Staurosporine_62996-74-1_DataSheet_MedChemExpress
国际化妆品原料标准中文名称、INCI名、CAS号查询表(2010年版)

序号
1681 5894 10462 12033 1493 1494 1682 1679 1685 8598 10441 5892 5893 5896 5897 5895 8599 6505 6506 8241 8602 6523 66 71 660 14686 8209 8210 1683 3847 8600 3852 3851 3854 3855 3856 3857 3848 4272 8206 14846 4080 3860 3858 3859
中文名称
1,2-丁二醇 1,2-己二醇 1,2-戊二醇 1,3-丙二醇 1,3-双-(2,4-二氨基苯氧基)丙烷 1,3-双-(2,4-二氨基苯氧基)丙烷 HCl 1,4-丁二醇 1,4-丁二醇/琥珀酸/己二酸/HDI 共聚物 1,4-丁二醇二(甲基丙烯酸)酯 1,5-萘二酚 1,5-戊二醇 1,6-己二胺 1,6-己二醇 1,6-己二醇二水杨酸酯 1,6-己二醇二硬脂酸酯 1,6-己二醇蜂蜡酸酯 1,7-萘二酚 10-羟基癸酸 10-羟基癸烯酸 1-甲基乙内酰脲-2-酰亚胺 1-萘酚 1-羟乙基-4,5-二氨基吡唑硫酸盐 1-乙酰萘 1-乙酰氧基-2-甲基萘 2-(2-氨基乙氧基)乙醇 2,2'-硫代双(4-氯苯酚) 2,2'-亚甲基双 4-氨基苯酚 2,2'-亚甲基双-4-氨基苯酚 HCl 2,3-丁二醇
国际化妆品原料标准中文名称目录(2010年版)
序号 INCI名称 中文名称
酸 (C10-18 脂酸甘油三酯类聚甘油-3酯类) 磷酸酯类 (动物)肝水解产物 (动物)睾丸水解产物 (动物)脊髓索带提取物 (动物)脊髓索带脂质 (动物)脊髓脂质提取物 (动物)脐带提取物 (动物)气管水解产物 (动物)乳房提取物 (动物)神经提取物 (动物)胎盘蛋白 (动物)胎盘酶 (动物)胎盘脂质 (动物)心脏水解产物 (动物)心脏提取物 (动物)胸腺水解产物 (动物)胸腺提取物 (镁/钾/硅)(氟化物/氢氧化物/氧化 物) (牛)肝提取物 (牛)睾丸提取物 (牛)骨髓类脂质 (牛)骨髓提取物 (牛)肌肉提取物 (牛)卵巢提取物 (牛)脾提取物 (牛)网膜类脂质 (牛/猪)脑提取物 (日用)香精 (三油酰氧甲基)甲氨基乙醇硫酸酯 (神经)鞘磷脂 (神经)鞘脂类 (四丁氧基)丙基三硅氧烷 (天冬氨酸/谷氨酸)金 (辛/癸)酸异辛酯 (椰油/葵花籽油)酰胺丙基甜菜碱 (月桂/肉豆蔻)基二醇羟丙基醚 1-(3,4-二甲氧基苯基)-4,4-二甲基1,3-戊二酮 1,10-癸二醇 1,2,4-苯三酚三乙酸酯 1,2,4-三羟基苯 1,2,6-己三醇 1
药用辅料中英文对照

药用辅料中英文对照1 阿拉伯胶(Acacia)2 乙酰舒泛钾(Acesulfame Potassium)3 冰醋酸(Acetic Acid,Glacial)4 乙酰枸橼酸三丁酯(AcetyltributylCitrate)5 乙酰枸橼酸三乙酯(AcetyltriethylCitrate)6 人血白蛋白(Albumin)7 乙醇(Alcohol)8 海藻酸(Alginic Acid)9 脂肪族聚酯(Aliphatic Polyesters)10 阿力糖(Alitame)11 杏仁油(Almond Oil)12 维生素E(Alpha Tocopherol)13 氨溶液(Ammonia Solution)14 维生素C(Ascorbic Acid)15 棕榈酸维生素C酯(Ascorbyl Palmitate)16 阿司帕坦(Aspartame)17 绿坡缕石(Attapulgite)18 皂土(Bentonite)19 苯扎氯铵(Benzalkonium Chloride)20 苄索氯铵(Benzethonium Chloride)21 苯甲酸(Benzoic Acid)22 苯甲醇(Benzyl Alcohol)23 苯甲酸苄酯(Benzyl Benzoate)24 溴硝丙二醇(Bronopol)25 丁羟茴醚(Butylated Hydroxyanisole)26 丁羟甲苯(Butylated Hydroxytoluene)27 羟苯丁酯(Butylparaben)28 碳酸钙(Calcium Carbonate)29 无水磷酸氢钙(Calcium Phosphate,Dibasic Anhydrous)30 磷酸氢钙二水合物(Calcium Phosphate,Dibasic Dihydrate)31 磷酸钙(Calcium Phosphate,Tribasic)32 硬脂酸钙(Calcium Stearate)33 硫酸钙(Calcium Sulfate)34 低芥酸菜籽油(Canola Oil)35 卡波姆(Carbomer)36 二氧化碳(Carbon Dioxide)37 羧甲纤维素钙(Carboxymethylcellulose Calcium)38 羧甲纤维素钠(Carboxymethylcellulose Sodium)39 角叉菜胶(Carrageenan)40 蓖麻油(Castor Oil)41 氢化蓖麻油(Castor Oil,Hydro-genated)42 微晶纤维素(Cellulose,Microcr ystalline)43 粉状纤维素(Cellulose,Powdered)44 微粉硅胶微晶纤维素(Cellulose, Silicified Microcrystalline)45 醋酸纤维素(Cellulose Acetate)46 纤维醋法酯(Cellulose Acetate Phthalate)47 角豆胶(Ceratonia)48 十八十六醇(Cetostearyl Alcohol)49 西曲溴铵(Cetrimide)50 十六醇(Cetyl Alcohol)51 壳聚糖(Chitosan)52 氯己定(Chlorhexidine)53 三氯叔丁醇(Chlorobutanol)54 氯甲酚(Chlorocresol)55 一氯二氟乙烷(Chlorodifluoroe-thane)56 氟里昂(Chlorofluorocabons)57 对氯间二甲酚(Chloroxylenol)58 胆固醇(Cholesterol)59 枸橼酸(Citric Acid Monohydrate)60 胶态二氧化硅(微粉硅胶)(Colloidal Silicon Dioxide)61 着色剂(Coloring Agents)62 玉米油(Corn Oil)63 棉籽油(Cottonseed Oil)64 甲酚(Cresol)65 交联羧甲纤维素钠(Croscarmellose Sodium)66 交联聚维酮(Crospovidone)67 环糊精(Cyclodextrins)68 环甲基硅酮(Cyclomethicone)69 苯甲地那铵(Denatonium Benzoate)70 葡萄糖结合剂(Dextrates)71 糊精(Dextrin)72 葡萄糖(Dextrose)73 邻苯二甲酸二丁酯(Dibutyl Phthalate)74 癸二酸二丁酯(Dibutyl Sebacate)75 二乙醇胺(Diethanolamine)76 邻苯二甲酸二乙酯(Diethyl Phthalate)77 二氟乙烷(Difluoroethane)78 二甲硅油(Dimethicone)79 二甲醚(Dimethyl Ether)80 邻苯二甲酸二甲酯(Dimethyl Phthalate)81 二甲亚砜(Dimethyl Sulfoxide)82 多库酯钠(Docusate Sodium)83 依地酸(乙二胺四乙酸)(Edetic Acid)84 乙酸乙酯(Ethyl Acetate)85 乙基麦芽酚(Ethyl Maltol)86 油酸乙酯(Ethyl Oleate)87 乙基香草醛(Ethyl Vanillin)88 乙基纤维素(Ethylcellulose)89 硬脂酸棕榈酸乙二醇酯(Ethylene Glycol Palmitostearate)90 羟苯乙酯(Ethylparaben)91 果糖(Fructose)92 富马酸(Fumaric Acid)93 明胶(Gelatin)94 液体葡萄糖(Glucose,Liquid)95 甘油(Glycerin)96 山萮酸甘油酯(Glyceryl Behenate)97 单油酸甘油酯(Glyceryl Monooleate)98 单硬脂酸甘油酯(Glyceryl Monostearate)99 硬脂酸棕榈酸甘油酯(Glyceryl Palmitostearate) 100 四氢呋喃聚乙二醇醚(Glycofurol)101 瓜耳胶(Guar Gum)102 七氟丙烷(HFC)(Heptafluoro-propane)103 海克西定(Hexetidine)104 烷烃类(HC) (Hydrocarbons)105 盐酸(Hydrochloric Acid)106 羟乙纤维素(Hydroxyethyl Cellulose)107 羟乙甲纤维素(Hydroxyethylmethyl Cellulose) 108 羟丙纤维素(Hydroxypropyl Cellulose)109 低取代羟丙纤维素(Hydroxypropyl Cellulose,Low-substituted)110 羟丙甲纤维素(Hypromellose)111 羟丙甲纤维素酞酸酯(Hypromellose Phthalate)112 咪唑烷脲(Imidurea)113 异丙醇(Isopropyl Alcohol)114 肉豆蔻酸异丙酯(Isopropyl Myristate)115 棕榈酸异丙酯(Isopropyl Palmitate)116 白陶土(Kaolin)117 乳酸(Lactic Acid)118 拉克替醇(Lactitol)119 乳糖(Lactose)120 羊毛脂(Lanolin)121 含水羊毛脂(Lanolin,Hydrous)122 羊毛醇(Lanolin Alcohols)123 卵磷脂(Lecithin)124 硅酸镁铝(Magnesium Aluminum Silicate) 125 碳酸镁(Magnesium Carbonate)126 氧化镁(Magnesium Oxide)127 硅酸镁(Magnesium Silicate)128 硬脂酸镁(Magnesium Stearate)129 三硅酸镁(Magnesium Trisilicate)130 苹果酸(Malic Acid)131 麦芽糖醇(Maltitol)132 麦芽糖醇溶液(Maltitol Solution)133 麦芽糖糊精(Maltodextrin)134 麦芽酚(Maltol)135 麦芽糖(Maltose)136 甘露醇(Mannitol)137 中链脂肪酸甘油三酯(Medium-chain Triglycerides) 138 葡甲胺(Meglumine)139 薄荷脑(Menthol)140 甲基纤维素(Methylcellulose)141 羟苯甲酯(Methylparaben)142 液体石蜡(Mineral Oil)143 轻质液体石蜡(Mineral Oil,Light)144 液体石蜡羊毛醇(Mineral Oil and Lanolin Alcohols) 145 单乙醇胺(Monoethanolamine)146 谷氨酸一钠(Monosodium Glutamate)147 硫代甘油(Monothioglycerol)148 氮(Nitrogen)149 一氧化二氮(Nitrous Oxide)150 油酸(Oleic Acid)151 橄榄油(Olive Oil)152 石蜡(Paraffin)153 花生油(Peanut Oil)154 凡士林(Petrolatum)155 凡士林羊毛醇(Petrolatum and Lanolin Alcohols) 156 苯酚(Phenol)157 苯氧乙醇(Phenoxyethanol)158 苯乙醇(Phenylethyl Alcohol)159 醋酸苯汞(Phenylmercuric Acetate)160 硼酸苯汞(Phenylmercuric Borate)161 硝酸苯汞(Phenylmercuric Nitrate)162 磷酸(Phosphoric Acid)163 波拉克林钾(Polacrilin Potassium)164 泊洛沙姆(Poloxamer)165 葡聚糖(Polydextrose)166 聚乙二醇(Polyethylene Glycol)167 聚氧乙烯(Polyethylene Oxide)168 聚(甲基)丙烯酸树脂(Polymethacr-ylates)169 聚氧乙烯烷基醚(Polyoxyethylene Alkyl Ethers)170 聚氧乙烯蓖麻油衍生物(Polyoxyeth-ylene Castor Oil Derivatives)171 聚山梨酯(Polyoxyethylene Sorbitan Fatty Acid Esters) 172 硬脂酸聚氧乙烯酯(Polyoxyethylene Stearates)173 聚醋酸乙烯酞酸酯(Polyvinyl Acetate Phthalate)174 聚乙烯醇(Polyvinyl Alcohol)175 苯甲酸钾(Potassium Benzoate)176 碳酸氢钾(Potassium Bicarbonate)177 氯化钾(Potassium Chloride)178 枸橼酸钾(Potassium Citrate)179 氢氧化钾(Potassium Hydroxide)180 焦亚硫酸钾(Potassium Metabisulfite)181 山梨酸钾(Potassium Sorbate)182 聚维酮(Povidone)183 丙酸(Propionic Acid)184 没食子酸丙酯(Propyl Gallate)185 碳酸丙烯酯(Propylene Carbonate)186 丙二醇(Propylene Glycol)187 海藻酸丙二醇酯(Propylene Glycol Alginate)188 羟苯丙酯(Propylparaben)189 糖精(Saccharin)190 糖精钠(Saccharin Sodium)191 芝麻油(Sesame Oil)192 虫胶(Shellac)193 二氧化硅二甲硅油(Simethicone)194 海藻酸钠(Sodium Alginate)195 抗坏血酸钠(Sodium Ascorbate)196 苯甲酸钠(Sodium Benzoate)197 碳酸氢钠(Sodium Bicarbonate)198 氯化钠(Sodium Chloride)199 枸橼酸钠二水合物(Sodium Citrate Dihydrate)200 环拉酸钠(Sodium Cyclamate)201 氢氧化钠(Sodium Hydroxide)202 月桂硫酸钠(十二烷基硫酸钠)(Sodium Lauryl Sulfate)203 焦亚硫酸钠(偏亚硫酸钠)(Sodium Metabisulfite)204 磷酸氢二钠(Sodium Phosphate,Dibasic)205 磷酸二氢钠(Sodium Phosphate ,Monobasic)206 丙酸钠(Sodium Propionate)207 羧甲淀粉钠(Sodium Starch Glycolate)208 硬脂富马酸钠(Sodium Stearyl Fumarate)209 山梨酸(Sorbic Acid)210 山梨坦酯Sorbitan Esters(Sorbitan Fatty Acid Esters) 211 山梨醇(Sorbitol)212 大豆油(Soybean Oil)213 淀粉(Starch)214 预胶化淀粉(Starch,Pregelatinized)215 灭菌玉米淀粉(Starch,Sterilizable Maize)216 硬脂酸(Stearic Acid)217 硬脂醇(Stearyl Alcohol)218 羟糖氯(Sucralose)219 蔗糖(Sucrose)220 可压性蔗糖(Sugar,Compressible)221 蔗糖粉(Sugar,Confectioner’s)222 蔗糖球形颗粒(Sugar Spheres)223 硫酸(Sulfuric Acid)224 葵花籽油(Sunflower Oil)225 氢化植物油(硬脂)栓剂基质(Sup-pository Bases,Hard Fat)226 滑石粉(Talc)227 酒石酸(Tartaric Acid)228 四氟乙烷(HFC)(Tetrafluoroe-thane)229 硫柳汞(Thimerosal)230 二氧化钛(Titanium Dioxide)231 西黄蓍胶(Tragacanth)232 海藻糖(Trehalose)233 三醋汀(Triacetin)234 枸橼酸三丁酯(Tributyl Citrate)235 三乙醇胺(Triethanolamine)236 枸橼酸三乙酯(Triethyl Citrate)237 香草醛(Vanillin)238 氢化植物油(Vegetable Oil,Hydrogenated) 239 水(Water)240 阴离子乳化蜡(Wax,Anionic Emulsifying) 241 巴西棕榈蜡(Wax,Carnauba)242 十六醇酯蜡(Wax,Cetyl Esters)243 微晶蜡(Wax,Microcrystalline)244 非离子乳化蜡(聚西托醇乳化蜡)(Wax,Nonionic Emulsifying)245 白蜡(Wax,White)246 黄蜡(Wax,Yellow)247 黄原酸胶(Xanthan Gum)248 木糖醇(Xylitol)796249 玉米朊(玉米蛋白)(Zein) 250 硬脂酸锌(Zinc Stearate)。
耐甲氧西林金黄色葡萄球菌恒温扩

体积 10ul 180ul 40ul 1.0ml -
数量 20 管 1管 1管 1管 1份
编号 1 2 3 4
试剂盒组成 一次性核酸检测装置 (3 号)
色卡 备用液泡 装置操作说明书
体积 -
数量 20 份 1份 2颗 1份
*装有反应液的反应管中包括除样本外的所有试剂。
上覆矿物油,以防止扩增中产生气溶胶,防止假阳性。
4.1.2 阳性对照出现两条红线:一条位于检测区(T),另一条位于质控区(C)。
4.1.3 每个测试样本至少出现一条质控线,有或无检测线。
4.2 结果描述及判定(如图 2)
4.2.1 仅在质控区 C 出现一条红线,表示样品中无耐甲氧西林的金黄色葡萄球菌或细菌拷贝数低于试剂盒最低检测限。
4.2.2 出现两条红线,一条检测线,一条质控线,表示样品中存在耐甲氧西林的金黄色葡萄球菌。将检测线强度与色卡
1.2 95℃-100℃水浴 10 分钟,然后冷却至室温;
注:水浴过程中离心管由于受热,盖子可能会弹开,所以要在操作过程中注意观察(建议选用防爆管)。
1.3 以>10,000rpm 的转速离心 5 分钟,取上清液作为反应模板;
1.4 处理后的样本应尽快使用,或将上清吸出后保存于-20℃~-80℃,最长不超过 1 个月(不宜反复冻融)。
3.2 按手柄至检测装置于关闭状态(听见清脆的“咔嚓”声);
3.3 将检测装置放置在操作台上,10-30 分钟内通过阅读窗判读结果,30 分钟后判读结果无效;
公司地址:杭州市滨江区南环路 3766 号新世纪大厦 8 层
电话:0571-88939368
技术支持:0571-88939360
公司网址:
【储运条件及有效期】
骨钙素N端中分子片段检测试剂盒(酶联免疫法)说明书

14.洗板机要经常保养,以免试验中由于机械问题导致 试验失败或交叉污染。 15.所有试剂和实验室设备应按传染性物品处理和丢弃。
注意事项:由于不同的实验方法和试剂在识别位点、特
异性及干扰因素等诸方面不尽相同,因此对于某一特定
样本,其测定结果也存在一定差异;所以实验室在向临
床医生提供检测结果的同时也必须包括相应的实验方
如果离失效期不足 8 周,则以有效期为准。 复溶后校准品和质控品应在–18°C 以下保存不超
过 3 个月,且只能冻融两次。当抗体试剂混合后,剩余 混合物应存放在 2-8°C 且不超过 1 个月,或在–18°C 以下冰冻保存。
浓缩洗液在稀释后室温放置可稳定 2 天,在 2~8 ℃保存可稳定 8 周,如果离失效期不足 8 周,则以有效 期为准。
软件 2. 试剂准备
1) 试剂盒内所有组分和样本平衡至室温(18-22 ℃);酶标板铝箔袋每次使用前也需平衡至室 温,以免在酶标板上凝集水珠,从而影响实验 结果。在取出所需的板条后,余下的须尽快放 回铝箔袋,封口后置于 2-8℃保存。
2) 校准品 0 用 5mL 蒸馏水复溶,校准品 1-5 和质 控品各用 0.5mL 蒸馏水复溶。复溶 15 分钟。
骨钙素n端中分子片段检测试剂盒酶联免疫法说明书酶联免疫试剂盒说明书酶免试剂盒说明书小片段dna回收试剂盒酶联免疫试剂盒降钙素原检测试剂盒碱性磷酸酶试剂盒碱性磷酸酶染色试剂盒胃蛋白酶原试剂盒胃蛋白酶检测试剂盒
骨钙素N端中分子片段检测试剂盒(酶联免疫法)说明书
【产品名称】 通用名称:骨钙素 N 端中分子片段检测试剂盒(酶 联免疫法) 英文名称:N-MID Osteocalcin ELISA
良好实验室管理规范(GLP)要求在每轮实验中使 用质控品以检测实验操作质量。质控品应以待测样本对
抗体公司

赛信通(上海)生物试剂有限公司
上海市浦东南路1101号远东大厦514室,200120 info@cst www.cst 2158356288 公司总部: 美国
Established in Beverly, MA in 1999, Cell Signaling Technology (CST) is a privatelyowned company with over 400 employees worldwide. We are dedicated to providing innovative research tools that are used to help define mechanisms underlying cell function and disease. Since its inception, CST has become the world leader in the production of the highest quality activationstate and total protein antibodies utilized to expand knowledge of cell signaling pathways. Our mission is to deliver the world's highest quality research tools that accelerate progress in biological research and personalized medicine. 总引用数为4670,来自于1966篇文章。最常引用的试剂包括: Akt, ERK2, ERK1, p38, Akt1。
AbD Serotec (BioRad)
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
希森美康血凝仪

CS-2100i/2000i根据SYSMEX在血栓与止血领域多年积累的经验,为全面满足各类实验室的需求率先开发CS-2100i/2000i。
四种方法学:凝固法/发色底物法/免疫比浊法/聚集法unique!多波长高精度光学检测:5种波长检测提供可靠数据新型智能监测:特有的HIL check 功能排除溶血/黄疸/脂血干扰更多新检测项目的选择:FXIII,vWF:Rco等更可靠的实验室质量保证:SNCS实时在线质控全面满足血栓与止血检测需求!CA-7000以全球最快的分析速度提供全面和高精准的止凝血项目检测结果,仪器集中了凝固法,发色底物法和免疫法于一体,设计高度人性化和智能化,操作简便,成为大规模实验室的首选。
● 多参数测试,500测试/小时高速分析能力● 操作简便,灵活对待各种需求● 优秀的试剂管理系统(SRS)● 安全、实用的系统设计● 大容量的数据管理能力,完整的质控系统CA-1500汇集了当今血栓/止血分析仪最新的各种先进功能于一身,是市场上少见的性能/价格比极高的一台仪器,是大型教学医院,综合医院实验室的首选。
它具有快速处理能力,最快180测试/小时,集多种检测功能于一身:凝固法、发色底物法、免疫法。
具有全能随机组合能力,两种方法测定纤维蛋白质,适合常规大量和急诊使用。
● 拥有高速处理能力、随机测试功能和自动再检查功能● 三种分析方式,包括多规则监视的广泛质控文件和平行线生物分析功能● 卓越的性能可以灵活适应实验室的多样化需求CA-500系列CA-500系列包含了六款机型,设计新颖、符合经济原则,是各中小型实验室开展血栓/止血实验的最佳选择,也是半自动升级到全自动的理想机型。
小型台式仪实用可靠,具备三种检测系统即凝固法、发色底物法、免疫法的自由组合用户可根据需要选择相应机型。
CA-50设计上完全沿用了全自动CA系列的检测原理,锁定人为误差因素的设计确保它有别于其他半自动血凝仪,达到全自动仪器的准确性与重复性效果,四通道即可批量检测又可单独检测,内置质控文件,适用于小标本量实验室使用。
细胞衰老特异性β-半乳糖苷酶检测试剂盒产品说明书(中文版)

细胞衰老特异性β-半乳糖苷酶检测试剂盒产品说明书(中文版)主要用途细胞衰老特异性β-半乳糖苷酶检测试剂是一种旨在以X-Gal为底物,通过细胞内β-半乳糖苷酶在酸性条件下稳定表达,催化生成深蓝色的沉积产物,从而在光学显微镜下观察到蓝色表达的细胞作为细胞复制性衰老(replicative senescence)的分子特征来识别和探测衰老细胞的权威而经典的技术方法。
该技术由大师级科学家精心研制、成功实验证明的。
广泛应用于细胞生物学研究。
其适用于各种人体和动物细胞或活体内(in vivo)检测。
产品即到即用,性能稳定,参数优化,着色敏感,堪称国际上同类产品最佳。
技术背景细胞复制性衰老是细胞控制其生长潜能的保障机制。
衰老细胞停滞在细胞周期的G1期,表现出细胞扁平、胀大、颗粒增多的形态特征,尤其在酸性条件下,细胞内β-半乳糖苷酶活性增加,而成为细胞衰老的生物学标记。
产品内容清理液(Reagent A)毫升固定液(Reagent B)毫升酸性液(Reagent C)毫升稀释液(Reagent D)毫升染色液(Reagent E)毫升产品说明书 1份保存方式保存染色液(Reagent E)在-20℃冰箱里;其余的保存在4℃冰箱里;稀释液(Reagent D)和染色液(Reagent E)用铝箔封裹,避免光照;有效保证6月用户自备2毫升离心管:用于配制染色工作液15毫升锥形离心管:用于配制染色工作液恒温培养箱:用于染色孵育光学显微镜:用于观察染色后的细胞实验步骤操作一、25cm2细胞培养瓶染色实验开始前,将试剂盒里的染色液(Reagent E)从-20℃的冰箱里取出,放进冰槽里等待溶化。
然后移取xx毫升稀释液(Reagent D)到2毫升离心管,加入xx微升染色液(Reagent E),混匀后,放进37℃恒温水槽里预热,标记为染色工作液。
然后进行下列操作:1.小心抽去25cm2细胞培养瓶里的培养液2.小心加入xx毫升清理液(Reagent A),清洗生长中的细胞表面3.小心抽去清理液4.小心加入xx毫升固定液(Reagent B),覆盖整个生长表面5.在室温下孵育5分钟6.小心抽去固定液7.小心加入xx毫升酸性液(Reagent C),清洗细胞表面8.小心抽去酸性液9.重复实验步骤7和8一次10.小心加入xx毫升染色工作液,覆盖整个细胞表面11.放进37℃培养箱,孵育3小时至16小时,或细胞呈现蓝色(注意:避免液体蒸发)12.在光学显微镜下观察和计数:表达衰老特异性β- 半乳糖苷酶的细胞为阳性细胞,呈现蓝色。
罗米司亭及艾曲波帕药品说明书

根据病人的病情选择:1.确定什么原因引起的血小板减少,至少要找到疾病的诱因。
免疫系统?骨髓造血系统?分泌〔激素〕?2.假设不能找到诱因,一定要使用该两种药品,先用6周,看看效果,查血小板计数,是否继续使用,再做评估。
罗米司亭【商品名】Nplate 【药品名称】罗米司亭/ romiplostim【适应症】治疗脾切除和脾未切除慢性免疫性血小板减少性紫癜〔ITP〕成人患者的血小板生成药。
【用法用量】(1)初始剂量1μg/kg每周1次皮下注射。
(2)因为需要减低出血的风险,通过增量1μg/kg调整每周剂量以到达和维持血小板计数50 ×109/L.(3)最大剂量不要超过每周10μg/kg。
如血小板计数达>400×109/L不要给药。
(4)如在最大剂量4周后血小板计数不增加中断Nplate。
(5)在配制期间不要震荡;避光保护配制好的Nplate;24小时给配制好的Nplate。
(6)注射容积可能非常小。
使用刻度0.01 mL的注射器。
(7)遗弃单次使用小瓶中未使用部份。
【本卷须知】〔1〕Nplate增加骨髓网硬蛋白(reticulin)沉积的风险;临床研究未除外网硬蛋白和其它纤维沉积导致有血细胞减少的骨髓纤维化的可能性。
监查外周血骨髓纤维化征象。
〔2〕中止Nplate可能导致血小板减少比Nplate治疗前更坏。
Nplate中止后监查全血细胞计数(CBCs),包括血小板计数至少2周。
〔3〕过量Nplate可能增加血小板计数至产生血栓形成/栓塞并发症的水平。
〔4〕如随Nplate初期反响后血小板计数严重减低评估患者中和抗体的形成。
〔5〕Nplate可能增加血液学恶性病的风险,尤其是有骨髓增生异常综合征患者。
〔6〕每周监查CBCs,包括血小板计数和外周血涂片,直至到达稳定的Nplate剂量。
其后,至少每月监查CBCs,包括血小板计数和外周血涂片。
〔7〕只能通过受限制的分配方案,称为Nplate NEXUS(了解和支持Nplate专家和患者网络)方案,才能获得Nplate。
IFCC Aspartate Aminotransferase 检测手册说明书

ASTAspartate Aminotransferase IFCCMANUAL RX MONZAINTENDED USEFor the quantitative in vitro determination of AspartateAminotransferase (AST) in serum and plasma. This product is suitable for manual use and on the Rx Monza analyser.Cat. No. AS 1202 R1a. Buffer/Substrate 1 x 70 ml 20 x 2 ml R1b. Enzyme/Coenzyme/ 20 x 2 ml α-oxoglutarate GTIN: 05055273200416AS 1204 R1a. Buffer/Substrate 1 x 105 ml 10 x 10 ml R1b. Enzyme/Coenzyme/ 10 x 10 ml α-oxoglutarate GTIN: 05055273200423AS 1267 R1a. Buffer/Substrate 1 x 105 ml 5 x 20 ml R1b. Enzyme/Coenzyme/ 5 x 20 ml α-oxoglutarate GTIN: 05055273200430AS 2359 R1a. Buffer/Substrate 5 x 100 ml 5 x 100 ml R1b. Enzyme/Coenzyme/ 5 x 100 ml α-oxoglutarate GTIN: 05055273200454UV METHODThis is an optimised standard method according to the concentrations recommended by the IFCC.CLINICAL SIGNIFICANCE (1,2,3,4)The aminotransferases are a group of enzymes that catalyse the inter conversions of amino acids and α-oxoacids by transfer of amino groups. AST (aspartate aminotransferase or glutamate oxaloacetatetransaminase) has been found in the cytoplasm and the mitochondria of cells that have been studied. In cases of mild tissue damage, e.g. liver, the predominant form of serum AST is that from the cytoplasm, with a smaller amount coming from the mitochondria. Severe tissue damage will result in more mitochondrial enzyme being released. Elevated levels of AST can signal myocardial infarction, hepatic disease, muscular dystrophy and organ damage.Although heart muscle is found to have the most activity of the enzyme, significant activity has also been seen in the brain, liver, gastric mucosa, adipose tissue and kidneys of humans.The IFCC has now recommended (1980) standardised procedures for AST determinations including:-1. optimization of substrate concentrations.2. Employment of Tris buffers (instead of phosphate, which has beenshown to inhibit recombination of the apoenzyme with pyridoxal phosphate).3. Pre-incubation of combined buffer and serum to allow sidereactions with NADH to occur. 4. Substrate start (α-oxoglutarate)5. Optional pyridoxal phosphate activation.This is an optimised standard method according to the recommendations of the IFCC.PRINCIPLEα-oxoglutarate reacts with L-aspartate in the presence of AST to form L-glutamate plus oxaloacetate. The indicator reaction utilises the oxaloacetate for a kinetic determination of NADH consumption. AST -oxoglutarate + L-aspartate L-glutamate + oxaloacetate MDH oxaloacetate + NADH + H + L-malate + NAD +SPECIMEN COLLECTION AND PREPARATION (5) Serum:- Use serum free from haemolysis.Plasma:- EDTA or heparin can be used as the anticoagulant.Plasma should be separated from cells within one hour after collection.Specimens should be refrigerated if not used immediately:-Specimens stored longer than 3 days should be frozen at -20︒C.REAGENT COMPOSITIONContents Concentrations in the TestR1a. Buffer/Substrate Tris buffer 80 mmol/l, pH 7.5 L-aspartate 240 mmol/l R1b. Enzyme/Coenzyme/α-oxoglutarate α-oxoglutarate 12 mmol/l MDH ≥420 U/l LD ≥600 U/l NADH 0.18 mmol/lSAFETY PRECAUTIONS AND WARNINGS For in vitro diagnostic use only. Do not pipette by mouth.Exercise the normal precautions required for handling laboratory reagents.Solution R1a contains Sodium Azide. Avoid ingestion or contact with skin or mucous membranes. In case of skin contact, flush affected area with copious amounts of water. In case of contact with eyes or if ingested, seek immediate medical attention.Sodium Azide reacts with lead and copper plumbing, to form potentially explosive azides. When disposing of such reagents flush with large volumes of water to prevent azide build up. Exposed metal surfaces should be cleaned with 10% sodium hydroxide.Health and Safety data sheets available on request.The reagents must be used only for the purpose intended by suitably qualified laboratory personnel, under appropriate laboratory conditions.STABILITY AND PREPARATION OF REAGENTS R1a. Buffer/SubstrateContents ready for use. Stable up to the expiry date when stored at +2 to +8︒C.R1b. Enzyme/Coenzyme/α-oxoglutarate Reconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with the appropriate volume of Buffer/Substrate R1a: 2 ml for the 20 x 2 ml kit (AS 1202) 10 ml for the 10 x 10 ml kit (AS 1204) 20 ml for the 5 x 20 ml kit (AS 1267) Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C. Cat. AS 2359 5 x 100 mlReconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with a portion of Buffer/Substrate R1a and then transfer the entire contents to bottle R1a rinsing bottle R1b several times. Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C.MATERIALS PROVIDED Buffer/SubstrateEnzyme/Coenzyme/ -oxoglutarateMATERIALS REQUIRED BUT NOT PROVIDEDRandox Assayed Multisera Level 2 (Cat. No. HN 1530) and Level 3 (Cat. No. HE 1532)Randox Calibration Serum Level 3 (Cat. No. CAL 2351) RX series Saline (Cat. No. SA 3854)PROCEDUREAspirate fresh ddH 2O and perform a new Gain Calibration in flow cell mode. Select AST in the Run Test screen and carry out a water blank as instructed.Pipette into a test tube:Sample 0.05 ml Reagent 0.5 mlMix and aspirate into the Rx Monza.CALIBRATION FOR RX MONZAThe use of Saline and Randox Calibration Serum Level 3 isrecommended for calibration. Calibration is recommended with change of reagent lot or as indicated by quality control procedures.FOR MANUAL USEWavelength: 340 nm (Hg 334 nm or Hg 365 nm) Cuvette: 1 cm light path Temperature: 25/30/37︒C Measurement: against airPipette into cuvette: Macro MicroSample 0.2 ml 0.1 ml Enzyme/Coenzyme/ α-oxoglutarate R1 2.0 ml 1.0 mlMix, read initial absorbance after 1 minute. Read again after 1, 2 and 3 minutes. Note: If the absorbance change per minute is between 0.11 and 0.16 at 340/Hg 334 nm 0.06 and 0.08 at Hg 365 nmuse only the values for the first 2 minutes for the calculation.MANUAL CALCULATIONTo calculate the AST activity, use the following formulae:U/l = 1746 x A 340 nm/min U/l = 1780 x A Hg 334 nm/min U/l = 3235 x A Hg 365 nm/minSTANDARDISATIONRandox Calibration Serum Level 3 is traceable to AST reference material JSCC TS01.QUALITY CONTROLRandox Assayed Multisera, Level 2 and Level 3 are recommended for daily quality control. Two levels of controls should be assayed at least once a day. Values obtained should fall within a specified range. If these values fall outside the range and repetition excludes error the following steps should be taken:1. Check instrument settings and light source.2. Check cleanliness of all equipment in use.3. Check water. Contaminants, i.e. bacterial growth, maycontribute to inaccurate results. 4. Check reaction temperature.5. Check expiry date of kit and contents.6. Contact Randox Laboratories Customer Technical Services, Northern Ireland +44 (0) 28 9445 1070.SPECIFICITY/INTERFERENCE (6,7)Gross haemolysis will produce falsely elevated test results. The effects of various drugs on AST activity should be taken intoconsideration in the case of patients receiving large doses of drugs.The analytes below were tested up to the following levels and were found not to interfere: Haemoglobin 250 mg/dl Free Bilirubin 25 mg/dl Conjugate Bilirubin 25 mg/dl Triglycerides 1000 mg/dlIntralipid ® 200 mg/dlA list of substances and conditions known to effect AST activity in vivo is given by both Young et al and Friedman et al. Norepresentation is made by Randox Laboratories Ltd regarding the completeness of these lists and the accuracy of the information contained therein.NORMAL VALUES IN SERUM (8,9) +25︒C +30︒C +37︒C Men up to 18 U/l up to 25 U/l up to 37 U/l Women up to 15 U/l up to 21 U/l up to 31 U/lIt is recommended that each laboratory establish its own reference range to reflect the age, sex, diet and geographical location of the population.SPECIFIC PERFORMANCE CHARACTERISTICS The following performance data were obtained using an Rx Monza analyser running at +37o C.LINEARITYThis method is linear up to 562 U/l. If the sample concentration exceeds this value, dilute the sample 1+9 with 0.9% NaCl solution and re-assay. Multiply the result by 10.SENSITIVITYThe minimum detectable concentration of AST with an acceptable level of precision was determined as 9.3 U/l.PRECISIONIntra AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.66 1.47CV(%) 4.65 0.96n 20 20Inter AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.77 7.10CV(%) 4.96 4.63n 20 20CORRELATIONThis method (Y) was compared with another commerciallyavailable method (X) and the following linear regression equationobtained:Y = 1.07X + 4.9and a correlation coefficient of r = 0.997543 patient samples were analysed spanning the range 28 to 559U/l.REFERENCES1. Wroblewski F, La Due J.S: Ann Intern Med. 1956; 45: 801.2. Wroblewski F, La Due J.S: Proc Soc Exp Biol Med 1956;91: 569.3. Bergmeyer HU, Bowers GN Jr, et al: Clin Chem 1977; 23:887.4. Bergmeyer HU, Bowers GN Jr, et al: J.Clin Chem ClinBiochem 1980; 18: 521-534.5. Tietz N W: Fundamentals of Clinical Chemistry ed 3.Philadelphia, WB Saunders Co. 1987, pg 372.6. Young D S, et al: Clin Chem 1975, 21; No5.7. Friedman RB, et al: Clin Chem 1980, 26; No4.8. Wallnofer H, Schmidt.E, Schmidt FW, eds: Synopsis derLeberkrankheiten Stuttgart, Georg Thieme Verlag, 1974.9. Thefeld W, et al: Dtsch Med Wschr 1974; 99: 343.Revised 26 Apr 16 biRev. 003THIS PAGE IS INTENTIONALLY BLANK。
三氟乙酸脱苄基O-debenzylation_of_ortho-substituted_phenols_with_trifluoroacetic_acid

Mild,efficient and rapid O-debenzylation of ortho -substituted phenols with trifluoroacetic acidSteven Fletcher *,Patrick T.Gunning *Department of Chemistry,University of Toronto,Mississauga,ON L5L 1C6,Canadaa r t i c l e i n f o Article history:Received 21May 2008Revised 2June 2008Accepted 4June 2008Available online 10June 2008a b s t r a c tThe mild and efficient deblocking of aryl benzyl ethers with TFA is reported.Cleavage was fastest with ortho -electron-withdrawing groups on the phenolic ring,which we have attributed to a proton chelation effect,furnishing the deprotected phenols in excellent yields.The corresponding para -methoxybenzyl,allyl and iso -propyl ethers were also cleanly removed under these conditions.In addition,the selective aryl benzyl ether debenzylation in the presence of benzyl ester,Cbz carbamate and Boc carbamate func-tionalities was also observed.Crown Copyright Ó2008Published by Elsevier Ltd.All rights reserved.Phosphotyrosines feature in the design of inhibitors of several protein targets,including protein tyrosine phosphatase 1B (PTP1B).1However,these moieties suffer from hydrolytic lability to cellular phosphatases and poor cell penetration due to the asso-ciated dianionic charge.1To address these issues,salicylic acid derivatives (and closely-related analogues)have become popular mimetics of phosphotyrosine in small molecule inhibitors.1–5Turk-son et al.have recently reported on NSC74859(1),a potent,sali-cylic acid-based inhibitor of the oncogenic protein Stat3.6As part of our structure–activity relationship (SAR)studies on NSC74859(1),we sought to debenzylate both the phenol ether and benzoate ester in 2without reducing the aryl-bromide bond,a common undesired side reaction that occurs with hydrogen gas and Pd/C catalyst.7O -Benzyl-protected phenols are known to undergo debenzyla-tion with trifluoroacetic acid (TFA)8by an initial protonation of the weakly basic phenol oxygen,although additives such as strongorganic acids (e.g.,trifluoromethanesulfonic acid 9)or a large excess of nucleophilic scavenger (e.g.,thioanisole,which accelerates the reaction by a ‘push–pull’mechanism 10)are typically required.Re-cent work by Ploypradith et al.describes the mild deprotection of aromatic ethers with sub-stoichiometric para -toluenesulfonic acid on solid support.11In a special case,O -benzyl-protected ortho -nitrophenol was cleaved rapidly (<5min)with neat TFA,12which we considered was due to the ability of the substrate to chelate a proton since the structurally-similar ortho -hydroxybenzoates (salicylates)are well-known to chelate copper ions and iron ions.We reasoned that 2(and indeed 3)may similarly undergo acceler-ated debenzylation with TFA.In fact,as shown in Scheme 1,treat-ment of 2(or 3)with a 1:1mixture of TFA/toluene led to rapid debenzylation (5min for 2;1h for 3)in 91%yield for 2(or 85%yield for 3).In this Letter,we will explore the structural require-ments of the phenol component that increase the lability of the O -benzyl phenol ether bond in the presence of TFA.In addition,0040-4039/$-see front matter Crown Copyright Ó2008Published by Elsevier Ltd.All rights reserved.*Tel.:+19058285354;fax:+19058285425(P.T.G.).E-mail addresses:steven.fletcher@utoronto.ca (S.Fletcher),patrick.gunning@utoronto.ca (P.T.Gunning).Tetrahedron Letters 49(2008)4817–4819Contents lists available at ScienceDirectTetrahedron Lettersj o ur na l h om e pa ge :w w w.e ls e v ie r.c o m/lo c at e/t et l e twe will explore the selectivity of this mild debenzylation tech-nique with respect to other aromatic ethers and examine the sta-bility of other benzyl-based protecting groups to these reaction conditions.A series of 12O -benzyl-protected phenols was prepared by standard procedures in near quantitative yields.Each of these ethers was then deprotected with a 1:1mixture of TFA/toluene;our observations are summarized in Table 1.In certain cases,O ?C benzyl migration (Friedel–Crafts reaction)by-products (610%)were occasionally inseparable from the product by silica gel flash column chromatography.Thus,several benzyl cation cap-tors were investigated for their abilities to improve yields and puri-ties of the debenzylation reactions.Three to ten equivalents of p -cresol,anisole and triethylsilane were employed,but these exerted little effects on reducing by-product formation.Conversely,we dis-covered that including the more nucleophilic scavenger thioanisole as an additive to the co-solvent toluene typically,after silica gel flash column chromatography,furnished products in P 95%puri-ties (and higher yields),as judged by 1H NMR.Nevertheless,we envisaged any Friedel–Crafts impurities would be more readily separable on slightly more complex aryl benzyl ethers,as we ob-served with the substrates shown in Scheme 1and Tables 3and 4(>99%purities (1H NMR)in each case).Whilst likely leading to even higher yields and purities,large excesses of thioanisole (50equiv)are also known to accelerate TFA-mediated debenzyla-tion.10However,in our hands just 3equiv of thioanisole had little effect on the rate of debenzylation,allowing us to attribute the deprotection rates solely to the structure of the phenol.Electron-rich phenols are good scavengers of benzyl cations,13and since preliminary experiments with electron-rich phenols generated complex mixtures of Friedel–Crafts by-products under these deb-enzylation conditions,we chose to investigate only electron-poor phenols in this study.O -Benzyl-protected phenols with p -ortho -electron-withdraw-ing groups (6a ,6b ,6d ,6f )were swiftly (several in less than 3h cf.24h for unsubstituted phenol 6l )and cleanly debenzylated,with less than 5%of the undesired C-benzylated phenol by-prod-ucts.In contrast,meta -and para -electron-withdrawing groups slo-wed down the debenzylation (e.g.,entries 6g and 6h ),relative to the control compound 6l ,which itself could only be obtained in moderate purity by this method.The r -withdrawing (and p -donating)bromophenols 6i –k were insufficiently deactivated to benzyl cation scavenging and were contaminated with several by-products.Importantly,n -butyl benzyl ether 8was unaffected by TFA under the reaction conditions,indicating this procedure is selective for aryl benzyl ethers.In addition,the results in Table 1suggest that this procedure is suitable only for phenols substituted with p -electron-withdrawing groups.Since the debenzylation mechanism with TFA proceeds via an initial protonation of the phenol ether oxygen,the more available the ether oxygen lone pairs are,the faster the reaction will be.Hence,the slower reaction times for the phenols bearing meta -and para -electron-withdrawing groups make sense,although this is not true for the ortho -functionalized aryl benzyl ethers.As hypothesized for the bis-benzyl salicylate derivative 2earlier,we considered these ortho -substituted phenols were capable of chelat-ing the acidic hydrogen atom from TFA which therein facilitated the acid-mediated debenzylation via a six-membered cyclic inter-mediate,as proposed in Scheme 2.A similar chelation intermediate has been put forward by Baldwin and Haraldsson to account for the Lewis acid MgBr 2-mediated debenzylation of aromatic benzyl ethers ortho to an aldehyde group.14Accordingly,to test this hypothesis we expanded this series of ortho -substituted aryl benzyl ethers,and the results from their deb-enzylation reactions with TFA are summarized in Table 2.These substrates have been listed in order of increasing approximateTable 1TFA-mediated debenzylation of O -benzyl-protected phenols aTFAtolueneOBnROHR67Substrate RTime (h)b Yield c (%)6a o -CO 2Me,m d -NHAc 5min 936b o -CO 2Me 5min 946c p -CO 2Me 36e 63(85f )6d o -CO 2Bn 5min 936e p -CO 2Bn 36e 58(79f )6f o -NO 23976g m -NO 236e 75(98f )6h p -NO 236e 66(98f )6i o -Br 16—g 6j m -Br 30—g 6k p -Br 36—g 6lH 24—gn -BuOBn (8)—24No reactionaThe reaction was carried out with 6(0.5mmol)in a 1:1mixture of TFA/toluene (5ml)at rt,with 3equiv of thioanisole.bTime taken for all starting material to be consumed.cIsolated yield after silica gel flash column chromatography.dmeta to phenol oxygen AND para to ester.eReaction was slow and incomplete after 3days.fYield based on recovered starting material.gComplex mixture of products.Table 2TFA-mediated debenzylation of O -benzyl-protected,ortho -substituted phenols aTFA tolueneOBnOH67RRSubstrate R p K aH b Time c (h)Yield d (%)Relative rate 6m CO 2NH 2À2248316n CHO À7 3.594e 6.96o CO 2H À8191246b CO 2Me À8.55min 942886d CO 2Bn À8.55min 932886p CN À10>4851(95f )—6f NO 2À1239786i Br —16—g 1.56lH—24—g1aThe reaction was carried out with 6(0.5mmol)in a 1:1mixture of TFA/toluene (5ml)at rt,with 3equiv of thioanisole.bApproximate p K aH of conjugate acid of R group.15cTime taken for all starting material to be consumed.dIsolated yield after silica gel flash column chromatography.eIncluding thioanisole in the deprotection of 6n led to further by-products,thus no scavenger was used and compound 7n could be obtained in only 90%purity.fYield based on recovered starting material.gComplex mixture of products.4818S.Fletcher,P.T.Gunning /Tetrahedron Letters 49(2008)4817–4819acidity of the conjugate acid (decreasing p K aH )of the ortho -elec-tron-withdrawing substituent.15There appears to be an optimal p K aH of around À8.5,that is exhibited by carboxylic esters,which lead to the fastest rate of debenzylation with TFA.In an approxi-mate bell-shaped distribution of reaction rate versus ortho -substi-tuent p K aH —that was interrupted only by ortho -cyanophenol 6p —protonatable groups with p K aH ’s <À8.5or >À8.5were less effective at accelerating the TFA-mediated debenzylation.These data concur with our chelation hypothesis:groups that are too ba-sic bind more strongly to the TFA proton making it less available for sharing with,and ultimately releasing to,the phenol ether oxygen;groups that are weakly basic do not bind the TFA proton as well,leading to reduced chelation and hence less rate enhancement.The anomalous result for ortho -cyanophenol 6p was anticipated since this compound was selected as a negative control.Phenol 6p is geometrically incapable of chelating a proton,because the lin-ear,sp -hybridized nitrile functionality directs its basic nitrogen atom (p K aH %À10)away from the phenol oxygen.As predicted,there was no rate enhancement for the TFA-mediated debenzyla-tion of 6p relative to phenol 6l .In fact,6p was only slowly deben-zylated,at a rate that was comparable with the m -nitro and p -nitro derivatives 6g and 6h ,respectively.We next wanted to investigate the selectivity for the deprotec-tion of the benzyl group over other phenol protecting groups.Accordingly,the benzyl group in salicylate derivative 9a was varied with para -methoxybenzyl (PMB;9b ),methyl (9c ),allyl (9d )and iso -propyl (i -Pr;9e ).These substrates were then debenzylated with a 1:1mixture of TFA/toluene;our findings are reported in Table 3.Any impurities this time were minor and readily separable from the products,eliminating the need for the additive thioanisole.The relative rates at which these protecting groups were removed was para -methoxybenzyl >benzyl >allyl >iso -propyl )methyl,which reflects the stability of the carbocations.These data suggest that in salicylates such as 9,the benzyl phenol protecting group (R =Bn)can be removed with TFA in the presence of the corres-ponding allyl,iso -propyl and methyl ethers.Finally,we explored the selectivity of this mild debenzylation technique over other benzyl-based protecting groups,as shown in Table 4.As the results demonstrate,it was possible to deblock the O -benzyl ether in the presence of a benzyl ester (6d )and in the presence of a benzyl carbamate (11b ),thereby increasing the orthogonality of O -benzyl phenol ethers of salicylate derivatives.Interestingly,it was even possible to cleave the benzyl group in 11c with TFA in the presence of an N -Boc-protected aniline.In summary,we have presented the mild,efficient and rapid deblocking of ortho -substituted aryl benzyl ethers with TFA.Deb-enzylation was fastest when the ortho group was a carboxylic ester,which we have attributed to a proton chelation effect.Other ortho groups that accelerated the TFA-mediated debenzylation included carboxylic acid,aldehyde and nitro.In addition,we have shown that in such ortho -functionalized phenols,benzyl could be removed in the presence of the corresponding iso -propyl,allyl and methyl ethers.Moreover,the benzyl ether could be selectively cleaved in the presence of benzyl ester,Cbz carbamate and Boc carbamate functionalities.AcknowledgementsThe authors gratefully acknowledge financial support for this work from the Canadian Foundation of Innovation and the Univer-sity of Toronto (Connaught Foundation).References and notes1.Zhang,S.;Zhang,Z.-Y.Drug Discov.Today 2007,12,373–381.2.(a)Pei,Z.;Li,X.;Liu,G.;Abad-Zapatero,C.;Lubben,T.;Zhang,T.;Ballaron,S.J.;Hutchins,C.W.;Trevillyana,J.M.;Jirouseka,M.R.Bioorg.Med.Chem.Lett.2003,13,3129–3132;(b)Xin,Z.;Liu,G.;Abad-Zapatero,C.;Pei,Z.;Szczepankiewicz,B.G.;Li,X.;Zhang,T.;Hutchins,C.W.;Hajduk,P.J.;Ballaron,S.J.;Stashko,M.A.;Lubben,T.H.;Trevillyana,J.M.;Jirouseka,M.R.Bioorg.Med.Chem.Lett.2003,13,3947–3950.3.Tautz,L.;Bruckner,S.;Sareth,S.;Alonso,A.;Bogetz,J.;Bottini,N.;Pellecchia,M.;Mustelin,T.J.Biol.Chem.2005,280,9400–9408.4.Shrestha,S.;Bhattarai,B.R.;Chang,K.J.;Leea,K.-H.;Choa,H.Bioorg.Med.Chem.Lett.2007,17,2760–2764.5.Liljebris,C.;Larsen,S.D.;Ogg,D.;Palazuk,B.J.;Bleasdale,J.E.J.Med.Chem.2002,45,1785–1798.6.Siddiquee,K.;Zhang,S.;Guida,W.C.;Blaskovich,M.A.;Greedy,B.;Lawrence,H.R.;Yip,M.L.R.;Jove,R.;Laughlin,M.M.;Lawrence,N.J.;Sebti,S.M.;Turkson,J.Proc.Natl.Acad.Sci.U.S.A.2007,104,7391–7396.7.Pandey,P.N.;Purkayastha,M.L.Synthesis 1982,876–878.8.(a)Greene,T.W.;Wuts,P.G.M.Protective Groups in Organic Synthesis ,3rd ed.;John Wiley &Sons:New York,1999;(b)Kocienski,P.J.Protecting Groups ,3rd ed.;Georg Thieme:Stuttgart,Germany,2003.9.Kiso,Y.;Isawa,H.;Kitagawa,K.;Akita,T.Chem.Pharm.Bull.1978,26,2562–2564.10.Kiso,Y.;Ukawa,K.;Nakamura,S.;Ito,K.;Akita,T.Chem.Pharm.Bull.1980,28,673–676.11.Ploypradith,P.;Cheryklin,P.;Niyomtham,N.;Bertoni,D.R.;Ruchirawat,.Lett.2007,9,2637–2640.12.Marsh,J.P.,Jr.;Goodman,.Chem.1965,30,2491–2492.13.(a)Eberle,A.N.J.Chem.Soc.,Perkin Trans.11986,361–367;(b)Bodanszky,M.;Tolle,J.C.;Deshmane,S.S.;Bodanszky,A.Int.J.Pept.Protein Res.1978,12,57–68.14.Haraldsson,G.G.;Baldwin,J.E.Tetrahedron 1997,53,215–224.15.(a)Ionization Constants of Organic Acids in Solution ;Serjeant,E.P.,Dempsey,B.,Eds.IUPAC Chemical Data Series No.23;Pergamon Press:Oxford,UK,1979;(b)see also:/labs/evans/pdf/evans_pKa_table.pdf .Table 3TFA-mediated deprotection of O-blocked phenol ether derivatives of methyl 4-acetamidosalicylate aTFAtolueneNHAcNHAcORO OMeOH OMeO 910Substrate R Time b (h)Yield c (%)9a Bn 5min 919b PMB 2min 909c Me 480d 9d Allyl 20919ei -Pr3692aThe reaction was carried out with 9(0.5mmol)in a 1:1mixture of TFA/toluene (5ml)at rt.bTime taken for all starting material to be consumed.cIsolated yield after silica gel flash column chromatography.dOnly starting material remained after 48h,at which point the reaction was aborted.Table 4Selectivity investigation into the TFA-mediated debenzylation of aryl benzyl ethers aTFA tolueneOBnOH2Bn2Bn1112RRSubstrate R Yield b (%)6d c H 9311a NHAc 9211b NHCbz 9311c dNHBoc54aThe reaction was carried out with 11(0.5mmol)in a 1:1mixture of TFA/toluene (5ml)at rt for 5min,then all solvents were evaporated.bIsolated yield after silica gel flash column chromatography.cFor compound 6d ,3equiv of thioanisole were also used.dAfter 5min,the reaction mixture was diluted with CH 2Cl 2and then immedi-ately neutralized with 1M NaOH.The organic layer was then separated and evaporated.S.Fletcher,P.T.Gunning /Tetrahedron Letters 49(2008)4817–48194819。
伟素产品知识(舒洛地特)

1
0
0
24
48 72
96 120 144 168 192 216 240 264 288 312 336 360
Time (h)
Breccia A. et al. , Eur J Clin Res 1992
伟素 独特的剂量单位
®
LSU或LRU (脂酶单位或释放脂蛋白脂酶单位) :在一定实验条件 下给SD大鼠注射本品,心脏取血并离心,然后在血浆中加入脂性 底物,室温下反应 15 分钟后,导致血浆 / 脂性底物系统吸收度 (D.O.) 值 下 降 50 % 的 本 品 的 效 价 为 1 脂 酶 单 位 ( LSU = Lipasemic Unit)。
舒洛地特可以与ACEI类药物合用
不同亚组(与ACEI合用组&不与ACEI合用组)AER的 降低比例:对照组vs治疗组
100 80 60
On ACEI
40 20 0 50 mg 100 mg 200 mg Sulodexide
J Am Soc Nephrol 2002;13:1615-1625
Not on ACEI
• GAG被证明可以有效治疗糖尿病肾病
适度抗凝
维持视网膜毛细血 管的正常通透性
伟素活性
血小板黏附聚集
减少血管渗漏
纤维蛋白原血症
伟素对糖尿病眼病的作用
Flavia Rubbi
Dipartimento di Discipline Chirurgiche, Rianimatorie e dei Trapianti Clinica Oculistica II
黄斑部位渗出物变化
荧光素泄漏
眼球后极部位出血
伟素的治疗显示,它显著地降低了早 期糖尿病眼病病人的硬渗出物, IRMA 和出血,并且对动脉压和代谢无影响 伟素显示可以恢复 I 型和 II 型糖尿病人 的视网膜内皮基膜的代谢和功能。
鲑鱼降钙素(SCT)ELISA试剂盒使用说明书

鲑鱼降钙素(SCT)ELISA试剂盒使用说明书鲑鱼降钙素(SCT)ELISA试剂盒使用说明书ELISA试剂盒规格:(1) 规格:96T 可以测90个样,5个标准孔,1个空白孔(2) 规格:48T 可以测42个样,5个标准孔,1个空白孔Elisa试剂盒种属:马铃薯,鹿,羊,鸡,鸭,鱼,人,大鼠,小鼠,豚鼠,仓鼠,裸鼠,兔子, 猪,犬,猴,马,牛等动植物。
鲑鱼降钙素(SCT)ELISA试剂盒使用说明书The experimental contents and methods1 in microtiter plates per hole adding test specimens of 50 L, with positive and negative control of the2 holes, each hole by adding positive (or negative) control of the 1 drops, and the blank control 1 hole.2 per hole adding enzyme conjugate 1 drops (except blank control), mixing, seal plate, 37 DEG C and then incubated for 30 minutes.3 discard hole liquid, washing liquid filled the hole, standing for 5 seconds, repeated 5 times after drying, pat dry.4 per hole Jiaxian reagent A and B solution 1 drops, mixing, sealing plate, 37 DEG C and then incubated for 15 minutes.5 per hole adding stop solution 1 drops, mixing.6 using the microtiter plate reader, the wavelength of 450nm, first with a blank hole zero, and then read the hole od.本试剂仅供研究使用标本:全血试验原理:HLA- B27试剂盒是固相夹心法酶联免疫吸附实验(ELISA).已知HLA-B27浓度的标准品、未知浓度的样品加入微孔酶标板内进行检测。
免疫组化实验--全套试剂耗材

免疫组化实验试剂耗材大全华越洋---------------------------- 0.1%胰蛋白酶消化液waryong 10ml 110多聚甲醛merk 25g 504%多聚甲醛waryong 500ml 22010X多聚赖氨酸waryong 10ml 260抗荧光衰减封片剂waryong 25ml 230防脱载玻片waryong 50片310mayer'苏木素染液(免疫组化)waryong 100ml 410封闭用正常绵羊/山羊/兔/人血清waryong 10ml 75弗氏不完全佐剂sigma 10ml 180弗氏完全佐剂sigma 10ml 200柠檬酸钠缓冲液0.01mol/L PH6.0 waryong 1L 10DAB amresco 1g 13520XDAB显色液 A,B液各1.5ml waryong 3ml 95NBT amresco 100mg 95BCIP amresco 100mg 310BCIP/NBT底物显色试剂盒waryong 25ml 210PBST(PH7.4)抗体稀释液waryong 1ml 25一抗稀释液waryong 100ml 390HRP标记抗体稀释液waryong 100ml 390AP标记抗体稀释液waryong 100ml 390荧光抗体稀释液waryong 50ml 110免疫组化名称规格价格Super Polymer-二步法IHC试剂盒3ml35818ml1598兔Streptavidin-HRP试剂盒3ml19818ml998鼠Streptavidin-HRP试剂盒3ml19818ml998兔∕鼠通用型Streptavidin-HRP试剂盒3ml25818ml1198山羊抗兔IgG,Biotin(IHC工作液)3ml6818ml298山羊抗鼠IgG,Biotin(IHC工作液)3ml6818ml298山羊抗兔∕鼠IgG,Biotin(IHC工作液)3ml9818ml498 Streptavidin-HRP(IHC工作液)3ml9818ml49860ml258 AEC底物显色试剂盒20ml98 BCIP∕NBT碱性磷酸酶显色试剂盒(40x)40ml198 BCIP/NBT碱性磷酸酶显色试剂40ml229改良型苏木素(IHC常用复染试剂)10ml68柠檬酸缓冲液(IHC抗原修复液,100x)100ml68EDTA缓冲液(IHC抗原修复液,50x)100ml68封闭用正常山羊血清工作液(免疫组化封闭液)10ml68内源性过氧化物酶封闭液10ml68内源性碱性磷酸酶封闭液10ml68Biotin标记抗体稀释液20ml中性树胶100g98水性封片剂10ml98 Super Polymer-二步法IHC试剂盒(带DAB显色液)3ml39818ml1698兔Streptavidin-HRP试剂盒(带DAB显色液)3ml25818ml1098鼠Streptavidin-HRP试剂盒(带DAB显色液)3ml25818ml1098兔∕鼠通用型Streptavidin-HRP试剂盒(带DAB显色液)3ml29818ml1298。
SignalBoost使用说明书

产品使用说明书SignalBoost TM 免疫信号增强试剂盒免疫信号增强试剂盒((目录号407207)请注意该操作流程并不针对某一具体产品,而是该产品的统一规范说明书。
具体信息请参照产品标签和COA (certificate of analysis )。
另请注意产品运输条件与储存条件并不相同。
详细信息请登录 。
包装大小包装大小::1 kit可检测次数或面积可检测次数或面积::20 Miniblots (10cm ×8cm 聚丙烯酰胺凝胶转移膜)或1600cm 2形式形式::液体,抗体稀释液检测方法检测方法::化学发光,比色法,荧光储存储存::货到后请将整个试剂盒4℃避光保存,打开包装后,样品可以在4℃稳定存放1年。
使用范围使用范围::SignalBoost TM 免疫信号增强试剂盒是一种用于免疫检测的活性试剂,可以用来解决免疫检测实验中(比如免疫印迹,ELISA 等)经常遇到的低灵敏度和高背景强度等问题。
背景背景::SignalBoost TM 可以促进抗原抗体反应,产生比传统方法高几倍或几十倍的信号强度。
这种增强效应对于低活力抗体的改善尤为显著。
该试剂盒包含针对一抗和二抗的优化组分,从而能够降低背景,提高信噪比。
它可以用于多种基于抗原抗体反应的检测实验,比如免疫印迹,免疫斑点和ELISA 。
试剂盒中各组分对标记抗体的酶的活性(过氧化物酶,碱性磷酸酶等)没有影响,不会干扰它们在这些检测实验中的应用。
另外,该试剂盒同样可以用于后续荧光检测和显色反应。
为了方便,SignalBoost TM 采用即用形式,无需稀释,只需用其替换传统的抗体稀释溶液即可,并不增加检测时间。
检测原理检测原理::SignalBoost TM 提供一套用于稀释一抗和二抗的试剂。
Solution 1用来稀释一抗到合适浓度,而二抗用Solution 2稀释。
这些溶液可以替代其他的稀释缓冲液,包括TBS ,TBS -T ,PBS ,PBS -T 等。
荷尔登生物医学检测产品说明书
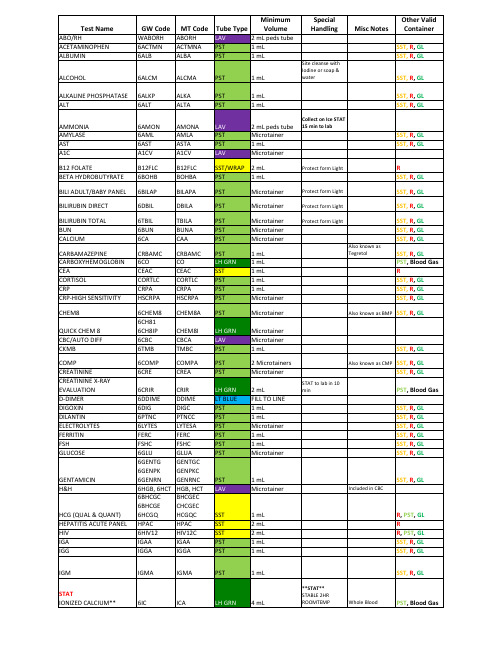
1mL
R
1mL
SST, R, GL
1mL
No capillary
1mL
collection
Order of Draw: 1. BLOOD CULTURES 2. LT BLUE 3. RED 4. SST 5. PST/LH GRN 6. LAV 7. GRAY
M2909 (1-19)
Microtainer Microtainer 1 mL
2 Microtainers Microtainer
2 mL FILL TO LINE 1 mL 1 mL Microtainer 1 mL 1 mL Microtainer
SST, R, GL
STAT to lab in 10 min
Also known as CMP SST, R, GL SST, R, GL
TOBRAMYCIN TOTAL PROTEIN TROPONIN TSH
TYPE AND CROSSMATCH
TYPE AND SCREEN URIC ACID
VANCOMYCIN VALPROIC ACID VITAMIN D
FEA
6LA LHC 6LIP
6LIVER 6LIPID LIA 6MG 6METX 6MONO PHNOC 6PFA 6PHOS 6PBNP 6PCAL 6KA 6PTIMEN PSAC 6PTT 6RETCT 6RENAL WHRIG 6SAL SYPHB TBSATA TT3C T4C TESTOC THEOC
CHEM8
QUICK CHEM 8 CBC/AUTO DIFF CKMB
COMP CREATININE CREATININE X-RAY EVALUATION D-DIMER DIGOXIN DILANTIN ELECTROLYTES FERRITIN FSH GLUCOSE
磷钨酸负染色液(2%,pH7.0)
版本:A4 修改日期:2023.12.18 磷钨酸负染色液(2%,pH7.0)产品简介:负染色又称阴性染色,是由Hall 发现的相对于普通染色(即正染色)而言的染色技术,其原理在于利用重金属盐包绕低电子密度的样品,增强样本四周的电子密度,造成细微结构之间的"质量-厚度”差异,增强散射吸收反差,使样品在黑暗的背景上呈现明亮的结构,负染色液有磷钨酸、钼酸铵、印度墨汁等,其中最常用的是1~3%磷钨酸。
Leagene 磷钨酸负染色液(2%,pH7.0)适用于显示大分子、细菌、病毒、原生动物、噬菌体、细胞器、核酸大分子、蛋白质晶体及其他大分子材料等,染色后的样品图像呈现透明的亮光,而背景图像呈黑色。
该试剂仅用于科研领域,不适用于临床诊断或其他用途。
产品组成:自备材料:1、 离心机2、 载网3、 显微镜操作步骤(仅供参考):(一)滴染法1、样品低速离心(2000g ,10min)或采用其他方法浓缩样品,制成悬浮液并且使其达到一定浓度和纯度。
2、将样品悬浮液直接滴于带有支持膜的载网上。
3、用滤纸条从液滴边缘吸去多余液体,稍干燥。
4、 滴加负染色液。
5、吸去多余染色液,自然干燥,进行显微镜观察。
(二)漂浮法1、样品低速离心(2000g ,10min)或采用其他方法浓缩样品,制成悬浮液并且使其达到一定浓度和纯度。
2、将带有支持膜的载网置于样品液滴上漂浮以沾取样品。
编号 名称 DZ0035 Storage 磷钨酸负染色液(2%,pH7.0) 100ml RT 避光 使用说明书 1份3、载网置于负染色液上漂浮1~2min。
4、吸去多余染色液,自然干燥,进行显微镜观察。
染色结果:样品透明的亮光背景黑色注意事项:1、目的样本尽量新鲜。
2、样品应为均匀的悬浮液,其纯度和浓度应适宜,否则无法与染色剂之间产生特异和清晰的结合反应。
3、为了您的安全和健康,请穿实验服并戴一次性手套操作。
4、试剂开封后请尽快使用,以防影响后续实验效果。
索莱宝 BC0605 蔗糖磷酸合成酶(SPS)活性检测试剂盒 说明书
蔗糖磷酸合成酶(SPS )活性检测试剂盒说明书微量法货号: BC0605 规格: 100T/48S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系索莱宝工作人员。
试剂名称 规格 保存条件 提取液 液体50 mL×1瓶 2-8℃保存 试剂一 液体2.5 mL×1瓶 -20℃保存 试剂二 粉剂10 mg×1支 2-8℃保存 试剂三 液体2 mL×1瓶 2-8℃保存 试剂四 液体25 mL×1瓶 2-8℃保存 试剂五液体10 mL×1瓶2-8℃保存溶液的配制:试剂二:临用前加1 mL 水,配制成10 mg/mL 蔗糖溶液,再将其用蒸馏水稀释为500 μg/mL 备用。
产品说明:蔗糖不仅是重要的光合产物,也是植物体内运输的主要物质,还是碳水化合物的贮存形式之一。
蔗糖磷酸合成酶(SPS )以果糖-6-磷酸为受体,形成的蔗糖磷酸在蔗糖磷酸酶的作用下形成蔗糖。
一般把蔗糖磷酸酯合成酶-蔗糖磷酸酶系统看作是蔗糖合成的主要途径。
蔗糖磷酸合成酶催化果糖-6-磷酸形成蔗糖磷酸,蔗糖与间苯二酚反应可呈现颜色变化,在480nm 下有特征吸收峰,酶活力大小与颜色的深浅成正比。
注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:可见分光光度计/酶标仪、水浴锅、离心机、移液器、微量玻璃比色皿/96孔板、研钵/匀浆器、冰 操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)按照组织质量(g ):提取液体积(mL)为1:5~10的比例(建议称取约0.1g 组织,加入1mL 提取液),进行冰浴匀浆。
8000g 4℃离心10min ,取上清,置冰上待测。
二、测定步骤1、 分光光度计/酶标仪预热30min 以上,调节波长至480nm ,蒸馏水调零。
2、 样本测定(在1.5mL EP 管中依次加入下列试剂):试剂名称(μL )测定管 对照管 标准管空白管 样本 10 10 蒸馏水 45 45 55 试剂一45Beijing Solarbio Science & Technology Co., LtdTel: 400-968-6088 混匀,80℃水浴保温20min,冷却后,12000rpm常温离心10min。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
P d t D t Sh t Product Name:
Staurosporine CAS No.:
62996-74-1Cat. No.:
HY-15141
Product Data Sheet
MWt:
466.53Formula:
C28H26N4O3Purity :>98%
Solubility:
DMSO
Mechanisms:
Biological Activity:
Staurosporine is a prototypical potent ATP-competitive kinase inhibitor with IC50 of 0.7, 7, 8.5, 6, 20 Pathways:TGF-beta/Smad; Target:PKC nM for PKC, PKA,PKG, p60v-src tyrosine protein kinase, CaM kinase II, respectively.
IC50 value:
Target: PKC The main biological activity of staurosporine is the inhibition of protein kinases through the prevention of ATP binding to the kinase. This is achieved through the stronger affinity of
staurosporine to the ATP-binding site on the kinase. Staurosporine is a prototypical ATP-competitive kinase inhibitor in that it binds to many kinases with high affinity, though with little selectivity. This lack of specificity has precluded its clinical use, but has made it a valuable research tool. In
research sta rosporine is sed to ind ce apoptosis The mechanism of ho it mediates this is not References:
[1]. Matsumoto H, et al. Staurosporine, a protein kinase C inhibitor interferes with proliferation of
arterial smooth muscle cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):105-9.[2]Karaman MW et al A quantitative analysis of kinase inhibitor selectivity Nat Biotechnol 2008research, staurosporine is used to induce apoptosis. The mechanism of how it mediates this is not well understood. It has been found that one way in which staurosporine induces ...
[2]. Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008
Jan;26(1):127-32.[3]. Chae HJ, et al. Molecular mechanism of staurosporine-induced apoptosis in osteoblasts.
Pharmacol Res. 2000 Oct;42(4):373-81.Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o
m。
