临床监查员专业术语、缩略语中英对照表
临床试验常用术语及缩略语

临床试验常用术语及缩略语监管机构和法规相关◆NMPA国家药品监督管理局(National Medical Products Administration)◆CDE国家药品监督管理局药品审评中心(CENTER FOR DRUG EVALUATION,NMPA)◆国家药品不良反应监测中心(National Center for ADR Monitoring,China)◆GCP药物临床试验质量管理规范(Good Clinical Practice)◆GMP药品生产质量管理规范(GoodManufacturingPractice)◆GLP药物非临床研究质量管理规范(Good Laboratory Practice of drug)◆ICH人用药品注册技术要求国际协调会议(InternationalConference on Harmonization)◆ICH GCP:指导原则的目的是为欧盟、日本和美国提供统一的标准,以促进这些管理当局在其权限内相互接受临床数据。
本指导原则的发展考虑了欧盟、日本、美国,以及澳大利亚、加拿大、北欧国家和世界卫生组织(GCP)的现行GCP。
◆WHO世界卫生组织(World Health Organization)◆FDA美国食品与药品管理局(Food and Drug Administration)文件相关◆CTP临床试验方案(Clinical Trial Protocol):指说明临床试验目的、设计、方法学、统计学考虑和组织实施的文件。
试验方案通常还应当包括临床试验的背景和理论基础,该内容也可以在其他参考文件中给出。
试验方案包括方案及其修订版。
◆SOP标准操作规程(Standard Operating Procedure):指为保证某项特定操作的一致性而制定的详细的书面要求。
◆TMF试验主文件夹/研究管理文件夹(Trial Master File)◆IB研究者手册(Investigator’s Brochure):指与开展临床试验相关的试验用药品的临床和非临床研究资料汇编。
常用临床试验缩写
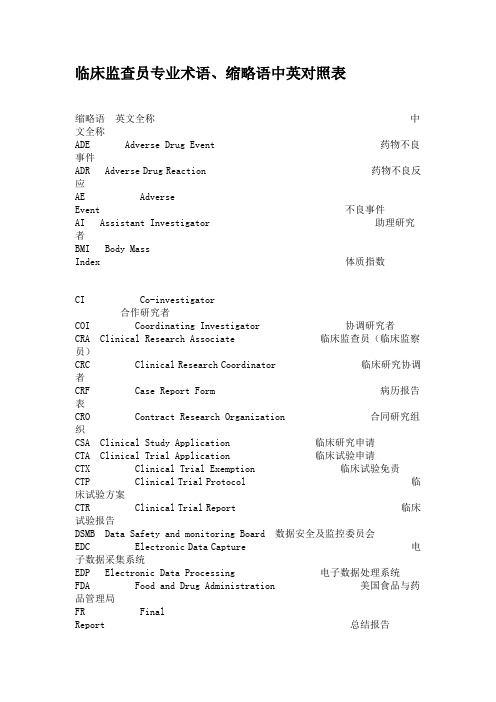
临床监查员专业术语、缩略语中英对照表缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE AdverseEvent 不良事件AI Assistant Investigator 助理研究者BMI Body MassIndex 体质指数CI Co-investigator合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate 临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FDA Food and Drug Administration 美国食品与药品管理局FR FinalReport 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规范GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure研究者手册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部NDA New Drug Application 新药申请NEC New DrugEntity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)PI PrincipalInvestigator 主要研究者PL ProductLicense 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QC QualityControl 质量控制RA RegulatoryAuthorities 监督管理部门SA SiteAssessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD SubjectDiary 受试者日记SFDA State Food and Drug Administration 国家食品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议ActiveControl 阳性对照、活性对照Audit 稽查Audit Report 稽查报告Auditor 稽查员Blank Control 空白对照Blinding/masking 盲法/设盲Case History 病历Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance依从性Coordinating Committee 协调委员会Cross-over Study 交叉研究Double Blinding 双盲Endpoint Criteria/measurement 终点指标Essential Documentation 必需文件Exclusion Criteria 排除标准Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection检察/视察Institution Inspection 机构检察Investigational Product 试验药物Investigator 研究者Monitor 监查员(监察员)Monitoring监查(监察)Monitoring Plan 监查计划(监察计划)Monitoring Report 监查报告(监察报告)Multi-center Trial 多中心试验Non-clinical Study 非临床研究Original Medical Record 原始医疗记录Outcome Assessment 结果评价Patient File 病人档案Patient History 病历Placebo 安慰剂Placebo Control 安慰剂对照Preclinical Study 临床前研究Protocol试验方案Protocol Amendments 修正案Randomization 随机Reference Product 参比制剂Sample Size 样本量、样本大小Seriousness 严重性Severity严重程度Single Blinding 单盲Sponsor 申办者StudyAudit 研究稽查Subject 受试者Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募StudySite 研究中心Subject Screening Log 受试者筛选表System Audit 系统稽查Test Product 受试制剂Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Triple Blinding 三盲Wash-out洗脱Wash-out Period 洗脱期。
临床试验缩略语中英文对照

ALB 白蛋白
ALT 丙氨酸氨基转移酶
AST 天门冬氨酸氨基转移酶
ATP 三磷酸腺苷
AUC 血浆药物浓度-时间曲线下面积BP 血压
bid 每日两次
BUN 尿素氮
CK-MB 肌酸激酶
Cr 肌酐
CR 完全缓解
CRF 病例报告表
CL/F 口服给药后血浆中的系统清除率Cmax 峰浓度
DBIL 直接胆红素
dL 分升
DLT 剂量限制毒性
EC 伦理委员会
EGFR 表皮生长因子受体
GCP 临床试验规范
GGT 谷氨酰转移酶
Hb 血红蛋白
HED 人体等效剂量
HR 心率
IB 研究者手册
IBIL 间接胆红素
INR 国际标准化值
ITT 治疗意图
IU 国际单位
LVEF 左心室射血分数
MTD 最大耐受药物剂量
NCI-CTC 国立癌症研究所通用毒性标准NSCLC 非小细胞肺癌
NOAEL 未见毒性反应的剂量
ORR 客观有效率
PFS 无进展生存期
PR 部分缓解
PK 药代动力学
qd 每日一次
SAE 严重不良事件
SAP 统计分析计划
TBIL 总胆红素
TP 总蛋白
TSH 促甲状腺激素
TKIs 酪氨酸激酶抑制剂Tmax 达峰时间
T1/2 血浆消除半衰期UNL 正常值上限WBC 白细胞计数。
CRA专业术语中英文对照

缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate 临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CSA Clinical Study Agreement 临床研究协议CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FA Financial Agreement 财务协议FDA Food and Drug Administration美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规范GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure 研究者手册IC Informed Consent 知情同意ICF Informed Consent Form知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SFDA State Food and Drug Administration国家食品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议Active Control 阳性对照、活性对照Audit稽查Audit Report 稽查报告Auditor 稽查员Blank Control 空白对照Blinding/masking 盲法/设盲Case History 病历Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance 依从性Coordinating Committee 协调委员会Cross-over Study 交叉研究Double Blinding双盲Endpoint Criteria/measurement 终点指标Essential Documentation 必需文件Exclusion Criteria 排除标准Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Investigational Product 试验药物Investigator 研究者Monitor 监查员(监察员)Monitoring 监查(监察)Monitoring Plan 监查计划(监察计划)Monitoring Report 监查报告(监察报告)Multi-center Trial 多中心试验Non-clinical Study 非临床研究Original Medical Record 原始医疗记录Outcome Assessment 结果评价Patient File 病人档案Patient History 病历Placebo 安慰剂Placebo Control 安慰剂对照Preclinical Study 临床前研究Protocol 试验方案Protocol Amendments 修正案Randomization 随机Reference Product 参比制剂Sample Size 样本量、样本大小Seriousness 严重性Severity 严重程度Single Blinding 单盲Sponsor申办者Study Audit 研究稽查Subject 受试者Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Study Site 研究中心Subject Screening Log 受试者筛选表System Audit 系统稽查Test Product 受试制剂Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Triple Blinding 三盲Wash-out 洗脱Wash-out Period 洗脱期。
临床英语术语缩写表

临床研究常用术语缩写表SOP 类型缩写表OP 操作规程Operating Procedures WI 工作指南Work InstructionsTP 模板TemplateFM 批准的标准表格Approved Standard FormsOD 其他文件Other Documents业务部门缩写表/ Functional Area Abbreviation Table:BS 生物统计BiostatisticsBD 业务拓展Business DevelopmentCM 临床监查/运营Clinical Monitoring/OperationDM 数据管理Data ManagementIT 信息技术Information TechnologyMS 医学科学服务Medical Science ServicePM 项目管理Project ManagementQA 质量保证Quality AssuranceRM 记录管理Records ManagementRA 注册事务Regulatory AffairsSM SOP 管理SOP ManagementST 研究中心管理服务Site Management ServiceTR 培训Training试验主文档:(TMF)Trial Master FilePMF 项目管理文件夹Project Management FileCCF 申办方临床研究文件夹Central Clinical FileCIF 申办方-研究者文件夹Central Investigator FileISF 研究者文件夹Investigator Site FileBSF 生物统计学文件夹Biostatistics Study FileDMSF 数据管理研究文件夹Data Management Study File。
CRA专业术语中英文对照

缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate 临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CSA Clinical Study Agreement 临床研究协议CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FA Financial Agreement 财务协议FDA Food and Drug Administration美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规范GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure 研究者手册IC Informed Consent 知情同意ICF Informed Consent Form知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SFDA State Food and Drug Administration国家食品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议Active Control 阳性对照、活性对照Audit稽查Audit Report 稽查报告Auditor 稽查员Blank Control 空白对照Blinding/masking 盲法/设盲Case History 病历Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance 依从性Coordinating Committee 协调委员会Cross-over Study 交叉研究Double Blinding双盲Endpoint Criteria/measurement 终点指标Essential Documentation 必需文件Exclusion Criteria 排除标准Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Investigational Product 试验药物Investigator 研究者Monitor 监查员(监察员)Monitoring 监查(监察)Monitoring Plan 监查计划(监察计划)Monitoring Report 监查报告(监察报告)Multi-center Trial 多中心试验Non-clinical Study 非临床研究Original Medical Record 原始医疗记录Outcome Assessment 结果评价Patient File 病人档案Patient History 病历Placebo 安慰剂Placebo Control 安慰剂对照Preclinical Study 临床前研究Protocol 试验方案Protocol Amendments 修正案Randomization 随机Reference Product 参比制剂Sample Size 样本量、样本大小Seriousness 严重性Severity 严重程度Single Blinding 单盲Sponsor申办者Study Audit 研究稽查Subject 受试者Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Study Site 研究中心Subject Screening Log 受试者筛选表System Audit 系统稽查Test Product 受试制剂Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Triple Blinding 三盲Wash-out 洗脱Wash-out Period 洗脱期ical Research Associatejing, Shanghai, Guangzhou, Wuhan, Chengdu, Shengshanges:otocol compliance, ICH-GCP and other regulatory obligations at trial sites by standardized ctivitiesccuracy and authenticity of clinical data, manage investigational products and other trial lies, maintain adequate clinical trial documentation etcctivities in line with milestones (i.e. start-up, monitoring, closeout, etc.) in compliance with Ps and ICH-GCPd communication between sponsor and investigator, report related issues to the line mediatelye timely reporting of study information, which includes safety reporting to relevant study er and regulatory authoritiesrelated to clinical trial monitorings:elor or above in medical or above, majoring in medicine or pharmacy or relatedcient in office software, e.g word, excel, PPT etc.d communications and coordination skillsd written and oral Englishrience in CRO is preferredHome | About H&J | News | Services | Regulations | Our clients | CRA | China CRO。
临床研究专业术语缩略语中英对照表

临床研究专业术语缩略语中英对照表为了方便专业人士在临床研究领域的交流和理解,本文提供了临床研究中常见的专业术语的缩略语中英对照表。
这些缩略语旨在简化长句和复杂术语,使他们更易于书写和快速交流。
以下是常见临床研究专业术语及其相应的缩略语及中英文对照:1. Adverse Event(AE)-- 不良事件2. Serious Adverse Event(SAE)-- 严重不良事件3. Placebo(PBO)-- 安慰剂4. Randomized Controlled Trial(RCT)-- 随机对照试验5. Informed Consent(IC)--知情同意6. Institutional Review Board(IRB)-- 伦理审查委员会7. Case Report Form(CRF)-- 病例报告表8. Data Monitoring Committee(DMC)-- 数据监测委员会9. Risk-Benefit Ratio(RBR)-- 风险-效益比10. Investigational New Drug(IND)-- 新药研究11. Good Clinical Practice(GCP)-- 良好临床实践12. Adverse Drug Reaction(ADR)-- 不良药物反应13. Intent-to-Treat(ITT)-- 治疗意向分析14. Protocol Violation(PV)-- 方案违规15. Sponsor-Investigator(SI)-- 研究发起人16. Standard Operating Procedures(SOPs)-- 标准操作规程17. Confidentiality Agreement(CA)-- 保密协议18. Data and Safety Monitoring Board(DSMB)-- 数据和安全监测委员会19. Clinical Research Coordinator(CRC)-- 临床研究协调员20. Declaration of Helsinki(DoH)--《赫尔辛基宣言》以上是临床研究专业术语的缩略语中英对照表。
临床研究专业术语缩略语中英对照表

临床研究专业术语缩略语中英对照表研究领域日新月异,医学领域也不例外。
临床研究作为医学研究的重要组成部分,涉及到众多专业术语和缩略语,对于研究人员和医学学生来说,了解和掌握这些术语的中英对照表具有重要意义。
下面将为大家介绍一些常见的临床研究专业术语缩略语的中英对照表。
1. 临床研究常用术语- Randomized Controlled Trial (RCT):随机对照试验- Informed Consent:知情同意- Double-blind:双盲- Placebo:安慰剂- Control Group:对照组- Experimental Group:实验组- Sample Size:样本量- Follow-up:随访- Protocol:方案- Ethics Committee:伦理委员会- Adverse Events:不良事件- Confidentiality:保密性2. 临床试验阶段术语临床试验一般分为四个阶段,每个阶段有其特定的目标和性质。
- Phase 1 Clinical Trial:一期临床试验- Phase 2 Clinical Trial:二期临床试验- Phase 3 Clinical Trial:三期临床试验- Phase 4 Clinical Trial:四期临床试验3. 常见疾病和症状在临床研究中,涉及到各种各样的疾病和症状的缩略语。
- AIDS:艾滋病- COPD:慢性阻塞性肺疾病- IBS:肠易激综合征- CAD:冠心病- MI:心肌梗死- CHF:充血性心力衰竭- TIA:短暂性脑缺血发作- PMS:经前紧张综合征- GERD:胃食管反流病- UTI:尿路感染- COPD:慢性阻塞性肺病- IBD:炎症性肠病- CVD:心血管疾病- CNS:中枢神经系统- RA:类风湿性关节炎4. 诊断工具缩略语在临床研究中,很多诊断工具和设备都有一些特定的缩略语。
- ECG:心电图- CT:计算机断层扫描- MRI:磁共振成像- PET:正电子发射计算机断层扫描- CBC:全血细胞计数- Pap Smear:宫颈抹片- EEG:脑电图- X-ray:X射线- EMG:肌电图- ABG:动脉血气分析- LFT:肝功能检查- BMP:基础血液检查- PSA:前列腺特异性抗原- CXR:胸部X射线- DEXA:双能X射线吸收法- MRA:磁共振血管成像5. 药物和治疗方法缩略语在临床研究中,药物和治疗方法也有很多缩略语。
CRA专业术语中英文对照

缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate 临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CSA Clinical Study Agreement 临床研究协议CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FA Financial Agreement 财务协议FDA Food and Drug Administration美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规范GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure 研究者手册IC Informed Consent 知情同意ICF Informed Consent Form知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部只有凭借毅力,坚持到底,才有可能成为最后的赢家。
CRA专业术语中英文对照

CRA专业术语中英文对照缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件是指药物治疗过程中所发生的任何不幸的医疗卫生事件,而这种事件不一定与药物有因果关系。
药品不良事件包括药品标准缺陷、药品质量问题、药品不良反应、用药失误以及药品滥用。
ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件在用药病人或临床试验受试者中发生的任何不幸医疗事件,他不一定要与治疗有因果关系AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator 合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate 临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CSA Clinical Study Agreement 临床研究协议CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FA Financial Agreement 财务协议FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规范GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure 研究者手册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive V oice Response System 互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SFDA State Food and Drug Administration 国家食品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议Active Control 阳性对照、活性对照Audit 稽查Audit Report 稽查报告Auditor 稽查员Blank Control 空白对照Blinding/masking 盲法/设盲Case History 病历Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance 依从性Coordinating Committee 协调委员会Cross-over Study 交叉研究Double Blinding 双盲Endpoint Criteria/measurement 终点指标Essential Documentation 必需文件Exclusion Criteria 排除标准Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Investigational Product 试验药物Investigator 研究者Monitor 监查员(监察员)Monitoring 监查(监察)Monitoring Plan 监查计划(监察计划)Monitoring Report 监查报告(监察报告)Multi-center Trial 多中心试验Non-clinical Study 非临床研究Original Medical Record 原始医疗记录Outcome Assessment 结果评价Patient File 病人档案Patient History 病历Placebo 安慰剂Placebo Control 安慰剂对照Preclinical Study 临床前研究Protocol 试验方案Protocol Amendments 修正案Randomization 随机Reference Product 参比制剂Sample Size 样本量、样本大小Seriousness 严重性Severity 严重程度Single Blinding 单盲Sponsor 申办者Study Audit 研究稽查Subject 受试者Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Study Site 研究中心Subject Screening Log 受试者筛选表System Audit 系统稽查Test Product 受试制剂Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Triple Blinding 三盲Wash-out 洗脱Wash-out Period 洗脱期。
临床监察员专业术语和职位英文描述

缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator 合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate 临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规范GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure 研究者手册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部 NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划药物临床试验网受试者招募SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SFDA State Food and Drug Administration 国家食品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议Active Control 阳性对照、活性对照Audit 稽查Audit Report 稽查报告Auditor 稽查员Blank Control 空白对照Blinding/masking 盲法/设盲Case History 病历Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance 依从性Coordinating Committee 协调委员会Cross-over Study 交叉研究Double Blinding 双盲Endpoint Criteria/measurement 终点指标Essential Documentation 必需文件Exclusion Criteria 排除标准Inclusion Criteria 入选标准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察 copyright Institution Inspection 机构检察Investigational Product 试验药物Investigator 研究者Monitor 监查员(监察员)Monitoring 监查(监察)Monitoring Plan 监查计划(监察计划) Monitoring Report 监查报告(监察报告) Multi-center Trial 多中心试验Non-clinical Study 非临床研究Original Medical Record 原始医疗记录Outcome Assessment 结果评价Patient File 病人档案Patient History 病历Placebo 安慰剂创始人袁旭Placebo Control 安慰剂对照Preclinical Study 临床前研究Protocol 试验方案Protocol Amendments 修正案Randomization 随机Reference Product 参比制剂Sample Size 样本量、样本大小Seriousness 严重性Severity 严重程度Single Blinding 单盲Sponsor 申办者Study Audit 研究稽查Subject 受试者Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Study Site 研究中心Subject Screening Log 受试者筛选表System Audit 系统稽查Test Product 受试制剂Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Triple Blinding 三盲Wash-out 洗脱Wash-out Period 洗脱期introductionQuintiles Transnational Corp. helps improve healthcare worldwide by providing a broad range of professional services, information and partnering solution s to the pharmaceutical, biotechnology and healthcare industries. Quintiles helps its customers compress the time it takes to bring a drug from discovery through regulatory approval; accelerate the launch of products to peak sales, build effective sales forces and manage product portfolios more efficiently; and achieve strategic and financial objectives by offering tailored alternatives to traditional fee-for-service product development and commercial services agreements. Headquartered near Research Triangle Park, North Carolina, Quintiles was founded in 1982 and hasmore than 16,000 employees and offices in over 50 countries. Quintiles Medical Development (Shanghai) Co., Ltd. is a wholly owned subsidiary of Quintiles Transnational Corp. Further information, please visit our global websiteJob title:Clinical ProjectManager(临床项目经理)RESPONSIBILITIESManage and co-ordinate efforts of cross-functional project teams to support milestone achievement and to manage study issues and obstacles and ensure consistent use of study tools and training materials and compliance with standard processes, policies and procedures.Develop study management plans, together with team assignments and accountabilities and oversight of database maintenance.Serve as primary project contact with Sponsor to ensure communication is maintained and reporting schedules are adhered to.Collect information on team performance against contract, customer expectations, and project baselines.Lead problem solving and resolution efforts to include management of risk, contingencies and issues.Identify quality issues within the study to implement appropriate corrective action plans. Escalate findings and action plans to appropriate parties.Provide input for the development of proposals for new work and manage project budgets.Provide input to line managers of their project team members' performance relative to project tasks.Prepare and present project information at internal and external meetings. Participate in proposal development and in the bid-defense process with guidance and supervision.Ensure high performance and efficiency of the clinical team through the scheduling of co-monitoring/accompanied site/visits and ongoing mentoring of CRA team. REQUIRED KNOWLEDGE, SKILLS AND ABILITIESIn depth knowledge of, and skill in applying, applicable clinical research regulatory requirements; i.e., Good Clinical Practice (GCP) and International Conference on Harmonisation (ICH) guidelinesGood therapeutic and protocol knowledgeStrong communication and interpersonal skills, including good command of English languageGood problem solving skillsDemonstrated ability to deliver results to the appropriate quality and timeline metricsGood teamwork skillsExcellent customer service skillsGood presentation skillsGood judgmentStrong software and computer skills, including MS Office applicationsAbility to establish and maintain effective working relationships with coworkers, managers and clientsMINIMUM REQUIRED EDUCATION AND EXPERIENCEBachelor's degree in life sciences or related field and 5 years' clinical research experience including 2 years' project management experience and experience in clinical operations; or equivalent combination of education, training and experience. Job title:Clinical Trials AssistantLocation:BeijingResponsibilitiesAssist Clinical Team Lead (CTL) and Clinical Research Associates (CRAs) with accurately updating and maintaining clinical systems that track site compliance and performance within project timelines.Assist the clinical team in the preparation, handling, distribution, filing, and archiving of clinical documentation and reports according to the scope of work and standard operating procedures. Assist with periodic review of study files for accuracy and completeness.Assist CTLs with preparation, handling and distribution of Clinical Trial Supplies and maintenance of tracking information.Assist with the tracking and management of Case Report Forms (CRFs), queries and clinical data flow. Act as a central contact for the clinical team for designated project communications, correspondence and associated documentation.May perform assigned administrative tasks to support team members with clinical trial execution.All responsibilities are essential job functions unless noted as nonessential (N).Required knowledge, skills and abilitiesAwareness of knowledge of applicable clinical research regulatory requirements; i.e., Good Clinical Practice (GCP) and International Conference on Harmonisation (ICH) guidelinesKnowledge of applicable protocol requirements as provided in company trainingComputer skills including proficiency in use of Microsoft Word, Excel and PowerPointStrong written and verbal communication skills including good command of English languageEffective time management and organizational skillsAttention to detail and accuracy in workAbility to establish and maintain effective working relationships with coworkers, managers and clients Minimum required education and experienceSchool diploma/certificate or educational equivalent; or equivalent combination of education, training and experienceJob title:Clinical research AssistantDescriptionQuintiles pioneered the idea of helping pharma companies conduct objective clinical trials to establish not only whether a drug is effective, but who can take it safely. Our customers have relied on us to design and conduct rigorous clinical research for decades, from small studies to multinational mega-trials. However, we continue to develop new ways of interpreting and evaluating data that allow us to create more effective trials and determine outcomes faster.If that is your passion, we have a place for you.Job Responsibilities:- Oversees the progress of a Clinical Trial, ensuring that it is conducted, recorded and reported in accordance with the protocol, Standard Operating Procedures (SOPs), Good Clinical Practice (GCP) and the applicable Regulatory requirements.- Administer protocol and related study training to assigned site.- Establish regular lines of communication with sites to manage ongoing project expectations and issues.QualificationsRequirements:- Degree in Science, with a major in Pharmacy, Biological / Life Sciences or Nursing from a recognized tertiary institution- Minimum 1 year of clinical monitoring experience in the pharmaceutical / CRO industry.- For Senior Research Associate position, minimum 3 years clinical monitoring experience, preferably with some experience in leading clinical teams in the region- Good knowledge of drug development process, clinical trial monitoring procedures, medical terminology and GCP/ICH guidelines- Excellent organizational and problem solving skills- Strong written and verbal communication skills- Ability to travel when requiredJob title:Senior Clinical Project ManagerDescriptionQuintiles pioneered the idea of helping pharma companies conduct objective clinical trials to establish not only whether a drug is effective, but who can take it safely. Our customers have relied on us to design and conduct rigorous clinical research for decades, from small studies to multinational mega-trials. However, we continue to develop new ways of interpreting and evaluating data that allow us tocreate more effective trials and determine outcomes faster.If that is your passion, we have a place for you.Responsibilities- Manage and co-ordinate efforts of cross-functional project teams to support milestone achievement and to manage study issues and obstacles. Ensure consistent use of study tools and training materials and compliance with standard processes, policies and procedures. Implement continuous improvement activities for assigned projects.- Develop study management plans, together with team assignments and accountabilities and oversight of database maintenance.- Serve as primary project contact with Sponsor to ensure communication is maintained and continuously improved and reporting schedules are adhered to.- Report on team performance against contract, customer expectations, and project baselines to management.- Lead problem solving and resolution efforts to include management of risk, contingencies and issues. Develop proactive contingency plans to mitigate clinical risk.- Identify quality issues within the study through regular review of site communications, monitoring visit reports, data flow information and quality assurance audit findings to implement appropriate corrective action plans. Escalate findings and action plans to appropriate parties.- Collaborate with other functional groups within the company where necessary to support milestone achievement and to manage study issues and obstacles.- Provide input for the development of proposals for new work and project budgets.- Provide input to line managers of their project team members performance relative to project tasks. Recommend team members further professional development. Support staff development. Mentor less experienced CPMs.- Ensure high performance and efficiency of the clinical team through the scheduling of co-monitoring/accompanied site visits and ongoing mentoring of CRA team.- Prepare and present project information at internal and external meetings.- Participate in proposal development. May lead bid defense presentations in partnership with Business Development and Senior Clinical Project Management staff.- Define project workloads and assignments. Develop and oversee maintenance of internal databases and project plansQualifications- Bachelors degree in life sciences or related field and 7 years clinical research experience including 4 years project management experience and experience in clinical operations; or equivalent combination of education, training and experience.- In depth knowledge of, and skill in applying, applicable clinical research regulatory requirements; i.e., Good Clinical Practice (GCP) and International Conference on Harmonisation (ICH) guidelines- In depth therapeutic and protocol knowledge- Strong communication and interpersonal skills, including good command of English language- Strong organizational and problem solving skills- Demonstrated ability to deliver results to the appropriate quality and timeline metrics- Good team leadership skills- Effective mentoring and training skills- Excellent customer service skills- Effective presentation skills- Ability to manage competing priorities- Strong software and computer skills, including MS Office applications- Ability to establish and maintain effective working relationships with coworkers, managers and clients.Job title:Manager, Clinical Operations (China - Beijing)DescriptionQuintiles pioneered the idea of helping pharma companies conduct objective clinical trials to establish not only whether a drug is effective, but who can take it safely. Our customers have relied on us to design and conduct rigorous clinical research for decades, from small studies to multinational mega-trials. However, we continue to develop new ways of interpreting and evaluating data that allow us to create more effective trials and determine outcomes faster.If that is your passion, we have a place for you.Job Responsibilities:- Responsible to lead and manage the project team on regional or global studies- Responsible for designing and overseeing the implementation of project specific procedures to ensure that the study goals are met- Participate in clinical operations quality or process initiatives.Qualifications- Degree in Science, with a major in Pharmacy, Biological / Life Sciences or Nursing from a recognized tertiary institution- At least 7 years of experience working on clinical trials with 3 years experience in a leadership capacity.- Advanced knowledge and ability to apply GCP/ICH and applicable regulatory guidelines- Strong leadership skills- Excellent organizational and problem solving skills- Effective time management skills and ability to manage competing priorities- Strong written and verbal communication skills- Able to travel when required临床实验助理Job Responsibilities:1、Assist Clinical Team Lead (CTL) and Clinical Research Associates (CRAs) with accurately updating and maintainingclinical systems that track site compliance and performance within project timelines.2、Assist the clinical team in the preparation, handling, distribution, filing, and archiving of clinical documentation and reports according to the scope of work and standard operating procedures. Assist with periodic review of study files for accuracy and completeness.3、Assist CTLs with preparation, handling and distribution of Clinical Trial Supplies and maintenance of tracking information.Assist with the tracking and management of Case Report Forms (CRFs), queries and clinical data flow.4、Act as a central contact for the clinical team for designated project communications, correspondence and associated documentation.5、Provide administrative support to team members with clinical trial execution. Requirements:1、Awareness of knowledge of applicable clinical research regulatory requirements;i.e.,Good Clinical Practice (GCP) and International Conference on Harmonisation (ICH) guidelines.2、Knowledge of applicable protocol requirements as provided in company training. Computer skills including proficiency in use of Microsoft Word, Excel and PowerPoint.3、Strong written and verbal communication skills including good command of English language4、Attention to detail and accuracy in work.5、Diploma or bachelor degree;or equivalent combination of education, training and experienceCRASkills• Strong computer and internet skills including knowledge of MS-Office products such as Excel,Word• Strong regulatory knowledge including GCP• Excellent interpersonal, verbal and written communication skills• Sound problem solving skills• Ability to successfully work in a (‘virtual’) team environment• Sound presentation skills• Consultative skills• Client focused approach to work with the ability to interact professionally within a clientorganization• Ability to prioritize multiple tasks and achieve project timelines• Able to take initiative and work independently• Sense of urgency in completing assigned tasks• Able to travel a minimum of 65% on average• Holds a driving license where required• Effective time management in order to meet daily metrics or team objectives• Shows commitment to and performs consistently high quality workEducation• Educated to degree level (biological science, pharmacy or other health-related discipline preferred), equivalent nursing qualification or other equivalent experienceLanguage Skills• Competent in written and oral English.Minimum Work Experience• Previous work experience (e.g. CR Assistant, nursing, laboratory, data management) would beadvisable药品安全经理岗位职责:1、建立药物安全工作的SOP体系1)建立与药物安全有关的不良事件收集、员工和研究者培训、严重不良事件报告、定期不良事件更新报告、临床方案和报告审阅、说明书审阅、文档管理等相关SOP体系;2)进行年度SOP回顾,必要时进行更新2、管理和培训1)对药物安全专员进行工作指导、检查、培训、辅导和业务管理;2)根据需要对公司相关部门员工、研究者和CRO公司CRA进行相关SOP培训,并记录培训结果;3)与公司相关部门协调建立建全不良事件收集渠道;4)制定ACN药物安全工作的发展规划和年度计划;3、审阅临床研究方案、ICF/CRF、定期安全更新报告、临床报告和说明书1)了解国内外药品临床开发的进展及相关资料;2)参与临床试验方案的讨论,并给出有关药物安全性方面的意见和建议;3)审阅临床试验方案(包括PMS)、ICF和CRF,给出合理的意见和建议,并签字确认;4)审阅并签发药物安全专员准备的定期安全更新报告5)审阅临床试验报告和说明书的安全性内容或信息,并给出合理的意见或修改建议;4、临床试验过程中不良事件收集和严重不良事件报告1)根据CRA(CRO公司)提供的严重不良事件报告表准备英文报告,并在规定时限内向API PV报告;2)对严重不良事件进行跟踪,并及时向API PV报告;3)对于ACN发起的临床研究,DSO应将方案中有关药品安全性的内容以英文报告给API PV, 在取得临床研究报告后,将报告中药物安全性部部分的内容以英文报告给API PV.4)对于由研究者发起并得到ACN支持的研究,督促研究者及时向DSO报告研究过程中的不良事件,DSO在规定的时间内向API PV提交AE报告;5)在临床试验过程中向SFDA, 试验基地及伦理委员会报告SUSAR Case; 6)与临床开发部门和CRO公司进行良好的沟通以取得支持;5、其他1)协助注册人员准备多中心临床试验的风险控制计划;2)与API PV部门进行有效的沟通与交流;3)接受API PV或监理管机构的审计4)定期接受有关药物安全的培训,了解有关药物安全的最新动态和知识;5)完成部门经理交办的其他任务工作要求:知识技能■药理学、或临床医学相关专业学士以上学位;■与临床有关的高等知识(病理生理学、诊断、内科学、临床药理学等);■优秀的领导和沟通协调能力■计划能力,分析和解决问题能力;■良好的计算机、人际关系技能;■能够清晰、简洁、有效地通过电话和书面进行沟通;■英语口语和书写流利;工作经验■ 5年及以上药物安全相关工作经验和管理经验;■跨国制药公司工作经历;■对药品安全有关法规和指南有正确的理解;■与药品警戒领域的专家能够进行良好的沟通和联络;。
临床监查员专业术语、缩略语中英对照表
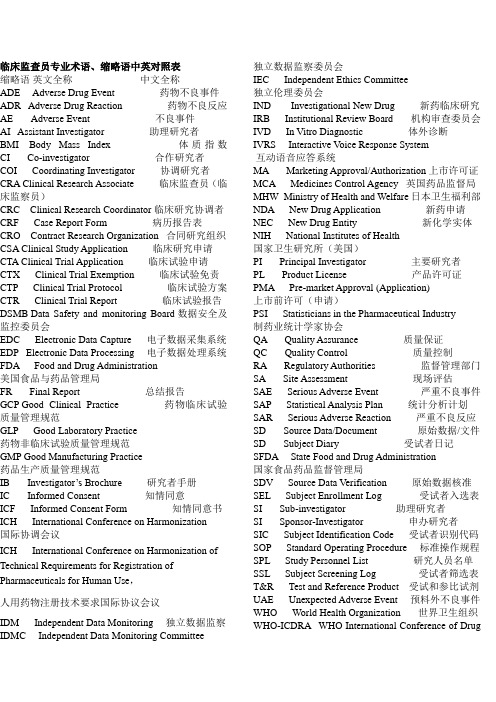
临床监查员专业术语、缩略语中英对照表缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator 合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate 临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FDA Food and Drug Administration美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice药物非临床试验质量管理规范GMP Good Manufacturing Practice药品生产质量管理规范IB Investigat or’s Brochure研究者手册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议ICH International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use,人用药物注册技术要求国际协议会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive V oice Response System互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare日本卫生福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health国家卫生研究所(美国)PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre-market Approval (Application)上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry制药业统计学家协会QA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SFDA State Food and Drug Administration国家食品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO-ICDRA WHO International Conference of DrugRegulatory Authorities WHO国际药品管理当局会议Active Control 阳性对照、活性对照Audit 稽查Audit Report 稽查报告Auditor 稽查员Blank Control 空白对照Blinding/masking 盲法/设盲Case History 病历Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance 依从性Coordinating Committee 协调委员会Cross-over Study 交叉研究Double Blinding 双盲Endpoint Criteria/measurement 终点指标Essential Documentation 必需文件 Exclusion Criteria 排除标准 Inclusion Criteria 入选表准 Information Gathering 信息收集 Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Investigational Product 试验药物 Investigator 研究者Monitor 监查员(监察员) Monitoring 监查(监察) Monitoring Plan 监查计划(监察计划) Monitoring Report 监查报告(监察报告) Multi-center Trial 多中心试验Non-clinical Study 非临床研究 Original Medical Record 原始医疗记录 Outcome Assessment 结果评价 Patient File 病人档案 Patient History 病历 Placebo 安慰剂 Placebo Control 安慰剂对照 Preclinical Study 临床前研究 Protocol 试验方案 Protocol Amendments 修正案 Randomization 随机 Reference Product 参比制剂 Sample Size 样本量、样本大小 Seriousness 严重性 Severity 严重程度 Single Blinding 单盲 Sponsor 申办者 Study Audit 研究稽查Subject 受试者Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表Subject Identification Code List 受试者识别代码表 Subject Recruitment 受试者招募Study Site 研究中心Subject Screening Log 受试者筛选表System Audit 系统稽查Test Product 受试制剂Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Triple Blinding 三盲Wash-out 洗脱Wash-out Period 洗脱期。
CRA专业术语中英文对照

缩略语缩略语 英文全称英文全称 中文全称中文全称ADE Adverse Drug Event 药物不良事件药物不良事件ADR Adverse Drug Reaction 药物不良反应药物不良反应 AE Adverse Event 不良事件不良事件AI Assistant Investigator 助理研究者助理研究者BMI Body Mass Index 体质指数体质指数CI Co-investigator 合作研究者合作研究者COI Coordinating Investigator 协调研究者协调研究者CRA Clinical Research Associate 临床监查员(临床监察员)临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者临床研究协调者CRF Case Report Form 病历报告表病历报告表CRO Contract Research Organization 合同研究组织合同研究组织CSA Clinical Study Application 临床研究申请临床研究申请CSA Clinical Study Agreement 临床研究协议临床研究协议CTA Clinical Trial Application 临床试验申请临床试验申请CTX Clinical Trial Exemption 临床试验免责临床试验免责CTP Clinical Trial Protocol 临床试验方案临床试验方案CTR Clinical Trial Report 临床试验报告临床试验报告DSMB Data Safety and monitoring Board数据安全及监控委员会数据安全及监控委员会数据安全及监控委员会 EDC Electronic Data Capture 电子数据采集系统电子数据采集系统EDP Electronic Data Processing 电子数据处理系统电子数据处理系统FA Financial Agreement 财务协议财务协议FDA Food and Drug Administration美国食品与药品管理局美国食品与药品管理局 FR Final Report 总结报告总结报告GCP Good Clinical Practice 药物临床试验质量管理规范药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规范GMP Good Manufacturing Practice 药品生产质量管理规范药品生产质量管理规范IB Investigator ’s Brochure 研究者手册IC Informed Consent 知情同意知情同意ICF Informed Consent Form 知情同意书知情同意书ICH International Conference on Harmonization 国际协调会议国际协调会议IDM Independent Data Monitoring 独立数据监察独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会独立伦理委员会IND Investigational New Drug 新药临床研究新药临床研究IRB Institutional Review Board 机构审查委员会机构审查委员会IVD In Vitro Diagnostic 体外诊断体外诊断IVRS Interactive Voice Response System 互动语音应答系统互动语音应答系统MA Marketing Approval/Authorization 上市许可证上市许可证MCA Medicines Control Agency 英国药品监督局英国药品监督局MHW Ministry of Health and Welfare日本卫生福利部日本卫生福利部 NDA New Drug Application 新药申请新药申请NEC New Drug Entity 新化学实体新化学实体NIH National Institutes of Health 国家卫生研究所(美国)国家卫生研究所(美国)PI Principal Investigator 主要研究者主要研究者PL Product License 产品许可证产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)上市前许可(申请) PSI Statisticians in the Pharmaceutical Industry制药业统计学家协会制药业统计学家协会 QA Quality Assurance 质量保证质量保证QC Quality Control 质量控制质量控制RA Regulatory Authorities 监督管理部门监督管理部门SA Site Assessment 现场评估现场评估SAE Serious Adverse Event 严重不良事件严重不良事件SAP Statistical Analysis Plan 统计分析计划统计分析计划SAR Serious Adverse Reaction 严重不良反应严重不良反应SD Source Data/Document 原始数据/文件文件SD Subject Diary 受试者日记受试者日记SFDA State Food and Drug Administration国家食品药品监督管理局国家食品药品监督管理局 SDV Source Data Verification 原始数据核准原始数据核准SEL Subject Enrollment Log 受试者入选表受试者入选表SI Sub-investigator 助理研究者助理研究者SI Sponsor-Investigator 申办研究者申办研究者SIC Subject Identification Code 受试者识别代码受试者识别代码SOP Standard Operating Procedure 标准操作规程标准操作规程SPL Study Personnel List 研究人员名单研究人员名单SSL Subject Screening Log 受试者筛选表受试者筛选表T&R Test and Reference Product 受试和参比试剂受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件预料外不良事件WHO World Health Organization 世界卫生组织世界卫生组织WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议Active Control 阳性对照、活性对照阳性对照、活性对照Audit 稽查稽查Audit Report 稽查报告稽查报告Auditor 稽查员稽查员Blank Control 空白对照空白对照Blinding/masking 盲法/设盲设盲Case History 病历病历Clinical study 临床研究临床研究Clinical Trial 临床试验临床试验Clinical Trial Report 临床试验报告临床试验报告Compliance 依从性依从性Coordinating Committee 协调委员会协调委员会Cross-over Study 交叉研究交叉研究Double Blinding 双盲双盲Endpoint Criteria/measurement 终点指标终点指标Essential Documentation 必需文件必需文件Exclusion Criteria 排除标准排除标准Inclusion Criteria 入选表准入选表准信息收集Information Gathering 信息收集启动会议Initial Meeting 启动会议视察 Inspection 检察/视察机构检察Institution Inspection 机构检察试验药物Investigational Product 试验药物研究者Investigator 研究者监查员(监察员) Monitor 监查员(监察员)监查(监察) Monitoring 监查(监察)监查计划(监察计划) Monitoring Plan 监查计划(监察计划)监查报告(监察报告) Monitoring Report 监查报告(监察报告)多中心试验Multi-center Trial 多中心试验非临床研究Non-clinical Study 非临床研究原始医疗记录Original Medical Record 原始医疗记录结果评价 Outcome Assessment 结果评价病人档案Patient File 病人档案病历Patient History 病历安慰剂Placebo 安慰剂安慰剂对照Placebo Control 安慰剂对照临床前研究Preclinical Study 临床前研究试验方案Protocol 试验方案修正案Protocol Amendments 修正案随机Randomization 随机参比制剂Reference Product 参比制剂样本量、样本大小Sample Size 样本量、样本大小严重性Seriousness 严重性严重程度Severity 严重程度单盲Single Blinding 单盲申办者Sponsor申办者研究稽查Study Audit 研究稽查受试者Subject 受试者受试者入选Subject Enrollment 受试者入选受试者入选表Subject Enrollment Log 受试者入选表受试者识别代码表 Subject Identification Code List 受试者识别代码表受试者招募Subject Recruitment 受试者招募研究中心Study Site 研究中心受试者筛选表Subject Screening Log 受试者筛选表系统稽查System Audit 系统稽查受试制剂Test Product 受试制剂试验启动会议Trial Initial Meeting 试验启动会议试验总档案Trial Master File 试验总档案试验目的Trial Objective 试验目的三盲Triple Blinding 三盲洗脱Wash-out 洗脱洗脱期Wash-out Period 洗脱期。
临床专业术语缩写

ICF Informed Consent Form知情同意书
ICH International Conference on Harmonization国际协调会议
IDM Independent Data Monitoring独立数据监察
IDMC Independent Data Monitoring Committee独立数据监察委员会
NIH National Institutes of Health国家卫生研究所(美国)
PI Principal Investigator主要研究者
PL Product License产品许可证
PMA Pre-market Approval(Application)上市前许可(申请)
PSI Statisticians in the Pharmaceutical Industry制药业统计学家协会
Multi—center Trial多中心试验
Non—clinical Study非临床研究
Original Medical Record原始医疗记录
Outcome Assessment结果评价
Patient File病人档案
Patient History病历
Placebo安慰剂
Placebo Control安慰剂对照
Wash—out洗脱
Wash—out Period洗脱期
FR Final Report总结报告
GCP Good Clinical Practice药物临床试验质量管理规范
GLP Good Laboratory Practice药物非临床试验质量管理规范
GMP Good Manufacturing Practice药品生产质量管理规范
临床试验常用的英文缩略语

临床试验常用的英文缩略语(全免费)TP: time-to-progression 疾病进展时间SAE: severity Adverse Event严重不良事件AE:Adverse Event不良事件SOP: Standard Operating Procedure标准操作规程CRF: Case Report form病例报告表DLT:剂量限制毒性MTD:最大耐受剂量KPS: Karnofsky Performance Status行为状态评分CR:complete response完全缓解PR:partial response部分缓解SD:病情稳定PD:progressive disease病情进展CTC:常用药物毒性标准IEC: independent ethics committee 独立伦理委员会IRB : institutional review board 伦理委员会CRA:临床研究助理CRO: Contract Research Organization合同研究组织DFS: Disease Free Survival无病生存期OS:(Overall Survival)总生存时间IC:Informed consent知情同意ADR: Adverse Drug Reaction不良反应GAP:Good Agricultural Practice 中药材种植管理规范GCP:Good Clinical Practice 药物临床试验质量管理规范GLP:Good Laboratory Practice 药品实验室管理规范GMP:Good Manufacturing Practice 药品生产质量管理规范GSP:Good Supply Practice 药品经营质量管理规范GUP:Good Use Practice 药品使用质量管理规范PI :Principal investigator主要研究者CI:Co-inveatigator合作研究者SI :Sub-investigator助理研究者COI :Coordinating investigtor协调研究者DGMP:医疗器械生产质量管理规范ICF:Informed consent form知情同意书RCT : randomized controlled trial, 随机对照试验NRCCT:non-randomized concurrent controlled trial, 非随机同期对照试验EBM: evidence-based medicine循证医学RCD:randomized cross-over disgn随机交*对照试验HCT:historial control trial, 历史对照研究RECIST:Response Evaluation Criteria In Solid Tumors. 实体瘤疗效反应的评价标准QC: Quality Control质量控制UADR: Unexpected Adverse Drug Reaction,非预期药物不良反应代表含义: 指对所有与有关的要求、GCP及现行管理法规的遵从。
临床试验常用术语缩写

专业术语缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator 合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织ECRF 电子化病历报告表CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure研究者手册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ECG Electrocardiogram心电图ICH International Conference on Harmonization 国际协调会IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断MA Marketing Approval/Authorization 上市许可证IVRS Interactive V oice Response System 互动语音应答系统MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国) PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记Subject identification code (SIC)受试者识别代码SFDA State Food and Drug Administration 国家食品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码pd pharmacodynamics药物效应动力学SOP Standard Operating Procedure 标准操作规程pk pharmacokinetics药物代谢动力学SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织Active Control 阳性对照、活性对照WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议Unexpected adverse event (UAE)预料外不良事件Audit 稽查Audit Report 稽查报告Auditor 稽查员Blank Control 空白对照Blinding/masking 盲法/设盲Case History 病历Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance 依从性Coordinating Committee 协调委员会Cross-over Study 交叉研究Double Blinding 双盲Endpoint Criteria/measurement 终点指标Essential Documentation 必需文件Exclusion Criteria 排除标准Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Investigational Product 试验药物Investigator 研究者Monitor 监查员(监察员) Monitoring 监查(监察) Monitoring Plan 监查计划(监察计划)Monitoring Report 监查报告(监察报告) Multi-center Trial 多中心试验Non-clinical Study 非临床研究Original Medical Record 原始医疗记录Outcome Assessment 结果评价Patient File 病人档案Patient History 病历Placebo 安慰剂Placebo Control 安慰剂对照Preclinical Study 临床前研究Protocol 试验方案Protocol Amendments 修正案Randomization 随机Reference Product 参比制剂Sample Size 样本量、样本大小Seriousness 严重性Severity 严重程度Single Blinding 单盲Sponsor 申办者Study Audit 研究稽查Subject 受试者Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Subject Screening Log 受试者筛选表System Audit 系统稽查Study Site 研究中心Test Product 受试制剂Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Wash-out 洗脱Trial Objective 试验目的Triple Blinding 三盲Wash-out Period 洗脱期Alb白蛋白ALD(Approximate Lethal Dose)近似致死剂量ALP碱性磷酸酶Alpha spending function消耗函数ALT丙氨酸氨基转换酶Approval批准Analysis sets统计分析的数据集Approval批准ATR衰减全反射法Assistant investigator助理研究者AST天门冬酸氨基转换酶AUCss稳态血药浓度-时间曲线下面积Standard operating procedure (SOP)标准操作规程Case report form/ case record form(CRF)病例报告表病例记录表Clinical trial application (CTA)临床试验申请Clinical trial exemption (CTX)临床试验免责Clinical trial protocol (CTP)临床试验方案Contract research organization (CRO)合同研究组织Computer-assisted trial design (CA TD)计算机辅助试验设计Source data (SD)原始数据Electronic data capture (EDC)电子数据采集系统Source data verification (SDV)原始数据核准Electronic data processing (EDP)电子数据处理系统Subject enrollment log受试者入选表Institution review board (IBR)机构审查委员会Intention-to –treat (ITT)意向性分析(-统计学)Interactive voice response system (IVRS)互动式语音应答系统Investigator’s brochure (IB)研究者手册Maximum Tolerated Dose (MTD)最大耐受剂量Principle investigator (PI)主要研究者Product license (PL)产品许可证Serious adverse event (SAE)严重不良事件Serious adverse reaction (SAR)严重不良反应 . ..。
临床监查员专业术语

临床监查员专业术语、缩略语中英对照表缩略语英文全称中文全称ADE Adverse Drug Event药物不良事件ADR Adverse Drug Reaction药物不良反应AE Adverse Event不良事件AI Assistant Investigator助理研究者BMI Body Mass Index体质指数CI Co-investigator合作研究者COI Coordinating Investigator协调研究者CRA Clinical Research Associate临床监查员(临床监察员)CRC Clinical Research Coordinator临床研究协调者CRF Case Report Form病历报告表CRO Contract Research Organization合同研究组织CSA Clinical Study Application临床研究申请CTA Clinical Trial Application临床试验申请CTP Clinical Trial Protocol临床试验方案CTR Clinical Trial Report临床试验报告DSMB Data Safety and monitoring Board数据安全及监控委员会EDC Electronic Data Capture电子数据采集系统FDA Food and Drug Administration美国食品与药品管理局FR Final Report总结报告GCP Good Clinical Practice药物临床试验质量管理规范GLP Good Laboratory Practice药物非临床试验质量管理规范GMP Good Manufacturing Practice药品生产质量管理规范IB Investigator’ s Brochure研究者手册IC Informed Consent知情同意ICF Informed Consent Form知情同意书IDM Independent Data Monitoring独立数据监察IDMC Independent Data Monitoring Committee独立数据监察委员会IEC Independent Ethics Committee独立伦理委员会IND Investigational New Drug新药临床研究IRB Institutional Review Board机构审查委员会MA Marketing Approval/Authorization上市许可证NDA New Drug Application新药申请PI Principal Investigator主要研究者QA Quality Assurance质量保证QC Quality Control质量控制SAE Serious Adverse Event严重不良事件SAR Serious Adverse Reaction严重不良反应SD Source Data/Document原始数据 / 文件SD Subject Diary受试者日记SFDA State Food and Drug Administration国家食品药品监督管理局SDV Source Data Verification原始数据核准SEL Subject Enrollment Log受试者入选表SI Sub-investigator助理研究者SI Sponsor-Investigator申办研究者SIC Subject Identification Code受试者识别代码SOP Standard Operating Procedure标准操作规程SSL Subject Screening Log受试者筛选表WHO World Health Organization世界卫生组织。
临床试验常用术语缩写

专业术语缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co—investigator 合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织ECRF 电子化病历报告表CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure研究者手册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ECGElectrocardiogram心电图ICH International Conference on Harmonization 国际协调会IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断MA Marketing Approval/Authorization 上市许可证IVRS Interactive Voice Response System 互动语音应答系统MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国) PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre—market Approval (Application)上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记Subject identification code (SIC)受试者识别代码SFDA State Food and Drug Administration 国家食品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SI Sub—investigator 助理研究者SI Sponsor—Investigator 申办研究者SIC Subject Identification Code 受试者识别代码pd pharmacodynamics药物效应动力学SOP Standard Operating Procedure 标准操作规程pkpharmacokinetics药物代谢动力学SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织Active Control 阳性对照、活性对照WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议Unexpected adverse event (UAE)预料外不良事件Audit 稽查Audit Report 稽查报告Auditor 稽查员Blank Control 空白对照Blinding/masking 盲法/设盲Case History 病历Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance 依从性Coordinating Committee 协调委员会Cross-over Study 交叉研究Double Blinding 双盲Endpoint Criteria/measurement 终点指标Essential Documentation 必需文件Exclusion Criteria 排除标准Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Investigational Product 试验药物Investigator 研究者Monitor 监查员(监察员)Monitoring 监查(监察)Monitoring Plan 监查计划(监察计划)Monitoring Report 监查报告(监察报告)Multi-center Trial 多中心试验Non—clinical Study 非临床研究Original Medical Record 原始医疗记录Outcome Assessment 结果评价Patient File 病人档案Patient History 病历Placebo 安慰剂Placebo Control 安慰剂对照Preclinical Study 临床前研究Protocol 试验方案Protocol Amendments 修正案Randomization 随机Reference Product 参比制剂Sample Size 样本量、样本大小Seriousness 严重性Severity 严重程度Single Blinding 单盲Sponsor 申办者Study Audit 研究稽查Subject 受试者Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Subject Screening Log 受试者筛选表System Audit 系统稽查Study Site 研究中心Test Product 受试制剂Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Wash—out 洗脱Trial Objective 试验目的Triple Blinding 三盲Wash—out Period 洗脱期Alb白蛋白ALD(Approximate Lethal Dose)近似致死剂量ALP碱性磷酸酶Alpha spending function消耗函数ALT丙氨酸氨基转换酶Approval批准Analysis sets统计分析的数据集Approval批准ATR衰减全反射法Assistant investigator助理研究者AST天门冬酸氨基转换酶AUCss稳态血药浓度-时间曲线下面积Standard operating procedure (SOP)标准操作规程Case report form/ case record form(CRF)病例报告表病例记录表Clinical trial application (CTA)临床试验申请Clinical trial exemption (CTX)临床试验免责Clinical trial protocol (CTP)临床试验方案Contract research organization (CRO)合同研究组织Computer-assisted trial design (CATD)计算机辅助试验设计Source data (SD)原始数据Electronic data capture (EDC)电子数据采集系统Source data verification (SDV)原始数据核准Electronic data processing (EDP)电子数据处理系统Subject enrollment log受试者入选表Institution review board (IBR)机构审查委员会Intention—to –treat (ITT)意向性分析(-统计学)Interactive voice response system (IVRS)互动式语音应答系统Investigator’s brochure (IB)研究者手册Maximum Tolerated Dose (MTD)最大耐受剂量Principle investigator (PI)主要研究者Product license (PL)产品许可证Serious adverse event (SAE)严重不良事件Serious adverse reaction (SAR)严重不良反应。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
临床监查员专业术语、缩略语中英对照表
缩略语英文全称中文全称
ADE Adverse Drug Event 药物不良事件
ADR Adverse Drug Reaction 药物不良反应
AE Adverse Event 不良事件
AI Assistant Investigator 助理研究者
BMI Body Mass Index 体质指数
CI Co-investigator 合作研究者
COI Coordinating Investigator 协调研究者
CRA Clinical Research Associate 临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表
CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请
CTA Clinical Trial Application 临床试验申请
CTX Clinical Trial Exemption 临床试验免责
CTP Clinical Trial Protocol 临床试验方案
CTR Clinical Trial Report 临床试验报告
DSMB Data Safety and monitoring Board 数据安全及监控委员会
EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统
FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告
GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规范GMP Good Manufacturing Practice 药品生产质量管理规范IB Investig ator’s Brochure研究者手册
IC Informed Consent 知情同意
ICF Informed Consent Form 知情同意书
ICH International Conference on Harmonization国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会
IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究
IRB Institutional Review Board 机构审查委员会
IVD In Vitro Diagnostic 体外诊断
IVRS Interactive Voice Response System 互动语音应答系统MA Marketing Approval/Authorization 上市许可证
MCA Medicines Control Agency 英国药品监督局
MHW Ministry of Health and Welfare 日本卫生福利部NDA New Drug Application 新药申请
NEC New Drug Entity 新化学实体
NIH National Institutes of Health 国家卫生研究所(美国)PI Principal Investigator 主要研究者
PL Product License 产品许可证
PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计
学家协会
QA Quality Assurance 质量保证
QC Quality Control 质量控制
RA Regulatory Authorities 监督管理部门
SA Site Assessment 现场评估
SAE Serious Adverse Event 严重不良事件
SAP Statistical Analysis Plan 统计分析计划
SAR Serious Adverse Reaction 严重不良反应
SD Source Data/Document 原始数据/文件
SD Subject Diary 受试者日记
SFDA State Food and Drug Administration 国家食品药品监
督管理局
SDV Source Data Verification 原始数据核准
SEL Subject Enrollment Log 受试者入选表
SI Sub-investigator 助理研究者
SI Sponsor-Investigator 申办研究者
SIC Subject Identification Code 受试者识别代码
SOP Standard Operating Procedure 标准操作规程
SPL Study Personnel List 研究人员名单
SSL Subject Screening Log 受试者筛选表
T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议
Active Control 阳性对照、活性对照
Audit 稽查
Audit Report 稽查报告
Auditor 稽查员
Blank Control 空白对照
Blinding/masking 盲法/设盲
Case History 病历
Clinical study 临床研究
Clinical Trial 临床试验
Clinical Trial Report 临床试验报告
Compliance 依从性
Coordinating Committee 协调委员会
Cross-over Study 交叉研究
Double Blinding 双盲
Endpoint Criteria/measurement 终点指标
Essential Documentation 必需文件
Exclusion Criteria 排除标准
Inclusion Criteria 入选表准
Information Gathering 信息收集
Initial Meeting 启动会议
Inspection 检察/视察
Institution Inspection 机构检察
Investigational Product 试验药物
Investigator 研究者
Monitor 监查员(监察员)
Monitoring 监查(监察)
Monitoring Plan 监查计划(监察计划)
Monitoring Report 监查报告(监察报告) Multi-center Trial 多中心试验
Non-clinical Study 非临床研究
Original Medical Record 原始医疗记录
Outcome Assessment 结果评价
Patient File 病人档案
Patient History 病历
Placebo 安慰剂
Placebo Control 安慰剂对照
Preclinical Study 临床前研究
Protocol 试验方案
Protocol Amendments 修正案
Randomization 随机
Reference Product 参比制剂
Sample Size 样本量、样本大小
Seriousness 严重性
Severity 严重程度
Single Blinding 单盲
Sponsor 申办者
Study Audit 研究稽查
Subject 受试者
Subject Enrollment 受试者入选
Subject Enrollment Log 受试者入选表
Subject Identification Code List 受试者识别代码表 Subject Recruitment 受试者招募
Study Site 研究中心
Subject Screening Log 受试者筛选表
System Audit 系统稽查
Test Product 受试制剂
Trial Initial Meeting 试验启动会议
Trial Master File 试验总档案
Trial Objective 试验目的
Triple Blinding 三盲
Wash-out 洗脱
Wash-out Period 洗脱期。
