盐酸哌甲酯缓释片说明书
专注达_百度百科

6 不良反应
临床试验数据
双盲试验数据——发生率≥1%的不良反应 在4个安慰剂对照、双盲试验中,评价639例注意缺陷多动障碍(ADHD)患者使用本品的安全性。本部分的数据来源于综合数 据。
表1列出了这些试验中本品组患者发生率≥1%的药物不良反应(ADR)。
/link?url=k5BfSVTIgBMJw3gUL5_NclfsAyr5TcAlm8K57OyJgQnjLrqtMirtUH3iTXRe-nltstr9EjVYbp7RT4y05C4pAifs1yl2kHahlWqME0Q3… 2/7
上市后数据
表4列出了本品上市后应用期间初步定为不良反应的不良事件,其频率按以下规定表示: 很常见 ≥ 1/10 常见 ≥1/100 且 < 1/10 少见 ≥1/1,000 且 < 1/100 罕见 ≥1/10,000 且 < 1/1000 极罕见 <1/10,000,包括个别病例。 根据自发报告的频率在表4中列出了不同发生率的不良反应。
尚未进行本品对驾驶和机械操作能力影响的研究。但本品可能引起头晕。所以当驾驶、操作机械或从事其它具有潜在危险性的活 动时应慎用本品。
本品在下列情况下慎用:
根据临床经验,给精神病患者服用哌甲酯可能使行为障碍和思维紊乱的症状加重。
在临床试验中,与安慰剂比较,本品和每天服用3次的哌甲酯速释片都可使安静状态的脉搏每分钟平均增加2~6次,收缩压和舒 张压平均升高1~4mmHg。所以,服药后可能因血压升高或心率加快而可能引起危险的患者应慎用本品。对服用本品的患者应定 期监测血压和心率,尤其是高血压患者。
/link?url=k5BfSVTIgBMJw3gUL5_NclfsAyr5TcAlm8K57OyJgQnjLrqtMirtUH3iTXRe-nltstr9EjVYbp7RT4y05C4pAifs1yl2kHahlWqME0Q3… 3/7
盐酸哌甲酯控释片
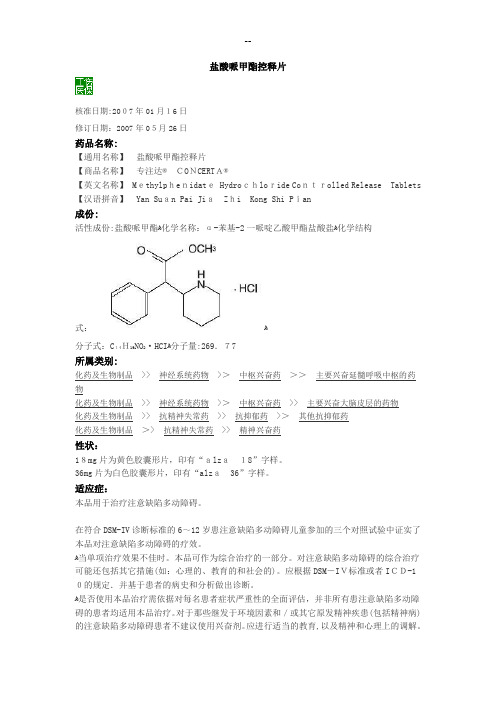
盐酸哌甲酯控释片核准日期:2007年01月16日修订日期:2007年05月26日药品名称:【通用名称】盐酸哌甲酯控释片【商品名称】专注达®CONCERTA®【英文名称】Methylphenidate Hydrochloride Controlled Release Tablets 【汉语拼音】Yan Suan Pai JiaZhi Kong Shi Pian成份:活性成份:盐酸哌甲酯ﻫ化学名称:α-苯基-2一哌啶乙酸甲酯盐酸盐ﻫ化学结构式:ﻫ分子式:C14H19NO2·HCIﻫ分子量:269.77所属类别:化药及生物制品>> 神经系统药物>>中枢兴奋药>>主要兴奋延髓呼吸中枢的药物化药及生物制品>> 神经系统药物>>中枢兴奋药>> 主要兴奋大脑皮层的药物化药及生物制品>> 抗精神失常药>> 抗抑郁药>>其他抗抑郁药化药及生物制品>> 抗精神失常药>> 精神兴奋药性状:18mg片为黄色胶囊形片,印有“alza18”字样。
36mg片为白色胶囊形片,印有“alza36”字样。
适应症:本品用于治疗注意缺陷多动障碍。
在符合DSM-IV诊断标准的6~12岁患注意缺陷多动障碍儿童参加的三个对照试验中证实了本品对注意缺陷多动障碍的疗效。
ﻫ当单项治疗效果不佳时。
本品可作为综合治疗的一部分。
对注意缺陷多动障碍的综合治疗可能还包括其它措施(如:心理的、教育的和社会的)。
应根据DSM-IV标准或者ICD-10的规定.并基于患者的病史和分析做出诊断。
ﻫ是否使用本品治疗需依据对每名患者症状严重性的全面评估,并非所有患注意缺陷多动障碍的患者均适用本品治疗。
对于那些继发于环境因素和/或其它原发精神疾患(包括精神病)的注意缺陷多动障碍患者不建议使用兴奋剂。
应进行适当的教育,以及精神和心理上的调解。
规格:本品为每日口服1次的控释片,每片含有18或36mg盐酸哌甲酯。
专注达说明书

专注达,盐酸哌甲酯控释片:治疗注意缺陷多动障碍(儿童多动症)作者:佚名来源:本站原创点击数: 1007 更新时间:2009年08月06日【字体:大中小】内容导读:专注达,盐酸哌甲酯控释片用于治疗注意缺陷多动障碍(儿童多动症)......【通用名】盐酸哌甲酯控释片【商品名】专注达片【英文名】MethylphenidateHydrochlorideControlled-ReleaseT ablets【汉语拼音】YansuanPaijiazhiKongshipian【主要成分】专注达只要成分及其化学名称为:本品为α-苯基-2-哌啶乙酸甲酯盐酸盐。
分子式为:C14H19NO2·HCl分子量为:269.77【形状】18mg片状为黄色胶囊形片,印有“alza18”字样;36mg片状为白色胶囊形片,印有“alza36”字样。
【药理毒理】盐酸哌甲酯是一个中枢神经兴奋剂,其治疗注意缺陷多动障碍的作用机制尚不清楚。
哌甲酯被认为通过阻断突触前神经元对去甲肾上腺素和多巴胺的再摄取,以及增加这些单胺物质释放至外神经元间隙。
哌甲酯是外消旋体,右旋异构体比左旋异构体更具药理活性。
【药代动力学】吸收:哌甲酯吸收迅速,成人口服专注达后,哌甲酯血浆浓度迅速升高,在1-2小时内达到初始最大值,随后几小时内平稳升高,6-8小时达到血浆浓度峰值。
然后其血浆浓度开始逐渐下降,与每日服用3次的盐酸哌甲酯速释制剂相比,成人每日服用1次专注达可使血药浓度的峰值与谷值之间的波动降到最小。
成人每日服用1次专注达和每日服用3次盐酸哌甲酯速释制剂的相对生物利用度相当.代谢和排泄:哌甲酯主要是通过去酯化作用代谢为α-苯基-哌啶乙酸,此代谢物几乎无药理活性,以哌甲酯代谢α-苯基-哌啶乙酸为衡量依据。
每日单次服用专注达和服用3次盐酸哌甲酯速释片在成人体内哌甲酯的代谢相似。
专注达单次或重复给药的代谢相同。
尚未对6岁以下儿童进行药动学研究。
肾功能不全的患者:尚用于肾功能不全患者的经验,口服放射标记的哌甲酯后,哌甲酯被广泛代谢,约80%的标记物以α-苯基-哌啶乙酸的形式由尿液排出。
我院儿科门诊使用盐酸哌甲酯药物处方的合理性分析

[ bt c] O j t e o v ut t tn i mn ttn f e y hn a yr h r e als A s at r be v T a a e aoat i a isao t l ei t hd eli b t c i e l eh r ly n d ir i i om hp de o od t e
d i1 .99 ji n 17 o: 3 6/.s .64—30 。0 10 . 1 0 s 8 62 1 。9 2
A a s nrt n lyo rsr t n o typ eiaeh d ohoietbe LU F , H O J n in . n l i o ai a t fp eci i f ys o i po meh lh nd t y r clr lt d a s I u Z A i -a g aj
《 新编药物学》 l -及 临床 实际使用情况 , 第 6版 1 确
定药品的 D D值 , D 并计算用药频数 ( D s 及 D I D D) U
值。若 D I 1 U > 则说 明医生处方 日剂量 > D , D D 若 D I 则 说 明医生 处 方 日剂 量 <D D D s U <1 D 。D D =用
注意力集 中, 活动量减少 , 对别人 的干扰减少 , 顺从 性增加。临床上用于注意缺 陷多动 障碍 (tn o aetn t i dfihpr ti i re, D D 即儿童多动症的 ec e cv y s drA H ) i t y ai td o
比较缓和的直接作用于中枢神经系统 的兴奋药 , 能 兴奋中枢的多种精神性活动 , 抑制儿童多动 , 使患儿
人 民共 和 国处方 管 理 办 法 》 20 (07年 5月 1日起 施
精神专科医院门诊盐酸哌甲酯缓释片使用情况分析

精神专科医院门诊盐酸哌甲酯缓释片使用情况分析摘要】目的:探讨精神专科医院门诊盐酸哌甲酯缓释片的使用情况。
方法:对本院2014年1月—2017年12月期间门诊盐酸哌甲酯缓释片1090张处方进行统计,分析近几年门诊盐酸哌甲酯缓释片的使用情况。
结果:在2014年1月至2017年12月期间,每年门诊盐酸哌甲酯缓释片的处方量增加,药品年消耗总量增加,患者人数增加。
在每年新增患者中,男性多于女性,6~10岁年龄阶段的儿童患者最多。
结论:在精神专科医院门诊,盐酸哌甲酯缓释片使用量逐年增加,患者年龄逐渐儿童化,医师和药师应该分别把好疾病诊疗关和处方审核关,社会也应该多关注儿童身心健康,降低多动症的发病率。
【关键词】精神专科医院;门诊;盐酸哌甲酯缓释片【中图分类号】R95 【文献标识码】A 【文章编号】2095-1752(2018)27-0392-02Analysis of the use of Methylphenidate HCl Extended-release Tablets in psychiatric outpatient clinics【Abstract】Objective To investigate the use of Methylphenidate HCl Extended-release Tablets in psychiatric outpatient clinics. Methods The 1090 prescriptions of outpatient Methylphenidate HCl Extended-release Tablets in our hospital from January 2014 to December 2017 were analyzed, and the use of Methylphenidate HCl Extended-release Tablets in the outpatient department in recent years was analyzed. Results From January 2014 to December 2017, the number of prescriptions of Methylphenidate HCl Extended-release Tablets in the outpatient department increased, the total annual consumption of drugs increased and the number of patients increased. Among the newly added patients, there are more males than females, and most of the children are 6-10 years old. Conclusion In the psychiatric hospital, the use of Methylphenidate HCl Extended-release Tablets is increasing yearby year, the age of the patients is becoming more and more children. The doctors and pharmacists should check the diagnosis, treatment and prescription of the disease well. The society should pay more attention to the physical and mental health of children and reduce the incidence of ADHD.【Key words】Psychiatric hospital; Outpatient service; Methylphenidate HCl Extended-release Tablets.作为一种中枢兴奋药,哌甲酯主要用于治疗儿童注意缺陷多动障碍(attention deficit hyperactivity disorder, ADHD)、成人注意缺陷多动障碍和发作性睡病,并且是该类疾病的一线药物。
控缓释肠溶制剂正确服用方法
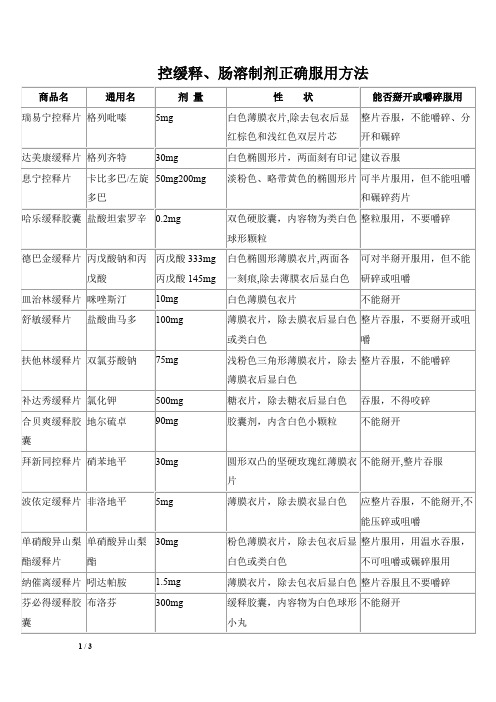
薄膜衣片,除去膜衣后显白色 整片吞服,不要掰开或咀 或类白色 嚼
扶他林缓释片 双氯芬酸钠
75mg
浅粉色三角形薄膜衣片,除去 整片吞服,不能嚼碎 薄膜衣后显白色
补达秀缓释片 氯化钾 合贝爽缓释胶 地尔硫卓 囊 拜新同控释片 硝苯地平
500mg 90mg
糖衣片,除去糖衣后显白色 胶囊剂,内含白色小颗粒
吞服,不得咬碎 不能掰开
美施康定缓释 硫酸吗啡 片 专注达缓释片 盐酸哌甲酯
30mg
为紫色薄膜衣片,除去包衣后 必须整片吞服,不可掰开、 显白色 碾碎或咀嚼 要整片用水送下、不能咀 嚼、掰开或压碎
18mg
为胶囊形片
奥施康定缓释 盐酸羟考酮 片 奥施康定缓释 盐酸羟考酮 片
2/3
10mg
为圆形、双凸薄膜包衣片,为 必须整片吞服,不得掰开、 白色片 咀嚼或研磨
纳催离缓释片 吲达帕胺 芬必得缓释胶 布洛芬 囊
1/3
薄膜衣片,除去包衣后显白色 整片吞服且不要嚼碎 缓释胶囊,内容物为白色球形 不能掰开 小丸
倍他乐克缓释 琥珀酸美托洛 47.5 mg 片 桑塔缓释片 尔 盐酸阿呋唑嗪 10mg
本品为白色或类白色薄膜衣 片,除去包衣后显白色
可掰开服用,但不能咀嚼 或压碎
为土黄色椭圆形肠溶衣片,除 必须用大量液体整片吞服 去包衣后显浅棕色 (不能嚼碎)
标准桃金娘肠 标准桃金娘油 每粒含 300mg 溶胶囊 (成人装) 波利特肠溶片 雷贝拉唑钠 10mg 标准桃金娘油
为微黄色透明肠溶软胶囊,内 宜在餐前 30 分钟用较多的 容物为无色至微黄色的油质液 凉开水送服。勿将胶囊掰 体,气芬香 开或咀嚼服用。
卡比多巴/左旋 50mg200mg 多巴
哈乐缓释胶囊 盐酸坦索罗辛 0.2mg
滋阴潜阳安神方联合盐酸哌甲酯缓释片治疗轻中度ADHD患儿的临床观察

滋阴潜阳安神方联合盐酸哌甲酯缓释片治疗轻中度ADHD患儿的临床观察王涛;陈礼贤;徐丹红;林忠辉【期刊名称】《中国中医药科技》【年(卷),期】2022(29)2【摘要】注意力缺陷多动障碍(attention deficit hyperactivity disorder,ADHD)是一种临床常见的神经精神类疾病,好发于学龄期间的患儿,数据统计我国儿童ADHD的发病率约为5.6%(其中男孩7.7%,女孩3.4%),并且呈慢性病程,约57%可持续到成年[1]。
临床主要表现为多动、冲动、情绪反应与社交障碍、注意力不集中等,严重影响患儿的生长发育、学习以及生活质量等[2]。
目前临床治疗以盐酸哌甲酯缓释片为首选,该药起效快、效果满意,但禁用于6岁以下儿童,并且长期应用不良反应突出,严重限制其临床的广泛使用。
研究显示中药复方及单味药对ADHD有较好的治疗作用,其与调节脑内单胺类神经递质、代谢产物、受体及酶的表达和DA、5-HT、NE等神经递质系统紧密相关[3]。
本研究笔者自拟滋阴潜阳安神方联合盐酸哌甲酯缓释片治疗轻中度ADHD患儿,现将详细情况报道如下。
【总页数】3页(P254-256)【作者】王涛;陈礼贤;徐丹红;林忠辉【作者单位】浙江省台州市玉环市第二人民医院【正文语种】中文【中图分类】R74【相关文献】1.小青龙汤联合激素治疗轻中度急性哮喘(外寒里饮证)患儿的临床观察2.氨氯地平片联合马来酸依那普利片缓释片治疗轻中度高血压临床疗效观察3.美沙拉秦栓联合自拟中药灌肠方治疗轻中度活动期溃疡性直肠炎33例临床观察4.心理-行为矫正疗法联合盐酸哌甲酯缓释片治疗注意力缺陷多动障碍患儿的临床疗效5.化瘀青娥方联合多奈哌齐治疗轻中度阿尔茨海默病的临床观察因版权原因,仅展示原文概要,查看原文内容请购买。
神经外科常用 麻醉药品

盒
北京萌蒂制药
13
盐酸羟考酮缓释片
40mg*10片
盒
北京萌蒂制药
14*
盐酸麻黄碱注射液
30mg/1ml
支
沈阳第一制药厂
第一类精神药品
15
盐酸氯胺酮注射液
100mg/2ml
支
福建古田制药
16
哌甲脂片
10mg*20片
盒
苏州一药
17
盐酸哌甲酯缓释片
18mg*15片
盒
美国ALZA公司
合计
17
支
沈阳第一制药厂
5
6
注射用盐酸瑞芬太尼
1mg
支
宜昌人福药业
7
枸橼酸芬太尼注射液
0.1mg/2ml
支
宜昌人福药业
8
芬太尼透皮贴剂
4.2mg
帖
西安杨森制药
9
枸橼酸舒芬太尼注射液
50ug/1ml
支
宜昌人福药业
10
枸橼酸舒芬太尼注射液
50ug/1ml
支
德国
11
磷酸可待因片
30mg*20片
盒
青海制药
12
盐酸羟考酮缓释片
神经外 科备用麻醉药品、第一类精神药品情况一览表
类别
序号
药品名称
规格
单位
产地
批号
效期
数量
麻醉药品
1
硫酸吗啡缓释片
30mg*10片
盒
北京萌蒂制药
2
硫酸吗啡注射液
10mg/1ml
支
青海制药
3
盐酸哌替啶注射液
50mg/2ml
支
青海制药
哌甲酯说明书
Once DailyMetadate CD®(methylphenidate HCl, USP)Extended-Release CapsulesRx onlyDESCRIPTIONMETADATE CD is a central nervous system (CNS) stimulant. The extended-release capsules comprise both immediate-release (IR) and extended-release (ER) beads such that 30% of the dose is provided by the IR component and 70% of the dose is provided by the ER component. METADATE CD is available in six capsule strengths containing 10 mg (3 mg IR; 7 mg ER), 20 mg (6 mg IR; 14 mg ER), 30 mg (9 mg IR; 21 mg ER), 40 mg (12 mg IR; 28 mg ER), 50 mg (15 mg IR; 35 mg ER), or 60 mg (18 mg IR; 42 mg ER) of methylphenidate hydrochloride for oral administration.Chemically, methylphenidate HCl is d,l (racemic)-threo-methyl α-phenyl-2-piperidineacetate hydrochloride. Its empirical formula is C14H19NO2•HCl. Its structural formula is:Methylphenidate HCl USP is a white, odorless, crystalline powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone. Its molecular weight is 269.77.METADATE CD also contains the following inert ingredients: Sugar spheres, povidone, hydroxypropylmethylcellulose and polyethylene glycol, ethylcellulose aqueous dispersion, dibutyl sebacate, gelatin, and titanium dioxide.The individual capsules contain the following color agents:10 mg capsules: FD&C Blue No. 2, FDA/E172 Yellow Iron Oxide20 mg capsules: FD&C Blue No. 230 mg capsules: FD&C Blue No. 2, FDA/E172 Red Iron Oxide40 mg capsules: FDA/E172 Yellow Iron Oxide50 mg capsules: FD&C Blue No. 2, FDA/E172 Red Iron OxideCLINICAL PHARMACOLOGYPharmacodynamicsMethylphenidate HCl is a central nervous system (CNS) stimulant. The mode of therapeutic action in Attention Deficit Hyperactivity Disorder (ADHD) is not known. Methylphenidate is thought to block the reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space. Methylphenidate is a racemic mixture comprised of the d-and l-threo enantiomers. The d-threo enantiomer is more pharmacologically active than the l-threo enantiomer.PharmacokineticsThe pharmacokinetics of the METADATE CD methylphenidate hydrochloride formulation have been studied in healthy adult volunteers and in children with Attention Deficit Hyperactivity Disorder (ADHD).Absorption And DistributionMethylphenidate is readily absorbed. METADATE CD has a plasma/time concentration profile showing two phases of drug release with a sharp, initial slope similar to a methylphenidate immediate-release tablet, and a second rising portion approximately three hours later, followed by a gradual decline. (See Figure 1 below.)Comparison Of Immediate Release (IR) And METADATE CD Formulations After Repeated Doses Of Methylphenidate HCl In Children With ADHDMETADATE CD was administered as repeated once-daily doses of 20 mg or 40 mg to children aged 7-12 years with ADHD for one week. After a dose of 20 mg, the mean (±SD) early C max was 8.6 (±2.2) ng/mL, the later C max was 10.9 (±3.9)* ng/mL and AUC0-9h was 63.0 (±16.8) ng•h/mL. The corresponding values after a 40 mg dose were 16.8 (±5.1) ng/mL, 15.1 (±5.8)* ng/mL and 120 (±39.6) ng•h/mL, respectively. The early peak concentrations (median) were reached about 1.5 hours after dose intake, and the second peak concentrations (median) were reached about 4.5 hours after dose intake. The means for C max and AUC following a dose of 20 mg were slightly lower than those seen with 10 mg of the immediate-release formulation, dosed at 0 and 4 hours.*25-30% of the subjects had only one observed peak (C max) concentration of methylphenidate.FIGURE 1Comparison of Immediate Release (IR) and METADATE CD FormulationsAfter Repeated Doses of Methylphenidate HCl in Children with ADHDDose ProportionalityFollowing single oral doses of 10-60 mg methylphenidate free base as a solution given to ten healthy male volunteers, C max and AUC increased proportionally with increasing doses. After the 60 mg dose, t max was reached 1.5 hours post-dose, with a mean C max of 31.8 ng/mL (range 24.7-40.9 ng/mL).Following one week of repeated once-daily doses of 20 mg or 40 mg METADATE CD to children aged 7-12 years with ADHD, C max and AUC were proportional to the administered dose.Food EffectsIn a study in adult volunteers to investigate the effects of a high-fat meal on the bioavailability of a dose of 40 mg, the presence of food delayed the early peak by approximately 1 hour (range -2 to 5 hours delay). The plasma levels rose rapidly following the food-induced delay in absorption. Overall, a high-fat meal increased the C max of METADATE CD by about 30% and AUC by about 17%, on average (see DOSAGE and ADMINISTRATION).After a single dose, the bioavailability (C max and AUC) of methylphenidate in 26 healthy adults was unaffected by sprinkling the capsule contents on applesauce as compared to the intact capsule. This finding demonstrates that a 20 mg METADATE CD Capsule, when opened and sprinkled on one tablespoon of applesauce, is bioequivalent to the intact capsule.Metabolism And ExcretionIn humans, methylphenidate is metabolized primarily via deesterification to alpha-phenylpiperidine acetic acid (ritalinic acid). The metabolite has little or no pharmacologic activity.In vitro studies showed that methylphenidate was not metabolized by cytochrome P450 isoenzymes, and did not inhibit cytochrome P450 isoenzymes at clinically observed plasma drug concentrations.The mean terminal half-life (t½) of methylphenidate following administration of METADATE CD (t½=6.8h) is longer than the mean terminal (t½) following administration of methylphenidate hydrochloride immediate-release tablets (t½=2.9h) and methylphenidate hydrochloride sustained-release tablets (t½=3.4h) in healthy adult volunteers. This suggests that the elimination process observed for METADATE CD is controlled by the release rate of methylphenidate from the extended-release formulation, and that the drug absorption is the rate-limiting process.Special PopulationsGenderThe pharmacokinetics of methylphenidate after a single dose of METADATE CD were similar between adult men and women.RaceThe influence of race on the pharmacokinetics of methylphenidate after METADATE CD administration has not been studied.AgeThe pharmacokinetics of methylphenidate after METADATE CD administration have not been studied in children less than 6 years of age.Renal InsufficiencyThere is no experience with the use of METADATE CD in patients with renal insufficiency. After oral administration of radiolabeled methylphenidate in humans, methylphenidate was extensively metabolized and approximately 80% of the radioactivity was excreted in the urine in the form of ritalinic acid. Since renal clearance is not an important route of methylphenidate clearance, renal insufficiency is expected to have little effect on the pharmacokinetics of METADATE CD.Hepatic InsufficiencyThere is no experience with the use of METADATE CD in patients with hepatic insufficiency. CLINICAL STUDIESMETADATE CD was evaluated in a double-blind, parallel-group, placebo-controlled trial in which 321 untreated or previously treated pediatric patients with a DSM-IV diagnosis of Attention Deficit Hyperactivity Disorder (ADHD), 6 to 15 years of age, received a single morning dose for up to 3 weeks. Patients were required to have the combined or predominantlyhyperactive-impulsive subtype of ADHD; patients with the predominantly inattentive subtype were excluded. Patients randomized to the METADATE CD group received 20 mg daily for the first week. Their dosage could be increased weekly to a maximum of 60 mg by the third week, depending on individual response to treatment.The patient’s regular school teacher completed the teachers’ version of the Conners’ Global Index Scale (TCGIS), a scale for assessing ADHD symptoms, in the morning and again in the afternoon on three alternate days of each treatment week. The change from baseline of the overall average (i.e., an average of morning and afternoon scores over 3 days) of the total TCGIS scores during the last week of treatment was analyzed as the primary efficacy parameter. Patients treated with METADATE CD showed a statistically significant improvement in symptom scores from baseline over patients who received placebo. (See Figure 2.) Separate analyses of TCGIS scores in the morning and afternoon revealed superiority in improvement with METADATE CD over placebo during both time periods. (See Figure 3.) This demonstrates that a single morning dose of METADATE CD exerts a treatment effect in both the morning and the afternoon.INDICATION AND USAGEAttention Deficit Hyperactivity Disorder (ADHD)METADATE CD (methylphenidate HCl, USP) Extended-Release Capsules are indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD).The efficacy of METADATE CD in the treatment of ADHD was established in one controlled trial of children aged 6 to 15 who met DSM-IV criteria for ADHD (see CLINICALPHARMACOLOGY).A diagnosis of Attention Deficit Hyperactivity Disorder (ADHD; DSM-IV) implies the presence of hyperactive-impulsive or inattentive symptoms that caused impairment and were present before age 7 years. The symptoms must cause clinically significant impairment, e.g., in social, academic, or occupational functioning, and be present in two or more settings, e.g., school (or work) and at home. The symptoms must not be better accounted for by another mental disorder. For the Inattentive Type, at least six of the following symptoms must have persisted for at least 6 months: lack of attention to details/careless mistakes; lack of sustained attention; poor listener; failure to follow through on tasks; poor organization; avoids tasks requiring sustained mental effort; loses things; easily distracted; forgetful. For the Hyperactive-Impulsive Type, at least six of the following symptoms must have persisted for at least 6 months: fidgeting/squirming; leaving seat; inappropriate running/climbing; difficulty with quiet activities; “on the go;” excessive talking; blurting answers; can’t wait turn; intrusive. The Combined Types requires both inattentive and hyperactive-impulsive criteria to be met.Special Diagnostic ConsiderationsSpecific etiology of this syndrome is unknown, and there is no single diagnostic test. Adequate diagnosis requires the use not only of medical but of special psychological, educational, and social resources. Learning may or may not be impaired. The diagnosis must be based upon a complete history and evaluation of the child and not solely on the presence of the required number of DSM-IV characteristics.Need For Comprehensive Treatment ProgramMETADATE CD is indicated as an integral part of a total treatment program for ADHD that may include other measures (psychological, educational, social) for patients with this syndrome. Drug treatment may not be indicated for all children with this syndrome. Stimulants are not intended for use in the child who exhibits symptoms secondary to environmental factors and/or other primary psychiatric disorders, including psychosis. Appropriate educational placement is essential and psychosocial intervention is often helpful. When remedial measures alone are insufficient, the decision to prescribe stimulant medication will depend upon the physician’s assessment of the chronicity and severity of the child’s symptoms.Long-Term UseThe effectiveness of METADATE CD for long-term use, i.e., for more than 3 weeks, has not been systematically evaluated in controlled trials. Therefore, the physician who elects to use METADATE CD for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient (see DOSAGE and ADMINISTRATION). CONTRAINDICATIONSAgitationMETADATE CD is contraindicated in patients with marked anxiety, tension and agitation, since the drug may aggravate these symptoms.Hypersensitivity To Methylphenidate Or Other ExcipientsMETADATE CD is contraindicated in patients known to be hypersensitive to methylphenidate or other components of the product.METADATE CD contains sucrose. Therefore, patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption, or sucrase-isomaltase insufficiency should not take this medicine.GlaucomaMETADATE CD is contraindicated in patients with glaucoma.TicsMETADATE CD is contraindicated in patients with motor tics or with a family history or diagnosis of Tourette’s syndrome (see ADVERSE REACTIONS).Monoamine Oxidase InhibitorsMETADATE CD is contraindicated during treatment with monoamine oxidase inhibitors, and also within a minimum of 14 days following discontinuation of a monoamine oxidase inhibitor (hypertensive crises may result).Hypertension And Other Cardiovascular ConditionsMETADATE CD is contraindicated in patients with severe hypertension, angina pectoris, cardiac arrhythmias, heart failure, recent myocardial infarction, hyperthyroidism or thyrotoxicosis (see WARNINGS).Halogenated AnestheticsThere is a risk of sudden blood pressure increase during surgery. If surgery is planned, METADATE CD should not be taken on the day of the surgery.WARNINGSSerious Cardiovascular EventsSudden Death And Pre-existing Structural Cardiac Abnormalities Or Other SeriousHeart ProblemsChildren And AdolescentsSudden death has been reported in association with CNS stimulant treatment at usual doses in children and adolescents with structural cardiac abnormalities or other serious heart problems. Although some serious heart problems alone carry an increased risk of sudden death, stimulant products generally should not be used in children or adolescents with known serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or other serious cardiac problems that may place them at increased vulnerability to the sympathomimetic effects of a stimulant drug (see CONTRAINDICATIONS).AdultsSudden deaths, stroke, and myocardial infarction have been reported in adults taking stimulant drugs at usual doses for ADHD. Although the role of stimulants in these adult cases is also unknown, adults have a greater likelihood than children of having serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other serious cardiac problems. Adults with such abnormalities should also generally not be treated with stimulant drugs (see CONTRAINDICATIONS).Hypertension And Other Cardiovascular ConditionsStimulant medications cause a modest increase in average blood pressure (about 2-4 mmHg) and average heart rate (about 3-6 bpm), and individuals may have larger increases. While the mean changes alone would not be expected to have short-term consequences, all patients should be monitored for larger changes in heart rate and blood pressure. Caution is indicated in treating patients whose underlying medical conditions might be compromised by increases in blood pressure or heart rate, e.g., those with pre-existing hypertension, heart failure, recent myocardial infarction, or ventricular arrhythmia (see CONTRAINDICATIONS).Assessing Cardiovascular Status In Patients Being Treated With Stimulant Medications Children, adolescents, or adults who are being considered for treatment with stimulant medications should have a careful history (including assessment for a family history of sudden death or ventricular arrhythmia) and physical exam to assess for the presence of cardiac disease, and should receive further cardiac evaluation if findings suggest such disease (e.g., electrocardiogram and echocardiogram). Patients who develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease during stimulant treatment should undergo a prompt cardiac evaluation.Psychiatric Adverse EventsPre-Existing PsychosisAdministration of stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a pre-existing psychotic disorder.Bipolar IllnessParticular care should be taken in using stimulants to treat ADHD in patients with comorbid bipolar disorder because of concern for possible induction of a mixed/manic episode in such patients. Prior to initiating treatment with a stimulant, patients with comorbid depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression.Emergence Of New Psychotic Or Manic SymptomsTreatment emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, or mania in children and adolescents without prior history of psychotic illness or mania can be caused by stimulants at usual doses. If such symptoms occur, consideration should be given to a possible causal role of the stimulant, and discontinuation of treatment may be appropriate. In a pooled analysis of multiple short-term, placebo-controlled studies, such symptoms occurred in about 0.1% (4 patients with events out of 3482 exposed to methylphenidate or amphetamine forseveral weeks at usual doses) of stimulant-treated patients compared to 0 in placebo-treated patients.AggressionAggressive behavior or hostility is often observed in children and adolescents with ADHD, and has been reported in clinical trials and the postmarketing experience of some medications indicated for the treatment of ADHD. Although there is no systematic evidence that stimulants cause aggressive behavior or hostility, patients beginning treatment for ADHD should be monitored for the appearance of or worsening of aggressive behavior or hostility.Long-Term Suppression Of GrowthCareful follow-up of weight and height in children ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated children over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated children (i.e., treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development. Published data are inadequate to determine whether chronic use of amphetamines may cause a similar suppression of growth, however, it is anticipated that they likely have this effect as well. Therefore, growth should be monitored during treatment with stimulants, and patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.SeizuresThere is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizures, in patients with prior EEG abnormalities in absence of seizures, and, very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of seizures, the drug should be discontinued.Visual DisturbanceDifficulties with accommodation and blurring of vision have been reported with stimulant treatment.Use In Children Under Six Years Of AgeMETADATE CD should not be used in children under six years, since safety and efficacy in this age group have not been established.Drug DependenceMETADATE CD should be given cautiously to patients with a history of drug dependence or alcoholism. Chronic abusive use can lead to marked tolerance and psychological dependence with varying degrees of abnormal behavior. Frank psychotic episodes can occur, especially with parenteral abuse. Careful supervision is required during withdrawal from abusive use since severe depression may occur. Withdrawal following chronic therapeutic use may unmask symptoms of the underlying disorder that may require follow-up.PRECAUTIONSHematologic MonitoringPeriodic CBC, differential, and platelet counts are advised during prolonged therapy.Drug TestingMETADATE CD contains methylphenidate which may result in a positive result during drug testing.Information For PatientsPatients should be instructed to take one dose in the morning before breakfast. The patients should be instructed that the capsule may be swallowed whole, or alternatively, the capsule may be opened and the capsule contents sprinkled onto a small amount (tablespoon) of applesauce and given immediately, and not stored for future use. The capsules and the capsule contents must not be crushed or chewed.Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with methylphenidate and should counsel them in its appropriate use. A patient Medication Guide is available for METADATE CD. The prescriber or healthcare professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document. The Medication Guide may also be found in the full prescribing information for METADATE CD on http://ucb/products/cns/equasym-metadate/ or by calling 1-866-822-0068.Drug InteractionsBecause of possible effects on blood pressure, METADATE CD should be used cautiously with pressor agents.Human pharmacologic studies have shown that methylphenidate may inhibit the metabolism of coumarin anticoagulants, anticonvulsants (e.g., phenobarbital, phenytoin, primidone), phenylbutazone and some antidepressants (tricyclics and selective serotonin reuptake inhibitors). Downward dose adjustment of these drugs may be required when given concomitantly with methylphenidate. It may be necessary to adjust the dosage and monitor plasma drug concentrations (or, in the case of coumarin, coagulation times), when initiating or discontinuing concomitant methylphenidate.Serious adverse events have been reported in concomitant use with clonidine, although no causality for the combination has been established. The safety of using methylphenidate in combination with clonidine or other centrally acting alpha-2 agonists has not been systematically evaluated.In theory, there is a possibility that the clearance of methylphenidate might be affected by urinary pH, either being increased with acidifying agents or decreased with alkalizing agents. This should be considered when methylphenidate is given in combination with agents that alter urinary pH.Halogenated AnestheticsThere is a risk of sudden blood pressure increase during surgery. If surgery is planned, METADATE CD should not be taken the day of the surgery.Carcinogenesis, Mutagenesis, And Impairment Of FertilityIn a lifetime carcinogenicity study carried out in B6C3F1 mice, methylphenidate caused an increase in hepatocellular adenomas and, in males only, an increase in hepatoblastomas, at a daily dose of approximately 60 mg/kg/day. This dose is approximately 30 times and 4 times the maximum recommended human dose of METADATE CD on a mg/kg and mg/m2 basis, respectively. Hepatoblastoma is a relatively rare rodent malignant tumor type. There was no increase in total malignant hepatic tumors. The mouse strain used is sensitive to the development of hepatic tumors, and the significance of these results to humans is unknown. Methylphenidate did not cause any increases in tumors in a lifetime carcinogenicity study carried out in F344 rats; the highest dose used was approximately 45 mg/kg/day, which is approximately 22 times and 5 times the maximum recommended human dose of METADATE CD on a mg/kg and mg/m2 basis, respectively.In a 24-week carcinogenicity study in the transgenic mouse strain p53+/-, which is sensitive to genotoxic carcinogens, there was no evidence of carcinogenicity. Male and female mice were fed diets containing the same concentration of methylphenidate as in the lifetime carcinogenicity study; the high-dose groups were exposed to 60 to 74 mg/kg/day of methylphenidate. Methylphenidate was not mutagenic in the in vitro Ames reverse mutation assay or in the in vitro mouse lymphoma cell forward mutation assay. Sister chromatid exchanges and chromosome aberrations were increased, indicative of a weak clastogenic response, in an in vitro assay in cultured Chinese Hamster Ovary cells. Methylphenidate was negative in vivo in males and females in the mouse bone marrow micronucleus assay.Methylphenidate did not impair fertility in male or female mice that were fed diets containing the drug in an 18-week Continuous Breeding study. The study was conducted at doses up to 160 mg/kg/day, approximately 80-fold and 8-fold the highest recommended human dose of METADATE CD on a mg/kg and mg/m2 basis, respectively.PregnancyTeratogenic EffectsPregnancy Category CMethylphenidate has been shown to have teratogenic effects in rabbits when given in doses of 200 mg/kg/day, which is approximately 100 times and 40 times the maximum recommended human dose on a mg/kg and mg/m2 basis, respectively.A reproduction study in rats revealed no evidence of teratogenicity at an oral dose of 58 mg/kg/day. However, this dose, which caused some maternal toxicity, resulted in decreased postnatal pup weights and survival when given to the dams from day one of gestation through the lactation period. This dose is approximately 30 fold and 6 fold the maximum recommended human dose of METADATE CD on a mg/kg and mg/m2 basis, respectively.There are no adequate and well-controlled studies in pregnant women. METADATE CD should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Nursing MothersIt is not known whether methylphenidate is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised if METADATE CD is administered to a nursing woman.Pediatric UseThe safety and efficacy of METADATE CD in children under 6 years old have not been established. Long-term effects of methylphenidate in children have not been well established (see WARNINGS).ADVERSE REACTIONSThe premarketing development program for METADATE CD included exposures in a total of 228 participants in clinical trials (188 pediatric patients with ADHD, 40 healthy adult subjects). These participants received METADATE CD 20, 40, and/or 60 mg/day. The 188 patients (ages 6 to 15) were evaluated in one controlled clinical study, one controlled, crossover clinical study, and one uncontrolled clinical study. Safety data on all patients are included in the discussion that follows. Adverse reactions were assessed by collecting adverse events, results of physical examinations, vital signs, weights, laboratory analyses, and ECGs.Adverse events during exposure were obtained primarily by general inquiry and recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse events without first grouping similar types of events into a smaller number of standardized event categories. In the tables and listings that follow, COSTART terminology has been used to classify reported adverse events.The stated frequencies of adverse events represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse event of the type listed. An event was considered treatment emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.。
盐酸哌甲酯缓释片联合感觉统合训练治疗儿童注意缺陷多动障碍临床

注意缺陷多动障碍(attentiondeficithyperactivi tydisorder,ADHD),俗称少儿多动症[1]或者错误认 为儿童好动、贪玩。目前已被重新认识并定义为:儿 童时期常见的特定的一类精神、心理障碍[2]。该病 主要临床表现为与患儿实际年龄及精神、心理发育 水平不相符合的对事物、事件表现注意力不集中;对 事物、事件关注时间过短或者缺失;心理、生理活动 过多、过度和性格冲动,经常伴有学习阅读困难、品 格德行障碍和适应能力低下等。国内外权威机构调 查发现,每 100个儿童中约 3~6个儿童患有不同轻 重程度的 ADHD,男性比例居多,是女性的两倍左
作者单位:515000 广东省汕头市妇幼保健院儿科
右。部分 ADHD患儿成长过程中仍残存或多或少 的注意力不集中,感觉、思维和行为状态容易波动等 临床症状。随着年龄的增长无法达到临床痊愈的目 标,影响患儿学习成绩、生理心理健康以及成家立业 后与其家庭成员相处生活能力和社会交际能力。中 枢神经兴奋剂盐酸哌甲酯(MPH)用于治疗 ADHD 的疗效和安全性已为临床医务工作者所接纳。然 而,单纯的药物治疗只是现代医学心身健康整体治 疗的一小部分,笔者为探讨更有效的综合、整体性治 疗方法,对在我院治疗的 100例 ADHD患儿进行了 分组研究,结果报告如下。 1 资料与方法 1.1 一般资料 选取 2017年 1~12月我院儿科收
[Abstract] Objective Toexploretheclinicalefficacyofmethylphenidatehydrochloridesustainedreleasetabletscombined withsensoryintegrationtraining(SIT)inthetreatmentofattentiondeficithyperactivitydisorder(ADHD).Methods Onehundredchil drenwithADHDwererandomlydividedintotwogroups,50casesineachgroup.Thecontrolgrouptookmethylphenidatehydrochloride sustainedreleasetabletsalone,andtheobservationgroupdidmethylphenidatehydrochloridesustainedreleasetabletsconcomitantuseof SIT,comparingthewechslerintelligencescaleforchildren(WISC)andtheConner’sbehaviorratingscaleinthechildrenwithADHD 12monthslater.Results Beforethetreatment,nosignificantdifferenceintheWISCandtheConner’sbehaviorratingscaleoccurred betweenthetwogroups(P>0.05).Twelvemonthslater,theWISCincreasedandtheConner’sbehaviorscalescoredecreasedsignifi cantlyinthetwogroups.Moreover,theincreasedanddecreasedrangesbothoftheWISCandtheConner’sbehaviorscalescoreofthe observationgroupshowedsignificantlygreaterthanthoseofthecontrolgroup(P<0.01).Conclusion Theefficacyofthedrugcon comitantuseofsensoryintegrationtrainingwasbetterthanthedrugaloneintherapyforchildrenADHD.
盐酸哌甲酯缓释片联合小儿智力糖浆治疗儿童多动症的临床疗效及对血清多巴胺水平的影响

盐酸哌甲酯缓释片联合小儿智力糖浆治疗儿童多动症的临床疗效及对血清多巴胺水平的影响摘要:目的:探讨盐酸哌甲酯缓释片联合小儿智力糖浆治疗儿童多动症的临床疗效及对血清多巴胺水平的影响。
方法:选择2023年1-6月医院收治的多动症儿童50例,均给予盐酸哌甲酯缓释片联合小儿智力糖浆治疗,观察患儿的临床疗效、治疗前后的血清多巴胺水平、治疗前后的Conners行为评分以及不良反应。
结果:该50例患儿经治疗后,痊愈、显效、有效、无效各有20例、17例、11例、2例,临床总疗效率为96.00%(48/50)。
且患儿治疗后的血清多巴胺水平为(880.47±157.42)μg/L,显著高于对照组(P<0.05)。
该50例患儿治疗后的Conners行为量表中学习、品行、冲动、心身、焦虑与多动等行为评分各为(1.34±0.28)、(1.42±0.30)、(1.48±0.47)、(0.75±0.22)、(1.30±0.33)、(1.34±0.35)分,均较对照组的低(P<0.05)。
在患儿治疗期间,共发生2例的恶心呕吐与1例的头痛头晕,药物不良反应总发生率为6.00%(3/50)。
结论:在多动症儿童治疗中盐酸哌甲酯缓释片联合小儿智力糖浆的疗效可靠,可以提高患儿的血清多巴胺水平,概述俺儿多动症行为,且不良反应少,安全性较高,值得推广。
关键词:盐酸哌甲酯缓释片;小儿智力糖浆;多动症;临床疗效;血清多巴胺水平多动症又称为注意力缺陷多动障碍,是儿童时期比较常见的精神神经类疾病,多动症主要的症状在临床上体现的就是注意力集中障碍,孩子不能够在一段时间内像正常孩子一样注意力集中地去完成一项任务,往往一会儿注意力就不集中了,而且很容易被旁边的事物所打扰[1]。
此外,患儿还可伴有易冲动、易激动等,对患儿的生活、学习等造成巨大干扰。
目前针对于儿童多动症来说,主要以综合治疗为主,包括药物、心理、饮食等多形式,其中药物治疗为本病的重要治疗方式,故本研究选择2023年1-6月医院收治的多动症儿童50例,分析了盐酸哌甲酯缓释片联合小儿智力糖浆治疗儿童多动症的价值,具体有下。
盐酸哌甲酯缓释片联合脑电生物反馈对多动症患儿的影响

盐酸哌甲酯缓释片联合脑电生物反馈对多动症患儿的影响邱佳静① 黄惠玲① 陈晓梅① 【摘要】 目的:评价盐酸哌甲酯缓释片联合脑电生物反馈对多动症患儿的影响。
方法:选取2020年6月—2022年6月泉州市妇幼保健院·儿童医院收治的100例多动症患儿。
根据简单随机化法将其分为对照组和试验组,各50例。
对照组给予盐酸哌甲酯缓释片,试验组给予盐酸哌甲酯缓释片联合脑电生物反馈。
比较两组临床疗效,治疗前后视听整合持续测试系统(IVA-CPT)评分、行为情况、生活质量及不良反应。
结果:试验组总有效率(96.00%)高于对照组(82.00%),差异有统计学意义(P<0.05)。
治疗后,试验组综合注意力商数、综合控制力商数评分均高于对照组,差异有统计学意义(P<0.05)。
治疗后,试验组学习问题、冲动多动、品行问题、身心问题评分均低于对照组,差异有统计学意义(P<0.05)。
治疗后,试验组生理功能、社会功能、生理职能、精神状态评分均高于对照组,差异有统计学意义(P<0.05)。
两组不良反应发生率比较,差异无统计学意义(P>0.05)。
结论:盐酸哌甲酯缓释片联合脑电生物反馈治疗多动症患儿效果较好,能更好地改善患儿的病情,改善其生活质量,且不会增加不良反应的发生率,安全有效。
【关键词】 盐酸哌甲酯缓释片 脑电生物反馈 多动症 不良反应 doi:10.14033/ki.cfmr.2023.22.029 文献标识码 B 文章编号 1674-6805(2023)22-0114-04 Effects of Methylphenidate Hydrochloride Sustained-release Tablets Combined with Brain Electrical Biofeedback on Children with Hyperactivity Disorder/QIU Jiajing, HUANG Huiling, CHEN Xiaomei. //Chinese and Foreign Medical Research, 2023, 21(22): 114-117 [Abstract] Objective: To evaluate the effects of Methylphenidate Hydrochloride Sustained-release Tablets combined with brain electrical biofeedback on children with hyperactivity disorder. Method: A total of 100 children with hyperactivity disorder admitted to Quanzhou Maternal and Child Health Hospital · Children's Hospital from June 2020 to June 2022 were selected. According to the simple randomization method, they were divided into control group and experimental group, with 50 cases in each group. The control group was given Methylphenidate Hydrochloride Sustained-release Tablets, and the experimental group was given Methylphenidate Hydrochloride Sustained-release Tablets combined with brain electrical biofeedback. The clinical efficacy, integrated visual and audio continuous performance test (IVA-CPT) scores, behavior condition, quality of life before and after treatment and adverse reactions were compared between the two groups. Result: The total effective rate of experimental group (96.00%) was higher than that of control group (82.00%), and the difference was statistically significant (P<0.05). After treatment, the scores of comprehensive attention quotient and comprehensive control quotient of experimental group were higher than those of control group, and the differences were statistically significant (P<0.05). After treatment, the scores of learning problems, impulsive hyperactivity, conduct problems and psychosomatic problems of experimental groups were lower than those of control group, and the differences were statistically significant (P<0.05). After treatment, the scores of physiological function, social function, physiological function and mental state of experimental groups were higher than those of control group, and the differences were statistically significant (P<0.05). There was no significant difference in the incidence of adverse reactions between the two groups (P>0.05). Conclusion: Methylphenidate Hydrochloride Sustained-release Tablets combined with brain electrical biofeedback have a good effect in the treatment of children with hyperactivity disorder, which can better improve the condition of children and improve their quality of life, and does not increase the incidence of adverse reactions, and is safe and effective. [Key words] Methylphenidate Hydrochloride Sustained-release Tablets Brain electrical biofeedback Hyperactivity disorder Adverse reactions First-author's address: Quanzhou Maternal and Child Health Hospital · Children's Hospital, Quanzhou 362000, China 多动症是患病率较高的一种疾病,属于心理行为障碍性疾病,疾病的发生因素较为复杂和多样,与环境、脑功能、神经系统失调、遗传等因素有关,患儿可出现自我控制能力差、活动过度、行为冲动、注意力不集中等症状,会对机体健康水平与生长发育造成影响,降低正常生活质量,因此,做好疾病的有效治疗意义重大[1]。
盐酸哌甲酯缓释片联合医教结合干预对注意缺陷多动障碍儿童的疗效分析

盐酸哌甲酯缓释片联合医教结合干预对注意缺陷多动障碍儿童的疗效分析陈礼贤;林辉;徐丹红;沈华斌【期刊名称】《中外医学研究》【年(卷),期】2023(21)3【摘要】目的:研究盐酸哌甲酯缓释片联合医教结合干预对注意缺陷多动障碍(ADHD)儿童的疗效。
方法:选取2019年3月—2022年5月玉环市第二人民医院收治的80例ADHD儿童,经随机数表法将其分为对照组(n=40)与研究组(n=40)。
两组均予以盐酸哌甲酯缓释片药物,对照组在此基础上予以行为干预,研究组在对照组的基础上予以医教结合干预,两组均干预6周。
观察两组干预前后血清多巴胺水平、儿童智力、儿童行为。
结果:干预后,两组血清多巴胺水平与干预前相比均升高,且研究组高于对照组(P<0.05)。
干预后,两组操作智商(PIQ)、言语智商(VIQ)、全智商(FIQ)评分与干预前相比均升高,且研究组均高于对照组(P<0.05)。
干预后,两组多动-冲动、注意力不集中、对立违抗评分与干预前相比均降低,且研究组均低于对照组(P<0.05)。
结论:盐酸哌甲酯缓释片联合医教结合干预应用于ADHD儿童,可增加血清多巴胺水平,提高儿童智力与行为。
【总页数】4页(P147-150)【作者】陈礼贤;林辉;徐丹红;沈华斌【作者单位】玉环市第二人民医院【正文语种】中文【中图分类】R74【相关文献】1.盐酸哌甲酯缓释片联合感觉统合训练治疗儿童注意缺陷多动障碍临床效果分析2.盐酸哌甲酯缓释片联合感觉统合训练治疗儿童注意缺陷多动障碍临床效果分析3.盐酸哌甲酯缓释片联合经颅磁刺激治疗对儿童注意缺陷多动障碍疗效的研究4.盐酸哌甲酯缓释片联合ω-3多不饱和脂肪酸治疗儿童注意缺陷多动障碍的临床研究5.盐酸哌甲酯缓释片联合脑电生物反馈加感统训练治疗儿童注意缺陷多动障碍的临床疗效研究因版权原因,仅展示原文概要,查看原文内容请购买。
认知行为疗法联合盐酸哌甲酯缓释片治疗对多动症患儿认知功能及血清多巴胺水平的影响

认知行为疗法联合盐酸哌甲酯缓释片治疗对多动症患儿认知功
能及血清多巴胺水平的影响
袁明珠;胡光华;毛旭鑫
【期刊名称】《临床医学工程》
【年(卷),期】2024(31)4
【摘要】目的探讨认知行为疗法(CBT)联合盐酸哌甲酯缓释片治疗多动症患儿的临床效果。
方法98例多动症患儿随机分为两组。
参照组予以盐酸哌甲酯缓释片治疗,观察组在参照组的基础上予以CBT干预。
比较两组的认知功能、血清多巴胺水平。
结果治疗后,观察组的Conner行为评定量表中学习行为、品行行为、身心行为和
冲动行为评分均低于参照组,血清多巴胺水平高于参照组(P<0.05)。
结论CBT联合盐酸哌甲酯缓释片治疗多动症可显著改善患儿的认知功能,提升血清多巴胺水平。
【总页数】2页(P467-468)
【作者】袁明珠;胡光华;毛旭鑫
【作者单位】郑州市第九人民医院心理科
【正文语种】中文
【中图分类】R749.94
【相关文献】
1.奥氮平联合丙戊酸钠缓释片对难治性精神分裂患者认知功能及血清催乳素水平的影响
2.认知行为疗法联合奥卡西平对癫痫患儿认知功能及血清BDNF、Hcy、IGF-1水平的影响
3.试论孔子音乐美学思想对当代电影音乐的影响——从电影《我的
父亲母亲》音乐谈起4.药物治疗联合网络认知行为疗法对精神分裂症患者行为症状认知功能及生命质量的影响5.吡贝地尔缓释片联合盐酸多奈哌齐治疗对帕金森病患者认知功能及血清sLAG3、RANTES水平的影响
因版权原因,仅展示原文概要,查看原文内容请购买。
盐酸哌甲酯缓释片与舒尔特方格法联合方案对多动症患儿临床症状及认知功能的影响

盐酸哌甲酯缓释片与舒尔特方格法联合方案对多动症患儿临床症状及认知功能的影响曹玲;赵艾红;唐为娟【期刊名称】《吉林医学》【年(卷),期】2024(45)6【摘要】目的:分析盐酸哌甲酯缓释片与舒尔特方格法联合方案治疗多动症患儿对临床症状及认知功能的影响。
方法:选取2020年1月~2023年1月盐城市建湖县人民医院儿科106例就诊的多动症患儿为研究对象,分为对照组与试验组各53例。
其中对照组给予盐酸哌甲酯缓释片治疗,试验组则采用盐酸哌甲酯缓释片联合舒尔特方格法治疗,比较两组患儿临床症状改善情况及认知功能。
结果:治疗后,试验组总有效率高于对照组,差异有统计学意义(P<0.05);试验组注意缺陷多动障碍SNAP-Ⅳ评定量表(父母版)总评分低于对照组,差异有统计学意义(P<0.05);试验组认知功能显著高于对照组,差异有统计学意义(P<0.05);试验组血清多巴胺、25-羟维生素D水平均高于对照组,差异有统计学意义(P<0.05)。
结论:将盐酸哌甲酯缓释片与舒尔特方格法联合治疗多动症疗效较好,能够有效改善患儿的临床症状,提高其认知功能,调节血清多巴胺及25-羟维生素D水平。
【总页数】4页(P1435-1438)【作者】曹玲;赵艾红;唐为娟【作者单位】盐城市建湖县人民医院儿科【正文语种】中文【中图分类】R748【相关文献】1.盐酸哌甲酯缓释片在多动症患儿中的应用2.舒尔特方格法联合盐酸哌甲酯缓释片用于儿童多动症的临床效果分析3.盐酸哌甲酯缓释片联合执行功能康复训练治疗注意缺陷多动障碍患儿的临床效果4.盐酸哌甲酯缓释片治疗多动症患儿临床效果观察及对神经递质的影响5.认知行为疗法联合盐酸哌甲酯缓释片治疗对多动症患儿认知功能及血清多巴胺水平的影响因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
盐酸哌甲酯缓释片以下内容仅供参考,请以药品包装盒中的说明书为准。
妊娠:C哺乳:L2儿童:本品不可用于6岁以下儿童盐酸哌甲酯缓释片说明书【说明书修订日期】核准日期:2007年01月16日修改日期:2007年05月26日2008年04月18日2009年05月19日2009年12月02日2011年01月10日2011年11月05日2012年02月27日2012年06月25日2012年08月28日【警告】本品应慎用于有药物依赖史或酒精依赖史的患者。
长期滥用会导致明显的耐受性和精神依赖,并伴随不同程度的行为失常。
尤其是在非肠道途径滥用药物时,可引起明显的精神病性发作。
因对滥用药物的停用可能引起严重的抑郁症,应对其停用过程进行监护。
长期治疗停药时,可能会使一些潜在疾病症状显现,需要进行随访。
【特殊标记】精神药品【药品名称】盐酸哌甲酯缓释片【英文名称】MethylphenidateHydrochloride Prolonged-Release Tablets【汉语拼音】YansuanPaijiazhi Huanshipian【成份】活性成份:盐酸哌甲酯【性状】本品为胶囊形片。
【适应症】本品用于治疗注意缺陷多动障碍。
在符合DSM-IV诊断标准的6-12岁患注意缺陷多动障碍儿童参加的三个对照试验中证实了本品对注意缺陷多动障碍的疗效。
当单项治疗效果不佳时,本品可作为综合治疗的一部分。
对注意缺陷多动障碍的综合治疗可能还包括其它措施(如:心理的、教育的和社会的)。
应根据DSM-IV标准或者ICD-10的规定,并基于患者的病史和分析做出诊断。
是否使用本品治疗需依据对每名患者症状严重性的全面评估,并非所有患注意缺陷多动障碍的患者均适用本品治疗。
对于那些继发于环境因素和/或其它原发精神疾患(包括精神病)的注意缺陷多动障碍患者不建议使用兴奋剂。
应进行适当的教育,以及精神和心理上的调解。
【规格】本品为每日口服1次的缓释片,每片含有18或36mg盐酸哌甲酯。
【用法用量】本品不可用于6岁以下儿童。
用法:口服。
用量:每日1次。
本品给药后作用可持续12小时,应在早晨服药。
本品要整片用水送下,不能咀嚼、掰开或压碎。
本品可于餐前或餐后服用。
剂量可根据患者个体需要及疗效而定。
每次可增加剂量18mg,直至最高剂量为54mg(每日1次、晨服)。
通常约每周调整剂量1次。
新接受哌甲酯治疗的患者对于目前未接受哌甲酯治疗的患者或正在接受其它兴奋剂治疗的患者,本品的推荐起始剂量为每日1次18mg。
正在接受哌甲酯治疗的患者对于正在接受每次5mg、每日2次盐酸哌甲酯速释片;20mg 盐酸哌甲酯缓释片(如:利他林缓释片)或每次5mg、每日3次盐酸哌甲酯速释片治疗的患者,本品的推荐剂量为18mg。
对于正在接受每次10mg、每日2次盐酸哌甲酯速释片;40mg 盐酸哌甲酯缓释片(如:利他林缓释片)或每次10mg、每日3次盐酸哌甲酯速释片治疗的患者,本品的推荐剂量为36mg。
在某些情况下,可使用54mg的剂量。
推荐剂量应基于目前的服药剂量及疗效。
对正在服用盐酸哌甲酯而剂量与上述不同的患者,应根据临床疗效确定剂量。
每日剂量不应超过54mg。
维持治疗尚无对照试验对本品的长期使用进行系统评价。
选择本品长期治疗时,医生应定期对患者长期用药的疗效进行再评价。
评价方法为停药后,在无药物治疗的情况下进行患者功能评价。
在暂时或永久停药后,对病情的改善有可能会持续。
减量或停药如果症状加重或发生其它不良事件,应减少药量或停药。
【不良反应】此项下的不良反应是对已获得的不良事件相关信息做全面评估后得出的与使用该药品有相关性的不良事件。
与该药品的相关性不是通过个例来确定。
此外,因为不同的临床试验开展条件差异较大,一种药物临床试验中观察到的不良反应发生率不能直接和另外一种药物临床试验中的不良反应发生率进行比较,而且可能反应不出实际临床应用中的不良反应发生率。
临床试验数据双盲试验数据-发生率少于1%的不良反应以下双盲试验中涉及的药物不良反应(ADR)均适用于儿童、青少年及成人。
儿童、青少年在4个安慰剂对照、双盲试验中,评价639例儿科(儿童和青少年)注意缺陷多动障碍(ADHD)受试者使用本品的安全性。
本部分的数据来源于综合数据。
表1列出了这些试验中本品组儿科及青少年受试者发生率不少于1%的药物不良反应(ADR)。
表1.在4个安慰剂对照、双盲试验中,本品组儿童及青少年受试者报告率不少于1%的不良反应系统/器官不良反应本品(n=321)%安慰剂(n=318)%感染鼻咽炎 2.8 2.2 精神病性障碍失眠* 2.8 0.3 神经系统头晕 1.9 0 呼吸系统、胸、膈咳嗽 1.9 0.9 口咽痛 1.2 0.9 胃肠道上腹部疼痛 6.2 3.8 呕吐 2.8 1.6 全身及给药部位发热 2.2 0.9*失眠包括:入睡困难型失眠症(本品组=0.6%)和失眠症(本品组=2.2%)多数不良反应的程度为轻中度。
成人在3项安慰剂对照、双盲临床试验中评价了905名成年ADHD受试者使用本品的安全性。
本部分内容来源于混合数据。
表2列出了在上述试验的本品组成年受试者中发生率不少于1%的药物不良反应。
表2.在3项成年受试者的安慰剂对照、双盲临床试验的本品组中发生率不少于1%的药物不良反应系统/器官不良反应本品(n=596)%安慰剂(n=309)%感染上呼吸道感染 1.7 1.0 鼻窦炎 1.3 1.0 代谢和营养食欲下降24.8 6.1 厌食症 4.2 1.3 精神病性障碍失眠13.3 7.8 焦虑8.4 2.9 入睡困难型失眠症 5.7 2.6 情绪低落 4.4 2.6 烦躁不安 4.0 0 激越 3.2 0.6神经质 2.3 0.6 磨牙症 1.5 0.6 抑郁 1.5 0.6 情绪波动 1.3 0.6 性欲下降 1.3 0.6 恐慌发作 1.3 0.3 紧张 1.3 0.3 攻击行为 1.2 0.6 意识模糊状态 1.0 0.3 神经系统头痛24.2 18.8 头晕7.4 5.5 震颤 3.4 0.6 感觉异常 1.2 0紧张性头痛 1.0 0.3 眼部调节障碍 1.3 0视力模糊 1.3 1.0 耳部及迷路眩晕 2.0 0.3 心脏心动过速 6.0 0心悸 4.5 0.6 血管高血压 2.2 1.6潮红 1.3 0.6 呼吸系统、胸、纵膈口咽痛 1.5 1.3 咳嗽 1.2 1.0 呼吸困难 1.2 0.6 胃肠道口干15.1 3.6 恶心14.3 4.9 消化不良 2.0 1.9 呕吐 1.8 0.6 便秘 1.5 0.6 皮肤及皮下组织多汗 5.7 1.3 骨骼肌和结缔组织肌肉紧缩 1.3 0 肌痉挛 1.0 0.3 生殖系统和乳腺勃起功能障碍 1.0 0.3 全身及给药部位易怒 5.2 2.9 疲乏 4.7 4.2 口渴 1.8 0.6 无力 1.2 0 检查指标体重下降8.7 3.6心率加快 3.0 1.9 血压升高 2.5 1.9 丙氨酸转氨酶升高 1.0 0 多数不良反应的程度为轻中度开放性试验数据-发生率不少于1%的不良反应在12个开放性临床试验中,评价3782例儿科和成年ADHD 受试者使用本品的安全性。
本部分的数据来源于混合数据。
表3列出了本品组受试者发生率不少于1%且未显示于表1和表2的药物不良反应。
表3.在12个开放性临床试验中,本品组受试者报告率不少于1%的不良反应系统/器官不良反应本品(n=3782) %精神病性障碍抽搐 2.0 心境不稳 1.1 神经系统嗜睡 1.0 胃肠道腹泻 2.4 腹部不适 1.3 腹痛 1.2 皮肤及皮下组织皮疹 1.3 全身及给药部位紧张不安感 1.4多数不良反应的程度为轻中度。
双盲和开放性试验数据-发生率小于1%的不良反应表4列出了在双盲和开放性试验中本品组儿科和成年受试者发生率小于1%的其他不良反应。
表4.在双盲或开放性临床试验中,本品组儿科和成年受试者发生率小于1%的不良反应系统/器官不良反应血液和淋巴系统白细胞减少症精神病性障碍易怒、警觉过度、心境改变、睡眠障碍、悲伤哭泣神经系统嗜睡、镇静、精神运动机能亢进眼部眼干皮肤及皮下组织斑疹检查指标心脏杂音多数不良反应的程度为轻中度上市后数据表5列出了从本品的上市后经验中判定的不良反应,其频率按以下规定表示:很常见≥1/10常见≥1/100且<1/10少见≥1/1,000且<1/100罕见≥1/10,000且<1/1000非常罕见<1/10,000,包括个别病例。
根据自发报告的频率在表5中列出了不同发生率的不良反应。
表5.根据自发报告的频率评估本品上市后的不良反应血液和淋巴系统非常罕见全血细胞减少症、血小板减少症、血小板减少性紫癜精神病性障碍非常罕见定向障碍、幻觉、幻听、幻视、躁狂、多言癖神经系统非常罕见惊厥、癫痫大发作、运动障碍眼部非常罕见复视、瞳孔散大、视力缺损心脏非常罕见心绞痛、心动过缓、期外收缩、室上性心动过速、室性期外收缩血管非常罕见雷诺现象皮肤及皮下组织非常罕见脱发、红斑骨骼肌及结缔组织非常罕见关节痛、肌肉痛、肌肉颤搐免疫系统罕见超敏反应如:血管性水肿、过敏性反应、耳部肿胀、大疱性皮肤病、表皮脱落、荨麻疹、瘙痒症NEC、皮疹、出疹和疹病NEC全身及给药部位罕见疗效降低非常罕见胸痛、胸部不适、药效减低、高热检查指标非常罕见血碱性磷酸酶升高、血胆红素水平升高、肝酶升高、血小板计数减少、白细胞计数异常【禁忌】本品禁用于:有明显焦虑、紧张和激越症状的患者(可能会使这些症状加重)。
已知对哌甲酯或本品其它成份过敏的患者。
青光眼患者。
运动性抽搐(motortics)或有家族史或诊断有抽动秽语综合征的患者。
正在或14天内使用过单胺氧化酶抑制剂治疗的患者(可能导致高血压危象)。
【注意事项】猝死和患结构性心脏病或其他严重心脏病患者儿童和青少年:曾有患结构性心脏病或其他严重心脏病的儿童和青少年正常使用中枢神经兴奋剂发生猝死的报告。
尽管一些严重心脏病使猝死的风险增加,但是总体上兴奋剂类药品不应用于已知患有结构性心脏病、心肌病、严重心律失常或可使兴奋剂拟交感神经效应增加的其他严重心脏病的儿童和青少年。
成人:已见成年患者正常使用盐酸哌甲酯治疗ADHD发生猝死、中风和心肌梗塞的报道。
尽管在这些成年病例中,未知兴奋剂的作用,但是成人比儿童患有严重结构性心脏异常、心肌病、严重心律失常、冠状动脉病或其他严重心脏问题的可能性更大。
患上述疾病的成人通常不应使用兴奋剂治疗。
兴奋剂药品治疗患者需心血管评估使用兴奋剂治疗的儿童、青少年或成人,应该有详细的病史(包括猝死或室律失常的家族史的评价)和体格检查来评价是否有心脏疾病,且应该进一步进行心脏评估是否有迹象暗示这些疾病(例如心电图和超声心动图)。
