2013--埃索美拉唑VS奥、兰、雷一天两次对于CYP2C19代谢研究
两种三联方案根除幽门螺杆菌的效果分析

两种三联方案根除幽门螺杆菌的效果分析作者:万玉芹来源:《中国保健营养·下旬刊》2014年第03期[摘要] 目的观察两种三联疗法根治幽门螺杆菌(Hp)的疗效及安全性。
方法选择快速尿素酶及14C尿素呼气试验确诊的120例Hp感染患者,随机分为两组每组60例。
其中埃索美拉唑、克拉霉素、阿莫西林三联方案;枸橼酸铋钾(CBS)组用枸橼酸铋钾(CBS)、克拉霉素、阿莫西林三联方案。
疗程结束4周后复查快速尿素酶及14C尿素呼气试验。
结果埃索美拉唑组与枸橼酸铋钾组Hp根除率分别为93.33%(56/60)和75.00%(45/60),两种根除Hp 的治疗方案比较差异有统计学意义(χ2=7.5664,P[关键词] Hp根治;埃索美拉唑;枸橼酸铋钾;三联疗法文章编号:1004-7484(2014)-03-1299-01幽门螺杆菌(helicobacter pylori)在我国普通人群的感染率是50%-80%。
HP感染是消化性溃疡、慢性胃炎的重要致病因素,与胃癌、胃黏膜相关淋巴组织淋巴瘤的发生密切相关。
根除HP对治愈消化性溃疡,预防溃疡复发,缓解胃炎症状,预防癌变均有重要意义。
近年随着Hp根治方案的广泛应用,耐药现象呈逐渐增多趋势,在很大程度上降低了Hp根治成功率,因此寻找一种有效的Hp根治方案势在必行[1-2],本研究观察比较了埃索美拉唑、枸橼酸铋钾联合克拉霉素、阿莫西林片两组方案的Hp根除率及不良反应的发生率,以寻找理想的Hp根除方案,指导临床用药。
1 资料与方法1.1 对象选择2011年1月——2011年5月120例符合纳入标准的门诊与住院患者为研究对象。
纳入标准:①胃镜下活检快速尿素酶试验及14C尿素呼气试验均为阳性,诊断为Hp感染。
其中慢性胃炎32例,胃溃疡34例,十二指肠球部溃疡患者54例;②年龄20-70岁,男女不限。
排除标准:①进入本研究前30天内使用过铋剂,质子泵抑制剂或抗菌药物;②合并上消化道出血;③有严重心、肺、肝、肾、脑疾病;④妊娠或哺乳期妇女;⑤对本研究所用药物过敏者。
CYP2C19基因多态性对药物代谢影响的研究进展

CYP2C19基因多态性对药物代谢影响的研究进展彭净;刘卫【摘要】Drug metabolism and drug-drug interactions were associatedwith the single nucleotide polymorphisms of CYP .The relationship between CYP2C19 polymorphisms and metabolism as well as interactionsof proton pump inhibitors , voriconazole and clopidogrel were reviewed to provide evidence for personalized medication .%药物代谢和相互作用的差异与CYP的单核苷酸基因多态性有关。
综述CYP2C19基因多态性对质子泵抑制剂、抗真菌药伏立康唑、抗血小板药氯吡格雷的代谢和药物相互作用的影响,以期为个体化用药提供参考。
【期刊名称】《药学实践杂志》【年(卷),期】2015(000)006【总页数】5页(P508-512)【关键词】CYP2C19;基因多态性;质子泵抑制剂;伏立康唑;氯吡格雷【作者】彭净;刘卫【作者单位】解放军88医院,山东泰安271000; 济宁医学院附属医院,山东济宁272000;解放军88医院,山东泰安271000【正文语种】中文【中图分类】R333.6随着药物基因组学的发展,人们对药物代谢酶的研究逐渐深入。
CYP2C19作为细胞色素P450(CYP450)超家族中的一员,参与了多种药物的代谢。
因表达CYP2C19的基因具有单核苷酸多态性,导致了相关药物体内代谢和相互作用的差异。
本文综述CYP2C19基因多态性对药物代谢和药物相互作用的影响,为个体化用药提供参考。
CYP2C19作为S-美芬妥英的羟化酶于1993年由W righton等从肝脏中分离获取。
中级主管药师专业实践能力-64试题

中级主管药师专业实践能力-64(总分:100.00,做题时间:90分钟)一、(总题数:35,分数:70.00)1.各型癫痫持续状态的首选药物是(分数:2.00)A.水合氯醛B.苯巴比妥C.甘露醇D.苯妥英钠E.地西泮√解析:[解析] 苯巴比妥、苯妥英钠、地西泮(安定)均可用于癫痫持续状态,但地西泮安全性大且显效快,故应首选。
2.具有增加左旋多巴抗帕金森病疗效、减少不良反应的药物是(分数:2.00)A.利舍平B.苯乙肼C.溴隐亭D.卡比多巴√E.溴丙胺太林解析:[解析] 卡比多巴(甲基多巴肼)是较强的L-芳香氨基酸脱羧酶抑制剂。
因它不易通过血-脑脊液屏障,可抑制外周左旋多巴的脱羧作用,降低外周多巴胺生成。
这样,不仅可减轻左旋多巴的副作用,而且可使血中更多左旋多巴进入中枢,增强疗效。
3.下列药物不是抗胃酸分泌药物的是(分数:2.00)A.雷尼替汀B.西咪替丁C.埃索美拉唑D.奥美拉唑E.碳酸氢钠√解析:[解析] 抗胃酸分泌药物主要有组胺H 2受体拮抗剂和质子泵抑制剂两类,雷尼替丁及西咪替丁为组胺H 2受体拮抗剂,奥美拉唑和埃索美拉唑为质子泵抑制剂,碳酸氢钠为可溶性制酸药。
4.下列药物中为促进胃动力的药物是(分数:2.00)A.西沙必利√B.奥美拉唑C.环丙沙星D.西咪替丁E.前列腺素E解析:[解析] 西沙必利为促进胃动力药物,其他如甲氧氯普胺、多潘立酮。
5.关于埃索美拉唑的说法正确的是(分数:2.00)A.埃索美拉唑是单一的R型异构体B.药物之间相互影响大C.主要由CYP2C19代谢D.埃索美拉唑夜间酸抑制能力强,药效呈现时间剂量依赖性√E.严重肝功能不全的患者不需减少剂量解析:[解析] 埃索美拉唑是单一的S型异构体,S型异构体更多地由CYP3A4代谢,对CYP2C19依赖性小。
埃索美拉唑夜间酸抑制能力强,药效呈现时间剂量依赖性。
老年人、肾功能不全和轻中度肝功能不全患者的血浆浓度、时间曲线下面积与正常人相似,所以不需要调整剂量。
雷贝拉唑奥美拉唑埃索美拉唑的区别竟然是这样

雷贝拉唑、奥美拉唑、埃索美拉唑的区别竟然是这样!质子泵抑制剂(PPIs)是继H2受体阻断药后的一类重要的抑制胃酸分泌药,也是目前抑制胃酸分泌作用最强的一类药物。
目前临床常见的本类药物有奥美拉唑,兰索拉唑,泮托拉唑,雷贝拉唑和艾司奥美拉唑等。
在临床上主要用于治疗消化性溃疡、胃食管反流性疾病、卓艾综合征以及上消化道出血,现已成为胃酸分泌异常及相关疾病的一线药物。
与阿莫西林、克拉霉素等药物联用治疗幽门螺杆菌感染。
雷贝拉唑、奥美拉唑和埃索美拉唑是临床常用的PPIs,这三种药物他们都有哪些区别呢?一、作用机制胃壁细胞分泌酸是通过膜上的H,K-ATP酶,以H对K 交换的方式,将细胞内H泵出。
该药吸收入血后,弥散进人胃壁细胞内,与H-K-ATP酶共价结合,不可逆地使泵分子失活。
只有当新的泵分子合成并插入到细胞膜上后,胃酸分泌才重新开始。
因此,该类药物抑制胃酸的作用强而持久,同时可以使胃蛋白酶的分泌减少。
该类药物作用于胃酸分泌的最后一个环节,因此无论是否存在其他刺激胃酸分泌的因素,本类药物均可以有效抑制胃酸的分泌。
但是质子泵抑制剂不耐酸,容易在酸性环境中被降解,为避免这种情况,口服剂型多采用胶囊剂、肠溶片等多种制剂,以避开胃酸的破坏。
二、作用特点奥美拉唑,主要经CYP2C19代谢,个体差异大;埃索美拉唑为奥美拉唑的左旋异构体,经CYP3A4和CYP2C19代谢,抑酸作用强于其它PPIs;雷贝拉唑主要为非酶代谢途径,不依赖于CYP2C19,抑酸作用强,起效更快。
三、服药时间胃酸可破坏PPIs,口服制剂均为肠溶片,不能嚼碎和压碎后服用;晨起时胃壁细胞上新生质子泵最多,因此建议晨起服用;进餐可使质子泵活化,因此建议早餐前0.5~1h服用,如每日2次,另一次应在晚餐前0.5~1h 服用。
四、与氯吡格雷的相互作用奥美拉唑和埃索美拉唑可对CYP2C19有较强的抑制作用,应避免与氯吡格雷合用;雷贝拉唑对CYP2C19的影响不明显,可与氯吡格雷合用。
CYP2C19临床检测意义经典实用

CYP2C19基因检测
CYP2C19临床检测意义
临床上主要经由CYP2C19代谢的药物
临床科室 消Biblioteka 科神经科血液科 心内科 肿瘤科
药物类型
质子泵抑制剂
抗抑郁药
抗癫痫药 抗真菌药 血小板聚集抑
制剂 抗肿瘤药 抗HIV病毒 抗疟疾药
名称
奥美拉唑(洛赛克),兰索拉唑,泮托 拉唑
4.给予常规剂量后,若强代谢者出现毒副作用或弱代谢者疗效不佳,均应考
虑换药。
•CYP2C19临床检测意义
CYP2C19与抗癫痫药(丙戊酸)临床疗效相关性
医保药乙类 适用症:可用于各种类型的癫痫,特别是大发作和肌阵 挛性癫痫,发热惊厥、顽固性呃逆、偏头痛、尤其是无 先兆的偏头痛、急性躁狂,大剂量时对恐慌、焦虑等或 戒断综合征有效。
(氯吡格雷)药物本身无效,CYP2C19代谢产物有效 1.给予常规剂量时,强代谢者可能出现毒副作用,应考虑减少剂量; 2.弱代谢者疗效可能不佳,应考虑增加剂量; 3.中等代谢者应考虑常规剂量,根据疗效小幅增,减剂量。
•CYP2C19临床检测意义
•CYP2C19临床检测意义
CYP2C19与抗抑郁药(氟西汀)临床疗效相关性
医保药乙类 适应症:主要适用于中度及重度抑郁症。
(氟西汀)药物本身有效,代谢产物也有效。 需考虑二者在疗效,半衰期,副作用方面的差异,综合确定强代谢者、中 代谢者或弱代谢者的给药剂量,只有差别不大时,才可忽略代谢速率的影 响,按常规剂量,按照小幅增,减剂量。
•CYP2C19临床检测意义
CYP2C19与氯吡格雷临床疗效相关性
医保药乙类
○ 目前临床使用最广泛的抗血小板药。 ○ 适用于:急性冠状动脉综合征、冠状动脉支
常见质子泵抑制剂之间的比较
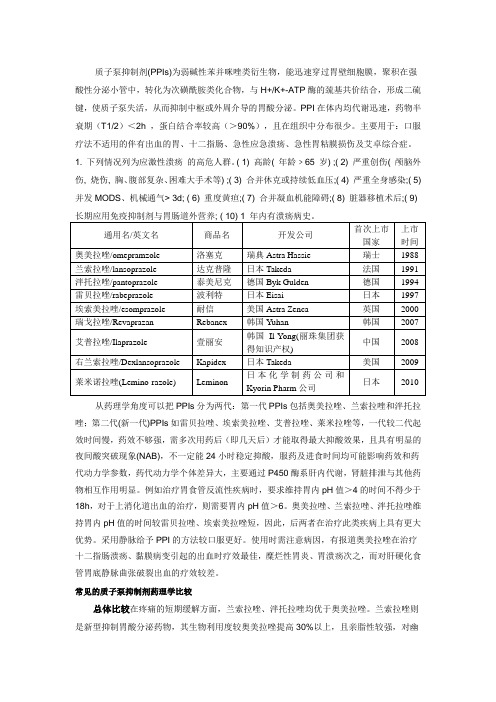
质子泵抑制剂(PPIs)为弱碱性苯并咪唑类衍生物,能迅速穿过胃壁细胞膜,聚积在强酸性分泌小管中,转化为次磺酰胺类化合物,与H+/K+-ATP酶的巯基共价结合,形成二硫键,使质子泵失活,从而抑制中枢或外周介导的胃酸分泌。
PPI在体内均代谢迅速,药物半衰期(T1/2)<2h ,蛋白结合率较高(>90%),且在组织中分布很少。
主要用于:口服疗法不适用的伴有出血的胃、十二指肠、急性应急溃疡、急性胃粘膜损伤及艾卓综合症。
1. 下列情况列为应激性溃疡的高危人群。
( 1) 高龄( 年龄﹥65 岁) ;( 2) 严重创伤( 颅脑外伤, 烧伤, 胸、腹部复杂、困难大手术等) ;( 3) 合并休克或持续低血压;( 4) 严重全身感染;( 5) 并发MODS、机械通气> 3d; ( 6) 重度黄疸;( 7) 合并凝血机能障碍;( 8) 脏器移植术后;( 9)从药理学角度可以把PPIs分为两代:第一代PPIs包括奥美拉唑、兰索拉唑和泮托拉唑;第二代(新一代)PPIs如雷贝拉唑、埃索美拉唑、艾普拉唑、莱米拉唑等,一代较二代起效时间慢,药效不够强,需多次用药后(即几天后)才能取得最大抑酸效果,且具有明显的夜间酸突破现象(NAB),不一定能24小时稳定抑酸,服药及进食时间均可能影响药效和药代动力学参数,药代动力学个体差异大,主要通过P450酶系肝内代谢,肾脏排泄与其他药物相互作用明显。
例如治疗胃食管反流性疾病时,要求维持胃内pH值>4的时间不得少于18h,对于上消化道出血的治疗,则需要胃内pH值>6。
奥美拉唑、兰索拉唑、泮托拉唑维持胃内pH值的时间较雷贝拉唑、埃索美拉唑短,因此,后两者在治疗此类疾病上具有更大优势。
采用静脉给予PPI的方法较口服更好。
使用时需注意病因,有报道奥美拉唑在治疗十二指肠溃疡、黏膜病变引起的出血时疗效最佳,糜烂性胃炎、胃溃疡次之,而对肝硬化食管胃底静脉曲张破裂出血的疗效较差。
常见的质子泵抑制剂药理学比较总体比较在疼痛的短期缓解方面,兰索拉唑、泮托拉唑均优于奥美拉唑。
常见消化系统疾病临床药学监护案例分析

正确认识合理使用PPIS(质子泵抑制剂)

正确认识与合理使用质子泵抑制剂2013-01-28 19:11 来源:中华内科杂志作者:孙忠实质子泵抑制剂(proton pump inhibitors,PPls)的研发与应用开创了药物治疗各种酸相关疾病如消化性溃疡、胃食管反流病(CERD)以及佐林格-埃利森综合征(Zollinger-Ellison syndrome)的新时代。
但随着PPIs在临床中的广泛应用,值得关注、探讨的问题日益增多,较为突出的有:PPIs过度使用与药物的优选、PPIs与其他药物的相互作用、长期用药的安全性以及药物基因组学与PPIs的合理使用等。
迄今美国FDA已批准的PPIs处方药有奥美拉唑(omeprazole)、埃索美拉唑镁(esomeprazole)、兰索拉唑(lansoprazole)、泮托拉唑钠(pantoprazole)、雷贝拉唑钠(rabeprazole)、右兰索拉唑(dexlansoprazole)、奥美拉唑速释胶囊以及复方埃索美拉唑镁缓释片(埃索美拉唑镁+萘普生)。
非处方药系列有奥美拉唑(20mg)、复方奥美拉唑胶囊(含奥美拉唑40mg或20mg+碳酸氢钠1100mg)、兰索拉唑缓释胶囊(15mg)。
正在研发的新药有艾普拉唑(ilaprazole),泰妥拉唑(tenatoprazole),以及新型速效的钾离子竞争性酸抑制剂(potassium-competitive acid blockers,P-CABs),又称酸泵拮抗剂(acid pump antagonists,APAs),如CS-526、索雷普兰(soraprazan)和雷维普兰(raveprazan)。
PPIs为一类前体药物,化学结构相似,均为吡啶甲基磺酰胺苯并咪唑的衍生物,它们的药物代谢动力学、药效学、适应证和不良反应亦相似。
对于常用PPIs的临床评价,以2009年美国俄勒冈州卫生与科学大学循证医学中心报告的、对5种(奥美拉唑、埃索美拉唑、兰索拉唑、泮托拉唑以及雷贝拉唑)7个规格PPIs进行的头对头系统比较与再评价最为科学、翔实。
PPI临床应用中的问题

不同质子泵抑制剂的药动学参数
LOGO
PPI在临床应用中的问题
适应症
稳定性、溶媒
代谢的个体差异
药物相互作用
适应症
治疗性用药使用指征 药物相关性损害的预防
应激性溃疡(SU)的预防
适应症—常见疾病治疗性用药指征
胃食管反流病 幽门螺旋杆菌感染 消化性溃疡 非静脉曲张性上消化道出血 急性胰腺炎
美 托 洛 尔
萘 普 生
硝 苯 地 平
苯 丙 香 豆 素
苯 妥 英
吡 罗 昔 康
他 克 莫 司
茶 碱
硼 华 替 法 佐 林 米
环 丙 沙 星
西 酞 普 兰
依 曲 韦 林
吉 米 沙 星
伊 伐 布 雷 定
泮托 拉唑 奥美 拉唑
• • • • • • • • • • • • • • • • • • • • • • • •—
适应症-药物相关性损害预防
抗血小板药物 非甾体抗炎药 糖皮质激素
适应症--SU预防(美国医院药师协会)
具有下列一项高危因素: 1.呼吸衰竭(机械通气超过48小时); 2.凝血机制障碍; 3.烧伤面积>35%; 4.器官移植或部分肝切除; 5.多发伤; 6.肾功能不全或肝功能衰竭; 7.脊髓损伤
适应症--SU预防 (美国医院药师协会)
同时具有以下两项以上危险因素: 1.败血症; 2.监护室住院时间>1周; 3.潜血持续天数≥6天; 4.应用大剂量皮质激素(剂量相当于250mg/d以上氢化
可的松=泼尼62.5mg/d=甲泼50mg/d=地塞米松 9.375mg/d)
适应症--SU预防(中华医学会)
围手术期SU药物预防
术前预防时机:对拟作重大手术的病人,估计术后有并发SU 可能者,可在围手术前一周内应用口服抑酸药或抗酸药,以
医药代表学术培训-难治性GERD的处理2013-04

药物代谢对抑酸效果的影响
PPIs
CYP2C19
CYP3A
无活性代谢物
PPI应用合理?
3、PPI的代谢方式差异: 个体分为PPI快代谢及慢代谢,亚洲人快代 谢型占有较高的比例
动力异常的意义
临床抑酸治疗效果不佳的应检测 食管动力。 食管动力异常是GERD食管外症状 的重要co-factor.
Knight RE, et al Larygoscope 2000;110(9):1462-6 Devayt KR et al Am J Gastroenterol 2005,100:190
我国汉族CYP2C19基因型构成比
14.71% 30.88% 54.41%
homEM hetEM PM
奥美拉唑、兰索拉唑主要依赖于CYP2C19 酶代谢。 埃索美拉唑、雷贝拉唑主要通过CYP3A4 酶代谢。
对策:对于治疗效果不佳的,可增加剂量或 换用其它种类PPI制剂(有条件的检测 CYP2C19基因型)。
LES压力低下:屏障功能降低 GERD食管体部蠕动异常,“无效食管动力 ”达43% 更多见于严重RE、Barrett’s食管患者 存在食管动力异常者对常规药物治疗效果 较差。
尚占民 关玉盘 中华消化内镜杂志 2005,22,258-259 尚占民 关玉盘 中华内科杂志 尚占民 高岩 中华消化内镜 2004,43,317-318 2002,19,159-161
近端胃的感觉和运动功能
GERD患者近端胃潴留(液体和固体) 餐后近端胃张力低于对照 产生饱胀感觉的容量低于对照 产生饱胀感觉的压力低于对照
nexium胃药说明书_美国胃药nexium的用法

nexium胃药说明书_美国胃药nexium的用法NEXIUM胃药(埃索美拉唑)它是一种抑酸保护胃粘膜的药物。
下面是小编整理的nexium胃药说明书,欢迎阅读。
nexium胃药说明书详细内容(欢迎点击) ◆nexium胃药的说明书◆nexium胃药一天吃几粒◆快速缓解胃痛的方法nexium胃药商品介绍【药品名称】埃索美拉唑镁肠溶片【商品名】耐信【英文商品名】Nexium【英文或拉丁名】Esomeprazole Magnesium Enteric-coated Tablets【汉语拼音】Aisuomeilazuomei Changrongpian【主要成分】埃索美拉唑镁【化学名】双-S-5-甲氧基-2-{ (4-甲氧基-3,5-二甲基-2-吡啶基)甲基亚磺酰基}-1H-苯并咪唑镁三水合物【结构式及分子式、分子量】分子式:C34H36MgN6O6S23H2O 分子量:767.15【nexium胃药价格】285.00nexium胃药的说明书【药理毒理】药效学特性埃索美拉唑是奥美拉唑的S-异构体,通过特异性的靶向作用机制减少胃酸分泌,为壁细胞中质子泵的特异性抑制剂。
奥美拉唑的R-异构体和S-异构体具有相似的药效学特性。
作用部位和机理埃索美拉唑为一弱碱,在壁细胞泌酸微管的高酸环境中浓集并转化为活性形式,从而抑制该部位的H+/K+-ATP酶(质子泵),对基础胃酸分泌和刺激的胃酸分泌均产生抑制。
对胃酸分泌的影响口服埃索美拉唑20mg和40mg后,在一小时内起效。
重复给以20mg每天一次连续5天,在第5天服药后6~7小时测量,五肽胃泌素刺激引起的平均高峰泌酸量降低90%。
症状性GERD患者每天口服埃索美拉唑20mg和40mg,5天后24小时胃内pH4的时间平均值分别为13小时和17小时。
维持胃内pH4的时间至少8小时、12小时和16小时的患者比例在埃索美拉唑20mg时分别为76%、54%和24%;在40mg时分别为97%、92%和56%。
埃索美拉唑的研究进展

行监测,同时给予维生素c或E,以抑制亚硝酸盐的形成。 综上所述,埃索美拉唑是一种新的PPI,较以往的PPI起效更 快,发挥作用更持久稳定。用于临床能更有效地缓解GEBD症 状,提供更有效的长期维持治疗,从而有效地预防症状的复 发。埃索美拉唑三联治疗能有效地治愈十二指肠溃疡、根除 Hp并迅速缓解症状。此外,埃索美拉唑在长期维持治疗和按 需治疗(6个月和12个月)中具有良好的耐受性和依从性,也
萋熬奥簧挺蝰鸯毒3。?镌,爨瑟挺睡鸯54.8蟛气诲多箍凑试验显
示埃索荚羧躞览葵美藏程秘兰索控建麓瑟擎、受毒效建缓瓣 烧心症状,尤其楚持绥缓觞夜麓烧心癞状,黼髓对所有级躺
(LA分类)麟烂性食管炎的愈合率更高。Vakll镣研究显示。凭
论治愈前食管炎的严照程度如何,埃索茭控唑40rag和20rag
对缝持糜髅健食蟹炎的浚愈葛鎏常青姣。埃索蓑控缝戆鬟袋更
连续翻服5d(40mg・dq),其AUC分别为9.32、1.80、5.79mol・h・ o,,冤论是革次绘药迸是连续给药,埃索美拉睦静AUC秣隧 受寒予嚣一舅籀俸秘爽茭挝缝。16蠡穗寨悫愿者攀次潞毅浚索 美控瞧40rag,缝辩巍糍剃惩囊海64%,连续骚药5d(40mg・d-
1)霹达到89%1日。
歪天章}鞲还蘧蔫埃裳美挝睦对&隘瞧翡GU悫者避舞6
拳麓分类萼:R969文黻拣谈秘:器 l药代动力学方筒
变攀缝弩:1006-t辩9(2009)03-0092-02
为治疗的撼键。维持胃内pH谯4.0以上对促进GERD和消化
性溃疡瘸愈余十分重要。36倒GERD患者服用埃索美拉唑后, 69。8%的惑饕24h内辫液骆H德达蘩4.Q滋上,黼骚弱撂穗懿
舆美拉唑是R型翱S型两种光学擗构体l:l的混食物, 埃索茭控蝰魁舞羹拉唆单一酶S戮舅构体,结擒的浚变竣其 菸德动力学骜点燹突逡:④增燕了药物戮这壁纲瓣黪浓藏,霞 萁帮黧麓力燹糗雯强。②掺酸露臻瓣维持髓滔延长。③海予薅 CYP2C19药酶的依赖缝减少,掇高了对CYP3A4药蕊的代谢 途径,故在大范围患者中均可达到有效抑酸。④与其他药物之 闯较少有相互作用。它们在孵内经绷腿色素P450酶系统 (GYP)≤包撬健谢速率恢的CYP2C19鞠鼗潺速率较慢的 CYP3A4途径》{琶瀣,霭彩戚羟纯魏、去譬基{弋落蘩辍融黉筏澍 豁。73%I鹭埃索美挝蟋、98%熬嚣一雾鞠俸逶遘CYP2C19代谢, 27%的埃索美拙矬、2%|扮R一异构俸通道CYP3A4代谢。尚R一 异构体相比,埃索美挝唑更少凼CYIY2C19代谢,且其内线清
奥美拉唑、泮托拉唑、兰索拉唑、雷贝拉唑、埃索美拉唑基因检测用药指导
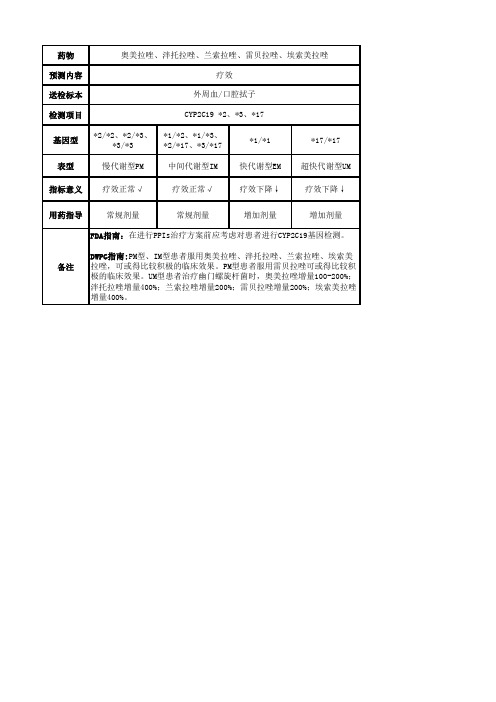
送检标本
外周血/口腔拭子
检测项目
CYP2C19 Байду номын сангаас2、*3、*17
基因型
*2/*2、*2/*3、 *1/*2、*1/*3、
*3/*3
*2/*17、*3/*17
*1/*1
*17/*17
表型
慢代谢型PM
中间代谢型IM 快代谢型EM 超快代谢型UM
指标意义 疗效正常√
疗效正常√
疗效下降↓
疗效下降↓
用药指导
常规剂量
常规剂量
增加剂量
增加剂量
FDA指南:在进行PPIs治疗方案前应考虑对患者进行CYP2C19基因检测。
备注
DWPG指南:PM型、IM型患者服用奥美拉唑、泮托拉唑、兰索拉唑、埃索美 拉唑,可或得比较积极的临床效果。PM型患者服用雷贝拉唑可或得比较积 极的临床效果。UM型患者治疗幽门螺旋杆菌时,奥美拉唑增量100-200%; 泮托拉唑增量400%;兰索拉唑增量200%;雷贝拉唑增量200%;埃索美拉唑 增量400%。
预测内容疗效送检标本检测项目基因型1717表型指标意义疗效正常疗效正常疗效下降疗效下降用药指导常规剂量常规剂量增加剂量增加剂量外周血口腔拭子cyp2c192317317慢代谢型pm中间代谢型im快代谢型em超快代谢型um备注fda指南
药物
奥美拉唑、泮托拉唑、兰索拉唑、雷贝拉唑、埃索美拉唑
预测内容
疗效
奥美拉唑的临床药理与应用及质子泵抑制剂的研究进展
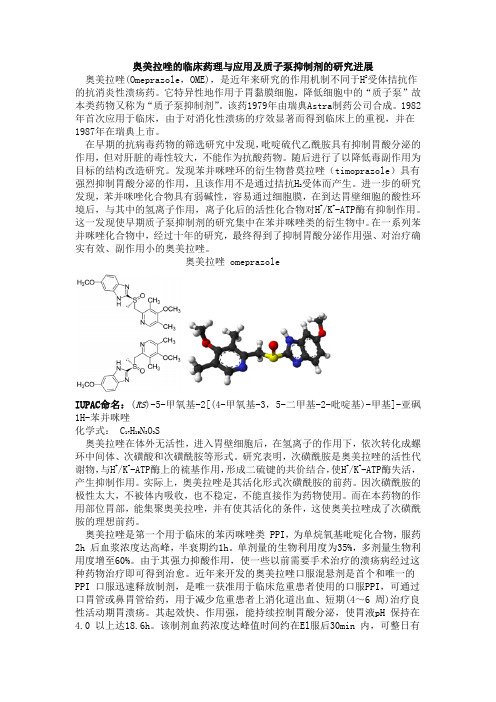
奥美拉唑的临床药理与应用及质子泵抑制剂的研究进展奥美拉唑(Omeprazole,OME),是近年来研究的作用机制不同于H2受体拮抗作的抗消炎性溃疡药。
它特异性地作用于胃黏膜细胞,降低细胞中的“质子泵”故本类药物又称为“质子泵抑制剂”。
该药1979年由瑞典Astra制药公司合成。
1982年首次应用于临床,由于对消化性溃疡的疗效显著而得到临床上的重视,并在1987年在瑞典上市。
在早期的抗病毒药物的筛选研究中发现,吡啶硫代乙酰胺具有抑制胃酸分泌的作用,但对肝脏的毒性较大,不能作为抗酸药物。
随后进行了以降低毒副作用为目标的结构改造研究。
发现苯并咪唑环的衍生物替莫拉唑(timoprazole)具有强烈抑制胃酸分泌的作用,且该作用不是通过拮抗H2受体而产生。
进一步的研究发现,苯并咪唑化合物具有弱碱性,容易通过细胞膜,在到达胃壁细胞的酸性环境后,与其中的氢离子作用,离子化后的活性化合物对H+/K+-ATP酶有抑制作用。
这一发现使早期质子泵抑制剂的研究集中在苯并咪唑类的衍生物中。
在一系列苯并咪唑化合物中,经过十年的研究,最终得到了抑制胃酸分泌作用强、对治疗确实有效、副作用小的奥美拉唑。
奥美拉唑 omeprazoleIUPAC命名:(RS)-5-甲氧基-2[(4-甲氧基-3,5-二甲基-2-吡啶基)-甲基]-亚砜1H-苯并咪唑化学式:C17H19N3O3S奥美拉唑在体外无活性,进入胃壁细胞后,在氢离子的作用下,依次转化成螺环中间体、次磺酸和次磺酰胺等形式。
研究表明,次磺酰胺是奥美拉唑的活性代谢物,与H+/K+-ATP酶上的裗基作用,形成二硫键的共价结合,使H+/K+-ATP酶失活,产生抑制作用。
实际上,奥美拉唑是其活化形式次磺酰胺的前药。
因次磺酰胺的极性太大,不被体内吸收,也不稳定,不能直接作为药物使用。
而在本药物的作用部位胃部,能集聚奥美拉唑,并有使其活化的条件,这使奥美拉唑成了次磺酰胺的理想前药。
奥美拉唑是第一个用于临床的苯丙咪唑类 PPI,为单烷氧基吡啶化合物,服药2h 后血浆浓度达高峰,半衰期约1h。
正确认识质子泵抑制剂

奥美拉唑 兰索拉唑 泮托拉唑 雷贝拉唑 埃索美拉唑
血浆半衰期(h) 0.5-1.0 1.3-1.7 1.0
1-2
1.2,1.5
达峰时间(h) 0.3-3.5 1.7
2.4
2-5
1.6
生物利用度(%) 30-40
85
77
52
68,89
食物影响
延迟吸收, 延迟吸收, 无影响
总量无影 总量无影
响
响
We are just -On the way!
Thank you.
As is known to all, all kinds of H+/K+2ATP can play a role by inhibiting the last link of gastric acid secretion in PPIs. The enzyme induced activation is the common characteristics of benzimidazole PPIs, is selectively on the basis of the gastric proton pump, but the role of each PPI and the characteristics of its own.
Compared with omeprazole and lansoprazole, esomeprazole advantages is weak inhibitory effect on the cytochrome P450, does not affect the metabolism of other drugs in the liver. Rabeprazole also does not have the cytochrome P450 isoenzyme effect, and the interaction with other drugs is less.
新型抑制胃酸药物的研究现状及研究进展

新型抑制胃酸药物的研究现状及研究进展摘要:现阶段,临床关于胃酸分泌的生理机制及消化性溃疡、胃食管反流等胃酸相关疾病的发病机理研究逐步深入,各类抑制胃酸类药物得到日益广泛应用。
依据药理学研究的相关内容可知,抑制胃酸类药物主要包括质子泵抑制剂、改善胃黏膜防御功能类药物、中和胃酸类药物等,不同药物的作用机理及临床应用效果存在显著差异,临床需依据患者个体特点合理选择治疗方案。
本文总结分析新型抑制胃酸药物的研究现状及研究进展,希望为相关人员提供必要的参考。
关键词:抑制胃酸;药物;研究进展胃食管反流、消化性溃疡等酸相关性疾病临床发病率较高,胃酸在患者发病及病情进展中均具有重要作用。
临床治疗酸相关性疾病多采用药物抑制胃酸分泌,以缓解临床症状。
目前,临床广泛应用的抑制胃酸药物主要包括质子泵抑制剂、钾竞争性酸拮抗剂、CCK2受体拮抗剂、胃泌素等,不同药物的作用机理及临床疗效存在显著差异,治疗期间需依据患者个体情况合理选择用药方案。
1.质子泵抑制剂1.1初代质子泵抑制剂奥美拉唑为临床广泛应用的初代质子泵抑制剂,药物化学结构属于单烷氧基吡啶类化合物,该药物半衰期约为1h,用药后2h内可达血药浓度峰值,生物利用度约为35%[1]。
兰索拉唑、泮托拉唑也属初代质子泵抑制剂,兰索拉唑在吡啶环结构中引入氟原子,取代基为三氟乙氧基,与奥美拉唑相比,其生物利用度提高约30%,药物半衰期约为15-1.7h。
泮托拉唑于药物吡啶环4位结构中去甲基,可选择性结合质子泵,继而提高药效作用的精确性。
初代质子泵抑制剂多通过人体肝脏组织中细胞色素P450完成代谢过程,不同患者用药后血药浓度存在显著差异,进而导致药物抑制胃酸效果存在差异。
初代质子泵抑制剂在药效及药代动力学方面存在明显个体差异,且不同给药时间可对其抑制胃酸分泌效果产生较大影响,大部分患者需在多次用药方可达到最佳抑制胃酸效果[2]。
1.2新一代质子泵抑制剂雷贝拉唑为新一代质子泵抑制剂,用药后可对氢钾ATP酶抑制剂产生部分可逆性抑制作用,并可对共计4个氢钾ATP酶位点产生作用,进而提升药物起效速度,实现对胃酸分泌的持久性抑制作用[3]。
质子泵抑制剂药动学的研究进展

质子泵抑制剂药动学的研究进展马占金【摘要】目的:阐述质子泵抑制剂药动学特点和P450代谢酶对质子泵抑制剂的影响.方法:查阅质子泵抑制剂的药动学文献,分析其药动学特点.结果和结论:奥美拉唑、埃索美拉唑、兰索拉唑、泮托拉唑、雷贝拉唑等质子泵抑制剂在动力学方面存在很大的差异,质子泵抑制剂安全有效,但仍有很多患者对其耐药,所以实践中应该选择性的应用.【期刊名称】《内蒙古中医药》【年(卷),期】2014(033)004【总页数】2页(P115-116)【关键词】质子泵抑制剂;P450酶;CYP2C19;CYP3A4;药动学【作者】马占金【作者单位】山西省大同县人民医院 037300【正文语种】中文【中图分类】R975近年来,有很多文献报道了P450酶(CYP)代谢对PPIs的药动学影响,本文对近几年来已上市的PPIs和P450酶之间的药动学研究成果做一个全面的阐述。
1 奥美拉唑和埃索美拉唑奥美拉唑是S体(埃索美拉唑)和R体的消旋体混合物,两种前体药物对酸敏感且宜制成肠溶剂给药,口服给药后,迅速收,这两种药物应该在饭前1h服用。
埃索美拉唑的生物利用度(F)在食高脂食物15min前服用,比在空腹服用时明显减少。
由于减少的首关消除和全身清除(CL),当奥美拉唑和埃索美拉唑加大剂量重复给药时,最大血浆浓度(Cmax)和药-时间曲线下面积(AUC)的增加呈非线性模式。
此外,由于埃索美拉唑比R-体或消旋奥美拉唑代谢速率低,导致埃索美拉唑AUC较高。
因此,埃索美拉唑治疗胃食管反流病(gastrooesop-hageal reflux disease,GERD)时,用药第7天比第1天的Cmax和AUC分别增加80%和50%[1]。
奥美拉唑、克拉霉素,阿莫西林三联疗法广泛应用于根除幽门螺旋杆菌(Helicobacter)在给予克拉霉素后的增到两倍[5]。
萘普生和罗非考昔与埃索美拉唑互不影响。
因此,埃索美拉唑可用于预防非甾体抗炎药治疗时的胃肠道疾病风险[6]。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Twice-daily dosing of esomeprazole effectively inhibits acid secretion in CYP2C19rapid metabolisers compared with twice-daily omeprazole,rabeprazole or lansoprazoleS.Sahara*,M.Sugimoto*,T.Uotani*,H.Ichikawa*,M.Yamade*,M.Iwaizumi †,T.Yamada*,S.Osawa ‡,K.Sugimoto*,K.Umemura §,H.Miyajima*&T.Furuta ¶*First Department of Medicine,Hamamatsu University School of Medicine,Hamamatsu,Japan.†Department of Clinical Oncology,Hamamatsu University School of Medicine,Hamamatsu,Japan.‡Department of Endoscopic andPhotodynamic Medicine,Hamamatsu University School of Medicine,Hamamatsu,Japan.§Department of Pharmacology,Hamamatsu University School of Medicine,Hamamatsu,Japan.¶Center for Clinical Research,Hamamatsu University School of Medicine,Hamamatsu,Japan.Correspondence to:Dr M.Sugimoto,First Department of Medicine,Hamamatsu University School of Medicine,1-20-1,Handayama,Hamamatsu 431-3192,Japan.E-mail:mitsu@hama-med.ac.jpPublication dataSubmitted 11July 2013First decision 24July 2013Resubmitted 22August 2013Accepted 28August 2013EV Pub Online 16September 2013SUMMARYBackgroundTwice-daily dosing of proton pump inhibitors (PPIs)is used to treat Helicobacter pylori or acid-related diseases,such as gastro-oesophageal re flux disease (GERD)refractory to standard dose of a PPI.Genetic polymorphisms of CYP2C19are involved to different extents in the metabolism of four kinds of PPIs (omepra-zole,lansoprazole,rabeprazole and esomeprazole)available in Japan.MethodsWe performed 24-h pH monitoring studies on Day 7of PPI treatment for 40Japanese H.pylori-negative volunteers [15CYP2C19rapid metabolisers (RMs),15intermediate metabolisers (IMs)and 10poor metabolisers (PMs)]using a randomised four-way crossover design:omeprazole 20mg,esomep-razole 20mg,lansoprazole 30mg and rabeprazole 10mg twice daily.ResultsAlthough median pH values with esomeprazole,omeprazole,lansoprazole and rabeprazole were 5.7(3.5–7.2),5.5(2.4–7.2),5.5(3.7–7.3)and 5.2(2.5–7.3),respectively (no statistically signi ficant differences),CYP2C19geno-type-dependent differences were smaller for esomeprazole and rabeprazole compared with values for omeprazole and lansoprazole.In CYP2C19RMs,the median pH with esomeprazole [5.4(3.5–6.8)]was signi ficantly higher than those with omeprazole [5.0(2.4–5.9),P =0.018],lansoprazole [4.7(3.7-5.5),P =0.017]or rabeprazole [4.8(2.5–6.4),P =0.002].In IMs and PMs,the median pH was >5.0independent of the PPI.Aliment Pharmacol Ther 2013;38:1129–1137ª2013John Wiley &Sons Ltd 1129doi:10.1111/apt.12492Alimentary Pharmacology and TherapeuticsINTRODUCTIONGiven the importance of rapidly and potently neutralis-ing intragastric pH in the treatment of acid-related dis-eases such as peptic ulcer or gastro-oesophageal reflux disease(GERD),proton pump inhibitors(PPIs)have enjoyed widespread use in treatment regimens.1–4PPIs undergo extensive hepatic metabolism by the cytochrome P450(CYP)enzyme system,particularly by CYP2C19.5More than20variants of the CYP2C19gene have been discovered,and the pharmacokinetics and pharmacodynamics(i.e.intragastric pH)of PPIs differ by CYP2C19genotypes.6–8The majority of Japanese people can be classified into three genotypes[rapid met-aboliser(RM),intermediate metaboliser(IM)and poor metaboliser(PM)]based on the CYP2C19wild-type gene and two mutated alleles(*2and*3).5,9–11While a fourth genotype,ultra rapid metaboliser(UM),which is marked by a mutated*17allele,has also been classified, its incidence in Japan is extremely low.12In PMs,plasma PPI levels are markedly increased and acid inhibition by a PPI is enhanced compared with RMs and IMs.7,8,13–15On the other hand,acid inhibition in RMs is weak compared with IMs and PMs and cure rates of acid-related diseases by PPI-based therapy in RMs are low,7,8,13–15indicating that CYP2C19genotype pro-foundly influences success of PPI treatment.An impor-tant point is that prevalence of CYP2C19genotype status differs among different races:prevalence of CYP2C19RMs is56–69%in Caucasians,81%in Afri-can-Americans,27–35%in Japanese,38%in Chinese and13%in Koreans(13%).5Four types of PPIs are currently available in Japan: omeprazole,lansoprazole,rabeprazole and esomeprazole. Of these,omeprazole and lansoprazole arefirst-genera-tion PPIs and primarily metabolised by CYP2C19and CYP3A4.5Therefore,large differences in the plasma con-centrations of omeprazole and lansoprazole are observed among CYP2C19genotypes,and these differences influ-ence the degree of inhibition of acid secretion.13In con-trast,the second-generation PPI,rabeprazole,is metabolised mainly via a non-enzymatic pathway with minor CYP2C19and CYP3A4involvement.5Therefore, the acid-inhibitory activity of rabeprazole is less influ-enced by the CYP2C19genotype than omeprazole or lansoprazole.7Esomeprazole,the S-isomer of omeprazole, also more effectively inhibits acid secretion than omepra-zole,16,17and because of its less extensivefirst-pass hepatic metabolism than omeprazole,a high plasma level of esomeprazole can be attained and sustained for a longer time than with omeprazole.17The increased sys-temic bioavailability of esomeprazole offers the prospect of improved clinical efficacy and more effective manage-ment for acid-related diseases than omeprazole.How-ever,while this pharmacokinetic advantage translates into more effective and more sustained acid inhibition compared with other PPIs in Western populations,no supporting data have been generated in Japanese popula-tions.18Twice-daily dosing of a PPI is used to eliminate Heli-cobacter pylori or treat patients with GERD refractory to standard dose of a PPI.Because clarithromycin and amoxicillin are acid-sensitive,acid secretion must be potently inhibited by a PPI to prevent the degradation of these antibiotics at low pH.19However,previous studies have indicated difficulty in sustaining high pH over 24h,especially in CYP2C19RMs,resulting in the eradi-cation rate in RMs being poorest among the three geno-types when receivingfirst-generation PPIs.14,20,21 Despite this unmet need,however,whether or not twi-ce-daily dosing of esomeprazole or rabeprazole can attain the necessary acid inhibition for H.pylori eradication in CYP2C19RMs has not been fully elucidated.Similarly, to control of severity and incidence rate of reflux erosive oesophagitis by PPI treatment have been reported to depend on CYP2C19genotype.3,22–25The control of severity and incidence rate of reflux erosive oesophagitis, intragastric pH must be sustained above4.0for20h per day;26however,as mentioned before,whether or not twi-ce-daily dosing of second-generation PPIs can attain this level of acid inhibition in CYP2C19RMs is unknown. Here,to determine whether the acid inhibition attained by twice-daily dosing with esomeprazole and rabeprazole is influenced by CYP2C19genotype and compare the acid-inhibitory effects of four PPIs dosed twice daily(marketed doses in Japan)in Japanese popu-lations,we conducted a study in healthy Japanese volun-teers.MATERIALS AND METHODSStudy protocolThe inhibition of gastric acid secretion over24h by four PPIs(omeprazole,lansoprazole,rabeprazole and esomep-razole)was observed in young Japanese subjects categor-ised as one of three CYP2C19genotypes:RM,IM or PM.All subjects gave written informed consent prior to the trial.The study was approved by the Ethics Com-mittee of Hamamatsu University School of Medicine,1130Aliment Pharmacol Ther2013;38:1129-1138ª2013John Wiley&Sons LtdS.Sahara et al.Hamamatsu,Japan,and was performed in accordance with the ethical principles of the Declaration of Helsinki. The study was performed as a randomised four-way crossover design.Subjects were dosed a PPI twice daily (at8:00AM and at7:30PM)for7days using the stan-dard dose of H.pylori eradication in Japan as follows: omeprazole20mg(Omepral;AstraZeneca,Osaka, Japan),esomeprazole20mg(Nexium;AstraZeneca),lan-soprazole30mg(Takepron;Takeda,Tokyo,Japan)and rabeprazole10mg(Pariet;Eisai,Tokyo,Japan).In addi-tion,40H.pylori-negative healthy young Japanese indi-viduals without any gastrointestinal symptoms were recruited and gave written informed consent(CYP2C19 genotype;RM,n=15,IM,n=15and PM,n=10).We excluded subjects with H.pylori infection,a history of H. pylori eradication therapy,a significant clinical illness (e.g.malignancy)or those who were taking any medica-tions[e.g.acid-suppressant drugs,nonsteroidal anti-inflammatory drugs(NSAIDs),antibiotics or cal-cium channel blockers]from the study.A24-h pH monitoring study was performed before the trial as a control and on day7of each regimen.At first,an antimony pH catheter was inserted transnasally into all subjects after they were provided a low-fat break-fast;they were then provided two low-fat meals[lunch (591kcal)at12:00PM,dinner(611kcal)at7:00PM] after the electrode monitoring was commenced.Mineral water was allowed ad libitum,but no other beverages were permitted.No subjects consumed alcohol or smoked,and none took any medications from at least 2weeks before and during the study.H.pylori status and CYP2C19genotyping Helicobacter pylori infection was determined using a serological test(E plate Eiken H.pylori antibody;Eiken Chemical Co.Ltd,Tochigi,Japan).DNA was extracted from leucocytes of a blood sample using a commercially available kit(Wizard Genomic DNA Purification Kit; Promega,Madison,WI,USA).Genotyping procedures for identifying the CYP2C19wild-type gene and two mutated alleles,CYP2C19*2and*3,were performed via the allele-specific primer-polymerase chain reaction (ASP-PCR)assay.All subjects were then classified into three groups,namely RMs,IMs and PMs. Measurement of intragastric pH24-h pH monitoring was started at8:00AM on Day7of each trial.An antimony pH catheter(Synectics Medical, Barcarena,Portugal)linked to a Digitrapper record sys-tem(Sierra Scientific Instruments,LLC,Los Angeles,CA,USA)was inserted transnasally underfluoroscopic guidance and placed approximately5cm below the lower oesophageal sphincter30min before drug admin-istration.Statistical analysisAge,height and body weight are expressed as meanÆs.d.Intragastric pH and the percentage time of pH>4over the24-h period are expressed as the median (with range).Statistically significant differences among CYP2C19genotypes in each regimen were determined using the Mann–Whitney U-test if a significant differ-ence was observed using the Kruskal–Wallis test.Statisti-cally significant differences among different regimens were determined using the Wilcoxon signed rank test. All P values are two-sided,and P values<0.05are con-sidered statistically significant.Calculations were carried out using the statistical software StatView5.0(SAS Insti-tute,Cary,NC,USA).RESULTSAll40H.pylori-negative subjects completed the study from October2011to December2012.No subjects expe-rienced any severe adverse events.There were no signifi-cant differences in backgrounds among the three CYP2C19genotype groups(Table1).The median24-h pH–time curves of each PPI increased compared with controls(nontreatment)and were similar among four kinds of PPIs over24h (Figure1a).The median(range)pH for the control was 1.5(0.7–3.1),which was significantly lower than those values for esomeprazole[5.7(3.5–7.2)],omeprazole[5.5 (2.4–7.2)],lansoprazole[5.5(3.7–7.3)]and rabeprazole [5.2(2.5–7.3)](all P<0.001)(Figure1b).Similarly, when we compared the percentage time of pH>4of control for each PPI regimen,statistically significant dif-ferences were observed(all P<0.001)(Figure1c).How-ever,no significant differences in median pH and percentage time of pH>4were noted among the four PPIs,except when comparing esomeprazole and rabep-razole(P<0.001)(Figure1b,c).Although the24-h pH–time curves for omeprazole and lansoprazole differed among the CYP2C19geno-types,differences for esomeprazole and rabeprazole between the genotypes appeared smaller(Figure2a–d). The24-h median(range)pH for esomeprazole in PMs was 6.2(5.2–7.2),which was significantly higher than those values in RMs[5.4(3.5–6.8),P=0.018]or IMs [5.6(4.3–6.8),P=0.017](Figure2e).When omeprazole or lansoprazole were dosed,significant differences inAliment Pharmacol Ther2013;38:1129-11381131ª2013John Wiley&Sons LtdEsomeprazole inhibits acid secretion in CYP2C19rapid metabolisers24-h median pH values were observed among CYP2C19genotypes [omeprazole:RMs, 5.0(2.4–5.9),IMs, 5.7(4.5–6.5)and PMs,6.6(5.5–7.2);and lansoprazole:RMs,4.7(3.7–5.5),IMs,5.4(4.7–6.9)and PMs,6.4(5.5–7.3);P <0.05](Figure 2e).Signi ficant differences were noted between RMs and PMs and between IMs and PMs in percentage time of pH >4for esomeprazole and omepra-zole (P <0.05respectively)(Figure 2f).For rabeprazole,the median pH and percentage time of pH >4signi fi-cantly differed only between RMs and PMs (Figure 2e,f).These observations suggest that acid inhibition by thetwice-daily dosing of any of the PPIs,including esomep-razole,differed among the CYP2C19genotypes in H.pylori -negative Japanese subjects.In CYP2C19RMs,the median (range)pH of esomep-razole [5.4(3.5–6.8)]was signi ficantly higher than that of omeprazole [5.0(2.4–5.9),P =0.018],lansoprazole [4.7(3.7–5.5),P =0.017]or rabeprazole [4.8(2.5–6.4),P =0.002](Figure 3a).Esomeprazole alone attained an intragastric pH higher than 5.0in RMs.In PMs,the median (range)percentage time of pH >4with omepra-zole [94.5%(84.8–100%)]was signi ficantly higherthanAge (years)21.7Æ1.722.1Æ1.521.7Æ2.021.2Æ1.80.473Height (cm)169.7Æ8.4168.7Æ10.9167.6Æ7.0167.2Æ6.80.853Weight (kg)59.7Æ9.960.4Æ12.259.5Æ8.158.7Æ9.30.969CYP2C19genotypes*1/*1:n =15*1/*2:n =11*1/*3:n =4*2/*2:n =5*2/*3:n =48161220424Time875630412875630412RPZOPZ LPZ EPZ Control Control EPZ LPZ OPZ RPZ Control EPZ LPZ OPZ RPZ1008060402001132Aliment Pharmacol Ther 2013;38:1129-1138ª2013John Wiley &Sons LtdS.Sahara et al.that with esomeprazole [87.2%(71.6–99.2%),P =0.017]or rabeprazole [86.6%(21.0–99.2%),P =0.047](Figure 3b).However,the median of intragastric pH was higher than 5.0in all IMs and PMs independent of the PPI.DISCUSSIONWe demonstrated that the 24-h pH values attained over-all with twice-daily dosing of PPIs were equivalent among the four kinds of PPIs available in Japan.How-ever,the variations in acid inhibition associated with the CYP2C19genotype were small for esomeprazole and rabeprazole compared with values for omeprazole and lansoprazole.In H.pylori -negative Japanese subjects with CYP2C19RMs,median pH was signi ficantly higher with esomeprazole than with the other three PPIs.Although median pH in PMs was signi ficantly higher with omep-razole than with other PPIs,including esomeprazole,acid inhibition attained by any of the four PPIs in IMs and PMs was suf ficient for treatment of GERD and H.pylorieradication.4,26–28Therefore,although in fluence of CYP2C19genotype status on acid inhibition cannot be ignored,we deem esomeprazole to be preferable PPI for use in treating acid-related diseases,particularly in Japa-nese with CYP2C19RMs.In RMs,PPIs were rapidly eliminated from the sys-temic circulation,and plasma PPI levels before and 3h after dosing were often below detectable levels,resulting in insuf ficient acid inhibition.Following the rapid elimi-nation of PPIs,newly generated or activated H +,K +-ATPases in gastric parietal cells are able to secrete gastric acid.6–8,13However,acid inhibition by PPIs has also been observed to occasionally be insuf ficient,even if dosed twice daily in CYP2C19RMs.8Because the phar-macokinetics and pharmacodynamics of PPIs differ by the dependence on CYP2C19in each metabolic path-way,7,8,13–15,29PPI with less in fluence of CYP2C19is more preferable.One attractive characteristic of esomep-razole is that it is metabolised by CYP2C19to a lesser extent than omeprazole and therefore achievespotentEsomeprazoleRabeprazoleLansoprazoleOmeprazoleR P I R P I R P I R P I 812 1620 24 4812 1620 24 4812 1620 24 4 812 1620 24 4 *******##R P I R P I R P I R P I 875630412875630412875630412875630412875630412100806040200Aliment Pharmacol Ther 2013;38:1129-11381133ª2013John Wiley &Sons LtdEsomeprazole inhibits acid secretion in CYP2C19rapid metabolisersacid inhibition over 24h,irrespective of CYP2C19geno-type.16,17,30However,because CYP3A4is also involved in the metabolism of esomeprazole,esomeprazole has the potential risk of drug –drug interaction with a variety of drugs metabolised by CYP3A4.31Physicians must pay attention to this problem.In a randomised crossover study using healthy H.pylori -negative subjects,the percentage time of pH >4with esomeprazole 20mg was signi ficantly longer than that with lansoprazole 15mg (50.4%vs.43.0%,P =0.03)or rabeprazole 10mg (59.8%vs.51.7%,P =0.01).32In the present study,the median 24-h pH with esomeprazole 20mg twice daily in H.pylori -nega-tive CYP2C19RMs was signi ficantly higher than that with omeprazole,rabeprazole or lansoprazole throughout the 24-h observation period.We therefore recommend the use of esomeprazole in patients with acid-related dis-eases,particularly those refractory to standard doses of a PPI (e.g.CYP2C19RMs).Several studies have examined the ef ficacy of twice-daily dosing of a PPI with relation to CYP2C19,with con flicting findings being reported.For example,while two studies noted CYP2C19-dependent differences inacid inhibition between lansoprazole 30mg and rabep-razole 20mg,no such differences were seen between rabeprazole 10mg and esomeprazole 20mg.33,34In the present study,the intragastric pH attained by twice-daily dosing of PPIs differed among CYP2C19genotypes.However,the CYP2C19genotype-dependent variance in intragastric pH attained by esomeprazole and rabepraz-ole was smaller than that attained by lansoprazole or omeprazole.Therefore,twice-daily dosing of second-gen-eration PPIs might overcome the in fluence of CYP2C19genotypes to some degree,although not completely.As an alternative,trials of four-times daily dosing of PPIs have also been reported,involving rabeprazole 10mg,lansoprazole 30mg and esomeprazole 10mg,all of which attained suf ficient acid inhibition in CYP2C19RMs.8,34,35Taken together,these present and previous findings therefore suggest that multiple dosing of a PPI decreases CYP2C19genotype-dependent differences in acid inhibition.Potent acid inhibition is important for the eradication of H.pylori.4Potent acid inhibition also increases the sta-bility and bioavailability of antibiotics in the stomach and the concentration of antibiotics in gastric mucosa.36–38**EPZ OPZ RPZLPZ EPZ OPZ RPZLPZ EPZ OPZ RPZLPZ EPZ OPZ RPZLPZ EPZ OPZ RPZLPZ EPZ OPZ RPZLPZ 8756304121008060402001134Aliment Pharmacol Ther 2013;38:1129-1138ª2013John Wiley &Sons LtdFurthermore,acid inhibition allows H.pylori to reach its growth phase,rendering the bacteria more sensitive to antibiotics.38We28previously reported that,in analysis of24-h pH monitoring using H.pylori-negative subjects, 24-h pH level was required to be higher than5.0,which was achieved in CYP2C19IMs with eradication rate of higher than95%.In addition,we demonstrated that the pH level over24h was significantly higher in patients who achieved successful eradication using lansoprazole-based triple therapy[6.4(5.0–7.6)]than those who did not[5.2(2.2–6.2)].4In this study,median pH with esomeprazole in H.pylori-negative CYP2C19RMs was[5.4(3.5–6.8)],but those attained with other PPIs were not higher than5.0, suggesting that esomeprazole-based eradication might be acceptable compared with other PPIs for CYP2C19RMs. We therefore will plan to show in our hypothesis that H.pylori eradication rate with esomeprazole-based regi-men is independent of the CYP2C19genotype and is higher than that with an omeprazole-,rabeprazole-or lansoprazole-based regimen in CYP2C19RM patients. PPIs improve acid reflux heartburn symptoms and oesophageal mucosal breaks.39–43Meta-analyses of treat-ments for erosive GERD patients have shown that PPIs are much more effective in curing oesophageal erosions and acid reflux-related symptoms than H2receptor antagonists.44,45Furthermore,twice-daily dosing of rab-eprazole was effective in treating patients with GERD refractory to standard doses of PPI therapy.46However, improvements in heartburn associated with non-erosive reflux disease(NERD)using standard PPI dosages are lower(ranging from30to60%)than those for erosive GERD(ranging from10to30%).47–49The development of such oesophageal mucosal damage is closely related to intraoesophageal and intragastric pH values.For effective treatment of GERD using acid-inhibitory drugs, intragastric pH over a24-h period should fall below<4.0 for no longer than2–4h.26In this study,in RMs,the median percentage time of pH<4ranged from26.6to 38.7%independent of the PPI used,suggesting that PPI twice‐daily dosing may be insufficient for treating GERD/NERD patients refractory to standard doses of a PPI.Several limitations to the present study warrant men-tion.First,the subjects were all young,healthy, H.pylori-negative volunteers,not patients.Because acid secretion and response to PPIs depend on age,the grade of gastric mucosal atrophy and H.pylori infection,our results should be extrapolated with caution to patients treated with a PPI for H.pylori eradication or GERD. Second,we did not measure the plasma PPI levels;there-fore,we could not directly correlate the metabolic dispo-sition of each PPI with the pH attainted with each PPI. Third,the sample size was small,particularly when strat-ifying data based on CYP2C19genotype group.Enrol-ment of a large population of H.pylori-positive patients will be required to show significant evidence that the cure rate of patients treated with esomeprazole-based H.pylori eradication or patients refractory to standard PPI treatment is better than that of therapy based on another stly,we investigated acid-inhibitory effects at twice-daily dosing of rabeprazole10mg(a marketed dose in Japan),not20mg(a standard dose worldwide).Therefore,the present study results cannot be extrapolated to Western patients.In general,because a dose-dependent increase in median pH and in percen-tage time of pH>4was observed for rabeprazole administered once daily in doses of5,10,20and 40mg,50further study to compare the efficacy for acid inhibition attained with twice-daily dosing of rabeprazole 20mg and esomeprazole20mg will be required.In conclusion,we demonstrated that,for Japanese people,esomeprazole is more effective than other PPIs tested for achieving potent acid inhibition when dosed twice daily in CYP2C19RMs who are at risk of being unresponsive to PPI therapy at the standard dose.The clinical potential of twice-daily dosing of esomeprazole must be verified in future studies under appropriate pro-tocols.AUTHORSHIPGuarantor of the article:None.Author contributions:All authors approved thefinal ver-sion of the manuscript.ACKNOWLEDGEMENTSDeclaration of personal interests:None.Declaration of funding interests:Grants-in-aid from the Ministry of Education,Culture,Sports,Science and Technology of Japan(23590912and23590913)and from Japan Research Foundation for Clinical Pharmacology. First Department of Medicine and The Center for Clini-cal Research at Hamamatsu University School of Medi-cine have received grants from Takeda Pharmaceutical Co.,Ltd.,AstraZeneca KK,Eisai Co.,Ltd.,Daiichi-Sankyo Co.Ltd.Aliment Pharmacol Ther2013;38:1129-11381135ª2013John Wiley&Sons LtdEsomeprazole inhibits acid secretion in CYP2C19rapid metabolisersREFERENCES1.Walsh JH,Peterson WL.The treatmentof Helicobacter pylori infection in themanagement of peptic ulcer disease.NEngl J Med1995;333:984–91.2.Furuta T,Sagehashi Y,Shirai N,et al.Influence of CYP2C19polymorphismand Helicobacter pylori genotypedetermined from gastric tissue sampleson response to triple therapy for Hpylori infection.Clin GastroenterolHepatol2005;3:564–73.3.Furuta T,Shirai N,Watanabe F,et al.Effect of cytochrome P4502C19genotypic differences on cure rates forgastroesophageal reflux disease bylansoprazole.Clin Pharmacol Ther2002;72:453–60.4.Sugimoto M,Furuta T,Shirai N,et al.Evidence that the degree and duration of acid suppression are related toHelicobacter pylori eradication bytriple therapy.Helicobacter2007;12:317–23.5.Ishizaki T,Horai Y.Review article:cytochrome P450and the metabolismof proton pump inhibitors–emphasis on rabeprazole.Aliment Pharmacol Ther1999;13(Suppl3):27–36.6.Horai Y,Kimura M,Furuie H,et al.Pharmacodynamic effects and kineticdisposition of rabeprazole in relation toCYP2C19genotypes.AlimentPharmacol Ther2001;15:793–803.7.Shirai N,Furuta T,Moriyama Y,et al.Effects of CYP2C19genotypicdifferences in the metabolism ofomeprazole and rabeprazole onintragastric pH.Aliment PharmacolTher2001;15:1929–37.8.Sugimoto M,Furuta T,Shirai N,et al.Different dosage regimens ofrabeprazole for nocturnal gastric acidinhibition in relation to cytochromeP4502C19genotype status.ClinPharmacol Ther2004;76:290–301.9.Chang M,Dahl ML,Tybring G,Gotharson E,Bertilsson e ofomeprazole as a probe drug forCYP2C19phenotype in SwedishCaucasians:comparison with S-mephenytoin hydroxylation phenotypeand CYP2C19genotype.Pharmacogenetics1995;5:358–63. 10.Kubota T,Chiba K,Ishizaki T.Genotyping of S-mephenytoin4’-hydroxylation in an extended Japanesepopulation.Clin Pharmacol Ther1996;60:661–6.11.Sim SC,Risinger C,Dahl ML,et al.Acommon novel CYP2C19gene variantcauses ultrarapid drug metabolismrelevant for the drug response toproton pump inhibitors andantidepressants.Clin Pharmacol Ther2006;79:103–13.12.Sugimoto K,Uno T,Yamazaki H,Tateishi T.Limited frequency of theCYP2C19*17allele and its minor rolein a Japanese population.Br J ClinPharmacol2008;65:437–9.13.Furuta T,Ohashi K,Kosuge K,et al.CYP2C19genotype status and effect ofomeprazole on intragastric pH inhumans.Clin Pharmacol Ther1999;65:552–61.14.Furuta T,Shirai N,Takashima M,et al.Effects of genotypic differences inCYP2C19status on cure rates forHelicobacter pylori infection by dualtherapy with rabeprazole plusamoxicillin.Pharmacogenetics2001;11:341–8.15.Sugimoto M,Furuta T,Shirai N,et al.Comparison of an increased dosageregimen of rabeprazole versus aconcomitant dosage regimen offamotidine with rabeprazole fornocturnal gastric acid inhibition inrelation to cytochrome P4502C19genotypes.Clin Pharmacol Ther2005;77:302–11.16.Abelo A,Andersson TB,Antonsson M,Naudot AK,Skanberg I,Weidolf L.Stereoselective metabolism ofomeprazole by human cytochromeP450enzymes.Drug Metab Dispos2000;28:966–72.17.Hassan-Alin M,Andersson T,Niazi M,Rohss K.A pharmacokinetic studycomparing single and repeated oraldoses of20mg and40mg omeprazoleand its two optical isomers,S-omeprazole(esomeprazole)and R-omeprazole,in healthy subjects.Eur JClin Pharmacol2005;60:779–84.18.Andersson T,Rohss K,Bredberg E,Hassan-Alin M.Pharmacokinetics andpharmacodynamics of esomeprazole,the S-isomer of omeprazole.Aliment Pharmacol Ther2001;15:1563–9.19.Peterson WL.The role of antisecretorydrugs in the treatment of Helicobacterpylori infection.Aliment PharmacolTher1997;11(Suppl1):21–5.20.Sugimoto M,Shirai N,Nishino M,et al.Rabeprazole10mg q.d.s.decreases24-h intragastric aciditysignificantly more than rabeprazole20mg b.d.or40mg o.m.,overcomingCYP2C19genotype.Aliment PharmacolTher2012;36:627–34.21.Furuta T,Shirai N,Takashima M,et al.Effect of genotypic differences inCYP2C19on cure rates for Helicobacterpylori infection by triple therapy with aproton pump inhibitor,amoxicillin,andclarithromycin.Clin Pharmacol Ther2001;69:158–68.22.Zendehdel N,Biramijamal F,Hossein-Nezhad A,Sarie H,Doughaiemoghaddam M,Pourshams A.Role of cytochrome P4502C19geneticpolymorphisms in the therapeuticefficacy of omeprazole in Iranianpatients with erosive reflux esophagitis.Arch Iran Med2010;13:406–12.23.Kawamura M,Ohara S,Koike T,et al.The effects of lansoprazole on erosivereflux oesophagitis are influenced byCYP2C19polymorphism.AlimentPharmacol Ther2003;17:965–73.24.Kawamura M,Ohara S,Koike T,et al.Cytochrome P4502C19polymorphisminfluences the preventive effect oflansoprazole on the recurrence oferosive reflux esophagitis.JGastroenterol Hepatol2007;22:222–6.25.Saitoh T,Otsuka H,Kawasaki T,et al.Influences of CYP2C19polymorphismon recurrence of reflux esophagitisduring proton pump inhibitormaintenance therapy.Hepatogastroenterology2009;56:703–6.26.Bell NJ,Burget D,Howden CW,Wilkinson J,Hunt RH.Appropriateacid suppression for the management ofgastro-oesophageal reflux disease.Digestion1992;51(Suppl1):59–67.benz J,Stolte M,Blum AL,et al.Intragastric acidity as a predictor of thesuccess of Helicobacter pylorieradication:a study in peptic ulcerpatients with omeprazole andamoxicillin.Gut1995;37:39–43.28.Furuta T,Shirai N,Kodaira M,et al.Pharmacogenomics-based tailoredversus standard therapeutic regimen foreradication of H.pylori.Clin PharmacolTher2007;81:521–8.29.Shirai N,Furuta T,Xiao F,et al.Comparison of lansoprazole andfamotidine for gastric acid inhibitionduring the daytime and night-time indifferent CYP2C19genotype groups.Aliment Pharmacol Ther2002;16:837–46.30.Bardhan K,Bayerdorffer E.VeldhuyzenVan Zanten SJ,Lind T,Megraud F,Delchier JC,et al.The HOMER Study:the effect of increasing the dose ofmetronidazole when given withomeprazole and amoxicillin to cureHelicobacter pylori infection.Helicobacter2000;5:196–201.31.McColl KE,Kennerley P.Proton pumpinhibitors–differences emerge in hepaticmetabolism.Dig Liver Dis2002;34:461–7.1136Aliment Pharmacol Ther2013;38:1129-1138ª2013John Wiley&Sons Ltd S.Sahara et al.。
