An electrochemicalDNAsensorforsequence-specific DNA
一种新型电化学DNA纳米生物传感器_CDS.

中国基础科学研究进展2007 3 Chi na B a si c Sci ence 21一种新型电化学DNA 纳米生物传感器 CDS!樊春海发展新型DNA 检测方法是后基因组时代的需求,诸如生物安全(生物恐怖袭击、SARS 等高致病性传染病以及健康(肝炎、H I V 等等领域都需要快速、便捷的DNA 或RNA 检测技术。
电化学技术具有快速、灵敏、低能耗、易于微型化和集成化等优点,被认为是在时效、成本等有较高限定要求的场合实现DNA 检测的首选技术之一。
我们课题组在国家自然科学基金委、中国科学院和上海市科委等相关项目支持下研制出一种新型的电化学DNA 纳米生物传感器 CDS(chronocoulo m etric DNA sensor。
该生物传感器具有高灵敏度和高特异性。
CDS 生物传感器有两个比较明显的特色。
其一是实现了DNA 探针分子在电极界面上的组装和精细调控。
研究表明,高密度的DNA 探针不利于其捕获靶标DNA 分子,这是由于DNA 分子带有负电荷,相互之间有静电斥力,同时过高的密度也增加了位阻效应。
当DNA 探针分子在金电极表面上的组装密度合适的情况下,可以实现接近80%的捕获效率,可以极大提高传感器的检测灵敏度。
其二是引入金纳米粒子进行电化学信号放大。
金纳米粒子可以与含巯基修饰的信号DNA 分子形成非常稳定的复合物,同时由于纳米粒子的高比表面积,一个20nm 的金纳米粒子表面可以负载数百条信号DNA 分子。
采用一种夹心式的检测方法,在靶标DNA 存在时可以把负载信号DNA 分子的金纳米粒子一起通过杂交反应连接到电极表面。
这样一次杂交事件就可以产生数百倍的信号放大,因而显著提高了DNA 检测的灵敏度。
该生物传感器可在1 2h 内快速检测到约2万多个DNA 分子,检测灵敏度达到10M f (飞摩尔/升,比常规荧光DNA 检测方法(约10p M 提高了约3个数量级。
同时,该生物传感器也具备高特异性,即使在大量干扰DNA 存在下仍然可以检测出靶标。
传感器与检测技术英文书籍英语

传感器与检测技术英文书籍英语Sensors and Detection Technologies: A Comprehensive Guide.Introduction.Sensors and detection technologies play a crucial role in various scientific, industrial, and commercial applications. These technologies enable us to measure, monitor, and analyze physical, chemical, and biological parameters in real-time or over time. This guide provides a comprehensive overview of the different types of sensors, their working principles, applications, and advancements in sensing technologies.Types of Sensors.1. Physical Sensors:Pressure sensors: Measure force or pressure applied toan object.Temperature sensors: Detect changes in temperature and provide real-time temperature readings.Position sensors: Determine the position or displacement of an object.Velocity and acceleration sensors: Measure the speed and acceleration of moving objects.2. Chemical Sensors:Gas sensors: Detect and measure the presence and concentration of gases in the environment.Biosensors: Utilize biological recognition elements to detect specific molecules or analytes.Chemical arrays: Employ multiple sensors to provide a comprehensive analysis of chemical composition.3. Biological Sensors:Biosensors: Detect and measure biological substancesor organisms.Microfluidic devices: Enable precise control and manipulation of small fluid volumes for biological analysis.Lab-on-a-chip: Integrate multiple analytical functions into a single portable device.4. Optical Sensors:Optical fiber sensors: Utilize optical fibers to transmit light signals and detect changes in thesurrounding environment.Fiber Bragg grating (FBG) sensors: Measure strain, temperature, and other parameters based on the wavelength shift of reflected light.Surface plasmon resonance (SPR) sensors: Utilize theinteraction of light with metal nanoparticles to detect changes in refractive index caused by specific molecules.Working Principles.Sensors convert physical, chemical, or biological signals into electrical or optical signals. The working principles vary depending on the sensor type:1. Physical Sensors:Piezoelectric sensors: Generate an electrical charge when subjected to mechanical stress or vibration.Thermistors and thermocouples: Change their electrical resistance or generate voltage in response to temperature changes.Potentiometers: Measure position or displacement by varying resistance as a movable contact slides along a resistive element.2. Chemical Sensors:Electrochemical sensors: Utilize electrochemical reactions to generate electrical signals proportional to the analyte concentration.Optical sensors: Detect changes in light absorption, reflection, or fluorescence caused by the presence of specific molecules.3. Biological Sensors:Antibody-based sensors: Employ specific antibodies to bind and detect target molecules or organisms.Nucleic acid-based sensors: Utilize DNA or RNA sequences to detect and analyze specific genetic material.Applications.Sensors and detection technologies find applications in a wide range of fields, including:Environmental monitoring: Air quality, water quality, and soil analysis.Industrial automation: Process control, robotics, and quality assurance.Medical diagnostics: Blood analysis, disease detection, and patient monitoring.Agricultural technology: Crop monitoring, soilnutrient analysis, and pest detection.Aerospace and defense: Navigation, guidance, andtarget detection.Advancements in Sensing Technologies.Miniaturization and integration: Development of smaller, more integrated sensors with improved portability and cost-effectiveness.Enhanced sensitivity and selectivity: Advancements in materials science and signal processing techniques to achieve higher detection limits and reduced false positives.Wireless connectivity: Integration of sensors with wireless communication technologies for remote monitoring and data transmission.Artificial intelligence (AI): Utilization of AI algorithms to enhance sensor performance, analyze data in real-time, and make predictions or recommendations.Conclusion.Sensors and detection technologies are essential tools for scientific research, industrial processes, and various commercial applications. The different types of sensors, their working principles, and recent advancements enable us to gather valuable information, monitor processes, and make informed decisions. Continued research and development in sensing technologies hold the promise of further innovation and expanded capabilities in the future.。
电信号分子在电化学功能核酸生物传感器中的研究进展

·综述与专论·2019, 35(5):157-169生物技术通报BIOTECHNOLOGY BULLETIN核酸除了作为遗传物质的载体之外,还可以与一些天然存在或人工合成的小分子或离子结合,并发生构象的改变;或是在这些靶分子的存在下,具有类似蛋白酶的催化活性,可以催化底物发生切割或连接反应,这一类核酸称为功能核酸(Functional nucleic acids,FNAs)[1-2]。
FNAs 主要有两类[3],一类是与靶物质结合时可发生构象改变的适配体(Aptamer);另一类是有酶催化活性的脱氧核酶(DNAzyme)和G -四链体(富含腺嘌呤的DNA 所形成的四链体结构)与氯化血红素的复合物收稿日期:2018-07-02基金项目:转基因重大专项(2018ZX08012-001-004)作者简介:谢银侠,女,硕士研究生,研究方向:核酸分子检测;E -mail :2913130957@ 通讯作者:许文涛,男,副教授,博士生导师,研究方向:功能核酸生物传感器检测技术;E -mail :xuwentao@电信号分子在电化学功能核酸生物传感器中的研究进展谢银侠 王蔚然 程楠 许文涛(中国农业大学食品科学与营养工程学院,北京 100081)摘 要: 电信号分子是应用于功能核酸电化学生物传感器中起着信号转换作用的具有电化学活性且能够和核酸相互作用或是可以标记在核酸链上的一类分子的统称。
电信号分子对于功能核酸电化学生物传感器是必不可少的一部分,它对于电化学生物传感器检测的灵敏度和应用的普及性都至关重要。
简要介绍了5大类电信号分子,即染料类电信号分子、金属有机配合物类电信号分子、纳米材料类电信号分子、类过氧化氢酶类电信号分子、有机小分子类电信号分子,详细阐述了这些电信号在功能核酸电化学生物传感器中的应用,主要从产生电信号的方式、实际应用以及每种电信号的使用优缺点进行分析,并对新的电信号分子的发现或设计进行了展望,以期对后续有关电信号的研究有借鉴作用。
1.项目名称生物分子界面作用过程的机制、调控及生物分析

1. 项目名称生物分子界面作用过程的机制、调控及生物分析应用研究2. 推荐单位意见该项目针对生物分析化学中的重大科学问题和应用前景,利用界面调控来发展新型生物分析技术,为生物分析提供了新的思路。
项目研究团队在国家杰出青年基金、科技部“重大科学研究计划”等资助下开展了系统的研究工作,提出了"生物识别-生物分子折叠-电子/能量传递"的耦联传感策略,并被国际国内同行广泛采用;通过研究界面上生物分子吸附、组装和识别等基本物理化学过程的机理及其与生物传感检测性能之间的关系,构建了有利于发生高效生物分子识别的多元、协同功能生物界面;构建了一系列基于功能生物界面的高性能生物传感器,并用于多种疾病相关基因和功能性小分子的高灵敏检测。
项目团队已在Nature Protocols、JACS/Angew. Chem./Adv.Mater.等领域顶尖学术刊物发表了系列研究论文,其中8篇代表性论文被SCI 他引2000余次,20篇核心论文被SCI 他引4000余次。
团队成员还多次应Acc. Chem. Res., Chem. Soc. Rev.等著名杂志邀请撰写相关综述。
我单位认真审阅了该推荐材料及完成人资格,确认推荐材料真实有效,相关栏目符合填写要求,公示期间无异议。
经评审,推荐该项目为国家自然科学奖二等奖。
3. 项目简介本项目属于“生物分析化学”与“界面物理化学”交叉的基础研究。
发展针对特定生物分子的灵敏、特异的分析方法不仅是生物分析化学学科的内在需求,而且也是健康、环境和生物安全等领域所面临的重要挑战,对于肿瘤等重要疾病的早期检测、环境和传染性疾病的监控等方面均具有重要意义。
两相交界的表界面是物质、能量交换和信号转化的场所。
本项目以生物传感“界面”为核心,针对生物分子在传感界面上的吸附、组装和识别过程这一关键科学问题开展了系统研究。
在国际上率先提出并发展了一种基于生物分子构象变化的“动态”生物传感检测新策略,通过构建一系列基于界面调控的生物传感器,实现了若干与重大疾病相关的生物分子的高灵敏、高选择性生物分析检测。
EA——精选推荐

EAELECTROCHIMICA ACTAGUIDE FOR AUTHORSGeneralThe Journal publishes full-length research papers, critical reviews and discussion papers in the field of pure and applied electrochemistry. Contributions from members and non-members of the International Society of Electrochemistry are equally welcome.Research Papers should be complete and authoritative accounts of work that has special significance and general interest, presented clearly and concisely.Critical Reviews are commissioned by the Editor-in-Chief. Authors intending to offer critical reviews are advised first to contact the Editor-in-Chief.Discussion papers contain critical comments on papers already published in the Journal. Comments will be submitted to the Authors of the paper under discussion. Eventual replies will be published jointly with the comments in a special section of the Journal labelled "Discussion Section" at the end of an ordinary issue. Publication will occur only on agreement of both parts. ISE recommends that Preliminary Notes (which are no longer published by Electrochimica Acta) should be sent to Electrochemistry Communications (Editor-in-Chief RG Compton, Oxford University, Physical and Theoretical Chemistry Laboratory, South Parks Road, Oxford OX1 3QZ, England, /doc/82c13e92daef5ef7ba0d3c36.html/locate/elecom ).Contributions will only be considered for publication if they are likely to be of interest to our readers and subscribers. Contents must be relevant to electrochemistry. Presentation and discussion should be at the level of the international status of the Journal. The language can be a reason for rejection at first sight if below an acceptable level of clarity.Detailed descriptions of apparatus etc. should only be given if such apparatus is new. Papers reporting experimental data without adequate interpretation are not acceptable. Papers devoted to applications of well-established techniques to technical problems are as a rule not accepted. Papers are expected to contain mechanistic analysis relevant to electrochemistry. The use of an electrochemical technique does not necessarily turn an otherwise non-electrochemical subject into an electrochemical piece of work.Contributions will be accepted for publication only on the recommendation of referees.Format of ManuscriptAll contributions should be typed, double-spaced, 12-pt font, with wide margins and on one side of the page only. They must be written in English. All pages must be numbered in sequence. Authors for whom English is a foreign language are recommended to have the manuscript thoroughly checked and corrected before submission. The Editors will warmly appreciate the co-operation of authors in preparing papers in a manner that will facilitate the complex work of publication. For research papers an abstract not exceeding 200 words should be provided. Authors must provide five keywords at the end of each abstract for future indexing. Keywords should not be general in nature such as: electrode.The corresponding author should be identified on the manuscript by an asterisk * and a footnote with phone and fax numbers and e-mail address. Authors who are ISE members should be identifiedon the title page with superscript 1 after the name and a footnote " 1 ISE member ".The following should be the main sections and related headings of papers: 1. Introduction, 2. Experimental (or Theory), 3. Results and Discussion, 4. Conclusions, (Acknowledgements), References. Any other section should be a subsection identified by subheadings (1.1., 1.2., 1.2.1., 2.1….and so on).Acronyms should be avoided and in any case explained in full the first time they are introduced. TablesTables should be kept to a minimum and must be numbered in sequence; no data should be recorded in both graphical and tabular form. All tables should be provided with headings and should be intelligible without reference to the text.FiguresFigures must be numbered in sequence and the number must be placed close to the figures. Figures should not beembedded in the text document; they should be placed at the end of the manuscript, with a maximum of two figures per page. Figures not identified by a number are not acceptable. Captions to figures should be grouped together.Nomenclature and UnitsEach paper should be consistent within itself as to abbreviations, symbols and units. Authors should use SI units wherever possible and when these are not used should provide a conversion factor to SI units. Axes for graphs and headings for tables should be given in quantity calculus form, eg times as t/s, potential as E/mV, current density as i/A cm-2. Any electrochemical abbreviations should be written in lower case without stops, for example emf, ac.ReferencesReferences should be indicated in the text in square brackets and listed at the end of the paper as follows:[1] R.A. Vargas, A. Garciá, M.A. Vargas, Electrochim. Acta 43 (1988) 1271.[2] J. Newman, Electrochemical Systems, 2nd ed., Prentice-Hall, Englewood Cliffs, NJ,1991.[3] A.R. Hillman, in: R.G. Linford (Ed.), Electrochemical Science and Technology of Polymers, vol. 1, Elsevier, Amsterdam, 1987, Ch. 5.[4] B. Miller, Proc. 6th Australian Electrochem. Conf., Geelong, Vic., 19-24 Feb., 1984; J. Electroanal. Chem., 168 (1984) 91.Abbreviations of journal titles should follow those in World List of Scientific Periodicals (Fourth Edition).Multiple references under a same number do not fit in with the standard of the Journal and should be avoided.Specific Instructions for online submission of papersSubmission to Electrochimica Acta proceeds totally online. To submit an article online, authors are requested to upload their article via the journal's online submission and reviewing system (EES) that can be reached at(/doc/82c13e92daef5ef7ba0d3c36.html /electacta/ ). At this page you will also find a detailed description on its use. After uploading your file the system generates an Adobe Acrobat PDF version of the article which is used for the reviewing process. Authors, Reviewers and Editors sendand receive all correspondence by e-mail and no paper correspondence is necessary. Please make sure that artwork files are in an acceptable format (TIFF, EPS or MS Office files) and with the correct resolution. If, together with your accepted article, you submit usable colour figures then Elsevier will ensure, at no additional charge, that these figures will appear in colour on the Web (e.g., ScienceDirect and other sites) regardless of whether or not these illustrations are reproduced in colour in the printed version. For colour reproduction in print, you will receive information regarding the costs from Elsevier after receipt of your accepted article. Please indicate your preference for colour in print or on the Web only. For further information on the preparation of electronic artwork, please see /doc/82c13e92daef5ef7ba0d3c36.html /artworkinstructions.Files should be uploaded in the following order: cover letter, manuscript, captions, tables, figures. The submitting Author (who will exchange correspondence with the system) may not necessarily be the corresponding Author identified by * on the front page of the manuscript.Refereeing of ManuscriptsThe Editors reserve the right to decline to publish manuscripts which they consider inappropriate for Electrochimica Acta.When submitting their paper authors should supply the names and addresses (including e-mail addresses) of 3 suitable referees. The editors usually ask up to 3 referees to review each paper. Guided by referees' reports, the editors will place each manuscript in one of four categories:a) to be accepted for publication,b) to be reconsidered, after the authors have had an opportunity to make recommended revisions and reply in detail to referees' comments,c) to be rejected, with an invitation to the authors to submit an extensively revised manuscript (reject provisionally or in the present form). The revision will be treated as a new manuscript, with the difference that it must be accompanied by a set of detailed replies to all issues raised by the referees of the original manuscript,d) to be rejected. Papers in this category should not be resubmitted, however revised.Proofs, Copyright and ReprintsAll questions arising after acceptance of a paper, especially those concerning proofs, should be directed to:Elsevier Ireland LtdElsevier HouseBrookvale PlazaEast ParkShannon Co. ClareIrelandTel: +353 61 709600Fax: +353 61 709114Proofs. One set of page proofs in PDF format will be sent by e-mail to the corresponding author (if we do not have an e-mail address then paper proofs will be sent by post). Elsevier now sends PDF proofs which can be annotated; for this you will need to download Adobe Reader version 7 available free from /doc/82c13e92daef5ef7ba0d3c36.html /products/acrobat/readstep2.html. Instructions on how to annotate PDF files will accompany the proofs. The exact system requirements are given at theAdobe site: /doc/82c13e92daef5ef7ba0d3c36.html /products/acrobat/acrrsystemreqs.html#70win.If you do not wish to use the PDF annotations function, you may list the corrections (includingreplies to the Query Form) and return to Elsevier in an e-mail. Please list your corrections quotingline number. If, for any reason, this is not possible, then mark the corrections and any other comments (including replies to the Query Form) on a printout of your proof and return by fax, orscan the pages and e-mail, or by post.Please use this proof only for checking the typesetting, editing, completeness and correctness of the text, tables and figures. Significant changes to the article as accepted for publication will only be considered at this stage with permission from the Editor. We will do everything possible to get your article published quickly and accurately. Therefore, it is important to ensure that all of your corrections are sent back to us in one communication: please check carefully before replying, as inclusion of any subsequent corrections cannot be guaranteed. Proofreading is solely your responsibility. Note that Elsevier may proceed with the publication of your article if no response is received.Copyright. Authors are requested to read the note on copyright published inside the covers of each issue. A form to authorise transfer of copyright will be sent out on acceptance of a paper or with the proofs. This form must be signed in the appropriate section and returned to the Publisher before the paper can be published.Offprints.The corresponding author, at no cost, will be provided with a PDF file of the article via e-mail. The PDF file is a watermarked version of the published article and includes a cover sheet with the journal cover image and a disclaimer outlining the terms and conditions of use. Additional paper offprints can be ordered by the authors. An order form with prices will be sent to the corresponding author.Electrochemical CalendarMaterial for the Calendar should be sent to the Editor-in-Chief, Conference organizers are asked to submit details at least six months before the date of the event.。
碳点荧光法测定羟基自由基和葡萄糖

碳点荧光法测定羟基自由基和葡萄糖于海萍;黄述朝;高吉刚;王晓艳【摘要】以柠檬酸和二聚氰胺为原料,水热法制出了发蓝绿色荧光的氮掺杂碳点,这种碳点的粒径小、水溶性好、荧光量子产率高.该碳点的荧光可被芬顿反应产生的羟基自由基猝灭,从而建立了羟基自由基的测定方法,测定的线性范围为9.5×10-7~7.5×10-5 mol·L-1,测定极限为9.5×10-7 mol·L-1.耦合葡萄糖在过氧化酶(GOD)作用下产生H2O2的反应,建立了碳点荧光法测定葡萄糖含量的新方法,该方法应用于人体尿液的葡萄糖含量的测定,取得了满意的结果.【期刊名称】《广州化工》【年(卷),期】2018(046)013【总页数】4页(P70-72,85)【关键词】碳点;二聚氰胺;羟基自由基;葡萄糖【作者】于海萍;黄述朝;高吉刚;王晓艳【作者单位】山东农业大学化学与材料科学学院,山东泰安 271018;山东农业大学化学与材料科学学院,山东泰安 271018;山东农业大学化学与材料科学学院,山东泰安 271018;山东农业大学化学与材料科学学院,山东泰安 271018【正文语种】中文【中图分类】O644.1羟基自由基(·OH)是一种对生物体毒性最强、危害最大的活性氧粒子之一,它可以与生物体内的糖类、氨基酸、蛋白质、核酸和脂类多种分子作用[1],造成细胞和组织的坏死或损伤,引发机体功能的衰退和许多疾病的产生[2],因此,及时准确的检测羟基自由基是非常必要的。
常见的检测羟基自由基的方法有分光光度法、荧光光度法、高效液相色谱和电化学检测等方法,它们各有特点,同时也存在着灵敏度不高、光稳定性差、前处理复杂等缺点[3-4]。
荧光碳点作为一种新型的荧光材料,除了具有高荧光性外,还具有较好的生物相容性、水溶性、光稳定性[5-7],以及原料易得、制备方法灵活多样[8]、易于修饰等特点[9-10],受到人们广泛的关注[11-12]。
好An electrochemical biosensor based on hairpin-DNA aptamer probe and restriction

An electrochemical biosensor based on hairpin-DNA aptamer probe and restriction endonuclease for ochratoxin A detectionJing Zhang a ,Jinghua Chen b ,⁎,Xi Zhang b ,Zhigao Zeng b ,Mei Chen b ,Shihua Wang a ,⁎a The Ministry of Education Key Laboratory of Biopesticide and Chemical Biology,and College of Life Sciences,Fujian Agriculture and Forestry University,Fuzhou,Fujian 350002,China bDepartment of Pharmaceutical Analysis,Faculty of Pharmacy,Fujian Medical University,Fuzhou,Fujian 350108,Chinaa b s t r a c ta r t i c l e i n f o Article history:Received 3August 2012Received in revised form 30August 2012Accepted 5September 2012Available online 13September 2012Keywords:Hairpin-DNARestriction endonuclease OTAAptamerA new electrochemical DNA biosensor based on hairpin anti-ochratoxin A (OTA)aptamer and site-speci fic DNA cleavage of restriction endonuclease TaqaI was reported in this paper.TaqaI is able to speci fically cleave onlydouble strand DNA,but not single strand DNA.The hairpin-DNA aptamer probe (Hap),thiolated single strand DNA labeled with biotin group,was immobilized on a gold electrode.In the presence of OTA,Hap bind with OTA to form Hap-OTA G-quadruplex.Meanwhile,the stem of Hap,which contains a TaqaI recognition site,is opened to form single strand and resistant to the cleavage of TaqaI.After reaction with the streptavidin-HRP,the resulting HRP-tagged Hap-OTA can effectively catalyze the hydrogen peroxide (H 2O 2)-mediated oxidation of 3,3′,5,5′-tetramethylbenzidine sulfate (TMB),accompanied by a change from colorless to blue in solution color and an increased electrochemical current signal.However,in the absence of OTA,the biotin group of the Hap can be easily cleaved by TaqaI due to the double-stranded stem,which makes the streptavidin-HRP cannot bind with the Hap and increase electrochemical current signal.By employing the above strategy,this DNA biosensor can detect as low as 0.4pg/mL OTA with ultrahigh selectivity.©2012Elsevier B.V.All rights reserved.1.IntroductionOTA is known to be carcinogenic,hepatotoxic,teratogenic,nephro-toxic and immunotoxic to a variety of mammalian species [1,2].There-fore,considering the severe toxic effects of OTA,it is urgently needed to develop a very sensitive analytical method for low level OTA detec-tion.Surprisingly,Cruz-Aguado and Penner first developed anti-OTA aptamer [3].Compared with antibodies,aptamers not only have similar high af finity and selectivity,but also have several signi ficant advantages including simple production,easy storage,great reproduc-ibility,target versatility,easy modi fication,convenient regeneration and commercially available [4].So many aptamer-based biosensors (aptasensors)for OTA detection have been described [5–7].However,these OTA aptasensors required multiple,complex and time con-suming steps prior to detection,and the sensitivities still need to be improved.Recently,several novel OTA electrochemical aptasensors have been developed due to fabrication easiness,operation conve-nience,low cost,fast,portable,aiming at the improvement of sensi-tivity and selectivity [8,9].Although these electrochemical methods were ultra-sensitive,required modi fications of nanoparticles was a complex process.Moreover,they may suffer from high background signals that are associated with low-speci fic binding of linear aptamer probe and OTA.In an attempt to improve the sensitivity and speci ficity of the sensors,hairpin-DNA,composed of a hairpin oligonucleotide that possesses a stem-and-loop structure,was applied in the fabrication of the electro-chemical DNA sensors [10–12].In comparison with similar sensors performed using linear ss-DNA,hairpin-DNA probe has been found to exhibit extraordinary stability,better selectivity,higher sensitivity,ex-cellent reproducibility,fast speed and convenience [13,14].In addition,different hairpin-DNA probes can be designed by choosing loop se-quence and size,namely,we could design hairpin-DNA probes as dif-ferent recognition elements according to various targets.Therefore,we herein propose a new electrochemical biosensor based on hairpin-DNA aptamer probe for detection of OTA.As far as we know,this is the first attempt to apply hairpin-DNA aptamer into the fabrication of electro-chemical sensor for OTA detection.At the same time,in order to improve the sensitivity of the sensor,TaqaI,which is a restriction endonuclease that cuts only double-stranded not single stranded DNA at speci fic rec-ognition nucleotide sequences known as restriction sites (5′-TCGA-3′)[15],is adopted here.The combination of the hairpin-DNA aptamer probe,restriction endonuclease and OTA-induced structure-switch pro-vides an ultra-high sensitivity and speci ficity assay for the OTA detection.2.Experimental2.1.Chemicals and apparatusHap:5′-biotin-T TC GA T CGG GTG TGG GTG GCG TAA AGG GAG CAT CGG A TC GA A AAA-HS-3′(The boldfaced portion is a TaqaI recognitionElectrochemistry Communications 25(2012)5–7⁎Corresponding authors.Tel./fax:+8659122862016.E-mail addresses:cjh_huaxue@ (J.Chen),wshyyl@ (S.Wang).1388-2481/$–see front matter ©2012Elsevier B.V.All rights reserved./10.1016/j.elecom.2012.09.006Contents lists available at SciVerse ScienceDirectElectrochemistry Communicationsj o ur n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /e l e c o msite,and italics indicated a OTA recognition site,the sequence with underline is the stem part of the Hap)was synthesized by TaKaRa biotechnology Co.,Ltd.(Dalian,China).TaqaI was obtained from New England BioLabs.TMB/H2O2solution was purchased from Neogen Corporation(USA).Other reagents were purchased from Sigma-Aldrich(USA).All solutions were prepared with MilliQ water (18.2MΩ/cm resistivity)from a Millipore system and all other chemicals were of analytical grade.All electrochemical measurements were performed by using CHI660D Electrochemical Workstation (CH Instrument,USA).The electrochemical system consisted of a working electrode(a2-mm-diameter Au disk electrode),a platinum wire as the auxiliary electrode,and a reference electrode(Ag/AgCl). The chronoamperometry was performed with incipient potential of+0.1V,the sample interval of0.1s and the experimental time of100s.2.2.Preparation of the proposed biosensorA gold disk electrode(GE)wasfirstly polished to obtain mirror surface with0.05μm alumina powder,followed by sonication in ethanol and water for5min respectively.After drying with nitrogen, the electrode was immediately used for DNA immobilization.Next, 5μL Hap solution wasfirst spread on the pre-cleaned gold electrode surface for12h in the100%humidity.Then,this electrode was immersed in1mM MCH for2h to remove the nonspecific DNA adsorption and optimize the orientation of the Hap to make binding easier[16].The Hap surface density could be measured with chronocoulometry(CC)as previously described[17].At low surface densities,the net current signal(ΔI p,the current obtained in the presence of OTA minus the background current)was very low.At the same time,at high surface densities,the binding efficiency of OTA with the Hap decreased and the cutting reaction rate of the TaqaI slowed down due to the steric effect.Thus,theΔI p was also low.Our results showed theΔI p reached to the maximum when the surface density was1.52×1012molecules/cm2.So,it was used for the further detection.Subsequently,the Hap-immobilized electrode was immersed in OTA for30min at37°C with stirring.Afterwards, the sensor was then immersed in a solution of TaqaI reaction buffer (0.1Units/μL)and rinsed.Prior to electrochemical measurement, the modified electrode was incubated with3μL of streptavidin-HRP for25min at room temperature and washed.Finally,the above elec-trode was detected in the TMB/H2O2solution by electrochemical measurement.3.Results and discussion3.1.Principle of the proposed sensorThe detailed principle of our sensor is illustrated in Fig.1.Hap was designed according to the structure of the anti-OTA aptamer.The loop and part of the stem of the Hap contains OTA recognition site(colored red),and the stem of the Hap contains a TaqaI recognition site (colored green).Firstly,the Hap,labeled with a biotin group at the 5′-end and a thiol group at the3′-end,was immobilized on a gold electrode through S\Au bonding(Fig1).In the presence of OTA, the red part of Hap bound with OTA to form the OTA-Hap G-quadruplex.Meanwhile,the stem of the Hap was opened to form single strand and resistant to the cleavage of TaqaI.Thus the streptavidin-HRP can attach to the biotinylated Hap through the biotin-avidin-system.Upon dipping the resulting modified electrode into the H2O2and TMB,the HRP attached to the Hap catalyzed the H2O2-mediated oxidation of TMB,accompanied by a change from colorless to blue in solution color and an increased electrochemical current signal.However,in the absence of OTA,the stem of the Hap exited predominantly in double-stranded form.So it can be easily cleaved by TaqaI,which leads the biotin group to leave the surface of the electrode by washing.Thus,streptavidin-HRP cannot bind to the Hap and increase electrochemical current signal.Electrochemical im-pedance spectrum(EIS)was employed to characterize the fabrication in whole process.The results indicated that our biosensor indeed worked as expected(data were not shown in).3.2.The selectivity of the sensorIn order to evaluate the selectivity of practical implementation of OTA detection,we compared the change of current intensity induced by three other mycotoxins(ochratoxinB(OTB),aflatoxin B1(AFB1) and zearalenone(ZEN)at the same concentration.As shown in Fig.2,the current signal to the AFB1or ZEN was almost neglectable while that to the OTB was a little high.As we all know,OTB,which is the structural analog of the OTA,still possesses the combination ability with OTA aptamer[8].Therefore,it is of great significance for the selectivity of a sensor to distinguish OTA from OTB.To quantita-tively evaluate the selectivity of our sensor,we defined the discrimi-nation factor as the ratio of theΔI p obtained with OTA to OTB.From the data shown in Fig.2,we calculated that the discrimination factor was18.6.In comparison with the other electrochemicalsensorsFig.1.The principle of the sensor.6J.Zhang et al./Electrochemistry Communications25(2012)5–7reported recently [8,18],of which the discrimination factors were about 5.0and 1.6,respectively,our sensor dramatically improve the discrimination ability of electrochemical biosensors.Furthermore,compared with the sensors based on OTA-antibody,our sensor also have higher selectivity because the difference in af finity between OTA and OTB with aptamer is far greater than with monoclonal anti-body or polyclonal antibodies [19].3.3.The sensitivity of the aptasensorThe sensitivity of the electrochemical DNA biosensor for detection of OTA was investigated by varying OTA concentration (see Fig.3).The average current showed a good linear correlation with the con-centration of OTA in the range of 1.0–20pg/mL.The calibration equa-tion obtained from this curve is I(nA)=37.295C(pg/mL)+196.995with a correlation coef ficient of 0.9961.The detection limit was calcu-lated to be 0.4pg/mL for OTA.The relative standard deviation (RSD)of the sensor for 10replicate determination of 10pg/mL was 5.8%.In contrast to prior electrochemical sensors based on linear ss-DNA aptamer probe or anti-OTA antibody [8,20],the proposed biosensor,which combined the merits of hairpin-DNA aptamer probe and restric-tion endonuclease,can get a better sensitivity because the background signal was too low to be ignored.In order to evaluate the practical applicability and accuracy,the sensor was used for determining the recoveries by spiking three different concentrations(5.0,10.0,15.0pg/mL)of OTA into the real wheat sample.The recoveries of the spiked samples and the relative standard derivations were in the range of 95.8–105.4%and 5.2–6.4%,respectively.The experimental results veri fied that the proposed aptasensor can be applied to the quantitative determination of OTA in wheat.4.ConclusionsIn summary,we have introduced here a novel electrochemical biosensor based on hairpin-DNA aptamer probe and site-speci fic DNA cleavage of restriction endonuclease for detection of OTA in wheat.The results illustrated that the proposed sensor had the advantages of higher sensitivity and lower background current.These features,as well as its other advantages,such as fabrication easiness,operation convenience and low cost,make it a promising candidate for routine detection of OTA.In addition,because the result of our assay will be clearly observable with the naked eye,this sensor will facilitate the construction of the optical biosensors,appropriate for deployment in the developing world.Most importantly,with more and more aptamer speci fic to other toxins explored,the sensor can be used in monitoring of other toxins only through designing hairpin-DNA aptamer probe and rational selecting endonuclease according the various toxins.Therefore,we believe that our strategy could offer a universal approach for field monitoring and large scale quality control of food safety.AcknowledgmentThe authors gratefully acknowledge the financial support of Na-tional Natural Science Foundation of China (21105012,21205015),Program for New Century Excellent Talents in University of China (NCET-10-0010),Agricultural Five New Projects of Fujian Develop-ment and Reform Commission,National Science Foundation of Fujian Province (2011J01028),Program for Fujian University Outstanding Youth Scienti fic Research (JA11105,JA10295),the Youth Foundation of Fujian Health Department (2010126)and the Foundation of Medi-cal Innovation of Fujian Health Department(2011-CX-22).References[1] E.O'Brien,D.R.Dietrich,Critical Reviews in Toxicology 35(2005)33.[2]I.Bazin,E.Nabais,M.Lopez-Ferber,Toxins 2(2010)2230.[3]J.A.Cruz-Aguado,G.Penner,Journal of Agricultural and Food Chemistry 56(2008)10456.[4]S.Jayasena,Clinical Chemistry 45(1999)1628.[5] C.Yang,Y.Wang,J.L.Marty,X.R.Yang,Biosensors and Bioelectronics 26(2011)2724.[6]L.F.Sheng,J.T.Ren,Y.Q.Miao,J.H.Wang,E.K.Wang,Biosensors and Bioelectronics26(2011)3494.[7]J.Chen,Z.Fang,J.Liu,L.Zeng,Food Control 25(2012)555.[8]H.Kuang,W.Chen,D.H.Xu,L.G.Xu,Y.Y.Zhu,L.Q.Liu,H.Q.Chu,C.F.Peng,C.L.Xu,S.F.Zhu,Biosensors and Bioelectronics 26(2010)710.[9]P.Tong,W.W.Zhao,L.Zhang,J.J.Xu,H.Y.Chen,Biosensors and Bioelectronics33(2012)146.[10]S.Tyagi,F.Kramer,Nature Biotechnology 14(1996)303.[11] C.H.Fan,K.W.Plaxco,A.J.Heeger,Proceedings of the National Academy of Sciencesof the United States of America 100(2003)9134.[12]J.Chen,J.Zhang,K.Wang,L.Huang,X.Lin,G.Chen,Electrochemistry Communi-cations 10(2008)1448.[13]J.J.Li,Y.Chu,B.Y.Lee,X.S.Xie,Nucleic Acids Research 36(2008)e36.[14]R.Miranda-Castro,P.De-Los-Santos-Alvarez,M.J.Lobo-Castanon,A.J.Miranda-Ordieres,P.Tunon-Blanco,Analytical Chemistry 79(2007)4050.[15] C.Kessler,V.Manta,Gene 92(1990)1.[16] A.K.H.Cheng,B.X.Ge,H.Z.Yu,Analytical Chemistry 79(2007)5158.[17]o,S.Song,H.Wu,L.Wang,Z.Zhang,L.He,C.H.Fan,Analytical Chemistry 77(2005)6475.[18] C.Yang,tes,B.Prieto-Simón,J.L.Marty,X.Yang,Biosensors and Bioelectronics32(2012)208.[19]J.A.Cruz-Aguado,G.Penner,Food Chemistry 56(2008)10456–10461.[20]M.A.Fernández-Baldo,F.A.Bertolino,G.A.Messina,M.I.Sanz,J.Raba,Talanta83(2010)651.50100150200-1500-1000-500C u r r e n t /n At/sFig.2.Selectivity of the sensor toward OTA (50pg/mL)against other mycotoxins (50pg/mL).Blank (a),AFB1(b),ZEN (c),OTB (d),OTA (e).Inset:the histogram of selectivity.Each data point represents the average value of three independent experi-ments with error bars indicated.4080120160200240-1500-1000-50051015202004006008001000C u r r e n t / n At/sa fI= 37.295C + 196.99r= 0.9961C(pg/mL)I (n A )Fig.3.Chronoamperometry for different OTA concentrations (pg/mL):(a –f)back-ground,1.0,5.0,10.0,15.0and 20.0.Inset:A calibration curve demonstrating peak current intensity versus OTA concentration.7J.Zhang et al./Electrochemistry Communications 25(2012)5–7。
纳米材料修饰电极
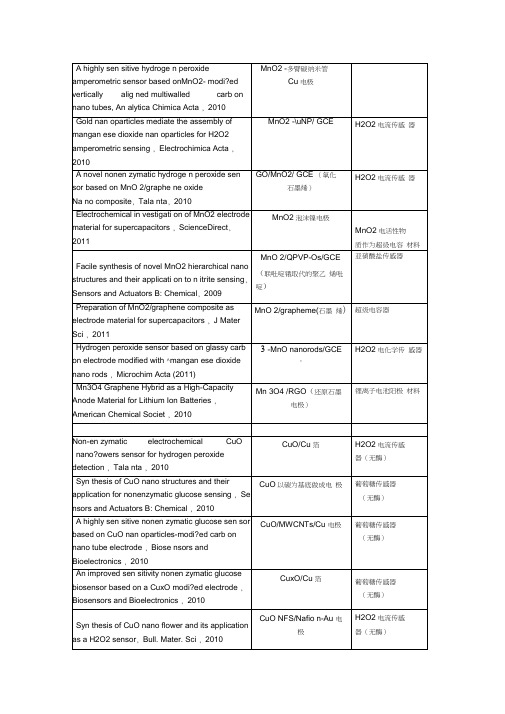
f-MWCNTs /GCESWCNH/GCE (单臂碳纳米管) graphe ne /GCEGR -CS/GCE,(石墨烯-壳聚糖)chitosan-graphene-GCE (壳聚糖-石墨烯) nano-Cu/PPy/GCE (聚吡咯) CPB/chitosan/GCE 溴化十六烷基吡啶 Chitosa n-CTAB /GCE亚硝酸盐MnO2/QPVP-Os/GCE (联吡啶锇取代的 聚乙烯吡啶) nano-Au/Ch/GCE (氯化胆碱)CR-GO/GCE (还原型氧化石墨烯) Nano-AI2O3 /GCEDAB /GCE (双十二烷基溴化铵) 对乙酰氨基酚 f-MWCNTs /GCE MWNT or SWNT/EPPGMWCNTs:graphite/GCE 多臂碳纳米管和石墨混合 Carbon nano tubes paste electrodes 碳纳米管糊电极 graphene /GCE (石墨烯)PAMAMPAMAM/Fe3O4 /GCE PAMAM/CoTe /GCE纳米AI2O3,对有机磷分子有较好的氧化还原活性。
其氮原子上一对孤对电子易于从溶液中结合一个氢质子•。
人们充分利用生物高分子壳聚糖的生物相容性、低 不断开发它的应用领域。
在分析化学上,己用于分离富Kazunori 等用壳聚糖修饰电极测定 北京大学叶宪曾研究组曾用壳聚糖修饰玻Au (lll ),Ag (l ),Pt (ll )和 Pd (ll )。
尿酸和乙酰氨基酚对葡萄糖检测的干扰。
多巴胺花状 ZnO/GCE多巴胺传感器同时检测多巴胺和对乙酰氨基酚 同时检测尿酸、多巴胺和抗坏血酸 抗坏血酸存在下检测多巴胺 抗坏血酸存在下检测多巴胺 同时检测抗坏血酸、多巴胺、尿酸 同时测定多巴胺和尿酸 同时检测多巴胺和抗坏血酸 同时检测多巴胺和抗坏血酸亚硝酸盐传感器 亚硝酸盐传感器亚硝酸盐传感器 对亚硝酸盐的检测检测水样中的亚硝酸盐同时检测多巴胺和对乙酰氨基酚 测定对乙酰基氨基酚 对乙酰氨基酚(扑热息痛) 对乙酰氨基酚对乙酰氨基酚的检测,不受多巴 胺和抗坏血酸的干扰测定牛奶中的双酚 A 测定水中的双酚A壳聚糖分子链上有许多游离的氨基, 而使壳聚糖成为带正电荷的聚电解质 毒性、生物可降解性以及可食用性, 集痕量Ni (ll ) , Cu (ll ) , Cd (ll )。
传感器技术在有机磷农药残留检测中的应用

传感器技术在有机磷农药残留检测中的应用【摘要】快速有效地检测农产品中有机磷农药残留是目前急需解决的问题。
本文分析了传感器技术在多种有机磷农残检测手段中的应用和存在的不足,特别是近年来发展迅猛的生物传感器技术呈现的多样化特点。
目前仍然缺乏一种简便、高效、快速、无损的检测技术,这已经成为当前研究者关注的热点。
【关键词】有机磷农药;传感器;检测1.引言有机磷农药是20世纪30年末问世的第二代人工合成农药,具有广谱、高效、品种多和残毒期短等特点,经常被用作杀虫剂喷洒在果树和蔬菜上。
如果残留在水果和蔬菜上的有机磷或环境中的有机磷进入到有机体内,大部分会对生物体内胆碱酯酶有抑制作用,使其失去分解乙酰胆碱的能力,造成乙酰胆碱积累,引起神经功能紊乱,从而导致肌体的损害。
因此,对农产品中的有机磷残留进行快速、高效的检测具有重要意义。
以理化方法为主的波谱法、色谱法、色质联用法等传统检测手段,操作复杂,耗时长。
在国内外近年来开展的快速、高效的检测方法研究中,传感器技术特别是生物传感器技术得到广泛应用,起到了重要作用。
2.常用传感器检测技术2.1 电子鼻(气敏传感器)检测技术电子鼻因模拟嗅觉系统而得名,是模仿生物鼻的一种电子系统,是二十世纪90年代发展起来的分析、识别和检测复杂嗅觉及大多数挥发性气体成分的仪器。
电子鼻主要是由气敏传感器阵列和模式识别系统两部分组成。
气敏传感器相当于人类嗅觉系统中的嗅觉细胞,是电子鼻检测性能优劣的基础。
单个气敏传感器的功能十分有限,目前还没有发现只对某种气体单一敏感的传感器材料,单个传感器对不同的响应可能会有变化,但它不具备自动识别气体种类和数量的能力。
因此由具有光谱响应特性、高灵敏度、对不同气体(气味)灵敏度不同的气敏传感器组成传感器阵列,利用其交叉敏感性,来提高电子鼻的检测性能。
利用信号预处理方法滤除模式采集过程中引入的噪声和干扰,提高信噪比,同时消除信号的模糊和失真,人为增强有用信号。
电化学脱氧核糖核酸传感器进展

・综 述・电化学脱氧核糖核酸传感器进展Ξ李 红a,b,计亮年a(a中山大学化学与化学工程学院 广州 510275;b华南师范大学化学系 广州 510631) 电化学DNA传感器的发展在过去短短几年里倍受重视[1]。
它是一种全新的特定DNA序列(基因)检测技术,它与通常的标记(放射性同位素标记,荧光标记等)探针技术相比,具有选择性好、灵敏度高、响应快、操作简便、价格低廉等特点,可望用于临床上基因疾病的快速检验,用于探讨各种因素引起DNA损伤的程度和可能的突变机理,用于以DNA为作用靶的药物研究。
因此,虽然电化学DNA 传感器才有几年的历史,但已有了很大的进展。
本文将对电化学DNA传感器研究进展进行评述。
1 电化学DNA传感器原理电化学DNA传感器是由一个支持DNA片段(探针)的电极和检测用的电活性杂交指示剂(hy2 bridization indicator)构成。
DNA探针是单链DNA (ssDNA)片段(或者一整条链),长度从十几个到上千个核苷酸不等,它与靶序列(target sequence)是互补的,一般多采用人工合成的短的寡聚脱氧核苷酸作为DNA探针。
通常是将ssDNA(探针分子)修饰到电极表面构成DNA修饰电极。
由于ssDNA与其互补靶系列杂交具有高度的序列选择性,使得这种ssDNA修饰电极呈现极强的分子识别功能。
在适当的温度、pH、离子强度下,电极表面的DNA探针分子能与靶序列选择性地杂交,形成双链DNA(dsD2 NA),从而导致电极表面结构的改变。
这种杂交前后的结构差异,可以通过具有电活性的杂交指示剂来识别,这样便达到了检测靶序列(或特定基因)的目的。
2 DNA修饰电极的制备及其特点DNA修饰电极所使用的基底电极有玻碳电极,金电极,碳糊电极和裂解石墨电极等。
目前制DNA 修饰电极主要有3种方法:吸附法、共价键合法和组合法。
211 吸附法21111 化学吸附法Joseph Wang等[1,2]发展了一种简单DNA修饰电极的方法。
501型氨气敏电极说明书

501型氨气敏电极说明书氨气敏电极是用于水中溶解氨,铵盐测定的电化学传感器,亦可用于硝酸盐氮、总氮、有机氮的测定。
其结构示意图见图一,在电极管底部,装有微孔气透膜(1),管内盛有中介液(内电解液)(5)内部敏感元件是由平头PH玻璃电极(12)及银/氯化银电极(9)组成的电极对,平头PH玻璃电极的敏感玻璃膜(3)紧贴于气透膜(1)上,二者之间形成一极薄的中介液层(2),当氨气敏电极浸入加有氢氧化钠的感性待测试液时,试液中的铵酸转化为溶解的氨,经气透膜(1)渗透入中介液薄层(2)发生如下变化。
NH3+2O NH4+OH —由于中介液本身的氨离子浓度较上述反应形成的NH4量显著为高故其变化可忽略不计,而中介液薄层的PH值则由于OH —的形成而升高,中介液中氯离子活度恒定,故银/氯化银参比电极电位值恒定,因此在恒定时,PH玻璃电极与银/氯化银电极这一电极对的电位值。
仅随中介液薄层的PH值变化,亦可测得的电位值将于外部试液中氨的浓度呈近似Nernst。
2.3RTE=常数- ————Log(NH3)F空白电位计+ 80mV左右本电极测定氨的线性范围为1×10-5M~1×10-1M,检测下限约为5×10-6M,适于室温(不高于40℃)使用。
氨气敏电极由电极外腔管(已装上一透气膜,未装入中解液)及内部电极对两部分组成。
并随同电极附有气透膜。
(一)电极组装与保存电极组装步骤如下:(1)装置前先将固定螺帽(13)松开。
取出内部玻璃电极,并浸泡在0.1M氯化铵溶液中1小时以上。
(2)配制中介液(0.1M氯化铵),亦可根据使用时的特殊条件。
采用其他成份的中介液,其要求是:氯离子活度恒定,铵离子浓度较测试过程中形成的NH3浓度高,PH缓冲容量尽可能小,总离子强度尽可能与外部被测试液接近,但应注意在浓度较大的碱金属盐溶液中PH玻璃电极响彻云响应较迟缓。
(3)装入气透膜,电极第一次使用时可用于原已装好的气透膜,如发现原装上的气透膜已破裂,或用久后不能使用时,可先将固定螺帽(13)松开并取出玻璃电极后,再将垫圈(8)处外腔管上。
DNA电化学传感器.
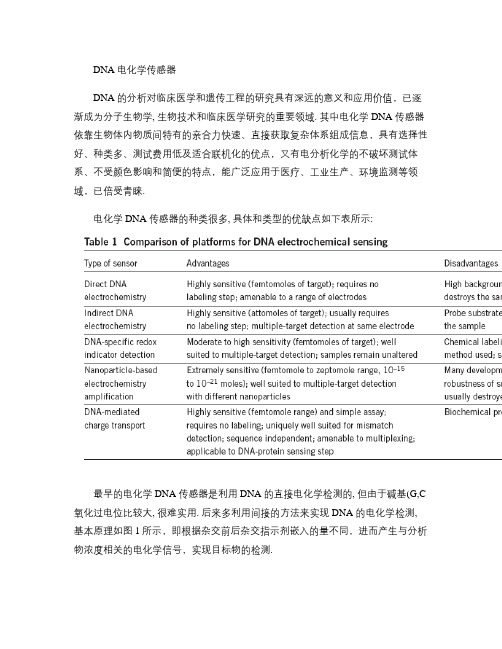
DNA 电化学传感器DNA 的分析对临床医学和遗传工程的研究具有深远的意义和应用价值,已逐渐成为分子生物学, 生物技术和临床医学研究的重要领域. 其中电化学DNA 传感器依靠生物体内物质间特有的亲合力快速、直接获取复杂体系组成信息,具有选择性好、种类多、测试费用低及适合联机化的优点,又有电分析化学的不破坏测试体系、不受颜色影响和简便的特点,能广泛应用于医疗、工业生产、环境监测等领域,已倍受青睐.电化学DNA 传感器的种类很多, 具体和类型的优缺点如下表所示:最早的电化学DNA 传感器是利用DNA 的直接电化学检测的, 但由于碱基(G,C 氧化过电位比较大, 很难实用. 后来多利用间接的方法来实现DNA 的电化学检测, 基本原理如图1所示,即根据杂交前后杂交指示剂嵌入的量不同,进而产生与分析物浓度相关的电化学信号,实现目标物的检测.图1 电化学DNA 传感器结构示意图接着为了提高灵敏度与选择性,很多研究者利用酶及纳米粒子, 量子点的放大效应,来满足低浓度的检测, 如图2所示, 就是利用典型的构建DNA 传感器的”三明治结构”与纳米Pt 的高效电化学催化性质来实现DNA 的低浓度检测.Kurt V. Gothelf 等就利用了PdS,CdS 与ZnS 量子点实现了DNA 的fM 级检测, 如图3所示: 首先图3.C1,2,3:capture DNA; r1,2,3:report DNA ;B: C1,2,3都存在; D: 仅C3@AuWE:汞膜玻碳电极(同为镀汞;RE:Ag/AgCl; CE: Pt在金基底上自组装5-SH- C1,2,3并用MCH 来惰化活性位点, 接着与固载了5-SH-r1,2,3的PdS,CdS, ZnS 量子点退火杂交, 经彻底洗涤后, 金基底上间接固载的金属硫化物纳米颗粒用0.10M HNO3溶解下来, 再利用阳极溶出伏安法(ASV的溶出峰(如图2 B 能很好的分离来定性(电位与定量(电流检测这些溶解的金属离子. 可以利用此装置才检测 target 3, 如图4所示:target 3与r3 有20bases 互补, 而C3与r3 只有15bases 互补, 故可利用前者杂交结合力的竞争优势(将已与C3杂交的PbS 竞争下来脱离金基底—Pd 的ASV 信号降低来实现target 3的检测. 图5就反映了target 3加入前后Pd图4. 竞争检测 DNA target 3 图5. 竞争前后Pb 的ASV 与 nano-PdS@Au的AFM 图的ASV 信号降低的情况及竞争前后金基底上相同区域PdS 纳米粒子的减少情况. 图6就是不同浓度的target3时,Pd 与Cd 的电流比值情况, 显然这是属于”signal off ”类型的电化学DNA 传感器, 但是由于此方法中有一”内标—CdS ”, 所以可以利用Pd 与内标Cd 的信号比值来反应target 3的多少, 进而消除了常规”signal off” 型传感器的缺点.图6. 竞争检测不同浓度的target 3另外Joseph Wang等也利用相似的原理实现了多个DNA 序列的同时检测, 原理和结构如图7,8所示:图7. 利用不同的纳米晶追踪者检测多个目标DNA 图8.SWASV of the metal traces 除了上面的利用纳米粒子的电化学来间接检测DNA 外, 还有一类研究较多的电化学DNA 传感器就是利用被标记(如MB,FC 等电活性物质的DNA 做report 序列, 根据其或probe 与target 作用前后, 电活性分子电信号的变化来反应target 的量. 如图9所示:target3入之前,MB-report 2 与probe 1一端杂交, 使得MB 离电极较远, 电信号弱, 当target 3加入后杂交碱基数多, 占优势,1与2变解旋, 使得MB 离电极很近, 发生电子转移容易, 电信号增加, 显然这是一个” signal-on”的DNA 电化学传感器, 比” signal-off”型占优势, 作者发现此图9. signal-on DNA sensor 图10. 不同浓度的target 3与mismatched 的伏安图传感器不仅稳定性好, 而且加入饱和浓度的5-base-mismatched target 3对测试信号没有任何影响, 如图10所示. 这种类型的电化学DNA 传感器, 设计的思路巧妙, 优势也很大, 现在研究的人员也较多, 类型也会越来越丰富.从上面的一些比较经典的电化学DNA 传感器的构建来看, 利用纳米粒子, 量子点等, 可以实现DNA 的高灵敏度检测, 利用活性物质标记的DNA 也可实现低浓度及有几个碱基错配的检测, 但是实现单碱基错配的检测都不是很容易, 报道也不是很多. 而关于单核苷酸多态性(SNP在DNA 的检测中很重要, 人体许多表型差异、对药物或疾病的易感性等等都可能与SNP 有关, 现在也普遍认为SNP 研究是人类基因组计划走向应用的重要步骤, 所以在电化学DNA 传感器的设计中, 应尝试更多新颖的思路, 实现不仅灵敏度高而且选择性高的多目标物的同时分析.参考文献:1. Naturebiotechnology ,2003,Vol 21,Number 10, 1192-1199Jacqueline BartonElectrochemical DNA sensor2. PNAS , 2006 , vol. 103 , 16677–16680Yi Xia, Arica A. Lubin, Brian R. Baker, Kevin W. Plaxco and Alan J. HeegerSingle-step electronic detection of femtomolar DNA by target-induced strand displacement in an electrode-bound duplex3. J. AM. CHEM. SOC. 2006, 128, 3860-3861Jacob A. Hansen, Rupa Mukhopadhyay, Jonas Ø. Hansen, and Kurt V. GothelfFemtomolar Electrochemical Detection of DNA Targets Using Metal Sulfide Nanoparticles4. J. AM. CHEM. SOC. 2003, 125, 3214-3215Electrochemical Coding Technology for Simultaneous Detection of Multiple DNA TargetsJoseph Wang, Guodong Liu, and Arben Merkocü i。
CurriculumVitae

Curriculum VitaeProfessor Li Mao Guo, Ph.D. College of Chemistry and Materials Science, Anhui Normal University Beijing East Road No.1, Wuhu 241000, ChinaTel.: +86 553 3869302; Fax: +86 553 3869303E-mail: ******************Full Professor of Analytical ChemistryB.S., Anhui Normal University, China (1997)M.S., Anhui Normal University, China (2002)Ph. D., Anhui Normal University, China (2008)Research Fellow (Postdoctoral), National University of Singapore, Singapore (2009.10-2011.5)Research InterestsUsing synthetic inorganic chemistry and analytical chemistry as major tools to study the important enzymes (proteins) and other biomolecules. Currently, I have three projects in my lab.1. Direct electrochemistry of heme proteins.2. Enzyme mimetics based on metal or metal oxide nanostructures.3. Biosensors based on polydopamine or derivatives.Social ServiceThe member of Chinese Chemical Society.The Editorial Board Member of Scientific Reports (Chemistry).All Publications1.Mengmeng Guo, Muping Yu, Xiang Li, Maoguo Li*, “Immobilised cytochrome c onthe carbon dots functionalised MWCNTs and its application to hydrogen peroxide detection” International Journal of Environmental Analytical Chemistry 2017, 97, 1107–1118.2.Ding Hou, Haisheng Tao*, Xuezhen Zhu, Maoguo Li*, “Polydopamine and MnO2core-shell composites for high-performance supercapacitors” Applied Surface Science 2017, 419, 580–585.3.Yinling Wang*, Shengye Dong, Xiaoqin Wu, Xiaowang Liu, Maoguo Li*, “Core-shellN-doped carbon spheres for high-performance supercapacitors”,Journal of Materials Science2017, 52, 9673–7682.4.Yinling Wang,* Xiaoqin Wu, Xuemei Zhang, Maoguo Li*, “Chitosan, EDTA and cobaltsalts derived metal-N-C sub-micrometer spheres for high-performance oxygen reduction”, Journal of the Electrochemical Society 2017, 164, H389–H395.5.Xiujuan Wu, Miaomiao Yu, Xiaowang Liu, Maoguo Li*, “Low potentialdetermination of NADH at 1-Hydroxypyrene/reduced graphene oxide modified electrode”, International Journal of Electrochemical Science2017, 12, 4488–4501.6.Mengmeng Guo, Qikang Wu, Miaomiao Yu, Yinling Wang, Maoguo Li*, “One-stepliquid phase chemical method to prepare carbon-based amorphous molybdenum sulfides: As the effective hydrogen evolution reaction catalysts”, Electrochimica Acta 2017, 236, 280–287.7.Anna Li, Yuzhe Hu, Muping Yu, Xiaowang Liu, Maoguo Li*, “In situ growth of MoS2on carbon nanofibers with enhanced electrochemical catalytic activity for the hydrogen evolution”, International Journal of Hydrogen Energy2017, 42, 9419–9427.8.Jian Pang, Xiujuan Wu, Anna Li, Xiaowang Liu, Maoguo Li*,“Detection of catechinin Chinese green teas at N-doped carbon modified electrode”,Ionics2017,23, 1889–1895.9.Yinling Wang*, Shengye Dong, Xiaoqin Wu, Maoguo Li,“One-StepElectrodeposition of MnO2@NiAl Layered Double Hydroxide Nanostructures on the Nickel Foam for High-Performance Supercapacitors”, Journal of the Electrochemical Society 2017 164, H56–H62.10.Yinling Wang, Zhangcui Wang, Xiaoqin Wu, Xiaowang Liu, Maoguo Li*, "SynergisticEffect between Strongly Coupled CoAl Layered Double Hydroxides and Graphene for the Electrocatalytic Reduction of Oxygen", Electrochimica Acta2016, 192, 196–204.11.Yinling Wang*, Fajun Li, Shengye Dong, Xiaowang Liu, Maoguo Li*, “A facileApproach for Synthesizing Fe-Based Layered Double Hydroxides with High Purity and Its Exfoliation”, Journal of Colloid and Interface Science2016, 467, 28–34. 12.Yinling Wang*, Xuemei Zhang, Anna Li, Maoguo Li*,"Intumescent FlameRetardant-Derived P,N co-Doped Porous Carbon As an Efficient Electrocatalyst for the Oxygen Reduction Reaction", Chemical Communications2015,51, 14801–14804.13.Xuemei Zhang, Yinling Wang*, Shengye Dong, Maoguo Li*, "Dual-SitePolydopamine Spheres/CoFe Layered Double Hydroxides for Electrocatalytic Oxygen Reduction Reaction", Electrochimica Acta2015, 170, 248–255.14.Yinling Wang*, Zhangcui Wang, Yeping Rui, Maoguo Li*,“Horseradish PeroxidaseImmobilization on Carbon Nanodots/CoFe Layered Double Hydroxides: Direct Electrochemistry and Hydrogen Peroxide Sensing",Biosensors & Bioelectronics 2015,64, 57–62.15.Xiaoli Jiang, Yinling Wang*, Maoguo Li*,“Selecting Water-Alcohol Mixed Solventfor Synthesis of Polydopamine Nano-spheres Using Solubility Parameter”, Scientific Reports 2014, 4, Article Number 6070. DOI:10.1038/srep06070.16.Chang Guo, Lin Wang, Maoguo Li*,“Functionalization of Carbon Nanotubes withCopper for Nonenzymatic Electrochemical Detection of Glucose”, Nanoscience and Nanotechnology Letters 2014, 6(6), 481–487.17.Chang Guo, Maoguo Li*,“Synthesis and Cell Imaging of LaF3Nanocrystals withSmall Particle Size and Novel Upconversion Luminescence”, Acta Chimica Sinica 2014, 72(2): 215-219.18.Li Huang, Shoufeng Jiao, Maoguo Li*, “Determination of uric acid in human urineby eliminating ascorbic acid interference on copper(II)-polydopamine immobilized electrode surface”, Electrochim. Acta 2014, 121, 233–239.19.Yinling Wang*, Yeping Rui, Fajun Li, Maoguo Li,“Electrodeposition of NickelHexacyanoferrate/Layered Doublehydroxide Hybrid Film on the Gold Electrode and Its Application in the Electroanalysis of Ascorbic Acid”, Electrochim. Acta2014, 117, 398–404.20.Qian Song, Maoguo Li*, Li Huang, Qikang Wu, Yunyou Zhou*, Yinling Wang,“Bifunctional Polydopamine@Fe3O4 Core-Shell Nanoparticles for Electrochemical Determination of Lead (II) and Cadmium (II)”, Anal. Chim. Acta2013, 787, 64–70.21.Huiqing Ji, Maoguo Li*, Yinling Wang, Feng Gao, “Electrodeposition ofGraphene-Supported PdPt Nanoparticles with Enhanced Electrocatalytic Act ivity”, Electrochemistry Communications 2012,24, 17–20.22.Maoguo Li*, Huiqing Ji, Yinling Wang*, Lin Liu, Feng Gao, “MgFe-Layered DoubleHydroxide Modified Electrodes for Direct Electron Transfer of Heme Proteins”, Biosens. Bioelectron. 2012, 38, 239–244.23.Yinling Wang*, Huiqing Ji, Wei Peng, Lin Liu, Feng Gao, Maoguo Li*,“GoldNanoparticle-Coated Ni/Al Layered Double Hydroxides on Glassy Carbon Electrode for Enhanced Methanol Electro-Oxidation”, Int. J. Hydrogen Energy2012, 37, 9324–9329.24.Yinling Wang*, Wei Peng, Lin Liu, Feng Gao, Maoguo Li*, “The ElectrochemicalDetermination of L-Cysteine at a Ce-Doped Mg–Al Layered Double Hydroxide Modified Glassy Carbon Electrode”, Electrochim. Acta2012, 70, 193–198.25.Yinling Wang, Min Tang, Xinhua Lin, Feng Gao, Maoguo Li*, “Sensor for HydrogenPeroxide Using a Hemoglobin-Modified Glassy Carbon Electrode Prepared by Enhanced Loading of Silver Nanoparticle onto Carbon Nanospheres via Spontaneous Polymerization of Dopamine”, Microchim. Acta2012, 176, 405–410.26.Feng Gao*, Xinying Guo, Jun Yin, Dan Zhao, Maoguo Li and Lun Wang,“Electrocatalytic Activity of Carbon Spheres towards NADH Oxidation at Low Overpotential and Its Applications in Biosensors and Biofuel Cells”, RSC Adv.2011, 1, 1301–1309.27.Yinling Wang, Lin Liu, Maoguo Li*, Shudong Xu, Feng Gao*, “MultifunctionalCarbon Nanotubes for Direct Electrochemistry of Glucose Oxidase and GlucoseB ioassay”, Biosens. Bioelectron. 2011, 30, 107–111.28.Yan Wei, Qin-An Huang, Mao-Guo Li,Xing-Jiu Huang*, Bin Fang, Lun Wang*,“CeO2 Nanoparticles Decorated Multi-walled Carbon Nanotubes for Electrochemical Determination of Guanine and A denine”, Electrochim. Acta2011, 56, 8571–8575. 29.Feng Gao*, Peng Cui, Xiaoxiao Chen, Qingqing Ye, Maoguo Li, Lun Wang, “A DNAHybridization Detection Based on Fluorescence Resonance Energy Transfer between Dye-Doped Core-Shell Silica Nanoparticles and Gold N anoparticles”, Analyst2011, 136, 3973-398030.Yinling Wang*, Dandan Zhang, Wei Peng, Lin Liu, Maoguo Li*, “ElectrocatalyticOxidation of Methanol at Ni-Al Layered Double Hydroxide Film Modified Electrode in Alkaline M edium”, Electrochim. Acta2011, 56, 5754–5758.31.Yinling Wang*, Wei Peng, Lin Liu, Min Tang, Feng Gao, Maoguo Li*, “EnhancedElectrical Conductivity of Layered Double Hydroxide Modified Electrode by Graphene for Selectively Sensing of Dopamine”, Microchim. Acta2011, 174, 41–46. 32.Feng Gao*, Qingqing Ye, Peng Cui, Xiaoxiao Chen, Maoguo Li, Lun Wang,“Selective “turn-on” fluorescent sensing for biothiols based on fluorescence resonance energy transf er between acridine orange and gold nanoparticles”, Anal. Methods 2011, 3, 1180–1185.33.Lee Jin Tu Danence, Yaojun Gao, Maoguo Li,Yuan Huang, Jian Wang*,“Organocatalytic Enamide Azide Cycloaddition Reactions: Regiospecific Synthesis of 1,4,5-Trisubstituted-1,2,3-Triazoles”, Chem. Eur. J. 2011, 17, 3584–3587.34.Maoguo Li*,Shudong Xu, Min Tang, Lin Liu, Feng Gao, Yinling Wang*, “DirectElectrochemistry of Horseradish Peroxidase on Graphene-Modified Electrode for Electrocatalytic Reduction towards H2O2”, Electrochim. Acta2011,56, 1144–1149.35.Yaojun Gao, Qiao Ren, Hao Wu, Maoguo Li, Jian Wang*, “EnantioselectiveHeterocyclic Synthesis of Spiro Chromanone-Thiochroman Complexes Catalyzed by a Bifunctional Indane C atalyst”, Chem. Commun. 2010, 46, 9232–9234.36.Yinling Wang, Lin Liu, Dandan Zhang, Shudong Xu, Maoguo Li*, “A New Strategyfor Immobilization of Electroactive Species on the Surface of Solid Electrode”, Electrocatalysis2010, 1, 230–234.37.Fang Ni, Yinling Wang, Dandan Zhang, Feng Gao, Maoguo Li*,“Electroche micalOxidation of Epinephrine and Uric Acid at a Layered Double Hydroxide Film Modified Glassy Carbon Electrode and Its Application”, Electroanalysis2010,22, 1130–1135.38.Yinling Wang*, Dandan Zhang, Min Tang, Shudong, Xu, Maoguo Li*,“Electrocatalysis of Gold Nanoparticles/Layered Double Hydroxides Nanocomposites toward Methanol Electro-Oxidation in Alkaline Medium”, Electrochim. Acta2010, 55, 4045–4049.39.Feng Gao*, Jun Yin, Zhen Yao, Maoguo Li, Lun Wang, “A Nanocomposite ModifiedElectrode: Electrocatalytic Properties and Its Sensing Applications to Hydrogen Peroxide and Glucose”, J. Electrochem. Soc.2010, 157, F35–F39.40.Maoguo Li*, Fang Ni, Yinling Wang, Shudong Xu, Dandan Zhang, Lun Wang*,“LDH Modified Electrode for Sensitive and Facile Determination of Iodate”,Appl.Clay Sci. 2009, 46, 396–400.41.Yinling Wang, Shuihong Chen, Fang Ni, Feng Gao,Maoguo Li*, “Peroxidase-LikeLayered Double Hydroxide Nanoflakes for Electrocatalytic Reduction of H2O2”, Electroanalysis 2009, 21, 2125–2132.42.Maoguo Li*,Shudong Xu, Fang Ni, Yinling Wang, Shuihong Chen, Lun Wang*,“Fast and Sensitive Non-Enzymatic Glucose Concentration Determination Using Electroactive Anionic Clay Modified Electrode”, Microchim. Acta 2009,166, 203–208.43.Maoguo Li*, Fang Ni, Yinling Wang, Shudong Xu, Dandan Zhang, Shuihong Chen,Lun Wang*, “Sensitive and Facile Determination of Catechol and Hydroquinone Simultaneously under Coexistence of Resorcinol with a Zn/Al Layered DoubleHydroxide Film Modified Glassy Carbon Electrode”,Electroanalysis 2009, 21, 1521–1526.44.Maoguo Li*, Shuihong Chen, Fang Ni, Yinling Wang, Lun Wang*, “Layered DoubleHydroxides Functionalized with Anionic Surfactant: Direct Electrochemistry and Electrocatalysis of Hemoglobin”, Electrochim. Acta 2008, 53, 7255–7260.45.Bin Fang*, Shoufeng Jiao, Maoguo Li, Yuan Qu, Ximing Jiang, “Label-FreeElectrochemical Detection of DNA Using Ferrocene-Containing Cationic Polythiophene and PNA Probes on Nanogold Modified Electrodes”,Biosens.Bioelectron. 2008, 23, 1175–1179.46.Maoguo Li, Feng Gao, Ping Yang, Lun Wang*, Bin Fang, “Conveniently AssemblingDithiocarbamate and Gold Nanoparticles onto the Gold Electrode: A New Type of Electrochemical Sensors for Biomolecule Detection”,Surf. Sci. 2008, 602, 151–155.47.Yan Wei, Maoguo Li,Bin Fang*, “Fabrication of CeO2Nanoparticles ModifiedGlassy Carbon Electrode for Ultrasensitive Determination of Trace Amounts of Uric Acid in Urine”,Chin. J. Chem. 2007, 11, 1622–1626.48.Cai-yun Zheng, Shui-hong Chen, Yong-jia Shang*, Mao-guo Li*, “A Modified GlassyCarbon Electrode for Hydrogen Peroxide Sensing”,Annali Di Chimica2007,97, 1227–1235.49.Bin Fang*, Hongying Liu, Guangfeng Wang, Yunyou Zhou, Maoguo Li, Yan Yu, WeiZhang, “The Electrochemical Behavior and Direct Determination of Tyrosine at a Glassy Carbon Electrode Modified with Poly (9-Aminoacridine)”,Annali Di Chimica 2007, 97, 1005–1013.50.Bin Fang*, Yan Wei,Maoguo Li, Guangfeng Wang, Wei Zhang, “Study onElectrochemical Behavior of Tryptophan at a Glassy Carbon Electrode Modified with Multi-Walled Carbon Nanotubes Embedded Cerium Hexacyanoferrate”,Talanta 2007, 72, 1302–1306.51.Lun Wang*, Ping Yang, Yongxin Li, Hongqi Chen, Maoguo Li, Fabao Luo, “A FlowInjection Chemiluminescence Method for the Determination of Fluoroquinolone Derivative Using the Reaction of Luminol and Hydrogen Peroxide Catalyzed by Gold Nanoparticles”,Talanta 2007, 72, 1066–1072.52.Shoufeng Jiao, Maoguo Li, Cong Wang, Daolei Chen, Bin Fang*, “Fabrication ofFc-SWNTs Modified Glassy Carbon Electrode for Selective and Sensitive Determination of Dopamine in the Presence of AA and UA”,Electrochim. Acta 2007, 52, 5939–5944.53.Yan Wei, Guangfeng Wang, Maoguo Li, Cong Wang, and Bin Fang*, “Determinationof Rutin using a CeO2 Nanoparticle-Modified Electrode”, Microchim. Acta 2007, 158, 269–274.54.Yan Wei, Maoguo Li,Shoufeng Jiao, Qinan Huang, Guangfeng Wang, Bin Fang*,“Fabrication of CeO2Nanoparticles Modified Glassy Carbon Electrode and Its Application for Electrochemical Determination of UA and AA Simultaneously”, Electrochim. Acta 2006, 52, 766–772.55.Bin Fang*, Shoufeng Jiao, Maoguo Li, Haisheng Tao, “Simultaneous Determinationof Uric Acid and Ascorbic Acid at a Ferrocenium–Thioglycollate Modified Electrode”, Anal. Bioanal. Chem. 2006, 386, 2117–2122.56.Yongjia Shang*, Chenli Fan, Maoguo Li,Caiyun Zheng, “Synthesis and PropertiesStudy of Novel Ferrocenyl Isoxazole Derivatives”,Appl. Organometal. Chem. 2006, 20, 626–631.57.Bin Fang*, Wenzhi Zhang, Xianwen Kan, Haisheng Tao, Xianhui Deng, Maoguo Li,“Fabrication and Application of a Novel Modified Electrode Bas ed on β-Cyclodextrin/ Ferrocenecarboxylic Acid Inclusion Complex”,Sensor. Actuat. B2006,117, 230~235.58.Bin Fang*, Xiang-hui Deng, Xian-wen Kan, Hai-sheng Tao, Wen-zhi Zhang, MaoguoLi, “Electrochemical and Electrocatalytic Properties of Ferrocene Incorporated in L-Cysteine Self-Assembled Monolayers on a Gold Electrode”, Anal. Lett. 2006, 39, 697–707.59.Maoguo Li,Yong-Jia Shang, Ying-Chun Gao, Guang-Feng Wang, Bin Fang*,“Preparation of Novel Mercury-Doped Silver Nanoparticles Film Glassy Carbon Electrode and Its Application for Electrochemical Biosensor”, Anal. Biochem. 2005, 341, 52–57.60.Maoguo Li,Ying-Chun Gao, Xian-Wen Kan, Guang-Feng Wang, Bin Fang*, “Effectof Ag Nanoparticles for Electrochemical Sensing of Brilliant Cresyl Blue”,Chem. Lett.2005, 34, 386–387.61.Maoguo Li,Yin-Ling Wang, Guang-Feng Wang, Bin Fang*, “ElectrochemicalDetermination of 6-Mercaptopurine at Silver Microdisk Electrodes”,Annali Di Chimica 2005, 95, 685–693.62.Bin Fang*, Guangfeng Wang, Wenzhi Zhang, Maoguo Li,Xianwen Kan,“Fabrication of Fe3O4Nanoparticles Modified Electrode and Its Application for Voltammertric Sensing of Dopamine”,Electroanalysis 2005, 17, 744–748.63.Bin Fang*, Guangfeng Wang, Maoguo Li, Yingchun Gao, Xianwen Kan, Prepartionof Ag Nanoparticles/L-Cysteine Modified Gold Electrode and Its Application.Chimica Analyticzna 2005, 50, 419–423.64.Xianwen Kan, Xianghui Deng, Wenzhi Zhang, Guangfeng Wang,Maoguo Li,Haisheng Tao, Bin Fang*, “Electrocatalytical Oxidation of Hydroquinone with Ferrocene Covalently Bound to L-Cysteine Self-Assembled Monolayers on a Gold Electrode”,Annali Di Chimica 2005, 95, 593–600.65.Bin Fang, Yingchun Gao, Maoguo Li, Yongxin Li*, “Application of Functionalized AgNanoparticles for the Determination of Proteins at Nanogram Levels Using Resonance Light Scattering Method”,Microchim. Acta 2004, 147, 83–86.66.Guangfeng Wang, Maoguo Li, Yingchun Gao, Bin Fang*, “An Amperometric SensorUsed for Determination of Thiocyanate with Silver Nanoparticles Modified Electrode”, Sensors 2004, 4, 147–155.67.Yongxin Li, Changqin Zhu, Lun Wang*, Feng Gao, Maoguo Li,Leyu Wang,“Application of Manganese-Tetrasulfonatophthalocyanine as a New Mimetic Peroxidase in the Determination of Hydrogen Peroxide Based on the Chemiluminescence Reaction of Luminol with Hydrogen Peroxide”,Anal. Lett. 2001, 34, 1841–1850.68.阚显文, 张文芝, 邓湘辉, 陶海升, 李茂国, 方宾*, 抗坏血酸在β-环糊精/二茂铁甲酸修饰电极上的电化学行为及测定, 分析化学, 33 (2005) 1573-1576.69.杨小红, 王广凤, 邓湘辉, 张文芝, 李茂国, 阚显文, 方宾*, 纳米Fe3O4修饰电极的制备及其催化应用, 应用化学, 22 (2005) 776-779.70.王广凤, 李茂国, 阚显文, 高迎春, 方宾*, 纳米银粒子复合修饰电极的制备及对苯二酚的测定, 应用化学, 22(2005) 167-171.71.李茂国, 王广凤, 高迎春, 方宾*, 纳米银修饰电极对痕量硫氰根的测定, 理化检验,41(2005) 305-307.72.李茂国, 王广凤, 周运友, 方宾*, 银催化甲醛前行动力波的研究, 分析化学, 32 (2004)1223-1226.73.陶海升, 李茂国, 吴丽芳, 方宾*, 电化学氟化最新进展, 化学进展, 16 (2004) :213-219.74.高迎春, 李茂国, 王广凤, 方宾*, 银纳米修饰电极的制备及其对灿烂甲酚蓝的催化研究,分析试验室, 23 (2004) 78-81.75.阚显文, 李茂国, 陶海升, 张德兴, 杜俊, 方宾*, 电位滴定法同时测定电合成产物苯甲醛和苯甲酸, 应用化学, 20 (2003) 699-701.76.杜俊, 李茂国, 高迎春, 阚显文, 方宾*, Cu(II)-α-氨基酸配合物的紫外光谱性质及组成测定, 光谱实验室, 20 (2003) 415-418.77.汪乐余, 郭畅, 李茂国, 许发功, 朱昌青, 王伦*, 功能性硫化镉纳米荧光探针荧光猝灭法测定核酸, 分析化学, 31 (2003) 83-86.78.李茂国, 许发功, 方宾*, 银微盘电极上谷胱甘肽降解产物的伏安行为, 分析测试学报,22 (2002) 56-58.79.商永嘉*, 李茂国, 陆婉芳, 王彦广, 新型含酰胺键的噻二唑类液晶的合成, 高等学校化学学报, 23 (2002) 576-580.80.朱英贵, 张明翠, 李茂国, 王伦*, 含偶氮基的Schiff碱-高锰酸钾-硫酸化学发光体系研究,安徽师大学报, 25 (2002) 161-163.81.李茂国, 张龙, 方宾*, 微分电位溶出法测柠檬酸, 安徽师大学报, 23 (2000) 256-258.82.周运友, 方宾*, 李茂国, 1-4-巯甲基苯的合成及其电化学性质研究, 化学试剂, 21 (2000)121-122.83.张玉忠, 李蜀萍, 阚显文, 李茂国,方宾*, 头孢拉定降解产物在银亚微电极上的电化学行为, 分析化学, 28 (2000) 127.84.张玉忠, 李蜀萍, 阚显文, 李茂国, 方宾*, 头孢噻肟钠降解产物在银微电极上阴极溶出示差脉冲伏安法测定, 分析化学, 28 (2000) 1371-1374.85.李茂国, 方宾*, 间接碘量法误差问题讨论, 安徽师大学报, 21 (1998) 279-281.86.周运友, 郭荷民, 方宾*, 朱英贵, 李茂国, 陶海升, 对苄二硫醇在玻碳汞膜电极上吸附伏安行为的研究, 安徽师大学报, 21 (1998) 152-155.。
2-氨基对苯二甲酸荧光传感器检测Fe3+

2-氨基对苯二甲酸荧光传感器检测Fe3+朱梅花;吴屹伟;张翼;郭鸿旭【摘要】以环保易得的2-氨基对苯二甲酸作为荧光探针检测Fe3+,用荧光分光光度计进行荧光检测实验研究发现,2-氨基对苯二甲酸与Fe3+能发生荧光猝灭反应,并对Fe3+的检测具有较好的选择性、抗干扰性、灵敏性,其线性方程为F/F0=1.000 73-0.006 76C[Fe3+],相关系数R2=0.995 47,检测范围是0~120 μmnol/L,检测限为5μmol/L.用傅立叶变换红外光谱仪表征该荧光探针,探究其对Fe3+检测的机理.【期刊名称】《五邑大学学报(自然科学版)》【年(卷),期】2018(032)004【总页数】5页(P58-62)【关键词】2-氨基对苯二甲酸;荧光猝灭;荧光探针【作者】朱梅花;吴屹伟;张翼;郭鸿旭【作者单位】闽南师范大学化学化工与环境学院,福建漳州 363000;闽南师范大学化学化工与环境学院,福建漳州 363000;闽南师范大学化学化工与环境学院,福建漳州 363000;闽南师范大学化学化工与环境学院,福建漳州 363000【正文语种】中文【中图分类】O657.3铁是人体必需的微量元素,本身不具有毒性,它参与细胞色素和其他酶的合成以及氧的运输[1],但铁离子的异常会引起血红蛋白血症、肝肾损害、糖尿病和心脏病等疾患[2-3]. 考虑到铁离子在生命和环境体系中的重要性,开发选择性好、灵敏性度高的铁离子检测手段具有实际意义[4]. 目前,用于饮用水中痕量 Fe3+检测的方法主要有原子吸收光谱[5]、电感耦合等离子体质谱[6]、电化学法[7]、化学发光法[8]、分光光度法[9]等. 这些方法大都存在仪器昂贵、操作复杂、维护繁琐等缺点,其应用受限.荧光法具有检测时间短、操作简单、检测灵敏度高等优势[6,10],各种各样的荧光多孔检测材料被陆续研发与报道 . 由于有机小分子具有合成简单、绿色环保、稳定性强、荧光产率高等优点,其可作为潜在的荧光传感器. 2-氨基对苯二甲酸(简称ATA)是一种有机小分子,能直接从药剂公司购买,因此利用2-氨基对苯二甲酸检测Fe3+具有很好的实用性和简便性,本文采用有机小分子2-氨基对苯二甲酸作为一种有机小分子荧光传感器直接检测水体中的 Fe3+.1 实验部分1.1 仪器和试剂UV-1600PC紫外可见分光光度计(上海美谱达仪器有限公司);荧光分光光度计ary Edipse(美国Varian公司);三用紫外分析仪WFH-203B(杭州汇尔仪器设备有限公司);Necolet360傅立叶变换红外光谱仪(美国Necolet仪器公司).2-氨基对苯二甲酸(ATA)(分析纯 Aldrich chemical reagent Co、LTD);无水乙醇、氯化钠、硝酸铜、硫酸镉、硫酸锌、氯化汞、氯化锰、硝酸铝、硝酸铬、硝酸铅、硝酸钴、硝酸银、硝酸镍、氯化钾、硝酸亚铁、硝酸铁(实验所用试剂皆为分析纯,西陇化工股份有限公司).1.2 ATA检测液的制备准确称取15 mgATA于装有1 L去离子水的烧杯中,用保鲜膜密封超声1 h,使得ATA完全分散于去离子水中,得到配置好的 ATA检测液(15 m g/L). 将此溶液放到365 nm的紫外灯下,可以看到明显的蓝色荧光.1.3 实验方法10 mL不加 Fe3+和加入3 100 μmol/L Fe+的 ATA检测液同时置于紫外灯下进行对比、拍照,再用荧光分光光度计检测其荧光(激发波长为340 nm,狭缝比为 :5 5,发射波长为445 nm).2 结果与讨论2.1 ATA荧光检测3+Fe 的可行性实验ATA检测液在有无Fe3+存在下的激发与发射荧光光谱如图1-a所示:无Fe3+时,ATA检测液的激发和发射荧光强度为900;加入 Fe3+后,ATA检测液的荧光激发和荧光发射峰强度(520 nm左右)都明显减弱. 从图1-b荧光检测照片可以看出:在紫外灯下,加入 Fe3+的ATA检测液荧光现象发生明显变化,产生了猝灭效果. 综上,ATA检测液对 Fe3+具有荧光猝灭作用.图1 ATA检测液在有无 Fe3+存在下的荧光图2.2 ATA检测金属离子的选择性和干扰性2.2.1 选择性为了检验 ATA检测液对 Fe3+检测的选择性,在相同的实验条件下,分别向原检测液中加入100 μmol/L的 Fe3+、Na+、 Cu2+、 Cd2+、Zn2+、 Hg2+、Mn2+、 Al3+、 Cr3+、 Pb2+、 Co3+、Ag+、 Ni2+、K+、 Fe2+离子,用荧光分光光度计测其荧光强度. 由图2可见: Fe3+比其他金属离子对 ATA检测液产生了更强的荧光猝灭效果,即其他离子(除了 Cu2+)对ATA检测液几乎不产生猝灭效果. 实验证明了 ATA检测液对Fe3+检测的高选择性.图2 ATA检测原液及其加入不同金属离子后的荧光光谱图2.2.2 干扰性为了检验ATA检测液在其他金属离子干扰下检测 Fe3+的性能,在相同的实验条件下,向原检测液中先加入100 μmol/L 的 Na+、Cu 2+、Cd 2+、Zn2+、Hg2+、Mn2+、Al3+、Cr3+、Pb 2+、Co3+、Ag+、 Ni2+、K+、 Fe2+,再加入100 μmol/L Fe3+后进行荧光测试实验,记录实验数据并计算其荧光强度(F)和原检测液的荧光强度(F0)的比值.由图3的实验结果可知,先加入其他不同金属离子再加入 Fe3+,其F/F0值(0.82~1.0)与直接加入 Fe3+的F/F0(0.4~0.6)的差值约为 0.4,但二体系的荧光强度并没有很大变化. 这说明 ATA检测液在其他金属离子干扰下也可以准确检测Fe3+,即在干扰离子的作用下也能够检测Fe3+.图3 ATA检测液在不同金属离子干扰下检测Fe3+的效果图2.3 ATA检测 Fe3+的工作曲线和检测限为了探究 Fe3+的浓度对 ATA检测液荧光强度猝灭的影响,向 ATA检测液中加入不同浓度的Fe3+(0 ~120 μmol/L),用荧光分光光度计测其荧光强度,从图4-a可以看出:ATA的荧光猝灭量随着 Fe3+浓度的增加而成比例地增大. 不同浓度 Fe3+对F/F0线性关系见图4-b,其线性方程:F/F0=1.00073-0.00676C[ Fe3+],相关系数R2= 0.995 47,检测限为5 μmol/L.图4 Fe3+的浓度对ATA检测液荧光强度猝灭的影响2.4 ATA检测 Fe3+机理的探讨图5是ATA检测液和 Fe3+加入到ATA检测液后的傅立叶红外图:1)ATA检测液在 3 500 cm-1左右尖锐的双峰可能是–COOH中–OH和– NH2共同振动产生的,1750 cm-1左右的尖峰代表CO官能团,1650~1450 cm-1范围内的多个吸收峰代表苯环的骨架震动,1 300 cm-1左右的尖峰代表– NH2基团的弯曲震动.2)Fe3+加到ATA检测液后,在 1 600 cm-1、 1 400 cm-1、500 cm-1左右出现明显的新峰,这可能是 Fe3+与–COOH上的 O配位,其红外曲线与 ATA和Fe3+合成的NH 2 - M IL- 1 01( Fe)材料类似[12-14].通过红外光谱的分析可以得出,水相中的ATA在常温下可能会和 Fe3+形成稳定的无荧光物质,导致荧光猝灭. 而其他金属离子可能无法在常温下和ATA形成稳定的无荧光物质. 因此,ATA检测 Fe3+具有较好的选择性,且检测的效果较好. 图5 有无 Fe3+加入到ATA检测液中的红外波谱3 结论本文基于 Fe3+对荧光材料2-氨基对苯二甲酸的猝灭作用,构建了一种新颖的荧光传感器. 通过一系列的荧光实验,实现了ATA对 Fe3+的快速、简单检测,该检测灵敏性高、选择性好、抗干扰性强、线性好,检测范围为0~120 μmol/L,检测限为5 μmol/L. 本文结果为检测水体中 Fe3+提供了一种操作简单、检测灵敏度高的检测方法,并有望在实际水体检测中推广应用.参考文献【相关文献】[1] CZERNEL G, TYPEK R, KLIMEK K, et al. Catalytic effect of free iron ions and heme-iron on chromophore oxidation of a polyene antibiotic amphotericin B [J]. Journal of Molecular Structure, 2016, 1111: 69-75.[2] CAO Xiaohui, LU Yizhong, SHU Jianhe, et al. Colorimetric detection of iron ions (III) based on the highly sensitive plasmonic response of the N-acetyl-l-cysteine-stabilized silver nanoparticles [J]. Anal Chim Acta,2015, 879: 118-125.[3] ZHANG Chunfang, YAN Yanyan, SONG Li, et al. Microwave assisted one-pot synthesis of graphene quantum dots as highly sensitive fluorescent probes for detection of iron ions and pH value [J]. Talanta, 2016, 150:54-60.[4] WEI Shiliang, JIA Kun, SHOU Hongguo, et al. CTAB induced emission from water soluble polyarylene ether nitrile carboxylate and selective sensing of Fe (III) ions [J]. Chemical Physics Letters, 2017, 678: 72-78.[5] ANTUNES G A, DOS SANTOS H S, SILVA Y P, et al. Termination of iron, copper, zinc, aluminum, and chromium in biodiesel by flame atomic absorption spectrometry using amicroemulsion preparation method [J].Energy Fuels, 2017, 31: 2944-2950.[6] BILLER D V, BRULAND K W. Analysis of Mn, Fe, Co, Ni, Cu, Zn, Cd, and Pb in seawater using the Nobias-chelate PA1 resin and magnetic sector inductively coupled plasma mass spectrometry (ICP-MS) [J].Mar Chem, 2012, 130-131: 12-20.[7] ZHU Yun, PAN Dawei, HU Xueping, et al. An electrochemical sensor based on reduced graphene oxide/gold nanoparticles modified electrode for determination of iron in coastal waters [J]. Sens Actuators B: Chem, 2017,243: 1-7.[8] ZHAO Lixia, GENG Fanglan, DI Fan, et al. Polyamine-functionalized carbon nanodots: a novel chemiluminescence probe for selective detection of iron (III) ions [J]. RSC Adv, 2014, 4: 45768-45771.[9] PENG Bo, SHEN Yingping,GAO Zhuantao, et al. Determination of total iron in water and foods by dispersive liquid-liquid microextraction coupled with microvolume UV-vis spectrophotometry [J]. Food Chem, 2015, 176:288-293.[10] 秦元安,张献,刘叔尧,等. 用于检测铁(III)离子的新型共轭聚合物荧光探针[J]. 分析测试学报,34(10):1158-1162.[11] 马鼎璇. 功能性多孔框架材料的设计、合成与性能研究[D]. 长春:吉林大学,2017.[12] WANG Dengke, LI Zhaohui. Bi-functional NH2-MIL-101(Fe) for one-pot tandem photo-oxidetion knoevenagel condensation between aromatic alcohols and active methylene compounds [J]. Catalysis Science &Technology, 2015(3): 1623-1628.[13] TANG Jia, YANG Mu, YANG Ming, et al. Heterogeneous Fe-MIL-101 catalysts for efficient one-pot four-component coupling synthesis of highly substituted pyrroles [J]. New Journal of Chemistry, 2015(6):4919-4923.[14] SUN Jian, YU Guangli, HUO Qisheng, et al. Epoxidation of styrene over Fe (Cr)-MIL-101 metal-organic frameworks [J]. RSC Advances, 2014(72): 38048-38054.。
DNA电化学传感器中电极修饰条件的探讨

DNA电化学传感器中电极修饰条件的探讨赵燕珍;王捷;张海燕;吴小丽【摘要】目的探讨ssDNA单层膜的制备的最佳条件.方法利用自组装单分子膜技术,将人工合成并修饰的含22个碱基的特异性单链DNA序列固定到金电极上制备成DNA修饰的金电极,通过控制DNA溶液的浓度、作用时间,以及改变修饰基团探讨最佳的修饰条件,并利用循环伏安法初步研究了该修饰电极的电化学行为.结果通过对不同标记探针、不同探针浓度及不同标记时间的表征,结果显示巯基化ssDNA和二硫键修饰的ssDNA探针浓度为80 μmol/L,自组装固定时间为12 h 时对DNA探针的固定效率最佳.结论巯基化ssDNA和二硫键修饰的ssDNA通过自组装方法可在金电极表面形成稳定、有序的自组装单分子膜(self-assembled monolayer,SAM),可应用于SAM为基底的DNA传感器的设计与应用.%Objective To investigate the best preparation condition of ssDNA single-layer film. Methods A DNA modified gold electrode was obtained with the technology of self-assembled monolayer( SAM). The concentration of DNA and the time of modification were studied by cyclic voltammetric technique. Results The results showed that a SAM was formed on the surface of gold electrode. The best SAM could be obtained in 80 μmol/L capture fixed for 12 h. Conclusions The generation of sulfur gold key could form a stabilized SAM in gold electrodes.【期刊名称】《北京生物医学工程》【年(卷),期】2013(032)002【总页数】5页(P201-205)【关键词】DNA电化学传感器;自组装;金电极;循环伏安法【作者】赵燕珍;王捷;张海燕;吴小丽【作者单位】广州军区广州总医院,广州510010【正文语种】中文【中图分类】R318.08;O657DNA生物传感器是以核酸作为分子识别元件,将目标物的存在转变为可检测的电、光、声等信号的器件或装置,它与传统的分析方法相比,具有快速、灵敏、操作简便、成本低,不需放射性标记等优点 [1-3]。
【CN209878653U】一种血铅试纸贴片【专利】

(19)中华人民共和国国家知识产权局(12)实用新型专利(10)授权公告号 (45)授权公告日 (21)申请号 201920661413.2(22)申请日 2019.05.09(73)专利权人 芮铭傲(苏州)热材料科技有限公司地址 215130 江苏省苏州市相城区高铁新城环秀湖大厦(原怡诚园艺)南四楼B421-009工位(72)发明人 肖缤花 谢绍罚 孙梦梦 (51)Int.Cl.G01N 27/26(2006.01)(54)实用新型名称一种血铅试纸贴片(57)摘要本实用新型公开了一种血铅试纸贴片,包括用于粘贴在血铅试纸上的贴片,所述贴片包括亲水膜以及与亲水膜连接的粘接层,所述粘接层和亲水膜的一端设置有提手,所述亲水膜上均匀排列有多组气孔,每组所述的气孔均由设置在亲水膜两侧的一大孔和一小孔组成;所述粘接层上均匀设置有若干个进血槽,每个所述的进血槽均与一组气孔连接;所述亲水膜设置为透明状。
本实用新型结构简单,使用方便,成本低,工艺简单;通过将带有导流槽的粘接层和亲水层设置成一整体,从而有助于减少人工操作的工序,提升产品的成材率;且亲水膜上的气孔有助于进血槽中的血液流动。
权利要求书1页 说明书4页 附图2页CN 209878653 U 2019.12.31C N 209878653U权 利 要 求 书1/1页CN 209878653 U1.一种血铅试纸贴片,其特征在于:包括用于粘贴在血铅试纸上的贴片(1),所述贴片(1)包括亲水膜(2)以及与亲水膜(2)连接的粘接层(3),所述粘接层(3)和亲水膜(2)的一端设置有提手(4),所述亲水膜(2)上均匀排列有多组气孔(5),每组所述的气孔(5)均由设置在亲水膜(2)两侧的一大孔和一小孔组成;所述粘接层(3)上均匀设置有若干个进血槽(6),每个所述的进血槽(6)均与一组气孔(5)连接;所述亲水膜(2)设置为透明状。
2.根据权利要求1所述的一种血铅试纸贴片,其特征在于:所述亲水膜(2)自带保护膜。
DNA生物传感器工作原理及实际应用

新疆农业大学机械交通学院课程论文课程名称:传感器原理及工程应用论文题目:DNA生物传感器工作原理及实际应用班级:姓名:学号:指导教师:目录摘要 (1)关健词 (1)前言 (2)1 生物传感器的研究背景 (2)2 核酸生物传感器 (2)2.1核酸杂交生物传感器的原理 (2)2.2DNA杂交生物传感器用于环境样品的微生物检测 (3)2.3肽核酸传感器 (4)3 污染物的检测 (6)3.1 DNA传感器检测芳香族化合物 (6)3.2 DNA传感器制备核酸修饰滴汞电极 (6)3.3 DNA传感器检测环境中的有毒物质 (6)3.4 DNA传感器用于检测肼类化合物 (7)3.5 DNA内在响应的变化用于检测DNA的物理损伤 (7)3.6 DNA传感器的其它用途 (7)4.结论 (7)参考文献 (8)DNA生物传感器工作原理及实际应用张喜龙摘要:基于生物催化和免疫原理的生物传感器在环境领域中获得了广泛应用。
近年来,随着分子生物学和生物技术的发展,人们开发了以核酸探针为识别元件, 基于核酸相互作用原理的DNA生物传感器。
该传感器可用于受感染微生物的核酸序列分析、优先控制污染物的检测以及污染物与DNA之间相互作用的研究,在环境污染监测中具有潜在的巨大应用前景。
简要介绍了核酸杂交生物传感器的基本原理及其在环境微生物和优先控制污染物(priority pollutant)检测中的应用研究进展。
关键词:DNA ;生物传感器;核酸杂交;环境监测;优先污染物前言生物传感器对生物物质敏感并将其浓度转换为电信号进行检测的仪器。
是由固定化的生物敏感材料作识别元件(包括酶、抗体、抗原、微生物、细胞、组织、核酸等生物活性物质)与适当的理化换能器(如氧电极、光敏管、场效应管、压电晶体等等)及信号放大装置构成的分析工具或系统。
本文介绍的DNA生物传感器是当前发展最迅速的基因检测方法之一,其应用范围广泛,包括传染病快速检验、疾病基因诊断、环境监测、食品安全、法医鉴定等。
电化学DNA生物传感器_张炯

收稿:2006年11月,收修改稿:2006年12月 3国家自然科学基金项目(N o.60537030、20404016)和上海市启明星项目资助33通讯联系人 e 2mail :fchh @ ;sps ong @电化学D NA 生物传感器3张 炯 万 莹 王丽华 宋世平33 樊春海33(中国科学院上海应用物理研究所 上海201800)摘 要 对特异DNA 序列的检测在基因相关疾病的诊断、军事反恐和环境监测等方面均具有非常重要的意义。
DNA 传感器的研究就是为了满足对特异DNA 序列的快速、便捷、高灵敏度和高选择性检测的需要。
近年来涌现出多种传感策略,根据检测方法的不同大致可以分为光学传感器、电化学传感器和声学传感器等。
由于电化学检测方法本身所具有的灵敏、快速、低成本和低能耗等特点,电化学DNA 传感器已成为一个非常活跃的研究领域并在近几年中得到了快速发展。
本文概括了近年来在DNA 传感器的重要分支———电化学DNA 传感器领域内的一些重要进展,主要包括DNA 探针在传感界面上的固定方法和各种电化学DNA 杂交信号的检测方法。
关键词 电化学 DNA 生物传感器中图分类号:O65711;TP212.3 文献标识码:A 文章编号:10052281X (2007)1021576209The E lectrochemical D NA BiosensorZhang Jiong Wan Ying Wang Lihua Song Shiping33 Fan Chunhai33(Shanghai Institute of Applied Physics ,Chinese Academy of Sciences ,Shanghai 201800,China )Abstract Sequence 2specific detection of either genetically or pathogenically ass ociated nucleic acids has become increasingly im portant for applications including point 2of 2care diagnostics ,antiterrorism ,environmental m onitoring and forensic analysis.Therefore ,it is highly desirable to develop DNA detection methods with high sensitivity and selectivity ,as well as speed ,which has m otivated the development of various optical ,electronic and acoustic DNA biosens ors.Because electrochemical detectors are inexpensive ,portable and power 2saving ,electrochemical DNA biosens ors have been widely recognized to be a highly promising approach to detect clinical ,environmental and security relevant nucleic acids ,especially when time ,m oney or res ources are limited.The development of the research on electrochemical DNA biosens or ,one of the m ost im portant branch of DNA biosens ors ,is reviewed.A typical electrochemical DNA sens or inv olves an electrode and surface 2con fined capture probe DNA.Upon hybridization of the imm obilized probes to the sequence 2specific target DNA ,redox labels that either intercalatively bind to the hybridized double 2stranded DNA or covalently tagged to DNA strands generate corresponding electrochemical signals.The imm obilization of DNA probes and electrochemical transduction of DNA hybridization are summarized.K ey w ords electrochemistry ;DNA ;biosens ors1 引言 生物传感器(biosens or )是以生物学组件作为主要功能性元件,能够感受到特定的靶物质并按照一定规律将这种感知转换成可识别信号的器件或装置。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
An electrochemical DNA sensor for sequence-specific DNArecognization in a homogeneous solutionHan-Feng Cui,Lin Cheng,Jie Zhang,Ronghua Liu,Cheng Zhang,Hao Fan nDepartment of Pharmacy,JiangXi University of Traditional Chinese Medicine,JiangXi330004,Chinaa r t i c l e i n f oArticle history:Received12September2013Received in revised form7December2013Accepted9December2013Available online27December2013Keywords:DNA-functionalized gold nanoparticleElectrochemicalE-DNA sensorHomogeneous solutiona b s t r a c tIn this work,a sensitive electrochemical DNA sensor based on an avidin modified electrode and a DNA-functionalized Au nanoparticle(DFNP)was developed.The DNA-functionalized Au nanoparticle con-tained two kinds of DNA,one is hairpin probe DNA with a biotin at the30terminal and a thiol at the50terminal,the other is methylene blue(MB)-labeled linear signal DNA.Without hybridizing with thetarget DNA,the loop of the hairpin impeded biotin linked with avidin on electrode.However,after targethybridization,the hairpin was opened and biotin was recognized by avidin which resulted in a DNA-functionalized Au nanoparticle brought on the electrode surface.Electrochemical signals of MB bound tosignal DNA were measured by differential pulse voltammetry(DPV).Taking advantage of amplificationeffects of the AuNP and binding specificity of the hairpin probe,this DNA biosensor greatly simplified theelectrochemical DNA detection method and displayed high specificity in DNA detection.By using thisnew method,we demonstrate that this prototype sensor has been able to detect as low as picomolar DNAtargets with excellent differentiation ability even for a single mismatch.&2013Elsevier B.V.All rights reserved.1.IntroductionNucleic acids are one of the most fundamental molecules for alllife forms,and as a result the specific nucleic acid sequencequantification is essential in biological and biomedical studies,such as medical diagnostics,gene expression analysis,and thedetection of infectious diseases.Until now,many approaches havebeen successfully developed for the sequence-selective DNAhybridization examination,includingfluorescence,electrochemi-cal and colorimetric etc.methods(Huang et al.,2012;Liu et al.,2013;Shen et al.,2012;Yang et al.,2013).The electrochemical DNA sensing technology has receivedparticular attention mostly due to its high sensitivity,selectivityand easy operation with simple instrumentations(Wang et al.,2001a,2001b;Cai et al.,2003a,2003b).The DNA recognition eventcan be detected by using different strategies,including intrinsicelectroactivity of the nucleic acid(Jelen et al.,2002;Wang et al.,2001a,2001b),enzyme labels(Lumley et al.,1996;Alfonta et al.,2001),DNA duplex intercalators(Kara et al.,2002;Erdem et al.,2001),electroactive markers(Wang et al.,2003a,2003b;Iharaet al.,1997),and metal nanoparticles/quantum dots(Cai et al.,2003a,2003b;Jacob Hansen et al.,2006;Wang et al.,2003a,2003b;Liu et al.,2005;Park et al.,2002).In recent years,theelectrochemical DNA(E-DNA)sensor due to its reagentless,simpleoperation and regeneration have attracted much attention(Fan et al.,2003;Zhang et al.,2007;Xiao et al.,2007;Immoos et al.,2004).E-DNA sensors are comprised of an oligonucleotide probe modified onone terminus with a redox reporter and attached to an electrode atthe other.Hybridization of this probe DNA to a target oligonucleotideinfluences the rate at which the redox reporter collides with theelectrode,leading to a detectable change in redox current.Thisdesign is in fact an electrochemical analog offluorescent“molecularbeacons”,i.e.,stem-loop(hairpin)probes with internally quenchedfluorescence(Tyagi and Kramer,1996;Tyagi et al.,1998).Broadly,thereagentless,single-step E-DNA sensors could be classified into“signal-on”and“signal-off”two types.In the“signal-off”sensors,target hybridization sequesters the redox moiety from the electrode,reducing the observed Faradaic current.However,“signal-off”sensorssuffer from false positives when either the probe DNA or the redoxtag become degraded and are limited in their signal gain(Xiao et al.,2007).In contrast,in the signal-on sensors,target hybridizationenhances the rate which the redox moiety approaches the electrode,avoids false positives and leads to a current increase.However,signal-on E-DNA sensor usually bears low sensitivity(Xiao et al.,2007;Immoos et al.,2004).For<!–td:hybridization‐basCompared with the sensors with hybri-dization happens in homogeneous solution,hybridization-based DNAelectrochemical sensors causes lower available hybridization efficiency,becausehybridization-based DNA electrochemical sensors need probeContents lists available at ScienceDirectjournal homepage:/locate/biosBiosensors and Bioelectronics0956-5663/$-see front matter&2013Elsevier B.V.All rights reserved./10.1016/j.bios.2013.12.027n Corresponding author.Tel.:þ867917118918;fax:þ867917118919.E-mail address:fanhao11@(H.Fan).Biosensors and Bioelectronics56(2014)124–128DNA pre-immobilization on the electrode surface.And then we are faced with the difficulty for real-time electrochemical monitoring PCR and recognizing specific sequence DNA in living cells,and thefluor-escent molecular beacons have been widely used forreal-time monitor-ing DNA in PCR and in cells with hybridization in homogeneous solution(Ram et al.,2008;Santangelo et al.,2004;Nitin et al.,2004). Therefore,developing the electrochemical DNA sensing technology directly in homogeneous solution is still a challenge.We herein reported a non-immobilizing E-DNA sensor with hybridization which occurred in a homogeneous solution that employed DNA-functionalized Au nanoparticle(DFNP)and avidin modified electrode.In the present study,we have developed a DNA-functionalized Au nanoparticle,integrated DNA recognition; signal amplification and specific biotin–avdin link functional section.They are used as probe for DNA detection and featured high sensitivity up to low picomolar.In the present study,we have developed a DNA-functionalized Au nanoparticle,integrated DNA recognition;signal amplification and a specific biotin–avdin link functional section,as a probe for DNA detection and featured high sensitivity up to low pmtomolar. In target DNA detection,as shown Fig.1,when the probe DNA of DFNP is under stem-loop state,a comparatively huge bulk of Au nanoparticle and the loop of probe DNA would prevent biotin on the probe DNA being captured by the avidin on the electrode for the spatial effects.After the DFNP solution is incubated in a buffer solution containing target DNA,and then target DNA would combine probe DNA by forming double strand with complemen-tary sequences,which caused hairpin open and eliminate steric hindrance to biotin.Afterwards,when avidin modified electrode was immersed in a solution after the hybridization event,the biotin of the DFNP could have attached to avidin on the electrode and thus was captured on the electrode.The electrochemical signals of MB of signal DNA were measured by differential pulse voltammetry(DPV).Taking advantage of amplification effects of the Au nanoparticle(AuNP)and binding specificity of the hairpin probe,this biosensor greatly simplifies the electrochemical detec-tion method of DNA and displays high specificity.2.Experimental section2.1.MaterialsAll the probes and target oligonucleotides were purchased from the Takara Biotechnology Company.The oligonucleotides were purified via the C18reversed-phase HPLC and polyacrylamide gel electrophoresis(PAGE)and their sequences are shown in Table1. The stem-loop probe oligonucleotide(oligo1)has a50-HS label and a30-biotin,which will form the stem at appropriate ionic strength.The MB-labeled signal DNA(oligo2).The sequence of the target(oligo3)is perfectly matched to the loop sequence of the probe;oligo4contains a one-base mismatch,while oligo5has three-base mismatches.Oligo6is a random sequence unrelated to the probe sequence.Unless otherwise noted,all chemicals were purchased from Dingguo Biotechnology Inc.(Shanghai,China)and were of analytical reagent grade.The biotin and avidin were purchased from Sangon Biotechology Inc.(Shanghai,China).All of the solutions were prepared with ultrapure water from a Millipore Milli-Q system.2.2.ApparatusAll voltammetric experiments were performed using a CHI660 electrochemical analyzer(CHI Instrument Inc,USA).Electroche-mical experiments were carried out in a3ml electrochemical cell at room temperature(251C)by using three electrode configura-tions.A platinum wire served as a counter electrode and an Ag/ AgCl as reference electrode with saturated KCl solution.2.3.Preparation of Au nanoparticlesAu nanoparticles were prepared by citrate reduction of HAuCl4 according to the literature(Doron et al.,1995).In brief,100mL of 0.01g HAuCl4solution was brought to reflux with stirring,and 2.5ml of1%sodium citrate solution was introduced into this HAuCl4solution.The mixture solution was kept boiling foranother Fig.1.Scheme for the DNA-functionalized Au nanoparticle-based E-DNA sensor.H.-F.Cui et al./Biosensors and Bioelectronics56(2014)124–12812530min and left to cool to room temperature.The diameter of such-prepared Au nanoparticle was determined ca.1673nm by using atomic force microscopy scanning with line scan mode and taking the tip convolution effects into account (The AFM image is in the Supporting Information Fig.S1).The obtained Au nanopar-ticles were then stored in a brown glass bottle at 41C for further use.2.4.Preparation of the DNA-functionalized Au nanoparticles The process of probe DNA and signal DNA labeling was performed according to paper (Taton et al.,2000)as follows:The mixture of 5.6nM probe DNA and 2.8nM signal DNA was activated with acetate buffer (pH 5.2)and 1.5μL of 10mM Tris(2-Carbox-yethyl)Phosphine Hydrochloride(TCEP)for 1h,then added to 1mL of freshly prepared Au nanoparticles,and shaken gently overnight.Over the course of 16h,the DNA-AuNP conjugates were aged in salts (0.1M NaCl,10mM acetate buffer)for another 24h.Excess reagents were removed by centrifuging at 16,000rpm for 30min.The red precipitate was washed and centrifuged repeatly for three times.The resulting nanoparticles were dispersed into a buffer solution (0.1M,containing 0.3M NaCl and 3mM Mg 2þ,pH 8.1)and stored at 41C.According to the method in previous reports (Zhang et al.,2008;Demers et al.,2000),it was found that there were 94single strands per AuNP,consisting of 21probe DNA and 73signal DNA on each AuNP (see Supporting information (SI)for details).2.5.Preparation of avidin-coated electrode surfacesThe gold electrode was exposed to an ethanolic 5mM solution of 3,30-dithiodipropionic acid for 30min,followed by water rin-sing.5μl of 100mg mL À1EDC solution was then placed on the surface,followed immediately by 5μL of 100mg mL À1NHS solution.These solutions were allowed to interact with the electrode with 3,30-dithiodipropionic acid modi fication for 30min in a 100%humid environment to prevent solution evapora-tion.The surface was then rinsed with water and immersed in an aqueous 0.2mg mL À1avidin solution for at least 120min,after which the surface was rinsed again.The electrode was then exposed to a 1mM 2-aminoethanol solution (pH 8.0,adjusted using HCl)for 60min,rinsed,and used in hybridization detection.2.6.DNA sequence sensing performanceThe DNA assay procedure was initiated in 10mM phosphate buffer (PBS)containing 3.0mM Mg 2þby adding 10μL of 0.43μM DFNP to target DNA and stirring the mixture for 30min at 37o C.During the process,the target DNA hybridized with probe DNA,and then one avidin-modi fied electrode was immersed in the solution for capturing DFNP-target DNA hybridization.After 40min,the avidin-modi fied electrode was washed with a 0.1%sodium dodecyl sulfate (SDS)phosphate buffer (pH 7.3)to remove the non-speci fic adsorption.The electrochemical investigation was carried out in a 3ml electrochemical cell with the resulted gold working electrode,an Ag/AgCl reference electrode and the platinum wire counter electrode by DPV.The electrochemical signal of the MB was obtained in the 10mM PBS buffer (pH 8.0)at about À0.20V (versus Ag/AgCl).2.7.Optimization of testing conditionsMany experimental parameters,such as hybridization tempera-ture,hybridization time,and Mg 2þion strength,capture time,can affect the detection sensitivity of the assay.A series of optimiza-tions of these parameters were carried out to improve the sensitivity and selectivity of the DNA assay.Supporting informa-tion Fig.S4shows the effect of the capture time on the signal of DNA detection.In this study,a 1.8Â10À11M complementary DNA target was incubated with DFNP for 30min at a series of temperatures ranging from 25to 45o C.As shown in Supporting information Fig.S5,the highest signal is obtained at 37o C.Therefore,a hybridization temperature of 37o C was selected in the following experiments.The effect of the hybridization time has also been investigated.The target DNA was incubated with DFNP at 37o C for different times from 10to 50min.The signal responses of the assay to the different hybridization times are shown in Supporting information Fig.S6.It can be seen from this figure that the signal increases rapidly as the hybridization time increases from 10to 30min and then increases slightly from 30to 50min,indicating that the hybridization reaches equilibrium at 30min.As a result,a reaction time of 30min was selected for the DNA hybridization.The cation Mg 2þplays an important role in DNA hybridization.For a hairpin DNA probe-based DNA assay,there is hybridization competition between intramolecular stem part hybridization and intermolecular target DNA-probe hybridization.Therefore,Mg 2þconcentration in the hybridization buffer should be balanced.Although a high concentration of Mg 2þcan enhance target DNA hybridized to the loop of the structure,it may also strengthen the binding of the stem part of the probe,which may adversely affect the performance of the assay.Supporting information Fig.S7shows the effect of Mg 2þconcentration in the hybridization buffer on the response signal of the assay.As shown in this figure,the assay produces the highest signal when the Mg 2þconcentration is 3mM.Accordingly,a 3mM Mg 2þconcentration was used throughout the experiment to obtain the highest signal.3.Results and discussionWe employed a stem-loop DNA probe dually labeled with HS and biotin at the 50-and the 30-end,respectively,which could be facilely immobilized at Au nanoparticle surfaces via the Au –S bridge and hybridized with target DNA.The MB-labelded signal DNA with HS at 50end could provide the electrochemical signal.Two kinds of DNA have been immobilized at Au nanoparticle to construct DFNP.The detection strategy is demonstrated in Fig.1.Before the hybridization,the DFNP remained in the stem-loop structure,which forced the biotin to close to the Au nanoparticle.Due to the steric effect of the Au nanoparticle,the biotin was prevented from conjugating with the avidin on the electrode and resulting in that the DFNP could not be captured by the electrode.After being hybridized with the target DNA,the DFNP 0s loop-stem structure opened and then the biotin molecule was easily bound to avidin modi fied electrode,resulting in the DFNP 0s being captured by the electrode.The capture ef ficiency was proportionate with the concentration of the target DNA.Table 1Oligonucleotides employed in this work.oligo 1(stem-loop probe)50-SH-CCACGCTTGTGGGTCAA-CCCCCGTGG-Biotin-30oligo 2(linear signal DNA)50-SH-GGTGGAGGGACGAGG-MB-30oligo 3(target)50-GGGGTTGAC CCACAAG-30oligo 4(one base-mismatched DNA )50-GGGGTCGACCCACAAG-30oligo 5(three base-mismatched DNA)50-GGGGTCTTCCCACAAG-30oligo6(non-complementary DNA)50-TTCGGCTCTATCAATC-30H.-F.Cui et al./Biosensors and Bioelectronics 56(2014)124–128126The target hybridization event can be sensitively transduced via detecting the electrochemical reduction current signal of MB at the DFNP.Of note,a single DFNP is loaded with about 70signal DNA strands;this offers a signi ficant ampli fication for DNA detection.Indeed,in the stem-loop state we only found a relatively small and stable background current (Fig.2curve a).As we challenged the sensor with 1.2Â10À11M target DNA,which was expected to open the stem-loop,a large signal (Fig.2curve b)was observed.We found that as the concentration of the complementary target DNA sequence continuously increased (Fig.3A),which was loga-rithmically related to the target protein concentration from 2.3Â10À12to 2.3Â10À9M (Fig.3B).The equation for the result-ing calibration plot was calculated as y ¼0.199log x þ0.143(x is the concentration of target DNA divided by pM,y is the DPV peak current value)with correlation coef ficient of 0.9953and detection limit of 1.7Â10À12M (43SD).This sensitivity re flected the high signal ampli fication of this method and the improved signal gain as a signal-on sensor used MB as indicators.In order to con firm the binding speci ficity of the DFNP to the target DNA,control experiments were performed by using differ-ent DNA sequences to hybridize with 0.43μM DFNP,including complementary DNA,one base-mismatched DNA,three base-mismatched DNA and non-complementary DNA.As shown in Fig.4the non-complementary DNA produced a neglectable DPV signal (signal (d)),which was similar to the background signal (signal (e)),and when compared to the complementary sequence (signal (a)),the three base-mismatched DNA produced a very low insigni ficant DPV signal (signal (c)),and the one base-mismatched DNA generated a one third signal (signal (b)).We propose that this sequence speci ficity of the DFNP is mainly due to the intrinsic recognition ability of DNA bases,and also to the reason that even if the probe is hybridized with mismatched target,the probe DNA 0s hairpin structure is partially opened,an obvious steric effect still exists for such “hybridizer ”to contact the electrode.In order to evaluate the applicability of using this method in real-sample detection,we tested the sensor for their ability to detect DNA targets in the presence of biological fluids and PCR environment respectively.As demonstrated in Fig.S10,while the signals obtained in sera (both diluted and undiluted)were slightly suppressed compared to those obtained in pure buffer,we could still easily differentiate the target from a large excess of noncognate DNA.This clearly showed that our DFNP could selectively identify DNA targets even in complexes such as undiluted pared with the signal obtained from the hybridization event in buffer,the signalobtained from the hybridization event in undiluted serum is reduced by one sixth.We think that the signal decrease is due to the protein adsorption on surface of DFNP which leads to reducing ef ficiency of hybridization inserum.Fig.2.The DPV response of MB when the DFNP was incubated in phosphate buffer (a)and target DNA(b).Fig.3.(A)DPV response of DNA-functionalized Au nanoparticle-based DNA sensor in the solution containing 2.3Â10À12M target DNA (a),2.3Â10À11M target DNA (b), 2.3Â10À10M target DNA (c),and 2.3Â10À9M target DNA (d).(B)The calibration plots of target DNA (2.3Â10À12–2.3Â10À9M).Fig.4.The comparison of the DPV signal of MB when the DFNP is hybridized with 2.8Â10À10M target DNA (a), 2.8Â10À8M one base-mismatched DNA (b),2.8Â10À8M three base-mismatched DNA (c),2.8Â10À8M non-complementary DNA (d)and only the hybridization buffer containing no DNA sequences (e).H.-F.Cui et al./Biosensors and Bioelectronics 56(2014)124–128127We selected the detection of PCR amplicons from the lycopene-β-cyclase(LYC)gene to test the real applicability of our new E-DNA sensor.By using PCR protocol,we obtained DNA targets of104bp that could be directly detected by the sensor.We found that this sensor could selectively identify as few as4.1Â10À11M LYC DNA in PCR environment,which suggested that the E-DNA sensor was a promising method for the rapid detection of PCR product(see Supporting information(SI)for details).This DNA-functionalized Au nanoparticle-based E-DNA sensor demonstrated several advantages.First,the DFNP integrated both DNA detection probe and signal carrier at the surface of AuNPs, thus obviating the use of additional capture DNA modification involved in conventional bioassays.Second,stem-loop probes inherently possessed high specificity due to their conformational constraints,and thus were superior to linear ones for DNA detection.Morever,comparing to pervious“signal-on”E-DNA sensor(Cheng et al.,2010;Tennico et al.,2010;Cho et al.,2008) that utilizes the oligonucleotide probe covalently linked with a redox label and one hybridization event could bring only one signal reporter.The present sensor has a lower detection limit due to the amplification effect of AuNP,which has a large surface,with which one hybridization event could bring multiple signal mole-cules,leading to larger signal amplification.4.ConclusionIn summary,we completed a non-immobilizing E-DNA sensing strategy,which allowed the hybridization between DNA probe and target DNA which occured in a homogeneous solution.This assay protocol is simple,convenient and cost-effective and by using this novel method,we could conveniently detect as few as1.7Â10À12M target DNA.We propose that it might be a promising approach to perform DNA-based diagnostics where resources are limited,such as small clinics in developing countries orfield detection.AcknowledgmentWe gratefully acknowledge thefinancial support from the NSF of China(Grant no.21265007)and JiangXi Science and Technology Committee Foundation(Grant nos.20122BAB215024 and20132BAB215032),JiangXi Education Science Foundation No. (GJJ12538).Appendix A.Supporting informationSupplementary data associated with this article can be found in the online version at /10.1016/j.bios.2013.12.027. ReferencesAlfonta,L.,Singh,A.K.,Willner,I.,2001.Anal.Chem.73,91–95.Cai,H.,Cao,X.N.,Jiang,Y.,He,P.G.,Fang,Y.Z.,2003a.Anal.Bioanal.Chem.375, 287–293.Cai,H.,Zhu,N.,Jiang,Y.,He,P.,Fang,Y.,2003b.Biosens.Bioelectron.18,1311–1315. Cheng,G.F.,Shen,B.J.,Zhang,F.,Wu,J.K.,Xu,Y.,He,P.G.,Fang,Y.Z.,2010.Biosens.Bioelectron.25,2265–2269.Cho,H.S.,Baker,B.R.,Wachsmann-Hogiu,S.,Pagba,C.V.,Laurence,T.A.,Lane,S.M., Lee,L.P.,Tok,J.B.H.,2008.Nano Lett.8,4386–4390.Demers,L.M.,Mirkin,C.A.,Mucic,R.C.,Reynolds,R.A.,Letsinger,R.L.,Elghanian,R., Viswanadham,G.,2000.Anal.Chem.72,5535–5540.Doron,A.,Katz,E.,Willner,I.,ngmuir11,1313–1317.Erdem,A.,Kerman,K.,Meric,B.,Ozsoz,M.,2001.Electroanalysis13,219–223. Fan,C.H.,Plaxco,K.W.,Heeger,A.J.,A100,9134–9137. Ihara,T.,Nakayama,M.,Murata,M.,Nakano,K.,Maeda,M.,mun.17,1609–1612.Immoos,C.E.,Lee,S.J.,Grinstaff,M.W.,2004.J.Am.Chem.Soc.126,10814–10815. Huang,J.H.,Su,X.F.,Li,Z.G.,2012.Anal.Chem.84,5939–5943.Jacob Hansen,A.,Mukhopadhyay,R.,Hansen,J.,Kurt Gothelf,V.,2006.J.Am.Chem.Soc.128,3860–3863.Jelen,F.,Yosypchuk,B.,Kourilova,A.,Novotny,L.,Palecek,E.,2002.Anal.Chem.74, 4788–4792.Kara,P.,Kerman,K.,Ozkan,D.,Meric,B.,Erdem,A.,Ozkan,Z.,Ozsoz,M.,2002.mun.4,705–709.Liu,G.,Lee,T.M.H.,Wang,J.,2005.J.Am.Chem.Soc.12,38–42.Lumley,T.,Campbell,C.,Heller,A.,1996.J.Am.Chem.Soc.118,5504–5508. Nitin,N.,Santangelo,P.J.,Kim,G.,Nie,S.,Bao,G.,2004.Nucl.Acids Res.32,e58. Liu,P.,Yang,X.H.,Sun,S.,Wang,Q.,Huang,J.,Liu,J.B.,He,L.J.,2013.Anal.Chem.85, 7689–7695.Park,S.J.,Taton,T.A.,Mirkin,C.A.,2002.Science295,1503–1507.Ram,S.,Vajpayee,P.,Shanker,R.,2008.Environ.Sci.Technol.42,4577–4582. Santangelo,P.J.,Nix,B.,Tsourkas,A.,Bao,G.,2004.Nucl.Acids Res.32,e57. Yang,T.,Guan,Q.,Guo,X.H.,Meng,L.,Du,M.,Jiao,K.,2013.Anal.Chem.85, 1358–1366.Taton,T.A.,Mirkin,C.A.,Letsinger,R.L.,2000.Science289,1757–1760.Tyagi,S.,Bratu,D.P.,Kramer,F.R.,1998.Nat.Biotechnol.16,49–53.Tyagi,S.,Kramer,F.R.,1996.Nat.Biotechnol.14,303–308.Tennico,Y.H.,Hutanu,D.,Koesdjojo,M.T.,Bartel,C.M.,Remcho,V.T.,2010.Anal.Chem.82,5591–5597.Shen,W.,Deng,H.M.,Gao,Z.Q.,2012.J.Am.Chem.Soc.134,14678–14681. Wang,J.,Kawde,A.N.,Erdem,A.,Salazar,M.A.,2001a.Analyst126,2020–2025. Wang,J.,Liu,G.,Merkoci,A.,2003a.J.Am.Chem.Soc.125,3214–3217.Wang,J.,Polsky,R.,Merkoci,A.,Turner,K.L.,ngmuir19,989–993. Wang,J.,Xu,D.,Kawde,A.N.,Polsky,R.,2001b.Anal.Chem.73,5576–5581. Xiao,Y.,Qu,X.G.,Plaxco,W.K.,Heeger,J.A.,2007.J.Am.Chem.Soc.129, 11896–11898.Zhang,J.,Song,S.P.,Wang,L.,Pan,D.,Fan,C.H.,2007.Nat.Prot.2,2888–2892. Zhang,S.,Zhong,H.,Ding,C.,2008.Anal.Chem.80,7206–7212.H.-F.Cui et al./Biosensors and Bioelectronics56(2014)124–128 128。
