化学专业英语试卷B
有机化学专业英语

烷基Alkyl [ˈælkil]芳基aryl [ˈæril]甲基methyl [ˈmeθil]亚甲基methylene[ˈmeθili:n]乙基ethyl [ˈeθil,ˈeθəl]丙基propyl [ˈprəupil]异丙基isopropyl [ˌaisəuˈprəupil]丁基butyl [ˈbju:til]戊基pentyl [ˈpentil]己基hexyl [ˈheksil]庚基heptyl [ˈheptil]辛基octyl [ˈɔktəl]壬基nonyl [ˈnɔnil]奎基decyl ['desəl][di:'s i l]叔丁基tert-butyl异丁基iso-butyl环戊基cyclopentyl []环己基cyclohexyl []甲氧基methoxyl ['metɒksɪl]乙氧基ethoxyl [eˈθɔksil]丁氧基butoxyl酰基acyl[ˈæsil]甲酰基formyl [ˈfɔ:mil]乙酰基acetyl [ˈæsitil]乙烯基vinyl [ˈvaɪnəl]或ethenyl丁烯基butenyl [ˈbjutənil]己烯基hexenyl庚烯基heptenyl [ˈheptəˌnil]烯丙基allyl [ˈælil]乙炔基ethinyl [eˈθainil] 或alkynyl硝基nitro [ˈnaitrəu]亚硝基nitroso [naiˈtrəusəu]氨基amino [əˈmi:nəʊ, ˈæməˌnəʊ]二氨基diamino亚氨基imino [ˈiminəu,iˈmi:nəu]重氮基diazo [daiˈæzəu]苯基phenyl [ˈfenəl,ˈfi:nəl,ˈfi:nil]苄基benzyl [ˈbenzil]或phenmethyl [ˌfinˈmeθil]苯乙基phenethyl [fenˈeθəl]乙氧苯基ethoxyphenyl苯胺基anilino [ˈænili:n]1羰基carbonyl [ˈkɑ:bənil]羧基carboxyl [kɑ:ˈbɔksil]联苯基biphenyl [baiˈfenl]甲酰基formyl [ˈfɔ:mil]苯酰,苯甲酰benzoyl ['benzəʊɪl]脒基guanyl [il]羟基hydroxyl [haiˈdrɔksil]烷氧基alkoxy [ælˈkɔksi]或alkoxyl group 芳基aryl group二芳基diaryl group [daiˈæril]吡啶基pyridyl[ˈpiridil]三苯甲基trityl['traɪtl]二苯甲基benzhydryl [benaɪd'raɪl]氨基甲酰基carbamoyl[kɑ:'bæməɪl]三甲基硅基trimethylsilyl炔丙基propargyl [prəʊ'pɑ:dʒɪl]丙酮基(乙酰甲基)acetonyl['æsɪtənɪl]正n,normal异iso邻位ortho-[ˈɔ:θəu]间位meta-['mɛtə]对位para-[ˈpɑ:rə]伯Primary ['praimәri]仲Secondary ['sekәndәri]叔Tertiary ['tә:ʃәri] tert-季碳quaternary [kwəˈtə:nəri] carbon一,单mono-二di-,双bis ,bi(化学中只有碳酸氢根才用bi,如bicarbonate [baiˈkɑ:bənit])三tri-,tris四tetra-四quadric-五penta-五quinque-六hexa-七hepta-七septi八octa-九nona-十deca-['dɛkə]十一undeca ,hendeca-十二dodeca-十三trideca-十四tetradeca十五pentadeca-十六hexadeca-2十七heptadeca-顺式,cis-同,共syn反式trans有机化合物类名Aliphatic compound 脂肪族化合物[]Hydrocarbon 碳氢化合物[ˌhaɪdrəˈk ɑ:bən] Alkane 烷[]Wax 蜡[]Paraffin wax 石蜡arene 芳烃[]Alkene 烯[]Alkyne 炔[ˈælkain]Acetylide 炔化物[] Active hydrogen compounds 活泼氢化合物acid [ˈæsid]Carbon acid 碳氢酸Super acid 超酸Diene 双烯[ˈdaii:n]Triene 三烯[ˈtraii:n]Allene 丙二烯[ˈæli:n]Propylene丙烯[] cumulene 累积多烯[] Enyne 烯炔[eˈni:n]Diyne 二炔Alkyl halide 卤代烷[ˈælkil ˈhælaid]Alcohol 醇[]Homoallylic alcohol 高烯丙醇Ether 醚[ˈi:θə]Ester 酯[ˈestə]Ketone 酮Aldehyde 醛[ˈældihaid]Epoxide 环氧化物[eˈpɔksaid]Sulfone 砜[ˈsʌlf əun]Sulfoxide 亚砜Sulfonic acid 磺酸Carboxylic acid 羧酸Cellosolve 溶纤剂Crown ether 冠醚Nitro compound 硝基化合物Amine 胺[] Quaternaryammonium compound 季铵化合物[] []Amine oxide 氧化胺Diazoalkane 重氮烷[daɪ,æzəʊ'ælkeɪn] Mercaptan 硫醇[] Aldehyde hydrate 醛水合物Ketone hydrate 酮水合物Hemiacetal 半缩醛[ˌhemiˈæsitæl]3Acetal 缩醛acetal [化]乙缩醛, 乙缩醛二乙醇[ˈæsitæl] Ketal 缩酮[ˈki:tæl]thiazole噻唑[ˈθaiəˌzəul]Dithiane 二噻烷[daiˈθaiən]Aminal 缩醛胺;动物imine 亚胺[]Aldimine 醛亚胺Oxime 肟[]nitroso compound 亚硝基化合物aldoxime 醛肟,乙醛肟[ælˈdɔksi:m] Hydrazone 腙[ˈhaidrəˌzəun]Azine 嗪[ˈæzi:n]Semicarbazone 缩氯基脲Cyanohydrin 羟腈,氰醇[ˌsaiənəuˈhaidrin] Pinacol 频哪醇Enol 烯醇[ˈi:nɔl]Enol ether 烯醇醚Enol ester 烯醇酯[ˈi:nɔl][ˈestə] Enamine 烯胺[iˈnæmin]Ynamine 炔胺Mannich base 曼尼希碱orthoester 原酸酯Acyl halide 酰卤[ˈæsil]Acyl fluoride 酰氟[]Acyl chloride 酰氯Acyl bromide 酰溴Acyl iodide 酰碘[ˈaiədaid]Carbobenzoxy chloride 苄氧甲酰氯Acyl tosylate 酰基对甲苯磺酸酐Ketene 乙烯酮[ˈki:ti:n]Peracid 过酸Perester 过酸酯Acyl peroxide 酰基过氧化物Nitrile 腈[ˈnaitrail]acetonitrile 乙腈[ˌæsitəuˈnaitril]或methyl cyanide [ˈsaɪəˌnaɪd]Nitrile oxide 氧化腈Isonitrile 异腈,异氰化物Amide 酰胺[ˈæmaid]Imide 二酰亚胺[ˈimaid]N-bromo compound N-溴化物Hydrazide 酰肼[]Azide 叠氮化物[ˈæzaid,ˈeizid]Acyl azide 酰基叠氮[ˈæsil][ˈæzaid,ˈeizid] Amidine 脒[ˈæmiˌdi:n]Keto ester 酮酸酯Acyl cyanide 酰腈[ˈæsil][ˈsaɪəˌnaɪd] Carbon suboxide 二氧化三碳Glycidic acid 环氧丙酸Carbammic acid 氨基甲酸Carbamate 氨基甲酸酯[ˈkɑ:bəmeit]Urea 脲,尿素[] Cyanamide 氨腈[saiˈænəmaid] Carbodiimide 碳二亚胺[,kɑ:bədai'imaid] Allophanate 脲基甲酸酯Thioester 硫代酸酯[ˌθaiəuˈestə]Thiol acid 硫羰酸[ˈθaiəu]Lactone 内酯[ˈlæktəun]Lactol 内半缩醛[ˈlæktəl]4Macrolide 大环内酯[ˈmækrəlaid] Amino acid 氨基酸Zwitterion两性离子[ˈtsvitəraiən]Inner salt 内盐Betaine 甜菜碱[ˈbi:təi:n]Lactam 内酰胺[ˈlæktæm]Hydantoin 或glycolylurea 乙内酰脲[haiˈdæntəwin]Hydration水合,水合作用[haɪ'dreʃən] Peptide 肽[ˈpepˌtaɪd]Glycol 乙二醇[]Aldol 羟醛[ˈældəul]Acyloin 偶姻,酮醇[əˈsiləuin]acyloin condensation 酮醇缩合Carbohydrate 碳水化合物Aldose 醛糖[ˈældəus]Ketose 酮糖[ˈki:təus]Furanose 呋喃糖[ˈfjuərəˌnəus] Pyranose 吡喃糖[ˈpaiərənəus] Glycoside 糖苷[ˈɡlaikəˌsaid]Glucoside 葡[萄]糖苷Aglycon 苷元[əˈɡlaikɔn]Saccharide 糖类[ˈsækəraid] Oligosaccharide 寡糖[ˌɔliɡəuˈsækəraid] Polysaccharide 多糖[pɔliˈsækəraid] Alditol 糖醇[ˈælditɔl]Osazone 脎[ˈəusəˌzəun]Alicyclic compound 脂环化合物[æliˈsiklik] Cycloalkane 环烷Cycloalkene 环烯Spirane 螺烷[ˈspaiərein]Cage compound 笼型化合物Propellane 螺桨烷Rotazane 轮烷Catenane 索烃[ˈkætnein]Fused ring 稠环[fju:zd riŋ]化学专业英语词汇常用前后缀-acetal 醛缩醇acetal- 乙酰acid 酸-al 醛alcohol 醇-aldehyde 醛alkali- 碱allyl 丙烯基 'alkoxy- 烷氧基Methoxy甲氧基的-amide 酰胺 []amino- 氨基的[əˈmi:nəʊ, ˈæməˌnəʊ]-amidine 脒[ˈæmiˌdi:n]-amine 胺-ane 烷anhydride 酐[ænˈhaidraid]anilino- 苯胺基[ˈænili:n]aquo- 含水aqueous水的,水成的[ˈeikwiəs] -ase 酶-ate 含氧酸的盐、酯-atriyne 三炔azo- 偶氮[ˈæzəu]azoxy-氧化偶氮-N=N(O)-hydrazo-氢化偶氮-NH-NH-5benzene 苯[ˈbenˌzi:n, benˈzi:n] bi- 在盐类前表示酸式盐bis- 双-borane 硼烷[ˈbəurein]bromo- 溴butyl 丁基 .-carbinol 甲醇carbonyl 羰基-caboxylic acid 羧酸centi- 10-2chloro- 氯代cis- 顺式condensed 缩合的、冷凝的cyclo- 环deca- 十deci 10-1di二-dine 啶dodeca- 十二-ene 烯epi- 表epoxy- 环氧 []-ester 酯-ether 醚ethoxy- 乙氧基[] ethyl 乙基fluoro-或fluor- 氟代-form 仿-glycol 二醇hemi- 半hendeca- 十一hepta- 七heptadeca- 十七hexa- 六hexadeca- 十六-hydrin 醇hydro- 氢或水hydroxyl 羟基hypo- 低级的,次-ic 酸的,高价金属-ide 无氧酸的盐,酰替胺,酐-il 偶酰-imine 亚胺 /iodine 碘[]iodo- 碘代iso- 异,等,同-ite 亚酸盐keto- 酮ketone 酮-lactone 内酯mega- 106meta- 间,偏methoxy- 甲氧基methyl 甲基micro- 10-6milli- 10-3mono- ( mon-) 一,单nano- 10-9nitro- 硝基nitroso- 亚硝基nona- 九nonadeca- 十九octa- 八octadeca- 十八6-oic 酸的-ol 醇9 a$ f! Q, H: [5 n& G-one 酮ortho- 邻,正,原-ous 亚酸的,低价金属oxa- 氧杂-oxide 氧化合物-oxime 肟 []oxo- 酮 []oxy- 氧化 []-oyl 酰para- 对位,仲penta- 五pentadeca- 十五per- 高,过petro- 石油phenol 苯酚[ˈfi:nəl]phenyl 苯基 []pico- 10-12poly- 聚,多quadri- 四quinque- 五semi- 半septi- 七sesqui 一个半sulfa- 磺胺 []sym- 对称syn- 顺式,同,共ter- 三 -tetra- 四tetradeca- 十四tetrakis- 四个thio- 硫代[]trans- 反式,超,跨tri- 三trans- 反式,超,跨tri- 三trideca- 十三tris- 三个undeca- 十一 .Alkylation 烷基化C- alkylation C-烷基化O- alkylation O-烷基化N-alkylation N-烷基化Silylation 硅烷[基]化Exhaustive methylation 彻底甲基化Seco alkylation 断裂烷基化Demethylation 脱甲基化Ethylation 乙基化Arylation 芳基化Acylation 酰化Formylation 甲酰化Carbalkoxylation 烷氧羰基化Carboamidation 氨羰基化Carboxylation 羧基化Amination 氨基化Bisamination 双氨基化Cine substitution 移位取代Transamination 氨基交换Hydroxylation 羟基化acyloxyation 酰氧基化7Decarboxylative nitration 脱羧卤化Allylic halogenation 烯丙型卤化Dehalogenation 脱卤Nitration 硝化Decarboxylative nitration 脱羧硝化Nitrosation 亚硝化Sulfonation 磺化Chlorosulfonation 氯磺酰化Desulfonation 脱磺酸基Sulfenylation 亚磺酰化Sulfonylation 磺酰化Chlorosulfenation 氯亚磺酰化Chlorocarbonylation 氯羰基化Diazotization 重氮化[daiˌæzətaiˈzeiʃən] Diazo transfer 重氮基转移Coupling reaction 偶联反应uni- 单,一unsym- 不对称的,偏位-yl 基-ylene 撑(二价基,价在不同原子上)-yne 炔Diazonium coupling 重氮偶联[ˌdaiəˈzəuniəm]Cross-coupling reaction 交叉偶联反应1,4-addition 1,4-加成C-C Pi-bond C-C π键Conjugate addition 共轭加成[ˈkɔndʒəˌgeɪt] Dimerization 二聚Trimefization 三聚Additive dimerization 加成二聚Sulfurize 使硫化[]sulfurization 硫化Selenylation 硒化Hydroboration 硼氢化[ˈhaidrəuˌbɔ:ˈreiʃən] Oxyamination 羟氨基化Insertion 插入carbonylation 羧基化Hydroformylation 加氢甲酰基化Hydroacylation 加氢酰化Oxo process 羰基合成Decarbonylation 脱羰[di:ˌkɑ:bənəˈleiʃən] Hydrocarboxylation 氢羧基化Homologization 同系化Cyanoethylation 氰乙基化Decyanoethylation 脱氰乙基Ring closure 环合,闭环Diene synthesis 双烯合成Dienophile 亲双烯体Endo addition 内型加成Exo addition 外型加成Diels-Alder reaction 第尔斯-尔德反应Retro Diels-Alder reaction 逆第尔斯-阿尔德反应Ene synthesis 单烯合成Anionic cycloaddition 负离子环加成Dipolar addition 偶极加成Dehydrohalogenation 脱卤化氢Deamination 脱氨基Pyrolytic elimination 热解消除Elimination-addition 消除-加成Decarboxylation 脱羧Decarboxamidation 脱酰胺8Decyanation 脱氰基Alkylolysis,alkyl cleavage 烷基裂解Acylolysis,acyl cleavage 酰基裂解Flash pyrolysis 闪热裂Fragmentation 碎裂Chiletropic reaction 螯键反应Chelation 螯环化Esterification 酯化Transesterification 酯交换Saponification 皂化Alcoholysis 醇解Ethanolysis 乙醇解Cyanomethylation 氰甲基化Aminomethylation 氨甲基化Hydroxymethylation 羟甲基化Hydroxyalkylation 羟烷基化Cholromethylation 氯甲基化Haloalkylation 卤烷基化Transacetalation 缩醛交换Enolization 烯醇化Haloform reaction 卤仿反应Condensation 缩合Aldol condensation 羟醛缩合Cross aldol condensation 交叉羟醛缩合Retrograde aldol condensation 逆羟醛缩合Acyloin condensation 偶姻缩合Cyclization 环化Annulation,annelation 增环反应Spiroannulation 螺增环Autoxidation 自氧化Allylic hydroperoxylation 烯丙型氢过氧化Epoxidation 环氧化Oxonolysis 臭氧解Electrochemical oxidation 电化学氧化Oxidative decarboxylation 氧化脱羧Aromatization 芳构化Catalytic hydrogenation 催化氢化Heterogeneous hydrogenation 多相氢化Homogeneous hydrogenation 均相氢化Catalytic dehydrogenation 催化脱氢Transfer hydrogenation 转移氢化Hydrogenolysis 氢解Dissolving metal reduction 溶解金属还原Single electron transfer 单电子转移Bimolecular reduction 双分子还原Electrochemical reduction 电化学还原Reductive alkylation 还原烷基化Reductive acylation 还原酰化Reductive dimerization 还原二聚Deoxygenation 脱氧Desulfurization 脱硫Deselenization 脱硒Mitallation 金属化Lithiation 锂化Hydrometallation 氢金属化Mercuration 汞化Oxymercuration 羟汞化Aminomercuration 氨汞化Abstraction 夺取[反应]Internal abstraction 内夺取[反应] Rearrangement 重排Prototropic rearrangement 质了转移重排Double bond migration 双键移位Allylic migration 烯丙型重排Allylic migration 烯丙型迁移Ring contraction 环缩小[反应]Ring expansion,ring enlargement 扩环[反应] -ketol rearrangement -酮醇重排Pinacol rearrangement 频哪醇重排Retropinacol rearrangement 逆频哪醇重排Semipinacol rearrangement 半频哪醇重排Benzilic rearrangement 二苯乙醇酸重排Acyl rearrangement 酰基重排Migratory aptitude 迁移倾向Transannular insertion 跨环插入Transannular rearrangement 跨环重排Migration 迁移Prototropy 质子转移Cationotropic rearrangement 正离子转移重排Anionotropy 负离子转移Anionotropic rearrangement 负离子转移重Sigmatropic rearrangement -迁移重排Homosigmatropic rearrangement 同迁移重排Electrophilic rearrangement 亲电重排Photosensitization 光敏化Forbidden transition 禁阻跃迁photooxidation 光氧化Photoisomerization 光异构化Photochemical rearrangement 光化学重排Angular methyl group 角甲基Alkylidene group 亚烷基[ælˈkiləˌdi:n]Methylene 亚甲基[ˈmeθili:n]Allyl group 烯丙基Allylic 烯丙型[的] [ˈæləˌlik]Phenyl group 苯基[ˈfenəl,ˈfi:nəl,ˈfi:nil]Aryl group 芳基Benzyl group 苄基Benzylic 苄型[的]Activating group 活化基团Chromophore 生色团[ˈkrəuməfɔ:] Auxochrome 助色团[ˈɔ:ksəkrəum] Magnetically anisotropic group 磁各向异性基团[əˌnaisəuˈtrɔpik]Smally ring 小环Common ring 普通环Medium ring 中环[ˈmi:djəm]Large ring 大环Bridged-ring system 桥环体系Spiro compound 螺环化合物Helical molecule 螺旋型分子Octahedral compound 八面体化合物Conjugation 共轭[]Conjugated-system 共轭体系Acyl cation 酰[基]正离子Benzylic cation 苄[基]正离子[ˈkætaiən] Arenium ion 芳[基]正离子或aryl cation Ketyl radical 羰自由基Radical ion 自由基离子[ˈaiən]Radical cation 自由基正离子Radical anion 自由基负离子[ˈænaiən] Isomerism 异构[现象]Acid form 酸式Fluxional structure 循变结构Stereochemistry 立体化学Optical activity 光学活性,旋光性Dextro isomer 右旋异构体[]Laevo isomer 左旋异构体[] Tetrahedral configuration 四面体构型Stereoisomerism 立体异构[现象] Asymmetric atom 不对称原子Asymmetric carbon 不对称碳Pseudoasymmetric carbon 假不对称碳10Phantom atom 虚拟原子[]Homotopic 等位[的]Heterotopic 异位[的]Enantiotopic 对映[异构体]的Diastereotopic 非对映异构体[的] [ˌdaiəstiəri əˈtɔpik]Configuration 构型[kənˌfiɡjuˈreiʃən] Absolute configuration 绝对构型Chirality 手性Chiral 手性[的] [英] [ˈtʃirəl][美] [ˈkaɪrəl] Chiral center 手性中心Chiral molecule 手性分子Achiral 非手性[的] [ei'kairəl]Fischer projection 费歇尔投影式Neoman projection 纽曼投影式D-L system of nomenclature D-L命名体系R-S syytem of nomenclature R-S命名体系Cahn-Ingold-Prelon sequence 顺序规则Symmetry factor 对称因素Plane of symmetry 对称面Mirror symmetry 镜面对称Enantiomer 对映[异构]体[]Diastereomer 非对映[异构]体[]Epimer 差向异构体[] Anomer 端基[差向]异构体Erythro configuration 赤型构型Erythro isomer 赤型异构体Threo configuration 苏型构型Threo isomer 苏型异构体Trigonal carbon 三角型碳Cis-trans isomerism 顺反异构E isomer E异构体Z isomer Z异构体Endo isomer 内型异构体Exo isomer 外型异构体Prochirality 前手性Pro-R group 前R基团Pro-S proup 前S基团Re face Re 面Si face Si 面Racemic mixture 外消旋混合物[]Racemic compound 外消旋化合物Racemic solid solution 外消旋固体溶液Meso compound 内消旋化合物Quasi recemate 准外消旋体[ˈkwɑ:zi(:),ˈkweis ai]Conformation 构象Conformational 构象的Torsion angle 扭转角Rotamer 旋转异构体Anti conformation 反式构象Bisecting conformation 等分构象Anti periplanar conformation 反叠构象Synperiplanar conformation 顺叠构象Synclinal conformation 反错构象Synclinal conformation 顺错构象Eclipsed conformation 重叠构象Gauche conformation, skew con-formation 邻位交叉构象Staggered conformation 对位交叉构象11Steric effect 空间效应[ˈstiərik,ˈsterik] Steric hindrance 位阻Atropismer 阻转异构体Puckered ring 折叠环Conformational inversion 构象反转Chair conformation 椅型构象Boat conformation 船型构象Twist conformation 扭型构象Skew boat conformation 扭船型构象Half-chair conformation 半椅型构象Pseudorotation 假旋转Envelope conformation 信封[型]构象Axial bond 直[立]键[ˈæksi:əl] Equatorial bond 平[伏]键[ˌi:kwəˈtɔ:ri:əl, -ˈtəʊr-, ˌekwə-] Cisoid conformation 顺向构象Transoid conformation 反向构象Retention of configuration 构型保持Regioselectivity 区域选择性Regiospecificity 区域专一性Stereocelectivity 立体选择性Stereospecificty 立体专一性Conformer 构象异构体Conformational effect 构象效应Cram’s rube 克拉姆规则Prelog’rule普雷洛格规则Stereochemical orientation 立体[化学]取向Conformational transmission 构象传递Homolog 同系物Ipso position 本位Ortho position 邻位Meta position 间位Para position 对位Amphi position 远位Peri position 近位Trigonal hybridization 三角杂化Molecular orbiral method 分子轨道法Valence bond method 价键法Delocalezed bond 离域键Cross conjugation 交叉共轭Vinylog 插烯物Mesomeric effect 中介效应Resonance 共振[ˈrezənəns]Resonance effect 共振效应Hyperconjugation 超共轭Isovalent hyperconjugation 等价超共轭No-bond resonance 无键共振Aromaticity 芳香性Aromatic sexter 芳香六隅Huckel’rule休克尔规则Paramagnetic ring current 顺磁环电流Diamagnetic ring cruuent 抗磁环电流Homoaromaticity 同芳香性Antiaromaticity 反芳香性Alternant hydrocarbon 交替烃Non-alternant hydrocarbon 非交替烷Pericyclic reaction 周环反应Electrocyclic rearrangement 电环[化]重排Conrotatory 顺旋Disroatatory 对旋Cycloaddition 环加成Symmetry forbidden-reaction 对称禁阻反应Synfacial reaction 同面反应Antarafacial reaction 异面反应Mobius system 默比乌斯体系Leois structure 路易斯结构Coordinate-covalent bond 配位共价键Banana bond 香蕉键Pauling electronegativity scale 鲍林电负性标度Polarizability 可极化性Inductive effect 诱导效应Field effect 场效应Electrical effect 电场效应tautomerism 互变异构Tautomerization 互变异构化Keto-enol tautomerism 酮-烯醇互变异构Phenol-keto tautomerism 酚-酮互变异构Imine-enamine atutomerism 亚胺-烯胺互变异构Ring-chain tautomerism 环-链互变异构Valence tautomerism 价互变异构Ambident 两可[的]Solvent effect 溶剂效应Acid-base catalyxed reaction 酸性溶剂Basic solvent 碱性溶剂Dielectric constant 介电常数Solvated electron 溶剂化电子Acid-base catalyzed reaction 酸碱催化反应Conjugate base 共轭酸Conjugate base 共轭碱Therm odynamic acidity 热力学酸度Kinetic acidity 动力学酸度Electron donof-acceptor complex,EDAcomplex 电子给[体]受体络合物Host 主体Guest 客体Primary isotope effect 一级同位素效应Secondary isotope effect 二级同位数效应Inverse isotope effect 逆同位素效应Kinetic control 动力学控制Thermodynamic control 热力学控制Substrate 底物Intermediate 中间体Reactive intermediate 活泼中间体Microscopic reversibility 微观可逆性Hammond postulate 哈蒙德假说Linear free energy 线性自由能Non-bonded interaction 非键相互作用Torsional effect 扭转效应Pitzer strain 皮策张力Restricted rotation 阻碍旋转Eclipsing effect 重叠效应Eclipsing strain 重叠张力Small-angle strain 小角张力Large angle strain 大角张力Transannular interaction 跨环相互作用Transannular strain 跨环张力I strain 内张力F strain 前张力B strain 后张力Anomeric effect 端基异构效应Walden inversion 瓦尔登反转Racemization 外消旋化Isoinversion 等反转Isoracemization 等消旋Homochiral 纯手性[的]Mechanism 机理Unimolecular nucleophilic 单分子亲核取代Bimolecular nucleophilic sub-stitution 双分子亲核取代Bimolecular nucleophilic substi-tution(with allyl ic rearrange-ment) 双分子亲核取代(含烯丙型重排)Internal nucleophilic substiru-tion 分子内亲核取代Aromatic nucleophilic substitu-tion 芳香亲核取代Unimolecular electrophilic sub-stitution 单分子亲电取代Bimolecular electrophilic substi-tution 双分子亲电取代Nucleophile-assisted unimolecu-lar electrophilic substitution 亲核体协助单分子亲电取代Unimolecular elimination 单分子消除Bimolecular elimination 双分子消除Unimolecular elimination through the conjugate base 单分子共轭碱消除Bimolecular elimination through the conjugate b ase 双分子共轭碱消除Bimolecular elimination with for-mation of a car bonyl group 双分子羰基形成消除Unimolecular acid-catalyzed acyl-oxygen cleava ge 单分子酸催化酰氧断裂Bimolecular base-catalyzed acyl-oxygen cleavag e 双分子碱催化酰氧断裂Unimolecular acid-catalyzed alkyl-oxygen cleav age 单分子酸催化烷氧断裂Bimllecular base-catalyzed alkyl-oxygen cleavag e 双分子碱催化烷氧断裂π-allyl complex mechanism π烯丙型络合机理Borderline mechanism 边理机理Homolysis 均裂Heterolysis 异裂Heterolytic michanism 异裂机理Counrer[gegen]ion 反荷离子Ion pair 离子对Carbocation 碳正离子[ˌkɑ:bəˈkeiʃən] Nonclassical carbocation 非经典碳正离子Carbanion 碳负离子[ˈkɑ:bənaiən]Masked carbanion 掩蔽碳负离子Carbenoid 卡宾体Carbene 卡宾[]Nitrene 氮宾[a] Carbine 碳炔[] Electrophilic addition 亲电加成Electrophile 亲电体Diaxial addition 双直键加成Markovnikov’s rube 马尔科夫尼科规则Anti-Markovnikov addition 反马氏加成Michael addition 迈克尔加成Substitution 取代Electrophilic substitution 亲电取代Addition-elimination mechanism 加成消除机理[ˈmekənizəm]Electrophilic aromatic substitu-tion 亲电芳香取代Electron transfer 电子转移Electron-donating group 给电子基团Electron-Withdrawing group 吸电子基团Deactivating group 钝化基团Orinentation 取向Ortho-para directing group 邻对位定位基Meta directing group 间位定位基Ortho effect 邻位效应Partial rate factor 分速度系数Nucleophilic reaction 亲核反应Internal return 内返Nucleophilicity 亲核体Nucleophilicity 亲核性α-effect α-效应Backside attack 背面进攻Inversion 反转Umbrella effect 伞效应Push-pull effect 推拉效应Leaving group 离去基团Electrofuge 离电体Nucleofuge 离核体14Phase-transfer catalysis 相转移催化Neighboring group participation 邻基基参与Neighboring proup assistance,anchimeric assista nce 邻助作用Neighboring group effect 邻基效应Apofacial reaction 反面反应Briddgehead displacement 桥头取代Aryl action 芳正离子Benzyne 苯炔Zaitsev rule 札依采夫规则Anti-Zaitsev orientation 反札依采夫定向Hofmann’s rule 霍夫曼规则Bredt rule 布雷特规则Initiation 引发Anionic cleavage 负离子裂解Partial bond fixation 键[的]部分固定化exothermic发热的,放出热量的[ˌeksəuˈθə:mik]各种反应类型Halogenations reaction卤化反应Hydrogenation reaction氢化反应Alkylation reaction烷基化(烃化)反应Hydrocarbylation 烃基化反应Oxidation and reduction reaction氧化还原反Reductive amination还原胺化反应Cross-coupling reaction交叉耦合反应Cycloaddition reaction环加成反应Rearrangement reaction重排反应Acylation reaction酰化反应Acetylization reaction乙酰化反应Amide reaction酰胺反应Sulfonylation磺酰化反应Nitration reaction硝化反应Esterification酯化反应Anhydride reaction酸酐反应Oximation reaction肟化反应Coupled(或coupling)reaction偶联反应1,3-dipolar cycloaddition 1,3-偶极环加成Pericyclic reaction周环反应Hydrolysis reaction 水解反应Ester hydrolysis 酯水解反应Hydrolytic-polymeric reaction水解聚合反应Dehydrogenation reaction脱氢反应Dehydrohalogenation reaction 脱卤化氢反应Dehydration reaction脱水反应Decarboxylation reaction脱羧反应Addition reaction加成反应Substitution reaction取代反应Cracking reaction 裂化反应Elimination reaction消除反应And metal response reaction与活泼金属反应Phase transfer catalytic reaction相转移催化反应Acid-base catalyzed reaction酸碱催化反应Polymerization reaction聚合反应Polycondensation reaction缩聚反应Condensation reaction 缩合反应Silver mirror reaction银镜反应Nucleophilic reaction亲核反应Electrophilic reaction亲电反应Nucleophilic cycloaddition reaction亲核环加成反应Nucleophilic substitution亲核取代反应Electrophilic substitution亲电取代反应Unimolecular electrophilic substitution单分子亲电取代反应Bimolecular electrophilic substitution双分子亲电取代反应Unimolecular elimination reaction单分子消除反应Bimolecular elimination reaction双分子消除反应Unimolecular nucleophilic substitution单分子亲核取代Bimolecular nucleophilic substitution双分子亲核取代反应Internal nucleophilic substitution分子内亲核取代Aromatic nucleophilic substitution reaction芳香亲核取代反应活化剂的中英文名称Bis(2-ethylhexyl) sebacate (癸二酸二仲辛酯;癸二酸二2-乙基己酯)Zinc stearate (硬脂酸锌)Suberic acid (辛二酸)Adipic acid, (己二酸)Hexanedioic acid, (己二酸)Sebacic acid, dibutyl ester (癸二酸二丁酯)Abietic acid (松香酸)Lactic acid (乳酸)Poly(ethylene glycol) (聚乙二醇)Glycerol stearate (硬脂酸甘油酯)Imidazoline (咪唑啉,间二氮杂环戊烯)β-Pinene (β-蒎烯,β-松油二环烯)Adipic acid (脂肪酸)Butyl acetate (乙酸丁酯)Ethylene glycol butyl ether (乙二醇丁醚)Sebacic acid, (癸二酸)Decanedioic acid, (癸二酸)Ethylene glycol ethyl ether (乙二醇乙醚)2-Butenedioic acid (E)-, (2-丁烯二酸)Succinic acid, (琥珀酸,丁二酸)Ethylene glycol methyl ether (乙二醇甲醚)Acetyl acetate (乙酸乙酰脂)1H-Benzotriazole (1-H-笨并三唑)α-Pinene (α-蒎烯,α-松香二环烯)Salicylic acid (水杨酸)Iso-Propanol (异丙醇) Ethanol (乙醇)Lysine (赖氨酸)Glutamic acid (谷氨酸,2-氨基戊二酸) Glyceroyl, (甘油酰)N,N,N',N'-Tetrakis-(2-hydroxypropyl)-ethylene-diamine(N,N,N,N-四(2-羟基丙基)乙烯二氨)Isoleucine, (异亮氨酸)Decamethylenedicarboxylic acid, disalicyloylhy drazide (Tris(2,3-dibromopropyl)isocyanurate(3(2,3-2溴丙基)异氰尿酸盐)3-(N-Salicyloyl)amino-1,2,4-triazole (3-(N-水杨酰)氨-1,2,4-三唑)Isocyanuric acid (异氰尿酸)Salicylamide (水杨酰胺)Polyethylene glycol (聚乙二醇)Diethylene glycol diethyl ether (二甘醇二乙醚,(一缩)二乙二醇二乙醚)Butyl carbitol (丁基卡必醇)Ethyl carbitol (乙基卡必醇)Methyl carbitol (甲基卡必醇)Ethylene glycol monobutyl ether (乙二醇单丁醚)Glutaric acid (戊二酸,谷酸)Succinic acid (琥珀酸,丁二酸)Citric acid (柠檬酸)Salicylic acid (水杨酸)Lactic acid (2-羟基丙酸,乳酸)Glycerin monostearate (甘油一硬脂酸)Pentaerythritol (季戊四醇)tetrakis[β-(3,5-di-tert-butyl-4-hydroxy-phenyl)pr opionate] 四[β-(3,5-二叔丁基-4-羟基-苯基)丙酸酯Dioctyl sebacate (癸二酸二辛酯)N-Methyl pyrrolidone, (N-甲基吡咯烷酮)Diethylene glycol ethyl ether (二甘醇乙醚)Propylene glycol (丙二醇)Octanedioic acid (辛二酸)Oleamide (油酸酰胺)[olamine 乙醇胺]2-Mercapto benzothiazole (2-巯基-苯并噻唑)Nonanedioic acid, (壬二酸)cis-9-Octadecenoic acid, (顺式-9-十八炭烯酸,油酸)Sebacic acid, uses (癸二酸)12-Hydroxy stearic acid (十二羟基硬脂酸)Phthalic acid (苯二甲酸)1,1,3-Tris(2-methyl-4-hydroxy-5-tert-butylpheny l)butane(1,1,3-三(2-甲基-4-羟基-5-叔丁基苯基)丁烷)1,3,5-Trimethyl-2,4,6-tris(3,5-di-tert-butyl-4-hyd roxybenzyl)benzene(1,3,5-三甲基-2,4,6-三(3,5-二叔丁基-4-羟基苯基)苯)1-Methyl-2-pyrrolidone, (1-甲基-2-吡咯烷酮)Carbonic acid, (碳酸)Phthalic acid, (苯二甲酸)Malic acid (苹果酸,羟基丁二酸)2,3-Dibromo-2-butene-1,4-diol (2,3-二溴-2-丙烯-1,4-二醇)Cetylpyridinium bromide (溴代十六烷基吡啶)Pentanedioic acid (戊二酸) pentanediol (戊二醇)pentanoic acid ( 戊酸) pentanol (戊醇) Butanedioic acid, (丁二酸)1,2-Dibromoethylbenzene (1,2-二溴乙基苯)Salicylic acid, (水杨酸)Stearic acid (硬脂酸)Pentanedioic acid (戊二酸)Maleic acid, (马来酸,失水苹果酸)Phthalic acid, (苯二甲酸)Tartaric acid, (酒石酸)Acetic acid, (乙酸)Polyoxyethylene octylphenol ether (聚氧乙烯辛基酚醚,聚氧化亚乙基辛基分醚)Ethanedioic acid, (乙二酸)Polyethylene glycol (聚乙二醇)Diethylene glycol butyl ether (二甘醇丁醚)Diethylene glycol monoethyl ether (二甘醇单乙醚)Ethylene glycol monobutyl ether (乙二醇单丁醚)Pentaerythritol, (季戊四醇)Diglycol, (二甘醇,一缩二乙二醇)Hexylene glycol (己二醇)Ethylene glycol, (乙二醇)Glycerol, (甘油,丙三醇)Cyclobutanediamine (环丁烷二胺)Dibromobutenediol (二溴丁二醇)Cyclohexanediamine (环己烷二胺)Succinamide (琥珀酰胺,丁二酸胺)Ethylenediamine, (乙二胺)Triethanolamine, (三乙醇胺)5-Aminoisophthalic acid (5-氨基间苯二甲酸)p-tert-Butylbenzoic acid (对叔丁基苯甲酸)Propionic acid, (丙酸)Benzoic acid, (安息香酸, 苯(甲)酸)Salicylamide (水杨酰胺)Aniline, (苯胺)Palmitic acid, (棕榈酸, 十六酸, 软脂酸)Glutamic acid, (谷氨酸) Glutaric acid (谷酸,戊二酸)Glycine, (甘氨酸,氨基乙酸)Malic acid (苹果酸,羟基丁二酸)Adipic acid, (己二酸)Diethanolamine (二乙醇胺)Triethylamine, (三乙胺)Malic acid (苹果酸)Oxalic acid, (草酸)Oleic acid, (油酸)Glutaric acid (谷氨酸)Sorbic acid (山梨酸) sorbic alcohol (山梨醇)=sorbit Reactant 反应物nProduct 产物nCatalyst 催化剂catalytic agent,catalyzer Degree Celsius摄氏度Sodium Borohydride硼氢化钠Lithium Aluminum Hydride (LAH)氢化铝锂Lithiumtrit-ButoxyaluminohydrideLiAlH(O t-C4H9)3叔丁氧基氢化铝锂Diisobutylaluminum Hydride AlH[CH2CH(CH3)2]2二异丙基氢化铝Diborane B2H6硼烷Reactive Metals 活泼金属如 Na, or Li, or K ,Mg or Al or Zn or FePotassium carbonate碳酸钾Sodium carbonate 碳酸钠Sodium bicarbonate 碳酸氢钠[]Sodium chloride氯化钠Sodium acetate 乙酸钠Sodium cyanide[ˈsaɪəˌnaɪd]氰化钠Sodium methoxide或Sodium methylate甲醇钠,甲氧基钠Sodium ethoxide[ˈsəʊdi:əm i:ˈθɔksaid]或Sodium ethylate[ˈsəʊdi:əm ˈeθileit]乙醇钠Sodium sulfate 硫酸钠Magnesium sulfate [mægˈni:zi:əm ]硫酸镁Acetic acid乙酸Formic acid甲酸Ammonium format甲酸铵Formamide[fɔ:ˈmæmid]或formyl amide 甲酰胺formaldehyde 甲醛[f ɔ:ˈmældəˌha ɪd]Methyl iodide碘甲烷Potassium iodide碘化钾[]Potassium chloride氯化钾Potassium cyanide[ˈsaɪəˌnaɪd]氰化钾Dimethyl sulfate硫酸二甲酯Palladium –carbon 钯碳Pd/C [pəˈleidiəm] Palladium chloride氯化钯Palladium diacetate醋酸钯Chloroacetone 氯丙酮Tetrabutyl ammonium bromide四丁基溴化铵Tetrabutyl ammonium fluoride四丁基氟化铵容器类:量杯measuring cup烧杯beaker 不锈钢杯stainless-steel beaker量筒measuring flask/measuring cylinder 量筒graduated flask/measuring cylinder坩埚crucible 坩埚钳crucible clamp 坩埚crucible pot, melting pot试管test tube 试管架test tube holder漏斗funnel 分液漏斗separatory funnel烧瓶flask 锥形瓶conical flask塞子stopper洗瓶plastic wash bottle滴定管burette玻璃活塞stopcock冷凝器condenser试剂瓶reagent bottles玻棒glass rod 搅拌棒stirring rod蒸馏烧瓶distilling flask碘量瓶iodine flask表面皿watch glass蒸发皿evaporating dish容量瓶volumetric flask/measuring flask移液管(one-mark) pipette刻度移液管graduated pipettes20称量瓶weighing bottle吸液管pipette滤管filter天平balance/scale分析天平analytical balance台秤platform balance游码crossbeams and sliding weights酒精灯alcohol burner酒精喷灯blast alcohol burner搅拌装置stirring device洗耳球rubber suction bulb研磨钵mortar 研磨棒pestle 玛瑙研钵agate mortar瓷器porcelain白细口瓶flint glass solution bottle with stopper 滴瓶dropping bottle 小滴管dropper蒸馏装置distilling apparatus蒸发器evaporator试验用器材:升降台lab jack铁架台iron support万能夹extension clamp蝴蝶夹double-buret clamp双顶丝clamp regular holder止水夹flatjaw pinchcock圆形漏斗架cast-iron ring移液管架pipet rack试管架tube rack沸石boiling stone橡胶管rubber tubing药匙lab spoon镊子forceps坩埚钳crucible tong剪刀scissor 打孔器stopper borer石棉网asbestos-free wire gauze电炉丝wire coil for heater脱脂棉absorbent cottonphph试纸universal ph indicator paper滤纸filter paper称量纸weighing paper擦镜纸wiper for lens秒表stopwatch量杯glass graduates with scale白滴定管(酸)flint glass burette with glass stopcock棕色滴定管(酸)brown glass burette with glass stopcock白滴定管(碱)flint glass burette for alkali棕色滴定管(碱)brown glass burette for alkali 比重瓶specific gravity bottle水银温度计mercury-filled thermometerph计ph meter折光仪refractometer真空泵vacuum pump冷、热浴bath离心机centrifuge口罩respirator防毒面具respirator、gasmask磁力搅拌器magnetic stirrer电动搅拌器power basic stirrer烘箱oven闪点仪flash point tester马弗炉furnace电炉heater微波炉电热套heating mantleBunsen burner 本生灯product 化学反应产物apparatus 设备。
化工专业英语练习题 参考答案

练习一参考答案1将下列句子或段落翻译成英语1)A process is any operation or series of operations that causes a physical or chemical change in asubstance or a mixture of substances .The material that enters a process is referred to as input or feed the process,and that which leaves is called output or product.2)As a chemical engineer,you might be called on to design individual process units (such as reactors,distillation columns,heat exchangers),supervise the operation of a process,or modify a process design to accommodate a change in the feed or in the desired product characteristics.As a rule,to any of these things you must know the amounts,compositions,and conditions of the materials that enter and leave each process unit,and if you are working with an existing units,you must be able to measure enough of these quantities to verify that the process is doing what it was designed to do.3)Founded in 1839from a small production firm for pharmaceutical products,B.Braun has grown steadilyinto a multinational company dealing with medical products,medical technology,pharmaceutical and biotechnology.2将下列句子或段落翻译成汉语1)包括的一系列操作,如混合、蒸发、过滤,无论产物是什么,这些操作都基本同,从而导致了单元操作的概念。
应用化学专业英语第二版万有志主编版(课后答案和课文翻译)

Unit 1 The Roots of ChemistryI. Comprehension.1.C2. B3. D4. C5. BII. Make a sentence out of each item by rearranging the words in brackets.1. The purification of an organic compound is usually a matter of considerable difficulty, and it is necessary to employ various methods for this purpose.2. Science is an ever-increasing body of accumulated and systematized knowledge and is also an activity by which knowledge is generated.3. Life, after all, is only chemistry, in fact, a small example of chemistry observed on a single mundane planet.4. People are made of molecules; some of the molecules in people are rather simple whereas others are highly complex.5. Chemistry is ever present in our lives from birth to death because without chemistry there is neither life nor death.6. Mathematics appears to be almost as humankind and also permeates all aspects of human life, although many of us are not fully aware of this.III. Translation.1. (a) chemical process (b) natural science (c) the technique of distillation2. It is the atoms that make up iron, water, oxygen and the like/and so on/andso forth/and otherwise.3. Chemistry has a very long history, in fact, human activity in chemistry goesback to prerecorded times/predating recorded times.4. According to/From the evaporation of water, people know/realized thatliquids can turn/be/change into gases under certain conditions/circumstance/environment.5. You must know the properties of the material before you use it.IV . Translation化学是三种基础自然科学之一,另外两种是物理和生物。
绍兴文理学院2021级大一化学专业英语上学期期末考试试卷附答案

2021级大一英语上学期期末考试试卷附答案Part II Reading Comprehension (30 %)Directions: There are four passages in this part. Each passage is followed by some questions or unfinished statements. For each of them there are four choices marked A), B), C) and D). You should decide on the best choice and mark the corresponding letter on the Answer Sheet with a single line through the center.Passage OneScience is not a set of unquestionable results but a way of understanding the world around us. Its real work is slow. The scientific method , as many of us learned in school, is a gradual process that begins with a purpose or problem or question to be answered. It includes a list of materials, a procedure to follow, a set of observations to make and, finally, conclusions to reach. In medicine, when a new drug is proposed that might cure or control a disease, it is first tested on a large random group of people, and their reactions are then compared with those of another random group not given the drug. All reactions in both groups are carefully recorded and compared, and the drug is evaluated. All of this takes time and patience.It’s the result of course, that makes the best news—not the years of quiet work that characterize the bulk of scientific inquiry. After anexperiment is concluded or an observation is made, the result continues to be examined critically. When it is submitted for publication, it goes to a group of the scientist’s colleagues, who review the work. Einstein was right when he said: “No amount of experimentation can ever prove me right, a single experiment can at any time prove me wrong.”In August 1996, NASA announced the discovery in Antarctica of a meteorite(流星) from Mars that might contain evidence of ancient life on another world. As President Clinton said that day, the possibility that life existed on Mars billions of years ago was potentially one of the great discoveries of our time.After the excitement wore down and initial papers were published, other researchers began looking at samples from the same meteorite. Some concluded that the “evidence of life”was mostly contamination from Antarctic ice or that there was nothing organic at all in the rock.Was this a failure of science, as some news reports trumpeted?No! It was a good example of the scientific method working the way it is supposed to. Scientists spend years on research, announce their findings, and these findings are examined by other scientists. That’s how we learn. Like climbing a mountain, we struggle up three feet and fall back two. It’s a process filled with disappointments and reverses, but somehow we keep moving ahead.21. The author’s main purpose in writing this passage is to state that ____________.A) most scientific discoveries are not reliableB) mass media is misleading because it looks at the research results onlyC) scientific research is a process filled with reverses and requires slow and patientworkD) repeated experiments are necessary before medicine can be used in patients22. Publication of a scientific finding signifies __________.A) a challenge to fellow scientists to prove it wrongB) the end of a processC) the beginning of a new scientific inquiryD) the soundness of the result23. Einstein’s words are used to show that he thought___________.A) experiments have proved him rightB) scientists do not need so many experimentsC) one experiment is not enough to prove him wrong.D) scientific ideas are never free from challenge24. NASA’s announcement of the discovery of evidence of ancient life on Mars shows _________.A) the way human beings learn about natureB) the failure of the scientific methodC) the fruitlessness of human search for life on another worldD) the excitement brought by scientific findings25. It can be inferred from the passage that the media is interested in __________.A) the process of scientific researchB) the results of scientific researchC) the scientists who do the researchD) the effects of scientific research on human lifePassage TwoNormally a student must attend a certain number of courses in order to graduate, and each course which he attends gives him a credit which he may count towards a degree. In many American universities the total work for a degree consists of thirty-six courses each lasting for one semester. A typical course consists of three classes per week for fifteen weeks; while attending a university a student will probably attend four or five courses during each semester. Normally a student would expect to take four years attending two semesters each year. It is possible to spread the period of work for the degree over a longer period. It is also possible for a student to move between one university and anotherduring his degree course, though this is not in fact done as a regular practice.For every course that he follows a student is given a grade, which is recorded, and the record is available for the student to show to prospective employers. All this imposes a constant pressure and strain of work, but in spite of this some students still find time for great activity in student affairs. Elections to positions in student organizations arouse much enthusiasm. The effective work of maintaining discipline is usually performed by students who advise the academic authorities. Any student who is thought to have broken the rules, for example, by cheating has to appear before a student court. With the enormous numbers of students, the operation of the system does involve a certain amount of activity. A student who has held one of these positions of authority is much respected and it will be of benefit to him later in his career.26. Normally a student would at least attend __________classes each week.A) 36B) 12C) 20D) 1527. According to the first paragraph an American student is allowed _______.A) to live in a different universityB) to take a particular course in a different universityC) to live at home and drive to classesD) to get two degrees from two different universities28. American university students are usually under pressure of work because_________.A) their academic performance will affect their future careersB) they are heavily involved in student affairsC) they have to observe university disciplineD) they want to run for positions of authority29.Some students are enthusiastic for positions in student organizations probably because_________.A) they hate the constant pressure and strain of their studyB) they will then be able to stay longer in the universityC) such positions help them get better jobsD) such positions are usually well paid30. The student organizations seem to be effective in _________.A) dealing with the academic affairs of the universityB) ensuring that the students observe university regulationsC) evaluating students’performance by bringing them before a courtD) keeping up the students’enthusiasm for social activitiesPassage ThreeDoreen Sykora is now a junior at Mcgill University. She had a difficult time when she first began college. She said, “I was always well prepared for my examinations. But I would go in to class to take the exam, and I would fall apart. I could not answer the questions correctly-----even though I knew the answers! I would just blank out because of nervousness and fear.”Hitoshi Sakamoto, an anthropology student at Temple University in Tokyo reports similar experiences.These two young students were experiencing something called test anxiety. Because a student worries and is stressed about a test, his or her mind does not work as well as it usually does. The student cannot write or think clearly because of the severe tension and nervousness.Now there are special university courses to help students. In these courses, advisors and psychologists try to help students by teaching them to manage test anxiety. Such a course helps students learn to live with stress and not fail because of it. First students take a practice test to measure their worry level. If the tests show that their stress level is high, the students can take a short course to manage the fear. These courses teach students how to relax their bodies. They get training to become calm in very tense situations. By controlling their nervousness, they can let their minds work more easily. Learned information then comes out without difficulty on a test.Doreen Sykora saw immediate results after taking such a course. She now has enthusiasm about the relaxation methods. “Mostly, what I do is imagine myself in a very calm place. Then I imagine myself picking up a pencil. I move slowly and carefully. I breathe easily and let all the tension out. With each breath, more worry leaves me. It really works too. My grades have improved greatly! I’m really doing well at McGill now. This relaxation method works not only on examinations, but it has improved the rest of my life as well.”For Hitoshi in Tokyo, the results were much the same. He is enjoying school a lot more and learning more.31. Doreen Sykora and Hitoshi Sakamoto were filled with nervousness and fear during examinations because they were__________.A) not ready and unaware of the answersB) physically so weak that they fell apartC) subject to test anxietyD) unable to write or think clearly32. The higher the students’worry level is, __________.A) the less calm and relaxing they areB) the more difficult they will be trained to manage fearC) the more stressed and tense they areD) the longer courses they will take to manage fear33. What’s the purpose of some special university student-help courses?A) To help students to reduce test anxiety.B) To show a stress level experienced by students.C) To learn more knowledge about test anxiety.D) To have a better understanding of test anxiety.34. What’s the meaning of “blank out”in paragraph one?A) To be like a blanket.B) To be sure of an answer.C) To be relaxed.D) To be unable to think clearly.35.Which of the following best sums up the organization of the passage?A) Examples----theories----ideas.B) Problem----strategy----examples----results.C) General statement----examples----result.D) Strategy----experiment----examples.Part III Vocabulary and Structure (15 %)Directions: There are 30 incomplete sentences in this part. For each sentence there are four choices marked A), B), C) and D). Choose the ONE that best completes the sentence. Then mark the corresponding letter on the Answer Sheet with a single line through the center.36. The president made a _______ speech at the opening ceremony of the sports meeting, which encouraged the sportsmen greatly.A) vigorousB) tediousC) flatD) harsh37. It is not easy to learn English well but if you _______, you will succeed in the end.A) hang upB) hang aboutC) hang onD) hang onto38. Remember that customers don’t _______ about prices in that city.A) debateB) bargainC) disputeD) consult39. The newcomers found it impossible to _______ themselves to the climate sufficiently to make permanent homes in the new country.A) suitB) adaptC) regulateD) coordinate40. A _______ to this problem is expected to be found before long.A) resultB) functionC) settlementD) solution41. You have nothing to _______ by refusing to listen to our advice.A) gainB) graspC) seizeD) earn42. One day I _______ a newspaper article about the retirement of an English professor at a nearby state college.A) came acrossB) came aboutC) came afterD) came at43. A peculiarly pointed chin is his memorable facial _______.A) markB) featureC) traceD) appearance44. I hope that you’ll be more careful in typing the letter. Don’t _______ anything.A) omitB) leakC) lackD) withdraw45. Our new house is very _______ for me as I can get to the office in five minutes.A) adaptableB) convenientC) availableD) comfortable46. Those gifts of rare books that were given to us were deeply_______.A) appreciatedB) approvedC) appealedD) applied47. The sale usually takes place outside the house, with the audience _______ on benches, chairs or boxes.A) having seatedB) seatingC) seatedD) having been seated48. He is _______ about his chances of winning a gold medal in the Olympics next year.A) optimisticB) optionalC) outstandingD) obvious49. The clothes a person wears may express his _______or social position.A) curiosityB) statusC) determinationD) significance50. I don’t know the word. I had to _______ a dictionary.A) throw upB) make outC) refer toD) take over51. Look at these beautiful Japanese stamps. Roger gave them to me in _____ for two sets of 1988 British special issue.A) exchangeC) shiftD) switch52. It is rather _____ that the research team as a whole still has little idea about the cause of that fatal disease.A) rewardingB) demandingC) embarrassingD) requiring53. The people of African interior began to _____ gold in exchange for the goods they needed from abroad.A) desireB) affordC) offerD) receive54. We should not blame her for what happened yesterday, because that was outside her _____of responsibility.A) fieldB) limitC) extentD) range55. The students put forward some suggestions _____ consideration.B) worthyC) worthD) worthy of56. The author of the report is well _____ with the problem in the hospital because he has been working there for many years.A) acquaintedB) informedC) enlightenedD) advised57. After years of hard work, he finally gained ______ to the university which he longed for many years.A) accessB) commitmentC) opportunityD) reward58. _____ you have passed the driving test successfully, you can drive on your own.A) By nowB) Now and againC) Now thenD) Now that59. Within first seven seconds of meeting, people will form their opinion about others through unspoken communication like _____, postures and attitudes.A) signsB) gesturesC) symptomsD) symbols60. It had never _____ to me that our football team won the game.A) struckB) occurredC) hitD) meant61. The students ______ in cleaning the classroom according to the arrangement.A) alternateB) adaptC) adoptD) admit62. Have you any ______plans about how to deal with these difficulties? We need to be practical-minded.A) abstractB) consistentC) concreteD) contrary63. The professor was afraid that unless the train speeded up he would miss his _______ to New York.A) junctionB) connectionC) seatD) carriage64. When writing about controversial topics, some authors try to be _______ without favoring either side.A) reflectiveB) persuasiveC) impressiveD) objective65. Many factors such as too much stress, bad living habits can lead to poor ______ and ill health.A) experienceB) appearanceC) performanceD) competencePart IV Cloze (10 %)Directions: There are 20 blanks in the following passage. For each blank there are four choices marked A), B), C) and D). You should choose the ONE answer that best fits into the passage. Then mark the corresponding letter on the Answer Sheet with a single line through the center.Language is a signaling system which operates with symbolic vocal sounds (语声), and which is used by a group of people for the purpose of communication.Let’s look at this 66______ in more detail, because it is language, more than anything else, 67_____ distinguishes man from the rest of the 68_____ world.Other animals, it is true, communicate with one another by 69_____ of cries: for example, many birds utter (发声) 70_____calls at the approach of danger; monkeys utter 71_____ cries, such as expressions of anger, fear and pleasure. 72_____ these various means of communication differ in important ways 73_____ human language. For instance, animals’cries do not 74_____ thought and feelings clearly. This means, basically, that they lack structure. They lack the kind of structure that 75_____ us to divide a human utterance (发声) into 76_____.We can change an utterance by 77_____ one word in it with 78_____: a good illustration of this is a soldier who can say, e.g., “tanks approaching from the north”, 79_____ who can change one word and say “aircraftapproaching from the north”or “tanks approaching from the west”; but a bird has a single alarm cry, 80_____ means “danger!”This is why the number of 81_____ that an animal can make is very limited: the great tit (山雀) is a case 82_____ point; it has about twenty different calls, 83_____ in human language the number of possible utterances is 84_____. It also explains why animal cries are very 85_____ in meaning.66. A) recognitionB) function67. A) itB) that68. A) nativeB) animal69. A) waysB) methods70. A) datingB) exciting71. A) identicalB) different72. A) ButB) Therefore73. A) fromB) about74. A) inferB) explain75. A) encouragesB) enforces76. A) soundsB) words77. A) spellingB) saying78. A) oursB) another79. A) soB) but80. A) thisB) that81. A) signsB) signals82. A) inB) at83. A) sinceB) while84. A) limitless85. A) ordinaryB) alikeC) classificationD) definitionC) asD) whatC) humanD) physicalC) meansD) approachesC) warningD) boringC) similarD) unfamiliarC) AfterwardsD) FurthermoreC) withD) inC) interpretD) express C) enablesC) voicesD) speechesC) replacingD) pronouncingC) theirsD) othersC) orD) andC) whichD) itC) gesturesD) marksC) ofD) forC) anyhowD) somehowC) changeableD) ceaselessC) commonD) generalPart V Writing (15%)Directions: For this part, you are allowed 30 minutes to write a composition on the topic My View on Online Self-access Learning. You should write at least 120 words. And you should base your composition on the outline (given in Chinese) below:1. 一些人认为上机自主学习方式好;2. 也有一些人认为传统的授课方式好;3. 我的看法。
(完整版)化学专业英语
一、元素和单质的命名“元素”和“单质”的英文意思都是“element”,有时为了区别,在强调“单质”时可用“free element”。
因此,单质的英文名称与元素的英文名称是一样的。
下面给出的既是元素的名称,同时又是单质的名称。
2过渡元素和单质Fe : iron Mn : manganese Cu: copper Zn: zinc Hg: mercury Ag: silver Au: gold二化合物的命名:化合物的命名顺序都是根据化学式从左往右读,这与中文读法顺序是相反的。
表示原子个数时使用前缀:mono-di -tri- tetra -penta- hexa-hepta- octa-,nona-, deca-,但是在不会引起歧义时,这些前缀都尽可能被省去。
1.化合物正电荷部分的读法:直呼其名,即读其元素名称。
如CO: carbon monoxide Al2O3: aluminium oxideN2O4:Di nitrogen tetroxide对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的氧化态,或用后缀-ous表示低价,-ic表示高价。
如FeO: iron(II) oxide 或ferrous oxide Fe2O3: iron (III) oxide或ferric oxide Cu2O: copper(I) oxide 或cuprous oxide CuO: copper(II) oxide或cupric oxide 2.化合物负电荷部分的读法:2.1二元化合物:常见的二元化合物有卤化物,氧化物,硫化物,氮化物,磷化物,碳化物,金属氢化物等,命名时需要使用后缀-ide,如:fluoride,chloride,bromide,iodide,oxide ,sulfide ,nitride, phosphide, carbide,hydride; OH -的名称也是用后缀-ide:hydroxide,非金属氢化物不用此后缀,而是将其看成其它二元化合物(见2。
化学专业英语化学专业英语课期末考试试卷含答案

化学专业英语试卷学号:姓名:成绩: 一:把下列单词或词组译成英文本题共 30 分,每小题 1 分1. NiClO42 nickel perchlorate3. FeCl2 iron2chloride5. AlNO33 aluminum nitrate7. MnO2 manganese dioxide9. N2O3 dinatrogen trioxide11. NaClO sodium hypochloride13. P2O5 diphosphorous pentaoxide15. KMnO4 patassium permangate17. 盐酸hydrochloric acid19. KCN patassium cyanide21. 5-甲基-4-丙基壬烷5-methyl-4-propylnonaane23. 四氯化碳carbon tetrachloride25. 中和neutralize27. 比热容specific heat capacity29. 酸酐anhytride 2. CuSO4 copper sulfate4. CoCO3 cobalt carbate6. CaC2H3O22 calcium acetate8. H2SO410. 六氰合铁Ⅱ酸钾12. Ag2SO3 sliver sulfite14. 草酸铅 lead cyanate16. ZnOH2 zinc hydroxide18. 磷酸根 phosphate20. 2,3-二甲基戊烷2,3-dimethylpentane22. 2,3,7-三甲基-5-乙基辛烷2,3,7-trimethyl-5-ethyloct ane24. 石蕊试纸litmus paper 26. 滴定titration28. 非电解质electrolyte 30. 配位化合物complex compound三. 把下列短文译成汉语本题共 40 分,每小题 10 分1. Without chemistry our lives would be unrecognisable, for chemistry is at work all around us. Think what life would be like without chemistry - there would be no plastics, no electricity and no protective paints for our homes. There would be no synthetic fibres to clothe us and no fertilisers to help us produce enough food. We wouldn’t be able to travel because there would be no metal, rubber or fuel for cars, ships and aeroplane. Our lives would be changed considerably without telephones, radio, television or computers, all of which depend on chemistry for the manufacture of their parts. Life expectancy would be much lower, too, as there would be no drugs to fight disease.没有化学反应我们的生活将会大变样,化学就在我们周围;没有化学生活会是什么样子——没有塑料,,家里没有电,也没有防护漆;不会给我们合成纤维,没有化肥帮助我们生产足够的食物;我们不能旅行,因为不会有金属、橡胶或燃料汽车、船只和飞机;我们的生活将会大大改变了没有电话、收音机、电视或电脑,所有这些依赖化学生产的部分;没有药物来抵抗疾病,预期寿命将低得多;2. The first and second laws of thermodynamics and the meaning of entropy will be discussed. and expanded upon in this lesson. It will be shown that energy transformations on a macroscopic scale — that is, between large aggregates of atoms and/or molecules —can be understood in terms of a set of logical principles. Thus thermodynamics provides a model of the behavior of matter in bulk. The power of such a model is that it does not depend on atomic or molecular structure. Furthermore, conclusions about a given process .based on this model, do not require details of how the process is carried out.探讨热力学第一和第二定律和熵的意义.和扩展在这个知识;也就是说它将表明能源在宏观上的转换,根据一组逻辑原则可以理解能量在大量的原子或分子内的转换;因此热力学定理提供了一个物质体积变化的模型;这样一个模型的能力在于它不依赖于原子或分子结构;此外,给定进程的结论依托于这种模式,不需要的详细说明过程是如何进行的3.Preparation of Cuen2cdaH2O: H2cda 4-羟基-2,6 吡啶二酸 g, mmol was dissolvedin water 10 mL and the pH value of the solution was adjusted to 7~8 with aqueous NaOH solution molL-1, then adding it dropwise to a methanol solution 10mL ofCuClO42·6H2O , and ethylenediamine mmol under stirring at room temperature.After the resulting small quantity of precipitates was filtered off, dark blue crystals suitable for X-ray structure analysis were obtained by slow evaporation of the filtrate at room temperature.制备CUen2cdaH2O:使克,的4 -羟基2、6吡啶二酸溶解在10ml水中加入氢氧化钠水溶液调整到pH值7 ~ 8,然后将它一滴一滴地添加到CuClO42·6H2O,的乙醇溶液和乙二胺,在室温下搅拌;在室温下,缓慢蒸发滤液,得到深蓝色晶体,用x射线分析它的结构4. Measure 50 ml of vinegar with a pipette and pour into a 250-ml beaker. Add 2 drops of phenolphthalein indicator. Fill a burette with a 1 N solution of sodium hydroxide NaOH and draw out the excess as described above. From the burette add NaOH to the beaker of vinegar until 1 drop of NaOH produces a pale pink color in the solution. Maintain constant stirring. The appearance of pink tells you that the acid has been neutralized by the base and there is now 1 drop of excess base which has turned the indicator. Read the burette and record this reading as the volume of base used to neutralize the acid. One molecule of NaOH neutralizes one molecule of acetic acid, or one gram-molecular weight of NaOH neutralizes one gram-molecular weight of acetic acid. Calculate the amount of acetic acid present in the vinegar. Report this amount as the percentage of acetic acid. 用移液管吸取50ml醋加入到250毫升烧杯,加2滴酚酞指示剂;在滴定管中加入1M的氢氧化钠溶液,去除刻度线以上的溶液,将氢氧化钠溶液加入到醋中,并不断震荡,至到加入一滴氢氧化钠溶液变成粉红色;出现粉红色的颜色,表示酸中和了碱,而且多余的一滴碱使指示剂变色;阅读并纪律中和酸消耗碱的体积;一个分子的氢氧化钠中和一个分子的醋酸,或一个分子重量的氢氧化钠中和一个分子重量的醋酸反应;计算醋酸在醋的量;报告醋酸的百分比;。
化学专业英语
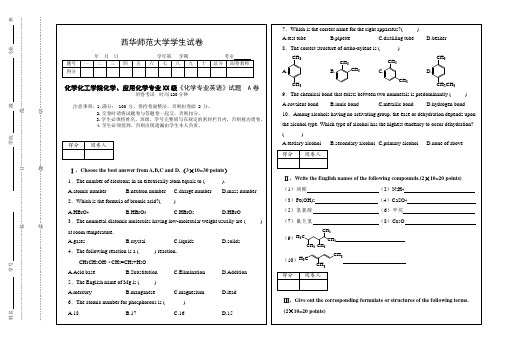
A.HBrO4B.HBrO3C.HBrO2D.HBrO
3、The nonmetal diatomic molecules having low-molecular weight usually are ()at room temperature.
西华师范大学学生试卷
年月日学年第学期考室
题号
一
二
三
四
五
六
七
八
九
十
总分
阅卷教师
得分
化学化工学院化学、应用化学专业XX级《化学专业英语》试题A卷
闭卷考试时间120分钟
注意事项:1.满分:100分。保持卷面整洁,否则扣卷面2分。
2.交卷时请将试题卷与答题卷一起交,否则扣分。
3.学生必须将姓名、班级、学号完整填写在规定的密封栏目内,否则视为废卷。
3、...
4、Water is widely used in various process applications in industry. Other major industrial uses are boiler feed water and cooling water.The kind and degree of treatment of water in these applications depends upon the end use. As examples, cooling water may require only minimal treatment, while water used in food processing must be free ofpathogens(病原体) and toxid substances.(5 points)
化学专业英语-Biochemistry
(2) Building molecules(构件分子)
(3) Biomacromolecules(生物大分子)
常 见 的 功 能 基 团
Functional groups
1) Functional groups are clusters of atoms with characteristic structure and functions. 2) Most biomolecules contain more than one functional group. 3) Different families of organic compounds result when hydrogen atoms on organic molecules are replaced by different functional groups. 4) The distinct chemical properties of each functional group contribute to the behavior of any molecule that contains it.
Fatty acids
Phospholipids 磷脂
Phospholipids are modified so that a phosphate group (PO4-) is added to one of the fatty acids. The addition of this group makes a polar "head" and two nonpolar "tails".
Polysaccharides
These classes perform a variety of
化学专业英语试卷B

2012—2013学年度第一学期应用化学专业专业英语课程试卷B 注意事项:1. 考生务必将自己姓名、学号、专业名称写在指定位置;2. 密封线和装订线内不准答题;一、词汇填空 写出下列每个词汇对应的英汉单词共20小题,每空1分,共20分1. 原子2. 镍3. 氦4. 元素5. 阴离子6. 钴7. 丙酮8. 碘9. 乙醚10. 钾11. 甲烷12. 乙醇13. chlorine14. nickel15. phosphorus16. potassium17. arsenic18. sulfur19. butane20. aluminum二、给下列无极化合物的英语名称共10小题,每小题2分,共20分1. HCl2. HBr3. CuSO 44. H 2SO 45. NaCl6. Na 2S7. KF8. Al 2O 39. KMnO 410. FeCl 3二、给下列有机化合物的英语名称共5小题, 每小题4分,共20分3322CH 3CH 2CH 32. CH 32C =CHCH 33. CH 2OHCH 2CHCH 2OHCH 2OH4. 3,4-二甲基苯酚5. 甲乙醚三、英译汉共10小题, 每小题4分,共40分1. The study of the properties of substances constitutes an important part of chemistry, because their properties determine the uses to which they can be put.2.The cleavage of the different crystals of salt is the same; when crushed, the crystals always break along planes parallel to the original faces, producing smaller crystals similar to the larger ones. 3.It is customary to say that under the same external conditions all specimens of a particular substance have the same physical properties density, hardness, color, melting point, crystalline form, etc. 4.Sodium chloride has the properties of changing into a soft metal, sodium, and a greenish-yellow gas, chlorine, when it is decomposed by passage of an electric current through it. 5. When biscuits are made with use of sour milk and baking soda there isa chemical reaction between the baking soda and a substance in the sourmilk, lactic acid, to produce the gas carbon dioxide, which leavens the dough by forming small bubbles in it.6.When an acid, base, or salt is dissolved in water the resulting solutionis a conductor of the electric current and is termed an electrolyte. If no conduction of current occurs, the compound is known as a nonelectrolyte.7.Green chemistry is the chemistry that aims to reduce the amount ofhazardous substance coming out in the process of producing chemical materials and to reduce the amount of resource and energy consumed in that process.8.Nonbenzenoid compounds containing rings of carbon atoms are calledalicyclic. These are carbocyclic compounds which resemble aliphatic compounds in many ways.9.The second group is composed of compounds derived from or related tobenzene, C6H6. Because the first known derivatives of benzene were naturalproducts extracted from balsams and impressed the discoverers because of their fragrant aromas, the group as a whole came to be known as aromatic compounds.10.Heterocyclic compounds are cyclic compounds with the ring containingcarbon and other elements, the commonest being oxygen, nitrogen and sulfur. There are a number of heterocyclic rings which are easily opened and do not posses any aromatic properties, e. g. , ethylene oxide, γ- and δ-lactones.。
化学专业英语

普通化合物分类 我们可以根据化学式按着下面的方法分类许多普
通化合物。
1. Acids, in the conventional sense, may be recognized by noting that the H is written first in the formula and that the rest of the compound is generally nonmetallic. Ex., HCl, H2SO4, HClO.
• become (be) familiar with…熟悉,通晓
例如,盐酸归类为酸,由于已熟悉作为不同类别 的酸的性质,我们就会立即知道这一化合物的一 般性质。
A great many of the compounds we are to study may be classified as acids, bases, salts, metallic oxides, or nonmetallic oxides. Of these five classes of compounds, the first three-acids, bases, and salts-are by far the most important.
• be aware of 知道,意识到……
那么,如果我们能够恰当地将一个化合物归类,我们立 刻就能从这类化合物的性质来了解这个化合物的一般性 质。
For example, HCl is classed as an acid, and by becoming familiar with the behavior of acids as a distinct class, we are at once aware of the general properties of the compound.
化学专业英语

化学专业英语Chemistry is the study of matter and its properties, composition, and structure. Chemistry majors learn toidentify, measure, and explain the structure, reactivity, and physical and chemical properties of elements and compounds.Chemistry is a core academic discipline that applies mathematics, physics, and explanation of scientificprinciples to the study of the properties of substances andthe interactions among them. It is the central science of natural and life sciences and is essential to many other disciplines and fields, including biochemistry, environmental science, engineering, and medicine.In general, a chemistry major can expect to take coursesin organic and analytical chemistry, physical chemistry, biochemistry, inorganic chemistry, and chemical biology, as well as math and physics courses. Most programs also require laboratory experience, which may involve hands-on experiments, data analysis, and synthesis of materials.At the graduate level, a chemistry student can specialize in a specific area, such as synthetic or physical chemistry, biochemistry, or analytical chemistry. Each of these areas requires the mastery of different principles and techniques,as well as knowledge of reaction mechanisms, thermodynamics, and quantum mechanics.Students pursuing a degree in chemistry often participate in internships or research projects in industrial, government, or university laboratories. Chemistry majors may also havethe opportunity to gain practical experience by working in astudent-run laboratory or lab tech position. These experiences can provide valuable insights into the real-world applications of chemistry and give access to potential job opportunities.Earning a degree in chemistry can lead to a wide range of career paths, from research to industry and regulatory. With the right skills and knowledge, graduates can work in pharmaceutical development, toxicology, pharmaceutical sales and marketing, teaching, consulting, forensics, energy production, medical diagnostics, and food science. Regardless of the field they pursue, chemistry majors develop strong problem-solving skills and a comprehensive understanding of how chemicals interact as well as a deep appreciation for the incredible diversity of life on Earth.。
化学专业英语

ⅣA
(carbon family )
9. Group ⅣA consists of a nonmetal, carbon, two metalloids, silicon and germanium, and two metals, tin and lead.
•except for 除……之外 •make up 组成 •alkali metal family 碱金属族 •reactive (活泼的)____inert (不活泼的) •the elemental state 游离态
除了氢(一种气体)外,ⅠA的元素组成了碱金属族。它 们是非常活泼的金属,在自然界中没有发现游离态。然而, 它们的化合物是广泛存在的。
•be known as 以……著称,就是通常说的 •Particularly 特别是 ⅡA元素就是通常所说的碱土金属。它们 的特征离子价态为+2价。这些金属,特 别是最后两种金属,几乎同碱金属一样 活泼。
IIB
(zinc family)
The group ⅡB elements-zinc, cadmium, and mercury are less reactive than are those of group ⅡA, but are more reactive than the neighboring elements of group ⅠB. The characteristic charge on their ions is also 2+.
ⅢB
(scandium family )
ⅢA
(boron family )
With the exception of boron, group ⅢA elements are also fairly reactive metals.
ecit化学工程与工艺专业英语试题

1-5 BACCD 6-10 DBDDD 11-15 CCBCDI.Choose the Best item to fill in the blanks.1. Modern chemical industry began around B .A.1860B. 1800C.1790D.18562. A made the Haber process for ammonia industrialized.A.Carl BuschB.Fritz HaberC.Willim Henry PerkinD.Calvin3. C is not included in the types of Industrial Research and Development.A. product developmentB.process developmentC.manufacturing improvementD.application development4. C kinds of chemical compounds are now known.A.nine millionB.one millionC.ten millionD.a hundred million5.The main constituents of plants are D .A.oxygenB.waterC.carbonD.carbonhydratesanic chemicals mainly come from oil,natural gas,and D .A.metal B air C water D coal7. B of all organic chemical is obtained from crude oil and natural gas.A 80%B 99%C 70%D 79%8. D is not categorized as high-volume sectors.A sulphuric acidB chlor-alkaliC polytheneD carbondioxide9. D sector provides the key intermediates to building block.A chlor-alkali productsB dyestuffsC pharmaceuticalsD petrochemicals10.Of all soda-ash, 50% is sold to the D industy.A buildingB paper-makingC transportationD glass –making11. C is the chemical that is produced in the largest tonnage.A carbonB oxygenC sulphuric acidD ammonia12. C makes up three quarters of the air we breathe.A hydrogenB oxygenC dinitrogenD nitrogen13. Almost all explosives are ultimately derive from B .A ureaB nitric acidC sulphuric acidD ammonia14.The most significant constituents of petroleum are C .A nitrogenB sulfurC hydrocarbonsD oxygen15. D showed that thermodynamically the reaction of nitrogen with hydrogen is feasible.A Friz HaberB NernstC BoschD MittaschII. Answer the following questions according to the texts.1.What is the definition of the chemistry industry?A:The chemical industry today is a very diverse sector of manufacturing industry, within which it plays a central role. It makes thousands of different chemicals which the general public only usually encounter as end or consumer products.今天的化学工业已经是制造业中有着许多分支的部门,并且在制造业中起着核心的作用。
化学专业英语考试

1.如果你决定进入农业,你需要知道fertilizersand农药、以及动物营养。
即使你参加一些职业,似乎没有什么联系化学,如法律,你会找到一个促进化学知识很有用的。
律师freguently要处理专利有关的化学的发明。
美国国会的一些成员有广泛的化工培训,这给了他们一个很大的好处,在讨论的环境污染、核能》、《食品与药物管理局和其他立法关注的科学问题。
2.一种物理量的转换到另一个从一个单位完成与转换因子的数值关系源自两个单位。
选择正确的转换因子允许取消不必要的单位。
转换的因素或物理常数应该包括足够数量的位数以免影响不确定的答案。
3.这些物质的变化,进行化学反应被称为反应物,形成新物质的产品。
化学发生的变化,这种变化与符号、公式表示在一个化学方程式。
所有化学方程式必须修正系数balanced-the必须用于每一个物种,以便所有原子中各元素的反应仍会在产品。
4.焓的变化,△H,对于一个反应是相等的,△H级逆向反应,但相对于标志。
如果数量的反应物和产品在反应将被改变。
△H为反应变化比例分配。
对于任何化学反应,△H值是否有相同的反应发生在一个步骤或几个步骤。
5. A是一种化学反应性官能团原子或一组原子特性对家庭给予的有机化合物。
现场的反应通常是一种有机分子官能团,多重共价键中,或一个极性单个的债券。
一个electron-poor原子或小组,他们将原子结合,对一个可用的电子称electrophile,一个electro-rich原子或小组,他们将一个electron-deficient结合原子叫做nucleophile。
6.铁暴露于潮湿的空气除锈的动作很快,形成了一层层硬且易碎的存款的氧化物。
氧化物不粘在表面的金属一样,氧化铝和其他某些金属氧化物,但皮off.露出一个干净的物体表面的铁的作用空气。
作为一个结果,一块铁生锈了完全在较短的时间内,除非我们采取措施防止腐蚀。
化学生物学 专业英语

化学生物学专业英语English:Chemical biology is an interdisciplinary field that combines the principles of chemistry and biology to address important biological questions at the molecular level. This field focuses on understanding the chemical processes and interactions that occur within living organisms, and how they can be manipulated for various applications. Chemical biologists use a wide range of techniques and tools, such as chemical synthesis, spectroscopy, and imaging, to study biological systems and elucidate the underlying molecular mechanisms. By gaining a deep understanding of the chemical aspects of biological processes, chemical biologists can contribute to the development of new drugs, the design of novel biomaterials, and the engineering of cellular systems for therapeutic purposes.中文翻译:化学生物学是一门跨学科领域,将化学和生物学的原理结合起来,以解决分子水平上重要的生物学问题。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
2012—2013学年度第一学期 应用化学专业《专业英语》课程试卷(B ) 注意事项:1. 考生务必将自己姓名、学号、专业名称写在指定位置; 2. 密封线和装订线内不准答题。
一、词汇填空 (写出下列每个词汇对应的英汉单词)(共20小题,每空1分,共20分) 1. 原子 ( ) 2. 镍 ( ) 3. 氦 ( ) 4. 元素 ( ) 5. 阴离子 ( )
6. 钴 ( )
7. 丙酮 ( )
8. 碘 ( )
9. 乙醚 ( ) 10. 钾 ( ) 11. 甲烷 ( ) 12. 乙醇 ( ) 13. chlorine ( ) 14. nickel ( ) 15. phosphorus ( ) 16. potassium ( ) 17. arsenic ( ) 18. sulfur ( )
19. butane ( )
20. aluminum ( )
二、给下列无极化合物的英语名称(共10小题, 每小题2分,共20分) 1.
HCl 2.
HBr 3.
CuSO 4 4.
H 2SO 4 5.
NaCl 6.
Na 2S 7.
KF 8.
Al 2O 3 9.
KMnO 4 10. FeCl 3
二、给下列有机化合物的英语名称(共5小题, 每小
题4分,共20分) 1. CH 3CH(CH 3)CH 2CH(CH 2CH 3)CH 2CH 3
2. (CH 3)2C =CHCH 3
3. CH 2(OH)CH 2CH(CH 2OH)CH 2OH
4. 3,4-二甲基苯酚
5.甲乙醚
三、英译汉(共10小题, 每小题4分,共40分)
1.The study of the properties of substances constitutes an important part of chemistry,
because their properties determine the uses to which they can be put.
2.The cleavage of the different crystals of salt is the same; when crushed, the crystals
always break along planes parallel to the original faces, producing smaller crystals similar to the larger ones.
3.It is customary to say that under the same external conditions all specimens of a
particular substance have the same physical properties (density, hardness, color, melting point, crystalline form, etc).
4.Sodium chloride has the properties of changing into a soft metal, sodium, and a
greenish-yellow gas, chlorine, when it is decomposed by passage of an electric current through it.
5.When biscuits are made with use of sour milk and baking soda there is a chemical
reaction between the baking soda and a substance in the sour milk, lactic acid, to produce the gas carbon dioxide, which leavens the dough by forming small bubbles
in it.
6.When an acid, base, or salt is dissolved in water the resulting solution is a conductor
of the electric current and is termed an electrolyte. If no conduction of current occurs, the compound is known as a nonelectrolyte.
7.Green chemistry is the chemistry that aims to reduce the amount of hazardous
substance coming out in the process of producing chemical materials and to reduce the amount of resource and energy consumed in that process.
8.Nonbenzenoid compounds containing rings of carbon atoms are called alicyclic.
These are carbocyclic compounds which resemble aliphatic compounds in many ways.
9.The second group is composed of compounds derived from or related to benzene,
C6H6. Because the first known derivatives of benzene were natural products extracted from balsams and impressed the discoverers because of their fragrant aromas, the group as a whole came to be known as aromatic compounds.
10.Heterocyclic compounds are cyclic compounds with the ring containing carbon and
other elements, the commonest being oxygen, nitrogen and sulfur. There are a number of heterocyclic rings which are easily opened and do not posses any aromatic properties, e. g. , ethylene oxide, γ- and δ-lactones.。
