食品添加剂欧盟编码纯英文版
EC-No 13332008

REGULATION(EC)No1333/2008OF THE EUROPEAN PARLIAMENT AND OF THE COUNCILof16December2008on food additives(Text with EEA relevance)THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EURO-PEAN UNION,Having regard to the Treaty establishing the European Commu-nity,and in particular Article95thereof,Having regard to the proposal from the Commission,Having regard to the opinion of the European Economic and Social Committee(1),Acting in accordance with the procedure laid down in Article251 of the Treaty(2),Whereas:(1)The free movement of safe and wholesome food is anessential aspect of the internal market and contributes sig-nificantly to the health and well-being of citizens,and totheir social and economic interests.(2)A high level of protection of human life and health shouldbe assured in the pursuit of Community policies.(3)This Regulation replaces previous Directives and Decisionsconcerning food additives permitted for use in foods witha view to ensuring the effective functioning of the internalmarket whilst ensuring a high level of protection of humanhealth and a high level of consumer protection,includingthe protection of consumer interests,via comprehensiveand streamlined procedures.(4)This Regulation harmonises the use of food additives infoods in the Community.This includes the use of foodadditives in foods covered by Council Directive89/398/EEC of3May1989on the approximation of thelaws of the Member States relating to foodstuffs intendedfor particular nutritional uses(3)and the use of certainfood colours for the health marking of meat and the deco-ration and stamping of eggs.It also harmonises the use offood additives in food additives and food enzymes thusensuring their safety and quality and facilitating their stor-age and use.This has not previously been regulated atCommunity level.(5)Food additives are substances that are not normally con-sumed as food itself but are added to food intentionally fora technological purpose described in this Regulation,suchas the preservation of food.All food additives should becovered by this Regulation,and therefore in the light of sci-entific progress and technological development the list offunctional classes should be updated.However,substancesshould not be considered as food additives when they areused for the purpose of imparting flavour and/or taste orfor nutritional purposes,such as salt replacers,vitaminsand minerals.Moreover,substances considered as foodswhich may be used for a technological function,such assodium chloride or saffron for colouring and food enzymesshould also not fall within the scope of this Regulation.However,preparations obtained from foods and othernatural source material that are intended to have a techno-logical effect in the final food and which are obtained byselective extraction of constituents(e.g.pigments)relativeto the nutritive or aromatic constituents,should be con-sidered additives within the meaning of this Regulation.Finally,food enzymes are covered by Regulation(EC)No1332/2008of the European Parliament and of theCouncil of16December2008on food enzymes(4),whichexcludes the application of this Regulation.(6)Substances not consumed as food itself but used intention-ally in the processing of foods,which only remain as resi-dues in the final food and do not have a technologicaleffect in the final product(processing aids),should not becovered by this Regulation.(1)OJ C168,20.7.2007,p.34.(2)Opinion of the European Parliament of10July2007(OJ C175E,10.7.2008,p.142),Council Common Position of10March2008(OJ C111E,6.5.2008,p.10),Position of the European Parliament of 8July2008(not yet published in the Official Journal)and Council Decision of18November2008.(3)OJ L186,30.6.1989,p.27.(4)See page7of this Official Journal.(7)Food additives should be approved and used only if theyfulfil the criteria laid down in this Regulation.Food addi-tives must be safe when used,there must be a technologi-cal need for their use,and their use must not mislead theconsumer and must be of benefit to the consumer.Mis-leading the consumer includes,but is not limited to,issuesrelated to the nature,freshness,quality of ingredients used,the naturalness of a product or of the production process,or the nutritional quality of the product,including its fruitand vegetable content.The approval of food additivesshould also take into account other factors relevant to thematter under consideration including societal,economic,traditional,ethical and environmental factors,the precau-tionary principle and the feasibility of controls.The useand maximum levels of a food additive should take intoaccount the intake of the food additive from other sourcesand the exposure to the food additive by special groups ofconsumers(e.g.allergic consumers).(8)Food additives must comply with the approved specifica-tions,which should include information to adequatelyidentify the food additive,including origin,and to describethe acceptable criteria of purity.The specifications previ-ously developed for food additives included in Commis-sion Directive95/31/EC of5July1995laying downspecific criteria of purity concerning sweeteners for use infoodstuffs(1),Commission Directive95/45/EC of26July1995laying down specific purity criteria concerningcolours for use in foodstuffs(2)and Commission Directive96/77/EC of2December1996laying down specific puritycriteria on food additives other than colours and sweeten-ers(3)should be maintained until the corresponding addi-tives are entered in the Annexes to this Regulation.At thattime,the specifications related to such additives should beset out in a Regulation.Those specifications should relatedirectly to the additives included in the Community lists inthe Annexes to this Regulation.However,considering thecomplex character and substance of such specifications,forthe sake of clarity they should not be integrated as such inthe Community lists but should be set out in one or moreseparate Regulations.(9)Some food additives are permitted for specific uses for cer-tain authorised oenological practices and processes.Theuse of such food additives should comply with this Regu-lation and with the specific provisions laid down in the rel-evant Community legislation.(10)In order to ensure harmonisation,the risk assessment andapproval of food additives should be carried out in accor-dance with the procedure laid down in Regulation(EC)No1331/2008of the European Parliament and of theCouncil of16December2008establishing a commonauthorisation procedure for food additives,food enzymesand food flavourings(4)(11)Under Regulation(EC)No178/2002of the European Par-liament and of the Council of28January2002layingdown the general principles and requirements of food law,establishing the European Food Safety Authority and lay-ing down procedures in matters of food safety(5),theEuropean Food Safety Authority(hereinafter referred to asthe Authority)is to be consulted on matters likely to affectpublic health.(12)A food additive which falls within the scope of Regulation(EC)No1829/2003of the European Parliament and of theCouncil of22September2003on genetically modifiedfood and feed(6)should be authorised in accordance withthat Regulation as well as under this Regulation.(13)A food additive already approved under this Regulationwhich is prepared by production methods or using start-ing materials significantly different from those included inthe risk assessment of the Authority,or different fromthose covered by the specifications laid down,should besubmitted for evaluation by the Authority.‘Significantlydifferent’could mean,inter alia,a change of the productionmethod from extraction from a plant to production by fer-mentation using a micro-organism or a genetic modifica-tion of the original micro-organism,a change in startingmaterials,or a change in particle size,including the use ofnanotechnology.(14)Food additives should be kept under continuous observa-tion and must be re-evaluated whenever necessary in thelight of changing conditions of use and new scientificinformation.Where necessary,the Commission togetherwith the Member States should consider appropriateaction.(15)Member States which maintained on1January1992pro-hibitions on the use of certain additives in certain specificfoods which are considered traditional and are producedon their territory should be permitted to continue to applythose prohibitions.Moreover,as regard products such as‘Feta’or‘Salame cacciatore’,this Regulation should be with-out prejudice to more restrictive rules linked to the use ofcertain denominations under Council Regulation(EC)No510/2006of20March2006on the protection of geo-graphical indications and designations of origin for agri-cultural products and foodstuffs(7)and Council Regulation(EC)No509/2006of20March2006on agricultural prod-ucts and foodstuffs as traditional specialities guaranteed(8).(1)OJ L178,28.7.1995,p.1.(2)OJ L226,22.9.1995,p.1.(3)OJ L339,30.12.1996,p.1.(4)See page1of this Official Journal.(5)OJ L31,1.2.2002,p.1.(6)OJ L268,18.10.2003,p.1.(7)OJ L93,31.3.2006,p.12.(8)OJ L93,31.3.2006,p.1.(16)Unless subject to further restrictions,an additive may bepresent in food,other than by direct addition,as a result ofcarry-over from an ingredient in which the additive waspermitted,provided that the level of the additive in thefinal food is no greater than would be introduced by theuse of the ingredient under proper technological condi-tions and good manufacturing practice.(17)Food additives remain subject to the general labelling obli-gations as provided for in Directive2000/13/EC of theEuropean Parliament and of the Council of20March2000on the approximation of the laws of the Member Statesrelating to the labelling,presentation and advertising offoodstuffs(1)and,as the case may be,in Regulation(EC)No1829/2003and in Regulation(EC)No1830/2003ofthe European Parliament and of the Council of22Septem-ber2003concerning the traceability and labelling ofgenetically modified organisms and the traceability of foodand feed products produced from genetically modifiedorganisms(2).In addition,specific provisions on the label-ling of food additives sold as such to the manufacturer orto the final consumer should be contained in thisRegulation.(18)Sweeteners authorised under this Regulation may be usedin table-top sweeteners sold directly to consumers.Manu-facturers of such products should make information avail-able to the consumer by appropriate means to allow themto use the product in a safe manner.Such informationcould be made available in a number of ways including onproduct labels,Internet websites,consumer informationlines or at the point of sale.In order to adopt a uniformapproach to the implementation of this requirement,guid-ance drawn up at Community level may be necessary.(19)The measures necessary for the implementation of thisRegulation should be adopted in accordance with CouncilDecision1999/468/EC of28June1999laying down theprocedures for the exercise of implementing powers con-ferred on the Commission(3).(20)In particular the Commission should be empowered toamend the Annexes of this Regulation and to adopt appro-priate transitional measures.Since those measures are ofgeneral scope and are designed to amend non-essential ele-ments of this Regulation,inter alia,by supplementing itwith new non-essential elements,they must be adopted inaccordance with the regulatory procedure with scrutinyprovided for in Article5a of Decision1999/468/EC.(21)On grounds of efficiency,the normal time-limits for theregulatory procedure with scrutiny should be curtailed forthe adoption of certain amendments to Annexes II and IIIrelating to substances already authorised under other Com-munity law as well as any appropriate transitional mea-sures related to these substances.(22)In order to develop and update Community law on foodadditives in a proportionate and effective way,it is neces-sary to collect data,share information and coordinate workbetween Member States.For that purpose,it may be usefulto undertake studies to address specific issues with a viewto facilitating the decision-making process.It is appropri-ate that the Community finance such studies as part of itsbudgetary procedure.The financing of such measures iscovered by Regulation(EC)No882/2004of the EuropeanParliament and of the Council of29April2004on officialcontrols performed to ensure the verification of compli-ance with feed and food law,animal health and animalwelfare rules(4).(23)Member States are to carry out official controls in order toenforce compliance with this Regulation in accordancewith Regulation(EC)No882/2004.(24)Since the objective of this Regulation,namely to lay downCommunity rules on food additives,cannot be sufficientlyachieved by the Member States and can therefore,in theinterests of market unity and a high level of consumer pro-tection,be better achieved at Community level,the Com-munity may adopt measures,in accordance with theprinciple of subsidiarity as set out in Article5of the Treaty.In accordance with the principle of proportionality,as setout in that Article,this Regulation does not go beyondwhat is necessary in order to achieve that objective.(25)Following the adoption of this Regulation the Commis-sion,assisted by the Standing Committee on the FoodChain and Animal Health,should review all the existingauthorisations for criteria,other than safety,such as intake,technological need and the potential to mislead the con-sumer.All food additives that are to continue to be autho-rised in the Community should be transferred to theCommunity lists in Annexes II and III to this Regulation.Annex III to this Regulation should be completed with theother food additives used in food additives and foodenzymes as well as carriers for nutrients and their condi-tions of use in accordance with Regulation(EC)No1331/2008[establishing a common authorisation pro-cedure for food additives,food enzymes and food flavour-ings].To allow a suitable transition period,the provisionsin Annex III,other than the provisions concerning carriersfor food additives and food additives in flavourings,shouldnot apply until1January2011.(26)Until the future Community lists of food additives areestablished,it is necessary to provide for a simplified pro-cedure allowing the current lists of food additives con-tained in the existing Directives to be updated.(1)OJ L109,6.5.2000,p.29.(2)OJ L268,18.10.2003,p.24.(3)OJ L184,17.7.1999,p.23.(4)OJ L165,30.4.2004,p.1.Corrected by OJ L191,28.5.2004,p.1.(27)Without prejudice to the outcome of the review referred toin recital25,within one year following the adoption of thisRegulation the Commission should set up an evaluationprogramme for the Authority to re-evaluate the safety ofthe food additives that were already approved in the Com-munity.That programme should define the needs and theorder of priorities according to which the approved foodadditives are to be examined.(28)This Regulation repeals and replaces the following acts:Council Directive of23October1962on the approxima-tion of the rules of the Member States concerning thecolouring matters authorised for use in foodstuffs intendedfor human consumption(1),Council Directive65/66/EECof26January1965laying down specific criteria of purityfor preservatives authorised for use in foodstuffs intendedfor human consumption(2),Council Directive78/663/EECof25July1978laying down specific criteria of purity foremulsifiers,stabilizers,thickeners and gelling agents foruse in foodstuffs(3),Council Directive78/664/EEC of25July1978laying down specific criteria of purity forantioxidants which may be used in foodstuffs intended forhuman consumption(4),First Commission Directive81/712/EEC of28July1981laying down Communitymethods of analysis for verifying that certain additives usedin foodstuffs satisfy criteria of purity(5),Council Directive89/107/EEC of21December1988on the approximationof the laws of the Member States concerning food additivesauthorised for use in foodstuffs intended for human con-sumption(6),Directive94/35/EC of the European Parlia-ment and of the Council of30June1994on sweetenersfor use in foodstuffs(7),Directive94/36/EC of the Euro-pean Parliament and of the Council of30June1994oncolours for use in foodstuffs(8),Directive95/2/EC of theEuropean Parliament and of the Council of20February1995on food additives other than colours and sweeten-ers(9),Decision No292/97/EC of the European Parliamentand of the Council of19December1996on the mainte-nance of national laws prohibiting the use of certain addi-tives in the production of certain specific foodstuffs(10)and Commission Decision2002/247/EC of27March2002suspending the placing on the market and import ofjelly confectionary containing the food additive E425kon-jac(11).However,it is appropriate that certain provisionsof those acts remain in force during a transitional periodto allow time for the preparation of the Community listsin the Annexes to this Regulation,HAVE ADOPTED THIS REGULATION:CHAPTER ISUBJECT MATTER,SCOPE AND DEFINITIONSArticle1Subject matterThis Regulation lays down rules on food additives used in foods with a view to ensuring the effective functioning of the internal market whilst ensuring a high level of protection of human health and a high level of consumer protection,including the protection of consumer interests and fair practices in food trade,taking into account,where appropriate,the protection of the environment.For those purposes,this Regulation provides for:(a)Community lists of approved food additives as set out inAnnexes II and III;(b)conditions of use of food additives in foods,including in foodadditives and in food enzymes as covered by Regulation(EC) No1332/2008[on food enzymes],and in food flavourings as covered by Regulation(EC)No1334/2008of the Euro-pean Parliament and of the Council of16December2008on flavourings and certain food ingredients with flavouring properties for use in and on foods(12);(c)rules on the labelling of food additives sold as such.Article2Scope1.This Regulation shall apply to food additives.2.This Regulation shall not apply to the following substances unless they are used as food additives:(a)processing aids;(b)substances used for the protection of plants and plant prod-ucts in accordance with Community rules relating to plant health;(c)substances added to foods as nutrients;(d)substances used for the treatment of water for human con-sumption falling within the scope of Council Directive 98/83/EC of3November1998on the quality of water intended for human consumption(13);(1)OJ115,11.11.1962,p.2645/62.(2)OJ22,9.2.1965,p.373.(3)OJ L223,14.8.1978,p.7.(4)OJ L223,14.8.1978,p.30.(5)OJ L257,10.9.1981,p.1.(6)OJ L40,11.2.1989,p.27.(7)OJ L237,10.9.1994,p.3.(8)OJ L237,10.9.1994,p.13.(9)OJ L61,18.3.1995,p.1.(10)OJ L48,19.2.1997,p.13.(11)OJ L84,28.3.2002,p.69.(12)See page34of this Official Journal.(13)OJ L330,5.12.1998,p.32.(e)flavourings falling within the scope of Regulation(EC)No1334/2008[on flavourings and certain food ingredients with flavouring properties for use in and on foods].3.This Regulation shall not apply to food enzymes falling within the scope of Regulation(EC)No1332/2008[on food enzymes],with effect from the date of adoption of the Commu-nity list of food enzymes in accordance with Article17of that Regulation.4.This Regulation shall apply without prejudice to any spe-cific Community rules concerning the use of food additives:(a)in specific foods;(b)for purposes other than those covered by this Regulation.Article3Definitions1.For the purposes of this Regulation,the definitions laid down in Regulations(EC)No178/2002and(EC)No1829/2003 shall apply.2.For the purposes of this Regulation the following definitions shall also apply:(a)‘food additive’shall mean any substance not normally con-sumed as a food in itself and not normally used as a charac-teristic ingredient of food,whether or not it has nutritive value,the intentional addition of which to food for a tech-nological purpose in the manufacture,processing,prepara-tion,treatment,packaging,transport or storage of such food results,or may be reasonably expected to result,in it or its by-products becoming directly or indirectly a component of such foods;The following are not considered to be food additives:(i)monosaccharides,disaccharides or oligosaccharides andfoods containing these substances used for their sweet-ening properties;(ii)foods,whether dried or in concentrated form,includ-ing flavourings incorporated during the manufacturingof compound foods,because of their aromatic,sapid ornutritive properties together with a secondary colour-ing effect;(iii)substances used in covering or coating materials,which do not form part of foods and are not intended to beconsumed together with those foods;(iv)products containing pectin and derived from dried apple pomace or peel of citrus fruits or quinces,or froma mixture of them,by the action of dilute acid followedby partial neutralisation with sodium or potassium salts(liquid pectin);(v)chewing gum bases;(vi)white or yellow dextrin,roasted or dextrinated starch, starch modified by acid or alkali treatment,bleachedstarch,physically modified starch and starch treated byamylolitic enzymes;(vii)ammonium chloride;(viii)blood plasma,edible gelatin,protein hydrolysates and their salts,milk protein and gluten;(ix)amino acids and their salts other than glutamic acid, glycine,cysteine and cystine and their salts having notechnological function;(x)caseinates and casein;(xi)inulin;(b)‘processing aid’shall mean any substance which:(i)is not consumed as a food by itself;(ii)is intentionally used in the processing of raw materials, foods or their ingredients,to fulfil a certain technologi-cal purpose during treatment or processing;and(iii)may result in the unintentional but technically unavoid-able presence in the final product of residues of the sub-stance or its derivatives provided they do not presentany health risk and do not have any technological effecton the final product;(c)‘functional class’shall mean one of the categories set out inAnnex I based on the technological function a food additive exerts in the foodstuff;(d)‘unprocessed food’shall mean a food which has not under-gone any treatment resulting in a substantial change in the original state of the food,for which purpose the following in particular are not regarded as resulting in substantial change: dividing,parting,severing,boning,mincing,skinning,par-ing,peeling,grinding,cutting,cleaning,trimming,deep-freezing,freezing,chilling,milling,husking,packing or unpacking;(e)‘food with no added sugars’shall mean a food without thefollowing:(i)any added monosaccharides or disaccharides;(ii)any added food containing monosaccharides or disac-charides which is used for its sweetening properties;(f)‘energy-reduced food’shall mean a food with an energy valuereduced by at least30%compared with the original food ora similar product;(g)‘table-top sweeteners’shall mean preparations of permittedsweeteners,which may contain other food additives and/or food ingredients and which are intended for sale to the final consumer as a substitute for sugars;(h)‘quantum satis’shall mean that no maximum numerical levelis specified and substances shall be used in accordance with good manufacturing practice,at a level not higher than is necessary to achieve the intended purpose and provided the consumer is not misled.CHAPTER IICOMMUNITY LISTS OF APPROVED FOOD ADDITIVESArticle4Community lists of food additives1.Only food additives included in the Community list in Annex II may be placed on the market as such and used in foods under the conditions of use specified therein.2.Only food additives included in the Community list in Annex III may be used in food additives,in food enzymes and in food flavourings under the conditions of use specified therein.3.Food additives in Annex II shall be listed on the basis of the categories of food to which they may be added.4.Food additives in Annex III shall be listed on the basis of the food additives,food enzymes,food flavourings and nutrients or categories thereof to which they may be added.5.Food additives shall comply with the specifications as referred to in Article14.Article5Prohibition of non-compliant food additives and/ornon-compliant foodNo person shall place on the market a food additive or any food in which such a food additive is present if the use of the food addi-tive does not comply with this Regulation.Article6General conditions for inclusion and use of food additivesin Community lists1.A food additive may be included in the Community lists in Annexes II and III only if it meets the following conditions and, where relevant,other legitimate factors,including environmental factors:(a)it does not,on the basis of the scientific evidence available,pose a safety concern to the health of the consumer at the level of use proposed;(b)there is a reasonable technological need that cannot beachieved by other economically and technologically practi-cable means;and(c)its use does not mislead the consumer.2.To be included in the Community lists in Annexes II and IIIa food additive must have advantages and benefits for the con-sumer and therefore serve one or more of the following purposes:(a)preserving the nutritional quality of the food;(b)providing necessary ingredients or constituents for foodsmanufactured for groups of consumers with special dietary needs;(c)enhancing the keeping quality or stability of a food orimproving its organoleptic properties,provided that the nature,substance or quality of the food is not changed in such a way as to mislead the consumer;(d)aiding in the manufacture,processing,preparation,treat-ment,packing,transport or storage of food,including food additives,food enzymes and food flavourings,provided that the food additive is not used to disguise the effects of the use of faulty raw materials or of any undesirable practices or techniques,including unhygienic practices or techniques, during the course of any such activities.3.By way of derogation from paragraph2(a),a food additive which reduces the nutritional quality of a food may be included in the Community list in Annex II provided that:(a)the food does not constitute a significant component of anormal diet;or(b)the food additive is necessary for the production of foods forgroups of consumers with special dietary needs.。
欧盟关于食物添加剂的代码

LIST OF ADDITIVES CURRENTLY PERMITTED IN FOODIN THE EUROPEAN UNION AND THEIR E NUMBERS.The additives below are listed in groups for ease of reference.The groups are as follows:1. Antioxidants2. Colours3. Emulsifiers, Stabilisers, Thickeners and Gelling agents.4. Preservatives5. Sweeteners6. OthersWhen shopping you can find an additive on the list by first looking for its category, such as colour or preservative, and then looking for its name or number.This list does not in any way supplement the law in this area, nor constitute legal guidance.For information on the specifications and likely sources of these additives, please contact Mark Willis (020 7276 8559). For further information on legislation controlling the use of food additives, please contact John Caseley (020 7276 8591) for sweeteners and Andy Spencer (020 7276 8560) for the other categories.ColoursE100CurcuminE101(i) Riboflavin(ii) Riboflavin-5'-phosphateE102TartrazineE104Quinoline yellowE110Sunset Yellow FCF; Orange Yellow SE120Cochineal; Carminic acid; CarminesE122Azorubine; Carmoisine.E123 AmaranthE124Ponceau 4R; Cochineal Red AE127ErythrosineE128Red 2GE129Allura Red ACE131Patent Blue VE132lndigotine; Indigo CarmineE133Brilliant Blue FCFE141Chlorophylls and chlorophyllinsE141Copper complexes of chlorophyll and chlorophyllins E142Green SE150a Plain caramelE150b Caustic sulphite caramelE150c Ammonia caramelE150d Sulphite ammonia caramelE151Brilliant Black BN; Black PNE153Vegetable carbonE154Brown FKE155Brown HTE160a CarotenesE160b Annatto; Bixin; NorbixinE160c Paprika extract; Capsanthian; CapsorubinE160d LycopeneE160e Beta-apo-8'-carotenal (C30)E160e Ethyl ester of beta-apo-8'-carotenoic acid (C30)E161b LuteinE161g CanthaxanthinE162Beetroot Red; Betanin.E163 AnthocyaninsE170Calcium carbonateE171Titanium dioxideE172Iron oxides and hydroxidesE173AluminiumE174SilverE175GoldE180Litholrubine BK.PreservativesE200Sorbic acidE202Potassium sorbateE203Calcium sorbateE210Benzoic acidE211Sodium benzoateE212Potassium benzoateE213Calcium benzoateE214Ethyl p-hydroxybenzoateE215Sodium ethyl p-hydroxybenzoateE216Propyl p-hydroxybenzoateE217Sodium propyl p-hydroxybenzoateE218Methyl p-hydroxybenzoateE219Sodium methyl p-hydroxybenzoate E220Sulphur dioxideE221Sodium sulphiteE222Sodium hydrogen sulphiteE223Sodium metabisuiphiteE224Potassium metabisulphiteE226Calcium sulphiteE227Calcium hydrogen sulphiteE228Potassium hydrogen sulphiteE230Biphenyl; diphenylE231Orthophenyl phenolE232Sodium orthophenyl phenolE234NisinE235NatamycinE239Hexamethylene tetramine.E242Dimethyl dicarbonateE249Potassium nitriteE250Sodium nitriteE251Sodium nitrateE252Potassium nitrateE280Propionic acidE281Sodium propionateE282Calcium propionateE283Potassium propionateE284Boric acidE285Sodium tetraborate; boraxE1105Lysozyme.AntioxidantsE300Ascorbic acidE301Sodium ascorbateE302Calcium ascorbateE304Fatty acid esters of ascorbic acid E306TocopherolsE307Alpha-tocopherolE308Gamma-tocopherolE309Delta-tocopherolE310Propyl gallateE311Octyl gallateE312Dodecyl gallateE315Erythorbic acidE316Sodium erythorbateE320Butylated hydroxyanisole (BHA)E321Butylated hydroxytoluene (BHT).SweetenersE420(i) Sorbitol(ii) Sorbitol syrupE421MannitolE953lsomaltE965(i) Maltitol(ii) Maltitol syrupE966LactitolE967XylitolE950Acesulfame KE951AspartameE952Cyclamic acid and its Na and Ca saltsE954Saccharin and its Na, K and Ca saltsE957ThaumatinE959Neohesperidine DC.Emulsifiers, Stabilisers, Thickenersand Gelling AgentsE322LecithinsE400Alginic acidE401Sodium alginateE402Potassium alginateE403Ammonium alginateE404Calcium alginateE405Propane-1,2-diol alginateE406AgarE407CarrageenanE407a Processed eucheuma seaweedE410Locust bean gum; carob gumE412Guar gumE413TragacanthE414Acacia gum; gum arabicE415Xanthan gumE416Karaya gumE417Tara gumE418Gellan gumE425KonjacE432Polyoxyethylene sorbitan monolaurate; Polysorbate 20E433Polyoxyethylene sorbitan mono-oleate; Polysorbate 80E434Polyoxyethylene sorbitan monopalmitate; Polysorbate 40 E435Polyoxyethylene sorbitan monostearate; Polysorbate 60E436Polyoxyethylene sorbitan tristearate; Polysorbate 65E440Pectins.E442Ammonium phosphatidesE444Sucrose acetate isobutyrateE445Glycerol esters of wood rosinsE460CelluloseE461Methyl celluloseE463Hydroxypropyl celluloseE464Hydroxypropyl methyl celluloseE465Ethyl methyl celluloseE466Carboxy methyl cellulose Sodium arboxy methyl celluloseE468Crosslinked sodium carboxy methyl celluloseE469Enzymatically hydrolysed carboxy methyl celluloseE470a Sodium, potassium and calcium salts of fatty acidsE470b Magnesium salts of fatty acidsE471Mono- and diglycerides of fatty acidsE472a Acetic acid esters of mono- and diglycerides of fatty acidsE472b Lactic acid esters of mono- and diglycerides of fatty acidsE472c Citric acid esters of mono- and diglycerides of fatty acidsE472d Tartaric acid esters of mono- and diglycerides of fatty acidsE472e Mono- and diacetyltartaric acid esters of mono- anddiglycerides of fatty acidsE472f Mixed acetic and tartaric acid esters of mono- anddiglycerides of fatty acidsE473Sucrose esters of fatty acidsE474SucroglyceridesE475Polyglycerol esters of fatty acidsE476Polyglycerol polyricinoleate.E477 Propane-1,2-diol esters offatty acidsE481Sodium stearoyl-2-lactylateE482Calcium stearoyl-2-lactylateE483Stearyl tartrateE491Sorbitan monostearateE492Sorbitan tristearateE493Sorbitan monolaurateE494Sorbitan monooleateE495Sorbitan monopalmitateE1103Invertase.OthersAcid, acidity regulators, anti-caking agents, anti-foaming agents, bulking agents, carriers and carrier solvents, emulsifying salts, firming agents, flavour enhancers, flour treatment agents, foaming agents,glazing agents, humectants, modifiedstarches, packaging gases, propellants, raising agents and sequestrants. E170Calcium carbonatesE260Acetic acidE261Potassium acetateE262Sodium acetateE263Calcium acetateE270Lactic acidE290Carbon dioxideE296Malic acidE297Fumaric acidE325Sodium lactateE326Potassium lactateE327Calcium lactateE330Citric acidE331Sodium citratesE332Potassium citratesE333Calcium citratesE334Tartaric acid (L-(+))E335Sodium tartratesE336Potassium tartratesE337Sodium potassium tartrateE338Phosphoric acidE339Sodium phosphates.E340Potassium phosphatesE341Calcium phosphatesE343Magnesium phosphatesE350Sodium malatesE351Potassium malateE352Calcium malatesE353Metatartaric acidE354Calcium tartrateE355Adipic acidE356Sodium adipateE357Potassium adipateE363Succinic acidE380Triammonium citrateE385Calcium disodium ethylene diamine tetra-acetate;calcium disodium EDTAE422GlycerolE431Polyoxyethylene (40) stearateE450DiphosphatesE451TriphosphatesE452PolyphosphatesE459Beta-cyclodextrinE479b Thermally oxidised soya bean oil interacted withmono and diglycerides of fatty acidsE500Sodium carbonatesE501Potassium carbonatesE503Ammonium carbonatesE504Magnesium carbonatesE507Hydrochloric acidE508Potassium chlorideE509Calcium chloride.E511Magnesium chlorideE512Stannous chlorideE513Sulphuric acidE514Sodium sulphatesE515Potassium sulphatesE516Calcium sulphateE517Ammonium sulphateE520Aluminium sulphateE521Aluminium sodium sulphateE522Aluminium potassium sulphateE523Aluminium ammonium sulphateE524Sodium hydroxideE525Potassium hydroxideE526Calcium hydroxideE527Ammonium hydroxideE528Magnesium hydroxideE529Calcium oxideE530Magnesium oxideE535Sodium ferrocyanideE536Potassium ferrocyanideE538Calcium ferrocyanideE541Sodium aluminium phosphateE551Silicon dioxideE 552Calcium silicateE553a(i) Magnesium silicate(ii) Magnesium trisilicateE553b TalcE554Sodium aluminium silicate.E555Potassium aluminium silicateE556Aluminium calcium silicateE558BentoniteE559Aluminium silicate; KaolinE570Fatty acidsE574Gluconic acidE575Glucono delta-lactoneE576Sodium gluconateE577Potassium gluconateE578Calcium gluconateE579Ferrous gluconateE585Ferrous lactateE620Glutamic acidE621Monosodium glutamateE622Monopotassium glutamateE623Calcium diglutamateE624Monoammonium glutamate E625Magnesium diglutamateE626Guanylic acidE627Disodium guanylateE628Dipotassium guanylateE629Calcium guanylateE630lnosinic acidE631Disodium inosinateE632Dipotassium inosinateE633Calcium inosinateE634Calcium 5'-ribonucleotidesE635Disodium 5'-ribonucieotides. E640Glycine and its sodium salt E650Zinc acetateE900DimethylpolysiloxaneE901Beeswax, white and yellowE902Candelilla waxE903Carnauba waxE904ShellacE905Microcrystalline waxE912Montan acid estersE914Oxidised Polyethylene waxE920L-CysteineE927b CarbamideE938ArgonE939HeliumE941NitrogenE942Nitrous oxideE943a ButaneE943b Iso-butaneE944PropaneE948OxygenE949HydrogenE999Quillaia extractE1200PolydextroseE1201PolyvinylpyrrolidoneE1202PolyvinylpolypyrrolidoneE1404Oxidised starchE1410Monostarch phosphateE1412Distarch phosphate.E1413Phosphated distarch phosphateE1414Acetylated starchE1420Acetylated StarchE1422 Acetylated distarch adipateE1440 Hydroxyl propyl starchE1442 Hydroxy propyl distarch phosphate E1450 Starch sodium octenyl succinateE1451Acetylated oxidised starchPolyethylene glycol 6000E1505Triethyl citrateE1518Glyceryl triacetate; triacetinE1520Propan-1,2-diol; propylene glycol。
食品添加剂中英文对照表

134 E 400 Alginic acid
135 E 401 Sodium alginate
136 E 402 Potassium alginate
137 E 403 Ammonium alginate
138 E 404 Calcium alginate 139 E 405 Propan-1,2-diol alginate 140 E 406 Agar 141 E 407 Carrageenan 142 E 407a Processed eucheuma seaweed 143 E 410 Locust bean gum or carob bean gum 144 E 412 Guar gum 145 E 413 Tragacanth 146 E 414 Acacia gum(gum arabic) 147 E 415 Xanthan gum 148 E 416 Karaya gum 149 E 417 Tara gum 150 E 418 Gellan gum 151 E 420 Sorbitol (i)Sorbitol (ii)Sorbitol syrup 152 E 421 Mannitol 153 E 422 Glycerin or glycerol 154 E 425 Konjac (i)Konjac gum (ii)Konjac glucomannae 155 E 431 Polyoxyethylene (40) stearate 156 E 432 Polyoxyethylene sorbitan monolaurate (polysorbate 20) 157 E 433 Polyoxyethylene sorbitan monooleate (polysorbate 80)
Potassium cetrates (i)Monopotassium citrate (ii)Tripotassium citrate
食品添加剂中英文对照表

130 E 357 Potassium adipate
131 E 363 Succinic acid
132 E 380 Triammonium cetrate
133 E 385
Calcium disodium ethylene diamine tetra-acetate (Calcium disodium EDTA)
83 E 281 Sodium propionate
84 E 282 Calcium propionate
85 E 283 Potassium propionate
86 E 284 Boric acid
87 E 285 Sodium tetraborate (borax)
88 E 290 Carbon dioxide
79 E 262
Sodium acetates (i)Sodium acetate (ii)Sodium hydrogen acetate(sodium diacetate)
80 E 263 Calcium acetate
81 E 270 Lactic acid
82 E 280 Propionic acid
59 E 222 Sodium hydrogen sulphite 60 E 223 Sodium metabisulphite 61 E 224 Potassium metabisulphite 62 E 226 Calcium sulphite 63 E 227 Calcium hydrogen sulphite 64 E 228 Potassium hydrogen sulphite 65 E 230 Biphenyl, diphenyl 66 E 231 Orthophenyl phenol 67 E 232 Sodium orthophenyl phenol 68 E 233 Thiabendazole 69 E 234 Nisin 70 E 235 Natamycin or pimaricin 71 E 239 Hexamethylene tetramine 72 E 242 Dimethyl dicarbonate 73 E 249 Potassium nitrite 74 E 250 Sodium nitrite 75 E 251 Sodium nitrate 76 E 252 Potassium nitrate 77 E 260 Acetic acid 78 E 261 Potassium acetate or potassium diacetate
95_2_EC 食品添加剂的指令

This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents ►B EUROPEAN PARLIAMENT AND COUNCIL DIRECTIVE No 95/2/ECof 20 February 1995on food additives other than colours and sweeteners (OJ L 61, 18.3.1995, p. 1)Amended by:Official Journal No page date ►M1 Directive 96/85/EC of the European Parliament and of the Council of19 December 1996 L 86 4 28.3.1997 ►M2 Directive 98/72/EC of the European Parliament and of the Council of15 October 1998 L 295 18 4.11.1998 ►M3 Directive 2001/5/EC of the European Parliament and of the Council of12 February 2001 L 55 59 24.2.2001 ►M4 Directive 2003/52/EC of the European Parliament and of the Council of 18 June 2003L 178 23 17.7.2003 ►M5 Regulation (EC) No 1882/2003 of the European Parliament and of the Council of 29 September 2003L 284 1 31.10.2003 ►M6 Directive 2003/114/EC of the European Parliament and of the Council of 22 December 2003L 24 58 29.1.2004 ►M7 Directive 2006/52/EC of the European Parliament and of the Council of 5 July 2006L 204 10 26.7.2006 ►M8 Commission Directive 2010/69/EU of 22 October 2010 L 279 22 23.10.2010Corrected by: ►C1 Corrigendum, OJ L 248, 14.10.1995, p. 60 (95/2/EC) ►C2 Corrigendum, OJ L 78, 17.3.2007, p. 32 (2006/52/EC)EUROPEAN PARLIAMENT AND COUNCIL DIRECTIVENo 95/2/ECof 20 February 1995on food additives other than colours and sweetenersTHE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION,Having regard to the Treaty establishing the European Community, and in particular Article 100a thereof,Having regard to the proposal from the Commission (1),Having regard to the opinion of the Economic and Social Committee (2),Acting in accordance with the procedure laid down in Article 189b of the Treaty (3),Having regard to the Council Directive 89/107/EEC of 21 December 1988 on the approximation of the laws of the Member States concerning food additives authorized for use in foodstuffs intended for human consumption (4), and in particular Article 3 (2) thereof,Whereas differences between national laws relating to preservatives, antioxidants and other additives and their conditions of use hinder the free movement of foodstuffs; whereas this may create conditions of unfair competition;Whereas the prime consideration for any rules on these food additives and their conditions of use should be the need to protect the consumer;Whereas it is generally recognized that unprocessed foodstuffs and certain other foodstuffs should be free from food additives;Whereas, having regard to the most recent scientific and toxicological information on these substances, some of them are to be permitted only for certain foodstuffs and under certain conditions of use;Whereas it is necessary to lay down strict rules for the use of food additives in infant formulae, follow-on formulae and weaning foods, as referred to in Council Directive 89/398/EEC of 3 May 1989 on the approximation of the laws of the Member States relating to foodstuffs intended for particular nutritional uses (5), and in particular Article 4 (1)(e) thereof;(1) OJ No C 206, 13. 8. 1992, p. 12, and OJ No C 189, 13. 7. 1993, p. 11.(2) OJ No C 108, 19. 4. 1993, p. 26.(3) Opinion of the European Parliament of 26 May 1993 (OJ No C 176, 28. 6.1993, p. 117), confirmed on 2 December 1993 (OJ No C 342, 20. 12. 1993), common position of the Council of 10 March 1994 (OJ No C 172, 24. 6.1994, p. 4) and decision of the European Parliament of 16 November 1994Whereas this Directive is not intended to affect rules relating tosweeteners and colours;Whereas, pending specific provisions pursuant to Council Directive91/414/EEC of 15 July 1991 concerning the placing of plant protectionproducts on the market (1), and pursuant to Council Directive90/642/EEC of 27 November 1990 on the fixing of maximum levelsfor pesticide residues in and on certain products of plant origin,including fruit and vegetables (2), certain substances belonging to thiscategory are provisionally covered by this Directive;Whereas the Commission is to adapt Community provisions to accordwith the rules laid down in this Directive;Whereas the Scientific Committee for Food has been consulted for thosesubstances which are not yet the subject of a Community provision;Whereas it is necessary to include in this Directive specific provisionsconcerning additives referred to in other Community provisions;Whereas it is desirable that when a decision is taken on whether aparticular foodstuff belongs to a certain category of foods, the consultation of the Standing Committee for Foodstuffs procedure is followed;Whereas modifications of existing purity criteria for food additives otherthan colours and sweeteners and new specifications for those where nopurity criteria exist will be adopted in accordance with the procedurelaid down in Article 11 of Directive 89/107/EEC;Whereas the Scientific Committee for Food has not yet given anopinion on flour treatment agents; whereas those agents will be thesubject of a separate Directive;(3),Whereas this Directive replaces Directives 64/54/EEC70/357/EEC (4), 74/329/EEC (5) and 83/463/EEC (6); whereas thoseDirectives are hereby repealed,HAVE ADOPTED THIS DIRECTIVE:Article 1▼M21. This Directive is a specific Directive forming a part of the comprehensive Directive, within the meaning of Article 3 of Directive89/107/EEC, and applies to additives other than colours and sweeteners.It does not apply to enzymes other than those mentioned in theAnnexes,(1) OJ No L 230, 19. 8. 1991, p. 1. Directive as last amended by CommissionRegulation (EEC) No 3600/92 (OJ No L 366, 15. 12. 1992, p. 10).(2) OJ No L 350, 14. 12. 1990, p. 71.(3) OJ No 12, 27. 1. 1964, p. 161/64.42. Only additives which satisfy the requirements laid down by theScientific Committee for Food may be used in foodstuffs.thisDirective:ofpurposethe3. For(a) ‘preservatives’ are substances which prolong the shelf-life offoodstuffs by protecting them against deterioration caused bymicro-organisms;(b) ‘antioxidants’ are substances which prolong the shelf-life offoodstuffs by protecting them against deterioration caused byoxidation, such as fat rancidity and colour changes;▼M7(c) ‘carriers’, including carrier solvents, are substances used todissolve, dilute, disperse or otherwise physically modify a foodadditive or flavouring without altering its function (and withoutexerting any technological effect themselves) in order to facilitateits handling, application or use;▼B(d) ‘acids’ are substances which increase the acidity of a foodstuffand/or impart a sour taste to it;(e) ‘acidity regulators’ are substances which alter or control the acidityor alkalinity of a foodstuff;(f) ‘anti-caking agents’ are substances which reduce the tendency ofindividual particles of a foodstuff to adhere to one another;(g) ‘anti-foaming agents’ are substances which prevent or reducefoaming;(h) ‘bulking agents’ are substances which contribute to the volume of afoodstuff without contributing significantly to its available energyvalue;(i) ‘emulsifiers’ are substances which make it possible to form ormaintain a homogenous mixture of two or more immisciblephases such as oil and water in a foodstuff;(j) ‘emulsifying salts’ are substances which convert proteins contained in cheese into a dispersed form and thereby bring about homogenous distribution of fat and other components;(k) ‘firming agents’ are substances which make or keep tissues of fruit or vegetables firm or crisp, or interact with gelling agents toproduce or strengthen a gel;(l) ‘flavour enhancers’ are substances which enhance the existing taste and/or odour of a foodstuff;(m) ‘foaming agents’ are substances which make it possible to form a homogenous dispersion of a gaseous phase in a liquid or solidfoodstuff;(o) ‘glazing agents’ (including lubricants) are substances which, when applied to the external surface of a foodstuff, impart a shinyappearance or provide a protective coating;(p) ‘humectants’ are substances which prevent foodstuffs from drying out by counteracting the effect of an atmosphere having a lowdegree of humidity, or promote the dissolution of a powder in anaqueous medium;(q) ‘modified starches’ are substances obtained by one or more chemical treatments of edible starches, which may haveundergone a physical or enzymatic treatment, and may be acid oralkali thinned or bleached;(r) ‘packaging gases’ are gases other than air, introduced into a container before, during or after the placing of a foodstuff in thatcontainer;(s) ‘propellants’ are gases other than air which expel a foodstuff froma container;(t) ‘raising agents’ are substances or combinations of substances which liberate gas and thereby increase the volume of a dough or a batter;(u) ‘sequestrants’ are substances which form chemical complexes with metallic ions;▼M6(v) ‘stabilisers’ are substances which make it possible to maintain the physico-chemical state of a foodstuff; stabilisers include substanceswhich enable the maintenance of a homogenous dispersion of twoor more immiscible substances in a foodstuff, substances whichstabilise, retain or intensify an existing colour of a foodstuff andsubstances which increase the binding capacity of the food,including the formation of cross-links between proteins enablingthe binding of food pieces into re-constituted food;▼B(w) ‘thickeners’ are substances which increase the viscosity of a foodstuff.thanaresubstanceswhichemulsifiers4. Flourothertreatmentagentsare added to flour or dough to improve its baking quality.5. For the purposes of this Directive the following are not consideredas food additives:(a) substances used for treatment of drinking water as provided for inDirective 80/778/EEC (1);(b) products containing pectin and derived from dried apple pomace orpeel of citrus fruits, or from a mixture of both, by the action ofdilute acid followed by partial neutralization with sodium orpotassium salts (‘liquid pectin’);(c) chewing gum bases;(d) white or yellow dextrin, roasted or dextrinated starch, starchmodified by acid or alkali treatment, bleached starch, physicallymodified starch and starch treated by amylolitic enzymes;(e) ammonium chloride;(f) blood plasma, edible gelatin, protein hydrolysates and their salts,milk protein and gluten;(g) amino acids and their salts other than glutamic acid, glycine,cysteine and cystine and their salts and having no additive function;(h) caseinates and casein;(i) inulin.Article 2▼M21. Only substances listed in Annexes I, III, IV and V may be used infoodstuffs for the purposes mentioned in Article 1(3) and Article 1(4),2. Food additives listed in Annex I are permitted in foodstuffs, forthe purposes mentioned in Article 1(3) and Article 1(4), with theexception of those foodstuffs listed in Annex II, following the‘quantum satis’ principle,▼B3. Except where specifically provided for, paragraph 2 does notapply to:(a) — unprocessed foodstuffs,— honey as defined in Directive 74/409/EEC (1)— non-emulsified oils and fats of animal or vegetable origin,— butter,▼M2— pasteurised and sterilised (including UHT) milk (including plain, skimmed and semi-skimmed) and plain pasteurised cream,▼B— unflavoured, live fermented milk products,— natural mineral water as defined in Directive 80/777/EEC (2) and spring water,— coffee (excluding flavoured instant coffee) and coffee extracts,— unflavoured leaf tea,— sugars as defined in Directive 73/437/EEC (1),▼M2— dry pasta, excluding gluten-free and/or pasta intended for hypoproteic diets, in accordance with Directive 89/398/EEC,▼B— natural unflavoured buttermilk (excluding sterilized buttermilk).Within the meaning of this Directive, the term ‘unprocessed’ meansnot having undergone any treatment resulting in a substantialchange in the original state of the foodstuffs; however, thefoodstuffs may have been, for example, divided, parted, severed,boned, minced, skinned, pared, peeled, ground, cut, cleaned,trimmed, deep-frozen or frozen, chilled, milled or husked, packedor unpacked;(b) foods for infants and young children as referred to in Directive89/398/EEC, including foods for infants and young children notin good health; these foodstuffs are subject to the provisions ofAnnex VI;(c) the foodstuffs listed in Annex II, which may contain only thoseadditives referred to in that Annex and those additives referred toin Annexes III and IV under the conditions specified therein.4. Additives listed in Annexes III and IV may only be used in thefoodstuffs referred to in those Annexes and under the conditionsspecified therein.5. Only those additives listed in Annex V may be used as carriers orcarrier solvents for food additives and must be used under theconditions specified therein.6. The provisions of this Directive shall also apply to the corresponding foodstuffs intended for particular nutritional uses inaccordance with Directive 89/398/EEC.7. Maximum levels indicated in the Annexes refer to foodstuffs asmarketed, unless otherwise stated.8. In the Annexes to this Directive, ‘quantum satis’ means that nomaximum level is specified. However, additives shall be used inaccordance with good manufacturing practice, at a level not higherthan is necessary to achieve the intended purpose and provided thatthey do not mislead the consumer.Article 3▼M6additivepermissible:isfoodapresence1. Theof(a) in a compound foodstuff other than one mentioned in Article 2(3),to the extent to which the food additive is permitted in one of theingredients of the compound foodstuff;(b) in a foodstuff where a flavouring has been added, to the extent towhich the food additive is permitted in the flavouring in compliancewith this Directive and has been carried over to the foodstuff via theflavouring, provided the food additive has no technological functionin the final foodstuff; or(c) if the foodstuff is destined to be used solely in the preparation of acompound foodstuff and to an extent such that the compoundfoodstuff conforms to the provisions of this Directive.▼Binfantformulae,formulaefollow-onto2. Paragraphapply1doesnotand►M7 processed cereal-based foods and baby foods ◄, as referred▼M63. The level of additives in flavourings shall be limited to theminimum necessary to guarantee the safety and quality of flavouringsand to facilitate their storage. Furthermore, the presence of additives inflavourings must not mislead consumers or present a hazard to theirhealth. If the presence of an additive in a foodstuff, as a consequence ofadding flavourings, has a technological function in the foodstuff, it shallbe considered as an additive of the foodstuff and not as an additive ofthe flavouring.▼BArticle 4This Directive shall apply without prejudice to specific Directivespermitting additives listed in the Annexes to be used as sweeteners orcolours.Article 5Where necessary, it may be decided by the procedure laid down inArticle 6 of this Directive:— whether a particular foodstuff not categorized at the moment this Directive was adopted belongs to a category of foodstuffs referred toin Article 2 or in one of the Annexes, or— whether a food additive listed in the Annexes and authorized at ‘quantum satis’ is used in accordance with the criteria referred toin Article 2, or— whether a substance is a food additive within the meaning of Article 1.▼M5Article 6theStandingCommitteeonbyassisted1. ThebeCommissionshallthe Food Chain and Animal Health, set up by Article 58 of Regulation(EC) No 178/2002 (1), hereinafter referred to as ‘the Committee’.2. Where reference is made to this Article, Articles 5 and 7 ofDecision 1999/468/EC (1) shall apply, having regard to the provisionsof Article 8 thereof.The period laid down in Article 5(6) of Decision 1999/468/EC shall beset at three months.rulesprocedure.itsofadoptCommittee3. Theshall▼BArticle 7Member States shall, within three years of the entry into force of thisDirective, establish systems to monitor the consumption and use of foodadditives and report their findings to the Commission.The Commission shall report to the European Parliament and theCouncil within five years of the entry into force of this Directive onthe changes which have taken place in the food additives market, thelevels of use and consumption.In accordance with the general criteria in point 4 of Annex II toDirective 89/107/EEC, within five years of the entry into force of thisDirective, the Commission shall review the conditions of use referred toin this Directive, and propose amendments where necessary.Article 8and83/463/EEC74/329/EEC70/357/EEC,1. Directives64/54/EEC,are hereby repealed.2. References to these repealed Directives and to the purity criteriafor certain food additives referred to in them shall henceforth beconstrued as references to this Directive.Article 9Member States shall bring into force the laws, regulations and administrative provisions necessary to comply with this Directive not later than25 September 1996 in order to:— allow, by 25 September 1996 at the latest, trade in and use of products conforming to this Directive,— prohibit by 25 March 1997 at the latest, trade in and use of products not conforming to this Directive; products put on the market orlabelled before that date which do not comply with this Directivemay, however, be marketed until stocks are exhausted.They shall forthwith inform the Commission thereof.1When Member States adopt these measures, they shall contain a reference to this Directive or shall be accompanied by such reference on the occasion of their official publication. The methods of making such reference shall be laid down by the Member States.Article 10This Directive shall enter into force on the seventh day following that of its publication in the Official Journal of the European Communities.Article 11This Directive is addressed to the Member States.ANNEX IFOOD ADDITIVES GENERALLY PERMITTED FOR USE IN FOODSTUFFS NOT REFERRED TO IN ARTICLE 2 (3) Note1. Substances on this list may be added to all foodstuffs with the exception ofthose referred to in Article 2 (3) following the quantum satis principle.▼M62. The substances listed under numbers E 407, E 407a and E 440 may bestandardised with sugars, on condition that this is stated in addition to thenumber and designation.▼B3. Explanation of symbols used:* The substances E 290, E 938, E 939, E 941, E 942, E 948 and►M3 E949 ◄ may also be used in the foodstuffs referred to in Article 2 (3).# The substances E 410, E 412, E 415 and E 417 may not be used toproduce dehydrated foodstuffs intended to rehydrate on ingestion.▼M74. The substances listed under numbers E 400, E 401, E 402, E 403, E 404, E406, E 407, E 407a, E 410, E 412, E 413, E 414, E 415, E 417, E 418 and E440 may not be used in jelly mini-cups, defined, for the purpose of thisDirective, as jelly confectionery of a firm consistence, contained in semi-rigid mini-cups or mini-capsules, intended to be ingested in a single bite byexerting pressure on the mini-cups or mini-capsule to project the confectionery into the mouth.▼B▼M6▼B▼M1▼M7▼B▼M6▼M2▼M6▼B▼M2▼M3▼M2▼B▼M2(1) May be used only as a flour treatment agent.ANNEX IIFOODSTUFFS IN WHICH A LIMITED NUMBER OF ADDITIVES OF ANNEX I MAY BEUSED▼M6▼B▼M2▼B▼B▼B▼B ▼M6▼B▼M2▼B▼B▼M2▼M8▼B(1) OJ No L 228, 16. 8. 1973, p. 23.Cocoa and chocolate products energy-reduced or with no added sugars are not covered by Annex II.(2) OJ No L 244, 30. 9. 1993, p. 23.(3) OJ No L 205, 13. 8. 1979, p. 5.(4) OJ No L 24, 30. 1. 1976, p. 49.(5) OJ No L 84, 27. 3. 1987, p. 1.(6) OJ No L 373, 31. 12. 1988, p. 59.(7) OJ No L 231, 13. 8. 1992, p. 1.(8) OJ No L 176, 3. 7. 1984, p. 6.ANNEX IIICONDITIONALLY PERMITTED PRESERVATIVES ANDANTIOXIDANTSPART ASorbates, benzoates and p-hydroxybenzoates▼M7▼B(1) Benzoic acid may be present in certain fermented products resulting from the fermentation process following good manufacturing practice.Note1. The levels of all substances mentioned above are expressed as the free acid.2. The abbreviations used in the table mean the following:— Sa + Ba: Sa and Ba used singly or in combination— Sa + PHB: Sa and PHB used singly or in combination— Sa + Ba + PHB: Sa, Ba and PHB used singly or in combination.3. The maximum levels of use indicated refer to foodstuffs ready forconsumption prepared following manufacturers' instructions.▼M2▼B▼M7▼B▼B▼M2▼M7▼M6▼M7▼M8▼B( 1 ) This entry does not include dairy-based drinks. ( 2 ) OJ No L 84, 27. 3. 1987, p. 1. ( 3) OJ No L 186, 30. 6. 1989, p. 27. ►M7 ( 4 ) Directive 2002/46/EC of the European Parliament and of the Council (OJ L 183, 12.7.2002, p. 51). ◄ ►M7 ( 5) Commission Directive 1999/21/EC (OJ L 91, 7.4.1999, p. 29). ◄ PART BSulphur dioxide and sulphitesNote1. Maximum levels are expressed as SO 2 in mg/kg or mg/l as appropriate andrelate to the total quantity, available from all sources. 2. An SO 2 content of not more than 10 mg/kg or 10 mg/l is not considered tobe present.▼B▼B▼M7▼M8▼B(1) In edible parts.PART COther preservatives▼M2▼M8▼B▼M8▼B►M8(1) This substance may be present in certain cheeses as a result of fermentation process. ◄(a) When labelled ‘for food use’, nitrite may be sold only in a mixture with salt or a salt substitute.(b) Fo-value 3 is equivalent to 3 minutes heating at 121o C (reduction of the bacterial load of one billion spores in each 1 000 cans toone spore in a thousand cans).(c) Nitrates may be present in some heat-treated meat products resulting from natural conversion of nitrites to nitrates in a low-acidenvironment.1 Meat products are immersed in curing solution containing nitrites and/or nitrates, salt and other components. The meat products mayundergo further treatments e.g. smoking.1.1 Meat is injected with curing solution followed by immersion curing for 3 to 10 days. The immersion brine solution also includesmicrobiological starter cultures.1.2 Immersion cured for 3 to 5 days. Product is not heat-treated and has a high water activity.1.3 Immersion cured for at least4 days and pre-cooked.1.4 Meat is injected with curing solution followed by immersion curing. Curing time is 14 to 21 days followed by maturation in cold-smoke for 4 to 5 weeks.1.5 Immersion cured for 4 to 5 days at 5 to 7o C, matured for typically 24 to 40 hours at 22o C, possibly smoked for 24 hrs at 20 to 25o Cand stored for 3 to 6 weeks at 12 to 14o C.1.6 Curing time depending on the shape and weight of meat pieces for approximately 2 days/kg followed by stabilisation/maturation.2 Dry curing process involves dry application of curing mixture containing nitrites and/or nitrates, salt and other components to thesurface of the meat followed by a period of stabilisation/maturation. The meat products may undergo further treatments e.g. smoking.2.1 Dry curing followed by maturation for at least 4 days.2.2 Dry curing with a stabilisation period of at least 10 days and a maturation period of more than 45 days.2.3 Dry cured for 10 to 15 days followed by a 30 to 45 day stabilisation period and a maturation period of at least 2 months.2.4 Dry cured for 3 days + 1 day/kg followed by a 1 week post-salting period and an ageing/ripening period of 45 days to 18 months.2.5 Curing time depending on the shape and weight of meat pieces for approximately 10 to 14 days followed by stabilisation/maturation.3 Immersion and dry cured processes used in combination or where nitrite and/or nitrate is included in a compound product or wherethe curing solution is injected into the product prior to cooking. The products may undergo further treatments e.g. smoking.3.1 Dry curing and immersion curing used in combination (without injection of curing solution). Curing time depending on the shape andweight of meat pieces for approximately 14 to 35 days followed by stabilisation/maturation.3.2 Injection of curing solution followed, after a minimum of 2 days, by cooking in boiling water for up to 3 hours.3.3 Product has a minimum 4-week maturation period and a water/protein ratio of less than 1,7.3.4 Maturation period of at least 30 days.3.5 Dried product cooked to 70o C followed by 8 to 12 day drying and smoking process. Fermented product subject to 14 to 30 daythree-stage fermentation process followed by smoking.3.6 Raw fermented dried sausage without added nitrites. Product is fermented at temperatures in the range of 18 to 22o C or lower (10 to12o C) and then has a minimum ageing/ripening period of 3 weeks. Product has a water/protein ratio of less than 1,7.▼M2▼B▼M6▼B(1) Propionic acid and its salts may be present in certain fermented products resulting from the fermentation process following goodmanufacturing practice.►M6(2) Council Regulation (EC) No 1493/1999 of 17 May 1999 on the common organisation of the market in wine (OJ L 179,14.7.1999, p.1). Regulation as last amended by Commission Regulation (EC) No 1795/2003 (OJ L 262, 14.10.2003, p. 1).(3) Commission Regulation (EC) No 1622/2000 of 24 July 2000 laying down certain detailed rules for implementing Regulation (EC) No1493/1999 on the common organisation of the market in wine and establishing a Community code of oenological practices andprocesses (OJ L 194, 31.7.2000, p.1). Regulation as last amended by Regulation (EC) No 1410/2003 (OJ L 201, 8.8.2003, p. 9). ◄PART DOther antioxidantsNote►M7The * in the table refers to the proportionality rule: when combinations of gallates, TBHQ, BHA and BHT are used, the individual levels must be reduced proportionally. ◄▼M7▼C2▼B▼M7ANNEX IVOTHER PERMITTED ADDITIVESThe maximum levels of use indicated refer to foodstuffs ready for consumption prepared following manufacturers' instructions.▼M2▼B▼M2▼M2▼M6▼M2▼B▼M7▼B▼M7▼M8。
欧盟食品级EC1935-2004中英文11

REGULATION (EC) No 1935/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCILof 27 October 2004on materials and articles intended to come into contact with food and repealing Directives 80/590/EECand 89/109/EEC2004年10月27日发布关于食品接触类原料和物料的欧盟1935/2004指令,同时撤销原欧洲共同体80/590和89/109指令THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION,欧洲议会与理事会Having regard to the Treaty establishing the European Community,and in particular Article 95 thereof,注意到建立欧洲共同体的《条约》,特别是第96条Having regard to the proposal from the Commission,注意到委员会的提案Having regard to the opinion of the European Economic andSocial Committee (1),注意到欧洲经济和社会委员会的意见(1)Acting in accordance with the procedure laid down in Article 251of the Treaty (2),按照《条约》第251条规定的程序(2)Whereas:鉴于(1) Council Directive 89/109/EEC of 21 December 1988 on the approximation of the laws of the Member States relating to materials and articles intended to come into contact with foodstuffs (3) established general principles for eliminating the differences between the laws of the Member States as regards those materials and articles and provided for the adoption of implementing directives concerning specific groups of materials and articles (specific directives). This approach was successful and should be continued.理事会1988年12月21日的指令89 /109 /EEC关于统一各成员国材料和制品用于接触食品的法律,为消除各成员国关于这些原料和物料制定的差异,以及为特定材料和物品(具体指令)提供通过执行的指令而建立的一般原则。
食品添加剂欧盟编码一览表

•E100 Curcumin•E101 (i) Riboflavin (ii) Riboflavin-5'-phosphate•E102 Tartrazine•E104 Quinoline Yellow•E110 Sunset Yellow FCF, Orange Yellow S•E120 Cochineal, Carminic acid, Carmines•E122 Azorubine, Carmoisine•E123 Amaranth•E124 Ponceau 4R, Cochineal Red A•E127 Erythrosine•E128 Red 2G•E129 Allura Red AC•E131 Patent Blue V•E132 Indigotine, Indigo carmine•E133 Brilliant Blue FCF•E140 Chlorophylis and Chlorophyllins: (i) Chlorophylls (ii) Chlorophyllins•E141 Copper complexes of chlorophylls and chlorophyllins (i) Copper complexes of chlorophylls (ii) Copper complexes of chlorophyllins •E142 Greens S•E150a Plain caramel•E150b Caustic sulphite caramel•E150c Ammonia caramel•E150d Sulphite ammonia caramel•E151 Brilliant Black BN, Black PN•E153 Vegetable carbon•E154 Brown FK•E155 Brown HT•E160a Carotenes: (i) Mixed carotenes (ii) Beta-carotene•E160b Annatto, bixin, norbixin•E160c Paprika extract, capsanthin, capsorubin•E160d Lycopene•E160e Beta-apo-8'-carotenal (C 30)•E160f Ethyl ester of beta-apo-8'-carotenic acid (C 30)•E161b Lutein•E161g Canthaxanthin•E162 Beetroot Red, betanin•E163 Anthocyanins•E170 Calcium carbonates•E171 Titanium dioxide•E172 Iron oxides and hydroxides•E173 Aluminium•E174 Silver•E175 Gold•E180 Latolrubine BK•E200 Sorbic acid•E202 Potassium sorbate•E203 Calcium sorbate•E210 Benzoic acid•E211 Sodium benzoate•E212 Potassium benzoate•E213 Calcium benzoate•E214 Ethyl p-hydroxybenzoate•E215 Sodium ethyl p-hydroxybenzoate•E216 Propyl p-hydroxybenzoate•E217 Sodium propyl p-hydroxybenzoate•E218 Methyl p-hydroxybenzoate•E219 Sodium methyl p-hydroxybenzoate•E220 Sulphur dioxide•E221 Sodium sulphite•E222 Sodium hydrogen sulphite•E223 Sodium metabisulphite•E224 Potassium metabisulphite•E226 Calcium sulphite•E227 Calcium hydrogen sulphite•E228 Potassium hydrogen sulphite•E230 Biphenyl, diphenyl•E231 Orthophenyl phenol•E232 Sodium orthophenyl phenol•[E233 Thiabendazole] - Item deleted by Directive 98/72/EC•E234 Nisin•E235 Natamycin•E239 Hexamethylene tetramine•E242 Dimethyl dicarbonate•E249 Potassium nitrite•E250 Sodium nitrite•E251 Sodium nitrate•E252 Potassium nitrate•E260 Acetic acid•E261 Potassium acetate•E262 Sodium acetates (i) Sodium acetate (ii) Sodium hydrogen acetate (sodium diacetate)•E263 Calcium acetate•E270 Lactic acid•E280 Propionic acid•E281 Sodium propionate•E282 Calcium propionate•E283 Potassium propionate•E284 Boric acid•E285 Sodium tetraborate (borax)•E290 Carbon dioxide•E296 Malic acid•E297 Fumaric acid•E300 Ascorbic acid•E301 Sodium ascorbate•E302 Calcium ascorbate•E304 Fatty acid esters of ascorbic acid (i) Ascorbyl palmitate (ii) Ascorbyl stearate•E306 Tocopherol-rich extract•E307 Alpha-tocopherol•E308 Gamma-tocopherol•E309 Delta-tocopherol•E310 Propyl gallate•E311 Octyl gallate•E312 Dodecyl gallate•E315 Erythorbic acid•E316 Sodium erythorbate•E320 Butylated hydroxyanisole (BHA)•E321 Butylated hydroxytoluene (BHT)•E322 Lecithins•E325 Sodium lactate•E326 Potassium lactate•E327 Calcium lactate•E330 Citric acid•E331 Sodium citrates (i) Monosodium citrate (ii) Disodium citrate (iii) Trisodium citrate•E332 Potassium citrates (i) Monopotassium citrate (ii) Tripotassium citrate•E333 Calcium citrates (i) Monocalcium citrate (ii) Dicalcium citrate (iii) Tricalcium citrate•E334 Tartaric acid (L(+)-)•E335 Sodium tartrates (i) Monosodium tartrate (ii) Disodium tartrate•E336 Potassium tartrates (i) Monopotassium tartrate (ii) Dipotassium tartrate•E337 Sodium potassium tartrate•E338 Phosphoric acid•E339 Sodium phosphates (i) Monosodium phosphate (ii) Disodium phosphate (iii) Trisodium phosphate•E340 Potassium phosphates (i) Monopotassium phosphate (ii) Dipotassium phosphate (iii) Tripotassium phosphate•E341 Calcium phosphates (i) Monocalcium phosphate (ii) Dicalcium phosphate (iii) Tricalcium phosphate•E343 Magnesium phosphates (i) monomagnesium phosphate (ii) Dimagnesium phosphate [Under discussion and may be included in a future amendment to the Directive on miscellaneous additives]•E350 Sodium malates (i) Sodium malate (ii) Sodium hydrogen malate •E351 Potassium malate•E352 Calcium malates (i) Calcium malate (ii) Calcium hydrogen malate•E353 Metatartaric acid•E354 Calcium tartrate•E355 Adipic acid•E356 Sodium adipate•E357 Potassium adipate•E363 Succinic acid•E380 Triammonium citrate•E385 Calcium disodium ethylene diamine tetra-acetate (Calcium disodium EDTA)•E400 Alginic acid•E401 Sodium alginate•E402 Potassium alginate•E403 Ammonium alginate•E404 Calcium alginate•E405 Propan-1,2-diol alginate•E406 Agar•E407 Carrageenan•E407a Processed eucheuma seaweed [Added in December 1996 by Directive 96/83/EC]•E410 Locust bean gum•E412 Guar gum•E413 Tragacanth•E414 Acacia gum (gum arabic)•E415 Xanthan gum•E416 Karaya gum•E417 Tara gum•E418 Gellan gum•E420 Sorbitol (i) Sorbitol (ii) Sorbitol syrup•E421 Mannitol•E422 Glycerol•E425 Konjac (i) Konjac gum (ii) Konjac glucomannane[Added in October 1998 by Directive 98/72/EC]•E426 Soybean hemicellulose [Listed in proposed amendment in COM(2004)650 published October 2004]•E431 Polyoxyethylene (40) stearate•E432 Polyoxyethylene sorbitan monolaurate (polysorbate 20)•E433 Polyoxyethylene sorbitan monooleate (polysorbate 80)•E434 Polyoxyethylene sorbitan monopalmitate (polysorbate 40) •E435 Polyoxyethylene sorbitan monostearate (polysorbate 60) •E436 Polyoxyethylene sorbitan tristearate (polysorbate 65)•E440 Pectins (i) pectin (ii) amidated pectin•E442 Ammonium phosphatides•E444 Sucrose acetate isobutyrate•E445 Glycerol esters of wood rosins•E450 Diphosphates: (i) Disodium diphosphate (Disodium dihydrogen diphosphate, Disodium dihydrogen pyrophosphate, Sodium acidpyrophosphate, Disodium pyrophosphate); (ii) Trisodiumdiphosphate (Acid trisodium pyrophosphate, Trisodium monohydrogen diphosphate); (iii) Tetrasodium diphosphate (Tetrasodiumpyrophosphate, Sodium pyrophosphate); (iv) Dipotassiumdiphosphate (v) Tetrapotassium diphosphate (Potassiumpyrophosphate, Tetrapotassium pyrophosphate); (vi) Dicalcium diphosphate (Calcium pyrophosphate); (vii) Calcium dihydrogen diphosphate (Acid calcium pyrophosphate, Monocalcium dihydrogen pyrophosphate)•E451 Triphosphates: (i) Pentasodium triphosphate (pentasodium tripolyphosphate, sodium tripolyphosphate); (ii) Pentapotassium triphosphate (Pentapotassium tripolyphosphate, Potassiumtriphosphate, Potassium tripolyphosphate)•E452 Polyphosphates: (i) Sodium polyphosphates (Sodium hexametaphosphate, Sodium tetrapolyphosphate, Graham's salt, Sodium polyphosphates, glassy, Sodium polymetaphosphate, Sodium metaphosphate, Insoluble sodium metaphosphate, Maddrell's salt, Insoluble sodium polyphosphate, IMP); (ii) Potassiumpolyphosphates (Potassium metaphosphate, Potassiumpolymetaphosphate, Kurrol salt); (iii) Sodium calciumpolyphosphate (Sodium calcium polyphosphate, glassy); (iv) Calcium polyphophates (Calcium metaphosphate, Calcium polymetaphosphate)•E459 Beta-cyclodextrine [Added in October 1998 by Directive 98/72/EC]•E460 Cellulose (i) Microcrystalline cellulose (ii) Powdered cellulose•E461 Methyl cellulose•E462 Ethyl cellulose [Listed in proposed amendment in COM(2004)650 published October 2004]•E463 Hydroxypropyl cellulose•E464 Hydroxypropyl methyl cellulose•E465 Ethyl methyl cellulose•E466 Carboxy methyl cellulose, Sodium carboxy methyl cellulose•E467 Ethyl hydroxyethyl cellulose [Number had been allocated in a proposed amendment to Directive 95/2 circulated in August 1999. However, it was not accepted and further evaluation was requested]•E468 Crosslinked sodium carboxymethyl cellulose [Added in October 1998 by Directive 98/72/EC]•E469 Enzymically hydrolysed carboxy methyl cellulose[Added in October 1998 by Directive 98/72/EC]•E470a Sodium, potassium and calcium salts of fatty acids•E470b Magnesium salts of fatty acids•E471 Mono- and diglycerides of fatty acids•E472a Acetic acid esters of mono- and diglycerides of fatty acids •E472b Lactic acid esters of mono- and diglycerides of fatty acids •E472c Citric acid esters of mono- and diglycerides of fatty acids •E472d Tartaric acid esters of mono- and diglycerides of fatty acids •E472e Mono- and diacetyl tartaric acid esters of mono- and diglycerides of fatty acids•E472f Mixed acetic and tartaric acid esters of mono- and diglycerides of fatty acids•E473 Sucrose esters of fatty acids•E474 Sucroglycerides•E475 Polyglycerol esters of fatty acids•E476 Polyglycerol polyricinoleate•E477 Propane-1,2-diol esters of fatty acids•E479b Thermally oxidized soya bean oil interacted with mono- and diglycerides of fatty acids•E481 Sodium stearoyl-2-lactylate•E482 Calcium stearoyl-2-lactylate•E483 Stearyl tartrate•E491 Sorbitan monostearate•E492 Sorbitan tristearate•E493 Sorbitan monolaurate•E494 Sorbitan monooleate•E495 Sorbitan monopalmitate•E500 Sodium carbonates (i) Sodium carbonate (ii) Sodium hydrogen carbonate (iii) Sodium sesquicarbonate•E501 Potassium carbonates (i) Potassium carbonate (ii) Potassium hydrogen carbonate•E503 Ammonium carbonates (i) Ammonium carbonate (ii) Ammonium hydrogen carbonate•E504 Magnesium carbonates (i) Magnesium carbonate (ii) Magnesium hydroxide carbonate (syn. Magnesium hydrogen carbonate)•E507 Hydrochloric acid•E508 Potassium chloride•E509 Calcium chloride•E511 Magnesium chloride•E512 Stannous chloride•E513 Sulphuric acid•E514 Sodium sulphates (i) Sodium sulphate (ii) Sodium hydrogen sulphate•E515 Potassium sulphates (i) Potassium sulphate (ii) Potassium hydrogen sulphate•E516 Calcium sulphate•E517 Ammonium sulphate•E520 Aluminium sulphate•E521 Aluminium sodium sulphate•E522 Aluminium potassium sulphate•E523 Aluminium ammonium sulphate•E524 Sodium hydroxide•E525 Potassium hydroxide•E526 Calcium hydroxide•E527 Ammonium hydroxide•E528 Magnesium hydroxide•E529 Calcium oxide•E530 Magnesium oxide•E535 Sodium ferrocyanide•E536 Potassium ferrocyanide•E538 Calcium ferrocyanide•E541 Sodium aluminium phosphate, acidic•E551 Silicon dioxide•E552 Calcium silicate•E553a (i) Magnesium silicate (ii) Magnesium trisilicate•E553b Talc•E554 Sodium aluminium silicate•E555 Potassium aluminium silicate•E556 Calcium aluminium silicate•E558 Bentonite•E559 Aluminium silicate (Kaolin)•E570 Fatty acids•E574 Gluconic acid•E575 Glucono-delta-lactone•E576 Sodium gluconate•E577 Potassium gluconate•E578 Calcium gluconate•E579 Ferrous gluconate•E585 Ferrous lactate•E586 4-hexylresorcinol [Listed in proposed amendment in COM(2004)650 published October 2004]•E620 Glutamic acid•E621 Monosodium glutamate•E622 Monopotassium glutamate•E623 Calcium diglutamate•E624 Monoammonium glutamate•E625 Magnesium diglutamate•E626 Guanylic acid•E627 Disodium guanylate•E628 Dipotassium guanylate•E629 Calcium guanylate•E630 Inosinic acid•E631 Disodium inosinate•E632 Dipotassium inosinate•E633 Calcium inosinate•E634 Calcium 5'-ribonucleotides•E635 Disodium 5'-ribonucleotides•E640 Glycine and its sodium salt•E650 Zinc acetate[Added in February 2001 by Directive 2001/5/EC]•E900 Dimethyl polysiloxane•E901 Beeswax, white and yellow•E902 Candelillla wax•E903 Carnauba wax•E904 Shellac•E905 Microcrystalline wax [Added in October 1998 by Directive 98/72/EC]•E907 Hydrogenated poly-1-decene [Added in December 2003 by Directive 2003/114/EC]•E912 Montanic acid esters•E914 Oxidized polyethylene wax•E920 L-Cysteine [Added in October 1998 by Directive 98/72/EC]•E927b Carbamide•E938 Argon•E939 Helium•E941 Nitrogen•E942 Nitrous oxide•E943a Butane[Added in February 2001 by Directive 2001/5/EC]•E943b Isobutane [Added in February 2001 by Directive 2001/5/EC]•E944 Propane [Added in February 2001 by Directive 2001/5/EC]•E948 Oxygen•E949 Hydrogen [Added in February 2001 by Directive 2001/5/EC]•E950 Acesulfame K•E951 Aspartame•E952 Cyclamic acid and its Na and Ca salts•E953 Isomalt•E954 Saccharin and its Na, K and Ca salts•E955 Sucralose [Added in December 2003 by Directive 2003/115]•E957 Thaumatin•E959 NeohesperidineDC•E962 Salt of aspartame - acesulfame [Added in December 2003 by Directive 2003/115]•E965 Maltitol (i) Maltitol (ii) Maltitol syrup•E966 Lactitol•E967 Xylitol•E968 Erythritol [Listed in proposed amendment in COM(2004)650 published October 2004]•E999 Quilllaia extract•E1103 Invertase [Added in October 1998 by Directive 98/72/EC]•E1105 Lysozyme•E1200 Polydextrose•E1201 Polyvinylpyrrolidone•E1202 Polyvinylpolypyrrolidone•E1404 Oxidized starch•E1410 Monostarch phosphate•E1412 Distarch phosphate•E1413 Phosphated distarch phosphate•E1414 Acetylated distarch phosphate•E1420 Acetylated starch•E1422 Acetylated distarch adipate•E1440 Hydroxy propyl starch•E1442 Hydroxy propyl distarch phosphate•E1451 Acetylated oxidised starch[Added in October 1998 by Directive 98/72/EC]•E1450 Starch sodium octenyl succinate•E1505 Triethyl citrate•E1517 Glyceryl diacetate (diacetin)•E1518 Glyceryl triacetate (triacetin)•E1519 Benzyl alcohol•E1520 Propan-1,2-diol (propylene glycol)[Added in February 2001 by Directive 2001/5/EC]。
食品添加剂的编码

食品添加剂的编码E编码是欧盟为各种食品添加剂而编订的食物标签。
不是所有有E编码的食品添加剂在所有国家都允许用于食品。
E编码的格式为“E”字加三位数字,细分项目在E XXX 后加i/ii/iii 或a/b/c/d,新项目用到四位数字: E XXXX,例如色素类为E100- 199,防腐剂类为E200-299,防氧化剂、酸度调节剂为E300- 399,增稠剂、稳定剂及乳化剂为E400-499,酸碱度平衡剂、防凝结剂为E500-599,增味剂及味精为E620-649等。
INS编码(international number system)是1989年7月食品法典委员会第18次会议通过以E-number为基础的食品添加剂国际编码系统。
大部分与E编号相同,同时对E编号中未细分的同类物作了补充。
有INS编号的物质只是作为参考并不表示食品添加剂法规委员会(CCFC)批准其作为食品添加剂使用。
INS编码包括3或4位数字,INS的编号从100至1521。
在一些INS编码数字后加有字母标注,例如,150a代表焦糖色素Ⅰ-普通型,150b代表焦糖色素Ⅱ-亚硫酸化腐蚀型,150c代表焦糖色素Ⅲ-氨化,150d代表焦糖色素Ⅳ-亚硫酸氨化,包含任意字母下标的数字都应标在食品标签中。
在INS编码数字或字母后再加(i)、(ii)、(iii)表示亚类,例如160a代表胡萝卜素,160a(ⅰ)代表β-胡萝卜素(人造),160a(ⅱ)代表天然提取胡萝卜素。
INS不包含食用香料、胶母糖基础剂和食品营养添加剂。
在INS 编号中也常常对具有相近作用的食品添加剂编组,但由于列表的扩大,经常出现三位的数字已经被分配完的情况,所以列表中的食品添加剂不再定位于对功能的描述。
CAS编码(Chemical Abstract Service No.简称CAS No. )是美国化学文摘服务社为化学物质登陆提供的登记号,美国化学会的下设组织CAS负责为每一种出现在文献中的物质分配一个CAS号,其目的是为了避免化学物质有多种名称的麻烦,使数据库的检索更为方便。
欧盟食品着色剂指令94/36/EC(中英文对照版)

欧洲议会和理事会指令94/36/EC食品着色剂指令(OJ L 237,10.9.1994,p.13)修订情况:修订 标注 修订指令 官方公报卷号 页码 日期M1 2003年9月29日欧洲议会和理事会法规(EC)No 1882/2003L 284 1 2003-10-3l 注:文中凡被修订的条款均加注T M1的上标,修订指令M1的生效日期为2003年11月20日。
欧洲议会和欧盟理事会,考虑到建立欧洲共同体的条约,特别是其第lOOa条,考虑到理事会1988年12月21日关于使各成员国食品添加剂法律趋于一致的指令S9/107/EEC(OJ NoI+40,11.2.1989,p.27。
指令已被指令94/34/EC修订),特别是其第3条第2款,考虑到委员会的提案(OJ No C 12,8.1.1992,p.7)考虑到经济与社会委员会的意见(OJ No C 313,30.11,1992,p.1)按照条约(1993年3月10日的欧洲议会意 (OJ NoCll5,26.4.1993,p.105),1993年12月2日确认(OJ Nol’342,20.12.1993),1994年3月9日理事会的共同见解(未在官方公报上发布)以及1994年3月9日欧洲议会的决议 (OJ No C 91,28.3.1.994,p.79)89b 条规定的程序采取措施,鉴于各国用于食品的着色剂使用条件法律的差异阻碍了食品的自由流通;鉴于这些差异可能会为不公平的竞争创造条件;鉴于针对这些食品添加剂的任何规则和使用条件主要考虑的应是保护和告知消费者的需求;鉴于一种食品添加剂只有在有证据表明是技术上的需求并且它的使用不会危害人类健康时,方可使用;鉴于着色剂是用来恢复那些色泽由于加工、储存、包装和分销受到影响,并因此可能会损坏其视觉可接受性的食品本身原来所具有的外观;鉴于着色剂是用来使食品视觉上更吸引人并帮助鉴别通常与特殊食品相关的风味以及赋予无色食品以颜色;鉴于有必要包含某些用于肉类卫生标记的着色剂,而加盖卫生标记是根据指令9l/497/EEC(OJ No 268,24.9.1991,p.69。
关于食品添加剂的欧洲代码EN

Polyoxyethylene sorbitan monopalmitate; Polysorbate 40
E435
Polyoxyethylene sorbitan monostearate; Polysorbate 60
E436
Polyoxyethylene sorbitan tristearate; Polysorbate 65
Xylitol
E950
Acesulfame K
E951
Aspartame
E952
Cyclamic acid and its Na and Ca salts
E954
Saccharin and its Na, K and Ca salts
E957
Thaumatin
E959
Neohesperidine DC.Emulsifiers, Stabilisers,
E406
Agar
E407
Carrageenan
E407a
Processed eucheuma seaweed
E410
Locust bean gum; carob gum
E412
Guar gum
E413
Tragacanth
E414
Acacia gum; gum arabic
E415
Xanthan gum
E150c
Ammonia caramel
E150d
Sulphite ammonia caramel
E151
Brilliant Black BN; Black PN
E153
Vegetable carbon
E154
Brown FK
常用食品添加剂中英文对照

131 E 363 Succinic acid 144 E 412 Guar gum 151 E 420 Sorbitol (i)Sorbitol (ii)Sorbitol syrup
苯甲酸钠 二氧化硫 乙酸 乳酸 丙酸 苹果酸 富马酸 抗坏血酸 抗坏血酸钠 异抗坏血酸钠 乳酸钙
序号
E编 号
食品添加剂中英文对照表
英文名称
中文名称
1 E 100 Curcumin or turmeric
姜黄素或姜黄
3 E 102 Tartrazine
柠檬黄
6 E 120 Cochineal, Carminic acid, Carmines 胭脂虫红,胭脂红酸,胭脂红
8 E 123 Amaranth
282 E 951 Aspartame 283 E 953 Isomalt 287 E 965 Maltitol (i)Maltitol (ii)Maltitol syrup 289 E 967 Xylitol
阿斯巴甜(天冬酰苯丙氨酸甲酯)
异麦芽糖醇 麦芽糖醇 i)麦芽糖醇 ii)麦芽 糖醇糖浆
木糖醇
EEC, 澳新
EEC, 新加坡, 澳新 EEC, 新加坡, 澳新 EEC, 新加坡, 澳新
EEC
EEC, 新加坡, 澳新
EEC
EEC, 日本, 新加坡, 澳新 EEC, 日本, 澳新 EEC, 日本 , 澳新
EEC, 澳新
EEC, 日本benzoate 57 E 220 Sulphur dioxide 77 E 260 Acetic acid 81 E 270 Lactic acid 82 E 280 Propionic acid 89 E 296 Malic acid 90 E 297 Fumaric acid 91 E 300 Ascorbic acid 92 E 301 Sodium ascorbate 103 E 316 Sodium erythorbate 109 E 327 Calcium lactate
欧盟食品添加剂法规
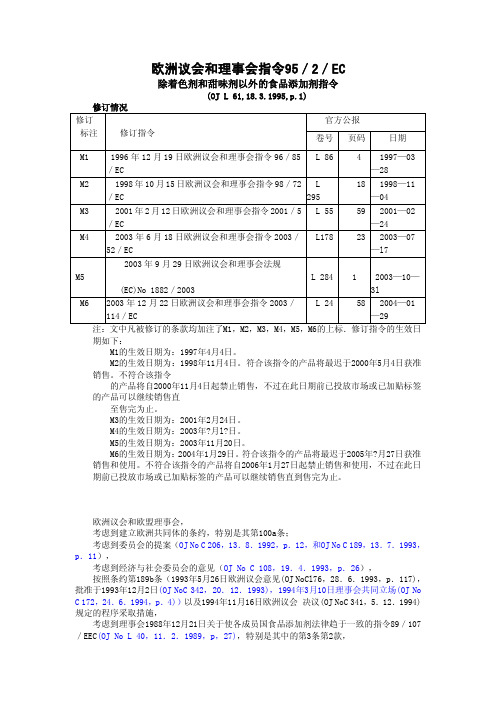
成同质分布状态的物质; (k)“固化剂”是指那些使水果或蔬菜的组织保持结实或脆的,或与胶凝剂彼此作用,
产生或加强凝胶体的物质; (1)“增味剂”是指那些增加食品现存味道和(或)气味的物质; (m)“发泡剂”是指在液体或固体食品中可使气态的物质形成同质弥散状态的物质; (n)“胶凝剂”是指那些通过形成凝胶体赋予食品一定质地的物质; (o)“上光剂”(包括润滑剂)是指那些用于食品外表时可产生光亮外表或提供保护膜的
的物质; (b)“抗氧化剂”是指那些防止因氧化引起的食品腐败变质,如脂肪变质以及颜色变化,
从而延长食品保存期的物质; (c)“载体”,包括载体溶剂,是指那些为了方便食品添加剂的处理,应用或使用,用
来溶解、稀释、分散或其他不改变食品添加剂的技术功能只产生物理变化的物质(并且它们 本身未发挥任何技术作用);
以外的气体; (s)“推进气体”是指那些把食品从容器中排出的气体(不是空气); (t)“膨松剂”是指能用来释放气体并借此增加面团或糊状物的体积的物质或某些物质
的混合物; (u)“螯合剂”是指与金属离子一起形成化学复合物的物质; (v)“6“稳定剂”是保持食品物理化学状态的物质;稳定剂包括使食品中两种或多种不
(d)“酸”是指增加食品酸度和(或)使食品具有酸味的物质; (e)“酸度调节剂”是改变或控制食品酸度或碱度的物质; (f)“抗结剂”是指那些减少食品个体微粒彼此粘合倾向的物质; (g)“消泡剂”是指那些预防或减少泡沫的物质; (h)“填充剂”是指那些有助于食品的体积而无益于其可获取的能量价值的物质; “乳化剂”是指那些可能使两种或更多的不可溶合状态的物质,例如食品中的油和水形 成或保持一种同质混合状态的物质; “乳化盐”是指那些使干酪中的蛋白质转化为一种分散状态,从而使脂肪和其他成分形
关于食品添加剂的欧洲代码EN

Enzymatically hydrolysed carboxy methyl cellulose
E470a
Sodium, potassium and calcium salts of fatty acids
E470b
Magnesium salts of fatty acids
E471
Mono- and diglycerides of fatty acids
Butylated hydroxytoluene (BHT).
Sweeteners
E420
(i) Sorbitol
(ii) Sorbitol syrup
E421
Mannitol
E953
lsomalt
E965
(i) Maltitol
(ii) Maltitol syrup
E966
Lactitol
E967
E464
Hydroxypropyl methyl cellulose
E465
Ethyl methyl cellulose
E466
Carboxy methyl cellulose Sodium arboxy methyl cellulose
E468
Crosslinked sodium carboxy methyl cellulose
E234
Nisin
E235
Natamycin
E239
Hexamethylene tetramine.
E242
Dimethyl dicarbonate
E249
Potassium nitrite
E250
Sodium nitrite
欧盟食用色素编号中英文对照

欧盟食用色素编号中英文对照欧盟食用色素编号E100- Curcumin 姜黄色素, 酸性黄E101- Riboflavin, Riboflavin-5'-phosphate 核黄素E102- Tartrazine 酒石黄E104- Quinoline Yellow 喹啉黄E107- Yellow 7G 黄7GE110- Sunset Yellow FCF, Orange Yellow S 日落黄E120- Cochineal, Carminic acid, Carmines 胭脂红、洋红、深红E122- Azorubine, Carmoisine 偶氮玉红、淡红E123- Amaranth 紫红色E124- Ponceau 4R, Cochineal Red A 丽春花色, 鲜红色E127- Erythrosine 赤藓红,四碘荧光素,新品酸性红E128- Red 2G 红色2GE129- Allura red AC 诱惑红E131- Patent blue V 专利兰紫VE132- Indigotine, Indigo carmine 靛蓝, 靛青E133- Brilliant blue FCFE140- Chlorophylis, Chlorophyllins 叶绿素、叶绿酸E141- Copper complexes of chloropyll and chlorophyllins 叶绿素铜钠盐E142- Green S 绿色E150(a)- Plain caramel 普通焦糖色E150(b)- Caustic sulphite caramel 苛性亚硫酸盐焦糖E150(c)- Ammonia caramel 氨法焦糖E150(d)- Sulphite ammonia caramel 亚硫酸铵焦糖E151- Brilliant Black BN, Black PN 亮黑色E153- Vegetable carbon 植物炭黑E154- Brown FK 棕色FKE155- Brown HT (Chocolate) 棕色HT(巧克力色)E160(a)- Carotene, alpha-, beta-, gamma- 胡萝卜素alpha-, beta-, gamma-E160(b)- Annatto (Arnatto, Annato), bixin, norbixin 胭脂树红, 果红;胭脂树橙,降胭脂树橙E160(c)- Paprika extract, capsanthin, capsorubin 辣椒红E160(d)- Lycopene 番茄红E160(e)- Beta-apo-8'-carotenal (C 30) β-衍-8'-胡萝卜醛(C 30) E160(f)- Ethyl ester of beta-apo-8'-carotenic acid (C 30) β-衍-8'-胡萝卜酸乙酯E161(b)- Xanthophylls - Lutein 黄色素—叶黄素E161(g)- Xanthophylls - Canthaxanthin 黄色素—角黄素、斑蝥黄E162- Beetroot Red, Betanin 甜菜红E163- Anthocyanins 花青素E170- Calcium carbonate 碳酸钙E171- Titanium dioxide 二氧化钛E172- Iron oxides and hydroxides 氧化铁及氢氧化铁E173- Aluminium 铝E174- Silver 银E175- Gold 金E180- Latolrubine BK 拉脱玉红E181- Tannic acid, tannins 丹宁酸。
食品添加剂中英文对照表

Calcium phosphates (i)Monocalcium phosphate (ii)Dicalcium phophate (iii)Tricalcium phosphate
59 E 222 Sodium hydrogen sulphite 60 E 223 Sodium metabisulphite 61 E 224 Potassium metabisulphite 62 E 226 Calcium sulphite 63 E 227 Calcium hydrogen sulphite 64 E 228 Potassium hydrogen sulphite 65 E 230 Biphenyl, diphenyl 66 E 231 Orthophenyl phenol 67 E 232 Sodium orthophenyl phenol 68 E 233 Thiabendazole 69 E 234 Nisin 70 E 235 Natamycin or pimaricin 71 E 239 Hexamethylene tetramine 72 E 242 Dimethyl dicarbonate 73 E 249 Potassium nitrite 74 E 250 Sodium nitrite 75 E 251 Sodium nitrate 76 E 252 Potassium nitrate 77 E 260 Acetic acid 78 E 261 Potassium acetate or potassium diacetate
欧盟食品添加剂法规05

制定实施欧洲议会和理事会(EC)No853/2004、854/2004和882/2004规章的过渡安排,及修订853/2004和854/2004
食品添加剂框架指令
89/107/EEC英文中文
2003/1882/EC英文
中文译文一包含修正案94/34/EC
食品着色剂
使用详细规定:94/36/EC英文中文
中文译文已包括修正案99/75/EC2001/50/EC2004/47/EC
质量规格规定:95/45/EC英文中文
2006/33/EC英文
食品甜味剂
使用详细规定:94/35/EC英文中文
中文译文已包括修正案:96/83/EC2003/115/EC
质量规格规定:95/31/EC(2004已修订整合)英文
关于对(EC)No852/2004规章(关于食品卫生)某些规定执行的指导文件
Regulation ( EC) No 882/2004英文(中文)
对用于人类消费的动物源性食品进行官方控制的组织的特定规则欧洲议会和理事会(EC)No882/2004规章
Regulation (EC) No 2076/2005英文(中文)
欧盟法规与标准
分类
欧盟有关ቤተ መጻሕፍቲ ባይዱ品添加剂法规标准
说明
相关法规
Regulation (EC) No 178/2002英文(中文)中文(目录)
关于一般食品法(EC)No178/2002规章的实施指南(第11,12,16,17,18,19,20条)
Regulation ( EC) No 852/2004英文(中文)
欧盟法规与标准分类欧盟有关食品添加剂法规标准说明相关法规regulationecno1782002英文中文中文目录关于一般食品法ecno1782002规章的实施指南第11121617181920条regulationecno8522004英文中文关于对ecno8522004规章关于食品卫生某些规定执行的指导文件regulationecno8822004英文中文对用于人类消费的动物源性食品进行官方控制的组织的特定规则欧洲议会和理事会ecno8822004规章regulationecno20762005英文中文对肉中旋毛虫官方控制的特殊规定委员会ecno20762005规章制定实施欧洲议会和理事会ecno85320048542004和8822004规章的过渡安排及修订8532004和8542004食品添加剂框架指令89107eec英文中文20031882ec英文中文译文一包含修正案9434ec食品着色剂使用详细规定
食品添加剂中英文对照表

10 E 127 Erythrosine
11 E 128 Red 2G
12 E 129 Allura Red AC
13 E 131 Patent Blue V
14 E 132 Indigotine, Indigo carmine
15 E 133 Brilliant Blue FCF
16 E 140 17 E 141 18 E 142
Potassium phosphates (i)Monopotassium phosphate (ii)Dipotassium phosphate (iii)Tripotassium phosphate
Calcium phosphates (i)Monocalcium phosphate (ii)Dicalcium phophate (iii)Tricalcium phosphate
83 E 281 Sodium propionate
84 E 282 Calcium propionate
85 E 283 Potassium propionate
86 E 284 Boric acid
87 E 285 Sodium tetraborate (borax)
88 E 290 Carbon dioxide
117 E 337 Sodium potassium tartrate
118 E 338 Phosphoric acid
119 E 339 120 E 340 121 E 341 122 E 343
Sodium phosphates (i)Monosodium phophate (ii)Disodium phosphate (iii)Trisodium phosphate
添加剂类别名称欧洲代码

添加剂类别名称、欧洲代码Klassennamen von Zusatzstoffen/ E-Nummern 欧洲代码 / 总概念/Oberbegriff E-Nummern德文 /Deutsch英文/Englisch中文/Chinesisch100-199 Farbstoffe colours200-299 Konservierungsstoffe , preservatives-mittelPackgase packaging gases300-399 Antioxidationsmittel antioxidants ,(Antioxidantien),Stabilisatoren , stabilisers (stabilizers),einzelne Emulgatoren some emulsifiers400-499 Verdickungsmittel , thickeners ,Geliermittel gelling agents(einschl. Gleitmittel), (including lubricants),einzelne Emulgatoren , some emulsifiers ,einzelne someZuckeraustauschstoffe sugar substitutes ,-polyolsSchaummittel foaming agents 500-1999 sonstige Zusatzstoffe other additivesGeschmacksverst?rker flavo(u)r enhancersS?uerungsmittel acid <EU> (acidifier,acidifying agent)S?ureregulatoren acidity regulatorsTrennmittel anti-caking agentsmodifizierte St?rke modified starchSü ?stoffe sweetenersBacktriebmittel raising agents色素防腐剂(保藏剂)包装瓦斯抗氧化剂,稳定剂,乳化剂(一部分)增稠剂,胶凝剂,(包括润滑剂)乳化剂 (一部分 ),代用糖 /糖替代品(一部分 )起泡剂,发泡剂其他附加剂增味剂;增香剂酸味剂,酸化剂酸度调节剂抗结剂(分离剂,防结块)变性淀粉(改性淀粉、改良淀粉)甜味剂(增甜剂)膨胀剂Schaumverhü ter anti-foaming agentsüberzugsmittel glazing agentsSchmelzsalze emulsifying salts Mehlbehandlungsmittel flour treatment agentsFestigungsmittel firming agentsFeuchthaltemittel humectants Fü llstoffe bulking agentsTreibgase propellent gasesAromen flavourings Komplexbildner sequestrantsTr?gerstoffe carriersPackgase packaging gases消泡剂上光剂乳化盐面粉处理剂(面粉改良剂)固化剂湿润剂,保湿剂填料推进剂、推进气体调味剂(芳香剂)络合剂载体包装瓦斯Fettdruck: Klassenname muss auf der Lebensmittelverpackung angegeben werden; vgl. u. a.: Richtlinie 2000/13/EG, Anhang II;Definitionen: Richtlinie 95/2/EG, Art. 1说明:如果某一个食品含上列以粗体写的添加剂的话,在这种产品的包装的说明上应该提供这个添加剂的类别名称。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
食品添加剂欧盟编码•E100 Curcumin•E101 (i) Riboflavin (ii) Riboflavin-5'-phosphate•E102 Tartrazine•E104 Quinoline Yellow•E110 Sunset Yellow FCF, Orange Yellow S•E120 Cochineal, Carminic acid, Carmines•E122 Azorubine, Carmoisine•E123 Amaranth•E124 Ponceau 4R, Cochineal Red A•E127 Erythrosine•E128 Red 2G•E129 Allura Red AC•E131 Patent Blue V•E132 Indigotine, Indigo carmine•E133 Brilliant Blue FCF•E140 Chlorophylis and Chlorophyllins: (i) Chlorophylls (ii) Chlorophyllins•E141 Copper complexes of chlorophylls and chlorophyllins (i) Copper complexes of chlorophylls (ii) Copper complexes of chlorophyllins•E142 Greens S•E150a Plain caramel•E150b Caustic sulphite caramel•E150c Ammonia caramel•E150d Sulphite ammonia caramel•E151 Brilliant Black BN, Black PN•E153 Vegetable carbon•E154 Brown FK•E155 Brown HT•E160a Carotenes: (i) Mixed carotenes (ii) Beta-carotene •E160b Annatto, bixin, norbixin•E160c Paprika extract, capsanthin, capsorubin•E160d Lycopene•E160e Beta-apo-8'-carotenal (C 30)•E160f Ethyl ester of beta-apo-8'-carotenic acid (C 30) •E161b Lutein•E161g Canthaxanthin•E162 Beetroot Red, betanin•E163 Anthocyanins•E170 Calcium carbonates•E171 Titanium dioxide•E172 Iron oxides and hydroxides•E173 Aluminium•E174 Silver•E175 Gold•E180 Latolrubine BK•E200 Sorbic acid•E202 Potassium sorbate•E203 Calcium sorbate•E210 Benzoic acid•E211 Sodium benzoate•E212 Potassium benzoate•E213 Calcium benzoate•E214 Ethyl p-hydroxybenzoate•E215 Sodium ethyl p-hydroxybenzoate •E216 Propyl p-hydroxybenzoate•E217 Sodium propyl p-hydroxybenzoate •E218 Methyl p-hydroxybenzoate•E219 Sodium methyl p-hydroxybenzoate •E220 Sulphur dioxide•E221 Sodium sulphite•E222 Sodium hydrogen sulphite•E223 Sodium metabisulphite•E224 Potassium metabisulphite•E226 Calcium sulphite•E227 Calcium hydrogen sulphite•E228 Potassium hydrogen sulphite•E230 Biphenyl, diphenyl•E231 Orthophenyl phenol•E232 Sodium orthophenyl phenol•[E233 Thiabendazole] - Item deleted by Directive 98/72/EC•E234 Nisin•E235 Natamycin•E239 Hexamethylene tetramine•E242 Dimethyl dicarbonate•E249 Potassium nitrite•E250 Sodium nitrite•E251 Sodium nitrate•E252 Potassium nitrate•E260 Acetic acid•E261 Potassium acetate•E262 Sodium acetates (i) Sodium acetate (ii) Sodium hydrogen acetate (sodium diacetate)•E263 Calcium acetate•E270 Lactic acid•E280 Propionic acid•E281 Sodium propionate•E282 Calcium propionate•E283 Potassium propionate•E284 Boric acid•E285 Sodium tetraborate (borax)•E290 Carbon dioxide•E296 Malic acid•E297 Fumaric acid•E300 Ascorbic acid•E301 Sodium ascorbate•E302 Calcium ascorbate•E304 Fatty acid esters of ascorbic acid (i) Ascorbyl palmitate (ii) Ascorbyl stearate•E306 Tocopherol-rich extract•E307 Alpha-tocopherol•E308 Gamma-tocopherol•E309 Delta-tocopherol•E310 Propyl gallate•E311 Octyl gallate•E312 Dodecyl gallate•E315 Erythorbic acid•E316 Sodium erythorbate•E320 Butylated hydroxyanisole (BHA)•E321 Butylated hydroxytoluene (BHT)•E322 Lecithins•E325 Sodium lactate•E326 Potassium lactate•E327 Calcium lactate•E330 Citric acid•E331 Sodium citrates (i) Monosodium citrate (ii) Disodium citrate (iii) Trisodium citrate•E332 Potassium citrates (i) Monopotassium citrate (ii) Tripotassium citrate•E333 Calcium citrates (i) Monocalcium citrate (ii) Dicalcium citrate (iii) Tricalcium citrate•E334 Tartaric acid (L(+)-)•E335 Sodium tartrates (i) Monosodium tartrate (ii) Disodium tartrate•E336 Potassium tartrates (i) Monopotassium tartrate (ii) Dipotassium tartrate•E337 Sodium potassium tartrate•E338 Phosphoric acid•E339 Sodium phosphates (i) Monosodium phosphate (ii) Disodium phosphate (iii) Trisodium phosphate•E340 Potassium phosphates (i) Monopotassium phosphate (ii) Dipotassium phosphate (iii) Tripotassium phosphate•E341 Calcium phosphates (i) Monocalcium phosphate (ii) Dicalcium phosphate (iii) Tricalcium phosphate•E343 Magnesium phosphates (i) monomagnesium phosphate (ii) Dimagnesium phosphate [Under discussion and may be included in a future amendment to the Directive on miscellaneous additives]•E350 Sodium malates (i) Sodium malate (ii) Sodium hydrogen malate•E351 Potassium malate•E352 Calcium malates (i) Calcium malate (ii) Calcium hydrogen malate•E353 Metatartaric acid•E354 Calcium tartrate•E355 Adipic acid•E356 Sodium adipate•E357 Potassium adipate•E363 Succinic acid•E380 Triammonium citrate•E385 Calcium disodium ethylene diamine tetra-acetate (Calcium disodium EDTA)•E400 Alginic acid•E401 Sodium alginate•E402 Potassium alginate•E403 Ammonium alginate•E404 Calcium alginate•E405 Propan-1,2-diol alginate•E406 Agar•E407 Carrageenan•E407a Processed eucheuma seaweed [Added in December 1996 by Directive 96/83/EC]•E410 Locust bean gum•E412 Guar gum•E413 Tragacanth•E414 Acacia gum (gum arabic)•E415 Xanthan gum•E416 Karaya gum•E417 Tara gum•E418 Gellan gum•E420 Sorbitol (i) Sorbitol (ii) Sorbitol syrup•E421 Mannitol•E422 Glycerol•E425 Konjac (i) Konjac gum (ii) Konjac glucomannane[Added in October 1998 by Directive 98/72/EC]•E426 Soybean hemicellulose [Listed in proposed amendment in COM(2004)650 published October 2004]•E431 Polyoxyethylene (40) stearate•E432 Polyoxyethylene sorbitan monolaurate (polysorbate 20) •E433 Polyoxyethylene sorbitan monooleate (polysorbate 80) •E434 Polyoxyethylene sorbitan monopalmitate (polysorbate 40) •E435 Polyoxyethylene sorbitan monostearate (polysorbate 60) •E436 Polyoxyethylene sorbitan tristearate (polysorbate 65)•E440 Pectins (i) pectin (ii) amidated pectin•E442 Ammonium phosphatides•E444 Sucrose acetate isobutyrate•E445 Glycerol esters of wood rosins•E450 Diphosphates: (i) Disodium diphosphate (Disodium dihydrogen diphosphate, Disodium dihydrogen pyrophosphate, Sodium acid pyrophosphate, Disodium pyrophosphate); (ii)Trisodium diphosphate (Acid trisodium pyrophosphate, Trisodium monohydrogen diphosphate); (iii) Tetrasodium diphosphate(Tetrasodium pyrophosphate, Sodium pyrophosphate); (iv)Dipotassium diphosphate (v) Tetrapotassium diphosphate(Potassium pyrophosphate, Tetrapotassium pyrophosphate); (vi) Dicalcium diphosphate (Calcium pyrophosphate); (vii) Calcium dihydrogen diphosphate (Acid calcium pyrophosphate,Monocalcium dihydrogen pyrophosphate)•E451 Triphosphates: (i) Pentasodium triphosphate (pentasodium tripolyphosphate, sodium tripolyphosphate); (ii) Pentapotassiumtriphosphate (Pentapotassium tripolyphosphate, Potassiumtriphosphate, Potassium tripolyphosphate)•E452 Polyphosphates: (i) Sodium polyphosphates (Sodium hexametaphosphate, Sodium tetrapolyphosphate, Graham's salt, Sodium polyphosphates, glassy, Sodium polymetaphosphate,Sodium metaphosphate, Insoluble sodium metaphosphate,Maddrell's salt, Insoluble sodium polyphosphate, IMP); (ii)Potassium polyphosphates (Potassium metaphosphate,Potassium polymetaphosphate, Kurrol salt); (iii) Sodium calcium polyphosphate (Sodium calcium polyphosphate, glassy); (iv)Calcium polyphophates (Calcium metaphosphate, Calciumpolymetaphosphate)•E459 Beta-cyclodextrine [Added in October 1998 by Directive 98/72/EC]•E460 Cellulose (i) Microcrystalline cellulose (ii) Powdered cellulose •E461 Methyl cellulose•E462 Ethyl cellulose [Listed in proposed amendment in COM(2004)650 published October 2004]•E463 Hydroxypropyl cellulose•E464 Hydroxypropyl methyl cellulose•E465 Ethyl methyl cellulose•E466 Carboxy methyl cellulose, Sodium carboxy methyl cellulose•E467 Ethyl hydroxyethyl cellulose [Number had been allocated in a proposed amendment to Directive 95/2 circulated in August 1999. However, it was not accepted and further evaluation was requested]•E468 Crosslinked sodium carboxymethyl cellulose [Added in October 1998 by Directive 98/72/EC]•E469 Enzymically hydrolysed carboxy methyl cellulose[Added in October 1998 by Directive 98/72/EC]•E470a Sodium, potassium and calcium salts of fatty acids•E470b Magnesium salts of fatty acids•E471 Mono- and diglycerides of fatty acids•E472a Acetic acid esters of mono- and diglycerides of fatty acids •E472b Lactic acid esters of mono- and diglycerides of fatty acids •E472c Citric acid esters of mono- and diglycerides of fatty acids •E472d Tartaric acid esters of mono- and diglycerides of fatty acids•E472e Mono- and diacetyl tartaric acid esters of mono- anddiglycerides of fatty acids•E472f Mixed acetic and tartaric acid esters of mono- anddiglycerides of fatty acids•E473 Sucrose esters of fatty acids•E474 Sucroglycerides•E475 Polyglycerol esters of fatty acids•E476 Polyglycerol polyricinoleate•E477 Propane-1,2-diol esters of fatty acids•E479b Thermally oxidized soya bean oil interacted with mono- and diglycerides of fatty acids•E481 Sodium stearoyl-2-lactylate•E482 Calcium stearoyl-2-lactylate•E483 Stearyl tartrate•E491 Sorbitan monostearate•E492 Sorbitan tristearate•E493 Sorbitan monolaurate•E494 Sorbitan monooleate•E495 Sorbitan monopalmitate•E500 Sodium carbonates (i) Sodium carbonate (ii) Sodium hydrogen carbonate (iii) Sodium sesquicarbonate•E501 Potassium carbonates (i) Potassium carbonate (ii) Potassium hydrogen carbonate•E503 Ammonium carbonates (i) Ammonium carbonate (ii) Ammonium hydrogen carbonate•E504 Magnesium carbonates (i) Magnesium carbonate (ii) Magnesium hydroxide carbonate (syn. Magnesium hydrogen carbonate)•E507 Hydrochloric acid•E508 Potassium chloride•E509 Calcium chloride•E511 Magnesium chloride•E512 Stannous chloride•E513 Sulphuric acid•E514 Sodium sulphates (i) Sodium sulphate (ii) Sodium hydrogen sulphate•E515 Potassium sulphates (i) Potassium sulphate (ii) Potassium hydrogen sulphate•E516 Calcium sulphate•E517 Ammonium sulphate•E520 Aluminium sulphate•E521 Aluminium sodium sulphate•E522 Aluminium potassium sulphate•E523 Aluminium ammonium sulphate•E524 Sodium hydroxide•E525 Potassium hydroxide•E526 Calcium hydroxide•E527 Ammonium hydroxide•E528 Magnesium hydroxide•E529 Calcium oxide•E530 Magnesium oxide•E535 Sodium ferrocyanide•E536 Potassium ferrocyanide•E538 Calcium ferrocyanide•E541 Sodium aluminium phosphate, acidic•E551 Silicon dioxide•E552 Calcium silicate•E553a (i) Magnesium silicate (ii) Magnesium trisilicate•E553b Talc•E554 Sodium aluminium silicate•E555 Potassium aluminium silicate•E556 Calcium aluminium silicate•E558 Bentonite•E559 Aluminium silicate (Kaolin)•E570 Fatty acids•E574 Gluconic acid•E575 Glucono-delta-lactone•E576 Sodium gluconate•E577 Potassium gluconate•E578 Calcium gluconate•E579 Ferrous gluconate•E585 Ferrous lactate•E586 4-hexylresorcinol [Listed in proposed amendment in COM(2004)650 published October 2004]•E620 Glutamic acid•E621 Monosodium glutamate•E622 Monopotassium glutamate•E623 Calcium diglutamate•E624 Monoammonium glutamate•E625 Magnesium diglutamate•E626 Guanylic acid•E627 Disodium guanylate•E628 Dipotassium guanylate•E629 Calcium guanylate•E630 Inosinic acid•E631 Disodium inosinate•E632 Dipotassium inosinate•E633 Calcium inosinate•E634 Calcium 5'-ribonucleotides•E635 Disodium 5'-ribonucleotides•E640 Glycine and its sodium salt•E650 Zinc acetate[Added in February 2001 by Directive 2001/5/EC]•E900 Dimethyl polysiloxane•E901 Beeswax, white and yellow•E902 Candelillla wax•E903 Carnauba wax•E904 Shellac•E905 Microcrystalline wax [Added in October 1998 by Directive 98/72/EC]•E907 Hydrogenated poly-1-decene [Added in December 2003 by Directive 2003/114/EC]•E912 Montanic acid esters•E914 Oxidized polyethylene wax•E920 L-Cysteine [Added in October 1998 by Directive 98/72/EC]•E927b Carbamide•E938 Argon•E939 Helium•E941 Nitrogen•E942 Nitrous oxide•E943a Butane[Added in February 2001 by Directive 2001/5/EC]•E943b Isobutane [Added in February 2001 by Directive 2001/5/EC]•E944 Propane [Added in February 2001 by Directive 2001/5/EC]•E948 Oxygen•E949 Hydrogen [Added in February 2001 by Directive 2001/5/EC]•E950 Acesulfame K•E951 Aspartame•E952 Cyclamic acid and its Na and Ca salts•E953 Isomalt•E954 Saccharin and its Na, K and Ca salts•E955 Sucralose [Added in December 2003 by Directive 2003/115]•E957 Thaumatin•E959 NeohesperidineDC•E962 Salt of aspartame - acesulfame [Added in December 2003 by Directive 2003/115]•E965 Maltitol (i) Maltitol (ii) Maltitol syrup•E966 Lactitol•E967 Xylitol•E968 Erythritol [Listed in proposed amendment in COM(2004)650 published October 2004]•E999 Quilllaia extract•E1103 Invertase [Added in October 1998 by Directive 98/72/EC]•E1105 Lysozyme•E1200 Polydextrose•E1201 Polyvinylpyrrolidone•E1202 Polyvinylpolypyrrolidone•E1404 Oxidized starch•E1410 Monostarch phosphate•E1412 Distarch phosphate•E1413 Phosphated distarch phosphate•E1414 Acetylated distarch phosphate•E1420 Acetylated starch•E1422 Acetylated distarch adipate•E1440 Hydroxy propyl starch•E1442 Hydroxy propyl distarch phosphate•E1451 Acetylated oxidised starch[Added in October 1998 by Directive 98/72/EC]•E1450 Starch sodium octenyl succinate•E1505 Triethyl citrate•E1517 Glyceryl diacetate (diacetin)•E1518 Glyceryl triacetate (triacetin)•E1519 Benzyl alcohol•E1520 Propan-1,2-diol (propylene glycol)[Added in February 2001 by Directive 2001/5/EC]。
