酸2
鲁教版化学九年级下册酸及其性质 第2课时 学案

7.1.2酸及其性质第二课时学案【学习目标】1. 通过阅读标签和观察思考,了解浓硫酸的物理性质;认识浓硫酸特性,初步学会浓硫酸稀释的方法。
2. 与盐酸对比,通过实验探究,掌握硫酸的化学性质,会写相关的化学方程式;(重点)了解硫酸的用途。
3. 通过盐酸、硫酸化学性质对比,总结出酸的通性,并了解酸具有通性的原因。
4. 通过盐酸与硫酸的区分,了解不同酸的个性。
【导学过程】一、知识链接,情境导入通过实验探究,我们了解了盐酸的化学性质。
硫酸又是怎样的呢?与盐酸相比有哪些相似的性质?有什么不同的性质?二、自主学习,合作探究学习任务一:认识浓硫酸(阅读观察)(知识梳理)浓硫酸具有性,可作为等气体的剂,但不能干燥碱性性气体如氨气(NH3);浓硫酸具有脱水性,可以使有机物发生碳化;具有强腐蚀性,若不慎将浓硫酸沾到皮肤上,处理方法是用冲洗,最后涂抹3%~5%的溶液;浓硫酸溶于水时放出大量的热,在稀释时,一定要把沿容器壁缓缓注入中,并不断,切不可将倒进里。
(交流共享)浓硫酸敞口放置一段时间,其溶液组成会发生怎样的变化?因此应保存。
学习任务二:硫酸的化学性质(问题引领)回忆盐酸的化学性质写出相对应的一个化学方程式:(1)盐酸能使紫色石蕊试液变;无色酚酞试液。
(2)盐酸+碱(3)盐酸+某些盐(4)盐酸+某些金属氧化物(5)盐酸+某些金属(实验探究)1.硫酸与指示剂:紫色石蕊;无色酚酞。
2.硫酸与氢氧化钠: H2SO4 + NaOH=3.硫酸与碳酸钠:现象;化学方程式 H2SO4+Na2CO3=硫酸与氯化钡:现象;化学方程式H2SO4+BaCl2=4.硫酸与铁锈(Fe2O3)现象:;化学方程式:5.硫酸与金属镁、铁、铜现象:Mg:;Fe ;Cu:无现象,不反应。
化学方程式:(知识梳理)1.盐酸与硫酸具有相似化学性质,因为它们溶于水时,都解离出共同的阳离子离子。
2.酸的通性(1)酸能使紫色石蕊试液变;无色酚酞试液。
(2)酸+碱→盐+水(反应)(生成物中有水)硫酸和氢氧化铜硫酸和氢氧化钡盐酸和氢氧化铁(3)酸+碳酸盐→新盐+碳酸(水+二氧化碳)( 反应)(生成物中有水和气体)盐酸和碳酸钙硫酸和碳酸钠盐酸和碳酸钠(4)酸+金属氧化物→盐+水( 反应)(生成物中有水)氧化铁和盐酸氧化铜和硫酸(5)酸+金属→盐+氢气( 反应)(条件:酸不能是浓硫酸或硝酸,有强氧化性;金属在金属活动性顺序中排在H前)镁和稀盐酸铝和稀盐酸锌和稀硫酸铁和稀硫酸3.金属活动性顺序:K Ca Na Mg Al Zn Fe Sn Pb(H)Cu Hg Ag Pt Au在金属活动性顺序中,排在氢前面的金属能与酸发生置换反应放出氢气,排在氢后面的金属不能与酸发生置换反应放出氢气。
邻苯二甲酸二(2-乙基己基)酯

【中文名称】邻苯二甲酸二(2-乙基己基)酯【英文名称】di(2-ethylhexyl)phthalate【中文同义词】邻苯二酸二异辛酯邻苯二甲酸二(2-乙基己基)酯邻苯二酸二异辛酯1,2-苯二甲酸二(2-乙基己基)酯邻苯二甲酸二(2-乙基已)酯邻苯二甲酸二(A-乙基己酯)邻苯二甲酸二(α-乙基己酯)邻苯二甲酸二辛酯(DOP)邻苯二酸二辛酯增塑剂DOP苯二甲酸二(异)辛酯邻苯二甲酸二(2-乙基)己基酯二辛基酞酸酯邻苯二甲酸二(α-乙基己)酯邻酞酸二辛酯酞酸双(2-乙基己基)酯鄰苯二甲酸二辛酯苯二甲酸二辛酯邻酞酸二正辛酯增塑剂DIOP邻苯二甲酸双(2-乙基己酯)邻苯二甲酸-2-乙基己酯二辛基鈉琥珀酸風【英文同义词】di-sec-octyl phthalate2-ETHYLHEXYL PHTHALATEBIS(2-ETHYLHEXYL) PHTHALATED1-2-ETHYLHEXYL PHTHALATEDI-2-ETHYLHEXYL PHTHALATE DIOCTYL PHTHALATE'DIOCTYL' PHTHALATEPHTHALIC ACID BIS(2-ETHYLHEXYL) ESTER PHTHALIC ACID BIS(2-ETHYLHEXYL) ESTERPHTHALIC ACID DI-2-ETHYLHEXYLPHTHALIC ACID DI(2-ETHYLHEXYL) ESTERPHTHALIC ACID DIOCTYL ESTER1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester1,2-Benzenedicarboxylic acid, bis(ethylhexyl) ester1,2-benzenedicarboxylicacid,bis(2-ethylhexyl)ester1,2-benzenedicarboxylicacid,bis(ethylhexyl)ester1,2-Benzenedicarboxylicacidbis(2-ethylhexyl)esterai3-04273Behp【CAS No.】117-81-7【分子式】C24H38O4【分子量】390.62危险性概述回目录【健康危害】吸入、摄入或经皮肤吸收后对身体有害。
2常见酸的酸性强弱的比较

2常见酸的酸性强弱的比较.doc
1. 盐酸和乙酸
盐酸是一种强酸,其酸性常数(pKa)约为-6.3,可以完全电离成氢离子和氯离子。
乙酸则是一种弱酸,其pKa约为4.8,只有一小部分分子会电离成氢离子和乙酰离子。
2. 硫酸和磷酸
硫酸是一种强酸,其pKa约为-3,可以完全电离成氢离子和硫酸根离子。
磷酸在水溶液中存在多种形式,其中H3PO4是一种弱酸,其pKa约为2.14,只有一小部分分子会电离成氢离子和磷酸根离子。
总体而言,强酸会更快地失去氢离子,因此其酸性会更强。
但是,弱酸的pKa值较低,也可能导致其在一定条件下具有比某些弱酸更强的酸性。
同时,该酸与其相应的碱的反应也会影响其酸性强度的表现。
第二章 酸碱理论

溶剂本身的酸碱性及其强弱,可从溶剂的亲质子性 能加以比较. 若以水为参照, 任何亲质子能力比水强的溶剂,就 是比水强的碱性溶剂;亲质子能力比水弱的溶剂, 就是比水强的酸性溶剂。
HAc
H2O
NH3
H2SO4
质子酸(碱)的强度通常可利用酸与参考碱(酸) 之间质子转移平衡进行定量比较: HB+B参考 HB参考+ B Ka
注意: 同一类硬酸(碱)或软酸(碱)中,软硬度也有差 别; 软酸: Hg>Cd>Zn 同一种元素,氧化态不同,则属于不同类酸碱; Fe3+, Fe2+, Fe 在分子或原子团中,取代基的电负性越大,给予体 或接受体原子的电子密度越小,有效核电荷增大, 对价电子抓得越紧,酸或碱的硬度也越大,反之, 则硬度越小。
[Co(NH3)5X]2+ [Co(CN)5X]3-
F-
I-
5.应用于催化体系 AlCl3硬酸
6.预测化学反应速度 通常生成硬-硬或软-软取代产物的反应速度比 较快。
2.3.3酸碱的软硬度
Ahland的酸碱软硬度标度和Pearson定性 分类的结论基本一致,只有H+,Fe3+和I-,CN有些例外,按H+和Fe3+的A值应该归入软酸 或交界酸,但按它们的性质,却属于硬酸。 I-和CN-有几乎同样的值,但从它们的化学性 质看, CN-的软度显然比I-大。 在应用酸碱的软硬度估计酸碱加合物稳定性 时必须同时考虑酸碱强度的影响。否则会对 酸碱反应的推动力得出错误的结论。
无机含氧酸的强度
无机含氧酸通式HOR,R可代表各种非金属原子,也 可代表一个多原子取代基。 HOR的酸性是由取代基R 来决定的。
周期表中同族元素(同氧化态)的电负性总是自下 而上依次递增,因此,任何一组氢氧化物的酸强度 将随原子序数的增大而减小,碱强度则随原子序数 的增大而增大。
2酸2碱4个盐用途+俗称
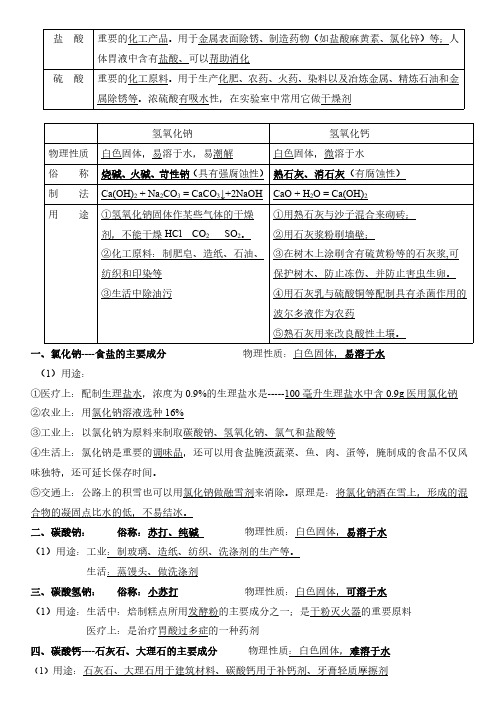
一、氯化钠----食盐的主要成分物理性质:白色固体,易溶于水(1)用途:①医疗上:配制生理盐水,浓度为0.9%的生理盐水是-----100毫升生理盐水中含0.9g 医用氯化钠②农业上:用氯化钠溶液选种16%③工业上:以氯化钠为原料来制取碳酸钠、氢氧化钠、氯气和盐酸等④生活上:氯化钠是重要的调味品,还可以用食盐腌渍蔬菜、鱼、肉、蛋等,腌制成的食品不仅风味独特,还可延长保存时间。
⑤交通上:公路上的积雪也可以用氯化钠做融雪剂来消除。
原理是:将氯化钠洒在雪上,形成的混合物的凝固点比水的低,不易结冰。
二、碳酸钠:俗称:苏打、纯碱物理性质:白色固体,易溶于水(1)用途:工业:制玻璃、造纸、纺织、洗涤剂的生产等。
生活:蒸馒头、做洗涤剂三、碳酸氢钠:俗称:小苏打物理性质:白色固体,可溶于水(1)用途:生活中:焙制糕点所用发酵粉的主要成分之一;是干粉灭火器的重要原料医疗上:是治疗胃酸过多症的一种药剂四、碳酸钙----石灰石、大理石的主要成分物理性质:白色固体,难溶于水(1)用途:石灰石、大理石用于建筑材料、碳酸钙用于补钙剂、牙膏轻质摩擦剂盐酸重要的化工产品。
用于金属表面除锈、制造药物(如盐酸麻黄素、氯化锌)等;人体胃液中含有盐酸、可以帮助消化硫酸重要的化工原料。
用于生产化肥、农药、火药、染料以及冶炼金属、精炼石油和金属除锈等。
浓硫酸有吸水性,在实验室中常用它做干燥剂氢氧化钠氢氧化钙物理性质白色固体,易溶于水,易潮解白色固体,微溶于水俗称烧碱、火碱、苛性钠(具有强腐蚀性)熟石灰、消石灰(有腐蚀性)制法Ca(OH)2+Na 2CO 3=CaCO 3↓+2NaOH CaO +H 2O =Ca(OH)2用途①氢氧化钠固体作某些气体的干燥剂,不能干燥HCl CO 2SO 2。
②化工原料:制肥皂、造纸、石油、纺织和印染等③生活中除油污①用熟石灰与沙子混合来砌砖;②用石灰浆粉刷墙壁;③在树木上涂刷含有硫黄粉等的石灰浆,可保护树木、防止冻伤、并防止害虫生卵。
邻苯二甲酸二(2-乙基已基)酯

鄰苯二甲酸二(2-乙基已基)酯[Bis (2-Ethylhexyl) Phthalate] 壹、物質確認(Substance Identification)一、物質名稱:鄰苯二甲酸二(2-乙基已基)酯二、CAS Number:117-81-7三、別名:(一) 1,2-BENZENEDICARBOXYLIC ACID, BIS(2-ETHYLHEXYL) ESTER(二) 1,2-BENZENEDICARBOXYLIC ACID, BIS(ETHYLHEXYL) ESTER(三) 2-ETHYLHEXYL PHTHALATE(四) AI3-04273(五) BEHP(六) BIS(2-ETHYLHEXYL)-1,2-BENZENEDICARBOXYLATE(七) BISOFLEX 81(八) BISOFLEX DOP(九) COMPOUND 889(十) DAF 68(十一) DEHP(十二) DI(2-ETHYLHEXYL)ORTHOPHTHALATE(十三) DI(ETHYLHEXYL) PHTHALATE(十四) DI-2-ETHYLHEXYLPHTHALATE(十五) DIETHYLHEXYL PHTHALATE(十六) DOF (RUSSIAN PLASTICIZER)(十七) DOP(十八) Di-sec-octyl phthalate(十九) Dioctyl phthalate(二十) ERGOPLAST FDO(二十一) ERGOPLAST FDO-S(二十二) ETHYLHEXYL PHTHALATE(二十三) EVIPLAST 80(二十四) EVIPLAST 81(二十五) FLEXIMEL(二十六) FLEXOL DOP(二十七) FLEXOL PLASTICIZER DOP(二十八) GOOD-RITE GP 264(二十九) HATCOL DOP(三十) HERCOFLEX 260(三十一) KODAFLEX DOP(三十二) MOLLAN O(三十三) NCI-C52733(三十四) NUOPLAZ DOP(三十五) Octoil(三十六) Octyl phthalate(三十七) PALATINOL AH(三十八) PHTHALIC ACID DIOCTYL ESTER(三十九) PHTHALIC ACID, BIS(2-ETHYLHEXYL) ESTER (四十) PITTSBURGH PX-138(四十一) PLATINOL AH(四十二) PLATINOL DOP(四十三) Pesticide Code 295200(四十四) RC PLASTICIZER DOP(四十五) REOMOL D 79P(四十六) REOMOL DOP(四十七) SICOL 150(四十八) STAFLEX DOP(四十九) TRUFLEX DOP(五十) VESTINOL AH(五十一) VINICIZER 80(五十二) WITCIZER 312四、分子式:C24-H38-O4五、美國環保署有害廢物編號(EPA Hazardous Waste Number):U028六、相關化學物質(Associated Chemicals):CAS 4376-20-9[Mono-(2-ethylhexyl)phthalate] 貳、製造及使用(Manufacturing/Use Information)一、製造方法(Methods of Manufacturing):1.製備方法(Preparation)︰GARNER, WATSON, US PATENT 2,508,911 (1950 TO SHELL); BRITISH PATENT 747,260 (1956 TO CHEMISCHE WERKE HULS).2.酯化(Esterification):2-乙基已基醇(2-Ethylhexanol) + 鄰苯二甲酸酐(Phthalic Anhydride)二、製劑/製備(Formulations/Preparations)︰USEPA/OPP 殺蟲劑代碼(Pesticide Code):295200。
2-磷酸甘油酸

2-磷酸甘油酸(英语:2-phosphoglycerate)是生物界中常见的化学பைடு நூலகம்子,一般出现在糖解作用与糖质新生作用的过程中。
在糖解作用中,2-磷酸甘油酸是3-磷酸甘油酸在磷酸甘油酸变位酶(Phosphoglycerate)的催化之下产生,2-磷酸甘油酸第2个碳上所接的磷酸根,是来自变位酶。这个反应需要镁离子或其他2价阳离子的参与。
1.3常见的酸(第2课时)(教学教学设计)-九年级科学上册同步高效课堂(浙教版)

针对本节课的教学反思与总结,我提出以下改进措施和建议:
1. 加强实验操作指导,确保每位学生都能熟练掌握实验技能。
2. 加强学生的表达训练,提高他们的语言组织和表达能力。
3. 注重课堂纪律,鼓励每位学生积极参与课堂讨论。
4. 继续丰富教学方法,提高学生的学习兴趣和参与度。
5. 关注学生的个体差异,因材施教,提高教学效果。
(4)盐酸和硝酸的工业制备方法:介绍盐酸和硝酸的工业制备方法,以及其在工业生产中的应用。
(5)酸碱中和反应在环境保护中的应用:让学生了解酸碱中和反应在处理工业废水、降低土壤酸碱度等方面的应用。
2. 鼓励学生进行课后自主学习和探究
(1)调查生活中常见的酸及其应用:让学生观察和记录生活中常见的酸,了解其用途,如醋酸在烹饪中的应用、柠檬酸在饮料制作中的应用等。
(4)拓展学习的创新性想法或建议:结合所学知识,提出在生活或工作中应用酸的新思路或改进方案。
学生应在课后充分利用网络资源、图书馆等途径,进行拓展学习,提高自身的综合素质和能力。教师应关注学生的学习需求,提供必要的支持和指导,帮助学生在拓展学习中取得更好的成果。
八、教学反思与总结
教学反思:
在教学方法方面,我采用了讲授法、案例研究法和实验教学法,力求让学生在理论学习与实践操作中深入理解酸的性质和应用。在课堂互动方面,通过提问、小组讨论和角色扮演等环节,激发学生的积极性和参与感。然而,在实验操作环节,我发现部分学生对实验仪器和操作方法不够熟悉,导致实验效果不理想。在今后的教学中,我需要加强实验操作的指导,确保每位学生都能熟练掌握实验技能。
食用油中邻苯二甲酸二(2-乙基)己酯(dehp)不合格项目小知识_概述

食用油中邻苯二甲酸二(2-乙基)己酯(dehp)不合格项目小知识概述1. 引言1.1 概述食用油是人们日常生活中必不可少的食品材料之一,其品质和安全关系到人们的健康。
然而,近年来,食用油中检出邻苯二甲酸二(2-乙基)己酯(DEHP)的不合格情况时有发生,引起了广泛关注。
DEHP是一种常见的增塑剂,被广泛应用于各类橡胶制品、塑料制品以及涂层等工业领域。
虽然DEHP在工业用途上具有一定价值,但其在食用油中超标存在一定的健康风险。
1.2 文章结构本文将围绕"食用油中DEHP不合格项目小知识"展开论述。
文章将分为以下几个部分:引言、食用油中DEHP介绍、健康风险、法规标准与监测方法、DEHP 来源与检测方法、影响因素及危害性评估、控制与预防措施以及实际案例分析等内容。
1.3 目的本文旨在概述食用油中邻苯二甲酸二(2-乙基)己酯(DEHP)不合格项目的相关知识,包括其基本介绍、健康风险、法规标准与监测方法,以及DEHP来源与检测方法、影响因素及危害性评估等内容。
通过对食用油中DEHP超标案例的分析和总结,提出对食品安全监管的启示和建议,以保障公众的食品安全与健康。
2. 食用油中邻苯二甲酸二(2-乙基)己酯(DEHP):2.1 基本介绍:邻苯二甲酸二(2-乙基)己酯(DEHP)是一种常见的塑化剂,广泛应用于软质聚氯乙烯(PVC)制品中。
它具有优良的柔软性和可塑性,并能提高产品的耐磨性和抗老化性能。
由于其广泛使用,DEHP也容易被食品接触材料中的食用油所污染。
2.2 健康风险:DEHP被认为是一种潜在的致癌物质,并且对生殖系统和内分泌系统有一定的影响。
动物实验表明,长时间暴露于高浓度的DEHP可能引起生殖毒性、肝脏损伤、免疫功能异常等问题。
此外,婴儿和儿童对DEHP的敏感度较高,他们在摄入含有DEHP的污染食品后,可能更容易受到影响。
2.3 法规标准与监测方法:针对食用油中DEHP含量的限制,在很多国家和地区都有相应的法规标准。
山西师范大学高等无机课件 第二章酸碱理论

③ 含有碳一碳双键的分子 碳一碳双键处具有 较高的电子密度。反应中可以提供π电子给金属 离子,以形成配位共价键。最熟悉的例子就是蔡 斯 盐 K[Pt(C2H4)Cl3] 。 在 蔡 斯 盐 中 , 乙 烯 提 供 的 共享电子是π电子,而不是σ电子。Pt2+跟乙烯结 合不是通过某个碳原子,而是通过π电子云,它 跟两个碳原子保持相等距离,即:
① 阴离子 常见的阴离子Lewis碱有 F-,C1—、 Br-、OH- 、CN—等。实际上只要Lewis酸具 有足够的强度,任何阴离子都可以是Lewis碱。
② 具有孤对电子的中性分子 最常见的例子有 氨 、 胺 和 水 等 。 此 外 象 CO 、 CHOH 、 CH3COCH3等也可用作Lewis碱。
加合物中的一个酸或一个碱也可以被另一酸或
碱所取代,如
Al(H2O)63+ + 6F-
AlF63- + 6H2O
加合物(1) + 碱(2)
加合物(2) + 碱(1)
两种无水氧化物生成盐的反应可以看作是Lewis酸
一 碱 反 应 生 成 加 合 物 的 过 程 。 如 CaO 与 SiO2 的 反 应,可以看作是SiO2作为酸与O2-作为碱的反应
“供给”电子对的粒子是碱,而“接受”电 子对的粒子是酸。酸碱通过电子对授受关系形 成配位共价键,生成酸碱加合物,即
A + :B → A :B(A←B) 酸 + 碱 → 酸碱加合物(配合物) H+ + OH- → H2O Ag+ + 2 :NH3 → Ag(NH3)2+ Ni + 4 :CO → Ni(CO)4 BF3 + :F— → BF4— BCl3 + NH3 → C13B—NH3
依地酸二钠化学式-概述说明以及解释

依地酸二钠化学式-概述说明以及解释1.引言1.1 概述概述:依地酸二钠,化学式为Na2EDTA(英文全称为Disodium Ethylenediaminetetraacetate),是一种重要的有机化合物。
它是由四个乙二胺羧酸基团与两个钠离子组成的配合物。
依地酸二钠在化学和生物学领域中广泛应用,具有多种特殊的化学性质和物理性质。
本文将详细介绍依地酸二钠的化学性质、物理性质以及其在各个应用领域的具体应用。
首先,将对依地酸二钠的化学性质进行探索,包括其化学结构、分子量、酸碱性等方面的特点。
然后,将阐述依地酸二钠的物理性质,如颜色、溶解性等。
最后,将重点介绍依地酸二钠在许多领域的应用,如工业生产、医药、环境保护等。
通过深入了解依地酸二钠的特性及其应用领域,可以更好地认识该化合物的价值和潜力。
本文的目的在于全面展示依地酸二钠的化学性质、物理性质和应用领域。
通过对该化合物的深入研究,有助于人们更好地理解和利用依地酸二钠。
同时,对依地酸二钠的未来发展进行展望,以期为相关领域的科研人员提供参考,推动依地酸二钠的应用进一步发展。
接下来,将分别详细介绍依地酸二钠的化学性质、物理性质以及其在各个应用领域的具体应用,从而全面了解依地酸二钠的特点和潜力。
1.2文章结构文章结构本文共分为引言、正文和结论三个部分。
引言部分将对依地酸二钠进行概述,介绍其化学性质、物理性质和应用领域等主要内容。
正文部分将详细探讨依地酸二钠的化学性质、物理性质和应用领域。
其中,2.1小节将介绍依地酸二钠的化学性质,包括其化学式、结构和化学反应等方面的内容;2.2小节将探讨依地酸二钠的物理性质,包括其外观、溶解性、熔点等方面的特点;2.3小节将对依地酸二钠的应用领域进行分析,包括其在医药领域、化工领域和农业领域的应用等方面。
结论部分将总结依地酸二钠的特点,归纳其主要的化学性质、物理性质和应用领域,并对其未来发展进行展望。
最后,以简洁的结束语结束全文。
二聚酸二缩水甘油酯结构式-概念解析以及定义

二聚酸二缩水甘油酯结构式-概述说明以及解释1.引言1.1 概述二聚酸二缩水甘油酯是一种重要的化合物,具有广泛的应用价值。
它具有良好的渗透性和吸附性能,能够在多种领域发挥作用。
本文将对二聚酸二缩水甘油酯的结构和性质进行深入探讨,通过对其化学特性和应用领域的研究,探讨其在材料科学、医药和化工等领域的潜在应用。
希望通过本文的介绍,能够加深对二聚酸二缩水甘油酯的认识,并为其进一步研究和应用提供参考。
1.2 文章结构文章结构部分内容:文章将主要分为引言、正文和结论三部分。
在引言部分,将介绍二聚酸二缩水甘油酯的概述、文章的结构以及文章的目的。
在正文部分,将详细介绍二聚酸的性质、二缩水甘油酯的结构以及二聚酸二缩水甘油酯的应用。
最后,在结论部分将总结二聚酸二缩水甘油酯的特点,展望未来研究方向并得出结论。
整篇文章将以系统、全面的方式介绍二聚酸二缩水甘油酯的相关内容,为读者提供深入了解该化合物的知识和应用价值。
1.3 目的本文旨在深入探讨二聚酸二缩水甘油酯的结构式及其性质,并对其在不同领域的应用进行详细分析。
通过对二聚酸二缩水甘油酯的研究,我们旨在全面了解其在化学、医药、食品等领域的潜在用途,并为未来的科研和工程应用提供重要参考。
同时,本文还将对二聚酸二缩水甘油酯的特点进行总结,并展望未来可能的研究方向,以期为相关领域的科学家和工程师提供有益的启示和建议。
通过本文的深入研究,我们希望能够全面了解二聚酸二缩水甘油酯的结构与性质,并为其在工业及科研领域的进一步应用指明方向。
2.正文2.1 二聚酸的性质二聚酸是一种具有特殊化学性质的化合物,其分子结构中含有两个羧基(-COOH)。
二聚酸具有以下主要性质:1. 酸性:二聚酸在水中能够释放出氢离子,呈酸性。
其酸性强弱取决于羧基的离子化程度,通常情况下,二聚酸呈现出中等到较强的酸性。
2. 稳定性:二聚酸在常温下具有一定的稳定性,不易发生分解反应。
但在高温、高压或强酸碱环境下,会发生分解或降解反应。
l-抗坏血酸-2-磷酸酯质量标准

l-抗坏血酸-2-磷酸酯质量标准下载提示:该文档是本店铺精心编制而成的,希望大家下载后,能够帮助大家解决实际问题。
文档下载后可定制修改,请根据实际需要进行调整和使用,谢谢!本店铺为大家提供各种类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by this editor. I hope that after you download it, it can help you solve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you! In addition, this shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts, other materials and so on, want to know different data formats and writing methods, please pay attention!l抗坏血酸2磷酸酯质量标准一、引言随着人们对健康意识的提高,l抗坏血酸2磷酸酯作为一种重要的抗氧化剂,在医药、食品和化妆品等领域有着广泛的应用。
四氢邻苯二甲酸二缩水甘油酯结构式_概述说明

四氢邻苯二甲酸二缩水甘油酯结构式概述说明1. 引言1.1 概述四氢邻苯二甲酸二缩水甘油酯,又称为加醚明,是一种重要的化学物质。
其化学名称为四氢邻苯二甲酸2,3-二(缩水)甘油酯,分子式为C20H34O8。
该物质具有特殊的结构和性质,在医药、工业和生物学等领域中具有广泛的应用前景。
1.2 文章结构本文将从以下几个方面对四氢邻苯二甲酸二缩水甘油酯进行全面介绍:第一部分是引言。
该部分将对四氢邻苯二甲酸二缩水甘油酯的概述、文章结构以及研究目的进行详细阐述;第二部分是关于四氢邻苯二甲酸二缩水甘油酯结构式的介绍。
包括化学名称和分子式、结构特点以及物理性质等内容;第三部分是探讨四氢邻苯二甲酸二缩水甘油酯在不同领域中的应用。
主要包括医药领域应用、工业领域应用和生物学领域应用等方面;第四部分是关于四氢邻苯二甲酸二缩水甘油酯合成方法与制备工艺的介绍。
包括常见的合成方法介绍、制备工艺条件说明以及反应机理的分析等内容;最后一部分是结论与展望,对本文进行总结,并对未来研究方向给予展望。
1.3 目的本文旨在详细介绍四氢邻苯二甲酸二缩水甘油酯的结构式、物理性质以及其在不同领域中的应用。
同时,将探讨该化合物的合成方法与制备工艺,并进行反应机理的分析。
通过本文,读者将能够了解到四氢邻苯二甲酸二缩水甘油酯这一重要化学物质的基本信息与潜在价值,为相关研究提供参考和指导。
2. 四氢邻苯二甲酸二缩水甘油酯的结构式:2.1 化学名称和分子式:四氢邻苯二甲酸二缩水甘油酯的化学名称是1,2,3,4-四羟基环戊基甲烷四酸二缩水戊醇酯,其分子式为C12H22O11。
2.2 结构特点:四氢邻苯二甲酸二缩水甘油酯是一种多环脂肪族化合物,由四个羟基环戊基甲烷单元与两个缩水戊醇单元通过酯键相连接而成。
其结构如下图所示:HOOC-CH(CO)O-CH₂-CH₂-O-CO-(CH)COOH|O-CH₂-CH₂其中,HOOC代表羧基,CO代表氧单键。
这种化合物具有稳定的脂肪族结构。
酸组词造句二年级简单

酸组词造句二年级简单
1. 小明尝了一口酸橙汁,觉得非常酸。
2. 妈妈给我买了一颗酸葡萄,我吃了一口,皱起了眉头。
3. 姐姐用酸奶做了一份酸甜可口的水果沙拉。
4. 小猫咬了一口酸柠檬,吃完后立刻皱起了嘴巴。
5. 弟弟喜欢吃酸糖,每次吃完都露出酸酸的表情。
6. 妹妹在超市里发现了一种酸酸的葡萄糖果,非常喜欢。
7. 奶奶做的酸菜鱼非常好吃,酸中带着一丝辣味。
8. 哥哥喜欢喝酸梅汤,觉得又酸又凉爽。
9. 弟弟不喜欢吃酸的水果,他更喜欢甜甜的苹果。
10. 妈妈给我买了一袋酸葡萄糖,我和朋友们一起分享了。
癸二酸二(2—乙基己)酯规范

G J B 中华人民共和国国家军用标准FL6810GJB 1967—94癸二酸二(2—乙基己)酯规范S pe cif ic ati on fo r di—2—e thy lh exy l seb ac ate1994—09—12发布 1995—04—01实施国防科学技术工业委员会批准中华人民共和国国家军用标准癸二酸二(2—乙基己)酯规范 GJB 1967—94Speci fica tio n fo r d i—2—e thyl hexy l s ebac ate1 范围1.1 主要内容本规范规定了癸二酸二(2—乙基己)酯的要求、质量保证规定、交货准备及说明事项。
1.2 使用范围本规范适用于工业癸二酸二辛脂经精制而成的特级品2 引用文件GB 191—90 包装储运图示标志GB 601—88 化学试剂滴定分析(容量分析)用标准溶液的制备GB 603—88 化学试剂试验方法中所用制剂及制品的制备GB 1664—88 增塑剂外观色泽的测定(铂—钴比色法)GB 1699—88 增塑剂加热减量的测定GB 4472—84 化工产品密度、相对密度测定通则GB 6283—86 化工产品中水分含量的测定卡尔·费休法(通用方法)GB 6489.2—86 工业用邻苯二甲酸酯类的检验方法酸度的测定GB 6678—86 化工产品采样总则GB 6680—86 液体化工产品采样通则GB 6682—92 分析实验室用水规格和试验方法ZBG 71006—89 工业癸二酸二辛脂1985年国家标准局发布《国家产品质量仲裁检验暂行办法》3 要求3.1 理化性能癸二酸二(2—乙基己)酯的物理和化学性能,按本规范规定的方法检验时,应符合表1的要求。
表13.2 外观微黄色透明液体,无可见机械杂质。
4 质量保证规定4.1 检验责任除合同或订单中另有规定外,承制方应负责完成本规范规定的所有检验。
必要时订购方或上级检定机构有权对本规范所述的任一检验项目进行检验。
二元酸酸值规律

二元酸酸值规律是一个化学概念,它描述了含有两个氢原子的酸的性质和行为。
在有机化学中,二元酸是指分子中含有一个羧基的酸,如乙酸、丁二酸等。
这些酸在有机合成、药物合成和材料科学等领域有着广泛的应用。
酸值是衡量有机物中羧酸含量的一项指标,它表示羧酸分子中羧基对氢离子(H+)的亲和能力。
具体来说,酸值是指中和一定体积的有机物溶液所需的氢氧化钾(KOH)的毫克数。
因此,酸值越大,说明羧酸的酸性越强,含有羧基的分子越多。
根据二元酸酸值规律,我们可以得出以下结论:1. 酸性递增性:随着分子中碳原子数的增加,二元酸的酸性逐渐增强。
这是因为羧酸的酸性取决于羧基中碳氧双键的稳定性,碳原子越多,双键越稳定,酸性越弱。
2. 酸性差异:不同二元酸的酸性差异很大。
一般来说,具有相同碳原子数的二元酸,脂肪酸的酸性比芳香酸的强,而具有相同脂肪酸结构的二元酸,直链状的比具有支链状的酸性更强。
这是因为脂肪酸的羧基中的氢原子可以更自由地与碱反应,而芳香酸的羧基中的氢原子受到共轭效应的影响,不易被碱所中和。
3. 酸值与二元酸浓度:在一定范围内,二元酸的浓度越高,其酸值也越高。
这是因为浓度较高的二元酸溶液中含有更多的羧酸分子,因此更容易与碱反应生成盐和水。
4. 多元酸的酸性:二元酸不是指含有一个以上羧基的酸的唯一类型。
实际上,许多有机物中含有多个羧基,如多元羧酸、多聚羧基化合物等。
这些物质通常具有很强的酸性,能够与碱发生强烈的反应。
在实际应用中,二元酸酸值规律对于合成化学品的生产、药物合成和材料科学等领域具有重要的指导意义。
例如,在生产某些有机物时,可以通过控制二元酸的浓度和种类来提高产品的质量和产量;在药物合成中,二元酸可以作为药物的活性成分之一,其种类和浓度会影响药物的疗效和安全性;在材料科学领域,二元酸可以用于制备具有特殊性能的有机材料,如具有生物相容性的药物载体等。
总之,二元酸酸值规律在化学领域具有重要的应用价值,它可以帮助我们更好地了解二元酸的性质和行为,指导化学合成、药物合成和材料科学等领域的研究和实践。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Benzoylation of Anisole over Silicotungstic Acid Modified Mesoporous AluminaS.Selvakumar ÆA.P.SinghReceived:15September 2008/Accepted:23October 2008/Published online:11November 2008ÓSpringer Science+Business Media,LLC 2008Abstract Mesoporous alumina (MA)molecular sieves were synthesized by using aluminum sec-butoxide as Al precursor and lauric acid as the structure directing agent.The synthesized MA was functionalized with silicotungstic acid (STA)via wet impregnation method and characterized by various physico-chemical techniques.The XRD patterns of a series of HPA functionalized MA are showing the ordered structures.N 2sorption analysis shows type IV isotherm.NH 3–TPD measurements revealed an increase in number of acid sites with an increase in loading of STA over MA.At the same time decrease in the acidity was observed with the increase in calcination temperature of the supported materials.Functionalization of STA were also carried out over different alumina supports such as catapol-B (CB)and alumina synthesized without surfactant (ASW)and their activities were evaluated by carrying out liquid phase Friedel–Craft acylation (FC)reaction of anisole with p -toluoyl chloride in a batch reactor at 120°C.Recycling was performed in the FC reaction using 30wt%STA–MA two times and no major deactivation of the catalyst was observed.Keywords Mesoporous alumina ÁSilicotungstic acid ÁFriedel–Craft acylation1IntroductionSynthesis of highly acidic inorganic materials by heterog-enization of homogeneous systems is currently the subjectof a great deal of research in green chemistry that aims to facilitate the recovery of the catalyst and to minimize the pollution.Many acidic catalysts have been developed using silica,titania,metal oxides,and microporous zeolites as supports [1–5].However,there are still many problems because of limited acidity and diffusion.After the suc-cessful synthesis of mesoporous silica by Mobil researchers,[6,7]many attempts have been made to pre-pare mesoporous non siliceous materials by using the concept of the surfactant-templating route.Niobium,haf-nium,and cerium based materials are now frequently cited [8–10].However,as noted in a review by Sayari and Liu [11],although a large number of elements are able to form such materials,only few of these exhibits ordered porous structures.Among the different non siliceous mesoporous materials porous alumina is attractive with broad applica-bility as adsorbents,catalyst supports,and as part of bifunctional catalysts in large-scale processes in the chemical and petrochemical industry.However,its non uniform pore size,low porosity,and low surface area limit its potential application for catalyzing reactions.The pos-sibility of obtaining such material with a mesoporous texture has made this oxide even more interesting.Due to the importance of alumina in catalysis,the ability to tailor its pore system is needed,and thus,several attempts have been made to synthesize mesoporous aluminas.Various surfactants have been used as template to direct the for-mation of mesostructures via interactions between the organic templates and the inorganic precursors,e.g.via hydrogen bonding or electronic interactions.Pinnavaia et al.were the first to report the preparation of meso-structured wormhole-like alumina from aluminum tri-sec-butoxide in the presence of electrically neutral,block-copolymer surfactants as structure-directing agents [12,13].Similar wormhole structures have also beenS.Selvakumar ÁA.P.Singh (&)Inorganic and Catalysis Division,National Chemical Laboratory,Pune 411008,Indiae-mail:ap.singh@ncl.res.inCatal Lett (2009)128:363–372DOI 10.1007/s10562-008-9755-3synthesized by the hydrolysis of aluminum alkoxides assisted by the anionic surfactant sodium dodecylben-zenesulfonate[14]or the cationic surfactant cetyltrimethyl-ammonium bromide[15].Friedel–Crafts acylation and benzylation of aromatic compounds is an important transformation in organic synthesis leading to aromatic ketones.Traditionally,these reactions are carried out by using homogeneous catalysts such as AlCl3,BF3,TiCl4,SnCl4or strong protic acids (such as HF and H2SO4).However,the commonly used homogeneous Lewis acid catalysts pose several problems, such as difficulty in separation and recovery from the reaction products,use in more than a stoichiometric amount of a Lewis acid is needed in many cases due to coordination of the Lewis acids to aromatic ketones pro-duced.The workup commonly requires hydrolysis of the complex,leading to the loss of the catalyst and giving large amounts of corrosive waste streams.Heteropoly acids(HPAs)are Bronsted acids composed of heteropoly anions and protons as countercations.HPAs are stronger than many conventional solid acids such as mixed oxides and zeolites.HPAs catalyze a wide variety of reactions in homogeneous phase offering strong option for efficient and cleaner processing.It has been recognized that a major drawback of HPA is its low thermal stability when applied to high-temperature reactions,low surface area (1–10m2/g)and separation problem from reaction mixture [16].Although the thermal stability depends on the struc-ture and composition of HPAs,the total decomposition of Keggin structure generally occurs at temperatures up to 723–823K to give the mixed oxides composed of HPAs [17].HPAs can be made ecofriendly insoluble solid acid with high thermal stability and high surface area by sup-porting them onto suitable supports.The support provides an opportunity to HPAs to be dispersed over a large surface area,which increases catalytic activity.Major factors contributing to the catalytic activity are nature of support, loading and conditions of pretreatment.Various supports like silica,[18–22]titania[23,24]active carbon[25–28] MCM-41[29–31],acidic ion exchange resins[32]have been used for supporting HPAs.Hence,we thought that the immobilization of HPAs over mesoporous alumina would develop a novel class of heterogeneous solid acid catalysts with enhanced acidity.Moreover,the use of a heteroge-neous HPAs system would offer ease of catalyst recovery and reuse,and minimize the production of waste currently formed during recovery.In this paper,we disclose the preparation of silico-tungstic acid(STA)functionalized mesoporous alumina using mesoporous alumina as solid support and STA as an acidic component.The nature of support and support-STA interaction was determined by different techniques.The activity of the catalyst was examined by Friedel–Craft(FC)acylation reaction of anisole with p-toluoyl chloride(p-TC) to4-methyl-40-methoxy benzophenone(4,40-MMBP).2Experimental2.1MaterialsAluminium tri-sec-butoxide,lauric acid,and1-propanol were procured from across organics,Aldrich and Merck, respectively.Silicotungstic acid(H4SiW12O40Áx H2O)was purchased from Loba chemie limited.Catapol-B is a boehmite form of Al2O3was purchased from Condea-German chemical company,having the surface area 240m2g-1.All the chemicals were research grade and used as received without further purification.2.2Preparation of Mesoporous Alumina(MA) Mesoporous alumina was prepared from the gel with the following composition,aluminium tri-sec-butoxide/lauric acid/1-propanol/H2O1:0.3:30:3.1[14].Typically,an alu-minium hydroxide suspension was prepared by the hydrolysis of54.8g of aluminium tri-sec-butoxide with12. 4g of deionised water in400g of1-propanol(99%).After stirring for1h,13.6g of lauric acid(99.5%)was added slowly to the gel mixture.The mixture was aged for24h at room temperature and then heated under static conditions at110°C in a round bottomedflask for2days.The solid material obtained was thenfiltered,washed with ethanol and dried at100°C for4–5h.Finally,the material was calcined at550°C with a temperature ramp of1°C/min from room temperature to thefinal temperature.The catalysts were prepared by wet impregnation method using mesoporous alumina as the support.To a methanolic solution of silicotungstic acid,mesoporous alumina powder was added and the mixture was stirred for 8–10h.The excess of methanol was evaporated to dryness and the obtained product was dried at120°C and calcined in air at250°C[33].A series of catalysts with different STA loading(5–40%)on mesoporous alumina was pre-pared and calcined at250°C.In order to study the influence of calcination temperature on the catalytic activity,catalyst with30wt%STA loading on mesoporous alumina was calcined at three different temperatures(250, 450and650°C).2.3CharacterizationSynthesized catalysts were characterized by X-ray dif-fraction using a Rigaku Miniflex powder diffractometer on finely powdered samples using Cu K a radiation and30kV and15mA.The XRD patterns were recorded for2h364S.Selvakumar,A.P.Singhbetween0.5and5then10–80,at a scan rate of2°/min. Adsorption of nitrogen was carried out at77K using a NOVA1200(Quanta chrome)apparatus for analyzing surface areas and pore-size distributions of the synthesized catalysts.Specific surface areas were calculated following the BET procedure.Pore-size distribution was obtained by using the BJH pore analysis applied to the desorption branch of the nitrogen adsorption/desorption isotherms. FT-IR spectra of solid samples were taken in the range of 4000–400cm-1on a Shimadzu FT-IR8201instrument. Thermo gravimetric analyses(TGA and DTA)were per-formed using a Diamond TG/DTA instrument,from30to 1000°C at a heating rate of10°C/min under airflow.NMR spectra were recorded on a DPX200Bruker spectrometer. Experiments on the saturation adsorption of ammonia and its subsequent temperature-programmed desorption(TPD–NH3)were performed on a Micromeritics Autochem2910 instrument.About0.1g of a fresh sample was placed in a U-shaped,flow-through,quartz micro reactor for each experiment.The catalyst was activated at220°C for2h under Heflow(30mL/min)and then cooled to100°C before being exposed to ammonia.The sample wasflushed again in He for1h to remove any physisorbed ammonia, and a desorption profile was then recorded by increasing the sample temperature from100to900°C at a ramp rate of10°C/min.2.4Friedel–Craft Acylation Reaction of Anisolewith p-toluoyl ChlorideAnisole and p-toluoyl chloride were used without further purification.The catalyst was activated at100°C for2h before use in the experiments,so as to maintain the dry conditions.The FC reaction was performed in a50mL round bottomflaskfitted with a condenser.The tempera-ture of the reaction vessel was maintained using an oil bath. In a typical run,a mixture of anisole(5mmol),p-toluoyl chloride(5mmol)and activated catalyst(0.1gm),was magnetically stirred and heated to attain the reaction tem-perature(120°C).The product samples were withdrawn at regular intervals of time and analyzed periodically on a gas chromatograph(Agilent6890N)equipped with aflame ionization detector and a capillary column(5l m thick cross-linked methyl silicone gum,0.2mm950m long). The main product,4-methyl-40-methoxy benzophenone is separated by column chromatography and confirmed by1H and13C NMR analysis.13C NMR(CDCl3,50MHz)d21.5 (–CH3),55.36(–OCH3),195.27(–CO–),135.36(1), 130.31(2,6),128.77(3,5),142.52(4),129.89(10),132.32 (20,60),113.37(30,50),162.92(40).1H NMR(CDCl3, 200MHz)d 2.43(S,3H), 3.87(S,3H), 6.95(D, J=8.84Hz,2H),7.26(D,J=7.83Hz,2H),7.67(D, J=8.21Hz,2H),7.81(D,J=8.85Hz,2H).3Results and Discussion3.1X-ray Diffraction(XRD)The powder X-ray diffraction(XRD)patterns of all the synthesized catalysts are shown in Fig.1.The small angle X-ray diffraction patterns of calcined and STA modified samples show an intense single peak at high‘d’spacing typically observed for mesoporous materials featuringBenzoylation of Anisole over Silicotungstic Acid365randomly order pores[34]whereas STA loaded catapol-B and alumina synthesized without surfactant didn’t show such peak.An increase in the intensity of the XRD line is observed upon removal of the surfactant from the MA (Fig.1A).This may be because of the electron density between the inorganic walls and the pores become pro-gressively larger as the organic is decomposed[14]. Figure1A shows that the mesoporous structure remained intact up to30wt%STA loading and further loading looses its mesoporosity subsequently.Figure1B,C show the wide angle XRD pattern of the as-synthesized and calcined mesoporous alumina along with STA loaded MA. The high angle reflection of mesoporous alumina is showing the c-phase[35]and STA modified materials start showing bulk STA peaks above30wt%loading.This indicates that30wt%loading of STA on MA is optimum and STA is well dispersed uniformly inside the pores of mesoporous channels.3.2Nitrogen Sorption StudiesThe textural property of plain,modified mesoporous alumina,catapol-B and alumina synthesized without sur-factant are shown in Table1.All the isotherms show type IV behaviour demonstrated for mesoporous materials.The surface area,pore volume and pore diameter of the cal-cined MA is found to be295m2g-1,0.56cm3g-1and 50A˚,respectively,which decreased to214m2g-1, 0.25cm3g-1and41.76A˚,respectively,after30wt%STA loading on MA.The decrease in pore volume and pore diameter of the STA modified MA shows the proper impregnation of the materials inside the MA.The pore volume and surface area of the30wt%STA loaded commercial alumina(catapol-B)is lower than that of the 30wt%STA loaded MA.The pore size distribution(PSD) curve of alumina synthesized without surfactant(ASW) and catapol-B(CB)(Fig.2A)are very broad and indicates that these materials are having a very broad range of mesopores.3.3FT-IR SpectraThe as-synthesized,calcined and supported mesoporous alumina with STA were analyzed by FTIR in order to confirm the presence of the Keggin anion on Al2O3 mesophase.Figure3A shows the FTIR spectra of as-syn-thesized,calcined and STA modified aluminas.All the samples show a strong and broad band in the region of 3600–3200cm-1corresponding to the hydrogen bonded–OH group.Similarly a broad band centered at1640cm-1is seen in all samples corresponding to the bending mode of the adsorbed water molecule.The as-synthesized material shows band in the region of2800–2900cm-1corre-sponding to the–C–H stretching frequency of the surfactant.The Al–O–Al bending modes(symmetric and asymmetric)are observed at1077and1165cm-1, respectively[36].Figure3B shows the FTIR spectra of(a) 30wt%STA loaded MA(b)calcined MA(c)20wt%STA loaded MA in the region between400and1600cm-1,finger print region of the metal oxygen bonds.The metalTable.1Summary of the catalyst property Sample BET surface area(m2g-1)Pore diameter(A˚)Pore volume(cm3g-1) Calcined MA29550.000.5630wt%STA–MA21441.760.2530wt%STA–ASW230Broad0.3230wt%STA–CB110Broad0.22366S.Selvakumar,A.P.Singhoxygen bands for pure STA(W=O t,W–O c–W and W–O e–W)normally appear at983,898and797cm-1, respectively,which are not seen after heterogenization of STA on MA due to the broadness of MA band in this region.3.4Thermal AnalysisFigure4represents the TGA(inset)and DTG pattern of as-synthesized MA,calcined MA,pure STA and30%STA loaded MA,respectively.The TGA curve of as-synthesized MA is showing two weight losses.Thefirst weight loss below150°C corresponds to the removal of physisorbed water molecule.The significant second weight loss that occurred in the region200–500°C is attributed to the removal of the surfactant molecule.The TG–DTG analysis of pure STA shows three stages of weight loss.Thefirst weight loss around2.8%occurred between room temper-ature and100°C corresponds to physisorbed water.The second one around 3.1%,in the range of100–250°C,accounted for the loss of crystallization water and the third weight loss*0.9%,in the range of250–500°C,was due to the loss of water molecule originating from all acidic protons[37].Calcined mesoporous alumina shows only one weight loss below150°C corresponds to the removal of physisorbed water molecule.The TGA behavior was similar in the case of30wt%STA supported MA com-pared to calcined materials which shows weight loss corresponds to physisorbed water molecule.The sup-ported sample didn’t show any appreciable change in weight loss.3.527Al CP/MAS NMR SpectraMAS NMR is an indispensable tool in characterization of the coordination of aluminum in mesoporous aluminas. The27Al MAS NMR spectra of the calcined mesoporous alumina and30%STA loaded mesoporous alumina are depicted in Fig.5.The calcined sample is showing two distinct aluminium sites at3.8and61.8ppm[14]indicat-ing octahedral and tetrahedral coordination,respectively. The similar peaks are observed in the case of STA loaded material also with minor shift.Benzoylation of Anisole over Silicotungstic Acid3673.6Acidity MeasurementThe process of ammonia adsorption–desorption on cata-lysts is widely applied for the determination of surface acidity.The amount of ammonia adsorbed at high tem-perature is usually taken as a measure of acidity.Ammonia was used as adsorbate because all acid sites on the catalyst surface are accessible for NH3molecules,and the mole-cules are more selectively adsorbed in the presence of sites of different strengths.Acidity measurement was performed for various STA loaded catalysts.In a typical run,0.1g of the catalyst sample was dehydrated at250°C for2h under Heflow(30mL/min)and then cooled down to100°C before being exposed to ammonia.The sample wasflushed again for6h by He to desorb any physisorbed ammonia.A desorption profile was then recorded by increasing the catalyst temperature from100to900°C at a ramp rate of 10°C/min in theflow of30mL/min helium.The total number of acid sites on the catalysts was found to increase proportionally with increased loading of STA over MA(Fig.6C).At the same time the total acidity value of30wt%STA loaded on ASW is more than the similar amount of STA loaded on MA(Fig.6A,B).It is also evident from these data that high acidity was observed with sample heated at250°C and further the acidity get decreased when the calcination temperature of the material increased beyond250°C.This may be due to decompo-sition of the Keggin structure of the heteropoly acid when the calcination temperature increased.This is evidenced by TGA spectra of the pure heteropoly acid(Fig.4C).3.7Catalytic Activity of Various CatalystsThe results of the catalytic activities in the Friedel–Crafts (FC)reaction of anisole with p-toluoyl chloride using pure MA support,pure STA,AlCl3,Al–MCM-41,W–meso-Al, STA loaded catapol-B(STA–CB),STA loaded alumina without surfactant(STA–ASW)and STA loaded meso-porous alumina(STA–MA)are depicted in Table2.The activities of various catalysts are compared under identical reaction conditions using data after6h run.The main product of the reaction is4-methyl-40-methoxy benzophe-none(4,40-MMBP).The selectivity of the product(4,40-MMBP)depends upon the type of the catalyst used in the reaction which may be attributed to the different reactivity368S.Selvakumar,A.P.Singhposition of anisole over these catalysts.The conversion of anisole over Al–MCM-41,30wt%STA–ASW,30wt% STA–MA and30wt%ST–CB were found to be6.50,72.68, 62.95and56.22wt%,respectively,whereas the corre-sponding para selectivity for4,40-MMBP were18.69,74.54, 94.92and85.91wt%,respectively.Among the STA loaded catalysts,alumina synthesized without surfactant gave higher conversion compared to STA loaded MA and STA–CB,however the selectivity for4,40-MMBP was found to be higher over STA–MA than STA–ASW and STA–CB. The catalyst used in this study,shows the following decreasing order of activity after6h of reaction time:pure STA[30-STA–ASW[30-STA–MA[30-STA–CB[ W-MA[AlCl3[Pure Support[Al–MCM-41.Whereas the selectivity to para product is in the order of30-STA–MA [pure MA support[pure STA[AlCl3[30-STA–CB [Al–MCM-41[W-MA[30-STA–ASW.The high selec-tivity of30-STA–MA may be due to uniform size of the mesopores than the other two alumina materials which is having wide range of pores which is clearly explained in the pore size distribution curve in the Fig.2.Acylation experiments were conducted using different STA loaded(5–40wt%)catalysts and different calcination temperature of30wt%-STA–MA catalysts(Fig.7).It is seen that the anisole conversion increased with increase in STA loading up to30wt%and then gets stabilized with further increase in loading.At the same time there is no appreciable change in para selectivity was observed with increase in STA loading.Further,to study the effect of calcination temperature,the catalyst was calcined at three different temperatures between250and650°C.The con-version of anisole decreases exactly half when theTable.2Catalytic activity of various catalystsS.No.Catalyst Conversion(wt%)Product distribution(wt%)Ortho Para Meta1Blank 5.4636.6560.92 2.422Pure STA a73.24 6.2487.89 5.923AlCl327.7147.1552.620.234Al–MCM-41 6.5080.4218.690.975W–meso-Al(W–MA)39.1019.6876.29 4.036Pure Support(MA)13.9294.69 5.090.22 730wt%STA–ASW72.6821.6974.54 3.60 830wt%STA–MA62.95 4.2594.920.78 930wt%STA–CB56.229.7485.91 4.36Conditions:temperature=120°C,catalysts=0.1g,anisole/p-toluoyl chloride molar ratio=1,time=6ha Amount of catalyst used=30mgBenzoylation of Anisole over Silicotungstic Acid369calcination temperature increased from 250to 650°C.But there is no appreciable change in the product distribution.This may be due to the decomposition of keggin ion when the calcination temperature of the catalyst increased.This result is highly supported by the ammonia TPD profile (Fig.6B),where the acidity of the catalyst decreases when the calcination temperature of the catalyst increased.This result clearly indicate that 30wt%STA is needed for complete coverage of the mesoporous alumina support and the calcination temperature should be 250°C for good conversion and selectivity.3.8Effect of Reaction TemperatureThe acylation of anisole was carried out in the range 90–130°C to know the effect of reaction temperature on the conversion of anisole and product selectivity by using 30wt%STA loaded MA calcined at 250°C.The results obtained are presented in Fig.8.The conversion of anisole is increasing very slowly from 90°C to 110°C.Once it reaches 120°C the conversion is increased sharply,and it gets nearly level off after this temperature.There is no appreciable change in the para selectivity observed.One of the reasons for the increased rates at higher temperature may be ascribed to an enhancement of the rate of diffusion of anisole inside the channel of the catalyst,however,reaction rates are usu-ally more temperature dependant than rate of diffusion.3.9Influence of Molar Ratios of the ReactantsThe results of the influence of anisole/p -toluoyl chloride molar ratios on the anisole conversion and product distri-bution is shown in Fig.9.The ratios were changed by keeping the amount of p -TC as constant.The data show at 120°C that,when anisole/p -TC ratio is increased from 1to 3,the conversion of anisole decrease linearly from 62.9to 10.9wt%,respectively.From this result we can conclude that 1:1molar ratio is good for high conversion of anisole and para selectivity for 4,40-MMBP.3.10Influence of Catalyst/Anisole (w/w)RatioTo study the effects of catalyst concentration on the con-version of anisole,rate of anisole conversion and product distribution,the catalyst (30%loaded)concentration (cat-alyst/anisole ratio (w/w))was increased from 0.04to 0.55and the results are depicted in Fig.10.The different ratios of catalyst/anisole were obtained by varying the amount of catalyst and keeping the concentration of anisole constant.The conversion of anisole increased from 25.4to 62.9wt%as the catalyst concentration increases from 0.04to 0.18.The conversion gets nearly saturated when the ratio increased further.There is no much difference in the product distribution against the change in catalyst concentration.370S.Selvakumar,A.P.Singh3.11Catalyst RecyclingRecycle of the synthesized catalysts was studied in the FC reaction of anisole with p-TC using30wt%STA–MA in order to check the stability and activity of recycled catalysts(Fig.11).Three reaction cycles (fresh and two recycles)were carried out under similar reaction condition,using the same catalyst.After workup of the reaction mixture,the catalyst was separated by filtration,washed with acetone and activated for10h at 100°C in the presence of air before use in the next experiment.The conversion of anisole over fresh,first and second recycling are found to be62.9,59.1, 55.7wt%,respectively.The corresponding selectivity for 4,40-MMBP are94.9,91.3,84.6%,respectively.The little decrease in the conversion may be due to the partial leaching of the STA from the mesoporous alumina support.4ConclusionIn conclusion,MA has been synthesized by the lauric acid and aluminum sec-butoxide as the precursor.The calcined MA was functionalized with STA by wet impregnation method.Different loadings of STA over MA was carried out and characterized by various physico-chemical tech-niques to know the structural integrity and nature of support STA interaction.The activities of all the synthe-sized catalysts along with the conventional catalyst (AlCl3)were checked in the FC acylation reaction of anisole with p-toluoyl chloride,which indicate that 30wt%STA loaded on MA is showing good selectivity towards4,40-MMBP(94.92%)with moderate conversion of anisole(62.95wt%).Among the different alumina support tested the anisole conversion is in the order ASW[MA[CB while the order of selectivity toward para product is MA[CB[ASW.The influence of the calcination temperature,catalyst concentration,reaction temperature and anisole/p-TC molar ratio on the catalyst performance is examined in order to optimize the con-version of anisole and selectivity to4,40-MMBP.The conversion of anisole using30-STA–MA increased sig-nificantly with an increase in reaction time,catalyst concentration,and reaction temperature and decreased for anisole to p-TC molar ratio and calcination temperature. The para-selectivity of the catalyst is correlated with the pore size distribution and the catalyst,with uniform mesopores is giving more selectivity than the wide range of mesopores.No major loss of activity was observed after two recycles.The conversion of anisole and selec-tivity towards para product were55.6and84.7wt%, respectively at the end of the second cycle by using 30wt%STA–MA as catalyst.Acknowledgments SK thanks Council of Scientific and Industrial Research,New Delhi,for Senior Research Fellowship.Benzoylation of Anisole over Silicotungstic Acid371References1.Xia YD,Hua WM,Tang Y,Goa Z(1999)Chem Commun18992.Jin T,Yamaguchi T,Tanabe K(1986)J Phys Chem90:47973.Hino M,Arata K(1979)J Chem Soc Chem Commun11484.Corma A(1995)Chem Rev95:5595.Liu Z,Ji W,Dong L,Chen Y(1998)Mater Chem Phys56:1346.Kresge CT,Leonowicz ME,Roth WJ,Vartuli JC,Beck JS(1992)Nature359:7107.Beck JS,Vartuli JC,Roth WJ,Leonowicz ME,Kresge CT,Schmitt KD,Chu CTW,Olson DH,Sheppard EE,McCullen SB, Higgins JB,Schlenker JL(1992)J Am Chem Soc114:108348.Antonelli DM,Ying JY(1996)Angew Chem Int Ed Engl35:4269.Antonelli DM,Ying JY(1996)Chem Mater8:87410.Terrible D,Trovarelli A,Llorca J,De Leitenburg C,Dolcettin G(1998)J Catal178:29911.Sayari A,Liu P(1997)Microporous Mater12:14912.Bagshaw SA,Pinnavaia TJ(1996)Angew Chem108:118013.Bagshaw SA,Pinnavaia TJ(1996)Angew Chem Int Ed Engl35:110214.Vaudry F,Khodabandeh S,Davis ME(1996)Chem Mater8:145115.Cabrera S,Haskouri JE,Alamo J,Beltron A,Beltran D,Men-dioroz S,Marcos MD,Amoros P(1999)Adv Mater11:37916.Kozhevnikov IV(1998)Chem Rev98:17117.Fournier M,Feumi-Jantou C,Rabia C,Herve G,Launay S(1992)J Mater Chem2:97118.Misono M(1987)Catal Rev Sci Eng29:26919.Misono M(1998)Catal Rev Sci Eng30:33920.Rocchiccioli-Deltcheff C,Amirouche M,Herve G,Founier M,Che M,Tatibouct JM(1990)J Mol Catal126:59121.Swanmi S,Shin-ichi N,Okuahar T,Misono M(1997)J Catal166:26322.Faming Z,Shenquing G,Shuoming S CN1,197,057(Cl,C07,C69/34)28October1998,Applied;97,104,061,23Apr1997, 6pp(Ch)23.Vazquez PG,Blanco MN,Caceres V(1999)Catal Lett60:20524.Pizzio LR,Cacares CV,Blanco MN(1998)Appl Catal A167:28325.Schwegier MA,Vinke P,Vijk M,Bekkum H(1992)Appl CatalA80:4126.Izumi Y,Hasebe R,Urabe K(1983)J Catal84:40227.Dupont P,Vedrine JC,Paumard E,Hecquet G,Lefebve F(1995)Appl Catal A129:21728.Dupont P,Lefebve P(1996)J Mol Catal A124:29929.Kozhevnikov IV,Sinnema A,Jansen RJ,Panin K,Bekkum KV(1995)Catal Lett30:24130.Jalil PA,Al-Daous MA,Al-Arfaj ARA,Al-Amer AM,BeltraminiJ,Barri SAI(2001)Appl Catal A207:15931.Verhoef MJ,Kooyamann PJ,Peters JA,Bekkum HV(1999)Microporous Mesoporous Mater27:36532.Nomiya K,Murasaki H,Miwa M(1986)Polyhedron5:103133.Devassy BM,Halligudi SB,Hegde SG,Halgeri AB,Lefebvre F(2002)Chem Commun107434.Chen CY,Li HY,Davis ME(1993)Microporous Mater2:1735.Potdar HS,Jun KW,Bae JW,Kim SM,Lee YJ(2007)Appl CatalA Gen321:10936.Colomban PH(1988)J Mater Sci Lett7:132437.Sawant DP,Vinu A,Jacob NE,Lefebvre F,Halligudi SB(2005)J Catal235:341372S.Selvakumar,A.P.Singh。
