Citalopram_hydrobromide_SDS_MedChemExpress
Tapentadol_hydrochloride_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Aug.-29-2017Print Date:Aug.-29-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Tapentadol (hydrochloride)Catalog No. :HY-70042ACAS No. :175591-09-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C14H24ClNOMolecular Weight:257.80CAS No. :175591-09-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
鲁米诺CAS521-31-3在蛋白质WB实验中的应用

鲁米诺CAS521-31-3在蛋白质WB实验中的应用
化学发光试剂鲁米诺,全称3-氨基苯二甲酰肼,俗名发光氨luminol,通常用于酶促化学发光实验以及刑侦上的微量血迹检测。
由于其结构简单、易合成、发光量子效率高的特点,现也被用于蛋白质印迹试验western blot中。
蛋白免疫印迹是将经过PAGE(聚丙烯酰胺凝胶电泳)分离的蛋白质样品,转移到固相载体NC膜(硝酸纤维素薄膜)或PVDF膜(聚偏二氟乙烯膜)上,固相载体以非共价键形式吸附蛋白质,且能保持电泳分离的多肽类型及其生物学活性不变。
以固相载体上的蛋白质或多肽作为抗原,与对应的抗体起免疫反应,再与酶或同位素标记的第二抗体起反应,经过底物显色或放射自显影以检测电泳分离的特异性目的基因表达的蛋白成分。
该技术也广泛应用于检测蛋白水平的表达。
鲁米诺发光反应
鲁米诺的发光反应是属于整个蛋白质Western Blot印迹实验中的最后一步,加入1:1的溶液A和溶液B,覆盖pvdf膜,可以显色或发光来观察实验结果。
一般使用的发光液A和B分别是鲁米诺和过氧化氢。
由于鲁米诺被过氧化氢催化氧化发光的反应需要过氧化物酶的参与,属于酶促化学发光,需要维持反应的pH值,实验中的匀浆缓冲液通常用我们Tris-HCL配成的TBS缓冲液,蛋白质转膜缓冲液也是用Tris加SDS、甘氨酸、甲醇缓冲体系,如果是高于20kd的蛋白则可以使用CAPS缓冲剂,也快吃用Tricine代替甘氨酸。
市场上通常鲁米诺是做成即用型的ECL发光液,德晟生产的鲁米诺是粉末试剂,因为发光试剂对光和温度敏感,粉末试剂更利于保存,需要使用时,现配现用即可,可以根据不同的实验配置不同的浓度,不用采购多种浓度试剂,减少浪费。
含有一个非平面杂环胺配体的新型反铂抗癌药物的水解机理

含有一个非平面杂环胺配体的新型反铂抗癌药物的水解机理赵亚华【期刊名称】《物理化学学报》【年(卷),期】2009(025)011【摘要】Hydrolysis processes of novel anticancer transplatin analogues, trans-[PtCl_2(NH_3)(Am)](Am: nonplanar heterocycle piperidine or piperazine), were explored using the B3LYP hybrid functional and isoclectric focusing polarized continuum model (IEF-PCM). Stationary points on the potential energy surfaces for the first and second hydrolysis steps that proceed via a general S_N2 pathway were fully optimized and characterized. The most remarkable structural variations in the hydrolysis process were found to occur in the equatorial plane of five-coordinate trigonal-bipyramidal (TBP) like structures of the intermediates and transition states. We obtained lower activation energies for trans-[PtCl_2(NH_3)(piperazine)] and a slightly higher activation energy for the first step and a slightly lower activation energy for the second step during the hydrolysis of trans-[PtCl_2(NH_3)(piperidine)] by comparison to previous work on the hydrolysis reactions of cisplatin. Our calculations suggest that this class of non-classical transplatin analogues with one nonplanar heterocyclic amine decreases the equatorial steric effect and the hydrolysis reaction barriers.%用B3LYP杂化泛函和等电子聚焦极化连续模型(IEF-PCM)研究了trans-[P[Cl_2(NH_3)(Am)](Am:非平面哌啶或哌嗪)新型反铂抗癌药物的水解反应机理.对经由一般的S_N2机理的第一步和第二步水解反应势能而上的稳定点进行了全优化和表征.在水解中,最显著的结构变化发生在反应过渡态和中间体的五配位三角双锥的赤道面上.与经典顺铂(cisplatin)比较,反式[PtCl_2(NH_3)(piperazine)]的第一步和第二步水解活化能均低于顺铂,而反式[PtCl_2(NH_3)(piperidine)]的第一步水解活化能稍高于顺铂,第二步水解活化能稍低于顺铂.计算表明,这些含有非平面杂环胺反铂的配合物减小了赤道面上的立体效应和水解势垒.【总页数】7页(P2350-2356)【作者】赵亚华【作者单位】华南农业大学生命科学学院,广州,510642【正文语种】中文【中图分类】O641【相关文献】1.抗癌药物——铂络合物中铂之测定 [J], 涂清和2.非对称反铂抗癌药物反-异丙胺·间羟甲基吡啶·二氯铂水解机理的理论研究 [J], 叶冰;许旋3.杂环反铂(Ⅱ)抗癌药物水解反应机理的DFT研究 [J], 李添;周立新;李娟4.以磷酸二氢根为轴向配体的铂(Ⅳ)前药的设计、合成和抗癌活性测试 [J], 高安丽;熊庆丰;姜婧;余娟;楼丽广;刘伟平5.铂配合物的抗癌作用及非铂抗癌物 [J], 高朝明因版权原因,仅展示原文概要,查看原文内容请购买。
气相色谱法测定富马酸卢帕他定中三乙胺的含量

201 8年 第 14期 第 45卷 总第 376期
广 东 化 工 www.gdchem com
蛋白裂解液
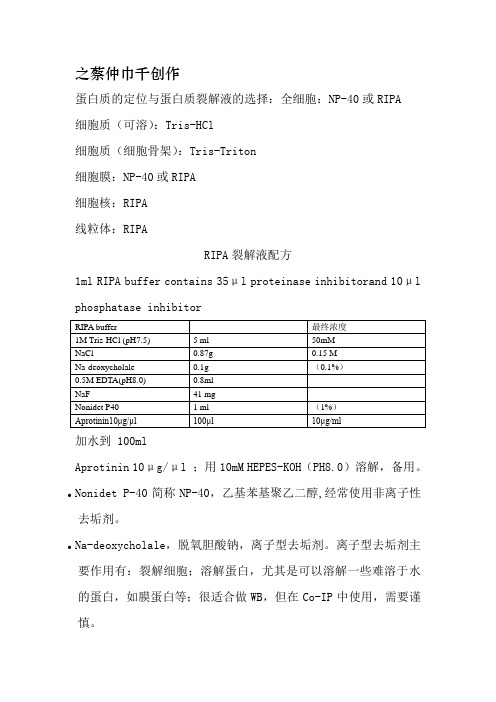
之蔡仲巾千创作蛋白质的定位与蛋白质裂解液的选择:全细胞:NP-40或RIPA细胞质(可溶):Tris-HCl细胞质(细胞骨架):Tris-Triton细胞膜:NP-40或RIPA细胞核:RIPA线粒体:RIPARIPA裂解液配方1ml RIPA buffer contains 35μl proteinase inhibitorand 10μl phosphatase inhibitor加水到 100mlAprotinin 10μg/μl ;用10mM HEPES-KOH(PH8.0)溶解,备用。
●Nonidet P-40简称NP-40,乙基苯基聚乙二醇,经常使用非离子性去垢剂。
●Na-deoxycholale,脱氧胆酸钠,离子型去垢剂。
离子型去垢剂主要作用有:裂解细胞;溶解蛋白,尤其是可以溶解一些难溶于水的蛋白,如膜蛋白等;很适合做WB,但在Co-IP中使用,需要谨慎。
●亮抑霉肽(Leupeptin):抑制丝氨酸蛋白酶包含胰蛋白酶、纤溶酶、猪胰激肽原酶)和半胱氨酸蛋白酶(包含木瓜蛋白酶和组织蛋白酶B)●抑肽素A(Pepstatin A)抑制各种天门冬氨酸蛋白酶,例如组织蛋白酶D、肾素、胃蛋白酶、细菌天门冬氨酸蛋白酶和HIV蛋白酶;●胰凝乳蛋白酶抑制剂(Chymostatin)抑制具有糜蛋白酶类特异性丝氨酸蛋白酶(包含糜蛋白酶、胃促胰酶、组织蛋白酶 G)和大多数丝氨酸蛋白酶(包含组织蛋白酶B,H,L)●抗木瓜蛋白酶(Antipain)抑制木瓜蛋白酶、胰蛋白酶和纤溶酶。
●氟化钠抑制酸性磷酸酶●正钒酸钠:抑制碱性磷酸酶和酪氨酸磷酸酶(tyrosine phosphatase)●焦磷酸钠(sodium pyrophosphate):抑制丝氨酸-苏氨酸磷酸酶(serine-threonine phosphatase)。
在与蛋白相关的检测中,首先最关键的一步即是蛋白质的提取。
蛋白质的提取过程中,我们要经常加和蛋白酶抑制剂以防止蛋白质的降解。
蛋白免疫印迹方法-康奈尔大学刘瑞海实验室实验方法

Western blot for all proteinsUpdated on 03/25/2013It’s all depends on my experience. Follow or not, it’s your call.Cell growth, treatment and cell collection1.Cells are plated in six well plates(六孔板) at a density(密度)of 6-7*106cells/well.(3.0-3.5*105 cells/mL)2.Depends on(取决于) time-connection results, treatment time would be various(不同).3.Treat cell with chemical, extraction or anything else with does-elevated concentration oncertain hours (based on time-kinetics(时间-动力学)).4.After cell culture, remove growth media(生长培养基), wash cell twice using 1mL of PBS,then add 1mL PBS and remove cell into centrifuge tubes(离心管) on ice.5.Centrifuge(离心) cells at 1000-1100 RPM(每分钟转数) for 5 mins.6.Pour all PBS into waste(废液桶) and keep cells dry in air about 0.5-1 mins.7.Freeze all cell at -20o C refrigerator. [细胞是保存在大离心管中]*Note: Protein would be degraded by(因...而损失) time. Please process your cell no longer than 1 week; you will lose most of your protein if freezing for more than 2 weeks.Cell extraction1. Lyse(裂解) the cells using RIPA buffer(细胞裂解液)[细胞裂解液配制见第2步], 100uL per tube (depends on how high your protein concentration designed), 30 mins on ice, vortex(在漩涡震荡器轻轻震荡5秒钟) every 5 mins.[加入细胞裂解液的体积是根据之前种的细胞的浓度来决定的,一般来讲,之前在六孔板上每种一个孔的细胞需要20uL的RIPA buffer,5-6孔可加100uL。
CAS1204313-51-8_Icotinib Hydrochloride_MedBio相关资料

在体外激酶测定中,将2.4 ng /μLEGFR蛋白与32 ng /μLCrk在含有1μM冷ATP和1μCi32P-γ-ATP的25μL激酶反应缓冲液中混合。将混合物与Icotinib在0,0.5,2.5,12.5或62.5nM下在冰上温育10分钟,然后在30℃温育20分钟。用SDS样品缓冲液在100℃猝灭4分钟后,通过在10%SDS-PAGE凝胶中电泳分离蛋白质混合物。然后暴露干燥的凝胶以检测放射性。量化由软件[1]执行。
CAS
1、产品物理参数:
常用名
凯美纳
英文名
Icotinib Hydrochloride
CAS号
1204313-51-8
分子量
427.881
密度
无资料
沸点
无资料
分子式
C22H22ClN3O4
熔点
无资料
闪点
无资料
2、技术资料:
体外研究
与Iconitib在0.5μM孵育导致激酶活性抑制分别为91%,99%,96%,61%和61%。 Iconitib抑制A431和BGC-823 A549,H460和KB细胞系的增殖,IC50分别为1,4.06,12.16,16.08,40.71μM。当用88种激酶进行分析时,Icotinib仅对EGFR及其突变体显示出有意义的抑制活性。 Icotinib阻断人表皮样癌A431细胞系中EGFR介导的细胞内酪氨酸磷酸化(IC50 = 45 nM)并抑制肿瘤细胞增殖[1]。
10mM (in 1mL DMSO)
≥98%
1172133-28-6
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
盐酸倍他司汀及其关键中间体的合成综述

盐酸倍他司汀及其关键中间体的合成综述盐酸倍他司汀(Escitalopram hydrobromide)是一种常用的抗抑郁药物,广泛用于治疗抑郁症和焦虑症。
它是一种选择性去甲肾上腺素再摄取抑制剂(SSRI),能有效增加大脑中去甲肾上腺素的水平,从而改善情绪和心理状态。
盐酸倍他司汀的合成工艺涉及多个中间体的合成步骤,下面将从中间体入手,对盐酸倍他司汀及其关键中间体的合成进行综述。
一、关键中间体的合成1. 3-氯-3-(二氯甲基)丙烯(3-Chloro-3-(dichloromethyl)propene)3-氯-3-(二氯甲基)丙烯是合成盐酸倍他司汀的关键中间体之一,它通常通过氯乙腈和三氯乙酸的反应制备。
首先将氯乙腈和三氯乙酸在二氯甲烷中反应,再加入三甲胺,使用活性碳吸附,最后蒸馏得到3-氯-3-(二氯甲基)丙烯。
2. 3-(二氯甲基)-7-氟-1,3-二氢-5-(4-甲基-1-哌啶基)-2H-1,4-苯并二氮在-3-酮(3-(Dichloromethyl)-7-fluoro-1,3-dihydro-5-(4-methyl-1-piperazinyl)-2H-1,4-be nzodiazepin-2-one)以上述3-氯-3-(二氯甲基)丙烯为起始原料,通过串联反应得到3-(二氯甲基)-7-氟-1,3-二氢-5-(4-甲基-1-哌啶基)-2H-1,4-苯并二氮在-3-酮。
将3-氯-3-(二氯甲基)丙烯和对氨基苯甲酮在三甲苯中加热反应,生成中间体,再用氢醌处理,发生串联反应,得到目标产物。
3. 盐酸倍他司汀通过将3-(二氯甲基)-7-氟-1,3-二氢-5-(4-甲基-1-哌啶基)-2H-1,4-苯并二氮在-3-酮与盐酸的反应,得到盐酸倍他司汀的合成。
二、盐酸倍他司汀的合成综述盐酸倍他司汀的合成工艺包括多个中间体的合成步骤,其合成路线如下:以上合成路线中间体的合成步骤相对繁琐,需要多步反应和纯化过程,针对每个中间体的合成过程进行优化是十分重要的。
CAS号847871-99-2_分子量_MedBio使用方法
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11283
雷特格韦钾盐
Raltegravir potassium salt
871038-72-1
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11246
NQDI-1
NQDI 1
175026-96-7
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11205
依达奴林RG7388129705-06-950mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11201
4-[[(4S,5R)-4,5-双(4-氯苯基)-4,5-二氢-2-[4-甲氧基-2-(1-甲基乙氧基)苯基]-1H-咪唑-1-YL]羰基]-2-哌嗪酮
CAS
1、Lenalidomide (hemihydrate)物理参数:
常用名
来那度胺半水合物
英文名
Lenalidomide (hemihydrate)
CAS号
847871-99-2
分子量
268.27
密度
无资料
沸点
无资料
分子式
C13H14N3O3.5
熔点
无资料
闪点
无资料
2Lenalidomide (hemihydrate)、技术资料:
高效液相色谱法测定注射用美罗培南的有关物质

第35卷第3期 长治医学院学报2021 年 6 月JOURNAL OF CHANGZHI MEDICAI COLLEGE167Vol. 35 No. 3Jun. 2021高效液相色谱法测定注射用美罗培南的有关物质李金格禹玉洪**作者单位山西医科大学药学院药剂教研室(030001)* 通信作者(E-mail :3024546064@ qq. com)摘要目的:探讨优化注射用美罗培南杂质A 、B 的测定方法。
方法:运用高效液相色谱法(HPLC) 进行检测,色谱柱以十八烷基硅烷键合硅胶为填充剂;流动相A :20. 0 mmol-L'1磷酸二氢钠-甲醇(89 :11, V/V),流动相B :甲醇,流速1.0 mL-min 1,检测波长220 nm,柱温30 P 。
结果:主成分峰与杂质峰可实现基线分离,杂质A 检测限和定量限分别为1. 62,5.15 ng,杂质B 检测限和定量限分别为0. 85,2. 51 ng ;1.2~24.0 ixg-mL -1的杂质A 具有良好的线性关系(r=0. 999 9) ,0.7-14.0 ixg-mL 1的杂质B 具有良好的线性 关系(r=0. 999 9);杂质A 平均加样回收率为101.2%(RSD= 1.38%,“ = 9),杂质B 平均加样回收率为100.2%(RSD=1.29%,n = 9)o 经破坏性试验,美罗培南可能的降解杂质A 、B 均不干扰美罗培南主峰的测定。
结论:检测限及定量限、精密度、稳定性、耐用性试验结果均符合HPLC 有关物质测定的方法学验证要求。
本HPLC 法专属性良好,可用于美罗培南的主要杂质A 、B 的定量控制。
关键词美罗培南;有关物质;高效液相色谱法中图分类号R97&1文献标识码 A 文章编号1006(2021)03-167-05Determination of Related Substances of Meropenem for Injection by High Performance Liquid ChromatographyLI Jinge , YU YuhongDepartment of Pharmacy , School of Pharmacy , Shanxi Medical UniversityAbstract Objective : To explore and optimize the determination method of impulity A andB of meropenem for injection. Meth ods :Using the high performance liquid chromatography ( HPLC ) to detection , Octadecylsilane-bonded silica gel was used as the fi ler ; The mobile phase A : 20. 0 mmol * L -1 sodium dihydrogen phosphate-methanol ( 89 : 11, V/V) . The mobile phase B : methanol ,the flow rate was 1. 0 mL *m in _1 and the detection wavelength was set at 220 nm. The column temperature was set at 30 % . Re sults :The principal component peak and impurity peak could achieve baseline separation. The detection limit and quantitative limit of impurity A were 1. 62 ng and 5. 15 ng respectively , and the detection limit and quantitative lim 让 of impurity B were 0. 85 ng and 2. 51 ng respectively. There was A good linear relationship between impurity A (r = 0. 999 9) and impurity B ( r= 0. 999 9 ) in therange of 1. 2-24. 0 |xg *m L _1 and 0. 7 ~ 14. 0 jig * mL -1. The average recovery of impurity A was 101. 2% ( RSD = 1. 38% , n = 9),and that of impurity B was 100. 2% ( RSD = 1. 29% , n= 9). After stressing test, both of impurities A and B of meropenem didn * tinterfere w 让h the determination of meropenem main peak. Conclusion : The test results of detection lim 让 and quant N ative lim 让,pre cision ,stabil 让y and durability all meet the methodological verification requirements of HPLC related substance determination. The HPLC method has good specificity and can be used for the quant N ative control of major impurities A and B.Key words meropenem ; related substances ; HPLC注射用美罗培南(Meropenem, C ”H25弘0申) 是由日本住友制药公司与英国ICI 制药公司共同 开发的第二代碳青霉烯抗生素,通过干扰细菌细胞壁的合成发挥杀菌作用,具有广谱耐酶的特 点[1_4]o 在美罗培南原料中常检测出杂质A 及杂质B,杂质A(C 17H 27N 3O 6S)为美罗培南四元内酰 胺环结构发生水解反应而形成,系美罗培南的降 解产物;杂质B(C 34H 50N 6O 10S 2)为美罗培南与杂质A 发生聚合反应而形成,系美罗培南的二聚体X 。
Dasatinib_hydrochloride_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Dasatinib hydrochloride is a potent and dual Abl WT /Src inhibitor IC 50 of 0.6 nM/0.8 nM respectively; also inhibits c–Kit WT /c–Kit D816V with IC 50 of 79 nM/37 nM.IC50 & Target: IC50: 0.6 nM/0.8 nM (Abl WT /Src)[1]IC50: 79 nM/37 nM (c–Kit WT /c–Kit D816V )[2]In Vitro: Dasatinib potently inhibits wild–type Abl kinase and all mutants except T315I over a narrow range (IC 50≤1.7 nM). Dasatinib (IC 50: 0.8 nM) displays 325–fold greater potency compared with Imatinib against cells expressing wild–type Bcr–Abl in Ba/F3 cells [1].In Vivo: Daily treatment with Dasatinib (50 mg/kg) is initiated on day 10. Using this approach, a significant inhibition of BCPAP orthotopic tumor growth is observed 6 days after treatment (day 16, P=0.014), which is sustained through days 23 and 29(P=0.0003), compared with vehicle–treated mice [3]. Metabolism studies of Dasatinib (50 mg/kg) in rat suggested that Dasatinib is the major circulating component, whereas multiple metabolites contributed to the remaining 40–60% of the sample radioactivity at 4 h post dose [4].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Kinase assays using wild–type and mutant glutathione S–transferase (GST)–Abl fusion proteins (c–Abl amino acids 220–498) are done with minor alterations. GST–Abl fusion proteins are released from glutathione–Sepharose beads before use; the concentration of ATP is 5 μM. Immediately before use in kinase autophosphorylation and in vitro peptide substrate phosphorylation assays, GST–Abl kinase domain fusion proteins are treated with LAR tyrosine phosphatase. After 1–hour incubation at 30°C, LAR phosphatase is inactivated by addition of sodium vanadate (1 mM). Immunoblot analysis comparing untreated GST–Abl kinase to dephosphorylated GST–Abl kinase is routinely done using phosphotyrosine–specific antibody 4G10 to confirm complete (>95%)dephosphorylation of tyrosine residues and c–Abl antibody CST 2862 to confirm equal loading of GST–Abl kinase. The inhibitor concentration ranges for IC 50 determinations are 0 to 5,000 nM (Imatinib and AMN107) or 0 to 32 nM (Dasatinib). The Dasatinib concentration range is extended to 1,000 nM for mutant T315I. These same inhibitor concentrations are used for the in vitro peptide substrate phosphorylation assays. The three inhibitors are tested over these same concentration ranges against GST–Src kinase and GST–Lyn kinase.Cell Assay: Dasatinib is dissolved in DMSO (10 mM) and stored, and then diluted with appropriate media before use [1].[1]Ba/F3 cell lines are plated in triplicate and incubated with escalating concentrations of Imatinib, AMN107, or Dasatinib for 72 hours.Proliferation is measured using a methanethiosulfonate–based viability assay (CellTiter96 Aqueous One Solution Reagent). IC 50 and IC 90 values are reported as the mean of three independent experiments done in quadruplicate. The inhibitor concentration ranges for IC 50 and IC 90determinations are 0 to 2,000 nM (Imatinib and AMN107) or 0 to 32 nM (Dasatinib). The imatinibconcentration range is extended to 6,400 nM for mutants with IC 50>2,000 nM. The Dasatinib concentration range is extended to 200Product Name:Dasatinib (hydrochloride)Cat. No.:HY-10181A CAS No.:854001-07-3Molecular Formula:C 22H 27Cl 2N 7O 2S Molecular Weight:524.47Target:Src; Bcr–Abl; Autophagy Pathway:Protein Tyrosine Kinase/RTK; Protein Tyrosine Kinase/RTK;Autophagy Solubility:DMSO: 15 mg/mLnM for mutant T315I.Animal Administration: Dasatinib is prepared in 80 mM sodium citrate buffer, pH 3.0 (Mice)[3].Dasatinib is prepared in 0.5% methylcellulose (Rat)[4].[3][4]Mice[3]Male athymic nude mice (25 grams; 5–week old) are used. Dasatinib (50 mg/kg) is prepared for daily oral gavage (5 d/wk) in 80 mM sodium citrate buffer, pH 3.0. For the orthotopic murine model, mice are randomized on day 10 based on bioluminescence activity to receive drug or vehicle. In the metastatic murine model, mice receives dasatinib or vehicle, as described earlier, starting 2 days before intracardiac injection (pretreatment), or on day 11 following randomization (posttreatment).Rat[4]Dasatinib is dosed to male Wistar–Han (WH) rats at 50 mg/kg orally in 0.5% methylcellulose. The automated rat blood collection device is programmed to collect 200 μL of blood at predetermined intervals. At each time point, the accusampler is programmed to directly spot 20 μL of blood twice (two spots) onto the DBS card. The remaining 160 μL of liquid blood is collected into sodium EDTA–containing tubes. Plasma samples are obtained after immediate centrifugation of blood at 11,000 rpm for 5 min. The plasma samples are stored at -80°C until analyses. The DBS samples are dried under room temperature for a minimum of 2 h and stored in a plastic bag in the dessicator until sample analysis.References:[1]. O'Hare T, et al. In vitro activity of Bcr–Abl inhibitors AMN107 and BMS–354825 against clinically relevant imatinib–resistant Abl kinase domain mutants. Cancer Res. 2005 Jun 1;65(11):4500–5.[2]. Shah NP, et al. Dasatinib (BMS–354825) inhibits KITD816V, an imatinib–resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006 Jul 1;108(1):286–91.[3]. Chan CM, et al. Targeted inhibition of Src kinase with dasatinib blocks thyroid cancer growth and metastasis. Clin Cancer Res. 2012 Jul 1;18(13):3580–91.[4]. Shen Z, et al. Metabolite profiling of dasatinib dosed to Wistar Han rats using automated dried blood spot collection. J Pharm Biomed Anal. 2012Aug–Sep;67–68:92–7.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
非诺贝特降胆固醇机制的研究进展

非诺贝特降胆固醇机制的研究进展翟婷;李世云;黄华安【摘要】非诺贝特是第三代苯氧芳酸衍生物调脂药,是目前市场上常见的甘油三酯(TG)调节剂之一,临床常与他汀联合用于调节血脂和降低心血管剩余风险.临床试验证明其除了具有确切的降低TG、低密度脂蛋白胆固醇(LDL-C)和载脂蛋白B(apo B)的作用外,还具有降低胆固醇(CHO)的作用,但针对其降低CHO的机制报道较为少见.该文从非诺贝特对CHO的合成、吸收、转运及其影响胰岛素敏感性等方面的作用调节机制进行综述.【期刊名称】《海南医学》【年(卷),期】2018(029)024【总页数】4页(P3526-3529)【关键词】非诺贝特;胆固醇;机制【作者】翟婷;李世云;黄华安【作者单位】遵义医学院,贵州遵义 563003;遵义医学院,贵州遵义 563003;成都大学附属医院,四川成都 610081;成都大学附属医院,四川成都 610081【正文语种】中文【中图分类】R972+.6在战胜动脉硬化所导致的心血管风险征程中,他汀类通过降低低密度脂蛋白胆固醇(LDL-C)降低动脉粥样硬化实现降低心血管风险已经成为共识[1]。
随着他汀类药物降低心血管主要风险达到一个较好状态后,心血管剩余风险越来越被重视[2-3]。
在心血管剩余风险认识的过程中,以非诺贝特为代表的贝特类药物逐步被更多地在研究和临床实践中被关注[1,4],但其具体机制尚不明了,本文拟对非诺贝特对胆固醇代谢的调节作用进行综述。
1 非诺贝特抑制胆固醇的合成乙酰辅酶A从头合成内源性胆固醇是体内胆固醇的主要来源之一,HMG-CoA还原酶(3-羟基-3-甲基戊二酰辅酶A还原酶,HMGR)为合成过程中的限速酶。
Schneider等[5]对纳入使用非诺贝特治疗8周以上的4例Ⅱa型和6例Ⅱb型高脂血症患者进行研究。
正式进入实验阶段时开始停用非诺贝特治疗,时间为8周;8周后再次恢复非诺贝特治疗,分别测定第0周、8周、22周、33周时患者胆固醇及单核细胞HMGR水平。
双能X线骨密度测定法联合骨转换标志物定量法在绝经后骨质疏松诊断中的临床应用价值

双能X线骨密度测定法联合骨转换标志物定量法在绝经后骨质疏松诊断中的临床应用价值发布时间:2021-06-01T07:19:07.245Z 来源:《健康世界》2021年4期作者:王小雨赵诗瑶耿静陈星雅杨赫男[导读] 目的探索双能X线骨密度测定法联合骨转换标志物对女性绝经后骨质疏松的诊断价值。
方法 2019年1月-2020年9月我院收治的100例绝经后女性患者,分别于初诊、初诊后6个月、12个月,用电化学发光仪检测绝经后患者血液中I型胶原氨基端延长肽(PINP)和β-胶原特殊序列(β-CTX)、N-骨钙素(N-MID)、甲状旁腺激素(PTH)、25羟维生素D[25(OH)D3]含量变化,双能X线骨密度仪检测绝经后患者1-4腰椎的骨密度值。
王小雨赵诗瑶耿静陈星雅杨赫男齐齐哈尔医学院附属第三医院黑龙江齐齐哈尔 161006摘要:目的探索双能X线骨密度测定法联合骨转换标志物对女性绝经后骨质疏松的诊断价值。
方法 2019年1月-2020年9月我院收治的100例绝经后女性患者,分别于初诊、初诊后6个月、12个月,用电化学发光仪检测绝经后患者血液中I型胶原氨基端延长肽(PINP)和β-胶原特殊序列(β-CTX)、N-骨钙素(N-MID)、甲状旁腺激素(PTH)、25羟维生素D[25(OH)D3]含量变化,双能X线骨密度仪检测绝经后患者1-4腰椎的骨密度值。
结果与初诊时相比,初诊后6个月、12个月绝经后患者同节段腰椎骨密度值持续降低。
初诊后6个月、12个月绝经后患者血液PINP、β-CTX、N-MID、PTH、D[25(OH)D3]水平均无明显变化。
绝经后女性一年内骨密度值变化情况与骨转换标志物值呈正相关。
结论双能X线骨密度测定法联合骨转换标志物检测对绝经后骨质疏松诊断及骨密度发展趋势判断具有较高的价值。
关键词:双能X线骨密度;I型胶原氨基端延长肽;β-胶原特殊序列;N-骨钙素;甲状旁腺激素;25羟维生素D;绝经后;骨质疏松 The value of diagnosing perimenopausal osteoporosis by dual-energy X-ray bone mineral density combined with Markers of bone turnover level Wang Xiaoyu;Zhao Shiya;Wang Lili;Shen Jianfei;Ren Lijun;Liu Zhijian;Yao Lan;Geng Jing;Chen Xingya Department of nuclear medicine,Third Affiliated Hospital of Qiqihar Medical College,Qiqihaer,Heilonghjiang,161006 Abstract Objective To explore the value of diagnosing perimenopausal osteoporosis by dual-energy X-ray bone mineral density measurement combined with Markers of bone turnover.Methods From May 2019 to June 2020,100 patients with perimenopausal osteoporosis admitted to our hospital were included in the experimental group,and 100 normal menstruation were included as the control group.The Electrochemiluminescence instrument test was used to detect the changes of blood N-terminal propeptide of typeⅠprecollagen(PINP),β cross-linked C-telopeptide of typeⅠcollagen(β-CTX),N-terminal in the middle osteocalcin(N-MID),parathyroid hormone(PTH)and 25-hydroxy vitamin D3 [(25(OH)D3)] levels in the two groups.The dual-energy X-ray bone densitometer was used to detect the bone mineral density of the 1-4 lumbar vertebrae in the two groups.Realtime Compared with the control group,the peak bone mineral density of the lumbar vertebrae of the same segment in the experiment group is lower(P<0.01).The blood PINP,β-CTX,N-MID,PTH levels in the experiment group were higher than those in the control group(P<0.01);The level [25(OH)D3] in the test group was lower than that in the control group(P<0.01).Conclusion The dual-energy X-ray bone mineral density measurement combined with Markers of bone turnover has a high diagnostic value for diagnosing perimenopausal osteoporosis.Key words Dual-energy X-ray bone mineral density;β-isomerized C-terminal telopeptides(β-CTx);Procollagen type 1 amino-terminal propeptide(tP1NP);Perimenopausal period;Osteoporosis绝经后骨质疏松症的特征是矿物质形成与吸收之间的不平衡,导致净骨矿物质流失,骨脆性增加的常见疾病[1]。
三甲基硅烷衍生化GC—MS研究延胡索中水溶性非生物碱类化学成分

三甲基硅烷衍生化GC—MS研究延胡索中水溶性非生物碱类化学成分[摘要]目的:研究延胡索Corydalis yanhusuo中水溶性非生物碱类化合物。
方法:延胡索的80%乙醇提取物经DA201大孔树脂柱吸附,收集水洗部位,该水洗脱部位再经732阳离子树脂交换,其水洗脱组分干燥后进行三甲基硅烷衍生化。
采用GCMS检测分析,NIST2005标准谱库结合具体衍生物的MS/MS 质谱图进行化合物结构推断。
结果:GCMS共检出约50个色谱峰,初步鉴定了其中的16个化合物,均为含有羟基或羧基的大极性化合物。
结论:16个化合物为首次从延胡索中发现,该研究结果为延胡索的深入开发提供了科学依据。
[关键词]延胡索;水溶性非生物碱类化学成分;三甲基硅烷衍生化;GCMS中药延胡索是罂粟科紫堇属植物延胡索Corydalis yanhusuo W.T.Wang 的干燥块茎,生长于低海拔旷野草地、丘陵、林缘。
分布于陕西,江苏,安徽,浙江,河南,湖北等地。
历代本草均有记载,性味辛、苦、温,归肝、脾经,具有活血、利气、止痛的功效。
主要用于胸胁、脘腹疼痛、经闭、痛经、产后瘀阻、跌扑肿痛等[1]。
文献报道延胡索所含的化合物多为生物碱类化合物,大极性的醇,酸,胺,单糖和低聚糖类等水溶性非生物碱类化合物报道的很少,为了进一步明确延胡索中该类化学成分组成,作者对延胡索80%乙醇提取物的大孔树脂水洗脱部位进行了系统的组分研究。
在前期研究的基础上,水洗脱部位通过732阳离子交换树脂,分别收集水洗脱部分和5%氨水洗脱部分。
这两部分的化学成分研究未见相关报道。
本文对732阳离子交换树脂水洗脱部分采取三甲基硅烷化衍生化,GCMS进行分析,通过与NIST2005标准谱库匹配,结合具体MS/MS 质谱图进行分析的方法,初步鉴定了其中的16个化学成分,并用质谱总离子流峰面积归一化法测定了各成分的相对含量。
1材料GCTPremier GCTOF气相色谱串接质谱仪(Waters公司);DA201大孔树脂(南开大学化工厂);732阳离子交换树脂(沧州宝恩化工有限公司);高纯氮(99.999%)。
CAS220953-69-5_Antalarminhydrochloride_技术资料_MedBio
1mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13430
(R,S)-Atenolol
(R,S)-Atenolol
29122-68-7
10g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12533
Talnetant
Talnetant
174636-32-9
CAS
1、产品物理参数:
常用名
N-丁基-N-乙基-2,5,6-三甲基-7-(2,4,6-三甲基苯基)-7H-吡咯并[2,3-d]嘧啶基-4-胺盐酸盐
英文名
Antalarmin hydrochloride
CAS号
220953-69-5
分子量
415.014
密度
无资料
沸点
无资料
分子式
C24H35ClN4
熔点
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13435
6-OAU
6-OAU
83797-69-7
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12509
NPS-2143 hydrochloride
NPS-2143 hydrochloride
324523-20-8
5mg
≥98%
10mg
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Citalopram (hydrobromide)Catalog No. :HY-B1287CAS No. :59729-32-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:(±)⁻Citalopram hydrobromide; Lu 10⁻171BFormula:C20H22BrFN2OMolecular Weight:405.30CAS No. :59729-32-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
