,范文斌,等.KNbO3多晶陶瓷晶界势垒和电阻率分析[J].云南大学学报(自然科学版),1998,20(S1):3-5.[12]Alexander T,Paula M V,Ana M R S,et al.Oxygen vacancies as a link between the grain growth and grain boundaryconductivity anomalies in titanium-rich strontium titanate[J].Journal of the European Ceramic Society,2018,38(6): 2547-2552.[13]He F,Lv M,Lu M H,et al.Direct current field induced asymmetrical DC-resistivity degradation in SrTiO3-based grainboundary layer ceramic[J].Ceramics International,2019,45(10):13546-13550.[14]Miho Uehara,Masakazu Tanahashi.Distribution of electric resistance of dielectric semiconductive ceramics[J].Journal ofthe Ceramic Society of Japan,2010,99(1155):1120-1123.[15]徐序,罗凌虹,程亮,等.Sc2O3稳定ZrO2多晶离子导体的晶界电导特性研究[J].陶瓷学报,2017,38(5):771-776.[16]甘国友,王静,严继康,等.SrTiO3双功能陶瓷表面层效应的研究[J].材料导报,2006,20(S1):354-355.[17]Lee E,Pee Jae Hwan,Lee Sung Min,et al.Enhanced high-temperature electrical resistivity of aluminum nitride obtainedby engineering a schottky barrier at grain boundaries[J].Journal of the Korean Physical Society,2020,77(8):673-679.[18]钟吉品,王鸿,殷之文.晶界势垒对晶界层电容器性能的影响[J].无机材料学报,1990,5(2):126-131.[19]Glot A B,Mazurik S V,Jones B J,et al.Current limiting and negative differential resistance in indium oxide basedceramics[J].Journal of the European Ceramic Society,2009,30(2):539-544.(责任编辑㊀郭定和)。
安徽省污水处理厂尾水湿地处理

安徽省污水处理厂尾水湿地处理技术导则(试行)安徽省住房和城乡建设厅安徽省城建设计研究院2015年2月前言安徽省城镇污水处理厂尾水大多数排放进入封闭性或缓流的河湖水体。
虽然省内污水处理水平及尾水排放标准逐步提高,但是污水处理厂尾水进入封闭河湖,难于满足水环境质量的要求。
因此,为改善封闭河湖以及城市水体的污染情况,利用湿地技术进一步提高城镇污水处理厂的尾水水质,制订本导则。
本导则规定了城镇污水处理厂尾水湿地处理技术工艺流程、技术措施、施工、验收、运行及管理的技术要求。
本导则由安徽省住房与城乡建设厅解释。
目录1 总则 (1)1。
0.1编制目的 (1)1。
0.2适用范围 (1)1.0。
3水质目标 (1)1.0。
4指导思想 (1)1.0.5其他 (1)2 术语 (2)3 工艺流程 (5)3。
1 一般规定 (5)3.2 工艺流程 (5)4 技术措施 (6)4.1一般规定 (6)4。
2表面流人工湿地 (7)4.3水平潜流人工湿地 (7)4.4垂直流人工湿地 (9)4。
5自然湿地强化措施 (10)4。
6湿地辅助技术 (10)4。
7植物 (11)4.8填料 (13)5 施工与验收 (15)5。
1 一般规定 (15)5。
2 施工 (15)5。
3 调试启动 (16)5。
4 工程验收 (16)6 运行与管理 (17)1.0。
1 编制目的为改善封闭或缓流水体的水环境质量,促进城镇生态环境建设,结合本地实际情况,采用湿地技术提高安徽省城市污水处理厂尾水水质,以减少污染负荷、改善城市河湖水质。
1.0.2 适用范围安徽省省内排入封闭水体的污水处理厂尾水,有可供利用的自然条件(土地、地形地貌、地质、气象、水文、土壤以及动植物生态等)时,宜采用湿地技术对污水处理厂尾水进一步处理。
1。
0。
3 水质目标污水处理厂尾水经过湿地处理后,主要的水质指标(COD Cr、BOD5、NH3—N、TP等)达到《地表水环境质量标准》(GB 3838—2002)中的Ⅳ类水标准:COD Cr≤30mg/L,BOD5≤6mg/ L,NH3—N≤1.5mg/ L,TP≤0。
