Criteria for Registration of B&PVs(NB-264)
ICH概述

CH共召开了10次国际性大会,具体情况见下表:
时间
举办地
参会人数
1200 1500 2400 1600 1700 1800
1991.11 布鲁塞尔,比利时 1993.10 佛罗里达,美国 1995.11 横滨,日本 1997.07 布鲁塞尔,比利时 2000.11 圣地亚哥,美国 2003.11 大阪,日本 2007.11 东京,日本 2008.11 华盛顿,美国 2009.06 东京,日本
4. ICH工作的特征和目标 (1)病人第一 一切从病人利益出发是ICH讨论和协商的基础,决定技术文件的准 则是:“是否有利于病人?如何才能更快地为病人提供高质量、安全有 效的药物?如何才能按国际标准进行高质量的临床试验?” (2)对话和协作 管理部门和工业部门的专家在同一原则下进行讨论,从不同角度提 出更合理的见解,避免片面性。在ICH会议中,管理部门和工业部门是 对话,不是对抗;是相互合作和相互信任,不是互相扯皮。
3.2 工作程序 ICH把需讨论专题的进展分为5个阶段: (1)阶段1:EWG技术讨论 专家工作组对新选题目进行初步讨论,并起草出初稿,初稿可以是 建议(Recommendation)、政策说明(Policy Statement)、指导原 则(Guide-line)或讨论要点(Points to Consider)等形式。由专家工 作组对初稿进行讨论、审查和修改,直到达成共识,提交指导委员会。 (2)阶段2:达成共识 由指导委员会的六个主办单位负责人对初稿进行审查讨论后签字, 提交欧、美、日三方药品管理部门正式讨论,在六个月内将意见汇总。
---日本厚生劳动省,Ministry of Health、Labour and Welfare,Japan (MHW)
---日本制药工业协会,Japan Pharmaceutical Manufacturers Association (JPMA)
2-1 WHO-PQ认证指导原则及提交资料的格式要求 2013.06(英文)

• Prequalification of generic products approved by Stringent Regulatory Authorities (SRAs)
7
7
Prequalification Guidelines (Quality)
• Guidelines on active pharmaceutical ingredient master file procedure
15
15
CTD- triangle
Regional Admin Information Module 1
Not Part of the CTD
Clinical Overview
Module 2
Quality Overall Summary
Nonclinical Overview
The CTD
Nonclinical Summary
information –Reginon specific • • • Module 2 – Quality Overall Summary – QOS-PD Module 3 - Quality Module 5 – Bioequivalent study Reports(or biawaiver)
• Provide clear and transparent guidance to applicants for preparing and submitting these dossiers and facilitate the subsequent assessment;
心脏器械,支架注册指导原则_斯里兰卡

心脏器械/支架注册指导原则(斯里兰卡)心脏器械/支架注册指导原则(Guidelines for registration of Cardiac device / stent)指导原则是由斯里兰卡CDDA(Cosmetic Devices and Drug Regulatory Authority)的医疗器械评价分委员会(MDESC,Medical Device Evaluation Sub Committee)制定的,它是对《斯里兰卡医疗器械注册指导原则》(Guideline for Registration of Medical Devices in Sri Lanka,CDDA 于2011年11月发布)的补充。
其具体内容如下:1. The FDA approval (which permits free sale in the USA) will be sufficientgrounds for registration of product.美国FDA的批准(允许在美国自由销售)可以作为产品注册的充分依据。
2. If only the CE mark (which permits free sale within the European Union) isavailable ANYONE of the additional criteria given in (3)below may be requested if the committee is not fully satisfied with the data provided.在只获得CE批准(允许在欧盟自由销售)的情况下,如果委员会(译注:指)对已提供的资料不完全满意,可能会要求提供以下第3条里所列的任意一项资料。
3. Where any three members of the committee are of the opinion that aproduct with neither FDA approval nor CE accreditation needs to be registered in Sri Lanka, both of the following could be requested and considered in order to further establish clinical efficacy and safety.需要在斯里兰卡注册而既没有获得FDA批准又没有获得CE认可的产品,如果有任何三个委员会成员达成共识,则可能要求提供以下两项资料以进一步确定其临床有效性和安全性:(I) Multi center clinical trial/s Published in medical journal/s which is/areincluded in the Index Medicals.在《Index Medicals》中收录的医学杂志上发表的多中心临床试验资料。
比赛规则的英语作文带翻译

比赛规则的英语作文带翻译In every competitive event, a set of rules is established to ensure fairness and sportsmanship. These rules are the backbone of any competition, providing a framework within which participants can showcase their skills and abilities. Understanding and adhering to these rules is crucial for the success of the event and the integrity of the sport.翻译:在每项竞技活动中,都会建立一套规则,以确保公平和体育精神。
这些规则是任何比赛的支柱,为参与者提供了一个展示他们技能和能力的框架。
理解和遵守这些规则对于活动的成功和运动的完整性至关重要。
1. Registration and Eligibility: Participants must register in advance and meet the eligibility criteria set by the organizers. This may include age, skill level, or other specific requirements.翻译:1. 注册和资格:参与者必须提前注册并满足组织者设定的资格标准。
这可能包括年龄、技能水平或其他特定要求。
2. Competition Format: The format may vary from one competition to another. It could be a single-elimination tournament, a round-robin, or a points-based system.Participants should familiarize themselves with the format to prepare adequately.翻译:2. 比赛形式:比赛形式可能因比赛而异。
ICH指导原则-术语

ICH指导原则为了严格管理药品,必须对药品的研制、开发、生产、销售、进品等进行审批,形成了药品的注册制度。
但是不同国家对药品注册要求各不相同,这不仅不利于病人在药品的安全性、有效性和质量方面得到科学的保证及国际技术和贸易交流,同时也造成制药工业和科研、生产部门人力、物力的浪费,不利于人类医药事业的发展。
因此,由美国、日本和欧盟三方的政府药品注册部门和制药行业在1990年发起的ICH(人用药物注册技术要求国际协调会议,International Conference on Harmonization of TechnicalRequirements for Registration of Pharmaceuticals for Human Use)就是这样应运而生的。
ICH的论题主要分为四类,因此ICH根据论题的类别不同而进行相应的编码分类:1. “Q”类论题:Q代表QUALITY,指那些与化工和医药,质量保证方面的相关的论题。
2. “S”类论题:S代表SAFETY,指那些与实验室和动物实验,临床前研究方面的相关的论题。
3. “E”类论题:E代表EFFICACY,指那些与人类临床研究相关的课题。
4. “M”类论题:M代表MULTIDISCIPLINARY, 指那些不可单独划入以上三个分类的交叉涉及的论题。
同时M又细分为5个小类M1: 常用医学名词(MedDRA)M2: 药政信息传递之电子标准M3: 与临床试验相关的临床前研究时间的安排M4: 常规技术文件(CTD)M5: 药物词典的数据要素和标准2005年11月ICH执行委员会接受了一套用于ICH指导原则的新编码法则,并与当月正式执行。
Quality质量:Q1: Stability稳定性Q1A(R2): Stability Testing of New Drug Substances and Products新原料药和制剂的稳定性试验Q1B: Photostability Testing of New Drug Substances and Products新原料药和制剂的光稳定性试验Q1C: Stability Testing for New Dosage Forms新剂型的稳定性试验Q1D: Bracketing and Matrixing Designs for Stability Testing of Drug Substances and Drug Products原料药和制剂稳定性试验的交叉和矩阵设计Q1E: Evaluation of Stability Data稳定性数据的评估Q1F: Stability Data Package for Registration Applications in Climatic Zones III and IV在气候带III和IV,药物注册申请所提供的稳定性数据Q2: Analytical Validation分析验证Q2(R1): Validation of Analytical Procedures: Text and Methodology分析程序的验证:正文及方法论Q3: Impurities 杂质Q3A(R2): Impurities in New Drug Substances新原料药中的杂质Q3B(R2): Impurities in New Drug Products (Revised Guideline)新制剂中的杂质Q3C(R3): Impurities: Guideline for Residual Solvents杂质:残留溶剂指南Impurities: Guideline for Residual Solvents (Maintenance) 杂质:残留溶剂指南(保留)PDE for Tetrahydrofuran (in Q3C(R3)) 四氢呋喃的PDEPDE for N-Methylpyrrolidone (in Q3C(R3)) N-甲基吡咯烷酮的PDEQ4: Pharmacopoeias药典Q4A: Pharmacopoeial Harmonisation 药典的协调Q4B: Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions药典内容的评估及推荐为用于ICH地区Q4B Annex1 Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regionson Residue on Ignition/Sulphated Ash General Chapter附录1 药典内容的评估及推荐为用于ICH地区关于灼烧残渣/灰分常规篇Q4B Annex2 Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions on Test for Extractable Volume of Parenteral Preparations General Chapter 附录2 药典内容的评估及推荐为用于ICH地区关于注射剂可提取容量测试常规篇Q4B Annex3 Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions on Test for Particulate Contamination: Sub-Visible Particles General Chapter 附录3 药典内容的评估及推荐为用于ICH地区关于颗粒污染物测试:不溶性微粒常规篇Q5: Quality of Biotechnological Products 生物技术制品质量Q5A(R1): Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or AnimalOrigin来源于人或者动物细胞系的生物技术产品的病毒安全性评估Q5B: Quality of Biotechnological Products: Analysis of the Expression Construct in Cells Used forProduction of r-DNA Derived Protein Products生物技术产品的质量:源于重组DNA的蛋白质产品的生产中所用的细胞中的表达构建分析Q5C: Quality of Biotechnological Products: Stability Testing of Biotechnological/Biological Products生物技术产品的质量:生物技术/生物产品的稳定性试验Q5D: Derivation and Characterisation of Cell Substrates Used for Production ofBiotechnological/Biological Products用于生产生物技术/生物产品的细胞底物的起源和特征描述Q5E: Comparability of Biotechnological/Biological Products Subject to Changes in Their ManufacturingProcess基于不同生产工艺的生物技术产品/生物产品的可比较性Q6: Specifications 规格Q6A: Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances (including decision trees)质量规格:新原料药和新制剂的检验程序和可接收标准:化学物质(包括决定过程)Q6B: Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products 质量规格:生物技术/生物产品的检验程序和可接收标准Q7: Good Manufacturing Practices (GMP)Q7A: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients活性药物成份的GMP指南Q8: Pharmaceutical Development药物研发Annex to Q8Q8附录Q9: Quality Risk Management质量风险管理Q10: Pharmaceutical Quality System 药物质量体系。
临床试验中英对照词汇表english vocabulary of clinical trials-yrn2019051011
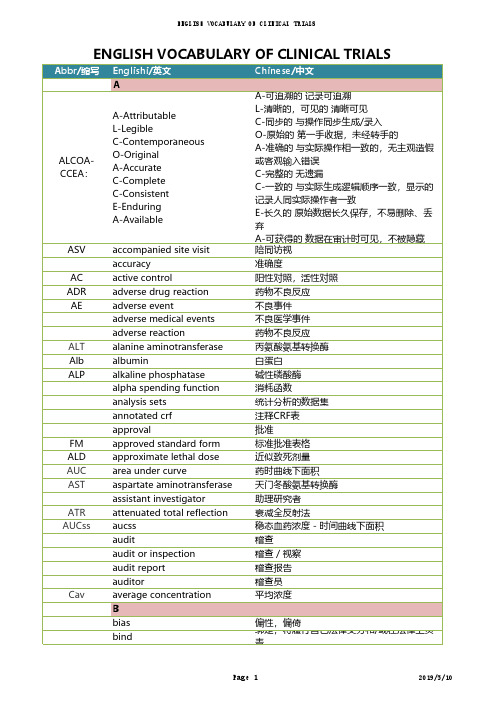
首席医学官 首席营销官
国家食品药品监督管理总局
圆二色谱 清除率 临床稽查员 临床数据管理系统 临床总监 临床等效应 临床研究监查助理 临床监查/运营 临床项目助理 临床研究监察员,临床研究助理 临床协调员 临床研究
Page 2
2019/5/10
ENGLISH VOCABULARY OF CLINICAL TRIALS
resolution
数据澄清和解决表
Page 3
2019/5/10
ENGLISH VOCABULARY OF CLINICAL TRIALS
DCF DCR DM DMP DMSF DQF DVP
DF
DSC DFS
DLT
eCRF EDC EDP EOS
EC ESH-ESC
EU EBM
data clarification form
标准批准表格
ALD
approximate lethal dose
近似致死剂量
AUC
area under curve
药时曲线下面积
AST
aspartate aminotransferase 天门冬酸氨基转换酶
assistant investigator
助理研究者
ATR
attenuated total reflection
国际医学科学组织理事会
sciences
close out visits
关闭访视
crea
肌酐
cross-over study
交叉设计
css, steady-state concentration 稳浓度
cure
痊愈
curriculum vitea
中英文-PDA TR70 无菌生产设施的清洁消毒程序原理

TR70 无菌清洁消毒原理
2 / 119
TR70 Fundamentals of Cleaning and Disinfection Programs for Aseptic Manufacturing Facilities
9.4.2 Work Surfaces 9.4.3 Nonstructural Clean Room and Hard-to-Clean Surfaces
21.0 APPENDIX V: EPA-RELATED SAFETY LABELING INFORMAT PROTOCOL TESTING FOR DISINFECTANT REGISTRATION 23.0 APPENDIX VII: EN TESTS FOR DISINFECTION EFFICACY 24.0 APPENDIX VIII: LARGE-SCALE GASSING OR FOGGING OF CLEAN ROOMS
消毒计划 知识评估和确认 实施与清洁和消毒有关的调 查 结论 附录1:消毒的历史 过去的消毒技术 化学时代的消毒技术 发现微生物是疾病的原因 今天的微生物污染控制 附录2:杀菌剂、消毒剂和杀 孢子剂的注册 附录3:美国环境保护署概述
附录4:欧洲生物杀灭产品法 规概览 附录5:EPA相关安全标识信 息 附录6:AOAC消毒剂注册测 试方案 附录7:EN消毒有效性测试 附录8:洁净间大容量气雾
14.8 Aspects of a Disinfection Program 14.9 Assessment of Understanding and Qualification 15.0 CONDUCTING INVESTIGATIONS RELATED TO CLEANING AND DISINFECTION 16.0 CONCLUSION 17.0 APPENDIX I: HISTORY OF DISINFECTION 17.1 Disinfecting Technologies ofthe Past 17.2 Disinfecting Technologies in the Age of Chemistry 17.3 Discovering Microorganisms as a Basis of Disease 17.4 Microbiological Contamination Control Today 18.0 APPENDIX II: REGISTRATION OF SANITIZERS, DISINFECTANTS AND SPORICIDES 19.0 APPENDIX III: OVERVIEW OF THE U.S. ENVIRONMENTAL PROTECTION AGENCY 20.0 APPENDIX IV: OVERVIEW OF THE EU BIOCIDAL REGULATIONS
报考英语报名流程

报考英语报名流程English:To apply for the English exam, you first need to visit the official website of the examination board or the institution organizing the exam. There, you will find detailed information about the exam, including eligibility criteria, exam dates, and registration procedures. Carefully read through all the instructions and requirements to ensure you meet the criteria for registration. Next, create an account on the website if you don't already have one. This usually involves providing basic personal information such as your name, contact details, and identification documents. Once your account is created, navigate to the exam registration section and fill out the online registration form. You may need to upload scanned copies of your identification documents and recent photographs, so make sure you have these ready beforehand. After completing the registration form, proceed to pay the exam fee, which is typically done online using a credit or debit card. Once the payment is processed successfully, you will receive a confirmation email or notification confirming your registration for the exam. Keep this confirmation safe as you may need it for future reference. Finally, make a note of the exam dateand any other relevant details provided by the examination board to ensure you are well-prepared on the day of the exam.中文翻译:要报考英语考试,首先需要访问考试委员会的官方网站或组织考试的机构。
RegistrationOrdinance(Cap165)

Guidance Notes for Application for First Registration of Nursing Homes under the Hospitals, Nursing Homes and Maternity HomesRegistration Ordinance (Cap. 165)1.Purpose1.1This document serves as a general guide for persons applying for firstregistration of a nursing home under the Hospitals, Nursing Homes and Maternity Homes Registration Ordinance(hereafter referred to as ‘Cap.165’). It should not be regarded as complete registration requirements. 2.Registration of a Nursing Home2.1Under Cap. 165, it is an offence for any person to carry on a hospitalwithout being duly registered in respect thereof. Examples of hospitals that fall within the jurisdiction of this ordinance are private hospitals, maternity homes, nursing homes for the elderly, renal dialysis centres, treatment centres for drug dependent persons and day surgery facilities. 2.2 Any person who intends to operate a healthcare institution in the form of anursing home must obtain prior approval from the Director of Health (DH).3.Criteria for Registration of a Nursing Home3.1Conditions relating to the accommodation, staffing or equipment of thenursing home under application shall be fit for the proposed purposes.3.2The “Code of Practice For Private Hospitals, Nursing Homes andMaternity Homes” (hereinafter referred as “the Code”) sets out the standards of good practice for healthcare institutions to adopt for patient safety and quality services. The Code constitutes the basis of assessment of the suitability of the healthcare institution for registration under Cap.165. Compliance with Cap. 165 and the requirements set out under the Code are the conditions for the registration and re-registration of healthcare institutions.3.3The applicant shall also ensure that the building and engineering workscomply with relevant ordinances, regulations and codes, including but notlimited to the following :♦Buildings Ordinance and its subsidiary legislation (Cap. 123).Codes of Practice, Practice Notes and Circulars published by theBuildings Department, Government of HKSAR♦Fire Safety (Commercial Premises) Ordinance (Cap. 502), Fire Safety (Buildings) Ordinance (Cap. 572). Codes of Practice andCirculars published by the Fire Services Department, Governmentof HKSAR♦Waterworks Ordinance and its subsidiary legislation (Cap. 102).Practice Notes, Circulars and the Hong Kong Waterworks StandardRequirements for Plumbing Installation in Buildings published bythe Water Supplies Department, Government of HKSAR♦Electricity Ordinance and its subsidiary legislation (Cap. 406).Code of Practice for the Electricity (Wiring) Regulations publishedby the Electrical and Mechanical Services Department, Governmentof HKSAR♦Lifts and Escalators Ordinance and its subsidiary legislation (Cap.618). Circulars, Codes of Practice published by the Electrical andMechanical Services Department, Government of HKSAR ♦Code of Practice for Prevention of Legionnaires’ Disease published by the Prevention of Legionnaires’ Disease Committee,Government of HKSAR4.Application Procedures for First Registration of a Nursing Home underCap. 1654.1 The person intending to be registered in respect of the nursing home(hereafter referred to as ‘the applicant’) or his/her authorized representative shall submit application for registration to DH.4.2 At least 4 months before the intended date of commencement of operation,the Applicant should submit to DH a “Letter of Intent for a new Nursing Home” with the documents as set out in Annex I.4.3 Within 15 working days, DH will send to the Applicant a list of specifieddocuments required in support of the application for registration of the nursing home for operating the proposed services. Basic documentsrequired for registration of nursing homes is at Annex II for reference.4.4 At least 2 months before the intended date of commencement ofoperation, the Applicant shall submit a completed “Application Form for First Registration / Re-Registration of Private Hospitals, Nursing Homes and Maternity Homes” and the required documents specified by DH.5.Inspection and Notification of Result of Application5.1When the site is ready for inspection and calibrated equipment are inplace, the Applicant should inform DH for arrangement of inspection.Upon receiving notification, complete set of completed application form and all required documents, inspection team of the Department of Health would conduct site inspection within 20 working days.5.2The Applicant may at any time be required to submit supplementaryinformation before the application is approved.5.3Upon completion of inspection(s) and receipt of all information requiredto show compliance with the registration requirements, the Certificate of Registration for the nursing home will be issued by DH within 14 working days.6.Submission of Application6.1“Letter of Intent for a new Nursing Home” and completed “ApplicationForm for First Registration/ Re-Registration of Private Hospitals, Nursing Homes and Maternity Homes” shall be submitted and signed by the Applicant or his/her authorised representative.6.2The application form can be downloaded at.hk/english/useful/useful_forms/files/or phf_reg_hospital.pdf. Application for first registration shall be accompanied by the prescribed fee ($6815).6.3All letters, application-related documents and completed application formshall be submitted to the Department of Health at the following address :Office for Reg ula tion of Private Hea l thcare FacilitiesDepartment of HealthRoom 402, 4/F, Cityplaza 314 Taikoo Wan RoadQuarry Bay, Hong Kong(Enquiry Number: 3107 8451)6.4For enquiry, the Applicant can contact Office for Regulationof Private Healthcare Facilities, Department of Health bytelephone Department of Health by telephone (3107 8451)or by email (************.hk).Office for Re gulation of Private Healthcare Facilit i esDepartment of HealthApril 2016Annex I Documents to be submitted with the “Letter of Intent for a new Nursing Home” for application for first registration of a Nursing Home under Cap.1651.Key information regarding the proposed Nursing Home(a)Name and address of the proposed Nursing Home(b)Name of the person to be registered in respect of the Nursing Home(c)Contact details of the applicant and/or name & contact details of theauthorized representative for the application for first registration of theNursing Home(d)List of service(s) to be provided by the Nursing Home(e)Expected date of commencement of service provision of the NursingHome (where service will be implemented in phases, the respectivedates should be provided)2.Documents to prove fitness for carrying out the service in respect ofaccommodation(a)Documentation showing compliance with the land grant/leaseconditions for the use of the building/premises as a nursing home(b)Layout plan of the premises(c)[For new buildings only] Occupation permit issued by the BuildingAuthority(d)Other approval document issued by the Building Authority and/or otherauthorised parties if there is structural change of premises(e)[For proposed services involving specialised ventilation area# only ](i) summary of the healthcare ventilation and air-conditioningstandard(s)/code(s) against which the key parameters of thespecialized ventilation areas will comply*(ii)Engineering calculation, air-side schematic diagram(s) and layout plan(s) which substantiate the appropriateness of air-conditioningand/or ventilation provisions for the intended use and showingcompliance with the proposed healthcare facility standard / codeand the indoor requirement of medical equipment(f)[For haemodialysis services only](i)Information on the proposed water treatment and distributionsystem, including schematic diagram(s) and as-built layoutplan(s);(ii)information on the piping and plumbing system with material used 3.Document to prove fitness for carrying out the service in respect ofequipment(a)List of major medical equipment (e.g. haemodialysis machine,defibrillators, medical gas cylinders, ceiling hoist, etc.) supportingproposed services.(b)List of other equipments essential for supporting the services.(c)Information on whether the equipment(s) will have means of alternativepower supply other than normal power source should be provided (e.g.built-in battery, external battery, etc.)Remarks :#Specialised ventilation areas are areas with special ventilation design for infection control and/or occupational safety.If the proposed change in services involves any specialised ventilation area, the Applicant shall submit ventilation system specifications of the involved specialised ventilation area and demonstrate the compliance to relevant international standard.Examples are isolation room with differential pressure from adjacent areas, procedure room, bronchoscopy room, plant room housing combustion equipment, welding facility, woodworking workshop, and area containing oxygen-displacing gases.*Examples of reference healthcare facility standards / codes for the following systems are:-Ventilation and Air-conditioning System:HTM 03-01 or ASHRAE 170 and “Infection Control Branch (ICB) Infection Control Guidelines” published by the Centre of Health Protection (CHP) for airborne infection, isolation rooms and protective environment (if applicable)If the applicant proposes to adopt alternative standard(s) / code(s) other than the standard(s) as stated for the corresponding areas, justifications such as best practices and technical capability for healthcare operational needs shall be submitted with substantial project reference and technical drawings together with engineering calculations.Annex II Documents required for the application forfirst registration of nursing homes under Cap. 165The person intending to be registered of a nursing home or his/her authorised representative shall submit the following documents together with the completed and signed “Application Form for First Registration / Re-Registration of Private Hospitals, Nursing Homes and Maternity Homes” to the Director of Health for the application for registration of the proposed new nursing home at least 2 months before the intended date of commencement of operation:1. A photocopy of the Hong Kong Identity Card of the applicant (applicationsby individual only)2. A photocopy of the Business Registration Certificate of the applicant issuedby the Commissioner of Inland Revenue3. A photocopy of Certificate of Incorporation issued by the Registrar ofCompanies (applications by incorporated company only)4.Authorization letter for the authorized representative of the applicant inmaking application for First Registration of the Nursing Home5. A crossed cheque payable to “Government of HKSAR” for payment of theprescribed fee HK$6815 for the first application for registration6.Duly completed Report for Registration of Nursing Homes7.The following documents to prove fitness for carrying out the service inrespect of accommodation :(a)Certificate(s) of fire services installation and equipment (FS251,FS314A or FS172, whichever applicable) and associated as-fitted fireservices schematic diagram(s) and layout plan(s)(b)[For proposed services involving specialised ventilation area#](i)As-fitted air-side schematic diagram(s) and layout plan(s)(ii)Proposed maintenance schedule (and maintenance record, if applicable) of the ventilation and air-conditioning installations(c)Proposed maintenance schedule (and maintenance record, if applicable)of the ventilation and air-conditioning installations(d)Work Completion Certificate (Form WR1) for fixed electricalinstallations including associated as-fitted electrical wiring schematicdiagram and lighting & power layout plan8.The following documents to prove fitness for carrying out the service inrespect of medical and other essential equipments :(a)Proof of fitness for safe operation of medical & other essentialequipments listed in the Report for Registration of nursing home (e.g.valid licences/certificates, satisfactory acceptance test reports)(b)Maintenance schedule of the medical & other essential equipment(s) asadvised by respective manufacturer and the record of last maintenance(c)[for haemodialysis service only] Analytical reports for representativewater samples for the water distribution system, showing quality fit forthe purpose(d)Certificate/gas analysis report showing fitness for use of medical gascylinders/pipeline system9.The following documents regarding staffing :(a)Person(s)-in-charge of proposed service(s) with their qualification andexperience(b)Proposed duty roster of nursing staff for the first two weeks from theservice commencement date(c)Relevant training records(d)Relevant drill records or proposed date10.Updated information in respect of previous submissions, if any11.Expected date of commencement of Services12.Expected date when the premises is ready for inspectionRemarks:#Specialised ventilation areas are areas with special ventilation design for infection control and/or occupational safety.If the proposed change in services involves any specialised ventilation area, the Applicant shall submit ventilation system specifications of the involved specialised ventilation area and demonstrate the compliance to relevant international standard.Examples are isolation room with differential pressure from adjacent area, procedure, bronchoscopy room, plant room housing combustion equipment, welding facility, woodworking workshop, and area containing oxygen-displacing gases.Examples of reference healthcare facility standards / codes for the following systems are:-Ventilation and Air-conditioning System:HTM 03-01 or ASHRAE 170 and “Infection Control Branch (ICB) Infection Control Guidelines” publishedby the Centre of Health Protection (CHP) for airborne infection, isolation rooms and protective environment (if applicable)If the applicant proposes to adopt alternative standard(s) / code(s) other than the standard(s) as stated for the corresponding areas, justifications such as best practices and technical capability for healthcare operational needs shall be submitted with substantial project reference and technical drawings together with engineering calculations.。
制药行业常用英语

Cross-Contamination:交叉污染(一种物料或产品对另一种物料或产品的污染)
Deviation:偏差(对批准的指令或规定的标准的偏离0
Drug (Medicinal) Product:药品(经最后包装准备销售的制剂-参见Q1A)
Batch (or Lot):批由一个或一系列工艺过程生产的一定数量的物料,因此在规定的限度内是均一的。在连续生产中,一批可能对应于与生产的某一特定部分。其批量可规定为一个固定数量,或在固定时间间隔内生产的数量。
Batch Number (or Lot Number):批号
用于标识一批的一个数字、字母和/或符号的唯一组合,从中可确定生产和销售的历史。
Process Aids:工艺辅料除溶剂外,在原料药或中间体生产中起辅助作用、本身不参与化学或生物学反应的物料(例如,助滤剂、活性炭)。Production:生产
在原料药制备过程中,从接收原料,到工艺加工和原料药包装所涉及的所有操作。
Qualification:确认证明设备或辅助系统,安装正确、工作正常、确实产生预期的结果,并以文件佐证。确认是验证的一部分,但单独的确认步骤不构成工艺验证。
Reference Standard, Secondary:二级参考标准品
与基准参考标准品比较显示具有规定的质量和纯度,并用作日常实验室分析的参考标准品。
Reprocessing:返工将不符合标准或规格的一个中间体或原料药返回工艺过程,重复规定的生产工艺中的某一结晶步骤或其它合适的化学或物理处理步骤(如蒸馏,过滤,层析,磨粉),这种做法通常是可以接受的。在中间控制的测试表明一工艺步骤没有完成,从而继续该步骤,是正常工艺的一部分,而不是返工。
ICH药品质量控制相关文件介绍

对每个起草文件的专题派若干专家参加,其中一名任专题 组长,负责该专题的工作。协调的专题共分四个类别:
20
(3)秘书处 秘书处设在日内瓦IFPMA总部。主要负责指导委员会及
专家工作组会议的准备工作和有关文件的起草,并负责与 各组的协调员联系,以保证将讨论的文件按时发送到有关 人员。
后来,随着工作的深入开展,认为电子通讯和术语的统 一,应作为互读文件的基础。因此,增加了“综合学科”, 并成立了子课题。
13
2.概况及组织机构 2.1 概况
ICH是由欧盟、美国和日本三方的药品注册部门和生产部门组成, 六个参加单位分别为: ---欧盟,European Union (EU)
---欧洲制药工业协会联合会,European Federation of Pharmaceutical Industries Associations (EFPIA)
21
3. 指责和工作程序 3.1 职责
(1)对在欧盟、美国和日本注册产品的技术要求中存 在的不同点,创造注册部门与制药部门对话的场所,以便 更及时将新药推向市场,使病人得到及时治疗;
(2)监测和更新已协调一致的文件,使在最大程度上 相互接受ICH成员国的研究开发数据;
(3)随着新技术进展和新治疗方法应用,选择一些课 题及时协调,以避免今后技术文件产生分歧;
2 - 7 June 2018, Kobe, Japan
19
2.2 组织机构
ICH由指导委员会、专家工作组和秘书处组成:
(1)指导委员会 (the Streering Committee, SC) 指导委员会共有14名成员,由六个参加单位和IFPMA各
山东省青岛市2021年高三年级期初调研考试 英语试题附答案

青岛市2021届高三上学期期初考试2020.09英语第二部分阅读(共两节,满分50分)第一节(共15小题:每小题2.5分,满分37.5分)阅读下列短文,从每题所给的A、B、C、D四个选项中,选出最佳选项。
AWe are Idea International Education. As a comprehensive education service institute, our mission is to assist native English Speaking Teachers in their quest of getting teaching positions which best suit them. We have school locations in small towns and big cities all over Guangdong. China and we would be happy to speak with those who are interested in the teaching opportunities we can provide! Below is a list of basic details about our positions:Key Responsibilities Involved:·Our average work schedule being Monday through Friday·Teachers being given 40 working bours per week with 2-day weekend off per week·Teaching periods from September through January and March through July, with the provided option of teaching during semester breaksThe Treatments:·Salaries range from 10, 000 to 18, 000 RMB and is based on qualification , degree , teaching experience,& teaching certification·5000 RMB contract finish bonus (Generally, a contract lasts 1 year)·Private Furnished Independent Apartment provided·Two free meals offered by the school per work day·Airport pick-up·Medical insurance and accident insuranceRequired Documentation:- A current resume or one-sheet, indicating your nationality- A copy of your passport / ID- A copy of your highest degree and / or other certification- Current Photo- References with contact informationPlease contact us for more details: 21. What’s the job description of the teaching positions?A. Teachers only work on Monday and Friday.B. Teachers should work more than 10 hours every day.C. Teachers are required to work 11 months per year.D. Teachers can choose to work during school holidays.22. How will you be treated if you get the teaching position?A. You will be paid at least 18,000 RMB monthly.B. You will get extra 5,000 yuan after a year’s teaching.C. You need to live in a shared dormitory with others.D. You are free to choose your insurance types.23. Which is unnecessary when you apply for the positions?A. A proof of previous working experience.B. A copy of your IDC.A copy of your certification.D. Your recent photo.BWith fewer races to take part in, and one more year to wait, Chinese sprinter(短跑运动员)Xie Zhenye is committed to staying patient and focused as he tries to achieve his Olympic dream.No matter what the future bolds, Xie’s expectation for the Tokyo Games remains the same become the first Chinese national to reach a hat-trick(三连胜) of sprint finals, in the 100m,200m and 4x100m events.“With the postponement of the Olympic Games, I need to be more patient and focused and make good use of this additional year to get better prepared for the Games,” said the 27-year-old, who is currently training at Beijing Sport University.Xie trained with his national teammates in the United States for four months during the winter. According to his original plan, he was due to take part in a series of outdoor races in Europe and the US to tune up for the Tokyo Games. That was until the COVID-19 pandemic changed everything.Xie flew to China’s coastal city of Qinhuangdao, Hebei province, at the end of March, and after a 28-day quarantine(隔离期), returned to Beijing to stan his lockdown training session.“Although most of the competitions have been canceled or postponed, I can still compete in some virtual events and testing events organized by the national team.” Xie said.“I am working closely with my coach and sticking to our new preparation plan. Our aim is to maintain my good form until the Tokyo Games next year.”Xie enjoyed a successful season in 2019, setting a new Asian record of 19.88 at the Diamond League London meeting. Later, at the world championships in Doha, the 2010 Youth Olympic champion became the first Chinese to reach the men’s 200m final.Xie has already met the Olympic entry standard for the 100m and 200m in Tokyo, while the Chinese relay team has also booked a berth(参赛权) in the 4x100m. Now, Xie wants to make more history in the finals of all three events in Tokyo next summer.“I have the confidence to pursue that. I will try my best and leave no regrets in Tokyo,” he said.24. What’s the main idea of the text?A. The pandemic has postponed the Tokyo Games.B. Xie is aiming for his Olympic dream through hard training.C. Xie has achieved great success in his sports life.D. The pandemic has caused great pressure to athletes.25. Which of the following can best replace the underlined phrase “tune up” in paragraph 4?A. Make up.B. Long.C. Leave.D. Prepare.26. What did Xie do in Qinhuangdao?A. He had a pleasant holiday with his team.B. He participated in the outdoor races.C. He had a quarantine for about a month.D. He started his training session.27. What does the underlined word “that” in the last paragraph probably refer to?A. Keeping good playing condition.B. Meeting the Olympic entry standard.C. Setting a new record of the men’s 200m.D. Winning the finals of all three events.C“Join our exciting wildlife watching tours and experience the holiday of a lifetime!” Eco-wildlife tours like this are becoming more and more popular with tourists. The opportunity to see whales and dolphins swimming in their natural habitat is so much better than seeing these great mammals in zoos and aquariums. As your boat edgesout into the blue water, a group of dolphins come to greet you and swim around the boat, jumping playfully around you. In some places, tour companies even encourage tourists to go swimming with the dolphins. These intelligent mammals seem to enjoy our company and interact with us. But is it possible that this kind of human activity putting their survival at risk?A recent study has shown that the behavior of whales and dolphins changes greatly when they are close to boats. Interpreting the boats as a possible danger, they start to breathe more to breathe more quickly. The boats drive them away from the places where they feed and interrupt their routine of resting and taking care of their young. All of these factors could have a very negative effect on their general health. Another factor that should be taken into account is the effect of human attention on the animals. As they become more used to interacting with humans, they become less afraid of them. There have been several cases of tour boats running into dolphins or whales and causing their deaths. As dolphins live within close communities, events like these cause a lot of stress.Perhaps the best way to protect these animals is to reduce our interaction with them. This we could do by keeping a minimum distance between the animals and the boats, and by limiting the number of boats out on the water at one time. Most importantly, instead of interrupting these animals’ routines, we should try to adapt to them. In this way, we could learn to exist in harmony with these wild animals and ensure our continued co-existence on the planet that we shall share.28. Why are Eco-wildlife tours increasingly popular?A. Because they are widely advertised.B. Because tourists can visit wild animals in nature.C. Because wild animals enjoy our company.D. Because visitors do not disturb wild animals.29. What’s the author’s attitude towards Eco-wildlife tours?A. Interested.B. Supportive.C. Critical.D. Confused.30. What can we learn from the recent study?A. The visiting boats excite whales and dolphins.B. Boats drive young whales and dolphins away from their parents.C. Human’s attention benefits whales and dolphins a lot.D. Getting too close to wild animals may cause trouble to them.31. What is suggested to stay in harmony with wild animals according to the text?A. Making less interaction with them.B. Keeping a minimum distance between boats.C. Banning boats entering their habitat.D. Making them adapt to our daily routine.DChengdu, the capital of Southwest China’s Sichuan province, is promoting innovation in the new economy to create a powerhouse for regional economic development.The city’s recent move to open up registration for companies as part of the first of innovation-application experimental labs and future scene labs is an important part of its efforts to promote innovation. Officials saidthis will help with the development of business in the new economy.In order to be selected into Chengdu’s innovation-application experimental labs, companies should have an investment in research and development that accounted for 3-5 percent of their total experimental labs include having no less than five professionals working full-time in science and technology research. They also must have a three-to-five-year development plan.The city government said companies will receive investment or subsidies(补贴) worth up to 2 million yuan ($285,695) upon approval.Yang Quan, an official in charge, said the move will play an important role in promoting new technologies while facilitating their practical uses in urban development. He said the two lab projects will offer more opportunities in the development of small and medium-sized companies in the new economy. This will help form relatively mature industry clusters(集群) to boost the local economy.The city’s supportive measures to boost the new economy have benefited numerous companies, with Chengdu Haier Sen Hospital being one of them.32. What can be the best title for the text?A. Chengdu boosts innovation efforts in new economy.B. Chengdu opens up registration for new companies.C. Chengdu announces strict criteria to select companies.D. Chengdu offers more chances for large companies.33. What is paragraph 3 mainly about?A. Procedures for companies to register.B. Criteria for companies to be selected in the project.C. Investment in research and development.D. Development plans of selected companies.34. What can we learn from Yang’s remarks?A. Companies will get financial support.B. New technologies play an important role in the move.C. The move will speed up the local economy.D. Small companies will decline due to the move.35. What will the author probably write about in the next paragraph?A. Newly founded industry clusters.B. Other measures to develop economy in Chengdu.C. The development of Chengdu Haier Sen Hospital.D. The praise for the measures from all aspects第二节(共5小题:每小题2.5分,满分12.5分)阅读下面短文,从短文后的选项中选出可以填入空白处的最佳选项,选项中有两项为多余选项。
食品添加剂的备案流程

食品添加剂的备案流程Food additives are substances added to food to improve its flavor, appearance, texture, or shelf life. In order to ensure the safety and quality of food additives, a rigorous registration process is in place.食品添加剂是为了改善食物的味道、外观、口感或保质期而添加的物质。
为了确保食品添加剂的安全性和质量,制定了严格的备案流程。
The registration process for food additives involves submitting detailed information about the additive, including its composition, intended use, and potential health effects. This information is then reviewed by food safety authorities to determine if the additive is safe for consumption.食品添加剂的备案流程包括提交有关添加剂的详细信息,包括其成分、预期用途以及潜在的健康影响。
这些信息随后由食品安全机构进行评审,以确定该添加剂是否安全可食用。
One of the key criteria for the registration of food additives is that they must be safe for human consumption. This involves conductingextensive safety studies to assess the potential risks associated with the additive, including its toxicity, allergenicity, and potential for causing adverse health effects.食品添加剂备案的关键标准之一是必须对人类消费安全。
GMP( 韩国)

5. “Manufacturing” means all services for producing Medical Devices, including packing and labeling.
5.“制造”指生产医疗器械的所有服务项目,包括包装与贴标。
3.“无菌医疗器械”是指在制造过程中经过灭菌消毒,在容器或包装上注明“无菌”字样、灭菌方法、灭菌日期等信息的无菌产品。
4. “Traceability” means check and management of raw materials and components of the product, and the quality control history, distributor, the location, etc. thereof.
8.“建议性通知”指医疗器械分销后制造商发布的器械使用、变更、退货、报废等方面的附加信息或措施。
9. A “Quality Auditor” means a reviewer in the field of medical devices asprovided in the Criteria for Registration of a Quality ManagementSystem Certification Auditor under Article 7 of the「Act on QualityManagement and Safety Management of Industrial Products」andArticle 15 of the「Enforcement Regulations of the IndustrialStandardization Act」, who belongs to a medical device qualitymanagement review agency designated by the KFDA Commissionerand performs quality management review in respect of medicaldevices(hereafter referred to as "Auditor").
江西住建云二级建造师初始注册流程

英文回复:The initial application for registration of the construction builder in Jiangxi is subject to the following steps。
Registered persons are required toplete their registration at the Jiangxi Housingand Urban and Rural Construction Office Global Information Station and, after successful registration, log in to their personal account number,click on the “Personal Information” column, and fill in their basic personal information, including their name, sex, date of birth, unique identification code,connection。
There is a subsequent need to upload certificate photographs, unique identification information photographs,etc。
Uponpletion of the personal information, an on—line fee,registration fees and examination fees are paid。
Once the fee has been paid, the time and place of the examination may be chosen。
看明白新药如何开发溶出方法,才能做好仿制药

看明白新药如何开发溶出方法,才能做好仿制药前言前文依托考昔为例:适度区分力的溶出方法开发与验证指出,任何具有区分力的溶出方法的开发需要以下步骤:设定目标溶出曲线(例如,15min内,溶出<50%,30min溶出>85%);绘制实验流程图,标明预期结果和下一步工作;优化溶出介质、转速、装置和介质体积;验证溶出方法的区分力;验证溶出方法的重复性和耐用性,满足变异要求(即第一个取样点RSD<20%,其他RSD<10%);如对溶出方法的区分力满意,可以不满足漏槽条件。
接下来的这篇文章是对该思路的完美诠释!该文章由达沙替尼片原研公司——百时美施贵宝发表。
该溶出方法开发于达沙替尼片一期临床结束后、二期临床开始前,约在2003年。
该文描述了在新药研发中如何系统的开发溶出方法。
难溶性药物是在研小分子药物的主流。
其仿制药处方工艺开发的难点在于找到“目标曲线”。
谢沐风老师为仿制难溶性药物设计的溶出路线图为:原研如没有一条曲线能在45~120min达到85%,则可适当放宽试验条件(以原研制剂样品批内/批间精密度为准,加大转速或加入低浓度表面活性剂)、且无突释和拐点,这样的曲线通常被认为是最具体内外相关性,也是最理想的曲线。
这是剖析原研,找到原研的那一根“筋”,指导仿制药处方工艺的开发。
达沙替尼(Dasatanib, BMS-354825)是一种强效的酪氨酸激酶多靶点抑制剂。
为继尼洛替尼之后另一个用于伊马替尼耐药和不耐受的慢性粒细胞白血病(CML)慢性期的药物。
与针对Bcr-Abl融合蛋白单靶点的伊马替尼和尼洛替尼相比,达沙替尼属于多靶点药物,对5种关键性致癌酪氨酸蛋白激酶,即BCR-ABL、SRC、c-KIT、PDGFR和Ephrin(EPH)均有作用。
该产品最早由百时美施贵宝公司研发,2006年6月在美国获准上市,11月在欧盟上市。
我国已进口,商品名施达赛?。
国产药品为正大天晴,于2013年上市。
达沙替尼为BCSⅡ类。
昆山招收残疾人的备案流程

昆山招收残疾人的备案流程英文回答:Procedure for Registration of Disabled Persons in Kunshan.Step 1: Eligibility Determination.Determine if the individual meets the eligibility criteria for registration as a disabled person in Kunshan. This includes meeting the definition of disability and being a resident of Kunshan.Obtain a disability certificate from a designated medical institution.Step 2: Application Submission.Complete the application form for registration as a disabled person.Submit the application form along with the following supporting documents:Disability certificate.Proof of identity (e.g., ID card)。
Proof of residence in Kunshan (e.g., household registration book)。
Step 3: Review and Approval.The Civil Affairs Bureau will review the application and supporting documents.The application will be approved if it meets all the eligibility requirements.Step 4: Disability Card Issuance.Upon approval, the Civil Affairs Bureau will issue adisability card to the individual.The disability card serves as proof of disability and entitles the holder to various benefits and services.Step 5: Registration Completion.The registration process is complete once the disability card is issued.Additional Notes:The registration process is free of charge.Individuals who are unable to apply in person can authorize a representative to act on their behalf.The Civil Affairs Bureau may conduct a home visit to verify the applicant's condition.The disability card should be renewed every three years.中文回答:昆山残疾人备案流程。
货物贸易对外支付备案流程

货物贸易对外支付备案流程英文回答:Procedure for Foreign Exchange Registration of Trade-in-Goods Payments.Step 1: Determine the Necessity for Registration.Determine if the trade-in-goods payment requires foreign exchange registration based on the following criteria:The total amount of the goods exceeds the specified threshold.The payment method is other than cash, check, or bank transfer.Step 2: Prepare Required Documents.Gather the necessary documents for registration, including:Trade contract.Invoice.Payment advice.Customs declaration form.Other supporting documents as required.Step 3: Submit Registration Application.Submit the completed registration application form and supporting documents to the designated bank. The application form typically includes information such as:Applicant's information.Trade transaction details.Currency and amount of payment.Payment method.Step 4: Bank Review and Approval.The bank reviews the application and supporting documents for completeness and accuracy. They may request additional information or clarification if necessary. Once the application is approved, the bank will issue a foreign exchange registration certificate.Step 5: Foreign Exchange Payment.Proceed with the foreign exchange payment using the approved registration certificate. Ensure that the payment details align with the information provided in the registration application.Step 6: Post-Transaction Reporting.Within 30 days of making the payment, submit a post-transaction report to the designated bank or authorized dealers. The report should include information about the actual payment made, such as:Amount.Currency.Date of payment.中文回答:货物贸易对外支付备案流程。
bluesign批准的化工产品和工业用物品-蓝标认证中文标准08

bluesign® criteria for bluesign® approved chemical products and articles for industrial use BlueSign®工业用经BlueSign®批准的化学产品和物品标准2 | 5Content 内容1 Purpose 目的3 2 Scope of bluesign® approved trademark 范围bluesign 批准商标 3 3Definitions 定义 3 3.1 Chemical product 化工产品 3 3.2 Chemical supplier 化工产品供应商 3 3.3 Rebrander3 3.4 Manufacturer 制造商 3 3.5 Converter 转换器3 4Requirements for chemical suppliers, manufacturers and converters 化学供应商、制造商和转换器的要求 4 4.1 Chemical supplier 化工产品供应商 4 4.2 Manufacturer 制造商 4 4.3Converter 转换器4 5 Requirements for chemical products and articles 化学制品和物品的要求 4 5.1 Chemical products 化工产品 4 5.2Articles 文章(条款)4 6 System integrity 系统的完整性5 7 Validity 有效性5 8Other applicable documents 其他适用文件53 | 51 Purpose 目的These bluesign® criteria define requirements for bluesign® approved articles and chemical products for industrial use which are placed o n t he m arket. 这些BlueSign®标准规定了投放市场的BlueSign®批准产品和工业用化学产品的要求。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Criteria for Registration ofBoilers, Pressure Vesselsand Pressure Retaining ItemsApproved by the Board of Trustees: August 4, 2009The National Board of Boiler & Pressure Vessel Inspectors1055 Crupper AvenueColumbus, OH 43229-1183Phone: (614)888-8320*Denotes Revised Section(s)NB-264, Revision 8Table of ContentsSubject Page2 Foreword3 Introduction Article 1 Overview Inspection 5Appendix A Authorization to Register 6Appendix B Registration of Pressure Vessels Manufactured to ASMESection VIII, Division 1, and marked with UM Stamp 7Appendix C Registration of Items after Fabrication is Completed 8*FOREW0RDThe National Board does not “approve”, “rate”, or “endorse” any item,construction, proprietary device, or activity.National Board does not take any position with respect to the validity of anypatent rights asserted in connection with any items mentioned in thisdocument, and does not undertake to insure anyone utilizing a code ofconstruction against liability for infringement of any applicable Letters Patent, nor assume any such liability. Users of this document are expressly advisedthat determination of the validity of any such patent rights, and the risk ofinfringement of such rights, is entirely their own responsibility.The above National Board symbols are registered withThe U.S. Patent & Trademark Office“National Board” is the abbreviation for The National Board of Boiler andPressure Vessel Inspectors.No part of this document may be reproduced in any form, in an electronic retrieval system or otherwise, without the prior written permission of the publisher.IntroductionThe purpose of registration is to provide owners, users, and jurisdictionalauthorities charged with public safety with certification by the manufacturers of boilers, pressure vessels and other pressure retaining items that thoseregistered items meet the requirements of a code of construction.In order to register a boiler, pressure vessel or other pressure retaining itemwith the National Board, the following criteria must be met:the boiler, pressure vessel or other pressure retaining item must be manufactured in accordance with the ASME Code or CanadianStandards Association (CSA) B51.The manufacturing organization must implement a quality system.The manufacturing organization must provide for third party inspection as required by the Code of Construction. (Article 1).Registration occurs following manufacturing, testing, inspection, and certification. The manufacturing organization submits the required documentation to the National Board for permanent filing. Documents may be retrieved at any time. An agreement between the manufacturing organization and the National Board is required which is evidenced by a Certificate of Authorization to Register issued by the National Board (Appendix A). The holder of a Certificate of Authorization to Register may then register boilers, pressure vessels and other pressure retaining items with the National Board.Many jurisdictions adopt and enforce laws that regulate the design and construction of boilers, pressure vessels and other pressure retaining items. These laws specify a code of construction that must be met by the manufacturing organization.American Society of Mechanical Engineers (ASME)Accreditation of a manufacturer’s quality program by ASME meets the requirements of this document. Similarly, data reports completed in accordance with Section I, III, IV, VIII, X, or XII of the ASME Code meet the requirements of this document, provided the data report is signed by an Authorized Inspector, when required by the Code.Items such as UM pressure vessel produced to ASME Section VIII, Div. 1, are acceptable for registration with the National Board. In such case, the Certificate of Compliance signed by the manufacturing organization is accepted in lieu of the manufacturer’s data report. The requirements for registration are described in Appendix B.Canadian Standards Association (CSA) B51Acceptance of the manufacturer’s quality program as described in CSA B51 meets the requirements of this document.Article 1 Overview InspectionSection 1 PurposeThe purpose of this article is to establish requirements for third partyinspection.Section 2 ScopeThird party inspection is a process by which boilers, pressure vessels and otherpressure retaining items are inspected during fabrication to determine theirconformity with the code of construction.Boilers, pressure vessels and other pressure retaining items registered with theNational Board must be subject to third party inspection, as provided for inthis article, except as permitted in Appendix B.Section 3 RequirementsA third party inspection agreement must be in force for the duration of theAuthorization to Register with the National Board. Inspectors shall hold a valid National Board Commission and appropriate endorsement. Inspectors shall beindependent from the manufacturing organization.The process for commissioning of Inspectors and their duties andresponsibilities are described in the National Board Rules for CommissionedInspectors.The manufacturing organization must:a.provide for the inspection of all boilers, pressure vessels and otherpressure retaining items to be registered with the National Board; andb.provide the Inspector with access to the manufacturing organization’sfacilities.Appendix A Authorization to RegisterSection 1 ComplianceA manufacturing organization that complies with the requirements of this document is eligible to apply for a Certificate of Authorization to Register with the National Board.Section 2 ValidityThe manufacturing organization’s Certificate of Authorization to Register is valid only while operating under the company name and location listed on the certificate and only until the expiration date listed on the certificate.Section 3 National Board NumberAll boilers, pressure vessels and other pressure retaining items stamped with a National Board number must be registered with the National Board on data reports as described in the Introduction. Improper or unauthorized use of any National Board symbol (or marking) is a violation of the National Board trademark.Section 4 Multiple Codes of ConstructionThe manufacturing organization’s Certificate of Authorization to Register is valid only for the codes of construction identified on the certificate. Additional codes of construction may be added provided the manufacturing organization’s inspection agency reviews the quality system and verifies that the requirements of this document are met. The manufacturing organization shall forward the inspection agency’s verification to the National Board.Appendix B Registration of Pressure Vessels Manufactured to ASMESection VIII, Division 1, and marked with UM StampSection 1 PurposeThe purpose of this Appendix is to establish the requirements for theregistration of pressure vessels manufactured to ASME Section VIII, Division 1,and marked with the UM Stamp (UM vessels).Section 2 ScopeUM vessels are produced by manufacturers in possession of an ASME UMstamp. There is no third party inspection of each vessel, but each vessel is produced in accordance with a quality system that is under the surveillance ofan Authorized Inspection Agency (AIA).Each UM vessel is documented on a certificate of compliance signed by a manufacturer’s representative and a Certified Individual. The National Board number must be recorded in the “Remarks” section of the certificate of compliance for each registered vessel. Nameplates and/or stamping for UM vessels must be in accordance with ASME Code requirements. The NB symbol and the National Board number must be stamped immediately above the required ASME data on each registered vessel.National Board numbers for UM vessels must be assigned by the manufacturer from the same series of numbers used for any other National Board registered items.Section 3 Additional Requirements to be Included in the Manufacturer’sSystemQualityThe following items are required to be included in the manufacturer’s quality system:a. A description of the control and issuance of National Board numbers,including the identity of the individual responsible for the activity.b.A description of the controls for submitting certificates of compliance,including requirements that:1.The certificate is signed by appropriate individual(s).2.An original Certificate of Compliance is submitted to the NationalBoard.3.The time frame in which the document will be submitted to theNational Board is specified.4.The individual responsible for submitting the document to the NationalBoard is identified.Appendix C Registration of Items after Fabrication is CompletedSection 1 PurposeThe purpose of this Appendix is to establish the requirements for theregistration of items after fabrication. Only the original manufacturer mayregister an item after fabrication. The term “original manufacturer” is definedas the original manufacturer or any successor organization that assumes the responsibility on behalf of the original manufacturer and who meets therequirements outlined in Section 3 below. The item may or may not have beenplaced into service.*Section 2 ScopeThe registration of items after fabrication is limited to items built and stampedin accordance with ASME Code or CSA B51 requirements. The preferredmethod of registration is for manufacturers to register items within 60 days of completion of fabrication, while the item is still in the control of the manufacturer. The procedure outlined in this document was created to provide a process for registration of items that were not registered during the original manufacturing process. Each request will be evaluated on a case-by-case basis in order to maintain the value and integrity of the registration program.*Section 3 Procedure3.1ASME Code Compliant StampingThe original nameplate or stamping must be sufficiently legible toidentify all required ASME Code markings.3.2Authorization to RegisterThe manufacturer must possess a valid Certificate of Authorization toRegister with the National Board. In the case of an item built andstamped in accordance with ASME Code, the Certificate of Authorization to Register must include the ASME stamp which appears on the item. 3.3Jurisdictional PrecedenceEvery jurisdiction has specific requirements for the acceptance of items installed for operation in that jurisdiction. Where any provision in thisdocument presents a conflict with any jurisdictional regulation, thejurisdictional regulation governs. It is the responsibility of themanufacturer to contact the jurisdiction where the item is located toensure that jurisdiction will allow operation of the item if it is registered with the National Board using this procedure.Criteria for Registration of Boilers, Pressure Vessels and Other Pressure Retaining Items Appendix C (Cont.)3.4National Board Markings3.4.1 The “NB” stamp and registration number must be added to theoriginal nameplate or stamping, or to a replacement nameplateshowing the original information.3.4.2 The “NB” stamp or a replacement nameplate may be transportedfrom the manufacturers location listed on the “Certificate ofAuthorization to Register” to the location of the item, provided thestamp or nameplate is continuously in the custody of arepresentative of the manufacturer.3.5Approval RequiredA request to “NB” stamp and register the item after fabrication must besubmitted by the manufacturer on form NB-406 (current effectiverevision) and approved by the Executive Director. The Executive Director may require additional supporting documentation in order to evaluatethe request.The request for approval must include NB-406 with Part 1 completed anda copy of the manufacturer’s data report (MDR). The NB-406 mayaddress multiple items if the associated MDR covers multiple items.Otherwise, there shall be a separate NB-406 for each item.If approved,the National Board will return a signed copy of NB-406 to the requester.Following the application of the “NB” stamp and registration number,Part 2 of NB-406 must be completed and submitted to the NationalBoard for registration, along with the “corrected copy” of the original orlegible copy of the MDR.3.6Authorized Inspector’s ResponsibilitiesThe Authorized Inspector (AI) who signed the original MDR for the itemmust accompany the manufacturer’s representative to the location of the item in order to verify the existing stamping with the MDR. The AI must witness application of the “NB” stamp and registration number or areplacement nameplate. If this AI is no longer employed by theAuthorized Inspection Agency (AIA), another AI employed by the sameAIA may perform this function. In the case of an ASME stamped itemwhere the original AIA is a jurisdiction, the application of the “NB” stamp and registration number or a replacement nameplate may be witnessedby an AI employed by the jurisdiction at the location of installation only if that jurisdiction is an ASME accredited AIA.3.7Registration FeeThe National Board will charge a fee for registration after fabrication.Criteria for Registration of Boilers, Pressure Vessels and Other Pressure Retaining Items NB-264, Revision 8 10*Figure -1(a)Stamping RequirementsBoiler or Pressure Vessel Nameplate for ASME and CSA B511 The National Board registration symbol shall be stamped.Notes: 1) Minimum height of lettering and numbering shall be 5/32”.2) The National Board registration symbol (or marking) and National Board number shall appear only on boilers, pressure vessels or other pressure retaining items registered with the National Board. ------------------------------------------------------------------------------------------------------- Stamping in this area must comply with the requirements of the appropriate ASME Code Section or CSA B51, as applicable100000000Form NB-406 Registration after FabricationPart 1 Request (To be completed by Manufacturer)1. Manufactured by ______________________________________________________________________________(name and address)2. Location of installation _________________________________________________________________________(address)3. Item identification (copy of Manufacturer’s Data Report must be attached to this form)Type ____________________________________________ Dimensions __________________________ Mfgr’s serial no. ___________________________________________ Year built _____________________Code stamp ___________ CRN __________________ MAWP ______________________________(if applicable)4. Requested by ____________________________________________________ Date _________________(Quality Manager or other authorized manufacturer’s representative)Stop here and submit to the National Board for approval._________________________________________________________________________________Approved by ________________________________________________________ Date _________________ (National Board Executive Director)_________________________________________________________________________________Part 2 Conclusion (To be completed by Manufacturer and Authorized Inspector)1. National Board Number assigned and stamped on item ___________________2. Attach a photograph, copy or facsimile of the revised nameplate or stamping to this form.I certify that to the best of my knowledge and belief, the statements in this report are correct, and that the information, data, and identification numbers are correct. Attached is a photograph, copy, or facsimile of the revised nameplate or stamping.Name of Manufacturer ____________________________________________________________________________Signature __________________________________________________________ Date _________________National Board Certificate of Authorization to Register expiration date ______________________________________Witnessed by ___________________________________________________________________________________ (name of Authorized Inspector)Employed by ____________________________________________________________________________________ Signature __________________________________________________________ Date _________________ NB Commission No. _______________________________________________ Endorsement __________________ When Part 2 is completed, submit this form with the “corrected copy” of the MDR, the facsimile of the revised nameplate or stamping, and any necessary attachments to the National Board for registration.NB-406 Rev. 1。
