脂联素演示教学
脂联素ppt课件

.
18
xx xx
脂联素具有调节葡萄糖转运的作用 。研究发现通过静脉 注射脂联素可以降低肝脏磷酸烯醇丙酮酸羧化酶和葡糖6-磷酸酶的mRNA 表达 , 抑制肝糖生成酶的表达 , 从而减 少了内源性糖的生成。
.
19
xx xx
脂联素能够影响机体处理糖类和脂肪的能力。脂联 素可能通过抑制TNF-α通路增强胰岛素PI-3K通路,增强胰 岛素的敏感性。同时β细胞是脂联素直接作用的靶细胞,血 清脂联素浓度和β细胞上脂联素受体的多少决定了脂联素对 β细胞的作用强弱,这暗示了脂联素对调节糖代谢有着双重 作用,即增强胰岛素敏感性及改善β细胞功能。
.
4
xx xx
Maeda 等oQ 的类似物, 因其与胶原质Ⅹ、Ⅷ和 补体因子C1q 有高度相似, 并且在脂肪组织中基因转录产 物最丰富, 故称为 apM1;
Nakano 等( 1996) 用明胶亲和层析分离人血浆蛋白时发 现了该蛋白质, 故命名为明胶结合蛋白( GBP28);
.
16
xx xx
在培养的 3T3-L1 脂肪细胞, 给予短期的胰岛素刺激, 可促进脂联素基因的表达和转录, 使脂联素的合成和 分泌增加。在 3T3-L1 脂肪细胞里, 生长激素( GH ) 可增加脂联素的表达水平, 且呈时间依赖性, 其作用 一般 30 h 后开始生效, 40 h达最大效应, 说明 GH 对 脂联素表达的调节具有迟缓性。
Arita 等( 1999) 将 apM 1基因产物命名为脂联素。
.
5
xx xx
人类脂联素由244个氨基酸组成(鼠为247个),翻 译后修饰为8种不同的同源蛋白,经胰蛋白酶裂解后得到 球形结构域,其活性远远大于脂联素,而且与Clq和TNFα家族具有结构同源性。
脂联素免疫层析法试纸条制作方法

脂联素免疫层析法试纸条制作方法(原创版3篇)《脂联素免疫层析法试纸条制作方法》篇1脂联素免疫层析法试纸条是一种用于检测脂联素水平的快速检测方法。
下面是脂联素免疫层析法试纸条的制作方法:1. 制备抗脂联素抗体:将脂联素与载体结合,制备成抗脂联素抗体。
2. 制备试纸条:将抗脂联素抗体与羊抗鼠抗体结合,制备成试纸条。
3. 制备标准品和样本:制备脂联素标准品和样本,并将其点在试纸条上。
4. 检测:将试纸条放入检测仪器中,通过免疫层析技术检测脂联素水平。
需要注意的是,脂联素免疫层析法试纸条的制作方法可能会因不同的实验需求而有所不同。
《脂联素免疫层析法试纸条制作方法》篇2脂联素免疫层析法试纸条是一种用于检测脂联素水平的快速检测方法。
下面是脂联素免疫层析法试纸条的制作方法:1. 制备抗脂联素抗体:将脂联素与载体结合,制备成抗脂联素抗体。
2. 制备试纸条:将抗脂联素抗体与羊抗兔IgG 抗体结合,制备成试纸条。
3. 制备标准品和样本:将脂联素标准品和样本分别与羊抗兔IgG 抗体结合,制备成标准品和样本。
4. 测定:将试纸条插入样本或标准品中,让它们缓慢渗透,然后在规定的时间内读取结果。
需要注意的是,脂联素免疫层析法试纸条的制作方法可能会因生产厂家和试剂盒的不同而有所差异。
《脂联素免疫层析法试纸条制作方法》篇3脂联素免疫层析法试纸条是一种应用免疫层析技术检测脂联素的快速检测方法。
下面是脂联素免疫层析法试纸条的制作方法:1. 制备抗脂联素抗体:将脂联素与载体结合,制备成抗脂联素抗体,备用。
2. 制备试纸条:将玻璃纤维膜剪切成一定大小的试纸条,并在一端固定。
3. 固定抗体:将制备好的抗脂联素抗体用某种方法固定在试纸条上。
一般采用喷涂、滴涂或印刷等方法将抗体固定在试纸条上。
4. 制备检测线:将抗体与一种显色剂(如辣根过氧化物酶)结合,制备成检测线。
检测线应该与试纸条上的固定抗体相对应。
5. 制备对照线:将抗体与另一种显色剂结合,制备成对照线。
脂联素医学课件
详细描述
脂联素可以与其他减肥药物或饮食疗法联合 使用,以增强减肥效果,改善肥胖症患者的 健康状况。此外,脂联素还可以通过调节脂 肪组织和肌肉组织的代谢,减少脂肪堆积和 肌肉萎缩,有助于预防和治疗肥胖症的并发 症。
脂联素在心血管疾病预防和治疗中的应用
总结词
脂联素有助于预防和治疗心血管疾病
详细描述
心血管疾病是一种常见的慢性疾病,与肥胖、糖尿病等代谢性疾病密切相关。脂联素可 以调节血脂代谢,降低低密度脂蛋白胆固醇和甘油三酯水平,同时提高高密度脂蛋白胆
脂联素对肥胖的治疗意义
通过提高肥胖患者的脂联素水平或增 强其活性,有助于改善肥胖相关的代 谢紊乱,减轻体重并降低心血管疾病 的风险。
脂联素与心血管疾病
脂联素与心血管疾病的关系
脂联素具有抗炎、抗氧化和抗动脉粥样硬化的作用,可以保护心血管系统。研究表明,脂 联素水平低下与心血管疾病的发生和发展有关。
脂联素对心血管疾病的治疗意义
03
脂联素与糖尿病并发症
脂联素还可能对糖尿病并发症具有保护作用。研究表明,脂联素可以减
轻糖尿病患者的血管病变和神经病变,降低心血管疾病的风险。
脂联素与肥胖症
脂联素与肥胖的关系
脂联素与肥胖并发症
肥胖患者通常存在脂联素水平下降的 情况,这与其脂肪组织功能障碍和胰 岛素抵抗有关。
脂联素还可能对肥胖患者的并发症具 有保护作用,如改善血脂异常、减少 泌的蛋白质,具有调节血糖和胰岛素敏感性的作用。研究表明,脂联素可以增加胰岛 素的敏感性,减少胰岛素抵抗,从而有助于改善糖尿病患者的血糖控制。
脂联素在糖尿病治疗中的应用
总结词
脂联素可以作为糖尿病辅助治疗手段
详细描述
脂联素可以与其他降糖药物联合使用,以增强降糖效果,改善糖尿病患者的血糖控制。此外,脂联素 还可以通过调节脂肪组织和肌肉组织的代谢,减少脂肪堆积和肌肉萎缩,有助于预防和治疗糖尿病的 并发症。
高中化学 专题3.3.2 酯教学案 新人教版选修5

教
学
目
的
知识
技能
掌握酯化反应的原理、实验操作及相关问题,进一步理解可逆反应、催化作用。
过程
方法
1、培养学生用已知条件设计实验及观察、描述、解释实验现象的能力,
2、培养学生对知识的分析归纳、概括总结的思维能力与表达能力。
情感
价值观
介绍同位素示踪法在化学研究中的使用,通过酯化反应过程的分析、推理、研究,培养学生从现象到本质、从宏观到微观、从实践到理论的科学思维方法。
2、羧酸的通性
(1)常见一元羧酸的酸性强弱顺序为:甲酸、苯甲酸、乙酸、碳酸
(2)酯化反应
R1—COOH + R2-CH2OH R1-COO—CH2-R2+H2O
[特例]无机含氧酸与醇作用也能生成酯,如C2H5OH+HONO2 C2H5-O-NO2+H2O
(3)还原反应
[讲]由于羟基的影响,羧基中的羰基比醛、酮分子中的羰基较难发生加成反应,但在特殊试剂如LiAlH4的作用下可将羧酸还原为醇。
三、酯
1、结构简式通式:RCOOR′分子式通式:CnH2nO2与同碳原子羧酸同分异构体。
2、酯类化合物的存在:水果、饮料、糖类、糕点等
3、化学性质酯的水解:酯+水 酸+醇
教学过程
备注
写出乙酸、甲酸分别和乙醇的酯化反应的化学方程式。此反应中浓H2SO4的作用是什么?思考、回答并找两名同学到黑板上板书。
HCOOH + 2Ag(NH3)2OH CO2↑+2H2O +4NH3↑+2Ag
甲酸与Cu(OH)2的反应:2HCOOH +Cu(OH)2 (HCOO)2Cu +2H2O
脂联素

胰岛素增敏激素
01 基本信息
03 及其受体 05 功效
目录
02 生化特性
04
风湿性疾病中的免疫 调节作用
06 其他信息Байду номын сангаас
脂肪组织(adipose tissue)主要由大量聚集成团的脂肪细胞构成,脂联素(Adiponectin/ADPN)是脂肪细 胞分泌的一种内源性生物活性多肽或蛋白质。脂联素是一种胰岛素增敏激素(An Insulin-sensitizing Hormone),能改善小鼠的胰岛素抗性(Insulin resistance)和动脉硬化症;对人体的研究发现,脂联素水平能 预示II型糖尿病和冠心病的发展,并在临床试验表现出抗糖尿病、抗动脉粥样和炎症的潜力。
Ouchi等发现脂联素可以降低单核细胞的粘附作用,单核细胞可以使THP-1细胞在人体大动脉内皮组织排列成 行,而这种粘附作用发生在动脉粥样硬化血管壁损伤的早期。脂联素还可以降低多种粘附分子(VCAM-1、ICAM-1、 选择素-E)在内皮细胞的表达。核转录因子NF-κB是参与由TNF-α刺激VCAM-1、ICAM-1、选择素-E转录调节的重 要因子。Ouchi和Kihara等报道,脂联素可能通过抑制NF-κB的信号通路调节内皮细胞的功能。NF-κB诱导激酶 (NIK)使IKB激酶(IKK)复合物磷酸化,激活NF-κB。TNF受体结合因子2是NIK和TNF-α信号通路连接蛋白,是 TNF-α诱导NIK– NF-κB激活和JNK或p38通路激活的分叉点,NIK不参与JNK和p38激酶的激活。据报道,cAMPPKA信号通路通过稳定IKB-α削弱 NF-κB的活性,虽然其精确的机制还未澄清。脂联素特异性地抑制TNF-α诱导 ΙκB-α-NF-κB通路的激活,不影响JNK、p38或 Akt激酶的磷酸化,表明脂联素在NIK和ΙκB-α之间降低了 TNF- α诱导NF-κB信号通路的转导。这些结果表明,脂联素不依赖 cAMP-PKA对TNF-α诱导的粘附分子表达产生 效应。
人中分子量脂联素MMWADP酶联免疫分析
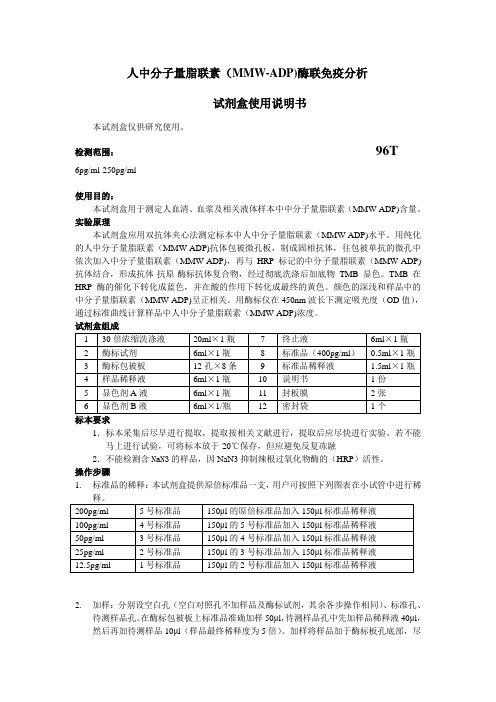
人中分子量脂联素(MMW-ADP)酶联免疫分析试剂盒使用说明书本试剂盒仅供研究使用。
检测范围:96T6pg/ml-250pg/ml使用目的:本试剂盒用于测定人血清、血浆及相关液体样本中中分子量脂联素(MMW-ADP)含量。
实验原理本试剂盒应用双抗体夹心法测定标本中人中分子量脂联素(MMW-ADP)水平。
用纯化的人中分子量脂联素(MMW-ADP)抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入中分子量脂联素(MMW-ADP),再与HRP标记的中分子量脂联素(MMW-ADP)抗体结合,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB显色。
TMB在HRP酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的中分子量脂联素(MMW-ADP)呈正相关。
用酶标仪在450nm波长下测定吸光度(OD值),通过标准曲线计算样品中人中分子量脂联素(MMW-ADP)浓度。
试剂盒组成标本要求1.标本采集后尽早进行提取,提取按相关文献进行,提取后应尽快进行实验。
若不能马上进行试验,可将标本放于-20℃保存,但应避免反复冻融2.不能检测含NaN3的样品,因NaN3抑制辣根过氧化物酶的(HRP)活性。
操作步骤1.标准品的稀释:本试剂盒提供原倍标准品一支,用户可按照下列图表在小试管中进行稀2.加样:分别设空白孔(空白对照孔不加样品及酶标试剂,其余各步操作相同)、标准孔、待测样品孔。
在酶标包被板上标准品准确加样50μl,待测样品孔中先加样品稀释液40μl,然后再加待测样品10μl(样品最终稀释度为5倍)。
加样将样品加于酶标板孔底部,尽量不触及孔壁,轻轻晃动混匀。
3.温育:用封板膜封板后置37℃温育30分钟。
4.配液:将30倍浓缩洗涤液用蒸馏水30倍稀释后备用5.洗涤:小心揭掉封板膜,弃去液体,甩干,每孔加满洗涤液,静置30秒后弃去,如此重复5次,拍干。
6.加酶:每孔加入酶标试剂50μl,空白孔除外。
1673[1]人脂联素
![1673[1]人脂联素](https://img.taocdn.com/s3/m/5a4be2dad15abe23482f4dc8.png)
Adiponectin Multimerization Is Dependent on Conserved Lysines in the Collagenous Domain:Evidence for Regulation of Multimerization by Alterations in Posttranslational ModificationsAyanthi A.Richards,Tim Stephens,Hayley K.Charlton,Alun Jones,Graeme A.Macdonald,Johannes B.Prins,and Jonathan P.WhiteheadCentre for Diabetes and Endocrine Research (A.A.R.,T.S.,H.K.C.,G.A.M.,J.B.P.,J.P.W.),School of Medicine,University of Queensland,Princess Alexandra Hospital,Brisbane,Queensland 4102,Australia;and Institute for Molecular Bioscience (A.J.),University of Queensland,Brisbane,Queensland 4072,AustraliaAdiponectin is a secreted,multimeric protein with insulin-sensitizing,antiatherogenic,and antiin-flammatory properties.Serum adiponectin con-sists of trimer,hexamer,and larger high-molecu-lar-weight (HMW)multimers,and these HMW multimers appear to be the more bioactive forms.Multimer composition of adiponectin appears to be regulated;however,the molecular mechanisms in-volved are unknown.We hypothesize that regula-tion of adiponectin multimerization and secretion occurs via changes in posttranslational modifica-tions (PTMs).Although a structural role for intertri-mer disulfide bonds in the formation of hexamers and HMW multimers is established,the role of other PTMs is unknown.PTMs identified in murine and bovine adiponectin include hydroxylation of multiple conserved proline and lysine residues and glycosylation of hydroxylysines.By mass spec-trometry,we confirmed the presence of these PTMs in human adiponectin and identified three additional hydroxylations on Pro71,Pro76,andPro95.We also investigated the role of the five modified lysines in multimer formation and secre-tion of recombinant human adiponectin expressed in mammalian cell lines.Mutation of modified ly-sines in the collagenous domain prevented forma-tion of HMW multimers,whereas a pharmacologi-cal inhibitor of prolyl-and lysyl-hydroxylases,2,2-dipyridyl,inhibited formation of hexamers and HMW multimers.Bacterially expressed human adi-ponectin displayed a complete lack of differentially modified isoforms and failed to form bona fide tri-mers and larger multimers.Finally,glucose-induced increases in HMW multimer production from human adipose explants correlated with changes in the two-dimensional electrophoresis profile of adiponectin isoforms.Collectively,these data suggest that adiponectin multimer composi-tion is affected by changes in PTM in response to physiological factors.(Molecular Endocrinology 20:1673–1687,2006)THE PLASMA PROTEIN adiponectin is an adipo-cyte-secreted peptide hormone with demon-strated antidiabetic,antiatherogenic,and antiinflam-matory properties (reviewed in Ref.1).Adiponectin is secreted,and exists in the circulation,as a series of multimers ranging from homotrimers to disulfide-dependent hexamers and high-molecular-weight (HMW)multimers (2).Early reports demonstrated that circulating total adiponectin levels were reduced in states of obesity,insulin resistance,and diabetes.More recently,it has emerged that HMW multimers are the more bioactive species (3)and that the ratio of HMW to total adiponectin,rather than total levels alone,provides the closest correlation with measures of insulin sensitivity,particularly hepatic insulin sensi-tivity (3–6).Furthermore,a number of naturally occur-ring mutations in adiponectin have been identified in patients with type 2diabetes.In transient transfection studies,several of these mutant forms of adiponectin exhibit defective formation and/or secretion of HMW multimers (2,7).Reduced serum levels of HMW adi-ponectin are also associated with coronary artery dis-ease (8),whereas the vascular protective effects of adiponectin were shown to be restricted to the HMW component (8).First Published Online February 23,2006Abbreviations:BFA,Brefeldin A;BMI,body mass index;CHO,Chinese hamster ovary;CSL,core-specific lectin;2DE,two-dimensional electrophoresis;DTT,dithiothreitol;ER,en-doplasmic reticulum;HA,hemagglutinin;HEK,human em-bryonic kidney;HMW,high molecular weight;IPTG,isopro-pyl--D -thiogalactopyranoside;LC,liquid chromatography;LMW,low molecular weight;MALDI-TOF,matrix-assisted laser desorption/ionization-time of flight;MS,mass spec-trometry;PTM,posttranslational modification;RT,room tem-perature;WT,wild type.Molecular Endocrinology is published monthly by The Endocrine Society (),the foremost professional society serving the endocrine community.0888-8809/06/$15.00/0Molecular Endocrinology 20(7):1673–1687Printed in U.S.A.Copyright ©2006by The Endocrine Societydoi:10.1210/me.2005-0390A number of agents are now recognized regulators of adiponectin expression and multimer composition. Thiazolidinediones,a class of peroxisome proliferator activated receptor␥agonists commonly used in an-tidiabetic therapy,increase adipocyte expression of adiponectin[via a peroxisome proliferator activated receptor␥response element(9)]and simultaneously increase the proportion of circulating HMW multimers (3).Testosterone,although not affecting intracellular production of adiponectin or multimer composition, appears to selectively retard secretion of HMW mul-timers from adipocytes(10).This is in keeping with the observed sexual dimorphism in circulating adiponec-tin levels and multimer composition,with males having reduced levels of total and HMW adiponectin(11,12). What remains to be elucidated are the molecular mechanisms underlying the regulation of adiponectin multimer composition and secretion.The adiponectin protein consists of three structural domains(Fig.1A);the N-terminal variable region(of greatest amino acid sequence divergence between species),the collagenous domain(named so because of its sequence and structural homology with colla-gen),and the C-terminal globular(receptor-binding/ effector)domain(13).Biochemical analyses of adi-ponectin multimers have revealed the importance of two kinds of molecular interaction that are integral to their structure(see Fig.1B).First,the protein forms homotrimers via the noncovalent interactions of the collagenous domains in a triple helix.Hydrophobic interactions between globular domains may also con-tribute to trimer stability and help to initiate triple helix formation(14).Early biochemical studies demon-strated that temperatures over70C resulted in break-down of intratrimer interactions(2).Second,intermo-lecular disulfide bonds,involving C39in thevariable Fig.1.Structure of Human AdiponectinA,Structural domains of human adiponectin.Known PTMs are indicated.B,Schematic representation of adiponectin multimers showing triple helix and disulfide bond interactions.C,Conserved,hydroxylated and glycosylated lysine residues(K)in the variable and collagenous domains of human adiponectin that were substituted with arginines.Nomenclature of the various mutants(K1R,K2–3R,K4–5R,and K1–5R)is shown.N,Amino terminus;C,carboxyl terminus,␣␣,amino acids.1674Mol Endocrinol,July2006,20(7):1673–1687Richards et al.•Regulation of Adiponectin Multimerizationdomain of the murine protein(C36of human adiponec-tin),provide the intertrimer links within hexamers and HMW species.Amino acid substitution of this Cys residue produces a protein unable to form multimers larger than trimer,and reduction of disulfide bonds in wild-type(WT)adiponectin similarly collapses hex-amer and HMW species to trimer(2,11,15).Further-more,boiling of purified trimers,hexamers,and HMW multimers,resulting in denaturation of each to their composite monomers and dimers,revealed that hex-amers and HMW species,unlike trimers,are com-posed almost exclusively of disulfide-linked dimers, whereas trimers displayed a1:1ratio of monomer to dimer(15).Adiponectin also undergoes posttranslational mod-ifications(PTMs)other than disulfide bonds.The pres-ence of an␣2,8-linked disialic acid moiety (Neu5Aca238Neu5Aca233Gal)has been reported, although the site(s)and structural and functional sig-nificance of this modification have not been deter-mined(16).Wang et al.(17,18)identified four con-served prolines in the collagenous domain that undergo hydroxylation,and five conserved lysines (one in the variable region and four in the collagenous domain)that are hydroxylated and subsequently gly-cosylated(see Fig.1A).The same investigators showed that conservative amino acid substitution of the collagenous domain lysines in murine adiponectin (17)produced a variant that was no longer glycosy-lated and was largely unable to enhance insulin action in hepatocytes.In collagen,proline hydroxylation and lysine hydroxylation and glycosylation are known to have an important structural role in triple helix forma-tion,secretion,and stability(19).This,together with the selective requirement of HMW forms for the he-patic effects of adiponectin,prompted us to examine the role of proline hydroxylation and lysine hydroxyla-tion/glycosylation in multimerization of human adiponectin.In the current study,we have employed site-directed mutagenesis and pharmacological approaches in combination with biochemical analyses to assess the role of PTMs,particularly lysine hydroxylation/ glycosylation and proline hydroxylation,in adiponectin multimerization and secretion.Our findings indicate an essential requirement for lysine hydroxylation and gly-cosylation in the formation of HMW multimers,and provide the first evidence that regulation of HMW mul-timer production by adipocytes may occur via alter-ations in PTM of adiponectin.RESULTSMass Spectrometry(MS)Analysis of Purified Recombinant Human AdiponectinTo directly investigate the PTMs in human adiponec-tin,and compare them with those identified in the mouse and bovine proteins,we subjected tryptic di-gests of purified human embryonic kidney(HEK)-pro-duced recombinant adiponectin to matrix-assisted la-ser desorption/ionization-time of flight(MALDI-TOF), MALDI-TOF-TOF,and liquid chromatography(LC)/ MS/MS analysis.An adiponectin mass fingerprint was produced spanning over74%of the protein sequence. Comparison of the experimentally derived mass spec-trum with the predicted spectrum revealed several modified peptides with mass changes ofϩ16and ϩ340Da corresponding to the addition of a hydroxyl or a glucosylgalactosyl-hydroxyl moiety,respectively. Using this approach,we have been able to confirm the presence of all of the hydroxy-proline and glucosyl-galactosyl hydroxy-lysine residues previously identi-fied in mouse and bovine adiponectin(17,18),except for modification of Lys33,which was not observed, probably due to poor representation of the N terminus of the protein in the peptide mass fingerprint(Fig.2,A and B).Importantly,we observed peptides resulting from differential cleavage at Lys65,Lys68,and Lys77 (Fig.2B;peptides B,C,D,and E).Trypsin cleavage occurs at C-terminal peptide bonds of lysine and hy-droxylysine but not glucosylgalactosyl hydroxy-lysine residues(20).Thus,our fingerprint data provide clear evidence of differential glycosylation of Lys65,Lys68, and Lys77.Lys101in peptide F was also variably glycosylated(Fig.2B).In addition to the previously described modifica-tions,MALDI-TOF analysis suggested the addition of hydroxyl groups at Pro71,Pro76,and Pro95(Fig.2C). MS/MS sequencing demonstrated that Pro95,situ-ated within the peptide GFPGIQGR,was present in both the unmodified and hydroxylated form(Fig.2C, peptide H).Residues Pro71and Pro76are located within the peptide GDPGLIGPK(Fig.2C,peptide G). Analysis of the fingerprint data for this peptide re-vealed masses of853Da(base),869Da(baseϩ16 Da),885Da(baseϩ32Da),and901Da(baseϩ48 Da),and MALDI-TOF-TOF peptide sequencing con-firmed the identity of the base peptide.Together, these data suggest that peptide GDPGLIGPK is differ-entially hydroxylated at Pro71,Pro76,and Lys77. Competent Expression and Assembly of Human Adiponectin Multimers in CHO and HEK Cells Waki et al.(2)first characterized the multimer compo-sition of adiponectin from mouse and human sera by SDS-PAGE of non-reduced and non-heat-denatured samples.These analyses clearly demonstrated the ex-istence of multiple HMW species of human adiponec-tin compared with a single HMW band in mouse se-rum.Subsequent studies have focused on the murine protein,and have often employed the well-character-ized3T3-L1murine adipocyte cell line.The lack of an analogous human adipocyte cell line necessitated the creation of a surrogate expression system that would also be easily amenable to genetic manipulation.WT human adiponectin cDNA was cloned from adipose tissue mRNA by RT-PCR and stably transfected intoRichards et al.•Regulation of Adiponectin Multimerization Mol Endocrinol,July2006,20(7):1673–16871675both HEK and Chinese hamster ovary (CHO)cell lines.HEK cells have been used previously for adiponectin production (3,11),whereas CHO cells were demon-strated to efficiently produce the core-specific lectin (CSL)or mannose-binding protein,a closely related,mul-timeric plasma protein secreted by hepatocytes (21,22).Analysis of secreted adiponectin from CHO and HEK stable cell lines revealed comparable multimer profiles (Fig.3A,lanes 3and 4).A C-terminally hemagglutinin (HA)-tagged construct was also generated,and the tagged protein was demonstrated to form similar mul-timers to untagged adiponectin (Fig.3A,lanes 4and 5).Comparison of secreted adiponectin from CHO (lane 3)and HEK (lane 4)stable lines with conditionedmediaFig.2.Mass Spectroscopy of Human AdiponectinA,Schematic of the collagenous domain of human adiponectin showing all modifications identified by MS analysis of tryptic fragments.In addition to the four known hydroxy-proline residues (44,47,53,and 91),three additional proline residues (71,76,and 95)were found to be similarly modified (boxed ).Additionally,the four known glycosylated hydroxy-lysines in the collagenous domain were identified,but not that in the variable domain (framed ).B,Identification,by peptide mass fingerprinting,of known modifications that are conserved in human adiponectin.Peptides A and E demonstrate hydroxylation of prolines 44,47,53,and 91,as shown previously for mouse and bovine adiponectin.Peptides C,D,and E are all derivatives of peptide B that result from differential glycosylation of hydroxylysines 65,68,and 77.Peptide F also demonstrates variable glycosylation of hydroxylysine 101.C,Identification of three novel hydroxyprolines in human adiponectin.The unmodified form of peptide G was identified by its mass fingerprint and then confirmed by sequencing using MALDI-TOF-TOF.Mass fingerprints were also identified for variants of this peptide containing one,two,or three hydroxyl groups (most probably at Pro71,Pro76,and Lys77).Proline 95in peptide H was similarly identified to be variably hydroxylated.Peptide sequence data were scored using the MASCOT ion score algorithm to produce a standardized probabilistic measure of confidence.1676Mol Endocrinol,July 2006,20(7):1673–1687Richards et al.•Regulation of Adiponectin MultimerizationFig.3.Conservative Substitution of Hydroxylated and Glycosylated Lysine Residues in the Collagenous Domain Impairs HMW Multimer Formation and SecretionA,Comparison of human adiponectin multimers in serum (lane 1)with those secreted by in vitro -differentiated human primary adipocytes (lane 2)and stably transfected CHO (lane 3)and HEK (lane 4)cells,after analysis by non-reducing SDS-PAGE on gradient ne 5shows C-terminally HA-tagged adiponectin,from stable HEK transfectants,which forms similar multimers to those of the untagged protein (lane 4).B,Adiponectin multimer composition of conditioned serum-free medium from CHO cells transiently expressing WT adiponectin,K2–3R,or K4–5R after analysis as in A.C,Adiponectin multimer composition of conditioned medium from CHO stable cell lines expressing WT (W),C36S (C),or K2–5R (K)analyzed as in A.D,Crude fractionation of LMW and HMW multimers by velocity sedimentation analysis of the samples in C.E,Secretion kinetics of HA-tagged WT,C36S,and K2–5R adiponectin from stably expressing HEK cell lines.Cells were allowed to secrete into serum-free medium for various periods of time before harvest of medium,precipitation (using trichloroacetic acid),and analysis by SDS-PAGE and immunoblotting for HA.A representative experiment (of three independent experiments)is shown.F,CHO cells stably expressing WT (W),C36S (C),or K2–5R (K)were cultured in the presence or absence of 5g/ml BFA for 5h.Monomer (M),dimer (D),and hexamer (H)are readily apparent in lysates of WT stables with the HMW species accumulating upon inhibition of secretion by BFA.Trimer failed to accumulate intracellularly under any condition even in C36S lysates.HMW species are lacking in K2–5R lysates even after inhibition of secretion with BFA.The lower panel shows longer exposure of the upper region of the main panel ,demonstrating HMW multimers in cells expressing WT adiponectin but not the two mutant forms.Asterisks mark nonspecific bands.Molecular weight markers are indicated.W,WT;C,C36S;K,K2–5R;H,hexamer;T,trimer;D,dimer;M,monomer.Richards et al.•Regulation of Adiponectin Multimerization Mol Endocrinol,July 2006,20(7):1673–16871677from in vitro-differentiated primary human adipocytes (lane2)and with human serum(lane1)revealed a similar profile and distribution of multimers,although the hex-amer species consistently appeared as a doublet in se-rum(Fig.3A).These stable lines were therefore em-ployed for our subsequent investigations.HMW Multimer Formation Is Dependent on Conserved,Hydroxylated,and Glycosylated Lysine Residues in the Collagenous Domain Although the structural role of the disulfide-forming cysteine residue in the variable region of adiponectin is now well established(2,11,15),the role of other PTMs in multimer formation has not been addressed.Con-served proline and lysine residues in the variable and collagenous domains of the protein are known to un-dergo hydroxylation,which,in the case of lysines,is followed by addition of a glucosylgalactosyl moiety (17,18).Four of the five conserved and modified ly-sines occur in the collagenous domain of the protein, and conservative substitution of these in murine adi-ponectin severely compromised activity of the protein in primary hepatocytes(17).However,the mechanism behind this loss of function was not examined.We therefore investigated the role of these four modified lysines,and the fifth modified lysine in the variable domain of the protein,in multimerization of human adiponectin.Mutants containing K-to-R substitution of the first(K1R),second and third(K2–3R),fourth and fifth(K4–5R),second to fifth(K2–5R),or first to fifth (K1–5R)residues(see Fig.1C)were generated by site-directed mutagenesis and expressed transiently or stably in CHO cells.Transfected cells were cultured in serum-free medium,and secreted adiponectin was analyzed for multimer composition on gradient gels. Conservative substitution of either the first two(K2–3R)or last two(K4–5R)lysines in the collagenous domain resulted in impaired HMW multimer produc-tion and a greater proportion of trimer than was seen in WT adiponectin from transient transfections(Fig. 3B).The phenotype was more severe in K2–3R,sug-gesting that these two lysines may serve a more crit-ical function than the fourth and fifth lysines.Mutation of all four lysines in the collagenous domain(K2–5R) had an additive effect,resulting in a complete loss of HMW multimers,and this was confirmed by velocity sedimentation on sucrose gradients(Fig.3,C and D). The disulfide-incompetent mutant,C36S,formed only trimers as has been reported for the corresponding mutant of murine adiponectin(C39S)(2,11,15).Com-parison of the secretion rates of WT and mutant adi-ponectin also revealed striking differences,the K2–5R being secreted with approximately50%reduced effi-ciency,whereas the C36S was secreted at1.5–2times the rate of WT(Fig.3E).It was therefore possible that the loss of HMW multimers in secreted K2–5R was due to a failure in secretion of these species rather than a failure in their intracellular formation.To address this, lysates from stable cell lines were analyzed for the presence of HMW multimers after5-h culture in the presence of brefeldin A(BFA)to inhibit secretion and accumulate the protein in the secretory pathway(23–25).Although an accumulation of HMW multimers was apparent after BFA treatment of WT-expressing cells, K2–5R cell lysates were devoid of HMW multimers in both BFA-treated and untreated cells(Fig.3F),indi-cating a defect in formation of these larger multimers by the mutant protein.A decrease in mobility of all species in lysates was routinely observed after culture in BFA and may result from altered processing due to redistribution of Golgi enzymes into the endoplasmic reticulum(ER)with BFA-induced fusion of the two organelles(24,25).Intriguingly,no intracellular accu-mulation of trimer was observed under any condition in the stable cell lines or in primary human adipocytes (data not shown)but did occur after overexpression by transient transfection(data not shown).Analysis of the KR1mutant containing substitution of the single lysine in the variable domain revealed no apparent effect on multimerization compared with WT(Fig.4).Similarly, no exacerbation of the quadruple mutant(K2–5R)phe-notype was observed upon mutation of all five lysines (K1–5R)(Fig.4).These observations demonstrate an important functional role for the conserved hydroxy-lated and glycosylated lysine residues,selectively in the collagenous domain of adiponectin,in the intra-cellular assembly and secretion of HMW multimers. Inhibition of Proline and Lysine Hydroxylation by 2,2-Dipyridyl Inhibits MultimerizationIn a complementary approach to the mutagenesis studies described above,we employed apharmaco-Fig.4.Substitution of a Hydroxylated and Glycosylated Ly-sine Residue in the Variable Domain Has No Effect on Mul-timerizationAdiponectin multimer composition of conditioned,serum-free medium from CHO cells transiently expressing WT adi-ponectin,K1R,K2–5R,or K1–5R as analyzed by SDS-PAGE on gradient gels.H,Hexamer;T,trimer.1678Mol Endocrinol,July2006,20(7):1673–1687Richards et al.•Regulation of Adiponectin Multimerizationlogical inhibitor of prolyl-and lysyl-hydroxylases,2,2Ј-dipyridyl.This agent has been used previously to dem-onstrate hydroxylation and subsequent glycosylation of lysine residues in the CSL(21).2,2Ј-Dipyridyl treat-ment of primary rat hepatocytes was shown to inhibit secretion of CSL,and this was thought to occur as a consequence of failed proline and lysine hydroxylation and,thus,subsequent glycosylation of hydroxylysines (26).CHO cells stably expressing WT adiponectin were depleted of intracellular adiponectin by treatment with cycloheximide for20h.The ribosomal inhibitor was then removed,and protein synthesis was allowed to resume for3h in the presence or absence of BFA or 2,2Ј-dipyridyl.Analysis of lysates revealed a complete absence of hexamer and HMW species in2,2Ј-dipyri-dyl-treated cells,although these multimers were seen in WT samples and accumulated upon inhibition of secretion with BFA(Fig.5A).Similar results were ob-tained in primary human adipocytes(data not shown). Analysis of medium samples from the same experi-ment,after reduction and heat denaturation,revealed a partial inhibition of adiponectin secretion by2,2Ј-dipyridyl(Fig.5A),in agreement with the previously observed effects of the drug on CSL secretion(26).By comparison,BFA completely inhibited secretion(Fig. 5A).Multimer analysis of medium after a longer(24h) period of secretion revealed that adiponectin secreted from2,2Ј-dipyridyl-treated cells is predominantly in the form of trimer(Fig.5B).Note that multimers in 2,2Ј-dipyridyl-treated cells migrate more rapidly than those from untreated and BFA-treated cells,consis-tent with a loss of PTMs with2,2Ј-dipyridyl(Fig.5B). Thus,inhibition of proline hydroxylation in addition to lysine hydroxylation resulted in a more severe impair-ment of multimer formation,with loss of both hexamer and HMW species.The observed inhibition of adi-ponectin secretion by2,2Ј-dipyridyl mirrors that ob-served for the K2–5R mutant and is likely due to the loss of hydroxyproline and glucosylgalactosyl hy-droxy-lysine residues.However,we cannot exclude the possibility that nonspecific/indirect effects of the drug may also compromise adiponectin multimeriza-tion and secretion.Indeed,fragmentation of the Golgi apparatus was observed by immunofluorescence la-beling for the cis/medial Golgi marker,GM130,and may well be accompanied by a widespread disruption to ER-to-Golgi transport and/or exocytic vesicle traffic (Fig.5C).However,dispersion of the Golgi marker was also apparent in BFA-treated cells(Fig.5C),where formation of HMW adiponectin multimers was uncompromised.Adiponectin Expressed in Bacteria Lacks PTMs and Fails to Form Trimers,Hexamers,andHMW MultimersTo further examine the role of PTMs in multimer for-mation of human adiponectin,we expressed the pro-tein in bacteria,which lack the capacity to perform many of the PTMs of eukaryotic cells.Human adi-ponectin lacking the18-amino acid mammalian signal peptide sequence was expressed in BL21(DE3)pLys-S cells after induction with isopropyl--D-thiogalactopy-ranoside(IPTG).Cell extracts were fractionated into soluble and particulate components,and the soluble fraction was analyzed under various conditions along-side CHO-produced WT adiponectin(CHO-Ad)(Fig.6).In all analyses,the bacterially produced protein exhibited significantly greater mobility than CHO-Ad, consistent with a lack of eukaryotic PTMs(Fig.6A, compare lanes4and6).When samples were boiled to disassemble trimers and larger multimers to their con-stituent monomers and dimers(2),CHO-Ad was present almost exclusively as dimer.In contrast,bac-terially produced adiponectin was largely monomeric with only a trace of dimer(Fig.6A).Analysis of the same samples after both reduction and boiling re-vealed complete conversion of the CHO-Ad dimerto Fig.5.2,2Ј-Dipyridyl-Mediated Inhibition of Prolyl-and Ly-syl-Hydroxylases Impairs Adiponectin Multimerization and SecretionA,CHO cells stably expressing WT human adiponectin were cultured in cycloheximide for20h to deplete intracel-lular adiponectin before washing to remove cycloheximide. Cells were then either left untreated(U)or treated with3.5m M 2,2Ј-dipyridyl(Dp)or5g/ml BFA(B)and allowed to secrete newly synthesized adiponectin into serum-free medium con-taining the same dose of drug.Lysates and medium were harvested after3h for analysis under non-reducing,non-heat-denaturing or reducing,denaturing conditions,respec-tively.Panel to the right shows a longer exposure of the corresponding region of the main blot.B,Multimer compo-sition of conditioned medium from cells treated as in A but allowed to secrete for24h.C,Cells treated as in A were immunofluorescently labeled for the cis/medial Golgi marker, GM130.U,Untreated;Dp,2,2Ј-dipyridyl-treated;B,BFA-treated;H,hexamer;T,trimer;D,dimer;M,monomer.Scale bar,10m.Richards et al.•Regulation of Adiponectin Multimerization Mol Endocrinol,July2006,20(7):1673–16871679monomer as well as loss of the bacterial adiponectin dimer species(Fig.6A).Thus,disulfide bond formation in the bacterially expressed protein appears to be inefficient,at least in the absence of periplasmic tar-geting.Furthermore,upon analysis of multimer com-position on gradient gels,trimer,hexamer,and HMW species were obviously lacking in the bacterially ex-pressed protein,despite the presence of some minor bands in the60-to250-kDa size range(Fig.6B).These species,although disulfide dependent,failed to dis-play the biochemical characteristics of adiponectin multimers from mammalian cells(CHO-Ad),because they were insensitive to boiling(Fig.6B).Instead,they may represent chaperone-bound intermediates or ag-gregates.The failure of bacterially expressed adi-ponectin to multimerize is consistent with the impor-tance of PTMs in this process and has notable implications for functional studies using recombinant adiponectin expressed in prokaryotic systems. Isoform Composition of WT,C36S,and K2–5R CHO-AdTwo-dimensional electrophoresis(2DE)of3T3-L1-produced(murine)adiponectin revealed eight differ-entially modified isoforms of the protein,of which six isoforms were glycosylated(17).Similarly,bovine se-rum adiponectin has also been shown to exist as eight to nine isoforms(18).We therefore employed the same technique to determine the isoform composition of WT and mutant human adiponectin secreted from stably expressing CHO cell lines.Western blotting for adi-ponectin revealed a train of seven isoforms of de-creasing pI and mobility in WT CHO-Ad(Fig.7).At least as many isoforms were apparent in the C36S and K2–5R mutants,as well as in WT CHO-Ad secreted from dipyridyl-treated cells.(Fig.7).While WT and C36S samples appeared as a single train of spots,a second train of spots was prominent in both K2–5R mutant and WT CHO-Ad upon dipyridyltreatment. Fig.6.Bacterially Expressed Human Adiponectin Fails toForm Multimers Despite Formation of Disulfide BondsThe soluble fraction of lysate from IPTG-induced bacterialcells expressing human adiponectin(Bact-Ad)was analyzedalongside the same lysate fraction from nontransformed,in-duced cells(Bact)and the CHO-expressed protein(CHO-Ad)after boiling alone or boiling and reduction with DTT(A).Whereas the CHO-Ad exists predominantly as dimer afterboiling alone,Bact-Ad is predominantly monomer with asmall proportion of dimer,which is lost upon treatment withDTT.Bands present in Bact samples were labeled with as-terisks as nonspecific.B,Multimer analysis of the same sam-ples under non-reducing and non-heat-denaturing conditionson gradient gels reveals much larger species in addition tomonomer and dimer in Bact-Ad.Unlike the multimers presentin CHO-Ad,the larger species in Bact-Ad were resistant toboiling,despite being similarly sensitive to DTT.Multimers ofCHO-Ad are indicated.H,Hexamer;T,trimer;D,dimer;M,monomer.Fig.7.2DE Analysis of WT and Mutant Human AdiponectinProduced by CHO Stable Cell Lines and Bacterially Ex-pressed WT AdiponectinConditioned serum-free medium from CHO stable celllines expressing WT adiponectin,C36S,K2–5R,or WT adi-ponectin after pretreatment with2,2Ј-dipyridyl(Dipyr),andbacterially expressed WT adiponectin(Bact-Ad)were ana-lyzed by2DE and Western blotting to determine the compo-sition of differentially modified isoforms.1680Mol Endocrinol,July2006,20(7):1673–1687Richards et al.•Regulation of Adiponectin Multimerization。
4脂联素

Downloaded from at University of Missouri-Columbia on July 18, 2010
The extracellular matrix (ECM) is composed largely of collagen fibers and glycosaminoglycans. Until recently, ECM was thought to serve only as a scaffold to stabilize the physical structure of tissues. Now it is becoming clear that ECM plays a more active and complex role in regulating the behavior of the cells that contact it. ECM secreted by fibroblasts and endothelial cells has been shown to function as a reservoir of growth factors, enzymes and inhibitors. Among glycosaminoglycans, heparan sulfate and heparin bind to many cytokines that affect cell growth and differentiation. Heparin affinity chromatography is hence commonly used for their purification {1). Gelatin affinity chromatography is commonly used for purification of matrix metalloproteinases, which are secreted by resident cells of tissues, like fibroblasts and epithelial cells, or by
防治糖尿病新途径——脂连素

防治糖尿病新途径——脂连素班级:02级口腔医学姓名:肖文美指导教师:贾竹青【摘 要】脂肪组织是人体内重要的内分泌组织,分泌多种重要的激素。
脂连素是一种新的由脂肪细胞特异性分泌的蛋白,具有调节糖脂代谢、抗炎症反应、抗动脉粥样硬化等多重效应。
肥胖、Ⅱ型糖尿病、冠心病患者脂连素水平降低。
脂连素的水平和胰岛素敏感性密切相关,是后者的独立预测因子。
【关键词】脂连素;胰岛素抵抗;肥胖;Ⅱ型糖尿病【Abstract】Fat tissue, which is an important endocrine tissue in our bodies, secretes various important hormone. Adiponectin, which is a new protein secreted specifically by fat cell, has various functions, such as regulating metabolism, anti-inflammtory, resisting atherosclerosis. Adiponectin decreased is common in obesity and type Ⅱdiabetes patients. Adiponectin associating closely with insulin sensitivity, is an independence forecast factor of the latter.【Key words】adiponectin;insulin resistance;obesity;type Ⅱ diabetes一、脂连素的结构脂连素是脂肪细胞特异性分泌的一种新型蛋白,包含244个氨基酸,一级序列分析提示其包含四个功能区:前18个氨基酸的信号肽,23个氨基酸组成的氨基端非螺旋功能区、一段22个胶原重复序列(其中包括8个完全重复Gly-X-Pro及14个不完全重复Gly-X-Y)和137个氨基酸组成的羧基端,后者可能参与形成羧基端的球形功能区。
脂联素医学课件

02
03
04
脂联素与神经系统疾病
帕金森病是一种常见的神经系统疾病,表现为肌肉僵硬、震颤和运动障碍等症状。脂联素可以减轻帕金森病的症状,改善神经细胞的生存和功能。
研究发现,脂联素可以激活脑内多巴胺能神经元及其通路,从而增加多巴胺的合成和释放,缓解帕金森病患者的症状。
脂联素与帕金森病
阿尔茨海默病是一种以认知障碍为特征的神经系统退行性疾病。脂联素可以减轻阿尔茨海默病的症状,并参与调节脑内炎症和氧化应激反应。
脂联素与其他神经系统疾病
05
脂联素的临床应用与展望
总结词
脂联素与心血管疾病
脂联素与糖尿病
脂联素基因检测
脂联素的临床检测与应用
01
02
03
04
脂联素可以与其他生物标志物联合应用,如BMI、腰围、血压、血糖和血脂等指标,共同评估个体的健康风险。
总结词
脂联素与其他生物标志物的联合应用
BMI(体重指数)和腰围是评估肥胖和中心型肥胖的重要指标,与脂联素水平密切相关。联合检测BMI和腰围可以更全面地评估个体的代谢状况和心血管疾病风险。
脂联素与糖尿病并发症
脂联素水平下降可导致微血管病变、神经病变等多种糖尿病并发症的发生。
脂联素与糖尿病
脂联素与血管内皮功能
脂联素对血管内皮功能具有保护作用,可减轻内皮损伤,抑制炎症反应,从而降低高血压的发病风险。
脂联素与高血压的关联
脂联素水平与高血压的发生呈负相关,低脂联素水平可能导致高血压的发生。
研究发现,脂联素可以抑制小胶质细胞的活化和炎症反应,从而减轻阿尔茨海默病的症状。此外,脂联素还可以抑制脑内氧化应激反应,保护神经细胞免受损伤。
脂联素与阿尔茨海默病
研究还发现,脂联素可能参与调节其他神经系统疾病的发病和进展,包括癫痫、多发性硬化和肌萎缩性侧索硬化症等。
脂联素与冠状动脉慢血流现象的关系

ma l Li ga 2 0 i: n to 0 6@ g i. o mal c r n
根据 Gbo 等 的报告 , T C中将造影剂 完全进 i n s CF 入 血管 的第 一 帧定 义 为 首 帧 , 影 剂开 始 进 入 血 管 造
( C 采用 左前 斜 位加 头 位 投 照 , 降 支 ( A 和 微 血管功 能 障碍有 关 。脂联 素是 由脂肪 细胞 合 成 R A) 前 L D) 回旋支 ( C 分 别采 用右 前斜 位或 左前 斜 位 加足 位 并 分 泌的 一 种 蛋 白质 激 素 , 能 量 代 谢 、 岛 素 抵 L X) 在 胰
r u in f a t d c e s d h c u n e o F n r a e mo gt s ai t i t e d c e s fpa go p sg i c nl e r a e .T eo c re c f S P i c e s d a n o ep t n sw t h e r a eo l maAP i y C h e h s N.Co cu i n n l so
i u oobn sa E IA) xmi t nW a ei r t ebodt t hpt n ea fnt n b o t cmpe e ts mm n sre t sy( LS .E a n i a m d ui l s, eai adrn lu ci , l df , o l estet a ao s no n e c o o a t s
床 的重 视 。Tmb … 于 17 a e等 92年 首 先 报 道 了 6例 11 研 究对 象 2 1 . 00年 6月 ~2 1 0 1年 6月 西 京 医 有胸 痛症 状 的患 者 , A 没 有 发 现 血 管 病 变 , 发 院心 内科 住 院 患者 , 校 正 TMI 计 数 ( T C) CG 但 经 I 帧 C F 诊
脂联素

内皮细胞、单核细胞、胰岛B细胞、巨噬细胞和受损血管内 皮细胞等均有AdipoR的表达。大动脉内皮细胞和胰岛B细胞 同时表达AdipoR1和AdipoR2两种受体,但是优先表达AdipoR1 的mRNA。
图4 两种脂联素受体的立体图
-
脂联素的编码基因
吴健雄学院 2015.10.21
脂联素的基因由apM1编码,不同种属其基因位点不同, 在人类定位于3q27染色体,全长约16 kb。apM1基因由3个 外显子(从18 bp到4 277 bp)和2个内含子(0.8 kb和12 kb)构 成。
新生儿血清脂联素水平与出生体重及头围呈正相关,儿童 青春发育前血清脂联素水平并无明显的性别差异 , 到了青 春发育期则与成年人相似。女童血清脂联素水平明显高于 男童, 男童脂联素水平呈V 字型 , 在10-12岁时达到低谷 ,这 可能与青春期性激素水平有关。
-
脂联素受体
吴健雄学院 2015.10.21
-
脂联素的表达
吴健雄学院 2015.10.21
在人、猴子、小鼠中研究结果发现, 脂肪组织脂联素基因表 达量和血浆脂联素水平随脂肪过度沉积而下降, 随体重下降 而上升。 体内脂联素表达与脂肪沉积密切相关, 但是两者之 间如何调控的机理还不清楚。
-
脂联素的表达
吴健雄学院 2015.10.21
调节脂联素在脂肪细胞表达的因素有生脂转录因子过氧化物 酶增殖激活受体-γ,激动剂(TZD)、β-肾上腺能受体、第2信 使cAMP、IL-6、IGF-I及皮质激素等。此外,影响脂联素基因 表达的还有生长激素、睾酮、内皮素-1及儿茶酚胺等。
脂联素结构
吴健雄学院 2015.10.21
脂联素通过3个球形结构域单体连接成三聚体,4-6个三 聚体通过胶原结构域链接形成低聚体或者高级结构,其在 血浆内的浓度为5-30μg/ml,有全长和球形两种循环形式。
脂联素含量检测的金标准_概述说明以及解释

脂联素含量检测的金标准概述说明以及解释1. 引言1.1 概述脂联素(adiponectin)是一种由脂肪组织分泌的重要激素,对机体的代谢调节和炎症反应具有重要作用。
近年来,随着对脂联素功能研究的深入和相关临床疾病的发现,脂联素含量检测逐渐成为关注的焦点。
准确测定脂联素含量是了解其生物学功能、评估机体健康状态以及辅助临床诊断的重要手段。
1.2 文章结构本文将从以下几个方面对脂联素含量检测进行探讨。
首先,我们将介绍脂联素的重要性和作用,以便更好地理解为什么脂联素含量检测具有如此大的意义。
接着,我们将评估目前现有脂联素检测方法存在的问题,并提出改进建议。
然后,我们将详细阐述针对血清和组织中脂联素的检测方法,并解释其原理与验证过程。
同时,我们也会探讨在进行脂联素含量检测时可能遇到的挑战,并提出解决方案。
最后,我们将总结文中所述要点,并对未来脂联素含量检测的发展方向进行展望。
1.3 目的本文的目的在于全面概述和说明脂联素含量检测的金标准。
通过详细介绍脂联素的重要性和作用,以及现有检测方法存在的问题,旨在提高人们对脂联素含量检测重要性的认识,并促进相关技术的发展。
通过明确脂联素含量检测的方法与原理,以及解决可能遇到挑战的方案,旨在为研究人员和临床专家提供可靠可行的指导。
最终,本文希望能为未来脂联素含量检测研究指引提供一定参考,并对其发展方向进行探讨。
2. 脂联素含量检测的金标准2.1 脂联素的重要性和作用脂联素是一种由脂肪细胞分泌的激素,它在机体内部起着重要调节代谢和整体健康状况的作用。
脂联素能够影响胰岛素敏感性、糖代谢、脂肪酸氧化和炎症反应等多个生理过程。
因此,在许多代谢性疾病如肥胖、2型糖尿病和心血管疾病中,脂联素含量成为了一个被广泛关注的指标。
2.2 现有脂联素检测方法的问题目前,存在各种各样的方法来检测脂联素含量,如酶联免疫吸附法(ELISA)、放射免疫分析法(RIA)以及质谱等。
然而,这些传统方法存在一些问题。
脂联素医学课件

脂联素在肥胖症治疗中的作用
能量代谢调节
脂联素可以调节能量代谢,减少脂肪 堆积和增加能量消耗。在肥胖治疗中 ,增加脂联素水平可以促进脂肪燃烧 和减少体重。
改善胰岛素抵抗
脂联素还具有改善胰岛素抵抗的作用 ,这对于肥胖伴糖尿病的治疗尤为重 要。通过增加脂联素水平,可以增强 胰岛素敏感性,降低血糖水平。
脂联素在心血管疾病预防中的应用
脂联素与代谢综合征
02
脂联素水平下降与代谢综合征的发生有关,增加心血管疾病的
风险。
脂联素与肥胖相关并发症
03
肥胖症患者常伴有高血压、血脂异常等问题,这些问题可能与
脂联素水平异常有关。
脂联素与心血管疾病
1 2
脂联素与动脉粥样硬化
脂联素参与了动脉粥样硬化斑块的稳定性和炎症 反应过程。
脂联素与心肌梗死
低脂联素水平可能增加心肌梗死的风险,因为它 与动脉粥样硬化的发生和发展有关。
脂联素最新研究成果
01
02
03
研究1
脂联素在糖尿病治疗中的 新进展
研究2
脂联素与心血管疾病预防 和治疗的新发现
研究3
脂联素在肥胖和代谢综合 征治疗中的突破性进展
THANKS
感谢观看
03
脂联素与代谢性疾病
脂联素与糖尿病
脂联素与胰岛素抵抗
脂联素能够提高胰岛素敏感性,降低胰岛素抵抗的风险。
脂联素与β细胞功能
脂联素可以保护β细胞免受炎症和氧化应激的损伤,维持其正常功 能。
脂联素与糖尿病并发症
脂联素水平下降与糖尿病并发症的发生和发展密切相关。
脂联素与肥胖症
脂联素与脂肪积累
01
脂联素水平降低可能导致脂肪在腹部和肝脏等部位的积累。
脂联素与动脉粥样硬化

脂联素与动脉粥样硬化作者:张闽杨晔文章来源:心血管论坛点击数:更新时间:2007-4-11 脂肪组织不仅作为能量贮存组织,而且是一个能分泌多种细胞因子的内分泌器官,脂联素为脂肪组织分泌的一种脂肪细胞因子,在动物和人类的实验中证实具有改善胰岛素抵抗、抗动脉粥样硬化、抗炎等作用。
在临床上,与肥胖、胰岛素抵抗、2型糖尿病及心血管疾病密切相关。
脂联素具有抗动脉粥样硬化的功能,其机理已有一定的阐述,但仍需要进一步研究。
一、脂联素概述脂联素(adiponectin)亦称Acrp30、GBP28或AdipoQ,是由脂肪细胞分泌一种激素蛋白,1995年Scherer等[1]首先从鼠的脂肪细胞分离出来,基因定位于3q27,全长17kb,由3个外显子2个内含子组成;其蛋白质结构由244个氨基酸组成,分为3个部分:氨基末端的信号肽、中间的胶原样结构域及羧基末端的球形结构域。
其球形结构域的三维结构与TNFa极其相似,是其发挥功能的主要结构,提示脂联素的作用与TNFa 存在一定的关联。
脂联素受体有两类基因,分别定义为AdipoR1和AdipoR2,AdipoR1表达广泛,以骨骼肌的表达最为丰富,而AdipoR2则主要表达于肝脏[2]。
脂联素是一种血浆含量相对丰富的蛋白,正常人血浆浓度范围为1.9~17.0mg/PL,平均血浆浓度为(8.9±1.5)mg /PL。
影响其血浆浓度的因素较多,从基因水平上看,R112C突变者,其血浆脂联素水平显著下降;肥胖症、胰岛素抵抗、心血管疾病等疾病状态的患者血浆脂联素水平较正常人明显降低;脂联素的分泌呈性别相关性,女性比男性高,目前认为主要与雄激素的影响有关;Pajvani UB[3]等研究认为与脂联素的分子构成有关,在男性以低分子量的脂联素为主,而女性则低分子量和高分子量成分均等;在各种脂肪细胞因子中,大部分认为TNFa最有可能起主要的反馈抑制作用。
脂联素具有改善胰岛素抵抗,增加胰岛素增敏性作用[2]:脂联素通过激活AMPK(AMP活化蛋白激酶),增加机体对脂质的摄取及氧化作用,降低肌肉组织及肝脏的FFA水平;增加肌肉组织的糖摄取;抑制糖异生的关键酶—烯醇式丙酮酸羧激酶(PEPCK)和葡萄糖六磷酸酶(G6Pase)使肝糖输出减少。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
脂联素的发现
吴健雄Байду номын сангаас院 2015.10.21
Maeda 等Q 的类似物, 因其与胶原质Ⅹ、Ⅷ和补体因 子C1q 有高度相似, 并且在脂肪组织中基因转录产物最丰富, 故称为 apM1;
Nakano 等( 1996) 用明胶亲和层析分离人血浆蛋白时发现 了该蛋白质, 故命名为明胶结合蛋白( GBP28);
脂联素结构
吴健雄学院 2015.10.21
脂联素通过3个球形结构域单体连接成三聚体,4-6个三 聚体通过胶原结构域链接形成低聚体或者高级结构,其在 血浆内的浓度为5-30μg/ml,有全长和球形两种循环形式。
图2 脂联素的三聚体和低聚体
脂联素含量在人群中的差异 吴健雄学院 2015.10.21
人类脂联素mRNA在胚胎发育的中晚期开始表达 , 起缘于中 胚层和外胚层。 分娩时脐带血中脂联素水平(11.7-35.7μg/ml) 明显高于年长儿和成年人。 脂联素的表达和分泌总体来说 是女性(11.7 μg/ml)高于男性(7.9 μg/ml)。
脂联素与能量代谢
吴健雄学院 2015.10.21
脂联素具有调节葡萄糖转运的作用 。研究发现通过静脉 注射脂联素可以降低肝脏磷酸烯醇丙酮酸羧化酶和葡糖6-磷酸酶的mRNA 表达 , 抑制肝糖生成酶的表达 , 从而减 少了内源性糖的生成。
脂联素与能量代谢
吴健雄学院 2015.10.21
脂联素能够影响机体处理糖类和脂肪的能力。脂联素 可能通过抑制TNF-α通路增强胰岛素PI-3K通路,增强胰岛素 的敏感性。同时β细胞是脂联素直接作用的靶细胞,血清脂 联素浓度和β细胞上脂联素受体的多少决定了脂联素对β细 胞的作用强弱,这暗示了脂联素对调节糖代谢有着双重作 用,即增强胰岛素敏感性及改善β细胞功能。
TZD可刺激脂联素基因表达,增强其启动子活性,升高循 环脂联素水平;IGF—I则在转录水平增加脂联素表达;而 IL-6、C反应蛋白、皮质激素和TNF-α抑制脂联素基因表达。 大量研究结果表明, 人和动物的脂肪细胞脂联素表达及其 血浆浓度与空腹血糖、空腹胰岛素、胰岛素抵抗程度呈负 相关, 与胰岛素敏感性呈正相关。
脂联素的表达
吴健雄学院 2015.10.21
调节脂联素在脂肪细胞表达的因素有生脂转录因子过氧化物 酶增殖激活受体-γ,激动剂(TZD)、β-肾上腺能受体、第2信 使cAMP、IL-6、IGF-I及皮质激素等。此外,影响脂联素基因 表达的还有生长激素、睾酮、内皮素-1及儿茶酚胺等。
脂联素的表达
吴健雄学院 2015.10.21
脂联素
脂联素介绍
吴健雄学院 2015.10.21
脂联素( adiponectin) 就是脂肪细胞分泌的一种脂肪细 胞因子, 是迄今发现的唯一与肥胖呈负相关的脂肪细胞特 异性蛋白。近几年的研究发现, 脂联素具有直接抗糖尿病、 抗动脉粥样硬化、抗炎和抗血管形成的特性。
脂联素的发现
吴健雄学院 2015.10.21
高度相关,两者有66.7%的同源性。
图3 脂联素受体结构
脂联素受体
吴健雄学院 2015.10.21
人体多种组织细胞表面均有脂联素受体的分布和表达, AdipoR1主要表达在骨骼肌细胞,对脂联素的球状结构域具 有高亲和力,但对全长脂联素亲和力低,而AdipoR2主要表 达在肝细胞,对两者具有中等亲和力。
脂联素受体
吴健雄学院 2015.10.21
内皮细胞、单核细胞、胰岛B细胞、巨噬细胞和受损血管内 皮细胞等均有AdipoR的表达。大动脉内皮细胞和胰岛B细胞 同时表达AdipoR1和AdipoR2两种受体,但是优先表达AdipoR1 的mRNA。
图4 两种脂联素受体的立体图
脂联素的编码基因
吴健雄学院 2015.10.21
脂联素是由4个研究小组分别用不同的方法独立鉴定出来的。
Scherer其与补体 C1q有很近的同源性, 且相对分子质 量为 30*103Da, 故命名为脂肪细胞补体相关蛋白( ACRP30);
Hu 等(1996) 用mRNA差异显示技术从鼠脂肪中分离并克隆出该 基因, 命名为 AdipoQ;
脂联素的基因由apM1编码,不同种属其基因位点不同, 在人类定位于3q27染色体,全长约16 kb。apM1基因由3个 外显子(从18 bp到4 277 bp)和2个内含子(0.8 kb和12 kb)构 成。
脂联素的表达
吴健雄学院 2015.10.21
在人、猴子、小鼠中研究结果发现, 脂肪组织脂联素基因表 达量和血浆脂联素水平随脂肪过度沉积而下降, 随体重下降 而上升。 体内脂联素表达与脂肪沉积密切相关, 但是两者之 间如何调控的机理还不清楚。
脂联素受体
吴健雄学院 2015.10.21
Yamauchi等(2003)首次克隆出人类和小鼠脂联素受体,发现
高度保守的脂联素受体(AdipoR)有两种构成:AdipoR1和
AdipoR2,并发现人和鼠AdipoR1基因有96.8%的同源性,
AdipoR2基因有95.2%的同源性,AdipoR1和AdipoR2结构
脂联素含量在人群中的差异 吴健雄学院 2015.10.21
新生儿血清脂联素水平与出生体重及头围呈正相关,儿童 青春发育前血清脂联素水平并无明显的性别差异 , 到了青 春发育期则与成年人相似。女童血清脂联素水平明显高于 男童, 男童脂联素水平呈V 字型 , 在10-12岁时达到低谷 ,这 可能与青春期性激素水平有关。
Arita 等( 1999) 将 apM 1基因产物命名为脂联素。
脂联素结构
吴健雄学院 2015.10.21
人类脂联素由244个氨基酸组成(鼠为247个),翻译
后修饰为8种不同的同源蛋白,经胰蛋白酶裂解后得到球
形结构域,其活性远远大于脂联素,而且与Clq和TNF-α家
族具有结构同源性。
图1 人与鼠的脂联素结构
脂联素的表达
吴健雄学院 2015.10.21
在培养的 3T3-
L1 脂肪细胞,
脂联素的表达
吴健雄学院 2015.10.21
肿瘤坏死因子α ( TNF-α) 对脂联素基因的表达起负调控作用, 并依赖于时间和剂量。原因是脂联素和 TNF-α的结构相似, 它 们能够与对方的受体结合, 通过同一个信号通道, 发挥各自的 生物学效应。去甲肾上腺素抑制 3T3-L1 细胞中脂联素 mRAN 表达水平, 并呈剂量依赖性 。
