IChO2016国际化学奥林匹克竞赛英文真题理论部分(包括答案)
国际化学奥林匹克竞赛-国际化学奥林匹克竞赛-第38届ICHO理论试题(中文版)答案

1-1.The mass of a water droplet:m = V ρ = [(4/3) π r3] ρ = (4/3) π (0.5x10-6 m)3 (1.0 g/cm3)= 5.2x10-16 kg⇒10 marksAverage kinetic energy at 27o C:KE = mv2/2 = (5.2x10-16 kg) (0.51x10-2 m/s)2/2= 6.9x10-21 kg m2/s2= 6.9 x10-21 J ⇒15 marks*.The average kinetic energy of an argon atom is the same as that of a water droplet.KE becomes zero at –273 o C.From the linear relationship in the figure, KE = aT (absolute temperature)where a is the increase in kinetic energy of an argon atom per degree.a = KE/T = 6.9x10-21 J/(27+273K) = 2.3x10-23 J/K⇒25 marksS: specific heat of argon N: number of atoms in 1g of argonS = 0.31 J/g K = a x NN = S/a = (0.31 J/g K) / (2.3x10-23 J/K)= 1.4x1022 ⇒30 marksAvogadro’s number (N A) : Number of argon atoms in 40 g of argonN A = (40)(1.4x1022)= 5.6 x1023⇒20 marks2-1. ⇒ 30 marksmass of a typical star = (4/3)(3.1)(7x108 m)3(1.4 g/10-6 m 3) = 2×1033 g mass of protons of a typical star = (2×1033 g)(3/4 + 1/8) = 1.8×1033 g number of protons of a typical star = (1.8×1033 g)(6×1023/g) = 1×1057number of stellar protons in the universe = (1×1057)(1023) = 1×1080Partial credits on principles:Volume = (4/3)(3.14)radius 3×density; 4 marks 1 mole = 6×1023; 4 marksTotal number of protons in the universe = number of protons in a star ×1023; 2 marks Mass fraction of protons from hydrogen = (3/4)(1/1); 5 marks Mass fraction of protons from helium = (1/4)(2/4); 10 marks2-2. ⇒ 30 marks∆E(2→3) = C(1/4 - 1/9) = 0.1389 C λ(2→3) = 656.3 nm ∆E(1→2) = C(1/1 - 1/4) = 0.75 Cλ(1→2) = (656.3)(0.1389/0.75) = 121.5 nmNo penalty for using Rydberg constant from memory. 15 marks penalty if answered in a different unit (Hz, etc.)2-3.T = (2.9×10-3 m K)/1.215×10-7 m = 2.4×104 K ⇒ 10 marks2-4..⇒ 20 marksλ = 3 × 108 m/1.42 × 109 = 0.21 mT = (2.9 × 10-3 m K)/0.21 m = 0.014 K2-5. ⇒ 10 marks14N + 4He → (17O ) + 1HO-17, O acceptable1783-1.k des = A exp(-E des/R T)= (1x1012 s-1)(5x10-32) = 5x10-20 s-1 at T = 20 K ⇒10 markssurface residence time, τresidence = 1 / k des = 2x1019 s = 6x1011 yr ⇒20 marks(full credit for τhalf-life = ln2 / k des = 1x1019 s = 4x1011 yr)residence time = 2x1019s3-2.The distance to be traveled by a molecule: x = πr = 300 nm.k mig = A exp(-E mig/R T)= (1x1012 s-1)(2x10-16 ) = 2x10-4 s-1 at T = 20 K ⇒ 5 marksaverage time between migratory jumps,τ = 1 / k mig = 5x103 sthe time needed to move 300 nm= (300 nm/0.3 nm) jumps x (5x103 s/jump) = 5x106 s = 50 days ⇒15 marks(Full credit for the calculation using a random-walk model. In this case:t = τ (x/d) 2 = 5 x 109 s = 160 yr. The answer is still (b).)(a) (b)(c) (d) (e)10 marks3-3.k(20 K) / k(300 K) = exp[(E/R) (1/T1 - 1/T2)]= e-112 = ~ 10-49 for the given reaction ).) ⇒15 marks The rate of formaldehyde production at 20 K= ~ 10-49 molecule/site/s = ~ 10-42 molecule/site/ yr⇒10 marks(The reaction will not occur at all during the age of the universe (1x1010 yr).)rate = 10-42molecules/site/yr3-4. circle one(a) (b) (c) (a, b) (a, c) (b,c)(a, b, c)(15 marks, all or nothing)4-1.H PNumber of atoms ( 11.3 ) 1⇒ 10 marksTheoretical wt % ( 3.43 )⇒ 10 marks4-2.adenineN NN NN H H guanineNN N NO N HH HNN O N H H cytosineNN H O O thymine(10 marks on each)4-3. 7 marks each, 20 marks for threeadenineNNNNNHHguanine NN NNON HHH NNH OOthymineNNONHH cytosine NNH OOthymineguanine NN NNON HHHcytosineNNONHHcytosineNNON HHNNHOO thyminethymineNNHOONNH OOthyminethymine NNHOONNONHH cytosineadenineNNNNNHH adenineNNNNNHHadenine NNNNNHHguanineguanine NNNNON HHHNNNNONHHH4-4. 2.5 marks for each bracketadenineN NN N HNH 2guanine N NH N N HO NH 2Uracil N H NH O cytosineN H N NH 2OOHCN ( 5 ) ( 5 ) ( 4 )( 4 )H 2O ( 0 ) ( 1 ) ( 2 ) ( 1 )5-1.(20 marks)1st ionization is complete: H2SO4→ H+ + HSO4-[H2SO4] = 02nd ionization: [H+][SO42-]/[HSO4-] = K2 = 1.2 x 10-2 (1)Mass balance: [H2SO4] + [HSO4-] + [SO42-] = 1.0 x 10-7 (2)Charge balance: [H+] = [HSO4-] + 2[SO42-] + [OH-] (3)Degree of ionization is increased upon dilution.[H2SO4] = 0Assume [H+]H2SO4 = 2 x 10-7From (1), [SO42-]/[HSO4-] = 6 x 104 (2nd ionization is **plete)[HSO4-] = 0From (2), [SO42-] = 1.0 x 10-7 [5 marks]From (3), [H+] = (2 x 10-7) + 10-14/[H+][H+] = 2.4 x 10-7(pH = 6.6) [8 marks][OH-] = 10-14/(2.4 x 10-7) = 4.1 x 10-8[2 marks]From (1), [HSO4-] = [H+][SO42-]/K2= (2.4 x 10-7)(1.0 x 10-7)/(1.2 x 10-2) = 2.0 x 10-12[5 marks]Check charge balance:2.4 x 10-7≈ (2.0 x 10-12) + 2(1.0 x 10-7) + (4.1 x 10-8)Check mass balance:0 + 2.0 x 10-12 + 1.0 x 10-7≈ 1.0 x 10-7Species Concentration** x 10-12HSO4-** x 10-7SO42-** x 10-7H+** x 10-8 OH-5-2. (20 marks)mmol H3PO4 = 0.85 ⨯ 3.48 mL ⨯ 1.69g/mL ⨯ 1 mol/98.00 g ⨯ 1000 = 51.0 [5 marks]The desired pH is above p K2.A 1:1 mixture of H2PO4- and HPO42- would have pH = p K2 = 7.20.If the pH is to be 7.40, there must be more HPO42- than H2PO4-.We need to add NaOH to convert H3PO4to H2PO4-and to convert to the right amount of H2PO4-to HPO42-.H3PO4 + OH-→ H2PO4- + H2OH2PO4- + OH-→ HPO42- + H2OThe volume of 0.80 NaOH needed to react with to to convert H3PO4 to H2PO4- is:51.0 mmol / 0.80M = 63.75 mL [5 marks]To get pH of 7.40 we need:H2PO4- + OH-→ HPO42-Initial mmol 51.0 x 0Final mmol 51.0-x 0 xpH = p K2 + log [HPO42-] / [H2PO4-]7.40 = 7.20 + log {x / (51.0-x)}; x = 31.27 mmol [5 marks]The volume of NaOH needed to convert 31.27 mmol is :31.27 mmol / 0.80 M = 39.09 mLThe total volume of NaOH = 63.75 + 39.09 =102.84 mL , 103 mL [5 marks]Total volume of 0.80 M NaOH (mL) 103 mL5-3. (20 marks)p K = 3.52pH = pK a + log ([A-]/[HA])[A-]/[HA] = 10(pH-pKa) [5 marks]In blood, pH =7.40, [A-]/[HA] = 10(7.40-3.52) = 7586Total ASA = 7586 +1 = 7587 [5 marks]In stomach, pH = 2.00, [A-]/[HA] = 10(2.00-3.52) = 3.02x10-2Total ASA = 1+ 3.02x10-2 = 1.03 [5 marks]Ratio of total aspirin in blood to that in stomach = 7587/1.03 = 7400 [5 marks]** ( 103Ratio of total aspirin in blood to that in stomach6-1. (5 marks)4 H2O + 4 e-→ 2 H2(g) + 4 OH- (or 2 H2O + 2 e-→ H2(g) + 2 OH-)6-2. (5 marks)2 H2O → O2 + 4 H+ + 4 e-(or H2O → 1/2 O2 + 2 H+ + 2 e- )6-3. (5 marks)Cu → Cu2+ + 2e-6-4. (20 marks)Reduction of sodium ion seldom takes place.It has a highly negative reduction potential of –2.710 V.Reduction potential for water to hydrogen is negative (water is very stable).But, it is not as negative as that for sodium ion. It is –0.830 V.Reduction of both copper ion and oxygen takes place readily and the reduction potentials for both are positive.In the present system, the reverse reaction (oxidation) takes place at the positive terminal. Copper is oxidized before water.Reduction potential for hydrogen ion is defined as 0.000 V.6-5. (15 marks)pOH = 14.00 – 4.84 = 9.16[OH-] = 6.92 x 10-10K sp = [Cu2+][OH-]2 = 0.100 x (6.92 x 10-10) = 4.79 x 10-206-6.E = E o Cu2+/Cu + (0.0592/2) log [Cu2+]= +0.340 + (0.0592/2) log [Cu2+]= +0.340 + (0.0592/2) log (K sp / [OH-]2)= +0.340 + (0.0592/2) log (K sp) - (0.0592/2) log [OH-]2= +0.340 + (0.0592/2) log (K sp) - 0.0592 log [OH-],3 marksBy definition, the standard potential for Cu(OH)2(s) + 2e-→ Cu(s) + 2OH- is the potential where [OH-] = 1.00.E = E o Cu(OH)2/Cu = +0.340 + (0.0592/2) log (K sp)= +0.340 + (0.0592/2) log (4.79 x 10-20)= +0.340 - 0.5722 marks= -0.232 V10 marks-------------------------------------------------------------------------------------------------------------- One may solve this problem as following.Eqn 1: Cu(OH)2(s) + 2e -→ Cu + 2OH-E+o = E o Cu(OH)2/Cu = ?Eqn 2: Cu(OH)2(s) → Cu2+ + 2OH-E o = (0.05916/n) logK sp= (0.05916/2) log(4.79×10-20)= -0.5715 V3 marksEqn 1 – Eqn 2 : Cu2+ + 2e-→ CuE-o = E+o - E o = E o Cu2+/Cu = 0.34 VTherefore, E+o = E-o + E o = + 0.34 + (-0.5715)2 marks= -0.232 V10 marks-0.232 V6-7.Below pH = 4.84, there is no effect of Cu(OH)2 because of no precipitation.Therefore,E = E Cu2+/Cu = +0.340 + (0.0592/2) log [Cu2+]= +0.340 + (0.0592/2) log 0.1003 marks= +0.340 – 0.0296 = +0.310 V7 marks** V6-8.** g graphite = 0.0833 mol carbon6 mol carbon to 1 mol lithium; 1 g graphite can hold 0.0139 mol lithiumTo insert 1 mol lithium, 96487 coulombs are needed.Therefore, 1 g graphite can charge 96487 × 0.0139 = 1340 coulombs. 5 marks1340 coulombs / g = 1340 A sec / g = 1340 x 1000 mA × (1 / 3600) h = 372 mA h / g 5 marks372 mA h / g7-1. (10 marks)n/V = P/RT = (80 x 106 / 1.013 x 105 atm)/[(0.082 atm L/mol/K)(298K)] = 32 mol/L5 marksdensity = mass/volume = d = 32 x 2 g/L = 64 kg/m 3 5 marks64 kg/m 37-2.** or 0.23H 2(g) + 1/2 O 2(g) → H 2O(l); ∆H rexn-1 = ∆H f [H 2O(l)] = -286 kJ/mol = -143 kJ/g 7 marksC(s) + O 2(g) → CO 2(g); ∆H rexn-2 = ∆H f [CO 2(g)] = -394 kJ/mol = -33 kJ/g 7 marks(-∆H rexn-1) / (-∆H rexn-2) = 4.3 or (-∆H rexn-2) / (-∆H rexn-1)= 0.236 marks7-3. (a) (-)1.2 x 105 kJ, (b) (-)6.9 x 104 kJ** x 108 sec or 3.3 x 104 hr or 1.4 x 103 days or 46 month or 3.8 yrI = 0.81 AH 2(g) + 1/2 O 2(g) → H 2O(l)∆H c = -286 kJ/mol = -143 kJ/g = -143 x 103 kJ/kg 5 marksΔG = ΔH – T ΔSΔS c= 70 – 131 – 205/2 = -163.5 J/K/mol5 marksΔG c = -286 kJ/mol + 298K x 163.5 J/K/mol = -237 kJ/mol = -1.2 x 105 kJ/kg 5 marks(a) electric motor W max = ΔG c ⨯ 1 kg = - 1.2 x 105 kJ 5 marks (b) heat engine W max = efficiency x ∆H c 5 marks= (1 – 298/573) x (-143 x 103 kJ) = -6.9 x 104 kJ 5 marks119 x 103 kJ = 1 W x t(sec)t = 1.2 x 108 sec = 3.3 x 104 hr = 1.4 x 103 days = 46 month = 3.8 yr 5 marksΔG = -nFE n = # of electrons involved in the reaction F = 96.5 kC/molH 2(g) + 1/2 O 2(g) → H 2O(l) n = 2 5 marksE = - ΔG/nF = 237 kJ/mol / 2 / 96.5 kC/mol = 1.23 V5 marksI = W/E = 0.81 A5 marks8-1-1. (5 marks on each)①C②C③CO8-1-2.③ Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g) 5marks① C(s) + O2(g) → CO2(g) ΔH①◦ = -393.51 kJ = ΔH f◦(CO2(g))② CO2(g) + C(s) → 2CO(g) ΔH②◦ = 172.46 kJFrom ① and ②,ΔH f◦(CO(g)) = (1/2){172.46 + (-393.51)} = -110.525 kJΔH f◦(Fe2O3) = -824.2 kJΔH③◦ = 3ⅹΔH f◦(CO2(g)) - ΔH f◦(Fe2O3) - 3ⅹΔH f◦(CO(g))= 3ⅹ(-393.51) – (-824.2) - 3ⅹ(-110.525) = -24.8 kJ 7 marks ΔS③°=2ⅹ27.28+3ⅹ213.74-87.4-3ⅹ197.674=15.36 J/K 3 marks ΔG③°=ΔH°-TΔS°=-24.8kJ-15.36J/Kⅹ1kJ/1000Jⅹ1473.15K=-47.43 kJ5 marksK = e(-ΔG°/RT)= e(47430J/(8.314J/Kⅹ1473.15K)) = 48 5 marksBalanced equation of ③:K = 48Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)8-2-1. (20 marks)One AB2O4 unit has available 4 (= 1 + (1/4)ⅹ12) octahedral sites.48-2-2. (20 marks)Since one face-centered cube in AB2O4 represents one Fe3O4 unit in this case, it has 8 available tetrahedral sites. In one Fe3O4 unit, 1 tetrahedral site should be occupied by either one Fe2+ (normal-spinel) or one Fe3+ (inverse-spinel). Therefore, in both cases, the calculation gives (1/8) ⅹ100% = 12.5% occupancy in available tetrahedral sites.**%8-2-3. (10 marks for d-orbital splitting, 10 marks for elec. distribution)9-1-1. 1 answer for 8 marks, two for 15 marksH 3CN NNH 3CNNN :::+_+::_:9-1-2. ( 10 marks)H 3CN::9-1-3.H 3CNCH 2CH 2:H 3CN HH CCH 2:(10 marks) (10marks )9-2-1. 5 marks eachHONN +_::ONN:H+:HH_O NN:H+:H_::::::9-2-2.( 10 marks)CH 2CO ::9-3-1.(40 marks)CH 3H 3CH 3C+BC H 2CCH 3CH 3CO 2DEOOO_9-3-2.(10 marks)O OH O n+F10-1. 10 marks eachNMLCH 2OHCH 2OHMeOOMeH HH HOMeMeO CHOCHOCH 2OHCH 2OHHHH H OHOMeMeO OH10-2. 8 marks each for correct structuresNumber of possible structures24 marks12OH(OH)OH(H)HH HHOMeOMeOH COOMeOH(OH)OH(H)HH HHOMeOMeOHCOOMe34OH(OH)OH(H)OH(OH)OHe(H)10-3. 10 marks eachGICH 2OHCH 2OHHHHHMeOOMeOHOMeCH 2OHCH 2OHHHHOMeOMeOMe10-4. 10 marksNumber of the correct structure for C from 10-2110-5.BOH(OH)OH(H)HHHH OHCOOHOHOH10 marks eachDJOH(OH)OH(H)HHHHOMeOMeCOOMeOMeOH(OMe)OMe(H)HHHHOMeOMeOMeCOOMe10-6. 20 marksHOOCOHHH OOOHOOH COOHOOHOHOH COOH11-1. 10 marks311-2. 30 marksCOOHHOOCOOH11-3. 2.5 marks eacha, c, d11-4 30 marksOOCOCOOOHTransition State11-5.For the enzyme-catalyzed reaction, Arrehnius equation could be applied.k cat/k uncat = A exp (-E a, cat/ RT) / A exp (-E a, uncat / RT)= exp [-∆E a, cat-uncat/ RT]= exp [-∆E a, cat-uncat(J/mol) / (2,480 J/mol)] = 106Therefore, -∆E a, cat-uncat = 34,300 J/mol 15 marksk uncat, T/k uncat, 298 = exp (-∆H≠ uncat/ RT) / exp (-∆H≠uncat / 298R)= exp [(-∆H≠ uncat/R)(1/T-1/298)]ln(k uncat, T/k uncat, 298 )= 13.8 = [(-86900/8.32)(1/T-1/298)]Therefore, T = 491 K, or 218o C 15 marks-E a, cat-uncat = 34,300 J/molT = 491 K, or 218o C。
国际化学奥林匹克竞赛-40thIchotheoreticalanswers

Yes, but only in quite dilute solutions can this happen. 1 pt for ticking yes
c = [HA] + [A–] = [H+]
(1 pt)
[H+] = [A–] + [OH–]
(1 pt)
This means that [HA] = [OH–]
(1 pt for reasonable guess – between 6 and 7)
A good approximation is: [H+ ] = 3 (KKw )
The full equation can be solved through iteration: [H+ ] = 3 (K + [H+ ])Kw
In equilibrium constant calculations all concentrations are referenced to a standard concentration of 1 mol/dm3. Consider all gases ideal throughout the exam.
4
Name:
Code: XXX-
c)
Could it be possible that the solution contained acetic acid?
Acetic acid: pKa = 4.76
Yes No
If yes, calculate the pH (or at least try to estimate it) and show your work.
Official English version
第36届国际化学奥林匹克竞赛理论试题

・ 2,・
对于确定 !"!) 在热力学上是否稳定, 不仅仅其晶格能0 - "& 很重要。为了确定对于分解成它的组成元素是否稳定, 必须 了解 !"!) 的标准生成焓0 . "& 。 / / 01 2( 3)根据波恩 * 哈伯循环 ( 4567 * 8"396 * :;:)9) 计算 !"!) 的0 . "& 。 熔化热 离子化焓 ( 即第一电离能) 离子化焓 ( 即第二电离能) 蒸发热 解离能 生成焓 电子亲和能 0 熔化 "( !") 0$1 0%1
04. ( ,1. ( 02. 4 2,. 2 42. 0 00. 6 ,1. /
8 8 公式 ’ ’ (’ , 9 ( : ( ) : !*)’ ( 9 " 墙 : !+ : ,
’(
ห้องสมุดไป่ตู้
一定时间 !* 的单位面积 ) 上沿温度梯度( 墙壁方向 -) 的热能流量 ( ./.012 "345 ( 634/1 6 *.7&.06*80. 106,9./*( 5633 ,90.:*94/ -)&.0 60.6 ) 6/, *97. !*) 墙壁厚度 8 8 " 墙 墙的热传导率8 8 !+ 室内外温度的差值
4,
第 ! 题:一价碱土金属化合物?
过去曾有几篇关于一价钙化合物的报导。虽然直到最近这些 “ 化合物” 的本质仍不清楚, 然而, 它们仍是固体化学家感兴 趣的课题。有人曾试图用 ( !) 钙、 ( :) 氢、 ( ;) 碳将 (!(<, 还原为 (!(<。 =% 5 写出由 (!(<, 制备 (!(< 的反应方程式。 7 7 尝试用化学计量的 (!( 摩尔比 5 : 5 ) 还原 (!(<, 之后, 得到非均匀的灰色物质。在显微镜下仔细观察, 它们是银色的金属 团粒和无色的晶体 ( ;>?-@!<-) 。 =% , 指出金属团粒和无色的晶体 ( ;>?-@!<-) 分别是什么物质? 7 7 当试图用氢还原 (!(<, 时, 生成白色产物。元素分析表明该产物含 0,% =A " ’ "B 的钙和 8A% =, " ’ "B 的氯。 =% = 推断生成的化合物的化学式 当试图用碳还原 (!(<, 时, 形成红色结晶物质。元素分析表明 (! 和 (< 的摩尔比为 C ( (!): C ( (<) & 5% 0 : 5 。在红色结晶物 质水解中, 放出的气体与 DE, (= 水解放出的气体相同。 =% 8( !) 写出水解中所生成的气体的两种非环状构造异构体的结构式。 =% 8( :)写出 (!(<, 与碳反应生成的化合物的化学式 ( 假定一价钙不存在) 。 7 7 由于上述这些企图都不能实现 (!(< 的生成, 必须更多地考虑 (!(< 的假想结构。我们可以假设 (!(< 为一种简单的晶体 结构。正负离子半径比 ( > D" . ) ’ ( > F # 6 )常常决定晶体的结构类型。下表列出了 DF 型化合物的有关情况。
第一届Chemy化学奥林匹克竞赛联赛试题答案精编版

第一届Chemy化学奥林匹克竞赛联赛答案(2016年8月28日9:00 ~ 12:00)·竞赛时间3小时。
迟到超过半小时者不能进考场。
开始考试后1小时内不得离场。
时间到,把试卷(背面朝上)放在桌面上,立即起立撤离考场。
·试卷装订成册,不得拆散。
所有解答必须写在指定的方框内,不得用铅笔填写。
草稿纸在最后一页。
不得持有任何其他纸张。
·姓名、报名号和所属学校必须写在首页左侧指定位置,写在其他地方者按废卷论处。
·允许使用非编程计算器以及直尺等文具。
第1题(8分)1-1 将明矾置于河水中搅拌,生成载带着悬浮物的氢氧化铝,然后沉降,使水澄清。
但若用蒸馏水代替河水,便不会发生以上现象,请解释该过程的原因,并写出生成氢氧化铝的方程1-2 在氯化汞溶液中逐滴加入碘化钾溶液,生成橘红色沉淀,继续滴加,发现沉淀溶解,写1-3 在含有四氨合铜离子的溶液中加入硫酸,溶液的颜色由绛蓝色变为蓝色,写出总反应方第2题(8分)有一个经验规则可以用于估算基元反应的活化能:BE是反应中断掉的键的键能之和。
①反应中没有键的断裂的反应;②自由基与分子的放热反应;③反应中键不完全断裂(如协同反应)的反应。
④反应中键完全断裂的反应。
根据上述经验规则,估算氢气与氯气反应中各步基元反应的活化能,并与实验值比较。
Cl2 = 2Cl·E a1 = 242.8 kJ·mol-1Cl· + H2 = HCl + H·E a2= 25.1 kJ·mol-1H· + Cl2 = HCl + Cl·E a3= 12.6 kJ·mol-1-1;BE(H-H) = 436 kJ·mol-1;BE(H-Cl) = 431 kJ·mol-1第3题(8分)3-1 Cr2,Mo2与W2分子中存在着六重键,金属的ns轨道与(n-1)d轨道参与成键。
国际化学奥林匹克竞赛——第32届IChO预备题

第32届IChO预备题中译本(简译本)说明:本译本只摘译了原文题面部分。
其他部分(包括答案)请阅原文,因答案基本上是国际通用符号,无须翻译即可读懂,国际竞赛知识点已有译本下发。
参加国家队选拔的集训队员应首先书面应答预备题,不要先看答案,但做完预备题后应仔细研读答案,以把握第32届国际竞赛试题涉及的知识基础的水平和应答要求。
预备题涉及的属于国际竞赛三级的知识点将在选拔赛前安排讲座,预备题实验也将在选拔赛前安排实践。
选拔赛将以模拟国际竞赛的方式进行。
由于原文以acrobat为界面,译文不得不舍弃原文中少数背景图, 欲知原文全部附图者请读原文。
原文可从网上下载,网址为::icho2000.gymfag.dk第1题酸雨纯水pH为7.0。
天然雨水因溶解大气二氧化碳而呈弱酸性。
但许多地区的雨水酸性更强,其原因有的是天然的,有的则是人为的。
大气中的二氧化硫和一氧化氮会被氧化为三氧化硫和二氧化氮,并分别与水反应生成硫酸和硝酸。
所谓“酸雨”的平均pH为4.5,最低可达1.7。
二氧化硫在水溶液中是一个二元酸,在25o C时酸式电离常数如下:SO2(aq) + H2O(l) ⇌ HSO3-(aq) + H+(aq) K a1= 10-1.92 MHSO3-(aq) ⇌ SO32-(aq) + H+(aq) K a2= 10-7.18 M注:“M”是原文用于代替国际符号mol·dm-3的欧洲国家中学教科书通用符号,请同时熟悉这两种符号。
本译文未将此符号改为国际符号。
下同。
请注意平衡常数的指数表达式和以SO2而非H2SO3为反应物。
a.在二氧化硫的分压为1bar时它在每升水中的溶解度为33.9升(25o C, 全题同)。
i)计算被二氧化硫饱和的水中的二氧化硫总浓度(忽略因溶解SO2引起的水的体积变化)。
ii)计算亚硫酸氢根离子的百分含量。
1iii)计算溶液的pH。
b.计算含0.0100 M亚硫酸钠的水溶液的氢离子浓度。
国际化学奥林匹克竞赛-36thIChOpracticalproblems

- use only the pen and calculator provided
- results
the number of significant figures in numerical answers must conform to the rules of evaluation of experimental error. Mistakes will result in penalty points even if your experimental technique is flawless.
95.94 74
W
183.84 106
Sg
263 59
Pr
140.91 91
Pa
231
25
Mn
54.94 43
Tc
98.91 75
Re
186.21 107
Bh
264 60
Nd
144.24 92
U
238
26
Fe
55.85 44
Ru
101.07 76
Os
190.23 108
Hs
265 61
Pm
144.92 93
Sn
118.71 82
Pb
207.19
67
Ho
164.93 99
Es
252
7
N
14.01 15
P
30.97 33
As
74.92 51
Sb
121.76 83
Bi
208.98
68
Er
167.26 100
Fm
257
8
O
16.00 16
IChO2016国际化学奥林匹克竞赛英文真题实践部分(包括答案)

48th InternationalChemistry Olympiad Practical TasksPart I.26 July 2016Tbilisi, GeorgiaInstructions∙Begin only when the START command is given. The exam contains two parts. You have 100 minutes to work on Part I (Task 1). After this you will have to leave the lab for 30 minutes.∙Part I of the exam (Task 1) contains 5 pages, its answer sheets have 3 pages.∙Follow the safety rules announced in the preparatory tasks. You get one warning for violations. On the second warning you will get disqualified.∙Wear your lab coat and safety goggles while in the lab. Ask your lab assistant for the gloves of your size when you need them.∙Use only the pen, marker pen and calculator provided. Do not write with the marker on paper; use it only to label glass or plastic labware.∙Make sure that your student code is on every answer sheet.∙All answers must be written in the appropriate boxes on the answer sheet.Anything written elsewhere will not be graded. Use the reverse of the examsheets if you need scratch paper.∙You have no access to sinks in the lab. You are provided with a sufficient quantity of labware. Only a few items need to be used again. Wash these carefully with an appropriate solvent into the waste container. Use the brush if needed. Distilledwater and paper tissues are freely available.∙Liquid waste is to be put into the container label ed “LIQUID WASTE”. Do not put rubbish (tissues, plastic, etc.) in this container, but into the waste baskets in thelab.∙Chemicals and labware are not supposed to be refilled or replaced. Each such incident (other than the first in the entire exam, which you will be allowed) willresult in the loss of 1 point from your 40 practical points.∙Raise your hand if you have a safety question or you need a restroom break or drinking water.∙When you have finished this part of the examination, put your answer sheet into the envelope provided and leave it on the table. Do not seal the envelope. You will not have further access to the answer sheets from this part.∙You must stop your work immediately when the STOP command is given. A delay in doing this may lead to cancellation of your exam. Do not leave your place until permitted by the lab assistants. You can keep the task text.∙The official English version of this examination is available on request only for clarification.LabwareChemicalsTask 1You have 10 different compounds dissolved in water in 5 unknown solutions. Each numbered container contains two of the following compounds in aqueous solution (every compound is used, and each compound is used only once):AgNO3, Al2(SO4)3, Ba(NO3)2, Fe(NO3)3, KI, KIO3, Na2CO3, Na2SO3, MgCl2, NH3You are given HNO3 solution, NaOH solution, hexane and the aqueous solutions of the 10 pure compounds listed above.You can use empty test tubes and any of the liquids provided (including the unknowns) to identify the unknown samples. A funnel and filter paper can be used for separation. Identify the compounds in the solutions 1-5. Give the number of the solution that contains the individual compounds on the answer sheet. Indicate twoobservations caused by a chemical reaction for each compound in yourunknown mixtures by giving the letter code of the appropriate observation(choose one or more from the list), and write appropriate balanced ionicequation(s) that explain the observation. At least one of the reactions has to bespecific for clearly identifying the compound from this selection of unknowns. Note: After the STOP signal close all the centrifuge test tubes containing the unknown mixtures with the blue caps labeled with the student code and leave these in the rack.48th InternationalChemistry Olympiad Practical Tasks Part I. Answer Sheets26 July 2016Tbilisi, GeorgiaTask 1 13% of the totalOnly fill out this table when you are ready with all your assignments. Use the following observation codes:A - Formation of white precipitate F - Brown color in the organic phaseB - Formation of colored precipitate (red, brown, yellow, black etc.) G - Purple color in the organic phaseC - Dissolution of precipitate H - Formation of colored gasD - Color change in the solution I - Formation of colorless and odorless gasE - Formation of colored solution J - Formation of colorless and odorous gasK – Change in the color of precipitateReplacements:5p for locating each compound, 1 p for each relevant equation. Altogether 10x7p.Subpoints for 1p: 0.4p for correct observation with appropriate reagent(s); 0.6p for the relevant balanced ionic equation (-0.1p for minor typos; maximum of -0.3p for poor balancing; maximum of 0.3p for an equation in other than ionic form)The unknowns are identical mixtures for every student in different order. Most are mixed in 1:1 ratio by volume.Characteristic reactions are marked with bold letters. One of these or equivalent has to be shown on the answer sheet.NH3Fe(NO3)3Al2(SO4)3AgNO3KIO3Na2CO3MgCl2Na2SO3Ba(NO3)2KI48th InternationalChemistry Olympiad Practical TasksPart II.26 July 2016Tbilisi, GeorgiaInstructions∙You have a 15 minute reading time before you start work. Begin reading only when the START command is given.∙Follow the safety rules announced in the preparatory tasks. You get one warning for violations. On the second warning you will get disqualified.∙Wear your lab coat and safety goggles while in the lab. Ask your lab assistant for the gloves of your size when you need them.∙Use only the pen, marker pen and calculator provided. Do not write with the marker on paper; use it only to label glass or plastic labware.∙Make sure that your student code is on every answer sheet.∙All answers must be written in the appropriate boxes on the answer sheet.Anything written elsewhere will not be graded. Use the reverse of the examsheets if you need scratch paper.∙You have no access to sinks in the lab. You are provided with a sufficient quantity of labware. Only a few items need to be used again. Wash these carefully withappropriate solvent into the waste container. Use the brush if needed. Distilledwater and paper tissues are freely available.∙Liquid waste is to be put into the container labeled “LIQUID WASTE”. Do not put rubbish (tissues, plastic, etc.) in this container, but into the waste baskets in the lab.∙Chemicals and labware are not supposed to be refilled or replaced. Each such incident (other than the first in the entire exam, which you will be allowed) willresult in the loss of 1 point from your 40 practical points.∙Raise your hand if you have a safety question or you need a restroom break or drinking water.∙When you have finished the examination, put your answer sheet into the envelope provided and leave it on the table. Do not seal the envelope.∙You must stop your work immediately when the STOP command is given. A delay in doing this may lead to cancellation of your exam. Do not leave your place until permitted by the lab assistants. You can keep the task text.∙The official English version of this examination is available on request only for clarification.Instructions specific for Part II∙The working time for Part II (Task 2 and 3) is 200 minutes.∙Start Part II with Task 2. When you are ready to start with Task 3, tell the lab assistant, and you will receive the chemicals and labware for Task 3. Reagents for Task 2 will be taken away from you at this point.∙Part II of the exam (Task 2-3) contains 10 pages, its answer sheets have 7 pages.∙Ask the lab assistants when you need your alcohol lamp lighted. Heat only glass test tubes. Close the alcohol lamp with the cap when finished.LabwareChemicalsPeriodic table with relative atomic massesTask 2Determination of fluoride and chloride content in mineral waterGeorgia is world famous for its splendid mineral waters. Many of these are used to cure various diseases. Manufacturers have to carefully control the ionic composition of waters, fluoride and chloride being among the most important ions.Visual colorimetric detection of fluorideThe method of fluoride determination is based on the decrease in the color intensity of zirconium(IV)-Alizarin Red S complex in the presence of fluoride ions due to formation of a more stable colorless complex. The equilibrium is achieved in about 20 minutes after the reagent addition. The fluoride concentration is determined visually by comparing the color developed in the sample with those in the calibration solutions. Transfer 9.0 cm3 of mineral water from the sample into the plastic test tube label ed “X”. Calculate how much of the 9.0 mg/dm3 standard fluoride solution you will need to prepare a set of calibration solutions with the following fluoride ion content: 0.0; 1.0; 2.0; 3.5; 5.0; 6.5; 8.0 mg/dm3 (calculate for 9.0 cm3 of each solution).Using the 1.0 cm3 and 10.0 cm3 graduated pipettes, add thecalculated amounts of the standard fluoride solution to the test-tubes, then add 1.0 cm3 of Zirconyl Alizarin indicator into each testtube, and bring the volume in each calibration test tube to the 10.0cm3 mark with distilled water (the mark is shown in the figure withthe arrow).2.1.1.Report the fluoride volumes used in your dilutions.Mix the obtained solutions in the test tubes. Set the tube rack aside for at least 20 minutes.pare the color of the sample and the calibration solutions looking on themfrom the top down and from the front. Select the concentration of the standardthat is closest to the fluoride concentration of the water sample.Note: the rack with the test tubes will be photographed by the lab staff after the whole exam is finished.Standardization of silver nitrate solution by the Mohr methodTransfer 10.0 cm3 of the standard 0.0500 mol/dm3 NaCl solution into an Erlenmeyer flask using the bulb (Mohr) pipette. Add approximately 20 cm3 of distilled water and10 drops of 10% aqueous K2СгО4 solution.Fill a burette with the silver nitrate solution. Titrate the contents of the flask with the silver nitrate solution while vigorously mixing the solution containing the precipitate formed. The final titrant drops are added slowly with vigorous swirling of the flask. The titration is complete when the faint color change visible on titrant addition does not disappear in the pure yellow suspension. Take the final burette reading. Repeat the titration as necessary.2.2.1.Report your volumes on the answer sheet.2.2.2.Write balanced chemical equations for the titration of NaCl with AgNO3 and forthe end-point indication reaction.2.2.3.Calculate the concentration of the AgNO3 solution from your measurement.2.2.4.The Mohr titration method requires a neutral medium. Write down equationsfor the interfering reactions that take place at lower and at higher pH. Chloride determination by the Volhard methodWash the bulb (Mohr) pipette with distilled water. Wash the Erlenmeyer flasks first with a small portion of the ammonia solution left over from Task 1 to help removing the silver salt precipitate and then with distilled water. (In case you used up all the ammonia solution in the first task, you can get a refill without penalty.)Transfer a 10.0 cm3 aliquot of the mineral water from the sample into an Erlenmeyer flask using the bulb (Mohr) pipette. Add 5 cm3 of 2 mol/dm3 nitric acid using a graduated cylinder. Add 20.00 cm3 of the silver nitrate solution from the burette and mix well the suspension. Add appr. 2 cm3 of the indicator (Fe3+) solution with the Pasteur pipette.Fill the second burette with the standard ammonium thiocyanate solution (see the exact concentration on the label). Titrate the suspension with this solution while vigorously swirling. At the end point one drop produces a faint brown color that is stable even after intense mixing. Take the final burette reading. Repeat the titration as necessary. Note. The AgCl precipitate exchanges Cl− ions with SCN– ions from the solution. If you titrate too slowly or with breaks, the brown color disappears with time, and too much titrant is spent for the titration. Therefore when approaching the endpoint you should add the titrant at a constant slow rate swirling the flask constantly so that the suspension would stay white. The appearance of faint brown color will mean reaching the endpoint.2.3.1.Report your volumes on the answer sheet.2.3.2.Write down balanced chemical equations for the back titration with NH4SCNand that for the end-point indication reaction.2.3.3.Calculate the chloride concentration (in mg/dm3) in the water sample from yourmeasurements.2.3.4.If Br−, I−, and F− ions are present in the sample in addition to chloride, theconcentration of which ion(s) will contribute to the result of the Volhardtitration?2.3.5.When trying to determine the concentration of Cl− in the presence of otherhalides, an analyst added some potassium iodate and sulfuric acid to the sample and boiled the solution. Afterwards he reduced the excess of iodate to iodine by boiling the sample with phosphorous acid H3PO3. What interfering anions wereremoved by this operation? Write the chemical equations for the reactions ofthese ions with iodate.Task 3Identifying flavors and fragrancesTourists coming to Georgia admire many specialties, local cuisine occupying one of the top positions in the list of adventures. Excellent meat, fresh vegetables and greens, ripe fruits, home-made jams… What else is needed to satisfy true gourmets? Of course, unique flavors and fragrances!You are given 8 samples of unknown organic compounds (labeled 1 to 8), which are industrially used as flavors and fragrances. All samples are pure individual compounds. Their possible structures are found among A-M given here.The organic compounds in your unknown samples are readily soluble in ether, and insoluble in dilute aqueous NaOH and HCl. These compounds, but the unknown No. 6. are insoluble in water, the latter being slightly soluble (3.5 g/dm3).3.1.Perform test reactions described below to identify the samples 1-8. Indicate theresults of the tests by giving the Roman numeral of the appropriate observation (choose one or more from the list). Fill in all cells of the table. Use + and – toindicate positive and negative tests.3.2. Identify the unknowns based on the test results and the information givenabove. Write the structure codes (of A to M) of the identified samples in theappropriate box.Test proceduresKMnO4 test (Baeyer test)Place appr. 1 cm3 of 95% ethanol in a plastic test tube and add 1 drop of an unknown. Add 1 drop of KMnO4 solution and shake the mixture. Treat the test as positive if the permanganate color disappears immediately after shaking.3.3.Write the reaction scheme for a positive Baeyer test with one of the compoundsA-M.Cerium(IV) nitrate testPlace 2 drops of the Ce(IV) reagent into a glass test tube, add 2 drops of acetonitrile and then 2 drops of an unknown (the sequence is important!). Shake the mixture. In the case of positive test the mixture color promptly changes from yellow to orange-red.Note 1. Use only glass test tubes to perform the test. In case you need to wash the glass test tubes, carefully choose the appropriate solvent. Use caps to prevent the strong odor.Note 2. Comparison with blank (no unknown) and reference (with ethanol) tests is recommended for adequate interpretation.Note 3. Ce(IV) ions initially form brightly colored coordination compounds with alcohols. Complexes formed from primary or secondary alcohols react further (within 15 seconds to 1 hour) with the disappearance of the color.2,4dinitrophenylhydrazine (2,4-DNPH) testAdd only 1 drop of an unknown to 1 cm3 of 95% ethanol in a plastic test tube. Add 1 cm3 of the DNPH reagent to the prepared solution. Shake the mixture and let it stand for1-2 min. Observe formation of yellow to orange-red precipitate if the test is positive.3.4.Write the reaction scheme for a positive 2,4-DNPH test with one of thecompounds A-M.Ferric hydroxamate testAsk a lab assistant to light up your alcohol lamp. Mix 1 cm3 of 0.5 mol/dm3 ethanolic hydroxylamine hydrochloride solution with 5 drops of 6 mol/dm3 sodium hydroxide aqueous solution in a glass test tube. Add 1 drop of an unknown and use the alcohol lamp to heat the mixture to boiling while gently swirling the test tube to avoid splashes of the reaction mixture. Allow it to cool down slightly and add 2 cm3 of 1 mol/dm3 HCl solution. Add 1 drop of 2.5% iron(III) chloride solution. Observe appearance of magenta color if the test is positive. Close the alcohol lamp with the cap when finished.Note 1. Use glass test tubes only to perform the test; use the test tube holder when heating. In case you need to wash the glass test tubes, use an appropriate solvent. Stopper the test tubes with a green cap after completing the test to prevent a strong odor.Note 2. Fe(III) ions form a colored 1:1 complex with hydroxamic acids (R-CO-NHOH).3.5.Write the reaction scheme for a positive ferric hydroxamate test with one of thecompounds A-M.Note: After the STOP signal reattach the corresponding needles on the syringes with the unknown compounds, and place them into the plastic cup and leave them on the table.48th InternationalChemistry Olympiad Practical Part II. Answer Sheets26 July 2016Tbilisi, GeorgiaTask 2 14% of the total2.1.1. Report the fluoride volumes used in your dilutions.2.1.2.2.2.1. Report your titration volumes.2.2.2. Write a balanced chemical equation for the titration of NaCl with AgNO 3 and that for the end-point indication reaction. 2.2.3. Calculate the concentration of the AgNO 3 solution from your measurement.2.2.4.The Mohr titration method requires a neutral medium. Write equations for theinterfering reactions that take place at lower and at higher pH.2.3.1.Report your volumes on the answer sheet.2.3.2.Write a balanced chemical equation for the back titration with NH4SCN and thatfor the end-point indication reaction.2.3.3.Calculate the chloride concentration (in mg/dm3) in the water sample from yourmeasurements.2.3.4. If Br −, I −, and F − ions are present in the sample in addition to chloride, theconcentration of which ion(s) will contribute to the result of the Volhard titration? Tick the appropriate box(es). ☐ Br ––☐ F –2.3.5. halogens, an analyst added some potassium iodate and sulfuric acid to the sample and boiled the solution. Afterwards he reduced the excess of iodate to iodine by boiling the sample with phosphorous acid H 3PO 3. What interfering anions were removed by this operation?☐ Br –☐ I –☐ F –☐ noneWrite the reaction equations of these ions with iodate.Replacements:Grading scheme for the titration resultsIf A< Value < B, then Grade = Max gradeIf Value < y, then Grade = 0, If Value > z, then Grade = 0If y < Value < A, then Grade = Max grade ⨯ (Value – y)/(A – y) If B < Value < z, then Grade = Max grade ⨯ (z – Value)/(z – B)MasterValue (M.V.)M a x g r a d eAByzTask 313% of the total3.1.Indicate the results and observations of tests by giving the Roman numerals of the appropriate observations in the table. Fill in all cells of the table. Use + and – to indicate positive and negative tests. Choose one or more codes from the list below.I – Immediate disappearance of purple color VI - Formation of a yellow or orange-red precipitateII – Slow disappearance of purple color VII - Appearance of orange or red color insolutionIII - Disappearance of yellow color VIII - Appearance of magenta color IV – Formation of a brown or black precipitate IX - The unknown compound is insoluble in ethanol V - Formation of a white precipitate X – no visible changes3.2. Write the structure codes (of A to M) of the identified samples in theappropriate boxes when you are certain in your assignments.3.3.Write the reaction scheme for a positive Baeyer test with one of the compoundsA-M.3.4.Write the reaction scheme for a positive 2,4-DNPH test with one of thecompounds A-M.3.5.Write the reaction scheme for a positive ferric hydroxamate test with one of thecompounds A-M.The problem can be approached in many ways. A systematic solution for one variant of the unknown compounds encoding is given below (other variants are processed similarly).Step 1. Solubility data analysis.The data given allow excluding compounds K (presence of phenol-like moiety) and M (presence of azine nitrogen) soluble in aqueous NaOH and aqueous HCl, respectively. Step 2. Tests for the functional groups.2.1 Unsatured compounds excluding those aromatic give positive Baeyer test, and those with keto- or aldehyde group give posi tive 2,4DNPH test2.2 For avoiding mistakes in true-positive / false-positive interpretation the precise description of true-positive tests is given in the test procedures.2.3 The information given in the Notes after the test procedures CLEARLY indicates the following prompts:Analysis of the table allows unanimously identifying A, B, E, C, and G. L(Ferric hydroxamate and 2,4DNPH), J (2,4-DNPH and Cerium(IV) nitrate test), and F (KMnO4 and 2,4DNPH tests) can be excluded from further consideration, since these provide for two positive tests each.Step 3. Choosing of right structures of the samples 6 and 7 based on additional data given. Among all compounds in the list, only H and I cannot give any positive test, the attribution requiring consideration of the solubility data. Sample 7 in the above table is soluble in ether only (attributed as I), whereas Sample 6 is partially soluble in water (attributed as H).The final assignment is given below.1 points for each test (1p ⨯ 4 tests ⨯ 8 unknowns=32p)2 points for each correct assignment (2p⨯ 8 unknowns=16p)Appendix AHazard codes, provided by Globally Harmonized System of Classification and Labeling of Chemicals (not to be printed for students)Appendix B Hazard Statement Descriptions。
国际化学奥林匹克竞赛试题汇编-第38届ICHO理论试题(中文版)答案

1-1.The mass of a water droplet:m = V ρ = [(4/3) π r3] ρ = (4/3) π (0.5x10-6 m)3 (1.0 g/cm3)= 5.2x10-16 kg⇒10 marksAverage kinetic energy at 27o C:KE = mv2/2 = (5.2x10-16 kg) (0.51x10-2 m/s)2/2= 6.9x10-21 kg m2/s2= 6.9 x10-21 J ⇒15 marks*.The average kinetic energy of an argon atom is the same as that of a water droplet.KE becomes zero at –273 o C.From the linear relationship in the figure, KE = aT (absolute temperature)where a is the increase in kinetic energy of an argon atom per degree.a = KE/T = 6.9x10-21 J/(27+273K) = 2.3x10-23 J/K⇒25 marksS: specific heat of argon N: number of atoms in 1g of argonS = 0.31 J/g K = a x NN = S/a = (0.31 J/g K) / (2.3x10-23 J/K)= 1.4x1022 ⇒30 marksAvogadro’s number (N A) : Number of argon atoms in 40 g of argonN A = (40)(1.4x1022)= 5.6 x1023⇒20 marks2-1. ⇒ 30 marksmass of a typical star = (4/3)(3.1)(7x108 m)3(1.4 g/10-6 m 3) = 2×1033 g mass of protons of a typical star = (2×1033 g)(3/4 + 1/8) = 1.8×1033 g number of protons of a typical star = (1.8×1033 g)(6×1023/g) = 1×1057number of stellar protons in the universe = (1×1057)(1023) = 1×1080Partial credits on principles:Volume = (4/3)(3.14)radius 3×density; 4 marks 1 mole = 6×1023; 4 marksTotal number of protons in the universe = number of protons in a star ×1023; 2 marks Mass fraction of protons from hydrogen = (3/4)(1/1); 5 marks Mass fraction of protons from helium = (1/4)(2/4); 10 marks2-2. ⇒ 30 marks∆E(2→3) = C(1/4 - 1/9) = 0.1389 C λ(2→3) = 656.3 nm ∆E(1→2) = C(1/1 - 1/4) = 0.75 Cλ(1→2) = (656.3)(0.1389/0.75) = 121.5 nmNo penalty for using Rydberg constant from memory. 15 marks penalty if answered in a different unit (Hz, etc.)2-3.T = (2.9×10-3 m K)/1.215×10-7 m = 2.4×104 K ⇒ 10 marks2-4..⇒ 20 marksλ = 3 × 108 m/1.42 × 109 = 0.21 mT = (2.9 × 10-3 m K)/0.21 m = 0.014 K2-5. ⇒ 10 marks14N + 4He → (17O ) + 1HO-17, O acceptable1783-1.k des = A exp(-E des/R T)= (1x1012 s-1)(5x10-32) = 5x10-20 s-1 at T = 20 K ⇒10 markssurface residence time, τresidence = 1 / k des = 2x1019 s = 6x1011 yr ⇒20 marks(full credit for τhalf-life = ln2 / k des = 1x1019 s = 4x1011 yr)residence time = 2x1019s3-2.The distance to be traveled by a molecule: x = πr = 300 nm.k mig = A exp(-E mig/R T)= (1x1012 s-1)(2x10-16 ) = 2x10-4 s-1 at T = 20 K ⇒ 5 marksaverage time between migratory jumps,τ = 1 / k mig = 5x103 sthe time needed to move 300 nm= (300 nm/0.3 nm) jumps x (5x103 s/jump) = 5x106 s = 50 days ⇒15 marks(Full credit for the calculation using a random-walk model. In this case:t = τ (x/d) 2 = 5 x 109 s = 160 yr. The answer is still (b).)(a) (b)(c) (d) (e)10 marks3-3.k(20 K) / k(300 K) = exp[(E/R) (1/T1 - 1/T2)]= e-112 = ~ 10-49 for the given reaction ).) ⇒15 marks The rate of formaldehyde production at 20 K= ~ 10-49 molecule/site/s = ~ 10-42 molecule/site/ yr⇒10 marks(The reaction will not occur at all during the age of the universe (1x1010 yr).)rate = 10-42molecules/site/yr3-4. circle one(a) (b) (c) (a, b) (a, c) (b,c)(a, b, c)(15 marks, all or nothing)4-1.H PNumber of atoms ( 11.3 ) 1⇒ 10 marksTheoretical wt % ( 3.43 )⇒ 10 marks4-2.adenineN NN NN H H guanineNN N NO N HH HNN O N H H cytosineNN H O O thymine(10 marks on each)4-3. 7 marks each, 20 marks for threeadenineNNNNNHHguanine NN NNON HHH NNH OOthymineNNONHH cytosine NNH OOthymineguanine NN NNON HHHcytosineNNONHHcytosineNNON HHNNHOO thyminethymineNNHOONNH OOthyminethymine NNHOONNONHH cytosineadenineNNNNNHH adenineNNNNNHHadenine NNNNNHHguanineguanine NNNNON HHHNNNNONHHH4-4. 2.5 marks for each bracketadenineN NN N HNH 2guanine N NH N N HO NH 2Uracil N H NH O cytosineN H N NH 2OOHCN ( 5 ) ( 5 ) ( 4 )( 4 )H 2O ( 0 ) ( 1 ) ( 2 ) ( 1 )5-1.(20 marks)1st ionization is complete: H2SO4→ H+ + HSO4-[H2SO4] = 02nd ionization: [H+][SO42-]/[HSO4-] = K2 = 1.2 x 10-2 (1)Mass balance: [H2SO4] + [HSO4-] + [SO42-] = 1.0 x 10-7 (2)Charge balance: [H+] = [HSO4-] + 2[SO42-] + [OH-] (3)Degree of ionization is increased upon dilution.[H2SO4] = 0Assume [H+]H2SO4 = 2 x 10-7From (1), [SO42-]/[HSO4-] = 6 x 104 (2nd ionization is **plete)[HSO4-] = 0From (2), [SO42-] = 1.0 x 10-7 [5 marks]From (3), [H+] = (2 x 10-7) + 10-14/[H+][H+] = 2.4 x 10-7(pH = 6.6) [8 marks][OH-] = 10-14/(2.4 x 10-7) = 4.1 x 10-8[2 marks]From (1), [HSO4-] = [H+][SO42-]/K2= (2.4 x 10-7)(1.0 x 10-7)/(1.2 x 10-2) = 2.0 x 10-12[5 marks]Check charge balance:2.4 x 10-7≈ (2.0 x 10-12) + 2(1.0 x 10-7) + (4.1 x 10-8)Check mass balance:0 + 2.0 x 10-12 + 1.0 x 10-7≈ 1.0 x 10-7Species Concentration** x 10-12HSO4-** x 10-7SO42-** x 10-7H+** x 10-8 OH-5-2. (20 marks)mmol H3PO4 = 0.85 ⨯ 3.48 mL ⨯ 1.69g/mL ⨯ 1 mol/98.00 g ⨯ 1000 = 51.0 [5 marks]The desired pH is above p K2.A 1:1 mixture of H2PO4- and HPO42- would have pH = p K2 = 7.20.If the pH is to be 7.40, there must be more HPO42- than H2PO4-.We need to add NaOH to convert H3PO4to H2PO4-and to convert to the right amount of H2PO4-to HPO42-.H3PO4 + OH-→ H2PO4- + H2OH2PO4- + OH-→ HPO42- + H2OThe volume of 0.80 NaOH needed to react with to to convert H3PO4 to H2PO4- is:51.0 mmol / 0.80M = 63.75 mL [5 marks]To get pH of 7.40 we need:H2PO4- + OH-→ HPO42-Initial mmol 51.0 x 0Final mmol 51.0-x 0 xpH = p K2 + log [HPO42-] / [H2PO4-]7.40 = 7.20 + log {x / (51.0-x)}; x = 31.27 mmol [5 marks]The volume of NaOH needed to convert 31.27 mmol is :31.27 mmol / 0.80 M = 39.09 mLThe total volume of NaOH = 63.75 + 39.09 =102.84 mL , 103 mL [5 marks]Total volume of 0.80 M NaOH (mL) 103 mL5-3. (20 marks)p K = 3.52pH = pK a + log ([A-]/[HA])[A-]/[HA] = 10(pH-pKa) [5 marks]In blood, pH =7.40, [A-]/[HA] = 10(7.40-3.52) = 7586Total ASA = 7586 +1 = 7587 [5 marks]In stomach, pH = 2.00, [A-]/[HA] = 10(2.00-3.52) = 3.02x10-2Total ASA = 1+ 3.02x10-2 = 1.03 [5 marks]Ratio of total aspirin in blood to that in stomach = 7587/1.03 = 7400 [5 marks]** ( 103Ratio of total aspirin in blood to that in stomach6-1. (5 marks)4 H2O + 4 e-→ 2 H2(g) + 4 OH- (or 2 H2O + 2 e-→ H2(g) + 2 OH-)6-2. (5 marks)2 H2O → O2 + 4 H+ + 4 e-(or H2O → 1/2 O2 + 2 H+ + 2 e- )6-3. (5 marks)Cu → Cu2+ + 2e-6-4. (20 marks)Reduction of sodium ion seldom takes place.It has a highly negative reduction potential of –2.710 V.Reduction potential for water to hydrogen is negative (water is very stable).But, it is not as negative as that for sodium ion. It is –0.830 V.Reduction of both copper ion and oxygen takes place readily and the reduction potentials for both are positive.In the present system, the reverse reaction (oxidation) takes place at the positive terminal. Copper is oxidized before water.Reduction potential for hydrogen ion is defined as 0.000 V.6-5. (15 marks)pOH = 14.00 – 4.84 = 9.16[OH-] = 6.92 x 10-10K sp = [Cu2+][OH-]2 = 0.100 x (6.92 x 10-10) = 4.79 x 10-206-6.E = E o Cu2+/Cu + (0.0592/2) log [Cu2+]= +0.340 + (0.0592/2) log [Cu2+]= +0.340 + (0.0592/2) log (K sp / [OH-]2)= +0.340 + (0.0592/2) log (K sp) - (0.0592/2) log [OH-]2= +0.340 + (0.0592/2) log (K sp) - 0.0592 log [OH-],3 marksBy definition, the standard potential for Cu(OH)2(s) + 2e-→ Cu(s) + 2OH- is the potential where [OH-] = 1.00.E = E o Cu(OH)2/Cu = +0.340 + (0.0592/2) log (K sp)= +0.340 + (0.0592/2) log (4.79 x 10-20)= +0.340 - 0.5722 marks= -0.232 V10 marks-------------------------------------------------------------------------------------------------------------- One may solve this problem as following.Eqn 1: Cu(OH)2(s) + 2e -→ Cu + 2OH-E+o = E o Cu(OH)2/Cu = ?Eqn 2: Cu(OH)2(s) → Cu2+ + 2OH-E o = (0.05916/n) logK sp= (0.05916/2) log(4.79×10-20)= -0.5715 V3 marksEqn 1 – Eqn 2 : Cu2+ + 2e-→ CuE-o = E+o - E o = E o Cu2+/Cu = 0.34 VTherefore, E+o = E-o + E o = + 0.34 + (-0.5715)2 marks= -0.232 V10 marks-0.232 V6-7.Below pH = 4.84, there is no effect of Cu(OH)2 because of no precipitation.Therefore,E = E Cu2+/Cu = +0.340 + (0.0592/2) log [Cu2+]= +0.340 + (0.0592/2) log 0.1003 marks= +0.340 – 0.0296 = +0.310 V7 marks** V6-8.** g graphite = 0.0833 mol carbon6 mol carbon to 1 mol lithium; 1 g graphite can hold 0.0139 mol lithiumTo insert 1 mol lithium, 96487 coulombs are needed.Therefore, 1 g graphite can charge 96487 × 0.0139 = 1340 coulombs. 5 marks1340 coulombs / g = 1340 A sec / g = 1340 x 1000 mA × (1 / 3600) h = 372 mA h / g 5 marks372 mA h / g7-1. (10 marks)n/V = P/RT = (80 x 106 / 1.013 x 105 atm)/[(0.082 atm L/mol/K)(298K)] = 32 mol/L5 marksdensity = mass/volume = d = 32 x 2 g/L = 64 kg/m 3 5 marks64 kg/m 37-2.** or 0.23H 2(g) + 1/2 O 2(g) → H 2O(l); ∆H rexn-1 = ∆H f [H 2O(l)] = -286 kJ/mol = -143 kJ/g 7 marksC(s) + O 2(g) → CO 2(g); ∆H rexn-2 = ∆H f [CO 2(g)] = -394 kJ/mol = -33 kJ/g 7 marks(-∆H rexn-1) / (-∆H rexn-2) = 4.3 or (-∆H rexn-2) / (-∆H rexn-1)= 0.236 marks7-3. (a) (-)1.2 x 105 kJ, (b) (-)6.9 x 104 kJ** x 108 sec or 3.3 x 104 hr or 1.4 x 103 days or 46 month or 3.8 yrI = 0.81 AH 2(g) + 1/2 O 2(g) → H 2O(l)∆H c = -286 kJ/mol = -143 kJ/g = -143 x 103 kJ/kg 5 marksΔG = ΔH – T ΔSΔS c= 70 – 131 – 205/2 = -163.5 J/K/mol5 marksΔG c = -286 kJ/mol + 298K x 163.5 J/K/mol = -237 kJ/mol = -1.2 x 105 kJ/kg 5 marks(a) electric motor W max = ΔG c ⨯ 1 kg = - 1.2 x 105 kJ 5 marks (b) heat engine W max = efficiency x ∆H c 5 marks= (1 – 298/573) x (-143 x 103 kJ) = -6.9 x 104 kJ 5 marks119 x 103 kJ = 1 W x t(sec)t = 1.2 x 108 sec = 3.3 x 104 hr = 1.4 x 103 days = 46 month = 3.8 yr 5 marksΔG = -nFE n = # of electrons involved in the reaction F = 96.5 kC/molH 2(g) + 1/2 O 2(g) → H 2O(l) n = 2 5 marksE = - ΔG/nF = 237 kJ/mol / 2 / 96.5 kC/mol = 1.23 V5 marksI = W/E = 0.81 A5 marks8-1-1. (5 marks on each)①C②C③CO8-1-2.③ Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g) 5marks① C(s) + O2(g) → CO2(g) ΔH①◦ = -393.51 kJ = ΔH f◦(CO2(g))② CO2(g) + C(s) → 2CO(g) ΔH②◦ = 172.46 kJFrom ① and ②,ΔH f◦(CO(g)) = (1/2){172.46 + (-393.51)} = -110.525 kJΔH f◦(Fe2O3) = -824.2 kJΔH③◦ = 3ⅹΔH f◦(CO2(g)) - ΔH f◦(Fe2O3) - 3ⅹΔH f◦(CO(g))= 3ⅹ(-393.51) – (-824.2) - 3ⅹ(-110.525) = -24.8 kJ 7 marks ΔS③°=2ⅹ27.28+3ⅹ213.74-87.4-3ⅹ197.674=15.36 J/K 3 marks ΔG③°=ΔH°-TΔS°=-24.8kJ-15.36J/Kⅹ1kJ/1000Jⅹ1473.15K=-47.43 kJ5 marksK = e(-ΔG°/RT)= e(47430J/(8.314J/Kⅹ1473.15K)) = 48 5 marksBalanced equation of ③:K = 48Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)8-2-1. (20 marks)One AB2O4 unit has available 4 (= 1 + (1/4)ⅹ12) octahedral sites.48-2-2. (20 marks)Since one face-centered cube in AB2O4 represents one Fe3O4 unit in this case, it has 8 available tetrahedral sites. In one Fe3O4 unit, 1 tetrahedral site should be occupied by either one Fe2+ (normal-spinel) or one Fe3+ (inverse-spinel). Therefore, in both cases, the calculation gives (1/8) ⅹ100% = 12.5% occupancy in available tetrahedral sites.**%8-2-3. (10 marks for d-orbital splitting, 10 marks for elec. distribution)9-1-1. 1 answer for 8 marks, two for 15 marksH 3CN NNH 3CNNN :::+_+::_:9-1-2. ( 10 marks)H 3CN::9-1-3.H 3CNCH 2CH 2:H 3CN HH CCH 2:(10 marks) (10marks )9-2-1. 5 marks eachHONN +_::ONN:H+:HH_O NN:H+:H_::::::9-2-2.( 10 marks)CH 2CO ::9-3-1.(40 marks)CH 3H 3CH 3C+BC H 2CCH 3CH 3CO 2DEOOO_9-3-2.(10 marks)O OH O n+F10-1. 10 marks eachNMLCH 2OHCH 2OHMeOOMeH HH HOMeMeO CHOCHOCH 2OHCH 2OHHHH H OHOMeMeO OH10-2. 8 marks each for correct structuresNumber of possible structures24 marks12OH(OH)OH(H)HH HHOMeOMeOH COOMeOH(OH)OH(H)HH HHOMeOMeOHCOOMe34OH(OH)OH(H)OH(OH)OHe(H)10-3. 10 marks eachGICH 2OHCH 2OHHHHHMeOOMeOHOMeCH 2OHCH 2OHHHHOMeOMeOMe10-4. 10 marksNumber of the correct structure for C from 10-2110-5.BOH(OH)OH(H)HHHH OHCOOHOHOH10 marks eachDJOH(OH)OH(H)HHHHOMeOMeCOOMeOMeOH(OMe)OMe(H)HHHHOMeOMeOMeCOOMe10-6. 20 marksHOOCOHHH OOOHOOH COOHOOHOHOH COOH11-1. 10 marks311-2. 30 marksCOOHHOOCOOH11-3. 2.5 marks eacha, c, d11-4 30 marksOOCOCOOOHTransition State11-5.For the enzyme-catalyzed reaction, Arrehnius equation could be applied.k cat/k uncat = A exp (-E a, cat/ RT) / A exp (-E a, uncat / RT)= exp [-∆E a, cat-uncat/ RT]= exp [-∆E a, cat-uncat(J/mol) / (2,480 J/mol)] = 106Therefore, -∆E a, cat-uncat = 34,300 J/mol 15 marksk uncat, T/k uncat, 298 = exp (-∆H≠ uncat/ RT) / exp (-∆H≠uncat / 298R)= exp [(-∆H≠ uncat/R)(1/T-1/298)]ln(k uncat, T/k uncat, 298 )= 13.8 = [(-86900/8.32)(1/T-1/298)]Therefore, T = 491 K, or 218o C 15 marks-E a, cat-uncat = 34,300 J/molT = 491 K, or 218o C。
第32届国际化学奥林匹克竞赛试题理论题
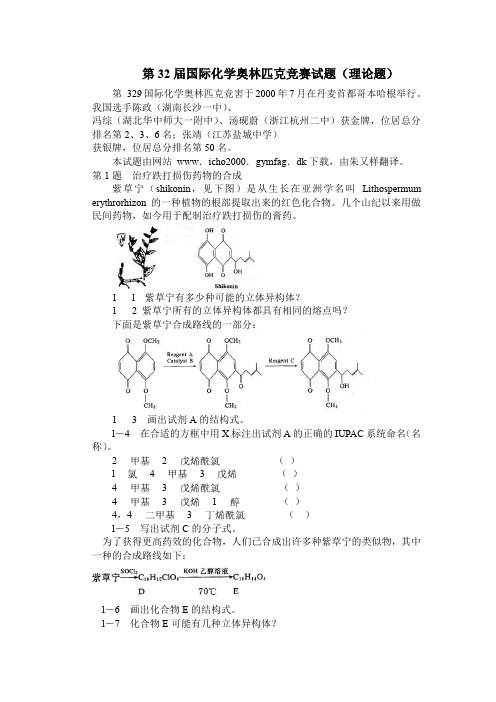
第32届国际化学奥林匹克竞赛试题(理论题)第329国际化学奥林匹克竞害于2000年7月在丹麦首都哥本哈根举行。
我国选手陈政(湖南长沙一中)、冯综(湖北华中师大一附中)、汤砚蔚(浙江杭州二中)获金牌,位居总分排名第2、3、6名;张靖(江苏盐城中学)获银牌,位居总分排名第50名。
本试题由网站www.icho2000.gymfag.dk下载,由朱又样翻译。
第1题治疗跌打损伤药物的合成紫草宁(shikonin,见下图)是从生长在亚洲学名叫Lithospermum erythrorhizon的一种植物的根部提取出来的红色化合物。
几个山纪以来用做民间药物,如今用于配制治疗跌打损伤的膏药。
1l 紫草宁有多少种可能的立体异构体?1 2 紫草宁所有的立体异构体都具有相同的熔点吗?下面是紫草宁合成路线的一部分:1 3 画出试剂A的结构式。
l-4 在合适的方框中用X标注出试剂A的正确的IUPAC系统命名(名称)。
22()l43()43()431()4,43()l-5 写出试剂C的分子式。
为了获得更高药效的化合物,人们已合成出许多种紫草宁的类似物,其中一种的合成路线如下:l-6 画出化合物E的结构式。
l-7 化合物E可能有几种立体异构体?合成紫草才有用类似物的另一路线如下:1-8画出化合物F 的结构式。
l -9画出化合物G 的结构式。
第2题丹麦-瑞典大桥连接丹麦和瑞典的隧道和桥梁复合交通设施于2000年7月 1日正式开通,它是由从哥本哈根通往一个人工岛的隧道和从人工岛通向马尔默的大桥组成。
该工程的主要建筑材料是钢筋混凝土。
本试题讨论与该工程建筑材料的生产和老化有关的化学反应问题。
混凝土是由水泥、水和砂石混合而成的。
水泥主要由经加热和研磨黏土和石灰石混合物而形成的硅酸钙和铝酸钙组成。
在水泥生产的最后几步,加入少量的石膏(CaSO 4·2H 2O )以改善混凝土的硬化性能。
在最后生产步骤中可能由于温度的升高导致不希望要的半水石膏(CaSO 4·l /2H 2O )的形成,其反应如下:CaSO 4·2H 2O (s )→CaSO 4·l /2H 2O (S )+211H 2O (g )0℃=273.15K 2-l 计算由l .00 ks CaSO 4·2H 2O (s )生成CaSO 4·l /2H 2O (s )的△H (单位为 kJ )。
国际化学奥林匹克竞赛-36thIChOtheoreticalanswers

(1 point) (1 point)
Eloss = 1556 MJ
Final
2
13
Name: ______________________ Student code: ___________
1.5 Total energy and costs:
total energy: Etot = Ewater + Eair + Eloss = 1316 MJ + 12 MJ + 1556 MJ = 2884 MJ
(4 points) (2)
slope:
1 = 1.9 cm −3
Va,max
⇒
Va,max = 0.53 cm3
(1)
intercept:
1 = 6·102 Pa cm-3 K ⋅Va,max
⇒
K·Va,max = 1.7⋅10-3 Pa-1 cm3
(1)
2.4 Equation for reaction rate:
Final
4
22
Name: ______________________ Student code: ___________
22
2.3 d) Gas volume Va,max and product K·Va,max:
1 = 1 + 1 = Va,max
⇒
θ K⋅p
Va
1 + p =p K ⋅Va,max Va,max Va
2 CO + O2 2 NO + 2 CO 2 C8H18 + 25 O2
→ → →
2 CO2 N2 + 2 CO2 16 CO2 + 18 H2O
2016年全国高中学生化学竞赛(决赛)理论试题、参考答案、评分细则

第30届中国化学奥林匹克(决赛)理论试题2016年11月26日长沙●本试卷共9道大题,总分100分,考试时间4小时。
迟到超过30分钟者不能进考场。
开始考试后1小时内不得离场。
●考试“结束铃声”响起后,立即停止答题,把试卷和答题纸放于桌面,由监考人员检查无缺,听到可以离开指令后方可离开考场。
●若发出停止答题指令后仍继续答题者,正在解答的试题(大题)以零分计。
●试卷已装订成册,不得拆散。
所有解答必须写在答卷纸的指定的框格内,写在其它地方的应答一律无效。
若有改动,需将新内容写于答卷的附页,并标明题号。
●用黑色水笔和黑色圆珠笔答题,试卷袋已附有草稿纸,因此不得携带纸张进入考场。
若另需草稿纸,可举手向监考人员索取,不得将草稿纸带出考场。
●将营员号及姓名写在试卷首页和每页答题卷指定位置,否则无效。
●允许使用非编程计算器以及直尺、橡皮等文具,不得携带铅笔盒、书籍、通讯工具入场。
●欲上卫生间者,可举手示意。
经监考人员允许方可离开座位,考场外由志愿者全程引领。
常数:R=8.31447 J·K-1·mol-1 F=96485 C·mol-1h=6.625×10-34 J·s 1 eV=1.602×10-19 J第1题(13分)1-1.简要解释为什么水溶液中HCN是弱酸,而液态HCN的酸性相比于其水溶液显著增强。
1-2.Hg2+离子具有亲硫性,可与二硫代氨基甲酸盐形成Hg(S2CNEt2)2的二聚体,请画出该二聚体的立体结构,并指出中心原子的杂化方式。
异烟酰腙的结构如下:它与2-乙酰基吡啶反应生成一种配体L。
将一定量的四水醋酸镍、配体L以及4,4’-联吡啶溶解在1︰1的乙醇-水的混合溶剂中回流2小时,冷却至室温,析出物质经洗涤干燥后得到褐色片状晶体M。
分析结果表明,M中N元素含量为21.0%。
1-3.画出配体L的结构,请在图中用*号标出配位原子。
1-4.通过计算,写出配合物M符合IUPAC规则的分子式,画出M的所有几何异构体的结构,在每个几何异构体下标注Ⅰ、Ⅱ、Ⅲ…等,指出哪些几何异构体存在旋光异构现象?哪几个几何异构体稳定性最高?请说明理由。
高中化学国际奥林匹克竞赛样题2016第一轮-参考答案

= 0.0120 g cm−3
2
Give credit if they use 24 dm3 for 1 mol of gas as a known value at STP.
(f) WF6 (g) + 4H2O (l) → H2WO4 (aq) + 6HF (aq)
1
WF6 (g) + 3H2O (l) → WO3 (s) + 6HF (aq) Accept either. State symbols not required.
48th INTERNATIONAL CHEMISTRY OLYMPIAD
2016 UK Round One MARK SCHEME
Although we would encourage students to always quote answers to an appropriate number of significant figures, do not penalise students for significant figure errors. Allow where a student’s answers differ slightly from the mark scheme due to the use of rounded/non-rounded data from an earlier part of the question.
(iii) B SF6 is kinetically stable but thermodynamically unstable
1
(h) Tungsten = 1 atom inside unit cell + 4 × atoms on face + 8 × atoms on corner
国际化学奥林匹克竞赛理论试题

科目 化学 年级 高三文件 aosai004.doc 考试类型 奥赛 考试时间 1999 关键词 化学竞赛标题 第31届国际化学奥林匹克竞赛理论试题 内容第一题化合物Q (摩尔质量 122.0 g mol -1) 含碳、氢、氧。
A 分题CO 2(g) 和 H 2O(l) 在 25.00 o C 的标准生成焓分别为 –393.51和–285.83 kJ ·mol -1。
气体常数R 为 8.314 J ·K -1·mol -1。
(原子量: H = 1.0, C = 12.0, O = 16.0)质量为0.6000g 的固体Q 在氧弹量热计内充分燃烧,在25.000o C 下开始反应, 此时量热计内含水710.0 g 。
反应完成后温度上升至27.250 o C, 反应生成1.5144 g CO 2 (g) 和 0.2656 g H 2O(l)。
问题1-1 确定Q 的分子式,并写出Q 燃烧反应的配平的化学方程式(标明物质状 态)。
问题1-2 若水的比热为4.184 J ·g -1·K -1,反应内能变化(∆U o )为–3079kJ ·mol -1,请计算量热计的热容(不包括水)。
问题1-3 计算Q 的标准生成焓(H f o )。
B 分题在6o C 时Q 在苯和水中的分配情况见下表,表中C B 和C w 分别表示Q 在苯和水中的平衡。
假定Q 在苯中的物种是唯一的,并与浓度与温度无关。
浓 度 (mol L -1) C B C W 0.0118 0.0478 0.0981 0.156 0.00281 0.00566 0.00812 0.0102问题1-4 假定Q 在水中是单体,通过计算说明Q 在苯中是单体还是双聚体。
问题1-5 理想稀溶液的凝固点表达式如下:fs 20f f 0f Δ.)(H X T R T T =-其中T f是溶液的凝固点,T f0是溶剂的凝固点,H f是溶剂的熔化热,X S是溶质的摩尔分数,苯的摩尔质量为78.0 g mol-1。
化学竞赛试题(含答案)
化学竞赛决赛试题(满分100分100分钟)可能用到的相对原子质量:H-1 C-12 N—l4O一l6F-19 Mg一24 P一31 S一32 Cl一35.5 K一39 Ca一40 Fe一56 Cu一64 Zn-65一、选择题(本题包括20个小题,每小题2分,共40分;每小题有1个或2个选项符合题意。
若有两个答案的错选l个不得分,漏选1个扣l分。
请将答案填在下方的表格内)1.世界环境日为每年的6月5日,它的确立反映了世界各国人民对环境问题的认识和态度,表达了人类对美好环境的向往和追求。
2011年世界环境日的主题是“森林:大自然为您效劳”,旨在配合联合国国际森林年,强调森林的生态价值,提高人们森林保护意识。
下列标记是2011年国际环境日标识的是2.2011年我国用“长征2号FT1”型运载火箭成功地将“天宫一号”送入指定轨道,其后,天宫一号与神舟八号实现了成功对接。
这是我国航天领域又一重大突破。
“长征2号FT1”型运载火箭在升空时发生的下列变化中,属于化学变化的是A.火箭点火B.导流槽内的水受热汽化C.隔热材料脱落D.整流罩脱落3.欧洲科研人员成功“抓住”反氢原子长达一千秒,是2011年世界十大科技新闻之一。
科学家认为,组成物质的基本粒子,如电子、质子、中子等,都有各自的反粒子,它们在质量上以及其他方面与它对应的粒子一模一样,但所带的电荷正负恰恰相反。
粒子与反粒子碰到一起会同归于尽,化作一束强光,这种现象称之为湮灭。
有反粒子就可能形成反物质、反世界。
较长时间“抓住”反氢原子,有利于对反物质性质进行精确研究。
下列关于反氢原子的叙述不正确的是A.反氢原子中有一个带负电荷的质子B.反氢原子的相对原子质量为lC.反氢原子中有一个带正电荷的电子D.反氢原子带一个单位负电荷4.我国科学家屠呦呦因为“发现青蒿素——一种用于治疗疟疾的药物,挽救了全球特别是发展中国家的数百万人的生命”而获拉斯克奖。
青蒿素是治疗疟疾的特效药,青蒿素分子式为C15H22O5。
2016年中国化学奥林匹克(初赛)试题、答案、评分标准与细则
第30届中国化学奥林匹克(初赛)试题、答案及评分标准第1题(8分)1-1离子化合物A2B由四种元素组成,一种为氢,另三种为第二周期元素。
正、负离子皆由两种原子构成且2242年才被用作牙膏的添加剂和补牙填充剂成分。
A是离子晶体,由NaF和NaPO3在熔融状态下反应得到。
它易溶于水,阴离子水解产生氟离子和对人体无毒的另一种离子。
1-4-1写出合成A的反应方程式。
第2题(9分)鉴定NO3–离子的方法之一是利用“棕色环”现象:将含有NO3–的溶液放入试管,加入FeSO4,混匀,然后顺着管壁加入浓H2SO4,在溶液的界面上出现“棕色环”。
分离出棕色物质,研究发现其化学式为[Fe(NO)(H2O)5]SO4。
该物质显顺磁性,磁矩为3.8 μB (玻尔磁子),未成对电子分布在中心离子周围。
2-1写出形成“棕色环”的反应方程式。
2-2推出中心离子的价电子组态、自旋态(高或低)和氧化态。
第3题(13分)3-1好奇心是科学发展的内在动力之一。
P2O3和P2O5是两种经典的化合物,其分子结构已经确定。
自然而然会有如下问题:是否存在磷氧原子比介于二者之间的化合物?由此出发,化学家合成并证实了这些中间化合物的存在。
3-1-1写出这些中间化合物的分子式。
3-1-2 画出其中具有2重旋转轴的分子的结构图。
根据键长不同,将P-O键分组并用阿拉伯数字标出(键长相同的用同一个数字标识)。
比较键角∠O-P(V)-O和∠O-P(III)-O的大小。
3-2NH3分子独立存在时H-N-H键角为106.7o。
右图是[Zn(NH3)6]2+离子的部分结构以及H-N-H键角的测量值。
解释配合物中H-N-H键角变为109.5o的原因。
3-3 量子化学计算预测未知化合物是现代化学发展的途径之一。
2016年2月有人通过计算预言铁也存在四氧化物,其分子构型是四面体,但该分子中铁的氧化态是+6而不是+8。
3-3-1 写出该分子中铁的价电子组态。
正确画出四个氧原子围绕中心铁原子形成四面体分布且示出两个氧之间的过氧键,得满分;若正确画出四面体分布但未示出过氧键,得2分;其他答案不得分。
第二届Chemy化学奥林匹克竞赛联赛试题答案

第二届Chemy化学奥林匹克竞赛联赛(2016年9月28日18:00 ~ 22:00)·竞赛时间4小时。
迟到超过半小时者不能进考场。
开始考试后1小时内不得离场。
时间到,把试卷(背面朝上)放在桌面上,立即起立撤离考场。
·试卷装订成册,不得拆散。
所有解答必须写在指定的方框内,不得用铅笔填写。
草稿纸在最后一页。
不得持有任何其他纸张。
·姓名、报名号和所属学校必须写在首页左侧指定位置,写在其他地方者按废卷论处。
·允许使用非编程计算器以及直尺等文具。
第1题(10分)天青石的主要成分为硫酸锶,有时也含有硫酸钙和硫酸钡。
我们用以下方法测定天青石中Sr和Ba的含量。
准确称取干燥的MgO 0.7971 g,用盐酸溶解,煮沸并冷却后稀释至1 L。
移取上述溶液25.00 mL,加入适量NH3-NH4Cl缓冲液和EBT指示剂,用待标定EDTA滴定,消耗23.19 mL。
准确称取天青石2.5555 g,加入碳酸钠和碳酸钾各5 g充分混匀,于马弗炉中1073 K下熔融20 min,取出冷却,用水充分浸取,过滤,用1 % Na2CO3洗涤沉淀8次。
用热盐酸将沉淀洗至500 mL容量瓶中,加1滴甲基红,滴加氨水至黄色,在滴加盐酸至红色并过量1~2滴,冷却后稀释至刻度,摇匀。
移取上述样品溶液100.00 mL,加热微沸,不断搅拌并滴入过量EDTA溶液和饱和K2Cr2O7溶液以沉淀Ba2+,继续加热至沸,静置,过滤,用1 % NH4Ac洗涤烧杯及沉淀,沉淀及滤纸转移至锥形瓶中,用盐酸溶解,加水稀释,加入足量KI溶液,摇匀,加入淀粉指示剂,用0.01085 mol·L-1 Na2S2O3溶液滴定至终点,消耗5.49 mL。
移取上述样品溶液25.00 mL,加入10 mL 0.02 mol·L-1 Zn-EDTA溶液和10 mL 1+1氨水,加2滴萘酚绿-达旦黄、4滴EBT,用上述标准EDTA溶液滴定至亮绿色,消耗25.19 mL。
2016第30届中国化学奥林匹克决赛理论试题含答案[精美word精校版]
![2016第30届中国化学奥林匹克决赛理论试题含答案[精美word精校版]](https://img.taocdn.com/s3/m/705ad404856a561253d36f6f.png)
2016 第 30 届中国化学奥林匹克 ( 决赛 ) 理论试题含答案 [ 精巧 word 精校版 ]第 30 届中国化学奥林匹克(决赛)理论试题2016年 11月 26日长沙●本试卷共 9 道大题,总分100 分。
考试时间 4 小时,迟到超出30 分钟者不可以进入考场。
开考后1 小时内不得走开考场。
●考试“结束铃声”响起后。
立刻停止答题,把试卷和答题纸放于桌面,由监考人员检查无缺。
听到能够走开指令后方可走开考场。
●发出停止答题指令后仍持续答题者,正在解答的试题(大题)以零分计。
●试卷已经装订成册,不得拆开。
全部解答一定写在答卷上指定的框格,写于其余地方无效。
如有变动需将新内容写于答卷的附页,并注明题号。
●用黑色墨水笔或黑色圆珠笔大题。
试卷袋已附有底稿纸,所以不得携带纸张进入考场。
若另需底稿纸,可举手向监考人员讨取。
不得将底稿纸带出考场。
●将营员号及姓名写在试卷首页和每页答卷指定地点,不然无效。
●同意使用非编程计算器以及直尺等文具。
不得携带铅笔盒、书本、通信工具入场a●欲上洗手间者,请举手表示。
经监考人员同意方可离开座位,考场外由志愿者全程引领。
H相对原子质量HeLiBe B C N O F NeNa MgAlSi P S Cl ArK Ca Sc TiVCr Mn Fe Co Ni Cu Zn GaGeAsSeBr Kr39.10 44.96 47.8850.9455.8572.61Rb SrYZr Nb Mo TcRu RhPdAgCd InSnSb TeIXeCs Ba HfTaWRe Os IrPt Au HgTlPb Bi132.9137.3La -Lu178.5 180.9 183.9 186.2FrRaRfDbSgBhHsMt[223] [226] Ac-LrPo At Rn[210][210] [222]常数:·mol -1·K-1F=96487 C ·mol -1×10-34J ·×10-19J第 1 题(13 分)1-1 简要解说为何水溶液中HCN 是弱酸,而液态 HCN 的酸性对比于其水溶液明显加强。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
48th InternationalChemistry Olympiad Theoretical Problems28 July 2016Tbilisi, GeorgiaInstructions∙Begin only when the START command is given. You have 5 hours to work on the problems.∙Use only the pen and calculator provided.∙The problem booklet has 23 pages, the answer sheet is 28 pages.∙Make sure that your code is on every page of the answer sheet.∙Questions are identical in the problem text and on the answer sheets.∙All results must be written in the appropriate boxes on the answer sheets.Anything written elsewhere will not be graded. Use the reverse of the problempages if you need scratch paper.∙Write relevant calculations in the appropriate boxes when necessary. If you provide only correct end results for complicated questions, you will receive noscore.∙Raise your hand if you need a restroom break.∙When you have finished the examination, put your answer sheets into the envelope provided. Do not seal the envelope.∙You can keep the problem booklet.∙You must stop your work immediately when the STOP command is given. A delay in doing this may lead to cancellation of your exam.∙Do not leave your seat until permitted by the supervisors.∙The official English version of this examination is available on request only for clarification.Constants and formulaePeriodic table with relative atomic massesNitrogen trifluoride is a surprisingly stable compound that was first prepared in the melt electrolysis of a mixture of ammonium fluoride and hydrogen fluoride.1.1.On which electrode does nitrogen trifluoride form? Write a balanced chemicalequation for the electrode half reaction for the formation of NF3.Interestingly the related fluoroamine (NH2F) and difluoroamine (NHF2) are very unstable materials; decomposition of either pure substance can even be explosive. This is dangerous as they are formed in the electrolysis as side products.1.2.Which of NF3, NHF2 or NH2F compound is expected to condense at the lowesttemperature?The N-F bond lengths in these molecules were determined to be 136, 140 and 142 pm. The change in the bond lengths can be explained with a simple electrostatic model taking into account the partial charges on the atoms.1.3.Assign the N-F bond lengths (136, 140, 142 pm) to the molecules.When NHF2 is bubbled through a solution of KF in HF, a binary nitrogen – fluorine compound can be obtained as a mixture of two geometric isomers.1.4.Write a balanced chemical equation for the formation of the binary nitrogen-fluorine compound.Tetrafluoroammonium ion (NF4+) and its corresponding salt can form from NF3 with elementary fluorine in the presence of an appropriate reagent.1.5.Propose a suitable reagent and write a balanced chemical equation for thereaction.NF4+ ions form stable salts with a number of anions. These are very sensitive to humidity, because NF4+ ion hydrolyzes forming NF3 and O2. Interestingly nitrogen trifluoride always forms quantitatively, while the quantity of oxygen is often less than expected due to side reactions.1.6.Write a balanced chemical equation for the hydrolysis of NF4+. Write a balancedchemical equation for a possible side reaction that can decrease thetheoretically expected O2:NF3 mole ratio.Tetrafluoroammonium salts were investigated for use as solid rocket fuels, because NF3 and F2 are released from them on heating. One of them has a fluorine content of65.6 m/m%, all of which is converted into NF3 and F2 upon decomposition. During the decomposition 2.5 times as many moles of F2 are formed as of NF3.1.7.Determine the formula of the salt in question.One of the first materials used in solid state electronics was red copper(I) oxide. Interest is renewed nowadays because it could be a non-toxic and cheap component of solar cells.ABThe two figures above depict the cubic unit cell of the Cu2O crystal. The lattice constant of the structure is 427.0 pm.2.1.1.Which of the atoms (A or B) is copper?Which basic structure (primitive cubic, face centered cubic, body centered cubic, diamond) is formed by the A atoms and which structure is formed by the Batoms?What are the coordination numbers of the atoms?2.1.2.Calculate the smallest O-O, Cu-O and Cu-Cu distances in the structure.2.1.3.What is the density of pure copper(I) oxide?A common defect in this crystal is some copper atoms missing with the oxygen lattice unchanged. The composition of one such crystal sample was studied, and 0.2% of all copper atoms were found to be in oxidation state +2.2.2.What percentage of normal copper sites are empty in the crystal sample? Whatis x in the empirical formula Cu2-x O of the crystal?Copper(I) oxide is insoluble in water. It is stable in dry air, but humidity in the air catalyzes a transformation (Reaction 1).When copper(I) oxide is dissolved in dilute sulfuric acid, a blue solution containing a precipitate is formed without evolution of a gas (Reaction 2). When hot, concentrated sulfuric acid is used, no precipitate remains, but an odorous gas forms (Reaction 3). The same gas forms when the precipitate from reaction 2 is dissolved in hot concentrated sulfuric acid.2.3.Write balanced chemical equations for reactions (1-3).Copper(I) oxide can be produced in a number of ways. Heating copper in air is a common method in the synthesis of semiconductor Cu2O. In a pure oxygen atmosphere, the three species containing copper (Cu(s), Cu2O(s) or CuO(s)) can potentially interconvert.Suppose that the Δf H o and S o data given for 105 Pa are independent of temperature:2.4.Determine the temperature ranges, if any, of thermodynamic stability of copperand its oxides between 500 and 1500 K in a 105 Pa oxygen atmosphere. Important data are given for 298 K. Use this temperature in the following calculations: K sp(Cu(OH)2)=2∙10−19Cu2O(s) + H2O(l) + 2 e−⟶2 C u(s) + 2 O H−(aq) E o= −0.360 VCu2+(aq) + e−⟶ C u+(aq) E o= +0.159 VCu2+(aq) + 2 e−⟶ C u(s)E o= +0.337 VOne possibility for producing Cu2O is the anodic oxidation of copper. Electrolysis of an aqueous basic solution (e.g. NaOH) with a copper anode and platinum cathode can lead to formation of copper(I) oxide on the anode.2.5.Write the half reaction equations for the electrode processes during the anodicproduction of Cu2O in NaOH solution with a platinum cathode and copperanode.Electrolytic reduction of copper(II) ions in solution is another possibility.2.6.1.Write the half reaction equation of the cathode process giving Cu2O in acidicmedium.Let us use 0.100 mol dm−3 Cu2+ solution and carry out electrolysis with platinum electrodes.2.6.2.What is the maximum pH at which the concentration of copper(II) can bemaintained at 0.100 mol dm−3?If the pH is too low, reduction to metallic copper is preferred to the formation of copper(I) oxide.2.6.3.What is the minimum pH at which the cathodic production of Cu2O in a 0.100mol dm−3 Cu2+ solution is still possible?Problem 3 9% of the total Iodine deficiency is of special concern in Georgia because it occupies a region where iodine is scarce in soil and water. Iodine deficiency can be effectively and inexpensively prevented if salt for human consumption is fortified with small amounts of iodine. Methods for analyzing salt for iodine content are thus important. Current regulations in Georgia are that iodized salt must contain between 25-55 ppm iodine (1 ppm =1 mg iodine/kg salt).Most salt is iodized by fortification with potassium iodate (KIO3). Iodate content can be determined in salt samples using iodometric titration. In a typical procedure, 10.000 g of an iodized salt sample is dissolved in 100 cm3 of 1.0 mol/dm3 aqueous HCl to which1.0 g KI has been added. The solution is then titrated with 0.00235 mol/dm3 aqueous sodium thiosulfate solution to a starch endpoint; this requires 7.50 cm3 of titrant.3.1.1.Write a balanced net ionic equation for the reaction when iodate reacts withexcess iodide in acidic solution.3.1.2.Write a balanced net ionic equation for the reaction taking place during thetitration with thiosulfate.3.1.3.Calculate the iodization level, in ppm, of this salt sample.A less common agent for iodizing salt is potassium iodide, which cannot be easily measured by iodometric titration.One possible method for analyzing iodide in the presence of chloride is potentiometric titration. However, this method is not very precise in the presence of large amounts of chloride.In this method, a silver wire is immersed in the solution (containing iodide and chloride) to be analyzed and silver ion is gradually added to the solution. The potential of the silver wire is measured relative to a reference electrode consisting of a silver wire in a 1.000 mol/dm3 solution of AgNO3. The measured potentials are negative and the absolute values of these potentials are reported. The solution to be analyzed has a volume of 1.000 dm3 (which you may assume does not change as silver ion is added), and T= 25.0°C.The results of this experiment are governed by three equilibria: the solubility of AgI(s) [K spI] and AgCl(s) [K spCl] and the formation of AgCl2−(aq) [K f]. (Iodide also forms complex ions with silver but this may be neglected at the very low concentrations of iodide present in this experiment).AgI(s) ⇌ Ag+(aq) + I−(aq) K spIAgCl(s) ⇌ Ag+(aq) + Cl−(aq) K spClAg+(aq) + 2 Cl−(aq) ⇌ AgCl2−(aq) K fBelow are shown the results of two experiments measuring the observed potential as a function of added number of moles of silver ion. Experiment A (solid circles) was carried out with 1.000 dm 3 of solution containing 1.00∙10−5 mol/dm 3 iodide and no chloride ion. Experiment B (open circles) was done using 1.000 dm 3 of solution containing 1.00∙10−5 mol/dm 3 iodide and 1.00∙10−1 mol/dm 3 chloride.μmol Ag + added3.2.1. Select an appropriate data point from the experiments and use it to calculate thesolubility product of AgI (K spI ). 3.2.2. Select an appropriate data point from the experiments and use it to calculate thesolubility product of AgCl (K spCl ). 3.2.3. Select an appropriate data point from the experiments and use it to calculate K f .You may need to use values of K spI or K spCl to do this calculation. If you wereunable to carry out the calculations in 3.2.1. or 3.2.2., you may use the arbitrary values of K spI = 1.00∙10−15 and K spCl = 1.00∙10−9 without penalty.An analytical method that is more practical, because it is not sensitive to the presence of chloride, uses the Sandell-Kolthoff reaction. This is the reaction of H3AsO3 with Ce(IV) to give Ce(III) in acidic solution, which is strongly catalyzed by iodide ion.3.3.1.Write balanced net ionic equations for the reaction of cerium(IV) with H3AsO3 inacidic solution, as well as reactions of cerium(IV) with a species containing theelement iodine and H3AsO3 with a species containing the element iodine, thatcould reasonably account for the catalysis of the net reaction by iodide.The reaction of Ce(IV) with H3AsO3 can be monitored by measuring the absorbance at 405 nm, as Ce(IV) is orange and absorbs significantly at 405 nm, while the other reactants and products are colorless and do not absorb appreciably. Three runs were carried out, all in 0.50 mol/dm3 H2SO4at 25.0°C using the following initial concentrations:An analyst initiated the reactions by mixing the reagents in a cuvette. After a short variable delay absorbance measurements were started, with the first measurement recorded at t=0 s. The data obtained are shown below:Under these conditions (0.5 mol/dm3 H2SO4, 25.0°C), the rate law for the reaction can be written asRate = k[H3AsO3]m[Ce(IV)]n[I−]p where m, n, and p are integers.3.3.2.Determine the values of m, n, and p and calculate the value of k (be sure tospecify its units).A 1.000 g sample of iodized salt is dissolved in water to give 10.00 cm 3 of solution. A 0.0500 cm 3 aliquot of this solution is added to a mixture of 1.000 cm 3 0.025 mol/dm 3 H 3AsO 3 in 0.5 mol/dm 3 H 2SO 4 and 0.800 cm 3 0.5 mol/dm 3 H 2SO 4. To this mixture is added 0.200 cm 3 0.0120 mol/dm 3 Ce(NH 4)2(NO 3)6 in 0.5 mol/dm 3 H 2SO 4 and the absorbance at 405 nm is measured as a function of time at 25.0°C:3.3.3.Calculate the iodization level, in ppm, of this salt sample.Problem 4 8% of the total Application of kinetic studies in water treatmentIndustrial waste is a major cause of water pollution and kinetic studies are carried out in a laboratory to design effluent treatment. 1,4-dioxane, more commonly known as dioxane (C4H8O2), an industrial solvent and by-product, is a significant water contaminant. It can be oxidised to hazard free chemicals using oxidants such as peroxodisulfate, ozone or hydrogen peroxide.The data obtained in the kinetic study of oxidation of dioxane with potassium peroxodisulfate (K2S2O8) as oxidant and AgNO3 as catalyst at T = 303.15 K are given below. The reaction was monitored by the estimation of unreacted peroxodisulfate. The concentration of AgNO3 used in this study was 1.00∙10−3 m mol∙dm−3.In many countries the accepted maximum level of dioxane in drinking water is specified as 0.35 μg dm−3.A water sample contains an initial dioxane concentration of 40.00 μg dm−3. Assume that1 mol dioxane requires 1 mol of peroxodisulfate for oxidation. The concentration of AgNO3 used in this study was 1.00∙10−3 m mol∙dm−3.4.1.1.Calculate the time in minutes the oxidation process has to continue in order toreach the accepted level of dioxane at 303.15 K if the initial concentration ofK2S2O8is 5.0∙10−6 mol dm−3. Assume that the rate law obtained from the dataabove is valid under these conditions.Various mechanisms have been proposed for the peroxodisulfate oxidation of dioxane. Misra and Ghosh (1963) proposed the following mechanism:k1S2O82− + Ag+⇄Ag3+ + 2SO42−k2k3Ag3+ + D (dioxane) ⟶D’ (dioxane oxidised) + 2H+ + Ag+4.1.2.Assuming Ag(III) to be in steady state, deduce the rate equation for theoxidation of dioxane.4.1.3.Which of the following is/are correct?A) The rate equation based on the mechanism given in 4.1.2, at very highconcentrations of dioxane, is consistent with the experimental data in 4.1.1.B) The rate equation based on the mechanism given in 4.1.2, at very lowconcentrations of dioxane, is consistent with the experimental data in 4.1.1.C) The units of the observed rate constant are dm3∙mol−1∙s−1 at very highconcentrations of dioxane.D) The units of the observed rate constant are dm3∙mol−1∙s−1 at very lowconcentrations of dioxane.Degradation of pharmaceutical products – a kinetic overviewKinetic studies are important in deciding the shelf life of a pharmaceutical product. Several chemical reactions can affect the shelf life of pharmaceutical products and the rates of these reactions depend on conditions such as pH, temperature, humidity. Lysine acetylsalicylate (LAS) is prescribed as a pain killer and anti-inflammatory drug under the brand name Aspegic. LAS on hydrolysis forms lysine salicylate and acetic acid.Hydrolysis of LAS can proceed via three different pathways (a) acid catalysed,(b) uncatalysed and (c) base catalysed.If [LAS] denotes the concentration of LAS at time ‘t’, the overall rate of the hydrolysis reaction can be written as−d[LAS]dt=k H[LAS][H+]+k0[LAS]+k OH[LAS][OH−]where k H, k0 and k OH are the rate constants of the acid catalysed, uncatalysed and base catalysed pathways of hydrolysis, respectively. The observed rate constant is defined by:−d[LAS]dt=k obs[LAS]4.2.1. Write an expression for k obs in terms of k H, k0, k OH and [H+].Hydrolysis of LAS was carried out at 298.15 K at various pH values (0.50 to 13.0). A very low initial concentration of LAS ensured that the pH did not change during the course of the reaction.The following graph shows the pH dependence of the hydrolysis of LAS.4.2.2.Which of the following is/are correct?A) k obs≌k0 at pH = 12B) k obs≌k0 at pH = 5.0C) The rate of the reaction increases when the pH is changed from 0.50 to 1.0.D) The rate of the reaction increases when the pH is changed from 10 to 12.ing the diagram and the data given below, calculate k H, k0 and k OH. Make sureto specify the units.Acetylsalicylic acid, more commonly known as aspirin, is a medicine often used for reducing fever, pain and inflammation. Like LAS, the hydrolysis of aspirin can also take different pathways depending on the pH. The pH rate profile of aspirin hydrolysis at 333.15 K is given below:The following are possible reactions for the hydrolysis of aspirin. Depending on the pH, one or more of these reactions will predominate.I. CH3COOC6H4COOH + H3O+⟶ HOC6H4COOH + CH3COOH + H+II. CH3COOC6H4COOH + H2O ⟶ HOC6H4COOH + CH3COOHIII. CH3COOC6H4COOH + OH−⟶ HOC6H4COO− + CH3COOHIV. CH3COOC6H4COO− + H3O+⟶ HOC6H4COOH + CH3COOHV. CH3COOC6H4COO− + H2O ⟶ HOC6H4COO− + CH3COOHVI. CH3COOC6H4COO− + OH−⟶ HOC6H4COO− + CH3COO−ing the pH-rate profile diagram and the reactions given above, state which of the following statements is/are correct. (pK a of aspirin = 3.57 at 333.15 K)a) In the region C-D, reaction IV is predominantb) In the region C-D, reaction V is predominantc) In the region D-E reaction VI is predominantd) In the region A-B, reaction II is predominantA separate plot of k obs vs pH for the hydrolysis of aspirin has been confirmed to show a minimum at a particular pH. At 290.15 K the following rate constants for reactions I, II and III were determined:The ionic product of water at 290.15 K can be taken as 1.0∙10−14.4.3.2.Assuming that only reactions I, II and III occur, calculate the value of the pH atthe minimum of k obs.Problem 5 8% of the total 5500 years ago in ancient Egypt people learned for the first time how to synthesize a blue pigment. Now we know this pigment as Egyptian blue. About 2000 years later in ancient China another pigment was widely used, which is now referred to as Chinese blue. The two pigments are similar in structure, but have different elemental compositions.Ushabti figurines from Egyptian pharaoh tomb covered with Egyptian blue anda Chinese blue soap dispenser sold at AlibabaThe ancient method of preparation for these pigments can be easily reproduced in a modern laboratory.When considering the amounts, assume that all of the compounds in this task are pure, and the yields are quantitative.To make Egyptian blue, one should heat 10.0 g of mineral A with 21.7 g of SiO2 and9.05 g of mineral B at 800–900°C for a prolonged time. 16.7 dm3 of a mixture of two gaseous products are released (the volume is measured at 850°C and 1.013∙105 Pa (1.013 bar) pressure. In result, 34.0 g of the pigment was obtained. No other products are formed. As the gas mixture is cooled, one component of the mixture condenses. As the remaining gas is further cooled to 0°C, the gaseous volume reduces to 3.04 dm3.5.1.1.Find the mass of the gaseous mixture formed upon heating of A with B and SiO2.5.1.2.Determine the quantitative composition of this gas mixture.When 10.0 g of mineral A is heated with 21.7 g of SiO2 in the absence of B, it forms8.34 dm3of gaseous products (measured at 850°C and 1.013∙105 Pa = 1.013 bar pressure). Mineral A contains only one metal.5.1.3. Calculate the molar mass and determine the formula of mineral B. Hint: it is anionic solid insoluble in water and containing no water of crystallization.In order to obtain Chinese blue, one should take 17.8 g of mineral C instead of mineral B (keeping the amounts of mineral A and SiO2 same as for Egyptian blue), and run the reaction at higher temperatures. Besides the pigment, the same gaseous products in the same quantities are formed as in the preparation of Egyptian blue.5.1.4.Determine the formula of mineral C.5.1.5.Determine the formulae of Egyptian blue and Chinese blue.5.1.6. Determine the formula of mineral A.Elemental analysis of some samples of Chinese blue shows traces of sulfur. This led to a conclusion that those were synthesized using another common mineral instead of C. 5.2.1.Suggest a formula for the mineral used in place of C.5.2.2. Could the temperature of synthesis of Chinese blue be decreased if this mineralis used instead of C?If during the synthesis of Chinese blue we take a smaller amount of silica than in the process above, we will obtain a purple pigment: Chinese violet. It was used, in particular, for coloring the famous Terracotta army soldiers.Terracotta army from Xian, China and reconstruction of its original coloring 5.3. Write down the formula of a binary compound that forms under the conditionsrequired for Chinese violet and is responsible for the change of the color.Problem 6 7% of the total Although there is currently no known cure for Alzheimer’s disease, there are medications available to manage the neurodegenerative disorder. Among these are acetylcholinesterase inhibitors, of which galantamine 1 is an example. This molecule can be isolated from the Caucasian snowdrop, a plant native to Georgia; however, the large amounts needed for therapy require a synthetic route. Shown below is the route used to prepare galantamine industrially.resolutionNotes about the synthesis:∙1H NMR of A indicates 2 aromatic protons in a para arrangement.∙C is labile in aqueous conditions, so it is not isolated, but rather reacted immediately with NaBH4 to convert it to D.6.1.1.Suggest structures for A, B, C, D, F, and G. None of the reactions except for thefinal transformation with L-selectride are stereoselective. Therefore,stereochemistry does not need to be indicated in your answers.6.1.2. Give the formula for a possible reagent, X , to convert compound D to E .The optical rotation of the material obtained by resolution was –400° cm 2 g -1, while that of the enantiomerically pure compound is –415° cm 2 g -1 when measured under the same conditions. You may assume that the only optical impurity is the other enantiomer. One way of describing optical purity is enantiomeric excess (ee ). It is defined as the difference in the percentages of the enantiomers in a mixture. For example in a mixture of 70% R and 30% S , the ee is 40%.6.2.1. What is the enantiomeric excess of the resolved compound as prepared by theindustrial route?L-selectride is a commercial reagent that performs the final reaction stereoselectively.6.2.2. Assign the labelled stereocentres ( ) in (–)-1as R or S .6.2.3. Give the formula for a reagent that carries out thesame reaction as L-selectride, converting H to 1.You need not worry about stereoselectivity.An alternative route to galantamine occurs with theseven-membered ring being the last ring to form.6.3.1. Give the formula for compound Y to carry out the first step of the route.6.3.2. Suggest structures for J and K. (4 equivalents)pH = 5Problem 78% of the totalThis question looks at the synthesis of dolasetronmesylate , Z (shown right), a drug sold under thetradename Anzemet and used to treat post-operative nausea and vomiting.The synthesis begins as shown below.First cyclic compound A is made, which contains C, H, and O only. Compound G is achiral and can be prepared directly from D using ozone under reductive conditions, or via stereoisomers E1 and E2 using OsO 4, or via stereoisomers F1 and F2 using the peracid shown.7.1.Determine the empirical formula of G from the percentage masses given. 7.2. Give the structures of A , B , C , D , E1, E2, F1, F2 and G .Compound G is used in the next stage of the synthesis, under buffered conditions, to form H (as a mixture of two achiral diastereoisomers). Reduction of H with NaBH4 gives alcohol I (as a mixture of four achiral diastereoisomers). I reacts with acidified dihydropyran to form J (as a mixture of even more diastereoisomers). J is then treated first with t-butoxide base, then refluxed with acid before finally extracting under weakly basic conditions to form K as a mix of two diastereomers, K1 (major product) and K2 (minor product). These could be separated, and K1 was used in the final stages of the synthesis.7.3.1.Give the structures of H, I, and J. There is no need to show the differentdiastereoisomers formed.7.3.2. Give the structures of diastereoisomers K1, and K2.In the final stage of the synthesis, L and M react to form intermediate N. N then reacts with K1 to form, after extraction, the neutral amine which gives the target compound upon protonation with CH3SO3H.7.4.Give the structure of N.Problem 8 7% of the totalAn exotic, but biologically relevant sugar analogue can be prepared from D -glucose in the following manner. Heating a mixture of D -glucose and acetone with a few drops ofconcentrated acid results in the formation of a diacetonide A . Then A can be hydrolyzed selectively to B .Acetone/H +XD -glucose A B8.1.1. Which of the following sentences is true?A is an α isomer. A is neither α nor β.A is a β isomer.A is a mixture of α and β isomers.8.1.2. Which of the following sentences is true?We can get product A only if we use α-D glucose as starting material. We can get product A only if we use β-D glucose as starting material.We can get product A either from α- or from β-D glucose as starting material.8.1.3. Which one of these reagents can be utilized as X for the selective hydrolysis ofA ?50% acetic acid concentrated H 2SO 4 6 M HCl in water1 M NaOH in water6 M HCl in acetic acid8.1.4. Which is the stereochemically correct structure for compound B ?Neither of theseB is treated with sodium metaperiodate to get C.C is then reacted with an aqueous solution of NaCN, then heated with 10% NaOH solution to get a mixture of two diastereomeric compounds D1 and D2. These compounds can be separated by column chromatography.IO4−1. NaCN2. NaOH/H2OB C188.2 g/molD1 + D2Reaction of D1 with LiAlH4 followed by heating with 1M HCl solution gives sugar F that is the hydrolysis product of the most abundant natural polysaccharide.LiAlH41M HCl/H2OD1 E F8.2.1.Draw the structures of C, D1, D2, E and F including stereochemical information.Show F as the more stable 6-membered ring containing isomer using the ringskeleton. Indicate with a wavy line if absolute chirality around a carbon is notknown.8.2.2.The reaction sequence from glucose to F does not seem to be useful. In somecases, however, this is the most economical way to produce F. In which case?13C labelling at carbon 6 of F13C labelling at carbon 5 of F13C labelling at carbon 1 of F15O labelling at glycosidic OH of Fsynthesis of an uncommon isomer of FNeutralization of D2 with HCl followed by heating in toluene results in dehydration and formation of G, which has a tricyclic structure in water-free solvents. Boiling G in 1M HCl solution gives H (C6H10O7), which is a natural sugar derivative containing a 6 membered ring. H is a building block of heparin, an anticoagulant polysaccharide produced by our bodies.1. Equimolar HCl2. Heat, toluene 1M HCl/H2OD2G H−H2O8.3.1.Draw the structure of G including the stereochemistry.Draw H as the more stable 6-membered ring containing isomer using the ringskeleton. Indicate with a wavy line if absolute chirality around a carbon is notknown.。
