供应商审核报告(中英文对照)
T.S3-33产品审核报告中英文版(含附属全套EXCEL表)

900
计算总缺陷点数(ΣFP)=发现的缺陷数× 系数;Total fault points (Σ FP) = Number of faults found×Factor; 计算质量特性值(QKZ)=(1-ΣFP/Σn)*100%=(1-总缺陷点数/所有特性已加权的抽样数之和)*100% 计算项次合格率
缺陷级别
Defect Classificatio n
缺陷程度
Severity Level
产品审核缺陷分级 Classification of Product Audit Defects 功能 Function 外观 Appearance 包装 Package 尺寸 Dimension
无法完成装配,产 品不能安装的,顾 客退货或索赔的。
Inspection: Appearance, color, surface states and stamp marking correspond to the requirements specified in the relevant drawings or inspection procedures.
Hale Waihona Puke 300400 500
从图纸和控制计划或检验基准书中选择尺寸。
Select dimensions from the drawings and control plan or inspection standards.
与检具或者对手件试装、匹配等
Match or trial assembly with inspection devices or mating parts
供应商资质调查表中英文

QMP7-4-1-01-00002SUPPLIER FACILITY SURVEY供应商(Supplier):地址(Address):评价日期Date of assessment:电话Phone:传真Fax:调查期间供应商联系人员名单/职务List of personnel contacted during audit / position title.联系人(Contact):职务(Title):蓝托考核小组(姓名及公司名称)Ranto Audit Team (Name & Ranto Facility)姓名(Name):单位/部门(Facility/Department)步骤Process:(1)不是所有问题都需填写. 因观察项(次要的不合格项)与风险项(主要的不合格项)之间有差异,因此考核人员要将考核要素归类并记下其状态。
All questions do not have to be completed. For those discrepancies found that are a "finding" (minorunacceptable condition) or a "risk" (major not acceptable condition); the auditor will rank the element and describe the condition. (2)所有将要被审核的供应商都应被通知(在审核前必须将该调查表传真或发邮件给供应商)In all cases the supplier being audited will be notified.(3)对于加“★”的项目必须要审核到,并且就审核发现做出具体的描述。
To“★”items must be audited and make the detail description of findings。
供应商审核检查表中英文版

Light bulbs, fixtures, skylights or other glass suspended over any ingredient or packaging material exposed in any step of the operation are protected to prevent contamination.
Fixtures, ducts and pipes that drip or form condensation are not suspended over working areas where a potential of contamination may occur.
生产区域上方,设备、管路上的冷凝水、滴漏将是ห้องสมุดไป่ตู้在的污染
无不符合要求的储存:垃圾、废品、未切割的种子或者草,适合昆虫繁殖和鼠类藏匿的场所
There is adequate drainage in all areas to prevent the contamination of components through seepage or foot-borne filth.
在原料和包装材料上方的灯具、天窗以及其他悬挂的玻璃制品,必须有防护
Adequate ventilation or control equipment is provided to minimize odors and noxious fumes or vapors in areas where they may contaminate components
Separation by partition, location or other effective means is provided for those operations that may cause contamination of food products with undesirable microorganisms, chemicals, filth or other materials.
供应商审核表中英文模板

□ Follow-up Audit
跟进审核
□
重新审核 Re-audit
业务员及联系方式 被审核公司所在地 Auditee's location 厂房面积 Floor space 办公面积 Office space 管理人员人数 No. of managerial Staff 销售人数 No. of sales staff 工人数量 No. of workers 打样所需时间 Sample leadtime 主要产品 Major products 常用材料 Frequently used materials 主要设备 Major equipment 主要市场 Major market 自有品牌 Self-owned brands OEM品牌 OEM brands 质量体系 Quality system 特别优势 Competitive advantage 成立于 Established in 生产厂房面积 Manufactural space 总人数 Total No. of employees 研发人数 No. of R&D staff 质检人数 No. of inspectors 旺季时间 Busy season 开模所需时间 Mold leadtime
评估结果 Evaf 2
Audit by Guangzhou Wellrich Co., Ltd.
2 of 2
Audit by Guangzhou Wellrich Co., Ltd.
Supplier Audit
工作单号 Work File No. 审核类型 Audit Type 被审核公司 Auditee Company 被审核公司地址 Auditee's Address 总经理联系方式 报告号 Report No. 审核日期 Audit Date
供应商审核报告
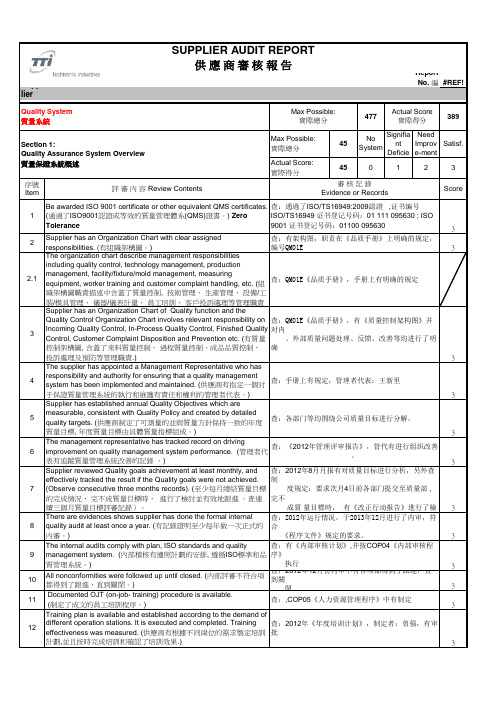
有
15 Quality Cost Control. (質量成本控製.)
有,COP20《质量成本控制程序》,有执行
3
a. Supplier has established detailed quality cost objectives, and
15.1
management has reviewed the achievement at least once a month. (建立了具體的質量成本目標 ,並且管理層至少每月一次評估了質量成
有产品生产许可证,证书号:XK13-0250-00425
3
4
Product performance validation includes customer specific requirements. (產品性能確認包括了客戶明確的要求。)
产品试验标准中(JS-WI-112A)明确参照客户要求及
国家标准
57
No System
Signifia nt
Deficie ncy
Need Improv e-ment
Satisf.
50
0
1
2
3
序號 Item
評 審 內 容 Review Contents
審核記錄 Evidence or Records
Score
There is a documented plan for new product development and also 1 for any change of it. (對全新產品或重大更改產品的開發和行動前, 以 产品开发按COP09《产品开发控制程序APQP》执行
查:,COP05《人力资源管理程序》中有制定
3
AO史密斯 供应商质量体系审核表(中英文)
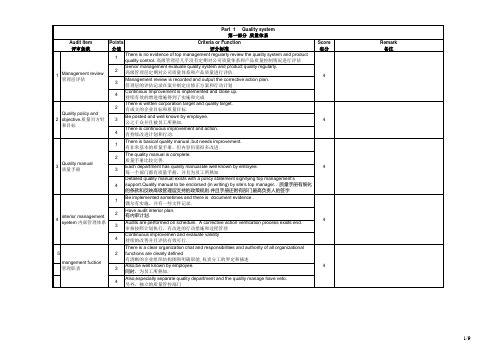
Points 分值Criteria or Function评分标准Score实分1There is no evidence of top management regularly review the quality system and product quality control. 高级管理层几乎没有定期对公司质量体系和产品质量控制情况进行评估2Senior management evaluate quality system and product quality regularly.高级管理层定期对公司质量体系和产品质量进行评估.3Management review is recorded and output the corrective action plan.管理层的评估记录在案并制定出修正方案和行动计划4Continious Improvement is implemented and close up.持续有效的增进措施得到了实施和完成2There is written corporation target and quality target.有成文的企业目标和质量目标.3Be posted and well known by employee.公之于众并且被员工所熟知.4There is continuous improvement and action.有持续改进计划和行动.1There is basical quality manual ,but needs improvement.有非常基本的质量手册,但内容仍需很多改进.2The quality manual is complete.质量手册比较完善.3Each department has quality manual.Be well known by emloyee.每一个部门都有质量手册,并且为员工所熟知4Detailed quality manual exists with a policy statement signifying top management's support.Quality manual to be enclorsed (in writing) by site's top manager.. 质量手册有细化的条款和反映高级管理层支持的政策规则.并且手册还附有部门最高负责人的签字1Be implemented sometimes and there is document evidence .偶尔有实施,并有一些文件记录.2Have audit interior plan.有内审计划.3Audits are performed on schedule. A corrective action verification process exists end.审核按照计划执行,有改进的行动措施和过程管理4Continuous improvemen and evaluate validity 持续的改善并且评估有效可行.52There is a clear organization chat and responsibilities and authority of all organizational functions are clearly defined有清晰的企业组织结构图和明确职能,权责分工的界定和描述3Also,be well known by employee.同时,为员工所熟知.4Also,especially separate quality department and the quality manage have veto.另外,独立的质量管控部门mangement fuction 管理职责4 4 4Quality manual 质量手册434interior managementsyetem 内部管理体系4Remark备注Management review管理层评估1Audit Item评审条款Quality policy and objective.质量目方针和目标2Points 分值Criteria or Function评分标准Total Points 实得分2There are written procedures for development system with responsibilities assigned.有明确责任的产品开发体系规则.4Including design review and validate.包括了设计评估和验证6There is evidence for implement.有显示表明对以上内容进行了实施.8Including feasibility,apqp and validate.同时还包括了可行性评审、先期评审和设计验证等1New product has been inspected for each item(appearance,size,performance etc.) .Haveinspecting record. 对新产品进行了各项检测(外观、尺寸、性能等),有检测报告.2New product has been inspected for each item(life,material,reliability etc.)Have completerecord. 对新产品进行各项性能测试(寿命、材料、可靠性等等),有完整的记录3Providing complete quality guarantee report.there is identical inspecting result .能提供完整的质量保证报告,并与检测结果一致4There is feasible production flow and quality control plan.制定了可行的产品流程和质量控制计划2Have experiential empoyee for similar product.具有类似产品开发经验的人员配置.3有关于人员任职资格的文件和人员认聘资料记录4Leading design sample and employee.具有行业领先的设计样本和设计人员2Adopt cad,ca and caq.采用了计算机辅助设计(CAD )、制造(CAM )和质量管理(CAQ )4Software and hardware satisfy our product requirement.软件和硬件能够满足我们的产品需求.2Can provide sample in short time.能在短期内提供样品3have reliability and life test ability for sample.对样品有可靠性和使用寿命等性能等方面的测试能力4Satisfactory delivery.理想的交付Software &hardware 软件和硬件providing sample 样品提供Audit Item 评分条款product development 产品开发product approvement 产品许可design employee 设计人员资格Remark 备注12345Audit Item 评分条款Points 分值Criteria or Function评分标准Total Points 实得分2developed设计了工艺流程图4catched by employees 员工能够掌握6been accurately reflect process flow 准确地反映了制造流程8even there are proceduresfor notification of customer for process material changes,changes in manufacturing locations,quality problems. 不仅如此,还制定了诸如在材料,生产地点变更后以及出现质量问题时的客户通知程序和制度2developed制定了作业指导书3showed on the manufacture-line and catched by employees 作业指导书展示在生产线上,并被作业人员所掌握4all documents show evidence of Control and are latest revisions.employee act according these totally. 所有的文件都能起到有效的控制作用并保持最新的校订版本,员工完全能够按照指导进行操作1have factory management system ,such as 5S.有诸如5S 的管理体系3gauges and inspection equipment are available on changeless place and accessible to operatiors and inspectors.量具和检测设备都放置在易于操作人员和检测人员接触到的固定的位置4preventive actions system to deal with gusty matter,such as machine undoing,toolvanishing,compo. 制定了预防诸如机器失效,工具vanish,compo等紧急情况的行动指南1there are different sign on the qualified product,unqualified product,and container.有区别地对合格品、不合格品及其容器具进行了标识2there are written procedures for dealing with unqualified product,such as rework, accept in particular, scrap, and acting actually.订立了对不合格品的处理规则(如返工、另作他用、直接报废等), 并在现实中执行3statistical methods are used for improvement activities, i.e. Pareto Analysis, Control Charts,Bar Charts, Line Graphs.对不合格品进行统计分析4corrective actions are taken to reduce scrap and rework. evidence is shown.有根据表明企业采取了有效的改进措施来降低报废率和返工率84343123Process Flow Charts 工艺流程图Work instruction 作业指导书Factory Management 工厂管理Control of non-conforming 不合格品控制Remark 备注Audit Item 评分条款Points 分值Criteria or Function评分标准Total Points 实得分1client's property are properly remarked and putted orderly.对顾客的资产进行了正确的标注并摆放有序2storehouse's circumstance is adopt to client property's characteristic.仓储环境符合客户资产的存放要求3maintain client's property termly.定期对客户资产进行保养4there are written procedures for storage, including all action above.制定了包含上述所有行动内容的库存规章制度1documented inspection procedures are available for inspectors that include identification of critical characteristics.供检验员参考的检验程序有案可查,并且文档对重要特性进行了特别的定义2these written procedures are easily get and understanded by employee.检验程序和标准容易被员工获取和掌握3inspection circumstance,such as light, temperature,noise, humidity are proper.检验环境符合条件(如光线、温度、噪音、湿度等)4documented inspection procedures should include identification of criticalcharacteristics,tolerance,mark and packing.检验程序和标准必须包含重要特性、tolerance 、标识、包装等内容2documented equipment maintain record 有设备维护记录档案4there are written procedures for equipment maintain.all action shall be done.制定了设备维护保养规程并有效地执行了2there are procedures for checking for process changing in manufacture, such as processmaterial changes, changes in manufacturing locations, quality problems. 制定了生产过程变更的检查规程,比如生产原料变更,制造地点变更以及质量问题等等3documented register of testing and aproving for the process changes shall be done before the process update. 在生产过程更新前已经对生产变更的测试和许可进行了记录和存档4there are procedure for notification and approbation of customers before the process update.制定了在生产过程更新前的客户通知和客户许可规程2written employee manual, post responsibility, employee training record.有成文的员工手册、岗位职责和岗位培训记录3personel configure chart and fill-in were confirm.已确认了人员配置和上岗计划4effective assessing and encouragement procedures for employee's action 制定了有效的员工考核和奖励制度4445employee 作业人员Remark 备注44Protection of client's property 顾客资产的保护Inspecte process 检验过程equipment maintain 设备维护process changes in manufacture 生产工艺变更6789。
供应商审核报告(中英文对照)

Year of foundation 贵司成立年份:Annual turnover 年销售额:Number of Employees 职工总人数:Sales department 销售部:Production 生产部:Finances 财务部:Quality assurance 品控部:Supplied product 供应产品:Complied standard 产品符合标准:Shelf life 保质期:Export countriy for this product 产品出口国:Compared with other factory, advantage of your factory and product 与同行相比,贵司产品的优势:Production flow chart or simple description(please attach a copy) 生产流程,请简单描述或附工艺流程图:Zip Code 邮编:3. QUESTIONNAIRE 问卷2. PRODUCT INFORMATION 产品信息Manufacture capability 生产能力:Other product 其他可供应产品:Company LogoSUPPLIER AUDIT REPORT供应商审核报告Address 地址:1. SUPPLIER DATA 供应商信息Supplier name 供应商名称:Guidlines for filling 填写指南1- The porpose filling of this questionnaire is to collect information about the Quality System of supplier. 填写该问卷的目的是了解供应商质量控制体系。
2- Mark with (X ) the alternative that matches replies to your answer. Please do not leave any question without answer. If necessary make observations. 在符合项下画“X ”,请回答所有问题,如需说明请备注。
供应商自我验货检查报告英文版

1
2
Defect Allow
0
Defect Found
Product Item Code
Defect Breakdown/Remark
Cri.
Maj.
Min.
1
2
Defect Allow
0
Defect Found
Product Item Code
Defect Breakdown/Remark
Actual Product with GS
Problematic Remark( if applicable)
Informative Remark( if applicable)
Defect (Cri / Maj / Min)
Defect (Cri / Maj / Min)
Ⅸ. PHOTO REFERENCE – ONSITE TESTING(Note: illustrateone type test once)
1) FOB price byond 10 ponds.
2) the sell package size lower 60cm in height X 40cm in depth X 40cm in Width, or heavy box weight lighter 20kg.
Ⅶ. PHOTO REFERENCE(Note: supplier can fine tunetemplateto fit the actual situation)
General test
Sample Size
Result
Comments
1
Packaging Check
2Pcs/item
Item No
审核指南中英文对照版

International Organization for Standardization International Accreditation ForumDate: 21 September 2004 ISO 9001 Auditing Practices GroupThe ISO 9001 Auditing Practices Group is an informal group of quality management system (QMS) experts, auditors and practitioners drawn from the ISO Technical Committee 176 Quality Management and Quality Assurance (ISO/TC 176) and the International Accreditation Forum (IAF).It has developed a number of guidance papers and presentations (see "QMS auditing topics" below) that contain ideas, examples and explanations about the auditing of QMSs. These reflect the process-based approach that is essential for auditing the requirements of ISO 9001:2000 Quality management systems - Requirements.The guidance is primarily aimed at QMS auditors, consultants and quality practitioners, but is not definitive. The papers and presentations reflect a number of different views in QMS auditing. As such, their content may not always be consistent. It is not intended that the guidance will be used as specified requirements, an industry benchmark, or as criteria that all QMS auditors, consultants or practitioners have to follow.QMS auditing topics•The need for a 2-stage approach to auditing 2阶段审核的必要性•Measuring QMS effectiveness and improvements测量QMS的有效性及其改进•Identification of processes 过程的识别•Understanding the process approach 过程方法的理解•Determination of the “where appropriate” processes 确定”适当的”过程•Auditing the “where appropriate” requirements审核”适当的”要求•Demonstrating conformity to the standard 证明符合标准•Linking an audit of a particular task, activity or process to the overall system•Auditing continual improvement 审核持续改进•Auditing a QMS which has minimum documentation审核QMS文件化的最低要求•How to audit top management processes 如何审核最高管理层•The role and value of the audit checklist 审核检查表的角色和价值•Scope of ISO 9001:2000, Scope of Quality Management System and Defining Scope of Certification ISO9001:2000的范围,质量管理体系的范围和定义认证范围•How to Add Value during the audit process审核过程如何增值•Auditing competence and the effectiveness of actions taken审核能力要求和采取措施的有效性•Auditing Statutory and Regulatory requirements审核法规和指令要求•Auditing the Quality Policy andQuality Objectives审核质量方针和质量目标•Auditing ISO 9001, Clause 7.6 Control of monitoring and measuring devices审核ISO9001, 7.6条款监视和测量装置的要求•Making effective use of ISO 19011ISO19011的有效应用•Auditing Customer Feedback processes审核顾客反馈过程•Documenting a Nonconformity文件化不符合项•Guidance for reviewing and closing nonconformities评审和关闭不符合项指南•Auditing Internal Communications审核内部沟通•Auditing Preventive Action审核预防措施•Auditing Service Organizations审核服务组织•Auditing the Effectiveness of the Internal Audit审核内部审核的有效性•Auditing Electronic Based Management Systems审核电子管理体系A "Zip" file of all the above documents is also available. 可获得以上文件的ZIP档。
供应商审核报告

Supplier Quality System Audit项次No项目 Item单项最高分Highest Score实际得分Real Score评鉴合格率Qualified Rate单项目标Target%质量系统Quality System Requirement设计管理Design Control文件管理与品质记录Document Control & Quality Record 供应商管理Supplier Managemant产品识别与追朔Product Identification and Lot Traceability 检验与测量Inspection and Test仪器校验Equipment Calibration制程控制Process Control不合格品控制Nonconforming Product Control仓库管理Warehouse Management生产排程管理Production Schedule合同评审Contract Review采购 PurchasingRoHS管理 RoHS management缺失的分布Defect Distribution Chart综合品分 Total评分标准 Standard有文件且执行良好Document and implementation are well-done有文件,绝大部份确实执行,少部份未执行Document ok but implementation with small part有文件,少部份执行,绝大部份未执行Document ok but implementation with most part无文件,亦无执行NO document and no implementation质量系统Quality System设计管理Design Control文件管理与品质记录Document Control & Quality Record供应商管理Supplier Managemant产品识别与追朔Product Identification and Lot Traceability检验与测量Inspection and Test仪器校验Equipment Calibration制程控制Process Control不合格品控制Nonconforming Product Control仓库管理Warehouse Management生产排程管理Production Schedule合同评审Contract Review采购Purchasing评鉴合格率Qualified Rate确保质量政策让组织内所有阶层了解并展开为各部门目标,从而实施推行Assuring the quality policy be understood by every member of the orgnization and work together to realize the target.客户满意度与质量目标之间,具有相关的客观资讯予以证实There is relative objective information to testify customer satisfaction and quality tare.从事质量管理、执行、验证人员,均需明文定义其责任、授权与相互关系Personal responsibility, authorization, inter-relationship should be explicitly identified a mong thosemembers responsible for quality management, policy carrying out and quality testing.指派管理代表之其中一员,完全授权推展质量系统运作并不受其它职务责任影响,并定期向管理阶层报告以供审查Appoint one member and fully qualify him to carry out quality system and report to supe rvisors periodically.质量手册应定义各项书面程序以符合质量系统与质量政策需求,并对于文件架构予以概要说明The quality handbook should include a written process document so as to conform to th e quality system and policy, and give a general instruction to the document frame.先期产品的管制计划应包括原型样品、试产、量产三个阶段(QC工程图)Procduct control plans should include three process: sample run, pilot Run and mass p roduction(QCflow chart).订定管制计划检讨、更新与确认程序,同时可依照程序提供客户产品管制状况。
供应商审核报告表中英文对照版

表格编号form code:A-PQEO-15/1-5/03Page页: 2Supplier/供应商: Date日期: QM audit/体系审核a = Requirements are fully satisfied/完全满意*) Please enclose copy附上复印件b = Requirements are satisfied with deviations/基本满意,但稍有偏差c = Requirements are not satisfied/不满意n.a. = Not applicable/不适用a = Requirements are fully satisfied/完全满意*) Please enclose copy附上复印件b = Requirements are satisfied with deviations/基本满意, 但稍有偏差c = Requirements are not satisfied/不满意n. a. = Not applicable/不适用a = Requirements are fully satisfied/完全满意*) Please enclose copy附上复印件b = Requirements are satisfied with deviations/基本满意,但稍有偏差c = Requirements are not satisfied/不满意n. a. = Not applicable/不适用Page页: 3Supplier供应商: Date日期:表格编号form code:A-PQEO-15/1-5/03a = Requirements are fully satisfied完全满意b = Requirements are satisfied with deviations/基本满意,但稍有偏差c = Requirements are not satisfied/不满意n.a. = Not applicable/不适用b = Requirements are satisfied with deviations/基本满意, 但稍有偏差c = Requirements are not satisfied/不满意n. a. = Not applicable/不适用b = Requirements are satisfied with deviations/基本满意,但稍有偏差c = Requirements are not satisfied/不满意n. a. = Not applicable/不适用Page页: 5Supplier/供应商: Date日期:EHS audit/环境健康安全审核表格编号form code:A-PQEO-15/1-5/03a = Requirements are fully satisfied/完全满意*) Please enclose copy附上复印件b = Requirements are satisfied with deviations/基本满意, 但稍有偏差c = Requirements are not satisfied/不满意n.a. = Not applicable/不适用Action plan Enclosure for audit conducted on:Signature Supplier (corrective actions started): ___________________表格编号form code:A-PQEO-15/1-5/03。
供应商调查表(中英文对照)

质量负责人Quality supervisor:
手机M.b.:
电话Tel:
邮箱E-mail:
销售负责人Sales supervisor:
手机M.b.:
电话Tel:
邮箱E-mail:
职工总数workforce total________:技术人员technical personnel________;管理人员management personnel________;生产工人production personnel________,检验人员inspection personnel________ 厂房面积Workshop area:
认证情况Certifications: □UL □VDE □EMC
□ISO9001
□TUV
□ISO14001
□CCEE
□CE
□其它other 技术与质量特点Technology and Quality Features:
主要生产工艺流程Main production process :
主要原料名称及检验标准Main raw materials and inspection standards :
将直接影响我 联系
,并在附页上
别审核、签字
review the nair is made.It comages:8
classes(8H) Day trips
□是YES
□否NO
月产量
Monthly production
原料采购周期
material purchase Production cycle time cycle time
生产周期
三、主要生产设备和检测仪器 Main production equipment and instrumentation (一)、主要生产设备 Main production equipment 名称 Model 规格 Spec. 数量 Q.T 生产哪类配件 what accessories production 单班产量
供应商评审中英文对照

GCLSolar Energy保利协鑫太阳能SUPPLIER :供应商Assessment Team :供应物名称ASSESSMENT DATE: 供应时间SARSupplier AuditReport供应商审核报告SUPPLIER ASSESSMENTGUIDELINE供应商评估指南2010SUPPLIER ASSESSEMENTMANUFACTURING制造类供应商评估INTRODUCTION引言GCL Solar Energy is committed to product excellence in the markets we serve. We intend to continuously demonstrate this commitment by providing defect-free products on time and at competitive prices.协鑫光伏能源致力于对产品精益求精,在我们服务的市场,我们将不断证明这一承诺,并提供有竞争力的价格,无缺陷的产品。
This requires that we exercise every possible means to assure quality and consistent on-time delivery of purchased goods, which in-turn, contributes to continuous Quality improvement. Through the application of Statistical Process Control (SPC) combined with the cooperation and commitment of our Suppliers and Supply Chain Teams, GCL Solar Energy will drive continuous improvement both in Technical and Commercial aspects of business.这就要求我们千方百计保证质量和购进一致好的部件,不断提高服务质量。
可口可乐供应商审核报告(中英对外)

CONFIDENTIALIngredient Supplier Audit ReportSummary of AuditAudit ScopeThis plant audit is a part of the Coca-Cola supplier authorization program. The purpose of this visit is to assess the capability of the supplier to deliver products according to the Coca-Cola standards / specifications and to build confidence on the quality of the supplied goods. The detailed audit scope includes:Production and warehousing operation for Orange, Kumquat and sugar cane juice concentratesAudit AttendeesConclusionThe facility receives an overall rating of Meets Requirements with Conditions for Kumquat Juice, Orange Juice and sugar cane Juice Concentrates.本厂获得“条件性通过”,此评级针对橘汁,橙汁和甘蔗浓缩汁This rating is based on the assessment of compliance to requirements, as stated in the Supplier Expectations Brochure, the General and Specific Audit Modules, US Juice HACCP 120 and US Bioterrorism laws.Corrective Action responseA correction action response to the findings noted in the report is due back to the auditor by March 3, 2008所有的在报告中的整改措施需要在零八年三月三日前反馈给审核员Comments∙The Jan 8, 2008 audit of the facility was TCCC’s second visit to the facility. A technical visit completed in November 2007 highlighted several improvements. As of this audit, all requested improvements have been completed.于一月八日对厂进行第二次审核。
Supplier_Assessment_Report供应商评估报告书(英文)

Potential Supplier Assessment潜在供方评审报告SUPPLIER DATA 供应商资料ASSESSMENT DATA 评审资料Name名称: Date日期:Address地址: Assessed by评审人:Commodity零件类别: Chem化工类 Elec 电器类 Met 金属类Product 产品:Assessment Criteria 评审标准Phone No.: Fax No.传真: Overall Rating Score 总分Supplier Contacts :联系人Position :职位: Overall Rating Result 总结果Comments:评语:Team Leaders’signature’s评审小组组长签字:Assessor Phone No.评审员:RESULTS评审结果** Questions问题Conforms符合Nonconformities不符合PointScoreCorrective Action O/L-DateNo. Element要素Minor轻微Major主要分数纠正措施O/L日期Documentation Review 文件审核All 4.1 Management Responsibility 管理职责 4 4.2 Quality System 质量体系8 4.3 Contract Review 合同评审 1 4.4 Design Control 设计控制 4 4.5 Document and Data Control 文件和资料控制2 4.6 Purchasing 采购 4 4.8 Product Identification and Traceability产品标识及可追溯性1 4.9 Process Control 过程控制 6 4.1Inspection and Testing 检验和试验 64.1 1 Inspection, Measuring and Test Equipment检验,测量和试验设备44.1 3 Control of Nonconforming Product不合格品的控制24.1 4 Corrective and Preventive Action预防和纠正措施54.1 5 Handling, Storage, Packaging and Delivery搬运,储存,包装及交付64.1 6 Control of Quality Records 质量记录的控制14.17Internal Quality Audits 部质量审核 54.18Training 培训 1General Motors Specific 通用汽车具体要求1Total score总分TARGET DATE FOR CORRECTION OF ALL NONCONFORMITIES纠正所有不符合项的目标日期:: ....../....../......Question Scoring问题评分标准Element Scoring要素评分标准分数计算> Supplier not familiar withrequirements of element and has no relevant documentation.>供应商不了解要素的要求并且没有相关文件0> Implementation 80 – 95% complete anddocumented evidence is available.已实施80-95%并且有记录在案的证据可查.6Total Points总分:➢Supplier is familiar withrequirements of element but thereis noevidence/documentation/implement. ➢供应商了解要素要求但没有证据/记录/实施. 1➢Full implementation and confirmedevidence of effectiveness-Supplier metminimum req.➢完全实施并且具有确认实施有效的证据-供应商满足最低要求.7No. ofapplicableelements适用要素数目:➢Supplier is familiarwith requirements ofelement and haspreliminary/draftdocumentation.➢供应商了解要素要求并具有初步/草案性的文件. 2> Analysis of results &continuous improvement can bedemonstrated.有证据表明对实施效果的分析和持续改进8 PointScore分数= Total PointsNo. of applicableelements= Overall Rating:> Documentation is available but implementation is only 0 – 30%complete.➢文件已建立但仅实施了0-30% 3 > Supplier has reached world classperformance and continuous improvement inall areas.供应商达到世界级的表现并在各个方面取得持进.9 分数=总分/合适的要素> Documentation is available andimplementation is 30 – 60% complete. ➢文件已建立并实施了解情况50-60% 4 > Supplier is best-in-class, demonstratesignif. Innovation beyond customerrequirements and sets the industrybenchmark.供应商是同类别中的最佳.证据表明供应商有超出客户要求的显著革新并确立该行业的基准.10➢Implementation 60 – 80%complete and there ispreliminary evidence of relevantresults.➢已实施了60-80%并且已有了相应实施效果的初步证据.5Note:注:(1) To Pass or be Recommended the score must >=7.通过评审或被推荐的要求为至少(7)分.(2)To conditional Pass or be Recommended the score must >=6. All nonconformances must becorrectedbefore PPAP and SQE will verify.有条件通过评审或被推荐的要求为至少(6)分.所有的不符合应在产品PPAP 前得到纠正并为SQE验证。
供应商选择和管理程序中英文对照

Supplier Selecti on and Man ageme nt Procedure 供应商选择和管理程序1 Purpose 目的:To defi ne the methodology to select, assess, approve new suppliers and man age supplier to en sure uni formity across the group and that all purchased product con forms to the purchaserequireme nts of NAILI.定义如何选择、评估、批准和管理供应商。
2 Scope 范围:All parts and services con tribute to products that sold to Customers in cludi ng Raw material, Subc on tract ing parts, Services and Other Products.所有作用于销售给客户产品的原材料、零部件和服务,包括外包合同、零件、服务和其他的产品。
3 Defin iti on 定义:None 无4. Resp on sibility 职责:Purchas ing departme nt is resp on sible for sourci ng, prelim in arily assess ing, recomme nding, audit ing, man age new suppliers and steer price determ in ati on. 采购部负责寻找,初评,推荐,审核,管理供应商及跟进价格确认流程。
R&D departme nt is resp on sible for audit new suppliers to con firm their tech ni cal capability.产品开发部负责审核新供应商以确认其技术能力.Quality departme nt is resp on sible for prelim in arily assess ing, audit new suppliers to confirm their quality assura nee capability and incoming con trol 。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Year of foundation 贵司成立年份:Annual turnover 年销售额:Number of Employees 职工总人数:Sales department 销售部:Production 生产部:Finances 财务部:Quality assurance 品控部:Supplied product 供应产品:Complied standard 产品符合标准:Shelf life 保质期:Export countriy for this product 产品出口国:Compared with other factory, advantage of your factory and product 与同行相比,贵司产品的优势:Production flow chart or simple description(please attach a copy) 生产流程,请简单描述或附工艺流程图:Zip Code 邮编:3. QUESTIONNAIRE 问卷2. PRODUCT INFORMATION 产品信息Manufacture capability 生产能力:Other product 其他可供应产品:Company LogoSUPPLIER AUDIT REPORT供应商审核报告Address 地址:1. SUPPLIER DATA 供应商信息Supplier name 供应商名称:Guidlines for filling 填写指南1- The porpose filling of this questionnaire is to collect information about the Quality System of supplier. 填写该问卷的目的是了解供应商质量控制体系。
2- Mark with (X ) the alternative that matches replies to your answer. Please do not leave any question without answer. If necessary make observations. 在符合项下画“X ”,请回答所有问题,如需说明请备注。
3- If any question on this questionnire is not applicable on the production procedure or at Quality System,please answer with N/A (not applicable). 如问题不适用贵司生产程序或质量体系,请在“N/A ”项下画“X ”YES 是NO 否N/A 不符Certificate (attached copy)证书(附件)Manegment System: please mark with (X) which is the System below your company 管理体系:请在贵司有的质量系统旁画“X ”,并附上证书复印件:ISO14000BRCComments 备注:System 体系ISO 9001ISO22000OthersI. Quality Manegment System 质量管理体系Comments 备注1- Does the company have a Quality Policy that reflects thecommitment of Management System? 贵司是否有质量控制体系?2- Is there a formal procedure for customers complaints? If yes,Please make a short description.贵司是否有客户投诉处理程序?如有,请简要描述。
3- Is there a training Program to GMP, HACCP, allergen andothers? If yes, What is the frequency? 贵司是否有GMP/HACCP 等体系的培训?如有请说明培训的频率。
4- Are there Internal Audit? If yes, What is the frequency? Who is responsible for auditing? 贵司是否有内部审核,如有,请说明频率及负责人。
5- Is there a Traceability program? If yes, Please make a short description. 贵司是否有追溯体系?如有,请简单描述。
6- Does the company have a Pest Control Program to control insects and rodents? 贵司是否有害虫控制程序?7- Does the Company do any analysis on raw materials/ packages received? If yes, how often? 贵司是否检测原料和包装材料?如有,检测频率?8- How to deal with the nonconformance raw material? 贵司如何处理不符合要求的原料?11- Does the company keep finished products samples? If yes,how long will it be kept? 贵司是否对成品留样?如有,保留多久?9- Does the all raw materials have Certicate of analysis? 所有原料是否有分析单?12- Is there a laboratory on site? Does it test chemical andmicrobiological items? If not, which external lab is used? 贵司是否有实验室?实验室能进行理化、微生物指标检测吗?如没有,请说明使用哪个外部实验室。
10- Does the company keep raw materials samples? If yes, how long will it be kept? 贵司是否对原料留样?如有,保留多久?13- Do you have your own research and devenlopment dept.? If yes, which research do you carry out? 贵司是否有自己的研发部门? 如有,通常进行哪些研发?YES 是NO 否N/A 不符YES 是NO 否N/A 不符YES 是NO 否N/A 不符4- Is high level cleaning undertaken? how often? Please specify the clean methods. 贵司是否有设备清洗程序?频率?请简要说明清洗方法。
5- Is the packing palnt according to GMP standard? 包装车间是否符合GMP 标准?3- Is packaging checked on deliver against the specification? How to avoid package damage on deliver? 包装在运输途中是否符合规格要求?如何避免运输途中的包装破损?4- Is the product pallet available? 产品能打托吗?2- Are magnets used on manufacturing process? What is the magnetic capacity, where is it installed and what is the inspection frequency? 生产过程中是否用到磁棒?如有,磁力是多少?安装位置?检测频率?Comments 备注1- Are packaging specifications kept on file? Please specify the package material, dimension and weight.贵司是否有包装规格文件?请说明包装材料、尺寸、重量。
5- Does Quality Control Department make audits to verify thecleaning process efficiency? What is the frequency? 品控部是否对清洗结果进行审核?频率?III. Packing 包装6- What's the origin of production water? Is there any watertratment facility? 生产用水的来源是什么?贵司是否有生产用水处理设备?2- How do you seal the package? 包装如何封口?8- How many times do you test the product from raw material to finished product? Please specify which steps. 从原料到成品有几次检测?请说明分别是哪几步?3- Is there metal detector in the process? What is the limit (ferrous,no ferrous, stainless steel) What is the inspection frequency? 生产过程中是否有金属探测仪?如有,它的灵敏度?(铁,非铁,不锈钢)检测频率?1- Is there a implemented HACCP Program? 贵司是否有HACCP 体系?IV. HACCPComments 备注14- Is there a written Product Recall System? If so, is it tested and how frequent? 贵司是否有召回体系? 如有,是否进行测试,频率?7- Does the product pass through by a filtering system? What is the last filter size? 产品是否有过滤系统?过最后一道筛的目数?II. Process 生产过程Comments 备注1- Is there a Equipment Calibration? If yes, made by whatorganization? 贵司是否有仪器校准?如有,由什么机构来完成?YES 是NO 否N/A 不符YES 是NO 否N/A 不符Name 姓名:Position 职位:Data 日期:Do you have any suggestions or requirements for us in our cooperation? 贵司与我司合作过程中有何建议和要求?1- Does the product have GMO in its formulation? If yes please discribe the controls. 产品是否含有转基因成分?如有,请说明控制方法。
