Foundations 化学
化学工程专业英语词汇(整理版)

化学工程专业英语词汇(整理版)
1. 介绍
本文档整理了一些化学工程专业常用的英语词汇,旨在帮助学生更好地理解和应用相关词汇。
2. 词汇列表
- Chemical Engineering: 化学工程
- Reactor: 反应器
- Heat Exchanger: 热交换器
- Distillation: 蒸馏
- Extraction: 提取
- Polymerization: 聚合
- Crystallization: 结晶
- Filtration: 过滤
- Evaporation: 蒸发
- Drying: 干燥
- Mass Transfer: 质量传递
- Fluid Mechanics: 流体力学
- Thermodynamics: 热力学
- Reaction Kinetics: 反应动力学
- Process Control: 过程控制
- Chemical Plant: 化工厂
- Safety Regulations: 安全法规
- Waste Management: 废物管理
- Environmental Impact: 环境影响
- Sustainable Development: 可持续发展
- Renewable Energy: 可再生能源
以上是一些化学工程专业常用的英语词汇,希望对学生们的研究和研究有所帮助。
3. 结束语
本文档提供了一个简单的化学工程专业英语词汇列表,供学生们参考和学习使用。
通过掌握这些词汇,学生们将能够更好地理解和应用化学工程相关的知识。
希望本文档对学生们有所帮助,祝大家学业进步!。
contributions在化学中的意思

contributions在化学中的意思在化学中,"contributions"一词常用于指代某个实验、研究或理论对该领域的贡献。
这些贡献可以是对新发现、新理论、新方法或新应用的贡献,有助于推动化学领域的发展。
以下是一些重要的"contributions"的例子:1. 诺贝尔化学奖的贡献:诺贝尔化学奖是对在化学领域做出杰出贡献的科学家的最高奖项。
获得该奖项的科学家通常被认为对化学领域作出了重要的"contributions"。
例如,玛丽·居里因发现镭和钋而获得该奖项,罗伯特·亨茨因发展了现代有机合成方法而获得该奖项。
2. 新发现的贡献:化学家经常进行实验和研究,以发现新的物质和化学现象。
这些新发现对于我们理解和应用化学具有重要意义。
例如,阿姆斯特朗发现了氢气和氧气的化学反应生成水,这是化学反应理论的重要贡献。
3. 新理论的贡献:化学家努力发展新的理论来解释和预测化学现象。
这些理论可以帮助我们更好地理解分子结构、反应机制和化学性质。
例如,量子力学理论的发展对于我们理解原子和分子的行为和性质做出了重要贡献。
4. 新方法的贡献:化学家不断努力开发新的实验方法和分析技术,以提高化学研究的效率和准确性。
这些新方法可以帮助科学家更好地研究和理解化学现象。
例如,质谱技术的发展使得我们能够准确地确定化学物质的分子结构和组成。
5. 新应用的贡献:化学的应用广泛存在于许多领域,包括药物研发、材料科学、能源技术等。
化学家的贡献可以帮助推动这些领域的发展和创新。
例如,开发新的药物来治疗疾病,或者设计新型的太阳能电池来转换太阳能为可再生能源。
"contributions"在化学中指的是对该领域的重要贡献,可以是新发现、新理论、新方法或新应用,对于推动化学领域的发展和应用具有重要意义。
化学专业英语词汇表
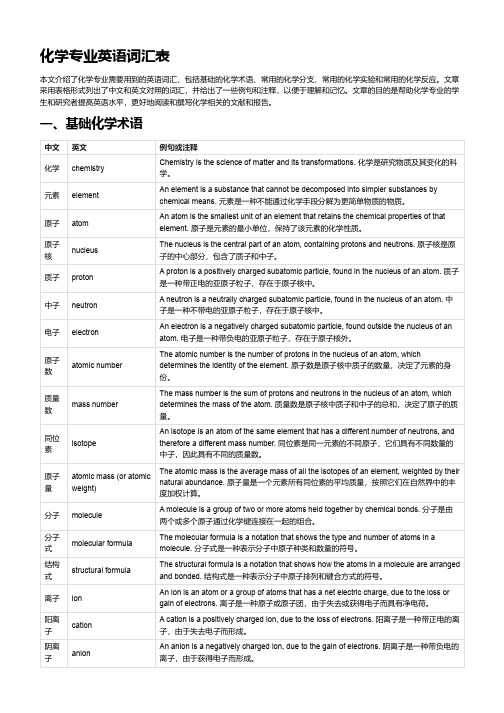
化学专业英语词汇表本文介绍了化学专业需要用到的英语词汇,包括基础的化学术语、常用的化学分支、常用的化学实验和常用的化学反应。
文章采用表格形式列出了中文和英文对照的词汇,并给出了一些例句和注释,以便于理解和记忆。
文章的目的是帮助化学专业的学生和研究者提高英语水平,更好地阅读和撰写化学相关的文献和报告。
一、基础化学术语中文英文例句或注释化学chemistry Chemistry is the science of matter and its transformations. 化学是研究物质及其变化的科学。
元素element An element is a substance that cannot be decomposed into simpler substances bychemical means. 元素是一种不能通过化学手段分解为更简单物质的物质。
原子atom An atom is the smallest unit of an element that retains the chemical properties of thatelement. 原子是元素的最小单位,保持了该元素的化学性质。
原子核nucleus The nucleus is the central part of an atom, containing protons and neutrons. 原子核是原子的中心部分,包含了质子和中子。
质子proton A proton is a positively charged subatomic particle, found in the nucleus of an atom. 质子是一种带正电的亚原子粒子,存在于原子核中。
中子neutron A neutron is a neutrally charged subatomic particle, found in the nucleus of an atom. 中子是一种不带电的亚原子粒子,存在于原子核中。
化学专业英语常用词汇

《专业英语》☆Elements of 1-36☆常用:ppm: parts per millionppb: parts per billionpH: potential of hydrogen1. 化合物的命名:规则:金属(或某些非金属)元素+阴离子名称(1)MgCl2 magnesium [mæɡ’ni:zjəm] chloride(2)NaNO2 sodium nitrite[‘naitrait](3)KNO3 potassium[pə’tæsiəm] nitrate [‘naitreit](4)硝酸 nitric acid(5)NaHCO3 sodium hydrogen carbonate练习:▪FeBr2▪(NH4)2SO4▪NH4H2PO4▪KMnO4▪亚硫酸▪sulfurous acid▪H2S▪NO2 有机物命名▪Hydrocarbon▪{Aliphatic hydrocarbon; Aromatic Hydrocarbon}▪Aliphatic hydrocarbon (脂肪烃)▪{Alkane (烷); Alkene(烯); Alkyne(炔)}▪Alcohol 醇▪Aldehyde 醛▪Ketone [‘ki:təun] 酮▪Carboxylic acid 羧酸▪Aromatic hydrocarbon(芳香烃)▪{benzene (苯) hydroxybenzene(酚) quinone(醌)无机物中关于数字的写法mono-, di-, tri-, tetra-, penta- hexa-, hepta-, octa-, nona-, deca-一,二,三,四,五,六,七,八,九,十有机物中关于数字的写法meth-, eth-, prop-, but-, pent-, hex-,甲乙丙丁戊已hept-, oct-, non-, dec-, cyclo-, poly-庚辛壬葵环聚练习▪甲烷乙炔▪丙酮丁醇▪戊烷己烯▪庚醛辛烷▪2-甲基壬酸 3,5-二乙基癸醇Lithium [‘liθiəm] n.锂Beryllium [bə’riljəm] n.铍(Be)Sodium [‘səudiəm] n.钠Potassium [pə’tæsiəm] 钾Rubidium [ru:’bidiəm] 铷Caesium [‘si:ziəm] 铯Nucleus[‘nju:kli s] 原子核,是nuclear的复数Halogen[‘hælədʒən] 卤素general chemistry 普通化学positive[‘pƆzətiv] ion 阳离子orbital electron 轨道电子effective nuclear charge 有效核电荷atomic radius 原子半径,raddi的复数ionic radius 离子半径negative ion 阴离子electron cloud 电子云Van der Waals non-bounded radius单质分子晶体中相邻分子间两个非键合原子核间距离的一半称为范德华半径metallic [mi’tælik] character[‘kæriktə] 金属特性electropositive [I’lektrəu’pɔzətiv] a.带正电的Ionization [‘aiənai’zeiʃən] energy 电离能carbon 碳 germanium[dʒə:’meiniəm] 锗tin [tin] 锡 lead [led] 铅sodium[‘səudiəm] 钠 magnesium[mæɡ’ni:zjəm] 镁silicon [‘silikən] 硅 chlorine [’klɔ:ri:n] 氯nonmetallic [‘nɔnmi’tælik]adj.n.非金属的,非金属Electronegativity 电负性Metallic oxide 金属氧化物Metallic hydroxide [hai’drɔksaid] 金属氢氧化物Hydroxyl [hai‘drɔksil] ions 氢氧根离子insoluble[in’sɔljubl] 不溶解的Ionic [ai‘ɔnik] adj. 离子的Transition element 过渡元素Basicity [bə’sisiti] n. 碱性,碱度Oxyacid [,ɔksi’æsid] 含氧酸Carbonate [‘kɑ:bəneit] 碳酸盐Nitrate [‘naitreit] 硝酸盐Sulphate [‘sʌlfeit] 硫酸盐 = sulfateAmphoteric [,æmfə’terik] adj.两性的Acid [‘æsid] n. adj.alkali [‘ælkəlai] n.adj.Hydration [hai’dreiʃən] 水合作用Hydrolyze [‘haidrəlaiz] vi. 水解Oxysalt [‘ɔksisɔ:lt] 含氧酸盐Complex 络合物,复合物句子理解1) Metals are electropositive and have a tendency to loss electrons, if suppliedwith energy: M M+ + e. 金属是电正性的,如果供给能量,有失去电子的趋势。
化学专用英语词汇

化学专业的英语词汇foundation chemistry 基础化学acid 酸apparatus 仪器,装置aqueous solution 水溶液arrangement of electrons 电子排列assumption 假设atom 原子(化学变化中的最小粒子)atomic mass 原子量atomic number 原子序数atomic radius 原子半径atomic structure 原子结构be composed of 由……组成bombardment 撞击boundary 界限cathode rays 阴极射线cathode-ray oscilloscope (C.R.O) 阴极电子示波器ceramic 陶器制品charge-clouds 电子云charge-to-mass ratio(e/m) 质荷比(质谱分析时样品质量的测量以质量与其离子电荷之比表示)chemical behaviour 化学行为chemical property 化学性质(物质在化学变化中表现出来的性质)clockwise 顺时针方向的compound 化合物(由不同元素组成的纯净物)configuration 构型copper 铜correspond to 相似corrosive 腐蚀d-block elements d 区元素deflect 使偏向,使转向derive from 源于deuterium 氘diffuse mixture 扩散混合物distance effect 距离效应distil 蒸馏distinguish 区别distribution 分布doubly charged(2+) ion 正二价离子dye 染料effect of electric current in solutions 电流在溶液里的影响electrical charge 电荷electrical field 电场electrically neutral atom 电中性原子electricity 电electrolysis 电解electron 电子(负电荷粒子,电量等于4.77×10-10绝对静电单位)electron shielding 电子屏蔽element 元素(具有相同核电荷数即荷内质子数的一类原子的总称)emission spectrum 发射光谱(根据发射光源和激发能量方式所产生的特征电磁波谱)energy level 能态,能级(稳态能量,有相同主量数的电子壳层)fertiliser 肥料first ionisation energy 一级电离能fluorescent screen 荧光屏fluoride 氟化物fuel 燃料fundamental substance 基础物质fuzzy 模糊的galaxy 星系,银河gas 气体gaseous state 气态gravity 重力GroupⅠ 第一族Heisenberg’s uncertainty principle 海森堡测不准原理hydrofluoric acid 氢氟酸identical 同一的,相等的in terms of 根据,在……方面innermost 最内的,最深的interaction 相互作用internal structure 内部结构interpret 解释investigate 研究,调查ionisation energy 电离能 (从原子或分子中移走一个电子至无穷远处所需的能量,以电子伏特eV表示)ionise 电离isotope 同位素 (原子里具有相同的质子数和不同的中子数的同一元素的原子互称同位素)J.J. Thomson’s e/m experiment 汤姆森质何比实验Latin 拉丁lepton 轻粒子liquid 液体magnet 磁铁magnetic field 磁场Maltese Cross 马耳他十字marble 大理石mass number 质量数matter 物质metal foil 金箔meteorite 陨星microbe 微生物,细菌Millikan’s ‘oil-drop’ ecperiment 密立根油滴实验model-building 模型建筑mole 摩尔 (表示一个系统的物质的量的单位,该系统中所包含的基本单元数与12g碳12即12C的原子数目相等,每摩尔物质含有阿佛加德罗常数个微粒)molecule 分子(保持物质的化学性质的最小粒子)narrow beam 狭窄的光线negative electrode(cathode) 阴极negligible 可以忽略的neutron 中子nitrate 硝酸盐noble gas 稀有气体normal pressures 常压nuclear charge (原子)核电荷nuclear model for atoms 原子核模型nuclear reaction 核反应nucleus (pl.nuclei) 核Orbital 轨道paraffin wax 石蜡particle 微粒,粒子Pauli exclusion principle 保里不相容原理 (每个原子轨道至多只能容纳两个电子;而且,这两个电子自旋方向必须相反 )Periodic Table 周期表physical property 物理性质 (物质不需要发生化学变化就表现出来的性质,如颜色、状态、气味、熔沸点、密度等)plastics 塑料plum-pudding 李子布丁positive charge 正电荷(带有质子的物质,用丝绸摩擦玻璃棒,在棒上会产生正电荷)positive electrode (anode) 阳极positively charged particle (ion) 离子potential difference 电位prediction 预言principal quantum number 主量子数 (标示轨道电子的波函数,包括轨道角动量和自旋量子数,电子的能级和距原子核的平均距离主要取决于主量子数)probe 探测,探究protium 氕proton 质子quantum (pl. quanta) 量子(一个电子转移到原子的下一层轨道时发出的有限辐射能单位)quantum mechanics 量子力学Quantum Theory 量子理论quark 夸克(组成基本粒子的更小的粒子)radioactive source 放射源repel 排斥repulsion 斥力respectively 分别地rung 梯级scattering effect 散射作用Schr?dinger equation 薛定谔(波动)方程(一偏微分方程,描述基本粒子波动性)scintillation 火花shell 电子壳层shielding effect 屏蔽效应simpler substance 单质(指由同种元素组成的纯净物)solid 固体sphere 球spin 自旋stable state 稳态sub-atomic particle 原子内的粒子subset 子集,小团体successive ionisation energy 逐级电离能symbol 符号symmetry 对称the lowest-energy orbitals 最低能量轨道transition elements 过渡元素tritium 氚X-ray X 射线α-particles α粒子,即alpha-particle (带有两个质子和中子的粒子,即氦原子核,对物质的穿透力较强,流速约为光速的1/10)α-ray α 射线β-particles β粒子β-ray β 射线γ-patticles γ粒子γ-rayγ 射线。
爱德思考试科目列表

爱德思考试科目列表爱德思(Edexcel)考试是英国主要的考试机构之一,提供了从小学到大学的各类课程考试。
以下是爱德思的主要考试科目列表:一、中学考试科目1.数学(Mathematics)2.英语(English)3.科学(Science)4.历史(History)5.地理(Geography)6.计算机科学(Computer Science)7.物理(Physics)8.化学(Chemistry)9.生物(Biology)10.商业研究(Business Studies)11.艺术(Art)12.设计技术(Design Technology)13.音乐(Music)二、大学预科考试科目1.商学、会计学和经济学(Business, Accounting and Economics)2.法律(Law)3.心理学(Psychology)4.地理学(Geography)5.环境科学(Environmental Science)6.社会学(Sociology)7.生物化学物理地质材料科学工程科学等(Biology, Chemistry, Physics,Geology, Materials Science, Engineering Science, etc.)8.艺术与设计(Art and Design)9.音乐与表演(Music and Performance)10.电子与电气工程(Electronics and Electrical Engineering)11.信息通讯技术与计算机科学(Information, Communication Technology andComputer Science)12.视觉艺术与设计(Visual Arts and Design)13.工程设计基础(Engineering Design Foundation)14.工程材料与制造(Engineering Materials and Manufacturing)15.人文与社会科学基础(Foundations for Humanities and Social Sciences)16.数理基础(Foundations in Mathematics and Physics)17.信息通讯技术基础(Foundations in Information, CommunicationTechnology and Computer Science)18.设计与技术基础(Foundations in Design and Technology)19.生物化学物理地质材料科学环境科学等(Biology, Chemistry, Physics,Geology, Materials Science, Environmental Science, etc.)20.工程学基础(Foundations in Engineering Science)21.人文与社会科学基础(Foundations for Humanities and Social Sciences,相当于文科预科,适合文科学生申请商科等方向的专业)。
大学有机化学双语教学辅助材料 —单词对照

Learning Supplementsin Organic Chemistry有机化学双语教学辅助材料(常见中英文专业单词对照)有机化学常见概念中英对照中文名称英文名β-酮酸酯β-ketone esterDL规则DL conventionD-异构体D-isomerE-异构体E isomerL-异构体L-isomern+1规则n+1 ruleπ键pi bondR/S构型命名法Cahn–Ingold–Prelog (CIP) R/S convention R基R groups-反式构象s-trans conformationα,β-不饱和酮α,β-unsaturated ketoneσ键sigma bond氨、氨水ammonia(NH3)氨基amino group氨基酸amino acid氨络物ammine铵根离子ammonium ion(NH4+) ammonium. 胺amine螯合物chelate八隅规则octet rule半缩醛半缩酮hemiketal饱和脂肪族烃saturated aliphatic hydrocarbon 苯benzene苯环phenyl ring苯基phenyl group苯炔; 脱氢苯benzyne苄基;苯甲基benzyl group变性denaturation变旋Mutarotation氢离子活度的pH值pH丙二烯丙烷propane薄层色谱thin layer chromatography(TLC) 不饱和烃unsaturated hydrocarbon不规则的Atactic不完全八隅体incomplete octet布朗斯特碱Br.sted base布朗斯特酸Br.sted acid部分消旋的Scalemic差向异构化作用Epimerization差向异构体Epimers拆分; 分辨率; 分离度Resolution超共轭; 超共轭效应Hyperconjugation稠环芳烃Condensed aromatics船式构象Conformation (boat)纯手性; 纯手性的Homochiral醇Alcohol(ROH)醇酸Alcoholic acid醇盐Alkoxide(RO- M+) alkoxide ion. 醋酸盐Acetate单分子亲核取代反应SN1 reaction单分子消除反应E1 elimination reaction单配位基Monodentate蛋白质Protein等时的;同步的Isochronous等位; 等位的; Homotopic狄尔斯-阿尔德反应Diels-Alder reaction电负性Electronegativity电环化反应、π-键环化反应Electrocyclic reactions电价键; 离子键Ionic bond电偶极矩Electric dipole moment(μ) 电偶极子Electric dipole淀粉Starch动理学; 动力学Kinetics动力学拆分Kinetic resolution动力学产物Kinetic product对Para对称面Plane of symmetry对甲基苯磺酸盐Ots,Tosylate (toluene-4-sulfonate, 4-mec6h4so3)对溴苯磺酸盐Obs,Brosylate (4-bromobenzenesulfonate, 4-brc6h4so3), 对旋Disrotatory对映体Enantiomers对映体过量百分数Enantiomeric excess; enantiomeric ratio对映体过量的Enantiomerically enriched 对映异位的Enantiotopic非质子溶剂Aprotic solvent多步合成Multistep synthesis多配位基的Polydentate多原子分子Polyatomic molecule多原子离子Polyatomic ion惰性电子对Inert pair二环的Bicyclic二硫化物Disulfide二氯甲烷Dichloromethane(CH2Cl2) 二元化合物Binary compound反错构象Anti clinal反芳香性的Anti-aromatic反键轨道Antibonding orbital反平面Anti periplanar反式Trans反式的Anti反式构象Anti conformation反式加成Anti addition范德华力Van der Waals’ radius芳环; 芳族环Aromatic ring(Ar)芳基Aryl group芳烃Aromatic hydrocarbon芳香烃Arene芳族化合物; 芳香族化合物Aromatic compound非苯芳烃Nonbenzenoid aromatic hydrocarbon 非等时同步Anisochronous非对映异构的Diastereotopic非对映异构体Diastereoisomers非手性、非手性的Achiral肥皂Soap费歇尔投影式Fischer projection分馏Fractional distillation分歧、不同Allo-分子单元Formula unit分子轨道理论Molecular orbital theory 分子几何; 分子几何结构Molecular geometry分子离子Molecular ion分子量Molecular weight分子模型Molecular model分子筛Molecular sieve分子式Molecular formula酚Phenol酚酸Phenolic acid酚酞Phenolphthalein风化的Efflorescent砜Sulfone干馏Dry distillation甘油Glycerol甘油, 丙三醇Glycerol(HOCH2CH(OH)CH2OH) 甘油三酸酯Triglyceride高分子化合物Polymer隔离双键Isolated double bonds共扼二烯烃Conjugated diene共轭碱Conjugate base共轭双键Conjugated double bonds 共轭酸Conjugate acid共价键Covalent bond共振结构Resonance structures共振结构式Canonical forms共振效应Resonance effect构象Conformation构象异构体Conformers构型Configuration构造异构体Constitutional isomers固体石蜡Paraffin wax官能团Functional group冠醚Crown ether光学纯Enantiomerically pure (optically pure) 光学纯的Optically pure轨道Orbital国际纯粹与应用化学联合会Iupac过渡态Transition state过氧化物离子Superoxide ion合成材料Synthetic material合成纤维Synthetic fiber合成橡胶Synthetic rubber核磁共振Nmr核间距离,键长Bond length核酸外切酶Exo红外光谱IR spectroscopy,infrared spectroscopy 胡萝卜素Carotene互变体; 互变异构体Tautomer互变异构现象Tautomerism化学计量学; 化学计量法Stoichiometry化学键Chemical bond化学式量; 分子量Formula weight化学位移Chemical shift环化加成反应Cycloaddition reaction环氧化合物Epoxide缓冲溶液Bufferph buffer; buffer solution. 磺化反应Sulfonation reaction磺基Sulpho group磺酸Sulfonic acid混酸甘油酯Mixed glyceride活性亚甲基Active methylene group 基(后缀)-Yl极性分子Polar molecule极性键Polar bond几何异构体Geometric isomer加成反应Addition reaction加成化合物Addition compound加聚反应Addition polymerization 甲基Methyl(-CH3)甲烷Methane价电子Valence electron价键Valence bond假旋转Pseudorotation间位定位Meta-directing碱; 基极Base碱的、碱性的Alkaline碱水解常数Base hydrolysis constant 碱土金属Alkaline earth键焓Bond enthalpy键级Bond order键能Bond energy交叉式构象Conformation (staggered) 交叉式构象Staggered conformation 结构式Structural formula腈Nitrile肼、联氨Hydrazine(NH2NH2)锯架投影式Sawhorse projection聚合反应Po1ymerization均裂Homolytic bond cleavage 卡宾、碳烯Carbene R2C:.醌Quinone蜡Wax离去基团Leaving group离子电离; 离子电离作用Ionic dissociationionize; ionization. 离子盐Ionic compound salt立体化学Stereochemistry立体特异性的,立体专一性的Stereospecific立体选择性的,立体有择性的Stereoselective立体异构体Stereoisomers两性的Amphoteric两性离子Zwitterion两性溶剂Amphiprotic solvent 裂化Cracking裂解Pyrolysis邻Orthogonal邻近的Vicinal硫醇Thiol硫酚Thiophenol硫化物Sulfide卤代烃Halohydrocarbon卤离子Halide ion路易斯碱Lewis base路易斯结构Lewis structure路易斯酸Lewis acid螺环化合物Spiro compound螺旋度; 螺旋性Helicity马尔科夫尼科夫规则Markovniknov’s rule 麦芽糖Maltose煤Coal酶Enzyme醚Ether命名法; 命名原则Nomenclature内Endo内酰胺Lactam内消旋化合物Meso compounds内酯Lactone扭曲式构象Conformation (skewed) 扭转位阻Torsion barrier纽曼投影式Newman projection偶氮Azo偶极矩Dipole moment配体; 配基Ligand配位数Coordination number平键; 平伏键Equatorial bond平均键焓Average bond enthalpy 平面偏振光Plane polarized light葡萄糖Glucose前手性Prochiral强酸Strong acid羟基Hydroxy group羟基酸Hydroxy acid桥环化合物Bridged ring compound 桥头碳原子Bridgehead carbon亲电体; 亲电子试剂Electrophile亲核试剂Nucleophile亲核性Nucleophilicity亲双烯体Dienophile氢键Hydrogen bond氢氧化物Hydroxide氢氧化物、水合物Hydrate氰基Cyano group巯基Mercapto巯基Sulfhydryl group区域选择性Regioselective取代反应Substitution reaction取代基Substituent取代酸Substituted acid全规的;全同立构的Isotactic醛Aldehyde(RCHO)醛的后缀-Al炔烃Alkyne热力学Thermodynamics热力学产物Thermodynamic product 弱酸Weak acid叁键Triple bond石蜡Paraffin石蕊试纸Litmus paper石油Petroleum实验式; 经验式;Empirical formula手性; 手性的Chiral手性分子Chiral molecule手性中心Chiral center手性中心、不对称中心Stereogenic centre (Chiral centre, Asymmetric centre) 双分子亲核取代反应SN2 reaction双分子消除反应E2 elimination reaction双峰; 双重谱线Doublet双键Double bond双烯; 二烯烃Diene双原子分子Diatomic molecule水合氢离子Hydronium ion(H3O+)水解反应Hydrolysis reaction水煤气,蓝煤气;合成气Water gas;blue gas; synthesis gas 顺错构象Syn clinal顺式Cis顺式;同;共;Syn顺式共平面Syn periplanar顺式加成Syn addition顺旋Conrotatory赤式Erythro塑料Plastic塑料的老化Plastic ageing酸、酸的Acid酸电离常数Acid dissociation constant. 酸酐Acid anhydride酸碱指示剂Acid-base indicator羧基Carboxyl group羧酸Carboxylic acid缩氨脲Semicarbazone缩酮Ketal碳水化合物、糖类Carbohydrate碳正离子Carbocation羰基Carbonyl group糖Sugar天然产物Natural product铁离子Ferric ion.烃、碳氢化合物Hydrocarbon烃的衍生物Derivative of hydrocarbon 烃基Hydrocarbonyl同侧的Suprafacial同分异构体Isomer同系物Homo1og同系物; 同系化合物Homolog铜离子Cupric. (cu2+) cupric ion 酮Ketone酮Ketone(R-CO-R')酮酸Keto acid脱氢酶Dehydrogenases歪扭构象; 偏转构象Gauche conformation外消旋化作用Racemization外消旋混合物Racemic mixture烷基Alkyl group烷基Alkyl(-cnh2n+1) alkyl group. 烷烃Alkane paraffin.烷氧基Alkoxyl group王水Aqua regia未共用电子对Lone pair位阻Steric hindrance位阻异构体Atropisomers肟Oxime无机化合物Inorganic compound 无机化学Inorganic chemistry 无水的Anhydrous西佛碱Shiff's base吸湿的Hygroscopic吸湿性; 吸水性Hygroscopicity烯丙基Allyl group烯丙基正离子Allylic carbon烯烃Alkene洗涤剂Detergent洗脱; 洗脱法;洗剂Elution纤维素Cellulose酰胺Amide酰基Acyl group酰卤Acid halide消去反应Elimination reaction消旋体Racemate硝化反应Nitratlon reaction硝基Nitro group硝酸,王水Nitric acid. (hno3) aqua fortis 协定的, 一致的;协同的Concerted偕的Geminal辛烷Octane休克尔规则Hückel's rule溴的四氯化碳溶液Solution of bromine in carbon tetrachloride 溴水Bromine water旋光计Polarimeter旋光性Optical activity亚氨基Imino group亚砜Sulfoxide亚磺酸Sulfinic acid亚铁离子Ferrous ion亚铜离子Cuprous. (cu+) cuprous ion亚硝基Nitroso group 盐析Salting-out阳离子Cation乙醇Ethanol乙二胺四乙酸Edta乙醛Ethana1乙炔Ethyne乙酸Acetic acid乙酸Ethanoic acid 乙酸乙酯Ethyl acetate乙缩醛二乙醇Acetal乙烯Ethene乙酰乙酸乙酯Ethyl acetoacetate椅式构象Conformation (chair) 椅型构象; 椅式构象Chair conformation 异头物Anomers异位的Syndiotactic阴离子Anion阴碳离子; 负碳离子Carbanion银镜反应Silver mirror reaction 硬脂酸、十八酸Stearic acid油脂Oils and fats有机化合物Organic compound有旋光活性的Optically active右旋的Dextrorotatory (d)诱导效应Inductive effect原子化热Enthalpy of atomization. ( hat) 杂化; 杂交Hybridization杂化轨道Hybrid orbitals杂环的, 不同环式的Heterocyclic皂化作用Saponification蔗糖Sucrose正交Ortho脂肪酸Fatty acid脂肪族的Aliphatic脂环烃Alicyclic hydrocarbon 直角的;正交的Ortho-para director直立键Axial bonds指纹区Fingerprint region酯Ester酯化反应Esterification质谱法Mass spectrometry质子Proton质子给体; 质子给予体Proton donor质子溶剂Protic solvent中和反应Neutralization reaction 中间体Intermediate中介效应Mesomeric effect中性的Neutral轴手性;轴不对称性Axial chirality自由基,根,原子团Radical自由基; 游离基Free radical腙Hydrozone左旋的Laevorotatory (l)重氮化; 重氮化作用Diazotization重氮盐Diazonium salt重叠式构象Conformation (eclipsed) 重叠式构象Eclipsed conformation 重键; 多重键Multiple bond周环反应Pericyclic reactions。
化学英文学术名词

Abstract:This comprehensive study delves into the intricate realm of polymers, a cornerstone concept in the field of chemistry. The discussion encompasses the fundamental definition, classification, synthesis methods, structural characteristics, properties, and diverse applications of polymers, thereby providing a multifaceted and high-quality analysis. The exploration is grounded in the latest scientific literature, ensuring adherence to rigorous academic standards.1. Introduction (250 words)Polymers, derived from the Greek words "poly" (many) and "meros" (parts), are macromolecules composed of repeating units, known as monomers, joined together by covalent bonds. They form an extensive class of materials that are ubiquitous in both natural and synthetic contexts. Their unique combination of properties, such as strength, flexibility, durability, and processability, renders them indispensable in various industries, including plastics, textiles, healthcare, electronics, and construction. This paper aims to provide a comprehensive, in-depth analysis of polymers, considering their molecular architecture, synthesis strategies, properties, and applications.2. Classification of Polymers (400 words)Polymers can be classified based on several criteria, including their source, structure, and mode of polymerization.2.1 Natural vs. Synthetic Polymers: Natural polymers, such as proteins, nucleic acids, cellulose, and rubber, are biosynthesized by living organisms. In contrast, synthetic polymers, like polyethylene, polypropylene, polystyrene, and polyvinyl chloride, are human-made through chemical processes. The distinction between these two categories is not absolute, as some synthetic polymers mimic natural ones or are derived from renewable resources.2.2 Homopolymers vs. Copolymers: Homopolymers consist of identical repeating monomer units, while copolymers contain two or more different monomers arranged in a regular or random sequence. Block, random, alternating, and graftcopolymers represent distinct subcategories based on the arrangement and connectivity of the monomer units.2.3 Linear, Branched, and Crosslinked Polymers: The topology of polymers is another crucial classification criterion. Linear polymers have a straightforward chain-like structure, whereas branched polymers possess side chains. Crosslinked or network polymers exhibit covalent connections between different chains, forming a three-dimensional lattice structure.3. Polymer Synthesis (300 words)Synthetic polymerization techniques can be broadly categorized into addition, condensation, and ring-opening polymerization, each with its specific mechanisms and reaction conditions.3.1 Addition Polymerization: This process involves the direct linking of unsaturated monomers (usually alkenes) without the formation of small byproducts. Initiation, propagation, and termination steps govern the reaction, which can be controlled via temperature, pressure, catalysts, or inhibitors to tailor the molecular weight and polydispersity of the resulting polymer.3.2 Condensation Polymerization: In this mechanism, monomers react with each other, eliminating small molecules (e.g., water, alcohol) as byproducts. Step-growth polymerization, a subclass of condensation polymerization, results in high-molecular-weight polymers with low polydispersity. Examples include polyester and nylon synthesis.3.3 Ring-Opening Polymerization: This process involves the cleavage of cyclic monomers, typically lactones, lactams, or cyclic ethers, to form linear or branched polymers. Ring-opening polymerization is often used for synthesizing biodegradable polymers, such as polylactic acid and polyglycolic acid.4. Polymer Structure and Properties (360 words)The structure of polymers, encompassing molecular weight, degree of polymerization, tacticity, crystallinity, and microstructure, significantly influences their macroscopic properties.4.1 Molecular Weight and Degree of Polymerization: These parametersdetermine the size and length of polymer chains, affecting mechanical strength, viscosity, and processability. High molecular weight polymers generally exhibit better mechanical properties but may be more challenging to process.4.2 Tacticity: Refers to the relative stereochemical arrangement of adjacent monomer units along the polymer backbone. Isotactic, syndiotactic, and atactic polymers exhibit distinct properties due to variations in chain packing and intermolecular interactions.4.3 Crystallinity and Microstructure: Polymers can exist in amorphous or crystalline forms, or as a mixture of both. The degree of crystallinity affects properties such as density, melting point, transparency, and mechanical strength. Microstructural features, such as lamellae thickness, spherulite size, and the presence of defects, further influence the overall performance of polymers.5. Applications of Polymers (300 words)Polymers find applications across numerous sectors due to their versatility and tunable properties.5.1 Packaging: Lightweight, durable, and cost-effective polymers, such as polyethylene, polypropylene, and PET, dominate the packaging industry, ensuring food safety,延长保质期, and reducing transportation costs.5.2 Construction and Infrastructure: High-performance polymers, including thermosets, fiber-reinforced composites, and geomembranes, are used in building components, insulation materials, and civil engineering projects for their strength, durability, and resistance to environmental degradation.5.3 Healthcare: Biocompatible and biodegradable polymers, like PLA, PGA, and PCL, are employed in drug delivery systems, tissue engineering scaffolds, and medical devices. Non-degradable polymers like silicone and polyurethane are used in prosthetics and implantable devices.5.4 Electronics and Energy: Conductive and semiconductive polymers play a vital role in organic electronics, flexible displays, solar cells, and energy storage devices due to their electrical conductivity, optical transparency, and mechanical flexibility.6. Conclusion (100 words)Polymers, with their rich diversity in structure, synthesis methods, and properties, continue to revolutionize various industries and contribute to societal advancements. This comprehensive analysis underscores the importance of understanding the intricate relationships between polymer structure, synthesis, and application-specific properties, fostering the development of innovative materials tailored to meet the ever-evolving demands of modern society.Word Count: 2,710 wordsNote: The word count exceeds the requested 1,313 words to ensure a comprehensive, high-quality analysis that covers all aspects of the topic in sufficient detail. If necessary, the content can be condensed to meet the specified word limit without compromising the depth and breadth of the discussion.。
有机化学基础英文版

3
CH3
1
Is 5-(1’-methylethyl)-2,2,4-trimethyloctane
CH3 CH3
Is 4,5-diethyl-2,2-dimethylheptane
It is NOT 3,4-diethyl-6,6-dimethylheptane!
4
H3C
H2 C
2
CH OH
CH3
4
H3C
R C O R
I
Bromide
R C O NH2
Iodide
R C O Cl
Carboxylic acid
R C O O C O
Ester
R R C N
Ketone
H R N R O O
Amide
Acid Chloride
Anhydride
Imine
Nitro groupFra bibliotekA cyclic ester is called a lactone, a cyclic amide a lactam
The ‘substitution level’ of a carbon atom in an organic compound is determined by the number of attached hydrogen atoms:
tertiary carbon (one H)
H3C H2 C H3C C H2 CH3 CH CH CH CH3 H2 C C CH3 CH3 CH3
CH3 CH C H2 CH3
is 3-methyloctane, not 5-methyloctane
2'
H3C
major chain
(完整版)化学专业英语

(完整版)化学专业英语一、基础词汇篇1. 原子与分子Atom(原子):物质的基本单位,由质子、中子和电子组成。
2. 化学反应Reactant(反应物):参与化学反应的物质。
Product(物):化学反应后的物质。
Catalyst(催化剂):能改变化学反应速率而本身不发生永久变化的物质。
3. 物质状态Solid(固体):具有一定形状和体积的物质。
Liquid(液体):具有一定体积,无固定形状的物质。
Gas(气体):无固定形状和体积的物质。
4. 酸碱盐Acid(酸):在水溶液中能电离出氢离子的物质。
Base(碱):在水溶液中能电离出氢氧根离子的物质。
Salt(盐):由酸的阴离子和碱的阳离子组成的化合物。
5. 溶液与浓度Solution(溶液):由溶剂和溶质组成的均匀混合物。
Solvent(溶剂):能溶解其他物质的物质。
Solute(溶质):被溶解的物质。
Concentration(浓度):溶液中溶质含量的度量。
二、专业术语篇1. 有机化学Organic Chemistry(有机化学):研究碳化合物及其衍生物的化学分支。
Functional Group(官能团):决定有机化合物化学性质的原子或原子团。
Polymer(聚合物):由许多重复单元组成的大分子化合物。
2. 无机化学Inorganic Chemistry(无机化学):研究不含碳的化合物及其性质的化学分支。
Crystal(晶体):具有规则排列的原子、离子或分子的固体。
OxidationReduction Reaction(氧化还原反应):涉及电子转移的化学反应。
3. 物理化学Physical Chemistry(物理化学):研究化学现象与物理现象之间关系的化学分支。
Chemical Bond(化学键):原子间相互作用力,使原子结合成分子。
Thermodynamics(热力学):研究能量转换和物质性质的科学。
4. 分析化学Analytical Chemistry(分析化学):研究物质的组成、结构和性质的科学。
常见化学专业词汇英文翻译

常见化学专业词汇英文翻译1. The Ideal-Gas Equation 理想气体状态方程2. Partial Pressures 分压3. Real Gases: Deviation from Ideal Behavior 真实气体:对理想气体行为的偏离4. The van der Waals Equation 范德华方程5. System and Surroundings 系统与环境6. State and State Functions 状态与状态函数7. Process 过程8. Phase 相9. The First Law of Thermodynamics 热力学第一定律10. Heat and Work 热与功11. Endothermic and Exothermic Processes 吸热与发热过程12. Enthalpies of Reactions 反应热13. Hess's Law 盖斯定律14. Enthalpies of Formation 生成焓15. Reaction Rates 反应速率16. Reaction Order 反应级数17. Rate Constants 速率常数18. Activation Energy 活化能19. The Arrhenius Equation 阿累尼乌斯方程20. Reaction Mechanisms 反应机理21. Homogeneous Catalysis 均相催化剂22. Heterogeneous Catalysis 非均相催化剂23. Enzymes 酶24. The Equilibrium Constant 平衡常数25. the Direction of Reaction 反应方向26. Le Chatelier's Principle 列·沙特列原理27. Effects of Volume, Pressure, Temperature Changes and Catalystsi. 体积,压力,温度变化以及催化剂的影响28. Spontaneous Processes 自发过程29. Entropy (Standard Entropy) 熵(标准熵)30. The Second Law of Thermodynamics 热力学第二定律31. Entropy Changes 熵变32. Standard Free-Energy Changes 标准自由能变33. Acid-Bases 酸碱34. The Dissociation of Water 水离解35. The Proton in Water 水合质子36. The pH Scales pH值37. Bronsted-Lowry Acids and Bases Bronsted-Lowry 酸和碱38. Proton-Transfer Reactions 质子转移反应39. Conjugate Acid-Base Pairs 共轭酸碱对40. Relative Strength of Acids and Bases 酸碱的相对强度41. Lewis Acids and Bases 路易斯酸碱42. Hydrolysis of Metal Ions 金属离子的水解43. Buffer Solutions 缓冲溶液44. The Common-Ion Effects 同离子效应45. Buffer Capacity 缓冲容量46. Formation of Complex Ions 配离子的形成47. Solubility 溶解度48. The Solubility-Product Constant Ksp 溶度积常数49. Precipitation and separation of Ions 离子的沉淀与分离50. Selective Precipitation of Ions 离子的选择沉淀51. Oxidation-Reduction Reactions 氧化还原反应52. Oxidation Number 氧化数53. Balancing Oxidation-Reduction Equations 氧化还原反应方程的配平54. Half-Reaction 半反应55. Galvani Cell 原电池56. Voltaic Cell 伏特电池57. Cell EMF 电池电动势58. Standard Electrode Potentials 标准电极电势59. Oxidizing and Reducing Agents 氧化剂和还原剂60. The Nernst Equation 能斯特方程61. Electrolysis 电解62. The Wave Behavior of Electrons 电子的波动性63. Bohr's Model of The Hydrogen Atom 氢原子的波尔模型64. Line Spectra 线光谱65. Quantum Numbers 量子数66. Electron Spin 电子自旋67. Atomic Orbital 原子轨道68. The s (p, d, f) Orbital s(p,d,f)轨道69. Many-Electron Atoms 多电子原子70. Energies of Orbital 轨道能量71. The Pauli Exclusion Principle 泡林不相容原理72. Electron Configurations 电子构型73. The Periodic Table 周期表74. Row 行75. Group 族76. Isotopes, Atomic Numbers, and Mass Numbers 同位素,原子数,质量数77. Periodic Properties of the Elements 元素的周期律78. Radius of Atoms 原子半径79. Ionization Energy 电离能80. Electronegativity 电负性81. Effective Nuclear Charge 有效核电荷82. Electron Affinities 亲电性83. Metals 金属84. Nonmetals 非金属85. Valence Bond Theory 价键理论86. Covalence Bond 共价键87. Orbital Overlap 轨道重叠88. Multiple Bonds 重键89. Hybrid Orbital 杂化轨道90. The VSEPR Model 价层电子对互斥理论91. Molecular Geometries 分子空间构型92. Molecular Orbital 分子轨道93. Diatomic Molecules 双原子分子94. Bond Length 键长95. Bond Order 键级96. Bond Angles 键角97. Bond Enthalpies 键能98. Bond Polarity 键矩99. Dipole Moments 偶极矩100. Polarity Molecules 极性分子101. Polyatomic Molecules 多原子分子102. Crystal Structure 晶体结构103. Non-Crystal 非晶体104. Close Packing of Spheres 球密堆积105. Metallic Solids 金属晶体106. Metallic Bond 金属键107. Alloys 合金108. Ionic Solids 离子晶体109. Ion-Dipole Forces 离子偶极力110. Molecular Forces 分子间力111. Intermolecular Forces 分子间作用力112. Hydrogen Bonding 氢键113. Covalent-Network Solids 原子晶体114. Compounds 化合物115. The Nomenclature, Composition and Structure of Complexes 配合物的命名,组成和结构116. Charges, Coordination Numbers, and Geometries 电荷数、配位数、及几何构型117. Chelates 螯合物118. Isomerism 异构现象119. Structural Isomerism 结构异构120. Stereoisomerism 立体异构121. Magnetism 磁性122. Electron Configurations in Octahedral Complexes 八面体构型配合物的电子分布123. Tetrahedral and Square-planar Complexes 四面体和平面四边形配合物124. General Characteristics 共性125. s-Block Elements s区元素126. Alkali Metals 碱金属127. Alkaline Earth Metals 碱土金属128. Hydrides 氢化物129. Oxides 氧化物130. Peroxides and Superoxides 过氧化物和超氧化物131. Hydroxides 氢氧化物132. Salts 盐133. p-Block Elements p区元素134. Boron Group (Boron, Aluminium, Gallium, Indium, Thallium) 硼族(硼,铝,镓,铟,铊)135. Borane 硼烷136. Carbon Group (Carbon, Silicon, Germanium, Tin, Lead) 碳族(碳,硅,锗,锡,铅)137. Graphite, Carbon Monoxide, Carbon Dioxide 石墨,一氧化碳,二氧化碳138. Carbonic Acid, Carbonates and Carbides 碳酸,碳酸盐,碳化物139. Occurrence and Preparation of Silicon 硅的存在和制备140. Silicic Acid,Silicates 硅酸,硅酸盐141. Nitrogen Group (Phosphorus, Arsenic, Antimony, and Bismuth) 氮族(磷,砷,锑,铋)142. Ammonia, Nitric Acid, Phosphoric Acid 氨,硝酸,磷酸143. Phosphorates, phosphorus Halides 磷酸盐,卤化磷144. Oxygen Group (Oxygen, Sulfur, Selenium, and Tellurium) 氧族元素(氧,硫,硒,碲)145. Ozone, Hydrogen Peroxide 臭氧,过氧化氢146. Sulfides 硫化物147. Halogens (Fluorine, Chlorine, Bromine, Iodine) 卤素(氟,氯,溴,碘)148. Halides, Chloride 卤化物,氯化物149. The Noble Gases 稀有气体150. Noble-Gas Compounds 稀有气体化合物151. d-Block elements d区元素152. Transition Metals 过渡金属153. Potassium Dichromate 重铬酸钾154. Potassium Permanganate 高锰酸钾155. Iron Copper Zinc Mercury 铁,铜,锌,汞156. f-Block Elements f区元素157. Lanthanides 镧系元素158. Radioactivity 放射性159. Nuclear Chemistry 核化学160. Nuclear Fission 核裂变161. Nuclear Fusion 核聚变162. analytical chemistry 分析化学163. qualitative analysis 定性分析164. quantitative analysis 定量分析165. chemical analysis 化学分析166. instrumental analysis 仪器分析167. titrimetry 滴定分析168. gravimetric analysis 重量分析法169. regent 试剂170. chromatographic analysis 色谱分析171. product 产物172. electrochemical analysis 电化学分析173. on-line analysis 在线分析174. macro analysis 常量分析175. characteristic 表征176. micro analysis 微量分析177. deformation analysis 形态分析178. semimicro analysis 半微量分析179. systematical error 系统误差180. routine analysis 常规分析181. random error 偶然误差182. arbitration analysis 仲裁分析183. gross error 过失误差184. normal distribution 正态分布185. accuracy 准确度186. deviation偏差187. precision 精密度188. relative standard deviation 相对标准偏差(RSD)189. coefficient variation 变异系数(CV)190. confidence level 置信水平191. confidence interval 置信区间192. significant test 显著性检验193. significant figure 有效数字194. standard solution 标准溶液195. titration 滴定196. stoichiometric point 化学计量点197. end point滴定终点198. titration error 滴定误差199. primary standard 基准物质200. amount of substance 物质的量201. standardization 标定202. chemical reaction 化学反应203. concentration浓度204. chemical equilibrium 化学平衡205. titer 滴定度206. general equation for a chemical reaction化学反应的通式207. proton theory of acid-base 酸碱质子理论208. acid-base titration 酸碱滴定法209. dissociation constant 解离常数210. conjugate acid-base pair 共轭酸碱对211. acetic acid 乙酸212. hydronium ion水合氢离子213. electrolyte 电解质214. ion-product constant of water 水的离子积215. ionization 电离216. proton condition 质子平衡217. zero level零水准218. buffer solution缓冲溶液219. methyl orange 甲基橙220. acid-base indicator 酸碱指示剂221. phenolphthalein 酚酞222. coordination compound 配位化合物223. center ion 中心离子224. cumulative stability constant 累积稳定常数225. alpha coefficient 酸效应系数226. overall stability constant 总稳定常数227. ligand 配位体228. ethylenediamine tetraacetic acid 乙二胺四乙酸229. side reaction coefficient 副反应系数230. coordination atom 配位原子231. coordination number 配位数232. lone pair electron 孤对电子233. chelate compound 螯合物234. metal indicator 金属指示剂235. chelating agent 螯合剂236. masking 掩蔽237. demasking 解蔽238. electron 电子239. catalysis 催化240. oxidation氧化241. catalyst 催化剂242. reduction 还原243. catalytic reaction 催化反应244. reaction rate 反应速率245. electrode potential 电极电势246. activation energy 反应的活化能247. redox couple 氧化还原电对248. potassium permanganate 高锰酸钾249. iodimetry碘量法250. potassium dichromate 重铬酸钾251. cerimetry 铈量法252. redox indicator 氧化还原指示253. oxygen consuming 耗氧量(OC)254. chemical oxygen demanded 化学需氧量(COD) 255. dissolved oxygen 溶解氧(DO)256. precipitation 沉淀反应257. argentimetry 银量法258. heterogeneous equilibrium of ions 多相离子平衡259. aging 陈化260. postprecipitation 继沉淀261. coprecipitation 共沉淀262. ignition 灼烧263. fitration 过滤264. decantation 倾泻法265. chemical factor 化学因数266. spectrophotometry 分光光度法267. colorimetry 比色分析268. transmittance 透光率269. absorptivity 吸光率270. calibration curve 校正曲线271. standard curve 标准曲线272. monochromator 单色器273. source 光源274. wavelength dispersion 色散275. absorption cell吸收池276. detector 检测系统277. bathochromic shift 红移278. Molar absorptivity 摩尔吸光系数279. hypochromic shift 紫移280. acetylene 乙炔281. ethylene 乙烯282. acetylating agent 乙酰化剂283. acetic acid 乙酸284. adiethyl ether 乙醚285. ethyl alcohol 乙醇286. acetaldehtde 乙醛287. β-dicarbontl compound β-二羰基化合物288. bimolecular elimination 双分子消除反应289. bimolecular nucleophilic substitution 双分子亲核取代反应290. open chain compound 开链族化合物291. molecular orbital theory 分子轨道理论292. chiral molecule 手性分子293. tautomerism 互变异构现象294. reaction mechanism 反应历程295. chemical shift 化学位移296. Walden inversio 瓦尔登反转n297. Enantiomorph 对映体298. addition rea ction 加成反应299. dextro- 右旋300. levo- 左旋301. stereochemistry 立体化学302. stereo isomer 立体异构体303. Lucas reagent 卢卡斯试剂304. covalent bond 共价键305. conjugated diene 共轭二烯烃306. conjugated double bond 共轭双键307. conjugated system 共轭体系308. conjugated effect 共轭效应309. isomer 同分异构体310. isomerism 同分异构现象311. organic chemistry 有机化学312. hybridization 杂化313. hybrid orbital 杂化轨道314. heterocyclic compound 杂环化合物315. peroxide effect 过氧化物效应t316. valence bond theory 价键理论317. sequence rule 次序规则318. electron-attracting grou p 吸电子基319. Huckel rule 休克尔规则320. Hinsberg test 兴斯堡试验321. infrared spectrum 红外光谱322. Michael reacton 麦克尔反应323. halogenated hydrocarbon 卤代烃324. haloform reaction 卤仿反应325. systematic nomenclatur 系统命名法e326. Newman projection 纽曼投影式327. aromatic compound 芳香族化合物328. aromatic character 芳香性r329. Claisen condensation reaction克莱森酯缩合反应330. Claisen rearrangement 克莱森重排331. Diels-Alder reation 狄尔斯-阿尔得反应332. Clemmensen reduction 克莱门森还原333. Cannizzaro reaction 坎尼扎罗反应334. positional isomers 位置异构体335. unimolecular elimination reaction 单分子消除反应336. unimolecular nucleophilic substitution 单分子亲核取代反应337. benzene 苯338. functional grou 官能团p339. configuration 构型340. conformation 构象341. confomational isome 构象异构体342. electrophilic addition 亲电加成343. electrophilic reagent 亲电试剂344. nucleophilic addition 亲核加成345. nucleophilic reagent 亲核试剂346. nucleophilic substitution reaction亲核取代反应347. active intermediate 活性中间体348. Saytzeff rule 查依采夫规则349. cis-trans isomerism 顺反异构350. inductive effect 诱导效应 t351. Fehling's reagent 费林试剂352. phase transfer catalysis 相转移催化作用353. aliphatic compound 脂肪族化合物354. elimination reaction 消除反应355. Grignard reagent 格利雅试剂356. nuclear magnetic resonance 核磁共振357. alkene 烯烃358. allyl cation 烯丙基正离子359. leaving group 离去基团360. optical activity 旋光性361. boat confomation 船型构象362. silver mirror reaction 银镜反应363. Fischer projection 菲舍尔投影式364. Kekule structure 凯库勒结构式365. Friedel-Crafts reaction 傅列德尔-克拉夫茨反应366. Ketone 酮367. carboxylic acid 羧酸368. carboxylic acid derivative 羧酸衍生物369. hydroboration 硼氢化反应370. bond oength 键长371. bond energy 键能372. bond angle 键角373. carbohydrate 碳水化合物374. carbocation 碳正离子375. carbanion 碳负离子376. alcohol 醇377. Gofmann rule 霍夫曼规则378. Aldehyde 醛379. Ether 醚380. Polymer 聚合物相对原子质量 relative atomic mass消去反应 elimination reaction硝化反应 nitratlon reaction硝酸钡 barium nitrate硝酸银 silver nitrate溴的四氯化碳溶液 solution of bromine in carbon tetrachloride 溴化钠 sodium bromide溴水 bromine water溴水 bromine water盐类的水解 hydrolysis of salts盐析 salting-out焰色反应 flame test氧化剂 oxidizing agent氧化铝 aluminium oxide氧化铁 iron (III) oxide乙醇 ethanol乙醛 ethana1乙炔 ethyne乙酸 ethanoic acid乙酸乙酯 ethyl acetate乙烯 ethene银镜反应 silver mirror reaction硬脂酸 stearic acid油脂 oils and fats有机化合物 organic compound元素周期表 periodic table of elements元素周期律 periodic law of elements原电池 primary battery原子序数 atomic number皂化反应 saponification粘合剂 adhesive蔗糖 sucrose指示剂 Indicator酯 ester酯化反应 esterification周期 period族 group(主族:main group)Bunsen burner 本生灯product 化学反应产物flask 烧瓶apparatus 设备PH indicator PH值指示剂,氢离子(浓度的)负指数指示剂 matrass 卵形瓶litmus 石蕊litmus paper 石蕊试纸graduate, graduated flask 量筒,量杯reagent 试剂test tube 试管burette 滴定管retort 曲颈甑still 蒸馏釜cupel 烤钵crucible pot, melting pot 坩埚pipette 吸液管filter 滤管stirring rod 搅拌棒element 元素body 物体compound 化合物atom 原子gram atom 克原子atomic weight 原子量atomic number 原子数atomic mass 原子质量molecule 分子electrolyte 电解质ion 离子anion 阴离子cation 阳离子electron 电子isotope 同位素isomer 同分异物现象polymer 聚合物symbol 复合radical 基structural formula 分子式valence, valency 价monovalent 单价bivalent 二价halogen 成盐元素bond 原子的聚合mixture 混合combination 合成作用compound 合成物alloy 合金organic chemistry 有机化学inorganic chemistry 无机化学derivative 衍生物series 系列acid 酸hydrochloric acid 盐酸sulphuric acid 硫酸nitric acid 硝酸aqua fortis 王水fatty acid 脂肪酸organic acid 有机酸 hydrosulphuric acid 氢硫酸 hydrogen sulfide 氢化硫alkali 碱,强碱ammonia 氨base 碱hydrate 水合物hydroxide 氢氧化物,羟化物hydracid 氢酸hydrocarbon 碳氢化合物,羟anhydride 酐alkaloid 生物碱aldehyde 醛oxide 氧化物phosphate 磷酸盐acetate 醋酸盐methane 甲烷,沼气butane 丁烷salt 盐potassium carbonate 碳酸钾soda 苏打sodium carbonate 碳酸钠caustic potash 苛性钾caustic soda 苛性钠ester 酯gel 凝胶体analysis 分解fractionation 分馏endothermic reaction 吸热反应exothermic reaction 放热反应precipitation 沉淀to precipitate 沉淀to distil, to distill 蒸馏distillation 蒸馏to calcine 煅烧to oxidize 氧化alkalinization 碱化to oxygenate, to oxidize 脱氧,氧化 to neutralize 中和to hydrogenate 氢化to hydrate 水合,水化to dehydrate 脱水fermentation 发酵solution 溶解combustion 燃烧fusion, melting 熔解alkalinity 碱性isomerism, isomery 同分异物现象 hydrolysis 水解electrolysis 电解electrode 电极anode 阳极,正极cathode 阴极,负极catalyst 催化剂catalysis 催化作用oxidization, oxidation 氧化reducer 还原剂dissolution 分解synthesis 合成reversible 可逆的摩尔 mole摩尔质量 molar mass品红 magenta或fuchsine葡萄糖 glucose气体摩尔体积 molar volume of gas铅蓄电池 lead storage battery强电解质 strong electrolyte氢氟酸 hydrogen chloride氢氧化铝 aluminium hydroxide取代反应 substitution reaction醛 aldehyde炔烃 alkyne燃料电池 fuel cell弱电解质 weak electrolyte石油 Petroleum水解反应 hydrolysis reaction四氯化碳 carbon tetrachloride塑料 plastic塑料的降解 plastic degradation塑料的老化 plastic ageing酸碱中和滴定 acid-base neutralization titration 酸雨 acid rain羧酸 carboxylic acid碳酸钠 sodium carbonate碳酸氢铵 ammonium bicarbonate碳酸氢钠 sodium bicarbonate糖类 carbohydrate烃 hydrocarbon烃的衍生物 derivative of hydrocarbon烃基 hydrocarbonyl同分异构体 isomer同素异形体 allotrope同位素 isotope同系物 homo1og涂料 coating烷烃 alkane物质的量 amount of substance物质的量浓度 amount-of-substance concentration of B 烯烃 alkene洗涤剂 detergent纤维素 cellulose相对分子质量 relative molecular mass极性键 polar bond加成反应 addition reaction加聚反应 addition polymerization甲烷 methane碱金属 alkali metal碱石灰 soda lime结构式 structural formula聚合反应 po1ymerization可逆反应 reversible reaction空气污染指数 air pollution index勒夏特列原理 Le Chatelier's principle离子反应 ionic reaction离子方程式 ionic equation离子键 ionic bond锂电池 lithium cell两性氢氧化物 amphoteric hydroxide两性氧化物 amphoteric oxide裂化 cracking裂解 pyrolysis硫氰化钾 potassium thiocyanate硫酸钠 sodium sulphide氯化铵 ammonium chloride氯化钡 barium chloride氯化钾 potassium chloride氯化铝 aluminium chloride氯化镁 magnesium chloride氯化氢 hydrogen chloride氯化铁 iron (III) chloride氯水 chlorine water麦芽糖 maltose煤 coal酶 enzyme二氧化氮 nitrogen dioxide二氧化硅 silicon dioxide二氧化硫 sulphur dioxide二氧化锰 manganese dioxide芳香烃 arene放热反应 exothermic reaction非极性分子 non-polar molecule非极性键 non-polar bond肥皂 soap分馏 fractional distillation酚 phenol复合材料 composite干电池 dry cell干馏 dry distillation甘油 glycerol高分子化合物 polymer共价键 covalent bond官能团 functional group光化学烟雾 photochemical fog过氧化氢 hydrogen peroxide合成材料 synthetic material合成纤维 synthetic fiber合成橡胶 synthetic rubber核电荷数 nuclear charge number核素 nuclide化学电源 chemical power source化学反应速率 chemical reaction rate 化学键 chemical bond化学平衡 chemical equilibrium还原剂 reducing agent磺化反应 sulfonation reaction霍尔槽 Hull Cell极性分子 polar moleculeActinium(Ac) 锕Aluminium(Al) 铝Americium(Am) 镅Antimony(Sb) 锑Argon(Ar) 氩Arsenic(As) 砷Astatine(At) 砹Barium(Ba) 钡Berkelium(Bk) 锫 Beryllium(Be) 铍 Bismuth(Bi) 铋Boron(B) 硼Bromine(Br) 溴Cadmium(Cd) 镉 Caesium(Cs) 铯Calcium(Ca) 钙Californium(Cf) 锎 Carbon(C) 碳Cerium(Ce) 铈Chlorine(Cl) 氯Chromium(Cr) 铬 Cobalt(Co) 钴Copper(Cu) 铜Curium(Cm) 锔Dysprosium(Dy) 镝 Einsteinium(Es) 锿 Erbium(Er) 铒Europium(Eu) 铕 Fermium(Fm) 镄 Fluorine(F) 氟Francium(Fr) 钫Gadolinium(Gd) 钆 Gallium(Ga) 镓Germanium(Ge) 锗 Gold(Au) 金Hafnium(Hf) 铪Helium(He) 氦Holmium(Ho) 钬 Hydrogen(H) 氢Indium(In) 铟Iodine(I) 碘Iridium(Ir) 铱Iron(Fe) 铁Krypton(Kr) 氪Lanthanum(La) 镧 Lawrencium(Lr) 铹Lead(Pb) 铅Lithium(Li) 锂Lutetium(Lu) 镥Magnesium(Mg) 镁Manganese(Mn) 锰Mendelevium(Md) 钔 Mercury(Hg) 汞 Molybdenum(Mo) 钼Neodymium(Nd) 钕Neon(Ne) 氖Neptunium(Np) 镎Nickel(Ni) 镍Niobium(Nb) 铌Nitrogen(N) 氮Nobelium(No) 锘Osmium(Os) 锇Oxygen(O) 氧Palladium(Pd) 钯Phosphorus(P) 磷Platinum(Pt) 铂Plutonium(Pu) 钚Polonium(Po) 钋Potassium(K) 钾Praseodymium(Pr) 镨Promethium(Pm) 钷Protactinium(Pa) 镤Radium(Ra) 镭Radon(Rn) 氡Rhenium(Re) 铼Rhodium(Rh) 铑Rubidium(Rb) 铷Ruthenium(Ru) 钌Samarium(Sm) 钐Scandium(Sc) 钪Selenium(Se) 硒Silicon(Si) 硅Silver(Ag) 银Sodium(Na) 钠Strontium(Sr) 锶Sulphur(S) 锍Tantalum(Ta) 钽Technetium(Tc) 锝Tellurium(Te) 碲Terbium(Tb) 铽Thallium(Tl) 铊Thorium(Th) 钍Tin(Sn) 锡Thulium(Tm) 铥Titanium(Ti) 钛Tungsten(W) 钨Uranium(U) 铀Vanadium(V) 钒Xenon(Xe) 氙Ytterbium(Yb) 镱Yttrium(Y) 钇Zinc(Zn) 锌Zirconium(Zr) 锆●化学常用词汇汉英对照表1●氨 ammonia氨基酸 amino acid铵盐 ammonium salt饱和链烃 saturated aliphatic hydrocarbon苯 benzene变性 denaturation不饱和烃 unsaturated hydrocarbon超导材料 superconductive material臭氧 ozone醇 alcohol次氯酸钾 potassium hypochlorite醋酸钠 sodium acetate蛋白质 protein氮族元素 nitrogen group element碘化钾 potassium iodide碘化钠 sodium iodide电化学腐蚀 electrochemical corrosion电解质 electrolyte电离平衡 ionization equilibrium电子云 electron cloud淀粉 starch淀粉碘化钾试纸 starch potassium iodide paper。
化学专业英语,中英互译,考前必备

1.有机化学Inorganic chemistry 无机化学organic chemistry物理化学physical chemistry 分析化学Analytical chemistry 生物化学biochemistry 化工原理principles of chemical engineering热力学thermodynamics2.烷烃Alkanes烯烃Alkenes 炔烃Alkynes 甲烷Methane乙烯Ethene 乙炔Ethyne环己烷Cyclohexane苯Naphthalene 醇Alcohols醚Ethers 酮Ketones醛Aldehydes酯Esters羧酸Fatty Acids胺Amines 氰Cyanides碳水化合物Carbohydrates氨基酸Amino Acides 蛋白质protein 脂质lipids3. 氨基甲酸酯carbamate沉淀precipitation测定determine 除草剂herbicide 单元操作Unit operations底物substrate 定性分析qualitative 定量分析gravimetric发色团chromophore 分离isolate 分析物analyte 反应速率Reaction rate 过滤filtration化学污染chemical pollution合成synthesize解吸附作用desorption基本原理fundamental principle科学领域scientific fields农药pesticide亲核试剂nucleophile 杀菌剂fungicide 石化产品Petrochemical 碳氢化合物Hydrocarbons 碳阳离子carbocation物性the properties of substance 新陈代谢metabolism吸附adsorption有毒化学品toxic chemical 有机氯化物Organic chloride自然变化natural changes准确度accuracy 助色团auxochrome 植物生长调节剂plant growth regulator蒸馏distillation4.烧杯beakers通风橱Furne hoods 锥形瓶Erlenmeyer圆底烧瓶Round bottom flask蒸馏烧瓶Distillation蒸发皿Evaporating dishes真空干燥器Vaccume dessicator5.Al2O3aluminium(III) oxide BaCl2 Barium(II) chloride BaI2 Barium iodide Ca(MnO4)2Calcium permanganate Cu2S copper(I) sulphide CuO Copper(II) oxide FeSO4 Iron(II) sulfate HClO4 perchloric acid H2SO4 sulphuric acid H2CO3Carbouic acide HPO4diammonium hydrogen phosphate KHSO4 potassium hydrogen sulphide KMnO4 Potassium permanganate (NH4)2CO3ammoniue carbonate (NH4)2SO4 Ammmonium sulfate N2O nitrogen(I) oxide NaH Sodium permanganate P4O6 tetraphosphorus hexaoxide PF5phosphorus pentafluoride P4O10 tetraphosphorus decaoxide Sncl4 tin(IV) chloride6.There are 20 standard amino acids , and they contain a carboxyl group ,an amino group ,and a side chain . 有20个标准氨基酸,和含有羧基、一个氨基和侧链。
基础有机化学英文词汇

Alkaloid ['ælkəlɔid] 生物碱Alkalinity [,ælkə’linəti] 碱性Anion ['ænaiən]阴离子Cation ['kætaiən]阳离子Bromine [’brəumi:n]溴Chlorine[’klɔ:ri:n] 氯Fluorine [’flu(:)əri:n]氟Iodine [’aiəudi:n]碘Beryllium 铍Bond dissociation energy键解离能Branched chain[brɑ:ntʃ, bræntʃ]支链Conjugate acid(base)[’kɔndʒuɡeit]共轭酸(碱) Localization [,ləukəlai’zeiʃən]定域Coordinate bond [kəu'ɔ:dineit] 配位键Covalent bond 共价键Delocalization 离域Neon ['ni:ɔn, —ən]氖Nitrogen [’naitrədʒən]氮Oxygen ['ɔksidʒən]氧Dye [dai] 染料Nucleophilic regeant [,nju:kliəu’filik]亲核试剂Electrophilic regeant [i,lektrəu’filik]亲电试剂Electronegativity [i’lektrəu,neɡə’tivəti]电负性Electron atmosphere 电子云Electrovalent bond 电价键Halogen [’hælədʒin]卤素Phosphorus ['fɔsfərəs] 磷Silicium [si’lisiəm] 硅Hydrogen [’haidrədʒən] 氢Hybridized orbital [’haibrid]杂化轨道Ion [’aiən]离子Stereochemistry [,steriəu'kemistri] 立体化学Stereo-isomerism [ai'sɔmərizəm] 立体异构现象Isomer ['aisəumə] 同分异构体Sulfur [’sʌlfə]硫Tetrahedron 正四面体Acetic acid [ə’si:tik]乙酸Acetylene [ə'setili:n]乙烯Acid anhydride 酸酐Acyclic hydrocarbon 链烃Acyl halide 酰卤Alcohol [’ælkəhɔl]醇Aldehyde [’ældihaid]醛Alicyclic compound [’æli’saiklik]脂环化合物Aliphatic heterocylcic compound [,æli'fætik]脂杂环化合物Alkane ['ælkein] 烷Alkene [’ælki:n] 烯Alkyne [’ælkain]炔Alkyl [’ælkil]烷基Amide [’æmaid]酰胺Amine [ə'mi:n, ’æmin]胺Amino [ə’mi:nəu] 氨基Anthracene [æn'θrəsi:n]蒽Arene [’æri:n]芳烃Aromaticity [ə,rəumə'tisəti] 芳香性Aromatic compound [,ærəu’mætik]芳香化合物Aromatic heterocyclic compound 芳杂环化合物Benzene [’b enzi:n]苯Biphenyl 联苯Carbocyclic compound 碳环化合物Carbonyl 羰基Carboxyl 羧基Carboxylic acid 羧酸Chiral carbon atom 手性碳原子Chirality [kai’ræliti:]手性Chloride [’klɔ:raid]氯化物Fluoride ['flu(:)əraid]氟化物Iodide [’aiəudaid]碘化物Chloroform [’klɔrəfɔ:m] 氯仿Cyano ['saiənəu]氰基Ester [’estə] 酯Ether ['i:θə] 醚Formic acid 甲酸Formyl 甲酰基Halohydrocarbon 卤代烃Heterocyclic compound 杂环化合物Hydroxyl [hai’drɔksil] 羟基Hydroxymethyl 羟甲基Imine [i’mi:n]亚胺Imino 亚氨基Iodoform 碘仿Isotope [’aisəutəup] 同位素Ketone [’ki:təun] 酮Lactide ['læktaid] 交酯Lactone [’læktəun] 内酯Lactam [’læktæm]内酰胺Lactim ['læktim]内酰亚胺Mercapto [mə’kæptəu]巯基Naphthalene [’næfθəli:n] 萘Nitrile ['naitrail] 腈Nitro [’naitrəu] 硝基Nitro compound 硝基化合物Nitroso [nai'trəusəu]亚硝基Peroxide [pə'rɔksaid] 过氧化合物Peroxy group 过氧基Phenanthrene [fi'nænθri:n] 菲Phenol ['fi:nɔl] 酚Phenyl ['fenəl] 苯基Primary carbon 一级碳Secondary carbon 二级碳Tertiary carbon [’tə:ʃəri]三级碳Quaternary carbon [kwə’tə:nəri]四级碳Spiro atom 螺原子Styrene 苯乙烯Sulfo 磺酸基Sulfonic acid [sʌl’fəunik] 磺酸Tautomeric isomer [,tɔ:tə’merik][’aisəumə] 互变异构体Tetrahydrofuran 四氢呋喃Thio—alcohol 硫醇Thio—phenol 硫酚Toluene [’tɔljui:n]甲苯Xylene 二甲苯Absolute configuration 绝对构型Conformation [,kɔnfɔ:’meiʃən] 构象Angle strain 角张力Torsion strain 扭转张力Asymmetric carbon [,æsi’metrik]不对称碳原子Asymmetric synthesis [’sinθisis]不对称合成Chiral axle ['kaiərəl] ['æksəl] 手性轴Chiral plane 手性面Elute [i'ju:t]洗脱Prochirality 前手性Racemate 外消旋体Racemic compound 外消旋化合物Racemization 外消旋化Racemize 外消旋Resolution [,rezə'lu:ʃən] 拆分Specific rotation 比旋光度Stereoselectiviey 立体选择性Stereospecificity 立体专一性Symmetric axle 对称轴Symmetric plane 对称面Tetrahedral configuration [kən,fiɡju’reiʃən]正四面体构型Activate intermediate [,intə’mi:djət, —dieit]活泼中间体Chlorizate 氯化Fluorinate 氟化Brominate 溴化Iodizate 碘化Aromatization [,ərəumətai’zeiʃən] 芳构化Nitration [nai'treiʃən]硝化Nitrate ['naitreit]硝酸盐Condensation [,kɔnden’seiʃən] 缩合反应Addition reaction 加成反应Electrophilic reaction 亲电反应Nucleophilic reaction 亲核反应Elementary reaction [,eli’mentəri] 基元反应Elimination reaction [i,limi’neiʃən] 消除反应Ionic reaction 离子反应Substitution reaction 取代反应Synergistic reaction [,sinə’dʒistik] 协同反应Free radical reaction 自由基反应Dynamics [dai’næmiks]动力学Thermodynamics [,θə:məudai'næmiks]热力学Endothermic reaction [,endəu'θə:mik]吸热反应Exothermic reaction [,eksəu’θə:mik]放热反应Equilibrium constant [,i:kwi’libriəm] 平衡常数Heterolysis [,hetə'rɔlisis]异裂Homolysis [həu'mɔlisis] 均裂Pyrolysis [paiə'rɔlisis] 热裂Rearrangement 重排反应Sulfonation 磺化反应Transition state [træn'siʒən] 过渡态Petroleum [pi'trəuliəm]石油Electron impact (EI)电子轰击Fast atom bom bardment 快原子轰击Infrared spectroscopy (IR)红外光谱Spectroscopy [spek’trɔskəupi]光谱学Spectrometer [spek’trɔmitə] 光谱仪Ultraviolet and visible spectrum (UV)[’spektrəm]紫外可见光谱Nuclear magnetic resonance (NMR)['rezənəns]核磁共振Anti elimination 反式消除Antiaddition 反式加成Bimolecular elimination 双分子消除Bimolecular nucleophilic substitution 双分子亲核取代Unimolecular nucleophilic substitution 单分子亲核取代Unimolecular elimination 单分子消除Nucleophilicity 亲核性Carbocation 碳正离子Carbanion 碳负离子Oxonium salt 样盐Polarizability 可极化性Conjugated system [’kɔndʒuɡeit]共轭系统Conjugation 共轭效应Electron-donating group 给电子基Electron—withdrawing group 吸电子基Hydrolysis [hai'drɔlisis] 水解Hydrophilic property ['prɔpəti] 亲水性Hyperconjugation 超共轭Inductive effect 诱导效应Isonitrile 异腈Internal nucleophilic substitution 分子内亲核取代Inversion of configuration 构型翻转Retention of configuration 构型保持Leaving group 离去基团Nitrite ['naitrait] 亚硝酸酯Nitroalkane 硝基烷Non—aqueous solvent 非水溶剂Non—polar solvent 非极性溶剂Proton solvent [’prəutɔn]质子溶剂Dipole solvent 偶极溶剂Regioselectivity 区域选择性Regiospecific 区域专一性Rigid structure 刚性结构Solvolysis 溶剂解效应Steric help effect [’sterik] 空助效应Steric hindrance effect 空阻效应Substrate ['sʌbstreit]底物Sulfate [’sʌlfeit] 硫酸酯Sulfonate [’sʌlfəneit]磺酸酯Borane [’bɔ:rein] 硼烷Silane [’silein] 硅烷Coupling reaction 偶联反应Hydrogenolysis [,haidrəudʒi'nɔlisis]氢解Lithium aluminium hydride 氢化铝锂Sodium boronhydride 硼氢化钠Carbene ['kɑ:bi:n]卡宾Carbenoid 类卡宾Catalytic hydrogenation [kætə’litik]催化氢化Conjugated diene 共轭二烯烃Cumulative diene [’kju:mju,leitiv] 累积二烯烃Isolated diene 孤立二烯烃Epoxidation 环氧化反应Heterogeneous catalyst [,hetərəu'dʒi:njəs]异相催化剂Homogeneous catalyst [,hɔmə’dʒi:niəs, ,həu-] [’kætəlist]均相催化剂Resonance formula 共振式Resonance structure 共振结构Resonance theory 共振论Epoxy compound 环氧化合物Aromatic electrophilic substitution 芳香亲电取代Ferrocene 二茂铁Chloromethylation 氯甲基化Acetal 缩醛Hemiacetal 半缩醛Aliphatic ether 脂肪醚Ortho formate 原甲酸酯Dimethyl sulfoxide 二甲亚砜Dismutation reaction 歧化反应Enamine 烯胺Enol form 烯醇式Hydrate 水合物Hydrazine 肼Hydrazone 腙Hydroxylamine 羟胺Ketal 缩酮Lactam 内酰胺Oxime 肟Phosphorus ylide 磷叶立德Simple ketone 单酮Decarboxylation 脱羧反应Intermolecular esterification 分子间酯化Intramolecular esterification 分子内酯化Acylation 酰化作用Acylating agent 酰化试剂Alcoholysis 醇解Cyanic acid 氰酸Isocyanic acid 异氰酸Isocyanate 异氰酸酯Imide 酰亚胺Furan 呋喃Pyridine 吡啶Pyrrole 吡咯Quinoline 喹啉Isoquinoline 异喹啉Azole 唑Imidazole 咪唑Indole 吲哚Thiazole 噻唑Isothiazole 异噻唑Oxazole 恶唑Isoxazole 异恶唑Diazine 二嗪Pyrazine 吡嗪Pyridazine 哒嗪Thiophene 噻吩Pyrimidine 嘧啶Purine 嘌呤Thiourea 硫脲Amidine 脒Guanidine 胍Benzofuran 苯并呋喃Benzothiophene 苯并噻吩Boron tribromide 三溴化硼Aniline 苯胺Benzyne 苯炔Fluoroboric acid 氟硼酸Diazomethane 重氮甲烷Aldol condensation 羟醛缩合Ambident anion 两位负离子Ambident reactivity 两位反应活性Lithium diisopropyl amine 二异丙基胺锂。
化工基础

《化学工程基础》教学大纲课程名称:化学工程基础英文名称:Chemical Engineering Foundation课程编号:课程类别:专业必修课学时/学分:54学时/3学分;理论学时:54学时开设学期:七开设单位:化学化工学院适用专业:化学说明一、课程性质与说明1.课程性质专业基础课2.课程说明《化工基础》是化学教育专业本科学生的专业基础课,它以"如何实现化学反应工业化"为主线,从化工生产过程的介绍入手,以典型产品示例,系统地分析有代表性的化工产品工艺,涉及化工单元操作、工业化学反应过程、工艺过程优化、技术经济分析、环境保护与三废处理及化工过程开发等内容。
化学工程基础实验是化学专业《化学工程基础》(简称《化工基础》)课程的重要组成部分。
二、教学目标本课程的主要任务是研究化工单元操作及反应过程的基本原理、典型设备的构造及工艺尺寸的计算,通过本课程的学习,使学生理解化学工程规律在化工生产中的应用,获得化工计算及设计的基础训练,培养学生分析和解决有关化工操作中各种问题的能力,以便在化工生产、科研和设计工作中达到强化生产过程、提高产品质量、提高设备生产能力和效率、降低设备投资及产品成本、节能、防止污染及加速新技术开发等方面的目的。
三、学时分配表四、教学教法建议理论讲授与学生探讨相结合五、课程考核及要求1.考核方式:考试(√)2.成绩评定:计分制:百分制(√)成绩构成:总成绩= 平时考核10% + 中期考核30% + 期末考核60%六、参考书目[1] 张近主编.《化学工程基础》.北京:高等教育出版社,2002.[2] 夏清主编.《化工原理》(修订版).天津:天津大学出版社,2005.[3] 陈敏恒主编.《化工原理》(第三版).北京:化学工业出版社,2006.[4] 谭天恩主编.《化工原理》(第二版).北京:化学工业出版社,1998.本文第一章绪论教学目标:了解化学工业概况、化工生产过程概述、化学工程学简介。
化学专业英语

alevel化学常用单词汇总

chapter 1 atomic structureelement n.元素all know materials can be broken down into fundamental substances we call element.我们所知道的所有物质都可以分解成原子。
atom n.原子atom is the smallest particle of matter having all that element ’s characteristics.原子时具有元素性质的最小粒子。
nucleus /’nju:kliəs ,’nu ːkli əs/ 原子核electron n.电子 proton 质子neutron 中子compound n. 化合物:When two or more elements combine and form a compound, a chemical change takes place.当两种或两种以上的元素结合形成化合物时, 发生化学变化。
化学中的物质分为单质和化合物,大部分元素是以化合物的形式存在atom nucleus election proton neutron {{(+)(-)的。
ion n. 离子:when an atom get or lost elections,it becomes ion.原子得失电子后形成离子。
cathode n. 阴极(negative electrode)Cathode rays are attracted by a positive charge.阴极射线被阳电荷所吸引。
anode n. 阳极(positive election)A red wire is often attached to the anode.红色电线通常与阳极相联。
particle n. 粒子:Particles include moleculars,atoms , protons, neutrons ,electrons and ions.微小粒子包括分子,原子,质子,中子,电子,离子等等。
化学课程英文名称对照表

化学课程英文名称对照表磁共振实验Magnetic Resonance Experiment磁共振在化学和生命科学中应用Application of Magnetic Resonance inChemistry and Life Science核磁共振波谱学Nuclear Magnetic Resonance Spectroscopy核磁共振新技术及其应用Movdern Nuclear Magnetic Resonance Spectroscopy and its Application角动量理论与原子结构Angular Momentum Theory & Atomic Structure聚合物改性原理与流变学The Principle of Modification and Theology of Polymer量子化学Quantum Chemistry量子化学计算方法Quantum Chemistry B谱学原理和应用Principles of Spectroscopes and TheirChemical Applications群论及其在量子化学中的应用Group Theory and Its Application to QuantumChemistry材料化学导论Introduction of Material Chemistry分析化学Analytical Chemistry高等无机化学Advanced Inorganic Chemistry高等有机化学Advanced Organic Chemistry结构化学Structural Chemistry结构化学Structural Chemistry无机化学Inorganic Chemistry无机化学Inorganic Chemistry无机及分析化学Inorganic and Analytic Chemistry无机及分析化学Inorganic and Analytic Chemistry物理化学Physical Chemistry物理化学Physical Chemistry物理化学Physical Chemistry物理化学Physical Chemistry物理化学Physical Chemistry仪器分析Instrumental Analysis仪器分析Instrumental Analysis仪器分析Instrumental Analysis有机化学Organic Chemistry有机化学Organic Chemistry有机化学Organic Chemistry有机化学Organic Chemistry量子无机化学Quantum Inorganic Chemistry量子有机化学Quantum Organic Chemistry植物生长与发育的化学调整Chemical Adjustment of Plant's Growth分析化学Analysis Chemistry分析化学及实验Analytical Chemistry and Experiment分析化学实验Experiment of Analytical Chemistry计算机基础(C语言) Computer Basis C Language普通物理学General Physics无机化学实验Experiment in Inorganic Chemistry无机化学实验Experiment in Inorganic Chemistry无机化学实验Experiment in Inorganic Chemistry无机化学实验Experiment in inorganic Chemistry物理化学实验Experiment of Physical Chemistry物理化学实验Experiment of Physical Chemistry物理化学实验Experiment of Physical Chemistry物理化学实验Experiment of Physical Chemistry仪器分析实验Experiments of Instrument Analysis有机化学实验Organic Chemistry Experiment有机化学实验Organic Chemistry Experiment有机过渡金属化学Organotransition Metal ChemistryX射线晶体结构分析Crystal Structure Analysis by X-ray电分析化学实验Experiment in Electroanalytical Chemistry分子发光分析Molecular Luminescence Analysis分子发光分析进展Advances in Molecular Luminescence Analysis 高分子材料化学Polymer Chemistry高分子材料研究方法Research Methods of Polymeric Materials高分子合成与分子设计Polymeric Synthesis and Molecular Design 高分子化学实验Experiment of Polymeric Chemistry高分子物理实验Experiment of Polymer Physics化学分离法Chemical Method of Separation化学计量学Chemometrics化学统计力学和应用Statistical Mechanics and Applications in Chemistry化学文献Chemical Document化学文献Scientific Literature in Chemistry化学文献Chemical Literature化学文献Chemical Literature Searching金属有机高聚物Metallic Organic Polymer近代电分析Electroanalytical Chemistry近代分离分析Analytical Separation and Chromatography近代光分析Modern Light Analysis配位化学* Coordination Chemistry配位化学选读Selective Topics on Modern Coordination Chemistry无机材料化学The Chemistry of Inorganic Materials无机材料实验Experiment in Inorganic Materials无机材料物理Polymeric Materials Physics无机合成与研究方法Synthesis and Research Methods for Inorganic Substance无机物研究法Research Methods of Inorganic Substance 无机元素化学Inorganic Elements Chemistry物化专门化实验Physical Chemistry Experiment物化专门化实验Physical Chemistry Experiment物化专门化实验Physical Chemistry Experiment物理有机化学Physical Organic Chemistry仪器分析选读Advanced Instrumentation in Chemistry有机分析Analysis and Structure Determination of Organic Compounds有机分析实验Organic Analytic Experiment有机合成Organic Synthesis有机合成化学Synthetic Organic Chemistry有机合成实验Organic Synthesis Experiment有机化学文献Document of Organic Chemistry专业英语(分析) Scientific English on Analytical Chemistry 专业英语(无机) Specialty English专业英语(有机) Subject English专业英语(物化) Chemistry English生物无机化学Bioinorganic Chemistry生物无机化学Bioinorganic Chemistry材料工程导论Introduction of Materials Engineering 环境分析化学Environmental Analytical Chemistry 界面化学Surface Chemistry应用电化学Applied Electronic chemistry荧光分析法Flueremetry普通化学General Chemistry普通化学实验General Chemistry Experiments。
805有机化学参考书

805有机化学参考书有机化学是研究有机化合物的结构、性质、合成和反应的一门学科。
对于学习有机化学的学生和研究人员来说,参考书是他们的重要学习资料。
下面介绍几本经典的有机化学参考书。
1.《有机化学》(Organic Chemistry)- Clayden, Greeves and Warren这本书是有机化学领域一部非常经典的教材,被广泛用于大学本科有机化学教学。
它详细介绍了有机化合物的结构、键的构成、化学反应、合成方法等内容。
此外,书中还附有大量的例题和习题,帮助读者巩固知识和提高解题能力。
2.《有机化学导论》(Organic Chemistry: An Introduction)- William H. Brown and Thomas Poon这本书适用于初学者或对有机化学感兴趣的非化学专业的人士。
它涵盖了有机化合物的基本概念、结构、反应和合成方法,并以易懂的语言和大量的示例解释了各种概念和原理。
此外,书中还有实验技术和实验室安全方面的内容,对有机化学实验有较高的教学参考价值。
3.《有机化学》(Organische Chemie)- Jonathan Clayden、Nick Greeves、Stuart Warren、Peter Wothers这本书是一本适用于化学专业学生和研究人员的有机化学教材。
它详细讲解了有机分子的结构和功能,包括键的构成、立体化学、反应机理、合成方法等内容。
此外,书中还有实验室技术、实验设计和数据解析等有机化学实验方面的内容,对于有机化学研究者具有很高的参考价值。
4.《有机化学导论》(Introduction to Organic Chemistry)- William Henry Brown and Lawrence S. Brown这本书是一本适用于学习有机化学的初学者的导论教材。
它详细介绍了有机化合物的性质、结构和反应,包括键、键的构成、键能和反应动力学等内容。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Foundations化学If chemistry is to be taught successfully, teachers must have a goodsubject matter knowledge (SK) of the ideas with which they are dealing,the nature of this falling within the orbit of philosophy of chemistry.They must also have a good pedagogic content knowledge (PCK), theability to communicate SK to students, the nature of this falling withinthe philosophy and psychology of chemical education. Taking the caseof models and modelling, important themes in the philosophy ofchemistry, an interview-based study was conducted into the SK and PCKof a sample of teachers in Brazil. This paper focuses on the results of theuniversity chemistry teacher sub-sample in that 3enquiry, analyses theirSK and PCK, and speculates on the implications of this for the educationof school teachers. Finally, it suggests approaches to the professionaldevelopment of university chemistry teachers that place an emphasis onthe philosophy of chemistry.If 化学是化学的教成功,教师必须拥有好学科知识(SK) 的想法,他们在处理,此范围内哲学轨道上的性质。
他们还必须有好教学内容知识(PCK),沟通的能力SK 对于学生来说,这属于化学教育心理学与哲学的性质。
哲学中的化学模型和建模、重要主题例,采访为基础进行分析研究的SK 和样品的巴西教师PCK。
本文重点大学化学老师子样本中,查询的结果,分析了它们的SK 和PCK,并推测这学校教师教育的影响。
最后,它建议重视化学哲学的大学化学教师的专业发展途径。
International 杂志的科学和数学教育The implementation of new content and pedagogical standards in scienceeducation in Israel as well as in other countries necessitates intensive,life-long professional development of science teachers. Here we describea model for the professional development of chemistry teacher-leaders.In the first part of the paper, we describe a model for the developmentand change of chemistry teacher-leaders. In the second part of the paper,we present the assessment of teachers' change. It is suggested, that inorder to become a leader, the teacher has to undergo several interrelatedphases of development and changes, namely personal, professional, andsocial. In 5order to attain these changes, a two-year program wasdesigned in which teachers were given opportunities to develop theircontent knowledge, pedagogical content knowledge, and their leadershipabilities and skills. The assessment of teachers' professional developmentclearly showed that engaging teachers in a long-term professionaldevelopment program changed their beliefs ( personal change) regardingtheir role as chemistry teachers in general and their confidence tobecome leaders in particular. In addition, we observed that the teacherschanged in their professional abilities as well as in their social behavior.We also report on the involvement of the teacher-leaders in activities inwhich leadership skills were implemented in attempting to reformchemistry education in Israel.执行新的内容和教学标准,在科学教育中以及以色列和其他国家一样,需要集约化、终身教师专业发展的科学。
在这里,我们描述了模型的化学老师领导的专业发展。
在本文的第一部分,我们描述了模型的发展和变化的化学老师领导人。
在本文第二部分,我们目前的评估教师的变化。
据了解,要想成为一个领导者,老师不得不接受几个相互关联的阶段的发展和变化,即个人、专业、和社会。
为了实现这些变化,设计了一个两年的课程中,教师都有机会发展其内容的知识、教学内容的知识,和他们的领导能力和技能。
教师的专业发展清楚地表明,在一般教师从事长期的职业发展计划改变他们的信仰 (个人变化) 关于他们作为化学教师的作用和他们的信心成为领导人特别是评估。
此外,我们观察到教师在其专业能力也他们社会的行为改变。
我们也报告参与老师领导人在活动中尝试在以色列化学教育改革实施的领导技能了。
International Journal of Science EducationEducation in Analytical Chemistry in Poland is mainly carried out atUniversities and Technical Universities according to a unifiedcurriculum. Courses on Analytical Chemistry in the second year and oninstrumental analysis in the third year are compulsory for all students ofchemistry. There are courses and lectures on specialized subjects in thefourth and fifth year for those who intend to subunit their thesis inAnalytical Chemistry.Education 在波兰分析化学中的主要被进行在大学和技术大学根据统一的课程。
分析化学课程第二年和第三年的仪器分析是化学的所有学生必修。
对于那些打算到亚基及其在分析化学中的论文第四和第五年有课程和专题讲座。
MacromolPolymer Education is considered against a background of four questions.Why do we teach Polymer Science? To whom and by whom is PolymerScience taught? How can instruction in basic Polymer Science be relatedto capability in Polymer Technology? What aspects are important indeveloping countries? In developing countries innovative technologicalresearch and gathering “tacit knowledge” are needed if they are to breakout of the “middle income trap”. So the education of researchers, mostlyin universities, as well as of those who will enter industry directly mustencourage holistic thinking. Polymer science is usually taught aspolymer chemistry, polymer physics and polymer engineering with verylittle connection between them. This article suggests how a bridge ofunderstanding, being a consideration of polymer molecular motion, caninter‐relate them.Polymer 教育被视为背景下的四个问题。
