The Office of Orphan Products Development Grant Program
FDA Orphan Drug Designation-PPT

EXAMPLE
Necrotizing Soft Tissue Infections
• For diagnostic claims, all who would be subjected to diagnosis per year Confirmatory Diagnostic for Anthrax EXAMPLE • For prevention claims, everyone who is at risk of the disease is counted per year Prevention of corneal transplant rejection
19
#1 – What is the Disease or Condition?
• Determine the disease/condition that would be treated, diagnosed or prevented by the drug/biologic • Challenging and can evolve
Benefits of Orphan Drug Designation
• If designated, eligible for the following financial incentives: o Tax Credits – 50% of clinical trials costs o Waiver of marketing application user fees – over $2 million o 7-year Marketing Exclusivity if first approved
2023届海南省乐东黎族自治县乐东中学等2校高三上学期模拟考试(三)英语试题(4)

2023届海南省乐东黎族自治县乐东中学等2校高三上学期模拟考试(三)英语试题(4)一、听力选择题1. What record did the woman just break?A.Her personal record.B.The school record.C.The national record.2. What will the man do tonight?A.Buy steak.B.Borrow books.C.Visit Cindy.3. What does Lacy usually play?A.The violin.B.The piano.C.The guitar.4.A.In a hotel.B.In a bank.C.In a classroom.D.In a box office.5. What does the woman think of her new job?A.Satisfactory.B.Low-paying.C.Tiring.二、听力选择题6. 听下面一段较长对话,回答以下小题。
1. What is the woman doing?A.Doing business.B.Hosting a programme.C.Eating in McDonald’s.2. When did McDonald’s have 7,063 restaurants in the world?A.In 1982.B.In 1980.C.In 1961.3. Which are probably the most popular according to the man?A.Sandwiches.B.The milk shakes.C.The French fries.4. Who bought the name McDonald’s?A.Richard.B.Maurice.C.Ray Kroc.7. 听下面一段较长对话,回答以下小题。
2025届 高中英语一轮复习语基默写课件选择性必修第三册UNIT 7 CAREERS

adj.有残疾的;丧失能力的
语境背诵
狂背单词
18.willing adj.乐意、愿意(做某事)的→unwilling adj.不愿意;不情愿→willingly adv.乐意地→willingness n.心甘情愿
语境背诵
狂背单词
7.manage vt. & vi.管理,负责→management n.经营,管理→manager n.经理 8.rely vi.信任;信赖;依赖;依靠→reliable adj.可信赖的,可靠的 9.impress vt.使铭记;给……留下深刻印象→impressive adj.给人深刻印象的,
合情合理地 22.motivate vt.激励→motivated adj.积极的,主动的→motivation n.激励;动机 23.profession n.专业,行业→professional adj.专业的
语境背诵
狂背单词
24.retire vi.退休,退职→retired adj.已退休的→retirement n.退休,退职 25.special adj.特殊的;重要的→specialist n.专家 26.occupy vt. 使 用 , 占 用 ; 侵 占 ; 占 领 →occupied adj. 忙 于 ; 使 用 中
令人钦佩的→impression n.印象;感想 10.intelligent adj.聪明的;有才智的;有智力的→intelligence n.智力,智慧,理
解力 11.employ vt. & n.雇用→employer n.雇主→employee n.雇员→employment n.工
USP–NFMonographDevelopmentProcess

Guideline for the Admission of Dietary Supplement Ingredients to the USP–NF Monograph Development ProcessG1.12-00Background The purpose of this Guideline is to set forth the criteria that USP Expert Committee uses to determine whether or not a dietary ingredient qualifies for admission to the process for the development of a quality monograph. The Guideline establishes a Class A and Class B system that categorizes dietary ingredients according to their level of safety concern, and describes the process by which the ingredients are classified. The USP Dietary Supplements Admission Evaluations Joint Standard Setting Subcommittee (DS AE JS3) makes a determination whether or not USP should proceed with monograph development.Subsequent approval of the monograph for inclusion into the United States Pharmacopeia and National Formulary (USP–NF ) is determined by the USP Balloting Process.Selection and Prioritization of Dietary IngredientsThe initial selection and prioritization of dietary ingredients for admission into the USP-NF compendial monograph development process is based on several considerations, including but not limited to the following:1. Extent of use, based upon market sales or other factors2. Historical use3. Knowledge of chemical composition4. Existence of other pharmacopeial standards5. Evidence of benefit6. Interest from a governmental body7. Potential risks to health associated with its use.Classification SystemUSP classifies dietary ingredients as follows:Class A: Admitted to the USP-NF compendial monograph development process Ingredients for which the available evidence does not indicate a serious risk to health* or other public health concern that precludes development of a USP-NF monograph and that could be approved for inclusion in the compendia with or without a cautionary label statement**.Guideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00Class B: Not admitted into the compendial monograph development process Ingredients for which the available evidence indicates a serious risk to health* or other public health concern that precludes development of a monograph for inclusion in the USP-NF .*Serious risk to health means that the use of the Ingredients could:(A) result in: (i) death; (ii) a life–threatening experience; (iii) inpatient hospitalization; (iv) a persistent orsignificant disability or incapacity; or (v) a congenital anomaly or birth defect; or (B) require, based on reasonable medical judgment, a medical or surgical intervention to prevent an outcome described under subparagraph (A).** Cautionary Label StatementIn certain cases the DSAE JS3 may recommend that monograph development proceed under Class A only if a cautionary label statement is included in the monograph. In such cases the monograph will not proceed to ballot until after the DSAE JS3 has had the opportunity to review the draft monograph to ensure that a suitable cautionary label statement is included. Examples of cases of approved monographs where cautionary label statements have been included are provided below. ArticleCautionary label statement that was included Black Cohosh Dosage forms prepared with this article should bear the followingstatement: Discontinue use and consult a healthcare practitioner ifyou have a liver disorder or develop symptoms of liver trouble,such as abdominal pain, dark urine, or jaundice.St. John’s Wort The label bears a statement indicating that “Rare cases of allergicreactions and photosensitivity have been reported with the use ofSt. John’s Wort. St. John’s Wort interacts with numerousmedications. Check with your healthcare provider before using.”Salix Species BarkThe label bears a statement indicating “Not for use in children,women who are pregnant or nursing, or by persons with knownsensitivity to aspirin”.Guideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00Comprehensive USP Admission EvaluationFor comprehensive USP admission evaluation, USP researches and evaluates diverse sources of safety information to determine the classification for dietary ingredients. Some articles may be exempt from comprehensive evaluation as outlined under “Criteria for Exemption from Comprehensive USP Admission Evaluation” below .The sources of information evaluated by USP include, but are not limited to the following:1. Human data: Although dietary ingredients are not required to undergo controlled clinicaltrials before they are marketed,1 the safety profile of an ingredient may be evaluatedusing the following information:a) Clinical safety studies: Sufficiently powered prospective observational studies,clinical trials, dose-escalation studies, systematic reviews, or retrospective meta-analysis of clinical studies provide useful information to evaluate the safety of aningredient.b) Other clinical studies: Although clinical studies may be limited by the small numberof study participants, observation of adverse events under controlled studyconditions generates useful safety information on the ingredient. Information fromrandomized, placebo-controlled, double-blind clinical studies, are valuable.c) Postmarket surveillance: Premarket safety studies sometimes are limited by thenumber of study subjects. When products are in wide use, detection of adverseevents provides a strong surrogate for safety monitoring in the general populationand in consumers who have chronic conditions. Postmarket surveillance alsoprovides valuable information about an ingredient’s safety profile in vulnerablepopulations, e.g., in pregnancy, lactation, the elderly, children, or prescriptionmedication users. Epidemiological reports might be helpful if the dietary ingredients are widely used as a traditional preparation.d) Adverse events: An adverse event associated with a supplement may be reportedby a healthcare practitioner (HCP) in a peer-reviewed journal or may be reportedby the HCP or a consumer to the local Poison Control Center or the United States1 Dietary Supplement Health and Education Act 1994.Available athttps:///About/DSHEA_Wording.aspx Accessed February 09, 2016Guideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00(U.S.) Food and Drug Administration’s (FDA) primary adverse event reportingportal, MedWatch. Since December 22, 2007, dietary supplement manufacturersand distributors are required to submit serious adverse event reports to FDAMedWatch. Valuable information about adverse events also is available from other international regulatory agencies such as Health Canada, the British Medicines and Healthcare Products Regulatory Agency (MHRA), and the Australian TherapeuticGoods Administration (TGA).e) Supplement interactions: Interactions with prescription drugs have significant safetyimplications because of their effects on bioavailability or induction/inhibition ofmetabolizing enzymes. Such interactions may lead to synergism or antagonism ofintended effects.f) Data from other relevant products: When long term safety information is not welldocumented, particularly for new dietary ingredients (NDIs) (ingredients introduced into the market after Oct. 15, 1994), any additional publicly available informationmay be useful in establishing the ingredient’s safety profile. Knowledge aboutchemical constituents may be used during investigations of equivalency amongcommercial dietary supplement products and their traditional counterparts. 2. Non-human data (pharmacological data): Carefully planned and justified in vivo animalstudies and in vitro experiments that investigate potential risks to human health.Information from animal experiments may bridge the knowledge gap regarding the safety of dietary supplement use.a) Reproductive toxicity: Animal experimental data regarding genotoxicity,reproductive and developmental toxicity, and carcinogenicity provide valuablesafety information for the use of the dietary ingredient by pregnant and lactatingmothers. In vitro studies such as the Ames test provide insights into likelygenotoxicity.b) Studies in experimental animals: Studies may provide insights into the mechanismof action of a substance, its purported efficacy, and its effect(s) on target organs. In vitro cell culture studies may provide information about effects at the cellular leveland molecular mediators involved in the observed effects.Guideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00c) Pharmacokinetics: Information about the absorption kinetics, bioavailability,accumulation in vital organs, plasma maximum concentration (C max ), time for half-life (t 1/2), and elimination kinetics, or marker compound kinetics identifies the dose or duration mediated toxicity of a dietary ingredient.d) Safety margin: Information from animal studies regarding the effective dose(ED 50), lethal dose (LD 50), no observed adverse effect level (NOAEL), and lowest observed adverse effect level (LOAEL) are used to determine the relative safetymargin in the use of the dietary ingredient.e) Presence of toxic constituents (or structurally related compounds with establishedtoxicity): Knowledge of chemical components of a dietary ingredient aids in safetyevaluation by identifying potentially toxic constituents, components containingtoxicophores, or constituents known to mimic or modulate endogenousintermediates. Structure-based computational prediction models and in silicoanalysis may be used if available, to make better determinations on toxicity whenaddressing uncertainty with safety data (such as equivocal evidence or missinginformation) from conventional in vivo or in vitro studies.3. Contemporaneous extent of use globally and in the U.S., including patterns of misuse,abuse, and fluctuations of use: Information collected from dietary supplement trade publications and regulatory bulletins helps generate signals of value. Informationregarding the extent of use (global market information) provides insights into the safety profile.4. Historical use: Traditional use documented in authoritative texts—including the context ofuse, dose, duration of use, method of preparation, and traditional cautions—provides valuable information about deviations of the commercial preparations from the traditional use, if any, and unexpected adverse effects. Traditional medical systems such asAyurveda and Traditional Chinese Medicine provide useful information about historical use.5. Regulatory status in the U.S. and other countries:a) Regulatory actions: Information about regulatory actions (including product recallsand safety alerts) from international regulatory agencies may indicate the extent ofadverse event reports, the incidence and methods of detection ofadulteration/contamination, the regional or global nature of adverse events, anddietary supplement–prescription drug interactions.Guideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00b) Regulatory status: The regulation of dietary supplements in the U.S. differs fromother countries. In some European countries, dietary supplement ingredients areregulated as OTC drugs or prescription drugs, which may require registration orpre-market approval. In the U.S. these same ingredients may be regulated asdietary supplements and thus do not require pre-marketing approval by FDA unless they are NDIs. Thus, the intervention of the HCP and the consumption patterns ofthe dietary ingredients by consumers are different in the U.S. and other countries.Accordingly, the qualitative and quantitative information on the safety profileobtained from different countries provides valuable information.c) GRAS/NDI status: FDA may be notified of Generally Recognized as Safe (GRAS)status for some dietary ingredients and the intended conditions of use. GRASnotifications to FDA of dietary ingredients may provide information about availablescientific data and basis of a product’s safety. Similarly, NDI notifications to FDAprovide information about the basis for a product’s safety.6. Existence of pharmacopeial monographs in other pharmacopeias may provide criticalinformation about the standards of purity, adulterants/contaminants, dosage, and caution statements intended to ensure the safe use of dietary ingredients. Examples of such authoritative information include but are not limited to, the World Health Organization (WHO), the European Scientific Cooperative on Phytotherapy (ESCOP), the German Commission E, Indian Pharmacopeia, Pharmacopoeia of the People’s Republic of China, and Health Canada monographs.In analyzing information from the above sources for a dietary ingredient, the limitations of dietary supplement specific issues (detailed in Gardiner et al., 20082,3) are considered. Reports of serious adverse events may be analyzed with an appropriate causality algorithm (such as the WHO-UMC system for standardized case causality assessment). The “odds ratio” for a serious adverse reaction and the signal of safety concern may be estimated from the extent of use, “number needed to harm” or proportional representation ratio.Commensurate with the signal of safety concern, USP may communicate the admission evaluation and classification through publications in peer–reviewed journals, public2 Gardiner P, Sarma DN, Low Dog T, Barrett ML, Chavez ML, Ko R, Mahady GB, Marles RJ,Pellicore LS, and Giancaspro GI. 2008. The state of dietary supplement adverse event reporting in the United States. Pharmacoepidemiology and Drug Safety. 17: 962–970. 3 Dietary Supplements: A Framework for Evaluating Safety. Institute of Medicine & National Research Council; The National Academies Press: Washington, DC, 2005.Guideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00communications, Pharmacopeial Forum notices, USP Dietary Supplement Compendium, or other appropriate means.The AE JS3 will determine the appropriate classification based on the totality of the evidence obtained above for each of the parameters examined. Criteria for Exemption from Comprehensive USP Admission EvaluationA comprehensive literature search and review may not be necessary for dietary ingredients for which a GRAS or an NDI notification was submitted to the FDA, for review and notobjected to by the Agency. USP Admission Classification for such dietary ingredients may be based on the information from the GRAS or NDI notifications, which usually contain themajority of the information that USP assesses as described above. A comprehensive review may be performed for dietary ingredients for which a GRAS or an NDI notification does not contain sufficient information to make an admission evaluation, or where the candidatedietary ingredient for admission is not exactly the same or equivalent to the subject of the NDI or GRAS notification. The following attributes will be reviewed to determine whether an exemption from a comprehensive admission evaluation applies:1. Equivalency of the candidate dietary ingredient to the subject of the NDI or GRASnotification. To determine equivalency, information on the following attributes will be reviewed:a. Identity of the ingredient;b. Physical characteristics of the ingredient i.e., the form of the ingredient reviewedwhether liquid, powder or other, including the particle size characteristics (micro ornanoparticle); and,c. The chemical profile, including the extraction procedure and compounds present inthe ingredient.2. Daily Intake: The maximum amount of the candidate dietary ingredient recommended orsuggested for use in a dietary supplement as determined from publicly availablepublications (for example, from the NIH Office of Dietary Supplements and NationalGuideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00Library of Medicine Dietary Supplement Label Database (DSLD)4), should not exceed the intake level stipulated in the GRAS or NDI notification.This Guideline is intended to allow USP to evaluate safety issues depending on the particular dietary ingredient involved, the level of available safety data, and other relevantconsiderations. For example, even where a dietary ingredient is classified as Class A and is eligible for admission into the USP-NF monograph development process, there may be specific safety concerns that will require future monitoring. USP monitors safety information for all dietary ingredients for which dietary supplement monographs have been developed on an ongoing basis for possible re–evaluation of an article’s admission status.USP’s evaluation of a dietary ingredient under this Guideline is performed for the sole purpose of determining admission into the compendial monograph development process and should not be relied upon as any finding about the intrinsic safety or effectiveness of the dietary ingredient under review.This Guideline supersedes any previous guideline issued by USP on safety criteria and admission classification for dietary supplements. 4 ODS-NLM Dietary Supplement Label Database (DSLD). Available at/dsld/ (Accessed February 09, 2016).。
The Science of Productivity Getting Things Done

The Science of Productivity GettingThings DoneThe science of productivity and getting things done is a topic that isrelevant to everyone, regardless of their profession or personal pursuits. Intoday's fast-paced world, the ability to effectively manage our time and tasks is crucial for success and well-being. From the workplace to the home, productivity plays a significant role in our daily lives. In this response, we will explore the science of productivity, the factors that influence it, and strategies forimproving it. Productivity can be defined as the measure of how efficiently resources are used to achieve a particular outcome. In the context of work, productivity is often associated with the ability to complete tasks and meet goals within a given time frame. However, productivity is not limited to the workplace– it also applies to our personal lives, such as managing household chores, pursuing hobbies, and maintaining relationships. One of the key factors that influence productivity is our ability to manage our time effectively. Time management involves prioritizing tasks, setting goals, and allocating time for different activities. Research has shown that individuals who are able toeffectively manage their time are more likely to be productive and achieve their desired outcomes. Time management skills can be developed through practice and the use of various tools and techniques, such as to-do lists, calendars, andscheduling software. Another important factor that influences productivity is our ability to focus and concentrate on the task at hand. In today's digital age, weare constantly bombarded with distractions, such as emails, social media, andother notifications. These distractions can have a significant impact on ourability to concentrate and complete tasks. Therefore, it is important to develop strategies for minimizing distractions and improving focus, such as settingspecific times for checking emails and social media, creating a designated workspace, and using techniques like the Pomodoro method to work in focused bursts. Furthermore, our physical and mental well-being also plays a crucial role in our productivity. Studies have shown that factors such as sleep, exercise, andnutrition can have a significant impact on our cognitive abilities and overallproductivity. Ensuring that we get an adequate amount of sleep, engage in regular physical activity, and eat a balanced diet can help improve our energy levels, focus, and overall productivity. In addition to individual factors, the environment in which we work and live also plays a significant role in our productivity. An organized and clutter-free workspace can help reduce distractions and improve focus, while a supportive and positive work culture can boost motivation and productivity. Similarly, our home environment can also impact our ability to be productive – for example, having a designated space for work or study can help create a conducive environment for productivity. In conclusion, the science of productivity and getting things done is a multifaceted topic that encompasses various factors, including time management, focus, well-being, and the environment. By understanding these factors and implementing strategies to improve them, we can enhance our productivity and achieve our goals more effectively. Whether in the workplace or in our personal lives, productivity is a crucial element for success and fulfillment. By continuously learning and applying the science of productivity, we can strive to lead more efficient and satisfying lives.。
济南“PEP”2024年小学第2次英语第2单元真题

济南“PEP”2024年小学英语第2单元真题考试时间:90分钟(总分:100)A卷考试人:_________题号一二三四五总分得分一、综合题(共计100题)1、填空题:The __________ is the capital city of Sweden. (斯德哥尔摩)2、听力题:The fruit is ___. (juicy)3、听力题:The ______ is known for her kindness.4、听力题:The cake is ___ (delicious/yummy).5、填空题:The _____ (采摘) of fruits is a fun summer activity.6、填空题:The ________ was a significant battle in World War II.7、听力题:The chemical formula for potassium bromide is __________.8、填空题:The heron is a _______ (优雅) bird.9、听力题:The main component of antioxidants is _____.10、填空题:The _______ (The Marshall Plan) provided aid to rebuild Europe after WWII.11、What do you call the study of rocks?A. BiologyB. ChemistryC. GeologyD. Astronomy答案:C12、填空题:The weather changes with the ______ (季节).13、填空题:My dad is a great __________ (支持者) of my interests.14、填空题:________ (草原动物) graze on grasses.15、填空题:My favorite _____ is a colorful bird.16、填空题:I like to run in the ______ (公园) every morning to stay fit.17、填空题:The famous battle where Napoleon was defeated took place at ______ (滑铁卢).18、听力题:The _____ (飞机) flies high.19、填空题:Flowers attract _____ (butterfly) and bees.20、填空题:The ______ (环境保护) includes planting trees.21、urban ecology) studies interactions in cities. 填空题:The ____22、听力题:My friend is a ______. He enjoys listening to music.23、听力题:The flowers are _______ (colorful) in the garden.24、填空题:The __________ is a famous river in Europe. (多瑙河)25、听力题:He is fishing in the ___. (river)My grandma loves to make ____ (sauces).27、听力题:He is ________ (running) in the race.28、听力题:The __________ is known for its diverse wildlife.29、What is the process of keeping food cold to preserve it?A. CookingB. RefrigerationC. FreezingD. Dehydration答案: B. Refrigeration30、听力题:The Mississippi River flows through __________.31、填空题:My sister has a ________ (毛绒玩具), and she takes it everywhere, even to ________ (学校).32、What do we call the animal that says "meow"?A. DogB. CatC. CowD. Sheep答案:B33、听力题:The Sahara Desert is located in _______.34、填空题:The squirrel's sharp claws help it climb ______ (树木).35、听力题:The ________ (train) moves very fast.36、填空题:Mount Kilimanjaro is found in _____ (14).37、听力题:A ______ is a graphical representation of results.Dogs are known for their _______ (忠诚).39、听力题:A __________ is formed by the accumulation of minerals over time.40、听力题:The flowers are _______ (blooming).41、听力题:Sodium chloride is commonly known as _____.42、填空题:The park is ________ (宽广).43、听力题:The horse is ___ (trotting) in the field.44、填空题:A __________ (溶胶) is a colloidal mixture with solid particles dispersed in a liquid.45、What do we call the game played on a board with black and white squares?A. Snakes and LaddersB. ChessC. CheckersD. Monopoly46、What is the largest organ in the human body?A. SkinB. LiverC. HeartD. Brain答案: A47、How many states are there in the United States?A. 50B. 48C. 52D. 51答案:A48、听力题:A compass shows _______.49、填空题:I have a collection of ________.50、What do you call a person who teaches others?A. EducatorB. InstructorC. MentorD. All of the above答案:D51、听力题:The stars are _____ (twinkling/shining).52、What do you call a type of music that is fast and upbeat?A. RockB. ClassicalC. JazzD. Slow答案:A53、What do we call a place where books are kept?A. LibraryB. BookstoreC. OfficeD. Classroom答案: A54、填空题:A horse is a majestic _______ that gallops.55、听力题:The _____ (forest/park) is quiet.56、填空题:I like to go hiking in the ______ (山) to see beautiful ______ (风景).57、填空题:The __________ (历史的视角变化) can illuminate new truths.58、听力题:There are ___ (three/four) books on the table.59、What do you call a piece of land surrounded by water on three sides?A. PeninsulaB. IslandC. LagoonD. Atoll答案:A60、Which instrument is used to measure temperature?A. BarometerB. ThermometerC. HygrometerD. Anemometer答案:B61、听力题:The chemical reaction that produces heat is called an _______ reaction.62、What do we call the process of water soaking into the ground?a. Infiltrationb. Percolationc. Absorptiond. Saturation答案:a63、听力题:My sister loves to ________ stories.64、填空题:I enjoy ______ (画画) in my sketchbook.65、填空题:In winter, I enjoy _______ (活动). The snow is _______ (形容词) and beautiful.66、听力题:My brother has a blue ______ (bike).67、What do you call a computer program that helps you find information on the internet?A. BrowserB. EditorC. SoftwareD. Application答案:A68、填空题:The capital of the Solomon Islands is ________ (霍尼亚拉).69、Which animal is known for its ability to swim?A. DogB. CatC. FishD. Horse答案:C70、填空题:The __________ (生态平衡) is crucial for survival.71、听力题:The __________ is a large area of rolling grassland.72、填空题:I have a toy _______ that can make bubbles in the air.73、What is the name of the fairy in Peter Pan?A. CinderellaB. TinkerbellC. ArielD. Belle答案:B74、听力题:An object in free fall experiences only the force of ______.75、听力题:The main type of carbohydrate is ______.76、填空题:The weather is _______ (晴朗) today.77、填空题:I want to learn to ________ (制作音乐).78、填空题:My cousin is very __________ (适应性强).79、What is the name of the famous lion in "The Lion King"?a. Simbab. Mufasac. Scard. Nala答案:a80、听力题:The ancient Egyptians used ________ to build their monuments.81、填空题:My cousin is a __________ (舞蹈家).82、Which vehicle runs on tracks?A. CarB. BusC. TrainD. Bicycle答案:C83、填空题:I love watching _______ (企鹅) slide on ice.84、听力题:I like to _____ (跳) rope.85、听力题:What is your favorite __________?86、What do you call a book of maps?A. AtlasB. EncyclopediaC. DictionaryD. Thesaurus答案:A87、听力题:The Earth's surface is constantly changing due to a variety of ______.88、听力题:The grass is ___. (green)89、听力题:Oxygen is essential for __________ to occur.90、填空题:My favorite game is ______ (象棋).91、选择题:What is 15 ÷ 3?A. 3B. 4C. 5D. 692、Which fruit is typically red and often used to make juice?A. OrangeB. GrapeC. AppleD. Lime答案: C93、填空题:I enjoy learning about ______ in science class.94、填空题:Planting trees can help combat _____ (全球变暖).95、填空题:The athlete trains for the _____ (比赛).96、ts can ______ (净化) air pollutants. 填空题:Some pla97、听力题:The Great Red Spot on Jupiter is a permanent ______.98、island) is surrounded by water on all sides. 填空题:The ____99、How many continents are there?A. FiveB. SixC. SevenD. Eight100、What is the currency used in the UK?A. EuroB. DollarC. PoundD. Yen答案:C。
美国《联邦规章典集》(CFR)第21篇“食品与药品”总目
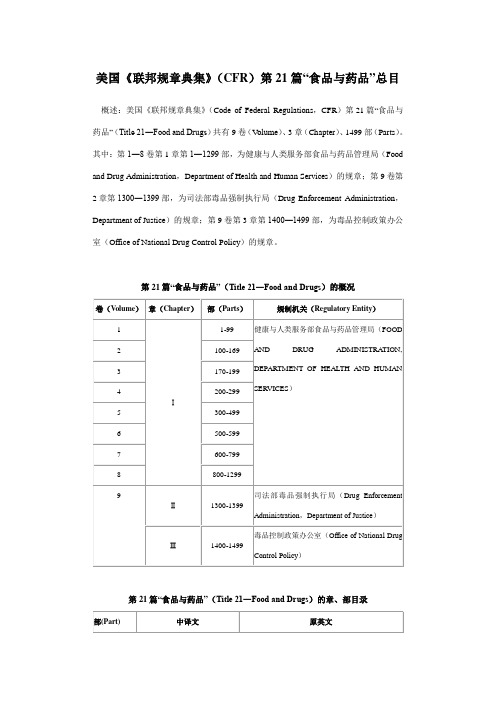
美国《联邦规章典集》(CFR)第21篇“食品与药品”总目概述:美国《联邦规章典集》(Code of Federal Regulations,CFR)第21篇“食品与药品”(Title 21―Food and Drugs)共有9卷(V olume)、3章(Chapter)、1499部(Parts)。
其中:第1―8卷第1章第1―1299部,为健康与人类服务部食品与药品管理局(Food and Drug Administration,Department of Health and Human Services)的规章;第9卷第2章第1300―1399部,为司法部毒品强制执行局(Drug Enforcement Administration,Department of Justice)的规章;第9卷第3章第1400―1499部,为毒品控制政策办公室(Office of National Drug Control Policy)的规章。
第21篇“食品与药品”(Title 21―Food and Drugs)的概况卷(Volume)章(Chapter)部(Parts)规制机关(Regulatory Entity)1Ⅰ1-99健康与人类服务部食品与药品管理局(FOODAND DRUG ADMINISTRA TION,DEPARTMENT OF HEALTH AND HUMANSERVICES)2100-1693170-1994200-2995300-4996500-5997600-7998800-12999Ⅱ1300-1399司法部毒品强制执行局(Drug EnforcementAdministration,Department of Justice)Ⅲ1400-1499毒品控制政策办公室(Office of National DrugControl Policy)第21篇“食品与药品”(Title 21―Food and Drugs)的章、部目录部(Part)中译文原英文第Ⅰ章―健康与人类服务部食品与药品管理局(CHAPTERADMINISTRATION, DEPARTMENT OF HEALTH AND HUMAN SERVICES)第A分章―总则(SUBCHAPTER A―GENERAL)1一般强制执行规章GENERAL ENFORCEMENT REGULA TIONS2一般行政规则与决定GENERAL ADMINISTRA TIVE RULINGS ANDDECISIONS3产品管辖权PRODUCT JURISDICTION5组织ORGANIZA TION7强制执行政策ENFORCEMENT POLICY10行政规范与程序ADMINISTRA TIVE PRACTICES ANDPROCEDURES11电子化记录;电子化签名ELECTRONIC RECORDS; ELECTRONICSIGNA TURES12正式证据的公众听证FORMAL EVIDENTIARY PUBLIC HEARING13在公众质询委员会前的公众听证PUBLIC HEARING BEFORE A PUBLIC BOARD OF INQUIRY14在公众咨询委员会前的公众听证PUBLIC HEARING BEFORE A PUBLIC ADVISORY COMMITTEE15在FDA局长前的公众听证PUBLIC HEARING BEFORE THECOMMISSIONER16在FDA前的规制性听证REGULA TORY HEARING BEFORE THE FOODAND DRUG ADMINISTRA TION17行政罚款听证CIVIL MONEY PENALTIES HEARINGS19行为标准与利益冲突STANDARDS OF CONDUCT AND CONFLICTSOF INTEREST20公共信息PUBLIC INFORMA TION21隐私保护PROTECTION OF PRIVACY25环境影响考虑ENVIRONMENTAL IMPACT CONSIDERA TIONS26药品良好制造规范报告、医疗器械质量体系核查报告以及某些医疗器械产品评价报告的互认:美国与欧共体MUTUAL RECOGNITION OFPHARMACEUTICAL GOOD MANUFACTURINGPRACTICE REPORTS, MEDICAL DEVICEQUALITY SYSTEM AUDIT REPORTS, ANDCERTAIN MEDICAL DEVICE PRODUCTEV ALUA TION REPORTS: UNITED STA TES ANDTHE EUROPEAN COMMUNITY50人类受试者的保护PROTECTION OF HUMAN SUBJECTS54临床试验者的财务公开FINANCIAL DISCLOSURE BY CLINICALINVESTIGA TORS56机构审查委员会INSTITUTIONAL REVIEW BOARDS58对非临床实验室研究的良好实验室规范GOOD LABORA TORY PRACTICE FOR NONCLINICAL LABORA TORY STUDIES60专利期恢复PA TENT TERM RESTORA TION70色素添加剂COLOR ADDITIVES71色素添加剂申请COLOR ADDITIVE PETITIONS73免除认证的色素添加剂的列表LISTING OF COLOR ADDITIVES EXEMPTFROM CERTIFICA TION74适用认证的色素添加剂的列表LISTING OF COLOR ADDITIVES SUBJECT TOCERTIFICA TION80色素添加剂认证COLOR ADDITIVE CERTIFICA TION81用于食品、药品和化妆品的临时性色素添加剂的一般规范和一般限制GENERAL SPECIFICA TIONS AND GENERALRESTRICTIONS FOR PROVISIONAL COLORADDITIVES FOR USE IN FOODS, DRUGS, ANDCOSMETICS82经认证的临时性列表的色素和规范的列表LISTING OF CERTIFIED PROVISIONALL Y LISTED COLORS AND SPECIFICA TIONS83-98[预留的][Reserved]99已上市的药品、生物制品和器械的未经批准的/新的用途的信息的发布DISSEMINA TION OF INFORMA TION ONUNAPPROVED/NEW USES FOR MARKETEDDRUGS, BIOLOGICS, AND DEVICES第B分章―用于人类消费的食品(SUBCHAPTER B―FOOD FOR HUMAN CONSUMPTION)100总则GENERAL101食品标识FOOD LABELING102非标准化食品的普通的或者通常的名称COMMON OR USUAL NAME FOR NONSTANDARDIZED FOODS104食品的营养质量指南NUTRITIONAL QUALITY GUIDELINES FORFOODS105特殊膳食用途的食品FOODS FOR SPECIAL DIETARY USE106婴儿配方母乳替代食品质量控制程序INFANT FORMULA QUALITY CONTROL PROCEDURES107婴儿配方母乳替代食品INFANT FORMULA108紧急许可控制EMERGENCY PERMIT CONTROL109在人类食品与食品-包装材料中的不可避免的污染物UNA VOIDABLE CONTAMINANTS IN FOODFOR HUMAN CONSUMPTION ANDFOOD-PACKAGING MA TERIAL110在制造、包装或者保存人类食品中的现行良好制造规范CURRENT GOOD MANUFACTURINGPRACTICE IN MANUFACTURING, PACKING, ORHOLDING HUMAN FOOD113装在密封容器中的热加工低酸食品THERMALL Y PROCESSED LOW-ACID FOODSPACKAGED IN HERMETICALL Y SEALEDCONTAINERS114酸化食品ACIDIFIED FOODS 115带壳蛋SHELL EGGS119存在显著或者不合理风险的膳食补充剂DIETARY SUPPLEMENTS THA T PRESENT A SIGNIFICANT OR UNREASONABLE RISK120危害分析与关键控制点(HACCP)体系HAZARD ANALYSIS AND CRITICAL CONTROL POINT (HACCP) SYSTEMS123鱼与渔业产品FISH AND FISHERY PRODUCTS129饮用水加工与装瓶PROCESSING AND BOTTLING OF BOTTLEDDRINKING WA TER130食品标准:总则FOOD STANDARDS: GENERAL131乳与奶油MILK AND CREAM133乳酪与相关乳酪产品CHEESES AND RELA TED CHEESE PRODUCTS 135冷冻点心FROZEN DESSERTS136烘焙产品BAKERY PRODUCTS137谷物粉与相关产品CEREAL FLOURS AND RELA TED PRODUCTS 139通心粉与面条产品MACARONI AND NOODLE PRODUCTS145罐装水果CANNED FRUITS146罐装水果汁CANNED FRUIT JUICES150水果黄油、果冻、防腐剂以及相关产品FRUIT BUTTERS, JELLIES, PRESERVES, AND RELA TED PRODUCTS152水果馅饼FRUIT PIES155罐装蔬菜CANNED VEGETABLES156蔬菜汁VEGETABLE JUICES158冷冻蔬菜FROZEN VEGETABLES160蛋与蛋制品EGGS AND EGG PRODUCTS161鱼与有壳的水生动物FISH AND SHELLFISH163可可制品CACAO PRODUCTS164树坚果与花生制品TREE NUT AND PEANUT PRODUCTS 165饮料BEVERAGES166人造黄油MARGARINE168增甜剂与餐桌糖浆SWEETENERS AND TABLE SIRUPS169食品敷料与调味料FOOD DRESSINGS AND FLA VORINGS 170食品添加剂FOOD ADDITIVES171食品添加剂申请FOOD ADDITIVE PETITIONS172允许直接加入用于人类消费食品的食品添加剂FOOD ADDITIVES PERMITTED FOR DIRECTADDITION TO FOOD FOR HUMANCONSUMPTION173在用于人类消费的食品中允许的次直接的食品添加剂SECONDARY DIRECT FOOD ADDITIVESPERMITTED IN FOOD FOR HUMANCONSUMPTION174间接食品添加剂:总则INDIRECT FOOD ADDITIVES: GENERAL175间接食品添加剂:胶粘剂与涂层的组分INDIRECT FOOD ADDITIVES: ADHESIVES AND COMPONENTS OF COA TINGS176间接食品添加剂:纸与纸板组分INDIRECT FOOD ADDITIVES: PAPER ANDPAPERBOARD COMPONENTS177间接食品添加剂:聚合体INDIRECT FOOD ADDITIVES: POL YMERS178间接食品添加剂:辅剂、生产助剂和消毒剂INDIRECT FOOD ADDITIVES: ADJUVANTS, PRODUCTION AIDS, AND SANITIZERS179在食品生产、加工和处理中的辐照IRRADIA TION IN THE PRODUCTION, PROCESSING AND HANDLING OF FOOD180在额外试验期间临时在食品或者在与食品接触中被允许的食品添加剂FOOD ADDITIVES PERMITTED IN FOOD OR INCONTACT WITH FOOD ON AN INTERIM BASISPENDING ADDITIONAL STUDY181先前核准的食品配料PRIOR-SANCTIONED FOOD INGREDIENTS182一般认为安全的物质SUBSTANCES GENERALL Y RECOGNIZED ASSAFE184被确认为一般认为安全的直接食品物质DIRECT FOOD SUBSTANCES AFFIRMED AS GENERALL Y RECOGNIZED AS SAFE186被确认为一般认为安全的间接INDIRECT FOOD SUBSTANCES AFFIRMED AS食品物质GENERALL Y RECOGNIZED AS SAFE 189禁止用于人类食品的物质SUBSTANCES PROHIBITED FROM USE INHUMAN FOOD190膳食补充剂DIETARY SUPPLEMENTS191-199[预留的][Reserved]第C分章―药品:总则(SUBCHAPTER C―DRUGS: GENERAL)200总则GENERAL201标识LABELING202处方药广告PRESCRIPTION DRUG ADVERTISING203处方药销售PRESCRIPTION DRUG MARKETING205对批发处方药销售商颁发州执照的指南GUIDELINES FOR STA TE LICENSING OFWHOLESALE PRESCRIPTION DRUGDISTRIBUTORS206人用固体口服剂型药品的印码IMPRINTING OF SOLID ORAL DOSAGE FORMDRUG PRODUCTS FOR HUMAN USE207药品生产者的登记与商业销售的药品的列表REGISTRA TION OF PRODUCERS OF DRUGSAND LISTING OF DRUGS IN COMMERCIALDISTRIBUTION208处方药的药物治疗指导MEDICA TION GUIDES FOR PRESCRIPTIONDRUG PRODUCTS210制造、加工、包装或者保存药品的现行良好制造规范;总则CURRENT GOOD MANUFACTURINGPRACTICE IN MANUFACTURING, PROCESSING,PACKING, OR HOLDING OF DRUGS; GENERAL211对完成的药品的现行良好制造规范CURRENT GOOD MANUFACTURINGPRACTICE FOR FINISHEDPHARMACEUTICALS216药房配药PHARMACY COMPOUNDING225对含药饲料的现行良好制造规CURRENT GOOD MANUFACTURING范PRACTICE FOR MEDICA TED FEEDS226对A型含药物品的现行良好制造规范CURRENT GOOD MANUFACTURINGPRACTICE FOR TYPE A MEDICA TEDARTICLES250对特殊人用药品的特殊要求SPECIAL REQUIREMENTS FOR SPECIFICHUMAN DRUGS290管制的药品CONTROLLED DRUGS299药品;正式名称与已确定的名称DRUGS; OFFICIAL NAMES AND ESTABLISHEDNAMES第D分章―人用药品(SUBCHAPTER D―DRUGS FOR HUMAN USE)300总则GENERAL310新药NEW DRUGS312试验用新药申请INVESTIGA TIONAL NEW DRUG APPLICA TION 314为FDA批准上市新药的申请APPLICA TIONS FOR FDA APPROV AL TOMARKET A NEW DRUG315诊断用放射性药品DIAGNOSTIC RADIOPHARMACEUTICALS316罕见病药ORPHAN DRUGS320生物利用度与生物等效性要求BIOA VAILABILITY AND BIOEQUIVALENCEREQUIREMENTS328含有酒精的预期用于口部摄入的非处方药品OVER-THE-COUNTER DRUG PRODUCTSINTENDED FOR ORAL INGESTION THA TCONTAIN ALCOHOL330一般认为安全与有效以及不错误标识的非处方人用药品OVER-THE-COUNTER (OTC) HUMAN DRUGSWHICH ARE GENERALL Y RECOGNIZED ASSAFE AND EFFECTIVE AND NOTMISBRANDED331用于非处方的人类使用的抗酸产品ANTACID PRODUCTS FOR OVER-THE-COUNTER (OTC) HUMAN USE332用于非处方的人类使用的抗胃肠气胀产品ANTIFLA TULENT PRODUCTS FOR OVER-THE-COUNTER HUMAN USE333用于非处方的人类使用的局部抗菌药品TOPICAL ANTIMICROBIAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE335用于非处方的人类使用的止泻药品ANTIDIARRHEAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE336用于非处方的人类使用的止吐药品ANTIEMETIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE338用于非处方的人类使用的帮助夜间睡眠的药品NIGHTTIME SLEEP-AID DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE340用于非处方的人类使用的兴奋药品STIMULANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE341用于非处方的人类使用的感冒、咳嗽、过敏症药、支气管扩张以及平喘药品COLD, COUGH, ALLERGY, BRONCHODILA TOR,AND ANTIASTHMA TIC DRUG PRODUCTS FOROVER-THE-COUNTER HUMAN USE343用于非处方的人类使用的内服的止痛、退热以及抗风湿药品INTERNAL ANALGESIC, ANTIPYRETIC, ANDANTIRHEUMA TIC DRUG PRODUCTS FOROVER-THE-COUNTER HUMAN USE344用于非处方的人类使用的局部的耳部药品TOPICAL OTIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE346用于非处方的人类使用的肛肠药品ANORECTAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE347用于非处方的人类使用的皮肤保护药品SKIN PROTECTANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE348用于非处方的人类使用的外部的止痛药品EXTERNAL ANALGESIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE349用于非处方的人类使用的眼科药品OPHTHALMIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE350用于非处方的人类使用的止汗药品ANTIPERSPIRANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE352用于非处方的人类使用的遮光药品SUNSCREEN DRUG PRODUCTS FOROVER-THE-COUNTER HUMAN USE [STAYEDINDEFINITEL Y]355用于非处方的人类使用的防龋药品ANTICARIES DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE357用于非处方的人类使用的其他内服药品MISCELLANEOUS INTERNAL DRUGPRODUCTS FOR OVER-THE-COUNTERHUMAN USE358用于非处方的人类使用的其他外用药品MISCELLANEOUS EXTERNAL DRUGPRODUCTS FOR OVER-THE-COUNTERHUMAN USE361一般认为安全与有效以及不错误标识的处方人用药品:用于研究的药品PRESCRIPTION DRUGS FOR HUMAN USEGENERALL Y RECOGNIZED AS SAFE ANDEFFECTIVE AND NOT MISBRANDED: DRUGSUSED IN RESEARCH369在用于非处方销售的药品与器械上关于警告的解释性声明INTERPRETA TIVE STA TEMENTS REW ARNINGS ON DRUGS AND DEVICES FOROVER-THE-COUNTER SALE370-499[预留的][Reserved]第E分章―动物药品、饮料和相关产品(SUBCHAPTER E―ANIMAL DRUGS, FEEDS, AND RELA TED PRODUCTS)500总则GENERAL501动物食品标识ANIMAL FOOD LABELING502非标准化的动物食品的普通的或通常的名称COMMON OR USUAL NAMES FOR NONSTANDARDIZED ANIMAL FOODS509在动物食品与食品-包装材料中UNA VOIDABLE CONTAMINANTS IN ANIMAL的不可避免的污染物FOOD AND FOOD-PACKAGING MA TERIAL510新动物药NEW ANIMAL DRUGS511作为试验用途的新动物药NEW ANIMAL DRUGS FOR INVESTIGA TIONALUSE514新动物药申请NEW ANIMAL DRUG APPLICA TIONS515含药饲料厂执照MEDICA TED FEED MILL LICENSE520口服剂型的新动物药ORAL DOSAGE FORM NEW ANIMAL DRUGS 522植入或者注射剂型的新动物药IMPLANTA TION OR INJECTABLE DOSAGEFORM NEW ANIMAL DRUGS524眼科和局部剂型的新动物药OPHTHALMIC AND TOPICAL DOSAGE FORMNEW ANIMAL DRUGS526乳房内的剂型INTRAMAMMARY DOSAGE FORMS529某些其他剂型的新动物药CERTAIN OTHER DOSAGE FORM NEWANIMAL DRUGS530在动物中的特别标签药品使用EXTRALABEL DRUG USE IN ANIMALS556在食品中新动物药残留的容许量TOLERANCES FOR RESIDUES OF NEW ANIMAL DRUGS IN FOOD558用于动物饲料的新动物药NEW ANIMAL DRUGS FOR USE IN ANIMALFEEDS564[预留的][Reserved]570食品添加剂FOOD ADDITIVES571食品添加剂申请FOOD ADDITIVE PETITIONS573在动物饲料与饮用水中允许的食品添加剂FOOD ADDITIVES PERMITTED IN FEED AND DRINKING WA TER OF ANIMALS579在动物饲料和宠物食品的生产、加工和处理中的辐照IRRADIA TION IN THE PRODUCTION,PROCESSING, AND HANDLING OF ANIMALFEED AND PET FOOD582一般认为安全的物质SUBSTANCES GENERALL Y RECOGNIZED ASSAFE584在动物饲料与饮用水中被确认为一般认为安全的食品物质FOOD SUBSTANCES AFFIRMED ASGENERALL Y RECOGNIZED AS SAFE IN FEEDAND DRINKING WA TER OF ANIMALS589禁止用于动物食品或者饲料的物质SUBSTANCES PROHIBITED FROM USE IN ANIMAL FOOD OR FEED590-599[预留的][Reserved]第F分章―生物制品(SUBCHAPTER F―BIOLOGICS)600生物制品:总则BIOLOGICAL PRODUCTS: GENERAL 601颁发执照LICENSING606对血液与血液组分的现行良好制造规范CURRENT GOOD MANUFACTURINGPRACTICE FOR BLOOD AND BLOODCOMPONENTS607对人类血液与血液制品的制造者的机构登记与产品列表ESTABLISHMENT REGISTRA TION ANDPRODUCT LISTING FOR MANUFACTURERS OFHUMAN BLOOD AND BLOOD PRODUCTS610普通生物制品标准GENERAL BIOLOGICAL PRODUCTSSTANDARDS630对血液、血液组分和血液衍生物的一般要求GENERAL REQUIREMENTS FOR BLOOD,BLOOD COMPONENTS, AND BLOODDERIVA TIVES640对人类血液和血液制品的附加标准ADDITIONAL STANDARDS FOR HUMAN BLOOD AND BLOOD PRODUCTS660对用于实验室检测的诊断物质的附加标准ADDITIONAL STANDARDS FOR DIAGNOSTIC SUBSTANCES FOR LABORATORY TESTS680对其他产品的附加标准ADDITIONAL STANDARDS FORMISCELLANEOUS PRODUCTS第G分章―化妆品(SUBCHAPTER G―COSMETICS)700总则GENERAL701化妆品标识COSMETIC LABELING710化妆品机构的自愿登记VOLUNTARY REGISTRA TION OF COSMETICPRODUCT ESTABLISHMENTS720化妆品配料构成声明的自愿存档VOLUNTARY FILING OF COSMETIC PRODUCT INGREDIENT COMPOSITION STA TEMENTS740化妆品警告声明COSMETIC PRODUCT WARNINGSTA TEMENTS741-799[预留的][Reserved]第H分章―医疗器械(SUBCHAPTER H―MEDICAL DEVICES)800总则GENERAL801标识LABELING803医疗器械报告MEDICAL DEVICE REPORTING806医疗器械;改正与移动的报告MEDICAL DEVICES; REPORTS OFCORRECTIONS AND REMOV ALS807对器械的制造者与首次进口者的机构登记与器械列表ESTABLISHMENT REGISTRA TION ANDDEVICE LISTING FOR MANUFACTURERS ANDINITIAL IMPORTERS OF DEVICES808对州和地方医疗器械要求的联邦优先权的豁免EXEMPTIONS FROM FEDERAL PREEMPTIONOF STA TE AND LOCAL MEDICAL DEVICEREQUIREMENTS809人用体外诊断产品IN VITRO DIAGNOSTIC PRODUCTS FORHUMAN USE810医疗器械召回权MEDICAL DEVICE RECALL AUTHORITY812试验用器械豁免INVESTIGA TIONAL DEVICE EXEMPTIONS813[预留的][Reserved]814医疗器械的上市前批准PREMARKET APPROVAL OF MEDICALDEVICES820质量体系规章QUALITY SYSTEM REGULA TION医疗器械跟踪要求MEDICAL DEVICE TRACKING 821REQUIREMENTS822上市后监视POSTMARKET SURVEILLANCE医疗器械分类程序MEDICAL DEVICE CLASSIFICA TION 860PROCEDURES性能标准制定程序PROCEDURES FOR PERFORMANCE 861STANDARDS DEVELOPMENT临床化学与临床毒理学器械CLINICAL CHEMISTRY AND CLINICAL862TOXICOLOGY DEVICES864血液学与病理学器械HEMA TOLOGY AND PA THOLOGY DEVICES 免疫学与微生物学器械IMMUNOLOGY AND MICROBIOLOGY866DEVICES868麻醉学器械ANESTHESIOLOGY DEVICES870心血管器械CARDIOVASCULAR DEVICES872牙科器械DENTAL DEVICES874耳、鼻和咽器械EAR, NOSE, AND THROA T DEVICES876胃肠病学-泌尿学器械GASTROENTEROLOGY-UROLOGY DEVICES878普通与整形外科器械GENERAL AND PLASTIC SURGERY DEVICES普通医院与个人使用器械GENERAL HOSPITAL AND PERSONAL USE880DEVICES882神经学器械NEUROLOGICAL DEVICES产科与妇科学器械OBSTETRICAL AND GYNECOLOGICAL 884DEVICES886眼科器械OPHTHALMIC DEVICES888矫形外科器械ORTHOPEDIC DEVICES890内科学器械PHYSICAL MEDICINE DEVICES892放射学器械RADIOLOGY DEVICES895禁止的器械BANNED DEVICES898电极铅线与患者电缆的性能标准PERFORMANCE STANDARD FOR ELECTRODE LEAD WIRES AND PA TIENT CABLES第I分章―乳房造影质量标准法(SUBCHAPTER I―MAMMOGRAPHY QUALITY STANDARDS ACT)900乳房造影法MAMMOGRAPHY第J分章―放射学的健康(SUBCHA PTER J―RADIOLOGICAL HEALTH)1000总则GENERAL1002记录与报告RECORDS AND REPORTS1003缺陷与未能守法的通报NOTIFICA TION OF DEFECTS OR FAILURE TOCOMPL Y1004电子产品的回购、修理或者置换REPURCHASE, REPAIRS, OR REPLACEMENTOF ELECTRONIC PRODUCTS1005电子产品的进口IMPORTA TION OF ELECTRONIC PRODUCTS1010电子产品的性能标准:总则PERFORMANCE STANDARDS FORELECTRONIC PRODUCTS: GENERAL 1020电离辐射发生产品的性能标准PERFORMANCE STANDARDS FOR IONIZINGRADIA TION EMITTING PRODUCTS1030微波与射电频率发生产品的性能标准PERFORMANCE STANDARDS FORMICROWA VE AND RADIO FREQUENCYEMITTING PRODUCTS1040发光产品的性能标准PERFORMANCE STANDARDS FORLIGHT-EMITTING PRODUCTS1050声波、次声波和超声波发生产品的性能标准PERFORMANCE STANDARDS FOR SONIC,INFRASONIC, AND ULTRASONICRADIA TION-EMITTING PRODUCTS第K分章―[预留的](SUBCHAPTER K―[RESERVED])第L分章―根据由食品与药品管理局行政执行的某些其他法的规章(SUBCHAPTERL―REGULA TIONS UNDER CERTAIN OTHER ACTS ADMINISTERED BY THE FOOD AND DRUG ADMINISTRA TION)1210根据《联邦进口乳法》的规章REGULA TIONS UNDER THE FEDERAL IMPORTMILK ACT1230根据《联邦腐蚀性毒物法》的规章REGULA TIONS UNDER THE FEDERAL CAUSTIC POISON ACT1240传染病的控制CONTROL OF COMMUNICABLE DISEASES1250州际运输卫生INTERSTA TE CONVEYANCE SANITA TION1251-1269[预留的][Reserved]1270预期用于移植的人体组织HUMAN TISSUE INTENDED FORTRANSPLANTA TION1271人体细胞、组织以及细胞的和基于组织的产品HUMAN CELLS, TISSUES, AND CELLULAR AND TISSUE-BASED PRODUCTS1272-1299[预留的][Reserved]第Ⅱ章―司法部毒品强制执行局(CHAPTER ADMINISTRATION, DEPARTMENT OF JUSTICE)1300定义DEFINITIONS1301管制物质的制造者、分销者和调剂者的登记REGISTRA TION OF MANUFACTURERS,DISTRIBUTORS, AND DISPENSERS OFCONTROLLED SUBSTANCES1302对管制物质的标识与包装要求LABELING AND PACKAGING REQUIREMENTSFOR CONTROLLED SUBSTANCES1303定额QUOTAS1304登记者的记录与报告RECORDS AND REPORTS OF REGISTRANTS 1305令的格式ORDER FORMS1306处方PRESCRIPTIONS1307杂项MISCELLANEOUS1308管制物质的表SCHEDULES OF CONTROLLED SUBSTANCES1309表I化学品的制造者、分销者、进口者和出口者的登记REGISTRA TION OF MANUFACTURERS,DISTRIBUTORS, IMPORTERS AND EXPORTERSOF LIST I CHEMICALS1310列入表的化学品和某些机器的记录与报告RECORDS AND REPORTS OF LISTED CHEMICALS AND CERTAIN MACHINES1311[预留的][Reserved]1312管制物质的进口与出口IMPORTA TION AND EXPORTA TION OFCONTROLLED SUBSTANCES1313前体与必要化学品的进口与出口IMPORTA TION AND EXPORTA TION OF PRECURSORS AND ESSENTIAL CHEMICALS1314-1315[预留的][Reserved]1316行政职能、规范和程序ADMINISTRA TIVE FUNCTIONS, PRACTICES,AND PROCEDURES第Ⅲ章―毒品控制政策办公室(CHAPTER Ⅲ―Off)1400[预留的][Reserved]1401信息的公众可及性PUBLIC A VAILABILITY OF INFORMA TION 1402强制性解密审查MANDATORY DECLASSIFICA TION REVIEW1403对给予州和地方政府资金和合作协议的统一行政要求UNIFORM ADMINISTRA TIVE REQUIREMENTSFOR GRANTS AND COOPERA TIVEAGREEMENTS TO STA TE AND LOCALGOVERNMENTS1404政府范围的排除与暂停(非获得)GOVERNMENTWIDE DEBARMENT AND SUSPENSION (NONPROCUREMENT)1405对无毒品工作场所的政府范围的要求(财政援助)GOVERNMENTWIDE REQUIREMENTS FORDRUG-FREE WORKPLACE (FINANCIALASSISTANCE)1406-1499[预留的][Reserved]。
FDA生物制品评价和研究中心(CBER)组织机构和主要职位清单

Division of Viral Products(病毒制品处)
Deputy Director(副主任)
Laboratory of Hepatitis Viruses(肝炎病毒实验室)
Deputy Director(副主任)
Blood and Plasma Branch(血液和血浆科)
Regulatory Project Management Branch(项目监管科)
Devices Review Branch(器械审查科)
七、OFFICE OF VACCINES RESEARCH AND REVIEW(疫苗研究和审查办公室)
Laboratory of Respiratory & Special Pathogens(呼吸系统和特殊病原体实验室)
Laboratory of Bacterial Toxins(细菌毒素实验室)
Lab. of Enteric & Sexually Transmitted Diseases(肠道和性传播疾病实验室)
Laboratory of Biophysics(生物物理学实验室)
Laboratory of Bacterial Polysaccharides(细菌多糖类实验室)
Laboratory of Mycobacterial Diseases & Cellular Immunology(分歧杆菌疾病和细胞免疫学实验室)
Division of Bacterial, Parasitic & Allergenic Products(细菌、寄生和变应原产品处)
安庆2024年小学六年级第3次英语第1单元真题

安庆2024年小学六年级英语第1单元真题考试时间:90分钟(总分:120)A卷考试人:_________题号一二三四五总分得分一、综合题(共计100题)1、听力题:The chemical symbol for technetium is ______.2、What do you call a fear of spiders?A. AgoraphobiaB. ArachnophobiaC. ClaustrophobiaD. Nyctophobia答案:B3、听力题:The __________ is a famous area known for its training grounds.4、填空题:Certain plants can ______ (增强) the local economy.5、听力题:The chemical formula for sodium oxalate is ______.6、What do we call a person who designs buildings?A. ArchitectB. EngineerC. ContractorD. Builder答案:A7、What is the name of the famous scientist who discovered the laws of motion?A. Albert EinsteinB. Isaac NewtonC. Galileo GalileiD. Charles Darwin答案: BThis ________ (玩具) teaches me about science.9、填空题:I like to eat _____ (水果) in summer.10、听力题:The ancient Egyptians used ________ to document their history.11、听力题:I need to ________ my lunch.12、填空题:My pet ___ (小鸟) sings every morning.13、填空题:A ______ (小径) can lead through a garden.14、听力题:We will _______ (hike) in the mountains.15、What is the term for a baby pig?A. CalfB. PigletC. LambD. Kid答案: B16、填空题:The __________ (历史的启迪) can motivate change.17、Which day comes after Monday?A. SundayB. TuesdayC. WednesdayD. Thursday18、填空题:The owl is wise and can turn its _________ (头) 180 degrees.19、听力题:The soup is ___ (hot/cold) today.20、听力题:The __________ is a region known for its historical significance.The _____ (植物生长周期) includes various stages.22、听力题:We need to ___ (clean) our room.23、听力题:Many galaxies are moving away from us, suggesting the universe is ______.24、填空题:The _______ (The Great Depression) led to significant changes in government policy.25、填空题:She has a beautiful _______ (声音).26、Which of these is a type of transportation?A. BicycleB. TreeC. HouseD. Mountain答案:A27、填空题:My ________ (祖父) loves to tell stories about his adventures.28、填空题:I saw a _______ (小刺猬) in the garden.29、填空题:I enjoy playing ________ (棋类游戏) with my family.30、What is the name of the first spacecraft to successfully land on Mars?A. Viking 1B. SpiritC. OpportunityD. Curiosity31、听力题:The chemical formula for strontium carbonate is _____.32、填空题:A ____(community safety initiative) promotes public well-being.33、Which one is a cold drink?A. CoffeeB. TeaC. LemonadeD. Soup34、填空题:I like to dance with my ________ (玩具名称).35、What is the process of water turning into vapor called?A. EvaporationB. CondensationC. PrecipitationD. Filtration答案:A36、What is the fastest land animal?A. CheetahB. LionC. HorseD. Gazelle答案:A37、What do we call the science of studying space?A. BiologyB. ChemistryC. AstronomyD. Geology38、填空题:The goldfish is one of the most popular ______ (宠物) in homes.39、Which animal has a long trunk?A. GiraffeB. ElephantC. RhinoD. Hippopotamus答案:B40、 (3) is known for its deserts and wildlife. 填空题:The ____41、What is the capital of Bangladesh?A. DhakaB. ChittagongC. KhulnaD. RajshahiI want to create a video game about my toy ____. (玩具名称)43、What do you call a place where animals are kept for public viewing?A. FarmB. ZooC. AquariumD. Park答案:B44、听力题:I have a ___ (cool) skateboard.45、填空题:I went to the zoo and saw a ______.46、听力填空题:I love reading mystery books. My favorite author is __________.47、What is the capital of Sweden?A. OsloB. CopenhagenC. StockholmD. Helsinki答案: C48、听力题:A horse gallops across the _____.49、What is the name of the famous bear who loves honey?a. Paddington Bearb. Winnie the Poohc. Balood. Smokey Bear答案:b50、填空题:The butterfly flutters from flower to ______ (花).51、填空题:The _______ (猫) likes to hide in boxes.52、听力题:A __________ is a substance made up of only one type of atom.I enjoy cooking with my _______ (家庭成员). It’s a fun way to spend time together.54、填空题:I _______ (想要) a new toy for my birthday.55、填空题:My favorite place is the ______ (海边).56、How many continents are there?A. FiveB. SixC. SevenD. Eight57、听力题:I love to ______ in the summer. (swim)58、What do we call the liquid part of blood?A. PlasmaB. SerumC. PlateletsD. Hemoglobin答案:A59、What is the name of the fairy tale character who had a red hood?A. CinderellaB. Sleeping BeautyC. Little Red Riding HoodD. Snow White答案: C60、填空题:A _____ (章鱼) can fit through small openings.61、填空题:The ferret is very _________. (好动)62、填空题:I have a kind . (我有一个善良的。
GenericDrugs

Generic Drugs: Overview of ANDA ReviewProcessTed SherwoodOffice of Pharmaceutical ScienceBrand vs. GenericG etF eelB etterB etterWhat is the Main ConsumerConcern Regarding Generics?z Do the quality and performance ofgeneric drugs compare to brand drugs?Often triggered by brand companiesand physiciansLegislative Historyz1906 Pure Food and Drug Act -establishes regulation of Food and Drugsz1938 Food, Drug and Cosmetic Act -introduced safety standardsz1962 Kefauver-Harris Amendments to the FDA&C Act -tightened safety standards and introduced requirement that drugs must be effectivez1984 Hatch-Waxman Act -created an abbreviated mechanism for approval of generic copies of all drugsoriginally approved after 1962, by stating that pre-clinical and clinical testing did not have to be repeated for genericsDefinition of a Generic DrugA drug product that is comparable to abrand/reference listed drug product in dosage form, strength, route of administration, quality and performance characteristics, and intended use.When can a Generic Drugbe Marketed?z After patent & exclusivity protection ends, or z patent owner waives its rights, andz FDA requirements are metPatent Protection• 17 years from the date the patent was issuedor• 20 years from the date the patent was submitted (to the Patent Office, not FDA)Equates to approximately 12 yearsof marketing protection.Patent FilingGranted by U.S. Patent and Trademark OfficeSubmitted to/for NDAs onlyCoversDrug Substance – Active IngredientMethod of Use – IndicationDrug Product – Formulation, CompositionPublished in Orange BookDelays final approval date of ANDAsApproximately 240 patents listed in Orange Bookwill expire in the next 5 yearsPatent CertificationI Patent Not Submitted to FDA -approval effective after OGD scientific determinationII Patent Expired -approval effective after OGD scientific determinationIII Patent Expiration Date (honored) – tentative approval after OGD scientific determination, final approval when patent expiresIV Patent Challenge – tentative approval after OGD science determination, final approval when challenge wonPatent Challenge ProcessParagraph IV certification by ANDA holder declaring patent invalid, not infringed, or not enforceableNotification provision on ANDA holder45-Day clockNo lawsuit – challenge successfulLawsuit – 30 months (risk of marketing aftermeeting FDA approval criteria) or final courtdecision, whichever earlierPatent Challenge Successful –Award of 180-Day ExclusivityPeriodAwarded to first ANDA holder to file acomplete application with patent challenge Protection from other generic competition – blocks approval of subsequent ANDAs Protection triggered by:First commercial marketingForfeiture provisionsExclusivityFDA controlled reward primarily tobrand name/new drug companiesfor continued developmentOrphan Drug Exclusivity (ODE) Orphan drug refers to a product that treats a raredisease -affecting fewer than 200,000 Americans7 years exclusivityGranted on approval of designated orphan drugOGD works with the Office of Orphan ProductsNew Chemical Entity (NCE)5 years exclusivityApplies to NCEs approved afterSeptember 24, 1984“Other”/Waxman-Hatch 3 years exclusivityApplies to “significant” approved change where new clinical studies (other than bioavailability studies) were conducted by the NDA holder and were essential for FDA’s approval.Changes include new: dosage form, strength, routeof administration, indication, dosing regimen, Rx to OTC switchPediatric6 months of exclusivityAdditive to patent or other exclusivity protectionApplies to all applications held by the NDAholder for that active moietyPatent and ExclusivityQuestionsPatent and Exclusivity Summary:Traditional TimelinesPatents: 17-20 years (Granted by PTO)P a t e n t S u b m i t t e d P a t e n t A p p r o v e dE x p i r e s !!!Exclusivity 3 years (Granted by FDA)A p p r o v e dE x p i r e s !!!A p p r o v e dE x p i r e s !!!Can be hereUsually hereN D A A p p r o v a lN D A S u b m i t t e d20 1712 year average-3P a t e n t S u b m i t t e d Patent and Exclusivity Summary: Traditional TimelinesN D A S u b m i t te dN D A A p p r o v a lA p p r o v e dE x p i r e s !!!A p p r o v e dExclusivity 3 years 200 mg 1x/dayCan be hereP a t e n t A p p r o v e dPatent: tied to 50 mg 4x/dayUsually hereE x p i r e s !!!E x p i r e s !!!Patent: tied to 50 mg 4x/dayUsually hereExclusivity 3 years 200 mg 1x/dayP a t e n t S u b m i t t e d N D A S u b m i t t e dE x p i r e s !!!200 mgP a t e n t A p p r o v e dGenerics can be approvedN D A A p p r o v a l50 mg A p p r o v e dE x p i r e s !!!Generics can be approved Indication 2 P a t e n tS u b m i t t e dP a t e n t A p pr o v e dN D AS u b m i tt ed N D AA p pr o v alA pp r o v e dE x pi r e s !!! Indication 1 E xpir e s!!!Exclusivity 3 years New Indicationcan beP a te n tS u bm i t t e d P a t e n tA p p r o ve d N D AS u b m i tt e dN D A A p p ro v alA N D As u b mi tt e dF D Ar e v i e wP er i od:16mon .Normal Cases (excludes NCEs)Generic Submission Timelines: E xp i r e s !!! Patents: 17-20 years (Granted by PTO) Full/FinalApprovalGeneric Drug Usually here Submissions grantedcan be here TentativeDay after NDA approval Trend Approvalcan be grantedWhat are the BasicGeneric Drug Requirements? z Same active ingredient(s)z Same route of administrationz Same dosage formz Same strengthz Same conditions of usez Inactive ingredients already approved in a similar NDANDA vs. ANDA Review Process (NDA) Requirements (ANDA) Requirements1. Labeling2. Pharm/Tox3. Chemistry4. Manufacturing5. Controls6. Microbiology7. Inspection8. Testing9. Animal Studies10. Clinical Studies 11. Bioavailability1. Labeling2. Pharm/Tox3. Chemistry4. Manufacturing5. Controls6. Microbiology7. Inspection8. Testing9. BioequivalenceLabeling“Same” as brand name labelingMay delete portions of labeling protected bypatent or exclusivity (i.e., an indication)May differ in excipients and product description(i.e., colors, shapes)NDA vs. ANDA Review Process (NDA) Requirements (ANDA) Requirements1. Labeling 1. Labeling2. Pharm/Tox 2. Pharm/Tox3. Chemistry 3. Chemistry4. Manufacturing 4. Manufacturing5. Controls 5. Controls6. Microbiology 6. Microbiology7. Inspection 7. Inspection8. Testing 8. Testing9. Animal Studies10. Clinical Studies 9. Bioequivalence11. BioavailabilityPharm/ToxAll inactive ingredients must be approved in either the reference listed drug or similar NDA in same or higher levels. (FDA publishes the ingredient and highest approved levels.) Generic focus – is there anything unique tousing this ingredient in the proposed genericNDA vs. ANDA Review Process (NDA) Requirements (ANDA) Requirements1. Labeling 1. Labeling2. Pharm/Tox 2. Pharm/Tox3. Chemistry 3. Chemistry4. Manufacturing 4. Manufacturing5. Controls 5. Controls6. Microbiology 6. Microbiology7. Inspection 7. Inspection8. Testing 8. Testing9. Animal Studies10. Clinical Studies 9. Bioequivalence11. BioavailabilityNDA vs. ANDA Review Process(NDA) Requirements(ANDA) Requirements1. Labeling 1. Labeling2. Pharm/Tox 2. Pharm/Tox3. Chemistry 3. Chemistry4. Manufacturing 4. Manufacturing5. Controls 5. Controls6. Microbiology 6. Microbiology7. Inspection 7. Inspection8. Testing 8. Testing9. Animal Studies10. Clinical Studies 9. Bioequivalence11. BioavailabilityChemistry, Manufacturing and Controls (CMC)Components and compositionManufacturing and ControlsBatch formulation and recordsDescription of facilitiesSpecifications and testingPackagingStabilityNDA vs. ANDA Review Process (NDA) Requirements (ANDA) Requirements1. Labeling 1. Labeling2. Pharm/Tox 2. Pharm/Tox3. Chemistry 3. Chemistry4. Manufacturing 4. Manufacturing5. Controls 5. Controls6. Microbiology 6. Microbiology7. Inspection 7. Inspection8. Testing 8. Testing9. Animal Studies10. Clinical Studies 9. Bioequivalence11. BioavailabilityInspections/TestingAssure adherence to and authenticity of data submitted in the applicationAssure manufacturing facilities are in compliance with current good manufacturing practices (CGMPs) Assure bioequivalence sites are in compliance with current good clinical practices (CGCPs)Conducted primarily by Field/Office of Regulatory Affairs with support from Center (Office of Compliance) and assigned geographicallyNDA vs. ANDA Review Process (NDA) Requirements (ANDA) Requirements1. Labeling 1. Labeling2. Pharm/Tox 2. Pharm/Tox3. Chemistry 3. Chemistry4. Manufacturing 4. Manufacturing5. Controls 5. Controls6. Microbiology 6. Microbiology7. Inspection 7. Inspection8. Testing 8. Testing9. Animal Studies10. Clinical Studies 9. Bioequivalence11. BioavailabilityWhat is Bioequivalence?z The rate and extent of absorption do not show a significant difference from listed drug, or z The extent of absorption does not show asignificant difference and any difference in rate is intentional or not medically significantA generic drug is considered to be bioequivalent to the brand name drug if:gCo nOGD Staff Total 240 Chemist 105 Bioequivalence/ 35 PharmacologistsProject Managers/ 65 Pharmacists Microbiologists 11 Medical Officers 2 Math Statisticians 2 IT Specialists 3 Admin/Support Staff 17Generic Drug Review Process Issuesz Consistency between reviews ofmultiple applications for the same drug z Fairness in timing of reviews z Patent/exclusivity issues z Demonstration of BioequivalenceCommunications withANDA Holders Acknowledgement of Receipt LetterStates date of application filingRefuse to Receive (82/year)Deficiency Actions (Bio and Labeling)Not Approvable Actions (CMC) (944/year)Minor deficiencies – 60-day review clockMajor deficiencies – 180-day review clockTentative Approval – approval pending patent expiration/resolutionApproval – drug product can be marketedPost MarketingChanges to an approved ANDA (21 CFR 314.70)Supplements (3500 received/year)Changes Being Effective (CBE)Changes Being Effective in 30-days (CBE-30)Prior Approval Supplement (PAS)Annual Report (6000 received/year)Summary of product(current) LabelingDistribution dataReporting of Adverse Drug Events(21 CFR 314.80 and 314.98)15-Day “Alert Reports” (both serious andunexpected)Periodic Adverse Drug Experience Reports quarterly for the first three years post-approval and annually thereafterPost Marketing (cont.)Manufacturing Compliance Programs Purpose to assure quality of marketed drug productsMechanismsSurveillanceManufacturing/testing plant inspections to assess ANDA holder’s continued compliance with good manufacturing practicesPost Marketing (cont.) Therapeutic Inequivalence Action Coordinating CommitteeEvaluates reports and related information on possible therapeutic failures and toxicity that are attributed to inequivalence for drug products Recommendations regarding appropriateregulatory actions to be taken based on a scientific evaluation and risk assessmentPost Marketing (cont.)Promotional Materials – for all brand and generic drug prescription productsProduct quality surveys – a recent review of 1,159 studies submitted to OGD revealed that the average difference between generics and their respective brand drugs was 3%Post Marketing (cont.)How is Generic DrugQuality Assured?z First 8 steps of review process identical to NDA processz Bioequivalence requirements for ANDA’s sameas NDA’sz FDA has experience with the productz Product is known to be safez Scientific literature publishedz Over half are produced by brand name manufacturesz Post-approval product surveysSpecial InitiativesCritical Path Initiative Medical product development path is becomingincreasingly challenging, inefficient and costly Need to update tools used to assess safety and efficacy“Toolkit” should contain powerful new scientific and technical methods to improve predictability and efficiency along the critical path from laboratory concept to commercial productQuestion Based Review Keep review up to date with advances inmanufacturing and formulation scienceQuality by DesignProcess Analytical TechnologySpecifications based on benefit to the consumer –eliminate non-scientific controls with no value to product qualityProduct specific risk assessmentReduce supplementsUse FDA resources effectivelyQuality by Design Understanding the product as it is developed anddesignedUnderstanding critical attributesDesigning product and process to be robust withregard to these attributesKnowing what happens to those attributes if changesare made in productionProvide the tools to utilize risk based approachesProcess AnalyticalTechnologyA system for designing, analyzing, andcontrolling manufacturing through timely measurements (i.e., during processing) of critical quality and performance attributes of raw and in-process materials and processes with the goal of ensuring final product quality.International Conference on Harmonization (ICH)To harmonize the interpretation and applicationof technical guidelines and requirementsTo reduce or eliminate duplicate testing duringresearch and development in participating countriesPresident's Emergency Plan for Acquired ImmunodeficiencySyndrome Relief (PEPFAR) Standard but expedited ANDA reviewSeveral ANDAs approvedEconomicsEstimated Savings Through Generic Drug Use$67 per retail prescriptionor$10.0 Billion a Year(just in the U.S.)DHHS Dec. 2004 "if consumers were to buy generic products whenever possible ... we estimate savings to be approximately $17 billion."GPhA May 2005Typical Price ComparisonsDrug Generic Price $/30 BrandPrice$/30 Lisinopril (Zestril®) 20 mg 20.69 46.69 Citalopram (Celexa®) 20 mg 52.99 100.99 Ciprofloxacin (Cipro®) 500 mg 88.59 215.99 Metformin (Glucophage®) 1000 mg 30.69 71.59 Fluconazole (Diflucan®) 200 mg 372.99 609.99 Fluoxetine (Prozac®) 20 mg 32.29 139.99Source: Washington metropolitan area pharmacies, March 2005. Drug Spending47.9 95.9 55.9 47.695.17450100 150 2002502005 2006Government ProgramsPrivateInsurance Out-of-Pocket Retail Rx Drugs In BillionsCMSFutureOver a $50 billion worth of drug products losingprotection in the next five yearsAugust 3, 2005: 5:52 PM EDTBy Aaron Smith, CNN/Money staff writerValue of Generics•Reduce Drug Costs•Increase Drug Use•Prevent Drug Shortages-Product rationalization-Supply disruptionBrand vs. GenericG etF eelB etterB etterSummaryU.S. DEPARTMENT OF HEALTH AND HUMAN SERVICESPUBLIC HEALTH SERVICEFOOD AND DRUG ADMINISTRATIONCENTER FOR DRUG EVALUATION AND RESEARCHOFFICE OF MANAGEMENTDIVISION OF DATABASE MANAGEMENTAPPROVEDDRUG PRODUCTS WITHTHERAPEUTIC EQUIVALENCE EVALUATIONS27 TH EDITIONTHE PRODUCTS IN THIS LIST HAVE BEEN APPROVED UNDERSECTIONS 505 AND 507 OF THE FEDERAL FOOD, DRUG, AND COSMETIC ACT. 2007“Orange Book”z All FDA approved drug products listed (NDA’s, ANDA’s and non-monograph OTC’s) z Therapeutic equivalence codes: NDAs & ANDAs -“A” = Substitutable-“B” = Inequivalent, NOT substitutable z Expiration dates: patent and exclusivity z Reference Listed Drugs -brand drugs identified by FDA for generic companies to compare their proposed products withOrange BookInternet Address/cder/orange/default.htmOther Generic Drug Links Office of Generic Drugs Home Page:/cder/ogd/index.htmOn line training program:/cder/learn/CDERLearn/gen DrugProcess/transcript.htmQuestions:Ted SherwoodSpecial Assistant to the DirectorOffice of Pharmaceutical Science, CDER White Oak, Bldg 21, Room 352810903 New Hampshire Ave.Silver Spring, MD 20903-0002Tel: 301-796-1605, Fax: 301-796-9733 ***********************.gov。
练习一 翻译以下句子,注意使用适当的衔接方式

练习一翻译以下句子,注意使用适当的衔接方式1.你太忙,没有时间打扫房间。
让我来吧。
2. A.你对他的看法怎样?B. 他聪明,勤奋,就是有点自私。
A. 我也这样看。
3.太阳刚刚下了地平线。
软风一阵一阵地吹上人面,怪痒痒的。
(茅盾《子夜》)4. 若是站在后山看下去,晴天里一片头巾、花帕、草帽,雨天里一片斗篷、纸伞、布伞。
(古华《芙蓉镇》)5. 横眉冷对千夫指,俯首甘为孺子牛。
(鲁迅)6.长江后浪推前浪,世上新人换旧人。
7.凡人不可貌相,海水不可斗量。
8.却当大雪之后,石是青的,雪是白的,树上枝条是黄的,又有许多松柏是绿的,一丛一丛,如画上点的苔一样。
(刘鹗《老残游记》)练习二翻译下面的词语和句子。
注意(1-12)项中动词的及物性;注意(13-30)项中动词的时态。
1.敲门2. 靠墙3.笑话她4. 想你5. 考虑问题6.因此在这个问题上我重复了这么多便以后,我今天实在不想再讲了。
7.如果今年再提出来,我想也不会有别的结果。
8.在亚洲的金融风暴中,对香港的影响在今年已经陆续显现了,尤其是在今年的上半年。
9. 咱们去吃饭吧。
10. 他们在读书。
11. 我们要在音乐会上唱歌。
12.你付钱了吗?13. 到2007年,这个比例将达到92%。
14. 我妹妹今天没去上学。
她得了重感冒,她头痛、发烧。
15. 你没有迟到,还有三分钟。
16.---- 你今天下午有空吗?--- 我四点以后才有空。
17. 我们一出发,天就下起雨来了。
18. 如果她更用功些,她就会学得更好。
19.---- 他入党多久了?---- 他入党二十多年了。
20. 电影已经开始十分钟了。
21. 已经上课十分钟了。
22. 那本英语书他已丢了三天了。
23. 图书馆已经开门半小时了。
24. 托马斯.爱迪生去世已经六十五年了。
25. 20年来,中国的科学家们为祖国做出了贡献。
26. 等我们的老师从国外回来,我们就要做完我们的工作了。
27. 中国人民不用多久就会变得富裕起来。
牡丹江2024年01版小学U卷英语第四单元自测题

牡丹江2024年01版小学英语第四单元自测题考试时间:80分钟(总分:110)A卷考试人:_________题号一二三四五总分得分一、综合题(共计100题)1、填空题:I love to watch the _____ in the sky.2、填空题:The teacher emphasizes the value of _____ (团队合作).3、听力题:A compound that has both acidic and basic properties is called an ______.4、Which animal is known as "man's best friend"?A. CatB. DogC. FishD. Bird答案: B5、听力题:The chemical formula for potassium oxalate is _____.6、What do we call the process of converting solid to liquid?A. FreezingB. MeltingC. BoilingD. Evaporating答案: B7、What do we call a group of lions?A. PackB. PrideC. HerdD. Gaggle答案:B8、How many months are there in a year?A. TenB. TwelveC. ElevenD. Nine答案:B9、Which of these is a type of tree?A. RoseB. OakC. DaisyD. Sunflower答案:B10、填空题:A turtle moves slowly and has a hard ______.11、听力题:The Earth's crust is composed of various geological ______.12、What is the frozen form of water?A. SteamB. IceC. SnowD. Rain答案:B13、填空题:I like to write stories about ______.14、听力题:The color of an object depends on the ______ (wavelength) of light it reflects.15、听力题:A covalent bond involves the sharing of ______.16、填空题:A ______ (植物的生长条件) can be manipulated for better results.17、填空题:The _____ (种植) season begins in spring.18、听力题:I have a _____ (玩具车) in my room.The soup is too _____ (spicy/bland).20、填空题:The country famous for sushi is ________ (日本).21、听力题:A ______ can help restore habitats.22、What do we use to tell time?A. RulerB. ClockC. ScaleD. Compass答案:B. Clock23、What is the name of the famous character in "The Little Mermaid"?A. ArielB. BelleC. JasmineD. Cinderella答案:A24、填空题:My pet rabbit has soft _______ (毛) that I like to pet.25、听力题:The chemical formula for aluminum sulfate is ______.26、听力题:A _______ is a chemical method of preserving food.27、听力题:The Earth's crust is constantly undergoing changes due to ______ and erosion.28、填空题:I like to ______ (参与) in local events.29、听力题:The _____ (river/ocean) is deep.30、填空题:My ________ (舅舅) works at a bank and always helps me with money.The __________ (峡谷) is deep and wide.32、What do you call a young rabbit?A. KitB. CalfC. KidD. Pup33、Which of these is not a fruit?A. BananaB. OrangeC. CarrotD. Apple答案:C34、填空题:My friend loves to engage in __________ (志愿活动).35、填空题:The teacher helps students develop _____ (技能).36、Which of these is a type of transportation?A. BicycleB. TreeC. HouseD. Mountain答案:A37、Which animal is known as the "king of the jungle"?A. TigerB. ElephantC. LionD. Bear答案:C38、填空题:I have a nickname that my friends call me, which is .(我有一个昵称,我的朋友称我为。
欧盟-罕见病

Orphan designation•Email•Print•Help•ShareThis section provides guidance and procedural information on applyingfor orphan designation for medicines for rare diseases in the European Union (EU). It also includes information on what happens after a designation has been granted, including the incentives available for sponsors developing orphanmedicines.Orphan designationTo qualify for orphan designation, a medicine must meet a number of criteria:•it must be intended for the treatment, prevention or diagnosis of a disease that is life-threatening or chronically debilitating;•the prevalence of the condition in the EU must not be more than 5 in 10,000 or it must be unlikely that marketing of the medicine would generate sufficient returns to justify the investment needed for its development;•no satisfactory method of diagnosis, prevention or treatment of the condition concerned can be authorised, or, if such a method exists, the medicine must be of significant benefit to those affected by the condition.Applications for orphan designation are examined by the European MedicinesAgency'sCommittee for Orphan Medicinal Products (COMP), using the network of experts that the Committee has built up. The evaluation process takes a maximum of 90 days from validation.For information on how to apply, see how to apply for orphan designation.After orphan designationSponsors who obtain orphan designation benefit from a number of incentives,includingprotocol assistance, a type of scientific advice specific for designated orphanmedicines, and market exclusivity once the medicine is on the market. Fee reductions are also available depending on the status of the sponsor and the type of service required.When planning the development of their medicinal product, sponsors should consult the relevant scientific guidelines.Sponsors must submit an annual report to the Agency summarising the status ofdevelopment of the medicine.Applications for marketing authorisation for designated orphan medicines are assessed by the Committee for Medicinal Products for Human Use (CHMP). Sponsors also need to submit an application for maintenance of the orphan designation in order to be eligible for the 10-year market exclusivity incentive. Sponsors may also need to submit anevaluation of orphan similarity.More information•Legal background•How to apply for orphan designation•Activities after orphan designation•Guidance and formsLegal background: orphan designation•Email•Print•Help•ShareThis page summarises the legal background to the procedure for orphandesignation in the European Union (EU). It includes the key milestones in EU legislation adopted since the Orphan Regulation was first adopted in 1999.Regulation (EC) No 141/2000 (the Orphan Regulation)On 16 December 1999, the European Parliament adopted Regulation (EC) No 141/2000 (the Orphan Regulation). This was published in the Official Journal of the EuropeanCommunities on 22 January 2000.The Regulation:•lays down the EU procedure for designation of orphan medicines;•defines incentives for the development and placing onto the market of designated orphan medicines;•establishes the Committee for Orphan Medicinal Products (COMP).Regulation (EC) No 847/2000On 27 April 2000, the European Commission adopted Regulation (EC) No 847/2000, which:•lays down implementing rules;•sets out definitions essential for the application of the Orphan Regulation.This Regulation entered into force on 28 April 2000. On this date, sponsors could begin to submit applications for orphan designation to the European Medicines Agency.European Commission Communication 2003/C 178/02On 29 July 2003, the European Commission adopted Communication 2003/C 178/02, which sets out the Commission's interpretation on:•matters relating to the implementation of the orphan designation;•provisions for market exclusivity.Regulation (EC) No 726/2004On 31 March 2004, the European Parliament adopted Regulation (EC) No 726/2004, which provides the legal framework for the centralised authorisation and supervision of medicines for human and veterinary use and establishes the European Medicines Agency (EMA). It determines that:•all marketing authorisations for orphan medicines in the EU should follow the centralised authorisation procedure;•the CHMP can issue guidance regarding compassionate-use programmes.Regulation (EC) No 507/2006On 29 March 2006, the European Parliament adopted Regulation (EC) No 507/2006, which provides the legal framework for the granting of a conditional marketingauthorisation to medicines that fall within the scope of Regulation (EC) No 726/2004. It establishes that orphan medicines can be granted a conditional marketingauthorisationwithin this legal framework.Regulation (EC) No 1901/2006On 12 December 2006, the European Parliament adopted Regulation (EC) No 1901/2006 on medicinal products for paediatric use. It establishes that the usual period of market exclusivity for orphan medicines may be extended to twelve years if study results are submitted in compliance with an agreed paediatric investigation plan at the timeofmarketing authorisation.Regulation (EC) No 2049/2005On 15 December 2005, the European Commission adopted Regulation (EC) No 2049/2005 regarding the payment of fees to, and receipt of assistance from, the EMA by micro, small and medium-sized enterprises (SMEs). It determines that scientific adviceandscientific services for designated orphan medicines shall be provided by the EMA to SMEs free of charge.European Commission guidelinesThe European Commission has also issued guidelines on aspects of the application of the Orphan Regulation:•Guideline on aspects of the application of Article 8(1) and (3) of Regulation (EC) No 141/2000: Assessing similarity of medicinal products versus authorised orphan medicinal products benefiting from market exclusivity and applying derogations from that market exclusivity;•Guideline on aspects of the application of Article 8(2) of Regulation (EC) No 141/2000 of the European Parliament and of the Council: Review of the period ofmarket exclusivity of orphan medicinal products.How to apply for orphan designation•Email•Print•Help•ShareThis page provides information for sponsors on how to apply to the European Medicines Agency for orphan designation for a medicine.Notification of intention to submitSponsors should notify the EMA of their intention to submit an application as early as possible, and at the latest two months prior to the planned submission date. Thisnotification should be sent by e-mail orphandrugs@ema.europa.eu. It should include: •the name of the active substance;•the proposed orphan indication (i.e. treatment, prevention or diagnosis of a rare disease); •the name and address of the sponsor;•the planned submission date for the designation application and the proposed date for a pre-submission meeting (if required);•the unique product identifier (UPI) number. If you already have a UPI number please quote it in all your correspondence. Otherwise, the number will be assigned automatically when we receive your intent to file and communicated to you by e-mail.Presubmission meetingsThe Agency strongly encourages sponsors to request a presubmission meeting with the Agency prior to filing an application.Presubmission meetings usually take place via teleconference, unless the sponsor has a strong preference to come to the Agency in person.Where possible, sponsors should request a pre-submission meeting at least two months prior to filing. Presubmission meetings for orphan designation are free of charge.Presubmission meetings are useful since the evaluation process has a fixed duration of 90 days and cannot be lengthened to accommodate for the lack of data or other omissions in the application. Experience has shown that they have a positive impact on the success rate of the applications.Application procedureSponsors should use the forms below to apply for orphan designation:•Application form for orphan-medicinal-product designation•Common European Medicines Agency / Food and Drug Administration (FDA) application form or application form for orphan medicinal product designation•Template for sections A to E for the scientific part of the application for orphan designation •Translations required with the submission of an application for orphan medicinal product designationRefer to these documents for assistance completing these forms:•Guideline on the format and content of applications for designation as orphan medicinal products and on the transfer of designations from one sponsor to another, 27 March 2014•Points to consider on the calculation and reporting of the prevalence of a condition for orphan designation•Recommendation on elements required to support the medical plausibility and the assumption of significant benefit for an orphan designation•Data providers and sources to identify existing authorised medicinal products in the European Union and European Economic AreaIn particular, when completing section A.3.2 'Plausibility of the orphan condition; rationale for use of the medicinal product', sponsors should clearly identify studies with thesubstance in a relevant model(s) of the condition and, if possible, preliminary clinical data in patients with the condition.Each application is assigned two coordinators:•one member of the Committee for Orphan Medicinal Products (COMP);•one scientific administrator from the Agency secretariat.Once the application form and sections A to E are complete, the sponsor should submit the complete application electronically to the Agency and to the assigned COMPcoordinator via Eudralink.The application should include full copies of the bibliographical references. The application form and sections A to E should be supplied in Word format (compatible with version 97-2003). References and the signed application form should be supplied as PDF files. In the application, sponsors should clearly substantiate the claims and support the statements made, with references where possible.Deadlines for submission of applications for orphan designation are availableunder submission deadlines.Applications for orphan designation are free of charge.The Agency will validate the application and will send the sponsor a validation issues letter explaining if the application is found to be invalid or incomplete. Once the validation is complete, the Agency will send a timetable for the evaluation procedure to the sponsor.The Agency advises sponsors developing advanced therapies to apply separately to the Committee for Advanced Therapies (CAT) to have their medicine classified as anadvanced-therapy medicine. For more information, see advanced-therapy medicinalproduct classification.Parallel application with international regulatorsThe Agency encourages parallel applications for orphan designation with regulatoryauthorities outside the EU.The Agency has special arrangements with regulators in the United States and Japan for this purpose:•The Agency advises sponsors to use the common orphan application form with the United States Food and Drug Administration (FDA) to apply for orphan designation,particularly if an application has not been submitted in the United States before.•If an application has not been submitted to the Japanese authorities before, the Agency also encourages the sponsor to seek orphan designation from the Ministry for Health, Labour and Welfare (MHLW) in Japan. Under the Japanese orphan designation system, the MHLW provides consultation on orphan designations before submission, whereasmarketing-authorisation applications submitted following anorphan designation areassessed by the and the Pharmaceuticals and Medical Devices Agency (PMDA). TheMHLW generally seeks scientific counsel from the PMDA on theorphan designation.Evaluation of applicationsAfter submission, the two coordinators prepare a summary report on the application, which is circulated to all COMP members and discussed at the COMP's next plenarymeeting.At this stage, the COMP will either adopt a positive opinion or raise a list of questionsand invite the sponsor to an oral explanation at the next COMP plenary meeting.The COMP should adopt an opinion by day 90 of the procedure. It forwards this tothe European Commission for adoption of a decision.If the COMP's opinion is negative, the sponsor can appeal.European Commission decisionThe European Commission will issue a decision on a COMP opinion within 30 days of receipt. Following a decision:•the Agency publishes information on the orphan designation under rare disease (orphan) designations;•the European Commission enters the orphan designation into the Community register of designated orphan medicinal products.More information•Submission deadlines for orphan designations•Questions and answers: orphan-designation application•Guidance and formsActivities after orphan designation•Email•Print•Help•ShareThis section provides information on the incentives available to sponsors ofmedicines that have obtained orphan designation and the activities that take place after a designation has been granted.Sponsors who obtain orphan designation benefit from a number of orphan incentives, including protocol assistance, a type of scientific advice specific for designated orphan medicines, and market exclusivity once the medicine is on the market. Fee reductions are also available depending on the status of the sponsor and the type of service required.Sponsors must submit an annual report on development to the Agency summarising the status of development of the medicine.Applications for marketing authorisation for designated orphan medicines are assessed by the Committee for Medicinal Products for Human Use (CHMP). Sponsors also need to submit an application for maintenance of the orphan designation in order to be eligible for the 10-year market exclusivity incentive. For more information, see marketingauthorisation and market exclusivity.Transfers of orphan designation from one sponsor to another are possible. Transfers are free of charge.Sponsors can also request removal of an orphan designation.Sponsors of medicines with orphan designation should also remember to apply fora paediatric investigation plan, deferral or waiver once phase-I clinical studies arecomplete. For more information, see paediatric medicine development.More information•Orphan incentives•Annual report on development•Marketing authorisation and market exclusivity•Transfers of orphan designation•Removal of an orphan designationOrphans: Regulatory and procedural guidance and forms•Email•Print•Help•ShareThis page lists the guidance documents on orphan designation.Table of contents•Orphan designation•Post-designation•General•Standard operating proceduresRelated links•Guideline on the format and content of applications for designation as orphan medicinal products and on the transfer of designations from one sponsor to another •Regulation (EC) No 141/2000 of the European Parliament and the Council of 16 December 1999 on orphan products•Commission Regulation (EC) No 847/2000 of 27 April 2000 laying down the provisions for implementation of the criteria for designation of a medicinal product as an orphan medicinal product and definitions of the concepts ‘similar medicinal product’and‘clinical superiority’•Commission Communication 2003/C 178/02 of 29 July 2003 setting out the Commission's interpretation on matters relating to the implementation of the orphan designation and provisions for market exclusivity•Guideline on aspects of the application of Article 8(1) and (3) of Regulation (EC) No 141/2000: Assessing similarity of medicinal products versus authorised orphanmedicinal products benefiting from market exclusivity and applying derogations from that market exclusivity•Guideline on aspects of the application of Article 8(2) of Regulation (EC) No 141/2000 of the European Parliament and the Council: Review of the period of marketexclusivity of orphan medicinal products•Inventory of Community and national incentive measures to aid the research, marketing, development and availability of orphan medicinal products•Register of designated orphan medicinal products•The Seventh Framework Programme•Meeting dates for the Committee for Orphan Medicinal Products (COMP)•FDA - Office of Orphan Products Development•Japanese National Institute for Biomedical Innovation: Services to promote development of medicinal products for rare diseases。
赣州2024年06版小学3年级第十四次英语第三单元全练全测

19、听力题: The cat is _____ (curious/lazy) and playful.
20、How many wheels does a unicycle have? A. 1 B. 2 C. 3 D. 4 答案:A
28、选择题: What is the name of the superhero who wears a cape and flies? A. Batman B. Superman C. Spider-Man D. Iron Man
29、听力题: My friend has a pet ____ (cat) that is very playful.
8、听力题: The chemical symbol for argon is ______.
9、听力题: He is a great ________.
10、听力题: My brother is a ______. He loves sports.
11、听力题: There is a __________ in the sky.
32、选择题: Which fruit is yellow and tropical? A. Banana B. Cherry C. Peach D. Plum
33、填空题: My uncle is a __________ (飞行员).
34、填空题: My mom loves __________ (参加艺术展).
51、What is the sound of a cow? A. Moo B. Quack C. Oink D. Bark 答案:A
2023年考研英语二考试真题及答案解析

2023年考研英语二考试真题及答案解析考研英语二考试真题及答案解析Section Ⅰ Use of EnglishHere’s a common scenario that any number of entrepreneurs face today: you’re the CEO of a small business and though youre making a nice 1 , you need to find a way to take it to the next level. what you need to do is 2 growth by establishing a growth team. A growth team is made up of members from different departments within your company, and it harnesses the power of collaboration to focus 3 on finding ways to grow.Lets look at a real-world 4 . Prior to forming a growth team, the software company BitTorrent had 50 employees.Working in the 5 departments of engineering, marketing and product development. This brought them good results until 2012, when their growth plateaued. The 6 was that too many customers were using the basic, free version of their product. And 7 improvements to the premium, paid version, few people were making the upgrade.Things changed, 8 , when an innovative project marketing manager came aboard, 9 a growth team and sparked the kind of 10 perspective they needed. By looking at engineering issues from a marketing point of view, it became clear that the 11 of upgrades wasnt due to a quality issue. Most customers were simply unaware of the premium version and what it offered.Armed with this 12 , the marketing and engineering teams joined forces to raise awareness by prominently 13 the premium version to users of the free version. 14 , upgrades skyrocketed, and revenue increased by 92 percent.But in order for your growth, team to succeed, it needs to a have a strong leader. It needs someone who can 15 the interdisciplinary team and keep them on course for improvement.This leader will 16 the target area, set clear goals and establish a time frame for the 17 of these goals. This growth leader is also 18 for keeping the team focus on moving forward and steer them clear of distractions. 19 attractive, new ideas can be distracting, the team leader must recognize when these ideas don’t 20 the current goal and need to be put on the back burner.1.A. purchase B. profit C. connection D. bet2.A. define B. predict C. prioritize D. appreciate3.A. exclusively B. temporarily C. potentially D. initially4.A. experiment B. proposal C. debate D. example5.A. identical B. marginal C. provisional D. traditional6.A. rumor B. secret C. myth D. problem7.A. despite B. unlike C. through D. besides8.A. moreover B. however C. therefore D. again9.A. inspected B. created C. expanded D. reformed10.A.cultural B. objective C. fresh D. personal11.A. end B. burden C. lack D. decrease12.A. policy B. suggestion C. purpose D. insight13.A. contributing B. allocating C. promoting D. transferring14.A. As a result B. At any rate C. By the way D. In a sense15.A. unite B. finance C. follow D. choose16.A. share B. identify C. divide D. broaden17.A. announcement B. assessment C. adjustment D. accomplishment18.A. famous B. responsible C. available D. respectable19.A. Before B. Once C. While D. Unless20.A. serve B. limit C. summarize D. alter【1】B. profit 原文提到“小公司的CEO也挣到了大钱”。
罕见疾病研究现状及展望

罕见疾病研究现状及展望韩金祥;崔亚洲;周小艳【摘要】通过对罕见病病谱及分布的调查,美国、日本等发达国家根据本国的情况给出了不同的界定标准,并已通过立法促进罕见病防治.近几年来,随着分子生物学技术的快速发展,遗传性罕见疾病的研究也进入快车道,其研究成果不仅在罕见疾病的临床诊断、药物开发、治疗中发挥了重要作用,而且还是研究复杂疾病或正常生命活动分子机制的捷径,我国对罕见疾病防治尚未立法,其研究的规模和深度与先进国家有相当的差距,但已引起各阶层的关注.本文提出我国罕见病面临的首要任务:(1)呼吁政府尽快立法 (2)开展RD全国病谱及分布调查,摸清我国RD的发病情况及规律(3) 在国家层面上大力开展RD学术研究.【期刊名称】《罕少疾病杂志》【年(卷),期】2011(018)001【总页数】6页(P1-6)【关键词】罕见疾病【作者】韩金祥;崔亚洲;周小艳【作者单位】山东省医药生物技术研究中心,卫生部生物技术药物重点实验室,山东省罕少见病重点实验室,山东省医学科学院,济南,250062;山东省医药生物技术研究中心,卫生部生物技术药物重点实验室,山东省罕少见病重点实验室,山东省医学科学院,济南,250062;山东省医药生物技术研究中心,卫生部生物技术药物重点实验室,山东省罕少见病重点实验室,山东省医学科学院,济南,250062【正文语种】中文【中图分类】R5111. 罕见疾病的定义目前,不同国家和地区对罕见疾病(Rare Diseases 简称RD)的认定标准存在一定的差异。
WHO将罕见疾病定义为患病人数占总人口的比例在0.65‰~1‰之间的疾病或病变。
美国规定RD是指患病人数少于20万人(约占总人口的0.65‰)或高于20万人但药物研制和生产无商业回报的疾病;日本则规定,RD为患病人数少于5万人(约占总人口的0.4‰)的疾病;澳大利亚规定,RD是指患病人数少于2000人(约占总人口的<0.1‰)的疾病;欧盟规定,RD是指患病率低于0.5‰并且是危及生命或长期困扰健康的疾病[1]。
高级口译教程词汇预习答案

megalopolis 特大型城市boast 以……为自豪unequalled 不能与……相媲美miraculous rise 奇迹般地迅速崛起financial giants 金融业的巨头business community商业界manufacturing industry 制造业IPR(intellectual property rights) 知识产权joint consultancy service 合资咨询服务机构transnational corporation 跨国公司last but not least 最后at one's earliest convenience 在其方便的时候,尽早…… cherish 珍惜economic recession 经济不景气ensure a sustained growth 确保持续增长on the occasion of 请允许我借……的机会……Unit 3 商务谈判进出口商品交易会import and export commodities fair销售部经理sales managersupply department 采购部brochure 宣传小册子scope of business 经营范围machine tool 机床workmanship 工艺make an inquiry 询价quotation 报价C.I.F Seattle 西雅图到岸价(*cost,insurance.freight)调整价格adjust the pricecompetitive 具有竞争力bulk 很大substantially 大大地展台exhibition stand经营的新品new line of business汽车零部件auto partsupdate 调整at the cost of 不惜以……为代价our part 我方发盘/报盘offer折扣discountsupplies 货物free sample 免费样品inspection 检验floor offer 底盘counter-offer 还盘合同格式format of contract规格specification单价unit price保险费由贵方承担the insurance premium should be born by your sidebusiness transaction 生意顺利成交Unit 4 旅游观光广袤无垠的中华大地the boundless expanse of the Chinese territory绚丽多姿的自然景观gorgeous and varied natural scenery如诗如画poetic and picturesque名胜古迹places of historic interest and scenic beauty兵马俑terra-cotta soldiers and horses故宫the Imperial Palace五岳之首the most famous of China's 5 great mountains峻拔突兀majestic and precipitous appeal山外有山mountains beyond mountains融自然与文化景观于一体embody natural scenery and cultural heritage奇石,清瀑,古松,亭阁grotesque rock formation, clear waterfalls, old-age pine trees and pavilions历代文人雅士书法家famous ancient writers, scholars and calligraphers of various dynasty石刻碑文stone inscription重峦叠嶂peaks rising one after another经典佳作great classics of ancient writers of various dynasty华夏祖先Chinese ancestors吉祥之地propitious place祭祀天地offer sacrifices to Heaven and Earth联合国教科文组织UNESCO(c=cultural,其他不必说了吧?)世界自然与文化遗产World heritage Commissiongeological accident 地质变化the earth's crust 地壳temperate climatic zone 热带地区unique fauna and flora 珍禽奇兽,奇花异草Great Barrier 大堡礁Ayer's Rock 阿叶尔斯石柱山Cacadu National Park 卡喀杜国家公园Sydney Opera House 悉尼歌剧院skiing resort 滑雪场gross domestic product(GDP) 国内生产总值camping park 野营公园caravan and cabin 汽车旅馆,公寓住所international cuisine 国际烹饪水准ethnic restaurant 风味餐厅departure tax stamp 离境印花税票American Express 美国运通信用卡Unit 6 宣传介绍地势平坦的冲积平原 a soil deposit plain land常住居民permanent residents慈悬浮列车the maglev train长江三角洲Yangtze River Delta龙头作用play a leading role清朝乾隆,嘉庆年间during the reigns of Qianlong and Jiaqing of Qing Dynasty石油化工产业the petrochemical industry精细化工产业the fine chemical家用电器产业the home electrical appliance industry生物医药产业the bioengineering and pharmaceutical industry支柱产业pillar industry历史文物保护单位sites of historical interest and cultural relics under protection 海派文化Shanghai regional culture美食家gourmet清真authentic Muslim万国建筑博览会exhibition of the world's architecture内环线高架道路elevated inner beltway野生动物园the Wildlife zoo迎新撞钟活动New year's Greeting Bell-striking庙会Temple Fair桂花节Sweet Osmanthus Festival海纳百川,有容乃大the sea admits hundreds of rivers for its capacity to hold 乘骐骥以驰骋兮on your steed galloping来吾道夫先路on my road pioneering聪明,精明,高明bright, smart, wiseBritish Commonwealth 英联邦physically spread out 布局分散predominant 主导conglomeration 聚结commute 外来工作者prominent landmark 显著的地貌标志Saint Paul's Cathedral 圣保罗大教堂Westminster Abbey 威斯敏斯特教堂monarchy 君主政体coronation 加冕礼Buckingham Palace 白金汉宫hub 中心slum 贫民窟lavish 豪华philharmonic orchestra 爱乐乐团venue 场所cornucopia 各类successive eras 各个阶段chronologically 从历史上repository 陈列馆premier art collection 最重要的美术作品striking portraits of Britons 不列颠人逼真的肖像Unit 7 参观访问学位点degree program国家级重点社科研究基地key social science research centers博士后科学研究流动站post-doctoral research stations国家级重点学科national key disciplines两院院士academicians of the Chinese academy of science and the Chinese academy of engineering网络教育online education科举制imperial examination日月光华,旦复旦兮brilliant are the sunlight and the moonlight after night the day dawns again人文精神humanistic spirit披荆斩棘,筚路蓝缕negotiate various impediment博学而笃志,切问而近思extensive scholarship with unyielding dedication and earnest inquiry with close examination治学态度educational philosophy取精用弘的学术思想the academic ideology of extracting the best and exploiting the greatest 怀抱超旷的才隽学人graduates with brilliant scholarship高等教育发展的重中之重priority among institutions of high learning承前启后inherit fine tradition and usher in the future mission精诚团结,共襄盛举strive together in good faith文理工医科综合性大学 a comprehensive university with a complete range disciplines in liberal arts, science, engineering and medicine全面提升知名度和影响力elevate influence and visibility in all dimensions社会转型时期 a period of social transition百年传承之名校a prestigious university with a century-long academic tradition and intellectual esteemVancouver 温哥华Canada’s gateway to the pacific 加拿大通往太平洋的门户The Panama Canal 巴拿马运河Natural ice-free harbor 天然不冻港Manufactured goods 制成品Lumber and paper milling 伐木、造纸Oil refining 炼油Metal fabricating 金属锻造Printed matter 印刷Real estate 房地产Triple 增至3倍Quadruple 增至4倍Quintuple 增至5倍High-rise office building 摩天办公楼Boutique 时装礼品店Ethnic group 少数民族团体Planetarium 天文馆Aquarium 水族馆Skating rink 溜冰场Botanical garden 植物园Conservatory of exotic plants 异国植物花房Maple tree 枫树Sap 树液syrup 糖浆Unit 8 人物访谈国际清算银行行长president of the Bank for international settlements 宏观经济macroeconomic浮动汇率floating foreign exchange rateworld economic projection 世界经济预计impetus 动力reassuring 让人放心command economy 计划经济fiscal policy 财政政策surplus and deficit 赢余和赤字deterioration 最坏;最低点without precedent 第一次pact 公约curb deficits 防止财政状况恶化pension commitments 养老金投入yields on nominal bonds 名义收益率deflation 紧缩exchange rate appreciation 货币升值domestic liquidity 国内流动资金precipitous move 突然变动stifle 葬送workable measures of transition 可行的过度措施stance 姿态新千年the new millennium新纪元the new age精髓essence陶器pottery京剧戏装Costumes of Peking Opera莫高窟复制品the replica of the Mogao Grottoes青铜战车the bronze chariot战国早期的礼仪乐器ritual musical instruments produced early in the Warring States Period 八音度 a range of octave音域宽wide range定音tone setting瑟,笙,箫,鼓se, sheng, xiao, drums整理collate骨哨bone flute摇篮cradle舞台服饰performance costumes夸张和象征的手法exaggeration and symbolic means名模famous modelUnit 9 文化交流民为贵people being the most important巨大活力the immense vitality生动写照vivid reflection生存权subsistence right立国之本the foundation to build the country不懈努力make unremitting endeavor相辅相成the two are complementary to each other民族先人ancestor初步繁荣昌盛initial prosperity吸收和借鉴absorb and draw upon fruits of祖国统一reunification of the country繁衍multiply伟大复兴the great rejuvenation先行者forerunner区域自治regional autonomy宪法保障protected by the Constitution崇高目标lofty goal亲仁善邻benevolence and good-neighborliness国之宝箴treasured maximmillennium 千年landmark 标志性reclusive 避世隐居Danish architect Jorn Utzon 丹麦设计师钧恩乌特松with media access 有机会接触媒体architectural icon 建筑业偶像in the pantheon of 在……的万神殿中pluck 淘汰a complete one-off 空前绝后was quite at odds with 相去甚远rectilinear 垂直式maverick genius 独树一帜的奇才promontory 海角backdrop 背景in high dudgeon 一怒之下manifold difficulties 各种各样的困难seductive beauty 有魅力的纯美patron 资助人Unit 10 科学报告中国古代药王神农氏Shennong, the celebrated herbal master of ancient China中医史上的萌芽阶段the embryo stage in the development of TCM战国时期the Warring States Period黄帝内经HuangDi’s Classic of internal Medicine神农本草经Shennong’s herbal classic主治、功用和毒性primary treatments, functions and toxic character药典pharmacopoeia救死扶伤healing the sick and saving the dying职业道德规范professional work ethic食补保健food treatment approach延缓衰老defer senility相互作用、互为依存be of mutual influence and interdependence有机的整体an organic whole诊断疾病diagnose disease阴阳对立制约yin and yang are mutually opposing and constraining互根互用be interdependent and mutually promoting消长平衡proportionally change with the decrease of one, resulting in, or from the increase of the other相互转化mutually transformational健康的要素be essential for the maintenance of good health指导思想guiding concept临床治疗方法clinical treatment针灸疗法acupuncture and moxibustion按摩推拿medical massage气功疗法deep breathing exercises赢得广泛赞誉win worldwide acclaim中医专业队伍TCM professionals综合医院general hospitalastrobiology 天体生物学nitrogen 氮hydrogen 氢oxygen 氧气photosynthesis 光合作用equilibrium 平衡meteor 流星embedded 埋植carbon compound 碳化合物hypothesis 假设Antarctic 南极的aesthetics 审美观the Leonid meteor showers 狮子座流星雨debris 碎片comet 彗星The Azores 亚速尔群岛Infrared spectrographs 红外线摄谱仪organic molecule 有机分子spectrographic 摄谱的disseminate 散布prebiotic life 前生物生命galaxy 银河系Unit 11 饮食文化烹饪艺术culinary art民以食为天food is the paramount necessity of the people 推陈出新creative efforts色、香、味color, aroma and taste摆放layout冷盘cold dishes原料raw material作料调配the blending of seasoning调味艺术the art of proper seasoning食物质地the texture of food刀功slicing technique乳猪suckling pig点心pastries黄酒yellow rice wine烈性白酒strong white liquor敬酒toast with小啜take a sip馒头steamed bread热卡calories主食staple foodfood style 饮食习惯solid diet 丰盛的食物health food 保健餐Little Italy 小意大利城Germantown 德国城native specialties 家乡特色菜Creole accent to the food 克里奥耳口味physical well-being 身体健康ironic 讽刺的preservative 防腐剂cheese 奶酪Unit 12 中国改革翻天覆地的变化earthshaking changes面貌焕然一新take on a brand-new look出/入境旅游outbound/inbound travel村/居委会village committee/urban neighborhood committee解决温饱问题solve the problem of food and clothing落实科学发展观follow a scientific approach of development以人为本,执政为民put people first in administration着力搞好宏观调空concentrate on doing macro-regulatory work well激发创造活力stimulate creativity实施稳健的财政政策follow prudent fiscal policy三农工作是重中之重work relating agriculture, rural areas and farmers remains top priority加强农田水利建设intensify development of irrigation and conservancy project多渠道转移农业富余劳动力transfer surplus rural labor to nonagricultural jobs推进产业结构优化升级optimize and upgrade the industrial structure加强生态建设strengthen ecological improvement推进财税体制改革promote the reform of fiscal and tax system加强精神文明建设promote social and ethical progress加强行政能力建设和政风建设improve the government’s administrative capacity and style of work建设服务型政府service-oriented government意气风发in high spirits同心同德、再接再厉united with one heart and one mind, continue our concerted and unyielding effortsoverstate 夸大turn one’s back on 对……封闭anarchy 政治混乱warlordism 军阀割据make up lost ground 收复失地springboard 跳板with gusto 满怀热情subsistence farming 自然经济marginal productivity 边际生产力tariff barrier 关税壁垒bolster 保持joint venture 合资企业incremental capital output ratio 资本产出比率reckon 估计purchasing power parity 购买力平价capital accumulation 资本积累demographic forecasts 人口统计学上的预见hiccup 磕磕碰碰forerunner 前驱dwarf 让……相形见绌Unit 13 信息时代筹备会议preliminary meeting处理程序性问题address procedure issue智能化intelligence computerization多样化diversification信息通信技术infocom technology结构调整architecture readjustment升级换代upgrading融语音、数据、图像于一体integrate voice date and image宽带高速信息网high-speed broadband information network全方位地满足业务需求meet various service requirements in all dimensions 制约因素reason宏观调控macroeconomic control市场管制market regulation规避市场风险avoid market risks创新的融资机智innovative financing mechanism资金的多元投入for more financing channels911事件September 11 terrorist attack应急系统emergency system数字鸿沟digital dividedon 穿上scaffold 框架thermostat 恒温计EKG 心电图仪telemetric system 遥测系统emulate 仿效symbiosis 共生现象software programmer 软件编程师collaborate 合作debug 调试neuron 神经元tackle 解决interstellar 星际microprocessor 微型处理器ad hoc 特别的cell phone 手机the heftiest desktop 最先进的台式机fight off an attacking wasp 击退发起进攻的黄蜂simpleton 傻子emergent behavior 突发性的行为mischievous and sinister 恶意antithetical 对立的resilience 应变能力seismic activity 地震活动geomagnetic storm 地磁风暴a worrisome spike 麻烦reroute traffic 改变行动路线Interplant 星际网asteroid 小行星unmanned probe 吾人驾驶探测器proprietary (信息)专有feel tingles on one’s spine 感觉到脊椎的震颤Unit 14 外交政策外国使节diplomatic envoy复杂而深刻的变化complex and profound changes各种问题相互交织various threats are intertwined指导国际关系的准则norms governing international relations切实履行implement in real earnest以强凌弱的霸权主义bully the weak and pursue hegemony文明的多样性the diversity of civilizations万物并育而不相害all living creature grow together without harming one another道并行而人不相悖ways run parallel without interfering with one another相互借鉴、取长补短learn from each other in mutual emulation相互包容、求同存异mutual tolerance, seek agreement while shelving differences减免债务reduce and forgive debts军事联盟military alliance动辄诉诸武力resort to use or threat of force摈弃冷战思维the Cold War mentality should be done away with核武器扩散nucleus weapons proliferation跨国犯罪trans-boundary crimes生态恶化environmental degradation永远不称霸never seek hegemony维护国家主权和领土完整safeguard national sovereignty and territorial integrity睦邻、安邻、富邻政策the policy of creating an amicable, secure and prosperous neighborhood奔腾不息的时代潮流irresistible tide of the timesdiplomacy 外交手段monetary structure 货币组织military deterrence 军事威慑utmost purpose 最高宗旨subordinate 服从于overshadow 黯然失色initiative 主动行动downright distrust 不信任的传统utility 利用authorization 授权sponsorship 操办intervention 干预take…into account 考虑到the IMF 国际货币基金组织trade deficits 贸易赤字commitment 致力于war-torn 遭受战争破坏elite 上层人物military alliance 军事联盟demobilization/remobilization 遣散军队/重组军队Unit 15 国际关系纪念……成立……周年commemorate ….anniversary of the founding of恪守承诺commitment to联合国宪章宗旨和原则the purpose and the principles of the UN Charter善邻之道live together in peace with one another as good neighbors划时代意义epoch-making里程碑milestone人类社会沧桑巨变stupendous changes in human society国际舞台风云变幻vicissitudes in the international arena地区热点问题regional hot spot issue民族分裂势力regional separatists极端宗教势力religious extremist毒品走私drug trafficking传染性疾病communicable disease坚持多边主义uphold multilateralism摈弃冷战思维abandon the Cold War mentality标本兼治address both symptoms and root causes裁军与军备控制disarmament and arms control防止核扩散prevent the proliferation of nuclear weapons包容精神the spirit of inclusiveness文明多样性diversity of civilization兼容并蓄的和谐世界harmonious world where all coexist and accommodate each other 休戚与共的命运interests and destiniessubsequent endeavor 此后的努力humanitarian 人道主义者refrain 不以non-intervention 不干涉domestic jurisdiction 内部事务the minimum doe of conduct 最起码的行为准则the Security Council 安理会paralysis 瘫痪veto right 否决权incapacitate 无所作为nuclear weapon proliferation 核武器扩散communicable disease 传染性疾病buffer conflicts 缓解冲突enforcement 强制meddle 管闲事manifold 多种多样permeate 渗透practice tolerance 宽容忍让transcend differences 超越差异convergence of interests 共同利益的汇合点coercion 高压政治tackle 处理。
good manufacturing practices

Good Manufacturing PracticesIntroductionGood Manufacturing Practices (GMP) refer to a set of guidelines and regulations that ensure the consistent quality and safety of products during their manufacturing processes. GMP is crucial across various industries, such as pharmaceuticals, food and beverages, cosmetics, and medical devices. This article explores the importance of GMP and the key elements involved in its implementation.Advantages of Implementing GMPImplementing GMP provides several benefits for manufacturers, consumers, and regulatory authorities. Some of the key advantages include:1. Ensures Product QualityGMP ensures that products are manufactured consistently and meetspecific quality standards. By implementing GMP, manufacturers can minimize the risk of product defects, contamination, and deviations from desired specifications. This, in turn, increases customer satisfaction and brand reputation.2. Enhances Consumer SafetyOne of the primary objectives of GMP is to ensure consumer safety by preventing the presence of harmful substances or contaminants in products. GMP guidelines encompass rigorous quality control measures, sanitation practices, and documentation requirements. This ensures that products are safe for consumption or use by the end-users.3. Facilitates International TradeAdherence to GMP is vital for manufacturers engaged in international trade. Many countries and regulatory bodies require compliance with GMPguidelines for imported products. By implementing GMP, manufacturers can access global markets, establish trust with foreign consumers, and avoid trade barriers and restrictions.4. Reduces Manufacturing RisksGMP includes comprehensive risk management strategies, such as hazard analysis and critical control points (HACCP). These measures helpidentify and mitigate potential risks in the manufacturing process, ensuring that products are safe and of high quality. By minimizing risks, manufacturers can avoid costly product recalls, legal issues, and reputational damage.Key Elements of GMP ImplementationTo comply with GMP guidelines, manufacturers need to establish and maintain certain key elements. These elements include:1. Corrective and Preventive Actions (CAPA)CAPA is an essential component of GMP, focusing on identifying and rectifying deviations from established procedures. It involves investigating, documenting, and addressing any non-conformities to prevent their recurrence. CAPA processes help manufacturers continuously improve their operations and maintain compliance with GMP standards.2. Personnel Training and QualificationProper training and qualification of personnel are vital to ensure GMP compliance. Manufacturers must provide comprehensive training programsto employees, covering topics such as hygiene practices, product handling, quality control techniques, and regulatory requirements. Regular assessments and certifications can help validate employees’ competence and knowledge.3. Documentation and Record KeepingAccurate and thorough documentation is a fundamental aspect of GMP. Manufacturers must maintain comprehensive records regarding various aspects of their operations, including raw materials, manufacturing processes, equipment maintenance, inspections, and quality control tests. These records facilitate traceability, enable effective audits, and demonstrate compliance with GMP requirements.4. Quality Control and Quality AssuranceGMP emphasizes the importance of robust quality control and quality assurance systems. This involves testing and analyzing raw materials,in-process samples, and finished products to ensure they meet specified quality standards. Regular inspections, audits, and validationactivities help identify any deviations from established procedures and ensure ongoing compliance.5. Facility and Equipment MaintenanceMaintaining appropriate facilities and equipment is crucial for GMP compliance. Manufacturers must establish and adhere to maintenance schedules, calibration procedures, and cleaning protocols to ensure that production environments and equipment are in optimal condition. Regular inspections and preventive maintenance activities help minimize the risk of contamination or equipment failure.ConclusionGood Manufacturing Practices (GMP) form the foundation for ensuring product quality, consumer safety, and regulatory compliance across various industries. By implementing GMP guidelines, manufacturers can establish consistent manufacturing processes, mitigate risks, and maintain high-quality standards. Adherence to GMP principles not only benefits manufacturers but also enhances consumer confidence and facilitates international trade. Through the diligent implementation of key elements, such as CAPA, personnel training, documentation, quality control, and facility maintenance, manufacturers can achieve and sustain GMP compliance in their operations.。
新编剑桥商务英语(高级)第三版2

可编辑修改精选全文完整版新编剑桥商务英语(高级)第三版2.1-CAL-FENGHAI.-(YICAI)-Company One1Growing the companyParts of a company1 Do you think this quotation is true all business?‘I think that our fundamental belief is that for us growth is a way of life and we have to grow at all times’.Mukesh Ambani, chairman of Reliance Industries2 Read this entry from a company website and use these words to label the digram.Subsidiary headquarters sales officesWarehouse R&D division main planWe are based in La Defense, the business district of Pairs, and new products are developed nearby at our labs in St Dense. Our principal manufacturing facility is just out side Lille and products go from there to be a cental distribution point at Compiegne.Three sales agencies cover the various regions of France with international offices in Frankfurt,Milan and Madrid. The London offices is run by our UK subsidiary.3 What is the diffidence between the following words and phrases?1 a sales office and a subsidiary2 a warehouse and a plant3 the headquarters and a divisionGrowth Strategy4 Find a synonym in the box of each of the underlined words.Go public sell off set up go out of business expandTake over make redundant shut down1 We acquired Everforce Ltd in 2005.2 Our target is to grow the business by 15% each year.3 We created a subsidiary to sell after-sales service.4 The company will be listed on the Stock Exchange next year.5 They went bankrupt last year.6 We laid 300 employees off in June.7 After a lot of discussion we decided to closed the plant.8 We have divested our shares in the logistic company.5 What is the diffidence between the following expressions?1 Laying people off and firing them2 taking over a company and merging with it3 organic growth and non-organic growth6SAP and Oracle are the world’s leading companies in providing the software solutions for business. But their business strategies are very diffident.Read the text about SAP’S growth strategy. Choose the best sentences from the list (A-H) below the complete each gap (1-6). Do not use any letter more than once.SAP competes with ‘organic growth’How do you stay at the top of the heap in the business software game If you’re SAP, you do it through ‘organic growth’, not blockbuster acquisitions. That is the world from SAP CEO Henning Kagermann. (0) H The second-best strategy is acquisition: Kagermann said. ‘The best is the organic growth. We are not just doing the organic growth because we have no other choice.The comment is aimed squarely at rival Oracle Corp; which spent nearly $20 billion between 2004 and 2006 expanding its core database business into the SAP-dominated business applications market.(1) . ‘We are the market leader,’he said.’It’s on surprise that a distant number-two player wants to catch u.’SAP was set up in 1972 by five former IBM employees.(2) . Although it has a growing number of subsidiary, there are complements to its main activities, as Shai Agassi, president’s of the company’s Products and technology Group explain, at the same time having a direct dig at Oracle. The key diffidence between the two companies, he says, lies in Oracle’s tendency to ‘acquire an industry solution that is at the heart’. ‘when we do an acquisition, it’t at the edge of the solutions. (3) Oracle is buying half body parts and trying to make a body out of it.’In fact, Agassi expects SPA to grow faster than the rest of the industry this year- 15% to 17% in sales of new software licences-through internal innovation and small-scale acquisition.(4) .SAP used to concentrate on large business customers, but increasingly pursuing sales in the market, a strategy that began in 2000.(5) .The company excepts to finish development of the mySAP suite within the next four years, as well as its Enterprise Services Achitecture (ESA). ESA is basically a platform that will allow SAP to provide consistent business services around it, in much the same way as Microsoft has built applications around its successful operating systems.Among the company’s other goals is the development of hundreds of additional services for the mySAP suite, a so-called‘ecosystems’ of supportive technologies.‘Business in the future is not business in an enterprise,‘Kagermann says.’ It’s business in an ecosystem. (6) . We try to invite others with great ideas to innovate on the platform.’Exam Success A In fact, it expects sales to companies with fewer than 25,000make sure that the employees to account for nearly half SAP’s total softwarephrase you choose fits sales this year.grammatically and in B They recently announced they had purchased Virsa System,meaning, both with a privately held supplier of regulatory compliance software.the sentence before C You just can’t do everything yourself if you want to remainand the sentence after. Competitive.Read the whole text D Competition in the market is fierce and only the big playersback to yourself at will survive.the end. E Since then, it has evolved from a small, regional enterpriseinto the global market leader in ERP software, employingmore than 34,000 people.F Kagermann was unimpressed with Oracle’s appetite for big,Headline-grabbing acquisition ( PeopleSoft Siebel System).G That is different from buying half of a heart.H He made the comments while he was talking with reportersLast week during his company’s annual Developer KickoffMeeting in Burlingame, CA.7 Summarise the growth strategies of SAP and Oracle. What is the key to SAP’s longer-term strategy?Past tenses1 Study these extracts from the text about SAP on page 17.Name each underline tenses (past simple, past continuous, past perfect, present perfect or used to )Say what you know about each tense’s use and why you think it is used here.1 He made the comments while he was talking with reporters last week.2 SAP was set up in 1972 by fine former IBM employees.3 Since then, it has evolved from a small, regional enterprise into the global market leader.4 They recently announced they had purchased Virsa System.5 SAP used to concentrate on large business customers,but is increasingly pursuing sales in the midmarket.6 it’s strategy that began in 2000.2 You receive this internal email. Follow the instruction it.Hi DeniseBelow is the short company history I’ve written for the ‘About us’entry on the English page of the new website. I think it’s generally OK but I’m so unconfident about my use of tenses in English that I’ve just left the verbs in the infinitive! Can you put them in theright form and send it backThanks and sorry for being so useless!BrigitteThis is the unusual of Raincoat Software, a company that (1) (come) into being accidentally because of the hobby of one man,Hans Meier.In 1998 Hans (2) (work) as a computer programmer for a large bank in Zurich. But he (3) (be) restless. Each evening he (4) (return) home and, just for fun, (5) (hack) into official websites on his personal computer (not the bank’s, of course!). The day after he (6) (hack) onto a particularly sensitive US government website, he (7) (receive) an email from them. Fearing that would the end of his career as a hack at the bank, he (8) (open) it. It (9) (be)a request from the US government, asking if he (10) (want) ajob as a security advisor.Rather than taking a job as a government employee, Hans Meier (11) (see) the opportunity to make a successful business out of acomputer security protection. Raincoat Software (12) (be) born.Since then, the company (13) (employ) over 50 ‘security expert’ - in other words, people write a similar background to ourfounder. We (14) (help) over 300 large companies andgovernment departments and are now a $100 million a year business.But did the US government think it (15) (take) a risk by employing Hans Meier all those years agoThe answer they (16) (give) then is still the company’s motto today:’Better safe than sorry’.Write about the past3 write a short piece (100 words approx) about a turning point in yourlife, work or studies: a moment when you decide to pursue adifferent route from the one you had up to that point. Use thefollowing to help you.1 What you were doing before that?2 What happen to change your life/3 What happen next?4 What happens when companies merge or acquire other companieslook at the table below and make notes.Opportunities threatsEmployeesShareholdersCustomersSuppliers5 In December 2009 Oracle, the world's second largest business software applications provider, took over PeopleSoft, the third largest. Read the letter that the CEO of PeopleSoft, Dave Duffield, wrote to his employees.1 Does he think the takeover will benefit employees or not?2 Which of these adjectives best describes his feelings about the takeover?bitter / resigned/angryThis is a sad day for me, and I'm sure; an equally sad day for you.It is now clear that Oracle will acquire our company. Over the past few weeks, our independent directors met with individual stockholders to get their views. We were told during these conversations that they believe Oracle's $24 wasn't adequate and did not reflect peopleSoft's real value. It become clear to us that the vast majority of our stockholders would accept $26.50 and Oracle was willing to pay for it.You should know, and I hope you would expect, that I am deeply saddened by this outcome. We have come so far under such trying circumstances over the past eighteen months, and especially the past two and a hall' months. PeopleSoft had gained significant momentum in all areas of our company, including with customers, prospects, and in the financial community.Over the next few weeks, we will be working with Oracle to ensure that you get answers to as many questions as possible that you have. I believe some of you will find interesting opportunities at Oracle, others will take your talents and work elsewhere the area that you live, while another group may have difficulties finding rewarding job experiences. It is to this last group that I offer my sincerest apologies for not figuring out a different conclusion to our 18-month saga.I know it is little comfort, but I am extraordinarily proud of what we have accomplished over the past 17一plus years, and longer in the case of JD Edwards.And I am even prouder of you for your perseverance and teamwork over the past eighteen months.I make a final request.And that is to continue our work with our heads held high.Whether it's serving customers, building products or working on internal operations, 1'eopleSoft and the people at peopleSoft have built their reputation as a company with class.Sincerely,DaveThe peoplesoft takeover1 2.1 Listen to two accounts of the takeover by a commentator and an industry analyst, both close to the takeover. What are the main differences in the working environment and the way employees were rewarded at the two companies?2 2.1 Listen again and answer the questions. Por each question (1-6), mark one letter (A, B or C) for the correct answer.1 What is said about how consultants are deployed in big IT consulting companies?A They are given jobs with a lot of responsibility.B They are often expected to learn on the job.C They only work on projects where they have proven experience.2 What does the commentator say about salaries at PeopleSoft?A At least the company was open about its pay policy.B They were at the market rate for the job.C They were unacceptably low.3 How did employees feel about their CEO, Dave Duffield?A That he respected them and looked after them.B That he was ready to leave the company.C That he developed good software applications.4 What does the commentator imply happened after the merger?A The company's reputation sufferedB People grew to respect the new CEOC A lot of people lost their jobs.5 How does the industry analyst defend the company's growth strategy?A He says size is very important in this industry.B If Oracle hadn't taken over PeopleSoft, someone else would have.C He says it will make them the biggest company in the sector.6 How does he explain the differences in company culture?A The two CEOs had a different philosophy.B The two companies were involved in different types of business.C There was no real difference.A prase releaseYou work in the press office of an insurance company that has recently taken over another company. Since the take-over there have been some negative reports about it. You decide to put out a press release. Include the following points.·Explain the business reasons for the take-over (to compete with other big insurance companies; to rationalise staffing)·Express your enthusiasm about the future opportun ities for the merged company.·Thank all the employees for their support.·Reassure people that there won't be major job cuts.Begin like this:PRESS RELEASEInsure COLast month Insure Co was pleased to announce the acquisition ofABC Insurance. The new company brings together two leading insurance providers to form the world's third largest insurance company…4 Read this extract from an article in Business Strategy magazine. Where do these four types of organisation belong in the text?stock brokers restaurants oil companies banksorganisational cultureRapid feedback and Low risk High risk Reward Work-hard, play-hard Tough-guy macho Slow feedback and culture cultureReward Process culture Bet-the-companycultureA of attempts have been made to categorise the organisation and culture of different companies, but only two things seem certain: 1)that many different cultures and types of organisation can exist within each company一and 2)that the activity and sector play a crucial role in determining how work is organised. Deal and Kennedy recognised this when they proposed four different types oforganisational culture: Work-hard, play一hard culture tends to apply to companieslike software developers or (1) which need to react quickly to changing circumstances and fo work of a high tempo. Creativity often plays an important part in their work so they tend to be organised in a project-based way grouping people in teams to solve particular tasks. Tough一guy macho culture concentrates power around key personnel,but it will also devolve a lot of responsibility to the individual and emphasise decisions that affect the present rather than the future. Examples are (2) sports teams, police, the military Process culture applies to companies which have strict hierarchies and strict job roles, such as insurance companies (3) and public services. Strategy and direction seem to take second place to organisation and so hey are often, maybe unfairly, associated with plodding and bureaucracy.Bet-the一company culture may also be present in companies with a hierarchical structure, but long-term planning and investment, involvinghigh risk, is also a key feature, so direction and goals are generally clearerExamples are aircraft manufacturers and (4)5 Describe an organisation you know or have worked or studied in.1 How many people worked there?2 What was its business/ speciality?3 How was it organised?4 How would you describe the culture?Did it fall into any of the categories described above In what waysAn employee survey6 Look at this survey from the same edition of Business Strategy magazine. Mark the six items that are most important to you (1 is the most important).I prefer an organisation which emphasises:A Individual responsibility and empowerment of employeesB Teamwork and consensusC Clear lines of reporting and areas of responsibilityD Quick decision-taking and actionE Long-term, careful planningF Creativity, innovation and taking risksG Clear and consistent proceduresH Job securityI Customer satisfactionJ Measurable resultsK Employee welfareL Financial reward for employees M Non-financial rewards (training, development)N Informal relationships between staff and management。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
FDA Office of Orphan Products Development
Grant Program Objective
To fund clinical research that will accelerate or assist in the approval of products which demonstrate promise for rare diseases/disorders. Basic Research Funding
FDA Office of Orphan Products Development
What is a HUD?
A Humanitarian Use Device (HUD) is a medical device intended to benefit patients in the diagnosis and/or treatment of a disease or condition that affects or is manifested in fewer than 4,000 patients per year in the United States.
FDA Office of Orphan Products Development
OPD Grant
>50/400 grants awarded to industry sponsors Grantees are US and abroad Orphan Designation not required New drug/product or new use of approved product
FDA Office of Orphan Products Development
Funding
FY 2003: 101 responsive applications 15 received funding Currently: 65 Active grants
FDA Office of Orphan Products Development
FDA Office of Orphan Products Development
Grant Support for Investigation of Rare Disease Treatment
Awarded to both academic and industry-sponsored research FDA funds approximately 20-30 new grants per year Provides $150,000 to $300,000 in direct costs per year - up to 3 years
FDA Office of Orphan Products Development
Grant Program Accomplishments
36 product approvals supported by OPD grants
Hundreds of publications, abstracts, and presentations have been produced as a result of orphan product grant studies.
October 2004 April 2005
FDA Office of Orphan Products Development
Responsibilities of the Office of Orphan Products Development in Evaluating Humanitarian Use Devices (HUD)
FDA Office of Orphan Products Development
Grant review: Responsive to Program Review Criteria?
Study is a clinical trial. Prevalence is fewer than 200,000 U.S. patients. Study must be performed under an active IND/IDE (except medical foods). Budget is within limits. Availability of sufficient product.
FDA Office of Orphan Products Development
Grant review: Scientific and Technical Merit?
Adequacy of resources and environment Budget Justification Informed consent documents and IRB approval Potential for completion of study within stated time and budget
FDA Office of Orphan Products Development
Grant review: Scientific and Technical Merit?
Soundness of the rationale Appropriateness of study design Statistical justification for proposed enrollment POWER Potential for patient accrual Qualifications of investigator & support staff
The Office of Orphan Products Development Grant Program
Janet Whitley, Ph.D. Office of Orphan Products Development Food and Drug Administration
FDA Office of Orphan Products Development
FDA Office of Orphan Products Development
HUDs vs HDEs
Two-step process:
1 – HUD: FDA will consider the use and size of the proposed patient population 2- HDE: Device is evaluated for safety and probable benefit in the CDRH Office of Device Evaluation.
FDA Office of Orphan Products Development
Grant Program Objective
To fund studies leading to publications in peerreviewed journals. Supports mainly Phase 1 and 2 trials Funding may be used to cover portion of larger trial Importance of funding
FDA Office of Orphan Products Development
Not funded because:
Non-responsive application Overall quality of application – clarity and detail Scientific merit Preliminary data, poor study design, weak rationale, sketchy analysis plan Compelling nature of study Resubmissions that don’t follow/address panel’s suggestions/concerns Feasibility Number of patients, drug supply, too much to do in too little time
FDA Office of Orphan Products Development
Orphan Grant Application Process
Issue Federal Register notice (RFA). Meets program review criteria. Review panels: Ad hoc panels of outside experts review scientific merit Regulatory review Prepare summary statements. Present to Advisory Council. Advise - Fund - Monitor.
FDA Office of Orphan Products Development
FY2005 Request for Applications (RFA)
Federal Register Announcement August OPD website Application Due Dates:
FDA Office of Orphan Products Development
Management of Funded Grants
Assignment to Project Officer Liaison between grantee and FDA review division. Enrollment goals/achievement. Quarterly progress updates. Site visits to assure grant compliance. Sponsor acquisition. Continuation funding dependent on progress.
