Ammonium hydroxide treatment of Amyloid beta
澳门版本食品添加剂中英文名称对照

第223/2005號行政長官批示
行政長官行使《澳門特別行政區基本法》第五十條賦予的職權,並根據經十一月二十一日第56/94/M號法令及第7/2004號行政法規修訂的八月十七日第50/92/M號法令第五條第三款的規定,作出本批示。
一、一般食品添加劑的特定名稱載於本批示附件一。
二、按食品添加劑的使用性質而定的功能分類載於本批示附件二。
三、以上兩款所指的附件為本批示的組成部分。
四、在不影響下款規定的情況下,本批示於公佈即日生效。
五、附件一則於本批示公佈後一年生效。
*
* 已廢止 - 請查閱:第177/2006號行政長官批示
二零零五年六月十六日
行政長官何厚鏵
附件一
一般食品添加劑的特定名稱
(1)食品法典委員會採用的食品添加劑國際編碼系統的“INS”識別編號(2)歐盟採用的以“E”為詞頭的識別編號
(3)中國採用的“GB”識別編號。
ammonium_hydroxide
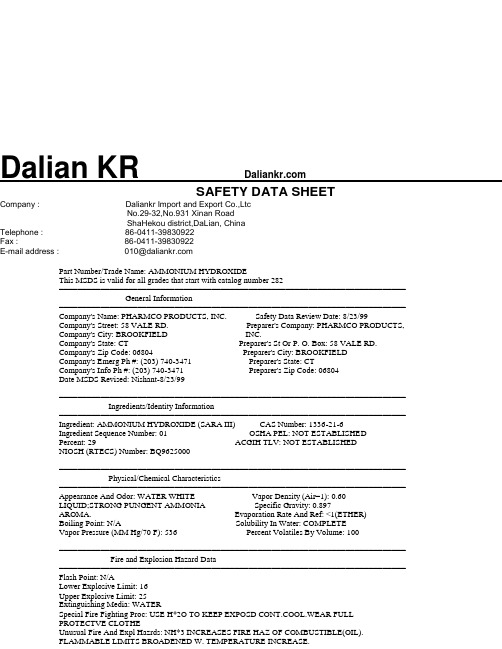
Dalian KR SAFETY DATA SHEETCompany : Daliankr Import and Export Co.,LtcNo.29-32,No.931 Xinan RoadShaHekou district,DaLian, ChinaTelephone : 86-0411-********Fax : 86-0411-********E-mail address : 010@Part Number/Trade Name: AMMONIUM HYDROXIDEThis MSDS is valid for all grades that start with catalog number 282=========================================================================== General Information=========================================================================== Company's Name: PHARMCO PRODUCTS, INC. Safety Data Review Date: 8/23/99 Company's Street: 58 VALE RD. Preparer's Company: PHARMCO PRODUCTS, Company's City: BROOKFIELD INC.Company's State: CT Preparer's St Or P. O. Box: 58 VALE RD. Company's Zip Code: 06804 Preparer's City: BROOKFIELDCompany's Emerg Ph #: (203) 740-3471 Preparer's State: CTCompany's Info Ph #: (203) 740-3471 Preparer's Zip Code: 06804Date MSDS Revised: Nishant-8/23/99=========================================================================== Ingredients/Identity Information=========================================================================== Ingredient: AMMONIUM HYDROXIDE (SARA III) CAS Number: 1336-21-6Ingredient Sequence Number: 01 OSHA PEL: NOT ESTABLISHED Percent: 29 ACGIH TLV: NOT ESTABLISHEDNIOSH (RTECS) Number: BQ9625000=========================================================================== Physical/Chemical Characteristics=========================================================================== Appearance And Odor: WATER WHITE Vapor Density (Air=1): 0.60LIQUID;STRONG PUNGENT AMMONIA Specific Gravity: 0.897AROMA. Evaporation Rate And Ref: <1(ETHER)Boiling Point: N/A Solubility In Water: COMPLETEVapor Pressure (MM Hg/70 F): 536 Percent Volatiles By Volume: 100=========================================================================== Fire and Explosion Hazard Data=========================================================================== Flash Point: N/ALower Explosive Limit: 16Upper Explosive Limit: 25Extinguishing Media: WATERSpecial Fire Fighting Proc: USE H*2O TO KEEP EXPOSD CONT.COOL.WEAR FULL PROTECTVE CLOTHEUnusual Fire And Expl Hazrds: NH*3 INCREASES FIRE HAZ OF COMBUSTIBLE(OIL). FLAMMABLE LIMITS BROADENED W. TEMPERATURE INCREASE.=========================================================================== Reactivity Data=========================================================================== MSDS 077, Rev 2.1, 12/05, DHAmmonium Hydroxide, Page 1 of 3Stability: YESCond To Avoid (Stability): HEAD DECOMPOSES W/O VIOLENCE IN ABSENCE OFCOMPATIBLE MATLMaterials To Avoid: STRONG OXIDIZERS,INCLUDING HALOGEN GASES(CL,BR,I, &ACIDS)Hazardous Decomp Products: GASEOUS NH*3 ON HEATNG.NITROGEN OXIDESEXPECTED.Hazardous Poly Occur: NOConditions To Avoid (Poly): N/A=========================================================================== Health Hazard Data=========================================================================== Signs/Symptoms Of Overexp: EYES,SKIN,INHALED:HIGHLY IRRITAT.INGEST: SEVEREBURNS TO GI TRAC. POSSIBLE DEATH FROM SHOCK/ASPHYXIA.Emergency/First Aid Proc: EYE:FLUSH W LG AMTS H*2O.SKIN:REMOVECLOTHES,WASH W LG AMTS H*2O INHALED:DON RESPIR PROTECTION,REMOVE TO FRESH AIR.GIVE CPR/O*2 IF NEED.KEEP PATIENT RECUMBENT.INGEST:DO NOT INDUCE VOMIT,GIV LG AMTS H*2O,OR DILUT VINEGAR,LEMON JUICE/OTHER.THEN DULCMN=========================================================================== Precautions for Safe Handling and Use=========================================================================== Steps If Matl Released/Spill: REMOVE UNPROTECTED PERSONS.PROTECTED PERSONSFLUSH W LG AMTS H*2O. LG SPILL:DIKE W SAND/INERT SOLID.PUMP INTO DRUMS,CLOSE,LABEL CORROSIVEWaste Disposal Method: DISPOSAL IS SUBJECT TO REGIONAL ERSSHOULD REVIEW OPERATIONS FOR COMPLIANCE,THEN CONSULT APPROP AGENCIES BEFORE DISPOSAL OF WASTE MATERIAL.Precautions-Handling/Storing: AVOID CONTACT W LIQ/GAS.AVOID HEAT/IGNITIONSOURCES.STORE OUTSIDE,OTHERWISE IN COOL,DRY,WELL VENTILATD.NON-COMBUSTIBLE AREA.PROTECT FROM PHYSICAL DAMAGEOther Precautions: FOR STORAGE CONSIDER:POPULATION,PROXIMITY TO H*2OSUPPLIES.CLOSED CONTAINERS PROVIDED W.SAFETY RELIEF VALVES.NEVER USE PRESSTO EMPTY.KEEP UPRIGHT.FOR SPILL:FLUSH W LG AMOUNTS OF WATER.=========================================================================== Control Measures=========================================================================== Respiratory Protection: GENERAL NOT REQD.SPILL:USE NIOSH APPRVD RESPIR FORNH*3,MIST,NO*2.Ventilation: LOCAL/MECHAN TO REDUCE AMMONIA LEVELS BELOW PERMISABLE LEVEL Protective Gloves: ALKALI RESISTNTEye Protection: SAFETY GLASSES NO CONTACTOther Protective Equipment: FULL PROTECTV CLOTHES,FACE SHIELD,EYE WASH,SAFETY SHOWER.Suppl. Safety & Health Data: CHEM NAME/SYNONYMS:AMMONIUM HYDROXIDE;AMMONIA,AQUEOUS;AMMONIA SOLN;AMMONIACAL LIQUOR.PER CONTACT W MFGR.P/N IS MFGR SPECIFIC DESIGNATION OF ITEM RATHER THAN GENERIC-AMMONIUM HYDROXIDE,25-30%=========================================================================== Transportation Data===========================================================================DOT Label: CORROSIVEIMO PSN Code: AWYDOT PSN Code: ANB IMO Proper Shipping Name: AMMONIADOT Proper Shipping Name: AMMONIA SOLUTION *SOLUTIONS IMO Regulations Page Number: 8111DOT Class: 8 IMO UN Number: 2672DOT ID Number: UN2672 IMO UN Class: 8DOT Pack Group: III IMO Subsidiary Risk Label: - MSDS 077, Rev 2.1, 12/05, DHAmmonium Hydroxide, Page 2 of 3IATA PSN Code: BKDIATA UN ID Number: 2672IATA Proper Shipping Name: AMMONIA SOLUTION *IATA UN Class: 8IATA Label: CORROSIVEAFI PSN Code: BKD AFI Prop. Shipping Name: AMMONIA SOLUTIONSAFI Class: 8AFI ID Number: UN2672AFI Pack Group: IIIAFI Label: CORROSIVEAFI Basic Pac Ref: 12-5===========================================================================Disposal Data=========================================================================== Landfill Ban Item: YESDisposal Supplemental Data: CHEM NAME/SYNONYMS:AMMONIUM HYDROXIDE;AMMONIA, AQUEOUS;AMMONIA SOLN;AMMONIACAL LIQUOR.PER CONTACT W MFGR.P/N IS MFGR SPECIFIC DESIGNATION OF ITEM RATHER THAN GENERIC-AMMONIUM HYDROXIDE, 25-30%IN CASE OF ACCIDENTAL EXPOSURE OR DISCHARGE, CONSULT HEALTH AND SAFETY FILE FOR PRECAUTIONS.1st EPA Haz Wst Code New: D0021st EPA Haz Wst Name New: CORROSIVE1st EPA Haz Wst Char New: CORROSIVITY1st EPA Acute Hazard New: NO===========================================================================Label Data=========================================================================== Special Hazard Precautions: CONTACT CAUSES BURNS TO SKIN AND EYES. IFINHALED, MAY BE HARMFUL. FIRE MAY PRODUCE IRRITATING OR POISONOUS GASES. RUNOFF FROM FIRE CONTROL OR DILUTION WATER MAY CAUSE POLLUTION.The information contained herein is based on data considered to be accurate. However, no warranty is expressed regarding the accuracy of these data or the results to be obtained from the use thereof. It is t he user’s obligation to determine the conditions of safe use of the product.MSDS 077, Rev 2.1, 12/05, DH Ammonium Hydroxide, Page 3 of 3。
奥托昆普哈贾瓦尔塔精炼厂镍电解工艺设计

Nickel electrolysis process at OutokumpuHarjavalta Metals Oy奥托昆普哈贾瓦尔塔金属公司镍电解工艺AbstractThis paper deals with the electrolysis of nickel from sulphate solution and its electrochemical principles. As an example, the nickel electrolysis process at Outokumpu Harjavalta Metals Oy is discussed in more detail. The leaching of nickel matte and the purification of the nickel sulphate solution prior to electrolysis is also discussed. In addition, a short review of other hydrometallurgical nickel matte treatment processes and nickel electrolysis technologies is given.摘要:本论文主要研究了硫酸镍溶液的电解过程及其电化学原理。
作为例子,本论文主要在细节方面讨论了奥托昆普哈贾瓦尔塔金属公司的镍电解过程。
同时也讨论了电解过程之前的镍浸出及净化过程。
除此之外,其它镍湿法冶金处理过程及电解技术也在文中涉及到。
Outokumpu has produced electrolytic nickel at Harjavaita works since 1960. Nickel is electrowon from a nickel sulphate solution using diaphragm cells where a diaphragm cloth is used to prevent the catholyte solution and the acidic anolyte frommixing. Nickel is deposited on thin nickel starter sheets and the anodes are of unalloyed lead. The current density is 200 A/m2 and the deposition time is seven days. The ready cathodes weigh about 65 kg and they are harvested, washed and cut into squares and strips and finally packed for delivery. Electrolytic nickel is supplied to the electroplating, melting and superalloying industry.早在1960年开始,奥托昆普便在哈贾瓦尔塔工厂开始生产电解镍。
欧盟关于食品添加剂的代码

Sodium stearoyl-2-lactylate
E482
Calcium stearoyl-2-lactylate
E483SteaΒιβλιοθήκη yl tartrateE491
Sorbitan monostearate
E492
Sorbitan tristearate
E493
Sorbitan monolaurate
Colours
E100
Curcumin
E101
(i) Riboflavin
(ii) Riboflavin-5'-phosphate
E102
Tartrazine
E104
Quinoline yellow
E110
Sunset Yellow FCF; Orange Yellow S
E120
Cochineal; Carminic acid; Carmines
E444
Sucrose acetate isobutyrate
E445
Glycerol esters of wood rosins
E460
Cellulose
E461
Methyl cellulose
E463
Hydroxypropyl cellulose
E464
Hydroxypropyl methyl cellulose
E283
Potassium propionate
E284
Boric acid
E285
Sodium tetraborate; borax
E1105
Lysozyme.
Antioxidants
E300
美国药典重金属检查法

美国药典重金属检查法231HEAVY METALSThis test is provided to demonstrate that the content of metallic impurities that are colored by sulfide ion, under the specified test conditions, does not exceed the Heavy metals limit specified in the individual monograph in percentage (by weight) of lead in the test substance, as determined by concomitant visual comparison (see Visual Comparison in the section Procedure underSpectrophotometry and Light-Scattering 851) with a control prepared from a Standard Lead Solution. [NOTE—Substances that typically will respond to this test are lead, mercury, bismuth, arsenic, antimony, tin, cadmium, silver, copper, and molybdenum.] Determine the amount of heavy metals by Method I, unless otherwise specified in the individual monograph. Method I is used for substances that yield clear, colorless preparations under the specified test conditions. Method II is used for substances that do not yield clear, colorless preparations under the test conditions specified for Method I, or for substances that, by virtue of their complex nature, interfere with the precipitation of metals by sulfide ion, or for fixed and volatile oils. Method III, a wet-digestion method, is used only in those cases where neither Method I nor Method II can be used.Special ReagentsLead Nitrate Stock Solution—Dissolve 159.8 mg of lead nitrate in 100 mL of water to which has been added 1 mL of nitric acid, then dilute with water to 1000 mL. Prepare and store this solution in glass containers free from soluble lead salts.Standard Lead Solution— On the day of use, dilute 10.0 mL of Lead Nitrate Stock Solution with water to 100.0 mL. Each mL of Standard Lead Solution contains the equivalent of 10 μg of lead. A comparison solution prepared on the basis of 100 μL of Standard Lead Solution per g of substance being tested contains the equivalent of 1 part of lead per million parts of substance being tested.METHOD IpH 3.5 Acetate Buffer—Dissolve 25.0 g of ammonium acetate in 25 mL of water, and add 38.0 mL of 6 N hydrochloric acid. Adjust, if necessary, with 6 N ammonium hydroxide or 6 N hydrochloric acid to a pH of 3.5, dilute with water to 100 mL, and mix.Standard Preparation— Into a 50-mL color-comparison tube pipet 2 mL of Standard Lead Solution (20 μg of Pb), and dilute with water to 25 mL. Using a pH meter or short-range pH indicator paper as external indicator, adjust with 1 N acetic acid or 6 N ammonium hydroxide to a pH between 3.0 and 4.0, dilute with water to 40 mL, and mix.Test Preparation— Into a 50-mL color-comparison tube place 25 mL of the solution prepared for the test as directed in the individual monograph; or, using the designated volume of acid where specified in the individual monograph, dissolve in and dilute with water to 25 mL the quantity, in g, of the substance to be tested, as calculated by the formula:2.0/(1000L),in which L is the Heavy metals limit, as a percentage. Using a pH meter or short-range pH indicator paper as external indicator, adjust with 1 N acetic acid or 6 N ammonium hydroxide to a pHbetween 3.0 and 4.0, dilute with water to 40 mL, and mix.Monitor Preparation— Into a third 50-mL color-comparison tube place 25 mL of a solution prepared as directed for Test Preparation, and add 2.0 mL of Standard Lead Solution. Using a pH meter or short-range pH indicator paper as external indicator, adjust with 1 N acetic acid or 6 N ammonium hydroxide to a pH between 3.0 and 4.0, dilute with water to 40 mL, and mix. Procedure— To each of the three tubes containing the Standard Preparation, the T est Preparation, and the Monitor Preparation, add 2 mL of pH 3.5 Acetate Buffer, then add 1.2 mL of thioacetamide–glycerin base TS, dilute with water to 50 mL, mix, allow to stand for 2 minutes, and view downward over a white surface *: the color of the solution from the Test Preparation is not darker than that of the solution from the Standard Preparation, and the color of the solution from the Monitor Preparation is equal to or darker than that of the solution from the Standard Preparation. [NOTE—If the color of the Monitor Preparation is lighter than that of the Standard Preparation, use Method II instead of Method I for the substance being tested.] METHOD IINOTE—This method does not recover mercury.pH 3.5 Acetate Buffer— Prepare as directed under Method I.Standard Preparation—Pipet 4 mL of the Standard Lead Solution into a suitable test tube, and add 10 mL of 6 N hydrochloric acid.Test Preparation— Use a quantity, in g, of the substance to be tested as calculated by the formula:4.0/(1000L),in which L is the Heavy metals limit, as a percentage. Transfer the weighed quantity of the substance to a suitable crucible, addsufficient sulfuric acid to wet the substance, and carefully ignite at a low temperature until thoroughly charred. (The crucible may be loosely covered with a suitable lid during the charring.) Add to the carbonized mass 2 mL of nitric acid and 5 drops of sulfuric acid, and heat cautiously until white fumes no longer are evolved. Ignite, preferably in a muffle furnace, at 500to 600, until the carbon is completely burned off (no longer than 2 hours). If carbon remains, allow the residue to cool, add a few drops of sulfuric acid, evaporate, and ignite again. Cool, add 5 mL of 6 N hydrochloric acid, cover, and digest on a steam bath for 10 minutes. Cool, and quantitatively transfer the solution to a test tube. Rinse the crucible with a second 5-mL portion of 6 N hydrochloric acid, and transfer the rinsing to the test tube.Monitor Preparation—Pipet 4 mL of the Standard Lead Solution into a crucible identical to that used for the Test Preparation and containing a quantity of the substance under test that is equal to 10% of the amount required for the Test Preparation. Evaporate on a steam bath to dryness. Ignite at the same time, in the same muffle furnace, and under the same conditions used for the Test Preparation. Cool, add 5 mL of 6 N hydrochloric acid, cover, and digest on a steam bath for 10 minutes. Cool, and quantitatively transfer to a test tube. Rinse the crucible with a second 5-mL portion of 6 N hydrochloric acid, and transfer the rinsing to the test tube.Procedure—Adjust the solution in each of the tubes containing the Standard Preparation, the Test Preparation, and the Monitor Preparation with ammonium hydroxide, added cautiously and dropwise, to a pH of 9. Cool, and adjust with glacial acetic acid, added dropwise, to a pH of 8, then add 0.5 mL in excess. Using a pH meter or short-range pH indicator paper asexternal indicator, check the pH, and adjust, if necessary, with 1 N acetic acid or 6 N ammonium hydroxide to a pH between 3.0 and 4.0. Filter, if necessary, washing the filter with a few mL of water, into a 50-mL color-comparison tube, and then dilute with water to 40 mL. Add 2 mL of pH 3.5 Acetate Buffer, then add 1.2 mL of thioacetamide–glycerin base TS, dilute with water to 50 mL, mix, allow to stand for 2 minutes, and view downward over a white surface*: the color of the solution from the Test Preparation is not darker than that of the solution from the Standard Preparation, and the color of the solution from the Monitor Preparation is equal to or darker than that of the solution from the Standard Preparation. [NOTE—If the color of the solution from the Monitor Preparation is lighter than that of the solution from the Standard Preparation, proceed as directed for Method III for the substance being tested.]METHOD IIIpH 3.5 Acetate Buffer— Prepare as directed under Method I.Standard Preparation— Transfer a mixture of 8 mL of sulfuric acid and 10 mL of nitric acid to a clean, dry, 100-mL Kjeldahl flask, and add a further volume of nitric acid equal to the incremental volume of nitric acid added to the Test Preparation. Heat the solution to the production of dense, white fumes; cool; cautiously add 10 mL of water; and, if hydrogen peroxide was used in treating the Test Preparation, add a volume of 30 percent hydrogen peroxide equal to that used for the substance being tested. Boil gently to the production of dense, white fumes. Again cool, cautiously add 5 mL of water, mix, and boil gently to the production of dense, white fumes and to a volume of 2 to 3 mL. Cool, dilute cautiously with a few mL of water, add 2.0 mL of Standard Lead Solution (20 μg of Pb), and mix. Transfer to a 50-mL color-comparison tube, rinse the flask with water, adding the rinsing to the tube until the volume is 25 mL, and mix.Test Preparation—Unless otherwise indicated in the individual monograph, use a quantity, in g, of the substance to be tested as calculated by the formula:2.0/(1000L),in which L is the Heavy metals limit, as a percentage.If the substance is a solid— Transfer the weighed quantity of the test substance to a clean, dry, 100-mL Kjeldahl flask. [NOTE—A 300-mL flask may be used if the reaction foams excessively.] Clamp the flask at an angle of 45, and add a sufficient quantity of a mixture of 8 mL of sulfuric acid and 10 mL of nitric acid to moisten the substance thoroughly. Warm gently until the reaction commences, allow the reaction to subside, and add portions of the same acid mixture, heating after each addition, until a total of 18 mL of the acid mixture has been added. Increase the amount of heat, and boil gently until the solution darkens. Cool, add 2 mL of nitric acid, and heat again until the solution darkens. Continue the heating, followed by addition of nitric acid until no further darkening occurs, then heat strongly to the production of dense, white fumes. Cool, cautiously add 5 mL of water, boil gently to the production of dense, white fumes, and continue heating until the volume is reduced to a few mL. Cool, cautiously add 5 mL of water, and examine the color of the solution. If the color is yellow, cautiously add 1 mL of 30 percent hydrogen peroxide, and again evaporate to the production of dense, white fumes and a volume of 2 to 3 mL. If the solution is still yellow, repeat the addition of 5 mL of water and the peroxide treatment. Cool, dilute cautiously witha few mL of water, and rinse into a 50-mL color-comparisontube, taking care that the combined volume does not exceed 25 mL.If the substance is a liquid— Transfer the weighed quantity of the test substance to a clean, dry, 100-mL Kjeldahl flask. [NOTE—A 300-mL flask may be used if the reaction foams excessively.] Clamp the flask at an angle of 45, and cautiously add a few mL of a mixture of 8 mL of sulfuric acid and 10 mL of nitric acid. Warm gently until the reaction commences, allow the reaction to subside, and proceed as directed for If the substance is a solid,beginning with ―add portions of the same acid mixture.‖Monitor Preparation— Proceed with the digestion, using the same amount of sample and the same procedure as directed in the subsection If the substance is a solid in the section Test Preparation, until the step ―Cool, dilute cautiously with a few mL of water.‖ Add 2.0 mL of Lead Standard Solution (20 μg of lead), and mix. Transfer to a 50-mL color comparison tube, rinse the flask with water, adding the rinsing to the tube until the volume is 25 mL, and mix. Procedure—Treat the Test Preparation, the Standard Preparation, and the Monitor Preparation as follows. Using a pH meter or short-range pH indicator paper as external indicator, adjust the solution to a pH between 3.0 and 4.0 with ammonium hydroxide (a dilute ammonia solution may be used, if desired, as the specified range is approached), dilute with water to 40 mL, and mix.To each tube add 2 mL of pH 3.5 Acetate Buffer, then add 1.2 mL of thioacetamide–glycerin base TS, dilute with water to 50 mL, mix, allow to stand for 2 minutes, and view downward over a white surface*: the color of the Test Preparation is not darker than that of the Standard Preparation, and the color of the Monitor Preparation is equal to or darker than that of the StandardPreparation.。
多孔硅纳米材料

Intracellular Degradation of Multilabeled Poly(Ethylene imine)−Mesoporous Silica−Silica Nanoparticles:Implications for Drug ReleaseLotta Bergman,†,‡Pasi Kankaanpa a,§Silja Tiitta,§Alain Duchanoy,†Ling Li,†Jyrki Heino,§and Mika Linde n*,‡†Laboratory for Physical Chemistry,Åbo Akademi University,Porthansgatan3-5,FI-20500Turku,Finland‡Inorganic Chemistry II,University of Ulm,Albert-Einstein-Allee11,D-89081Ulm,Germany§Department of Biochemistry and Food Chemistry,Vatselankatu2,Arcanum thirdfloor,University of Turku,FI-20014Turku, Finland*Supporting Informationlinked to different regions of the particles in order to study thehuman SAOS-2cells.A novel,quantitative method for nanoparticlemicroscopy is applied.Our results suggest that the core−shell−shelloutside cells,which is of high importance for further application of thisuorescence microscopy,surface functionalizationTargeted delivery of drugs is one of the most promising approaches for the delivery of drugs associated with severe side effects,especially of importance for chemotherapy-based cancer treatment.1−3Different nanoparticle-based drug delivery systems have been shown to accumulate in tumors either by passive or active targeting and to be taken up by target cells. While passive targeting is based on nanoparticle accumulation in tumors through the leaky nature of the tumor vasculature, active targeting is based on the attachment of cell-specific ligands onto the nanoparticle surface that are recognized by receptors overexpressed on the target cells.4,5Nanoparticle-based drug delivery has several attractive features in addition to cellular targeting,the most important being the possibility to achieve high drug-loading levels and controlled release profiles.A promising new nanoparticle platform attracting wide current interest is based on amorphous mesoporous silica nanoparticles (MSNs).6−8MSNs can be synthesized with controlled particle sizes and shapes,9and the pore dimensions can be tuned within a range of some nanometers to tens of nanometers,allowing both small molecular drugs and proteins or genes to be accommodated inside the mesopores.10−12Furthermore,sur-face functionalization is relatively straightforward13−15which allowsfine-tuning of the drug−support and particle−bioenvir-onment interactions.In most cases,active targeting of MSNs has been demonstrated under in vitro conditions but has also been shown to work in vivo.16−19However,in order for a nanoparticulate carrier system to be effective,premature leakage of the drug before reaching the target cells has to be kept at a minimum.This is especially important for MSNs,as the release rate from carriers where the drug release is diffusion-controlled is highest initially and also often connected with an initial burst release.20The extent of drug leakage is naturally also dependent on the physicochemical properties of the drug, such as drug solubility(often pH-dependent),and the degradation rate of the MSNs,the circulation time before reaching the target cells.Much recent focus has been put on developing means for drug release that can be triggered by intracellular processes or by external stimuli.There are two Received:October13,2012Revised:February21,2013Accepted:March19,2013Published:March19,2013main approaches for achieving triggerable drug release;covalent linking of the drug to the support through cleavable bonds,or functionalization of the outer surface of the MSNs using sheddable coatings or coatings which change conformation upon environmental changes,typically pH,redox level,or temperature.Several in vitro studies have demonstrated that such strategies do indeed decrease the level of premature drug release and also allow for subsequent intracellular release of the cargo.21−24Examples are bonds that can be cleaved at pH values lower than that in the plasma,as is the case in intracellular compartments and also within the interstitial space of solid tumors and within inflammatory tissues.Recently,we studied the therapeutic efficiency of MSNs surface function-alized by hyperbranched poly(ethylene imine),PEI,to which the cancer drug methotrexate(MTX)had been covalently linked.25MTX is an antimetobolite which inhibits the enzymatic activity of dihydrofolate reductase(DHFR),thus blocking the biosynthetic pathway of nucleotides and proteins.26Here,MTX served both as a targeting ligand and the drug,as MTX is structurally very similar to folic acid,an often employed targeting ligand,and both molecules are taken up by similar cellular routes.However,MTX has to reach the cytoplasm in order to be therapeutically active.Based on our findings we tentatively suggested that MTX predominantly remained covalently attached to the particles when outside the cells,and that MTX detachment occurred readily after particle endocytosis.Several possible explanations could account for this observation,including particle degradation through silica dissolution,27−29detachment of the PEI layer to which MTX is attached from the MSNs after particle endocytosis by the cells, enzymatic degradation of the peptide bond30linking MTX to the particles once the particles are inside endosomal vesicles,or a combination of these.In any case,the kinetics of detachment remains an open question.Furthermore,it was recently suggested that MSNs are exocytosed by cancer cells and that these are subsequently taken up by other cancer cells,31and one important question is if there would still be drug molecules present in MSNs that can be released in such“second generation”cells or if all the cargo was already released in the cell offirst entry.PEI-coated MSNs have also been suggested to preferentially lead to intracellular release of hydrophobic drugs or drug models physically adsorbed into the mesopores of the MSNs.32Clearly,the answer to such questions is bound to be strongly system dependent,but a question is also how this could be studied experimentally.Here we present data where nonporous silica core−mesoporous silica shell particles have been synthesized with a PEI layer attached to the mesoporous shell of the particles,thus creating core−shell−shell MSNs.All three parts of the particle were covalently labeled with different fluorescent dyes so that the detachment of different parts of the particle could be studied and evaluated semiquantitatively.A three-layer model particle design was chosen to ensure that we would be able to track the main particle independently from that of the mesoporous portion of the MSNs,as nonporous silica is degrading at a slower rate than its mesoporous counterparts.Confocalfluorescence microscopy studies were carried out on human osteosarcoma SAOS-2cells as a function of incubation time,and the images were analyzed using the BioimageXD software,33allowing particles being located outside of the cells to be distinguished from particles internalized by the cells.The data suggests that,even after four hours,particles inside cells still do partly contain the PEI-fluorophore layer representing the drug model,while a fair amount of the drug model has been detached from the particles mainly in the form of a PEI-mesoporous silica complex.The study represents afirst step toward reaching a better understanding about the intracellular decomposition of functionalized MSNs.2.EXPERIMENTAL SECTION2.1.Synthesis of Three-Layer Fluorescent Silica Nanoparticles.The solid silica particle core was prepared based on the procedure described by Sto b er et al.34In a typical synthesis,250μg(1mg/mL)offluorescein isothiocyanate isomer I,FITC(minimum90%HPLC,Sigma-Aldrich),was mixed with3-aminopropyltriethoxysilane,APTS(Sigma-Aldrich),under inert atmosphere and added to an alkaline (ammonium hydroxide solution,max33%NH3,puriss.,Sigma-Aldrich)solution together with tetraehoxyothosilicate TEOS (purum≥98%GC,Fluka).The resulting synthesis mixture had molar ratios of0.1FITC:242APTS:4630TEOS:1892 NH4OH:129684H2O:266744EtOH.The solution was stirred overnight350rpm at RT.The mesoporous surface layer was then introduced based on the method described by Kim et al.35The nonporous silica nanoparticles were separated,washed carefully,and dispersed into basic reaction solution.The structure-directing agent CTAB(Sigma-Aldrich)andfinally TEOS together with tetramethylrhodamine isothiocyanate TRITC(Sigma-Aldrich)fluorophore conjugated with APTS were continuously added to the synthesis,here proceeding step by step according to the reference.The resulting solution had molar ratios of TRITC 0.01:APTS0.025:CTAB158:TEOS440:NH4OH2882:EtOH 152574:H2O615220.Synthesis was stirred overnight at500 rpm,and particles were separated,washed,and dried in vacuo at298K.To remove surfactant,particles were extracted under sonication;30min in acidic ethanol(1:8-mixture of HCl and absolute ethanol).This treatment was repeated three times to ensure complete surfactant removal.Particles were further separated by centrifugation and carefully vacuum-dried over-night at298K.The so-synthesized core−shell particles were further surface-modified by hyperbranching polymerization of polyethyleneimine(PEI),using aziridine as a precursor. Aziridine was synthesized from aminoethylsulfuric acid (Sigma-Aldrich,Miss,USA)according to the procedure described by Allen et al.36The surface polymerization of PEI was performed in one step under argon as the protective gas. Particles were dispersed in toluene,and catalytic amounts of acetic acid were added,after which aziridine was added.In a typical conjugation,35μL of aziridine for100mg particles was used.The suspension was refluxed under stirring overnight at 348K,filtered,washed,and vacuum-dried at298K.The third fluorophor,Alexa633,was linked to the particle described above by attaching it to the surface amino groups.In a typical procedure,100mg of particles were dispersed in toluene. Surface primary amino groups were activated with DIPEA(100μL,1μL/mL in DMF)and further conjugated with Alexa633fluorophore(200μL,1μL/mL in DMF)under shaking for2h at RT(298K).Finally the particles were separated,washed with ethanol,and vacuum-dried at298K.2.2.Particle Characterization.The structure of the nanoparticles was confirmed by low angle,main reflection at 2.2°2Θ,powder-XRD using a Kratky compact small-angle system(Hecus Braun,Austria),Seifert ID-300X-ray generator with maximum intensity of50kV and40mA,and sample-to-detector distance of267mm.Thermogravimetric analysis was performed in air with a Netzsch STA449C cell setup with a heating rate of10K/min. Dynamic light-scattering(DLS)and zeta-potential measure-ments were performed using a Nano ZS(Malvern,Worcester-shire,UK)setup in a HEPES buffer.Measurements were performed at298K,using a monochromatic laser,with a working wavelength of632.8nm and using non-invasive back-scatter(NIBS),with the detector positioned at173°relative to the laser beam.Scanning electron microscopy(Jeol JSM-6335F,Jeol Ltd., Japan)was performed using an acceleration voltage of10kV and working distance of9.6mm.Transmission electron microscopy(TEM)micrographs were measured using a Zeiss Libra120TEM setup operated at80 kV.Nitrogen adsorption−desorption experiments(ASAP2010 sorptometer,Micromeritics)were carried out at77K.All samples were degassed for8h at323K before measurements. The specific surface area was determined by the BET method, and the pore dimensions were determined using the BJH method(desorption branch).2.3.Confocal Imaging and Image Analysis/Quantifi-cation.Particles were dispersed(1mg/mL)in water one hour before their delivery to cells.As silica particles do not hold intact in aqueous solvents and a relatively fast process of dissolution is reported for contact times below1h,the delay has to be taken into account when interpreting the imaging results.In this study,we concentrate on what happens to the particles in in vitro conditions at the exposion time point and1 and4h thereafter.Human SAOS-2cells were incubated in Dulbecco’s modified Eagle’s medium with the particles at a concentration of125μg/ mL.The cells werefixed with4%paraformaldehyde(20min RT)after5min,1h,or4h,and embedded in Mowiol. Thickness-selected cover glasses(0.17±0.01mm/Assistent) were used to minimizefluorescence intensityfluctuations.2D confocal images were acquired with a Zeiss AxioObserver Z1 (objective63x,1.4)equipped with LSM510,both near the glass surface and from the upper half of the cell.All three wavelengths(488,543,and633nm)were excited and recorded separately(multitracking)line-by-line,with pinhole-calibrated dichroics andfilters optimized for minimum bleed-through,and pinholes were adjusted for an optical slice thickness of700nm and pixel size of94nm×94nm for every wavelength. Acquisition settings and conditions were kept as constant as possible to enable the comparison offluorescence intensities. Importantly,images were always acquired with the same pixel density,roughly following the Nyquist theorem,and the detector sensitivity and background offset were kept constant at such values that the whole intensity range of the samples was recorded without saturation.Regions of interest(ROI)were drawn onto the images so that particles outside cells were analyzed separately from particles inside cells.In total approximately40images from inside and40from outside the cells were analyzed for each time point.Segmentation-based analyses were done byfirstfiltering the images with hybrid median2D and Gaussian smoothing (dimensionality3:4,4,2),then thresholding them for maximum object number(minimum object size10)andfinally running object separation(level1,image spacing used).The segmented objects were then analyzed for the intensities of each of the three wavelengths as well as average object size. Colocalization studies were performed to confirm the relation-ships of the different colors inside the cells,and all pixels in the images were analyzed,unlike in segmentation-based analyses. Colocalization analyses were done by using automatic thresh-olding and then recording all six possible Manders’colocalization coefficients,and observing changes in these between the three time points;5min,1h,and4h.All image analysis was done with the BioImageXD software.33The results were statistically analyzed with a t test for unequal variances and sample sizes,and significance was marked onto graphs as follows:ns=p>0.05,*=p<0.05,**=p<0.01,***=p<0.001.■RESULTS AND DISCUSSIONAn scanning electron microscopy(SEM)image of the core−shell−shell MSNs is shown in Figure1a.The particles have a diameter of about240nm and have a very narrow size distribution.A TEM image of a native silica core−shell MSN is shown in Figure1b.The core−shell structure of the MSNs is clearly seen,and the mesoporous shell is uniform.From the TEM image a mesoporous silica layer thickness of about12nm can be estimated,which corresponds to about30%of the total volume of the MSN.A nitrogen sorption isotherm measured for the native core−shell MSNs is shown in Figure2.A pronounced uptake at a relative pressure close to0.3p/p o is characteristic for MSNs synthesized using C16TAB as a structure-directing agent and corresponds tofilling of mesopores with a diameter of about4nm with a narrow pore size distribution.The BET specific surface area was344 m2/g,and the specific pore volume of the primary mesopores was0.34cm3/g,which is slightly higher than that expected from the relative contributions of the mesoporous surface layer and the solid core,suggesting that the thickness of the surface layer based on TEM might be underestimated.The pronounced uptake at higher p/p o is due tofilling of interparticulate porosity.The low-angle XRD pattern(Figure 3c)exhibited a main reflection at2.2°2Θand additional intensity in the regions expected for(11)and(20)reflections of a2D hexagonal mesophase,suggesting that the mesoporous surface layer is not disordered but has a structural motif similar to that observed for corresponding MSNs void of the solid core.Assuming a2D hexagonal arrangement of pores,the lattice spacing derived from the(10)reflection is4.83nm. Before PEI functionalization,the core−shell MSNs have a zeta-potential of−27mV in25mM HEPES pH7.2,which increases to+52mV after PEI functionalization,reflecting successful attachment of the highly cationic PEI layer.Thermogravimetric analysis shows a mass loss of7.1wt%in comparison to reference particles lacking PEI.Upon attachment of the Alexa 633dye to PEI,the zeta-potential increased slightly to55mV. The particles could be fully dispersed in HEPES,as evidenced by dynamic light-scattering(Figure S1).Thefluorescence emission colors of the different parts of the core−shell MSNs are schematically shown in Figure1a.The total amounts of fluorophores covalently attached to the PEI-MSNs were analyzed by dissolving the particles in2M NaOH-solution1 mg/mL and analyzing the dye content in the supernatant by fluorescence spectrometry.The PEI-MSNs contained Alexa633 1.09μg/mg particles(attached to the PEI layer),TRITC0.12μg/mg particles(attached to the mesoporous silica layer),and FITC0.04μg/mg particles(attached to the nonporous silica core).Fluorophore leakage was studied under in sink conditions (particle concentration75μg/mL)in HEPES buffer(pH7.2) at37°C,and no leakage of FITC or Alexa633was observed,but about 10%of the TRITC fluorophore was detected in the supernatant after the 4h incubation time.It was also ensured that the free fluorophore,de fined by the maximum concentration of FITC used in the study,could not be detected by confocal fluorescence microscopy under the applied imaging conditions.In the presence of cells no clumps or clusters of free fluorophore could be detected,and thereforeany detectable fluorescence signals could positively be attributed to fluorescence originating from the particles.While Alexa633and TRITC show pH-independent fluorescence intensities in the pH range of interest in our study (pH about 5−7.2),the fluorescence of FITC is known to be strongly pH dependent due to the p K a of FITC of 6.4.Tests using free FITC in bu ffers showed a decrease in the fluorescence intensity of about 50%at pH 6.5and of about 85%at pH 5.5as compared to the intensities measured for the same FITC concentration at pH 7.5,in good agreement with literature values.37Performing similar experiments using the core −shell particles void of PEI resulted in a FITC fluorescence intensity decrease of 60%at pH 5.5as compared to pH 7.5;that is,the decrease in emission intensity was lower than that of the free fluorophore.The smaller pH dependency of the FITC fluorescence emission intensity when incorporated into Sto b er-type silica is tentatively attributed to di fferent local pH values experienced by the probe inside the Sto b er particles(silicaFigure 1.Three color core −shell particles.(a)Schematic representation of the particle structure.Fluorescent dyes are covalently linked inside the silica network and likewise covalently linked to the surface polyethylene imine amino groups.(b)Scanning electron microscope image (SEM),scale bar corresponds to 1μm.(c)Transmission electron microscope image (TEM),scale bar corre-sponds to 20nm.Figure 2.(a)SAXS graph shows the ordered porosity of the mesoporous surface layer.(b)Nitrogen physisorption isotherm for prepared core −shell particles.(c)Nitrogen physisorption BJH analysis shows narrow pore size distribution in the mesoporous layer.contains Bro s tedt acidic silanol groups),38but the results also highlight that the Sto b er particles are not completely nonporous,as external pH changes can indeed be felt by the dye.Importantly,no pH-dependent changes in the fluorescence intensity of FITC were observed for PEI-coated core −shell particles,suggesting that the presence of PEI bu ffered the pH inside the core −shell particles.These results will be discussed below as a means for investigating the detachment kinetics of PEI from the MSNs,in addition to the analysis of the locus of the di fferent fluorescent dyes.For clarity,the di fferent fluorescence emission colors will be indicated together with their locus in the particles as follows;green (C)for FITC-core,yellow (MP)for TRITC-mesoporous shell,and red (PEI)for Alexa 633-PEI.3.3.Confocal Image Analysis. 3.3.1.Segmentations.Representative segmentations of the SAOS-2cells as a function of particle incubation times ranging from 5min to 4h are shown in Figure 3.The images were acquired in such a way that it could be distinguished whether fluorescence was originating from particles located outside or inside the cells.When close to glass surface (but still slightly above it)and inside the cell perimeter,one can specify from the 3D imaging characteristics of the confocal microscope the objects located inside and outside the cell.The same is true higher up,close to the summits of the cells,when images were taken slightly below the summit and also inside the cell perimeter.The cell surface is clearly identi fiable in the microscopy images shown in Figure 3,and regions of interest (ROI)were drawn to allow independent analyses of intracellular and extracellular regions.As can be seen in Figure 4(5′),MSNs are internalized already at the 5min time point,and the number of internalized MSNs increaseswith time.The MSNs are compartmentalized inside the cells,in agreement with the well-established endosomal uptake of this type of particles,while particles located outside the cells appear to be well-dispersed.2D image analysis of particle cluster size inside and outside of the cells (Figure 5)appreciates a decrease of cluster size inside the cells with time.Here,cluster sizes de fined by confocal microscopy are to be evaluated for relative di fferences only,not as absolute values,because of light scattering as de fined by the point spread function.Fast initial (5′)internalization leadstoFigure 3.Typical examples of the confocal fluorescence microscopy images analyzed,here from the green channel at the 1h time point.Left:original images,middle:original images with regions of interests (ROIs)drawn,right:segmentation results within the ROIs.Regions marked with A:segmented objects analyzed as intracellular.Regions marked with B:segmented objects analyzed as extracellular.Image intensities have been enhanced by linear intensity transfer functions to improve the visualization.Upper row:Image taken close to the upper part of the cells.Lower row:Image taken close to the glass substrate.The scale bar corresponds to 10μm.Pseudocolored versions of all of the three fluorescence channels observed is available as Supporting Information for the image in the lowerrow.Figure 4.Confocal images taken from the yellow channel from the top part of thecell.Figure 5.Segmented object size (pixels)from the yellow channel,inside cells (■)and outside cells (▲)as a function of incubation time.The analysis is based on the yellow channel (TRITC-MP),as it showed highest fluorescence intensity,but qualitatively similar results were obtained also when performing the analysis based on the green or the red channel.high particle concentration in intracellular compartments.Our earlier internalization studies with SAOS-2cells show an increase in integrin cluster size up to 45min,whereafter the cluster size starts to decline.39Similarly,in the present study,the point measurement at 1h shows a decrease of intracellular cluster size.Confocal images from the yellow channel at all three time points (Figure 4)support the numerical data:initially (5′)particles are seen to form larger clumps.At the 1h time point,the clusters are smaller and fainter,and they are perhaps closer to the nucleus as internalization has progressed.This is a trend that continues further at the 4h time point.A more detailed discussion about the potential locus of MSNs as a function of time is given below,in connection with a more detailed analysis of the time-dependent fluorescence measure-ments of the di fferent colors.All three fluorescent colors were segmented separately,and the absolute intensity of each fluorescent color was plotted over time.Interestingly,outside the cells no signi ficant changes in the fluorescence intensities of single fluorophores from individual channel-spesi fic segmentations were observed (Figure 6a).Additionally,the fluorescence intensities of all colors in relation to the yellow channel were calculated (Figure 6b).Intensities of all colors were then measured based on the yellow channel segmentations;that is,pixels having yellow fluorescence were used as the basis of the total intensity of the green and red fluorescence,and the intensities were normalizedagainst the total yellow fluorescence intensity.Also in these measurements no signi ficant changes in the dye intensities outside the cells over time were detected.This suggests that particles located extracellularly remained stable or dissolved in a homogeneous manner.The time-dependency of the fluores-cence intensity measured for the three di fferent fluorophores originating from particles located inside the cells followed a distinctively di fferent pattern,as shown in Figure 6c and d.While the total fluorescence emission intensity of the yellow (MP)remained virtually constant over time,a clear decrease in the corresponding total intensities of the red (PEI)and green (C)was observed signi ficantly with increasing incubation time.The decrease in the red (PEI)intensity,both in the individual segmentations and when normalized against the intensity of the yellow fluorescence,is consistent with a partial detachment of PEI from the particles with time together with dilution of the detached PEI,possibly due to endosomal escape.40,41This leads to a decrease of the red fluorescence observed in these pixels to values below the set detection limits.The decrease in the green (C)fluorescence intensities with time supports this suggestion,due to the observed decrease in the FITC fluorescence emission intensities with decreasing pH in the absence of pH-bu ffering PEI on the particles.3.3.2.Colocalization Analyses.Colocalization analyses of the three di fferent fluorophores were carried out in order to get a more detailed picture of the particle degradation process.TheFigure 6.Intensity of the segmented objects over time,measured both inside and outside the cells.(a)Separate segmentations outside the cells.(b)All colors segmented in relation to yellow channel outside the cells.(c)Separate segmentations inside the cells.(d)All colors segmented in relation to yellow channel inside the cells.[ns]indicates nonsigni ficant change,and stars (*),up to three,describe the degree of a signi ficant change.(yellow ■,red ●,green ▲).results are expressed as Manders’coefficients,always for two colors at a time.First,the number of pixels that contain both colors are determined,and the total intensity of a given color within these pixels is divided by the total intensity of one or the other color within that segment.Thus,the Manders’coefficients of,for example,red toward yellow and yellow toward red,are not necessarily the same.For example,red could be completely colocalized with yellow,which would correspond to a Manders’coefficient of1,while there could be yellow pixels which would not coincide with pixels showing both red and yellow,thus resulting in a Manders’coefficient smaller than1.It should also be noted that the determined Manders’coefficients are more sensitive to the stable particles as compared to potentially detachedfluorophores,as detached fluorophores are diluted and may fall below the set threshold values.This also implies that intracellular analyses are more sensitive to colors remaining inside cellular compartments rather than being located in the cytoplasm for the same reasons. The analyses of the Manders’coefficients were carried out separately for particles located inside or outside of the cells. Interestingly,the changes in the Manders’coefficients for particles located outside the cells was within experimental error for the time-period studied(data not shown),indicating that the particles contained allfluorophores up to the time-point of 4h.Importantly,this does not mean that there was no particle dissolution,but it indicates that the particles did at least not completely disintegrate nor did any of the layers completely detach from the main particle during the time of observation. Inside the cells,the situation was quite different.The Manders’coefficients of all possible color-combinations inside the cells are shown in Figure7a−c.Depending on the combinations,the Manders’coefficients,that is,the colocalization of different colors,are remarkably different when followed as a function of incubation time.The Manders’coefficient for green(C)−yellow(MP)remain high over the time of observation,while the corresponding values for yellow(MP)−green(C)decrease significantly over time.This suggests that the mesoporous silica layer is detaching from the main particles,leaving some of the mesoporous layer behind.Also the Manders’coefficient for red(PEI)/yellow (MP)remain high throughout the experiment,while the Manders’coefficient for yellow(MP)/red(PEI)decreases significantly over time.This result is consistent with a dissolution process where the mesoporous layer is dissolved in a way that some of the mesoporous silica layer is detaching together with the PEI layer from the particles,while a portion of the mesoporous silica layer is still associated with the main particle.This is also seen in the high Manders’coefficient for green(C)/yellow(MP)throughout the experiments.Interest-ingly,the corresponding Manders’coefficients for red(PEI)/ green(C)and green(C)/red(PEI)cannot be explained using this simple dissolution model.The Manders’coefficients indicate that the colocalization of green(C)/yellow(MP) remain high throughout the experiment,as expected based onthe values of the discussed Manders’coefficients,while significantly decreasing colocalization is seen in the decreasing values of the Manders’coefficient for yellow(MP)/green(C) with time.This suggests that the core is separating from the PEI layer,while the PEI layer remains on the Sto b er particle core.Thisfinding can be explained by a coexistence of particles where some fraction of the particles have lost the PEI layer, while some particles still have a PEI layer on them.As discussed above,the detachment of PEI,also supported by the time-dependent totalfluorescence intensity analysis,together with the escape of PEI into the cytoplasm,will lead to an overestimation of the amount of PEI present in intracellular compartments.Here PEI may be attached to particles or to fragments of mesoporous silica still being present within intracellular compartments.However,the results clearly suggest that the PEI layer is detaching from the particles,most probably both in the form of free PEI and PEI attached to fragments of mesoporous silica,and that this process preferentiallyoccurs Figure7.(a)Manders’colocalization coefficients as a function of incubation time(red/green■and green/red⧫),(b)Manders’colocalization coefficients as a function of incubation time(green/ yellow■,yellow/green⧫),and(c)Manders’colocalization coefficients as a function of incubation time(red/yellow■,yellow/ red⧫).。
氨氮对厌氧发酵的影响

氨氮对厌氧发酵的影响厌氧发酵是处理有机废弃物并实现其资源化利用的有效手段,然而厌氧发酵作为生物处理技术一种,必然存在着生化抑制反应。
存在的生化抑制反应主要有:pH抑制、氢抑制、挥发性有机酸(VFA)和氨氮的抑制等。
高浓度的氨氮就是有机废弃物厌氧生物处理中常遇到的一个难题。
本文阅读大量文献,集中研究氨氮在厌氧发酵过程中的产生机理、抑制浓度等规律,以期待解决或者避免氨氮在产甲烷发酵过程中的抑制反应情况,为今后的厌氧发酵提供理论和技术支持。
1氨氮的产生机理在有机垃圾厌氧消化的过程中,氮的平衡是非常重要的因素,尽管进入消化系统中的硝酸盐能被还原成氮气,但其仍将存在于系统中。
由于厌氧微生物细胞的增殖很少,只有很少的氮转化为细胞,大部分可生物降解的有机氮在厌氧发酵降解过程中形成水解产物-氨氮,主要以铵离子NH4+-N和游离氨NH3形式存在。
因此消化液中氨氮的浓度都高于进料的氨氮浓度,系统中的总氮是守恒的。
氨态氮主要是通过氨基酸的降解产生,其分解主要通过偶联进行氧化还原脱氮反应,这需要两种氨基酸同时参与,其中一个氨基酸分子进行氧化脱氮,同时产生的质子使另外一个氨基酸的两个分子还原,两个过程同时伴随着氨基酸的去除。
如丙氨酸和甘氨酸的降解:CH3CHNH2COOH(丙氨酸)+2H2O→CH3COOH+CO2+NH3+4H+CH2NH2COOH(甘氨酸)+4H+→2CH3COOH+2NH3两个反应合并即为:CH3CHNH2COOH+2CH2NH2COOH+2H2O→3CH3COOH+CO2+3NH3由于氨基酸的降解的能够产生NH3,因此在这一过程会影响到溶液的pH值。
NH3的存在对厌氧过程非常重要,一方面,NH3是微生物的营养物质,细菌利用氨氮作为其氮源,另一方面,NH3如果其浓度过高就会快速抑制甲烷菌的活性。
氨的存在形式有NH3和NH4+,两者的浓度决定于pH值。
NH3+H2O→NH4++OH-35℃时,K1×10-5 (1-1)K2×10-14 (1-2)两式相除,[NH3]=1.13×10-9有机酸积累,pH值降低,平衡向右移动,NH3离解为NH4+。
环氧氯丙烷和大位阻脂肪醇之间的合成反应Reaction_08_28_2013_103408

1. Single Step90%OverviewSteps/Stages Notes1.1R:NaOH, C:Bu4N+ •Br-Reactants: 2, Reagents: 1, Catalysts: 1, Steps:1, Stages: 1, Most stages in any one step: 1ReferencesPreparation of glycidyl ethers in the absenceof water and organic solventsBy Lee, Hyung Min et alFrom Jpn. Kokai Tokkyo Koho, 2002003428,09 Jan 2002Experimental ProcedureGeneral/Typical Procedure: (Example) Octanol (132g, 1mol) was introduced in to a 4-necked round-bottom flask equipped with stirrer, reflux condenser and thermometer and 30°C was maintained.Further, tetrabutyl ammonium bromide (16.1g, 0.050 mol) and sodium hydroxide (61.9g, 1.5 mol) wereadded to flask and stirred. After that, epichlorohydrin (140.1 g, 1.5 mol) was dropped over 10 minutes.Further, it was stirred at the same temperature for 3 hours. By-products such as sodium chloride andsodium hydroxide were removed by filtration. Unreacted epichlorohydrin was recovered by theprocesses such as distillation etc. And it was reused. Further, the target product, glycidyl ether wasobtained by simple filtration.CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.2. 2 Steps[Step 2.2]OverviewSteps/Stages Notes1.1R:KOH, C:Pd, S:H2O, heated; 6 h, reflux1.2R:H2, C:Ni, 4 h, 110°C, 4 MPa2.1R:Al isopropoxide, C:H2SO4, 90°C2.2 2 h, 90°C; 2 h, 90°C; 90°C → 50°C2.3R:NaOH, S:H2O, 50°C → 80°C; 8 h, 80°C1) Raney nickel used, Reactants: 2, Reagents:4, Catalysts: 3, Solvents: 1, Steps: 2, Stages:5, Most stages in any one step: 3ReferencesPreparation of glyceryl 9-methylpentadecan-7-yl etherBy Hirusaki, Shutoku and Shimozato,YasuyukiFrom Jpn. Kokai Tokkyo Koho, 2008162980,17 Jul 2008CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.3. Single StepOverviewSteps/Stages Notes1.1R:KOH, C:Bu4N+ •HSO4-Reactants: 2, Reagents: 1, Catalysts: 1, Steps:1, Stages: 1, Most stages in any one step: 1ReferencesSynthesis of glycol diglycidyl ethers usingphase-transfer catalysisBy Gu, Xue Ping et alFrom Synthesis, (6-7), 649-51; 1985 CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.4. Single StepOverviewSteps/Stages Notes1.1 -Reactants: 2, Steps: 1, Stages: 1, Most stagesin any one step: 1ReferencesCycloalkyloxyisopropanolaminesBy Mouzin, Gilbert et alFrom Eur. Pat. Appl., 37777, 14 Oct 1981 CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.5. Single StepOverviewSteps/Stages Notes1.1 -Reactants: 2, Steps: 1, Stages: 1, Most stagesin any one step: 1ReferencesOlefinic epoxy compoundsBy Massingill, John L.From U.S., 4579959, 01 Apr 1986 CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.6. Single StepOverviewSteps/Stages Notes1.1R:Al isopropoxide, C:H2SO4, 90°C1.2 2 h, 90°C; 2 h, 90°C; 90°C → 50°C1.3R:NaOH, S:H2O, 50°C → 80°C; 8 h, 80°CReactants: 2, Reagents: 2, Catalysts: 1,Solvents: 1, Steps: 1, Stages: 3, Most stagesin any one step: 3ReferencesPreparation of glyceryl 9-methylpentadecan-7-yl etherBy Hirusaki, Shutoku and Shimozato,YasuyukiFrom Jpn. Kokai Tokkyo Koho, 2008162980,17 Jul 2008CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.7. Single Step56%OverviewSteps/Stages Notes1.1R:NaH, S:THF 1.2S:THF Reactants: 2, Reagents: 1, Solvents: 1, Steps: 1, Stages: 2, Most stages in any one step: 2 Referencesβ-Adrenergic antagonists: N-alkyl and N-amidoethyl(arylalkoxy)propanolamines related to propranololBy Mauleon, David et alFrom European Journal of Medicinal Chemistry, 23(5), 421-6; 1988CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.8. Single Step42%OverviewSteps/Stages Notes1.1R:NaOH, C:Bu4N+ •HSO4-, S:H2O, 18 h, rt Reactants: 2, Reagents: 1, Catalysts: 1,Solvents: 1, Steps: 1, Stages: 1, Most stagesin any one step: 1ReferencesPreparation of filamin A-binding heterocyclesas analgesicsBy Burns Barbier, Lindsay et alFrom PCT Int. Appl., 2010051374, 06 May2010Experimental Procedurea. Synthesis of compound 4 To a mixture of compound epichlorohydrin (145 mg, 1.56 mmol) in NaOH(50% w/w) (1.04 g, 13 mmol) was added compound 2, 5-dimethylcyclohexanol (200 mg, 1.56 mmol)and Bu4HSO4 (22 mg, 0.06 mmol), the mixture was stirred at room temperature overnight (about 18hours) . Then, 3 mL of H2O was added into the mixture, extracted with ethyl acetate (5 mL x 3) and thecombined organic phase was dried and concentrated to get crude product which was purified by silicagel column (eluted with dichloromethane) to afford 120 mg of title product (yield: 41.7 %, confirmed bythin-layer chromatography) . Mol. Wt.: 184.28. Molecular formula: C11H20O2.CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.9. Single Step42%OverviewSteps/Stages Notes1.1R:NaOH, C:Bu4N+ •HSO4-, S:H2O, 18 h, rt Reactants: 2, Reagents: 1, Catalysts: 1,Solvents: 1, Steps: 1, Stages: 1, Most stagesin any one step: 1ReferencesPreparation of 2,3-dihydroxypropanaminederivatives as µ opioid receptor (MOR)agonists that binds filamin A for the treatmentof pain and inflammationBy Burns Barbier, Lindsay et alFrom U.S. Pat. Appl. Publ., 20100279997, 04Nov 2010Experimental Procedurea. Synthesis of Compound 4 To a mixture of compound epichlorohydrin (145 mg, 1.56 mmol) in NaOH(50% w/w) (1.04 g, 13 mmol) was added compound 2,5-dimethylcyclohexanol (200 mg, 1.56 mmol)and Bu4HSO4 (22 mg, 0.06 mmol), the mixture was stirred at room temperature overnight (about 18hours). Then, 3 mL of H2O was added into the mixture, extracted with ethyl acetate (5 mL×3) and thecombined organic phase was dried and concentrated to get crude product which was purified by silicagel column (eluted with dichloromethane) to afford 120 mg of title product (yield: 41.7%, confirmed bythin-layer chromatography).CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.10. Single Step86%OverviewSteps/Stages Notes1.10-5°C1.25°C → 25°C; 4 h, 22-25°C; 25°C → 13°C1.3R:KOH, S:Et2O, 5 h, 13-15°C; 15°C → rtReactants: 2, Reagents: 1, Solvents: 1, Steps:1, Stages: 3, Most stages in any one step: 3ReferencesSynthesis and some chemical reactions offunctionally substituted unconjugated diynesBy Veliev, M. G. et alFrom Maruzalar - Azarbaycan Milli ElmlarAkademiyasi, 63(6), 71-78; 2007CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.11. Single Step80%OverviewSteps/Stages Notes1.1R:NaOH, C:Bu4N+ •Br-, S:H2O, 2 h, 40°C deionized water used, alternative preparationgave lower yield, Reactants: 2, Reagents: 1,Catalysts: 1, Solvents: 1, Steps: 1, Stages: 1,Most stages in any one step: 1ReferencesBisphenol A-free epoxy resins, adhesives,coatings and coated food containersBy Schmidt, DanielFrom PCT Int. Appl., 2012149340, 01 Nov2012CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.12. 2 StepsOverviewSteps/Stages Notes1.1R:NaOH, C:Bu4N+ •Br-, S:H2O, 2 h, 40°C2.1R:NaOH, C:Bu4N+ •Br-, 30 min, 100°C 1) deionized water used, alternative preparation gave lower yield, 2) regioselective, Reactants: 2, Reagents: 1, Catalysts: 1, Solvents: 1, Steps: 2, Stages: 2, Most stages in any one step: 1ReferencesBisphenol A-free epoxy resins, adhesives, coatings and coated food containersBy Schmidt, DanielFrom PCT Int. Appl., 2012149340, 01 Nov 2012CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.13. Single Step94%OverviewSteps/Stages Notes1.1C:AlCl3, S:PhMe, 1 h, 160°C1.2R:NaOH, S:H2O, 18 h, 75°CReactants: 2, Reagents: 1, Catalysts: 1,Solvents: 2, Steps: 1, Stages: 2, Most stagesin any one step: 2ReferencesPreparation of 2,3-dihydroxypropanaminederivatives as µ opioid receptor (MOR)agonists that binds filamin A for the treatmentof pain and inflammationBy Burns Barbier, Lindsay et alFrom U.S. Pat. Appl. Publ., 20100279997, 04Nov 2010Experimental ProcedurePreparation of Compound 1 A flask was charged with D-menthol (10 g, 64 mmol), 40 mL toluene, andAlCl3 (0.68 g, 5.12 mmol). The temperature of the mixture was raised to 160° C. Then, epichlorohydrin(5.9 g, 64 mmol) was added with stirring for 1 hour. Next, NaOH (50%) (10.24 g, 128 mmol) wasadded with stirring at a temperature of 75° C. overnight (about 18 hours). Following this treatment, 5mL of water was added into the mixture. Next, the mixture was extracted with ethyl acetate three times(15 mL total of ethyl acetate) and the extracted organic phase was combined, dried, and concentratedto obtain the crude product. The crude product was purified by silica gel column to obtain the purifiedproduct, a colorless oil (TLC confirmed, 12.8 g, yield: 94%). 1H NMR (400 MHz, CDCl3) δ: 0.793~1.019(m, 13H), 1.237~1.402 (m, 3H), 1.602~1.684 (m, 1H), 2.054~2.124 (m, 1H), 2.227~2.275 (m, 1H),2.605~2.644 (m, 1H), 2.801~2.839 (m, 1H),3.098~3.185 (m, 2H), 3.367~3.409 (m, 0.5H), 3.583~3.603(m, 1H), 3.801~3.837 (m, 0.5H).CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.14. Single StepOverviewSteps/Stages Notes1.1R:LiH, S:PhMe, 4 h, 60-140°C; 140°C → 70°C1.2 2 h, 70°C; 7 h, 70°CReactants: 2, Reagents: 1, Solvents: 1, Steps:1, Stages: 2, Most stages in any one step: 2ReferencesMethod for preparation of new type ofmenthol derivativesBy Huang, Zhengliang et alFrom Faming Zhuanli Shenqing, 102153475,17 Aug 2011CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.15. Single StepOverviewSteps/Stages Notes1.1R:KOH Reactants: 2, Reagents: 1, Steps: 1, Stages:1, Most stages in any one step: 1ReferencesCatalytic hydrosilylation of 1-(2-furyl)-1-glycidyloxy-3-butyneBy Israfilov, Ya. M. et alFrom Kimya Problemlari Jurnali, (2), 390-392;2006CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.16. 2 StepsOverviewSteps/Stages Notes1.1R:ZnCl2, S:PhMe, 5 h, 100°C2.1R:NaOH, C:TEBAC, S:PhMe, S:H2O, 3 h, 70°CReactants: 2, Reagents: 2, Catalysts: 1,Solvents: 2, Steps: 2, Stages: 2, Most stagesin any one step: 1ReferencesSynthesis of new cooling agent 3-menthoxypropane-1,2-diolBy Li, Chunrong and Wang, SanyongFrom Xiangliao Xiangjing Huazhuangpin, (2),10-11; 2004CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.17. 2 StepsOverviewSteps/Stages Notes1.1R:NaH, C:ZnCl2, S:PhMe2.1R:NaOH, C:TEBAC, S:H2O, S:PhMe1) alternative catalyst gave lower yields,Reactants: 2, Reagents: 2, Catalysts: 2,Solvents: 2, Steps: 2, Stages: 2, Most stagesin any one step: 1ReferencesProcess for preparing 3-l-menthoxypropane-1,2-diolBy Amano, Akira et alFrom Eur. Pat. Appl., 1201635, 02 May 2002 Experimental ProcedureStep 1General/Typical Procedure: Synthesis of 1-chloro-3-1-menthoxypropan-2-ol Under a nitrogenatmosphere, into a reaction flask (volume: 300 ml) were added 1-menthol (10 g, 64.1 mmol) andtoluene (50 ml), and the whole was dissolved at room temperature and then the inner temperature waslowered to 5°C by ice-cooling. Thereafter, 60% sodium hydride (2.82 g, 70.5 mmol) was added theretoand then the temperature was raised to 100°C. Into the solution was added dropwise epichlorohydrin(5.93 g, 64.1 mmol) over a period of 1 hour. After the addition, they were reacted at the sametemperature for 3 hours, but the adducts (1-chloro-3-1-menthoxypropan- 2-ol or 1,2-epoxy-3-1-menthoxypropane) were not formed at all. Furthermore, as is apparent from the result of ComparativeExample 5, when the addition of 1-menthol to epichlorohydrin was carried out using a base (sodiumhydride), no adducts (1-chloro-3-1-menthoxypropan-2-ol or 1,2-epoxy-3-1-menthoxypropane) wereformed. 1-chloro-3-1-menthoxypropan-2-olStep 2Synthesis of 1,2-epoxy-3-1-menthoxypropane (1 ) Under a nitrogen atmosphere, into a reaction flask(volume: 200 ml) were added 1-chloro-3-1-menthoxypropan-2-ol (50 g, chemical purity: 97.8%, 0.1968mol) obtained in Example 1, toluene (75 ml), a 50% aqueous sodium hydroxide solution (31.49 g,0.3936 mol) and a 50% aqueous benzyltrimethylammonium chloride solution (1.46 g. 4.26 mmol), andthey were reacted at 75°C for 2 hours. After completion of the reaction, the organic layer was washedwith water and then the solvent (toluene) was removed to obtain an oily substance. The oily substancewas distilled under reduced pressure to obtain 1,2-epoxy-3-1-menthoxypropane (34.6 g, chemicalpurity: 98.25%) as a colorless transparent oily substance (yield: 97.0% based on 1-chloro-3-1-menthoxypropan-2-ol). 1,2-epoxy-3-1-menthoxypropane, yield 97.0% [boiling point: 75-80°C/10.7 Pa(0.08 mmHg)] (2) The analytical results of 1,2-epoxy-3-1-menthoxypropane obtained in the above (1)were as follows: [α]D25 :-90.95° (c =1.05, EtOH). MS(m/e, %): 212 (M+), 155, 138, 127, 123, 109, 95,81, 71 ,69, 67, 57, 55, 43, 41, 31, 29, 27. IR (neat, cm-1): 3050, 2960, 2925, 2875, 1460, 1370, 1095,910, 845, 765. 1H-NMR (CDCl3; δ ppm): 0.78 (3H, d, J=6.9), 0.81-0.88 (2H, m), 0.90 (3H, d, J=7.0),0.92 (3H, d, J=6.6), 0.95-1.00 (1H, m), 1.24(1H, m), 1.36 (1H, m), 1.59-1.67 (2H, m), 2.08(1H, m),2.14(1H, m), 2.38(1 H, broad),3.06-3.12 (1H, m), 3.38-3.44 (1H, m), 3.57-3.66 (2H, m), 3.71 -3.75(1H, dd), 3.90-3.96 (1H, m).CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.18. 2 Steps[Step 2.1]OverviewSteps/Stages Notes1.1R:NaOH, C:Bu4N+ •Br-, S:H2O, 2 h, 40°C2.1R:NaOH, C:Bu4N+ •Br-, 30 min, 100°C1) deionized water used, alternativepreparation gave lower yield, 2) regioselective,Reactants: 3, Reagents: 1, Catalysts: 1,Solvents: 1, Steps: 2, Stages: 2, Most stagesin any one step: 1ReferencesBisphenol A-free epoxy resins, adhesives,coatings and coated food containersBy Schmidt, DanielFrom PCT Int. Appl., 2012149340, 01 Nov2012CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.19. Single Step70%OverviewSteps/Stages Notes1.1R:NaOH, C:Bu4NCl, S:H2O Reactants: 2, Reagents: 1, Catalysts: 1,Solvents: 1, Steps: 1, Stages: 1, Most stagesin any one step: 1ReferencesFluorine-containing unsaturated glycidyl etheras a material for polymers or functionalsilanes and its preparationBy Takaai, Toshio et alFrom Jpn. Kokai Tokkyo Koho, 05032651, 09Feb 1993CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.20. 2 Steps[Step 2.1]OverviewSteps/Stages Notes1.1R:NaOH, C:Bu4N+ •Br-, S:H2O, 2 h, 40°C2.1 1 h, 140°C 1) deionized water used, alternative preparation gave lower yield, 2) regioselective, Reactants: 3, Reagents: 1, Catalysts: 1, Solvents: 1, Steps: 2, Stages: 2, Most stages in any one step: 1ReferencesBisphenol A-free epoxy resins, adhesives, coatings and coated food containersBy Schmidt, DanielFrom PCT Int. Appl., 2012149340, 01 Nov 2012CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.21. Single Step75%OverviewSteps/Stages Notes1.1R:KOH, S:MeOH stereoselective, KOH/MeOH, r.t., Epoxideformation, Heterocycle formation,Rearrangement, Ring cleavage, Reactants: 1,Reagents: 1, Solvents: 1, Steps: 1, Stages: 1,Most stages in any one step: 1ReferencesEpoxide migration (Payne rearrangement)and related reactionsBy Hanson, Robert M.From Organic Reactions (Hoboken, NJ,United States), 60, No pp. given; 2002 CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.22. Single Step65%OverviewSteps/Stages Notes1.1R:Na, S:PhMe1.2 -Reactants: 2, Reagents: 1, Solvents: 1, Steps:1, Stages: 2, Most stages in any one step: 2ReferencesIsoquinoline derivatives. Synthesis and β-adrenoblocking and antiarrythmic activities of4-(aminoalkanol) derivatives of 4-hydroxy-2,3,3-trimethyl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolineBy Markaryan, E. A. et alFrom Khimiko-Farmatsevticheskii Zhurnal,31(2), 20-21; 1997CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.23. Single StepOverviewSteps/Stages Notes1.1R:DBU stereoselective, alternative preparation shown,Reactants: 1, Reagents: 1, Steps: 1, Stages:1, Most stages in any one step: 1ReferencesPreparation of triptolide C-ring derivatives asanticancer agents and immune modulatorsBy Musser, John H.From PCT Int. Appl., 2010091193, 12 Aug2010Experimental Procedurering-opened analog, PG757, was obtained as a side product, it can be converted to PG762 using abase such as DBU (1,8-diazabicyclo[5.4.0]undec-7-ene). PG762CASREACT ®: Copyright © 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.。
依地酸二钠美国药典34版标准

Edetate Disodium(ed' e tate dye soe' dee um).C10H14N2Na2O8·2H2O 372.24Glycine, N,N¢-1,2-ethanediylbis[N-(carboxymethyl)-, disodium salt, dihydrate.Disodium (ethylenedinitrilo)tetraacetate dihydrate [6381-92-6].Anhydrous 336.21 [139-33-3].» Edetate Disodium contains not less than 99.0 percent and not more than 101.0 percent of C10H14N2Na2O8, calculated on the dried basis.Packaging and storage— Preserve in well-closed containers.USP Reference standards 11—USP Edetate Disodium RSIdentification—A: Infrared Absorption 197K: undried.B: To 5 mL of water in a test tube add 2 drops of ammonium thiocyanate TS and 2 drops of ferric chloride TS, and mix. To the deep red solution add about 50 mg of Edetate Disodium, and mix: the red color is discharged, leaving a yellowish solution.C: It responds to the flame test for Sodium 191.pH 791: between 4.0 and 6.0, in a solution (1 in 20).Loss on drying 731— Dry it at 150 for 6 hours: it loses not less than 8.7% and not more than 11.4% of its weight.Calc ium—To a solution (1 in 20) add 2 drops of methyl red TS, and neutralize with 6 N ammonium hydroxide. Add 3 N hydrochloric acid dropwise until the solution is just acid, and then add 1 mL of ammonium oxalate TS: no precipitate is formed.Heavy metals, Method II 231: 0.005%.Limit of nitrilotriacetic acid—Mobile phase— Add 10 mL of 1.0 M tetrabutylammonium hydroxide in methanol to 200 mL of water, and adjust with 1 M phosphoric acid to a pH of 7.5 ±0.1. Transfer the solution so obtained to a 1000-mL volumetric flask, add 90 mL of methanol, dilute with water to volume, mix, pass through a filter having a 0.5-µm or finer porosity, and degas.Cupric nitrate solution—Prepare a solution containing about 10 mg of cupric nitrate(Cu(NO3)2) per mL.Standard stock solution— Transfer about 100 mg of nitrilotriacetic acid, accurately weighed, to a 10-mL volumetric flask, add 0.5 mL of ammonium hydroxide, and mix. Dilute with water to volume, and mix.Resolution solution—Transfer 10 mg of Edetate Disodium to a 100-mL volumetric flask, add 100 µL of Standard stock solution, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to dissolve.Standard solution— Transfer 1.0 g of Edetate Disodium to a 100-mL volumetric flask, add 100 µL of Standard stock solution, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to dissolve.Test solution— Transfer 1.0 g of Edetate Disodium to a 100-mL volumetric flask, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to dissolve.Chromatographic system (see Chromatography 621)— The chromatograph is equipped witha 254-nm detector and a 4.6-mm × 15-cm column that contains packing L7. The flow rate is about2 mL per minute. Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.35 for nitrilotriacetic acid, 0.65 for copper, and 1.0 for edetate; and the resolution, R, between nitrilotriacetic acid and copper is not less than 3. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.Procedure— Separately inject equal volumes (about 50 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks. The response of the nitrilotriacetic acid peak obtained from the Test solution does not exceed the difference between the nitrilotriacetic acid peak responses obtained from the Standard solution and the Test solution: not more than 0.1% of nitrilotriacetic acid is found.Assay—Assay preparation— Dissolve about 5 g of Edetate Disodium, accurately weighed, in about 100 mL of water contained in a 250-mL volumetric flask, add water to volume, and mix.Procedure— Place about 200 mg of chelometric standard calcium carbonate, previously dried at 110 for 2 hours, cooled in a desiccator, and accurately weighed, in a 400-mL beaker, add 10 mL of water, and swirl to form a slurry. Cover the beaker with a watch glass, and without removingthe latter, add 2 mL of 3 N hydrochloric acid from a pipet. Swirl the contents of the beaker, and dissolve the calcium carbonate. Wash down the sides of the beaker, the outer surface of the pipet, and the watch glass with water, and dilute with water to about 100 mL. While stirring the solution, preferably with a magnetic stirrer, add about 30 mL of the Assay preparation from a 50-mL buret. Add 15 mL of 1 N sodium hydroxide and 0.30 g of hydroxy naphthol blue, and continue the titration with the Assay preparation to a blue endpoint. Calculate the weight, in mg, of C10H14N2Na2O8 in the portion of Edetate Disodium taken by the formula:(336.21/100.09)W(VT /V)in which 336.21 and 100.09 are the molecular weights of edetate disodium and calcium carbonate, respectively; W is the weight, in mg, of calcium carbonate; VT is the volume, in mL, of the Assay preparation; and V is the volume, in mL, of the Assay preparation consumed in the titration.Auxiliary Information— Please check for your question in the FAQs before contacting USP.Topic/Question Contact Expert CommitteeMonograph Elena Gonikberg, Ph.D.Principal Scientific Liaison1-301-816-8251 (SM32010) Monographs - Small Molecules 3Reference Standards RS Technical Services1-301-816-8129**************USP34–NF29 Page 2663Pharmacopeial Forum: V olume No. 32(4) Page 1070。
黑曲霉生产柠檬酸的发酵工艺流程

黑曲霉生产柠檬酸的发酵工艺流程1.首先,选择适当的黑曲霉菌种作为发酵的起始种。
First, choose the appropriate Aspergillus Niger strain as the starting culture for fermentation.2.将黑曲霉菌种接种到含有适量碳源和氮源的发酵基质中。
Inoculate the Aspergillus Niger strain into a fermentation medium containing suitable amounts of carbon and nitrogen sources.3.确保发酵基质的pH值在合适的范围内,通常为3.0至6.0。
Ensure that the pH of the fermentation medium is within the appropriate range, typically between 3.0 and 6.0.4.控制发酵温度在25°C至35°C之间,提供适宜的温度条件。
Control the fermentation temperature between 25°C and 35°C to provide suitable conditions for growth.5.确保发酵过程中的通气充足,以促进微生物的生长和代谢活动。
Ensure adequate aeration during the fermentation processto promote microbial growth and metabolic activity.6.在发酵过程中定期监测黑曲霉的生长情况和产酸量。
Regularly monitor the growth of Aspergillus Niger and the production of citric acid during the fermentation process.7.当黑曲霉的生长达到高峰并且产酸量稳定时,进行收获。
石墨烯二氧化锰的制备及其电催化氧化性能研究

重庆大学本科学生毕业设计(论文)石墨烯/二氧化锰的制备及其电催化氧化性能研究学生:万梦学号:20116568指导教师:刘成伦专业:应用化学重庆大学化学化工学院二〇一五年六月Graduation Design(Thesis) of Chongqing UniversityThe preparation and electrochemical catalytic properties research ofGraphene/MnO2Undergraduate: Wan MengSupervisor: Prof. Liu ChenglunMajor: Applied ChemistryCollege of Chemistry and Chemical EngineeringChongqing UniversityJune 2015重庆大学本科学生毕业设计(论文)中文摘要摘要我国城市化进程迅速发展,城市人口不断增多,产生了大量的城市垃圾,处理垃圾渗滤液成为治理环境过程中面临的一大难题,而电催化氧化法对垃圾渗滤液的处理有显著成效。
本文通过氧化还原法成功合成了MnO2,并通过浸渍法制备出了电催化活性更好的石墨烯(RGO)/MnO2复合材料。
运用XRD和SEM表征了复合材料的组成和形貌,并以苯酚溶液为模拟污染物,通过循环伏安检测和差分脉冲检测表征了复合材料的电催化氧化性。
对石墨烯/MnO2复合材料的合成方法进行了优化,探讨了石墨烯的掺杂量和还原时间对石墨烯/MnO2复合材料性能的影响。
实验结果指出,当增加石墨烯的掺杂量时,石墨烯/MnO2复合材料的电催化活性先增加然后减小,掺杂15wt%的石墨烯时,石墨烯/MnO2复合材料的电催化活性最好,通过差分脉冲法检测,当电解液为100mg/L的苯酚溶液时,其峰值电流相比于MnO2提高了54%,其电荷转移电阻降低了73%。
合成复合材料时,以氨水为还原剂,反应时间为2h时合成的石墨烯/MnO2复合材料具有较好的电催化活性,其电荷转移电阻比未还原的复合材料的降低了85%,比还原4h的降低了20%。
石油英语词汇a4

石油英语词汇a4 -CAL-FENGHAI.-(YICAI)-Company One1石油英语词汇(A4)ammendment 修改ammeter 安培计ammine 氨络物ammino 氨络ammite 鲕状岩ammocolous 砂生生物;栖砂的Ammocypris 瘤星介属ammonal 阿芒拿ammonia buffer 氨缓冲溶液ammonia condenser 氨冷凝器ammonia cooler 氨冷却器ammonia oil 氨压缩机汽缸油ammonia pump 氨泵ammonia refrigerator 氨冷冻机ammonia stripping urea process 氨气提尿素工艺ammonia stripping 氨洗涤ammonia suit 防氨服ammonia synthesis catalyst 氨合成催化剂ammonia synthesis loop 氨合成回路ammonia water 氨水ammonia 氨ammoniation 氨化ammonibacteria 氨细菌类ammonification 生氨;加氨ammonio 胺基ammonioborite 水铵硼石ammonite 菊石;阿芒奈特炸药ammonium acid phosphate 酸性磷酸铵ammonium bicarbonate 碳酸氢铵ammonium bifluoride 氟化氢铵ammonium bisulfite 亚硫酸氢铵ammonium carbonate 碳酸铵ammonium chloride brine 氯化铵盐水ammonium chloride spacer 氯化铵隔离液ammonium chloride 氯化铵ammonium chloroplatinate 氯铂酸铵ammonium chloroplatinite 氯亚铂酸铵ammonium fluoride 氟化铵ammonium hydroxide 氢氧化铵ammonium molybdate 钼酸铵ammonium nitrate 硝酸铵ammonium persulfate 过硫酸铵ammonium salt 铵盐ammonium sulfate 硫酸铵ammonium sulphonate 磺酸铵ammonium thiocyanate 硫氰酸铵ammonium vanadate 钒酸铵ammonium 铵ammonolysis 氨解作用ammonpulver 硝氨炸药Ammosiphonia 砂管口虫属ammoxidation catalyst 氨氧化催化剂ammunition protection grease 弹药保护脂ammuntion 弹药;装弹药AMNH 美国自然博物馆amnicolous 沙岸生物Amnuralithus 暗弯角石Amoco International Oil Co. 阿莫科国际石油公司amoebae amoeba的复数Amonotis 无耳髻蛤属amorce 起爆剂Amorican Orogeny 阿莫利坎造山运动amorization of intangible assets 无形资产摊销amorphism 无定形性;不结晶性;无定向;无目的Amorphognathus 变形颚牙形石属amorphous bitumen 无定形沥青amorphous carbon 非晶碳amorphous catalyst 无定形催化剂amorphous kerogen 无定形干酪根amorphous liptinite 无定形类脂体amorphous matrix 无定形基体amorphous solid 非晶固体amorphous 无定形的;非晶的;无一定方向的amortisseur 阻尼器;减震器amortization fund 摊提资金amortization period 清偿期amortization rate 摊销系数amortization 阻尼amortize 分期偿还;防止;阻尼amount insured 保险额amount of deflection 挠度amount of precipitation 降水量amount of scale buildup 结垢量amount remitted 汇出额amount 量;金额;合计amp gauge 安培表amp 安培amp 安培数AMP 放大器AMP 可利用的马达压降AMP 视骨架剖面amp 振幅AMP 自动做记号面板amp.in 放大器输入端ampacity 可容体的电流强度;载流量ampasimenite 辉霞斑岩ampelite 黄铁碳质页岩ampelitic limestone 碳质灰岩amperage 安培数ampere density 电流密度ampere hour 安培小时ampere winding 安匝ampere 安培amperemeter 电流计ampere hour capacity 安培小时容量ampere turn 安匝amperite 镇流管amperometric 测量电流的amperometry 电流分析amph 无定形的Amphechinus 双猬属amphiaster 两星骨针amphibian 水陆飞机;水陆两栖战车;两栖的amphibious bulldozer 水陆两用推土机amphibious operation 水陆联合作业amphibious vehicle 水陆两用车amphibious 两栖的amphibole 角闪石amphibolite facies 角闪岩相amphibolite 角闪岩amphibolization 角闪石化amphiboly 多义语句amphicar 水陆两用车Amphicoelia 双腔蛤属Amphicrinus 双面海百合属Amphicythere 双花介属amphidetic ligament 全韧式韧带amphidisc 双盘骨针Amphidontes 双齿砺属amphidromic point 无潮点amphidromic region 无潮区amphidromic system 无潮体系amphigenite 白榴熔岩amphigenous 遍生的Amphileberis 双面介属amphimorphic 二重的amphioxea 两头尖骨针amphipathic molecule 两性分子amphipathic 两性分子团的amphiphilic surfactant 两亲型表面活性剂Amphiporella 小双管苔藓虫属amphiprotic solvent 两性溶剂amphirhinal 双鼻孔Amphiroa 蟹手藻属amphisapropel 含植屑腐泥Amphishaenidae 穴蜥科Amphitherium 双兽属Amphizygus 双轭颗石amphi两种ampholyte 两性电解质ampholytic surface active agent 两性表面活性剂ampholytics 两性表面活性剂ampholytoid 中性胶体Amphorachitina 耳瓶几丁虫属amphoteric metal 两性金属amphoteric polymeric material 丙烯聚合物材料amphoteric surfactant 两性活性剂amphoteric 两性的amphoterism 两性ampl. 放大器ampl. 振幅ample 宽大的;充足的Amplexiphyllum 包合珊瑚属Amplexocarinia 脊板包珊瑚属Amplexoides 似包珊瑚属amplidyne drive 电机放大机传动装置amplidyne 交磁放大机amplification coefficient 放大系数amplification factor 放大因数amplification matrix 放大矩阵amplification ratio 放大率amplification 放大amplified curve 放大曲线amplifier band width 放大器带宽amplifier block 放大器组件amplifier gain 放大器增益amplifier modulation 放大器调制amplifier noise 放大器噪声amplifier stage 放大级amplifier 放大器;放大镜amplifier inverter 放大器换流器amplify 放大amplistat 自反馈式磁放大器amplitron 特高频功率放大管amplitude absorption 振幅吸收amplitude adder 幅度加法器amplitude balance 幅度平衡amplitude bistable circuit 振幅双稳电路amplitude calibrator 振幅校准器amplitude characteristic 振幅特性amplitude coefficient 振幅系数amplitude coherency filtering 振幅相干滤波amplitude color coding 振幅彩色编码amplitude comparator 振幅比较器amplitude compression 振幅压缩amplitude contraction 振幅压缩amplitude curve 振幅曲线amplitude decay function 振幅衰减函数amplitude distortion 振幅失真amplitude editing 振幅编辑amplitude envelope 振幅包络amplitude equalization 振幅均衡amplitude excursion 振幅偏差amplitude fluctuation 振幅摆动amplitude gain function 振幅增益函数amplitude hologram 振幅全息图amplitude limit 限幅amplitude log 声幅测井amplitude modulation 调幅amplitude modulator 调幅器amplitude normalization 振幅标准化amplitude of wave 波幅amplitude permeability 振幅磁导率amplitude processing 振幅处理amplitude ratio 振幅比amplitude recovery 振幅恢复amplitude residual curve 振幅剩余曲线amplitude resolution 振幅分辩率amplitude resonance 振幅谐振amplitude response 振幅响应amplitude restoration 振幅恢复amplitude selector 振幅选择器amplitude separator 振幅分离器amplitude spectrum shaping 振幅谱整形amplitude spectrum 振幅谱amplitude standout 振幅超出amplitude taper 振幅削减amplitude to period ratio 振幅周期比率amplitude uniformity 振幅一致性amplitude 广阔;丰富;振幅amplitude adjusted indices 幅度调节指数法amplitude frequency characteristic 振幅频率特性amplitude modulated transmitter 调幅发射机amplitude phase characteristic 振幅相位特性amplitude shift keying 振幅键控制Amplogladius 宽剑螺属Amploualvata 大盘螺属ampoule 安瓿;小瓶AMPS 航空多光谱摄影系统AMQUA 美国第四纪协会AMS 辅助测量短节AMS 辅助监视子系统AMS 美国冶金学会AMS 声学测量系统Amsassia 阿姆塞士珊瑚属AMSL 平均海平面以上Amstrat 美国地层数据库AMT 声频大地电磁法amt 总计AMU 声学测量单元amu 天线匹配器AMU 原子质量单位amygdaloid 杏仁岩;杏仁状amygdaloidal 杏仁状的Amygdalophyllum 杏仁珊瑚属amygdalophyre 杏仁状玢岩amyl alcohol 戊醇amyl butyrate 丁酸戊酯amyl cinnamic aldehyde 戊基肉桂醛amyl laurate 月桂酸戊酯amyl mercaptan 戊基硫醇amyl nitrate 硝酸戊酯amyl 戊基amylamine 戊胺amylan 淀粉胶amylase 淀粉酶amylene 戊烯amyloid 淀粉的;淀粉状朊;类淀粉物;胶化纤维素amylopectin 支链淀粉amylose 直链淀粉amylosuria 淀粉脲amylum 淀粉amyrin 香树精an integral part 不可分割的部分An 苯胺AN 氮气中退火AN 空气自然冷却an 年度的an 上述的AN 硝酸铵An 锕射气Anabaria 阿纳巴尔叠层石属Anabasis salsa 盐生假木贼anabatic differentiation 上升分异作用anabatic 上升的anaberrational telescope 消象差望远镜anaberrational 消象差的anabohitsite 铁橄苏辉岩anabolism 组成代谢anaboly 加速个体发育anabranch 汊河anacamptics 光、声反射学anachoric crust of weathering 氧化铁风化壳anaclastics 屈光学anaclinal stream 逆向河anaclinal valley 逆向谷anacline 正倾斜Anacolosidites 铁青树粉属ANACOM 模拟计算机anacoustic 隔音的anadiagenesis 深埋成岩作用anadiagenetic stage 深埋成岩期anaerobe 厌氧菌;厌氧生物anaerobes 厌氧菌anaerobia anaerobion的复数anaerobic bacteria 厌氧菌anaerobic culture 厌气培养anaerobic decay 缺氧分解作用anaerobic decomposition 缺氧分解anaerobic environment 缺氧环境anaerobic halophiles 厌氧嗜盐微生物anaerobic microbe 厌氧菌anaerobic micro organism 厌氧微成物anaerobic reduction 厌氧还原作用anaerobic sulfate reducer 厌氧硫酸盐还原菌anaerobic treatment 厌氧处理anaerobic water 无氧水anaerobic 厌氧的anaerobion 厌氧菌anaerobiont 嫌气生物;厌氧生物anaerobiosis 嫌气生活anaerobism 缺氧anaesthesia 麻醉anaesthetic 麻醉的;麻醉剂anagenesis 前进演化anagenite 杂砾岩;石英质砾岩anaglacial 始冰期anaglyph 补色立体图anaglyphic method 补色立体观测法anaglyphic projection 补色立体投影anaglyphic spectacles 补色立体眼镜anaglyphic viewing system 补色立体观察系统anaglyphoscope 补色立体镜anaglyphy 补色立体法analcime 方沸石analcime tephrite 方沸岩碱玄岩analcimite 方沸岩analcimolith 方沸石岩analcite 方沸石analcitite 方沸岩analcitization 方沸石化analecta 文选anallatic 光学测距的;光学测距仪analog adder 模拟加法器analog algorithm 模拟算法analog approach 相似法解题analog circuit 模拟电路analog compiler system 模拟编译程序系统analog computer 模拟计算机analog control 模拟控制analog controller 模拟控制器analog converter 模拟变换器analog correlator 模拟相关器analog data 模拟数据analog display 模拟显示analog film 模拟照相记录胶片analog filter 模拟滤波器analog formatter 模拟曲线格式器analog generator 模拟发生器analog gravity chart 模拟量重力图analog indicator 模拟指示器analog information 模拟信息analog machine 模拟机analog magnetic recording 模拟磁带记录analog magnetic tape 模拟磁带analog memory 模拟存储器analog model 模拟模型analog multiplexer 模拟多路转换器analog oscilloscope 模拟示波器analog playback system 模拟回放系统analog plotter 模拟绘图仪analog preamplifier 模拟前置放大器analog pulse 模拟脉冲analog quantity 模拟量analog recorder 模拟记录仪analog scanning 模拟扫描analog signal 模拟信号analog solution 模拟解analog strip chart 模拟窄带曲线图analog switch 模拟开关analog tape format 模拟磁带格式analog transmission 模拟传输analog variable 模拟变量analog 模拟的analoghybrid computer programming 模拟混合计算机程序设计analogous 模拟的analogue flow rate 模拟流量analogue simulation 相拟模拟analogue =analog 模拟analogy analysis 类比分析analogy method 类比法analogy model 模拟模型analogy procedure 类推方法analogy 类似analog digital computing system 模拟数字计算系统analog digital converter 模拟数字转换器analog to digital conversion 模数转换analog to digital converter 模数转换器analyse 分析analyser 分析仪analyses analysis的复数analysis of cash flow 现值流量分析analysis of covariance 协方差分析analysis of regression 回归分析analysis of variance 方差分析analysis 分析analyst 分析者;化验员;系统分析学家analytic centre 分析中心analytic density 解析密度analytic function 解析函数analytic geometry 解析几何analytic method 解析法analytic model 解析模型analytic operator 解析算子analytic signal 解析信号analytic solution 解析解analytic stratigraphy 分析地层学analytic system 分析系统analytic 分析的analytical algorithm 分析算法analytical approach 分析方法analytical balance 分析天平analytical column 分析柱analytical continuation 解析延拓analytical error 分析误差analytical extrapolation 解析外推法analytical function 解析函数analytical geochemistry 分析地球化学analytical geomorphology 分析地貌学analytical hierarchy process 层次分析法analytical liquid chromatograph 分析液相色谱仪analytical method 解析法analytical model 分析模型analytical photogrammetry 解析摄影测量analytical photography 解析摄影学analytical plate number 分析塔板数analytical plotter 解析绘图仪analytical proof 分析证明analytical pure 分析纯analytical reagent 分析纯试剂analytical separation 分析分离analytical set 解析集analytical solution 分析解analytical standard 分析标准analytical stereoplotter 解析立体绘图仪analytical structural geology 解析构造地质学analytical trend 解析趋势analytical 分析的analyticity 解析性analytics 逻辑分析的方法;分解学;解析法analyze 分析analyzer 分析器;检偏镜;模拟装置;试验资料处理仪;分析员analyzing prism 分析镜analyzing spot 扫描点anamesite 细玄岩anamorphic 深带合成变质的anamorphism 深带合成变质;深带复合变质anamorphosis 变形anamorphotic 畸形的ananthous 无花的anaphylactic shock 过敏性休克anaphylactic 过敏性的anaphylatoxin 过敏毒素Anapiculatisporites 背锥疣孢属anarchy 无政府状态;混乱;无秩序anaseism 背震中运动anaseismic 离震源的anastatic water 边缘水anastigmat 去象散透镜anastigmatic lens 去象散透镜;正光镜头anastigmatism 消象散性anastomosed stream 网状河anastomosing branch 汊河anastomosing drainage 交织水系anastomosing river 交织河流anastomosing stream pattern 交织河网anastomosing 河道交织作用anastomosing deltoidal branch 交织三角洲汊河anastomsis 吻合anatase 锐钛矿anatectic batholith 深源岩基anatectic earthquake 深源地震anatectic magma 重熔岩浆anatectic melting 深熔作用anatexis 深熔作用anatexite 深熔岩Anathermal 冰后升温期;冰后升温的anatomic 解剖的;结构上的anatomy 解剖;解剖学Anatosaurus 鸭嘴龙属ana上ancestor 祖先;最初效应;原始粒子ancestral petroleum 原石油ancestral structural belt 古老构造带ancestral 原始的anch 锚Ancheilotheca 无唇螺属Anchicodium 近松藻属anchieutectic magma 近共结岩浆anchieutectic rock 近共结岩anchi metamorphism 近地表变质作用anchi monomineralic rock 近单矿物岩anchor agitator 锚式搅拌器anchor arm 锚臂anchor block 固定墩anchor bolster 锚架anchor bolt 地脚螺丝anchor buoy 锚浮标anchor cable 锚缆anchor casing packer 锚定式套管封隔器anchor catcher 锚捕捉器anchor chain 锚链anchor clamp 锚卡anchor fluke 锚爪anchor gap 火花隙anchor gear 起锚设备anchor head 锚头anchor holding capacity 锚抓力anchor jaws 锚爪anchor lantern 锚灯anchor latch 锚锁销anchor lift 抓钩提升绳器anchor line 绷绳anchor mechanism 锚定机构anchor packer 卡瓦封隔器anchor palm 锚爪anchor pattern 底材表面粗糙类型anchor peg 套栓anchor pile 锚桩anchor pipe 锚管anchor plate 锚定板anchor post 锚柱;路线标桩anchor projectile 投射锚anchor rack 锚架anchor rod 锚杆anchor rope 锚索;绷绳anchor screw 地脚螺丝;固定螺丝anchor seal assembly 锁紧式密封总成anchor shank 锚柄;锚身anchor shoe 锚式鞋anchor slip 固定卡瓦anchor spike 锚尖端anchor stock 锚杆anchor stone 底砾anchor swivel 锚转环anchor tie 锚拉杆anchor trial 起锚试验anchor washpipe spear 固定冲管式打捞矛anchor winch 起锚绞车anchor windlass 起锚机anchor 套管固定器;射孔弹固定器;锚anchorage force 支撑力anchorage stone 底砾anchorage system 锚定系统anchorage 固定anchored buoy 锚泊浮标anchored guy line 锚定绷绳anchored joint 锚定接头anchored peg 套栓anchored tension cable 锚定拉张钢缆anchoring agent 结合剂;增粘剂anchoring berth 锚泊泊位;系泊浮筒处anchoring strength 锚固强度;结合强度anchoring system 铺管驳船抛起锚系统anchoring 系紧anchoring organ 固着器官anchorite 脉状闪长岩ancient contourite 古等深积岩ancient land 古陆ancient landform 古地形ancient magnetic field 古磁场ancient plate 古板块ancient shoreline 古海岸线ancient strand line 古滨线ancient stream channel 古河道ancient triple junction 古三联点ancient turbidite 古浊流层ancillary depocenter 辅助沉积中心ancillary equipment 辅助设备ancillary outfit 辅助设备ancillary 辅助的ancudite 高岭石Ancyrodella 小锚牙形石属Ancyrognathus 锚颚牙形石属Ancyroides 拟锚牙形石属Ancyrolepis 锚鳞牙石属Ancyropenta 五角锚牙形石属AND gate expander 与门扩展器AND NOT gate 与非门AND output 与输出AND tube 与门管AND unit 与单元andaluzite 红柱石Andean orogenesis 安第斯山型造山作用andelatite 二长安山岩andendiorite 英辉闪长岩andengranite 云闪花岗岩andesilabradorite 安山拉长岩andesine 中长石andesinfels 中长角斑岩andesinite 中长岩andesite 安山岩andesitic tuff 安山凝灰岩andesitoid 似安山岩andosoil 火山灰土壤andradite 钙铁榴石Andrewsarchus 安氏兽属androstane 雄甾烷AND circuit 与电路AND gate 与门;与门逻辑电路AND OR expander 与或扩展器AND OR AND gate 与或与门AND OR circuit 与或电路AND OR NOT gate 与或非门AND to OR function 与或作用anechoic 无回声的anelastic absorption 滞弹性吸收anelastic deformation 滞弹性形变anelastic property 滞弹性anelastic 滞弹性的;弹性后效anelasticity 滞弹性anelectric 非电化的;非电化体anelectrode 负极anemoarenyte 风成砂岩anemobarometer 风速风压计anemobiagraph 风速风压记录器anemoclast 风成碎屑anemoclastic rock 风成碎屑岩anemoclastic 风成碎屑的anemoclinometer 风斜表anemogenic sediment 风成沉积物anemogram 风速记录表anemograph 风速计anemolite 弯钟乳石anemometer 风力表anemometry 风速和风向测定法anemoscope 风速仪anemosilicarenite 风成硅质砂屑岩anemo风anergy 无效能anerobic corrosion 厌气菌腐蚀anerobic 厌氧的anerobiosis 厌氧生活aneroid barometer 无液气压计aneroid 无液的anesthetic action 麻醉作用anesthetic effect 麻醉效用anesthetic =anaestheticANFO 铵硝柴油混合炸药anfractuosity 曲折ang 角ANG 美国报业公会ANGA 美国天然气协会Angara flora 安加拉植物群Angara shield 安加拉地盾Angara 安加拉古陆Angaris 安加拉地盾Angarophycus 安加拉藻属angel 天使;寄生目标;假目标Anglaspis 环甲鱼属angle bar 角钢angle beam method 斜射法angle bend 弯头angle block 角铁angle brace 斜撑angle bracket 角形托座angle build 增斜angle buildup interval 造斜井段angle build up 造斜angle catch 窗插销angle cock 弯角旋塞阀angle compressor 角度式压缩机angle crane 斜座起重机angle cutter 角铁切割机;角铣刀angle detector 角检测器angle displacement 角位移angle drive 角驱动angle drop 降斜angle factor 角系数angle file 三角锉刀angle fishplate 角钢鱼尾板angle gain 增斜angle gate valve 角闸阀angle gauge block 量角规angle gauge 角度计angle head 角机头angle holding assembly 稳斜钻具组合angle holding BHA 稳斜底部钻具组合angle in 下钻时的井斜角angle iron stiffening 角钢支撑angle iron 角铁angle joint 隅接angle meter 测角器angle modulation 调角angle momentum 角动量angle needle valve 弯角针形阀angle noise 角度噪声angle of advance 提前角angle of altitude 仰角angle of approach 接近角angle of arrival 入射角angle of attack 迎角angle of backing off 刃后角angle of bank 倾斜角angle of circumference 圆周角angle of contact 接触角angle of convergence 收敛角angle of crossing 交会角angle of curvature 曲率角angle of cutting edge 刃前角angle of cutting 切削角angle of cut off 截止角angle of declination 偏角angle of deflection 偏转角angle of departure 射角angle of depression 俯角angle of deviation 偏角angle of diffraction 衍射角angle of dip 倾角angle of displacement 位移角angle of divergence 发射角angle of elevation 仰角angle of emergence 出射角angle of entry 入射角angle of firing 射角angle of flare 承口角angle of friction 摩擦角angle of gradient 倾度角angle of hade 断层余角angle of heel 横倾角angle of incidence 入射角angle of inclination 倾角angle of internal friction 内摩擦角angle of intromission 全透射角angle of lag 滞后角angle of lead 超前角angle of lean 倾角angle of minimum resolution 最小分辨角angle of missetting 误置角angle of obliquity 斜角angle of orientation 方位角angle of pitch 倾伏角;俯仰角;螺距角angle of pull 牵引角angle of rake 后倾角angle of reflection 反射角angle of refraction 折射角angle of repose 休止角angle of rotation 旋转角angle of run 去流角angle of rupture 破裂角angle of scattering 散射角angle of shear 剪切角angle of shearing resistance 抗剪角angle of sight 视线角angle of skew back 侧倾角angle of slide 滑动角angle of slip 位移角angle of slope 倾角angle of spread 展开角angle of strike 走向方位角angle of taper 锥削角angle of thread 螺纹牙形角angle of tilt 倾角angle of torsion 扭角angle of trim 平衡角angle of trimming 纵倾角angle of twist 扭转角angle of unconformity 不整合角angle of view 视角angle of visibility 可视角angle of wetting 湿润角angle of yaw 船头摆角angle out 起钻时的井斜角angle pipe 角管angle plate 角型板angle plug 弯插头angle point 角点angle press 角压塑机angle protractor 量角规angle pulley 换向滑轮angle purlin 角檩angle rafter 角椽angle rule 角尺angle section 角形截面angle sheave 导向轮angle splice bar 角型鱼尾板angle stake 斜接口angle steel 角钢angle table 角钢托座;三角桌;角撑架angle test tube 氟氢酸测斜管angle thermometer 直角温度计angle tie 斜接angle tolerance 角度公差angle transducer 角传感器angle valve 角阀angle wheel 斜齿轮angle 角;棱;角度angled 角的angledozer 侧推式推土机angles back to back 背对背角钢anglesite 硫酸铅矿angle averaging 角平均法angle azimuth indicator 倾斜度及方位指示器angle beam 隅梁angle bender 钢筋弯曲机angle finding position fixing 测角定位angle indicating unit 角度指示部件angle phase digital converter 轴角相移数字转换器angle to digit converter 角数转换器angle true net 等角网angling dozer 斜板推土机Angola abyssal plain 安哥拉深海平原angstrom 埃angular acceleration 角加速度angular accuracy 角精度angular adjustment 角度调整angular altitude 高低角angular aperture 孔径角angular belting 斜角皮带传动装置angular bevel gear 斜交伞齿轮angular bisector 角平分线angular blocky structure 角块状构造angular breadth 角宽度angular brightness 角亮度angular coarse aggregate 有棱角粗骨料angular conglomerate 棱角状砾岩angular contact ball bearing 向心止推滚珠轴承angular contact 斜角接触angular coordinate 角坐标angular cross bedding 倾斜交错层angular deflection 角度偏移angular deformation 角形变angular dependence 角关系angular derivative 角微商angular deviation 角偏向angular discordance 角度不整合angular distribution 角分布angular domain 角域angular drainage pattern 角状水系angular error 角度误差angular excess 角盈angular fold 尖棱褶皱angular force 角向力angular frequency 角频率angular gap 角形裂隙angular gear 斜齿齿轮angular grain chipping 角砾碎屑angular harmonic motion 角谐运动angular instrument 测角仪angular jointing 斜节理angular magnification 角度放大率angular misalignment 角度错位angular modulation 角度调制angular moment 角矩angular momentum 角动量angular motion 角运动angular orientation 角定向angular oscillation 角摆动angular perspective 成角透视angular phasing 角相位angular pitch 倾斜角angular point 角点angular rate 角速度angular resolution 角分辨率angular sand 角粒砂angular setting 角度调整angular shear 角度剪切angular speed 角速度angular spreading 角扩散angular strain 角应变angular surface 棱角表面angular thread 三角螺纹angular tolerance 角容限angular transformation 角变换angular travel 角行程angular unconformity 角度不整合angular variance 角方差angular velocity 角速度angular vertex 角的顶点angular 角的angularity correction 角度校正angularity 尖angular grained 尖角颗粒状的angulate 有角的angulation 回转angulator 变角器Angulisporites 菱环孢属Angulodus 角牙形石属Angulolithina 角颗石angulometer 量角器Angustella 狭荚蛤属anh 无水的anharmonic coupling 非谐耦合anharmonic ratio 非谐比例anharmonic resonance 非谐共振anharmonic vibration 非谐振动anharmonic 非谐的anharmonicity 非谐性anhedral crystal 他形晶anhedral grain 他形晶粒anhedral texture 他形结构anhedritite 硬石膏anhydration 干化anhydride 酐anhydrite cement 无水石膏水泥anhydrite rock 硬石膏岩anhydrite sheath 硬石膏鞘anhydrite 无水石膏anhydritic dolomite 硬石膏白云岩anhydrock 硬石膏岩anhydroferrite 赤铁矿anhydrone 无水高氯酸镁anhydrous alcohol 无水酒精anhydrous ammonia 无水氨anhydrous facies 无水相anhydrous isopropyl alcohol 无水异丙醇anhydrous lime 无水石灰anhydrous plaster 无水粉饰;无水石膏粉饰anhydrous solvent 无水溶剂anhydrous 无水的anhysteretic remanent magnetization 非滞后剩余磁化anhysteretic 非磁滞;非滞后的anil 靛蓝anilide N酰苯胺aniline equivalent 苯胺当量aniline point 苯胺点aniline process 苯胺法aniline 苯胺aniline formaldehyde resin 苯胺甲醛树脂aniline gravity constant 苯胺点比重常数animal plankton 浮游动物animal remains 动物遗体animal theory 动物说animation sequence 动画序列animation 生动Animikian system 安尼米克系anion exchange resin 阴离子交换树脂anion exchange resins 阴离子交换树脂anion exchange 阴离子交换anion exchanger 阴离子交换器anion 阴离子anionic catalytic polymerization 阴离子催化聚合anionic coordinate polymerization 阴离子配位聚合anionic detergent 阴离子洗涤剂anionic emulsifier 阴离子乳化剂anionic grafting 阴离子接枝anionic polyelectrolyte 阴离子聚电解质anionic polymer 阴离子聚合物anionic polymerization 负离子聚合anionic surface active agent 阴离子型表面活性剂anionic surfactant 阴离子表面活性剂aniontropy 阴离子移变aniseikon 目标移动电子显示器;电子照相仪;电子显微仪aniseikonia 物象不等Anisian stage =Anisic stageAnisian 安尼西阶Anisic stage 安尼西阶anisobaric 不等压的anisoelastic 非弹性的anisomeric rock 复杂结晶岩anisomeric 非异构的anisomerism 非异构性anisomerous texture 不等粒结构anisometric breccia 不等粒角砾岩anisometric crustal 非等轴晶体anisometric deposit 不等粒沉积anisometric growth 不等量生长;非等比生长;非同形生长anisometric 不等轴的;不等粒的Anisostoma 反口螺属anisotropic absorption 非均质吸收anisotropic body 各向异性体anisotropic conductivity 各向异性传导率anisotropic effective mass 各向异性有效质量anisotropic filter 方向滤波器anisotropic formation 各向异性地层anisotropic index 各向异性指数anisotropic material 各向异性材料anisotropic permeability 各向异性渗透率anisotropic scattering 各向异性散射anisotropic 各向异性的anisotropisation 各向异性化anisotropism 各向异性anisotropy coefficient 各向异性系数anisotropy dipmeter 各向异性地层倾角仪anisotropy energy 各向异性能anisotropy paradox 各向异性交错anisotropy ratio 各向异性比anisotropy 各向异性Anisotrypa 不等苔藓虫属anis不同ankaramite 橄榄辉玄岩ankaratrite 黄橄霞玄岩ankerite 铁白云石Ankylosaurus 甲龙属ANL 美国阿贡国立实验室ANL 退火ANL 自动噪音限制器Ann Rep 年报ann 年鉴ANN 人工神经网络ann 退火的annals 史册anneal 使退火;锻烧;韧炼annealing oil 退火油annealing 退火;缓冷;锻烧;韧化annex 附加物;附件;附录;附属建筑物;增建部分annexation 附加;合并;附加物annihilation 歼灭;湮灭annihilator 减震器;熄灭器;零化子annite 铁云母anniversary wind 季节风anniversary 周年纪念;周年的annotated mosaic 注记镶嵌图annotated photograph 已调绘相片annotation 注记;调绘annual accounting statement 年度会计决算annual compound rate 年复利率annual decline rate 年递减率annual financial report 年度财务报告annual heat budget 年热收支量annual lamination 年纹理annual magnetic variation 周年磁变annual output 年产量annual overhaul 年度检修annual parallax 周年视差annual plan 年度计划annual precipitation 年降水量annual profit 年利润总额annual rainfall 年降雨量annual rate 年利率annual recharge 年补给annual report 年度报告annual reserve hearing 年度储量审查annual rhythm 年韵律层annual runoff 年径流量annual sales revenue 年销售收入annual sales tax 年销售税金annual snowline 年雪线annual variation 年变化annual yield 年产量annual 周年的annually frozen layer 年冻结层annuity method of depreciation 年金折旧法annuity 年金;年金法annular anomaly 环状异常annular auger 环孔钻annular ball bearing 径向轴承annular bearing 环形轴承annular bit 环状钻头annular blowout preventer 套筒式环空防喷器annular boring 环孔钻annular bridging 环状桥堵annular burner 环状燃烧器annular bypass port 环空旁通孔annular bypass 水泥环内的窜槽annular capacity 环空单位长度容积annular casing 环形壳体annular clearance 环空间隙annular complex 环形聚合体annular drainage pattern 环状水系annular flow 环空液流;环状流annular gear 内齿轮annular mud velocity 环空泥浆速度annular nozzle 环状喷嘴annular orifice 环形孔板annular pack 环空充填annular packoff 环形封隔annular phasing 圆周相位角annular piston 环状活塞annular plate 边缘底板annular pressure 环空压力annular region 环形区域annular saw 圆锯annular space 环形空间annular vault 环形穹顶annular velocity 环隙流速annular volume 环空体积annular wheel 内齿轮annular 环形的annulation 环;环形物;环的形成annulet 小环;轮缘;圆箍线annuli annulus的复数Annulithus 环颗石annulus access flow test riser 环空测流立管annulus access line 环空连通管annulus access 环空通道annulus flow line 环空出油管annulus flow profile 环空流前annulus fluid 环空液体annulus line 环空管annulus logging 环空测试annulus pack off 环空封隔装置annulus pressure 环空压力annulus profile 环形剖面annulus scraper 环形刮蜡器annulus test 环空试压annulus top up cementation 环空满眼注水泥annulus unloading 环形空间卸压annulus valve 环形阀annulus water injection 环空注水annulus wing valve 环空翼阀annulus 环annulus tubing crossover 环空油管转换接头annum 年annunciator 信号器;报警器anodal 阳极的anode battery 板极电池anode bed 阳极地床anode cap 阳极罩anode capacitance 阳极电容anode coating 阳极涂层anode converter 阳极电源整流器anode corrosion efficiency 阳极腐蚀效率anode follower 阳极输出器anode groundbed 阳极地床anode loss protection 牺牲阳极保护anode polarization 阳极极化anode scrap 残阳极anode sleds 阳极栅anode slime 阳极残渣anode strap 阳极条anode 阳极anode bend detection 阳极检波anodic acid pickling 阳极酸洗anodic area 阳极区anodic inhibitor 阳极钝化剂anodic oxidation 阳极氧化anodic passivation 阳极钝化anodic passivity 阳极钝化anodic protection 阳极保护anodic site 阳极区anodic =anodalanodizing 阳极处理anogene 深成变质的anogenic 深成变质的anolyte 阳极电解液anomalistic point 反常点anomalistic 异常的Anomalotoechus 差壁苔藓虫属anomalous amplitude 异常振幅anomalous body 异常体anomalous dispersion 不规则弥散anomalous field 异常场anomalous free air gradient 异常自由空气梯度anomalous mass 异常质量anomalous occurrence 异常产状anomalous refraction 异常折射anomalous rotatory dispersion 异常旋光色散anomalous spectral reflectance 异常光谱反射率anomalous stream sediment 异常水系沉积物anomalous structure 反常结构anomalous surface 异常面anomalous transmission 反常透射anomalous underflow 异常暗流anomalous 异常的anomaly contour 等异常线anomaly of gravity gradient 重力梯度异常anomaly pattern 异常形式anomaly peak 异常峰anomaly resolution 异常分辨anomaly source 异常源anomaly threshold 异常下限anomaly trend 异常走向;异常趋势anomaly 异常;不规则;变态;破格;近点角;近点距离anomaly seeking method 异常调查法Anoplotheres 无防兽属anorak 防水布;防水衣anorganic 非有机的anorganogene 无机成因的anorganolite 无机岩anorganolith 无机岩anorge 不有机性anorogenic granite 非造山花岗岩anorogenic time 非造山期anorogenic 非造山期的anorthic 三斜的anorthite 钙长石anorthitissite 钙长角闪石岩anorthitite 钙长岩anorthoclase 歪长石anorthose 斜长石anorthosite 斜长岩anorthositic gabbro 钙长辉长岩Anosteira 无盾龟属anotron 冷阴极充气整流管ANOVA 方差分析anoxia 缺氧anoxic layer 缺氧层anoxic marine pore water 缺氧海相孔隙水anoxic 缺氧的anoxicity 缺氧度anoxybiosis 绝氧生活anoxybiotic 厌氧的anoxygenous 缺氧的ANS 美国国家标准ANS 自动噪声抑制器ANSAC 自动表层时间异常校正ANSI 美国国家标准学会answer 回答;答案answerable 应负责的;可以答复的answerback 应答ANT 天线anta 避角柱Antaciron 硅铁合金antagonism 对抗antagonist 对抗者antagonistic symbiosis 对抗共生antagonistic 有对抗作用的antalkali 解碱剂antapex 背点antapical born 底角antapical plate 底板Antarctic Circle 南极圈Antarctic pole 南极Antarctic realm 南极区antarctic 南极的;南极区Antarctica plate 南极板块Antarctica 南极洲Antartosaurus 北方龙属antecedence 先行antecedency =antecedenceantecedent money 押金antecedent sedimentary basin 先成沉积盆地antecedent stream 先成河。
MEMBRANE SEPARATION OF AMMONIUM ACETATE AND AMMON

专利名称:MEMBRANE SEPARATION OF AMMONIUM ACETATE AND AMMONIUM HYDROXIDE 发明人:WANG, Ying,YANG, Qilian申请号:CA1990000089申请日:19900316公开号:WO90/011119P1公开日:19901004专利内容由知识产权出版社提供摘要:A separation member is provided for use in the separation of ammonium acetate and ammonium hydroxide from a liquid containing same. The separation member comprises a porous hollow support member and a membrane which is integrally associated with the support member. The membrane is formed by subjecting the support member to a solution containing an effective amount of a polysiloxane and a silane, and if required a crosslinking agent, for a period of time sufficient to form a membrane capable of separating ammonium hydroxide and ammonium acetate. A method is also provided for separating ammonium acetate from ammonium hydroxide using the separation member. The method includes the steps of flowing through and about and in contact with the porous hollow support member (12) an aqueous solution containing ammonium hydroxide and ammonium acetate and passing air through the interior of the support member. The temperature of the air is controlled so that the temperature difference between the interior and exterior of the porous hollow suppport member is such that water and ammonium vapours are allowed to pass from the exterior of the support member to the interior of the support member. The water vapour is condensed and the ammonium vapour is converted to ammonium hydroxide. A method for preparing theseparation member and a membrane module containing one or more separation modules are further provided.申请人:WANG, Ying,YANG, Qilian地址:US,CA,CA国籍:US,CA,CA代理机构:ROGERS, BERESKIN & PARR更多信息请下载全文后查看。
水吸收氨气工艺流程

水吸收氨气工艺流程英文回答:Water absorption of ammonia is a common process used in various industries, such as wastewater treatment plants and chemical production facilities. This process involves the removal of ammonia gas from a gas stream by dissolving itin water. The resulting solution, often referred to as an ammonium hydroxide solution, can then be further processed or treated as needed.The basic principle behind water absorption of ammonia is the solubility of ammonia gas in water. Ammonia is highly soluble in water, and this property can be utilized to effectively remove it from a gas stream. The gas stream containing ammonia is brought into contact with water, either in a packed column or a scrubber, where the ammonia gas is absorbed into the water.The efficiency of the absorption process depends onvarious factors, such as the temperature and pressure ofthe gas stream, the concentration of ammonia in the gas stream, and the contact time between the gas and water. Higher temperatures and pressures generally enhance the solubility of ammonia in water, leading to a more efficient absorption process. Additionally, increasing the contact time between the gas and water, for example by using alarger packed column or increasing the flow rate of the gas stream, can also improve the absorption efficiency.Once the ammonia gas is absorbed into the water, it forms ammonium hydroxide, which is a weak base. The concentration of ammonium hydroxide in the resultingsolution depends on the initial concentration of ammonia in the gas stream and the efficiency of the absorption process. This solution can then be further processed or treated, depending on the specific requirements of the application.For example, in a wastewater treatment plant, the ammonium hydroxide solution obtained from the water absorption of ammonia process can be used as a nutrient source for biological treatment processes. The ammoniumions in the solution can be converted by bacteria into nitrate ions through a process called nitrification. This helps in the removal of ammonia from the wastewater, as nitrate ions are less toxic and easier to remove.中文回答:水吸收氨气是一种常见的工艺流程,广泛应用于废水处理厂和化工生产设施等各个行业。
氯化铵氢氧化钙共热制氨气 方程式

氯化铵氢氧化钙共热制氨气方程式1.氯化铵和氢氧化钙共热可以生成氨气。
Ammonia gas can be produced by the thermal decomposition of ammonium chloride and calcium hydroxide.2.氯化铵的化学式是NH4Cl。
The chemical formula of ammonium chloride is NH4Cl.3.氢氧化钙的化学式是Ca(OH)2。
The chemical formula of calcium hydroxide is Ca(OH)2.4.氨气的化学式是NH3。
The chemical formula of ammonia gas is NH3.5.氯化铵在加热的过程中分解成氨气和盐酸。
Ammonium chloride decomposes into ammonia gas and hydrochloric acid upon heating.6.氢氧化钙在加热的过程中分解成氧化钙和水。
Calcium hydroxide decomposes into calcium oxide and water upon heating.7.氨气是一种无色、有刺激气味的气体。
Ammonia gas is a colorless gas with a pungent odor.8.氨气可溶于水,形成氨水。
Ammonia gas is soluble in water, forming ammonia water.9.氯化铵和氢氧化钙的共热方法是制备氨气的一种常见方法。
The thermal decomposition of ammonium chloride and calcium hydroxide is a common method for producing ammonia gas.10.氨气是一种重要的化工原料,广泛用于制造化肥和合成其他化合物。
水吸收氨气工艺流程

水吸收氨气工艺流程英文回答:Water absorption of ammonia gas is a common industrial process used to remove ammonia from gas streams. This process is widely used in industries such as chemical plants, refineries, and wastewater treatment facilities. In this process, water is used as a solvent to absorb the ammonia gas, resulting in a solution of ammonia in water.The water absorption of ammonia gas process typically involves several steps. First, the gas stream containing ammonia is brought into contact with water. This can be done using various equipment such as packed towers, spray towers, or bubble columns. The gas and water are brought into intimate contact, allowing the ammonia to dissolveinto the water.The solubility of ammonia in water depends on various factors such as temperature, pressure, and theconcentration of other substances in the water. Generally, higher temperatures and lower pressures favor the absorption of ammonia into water. However, the presence of impurities or other gases in the gas stream can also affect the solubility of ammonia.Once the ammonia gas is absorbed into water, the resulting solution is typically referred to as an ammonium hydroxide solution or ammonia water. This solution can then be further processed or treated depending on the specific application. For example, in wastewater treatment facilities, the ammonia water may undergo further treatment to remove impurities before being discharged.One common application of water absorption of ammonia gas is in the production of ammonium nitrate, a widely used fertilizer. In this process, ammonia gas is absorbed into water to form ammonium hydroxide, which is then reacted with nitric acid to produce ammonium nitrate. This process is known as the Ostwald process and is an important industrial process for the production of ammonium nitrate.中文回答:水吸收氨气是一种常见的工业过程,用于从气体流中去除氨气。
石油词汇英语翻译(A19篇)2

aminosilane coupling agent 氨基硅烷偶合剂 amino acid 氨基酸 amino alcohol 氨基醇 amino group 氨基 amino phenol 氨基酚 ammendment 修改 ammeter 安培计 ammine 氨络物 ammino 氨络 ammite 鲕状岩 ammocolous 砂⽣⽣物;栖砂的 Ammocypris 瘤星介属 ammonal 阿芒拿 ammonia buffer 氨缓冲溶液 ammonia condenser 氨冷凝器 ammonia cooler 氨冷却器 ammonia oil 氨压缩机汽缸油 ammonia pump 氨泵 ammonia refrigerator 氨冷冻机 ammonia stripping urea process 氨⽓提尿素⼯艺 ammonia stripping 氨洗涤 ammonia suit 防氨服 ammonia synthesis catalyst 氨合成催化剂 ammonia synthesis loop 氨合成回路 ammonia water 氨⽔ ammonia 氨 ammoniation 氨化 ammonibacteria 氨细菌类 ammonification ⽣氨;加氨 ammonio 胺基 ammonioborite ⽔铵硼⽯ ammonite 菊⽯;阿芒奈特炸药 ammonium acid phosphate 酸性磷酸铵 ammonium bicarbonate 碳酸氢铵 ammonium bifluoride 氟化氢铵 ammonium bisulfite 亚硫酸氢铵 ammonium carbonate 碳酸铵 ammonium chloride brine 氯化铵盐⽔ ammonium chloride spacer 氯化铵隔离液 ammonium chloride 氯化铵 ammonium chloroplatinate 氯铂酸铵 ammonium chloroplatinite 氯亚铂酸铵 ammonium fluoride 氟化铵 ammonium hydroxide 氢氧化铵 ammonium molybdate 钼酸铵 ammonium nitrate 硝酸铵 ammonium persulfate 过硫酸铵 ammonium salt 铵盐 ammonium sulfate 硫酸铵 ammonium sulphonate 磺酸铵 ammonium thiocyanate 硫氰酸铵 ammonium vanadate 钒酸铵 ammonium 铵 ammonolysis 氨解作⽤ ammonpulver 硝氨炸药 Ammosiphonia 砂管⼝⾍属 ammoxidation catalyst 氨氧化催化剂 ammunition protection grease 弹药保护脂 ammuntion 弹药;装弹药 AMNH 美国⾃然博物馆 amnicolous 沙岸⽣物 Amnuralithus 暗弯⾓⽯ Amoco International Oil Co. 阿莫科国际⽯油公司 amoebae amoeba的复数 Amonotis ⽆⽿髻蛤属 amorce 起爆剂 Amorican Orogeny 阿莫利坎造⼭运动 amorization of intangible assets ⽆形资产摊销 amorphism ⽆定形性;不结晶性;⽆定向;⽆⽬的 Amorphognathus 变形颚⽛形⽯属 amorphous bitumen ⽆定形沥青 amorphous carbon ⾮晶碳 amorphous catalyst ⽆定形催化剂 amorphous kerogen ⽆定形⼲酪根 amorphous liptinite ⽆定形类脂体 amorphous matrix ⽆定形基体 amorphous solid ⾮晶固体 amorphous ⽆定形的;⾮晶的;⽆⼀定⽅向的 amortisseur 阻尼器;减震器 amortization fund 摊提资⾦ amortization period 清偿期 amortization rate 摊销系数 amortization 阻尼 amortize 分期偿还;防⽌;阻尼 amount insured 保险额 amount of deflection 挠度 amount of precipitation 降⽔量 amount of scale buildup 结垢量 amount remitted 汇出额 amount 量;⾦额;合计 amp gauge 安培表 amp 安培 amp 安培数 AMP 放⼤器 AMP 可利⽤的马达压降 AMP 视⾻架剖⾯ amp 振幅 AMP ⾃动做记号⾯板。
Milllex-FG水分离滤芯用户指南说明书

User GuideMillex®-FG Hydrophobic Filter• Single use only• Sterile• Non-pyrogenicSLFGR25LSFor research use only.Indications for Use/PurposeThe bidirectionally supported Millex®-FG is a device intended for use as a syringe filter to sterilize and/or clarify low volume solutions to remove fine particles from organic and aqueous solutions or for air and gas venting and filtration applications.IntroductionThe bidirectionally supported Millex®-FG device isa sterilizing filter for gases and liquids that are not compatible with standard membrane filters. It will remove microorganisms, particles, precipitates, and undissolved powders larger than 0.2 micron (μm). The sterile Millex®-FG filter is non-pyrogenic andnon-toxic. This single-use product consists of a 0.2 μm hydrophobic Fluoropore™ membrane filter sealed in a polyvinyl chloride (PVC) housing. Typical applications include the sterile filtration of alcohols, concentrated acids and bases, and air or gas.Millex® filters with hydrophobic Fluoropore TM membrane are ideal for sterilizing gases, venting sterile containers, and sterilizing or clarifying organic solutions.Chemical CompatibilityThe Millex®-FG filter is compatible with mild organic and organic aqueous solutions or air and gas. It may be used to filter the agents listed below. This guide has been developed from technical publications, materials suppliers, and laboratory tests, and is believed to be reliable. However, because of variability in temperature, concentrations, duration of exposure, and other factors outside of our control, no warranty is given or is to be implied with respect to such information. Agents not listed below should be tested with the Millex®-FG filter prior to use.Acetic acid (glacial)Acetic acid (5%)Aliphatic ethersAmmonium hydroxide (6 N)Amyl acetateAmyl alcoholBenzyl alcohol (1%)Boric acidBrine (sea water)Butyl alcoholCarbon tetrachlorideEthyl alcoholEthylene glycolFormaldehydeFreon® solvent,Trichlorotrifluoroethane (TF) orPrecision Cleaning Agent (PCA)Glycerine (glycerol)Helium (gas)HexaneHydrofluoric acidHydrogen (gas)Hydrogen peroxide (3%)Hypo (photo)Isobutyl alcoholIsopropyl alcoholMethyl alcoholMineral spiritsNitrogen (gas)ParaldehydePentanePetroleum based oilsPetroleum etherPhenol (0.5%)PyridineSilicone oilsDirections for UseWARNINGS• To ensure sterility, do not use this product if the package is damaged.• Do not use the Millex® 25 mm syringe filter for direct patient care applications; it is designed for laboratory use only.• Do not use with syringes smaller than 10 mL because pressures in excess of the maximum pressure rating may be reached, potentially causing damage to the filter unit and/or personal injury. CAUTIONS• Do not resterilize or reuse the Millex®-FG filter, as the company cannot assure the sterility, integrity, and performance beyond a single use.• Do not use the Millex®-FG filter at temperatures above 45 °C (113 °F).• Do not use the same Millex®-FG filter to filter solutions in both directions.• Do not use the Millex®-FG filter to filter emulsionsor suspensions.• Do not use the Millex®-FG filter to filter 5 mg or less of protein-containing solutions or reactive materials unless binding studies have been performed.• Do not reuse the syringe filter.• When used to protect a vacuum system,the Millex®-FG filter may become momentarily inoperable if aqueous fluid completely fills the housing. To clear the unit, disconnect it fromthe upstream Luer connection and asepticallyapply air pressure with a syringe.• If using the Millex®-FG filter with a vacuum or air system, attach the appropriate Luer adapters to the vacuum or air system tubing.If using the Millex®-FG filter as a syringe filter, follow the directions below. When filtering aqueous solutions (acids, bases), filter 10 mL of ethanol or methanol through the unit to wet the hydrophobic filter. Discard the first 2–3 mL of filtered solution to prevent alcohol contamination.1.Fill syringe with solution to be filtered.2. Aseptically remove coverfrom package.3. Attach syringe to filter and removeassembly from package. Attach needleto Luer-slip outlet if necessary.4. Hold syringe with filter (and needleif attached) pointing up and top offby pushing a few drops through.Do not contaminate underside offilter with fingers.5. Insert needle (if attached) and pushplunger to deliver filtered solution.The life science business of Merck operates as MilliporeSigma in the U.S. and Canada.Merck, Millipore, Fluoropore, Millex and Sigma-Aldrich are trademarks of Merck KGaA,Darmstadt, Germany or its affiliates. All other trademarks are the property of their respective owners. Detailed information on trademarks is available via publicly accessible resources.© 2021 Merck KGaA, Darmstadt, Germany and/or its affiliates. All Rights Reserved.SpecificationsMaterialsMembrane Hydrophobic Fluoropore™ (PTFE) polytetrafluoroethylene, type FG Pore size 0.2 µmHousing Polyvinyl chloride (PVC)DimensionsInlet to outlet 25 mmDiameter 29 mm (1.14 in.)Filtration area 4 cm 2 (0.62 in 2)Temperature limit 45 °C (113 °F)Pressure limit at 21 °C5.2 bar (75 psi) inlet and differential maximum Filtration volume 1 mL to 100 mLHold-up volume ≤ 0.1 mL after air purge Sterilization method Ethylene oxide gas ConnectionsFemale Luer-Lok™ inlet Male Luer-slip outletSymbol DefinitionsSymbol DefinitionSymbol DefinitionhCatalogue number N Date of manufacture D Do not reuse M Manufacturer HUse-by date LDo not use ifpackage is damagedgBatch codeIQ Sterilized using ethylene oxideNoticeWe provide information and advice to our customers on application technologies and regulatory matters to the best of our knowledge and ability, but without obligation or liability. Existing laws and regulations are to be observed in all cases by our customers. This also applies in respect to any rights of thirdparties. Our information and advice do not relieve our customers of their own responsibility for checking the suitability of our products for the envisaged purpose.The information in this document is subject to change without notice and should not be construed as acommitment by the manufacturing or selling entity, or an affiliate. We assume no responsibility for any errors that may appear in this document.Contact InformationFor the location of the office nearest you, go to /offices .Technical AssistanceVisit the tech service page on our web site at /techservice .Standard WarrantyThe applicable warranty for the products listed in this publication may be found at /terms .。
科技英语单词

P16 Biochemical 生化的Surface water 地表水Physicochemical 物理化学的Total solid 总固体Organic and nitrogen loading 有机Groundwater 地下水和氮负荷Scale-forming 结垢Ammonia 氨气Finished waters 办理后的水Dissolved oxygen 溶解氧Per-capita usage 人均用量Pollutional load 污染负荷Color 色度Stream purification capacity 河流Turbidity 浊度净化容量Taste 滋味Effluent quality or effluentOdor 臭味standard 出水质量或出水标准Nitrate 硝态氮Nitrogen fertilizer 氮肥Harmful metal ions 有害金属离子Organic chemicals 有机化学物质Organic chemicals 有机化学物质Municipal wastewater treatment Pesticide 农药plant (MWTP) 市政污水办理厂Chlorinated solvent 氯代溶剂P18. Environmental Protection Agency Inorganic chemistry 无机化学美国环保局Colloidal chemistry 胶体化学Hardness 硬度Radiochemistry 放射化学P17 Contamination 污染disposal 处理Cleanup 清理Drainage or sewer system 排水或污Analytical chemistry 剖析化学水系统Sampling 采样Purification or treatment Separation 分别facilities 净化或办理设备Quantification 量化effectiveness of treatment Leachate 渗滤液processes 办理工艺的有效性Aquifer 水体P19 by-product 副产物Urban population 城市人口impurities 杂质Population density 人口密度organic load 有机负荷combustion 焚烧formaldehyde 甲醛Incinerator 焚烧炉methanol 甲醇Particulate matter 颗粒物质vital-force theory 生命力理论Visible 可见的ammonium cyanate 氰化胺Atmospheric inversion 大气逆温urea 尿素Respiratory tract 呼吸道P24Photochemical 光化学的Elements ( 元素 )Unsaturated hydrocarbon 不饱和烃hydrocarbon 烷烃Automobile exhaust gases 汽车尾气nitrogen 氮Ozone 臭氧phosphorous 磷Formaldehyde 甲醛sulfur 硫Radioactive materials 放射性物质halogen 卤素Nuclear power plant 核电厂P25Solar energy 太阳能Properties ( 性质 )Biomass 生物质combustible 可燃的Ultraviolet radiation 紫外辐射melting point 熔点Carbon dioxide 二氧化碳boiling point 沸点Methane甲烷soluble 可溶的Chlorofluorocarbons 氟利昂formula 分子式Greenhouse gases 温室气体isomerism 同分异构P23 molecular 分子的organic chemistry 有机化学ionic 离子的synthesis 合成molecular weight 分子量liquid 水体中Bacteria 细菌Sources ( 根源 ) Marsh gas 沼气fibers 纤维Natural gas 天然气cellulose 纤维素Gas engines 燃气发动机starch 淀粉Firedamp 瓦斯alkaloids 生物碱Greenhouse gas 温室气体fermentation 发酵Stratosphere 同温(平流)层acetone 丙酮P28glycerol 甘油methane 甲烷antibiotics 抗生素ethane 乙烷acid 酸propane 丙烷microorganism 微生物butane 丁烷P27 normal 正Isomerism ( 同分异构现象 ) N-butane 正丁烷isomer 同分异构体Prefix iso- 前缀hydroxy acid 羟基酸isobutane 异丁烷structural formula构造分子式Straight chain 直链aliphatic 脂肪族Branched-chain 支链aromatic 芬芳族Room temperature 室温heterocyclic 杂环的Homologous series 同族系列Hydrocarbon ( 烃) General formula 通用分子式Saturated hydrocarbons 饱和烃P30Unsaturated hydrocarbons 不饱和烃nomenclature 系统命名法Paraffin series 链烷烃系列IUPAC(International Union of Pure petroleum 石油and Applied Chemistry)gasoline 汽油国际纯粹化学和应用化学联合会Diesel fuel 柴油strong base 强碱Methane 甲烷strong acid 强酸oxidizing agent 氧化剂P34concentrated sulfuric acid 浓缩的synthetic rubber 合成橡胶硫酸oxidation 氧化反响Oxidation 氧化aqueous solution 水溶液substitution 代替potassium permanganate 高锰酸钾pyrolysis 高温裂解reduction 复原反响cracking 裂化glycol 乙二醇high-molecular-weight高分子量catalysis 催化剂petroleum industry 石油工业addition 加成反响chemical synthesis 化学合成halogen acid 卤酸terminal carbon atoms 尾端碳原子hypochlorous acid 高氯酸omega oxidation 尾端氧化polymerization 聚合反响intermediate steps 中间步骤polymer 聚合物P32 resin 树脂Unsaturated hydrocarbons fiber 纤维ethylene series 烯类rubber 橡胶double bond 双键detergent 清洗剂alkenes 链烯bacterial oxidation 细菌氧化ethylene 乙烯P35propylene 丙烯Alcohols ( 醇类 )butylene 丁烯alcohol 醇类pyrolysis of petroleum 石油高温裂aerobic condition 好氧条件解degradation 降解diolefins 二烯烃hydroxy alkyl 氢氧烷基polyenes 多烯ionize 离子化acetylene series 炔类neutral 中性的triple bond 三键classification 分类primary alcohol 伯醇steam power plant 蒸汽发电机secondary alcohol 仲醇internal combustion engines内燃tertiary alcohol 叔醇机P36 embalming 防腐isopropyl alcohol 异丙醇biological specimens 生物标本methyl alcohol 甲醇dilution 稀释ethyl alcohol 乙醇toxicity thresholds 毒性阈值n-butyl alcohol 正丁醇nontoxic 无毒的carbon monoxide 一氧化碳activated sludge活性污泥enzyme 酶P42-P44glucose 葡萄糖acetaldehyde 乙醛industrial alcohol 工业酒精condensation 缩合distillation 蒸馏 hydration 水合aldehyde resin paint 醛类树脂颜料反响dimethyl ketone 双甲基酮cornstarch 玉米淀粉 waxy solid 蜡methyl ethyl ketone 甲乙酮状固体2-butanol 2- 丁醇hexadecanol 十六醇solvent 溶剂alkyl group 烷基团 hydroxyl group monocarboxylic acid 单羧基酸羟基团polycarboxylic acid 多羧基酸2-methyl-1-propanol 2- 甲基 -1- 丙醇hydroxyl group 羟基polyhydroxy alcohol 多羟基醇P48p ethylene glycol 乙烯二醇Ester 酯aldehydes 醛hydrolysis 水解ketones 酮reversible reaction 逆反响carbonyl group 羰基general formula 通用分子式P41 butyl acetate 乙酸丁酯Ozonide 臭氧化物perfumes 香水flavoring extracts 调味料vinyl chloride 氯乙烯odor 气味phosphorous 磷immiscible 难溶的bromide 溴化物separation 分别potassium cyanide 氰化钾purification 纯化nitrile 腈antibiotics 抗生素carbon chain 碳链hydrolyze 水解methyl chloride 一氯甲烷P49 ethyl chloride 一氯乙烷ether 醚refrigerant 制冷剂strong dehydrating agents 强脱水P51剂lead 铅fragments 断裂polyvinyl chloride 聚氯乙烯flammable 可燃的contaminated groundwater 受污染的diethyl ether乙醚地下水anesthetic 麻醉剂sanitary 卫生的flotation 上调carcinogen 致癌的decantation 积淀air pollutant 空气污染alkyl halide 卤化烃ethylene dibromide (EDB) 1 ,2- 二halogenated aliphatic compounds 溴乙烷卤代脂肪化合物pesticide 杀虫剂industrial wastewater 工业废水ingredient 成分municipal wastewater 市政污水dichloromethane 二氯甲烷maximum contaminant levels ( MCLS)chlorination 氯化反响最大污染水平P52volatile 挥发性chloroform 氯仿pollutants 污染物nonflammable 不行燃的P50 microgram 微克disinfection 消毒P62natural water 天然水combustion product 焚烧产物phosgene(COCl2)光气chimney sweeps 烟道打扫halogenated ethanes 卤乙烷creosote 防腐油industrial solvent 工业溶剂chlorinated aromatic compound含halogenated ethenes 卤乙烯氯芬芳烃化合物degreaser 除油器polychlorinated biphenyls 多氯联P53-P56 苯dibromochloropropane (DBCP)chlorinated benzene 氯苯chloroflurocarbon 氟氯烃P63Trade name 商品名hydrophobic 疏水性Freon 氟利昂dichlorobenzene 二氯苯fluorine 氟congener 同类ultraviolet radiation 紫外光辐射vapor pressure 蒸气压greenhouse gas 温室气体capacitor 电容器ozone destruction 臭氧层损坏plasticizer 增塑性greenhouse effect 温室效应biodegrade 生物降解P57-P61 anaerobic microorganisms 厌氧微生Aromatic compounds 物cyclic group 环基P64aromatic compound 芬芳族化合物phenols 酚aliphatic compound 脂肪族化合物monohydric phenol 一羟基酚benzene ring 苯环carbolic acid 碳酸parent compound 母体化合物ionize 电离kekule formula 凯库勒分子式germicide 杀菌剂polycyclic aromatic hydrocarbons hemicellulose 半纤维( PAHs)多环芳烃Heterocyclic compounds 杂环化合物Dye 染料digestive tract 消化系统P71 ruminant 反刍动物Sugar 糖P77-P84Carbohydrate 碳水化合物suspension 悬浮液monosaccharide 单糖preliminary treatment 预办理Disaccharide 双糖sedimentation 堆积作用polysaccharide 多糖anaerobic digestion 厌氧消化hexose 乙糖filtration 过滤glucose 葡萄糖centrifugation 离心xylose 木糖incineration 焚烧Galactose 半乳糖pentose 戊糖Lactic acid 乳酸lignin 木质素P75 resin 树脂Condensation product 浓缩产品pitch 柏油脂Starch 淀粉pulping process 造纸工艺Cellulose 纤维derivation 衍生物Hemicellulose 半纤维yeast 酵母菌amylase 直链淀粉P85-P86amylopectin 支链淀粉protein 蛋白质iodine 碘insulin 胰岛素indicator 指示剂amino 氨基mammals 哺乳动物ribonuclease 核糖核酸structural fiber 构造纤维hemoglobin 血红蛋白sulfite 亚硫酸盐nitrogen 氮sulfate 硫酸盐oxygen 氧pulping 纸浆iron 铁subunit 亚单位hydrolytic enzyme 水解酶P87-P96 liquid chromatography (LC)液相detergent 清洗剂色谱ingredient 成分hexane 正乙烷surface-active agent 表面活性剂immune 免疫的surfactant 表面活性物质exudate 溢出物polar 极性decomposition 分解sodium 钠polymeric 聚合potassium 钾phenolic 苯酚的rodent 利齿动物humic substance 腐殖质insect 昆虫excneta 排泄物categorize 分类aquatic plant 水生植物herbicide 除草剂potential hazard 潜伏危险algicide 除藻剂P100fungicide 除菌剂Solubility 溶解性chemical properties 化学特征Hydrophobicity 疏水性toxic properties 毒性Polarity 极性trace organics 微量有机物Volatility 挥发性concentration 浓度Density 密度impurity 杂质Sorption 吸附epidemiological 流行病学Transformation 变换statistical study 统计学Partitioning分派P97 Photochemical 光化学的organ 器官P125kidney 肾Standard method 标准方法liver 肝Parts per million (ppm)extraction 萃取Parts per billion (ppb)gas chromatography (GC)气相色谱Parts per trillion (ppt)International system of units (SI) ignition 灼烧Milligrams per liter (mg/L) purification 纯化Micrograms per liter ( μg/L) P136nanograms per liter (ng/L) weight analysis 重量剖析P133 membrane 膜general operation 一般操作bound water 联合水keystone 重点filter paper 滤纸field investigation 野外检查glass fiber filter 玻璃纤维滤纸specialized course 专业课程porosity 孔隙P134 barium sulfate 硫酸钡sample 采样ferric hydroxide 氢氧化铁instantaneously 瞬时的vessel 容器frequency of sample 采样频次drainage 排水grab sample 随机采样drying 烘干Composited sample 组合样品free water 自由水at regular intervals 必定间隔crystallization 结晶detention time 逗留时间P137P135 volatilization 挥发influent 进水decomposition 分解effluent 出水desiccation 干燥apparatus 装置evaporation dish 蒸发皿reagent 化学试剂crucible 坩埚solubility 溶解度moisture 湿度coefficient of expansion 膨胀系数relative humidity 相对湿度specification 规格desiccator 干燥器precipitation 积淀anhydrous calcium chloride无水氯purity 纯度化钙analytical balance 剖析天平normal solution 当量溶液P139 primary standard 一级标准fixed solid 固定固体secondary standard 二级标准volatile solid 挥发固体acid titration 酸滴定time consuming 耗费时间的base titration 碱滴定constant weight 恒重oxidation reduction methods 氧化gross weight 毛重复原方法net weight 净重calculation 计算porcelain 陶瓷colorimetry 比色法platinum ware 铂制器皿spectrophotometer 分光光度计pretreatment 预办理monochromatic light 单色光P140 wavelength 波长semisolid sample 半固体样品linear coordinate paper 直角坐标volumetric analysis 容积剖析纸standard solution 标准溶液nitrite亚硝酸根glassware 玻璃器皿calibration 校订suitable strength 适合强度turbidimetry 浊度剂法stoichiometric end point 化学当量nephelometry 悬浮法终点precision 精度calibrate 校订Accuracy 精准度buret 滴定管P160cylinder 量筒Regulatory 法例calibrated glassware 标正玻璃器皿acid rain 酸雨Indicator 指示剂ozone layer 臭氧层P141-P157 World Health Organization 世界卫pipet 移液管生组织chromic acid 铬酸Pollution awareness 污染意识spill 泄露点界线值emission 发散P174-P177sulfur dioxide 二氧化硫elimination 除去genetic code 基因代码selenium 硒reaction sequence 反响次序nutrient 营养物analogous 相像的contracting 减小P171-P172 epidemiological 传染学的global climate 全世界天气aerosol 气溶胶visibility 可见度ozone group 臭氧组release 开释control group 控制组methyl isocyanate 甲基异氰酸盐mortality 致命leakage 渗漏inanimate object 无生命物质hydrogen sulfide硫化氢ozone concentration 臭氧浓度industrial accident 工业事故Infection 感染dosage 用量P178-P184chronic effect 慢性作用autopsy 尸体解剖acute effect 急性作用inbred 同系生殖的episodes 事件human volunteer 人类志愿者dose-response curve 用量 - 响应曲线asthmatics 气喘病患者P173 parameter 参数synergism 共同作用community health 大众健康hypothetical 假设的unambiguous 显然的homogeneous 同种的proxy 代表pharmacology 药理学meteorological situation 气象条件origin 起点circulatory 循环hygiene 卫生学,保健法mortality 致死threshold limit values (TLVs)起morbidity 致病demographic 人口统计P184-P193regulation法例curve曲线radon 氡mitigate缓解acidic deposition酸堆积transparent透明的light photon 光电子plume opacity 烟气上涨haze 薄雾extrapolation 概括。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Victoria, Australia
2 Materials Science and Engineering, Preventative Health Flagship, Commonwealth Scientific and
Ammonium hydroxide treatment of Aβ produces an aggregate free solution suitable for biophysical and cell culture characterization
Timothy M. Ryan1 , Joanne Caine2 , Haydyn D.T. Mertens3 , Nigel Kirby3 , Julie Nigro2 , Kerry Breheney2 , Lynne J. Waddington2 , Victor A. Streltsov2 , Cyril Curtain1 , Colin L. Masters1 and Blaine R. Roberts1
major constituent of the plaques that are symptomatic of Alzheimer’s disease (Glenner & Wong, 1984; Masters et al., 1985). However, the formation of these fibrillar structures is not well correlated with disease severity (McLean et al., 1999), indicating that fibrils represent a relatively non-toxic endpoint in a toxic aggregation pathway (McLean et al., 1999). To reconcile these observations several theories suggesting the presence of soluble, small, oligomeric species of Aβ have been developed (Kirkitadze, Bitan & Teplow, 2002). There are a number methods for generating small toxic oligomers in vitro (for examples see (Sahoo et al., 2009; Yu et al., 2009; Ahmed et al., 2010; Ryan et al., 2010)), but typically most of these preparations involve the preliminary generation of a stock of Aβ which is largely monomeric (Stine et al., 2003). Generally this is a critical requirement for many kinetic studies, as solutions which contain a large number of aggregates will have adverse impacts on understanding how an unfolded form of Aβ self-associates into a small nucleus which then elongates into fibrillar structures (Stine et al., 2003; Teplow, 2006). Unfortunately, this is not a simple process of resuspending lyophilised peptide in the desired buffer, as the hydrophobic nature of the peptide makes the initial dissolution very difficult (Teplow, 2006). Furthermore Aβ appears to have a structural memory, reforming the conformations that it had adopted prior to lyophilisation (Stine et al., 2003). To avoid these issues many researchers resort to a variety of solubilisation agents, including strong alkali and acids (Zagorski et al., 1999; Fezoui et al., 2000; Teplow, 2006), dimethylsulfoxide (DMSO) (Shen & Murphy, 1995; Lambert et al., 2001; Broersen et al., 2011), chaotropic salts and fluorinated alcohols, such as trifluroethanol (Zagorski & Barrow, 1992) and hexafluoroisopropanol (HFIP) (Stine et al., 2003; Broersen et al., 2011). The most commonly adopted approach is to use HFIP (thought to induce alpha helical conformations, produce monomeric solutions and remove the conformational memory from the peptide) followed by removal of HFIP and resuspension using an aqueous compatible resuspension solvent such as DMSO (Walsh et al., 1997; Stine et al., 2003; Williams, Day & Serpell, 2010). Recently, the use of HFIP has been suggested by a number of groups to cause heterogeneity in the starting peptide self-association state (Nichols et al., 2005a; Nichols et al., 2005b; Pachahara et al., 2012), which may cause enhanced aggregation. We show using a variety of methods, including thioflavin T (ThT) binding, Small Angle X-ray scattering (SAXS), dynamic light scattering (DLS) and size exclusion chromatography measurements, that HFIP pretreatment affects the aggregation state and kinetics of amyloid formation of the resuspended peptide. We also show that the use of ammonium hydroxide, as an initial treatment, greatly aids solubility and produces a relatively less aggregated and seed free peptide solution, which is important for understanding the process of Aβ self-association and hence neuronal cell toxicity.
Industrial Research Organization, Parkville, Victoria, Australia
3 SAXS/WAXS Beamline, Australian Synchrotron, Clayton, Victoria, Australia
ABSTRACT
Alzheimer’s disease is the leading cause of dementia in the elderly. Pathologically it is characterized by the presence of amyloid plaques and neuronal loss within the brain tissue of affected individuals. It is now widely hypothesised that fibrillar structures represent an inert structure. Biophysical and toxicity assays attempting to characterize the formation of both the fibrillar and the intermediate oligomeric structures of Aβ typically involves preparing samples which are largely monomeric; the most common method by which this is achieved is to use the fluorinated organic solvent 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP). Recent evidence has suggested that this method is not 100% effective in producing an aggregate free solution. We show, using dynamic light scattering, size exclusion chromatography and small angle X-ray scattering that this is indeed the case, with HFIP pretreated Aβ peptide solutions displaying an increased proportion of oligomeric and aggregated material and an increased propensity to aggregate. Furthermore we show that an alternative technique, involving treatment with strong alkali results in a much more homogenous solution that is largely monomeric. These techniques for solubilising and controlling the oligomeric state of Aβ are valuable starting points for future biophysical and toxicity assays.
