chapter 5 Cycloalkanes
Cycloalkane-ye

I. Nomenclature
1. Monocycloalkanes 2. Bicyclic bridged hydrocarbons
4
1. Monocycloalkanes Prefix: cyclo;
Cyclopropane Cyclobutane Cyclopentane Cyclohexane Cyclooctane
12
1. Ring Strain
unstable stable [109.5°- 60°] =49.5° [109.5°- 90°] =19.5°
Heats of combustion:
(CH2)n +
3 2
n
O2
n CO 2 + n H2O + heat
Table Heats of Combustion of Cycloalkanes(kJ·mol-1)
Chapter 2Alkanes & Cycloalkanes
Section 2 Cycloalkanes
Cycloalkanes contain rings of carbon atoms.
Ⅰ. Nomenclature II. Structure III. Properties
1
Classification of cycloalkanes
18
2. Conformational isomerism in cyclohexane
Chair form Boat form
5 6
4
3
1 2
5 4
3
6 1
2
chair form
H
5
HH H
4
3
烃类热裂解

petrochemical industry
Boiling point and its use of various petroleum products
Petroleum gas Crude gasoline Petroleum ether Gasoline Solvent oil Kerosene Aviation kerosene Kerosene Diesel Machine oil Vaseline Paraffin wax Fuel oil Asphalt Petroleum coke
异构烷烃裂解规律 Cracking rules of isoalkanes
(1)比正构烷烃容易裂解或脱氢 The pyrolysis or dehydrogenation of the isoalkane is easier than that of the normal alkane. (2)脱氢能力与分子结构有关,难易顺序为叔氢>仲氢>伯氢
We studied the catalyst performance and utilization, and the mass balance calculation of the reaction process.
石油加工的两大体系 Two system of petroleum processing
3.1 热裂解过程的化学反应
(chemical reaction of thermal cracking process)
一、烃类裂解的反应规律 (Reaction rules of thermal cracking of hydrocarbon)
烃类热裂解过程是十分复杂的,即使是纯组分裂解,得到的产物也是很 复杂的。包括:脱氢、断裂、异构化、环化、岐化、聚合、焦化、二烯 合成等。 The process of hydrocarbon pyrolysis is very complex, and products are also very complex even if pure component is cracked. Thermal cracking of hydrocarbon contains the reactions of dehydrogenation, chain scission, isomerization, cyclization, disproportionation, polymerization, diene synthesis, etc.
chapter 5 nomenclature

As an introduction to the IUPAC nomenclature system, we shall first consider compounds that have no specific functional groups. Such compounds are composed only of carbon and hydrogen atoms bonded together by sigma bonds (all carbons are sp3 hybridized). 5-3 Alkanes Hydrocarbons having no double or triple bond functional groups are classified as alkanes or cycloalkanes, depending on whether the carbon atoms of the molecule are arranged only in chains or also in rings. Although these hydrocarbons have no functional groups, they constitute the framework of other classes of compounds, and provide an ideal starting point for studying and naming organic compounds. The alkanes and cycloalkanes are also members of a larger class of compounds referred to as aliphatic. Simply put, aliphatic compounds are compounds that do not incorporate any aromatic rings in their molecular structure.
化学第一章org2alkanes.cycloalkanes
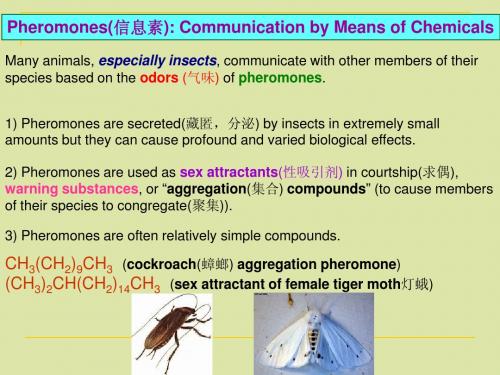
Sequence rule(次序规则)
a system of rules based on atomic number. CH3 4-甲基-8-异丙基十一烷 CH3CH2CH2CHCH2CH2CH2CHCH2CH2CH3 CH(CH3)2
about single bonds
H
H
CH4
H
C H
H
H H H
constitutional formula configurational formula
H
H C H
H C H
H
C 2 H6
C 3 H8
H
C H
H
H
CH3-CH3
H
C H
C
H
H
CH3-CH2-CH3
CH3-CH2-CH2-CH3
7 6 5 H3C CH 2 CH
4 CH 3 2 1 CH CH CH CH 3 3 CH 3 CH 35' 2 CH 2' CH 2CH 3 6' 7'
1
2,3,5-三甲基-4-丙基庚烷
CH3
2,7,8 3,4,9
H3C C C CH2 C H2C CH CH CH2 CH3 2,7,8-三甲基癸烷 10 9 H 8 3 7 6H2 5 4 2 1 CH3 CH3
Nanotubes, a new class of carbon-based materials with strength roughly one hundred times that of steel, also have an exceptional toughness.
有机化学课件Chapter环烷烃和芳香烃

9
2
1
3
8 6
4
7
5
8-甲基二环[4. 3. 0]壬烷
8-methylbicyclo[4. 3. 0]nonane
7
6 54
1 2
3
2, 7, 7-三甲基二环[2. 2. 1]庚烷
2, 7, 7-trimethylbicyclo [2. 2. 1]heptane
7
4
5
3
1
6
2 用","隔开
三环[2. 2. 1. 02, 6]庚烷
23
第四章 环烷烃和芳香烃
有机化学课件
24
第四章 环烷烃和芳香烃
4
Boat Conformer
Conformational Energy
有机化学课件
25
第四章 环烷烃和芳香烃
有机化学课件
26
第四章 环烷烃和芳香烃
直立键和平伏键(Axial and Equatorial Bonds)
四、单取代环己烷
Long-chain
有机化学课件
14
第四章 环烷烃和芳香烃
二、环大小与化学性质
五元以上 环烷烃
性质相似
链状烷烃
Cl2 / hv
Cl 自由基取代反应
H2 / Pt 催化加氢
HI
不反应 不反应
有机化学课件
15
第四章 环烷烃和芳香烃
三、小环化合物的特殊性质 —— 开环加成
1. 催化加氢
H2 / Pt, 50oC or Ni, 80oC
椅式构象的画法
有机化学课件
35
第四章 环烷烃和芳香烃
有机化学课件
36
烷烃

3-Ethyl-5-methylh eptane
(n ot 3-methyl-5-ethylheptane)
2-14
Nomenclature - IUPAC
7. The prefixes di-, tri-, tetra-, etc. are not included in alphabetization • alphabetize the names of substituents first and then insert these prefixes
2-6
Constitutional Isomerism
Constitutional
isomers: compounds with the same molecular formula but a different connectivity of their atoms
• example: C4H10
Alk enes (Chap ters 5-6) One or more carb on -carbon double b on ds H C C H H Eth ene H
A lkynes (Ch apter 7) One or more carbon-carb on trip le bonds H-C C-H Acetylen e
Primary
(1°) C: a carbon bonded to one other
carbon
• 1° H: a hydrogen bonded to a 1° carbon
Secondary
(2°) C: a carbon bonded to two other
第四章 烷烃和环烷烃

构造异构
(一)、碳链异构 (isomerism): 具有相同分子式,仅由于碳链结构不同而产
生的同分异构现象称为碳链异构。
由于碳链的连接次序不同而产生的 异构现象称为碳链异构
CH3 CH2 CH2 CH2 CH3
CH3 CH CH2 CH3 CH3 CH3
2, 4, 5 2, 3, 5
1. 确定主链: 最长链为主链。 号: 第一行 取代基编号为2, 4, 5; 第二行 取代基编号为2, 3, 5; 根据最低系列原则, 用第二行编号。 3. 命 名: 中文名称:2,3,5-三甲基己烷 英文名称:2,3,5-trimethylhexane
8 例1 CH3-CH2
4 3
43
2 5
61
2,4-二甲基已烷
(最长碳链;编号方向,从先遇到取代基 的一端开始编号;相同的取代基合并) 命名书写中常见错误: 2-二甲基己烷、 2,4-甲基己烷、 2,4-2甲基己烷、 2,4二甲基己烷
实 例 一
2. 编
1 2 3 4 5 6 6 5 4 3 2 1 CH3CHCH2CHCHCH3 CH3 H3C CH3
在英文命名中,取代基按词首的字母排列顺序先后列出
3-ethyl-4-isopropyl-3-methyl-5-propyloctane
课堂练习:写出化合物的名称,并指出各碳原子的类型。
C2H5 CH3 CH3 CH3 (2) CH3 CH3 CH3CCH2CCH3 CH3 CH3
(1) H3C
四、烷烃的同分异构现象 (The phenomenon of isomerism)
支链
CH3CH2CH2CH3
烷烃和环烷烃
烷烃分子中只有σ键,化学性质比较稳定, 不易发生化学反应。可以燃烧,也可以发生 卤代反应。
1、 燃烧
CnH2n+2
+
3n 1
(
2
) O2
n CO2 + (n+1)H2O
陈明
2、 卤代反应
(1) 甲烷的氯代
H
H
D or hv
H C H + Cl2
HC
H
H
H
D or hv
D or hv
H C Cl
H
根据碳原子数称某烷,前面不加“正”。
CH3CH2CH2CH3 丁烷 CH3(CH2)13CH3 十五烷
2) 支链烷烃 (1)把其看作直链烷烃的衍生物,把支链 作为取代基。在整个名称中包括母体和取代 基两部分,取代基部分在前,母体部分在后。
陈明
CH3CHCH2CH2CH3
CH3
2-甲基戊烷
(2)主链的选择: a、最长碳链为主链
陈明
Step 1 链的引发
D or hv
Cl Cl
Cl + Cl
Step 2 链的增长
H
H
H C H + Cl
H C + HCl
H
H
H
H
H C + Cl Cl
H C Cl+ Cl
H
H
陈明
Step 1 链的中止
H
HC
H H
+ Cl H
HC H
+ CH H
Cl + Cl
H H C Cl
H HH H CC H HH
陈明
二、命名( Nomenclature)
1、烷基 (alkyl) 的名称
烷基是烷烃去掉一个或几个 H 后剩下的 原子基团,用 R-表示,通式:CnH2n+1; 甲基常表示为 Me、乙基表示为 Et 。
有机化学习题集

第一章 绪 论Chaper1 intrduction1.将下列凯库勒式改写成路易斯式。
(1)(2)(3)(4)HH H H HH H HHHH HC C CC C C N OOO O ClO OO C HHHHH(5)(6)C 2H 5OH浓 H 2SO 4CH 2=CH 2 + H 2O 的反应热试计算23. 某化合物仅含碳、氢、氧元素,燃烧纯的该化合物 2.642mg ,得到CO 2 7.638mg,H 2O2.518mg ,试求其经验式。
4. 某化合物含碳45.44%、氢9.11%、氮21.20%、分子量132,求其分子式。
5. 试举例说明有机化合物在日常生活中的应用。
第二章 烷烃 Chapter 2 alkanes1、 写出下列化合物的构造式:1、 正戊烷 异戊烷 新戊烷2、 2,2,3-三甲基戊烷3、四甲基丁烷4、3-甲基-4-乙基己烷5、3-甲基-3-乙基-6-异丙基壬烷6、 2,5-二甲基-3,4-二氯己烷 2、 写出下列烷基的名称:CH 3CHCH 231.CH 3CH 2CH32.CH 3CHCH 2CH 2CH 233.(CH 3)3C CH 24.(CH 3)3C5.CH 33CH 36.3、 将下列化合物用系统命名法命名:(CH 3CH 2)22CH 22CH 3CH 3CH(CH 3)21. 2.CH 3CH 22CH 2CH 3CH(CH 3)23.(CH 3)2CCHCH 2CH 3CH 2CH 2CH 3CH 2CH 2CH 3(CH 3)2CHCHCHCCH 2CH 3CH 3CH 32CH 2CH 34.5.CH 3CH 2CHC 2CH 3CH 3CH 32CH 2CH 3CH 36.(CH 3CH 2CCH 2CH 2CH 3)3CHCH 3CH 37.CH 3CH 2CH 2CHCH 2CH 2CH 33)3(CH 3)3CCH 2CH(CH 2CH 3)28.4、 将下列烷烃的沸点按从高到低的顺序排列,然后与手册核对。
5 pigments and colorants print
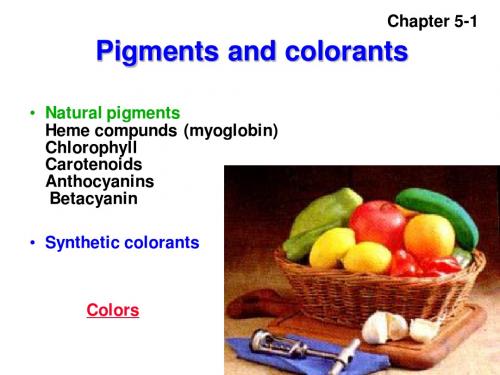
Yellow
Red
Violet or black (crystaline forms) Blue or green (associated with a protei来自)Chapter 5-18
• Two main groups of carotenoids:
1) The carotenes(烃类胡萝卜素), which contain only (烃类胡萝卜素) hydrogen and carbon, and may be cyclic or linear. Of the carotenes, beta-carotene is well known as a vitamin A precursor. Lycopene(番茄红素), one of (番茄红素) the red pigment in tomatoes, is a carotene. 2) Oxycarotenoids(氧合叶黄素), which contain oxygen (氧合叶黄素) in the form of hydroxy, epoxy(环氧), or oxy groups, (环氧) as well as hydrogen and carbon. These compounds are also referred to as xanthophylls(叶黄素). Lutein (叶黄素) 叶黄素) (叶黄素), found in egg yolk, is a modified betacarotene that is di-hydroxylated.
H3C CH3 CH2 H N CH3 HN H3C N O
Chapter 5-10
Mg+2
NH
H3C
O
O
有机化学英文命名

Alkanes
Methane CH3CH2CH3 Propane CH3CH3 Ethane
CH3CH2CH2CH3
Alkyl group
Methyl CH3CH2CH2 Propyl CH3CH2 Ethyl
CH3
Abbreviation
Me
Pr Et
CH4
Butane
CH3CH2CH2CH2 Butyl
CH2 CH CH2
Vinyl Allyl (Allylic group)
烯丙基
CH3 CH CH
CH2 C CH3
Propenyl
e i
丙烯基
Isopropenyl
3.2 Structure of Alkenes
Ethylene: sp2 Hybrid orbitals
Ground state 2p 2s 1s Exited state 2p
Step 2 Number the atoms in the main chain
Beginning at the end nearer the first branch point, number each C atom in the parent chain.
1 CH3 2 CH2 4 NOT
(II)
Constitutional isomers
构造异构: 原子连接顺序
(I)
(II) (III) (IV)
(III)
Stereoisomers
立体异构: 空间
(IV)
Cis-trans isomers
Configuration
cis-2-Butene Rotation about C-C double bond is restricted trans-2-Butene
6 Simple Org Comp-Alkanes Cycloalkanes

2. Naming Alkanes
In earlier times, when relatively few pure organic chemicals were known, new compounds were named at the whim of their discoverer. Thus, urea (CH4NO2) is a crystalline substance isolated from urine; morphine (C17H19NO3) is an analgesic (painkiller) named after Morpheus, the Greek god of dreams; and barbituric acid is a tranquilizing agent named by its discoverer in honor of his friend Barbara.
Chapter 6
Organic Compounds: Alkanes and Cycloalkanes
1
According to Chemical Abstracts, the publication that abstracts and indexes the chemical literature, there are more than 19 million known organic compounds. Each of these compounds has its own physical properties, such as melting point and boiling point, and each has its own chemical reactivity.
化学名词

acid-dissociation constant 酸解离常数 acid 酸base 碱 conjugate acid 共轭酸 conjugate base 共轭碱 covalent bonding 共价键 single bond 单键 double bond 双键triple bond 三件 curved-arrow formalism 弯键形式 degenerate orbital 兼并轨道 dipole moment 偶极距 electron density 电子密度 electronegativity 亲电性 electrophile 亲电试剂 electrostatic potential map 电势能图 empirical formula 经验公式 formal charges 形式电荷 Hund's rule 洪特规则 ionic bonding 离子键 isotopes 同位素 Lewis acid/base 路易斯酸 Lewis structure 路易斯结构 line-angle formula 线键形式 lone pair 孤对电子 molecular formula 分子式 node 节点nodal plane 节面 nonbonding electron 非成键电子 nucleophile 亲核试剂 octet rule 八耦体规则 orbital 轨道 organic chemitry 有机化学 polar covalent bond 平面共价键 resonance forms 共振形式 structural formular 结构式 valence 化合价 valence electron 价电子 Vitalism 生命力学说acid chloride 酰氯alcohol 醇aldehyde 醛alkane 烷烃alkene 烯烃alkyne 炔烃alkyl groups 烷基amide 酰胺amine 胺aromatic hydrocarbons 芳香碳氢化合物 carbonyl group 羰基carbonxyl group 羧基carboxylic acid 羧酸cis-trans isomers 顺反异构 constitutional isomers 结构异构cyano group 氰基dipole-dipole forces 偶极-偶极作用力 doudle bonds 双键ester 酯ether 醚functional group 官能团geometric isomers 几何异构hybrid atomic orbital 杂化原子轨道 hydrocarbons 碳氢化合物 cycloalkanes 环烷烃 cycloalkenes 环烯烃 cycloalkynes 环炔烃hydrogen bond 氢键hydroxyl group 羟基 stereoisomers 立体异构体 stereochemistry 立体化学ketone 酮London dispersion forces 色散力molecular dipole moment 分子偶极距 molecular orbital 分子轨道nitrile 腈sigma bond 西格玛键valence-shell electron-pair repulsion theory 价层电子对排斥理论 van der Waals forces 范德华力acyclic 非环的angle strain 角张力anti conformation 反交叉构象 aromatic hydrocarbon 芳香烃axial bond 轴向键 bridged bicyclic compound 桥环化合物 chair-chair interconversion 椅式椅式构象 combusion 燃烧 common names 普通命名 conformational analysis 构象分析 conformations 构象体chair comformation 椅式构象 flagpole hydrogens 旗键氢half-chair conformation 半椅式构象 cracking 裂解 catalytic cracking 催化裂解 hydrocracking 氢解cyclic 环 cycloalkane 环烷烃 degree of alkyl substitution 烷基取代度 eclipsed conformation 重叠构象 equatorial bond 平伏键fused-ring system 稠环gauche conformation 邻位交叉构象 geometric isomers 几何异构体 halogenation 卤代heat of combustion 燃烧热 homologs 同系物 hydrophlic 亲水的 kerosene 疏水的 methine group 次甲基 methylene group 亚甲基 methyl group 甲基n-alkane 正构烷烃 straight-chain alkane 直链烷烃 Newman projections 纽曼投影式 octane number 辛烷值 paraffins 石蜡ring strain 环张力angle strain 角张力 torsional strain 扭应变 sawhorse structures 锯架结构skew conformation 斜交叉构象 spirocyclic compounds 螺环化合物 staggered conformation 邻交叉构象 steric strain 空间位阻 substituent 取代基activation energy 活化能bond-dissociation enthalpy 键解离能carbnion 碳负离子carbene 碳烯carbocation 碳正离子catalyst 催化剂chain reaction 链反应initiation step 引发步骤propagation step 增长步骤termination step 终止步骤enthaply 焓endothermic 吸热exothermic 放热entropy 熵equilibrium 平衡equilibrium constant 平衡常数free energy 自由能standard Gibbs free energy change 标准吉布斯自由能改变 Hammond postulate Hammond 假设 heterolytic cleavage 异裂homolytic cleavage 均裂inductive effect 诱导效应intermidiate 中间体reactive intermidiate 活性中间体kinetices 动力学mechanism 机理potential-energy diagram 势能图radical 基radical inhibitor 自由基抑制剂rate equation 速率控制步骤 reaction-energy diagram 反应-能量曲线 resonance stabilizition 共振稳定性 substitution reaction 取代反应 thermodynamics 热力学transition state 过渡态absolute configuration 绝对构型achrial 非手性asymmetric carbon atom 不对称碳原子Cahn-Ingold-Prelog conversion Cahn-Ingold-Prelog 规则 chiral 手性chiral carbon atom 手性碳原子chirality center 手性中心chiral probe 手性探针configuration 构型configurational isomers 构型异构体 dextrorotatory 右旋diastereomer 非对映异构体 enantiomers 对映异构体Fischer projection 费谢尔投影式internal mirror plane 内部对称面Leftorium 左旋meso compound 内消旋化合物optical isomers 光旋异构体optical activity 旋光性optical purity 光学纯度plane-polarized light 平面偏振光polarimeter 旋光仪racemic mixture 外消旋化合物relative configuration 相对构型resolution 拆分resolving agent 拆分剂specific rotation 比旋光度stereocenter 立构中心 stereochemistry 立体化学stereoisomers 立体异构体 superimposable 不可重叠acid 酸acidity 酸性alkyl halide 卤代烃allylic 烯丙基allylic halogenation 烯丙基卤化反应 anti 反式anti-coplanar 反式共面syn-coplanar 顺式共面 aprotic solvent 非质子溶剂aryl halide 芳基卤代物 base 碱basicity 碱性concerted reation 协同反应 dehydrohalogenation 脱卤化氢的反应 electrophlie 亲电试剂 electrophlicity 亲电性 elimination 消除反应 freons 氟利昂geminal dihalide 谐二卤代物 halogen exchange reaction 卤素交换反应 hydride shift 氢迁移 hydroxylic solvent 羟基溶剂 inversion of configuration 构型转化 leaving group 离去集团 methyl shift 甲基迁移 nucleophile 亲核试剂 nucleophilicity 亲核性 nucleophilic substitution 亲核取代 organic synthesis 有机合成 polarizable 可极化primary halide 一级氯代物 secondary halide 次级卤代物 tertiary halide 三级氯代物 protic solvent 质子性溶剂 reagent 试剂 rearrangement 重排反应 retention of configuration 构型保持 solvolysis 溶剂解作用 stereospecific reaction 立体选择反应 steric hindrance 立体阻碍 vicinal dihalide 邻二卤取代 vinyl halide 乙烯基卤代物 Walden inversion 瓦尔登反转Zaitsev's rule Zaitsev's规则diene 二烯烃triene 三烯烃 tetraene 四烯烃bicyclic 双环bridged bicyclic 桥式双环 bridgehead carbons 桥头碳 catalytic cracking 裂解催化 dehydration 脱水反应 dehydrogenation 脱氢反应 dehydrohalogenation 脱卤化氢反应 double-bond isomers 双键异构体 element of unsaturation 不饱和度 geminal dihalide 邻二卤代物 heteroatom 杂原子 Hofmann product 霍夫曼产物 hydrogenation 氢化反应 polymer 聚合物addition polymer 加成聚合 saturated 饱和的 stereospecific reaction 立体专一反应 trans-diaxial 反式二轴 unsaturated 不饱和Zaitsav's elimination Zaitsav's 消除 Zaitsav's product Zaitsav's 产物addition 加成反应anti-addition 反式加成 electrophilic addition 亲电加成syn addition 顺式加成alkoxy group 烷氧基 alkoxymercuration 烷氧汞化作用alpha eliminayion alpha 消除反应 anionic polymerization 阴离子聚合反应 asymmetric induction 不对称诱导beta elemination beta 消去carbene 卡宾cationic polymerization 阳离子聚合反应 chain-growth polymerzation 链增长聚合 demercuration 脱汞作用epoxide 烷氧化物 epoxidation 环氧化作用free-radical polymerization 自由基聚合glycol 乙二醇 halogenation 卤化反应 halohydrin 卤代醇halonium inon 卤鎓离子 heterogeneous catalysis 多相催化hydration 水和作用 homogeneous catalysis 均相催化 hydroboration 硼氢化作用 hydrogenation 氢化作用 markovnikov's rule 马尔可夫尼科夫规则 markovnikov orientation 马尔可夫尼科夫取向 monomer 单体organic synthesis 有机合成oxidative cleavage 氧化裂解 oxymercuration 羥汞化作用 ozonolysis 臭氧分解peroxide effect 过氧化作用 peroxyacid 过氧酸polymer 聚合物 polymerization 聚合regioselective reaction 区域选择性反应 retrosynthetic analysis 反合成分析 Simmons-Smith reaction Simmon-Smith 反应 stereopecific reaction 立体选择反应acetylene 乙炔acetylide ion 炔碳负离子 alkoxide ion 烷氧负离子 alkyne 炔a terminal alkyne 端炔烃a interminal alkyne 内炔烃amyl 戊烯的旧名enol 烯醇Lindlar's catalyst Lindlar's 催化剂 s character S 成分siamyl group 二级异戊基 Tautomers 互变异构体vinyl cation 乙烯基阳离子acid derivates 羧酸衍生物 acid chlorides 酰氯esters 酯amides 酰胺alcohol 醇aldehyde 醛 formaldehyde 甲醛alkoxide ion 烷氧负离子 azeotrope 恒沸物carbinol carbon atom 甲醇碳原子 denatured alcohol 变性酒精diol 二醇disulfide 二硫化物 expoxides 环氧化物glycol 乙二醇vicinal diol 邻二醇grain alcohol 粮食醇 Grignard reagenthydride reagent 氢化试剂 hydrophilic 亲水的 hydrophobic 憎水的hydroxy group 羟基ketone 酮lithium dialkylcuprate 二烷基铜锂试剂 mercaptan (thiol) 硫醇miscible 互溶的 organometallic copounds 有机金属试剂 phenol 酚Raney nickel 瑞尼镍rubbing alcohol 外用酒精stunk 臭鼬sulfonic acid 磺酸thiolate ion 硫醇盐离子 wood alcohol 木醇alcohol dehydrogenase(ADH) 醇的脱氢酶 alkoxide ion 烷氧离子chromic acid reagent 铬酸试剂chromic acid test 铬酸 鉴定Collins reagent Collins 试剂 ester 酯ether 醚inorganic esters 无机酯Jones reagent Jones 试剂Lucas test Lucas 鉴定 nicotinamide adenine dinucleotide (NAD) 烟酰胺oxidation 氧化pinacol rearrangement 频哪醇重排 pyridinium chlorochromate (PCC) 吡啶氯化铬盐 reduction 还原Swern oxidation Swern 氧化 tosylate ester 对甲苯磺酸酯 Williamson ether synthesis Williamson 醚合成absorption spectroscopy 吸收光谱base peak 基峰conjugated double bonds 共轭双键 electronmagnetic spectrum 电磁光谱fingerprint region 指纹区Fourier transform infared spectrometer (FT-IR)Fourier 变换红外光谱 fragmentation 碎片frequency 频率high-resolution mass spectrometer 高分辨质谱仪infared spectrometer 红外光谱仪 interferometer 干涉仪IR active IR 活性的IR inactive IR非活性的mass spectrum 质谱仪molecular ion,M+(parent ion) 分子离子M+1 peak M+1 峰M+2 peak M+2 峰photon 光子radical cation 自由基阳离子source 离子源wavelength 波长wavenumber 波数accidentally equivalent nuclei 偶然等价原子核 chemically equivalent atoms 化学等性原子核 chemical shift 化学位移 complex splitting 复杂裂分 coupling constant (J) 偶合常数 deshielded 去屏蔽 diastereotopic atoms 非对映原子 downfield 低场Fourier transform spectroscopy Fourier变换波谱 transient 瞬间变化 gyromagnetic ratio 磁旋比induced magenetic field 诱导磁场 integration 积分magnetic coupled 磁耦合magnetic moment 磁矩magnetic resonance imaging (MRI) 磁共振影像 nuclear magnetic resonance spectroscopy (NMR) 核磁共振波谱 carbon magnetic resonance 碳核磁共振 proton magnetic resonance 质子核磁共振off-resonance decoupling 偏共振去偶 relaxation time 驰豫时间 shielded 屏蔽spin decoupling 自旋去耦spin-spin splitting 自旋-自旋裂分 TMS 四甲基硅烷 upfield 高场alkoxy group 烷氧基 alkoxymercuration 烷氧汞化ɑ-cleavage ɑ断裂autoxidation 自动氧化concerted reaction 协同反应crown ether 冠醚dioxane 二氧己烷 epoxidation 环氧化expoxide 环氧化合物epoxy resins 环氧树脂ether 醚symmetrical ether 对称的醚 unsymmetrical ether 不对称的醚furan 呋喃halohydrin 邻卤代醇 heterocyclic compound 杂环化合物 heterocyclic ethers 杂环醚MCPBA 间氯过氧化苯甲酸 MMPP 单过氧邻苯二甲酰镁 oxane 氧丙烷oxetane 氧杂环丁烷oxirane 环氧乙烷oxolane 氧杂环戊烷 peroxide 过氧化物 peroxyacid 过氧酸pyran 吡喃sulfide (thioether) 硫醚sulfone 砜sulfonium salt 硫鎓盐sulfoxide 亚砜Williamson ether synthesis Williamson 醚合成1.yoke: n. 轭, 轭状物, 套, 束缚, 支配v.把...套上轭, 结合, 连接2.cumulated: 累积的3.alumina: n.氧化铝4.carotene: n.胡萝卜素5.perpendicular: adj.垂直的, 正交的;n.垂线6.allene: 丙二烯; [pl. ]二烯烃; 丙二烯衍生物7.axis: n.轴8.corollary: n.必然的结果,推论9.rational: adj.理性的, 合理的, 推理的;n.[数]有理数10.inert: adj.无活动的, 惰性的, 迟钝的11.kinetic: adj.(运)动的, 动力(学)的12.thermodynamic: adj.热力学的, 使用热动力的13.proximity: n.接近, 亲近14.Diels: 狄尔斯(①姓氏 ②Otto Paul Hermann, 1876‐1954, 德国化学家, 曾获1950年诺贝尔化学奖)15.Alder: 阿尔德(①姓氏 ②Kurt, 1902‐1958, 德国化学家, 曾获1950年诺贝尔化学奖)16.acetylenic: 乙炔的17.radii: n.半径18.tuck: n.缝摺, 活力, 鼓声, 船尾突出部下方, 食物(尤指点心、蛋糕)vt.打摺, 卷起, 挤进, 塞, 使隐藏vi.折成摺子, 缩拢19.exudate: n.流出物, 分泌液20.chromia: 氧化铬绿21.pericyclic: 周环的1.naphthalene: n.萘(球), 卫生球2.coal tar: n.煤焦油3.Clapham: 克拉彭[英国伦敦西南部一地区]4.reverie : n.幻想5.hitherto: adv.迄今, 至今6.diminutive : adj.小的, 指小的, 小型的n.小的人, 指小辞, 指小词, 爱称7.whirl: v.(使)旋转, 急动, 急走n.旋转, —连串快速的活动8.evocative: adj.唤出的, 唤起的9.aromaticity: n.芳香性, [化]芳香族化合物的结构(特性)10.node: n.节点11.tub: n.浴盆12.tropylium: n.[化]卓鎓13.thiophene: n.[化]噻吩14.quinoline: n.喹啉, 喹啉衍生物15.nonaromatic : 非芳香性的16.anthracene: n.[化]蒽17.phenanthrene: n.[化]菲( 用于合成染料和药物)18.tetracene: 并四苯19. aliphatic: adj.脂肪族的, 脂肪质的20.pyrene: n.芘21.chrysene: n.屈22.Azulene: n. 奥, 茂并芳庚23.zwitlerionic: n.两性离子的24.benzopyrene: n.[化]苯并芘25.allotrope: n.同素异形体26.graphite: n.石墨27.toluidine: n.[化]甲苯胺28.aniline: n.[化]苯胺adj.苯胺的29.anisole: n.[化]茴香醚, 苯甲醚30.xylene: n.[化] 二甲苯31.cresol: n.[化]甲酚32. aliphatic: adj.脂肪族的, 脂肪质的1.Friedel‐Craft: 弗里德(Alfred Hermann, 1864‐1921, 奥地利著述家、记者, 曾获1911年诺贝尔和平奖)2.endothermic: adj.吸热(性)的,[动]温血的3.aromaticity: n.芳香性, [化]芳香族化合物的结构(特性)4.Partial Rate Factor: 分速度因素5.ERG: 供电子基团6.EWG: 拉电子基团7.quinone: n.[化]醌; 苯醌8.Bombardier: n.投弹手, 炮兵下士9.hydroquinone: n.对苯二酚10.glycerol: n.甘油, 丙三醇11.glycol: n.乙二醇12.pyrocatechol: n.[化]焦儿茶酚,邻苯二酚13.resorcinol: n.[化] 雷琐酚, 间苯二酚(=resorcin)14.oxonium: n.[化]氧鎓,钖promise: n.妥协, 折衷v.妥协, 折衷, 危及...的安全16.obligatory: adj.义不容辞的, 必须的1.citral: n.[化]柠檬醛2.Almond: n.[植]杏树, 杏仁3.cinnamon: n.[植物]肉桂, 桂皮, 肉桂色4.vanilla: n.[植]香草, 香子兰5.bactericidal: adj.杀菌的6.pyruvic acid: n.[化]丙酮酸7.imine: n.[化]亚胺8.customary: adj.习惯的, 惯例的9.cannizzaro: 坎尼扎罗(Cannizzaro,Stanislao,1826—1910)意大利化学家(themist,Italy)10.adenine: n.[生化]腺嘌呤11.pyridoxal: n.[生化]吡哆醛,维生素B12.cyanohydrin: n.[化]氰醇13.hemoglobin: n.血色素14.Rhodopsin: 视紫红质, 视网膜色素15.opsin: n.[生化]视蛋白16.oxime: n.[化]肟17.carbazide: (=carbohydrazide) 卡巴肼,卡巴脲,卡巴氮。
有机化学-陆阳主编8版-第四章 烷烃和环烷烃 Chapter-4-Alkanes

CH2CH3 -C(C,H,H)
CH3
-C(H,H,H)
CH2CH3
C1 C2
C1
CH2CH2CH3
CH2Cl C HF 2
C2
(3)含有双键或叁键的基团,可认为连有两个或叁 个相同的原子。
eg
C CH
(C) (C) C CH (C) (C)
C HC CH
(C) (C)
如果两个不同取代基所取代的位置按两种编号法位号相同, 则从顺序较小基团的一端开始编号。
碳的其余价键完全被氢原子饱和,因此又称为饱和 烃。
HHHH HCCCCH
HHHH
甲烷分子中的C是sp3杂化。甲烷分子中的每个C-H
都是碳的一个sp3杂化轨道与氢的1s轨道重叠形成 的,形成的这种键称为σ键,σ键的特点:是轴对 称,可以自由旋转,较稳定。
sp3杂化:
激发态
头碰头重叠 形成C—Hσ键
直链烷烃:去掉“正”字称为某烷 支链烷烃:看作是直链烷烃的取代衍生物,把 支链看作取代基。整个名称包括母体和取代基
两部分。
分为三步:一选二编三配基
1)选主链: a.选取一条连续最长的碳链作为主链 b.当有等长的碳链作主链时,选取代 基最多的那条。
C3 HC2 HCHCHC2 HC3 H H3CCHCH C3 H C3 HC3 H
同系物具有相似的化学性质,其物理性质一般随分 子量的改变而规律性变化。 系差: CH2为同系列的系差
构造式:代表分子中原子的种类、数目和排列次序的 式子。
结构式:除了代表分子中原子的种类、数目和排列次 序的之外,还包括了空间及原子、电子、构 型、构象等信息的式子。
同分异构:分子式相同而结构不同的现象。
H CH2 CH2 CH2 H 丙 烷
油层物理学课件双语

There is a definite bounding surfaces between different phases. A phase can consist of several components.
(3) Components(组分) is the substance which consist of the
At normal T and P:
C1~C4: Gas C5~C16: Liquid >C16: Solid (paraffin 石蜡)
paraffinsa (石蜡)
Nonhydrocarbons(非烃) are compounds of oxygen, sulfur, nitrogen of alkanes(是胶质、沥青质的主要成分)。
Physical properties of the reservoir fluids are different from those of the fluids at the surface.
随油藏的开采→地下流体的相态发生变化→影 响最终采收率。为合理开发油藏,就必需搞清地 下流体的相态、物性随压力的变化。
参考书: 1、洪世铎 «油层物理基础»; 2、何更生 «油层物理»; 3、罗蛰谭 «油层物理学»;
第一章油藏流体的物理性质
油层(formation):能储集油气、并能让油气在 其中流动的多孔介质。
油藏:深埋在地下的油气聚集的场所。 单一圈闭、统一的水动力系统、统一的油水界面。
Reservoir(油藏) is a porous and permeable
(3)饱和多相流体的油藏岩石的物理性质;
Properties of Porous Medium Containing Multiple Fluids
Solomons Organic Chemistry chapter 5

5
6
Chapter 5
Chapter 5
26. Which structure(s) represent(s) diastereomer(s) of I? 23. The molecules below are:
H H H H H F F H F H H H H H H F
H HO HO CH3 OH H H CH3 CH3 OH H HO OH H CH3 H H H H CH3 OH OH OH CH3 HO HO H CH3 H H OH CBr CH3 Br H H CH3 H CH3 Br H H CH3 H Br
5. Pairs of enantiomers are:
CH3 H3C Cl CH2CH2CH3 Cl CH3 CH3 CH2CH2CH3 H3C H Cl CH2CH2CH3
I
CH2CH2CH3 H3C H Cl
3. Hexane and 3-methylpentane are examples of: A) enantiomers. B) stereoisomers. C) diastereomers. D) constitutional isomers. E) None of these 4. I and II are:
A) B) C) D) E)
I
II constitutional isomers. enantiomers. diastereomers. identical. None of these
I
II
III
IV
V
A) B) C) D) E)
H CH3
Cl H
II II and III II and IV III and V IV and V
3环烷烃
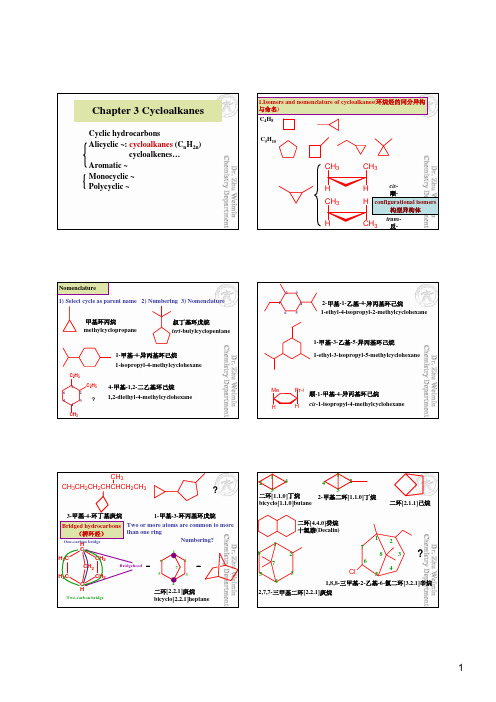
H
H
The least stable
Conformational inversion (ring flipping) in cyclohexane
The most important result of ring inversion is that any substituent (取 代基) that is axial in the original chair conformation becomes equatorial in the ring-flipped form and vice versa (反之亦然).
Start here or start here Then alternate to give
in which all the axial bonds are parallel to one another
(3) Place the equatorial bonds so as to approximate a tetrahedral arrangement of the bonds to each carbon. The equatorial bond of each carbon should be parallel to the ring bonds of its two nearest neighbor carbons.
A ring need not be planar!
CnH2n Small rings (小环): n=3,4 Normal rings (正常环): n=5,6,7 Medium rings (中环): n=8~11 Large rings (大环): n > 12
5.2 The sources of strain 1) Bond length distortion (键长伸缩张力): destabilization of a molecule that results when one or more of its bond distances are different from the normal values.
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Ring Strain and Stability
• Although the cycloalkanes show many of the same properties and chemistry as the acyclic alkanes, there are two additional features that result from the constraint of binding the chains into a ring: • Ring strain and conformational effect.
cyclobutane cyclopropane cyclopentane cyclohexane
Complex Cycloalkanes
• Naturally occurring materials contain cycloalkane structures • Examples: chrysanthemic acid (cyclopropane), prostaglandins (cyclopentane), steroids (cyclohexanes and cyclopentane)
Stereoisomers
• Compounds with atoms connected in the same order but which differ in three-dimensional orientation, are stereoisomers • The terms “cis” and “trans” should be used to specify stereoisomeric ring structures • Recall that constitutional isomers have atoms connected in different order
bicyclo[4.3.0]nonane
Spiro[4.5]decane
Bicyclic hydrocarbons
one-carbon bridge bridgehead carbons two-carbon bridge three-carbon bridge bicyclo[3.2.1]octane
Heats of formation per-CH2- for the cycloalkanes
n compound ΔHfo/n kcal/mol n 8 compound Cyclooctane ΔHfo/n kcal/mol -3.75
3 cyclopropane 4.25
4 cyclobutane
60o
90o
108o
120o
128.6o
135o
Conformation of Cycloalkane
Conformation of Cyclopropane
• 3-membered ring must have planar structure • Symmetrical with C–C–C bond angles of 60° • Requires that sp3 based bonds are bent (and weakened) – severe angle strain • All C-H bonds are eclipsed – torsional strain
Bicyclic and polycyclic compounds
• If two rings share two or more common atoms, the compound is called a bicyclic compound(桥环化合物). • If two rings have a single common atom, the result is called a spirocyclic compound (螺环化合物).
Conformations of Cyclobutane
• Cyclobutane has less angle strain than cyclopropane but more torsional strain because of its larger number of ring hydrogens • Cyclobutane is slightly bent out of plane - one carbon atom is about 25° above
5,7,7-Trimethyl-bicyclo[2.2.1]hept-2-ene
bicyclo[4.4.0]dec-1(6)-ene
Spirocyclic hydrocarbons
• The numbering of substituted bicyclic ring systems always assigns the 1 to one next to spiro-carbons in smaller ring. • Numbering proceeds around each bridge in turn, beginning with the smaller bridge. • Only after this rule is taken into account are the lowest numbers then assigned to double bonds and substituents
Bent bond
The Nature of Ring Strain
• Rings larger than 3 atoms are not flat • Cyclic molecules can assume nonplanar conformations to minimize angle strain and torsional strain by ring-puckering • Larger rings have many more possible conformations than smaller rings and are more difficult to analyze
bicyclo[4.3.0]nonane
carbon number of whole bicyclic ring carbon number of each bridge according bigger to small
• The numbering of substituted bicyclic ring systems always assigns the 1 to one of the bridgehead carbons. • Numbering proceeds around each bridge in turn, beginning with the largest bridge. • Only after this rule is taken into account are the lowest numbers then assigned to double bonds and substituents.
1.65
9
Cyclononane
-3.55
-4.95
5 cyclopentane -3.65
10 Cyclodecane
6 cyclohexane
-4.95
11 Cycloundecane
12 cyclododecane
-4.65
-4.55
7 cycloheptane -4.05
• Stability of cycloalkanes: 3<4<5<6>7>8>9
菊酸
前列腺素
可的松
Naming Cycloalkanes
• Rules are similar to those for open-chain alkanes • Count the number of carbons in the ring, and add the prefix cyclo- to the name of the corresponding alkane • If a substituent is present on the ring, count the number of carbon atoms in the ring and the number in the largest substituent chain. If the number of carbon atoms in the ring is equal to or greater than the number in the substituent, the compound is named as an alkyl-substituted cycloalkane • For substituted cycloalkane, start at a point of attachment as C1 and number the substituents on the ring. • If there are two or more substituents, begin numbering at the group with alphabetical priority, and give the other substituents the lowest possible number
Chapter 5 Cycloalkanes
Cycloalkanes
• Cycloalkanes are alkanes that have carbon atoms that form a ring (called alicyclic compounds) • Simple cycloalkanes rings of CH2 units, (CH2)n, or CnH2n • Structure is shown as a regular polygon with the number of vertices equal to the number of C’s (a projection of the actual structure)
